Abstract
The current experiment is one of a series of comparative studies in our laboratory designed to determine the network of somatosensory areas that was present in the neocortex of the mammalian common ancestor. Such knowledge is critical for appreciating the basic functional circuitry that all mammals possess and how this circuitry was modified to generate species specific, sensory mediated behavior. Our animal model, the gray short-tailed opossum (Monodelphis domestica) is a marsupial that is proposed to represent this ancestral state more closely than most other marsupials and to some extent, even monotremes. We injected neuroanatomical tracers into the primary somatosensory area (S1), rostral and caudal somatosensory fields (SR and SC, respectively), and multimodal cortex (MM) and determined their connections with other architectonically defined cortical fields. Our results show that S1 has dense intrinsic connections, dense projections from the frontal myelinated area (FM), and moderate projections from S2 and SC. SR has strong projections from several areas, including S1, SR, FM and piriform cortex. SC has dense projections from S1, moderate to strong projections from other somatosensory areas, FM, along with connectivity from the primary (V1) and second visual areas. Finally, MM had dense intrinsic connections, dense projections from SC and V1, and moderate projections from S1. These data support the proposition that ancestral mammals likely had at least four specifically interconnected somatosensory areas, along with at least one multimodal area. We discuss the possibility that these additional somatosensory areas (SC and SR) are homologous to somatosensory areas in eutherian mammals.
Keywords: Marsupial, cortical evolution, multimodal cortex, 3a, 3b, posterior parietal
Introduction
The emergence of the neocortex and its expansion is the hallmark of mammalian evolution (Krubitzer, 2007). While both comparative paleontology and modern genomic analysis have provided exciting new insights into the chronology (Luo et al., 2003; Luo et al., 2011; O’Leary et al., 2013), brain size and encephalization (Rowe et al., 2011), and interordinal relationships (Hallstrom and Janke, 2008) of early mammals, our understanding of ancestral brain organization remains limited; brain tissue does not fossilize, and cranial endocasts provide information only on gross brain morphology and size. Yet brain organization is a window into the behavior of our earliest ancestors, not only providing a view of how they perceived and interacted with the world, but also what types of sensory-mediated behaviors were subject to selection (Krubitzer and Seelke, 2012). The goal of the present investigation is to illuminate the basic plan of cortical organization and connectivity of early mammals by examining the gray short-tailed opossum, proposed to resemble this early ancestor more than other living species.
Although monotremes form the earliest mammalian radiation whose ancestors diverged approximately 207–237 million years ago (MYA), they are highly derived, so studies of their brain organization and connectivity provide useful, but somewhat limited information on the brains of early, ancestral mammals (Woodburne et al., 2003; Pereira and Baker, 2006; van Rheede et al., 2006; Hugall et al., 2007). Metatherians, including marsupials, diverged later than monotremes (127.5–190 MYA Meredith et al., 2011; O’Leary et al., 2013) and unlike monotremes, many small brained, less derived members of this clade are thought to be morphologically similar to ancestral mammals (Wong and Kaas, 2009; Rowe et al., 2011). There are well over 300 species of marsupials in Australia and the Americas, and their external morphology and behavior evolved in parallel to that of eutherian mammals to adapt to a multitude of terrestrial, arboreal and aquatic environments (Karlen and Krubitzer, 2007), suggesting that a basic plan of body and brain organization transformed independently, but in a similar manner, in these two major lineages. Despite their prolificacy and their importance for understanding brain evolution, relatively little is known about the cortical sensorimotor circuitry that drives their behavior.
The gray short-tailed opossum (Monodelphis domestica) is a small crepuscular marsupial native to South America, and one of the few marsupial species currently bred in laboratory conditions. We study Monodelphis for several critical reasons. First, as noted above, because marsupials’ ancestors arose very early in mammalian evolution, examining the cortical organization and connectivity of modern marsupials could provide important insights into the brains of the first mammals, allowing us to appreciate the basic cortical circuitry common to all mammals. A second reason is that studies of marsupial motor cortex indicate that it is completely embedded in or overlapping with somatosensory cortex (e.g. Lende, 1963a; Lende, 1963b; Lende, 1963c; Rees and Hore, 1970; Magalhaes-Castro and Saraiva, 1971; Beck et al., 1996; Frost et al., 2000; Karlen and Krubitzer, 2007). This feature of the marsupial cortex is reflected in patterns of connectivity of S1, which receives inputs associated with both somatic and motor cortex from the thalamus (Killackey and Ebner, 1973; Joschko and Sanderson, 1987). How this type of organization ultimately effects behavior is unclear since basic patterns of behavior in marsupials are remarkably similar to their morphologically equivalent eutherian counterparts (e.g. Australian striped possum vs. Malagsy aye aye; Erickson et al., 1998; Rawlins and Handasyde, 2002). Third, marsupials are born extremely immature, at developmental time points that correspond to embryonic events in eutherian mammals. These animals therefore make excellent models of cortical development. Fourth, apart from this methodological advantage is the overlooked effect of this premature birth on the development of the somatosensory and motor systems. Unlike most eutherian mammals, marsupials (particularly those that develop in a pseudopouch like gray short-tailed opossums) have access to extremely early tactile inputs before and during the development of central somatosensory pathways. How this impacts the organization, connectivity and ultimate sensory mediated behavior of marsupials is an important issue that has yet to be addressed.
Marsupials including Monodelphis domestica have been shown to have two complete somatotopic representations within the cortex, the primary somatosensory area, S1, and the second somatosensory area, S2 (Huffman et al., 1999; Catania et al., 2000; Frost et al., 2000; Karlen and Krubitzer, 2007). Based on electrophysiological recordings, architectonic analysis and patterns of connections, some studies indicate that marsupials have two to three additional fields associated with somatosensory processing; a rostral field termed R or SR, a caudal field termed C or SC, and a parietal ventral area, PV (Beck et al., 1996; Elston and Manger, 1999; Huffman et al., 1999; Wong and Kaas, 2009; Anomal et al., 2011). Despite the important phylogenetic position of marsupials, and the implications that cortical processing networks of early mammals may be more complex than were previously thought, little is known about the cortical connectivity of somatosensory areas in this group of mammals (see discussion).
The specific aim of the present investigation was to examine the cortical connections of three somatosensory fields in Monodelphis domestica, S1, SR and SC. This was done by injecting anatomical tracers into architectonically and/or electrophysiologically defined locations within these fields, and relating patterns of connectivity to architectonically defined cortical fields in the ipsilateral and contralateral hemisphere. These data from each hemisphere were then quantified so that relative density of connections could be assessed. Comparisons of opossum cortical circuitry with other marsupials, monotremes and eutherian mammals indicate that its cortical network likely reflects that of the common mammalian ancestor better than that of the highly derived extant monotremes.
Methods
Subjects
A total of 7 adult opossums (Monodelphis domestica), including 4 males and 3 females, were used for these experiments. Animals ranged in age from 7 to 19 months old and weighed between 73 and 128 g. The animals were housed in standard laboratory cages in which food and water were available ad libitum, and maintained on a 12-hour light/dark cycle. All experiments were performed under National Institutes of Health guidelines for the care of animals in research and all protocols were approved by the Institutional Animal Care and Use Committee of the University of California, Davis.
Neuroanatomical Tracer Injections
Animals were placed in an induction chamber and anesthetized with the inhalant anesthetic isoflurane (1–3%). After induction, a specially fitted mask was placed over the animal’s snout and the surgical plane of anesthesia was maintained with 1–2% isoflurane. Once anesthetized, 2% lidocaine was injected subcutaneously at the scalp and around the ears, and the animals were placed in a stereotaxic apparatus. Animals were given dexamethasone (0.4–2.0 mg/kg, intramuscularly, IM) and atropine (0.04 mg/kg, IM) at the start of surgery. Temperature was maintained by placing the animal on a heating pad, and body temperature and respiration were monitored throughout the experiment. An incision was made at the midline of the scalp and a small craniotomy was performed over cortical fields S1, SR, SC or MM. The exposed cortex was photographed and this image was used to record the position of tracer injections relative to blood vessels. A custom-beveled Hamilton syringe was lowered approximately 300 μm into the cortex, and 0.3–0.4 μL of 10% fluoro-emerald (FE), fluoro-ruby (FR), or biotinylated dextran amine (BDA; Molecular Probes, Eugene, OR) was injected into the cortex. Several animals received multiple tracer injections, resulting in a total of eleven injections in seven animals (see Table 2). Two injections spread into surrounding areas, and were not included in quantitative analysis, however were included as figures. The opening was then covered with an acrylic skull cap or bone wax, and the temporal muscle and skin were sutured. Following the injection, antibiotics (Baytril, 5 mg/kg, IM) and analgesics (buprenorphine, 0.03 mg/kg, IM) were administered. The animal was allowed to recover for 5–7 days, enabling transport of the tracer. Following this recovery period, animals were euthanized with an overdose of sodium pentobarbital (Beuthanasia; 250 mg/kg, IP) and transcardially perfused with 0.9% saline, followed by 4% paraformaldehyde in phosphate buffer (pH 7.4), and then 4% paraformaldehyde in 10% phosphate buffered sucrose.
Table 2.
Tracer injections
| Case | Area injected + halo | Hemisphere injected | Tracer | Amount Injected (μl) | Injection area (mm2) | Recording sites | Injection receptive field | Figure Number |
|---|---|---|---|---|---|---|---|---|
| 12-18 | S1 | Right | FR | 0.3 | 0.06 | 53 | Vibrissae | 2 |
| 12-13 | S1 | Right | FR | 0.3 | 0.11 | 0 | 3 | |
| 09-18 | S1 | Left | FR | 0.3 | 0.07 | 0 | ||
| 12-19 | S1 | Right | FR | 0.32 | 0.10 | 12 | ||
| 08-80 | S1+SR, SC | Left | BDA | 0.3 | 1.61 | 20 | Vibrissae | 4 |
| 08-80 | SR | Left | FR | 0.3 | 0.07 | 20 | Lips | 6 |
| 09-32 | SR+FM,S1 | Right | BDA | 0.3 | 1.02 | 41 | Vib/Lip/Naris | 7 |
| 09-18 | SC | Left | FE | 0.3 | 0.14 | 0 | 8 | |
| 08-29 | SC | Left | FE | 0.3 | 0.08 | 0 | 8 | |
| 12-18 | MM | Right | FE | 0.4 | 0.04 | 53 | unresponsive | 9 |
| 08-80 | MM | Left | FE | 0.3 | 0.07 | 20 | unresponsive | 9 |
Electrophysiological Recordings
In three cases (08-80, 09-32, and 12-18) electrophysiological recording experiments were performed following the recovery period to confirm the placement of the injections. For these terminal mapping procedures, subjects were anesthetized using either 1–2% isoflurane or 30% urethane in propylene glycol (1.25 g/kg, IP). All other surgical procedures are like those described above for neuroanatomical injection experiments, except a larger craniotomy was made and the dura over S1 and the surrounding cortical regions was retracted. Digital images were taken so that electrophysiological recording sites could be directly related to cortical vasculature.
Multiunit electrophysiological recordings of somatosensory cortex were performed using tungsten microelectrodes (0.010 inches, 5 MΩ; A-M Systems, Inc., Sequim, WA). The electrode was lowered into cortical layer 4, at a depth of approximately 200–400 μm below the pial surface. Multi-unit activity was amplified and filtered (100–5000 Hz; A-M Systems Model 1800 Microelectrode AC Amplifier; A-M Systems, Carlsborg, WA), captured and quantified (CED Power1401 mk II hardware with Spike2 software), monitored through a loudspeaker, and visualized on a computer monitor. At each recording site responses to somatosensory stimulation were identified. Somatosensory stimulation consisted of light taps, displacement of hairs, light brushing of skin with a paintbrush, hard taps, and manipulation of muscles and joints. Descriptions of the receptive fields and the type of stimulus required to elicit a response were documented and drawn on illustrations of the opossum body. Responses were recorded at multiple recording sites (approximately 500 μm apart). The location of each recording site was marked on the digital image of the cortical surface relative to the vascular pattern, and was used to aid in the process of tissue reconstruction and receptive field quantification. Following electrophysiological recording, animals were euthanized with an overdose of sodium pentobarbital (Beuthanasia; 250 mg/kg IP) and transcardially perfused with 0.9% saline, followed by 4% paraformaldehyde in phosphate buffer, and then 4% paraformaldehyde in 10% phosphate buffered sucrose.
Histology
Following perfusion, the brain was extracted, weighed, and photographed, and the cortex was removed from the subcortical structures and manually flattened between glass slides. The flattened cortex was sectioned at 30 μm using a freezing microtome. Alternating cortical sections were stained for myelin or mounted for fluorescent microscopy. In cases in which BDA was injected, tissue was divided into three series, one of which was reacted for BDA (Vectastain Elite; Vector Laboratories, Burlingame, CA). In one case, the brain was cut in the coronal plane at 40 μm, and alternate sections were stained for cytochrome oxidase (CO) or mounted for fluorescent microscopy. In this coronally sectioned case, block face images were taken of each section to aid in 3D reconstruction (see Data analysis).
Data analysis
In each case in which the cortex was flattened, camera lucida reconstructions of individual myelin sections were made on a stereomicroscope (Fig. 1A–B). As described previously (Seelke et al., 2012), although individual sections can contain many partial anatomical boundaries, the entire series of sections must be examined and combined into a single comprehensive reconstruction to determine the full extent of cortical field boundaries. Each reconstructed section contained the outline of the section, blood vessels, tissue artifacts, probes, visible electrode tracks, and architectonic borders of cortical fields. Sections were aligned using these landmarks and compiled into one composite image. When necessary, brightness and contrast were adjusted of photomicrographs using Adobe Photoshop.
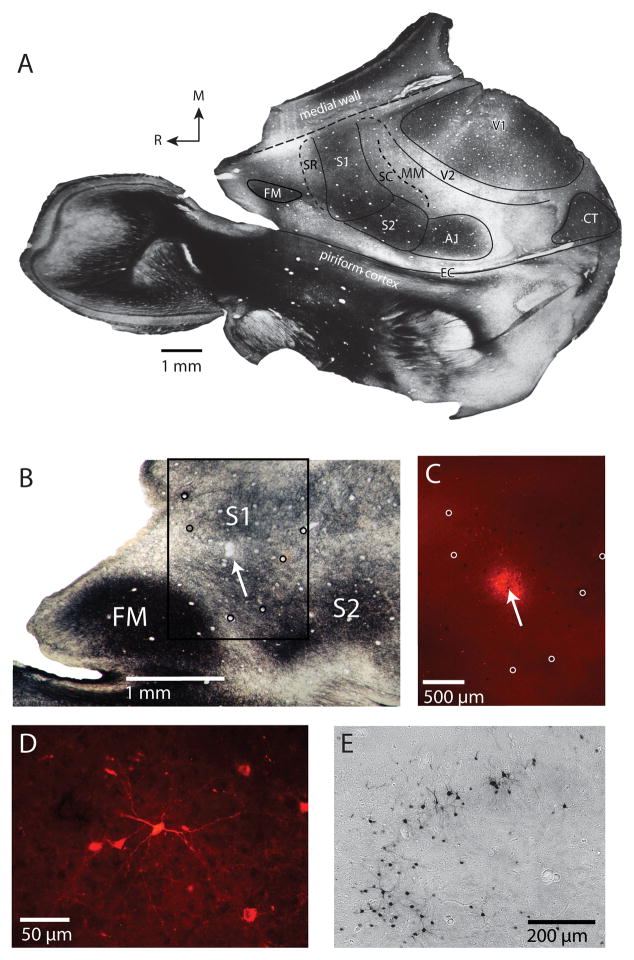
Figure 1.
(A) A light field digital image from case 09-32 of a left hemisphere that has been flattened, sectioned parallel to the cortical surface, and stained for myelin. Cortical field boundaries have been drawn based on myeloarchitecture (solid black lines) or estimated (dashed black lines). Where the medial wall has been unfolded, the junction with surface cortex has been indicated with longer dashed lines. Although many cortical field boundaries can be determined from a single section, the entire series of sections is used to make a comprehensive reconstruction. (B) Light field image from case 12-18 shows an injection site in S1 (arrow) in relation to myeloarchitectonic boundaries. (C) The same injection site under fluorescent illumination from the boxed region in B. The injection is small (~300–400 μm in diameter) and confined to the boundaries of S1. Circles in B and C mark the same blood vessels used to align sections. FR-labeled cell bodies from this case could be readily identified (D) within S1 and other regions. Cell bodies and processes were also well labeled following injections of BDA into S1 in case 08-80 (E). In all sections, rostral is to the left and medial is to the top. See Table 1 for abbreviations.
Injection sites (Fig. 1C) and retrogradely labeled cell bodies (Fig. 1D–E) were plotted using an X/Y stage encoding system (MD Plot, Minnesota Datametrics, MN) that was mounted to a fluorescent microscope and connected to a computer. Blood vessels and tissue artifacts were used to align connectional data from multiple sections and architectonic data from tissue stained for myelin; all data were combined into one comprehensive reconstruction that contained injection sites, retrogradely labeled cells and architectonic boundaries of cortical fields. These methods have been described previously (Campi and Krubitzer, 2010; Cooke et al., 2012).
In the coronally sectioned case (12-19) alternating sections were stained for CO and fluorescence, and fluorescent sections containing labeled cells were reconstructed as described above. However, rather than a compressed, flattened reconstruction, all sections were then aligned to the section outlines from block face images, and imported to 3D reconstruction software (Amira 3.1, Visage Imaging). Cortical field boundaries were reconstructed onto the adjacent fluorescent sections, and the total number of labeled cells within a cortical area across all sections was calculated.
Connectional data were quantified by calculating the percentage of labeled cells in each cortical field. This was calculated separately for each hemisphere: in any given cortical field this value was expressed as the percentage of total cells counted in that hemisphere. Labeled cells inside the injection halo where extracellular label indicates the extent of passive tracer spread were not included. This allowed us to normalize the data across cases and across different size injections. Means were calculated from multiple injections in different animals using only injections whose halos were entirely contained within the injected field. We used this data to assign connection strength when describing our connections for each cortical field. The criteria used are:
Strong: >10%
Moderate: 9% to 3%
Weak: 3% to 1%
Intermittent: >3% and inconsistent
Results
The goal of this study was to examine the corticocortical connections of S1 and surrounding fields in Monodelphis domestica. Regardless of which hemisphere was injected (see Table 2 for details), all cases were illustrated with injections shown in the left hemisphere for ease of comparison across cases. Below we describe the architectonic boundaries of cortical fields in Monodelphis followed by descriptions of ipsilateral and contralateral connections of S1, SR, SC and cortex immediately caudal to SC termed MM (see Table 1 for abbreviations).
Table 1.
| 3a | Somatosensory area (deep) |
| A1 | Primary auditory cortex |
| BDA | Biotinylated dextran amine |
| CO | Cytochrome oxidase |
| Contra | Contralateral |
| CT | Caudal temporal area |
| DZ | Dysgranular zone |
| EC | Entorhinal cortex |
| FE | Fluoro-emerald |
| fl | forelimb |
| FM | Frontal myelinated area |
| fp | forepaw |
| FR | Fluoro-ruby |
| IM | Intramuscular |
| IP | Intraperitoneal |
| Ipsi | Ipsilateral |
| ll | lower lip |
| M1 | Primary motor cortex |
| MM | Multimodal cortex |
| PM | Parietal medial area |
| PP/PPC | Posterior parietal cortex |
| PV | Parietal Ventral area |
| Pir | Piriform cortex |
| R | Rostral somatosensory field |
| S1 | Primary somatosensory area |
| S2 | Secondary somatosensory area |
| SC | Somatosensory caudal area |
| S.D. | Standard Deviation |
| SR | Somatosensory rostral area |
| ul | upper lip |
| V1 | Primary visual area |
| V2 | Secondary visual area |
| vib | vibrissae |
Cortical Field Boundaries
Cortical field boundaries determined with myelin and other stains have been described previously in Monodelphis by our own and other laboratories (Huffman et al., 1999; Catania et al., 2000; Frost et al., 2000; Kahn et al., 2000; Karlen and Krubitzer, 2006; Wong and Kaas, 2009). In several of these studies, cortical architecture was directly related to electrophysiologically identified boundaries of S1, S2, SR, SC, V1 and V2. As in previous studies, primary sensory areas including S1, V1, and A1 were densely myelinated and their boundaries were readily determined in all animals (Fig. 1A). In Monodelphis, the extrastriate region termed V2 consists of a lightly myelinated area immediately rostral to V1. While V2 has not been extensively mapped, previous studies have shown that, as in other species, at the V1/V2 border there is a reversal in the progression of receptive field locations. Both functional and architectonic evidence indicate it is approximately 0.5 mm wide (Kahn et al., 2000). The densely myelinated S1 has been shown to be co-extensive with a complete representation of the contralateral body, and neurons in this field respond to cutaneous stimulation (Huffman et al., 1999; Catania et al., 2000; Frost et al., 2000). The lateral portion of this field also contains a motor map of the face (Frost et al., 2000). The darkly myelinated V1 has been shown to be coextensive with a retinotopic map of the contralateral hemifield (Kahn et al., 2000), as described in all mammals studied. Although A1 was not defined eletrophysiologically in Monodelphis, its functional organization has been described in Australian quolls (Aitkin et al., 1986) and other small brained eutherian mammals such as mice (Stiebler et al., 1997), and it is in the location and has the appearance of the A1 we describe in Monodelphis. Further, in favorable preparations neurons in this densely myelinated region in Monodelphis respond well to auditory stimulation (Kahn and Krubitzer, 2002). For these reasons we term this field A1 in Monodelphis. Other areas that were darkly myelinated include S2, the caudotemporal areas (CT), and the frontal myelinated area (FM). Of these, S2 has been shown to be co-extensive with a functional map of the body in a range of marsupials (Beck et al., 1996; Huffman et al., 1999). SC was a moderately myelinated field bounded rostrally by S1 and laterally by S2. Caudally, SC borders a slightly less myelinated field that previous studies have shown to be co-extensive with multimodal (MM) cortex (Kahn and Krubitzer, 2002; Karlen and Krubitzer, 2006). MM is bounded caudally by V2 (Fig. 1). The moderately myelinated SR is bounded caudally by S1 and rostrolaterally by FM. Without functional data, the rostromedial boundary of SR is difficult to determine because it abuts cortex that is similar in appearance (i.e. moderately myelinated).
In three cases, electrophysiological recordings were made in and around the injections sites (e.g. see Figs. 2, 4, and 7). Consistent with previous studies in Monodelphis domestica and other marsupials, S1 contained neurons that were responsive to cutaneous stimulation of the contralateral body. Although our mapping density was low, a topographic organization was found within S1 similar to other studies in which S1 was mapped in detail (Catania et al., 2000; Frost et al., 2000). The goal of our mapping experiments was to define the body part representation injected and the functional borders of S1, not to generate comprehensive maps. This was possible in three cases in which anesthetic conditions were optimal.
Figure 2.
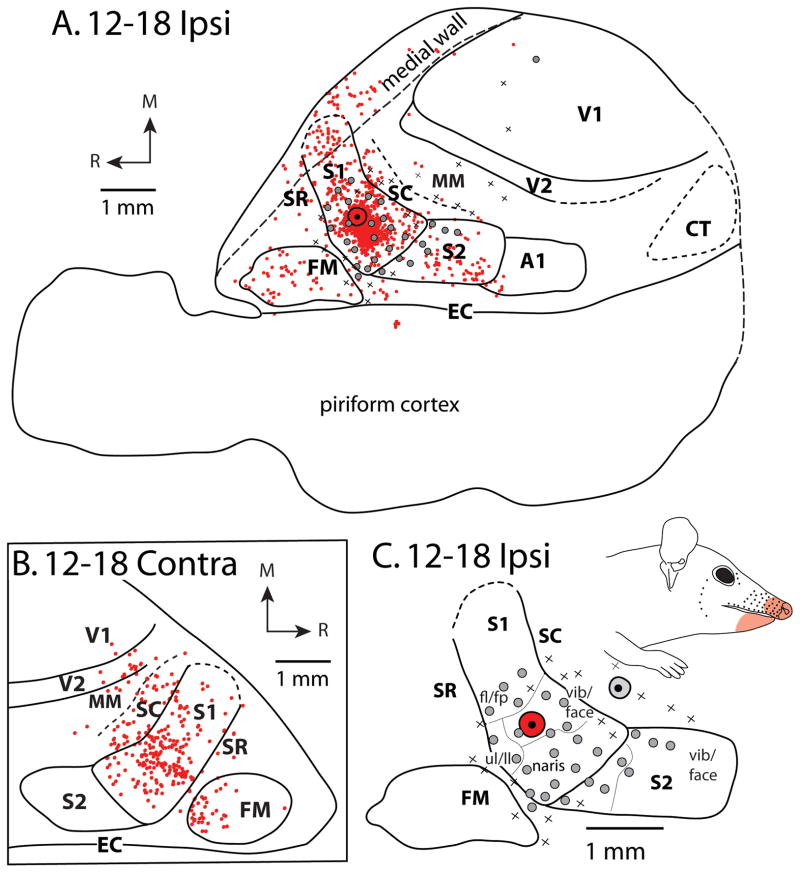
Patterns of ipsilateral (A) and contralateral (B) cortical connections resulting from an injection of FR in the vibrissae/snout representation of S1 for case 12-18. Black dot and surrounding black disc (A, C) are the FR injection site and halo, respectively. (Black dot surrounded by gray disc in C is a separate injection – see Fig. 9C). In both A and B, red dots indicate individual cells labeled by the neuroanatomical tracer. Most ipsilateral connections are intrinsic to S1. Moderate label is also observed in ipsilateral S2 and FM, with weak label in SC and SR. In the contralateral hemisphere, homotopic locations within S1 are most densely labeled. Moderate contralateral connections are observed with SC, FM, and V2 and sparse connections are observed in SR, MM, and V1. For quantification of labeled cells in each cortical area, see Table 3 and Figure 5A. Details of neuroanatomical tracers used and injection parameters are found in Table 2. In this case, electrophysiological recordings (A, C) were performed to confirm the placement of the tracer injection. Grey dots indicate recording sites at which neurons responded to somatosensory stimulation while Xs indicate sites at which no clear response was elicited. (C) Expanded view of injection site and electrophysiological map from A, showing different body part representations (separated by thin lines), and the receptive field location (light red shaded region on face) of neurons at sites surrounding the injection site. Solid lines mark cortical field boundaries determined from the entire series of myelin stained sections. Medial (M) and rostral (R) directions are indicated by arrows. Other conventions as in the previous figure.
Figure 4.
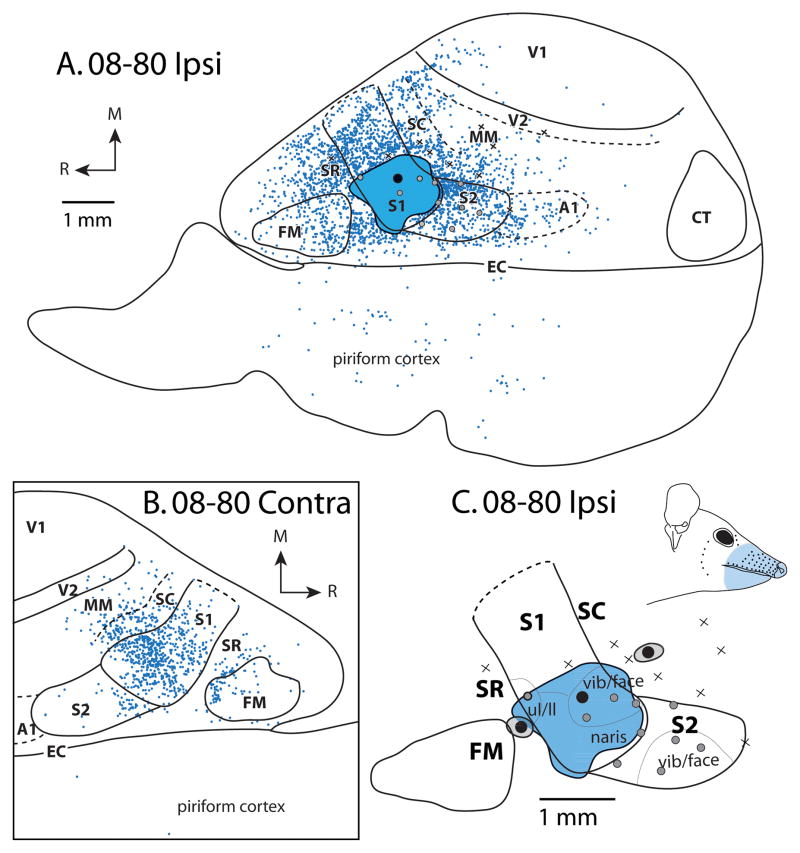
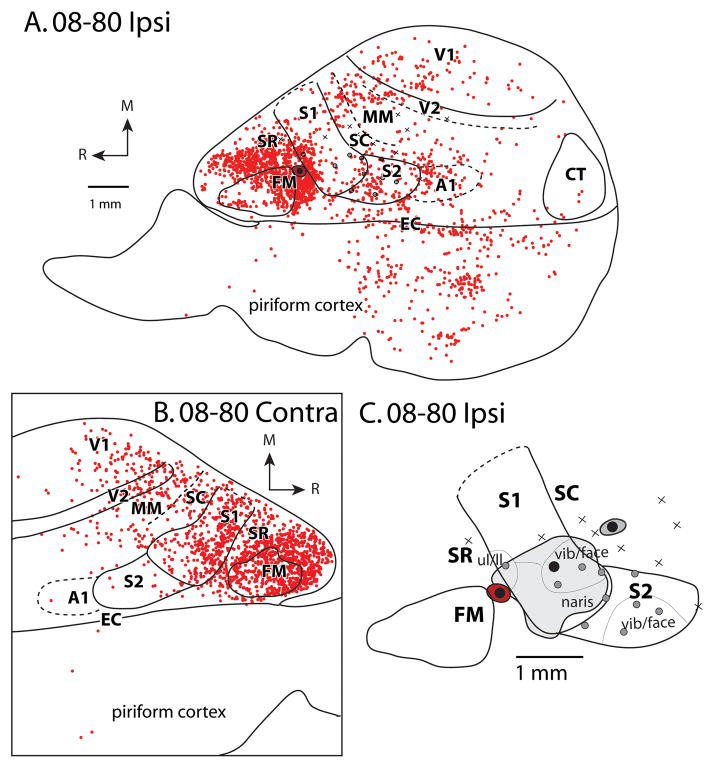
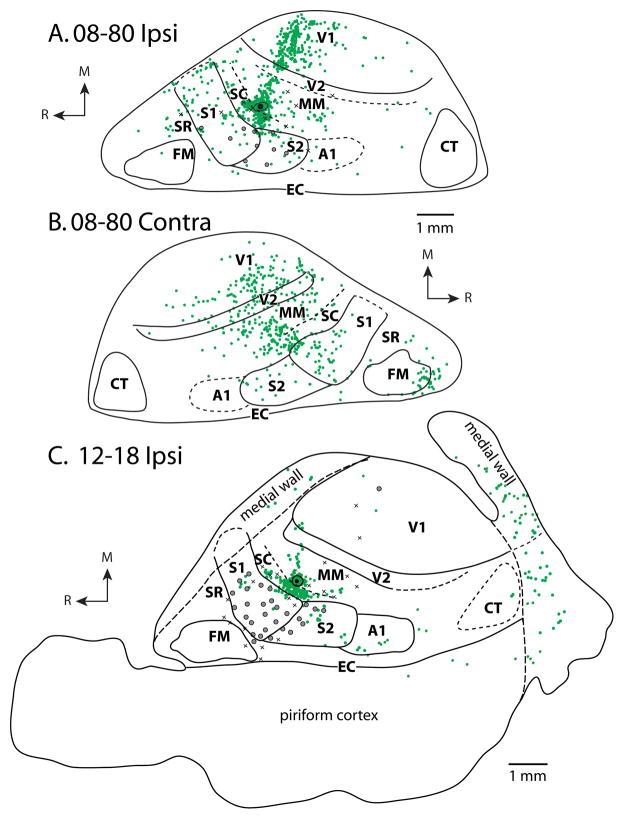
Patterns of ipsilateral (A) and contralateral (B) cortical connections resulting from a large injection of BDA in the vibrissae/face representation of S1 for case 08-80. This injection spread slightly into SR and SC. In both A and B, blue dots indicate individual labeled cells. Most ipsilateral connections are intrinsic to S1. Strong label is observed in S2. Moderate label is observed in FM, SC, SR, and MM. Homotopic locations within S1 in the contralateral hemisphere are most densely labeled; dense label is also observed in SC and moderate label is in SR, S2, MM and FM. In this case, electrophysiological recordings were performed to confirm the placement of the tracer injection. (C) Expanded view of electrophysiological map from A. Black dots surrounded by gray disk represent separate injections (see Fig. 6 and Fig. 9A, 9B). Conventions as in previous figures.
Figure 7.
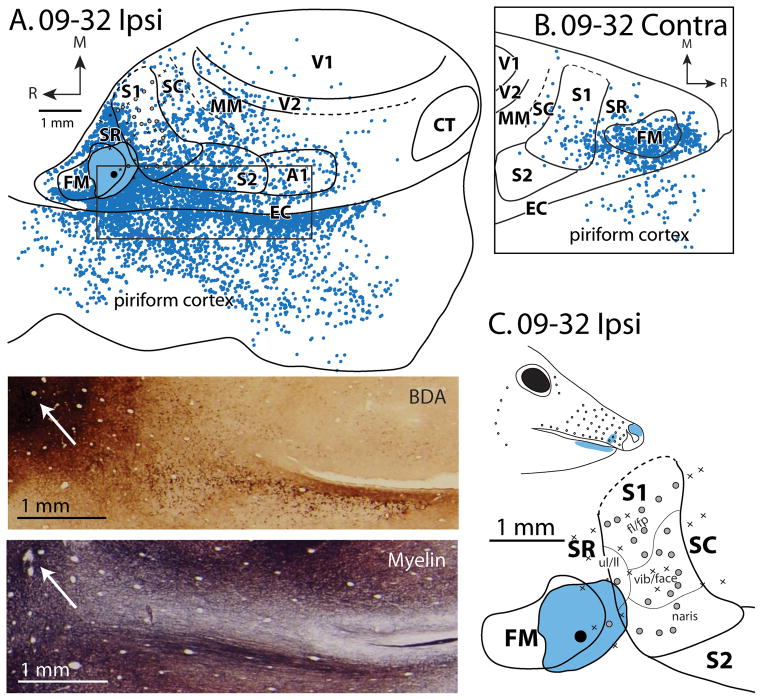
Patterns of ipsilateral (A) and contralateral (B) cortical connections resulting from a large injection of BDA that contained portions of FM, SR, and S1 for case 09-32. Moderate label is widely distributed across multiple cortical fields including FM, S1, S2, SR. One of the most dense and widespread labeled areas is in EC and piriform cortex. Sparse label was also widely distributed and found is SC, MM, A1, V2 and V1. The black box shows the region of cortex in the inset BDA and myelin stained sections. The arrow points to the injection site. In the contralateral hemisphere dense label was observed in FM and cortex just rostral to FM and moderate label was observed in SR and S1, SC and piriform cortex. Sparse label was observed in SC. In this case, electrophysiological recordings were performed to confirm the placement of the tracer injection. (C) Injection site and adjacent electrophysiological map of S1 as well as the location of a receptive field for neurons at a recording site located within the injection zone (light blue shading on animal face). Conventions as in previous figures.
Connections of S1
In 5 cases, injections were placed within S1 (Table 2, Figs 2–4; two cases not shown). In 4 of these cases, injection sites and their halo were under 0.15 mm2 while the final case, the injection site was 1.6 mm2 as measured in the flattened cortical sections (Fig. 4). The first 4 injections were entirely contained within S1, while the final injection slightly extended into cortex at the rostral and caudal boundaries of S1 (in SR and SC). For this reason this final case (08-80) was not included in the statistical analysis for S1.
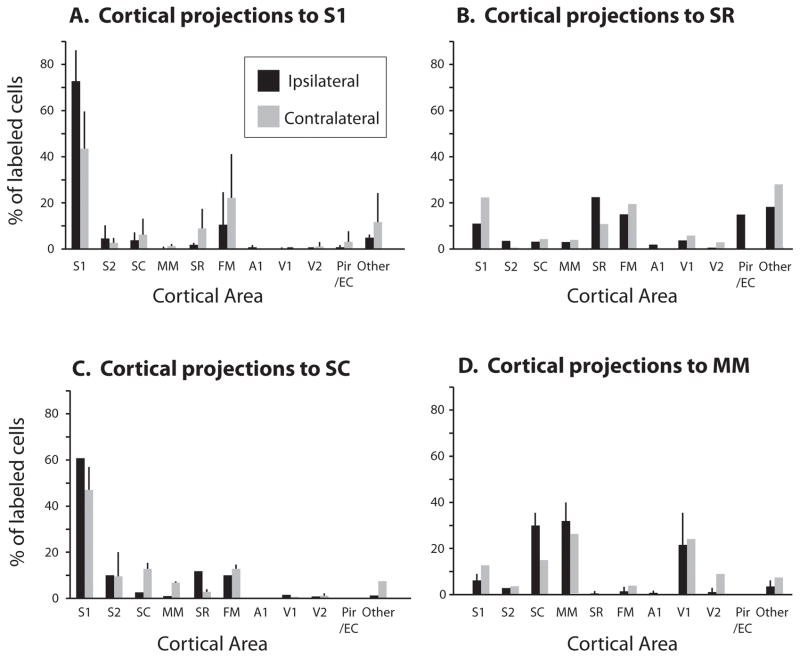
In two cases, injections were placed in lateral portions of S1, and electrophysiological mapping indicated that these injections were within portions of the face representation (Figs. 2C and 4C). In the other two cases, injections were placed in more medial portions of the field, in the expected locations of the forelimb representation (Fig. 3; other case not shown). The overall patterns of connections were similar in all cases. Most labeled cells were found within S1 (mean = 72.6%, Fig. 5A, Table 3). Much of this intrinsic labeling was concentrated close to the injection site, however in all cases labeled cells were seen throughout S1 (Figs. 2–4) and was not exclusively localized to the representation of a particular region of the body. This result was consistent for all injections, regardless of whether they were placed into the medially located forepaw/body representation or the laterally located face representation. Moderate label was also found in FM (mean 10.6%), and sparse labeling was observed in S2, SC and SR (mean 4.8%, 3.9% and 1.6% respectively). In three cases (Figs. 2A; 4A; case 12-19) sparse labeling was observed in MM, A1, V1, and V2, but each of these regions contained less than 1% of labeled cells (Fig. 5A). In two cases (Fig. 2A; case 12-19), sparse labeling was observed on the medial wall, just caudal to S1.
Figure 3.
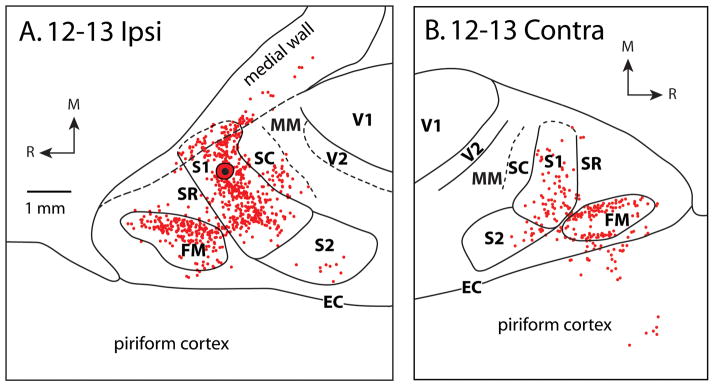
Patterns of ipsilateral (A) and contralateral (B) cortical connections resulting from an injection of FR centered in a medial portion of S1 in case 12-13. As in the previous case (12-18), most ipsilateral connections are intrinsic to S1. Moderate to dense label is also observed in SC and FM and sparse label is observed in SR and S2. In the contralateral hemisphere, dense connections are observed in S1 and FM, and moderate connections are seen in SR, S2, and piriform cortex. Other conventions as in previous figures.
Figure 5.
Percentage of labeled cells in ipsilateral (black) and contralateral (gray) cortex resulting from injections in S1 (A), SR (B), SC (C) and MM (D). The distribution of labeled cells across different cortical areas is different for each cortical field injected. Results are mean + standard deviation (when applicable) of injections from different animals. For results from individual cases, see Table 3. For a list of abbreviations, see Table 1.
Table 3.
| Ipsilateral connections | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Case | Area Injected | S1 | S2 | SC | SR | MM | FM | A1 | V1 | V2 | CT | Pir/EC | Other | |
| 12-13 | S1 | 58.2 | 0.5 | 7.8 | 2.3 | 0.3 | 26.8 | 0 | 0 | 0 | 0 | 0 | 4.1 | |
| 12-18 | S1 | 83.8 | 3.3 | 1.8 | 1.3 | 0.2 | 3.2 | 0.6 | 0.1 | 0.1 | 0 | 0 | 5.5 | |
| 12-19 | S1 | 75.9 | 10.7 | 2.1 | 1.1 | 0.2 | 1.7 | 0.5 | 0 | 0.1 | 0 | 1.6 | 6.1 | |
| Avg. | 72.6 | 4.8 | 3.9 | 1.6 | 0.2 | 10.6 | 0.4 | 0 | 0 | 0 | 0.5 | 5.3 | ||
| S.D. | 13.1 | 5.2 | 3.4 | 0.6 | 0 | 14 | 0.3 | 0.1 | 0 | 0 | 0.9 | 1 | ||
| 08-80 | SR | 11.1 | 3.6 | 3.4 | 22.5 | 3.2 | 15.1 | 2.4 | 4 | 1.1 | 0.3 | 15 | 18.3 | |
| 08-29 | SC | 60.4 | 9.9 | 3.9 | 12.6 | 0.4 | 10.1 | 0.1 | 1.2 | 0.6 | 0 | 0 | 0.8 | |
| 12-18 | MM | 4.4 | 3.1 | 33 | 0 | 38.1 | 3.1 | 1.8 | 9.9 | 0.2 | 0.4 | 0.9 | 5.1 | |
| 08-80 | MM | 8.2 | 2.8 | 25.8 | 1.4 | 27.6 | 0.1 | 0.4 | 30.8 | 2.5 | 0 | 0.1 | 0.4 | |
| Avg. | 6.3 | 2.9 | 29.4 | 0.7 | 32.9 | 1.6 | 1.1 | 20.4 | 1.3 | 0.2 | 0.5 | 2.7 | ||
| S.D. | 2.7 | 0.2 | 5.1 | 1 | 7.4 | 2.1 | 1 | 14.8 | 1.6 | 0.3 | 0.5 | 3.3 | ||
| 08-80 | S1+SR, SC | 46.7 | 10.9 | 9.6 | 9.9 | 4.5 | 4.8 | 2.1 | 0.9 | 0.5 | 0 | 1.5 | 8.4 | |
| 09-32 | SR+FM, S1 | 8.7 | 5.9 | 2.8 | 8.5 | 1.7 | 9.1 | 1.3 | 0.3 | 0.6 | 0 | 38.9 | 22.3 | |
| 09-18 | SC | 65.5 | 6 | 6.4 | 0 | 4.6 | 0 | 0 | 10.3 | 5 | 0 | 0.4 | 1.8 |
| Contralateral connections | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Case | Area Injected | S1 | S2 | SC | SR | MM | FM | A1 | V1 | V2 | CT | Pir/EC | Other | |
| 12-13 | S1 | 26 | 5.8 | 0 | 4.3 | 0 | 44.6 | 0 | 0 | 0 | 0 | 11.6 | 7.8 | |
| 09-18 | S1 | 35.7 | 0.2 | 2.9 | 21.4 | 0.7 | 30 | 0 | 0 | 0.5 | 0 | 0 | 8.6 | |
| 12-18 | S1 | 61.3 | 0 | 15.4 | 2.5 | 2.8 | 12.3 | 0 | 0.6 | 4.4 | 0 | 0 | 0.6 | |
| 12-19 | S1 | 50.9 | 1.9 | 6.6 | 5.1 | 2.3 | 1.9 | 0.2 | 0.2 | 0 | 0 | 1.1 | 29.8 | |
| Avg. | 43.5 | 2 | 6.2 | 8.3 | 1.5 | 22.2 | 0 | 0.2 | 1.2 | 0 | 3.2 | 11.7 | ||
| S.D. | 15.7 | 2.7 | 6.7 | 8.8 | 1.3 | 18.9 | 0.1 | 0.3 | 2.1 | 0 | 5.6 | 12.6 | ||
| 08-80 | SR | 22.4 | 0.9 | 4.4 | 10.9 | 4.3 | 19.6 | 0.1 | 5.9 | 3.2 | 0 | 0.2 | 28 | |
| 09-18 | SC | 53.8 | 1.4 | 10.6 | 3.6 | 6.3 | 14 | 0 | 0.9 | 2.1 | 0 | 0 | 7.5 | |
| 08-29 | SC | 39.8 | 16.9 | 14.7 | 1.7 | 6.9 | 11.2 | 0 | 0.3 | 0.9 | 0 | 0 | 7.6 | |
| Avg. | 46.8 | 9.1 | 12.6 | 2.7 | 6.6 | 12.6 | 0 | 0.6 | 1.5 | 0 | 0 | 7.5 | ||
| S.D. | 9.9 | 10.9 | 2.9 | 1.3 | 0.4 | 2 | 0 | 0.4 | 0.8 | 0 | 0 | 0.1 | ||
| 08-80 | MM | 12.8 | 3.6 | 14.1 | 0.2 | 24.2 | 4 | 0.2 | 24.2 | 9.1 | 0 | 0 | 7.6 | |
| 08-80 | S1+SR, SC | 61.4 | 5.4 | 13.1 | 3.8 | 5.6 | 5.9 | 0 | 0.1 | 0.7 | 0 | 0.2 | 3.8 | |
| 09-32 | SR+FM, S1 | 8.7 | 0.2 | 1.2 | 7.3 | 0 | 39.2 | 0 | 0 | 0 | 0 | 8.4 | 35 |
Within the contralateral hemisphere, in all cases the majority of labeled cells were found in locations that were homotopic to both the injection site in the opposite hemisphere and to areas that had dense ipsilateral label. Further, the overall patterns of interhemispheric connectivity were similar across cases (Figs. 2B, 3B and 4B). As in the ipsilateral hemisphere, the majority of label was in S1 (mean = 43.5%), and was not restricted to representation of a particular region of the body. Other regions projecting contralaterally to S1 included dense label in FM (mean = 22.2%), and moderate label in SC and SR (mean = 6.2% and 8.3%, respectively). S2 contained sparse label (mean = 2.0%), and less than 1% of label was observed in other sensory areas including A1, V1 and V2. Unsurprisingly, the most widespread and dense patterns of ipsilateral and contralateral connections were observed in the case which had the largest injection, which spread into adjacent fields (Fig. 4), however this injection also showed some characteristics of connections found to SC and SR (i.e., Figs. 6, 7, and 8). Specifically, the injected hemisphere showed higher than expected label in SC (9.6%), SR (9.9%), MM (4.5%) and A1 (2.1%) compared to injections entirely contained within S1. Similarly, the contralateral hemisphere showed increased label to SC (13.1%) and MM (5.6%, see Table 3).
Figure 6.
Patterns of ipsilateral (A) and contralateral (B) cortical connections resulting from an injection of FR in SR for case 08-80. Most ipsilateral connections are intrinsic to SR; dense label is also observed in FM, S1, and EC/piriform cortex. Moderate to sparse label is widely distributed and found is S2, SC, MM, A1, V2 and V1. In the contralateral hemisphere, dense label is observed in SR, S1, FM; moderate label is observed in SC, MM, V2 and V1. In this case, electrophysiological recordings were performed to confirm the placement of the tracer injection. (C), Injection site (black circle surrounded by red halo) and adjacent electrophysiological map of S1 as well as the injection sites in other cortical areas (BDA in S1, Fig. 4, and FE in SC, Fig. 9A, 9E, black circles surrounded by gray halos). Conventions as in previous figures.
Figure 8.
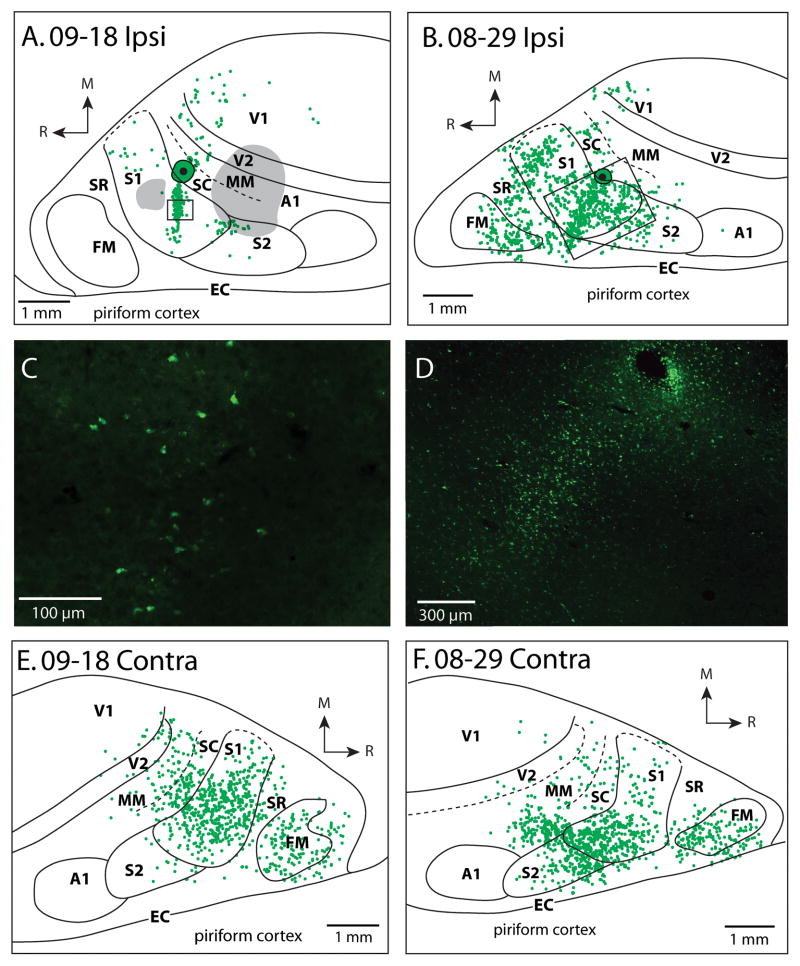
Patterns of ipsilateral (A, B) and contralateral (D, E) cortical connections resulting from an injection of FE into SC in cases 09-18 and 08-29. Green dots indicate individual cells labeled by the neuroanatomical tracer. In A, gray areas are regions of cortex with superficial cortical damage. In both (A) and (B), black boxes show areas from which pictures were taken in (C) and (D). In the case without cortical damage (B), most of the labeled cells were observed in S1, with strong label also in SR and FM and moderate label in S2 and SC. Weak label was observed in V1. In the contralateral hemispheres (E, F), dense label was observed in S1, SC and FM and moderate label was observed in S2 and MM, and weak label was observed in SR and V2. Conventions as in previous figures.
Connections of SR
Two cases had injections in SR; one injection was restricted to the boundaries of SR (case 08-80; Fig. 6) and one injection spread into S1 and FM (case 09-32; Fig. 7). The calculation of cell percentage illustrated in Figure 5, cited below, and listed in Table 3 is taken only from the case in which the injection was clearly restricted to SR (08-80; Fig. 6), however patterns of connections were largely similar between the two cases, and both clearly differed from the patterns of S1 connections (09-32; Fig. 7). Specifically, both cases show extensive projections from frontal cortex, dense connections with the rostral portion of S1, piriform and entorhinal cortex, and distributed connections with areas caudal to S1 including visual cortex. The primary difference between these two cases is an increase in projections from entorhinal and piriform cortex in the case not restricted to SR. For the case restricted to SR, intrinsic connections to SR accounted for 22.5% of labeled cells, while area FM contained 15.1% of labeled cells (Fig. 5B). Both S1 and piriform cortex also contained significant proportions of labeled cells (11.1% and 15.0%, respectively). We found additional connections with S2 (3.6%), SC (3.4%), V1 (4.0%) and A1 (2.4%). In general, the pattern of connectivity of SR was much more distributed compared to connections of S1 and included projections from auditory, visual and multimodal areas as well as piriform cortex.
Contralateral connectivity was also broadly distributed and appeared to be most dense in the homotopic locations that had dense ipsilateral connections (Figs 5B, 6B, 7B). Thus, label was most dense in areas SR (10.9%), FM (19.6%), and S1 (22.4%) and moderate in SC (4.4%). However, there were two notable trends in which contralateral connectivity differed. In both cases there were proportionately fewer contralateral projections from piriform cortex (0.2%) compared to ipsilateral connections (15%, Fig. 6, 7). Additionally, one case (08-80) showed moderate projections from contralateral MM (4.3%), V1 (5.9%), and V2 (3.2%), increased connectivity than was found in the ipsilateral hemisphere.
Connections of SC and MM
Four injection sites were located caudal to S1, two of these cases were primarily located in SC, with the injection halos partially extending into S1 (Fig. 8A; Fig. 8B) and two cases were placed caudal to this in MM (Fig. 9). While both injections into SC show similar patterns of connections, the results from one of these cases were not included in quantitative analysis due to damage to the cortical surface. For the SC injections, the majority of labeled cells were in S1 (60.4%). These labeled cells were not evenly distributed throughout S1, but were located in a dense focus adjacent to the injection site, with moderate labeling scattered throughout S1 (Fig. 8A, 8B). Moderate to sparse labeling was also observed intrinsically within SC (3.9%), S2 (9.9%), MM (0.4%), V2 (0.6%) and V1 (1.2%; see Fig. 5C). In one case label was observed in SR and FM (12.6 and 10.1, respectively) along with more extensive labeling throughout S1, but this may be due to the slight spread of the injection site into S1 (Fig. 8B).
Figure 9.
Patterns of ipsilateral and contralateral cortical connections resulting from an injection of FE in MM in case 08-80 (A and B) and case 12-18 (C). In both cases, the majority of labeled cells in the hemisphere ipsilateral to the injection site (A and C) are in MM and SC with additional dense labeling in V1. Moderate label was observed in S1, and weak label was observed in S2, FM, A1 and V2. In the contralateral hemisphere of 08-80 (B), strong label was found in MM, SC, S1 and V1. Moderate label was seen in S2, FM and V2. Conventions as in previous figures.
Contralateral label resulting from SC injections was most dense in mediolateral locations in S1 (46.8%), SC (12.6%), and S2 (9.1%; Fig. 8E, 8F) that matched the mediolateral location of the injection site in SC. In both cases, relatively dense label was also observed in FM (12.6%) in the contralateral hemisphere (see Fig. 5C), although label in FM was only observed in the ipsilateral hemisphere in one case.
In two cases, the injection sites were located more caudal compared to the previously described injections. Previous studies in which electrophysiological recordings were made in this region indicate that neurons are multimodal, responding to somatosensory, auditory or visual stimuli, and this field was termed MM. These connections differed from connections of SC in that the majority of labeled cells were observed in SC (29.4%), MM (32.9%) and V1 (20.4%; Figs. 5D, 9A, 9C). Moderate to sparse label was observed in S1 (6.3%), S2 (2.9%) and A1 (1.1%), and in one case, SR (1.4%). Contralateral projections were only determined in one case, due to lack of transport in the other case (Fig. 9B). Label was most dense in MM (24.2%), SC (14.1%), S1 (12.8%) and V1 (24.2%; Fig. 5D; 9B). Moderate to sparse label was observed in other cortical fields including S2 (3.6%), FM (4.0%) and SR (0.2%).
Discussion
The present investigation is part of a series of ongoing studies in our laboratory designed to understand how the brains of our common ancestors were organized. Our study builds upon recent developments exploring the issue of gross brain morphology in both mammaliaforms and early crown mammals that have been discussed in current literature, particularly in genomic and comparative paleontological studies (Luo et al., 2011; Rowe et al., 2011; O’Leary et al., 2013). While the issue of brain organization of early mammals has been deliberated by our own and other laboratories, the present study is the first comprehensive study of connectivity of multiple cortical fields in any marsupial. Our study, interpreted in the context of studies in other mammals, represents the only available avenue for understanding how the soft tissue of the brain was organized and interconnected in early mammals. In turn, this prototypical organization informs our understanding of how modifications to this organization ultimately generated the remarkable diversity in behavior of extant species including humans.
Cortical Connections of Somatosensory Cortex in Marsupials
While several studies have used electrophysiological recording methods to examine the functional organization of somatosensory cortex in marsupials (Huffman et al., 1999; Catania et al., 2000; Frost et al., 2000; for review see Karlen and Krubitzer, 2007), studies of corticocortical connections of somatosensory cortex are more limited. To date, the cortical connectivity of primary somatosensory cortex has only been investigated in three marsupials (Fig. 10). These include the North American Virginia opossum, Didelphis virginiana (Beck et al., 1996), the Australian brush-tailed possum, Trichosurus vulpecula (Elston and Manger, 1999), and the South American big-eared opossum, Didelphis aurita (Anomal et al., 2011). All of these studies report dense ipsilateral connections with other somatosensory areas, including S2, PV, SR, SC, and a region of cortex immediately lateral to S1, in and around the rhinal sulcus (we term this entorhinal cortex; EC). In the big-eared opossum and the brush-tailed possum, moderate to sparse connections were also observed with cortex immediately caudal to SC (PS, peristriate cortex in D. aurita and PP, posterior parietal cortex in T. vulpecula), with cortex immediately rostral to SR, and D. aurita, in cortex in the location of auditory cortex in other marsupials and other mammals in. Interhemispheric connections via the anterior commissure where only described for S1 in the brush-tailed possum and these were in a homotopic location with S1 and with SR, PV and EC.
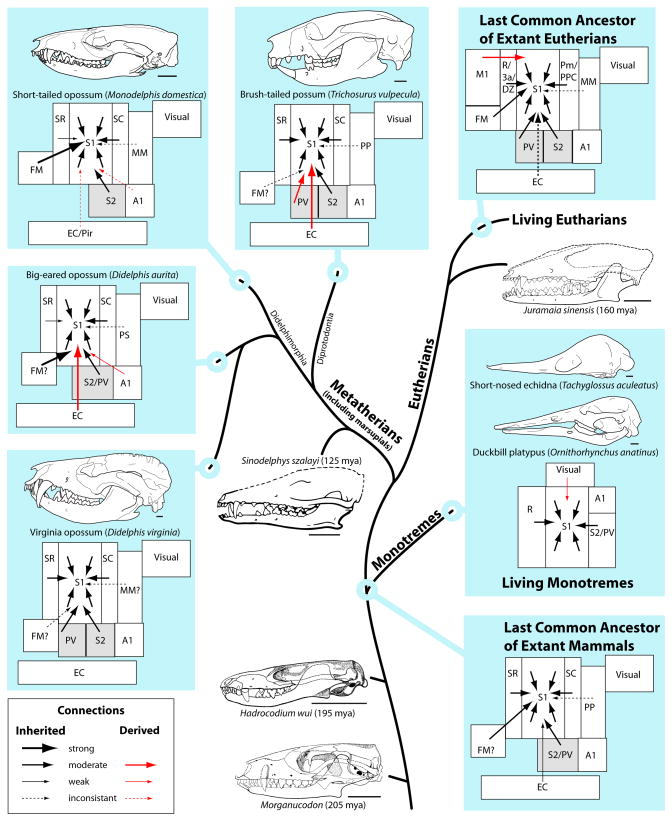
Figure 10.
Stylized cladogram summarizing cortical connections of S1 in mammals. Despite some differences, all marsupials share a basic pattern of connectivity that we propose represents that of the common ancestor of all mammals. Monotremes diverged earlier than marsupials and eutherians, and retain some basal traits like egg-laying, but have highly derived oral facial specializations, skulls, brains and lifestyles. Fossils of early mammals (Morganucodon, Hadrocodium wui, bottom center), early marsupials (Sinodelphis szalayi, center) and early eutherians (Juramaia sinensis, upper right) share many characteristics with skulls of some extant marsupials (left and top), particularly didelphids, while differing sharply from extant monotremes (right). The orafacial configuration, body size, and brain size of present day Monodelphis domestica (top left) indicate that it may be the best extant model for early marsupials and early mammals. The brain organization and connectivity of didelphids and especially Monodelphis may therefore reflect that of the common ancestor of all marsupials and all mammals. Inherited connections are drawn in black arrows; derived (independently acquired) connections are drawn in red arrows. The thickness of the arrow lines indicates the strength of connections. Cortical fields are outlined in boxes positioned such that the spatial relationships of fields have been preserved. The status of the presence of a separate S2 and PV (gray box) in the common ancestor is difficult to deduce since some marsupials have separate fields and some marsupials have a single field that could be either S2 or PV. mya, million years ago. Scale bars, 5 mm. Cladogram branch lengths are not to scale. Skull drawings adapted with permission from Macrini (2001, M. domestica; 2004, T aculeatus; 2005a, D. Virginiana; 2005b, O. anatinus; 2007, T. vulpecula), Luo and colleagues (2001, H. wui; 2003, S. szalavi; 2011, J. sinensis), and Kermack and colleagues (1981; Morganucodon). Connectional data adapted from Beck and colleagues (1996; Didelphis virginiana), Elston and Manger (1999; Trichosurus vulpecula), and Anomal and colleagues (2011; Didelphis aurita).
Our data in Monodelphis are similar to those reported in these previous studies of marsupials with a few important exceptions. First, unlike other marsupials, intrinsic connections of S1 were not restricted to the representation of the body part injected, but instead extended throughout S1 and this will be discussed below. Additionally, in contrast to most other mammals, electrophysiological recording studies indicate that Monodelphis does not have a parietal ventral area (Catania et al., 2000; Frost et al., 2000), and connections did not appear to differentiate S2 from other cortical fields in this region. While we did observe projections from S2 (as in other marsupials), the degree of projections from S2 varied considerably between cases. This is likely due to the location of injections in S1 and the nature of topographic interactions between S1 and S2. The laterally placed S1 injection was made into the vibrissae/naris representation of the animal (see Fig. 2C), and the face representation occupies a large portion of S2 (Catania et al., 2000; Frost et al., 2000). However, the medially placed injection was in the expected representation of the forepaw or body; this representation occupies only a very small lateral portion of S2 (Fig. 3A). Thus, these data suggest that unlike intrinsic connections of S1, connections with S2 are more tightly topographically organized. Further, we only saw very sparse connections with EC in a few animals, and moderate to dense connectivity with area FM, a heavily myelinated area rostral to the lateral portion of S1 and lateral to SR. The function of FM is not known. As with S2, the percent of labeled neurons in FM projecting to S1 differed between cases, and the ambiguous function of this area in opossums make it difficult to speculate why this difference was found. However, this observed difference may be due to the location of the injection within S1, an idea supported by the observation that medial portions (representing the body and forepaws) of S1 showed greater connectivity with FM than lateral (representing the face) portions of S1. While previous marsupial studies demonstrate a similar darkly myelinated region of cortex in the location of our FM (e.g. see Fig 13B of Beck et al., 1996; and Fig. 5A of Elston and Manger, 1999; and Fig. 2A of Anomal et al., 2011), only the study in D. aurita (Anomal et al., 2011) demonstrates dense projections with cortex in this region (termed the frontral region rather than FM). Other studies show sparse connections with this field. As in the brush-tailed possum, dense interhemispheric projections were from S1 and SR of the opposite hemisphere. However, we also observed interhemispheric projections from S2 (rather than PV), and from SC and FM (not reported in possums) to S1.
There are several factors that may contribute to the differences and similarities in organization and connectivity observed between Monodelphis and other marsupials investigated. The first is phylogeny. Virginia opossums are relatively closely related to the gray short-tailed opossum (both from the Didelphidae family) and both appear to have similar brain organization and patterns of connectivity of S1, while the more distantly related brush-tailed possum differs more significantly from these didelphids in both cortical organization, connections and cortical sheet size. Another possibility is that of brain size. The gray short-tailed opossum has a very small brain (~0.8 g) compared to the brush-tailed possum (11 g) and Virginia opossum (6.7 g). Several studies have shown that smaller brains often have more widespread connections compared to larger brains (Burkhalter and Charles, 1990), and that more local processing occurs in larger brains (Manger et al., 1998; for review see Kaas, 2012). Thus, some differences in connection patterns may be due to differences in processing strategies evolved in larger versus small brains. Finally, it is possible that differences in connectivity are due to differences in lifestyle and geographic dispersion. For example, the big-eared and gray short-tailed opossums have remarkably similar patterns of connectivity. While phylogeny may explain some of this similarity, it is important to consider that both species are omnivorous marsupials (primarily carnivorous in the wild), have partially overlapping habitats in Brazil, Paraguay, and Argentina, and are both predominantly terrestrial foragers and hunters that are also scansorial (have adapted the ability to climb). Thus, adaptations associated with niche and lifestyle may also contribute to similarities and differences in patterns of connectivity.
Cortical Connections of S1 in other mammals
In monotremes, an order of mammals whose ancestors branched off early in evolution, connectional data are extremely limited. In both platypus and echidnas, several different somatosensory areas have been identified, including S1, a second somatosensory area, which may correspond to S2 or PV or some combination of both fields, and a rostral area in which neurons are responsive to stimulation of deep receptors (R or SR; Krubitzer et al., 1995). Injections in S1 in the echidna result in a similar basic pattern of connectivity to that described for marsupials, but connections are more limited in extent. S1 has dense intrinsic connections and moderate connections with a field immediately rostral, (R/SR) and caudal to S1, although, due to its organization, the caudal field is likely to be S2/PV rather than SC. S1 receives moderate projections from cortex immediately rostral to SR, and light connections with V1 (See Fig. 10).
In contrast to monotremes, there are numerous studies on connections of S1 in eutherian mammals, and a complete review is beyond the scope of this discussion. However, comparisons of eutherian mammals with small to moderate sized brains, as well as those that occupy a variety of ecological niches indicate that a similar basic pattern of connectivity of S1 is present (Manzoni et al., 1989; Krubitzer et al., 1993; Catania and Kaas, 2001; Kaas, 2011). In these species S1 has dense intrinsic connections, and extrinsic connections with one or more lateral fields corresponding to S2 and PV, with cortex immediately caudal to S1, connections with cortex immediately rostral to S1, and often with cortex located far rostrally (Krubitzer et al., 1986, squirrel; Chapin et al., 1987, rat; Weller et al., 1987, tree shrew; Schwark et al., 1992, cat; Juliano et al., 1996, ferret).
Although the field immediately caudal to S1 is known by a variety of names in different species, such as PPC (Reep et al., 1994) and PM (Slutsky et al., 2000), neurons here often respond to deep somatic stimulation or are multimodal (Slutsky et al., 2000). Terminology for cortex immediately rostral to S1 has also varied (DZ, Chapin and Lin, 1984; R, Slutsky et al., 2000; 3a, Wong and Kaas, 2008; Cooke et al., 2012), but most studies indicate that like the marsupials in which this region of cortex has been explored extensively (Huffman et al., 1999), neurons in this region respond to stimulation of deep receptors of the skin, muscles and joints (Chapin and Lin, 1984; Slutsky et al., 2000; Cooke et al., 2012; see Krubitzer et al., 2011 for review).
One obvious difference in connectivity is the presence of an architectonically distinct M1 in eutherian mammals with extensive projections to S1. In some eutherians such as rats, S1 and M1 partially overlap at the representation of the hindlimb and forelimb (Donoghue and Wise, 1982). In contrast, in other rodents such as squirrels (Cooke et al., 2012) and in other eutherians such as primates (Powell and Mountcastle, 1959; Stepniewska et al., 1993; Huffman and Krubitzer, 2001) the motor map of M1 is a distinct field, separated from S1 by area 3a (see Krubitzer et al., 2011 for review). On the other hand, S1 and the presumptive M1 in marsupials almost completely overlap (Beck et al., 1996; see Karlen and Krubitzer, 2007 for review) and in gray short-tailed opossums there is not even evidence for a complete motor representation, since microstimulation in S1 only elicits movements of the face (Frost et al., 2000). Further, there is still some debate about whether marsupials even have an M1 that is homologous to M1 described in eutherian mammals (Beck et al., 1996). Thus, direct comparisons of connectivity to S1, even between different species of marsupials, can be difficult to interpret, because the presence of an M1, its location, and its extent is still contentious in marsupials.
Topography of intrinsic connections of S1 in mammals
One of the most novel findings in the gray short-tailed opossum was broadly distributed intrinsic connections of S1. This was true both for laterally placed injections in the face representation (Fig. 2A) as well as medially placed injections in the forepaw representation (Fig. 3A) suggesting that information across the body is highly integrated within S1. This pattern of intrinsic connectivity does not appear to be a general feature of marsupials. A single injection in the forepaw representation of S1 in the big-eared opossum shows that, despite a large injection site, intrinsic connections are mostly restricted to the medially located forepaw/body representations within S1 (see Fig. 8A of Anomal et al., 2011). Additionally, injections placed both medially and laterally in the Virginia opossum show similar topographic restriction between body and face representations (see Fig. 16 and 17 of Beck et al., 1996). The more distantly related Brush-tailed possum’s S1 also has topographically restricted intrinsic projections (Elston and Manger, 1999).
A more extensive comparative analysis of non-marsupial mammals provides further support that the pattern of intrinsic connectivity of S1 observed in the present investigation is not a general mammalian feature.. Small-brained eutherian mammals such as rats, star-nosed moles (Catania and Kaas, 2001), and naked mole rats (Henry and Catania, 2006) largely have restricted intrinsic connectivity of S1 suggesting the pattern observed in the present study is not simply a feature of a small brains. Other mammals, including squirrels (Krubitzer et al., 1986), tree shrews (Weller et al., 1987), cats (Schwark et al., 1992), and a variety of primates (e.g. Jones et al., 1978; Krubitzer and Kaas, 1990; Fang et al., 2002) show similar topographic restriction of intrinsic connection of S1 (3b). Interestingly, injections of S1 in monotremes (echidna) demonstrate scattered connections throughout S1 (Krubitzer, 1998). Thus, broadly distributed intrinsic connections of S1 across topographically mismatched body part representations appear to be observed in only two species examined (echidna and short-tailed opossum), both of which may have retained aspects of organization from their shared common ancestor.
While the limited data in monotremes indicates that broadly distributed intrinsic connection are, in part, due to retention of a primitive form, there are several additional factors which may contribute to this type of intrinsic processing within S1 in Monodelphis. Of all the marsupial species studied, the gray short-tailed opossum is the only one not reared in a pouch. Thus, during development these pups naturally have access to more tactile stimulation than other marsupials, and certainly experience more early tactile stimulation for all body parts than eutharian mammals. This could lead to an enhanced integration of information across the body compared to integration of information only for closely related body parts. Another possibility is that these inputs serve to modulate response on the snout. Electrophysiological studies have shown that the facial representation of the gray short-tailed opossum is expanded relative to the representation of the rest of the body (Catania and Kaas, 2000; Frost et al., 2000), suggesting functional importance of this area. Thus, it is possible that these inputs from different parts of S1 may be modulating the response of the snout. Finally, while the gray short-tailed opossum has cortical somatic representation of its entire body, as mentioned previously motor responses have only been elicited through microstimulation in lateral portions of S1, and have only resulted in movements of the face (Frost et al., 2000). Thus, these widespread inputs across S1 may, to some extent, represent connectivity with the gray short-tailed opossum “motor cortex”. This idea is particularly intriguing, as it could explain a difference in connectivity with the Virginia opossum, which has been shown to have a motor map which overlaps completely with somatosensory cortex (Beck et al., 1996).
Cortical connections of SR and SC: Evidence for multiple areas associated with somatosensory processing in marsupials
Based on receptive field characteristics and stimulus preference, electrophysiological recording studies have identified fields SC and SR in the Virginia opossum (Beck et al., 1996), the brush-tailed possum (Elston and Manger, 1999), the northern quoll, the striped possum (Huffman et al., 1999) and the big-eared opossum (Anomal et al., 2011). While other electrophysiological recording studies in the gray short-tailed opossum have not differentiated SR and SC based on neural response properties (Catania et al., 2000; Frost et al., 2000; present study), this is likely due to anesthetic recording conditions. When anesthetic preparation was optimal and responses could be studied in SR and SC in different marsupials, neurons in these areas responded to stimulation of deep receptors in muscles and joints, had large receptive fields and contained course topography of the contralateral body. A smaller percentage of recording sites in SR and SC contained neurons that responded to cutaneous stimulation. Further, these previous studies describe SC and SR as moderately to lightly myelinated fields compared to S1, and studies of connections of S1 add additional evidence to support a role in somatosensory processing for these two fields. However, to date there are no studies of connections of SR in any marsupial and only one study has described the connections of SC by injecting neuroanatomical tracers into this field (Anomal et al., 2011). In the big-eared opossum, connections of SC were similar to those described in the present investigation for Monodelphis (compare Fig. 6A of Anomal et al., 2011 with Fig. 8A of the present study). They found strong intrinsic connectivity, as well as connections to S2/PV and a region just caudal to SC termed peristriate cortex. While peristriate cortex generally refers to cortex surrounding V1 (such as V2), the region of cortex labeled as “peristriate” in the big-eared opossum was large, stretching from the rostral boundary of V1 to the caudal boundary of SC, and likely contained V2 caudally as well as cortex that may be homologous to MM in this study (see below). Anomal and colleagues (2011) also reported weak projections to FM, SR, V1 and A1. The presence of visual and auditory projections to SC in these marsupials indicates that SC is also involved in multimodal processing.
In the present study, injections in MM produced a pattern of connections that was distinct from that of the more rostrally-located SC. Most noteworthy were the relatively strong projections from non-somatosensory areas including V1 and V2. There is only one other study of marsupials in which one injection was placed in a similarly located region (termed PP) in brush-tailed possums (Elston and Manger, 1999). The patterns of connections observed in brush-tailed possums were similar to those of gray short-tailed opossums in that dense projections were observed from visual and auditory areas, and sparse projections were observed from S1, S2, PV, and cortex rostral to S1 in what may be equivalent to SR and FM in the current investigation. In terms of contralateral connectivity, in both gray short-tailed opossum’s MM and brush-tailed opossum’s PP, projections were seen from V1 and V2, SC, along with sparse projections from S1 and S2. In small eutherians like rats, a field in a similar location (PPC) also receives convergent sensory inputs from visual and somatosensory areas (Reep et al., 1994) suggesting that this area may have a similar integrative role in sensory processing. Further, although more data need to be collected from eutherian mammals other than mice and rats, it is possible that this multimodal region is a primitive form of the greatly expanded posterior parietal cortex of primates.
As mentioned previously, this is the first study investigating cortical connections of SR in marsupials. In squirrels, the field immediately rostral to S1 (termed 3a) is densely connected to motor cortex, S1 and PP, and sparsely connected to PV/S2, but does not have connections with F (likely equivalent to marsupial FM; Cooke et al., 2012). Other studies in this rostral field in rodents show this pattern of connectivity with S1, PP and motor cortex (Lee et al., 2011; for review see Krubitzer et al., 2011). While Monodelphis SR receives projections from S1 as well as SC and MM, unlike rodents, they also have additional projections from FM and piriform cortex. In both Monodelphis and other mammals, this rostral strip (SR and 3a, respectively) is poorly myelinated and poorly laminated (Wong and Kaas, 2009). Thus, while marsupials and rodents have areas rostral to S1 that contain neurons with similar somatosensory response properties, architectonic appearance, and connections with S1, the marked differences in connectivity between these areas suggest differences in function. Further, the issue of homology of cortical areas rostral to S1 in marsupials and eutherian mammals is complicated by the current uncertainty on the status of motor cortex in marsupials.
One of our SR injections included portions of FM and S1 (Fig. 7), the pattern of connectivity of this injection was remarkably similar, with the exception of a larger percentage of connectivity with EC and piriform cortex (15% in the restricted injection vs. 38.9% for the injection containing FM). Since the primary difference between these injections is the inclusion of FM, we would therefore hypothesize that FM is extensively connected with EC and piriform cortex. While further investigation of this hypothesis is needed, it is supported by an injection restricted to the heavily myelinated area F in squirrel, which also projects to a rhinal region of cortex (termed the parietal rhinal area; Cooke et al., 2012).
Somatosensory processing in early mammals
Comparisons across major groups of mammals indicate that there is a common plan of somatosensory cortex, composed of four interconnected fields, which to some degree is represented in marsupials, monotremes, and eutherian mammals (Fig 10). Further, the present study provides evidence suggesting additional fields such as FM and MM are components of this plan as well. Our connectional data for multiple cortical fields associated with somatosensory processing are supported by electrophysiological recording data from these different fields in most marsupials examined. On the other hand, monotremes appear to have a relatively simple network of somatosensory areas (S1, SR and S2 or S2/PV) compared to marsupials. While monotremes radiated earlier in evolution than marsupials, due to specializations like the bill, extant monotremes may have lost cortical areas (e.g. SC, MM, FM) and associated connections to support the enormous expansion of the bill representation in S1 and the acquisition of electrosensory reception. Because of these derivations, we propose that the organization and connectivity of marsupial neocortex, particularly those that are terrestrial/scansorial with small brains, may better reflect the ancestral state than that of monotremes, and certainly better represents the common ancestor of marsupials and placentals. Finally, data from eutherian mammals also provide support for the functionally interconnected somatosensory network of cortical areas present in marsupials [S1, S2, PV, a rostral (R, 3a, or DZ), and a caudal (PM/PPC) field], as well as for of multisensory cortex between caudal somatosensory areas and V1.
Based on these data, one can infer that the common mammalian ancestor had aspects of this plan. Rather than a simple network with one or two fields, the cortical networks involved in processing somatic inputs (both cutaneous and proprioceptive) were likely more complex than previously thought and included at least four interconnected cortical fields, as well as a primitive form of posterior parietal cortex involved in higher-order functions such as multisensory integration. Further, the large proportion of cortex devoted to somatosensory processing suggests that this sensory system was essential for survival in the Jurassic niche inhabited by early mammals.
Acknowledgments
Support: This project was supported by funds to Leah Krubitzer from NINDS (R21NS071225) and NEI (R01 EY022987) to Joao Franca from CNPq – Brazil (proc. no. 200713/2008-6) and James Dooley from NEI (T32-EY015387-05).
The authors thank Cindy Clayton, DVM and the rest of the animal care staff at the UC Davis Psychology Department Vivarium. Additional thanks to Becky Grunewald for technical assistance, Conor Weatherford for assistance in XY stage encoding, and Anika K Colopy for additional data collection and analysis.
Footnotes
Conflict of Interest Statement:
The authors JCD, JGF, AMHS, DFC, and LAK, have no conflicts of interest.
Role of Authors:
All authors had full access to all data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: JGF, DFC, and LAK. Acquisition of data: JCD, JGF, DFC, and LAK. Analysis and interpretation of data: JCD, JGF, AMHS, DFC, and LAK. Drafting of the manuscript: JCD and LAK. Critical revision of the manuscript for important intellectual content: JCD, JGF, AMHS, DFC, and LAK. Obtained funding: LAK. Study supervision: LAK.
Literature Cited
- Aitkin LM, Irvine DR, Nelson JE, Merzenich MM, Clarey JC. Frequency representation in the auditory midbrain and forebrain of a marsupial, the northern native cat (Dasyurus hallucatus) Brain, behavior and evolution. 1986;29(1–2):17–28. doi: 10.1159/000118669. [DOI] [PubMed] [Google Scholar]
- Anomal RF, Rocha-Rego V, Franca JG. Topographic Organization and Corticocortical Connections of the Forepaw Representation in Areas S1 and SC of the Opossum: Evidence for a Possible Role of Area SC in Multimodal Processing. Front Neuroanat. 2011;5 doi: 10.3389/fnana.2011.00056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck PD, Pospichal MW, Kaas JH. Topography, architecture, and connections of somatosensory cortex in opossums: evidence for five somatosensory areas. J Comp Neurol. 1996;366:109–133. doi: 10.1002/(SICI)1096-9861(19960226)366:1<109::AID-CNE8>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Burkhalter A, Charles V. Organization of local axon collaterals of efferent projection neurons in rat visual cortex. The Journal of comparative neurology. 1990;302:920–934. doi: 10.1002/cne.903020417. [DOI] [PubMed] [Google Scholar]
- Campi KL, Krubitzer L. Comparative studies of diurnal and nocturnal rodents: differences in lifestyle result in alterations in cortical field size and number. J Comp Neurol. 2010;518:4491–4512. doi: 10.1002/cne.22466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catania KC, Jain N, Franca JG, Volchan E, Kaas JH. The organization of somatosensory cortex in the short-tailed opossum (Monodelphis domestica) Somatosens Mot Res. 2000;17:39–51. doi: 10.1080/08990220070283. [DOI] [PubMed] [Google Scholar]
- Catania KC, Kaas JH. Areal and callosal connections in the somatosensory cortex of the star-nosed mole. Somatosensory & motor research. 2001;18:303–311. doi: 10.1080/01421590120089686. [DOI] [PubMed] [Google Scholar]
- Chapin JK, Lin CS. Mapping the body representation in the SI cortex of anesthetized and awake rats. The Journal of comparative neurology. 1984;229:199–213. doi: 10.1002/cne.902290206. [DOI] [PubMed] [Google Scholar]
- Chapin JK, Sadeq M, Guise JL. Corticocortical connections within the primary somatosensory cortex of the rat. The Journal of comparative neurology. 1987;263:326–346. doi: 10.1002/cne.902630303. [DOI] [PubMed] [Google Scholar]
- Cooke DF, Padberg J, Zahner T, Krubitzer L. The functional organization and cortical connections of motor cortex in squirrels. Cereb Cortex. 2012;22:1959–1978. doi: 10.1093/cercor/bhr228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donoghue JP, Wise SP. The motor cortex of the rat: cytoarchitecture and microstimulation mapping. The Journal of comparative neurology. 1982;212:76–88. doi: 10.1002/cne.902120106. [DOI] [PubMed] [Google Scholar]
- Elston GN, Manger PR. The organization and connections of somatosensory cortex in the brush-tailed possum (Trichosurus vulpecula): evidence for multiple, topographically organized and interconnected representations in an Australian marsupial. Somatosens Mot Res. 1999;16:312–337. doi: 10.1080/08990229970384. [DOI] [PubMed] [Google Scholar]
- Erickson CJ, Nowicki S, Dollar L, Goehring N. Percussive Foraging: Stimuli for Prey Location by Aye-Ayes (Daubentonia madagascariensis) International Journal of Primatology. 1998;19:111–122. [Google Scholar]
- Fang PC, Jain N, Kaas JH. Few Intrinsic Connections Cross the Hand-Face Border of Area 3b of New World Monkeys. The Journal of Comparative Neurology. 2002;454:310–319. doi: 10.1002/cne.10433. [DOI] [PubMed] [Google Scholar]
- Frost SB, Milliken GW, Plautz EJ, Masterton RB, Nudo RJ. Somatosensory and motor representations in cerebral cortex of a primitive mammal (Monodelphis domestica): a window into the early evolution of sensorimotor cortex. J Comp Neurol. 2000;421:29–51. doi: 10.1002/(sici)1096-9861(20000522)421:1<29::aid-cne3>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Hallstrom BM, Janke A. Resolution among major placental mammal interordinal relationships with genome data imply that speciation influenced their earliest radiations. BMC evolutionary biology. 2008;8:162. doi: 10.1186/1471-2148-8-162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry EC, Catania KC. Cortical, callosal, and thalamic connections from primary somatosensory cortex in the naked mole-rat (Heterocephalus glaber), with special emphasis on the connectivity of the incisor representation. The anatomical record Part A. Discoveries in molecular, cellular and evolutionary biology. 2006;288:626–645. doi: 10.1002/ar.a.20328. [DOI] [PubMed] [Google Scholar]
- Huffman KJ, Krubitzer L. Thalamo-cortical connections of areas 3a and M1 in marmoset monkeys. The Journal of comparative neurology. 2001;435:291–310. doi: 10.1002/cne.1031. [DOI] [PubMed] [Google Scholar]
- Huffman KJ, Nelson J, Clarey J, Krubitzer L. Organization of somatosensory cortex in three species of marsupials, Dasyurus hallucatus, Dactylopsila trivirgata, and Monodelphis domestica: neural correlates of morphological specializations. J Comp Neurol. 1999;403:5–32. doi: 10.1002/(sici)1096-9861(19990105)403:1<5::aid-cne2>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Hugall AF, Foster R, Lee MS. Calibration choice, rate smoothing, and the pattern of tetrapod diversification according to the long nuclear gene RAG-1. Systematic biology. 2007;56:543–563. doi: 10.1080/10635150701477825. [DOI] [PubMed] [Google Scholar]
- Jones EG, Coulter JD, Hendry SH. Intracortical connectivity of architectonic fields in the somatic sensory, motor and parietal cortex of monkeys. Journal of Comparative Neurology. 1978;181:297–347. doi: 10.1002/cne.901810206. [DOI] [PubMed] [Google Scholar]
- Joschko MA, Sanderson KJ. Cortico-cortical connections of the motor cortex in the brushtailed possum (Trichosurus vulpecula) J Anat. 1987;150:31–42. [PMC free article] [PubMed] [Google Scholar]
- Juliano SL, Palmer SL, Sonty RV, Noctor S, Hill GF., 2nd Development of local connections in ferret somatosensory cortex. The Journal of comparative neurology. 1996;374:259–277. doi: 10.1002/(SICI)1096-9861(19961014)374:2<259::AID-CNE8>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Kaas JH. Reconstructing the areal organization of the neocortex of the first mammals. Brain Behav Evol. 2011;78:7–21. doi: 10.1159/000327316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaas JH. Evolution of columns, modules, and domains in the neocortex of primates. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(Suppl 1):10655–10660. doi: 10.1073/pnas.1201892109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn DM, Huffman KJ, Krubitzer L. Organization and connections of V1 in Monodelphis domestica. J Comp Neurol. 2000;428:337–354. [PubMed] [Google Scholar]
- Kahn DM, Krubitzer L. Massive cross-modal cortical plasticity and the emergence of a new cortical area in developmentally blind mammals. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:11429–11434. doi: 10.1073/pnas.162342799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlen SJ, Krubitzer L. Phenotypic diversity is the cornerstone of evolution: variation in cortical field size within short-tailed opossums. J Comp Neurol. 2006;499:990–999. doi: 10.1002/cne.21156. [DOI] [PubMed] [Google Scholar]
- Karlen SJ, Krubitzer L. The functional and anatomical organization of marsupial neocortex: evidence for parallel evolution across mammals. Prog Neurobiol. 2007;82:122–141. doi: 10.1016/j.pneurobio.2007.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kermack KA, Mussett F, Rigney HW. The skull of Morganucodon. Zoological Journal of the Linnean Society. 1981;71:1–158. [Google Scholar]
- Killackey H, Ebner F. Convergent projection of three separate thalamic nuclei on to a single cortical area. Science. 1973;179:283–285. doi: 10.1126/science.179.4070.283. [DOI] [PubMed] [Google Scholar]
- Krubitzer L. What can monotremes tell us about brain evolution? Philosophical Transactions of the Royal Society. 1998;353:1126–1146. doi: 10.1098/rstb.1998.0271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krubitzer L. The magnificent compromise: cortical field evolution in mammals. Neuron. 2007;56:201–208. doi: 10.1016/j.neuron.2007.10.002. [DOI] [PubMed] [Google Scholar]
- Krubitzer L, Campi KL, Cooke DF. All rodents are not the same: a modern synthesis of cortical organization. Brain, behavior and evolution. 2011;78:51–93. doi: 10.1159/000327320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krubitzer L, Manger P, Pettigrew J, Calford M. Organization of somatosensory cortex in monotremes: in search of the prototypical plan. The Journal of comparative neurology. 1995;351:261–306. doi: 10.1002/cne.903510206. [DOI] [PubMed] [Google Scholar]
- Krubitzer LA, Calford MB, Schmid LM. Connections of somatosensory cortex in megachiropteran bats: the evolution of cortical fields in mammals. The Journal of comparative neurology. 1993;327:473–506. doi: 10.1002/cne.903270403. [DOI] [PubMed] [Google Scholar]
- Krubitzer LA, Kaas JH. The organization and connections of somatosensory cortex in marmosets. The Journal of Neuroscience. 1990;10:952–974. doi: 10.1523/JNEUROSCI.10-03-00952.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krubitzer LA, Seelke AMH. Cortical evolution in mammals: the bane and beauty of phenotypic variability. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(Suppl 1):10647–10654. doi: 10.1073/pnas.1201891109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krubitzer LA, Sesma MA, Kaas JH. Microelectrode maps, myeloarchitecture, and cortical connections of three somatotopically organized representations of the body surface in the parietal cortex of squirrels. The Journal of comparative neurology. 1986;250:403–430. doi: 10.1002/cne.902500402. [DOI] [PubMed] [Google Scholar]
- Lee T, Alloway KD, Kim U. Interconnected cortical networks between primary somatosensory cortex septal columns and posterior parietal cortex in rat. The Journal of comparative neurology. 2011;519:405–419. doi: 10.1002/cne.22505. [DOI] [PubMed] [Google Scholar]
- Lende RA. Cerebral cortex: a sensorimotor amalgam in the marsupiala. Science. 1963a;141:730–732. doi: 10.1126/science.141.3582.730. [DOI] [PubMed] [Google Scholar]
- Lende RA. Motor representation of the cerebral cortex of the opossum (Didelphis virginiana) J Comp Neurol. 1963b;121:405–415. doi: 10.1002/cne.901210308. [DOI] [PubMed] [Google Scholar]
- Lende RA. Sensory representation in the cerebral cortex of the opossum (Didelphis virginiana) J Comp Neurol. 1963c;121:395–403. doi: 10.1002/cne.901210307. [DOI] [PubMed] [Google Scholar]
- Luo Z-X, Crompton AW, Sun AL. A new mammaliaform from the early Jurassic and evolution of mammalian characteristics. Science. 2001;292:1535–1540. doi: 10.1126/science.1058476. [DOI] [PubMed] [Google Scholar]
- Luo Z-X, Ji Q, Wible JR, Yuan C-X. An Early Cretaceous tribosphenic mammal and metatherian evolution. Science. 2003;302:1934–1940. doi: 10.1126/science.1090718. [DOI] [PubMed] [Google Scholar]
- Luo Z-X, Yuan C-X, Meng Q-J, Ji Q. A Jurassic eutherian mammal and divergence of marsupials and placentals. Nature. 2011;476:442–445. doi: 10.1038/nature10291. [DOI] [PubMed] [Google Scholar]
- Macrini T. “Monodelphis domestica” (On-line) [Accessed March 4, 2013];Digital Morphology. 2001 at http://digimorph.org/specimens/Monodelphis_domestica/adult/
- Macrini T. “Tachyglossus aculeatus” (On-line) [Accessed March 4, 2013];Digital Morphology. 2004 at http://digimorph.org/specimens/Tachyglossus_aculeatus/skull/
- Macrini T. “Didelphis virginiana” (On-line) [Accessed March 4, 2013];Digital Morphology. 2005a at http://digimorph.org/specimens/Didelphis_virginiana/
- Macrini T. “Ornithorhynchus anatinus” (On-line) [Accessed March 4, 2013];Digital Morphology. 2005b at http://digimorph.org/specimens/Ornithorhynchus_anatinus/adult/
- Macrini T. “Trichosurus vulpecula” (On-line) [Accessed March 4, 2013];Digital Morphology. 2007 at http://digimorph.org/specimens/Trichosurus_vulpecula/
- Magalhaes-Castro B, Saraiva PE. Sensory and motor representation in the cerebral cortex of the marsupial Didelphis azarae azarae. Brain Res. 1971;34:291–299. doi: 10.1016/0006-8993(71)90282-4. [DOI] [PubMed] [Google Scholar]
- Manger P, Sum M, Szymanski M, Ridgway SH, Krubitzer L. Modular subdivisions of dolphin insular cortex: does evolutionary history repeat itself? Journal of cognitive neuroscience. 1998;10:153–166. doi: 10.1162/089892998562627. [DOI] [PubMed] [Google Scholar]
- Manzoni T, Barbaresi P, Conti F, Fabri M. The callosal connections of the primary somatosensory cortex and the neural bases of midline fusion. Experimental brain research Experimentelle Hirnforschung Expérimentation cérébrale. 1989;76:251–266. doi: 10.1007/BF00247886. [DOI] [PubMed] [Google Scholar]
- Meredith RW, Janecka JE, Gatesy J, Ryder OA, Fisher CA, Teeling EC, Goodbla A, Eizirik E, Simao TL, Stadler T, Rabosky DL, Honeycutt RL, Flynn JJ, Ingram CM, Steiner C, Williams TL, Robinson TJ, Burk-Herrick A, Westerman M, Ayoub NA, Springer MS, Murphy WJ. Impacts of the Cretaceous Terrestrial Revolution and KPg extinction on mammal diversification. Science. 2011;334:521–524. doi: 10.1126/science.1211028. [DOI] [PubMed] [Google Scholar]
- O’Leary MA, Bloch JI, Flynn JJ, Gaudin TJ, Giallombardo A, Giannini NP, Goldberg SL, Kraatz BP, Luo Z-X, Meng J, Ni X, Novacek MJ, Perini FA, Randall ZS, Rougier GW, Sargis EJ, Silcox MT, Simmons NB, Spaulding M, Velazco PM, Weksler M, Wible JR, Cirranello AL. The placental mammal ancestor and the post-K-Pg radiation of placentals. Science. 2013;339:662–667. doi: 10.1126/science.1229237. [DOI] [PubMed] [Google Scholar]
- Pereira SL, Baker AJ. A mitogenomic timescale for birds detects variable phylogenetic rates of molecular evolution and refutes the standard molecular clock. Molecular biology and evolution. 2006;23:1731–1740. doi: 10.1093/molbev/msl038. [DOI] [PubMed] [Google Scholar]
- Powell TP, Mountcastle VB. Some aspects of the functional organization of the cortex of the postcentral gyrus of the monkey: a correlation of findings obtained in a single unit analysis with cytoarchitecture. Bulletin of the Johns Hopkins Hospital. 1959;105:133–162. [PubMed] [Google Scholar]
- Rawlins DR, Handasyde KA. The feeding ecology of the striped possum Dactylopsila trivirgata (Marsupialia: Petauridae) in far north Queensland, Australia. Journal of Zoology. 2002;257:195–206. [Google Scholar]
- Reep RL, Chandler HC, King V, Corwin JV. Rat posterior parietal cortex: topography of corticocortical and thalamic connections. Experimental brain research Experimentelle Hirnforschung Expérimentation cérébrale. 1994;100:67–84. doi: 10.1007/BF00227280. [DOI] [PubMed] [Google Scholar]
- Rees S, Hore J. The motor cortex of the brush-tailed possum (Trichosurus vulpecula): motor representation, motor function and the pyramidal tract. Brain research. 1970;20:439–451. doi: 10.1016/0006-8993(70)90173-3. [DOI] [PubMed] [Google Scholar]
- Rowe TB, Macrini TE, Luo Z-X. Fossil evidence on origin of the mammalian brain. Science. 2011;332:955–957. doi: 10.1126/science.1203117. [DOI] [PubMed] [Google Scholar]
- Schwark HD, Esteky H, Jones EG. Corticocortical connections of cat primary somatosensory cortex. Experimental brain research Experimentelle Hirnforschung Expérimentation cérébrale. 1992;91:425–434. doi: 10.1007/BF00227839. [DOI] [PubMed] [Google Scholar]
- Seelke AM, Dooley JC, Krubitzer LA. The emergence of somatotopic maps of the body in S1 in rats: the correspondence between functional and anatomical organization. PLoS One. 2012;7 doi: 10.1371/journal.pone.0032322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slutsky DA, Manger PR, Krubitzer L. Multiple somatosensory areas in the anterior parietal cortex of the California ground squirrel (Spermophilus beecheyii) The Journal of comparative neurology. 2000;416:521–539. doi: 10.1002/(sici)1096-9861(20000124)416:4<521::aid-cne8>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Stepniewska I, Preuss TM, Kaas JH. Architectonics, somatotopic organization, and ipsilateral cortical connections of the primary motor area (M1) of owl monkeys. The Journal of comparative neurology. 1993;330:238–271. doi: 10.1002/cne.903300207. [DOI] [PubMed] [Google Scholar]
- Stiebler I, Neulist R, Fichtel I, Ehret G. The auditory cortex of the house mouse: left-right differences, tonotopic organization and quantitative analysis of frequency representation. Journal of comparative physiology A, Sensory, neural, and behavioral physiology. 1997;181:559–571. doi: 10.1007/s003590050140. [DOI] [PubMed] [Google Scholar]
- van Rheede T, Bastiaans T, Boone DN, Hedges SB, de Jong WW, Madsen O. The platypus is in its place: nuclear genes and indels confirm the sister group relation of monotremes and Therians. Molecular biology and evolution. 2006;23:587–597. doi: 10.1093/molbev/msj064. [DOI] [PubMed] [Google Scholar]
- Weller RE, Sur M, Kaas JH. Callosal and ipsilateral cortical connections of the body surface representations in SI and SII of tree shrews. Somatosensory research. 1987;5:107–133. doi: 10.3109/07367228709144622. [DOI] [PubMed] [Google Scholar]
- Wong P, Kaas JH. Architectonic subdivisions of neocortex in the gray squirrel (Sciurus carolinensis) Anatomical record. 2008;291:1301–1333. doi: 10.1002/ar.20758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong P, Kaas JH. An architectonic study of the neocortex of the short-tailed opossum (Monodelphis domestica) Brain Behav Evol. 2009;73:206–228. doi: 10.1159/000225381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodburne MO, Rich TH, Springer MS. The evolution of tribospheny and the antiquity of mammalian clades. Molecular phylogenetics and evolution. 2003;28:360–385. doi: 10.1016/s1055-7903(03)00113-1. [DOI] [PubMed] [Google Scholar]