Abstract
Sleep disturbance is a key behavioral risk factor for chronic medical conditions observed at high rates among overweight and obese individuals. Systemic inflammation, including that induced by stress, may serve as a common biological mechanism linking sleep, adiposity, and disease risk. To investigate these relationships, 48 postmenopausal women (mean age=61.8) completed a standardized laboratory stress task during which time blood was collected at baseline and 30+, 50+ and 90+ minutes after stressor onset to assess circulating levels of interleukin (IL)-6, IL-10, and IL-6/IL-10 ratio. Self-reported global sleep quality was assessed using the Pittsburgh Sleep Quality Index (PSQI) while adiposity was estimated by body mass index. Sagittal diameter was obtained in clinic to estimate visceral abdominal adiposity. Multi-level growth curve models revealed that poorer self-reported sleep quality was associated with greater stress-induced increases in IL-6/IL-10 ratio. In terms of adiposity, higher sagittal diameter, but not BMI, was associated with greater IL-6 reactivity (p’s<0.05). Further, associations between sleep quality and cytokine reactivity varied as a function of sagittal diameter. Among poor sleepers (1 SD above mean of PSQI score), stress-induced increases in IL-6 and IL-6/IL-10 ratio were significantly steeper in those with high visceral adiposity (1 SD above the mean of sagittal diameter) compared to those with low visceral adiposity (1 SD below the mean of sagittal diameter). In sum, poorer sleep quality and greater visceral adiposity, separately and especially in combination, are associated with greater stress-related increases in systemic inflammation. This research may help elucidate the complex link between sleep, obesity and inflammatory disease risk.
Introduction
Sleep disturbance, characterized by short sleep duration and/or poor subjective sleep quality, is an important behavioral predictor of several physical health outcomes, including obesity (Gangwisch et al., 2005; Mozaffarian et al., 2011; Taheri et al., 2004), cardiovascular disease (Gangwisch et al., 2006; Gottlieb et al., 2006; King et al., 2008; Unruh et al., 2008), and type 2 diabetes (Cappuccio et al., 2010; Gangwisch et al., 2007). While the specific pathways linking sleep and disease risk are unclear, emerging evidence suggests inflammatory processes may serve as key biological mechanisms.
Inflammation, mediated by elevations in pro-inflammatory proteins such as interleukin (IL)-6, is implicated in the pathogenesis of many of the diseases observed at high prevalence among poor sleepers (Libby, 2002; Pradhan et al., 2001; Ridker et al., 2000). Recent studies show that short sleep duration and poor subjective sleep quality are associated with increased concentrations of IL-6 (Miller et al., 2009; Mullington et al., 2010; Suarez, 2008). Elevated systemic levels of IL-6 have also been found among patients with clinical sleep disorders, such as primary insomnia (Burgos et al., 2006). Further, several laboratory experiments, though not all (Born et al., 1997; Dimitrov et al., 2006; reviewed in Solarz et al., 2012), demonstrate that sleep restriction of healthy sleepers results in elevations in plasma concentrations of IL-6 compared to a normal sleep condition (Shearer et al., 2001; Vgontzas et al., 1999; Vgontzas et al., 2004).
One pathway through which poor sleep may modulate inflammatory activity is via its impact on stress reactivity. An intriguing literature suggests that under conditions of sleep restriction individuals may be more reactive physiologically to stress. For instance, individuals deprived of a single night of sleep display greater threat responsiveness the following day, as marked by heightened amygdala activation (Yoo et al., 2007) and exaggerated systolic blood pressure reactivity in response to a social evaluative stressor (Franzen et al., 2011). Greater sleep disruption has also been associated with attenuated increases in natural killer (NK) cell number and slower recovery following a laboratory stressor compared to more sound sleepers (Wright et al., 2007a). However, another study found no consistent immune system differences, including morning levels of IL-6 and IL-10, in students exposed to 30-hours of sleep deprivation and an acute psychological stressor compared to a normal sleeping/no stress condition (Matzner et al., 2013). To date, only one prior study has investigated the influence of sleep quality on IL-6 reactivity to laboratory stress. In this regard, poor sleepers (i.e., participants with a Pittsburgh Sleep Quality Index (PSQI) global sleep quality score >5) showed greater task-related increases in systemic levels of IL-6 in response to a battery of cognitively demanding neuropsychological tests compared to good sleepers (i.e., PSQI global score ≤5) (Heffner et al., 2012).
An emerging feature in the link between sleep and inflammation has been the role of adiposity. Indeed, obesity is a strong correlate of poor sleep (Beccuti and Pannain, 2011; Cappuccio et al., 2008) and adipose tissue, particularly visceral fat, is a well-known contributor to systemic inflammation (Ouchi et al., 2011). It is estimated that 30% of inflammatory mediators in peripheral circulation originate from adipose tissue (Mohamed-Ali et al., 1997). Not only do adipocytes produce and release proinflammatory mediators, excess adipose is associated with an accumulation of migratory macrophages and a related shift towards a more pro-inflammatory local environment (Chawla et al., 2011; Neels and Olefsky, 2006; Odegaard and Chawla, 2008; Weisberg et al., 2003; Xu et al., 2003). Excess abdominal adiposity is associated with greater physiological reactivity to a laboratory stressor in several studies (Davis et al., 1999; Epel et al., 2000; Waldstein et al., 1999), including those measuring stress-induced cytokine reactivity (Brydon, 2011; Brydon et al., 2008); however, the effect of sleep on inflammation at varying levels of adiposity has not been investigated in response to laboratory stress.
The aims of the present study are to investigate the influences of self-reported global sleep quality and adiposity on cytokine reactivity to a laboratory-based stressor. We focus on the pro-inflammatory cytokine IL-6 because it is consistently increased in response to acute laboratory stress (Steptoe et al., 2007), associated with excess adiposity (Ouchi et al., 2011), and modulated by poor sleep (Motivala, 2011). In addition, we assessed levels of IL-10, an immunoregulatory anti-inflammatory cytokine whose secretion can be stimulated by IL-6 and can facilitate the resolution of inflammation (Daftarian et al., 1996). The ratio of IL-6 to IL-10 (IL-6/IL-10 ratio) provides an index for the balance between pro- and anti-inflammatory cytokines and displays significant variability in response to acute laboratory stress (Fredericks et al., 2010). Based on the prior literature, we hypothesize that poorer self-reported sleep quality and higher levels of adiposity will be associated with higher IL-6 concentrations and IL-6/IL-10 ratio in response to acute stress. Further, given the common association between poor sleep and obesity, we hypothesize that associations between poor sleep and stress-related cytokine reactivity will be stronger among those with higher levels of adiposity, particularly visceral abdominal adiposity.
Methods
Participants
The study sample was drawn from a larger cohort of post-menopausal women who participated in a prospective study of caregiving stress on immunological aging. The sample was comprised of healthy women providing a minimum of 4 hours of daily care to a family member with dementia and age-matched non-caregiving controls. In total, 50 women took part in the acute laboratory stress task, completed psychological questionnaires and body measurements, and underwent blood sampling for the assay of IL-6 and IL-10. Participants were excluded from this study if they reported major medical conditions, including cardiovascular disease, cancer, and diabetes, medication use known to affect stress hormones, and regular smoking. To confirm health status at baseline, participants were screened by self-report and also passed a physical exam by a physician. The study was approved by the Institutional Review Board of the University of California, San Francisco.
Procedures
Women interested in participating in this study were initially screened for eligibility by telephone. Next, they received a physical exam, including body measurements, a fasting blood draw, and provided written, informed consent at the UCSF Clinical and Translational Science Institute’s Clinical Research Center (CCRC). They were scheduled to return on a separate afternoon one week later to participate in the acute laboratory stress task. On the day of the laboratory task, participants ate a standardized lunch provided by the CCRC metabolic kitchen, and had an intravenous forearm catheter inserted at 1300 hr. After a one-hour baseline period with relaxation music in headphones, blood was drawn to assess resting levels of IL-6 and IL-10. Participants were then exposed to a modified form of the Trier Social Stress Test (TSST) (Kirschbaum et al., 1993) in which they were asked to give a speech about their strengths and weaknesses and to perform a difficult serial subtraction math task aloud. The phases of the stressor were comprised of four 5-minute stressful periods (20 minutes in total), including introduction of the two trained evaluators who described the task, a preparatory period for the speech, the speech task, and lastly the math task. All tasks were performed in front of an evaluative audience who maintained neutral facial expressions and tone of voice throughout the task period.
Measures
Self-reported sleep quality
Participants completed a modified version of the Pittsburgh Sleep Quality Index (PSQI)(Buysse et al., 1989). The PSQI is a widely used and reliable measure of global sleep quality. The original 19-items yield 7 component-scores that reflect the frequency of sleep problems in several areas including, subjective sleep quality; sleep latency; sleep duration; habitual sleep efficiency; sleep disturbance; use of sleep medication and daytime dysfunction. A higher PSQI global score is indicative of poorer overall sleep quality. In this study, the items comprising the “sleep disturbance” component of this scale were modified to better reflect disturbances common to our caregiver population (e.g., “Having to help my partner or parent who is ill”). These items were weighted in a manner to yield a scoring range identical to the original version. PSQI scores were missing for two participants, yielding 48 participants for these analyses.
Anthropometric Measurements
Body weight was measured on a digital scale with subjects in light clothing without shoes. Height was measured to the nearest 0.1 cm using a Harpenden stadiometer. BMI was calculated as weight in kilograms divided by height in meters squared. Sagittal diameter was used as our measure of abdominal adiposity. This was measured as the horizontal length (in centimeters) from the back to the belly using an anthropometer measuring stick while the participant was standing. This measure is widely used and reliable indicator of visceral abdominal fat (Pouliot et al., 1994; Zamboni et al., 1998).
Circulating Interleukin (IL)-6 and IL-10
Whole blood was collected into 10 ml SST tubes (Becton Dickinson, Franklin Lakes, and NJ) at baseline, +30, +50, and +90 minutes from the start of the TSST. Blood was allowed to clot for 30 minutes at room temperature and centrifuged at 1300 rpm for 15 minutes. Serum samples were aliquotted, frozen, and stored at −80°C until they were assayed for IL-6 and IL-10 in batch. A high sensitivity sandwich immunoassay was used to quantify circulating levels of IL-6 and IL-10 (Meso Scale Discovery, Gaithersburg, MD). The assay sensitivity for IL-6 is 0.46 pg/ml, and the average intra- and inter-assay coefficients are 4% and 6%, respectively. For IL-10, assay sensitivity is 1.05 pg/ml, and average intra- and inter-assay coefficients of variation are 7% and 8% respectively.
Assessment of general health and medication use
Participant health was assessed by self-reported physician-diagnosed illness at study entry on the following variables: hypertension (n=14), hypercholesterolemia (n=17), arthritis/osteoarthritis (n=11), and osteoporosis (n=5). No participants had autoimmune disorders. Use of the following classes of medications was also recorded: non-steroidal anti-inflammatory drugs (NSAIDs) (n=12), anti-hypertensives (n=16), statins (n=11), and anti-depressants (n=5). Sum scores of medical comorbidities and medication use were calculated for analyses. Depressive symptoms were assessed using the Inventory of Depressive Symptomatology (IDS;(Rush et al., 1996)), a well-validated measure of depression severity. Basic biochemistry panels of typical clinical markers such as albumin and complete blood count were also measured to confirm normal values.
Statistical Analyses
Statistical analyses were completed using IBM’s Statistical Software, SPSS 18.0. Pearson product-moment correlations and independent t-tests were conducted to examine associations between sociodemographic characteristics, predictor variables, and baseline concentrations of cytokines. Task-related changes in cytokine outcomes were initially estimated using repeated-measures analysis of variance (see Table 1). Primary hypotheses were tested using growth curve modeling (Singer and Willett, 2003).
Table 1.
Cytokine levels (mean and standard error) at baseline, +30 minutes, +50 minutes, and +90 minutes after the onset of the acute laboratory stressor. Repeated measures ANOVAs and post hoc bonferroni comparisons were conducted to determine changes in levels of IL-6, IL-10, and IL-6/IL-10 in response to the acute laboratory stressor. In this regard, levels of IL-6 and IL-6/IL-10, but not IL-10, displayed a significant time-related increase in response to acute psychological stress.
| Baseline | + 30 minutes | + 50 minutes | + 90 minutes | F ratio | p-value | |
|---|---|---|---|---|---|---|
| IL-6 pg/ml | 1.73 (0.16) | 2.17 (0.20)* | 2.54 (0.22)* | 3.44 (0.32)* | 32.49 | <0.001 |
| IL-10 pg/ml | 2.79 (0.40) | 3.03 (0.46) | 2.79 (0.38) | 2.74 (0.39) | 2.55 | 0.07 |
| IL-6/IL-10 ratio | 0.95 (0.13) | 1.22 (0.19) | 1.38 (0.20)* | 1.96 (0.28)* | 12.52 | <0.001 |
significantly different from the baseline value, p<0.05.
In growth curve models, the repeatedly measured outcome is regressed on time, simultaneously providing estimates for baseline (B0) and rate of change (Btime). In terms of the TSST, the B0 and Btime are estimates of cytokine levels at time 0 and its growth (or trajectory) over the next 90 minutes until study completion. A significant B0 designates that the cytokine of interest is at levels different from 0 pg/ml before the TSST begins, and a significant Btime designates that its rate of change over time is significant in response to the TSST. Without covariates or predictors in the model, these estimations are called the unconditional growth curve model.
To test the effects of PSQI global sleep quality (Bsleep) and adiposity (BMI, BBMI; sagittal diameter, Bsagittal) on cytokine reactivity, we examined the interactions between (1) PSQI global sleep quality (mean-centered) and time (Bsleep*time), (2) BMI (mean-centered) and time (BBMI*time), and (3) sagittal diameter (mean-centered) and time (Bsagittal*time) in separate models. A significant interaction between PSQI global sleep quality and time (Bsleep*time), for example, suggests that IL-6 growth over the course of the TSST significantly varies as a function of PSQI global sleep quality and the direct effect of PSQI global sleep quality (Bsleep) estimates whether baseline IL-6 is associated with sleep quality. The standard follow-up approach (Cohen et al., 2003; Singer and Willett, 2003) is to test simple slopes; whether the growth in IL-6 over time is significant at one standard deviation above the mean of PSQI global sleep quality and then again at one standard deviation below the mean of PSQI global sleep quality.
A significant three-way interaction between PSQI global sleep quality, adiposity measure, and time (Bsleep*sagittal*time) indicates that cytokine reactivity varies as a function of PSQI global sleep quality at different levels of adiposity. Accordingly, this is followed up with simple interaction tests between PSQI global sleep quality and time at 1 SD above and below mean adiposity. Significant simple interactions would indicate that PSQI global sleep quality predicts cytokine changes over time at that level of adiposity.
A random-intercept model with restricted maximum likelihood (REML) estimation was fitted with an unstructured covariance matrix in all models, allowing the proper handling of missing and skewed data, producing unbiased estimates (Singer and Willett, 2003). All models were tested adjusting for age, number of medical comorbidities, and caregiver status; however, findings were replicated with models that included depressive symptoms and medication use as additional covariates.
Results
Descriptive Statistics
Participants were 48 post-menopausal women (87% Caucasian) with a mean age of 61.8 years (SD=6.3). The mean body mass index (BMI) and sagittal diameter for the sample were 26.0 kg/m2 (SD=5.1; range 17.7 to 37.5) and 24.8 cm (SD=4.3; range 18.5 to 36.0). Depressive symptoms in this sample, as indexed by the scores on the IDS, ranged from 2 to 38 (mean score= 12.8, SD=8.9), while the mean PSQI global sleep quality score was 4.5 (SD=3.4). Because the goal of the larger study focused on caregiving stress, 21 of the 48 participants were providing care of a family member with dementia, while the remaining participants were not (n=27). Preliminary analyses revealed the stress-related changes in IL-6, IL-10, and IL-6/IL10 were similar in caregivers and non-caregivers (p’s values for the caregiver status by time interactions ranged 0.21 to 0.68). Consequently, caregiver status was used as a covariate in all subsequent analyses.
Bivariate correlations were calculated to test associations of sociodemographic variables, PSQI global sleep quality, and adiposity with baseline levels of circulating IL-6, IL-10, and IL-6/IL-10 ratio. In this regard, higher levels of IL-6 at baseline were associated with higher BMI scores (r=0.35, p=0.02) and greater sagittal diameter (r=0.45, p=0.002). There were no significant correlations between cytokine levels (i.e., IL-6, IL-10, IL-6/IL-10 ratio) and age, depressive symptoms, medication use, or medical comorbidities. PSQI global sleep quality was unrelated to cytokine measures at baseline but was associated with depressive symptoms, such that poorer sleep quality (i.e., higher PSQI global sleep quality score) was positively related to depressive symptoms (r=0.63, p<0.001).
Unconditional Growth Models: stress-induced cytokine reactivity
Table 1 displays the raw cytokine values at baseline and 30+, 50+, and 90+ minutes after the start of the laboratory stress task. Linear growth models fit for time were calculated for each cytokine outcome (IL-6, IL-10, and IL-6/IL-10 ratio). Results indicated that circulating IL-6 at baseline was significantly different than 0 and followed a linear increase across the TSST (B0=2.15, SE=0.41, p<0.001; Btime= 1.17, SE= 0.12, p<0.001). A similar significant baseline and linear increase was observed for IL-6/IL-10 ratio (B0=1.10, SE=0.37, p=0.005; Btime=0.67, SE=0.11, p<0.001). Circulating levels of IL-10 did not show a significant stress effect on average across the group (B0=2.92, SE=0.77, p<0.001; Btime= −0.05 SE=0.08, p=0.49). As such, we did not carry out further analyses to test the influences of sleep quality, adiposity and their interaction on trajectories of circulating levels of IL-10.
Effects of PSQI global sleep quality and adiposity on cytokine reactivity
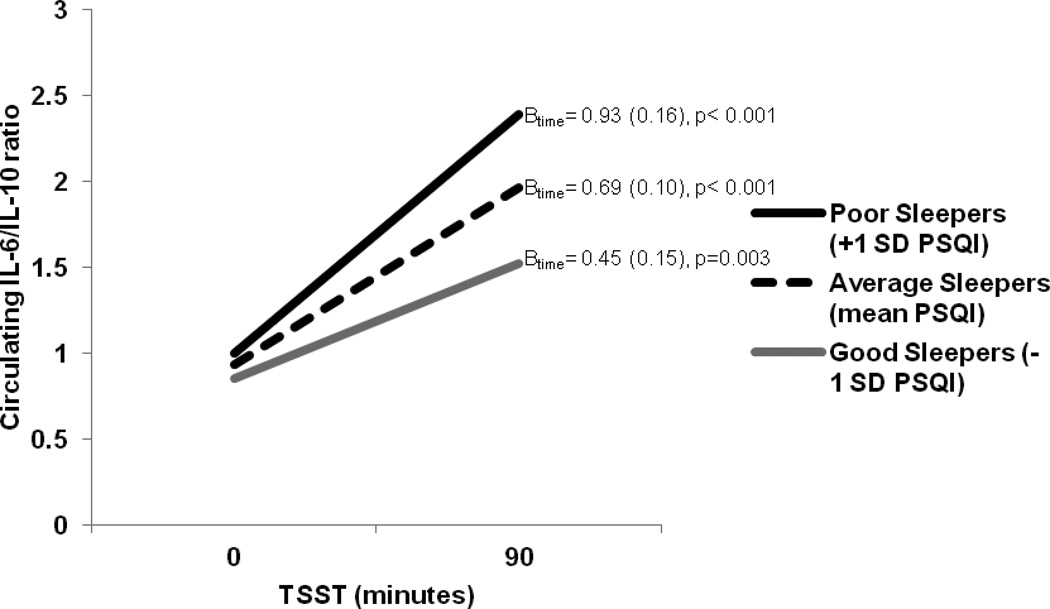
We investigated the effects of PSQI global sleep quality alone, adiposity alone (BMI and sagittal diameter, separately), and their interactions with time in predicting estimated IL-6 and IL-6/IL-10 ratio at baseline levels and trajectories in response to acute laboratory stress. In this regard, PSQI global sleep quality was unrelated to baseline levels of IL-6 (Bsleep=0.02, SE=0.08, p=0.82) or IL-6/IL-10 ratio (Bsleep=0.02, SE=0.08, p=0.76); however, poorer PSQI global sleep quality was significantly associated with IL-6/IL-10 ratio reactivity but not IL-6 reactivity in response to the TSST (IL-6: Bsleep*time=0.04, SE=0.04, p=0.23; IL-6/IL-10: Bsleep*time=0.07, SE=0.03, p=0.03). Visceral adiposity, as indexed by sagittal diameter, was associated with stress-induced IL-6 reactivity, but not IL-6/IL-10 ratio reactivity (IL-6: Bsagittal*time=0.06 SE=0.03, p=0.04; IL-6/IL-10: Bsagittal*time=0.03, SE=0.03, p=0.25). There were no associations between sagittal diameter and baseline values of IL-6 (Bsagittal=0.10, SE=0.06, p=0.09) or IL-6/IL-10 (Bsagittal=0.03, SE=0.05, p=0.60). BMI was unrelated to IL-6 and IL-6/IL-10 ratio baseline levels or increases (p’s>0.30). To better understand the pattern of results, simple slopes were calculated (Table 2). As displayed in Figure 1, poor sleepers (i.e., those one standard deviation above the mean PSQI global sleep quality score) were estimated to have a steeper increase in IL-6/IL-10 ratio to the laboratory stressor relative to average sleepers (i.e., those at the mean PSQI global sleep quality score) and better sleepers (i.e., those one standard deviation below the mean PSQI global sleep quality score). Similarly, participants with a higher sagittal diameter displayed a steeper stress-related increase in IL-6 concentration relative to those with average or below average estimates of sagittal diameter (Table 2).
Table 2.
Simple slope analyses reveal that stress-related IL-6 and IL-6/IL-10 ratio increases vary as a function of sagittal diameter and PSQI global sleep quality scores, respectively. All analyses adjusted for age, number of medical comorbidities, and caregiver status.
| Outcome: IL-6 trajectory | B0(SE) | p-value | Btime (SE) | p-value |
|---|---|---|---|---|
| High visceral adiposity (+1 SD sagittal diameter) | 2.65 (0.50) | <0.001 | 1.46 (0.18) | <0.001 |
| Average visceral adiposity (mean sagittal diameter) | 2.21 (0.41) | <0.001 | 1.19 (0.13) | <0.001 |
| Low visceral adiposity (-1 SD sagittal diameter) | 1.78 (0.47) | <0.001 | 0.92 (0.18) | <0.001 |
| Outcome: IL-6/IL-10 trajectory | ||||
| Poor sleepers (+1 SD PSQI) | 1.00 (0.39) | 0.014 | 0.93 (0.16) | <0.001 |
| Average sleepers (mean PSQI) | 0.93 (0.41) | 0.027 | 0.69 (0.10) | <0.001 |
| Good sleepers (−1 SD PSQI) | 0.85 (0.56) | 0.133 | 0.45 (0.15) | 0.003 |
Figure 1.
IL-6/IL-10 ratio reactivity over the course of the Trier Social Stress Task (TSST) differs as a function of self-reported global sleep quality (PSQI). Analyses adjusted for participant age, number of medical comorbidities, and caregiver status. Betas (standard errors) are provided for each simple slope regression line.
The moderating influence of visceral adiposity
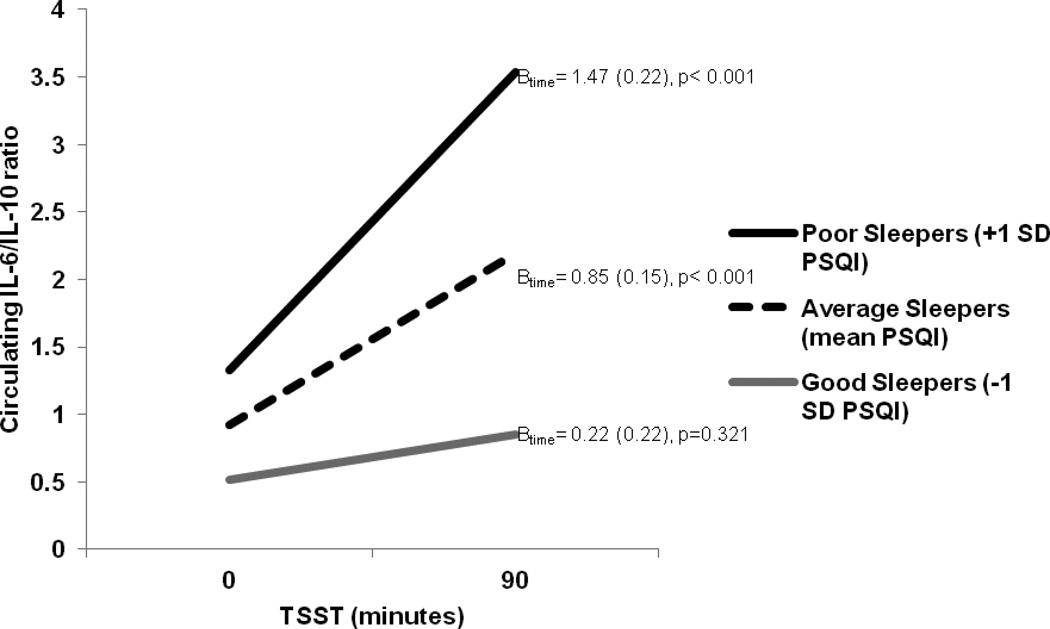
Because both visceral adiposity and PSQI global sleep quality alone were associated with cytokine reactivity, we tested the three-way interaction (PSQI global sleep quality X sagittal diameter X time) in predicting stress-related increases in IL-6 and IL-6/IL-10 ratio. Consistent with our hypotheses, both three-way interactions were Significant (IL-6: Bsleep*sagittal*time=0.03, SE=0.01, p=0.009; IL-6/IL-10: Bsleep*sagittal*time=0.02, SE=0.01, p=0.02) suggesting that the association between sleep quality and stress-related inflammatory activity varies as a function of sagittal diameter. To clarify these interactions, we next examined the simple interactions between PSQI global sleep quality and cytokine reactivity at one standard deviation above and below the mean of sagittal diameter. In this regard, at one standard deviation below the mean of sagittal diameter (i.e., low visceral adiposity) PSQI global sleep quality was unrelated to cytokine reactivity (IL-6: Bsleep*time= −0.10, SE=0.07, p=0.13; IL-6/IL-10: Bsleep *time=0.00, SE=0.06, p=0.97). In contrast, analyses revealed that PSQI global sleep quality was significantly associated with IL-6 and IL-6/IL-10 ratio reactivity among participants estimated to be one standard deviation above the mean of sagittal diameter (i.e., high visceral adiposity) (IL-6: Bsleep *time=0.13, SE=0.05 p=0.01; IL-6/IL-10: Bsleep*time=0.19, SE=0.05, p<0.001). Given the significant simple interaction at high levels of visceral adiposity, we next examined the simple slopes at one standard deviation above and below the means of PSQI global sleep quality. At high visceral adiposity, PSQI global sleep quality was unrelated to baseline levels of IL-6 (Bsleep=0.11, SE=0.10, p=0.31) and IL-6/IL-10 (Bsleep=0.11, SE=0.11, p=0.32); however, those reporting poorer PSQI global sleep quality displayed greater IL-6 and IL-6/IL-10 ratio reactivity relative to average and above average sleepers (Table 3; Figure 2).
Table 3.
Simple slope analyses revealed that stress-related increases in IL-6 and IL-6/IL-10 ratio varied as a function of PSQI global sleep quality scores at high visceral adiposity (+1 SD mean sagittal diameter) with steeper increases observed among poor sleepers. All analyses adjusted for age, number of medical comorbidities, and caregiver status.
| Outcome: IL-6 trajectory | B0(SE) | p-value | Btime (SE) | p-value |
|---|---|---|---|---|
| Poor sleepers (+1 SD PSQI) | 2.79 (0.50) | <0.001 | 1.86 (0.24) | <0.001 |
| Average sleepers (mean PSQI) | 2.43 (0.50) | <0.001 | 1.42 (0.18) | <0.001 |
| Good sleepers (−1 SD PSQI) | 2.08 (0.69) | 0.004 | 0.97 (0.26) | <0.001 |
| Outcome: IL-6/IL-10 trajectory | ||||
| Poor sleepers (+1 SD PSQI) | 1.33 (0.50) | 0.011 | 1.47 (0.22) | <0.001 |
| Average sleepers (mean PSQI) | 0.92 (0.51) | 0.076 | 0.85 (0.15) | <0.001 |
| Good sleepers (−1 SD PSQI) | 0.52 (0.74) | 0.489 | 0.22 (0.22) | 0.321 |
Figure 2.
IL-6/IL-10 ratio reactivity over the course of the Trier Social Stress Task (TSST) differs as a function of self-reported global sleep quality (PSQI) at high levels of visceral adiposity (i.e., one standard deviation above the mean on sagittal diameter). Analyses adjusted for participant age, number of medical comorbidities, and caregiver status. Betas (standard errors) are provided for each simple slope regression line.
Discussion
Individuals with poorer overall sleep quality and greater visceral adiposity displayed greater stress-induced increases in inflammatory activity than better sleepers and leaner individuals. Further, an interactions revealed that the influences of poor sleep quality on stress-induced concentrations of IL-6 and IL-6/IL-10 ratio were potentiated by high visceral adiposity, providing novel evidence for the complex link between sleep, adiposity, and inflammatory activity during periods of acute stress
The present study supports prior research demonstrating links between sleep and the immune system (Besedovsky et al., 2012; Bryant et al., 2004), and is consistent with a recent study that found poorer sleepers showed greater task-related increases in circulating IL-6 compared to better sleepers (Heffner et al., 2012). While in the hypothesized direction, the association between PSQI global sleep quality and stress-induced levels of IL-6 was below statistical significance in the present study (p=0.17). This may be attributable to a smaller sample size (n=48) compared to that employed by Heffner et al. (n=83). Nevertheless, the present study improves on this earlier finding by extending the investigation to another cytokine outcome- IL-6/IL-10 ratio, an index of proinfammatory bias, and by evaluating the modulatory effect of visceral adiposity. Together these preliminary findings raise the possibility that sleep quality may be a modulator of stress responsiveness with consequences for putative inflammatory mechanisms related to chronic disease risk.
In bivariate analyses, PSQI global sleep quality scores were unrelated to baseline cytokine levels. This is in contrast to some prior research (Prather et al., 2009b; Suarez, 2008) but consistent with others (Okun et al., 2009; Valentine et al., 2011). In addition, it is in line with Heffner et al., 2012 who also failed to observe baseline associations but, as noted, found that poorer overall sleep quality predicted stress-induced increases in IL-6. It is possible that the fact that PSQI global sleep quality is a composite measure and may not necessarily reflect objective sleep may help explain these equivocal findings. Accordingly, the use of objective measures of sleep, including home-polysomnography and actigraphy, would improve our understanding of what specific sleep parameters may uniquely contribute to baseline and stress-related inflammatory activity.
Participants with greater BMI and visceral adiposity displayed elevated baseline levels of IL-6 and IL-6/IL-10 ratio and levels of visceral adiposity were positively associated with stress-induced IL-6 increases in growth models. This is in line with a prior study showing that a higher waist circumference, a measure of visceral adiposity, but not BMI was associated with stress-induced increases in plasma levels of IL-1receptor antagonist (IL-1RA) (Brydon et al., 2008). However, unlike the current findings, this prior study failed to observe an effect of adiposity on concentrations of IL-6 following the acute laboratory stress. One possible explanation for this discrepancy may be the documented delayed response IL-6 shows compared to IL-1RA (Steptoe et al., 2001). Because the present study sampled IL-6 out as far as 90 minutes post-stressor (compared to 55 minutes in the Brydon et al. 2008 study), there was greater opportunity for adiposity to explain variability in IL-6 responsiveness. Another explanation may be that the present sample was comprised of only postmenopausal women (aged 51 to 79 years) compared to the young, premenopausal sample (aged 18 to 25 years) employed in the prior study. Cellular production of IL-6 in response laboratory stress has been shown to be greater in postmenopausal women compared premenopausal women (Prather et al., 2009a), a difference that may be due to levels of estradiol, which can inhibit inflammatory gene expression in vitro (Deshpande et al., 1997).
The biological pathways linking sleep and adiposity to stress-related increases in inflammation need clarification. It is well documented that acute laboratory stress results in activation of the sympathetic nervous system and hypothalamic-pituitary-adrenal (HPA) axis, both of which have been implicated in regulating inflammatory activity (Irwin and Cole, 2011). Enhanced sympathetically mediated blood pressure reactivity has been observed among sleep-deprived participants (Franzen et al., 2011). In addition, poorer sleep quality, measured objectively by actigraphy, is associated with a blunted cortisol response to acute laboratory stress (Wright et al., 2007b), which in turn may contribute to the slowed decline in NK cell numbers observed in prior research (Wright et al., 2007a) and the prolonged increase in inflammation observed in the present study. Visceral adipose tissue is replete with nerve innervation and β-adrenergic and glucocorticoid receptors (Black, 2006), raising the possibility that the elevated levels of inflammation may have originated from adipocytes (Mohamed-Ali et al., 1997). Macrophages migrate into adipose tissue in greater abundance in visceral fat than other types of adipose tissue (e.g., subcutaneous). In addition, resident macrophages in visceral fat often tend to express genes associated with a proinflammatory phenotype (i.e., M1). (Lumeng et al., 2007). Finally, dysregulation of the HPA axis and excess levels of cortisol have been observed among obese individuals (Pasquali et al., 2006), which may give rise to glucocorticoid receptor insensitivity and unregulated inflammatory activity in response to acute stress (Rohleder, 2012). Future studies are needed to substantiate these various mechanistic possibilities.
There are several limitations that should be considered when interpreting the present findings. First, the study sample was comprised of both chronically stressed female caregivers and low stress female non-caregivers. While preliminary analyses suggested that stress-related changes in cytokines were similar between these groups, the influence of unmeasured confounders cannot be ruled out. Further, the sample was limited to a small sample of postmenopausal women and should be replicated in other larger populations at different stages of the life course. Second, this study relied solely on sagittal diameter to provide a proxy for visceral fat, and as such further investigation using more sensitive measures of adipose composition, such as dual-energy X-ray absorptiometry (DEXA) or computed tomography (CT) is warranted. Third, this study did not assess the presence of obstructive sleep apnea (OSA), an important correlate of poor sleep quality, visceral adiposity, and inflammation. In general, women are at lower risk of OSA compared to men and while our sample ranged in BMI scores (17.7 to 37.5) less than 25% of the sample was obese, decreasing the likelihood that clinically significant OSA existed at a high rate in this sample. Nevertheless, it will be important to rule out the influence of OSA in future investigations. Fourth, this study lacked a control condition (i.e., a no-stress condition). While care was taken to standardize the timing of the stressor, so as to limit the effects of circadian variation, prolonged catheter insertion has been shown to result increased local inflammatory activity, including levels of IL-6 (Haack et al., 2002). Fifth, the cellar sources of inflammation in this study need to be elucidated. Multiple immune cell types as well as adipocytes have the capacity to produce proinflammatory mediators; accordingly, future studies are needed to identify which cell types account for the present findings. Finally, global sleep quality was based on a well-validated self-report measure that reflects overall sleep over the past month. As noted, use of objective sleep measures would improve our understanding of sleep effects on inflammation. Additionally, objective measures would aid in characterizing circadian disruption, which has been related to enhanced inflammatory responses (Castanon-Cervantes et al., 2010).
In summary, these findings provide preliminary evidence for the influence of poor overall sleep quality and visceral adiposity alone and especially in combination on the magnitude of pro-inflammatory cytokine reactivity to an acute stressor. This work supports prior research and provides novel evidence for the interaction between sleep and visceral adiposity as important factors in the understanding stress-related inflammatory disease risk.
Research Highlight.
Poor sleep quality and greater visceral adiposity, especially in combination, are associated with greater stress-related increases in circulating IL-6 and IL-6/IL-10.
Acknowledgement
The research study was supported by the Division of Behavioral and Social Research at the National Institute of Health/National Institute of Aging R56 Grant (AG030424) (ESE), a K08 grant (MH64110) from the National Institute of Mental Health (ESE), a K08 grant (HL112961) from the National Heart, Lung & Blood Institute (AAP), and The Carl & Elizabeth Naumann Fund Startup Grant (FSD). We thank Jean M. Tillie for running the cytokine assays.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Beccuti G, Pannain S. Sleep and obesity. Curr Opin Clin Nutr Metab Care. 2011;14:402–412. doi: 10.1097/MCO.0b013e3283479109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besedovsky L, Lange T, Born J. Sleep and immune function. Pflugers Archiv : European journal of physiology. 2012;463:121–137. doi: 10.1007/s00424-011-1044-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black PH. The inflammatory consequences of psychologic stress: relationship to insulin resistance, obesity, atherosclerosis and diabetes mellitus, type II. Med Hypotheses. 2006;67:879–891. doi: 10.1016/j.mehy.2006.04.008. [DOI] [PubMed] [Google Scholar]
- Born J, Lange T, Hansen K, Molle M, Fehm HL. Effects of sleep and circadian rhythm on human circulating immune cells. J Immunol. 1997;158:4454–4464. [PubMed] [Google Scholar]
- Bryant PA, Trinder J, Curtis N. Sick and tired: Does sleep have a vital role in the immune system? Nat Rev Immunol. 2004;4:457–467. doi: 10.1038/nri1369. [DOI] [PubMed] [Google Scholar]
- Brydon L. Adiposity, leptin and stress reactivity in humans. Biol Psychol. 2011;86:114–120. doi: 10.1016/j.biopsycho.2010.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brydon L, Wright CE, O’Donnell K, Zachary I, Wardle J, Steptoe A. Stress-induced cytokine responses and central adiposity in young women. Int J Obes (Lond) 2008;32:443–450. doi: 10.1038/sj.ijo.0803767. [DOI] [PubMed] [Google Scholar]
- Burgos I, Richter L, Klein T, Fiebich B, Feige B, Lieb K, Voderholzer U, Riemann D. Increased nocturnal interleukin-6 excretion in patients with primary insomnia: a pilot study. Brain, behavior, and immunity. 2006;20:246–253. doi: 10.1016/j.bbi.2005.06.007. [DOI] [PubMed] [Google Scholar]
- Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry research. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- Cappuccio FP, D’Elia L, Strazzullo P, Miller MA. Quantity and quality of sleep and incidence of type 2 diabetes: a systematic review and meta-analysis. Diabetes Care. 2010;33:414–420. doi: 10.2337/dc09-1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cappuccio FP, Taggart FM, Kandala NB, Currie A, Peile E, Stranges S, Miller MA. Meta-analysis of short sleep duration and obesity in children and adults. Sleep. 2008;31:619–626. doi: 10.1093/sleep/31.5.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castanon-Cervantes O, Wu M, Ehlen JC, Paul K, Gamble KL, Johnson RL, Besing RC, Menaker M, Gewirtz AT, Davidson AJ. Dysregulation of inflammatory responses by chronic circadian disruption. J Immunol. 2010;185:5796–5805. doi: 10.4049/jimmunol.1001026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chawla A, Nguyen KD, Goh YP. Macrophage-mediated inflammation in metabolic disease. Nat Rev Immunol. 2011;11:738–749. doi: 10.1038/nri3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J, Cohen P, West SG, Aiken LS. Applied multiple regression/correlation analysis for the behavioral sciences. Mahwah, NJ: Lawrence Erlbaum Associates, Inc; 2003. [Google Scholar]
- Daftarian PM, Kumar A, Kryworuchko M, Diaz-Mitoma F. IL-10 production is enhanced in human T cells by IL-12 and IL-6 and in monocytes by tumor necrosis factor-alpha. J Immunol. 1996;157:12–20. [PubMed] [Google Scholar]
- Davis MC, Twamley EW, Hamilton NA, Swan PD. Body fat distribution and hemodynamic stress responses in premenopausal obese women: a preliminary study. Health Psychol. 1999;18:625–633. doi: 10.1037//0278-6133.18.6.625. [DOI] [PubMed] [Google Scholar]
- Deshpande R, Khalili H, Pergolizzi RG, Michael SD, Chang MD. Estradiol down-regulates LPS-induced cytokine production and NFkB activation in murine macrophages. Am J Reprod Immunol. 1997;38:46–54. doi: 10.1111/j.1600-0897.1997.tb00275.x. [DOI] [PubMed] [Google Scholar]
- Dimitrov S, Lange T, Benedict C, Nowell MA, Jones SA, Scheller J, Rose-John S, Born J. Sleep enhances IL-6 trans-signaling in humans. FASEB J. 2006;20:2174–2176. doi: 10.1096/fj.06-5754fje. [DOI] [PubMed] [Google Scholar]
- Epel ES, McEwen B, Seeman T, Matthews K, Castellazzo G, Brownell KD, Bell J, Ickovics JR. Stress and body shape: stress-induced cortisol secretion is consistently greater among women with central fat. Psychosom Med. 2000;62:623–632. doi: 10.1097/00006842-200009000-00005. [DOI] [PubMed] [Google Scholar]
- Franzen PL, Gianaros PJ, Marsland AL, Hall MH, Siegle GJ, Dahl RE, Buysse DJ. Cardiovascular reactivity to acute psychological stress following sleep deprivation. Psychosomatic medicine. 2011;73:679–682. doi: 10.1097/PSY.0b013e31822ff440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredericks CA, Drabant EM, Edge MD, Tillie JM, Hallmayer J, Ramel W, Kuo JR, Mackey S, Gross JJ, Dhabhar FS. Healthy young women with serotonin transporter SS polymorphism show a pro-inflammatory bias under resting and stress conditions. Brain Behav Immun. 2010;24:350–357. doi: 10.1016/j.bbi.2009.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gangwisch JE, Heymsfield SB, Boden-Albala B, Buijs RM, Kreier F, Pickering TG, Rundle AG, Zammit GK, Malaspina D. Short sleep duration as a risk factor for hypertension: analyses of the first National Health and Nutrition Examination Survey. Hypertension. 2006;47:833–839. doi: 10.1161/01.HYP.0000217362.34748.e0. [DOI] [PubMed] [Google Scholar]
- Gangwisch JE, Heymsfield SB, Boden-Albala B, Buijs RM, Kreier F, Pickering TG, Rundle AG, Zammit GK, Malaspina D. Sleep duration as a risk factor for diabetes incidence in a large U.S. sample. Sleep. 2007;30:1667–1673. doi: 10.1093/sleep/30.12.1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gangwisch JE, Malaspina D, Boden-Albala B, Heymsfield SB. Inadequate sleep as a risk factor for obesity: analyses of the NHANES I. Sleep. 2005;28:1289–1296. doi: 10.1093/sleep/28.10.1289. [DOI] [PubMed] [Google Scholar]
- Gottlieb DJ, Redline S, Nieto FJ, Baldwin CM, Newman AB, Resnick HE, Punjabi NM. Association of usual sleep duration with hypertension: the Sleep Heart Health Study. Sleep. 2006;29:1009–1014. doi: 10.1093/sleep/29.8.1009. [DOI] [PubMed] [Google Scholar]
- Haack M, Kraus T, Schuld A, Dalal M, Koethe D, Pollmacher T. Diurnal variations of interleukin-6 plasma levels are confounded by blood drawing procedures. Psychoneuroendocrinology. 2002;27:921–931. doi: 10.1016/s0306-4530(02)00006-9. [DOI] [PubMed] [Google Scholar]
- Heffner KL, Ng HM, Suhr JA, France CR, Marshall GD, Pigeon WR, Moynihan JA. Sleep disturbance and older adults' inflammatory responses to acute stress. Am J Geriatr Psychiatry. 2012;20:744–752. doi: 10.1097/JGP.0b013e31824361de. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin MR, Cole SW. Reciprocal regulation of the neural and innate immune systems. Nat Rev Immunol. 2011;11:625–632. doi: 10.1038/nri3042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King CR, Knutson KL, Rathouz PJ, Sidney S, Liu K, Lauderdale DS. Short sleep duration and incident coronary artery calcification. JAMA. 2008;300:2859–2866. doi: 10.1001/jama.2008.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschbaum C, Pirke KM, Hellhammer DH. The ‘Trier Social Stress Test’--a tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology. 1993;28:76–81. doi: 10.1159/000119004. [DOI] [PubMed] [Google Scholar]
- Libby P. Inflammation in atherosclerosis. Nature. 2002;420:868–874. doi: 10.1038/nature01323. [DOI] [PubMed] [Google Scholar]
- Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest. 2007;117:175–184. doi: 10.1172/JCI29881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matzner P, Hazut O, Naim R, Shaashua L, Sorski L, Levi B, Sadeh A, Wald I, Bar-Haim Y, Ben-Eliyahu S. Resilience of the immune system in healthy young students to 30-hour sleep deprivation with psychological stress. Neuroimmunomodulation. 2013;20:194–204. doi: 10.1159/000348698. [DOI] [PubMed] [Google Scholar]
- Miller MA, Kandala NB, Kivimaki M, Kumari M, Brunner EJ, Lowe GD, Marmot MG, Cappuccio FP. Gender differences in the cross-sectional relationships between sleep duration and markers of inflammation: Whitehall II study. Sleep. 2009;32:857–864. [PMC free article] [PubMed] [Google Scholar]
- Mohamed-Ali V, Goodrick S, Rawesh A, Katz DR, Miles JM, Yudkin JS, Klein S, Coppack SW. Subcutaneous adipose tissue releases interleukin-6, but not tumor necrosis factor-alpha, in vivo. J Clin Endocrinol Metab. 1997;82:4196–4200. doi: 10.1210/jcem.82.12.4450. [DOI] [PubMed] [Google Scholar]
- Motivala SJ. Sleep and Inflammation: Psychoneuroimmunology in the Context of Cardiovascular Disease. Ann Behav Med. 2011 doi: 10.1007/s12160-011-9280-2. [DOI] [PubMed] [Google Scholar]
- Mozaffarian D, Hao T, Rimm EB, Willett WC, Hu FB. Changes in diet and lifestyle and long-term weight gain in women and men. N Engl J Med. 2011;364:2392–2404. doi: 10.1056/NEJMoa1014296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullington JM, Simpson NS, Meier-Ewert HK, Haack M. Sleep loss and inflammation. Best practice & research. Clinical endocrinology & metabolism. 2010;24:775–784. doi: 10.1016/j.beem.2010.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neels JG, Olefsky JM. Inflamed fat: what starts the fire? J Clin Invest. 2006;116:33–35. doi: 10.1172/JCI27280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odegaard JI, Chawla A. Mechanisms of macrophage activation in obesity-induced insulin resistance. Nature clinical practice. Endocrinology & metabolism. 2008;4:619–626. doi: 10.1038/ncpendmet0976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okun ML, Coussons-Read M, Hall M. Disturbed sleep is associated with increased C-reactive protein in young women. Brain, behavior, and immunity. 2009;23:351–354. doi: 10.1016/j.bbi.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouchi N, Parker JL, Lugus JJ, Walsh K. Adipokines in inflammation and metabolic disease. Nature reviews. Immunology. 2011;11:85–97. doi: 10.1038/nri2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasquali R, Vicennati V, Cacciari M, Pagotto U. The hypothalamic-pituitary-adrenal axis activity in obesity and the metabolic syndrome. Ann N Y Acad Sci. 2006;1083:111–128. doi: 10.1196/annals.1367.009. [DOI] [PubMed] [Google Scholar]
- Pouliot MC, Despres JP, Lemieux S, Moorjani S, Bouchard C, Tremblay A, Nadeau A, Lupien PJ. Waist circumference and abdominal sagittal diameter: best simple anthropometric indexes of abdominal visceral adipose tissue accumulation and related cardiovascular risk in men and women. Am J Cardiol. 1994;73:460–468. doi: 10.1016/0002-9149(94)90676-9. [DOI] [PubMed] [Google Scholar]
- Pradhan AD, Manson JE, Rifai N, Buring JE, Ridker PM. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA. 2001;286:327–334. doi: 10.1001/jama.286.3.327. [DOI] [PubMed] [Google Scholar]
- Prather AA, Carroll JE, Fury JM, McDade KK, Ross D, Marsland AL. Gender differences in stimulated cytokine production following acute psychological stress. Brain Behav Immun. 2009a;23:622–628. doi: 10.1016/j.bbi.2008.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prather AA, Marsland AL, Hall M, Neumann SA, Muldoon MF, Manuck SB. Normative variation in self-reported sleep quality and sleep debt is associated with stimulated pro-inflammatory cytokine production. Biol Psychol. 2009b;82:12–17. doi: 10.1016/j.biopsycho.2009.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridker PM, Rifai N, Stampfer MJ, Hennekens CH. Plasma concentration of interleukin-6 and the risk of future myocardial infarction among apparently healthy men. Circulation. 2000;101:1767–1772. doi: 10.1161/01.cir.101.15.1767. [DOI] [PubMed] [Google Scholar]
- Rohleder N. Acute and chronic stress induced changes in sensitivity of peripheral inflammatory pathways to the signals of multiple stress systems --2011 Curt Richter Award Winner. Psychoneuroendocrinology. 2012;37:307–316. doi: 10.1016/j.psyneuen.2011.12.015. [DOI] [PubMed] [Google Scholar]
- Rush AJ, Gullion CM, Basco MR, Jarrett RB, Trivedi MH. The Inventory of Depressive Symptomatology (IDS): psychometric properties. Psychol Med. 1996;26:477–486. doi: 10.1017/s0033291700035558. [DOI] [PubMed] [Google Scholar]
- Shearer WT, Reuben JM, Mullington JM, Price NJ, Lee BN, Smith EO, Szuba MP, Van Dongen HP, Dinges DF. Soluble TNF-alpha receptor 1 and IL-6 plasma levels in humans subjected to the sleep deprivation model of spaceflight. The Journal of allergy and clinical immunology. 2001;107:165–170. doi: 10.1067/mai.2001.112270. [DOI] [PubMed] [Google Scholar]
- Singer JD, Willett JB. Applied Longitudinal Data Analysis: Modeling change and event occurrence. New York: Oxford University Press; 2003. [Google Scholar]
- Solarz DE, Mullington JM, Meier-Ewert HK. Sleep, inflammation and cardiovascular disease. Front Biosci (Elite Ed) 2012;4:2490–2501. doi: 10.2741/e560. [DOI] [PubMed] [Google Scholar]
- Steptoe A, Hamer M, Chida Y. The effects of acute psychological stress on circulating inflammatory factors in humans: a review and meta-analysis. Brain Behav Immun. 2007;21:901–912. doi: 10.1016/j.bbi.2007.03.011. [DOI] [PubMed] [Google Scholar]
- Steptoe A, Willemsen G, Owen N, Flower L, Mohamed-Ali V. Acute mental stress elicits delayed increases in circulating inflammatory cytokine levels. Clin Sci (Lond) 2001;101:185–192. [PubMed] [Google Scholar]
- Suarez EC. Self-reported symptoms of sleep disturbance and inflammation, coagulation, insulin resistance and psychosocial distress: evidence for gender disparity. Brain, behavior, and immunity. 2008;22:960–968. doi: 10.1016/j.bbi.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taheri S, Lin L, Austin D, Young T, Mignot E. Short sleep duration is associated with reduced leptin, elevated ghrelin, and increased body mass index. PLoS Med. 2004;1:e62. doi: 10.1371/journal.pmed.0010062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unruh ML, Redline S, An MW, Buysse DJ, Nieto FJ, Yeh JL, Newman AB. Subjective and objective sleep quality and aging in the sleep heart health study. Journal of the American Geriatrics Society. 2008;56:1218–1227. doi: 10.1111/j.1532-5415.2008.01755.x. [DOI] [PubMed] [Google Scholar]
- Valentine RJ, Woods JA, McAuley E, Dantzer R, Evans EM. The associations of adiposity, physical activity and inflammation with fatigue in older adults. Brain Behav Immun. 2011;25:1482–1490. doi: 10.1016/j.bbi.2011.06.002. [DOI] [PubMed] [Google Scholar]
- Vgontzas AN, Papanicolaou DA, Bixler EO, Lotsikas A, Zachman K, Kales A, Prolo P, Wong ML, Licinio J, Gold PW, Hermida RC, Mastorakos G, Chrousos GP. Circadian interleukin-6 secretion and quantity and depth of sleep. J Clin Endocrinol Metab. 1999;84:2603–2607. doi: 10.1210/jcem.84.8.5894. [DOI] [PubMed] [Google Scholar]
- Vgontzas AN, Zoumakis E, Bixler EO, Lin HM, Follett H, Kales A, Chrousos GP. Adverse effects of modest sleep restriction on sleepiness, performance, and inflammatory cytokines. J Clin Endocrinol Metab. 2004;89:2119–2126. doi: 10.1210/jc.2003-031562. [DOI] [PubMed] [Google Scholar]
- Waldstein SR, Burns HO, Toth MJ, Poehlman ET. Cardiovascular reactivity and central adiposity in older African Americans. Health Psychol. 1999;18:221–228. doi: 10.1037//0278-6133.18.3.221. [DOI] [PubMed] [Google Scholar]
- Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright CE, Erblich J, Valdimarsdottir HB, Bovbjerg DH. Poor sleep the night before an experimental stressor predicts reduced NK cell mobilization and slowed recovery in healthy women. Brain Behav Immun. 2007a;21:358–363. doi: 10.1016/j.bbi.2006.08.007. [DOI] [PubMed] [Google Scholar]
- Wright CE, Valdimarsdottir HB, Erblich J, Bovbjerg DH. Poor sleep the night before an experimental stress task is associated with reduced cortisol reactivity in healthy women. Biol Psychol. 2007b;74:319–327. doi: 10.1016/j.biopsycho.2006.08.003. [DOI] [PubMed] [Google Scholar]
- Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, Sole J, Nichols A, Ross JS, Tartaglia LA, Chen H. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112:1821–1830. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo SS, Gujar N, Hu P, Jolesz FA, Walker MP. The human emotional brain without sleep--a prefrontal amygdala disconnect. Current biology : CB. 2007;17:R877–R878. doi: 10.1016/j.cub.2007.08.007. [DOI] [PubMed] [Google Scholar]
- Zamboni M, Turcato E, Armellini F, Kahn HS, Zivelonghi A, Santana H, Bergamo-Andreis IA, Bosello O. Sagittal abdominal diameter as a practical predictor of visceral fat. Int J Obes Relat Metab Disord. 1998;22:655–660. doi: 10.1038/sj.ijo.0800643. [DOI] [PubMed] [Google Scholar]