Abstract
The phosphatidylinositol-3-kinase/phosphatase and tensin homolog (PTEN)-mammalian target of rapamycin (mTOR) pathway regulates a variety of neuronal functions, including cell proliferation, survival, growth, and plasticity. Dysregulation of the pathway is implicated in the development of both genetic and acquired epilepsies. Indeed, several causal mutations have been identified in patients with epilepsy, the most prominent of these being mutations in PTEN and tuberous sclerosis complexes 1 and 2 (TSC1, TSC2). These genes act as negative regulators of mTOR signaling, and mutations lead to hyperactivation of the pathway. Animal models deleting PTEN, TSC1, and TSC2 consistently produce epilepsy phenotypes, demonstrating that increased mTOR signaling can provoke neuronal hyperexcitability. Given the broad range of changes induced by altered mTOR signaling, however, the mechanisms underlying seizure development in these animals remain uncertain. In transgenic mice, cell populations with hyperactive mTOR have many structural abnormalities that support recurrent circuit formation, including somatic and dendritic hypertrophy, aberrant basal dendrites, and enlargement of axon tracts. At the functional level, mTOR hyperactivation is commonly, but not always, associated with enhanced synaptic transmission and plasticity. Moreover, these populations of abnormal neurons can affect the larger network, inducing secondary changes that may explain paradoxical findings reported between cell and network functioning in different models or at different developmental time points. Here, we review the animal literature examining the link between mTOR hyperactivation and epileptogenesis, emphasizing the impact of enhanced mTOR signaling on neuronal form and function.
Keywords: granule cells, epilepsy, mTOR, neurogenesis, PTEN, TSC, hippocampus, autism
INTRODUCTION
The mammalian target of rapamycin (mTOR) is a serine/threonine kinase involved in the highly conserved phosphatidylinositol-3-kinase (PI3K)-Akt signaling pathway. mTOR regulates neuronal proliferation, survival, growth, and functioning (Sarbassov et al., 2005). Since this pathway is critical to development, dysregulation at any stage can have deleterious consequences (Tokuda et al., 2011). Mutations or deletions of phosphatase and tensin homolog (PTEN) and the heterodimeric complex of tuberous sclerosis proteins 1 and 2 (TSC1/hamartin, TSC2/tuberin) can dramatically upregulate mTOR signaling and contribute to a class of human neurological diseases characterized as “TORopathies” (Crino, 2009; Wong and Crino, 2012). PTEN dysregulation has been linked to disorders that include Lhermitte–Duclos disease (Liaw et al., 1997), Cowden syndrome (Goffin et al., 2001), Proteus syndrome (Zhou et al., 2001), and Bannayan–Riley–Ruvalcaba syndrome (Arch et al., 1997; Eng, 2003). Additionally, PTEN mutations have been associated with a number of neurological conditions, such as epilepsy, macrocephaly, mental retardation, and autism spectrum disorders (Marsh et al., 1999; Goffin et al., 2001; Zhou et al., 2003; Butler et al., 2005; Herman et al., 2007; O’Roak et al., 2012; Epi4K Consortium and Epilepsy Phenome/Genome Project, 2013). Dysregulation of either TSC1 or TSC2 leads to TSC, a disorder characterized by the widespread development of non-malignant tumors in multiple organ systems, including the brain, eyes, heart, skin, and lungs (for review, see Franz et al., 2010). However, the central nervous system (CNS) manifestations of TSC are the most disabling and include seizures, mental retardation, and behavioral disorders like autism (Sparagana and Roach, 2000). Epilepsy is the most common neurological symptom of TSC, often developing within a year of birth and affecting 60–90% of individuals over their lifetime (Holmes and Stafstrom, 2007). Promisingly, the mTOR antagonist rapamycin and its analogs have already entered clinical trials for TSC, with initial studies showing encouraging results (Krueger and Franz, 2008; Muncy et al., 2009; Wong, 2010; Kotulska et al., 2013; Krueger et al., 2013). TORopathies may also include disorders with evidence of enhanced mTOR signaling, but lacking known mTOR pathway mutations. For example, enhanced mTOR signaling has been observed in focal cortical dysplasia (Chen et al., 2012; Lim and Crino, 2013; Wong, 2013b; Yasin et al., 2013) and mesial temporal sclerosis (Sha et al., 2012; Sosunov et al., 2012); although the role of enhanced signaling in these conditions remains uncertain.
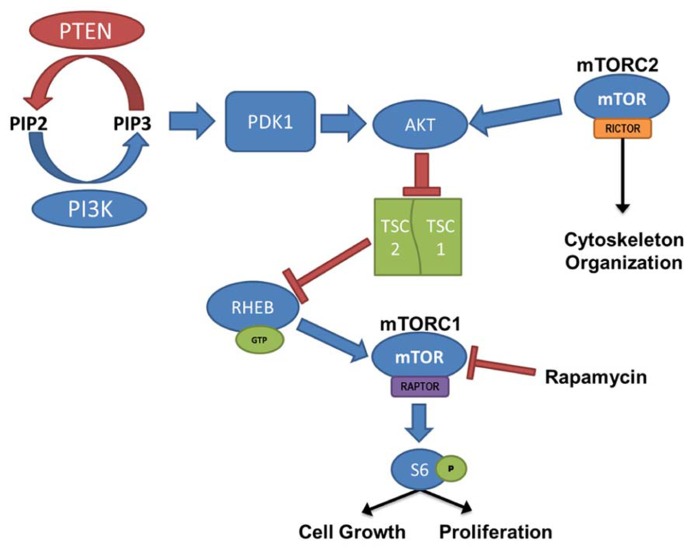
Mammalian target of rapamycin activation is controlled by the PI3K/Akt signaling pathway, where PTEN and the combined TSC1 and TSC2 complex act as negative regulators (Figure 1). PI3K is responsible for the production of phosphatidylinositol (3,4,5)-triphosphate (PIP3) through the phosphorylation of the biphosphate PIP2. PTEN acts in opposition to PI3K, dephosphorylating PIP3, resulting in the production of the PIP2. PIP3 binds PDK-1, allowing PDK-1 to activate Akt; thus, PTEN prevents Akt activation through the depletion of available PIP3. When Akt is activated it binds to TSC1/TSC2 complex. Normally when TSC1 and TSC2 are dimerized, the complex binds to RAS homolog enriched in brain (RHEB); however, activated Akt phosphorylates TSC2 and prevents RHEB from binding. In this case, GTP-bound RHEB activates mTOR. Therefore, loss-of-function mutations or removal of PTEN, which leads to hyperactivation of Akt, or loss of TSC1/TSC2, which leads to increased unbound RHEB, can cause an upregulation of mTOR signaling. Furthermore, the mTOR pathway contains two “arms” in which signaling is mediated either by mTOR complex 1 (mTORC1) or mTOR complex 2 (mTORC2). mTORC1 signaling requires activation of the adaptor protein raptor, while mTORC2 signaling requires the rictor adaptor protein. Studies implicate mTORC1 in regulating cell growth and proliferation(for review, see Fingar and Blenis, 2004; Magri et al., 2011), while mTORC2 is implicated in regulating the actin cytoskeleton, soma size, dendritic growth, and dendritic tiling (Jacinto et al., 2004; Koike-Kumagai et al., 2009; Urbanska et al., 2012; Thomanetz et al., 2013).
FIGURE 1.
PI3K/PTEN–mTOR pathway. mTOR is activated by signaling through the PI3K–Akt pathway, regulating cell growth and proliferation. PTEN, TSC1, and TSC2 act as negative regulators of the mTOR pathway, and removal leads to hyperactivation of mTOR. mTOR complex 1 (mTORC1) signaling requires activation of the adaptor protein raptor, and mTOR complex 2 (mTORC2), which is largely insensitive to acute rapamycin treatment, requires activation of the rictor protein. Although regulation of mTORC2 is less clearly defined, it has been linked to cytoskeleton organization.
INVOLVEMENT OF mTOR IN EPILEPTOGENESIS
EPILEPSY IS CHARACTERIZED BY RESTRUCTURING OF NEURONAL CIRCUITS IN THE BRAIN
It has been known since the 1980s that epilepsy can be associated with dramatic restructuring of neuronal pathways. Some of the best evidence of this comes from studies of the hippocampus. In tissue surgically resected from patients with temporal lobe epilepsy, hippocampal dentate granule cell mossy fiber axons were found to form de novo projections into the dentate inner molecular layer (IML) (Sutula et al., 1989). Subsequent studies revealed that these sprouted axons form excitatory synaptic connections with granule cell dendrites projecting through the IML, creating recurrent loops which have been hypothesized to promote hyperexcitability (for review, see Nadler, 2003). More recently, studies using models of temporal lobe epilepsy have identified ectopic granule cells (Parent et al., 1997; Scharfman et al., 2000; Overstreet-Wadiche et al., 2006), hypertrophied granule cells (Suzuki et al., 1995; Murphy et al., 2011, 2012), granule cells with basal dendrites (Ribak et al., 2000, 2012; Overstreet-Wadiche et al., 2006; Murphy and Danzer, 2011), and granule cells with altered synaptic structure (Pierce and Milner, 2001; Danzer et al., 2010; McAuliffe et al., 2011; Upreti et al., 2012) as common pathologies of the disorder. Interestingly, the dentate gyrus is one of only two regions exhibiting persistent neurogenesis in adulthood, and a majority of the granule cells exhibiting these pathological abnormalities appear to be newborn (Parent et al., 2006; Walter et al., 2007; Kron et al., 2010; Santos et al., 2011). Moreover, these post-seizure born dentate granule cells exhibit accelerated maturity and functional integration into the network; receiving perforant-path input sooner than in a normal brain (Overstreet-Wadiche et al., 2006). While the continued generation of neurons in the dentate may make the region particularly vulnerable to epileptogenic rewiring (Jessberger et al., 2007; Danzer, 2008), restructuring of neuronal circuits is also evident in other brain regions. Rewiring is evident in cortical epilepsy models (for example, Graber and Prince, 2004), and histological and functional mapping studies in humans with a range of epilepsy syndromes support the conclusion that restructuring is a recurring feature of the disease (Bonilha et al., 2012; Fang et al., 2013; Woodward et al., 2013; Xu et al., 2013; Haneef et al., 2014). Changes in neuronal circuitry in epilepsy can be widespread and profound, ranging from alterations in synaptic protein expression and synaptic strength at the subcellular level, to neuronal loss and addition at the cellular level, to altered connectivity between brain regions at the systems level. While such changes are likely to involve many signaling pathways, the mTOR pathway stands out for its ability to regulate cell proliferation, cell survival, cell growth, and synaptic strength. These features make it an appealing candidate for involvement in epileptogenesis.
HYPERACTIVATION OF THE mTOR SIGNALING PATHWAY CONSISTENTLY PRODUCES EPILEPSY IN ANIMAL MODELS
Numerous laboratories have examined the impact of PTEN, TSC1, or TSC2 deletions in mouse models. Initial studies examining germline deletions of PTEN (Suzuki et al., 1998; Podsypanina et al., 1999), TSC1 (Kwiatkowski et al., 2002), or TSC2 demonstrated that loss of gene function produced early mortality (Kobayashi et al., 1999; Onda et al., 1999). CNS deletion using conditional approaches also reduced survival in mice (Groszer et al., 2001; Zhou et al., 2009). Therefore, in order to generate viable animals that could be used to study cellular consequences of mTOR hyperactivation, smaller neuronal populations have been targeted with more specific Cre-loxP promoters (Kwon et al., 2001; Marino et al., 2002; Uhlmann et al., 2002; Fraser et al., 2004; Yue et al., 2005; Erbayat-Altay et al., 2007; Meikle et al., 2007; Wang et al., 2007; Zeng et al., 2008, 2011; Way et al., 2012), developmentally timed promoters (Kwon et al., 2006; Zhou et al., 2009), tamoxifen-inducible promoters in which gene removal can be temporally controlled by the researcher (Amiri et al., 2012; Pun et al., 2012), or a combination of these techniques (Abs et al., 2013). As illustrated by Figure 2, PTEN removal from dentate granule cells leads to hyperactivation of mTOR, evident as an increase in phosphorylated ribosomal protein S6 (pS6). Rapamycin treatment to prevent mTOR activation blocks the increase in pS6. Although many Cre promoters have been utilized to create the different mouse models, one of the commonalities between these transgenic mice is the incidence of seizures. For example, mice with PTEN removed from 30–60% of mature CA3 neurons, dentate granule cells, and layer III–V cortical neurons developed spontaneous seizures (Kwon et al., 2006), and 40% of mice with PTEN deleted from subgranular and subventricular zone progenitors developed spontaneous seizures (Amiri et al., 2012). Work by Pun et al. (2012) produced the most selective deletion to date, with PTEN removal from as little as 9% of the dentate granule cell population, producing a profound epilepsy syndrome in almost all animals. While PTEN is further upstream from mTOR than TSC1 and TSC2, deletion of either of these regulatory proteins produces similar consequences; although the time course of epileptogenesis varies between models. Removal of TSC1 using Synapsin-Cre mice produced knockout (KO) cells throughout the cortex, subcortical gray matter, and hippocampal CA3 and hilar neurons. These animals developed spontaneous seizures by post-natal day 5 and died between 3 and 5 weeks of age (Meikle et al., 2007; Choi et al., 2008). Removal of TSC1 from cortical and subventricular zone neurons in Emx1-Cre mice was associated with cortical seizures at post-natal day 13 (Magri et al., 2011; Carson et al., 2012). Similarly, GFAP-Cre directed TSC1 or TSC2 deletion from astrocytes and some neurons (Bajenaru et al., 2002; Su et al., 2004) produced seizures between 3 weeks and 2 months of age (Uhlmann et al., 2002; Erbayat-Altay et al., 2007; Zeng et al., 2008, 2010, 2011), while mice with TSC1 or TSC2 removal from radial glial cells commonly had seizures before their death at 3–4 weeks of age (Way et al., 2009; Magri et al., 2013). Finally, recent work by Abs et al. (2013) produced the intriguing result that global deletion of TSC1 from adult animals could result in the onset of spontaneous seizures within 2–9 days. Such rapid epileptogenesis would favor changes that could occur quickly, such as altered neuron physiology. Some “leaky” expression from their Cag-CreERT driver line, however, lead to premature TSC1 deletion from a small number of cells, leaving open the possibility longer term changes among these TSC1 KO cells may accelerate epileptogenesis in the animals. Taken together, these data suggest that mTOR hyperactivation among a wide range of cell types can drive epileptogenesis.
FIGURE 2.
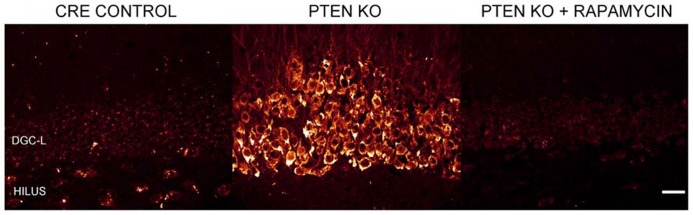
Dentate granule cells lacking PTEN have hyperactive mTOR signaling. Confocal maximum projections of the dentate gyrus from control mice, Gli1-CreERT2 × PTENfl/fl (PTEN KO) mice, and rapamycin treated PTEN KO mice immunostained for phosphorylated ribosomal protein S6 (pS6; red). pS6, the downstream target of mTOR complex 1, is greatly increased in the dentate granule cell layer (DGC-L) in the PTEN KO animal, demonstrating the upregulation of mTOR signaling with the removal of PTEN. Rapamycin treatment, and thus inhibition of mTOR, prevents the increase in pS6 in PTEN KO mice. Scale bar = 25 μm. Images courtesy of Isaiah Rolle.
ENHANCED mTOR SIGNALING IN ACQUIRED EPILEPSY MODELS
Mammalian target of rapamycin pathway upregulation has been described in several animal models of acquired epilepsy. In acquired epilepsy models, epileptogenesis is induced by experimentally injuring the brain using, for example, convulsant drugs or hypoxia. The mTOR pathway is not genetically manipulated in these models, as is the case for TSC and PTEN mutants, so changes in mTOR signaling reflect physiological responses to the injury. It is notable, therefore, that hyperactivation of mTOR signaling is present in acute seizure (Zhang and Wong, 2012), status epilepticus (Okamoto et al., 2010; Macias et al., 2013), traumatic brain injury (Chen et al., 2007), hypoxia (Talos et al., 2012; Sun et al., 2013), and in vitro (Berdichevsky et al., 2013) models of epilepsy. Following traumatic brain injury, phosphorylated mTOR was significantly increased after 30 min and remained elevated for 4–24 h (Chen et al., 2007). In the kainic acid status epilepticus model of epilepsy, mTOR signaling increased 1 h after injury, returned to baseline at 24 h, and increased again beginning at 3 days. This second wave lasted for several weeks (Zeng et al., 2009). Persistent upregulation of the pathway is hypothesized to initiate changes in neuronal structure and physiology that contribute to epileptogenesis. Consistent with this idea, administration of the mTOR antagonist rapamycin has been found to inhibit mossy fiber sprouting and mitigate the development of spontaneous seizures in several models of epilepsy (for review, see Wong, 2013a). On the other hand, work by Buckmaster and colleagues found that rapamycin was ineffective at reducing spontaneous seizure frequency in mice (Buckmaster and Lew, 2011; Heng et al., 2013); although mossy fiber sprouting was still blocked (Buckmaster et al., 2009). Whether the discrepant results reflect species differences (mouse vs. rat), model differences, or other factors is not known. Nonetheless, the subset of positive animal findings, combined with promising clinical data in children with TSC (Krueger and Franz, 2008; Muncy et al., 2009; Wong, 2010; Kotulska et al., 2013; Krueger et al., 2013), provide a strong rationale for further investigations.
REGULATION OF NEURONAL FORM AND FUNCTION BY mTOR
Given the extensive literature demonstrating neuronal rewiring in epilepsy, the ability of mTOR hyperactivation in the CNS to cause epilepsy, the evidence that the mTOR pathway is activated during epileptogenesis, and the role played by mTOR signaling in regulating neuronal plasticity, we decided to explore the consequences of enhanced mTOR signaling on neuronal form and function in the second part of this review. While the exact mechanisms by which excess mTOR signaling promotes epilepsy remain uncertain, we believe that understanding the cellular impacts of this signaling provides novel insights.
CELL PROLIFERATION
The mTOR signaling pathway is an important regulator of developmental and adult neurogenesis. Cell proliferation is impaired in mTOR KO mice, as well as RAPTOR and RICTOR KO mice, in which mTORC1 or mTORC2 signaling, respectively, is blocked. Loss of either mTOR, raptor, or rictor results in embryonic lethality, possibly in part due to impaired cell proliferation (Gangloff et al., 2004; Murakami et al., 2004; Guertin et al., 2006; Shiota et al., 2006; Cloetta et al., 2013). Conversely, embryonic deletion of PTEN from CNS stem/progenitor cells decreases apoptosis and increases neuron numbers compared to controls (Groszer et al., 2001). TSC deletions also promote cell proliferation, with the hallmark feature of the human disease being cortical tubers. The tubers are accumulations of non-malignant cells that exhibit abnormal proliferation, differentiation, and migration. In developing animals, TSC1 deletion from radial glial cells disrupts the subventricular proliferative zones and leads to megalencephaly (Magri et al., 2013). In the adult brain, effects of mTOR dysregulation on neuronal proliferation are less pronounced since adult neurogenesis is limited to olfactory and hippocampal granule neurons, but it is clear that mTOR plays an important role in regulating the generation of these cells (Hartman et al., 2013; Magri and Galli, 2013). Conditional deletion of PTEN from the subventricular zone progenitors, for example, increased proliferation of neurons populating the olfactory bulb (Gregorian et al., 2009), while inhibiting the PI3K/PTEN–mTOR pathway (using rapamycin and the PI3K inhibitor LY294002) led to reduced subventricular zone proliferation (Peltier et al., 2007; Paliouras et al., 2012). Rapamycin had a similar effect on subgranular zone production of granule cells (Raman et al., 2013). These data suggest that proliferation of adult-generated dentate granule cells would be increased with mTOR hyperactivation due to PTEN, TSC1, or TSC2 deletion. Consistent with this, investigators examining PTEN KO mice, including the GFAP-Cre and Nestin-CreERT2 lines, have reported increased cell proliferation in vivo at early time points (up to 4 months; Fraser et al., 2004; Amiri et al., 2012). However, increased production of granule cells may be temporary, as proliferation rates declined at later time points (Amiri et al., 2012). It is possible that the accelerated differentiation, which contributes to increased proliferation at early time points, depletes the subgranular zone stem cell pool at later time points. Ventricular zone progenitors, on the other hand, do not appear to exhibit the same limitation (Gregorian et al., 2009).
Increased neurogenesis from enhanced mTOR signaling can have profound effects when the altered signaling occurs early in development and is widespread, with the excess neurons contributing to gross brain abnormalities like hemimegalencephaly. Altered neurogenesis may also contribute to more subtle defects, like heterotopias and dysplasias. These small clusters of abnormal neurons often develop into epileptic foci, as evidenced by electroencephalography (EEG) studies and the abolition of seizures in many patients following surgical removal of the abnormal cells (for review, see Hauptman and Mathern, 2012). Interestingly, evidence has recently emerged suggesting that prenatal infection with human papillomavirus (HPV) may disrupt mTOR signaling, leading to the development of focal cortical dysplasia type II. The HPV oncoprotein E6 was found in resected human dysplasias, but not surrounding tissue, and transfection of fetal mouse brain with an E6 expression vector potently activated mTOR targets (Chen et al., 2012; Lim and Crino, 2013; Wong, 2013b; Yasin et al., 2013). Additional subtle effects of increased mTOR signaling in acquired epilepsy may include the burst of neurogenesis observed following epileptogenic brain injury in rodents (Parent et al., 1997). mTOR signaling promotes neurogenesis, and large numbers of these newborn cells integrate abnormally, raising the possibility that these new neurons may by epileptogenic (Scharfman and Hen, 2007; Scharfman and McCloskey, 2009; Parent and Kron, 2012). If true, it is possible that reduced seizure frequency following mTOR inhibition (for review, see Ryther and Wong, 2012) may be partly mediated by reduced neurogenesis. Finally, evidence that subgranular zone progenitors can be depleted following prolonged mTOR hyperactivation raises the possibility that epileptogenesis and seizures could accelerate the decline of this important cell population (Amiri et al., 2012). In some rodent epilepsy models, granule cell neurogenesis is increased in the first few weeks and months after the insult, but persistent decreases in neurogenesis have been observed when the animals are examined months later (Hattiangady and Shetty, 2010; Danzer, 2012).
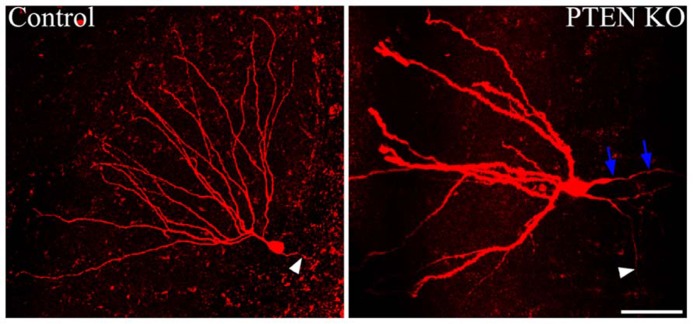
SOMATIC HYPERTROPHY
Somatic hypertrophy among neurons has been one of the most consistent findings in cells with increased mTOR signaling (Figure 3). In animal models, somatic hypertrophy has been observed following deletion of TSC1 (Meikle et al., 2007; Zeng et al., 2008; Zhou et al., 2009; Tsai et al., 2012), TSC2 (Zeng et al., 2011; Tsai et al., 2014), and PTEN (Backman et al., 2001; Groszer et al., 2001; Kwon et al., 2001, 2003, 2006; Fraser et al., 2004; Ljungberg et al., 2009; Amiri et al., 2012; Pun et al., 2012). Hypertrophy has been detected in hippocampal granule cells (Backman et al., 2001; Kwon et al., 2001, 2003, 2006; Fraser et al., 2004; Amiri et al., 2012; Pun et al., 2012), cortical neurons (Fraser et al., 2004; Kwon et al., 2006; Meikle et al., 2007; Ljungberg et al., 2009; Zhou et al., 2009), and purkinje cells (Tsai et al., 2012; Thomanetz et al., 2013). Treatment with rapamycin or CCI-779 to inactivate mTOR prevents the somatic hypertrophy (Kwon et al., 2003; Zeng et al., 2008, 2011; Zhou et al., 2009), and similarly, inhibition of mTORC2 signaling by deletion of RICTOR reduces neuronal size (Thomanetz et al., 2013). Somatic enlargement associated with mTOR hyperactivation is seen in human TORopathies, and is particularly prominent in patients with TSC, who exhibit giant cells within the tubers that characterize the disease (White et al., 2001). Enlarged neurons are also present in focal cortical dysplasia, in which cytomegalic neurons and “balloon cells” are characteristic. Altogether, these data support the conclusion that mTOR dysregulation promotes abnormal cell growth.
FIGURE 3.
Dentate granule cells lacking PTEN exhibit structural abnormalities. Confocal maximum projections of control and Gli1-CreERT2 × PTENfl/fl (PTEN KO) granule cells, filled with biotin and labeled with Alexa-Fluor 594-conjugated streptavidin. The PTEN KO cell exhibits somatic hypertrophy and dramatically increased dendritic thickness compared to the control dentate granule cell. Additionally, basal dendrites are commonly found on the PTEN-negative cells, here indicated by blue arrows. White arrowheads highlight the mossy fiber axon extending toward the hilus from each of the two cells. Scale bar = 50 μm. Images courtesy of Victor R. Santos and Dr. Raymund Y. K. Pun.
Increased soma size can alter neuronal function at both microscopic and macroscopic levels. At the single cell level, increased soma area would be predicted to decrease cellular input resistance and increase total cell capacitance. These predictions were upheld by Luikart et al. (2011) in work examining hippocampal granule cells following small hairpin RNA (shRNA)-mediated PTEN knockdown. Knockdown cells exhibited neuronal hypertrophy, decreased input resistance, and increased capacitance. Similar findings were reported for TSC1 KO cells (Bateup et al., 2011, 2013b). At the macroscopic level, increased soma size in PTEN, TSC1, or TSC2 KO mice contributes to gross distortion of brain structure. Specifically, cellular hypertrophy (potentially combined with increased neurogenesis) leads to progressive macrocephaly with increased brain size in mouse models, usually accompanied by disorganization of cell layers (Groszer et al., 2001; Kwon et al., 2001, 2003, 2006; Fraser et al., 2004; Zeng et al., 2008, 2011; Zhou et al., 2009; Amiri et al., 2012). The increase in cell size and cell layer disorganization can cause compression of other brain areas, such as hippocampal CA1 in the NSE-Cre PTEN KO mouse (Kwon et al., 2006). These dramatic disruptions likely contribute to functional deficits in the animal models and humans with related conditions like hemimegalencephaly.
CELL POLARIZATION
Establishing proper polarity is critical step in neuronal differentiation, and mTOR signaling appears to play a modulatory role in this process. In the rodent hippocampal dentate gyrus, granule cells typically possess only apical dendrites, which extend from the soma into the dentate molecular layer. When the mTOR pathway is hyperactivated in granule cells by PTEN deletion, however, large numbers of granule cells develop aberrant basal dendrites (Figure 3; Kwon et al., 2006; Pun et al., 2012). Basal dendrites are also a feature of rodent epilepsy models, where the abnormal processes project into the dentate hilus. These hilar-projecting dendrites are innervated by the mossy fiber axons of neighboring granule cells, creating recurrent excitatory loops which may contribute to hyperexcitability (Ribak et al., 2000, 2012; Thind et al., 2008). Granule cell basal dendrites that develop after PTEN deletion have spines apposed to presynaptic terminals of mossy fiber axons, suggesting they also mediate the formation of recurrent circuits (Pun et al., 2012). These recurrent connections are thought to contribute to hyperexcitability and seizure generation in PTEN KO animals.
Whether PTEN deletion disrupts cell polarization directly or indirectly is not clear. Immature granule cells frequently exhibit transient basal dendrites, which are eliminated as the cells mature (for review, see Rahimi and Claiborne, 2007). Retraction of these basal dendrites appears to be regulated by activity, since increasing neuronal activity in vitro can lead to their persistence (Nakahara et al., 2009). It is possible, therefore, that basal dendrites in PTEN KO mice are a consequence of increased neuronal activity in these animals (or the seizures that ultimately develop), although additional studies will be needed to test the idea.
AXON GROWTH
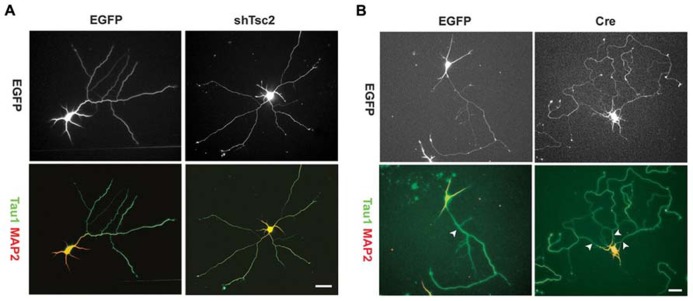
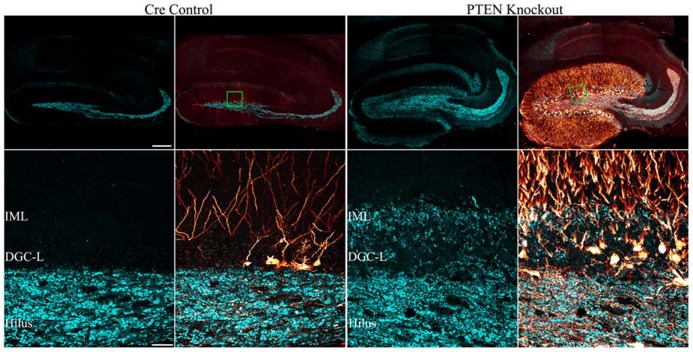
Axon growth appears to be promoted by the mTOR pathway, and disrupted mTOR activation can lead to irregular axon development. Overexpression of TSC1 or 2 in vitro – thus decreasing mTOR signaling – results in fewer neurons possessing an axon. Conversely, KO of TSC1 or knockdown of TSC2 increases the number of neurons with multiple axons (Figure 4), and rapamycin treatment was found to mitigate this effect in the TSC2 knockdown cells (Choi et al., 2008). Consistent with this in vitro work, deletion of TSC1 in a Synapsin-Cre TSC1 KO mouse model led to ectopic axon growth in cortex (Choi et al., 2008). PTEN removal in vivo also increased axon growth. PTEN deletion from dentate granule cells, occurring during development or after the cells mature, led to progressive enlargement of the granule cell mossy fiber axon tract (Kwon et al., 2006; Amiri et al., 2012). Moreover, NSE-Cre TSC1 KO mice exhibited increased thickness of the mossy fiber tract, while rapamycin administration reduced tract thickness (Zhou et al., 2009). Finally, work by Pun et al. (2012) demonstrated that selective deletion of PTEN from granule cells provoked aberrant axon sprouting into the dentate IML (mossy fiber sprouting, Figure 5). Together, these data suggest that mTOR signaling is involved in axon outgrowth, and disruption of this pathway can lead to axon path thickening (presumably due to larger numbers of axons/axon collaterals).
FIGURE 4.
Tuberous sclerosis complex deficiency induces multiple axons in culture. Knockdown of Tsc2 by shRNA (shTsc2) or knockout of Tsc1 (Cre) induced multiple axons in vitro. (A) E18 rat hippocampal neurons transfected with either enhanced green fluorescent protein (EGFP) alone or EGFP together with Tsc2 shRNA. Tsc2 knockdown induced multiple axons, all of which are positive for Tau1. (B) E17 mouse hippocampal neurons from Tsc1lox/flox embryos transfected with EGFP alone or EGFP together with Cre. Arrowheads indicate axons positive for Tau1. Scale bars = 20 μm. Reprinted with permission from Choi et al. (2008).
FIGURE 5.
Mossy fiber sprouting is present in mice with PTEN removal from dentate granule cells. Confocal maximum projections of hippocampi from tamoxifen-treated control and Gli1-CreERT2 × PTENfl/fl (PTEN KO) mice immunostained for GFP (red) and zinc-transporter 3 ZnT3 (cyan) are shown. ZnT3-labeling reveals the normal dentate granule cell mossy fiber axon terminal field (hilus and stratum lucidum) in the control animal, while mossy fiber sprouting into the dentate granule cell layer (DGC-L) and inner molecular layer (IML) is evident in the knockout animal (green boxes in the top row correspond to high resolution images shown in the bottom row). Scale bars = 200 μm (top row) and 30 μm (bottom row). Reprinted with permission from Pun et al. (2012).
The mechanism by which mTOR signaling promotes the sprouting of granule cell mossy fiber axons into the dentate IML remains unclear. In acquired epilepsy models, rapamycin has been consistently found to block mossy fiber sprouting, suggesting that increased mTOR signaling may mediate this pathology directly (Zeng et al., 2009; Huang et al., 2010; Buckmaster and Wen, 2011). However, deletion of PTEN from dentate granule cells does not always produce mossy fiber sprouting. In fact, mossy fiber sprouting was only detected in Gli-CreERT2 PTEN KO mice when the number of granule cells without PTEN exceeded ≈15% of the entire granule cell population (Pun et al., 2012). Interestingly, some animals with fewer PTEN KO cells developed seizures, but not mossy fiber sprouting: evidenced both by a lack of mossy fiber terminal immunolabeling in the IML, and an absence of green fluorescent protein (GFP)-labeled PTEN KO cells axons in this same region (Pun et al., 2012). These latter findings suggest that mossy fiber sprouting is an emergent property of mTOR hyperactivation. Restated, it would appear than mTOR hyperactivation in individual granule cells is not sufficient to drive mossy fiber sprouting, but large numbers of PTEN KO granule cells acting together do produce mossy fiber sprouting, suggesting that secondary processes are involved. It has been proposed that mossy fiber sprouting is triggered by mossy cell loss, as the degree of sprouting is positively correlated with the extent of mossy cell loss (Jiao and Nadler, 2007). Mossy cell loss in the absence of seizures, however, does not cause mossy fiber sprouting (Jinde et al., 2012), suggesting that while the two phenomena are correlated, they may not be causally linked. The ability of rapamycin to block mossy fiber sprouting in acquired epilepsy models, and the apparent inability of increased mTOR signaling to drive mossy fiber sprouting unless a threshold level of KO cells is met, raises interesting questions about when and where rapamycin is acting.
The net effect of increased axon sprouting following increased mTOR activation is uncertain (Buckmaster, 2012). Although granule cell mossy fiber sprouting is not required to produce epilepsy in mTOR hyperactivation models, increased sprouting of granule cells may contribute to epilepsy progression in the animals by promoting the formation of recurrent excitatory circuits. Alternatively, mossy fiber sprouting may play a compensatory role by promoting feedback inhibition. In addition to contacting excitatory granule cells, sprouted mossy fibers have also been observed to innervate inhibitory interneurons in animal models of epilepsy (Frotscher et al., 2006; Sloviter et al., 2006). Enhanced mTOR signaling may also increase inhibition by promoting sprouting of other neuronal types. Work by Buckmaster and Wen (2011) demonstrated that inhibiting mTOR with rapamycin in the pilocarpine model of epilepsy can reduce axon sprouting of somatostatin-expressing GABAergic interneurons, raising the possibility that mTOR-mediated sprouting in epilepsy may exert both pro-excitatory and homeostatic effects, depending on the cell type affected.
DENDRITIC GROWTH
Signaling through the PI3K/PTEN–mTOR pathway promotes dendritic growth and branching. In cultured hippocampal neurons, constitutively activating PI3K, or RNA interference (RNAi) knockdown of PTEN, resulted in increased dendrite number, branching complexity, and total dendrite length (Jaworski et al., 2005). Moreover, the effects appear to be mediated by mTOR, as rapamycin administration blocked abnormal dendritic growth following constitutive PI3K activation and PTEN knockdown (Jaworski et al., 2005). In vitro, the effects of PI3K activation on dendritic length and complexity appear to be limited to immature neurons, since mature neurons did not exhibit changes in dendritic structure (Cuesto et al., 2011). Removal of PTEN in vivo supports in vitro data; PTEN removal from dentate granule cells resulted in dendritic hypertrophy, elongated dendrites, and increased arborization among developing neurons (Figure 3; Kwon et al., 2006; Amiri et al., 2012; Pun et al., 2012). Removal of TSC1 from dentate granule cells increased molecular layer thickness, suggesting dendritic hypertrophy akin to the PTEN KO mice (Zhou et al., 2009). Rapamycin can reduce dendritic overgrowth in PTEN KO mice, further specifying mTOR signaling in dendritic size and branching (Zhou et al., 2009). Thus, it appears that mTOR controls growth of the soma and neuronal processes, and continued activation causes processes to thicken and elongate.
The structure of the dendritic tree is thought to be critical for precise signal processing. Therefore, changes in dendritic thickness associated with mTOR hyperactivation have the potential to alter synaptic potential integration. Theoretical models of synaptic potential attenuation use dendrite diameter in the estimation of the current flow. Thicker dendrites facilitate signal spread toward the soma compared to thinner dendrites, although thicker dendrites would also predict smaller excitatory post-synaptic potentials (EPSPs), as the larger processes should have lower input resistance (for review, see Rall, 2011). The increased surface area provided by larger dendrites increases the post-synaptic “real estate” available for innervation, potentially constituting one mechanism by which neurons regulate their synaptic input. Elucidating the net effect of thicker dendrites will require additional studies, and might vary by neuron and condition.
CELL INTRINSIC EFFECTS, PRIMARY NETWORK EFFECTS, AND SECONDARY NETWORK EFFECTS
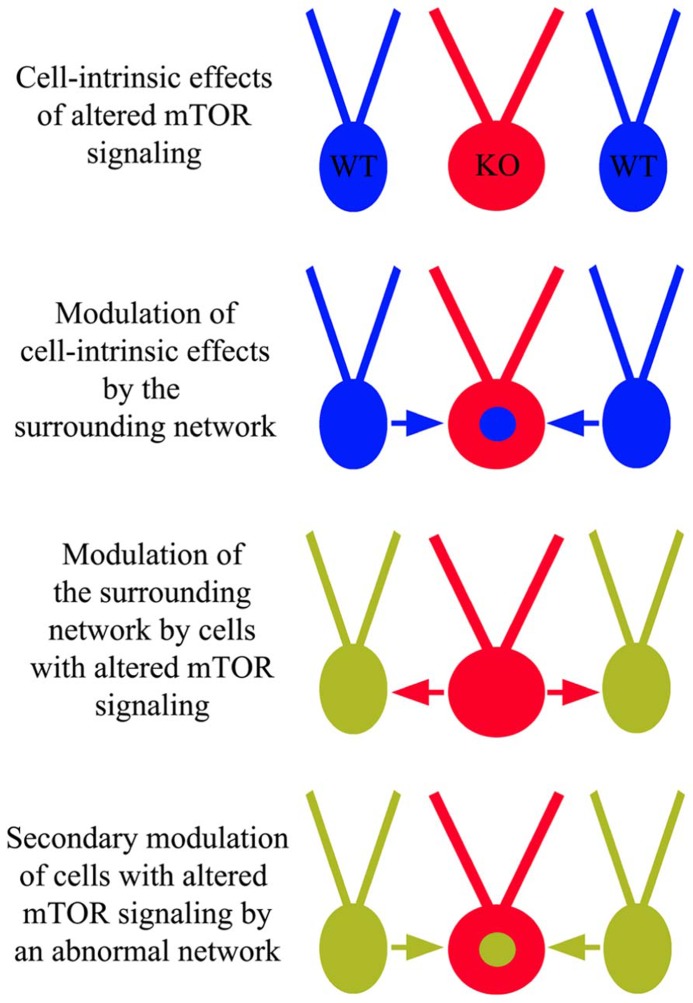
Anatomical and physiological studies examining the impact of mTOR hyperactivation following PTEN or TSC deletion have produced a number of conflicting findings. Some of these discrepancies likely involve the usual suspects, such as cell and gene-specific effects, but an emerging story suggests much of this complexity may reflect a role for the mTOR pathway in regulating network homeostasis and plasticity. The ability of mTOR to regulate synaptic structure and function clearly places the pathway in a position to impact how neurons respond to activity levels in their environment. This observation has two important implications. Firstly, it suggests that cells with disrupted mTOR signaling may respond inappropriately to input from other neurons in a fashion that is context dependent. Restated, different environments may produce different types of changes in PTEN or TSC KO cells. Secondly, since PTEN and TSC KO cells exhibit altered activity patterns, they can influence the activity of surrounding neurons, and these activity changes in the network may in turn alter the behavior of the KO cells (Figure 6). If correct, it may be more useful to view the mTOR signaling pathway as a means by which a neuron regulates its “social” behavior in a network. In the following sections we will present examples that demonstrate typical cell and gene-specific differences that account for variability, and more intriguing evidence of primary and secondary network effects on KO cell behavior.
FIGURE 6.
Cell-intrinsic effects of altered mTOR signaling and network influence. In addition to cell-intrinsic effects from disrupted mTOR signaling in cells lacking PTEN, TSC1, or TSC2 (red “KO” cells), these cells may respond inappropriately to surrounding wild type (WT) cells and/or cause WT cells to behave abnormally. These altered dynamics may produce an abnormal network, facilitating epileptogenesis.
CELL TYPE AND GENE-SPECIFIC EFFECTS: LONG-TERM DEPRESSION
In addition to changes in cell structure, mTOR hyperactivation is associated with changes in synaptic plasticity. Physiological changes that have been detected include a decrease in long-term depression (LTD). LTD is defined by a reduction in the efficacy of a synapse and is typically induced experimentally by low frequency stimulation. Two mechanistically distinct types of LTD co-exist at hippocampal synapses: a metabotropic glutamate receptor (mGluR)-dependent form and a N-methyl-D-aspartate (NMDA) receptor (NMDAR) dependent form (Mulkey and Malenka, 1992; Bolshakov and Siegelbaum, 1994; Oliet et al., 1997). mGluR-LTD, which requires dendritic protein synthesis, can be selectively induced by (S)-3,5-dihydroxyphenylglycine (DHPG), an agonist that activates group I mGluRs (Palmer et al., 1997; Fitzjohn et al., 1999; Schnabel et al., 1999; Huber et al., 2001). Activation of group I mGluRs triggers the PI3K–Akt–mTOR signaling cascade, which increases Akt and mTOR phosphorylation in CA1 dendrites. Moreover, treatment with rapamycin or PI3K inhibitors block DHPG-induced LTD (Hou and Klann, 2004), clearly implicating mTOR signaling in the process. Paradoxically, hyperactivation, like inhibition, of mTOR seems to mostly impair LTD; possibly as a secondary consequence of seizures in the animals (Kirschstein et al., 2007). The targeted cell type also appears to be important. Specifically, Takeuchi et al. (2013) showed mGluR-LTD was impaired at all ages in PTEN KO mice at the perforant path ≫ granule cell synapse, but not CA3 ≫ CA1 synapses. Interestingly, PTEN was deleted from dentate granule cells in these animals, whereas the PTEN gene was left intact in CA1 pyramidal cells, suggesting that mTOR hyperactivation in the post-synaptic cell may be responsible for the impairment in mGluR-LTD. Supporting this conclusion, TSC1 deletion specifically in CA1 neurons did impair mGluR-LTD in these same cells (Bateup et al., 2011). The location of mTOR hyperactivation, therefore, can be an important modulator of effect.
Interestingly, although PTEN is best known for its ability to regulate mTOR signaling, some of its effects on LTD may be mTOR independent. PTEN is required for the expression of NMDAR-LTD (Wang et al., 2006; Jurado et al., 2010), but TSC1 does not appear to be important for this form of LTD. Specifically, Sperow et al. (2012) found NMDAR-LTD to be substantially reduced at the CA3 ≫ CA1 synapse when PTEN deletion was targeted to post-synaptic CA1 neurons, while Bateup et al. (2011) demonstrated that NMDAR-dependent LTD was normal in mice in which TSC1 was deleted from CA1 neurons. Both mouse models exhibit hyperactivation of mTOR. PTEN, however, is much further upstream in the mTOR signaling pathway, with potentially distinct consequences. In fact, Jurado et al. (2010) demonstrated the NMDAR activation triggers an association between PTEN and the synaptic scaffold, anchoring PTEN at the post-synaptic terminal. Furthermore, PTEN removal, and not TSC1 deletion, would affect the PIP3 and PI3K influenced pathways. PI3Kγ is critical for NMDAR-LTD, and it is thought to signal via the activation of p38 MAPK and not the mTOR pathway, potentially explaining why NMDAR-LTD is normal in TSC1 KO mice and impaired in PTEN KO mice (Kim et al., 2011). mTOR hyperactivation by TSC1 deletion likely facilitates changes in network dynamics through the loss of mGluR-LTD; however, it appears that PTEN deletion would have more serious consequences for a neuron and its network due to impairments in both mGluR-LTD and NMDAR-LTD. Gene specific effects, therefore, are likely to account for some of the controversy in the mTOR field.
CELL INTRINSIC vs. SECONDARY EFFECTS: DENDRITIC SPINES
Dendritic spines are the major points of excitatory contact between neurons, and measures of spine density are frequently used as an indirect measure of excitatory input. Notably, inhibitory neuron synapses typically form on dendritic shafts or somata, and therefore are not revealed by light microscopy approaches. Studies examining mTOR regulation of dendritic spines and synapses support a pro-excitatory role for the signaling pathway; although complex mechanisms may be involved. PTEN deletion has been associated with an increase in spine density in most studies (Kwon et al., 2006; Pun et al., 2012), however, Haws et al. (2014) recently observed no change in spine density following PTEN deletion from hippocampal granule cells, and actually observed a decrease in spine density following PTEN knockdown in basolateral amygdala. Despite the reduction in spine density, the changes were associated with an increase in miniature EPSC (mEPSC) frequency and amplitude (Haws et al., 2014). The authors suggest that the discrepancy reflects a shift from thin spines to mushroom spines, the latter being associated with stronger synapses. Similarly, Luikart et al. (2011) found that PTEN knockdown using shRNA injection in mice caused increased mEPSCs and spontaneous post-synaptic currents in dentate granule cells. Deletion of TSC1 and TSC2 has been shown to reduce dendritic spine density among cortical neurons in vivo (Tavazoie et al., 2005; Meikle et al., 2008) and hippocampal pyramidal cells in organotypic explant cultures (Tavazoie et al., 2005; Meikle et al., 2008); Since both TSC and PTEN KO mice develop epilepsy, however, many of the changes observed in these animals likely reflect phenomena secondary to the occurrence of increased activity and spontaneous seizures (Wong and Guo, 2013). Indeed, it is well established that seizures alone can induce spine loss (Swann et al., 2000; Brewster et al., 2013; Singh et al., 2013), perhaps accounting for the cortical spine loss observed by Meikle et al. (2008) in TSC1 KO mice. Consistent with this idea, when Bateup et al. (2011) induced sparse TSC1 deletion from CA1 pyramidal cells in vivo, which did not induce epilepsy, spine reductions were absent.
CELL INTRINSIC vs. SECONDARY EFFECTS: BASAL SYNAPTIC TRANSMISSION
At entorhinal cortex ≫ dentate granule cell synapses, basal synaptic transmission has been found to exhibit complex temporal changes in Nse-Cre PTEN KO mice, strongly suggesting that secondary changes are having a significant impact on physiology in these animals. The Nse-Cre transgene produces robust PTEN deletion from dentate granule cells and CA3 pyramidal cells, with relative sparing of CA1 (Takeuchi et al., 2013). In young Nse-Cre PTEN KO mice, perforant path stimulation to evoke EPSCs in the dentate gyrus produced input/output relationships that were indistinguishable from controls. Middle-aged animals, on the other hand, exhibited enhanced input/output relationships (Kwon et al., 2006; Takeuchi et al., 2013). As the animals aged, however, the differences resolved, with responses again becoming indistinguishable from controls. Although the mechanism is uncertain, the return to baseline may reflect compensatory modifications to the network (Takeuchi et al., 2013). The temporal dynamics of the physiological changes parallel morphological changes in these animals, with young animals appearing relatively normal, middle aged animal beginning to show subtle changes in structure such as increased spine density, and older animals exhibiting gross structural changes and seizures.
CELL INTRINSIC vs. SECONDARY EFFECTS: LONG-TERM POTENTIATION
Long-term potentiation (LTP) is considered to be one of the cellular mechanisms that underlies learning. During LTP, synaptic strength is increased between connected neurons, and is typically induced by high frequency stimulation. mTOR activation appears to be involved in late phase LTP (L-LTP), which is long-lasting and dependent on protein synthesis and gene transcription, but not in early phase LTP, which is short-lasting and requires activation of protein kinase C. Inhibiting mTOR with rapamycin has consistently been shown to decrease L-LTP (Terashima et al., 2000; Tang et al., 2002; Cammalleri et al., 2003; Cracco et al., 2005; Tsokas et al., 2005; Vickers et al., 2005; Stoica et al., 2011) and a recent study of CamKIIα-Cre Rictor KO mice found a similar L-LTP deficit, implicating mTORC2 (Huang et al., 2013). Paradoxically, although inhibition of mTOR blocks L-LTP, the majority of studies examining PTEN and TSC KO mice have not found that hyperactivation of mTOR facilitates it. For example, L-LTP was decreased in both GFAP-Cre and CamKIIα-Cre PTEN KO mice (Fraser et al., 2008; Sperow et al., 2012), and TSC1 KO mice exhibited a similar phenotype (Zeng et al., 2007; Abs et al., 2013). These discordant results, however, may be explained by the occurrence of seizures in the animals. Seizure activity has been shown to impair LTP by shifting the majority of synapses to the “potentiated” state, precluding further enhancement (Beck et al., 2000; Goussakov et al., 2000; Meador, 2007). Consistent with this idea, Zeng et al. (2007) were able to partially reverse the LTP deficit in their TSC1 KO mice by applying the NMDA antagonist APV, suggesting that excessive activation of NMDARs contributes to this impairment. The study from Takeuchi et al. (2013), in which Nse-Cre PTEN KO mice were examined at multiple time points, provides further support for the idea that LTP impairment may be a secondary effect of mTOR hyperactivation. Similar to the prior studies, in middle-aged animals, PI3K-dependent LTP was impaired at perforant path ≫ dentate granule cells synapses and Schaffer collateral ≫ CA1 synapses (Takeuchi et al., 2013). In contrast to the prior studies, however, when young animals were examined, they were found to have elevated LTP at perforant path ≫ dentate granule cell synapses. A pre-seizure increase in neuronal excitability of CA1 pyramidal cells and a reduced threshold for L-LTP in the Schaffer collateral pathway has also been noted in TSC1 KO mice following an acute deletion (Abs et al., 2013). Enhanced LTP, therefore, may be a primary effect of increased mTOR activation, while LTP impairments may reflect the consequences of secondary phenomena, such as seizures or structural abnormalities that alter network properties and KO cell behavior.
CELL INTRINSIC vs. PRIMARY NETWORK EFFECTS
Approaches in which PTEN or TSC deletion is sparsely induced in neuronal populations have significant advantages over more widespread deletions, as secondary changes, such as seizures, can be avoided. A “sparse deletion” approach was used by Sabatini’s group in an elegant series of papers in which TSC1 was deleted from brain and tissue of TSC1flox/flox mice using a Cre-expressing virus. In the first study, TSC1 was deleted from pyramidal cells in organotypic hippocampal explant cultures (Tavazoie et al., 2005). TSC1 KO cells in this tissue exhibited increased spine length and spine head width, decreased spine density and increased mEPSC amplitude with no change in frequency. In the follow-up study, a similar approach was used in vivo, but with very different results (Bateup et al., 2011). Pyramidal cells in this study did not exhibit any changes in spine morphology or density, mEPSC amplitude was unchanged, and mEPSC frequency was increased. Interestingly, mGluR-LTD was impaired, removing an important mechanism for mitigating excess excitation from TSC1 KO cells. Organotypic hippocampal cultures exhibit much higher activity levels than hippocampus in vivo, and develop seizure-like activity over time (Dyhrfjeld-Johnsen et al., 2010). TSC1 KO cells in vitro, therefore, experience a very different environment than similar KO cells in vivo, potentially explaining the contrasting behavior of the two populations. Consistent with this idea, Bateup et al. (2013a) were able to demonstrate in dissociated cultures of TSC1 KO neurons that pharmacologically blocking activity prevented many of the gene expression changes in these cells, suggesting that they occur secondarily to the increased activity levels evident in the TSC1 KO cultures.
While the majority of studies examining mTOR effects on neuronal function have examined excitatory signaling, it is important not to downplay the potential significance changes in inhibitory signaling might have. As a case in point, Bateup et al. (2013b) recently demonstrated that although isolated TSC1 KO CA1 pyramidal cells exhibit enhanced mEPSC frequency and impaired mGluR-LTD in vivo, the KO cells were intrinsically less excitable, leading to little net change in excitatory synaptic drive. Rather, they found that network hyperexcitability was likely produced by a significant reduction in inhibitory drive. TSC1 KO cells in these animals exhibited decreased frequency and amplitude of miniature inhibitory post-synaptic currents (mIPSCs) and reduced amplitude of evoked IPSCs. These effects could be blocked with rapamycin treatment, confirming that they are mediated by mTOR (Bateup et al., 2013b). Altered excitatory/inhibitory balance in the brain, in this case favoring increased excitability via reduced inhibition, might be an important mechanism by which changes in mTOR signaling promote epileptogenesis.
SUMMARY
Hyperactivation of the mTOR pathway produces a constellation of changes predicted to promote epileptogenesis and hyperexcitability. mTOR signaling can promote adult neurogenesis in the hippocampus, and many of these new cells can integrate abnormally. Increased neurogenesis occurs in most animal models of temporal lobe epilepsy and is suspected of contributing to epileptogenesis (Jung et al., 2004, 2006; Hester and Danzer, 2013). In transgenic mice with inactivation or removal of PTEN, TSC1, or TSC2, cell populations with the resulting mTOR hyperactivation have many common structural abnormalities, including somatic and dendritic hypertrophy, aberrant basal dendrites, and enlargement of axon tracts. Morphological changes are consistent with increased synaptogenesis and recurrent circuit formation – both changes predicted to be epileptogenic. At the synaptic level, transgenic mouse models with mTOR hyperactivation frequently, but not always, exhibit enhanced synaptic transmission and plasticity. The mTOR signaling pathway plays a critical role in regulating how a neuron responds to the network in which it is embedded, and correspondingly, the impact of disrupting the pathway appears to exhibit network specific effects. Moreover, large populations of PTEN and TSC KO cells have the ability to induce secondary changes among the networks they influence, which in turn may produce tertiary, feedback changes among the KO cells. The complexity of this system creates significant hurdles for understanding the mTOR pathway, and is likely responsible for many of the paradoxical findings, but future studies are likely to provide important new insights into the acute and chronic effects of disrupted mTOR signaling in the many diseases in which the path is now implicated.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was supported by the National Institute of Neurological Disorders and Stroke (SCD, Award Numbers R01NS065020 and R01NS062806; CLL, NRSA F32NS083239). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Neurological Disorders and Stroke or the National Institutes of Health. We would also like to thank Keri Kaeding for useful comments on earlier versions of this manuscript and Victor R. Santos, Raymund Y. K. Pun, and Isaiah Rolle for contributing images for figures.
REFERENCES
- Abs E., Goorden S. M., Schreiber J., Overwater I. E., Hoogeveen-Westerveld M., Bruinsma C. F., et al. (2013). TORC1-dependent epilepsy caused by acute biallelic Tsc1 deletion in adult mice. Ann. Neurol. 74 569–579 10.1002/ana.23943 [DOI] [PubMed] [Google Scholar]
- Amiri A., Cho W., Zhou J., Birnbaum S. G., Sinton C. M., McKay R. M., et al. (2012). Pten deletion in adult hippocampal neural stem/progenitor cells causes cellular abnormalities and alters neurogenesis. J. Neurosci. 32 5880–5890 10.1523/JNEUROSCI.5462-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arch E. M., Goodman B. K., Van Wesep R. A., Liaw D., Clarke K., Parsons R., et al. (1997). Deletion of PTEN in a patient with Bannayan–Riley–Ruvalcaba syndrome suggests allelism with Cowden disease. Am. J. Med. Genet. 71 489–493 [DOI] [PubMed] [Google Scholar]
- Backman S. A., Stambolic V., Suzuki A., Haight J., Elia A., Pretorius J., et al. (2001). Deletion of Pten in mouse brain causes seizures, ataxia and defects in soma size resembling Lhermitte–Duclos disease. Nat. Genet. 29 396–403 10.1038/ng782 [DOI] [PubMed] [Google Scholar]
- Bajenaru M. L., Zhu Y., Hedrick N. M., Donahoe J., Parada L. F., Gutmann D. H. (2002). Astrocyte-specific inactivation of the neurofibromatosis 1 gene (NF1) is insufficient for astrocytoma formation. Mol. Cell. Biol. 22 5100–5113 10.1128/MCB.22.14.5100-5113.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bateup H. S., Denefrio C. L., Johnson C. A., Saulnier J. L., Sabatini B. L. (2013a). Temporal dynamics of a homeostatic pathway controlling neural network activity. Front. Mol. Neurosci. 6:28 10.3389/fnmol.2013.00028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bateup H. S., Johnson C. A., Denefrio C. L., Saulnier J. L., Kornacker K., Sabatini B. L. (2013b). Excitatory/inhibitory synaptic imbalance leads to hippocampal hyperexcitability in mouse models of tuberous sclerosis. Neuron 78 510–522 10.1016/j.neuron.2013.03.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bateup H. S., Takasaki K. T., Saulnier J. L., Denefrio C. L., Sabatini B. L. (2011). Loss of Tsc1 in vivo impairs hippocampal mGluR-LTD and increases excitatory synaptic function. J. Neurosci. 31 8862–8869 10.1523/JNEUROSCI.1617-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck H., Goussakov I. V., Lie A., Helmstaedter C., Elger C. E. (2000). Synaptic plasticity in the human dentate gyrus. J. Neurosci. 20 7080–7086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berdichevsky Y., Dryer A. M., Saponjian Y., Mahoney M. M., Pimentel C. A., Lucini C. A., et al. (2013). PI3K–Akt signaling activates mTOR-mediated epileptogenesis in organotypic hippocampal culture model of post-traumatic epilepsy. J. Neurosci. 33 9056–9067 10.1523/JNEUROSCI.3870-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolshakov V. Y., Siegelbaum S. A. (1994). Postsynaptic induction and presynaptic expression of hippocampal long-term depression. Science 264 1148–1152 10.1126/science.7909958 [DOI] [PubMed] [Google Scholar]
- Bonilha L., Nesland T., Martz G. U., Joseph J. E., Spampinato M. V., Edwards J. C., et al. (2012). Medial temporal lobe epilepsy is associated with neuronal fibre loss and paradoxical increase in structural connectivity of limbic structures. J. Neurol. Neurosurg. Psychiatry 83 903–909 10.1136/jnnp-2012-302476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewster A. L., Lugo J. N., Patil V. V., Lee W. L., Qian Y., Vanegas F., et al. (2013). Rapamycin reverses status epilepticus-induced memory deficits and dendritic damage. PLoS ONE 8:e57808 10.1371/journal.pone.0057808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckmaster P. S. (2012). “Mossy fiber sprouting in the dentate gyrus,” in Jasper’s Basic Mechanisms of the Epilepsies [Internet] eds Noebels J. L., Avoli M., Rogawski M. A., Olsen R. W., Delgado-Escueta A. V. (Bethesda, MD: National Center for Biotechnology Information; ). [PubMed] [Google Scholar]
- Buckmaster P. S., Ingram E. A., Wen X. (2009). Inhibition of the mammalian target of rapamycin signaling pathway suppresses dentate granule cell axon sprouting in a rodent model of temporal lobe epilepsy. J. Neurosci. 29 8259–8269 10.1523/JNEUROSCI.4179-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckmaster P. S., Lew F. H. (2011). Rapamycin suppresses mossy fiber sprouting but not seizure frequency in a mouse model of temporal lobe epilepsy. J. Neurosci. 31 2337–2347 10.1523/JNEUROSCI.4852-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckmaster P. S., Wen X. (2011).Rapamycin suppresses axon sprouting by somatostatin interneurons in a mouse model of temporal lobe epilepsy. Epilepsia 52 2057–2064 10.1111/j.1528-1167.2011.03253.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler M. G., Dasouki M. J., Zhou X. P., Talebizadeh Z., Brown M., Takahashi T. N., et al. (2005). Subset of individuals with autism spectrum disorders and extreme macrocephaly associated with germline PTEN tumour suppressor gene mutations. J. Med. Genet. 42 318–321 10.1136/jmg.2004.024646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cammalleri M., Lutjens R., Berton F., King A. R., Simpson C., Francesconi W., et al. (2003). Time-restricted role for dendritic activation of the mTOR-p70S6K pathway in the induction of late-phase long-term potentiation in the CA1. Proc. Natl. Acad. Sci. U.S.A. 100 14368–14373 10.1073/pnas.2336098100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carson R. P., Van Nielen D. L., Winzenburger P. A., Ess K. C. (2012). Neuronal and glia abnormalities in Tsc1-deficient forebrain and partial rescue by rapamycin. Neurobiol. Dis. 45 369–380 10.1016/j.nbd.2011.08.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J., Tsai V., Parker W. E., Aronica E., Baybis M., Crino P. B. (2012). Detection of human papillomavirus in human focal cortical dysplasia type IIB. Ann. Neurol. 72 881–892 10.1002/ana.23795 [DOI] [PubMed] [Google Scholar]
- Chen S., Atkins C. M., Liu C. L., Alonso O. F., Dietrich W. D., Hu B. R. (2007). Alterations in mammalian target of rapamycin signaling pathways after traumatic brain injury. J. Cereb. Blood Flow Metab. 27 939–949 [DOI] [PubMed] [Google Scholar]
- Choi Y. J., Di Nardo A., Kramvis I., Meikle L., Kwiatkowski D. J., Sahin M., et al. (2008). Tuberous sclerosis complex proteins control axon formation. Genes Dev. 22 2485–2495 10.1101/gad.1685008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cloetta D., Thomanetz V., Baranek C., Lustenberger R. M., Lin S., Oliveri F., et al. (2013). Inactivation of mTORC1 in the developing brain causes microcephaly and affects gliogenesis. J. Neurosci. 33 7799–7810 10.1523/JNEUROSCI.3294-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cracco J. B., Serrano P., Moskowitz S. I., Bergold P. J., Sacktor T. C. (2005). Protein synthesis-dependent LTP in isolated dendrites of CA1 pyramidal cells. Hippocampus 15 551–556 10.1002/hipo.20078 [DOI] [PubMed] [Google Scholar]
- Crino P. B. (2009). Focal brain malformations: seizures, signaling, sequencing. Epilepsia 50 3–8 10.1111/j.1528-1167.2009.02289.x [DOI] [PubMed] [Google Scholar]
- Cuesto G., Enriquez-Barreto L., Carames C., Cantarero M., Gasull X., Sandi C., et al. (2011). Phosphoinositide-3-kinase activation controls synaptogenesis and spinogenesis in hippocampal neurons. J. Neurosci. 31 2721–2733 10.1523/JNEUROSCI.4477-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danzer S., He X., Loepke A., McNamara J. (2010). Structural plasticity of dentate granule cell mossy fibers during the development of limbic epilepsy. Hippocampus 20 113–124 10.1002/hipo.20589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danzer S. C. (2008). Postnatal and adult neurogenesis in the development of human disease. Neuroscientist 14 446–458 10.1177/1073858408317008 [DOI] [PubMed] [Google Scholar]
- Danzer S. C. (2012). Depression, stress, epilepsy and adult neurogenesis. Exp. Neurol. 233 22–32 10.1016/j.expneurol.2011.05.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyhrfjeld-Johnsen J., Berdichevsky Y., Swiercz W., Sabolek H., Staley K. J. (2010). Interictal spikes precede ictal discharges in an organotypic hippocampal slice culture model of epileptogenesis. J. Clin. Neurophysiol. 27 418–424 10.1097/WNP.0b013e3181fe0709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eng C. (2003). PTEN: one gene, many syndromes. Hum. Mutat. 22 183–198 10.1002/humu.10257 [DOI] [PubMed] [Google Scholar]
- Epi4K Consortium and Epilepsy Phenome/Genome Project. (2013). De novo mutations in epileptic encephalopathies. Nature 501 217–221 10.1038/nature12439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erbayat-Altay E., Zeng L. H., Xu L., Gutmann D. H., Wong M. (2007). The natural history and treatment of epilepsy in a murine model of tuberous sclerosis. Epilepsia 48 1470–1476 10.1111/j.1528-1167.2007.01110.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang T., Fang F., Zhu H., Zhang G., Liu Z., Li Y. (2013). Functional connectivity changes in patients with absence epilepsy studied using resting-state functional MRI. J. Clin. Neurosci. 20 413–418 10.1016/j.jocn.2012.02.044 [DOI] [PubMed] [Google Scholar]
- Fingar D. C., Blenis J. (2004). Target of rapamycin (TOR): an integrator of nutrient and growth factor signals and coordinator of cell growth and cell cycle progression. Oncogene 23 3151–3171 10.1038/sj.onc.1207542 [DOI] [PubMed] [Google Scholar]
- Fitzjohn S. M., Kingston A. E., Lodge D., Collingridge G. L. (1999). DHPG-induced LTD in area CA1 of juvenile rat hippocampus; characterisation and sensitivity to novel mGlu receptor antagonists. Neuropharmacology 38 1577–1583 10.1016/S0028-3908(99)00123-9 [DOI] [PubMed] [Google Scholar]
- Franz D. N., Bissler J. J., McCormack F. X. (2010). Tuberous sclerosis complex: neurological, renal and pulmonary manifestations. Neuropediatrics 41 199–208 10.1055/s-0030-1269906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser M. M., Bayazitov I. T., Zakharenko S. S., Baker S. J. (2008). Phosphatase and tensin homolog, deleted on chromosome 10 deficiency in brain causes defects in synaptic structure, transmission and plasticity, and myelination abnormalities. Neuroscience 151 476–488 10.1016/j.neuroscience.2007.10.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser M. M., Zhu X., Kwon C.-H., Uhlmann E. J., Gutmann D. H., Baker S. J. (2004). Pten loss causes hypertrophy and increased proliferation of astrocytes in vivo. Cancer Res. 64 7773–7779 10.1158/0008-5472.CAN-04-2487 [DOI] [PubMed] [Google Scholar]
- Frotscher M., Jonas P., Sloviter R. S. (2006). Synapses formed by normal and abnormal hippocampal mossy fibers. Cell Tissue Res. 326 361–367 10.1007/s00441-006-0269-2 [DOI] [PubMed] [Google Scholar]
- Gangloff Y. G., Mueller M., Dann S. G., Svoboda P., Sticker M., Spetz J. F., et al. (2004). Disruption of the mouse mTOR gene leads to early postimplantation lethality and prohibits embryonic stem cell development. Mol. Cell. Biol. 24 9508–9516 10.1128/MCB.24.21.9508-9516.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goffin A., Hoefsloot L. H., Bosgoed E., Swillen A., Fryns J. P. (2001). PTEN mutation in a family with Cowden syndrome and autism. Am. J. Med. Genet. 105 521–524 10.1002/ajmg.1477 [DOI] [PubMed] [Google Scholar]
- Goussakov I. V., Fink K., Elger C. E., Beck H. (2000). Metaplasticity of mossy fiber synaptic transmission involves altered release probability. J. Neurosci. 20 3434–3441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graber K. D., Prince D. A. (2004). A critical period for prevention of posttraumatic neocortical hyperexcitability in rats. Ann. Neurol. 55 860–870 10.1002/ana.20124 [DOI] [PubMed] [Google Scholar]
- Gregorian C., Nakashima J., Le Belle J., Ohab J., Kim R., Liu A., et al. (2009). Pten deletion in adult neural stem/progenitor cells enhances constitutive neurogenesis. J. Neurosci. 29 1874–1886 10.1523/JNEUROSCI.3095-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groszer M., Erickson R., Scripture-Adams D. D., Lesche R., Trumpp A., Zack J. A., et al. (2001). Negative regulation of neural stem/progenitor cell proliferation by the Pten tumor suppressor gene in vivo. Science 294 2186–2189 10.1126/science.1065518 [DOI] [PubMed] [Google Scholar]
- Guertin D. A., Stevens D. M., Thoreen C. C., Burds A. A., Kalaany N. Y., Moffat J., et al. (2006). Ablation in mice of the mTORC components raptor, rictor, or mLST8 reveals that mTORC2 is required for signaling to Akt-FOXO and PKCalpha, but not S6K1. Dev. Cell 11 859–871 10.1016/j.devcel.2006.10.007 [DOI] [PubMed] [Google Scholar]
- Haneef Z., Lenartowicz A., Yeh H. J., Levin H. S., Engel J. Jr., Stern J. M. (2014). Functional connectivity of hippocampal networks in temporal lobe epilepsy. Epilepsia 55 137–145 10.1111/epi.12476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartman N. W., Lin T. V., Zhang L., Paquelet G. E., Feliciano D. M., Bordey A. (2013). mTORC1 targets the translational repressor 4E-BP2, but not S6 kinase 1/2, to regulate neural stem cell self-renewal in vivo. Cell Rep. 5 433–444 10.1016/j.celrep.2013.09.017 [DOI] [PubMed] [Google Scholar]
- Hattiangady B., Shetty A. K. (2010). Decreased neuronal differentiation of newly generated cells underlies reduced hippocampal neurogenesis in chronic temporal lobe epilepsy. Hippocampus 20 97–112 10.1002/hipo.20594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauptman J. S., Mathern G. W. (2012). Surgical treatment of epilepsy associated with cortical dysplasia: 2012 update. Epilepsia 53(Suppl. 4) 98–104 10.1111/j.1528-1167.2012.03619.x [DOI] [PubMed] [Google Scholar]
- Haws M. E., Jaramillo T. C., Espinosa F., Widman J. A., Stuber G. D., Sparta D. R., et al. (2014). PTEN knockdown alters dendritic spine/protrusion morphology, not density. J. Comp. Neurol. 522 1171–1190 10.1002/cne.23488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heng K., Haney M. M., Buckmaster P. S. (2013). High-dose rapamycin blocks mossy fiber sprouting but not seizures in a mouse model of temporal lobe epilepsy. Epilepsia 54 1535–1541 10.1111/epi.12246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman G. E., Butter E., Enrile B., Pastore M., Prior T. W., Sommer A. (2007). Increasing knowledge of PTEN germline mutations: two additional patients with autism and macrocephaly. Am. J. Med. Genet. A 143 589–593 10.1002/ajmg.a.31619 [DOI] [PubMed] [Google Scholar]
- Hester M. S., Danzer S. C. (2013). Accumulation of abnormal adult-generated hippocampal granule cells predicts seizure frequency and severity. J. Neurosci. 33 8926–8936 10.1523/JNEUROSCI.5161-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes G. L., Stafstrom C. E. (2007). Tuberous sclerosis complex and epilepsy: recent developments and future challenges. Epilepsia 48 617–630 10.1111/j.1528-1167.2007.01035.x [DOI] [PubMed] [Google Scholar]
- Hou L., Klann E. (2004). Activation of the phosphoinositide 3-kinase-Akt-mammalian target of rapamycin signaling pathway is required for metabotropic glutamate receptor-dependent long-term depression. J. Neurosci. 24 6352–6361 10.1523/JNEUROSCI.0995-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W., Zhu P. J., Zhang S., Zhou H., Stoica L., Galiano M., et al. (2013). mTORC2 controls actin polymerization required for consolidation of long-term memory. Nat. Neurosci. 16 441–448 10.1038/nn.3351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X., Zhang H., Yang J., Wu J., McMahon J., Lin Y., et al. (2010). Pharmacological inhibition of the mammalian target of rapamycin pathway suppresses acquired epilepsy. Neurobiol. Dis. 40 193–199 10.1016/j.nbd.2010.05.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber K. M., Roder J. C., Bear M. F. (2001). Chemical induction of mGluR5- and protein synthesis-dependent long-term depression in hippocampal area CA1. J. Neurophysiol. 86 321–325 [DOI] [PubMed] [Google Scholar]
- Jacinto E., Loewith R., Schmidt A., Lin S., Ruegg M. A., Hall A., et al. (2004). Mammalian TOR complex 2 controls the actin cytoskeleton and is rapamycin insensitive. Nat. Cell Biol. 6 1122–1128 10.1038/ncb1183 [DOI] [PubMed] [Google Scholar]
- Jaworski J., Spangler S., Seeburg D. P., Hoogenraad C. C., Sheng M. (2005). Control of dendritic arborization by the phosphoinositide-3′-kinase-Akt-mammalian target of rapamycin pathway. J. Neurosci. 25 11300–11312 10.1523/JNEUROSCI.2270-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessberger S., Zhao C., Toni N., Clemenson G., Li Y., Gage F. (2007). Seizure-associated, aberrant neurogenesis in adult rats characterized with retrovirus-mediated cell labeling. J. Neurosci. 27 9400–9407 10.1523/JNEUROSCI.2002-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao Y., Nadler J. V. (2007). Stereological analysis of GluR2-immunoreactive hilar neurons in the pilocarpine model of temporal lobe epilepsy: correlation of cell loss with mossy fiber sprouting. Exp. Neurol. 205 569–582 10.1016/j.expneurol.2007.03.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jinde S., Zsiros V., Jiang Z., Nakao K., Pickel J., Kohno K., et al. (2012). Hilar mossy cell degeneration causes transient dentate granule cell hyperexcitability and impaired pattern separation. Neuron 76 1189–1200 10.1016/j.neuron.2012.10.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung K.-H., Chu K., Kim M., Jeong S.-W., Song Y.-M., Lee S.-T., et al. (2004). Continuous cytosine-b-D-arabinofuranoside infusion reduces ectopic granule cells in adult rat hippocampus with attenuation of spontaneous recurrent seizures following pilocarpine-induced status epilepticus. Eur. J. Neurosci. 19 3219–3226 10.1111/j.0953-816X.2004.03412.x [DOI] [PubMed] [Google Scholar]
- Jung K. H., Chu K., Lee S. T., Kim J., Sinn D. I., Kim J. M., et al. (2006). Cyclooxygenase-2 inhibitor, celecoxib, inhibits the altered hippocampal neurogenesis with attenuation of spontaneous recurrent seizures following pilocarpine-induced status epilepticus. Neurobiol. Dis. 23 237–246 10.1016/j.nbd.2006.02.016 [DOI] [PubMed] [Google Scholar]
- Jurado S., Benoist M., Lario A., Knafo S., Petrok C. N., Esteban J. A. (2010). PTEN is recruited to the postsynaptic terminal for NMDA receptor-dependent long-term depression. EMBO J. 29 2827–2840 10.1038/emboj.2010.160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J. I., Lee H. R., Sim S. E., Baek J., Yu N. K., Choi J. H., et al. (2011). PI3Kgamma is required for NMDA receptor-dependent long-term depression and behavioral flexibility. Nat. Neurosci. 14 1447–1454 10.1038/nn.2937 [DOI] [PubMed] [Google Scholar]
- Kirschstein T., Bauer M., Muller L., Ruschenschmidt C., Reitze M., Becker A. J., et al. (2007). Loss of metabotropic glutamate receptor-dependent long-term depression via downregulation of mGluR5 after status epilepticus. J. Neurosci. 27 7696–7704 10.1523/JNEUROSCI.4572-06.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi T., Minowa O., Kuno J., Mitani H., Hino O., Noda T. (1999). Renal carcinogenesis, hepatic hemangiomatosis, and embryonic lethality caused by a germ-line Tsc2 mutation in mice. Cancer Res. 59 1206–1211 [PubMed] [Google Scholar]
- Koike-Kumagai M., Yasunaga K., Morikawa R., Kanamori T., Emoto K. (2009). The target of rapamycin complex 2 controls dendritic tiling of Drosophila sensory neurons through the tricornered kinase signalling pathway. EMBO J. 28 3879–3892 10.1038/emboj.2009.312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotulska K., Borkowska J., Jozwiak S. (2013). Possible prevention of tuberous sclerosis complex lesions. Pediatrics 132 e239–e242 10.1542/peds.2012-3607 [DOI] [PubMed] [Google Scholar]
- Kron M. M., Zhang H., Parent J. M. (2010). The developmental stage of dentate granule cells dictates their contribution to seizure-induced plasticity. J. Neurosci. 30 2051–2059 10.1523/JNEUROSCI.5655-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger D. A., Franz D. N. (2008). Current management of tuberous sclerosis complex. Paediatr. Drugs 10 299–313 10.2165/00148581-200810050-00004 [DOI] [PubMed] [Google Scholar]
- Krueger D. A., Wilfong A. A., Holland-Bouley K., Anderson A. E., Agricola K., Tudor C., et al. (2013). Everolimus treatment of refractory epilepsy in tuberous sclerosis complex. Ann. Neurol. 74 679–687 10.1002/ana.23960 [DOI] [PubMed] [Google Scholar]
- Kwiatkowski D. J., Zhang H., Bandura J. L., Heiberger K. M., Glogauer M., el-Hashemite N., et al. (2002). A mouse model of TSC1 reveals sex-dependent lethality from liver hemangiomas, and up-regulation of p70S6 kinase activity in Tsc1 null cells. Hum. Mol. Genet. 11 525–534 10.1093/hmg/11.5.525 [DOI] [PubMed] [Google Scholar]
- Kwon C. H., Luikart B. W., Powell C. M., Zhou J., Matheny S. A., Zhang W., et al. (2006). Pten regulates neuronal arborization and social interaction in mice. Neuron 50 377–388 10.1016/j.neuron.2006.03.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon C. H., Zhu X., Zhang J., Baker S. J. (2003). mTor is required for hypertrophy of Pten-deficient neuronal soma in vivo. Proc. Natl. Acad. Sci. U.S.A. 100 12923–12928 10.1073/pnas.2132711100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon C. H., Zhu X., Zhang J., Knoop L. L., Tharp R., Smeyne R. J., et al. (2001). Pten regulates neuronal soma size: a mouse model of Lhermitte–Duclos disease. Nat. Genet. 29 404–411 10.1038/ng781 [DOI] [PubMed] [Google Scholar]
- Liaw D., Marsh D. J., Li J., Dahia P. L., Wang S. I., Zheng Z., et al. (1997). Germline mutations of the PTEN gene in Cowden disease, an inherited breast and thyroid cancer syndrome. Nat. Genet. 16 64–67 10.1038/ng0597-64 [DOI] [PubMed] [Google Scholar]
- Lim K. C., Crino P. B. (2013). Focal malformations of cortical development: new vistas for molecular pathogenesis. Neuroscience 252 262–276 10.1016/j.neuroscience.2013.07.037 [DOI] [PubMed] [Google Scholar]
- Ljungberg M. C., Sunnen C. N., Lugo J. N., Anderson A. E., D’Arcangelo G. (2009). Rapamycin suppresses seizures and neuronal hypertrophy in a mouse model of cortical dysplasia. Dis. Model. Mech. 2 389–398 10.1242/dmm.002386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luikart B. W., Schnell E., Washburn E. K., Bensen A. L., Tovar K. R., Westbrook G. L. (2011). Pten knockdown in vivo increases excitatory drive onto dentate granule cells. J. Neurosci. 31 4345–4354 10.1523/JNEUROSCI.0061-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macias M., Blazejczyk M., Kazmierska P., Caban B., Skalecka A., Tarkowski B., et al. (2013). Spatiotemporal characterization of mTOR kinase activity following kainic acid induced status epilepticus and analysis of rat brain response to chronic rapamycin treatment. PLoS ONE 8:e64455 10.1371/journal.pone.0064455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magri L., Cambiaghi M., Cominelli M., Alfaro-Cervello C., Cursi M., Pala M., et al. (2011). Sustained activation of mTOR pathway in embryonic neural stem cells leads to development of tuberous sclerosis complex-associated lesions. Cell Stem Cell 9 447–462 10.1016/j.stem.2011.09.008 [DOI] [PubMed] [Google Scholar]
- Magri L., Cominelli M., Cambiaghi M., Cursi M., Leocani L., Minicucci F., et al. (2013). Timing of mTOR activation affects tuberous sclerosis complex neuropathology in mouse models. Dis. Model. Mech. 6 1185–1197 10.1242/dmm.012096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magri L., Galli R. (2013). mTOR signaling in neural stem cells: from basic biology to disease. Cell. Mol. Life Sci. 70 2887–2898 10.1007/s00018-012-1196-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marino S., Krimpenfort P., Leung C., van der Korput H. A. G. M., Trapman J., Camenisch I., et al. (2002). PTEN is essential for cell migration but not for fate determination and tumourigenesis in the cerebellum. Development 129 3513–3522 [DOI] [PubMed] [Google Scholar]
- Marsh D. J., Kum J. B., Lunetta K. L., Bennett M. J., Gorlin R. J., Ahmed S. F., et al. (1999). PTEN mutation spectrum and genotype-phenotype correlations in Bannayan–Riley–Ruvalcaba syndrome suggest a single entity with Cowden syndrome. Hum. Mol. Genet. 8 1461–1472 10.1093/hmg/8.8.1461 [DOI] [PubMed] [Google Scholar]
- McAuliffe J. J., Bronson S. L., Hester M. S., Murphy B. L., Dahlquist-Topala R., Richards D. A., et al. (2011). Altered patterning of dentate granule cell mossy fiber inputs onto CA3 pyramidal cells in limbic epilepsy. Hippocampus 21 93–107 10.1002/hipo.20726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meador K. J. (2007). The basic science of memory as it applies to epilepsy. Epilepsia 48 (Suppl. 9) 23–25 10.1111/j.1528-1167.2007.01396.x [DOI] [PubMed] [Google Scholar]
- Meikle L., Pollizzi K., Egnor A., Kramvis I., Lane H., Sahin M., et al. (2008). Response of a neuronal model of tuberous sclerosis to mammalian target of rapamycin (mTOR) inhibitors: effects on mTORC1 and Akt signaling lead to improved survival and function. J. Neurosci. 28 5422–5432 10.1523/JNEUROSCI.0955-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meikle L., Talos D. M., Onda H., Pollizzi K., Rotenberg A., Sahin M., et al. (2007). A mouse model of tuberous sclerosis: neuronal loss of Tsc1 causes dysplastic and ectopic neurons, reduced myelination, seizure activity, and limited survival. J. Neurosci. 27 5546–5558 10.1523/JNEUROSCI.5540-06.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulkey R. M., Malenka R. C. (1992). Mechanisms underlying induction of homosynaptic long-term depression in area CA1 of the hippocampus. Neuron 9 967–975 10.1016/0896-6273(92)90248-C [DOI] [PubMed] [Google Scholar]
- Muncy J., Butler I. J., Koenig M. K. (2009). Rapamycin reduces seizure frequency in tuberous sclerosis complex. J. Child Neurol. 24 477 10.1177/0883073808324535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami M., Ichisaka T., Maeda M., Oshiro N., Hara K., Edenhofer F., et al. (2004). mTOR is essential for growth and proliferation in early mouse embryos and embryonic stem cells. Mol. Cell. Biol. 24 6710–6718 10.1128/MCB.24.15.6710-6718.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy B. L., Danzer S. C. (2011). Somatic translocation: a novel mechanism of granule cell dendritic dysmorphogenesis and dispersion. J. Neurosci. 31 2959–2964 10.1523/JNEUROSCI.3381-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy B. L., Hofacer R. D., Faulkner C. N., Loepke A. W., Danzer S. C. (2012). Abnormalities of granule cell dendritic structure are a prominent feature of the intrahippocampal kainic acid model of epilepsy despite reduced postinjury neurogenesis. Epilepsia 53 908–921 10.1111/j.1528-1167.2012.03463.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy B. L., Pun R. Y., Yin H., Faulkner C. R., Loepke A. W., Danzer S. C. (2011). Heterogeneous integration of adult-generated granule cells into the epileptic brain. J. Neurosci. 31 105–117 10.1523/JNEUROSCI.2728-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadler J. V. (2003). The recurrent mossy fiber pathway of the epileptic brain. Neurochem. Res. 28 1649–1658 10.1023/A:1026004904199 [DOI] [PubMed] [Google Scholar]
- Nakahara S., Tamura M., Matsuki N., Koyama R. (2009). Neuronal hyperactivity sustains the basal dendrites of immature dentate granule cells: time-lapse confocal analysis using hippocampal slice cultures. Hippocampus 19 379–391 10.1002/hipo.20529 [DOI] [PubMed] [Google Scholar]
- Okamoto O. K., Janjoppi L., Bonone F. M., Pansani A. P., da Silva A. V., Scorza F. A., et al. (2010). Whole transcriptome analysis of the hippocampus: toward a molecular portrait of epileptogenesis. BMC Genomics 11:230 10.1186/1471-2164-11-230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliet S. H., Malenka R. C., Nicoll R. A. (1997). Two distinct forms of long-term depression coexist in CA1 hippocampal pyramidal cells. Neuron 18 969–982 10.1016/S0896-6273(00)80336-0 [DOI] [PubMed] [Google Scholar]
- Onda H., Lueck A., Marks P. W., Warren H. B., Kwiatkowski D. J. (1999). Tsc2(+/-) mice develop tumors in multiple sites that express gelsolin and are influenced by genetic background. J. Clin. Invest. 104 687–695 10.1172/JCI7319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Roak B. J., Vives L., Fu W., Egertson J. D., Stanaway I. B., Phelps I. G., et al. (2012). Multiplex targeted sequencing identifies recurrently mutated genes in autism spectrum disorders. Science 338 1619–1622 10.1126/science.1227764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overstreet-Wadiche L. S., Bromberg D. A., Bensen A. L., Westbrook G. L. (2006). Seizures accelerate functional integration of adult-generated granule cells. J. Neurosci. 26 4095–4103 10.1523/JNEUROSCI.5508-05.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paliouras G. N., Hamilton L. K., Aumont A., Joppe S. E., Barnabe-Heider F., Fernandes K. J. (2012). Mammalian target of rapamycin signaling is a key regulator of the transit-amplifying progenitor pool in the adult and aging forebrain. J. Neurosci. 32 15012–15026 10.1523/JNEUROSCI.2248-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer M. J., Irving A. J., Seabrook G. R., Jane D. E., Collingridge G. L. (1997). The group I mGlu receptor agonist DHPG induces a novel form of LTD in the CA1 region of the hippocampus. Neuropharmacology 36 1517–1532 10.1016/S0028-3908(97)00181-0 [DOI] [PubMed] [Google Scholar]
- Parent J. M., Elliott R. C., Pleasure S. J., Barbaro N. M., Lowenstein D. H. (2006). Aberrant seizure-induced neurogenesis in experimental temporal lobe epilepsy. Ann. Neurol. 59 81–91 10.1002/ana.20699 [DOI] [PubMed] [Google Scholar]
- Parent J. M., Kron M. M. (2012). “Neurogenesis and Epilepsy,” in Jasper’s Basic Mechanisms of the Epilepsies eds Noebels L., Avoli M., Rogawski M. A., Olsen R. W., Delgado-Escueta A. V. (Bethesda, MD: National Center for Biotechnology Information; ). [PubMed] [Google Scholar]
- Parent J. M., Yu T. W., Leibowitz R. T., Geschwind D. H., Sloviter R. S., Lowenstein D. H. (1997). Dentate granule cell neurogenesis is increased by seizures and contributes to aberrant network reorganization in the adult rat hippocampus. J. Neurosci. 17 3727–3738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peltier J., O’Neill A., Schaffer D. V. (2007). PI3K/Akt and CREB regulate adult neural hippocampal progenitor proliferation and differentiation. Dev. Neurobiol. 67 1348–1361 10.1002/dneu.20506 [DOI] [PubMed] [Google Scholar]
- Pierce J. P., Milner T. A. (2001). Parallel increases in the synaptic and surface areas of mossy fiber terminals following seizure induction. Synapse 39 249–256 [DOI] [PubMed] [Google Scholar]
- Podsypanina K., Ellenson L. H., Nemes A., Gu J., Tamura M., Yamada K. M., et al. (1999). Mutation of Pten/Mmac1 in mice causes neoplasia in multiple organ systems. Proc. Natl. Acad. Sci. U.S.A. 96 1563–1568 10.1073/pnas.96.4.1563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pun R. Y., Rolle I. J., Lasarge C. L., Hosford B. E., Rosen J. M., Uhl J. D., et al. (2012). Excessive activation of mTOR in postnatally generated granule cells is sufficient to cause epilepsy. Neuron 75 1022–1034 10.1016/j.neuron.2012.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahimi O., Claiborne B. J. (2007). Morphological development and maturation of granule neuron dendrites in the rat dentate gyrus. Prog. Brain Res. 163 167–181 10.1016/S0079-6123(07)63010-6 [DOI] [PubMed] [Google Scholar]
- Rall W. (2011). Core Conductor Theory and Cable Properties of Neurons. Comprehensive Physiology. New York: John Wiley & Sons Inc; 10.1002/cphy.cp010103 [DOI] [Google Scholar]
- Raman L., Kong X., Kernie S. G. (2013). Pharmacological inhibition of the mTOR pathway impairs hippocampal development in mice. Neurosci. Lett. 541 9–14 10.1016/j.neulet.2013.01.045 [DOI] [PubMed] [Google Scholar]
- Ribak C. E., Shapiro L. A., Yan X. X., Dashtipour K., Nadler J. V., Obenaus A., et al. (2012). “Seizure-induced formation of basal dendrites on granule cells of the rodent dentate gyrus,” in Jasper’s Basic Mechanisms of the Epilepsies eds Noebels J. L., Avoli M., Rogawski M. A., Olsen R. W., Delgado-Escueta A. V. (Bethesda, MD: National Center for Biotechnology Information; ). [PubMed] [Google Scholar]
- Ribak C. E., Tran P. H., Spigelman I., Okazaki M. M., Nadler J. V. (2000). Status epilepticus-induced hilar basal dendrites on rodent granule cells contribute to recurrent excitatory circuitry. J. Comp. Neurol. 428 240–253 [DOI] [PubMed] [Google Scholar]
- Ryther R. C., Wong M. (2012). Mammalian target of rapamycin (mTOR) inhibition: potential for antiseizure, antiepileptogenic, and epileptostatic therapy. Curr. Neurol. Neurosci. Rep. 12 410–418 10.1007/s11910-012-0276-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos V. R., de Castro O. W., Pun R. Y., Hester M. S., Murphy B. L., Loepke A. W., et al. (2011). Contributions of mature granule cells to structural plasticity in temporal lobe epilepsy. Neuroscience 197 348–357 10.1016/j.neuroscience.2011.09.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarbassov D. D., Ali S. M., Sabatini D. M. (2005). Growing roles for the mTOR pathway. Curr. Opin. Cell Biol. 17 596–603 10.1016/j.ceb.2005.09.009 [DOI] [PubMed] [Google Scholar]
- Scharfman H. E., Goodman J. H., Sollas A. L. (2000). Granule-like neurons at the hilar/CA3 border after status epilepticus and their synchrony with area CA3 pyramidal cells: functional implications of seizure-induced neurogenesis. J. Neurosci. 20 6144–6158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharfman H. E., Hen R. (2007). Neuroscience. Is more neurogenesis always better? Science 315 336–338 10.1126/science.1138711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharfman H. E., McCloskey D. P. (2009). Postnatal neurogenesis as a therapeutic target in temporal lobe epilepsy. Epilepsy Res. 85 150–161 10.1016/j.eplepsyres.2009.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnabel R., Kilpatrick I. C., Collingridge G. L. (1999). An investigation into signal transduction mechanisms involved in DHPG-induced LTD in the CA1 region of the hippocampus. Neuropharmacology 38 1585–1596 10.1016/S0028-3908(99)00062-3 [DOI] [PubMed] [Google Scholar]
- Sha L. Z., Xing X. L., Zhang D., Yao Y., Dou W. C., Jin L. R., et al. (2012). Mapping the spatio-temporal pattern of the mammalian target of rapamycin (mTOR) activation in temporal lobe epilepsy. PLoS ONE 7:e39152 10.1371/journal.pone.0039152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiota C., Woo J.-T., Lindner J., Shelton K. D., Magnuson M. A. (2006). Multiallelic disruption of the rictor gene in mice reveals that mTOR complex 2 is essential for fetal growth and viability. Dev. Cell 11 583–589 10.1016/j.devcel.2006.08.013 [DOI] [PubMed] [Google Scholar]
- Singh S. P., He X., McNamara J. O., Danzer S. C. (2013). Morphological changes among hippocampal dentate granule cells exposed to early kindling-epileptogenesis. Hippocampus 23 1309–1320 10.1002/hipo.22169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloviter R. S., Zappone C. A., Harvey B. D., Frotscher M. (2006). Kainic acid-induced recurrent mossy fiber innervation of dentate gyrus inhibitory interneurons: possible anatomical substrate of granule cell hyper-inhibition in chronically epileptic rats. J. Comp. Neurol. 494 944–960 10.1002/cne.20850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sosunov A. A., Wu X., McGovern R. A., Coughlin D. G., Mikell C. B., Goodman R. R., et al. (2012). The mTOR pathway is activated in glial cells in mesial temporal sclerosis. Epilepsia 53 (Suppl. 1) 78–86 10.1111/j.1528-1167.2012.03478.x [DOI] [PubMed] [Google Scholar]
- Sparagana S. P., Roach E. S. (2000). Tuberous sclerosis complex. Curr. Opin. Neurol. 13 115–119 10.1097/00019052-200004000-00001 [DOI] [PubMed] [Google Scholar]
- Sperow M., Berry R. B., Bayazitov I. T., Zhu G., Baker S. J., Zakharenko S. S. (2012). Phosphatase and tensin homologue (PTEN) regulates synaptic plasticity independently of its effect on neuronal morphology and migration. J. Physiol. 590 777–792 10.1113/jphysiol.2011.220236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoica L., Zhu P. J., Huang W., Zhou H., Kozma S. C., Costa-Mattioli M. (2011). Selective pharmacogenetic inhibition of mammalian target of rapamycin complex I (mTORC1) blocks long-term synaptic plasticity and memory storage. Proc. Natl. Acad. Sci. U.S.A. 108 3791–3796 10.1073/pnas.1014715108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su M., Hu H., Lee Y., d’Azzo A., Messing A., Brenner M. (2004). Expression specificity of GFAP transgenes. Neurochem. Res. 29 2075–2093 10.1007/s11064-004-6881-1 [DOI] [PubMed] [Google Scholar]
- Sun H., Kosaras B., Klein P. M., Jensen F. E. (2013). Mammalian target of rapamycin complex 1 activation negatively regulates Polo-like kinase 2-mediated homeostatic compensation following neonatal seizures. Proc. Natl. Acad. Sci. U.S.A. 110 5199–5204 10.1073/pnas.1208010110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutula T., Cascino G., Cavazos J., Parada I., Ramirez L. (1989). Mossy fiber synaptic reorganization in the epileptic human temporal lobe. Ann. Neurol. 26 321–330 10.1002/ana.410260303 [DOI] [PubMed] [Google Scholar]
- Suzuki A., de la Pompa J. L., Stambolic V., Elia A. J., Sasaki T., del Barco Barrantes I., et al. (1998). High cancer susceptibility and embryonic lethality associated with mutation of the PTEN tumor suppressor gene in mice. Curr. Biol. 8 1169–1178 10.1016/S0960-9822(07)00488-5 [DOI] [PubMed] [Google Scholar]
- Suzuki F., Junier M. P., Guilhem D., Sorensen J. C., Onteniente B. (1995). Morphogenetic effect of kainate on adult hippocampal neurons associated with a prolonged expression of brain-derived neurotrophic factor. Neuroscience 64 665–674 10.1016/0306-4522(94)00463-F [DOI] [PubMed] [Google Scholar]
- Swann J. W., Al-Noori S., Jiang M., Lee C. L. (2000). Spine loss and other dendritic abnormalities in epilepsy. Hippocampus 10 617–625 [DOI] [PubMed] [Google Scholar]
- Takeuchi K., Gertner M. J., Zhou J., Parada L. F., Bennett M. V., Zukin R. S. (2013). Dysregulation of synaptic plasticity precedes appearance of morphological defects in a Pten conditional knockout mouse model of autism. Proc. Natl. Acad. Sci. U.S.A. 110 4738–4743 10.1073/pnas.1222803110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talos D. M., Sun H., Zhou X., Fitzgerald E. C., Jackson M. C., Klein P. M., et al. (2012). The interaction between early life epilepsy and autistic-like behavioral consequences: a role for the mammalian target of rapamycin (mTOR) pathway. PLoS ONE 7:e35885 10.1371/journal.pone.0035885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang S. J., Reis G., Kang H., Gingras A. C., Sonenberg N., Schuman E. M. (2002). A rapamycin-sensitive signaling pathway contributes to long-term synaptic plasticity in the hippocampus. Proc. Natl. Acad. Sci. U.S.A. 99 467–472 10.1073/pnas.012605299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavazoie S. F., Alvarez V. A., Ridenour D. A., Kwiatkowski D. J., Sabatini B. L. (2005). Regulation of neuronal morphology and function by the tumor suppressors Tsc1 and Tsc2. Nat. Neurosci. 8 1727–1734 10.1038/nn1566 [DOI] [PubMed] [Google Scholar]
- Terashima A., Taniguchi T., Nakai M., Yasuda M., Kawamata T., Tanaka C. (2000). Rapamycin and FK506 induce long-term potentiation by pairing stimulation via an intracellular Ca(2+) signaling mechanism in rat hippocampal CA1 neurons. Neuropharmacology 39 1920–1928 10.1016/S0028-3908(00)00015-0 [DOI] [PubMed] [Google Scholar]
- Thind K. K., Ribak C. E., Buckmaster P. S. (2008). Synaptic input to dentate granule cell basal dendrites in a rat model of temporal lobe epilepsy. J. Comp. Neurol. 509 190–202 10.1002/cne.21745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomanetz V., Angliker N., Cloetta D., Lustenberger R. M., Schweighauser M., Oliveri F., et al. (2013). Ablation of the mTORC2 component rictor in brain or Purkinje cells affects size and neuron morphology. J. Cell Biol. 201 293–308 10.1083/jcb.201205030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokuda S., Mahaffey C. L., Monks B., Faulkner C. R., Birnbaum M. J., Danzer S. C., et al. (2011). A novel Akt3 mutation associated with enhanced kinase activity and seizure susceptibility in mice. Hum. Mol. Genet. 20 988–999 10.1093/hmg/ddq544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai P. T., Hull C., Chu Y., Greene-Colozzi E., Sadowski A. R., Leech J. M., et al. (2012). Autistic-like behaviour and cerebellar dysfunction in Purkinje cell Tsc1 mutant mice. Nature 488 647–651 10.1038/nature11310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai V., Parker W. E., Orlova K. A., Baybis M., Chi A. W., Berg B. D., et al. (2014). Fetal brain mTOR signaling activation in tuberous sclerosis complex. Cereb. Cortex 24 315–327 10.1093/cercor/bhs310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsokas P., Grace E. A., Chan P., Ma T., Sealfon S. C., Iyengar R., et al. (2005). Local protein synthesis mediates a rapid increase in dendritic elongation factor 1A after induction of late long-term potentiation. J. Neurosci. 25 5833–5843 10.1523/JNEUROSCI.0599-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlmann E. J., Wong M., Baldwin R. L., Bajenaru M. L., Onda H., Kwiatkowski D. J., et al. (2002). Astrocyte-specific TSC1 conditional knockout mice exhibit abnormal neuronal organization and seizures. Ann. Neurol. 52 285–296 10.1002/ana.10283 [DOI] [PubMed] [Google Scholar]
- Upreti C., Otero R., Partida C., Skinner F., Thakker R., Pacheco L. F., et al. (2012). Altered neurotransmitter release, vesicle recycling and presynaptic structure in the pilocarpine model of temporal lobe epilepsy. Brain 135(Pt 3) 869–885 10.1093/brain/awr341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbanska M., Gozdz A., Swiech L. J., Jaworski J. (2012). Mammalian target of rapamycin complex 1 (mTORC1) and 2 (mTORC2) control the dendritic arbor morphology of hippocampal neurons. J. Biol. Chem. 287 30240–30256 10.1074/jbc.M112.374405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vickers C. A., Dickson K. S., Wyllie D. J. (2005). Induction and maintenance of late-phase long-term potentiation in isolated dendrites of rat hippocampal CA1 pyramidal neurones. J. Physiol. 568(Pt 3) 803–813 10.1113/jphysiol.2005.092924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter C., Murphy B. L., Pun R. Y. K., Spieles-Engemann A. L., Danzer S. C. (2007). Pilocarpine-induced seizures cause selective time-dependent changes to adult-generated hippocampal dentate granule cells. J. Neurosci. 27 7541–7552 10.1523/JNEUROSCI.0431-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Cheng A., Mattson M. P. (2006). The PTEN phosphatase is essential for long-term depression of hippocampal synapses. Neuromol. Med. 8 329–336 10.1385/NMM:8:3:329 [DOI] [PubMed] [Google Scholar]
- Wang Y., Greenwood J. S., Calcagnotto M. E., Kirsch H. E., Barbaro N. M., Baraban S. C. (2007). Neocortical hyperexcitability in a human case of tuberous sclerosis complex and mice lacking neuronal expression of TSC1. Ann. Neurol. 61 139–152 10.1002/ana.21058 [DOI] [PubMed] [Google Scholar]
- Way S. W., McKenna J. III. Mietzsch U., Reith R. M., Wu H. C., Gambello M. J. (2009). Loss of Tsc2 in radial glia models the brain pathology of tuberous sclerosis complex in the mouse. Hum. Mol. Genet. 18 1252–1265 10.1093/hmg/ddp025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Way S. W., Rozas N. S., Wu H. C., McKenna J. III, Reith R. M., Hashmi S. S., et al. (2012). The differential effects of prenatal and/or postnatal rapamycin on neurodevelopmental defects and cognition in a neuroglial mouse model of tuberous sclerosis complex. Hum. Mol. Genet. 21 3226–3236 10.1093/hmg/dds156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White R., Hua Y., Scheithauer B., Lynch D. R., Henske E. P., Crino P. B. (2001). Selective alterations in glutamate and GABA receptor subunit mRNA expression in dysplastic neurons and giant cells of cortical tubers. Ann. Neurol. 49 67–78 [DOI] [PubMed] [Google Scholar]
- Wong M. (2010). Mammalian target of rapamycin (mTOR) inhibition as a potential antiepileptogenic therapy: from tuberous sclerosis to common acquired epilepsies. Epilepsia 51 27–36 10.1111/j.1528-1167.2009.02341.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong M. (2013a). A critical review of mTOR inhibitors and epilepsy: from basic science to clinical trials. Expert Rev. Neurother. 13 657–669 10.1586/ern.13.48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong M. (2013b). Mammalian target of rapamycin (mTOR) activation in focal cortical dysplasia and related focal cortical malformations. Exp. Neurol. 244 22–26 10.1016/j.expneurol.2011.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong M., Crino P. B. (2012). “mTOR and epileptogenesis in developmental brain malformations,” in Jasper’s Basic Mechanisms of the Epilepsies eds Noebels J. L., Avoli M., Rogawski M. A., Olsen R. W., Delgado-Escueta A. V. (Bethesda, MD: National Center for Biotechnology Information; ). [PubMed] [Google Scholar]
- Wong M., Guo D. (2013). Dendritic spine pathology in epilepsy: cause or consequence? Neuroscience 251 141–150 10.1016/j.neuroscience.2012.03.048 [DOI] [PubMed] [Google Scholar]
- Woodward K. E., Gaxiola-Valdez I., Goodyear B., Federico P. (2013). Frontal lobe epilepsy alters functional connections within the brain’s motor network: a resting-state fMRI study. Brain Connect. 10.1089/brain.2013.0178 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu C. P., Zhang S. W., Fang T., Manxiu M., Chencan Q., Huafu C., et al. (2013). Altered functional connectivity within and between brain modules in absence epilepsy: a resting-state functional magnetic resonance imaging study. Biomed. Res. Int. 2013 734893 10.1155/2013/734893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasin S. A., Ali A. M., Tata M., Picker S. R., Anderson G. W., Latimer-Bowman E., et al. (2013). mTOR-dependent abnormalities in autophagy characterize human malformations of cortical development: evidence from focal cortical dysplasia and tuberous sclerosis. Acta Neuropathol. 126 207–218 10.1007/s00401-013-1135-4 [DOI] [PubMed] [Google Scholar]
- Yue Q., Groszer M., Gil J. S., Berk A. J., Messing A., Wu H., et al. (2005). PTEN deletion in Bergmann glia leads to premature differentiation and affects laminar organization. Development 132 3281–3291 10.1242/dev.01891 [DOI] [PubMed] [Google Scholar]
- Zeng L. H., Bero A. W., Zhang B., Holtzman D. M., Wong M. (2010). Modulation of astrocyte glutamate transporters decreases seizures in a mouse model of Tuberous Sclerosis Complex. Neurobiol. Dis. 37 764–771 10.1016/j.nbd.2009.12.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng L. H., Ouyang Y., Gazit V., Cirrito J. R., Jansen L. A., Ess K. C., et al. (2007). Abnormal glutamate homeostasis and impaired synaptic plasticity and learning in a mouse model of tuberous sclerosis complex. Neurobiol. Dis. 28 184–196 10.1016/j.nbd.2007.07.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng L.-H., Rensing N. R., Wong M. (2009). The mammalian target of rapamycin signaling pathway mediates epileptogenesis in a model of temporal lobe epilepsy. J. Neurosci. 29 6964–6972 10.1523/JNEUROSCI.0066-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng L. H., Rensing N. R., Zhang B., Gutmann D. H., Gambello M. J., Wong M. (2011). Tsc2 gene inactivation causes a more severe epilepsy phenotype than Tsc1 inactivation in a mouse model of tuberous sclerosis complex. Hum. Mol. Genet. 20 445–454 10.1093/hmg/ddq491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng L. H., Xu L., Gutmann D. H., Wong M. (2008). Rapamycin prevents epilepsy in a mouse model of tuberous sclerosis complex. Ann. Neurol. 63 444–453 10.1002/ana.21331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B., Wong M. (2012). Pentylenetetrazole-induced seizures cause acute, but not chronic, mTOR pathway activation in rat. Epilepsia 53 506–511 10.1111/j.1528-1167.2011.03384.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J., Blundell J., Ogawa S., Kwon C. H., Zhang W., Sinton C., et al. (2009). Pharmacological inhibition of mTORC1 suppresses anatomical, cellular, and behavioral abnormalities in neural-specific Pten knock-out mice. J. Neurosci. 29 1773–1783 10.1523/JNEUROSCI.5685-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X., Hampel H., Thiele H., Gorlin R. J., Hennekam R. C., Parisi M., et al. (2001). Association of germline mutation in the PTEN tumour suppressor gene and Proteus and Proteus-like syndromes. Lancet 358 210–211 10.1016/S0140-6736(01)05412-5 [DOI] [PubMed] [Google Scholar]
- Zhou X. P., Waite K. A., Pilarski R., Hampel H., Fernandez M. J., Bos C., et al. (2003). Germline PTEN promoter mutations and deletions in Cowden/Bannayan–Riley–Ruvalcaba syndrome result in aberrant PTEN protein and dysregulation of the phosphoinositol-3-kinase/Akt pathway. Am. J. Hum. Genet. 73 404–411 10.1086/377109 [DOI] [PMC free article] [PubMed] [Google Scholar]