Abstract
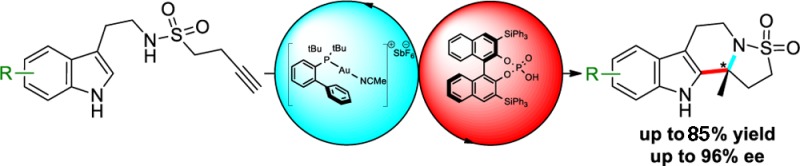
A highly enantioselective hydroamination/N-sulfonyliminium cyclization cascade is reported using a combination of gold(I) and chiral phosphoric acid catalysts. An initial 5-exo-dig hydroamination and a subsequent phosphoric acid catalyzed cyclization process provide access to complex sulfonamide scaffolds in excellent yield and high enantiocontrol. The method can be extended to lactam derivatives, with excellent yields and enantiomeric excesses of up to 93% ee.
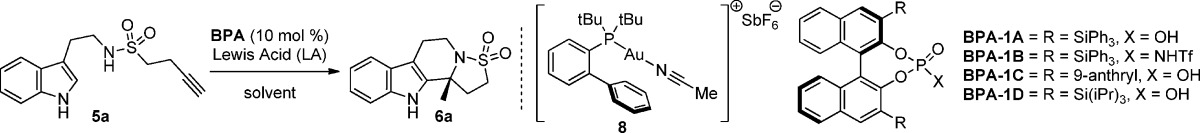
Enantioselective cascade processes exploiting one-pot combinations of gold and chiral binol phosphoric acid (BPA) catalysts to govern both the reaction pathway and stereocontrol are becoming useful in synthesis. Recently, reactions exploiting the ability of gold to facilitate cycloisomerization, isomerization, and tautomerism in combination with phosphoric acid or phosphate catalyzed hydrogenation, Pictet–Spengler, or (N-acyl) iminium ion cyclization processes have been reported.1,2 To this end, our group recently described a cascade process comprising alkynoic acid and tryptamine starting materials giving rise to polycyclic products with high enantioselectivities.3 In continuation of this work, we wished to examine the compatibility of a gold catalyzed hydroamination with an enantioselective phosphoric acid catalyzed N-sulfonyliminium cyclization, a sequence that has no precedent.
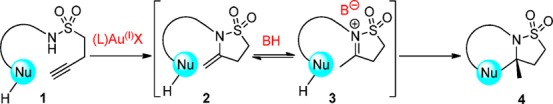
The proposed concept is depicted in Scheme 1 for an internal alkyl sulfonamide residue possessing a terminal alkyne 1. Our initial choice of sulfonamide over carboxylic acid amide was influenced in part by their abundance in medicinally relevant compounds4 and by the lack of such motifs in enantioselective cascade processes. We envisaged a novel alkyne hydroamination process to form isothiazolidine-1,1-dioxide (2), which could take place under gold(I) catalysis.5,6 Protonation would then afford the N-sulfonyliminium intermediate 3, which through tight ion pairing/general base catalysis with the conjugate base of the chiral phosphoric acid would facilitate an enantiofacially selective cyclization, thus providing a novel route to polycyclic isothiazolidine-1,1-dioxide moieties of type 4 (Scheme 1).7−10 Herein we report our findings.
Scheme 1. Concept of Dual Catalytic Gold and Organic Phosphoric Acid Enantioselective Cyclization Cascade.
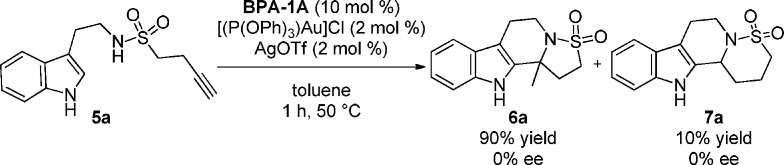
Proof of principle was first established after a brief screen of metal complexes by adding [P(OPh)3]AuCl (2 mol %) and AgOTf (2 mol %) in one portion to a mixture of sulfonamide 5a and BPA-1A (10 mol %, Table 1) in toluene at 50 °C (Scheme 2). The reaction furnished the cascade products 6a and 7a (5-exo and 6-endo respectively in 9:1 ratio) with quantitative mass return at a faster rate than previously reported in N-acyliminium cyclization cascades.3 Unfortunately, no enantioselectivity was witnessed. However, we later confirmed that this was due to a competing gold catalyzed background reaction;11 treatment of sulfonamide 5a with [P(OPh)3]AuCl (3 mol %) and AgOTf (3 mol %) at 60 °C in toluene afforded cyclization products 6a and 7a in quantitative yield as a 9:1 mixture of regioisomers respectively.12 A range of alternative alkynophilic Lewis acidic metal complexes were screened in an attempt to slow the undesirable background process. Pleasingly, with Echavarren’s catalyst13 (8), 6a was afforded in 56% yield and 73% ee (Table 1, entry 4). A brief solvent screen (Table 1, entries 4–8) identified toluene as the best solvent for enantioselectivity when compared with other aprotic solvents.14 To further minimize the competitive gold catalyzed background reaction and improve efficiency, the loading of gold catalyst 8 was decreased to 2 mol % (Table 1, entry 9), which indeed resulted in an increase in product enantiomeric excess. Performing the reaction at 60 °C, rather than at 100 °C, afforded higher yields of the desired reaction product but with reduced enantiomeric excess (Table 1, entries 9 and 11). However, due to the increased lifespan of the gold catalyst under these conditions we were able to lower the gold catalyst loading even further to 0.5–1.0 mol %, which proved beneficial to both reaction yield and enantioselectivity (Table 1, entries 12 and 13). A subsequent screen of chiral BPA derivatives provided no enhancement in enantioselectivity (Table 1, entries 15–17) and confirmed BPA-1A was optimal for the enantioselective cascade process.
Table 1. Optimization Studies.
| entrya | LA cat. | LA (mol %) | BPA (mol %) | acid (mol %) | solvent | temp (°C) | yield (%)b | ee (%) |
|---|---|---|---|---|---|---|---|---|
| 1 | [P(OPh)3]AuOTf | 2 | 1A | 10 | toluene | 100 | 26 | 17 |
| 2c | [NHC]AuOTfd | 7 | 1A | 10 | toluene | 100 | 71 | 52 |
| 3e | Cu(OTf)2 | 20 | 1A | 10 | toluene | 100 | 54 | 17 |
| 4f | 8 | 5 | 1A | 10 | toluene | 100 | 56 | 73 |
| 5 | 8 | 5 | 1A | 10 | (CH2Cl)2 | 60 | 83 | 46 |
| 6 | 8 | 5 | 1A | 10 | MeCN | 60 | 77 | 53 |
| 7 | 8 | 5 | 1A | 10 | hexane | 60 | 14 | 27 |
| 8 | 8 | 5 | 1A | 10 | THF | 60 | 83 | 0 |
| 9 | 8 | 2 | 1A | 10 | toluene | 100 | 68 | 87 |
| 10 | 8 | 2 | 1A | 10 | toluene | 95 | 68 | 85 |
| 11 | 8 | 2 | 1A | 10 | toluene | 60 | 82 | 81 |
| 12 | 8 | 1 | 1A | 10 | toluene | 60 | 84 | 88 |
| 13 | 8 | 0.5 | 1A | 10 | toluene | 60 | 78 | 88 |
| 14 | 8 | 0.1 | 1A | 10 | toluene | 60 | 21 | 92 |
| 15 | 8 | 1 | 1B | 10 | toluene | 60 | 83 | 45 |
| 16 | 8 | 1 | 1C | 10 | toluene | 60 | 85 | 71 |
| 17 | 8 | 1 | 1D | 10 | toluene | 60 | 79 | 45 |
All reactions proceeded for 20 h at 7 mM concentration (with respect to 5a) unless otherwise stated. No change in regioselectivity (9:1, 6a:7a) was witnessed throughout optimization.
Isolated yield.
Initial LA catalyst loading of 2 mol %; after 48 h additional LA was charged (5 mol %) and reacted for a further 12.5 h.
[NHC]AuOTf = {Au[1,3-bis(2,6-diisopropylphenyl)imidazol-2-ylidene]OTf}.
52 h reaction.
Reaction complete after 1 h.
Scheme 2. Proof of Principle Study in the N-Sulfonyliminium Cyclization Cascade.
With optimized conditions in hand a variety of substituted indole sulfonamide derivatives were cyclized with good to excellent yields and enantioselectivities up to 96% ee (Figure 1). The reaction was found to tolerate electron-withdrawing halides at various positions around the ring (6b to 6f) and the electron-deficient 5-nitrile derivative (6g), albeit at a slower reaction rate in the latter case due to its poor solubility in the reaction solvent. Substrates containing electron-donating groups (6h to 6k) were also found to work well. These derivatives demonstrated that all the possible positions around the carbocycle of the indole ring could be substituted without significant detriment to enantioselectivity or yield.
Figure 1.
Substrate scope. aIn all examples products of 5-exocyclization (6) were separated from 6-endo cyclization regioisomers (7) by FCC (9:1 crude ratio). Compound 7a was fully characterized in order to confirm the structure of the minor regioisomer. b Reaction time 60 h.
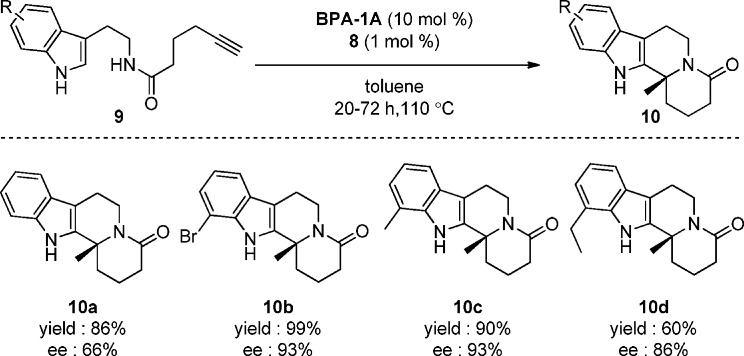
To further test the flexibility of this new methodology, attempts were made to perform the reaction cascade on the chain extended amide analogue 9a. Pleasingly, using 5 mol % of 8 and 10 mol % BPA-1A in refluxing toluene gave desired product 10a in 50% ee and 79% yield as a single regioisomer. Successful optimization of the enantioselectivity was achieved through lowering the loading of the gold catalyst (see Supporting Information); with 1 mol % of gold catalyst 8, lactam 10a was formed in 86% yield and 66% ee.
A collection of examples were examined to test the effects of electron withdrawing (10b) and donating (10c and 10d) substituents in this variant of our cascade process. Pleasingly the reactions proceeded with good to excellent yields and with enantiomeric excesses up to 93% (Figure 2).
Figure 2.
Application to δ-lactam synthesis via a 6-exo-cyclization cascade.
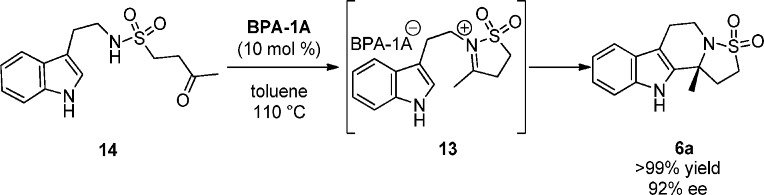
From the data collected during the development of our method (Table 1) and literature precedent, we propose the mechanism of the reaction proceeds through two sequential and independent catalyzed transformations (Scheme 3). A gold(I) complex activates alkyne 5 through π acid/base interactions lowering the energy of the alkyne LUMO,15 which allows the sulfonamide to attack selectively in a 5-exo fashion, presumably due to the gold alkyne bond being skewed toward the terminal end of the alkyne due to sterics.16 Protodeauration forms enesulfonamide 11. At this juncture intermediate 11 can take two potential pathways: (i) gold catalyzed nonenantioselective N-sulfonyliminium cyclization via 12(17,18) or (ii) the enantioselective BPA catalyzed N-sulfonyliminium cyclization via 13. Evidence that the enantioselective reaction pathway passes through an N-sulfonyliminium intermediate (not a gold(I) phosphate catalyzed intermediate19) came from an independent study (Scheme 4). Ketone 14 was prepared and treated with BPA-1A at 10 mol % in refluxing toluene. Product 6a was obtained in quantitative yield in 92% ee. That the sense and magnitude of the enantioselectivity in this reaction were the same as those in the cascade (Table 1, entry 14) points to a common intermediate 13.
Scheme 3. Proposed Cascade Mechanism.
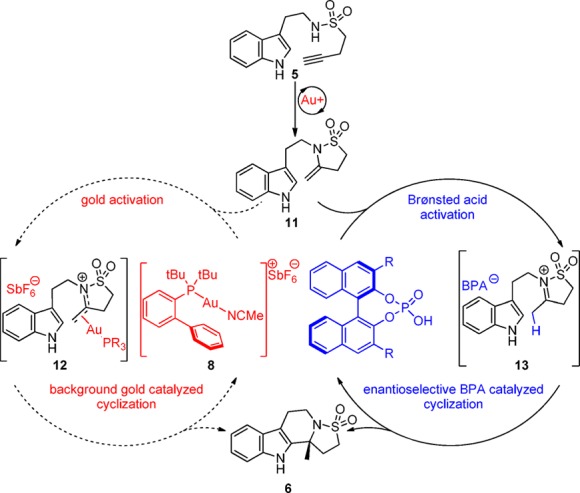
Scheme 4. Evidence for N-Sulfonyliminium Intermediate.
The absolute stereochemistry was assigned by single crystal X-ray diffraction of the 3-bromobenzylated compound, 15a (derived from 6a) and the lactam 10b (Figure 3). The absolute stereochemical configurations of the remaining derivatives were assigned by analogy and match the configuration of our previous findings.3
Figure 3.
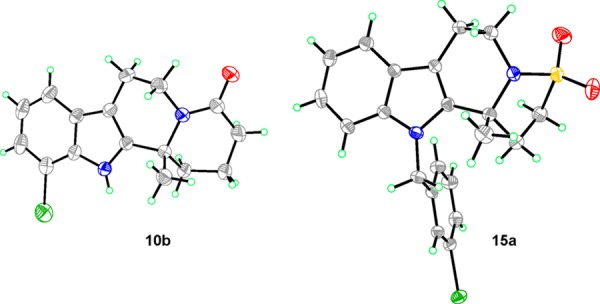
Thermal ellipsoid plot of 15a and 10b determined using single crystal X-ray diffraction data (1 equiv of 15a and solvent (10b) omitted for clarity). C, gray; Br, green; N, blue; O, red; S, yellow.
In conclusion we have developed a highly enantioselective N-sulfonyliminium cyclization cascade that allows access to complex and unusual sulfonamide scaffolds in excellent yield. We have also proven that this methodology can be used to create different ring systems of various sizes with most cases providing excellent yields and enantiomeric excesses. Mechanistic understanding has allowed us to control the background gold catalyzed reaction. Investigations into the development of more diverse cores possessing a range of different tethered nucleophiles are underway in our laboratory, and the results will be disclosed in due course.
Acknowledgments
We gratefully acknowledge the EPSRC (Leadership fellowship to D.J.D.; studentship to A.W.G.; postdoctoral fellowship to P.J.) and AstraZeneca for funding. We thank David M. Barber and Alison Hawkins of the Department of Chemistry for X-ray structure determination and the Oxford Chemical Crystallography Service for the use of the instrumentation.
Supporting Information Available
Experimental procedures and characterization of compounds are available with copies of 1H and 13C NMR spectra for all new compounds, as well as HPLC and X-ray data where applicable. This material is available free of charge via the Internet at http://pubs.acs.org.
The authors declare no competing financial interest.
Supplementary Material
References
- a Hashmi S. K.; Hubbert C. Angew. Chem., Int. Ed. 2010, 49, 1010. [DOI] [PubMed] [Google Scholar]; b Wang C.; Han Z.-Y.; Luo H.-W.; Gong L.-Z. Org. Lett. 2010, 12, 2266. [DOI] [PubMed] [Google Scholar]; c Liu X.-Y.; Che C.-M. Org. Lett. 2009, 11, 4204. [DOI] [PubMed] [Google Scholar]; d Rueping M.; Antonchick A. P.; Brinkmann C. Angew. Chem., Int. Ed. 2007, 46, 6903. [DOI] [PubMed] [Google Scholar]; e Patil N. T.; Mutyala A. K.; Konala A. K.; Tella R. B. Chem. Commun. 2012, 48, 3094. [DOI] [PubMed] [Google Scholar]; f Liu X.-Y.; Xiao Y.-P.; Siu F.-M.; Ni L.-C.; Chen Y.; Wang L.; Che C.-M. Org. Biomol. Chem. 2012, 10, 7208. [DOI] [PubMed] [Google Scholar]; g Wu H.; He Y.-P.; Gong L.-Z. Adv. Synth. Catal. 2012, 354, 975. [Google Scholar]; h Cai Q.; Liang X.-W.; Wang S.-G.; Zhang J.-W.; Zang X.; You S.-L. Org. Lett. 2012, 14, 5022. [DOI] [PubMed] [Google Scholar]; i Ren L.; Lei T.; Ye J.-X.; Gong L.-Z. Angew. Chem., Int. Ed. 2012, 51, 771. [DOI] [PubMed] [Google Scholar]; j Liu X.-Y.; Che C.-M. Org. Lett. 2009, 11, 4204. [DOI] [PubMed] [Google Scholar]; k Han Z.-Y.; Xiao H.; Chen X.-H.; Gong L.-Z. J. Am. Chem. Soc. 2009, 131, 9182. [DOI] [PubMed] [Google Scholar]; l Muratore M. E.; Shi L.; Pilling A. W.; Storer R. I.; Dixon D. J. Chem. Commun. 2012, 48, 6351. [DOI] [PubMed] [Google Scholar]; m Enomoto T.; Girard A.-L.; Yasui Y.; Takemoto Y. J. Org. Chem. 2009, 74, 9158. [DOI] [PubMed] [Google Scholar]; n Cai Q.; Liu C.; Liang X.-W.; You S.-L. Org. Lett. 2012, 14, 4588. [DOI] [PubMed] [Google Scholar]; o Zhang J.-W.; Xu Z.; Gu Q.; Shi X.-X.; Leng X.-B.; You S.-L. Tetrahedron. 2012, 68, 5263. [Google Scholar]; p Gómez-SanJuan A.; Sotomayor N.; Lete E. Tetrahedron Lett. 2012, 53, 2157. [Google Scholar]; q Aranzamendi E.; Sotomayor N.; Lete E. J. Org. Chem. 2012, 77, 2986. [DOI] [PubMed] [Google Scholar]; r Rueping M.; Volla C. M. R.; Bolte M.; Raabe G. Adv. Synth. Catal. 2011, 353, 2853. [Google Scholar]; s Duce S.; Pesciaioli F.; Gramigna L.; Bernard L.; Mazzanti A.; Ricci A.; Bartoli G.; Bencivennia G. Adv. Synth. Catal. 2011, 353, 860. [Google Scholar]; t Rueping M.; Volla C. M. R. RSC Adv. 2011, 1, 79. [Google Scholar]; u Rueping M.; Raja S.; Nunez A. Adv. Synth. Catal. 2011, 353, 563. [Google Scholar]; v Patil N. T.; Raut V. S.; Tella R. B. Chem. Commun. 2013, 49, 570. [DOI] [PubMed] [Google Scholar]; w Wu H.; He Y.-P.; Gong L.-Z. Org. Lett. 2013, 15, 460. [DOI] [PubMed] [Google Scholar]; x Courant T.; Kumarn S.; He L.; Retailleau P.; Masson G. Adv. Synth. Catal. 2013, 355, 836. [Google Scholar]; y Cai Q.; Liang X.-W.; Wang S.; You S.-L. Org. Biomol. Chem. 2013, 11, 1602. [DOI] [PubMed] [Google Scholar]; z Aillaud I.; Barber D. M.; Thompson A. L.; Dixon D. J. Org. Lett. 2013, 15, 2946. [DOI] [PMC free article] [PubMed] [Google Scholar]; aa Cala L.; Mendoza A.; Fananas F. J.; Rodriguez F. Chem. Commun. 2013, 49, 2715. [DOI] [PubMed] [Google Scholar]
- For recent reviews on cascade and dual catalysis, see:; a Rueping M.; Koenigs R. M.; Atodiresei I. Chem.—Eur. J. 2010, 16, 9350. [DOI] [PubMed] [Google Scholar]; b Shao A.; Zhang H. Chem. Soc. Rev. 2009, 38, 2745. [DOI] [PubMed] [Google Scholar]; c Parra A.; Reboredo S.; Martín Castro A. M.; Alemán J. Org. Biomol. Chem. 2012, 10, 5001. [DOI] [PubMed] [Google Scholar]; d Zhong C.; Shi X. Eur. J. Org. Chem. 2010, 16, 2999. [Google Scholar]; e Loh C. C. G.; Enders D. Chem.—Eur. J. 2012, 18, 10212. [DOI] [PubMed] [Google Scholar]; f Grondall C.; Jeanty M.; Enders D. Nat. Chem. 2010, 2, 167. [DOI] [PubMed] [Google Scholar]; g Du Z.; Shao Z. Chem. Soc. Rev. 2013, 42, 1337. [DOI] [PubMed] [Google Scholar]
- a Muratore M. E.; Holloway C. A.; Pilling A. W.; Storer I.; Trevitt G.; Dixon D. J. J. Am. Chem. Soc. 2009, 131, 10796. [DOI] [PubMed] [Google Scholar]; b Holloway C. A.; Muratore M. E.; Storer R. I.; Dixon D. J. Org. Lett. 2010, 12, 4720. [DOI] [PubMed] [Google Scholar]; c Raheem I. T.; Thiara P. S.; Peterson E. A.; Jacobsen E. N. J. Am. Chem. Soc. 2007, 129, 13404. [DOI] [PubMed] [Google Scholar]
- a Alemán J.; Solar V.; Alvarez-Valdés A.; Ríos-Luci C.; Padrón J. M.; Navarro-Ranninger C. Med. Chem. Commun. 2011, 2, 789. [Google Scholar]; b Supuran C. T.; Scozzafava A. Exp. Opin. Ther. Pat. 2002, 12, 217. [Google Scholar]
- a Wang H.-F.; Yang T.; Xu P.-F.; Dixon D. J. Chem. Commun. 2009, 3916. [DOI] [PubMed] [Google Scholar]; b Kothandaraman P.; Rao W.; Foo S. J.; Chan P. W. H. Angew. Chem., Int. Ed. 2010, 49, 4619. [DOI] [PubMed] [Google Scholar]; c Cheung F. K.; Hayes A. M.; Morris D. J.; Wills M. Org. Biomol. Chem. 2007, 5, 1093. [DOI] [PubMed] [Google Scholar]; d Barber D. M.; Sanganee H.; Dixon D. J. Chem. Commun. 2011, 47, 4379. [DOI] [PubMed] [Google Scholar]; e Barber D. M.; Sanganee H. J.; Dixon D. J. Org. Lett. 2012, 14, 5290. [DOI] [PubMed] [Google Scholar]; f Patil N. T.; Shinde V. S.; Sridhar B. Angew. Chem., Int. Ed. 2013, 52, 2251. [DOI] [PubMed] [Google Scholar]
- For recent reviews on gold catalysis, see:; a Hashmi S. K. Chem. Rev. 2007, 107, 3180. [DOI] [PubMed] [Google Scholar]; b Li Z.; Brouwer C.; He C. Chem. Rev. 2008, 108, 3239. [DOI] [PubMed] [Google Scholar]; c Arcadi A. Chem. Rev. 2008, 108, 3266. [DOI] [PubMed] [Google Scholar]; d Gorin D. J.; Sherry B. D.; Toste F. D. Chem. Rev. 2008, 108, 3351. [DOI] [PMC free article] [PubMed] [Google Scholar]; e Corma A.; Leyva-Pérez A.; Sabater M. J. Chem. Rev. 2011, 111, 1657. [DOI] [PubMed] [Google Scholar]; f Rudolph M.; Hashmi A. S. K. Chem. Commun. 2011, 47, 6536. [DOI] [PubMed] [Google Scholar]; g Hashmi A. S. K.; Hutchings G. J. Angew. Chem., Int. Ed. 2006, 45, 7896. [DOI] [PubMed] [Google Scholar]; h Toste F. D. Nature 2007, 446, 395–403. [DOI] [PubMed] [Google Scholar]; i Modern Gold Catalyzed Synthesis; Hashmi A. S. K., Toste F. D., Eds.; WILEY-VCH: Weinheim, 2012. [Google Scholar]; j Furstner A. Chem. Soc. Rev. 2009, 38, 3208. [DOI] [PubMed] [Google Scholar]
- For recent reviews on phosphoric acid catalysis, see:; a Rueping M.; Nachtsteim B. J.; Ieawsuwan W.; Atodiresei I. Angew. Chem., Int. Ed. 2011, 50, 6706. [DOI] [PubMed] [Google Scholar]; b Terada M. Synthesis 2010, 12, 1929. [Google Scholar]; c Terada M. Chem. Commun. 2008, 4097. [DOI] [PubMed] [Google Scholar]; d Akiyama T. Chem. Rev. 2007, 107, 5744. [DOI] [PubMed] [Google Scholar]; e Akiyama T.; Itoh J.; Fuchibe K. Adv. Synth. Catal. 2006, 348, 999. [Google Scholar]; f de Vries J. G.; Mrsic N. Catal. Sci. Technol. 2011, 1, 727. [Google Scholar]
- a Uraguchi D.; Terada M. J. Am. Chem. Soc. 2004, 126, 5356. [DOI] [PubMed] [Google Scholar]; b Uraguchi D.; Sorimachi K.; Terada M. J. Am. Chem. Soc. 2004, 126, 11804. [DOI] [PubMed] [Google Scholar]; c Akiyama T.; Itoh J.; Yokota K.; Fuchibe K. Angew. Chem., Int. Ed. 2004, 43, 1566. [DOI] [PubMed] [Google Scholar]
- a Wang B.-L.; Zhang J.-X.; Li N.-K.; Liu G.-G.; Shen Q.; Wang X.-W. Tetrahedron Lett. 2011, 52, 4671. [Google Scholar]; b Kang Q.; Zheng X.-J.; You S.-L. Chem.—Eur. J. 2008, 14, 3539. [DOI] [PubMed] [Google Scholar]; c Kang Q.; Zhao Z.-A.; You S.-L. J. Am. Chem. Soc. 2007, 129, 1484. [DOI] [PubMed] [Google Scholar]; d Chen L.-Y.; He H.; Chan W.-H.; Lee A. W. M. J. Org. Chem. 2011, 76, 7141. [DOI] [PubMed] [Google Scholar]; e Xing C.-H.; Liao Y.-X.; Ng J.; Hu Q.-S. J. Org. Chem. 2011, 76, 4125. [DOI] [PubMed] [Google Scholar]; f Xu F.; Huang D.; Han C.; Shen W.; Lin X.; Wang Y. J. Org. Chem. 2010, 75, 8677. [DOI] [PubMed] [Google Scholar]
- Fleischmann M.; Drettwan D.; Sugiono E.; Rueping M.; Gschwind R. M. Angew. Chem., Int. Ed. 2011, 50, 6364. [DOI] [PubMed] [Google Scholar]
- a Bandini M.; Bottoni A.; Chiarucci M.; Cera G.; Miscione G. P. J. Am. Chem. Soc. 2012, 134, 20690. [DOI] [PubMed] [Google Scholar]
- Treatment of alkyne 5a with BPA-1A in refluxing toluene resulted in no reaction and showed only pure starting material.
- a Nieto-Oberhuber C.; Lopez S.; Munoz M. P.; Cardenas D. J.; Bunuel E.; Nevado C.; Echavarren A. M. Angew. Chem., Int. Ed. 2005, 44, 6146. [DOI] [PubMed] [Google Scholar]; b Perez-Galan P.; Delpont N.; Herrero-Gomez E.; Maseras F.; Echavarren A. M. Chem.—Eur. J. 2010, 16, 5324. [DOI] [PubMed] [Google Scholar]; c Herrero-Gomez E.; Nieto-Oberhuber C.; Lopez S.; Benet-Buchholz J.; Echavarren A. M. Angew. Chem., Int. Ed. 2006, 45, 5455. [DOI] [PubMed] [Google Scholar]
- This may be due to its ability to increase tight ion pairing:Brak K.; Jacobsen E. N. Angew. Chem., Int. Ed. 2013, 52, 534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidbaur H.; Schier A. Organometallics 2010, 29, 2. [Google Scholar]
- a Soriano E.; Marco-Contelles J. Chem.—Eur. J. 2008, 14, 6771. [DOI] [PubMed] [Google Scholar]; b Soriano E.; Marco-Contelles J. Acc. Chem. Res. 2009, 42, 1026. [DOI] [PubMed] [Google Scholar]; c Li G.; Zhang Z.; Zhang L. J. Am. Chem. Soc. 2008, 130, 3740. [DOI] [PubMed] [Google Scholar]
- a LaLonde R. L.; Brenzovich W. E. Jr.; Benitez D.; Tkatchouk E.; Kelley K.; Goddard W. A. III; Toste F. D. Chem. Sci. 2010, 1, 226. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Liu L.; Hammond G. B. Chem. Soc. Rev. 2012, 41, 3129. [DOI] [PubMed] [Google Scholar]
- We cannot rule out Lewis acid assisted Brønsted acid catalysis:Yang T.; Campbell L.; Dixon D. J. J. Am. Chem. Soc. 2007, 129, 12070. [DOI] [PubMed] [Google Scholar]
- Raducan M.; Moreno M.; Boura C.; Echavarren A. M. Chem. Commun. 2012, 48, 52. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.