Abstract
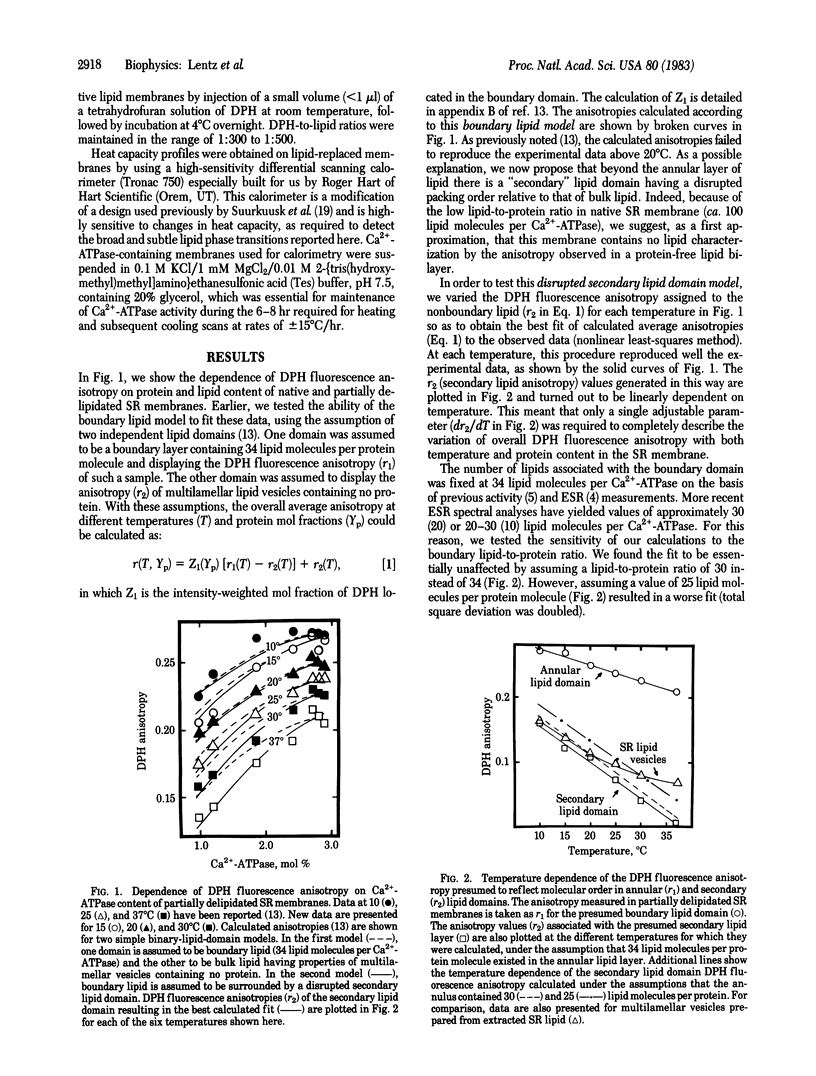
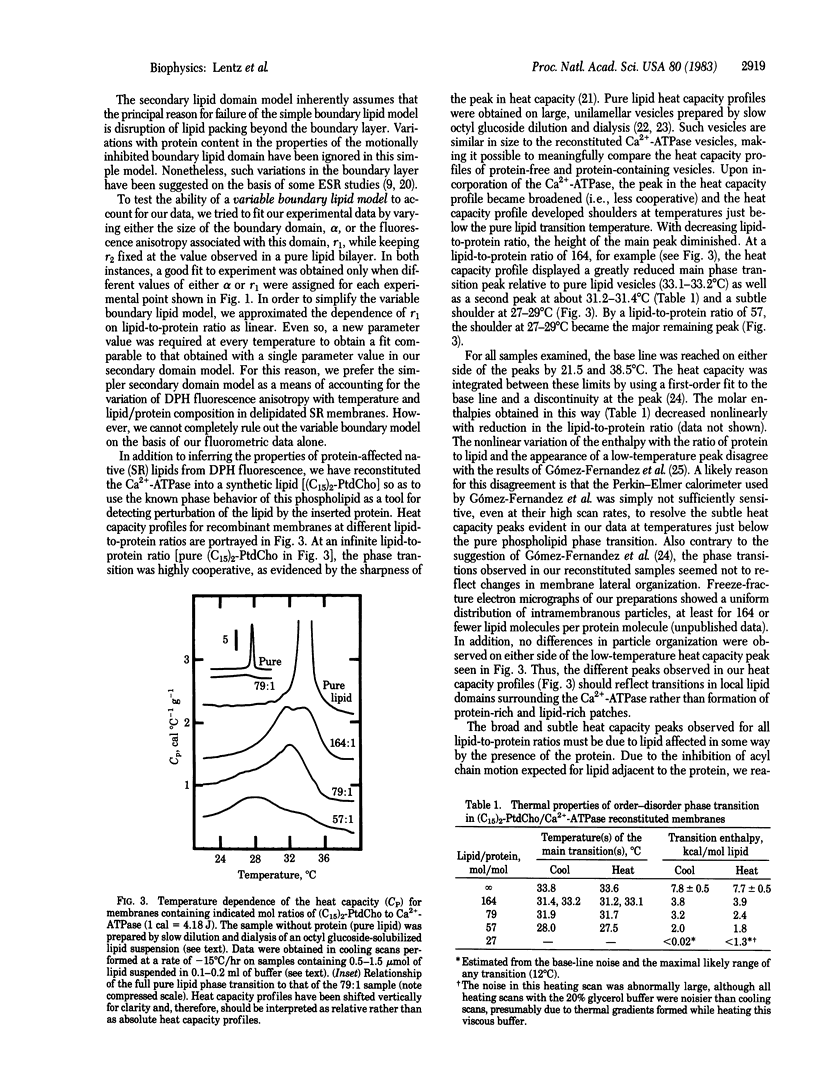
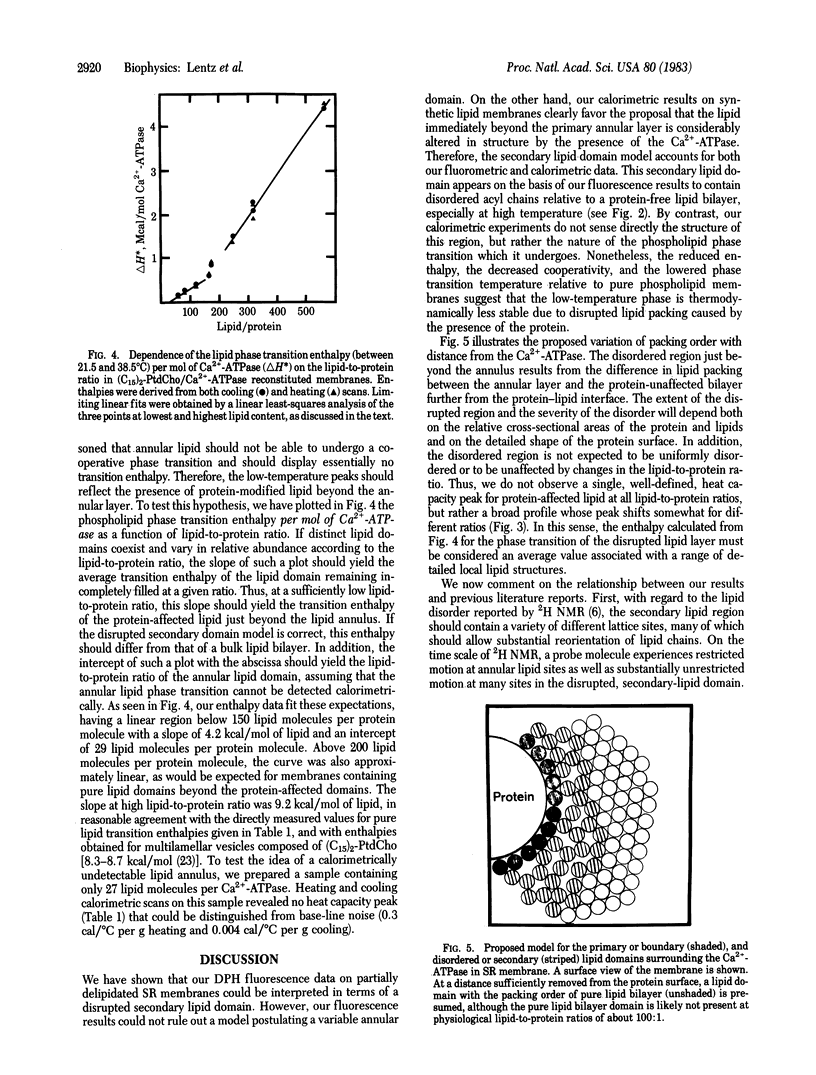
Data are presented that lead to an alternative model for the organization and molecular dynamics of lipid molecules near the Ca2+-stimulated, Mg2+-dependent adenosinetriphosphatase (Ca2+-ATPase; ATP phosphohydrolase, EC 3.6.1.3) of sarcoplasmic reticulum. Measurements of the steady-state fluorescence anisotropy of 1,6-diphenyl-1,3,5-hexatriene in progressively delipidated sarcoplasmic reticulum membranes have been quantitatively interpreted in terms of a layer of lipid of high anisotropy (the lipid annulus) coexisting with lipid layers of very low anisotropy. In addition, the Ca2+-ATPase has been reconstituted into pure 1,2-dipentadecanoyl 3-sn-phosphatidylcholine membranes over a range of lipid-to-protein ratios. High-sensitivity differential scanning calorimetry has demonstrated that roughly 30 lipid molecules per Ca2+-ATPase molecule (annular lipids) fail to undergo a calorimetrically detectable phase transition in the temperature range 4-44 degrees C. Roughly 100 lipid molecules beyond the annulus undergo a detectable phase transition at a temperature below the phase transition of pure lipid and with an enthalpy change [4.2 kcal/mol (1 kcal = 4.18 kJ)] about half that observed for pure lipid vesicles (7.7-7.8 kcal/mol). We propose that both the fluorometric and calorimetric data are consistent with a model in which a motionally inhibited lipid annulus is surrounded by a more extensive region of disrupted lipid packing order, which we have called the secondary lipid domain.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brenner J. I., Ringel R., Green D., Berman M. A., Whitley N. Use of computed axial tomography to diagnose vascular ring in an infant. Tex Heart Inst J. 1982 Mar;9(1):53–56. [PMC free article] [PubMed] [Google Scholar]
- Chapman D., Gómez-Fernández J. C., Goñi F. M. Intrinsic protein--lipid interactions. Physical and biochemical evidence. FEBS Lett. 1979 Feb 15;98(2):211–223. doi: 10.1016/0014-5793(79)80186-6. [DOI] [PubMed] [Google Scholar]
- Davoust J., Bienvenue A., Fellmann P., Devaux P. F. Boundary lipids and protein mobility in rhodopsin-phosphatidylcholine vesicles. Effect of lipid phase transitions. Biochim Biophys Acta. 1980 Feb 15;596(1):28–42. doi: 10.1016/0005-2736(80)90168-6. [DOI] [PubMed] [Google Scholar]
- Dean W. L., Tanford C. Properties of a delipidated, detergent-activated Ca2+--ATPase. Biochemistry. 1978 May 2;17(9):1683–1690. doi: 10.1021/bi00602a016. [DOI] [PubMed] [Google Scholar]
- Gomez-Fernandez J. C., Goni F. M., Bach D., Restall C. J., Chapman D. Protein-lipid interaction. Biophysical studies of (Ca2+ + Mg2+)-ATPase reconstituted systems. Biochim Biophys Acta. 1980 Jun 6;598(3):502–516. doi: 10.1016/0005-2736(80)90031-0. [DOI] [PubMed] [Google Scholar]
- Hesketh T. R., Smith G. A., Houslay M. D., McGill K. A., Birdsall N. J., Metcalfe J. C., Warren G. B. Annular lipids determine the ATPase activity of a calcium transport protein complexed with dipalmitoyllecithin. Biochemistry. 1976 Sep 21;15(19):4145–4151. doi: 10.1021/bi00664a002. [DOI] [PubMed] [Google Scholar]
- Hinz H. J., Sturtevant J. M. Calorimetric studies of dilute aqueous suspensions of bilayers formed from synthetic L- -lecithins. J Biol Chem. 1972 Oct 10;247(19):6071–6075. [PubMed] [Google Scholar]
- Jost P. C., Capadil R. A., Vanderkooi G., Griffith O. H. Lipid-protein and lipid-lipid interactions in cytochrome oxidase model membranes. J Supramol Struct. 1973;1(4):269–280. doi: 10.1002/jss.400010404. [DOI] [PubMed] [Google Scholar]
- Jost P. C., Griffith O. H. Lipid-lipid and lipid-protein interactions in membranes. Pharmacol Biochem Behav. 1980;13 (Suppl 1):155–165. doi: 10.1016/s0091-3057(80)80025-6. [DOI] [PubMed] [Google Scholar]
- Jähnig F. Structural order of lipids and proteins in membranes: evaluation of fluorescence anisotropy data. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6361–6365. doi: 10.1073/pnas.76.12.6361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles P. F., Watts A., Marsh D. Spin-label studies of head-group specificity in the interaction of phospholipids with yeast cytochrome oxidase. Biochemistry. 1981 Sep 29;20(20):5888–5894. doi: 10.1021/bi00523a036. [DOI] [PubMed] [Google Scholar]
- Knowles P. F., Watts A., Marsh D. Spin-label studies of lipid immobilization in dimyristoylphosphatidylcholine-substituted cytochrome oxidase. Biochemistry. 1979 Oct 16;18(21):4480–4487. doi: 10.1021/bi00588a005. [DOI] [PubMed] [Google Scholar]
- Lentz B. R., Freire E., Biltonen R. L. Fluorescence and calorimetric studies of phase transitions in phosphatidylcholine multilayers: kinetics of the pretransition. Biochemistry. 1978 Oct 17;17(21):4475–4480. doi: 10.1021/bi00614a018. [DOI] [PubMed] [Google Scholar]
- Lentz B. R., Moore B. M., Barrow D. A. Light-scattering effects in the measurement of membrane microviscosity with diphenylhexatriene. Biophys J. 1979 Mar;25(3):489–494. doi: 10.1016/S0006-3495(79)85318-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lentz B. R., Moore B. M., Kirkman C., Meissner G. Lipid-Protein Interactions in Sarcoplasmic Reticulum: A Disrupted Secondary Lipid Layer Surrounds the Ca-ATPase. Biophys J. 1982 Jan;37(1):30–32. doi: 10.1016/S0006-3495(82)84584-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendelsohn R., Dluhy R., Taraschi T., Cameron D. G., Mantsch H. H. Raman and Fourier transform infrared spectroscopic studies of the interaction between glycophorin and dimyristoylphosphatidylcholine. Biochemistry. 1981 Nov 10;20(23):6699–6706. doi: 10.1021/bi00526a027. [DOI] [PubMed] [Google Scholar]
- Mimms L. T., Zampighi G., Nozaki Y., Tanford C., Reynolds J. A. Phospholipid vesicle formation and transmembrane protein incorporation using octyl glucoside. Biochemistry. 1981 Feb 17;20(4):833–840. doi: 10.1021/bi00507a028. [DOI] [PubMed] [Google Scholar]
- Moore B. M., Lentz B. R., Hoechli M., Meissner G. Effect of lipid membrane structure on the adenosine 5'-triphosphate hydrolyzing activity of the calcium-stimulated adenosinetriphosphatase of sarcoplasmic reticulum. Biochemistry. 1981 Nov 24;20(24):6810–6817. doi: 10.1021/bi00527a011. [DOI] [PubMed] [Google Scholar]
- Moore B. M., Lentz B. R., Meissner G. Effects of sarcoplasmic reticulum Ca2+-ATPase on phospholipid bilayer fluidity: boundary lipid. Biochemistry. 1978 Nov 28;17(24):5248–5255. doi: 10.1021/bi00617a026. [DOI] [PubMed] [Google Scholar]
- Nakamura M., Onishi S. Organization of lipids in sarcoplasmic reticulum membrane and Ca2+-dependent ATPase activity. J Biochem. 1975 Nov;78(5):1039–1045. doi: 10.1093/oxfordjournals.jbchem.a130981. [DOI] [PubMed] [Google Scholar]
- Paddy M. R., Dahlquist F. W., Davis J. H., Bloom M. Dynamical and temperature-dependent effects of lipid-protein interactions. Application of deuterium nuclear magnetic resonance and electron paramagnetic resonance spectroscopy to the same reconstitutions of cytochrome c oxidase. Biochemistry. 1981 May 26;20(11):3152–3162. doi: 10.1021/bi00514a026. [DOI] [PubMed] [Google Scholar]
- Rice D. M., Meadows M. D., Scheinman A. O., Goñi F. M., Gómez-Fernández J. C., Moscarello M. A., Chapman D., Oldfield E. Protein-lipid interactions. A nuclear magnetic resonance study of sarcoplasmic reticulum Ca2,Mg2+-ATPase, lipophilin, and proteolipid apoprotein-lecithin systems and a comparison with the effects of cholesterol. Biochemistry. 1979 Dec 25;18(26):5893–5903. [PubMed] [Google Scholar]
- Seelig J., Tamm L., Hymel L., Fleischer S. Deuterium and phosphorus nuclear magnetic resonance and fluorescence depolarization studies of functional reconstituted sarcoplasmic reticulum membrane vesicles. Biochemistry. 1981 Jun 23;20(13):3922–3932. doi: 10.1021/bi00516a040. [DOI] [PubMed] [Google Scholar]
- Suurkuusk J., Lentz B. R., Barenholz Y., Biltonen R. L., Thompson T. E. A calorimetric and fluorescent probe study of the gel-liquid crystalline phase transition in small, single-lamellar dipalmitoylphosphatidylcholine vesicles. Biochemistry. 1976 Apr 6;15(7):1393–1401. doi: 10.1021/bi00652a007. [DOI] [PubMed] [Google Scholar]
- Thomas D. D., Bigelow D. J., Squier T. C., Hidalgo C. Rotational dynamics of protein and boundary lipid in sarcoplasmic reticulum membrane. Biophys J. 1982 Jan;37(1):217–225. doi: 10.1016/S0006-3495(82)84671-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts A., Davoust J., Marsh D., Devaux P. F. Distinct states of lipid mobility in bovine rod outer segment membranes. Resolution of spin label results. Biochim Biophys Acta. 1981 May 20;643(3):673–676. doi: 10.1016/0005-2736(81)90365-5. [DOI] [PubMed] [Google Scholar]


