Abstract
Purpose
To test whether long-term multivitamin supplementation affects the incidence of cataract and/or age-related macular degeneration (AMD) in a large cohort of men.
Design
Randomized, double-blind, placebo-controlled trial.
Participants
Fourteen-thousand six hundred forty one United States male physicians aged ≥50 years.
Intervention
Daily multivitamin or placebo.
Main Outcome Measures
Incident cataract and visually-significant AMD responsible for a reduction in best-corrected visual acuity to 20/30 or worse based on self-reports confirmed by medical record review.
Results
During an average of 11.2 years of treatment and follow-up, a total of 1,817 cases of cataract and 281 cases of visually-significant AMD were confirmed. There were 872 cataracts in the multivitamin group and 945 in the placebo group (hazard ratio [HR], 0.91; 95% confidence interval [CI], 0.83 to 0.99; p=0.04). For visually-significant AMD, there were 152 cases in the multivitamin group and 129 in the placebo group (HR, 1.19; 95% CI, 0.94 to 1.50; p=0.15).
Conclusions
These randomized trial data from a large cohort of middle-aged and older US male physicians indicate that long-term daily multivitamin use modestly and significantly decreased the risk of cataract, but had no significant effect on visually-significant AMD.
Trial registration
clinicaltrials.gov Identifier: NCT00270647
Nutritional factors are postulated to play a causal role in the development of cataract and age-related macular degeneration (AMD), two leading causes of visual impairment in older Americans.1 Considerable observational evidence suggests that persons with higher dietary intake or blood levels of nutrients with antioxidant capabilities have lower rates of cataract and AMD.2–7 Moreover, animal studies show that supplementation with antioxidants and other micronutrients can prevent or delay the formation of lens opacities,3,8,9 and can reduce the damaging effects of reactive oxygen species on the retina.10–12 However, results of nutritional supplementation trials in humans have been inconsistent.
For cataract, randomized trials conducted among older, generally well-nourished populations indicate that high-dose supplements of selected nutrients (most commonly vitamin E, vitamin C, and beta carotene), alone or in combination, for up to 10 years have little material impact on cataract occurrence.13–21 Conversely, two trials testing a multivitamin supplement, one in a nutritionally-deficient population in China22 and the other in a well-nourished population in Italy,23 reported significantly lower rates of cataract in the multivitamin group. For AMD, the Age-related Eye Disease Study (AREDS) demonstrated that daily supplementation with zinc and high-dose antioxidants vitamin E, vitamin C, and beta carotene could reduce the risk of advanced AMD by 25% in persons with intermediate AMD or advanced AMD in one eye.24 A second trial, in a population at high risk of cardiovascular disease (CVD), showed that daily folic acid, vitamin B6, and vitamin B12 reduced AMD incidence by 35%–40%.25 Five other trials testing high-dose vitamin E, vitamin C, and beta carotene, alone or in combination, reported no benefit on AMD.26–30
Herein, we present the final results for cataract and AMD from the multivitamin component of Physicians’ Health Study II (PHS II).31
Methods
Study Design
The Physicians’ Health Study (PHS II) was a randomized, double-blind, placebo-controlled, factorial trial evaluating a daily multivitamin (Centrum Silver), alternate day vitamin E (400 IU synthetic α-tocopherol), and daily vitamin C (500 mg synthetic ascorbic acid) in the prevention of cancer and CVD among 14,641 male physicians aged 50 years and older.31 A fourth randomized component, alternate day beta-carotene (50 mg Lurotin), was terminated in March, 2003. The vitamin E and vitamin C components of the trial ended as scheduled on August 31, 2007.18,30,32,33 Cataract and AMD were pre-specified secondary endpoints of PHS II.
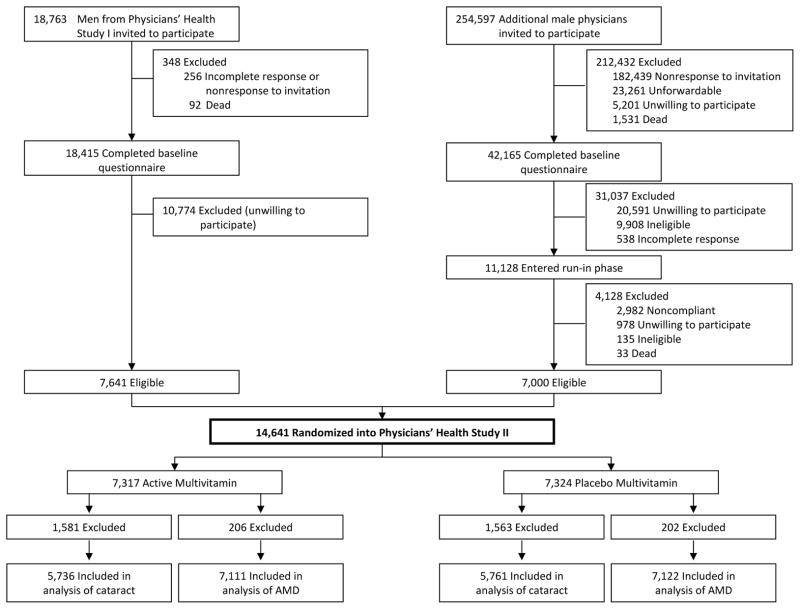
The study design for PHS II has been described previously.31 Briefly, recruitment, enrollment, and randomization of men into PHS II occurred in two phases (Figure 1). Phase 1 began in 1997 and included 7,641 willing and eligible participants from PHS I20,29,34,35 who retained their original beta-carotene treatment assignment and were newly randomized to a multivitamin, vitamin C, and vitamin E. Phase 2 began in 1999 and consisted of 7,000 new physician participants who were independently randomized to each of a multivitamin, beta-carotene, vitamin C, and vitamin E, or their matching placebos. All participants provided written informed consent, and PHS II was approved by the institutional review board of the Brigham and Women’s Hospital, Boston, Massachusetts.
Figure 1.
Flow diagram of the multivitamin component of the Physicians’ Health Study II. A total of 3,144 participants who reported a diagnosis of cataract at baseline, and 408 participants who reported a diagnosis of age-related macular degeneration at baseline, were excluded. Abbreviation: AMD, age-related macular degeneration.
Participants completed annual questionnaires providing information on compliance with pill taking, potential adverse events, updated risk factors, and the occurrence of any new endpoints including cataract and AMD. Treatment and follow-up continued in blinded fashion through June 1, 2011, the scheduled end of the multivitamin component. Morbidity and mortality follow-up were 98.2% and 99.9%, respectively.
Compliance with pill taking was based on self-report and defined as taking at least two thirds of the study agents. Adherence at 6 years was 73.6% for active multivitamin and 73.3% for placebo (P=0.68).
Ascertainment and Confirmation of Study Endpoints
Participants who reported cataract (n= 3,144) or AMD (n= 408) at baseline were excluded from analyses of the respective endpoint. For new reports of cataract or AMD, participants were asked to provide written consent to obtain medical records. Ophthalmologists/optometrists were contacted by mail and asked to provide information about the reported endpoint by completing a questionnaire or, alternatively, supplying copies of relevant medical records.
Cataract
The cataract questionnaire asked about the presence of lens opacities, diagnosis date, best-corrected visual acuity, cataract extraction, other ocular abnormalities, and cataract type and etiology.
The co-primary vision endpoint was incident cataract, defined as a confirmed lens opacity, diagnosed after randomization, but before June 1, 2011, age-related in etiology, and responsible for reduced best-corrected visual acuity to 20/30 or worse. Cataract extraction was a pre-specified secondary endpoint and was defined as the surgical removal of an incident cataract. A total of 11,497 participants did not report cataract at baseline and are included in this analysis. Of these, 5,736 men were in the multivitamin group and 5,761 in the placebo group (Figure 1).
Age-related Macular Degeneration
The AMD questionnaire requested information about diagnosis date, best-corrected visual acuity at diagnosis, and date when best-corrected visual acuity reached 20/30 or worse. Information was also requested about signs of AMD observed (drusen, retinal pigment epithelium [RPE] hypo/hyperpigmentation, geographic atrophy, RPE detachment, subretinal neovascular membrane, or disciform scar) when visual acuity was first noted to be 20/30 or worse, and the date exudative neovascular disease (defined by presence of RPE detachment, subretinal neovascular membrane, or disciform scar) was first noted. The questionnaire also asked whether there were other ocular abnormalities and, if so, whether the AMD, by itself, was significant enough to reduce best-corrected visual acuity to 20/30 or worse.
The co-primary vision endpoint was visually-significant AMD defined as confirmed AMD diagnosed after randomization, but before June 1, 2011, and best-corrected visual acuity loss to 20/30 or worse attributable to AMD. Two secondary endpoints were total AMD, comprised of all incident cases with or without vision loss, and advanced AMD, comprised of cases of exudative neovascular AMD plus geographic atrophy.
A total of 14,233 participants did not report AMD at baseline and are included in this analysis. Of these, 7,111 men were in the multivitamin group and 7,122 in the placebo group (Figure 1).
Statistical Analysis
In separate analyses of cataract and AMD, participants were classified according to randomized multivitamin treatment assignment and followed until the occurrence of that endpoint, death, or the end of the multivitamin component of PHS II, whichever came first. Estimated power for the primary study endpoints of incident cataract and visually-significant AMD was based on event rates observed in PHS I, and was estimated to be greater than 80% to detect a 10% reduction in cataract, and a 20% reduction in visually-significant AMD.
The distributions of baseline characteristics in the multivitamin and placebo groups were compared using 2-sample t-tests, chi-square tests for proportions, and tests for trend for ordinal categories. Kaplan-Meier curves estimated cumulative incidence over time by randomized group, and were compared using a crude log-rank test. Cox proportional-hazards models were used to estimate the hazard ratio (HR) of cataract and AMD among those in the multivitamin group compared to placebo after adjustment for age (years) at baseline, PHS cohort (original PHS I participant, new PHS II participant), and randomized beta carotene, vitamin E and vitamin C assignments. Models were also fit separately within three baseline age groups: 50–59, 60–69, ≥70 years. Tests of trend for the effect of age on the association between multivitamins and cataract or AMD were calculated by including a term for the interaction of multivitamins and age (with values 1 to 3 corresponding to the three age groups) in a proportional hazards model. The proportionality assumption was not violated for any cataract (diagnosis, p=0.99; extraction, p=0.75) or AMD endpoint (visually-significant AMD, p=0.40; total AMD, p=0.86; advanced AMD, p=0.98). Ninety-five percent confidence intervals (CI) and two-sided P value were calculated.
We analyzed subgroup data by categories of baseline variables, and by the other randomized treatment assignments. We explored possible effect modification by using interaction terms between subgroup indicators and multivitamin assignment.
We also considered the possibility that the apparent effect of multivitamins on one endpoint (e.g., cataract) reflected, at least in part, the effect of the intervention on the second endpoint (e.g., AMD). To address this, two separate proportional hazards models were fitted to estimate the effect of the intervention on one endpoint while adjusting for a diagnosis of the other as a time-varying covariate. Models were also fitted to estimate the effect of the intervention on one endpoint prior to, and after, diagnosis of the second endpoint.
Individuals were considered the unit of analysis because eyes were not examined independently, and were classified according to the status of the worse eye as defined by disease severity.36,37
Results
As expected in this large randomized trial, baseline characteristics had comparable distributions between the multivitamin and placebo groups (Table 1).
Table 1.
Baseline Characteristics by Multivitamin Randomized Treatment Assignment in Physicians’ Health Study II.a
| Cataract | Visually-significant AMD | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| No. Of Participantsb | Multivitamin (n=5,736) | Placebo (n=5,761) | No. Of Participants | Multivitamin (n=7,111) | Placebo (n=7,122) | |
| Age (mean [SD], y) | 61.9 (7.9) | 62.0 (7.9) | 63.9 (8.9) | 64.0 (9.0) | ||
| Age, y | ||||||
| 50–59 years | 5,6 06 | 49.1 | 48.5 | 5,865 | 41.2 | 41.2 |
| 60–69 years | 3,914 | 33.8 | 34.3 | 4,647 | 32.8 | 32.5 |
| ≥70 years | 1,977 | 17.2 | 17.2 | 3,721 | 26.0 | 26.3 |
| Cigarette smoking | ||||||
| Never | 6,824 | 60.0 | 58.8 | 8,072 | 57.1 | 56.4 |
| Former | 4,264 | 36.7 | 37.6 | 5,642 | 39.4 | 39.9 |
| Current | 400 | 3.4 | 3.6 | 507 | 3.5 | 3.6 |
| Alcohol use | ||||||
| Rarely/never | 2,108 | 18.9 | 18.0 | 2,650 | 19.2 | 18.3 |
| ≥1 drink/month | 9,317 | 81.1 | 82.0 | 11,492 | 80.8 | 81.7 |
| Body mass index (kg/m2) | ||||||
| <25 | 4,717 | 41.3 | 40.8 | 5,929 | 41.8 | 41.5 |
| 25 to <30 | 5,551 | 47.8 | 48.7 | 6,817 | 47.8 | 48.0 |
| ≥30 | 1,228 | 10.9 | 10.5 | 1,484 | 10.4 | 10.5 |
| History of hypertensionc | ||||||
| Yes | 4,426 | 38.1 | 39.4 | 5,896 | 41.0 | 42.3 |
| No | 6,998 | 61.9 | 60.6 | 8,258 | 59.0 | 57.7 |
| History of high cholesterold | ||||||
| Yes | 3,979 | 35.5 | 36.5 | 5,048 | 36.1 | 37.3 |
| No | 7,076 | 64.5 | 63.5 | 8,706 | 63.9 | 62.7 |
| History of diabetes | ||||||
| Yes | 547 | 4.8 | 4.8 | 853 | 6.3 | 5.7 |
| No | 10,940 | 95.2 | 95.2 | 13,365 | 93.7 | 94.3 |
| Aspirin use | ||||||
| Yes | 8,742 | 77.7 | 76.6 | 10,868 | 77.7 | 77.2 |
| No | 2,589 | 22.3 | 23.4 | 3,158 | 22.3 | 22.8 |
| Exercise ≥1 time/wk | ||||||
| Yes | 7,023 | 63.3 | 61.9 | 8,565 | 62.5 | 60.9 |
| No | 4,194 | 36.7 | 38.1 | 5,319 | 37.5 | 39.1 |
| Self-reported history of CVDe | ||||||
| Yes | 454 | 3.7 | 4.2 | 717 | 5.0 | 5.0 |
| No | 11,043 | 96.3 | 95.8 | 13,516 | 95.0 | 95.0 |
Abbreviations: AMD, age-related macular degeneration; SD, standard deviation; CVD, cardiovascular disease.
Data are given as percentage of participants unless otherwise noted
For some variables, numbers do not total 11,497 for cataract and 14,233 for AMD because of missing data for that variable.
History of hypertension was defined as self-reported systolic blood pressure of at least 140 mmHg, diastolic blood pressure of at least 90 mmHg, or past or current treatment for hypertension.
History of high cholesterol was defined as self-reported total cholesterol level of at least 240 mg/dL or past or current treatment for high cholesterol.
History of CVD included nonfatal myocardial infarction or nonfatal stroke.
During a mean follow-up of 11.2 years (median [interquartile range], 11.2 [10.7–13.3] years; maximum, 13.8 years), 1,817 cataracts and 1,337 cataract extractions were confirmed. We also confirmed 538 cases of AMD, including 281 cases of visually-significant AMD and 144 cases of advanced AMD.
Cataract
Overall, there was a significant 9% lower risk of cataract in the multivitamin group compared to placebo (872 versus 945 cases; HR, 0.91; 95% CI, 0.83–0.99; p=0.04) (Table 2). For subtypes, there was a significant 13% reduced risk of nuclear sclerosis (NS) in the multivitamin group (800 versus 900 cases; HR, 0.87; 95% CI, 0.79–0.96; p=0.005). There was a non-significant reduction in cortical cataract (356 versus 387 cases; HR, 0.90; 95% CI, 0.78–1.04; p=0.17). There were similar numbers of posterior subcapsular (PSC) cataracts in the multivitamin and placebo groups (247 versus 248 cases; HR, 0.98; 95% CI, 0.82–1.17; p=0.85). The findings were similar for extraction of cataract and subtypes.
Table 2.
Confirmed Cases of Age-related Cataract and Cataract Extraction According to Multivitamin Randomized Treatment Assignment in Physicians’ Health Study II.
| Cataract | Cataract Extraction | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Active (n=5,736) | Placebo (n=5,761) | HRa | 95% CI | P Value | P Trendb | Active (n=5,736) | Placebo (n=5,761) | HRa | 95% CI | P | P Trendb | |
| Total Cataract | ||||||||||||
| 50–59 years | 203 | 209 | 0.97 | 0.80–1.17 | 0.74 | 0.29 | 151 | 145 | 1.04 | 0.83–1.31 | 0.73 | 0.18 |
| 60–69 years | 407 | 436 | 0.94 | 0.82–1.07 | 0.33 | 291 | 329 | 0.88 | 0.75–1.03 | 0.12 | ||
| ≥70 years | 262 | 300 | 0.85 | 0.72–1.00 | 0.06 | 194 | 227 | 0.84 | 0.69–1.02 | 0.08 | ||
| Subtotal | 872 | 945 | 0.91 | 0.83–0.99 | 0.04 | 636 | 701 | 0.89 | 0.80–0.99 | 0.04 | ||
| Any NS | ||||||||||||
| 50–59 years | 175 | 187 | 0.93 | 0.76–1.15 | 0.51 | 0.46 | 132 | 129 | 1.02 | 0.80–1.30 | 0.85 | 0.29 |
| 60–69 years | 374 | 425 | 0.88 | 0.77–1.01 | 0.08 | 272 | 319 | 0.85 | 0.72–1.00 | 0.05 | ||
| ≥70 years | 251 | 288 | 0.85 | 0.72–1.01 | 0.06 | 186 | 215 | 0.85 | 0.70–1.04 | 0.11 | ||
| Subtotal | 800 | 900 | 0.87 | 0.79–0.96 | 0.005 | 590 | 663 | 0.87 | 0.78–0.98 | 0.018 | ||
| Any Cortical | ||||||||||||
| 50–59 years | 75 | 79 | 0.94 | 0.69–1.30 | 0.72 | 0.27 | 56 | 54 | 1.03 | 0.71–1.50 | 0.86 | 0.11 |
| 60–69 years | 169 | 169 | 1.00 | 0.81–1.24 | 0.98 | 123 | 130 | 0.95 | 0.74–1.21 | 0.66 | ||
| ≥70 years | 112 | 139 | 0.79 | 0.61–1.01 | 0.06 | 78 | 107 | 0.72 | 0.54–0.96 | 0.027 | ||
| Subtotal | 356 | 387 | 0.90 | 0.78–1.04 | 0.17 | 257 | 291 | 0.87 | 0.73–1.03 | 0.10 | ||
| Any PSC | ||||||||||||
| 50–59 years | 84 | 69 | 1.21 | 0.88–1.66 | 0.24 | 0.18 | 75 | 55 | 1.36 | 0.96–1.92 | 0.08 | 0.13 |
| 60–69 years | 102 | 112 | 0.91 | 0.70–1.20 | 0.51 | 85 | 96 | 0.89 | 0.66–1.19 | 0.42 | ||
| ≥70 years | 61 | 67 | 0.89 | 0.63–1.26 | 0.50 | 53 | 56 | 0.93 | 0.64–1.35 | 0.69 | ||
| Subtotal | 247 | 248 | 0.98 | 0.82–1.17 | 0.85 | 213 | 207 | 1.02 | 0.84–1.23 | 0.86 | ||
Abbreviations: HR, hazard ratio; CI, confidence interval; NS, nuclear sclerosis; PSC, posterior subcapsular.
Adjusted for age, Physicians’ Health Study cohort, and vitamin C, vitamin E, and beta-carotene treatment assignment.
Test for trend of the effect of age on the association between randomized treatment assignment and cataract.
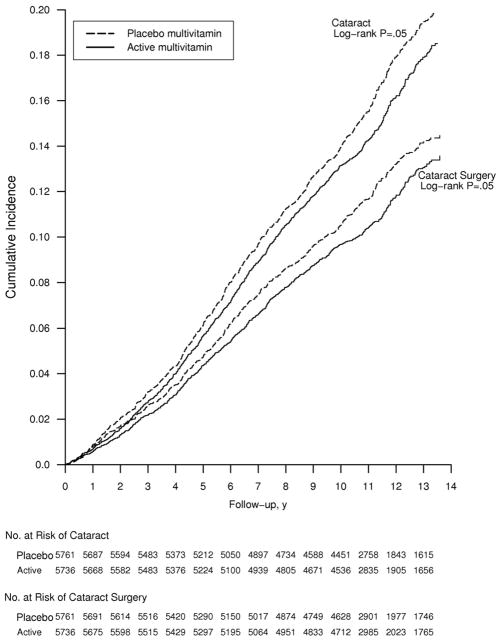
The benefits of daily multivitamin use were greater in older men, although no test of trend attained statistical significance for either cataract diagnosis or extraction (Table 2). For both endpoints, a beneficial effect of multivitamins began to emerge midway through follow-up and persisted throughout the remainder of the trial (crude log-rank P=0.05 for both endpoints) (Figure 2).
Figure 2.
Cumulative incidence rates of cataract in the multivitamin and placebo groups in the Physicians’ Health Study II.
The effect of multivitamins on cataract did not differ markedly within categories of baseline characteristics (Table 3). However, the effect did appear to vary according to vitamin C treatment assignment (p interaction=0.04) with a significant benefit observed only among men in the vitamin C placebo group.
Table 3.
Relative Rates of Cataract and Visually-Significant Age-related Macular Degeneration by Randomized Treatment Assignment Within Subgroups in Physicians’ Health Study II.a
| Cataract | Age-related Macular Degeneration | |||||||
|---|---|---|---|---|---|---|---|---|
| No.of Cataract/Total | No.of AMD/Total | |||||||
| Active | Placebo | HR (95% CI)b | P Interactionc | Active | Placebo | HR (95% CI)b | P Interactionc | |
| Cigarette smoking | ||||||||
| Never | 477/3437 | 504/3387 | 0.93 (0.82–1.05) | 0.45 | 64/4055 | 57/4017 | 1.08 (0.76–1.55) | 0.75 |
| Former | 366/2100 | 401/2164 | 0.91 (0.79–1.05) | 82/2799 | 67/2843 | 1.30 (0.94–1.79) | ||
| Current | 29/192 | 40/208 | 0.70 (0.43–1.13) | 6/249 | 5/258 | 1.16 (0.35–3.83) | ||
| Alcohol use | ||||||||
| Rarely/never | 180/1079 | 164/1029 | 0.99 (0.80–1.23) | 0.43 | 37/1356 | 20/1294 | 1.62 (0.94–2.80) | 0.17 |
| ≥1 drink/month | 687/4618 | 776/4699 | 0.89 (0.80–0.99) | 115/5705 | 107/5787 | 1.11 (0.85–1.44) | ||
| Body mass index (kg/m2) | ||||||||
| <25 | 374/2369 | 411/2348 | 0.89 (0.77–1.02) | 0.71 | 66/2976 | 59/2953 | 1.11 (0.78–1.58) | 0.87 |
| 25 to <30 | 399/2744 | 443/2807 | 0.90 (0.79–1.03) | 73/3396 | 60/3421 | 1.24 (0.88–1.75) | ||
| ≥30 | 99/623 | 91/605 | 1.02 (0.77–1.35) | 13/739 | 10/745 | 1.27 (0.56–2.91) | ||
| History of hypertension | ||||||||
| Yes | 399/2167 | 434/2259 | 0.94 (0.82–1.07) | 0.63 | 75/2899 | 58/2997 | 1.33 (0.94–1.87) | 0.35 |
| No | 470/3525 | 506/3473 | 0.89 (0.79–1.01) | 77/4163 | 71/4095 | 1.07 (0.78–1.48) | ||
| History of high cholesterol | ||||||||
| Yes | 349/1958 | 361/2021 | 0.98 (0.84–1.13) | 0.19 | 51/2485 | 45/2563 | 1.17 (0.78–1.75) | 0.99 |
| No | 508/3560 | 572/3516 | 0.86 (0.76–0.97) | 98/4395 | 83/4311 | 1.16 (0.87–1.56) | ||
| History of diabetes | ||||||||
| Yes | 56/273 | 55/274 | 0.98 (0.68–1.43) | 0.41 | 8/446 | 9/407 | 0.85 (0.33–2.22) | 0.56 |
| No | 816/5458 | 890/5482 | 0.90 (0.82–0.99) | 144/6658 | 120/6707 | 1.21 (0.95–1.54) | ||
| Exercise ≥1 time/wk | ||||||||
| Yes | 503/3544 | 543/3479 | 0.91 (0.80–1.02) | 0.98 | 83/4340 | 85/4225 | 0.97 (0.72–1.31) | 0.055 |
| No | 361/2055 | 394/2139 | 0.91 (0.79–1.05) | 67/2604 | 44/2715 | 1.56 (1.06–2.28) | ||
| Self-reported history of CVD | ||||||||
| Yes | 40/212 | 45/242 | 0.98 (0.64–1.50) | 0.91 | 10/358 | 10/359 | 0.93 (0.39–2.24) | 0.59 |
| No | 832/5524 | 900/5519 | 0.91 (0.82–1.00) | 142/6753 | 119/6763 | 1.21 (0.95–1.54) | ||
| Randomized to vitamin C | ||||||||
| Yes | 455/2889 | 452/2879 | 1.00 (0.88–1.14) | 0.04 | 75/3574 | 65/3575 | 1.15 (0.82–1.60) | 0.79 |
| No | 417/2847 | 493/2882 | 0.82 (0.72–0.94) | 77/3537 | 64/3547 | 1.22 (0.88–1.71) | ||
| Randomized to vitamin E | ||||||||
| Yes | 437/2869 | 479/2871 | 0.89 (0.78–1.01) | 0.67 | 75/3537 | 69/3574 | 1.12 (0.81–1.55) | 0.61 |
| No | 435/2867 | 466/2890 | 0.93 (0.81–1.06) | 77/3574 | 60/3548 | 1.26 (0.90–1.77) | ||
| Randomized to beta-carotene | ||||||||
| Yes | 421/2885 | 439/2872 | 0.94 (0.82–1.07) | 0.53 | 71/3591 | 60/3580 | 1.20 (0.85–1.69) | 0.92 |
| No | 451/2851 | 506/2889 | 0.88 (0.78–1.00) | 81/3520 | 69/3542 | 1.17 (0.85–1.62) | ||
| Aspirin use | ||||||||
| Yes | 708/4403 | 759/4339 | 0.89 (0.80–0.99) | 0.50 | 121/5460 | 100/5408 | 1.22 (0.93–1.58) | 0.61 |
| No | 162/1265 | 183/1324 | 0.97 (0.78–1.20) | 30/1564 | 29/1594 | 1.03 (0.62–1.72) | ||
Abbreviations: HR, relative risk; CI, confidence interval; CVD cardiovascular disease; AMD, age-related macular degeneration.
Baseline factors are defined as in Table 1.
Adjusted for age, Physicians’ Health Study cohort, and vitamin C, vitamin E, and beta-carotene treatment assignment.
Test of the null hypothesis of no difference in treatment effect across risk factor subgroups.
Age-related Macular Degeneration
Men in the multivitamin group had a 19% increased risk of visually-significant AMD that was not statistically significant (152 versus 129 cases; HR, 1.19; 95% CI, 0.94 to 1.50; p=0.15) (Table 4). There was also a significant 22% increased risk of total AMD (with or without vision loss) (294 versus 244 cases; HR, 1.22; 95% CI, 1.03 to 1.44; p=0.02), and a non-significant 22% increased risk of advanced AMD (79 versus 65 cases; HR, 1.22; 95% CI, 0.88 to 1.70; p=0.23) in the multivitamin group.
Table 4.
Confirmed Cases of Age-related Macular Degeneration According to Multivitamin Randomized Treatment Assignment in Physicians’ Health Study II.
| Active (n=7,111) | Placebo (n=7,122) | HRa | 95% CI | P | P-Trendb | |
|---|---|---|---|---|---|---|
| Visually-Significant AMD | ||||||
| 50–59 years | 6 | 8 | 0.76 | 0.26–2.18 | 0.61 | 0.43 |
| 60–69 years | 41 | 36 | 1.14 | 0.73–1.78 | 0.58 | |
| ≥70 years | 105 | 85 | 1.24 | 0.93–1.65 | 0.14 | |
| Total | 152 | 129 | 1.19 | 0.94–1.50 | 0.15 | |
| Total AMD | ||||||
| 50–59 years | 28 | 29 | 0.98 | 0.58–1.64 | 0.93 | 0.13 |
| 60–69 years | 97 | 90 | 1.07 | 0.80–1.43 | 0.64 | |
| ≥70 years | 169 | 125 | 1.38 | 1.09–1.73 | 0.007 | |
| Total | 294 | 244 | 1.22 | 1.03–1.44 | 0.02 | |
| Advanced AMD | ||||||
| 50–59 years | 3 | 7 | 0.43 | 0.11–1.67 | 0.22 | 0.14 |
| 60–69 years | 21 | 18 | 1.15 | 0.61–2.17 | 0.66 | |
| ≥70 years | 55 | 40 | 1.38 | 0.92–2.08 | 0.12 | |
| Total | 79 | 65 | 1.22 | 0.88–1.70 | 0.23 | |
Abbreviations HR, hazard ratio; CI, confidence interval; AMD, age-related macular degeneration.
Adjusted for age, Physicians’ Health Study cohort, and vitamin C, vitamin E, and beta-carotene treatment assignment.
Test for trend of the effect of age on the association between randomized treatment assignment and AMD.
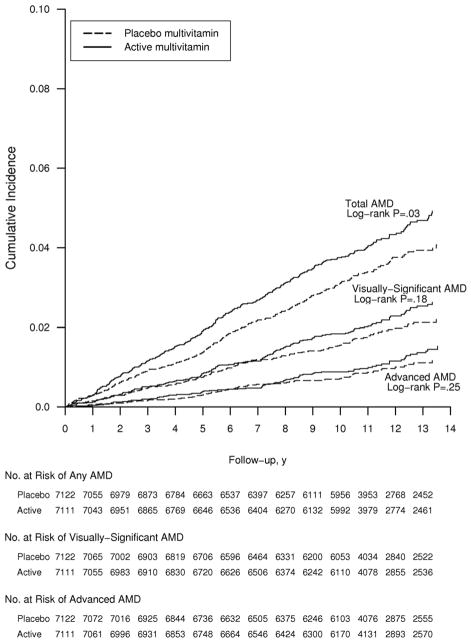
Hazard ratios for all AMD endpoints tended to be highest in the oldest age group, although no test of trend attained statistical significance. For the primary endpoint of visually-significant AMD, the curves appeared to diverge midway through follow-up but never attained statistical significance (crude log-rank P=0.18) (Figure 3). Curves for total AMD began to diverge earlier in the trial, and persisted throughout the trial (log-rank P=0.03). For advanced AMD, there was no apparent effect of a daily multivitamin at any point during the trial (crude log-rank P=0.25).
Figure 3.
Cumulative incidence rates of age-related macular degeneration in the multivitamin and placebo groups in the Physicians’ Health Study II. Abbreviation: AMD, age-related macular degeneration.
The effect of multivitamins on visually-significant AMD did not differ appreciably within categories of baseline characteristics (Table 3).
For both cataract and AMD, HR estimates for multivitamin treatment were not materially altered in analyses that accounted for diagnosis of the second endpoint. For example, the HR for cataract changed little (0.90; 95% CI, 0.82–0.99; P=0.03) after adjustment for a diagnosis of visually-significant AMD as a time-varying covariate. Similarly, the HR for visually-significant AMD changed little (1.18; 95% CI, 0.94–1.50; P=0.16) after adjustment for diagnosed cataract. We also compared HRs prior to and after diagnosis of the second endpoint. The HR for cataract was 0.92 (95% CI, 0.84–1.01) with no prior diagnosis of visually-significant AMD, and 0.69 (95% CI, 0.41–1.17) after a diagnosis of visually-significant AMD. For visually-significant AMD, the HR was 1.31 (95% CI, 0.92–1.87) with no prior diagnosis of cataract, and 1.08 (95% CI, 0.79–1.48) after a diagnosis of cataract.
Discussion
In this large-scale randomized trial of middle-aged and older men, long-term daily multivitamin use modestly and significantly reduced the co-primary vision endpoint of cataract after more than ten years of treatment and follow-up. There was no significant benefit or risk of daily multivitamin use on visually-significant AMD, the second co-primary vision endpoint, although the HRs tended to be modestly elevated.
Our findings for cataract are consistent with results of two prior trials of multivitamin use in cataract prevention. In the Linxian Cataract Study, conducted in a nutritionally-deficient population in China, persons aged 65 to 74 years randomized to a daily supplement of 14 vitamins and 12 minerals at 2 to 3 times the United States (U.S) recommended dietary allowance (RDA), compared to placebo, had a significant 36% lower prevalence of nuclear cataract after 6 years of treatment.22 There was no difference in the prevalence of cortical or PSC cataract in those aged 65 to 74 years, nor was there any difference in the prevalence of any cataract type in those aged 54–64 years. In the Italian American Clinical Trial of Nutritional Supplements and Age-related Cataract (CTNS), persons aged 55–75 years randomized to a daily multivitamin (Centrum), compared to placebo, had a significant 18% reduction in cataract development or progression after 9-years of treatment and follow-up.23 Analyses of subtypes indicated a significant 34% reduction in nuclear (HR, 0.66; 95% CI, 0.50–0.88), a non-significant 22% reduction in cortical (HR, 0.78; 95% CI, 0.60–1.02), and a significant two-fold increased risk of PSC cataract (HR, 2.00; 95% CI, 1.35–2.98) in the multivitamin group. Neither the Linxian nor the CTNS study examined the effect of the intervention on AMD.
Taken together, our findings in PHS II and two prior trials indicate that long-term daily multivitamin use may have a small to moderate beneficial effect on risk of cataract, and particularly nuclear cataract. Given that an estimated 10 million adults in the U.S. have impaired vision due to cataract,38,39 even a modest reduction in risk of cataract would have a large public health impact. It should also be noted that while the main trial results in AREDS indicated no benefit on lens opacity progression for daily treatment with high dose vitamin E (400 IU), vitamin C (500 mg), and beta carotene (15 mg), a propensity score analysis showed that self-selection for Centrum use (provided by AREDS) by approximately two-thirds of AREDS participants was associated with a significant reduction in lens opacity progression, particularly for nuclear opacities.40
PHS II is the first large-scale randomized trial to test a multivitamin supplement in AMD prevention. Our finding of no significant benefit appears to contrast with the benefits for other vitamin/mineral combinations in prior trials of AMD. For example, AREDS demonstrated that daily zinc (80 mg) and a high-dose antioxidant combination of vitamin E (400 IU), vitamin C (500 mg), and beta carotene (15 mg) significantly slowed the progression of AMD.24 The multivitamin in PHS II included these nutrients at RDA levels (zinc [15 mg], vitamin E [45 IU], vitamin C [60 mg], beta-carotene [5000 IU vitamin A, 20% as beta carotene]), rather than the high-dose formulation tested in AREDS. In addition, AREDS tested a higher-risk population than PHS II (no reported diagnosis of AMD at baseline), and their primary endpoint was advanced AMD whereas in PHS II, the primary endpoint was visually-significant AMD, most commonly characterized by some combination of drusen and RPE changes. Thus, our endpoint represented an earlier stage of disease development than the advanced AMD endpoint in AREDS. It is worth noting that AREDS reported no benefit of the zinc and antioxidant combination in participants with early stage AMD at baseline.24
Our findings also appear to contrast with the findings in the Women’s Antioxidant and Folic Acid Cardiovascular Study (WAFACS), where combined treatment with folic acid, vitamin B6, and vitamin B12 for 7.3 years reduced the risk of a diagnosis of AMD by 35% to 40%.25 Both WAFACS and PHS II employed the same method of case ascertainment and the same diagnostic criteria for AMD, but differed in other important respects. WAFACS tested pharmacologic doses of folic acid (2.5 mg/d), vitamin B6 (50 mg/d), and vitamin B12 (1 mg/d), whereas the multivitamin in PHS II contained RDA levels of these nutrients (folic acid [400 μg], vitamin B6 [3 mg], vitamin B12 [25 μg]). In addition, WAFACS was comprised of women with preexisting CVD or 3 or more CVD risk factors, whereas PHS II was comprised primarily of apparently healthy men. It therefore seems possible that our finding of no benefit (and possible harm) may reflect, at least in part, the lower dosage of the PHS II multivitamin plus the generally lower risk profile of the PHS II population.
Several possible limitations of our study need to be considered. At the initiation of PHS II in 1997, we selected a commonly used multivitamin, Centrum Silver, to increase the potential generalizability of study findings. The same formulation was used throughout PHS II. However, after the inception of PHS II, the doses of several nutrients in Centrum Silver were changed and several nutrients were added including lutein (250 μg). Lutein has been shown to be of possible benefit against cataract and AMD in observational studies,41–49 and in several small randomized trials,50–55 although the recently completed Age-Related Eye Disease Study 2 reported no overall benefit on either endpoint.56,57 However, the effect of adding lutein to the Centrum Silver formulation could not be addressed in our study. Compliance with study medication is a concern in any long-term randomized trial; however, compliance with the daily multivitamin remained high throughout follow-up. Finally, the PHS II population is comprised of generally well-nourished male physicians and our findings may not apply to women or to less well-nourished populations.
Several aspects of our methodology also deserve consideration. Case identification was based on participant reports, and thus some underascertainment of cataract and AMD is plausible. Such underascertainment would likely reduce study power, but is not associated with bias in randomized comparisons. Random misclassification was reduced by the use of medical records to confirm the participant reports. Non-random misclassification was unlikely since medical records were reviewed by an investigator (WGC) masked to treatment assignment, and study participants and treating ophthalmologists and optometrists were similarly unaware of treatment assignment. Finally, the equal distribution of baseline characteristics between the multivitamin and placebo groups indicates that confounding by measured factors is unlikely, and that other potential confounders, which were either unmeasured or unknown, were also likely to be evenly distributed between the two treatment groups.
In summary, the finding in this large-scale randomized trial of middle-aged and older men that long-term daily multivitamin use is associated with a modest but significant reduction in cataract, and in particular nuclear cataract, is consistent with results of previous trials of multivitamin use in cataract prevention. The finding of no significant benefit or harm for multivitamin use in prevention of visually-significant AMD needs to be confirmed in other populations of men and women.
Acknowledgments
Supported by grants CA 097193 (which included funding from the National Eye Institute and the National Institute on Aging), CA 34944, CA 40360, HL 26490, and HL 34595 from the National Institutes of Health (Bethesda, MD), and an investigator-initiated grant from BASF Corporation (Florham Park, NJ). Study agents and packaging were provided by BASF Corporation and Pfizer (formerly Wyeth, American Home Products, and Lederle) (New York, NY), and study packaging was provided by DSM Nutritional Products, Inc. (formerly Roche Vitamins) (Parsippany, NJ).
Role of the Sponsors: NIH, BASF, Pfizer, and DSM Nutritional Products Inc had no role in the study design; conduct of the study; collection, management, analysis, and interpretation of the data; or preparation, review, or approval of the manuscript.
Footnotes
Dr. Christen had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analyses.
Relevant Financial Disclosures:
Dr. Christen reported receiving research funding support from the National Institutes of Health and DSM Nutritional Products Inc. (formerly Roche Vitamins).
Dr. Glynn reported receiving investigator-initiated research funding from the National Institutes of Health, Bristol-Meyers Squibb, AstraZeneca, and Novartis, and signed a consulting agreement with Merck to give an invited talk.
Dr. Manson reported receiving investigator-initiated research funding from the National Institutes of Health, and assistance with study pills and packaging from BASF and Cognis Corporations for the Women’s Antioxidant and Folic Acid Cardiovascular Study and from Pronova BioPharma and Pharmavite for the VITamin D and OmegA-3 TriaL, and funding from the non-profit Aurora Foundation.
Dr. Buring reported receiving investigator-initiated research funding from the National Institutes of Health, and assistance with study pills and packaging from Natural Source Vitamin E Association and Bayer Healthcare for the Women’s Health Study.
Dr. Sesso reported receiving investigator-initiated research funding from the National Institutes of Health, the Tomato Products Wellness Council, and Cambridge Theranostics, Ltd.
Dr. Gaziano reported receiving investigator-initiated research funding from the National Institutes of Health the Veterans Administration, and the BASF Corporation, assistance with study agents and packaging from BASF Corporation and Pfizer (formerly Wyeth, American Home Products, and Lederle), and assistance with study packaging provided by DSM Nutritional Products, Inc. (formerly Roche Vitamins).
No other authors reported financial disclosures.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Congdon NG, West KP., Jr Nutrition and the eye. Curr Opin Ophthalmol. 1999;10:464–73. doi: 10.1097/00055735-199912000-00016. [DOI] [PubMed] [Google Scholar]
- 2.Chiu CJ, Taylor A. Nutritional antioxidants and age-related cataract and maculopathy. Exp Eye Res. 2007;84:229–45. doi: 10.1016/j.exer.2006.05.015. [DOI] [PubMed] [Google Scholar]
- 3.Agte V, Tarwadi K. The importance of nutrition in the prevention of ocular disease with special reference to cataract. Ophthalmic Res. 2010;44:166–72. doi: 10.1159/000316477. AQ: duplicated with 10. [DOI] [PubMed] [Google Scholar]
- 4.Fletcher AE. Free radicals, antioxidants and eye diseases: evidence from epidemiological studies on cataract and age-related macular degeneration. Ophthalmic Res. 2010;44:191–8. doi: 10.1159/000316476. [DOI] [PubMed] [Google Scholar]
- 5.Krishnadev N, Meleth AD, Chew EY. Nutritional supplements for age-related macular degeneration. Curr Opin Ophthalmol. 2010;21:184–9. doi: 10.1097/ICU.0b013e32833866ee. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Seddon JM. Multivitamin-multimineral supplements and eye disease: age-related macular degeneration and cataract. Am J Clin Nutr. 2007;85:304S–7S. doi: 10.1093/ajcn/85.1.304S. [DOI] [PubMed] [Google Scholar]
- 7.Cui YH, Jing CX, Pan HW. Association of blood antioxidants and vitamins with risk of age-related cataract: a meta-analysis of observational studies. Am J Clin Nutr. 2013;98:778–86. doi: 10.3945/ajcn.112.053835. [DOI] [PubMed] [Google Scholar]
- 8.Gerster H. Antioxidant vitamins in cataract prevention. Z Ernahrungswiss. 1989;28:56–75. doi: 10.1007/BF02025566. [DOI] [PubMed] [Google Scholar]
- 9.Taylor A. Role of nutrients in delaying cataracts. Ann N Y Acad Sci. 1992;669:111–23. doi: 10.1111/j.1749-6632.1992.tb17093.x. discussion 123–4. [DOI] [PubMed] [Google Scholar]
- 10.Organisciak DT, Wang HM, Li ZY, Tso MO. The protective effect of ascorbate in retinal light damage of rats. Invest Ophthalmol Vis Sci. 1985;26:1580–8. [PubMed] [Google Scholar]
- 11.Ham WT, Jr, Mueller HA, Ruffolo JJ, Jr, et al. Basic mechanisms underlying the production of photochemical lesions in the mammalian retina. Curr Eye Res. 1984;3:165–74. doi: 10.3109/02713688408997198. [DOI] [PubMed] [Google Scholar]
- 12.Tso MO. Experiments on visual cells by nature and man: in search of treatment for photoreceptor degeneration. Friedenwald lecture. Invest Ophthalmol Vis Sci. 1989;30:2430–54. [PubMed] [Google Scholar]
- 13.Teikari JM, Rautalahti M, Haukka J, et al. Incidence of cataract operations in Finnish male smokers unaffected by alpha tocopherol or beta carotene supplements. J Epidemiol Community Health. 1998;52:468–72. doi: 10.1136/jech.52.7.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Age-Related Eye Disease Study Research Group. A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E and beta carotene for age-related cataract and vision loss: AREDS report no. 9. Arch Ophthalmol. 2001;119:1439–52. doi: 10.1001/archopht.119.10.1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chylack LT, Jr, Brown NP, Bron A, et al. REACT Group. The Roche European American Cataract Trial (REACT): a randomized clinical trial to investigate the efficacy of an oral antioxidant micronutrient mixture to slow progression of age-related cataract. Ophthalmic Epidemiol. 2002;9:49–80. doi: 10.1076/opep.9.1.49.1717. [DOI] [PubMed] [Google Scholar]
- 16.McNeil JJ, Robman L, Tikellis G, et al. Vitamin E supplementation and cataract: randomized controlled trial. Ophthalmology. 2004;111:75–84. doi: 10.1016/j.ophtha.2003.04.009. [DOI] [PubMed] [Google Scholar]
- 17.Christen WG, Glynn RJ, Chew EY, Buring JE. Vitamin E and age-related cataract in a randomized trial of women. Ophthalmology. 2008;115:822–9. doi: 10.1016/j.ophtha.2007.06.040. [DOI] [PubMed] [Google Scholar]
- 18.Christen WG, Glynn RJ, Sesso HD, et al. Age-related cataract in a randomized trial of vitamins E and C in men. Arch Ophthalmol. 2010;128:1397–405. doi: 10.1001/archophthalmol.2010.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Christen W, Glynn R, Sperduto R, et al. Age-related cataract in a randomized trial of beta-carotene in women. Ophthalmic Epidemiol. 2004;11:401–12. doi: 10.1080/09286580490515152. [DOI] [PubMed] [Google Scholar]
- 20.Christen WG, Manson JE, Glynn RJ, et al. A randomized trial of beta carotene and age-related cataract in US physicians. Arch Ophthalmol. 2003;121:372–8. doi: 10.1001/archopht.121.3.372. [DOI] [PubMed] [Google Scholar]
- 21.Gritz DC, Srinivasan M, Smith SD, et al. The Antioxidants in Prevention of Cataracts Study: effects of antioxidant supplements on cataract progression in South India. Br J Ophthalmol. 2006;90:847–51. doi: 10.1136/bjo.2005.088104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sperduto RD, Hu TS, Milton RC, et al. The Linxian cataract studies. Two nutrition intervention trials. Arch Ophthalmol. 1993;111:1246–53. doi: 10.1001/archopht.1993.01090090098027. [DOI] [PubMed] [Google Scholar]
- 23.Clinical Trial of Nutritional Supplements and Age-Related Cataract Study Group. A randomized, double-masked, placebo-controlled clinical trial of multivitamin supplementation for age-related lens opacities: Clinical Trial of Nutritional Supplements and Age-Related Cataract report no. 3. Ophthalmology. 2008;115:599–607. doi: 10.1016/j.ophtha.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 24.Age-Related Eye Disease Study Research Group. A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss: AREDS report no. 8. Arch Ophthalmol. 2001;119:1417–36. doi: 10.1001/archopht.119.10.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Christen WG, Glynn RJ, Chew EY, et al. Folic acid, pyridoxine, and cyanocobalamin combination treatment and age-related macular degeneration in women: the Women’s Antioxidant and Folic Acid Cardiovascular Study. Arch Intern Med. 2009;169:335–41. doi: 10.1001/archinternmed.2008.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Teikari JM, Laatikainen L, Virtamo J, et al. Six-year supplementation with alpha-tocopherol and beta-carotene and age-related maculopathy. Acta Ophthalmol Scand. 1998;76:224–9. doi: 10.1034/j.1600-0420.1998.760220.x. [DOI] [PubMed] [Google Scholar]
- 27.Taylor HR, Tikellis G, Robman LD, et al. Vitamin E supplementation and macular degeneration: randomised controlled trial. BMJ. 2002;325:11. doi: 10.1136/bmj.325.7354.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Christen WG, Glynn RJ, Chew EY, Buring JE. Vitamin E and age-related macular degeneration in a randomized trial of women. Ophthalmology. 2010;117:1163–8. doi: 10.1016/j.ophtha.2009.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Christen WG, Manson JE, Glynn RJ, et al. Beta carotene supplementation and age-related maculopathy in a randomized trial of US physicians. Arch Ophthalmol. 2007;125:333–9. doi: 10.1001/archopht.125.3.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Christen WG, Glynn RJ, Sesso HD, et al. Vitamins E and C and medical record-confirmed age-related macular degeneration in a randomized trial of male physicians. Ophthalmology. 2012;119:1642–9. doi: 10.1016/j.ophtha.2012.01.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Christen WG, Gaziano JM, Hennekens CH. Design of Physicians’ Health Study II--a randomized trial of beta-carotene, vitamins E and C, and multivitamins, in prevention of cancer, cardiovascular disease, and eye disease, and review of results of completed trials. Ann Epidemiol. 2000;10:125–34. doi: 10.1016/s1047-2797(99)00042-3. [DOI] [PubMed] [Google Scholar]
- 32.Gaziano JM, Glynn RJ, Christen WG, et al. Vitamins E and C in the prevention of prostate and total cancer in men: the Physicians’ Health Study II randomized controlled trial. JAMA. 2009;301:52–62. doi: 10.1001/jama.2008.862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sesso HD, Buring JE, Christen WG, et al. Vitamins E and C in the prevention of cardiovascular disease in men: the Physicians’ Health Study II randomized controlled trial. JAMA. 2008;300:2123–33. doi: 10.1001/jama.2008.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hennekens CH, Buring JE, Manson JE, et al. Lack of effect of long-term supplementation with beta carotene on the incidence of malignant neoplasms and cardiovascular disease. N Engl J Med. 1996;334:1145–9. doi: 10.1056/NEJM199605023341801. [DOI] [PubMed] [Google Scholar]
- 35.Steering Committee of the Physicians’ Health Study Research Group. Final report on the aspirin component of the ongoing Physicians’ Health Study. N Engl J Med. 1989;321:129–35. doi: 10.1056/NEJM198907203210301. [DOI] [PubMed] [Google Scholar]
- 36.Ederer F. Shall we count numbers of eyes or numbers of subjects? Arch Ophthalmol. 1973;89:1–2. doi: 10.1001/archopht.1973.01000040003001. [DOI] [PubMed] [Google Scholar]
- 37.Glynn RJ, Rosner B. Accounting for the correlation between fellow eyes in regression analysis. Arch Ophthalmol. 1992;110:381–7. doi: 10.1001/archopht.1992.01080150079033. [DOI] [PubMed] [Google Scholar]
- 38.Eye Diseases Prevalence Research Group. Prevalence of cataract and pseudophakia/aphakia among adults in the United States. Arch Ophthalmol. 2004;122:487–94. doi: 10.1001/archopht.122.4.487. [DOI] [PubMed] [Google Scholar]
- 39.National Eye Institute. [Accessed September 15, 2013.];National Plan for Eye and Vision Research: Lens and Cataract Program. Available at: http://www.nei.nih.gov/strategicplanning/np_lens.asp.
- 40.Age-Related Eye Disease Study Research Group. Centrum use and progression of age-related cataract in the Age-Related Eye Disease Study: a propensity score approach. AREDS report no. 21. Ophthalmology. 2006;113:1264–70. doi: 10.1016/j.ophtha.2006.02.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chasan-Taber L, Willett WC, Seddon JM, et al. A prospective study of carotenoid and vitamin A intakes and risk of cataract extraction in US women. Am J Clin Nutr. 1999;70:509–16. doi: 10.1093/ajcn/70.4.509. [DOI] [PubMed] [Google Scholar]
- 42.Moeller SM, Voland R, Tinker L, et al. CAREDS Study Group. Associations between age-related nuclear cataract and lutein and zeaxanthin in the diet and serum in the Carotenoids in the Age-Related Eye Disease Study, an ancillary study of the Women’s Health Initiative. Arch Ophthalmol. 2008;126:354–64. doi: 10.1001/archopht.126.3.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mares-Perlman JA, Brady WE, Klein BE, et al. Serum carotenoids and tocopherols and severity of nuclear and cortical opacities. Invest Ophthalmol Vis Sci. 1995;36:276–88. [PubMed] [Google Scholar]
- 44.Brown L, Rimm EB, Seddon JM, et al. A prospective study of carotenoid intake and risk of cataract extraction in US men. Am J Clin Nutr. 1999;70:517–24. doi: 10.1093/ajcn/70.4.517. [DOI] [PubMed] [Google Scholar]
- 45.Vu HT, Robman L, Hodge A, et al. Lutein and zeaxanthin and the risk of cataract: the Melbourne visual impairment project. Invest Ophthalmol Vis Sci. 2006;47:3783–6. doi: 10.1167/iovs.05-0587. [DOI] [PubMed] [Google Scholar]
- 46.Lyle BJ, Mares-Perlman JA, Klein BE, et al. Antioxidant intake and risk of incident age-related nuclear cataracts in the Beaver Dam Eye Study. Am J Epidemiol. 1999;149:801–9. doi: 10.1093/oxfordjournals.aje.a009895. [DOI] [PubMed] [Google Scholar]
- 47.Christen WG, Liu S, Glynn RJ, et al. Dietary carotenoids, vitamins C and E, and risk of cataract in women: a prospective study. Arch Ophthalmol. 2008;126:102–9. doi: 10.1001/archopht.126.1.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bone RA, Landrum JT, Mayne ST, et al. Macular pigment in donor eyes with and without AMD: a case-control study. Invest Ophthalmol Vis Sci. 2001;42:235–40. [PubMed] [Google Scholar]
- 49.Moeller SM, Parekh N, Tinker L, et al. CAREDS Research Study Group. Associations between intermediate age-related macular degeneration and lutein and zeaxanthin in the Carotenoids in Age-related Eye Disease Study (CAREDS): ancillary study of the Women’s Health Initiative. Arch Ophthalmol. 2006;124:1151–62. doi: 10.1001/archopht.124.8.1151. [DOI] [PubMed] [Google Scholar]
- 50.Olmedilla B, Granado F, Blanco I, Vaquero M. Lutein, but not alpha-tocopherol, supplementation improves visual function in patients with age-related cataracts: a 2-y double-blind, placebo-controlled pilot study. Nutrition. 2003;19:21–4. doi: 10.1016/s0899-9007(02)00861-4. [DOI] [PubMed] [Google Scholar]
- 51.Arnold C, Winter L, Frohlich K, et al. Macular xanthophylls and omega-3 long-chain polyunsaturated fatty acids in age-related macular degeneration: a randomized trial. JAMA Ophthalmol. 2013;131:564–72. doi: 10.1001/jamaophthalmol.2013.2851. [DOI] [PubMed] [Google Scholar]
- 52.Piermarocchi S, Saviano S, Parisi V, et al. CARMIS Study Group. Carotenoids in Age-related Maculopathy Italian Study (CARMIS): two-year results of a randomized study. Eur J Ophthalmol. 2012;22:216–25. doi: 10.5301/ejo.5000069. [DOI] [PubMed] [Google Scholar]
- 53.Ma L, Yan SF, Huang YM, et al. Effect of lutein and zeaxanthin on macular pigment and visual function in patients with early age-related macular degeneration. Ophthalmology. 2012;119:2290–7. doi: 10.1016/j.ophtha.2012.06.014. [DOI] [PubMed] [Google Scholar]
- 54.Beatty S, Chakravarthy U, Nolan JM, et al. Secondary outcomes in a clinical trial of carotenoids with coantioxidants versus placebo in early age-related macular degeneration. Ophthalmology. 2013;120:600–6. doi: 10.1016/j.ophtha.2012.08.040. [DOI] [PubMed] [Google Scholar]
- 55.Berrow EJ, Bartlett HE, Eperjesi F, Gibson JM. The effects of a lutein-based supplement on objective and subjective measures of retinal and visual function in eyes with age-related maculopathy -- a randomised controlled trial. Br J Nutr. 2013;109:2008–14. doi: 10.1017/S0007114512004187. [DOI] [PubMed] [Google Scholar]
- 56.Chew EY, SanGiovanni JP, Ferris FL, et al. Age-Related Eye Disease Study 2 (AREDS2) Research Group. Lutein/zeaxanthin for the treatment of age-related cataract: AREDS2 randomized trial report no. 4. JAMA Ophthalmol. 2013;131:843–50. doi: 10.1001/jamaophthalmol.2013.4412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Age-Related Eye Disease Study 2 Research Group. Lutein + zeaxanthin and omega-3 fatty acids for age-related macular degeneration: the Age-Related Eye Disease Study 2 (AREDS2) randomized clinical trial. JAMA. 2013;309:2005–15. doi: 10.1001/jama.2013.4997. [DOI] [PubMed] [Google Scholar]