Abstract
Interleukin-1 (IL-1) is a central mediator of innate immunity and inflammation. The IL-1 family includes 7 ligands with agonist activity (IL-1α and β, IL-18, IL-33, IL-36α, β, γ), three receptor antagonists (IL-1Ra, IL-36Ra, IL-38) and an anti-inflammatory cytokine (IL-37). Members of the IL-1 Receptor (IL-1R) family include 6 receptor chains forming 4 signaling receptor complexes, two decoy receptors (IL-1R2, IL-18BP) and two negative regulators (TIR8 or SIGIRR, IL-1RAcPb). A tight regulation via receptor antagonists, decoy receptors and signaling inhibitors ensures a balance between amplification of innate immunity and uncontrolled inflammation. All cells of the innate immune system express and/or are affected by IL-1 family members. Moreover, IL-1 family members play a key role in the differentiation and function of polarized innate and adaptive lymphoid cells. Here we will review the key properties of IL-1 family members, with emphasis on pathways of negative regulation and orchestration of innate and adaptive immunity.
INTRODUCTION
Interleukin-1 (IL-1) was the first interleukin to be identified (for recent review, (Dinarello, 2009, 2010; Dinarello et al., 2012; Gabay et al., 2010; Sims and Smith, 2010) and has served as a groundbreaking molecule with implications extending far beyond its extended family. The original description of a cytokine acting at vanishingly low concentrations on cells and organs as diverse as the hypothalamus and T lymphocytes was without precedent in biology (for review (Dinarello, 2009): pleiotropism turned out to be a common property among cytokines. Early on it was realized that IL-1 was responsible for resistance against microbes (van der Meer et al., 1988) a discovery upstream of the identification of the TIR domain (originally standing for Toll-IL-1 resistance; shared by the IL-1 receptor (IL-1R) and Toll-like receptors (TLR)), and of the inflammasome. Along the same line the function of MyD88 as a key adaptor was first discovered for IL-1R and then extended to TLR (Muzio et al., 1998; Muzio et al., 1997; Sims and Smith, 2010). The IL-1R2 receptor was identified as a decoy for IL-1 (Colotta et al., 1993), a paradigm shift since the original definition of “receptor” by Langley in the XIX century (Langley, 1906). Decoy receptors have since emerged as a general strategy conserved in evolution to limit the action of cytokines, chemokines and growth factors. Thus, IL-1 has served as a forerunner for intercellular molecules and paradigms, which have had a broad impact in immunology and medicine at large.
The IL-1 and IL-1R families have grown impressively in size, complexity, and division of labour (Dinarello et al., 2010; Dinarello, 2009, 2010; Dinarello et al., 2012; Gabay et al., 2010). The discovery of innate lymphoid cells and the dissection of pathways of T cell differentiation have revealed essential functions for IL-1, IL-18 and IL-33 and have opened vistas on their functions (O’Shea and Paul, 2010; Spits et al., 2013). No less important have been the clinical implications of IL-1 research. Autoinflammatory diseases are uniquely IL-1 mediated disorders and anti-IL-1 therapies have had a tremendous impact on inflammatory diseases (Dinarello, 2010; Dinarello et al., 2012). Here we will review the common characteristics of IL-1 family members and specific receptors. Emphasis will be on the immunobiology of IL-1, its relatives and their receptors with a focus on selected cytokines (e.g. IL-33, IL-18, IL-36), pathways of negative regulation, orchestration of innate and adaptive lymphoid cells, and clinical implications.
AN OVERVIEW OF IL-1 AND IL-1R FAMILY MEMBERS
As shown in Fig.1A and B and Table 1, IL-1 family ligands include 7 molecules with agonist activity (IL-1α, IL-1β, IL-18, IL-33, IL-36α, β, and γ), three receptor antagonists (IL-1Ra, IL-36Ra and IL-38), and an anti-inflammatory cytokine (IL-37). The IL-1R family members include 11 molecules. A simplified nomenclature for IL-1R members is proposed here: IL-1R1 (IL-1RI); IL-1R2 (IL-1RII), IL-1R3 (IL-1RAcP), IL-1R4 (ST2), IL-1R5 (IL-18Rα), IL-1R6 (IL-1Rrp2, IL-36R), IL-1R7 (IL-18Rβ), IL-1R8 (TIR8, also known as SIGIRR), IL-1R9 (TIGIRR-2), IL-1R10 (TIGIRR-1).
Fig. 1.
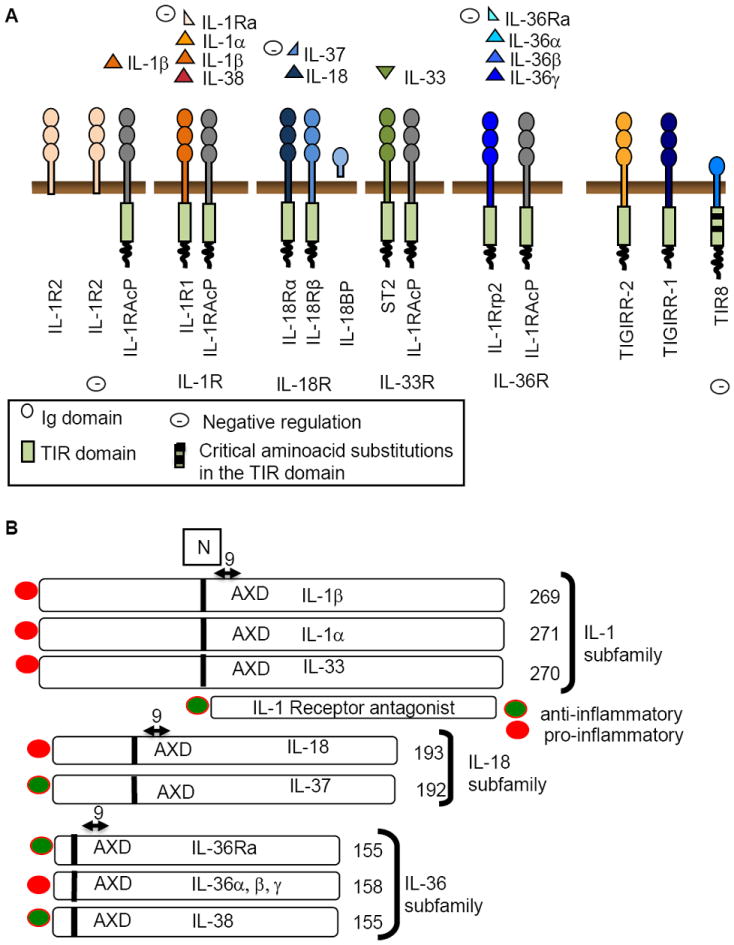
Panel A. A schematic representation of ligands and receptor chains in the IL-1 family. The minus sign indicates inhibition. TIR8 (also known as SIGIRR) has two aminoacid substitutions (Cys222 and Leu305 for canonical Ser447 and Tyr536). Panel B. Subfamilies among IL-1 ligands, divided based on the length of the N-terminal prodomain. Numbers refer to aminoacids. The cleavage site (N) is located 9 amino acids N-terminal to AXD, a conserved motif, where A is an aliphatic amino acid. IL-37 has been identified only in man.
Table 1.
Nomenclature and main functions of IL-1 family members
| Cytokine | Alternative name | Receptor | Co-receptor | Proposed nomenclature: | Activity | |
|---|---|---|---|---|---|---|
| Receptor | Co-receptor | |||||
|
| ||||||
| IL-1α | IL-1F1 | IL-1R1 | IL-1RAcP | IL-1R3 | Alarmin, inflammation, Th17 cell responses | |
| IL-1R2 | ||||||
|
| ||||||
| IL-1β | IL-1F2 | IL-1R1 | IL-1RAcP | IL-1R3 | Antimicrobial resistance, inflammation, Th17 cell responses | |
| IL-1R2 | ||||||
|
| ||||||
| IL-1Ra | IL-1F3 | IL-1R1 | Inhibition of inflammation | |||
|
| ||||||
| IL-18 | IL-1F4 | IL-18Rα | IL-18Rβ | IL-1R5 | IL-1R7 | Inflammation, Th1 cell responses |
|
| ||||||
| IL-33 | IL-1F11 | ST2 | IL-1RAcP | IL-1R4 | IL-1R3 | Inflammation, Th2 cell responses |
|
| ||||||
| IL-36α | IL-1F6 | IL-1Rrp2 (IL-36R) | IL-1RAcP | IL-1R6 | IL-1R3 | Skin and lung inflammation |
|
| ||||||
| IL-36β | IL-1F7 | IL-1Rrp2 (IL-36R) | IL-1RAcP | IL-1R6 | IL-1R3 | |
|
| ||||||
| IL-36γ | IL-1F8 | IL-1Rrp2 (IL-36R) | IL-1RAcP | IL-1R6 | IL-1R3 | |
|
| ||||||
| IL-36Ra | IL-1F5 | IL-1Rrp2 (IL-36R) | IL-1R6 | Inhibition of inflammation | ||
|
| ||||||
| IL-37 | IL-1F7 | IL-18Rα | Inhibition of inflammation | |||
|
| ||||||
| IL-38 | IL-1F10 | IL-1Rrp2 (IL-36R) | Inhibition of inflammation | |||
|
| ||||||
| TIR8 (SIGIRR) | IL-1R8 | Inhibition of inflammation | ||||
|
| ||||||
| TIGIRR-1 (IL1RAPL2) | IL-1R9 | not known | ||||
|
| ||||||
| TIGIRR-2 | IL-1R10 | not known | ||||
|
| ||||||
| IL-1R1 (?) | IL-1RAcPb | IL-1R3b | Inhibition of inflammation | |||
?: not formally proven to interact with IL-1RAcPb.
The receptor chains are generally characterized by an extracellular portion consisting of three Ig-like domains. Notable exceptions are the IL-18 binding protein (IL-18BP) and TIR8, which have a single Ig domain. The intracellular portions are characterized by a TIR domain essential for signaling via the MyD88 adaptor. The canonical TIR domain present in signaling receptors of the IL-1 family is shared by TLR. As discussed below, at least IL-1α and possibly IL-33 are preformed and released upon tissue damage, acting as bona fide alarmins. Thus, the TIR domain is a key transducer in sensing of microbes, tissue damage, driving amplification of innate immunity and inflammation. IL-1R signaling will not be reviewed here and the reader is referred to (Sims and Smith, 2010). Four signaling receptor complexes are formed: the IL-1 receptor (IL-1R1 and IL-1RAcP); the IL-33 receptor (ST2 and IL-1RAcP); the IL-18 receptor (IL-18Rα and IL-18Rβ); the IL-36 receptor (IL-1Rrp2 and IL-1RAcP). Two members of the family are decoy receptors (IL-R2 and IL-18BP). TIR8 has no ligand and acts as a negative regulator.
IL-1
IL-1 affects virtually all cells and organs and is a major pathogenic mediator of autoinflammatory, autoimmune, infectious and degenerative diseases (Dinarello, 2009, 2010; Dinarello et al., 2012; Gabay et al., 2010; Sims and Smith, 2010). Here we will summarize functions related to immunity and then focus on selected aspects (e.g. why two IL-1s; cancer; metabolism). Fig. 2A highlights actions of IL-1 which are directly related to immunity. The effects of IL-1 on the central nervous system include fever (IL-1 is the classic endogenous pyrogen (Dinarello, 2009, 2010; Sims and Smith, 2010)) and activation of the hypothalamus-pituitary-adrenal (HPA) axis. At elevated temperature, leukocyte migration is increased. Cortisol downstream of the HPA axis has a regulatory function on innate immunity and inflammation. The general significance of the acute phase response, triggered via IL-6 in the liver, is to amplify innate resistance mediated by the humoral arm of innate immunity (e.g. C-reactive protein (CRP); mannose binding lectin; complement components) and to regulate tissue damage (e.g. α1-antitrypsin). Induction of adhesion molecules in endothelial cells and chemokines results in amplification of leukocyte recruitment and innate resistance to infection. IL-1 markedly prolongs the lifespan and stimulates the effector function of neutrophils and macrophages (Mantovani et al., 2011). In addition, IL-1 orchestrates the differentiation and function of innate and adaptive lymphoid cells (see below).
Fig. 2.
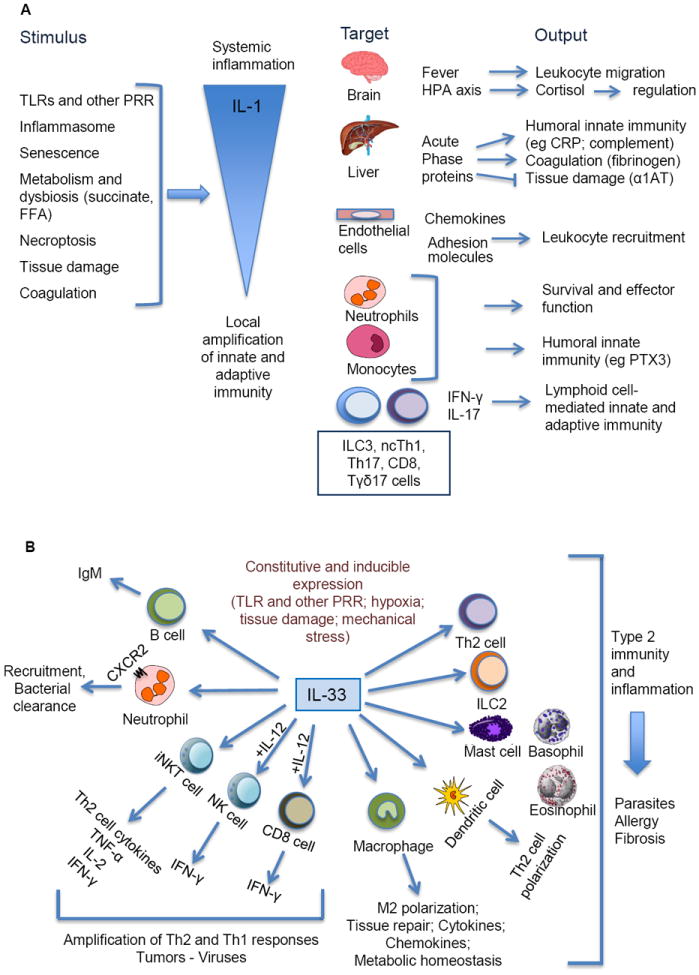
A schematic representation of role of IL-1 (panel A) and IL-33 (panel B) in innate and adaptive immunity. “+IL-12” refers to costimulation by IL-33 + IL-12 of target cells. PRR, pattern recognition receptor; Nc Th1, non-conventional Th1; α1AT, α1 antitrypsin inhibitor; CRP, C reactive protein; PTX3, pentraxin 3; HPA, hypothalamus-pituitary-adrenal axis; FFA, free fatty acid.
IL-1α and IL-1β, are encoded by distinct genes, bind to the same receptor (IL-1R1) and have similar biological properties. However, distinctions do exist and impact on immunity, inflammation and cancer. The IL-1α precursor is constitutively present in epithelial layers of the entire gastrointestinal tract, lung, liver, kidney, endothelial cells, and astrocytes. Upon cell death by necrosis, as occurs in ischemic diseases such as myocardial infarction, stroke, acute renal failure and tumor necrosis, the IL-1α precursor is released. Unlike the IL-1β precursor, the IL-1α precursor is fully active and functions as an “alarmin” by rapidly initiating a cascade of inflammatory cytokines and chemokines, which accounts for sterile inflammation (Chen et al., 2007; Rider et al., 2011). Thus, IL-1α mediates the early phases of sterile inflammation. In addition to the IL-1α precursor released from necrotic cells, there is a membrane form of IL-1α present on activated monocytes. However, circulating IL-1α is rarely detected even in persons with severe infections but is contained in apoptotic bodies released from endothelial cells (Berda-Haddad et al., 2011).
In contrast, IL-1β is produced by hematopoietic cells such as blood monocytes, tissue macrophages, skin dendritic cells and brain microglia in response TLR, activated complement components, other cytokines (such as TNF-α) and IL-1 itself (Dinarello, 2011). Unlike the IL-1α precursor, the IL-1β precursor is not active but is cleaved by caspase-1, releasing the active cytokine into the extracellular space. Although caspase-1 is abundant in hematopoietic cells, the proenzyme (procaspase-1) first requires cleavage by the inflammasome. The key component of this macromolecular complex is the protein-nucleotide-binding domain and leucine-rich repeat pyrin containing protein-3 (NLRP3) (Hoffman et al., 2001), also called cryopyrin. Single amino acid gain of function mutations in cryopyrin result in high amounts of actively secreted IL-1β. Elevated secretion of IL-1β is linked to inflammation in patients with these mutations, termed autoinflammatory diseases. However, not all IL-1β-mediated inflammation is due to NLRP3 or caspase-1 activity. Mice deficient in caspase-1 develop the same IL-1β-mediated disease as do wild-type mice (Dinarello, 2009, 2011). Extracellular cleavage of the inactive IL-1β precursor by neutrophil enzymes such a proteinase-3 and elastase generate active IL-1β because the cleavage site is close to that of caspase-1 (Dinarello, 2011). In addition, caspase-11 provides a non-canonical inflammasome component important for the response to cholera toxin B or selected microbes (Kayagaki et al., 2011).
Another distinction between IL-1α and IL-1β can be found in carcinogenesis; mice deficient in IL-1β develop fewer tumors compared IL-1α-deficient or wild-type mice (Krelin et al., 2007). IL-1β induces tumor angiogenesis and metastatic spread of tumors (Carmi et al., 2013). IL-1 is indeed an important component of the inflammatory microenvironment of tumors (Mantovani et al., 2008). Early observations on augmentation of metastasis by IL-1 were subsequently extended to primary carcinogenesis in different organs (Apte et al., 2006; Salcedo et al., 2013). IL-1α is a component of the intrinsic pathway linking genetic events causing cancer (Ras mutation) and the orchestration of cancer-related inflammation (Salcedo et al., 2013). Moreover, IL-1 plays a critical role in inflammatory conditions which increase cancer incidence (extrinsic pathway) as revealed by carcinogenesis in the pancreas, skin and liver (Salcedo et al., 2013). Given the diversity of cancer-related inflammation in different organs, exploitation of anti-IL-1 strategies in human cancer will require careful dissection of its role in different human neoplasms. Neutralizing antibodies to IL-1α have entered clinical trial in cachectic patients with terminal colon cancer with encouraging results (Hong et al., 2011).
Differences between IL-1α and IL-1β are for the most part differences due to cell sources of each cytokine and release mechanisms but not to differences in downstream events following receptor engagement. Having said that, IL-1α localizes to the nucleus and functions as a component of transcription whereas IL-1β has never been observed in the nucleus. The propiece of IL-1α contains a string of basic amino acids termed the nuclear localization sequence (NLS). The NLS accounts for the binding of the entire IL-1α precursor to DNA. The IL-1α propiece itself may act as an oncoprotein and expression of the propiece induces neoplastic changes in cells. The IL-1α precursor shuttles between the cytosol and nucleus with amazing rapidity (Cohen et al., 2010). Upon a signal to initiate apoptosis, cytosolic IL-1α moves to the nucleus and remains tightly bound to chromatin. In contrast, with a signal to undergo necrosis, for example due to hypoxia, IL-1α leaves the nucleus and resides in the cytosolic compartment (Cohen et al., 2010). With necrotic cell death, the IL-1α precursor is released (Rider et al., 2011) initiating neutrophilic inflammation (Rider et al., 2011), whereas in cells dying of apoptosis, chromatin-bound IL-1α is unavailable for initiating inflammation.
IL-1 production can be a consequence of alterations in the homeostatic metabolic state as observed in obesity (dysbiosis) (Tack et al., 2012) (Fig. 2A). Recent results have highlighted differential induction and role of IL-1α and IL-1β in fat induced vascular responses and atherosclerosis (Freigang et al., 2013). Atheroma formation has long been associated with IL-1β. In an unexpected twist, fatty acids were found to elicit release of IL-1α but not of IL-1β. This selective induction was secondary to mitochondrial uncoupling which blocked cholesterol crystal-elicited IL-1β production. Thus metabolic stress triggers a selective IL-1α-sustained pathway of vascular inflammation and pathology.
IL-1 is likely to orient macrophages to aerobic glycolysis as lipopolysaccharide (LPS) does (Rodriguez-Prados et al., 2010) and in turn it is downstream of metabolic responses elicited by microbial sensing. Accumulation of succinate in classically activated M1 macrophages (Tannahill et al., 2013) stabilizes hypoxia-inducible factor α (HIF-1α) which in turn induces IL-1β. Thus, a metabolic signal represented by succinate serves as a selective trigger of HIF-1α-dependent IL-1β production and as an amplifier of innate immunity and inflammation.
IL-33
IL-33 is a cytokine mainly involved in type 2 immunity and inflammation. Its main effects on innate and adaptive cells, including innate lymphoid cell-2 (ILC2), T helper-2 (Th2) cells and alternatively activated M2 polarized macrophages, are consistent with this general function (Fig.2B).
IL-33 is a 30 kDa protein, composed of an N-terminal domain containing a chromatin binding motif and a 18 kDa C-terminal region rich in β-sheets, expressed in a constitutive or inducible manner by several stromal, parenchymal and hematopoietic cell types. IL-33 is inactivated by caspase-1 (Cayrol and Girard, 2009) and the 30KDa protein is one of its bioactive forms. IL-33 is released as a bioactive molecule mainly upon necrotic cell death or secreted by unconventional mechanisms. As for other IL-1 family members, calpain and the neutrophil serine proteases cathepsin G and elastase cleave human IL-33 and generate more potent mature forms (Lefrancais et al., 2012).
Fig. 2B presents in a schematic way the spectrum of action of IL-33. Mature IL-33 signals through the ST2 receptor, which associates with IL-1AcP to induce MyD88-dependent signalling (Fig. 1A). The crystal structure of IL-33 in complex with the ectodomain of ST2 has recently been solved (Liu et al., 2013). ST2 is expressed on various innate and adaptive immune cell types, and drives the production of type 2 cytokines, which are responsible of protective type 2 inflammatory responses in infection and tissue repair, as well as of detrimental allergic responses. Genetic variation in the coding region at the IL1RL1, the gene coding for ST2, results in different concentrations of soluble ST2 (Ho et al., 2013).
Negative regulation of IL-33 activity is provided by soluble ST2 and soluble IL-1RAcP which together bind IL-33 and prevent its activity. In addition, TIR8 has been reported to negatively regulate IL-33-dependent signalling and IL-33-driven allergic responses (Bulek et al., 2009). Finally, negative regulation of IL-33-dependent lung inflammation is exerted through post-translational regulation of IL-33R expression by polyubiquitinilation and degradation (Zhao et al., 2012).
IL-33-activated DC favour polarization of Th2 cells (Besnard et al., 2011). By inducing IL-5 production in several cell types, ILCs in particular, and by promoting eosinophil maturation, IL-33 causes eosinophilia in vivo (Bouffi et al., 2013; Ikutani et al., 2012; Pecaric-Petkovic et al., 2009).
IL-33 amplifies innate immunity and inflammatory responses, not necessarily dependent on the development of adaptive Th2 cell responses (Oboki et al., 2010). In macrophages, IL-33 favours LPS-dependent cytokine and chemokine production, and M2 macrophage polarization (Espinassous et al., 2009; Kurowska-Stolarska et al., 2009). Macrophage-derived IL-33 has been shown to be a regulator of trophoblast proliferation and placental growth during early pregnancy (Fock et al., 2013). In a murine model of sepsis, IL-33 has been found to enhance neutrophil recruitment to the site of infection and bacterial clearance by preventing TLR-mediated downregulation of the CXCR2 chemokine receptor (Alves-Filho et al., 2010).
IL-33 is a driver of type 2 inflammatory conditions such as asthma, fibrosis and response to parasites (Liew et al., 2010; McHedlidze et al., 2013; Sica et al., 2013). IL-33 plays a key role in models of allergic lung inflammation, influenza virus or A. fumigatus induced airway hyperreactivity (Albacker et al., 2013; Tjota et al., 2013). Sources of IL-33 include epithelial cells, endothelial cells and fibroblasts. IL-33 targets a wide range of immunocompetent cells (Fig. 2B) and drives Th2 cell polarization, eosinophil recruitment, goblet cell hyperplasia and mucus secretion.
In humans, genome wide association studies (GWAS) have identified single nucleotide polymorphisms (SNPs) in the genes encoding ST2 and IL-33 associated with asthma (Moffatt et al., 2010). In chronic obstructive pulmonary disease (COPD), IL-13-dependent lung disease has been shown to depend on long-term epithelial progenitor cells programmed for excess IL-33 production (Byers et al., 2013).
IL-33 plays protective role against parasites ranging from nematode expulsion, to control of Toxoplasma gondii encephalitis (Moro et al., 2010; Neill et al., 2010). IL-13 produced by ILCs is responsible of this protective response (Moro et al., 2010; Neill et al., 2010). Interestingly, IL-1β suppresses IL-25 and IL-33 production maintaining chronicity of helminthic infection (Zaiss et al., 2013).
IL-33 has also been shown to be involved in a variety of pathological conditions unrelated to type 2 polarization including arthritis, sepsis, fungal and viral infections. In cardiovascular disease models, IL-33 plays a protective role, by reducing atherosclerotic plaque formation (Miller et al., 2008), or by reducing cardiac hypertrophy secondary to pressure overload (Sanada et al., 2007). IL-33 is also involved in inducing ILC2-dependent increases in eosinophils and M2 polarized macrophages of visceral adipose tissue, which are implicated in metabolic homeostasis (Molofsky et al., 2013). A reflection of the role of IL-33 in diverse pathologies is the finding of the predictive value of increased ST2 serum concentrations in Graft versus Host Disease after allogeneic stem cell transplantation (Vander Lugt et al., 2013).
Thus, IL-33 is a key player in pathological conditions related to type 2 responses, as well as in unrelated conditions, by affecting innate and adaptive lymphoid cells (ILC2 and Th2 cells) and by regulating the function of innate effectors including macrophages and mast cells.
IL-18
First described in 1989 as “interferon-γ (IFN-γ)-inducing factor”, IL-18 is closely related to IL-1β in that both are first synthesized as inactive precursors, both require caspase-1 for cleavage, both can be processed extracellularly by proteinase-3 and both have decoy receptors. Similar to IL-1α, the IL-18 precursor is found in nearly all the same mesenchymal cells in health in humans and mice (Puren et al., 1999) and IL-18 is associated with the monocyte membrane, as is IL-1α (Bellora et al., 2012). The tertiary structure of the IL-18 precursor is closely related to that of the IL-37 precursor and the intron-exon borders of the IL-18 and IL-37 genes suggest a close relationship. Indeed, IL-37 binds to the IL-18 receptor.
As discussed below, IL-18 is an important component of polarized Th1 cell and NK cell responses and of the interplay between macrophages and NK cells (Fig. 3A). IL-18 has been implicated in several autoimmune diseases, myocardial pathology, emphysema, metabolic syndrome, psoriasis, inflammatory bowel disease, macrophage activation syndrome, sepsis and acute kidney injury. In some models of disease as in age-related macular degeneration, IL-18 is protective (Doyle et al., 2012). IL-18 and IL-18R-deficient aged mice, spontaneously develop a metabolic syndrome similar to that of humans with diabetes, insulin resistance and atherosclerosis (Netea et al., 2006).
Fig. 3.
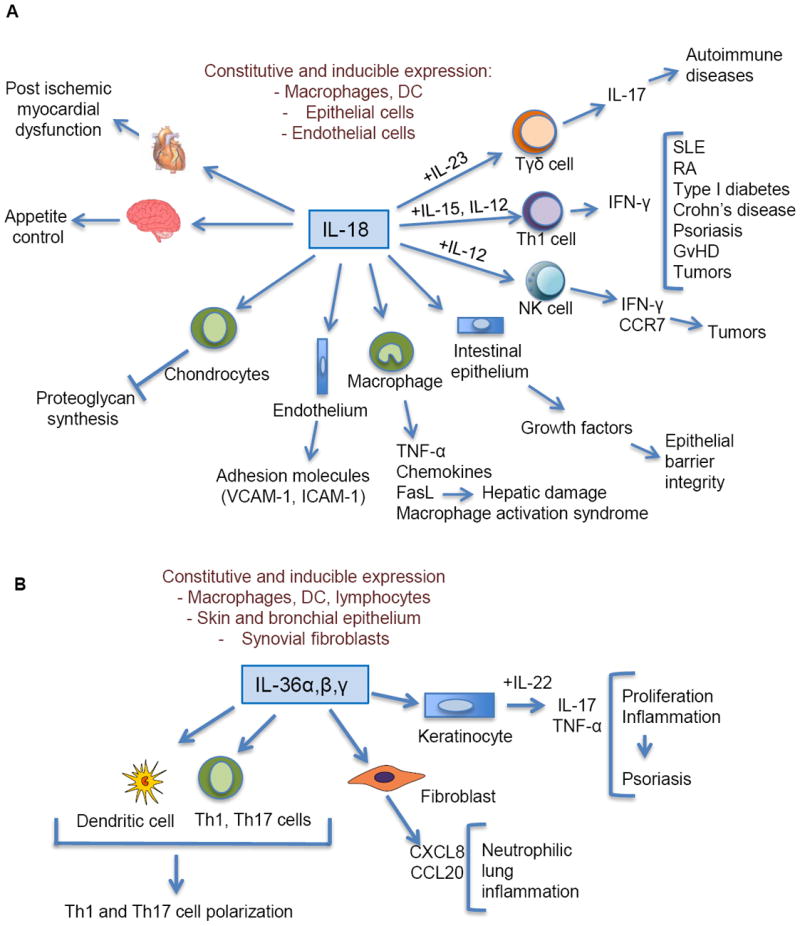
A schematic representation of role of IL-18 (Panel A) and IL-36 (Panel B) in innate and adaptive immunity. “+” refers to costimulation by IL-12, IL-23 or IL-15 of the action of IL-18 on target cells. SLE, systemic lupus erythematosus; RA, rheumatoid arthritis; GvHD, graft versus host disease.
Induction of IFN-γ by IL-18 requires caspase-1 processing (Siegmund et al., 2001b). Interestingly, Fas sustained liver injury is IL-18 dependent but caspase-1 independent, implying an alternative processing pathway (Brydges et al., 2009). IL-18 can contribute to some of the manifestations of inflammasome- mediated caspase-1 activation in autoinflammatory diseases (Towne et al., 2011).
Any phenotypic characteristic of caspase-1-deficient mice should be studied to assess whether it is due to reduced IL-1β or IL-18 activity. The caspase-1-deficient mouse is resistant to dextran sulfate sodium (DSS)-induced colitis (Siegmund et al., 2001b) but the IL-1β-deficient mouse is susceptible in the same disease model (Bersudsky et al., 2013). Since neutralizing antibodies to IL-18 are protective in the DSS colitis model, caspase-1 deficiency appears to prevent processing of IL-18 (Siegmund et al., 2001a) rather than IL-1β. On the other hand, there are examples where caspase-1 processing of IL-18 is not required. For example, Fas ligand stimulation results in release of biologically active IL-18 in caspase 1-deficient murine macrophages.
Mice harboring the mutations prevalent in cryopyrin associated periodic syndrome (CAPS) spontaneously develop inflammatory characteristics such as neutrophilia and elevated circulating concentrations of IL-1β and IL-18 and die in the neonatal period. Genetic analysis suggests that IL-18 and IL-1β have complementary roles in the pathogenesis of cutaneous and systemic manifestations of CAPS (Brydges et al., 2013).
In heart ischemia, the inflammatory cascade begins with activation of caspase-1, which leads to increased release of IL-1β; IL-1β then increases the processing and release of active IL-18 from the stored pools of the inactive IL-18 precursor (Pomerantz et al., 2001) and to counter the inflammatory cascade, IL-18BP reduces the activity of IL-18. Damage to the heart is likely due to Fas signaling. Fas induces inflammatory cytokine production, including IL-18. In addition to inducing IL-18, Fas signaling activates caspase-8 in macrophages and dendritic cells, which results in processing and release of mature IL-1β and IL-18 (Bossaller et al., 2012). Thus, IL-18 is a key component of polarized type 1 innate and adaptive responses. However, its role extends beyond type 1 immunity to include autoinflammation and tissue damage.
IL-36
IL-36 family members IL-36α (IL-1F6), IL-36β (IL-1F8) and IL-36γ (IL-1F9) bind to IL-1Rrp2 and use IL-1RAcP as a coreceptor (Fig. 1A) (Dinarello, 2011; Sims and Smith, 2010). IL-36Ra (IL-1F5), which shares more than 50% homology with IL-1Ra, is a receptor antagonist. IL-36α, IL-36β, IL-36γ, and IL-36Ra do not contain caspase cleavage sites and N-terminal truncation at the level of the A-X-Asp motif conserved in all IL-1 family members dramatically increases their pro-inflammatory activity (Towne et al., 2011).
IL-36 is produced by innate immune cells and lymphocytes inducing the production of pro-inflammatory cytokines, chemokines, and costimulatory molecules, thus promoting Th1 and Th17 cell polarization (Vigne et al., 2011; Vigne et al., 2012) (Fig. 3B). There is evidence that IL-36 is involved in innate immunity and inflammation in the skin and lung where epithelial cells can be a source of this cytokine. Here IL-36 is involved in pathological conditions including psoriasis and A. fumigatus infection (Blumberg et al., 2007; Gresnigt et al., 2013; Tortola et al., 2012). In general, IL-36 mirrors IL-1 and may serve as an amplifier of innate immunity and a mediator of inflammation in selected tissues, such as skin and lung.
IL-37
IL-37 (IL-1F7) has 5 splice variants and isoform IL-37b is the most complete. An instability sequence limits the half-life of IL-37 mRNA. Although IL-1β or TLRs increase IL-37, the anti-inflammatory cytokine transforming growth factor-β (TGF-β) was the most effective stimulus (Nold et al., 2010). Gene databases do not contain a murine homologue for IL-37, but human IL-37 is functional in mouse cells (Bufler et al., 2002; Sharma et al., 2008). Silencing of IL-37 in human blood monocytes with siRNA results in a 2-3 fold increase in LPS and IL-1β-induced cytokines (Nold et al., 2010), suggesting that endogenous IL-37 serves as a natural brake of inflammation.
Similar to IL-1α and IL-33, IL-37 is found in the nucleus where the cytokine functions in transcription suppressing gene expression (Sharma et al., 2008). The nuclear translocation and anti-inflammatory properties of IL-37 appear to be linked to binding to the Smad3 (Grimsby et al., 2004) transcription factor for the anti-inflammatory and immunosuppressive properties of TGF-β. Recombinant IL-37 binds the IL-18Rα chain (Kumar et al., 2002; Nold et al., 2013). Despite binding to the IL-18Rα chain, IL-37 does not act as a classical receptor antagonist (Bufler et al., 2002; Nold-Petry et al., 2009). Available evidence suggests that IL-37 engages the IL-18Rα to deliver an inhibitory signal by using TIR8 (Nold et al., 2013).
To define the in vivo function of IL-37, transgenic mice expressing the full-length IL-37b isoform (IL-37Tg) have been generated (Nold et al., 2010). IL-37Tg mice are protected against LPS challenge with less hypothermia, acidosis, hyperkalemia, hepatitis, dehydration and inflammatory cytokines (Nold et al., 2010).
IL-37Tg mice are protected against inflammatory conditions involving the gut (DSS-induced colitis) (McNamee et al., 2011), the heart (ischemic heart injury) (Yousif et al., 2011) and the liver (ischemia and reperfusion) (Sakai et al., 2012). Protection is associated with decreased concentrations of inflammatory cytokines and chemokines, and increased IL-10 (colitis). In addition, there is evidence that IL-37 expressing DC have defective capacity to activate effective T cell responses and induce T regulatory cells (Luo et al., 2013). IL-37 thus emerges as an inhibitor of adaptive immunity.
IL-38
IL-38 (IL-1F10) was originally identified in silico. The definition of its precise function requires continued investigation. The IL-38 gene is located in the IL-1 family cluster on chromosome 2 next to the genes encoding the IL-1 receptor antagonist (IL-1Ra) and IL-36Ra. IL-38 shares 41% homology with IL-1Ra, a 43% homology with IL-36Ra and has a three-dimensional structure similar to IL-1Ra. By immunohistochemistry, the IL-38 protein is expressed in the basal epithelia of skin and in proliferating B cells of the tonsil.
The primary translated product, the IL-38 precursor of 152 amino acids, lacks a signal peptide. The natural N-terminus is unknown and there is no caspase-1 consensus cleavage site for IL-38. The putative antagonistic role of IL-38 is based on its amino acid homology to the naturally occurring IL-1Ra as well as the observation that IL-38 binds to the extracellular domain of recombinant IL-1R1. However, in that study, the binding affinity of recombinant IL-38 was lower than those of IL-1Ra and IL-1β. IL-38 binds to the extracellular domain of recombinant IL-1Rrp2 (IL-36R) expressed as an IgG1 fusion protein (van de Veerdonk et al., 2012). IL-38 does not bind to IL-1R1, IL-1R3, or to IL-18Rα but only to the IL-36R. In human peripheral blood mononuclear cells (PBMC) stimulated with IL-36γ in the presence of IL-38, the production IL-8 is reduced by 42%. By comparison, the effect of IL-36Ra on IL-36-driven IL-8 is decreased by 75% (van de Veerdonk et al., 2012). It has been concluded that the ability of IL-38 to act as a receptor antagonist for the IL-36R has a biological basis but this property of IL-38 is weak, therefore, IL-38 appears to act as a partial receptor antagonist of the IL-36R. In the case of the IL-36Ra, its antagonist property is determined by generating different N-termini and testing for biological activity (see above). Thus it remains unclear if an alternate N-terminus may reveal a more robust function for recombinant IL-38.
Genetic association studies indicate that IL-38 may be involved in human inflammatory diseases. In a GWAS of 66,185 subjects with elevated C-reactive protein, loci implicated in the metabolic syndrome include NLRP3, IL-6R and IL-38 (Dehghan et al., 2011). Allele combinations that include IL-38 polymorphisms are associated with psoriatic arthritis and ankylosing spondylitis (Maksymowych et al., 2006; Rahman et al., 2006), suggesting that IL-38 plays an important role in the pathogenesis of these inflammatory diseases. Because of the role that Th17 cells play in the pathogenesis of autoimmune diseases, it is possible that IL-38 is involved in the regulation of IL-17 production. Indeed, IL-38 inhibits Candida albicans-induced IL-17 as well as IL-22 production from human memory T-cells. In the presence of IL-38, the production of IL-17A is reduced by 37 %, and of IL-22 by 39%. In the presence of IL-1Ra, the reduction of IL-17A and IL-22 is 82 % and 71 %, respectively (van de Veerdonk et al., 2012). Therefore, if IL-38 is blocking the IL-1 receptor, the effect is weak compared to IL-1Ra. Thus, available information suggests that IL-38 is a negative regulator structurally and functionally related to receptor antagonists, involved in human inflammation and autoimmunity. Further work is required to validate this view and to dissect the mode of action of this cytokine.
NEGATIVE REGULATORS
The IL-1 system is tightly regulated at multiple levels by diverse mechanisms including receptor antagonists, decoy receptors, dominant negative receptor complexes, and negative regulators (Fig. 4). In addition, soluble forms of signalling receptors or accessory proteins (e.g. ST2 and IL-1RAcP) may act as decoys or negative regulators by trapping the ligands. The existence of a wide range of negative regulators emphasizes the need for tight control of the IL-1 system, which mediates potentially devastating local and systemic inflammatory reactions. Here we will focus on mechanisms of negative regulation intrinsic to the IL-1 family, including decoy receptors, receptor antagonists and TIR8.
Fig. 4.
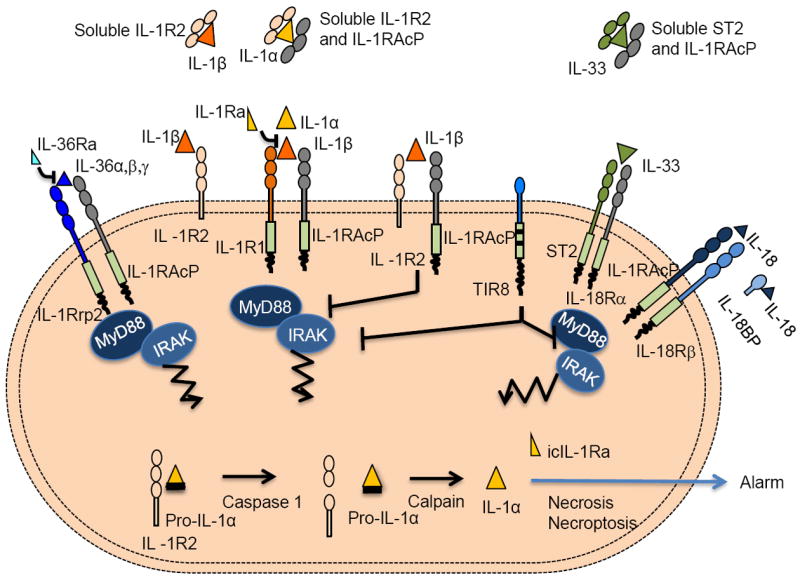
Negative regulators of IL-1, IL-33, IL-18 and IL-36.
icIL-1Ra, two isoforms of intracellular IL-1Ra.
The IL-1 receptor family includes two decoy receptors, IL-1R2 and IL-18BP (Fig. 1 and 4). IL-1R2 has a short cytoplasmic tail and no TIR domain, and does not signal. It is released in a soluble form (Kuhn et al., 2007; Lorenzen et al., 2012) as a consequence of proteolytic processing or alternative splicing. The overall structure of the IL-1β-IL-1R2-IL-1RAcP complex is similar to that of the signalling ligand-receptor complex (Wang et al., 2010).
IL-1R2 negatively regulates IL-1 activity by different mechanisms (Fig. 4). By binding with high affinity, IL-1R2 acts as a molecular trap for IL-1 (Colotta et al., 1993; Re et al., 1996). Interestingly, IL-1R2 binds IL-1Ra at least 100 times less efficiently than the agonists. Moreover, IL-1R2 forms a complex with IL-1 and the IL-1RAcP, exerting a dominant-negative effect. Finally, soluble IL-1R2 and soluble IL-1RAcP bind pro-IL-1β with high affinity and block its processing by caspase-1 (Smith et al., 2003). IL-1R2 is present in the cytoplasm and interacts with pro-IL-1α preventing cleavage and activation by different enzymes (calpain, granzyme B, chymase, and elastase) (Zheng et al., 2013). Caspase-1 cleaves IL-1R2 causing dissociation from IL-1α, calpain processing, and complete restoration of IL-1α activity after necrosis or during regulated secretion. Since IL-1R2 is expressed by a limited set of cells, this would represent a further mechanism of negative control of IL-1α by IL-1R2 during necrosis, restricted to specific cell types (Zheng et al., 2013).
In contrast to IL-1R1, which is expressed by a large variety of cell types, IL-1R2 is expressed by a more limited set of cell types, including monocytes, polarized M2 macrophages, microglial cells, neutrophils, B cells, and T regulatory (Treg) cells (Colotta et al., 1993; Martin et al., 2013; Mercer et al., 2010; Re et al., 1996).
Anti-inflammatory signals enhance IL-1R2 expression, including glucocorticoid hormones (GCs), prostaglandins, Th2 cell-associated cytokines (IL-4 and IL-13), IL-27 (Colotta et al., 1993), suggesting that induction of IL-1R2 contributes to the anti-inflammatory effect of these mediators. During pregnancy, chorionic gonadotropins down-regulate the synthesis and release of IL-1R2 by endometrial epithelial cells thus favouring embryonic IL-1β activities essential for attachment of the blastocyst. Increased blood concentrations of IL-1R2 have been detected in a wide range of human disorders and may reflect the activation of endogenous pathways of negative regulation of inflammation.
IL-18BP is comprised of only one Ig-like domain and is structurally and functionally similar to IL-1R2 (Novick et al., 1999). By preventing the binding of the agonist to IL-18R, and neutralizing IL-18 activity, IL-18BP dampens IFN-γ production and consequently limits Th1 cell responses (Kim et al., 2000; Novick et al., 1999) (Fig. 4). The gene is induced by IFN-γ, thus indicating that IL-18BP is part of a negative feedback loop controlling IFN-γ-dependent inflammation, and by IL-27. In homeostatic conditions, IL-18BP is present in the circulation at concentrations of 20-fold molar excess to IL-18, thus representing a default mechanism limiting IL-18 activity. In several IFN-γ-mediated autoimmune conditions, the concentrations of free IL-18 compared to IL-18 bound to IL-18BP is important in determining the severity of the disease (Novick et al., 2010). IL-18BP also binds IL-37 complex and it may act as a dominant negative.
The IL-1 ligand family includes two receptor antagonists, IL-1Ra and IL-36Ra (Dinarello, 2010). IL-1Ra binds IL-1R1 with an affinity higher than that of IL-1 but fails to recruit the IL-1RAcP. In addition to secreted soluble IL-1Ra, there are the two intracellular isoforms which are considered a reservoir of IL-1Ra, to be released upon cell death, limiting the pro-inflammatory action of tissue damage.
Deficiency of IL-1Ra in mice results in spontaneous and lethal arteritis, destructive arthritis and psoriatic-like skin lesions, as well as increased susceptibility to carcinogenesis (Horai et al., 2000; Nicklin et al., 2000). Children born with a genetic deficiency of IL-1Ra or functional inactive IL-1Ra suffer from severe systemic and local inflammation, including pustular skin eruptions, vasculitis, osteolytic lesions and sterile osteomyelitis (Aksentijevich et al., 2009; Reddy et al., 2009).
IL-36Ra acts as a specific receptor antagonist for IL-1Rrp2 and prevents the activity of IL-36. IL-36Ra negatively regulates the IL-36-elicited pathway comprising IL-23, IL-17 and IL-22 and development of psoriasiform dermatitis (Blumberg et al., 2007; Tortola et al., 2012). Mutations of the IL-36Ra gene are associated to a rare life-threatening form of psoriasis (Marrakchi et al., 2011).
TIR8 (also known as SIGIRR) is an atypical receptor characterized by a single Ig domain and a TIR domain with two substitutions (Ser447 and Tyr536 replaced by Cys222 and Leu305) which early on suggested non-conventional signalling (Fig. 1A and 4). TIR8 is expressed in several tissues, particularly in the kidney, digestive tract, liver, lung and lymphoid organs (Garlanda et al., 2009).
TIR8 inhibits NF-kB and JNK activation following stimulation of IL-1R family members or TLRs (IL-1RI, IL-18R, ST2, TLR1, TLR2, TLR3, TLR4, TLR7 and TLR9) (Bulek et al., 2009; Garlanda et al., 2004; Lech et al., 2008; Wald et al., 2003), by interfering with the recruitment of TIR-containing adaptor molecules (Wald et al., 2003). TIR8 can also regulate mTOR kinase activity in Th17 lymphocytes (Gulen et al., 2010) and in intestinal epithelial cells (Xiao et al., 2010).
Studies with deficient mice have shown that TIR8 plays non-redundant roles in regulating potentially detrimental inflammatory responses associated to infections, such as tuberculosis, candidiasis, aspergillosis, P. aeruginosa infection (Bozza et al., 2008; Chen et al., 2011; Garlanda et al., 2007a; Huang et al., 2006). TIR8-deficiency causes more severe gut inflammation during DSS colitis (Garlanda et al., 2004; Xiao et al., 2007) and increased susceptibility to intestinal carcinogenesis (Garlanda et al., 2007b; Xiao et al., 2007; Xiao et al., 2010). In a murine model of Chronic Lymphocytic Leukemia (CLL), TIR8-deficiency leads to a more severe and earlier appearance of monoclonal B-cell expansions and to shortened life span, in agreement with TIR8 downmodulation in human malignant B cells (Bertilaccio et al., 2011).
In kidney ischemia or transplantation, TIR8-deficiency causes increased renal injury or severe graft rejection, associated with excessive inflammation and amplified adaptive immune responses against donor antigens (Lech et al., 2009; Noris et al., 2009). TIR8 modulated LPS-induced microglia activation and neuroinflammation, cognitive and synaptic functions in hippocampal tissue in response to IL-1α and high mobility group box 1.
TIR8-deficiency is also associated with increased susceptibility to develop allergy and autoimmunity in various models including systemic lupus erythematosus, lupus nephritis, (Lech et al., 2008; Lech et al., 2010), arthritis (Drexler et al., 2010), experimental autoimmune encephalomyelitis, and psoriasis (Russell et al., 2013). In the latter cases, TIR8 dampens IL-1-dependent differentiation of Th17 and Tγδ17 cells (Gulen et al., 2010; Russell et al., 2013).
IL-1RAcPb is an alternative form of IL-1RAcP restricted to the central nervous system (CNS), generated by alternative splicing, in which the prototypical IL-1RAcP C-terminal exon 12 is skipped and an alternative exon 12b is used (Smith et al., 2009). The exon 12b encodes a sequence of approximately 140 additional amino acids in the C-terminal of the TIR domain causing a changed configuration in the DD loop and aD helix regions of the IL-1RAcPb TIR domain, and affecting the interaction with MyD88 and IRAK4. IL-1RAcPb could also be recruited to other IL-1RAcP-utilizing receptors, such as ST2 and IL-1Rrp2, which are expressed in the CNS. IL-1RAcPb-deficiency is associated with neuronal loss, suggesting that it may dampen the neurotoxic effects of IL-1 by modulating the intracellular signalling and gene expression response to LPS-induced IL-1, or possibly to other cytokines acting through IL-1RAcP (Smith et al., 2009).
ORCHESTRATION OF INNATE AND ADAPTIVE IMMUNOCOMPETENT CELLS
IL-1 family members affect virtually all cells of the innate immune system, including macrophages, neutrophils, eosinophils, basophils and mast cells (Fig. 2A). This aspect has previously been reviewed (Dinarello, 2009; Dinarello et al., 2012; Gabay et al., 2010; Sims and Smith, 2010) and specific topics (e.g. M2 macrophage polarization for IL-33) are discussed above. Here we will focus on interaction with lymphoid cells.
One of the early descriptions of, and bioassay for, IL-1 was based on costimulation of T lymphocyte proliferation, the so-called lymphocyte activation factor (LAF) activity and assay (Dinarello, 2009). The perception that IL-1 affects lymphoid cells has waned for a long time to be revisited with the realization that polarized T cell and ILC differentiation and function involves members of the IL-1 family (Fig. 5) (O’Shea and Paul, 2010; Spits et al., 2013).
Fig. 5.
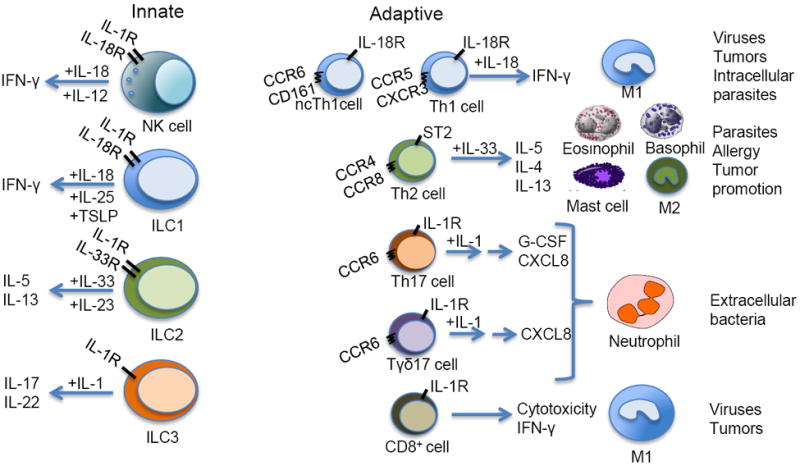
IL-1 family members in the differentiation and function of innate and adaptive lymphoid cells. “+” above arrows refers to stimulation of target cells by IL-1 family members with or without other cytokines (IL-12, IL-25, thymic stromal lymphopoietin (TSLP), IL-23). Arrows point to products of stimulated cells and cellular effectors (on the right part). ncTh1, non-conventional Th1 cells (human). The → → sign for the Th17 cells and Tγδ17 cells indicates the presence of intermediate cells (endothelium, epithelium) which respond to IL-17.
ILC are a complex and heterogeneous group of lymphoid cells involved in innate immunity and tissue remodeling (Spits et al., 2013). ILCs have been recently divided in different functional subsets: group 1 ILCs includes NK and other IFN-γ-producing ILCs; group 2 ILCs includes cells that produce type 2 cytokines (IL-4, IL-5, IL-9 and IL-13); group 3 ILCs produce IL-17 and/or IL-22. In analogy with T cell polarization, ILCs depend on γ chain cytokines and IL-1 family members for their specific development and activity. IL-18, together with IL-15 is a major stimulus for IFN-γ production by group 1 ILCs; IL-33, acting in synergy with IL-25, is a potent inducer of the expansion of type 2 ILCs and of the production of extremely high amounts of Th2 cell associated cytokines, in particular IL-5 and IL-13; IL-1β and IL-23 activate group 3 ILCs to produce IL-17 and IL-22 (Fig. 5).
NK cells are prototypic ILC1 cells. IL-18 is a key mediator in the interaction between NK cells and DC or macrophages (e.g. (Bellora et al., 2012; Mailliard et al., 2005). Macrophage-derived IL-18 promotes NK cell activation and, via DC, activation of adaptive responses. In secondary lymphoid tissues, DC-derived IL-1β preferentially promotes ILC3 phenotype and expansion from immature NK cells, at the expense of development and maturation of conventional IFN-γ-producing NK cells, which express lower amounts of IL-1RI (Hughes et al., 2010).
In an interesting unexpected twist, IL-33 has been recently involved in the response to bee venom phospholipase A2 (PLA2) (Palm et al., 2013). PLA2, a conserved component of many venoms, triggers release of IL-33 and activation of ILC2s. The adaptive Th2 response against PLA2 is also IL-33 and ST2 dependent. Thus IL-33 is a key element of the innate response to a conserved component of venoms.
IL-1 family members are key components of T cell polarization and function (O’Shea and Paul, 2010). IL-18 was originally identified based on its capacity to costimulate IFN-γ production. It is therefore not surprising that IL-18 has been associated with Th1 cell differentiation (Santarlasci et al., 2013). IL-18 is dispensable for recruitment of naïve T cells to become Th1 cells. The IL-18R is induced by IL-12 in Th1 cells and this cytokine amplifies their expansion and IFN-γ production.
It has long been held that IL-1R1 is not expressed in Th1 cells. Recent results with human lymphocytes dispel this oversimplification. It has found that a subset of Th1 clones, in particular those characterized by expression of CD161, expresses IL-1R1 (Cosmi et al., 2008; Maggi et al., 2012). These “non-classical” Th1 cells derive from Th17 cells. Thus, expression of IL-1R is a feature of “non-classical” Th1 cells ontogenetically related to Th17 cells. The actual role of IL-1 in the function of this Th1 subset remains to be elucidated.
The IL-1 family member associated with Th2 cell differentiation and function is IL-33 (see above). Unlike Th1 cells, Th2 cells express the IL-33 receptor chain ST2 (Guo et al., 2009). IL-33 is dispensable for Th2 cell differentiation but it amplifies cytokine production (in particular IL-5 and IL-13) by these cells.
Analysis of the differentiation of human naïve T cells to Th17 cells originally revealed a key role of IL-1 (Acosta-Rodriguez et al., 2007; Annunziato et al., 2007). Accordingly, IL-1 is required for development of Th17 cell-sustained autoimmunity (e.g. (Chung et al., 2009; Sutton et al., 2006; Veldhoen et al., 2006).
IL-1 has more subtle actions in Th17 cells than promotion of differentiation. Candida albicans and Staphylococcus aureus specific human Th17 cells produce IL-17, but differ in terms of IL-10 and IFN-γ. IL-1 is essential to generate C. albicans-induced cells capable of producing both IL-17 and IFN-γ. IL-1 also inhibits IL-10 production, which otherwise dampens IFN-γ secretion (Zielinski et al., 2012). Thus IL-1 plays a role in fine tuning the cytokine repertoire of Th17 cells specific for different pathogens.
Tγδ cells are the major source of IL-17 early after infection (Chien et al., 2013). IL-1 is not required for thymic differentiation of Tγδ17. However, Tγδ17 express the IL-1R and IL-1 in concert with IL-23 drives their activation and IL-1 production, thus setting in motion an amplification circuit (Duan et al., 2010; Zeng et al., 2012).
Methicillin-resistant Staphilococcus aureus represents a serious clinical problem. In a mouse model of skin infection, resistance to S. aureus depends on IL-1, which promotes IL-17 production by Tγδ cells (Myles et al., 2013). Keratinocytes and possibly myeloid cells are the major source of IL-1β. The same cells produce IL-19, IL-20 and IL-24, three members of the IL-10 family. These cytokines act via the IL-20 receptor and elicit degradation of the transcription factor cEBP, thus resulting in feedback inhibition of IL-1.
iNKT cells respond to IL-1 and IL-18. IL-1, together with other cytokines, has been reported to induce IL-17 production by iNKT cells and, in the context of viral infection, IL-22 (Doisne et al., 2011; Monteiro et al., 2013; Paget et al., 2012).
IL-1R1 deficient mice show defective capacity to generate effective CD8+ T cell responses to viruses (e.g. (Joeckel et al., 2012)) and tumors (Ghiringhelli et al., 2009). Using the OT-1 model, it has recently reported that IL-1β results in increased numbers and effector function of antigen-specific T cells. The effect of IL-1 includes the amplification of the memory response (Ben-Sasson et al., 2013). This finding raises the issue of the possible use of IL-1 as an adjuvant to stimulate CD8+ T cell response.
THERAPEUTIC STRATEGIES FOR IL-1 BLOCKADE IN HUMAN DISEASES
Anakinra is the generic name for the recombinant form of the naturally occurring IL-1Ra, and has been approved in 2001 to treat rheumatoid arthritis and recently to treat CAPS. However, Anakinra has proved efficacious in a broad spectrum of diseases and is currently in several clinical trials (Dinarello et al., 2012). These include autoimmune hearing loss, hydradenitis suppurativa, stroke and osteoarthritis of the hand. The responses to anakinra are rapid and sustained and in many conditions, treatment with anakinra allows for a reduction in steroid use, particularly in children with systemic juvenile idiopathic arthritis. Anakinra is also used to treat common diseases such as recurrent gout attacks unresponsive to standards of therapy.
There is a large body of pre-clinical evidence supporting the rationale for specifically targeting IL-1β with neutralizing antibodies. In autoinflammatory diseases, IL-1β is released from the activated monocyte due to disregulation of caspase-1. Canakinumab is a human monoclonal antibody specifically targeting IL-1β, which is approved for the treatment of CAPS, systemic onset juvenile idiopathic arthritis and refractory gout. It will be tested in a large trial in cardiovascular pathology. A neutralizing monoclonal anti-IL-1α antibody has been tested in Type 2 diabetes, cancer cachexia, pustular psoriasis, occlusive vascular disease and scarring acne vulgaris and in each condition, reduced disease severity has been observed in limited trials (Hong et al., 2011).
CONCLUDING REMARKS AND PERSPECTIVES
Evidence obtained in the last few years indicates that members of the IL-1 family are key players in the differentiation and function of innate and adaptive lymphoid cells. Thus, in a way, the long overlooked costimulating activity of IL-1 (LAF) has now been vindicated by the discovery of its role in innate and adaptive lymphoid cell differentiation and function.
Early work had shown that IL-1 has adjuvant activity (Dinarello, 2009) and activation of the inflammasome may contribute to the function of adjuvants in current clinical use. The identification of the role of IL-1 family members in lymphoid differentiation raises the issue as to whether this aspect of the function of IL-1 family members can be harnessed for better vaccines (e.g. (Ben-Sasson et al., 2013).
The IL-1 system is characterized by the recurrent theme of balancing accelerators and brakes (receptor antagonists, decoy receptors, TIR8). Scattered evidence suggests that negative regulators are key components of resolution of inflammation. It is tempting to speculate that these components of the IL-1 family may represent therapeutic targets in pro-resolving strategies.
Anti-IL-1 strategies have had a tremendous impact in the therapy of autoinflammatory disorders sustained by inflammasome activation and, to a lesser extent, autoimmune diseases (Dinarello, 2010). Ongoing studies suggest that blocking IL-1 may have a broader clinical impact on relatively rare (e.g. Behcet uveitis) and common (e.g. cardiovascular) diseases. Better understanding of the pathophysiology of IL-1 and its relatives holds promise of innovative therapeutic tools and targets: hic sunt leones.
Table 2.
Main phenotype of IL-1 family gene alterations in mice and humans.
| Gene modification | Main phenotypes | Selected references |
|---|---|---|
| Il-1ra-/- mice | Spontaneous arteritis, arthritis, psoriatic-like skin eruption, increased susceptibility to LPS and to carcinogenesis | (Horai et al, 2000; Krelin et al, 2007; Nicklin et al, 2000) |
| IL-1Ra-deficient humans | Severe systemic and local inflammation, including pustular skin eruptions, vasculitis, osteolytic lesions and sterile osteomyelitis | (Aksentijevich et al, 2009; Reddy et al, 2009) |
| Il-1α-/- mice | Increased susceptibility to LPS, defective immunoediting in carcinogenesis, reduced necrosis-induced inflammation and colitis, reduced atherosclerosis | (Chen et al, 2007; Krelin et al, 2007) |
| Il-1β-/- mice | Reduced acute phase response and fever, increased or decreased susceptibility to specific infections, reduced susceptibility to carcinogenesis | (Horai et al, 1998; Krelin et al, 2007) |
| Il-1r1-/- mice | Increased or decreased susceptibility to specific infections, reduced susceptibility to EAE, reduced inflammatory responses and delayed type hypersensitivity, defective adaptive immunity to tumors | (Sutton et al, 2006; Joeckel et al, 2012; Ghiringhelli et al, 2009) |
| Il-18-/- mice | Spontaneous metabolic syndrome, reduced susceptibility to LPS, arthritis, Fas ligand–mediated hepatitis, graft-versus-host disease, reduced susceptibility to EAE | (Netea et al, 2006; Dinarello, 2009) |
| Il-18rα -/- mice | Increased susceptibility to EAE | (Dinarello, 2009) |
| Il-33-/- mice | Decreased resistance to parasites, reduced inflammatory and allergic responses, reduced colitis | (Oboki et al, 2010; Liew et al, 2010) |
| St2-/- mice | Decreased resistance to parasites and bacteria, reduced arthritis and colitis, decreased Th2 responses, reduced endotoxin tolerance | (Neill et al, 2010; Liew et al, 2010) |
| IL-37Tg mice | Decreased susceptibility to LPS, DSS-induced colitis, ischemic heart injury, liver ischemia and reperfusion, defective adaptive immunity. | (Nold et al, 2010; McNamee et al, 2011; Yousif et al, 2011; Sakai et al, 2012; Luo et al, 2013) |
| IL-36αTg mice | Psoriasis | (Blumberg et al, 2007) |
| Il-36ra-/- mice | Psoriasis | (Tortola et al, 2012) |
| IL-36Ra-deficient humans | Severe psoriasis | (Marrakchi et al, 2011) |
| Il-36r-/- mice | Reduced psoriasiformis dermatitis | (Tortola et al, 2012) |
| TIR8-/- mice | Increased susceptibility to DSS-induced colitis, colon carcinogenesis, bacterial and fungal infections, autoimmunity including LES, arthritis, psoriasis and EAE, leukaemia, allergy, kidney ischemia/reperfusion | (Garlanda et al, 2004; Xiao et al, 2007; Wald et al, 2003; Garlanda et al, 2007; Lech et al, 2008; Bulek et al, 2009; Gulen et al, 2010; Bertilaccio et al, 2011) |
Acknowledgments
Alberto Mantovani and Cecilia Garlanda are supported by European Research Council (Project HIIIS), European Commission (FP7-HEALTH-2011- ADITEC 280873) and Associazione Italiana per la Ricerca sul Cancro (AIRC). Charles A. Dinarello is supported by NIH Grants A115614 and AR-45584
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Acosta-Rodriguez EV, Napolitani G, Lanzavecchia A, Sallusto F. Interleukins 1beta and 6 but not transforming growth factor-beta are essential for the differentiation of interleukin 17-producing human T helper cells. Nat Immunol. 2007;8:942–949. doi: 10.1038/ni1496. [DOI] [PubMed] [Google Scholar]
- Aksentijevich I, Masters SL, Ferguson PJ, Dancey P, Frenkel J, van Royen-Kerkhoff A, Laxer R, Tedgard U, Cowen EW, Pham TH, et al. An autoinflammatory disease with deficiency of the interleukin-1-receptor antagonist. N Engl J Med. 2009;360:2426–2437. doi: 10.1056/NEJMoa0807865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albacker LA, Chaudhary V, Chang YJ, Kim HY, Chuang YT, Pichavant M, Dekruyff RH, Savage PB, Umetsu DT. Invariant natural killer T cells recognize a fungal glycosphingolipid that can induce airway hyperreactivity. Nat Med. 2013;19:1297–1304. doi: 10.1038/nm.3321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alves-Filho JC, Sonego F, Souto FO, Freitas A, Verri WA, Jr, Auxiliadora-Martins M, Basile-Filho A, McKenzie AN, Xu D, Cunha FQ, Liew FY. Interleukin-33 attenuates sepsis by enhancing neutrophil influx to the site of infection. Nat Med. 2010;16:708–712. doi: 10.1038/nm.2156. [DOI] [PubMed] [Google Scholar]
- Annunziato F, Cosmi L, Santarlasci V, Maggi L, Liotta F, Mazzinghi B, Parente E, Fili L, Ferri S, Frosali F, et al. Phenotypic and functional features of human Th17 cells. J Exp Med. 2007;204:1849–1861. doi: 10.1084/jem.20070663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apte RN, Dotan S, Elkabets M, White MR, Reich E, Carmi Y, Song X, Dvozkin T, Krelin Y, Voronov E. The involvement of IL-1 in tumorigenesis, tumor invasiveness, metastasis and tumor-host interactions. Cancer Metastasis Rev. 2006;25:387–408. doi: 10.1007/s10555-006-9004-4. [DOI] [PubMed] [Google Scholar]
- Bellora F, Castriconi R, Doni A, Cantoni C, Moretta L, Mantovani A, Moretta A, Bottino C. M-CSF induces the expression of a membrane-bound form of IL-18 in a subset of human monocytes differentiating in vitro toward macrophages. Eur J Immunol. 2012;42:1618–1626. doi: 10.1002/eji.201142173. [DOI] [PubMed] [Google Scholar]
- Ben-Sasson SZ, Hogg A, Hu-Li J, Wingfield P, Chen X, Crank M, Caucheteux S, Ratner-Hurevich M, Berzofsky JA, Nir-Paz R, Paul WE. IL-1 enhances expansion, effector function, tissue localization, and memory response of antigen-specific CD8 T cells. J Exp Med. 2013;210:491–502. doi: 10.1084/jem.20122006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berda-Haddad Y, Robert S, Salers P, Zekraoui L, Farnarier C, Dinarello CA, Dignat-George F, Kaplanski G. Sterile inflammation of endothelial cell-derived apoptotic bodies is mediated by interleukin-1alpha. Proc Natl Acad Sci U S A. 2011;108:20684–20689. doi: 10.1073/pnas.1116848108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bersudsky M, Luski L, Fishman D, White RM, Ziv-Sokolovskaya N, Dotan S, Rider P, Kaplanov I, Aychek T, Dinarello CA, et al. Non-redundant properties of IL-1alpha and IL-1beta during acute colon inflammation in mice. Gut. 2013 doi: 10.1136/gutjnl-2012-303329. In press. [DOI] [PubMed] [Google Scholar]
- Bertilaccio MT, Simonetti G, Dagklis A, Rocchi M, Rodriguez TV, Apollonio B, Mantovani A, Ponzoni M, Ghia P, Garlanda C, et al. Lack of TIR8/SIGIRR triggers progression of chronic lymphocytic leukemia in mouse models. Blood. 2011;118:660–669. doi: 10.1182/blood-2011-01-329870. [DOI] [PubMed] [Google Scholar]
- Besnard AG, Togbe D, Guillou N, Erard F, Quesniaux V, Ryffel B. IL-33-activated dendritic cells are critical for allergic airway inflammation. Eur J Immunol. 2011;41:1675–1686. doi: 10.1002/eji.201041033. [DOI] [PubMed] [Google Scholar]
- Blumberg H, Dinh H, Trueblood ES, Pretorius J, Kugler D, Weng N, Kanaly ST, Towne JE, Willis CR, Kuechle MK, et al. Opposing activities of two novel members of the IL-1 ligand family regulate skin inflammation. J Exp Med. 2007;204:2603–2614. doi: 10.1084/jem.20070157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossaller L, Chiang PI, Schmidt-Lauber C, Ganesan S, Kaiser WJ, Rathinam VA, Mocarski ES, Subramanian D, Green DR, Silverman N, et al. Cutting edge: FAS (CD95) mediates noncanonical IL-1beta and IL-18 maturation via caspase-8 in an RIP3-independent manner. J Immunol. 2012;189:5508–5512. doi: 10.4049/jimmunol.1202121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouffi C, Rochman M, Zust CB, Stucke EM, Kartashov A, Fulkerson PC, Barski A, Rothenberg ME. IL-33 Markedly Activates Murine Eosinophils by an NF-kappaB-Dependent Mechanism Differentially Dependent upon an IL-4-Driven Autoinflammatory Loop. J Immunol. 2013;191:4317–4325. doi: 10.4049/jimmunol.1301465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozza S, Zelante T, Moretti S, Bonifazi P, DeLuca A, D’Angelo C, Giovannini G, Garlanda C, Boon L, Bistoni F, et al. Lack of Toll IL-1R8 exacerbates Th17 cell responses in fungal infection. J Immunol. 2008;180:4022–4031. doi: 10.4049/jimmunol.180.6.4022. [DOI] [PubMed] [Google Scholar]
- Brydges SD, Broderick L, McGeough MD, Pena CA, Mueller JL, Hoffman HM. Divergence of IL-1, IL-18, and cell death in NLRP3 inflammasomopathies. J Clin Invest. 2013 doi: 10.1172/JCI71543. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brydges SD, Mueller JL, McGeough MD, Pena CA, Misaghi A, Gandhi C, Putnam CD, Boyle DL, Firestein GS, Horner AA, et al. Inflammasome-mediated disease animal models reveal roles for innate but not adaptive immunity. Immunity. 2009;30:875–887. doi: 10.1016/j.immuni.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bufler P, Azam T, Gamboni-Robertson F, Reznikov LL, Kumar S, Dinarello CA, Kim SH. A complex of the IL-1 homologue IL-1F7b and IL-18-binding protein reduces IL-18 activity. Proc Natl Acad Sci U S A. 2002;99:13723–13728. doi: 10.1073/pnas.212519099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulek K, Swaidani S, Qin J, Lu Y, Gulen MF, Herjan T, Min B, Kastelein RA, Aronica M, Kosz-Vnenchak M, Li X. The essential role of single Ig IL-1 receptor-related molecule/Toll IL-1R8 in regulation of Th2 immune response. J Immunol. 2009;182:2601–2609. doi: 10.4049/jimmunol.0802729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byers DE, Alexander-Brett J, Patel AC, Agapov E, Dang-Vu G, Jin X, Wu K, You Y, Alevy Y, Girard JP, et al. Long-term IL-33-producing epithelial progenitor cells in chronic obstructive lung disease. J Clin Invest. 2013;123:3967–3982. doi: 10.1172/JCI65570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmi Y, Dotan S, Rider P, Kaplanov I, White MR, Baron R, Abutbul S, Huszar M, Dinarello CA, Apte RN, Voronov E. The role of IL-1beta in the early tumor cell-induced angiogenic response. J Immunol. 2013;190:3500–3509. doi: 10.4049/jimmunol.1202769. [DOI] [PubMed] [Google Scholar]
- Cayrol C, Girard JP. The IL-1-like cytokine IL-33 is inactivated after maturation by caspase-1. Proc Natl Acad Sci U S A. 2009;106:9021–9026. doi: 10.1073/pnas.0812690106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CJ, Kono H, Golenbock D, Reed G, Akira S, Rock KL. Identification of a key pathway required for the sterile inflammatory response triggered by dying cells. Nat Med. 2007;13:851–856. doi: 10.1038/nm1603. [DOI] [PubMed] [Google Scholar]
- Chen X, Zhao Y, Wu X, Qian G. Enhanced expression of single immunoglobulin IL-1 receptor-related molecule ameliorates LPS-induced acute lung injury in mice. Shock. 2011;35:198–204. doi: 10.1097/SHK.0b013e3181f226f3. [DOI] [PubMed] [Google Scholar]
- Chien YH, Zeng X, Prinz I. The natural and the inducible: interleukin (IL)-17-producing gammadelta T cells. Trends Immunol. 2013;34:151–154. doi: 10.1016/j.it.2012.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung Y, Chang SH, Martinez GJ, Yang XO, Nurieva R, Kang HS, Ma L, Watowich SS, Jetten AM, Tian Q, Dong C. Critical regulation of early Th17 cell differentiation by interleukin-1 signaling. Immunity. 2009;30:576–587. doi: 10.1016/j.immuni.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen I, Rider P, Carmi Y, Braiman A, Dotan S, White MR, Voronov E, Martin MU, Dinarello CA, Apte RN. Differential release of chromatin-bound IL-1alpha discriminates between necrotic and apoptotic cell death by the ability to induce sterile inflammation. Proc Natl Acad Sci U S A. 2010;107:2574–2579. doi: 10.1073/pnas.0915018107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colotta F, Re F, Muzio M, Bertini R, Polentarutti N, Sironi M, Giri JG, Dower SK, Sims JE, Mantovani A. Interleukin-1 type II receptor: a decoy target for IL-1 that is regulated by IL-4. Science. 1993;261:472–475. doi: 10.1126/science.8332913. [DOI] [PubMed] [Google Scholar]
- Cosmi L, De Palma R, Santarlasci V, Maggi L, Capone M, Frosali F, Rodolico G, Querci V, Abbate G, Angeli R, et al. Human interleukin 17-producing cells originate from a CD161+CD4+ T cell precursor. J Exp Med. 2008;205:1903–1916. doi: 10.1084/jem.20080397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehghan A, Dupuis J, Barbalic M, Bis JC, Eiriksdottir G, Lu C, Pellikka N, Wallaschofski H, Kettunen J, Henneman P, et al. Meta-analysis of genome-wide association studies in >80 000 subjects identifies multiple loci for C-reactive protein levels. Circulation. 2011;123:731–738. doi: 10.1161/CIRCULATIONAHA.110.948570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinarello C, Arend W, Sims J, Smith D, Blumberg H, O’Neill L, Goldbach-Mansky R, Pizarro T, Hoffman H, Bufler P, et al. IL-1 family nomenclature. Nat Immunol. 2010;11:973. doi: 10.1038/ni1110-973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinarello CA. Immunological and inflammatory functions of the interleukin-1 family. Annu Rev Immunol. 2009;27:519–550. doi: 10.1146/annurev.immunol.021908.132612. [DOI] [PubMed] [Google Scholar]
- Dinarello CA. Anti-inflammatory Agents: Present and Future. Cell. 2010;140:935–950. doi: 10.1016/j.cell.2010.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinarello CA. Interleukin-1 in the pathogenesis and treatment of inflammatory diseases. Blood. 2011;117:3720–3732. doi: 10.1182/blood-2010-07-273417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinarello CA, Simon A, van der Meer JW. Treating inflammation by blocking interleukin-1 in a broad spectrum of diseases. Nature Rev Drug Discov. 2012;11:633–652. doi: 10.1038/nrd3800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doisne JM, Soulard V, Becourt C, Amniai L, Henrot P, Havenar-Daughton C, Blanchet C, Zitvogel L, Ryffel B, Cavaillon JM, et al. Cutting edge: crucial role of IL-1 and IL-23 in the innate IL-17 response of peripheral lymph node NK1.1- invariant NKT cells to bacteria. J Immunol. 2011;186:662–666. doi: 10.4049/jimmunol.1002725. [DOI] [PubMed] [Google Scholar]
- Doyle SL, Campbell M, Ozaki E, Salomon RG, Mori A, Kenna PF, Farrar GJ, Kiang AS, Humphries MM, Lavelle EC, et al. NLRP3 has a protective role in age-related macular degeneration through the induction of IL-18 by drusen components. Nat Med. 2012;18:791–798. doi: 10.1038/nm.2717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drexler SK, Kong P, Inglis J, Williams RO, Garlanda C, Mantovani A, Yazdi AS, Brennan F, Feldmann M, Foxwell BM. SIGIRR/TIR-8 is an inhibitor of Toll-like receptor signaling in primary human cells and regulates inflammation in models of rheumatoid arthritis. Arthritis Rheum. 2010;62:2249–2261. doi: 10.1002/art.27517. [DOI] [PubMed] [Google Scholar]
- Duan J, Chung H, Troy E, Kasper DL. Microbial colonization drives expansion of IL-1 receptor 1-expressing and IL-17-producing gamma/delta T cells. Cell Host Microbe. 2010;7:140–150. doi: 10.1016/j.chom.2010.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espinassous Q, Garcia-de-Paco E, Garcia-Verdugo I, Synguelakis M, von Aulock S, Sallenave JM, McKenzie AN, Kanellopoulos J. IL-33 enhances lipopolysaccharide-induced inflammatory cytokine production from mouse macrophages by regulating lipopolysaccharide receptor complex. J Immunol. 2009;183:1446–1455. doi: 10.4049/jimmunol.0803067. [DOI] [PubMed] [Google Scholar]
- Fock V, Mairhofer M, Otti GR, Hiden U, Spittler A, Zeisler H, Fiala C, Knofler M, Pollheimer J. Macrophage-Derived IL-33 Is a Critical Factor for Placental Growth. J Immunol. 2013;191:3734–3743. doi: 10.4049/jimmunol.1300490. [DOI] [PubMed] [Google Scholar]
- Freigang S, Ampenberger F, Weiss A, Kanneganti TD, Iwakura Y, Hersberger M, Kopf M. Fatty acid-induced mitochondrial uncoupling elicits inflammasome-independent IL-1alpha and sterile vascular inflammation in atherosclerosis. Nat Immunol. 2013;14:1045–1053. doi: 10.1038/ni.2704. [DOI] [PubMed] [Google Scholar]
- Gabay C, Lamacchia C, Palmer G. IL-1 pathways in inflammation and human diseases. Nature Rev Rheumatol. 2010;6:232–241. doi: 10.1038/nrrheum.2010.4. [DOI] [PubMed] [Google Scholar]
- Garlanda C, Anders HJ, Mantovani A. TIR8/SIGIRR: an IL-1R/TLR family member with regulatory functions in inflammation and T cell polarization. Trends Immunol. 2009;30:439–446. doi: 10.1016/j.it.2009.06.001. [DOI] [PubMed] [Google Scholar]
- Garlanda C, Di Liberto D, Vecchi A, La Manna MP, Buracchi C, Caccamo N, Salerno A, Dieli F, Mantovani A. Damping excessive inflammation and tissue damage in Mycobacterium tuberculosis infection by Toll IL-1 receptor 8/single Ig IL-1-related receptor, a negative regulator of IL-1/TLR signaling. J Immunol. 2007a;179:3119–3125. doi: 10.4049/jimmunol.179.5.3119. [DOI] [PubMed] [Google Scholar]
- Garlanda C, Riva F, Polentarutti N, Buracchi C, Sironi M, De Bortoli M, Muzio M, Bergottini R, Scanziani E, Vecchi A, et al. Intestinal inflammation in mice deficient in Tir8, an inhibitory member of the IL-1 receptor family. Proc Natl Acad Sci U S A. 2004;101:3522–3526. doi: 10.1073/pnas.0308680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garlanda C, Riva F, Veliz T, Polentarutti N, Pasqualini F, Radaelli E, Sironi M, Nebuloni M, Zorini EO, Scanziani E, Mantovani A. Increased susceptibility to colitis-associated cancer of mice lacking TIR8, an inhibitory member of the interleukin-1 receptor family. Cancer Res. 2007b;67:6017–6021. doi: 10.1158/0008-5472.CAN-07-0560. [DOI] [PubMed] [Google Scholar]
- Ghiringhelli F, Apetoh L, Tesniere A, Aymeric L, Ma Y, Ortiz C, Vermaelen K, Panaretakis T, Mignot G, Ullrich E, et al. Activation of the NLRP3 inflammasome in dendritic cells induces IL-1beta-dependent adaptive immunity against tumors. Nat Med. 2009;15:1170–1178. doi: 10.1038/nm.2028. [DOI] [PubMed] [Google Scholar]
- Gresnigt MS, Rosler B, Jacobs CW, Becker KL, Joosten LA, van der Meer JW, Netea MG, Dinarello CA, van de Veerdonk FL. The IL-36 receptor pathway regulates Aspergillus fumigatus-induced Th1 and Th17 responses. Eur J Immunol. 2013;43:416–426. doi: 10.1002/eji.201242711. [DOI] [PubMed] [Google Scholar]
- Grimsby S, Jaensson H, Dubrovska A, Lomnytska M, Hellman U, Souchelnytskyi S. Proteomics-based identification of proteins interacting with Smad3: SREBP-2 forms a complex with Smad3 and inhibits its transcriptional activity. FEBS Lett. 2004;577:93–100. doi: 10.1016/j.febslet.2004.09.069. [DOI] [PubMed] [Google Scholar]
- Gulen MF, Kang Z, Bulek K, Youzhong W, Kim TW, Chen Y, Altuntas CZ, Sass Bak-Jensen K, McGeachy MJ, Do JS, et al. The receptor SIGIRR suppresses Th17 cell proliferation via inhibition of the interleukin-1 receptor pathway and mTOR kinase activation. Immunity. 2010;32:54–66. doi: 10.1016/j.immuni.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo L, Wei G, Zhu J, Liao W, Leonard WJ, Zhao K, Paul W. IL-1 family members and STAT activators induce cytokine production by Th2, Th17, and Th1 cells. Proc Natl Acad Sci U S A. 2009;106:13463–13468. doi: 10.1073/pnas.0906988106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho JE, Chen WY, Chen MH, Larson MG, McCabe EL, Cheng S, Ghorbani A, Coglianese E, Emilsson V, Johnson AD, et al. Common genetic variation at the IL1RL1 locus regulates IL-33/ST2 signaling. J Clin Invest. 2013;123:4208–4218. doi: 10.1172/JCI67119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman HM, Mueller JL, Broide DH, Wanderer AA, Kolodner RD. Mutation of a new gene encoding a putative pyrin-like protein causes familial cold autoinflammatory syndrome and Muckle-Wells syndrome. Nat Genet. 2001;29:301–305. doi: 10.1038/ng756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong DS, N A, Falchook GS, Piha-PAul S, Wheler JJ, Fu S, Tsimberidou AM, Hui D, Scott R, K R, et al. A phase I study of MABp1, a first-in-human, first-true human monoclonal antibody against the IL-1 in patients with advanced cancers. Mol Cancer Ther. 2011;10:A211. [Google Scholar]
- Horai R, Saijo S, Tanioka H, Nakae S, Sudo K, Okahara A, Ikuse T, Asano M, Iwakura Y. Development of chronic inflammatory arthropathy resembling rheumatoid arthritis in interleukin 1 receptor antagonist-deficient mice. J Exp Med. 2000;191:313–320. doi: 10.1084/jem.191.2.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X, Hazlett LD, Du W, Barrett RP. SIGIRR promotes resistance against Pseudomonas aeruginosa keratitis by down-regulating type-1 immunity and IL-1R1 and TLR4 signaling. J Immunol. 2006;177:548–556. doi: 10.4049/jimmunol.177.1.548. [DOI] [PubMed] [Google Scholar]
- Hughes T, Becknell B, Freud AG, McClory S, Briercheck E, Yu J, Mao C, Giovenzana C, Nuovo G, Wei L, et al. Interleukin-1beta selectively expands and sustains interleukin-22+ immature human natural killer cells in secondary lymphoid tissue. Immunity. 2010;32:803–814. doi: 10.1016/j.immuni.2010.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikutani M, Yanagibashi T, Ogasawara M, Tsuneyama K, Yamamoto S, Hattori Y, Kouro T, Itakura A, Nagai Y, Takaki S, Takatsu K. Identification of innate IL-5-producing cells and their role in lung eosinophil regulation and antitumor immunity. J Immunol. 2012;188:703–713. doi: 10.4049/jimmunol.1101270. [DOI] [PubMed] [Google Scholar]
- Joeckel LT, Wallich R, Metkar SS, Froelich CJ, Simon MM, Borner C. Interleukin-1R signaling is essential for induction of proapoptotic CD8 T cells, viral clearance, and pathology during lymphocytic choriomeningitis virus infection in mice. Journal Virol. 2012;86:8713–8719. doi: 10.1128/JVI.00682-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayagaki N, Warming S, Lamkanfi M, Vande Walle L, Louie S, Dong J, Newton K, Qu Y, Liu J, Heldens S, et al. Non-canonical inflammasome activation targets caspase-11. Nature. 2011;479:117–121. doi: 10.1038/nature10558. [DOI] [PubMed] [Google Scholar]
- Kim SH, Eisenstein M, Reznikov L, Fantuzzi G, Novick D, Rubinstein M, Dinarello CA. Structural requirements of six naturally occurring isoforms of the IL-18 binding protein to inhibit IL-18. Proc Natl Acad Sci U S A. 2000;97:1190–1195. doi: 10.1073/pnas.97.3.1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krelin Y, Voronov E, Dotan S, Elkabets M, Reich E, Fogel M, Huszar M, Iwakura Y, Segal S, Dinarello CA, Apte RN. Interleukin-1beta-driven inflammation promotes the development and invasiveness of chemical carcinogen-induced tumors. Cancer Res. 2007;67:1062–1071. doi: 10.1158/0008-5472.CAN-06-2956. [DOI] [PubMed] [Google Scholar]
- Kuhn PH, Marjaux E, Imhof A, De Strooper B, Haass C, Lichtenthaler SF. Regulated intramembrane proteolysis of the interleukin-1 receptor II by alpha-, beta-, and gamma-secretase. J Biol Chem. 2007;282:11982–11995. doi: 10.1074/jbc.M700356200. [DOI] [PubMed] [Google Scholar]
- Kumar S, Hanning CR, Brigham-Burke MR, Rieman DJ, Lehr R, Khandekar S, Kirkpatrick RB, Scott GF, Lee JC, Lynch FJ, et al. Interleukin-1F7B (IL-1H4/IL-1F7) is processed by caspase-1 and mature IL-1F7B binds to the IL-18 receptor but does not induce IFN-gamma production. Cytokine. 2002;18:61–71. doi: 10.1006/cyto.2002.0873. [DOI] [PubMed] [Google Scholar]
- Kurowska-Stolarska M, Stolarski B, Kewin P, Murphy G, Corrigan CJ, Ying S, Pitman N, Mirchandani A, Rana B, van Rooijen N, et al. IL-33 amplifies the polarization of alternatively activated macrophages that contribute to airway inflammation. J Immunol. 2009;183:6469–6477. doi: 10.4049/jimmunol.0901575. [DOI] [PubMed] [Google Scholar]
- Langley NJ. On nerve endings and on special excitable substances in cells. Proc R Soc London Ser B. 1906;78:170. [Google Scholar]
- Lech M, Avila-Ferrufino A, Allam R, Segerer S, Khandoga A, Krombach F, Garlanda C, Mantovani A, Anders HJ. Resident dendritic cells prevent postischemic acute renal failure by help of single Ig IL-1 receptor-related protein. J Immunol. 2009;183:4109–4118. doi: 10.4049/jimmunol.0900118. [DOI] [PubMed] [Google Scholar]
- Lech M, Kulkarni OP, Pfeiffer S, Savarese E, Krug A, Garlanda C, Mantovani A, Anders HJ. Tir8/Sigirr prevents murine lupus by suppressing the immunostimulatory effects of lupus autoantigens. J Exp Med. 2008;205:1879–1888. doi: 10.1084/jem.20072646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lech M, Skuginna V, Kulkarni OP, Gong J, Wei T, Stark RW, Garlanda C, Mantovani A, Anders HJ. Lack of SIGIRR/TIR8 aggravates hydrocarbon oil-induced lupus nephritis. J Pathol. 2010;220:596–607. doi: 10.1002/path.2678. [DOI] [PubMed] [Google Scholar]
- Lefrancais E, Roga S, Gautier V, Gonzalez-de-Peredo A, Monsarrat B, Girard JP, Cayrol C. IL-33 is processed into mature bioactive forms by neutrophil elastase and cathepsin G. Proc Natl Acad Sci U S A. 2012;109:1673–1678. doi: 10.1073/pnas.1115884109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liew FY, Pitman NI, McInnes IB. Disease-associated functions of IL-33: the new kid in the IL-1 family. Nature Rev Immunol. 2010;10:103–110. doi: 10.1038/nri2692. [DOI] [PubMed] [Google Scholar]
- Liu X, Hammel M, He Y, Tainer JA, Jeng US, Zhang L, Wang S, Wang X. Structural insights into the interaction of IL-33 with its receptors. Proc Natl Acad Sci U S A. 2013;110:14918–14923. doi: 10.1073/pnas.1308651110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzen I, Lokau J, Dusterhoft S, Trad A, Garbers C, Scheller J, Rose-John S, Grotzinger J. The membrane-proximal domain of A Disintegrin and Metalloprotease 17 (ADAM17) is responsible for recognition of the interleukin-6 receptor and interleukin-1 receptor II. FEBS Lett. 2012;586:1093–1100. doi: 10.1016/j.febslet.2012.03.012. [DOI] [PubMed] [Google Scholar]
- Luo Y, Cai X, Wang S, Nold-Petry C, Nold MF, Bulfer P, Norris D, Dinarello CA, Fujita M. IL-37 suppresses contact hypersensitivity by inducing tolerogenic dendritic cells. Cytokine. 2013;63:283. [Google Scholar]
- Maggi L, Santarlasci V, Capone M, Rossi MC, Querci V, Mazzoni A, Cimaz R, De Palma R, Liotta F, Maggi E, et al. Distinctive features of classic and nonclassic (Th17 derived) human Th1 cells. Eur J Immunol. 2012;42:3180–3188. doi: 10.1002/eji.201242648. [DOI] [PubMed] [Google Scholar]
- Mailliard RB, Alber SM, Shen H, Watkins SC, Kirkwood JM, Herberman RB, Kalinski P. IL-18-induced CD83+CCR7+ NK helper cells. J Exp Med. 2005;202:941–953. doi: 10.1084/jem.20050128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maksymowych WP, Rahman P, Reeve JP, Gladman DD, Peddle L, Inman RD. Association of the IL1 gene cluster with susceptibility to ankylosing spondylitis: an analysis of three Canadian populations. Arthritis Rheum. 2006;54:974–985. doi: 10.1002/art.21642. [DOI] [PubMed] [Google Scholar]
- Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- Mantovani A, Cassatella MA, Costantini C, Jaillon S. Neutrophils in the activation and regulation of innate and adaptive immunity. Nat Rev Immunol. 2011;11:519–531. doi: 10.1038/nri3024. [DOI] [PubMed] [Google Scholar]
- Marrakchi S, Guigue P, Renshaw BR, Puel A, Pei XY, Fraitag S, Zribi J, Bal E, Cluzeau C, Chrabieh M, et al. Interleukin-36-receptor antagonist deficiency and generalized pustular psoriasis. N Engl J Med. 2011;365:620–628. doi: 10.1056/NEJMoa1013068. [DOI] [PubMed] [Google Scholar]
- Martin P, Palmer G, Vigne S, Lamacchia C, Rodriguez E, Talabot-Ayer D, Rose-John S, Chalaris A, Gabay C. Mouse neutrophils express the decoy type 2 interleukin-1 receptor (IL-1R2) constitutively and in acute inflammatory conditions. J Leukoc Biol. 2013;94:791–802. doi: 10.1189/jlb.0113035. [DOI] [PubMed] [Google Scholar]
- McHedlidze T, Waldner M, Zopf S, Walker J, Rankin AL, Schuchmann M, Voehringer D, McKenzie AN, Neurath MF, Pflanz S, Wirtz S. Interleukin-33-dependent innate lymphoid cells mediate hepatic fibrosis. Immunity. 2013;39:357–371. doi: 10.1016/j.immuni.2013.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNamee EN, Masterson JC, Jedlicka P, McManus M, Grenz A, Collins CB, Nold MF, Nold-Petry C, Bufler P, Dinarello CA, Rivera-Nieves J. Interleukin 37 expression protects mice from colitis. Proc Natl Acad Sci U S A. 2011;108:16711–16716. doi: 10.1073/pnas.1111982108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercer F, Kozhaya L, Unutmaz D. Expression and function of TNF and IL-1 receptors on human regulatory T cells. PLoS One. 2010;5:e8639. doi: 10.1371/journal.pone.0008639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AM, Xu D, Asquith DL, Denby L, Li Y, Sattar N, Baker AH, McInnes IB, Liew FY. IL-33 reduces the development of atherosclerosis. J Exp Med. 2008;205:339–346. doi: 10.1084/jem.20071868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffatt MF, Gut IG, Demenais F, Strachan DP, Bouzigon E, Heath S, von Mutius E, Farrall M, Lathrop M, Cookson WO. A large-scale, consortium-based genomewide association study of asthma. N Engl J Med. 2010;363:1211–1221. doi: 10.1056/NEJMoa0906312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molofsky AB, Nussbaum JC, Liang HE, Van Dyken SJ, Cheng LE, Mohapatra A, Chawla A, Locksley RM. Innate lymphoid type 2 cells sustain visceral adipose tissue eosinophils and alternatively activated macrophages. J Exp Med. 2013;210:535–549. doi: 10.1084/jem.20121964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteiro M, Almeida CF, Agua-Doce A, Graca L. Induced IL-17-producing invariant NKT cells require activation in presence of TGF-beta and IL-1beta. J Immunol. 2013;190:805–811. doi: 10.4049/jimmunol.1201010. [DOI] [PubMed] [Google Scholar]
- Moro K, Yamada T, Tanabe M, Takeuchi T, Ikawa T, Kawamoto H, Furusawa J, Ohtani M, Fujii H, Koyasu S. Innate production of T(H)2 cytokines by adipose tissue-associated c-Kit(+)Sca-1(+) lymphoid cells. Nature. 2010;463:540–544. doi: 10.1038/nature08636. [DOI] [PubMed] [Google Scholar]
- Muzio M, Natoli G, Saccani S, Levrero M, Mantovani A. The human toll signaling pathway: divergence of nuclear factor kappaB and JNK/SAPK activation upstream of tumor necrosis factor receptor-associated factor 6 (TRAF6) J Exp Med. 1998;187:2097–2101. doi: 10.1084/jem.187.12.2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muzio M, Ni J, Feng P, Dixit VM. IRAK (Pelle) family member IRAK-2 and MyD88 as proximal mediators of IL-1 signaling. Science. 1997;278:1612–1615. doi: 10.1126/science.278.5343.1612. [DOI] [PubMed] [Google Scholar]
- Myles IA, Fontecilla NM, Valdez PA, Vithayathil PJ, Naik S, Belkaid Y, Ouyang W, Datta SK. Signaling via the IL-20 receptor inhibits cutaneous production of IL-1β and IL-17A to promote infection with methicillin-resistant Staphylococcus aureus. Nat Immunol. 2013:804–811. doi: 10.1038/ni.2637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neill DR, Wong SH, Bellosi A, Flynn RJ, Daly M, Langford TK, Bucks C, Kane CM, Fallon PG, Pannell R, et al. Nuocytes represent a new innate effector leukocyte that mediates type-2 immunity. Nature. 2010;464:1367–1370. doi: 10.1038/nature08900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Netea MG, Joosten LA, Lewis E, Jensen DR, Voshol PJ, Kullberg BJ, Tack CJ, van Krieken H, Kim SH, Stalenhoef AF, et al. Deficiency of interleukin-18 in mice leads to hyperphagia, obesity and insulin resistance. Nat Med. 2006;12:650–656. doi: 10.1038/nm1415. [DOI] [PubMed] [Google Scholar]
- Nicklin MJ, Hughes DE, Barton JL, Ure JM, Duff GW. Arterial inflammation in mice lacking the interleukin 1 receptor antagonist gene. J Exp Med. 2000;191:303–312. doi: 10.1084/jem.191.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nold-Petry CA, Nold MF, Nielsen JW, Bustamante A, Zepp JA, Storm KA, Hong JW, Kim SH, Dinarello CA. Increased cytokine production in interleukin-18 receptor alpha-deficient cells is associated with dysregulation of suppressors of cytokine signaling. J Biol Chem. 2009;284:25900–25911. doi: 10.1074/jbc.M109.004184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nold M, Nold-Petry C, Lo C, Li S, Zepp J, Azam T, Bulfer P, Garlanda C, Mantovani A, Dinarello CA. Interleukin 37 employs the IL-1 family inhibitory receptor SIGIRR and the alpha chain of the IL-18 receptor to suppress innate immunity. Cytokine. 2013;63:287. [Google Scholar]
- Nold MF, Nold-Petry CA, Zepp JA, Palmer BE, Bufler P, Dinarello CA. IL-37 is a fundamental inhibitor of innate immunity. Nat Immunol. 2010;11:1014–1022. doi: 10.1038/ni.1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noris M, Cassis P, Azzollini N, Cavinato R, Cugini D, Casiraghi F, Aiello S, Solini S, Cassis L, Mister M, et al. The Toll-IL-1R member Tir8/SIGIRR negatively regulates adaptive immunity against kidney grafts. J Immunol. 2009;183:4249–4260. doi: 10.4049/jimmunol.0803549. [DOI] [PubMed] [Google Scholar]
- Novick D, Elbirt D, Miller G, Dinarello CA, Rubinstein M, Sthoeger ZM. High circulating levels of free interleukin-18 in patients with active SLE in the presence of elevated levels of interleukin-18 binding protein. J Autoimmunity. 2010;34:121–126. doi: 10.1016/j.jaut.2009.08.002. [DOI] [PubMed] [Google Scholar]
- Novick D, Kim SH, Fantuzzi G, Reznikov LL, Dinarello CA, Rubinstein M. Interleukin-18 binding protein: a novel modulator of the Th1 cytokine response. Immunity. 1999;10:127–136. doi: 10.1016/s1074-7613(00)80013-8. [DOI] [PubMed] [Google Scholar]
- O’Shea JJ, Paul WE. Mechanisms underlying lineage commitment and plasticity of helper CD4+ T cells. Science. 2010;327:1098–1102. doi: 10.1126/science.1178334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oboki K, Ohno T, Kajiwara N, Arae K, Morita H, Ishii A, Nambu A, Abe T, Kiyonari H, Matsumoto K, et al. IL-33 is a crucial amplifier of innate rather than acquired immunity. Proc Natl Acad Sci U S A. 2010;107:18581–18586. doi: 10.1073/pnas.1003059107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paget C, Ivanov S, Fontaine J, Renneson J, Blanc F, Pichavant M, Dumoutier L, Ryffel B, Renauld JC, Gosset P, et al. Interleukin-22 is produced by invariant natural killer T lymphocytes during influenza A virus infection: potential role in protection against lung epithelial damages. J Biol Chem. 2012;287:8816–8829. doi: 10.1074/jbc.M111.304758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palm NW, Rosestein RK, Yu S, Schenten DD, Florsheim E, Medzhitov R. Bee venom phospholipase A2 induces a primary type 2 response that is dependent on the receptor ST2 and confers protective immunity. Immunity. 2013;39:976–985. doi: 10.1016/j.immuni.2013.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pecaric-Petkovic T, Didichenko SA, Kaempfer S, Spiegl N, Dahinden CA. Human basophils and eosinophils are the direct target leukocytes of the novel IL-1 family member IL-33. Blood. 2009;113:1526–1534. doi: 10.1182/blood-2008-05-157818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomerantz BJ, Reznikov LL, Harken AH, Dinarello CA. Inhibition of caspase 1 reduces human myocardial ischemic dysfunction via inhibition of IL-18 and IL-1beta. Proc Natl Acad Sci U S A. 2001;98:2871–2876. doi: 10.1073/pnas.041611398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puren AJ, Fantuzzi G, Dinarello CA. Gene expression, synthesis, and secretion of interleukin 18 and interleukin 1beta are differentially regulated in human blood mononuclear cells and mouse spleen cells. Proc Natl Acad Sci U S A. 1999;96:2256–2261. doi: 10.1073/pnas.96.5.2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman P, Sun S, Peddle L, Snelgrove T, Melay W, Greenwood C, Gladman D. Association between the interleukin-1 family gene cluster and psoriatic arthritis. Arthritis Rheum. 2006;54:2321–2325. doi: 10.1002/art.21928. [DOI] [PubMed] [Google Scholar]
- Re F, Sironi M, Muzio M, Matteucci C, Introna M, Orlando S, Penton-Rol G, Dower SK, Sims JE, Colotta F, Mantovani A. Inhibition of interleukin-1 responsiveness by type II receptor gene transfer: a surface “receptor” with anti-interleukin-1 function. J Exp Med. 1996;183:1841–1850. doi: 10.1084/jem.183.4.1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy S, Jia S, Geoffrey R, Lorier R, Suchi M, Broeckel U, Hessner MJ, Verbsky J. An autoinflammatory disease due to homozygous deletion of the IL1RN locus. N Engl J Med. 2009;360:2438–2444. doi: 10.1056/NEJMoa0809568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rider P, Carmi Y, Guttman O, Braiman A, Cohen I, Voronov E, White MR, Dinarello CA, Apte RN. IL-1α and IL-1β recruit different myeloid cells and promote different stages of sterile inflammation. J Immunol. 2011;187:4835–4843. doi: 10.4049/jimmunol.1102048. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Prados JC, Traves PG, Cuenca J, Rico D, Aragones J, Martin-Sanz P, Cascante M, Bosca L. Substrate fate in activated macrophages: a comparison between innate, classic, and alternative activation. J Immunol. 2010;185:605–614. doi: 10.4049/jimmunol.0901698. [DOI] [PubMed] [Google Scholar]
- Russell SE, Stefanska AM, Kubica M, Horan RM, Mantovani A, Garlanda C, Fallon PG, Walsh PT. Toll IL-1R8/single Ig IL-1-related receptor regulates psoriasiform inflammation through direct inhibition of innate IL-17A expression by gammadelta T cells. J Immunol. 2013;191:3337–3346. doi: 10.4049/jimmunol.1300828. [DOI] [PubMed] [Google Scholar]
- Sakai N, Van Sweringen HL, Belizaire RM, Quillin RC, Schuster R, Blanchard J, Burns JM, Tevar AD, Edwards MJ, Lentsch AB. Interleukin-37 reduces liver inflammatory injury via effects on hepatocytes and non-parenchymal cells. J Gastroenterol Hepatol. 2012;27:1609–1616. doi: 10.1111/j.1440-1746.2012.07187.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salcedo R, Cataisson C, Hasan U, Yuspa SH, Trinchieri G. MyD88 and its divergent toll in carcinogenesis. Trends Immunol. 2013;34:379–389. doi: 10.1016/j.it.2013.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanada S, Hakuno D, Higgins LJ, Schreiter ER, McKenzie AN, Lee RT. IL-33 and ST2 comprise a critical biomechanically induced and cardioprotective signaling system. J Clin Invest. 2007;117:1538–1549. doi: 10.1172/JCI30634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santarlasci V, Cosmi L, Maggi L, Liotta F, Annunziato F. IL-1 and T helper immune responses. Frontiers in Inflammation. 2013;4 doi: 10.3389/fimmu.2013.00182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma S, Kulk N, Nold MF, Graf R, Kim SH, Reinhardt D, Dinarello CA, Bufler P. The IL-1 family member 7b translocates to the nucleus and down-regulates proinflammatory cytokines. J Immunol. 2008;180:5477–5482. doi: 10.4049/jimmunol.180.8.5477. [DOI] [PubMed] [Google Scholar]
- Sica A, Invernizzi P, Mantovani A. Macrophage plasticity and polarization in liver homeostasis and pathology. Hepatology. 2013 doi: 10.1002/hep.26754. in press. [DOI] [PubMed] [Google Scholar]
- Siegmund B, Fantuzzi G, Rieder F, Gamboni-Robertson F, Lehr HA, Hartmann G, Dinarello CA, Endres S, Eigler A. Neutralization of interleukin-18 reduces severity in murine colitis and intestinal IFN-gamma and TNF-alpha production. Am J Physiol Regul Integr Comp Physiol. 2001a;281:R1264–1273. doi: 10.1152/ajpregu.2001.281.4.R1264. [DOI] [PubMed] [Google Scholar]
- Siegmund B, Lehr HA, Fantuzzi G, Dinarello CA. IL-1 beta -converting enzyme (caspase-1) in intestinal inflammation. Proc Natl Acad Sci U S A. 2001b;98:13249–13254. doi: 10.1073/pnas.231473998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sims JE, Smith DE. The IL-1 family: regulators of immunity. Nat Rev Immunol. 2010;10:89–102. doi: 10.1038/nri2691. [DOI] [PubMed] [Google Scholar]
- Smith DE, Hanna R, Della F, Moore H, Chen H, Farese AM, MacVittie TJ, Virca GD, Sims JE. The soluble form of IL-1 receptor accessory protein enhances the ability of soluble type II IL-1 receptor to inhibit IL-1 action. Immunity. 2003;18:87–96. doi: 10.1016/s1074-7613(02)00514-9. [DOI] [PubMed] [Google Scholar]
- Smith DE, Lipsky BP, Russell C, Ketchem RR, Kirchner J, Hensley K, Huang Y, Friedman WJ, Boissonneault V, Plante MM, et al. A central nervous system-restricted isoform of the interleukin-1 receptor accessory protein modulates neuronal responses to interleukin-1. Immunity. 2009;30:817–831. doi: 10.1016/j.immuni.2009.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spits H, Artis D, Colonna M, Diefenbach A, Di Santo JP, Eberl G, Koyasu S, Locksley RM, McKenzie AN, Mebius RE, et al. Innate lymphoid cells--a proposal for uniform nomenclature. Nat Rev Immunol. 2013;13:145–149. doi: 10.1038/nri3365. [DOI] [PubMed] [Google Scholar]
- Sutton C, Brereton C, Keogh B, Mills KH, Lavelle EC. A crucial role for interleukin (IL)-1 in the induction of IL-17-producing T cells that mediate autoimmune encephalomyelitis. J Exp Med. 2006;203:1685–1691. doi: 10.1084/jem.20060285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tack CJ, Stienstra R, Joosten LA, Netea MG. Inflammation links excess fat to insulin resistance: the role of the interleukin-1 family. Immunol Rev. 2012;249:239–252. doi: 10.1111/j.1600-065X.2012.01145.x. [DOI] [PubMed] [Google Scholar]
- Tannahill GM, Curtis AM, Adamik J, Palsson-McDermott EM, McGettrick AF, Goel G, Frezza C, Bernard NJ, Kelly B, Foley NH, et al. Succinate is an inflammatory signal that induces IL-1beta through HIF-1alpha. Nature. 2013;496:238–242. doi: 10.1038/nature11986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tjota MY, Williams JW, Lu T, Clay BS, Byrd T, Hrusch CL, Decker DC, de Araujo CA, Bryce PJ, Sperling AI. IL-33-dependent induction of allergic lung inflammation by FcgammaRIII signaling. J Clin Invest. 2013;123:2287–2297. doi: 10.1172/JCI63802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tortola L, Rosenwald E, Abel B, Blumberg H, Schafer M, Coyle AJ, Renauld JC, Werner S, Kisielow J, Kopf M. Psoriasiform dermatitis is driven by IL-36-mediated DC-keratinocyte crosstalk. J Clin Invest. 2012;122:3965–3976. doi: 10.1172/JCI63451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towne JE, Renshaw BR, Douangpanya J, Lipsky BP, Shen M, Gabel CA, Sims JE. Interleukin-36 (IL-36) ligands require processing for full agonist (IL-36alpha, IL-36beta, and IL-36gamma) or antagonist (IL-36Ra) activity. J Biol Chem. 2011;286:42594–42602. doi: 10.1074/jbc.M111.267922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Veerdonk FL, Stoeckman AK, Wu G, Boeckermann AN, Azam T, Netea MG, Joosten LA, van der Meer JW, Hao R, Kalabokis V, Dinarello CA. IL-38 binds to the IL-36 receptor and has biological effects on immune cells similar to IL-36 receptor antagonist. Proc Natl Acad Sci U S A. 2012;109:3001–3005. doi: 10.1073/pnas.1121534109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Meer JW, Barza M, Wolff SM, Dinarello CA. A low dose of recombinant interleukin 1 protects granulocytopenic mice from lethal gram-negative infection. Proc Natl Acad Sci U S A. 1988;85:1620–1623. doi: 10.1073/pnas.85.5.1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vander Lugt MT, Braun TM, Hanash S, Ritz J, Ho VT, Antin JH, Zhang Q, Wong CH, Wang H, Chin A, et al. ST2 as a marker for risk of therapy-resistant graft-versus-host disease and death. N Engl J Med. 2013;369:529–539. doi: 10.1056/NEJMoa1213299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24:179–189. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- Vigne S, Palmer G, Lamacchia C, Martin P, Talabot-Ayer D, Rodriguez E, Ronchi F, Sallusto F, Dinh H, Sims JE, Gabay C. IL-36R ligands are potent regulators of dendritic and T cells. Blood. 2011;118:5813–5823. doi: 10.1182/blood-2011-05-356873. [DOI] [PubMed] [Google Scholar]
- Vigne S, Palmer G, Martin P, Lamacchia C, Strebel D, Rodriguez E, Olleros ML, Vesin D, Garcia I, Ronchi F, et al. IL-36 signaling amplifies Th1 responses by enhancing proliferation and Th1 polarization of naive CD4+ T cells. Blood. 2012;120:3478–3487. doi: 10.1182/blood-2012-06-439026. [DOI] [PubMed] [Google Scholar]
- Wald D, Qin J, Zhao Z, Qian Y, Naramura M, Tian L, Towne J, Sims JE, Stark GR, Li X. SIGIRR, a negative regulator of Toll-like receptor-interleukin 1 receptor signaling. Nat Immunol. 2003;4:920–927. doi: 10.1038/ni968. [DOI] [PubMed] [Google Scholar]
- Wang D, Zhang S, Li L, Liu X, Mei K, Wang X. Structural insights into the assembly and activation of IL-1beta with its receptors. Nat Immunol. 2010;11:905–911. doi: 10.1038/ni.1925. [DOI] [PubMed] [Google Scholar]
- Xiao H, Gulen MF, Qin J, Yao J, Bulek K, Kish D, Altuntas CZ, Wald D, Ma C, Zhou H, et al. The Toll-interleukin-1 receptor member SIGIRR regulates colonic epithelial homeostasis, inflammation, and tumorigenesis. Immunity. 2007;26:461–475. doi: 10.1016/j.immuni.2007.02.012. [DOI] [PubMed] [Google Scholar]
- Xiao H, Yin W, Khan MA, Gulen MF, Zhou H, Sham HP, Jacobson K, Vallance BA, Li X. Loss of single immunoglobulin interlukin-1 receptor-related molecule leads to enhanced colonic polyposis in Apc(min) mice. Gastroenterology. 2010;139:574–585. doi: 10.1053/j.gastro.2010.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yousif NG, Li J, Yousif F, Ao L, Nold MF, Nold-Petry C, Fullerton DA, Dinarello CA, Meng X. Expression of IL-37 in mouse protects the myocardium against ischemic injury via modulation of NF-κB activation. Circulation. 2011;124(Suppl 21):A8603. [Google Scholar]
- Zaiss MM, Maslowski KM, Mosconi I, Guenat N, Marsland BJ, Harris NL. IL-1beta Suppresses Innate IL-25 and IL-33 Production and Maintains Helminth Chronicity. PLoS Pathog. 2013;9:e1003531. doi: 10.1371/journal.ppat.1003531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng X, Wei YL, Huang J, Newell EW, Yu H, Kidd BA, Kuhns MS, Waters RW, Davis MM, Weaver CT, Chien YH. gammadelta T cells recognize a microbial encoded B cell antigen to initiate a rapid antigen-specific interleukin-17 response. Immunity. 2012;37:524–534. doi: 10.1016/j.immuni.2012.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Wei J, Mialki RK, Mallampalli DF, Chen BB, Coon T, Zou C, Mallampalli RK, Zhao Y. F-box protein FBXL19-mediated ubiquitination and degradation of the receptor for IL-33 limits pulmonary inflammation. Nat Immunol. 2012;13:651–658. doi: 10.1038/ni.2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y, Humphry M, Maguire JJ, Bennett MR, Clarke MC. Intracellular interleukin-1 receptor 2 binding prevents cleavage and activity of interleukin-1alpha, controlling necrosis-induced sterile inflammation. Immunity. 2013;38:285–295. doi: 10.1016/j.immuni.2013.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zielinski CE, Mele F, Aschenbrenner D, Jarrossay D, Ronchi F, Gattorno M, Monticelli S, Lanzavecchia A, Sallusto F. Pathogen-induced human TH17 cells produce IFN-gamma or IL-10 and are regulated by IL-1beta. Nature. 2012;484:514–518. doi: 10.1038/nature10957. [DOI] [PubMed] [Google Scholar]


