Abstract
Phosphoinositides (PIs) make up only a small fraction of cellular phospholipids, yet they control almost all aspects of a cell's life and death. These lipids gained tremendous research interest as plasma membrane signaling molecules when discovered in the 1970s and 1980s. Research in the last 15 years has added a wide range of biological processes regulated by PIs, turning these lipids into one of the most universal signaling entities in eukaryotic cells. PIs control organelle biology by regulating vesicular trafficking, but they also modulate lipid distribution and metabolism via their close relationship with lipid transfer proteins. PIs regulate ion channels, pumps, and transporters and control both endocytic and exocytic processes. The nuclear phosphoinositides have grown from being an epiphenomenon to a research area of its own. As expected from such pleiotropic regulators, derangements of phosphoinositide metabolism are responsible for a number of human diseases ranging from rare genetic disorders to the most common ones such as cancer, obesity, and diabetes. Moreover, it is increasingly evident that a number of infectious agents hijack the PI regulatory systems of host cells for their intracellular movements, replication, and assembly. As a result, PI converting enzymes began to be noticed by pharmaceutical companies as potential therapeutic targets. This review is an attempt to give an overview of this enormous research field focusing on major developments in diverse areas of basic science linked to cellular physiology and disease.
I. INTRODUCTION
It is hard to define the research interest of people who study polyphosphoinositides (PPIs). Naturally, PPIs are lipid molecules, yet many researchers who study PPIs did not initially have a primary interest in lipids. Many of us have gotten interested in PPIs when these lipids became known as the source of second messengers in transducing signals from cell surface receptors. The spectacular progress in the 1980s in defining the pathways by which G protein-coupled receptors (GPCRs) and receptor tyrosine kinases (RTKs) activated phospholipase C (PLC) enzymes had a major impact on many scientists who showed interest in transmembrane signaling. However, cell biologists also developed immense interest in PPIs because of the importance of PPIs in shaping the membranes and controlling vesicular trafficking and organelle physiology. The attention of scientists who study ion channels also turned toward PPIs as it became obvious that many channels or transporters require PPIs for their activity or control. The discovery of phosphatidylinositol 3-kinases (PI3Ks) has set the stage to widen research interest in PPIs: association of PI3K with oncogenic as well as RTKs and their strong ties with cancer biology has won over cancer researchers, while the importance of PPIs in immune cell functions, chemotaxis, and secretion brought immunologists to the field. If this had not been enough, researchers working with infectious diseases noted that many pathogenic organisms possess enzymes essential for their pathogenic nature that act upon PPIs to invade cells or use the host cells' PPI machinery to evade natural defense mechanisms or reprogram cells to produce the pathogen. Neuroscientists also discovered that synaptic vesicle exocytosis and recycling requires phosphoinositides at multiple steps and that brain development, including neurite outgrowth and axon guidance, is highly dependent on PPIs. Even the invertebrate photo-sensing and signal transduction is dependent on PPIs, further extending the group of scientists showing interest in PPIs. This selected and probably incomplete list increases every day as more and more cellular processes are linked to these universal lipid regulators.
Such an ever-expanding list of processes regulated by PPIs begs an answer to the fundamental question of how and why these lipids gained such a pivotal role in eukaryotic cell regulation during evolution? What structural and functional features make these molecules so widely used and so adaptable to support the functions of a variety of signaling complexes? We have only begun to ask, let alone answer these questions for which evolution may give us some clues. Although PIs have been detected in mycobacteria, their appearance in evolution coincides with the development of internal membranes and organelles. Remarkably, PI kinases surfaced earlier in evolution than tyrosine kinases (190, 986) with common ancestors being a group of serine-threonine kinases, called the PI-kinase related kinases (190, 669). The latter enzymes are all functionally linked to DNA damage control and repair (190, 1350, 1422). PtdIns is unique among phospholipids in that it is a rich phosphorylation target at the cytoplasmic surface of any cellular membrane. In their phosphorylated forms, PPIs can serve as critical reference points for a great variety of proteins to find their docking destinations and/or change their conformation. This is true for cytosolic proteins that are recruited to the membrane by PPIs, as well as for peripheral or integral membrane proteins whose membrane adjacent regions or cytoplasmic “tails” show interaction with PPIs.
With the spectacular expansion of the PI field, it has become impossible to cover all aspects of PPI regulation at great depth in a comprehensive review. In the following overview I will attempt to describe the most basic features of the enzymes that synthesize and degrade PPIs and focus on aspects of this diverse research field that highlight general principles that govern PI-mediated regulation of the many different processes. For a more comprehensive analysis and deeper understanding of the details of the individual processes and their PPI regulation, the reader is referred to the many excellent reviews that have been published over the years and ones that are likely still in preparation. Soluble inositol phosphates partly liberated from inositol lipids and further phosphorylated to highly charged inositol polyphosphates also represent a research topic of great interest but will not be discussed here. The interested reader will find excellent reviews on that topic (29, 1079, 1390). Another aspect of PtdIns function not covered here is related to PtdIns serving as an anchor for a selected group of proteins found on the cell surface linked to the 6-position of the inositol ring through glycan linkages. More about the biology of these GPI-anchored proteins can be found in several comprehensive reviews (473, 1200).
II. THE BASICS: PHOSPHOINOSITIDE STRUCTURES AND ENZYMOLOGY
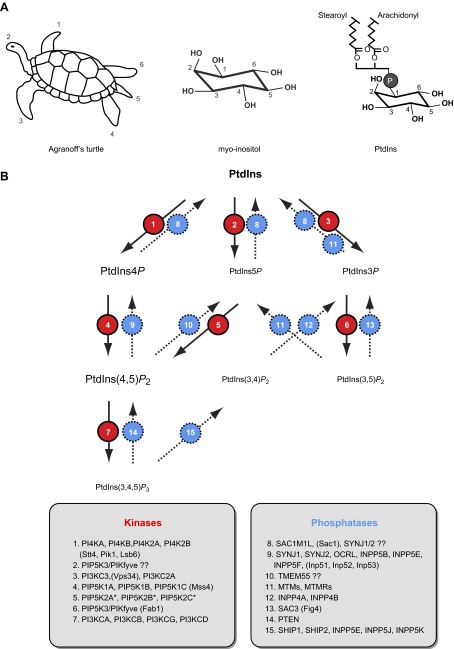
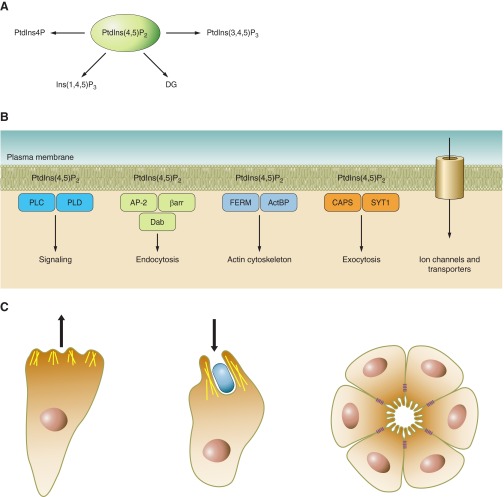
Polyphosphoinositides are phosphorylated derivatives of PtdIns generated by a number of kinases and phosphatases that act upon their membrane-bound lipid substrates (Figure 1). Phosphorylation occurs in one of the -OH groups of the inositol ring of PtdIns that is linked to the diacylglycerol (DG) backbone via a phosphodiester linkage utilizing the -OH group of the ring at the D1 position. PtdIns contains myo-inositol that assumes a “chair” conformation with five of its six -OH groups being equatorial and the one at position 2 being axial. The best visual representation of the myo-inositol structure and ring numbering was introduced by Bernard William Agranoff in 1978, who likened the “chair” structure of myo-inositol to a turtle whose body represents the inositol ring and the numbering starts at the right front flipper and proceeds counterclockwise through the head and the other appendages (9) (Figure 1A). Since the turtle is taken through its right flipper by the DG backbone, it leaves five hydroxyls for phosphorylation, but only three of these (positions -3, -4, and -5) are actually phosphorylated in naturally occurring PPIs according to current knowledge. The combination of these phosphorylations gives rise to the seven known PPIs (Figure 1B). A distinctive feature of PPIs is their enrichment in arachidonic acid at the sn-2 position of their glycerol backbone. The majority of the PPIs is the 1-stearoyl-2 arachidonyl form, and it has been a puzzling question where this enrichment takes place and what role deacylation-reacylation cycles play in determining this composition. It has been suggested that enzymes responsible for PtdIns synthesis and phosphorylation may show preference to the form(s) of lipids esterified with arachidonic acid at the sn-2 position (405). Arachidonate-rich phosphoinositides are also believed to be the source of PLA2-mediated arachidonate release for the synthesis of prostaglandins and leukotrienes.
Figure 1.
Phosphoinositide basics. A: Agranoff's turtle demonstrating the orientation of the hydroxyl groups in myo-inositol. B: interconversions between various phosphoinositides and the enzymes catalyzing these reactions. The yeast enzymes are listed in parentheses. Where there is some ambiguity it is indicated by “??”. *It is worth pointing out that contrary to their designation, PIP5K2s are 4-kinases that act on PtdIns5P.
The amounts of PPIs within cells have been estimated in different cells and tissues (1121, 1703). These estimates and measurements show significant variations. PtdIns represents ∼10–20% (mol%) of total cellular phospholipids, whereas PtdIns4P and PtdIns(4,5)P2 constitute ∼0.2–1%. Based on long-term [3H]inositol labeling, PtdIns4P and PtdIns(4,5)P2 have about 2–5% of the labeling relative to PtdIns (e.g., Ref. 87). Recent estimates of PtdIns(4,5)P2 density in the plasma membrane (PM) ranged between 5,000–20,000 molecules/μm2 (421). The other PPIs contribute even smaller amounts, PtdIns(3,4,5)P3 being about 2–5% of PtdIns(4,5)P2 and PtdIns3P about 20–30% of PtdIns4P. It is noteworthy that these ratios show great tissue variations and they are also very different in yeast and plants. Yeast do not have detectable amounts of PtdIns(3,4,5)P3 and some plants have a lot more PtdIns4P relative to a smaller pool of PtdIns(4,5)P2, and it is not clear if PtdIns(3,4,5)P3 is at all present in plants (1093, 1469). Remarkably, yeast and plant orthologs of the mammalian PTEN enzyme that has a critical role in PtdIns(3,4,5)P3 dephosphorylation have been found in spite of the apparent lack of detectable PtdIns(3,4,5)P3 in these organisms (611).
PtdIns is synthesized in the endoplasmic reticulum from CDP-DAG and myo-inositol by a PtdIns synthase (PIS) enzyme (11) (see sect. IV) and is then distributed throughout the cell presumably by several PI transfer proteins (PITPs) (277, 689) and possibly via vesicular trafficking. Our recent studies identified the PIS enzyme in an ER-derived highly mobile “organelle” that may serve as a dynamic PtdIns distribution device (796). Early studies already detected the phosphorylation reaction generating two “polyphosphoinositides” that had been previously described in the brain and determined to be PtdIns4P and PtdIns(4,5)P2 (186), thereby postulating PtdIns kinase (PI-kinase) and PtdInsP kinase (PIP kinase) activities associated with membranes (1044). Although these enzymatic activities were associated with various membrane fractions in fractionated tissues, and they showed even some unique features (like sensitivity to different detergents), the general consensus that emerged from these studies was that PI- and PIP-kinase activities were primarily present in the PM, serving what has become known as the signaling pool of PPIs (see sect. III). However, by now it is apparent that multiple isoforms of almost all of the kinase and phosphatase enzymes act upon PPIs, and they do so in different cellular compartments. This explains why in most cases these enzymes are not functionally redundant even if they catalyze the same biochemical reaction. The mechanism(s) that determine the intracellular localization and regulation of the PI kinase and phosphatase enzymes became a central question for each family of these proteins. Generally speaking, most PtdIns mono-phosphorylations (by the PI4Ks and Class III PI3K) occur in endomembranes, such as the endosomes and the Golgi/trans-Golgi network, whereas the phosphorylation of PtdIns4P to PtdIns(4,5)P2 by PIP 5-kinases and further to PtdIns(3,4,5)P3 by class I PI3Ks occurs primarily at the PM.
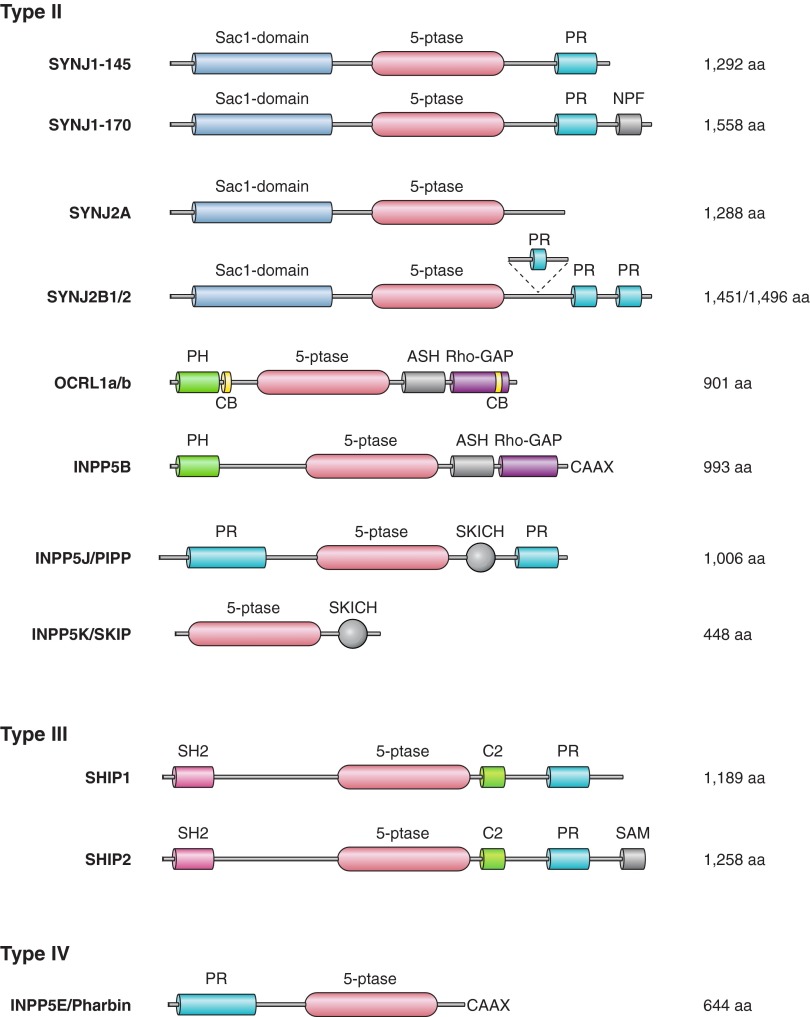
The two main routes of PPI elimination are through dephosphorylation by PPI phosphatases and hydrolysis by phosphoinositide-specific phospholipase C enzymes (PLCs). Some of the PPI phosphatases are quite specific for the position of the phosphate group that they remove, while others, mainly the ones that dephosphorylate monophosphorylated PPIs, are more promiscuous and their functional specificity lies in their localization. The diversity of the PPI phosphatases parallels, in fact, exceeds that of the kinases (Figure 1B), and several human diseases have been traced to the dysfunction of PPI phosphatases (see sect. VI). The diversity of phosphoinositide-specific PLCs is also remarkable (see sect. VII). The preferred in vivo substrate of the mammalian PLC enzymes is believed to be PtdIns(4,5)P2, although this question has not been satisfactorily answered in whole cell studies and purified PLC enzymes can also hydrolyze PtdIns4P and PtdIns in vitro.
Although individual groups of PPI metabolizing enzymes will be featured in more details below, a few important general questions are worth highlighting here. The first is related to their substrate recognition. Most, if not all, of the PPI kinase and phosphatase enzymes are loosely membrane-associated peripheral membrane proteins that utilize a substrate that is part of the membrane with the inositol headgroup facing the cytoplasmic leaflet of the membrane. When enzyme activities of these proteins are measured in vitro, the assay usually contains the lipid substrate in some form of lipid micelle, and the type and amount of detergent yielding optimal activity greatly differ for each of these enzymes. For example, the in vitro activity of PI 4-kinases depends on the presence of detergents such as Triton X-100, while those of the class I PI 3-kinases are inhibited by detergents and the activity of class III PI 3-kinases are usually assayed in the presence of Mn2+ instead of the usual Mg2+. Overexpression in cells of some of the PI kinases (such as the type III PI 4-kinases) hardly yields an increase in the phosphorylation of their endogenous lipid substrates. This indicates that the mechanism(s) that ensure recruitment of the enzymes to the membrane and their access to the membrane-bound PtdIns substrate are major determinants of their in situ activities. Few studies have been designed to understand the exact nature of the lipid substrate PI kinase interactions, and most of the solved structures of the kinase enzymes do not resolve the activation loop (a mobile part of the molecule that is critical for substrate presentation) within their catalytic center. Similarly, the lipid substrate in these structures is either missing or, if present, was provided in the form of a soluble inositol-phosphate headgroup. Therefore, there is a major gap in our understanding of how these enzymes work in their natural membrane environment.
Phosphoinositides affect cellular functions by interacting with molecules that either reside in the membrane, such as ion channels and transporters, or get recruited to the membrane by reversible mechanisms. Several signaling molecules are recruited to the membrane through interaction with PPIs via inositide-binding protein modules (see Table 1). The first such protein module was identified in pleckstrin (574), and ever since, the homologous modules have been termed pleckstrin homology (PH) domains. PH domains are present in a large number of regulatory molecules (296). It is important to note, however, that not all PH domains bind lipids and probably all PH domains also bind proteins (880). Often simultaneous protein and lipid binding are required for the membrane recruitment or conformational change of PH domains; hence, these (and other PI binding modules) are called coincidence detectors (214). Most frequently the protein input for PH domains come from interaction with small GTP binding proteins. Other domains, such as the FERM domains link the actin cytoskeleton to the PM (255). Both FERM and GLUE-domains contain a structural fold similar to that of PTB (phosphotyrosine binding) or PH domains (1062, 1416). EHD domains (1120) and BAR domains also bind anionic lipids including inositol lipids and also sense and/or generate membrane curvatures (466). This list is ever expanding and now also includes PDZ domains (1825) and the KA1 domain, a novel fold found at the COOH terminus of a range of proteins and which binds PtdIns(4,5)P2 but also other anionic phospholipids such as PS (1083). A recently described PtdIns(3,4,5)P3 binding domain found at the COOH termini of some IQ domain containing GAP proteins has a structure reminiscent of the integral fold of C2 domains (363). In addition, several proteins contain polybasic stretches that do not amount to a characteristic domain, but also interact with acidic phospholipids with electrostatic interaction. Good examples are the MARCKS proteins (1670) and the K-Ras COOH terminus (601), but many other proteins show membrane association using this mechanism. Importantly, many of these targeting sequences can use PtdIns4P as well as PtdIns(4,5)P2 as their membrane anchor lipid (559).
Table 1.
Inositide binding domains
| Name of Domain | Lipid Species | Reference Nos. |
|---|---|---|
| Pleckstrin homology (PH) domains | PtdIns(4,5)P2 | 574, 882 |
| PtdIns4P | 381, 895 | |
| PtdIns(3,4,5)P3 | 478, 1252, 1627 | |
| PtdIns(3,4)P2 | 381, 460 | |
| FYVE domains | PtdIns3P | 1457 |
| (named after the four proteins in which they were first identified: Fab1,YOTB/ZK632, Vac1p, and EEA1) | ||
| Phox homology (PX) domains | PtdIns3P | 1721 |
| ENTH (epsin NH2-terminal homology) | PtdIns(4,5)P2 | 877 |
| ANTH (AP180 NH2-terminal homology) | PtdIns(4,5)P2 | 877 |
| FERM domains | PtdIns(4,5)P2 | 255 |
| (named after the first proteins in which they were found: 4.1-ezrin-radexin-moiesin) | ||
| PTB domain | PtdIns(4,5)P2 | 1062 |
| GLUE domain | PtdIns(3,4,5)P3 | 1416 |
| Eps15 homology (EHD) domains | PtdIns(4,5)P2, PtdIns4P | 1120 |
| BAR (Bin–Amphiphysin–Rvs) | PtdIns(4,5)P2, other lipids | 1211, 1607 |
| PDZ domain | PtdIns(4,5)P2 | 1825 |
| KA1 (kinase-associated 1) domain | Acidic phospholipids, PS | 1083 |
III. HISTORICAL OVERVIEW
Although one can argue that a newcomer to a field can benefit from not being biased by existing dogmas, ignorance of the history of a research field and lack of understanding of the milestones and breakthroughs are making it difficult, if not impossible, to put any new research findings into perspective. One also has to know and respect the contribution of the scientists whose findings serve as the foundation upon which new knowledge can be built. Therefore, I give a short overview of the field that is certainly biased by my experiences, but can still give some ideas about the major milestones as this research field has evolved. Several reviews and recollections have been published on the historical aspects of this huge research field, and they are highly recommended for interested readers (10, 634, 701, 1039).
Inositol had already been discovered as a component of muscle by the end of the 19th century and its structure established as a hexa-hydroxyl-cyclohexane (990). Nine different stereoisomers of inositol were described in the 1940s, and myo-inositol was found to be the main eukaryotic isomer (1234). The importance of inositol was recognized in the era of vitamins when it was realized that inositol was an important dietary ingredient for rodents, especially when animals were kept in germ-free conditions. Animals kept on inositol-free diets developed alopecia and “fatty liver” (reviewed in Ref. 642). Subsequently, it was found that mammalian cells required myo-inositol to grow properly in culture (394). In the fungus neurospora, lack of inositol caused a defect in lysosomal membrane integrity and autolysis (1006). The notion that inositol was a component of lipid membranes was first recognized in mycobacteria [the lipid was phosphatidylinositol mannoside (96)] and a phosphoinositide was also described in soybean (803).
A. Identification of Phosphoinositides and Their Metabolic Fate
The real beginning of the “modern” era of PI research was marked by a series of ground-breaking studies in the 1940s by Jordi Folch who identified inositol in the ethanol-insoluble phospholipid fraction of bovine brain and determined that it contained phosphates and inositol in a molar ratio of 2:1 (450). This lipid, termed diphosphoinositide or DPI, was found primarily in myelin in tight association with proteins (“neurokeratin”) (449) and showed rapid metabolic labeling when guinea pigs were injected with [32P-\]phosphate (328, 329). Subsequent work mainly by three groups (led by Clinton Ballou, Rex Dawson, and Tim Hawthorne) identified the structures of mono-, di-, and tri-phosphoinositides (abbreviated at the time as MPI, DPI, and TPI, respectively) as glycerophospholipids with an inositol ring linked to an sn-1,2-DG backbone via the D1-OH group of myo-inositol, and containing a phosphate at the 4- and both the 4- and 5-positions, in DPI and TPI, respectively (359, 581, 1563). Although these lipids had been isolated and identified primarily from brain, where they are most abundant, it had become evident by the early 1960s that they were present in small amounts in all eukaryotic tissues (1651).
To understand the meaning of their 32P labeling, it was essential to understand the synthetic and degradation pathways of PPIs. From the pioneering work of Eugene Kennedy and his colleagues on the synthesis of glycerolipids, it had been understood that cytidine nucleotide intermediates (such as CDP-choline and CDP-ethanolamine) donate the headgroups to the sn-1,2-DG backbone during phosphatidylcholine and -ethanolamine synthesis. However, Bernard Agranoff and his colleagues found that this was not the case for PtdIns. Here, the lipid backbone itself was “activated” by the cytidine nucleotide in the form of CDP-DG (11). Since the precursor of this intermediate is phosphatidic acid (PtdOH), which is produced by phosphorylation of sn-1,2-DG by a DG kinase using the terminal phosphate of ATP, described by Hokin and Hokin (640), an alternative name of CMP-PtdOH was suggested, to indicate that the phosphate was carried over to PtdIns not from CTP but ATP. PtdIns synthesis was found primarily associated with “microsomal” fractions and hence attributed to the ER, but CDP-DG is also a precursor of the mitochondrial lipids phosphatidylglycerol and cardiolipin (324), suggesting an important compartmentalization of these metabolic pathways.
Several studies then indicated that DPI was a phosphorylation product by PtdIns kinase activities associated with various membrane fractions (283, 636, 1044), including the PM (1043) and, importantly, similar activities were also present in red blood cell membranes where it was possible to show generation of PtdIns4P and PtdIns(4,5)P2 by presumed sequential phosphorylations of PtdIns (636). These observations, together with the notion that PPIs were highly enriched in myelin sheets that are essentially rolled up plasma membranes, gave strong support to the idea that PPIs were primarily associated with the PM. The metabolism of DPI and TPI was also explored in early studies. Sloane-Stanley identified a phospholipase C (PLC) activity (although not called it PLC yet) capable of hydrolyzing brain phosphoinositides (1420), and Rodnight found this activity increased by Ca2+ (1276). These early observations were followed by the realization that TPI is metabolized in two different ways: one route with dephosphorylation to DPI and PtdIns and another, via hydrolysis to InsP3 and diacylglycerol (what is now known as PLC) (1554).
The very rapid labeling kinetics of PtdIns(4,5)P2 and PtdIns4P in erythrocyte membrane and in intact cells relative to the much slower labeling kinetics of PtdIns and other phospholipids suggested high turnovers of the phospho-monoester groups due to rapid “futile” phosphorylation-dephosphorylation cycles (reviewed in Ref. 385). This also indicated that dephosphorylation of the monophosphate groups and rephosphorylation can all take place in the PM. PLC activities were then found in several tissues both in soluble and membrane fractions (464, 775), and association of PLC activity with the PM was also described (852, 1047). The distribution of PLC activities between soluble and membrane fractions and the enzymatic characteristics of the activities associated with different fractions showed significant variations between various laboratories (e.g., see Ref. 702). These discrepancies are now better understood knowing how many PLC enzymes and membrane-recruitment mechanisms exist (see sect. VII).
B. Agonists Stimulate Phosphoinositide Metabolism
In 1953 Mabel and Lowell Hokin (638) reported that [32P]phosphate incorporation into a phospholipid fraction was strikingly increased in the exocrine pancreas when the tissue was stimulated with secretagogues that induced protein secretion. In a series of subsequent work (635), the Hokins found that the increased 32P-labeling was limited to the two lipids, “DPI” and PtdOH that were identified by Dawson as acutely 32P-labeled in the brain slices (328). The Hokins also found that this increase was a general phenomenon observed in various cells associated with a stimulated secretion response (635). They suggested that the primary response was an increased phosphoinositide-specific PLC-catalyzed hydrolysis of PtdIns with production of DG, which was then converted to PtdOH (a step where the 32P incorporation took place) and then back to PtdIns to complete a cycle that was dubbed the “PI cycle” (639) (Figure 2A). These studies implicated PIs in secretion, but how increased inositide labeling was linked to any specific biochemical process in secretion remained elusive. In fact, more and more studies indicated that the increased PI turnover could be dissociated from secretion: it was observed in cells and with stimuli that did not evoke secretion, such as in postganglionic neurons (632) or lymphocytes (441). Also, the increased PI turnover was preserved in Ca2+-free medium, whereas secretion was eliminated under those conditions (633). Moreover, increased PI labeling needed higher concentrations of agonists than those for secretion (637), and it was not evoked by some agonists that increased secretion via cAMP (1337). In the meantime, a series of important experiments linked the PI turnover to cell proliferation. Fisher and Muller (442) found that lymphocytes stimulated with the mitogen phytohemagglutinin increased 32P- or [3H]inositol labeling of PtdIns and a rapid appearance of PtdOH, consistent with increased turnover of PI. A close correlation between cell proliferation and specifically inositol lipid turnover was found in cells subjected to various stimuli, including transformation with viruses, such as Rous sarcoma or SV40 (358). A dramatic drop in PtdIns turnover was shown to correlate with the transition from proliferation to differentiation during lens development in chicken embryos (1791).
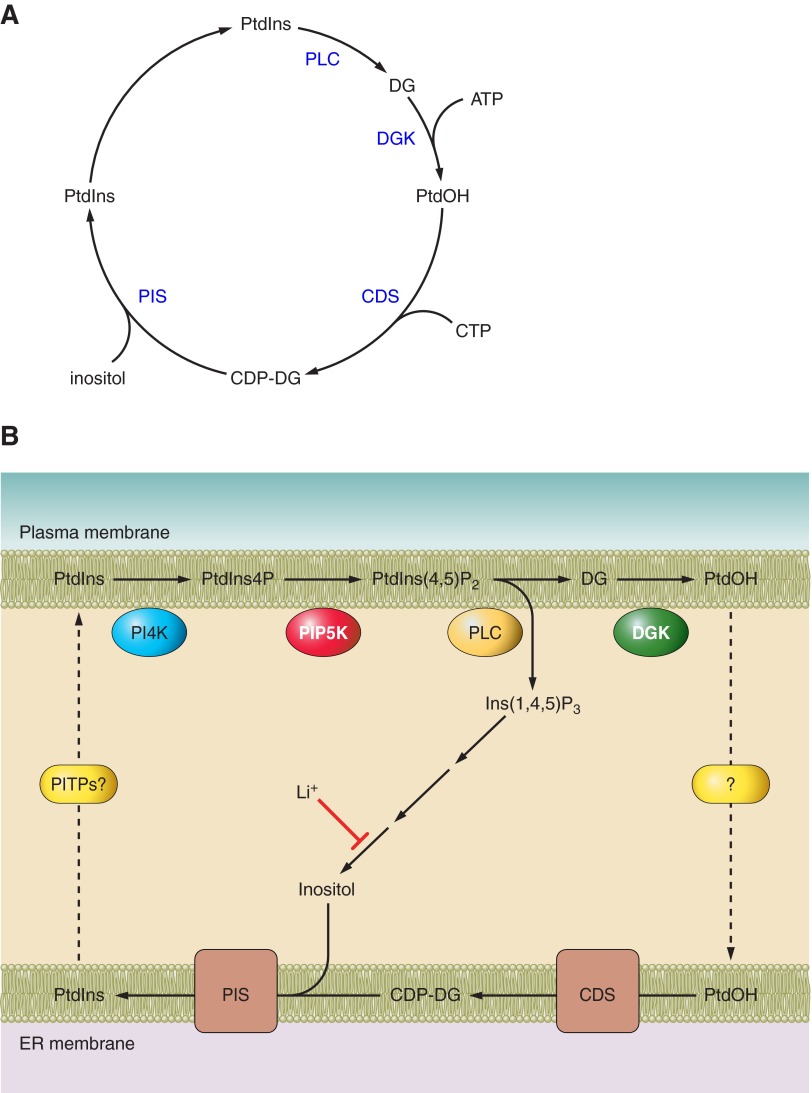
Figure 2.
The phosphoinositide cycle as originally perceived (A) and the updated version also showing the polyphosphoinositides, PtdIns4P and PtdIns(4,5)P2 (B). The primary event in triggering the cycle is the agonist-induced PLC activation. Note that all of the products of PtdIns(4,5)P2 hydrolysis are recycled. Diacylglycerol (DG) is converted to phosphatidic acid (PtdOH) by one of many DG-kinase enzymes (DGK). PtdOH then has to be transferred from the PM to the endoplasmic reticulum by a mechanism that has not been identified. In the ER, one of two CDP-DG synthase (CDS) enzymes conjugates PtdOH with CTP, and the CDP-DG is then conjugated with myo-inositol to phosphatdylinositol (PtdIns). PtdIns synthesis takes place mainly in a highly dynamic subcompartment of the ER. Much of the inositol used for PtdIns synthesis is derived from the sequential dephosphorylation of inositol 1,4,5-trisphosphate [Ins(1,4,5)P3], the other product of PLC-mediated PtdIns(4,5)P2 hydrolysis. Several of the dephosphorylation steps are inhibited by Li+, including the final dephosphorylation of inositol monophosphates by the enzyme inositol monophosphatase (IMP). The newly synthesized PtdIns has to reach the PM by a still obscure mechanism, perhaps mediated by PtdIns/PtdCho transfer proteins (PITPs).
These studies prepared the field for the notion that the PI turnover was somehow related to receptor action, although the link was not clearly defined (338, 392). This prompted Michell and Lapetina to consider that the PI response was a critical intermediate during stimulation of cell surface receptors that did not act via cAMP. They suggested that a product of PLC action on PtdIns, such as 1,2-cyclic inositol monophosphate, perhaps acted as a second messenger (852, 853). In parallel developments, cytoplasmic Ca2+ increase and, in many instances, cGMP elevations were increasingly recognized as possible triggering signals not only in excitable tissues, such as muscle cells, but in secretory cells as well. Intriguingly, the kind of receptors and agonists that evoked the Ca2+ responses were almost always identical to those that elicited a PI response. A theory was then introduced by Bob Michell in his seminal 1975 review (1040), where he proposed that the increased PI turnover was an early receptor-triggered event initiated by PLC activation characteristic of receptors that used Ca2+ or cGMP as second messengers. There were, however, some cracks in this picture that hampered its immediate endorsement and prompted further research. One of these points was related to the “speed” of the response relative to that of Ca2+ elevations, and the other was due to a few reports that found the enhanced PI labeling dependent on the presence of Ca2+ (21, 276) or perhaps Ca2+-controlled (32). This latter, together with the Ca2+ sensitivity of PLC activity appeared to support the views that the PI response was secondary or parallel to Ca2+ elevations (1174). Most studies, however, found the response independent of Ca2+, arguing against this idea (1041). Finally, Fain and Berridge (417) presented clear evidence that inositol lipid turnover was upstream of calcium fluxes during stimulation with 5-hydroxytryptamine in the blowfly salivary gland.
Another question raised was whether PtdIns or its phosphorylated derivatives (“DPI or TPI”) were the primary subject of PLC-mediated hydrolysis. Durrell (393) noted first that inositol-bisphosphate was one of the products that accumulated during stimulation, which can be derived only from hydrolysis of PtdInsP or PtdInsP2. Other reports described earlier that radiolabeled PtdInsP and PtdInsP2 were rapidly decreased upon stimulation with acetylcholine in avian salt gland (1339) and in iris smooth muscle (1), but the latter response was found Ca2+ sensitive and its connection to the increased PI labeling was not all that clear. The time must have been right for clarifying this question in the early 1980s when three groups reported that PtdIns(4,5)P2 was the initial target of PLC-mediated hydrolysis yielding Ins(1,4,5)P3 as the primary water-soluble hydrolytic product (131, 132, 299, 800, 1046). This was almost instantly followed by the recognition that Ins(1,4,5)P3 released Ca2+ from nonmitochondrial Ca2+ stores, finally unequivocally linking inositol lipid hydrolysis and Ca2+ signaling (1481). The other product of PtdIns(4,5)P2 hydrolysis, sn-1,2-DG, also found its targets when a DG-regulated phospholipid-dependent kinase family, named protein kinase C (PKC) was identified by Nishizuka. These findings opened a whole new research direction for the characterization and functional analysis of the PKC enzymes (1141, 1142). An extended view of the “PI cycle” has then been established by the mid 1980s (Figure 2B).
C. Expansion of Phosphoinositides
With these developments, the phosphoinositide-Ca2+ signaling paradigm became consolidated, and the efforts were concentrated on isolating and characterizing the enzymes that catalyzed these reactions. Particularly important was to identify the PLC enzymes that were activated by the Ca2+ mobilizing receptors. A significant achievement of this time was the purification (763, 1319, 1521) and subsequent molecular cloning (402, 762, 1493) of several forms of PLC pursued in the laboratories of Sue Goo Rhee, Matilda Katan, and Tadaomi Takenawa. It was also an important revelation that two major routes of PLC activation exist: one via the α and βγ subunits of heterotrimeric G proteins, in the case of PLCβ enzymes (207, 866, 1194, 1195), and the other via association and activation by RTKs, in the case of PLCγ (786, 995). Similar developments occurred in the inositide kinase (167, 362) and phosphatase (801, 1065, 1775) fields, and the former also produced some unanticipated and surprising results that were forerunners of an entirely new research field. These began with the discovery that several transforming oncogenes such as the Rous sarcoma virus, polyoma middle T antigen, or avian src were associated with PtdIns kinase activities (963, 1486, 1713), and it was simultaneously noted that more than one kind of PtdIns kinase was present in mammalian cells (403, 1712). Since all previous studies assumed that PtdInsP was a 4-phosphorylated PtdIns and hence PtdIns kinases were, by default, believed to be 4-kinases, it came as a surprise when the lipid product of the so-called type I PtdIns kinase was identified as a 3-phosphorylated PtdIns (1462, 1711). Soon it was also shown that growth factor or GPCR stimulation evoked the production of novel phosphoinositides PtdIns(3,4)P2 and PtdIns(3,4,5)P3 (66, 1572), while PtdIns3P was constitutively present in cells and did not respond to similar stimuli (1462).
Interest in PI 3-kinases was rapidly propelled by the association of PI 3-kinase activities with growth factor and oncogenic signaling, ultimately unraveling the various subclasses and primary structures of these enzymes and their adaptors (192, 614, 1178, 1367, 1465, 1476, 1638). Moreover, PI 3-kinase research was greatly aided by the discovery that the fungal drug wortmannin (Wm) was a potent PI 3-kinase inhibitor (52, 1761) and was further motivated by the revelation, with the help of large-scale genomic sequencing, that oncogenic mutations within the catalytic subunit of PI 3-kinase class Iα are found in a large number of human cancers (1334). However, PI 3-kinase research had a large impact on the overall PI field even beyond its close association with cancer. The fact that PtdIns(3,4,5)P3 was a poor substrate of PLC enzymes (1384) has directed research toward the notion that the lipid itself within the membrane must function as a signaling entity and that molecules must exist that respond to these lipid changes (1467). This concept has been proven with the discovery of the Akt kinase (also known as protein kinase B, PKB) as a major signaling route downstream of PI 3-kinases and PtdIns(3,4,5)P3 (202, 461). This, together with the identification of pleckstrin homology domains (1014, 1584) as protein modules that directly bind phosphoinositides (768, 881, 882), exposed the side of phosphoinositides as membrane-bound regulatory molecules as opposed to just being precursors of the two messengers Ins(1,4,5)P3 and DG. This has been a monumental paradigm shift that hugely expanded the functional versatility of PPIs and gave new meaning to the diversity of the PI kinases and phosphatases. These enzymes turned out to be regulators of a whole range of cellular functions with a still poorly understood contribution to the increased metabolic labeling of inositides described in early studies. More detailed description of how the PI3K field was established and expanded can be found in (1559, 1612).
D. Soluble Inositol Phosphates
Another direction to which the expansion took place was marked by the discovery that eukaryotic cells contained water-soluble inositol phosphates other than those generated by PLC-mediated hydrolysis of the PPIs and their dephosphorylated metabolites. This started when Robin Irvine's group discovered that the majority of the InsP3 increase after stimulation was not due to the active Ins(1,4,5)P3 isomer but another form, Ins(1,3,4)P3 (707) that was inactive as a Ca2+ mobilizing agent (706). The source of this isomer was soon clarified with the discovery of the “inositol tris/tetrakisphosphate pathway” whereby Ins(1,4,5)P3 was phosphorylated by a 3-kinase yielding Ins(1,3,4,5)P3, which was then converted to Ins(1,3,4)P3 (705). A long quest has followed to find a messenger function for Ins(1,3,4,5)P4 with suggestions that it would link Ins(1,4,5)P3-sensitive Ca2+ pools with sustained Ca2+ entry (235), and led to the isolation of an Ins(1,3,4,5)P4-binding protein identified as a Ras-GAP1 protein family member (306). However, the biological functions (if any) of Ins(1,3,4,5)P4 still remain elusive. In the meantime, additional, highly phosphorylated inositols, such as InsP5 and InsP6 have been described by HPLC analysis of myo-[3H]inositol-labeled cells (610) and a new pathway was identified by which Ins(1,3,4)P3 could be converted to Ins(1,3,4,6)P4 and then to InsP5 (91, 92, 1391). Although there were significant agonist-induced changes observed in the newly discovered InsP4 isomers, InsP5, and even InsP6 (86, 93, 1032), the significance and functions of these inositol phosphates remained unknown for a period of time until the discovery of a pathway in yeast that took Ins(1,4,5)P3 all the way to InsP6 with a functional link to messenger RNA export (1777). This was the beginning of a new era in inositol polyphosphate research with the discovery of pyro-phosphorylated inositol polyphosphates (954, 1031) and possible roles of several of these compounds (1068) in cell regulation. These new developments will not be detailed further in this review as they deviated from the PPI lipids themselves. However, these exciting findings deserve attention and have been reviewed recently elsewhere (1375, 1389, 1390).
IV. PHOSPHATIDYLINOSITOL SYNTHESIS
PtdIns synthesis takes place in the ER and, as recently suggested, in an ER-derived highly mobile subcompartment that may serve as a means to supply the lipid to other membranes (796). Since the increased PI synthesis after agonist stimulation is believed to be secondary to PPI breakdown that occurs in the PM, there have been speculations that PtdIns synthesizing activities might have separate ER and PM components and perhaps more than one phosphatidylinositol synthase (PIS) enzymes were responsible for them. Indeed, the whole PI synthesizing machinery was found in turkey erythrocyte membranes (1021, 1622), and PIS activities were found associated with PM as well as ER membranes after cell fractionation (694, 695, 1407). Molecular identification of the PIS enzyme was first achieved in yeast (1133, 1134) as a result of complementation cloning using a yeast strain that had been previously found defective in PIS enzyme activity (1135). Mammalian PIS was then cloned and characterized (957, 1528), showing that the same enzyme was responsible for both PtdIns synthase and myo-inositol exchange activities, two reactions that had been formerly attributed to distinct molecular entities (1522). There appears to be no other gene for PtdIns synthesis, so the association of the enzyme activity with PM fractions may be related to their contamination with ER or with the lighter mobile PIS membrane compartment (796). Two studies reported defects in PIS activity in zebrafish, one describing ER stress and hepatic steatosis (1547), while the other observed lens structural defects and reduced number of photoreceptors (1098).
The two substrates of PtdIns synthesis are myo-inositol and CDP-DG (Figure 2B). The latter is synthesized from CTP and PtdOH by CDP-DG synthase (CTP:phosphatidate cytidylyltransferase, or CDS for short) enzymes (11, 119). Early studies described two CDS activities: one associated with the outer surface of the ER (95) and another located in the matrix of mitochondria that was responsible for feeding into cardiolipin synthesis (1360). Mammalian CDS enzymes have been cloned (589, 1325, 1688), and two, highly similar but different genes, cds1 and cds2 are found in the human and other mammalian genomes (552, 1645). Both CDS proteins localize to the ER, but they do not enter the PIS positive mobile compartment (796). Importantly, none of the cloned CDS enzymes is found in the mitochondria (796) or showed the unique characteristics of the mitochondrial activity (1522). Therefore, the mitochondrial enzyme still remains elusive. A photoreceptor-specific isoform of Drosophila CDS was shown to be necessary for the maintenance of PtdIns(4,5)P2 levels and hence for a sustained light response, and CDS mutant flies developed light-dependent retinal degeneration (1736). Recent studies also identified mutations in one of the CDS genes in zebrafish causing specific defects in blood vessel formation and angiogenesis, and these effects were attributed to the rundown of PtdIns(4,5)P2 levels in the VEGF-stimulated endothelial cells (1188). These studies also concluded that CDS enzyme activities are necessary for maintaining the signaling pool of PtdIns(4,5)P2.
The other substrate of PtdIns synthesis is myo-inositol, and cells have three ways of providing this substrate for the PIS enzyme. Some organisms and cells can synthesize myo-inositol de novo from glucose-6-phosphate via Ins3P. [Many studies use Ins1P to designate this product. This refers to the l-enantiomer, whereas we use the d-designation for all inositol phosphates. Accordingly, l-Ins1P corresponds to d-Ins3P (1376)]. Otherwise, cells can take up myo-inositol from their surroundings or, importantly, they can recycle the myo-inositol from the inositol phosphates liberated during PLC activation (Figure 2B). Inositol uptake in mammalian cells is mediated by three different proteins: SMIT1 (841), SMIT2 (274), and HMIT (1589). SMIT1 and -2 are Na+/myo-inositol cotransporters that use the Na+ electrochemical gradient to transport myo-inositol into the cells. SMIT1 has a Km of ∼30 μM for myo-inositol, and it shows highest expression in kidney medulla and MDCK cells where it responds to osmotic challenge with increased expression (841). This already suggests that the protein functions in osmoregulation since myo-inositol serves as an important osmolyte in both neurons and the kidney. SMIT-2 has higher Km (∼150 μM) (274), and it shows wider tissue distribution (1292). HMIT is a H+/myo-inositol cotransporter that has a lower affinity (Km of ∼ 100 μM) but high capacity. It shows highest expression in the brain (1589). Interestingly, HMIT is mostly intracellular due to internalization and ER-retention signals within its sequence (1589), and its membrane insertion is regulated by activity in neurons (1590).
The question of how cells supply myo-inositol for PtdIns synthesis has attracted a lot of attention when it was discovered that treatment of rats with Li+ at doses that are used in the treatment of manic-depressive disorders caused brain inositol levels to drop with accumulation of inositol monophosphate (34, 35). Subsequent studies showed that Li+ inhibited the dephosphorylation of inositol monophosphates and that it was d-Ins1P that accumulates in the rat brains after prolonged Li+ treatment (1396). This latter finding was important because it showed that the accumulating inositol phosphate was not the isomer synthesized de novo by the cells (which is Ins3P or l-Ins1P) but it was originated from the PLC-liberated Ins-phosphates that yield Ins1P and Ins4P (87, 88). Indeed, Li+ was shown to enhance the accumulation of several inositol phosphate isomers upon stimulation by agonists that activate PLC enzymes (88, 133). Based on these findings it was suggested that the beneficial effects of Li+ treatment in manic-depressive disease are related to a deficient recycling of the inositol phosphates liberated by PLC activity, and this would affect neurons that display the highest activity (134). These observations and the proposed theory draw attention to the question of why brain cells are unable to supply inositol from the CSF and brought the inositol uptake pathways into the focus of intense research. Many studies have since been devoted to study the effects of mood-stabilizing drugs on myo-inositol uptake and inositol phosphate generation and recycling (60, 350, 351, 1258).
In light of these observations, it was a remarkable finding that SMIT1 knockout mice develop normally but die from congenital central apnea due to abnormal respiratory rhythmogenesis (138, 195). These mice have more than 90% reduction in brain and more than 80% in whole body myo-inositol content. Yet, their brain PtdIns levels are completely normal (137, 195). These findings cast some doubts about the “inositol-depletion hypothesis” of Li+ action and suggested that myo-inositol uptake by SMIT1 serves a different purpose, perhaps contributing to osmotic control. These findings also showed that PtdIns synthesis could go on undisturbed even at greatly reduced myo-inositol levels. However, Li+ treatment combined with strong PLC activation can drive down PtdIns levels in cultured cells (87, 727), suggesting that without sufficient external inositol it is possible to deplete cellular myo-inositol levels to the point where PtdIns synthesis will suffer. Concerning Li+ effects, it has also been hypothesized that some of the accumulating metabolites, such as CDP-DG or PtdOH, exert an inhibitory effect on signaling from PLC-coupled receptors (87, 90, 727). There are a number of other effects of Li+ unrelated to PtdIns synthesis, such as the inhibition of the GSK3β enzyme (739), but those are not subject to this review.
V. PHOSPHOINOSITIDE KINASES
As mentioned above in the historical overview, PI kinases were described as early as the 1960s, and it was already understood that separate activities converted PtdIns to PtdIns4P and PtdIns4P to PtdIns(4,5)P2, defining these two activities as “PI-kinases” and “PIP kinases.” This historical fact determines the terminology of these enzymes and their classification. Some of the enzymes can phosphorylate both PtdIns and phosphorylated PPIs in vitro, but in the following sections they will be discussed according to what is (or was) believed to be their primary enzymatic function within the intact cells. This may be a deviation from other reviews that discussed the enzymes only according to the position of the inositol ring that they can phosphorylate.
A. PtdIns Kinases
1. PtdIns 4-kinases
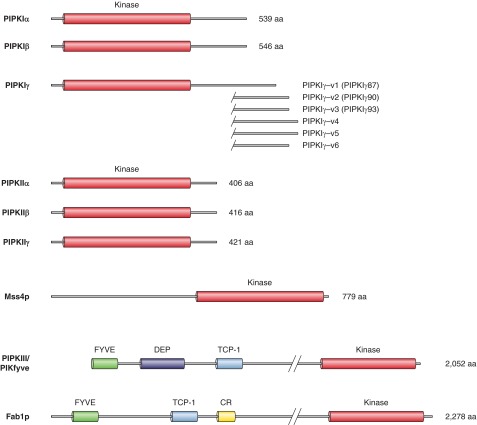
PtdIns 4-kinases (PI4Ks) were initially believed to be the only PtdIns kinases. Only after discovering that type I PI kinases were actually PI 3-kinases, had the PI4Ks been so named. That is why PI4Ks were left with the type II and type III PI4K designations. Initial reports on PI4K activities suggested that the enzymes were associated with the PM (1043), and consistent with this notion, they were also present in red blood cell membranes (636). However, distinct activities present in ER membranes showing differential sensitivities to detergents such as cutscum were already noted by early studies (280, 283, 577, 1043). Purification of these activities from a variety of membranes was then carried out (525, 651, 1233, 1658, 1705). This required solubilization of the membranes with detergents yielding an activity of ∼55 kDa termed type II PI4K. This tightly membrane-bound activity could be reactivated after separation and extraction from SDS gels and showed high affinity for ATP (Km ∼10–50 μM), potent inhibition by adenosine (Ki ∼10–70 μM), stimulation by detergents, and inhibition by Ca2+. Another PI4K activity was described in cholate extracts of bovine brain with larger size, lower ATP affinity, and lower adenosine sensitivity, and it was termed type III PI4K (403). A soluble, PI4K activity was also identified based on its sensitivity to PI 3-kinase inhibitors (1112), which showed the enzymatic properties of type III PI4Ks (386) and was then separable to a larger (210–230 kDa) and smaller (110 kDa) entity (89). The first PI4Ks were cloned from Saccharomyces cerevisiae and were designated as Pik1p and Stt4p (444, 1778). The mammalian homologs of these enzymes cloned from various species (89, 504, 1036, 1106, 1107, 1725) were then identified as PI4K type IIIβ and -α, respectively. Although the type II PI4Ks were known first with several attempts for isolation, their successful purification and cloning happened only later when two groups independently cloned PI4K type IIα (108, 1058) followed by the identification of another closely related form, PI4KIIβ (83, 1058). The structural features of PI4K enzymes are shown in Figure 3.
Figure 3.
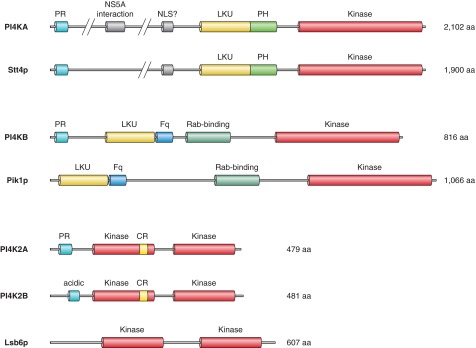
The family of PI 4-kinase enzymes. PI 4-kinases (PI4Ks) have two major types, the type III and type II enzymes [the type I enzyme(s) turned out to be the PI 3-kinases]. The type III enzymes are comprised of two proteins: the larger (∼210–230 kDa) PI4KA (Stt4p in yeast) and the smaller (∼92–110 kDa) PI4KB (Pik1p in yeast). These enzymes are relatives of PI 3-kinases and the PIK-related protein kinases, with a highly conserved COOH-terminal catalytic domain. They also have lipid-kinase unique (LKU) domains also found in PI 3-kinases. Other domains include proline-rich sequences (PR) and a frequennin-binding (Fq) domain in the PI4KB form. The smaller sized (∼56 kDa) type II PI4Ks exist in two forms in vertebrates: PI4K2A and PI4K2B that are highly homologous except at their very NH2 termini. [Only one form is found in S. cerevisiae (Lsb6) and in D. melanogaster]. The signature feature of these enzymes is a cysteine-rich (CR) sequence that is palmitoylated in the vertebrate enzymes providing stronger membrane association.
2. PI4KIIIα/PI4KA
Most of what we know about this enzyme was derived from studies in S. cerevisiae (1479). The yeast ortholog STT4 was initially identified in a screen that sought staurosporine-sensitive mutants (1778). Deletion of STT4 results in an osmo-remediable phenotype with defects in cell wall integrity (64, 1778). The connection to PKC1 has been revealed in that Stt4p was found to be essential to supply the Mss4p PIP 5-kinase with its substrate, PtdIns4P, to synthesize the PM pool of PtdIns(4,5)P2 (63, 64). The PM pool of PtdIns(4,5)P2 anchors Rom2p, an exchange factor that activates the Rho1p GTPase, which, in turn, is an activator of Pkc1p (63). Pkc1p kinase is a well-documented hub for several signaling pathways that are essential for cell wall integrity (892). Synthetic genetic array analysis of Stt4p revealed a strong connection to sphingolipid metabolism, again via the control of PM PtdIns(4,5)P2 pools and recruitment of Slm1p and Slm2p proteins (1510). Further defect related to an insufficient supply of the PM with PtdIns4P in temperature-sensitive Stt4 alleles was the failure of the actin cytoskeleton to properly organize (64). Such cells are also unable to recruit the p21-activated kinase, Cla4p, a direct downstream effector of Cdc42, to the sites of polarized growth (1717) and to assemble septin at the bud neck (140).
However, in addition to the roles of Stt4p in supplying the PM with PtdIns4P, several observations suggest that the kinase also affects processes linked to internal membranes. First, Stt4p generates a pool of PtdIns4P that is degraded by the ER resident Sac1p phosphatase (455). Although recent elegant studies showed that Sac1 in the ER still can access the PM PtdIns4P pool in trans (1454), it is likely that Stt4p is also involved in the synthesis of additional PtdIns4P pools found in the ER membranes. This possibility is supported by studies in which Stt4p could rescue defects in aminophospholipid transfer between the Golgi and the ER (1580) and that temperature-sensitive Stt4p mutants also display lysosomal defects (64). Moreover, Stt4p function was genetically linked to some of the Sec14 homologs (SFHs), which are lipid transfer proteins (1303), some of them connected to aminophospholipid transport (1737). Interestingly, Stt4p is also the major target of Wm in yeast, since neither the yeast PI 3-kinase, Vps34p, nor the other PI 4-kinase, Pik1p is particularly sensitive to this inhibitor (311).
As far as regulators of Stt4p are concerned, temperature-sensitive alleles of Stt4p can be rescued by overexpression of Sfk1 (suppressor of four kinase 1), a multispanning membrane protein with yet unidentified functions (63). Intriguingly, two other proteins, Ypp1p (79, 1792) and Efr3p, were described as regulators of Stt4p and organizers of the kinase into special signaling domains at contact zones between the ER and the PM (79, 980). Ypp1 also targets the Parkinson-related protein, A30P α-synuclein to the vacuole for degradation in yeast, again pointing out the possible role of Stt4p at internal membranes.
The biology of Stt4p orthologs (PI4KA or PI4KCA) in higher eukaryotes is much less understood. This enzyme also seems to be primarily responsible for the generation of the PM pool of PtdIns4P in cultured cells (82, 84), even though its primary location was originally described at the ER/Golgi interface (1107, 1726). Recent studies, however, showed that PI4KA was localized to the PM with the aid of the TTC7 and EFR3 proteins, which are homologs of the yeast Ypp1 and Efr3 proteins (1114). Importantly, this feature was linked to the presence of an NH2-terminal short sequence in PI4KA that has been missed in previous studies. Morpholino-induced downregulation of PI4KA in zebrafish embryos caused massive and complex developmental defects especially affecting the brain and were characterized by lack of pectoral fins. This latter defect could be traced to impaired Fgf signaling and was phenocopied by PI 3-kinase inhibitors consistent with a decreased supply of PtdIns(4,5)P2 at the PM (959). It is notable that PI4KA shows the highest expression in the brain both during development and in adult rodents (1107, 1828). At the cellular level, the enzyme was also found associated with nucleoli, but the biological significance of this finding is not known (748). A series of recent studies using RNAi screens to search for cellular host factors required for the infection cycle of hepatitic C virus (HCV), identified PI4KA as a critical protein for HCV replication in the liver (122, 166, 1513, 1577, 1597). Since HCV-infected cells develop a special replication organelle, the “membranous web,” it is possible that the PI4KA is needed for the formation of this organelle (1082). This is supported by the finding that PI4KA interacts with the HCV viral protein NS5A (13), which by itself can generate membrane structures resembling the “membranous web” (1082). Since PI4KA has also been linked to COPII positive ER exit sites (154, 425), it is a possibility that the “membranous web” originates from the ER. Studies are underway in several laboratories to untangle this process and to test whether inhibition of the kinase is a viable strategy to combat HCV infections.
3. PI4KIIIβ/PI4KB
The yeast homolog of PI4KB, called Pik1p, was the first PI4K purified and cloned in the Thorner laboratory (444, 445). Interestingly, the same gene was cloned using a monoclonal antibody that was raised against NUO135, a protein part of the nuclear pore complex (493). The PIK1 gene is essential, which clearly demonstrates that Stt4p and Pik1p assume nonredundant functions in yeast. Pik1p inactivation by temperature-sensitive alleles causes ∼50% decrease in cellular PtdIns4P and PtdIns(4,5)P2 levels (64, 1655). Pik1ts strains show greatly distorted and exaggerated Golgi membranes, vacuole fragmentation, and defective actin polarization at the budding pole (64, 1655). Certain Pik1ts alleles also show cytokinesis defects (493, 1655). Pik1p localizes to the Golgi and the nucleus (493, 1478, 1655) and regulates trafficking in the late secretory pathway (64, 555, 1655). Pik1p is localized to the Golgi in the yeast by interaction with Frq1p, the yeast ortholog of the small Ca2+ binding protein frequenin (also called NCS-1 in mammalian cells) (reviewed in Ref. 615) (599). Frq1p is essential for Pik1p Golgi localization (1478), and the Frq1p binding site was mapped between residues 125–169 of Pik1p, a region adjacent and downstream of the lipid kinase unique (LKU) domain characteristic of PI3Ks and type III PI4Ks (672). The Golgi recruitment of Pik1p is also controlled by Arf1, and the enzyme also interacts with the Arf1 exchange factor Sec7 (512). Pik1p shuttles between the nucleus and the cytoplasm, and both the nuclear and Golgi localizations of the enzyme are required for viability (1478). Phosphorylated Pik1p interacts with 14-3-3 proteins, and this interaction keeps Pik1p out of the nucleus (343). It is not clear why PtdIns(4,5)P2 levels are decreased in Pik1ts strains at the permissive temperature, since the yeast PIP 5-kinase Mss4p does not show Golgi localization and the only place where the two enzymes are found together is the nucleus. This may suggest that the Pik1p-dependent PtdIns(4,5)P2 has a nuclear function. A synthetic lethality screen identified the Golgi-associated Rab-GTPase Ypt31p (a Rab11 homologue) as a downstream target of Pik1p (1369) and Drs2, an aminophospholipid translocase that is also important for post-Golgi trafficking (240). Recent studies also showed that Ypt31p interacts with the Sec2/Sec4 complex in a Pik1p-dependnent manner and is replaced by the Sec4p effector Sec15p (a component of the exocyst) as PtdIns4P levels decrease, thereby establishing a link between Pik1p-mediated PtdIns4P generation and vesicular secretion (1072).
Mammalian PI4KB is also primarily Golgi-localized (514, 1726) and shuttles between the cytoplasm and the nucleus (332). In Madin-Darby canine kidney (MDCK) cells, PI4KB regulates Golgi to PM trafficking, both intra-Golgi (for influenza hemagglutinin) and for basolateral delivery (for vesicular stomatitis virus VSV-G protein) (193). Kinase-inactive forms of PI4KB also inhibit the Golgi-to-PM delivery of the VSV-G protein in nonpolarized cells (513). Recruitment of PI4KB to the Golgi is regulated by the small GTP binding protein Arf1 (514). The enzyme also interacts with NCS-1 (586, 1695), but unlike yeast Frq1, NCS-1 does not primarily localize to the Golgi, and it is not known to what extent (if any) NCS-1 contributes to the recruitment of PI4KB to the Golgi in mammalian cells (615). The GTP-bound form of Rab11 also binds mammalian PI4KB (at residues 401–516), but this interaction does not seem to regulate PI4KB activity or to recruit the enzyme to the Golgi. Conversely, PI4KB appears to be required to recruit Rab11 (333), and similar interactions between these proteins were demonstrated in the plant Arabidopsis thaliana (1237) and in Drosophila (1231). Notably, this latter study suggested that this function of PI4KB [called four wheel drive (Fwd) in the fly] is partially independent of the enzyme's catalytic activity. PI4KB is phosphorylated at multiple sites (1485), and phosphorylation of Ser258 and Ser266 affects the Golgi recruitment of the protein during recovery from brefeldin A treatment(590). A PKD-mediated phosphorylation of PI4KB at Ser268 regulates PI 4-kinase activity and is critical for the post-Golgi transport of the VSV-G protein, but not for Golgi recruitment of the enzyme (579). PKD phosphorylation of PI4KB also promotes interaction with 14-3-3 proteins, and this interaction stabilizes the protein in its active conformation (578). Mammalian PI4KB also shuttles between the cytoplasm and the nucleus, but the nuclear function(s) of the enzyme remains to be determined (332).
Recent studies reported that PI4KB is a key host enzyme in the replication cycle of small RNA viruses that reorganize the host cell ER-Golgi membrane structure to establish a replication platform (656). Intriguingly, some HCV strains also require this enzyme for replication and inhibitors of PI4KB potently slow down viral replication (656). This identifies PI4KB as a potential therapeutic target in antiviral strategies.
No studies have been published with targeted deletion of PI4KB in mammalian organisms. Disruption of the gene Fwd encoding PI4KB in Drosophila results in male infertility due to a cytokinesis defect during spermatogenesis (182). Since PtdIns(4,5)P2 is important in the formation of the cleavage furrow during cytokinesis (436, 1728), it seems that fwd is the only PI4K that can supply the PtdIns4P for cytokinesis during spermatogensis in Drosophila. Gene disruption of both PI4KB (AtPI4Kβ1 and its sister, AtPI4Kβ2) in Arabidopsis thaliana manifests in a defect in root hair growth (1237). These enzymes facilitate budding of vesicles from the TGN and associate with the TGN-localized RabA4b protein (a Rab11 homolog of the plant) at the region corresponding to the Rab11 interaction site in PI4KIIIβ. This process seems to be essential for polarized secretion and hence for root hair extension. Similarly to yeast and mammalian cells, the Arabidopsis homolog of frequenin interacts with the NH2-terminal domain of AtPI4Kβ1 to add Ca2+ regulation to the enzyme (1237).
4. Type II PI 4-kinases/PI4K2s
Early biochemical studies using mammalian tissues characterized what has become known as the type II PI 4-kinases. However, cloning of the genes for this group of proteins had to wait for a while (108, 1058). Two forms of the enzymes exist in vertebrates, the type IIα (PI4K2A) and type IIβ (PI4K2B) enzymes, with very similar features. Little information unique to PI4K2B is available as of to date; therefore, the two isoforms will be discussed together. Yeast and other lower organisms only have one form of the protein.
Type II PI4Ks are tightly membrane-bound proteins, due to the palmitoylation of a conserved stretch of cysteines within their catalytic domains (108, 109). In spite of a similar palmitoylation sequence within the two isoforms, a larger fraction of PI4K2B than PI4K2A is found in the cytosol (83, 1690) and the cytoplasmic fraction of PI4K2B is not palmitoylated (743). Based on early studies in which the type II PI4K activity was found in PM fractions, and in red blood cell membranes, it was logically assumed that the type II PI 4-kinase produces PtdIns4P in the PM. Therefore, it was surprising to find the majority of PI4K2A and PI4K2B enzymes in intracellular membranes, mostly associated with the TGN and endosomes by immunocytochemical analysis (83, 1060, 1680). It has been shown that PtdIns4P in the Golgi/TGN is critical to the membrane recruitment of various clathrin adaptors, such as the heterotetrameric AP-1 (1680) and monomeric GGAs (1509, 1671) and that PI4K2A, rather than the type III PI4KB, was the important enzyme in this process (1671, 1680). PI4K2A also shows association with a vesicular pool rich in the adaptor protein AP-3 (1330), and it directly interacts with AP-3 via a sorting motif found in the enzyme, which then acts both as a regulator and a cargo (298). Both the catalytic activity of the kinase and the direct interaction with AP-3 are important in supporting AP-3-mediated trafficking (298). An additional role of PI4K2A in EGF receptor trafficking was indicated by studies showing that EGFR targeting and degradation in lysosomes were found to be impaired after RNAi-mediated knock-down of the enzyme (1060). Similarly, PI4K2A was found important for the lysosomal trafficking of the glucorebrosidase enzyme (741). Undoubtedly, type II enzymes are also present in the PM, either under unstimulated conditions (PI4K2A) or after stimulation with PDGF (PI4K2B) (1690). Based on mass measurements, ∼50% of the total cellular PtdIns(4,5)P2 pool is synthesized via Wm-sensitive PI4Ks (1615), but what fraction of this is found in the different membranes has yet to be determined.
Regulation of the type II PI4Ks is poorly understood. An important feature of the type II PI4Ks is their association with cholesterol and sphingolipid-rich membrane domains (1685). The cholesterol content of these membranes has a big impact on both enzyme activity and interaction of the protein with regulatory factors (1059, 1686). Recent studies found that the palmitoylation of the PI4K2A enzyme in the Golgi is regulated by cholesterol (949). Calcium inhibits both type II PI4Ks, and membrane association increases PI4K2B activity (1690). Remarkably, the activity of type II PI4Ks varies between the isoforms, the PI4K2A enzyme being most active, while the PI4K2B form has much lower activity (83). The enzyme activity shows good correlation with palmitoylation and membrane association. In addition to palmitoylation, membrane attachment of these enzymes is also determined by the COOH-terminal part of the catalytic domain (109). The yeast ortholog LSB6 (also termed PIK2) (562, 1392) shows only very moderate PI4K activity and makes minor contribution to the overall PtdIns4P production of yeast cells under normal growth conditions. It also lacks most of the cysteines that are palmitoylated in the mammalian enzymes (562, 1392). Importantly, inactivation of LSB6 causes only a mild alteration in the trafficking of the endocytosed mating factor receptor. This defect is rescued by a construct that does not contain the catalytic domain but requires regions that interact with Las17p, the yeast homolog of WASP, a protein that is important for regulation of actin polymerization (230). It is possible that LSB6 acts as a scaffold and regulates the movements of vesicles; the lipid kinase activity of LSB6 may not be crucial for normal functions in the yeast.
A gene-trapped mouse with a truncated PI4K2A has been thoroughly investigated. The homozygous mice develop and are born normal in spite of the lack of detectable PI4K2A protein, but they succumb to late-onset spinocerebellar degeneration (1408). These animals develop severe lipofuscin-like depositions in the cerebellum associated with gliosis, and lose most of their Purkinje cells. They also show massive axonal degeneration in the spinal cord and die prematurely (1408). However, a screening study on patients suffering from autosomal recessive hereditary paraplegia, a human disease highly similar to the pathologies of PI4K2A-deficient mice, failed to identify pathogenic changes in the PI4K2A gene (270). Little is known about type II PI4Ks in other organisms. Zebrafish already contain both isoforms of the type II enzymes (959), while there is only one gene found in Drosophila (110). Arabidopsis contains eight genes encoding proteins with homology to the type II PI4Ks, six of which contain ubiquitin within their coding sequences (1092). Since ubiquitination is an important means of tagging proteins, including EGF receptors destined for endocytosis and degradation (547), this observation is a further hint that type II PI4Ks are regulators of endocytosis and they may direct endocytosed proteins for degradation.
5. Class III PI 3-kinase/PI3KC3
These enzymes are usually discussed with the PI 3-kinases, but because of their substrate restriction they are, in fact, PtdIns kinases and, therefore, will be discussed in this paragraph. The yeast enzyme, VPS34 was the first cloned PtdIns 3-kinase (1367). As indicated by its name, this protein had been initially identified in screens for S. cerevisiae mutants that develop vesicular sorting defects (Vps) (101, 1300). Only after the realization that the COOH-terminal half of Vps34p shows very high sequence homology with the mammalian type I PI 3-kinase 110 kDa catalytic subunit (614) was it understood that Vps34p is a PI 3-kinase (1367). Yeast cells with deleted Vps34p lack PtdIns 3-kinase activity and the lipid PtdIns3P, indicating that this enzyme is the sole source of this lipid in yeast (1367, 1446). The enzyme is unique among PI 3-kinases in that it can only phosphorylate PtdIns but not further phosphorylated PPIs (1446). Cells with Vps34 mutations or deletions have morphologically intact vacuoles and a normal secretory pathway, but fail to sort certain cargoes, such as the enzyme carboxypeptidase Y (CPY), to the vacuole (604). Vps34p tightly associates with a serine/threonine kinase, Vps15p, that greatly stimulates its kinase activity (1445), and Vps15 mutants display vacuolar sorting defects very similar to those described in Vps34p mutants (605). The production of PtdIns3P by Vps34p is important for transport from the Golgi to a prevacuolar compartment and subsequently to the vacuole (1445). Vps34p is also important for the membrane invaginations in multivesicular bodies (MVB) (70, 71, 765) and for various forms of autophagy (779). In each of these functions, different proteins are associated with the Vps34p-Vps15 complex (779). Accordingly, PtdIns3P exerts its effects by recruiting a variety of proteins that contain PI binding domains that are attracted to the membrane by this particular PPI species. These include FYVE domains (584, 766, 836), PX domains (239), and perhaps others that are less characterized (486, 1739). The specificity of these interactions is not solely determined by the interaction with PtdIns3P but also by the protein-protein interactions that are unique for the functionally distinct signaling complexes. It is noteworthy that Vps34p is less sensitive to the PI 3-kinase inhibitor Wm (IC50: ∼3 μM) than the mammalian PI 3-kinases (∼2–5 nM), especially the class I enzymes (1446). Vps34p not only regulates the trafficking of proteins to the vacuole but is also important for autophagy (74, 751), a starvation-induced protein-degradation process targeting cytosol and organelles to the lysosome (1073). Vps34p serves the secretory and autophagy pathways in two different protein complexes: both complexes I and II contain Vps34p, Vps15, and Vps30p, but complex I also has Atg14p and complex II has Vps38p. Complexes I and II regulate autophagy and CPY sorting to vacuoles, respectively (779).
The mammalian ortholog of Vps34p, called hVps34, PtdIns-specific PI 3-kinase or class III PI3K and its adaptor protein p150 have been also cloned (1189, 1642). The mammalian class III PI3K also has a role in endocytic sorting, and the hVps34/p150 complex is recruited to Rab5 positive endosomes (254, 1100) where it produces PtdIns3P, which attracts proteins that contain FYVE domains and PX domains (509, 752, 1409, 1750). hVps34 is also central to autophagy in mammalian cells (1532, 1631), but there is less distinction between the roles of complex I and complex II in this process. This is due to multiple interactions of the hVps34-associated Beclin-1 (the homolog of yeast Vps30p) with distinct protein complexes that regulate endocytosis as well as different steps of autophagy (709, 1011, 1818). One of these proteins is UVRAG (functionally related to Vps38) (709), which promotes autophagy either by recruiting curvature-sensing BAR-domain containing proteins (317, 1517) or by enhancing fusion of autophagosomes with lysosomes (908). An additional Beclin 1-interacting protein, Rubicon, is a negative regulator of autophagy that competes with Atg14L (the mammalian Atg14p) for binding Beclin 1 (1011, 1818). In addition, hVps34 is also involved in mediating mTOR activation during amino acid starvation (203). The mammalian enzyme is also less sensitive to Wm than the ClassI PI3Ks (1464). The structural basis of this difference as well as the potential for isoform specific inhibition of the enzyme has been highlighted in the recently published structure of the protein (1054).
B. PtdInsP Kinases
As mentioned above, PtdInsP kinase (PIPK) activities associated with membranes including the red blood cell membrane have been detected by early studies. These activities were thought to produce PtdIns(4,5)P2 from PtdIns4P in the PM as part of the PI signaling cycle, but comprised of two clearly distinguishable activities (type I and type II) with unique immunological and catalytic properties (114, 361, 1504). One of the distinctive features of the type I PtdInsP 5-kinase activity was its strong stimulation by PtdOH (726). It was also a curious early observation that type II PtdInsP kinase was ineffective against native membrane PPIs even though it was active against PtdIns4P substrates presented as micelles (although with higher Km for the lipid)(114). The first PtdInsP kinase cloned was the type II form (167, 362) (called C-isoform in Ref. 362) followed by the type I enzymes with a high degree of sequence similarity between the two forms (933). Only later was it discovered that the type II enzymes actually use PtdIns5P as substrate adding a phosphate to the 4-position to yield PtdIns(4,5)P2 and that their “5-kinase activity” was due to contamination of the commercial PtdIns4P preparations with PtdIns5P (1254). This explains why the type II enzymes were unable to make PtdIns(4,5)P2 from PtdInsPs of natural membranes.
PIP kinases are soluble peripherally membrane-bound proteins that show a highly conserved catalytic core and differ only in their COOH termini (Figure 4). The substrate specificity of the enzymes is determined by the activation loop within their catalytic domains and can be reversed by swapping activation loops between the type I and type II forms (832). The first reports on the cloning of the mammalian type II enzymes also identified two homologous sequences in the S. cerevisiae genome, Mss4 and Fab1 (167, 362). Mss4p is important for actin organization and membrane morphogenesis (348, 644) while Fab1p is essential for normal vacuolar morphology and functions (498, 1757). Since Fab1, as well as its mammalian homolog PIKfyve (1404), turned out to phosphorylate PtdIns3P to PtdIns(3,5)P2, these enzymes were classified as type III PIP kinases.
Figure 4.
The family of PIP kinase enzymes. Type I PIP kinases phosphorylate PtdIns4P to PtdIns(4,5)P2. They have three forms: α, β, and γ; the latter has five splice variants in humans that differ in their very COOH termini (alternative but still commonly used names are shown in parentheses). The type II PIP kinases phosphorylate PtdIns5P to PtdIns(4,5)P2 and also exist in three forms, of which the γ form has very low catalytic activity. Yeast only has one enzyme, Mss4p, that makes PtdIns(4,5)P2 from PtdIns4P. A third group of PIP kinases are called type III PIPkins, and they phosphorylate PtdIns3P to PtdIns(3,5)P2. The mammalian enzyme is called PIKfyve, while the yeast ortholog is Fab1p. The typical feature of these enzymes is the presence of a FYVE domain close to their NH2 termini that binds PtdIns3P and a Cpn60/TCP-1 chaperonin family domain (TCP-1).
1. Type I PIP-kinases
These enzymes are the true PtdIns4P 5-kinases, and several forms have been described in mammalian tissues encoded by three genes (PIPKIα, -β, and -γ) and a number of splice forms within PIPKIγ. Although all three major forms are widely expressed, the relative tissue distribution and the cellular localization of the various isoforms are unique, and it is generally agreed that they generate functionally distinct and specialized pools of PtdIns(4,5)P2 (375). However, the plasticity and redundancy of the PIP5Ks is really enormous, and a single copy of the PIP5KIγ gene is able to support normal mouse development and life to adulthood (1644) in spite of clearly distinguishable roles of these enzymes in specific cellular functions (see below).
Yeast possesses only one type I PtdInsP kinase, Mss4, that was first isolated as a multicopy suppressor of the stt4–1ts growth defect (1779), and subsequently shown to be a PtdIns4P 5-kinase (348, 644). Cells lacking Mss4 are not viable, and inactivation of the enzyme results in enlarged cells with rounded shape. This phenotype is very reminiscent of the defects seen in mutants of actin binding proteins (40, 546). Indeed, actin organization is severely impaired in mss4ts mutants at nonpermissive temperature (40, 65, 546). Interestingly, mss4ts and stt4ts mutants have overlapping but not identical phenotypes, suggesting that not all Mss4 functions are mediated by Pkc1 (1779). Remarkably, some (but not all) temperature-sensitive calmodulin (Cmd1) alleles confer defects in actin organization that can be rescued by Mss4 (347). In addition, Mss4 also has a role in endocytosis (347) and is linked to TOR2 (594), which is a Ser/Thr kinase with a role in cell cycle-dependent polarized actin distribution (1363). Further effectors of the PtdIns(4,5)P2 generated by Mss4 are the Slm1/2 proteins that are not only implicated in actin polarization (65, 415) but also are linked to sphingolipid metabolism (321, 486, 1510). Inhibition of sphingolipid synthesis also interferes with the signaling function of Mss4 and releases the protein from the PM (806), further supporting a connection between PtdIns(4,5)P2 synthesis and sphingolipid metabolism. The yeast phosphatidylcholine-specific PLD, Spo14 is also regulated by the PtdIns(4,5)P2 generated by Mss4 (284), and Mss4 was also found to have a role in late stages of secretion probably at the fusion step. In this capacity, Mss4 is tied to the components of the exocyst, the PM t-SNARE, Sec9, and the syntaxin-binding protein Sec1 (1303). Finally, a fraction of Mss4 also localizes to the nucleus, and temperature-sensitive mutants of Mss4 have been isolated that either accumulate in the nucleus or remain PM-associated (62). The functional significance of the nuclear Mss4 is not clear at this point, but it is worth noting that Mss4 can be phosphorylated by the yeast casein kinase I orthologs, and phosphorylation is required for PM binding and optimal functions (62).
From this description it is clear that Mss4 controls PtdIns(4,5)P2 synthesis in the context of a number of diverse biological functions, all of which are interfaced with unique regulatory molecular complexes. These functionally distinct roles have been diversified during evolution and are assumed by the various isoforms of the mammalian PIP 5-kinases.
2. PIPKIγ/PIP5K1C
PIPKIγ is the most versatile PIP 5-kinase, as it comes in several splice forms: PIPKIγ87, -γ90 (708), and a neuronal-specific -γ93 (510, 1741) (Figure 4). Aditional forms of 700 and 707 amino acid forms have been found in human (1359) and another 90-kDa form in the rat (1741). Since the exon structure differs between the rodent and human genes, and different splice forms have similar apparent molecular weights, the designation of these splice forms becomes somewhat complicated (1359, 1741). The various PIPKIγ forms are now named PIPKIγ-v1–6 in human and PIPKIγ-v1,2,3,6 in the rat (1359, 1741) (it is not clear if the Iγ-4,5 forms exist in rodents). In most tissues the 87-kDa form (PIPKIγ-v1) is prevalent, but brain expresses mostly the 90-kDa (PIPKIγ-v2) variant. Both the 87- and 90-kDa forms localize to the PM, but the 90-kDa form also localizes to focal adhesions via interaction with talin (353, 913). The interaction with talin is controlled by a balance of Tyr and Ser phosphorylations at the COOH terminus of the 90-kDa form of the kinase (875, 914). PIPKIγ93 (PIPKIγ-v3) is also localized to the PM, but the 707 amino acid human splice form (PIPKIγ-v5) shows both PM and intracellular vesicular localization (511, 1359), and the 700 amino acid form (PIPKIγ-v4) is found in nuclear speckles (1359). These localization patterns cover all the compartments where the α and β forms of type I PIPKs are found. Substrate (PtdIns4P) binding is a major factor in the PM localization of PIPKIγ (as well as Iα and -Iβ) (832), although there are other protein-protein interactions that are suspected to contribute to membrane localization (511). For example, Rac binding makes a significant contribution to the PM binding of the PIPKIβ (553). Functionally, PIPKIγ has been linked to endocytosis via interaction with the AP-2 adaptor and clathrin (80, 1550) and by promoting actin polymerization at the PM (398). A similarly important role of the enzyme was found in establishing adherent junctions via regulation of E-cadherin trafficking (912) and for EGF-driven directional migration (1498). Both the invasion and proliferation of breast cancer cells was found highly dependent on PIPKIγ (1499). PIPKIγ is the major PtdIns(4,5)P2 synthesizing activity in the brain and the synapse in particular (1704), and it plays critical roles at several steps in the synaptic vesicle exocytosis-retrieval cycle (352). Outside the brain, expression of PIPKIγ90 increases the pool of rapidly releasable dense core vesicles in chromaffin cells (1056), while elimination of the enzyme decreases the same pool and causes a defect in vesicle priming (520). Finally, PIPKIγ87 was found uniquely responsible for the generation of the PtdIns(4,5)P2 pools for agonist-stimulated Ins(1,4,5)P3 increases and Ca2+ signaling in HeLa cells (1679), and mast cells lacking PIPKIγ (both the 87- and 90-kDa forms) showed greatly reduced response to IgE receptor cross-linking (1621).
The activity of PIPKIγ is regulated by the small GTP-binding protein Arf6 (824) and by binding to AP-2 adaptor complexes (80, 1113). Stimulation of the enzyme by PtdOH has also been well documented, but it needs further studies to clarify if this regulation occurs in a physiological setting. PIPKIγ knockout mice develop normally but die soon after birth probably because of lack of suckling due to generalized neuronal defects (352). Interestingly, in another study, in which PIPKIγ was inactivated by a gene-trap method, the mouse exhibited embryonic lethality at E11.5 and showed cardiovascular and neural tube closure defects (1677). The lack of this enzyme also impairs anchoring the membrane to the cytoskeleton in megakaryocytes and platelets (1678). A mutation abrogating the kinase activity of PIPKIγ (PIP5K1C) has been identified as the cause of Lethal Contractual Syndrome 3, a severe developmental defect with early perinatal lethality (1119).
3. PIPKIα and -β/PIP5K1A and -B
The terminology of the -α and -β forms of the type I PIP kinases is rather confusing as the human α form corresponds to the mouse β, and conversely, the homolog of human β is the mouse α. As most reviews do, I will also follow the human nomenclature. There are only few functions that are specifically assigned to the α and β isoforms. In fact, overexpression of any of the type I PIP kinases affects actin dynamics and increases membrane activity, but the effects depend on the enzyme, the expression level, and the cell type. For example, PIPKIβ expression manifests in actin comet formation (1305), increased number of stress fibers (1759), increased membrane ruffling (646) and forced the appearance of internal vesicles that are coated with actin and unable to recycle to the PM (189). There have been reports, however, on changes specific to particular isoforms. For example, PIPKIβ, but not PIPKIα or PIPKIγ, was shown to localize at the trailing appendage of differentiated HL60 cells and knockdown of PIPKIβ interfered with polarization and movement of these cells during chemotaxis (845). Another special case of highly coordinated membrane activity where specific PIP5Ks are important is FcγR-mediated phagocytosis (172, 289). Here, PIPKIα is recruited to the phagocytic cup, and a kinase dead version of the enzyme blocks the process as well as the PtdIns(4,5)P2 accumulation associated with it (172, 289). It has been suggested that PtdIns(4,5)P2 is required for the actin polymerization during the pedestal formation to engulf and initiate ingestion of the phagocytic particle (172), but PtdIns(4,5)P2 needs to be eliminated for closure of the phagocytic cup (1370, 1766). However, recent studies suggest a more complicated mechanism, where PIPKIγ-deficient bone marrow-derived macrophages (BMM) showed the presence of highly polymerized actin and impaired attachment of the opsonized particles. In contrast, BMMs from PIPKIα-deficient mice showed no attachment defect, but instead a problem with particle ingestion (989). Remarkably, PIPKIγ-deficient cells showed increased RhoA and decreased Rac1 activation, and correction of the distorted activated RhoA/Rac1 ratio remedied the attachment defect (989). PIPKIα-deficient BMMs, on the other hand, had problems extending their pseudopods due to impaired WASP and Arp 2/3-dependent actin polymerization. These studies clearly showed that distinct PtdIns(4,5)P2 pools delivered by distinct kinases had functionally unique roles in a highly orchestrated process of membrane remodeling.
Not all of the effects of type I PIPKs are linked to actin polymerization. PIP5KIβ promotes transferrin receptor endocytosis in HeLa cells unrelated to its effects on actin polymerization by increasing the membrane association of AP-2 adaptor protein complexes (1185). Conversely, PIP5KIβ knockdown by RNAi (but not the other forms) inhibits the same process (1185). In another study, PIPKIα was found to regulate EGF receptor endocytosis (106) and an N-terminally truncated PIPKIα was described as a potent inhibitor of the removal of colony stimulating factor-1 receptors from the cell surface (326). As mentioned above, it is the PIPKIγ isoform that regulates clathrin-mediated endocytosis in neurons (80, 824).
In most of these actions, PIP5Ks are working tightly coupled with small GTP binding proteins, which can act both upstream and downstream of the PIP5K. RhoA was found to mediate the effects of PIP5K on stress fibers (1759) but was also found to act via a PIP5K (1010). Similarly, both PIPKIα and -β were found to associate with Rac1 regardless of the GTP-GDP status of the latter, but only PIPKIβ association regulated actin assembly (1560). On the other hand, the effects of PIP5K on ezrin membrane localization were found to be Rac1 dependent (68). Arf6 was also found to recruit both PIPKIα and -β to the PM and stimulate PIPKIβ activity (646), and constitutively active Arf6 mimicked many of the effects of overexpressed PIP5K (189). Arf6 also can indirectly regulate PIP5K by recruiting PLD2 to membrane ruffles where production of PtdOH by PLD can further activate PIP5K activity (1415). Type Iβ PIP kinases are also regulated by phosphorylation. Oxidative stress decreased PtdIns(4,5)P2 levels by releasing PIPKIβ from the PM (244, 554). This effect was due to Tyr phosphorylation of PIPKIβ by a Src family kinase (554) that was later identified as Syk and the phosphorylated residue as Y105 (244). Syk also phosphorylates PIPKIγ87 (but not the α form) during phagocytosis (989). In another paradigm, hypertonic osmotic stress increases PIPKIβ activity by Ser/Thr dephosphorylation (1758).
In addition to being associated with membrane dynamics, an exciting role of PIPKIα in the nucleus has recently been revealed. The existence of a nuclear PI system has been appreciated and studied for quite some time (see sect. X), but the exact processes that are regulated by the PI lipids and their modifying enzymes have been elusive. The presence of endogenous PIPKIα in nuclear speckles at the sites of pre-mRNA processing has been described earlier (168). Recent studies described the association of a noncanonical poly(A) polymerase, named Star-PAP with the PIPKIα enzyme in nuclear speckles (1029). Star-PAP was found strongly stimulated by PtdIns(4,5)P2 and responsible for the control of the expression of selected group of genes (1029).
PIPKIα knockout mice (lacking the mouse PIPKIβ) appear normal and develop to adulthood, although the number of offspring is lower than expected by the Mendelian ratio (1676). Interestingly, the platelets of these mice showed a defect in thrombin-induced InsP3 increases and aggregation that could be overcome at high agonist concentrations (1676). Because of the lack of any major abnormalities, it is likely that the other type I PIPK can assume the membrane-associated functions normally performed by PIPKIα, and as for nuclear functions, the recently described PIPKIγ 700 amino acid form found in nuclear speckles (1359) may be able to substitute for the PIPKIα enzyme. PIPKIβ knockout mice (lacking mouse PIPKIα) are also born and develop normally but show highly enhanced degranulation response of their mast cells after FceR-I crosslinking and enhanced cutaneous and systemic anaphylaxis (1344). This effect is explained by a defect in the stability of the actin cytoskeleton.
4. Type II PIP kinases PIP5K2s
Although the type II form was the first member cloned from the PIP kinase family (167, 362), the role(s) of these enzymes has remained largely elusive. Structurally, they are highly homologous to the type I enzymes (Figure 4) but, as pointed out above, they use PtdIns5P as substrate and convert it to PtdIns(4,5)P2 (1254). Understanding the function of the kinases is closely coupled to understanding the distribution, the pathway of production, and function(s) of the minor PI species PtdIns5P. There is much to be learned in this area, but the picture emerging from recent studies indicates that type II PIPKs (also called PI5P 4-kinases) may be more important in controlling PtdIns5P levels than to contributing to PtdIns(4,5)P2 production (267, 737). Mammalian type II PIP kinases also exist in three forms: -α, -β, and -γ (710, 934). Functional data suggest that PIPKIIα is enzymatically the most active, while -β has moderate and -γ very low catalytic activity using the standard in vitro assay conditions (197, 266). This is relevant as PIPKII enzymes form dimers, and it has been speculated that the less active forms may just serve as targeting devices to localize the active PIPKIIα enzyme to specific cellular locations (197, 263, 1672). The X-ray analysis of PIPKIIβ crystals also shows the protein in a dimeric form (1257). In fact, PIPKIIβ is localized both in the cytosol and to nuclear speckles, and it can bring PIPKIIα into the same nuclear location (197, 1672). PIPKIIγ, on the other hand, shows special tissue distribution being highly enriched in the kidney (266, 710) where it is mostly found in the epithelial cells of the thick ascending limb associated with intracellular vesicles (266). Notably, in other reports, the expressed enzyme was found ER-associated (710). The enzyme is also expressed in nervous tissues in neurons, again associated with endomembranes (265). Given the very low enzymatic activity of this form, it has been speculated that PIPKIIγ is a scaffold that brings the active -α enzyme to special membrane compartments (267).
Functionally speaking, there are very few studies addressing the cellular role(s) of PIPKII enzymes. RNAi-mediated knockdown and overexpression studies showed that PIPKIIα-mediated PtdIns(4,5)P2 formation was important for secretion in platelets (1307, 1308) and that the enzyme was also involved in platelet formation during development of the demarcation membranes in megakaryocytes (1368). Membrane recruitment of PIPKIIα could be mediated by interaction of the kinase with type I PIP kinases (620). A cytosolic and membrane-related role of PIPKIIβ was suggested by association of the protein with the TNF (223) and EGF (222) receptors. Deletion of PIPKIIβ in mice increases insulin sensitivity by enhancing Akt activation in muscle (848), and overexpression studies suggest that the enzyme attenuates insulin signaling (218). In agreement with these data, generation of PtdIns5P in the PM by the bacterial phosphatase IpgD enhances Akt activity (1206) presumably by altering EGF receptor trafficking and signaling (1255). The nuclear functions of PIPKIIβ have also been emerging. Ultraviolet irradiation increased nuclear PtdIns5P levels by a cascade of events that included p38 MAP-kinase mediated phosphorylation of PIPKIIβ at Ser236 leading to decreased activity. Decreased activity of the kinase led to the elevation of PtdIns5P, which, in turn, interacts with the ING2 protein via a PHD domain. PHD2, a nuclear adaptor protein controls p53 and histone acetylation, and PtdIns5P binding to PHD2 changes its association with the chromatin (737). In light of the recent discovery that the majority of the kinase activity associated with PIPKIIβ can be attributed to the -α enzyme as part of a heterodimer (197, 1672), it was important to show that Ser236 phosphorylation of -β does not affect its association with -α, and it is likely that the decreased activity of the complex is due to an allosteric regulatory mechanism (197).
5. Type III PIP kinases/PIP5K3
Type III PIP kinases phosphorylate PtdIns3P to PtdIns(3,5)P2. The yeast ortholog, named Fab1, was first described based on a genetic screen for defects in nuclear segregation (1757). The cloning of the 257-kDa protein revealed a strong sequence similarity with the first cloned type II PIPK within its putative catalytic region (1757). Fab1 contains a PtdIns3P binding FYVE domain close to its NH2 terminus (Figure 4). Although FAB1 is not an essential gene, fab1 null or temperature-sensitive mutant cells grow slowly and show defective spindle orientation resulting in haploid and binucleated daughter cells (1757). Fab1 null cells also develop huge vacuoles and display a block in the transport of certain hydrolyses (such as CPY) to the vacuole (498). It is speculated that the chromosomal segregation defect is secondary to the vacuolar defect possibly because of steric interference from the enlarged vacuoles (1757). That Fab1 is a PtdIns3P 5-kinase was revealed when it was found that osmotic stress led to a rapid increase in the level of a novel phospholipid, PtdIns(3,5)P2, in yeast (376), and it was shown that this increase was dependent on functional Fab1 (287). Simultaneously, it was demonstrated that Fab1 deletion or Fab1 mutant cells lacked PtdIns(3,5)P2 (498). Finally, in vitro Fab1 was shown to phosphorylate PtdIns3P to PtdIns(3,5)P2 (287, 1017), and consistent with its roles in vacuole physiology, Fab1 was localized to the vacuolar membrane (162, 378, 498). Interestingly, deletion of two other genes, Vac7 and Vac14, also eliminates detectable PtdIns3P 5-kinase activity (162, 378, 498), suggesting that they are upstream regulators of Fab1. Counterintuitively, deletion of Fig4, the phosphatase that converts PtdIns(3,5)P2 back to PtdIns3P (390, 497, 1311), does not increase the level of PtdIns(3,5)P2. Quite to the contrary, it decreases both the basal and osmotic stress-induced increase in the level of this lipid (389, 1311). This curious finding is due to the fact that Vac14 acts as a scaffold that holds Fig4 and Fab1 in a signaling complex (that also contains Vac7 and Atg18) (734), and removal of Fig4 leads to the destabilization of this complex with impaired Fab1 activity (170). Functionally speaking, all these proteins via the control of PtdIns(3,5)P2 have an important role in the multivesicular body (MVB) sorting pathway (378, 1159). This pathway is also important in autophagy via connection to the Atg18 protein (379) (see more on this in sect. X).
The mammalian type III PIP kinase is an ortholog of Fab1 and is named PIKfyve (1404). PIKfyve shows many similarities to Fab1 both structurally and functionally. It also has a PtdIns3P recognizing FYVE domain and interacts with several proteins that are homologues of the yeast proteins found in complex with Fab1. The mammalian version of the Vac14 scaffold is called ArPIKfyve that holds PIKfyve and Sac3 in one complex (734, 1351). Sac3 is the homolog of Fig4, the 5-phosphatase that converts PtdIns(3,5)P2 back to PtdIns3P (685). Intriguingly, the presence of Sac3 in the complex not only ensures that the kinase is balanced, but curiously, it also keeps PIKfyve in a highly active conformation (683). The biological advantage of this unique phenomenon that maintains a high turnover rate of PtdIns(3,5)P2 is not fully understood. Nevertheless, inhibition of PIKfyve function either by RNAi-mediated depletion (1316), expression of a dominant negative version (684), or pharmacological inhibition (with a selective PIKfyve inhibitor, YM2016636; Ref. 723) leads to massive vacuolization. These vacuoles represent swollen endocytic compartments and are associated with defects in fluid phase endocytosis (684) and in retrograde transport from the endosomes to the TGN (1316). Importantly, while the trafficking and degradation of transferrin and EGF receptors were found unaffected by knockdown studies (684, 1316), acute pharmacological blockade of PIKfyve did affect the degradation of EGF receptors with their accumulation in the membrane of the enlarged vesicles (336, 723). PIKfyve was also found functionally coupled to insulin-induced GLUT4 translocation and glucose uptake (618, 686), and PIKfyve inhibitors led to the accumulation of the autophagy marker LC3-II in endocytic structures (336). This latter finding also indicates a defect in the fusion of prelysosomal compartments and MVB with lysosomes. PIKfyve-depleted or -inhibited cells also fail to properly acidify their endocytic compartments (723), but it is not clear whether PtdIns(3,5)P2 directly or indirectly affects the activity of the V-ATPases. This latter effect may be related to the recently described PtdIns(3,5)P2 dependence of the lysosomal cation channel, TRPML1 or mucolipin (371). Most recently, PtdIns3P and PtdIns(3,5)P2 generation were found to control the activation and localization of the mTORC1 complex via a direct association between Raptor and PtdIns(3,5)P2 (180). Other recent studies indicated that PIKfyve, together with some of the myotubularin phosphatases that remove the 3-phosphate from PtdIns(3,5)P2 (see sect. VI), are important for PtdIns5P generation in mammalian cells (1173, 1352, 1827). One of these studies also found that PtdIns5P generated via this pathway is a regulator of cell migration (1173).
PIKfyve inactivation in Caenorhabditis elegans (1129) and Drosophila (1315) also leads to swollen endocytic compartments. Germline deletion of PIKfyve in mice is embryonic lethal in the preimplantation stage, and MEF cells from heterozygotes show an increased sensitivity to the PIKfyve inhibitor YM201636 (682). A debilitating mutation in one allele of PIKfyve causes Francois-Neetens mouchetée corneal fleck dystrophy in humans characterized by multiple focal vacuolization in the cornea (901). The evolutionary conservation of the tripartite PIKfyve/Fig4/Vac14 complex that is required for proper PIKfyve function is also underscored by mouse and human genetics. The genetic aberration responsible for the “pale-tremor mouse” phenotype has been identified as an early transposon insertion into the mouse Fig4/Sac3 gene. This also resulted in a decrease rather than an increase in PtdIns(3,5)P2 levels and caused a prominent defect at the late-endosome to lysosome transition (and probably in many other trafficking steps) responsible for the pigmentation defect and a progressive neurodegeneration (253). Pathogenic mutations in the human FIG4 gene have also been identified in a novel form of autosomal recessive Charcot-Marie-Tooth disorder, CMT4J, characterized by motor and sensory neuropathy (253) and in a small percentage of patients with amyotrophic lateral sclerosis (ALS) (252). Similarly, a Vac14 gene-trap mouse showed early newborn lethality associated with rapid neurodegeneration with massive vacuolization and cell death in certain brain areas. Since the development of these animals appears to be normal and their other organs are not as severely affected, the lack of Vac14 only compromises but does not completely eliminate PIKfyve function (1810). It is a fascinating question why only certain neurons mainly after birth are so dependent on PtdIns(3,5)P2 function.
C. PtdIns(4,5)P2 Kinases
The first indication that PtdIns(4,5)P2 can be further phosphorylated was the detection of novel lipids, PtdIns(3,4,5)P3 and PtdIns(3,4)P2, in 1989 by Taynor-Kaplan in human neutrophil cells (1572). In parallel studies it was discovered that type I PtdIns kinases could phosphorylate the 3-position of PtdIns (1711) and that the PI 3-kinase activity associated with oncogenes and stimulated growth factor receptors primarily produced PtdIns(3,4,5)P3 and, in fact, was a PtdIns(4,5)P2 3-kinase (66). These early reports have marked the beginning of the explosion of the PI 3-kinase field that unraveled the existence of several classes of PI 3-kinase enzymes and their regulatory adaptor proteins (Figure 5). The 110-kDa catalytic domains of class I PI 3-kinases are encoded by four distinct but highly related genes in mammals (-α, -β, -γ, and -δ). In addition, five distinct genes encode tightly associated PI 3-kinase regulatory proteins, p85α (with several splice variants), p85β, p55γ, p101 and p84/p87, the latter two serving as adaptors for the p110γ enzyme. Class II PI 3-kinases also exist in three forms (-α, -β, -γ). These are larger proteins (150–180 kDa) with relatively little known about their cellular regulation and functions. These enzymes were believed to phosphorylate PtdIns4P to PtdIns(3,4)P2 until recently, when increasing evidence suggests that their primary product in vivo is PtdIns3P (418, 1700) and, hence, they function as PtdIns 3-kinases rather than PtdIns4P or PtdIns(4,5)P2 kinases. However, because of the still lingering uncertainty about their in vivo substrate preference, these enzymes will be discussed here. The only class III PI 3-kinase, hVps34, is usually discussed with the PI 3-kinases, but because of its only substrate being PtdIns, it was already covered with the PtdIns kinases.
Figure 5.
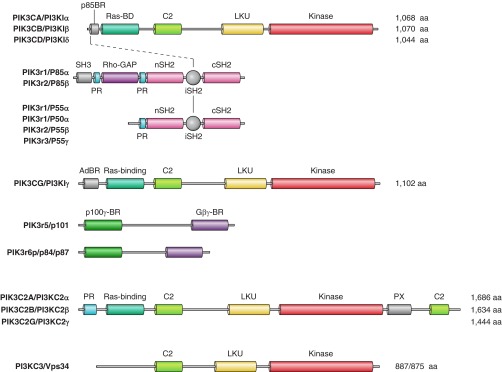
The family of PI 3-kinase enzymes. Class I PI 3-kinases phosphorylate PtdIns(4,5)P2 to PtdIns(3,4,5)P3. There are four different genes coding for the catalytic subunits of PI3Ks, called p110α, -β, -γ, and -δ. They all have a conserved COOH-terminal catalytic domain preceded by lipid kinase unique (LKU, also called “helical”), C2 and Ras binding domains (Ras-BD). These enzymes have tightly associated regulatory subunits: p110α, -β, and -δ associate with p85 and p55/50 regulatory subunits encoded by three different genes. Their association is mediated by an interaction between the very NH2-terminal p85-binding region (p85BR) of the catalytic chains with the inter-SH2 (iSH2) region of the regulatory subunits. These enzymes are also called class IA enzymes. The catalytic p110γ differs from the previous forms (hence it is called class IB) in that it associates with either of two adaptors, p101 and p84/p87. These different adaptors interact with NH2-terminal adaptor binding region (AdBR) of p110γ and lend regulation by G protein βγ subunits (p101) and perhaps by Ras (p84/p87) to the enzyme. Class II PI3Ks also come in three forms (α, β, and γ) and contain phox-homology (PX) and C2 domains placed COOH terminally to their catalytic domains. These enzymes can phosphorylate PtdIns and PtdIns4P in vitro, but their in vivo substrate preference is still debated. The enzymes most likely form PtdIns3P in the cell. The single class III PI3K and its yeast ortholog, Vps34p, phosphorylates PtdIns to PtdIns3P. These enzymes associate with a larger regulatory protein Vps15p in yeast and p150 in mammalian cells (not shown).
Class I PI 3-kinase activation leads to the production of PtdIns(3,4,5)P3 in the PM, and this molecule is the ultimate initiator of a great variety of cellular signaling responses and cascades of molecular events. A chief mediator of PtdIns(3,4,5)P3 actions is the protein kinase Akt (also called PKB) that is recruited to the PM by PtdIns(3,4,5)P3 via its PH domain where it gets phosphorylated on Thr308 and Ser473 by PDK1 (1081) and mTORC2 (1830), respectively, leading to its increased kinase activity. Interestingly, PDK1 also has a PH domain, and its recruitment to the membrane is also aided by PtdIns(3,4,5)P3 (816). Recent data suggest that mTORC2 may also be regulated by PtdIns(3,4,5)P3 (489, 1430). Activated Akt phosphorylates a whole range of substrates to regulate energy metabolism, survival, cell cycle progression, or differentiation depending on the tissue and the signaling context under which it is activated. These pathways will be detailed in section IX. Additional molecules regulated by PtdIns(3,4,5)P3 are guanine exchange factors (GEFs) for a range of small GTP binding proteins, such as Arfs (1627) and Rac/Rho/Cdc42 (608, 1699) or the Tec family tyrosine kinases such as Bruton's tyrosine kinase (Btk) (1261) or Itk (44), key components of B- and T-cell activation, respectively. PtdIns(3,4,5)P3 gradients have a major role in the migrational response of cells to chemotactic stimuli (459), a response not only serving phagocytic cells, but also being critical for morphogenic patterning, axon extension, blood vessel formation, and for invasion and metastasis by cancer cells. The contribution of the individual isoforms and their regulatory subunits to these processes during development and to the functions of various cells and tissues is only beginning to unfold with the help of genetic deletion of the individual enzymes in whole animals or in selected tissues and with the availability of subtype-specific PI 3-kinase inhibitors.
Class I PI 3-kinases control several cellular processes such as cell energetics and metabolism or immune cell functions, and they are also key players in cancer development, propagation, and metastasis. These important roles have propelled PI 3-kinase research into ever-increasing heights. In light of the incredible progress made in this research area, it seems ironic that PI 3-kinases were initially deemed nondruggable targets because of their fundamental roles in supporting almost every aspect of a cell's life. Contrast this with the fact that several subtype-specific PI 3-kinase inhibitors are now in development and some are being tested in clinical trials. Due to the enormity of the PI 3-kinase literature, its coverage in this review will be inevitably limited and focused only on fundamentals. Several excellent reviews have summarized various aspects of this topic in greater details in recent years (75, 221, 467, 814, 996, 1609, 1612, 1613, 1640, 1727, 1785).
1. Class I PI 3-kinases
PI3Kα/PI3KCA is a widely expressed and most studied member of the class I PI3K family. The 110-kDa protein contains a COOH-terminal catalytic domain that is highly homologous to those found in other PI 3-kinases, in type III PI 4-kinases (190, 505), and in PI kinase-related kinases (669). This group of catalytic domains is also highly conserved from yeast to human, although yeast has only a class III PI 3-kinase, Vps34. The domain architecture of these enzymes is shown in Figure 5. PI3Kα is tightly associated with a regulatory subunit encoded by the p85α gene with three splice forms: p85α, p55α, and p50α (468, 699, 1178). p85α has an NH2-terminal SH3 domain, a BH (BCR homology) domain, proline-rich regions, and two SH2 domains linked with a coiled-coil iSH2 (inter-SH2) region, which is one of the major sites of interaction with the NH2 terminus (1053) and C2 domains (660) of the catalytic p110α subunit. The shorter variants, p55α and p50α, lack the NH2 terminus of p85α including the SH3 and BH domains. The classical activation sequence of PI3Kα occurs during receptor tyrosine kinase activation, with recruitment of the p85/p110α complex via direct binding of the SH2 domains of p85 to the tyrosine phosphorylated motifs of the receptors (1435) such as the PDGF receptor (770) or the immunoglobulin family tyrosine-based activation motif (ITAM) of T- and B-cell receptors (943, 1078). In some cases, the recruitment is indirect, involving docking proteins, such as Grb2 and Gab1 for EGF receptors (1277, 1669) or IRS-1/2, in the case of insulin or IGF1 receptors (76, 1102). During this process, the p85 subunit also gets tyrosine phosphorylated and renders the catalytic subunit active via molecular rearrangements, the details of which are just beginning to unfold thanks to elegant structural studies (1053, 1807). Early reports showed that the iSH2 domain of p85 fused to the NH2 terminus of p110α yielded a constitutively active PI 3-kinase (657), but p85 interaction actually stabilizes the catalytic domain and keeps it in an inactive state (1781) as indicated by more recent structural studies (660, 978, 1807). As with all class I PI 3-kinases, the p110α subunit also has a Ras binding domain, and GTP-bound form of Ras is capable of directly activating PI3Kα (221, 657, 809, 1280). Several oncogenes like Src and polyoma middle T or the avian Rous sarcoma virus associate with the p85/p110α complex also leading to its strong activation (228, 292, 479, 760).
Homozygous deletion of PI3Kα in mouse is embryonic lethal (143) and so is the homozygous replacement (“knock-in”) of the kinase with a catalytically inactive mutant, D933A (457). Mice heterozygous for this mutation showed reduced growth, hyperinsulinemia with glucose intolerance, hyperphagia, and adiposity, all signs of reduced insulin sensitivity, demonstrating that IRS-mediated recruitment and activation of PI3Kα is a major pathway in insulin/IGF1-mediated regulation of growth and metabolism (457). Targeted deletion of PI3Kα in endothelial cells showed that this isoform has a cell-autonomous function critical for angiogenesis, and the embryonic lethality of PI3Kα deficiency is likely caused by defects in vascular remodeling (524). The complete deletion of PI3Kα also changes the balance between the p85 and p110 subunits resulting in excess free p85 subunits that can associate with other proteins affecting their functions (899). The best example for this is the association of the free p85α subunit with IRS-1 after insulin stimulation, which competes with the recruitment of the p85/p110 heterodimer, therefore limiting insulin signaling (955). These findings also highlight the complex changes that can indirectly result from a complete deletion of a protein in a knockout animal.
Several studies reported on the knockout of the p85α regulatory subunit, either eliminating all three splice forms (469, 470, 1585) or either the long (85 kDa) (1503, 1543) or short (p55 and p50) (241) forms. These studies surprisingly found that all these mice showed increased insulin sensitivity and hypoglycemia. Moreover, the MEF cells isolated from these animals showed enhanced rather than decreased PtdIns(3,4,5)P3 response after stimulation. Since p85α is the major adaptor protein that is needed to recruit PI3Kα to the insulin receptor, these paradoxical changes suggested that either upregulation of p85β, or more likely the presence of “free” p110α catalytic subunits that are more active because of the lack of their inhibition by the associated p85. More recently, it was also found that the p85α regulatory subunit also interacts and activates PTEN (226), the phosphatase that counters PI 3-kinase action, and this negative regulation is lost in p85α knockout mice. Therefore, the abundance or shortage of p85 relative to p110α could be an important factor in determining the extent of PI 3-kinase activation and hence is also a factor in tumorigenesis (1530). However, a detailed recent study casts doubt on the role of “free” p85α or p110α subunits in normal cells as they appear to exist as obligate heterodimers (503). Interestingly, elimination of p85α causes a severe defect in B-cell development and decreased B-cell proliferation in response to mitogens and in this case PI 3-kinase signaling is decreased rather than increased (470, 1503).
Mutations within the human PI3KCA (coding for PI3Kα) as well as in the PIK3r1 (coding for p85α) genes have been identified in a variety of cancers, at especially high percentage in colorectal cancers and glioblastomas, but also in other cancer forms (247, 716, 1198, 1219, 1334). It was also demonstrated that oncogenic mutants of PI3Kα are sufficient to promote cell proliferation and dissemination of cancer cells when injected into nude mice (541, 1190, 1333). It has been noted that the mutations cluster mostly in two regions that code for the helical and kinase domains of PI3Kα and some other ones within the NH2-terminal and C2 domains (1334). The X-ray structures of the p110α complexed with the iSH2 domain (660, 978) and the p110β/p85β complex (1807) has shed light on the possible mechanism of how these mutations lead to constitutive activation of the kinase. Most of these mutations are located in regions where the catalytic domain interacts with the iSH2 domain. These mutations probably weaken the interaction between the p85 and p110 subunits, therefore releasing the catalytic domain from the inhibitory effect exerted by the iSH2 domain. A unique role of the p85 regulatory subunits in stabilization of the PTEN phosphatase has been reported recently as well as mutations within these subunits causing destabilization and loss of PTEN in endometrial cancers (247).
2. PI3Kβ
The roles of PI3Kβ appear to be more restricted or specialized than those of PI3Kα, but this isoform has also been less studied. PI3Kβ forms a heterodimer with p85β and was expected to function in a similar manner as PI3Kα and thought to be functionally redundant with the latter. However, this idea was quickly repudiated when the embryonic lethality of the PI3Kα deleted homozygous animals was reported. An initial study reported early embryonic lethality in mice after deletion of PI3Kβ (142). Later studies using different strategies, either replacing the enzyme with a catalytically inactive (K805R) form (261) or deleting exons critical for catalytic activity (534), unexpectedly showed that these animals were viable and developed to adulthood. However, heterozygous mice in either case were born at a sub-Mendelian ratio, and some of the early embryos were small and moribund. Remarkably, the severity of the developmental defect of the embryos in one study correlated with the low expression levels of the kinase-dead PI3Kβ alleles and not with compensation from other forms of PI3K or regulatory subunits (261). This suggested a noncatalytic function of the protein that was attributed to a scaffolding role supporting clathrin-mediated endocytosis (261). Analysis of MEF cells from these animals revealed unexpected enzymatic roles of the kinase in mediating signals from certain GPCRs such as the sphingosine 1-phosphate receptor (261) or the LPA receptor (534). In contrast, PI3Kβ was not a major contributor to growth factor-induced signaling (534) but made some contribution to insulin-mediated signals (261). Similar conclusions were reached when using PI3Kβ-specific inhibitors (534, 804). These studies were consistent with early observations that PI3Kβ can be activated by both RTKs and Gβγ subunits (835).
PI3Kβ was also found essential in male fertility (262), and in platelet functions, especially related to platelet integrin-mediated signaling (209, 1003). Recent studies reported that a majority of PI3Kβ was nuclear where it contributed to the control of DNA replication (998) and also to double-stranded break DNA repair (831), but it is doubtful that the functions of the enzyme would be only nuclear. PI3Kβ-deficient MEF cells showed impaired autophagy, and expression of the enzyme enhanced autophagy. Surprisingly, PI3Kβ was not acting via the Akt-mTOR pathway but was associated with the Vps34-Vps15-Beclin-1-Atg14L complex and promoted PtdIns3P synthesis (374). Reactive oxygen species production by neutrophil cells in response to fungal antigens mediated by integrins was also found to require either the PI3Kβ or the PI3Kδ isoforms (175). These studies suggest a clear specialization of PI3Kβ for certain tissues and processes, but more studies are needed to define the exact role(s) of this isoform under more acute inhibitory conditions and not only by genetic manipulations.
Deletion of the p85β regulatory subunit enhanced T-cell proliferation and decreased cell death (342). On the other hand, p85β showed redundant functions with p85α in early embryonic development and either one was necessary and sufficient to support membrane-ruffling responses to PDGF (176). The two p85 regulatory subunits were also found to be required but redundant in B-cell development (1156).
Curiously, PI3Kβ mutations are not commonly found in human cancers even though this isoform also has oncogenic potential (346, 758, 1813). There are, however, conditions under which PI3Kβ can also be a factor in tumorigenesis especially under PTEN loss (731, 1687). The transforming ability of PI3Kβ was dependent on Gβγ interaction (331). PI3Kβ appears to be more “constitutively active” than the wild-type PI3Kα behaving similarly to one of the oncogenic mutants of PI3Kα (N345K) (330). Comparison of the solved structures of the PI3Kα (660, 978, 1733) and PI3Kβ (1807) complexed with their respective regulatory subunits (or pieces of it) may explain some of these differences, although the conclusions of the different studies differ as to whether the catalytic domain of PI3Kβ is restrained more or less by interactions from p85β (nSH2, iSH2, and cSH2). Also interesting is the question of why the discrepancy between these highly homologous enzymes in their responses to tyrosine-phosphorylated peptides or Gβγ subunits liberated during stimulation of a restricted group of GPCRs. Recent identification of the Gβγ subunit interaction region in PI3Kβ is a major step toward understanding the unique regulation of this PI3K isoform (331).
3. PI3Kγ/PI3KCG
The earliest indications that there was an alternative way of PI3K activation via GPCRs and heterotrimeric G proteins were the PtdIns(3,4,5)P3 increases found in neutrophils after stimulation with GPCR ligands (1461, 1572). It was also observed that PI3K inhibition blocked chemotaxis and oxidative burst in neutrophils, and the GPCR-stimulated degranulation in basophilic leukemia cells (52, 1484, 1761). This was followed by the detection of a Gβγ-regulated distinct PI3K activity in myeloid cells (1468) and the subsequent cloning of the 110-kDa catalytic (1465, 1476) and 101-kDa regulatory subunits (1465) of what was named PI3Kγ. More recently, another regulatory subunit of PI3Kγ, called p84 or p87PIKAP, has been identified (1496, 1641). The tissue distribution of PI3Kγ and its adaptors is more restricted than those of other PI3K isoforms, showing highest expression in cells of myeloid origin, such as macrophages, neutrophils, eosinophils, and mast cells (128). However, these enzymes are also found in lower levels in other tissues such as the heart or the exocrine pancreas (128, 1476). The p84/87 regulatory subunit is especially enriched in mast cells, macrophages, and heart (157, 1641), whereas the p101 subunit is more abundant in neutrophils (1497).
PI3Kγ can be activated by both Ras and Gβγ subunits, and both pathways are important for the full repertoire of neutrophil responses, such as chemotaxis and respiratory burst in response to chemotactic stimuli (1497). The relative roles of the two regulatory adaptors p101 and p84 in mediating Gβγ and Ras stimulation were uncovered relatively recently. Early studies showed that the isolated p110γ catalytic domain of PI3Kγ can be directly stimulated by Gβγ subunits (887, 1476) as well as by Ras (1184, 1309). The direct stimulation of p110γ lipid kinase activity by Ras was found only modest in one study (1309), but oncogenic Ras mutants potently stimulated p110γ both in vitro and in transfected cells in another (1184). Importantly, the Gβγ sensitivity of PI3Kγ stimulation is greatly enhanced by the p101 regulatory subunit in vitro or in cells (833, 1465), yet p101 does not change the Ras sensitivity of the kinase. Accordingly, a mutant p110γ with impaired Ras binding still responds to Gβγ, and this response is still enhanced by p101 (1184, 1497). In contrast, p84/p110γ complexes are not sensitized to Gβγ stimulation. Instead, the p84/p110γ complex requires Ras binding for membrane recruitment and activation by GPCRs (833). Therefore, it appears that p110γ complexed to the two distinct adaptors is regulated in a clearly distinct manner and participates in distinct cellular responses. In support of this idea, recent studies showed that mast cell degranulation that depends on simultaneous engagement of FcϵRI and the GPCR adenosine receptors relied on the p84/p110γ, while chemotaxis or Akt activation were also supported by the p101/p110γ complex. Moreover, the fate of the PtdIns(3,4,5)P3 made by the distinct complexes also differed; the one made by p101/p110γ quickly internalized while those made by p84/p110γ remained in the PM (157).
In addition to its lipid kinase function, PI3Kγ can also phosphorylate proteins such as MEK and activate the MAP kinase pathway through its protein kinase activity (1477). In elegant studies using activation loop swapping, PI3Kγ enzymes defective in either lipid or protein kinase activities were created to test the relative importance of lipid vs. protein kinase activities of the enzymes in initiating downstream responses (163).
The phenotypes observed after PI3Kγ deletion in mouse also supported the pivotal role of this enzyme in host defense and inflammation, consistent with the roles of this enzyme in chemotaxis of neutrophils and macrophages (624, 906, 1346). Analysis of PI3Kγ-deficient animals also revealed that the enzyme was critical in amplifying degranulation responses in mast cell and, accordingly, the knockout animals were protected from systemic anaphylaxis (847). Several other pathological conditions in which recruitment of neutrophils and macrophages by inflammatory chemokines is an aggravating factor have been alleviated by genetic or pharmacological inactivation of PI3Kγ. For example, atherosclerotic lesions are attenuated in apoE-deficient mice by inhibitors of PI3Kγ, or by replacing the macrophages of the apoE mice with ones originated from PI3Kγ-deficient animals (231, 456). Sepsis-induced multiorgan damage was also significantly reduced in mice with genetic or pharmacological inactivation of PI3Kγ (1002), and PI3Kγ deleted mice were largely protected in mouse models of rheumatoid arthritis (208). PI3Kγ inhibition also alleviated the severity of glomerulonephritis in a mouse model of systemic lupus (104). These studies and many others reviewed in more details (996, 1294) have identified PI3Kγ as a very desirable drug target for the control of a variety of inflammatory conditions, and several companies are developing subtype-specific PI3Kγ inhibitors (1294, 1310).
Even if the role of PI3Kγ in myeloid cell functions, chemokine responses, and inflammation are dominating themes, PI3Kγ has also been identified in other tissues and biological processes. These include a specific role of the kinase in platelet aggregation in response to Gi-coupled receptors, such as the ADP-receptor P2Y12 (623, 907), or in T-cell signaling (1346) and T-cell development resulting in a decreased CD4+/CD8+ T-cell differentiation ratio in PI3Kγ-deficient mice (1279, 1346). Moreover, PI3Kγ function is also important in the heart, especially under pathological conditions, as PI3Kγ deficiency protected mice from hypetrophy, fibrosis, and related dysfunctions caused by prolonged β-adrenergic exposure (1182). Strangely, PI3Kγ-deficient and “knock-in” mice expressing the kinase-dead version showed opposite responses to pressure overload following aortic constriction: knockout mice developed myocardial damage and heart failure while the knock-in mice were protected from all of these effects and even showed protection from fibrosis (1199). This striking difference revealed a kinase-independent function of PI3Kγ in the heart through its association with the phosphodiesterase PDE3B and stimulation of its PDE activity (1199). A PI3Kγ-mediated effect of angiotensin II (ANG II) on l-type Ca2+ channels in vascular smooth muscle cells has also been described (1244), and PI3Kγ-deficient mice were protected from the vascular damage and hypertensive effects of ANG II administration (1623). An initial report found an increased incidence of colorectal cancer in PI3Kγ-deficient mice (1345), but this phenotype was not reproduced in other studies using independently developed PI3Kγ-deficient mice and has been attributed to some unrelated and yet unclear genetic constellations (105).
4. PI3Kδ/PI3KCD
The last member of the class I PI 3-kinase family, named PI3Kδ, was cloned from a U937 monocyte cDNA library and showed an expression pattern restricted to white blood cells. The 110-kDa protein was able to associate with the SH2-domain-containing adaptors, p85α (as well as its smaller forms), p85β, and also with the Ras proteins (1614). Recruitment of this enzyme to the membranes of cytokine-stimulated leukocytes was similar to those of p110α, but a distinctive feature of the PI3Kδ enzyme was its ability to autophosphorylate (1611, 1614). Distinctive functions of this kinase were revealed by genetic ablation of the protein or “knock-in” studies that replaced the wild-type allele with a kinase-dead version (D910A) of the protein. More recently, the development and testing of subtype-specific PI 3-kinase inhibitors also shed light on the unique functions of this kinase in physiology and disease. These studies showed a nonredundant role of PI3Kδ in B-cell development and an abrogated proliferative response of B cells to B-cell receptor stimulation (268, 740, 1165). Accordingly, both T-cell-dependent and -independent humoral responses were strongly impaired in both the PI3Kδ deleted and the kinase-dead “knock-in” animals (268, 740, 1165).
Importantly, PI3Kδ often works together with PI3Kγ to support immune cell functions. One of the best examples of the complementary roles of the two enzymes is mast cells. Here the activation requires triggering of both the tyrosine kinase pathways (via either the KIT receptor or FcRϵI engagement) with PI3Kδ participation and sensitization via adenosine receptors that activate PI3Kγ (157, 847). This explains why inactivation of either enzyme impairs mast cell-mediated allergic responses and attenuates passive anaphylaxis (30, 847). Similarly, neutrophil cells also showed complementary roles of PI3Kγ and -δ isoforms in their ROS generation response under certain conditions (175, 285). These studies identified the PI3Kγ and -δ isoforms as potential targets to treat allergic diseases and also some autoimmune diseases including rheumatoid arthritis (1294, 1295, 1549). Moreover, PI3Kδ-specific inhibitors have shown some promise in the treatment of chronic lymphocytic leukemia (606) and multiple myeloma (681), and they were found potentially beneficial in reversing the glucocorticoid resistance in chronic obstructive pulmonary disease (1005, 1558). The recently solved structure of PI3Kδ (125) will certainly aid the development of PI3Kδ-specific inhibitors (41) that can be tested in a variety of immunological disease models.
5. Class II PI 3-kinases/PI3KC2s
Class II PI 3-kinases were discovered based on their sequence homology with class I PI 3-kinases (192, 369, 965, 1638). These enzymes also exist in three forms in mammalian cells: PI3KC2α, PI3KC2β, and PI3KC2γ (1347, 1610). Class II PI 3-kinases are larger proteins (170–200 kDa) that have highly homologous domains with other PI 3-kinases, such as the Ras binding domain, the helical and catalytic domains, but also have some distinctive features, such as a PX and C2 domains at their COOH termini. They are most dissimilar at their NH2 termini that differ in lengths but all contain proline-rich segments (Figure 5). Class II PI 3-kinases can phosphorylate PtdIns as well as PtdIns4P but not PtdIns(4,5)P2 in vitro, and based on this feature, it was initially believed that these enzymes produce primarily PtdIns(3,4)P2 from PtdIns4P in cells (369). However, increasing evidence suggests that class II PI 3-kinases also make PtdIns3P for specific processes (420). Since relatively little is known about the physiology of these enzymes, the three isoforms will be discussed together.
PI3KC2α and PI3KC2β are ubiquitously expressed, whereas PI3KC2γ shows more restricted expression mainly in liver and some other tissues depending on the species (627, 1061, 1171). PI3KC2α is found primarily in the trans-Golgi and in clathrin-coated vesicles (367) but was also localized to nuclear speckles (356). PI3KC2β, on the other hand, was found in the cytoplasm and in perinuclear puncta (1706), while PI3KC2γ was described in the Golgi (1171). Both PI3KC2α (484) and PI3KC2β (1706) were shown to bind clathrin, and clathrin stimulated the kinase activity of PI3KC2α (484, 485, 1583). These studies suggested a role of these kinases in clathrin-mediated endocytosis and trafficking. Although class II PI 3-kinases do not have any known adaptor proteins, several studies have indicated that PI3KC2α can respond to EGF (53), insulin (191), chemokine (1583), or TNF and leptin (829) receptor stimulation. Similarly, PI3KC2β associates with the activated PDGF and EGF receptors (53) and is stimulated by stem cell factor (51) and integrin engagement (1796).
As to the roles of these enzymes in cellular processes, several studies suggested that PI3KC2α is involved at multiple levels in the secretion and actions of insulin (418). PI3KC2α was found to regulate exocytosis of insulin in pancreatic beta cells (370), but was also activated by insulin via the small GTP binding protein TC10 (970) and played a role in insulin-induced Glut4 translocation (370). A role of PI3K-C2α was also suggested in neurosecretion (1034), and in this context, it is notable that TC10 was recently found to regulate axon cone specification by inducing translocation of the exo70 exocyst complex to membranes (391). PI3K-C2β, on the other hand, was found to regulate cell migration (368, 764, 971) via a Rac-dependent signaling pathway also involving PtdIns3P production. PI3KC2γ was claimed to play a role in regenerating liver (1171), but there is little knowledge concerning the involvement of this enzyme in any biological process. As pointed out above, it is increasingly evident that class II PI 3-kinases make PtdIns3P as their signaling molecule (370, 970, 1700). If the enzyme indeed makes primarily PtdIns3P, it is likely that it does so in very specific molecular contexts that differ from those regulated by the class III Vps34 enzymes. This has initiated a discussion as to what extent class II enzymes contribute to the bulk amount of PtdIns3P found in cells (418, 1034). It is worth pointing out that classII PI3Ks are less sensitive to the PI3K inhibitor Wm (369), and some of the PtdIns3P in cells is more resistant to inhibition by Wm (1464).
Genetic ablation of PI3KC2β in mice has been reported, but the mice are viable and fertile with no obvious specific phenotype reported (569). Inactivation of the enzyme's ortholog in C. elegans causes fat accumulation mimicking the effects of inactivation of the insulin receptor homolog (57). Ectopic expression of the PI3KC2 ortholog or its kinase-dead version in fly epithelium caused pattern formation defects (966).
VI. PHOSPHOINOSITIDE PHOSPHATASES
Initial interest toward the inositol lipid phosphatases in the early 1980s paled compared with the excitement for lipid kinases and PLC enzymes, and only a few groups pursued research in this direction. PI phosphatase research was motivated primarily by the interest in the metabolism of the second messenger Ins(1,4,5)P3 (286, 1474) by 5-dephosphorylation and by the prominent effect of lithium on inositol phosphate metabolism causing an accumulation of Ins monophosphates and depletion of myo-inositol (34), with a possible link to the effectiveness of Li+ in manic-depressive disease (134) (see sect. III). Therefore, identification of the Ins(1,4,5)P3 5-phosphatase (that showed no lithium sensitivity) and the lithium sensitive inositol monophosphatase(s) were top priorities in the mid 1980s. However, Phil Majerus' group had already focused on every form of inositol phosphate or -lipid phosphatases from early on and identified several enzymes that acted upon various inositol phosphate isomers and some on the lipids themselves (see Ref. 972 for a review on these early studies). The interest level was significantly raised when the gene causing the X-linked human disease Oculo-Cerebro-Renal Syndrome of Lowe (OCRL) was mapped and found to have significant homology to an already known 75-kDa inositol polyphosphate 5-phosphatase (61). Subsequent research showed that OCRL was a PtdIns(4,5)P2 rather than an inositol polyphosphate 5-phosphatase (1166, 1805). Further momentum was gained when a PtdIns(4,5)P2 5-phosphatase associated with presynaptic membranes and named synaptojanin (Snj) was described and cloned (1022). In parallel studies, the same enzyme was purified and cloned as a PLD inhibitor (259), a feature that was due to its PtdIns(4,5)P2 phosphatase activity since PLD requires PtdIns(4,5)P2 for optimal activity (918). These studies helped disseminate the notion that inositol lipid dephosphorylation was an important regulator of inositol lipid signaling and that it was essential to determine whether a phosphatase acted on lipids or on soluble inositol phosphates in its natural cellular location. The next huge development of the phosphatase field was marked by the realization that the tumor suppressor gene PTEN (phosphatase and tensin homolog located on chromosome 10) was actually a PtdIns(3,4,5)P3 3-phosphatase (and not only a protein tyrosine phosphatase as previously believed) and that it countered the actions of PI 3-kinases (968, 969, 1104). Fortuitously, another group of enzymes initially thought to be phosphotyrosine phosphatases, called the myotubularins, were also discovered by identification of a gene mutated in human X-linked centromyotubular myopathy (858). This enzyme, called MTMR1, turned out to be a PtdIns3P 3-phosphatase (152, 1536) and so did its sister protein, named MTMR2, that was mutated in the neurodegenerative disorder Charcot-Marie-Tooth disease type 4B (792). So, in the case of the phosphatases, human genetics played a pivotal role in unearthing several of the enzymes, and the usually pioneering yeast studies can be credited with the first identification of only one of the phosphatases, namely Sac1, with connection to actin polymerization (273), inositol lipids (1714) and which was later proven to be a phosphoinositide monophosphatase (538).
A. 5-Phosphatases
This group of enzymes can remove the phosphate from the 5-position from PtdIns(3,4,5)P3, PtdIns(4,5)P2, and PtdIns(3,5)P2. They are divided into four types (I-IV), but the 43-kDa type I enzyme (INPP5A), which was the first 5-phosphatase isolated and cloned, does not act on lipids but dephosphorylates Ins(1,4,5)P3 and Ins(1,3,4,5)P4 (340, 862, 863). The type II 5-phosphatases include the synaptojanins, OCRL, INPP5B, INPP5J, and SKIP, while the two type III enzymes are SHIP-1 and -2. The only type IV enzyme is INPP5E. All of these enzymes belong to the AP family of endonucleases and have two signature motifs (F/Y)WXGDXN(F/Y)R and P(A/S)(W/Y)(C/T)DR(I/V)L(W/Y) separated by ∼60–75 residues (973). The structure of the 5-phosphatase domain of one of the S. pombe 5-phosphatases has been solved, and it revealed the positions of His/Asp active site pairs within the core of the catalytic domain (1581). In addition to their catalytic domain, 5-phosphatases contain several additional protein motifs that determine their cellular localization and interactions with other proteins (Figure 6).
Figure 6.
The inositol lipid 5-phosphatase family. These enzymes dephosphorylate PtdIns(4,5)P2 and/or PtdIns(3,4,5)P3 in the 5-position. There are three major subgroups called type II, III, and IV. [The type I enzyme is a smaller, 43-kDa protein that hydrolyzes the water-soluble Ins(1,4,5)P3 molecule but not the membrane-bound lipids.] The type II 5-phosphatase enzymes are encoded by six genes. Two of these, synaptojanin-1 and synaptojanin-2, exist in multiple splice forms differing in their respective COOH termini (only the two main forms are shown). The characteristic feature of these enzymes is the presence of a Sac1 homology domain upstream of their conserved 5-phosphatase domains. The NPF repeats of the longer form of SYNJ1 binds to the endocytic protein Eps15. OCRL and INPP5B are also very similar to one another, with a whole set of domains providing multiple interactions in addition to the catalytic 5-ptase domain. These include an NH2-terminal PH domain and COOH-terminal ASH and Rho-GAP domains. The ASH domain binds Rab5 and the adaptor protein APPL1, while Rho-GAP domain binds the small GTP binding proteins, Rac1 and Cdc42. OCRL1a contains two clathrin binding (CB) domains, the second of which is not present in OCRL1b. INPP5B has very similar structure, but it lacks the clathrin-binding domains and has a COOH-terminal CAAX domain. INPP5J and INPP5K are smaller proteins that have a SKICH domain downstream of their 5-phosphatase domain. This core structure is surrounded by proline-rich sequences in INPP5J. The type III 5-phosphatases are represented by the SH2-domain-containing enzymes SHIP1 and SHIP2. They have an NH2-terminal SH2 domain and C2 and proline-rich sequences downstream of the 5-phosphatase domain. SHIP2 also has a sterile alpha motif (SAM) at its very COOH terminus. The single type IV enzyme, INPP5E has a proline-rich sequence and a COOH-terminal CAAX box in addition to the 5-phosphatase domain.
1. Type II 5-phosphatases
a) synaptojanins. As described above, synaptojanin-1 (Synj1) was discovered by the De Camilli group as a 5-phosphatase that functions in synaptic vesicle exocytosis and recycling (1022). SYNJ1 has multiple splice forms that differ in their COOH termini. SYNJ1–145 shows very high expression in the brain while SYNJ1–170 shows wider tissue distribution. A highly homologous sister enzyme, synaptojanin-2 (SYNJ2), is more widely expressed and also exists in three splice forms differing in their COOH termini (1123). Two of the SYNJ2 isoforms (SYNJ2B1 and -2) are highly expressed in nerve terminals and in the testes (1126). The hallmark of all SYNJs is a Sac1-like phosphatase domain located in the NH2 termini of the proteins. The isolated Sac1 domain of SYNJs can also dephosphorylate PtdIns3P and PtdIns(3,5)P2 in vitro (538, 1126). On the basis of these findings, it is believed that the Sac1-domain takes the 5-phosphatase product (mainly PtdIns4P) and further dephosphorylates it to PtdIns. Indeed, it has been shown that the activities of both the 5-phosphatase domain and the Sac1 domain of SYNJ1 were required to support synaptic vesicle recycling (981).
Both SYNJs were found to be critical in determining the fate of the endocytosed clathrin-coated vesicles (CCVs). SYNJ1 knockout mice develop and are born seemingly normally but die shortly after birth. These newborns show accumulation of CCVs in their nerve termini due to a defect in “uncoating” of the vesicles (301). This also causes poor recycling of vesicles into a fusion competent synaptic vesicle pool (794). SYNJ1 associates via its COOH-terminal domain with several proteins enriched in the nerve terminal such as amphyphysin (335) and with proteins that play important roles in the endocytic pathways, such as endophilin, intersectin, syndapin, and Eps15 (1418). SYNJ1 is constitutively phosphorylated and undergoes activity-dependent dephosphorylation by calcineurin, which, in turn, affects both its catalytic activity and interactions with other proteins (876, 1023, 1419). Both SYNJs interact with the SH3-domain containing adaptor protein, Grb2 and SYNJ2, but not SYNJ1, participates in clathrin-mediated EGF receptor and transferrin receptor endocytosis (974, 1314). These results suggest nonredundant functions of the SYNJ phosphatases in controlling PtdIns(4,5)P2 levels in the membranes of vesicles along the endocytic pathway. Interestingly, one isoform of SYNJ2, SYNJ2A, is targeted to the mitochondria via binding to the outer mitochondrial membrane protein OMP25 (1124). It is an intriguing possibility that membrane deformations during mitochondrial fission and fusion are regulated by this enzyme in a manner similar to what occurs during endocytosis. An extra copy of SYNJ1 due to a trisomic arrangement found in the Ts65Dn mice, a model for Down's syndrome, is associated with neurological defects and PtdIns(4,5)P2 dysregulation, and these aberrations are corrected by restoring SYNJ1 to disomy (1647). Since SYNJ1 is located on human Chr21 that is trisomic in Down's syndrome patients, it is an attractive possibility that SYNJ1 is a major factor in the neurological dysfunctions found in these patients (1647). Recent studies also linked SYNJ2 to hearing and cochlear function. In a mouse strain, called Mozart, which exhibits progressive hearing loss, the causative mutation was mapped to a critical region within the phosphatase domain of the Synj2 gene (N539K) (983). Whether this gene is affected in human hereditary hearing disorders has yet to be investigated. SYNJ2 deleted mice have not been reported yet in the literature.
S. cerevisiae cells also express three phosphatases that show homology to mammalian SYNJs and contain both a Sac1-like and 5-phosphatase domains (1440, 1473). These enzymes, named Inp51, Inp52, and Inp53 (or “synaptojanin-like,” Sjl1, -2, and -3, respectively) can be individually deleted, suggesting their significant functional redundancy, but triple deleted cells are nonviable (1440, 1473). Interestingly, a mammalian type II 5-phosphatase (75-kDa phosphatase, or Inpp5B) that does not contain a Sac1 domain still can restore viability in triple Sjl inactivated cells or alleviate the phenotypic changes observed in double-deleted strains (1155). The significance of this finding is that a functional Sac1 domain may not be necessary for the functions of the yeast Sjl proteins. This conclusion was supported by further reports that Inp52 (1453) or Inp53 (545) enzymes with mutated, inactive Sac1 but not inactive 5-phosphatase domains were able to restore the viability of triple Sjl inactivated strains. Nevertheless, the isolated Sac1 domains of Inp52 and Inp53 are able to hydrolyze PtdIns4P and PtdIns3P (538) and rescue defects caused by inactivation of the yeast Sac1 protein (667). Mutations in key residues within the Sac1 domain already render this domain of the Inp51 protein inactive. Individual or double mutant Sjl strains display functional defects that still suggest some specialization among the three Sjl proteins. The general conclusion reached based on a number of studies analyzing these mutants is that Inp51/Sjl1 and Inp52/Sjl2 have partially overlapping functions and that Slj1, in particular, is involved in the cell-wall integrity pathways and actin organization both of which requires PM PtdIns(4,5)P2 produced by the Stt4 PI4K and the Mss4 PIP 5-kinase (see Ref. 1479 for a detailed review). In contrast, Inp53/Sjl3 deleted strains display defects that suggest unique functions linked to clathrin-mediated trafficking both at the PM, such as endocytosis, or at the Golgi and TGN (544, 545, 1473) (and see Ref. 1479 for more details). The complicated phenotypes of the inactivation of the three Inp51–53 proteins in various combinations are due to several factors. First, the 5-phosphatase domains of the three enzymes control specific but perhaps overlapping pools of PtdIns(4,5)P2 and because of this the severity of the functional defects do not always correlate with overall PtdIns(4,5)P2 elevations. Second, the Sac1 phosphatase activity of Inp52 and Inp53 also contributes to the control of specific PtdIns4P and PtdIns3P pools and in most of these locations the Sac1 phosphatase has a redundant function with the Sjl enzymes (1479).
b) ocrl. As mentioned before, mutations in the OCRL gene were found responsible for the X-linked human disease OCRL (61). OCRL patients present with congenital cataract, renal tubular acidosis, aminoaciduria, and mental retardation (944). However, some patients with mutations of the same gene can also present with Dent's disease with milder symptoms that are mostly confined to the kidney (647). The similarity of the OCRL protein to the 75-kDa inositol lipid 5-phosphatase (INPP5B) gave a clue that this protein was a 5-phosphatase, and subsequent studies showed that PtdIns(4,5)P2 was the preferred substrate for OCRL over the soluble Ins(1,4,5)P3 or Ins(1,3,4,5)P4, establishing OCRL as an inositol lipid 5-phosphatase (1805). OCRL prefers PtdIns(4,5)P2 over PtdIns(3,4,5)P3 and was also shown to hydrolyze PtdIns(3,5)P2 (1361), but it remains to be seen whether this lipid is indeed regulated by OCRL within the cells. The OCRL protein shows wide tissue expression, and its localization to the Golgi and TGN originally suggested that the OCRL disease is a result of a Golgi-originated trafficking defect (388, 1166, 1483). An attempt to make a mouse model of OCRL was complicated by the fact that OCRL deletion in mice does not reproduce the phenotypes associated with the human disease, apparently because of the overlapping function(s) of the homologous 75-kDa 5-phosphatase Inpp5b (722). Combined inactivation of both genes, on the other hand, manifests in early embryonic lethality (722). The difference between humans and mice in the ability of the 75 kDa phosphatase to compensate for OCRL functions must lie within the 75 kDa phosphatase itself, which differs between the two species in expression level and splicing. Indeed, replacement of the mouse Inpp5b with the human INPP5B version in an Ocrl (−/−) background created mice that show some of the symptoms found in the human disease (173). This mouse model should greatly facilitate the progress in understanding the molecular pathology of OCRL and Dent's disease.
Since OCRL is only one of numerous 5-phosphatases within the cell, it is likely that it affects localized pool(s) of PtdIns(4,5)P2. Not surprisingly, it has been difficult to show increased levels of total PtdIns(4,5)P2 in cells obtained from OCRL patients, although in some cases such increases have been reported (1804). Other studies showed only indirect signs of aberrant PtdIns(4,5)P2 levels, such as altered F-actin distribution and dynamics in fibroblasts obtained from OCRL patients (1482). Although the bulk of the OCRL protein is associated with the Golgi/TGN (388, 1166, 1483), an increasing body of evidence suggests that its functions are more widespread. Localized functions of OCRL rely on the recruitment and regulation of the protein to different membranes and, therefore, the domain structure and multiple interactions of the protein with various regulatory proteins have attracted significant attention. In addition to its conserved 5-phosphatase domain, OCRL contains an NH2-terminal PH domain (988), an ASH (ASPM-SPD2-Hydin) domain (1232), and a COOH-terminal Rho-GAP-like domain that is catalytically inactive (407). OCRL has two clathrin binding domains: one located within the NH2-terminal PH domain (251, 988), while the other is found within the COOH-terminal Rho-GAP domain (250, 1594). None of the clathrin binding sites is present in the otherwise similarly built INPP5B protein (988). OCRL has two spliceoforms that only differ in a small eight-amino acid stretch within the Rho-GAP-like domain close to the clathrin binding site; the longer form binds clathrin with higher affinity (251) (Figure 6).
The OCRL protein associates with clathrin-coated transport intermediates and regulates trafficking between endosomes and the TGN (250). However, somewhat surprisingly, a prominent role of OCRL in the early steps of the endocytic pathway has been also described (407, 1634). Membrane recruitment and activation of the OCRL protein occurs via interaction with multiple Rab proteins (674) and the Rab5 effector APPL1 (adaptor protein containing PH domain, PTB domain and leucine zipper motif 1) protein (407). Interaction of OCRL with another Rab protein, Rab35, connects the phosphatase with abscission, the last step in cytokinesis (318). The structural details of the various domains of OCRL and their possible roles in activating and orienting of the phosphatase domain have begun to emerge based on the crystal structures of these domains with possible associated proteins (407, 652, 988, 1223, 1224). Taking all these data into consideration, the OCRL protein plays multiple roles in endocytic trafficking by its interaction with clathrin and Rab proteins (1223). It has been more difficult to connect the molecular details and cellular functions of OCRL to the defects underlying the pathophysiology of the disease. The similarity of the kidney symptoms of OCRL patients to those of Fanconi syndrome (236) led to the suggestion that the large LDL receptor family protein megalin, which recycles between the apical membrane and the TGN in proximal tubule cells, displays aberrant trafficking in OCRL patients (945, 1146). However, one study found no signs of aberrant megalin trafficking in human and canine renal epithelial cells after siRNA-mediated knockdown of OCRL, even though there was increased secretion of lysosomal hydrolyses (303). Recent studies also suggested that OCRL is important for the assembly and functions of the primary cilium, and this may be an important clue to understand the renal pathology of the disease (288, 956, 1263).
c) inpp5b. This enzyme is highly similar to the OCRL protein (45% identical at the amino acid level) and has a similar domain organization. In fact, INPP5B was identified before OCRL (1065). The enzyme, also called 75-kDa type II 5-phosphatase, is widely expressed, especially enriched in the kidney, lung, and testes (1438). Although initial studies claimed mitochondrial localization (1438), subsequent studies found the enzyme to be associated with the Golgi and ER exit sites (1718), and Rab proteins also affect the localization of the enzyme (1401, 1718). While INPP5B shows a similar domain structure to the OCRL 5-phosphatase, there are notable differences between the two enzymes. Unlike OCRL, INPP5B does not contain clathrin binding sites, and it has a COOH-terminal CAAX prenylation sequence (724) (Figure 6). The mouse has three splice variants of Inpp5B as 87-, 104-, and 115-kDa proteins, the expression of which are tissue and developmental stage specific (1013). INPP5B hydrolyzes equally PtdIns(4,5)P2 and PtdIns(3,4,5)P2 [and Ins(1,4,5)P3 and Ins(1,3,4,5)P4], but not PtdIns(3,5)P2 (724, 1361).
Homozygous mice deficient in Inpp5B are viable and appear normal, but do have testicular degeneration and defective sperm functions that manifest after sexual maturation (722). The sperm of these mice show reduced motility and reduced ability to fertilize eggs (722, 992). These defects can be the consequence of the impaired proteolytic processing of a sperm cell surface protein, fertilin-β, which is important for egg binding (596). In addition, there is a defect in the apical endocytosis in Sertoli cells causing swollen vacuoles (595).
d) inpp5j. This 5-phosphatase, also called proline-rich inositol polyphosphate 5-phosphatase (PIPP), was identified due to the presence of consensus 5-phosphatase sequences (1076). The enzyme is widely expressed and contains proline-rich regions both at the NH2 and COOH terminus and several 14-3-3 protein-binding motifs (1076). The enzyme also contains a SKICH (SKIP COOH-terminal homolgy) domain (540) that mediates PM association (1172) (Figure 6). In vitro, the 108-kDa enzyme can hydrolyze PtdIns(4,5)P2 and PtdIns(3,4,5)P3 as well as Ins(1,4,5)P3 and Ins(1,3,4,5)P4 (1076), but it is believed to regulate PtdIns(3,4,5)P3 levels in cells (1172). Overexpression of the enzyme in PC12 cells decreased PtdIns(3,4,5)P3 levels, Akt activation, and neurite outgrowth (1172). Recent studies identified collapsing response mediator protein 2 (CRMP2) as an INPP5J interacting partner and showed that the two proteins have opposing roles in regulation of nerve cell polarization, axon selection, and neurite elongation (59). Importantly, higher INPP5J expression levels have been correlated with better prognosis and disease outcome in breast cancer (1515, 1600).
d) skip/inpp5k. This enzyme is enriched in skeletal muscle and kidney (hence the name skeletal muscle and kidney enriched inositol polyphosphate phosphatase) and was identified also because of the presence of the two conserved regions found in 5-phosphatases (540). SKIP also contains a COOH-terminal SKICH domain that mediates its translocation from the Golgi/ER region to the PM after insulin stimulation (540) (Figure 6). It was shown that SKIP limits the insulin-mediated PtdIns(3,4,5)P3 accumulation at the PM, decreasing Akt activation, GLUT4 translocation, and glucose uptake (679). The notion that SKIP might control the insulin sensitivity of skeletal muscles is supported by studies on SKIP +/− mice that show increased insulin sensitivity associated with increased Akt activation and glucose uptake in skeletal muscles of the mice (680). Homozygous SKIP −/− mice exhibit embryonic lethality (680).
2. Type III 5-phosphatases
a) ship1 and -2. These 5-phosphatases were named because of their NH2-terminal SH2-domains. SHIP1 (also called INPP5D) was initially identified as an inositol lipid phosphatase associated with a number of adaptor proteins, such as Shc, Grb, and DOK (924), as well as with immunoreceptor tyrosine-based inhibitory (ITIM) or activation (ITAM) motifs of immune receptors (798, 1175). SHIP1 has several splice variants, a full-length (SHIP1α) and three shorter forms (SHIP1β, SHIP1δ and s-SHIP1) (not shown in Figure 6) (1290). Structurally, SHIP1 and -2 contain a central 5-phosphatase domain followed by a C2 domain and two NPXY sequences in which the Tyr can be phosphorylated to interact with PTB domains. All SHIP variants except SHIP1δ contain a COOH-terminal proline-rich sequence, and SHIP2 also has a COOH-terminal SAM (sterile alpha motif) sequence, whereas s-SHIP1 lacks the NH2-terminal SH2 domain. The mechanism of SHIP1/2 activation is based on recruitment of the phosphatase from the cytosol to the membrane via the various protein-protein interaction domains present in the molecule. These proteins function as part of multi-protein complexes, and their cell- and tissue-specific functions are probably dictated by their binding partners. Initially, the SHIP proteins were believed to have a restricted substrate recognition only able to dephosphorylate the 5-position in PtdIns(3,4,5)P3 [and Ins(1,3,4,5)P4 and perhaps other soluble higher inositol phosphates] (248, 1722). Recent studies, however, suggested that they can also hydrolyze PtdIns(4,5)P2 and control clathrin-mediated endocytosis (1115). Nevertheless, SHIP proteins are still considered as key enzymes in the control of the PI 3-kinase products PtdIns(3,4)P2 and PtdIns(3,4,5)P3 (112). It is important to note that the reaction product PtdIns(3,4)P2 can also activate certain downstream targets, such as Akt (460); hence, SHIP1/2 can selectively inactivate signaling pathways initiated by PI 3-kinases (1290). The enzymatic activity of SHIP1 does not appear to be regulated by Tyr phosphorylation or protein-protein interaction (1213), but it is enhanced by binding of the product PtdIns(3,4)P2 to the C2 domain (1170). Recent studies suggested that PLCβ3 and Lyn controls SHIP1 activity via phosphorylation of Tyr536 and Tyr564 (1742). Other studies identified a PKA phosphorylation site (Ser440) in SHIP1, which, when phosphorylated, significantly enhances the activity of the enzyme. This could contribute to the well-documented negative effects of cAMP-mobilizing signals on PI 3-kinase signaling in immune cells (1798). Some meroterpenoid compounds are also able to activate SHIP1 and exert strong anti-inflammatory effects that could represent a means of controlling PI 3-kinase downstream effectors in hematopoietic cells (1170, 1459, 1460).
SHIP1 and its splice variants are expressed exclusively in hematopoietic and spermatogenic cells (928), while SHIP2 (also called INPP5L1) shows ubiquitous expression (1095). Recent studies showed that SHIP1 expression is repressed by the human MIR155 microRNA (1154). Consistent with its expression pattern and enzymatic functions, SHIP1 deficiency results in enhanced PI 3-kinase signaling affecting cells and organs with hematopoietic cell involvement (B and T cells, macrophages, osteoclasts, mast cells, neutrophil cells, and platelets). Ship1 knockout mice develop a chronic myelogenic leukemia (CML)-like disease with myeloid proliferation, splenomegaly, and vast myeloid infiltration of the lungs causing premature death (593, 927). The significantly elevated PtdIns(3,4,5)P3 levels induce a hyperproliferative response of hematopoietic progenitors to a variety of growth factors (593) and increased Akt activation in mature neutrophils and mast cells (927). Since PtdIns(3,4,5)P3 has a pivotal role in amplifying polarization during chemotaxis both in phagocytic cells (1385, 1463) and lower organisms, such as Dictyostelium (976), there is a massive dysregulation of neutrophil polarization in Ship1 null mice (1140). The severe osteoporosis reported in Ship1 −/− mice is due to the hyperactivation of osteoclasts that are more numerous, enlarged, and hypernucleated (1523), resembling those in patients with Paget disease. Ship1 −/− mice also show deficient platelet aggregation (1386), an increased number of splenic dendritic cells that are hyperresponsive to GMCSF in vitro but have an immature phenotype (47).
The more ubiquitously expressed SHIP2 enzyme serves as a negative regulator of insulin signaling. Overexpression of SHIP2 antagonizes the PI3K angle of insulin signaling in 3T3-L1 adipocytes (1650) and cerebellar granule cells (1429). Additionally, transgenic mouse, overexpressing SHIP2, showed signs of mild insulin resistance (744). Higher levels of SHIP2 expression were found in the cerebral cortex of the db/db obese mice (1429), and SHIP2 gene polymorphisms have been linked to metabolic syndrome (746, 991). A mouse strain lacking Ship2 (and the neighboring Phox2a gene) shows insulin hypersensitivity and severe hypoglycemia and has early neonatal mortality (271). Curiously, another study reported no such problems with a SHIP2 knockout strain and, to the contrary, reported a resistance of these mice to weight gain in a high-fat diet (1417). A recent study identified Ser132 phosphorylation of SHIP2 that regulates its nuclear localization in nuclear speckles in a cell cycle-dependent manner. This study also found SHIP2 interaction with nuclear lamin A/C and that Ser132-phosphorylated SHIP2 also hydrolyzed PtdIns(4,5)P2 (400). Understanding the relationship between these novel features of SHIP2 and the phenotypic changes listed above will require further investigations. More details about SHIP2 can be found in Reference 401.
3. Type IV 5-phosphatase
The only type IV 5-phosphatase, also called INPP5E, 72-kDa polyphosphate 5-phosphatase, and Pharbin (801, 818), contains an NH2-terminal proline-rich segment, a central 5-phosphatase domain, and a COOH-terminal farnesylation CAAX motif (Figure 6). This enzyme can remove the 5-phosphate from PtdIns(4,5)P2, PtdIns(3,4,5)P3, and PtdIns(3,5)P2 and has about a 10 times higher affinity against PtdIns(3,4,5)P3 than SHIP1 (801, 818). INPP5E is located mostly in the Golgi and partially in the PM (818), but its distribution could be more specialized in specific cell types. Notably, INPP5E does not seem to be a general antagonist of PtdIns(3,4,5)P3 signaling. In macrophages, INPP5E is recruited to the phagocytic cup where it controls the conversion of PtdIns(3,4,5)P3 to PtdIns(3,4)P2, but unlike SHIP1 (294, 750), it only affects the FcγR- and not the complement receptor 3 (CR3)-mediated phagocytosis (649). Interestingly, overexpression of INPP5E in adipocytes does not antagonize insulin signaling but instead hydrolyzes PtdIns(3,5)P2 to PtdIns3P, which in turn increases GLUT4 translocation (817). Two recent studies linked INPP5E to the primary cilium. In one of these studies, mutations clustering within the 5-phosphatase domain of INPP5E were identified in patients with Joubert syndrome (145). These patients present with specific midbrain-hindbrain malformation, and variably with retinodystrophy, nephronophthisis, liver cirrhosis, and polydactyly and are considered as an emerging group of ciliopathies (1599). In this study, INPP5E was found to localize to the primary cilium in cells of the affected organs and the mutations caused destabilization of the cilia during stimulation (145). The other study found that disruption of the Inpp5e gene in mice caused multiorgan failure associated with defects in primary cilia (714). This latter study (714) also found destabilization of cilia in response to growth factor stimulation and identified a mutation in the human INPP5E gene that affected cilia localization in a family with MORM syndrome. The physical targeting of INPP5E to the cilia was recently shown to be dependent on a protein complex that included phosphodiesterase 6D, the Arf-like protein, ARL13B, and the centrosomal protein, CEP164, all of which had been linked to ciliopathies (668). These interesting discoveries should facilitate further studies to better understand the role of phosphoinositides in ciliary function and how INPP5E controls this process.
B. 3-Phosphatases
The phosphoinositide 3-phosphatases are comprised of two major groups. The first group contains PTEN (phosphatase and tensin homolog located on chromosome TEN), the voltage-sensitive phosphatases (VSPs) first isolated from the ascidia, Ciona intestinales (1096) but also found in zebrafish (650) and Xenopus (1259), and the mammalian VSP homolgs, the TPIP proteins (α, β and γ) (1163). These enzymes all share similar catalytic phosphatase domains (Figure 7), but differ in their substrate preference and what phosphates they remove. PTEN is one of the key enzymes that antagonizes PI 3-kinase action by removing the 3-phosphate from PtdIns(3,4,5)P3. In contrast, the nonmammalian VSPs are 5-phosphatases that act on PtdIns(4,5)P2 and PtdIns(3,4,5)P3 (550, 713). The mammalian TPIP proteins can dephosphorylate 3-phosphorylated inositides (1662), but it is not clear whether they can also act on PtdIns(4,5)P2. It is also questionable whether any of the mammalian VSP homologs are also voltage regulated (1163). In spite of the differences in their catalytic targets, these enzymes will be discussed in this section because of their close structural relationship to PTEN, an enzyme that has risen to prominence as a PtdIns(3,4,5)P3 3-phosphatase and a tumor suppressor gene. The second group of the 3-phosphatases are made up by the myotubularins (MTMs) with 15 structurally related members, 9 of which have phosphatase activity removing the 3-phosphate from PtdIns(3,5)P2 and PtdIns3P. The other six members are catalytically inactive proteins (1273) (Figure 7).
Figure 7.
The inositol lipid 3-phosphatases. A: the PTEN family of enzymes dephosphorylate PtdIns(3,4,5)P3 at the 3-position and hence “antagonize” the class I PI3Ks. The conserved phosphatase domain is followed by a C2 domain and a PDZ binding sequence in PTEN. The PBM is a phospholipid binding domain present in PTEN and also in the voltage-dependent phosphatases (these latter enzymes are actually 5-phosphatases). The TPIPα and -γ enzymes have an NH2-terminal putative transmembrane domain (TM) that is lacking in the β form. The TPTE enzyme is catalytically inactive. B: the myotubularin family of 3-phosphatases dephosphorylate PtdIns3P and PtdIns(3,5)P2. They fall into three subgroups. All of these enzymes (except for MTMR14) have a PHG (PH and GRAM) domain and coil-coil (CC) regions in addition to their phosphatase domain. The phosphatase domain also contains a SET interaction domain (SID). MTMR3 and -4 also has a FYVE domain in their COOH termini. No specific domains beyond the phosphatase domain have been described in MTMR14. There are additional MTM-related proteins that lack catalytic activity. They are not shown in this figure.
1. PTEN
This enzyme (also known as MMAC1 and TEP1) was first identified as a tumor suppressor gene after mapping its homozygous deletions in several human advanced cancers on chromosome 10 (897, 898, 1452). Due to its similarity to phosphatases and weak homology to tensin (there lies the origin of its name), PTEN was initially believed to be a dual-specificity protein phosphatase (1104). However, the enzyme shows low activity against protein and peptide substrates, and subsequent studies revealed that it was a PtdIns(3,4,5)P3 3-phosphatase (968, 969). Although able to dephosphorylate PtdIns(3,4)P2, PTEN is a lot more active against PtdIns(3,4,5)P3 (1015) and, therefore, is a functional antagonist of the class I PI 3-kinases. The protein and lipid phosphatase activities of PTEN can be dissociated, and a G129E mutation identified in a family with Cowden disease eliminates lipid but spares the protein phosphatase activity of the enzyme (481, 1103). Since Cowden disease is one of the cancer predisposition syndromes (641), these studies showed that the tumor suppressive function of PTEN is primarily associated with its lipid phosphatase activity. A mutant form of PTEN impairing protein but not lipid phosphatase activity was recently identified showing that together with the lipid phosphatase activity, the protein phosphatase activity of PTEN was also necessary to maintain proper migration of glioblastoma cells (325).
PTEN has a catalytic phosphatase domain, with a conserved C-(X)5-R signature motif, an NH2-terminal phosphoinositide binding segment (PBM), a C2 domain toward the COOH terminus followed by two PEST (proline, glutamic acid, serine, threonine) sequences, and a COOH-terminal PDZ binding motif (Figure 7). The structure of the catalytic domain of PTEN has been solved showing similarities to those of phosphotyrosine and dual-specificity phosphatases (870). However, PTEN has a deeper substrate binding pocket that allows the bulkier PtdIns(3,4,5)P3 headgroup to be used as a substrate. The C2 domain is important for membrane targeting and regulation of the enzyme. Unlike many other C2 domains, the C2 domain of PTEN does not bind Ca2+, but it contributes to membrane binding through electrostatic interactions (323). However, membrane binding is not the sole function of the PTEN C2 domain (507), which also makes several contacts with the catalytic domain via hydrogen bonds (870). Truncation or mutations within the C2 domain of PTEN make the protein unstable (323, 506, 507). The NH2-terminal phosphoinositide binding domain (PBM) of PTEN also contributes to membrane binding and localization. This domain in isolation can bind PtdIns(4,5)P2, and mutation of its key basic residues (such as K13E) abrogates its lipid binding (1266). Basic residue mutations within the PBM in the full PTEN molecule or PtdIns(4,5)P2 depletion prevent PTEN from efficiently localizing to the membrane (1249). Interestingly, PtdIns(4,5)P2 binding to the PBM domain, is also present in the Ciona VSP, and seems to be important for the coupling of the voltage sensor to the phosphatase domain (846). A phosphorylation-dependent autoinhibitory role of the PBM has also been suggested for the mammalian PTEN enzyme(1249). The COOH-terminal tail contains a stretch of ∼60 amino acids that can be phosphorylated by several protein kinases (22, 905) and phosphorylation renders PTEN inactive (1160, 1249) through interaction of the phosphorylated COOH terminus with the membrane interaction site of the C2 domain (1160). Since this COOH-terminal segment of PTEN is quite acidic and the protein phosphatase activity of PTEN is strongest against acidic peptides (1104), it was suggested that PTEN autocatalytically can activate itself through dephosphorylation of its COOH terminus (1247). The role of the PEST sequence within PTEN is not clear, but it may be related to the degradation of PTEN by proteosomes. Phosphorylation also affects the stability of PTEN (1562), and polyubiquitylation has been proposed to mark PTEN for proteosomal degradation (1561). The identity of the E3 ubiquitin ligase responsible for polyubiquitylation of PTEN has been controversial (458, 1674), but most recent studies suggest that WWP2 that belongs to the NEDD4-like family of E3 ligases is the elusive PTEN E3 ubiquitin ligase (967). Monoubiquitylation of PTEN, on the other hand, was found important for nuclear localization and tumor suppression (1579), at the same time mono- or poly-ubiquitylation was shown to inhibit PTEN phosphatase activity (964). A recent study found that PTEN gets sumoylated at residues K254 and K266 and that sumoylation is essential for the tumor suppressor function of the protein, presumably because of its contribution to membrane recruitment (521, 663).
The most important known function of PTEN is to serve as a negative regulator of PtdIns(3,4,5)P3 and hence serving as a tumor suppressor. As detailed under class I PI 3-kinases, PtdIns(3,4,5)P3 promotes carcinogenesis in a number of ways, both in a tumor cell autonomous manner, and also within the tumor cell environment. In the former, it stimulates proliferation, and protects cells from apoptosis, and it also increases the tumor cells' ability to migrate and break down tissue barriers including those of capillaries, thereby promoting metastasis. In the tumor environment, PtdIns(3,4,5)P3-dependent signaling has a crucial role in angiogenesis, stromal cell function, or immune surveillance. It has been very clear that germline mutations of PTEN cause increased incidence of multiple hamartomas and high risk of tumorigenesis observed in a number of human syndromes such as Cowden disease, Bannayan-Riley-Ruvalcaba syndrome, Lhermitte-Duclos disease, and Proteus- and Proteus-like syndrome (349, 1434). PTEN mutations have been detected in a variety of human cancers such as glioblastoma, breast, lung, colon, kidney, and uterine carcinomas or melanomas. Very often monoallelic mutations of PTEN are present in cancer tissues, but late-stage aggressive metastatic cancers show biallelic loss of PTEN (210, 349, 1434).
Most PTEN mutations cluster within the phosphatase domain making the enzyme catalytically inactive, but many mutations are found in other regions that affect the cellular localization or stability of the enzyme (999). It is also of great interest that PTEN is very sensitive to oxidative damage, and hydrogen peroxide rapidly inactivates its enzymatic activity (384, 842, 874, 888, 1297). One interesting and poorly understood aspect of PTEN tumor suppressor function is linked to its nuclear localization where a noncatalytic role has been recently proposed (1433). Clearly, there is more to PTEN function than PtdIns(3,4,5)P3 signaling, and its link to nuclear proteins, chromosomal instability, DNA repair, and kinetochore assembly (1394) suggests a complexity that has yet to be fully understood.
Mouse models of PTEN inactivation have been providing more detailed information on the tumor suppressor as well as the developmental and tissue-specific roles of PTEN. Homozygous PTEN deficiency causes early embryonic lethality, and heterozygote mice also develop tumors (838, 1450, 1500). Remarkably, a dose-dependent reduction in PTEN even above monozygocity is associated with increased tumor formation (31, 1578). In addition, conditional and tissue specific PTEN knockout mice revealed a plethora of PTEN-associated functions in virtually all tissues and organ systems. This is not unexpected based on the multitude of processes regulated by PtdIns(3,4,5)P3. More on the variety of the PTEN-related defects can be found in excellent reviews focusing on PTEN (805, 1434, 1501).
2. PTEN-related phosphatases
a) tpte/tpip proteins. Humans express one TPTE (transmembrane phosphatase with tensin homology) (242, 1662) and several splice variants of TPIP (TPTE and PTEN homologous inositol lipid phosphatase) (α, -β and γ) (1662) (Figure 7A). Of these, TPTE is specifically expressed in the testis and lacks phosphatase activity, while TPIPs are expressed in the testis, brain, and stomach and are enzymatically active against 3-phosphorylated inositides (1531, 1662). Mice also have a similar testis-specific protein called PTEN2, but it does not seem to be the ortholog of the human TPTE or TPIP enzymes (535, 1738). TPIPα and -γ have NH2-terminal sequences suggestive of three to four transmembrane domains. Expressed TPIPα localizes to the ER, but TPIPβ, which lacks transmembrane segments, is cytosolic and has no significant phosphatase activity (1662). The transmembrane domains of TPIPγ show weak similarity to the voltage sensor of the ci-VSP (713, 1096). The functions of these proteins are presently unknown.
b) voltage-sensitive phosphatases. Voltage-sensitive phosphatases (VSPs) were first identified in Ciona intestinales based on their sequence similarity to PTEN and the presence of a voltage sensing domain within their transmembrane segments (1096). These enzymes are remarkable in that they hydrolyze phosphoinositides and increase their catalytic activity in response to depolarization. Similar enzymes have been found in other species, notably in Xenopus laevis (1259) and in zebrafish (650) with slightly different voltage characteristics, and the human TPIPs are likely to be their mammalian homologs (1163). The catalytic domains of VSPs are similar to that of PTEN, but they lack the COOH-terminal PEST sequences and the PDZ binding motif. However, in spite of their sequence homology to PTEN, these enzymes function as 5-phosphatases (as opposed to 3-phosphatases) acting on PtdIns(3,4,5)P3 and PtdIns(4,5)P2 (550, 713, 1097). The substrate specificity of VSP at least partially depends on a single Gly residue within the conserved C-(X)5-R sequence, and an Ala substitution at this position (G365A) abolishes the activity against PtdIns(4,5)P2 (713). Comparison of the structures of the catalytic domains of PTEN and ciVSP showed a reduced size of the catalytic pocket of ciVSP due to a protruding Glu411 residue (1009).
The voltage-sensing domain of VSPs shows significant homology to those of voltage-gated ion channels, and its voltage sensing is manifested as a “sensing current” even in isolation (650, 1096). Mutations within the sensor module can shift the voltage dependence of the molecule (813). The exact mechanism of how the voltage-sensing unit couples to increased enzymatic activity is not yet understood. However, there is a close correlation between the conformational change of the voltage sensor and the activity of the enzyme (846, 1096, 1097). Also, the enzymatic activity of the phosphatase module has a retrograde effect on the voltage-sensing unit and phosphatase inhibitors and catalytically inactive Cys mutant catalytic modules alter the speed of the “sensing current” (650, 812). Recent studies found that the PBM domain of VSP has a critical role in coupling the voltage sensor to increased phosphatase activity (812, 846, 1637). This domain, which is homologous to the similar domain found at the NH2 terminus of PTEN, is a phosphoinositide binding module, and its deletion or mutations as well as PtdIns(4,5)P2 depletion affects the coupling between the two functional modules (812, 846, 1637).
The biological functions of VSP or its orthologs are not known, but their primary expression in the testes and its localization in the sperm tail in Ciona intestinales (1096) suggests a role in fertilization. In spite of the limited understanding of its physiology, VSPs have become invaluable experimental tools. To study the multiple roles of PM PtdIns(4,5)P2, our ability to rapidly manipulate the level of this lipid in intact cells has been a highly desirable methodological invention. A major breakthrough in this area was the introduction of the rapamycin-induced translocation of 5-phosphatase enzymes to rapidly deplete PtdIns(4,5)P2 (1489, 1617). However, the VSP enzymes offer several further advantages over the rapamycin-inducible changes: they allow induction of extremely rapid, quantitatively precise, and reversible changes in PM PtdIns(4,5)P2. Therefore, VSP is becoming an important experimental tool in phosphoinositide research for the controlled manipulation of PM phosphoinositides(422, 609, 953).
c) plip/ptpmt1. A PTEN-like phosphatase (PLIP) has been described with unique preference to PtdIns5P as a substrate in vitro (1187). However, subsequent studies identified the same enzyme as a mitochondrion-localized protein tyrosine phosphatase (PTPMT1) that dephosphorylates phosphatidylglycerol-phosphate, an essential intermediate of cardiolipin synthesis (1797). Therefore, it is quite unlikely that this enzyme works on any phosphorylated inositide and its deletion does not affect PtdIns5P levels (1186).
3. Myotubularins
The myotubularin (MTM) family includes 15 members that share a distinctive phosphatase domain (Figure 7B) (776, 855, 856, 1536). Nine of these proteins possess 3-phosphatase activity against PtdIns3P and PtdIns(3,5)P2 and share a CXXGWDR sequence motif within their catalytic core. The other six homologs are catalytically inactive proteins often referred to as “pseudophosphatases” that associate mostly with and regulate the active MTM enzymes. The phosphatase active forms can be divided into four subgroups: the MTM1 group contains MTM1, MTMR1, and MTMR2; the MTMR3 group includes MTMR3 and MTMR4; and the MTMR6 subgroup has MTMR6, -7, and -8. MTMR14 stands alone in a separate group. The pseudophosphatases consist of MTMR5, -9, -10, -11, -12, and -13. In addition to their catalytic domains, these proteins contain several protein- and lipid-interacting modules, such as PH-GRAM domains (364), FYVE domains, coiled-coil domains, and COOH-terminal PDZ binding sequences (see Figure 7B for details). Most of the family members show expression in a wide range of tissues except MTMR5 and MTMR7 that are expressed exclusively in the testes and brain, respectively (440, 855, 856).
a) mtm1. This was the first MTM described as the gene mutated in X-linked myotubular myopathy (XLMTM) (858). MTM1 was originally believed to be a dual specificity phosphotyrosine phosphatase (858), but its lipid phosphatase activity against PtdIns3P (152, 1536, 1814) was later demonstrated both as a GST-fusion protein and in cell expression studies. Many of the disease-causing mutations as well as mutation of the conserved Cys (C375S) residue render the enzyme catalytically inactive, and expression of such phosphatase inactive enzymes has dominant negative effects elevating PtdIns3P levels (1536). However, MTM1 can also dephosphorylate PtdIns(3,5)P2 and, therefore, increase PtdIns5P levels (1576). MTM1 knockout mice show perinatal mortality, and the pups that are born alive quickly succumb into muscle weakness and develop centronuclear myopathy with histological findings reminiscent of those seen in human XLMTM disease (196). Conditional and tissue-specific MTM1 knockout mice showed that MTM1 deficiency in skeletal muscle is the underlying cause of the disease, and it is not the development, but the maintenance of the skeletal muscle that requires this enzyme (196). MTM1 mutations were also identified in dogs suffering from X-linked myotubular myopathies (116). The intracellular localization of MTM1 has been controversial. Overexpressed MTM1 is mostly cytoplasmic with some perinuclear association, and it also shows up in membrane ruffles generated by overactive Rac (857). In other studies, endogenous MTM1 was found in early endosomes in association with the class III PI 3-kinase complex (212). It is likely that interaction with other proteins perhaps via phosphorylation-dephosphorylation cycles determine the location and therefore the localized regulatory function of the MTM1 protein. In support of this idea, MTMR12, a phosphatase-inactive MTM family protein, originally identified as an MTM1 adaptor protein (3-PAP), tightly associates with MTM1 (1117) and determines its localization (1118). MTM1 also associates with the intermediate filament desmin, and XLMTM-causing mutations in MTM1 disrupt this association leading to altered intermediate filaments, and abnormal mitochondrial positioning and functions (626). Recent data also suggest that MTM homologs in lower organisms control discrete pools of PtdIns3P specifically involved in endocytosis (1127, 1625).
b) mtmr1 and -2. MTMR (R stands for -related) proteins are close homologs of MTM1. MTMR1 is in fact located next to MTM1 on the X-chromosome both in humans and mice as a result of gene duplication (799). Accordingly, the structure and enzyme activity of MTMR1 is very close to those of MTM1 (1576). The function(s) of MTMR1 is not known, but it is likely that the restricted phenotype of MTM1 mutations are due to the fact that MTMR1 can substitute for MTM1 functions in all but a few tissues. MTMR2, on the other hand, was discovered as the gene, whose mutations cause the hereditary recessive disorder Charcot-Marie-Tooth disease type 4B1 (CMT4B1), which is characterized by peripheral neuropathy due to demyelination (124, 159, 653, 792). Disease-causing mutations severely affect the ability of MTMR2 to dephosphorylate PtdIns3P and PtdIns(3,5)P2 (123, 792). The structure of MTMR2 has been solved and found to have a larger phosphatase domain than other PtdIns3P phosphatases (117, 118). This is because of the presence of a “SID” [SET interaction domain (36)] domain within the phosphatase domain of MTMR2 (118). These studies also found that the NH2-terminal GRAM domain of MTMR2 has a similar architecture to PH domains, and it also binds phosphoinositides, primarily PtdIns(3,5)P2 (118). Expressed MTMR2 has been reported to be cytosolic or associated with perinuclear membranes or the nucleus (793, 1274). However, endogenous MTMR2 localizes to late endosomes (211). Recent studies showed that dephosphorylation of the NH2 terminus of MTMR2 facilitates its binding to early endosomes (462). MTMR2-deficient mice develop symptoms very reminiscent of those found in CMTB4B1 patients with peripheral neuropathy and impaired spermatogenesis (158, 164). The myelin unfolding seen in these mice can be reproduced by selective deletion of the gene in Schwann cells (160, 161), confirming the primary defect in myelination. MTMR2 interacts with the catalytically inactive pseudophosphatases MTMR5 (also named Set binding factor 1 or SBF1) (793) and MTMR13 (also called SBF2), and these interactions greatly enhance the phosphatase activity of MTMR2, as well as determine its cellular localization (123, 1274). Mutations in MTMR13 cause Charcot-Marie-Tooth disease type 4B2 (CMT4B2) with similar defects to CMT4B1 (69, 1381), and these deficiencies can be replicated in mice with MTMR13 gene disruption (1275, 1545). In contrast, MTMR5 deletion in mice causes defective spermatogenesis without any peripheral neuropathy (440). Therefore, it appears that the MTMR2-MTMR5 complex functions in spermatogenesis, whereas the MTMR2-MTMR13 complex is essential for myelination in Schwann cells (1273).
c) mtmr3 and -4. These widely distributed phosphatases are characterized by the presence of a FYVE domain in their extended COOH termini (1659, 1814). Interestingly, however, the FYVE domain of MTMR3 does not bind PtdIns3P, nor is it responsible for the endosomal localization of MTMR3 (941). MTMR3, on the other hand, binds PtdIns5P at its GRAM domain (941), which may be important for allosteric activation of the enzyme by this rare lipid species (1355). Only few data are available on the functions of these phosphatases. Expression of human MTMR3 in yeast induces enlargement of vacuoles and increases yeast PtdIns5P levels (1659) consistent with its ability to dephospharylate PtdIns3P and PtdIns(3,5)P2 (1659). Recent genome-wide association studies identified MTMR3 as a susceptibility gene in lung cancer in a large Chinese population (659) and also in early-onset inflammatory bowel disease in another study (598). It is not clear yet how the functions of these genes contribute to the respective pathologies. Nevertheless, evidence is accumulating that these phosphatases also regulate endocytic sorting (1122), routing, and inactivation of receptors (1780) and also can be ubiquitylated (1227).
d) mtmr6, -7, and -8. MTMR6 and MTMR7 are both capable of hydrolyzing PtdIns3P and PtdIns(3,5)P2 (1355), but MTMR7, which is mainly expressed in the brain, also acts on water-soluble Ins3P (1075). MTMR6 has been shown to inhibit the Ca2+-activated potassium channel KCa3.1 (1444), which requires PtdIns3P for its optimal activity (1442). Through this action, MTMR6 expression depolarizes T cells, thereby decreasing the driving force for the ICRAC channels, and hence, inhibiting T-cell activation (1443). This action of MTMR6 is not mimicked by MTMR8 and, conversely, MTMR6 does not regulate other, highly related KCa channels (1442). Recent studies reported that the right balance between PI3K and MTMR8 was important for muscle (1024) and vascular development (1025) in zebrafish. Of the pseudophosphatases, MTMR9 interacts with both MTMR6 and MTMR7 and activates the phosphatase activity of the respective enzymes (942, 1075, 1832).
e) mtmr14. This member of the MTMR family was identified in silico (1566) based on its homology to MTMRs and its high sequence homology to a Drosophila protein named “egg-derived tyrosine phosphatase” (EDTP) (1756). Disruption of the gene in flies produced the “JUMPY” phenotype characterized by progressive loss of muscle control and shaky movements (1382). Importantly, the human Jumpy/MTMR14, which displayed PtdIns3P and PtdIns(3,5)P3 3-phosphatase activity, harbored catalytically compromising mutations in two patients suffering from centronuclear myopathy (1566). The same phosphatase was also identified as an inositol lipid phosphatase showing highest activity against PtdIns(3,5)P2 with prominent expression in skeletal muscle and heart and hence also named muscle-specific inositol phosphatase (MIP) (1393). MTMR14-deficient mice show muscle weakness and fatigue that develops with aging, due to a Ca2+ leakage from the SR-cisternae apparently because of increased PtdIns(3,5)P2 levels (1393). The progressive muscle weakness is attributed to an increased activity of ryanodine receptors by the PtdIns(3,5)P2 (1570), altered t-tubule morphology observed in MTMR14-deficient mouse (625), and impaired excitation-contraction coupling in MTMR14-depleted zebrafish embryos (382). MTMR14 levels are also decreasing with age in parallel to weakened muscle function, and deletion of the gene in mouse significantly speeds up this process (1293). Since MTMR14 was also shown recently as a negative regulator of autophagy (1631, 1632), the rapid aging of muscles might be also related to an altered process of autophagy in MTMR14-deficient animals (625).
C. 4-Phosphatases
Phosphoinositide 4-phosphatases can remove the phosphate from the 4-position of PtdIns(3,4)P2 or PtdIns(4,5)P2. The enzymes that hydrolyze PtdIns(3,4)P2 are called INPP4A and INPP4B, while those that use PtdIns(4,5)P2 are TMEM55A and -B (the A and B forms of both groups are also called types I and II, respectively) (Figure 8A). The mammalian Rac guanine nucleotide exchange factors P-Rex1 and -2 also contain a domain homologous to the phosphatase domains of INPP4s, but these proteins do not have phosphatase activity (1317, 1698). There are two bacterial virulence toxins, IpgD from Shigella flexneri and SigD/SopB from Salmonella, that possess a wide range of phosphoinositide phosphatase activity in vitro but appear to act on PtdIns(4,5)P2 as a 4-phosphatase when expressed in cells, yielding the rare lipid PtdIns5P. Therefore, they are often used to study the functions and metabolism of PtdIns5P (1130, 1255, 1544). The Sac1 phosphatase dephosphorylates PtdIns4P, but it can also dephosphorylate PtdIns3P and will be discussed separately. No PtdIns(3,4,5)P3-directed 4-phosphatase activity has been reported.
Figure 8.
The inositol lipid 4-phosphatases. INPP4A and -B dephosphorylate PtdIns(3,4)P2 at the 4-position. They have a C2 domain in their NH2 termini and a phosphatase domain at their COOH termini. INPP4A also has a PEST sequence. The TMEM proteins have lower activity, and they act on PtdIns(4,5)P2 to generate PtdIns5P. These enzymes have putative transmembrane domains (TM) at their COOH termini. B: the Sac1 phosphatases can dephosphorylate any monophosphorylated PtdIns, but in the cell they primarily function as PtdIns4P 4-phosphatases. The yeast and human Sac1 are tail-anchored proteins with a COOH-terminal TM domain. Human Sac3 and yeast Fig4p work as PtdIns(3,5)P2 5-phosphatases. hSac2 is a 5-phosphatase acting on PtdIns(4,5)P2 and PtdIns(3,4,5)P3 yet, structurally is a homolog of the Sac1 family.
1. INPP4s
Two enzymes belong here, INPP4A and INPP4B. INPP4A, first isolated and cloned from rat brains, is ∼100 kDa in size and dephosphorylates the 4-position in PtdIns(3,4)P2 and to a much lesser extent Ins(3,4)P2 and Ins(1,3,4)P3 (1149, 1150). Another form of the enzyme, INPP4B, was cloned subsequently showing highest expression in the heart and skeletal muscle (originally termed type II inositol polyphosphate 4-phosphatase) (1147). In addition to their conserved phosphatase domains, both enzymes have a C2 domain at their NH2 termini that show distinct lipid binding specificities. While the C2 domain of INPP4A prefers binding PtdIns(3,4)P2, PtdIns3P and PtdSer (712), that of INPP4B binds PtdOH and PtdIns(3,4,5)P3 (434). In addition, there is a PEST sequence in INPP4A (but not INPP4B) that is cleaved by calpain, thereby inactivating the enzyme, as shown in stimulated platelets (1148). Expressed INPP4A is localized to early and recycling endosomes and is activated by Rab5 (1401). Consistent with an endosomal function, knockdown of INPP4A decreases transferrin uptake (1401). Conversely, overexpression of the enzyme restores the morphology of enlarged endosomes caused by PtdIns3P deficiency (712), suggesting that INPP4A can produce PtdIns3P. INPP4A acts as a negative signal via decreasing the levels of PtdIns(3,4)P2 and attenuates Akt activation and also exerts an antiproliferative effect (712, 1649). Decreased levels of INPP4A and loss of heterozygocity of the enzyme are observed in a variety of cancers, suggesting that the enzyme acts as a tumor suppressor (428). No such functions have been attributed to INPP4B. A frame-shift mutation that eliminates a functional Inpp4a in mice was found to cause the weeble phenotype (1153). These mice develop early onset ataxia due to the loss of Purkinje cells from the cerebellum. Interestingly, islands of Purkinje cells remain normal, and these express Eaat4, a glutamate transporter, suggesting that the lack of Inpp4a is associated with glutamate toxicity that is remedied by glutamate removal by the Eaat4 transporter (1321). Recently, an Inpp4a knockout mouse was generated and showed rapid neurodegeneration in the striatum and basal ganglia, causing a phenotype similar to involuntary movement disorders such as Parkinson disease. The neurons of these mice undergo NMDA receptor-mediated cell death due to the increased levels of NMDA receptors at the postsynaptic density (1343). There is little known about the connection between Inpp4s and human disease. A polymorphism in INPP4A has been associated with severe asthma (1388), and a loss of INPP4A and -B has been reported in various human malignancies (107, 1317).
2. TMEM55
The two forms of these enzymes, A and B, originally called type I and type II PtdIns(4,5)P2 4-phosphatases, were cloned based on the presence of a conserved CX5R motif also found in the Burkholderia pseudomallei virulence factor Bop, a putative phosphatase (1593). Both enzymes are expressed ubiquitously and act exclusively on PtdIns(4,5)P2. The TMEM55 name refers to the two putative transmembrane domains found in the COOH termini of these proteins following their catalytic domains (1317). Overexpression of TMEM55A increases EGF receptor degradation (1593). TMEM55B, on the other hand, was shown to control nuclear PtdIns5P levels and respond to DNA damage by nuclear translocation, activating the ING2 protein (522) and promoting p53-mediated cell death (1833).
D. The SAC1 Family of Phosphatases
The prototypical Sac1 phosphatase was discovered independently in two laboratories as a mutant allele that rescued defects associated with temperature-sensitive actin mutants (1152) or secretion defects caused by sec14ts mutations in yeast (273). The connection between SAC1 being linked to actin dynamics on the one hand, and to the secretion process on the other, had not been fully realized for a long period, nor was it known what the Sac1p protein did in yeast cells. Eight years had passed from these original discoveries before it was realized that Sac1 is a phosphoinositide phosphatase (538, 667). On the basis of sequence similarities within their phosphatase domains, additional members of the SAC1 phosphatase family include the yeast PtdIns(3,5)P2 5-phosphatase Fig4 (497) and its mammalian homolog Sac3, as well as the mammalian Sac2 protein, which does not have a yeast counterpart. In addition, a Sac1 homology domain is found in the synaptojanin family of 5-phosphatases that were discussed above. These enzymes all have a conserved signature C(X)5R(T/S) motif within their catalytic domains (Figure 8B).
1. Sac1/SAC1M1L
The yeast Sac1p is capable of removing a phosphate from phosphoinositide monophosphates, such as PtdIns4P, PtdIns3P, and PtdIns5P, and also from bisphosphorylated PtdIns if the phosphates are not in vicinal positions [such as in PtdIns(3,5)P2, but not PtdIns(3,4)P2 or PtdIns(4,5)P2] in vitro (538, 667). Sac1-deleted yeast strains are viable, but show defects in actin dynamics, vacuolar sorting, and secretion and have cell wall abnormalities (273). They also show large (8- to 10-fold) accumulation of PtdIns4P with moderate increases in PtdIns3P and PtdIns(3,5)P2 (455, 538, 667). Paradoxically, PtdIns4P increases are not associated with increased PtdIns(4,5)P2; in fact, the latter shows moderate to substantial decreases depending on the study (455, 538, 667). The large increase in PtdIns4P in Sac1-deleted yeast requires a functional Stt4 but not Pik1, suggesting that Sac1 controls primarily the PtdIns4P pools generated by the Stt4p PI4K (455). This finding raised important questions since Sac1 is primarily found in the ER while Stt4 is in the PM, enriched in ER-PM junctional zones (79). Recent studies solved this apparent conundrum showing that ER-tethered Sac1 can act in trans to control PM PtdIns4P that is generated by Stt4 at ER-PM junctional zones (1454). However, it is still puzzling why the increased PM PtdIns4P is not reflected in increased PtdIns(4,5)P2 levels. Sac1 is tethered to the ER by its COOH-terminal hydrophobic motif that binds to the ER dolicholphosphate-mannose synthase enzyme Dpm1 (427). However, Sac1 cycles between the Golgi and the ER, and under nutrition deprivation, it is mostly localized to the Golgi and it is suggested that this relocalization limits PtdIns4P levels in the Golgi and, hence, inhibits secretion (427). Sac1 also associates with Orm proteins at the ER that are negative regulators of serine palmitoyltransferase, the rate-limiting enzyme in sphingolipid synthesis (178). Sac1 is also linked to the synthesis of complex sphingolipids in additional ways as it was found to be important for the synthesis of inositol phosphoceramide (IPC) (179). Accordingly, Sac1 deletions increase the sensitivity of yeast cells to Aerobasidin, an inhibitor of IPC synthesis (1529). All of these studies suggest that Sac1 performs multiple functions as part of numerous distinct protein complexes linked to different pathways.
The mammalian Sac1 enzyme is a ubiquitously expressed protein, first isolated from rat brain and showing the same substrate specificity profile as the yeast ortholog, capable of hydrolyzing all PtdIns monophosphates and PtdIns(3,5)P2 but not PtdIns(4,5)P2 (1125). Curiously, human Sac1 expressed as a GST fusion protein only acts on PtdIns3P and PtdIns4P in spite of 95% homology with the rat enzyme (1289). Mammalian Sac1 is mostly ER localized in steady state, but it cycles between the Golgi and the ER. A basic stretch of amino acids found at the Sac1 COOH terminus mediates its interaction with the coatomer COPI complex that mediates its retrieval to the ER (147). Under starvation, Sac1 interacts with COPII and undergoes oligomerization mediated by its NH2-terminal leucine-zipper motif, and a significant fraction of Sac1 is then found in the Golgi (147). This process is reversed by growth factor stimulation via activation of p38 MAPK (147). Mammalian Sac1 is an essential protein, and deletion of the gene in mouse causes preimplantation lethality, and even in cells, knockdown of Sac1 leads to cell death, making it difficult to study the functions of the protein (929). Sac1-deficient cells show a major disorganization of their Golgi morphology without a gross defect in secretion. Importantly, using PtdIns4P-recognizing PH domains, no sign of PtdIns4P accumulation in any cellular compartment could be detected, although an increased level of [3H]PtdIns4P was demonstrable in metabolically labeled cells (929). Sac1 knockdown also caused a high level of spindle defects, creating aberrant chromosomal segregation and a block in exit from mitosis (929). All of these defects can be rescued by overexpression of Sac1, and both its catalytic activity and ability to cycle between the ER and the Golgi were required for complementation (929). Sac1 mutant flies also die in embryonic stage and develop dorsal closure defects (1689). The structure of yeast Sac1 has been recently solved at 2 Å resolution. The enzyme consists of an NH2-terminal lobe of yet unknown function and a COOH-terminal catalytic lobe showing both similarities and notable differences with other phosphoinositide phosphatases, such as PTEN and MTMR2 (979).
2. Fig4/SAC3
Yeast Fig4 was first identified in a screen looking for genes that are activated by pheromone-induced mating pathway (406). FIG4 was found as a nonessential gene that was required for cell and actin polarization and notably had a Sac1 homologous region (406). It took several years again before it was discovered that Fig4p is a phosphoinositide phosphatase that dephosphorylates PtdIns(3,5)P2 at the 5-position (497, 1045). As already discussed in the Fab1p PtdIns3P 5-kinase section (sect. 5), Fig4p forms and stabilizes a trimeric complex with Fab1p and the scaffold protein, Vac14p located on the vacuolar membrane (390, 1042). This explains why deletion of Fig4 paradoxically decreases, rather than increases, PtdIns(3,5)P2 levels (389, 390, 1311) as it is needed to keep Fab1p in a functional form. Therefore, Fig4 deletions cause vacuolar fission defects with enlarged vacuoles and defective retrograde transport (1311).
The mammalian homolog of Fig4 is called Sac3, and it should not be confused with the yeast Sac3 protein that is a member of the nuclear pore complex involved in mRNA export (878). The first mammalian Sac3 was identified as a human KIAA 00274 clone (1057) and characterized as the mammalian homolog of Fig4 (1353). Human Sac3 displayed features similar to its yeast counterpart: it hydrolyzed PtdIns(3,5)P2, formed a complex with the mammalian homologs of Fab1 (PIKfyve) and Vac14 (ArPIKfyve), and localized to endosomes (1353). Interestingly, in a parallel study, Sac3 was characterized in rats as a ubiquitously expressed protein that localized to the ER, Golgi, and recycling endosomes and played a role in neurite extension in response to NGF (1786). This latter report found Sac3 displaying a wider substrate profile of phosphatase activity acting on PtdIns4P, PtdIns3P, and PtdIns(3,5)P2. Genetic inactivation of Sac3 by an early transposon insertion into the mouse Fig4/Sac3 gene is responsible for the “pale-tremor mouse.” This Fig4−/− mouse is characterized with pigmentation defects and progressive spongiform neurodegeneration. Fibroblasts obtained from Fig4−/− mice show a decrease rather than an increase in PtdIns(3,5)P2 levels with prominent defects at the late-endosome to lysosome transition (and probably in many other trafficking steps). These are probably the underlying causes for the pigmentation defect and neurodegeneration (253). Pathogenic mutations in the human FIG4 gene have also been linked to a special form of autosomal recessive Charcot-Marie-Tooth disorder, CMT4J, characterized by motor and sensory neuropathy (253) and in a small percentage of patients with amyotrophic lateral sclerosis (ALS) (252). The most common mutation of Sac3 in CMT4J patients is I41T, which decreases Sac3 association with the PIKfyve-ArPIKfyve (Fab1-Vac14) complex and enhances its proteosomal degradation (683, 884). However, the Fig4I41T transgene overexpressed in Fig4−/− mice rescues the most severe phenotypes showing that this mutant protein still retains most of its functions (884). FIG4−/− mice also develop severe myelination defects that are especially pronounced in smaller caliber axons, and the number of myelinating oligodendrocytic progenitors are greatly reduced (1720). Remarkably, this defect can be corrected with neuron-targeted expression of Sac3/Fig4, suggesting that the defective oligodendrocytic progenitors are secondary to a neuronal defect. In this study, again the Fig4I41T mutant enzyme was able to rescue the hypomyelination (1720).
3. hSac2/INPP5F
There is no yeast homolog of human Sac2. The human Sac2 enzyme was identified as a phosphatase showing significant homology to the yeast Sac1 and Fig4 proteins (1057). Strikingly, this enzyme is a PtdIns(4,5)P2 5-phosphatase capable of acting also on PtdIns(3,4,5)P3 but not on any of the other phosphoinositides or soluble inositol phosphates (1057). A genetrap mouse lacking a functional Sac2 shows no major abnormalities but is more susceptible to isoproterenol-induced cardiac hypertrophy, whereas overexpression of Sac2 in hearts protects animals from developing cardiac hypertrophy (1823). These studies suggested that Sac2 reduces PtdIns(3,4,5)P3 levels in mouse heart and hence antagonizes the activation of the fetal gene programs that underlie cardiac hypertrophy.
VII. PHOSPHOLIPASE C ENZYMES
PLC enzymes hydrolyze the phosphodiester at the sn-3 position of phosphoinositides, yielding inositol phosphates and DAG. Other PLC enzymes (e.g., ones that hydrolyze PC) also exist justifying the term “phosphoinositide-specific” or PI-PLC to designate the substrate specificity of these proteins. Several bacteria also use PLC enzymes as virulence factors (528, 1646), and the bacterial PLCs do not hydrolyze phosphorylated PtdIns and act primarily on GPI linkages at the outer surface of cells. However, bacterial PLCs can also act on PtdIns itself. Because of this substrate restriction, the bacterial PLCs are also referred to as PtdIns (or PI) specific PLCs, which can cause some confusion. The eukaryotic PI-PLCs can use PtdIns, PtdIns4P, and PtdIns(4,5)P2 but not 3-phosphorylated inositides as substrates in vitro, but they are believed to act primarily on PtdIns(4,5)P2 in their native cellular environment. Only a few studies addressed this question rigorously, and a few reports suggested that PLCs act on PtdIns (1066) and that they are present in intracellular locations (149, 354), where they could act on PtdIns or PtdIns4P.
Initially, PLC enzymes were studied as activities responsible for phospholipid catabolism in the lysosome (703, 1272). After discovering that PLC was a key component of receptor-regulated signal transduction (1040) (see sect. III on the history of phosphoinositides), these enzymes were sought after as important receptor-regulated signal transducers. Having membrane-bound substrates, PLCs were studied in membranes, the red blood cell membrane being the purest case (383), but they were also detected and characterized in soluble fractions obtained from sheep seminal vesicle (630) or from rat and bovine brain (704, 1319, 1555). It was an important question to understand how PLCs were regulated by receptors. The Ca2+ requirement of PLC enzymatic activity and its strong stimulation by Ca2+ supported views that Ca2+ elevations might be the primary signals linking receptors to PLCs. These claims were later settled as PLC activation clearly precedes the cytoplasmic Ca2+ increases (see sect. III for more details). Also, several studies showed that the physicochemical state of the membrane determines the access of PLC to phosphoinositide substrates, raising the possibility that substrate presentation would be an effective way to regulate the enzyme (e.g., Refs. 631, 704). Soon it became clear that PLC enzymes are regulated by GPCRs via heterotrimeric G protein subunits (174, 571, 602, 1539). It was an important discovery in the late 1980s that GPCRs and RTKs generate Ins(1,4,5)P3 and evoke cytosolic Ca2+ increases with notably different kinetics (939, 1116), suggesting that the two kinds of receptors activate PLC enzymes with different mechanisms and possibly they act upon distinct PLC isoforms (67, 603, 1236). By that time two or three major forms of PLCs have been purified from various sources including the sheep seminal vesicle (630), liver (1521), and bovine brain (763, 1265, 1318, 1319). These activities differed in molecular size and immunoreactivity. These advances were rapidly followed by the molecular cloning of the first mammalian PLC enzymes (1493, 1494) that later became known as PLCβ, PLCγ, and PLCδ (1271). The protein initially designated as PLCα turned out to be an ER resident Cys-protease (1179, 1595), so there is no PLCα enzyme. The genomic era has led to the subsequent identification of several forms of each of the three classes and revealed additional families such as PLCε, PLCζ, and PLCη with a total of 13 isoforms belonging to six PLC families (572) (Figure 9).
Figure 9.
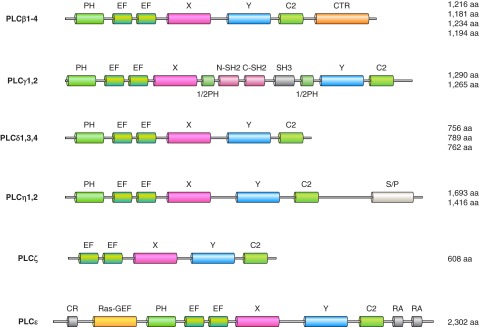
The phospholipase C family. These enzymes hydrolyze PtdIns(4,5)P2 to Ins(1,4,5)P3 and diacylglycerol, hence are called phosphoinositide-specific (PI)-PLCs. They should not be mistaken for the bacterial PLCs that are specific to PtdIns, unable to hydrolyze phosphorylated PtdIns, and hence called PI-specific PLC (unfortunately similarly abbreviated to PI-PLC). Mammalian PLCs have a core structure consisting of a PH domain, followed by EF hands, a catalytic domain formed from X and Y conserved regions and a C2 domain. PLCδ enzymes show this minimal domain organization. The only exemption is PLCζ that lacks the PH domain. This basic core is then extended in the various PLCs: the β enzymes have a characteristic COOH-terminal extension (CTR) that lends regulation by heterotrimeric G protein alpha subunits. The X and Y domains are separated with a long insert in the PLCγ enzymes consisting of two SH2 domains, an SH3 domain, and this whole insert is sandwiched in between two half PH domains. The PLCη enzymes have a long serine-proline-rich (S/P) segment in their COOH termini. The largest PLC enzyme is PLCε, which has a cysteine-reach region (CR) and a RAS-GEF domain at the NH2 terminus and two Ras association domains (RA) at the COOH terminus.
Each PLC contains common basic building blocks, such as a PH domain, four EF-hands, a triose phosphate isomerase (TIM) barrel-like catalytic domain formed from X and Y conserved regions, and a C2 domain (found in this order from NH2 to COOH terminus). In addition, most PLC families feature additional unique regulatory regions (Figure 9). PLC enzymes work mostly at the PM, and they are recruited to the membrane via multiple interactions with proteins and lipids mediated by the PH domain, the C2 domain, and a polybasic stretch within the CT-sequence found in PLCβ forms (419, 1214, 1673, 1760). The exact mechanism of membrane recruitment, however, is mostly obscure. The first mammalian PLC structure was that of PLCδ1 (408) followed by those of PLCβ2 (729), PLCβ3 (1657), and an invertebrate PLCβ (958). These structural studies revealed the molecular anatomy of the catalytic core and its relationship to the substrate PtdIns(4,5)P2 (408). They also showed the mode of interaction of PLC enzymes with regulators such as Rac1 (729) and the Gαq subunit (958, 1657). These structural studies also helped identify an acidic autoinhibitory loop located between the X and Y conserved domains, which may act as a pseudosubstrate to occlude the catalytic active site of the enzymes (612).
A thiol-reactive alkylating compound, U73122, has been widely used as an inhibitor of PLC (150). However, how this compound works is quite puzzling. It does behave as a PLC inhibitor in many cellular systems with a very narrow concentration range (2–5 μM) between effective and toxic doses (1423, 1552, 1732). However, it either has no effect on purified PLC enzymes or stimulates PLC activity in vitro at somewhat higher concentrations (∼10 μM) (802). U73122 also inhibits a number of Ca2+-regulated processes independent of its putative effects on PLC (169, 1240, 1660, 1817). The fact that its nonreactive control compound U-73343 does not reproduce the effects of the active compound, by no means is a satisfying criterion to indicate specificity on PLC. Newly developed PLC inhibitors will be extremely helpful to better understand the role of PLC enzymes in various cellular settings and also to understand the mode of action of U73122 (664).
PLC enzymes have also been identified in lower eukaryotes such as the PLCδ-like Plc1 in S. cerevisiae (447, 1771) and Dictyostelium (387). A PLCβ-like protein called NorpA was found in Drosophila (153), and a homologous G protein-regulated PLC is present in the squid eye (1067). The latter two play a critical role in invertebrate photo-signal transduction. Deletion of Plc1 in yeast causes several abnormalities with severe growth defects, impaired cell wall integrity, decreased osmotic resistance, and an inability to use carbon sources other than glucose (447). Hyposmotic stress activates Plc1 in yeast, and the produced Ins(1,4,5)P3 is rapidly converted to InsP6 (1208). Intriguingly, PLC deletion in Dictyostelium does not cause any major abnormalities, but PLC null cells fail to properly respond to chemorepellents while they do respond to chemoattractants, suggesting a modulatory role of PLC in chemosensing (771). NorpA mutant flies are viable, but they have defective light response in the retina and develop retinal degeneration (153). NorpA also serves as a downstream effector of the fly rhodopsin (ninaE) in temperature discrimination (1395).
A. PLCβ
There are four members of the PLCβ family designated as PLCβ1–4. All PLCβ enzymes are activated by Gα subunits of the Gq/11 class (1425, 1538, 1656) and PLCβ2 and PLCβ3 also by βγ subunits (174, 207). Therefore, PLCβ is a major downstream effector of GPCRs. It is now understood that GPCR activation of PLC that is resistant to pertussis toxin is mediated by the Gα subunits of Gq/11 (mostly PLCβ1, -β3, and -β4), while pertussis toxin-sensitive PLC activation by GPCRs occurs via βγ subunits liberated from Gi and Go proteins (mostly PLCβ2 and -β3) (183). PLCβ1 (and probably all other β isoforms) also acts as GTPase activating proteins (139). For a time it was believed that the characteristic COOH-terminal conserved coiled-coil (CT) domain found in all PLCβ is the site of activation by G protein α-subunits and is also the region that confers GAP activity (690, 1196, 1201, 1410). Structural studies allowed a more precise insight into the molecular details of Gq activation. They identified a unique, highly conserved helical extension of the C2 domain not found in any other PLC forms as the major site of Gq interaction (958, 1657). Most recent studies using expressed GFP-tagged enzymes in intact cells found that only PLCβ1 and PLCβ4 were enriched in the PM while PLCβ2 and PLCβ3 were mostly cytosolic and that the isolated CT domains of all but PLCβ2 were also localized to the PM (7). The importance of the CT domain was also highlighted in this study by the ability of the expressed isolated CT domains to inhibit Gq-mediated PLC activation and Ca2+ signaling apparently by interacting with the Gq alpha protein (7).
1. PLCβ1
This enzyme is broadly expressed but especially enriched in brain cortex and hippocampus. In fixed cells, the endogenous enzyme is found in the cytoplasm presumably associated with a variety of membranes (844), but an overexpressed PLCβ1 tagged with GFP associates with the PM (7, 8, 380). Two splice variants of PLCβ1 (PLCβ1a and -b), first described in heart, differ in their COOH termini and show different membrane localization (532). Both isoforms of PLCβ1 were also found in the nucleus (451, 987) and have a role in cell cycle control, lamin B1 phosphorylation, and G2/M progression (416, 443). PLCβ1 was also linked to myelodysplastic syndrome and its progression to acute myeloid leukemia (452). PLCβ1 −/− mice show growth retardation, and although viable at birth, they start to die at the third week after severe generalized seizures without any sign of gross brain structural abnormality. The similarity of these seizures to those developing after GABAA receptor inhibition suggested that PLCβ1 deletion affects the function of inhibitory neurons (784). Interestingly, impairment of receptor-mediated increase in PLC activity showed remarkable selectivity in brain slices from PLCβ1 −/− mice in that the response to muscarinic stimuli was greatly reduced, while that to mGluR agonist was enhanced and the response to serotonin receptor stimulation was unchanged (784). However, in another report, the cortical layering of neurons in the somatosensory cortex of PLCβ1 −/− mice was found impaired and the InsP response to mGluR stimulation significantly reduced (565). Other studies found these mice still reaching normal age but showing memory deficits, hyperactivity, reduced acustic startle response, attributed to an imbalance between muscarinic and dopaminergic or serotoninergic systems, reminiscent of animal models of schizophrenia (1020).
2. PLCβ2
This isoform is mostly expressed in hematopoetic cells (1194), but it is also found in other tissues such as in taste buds (787, 1070, 1299). PLCβ2 is primarily regulated by βγ subunits released from pertussis toxin-sensitive Gi/Go proteins (174, 207, 1426). PLCβ2 is also activated by small GTP binding proteins of the Rho family (691, 692), and this regulation requires the PH domain (693, 1428). The structure of PLCβ2 with a bound Rac1 showed a direct interaction between the PH domain of PLCβ2 and the switch region of Rac1 (729), but no major confromational change in the catalytic site compared with the PLCβ2 structure alone. Rac1 was also shown to recruit the enzyme into more specialized membrane compartments relative to the more general membrane recruitment by Gβγ subunits (542, 693). PLCβ2 −/− mice are viable but, contrary to expectations (being enriched in hematopoetic cells), their immune responses to bacterial and viral pathogens are not impaired, if anything they are enhanced. At the same time, neutrophil cells of these animals showed a greatly reduced InsP3, Ca2+, and superoxide responses, but a variety of bone marrow-derived cells (including neutrophils) showed enhanced chemotaxis, suggesting that PLCβ2 may exert a negative rather than positive influence on chemotactic signaling (732, 906). This may be due to more PtdIns(4,5)P2 being available for PI 3-kinases. Interestingly and conversely, another study found that PLCβ2 and -β3 are critical for T-cell chemotaxis (72). PLCβ2 −/− mice are also impaired in sweet, amino acid, and bitter taste reception (1091, 1808).
3. PLCβ3
This broadly expressed isoform can be activated by Gαq/11 as well as Gβγ subunits (1215). The recent structure of PLCβ3 in complex with activated Gαq defined the interface between the two proteins (1657). This enzyme has also been claimed to have an intracellular role to mediate the Gβγ/PKCη/PKD-mediated regulation of TGN to PM transport (354). PLCβ3 −/− mice have a shorter life span and succumb to myeloproliferative diseases, lymphomas, and other forms of cancer (1742, 1743). These animals do not respond to stimuli that evoke an itching response apparently due to impaired signaling from H1 histamine receptors in C-fiber nociceptive neurons (563). Smooth muscle cells obtained from PLCβ3 −/− mice failed to show Ca2+ elevations in response to opiate receptor stimulation. However, these mice showed high sensitivity to morphine and increased voltage-gated Ca2+ channel activity in response to opiates in their dorsal root ganglion neurons arguing against impaired opioid receptor signaling (1746). In zebrafish, PLCβ3 was found critical for endothelin-1-driven regulation of pharyngeal arch patterning (1661) and a negative regulator of VEGF-mediated vascular permeability (629).
4. PLCβ4
This enzyme is mostly expressed in specific areas in the brain, especially in the cerebellum (789, 868, 1526). A shorter splice variant of the enzyme has also been described showing high expression in the rat retina (5) but not responding to Gαq stimulation (790). PLCβ4 is regulated exclusively through Gαq and not via Gβγ or Rac proteins (570, 867). Because of the closest homology of PLCβ4 to the Drosphila NorpA protein that has key roles in photo-signal transduction, and the presence of the enzyme in retina, it was assumed that this isoform had important roles in visual processing in mammals. PLCβ4 −/− mice do, indeed, have impaired visual processing, but it is not related to defective photoreceptor light responses or structural aberrations of the retina but probably due to impaired signal processing (733). PLCβ4 −/− mice develop normally but show ataxia with slower cerebellar development (784). The InsP3 responses to mGluR or muscarinic receptor stimulation were reduced in cerebellar slices obtained from PLCβ4 −/− mice (784), and the process of climbing fiber synapse elimination in the developing cerebellum was also found impaired (759).
B. PLCδ
There are three different PLCδ enzymes, named PLCδ1, PLCδ3, and PLCδ4 (570) (PLCδ2 turned out to be the bovine version of human PLCδ4; Ref. 700). These enzymes all show broad tissue distribution but differ in cellular localization. PLCδ enzymes possess the minimal PLC building blocks: the NH2-terminal PH domain, the EF hands, the conserved X and Y domains, and the C2 domain at the COOH terminus (Figure 9). The PLC enzymes of lower organisms (yeast and Dictyostelium) are orthologs of PLCδ. The regulation of this isoform is the least understood. The activity of the enzyme is increased with Ca2+ elevations in the physiological range (33) but not by the G proteins that activate other PLCs (neither the heterotrimeric, nor the small forms). However, it was suggested that PLCδ1 is activated by the atypical G protein, transglutaminase II, or GαH (429) or the small GTP binding protein RalA (515, 1406). According to current views, PLCδ1 is regulated via its PH domain that recruits the enzyme to the PM. The isolated PH domain of PLCδ1 was one of the first PH domains to be structurally characterized showing binding to both PtdIns(4,5)P2 and the soluble Ins(1,4,5)P3 (431, 882). Because of its binding to PtdIns(4,5)P2, the PH domain of PLCδ1 is widely used as a PtdIns(4,5)P2 reporter in live cells (1451, 1615). Therefore, the enzyme is recruited to the PM via binding to its own substrate, PtdIns(4,5)P2, and the production of Ins(1,4,5)P3 would displace the enzyme from the membrane providing with a self-limiting regulatory mechanism (936). However, PM recruitment may not be the sole regulation of the enzyme by PtdIns(4,5)P2, since the enzyme was strongly stimulated by the lipid even in cell free systems (936). Elimination of the PH domain, or single residue mutations within, strongly influence the activity of the enzyme but not its catalytic site suggesting an allosteric regulation (187, 1754). The C2 domain of PLCδ enzymes has also been implicated in Ca2+ regulation and membrane association via nonspecific electrostatic interactions and PS binding (43, 409, 935).
1. PLCδ1
In addition to its role in hydrolyzing PtdIns(4,5)P2 at the PM, PLCδ1 shuttles between the cytosol and the nucleus (1755). The enzyme accumulates in the nucleus during the G1/S1 boundary (1448, 1753) following an increase in nuclear PtdIns(4,5)P2 levels (1448), and it also regulates cell cycle progression (1449). It is not clear how these nuclear changes contribute to the overall functions of the enzyme. PLCδ1 −/− mice are viable but lose hair within 10 days of birth and are susceptible to skin inflammation and also develop skin tumors (1109). These mice have a defect in keratinocyte differentiation in hair follicles and show an increased number and highly proliferative interfollicular epidermal cells and sebaceous glands. Keratinocytes from PLCδ1-deficient mice showed an impaired Ca2+ signal, PKC activation, and NFAT transcriptional activity (1109). PLCδ1 is also a downstream intermediate regulated by the keratinocyte-specific transcription factor Foxn1, the gene that is mutated in nude mice (1111). It is interesting to note that Orai1-deficient mice also develop hair loss and skin abnormalities and show defective keratinocyte differentiation (543). Orai1 is the channel component of the STIM1-regulated Ca2+ entry pathway that is activated upon ER Ca2+ depletion (435) and is highly expressed in epidermal keratinocytes (543). Extracellular Ca2+ is important to trigger keratinocyte differentiation, and it increases the expression of PLCγ1 and PLCδ1 (1241). PLCδ1 was also shown to be regulated by store-operated Ca2+ entry (795). Therefore, it is reasonable to assume that PLCδ1 and Orai1-mediated Ca2+ entry work in synergism to control keratinocyte differentiation. PLCδ1 −/− mice also develop skin inflammation due to the production of proinflammatory cytokines by the epidermis (676). Most recent studies reported that PLCδ1 −/− mice are protected from obesity when put on a high-fat diet apparently due to a higher metabolic rate and altered adipogenesis (622). The mechanism of how this is achieved is still under investigation.
PLCδ1 has been linked to several human diseases. Its levels were shown to increase and accumulate in neurofibrillary tangles in the brains of Alzheimer's patients (1399, 1400). Reduced PLCδ1 levels due to hypermethylation of its gene have been reported in esophageal, gastric, and colorectal cancers (320, 471, 658), and the metastatic potency of breast cancer cells showed correlation with the expression levels of PLCδ1 and PLCδ3 (1264).
2. PLCδ3
This isoform showed a few notable differences from its sister, PLCδ1, in cellular distribution and sensitivity to lipids and detergents and in the importance of its PH domain in regulation (1202, 1203). Recent data indicated that PLCδ3 downregulates RhoA and promotes neurite outgrowth in primary cortical neurons (823). PLCδ3 was found to interact with myosin VI (Myo6) and contribute to inner-ear hair cell stereocilia stabilization and possibly to microvilli maintenance in the colon epithelium (1328). A uniquely tight coupling between Ca2+ entering via arachidonate-activated ARC channels and PLCδ3-mediated PtdIns(4,5)P2 hydrolysis was also reported (1553). Knockout studies suggest that PLCδ1 and PLCδ3 have overlapping functions. PLCδ3 −/− mice show no obvious abnormalities, whereas a combined PLCδ1/PLCδ3 −/− knockout results in midgestational lethality caused primarily by a placental defect (1110).
3. PLCδ4
This enzyme has several isoforms in the rat, a smaller, 90-kDa form that is expressed in various tissues and a larger 93-kDa splice form that is specific to the testis (871). A third splice variant was also reported as a negative regulator of PLC activity (1105). There are four alternatively spliced forms in the mouse showing differential tissue expression (475). PLCδ4 is located primarily in the nucleus and shows changes with the cell cycle (925) and was shown to be increased in fast-growing human hepatoma cells (1338). The PH domain of PLCδ4 was reported not to bind Ins(1,4,5)P3 (1105), and it was tested whether it would be a better PtdIns(4,5)P2 reporter (872). However, this PH domain also has a lower PtdIns(4,5)P2 affinity, and it does not contribute to the localization of the enzyme that this study found primarily in the ER (872). Overexpression of the enzyme upregulates the ErB1/2-Erk signaling pathway and promotes cell proliferation in breast cancer cells (889). PLCδ4 −/− mice develop normally, but the males are sterile due to the inability of their sperm to induce normal acrosome reaction and fertilization (476, 477).
C. PLCγ
PLCγ exists in two forms: PLCγ1, which is widely expressed, and PLCγ2 that is mostly expressed in hematopoetic cells (645). These enzymes have the most complex structure among PLCs in that they contain a split PH domain, two SH2 and one SH3 domains sandwiched between their catalytic X and Y domains (Figure 9). These enzymes are regulated by tyrosine phosphorylation by RTKs that occurs following interaction of the SH2 domains of the PLCγ enzymes with specific tyrosine-phosphorylated motifs of a variety of activated RTKs including receptors for PDGF, EGF, and FGF (and many others) (749, 994, 1008, 1027, 1077, 1086, 1654). It is particularly important for T- and B-cell activation that PLCγ associates with large cytoplasmic molecular complexes that are recruited to the PM and which contain non-receptor tyrosine kinases that activate the enzyme (165, 1101, 1197, 1291, 1374, 1694). In some cases, PLCγ uses intermediary adaptor proteins to associate with the macromolecular complex, such as in T cells where the LAT (439, 1803) and SLP-76 (1751, 1752) proteins bring the PLCγ1 enzyme to the activated TCR complex. Recruitment of the PLCγ enzymes to the membrane via these protein-protein interactions is essential for the subsequent tyrosine phosphorylation and activation of the enzymes (786, 1008, 1139, 1653). Five Tyr residues in PLCγ1 were reported to be phosphorylated by RTKs: Tyr472, -771, -775, -783, and -1254 (786, 788, 797, 1383, 1652). Although conflicting reports have been published about the importance of some of these Tyr phosphorylations (1380, 1383), there is a clear consensus that phosphorylation of Tyr783 and perhaps Tyr775 are critical for the stimulated activity of PLCγ1 (526, 797, 1380). Phosphorylation of the equivalent residues in PLCγ2 (Tyr753 and Tyr759) are also critical in B-cell activation (797, 1278). How tyrosine phosphorylation affects enzymatic activity is a question that is just beginning to unfold (526). An intramolecular interaction between the COOH-terminal SH2 domain and the phosphorylated Tyr783 evokes a conformational change that relieves autoinhibition of the enzyme (526, 658). In addition to Tyr-phosphorylation, PLCγ1 was shown to be directly regulated by PtdIns(3,4,5)P3 either via interaction with the PH domain (419) or the SH2 domain (77, 1253). In addition, PtdIns(3,4,5)P3 also regulates PLCγ activity via activation of Tec-family tyrosine kinases, an effect that is most pronounced in immune cells and controlled by SHIP1 (448, 1356, 1357). Recent studies suggest that the sprouty proteins, negative regulators of RTK signaling (220), can associate with both PLCγ isoforms and inhibit their activation by RTKs (19).
1. PLCγ1
Homozygous PLCγ1 deletion in mice causes embryonic lethality at day 9 (730), apparently due to a loss of vasculogenesis and erythropoiesis (909). This is consistent with zebrafish studies that showed impaired vasculogenesis and ventricular contractility upon loss of PLCγ function (861, 1301). These phenotypes suggest that the effects of VEGF on vascular endothelium are mediated mostly by PLCγ-mediated pathways (1035, 1301, 1516). Since PLCγ1 activation is a major signaling route initiated by RTKs, it has been assumed, and in many cases shown, to be critical for the mitogenic, pro-proliferative effects during stimulation of these receptors. Indeed, elevated levels of PLCγ1 were found in aggressive breast cancers (56), and the enzyme was found critical for the metastatic properties of breast cancer cells (1329). However, even early studies suggested that RTKs, such as the FGFR, can induce mitogenesis without activation of PLCγ (1212), and this is consistent with the relatively normal development of mice up to day 8 without PLCγ1 (730). In Drosphila, PLCγ defects cause impaired wing and eye development (984).
2. PLCγ2
An additional and unique regulation of PLCγ2 is via interaction with Rac proteins (1221). Rac interacts with the split PH domain of PLCγ2 (199, 1663) and regulates the membrane recruitment and activity of the enzyme (413, 1221). It is assumed that Rac interaction with the split PH domain relieves an autoinhibition, independently of Tyr phosphorylation (413). PLCγ2 −/− mice are viable but are immune-compromised because of defective B-cell responses to immunoglobulins (1666). In addition to B cells, PLCγ2 deficiency also affects neutrophils (717), platelets (698), and dendritic cells (300). Recently, activating mutations of PLCγ2 were found in patients with spontaneous inflammations and autoimmunity (414), and activating deletions in PLCγ2 were found in families with cold urticaria, immunodeficiency, and autoimmunity (1169).
D. PLCε
This enzyme was a late-comer as it was discovered as a novel, Ras-regulated PLC enzyme identified in C. elegans and named PLC210 (1398). The mammalian homologs were then cloned independently in three laboratories (773, 938, 1431) and named PLCε. Two splice variants differing in their NH2 termini, called PLCε1a and PLCε1b, exist, but no functional difference between them has been reported (1436). It is generally accepted that the enzyme is widely expressed, although only at low levels making its detection difficult (1668). PLCε has several regulatory domains in addition to the classical building blocks, namely, an NH2-terminal cysteine-rich region followed by a Ras GEF domain (called here CDC25 GEF), and two Ras-association (RA) domains at the COOH terminus (Figure 9). The Ras GEF domain can activate Rap1 (736), while the two RA domains bind H-Ras and Rap1, with the second one (R2) being more important in this regard (1431). Therefore, PLCε is both an activator and a downstream target of Ras/Rap1 possibly representing a feed-forward activation loop. Receptor-induced activation of PLCε can be achieved from RTKs, such as the EGF receptor that requires H-Ras (1431) and probably involves translocation of the lipase from the cytosol to the PM (736, 1431). PLCε also translocates to the perinuclear area after Rap1 activation (736). It was proposed that Ras is responsible for the rapid phase of PLCe activation, while Rap1 is for a prolonged activation of the enzyme (1432). In addition, GPCR stimulation with LPA, or thrombin also activates PLCε, and this activation involves the G12/13 family of heterotrimeric G proteins (549, 774). The G12/13-mediated activation of PLCε is not direct; it goes through the Rho GEF proteins p115RhoGEF or LARG yielding GTP-bound Rho (549). Interestingly, while the EGF/Ras-mediated activation requires the COOH-terminal RA domains, the Rho-mediated activation does not require this segment but one within the conserved Y catalytic domain (1378). Rho-mediated activation of PLCε can also be achieved via Gq-coupled receptors, but in this case it is the p63RhoGEF that mediates Rho activation (18). The Ras and Rho-mediated activation of PLCε via the two different regions of the protein can work in a synergistic manner (1379). PLCε can also be activated via increases in cAMP levels mediated by the guanine exchange factor Epac, which acts on Rap2B (412, 1161, 1364).
Since a whole set of other PLCs can respond to stimulation of a variety of cell surface receptors, it is of particular interest to what extent PLCε contributes to the overall PLC activity during activation of specific receptors. This question was examined after RNAi-mediated knockdown of PLCε or probably more reliably in cells derived from PLCε −/− mice (1511, 1668). RNAi studies in Rat1 fibroblasts suggested that PLCβ3 was more important in the rapid phase, while PLCε in the prolonged phase of InsP3/DAG formation during endothelin, ET-1 receptor, LPA, or thrombin stimulation (772). Studies in astrocytes from PLCε −/− mice showed that PLCε is a major contributor to InsP3/DAG generation after LPA, thrombin, and S1P receptor stimulation and that LPA and S1P uses pertussis toxin-sensitive Gi/o proteins, while thrombin uses RhoA via G12/13 (264). Two groups have generated PLCε-deficient mice, one eliminating the entire PLCε protein (PLCε −/−; Ref. 1668), the other making a truncated protein that is catalytically inactive missing the EF-hands and part of the catalytic domain (PLCε Δx/Δx; Ref. 1511). These mice show some differences in their phenotypes (detailed in Ref. 1424), but they both highlighted critical roles of the enzyme in cardiac development and functions. PLCε Δx/Δx mice have developmental defects in the aortic and pulmonary semilunal valves with sequential ventricular dilation and hypertrophy (1511). This pathology is not seen in PLCε −/− mice (1668). PLCε −/− mice, on the other hand, are more susceptible to cardiac hypertrophy, possibly because of upregulation of the Gq-mediated PLC pathways, since downregulation of PLCε in vitro in ventricular cardiomyocytes greatly blunts their “hypertrophic” response, which is the opposite of what the knockout mice would predict (1424). PLCε −/− mice also have impaired cardiac contractility that may be related to scaffolding functions with muscle-specific AKAP proteins as well as the ryanodine receptors (1424, 1799). In addition, PLCε has been linked to inflammation and tumorigenesis in skin (687) and intestine (900) in mouse models. As for human diseases, PLCε mutations have been found in patients presenting with childhood nephrotic syndrome probably linked to altered podocyte functions (621). Interestingly, no kidney pathology was found in PLCε mice, but PLCε deletion in zebrafish causes developmental problems with the filtration barrier of the fish “kidney” (621). PLCε was found significantly downregulated in patients with sporadic colorectal cancers (1675), and the PLCE1 gene was found as a susceptibility locus for gastric and esophageal squamous cell carcinoma (3).
E. PLCζ
This is the smallest PLC enzyme that even lacks a PH domain (Figure 9). It was first isolated from mouse testis and is exclusively expressed in the spermatids (1349). PLC activation during the acrosome reaction has been described earlier (575), and injection of PLCζ cRNA into mouse eggs initiates Ca2+ oscillations and triggers egg activation, suggesting that it is the “sperm factor” that initiates egg activation upon fertilization (822, 1349). Human sperm devoid of PLCζ fails to trigger Ca2+ oscillations and egg activation (1774). The enzyme is localized at the equatorial region of human sperm (1774) and is found in the pronucleus during mouse egg activation (1770). PLCζ is the most Ca2+-sensitive PLC already being activated at basal levels (100 nM) of Ca2+ concentrations (822). Regulation of PLCζ appears to be different from the other PLCs. First, expression of the enzyme in CHO cells failed to induce any Ca2+ oscillations in spite of good expression and high level of PLC activity measured from the cytosol in vitro (1218). Most recent studies showed that PLCζ acts on a distinct vesicular pool of PtdIns(4,5)P2 within the egg cortex as opposed to the PM, showing clear distinction from PLCδ1 which affected the PM pool of the same lipid (1784). Also, unlike in other PLCs in which the linker region between the X-Y domains contains several acidic residues and exerts autoinhibition (612), in PLCζ this region contains a stretch of basic residues that mediate binding to membrane PtdIns(4,5)P2 (1144), and removal of this region inhibits rather than activates the enzyme (1145). A basic stretch found within the X-domain has also been implicated in nuclear localization and nucleo-cytoplasmic shuttling of the enzyme during Ca2+ oscillations (834).
F. PLCη
These enzymes have two isoforms, PLCη1 and PLCη2. They were identified independently in four laboratories in 2005 (673, 1108, 1471, 1820). They show highest expression in the brain and they are structurally related to PLCδ1 (Figure 9). In addition to the usual domains present in PLCs, they have a serine- and proline-rich region and a PDZ-domain interacting sequence in their COOH termini (1470). Relatively little is known about the regulation of these enzymes. PLCη2 can be activated by G protein βγ subunits shown both in expression studies in COS-7 cells (1820) and in reconstitution assays (1819). Neither the PH domain nor the COOH-terminal unique sequence is required for Gβγ activation of PLCη2 (1819), but the PH domain was shown to be needed for membrane localization (1108). PLCη2 also shows high Ca2+ sensitivity even more so than PLCδ1 (1108). A PLCη2 knockout mouse with a LacZ knockin has revealed high expression of the enzyme in the habenula and retina and suggested a possible role in retina development or maturation, but the mice showed no major abnormalities (753).
G. PLC-Related Proteins
In 1992 the Hirata laboratory isolated a protein from rat brain cytosol that showed high-affinity binding to Ins(1,4,5)P3 (756). This protein, initially named p130, turned out to be identical to a PLC-like protein called PLC-L that was deleted in the cancer tissue of a patient suffering from small cell lung carcinoma (755, 811). Another homolog named PLC-L(2) was also described and showed that these enzymes have high sequence homology to PLCδ1 but are inactive because of a mutation of a conserved His within their catalytic domains (755, 1181). These proteins were later named PRIP-1 and -2 (for PLC-related but catalytically inactive protein), the former being highly expressed in brain, while the latter showing wider tissue distribution (1007, 1181). PRIP-2 localizes to the ER and perinuclear region (1181). The isolated PH domains of both proteins showed high-affinity binding to Ins(1,4,5)P3 and PtdIns(4,5)P2 and in the case of PLC-L(2)/PRIP-2 it also localized to the perinuclear ER (1181), while in the case of PLC-L/p130/PRIP-1 it was found cytosolic (1616). Contrary to these findings, the PH domains of PRIP-1 and -2 both showed PM localization (490). PRIP-1 has a role in GABA receptor function indirectly by affecting the trafficking and phosphoregulation of GABA receptors (757, 1546). PRIP-1 interacts with the β-subunit of GABAA receptors and the associated protein GABARAP (754), and it augments the transport of γ2-containing GABAA receptors to the cell surface (1071). PRIP-1 knockout mice show anxiety-related behavior and reduced benzodiazepine sensitivity (754) and also exhibit seizurelike EEG activities (1822) indicating dysfunction of the GABAA receptors. PRIP-2 knockout mice develop normally and show no obvious abnormalities, but their B cells are hyperresponsive and display increased Ca2+ influx and NFAT activity after receptor crosslinking (1519). This may be related to the inhibitory effect of PRIP-1 on endogenous PLC activation and quenching some of the Ins(1,4,5)P3 generated (1524).
VIII. PHOSPHATIDYLINOSITOL TRANSFER PROTEINS
The first functional studies on a phosphatidylinositol transfer protein (PITP) were described in yeast, when the Sec14p protein was found to be important for the transport of secretory proteins from the Golgi (98, 102). Sec14p is a cytosolic protein that associates with the Golgi and is capable of either PtdIns or PtdCho transfer (98). Rescue studies have indicated that expression of Sec14 mutants defective in PtdIns but not PtdCho transfer are able to rescue the secretory defect of Sec14-deleted strains (1217), questioning whether PtdIns transfer is the primary role of the protein. Remarkably, genetic interference with PtdCho synthesis rescues Sec14p secretory defects (272), and Sec14p itself is an inhibitor of the CDP-Cho pathway (1018, 1414). Deletion of Kes1p, one of the oxysterol binding proteins of yeast also “bypasses” Sec14 defects initially raising the possibility that Kes1p mediates the PtdCho “toxicity” seen in Sec14-deleted strains (424). More recent studies indicate that Sec14p is essential for the maintenance of a Golgi pool of DG, which is spared when PtdCho syntheis is limited. Also, deletion of Kes1p, which can “extract” PtdIns4P and exchanges it for ER-derived sterols, spares the Golgi PtdIns4P pool (339). Importantly, “Sec14 bypass” conditions require a functional yeast PLD enzyme, Spo14p, presumably to supply DG from PtdCho (1748). More on Sec14 and its possible modes of action can be found in several recent reviews (100, 103).
The first mammalian PITP was isolated from bovine brain as two proteins of ∼30 kDa size capable of transferring PtdIns from labeled liver microsomes to PtdCho vesicles (597) and subsequently cloned from rat (355). PITPs were also isolated independently by two separate efforts purifying cytosolic factors that were required for G protein-mediated PLC activation (767, 1551) and ATP-dependent priming of exocytic vesicles (583). The mammalian PITPs [PITPα and its sister, PITPβ (1527), now called class I PITPs] show little sequence homology to the yeast Sec14p protein, yet mammalian PITPs can partially rescue yeast Sec14–1ts defects (but not SEC14 deletions) (1413), and conversely, Sec14p can restore PLC activity in mammalian cells (309). Curiously, unlike in the case of the yeast Sec14p (1217), the PtdIns transfer activity of mammalian PITPs was found to be important for complementation of the Sec14 defect is yeast (24, 1413). Another group of PITPs were initially identified in Drosophila, as one of the genes responsible for light-induced retinal degeneration phenotype, called RdgB (1636). Mammalian homologs of RdgB (variably named PITPnm or RdgBα) have been subsequently found (15, 232, 537), and it was recognized that these larger proteins possess at their NH2 termini a PITP-domain that is highly homologous to the class I mammalian PITPα and PITPβ. The same group of proteins was also isolated as interactors with the Ca2+-regulated tyrosine kinase, PYK2 and called Nir1–3 (891). Nir1, however, lacks the NH2-terminal PITP domain but shows homology to the other Nir proteins in the rest of their sequences (891). Finally, a PITP that is most homologous to the PITP domain of RdgBs but lacks all the other domains of the RdgB homologs was cloned and named RdgBβ (480). Nir2 and Nir3 are now called class IIA, while RdgBβ is named class IIB PITP (1575) (see Figure 10). Given the functional similarity between mammalian PITPs and Sec14p, it is noteworthy that both yeast and mammalian cells have Sec14 homologs that are lipid transfer proteins with cargoes other than PtdIns (1088, 1136). This raises the question of why the Sec14 scaffold was replaced by that of PITPs in metazoans for PtdIns/PtdCho transfer while still kept for other lipid transport purposes.
Figure 10.
The mammalian phosphatidylinositol transfer proteins. The PITP domain is capable of transferring PtdIns and PtdCho between natural membranes and artificial liposomes and hence are functionally similar to the yeast Sec14 protein. The class I PITPs are encoded by two genes, PITPα and PITPβ, the latter having two splice forms differing at the very COOH termini. A highly similar PITP domain is found in the NH2 terminus of a group of larger proteins that were first described in Drosophila as the RdgB proteins. These proteins were later identified in mammalian cells as membrane-associated PITPs (hence the name PITPnm) or as a family of proteins that interact with the Ca2+-regulated tyrosine kinase Pyk2 and named Nir1–3. In fact, one of these family members, Nir1/PITPnm3/RdgBαIII, lacks the PITP domain, so it is not a PITP. These proteins (called class IIA proteins) have an acidic region, initially defined as a Ca2+ binding domain but now recognized as a region containing an FFAT domain that provides ER localization through interaction with the ER resident VAP proteins. The DDHD domain is similar to a domain found in some PLA1 proteins, whereas the LNS2 domain is found in lipins that are PtdOH phosphohydrolases. The Pyk2 binding site, which is also located in the COOH terminus, probably overlaps with the LNS2. A third form of PITPs, called class IIB, is similar to the PITP region of the class IIA forms but lacks all other domains and has two splice variants differing in their COOH termini.
A. Class I PITPs
PITPα and PITPβ belong to this group, the latter having two splice variants that differ in their very COOH termini (277). Several details on the structure and PI-transfer characteristics of these PITPs have been uncovered, yet we still do not fully understand what these proteins do and how they work inside the cells. The structure of PITPα has been solved both with and without a PtdIns cargo molecule within the binding cavity (1366, 1557), and the headgroup of the lipid faces the interior of the cavity. Notably, this is the opposite orientation from what is found in the structure of the Sac14p protein (1387). The COOH-terminal last 10 amino acids are important to close onto the bound lipid, and PITPα mutants lacking the 5 or 10 last residues at the COOH terminus exert dominant negative effects when expressed in cells, while they still retain PI transfer activity (568). PITPα is mostly cytosolic, but PITPβ is found Golgi-associated (1216). Membrane association of PITPβ depends on specific COOH-terminal sequences unique to the PITPβ form (1216) but also require two conserved hydrophobic residues (Trp202, Trp203) found in all PITPs (1557). Since PITPs can transfer PtdIns (and to a smaller extent PtdCho) between membranes, it was initially believed that they transfer the ER-synthesized PtdIns to the PM (and perhaps to other membranes) for phosphorylation by PI4Ks and PIP5Ks, and hence supply PLC with its substrate PtdIns(4,5)P2. However, it appears that the mode of actions of PITPs is more complex (279, 1136), and recent models suggest that they can also present PtdIns for enzymes that use it as substrate (PI kinases or PLC enzymes) (830, 1354). Recent models posit that the cycling of the lipid through PITPs is an integral part of the ability of lipid modifying enzymes to act upon PtdIns. The term nanoreactor has been introduced to emphasize this catalytic/chaperone rather than transport function (689, 1136, 1354).
The vibrator mouse that shows early-onset action tremor, progressive spinocerebellar neurodegeneration, and juvenile death expresses very low level of PITPα (557, 1692). PITPα deleted mice are born normal, but develop spinocerebellar degeneration and soon succumb into hypoglycemia as well as intestinal and hepatic steatosis and die by day 12, apparently due to their inability to handle the milk fat during suckling (23). Cells obtained from these mice showed no significant change in phosphoinositide content, or problems with ER to PM secretion, exocytosis, endocytosis, or signaling, and therefore, the defects seemed to be confined to the defects in neurons, enterocytes, and liver cells, probably all related to problems with lipid handling (25, 99). The PtdIns binding of PITPα is critical for its function, as a PtdIns binding-deficient mutant protein cannot rescue the null phenotype (26). A detailed analysis of the pathology of mice expressing various levels of PITPα suggested that the lifespans of these animals were proportionally shortened with decreased level of the protein. These studies also showed a close relationship between the steatotic defect, hypoglycemia, and cerebellar inflammation, but the neuronal defects and early lethality still are maintained even when the lipid defects are corrected in the intestine (26). At the cellular level, PITPα was shown to associate with the netrin receptor and to play a role in neurite outgrowth (1747). Morpholino-mediated knockdown of PITPα in zebrafish causes early developmental arrest (688). In Drosophila, mutation of the single class I PITP Giotto causes defects in male germline cytokenesis (500, 508), with a phenotype, similar to the defect in the PI4KB homolog fwd (182).
PITPβ deletion is incompatible with life resulting in an early implantation defect in mice (25). The zebrafish homolog PITPβ is required for proper development of double cone cell outer segments in the retina (688). Knockdown of PITPβ in HeLa cells causes altered Golgi morphology and distorted nuclei and a retrograde transport defect between the Golgi and the ER (219). A few studies suggested that the sp1 splice variant of PITPβ is capable of sphingomyelin binding and transport in vitro and inside cells (341, 1605). However, other studies concluded that this feature of the protein might not be critical for its cellular functions (219, 1377). Given its essential role in mammalian organisms, it is generally believed that PITPβ delivers PtdIns for PI kinases and hence is necessary to maintain the PtdIns(4,5)P2 pools available for PLC enzymes (279, 310). However, a direct proof of this concept is still to be obtained.
B. Class II PITPs
The RdgB proteins contain several domains other than the PITP domain: their NH2-terminal PITP domain is followed by an acidic stretch and several hydrophobic domains initially believed to be membrane-spanning segments. The acidic region also includes an FFAT domain that anchors the protein to the ER via binding of VAP proteins. The RdgB proteins also contain a DDHD domain with homology to some of the PLA1 enzymes. Another conserved section, called LNS2, is homologous to that found in lipins, the phosphatidic acid phosphohydrolases. It is located close to the COOH terminus which also was identified as the site of PYK2 interaction (890) (Figure 10). The founding member of this family, the RdgB protein of Drosphila, has a uniquely important role in invertebrate photosignal transdcution. Flies mutated in this gene develop light-induced retinal degeneration and show impaired electric response to light evident in electroretinograms (1636). These defects can be fully remedied by expression of the full-length RdgB protein or its isolated PITP domain, but not by rat PITPα or a chimeric protein with a rat PITPα fused to the rest of Drosophila RdgB (1055). The two mammalian homolgs of RdgB (called RdgBα1, Nir2 or PITPnm1 and RdgBα2, Nir3 or PITPnm2) are also able to rescue the visual defects of the RdgB fly, although Nir2 works better than Nir3 (232). The Drosophila protein is localized to submicrovillar cisternae that are special regions of the ER at the base of the rhabdomeres (1502). It is currently assumed that RdgB is essential for the transfer of PtdIns from the ER to the PM (rhabdomeres) in the fly retina, and its defects interrupt the supply of this lipid to the PM and hence rapidly deplete the PtdIns(4,5)P2 pools that are important for proper PLC-generated signals during light exposure (1575).
Less is known about the mammalian RdgB homologs. In the mouse, Nir2 is ubiquitously expressed, while Nir3 is primarily found in the retina and some regions in the brain (948). Nir3 knockout mice show no obvious phenotype while Nir2 knockout is embryonic lethal (947). At the cellular level, Nir2 is Golgi localized (16, 921) where it helps maintain DG levels (919). However, Nir2 also gets associated with lipid droplets after oleic acid treatment (920), and it changes localization with the cell cyle, being found in the midbody and the cleavage furrow in telophase and anaphase, respectively (921). Functionally speaking, Nir2 was shown to regulate protein export from the ER and contributes to the structural integrity of the ER (39). The interaction of the Nir2 and Nir3 proteins with the ER is mediated via the acidic FFAT domain that is responsible for interaction with the ER-resident VAP-B proteins (39). It is very likely that Nir2 proteins work at membrane contact zones between the ER and the Golgi, and the interaction of Nir2 with PI4KA suggests that it may help deliver PtdIns for this PI 4-kinase to regulate exit of proteins from the Golgi (16). The small RdgBβ protein also having two splice forms is mostly found in the cytosol (480). Although the function of this protein is still unknown, it was shown to interact with the angiotensin II AT1 receptor interacting protein ATRAP with its lipid-binding domain, whereas it interacts with 14-3-3 proteins at its COOH terminus, and this latter interaction affects the rapid degradation of the protein (278, 495). Most recent studies found that RdgBβ is capable of PtdOH binding and hence may function as a PtdOH transfer protein (494).
IX. PHOSPHOINOSITIDES AT THE PLASMA MEMBRANE
It is hard not to notice the hierarchical organization of phosphoinositide distribution between the various membrane compartments. As one moves outward from the nucleus toward the PM, the extent of inositol lipid phosphorylation is increased in the membranes: the ER and nuclear envelope mainly contain PtdIns and the Sac1 phosphatase that localizes to these membranes ensures that any phosphorylation of PtdIns would be properly counteracted. The endosomal compartments, including the Golgi, contain monophosphorylated PtdIns, such as PtdIns4P or PtdIns3P, and have phosphatase enzymes that act both on mono- and bis-phosphorylated PtdIns, such as the MTMs and the 5- or 4-phosphatases. The PM has the bulk of PtdIns(4,5)P2 and PtdIns(3,4,5)P3 and the phosphatases that dephosphorylate them. The one exception to this rule is PtdIns(3,5)P2 that is formed at the multivesicular body, which is an endomembrane compartment. Yet, one can argue that MVB invaginating membranes [where PtdIns(3,5)P2 function is critical] are destined to the lysosome, the content of which can be viewed as “external” to the cell (Figure 11). The existence of the same process supporting the externalization of membranes at the PM (as in the case for virus budding) lends some support to these views (1016). Based on this distribution, PtdIns4P, PtdIns(4,5)P2, and PtdIns(3,4,5)P3 are the major inositol lipids in the PM. PtdIns4P in the PM is used to be considered only as an intermediate of PtdIns(4,5)P2 synthesis, but this simplistic view is increasingly challenged by solid data that suggest that PtdIns4P can support the functions of ion channels (see Ref. 559 and discussions below) and also contributes to the anchoring of lipid-modified targeting sequences that contain polybasic domains (559). We still need to learn more about PM PtdIns4P, but currently PtdIns(4,5)P2 is recognized as the most important inositide at the PM with diverse processes being controlled by changes in its levels (Figure 12). PtdIns(4,5)P2 is the precursor of Ins(1,4,5)P3 and DAG after PLC activation and hence is pivotal for early signaling from cell surface receptors. Second, PtdIns(4,5)P2 is a substrate of class I PI3Ks and the precursor of PtdIns(3,4,5)P3, the latter being critical for growth factor signaling, and also for PM polarization and membrane dynamics. Third, PtdIns(4,5)P2 itself is probably important for the proper operation of ion channels and transproters and plays roles in exo- and endocytosis. These processes will be briefly discussed below.
Figure 11.
Distribution of phosphoinositides in various membrane compartments in a generic cell. The majority of PtdIns(4,5)P2 is located in the plasma membrane (PM), and that is where it is converted to PtdIns(3,4,5)P3. PtdIns4P is also found in the PM, but it is also highly enriched in the Golgi and in some endosomes that are part of the TGN. PtdIns3P is formed on endosomes such as the early endosmes (EE) and their precursors and is converted to PtdIns(3,5)P2 at the level of sorting endosomes (SE) at regions that form the invaginating membranes destined to become luminal in multivesicular bodies (MVB). PtdIns is generated in ER-derived compartments that can reach every other membrane, but the mechanism of lipid transfer between these compartments is still unknown. It is important to note that this distribution does not rule out that each of these lipids can be present in small, undetected amounts in other membrane compartments.
Figure 12.
The multiple functions of PtdIns(4,5)P2 in the plasma membrane. A: this lipid serves as a precursor of the second messengers, Ins(1,4,5)P3 and DG upon PLC activation, or of PtdIns(3,4,5)P3 during PI3K action. It can also be dephosphorylated by 5-phosphatases to PtdIns4P. B: in addition to this “precursor” role, PtdIns(4,5)P2 can 1) regulate the activity of enzymes such as PLCδ1 or PLD; 2) contribute to the recruitment of endocytic clathrin adaptors; 3) anchor the actin cytoskeleton to the PM; 4) help dock secretory vesicles and presynatptic vesicles to the membrane during exocytosis; and 5) maintain or regulate the active conformation of a variety of ion channels and transporters. C: polarized or localized distribution of PtdIns(4,5)P2 and PtdIns(3,4,5)P3 play important roles in establishing and/or maintaining polarity, such as in directional migration, in local events, such as in phagocytosis, or in polarized epithelial cells that form barriers between their luminal and basolateral sides.
A. Canonical PI Signaling Triggered From Cell Surface Receptors
This topic has been in the frontiers of inositide research for some time, receiving enormous coverage and could fill a book easily by itself. This will be summarized only briefly just for the sake of completeness. Studies unraveling the connection between receptor-mediated stimulation of “PI-turnover” and Ca2+ signaling placed phosphoinositides in the center of attention in the early 1980s and generated knowledge that has become part of every textbook on cell physiology. The basic principle of receptor-initiated PLC activation with generation of Ins(1,4,5)P3 and DAG, two very different messenger molecules, has been firmly consolidated (130, 135). Yet, the variations of how many different PLC enzymes can be activated in how many different ways could not have been foreseen even with the wildest imagination (see sect. VII on PLCs). There are a large number of molecules regulated by DAG, starting with numerous PKC isoforms (1270, 1298) and extending beyond them (216, 1306). Similarly, the details of how Ins(1,4,5)P3 releases Ca2+ from the ER via three different types of InsP3 receptors (1050) and the complex regulation of the gating of these receptor/channels by Ca2+ and Ins(1,4,5)P3 (1535) now represents a whole new research field. The same features of the InsP3 receptors underlie Ca2+ waves and oscillations (136). A new chapter in PI signaling from the PM has begun with the identification of PI 3-kinases. Their close association with growth factor and oncogenic signaling has propelled this field and the literature on various isoforms of PI3Ks, and the variety of their activation mechanisms have been overwhelming (see sect. VC). This diversity is only paralleled by the number of effector proteins that bind and are regulated by the 3-phsophorylated PIs. The list includes guanine exchange factors and GTPase activating proteins for a variety of small GTP binding proteins, serine/threonine and soluble tyrosine kinases, and scaffolding proteins that organize signaling complexes. Through these pathways, inositides play pivotal roles in transmembrane signaling of every cell in an organism. These processes have been touched upon in the respective sections describing the enzymes and will not be further discussed here. However, there are several processes regulated by PIs in various membranes that historically are not covered under the canonical PI signaling umbrella but rather belong to membrane trafficking or to the ion channel fields. These are the areas that will be reviewed in some detail in the subsequent sections.
B. Ion Channels and Other Transporters
Early observations already hinted that phosphoinositides can directly influence ion transport pathways. A stimulatory effect of membrane PtdIns(4,5)P2 on PM Ca2+ pump activity was found as early as 1981 (1132), and the sarcoplasmic reticulum Ca2+-ATPase was shown to tightly associate with phosphoinositides (1619). However, the phosphoinositide regulation of ion channels/transporters has been first clearly postulated in the case of Na+/Ca2+ exchanger (NCX1) and KATP potassium channels studied in giant excised patches of the heart (616). In this and many subsequent studies, the lipid regulation was noted in excised patch recordings as a “rundown” of the current with time, which then was reversed by the inclusion of ATP (to resynthesize PPIs) or inositol lipids themselves to the cytosolic face of the membrane. The explanation for this phenomenon was that inositol lipids slowly disappear via dephosphorylation in the excised membrane patches. Most of our knowledge on the direct regulation of ion channels by inositides has originated from studies on excised patches taken from Xenopus oocytes expressing the channels in question (1282). Only recently had it become possible to test the regulation of these channels in intact cells with the aid of drug-induced inositol lipid manipulations (1489, 1617). There is ample literature on the regulation of ion channels and transporters by phosphoinositides (488, 617, 932, 1138, 1285, 1487). Here we only provide a summary of these studies in Table 2, which might be more useful for a quick overview. Interested readers can turn to more detailed reviews on this topic.
Table 2.
Effects of phosphoinositides on ion channels and transporters
| Channel | Ins Lipid Regulator | Reference Nos. |
|---|---|---|
| Voltage-gated Ca2+ channels | ||
| T-type channels | ??? | No reports |
| L-type channels | PtdIns(4,5)P2 (+) | 1038, 1490, 1491, 1815 |
| N-type channels | PtdIns(4,5)P2 (+) | 487, 865 |
| P/Q-type channels | PtdIns(4,5)P2(±) | 1302, 1735 |
| TRP channels | ||
| TrpL (Drosophila)* | PtdIns(4,5)P2(−) | 410, but (+) 662 |
| TrpC1 | PtdIns(4,5)P2(+) | 1331 |
| TrpC3 | PtdIns(4,5)P2(+) | 883 |
| TrpC4a | PtdIns(4,5)P2(−) | 1180 |
| TrpC5 | PtdIns(4,5)P2(±) | 782, 1573 |
| TrpC6 | PtdIns(4,5)P2(+) | 883, but (−) 27, 742 |
| TrpC7 | PtdIns(4,5)P2(+) | 883, but (−) 742 |
| TrpM2 | PtdIns(4,5)P2(+) | 1568 |
| TrpM4 | PtdIns(4,5)P2(+) | 1137, 1811 |
| TrpM5 | PtdIns(4,5)P2(+) | 923, 1284 |
| TrpM6 | PtdIns(4,5)P2(+) | 1745 |
| TrpM7 | PtdIns(4,5)P2(+) | 1312, but see 850, 1515 |
| TrpM8 | PtdIns(4,5)P2(+) | 319, 922, 1284 |
| TrpV1** | PtdIns(4,5)P2, PtdIns4P (±) | 257, 559, 951, 1587 |
| TrpV2 | PtdIns(4,5)P2(+) | 1033 |
| TrpV3 | PtdIns(4,5)P2(−) | 365 |
| TrpV5 | PtdIns(4,5)P2(+) | 869, 1284 |
| TrpV6 | PtdIns(4,5)P2(+) | 1556, 1789 |
| TrpA1 | PtdIns(4,5)P2(−) | 783, but (+) 761 |
| TrpP2 | PtdIns(4,5)P2(−) | 961 |
| Cyclic nucleotide-gated channels | ||
| Hyperpolarization-activated HCN2 channels | PtdIns(4,5)P2(+) | 1220, 1826 |
| Cyclic nucleotide-gated (CNG) | PtdIns(3,4,5)P3 (−) | 177, 181, 1724, 1793 |
| ICRAC/Orai1 channels | PtdIns4P ???(+) | 184, 820 |
| Potassium channels | ||
| ROMK1 (Kir1.1) | PtdIns(4,5)P2(+) | 661, 915 |
| IRKs (Kir2.x) | PtdIns(4,5)P2(+) | 807, 1281, 1283, 1795 |
| GIRK (Kir3.x) | PtdIns(4,5)P2(+) | 661, 807, 1495 |
| KATP (Kir6.x) | PtdIns(4,5)P2, PtdIns(3,4,5)P3, PtdIns(3,4)P2 | 423, 616, 1283 |
| Two pore | PtdIns(4,5)P2(+) | 937, but see 245, 911 |
| Voltage-gated (Kv1.3) | PtdIns(4,5)P2, PtdIns(3,4,5)P3 (−) | 1012 |
| M-type (KCNQ2,3,5) | PtdIns(4,5)P2(+) | 422, 1488, 1794 |
| BK (Ca2+ activated) | PtdIns(4,5)P2(+) | 1598 |
| Ion exchangers and transporters | ||
| Na+/Ca2+ exchanger | PtdIns(4,5)P2(+) | 58, 588, 616, 1763 |
| Na+/H+ exchanger | PtdIns(4,5)P2(+) | 12 |
| Na+/HCO3− cotransporter | PtdIns(4,5)P2(+) | 1734 |
| Epithelial sodium channel (ENaC) | PtdIns(4,5)P2, PtdIns(3,4,5)P3 (+) | 960, 1229, 1230, 1564, 1787 |
| CFTR | PtdIns(4,5)P2(+) | 619 |
| Ion pumps | ||
| PM Ca2+-ATPase | PtdIns(4,5)P2(+) | 249, 438, 1132, 1192 |
| Ligand-gated channels | ||
| P2X1 | PtdIns(4,5)P2(+) | 126 |
| P2X2 | PtdInsP, PtdIns(4,5)P2(+) | 474 |
| P2X2/3 | PtdIns(4,5)P2, PtdIns(3,4,5)P3 (+) | 1074 |
| P2X4 | PtdIns(4,5)P2, PtdIns(3,4,5)P3 (+) | 126 |
| P2X5 | PtdIns(4,5)P2(+) | 127 |
| P2X7 | PtdIns(4,5)P2(+) | 127, 291 |
| NMDA | PtdIns(4,5)P2 (+) | 977 |
| NR1/NR2 | PtdIns(4,5)P2 (+) | 1037 |
The Drosophila TrpL channels are stimulated after PLC activation, but the signal responsible for the stimulation is highly debated. DAG and polyunsaturated fatty acids (PUFA) have been the most accepted stimuli (573), and in some cases it is speculated that the PtdIns(4,5)P2 decrease and proton accumulation contributes to their activation (662). However, several data indicate that these channels still need PtdIns(4,5)P2 for optimal activity.
TrpV1 channels also called vanilloid receptors that respond to heat and capsaicin (224) are strongly sensitized by bradykinin and other PLC activators, and it was suggested that PtdIns(4,5)P2 breakdown relieves a tonic inhibition of these channels by PtdIns(4,5)P2 (257). However, the lipid regulation of these channels appears to be more complicated as they need phosphoinositides for their activity, and the effects of PtdIns(4,5)P2 also depend on the extent of stimulation by capsaicin (951, 1286, 1587) or association with other proteins (728, 780) and PtdIns4P may also support their activities (559, 951).
Calcium signaling has always been very closely linked to PLC-mediated phosphoinositide breakdown, namely through InsP3-induced Ca2+ release from the ER. The enormous progress concerning the details of InsP3-induced Ca2+ signals will not be discussed here but have been the subject of numerous great reviews (129, 1050, 1051, 1534). However, less is known about how phosphoinositides control Ca2+ influx, a topic I will dicuss in some detail.
1. Store-operated Ca2+ entry (SOCE)
It was recognized in the late 1970s and early 1980s that Ca2+ influx increases after stimulation of so-called “Ca2+-mobilizing receptors,” which led James Putney to postulate a Ca2+ entry pathway that is activated by emptying the ER Ca2+ stores (capacitative Ca2+ entry) and thus currently named store-operated Ca2+ entry (SOCE) (1242). The molecular entities that constitute this pathway had been enigmatic for a long period, with the nonspecific cation Trp (transient receptor potential) channels taking center stage (1193). The breakthrough in this field came when large-scale small interfering RNA (RNAi) studies identified the STIM1 protein as the ER Ca2+ sensor necessary for SOCE (916, 1296). This was followed within a year by the discovery of the Orai1 protein as the channel component of ICRAC, the highly Ca2+-selective current representing the major form of SOCE (435, 1635, 1802). A lot of progress has been made in understanding the mechanism of STIM1-Orai1 activation during ER Ca2+-store depletion covered by several recent reviews (345, 896, 1427). For this summary it is enough to say that the ER-resident single transmembrane STIM1 protein has a luminal Ca2+-sensing EF hand domain that triggers the oligomerization of the protein upon Ca2+ unbinding. This, in turn, induces a conformational change in the cytoplasmic aspect of STIM1 allowing it to interact and activate the Orai1 channels at junctions where the ER and the PM are in close proximity. The connection of this form of Ca2+ entry to inositol lipids was first noted when Wm was shown to inhibit the ICRAC channels, and curiously the inhibition seemed to be related to changes in PtdIns4P rather than PtdIns(4,5)P2 (184). Since STIM1 has a polybasic tail at its very COOH terminus, it has been suggested that it interacts with the acidic phospholipids possibly with PtdIns(4,5)P2 in the PM, a view now generally accepted (217, 1664). In contrasting findings, PtdIns(4,5)P2 was found not to play a significant role in STIM1 movements or activation of Orai1, and instead, PtdIns4P seemed to be implicated in the regulation of Orai1 itself (820, 1618). More studies will clarify which, if any, of these inositides and by what mechanism regulate this important Ca2+ entry pathway.
2. Voltage-regulated Ca2+ entry
The old classification that “excitable” cells contain voltage-gated Ca2+ channels and “nonexcitable” cells use SOCE has now become outdated, since most excitable cells do contain channels formerly thought to operate in nonexcitable cells (e.g., CRAC and Trp channels) and, conversely, many cells formerly considered nonexcitable do have Ca2+ entry channels that are regulated by voltage changes (e.g., most endocrine cells). As detailed in the next paragraph, a great number of ion channels and transporters require phosphoinositides for proper function or are even regulated by physiological changes in the PM levels of these lipids, most notably PtdIns(4,5)P2 or PtdIns4P. These can have direct effects on Ca2+ entry when the affected channels conduct Ca2+, but they can also indirectly influence Ca2+ influx if they change the membrane potential through modification of potassium channels or chloride channels. Membrane potential changes not only influence the gating of channels (such as the voltage-gated Ca2+ or Na+ channels), they also change the electrical driving force for Ca2+ (as in the case for CRAC channels).
3. Phosphoinositides: regulators or permissive factors for channels?
An important question often debated about inositol lipids and channel/transporter function is whether the lipids are permissive for optimal function of the proteins, or indeed are regulatory within the range of inositol lipid changes that occur under physiological conditions. One can easily envision that highly acidic inositol lipids in the inner leaflet of the PM engage positive residues at the cytoplasmic aspect of the TM helices of channels, thereby serving as structural reference points keeping the protein in the right conformation. This would ensure that channels are not active during their transport in the internal membranes and become functionally competent only in the PM. On the other hand, it has also been shown convincingly that PtdIns(4,5)P2 changes evoked by agonist stimulation are faithfully followed by changes in the activity of several potassium channels to the extent that these channels are used as “PtdIns(4,5)P2 reporters” (422). It is worth pointing out, however, that we often use very high supraphysiological concentrations of agonists in our cellular studies and at normal level of receptor occupancy the overall lipid changes in the PM may not be very significant. Whether a channel responds to small changes in phosphoinositides also depends on the binding affinity of the channel to the lipid; in fact, the activity of a channel with very high affinity to an inositide may depend on this lipid, yet even large lipid changes will not cause a change in channel activity.
The molecular mechanisms by which inositol lipids can control ion channels mostly remain elusive. Almost all ion channels and transporters contain clusters of basic residues in their membrane-adjacent regions facing the cytosol or within their COOH-terminal tails. For example, inositol lipid regulation was mapped to the TRP domains of the cytoplasmic tails of TrpM8 channels (1138, 1284). An interesting and common feature of the PtdIns(4,5)P2 regulation of many potassium and TRP channels is that the lipid alters the interaction of the channels with other specific regulators such as calmodulin (843), βγ-subunits (661), or ligands such as ATP (113). In addition, indirect regulation of channels via inositide-binding and channel-interacting proteins is also possible as suggested for Pirt, a molecule that interacts with TRPV1 channels and also binds phosphoinositides (780), or AKAP150, an A-kinase anchor protein that associates with TrpV1 (728). Two recent reports on the structures of two closely related Kir channels bound to PtdIns(4,5)P2 began to shed light on the structural basis of lipid regulation of these channels: in the case of the Kir2.2 K+ channel, PtdIns(4,5)P2 binds at an interface between the transmembrane domain (TMD) and the cytoplasmic domain of the channel such that the fatty acid chains interact with the TMD and the phosphorylated headgroup with the cytoplasmic domain bringing the latter closer to the membrane and helping to open the inner gate (566). In the case of the GIRK2 channel, PtdIns(4,5)P2 occupies a similar position at the interface between the TMD and the cytoplasmic domain. However, here the position of the cytoplasmic domain already being closer to the inner membrane interface is not affected dramatically with lipid binding. Instead, PtdIns(4,5)P2 has a more profound effect on the opening of the inner gates in a mutant channel that mimics the conformation of the Gβγ-activated state (1715). In other words, the lipid had an important effect on G protein-induced activation, consistent with previous conclusions based on functional analysis (661, 1795).
Another form of channel regulation by inositol lipids is the stimulated insertion of the channel from intracellular inactive compartments to the PM. This process is usually regulated by PtdIns(3,4,5)P3 made by PI 3-kinases, such as reported for L-type Ca2+ channels (1633) or for the maintenance of AMPA receptors at the postsynaptic density (54). Since this form of regulation is essentially a trafficking process, it will not be further discussed here.
C. Exocytosis
The earliest indications that phosphoinositides may be important for membrane fusion came from studies on dense core vesicle (DCV) exocytosis, where it was recognized that phosphoinositides were important for priming exocytic vesicles (395, 582, 583). In the process of regulated secretion, DCVs undergo maturation that increases their competence to dock and fuse with the PM when a rapid rise in Ca2+ concentration triggers the fusion process. Some of the mature granules under the membrane will dock to the PM, but these predocked vesicles still have to undergo “priming” to become the “readily releasable pool” that is first fused upon stimulation (312, 715, 1191). During isolation of cytosolic factors needed for “priming” of DCVs, the mammalian PITP and PIP 5-kinase proteins have been identified (582, 583). Moreover, it was shown that PLC-mediated PtdIns(4,5)P2 hydrolysis was necessary for exocytosis to take place (558, 1707, 1708). These observations then led to the notion that PtdIns(4,5)P2 is an obligatory factor required for generating competent exocytic vesicles, and increased PtdIns(4,5)P2 turnover promotes Ca2+-triggered fusion of vesicles with the PM (643). However, for a long period, the question of whether PtdIns(4,5)P2 was necessary for priming at the PM membrane or at the surface of the exocytic vesicle has not been clarified. In the last 10 years or so, several proteins relevant to exocytosis have been found to bind and to be possibly regulated by PtdIns(4,5)P2 (see Table 3). There are other proteins important for exocytosis that bind phospholipids, such as the DOC2 protein that supports slow asynchronous exocytosis (1762). However, DOC2 binds PtdSer via its C2 domain but does not bind phosphoinositides.
Table 3.
PtdIns(4,5)P2-interacting proteins related to exocytosis
It is not clear at present how PtdIns(4,5)P2 binding to any of these proteins regulate the exocytic process. The best studied of these is the Ca2+-sensitive phosphoinositide-binding regulator protein of exocytosis CAPS (46, 1313). This protein acts at a Ca2+-dependent prefusion step that is rate limiting and which requires the ATP-dependent priming of the DCVs (530) to promote the assembly of SNARE complexes (718, 719). CAPS requires PtdIns(4,5)P2 at the PM side for its activity (718), and it has a central PH domain capable of binding PtdIns(4,5)P2 and essential for CAPS' ability to facilitate exocytosis (529). Although there have been some ambiguities whether PtdIns(4,5)P2 binding of the PH domain is central to the function of the CAPS protein (529), the current view is that PtdIns(4,5)P2 at the PM supports CAPS function via interaction with the CAPS PH domain (778). Single-cell analysis of exocytic events in bovine chromaffin cells also concluded that PM PtdIns(4,5)P2 was the relevant lipid that determined the size of the readily releasable pool (1056). With the use of reporters for PtdIns(4,5)P2, such as PLCδ1PH-GFP or fluorescent neomycin analogs, it was possible to show that there is an increased concentration of PtdIns(4,5)P2 at the sites of exocytosis (258, 551, 643, 718, 1056). A recent study found that PtdIns(4,5)P2 binding to synaptotagmin-I increases the Ca2+ binding affinity of the protein 40-fold (1602), providing yet another mechanism to promote the fusion process. It would be of interest to know which PI and PIP kinase(s) contribute to the generation of PtdIns(4,5)P2 at the PM during exocytosis. PIP5Kγ is the enzyme that is important for synaptic vesicle exocytosis in the brain (1704) and is also critical in DCV exocytosis in neuroendocrine cells (520). Less clear is the identity of the PI4K enzyme. Isolated chromaffin granules were found to contain a PAO-sensitive PI4K (1716), and PAO-sensitive, type III PI4Ks were found to be crucial to determine the size of the readily releasable pool of exocytic vesicles in pancreatic beta cells (1168). However, the PI4K purified from chromaffin granules turned out to be the type IIα PI4K enzyme (108) that is not particularly PAO sensitive (83). In a number of studies, secretion was inhibited by Wm or LY294002 at higher concentrations, which would be consistent with inhibition of type III PI4Ks (492, 1256, 1480, 1682). Several data suggest that PI4KΒ is important for regulated exocytosis: neuronal calcium sensor 1 (NCS-1), a homolog of the Drosophila and yeast frequenin, which interacts with PI4KB (see sect. V), increases glucose-induced insulin secretion by increasing the readily releasable vesicular pool in pancreatic beta cells, an effect that requires PI4KB (531). In another study, reducing the level of PI4KB with RNA interference inhibited nutrient-induced insulin secretion (1683). Since NCS-1 was discovered as a protein regulating synaptic development and plasticity (reviewed in Ref. 615), it is a reasonable assumption that PI4KB also functions in the genesis, transport, and/or exocytosis of synaptic vesicles (1702).
The role of PI3Ks in exocytosis and secretion has also been explored. PI 3-kinase inhibition was found to increase exocytosis in adrenal chromaffin cells (1056, 1701) or insulin-secreting beta cells (411, 1790) apparently due to a transient increase in PtdIns(4,5)P2 level. In contrast, a significant amount of literature documents the need for PI3K in secretion from immune cells, most notably for histamine release from mast cells following FcR stimulation (997, 1164, 1207, 1761). However, in this case, PI3K, specifically the PI3Kδ (30) and PI3Kγ isoforms (157, 847), act as important amplifiers to control PLCγ activation, and hence, Ca2+ signaling and PtdIns(3,4,5)P3 may not be directly involved in the control of the exocytic machinery (30, 157, 847). Recent studies implicated PI3K-C2α in the control of exocytosis of insulin-containing vesicles in pancreatic β-cells (370, 879) and neurosecretory cells (1034, 1700). It should be remembered that PI 3-kinase inhibitors can also indirectly affect secretion by altering the various Ca2+ influx pathways (see above) or actin dynamics as shown in pancreatic β-cells (282, 1222).
D. Endocytosis
Most cell surface molecules undergo endocytosis and recycling, a process that ensures delivery of nutrients to the cell interior, and one that controls receptor number at the cell surface and also serves as a quality control mechanism. There are a number of forms of endocytosis, such as the clathrin-mediated and clathrin-independent endocytosis and endocytosis via caveolae as described in many detailed reviews (366, 655, 1019). The most studied and hence understood form of endocytosis is mediated by clathrin using a process that requires the participation of a great number of proteins that form the clathrin lattice and bring cargo molecules to this structure (1019, 1243, 1537). Endocytosis is also regulated by inositol lipids at several biochemical steps along the formation, maturation, and fission of the endocytic particle. One of the first indications that inositides have to do with endocytosis was the finding that the tetrameric clathrin adaptor protein AP-2, a key component of the clathrin-mediated endocytic machinery, was a “receptor” for InsP6 (1639). Subsequently, it was shown that the natural binding partner of this protein is PtdIns(4,5)P2 (482, 1288). Studies on synaptic vesicles revealed that the PtdIns(4,5)P2 5-phosphatase synaptojanin (981, 1022) and the PtdIns4P 5-kinase PIP5Kγ (352, 1704) are crucial regulators of synaptic vesicle cycling and neurotransmission (1702). Recent studies using rapid depletion of PM PtdIns(4,5)P2 in intact cells have unequivocally proven that clathrin-mediated endocytosis (CME) of nutrient receptors and GPCRs require PtdIns(4,5)P2 (2, 1569, 1617, 1831). Moreover, Arf6-regulated non-clathrin-mediated endocytic pathways also depend on phosphoinositides, although in this case the molecular identities of the lipid-dependent steps are less clear (17, 189, 646).
It has been shown that endocytic vesicles normally lose PtdIns(4,5)P2 by 5-dephosphorylation during separation from the PM, and if they do not, they will be coated with actin and accumulate and aggregate in the cell interior (e.g., Ref. 189). This observation fits with the notion that PtdIns(4,5)P2 is an identity code for PM. However, as more and more fine details are emerging on the hierarchy of the molecular events that control CME, it appears that several rounds of dephosphorylation and rephosphorylation cycles of PtdIns(4,5)P2 are necessary to maintain the flow of sequential steps in the initiation, maturation, invagination, and fission of internalizing vesicles (48). Moreover, in these different steps, distinct PtdIns4P 5-kinases and PtdIns(4,5)P2 5-phosphatases may have specific roles. Overexpression or knockdown of PIP5Kα or -β were shown to alter internalization of transferrin or EGF receptors (106, 1185). At the same time, several 5-phosphatases, such as SHIP1 (1115), OCRL (251, 988), and Sjn1 (1209), were all found to associate with components of the clathrin-coated pits (CCPs). A detailed TIRF analysis of different steps in CME during manipulation of PM PtdIns(4,5)P2 found no recruitment of PIP 5-kinases to CCP, yet their overexpression increased the number and size of CCPs (433). In the same study, several 5-phosphatases did show association with CCPs (although to significantly different extent and not to all CCPs), and the most important of these, Sjn1 inhibited the initiation and stabilization of early CCPs, but promoted the maturation of CCVs at later stages (433).
So how does PtdIns(4,5)P2 regulate endocytosis? Several proteins of the endocytic pathways bind phosphoinositides, primarily PtdIns(4,5)P2 with low affinity (see Table 4). Lipid binding contributes to the recruitment of such proteins to the PM. Most of these molecules are adaptor proteins that link cargo to clathrin, but they also include proteins that contain BAR (Bin-Amphiphysin-Rsv), F-BAR, or I-BAR domains (466, 1520, 1606). The latter three domains consist of dimers of long helical segments that generate a rigid banana shape with radiuses that differ between the three families. They have positive residues on the concave (BAR and F-BAR) or convex (I-BAR) surface that interact with acidic phospholipids in curved membranes (1211) and also have membrane-bending and curvature-generating activities (465). Some BAR domain-containing proteins also possess other phosphoinositide binding modules, such as PX domains that are found in a group of sortin nexin (SNX-BAR) proteins (304). Most of these proteins play important roles in tubulation and scission at different endomembranes (see below), but SNX9, in particular, is important during the formation of endocytic tubes (1437, 1537, 1765) and also interacts with dynamin 1 and N-WASP, a protein that controls actin polymerization (1402). Dynamin is the GTPase that is essential for the scission of the endocytic vesicle, and its PtdIns(4,5)P2 binding PH domain is critical for its function (1362). Although the PH domain of dynamin has low affinity to PtdIns(4,5)P2, the assembling dynamin molecules on the neck of endocytic vesicles form a ring of multiple lipid binding modules (1475, 1506, 1816). How PtdIns(4,5)P2 affects dynamin is not fully understood, but it can recruit the molecule to CCPs, and it also increases dynamin's GTPase activity (1816). It is clear, however, that dynamin interacts with a host of other proteins in the forming of endocytic vesicles via its other domains, and it controls not only the last step of CCPs life, i.e., its scission from the PM, but also regulates early steps during the formation and maturation of CCPs (see Refs. 432, 1362 for recent reviews on dynamin).
Table 4.
PtdIns(4,5)P2-interacting proteins related to endocytosis
E. Plasma Membrane Polarization
Most cells in an organism develop a lateral organization of their PM with significant differences in membrane compositions and functions as they make contacts with other cells and form tissues and organs. The best examples are epithelial cells that have apical and basolateral membranes or neurons with axons and dendrites with distinct features. Even solitary cells of the innate and adaptive immune system show significant polarization as cells chemotax and phagocytose or communicate with one another. Cell migration and the invasive properties of cancer cells also depend on cell polarization and make all the difference between curable and deadly forms or stages of the disease. In all of these processes phosphoinositides play fundamental roles in multiple ways. There are three areas that will be discussed in some detail: chemotaxis, phagocytosis, and the organization of polarized cells as they best show the principles that apply to many other polarization processes.
1. Chemotaxis
A significant portion of our knowledge came from studies using the model species Dictyostelium discoideum, a unicellular organism that responds with chemotaxis to cAMP signals originating from neighboring cells (reviewed at great detail in Refs. 206, 815, 1505, 1691). How cells sense and respond to a chemotactic gradient are exciting problems since the concentration difference between the cells leading and trailing edges is extremely low. This makes a tiny difference in signaling strength when considering the dose-response relationship of the chemotactic receptors (usually GPCRs) and their G protein activation. These small differences have to be amplified for the cell to be able to respond with robust polarization. Accordingly, it has been found that distribution of GPCRs or their G protein activation do not show prominent polarization (735, 1744). However, it was recognized for the first time in Dictyostelium that the PI3K product PtdIns(3,4,5)P3 localizes prominently to the leading edge of the cell (373, 666, 1026). Deletion of the three PI3Ks in Dictyostelium indeed compromises although does not eliminate the ability of cells to respond to chemotactic stimuli (1518). Importantly, the PtdIns(3,4,5)P3 phosphatase PTEN was found to localize to the trailing end of the cell (677), and its deletion increases overall PtdIns(3,4,5)P3 levels and dissipates the PtdIns(3,4,5)P3 gradient interfering with efficient chemotaxis (677). Since PtdIns(4,5)P2 binding of PTEN is important for PTEN localization (678), a decreased PtdIns(4,5)P2 level at the leading edge because of the active PI3Ks favors PTEN accumulation at the trailing end. PLC activity can also modify the distribution of PtdIns(4,5)P2 and PTEN, affecting the PtdIns(3,4,5)P3 gradient (771). These observations together led to the suggestion that localized stimulation (by PI3Ks) together with a global inhibition (by PTEN) form the basis of the amplification of gradient sensing in which the lipid PtdIns(3,4,5)P3 has a central role (815, 1505, 1626). Several other molecules also show polarized distribution in Dictyostelium. These include activated RasC and RasG; the TorC2 complex; a number of downstream PtdIns(3,4,5)P3 effectors that contain PH domains, such as Crac, PKBA, and PhdA (1505); and several recently identified proteins with unknown function (1801). RasG acivates PI3Ks, while RasC is is linked to TorC2, and it is part of the regulatory loops that control chemotaxis (205, 1342).
Very similar principles were found to drive the chemotactic responses of mammalian cells, such as neutrophils and macrophages (1463, 1691). Accumulation of PtdIns(3,4,5)P3 in the leading edge of differentiated HL60 cells have been described parallel to the findings in Dictyostelium (1385) and confirmed in many subsequent studies (430, 1667) including mouse neutrophils (1140). In this case, however, there are some differences in the players that form the positive and negative feedback loops in building up the PtdIns(3,4,5)P3 gradient. In mouse neutrophils, it was shown that SHIP1 rather than PTEN is important to serve as the negative regulator that keeps overall PtdIns(3,4,5)P3 levels down (1140), and that G βγ (1497) subunits liberated by the activated chemotactic receptors together with Ras (1342, 1497) strongly stimulate PI3Kγ at the leading edge of the cell. It has also been shown that a positive feedback loop between PtdIns(3,4,5)P3 and the small GTPases, Rho, Rac and Cdc42 and actin polymerization also contributes to the sharp polarization (1441, 1667, 1693). An additional positive feedback is provided by the secondary recruitment and activation of class IA PI3Ks by PI3Kγ-mediated increase in PtdIns(3,4,5)P3 (285, 1131). Although PTEN may not be the major phosphatase to influence the PtdIns(3,4,5)P3 gradient, it has been shown that PTEN −/− neutrophils are easily “distracted” and cannot properly “prioritize” their chemotactic cues (591). The complexity of the various ways PtdIns(3,4,5)P3 influences chemotaxis, and migration is demonstrated by the enhanced transendothelial migratory ability of PTEN (−/−) neutrophils which is counterintuitive to the concept of too much PtdIns(3,4,5)P3 causing a problem with directional sensing (904, 1341). This is related to the fact that PtdIns(3,4,5)P3 not only controls the polarization and amplifies the directional sensing of the cells, but also affects their adhesion, rolling, and motility, via other effectors including integrins and the actin cytoskeleton (1463) (and see below). It is important to note, however, that PtdIns(3,4,5)P3 is not the molecule that sets up the direction, as chemotaxis in Dictyostelium cells still occurs without PI3Ks (45, 628) and neutrophil cells will still migrate toward gradients without PI3Kγ (430, 1140). Nevertheless, PtdIns(3,4,5)P3 is definitely a signal that amplifies and increases the precision of directional sensing, and engages the mechanisms that move cells along chemotactic gradients. There have been a number of proteins identified in neutrophils that are regulated by PtdIns(3,4,5)P3 (827). These included serine/threonine kinases, such as PDK-1, mTORC2, and Akt/PKB; the tyrosine kinase Btk; GAP and GEF proteins for Arf; Cdc42; Rho and Rac; and cytoskeletal proteins such as WAVE2 and ezrin (827, 1463). Moreover, motor proteins, such as myosin X that has three PH domains (one is a split one), can bind PtdIns(3,4,5)P3 and be recruited or conformationally regulated by this lipid species (293, 950, 1228, 1591). All these proteins mediate a different aspect of the migrational response, and it is very likely that they may have their own dedicated source of PtdIns(3,4,5)P3 produced at different phases of the cell movement.
2. Phagocytosis
Phagocytosis is a critically important process for innate immunity and for clearance of cell debris during inflammation and tissue remodeling (6, 1821). It is one of the most spectacular membrane polarization events that one can observe under the microscope. Particles or cells to be phagocytosed initially attach to the cell surface, mostly via FcγRs or CR3 complement receptors (1592). This binding initiates a whole set of signaling events that induce the raising of the PM around the object (called pseudopods) until this membrane ring is high enough to close above the particle thereby engulfing it. The engulfed particle surrounded by a membrane then undergoes maturation upon which various endocytic compartments fuse with the phagosome lowering its pH, changing its membrane composition, and supplying degradative enzymes that eventually degrade the particle. This complex coordinated membrane deformation sequence is associated with characteristic changes in membrane phosphoinositides at the different stages of the phagocytic process (1766). After the particle is bound to the PM, increased synthesis of PtdIns(4,5)P2 was found to occur during formation of the phagocytic cup due to the recruitment and activation of PIPKIα (172). However, PtdIns(4,5)P2 suddenly disappears upon sealing of the phagosome caused partially by its PLCγ-mediated hydrolysis (172), and this latter step is essential for proper particle ingestion (1370). The PtdIns(4,5)P2 decrease is the result of several additional processes: a drop in the surface charge of the formed phagosome releases PIPKIα (1767), PtdIns(4,5)P2 is consumed by PI3Ks that form PtdIns(3,4,5)P3 on the bottom of the phagocytic cup as the pseudopod is raising (295, 1000, 1094), and several phosphatases that act both on PtdIns(4,5)P2, such as OCRL and INPP5B (155), or on PtdIns(3,4,5)P3, such as SHIP (14, 294) and Pharbin (649) are recruited to the phagosome. The sequential recruitment of the multiple inositol lipid metabolizing enzymes and the resulting localized and temporally organized spectacular changes in the various PI species during phagocytic membrane remodeling raises the question of what importance these lipid changes have and how they participate in the organization of this process.
As for PtdIns(4,5)P2, the changes in this lipid correlate very closely with the formation of actin during the pseudopod extension and with actin disappearance after particle engulfment (1370). The different type I PIP kinases appear to have subtle and distinct roles in the process: deletion of PIPKIγ from bone marrow-derived macrophages yields defective attachment of IgG-coated particles to the cells (989). These cells have increased amount of highly polymerized actin and fail to cluster their FcγR upon engagement (989). They also show enhanced RhoA and reduced Rac1 activation. In contrast, PIPKIα deletion inhibits the ingestion process and prevents activation of WASP and actin polymerization without altering attachment (989). The connection between PtdIns(4,5)P2 and actin dynamics has long been appreciated not only in phagocytosis but in virtually any membrane deforming events, including chemotaxis (1505, 1691), endocytosis (1320, 1768, 1800), and exocytosis (1574, 1681). Several actin capping and severing proteins were shown to bind PtdIns(4,5)P2, and therefore, the lipid can affect actin polymerization in a number of ways. It can dissociate the capping protein gelsolin from the (+) end of actin (720, 721), it can increase actin nucleation by the Arp2/3 complex via binding to the N-WASP protein (1048, 1287), and it can inhibit the actin-severing activities of cofilin and the actin depolimerizing factor ADF (1162, 1772, 1812). Profilin and α-actinin, two additional proteins promoting actin assembly and crosslinking, are also regulated by PtdIns(4,5)P2 (237, 516, 1412). Moreover, several actin bindin proteins containing FERM (four-point one-ezrin-radixin-moesin) domains contain PtdIns(4,5)P2 binding modules, and their PM association is determined by lipid binding to these domains (255, 556, 982). Hydrolysis of PtdIns(4,5)P2 by PLCγ is necessary for completion of phagosome formation (172, 1370). This not only allows the drop in PtdIns(4,5)P2 levels and hence facilitates actin disassembly, but also generates DAG to activate other downstream effectors, such as PKCϵ (238, 859) and Ras/Rap1 (171).
PI 3-kinase inhibition by Wm or LY294002 inhibits phagocytosis of large (>3 μm) particles but has a relatively small effect on the smaller ones (50, 295). Yet, localized prominent PtdIns(3,4,5)P3 increases in the phagocytic cup have been observed (750, 1000). A recent study found a close connection between PI3K activation and Cdc42 cycling during development of the phagosome. Cdc42, which promotes actin polymerization, also activates PI3Ks in the phagocytic cup; the 3-phosphorylated lipids, in turn, inactivate Cdc42 allowing actin depolimerization and recycling, which is important for phagosome formation (115). Relatively little is known about which PI3K isoform(s) are responsible for the generation of PtdIns(3,4,5)P3, but the class IA enzyme, via recruitment through p85 adaptors, is believed to be the most important for this process (750). In contrast, PI3Kγ, which is abundant in phagocytic cells, plays little if any role in phagocytosis of opsonized particles (1740). However, the role of the PI3K subclasses can be more specialized, as recent studies found that phagocytosis of the parasite Leishmania mexicana is strongly impaired in PI3Kγ-deficient cells or by specific inhibitors of this PI3K isoform (308). As for PtdIns(3,4,5)P3 effectors, the small GTP binding proteins of the Rho/Rac family have well-established roles in phagocytosis (215, 648) and GEFs for these proteins, such as VAV or the Ras GEF protein SOS, are activated by PtdIns(3,4,5)P3 (322, 696, 1588, 1624). PtdIns(3,4,5)P3 also can recruit protein kinases, such as Akt and Btk, and while the former is not desirable and hence being limited by several mechanisms (155), the latter serves as a positive regulator of PLCγ activation and helps complete phagosome formation (42, 738).
Recruitment of PI 5-phosphatases that act upon PtdIns(4,5)P2 and PtdIns(3,4,5)P3 is necessary to terminate the processes described above, and prepare the completely ingested phagosome to its intracellular jurney and maturation. It is not clear why phagocytic cells need four different 5-phosphatases [OCRL, INPP5B (155), SHIP (14, 294), and Pharbin (649)], but it is likely that each of these is specialized to disassamble specific protein-lipid signaling complexes. Accordingly, Pharbin (INPP5E) inhibited FcγR-mediated, but not CR3 complement receptor-mediated phagocytosis, while SHIP1 was found to be more important for CR3-mediated phagosome fromation (649). OCRL and INPP5B, on the other hand, were important at a later stage to limit Akt activation in the already ingested phagosome (155). The internalized phagosome is then mostly stripped of PtdIns(4,5)P2 and PtdIns(3,4,5)P3, and the major inositol lipid associated with it is PtdIns3P generated by the class III PI3K and possibly PtdIns4P, the product of PtdIns(4,5)P2 dephosphorylation but also produced by type II PI4ks (156). In this regard, the limiting membrane of the phagosome is similar to that of other endocytic vesicles. There is, however, an interesting recent observation that suggests that even the endocytosed phagocytic vacuole has a small amount of PtdIns(4,5)P2 and PtdIns(3,4,5)P3 associated with it, which helps generate actin comets that propel the vacuoles inside the cells. This study suggested the class III PI3K via generation of PtdIns3P helped dislodge Inpp5B from the nascent phagosome and allow class I PI3Ks to generate PtdIns(3,4,5)P3 (156). The subsequent fate of the maturing phagosome is a complex process involving a number of Rab proteins and the PI lipids PtdIns3P and PtdIns(3,5)P2. Although phagosome maturation has its unique features reviewed recently (446), from a PI lipid standpoint, it is not too dissimilar to other endocytic processes that will be discussed below.
An important trivial question rarely asked is what keeps these very local lipid gradients from spreading given the rapid rate of lateral diffusion of PIs within the PM. Such gradients can be maintained only if the lipid production is highly concentrated at these sites against a high background of degradative activity. Alternatively, and probably complementary to the former, the engagement of the lipid by protein binding partners could shield the lipids from degradation. To answer these questions, one would need to know the diffusion speed of lipids when they are free and when proteins are bound to them, or the average time a specific protein module spends bound to a PI species. A few studies have addressed these issues using fluorescent tools in living cells (518, 561, 840). They demonstrated that lipids indeed diffuse rapidly, so there is a need for localized synthesis/degradation or physical diffusion barriers, to maintain gradients. Second, protein effectors interact only transiently and can only act over micrometer distances. Recent studies using similar techniques found that significant diffusion barriers exist around the phagocytic cup keeping PtdIns(4,5)P2 in place (517).
3. Epithelial cell polarity
The classical example of membrane polarization is found in epithelial cells that form a barrier separating the inner millieu from the external world or line cavities in multicellular organisms. In the classical case, these cells have apical and basolateral membranes separated by tight junctions that link the cells together and the protein and lipid compositions of the apical and basolateral membranes are significantly different. How cells maintain this polarity has been the subject of intense research as newly synthesized molecules traffic to their proper destination, and to do so, they must use molecular codes for apical and basolateral targeting (194, 1030, 1696). Since different membrane compartments within the cells have characteristic PPI compositions, it is an important question whether the apical and basolateral membranes have unique PPIs associated with them. Indeed, it has been reported that when MDCK cells from an epithelial layer around a cyst in a three-dimensional culture, their apical mambranes are enriched in PtdIns(4,5)P2, while their basolateral membranes are enriched in PtdIns(3,4,5)P3, and this lipid is excluded from the apical membrane (499, 1004). These studies also found that this lipid distribution is important for proper targeting of proteins to the respective membranes, and this process can be disturbed by exogenous delivery of the lipids to the “inappropriate” membranes (499, 1004). In subsequent studies, also using human colon cancer cells, it was found that the PtdIns(3,4,5)P3 3-phosphatase PTEN localizes to the apical membrane explaining the lack of PtdIns(3,4,5)P3 in that compartment. Indeed, PTEN downregulation disrupted intestinal epithelial polarity and increased the invasiveness of human colon cancer cells (851). A possible link between PtdIns(4,5)P2 and the apical polarity was established when annexin2, which had been previously shown to bind PtdIns(4,5)P2 (1268), was found to be enriched in the apical membranes and also to recruit Cdc42, a small GTP binding protein. Cdc42, in turn, is critical in defining the apical membrane as it targets and enriches the Par6/aPKC polarity complex at the apical membrane (1004). What is not clear is which PI3Ks maintain the high PtdIns(3,4,5)P3 levels at the basolateral membranes during epithelial morphogenesis, but integrins interacting with matrix components are probably part of the PI3K activation mechanism.
Another role of PtdIns(4,5)P2 in the formation of adherens junctions has been recently described. One study showed increased PtdIns(4,5)P2 production by PIPKIγ at the adherens junctions, which was required to recruit the Exo70 exocyst subunit to the newly formed E-cadherin junctions (1749). Another study described that OCRL1 5-phosphatase was part of junctional complexes and was critical for the maturation of polarized epithelial cells (527). Consistent with the role of PtdIns(4,5)P2 in adherens junctions, an elegant EM study on PtdIns(4,5)P2 distribution found the lipid highly enriched in gap junctions, but otherwise showed an even distribution between the apical and basolateral membranes (1183). PIs certainly play additional roles in intracellular trafficking decisions, driving the selective delivery of cargoes to the respective PMs, but those processes belong to the intracellular roles of PIs and will be touched upon later. The aforementioned studies just began to explore the PM phosphoinositide subcompartments in epithelial cell polarity. Clearly, these lipids must have a role not only in targeting proteins to the right PM domains, but also in the regulation of channels and transporters that are so critical to the barrier and transport functions of epithelial layers. It is also important to note that the PtdIns(3,4,5)P3/PtdIns(4,5)P2 distribution described above may not be a general feature of all epithelial cells and could also change with the developmental stage (1397). It is expected and certainly hoped that research in this area will significantly increase and will be extended to planar cell polarity. This latter refers to the alignment of a collection of cells within a cell sheet, another exciting form of polarization that has not yet set an eye on phosphoinositides.
F. How Do Cells Maintain Their PM PtdIns(4,5)P2 Pools?
The key role of the PM pool of PtdIns(4,5)P2 to so many cellular functions makes it essential for the cells to control the level of this lipid in the membrane. Since PtdIns(4,5)P2 represents only a small fraction of the total cellular PtdIns, cells have to replenish this pool especially during prolonged and robust PLC activation. Early studies have already established that the entire PtdIns(45)P2 pool is turning over several times during even a 10-min stimulation (e.g., Ref. 299). This happens as the larger PM pool of PtdIns undergoes sequential phosphorylations by PI 4-kinases and type I PIP 5-kinases (see sects. II and III). It has been observed that Li+ inhibits several inositol phosphate phosphatases and ultimately the inositol monophosphatase that converts inositol monophosphates to inositol (34). Since recycling of inositol is essential for the maintenance of the “PI turnover,” Li+ ions are able to disrupt the cycle leading to the accumulation of inositol phosphates and CDP-DG and an eventual rundown of the PtdIns pools (87, 90, 133, 727) (see sect. IV and Figure 2B). Although it takes a longer period for the smaller PtdIns(4,5)P2 pools to be depleted in this manner, it has been proposed that the therapeutic benefits of Li+ treatment in manic-depressive disease might be related to the preferential effects of Li+ on neurons that show the highest activity (134). Since Li+ also affects other enzymes, most prominently GSK-3 that acts on the Wnt signaling pathway (739), this attractive Li+ hypothesis has never been unequivocally proven or disproven.
Another important and unresolved issue is which PI4Ks are involved in this process and how PtdIns gets to the PM since it is synthesized in the ER. It has been shown that inhibition of type III PI4Ks by higher concentrations of pan-PI3K inhibitors, such as Wm, are able to block PtdIns 4-phosphorylation and PtdIns(4,5)P2 maintenance in highly stimulated cells (1112). Since type II PI4Ks are insensitive to Wm (386, 1112), and PI4KB-specific inhibitors do not have the same effects, by way of exclusion it was concluded that PI4KA is the enzyme responsible for this process (82, 84). This was corroborated by earlier findings that the yeast homolog of PI4KA, Stt4p is responsible for PM PtdIns(4,5)P2 maintenance in yeast (63). However, the primarily ER localization of the mammalian PI4KA enzyme (1107, 1726) and the modest effects of its cellular knockdown on PtdIns(4,5)P2 maintenance in stimulated cells made these conclusions somewhat circumstantial (82, 84). A recent study shed new light on this question: this study found that the EFR3 and TTC7 proteins are capable of recruiting PI4KA to the PM and confirmed that this kinase is the primary source of PtdIns4P in the PM (1114). That still leaves us with the question of how PtdIns gets to the PM. Although PI transfer proteins (PITPs) are capable of PtdIns transfer between membranes (1551) and have been shown to present the lipid for PI4Ks (1354) and PI3Ks (1189), there are no solid evidence that these proteins are indded mediate PtdIns transfer from the ER to the PM. In fact, PITPs were mostly implicated in the synthesis of PtdIns4P at the Golgi (1354) and not at the PM. In a recent study we found that the PI synthase enzyme that was formerly believed to reside in the ER, can associate with a highly mobile membrane compartment that originates from but is not identical to the ER intermediate compartment (ERGIC). This mobile platform might be able to deliver PtdIns to various membranes via transient contacts (796). However, how lipid transfer takes place during these membrane contacts, and how the system senses and adapts to the PtdIns(4,5)P2 needs of the PM still needs to be clarified.
Another related question is how the PtdIns(4,5)P2 5-phosphatase-PIP5-kinase cycle affects PM PtdIns(4,5)P2 levels. The rapid turnover of the 5-phosphate in PtdIns(4,5)P2 has long been described, and it was attributed to “futile cycles” of dephosphorylation and rephosphorylation believed to take place in the PM as it happens in erythrocyte membranes (580). However, it must be clear by now that the multiple 5-phosphatases and 5-kinases that are often part of the same signaling complex and regulate several steps at the endocytic cylcle, must be, in big part, responsible for the high turnover of the 5-phosphate. This means that what appeared to be “futile cycle” might be the sum of several processes along the endocytic pathway in which PIP5Ks and 5-phosphatases generate a local turnover feeding specific signaling steps. This raises the question of whether a pathway exists by which the bulk of uncoated endocytic vesicles recycle back to the PM before reaching the early endosome/sorting endosome stage allowing their PtdIns4P content to be rephosphorylated by PIP5Ks? Another recent puzzling observation was that PtdIns(4,5)P2 levels can be maintained at apparently very different and greatly reduced PM PtdIns4P levels, a finding that is hard to reconcile with the high turnover rate of the phosphate in the 5-position (559). We do not have a clear answer to these observations, nor do we undertsand what happens to the PtdIns4P that is left behind on the early endocytic compartments, since another lipid, namely, PtdIns3P, is found to be important for the formation of early endosomes from the CCVs. Lastly, it is completely unknown what mechanism delivers the PtdOH generated at the PM by the phosphorylation of DG to the ER-localized CDS enzyme(s) for the recycling of the DG for PtdIns synthesis (see Figure 2B).
X. PHOSPHOINOSITIDES AT INTERNAL MEMBRANES
The ultimate precursor of all phosphoinositides is PtdIns, a lipid synthesized in the ER and in an ER-derived subcompartment (796). PtdIns is first phosphorylated to PtdIns3P and PtdIns4P in endosomes and Golgi, respectively, and then further to PtdIns(3,5)P2 and PtdIns(4,5)P2 in late endosomes/MVB and the PM. Parallel to these “synthetic” intermediates exist the “degradative” intermediates that are derived from the dephosphorylation of PtdIns(4,5)P2 and PtdIns(3,4,5)P3 producing PtdIns(3,4)P2 and PtdIns4P in membranes that are moving inside from the PM. As a result, internal membranes contain a variety of PI lipids, most of which have been linked to the regulation of specific trafficking pathways. Some authors refer to PI lipids as identity codes marking specific membrane compartments. While these lipids, indeed, can be characteristic of specific intracellular membranes, it may be misleading to assign their functions to those membranes as much as it can be misleading to draw conclusions from the steady-state distribution of proteins concerning their functions when they cycle between various compartments (e.g., transferrin receptors are mostly found in ER, yet their function clearly requires visiting the PM). Also, using our imaging and antibody tools, we make conclusions about the lack of lipids in internal compartments just as much as we do about their presence. This can also be misleading. It is quite possible that what we see is a larger “precursor” pool that is phosphorylated and dephophorylated with a very short-lived intermediate, and the invisible, short-lived lipid is in fact the important regulator. Contrast this with the opposite view that the lipid what we see is the “product” that must be the regulatory entity. There are probably cases for both, as we can draw parallels from the PM, where PtdIns(4,5)P2 is a regulatory lipid, a product of PIP5Ks, but it also is a precursor of PtdIns(3,4,5)P3 made by the PI3Ks and PtdIns(3,4,5)P3 is rapidly removed by 3- or 5-phosphatases. Therefore, one should be careful making strong inferences just from the localization of inositol lipids or the lack thereof, and also consider the location of the enzymes both for the production and elimination. Even though it seems counterintuitive to have both the kinase and the phosphatase enzymes at the same place apparently working against one another, it makes perfect sense if one sees this arrangement as a rapid cycling between active and inactive forms of a regulator (a lipid, in this case) just as it happens with small GTP binding proteins with their GEFs and GAPs (see this analogy discussed in Ref. 85) (Figure 13).
Figure 13.
The general principle of signaling by phosphoinositides. There are a large number of molecules that possess domains capable of recognizing phosphoinositides (effectors). These domains usually also detect the GTP-bound form of small GTP-binding proteins. The coincidental detection of the G protein and the inositol lipid either recruits the effector to the membrane or induces a conformational change in the molecule. The effector is usually found in a macromolecular complex that also contains the lipid kinase (and probably the phosphatase) as well as the GEF and GAP proteins of the small GTP binding protein. The “regulation” of the effector then depends on the speed of the respective activation-inactivation cycles on phosphoinositides and G proteins. In this model, the strict lipid-specificity of the effector is less important than the identity of the enzymes that are part of the protein signaling complex. This model also explains why enzymes producing or eliminating the same lipid cannot substitute for one another's functions. Note that there is no need to substantially increase the level of the lipids in the membrane where these processes take place. This model assumes some sort of “channeling” lipids to the effectors. It is important to note, however, that this may not be the sole mode of regulation by inositol lipids, and there are other situations where the overall lipid levels show larger changes, and the “free lipid” produced is available for multiple inositol lipid interacting proteins. These latter ones might be more easily detectable with our current molecular tools. The question of how to limit the diffusion of lipids is a critical one to maintain specificity and local control in either case.
A. ER
As described in earlier sections, PtdIns is produced in the ER and in a highly mobile organelle that still awaits further characterization (796). Further phosphorylated PtdIns are not detectable in the ER, probably due to the high enrichment of the PtdInsP monophosphatase enzyme Sac1 in the ER. In fact, Sac1 cycles between the ER and the cis-Golgi compartments (147, 426, 427) and according to recent studies it can also act in trans on PtdIns4P located in the PM at ER-PM contact zones (1454). There are, however, PI kinases found associated with the ER, namely, PI4KA (and possibly its yeast ortholog, Stt4p), which has been shown to regulate ER exit sites in mammalian cells (154, 425). In other studies, PtdIns4P made by the PI4KB (or Pik1p in yeast) was found necessary for fusion of COPII vesicles with the early Golgi compartment but not for budding from the ER (940). The inhibition of HCV repication by PI4KA knock-down or inhibitors revealed that this enzyme is important for the generation of the membranous webb, the membrane platform of HCV replication that is likely to be of ER origin (122, 1267, 1513, 1514, 1577, 1597). Our knowledge is still very limited on the question of what proteins and by what mechanism respond to PtdIns4P made in ER membranes or on the surface of vesicles that leave the ER. PtdIns4P is also targeted by the pathogen Legionella pneumophila, which uses its SidC (1248) and SidM (188, 1824) proteins, together with Rab1 to form a replication-permissive vacuole from ER-derived vesicles (1248).
B. Golgi
It has been the identification and cloning of the first yeast PI4K, Pik1p, that drew attention to the fact that the Golgi is an important site of PtdIns4P production and actions (444, 555, 1479, 1655). This was soon followed by studies showing the presence of mammalian PI4Ks in the Golgi (1036, 1106, 1107) and the role of Arf1 in their Golgi recruitment (514). Moreover, several PH domains with PtdIns4P binding were identified in proteins that showed Golgi localization (381, 893–895, 1783), strongly suggesting that PtdIns4P is an important regulator of Golgi functions (337). Functional studies showed that PI4KB (or Pik1p in yeast) is responsible for post-Golgi trafficking of several protein cargoes (64, 193, 514, 555, 1509, 1655). Since all four mammalian PI4Ks are found in the Golgi/TGN compartment (81), the question arises as to what extent can PI4Ks be linked to specific Golgi-related functions and how much functional overlap and redundancy exist among these enzymes. PI4KA is found in the ER and probably the cis-side of the Golgi (1107, 1726), PI4KB is localized along the whole Golgi compartments (337), while PI4K2A and PI4K2B are found in the trans-side of the Golgi and the TGN (83, 1680, 1690, 1697). This distribution, however, probably shows some changes depending on the status of the cell. More on the regulation of the PI4Ks in the Golgi can be found in section V.
The known effectors regulated by PtdIns4P at the Golgi can be grouped into three categories: the first includes clathrin adaptors, both the tetrameric forms, such as AP-1 and AP-3 (1330, 1680) and the monomeric GGA proteins (344, 1671). These proteins can bind PtdIns4P, and in mammalian cells their Golgi/TGN recruitment is closely associated with the functions of the type II PI4Ks (344, 1671). However, in yeast, the Golgi binding of the AP-1 and Gga2p proteins is functionally linked to Pik1p (316, 344). A significant complexity is layered on this process by the fact that the Golgi recruitment of either the clathrin adaptors or the Pik1p/PI4KB enzymes depends on the activation of Arf1 proteins (512, 514). This is clearly illustrated in a recent study showing in yeast that AP-1 and Gga2p are sequentially recruited to the TGN membrane and that PdIns4P made by Pik1p determines how tightly these two events are coupled. Moreover, Pik1p itself is helped in its recruitment by direct bidning to Gga2p (316) and also to the Arf1 GEF protein, Sec7p (512). It is clear from these yeast studies that Arf1- and PtdIns4P cycling is tightly coordinated at the level of both the regulators (Arf1 GEFs and PI4Ks) and the effectors (in this case the clathrin adaptors) at the Golgi/TGN compartment. The mutual reliance on a physical association between PI4K2A with AP-3 in the localization and functions of these two proteins has also been shown in mammalian cells. Here, PI4K2A is both a regulator of AP-3 and a “cargo” via its dileucine-based sorting motif, and these two proteins are mutually required for correct localization and for proper sorting of cargos to the lysosome (298). More details on the dual requirement for Arf1 and PtdIns4P of clathrin-dependent sorting out of the Golgi can be found in several recent reviews (314, 315, 523).
The second group of effectors is comprised of lipid binding proteins. Notably, most PH domains that recognize PtdIns4P are found in proteins that transfer lipids between membranes (381, 894, 1783). These include the mammalian oxysterol binding protein (OSBP) (894), some of its mammalian homolog ORPs (1167), and the yeast homolog OSH proteins (893, 1304, 1783). Further members of this group are the ceramide transfer protein CERT (564, 895) and the glycosyl-ceramide transfer protein FAPP2 (381, 513) and its truncated sister protein, FAPP2 (381). All of these proteins contain PtdIns4P binding PH domains that require both Arf1-GTP and PtdIns4P for Golgi association (895). The recently solved structure of the FAPP1 PH domain identified separate binding sites for binding of these two regulatory components (587, 885). An important common feature of all of these lipid-transfer proteins is the presence of a FFAT [two phenylalanines (FF) in an acidic tract] motif that provides an ER-association signal via interaction with ER-localized VAP (VAMP-associated) proteins (747, 931, 1049). Both the FFAT domain and the PH domain is essential for the lipid transfer (but not lipid cargo binding) functions of the proteins (564, 769, 1210), although the exact mechanism of how PtdIns4P regulates this process remains obscure (1262). The obvious question regarding the physiology of these proteins is which PI4K(s) are associated with their functions. Based on studies using the isolated PH domains of these proteins, their Golgi localization is supported by both PI4KB and PI4K2A (84), but the OSBP and FAPP1 PH domains show slightly different localization, and more importantly, their overexpression distorts Golgi structure differently (94). This probably reflects their PtdIns4P-dependent binding to additional and different proteins. When studying the lipid transfer functions of these proteins, it was found that ceramide transfer by CERT (that is needed for SM synthesis) relies on PI4KB more than PI4K2A (313, 1567), whereas FAPP2-mediated GlcCer transfer (required for synthesis of complex glycosphingolipids) is linked to PI4K2A more than to PI4KB (313). However, this kinase preference may not be absolute as the upregulation of SM synthesis by oxysterols was shown to depend on PI4K2A (97). In yeast, where the type II PI4K homolog Lsb6p is not a major Golgi regulator, the Golgi recruitment of all of the OSH proteins studied were dependent on Pik1p (1783). Moreover, the unique OSBP homolog Kes1p, the deletion of which alleviates the need for the PI transfer protein Sec14p (902), has a complex sterol-regulated antagonistic relationship with TGN/endosomal PtdIns4P signaling (1089). Recent studies have shown that Kes1p (also called Osh4p) exchanges sterols picked up from the ER for PtdIns4P at the Golgi membrane and, hence, the Golgi PtdIns4P made by Pik1p drives Kes1p-mediated sterol transport between the ER and Golgi membranes (339). This suggests a reciprocal relationship between PtdIns4P controlling lipid transfer and lipid transfer proteins regulating PtdIns4P-mediated signaling. The existence of additional links between PI4Ks and lipid metabolism is revealed by yeast studies finding genetic interactions between Stt4p and sphingolipid synthesis (1510). Although this connection is more closely related to the role of Stt4p in producing PtdIns(4,5)P2 at the PM (415), an ER-component is indicated by the synthetic lethality (1510) and physical association of Stt4p (502) with the ER-localized Scs2p protein, the yeast homolog of the mammalian VAP proteins. Moreover, Scs2p was shown to bind phosphoinositides, and this binding was found important for Scs2p function (745). There have been no similar studies described for PI4KA in mammalian cells. Finally, if PI4Ks are important for lipid transport and maintenance of lipid homeostasis, it is expected that the lipid composition of membranes have an impact on PtdIns4P levels. Indeed, spingolipids were found to be important for the PM association of the yeast PIP 5-kinase Mss4p (806). Similarly, an association was found between the Sac1 PtdIns4P phosphatase and the Orm proteins that control the rate-limiting step in sphingolipid syntheis in yeast. This also suggests a link between sphingolipid status and PtdIns4P regulation (178). Although much less is known about lipid regulation of PI synthesis in mammalian cells, it is notable that the distribution, palmitoylation, and activity of PI4K2A is regulated by the cholesterol content of internal membranes (949, 1686).
A third and different kind of PtdIns4P effector was discovered by a study that identified novel PtdIns4P-binding domains (357). This study found that the mammalian Golgi protein GOLPH3 binds specifically to PtdIns4P and controls Golgi morphology by forming a bridge between the Golgi membranes and the actin cytoskeleton through its binding to unconventional myosin, MYO18A (357). This finding that linked Golgi morphology to the actin cytoskeleton was somewhat surprising in light of the well-documented importance of the microtubular rather than the actin network in the maintenance of Golgi structure. The yeast homolog of GOLPH3, Vps74p, also binds PtdIns4P, and its steady-state Golgi localization requires Pik1p (357, 1731). The Vps74p protein was found necessary to retain several gycosyl-transferases in the Golgi (1365) and was identified as an essential component of the retrograde trafficking of these enzymes (1731). These studies also suggested that in addition to regulating anterograde trafficking, Pik1p was also important for the retrieval of specific cargoes, namely, Golgi glucosyltransferases. Curiously, the GOLPH3 locus is amplified in a number of human cancers and was linked to oncogenesis through enhanced mTOR1 signaling (1371). How these three aspects of GOLPH3/Vps74 biology are linked together is an exciting question that is surely being studied in several laboratories.
C. Endosomes and Lysosomes
Endosomal function is a term that covers a lot of processes. Newly synthesized molecules leaving either the ER or the Golgi (where PtdIns4P is the critical regulatory PI lipid) have to be sorted either toward the PM or the lysosome. At the same time, the PM undergoes constant internalization [a process where PtdIns(4,5)P2 is a major controlling PI lipid] and the internalized membranes and luminal contents have to be sorted either to the lysosome for degradation or back to the PM (Figure 11). During these processes membrane vesicles fuse, their membranes and luminal contents can mix, and then the membranes get separated segregating both luminal and membrane components to form new vesicular entities. At all of these steps, PI lipids, namely, PtdIns3P, PtdIns(3,5)P2, and PtdIns4P have fundamental roles. This is a huge research area that will be summarized here only with broad brush strokes.
1. PtdIns3P and PtdIns(3,5)P2 in endomembranes
Identification of the yeast Vps34p protein as a PI 3-kinase was a major milestone in highlighting the importance of PtdIns3P in intracellular trafficking (1367). It was soon followed by the recognition that a unique cysteine-finger protein module present in several proteins, namely, the yeast Fab1, the C. elegans YOTB/ZK632, the yeast Vac1, and the mammalian EEA1 (hence comes the name FYVE domain) (1090), is necessary to target the EEA1 protein to endocytic membranes (1458), and it is a PtdIns3P recognition module (200, 501, 836, 1064). FYVE domains have since been found in a great number of proteins (1457), and it has been firmly established that PtdIns3P, together with Rab5 proteins controls endocytic vesicle fusion (1100, 1409). The yeast PI3K Vps34p is essential for sorting proteins to the yeast vacuole (605, 1367), and among the FYVE domain containing proteins, the yeast Fab1p turned out to be a PtdIns3P 5-kinase that was necessary for maintaining vacuolar morphology and function (498, 1757). These studies have established PtdIns3P and PtdIns(3,5)P2 as key lipid regulators of endocytic trafficking. Another PtdIns3P binding module called phox-homology (PX) domain was later found in several proteins such as the sorting nexins (SNX) (304, 1540). Although the first PX domains were found to bind PtdIns3P (752, 1750), and all yeast PX domains bind this lipid (1782), some metazoan PX domains bind PtdIns(4,5)P2 (327, 810), and the lipid-binding specificity of many mammalian PX domains remains unknown. It is also important to note that PX domains also bind proteins (1540, 1643). Recent studies also suggest that class II PI3Ks produce some of the PtdIns3P in higher eukaryotes (370, 970, 1700), and the molecular cloning of the type II PI4Ks (108, 1058) revealing their presence in endosomes (83, 1060) also suggested the involvement of these latter enzymes in endocytic trafficking in mammalian cells (83, 1060). PtdIns3P controls several aspects of the biology of endosomes. It has a role in targeting newly synthesized lysosomal proteins to the lysosomes (vacuole in yeast), and it also controls retrieval of proteins from the lysosome to endosomes. It regulates the sorting of endocytosed cargoes for recycling or degradation and also plays a critical role in autophagy, a process by which starving cells degrade their own cytoplasm and organelles (1631). For all these different processes, a whole set of regulatory proteins are recruited to the endocytic membrane in specific sequences.
For example, soluble proteases have to reach their destination, the lysosomes. For these proteins, special receptors exist such as the yeast Vps10p and the mammalian mannose-6-phosphate receptor (M6PR). These transmembrane proteins cycle between the Golgi and prevacuolar compartments, with each round delivering a batch of enzymes for further sorting to the lysosomes. As pointed out in earlier sections, PtdIns4P is important for the exit of these transport carriers from the Golgi using clathrin-coated vesicles. However, retrieval of Vps10 from the endosomes relies on other inositol lipids and proteins that form a complex called the “retromer” (1373). In yeast, Vps29, Vps30, and Vps35 are important components of the retromer (1373), and one of these, Vps30, as well as another protein, Vps38, are both accessory proteins to the PI 3-kinase Vps34 (201). Moreover, two proteins, important for vesicle budding, Vps5 and Vps17 that are also part of the retromer (1372) possess PX domains that bind PtdIns3P (201). The mammalian Vps5 homologs SNX1 and SNX2 play similar roles in mammalian cells as part of the mammalian retromer complex. SNX1 shows PtdIns3P and PtdIns(3,5)P2 binding via its PX domains (297) and was found responsible for retrograde trafficking of the M6PR (213, 307).
Another role of PtdIns3P is to promote homotypic fusion of endocytosed uncoated vesicles. For this, tethering by the EEA1 proteins and Rab5 are both essential (1409). However, not all endocytic vesicles are equal, and different cell surface receptors are already routed to different destinations even at the early steps of the endocytic process. FYVE domain containing proteins other than EEA1, such as WDFY2 (585), can define special endocytic compartments with unique signaling properties (1665). The recycling of several receptors back to the cell surface requires PI3Ks (2, 670, 886, 1601). In this process, proteins are retrieved at different stages of vesicle maturation as they progress from early endosomes to late endosomes. A whole set of PtdIns3P binding proteins participate in this process that seems to be different for specific groups of molecules. Various PX domain-containing SNX proteins were identified as uniquely important for these processes (305, 307). For example, SNX4 controls trafficking of TfRs between the early and recycling endosomes (1571), and SNX3 is central to a newly recognized unique retromer pathway by which the Wnt sorting receptor Wntless is retrieved from the endosomes to the TGN (307, 576). Yet another SNX, SNX17, which has a FERM domain that recognizes NPxY sequences on transmemembrane proteins, is important for LDL-like receptor cycling (372, 1472) as well as the maintenance of integrins in the PM (1456).
Molecules that are not recycled back to the PM are taking a route toward degradation. This process uses another form of cargo selection that is based on poly-ubiquitylation, which can also serve as part of ER quality control of misfolded proteins. Many PM proteins such as RTKs that undergo endocytosis and are destined for degradation become multi- or polyubiquitylated and recognized by proteins that possess Ubi-binding domains. Here the Vps27 protein and its mammalian homolog Hrs are master regulators, both having a FYVE domain and being regulated by PtdIns3P (73, 146, 766, 1250, 1447). The central compartment where proteins are sorted for degradation is the MVB where the sequential assembly of a protein complex called the ESCRT machinery (ESCRT-0, -I, -II, and -III complexes) is essential for segregation of the ubiquitylated proteins into inwardly invaginating membranes (70, 71, 671, 765). PtdIns3P and Vps27/Hrs are necessary for the recruitment of the ESCRT-0 and hence the subsequent members of the complex to the MVB (73, 766, 1251, 1542). ESCRT-I does not show PtdIns3P binding on its own (821), but ESCRT-II does via the PH domain-like GLUE domain of Eap45 (the mammalian Vps36) (1416). ESCRT-III complexes are the ones that deform membranes of the MVB and generate the inwardly budding vesicles (567, 1327), and several proteins of this complex, such as CHMP3 (and its yeast homolog Vps24p) (1710) and CHMP2a and CHMP4b (225) can bind PtdIns3P, PtdIns(3,5)P2, or both. Recent studies reconstituted the assembly and lipid deforming effects of the ESCRT machinery in giant unilammellar vesicles in vitro and only found a moderate enhancement of intraluminal vesicle release by PtdIns3P or PtdIns(3,5)P2 (1723). Although this finding suggests that these lipids promote but are not essential for this reaction, they probably play a significantly more important facilitating role within the cell where these proteins are not supplied in such abundance.
Another important endomembrane process that requires PtdIns3P is autophagy (more precisely, macroautophagy) (281, 1073). Starving cells use this process to degrade a fraction of their cytoplasmic proteins and organelles, such as mitochondria. The hallmark of this process is the formation of a double membrane from internal membranes (also called phagophore), the origin of which is somewhat debated but likely being the ER with mitochondrial and possibly other membrane participation (548, 1565). This membrane gradually engulfs a piece of the cytosol and organelles to be degraded and eventually fuses with the lysosome. The phagophore can be recognized because of its high enrichment of the protein LC3 (microtubule-associated protein light chain 3, Atg8p in yeast) that gets phosphatidylethanolaminated, a unique lipid modification characteristic of this process (675). The initiation of the phagophore requires the class III PI3K, Vps34, its regulator, Vps15, and Atg6/Beclin1 forming a core complex. There are several additional proteins associated with this complex that play a role in the maturation and subsequenct fusion of the autophagosome with the lysosome, and some are shared with the vacuolar sorting pathway. For example, the Vps34/Vps15/Atg6 complex related to autophagy (also called complex I) contains Atg14 (Barkor/Atg14L in mammalian cells), whereas complex II that functions in vacuolar sorting contains Vps38 (UVRAG in mammals) instead of Vps14/Barkor (234, 1157). It is not surprising that some of the steps in phagophore maturation and lysosomal fusion show the same PI3K involvement as the vacuolar sorting pathway. What was surprising, though, was the finding that autophagosome formation also requires PtdIns 3-phosphatases, such as Jumpy (MTMR14) (1631, 1632), which suggests that the turnover of PtdIns3P must be more important than the actual levels during this process. Although the literature of autophagy is increasing every day with intimidating complexity (281, 1073), the exact role(s) of PtdIns3P in this process remains to be understood.
An important point to be emphasized is the close connection between autophagy and nutrient sensing, both of which have 3-phosphorylated inositide regulatory components (Figure 14). All metabolic processes in the cell have to be harmonized with the supply of nutrients, and the central hubs for this information network are the TOR proteins identified in 1991 as the yeast targets of the drug rapamycin (592). The TOR protein (TOR1 and TOR2 in yeast, and mTOR in mammalian cells) is a Ser/Thr kinase that belongs to the PI kinase-related kinase family and is evolutionary highly conserved. The mTOR catalytic subunit exists in two functionally distinct protein complexes, mTORC1 and mTORC2 (454). Each of these complexes contains several proteins, some of which are shared, but mTORC1 contains Raptor, while mTORC2 contains Rictor as unique scaffolding protein components. Rapamycin binding to FKBP12 induces a complex formation with mTORC1 (but not mTORC2), which, in turn, allosterically inhibits mTORC1 activity, explaining why acute rapamycin treatment only inhibits mTORC1. mTORC1 collects information through various pathways on cellular stress, energy levels, amino acids, and growth factors and controls cell growth, metabolism, cell cycle progression, and macroautophagy (854). In contrast, mTORC2 is regulated by growth factors and acts on several downstream AGC protein kinases to control actin cytoskeleton, apoptosis, and cell proliferation (454). It is beyond the scope of this review to get a detailed summary of the mTOR literature (for reviews, see Refs. 454, 854, 1830), but there are important connections between mTOR and PI3Ks that are worth mentioning. Most, if not all, growth factors activate the PI 3-kinase signaling cascade at the cell surface, primarily the class I p85/p110α complex. The resulting PtdIns(3,4,5)P3 engages a range of proteins that signal to mTORs. PtdIns(3,4,5)P3 recruits to the membrane both the PDK1 protein kinase and its downstream target, the Akt/PKB kinase, via their PH domains that bind PtdIns(3,4,5)P3 [and PtdIns(3,4)P2]. PDK1 phosphorylates Akt/PKB at position Thr308, and another phosphorylation at Ser473 within the hydrophobic motif, carried out by mTORC2, is needed for full activation. PtdIns(3,4,5)P3 also activates mTORC2 kinase activity by a poorly understood mechanism (489, 1430). Akt/PKB have several downstream targets that are part of the cell survival pathway (860, 1620, 1806). Akt/PKB inactivates the TSC1-TSC2 (hamartin-tuberin) complex, which is a critical inhibitory regulator of mTORC1 (491, 697, 1541). The TSC1/2 complex is a GTPase activating protein for the Rheb (Ras homologue enriched in brain) GTP binding protein that integrates signals from energy levels, O2 supply, and growth factors (985). The amino acid sensing, however, is mediated by a different pathway that involves the Rag GTPases (785, 1336) and one that requires translocation of the mTORC1 complex to the surface of the lysosomes (1335, 1829). Curiously, the Class III PI3K hVPS34 was found to be important for the amino acid sensing pathway for mTORC1 activation (536, 1143). It has also been shown that this pathway works parallel to the Rag GTPases and requires PLD1 and binding of PtdIns3P made by hVPS34 to the PX domain of PLD1 (1773). Since mTORC1 inhibits autophagy, and autophagy also needs VPS34, it is most likely that the VPS34 that serves as a mediator of mTORC1 activation by amino acids is found in a different complex and location than the one that promotes autophagy. Another study found that inositol phosphate multikinase (IPMK) also has a noncatalytic role keeping the mTORC1 complex together during its translocation to the lysosomal surface (791). More studies are needed to explore the complex PPI regulation of the mTOR-metabolic network with special emphasis on the compartmental organization and membrane association of these complexes.
Figure 14.
Integration of metabolic control, cell growth and proliferation, and their regulation by phosphoinositides. One of the main effectors of PI3K activation is the Akt/PKB protein kinase that is activated by translocation to the membrane by binding via its PH domain to PtdIns(3,4,5)P3 [and PtdIns(3,4)P2]. This recruitment is important for phosphorylation of Akt/PKB by two protein kinases, PDK1 (on Thr308) and the mTORC2 complex (on Ser473). These two kinases are also activated by PtdIns(3,4,5)P3. Although Akt signals in a number of other directions (not shown here for simplicity), one of its major functions is to control the activity of the mTORC1 complex. Active Akt stimulates mTORC1 by inhibiting the GAP activity of the TSC1/2 complex, the latter limiting the activity of the Rheb GTP binding proteins that are upstream activators of the mTORC1 complex. Active mTORC1 is a signal for cell growth and proliferation and inhibits apoptosis and autophagy. In case of starvation, energy depletion, stress, or hypoxia, mTORC1 is inhibited, and these signals activate the TSC1/2 complex. Under these conditions autophagy is stimulated. A different pathway is used during amino acid sensing. Amino acids activate the Rag GTPases and induce translocation of the mTORC1 complex to the surface of the lysosomes. The classIII PI3K hVPS34 is also important for the amino acid sensing pathway for mTORC1 activation using a pathway that works parallel to the Rag GTPases and which requires PLD1, and PtdIns3P binding to the PX domain of PLD. Recently, the class II PI3Ks and PtdIns(3,5)P2 production was also shown to stimulate mTORC1. Paradoxically, autophagy also requires PtdIns3P and VPS34, and it is most likely that the VPS34 that serves as a mediator of mTORC1 activation by amino acids works in a different complex and location than the one that promotes autophagy.
Significantly less clear are the mechanistic details of how the PtdIns3P to PtdIns(3,5)P2 conversion controls the lysosomal sorting process and the relevant downstream targets of the lipid PtdIns(3,5)P2 (377). It is well established that Fab1p deleted yeast strains have huge vacuoles and impaired transport of CPY to the vacuole (498). These defects are also associated with weakened vacuole acidification (1159) and chromosomal segregation defects, the latter probably secondary to the enlarged vacuoles (1757). However, autophagy in yeast does not require PtdIns(3,5)P2 (379). The limited known PtdIns(3,5)P2 targets include the PROPPINs that are seven β-propeller containing proteins, such as the yeast Atg18p (also known as Svp1p) (379) or its mammalian homolog WIPI1(725). Atg18p and WIPI1 and the other PROPPINs (Atg21p and WIPI1–4) are important in autophagy (397, 533, 825, 1238). Atg18p depletion leads to PtdIns(3,5)P2 accumulation and to enlarged vacuoles, a phenotype similar to that of Fab1p deletion, indicating a role in determining vacuolar size (397). However, as pointed out above, the sorting of hydrolyses and initiation of autophagy are two separable functions of Vps34p linked to complex II and complex I, respectively (779, 825). Since autophagy in yeast does not require PtdIns(3,5)P2 (379), Atg18p may have a dual role: it could be important for autophagy as a PtdIns3P effector and for vacuolar morphology as a PtdIns(3,5)P2-regulated protein. A recent study also found that PtdIns(3,5)P2 regulates the activation and localization of mTORC1 in mammalian cells and that the lipid interacts with the mTORC1 component raptor (180). Another group of effectors of PtdIns(3,5)P2 was recently identified as the mammalian TRPML proteins or mucolipins and their yeast homolog, Yvc1/TRPY1 (371). These are endolysosomal cation channels that are mutated in various forms of mucolipidosis (233, 246). In yeast they are regulated by cytoplasmic Ca2+ and activated by hyperosmotic shock (233), the latter also being a strong inducer of PtdIns(3,5)P2 (376). This is a new area of research that will rapidly expand and help us understand the conrtrol of lysosomal ion composition and function and the role of PtdIns(3,5)P2 in the process.
2. The role of PtdIns4P in endomembranes
Although PtdIns4P has been primarily linked with Golgi and PM functions, the endosomal localization of type II PI4Ks suggests that this lipid may also be important for certain trafficking steps in endosomes. Knock-down of PI4KIIα abolished the Golgi recruitment of the heterotetrameric adaptor AP-1 (1680), and also impaired the recruitment of AP-3 in endosomes (1330). PI4KIIα was also shown to regulate the late-Golgi transport of VSV-G and influenza hemagglutinin (1680). The type II PI4Ks were implicated in transferrin receptor endocytosis and recycling (83), and PI4KIIα has a major role in EGF receptor degradation in the late endosomal pathway (1060). Recent studies showed that knock-down of type II PI4Kα causes enlargement of prelysosomal/lysosomal compartments (298, 741, 1128), and PI4Kα is required for lysosomal delivery of the Gaucher disease enzyme β-glucocerebrosidase and its receptor, LIMP-2 (741). It will be important to understand where and how the PtdIns4P to PtdIns3P switch happens in the various endocytic compartments. Transport vesicles coming from the ER and the Golgi contain PtdIns4P. Moreover, the endocytosed vesicles also should have PtdIns4P as a result of the action of numerous 5-phosphatases. All of these internal vesicular intermediates will have to lose their PtdIns4P and acquire PtdIns3P. How this process is regulated enzymatically and why this switching is important are questions still to be investigated.
D. Nucleus
The presence of several enzymatic activities related to the “PI cycle,” such as PLC, DG kinase, and PI kinase in nuclear matrix, raised the possibility that a separate nuclear phosphoinositide system exists (275, 1205). Since PPIs are presumed to be membrane associated, the presence of the lipid as well as the enzymes in the nucleus initially raised suspicions that contamination with nuclear membranes might be responsible for these activities. However, phosphoinositide changes in the nuclei of Swiss 3T3 cells stimulated with IGF-I were found significantly different from those elicited at the PM, and the nuclear lipids were identified in the nuclear lamina rather than in the membranes (360, 1001). With the cloning of the various PI lipid metabolizing enzymes and the availability of antibodies for immunofluorescence, it has become clear that many enzymes of the PI cycle do localize to the nucleus. These include several isoforms of PLC enzymes [β1 (451, 952, 987), δ1 (302, 1448), and δ4 (925)], both forms of the type III PI4Ks [PI4KA (748) and PI4KB/Pik1 (332, 343, 1478)], the yeast PIP 5-kinase Mss4 (62) and the mammalian PIP 5-kinase Iα (168, 1029), and the type II PIPKβ (263). In addition to the kinases, inositide phosphatases were also shown to move in and out of the nucleus. An increased nuclear retention of PTEN was shown after oxidative stress (229), and the Ser132-phosphorylated SHIP2 was found localized to the nucleus and also becoming active against PtdIns(4,5)P2 (400). Moreover, the lipid PtdIns(4,5)P2 itself could be localized by antibodies or by PtdIns(4,5)P2 recognizing PH domains in the nuclear matrix, especially enriched in nuclear speckles (1087, 1176, 1684), the dynamic organelles that have high pre-mRNA splicing activity (849).
There are two major questions raised by these findings; one is related to the physical state of the lipids and another to the nuclear proteins that are regulatd by the nuclear lipids. Although we have yet to find answers to the first question, our mind has to be open to the possibility that nuclear lipids may exist in association with proteins, or forming a unique lipid phase like a micelle or liposome. For example, phospholipids, including phosphoinositides, have been found tightly bound to nuclear receptors (151, 828, 903). This does not rule out that phosphoinositides are functionally important in the inner nuclear membrane, where several studies showed their presence (204, 496, 1421). This latter might be significant in light of the nuclear Ins(1,4,5)P3 (519) and Ca2+ signals (396) and the high transcriptional activity located in juxtamembrane regions of the nucleus (20, 1512). There have been more advances in the identification of nuclear proteins that respond to lipid changes. A noncanonical poly(A) polymerase, named Star-PAP, has been identified as a protein that interacts with PIPKIα in nuclear speckles (1029). This protein adds poly(A) tails to a selected group of mRNAs, and its activity is highly stimulated by PtdIns(4,5)P2 (1028, 1029). Curiously, a low activity form of a DNA polymerase was also shown to be activated by PtdIns4P or its hydrolytic product, Ins(1,4)P2 (1507, 1508).
Although PtdIns(4,5)P2 appears to be the most abundant and recognized nuclear phosphoinositide, studies on type II PIPKs that phosphorylate PtdIns5P to PtdIns(4,5)P2 revealed an unexpected twist in their nuclear functions. The nucleus-localized PIPKIIβ (263), most likely in the form of a heterodimer with the catalytically more active PIPKIIα (1672), has an equally, if not more, important role in regulating PtdIns5P levels than controlling nuclear PtdIns(4,5)P2 (522). PtdIns5P has been linked to stress-induced nuclear signaling and apoptosis (737, 1833). The protein that responds to nuclear PtdIns5P is ING2, which specifically binds this lipid with its PHD domain. The ING2 protein associates with histone deacetylases and the chromatin (522, 837) and, hence, regulates histone acetylation. ING2 induces apoptosis via increased p53 acetylation both of which requires PtdIns5P (522). PtdIns5P levels are then regulated either by inhibition of PIPKIIβ (such as in stress responses where p38 MAPK phosphorylates PIPKIIβ) (522) or by stimulation of the type I PtdIsn(4,5)P2 4-phosphatase (1833). Another mechanism by which PIPKIIβ and PtdIns5P regulates nuclear functions is the association of the kinase with a nuclear ubiquitin ligase, Cul3-SPOP and stimulation of its activity (198).
In addition to these mechanisms, PtdIns(4,5)P2 may have an indirect role as a precursor of other regulators. The PtdIns(3,4,5)P3-Akt pathway was shown to regulate mRNA splicing, although it is not clear at what location Akt needs to be activated (148, 1709). It is generally believed that the classical PI3Ks are not associated with the nucleus (but see Refs. 831, 998), even though ample evidence suggests that PI3Ks control nuclear signaling (e.g., Ref. 781, 1809). However, the nucleus-localized inositol polyphosphate multikinase (IPMK) was shown to function as a PtdIns(4,5)P2 3-kinase (1269) capable of activating Akt (962). Still, in a somewhat convoluted manner, the nuclear IPMK lipid kinase activity requires the formation of PtdIns(3,4,5)P3 in the PM by the class I PI3Ks, presumably controlling the phosphorylation state of IPMK (962). Recent studies showed that IPMK-phosphorylated PtdIns(4,5)P2 was bound to the nuclear receptor steroidogenic factor 1 (SF-1) and PTEN was able to reverse this reaction (151). In the same study, IPMK was unable to phosphorylate free PtdIns(4,5)P2 and, conversely, class I PI3K could not phosphorylate PtdIns(4,5)P2 bound to SF-1. These results also speak to the question of the physical form(s) the lipids may take up in the nucleus.
The presence of PLC and DG kinase enzymes in the nucleus also suggest that Ins(1,4,5)P3 and DG formation both can initiate further nuclear signaling events. Nuclear Ca2+ signaling initiated from the nuclear membrane-derived intranuclear canaliculi has been demonstrated (396). However, this is not the only way Ins(1,4,5)P3 can be utilized. Ins(1,4,5)P3 can be phosphorylated to higher inositol phosphates, and these soluble, highly charged molecules have been found to be critical for mRNA export (1777). The enzymatic pathways of InsP6 formation and their importance have been first described and delineated in yeast studies (1158, 1455), but these metabolites had already been described earlier in mammalian cells (86, 610, 1466, 1776). By now all the enzymes producing them have been identified and cloned (1629, 1719). These pathways are essential in development of higher organisms (463, 1628). The higher inositol phosphates have multiple regulatory functions in the nucleus that have been recently reviewed and will not be further detailed here (28, 1324, 1375, 1390, 1455).
XI. PHOSPHOINOSITIDES IN DISEASE
A. Somatic Diseases
It may be an exaggeration, but with some efforts every human disease can be linked to altered inositol lipid metabolism. Although phosphoinositides are likely to be important in multifactorial diseases, it is easier to define the role of these lipids in disorders caused by a clearly identifiable single defect in inositol lipid metabolizing enzymes or effector proteins. Some of these are profoundly apparent, such as the combined immunodeficiency seen in Bruton's tyrosine kinase patients, due to a single mutation within the PH domain of the Btk tyrosine kinase that prevents its interaction with PtdIns(3,4,5)P3 (1260). Others cause more subtle aberrations such as the activating mutations of PLCγ2 in patients with spontaneous inflammations, cold urticaria, and autoimmunity (414, 1169).
A lot more human diseases are caused by excess phosphoinositides than the lack of them as exemplified by the cancer-causing elevations of PtdIns(3,4,5)P3. As detailed in section V, class I PI3Kα mutations are found in a large percentage of human cancers (1190, 1333), and the same is true for the loss of PTEN (210, 898), the phosphatase that converts PtdIns(3,4,5)P3 back to PtdIns(4,5)P2 (968). There is general agreement that PtdIns(3,4,5)P3 is an oncogenic lipid, and enormous information is available on how PtdIns(3,4,5)P3 can help tumor cells to survive and metastasize. First and most importantly, PtdIns(3,4,5)P3 promotes both proliferation and protects cells from apoptosis, two processes that are hallmarks of tumor cells. PtdIns(3,4,5)P3 activates numerous GEF proteins that act on small GTP binding proteins such as Ras, Rac, Rho, and Cdc42 and, therefore, can activate all of the processes that are used for invasion and metastasis, such as cell migration and attachment. The effects of PtdIns(3,4,5)P3 can be cell autonomous, but they can also affect the interaction of the cells with their tissue environment, something we still have to learn more about. This may explain why certain cancers have preferential sites of metastasis and avoid other organs. A fundamental question, however, that we still do not fully understand is whether PI3K activation per se is sufficient to transform cells. Several studies have shown that activating class I PI3K mutations are sufficient to transform cells or generate tumors in mouse models (541, 758, 873). However, often in these cancers other mutations show up that drive tumorigeneseis. It appears that cells need a driver for the transformation process, which will then be greatly aided by elevated PI3K activation. Therefore, activating PI3K mutations may just represent a sensitized state upon which any other oncogenic event can easily initiate the transformation process (1785). Especially important is the connection between K-Ras mutations and PI3Ks in cancer cell growth. K-Ras can activate all forms of class I PI3Ks, and hence is always associated with increased PI3K activity. However, PI3K inhibition alone is not very effective in K-Ras mutant-driven cancer cell lines (539), although it has been shown that K-Ras-p110α interaction (539) or the presence of p85 subunits is important for K-Ras-induced carcinogenesis (404). These data suggest that there could be a difference in PI3K participation at the onset of cell transformation and during the later phase of cancer expansion and maintenance (404). Nonetheless, ample evidence suggests that PI3K inhibition is a promising way of complementing cancer therapy. The close connection between PI3K activation and mTORC1 (and -2) (1830) and the characteristic metabolic realignment of cancer cells (930, 1608, 1769) explain why PI3K inhibitors and mTOR inhibitors are both in the frontiers as potential cancer therapies and that combined PI3K/mTOR inhibitors are also gaining significant momentum (600, 926, 1727).
Apart from the class IA PI3Ks, human diseases are rarely caused by activating mutations of inositide lipid kinases. In contrast, an increasing number of rare human diseases are linked to phosphoinositide phosphatase defects. These have been discussed in the respective individual sections but will be listed here in Table 5.
Table 5.
Human diseases caused by defective inositol lipid phosphatases
| Disease | Enzyme Defect | Main Symptoms and Pathology | Reference Nos. |
|---|---|---|---|
| OCRL | OCRL 5-phosphatase | Early cataract, mental retardation, renal tubular acidosis, aminoaciduria | 61, 1483 |
| Dent disease | OCRL 5-phosphatase | Variable, milder forms of Lowe's syndrome | 647 |
| ALS, PLS (small %) | Sac3/Fig4 | Progressive degeneration of motor neurons | 252 |
| Charcot-Marie-Tooth type CMT4J | Sac3/Fig4 | Peripheral sensorimotor neuropathy | 253 |
| Downs syndrome?? | Synaptojanin 1 trisomy? | Multiple neurological symptoms in CNS | 1647 |
| Joubert syndrome | INPP5E/Pharbin | Midbrain-hindbrain malformation, retinodystrophy, nephronophthisis, liver cirrhosis, polydactyly | 145 |
| X-linked myotubular myopathy | MTM1 | Skeletal muscle weakness, centronuclear myopathy | 858 |
| Charcot-Marie-Tooth disease type CMT4B1 | MTMR2 | Peripheral neuropathy with demyelinization | 159, 653 |
| Charcot-Marie-Tooth disease type CMT4B1 | MTMR13 | Peripheral neuropathy with demyelinization | 69 |
| Autosomal recessive centronuclear myopathy | MTMR14/hJUMPY | Centronuclear myopathy | 1566 |
The general lesson learned from these studies is that these phosphatase defects cause very specific phenotypes because the affected enzymes control a very specific pool of the inositol lipid, and for these narrowly defined biochemical events, the other enzymes do not provide alternative coverage in spite of their identical enzymatic activities. These susceptibilities may depend on species and probably even on other genetic modifiers as perfectly exemplified by the OCRL disease that is not easily reproducible in mice (173) and in which the apparently same enzyme defect can produce a range of symptoms with different severities (647).
It is notable that loss of function of inositol lipid kinases is rarely seen (or have not been yet recognized) in human diseases. This either means that such mutations are incompatible with normal development [see the only report on an inactivating mutation within the PIP 5-kinase Iγ gene that causes severe developmental abnormalities with perinatal lethality (1119)], or there is enough redundancy in the system to overcome such defects. However, it is quite likely that more subtle functional defects, either structural or regulatory, contribute to the development of human diseases. Given the role of the PI4Ks and classs III PI3K in ER-Golgi and endosomal functions, respectively, it would be almost surprising if they did not have a role in diseases where protein misfolding and quality control problems are part of the etiology such as in neurodegenerative diseases. A few examples have started to appear in the literature: for example, mutations in one allele of the Sac3/Fig4 phosphatase have been detected in 2% of ALS (amyotrophic lateral sclerosis or Lou Gehrig's disease) and PLS (primary lateral sclerosis) patients (252). As discussed in section VI, this phosphatase also impacts the function of the type III PIP kinase PIKfyve. PI4K2A gene-trapped mice develop late-onset spinocerebellar degeneration (1408). Notably, though, no mutations in the human PI4K2A gene were found in patients suffering from a similar condition (270). Given the slow onset of many of these diseases (like Alzheimer's or Parkinson's disease) and their multifactorial backround, one would not expect to see a very drastic change attributed to a phosphoinositide signaling defect but rather a subtle one that will have a gradual and compounding effect on protein trafficking and folding. Other subtle differences could cause developmental alterations in the brain that do not manifest in any recognizable anatomical abnormality, but manifests in diseases such as schizophrenia, depression, or autism. For example, the PI4KA gene is located in chromosome 22 in the q11.2 region that is often deleted in “22q11.2 deletion syndromes” such as in DeGeorge syndrome or Velocardiofacial syndrome (808). A strong association has been observed between 22q11.2 deletion syndromes and schizophrenia (55, 111). Several obvious schizophrenia susceptibility genes can be found in this chromosomal region, such as the catecholamine metabolizing COMT, the DeGeorge critical region gene (DGCR6) and the proline dehydrogenase gene (PRODH2) (227, 1730) or SNAP29 whose promoter region also showed association with schizophrenia (1729). However, some studies also found association with an SNP in the PI4KA promoter region with the schizophrenia phenotype (1326, 1648) and suggested that the PI4KA gene could be a modifier in cases of schizophrenia associated with 22q11.2 deletion syndromes (1648). It is almost certain that an increasing number of neurological and psyhiatric diseases will be linked to altered phosphoinositide metabolism (269).
B. Infectious Diseases
It has been increasingly recognized that various intracellular parasites utilize the host's intrinsic cellular machinery to enter the cells, move around, and evade the natural protective mechanisms (1204, 1225). In addition, many organisms, especially viruses, generate a platform for the reproduction of the organism and then get released from the cell interior (38). Identification of host proteins that these pathogens require at any point of their life cycle is an important goal that may uncover new therapeutic opportunities (777, 1239). Phosphoinositides, again, are important regulators of many of these processes. For example, the endocytosis of several pathogens uses the same routes by which cell surface proteins enter the endocytic pathways and hence rely upon PtdIns(4,5)P2 as detailed in section IXD. Examples include the entry of bacteria such as Listeria (1226, 1548), Yersinia (1340), enterohepatic E. coli (1348), Salmonella (1544), or viruses such as hepatitis C (1577), HIV (1069), or influenza (472), just to name a few from an ever-increasing list. Another role of PtdIns(4,5)P2 and PtdIns(3,4,5)P3 has been reported in filopod formation, actin polymerization, and movement of Listeria within the infected cells (1405). PtdIns4P is also utilized by several pathogens to shape their intracellular milieu. Legionella pneumophila uses several of its virulence proteins, such as SidC and SidM that bind PtdIns4P, to form its replicative vacuole within the cell (188, 613, 1248, 1492, 1824). Another intracellular bacteria Clamydia uses both type II PI4Ks and the OCRL 5-phosphatase to modify its vacuole for production of infectious progeny (1080). Most recently it was discovered that hepatitis C virus requires PtdIns4P, specifically made by PI4KA for replication (122, 1513, 1577, 1597). It is not clear for what purpose PI4KA is needed, but most likely generation of the “membranous webb,” which serves as the replication platform of the virus (1082), requires the PI4KA enzyme whose activity is stimulated by one of the viral proteins, NS5A (38, 910, 1267, 1514). Some enteroviruses, such as Polio or Coxsackie, need PtdIns4P made by PI4KB to generate their replication organelle (656). Development of specific PI4K inhibitors now attracts a lot more interest than it did a few years ago. Another process where phosphoinositides can be important is the budding of viruses when they leave the cell. This process uses several of the same lipid and protein regulators that are required for the biogenesis of the MVB during the formation of inwardly budding vesicles (1016), and inhibition of PIKfyve-catalyzed formation of PtdIns(3,5)P2 was found to inhibit retroviral budding (686).
Other pathogens do not rely upon the host cell's phosphoinositide metabolizing enzymes to create the lipid environment that is required for infection or survival. For example, Listeria produces a PLC enzyme as an important virulence factor that helps the bacterium escape from the vacuole of infected cells (1235). Salmonella enterica and Shigella flexneri inject an enzyme, called SigD/SopB and IpgD in the two strains, respectively, into the prospective host cells that induce active membrane remodeling to help the bacterial invasion at the entry process and, in the case of Shigella, its intracellular release from the endocytic compartment. Both of these enzymes show homology to mammalian inositol lipid phosphatases with a relatively broad in vitro specificity (993, 1151). However, inside the cells, these enzymes appear to hydrolyze primarily PtdIns(4,5)P2 with production of the rare and enigmatic PtdIns5P (607, 1130, 1544). These enzymes also lead to the production of 3-phosphorylated lipids (1130, 1206) by mechanisms that are not all that clear and, curiously, are not inhibited by classical PI3K inhibitors in the case of Salmonella SigD/SopB (975, 993). A similar phosphatase acting upon PtdIns(4,5)P2, called VPA0450, was identified in Vibrio parahaemolyticus (185), and a previously described acid phosphatase of Legionella that inhibits superoxide production of neutrophil cells is also capable of PtdIns(4,5)P2 hydrolysis (1323). The intracellular parasite, Plasmodium falciparum that causes malaria also produces its own inositol lipid kinases, a class III PI3K ortholog PfPI3K (1533, 1596), a PI4K ortholog PFE0485w (826), and a PIP 5-kinase (864) and also increases the levels of several inositol lipid isomers in the infected red blood cells (399, 1533). A plasmodium PLC enzyme has also been described (1245), and activation of Ca2+ signaling within the parasite has also been observed (37). Mycobacterium tuberculosis uses its own tricks to stay dormant in macrophages and evade the lysosome. It uses its own PtdIns-mannoside to inhibit PtdIns3P production (256) but also secretes a phosphatase, called SapM capable of dephosphorylating PtdIns3P (1630). Another phosphatase, MptpB, which is an important virulence factor of Mycobacterium tuberculosis (1411), is a trifunctional enzyme that hydrolyzes phosphoserine/threonines, phosphotyrosines, and phosphoinositides (120, 121). While inhibiting PtdIns3P production of the host, Mycobacteria also synthesize PtdIns3P (1085), apparently as an intermediate of PtdIns synthesis and not as a result of PtdIns phosphorylation (1084).
A lot more details about these processes can be found in several recent reviews (290, 613, 1204). Nevertheless, even this short summary makes it clear that pharmacological targeting of phosphoinositide metabolizing enzymes could be beneficial in fighting a variety of infectious agents and, indeed, has already attracted significant attention (38, 144, 243).
XII. CONCLUDING REMARKS
Even a lengthy review like this is unable to cover all of what has been learned about phosphoinositides over the last 50 years. It is clear that these lipids are fundamental in the regulation of all aspects of membrane dynamics in eukaryotic cells. However, it is also clear that we do not have a clear understanding of the overall underlying principles of how these lipids work and why we run into them wherever we look in biological research and medicine. The arrival of such a fundamental understanding will be marked when a single short paper can summarize much of what is covered here in hundreds of pages. From an evolutionary and teleological standpoint, phosphorylation of lipids (as well as proteins) is a way for simple organisms to store phosphate, a uniquely important commodity, whether a component of DNA or of the energy currency ATP. The inositol ring is a very useful structure for this purpose as it can take several phosphate molecules, especially in its soluble form, and indeed, it has been recognized as a phosphate storage molecule in seeds (1246). Phosphorylated inositides, therefore, must have served as signals to indicate the phosphate status of simple organisms and molecules capable of recognizing the phosphorylated headgroups must have developed to communicate this information to the processes that responded to the availability of phosphate from external sources. These primitive information circuits have evolved and been utilized in a number of new ways creating the seven inositol lipid isomers, produced by the multiple kinases and phosphatases and talking to the myriad of effector molecules. The permutation of these elements provides ample variations to serve the signaling needs of a variety of cellular processes discussed in this review.
As in any other scientific discipline, the progress in inositide research has been tightly linked with methodological advances. Much of what was learned from the metabolic studies using radioactive tracers and bulk lipid separation techniques paved the road for subsequent work on isolation and cloning of the enzymes. Progress in molecular biology techniques and the sequencing of the various genomes gave an enormous boost to these efforts and helped identify many of the inositide converting enzymes known today. Advances in genetics and positional cloning identified enzyme defects in inositide metabolism in several human diseases, and gene knockout and transgenic technologies allowed the creation of animal models for better understanding the physiological and pathological roles of these lipids and their selected metabolic enzymes. As we understand more and more about the importance of spatial and temporal organization of inositol lipid signals, it has become clear that we needed to follow lipid changes in live cells with subcellular resolution (94). Moreover, now we want to artificially induce inositol lipid changes in localized compartments to see the consequences for specific cellular processes (437, 1489, 1586, 1617). These techniques are now being extended to achieve superresolution level imaging (141, 917). These advances will surely help us better understand the biology of phosphoinositides.
Lastly, a word about how public health has benefitted from phosphoinositide research. As we look back on the long road that has been traveled from the first description of phosphoinositides in the brain to the PI3K inhibitors that are currently in clinical trials as anti-cancer or anti-inflammatory drugs, we cannot help but recognize the principles of how humankind benefits from basic research. It is quite unlikely that those early scientists thought about the “usefulness” of their research for immediately palpable results to improve human conditions. They were driven primarily by scientific curiosity and, sadly, might not have been funded in the current milieu of short-sighted demand for foreseeable immediate return by funding agencies. The impact of their research was not predictable, and that is the essence of basic research. And yet, wanted or not, the moments have come when those efforts, pieced together, outlined a larger picture that had tremendous relevance to human physiology and disease. Miraculously, without much organized intervention, the Pharma industry reacted and a translational aspect of phosphoinositide research has naturally evolved, yielding drugs that may see the market really soon, and this process is being extended to other inositides and diseases. We can only hope that these examples will serve as reminders that high basic quality basic research is worth pursuing and funding even when the outcome is less than predictable or exatly because of its unpredictability.
GRANTS
The author's research is supported by the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development of the National Institutes of Health.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author.
ACKNOWLEDGMENTS
I thank Dr. Robert Michell for his kind reading and suggestions on the historical overview and Drs. Tibor Rohacs and Gerald Hammond for their valuable comments. I am grateful to Anna Mezey-Brownstein for proofreading the article.
Address for reprint requests and other correspondence: T. Balla, National Institutes of Health, Bldg. 49, Rm. 5A22, 49 Convent Dr., Bethesda, MD 20892 (e-mail: ballat@mail.nih.gov).
REFERENCES
- 1. Abdel-Latif AA, Akhtar RA, Hawthorne JN. Acetylcholine increases the breakdown of triphosphoinositide in rabbit iris muscle prelabelled with [32P]phosphate. Biochem J 162: 61–73, 1977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Abe N, Inoue T, Galvez T, Klein L, Meyer T. Dissecting the role of PtdIns(4,5)P2 in endocytosis and recycling of the transferrin receptor. J Cell Sci 121: 1488–1494, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Abnet CC, Freedman ND, Hu N, Wang Z, Yu K, Shu XO, Yuan JM, Zheng W, Dawsey SM, Dong LM, Lee MP, Ding T, Qiao YL, Gao YT, Koh WP, Xiang YB, Tang ZZ, Fan JH, Wang C, Wheeler W, Gail MH, Yeager M, Yuenger J, Hutchinson A, Jacobs KB, Giffen CA, Burdett L, Fraumeni JF, Jr, Tucker MA, Chow WH, Goldstein AM, Chanock SJ, Taylor PR. A shared susceptibility locus in PLCE1 at 10q23 for gastric adenocarcinoma and esophageal squamous cell carcinoma. Nat Genet 42: 764–767, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Achiriloaie M, Barylko B, Albanesi JP. Essential role of the dynamin pleckstrin homology domain in receptor-mediated endocytosis. Mol Cell Biol 19: 1410–1415, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Adamski FM, Timms KM, Shieh BH. A unique isoform of phospholipase Cbeta4 highly expressed in the cerebellum and eye. Biochim Biophys Acta 1444: 55–60, 1999 [DOI] [PubMed] [Google Scholar]
- 6. Aderem A, Underhill DM. Mechanisms of phagocytosis in macrophages. Annu Rev Immunol 17: 593–623, 1999 [DOI] [PubMed] [Google Scholar]
- 7. Adjobo-Hermans MJ, Crosby KC, Putyrski M, Bhageloe A, van Weeren L, Schultz C, Goedhart J, Gadella TW., Jr PLCbeta isoforms differ in their subcellular location and their CT-domain dependent interaction with Galphaq. Cell Signal 25: 255–263, 2013 [DOI] [PubMed] [Google Scholar]
- 8. Adjobo-Hermans MJ, Goedhart J, Gadella TW., Jr Regulation of PLCbeta1a membrane anchoring by its substrate phosphatidylinositol (4,5)-bisphosphate. J Cell Sci 121: 3770–3777, 2008 [DOI] [PubMed] [Google Scholar]
- 9. Agranoff BW. Cyclitol confusion. Trends Biochem Sci 3: N283–N285, 1978 [Google Scholar]
- 10. Agranoff BW. Turtles all the way: reflections on myo-inositol. J Biol Chem 284: 21121–21126, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Agranoff BW, Bradley RM, Brady RO. The enzymatic synthesis of inositol phosphatide. J Biol Chem 233: 1077–1083, 1958 [PubMed] [Google Scholar]
- 12. Aharanovitz O, Zaun HC, Balla T, York JD, Orlowski J, Grinstein S. Intracellular pH regulation by Na+/H+ exchange requires phosphatidylinositol 4,5-bisphosphate. J Cell Biol 150: 213–224, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ahn J, Chung KS, Kim DU, Won M, Kim L, Kim KS, Nam M, Choi SJ, Kim HC, Yoon M, Chae SK, Hoe KL. Systematic identification of hepatocellular proteins interacting with NS5A of the hepatitis C virus. J Biochem Mol Biol 37: 741–748, 2004 [DOI] [PubMed] [Google Scholar]
- 14. Ai J, Maturu A, Johnson W, Wang Y, Marsh CB, Tridandapani S. The inositol phosphatase SHIP-2 down-regulates FcgammaR-mediated phagocytosis in murine macrophages independently of SHIP-1. Blood 107: 813–820, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Aikawa Y, Hara H, Watanabe T. Molecular cloning and characterization of mammalian homologues of the Drosophila retinal degeneration B gene. Biochem Biophys Res Commun 236: 559–564, 1997 [DOI] [PubMed] [Google Scholar]
- 16. Aikawa Y, Kuraoka A, Kondo H, Kawabuchi M, Watanabe T. Involvement of PITPnm, a mammalian homologue of Drosophila rdgB, in phosphoinositide synthesis on Golgi membranes. J Biol Chem 274: 20569–20577, 1999 [DOI] [PubMed] [Google Scholar]
- 17. Aikawa Y, Martin TF. ADP-ribosylation factor 6 regulation of phosphatidylinositol-4,5-bisphosphate synthesis, endocytosis, and exocytosis. Methods Enzymol 404: 422–431, 2005 [DOI] [PubMed] [Google Scholar]
- 18. Aittaleb M, Boguth CA, Tesmer JJ. Structure and function of heterotrimeric G protein-regulated Rho guanine nucleotide exchange factors. Mol Pharmacol 77: 111–125, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Akbulut S, Reddi AL, Aggarwal P, Ambardekar C, Canciani B, Kim MK, Hix L, Vilimas T, Mason J, Basson MA, Lovatt M, Powell J, Collins S, Quatela S, Phillips M, Licht JD. Sprouty proteins inhibit receptor-mediated activation of phosphatidylinositol-specific phospholipase C. Mol Biol Cell 21: 3487–3496, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Akhtar A, Gasser SM. The nuclear envelope and transcriptional control. Nat Rev Genet 8: 507–517, 2007 [DOI] [PubMed] [Google Scholar]
- 21. Akhtar RA, Abdel-Latif AA. A calcium ion requirement for acetylcholine-stimulated breakdown of triphosphoinositide in rabbit iris smooth muscle. Pharmacol Exp Ther 204: 655–668, 1978 [PubMed] [Google Scholar]
- 22. Al-Khouri AM, Ma Y, Togo SH, Williams S, Mustelin T. Cooperative phosphorylation of the tumor suppressor phosphatase and tensin homologue (PTEN) by casein kinases and glycogen synthase kinase 3beta. J Biol Chem 280: 35195–35202, 2005 [DOI] [PubMed] [Google Scholar]
- 23. Alb JG, Jr, Cortese JD, Phillips SE, Albin RL, Nagy TR, Hamilton BA, Bankaitis VA. Mice lacking phosphatidylinositol transfer protein-alpha exhibit spinocerebellar degeneration, intestinal and hepatic steatosis, and hypoglycemia. J Biol Chem 278: 33501–33518, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Alb JG, Jr, Gedvilaite A, Cartee RT, Skinner HB, Bankaitis VA. Mutant rat phosphatidylinositol/phosphatidylcholine transfer proteins specifically defective in phosphatidylinositol transfer: implications for the regulation of phospholipid transfer activity. Proc Natl Acad Sci USA 92: 8826–8830, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Alb JG, Jr, Phillips SE, Rostand K, Cui X, Pinxteren J, Cotlin L, Manning T, Guo S, York JD, Sontheimer H, Collawn JF, Bankaitis VA. Genetic ablation of phosphatidylinositol transfer protein function in murine embryonic stem cells. Mol Biol Cell 13: 739–754, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Alb JG, Jr, Phillips SE, Wilfley LR, Philpot BD, Bankaitis VA. The pathologies associated with functional titration of phosphatidylinositol transfer protein alpha activity in mice. J Lipid Res 48: 1857–1872, 2007 [DOI] [PubMed] [Google Scholar]
- 27. Albert AP, Saleh SN, Large WA. Inhibition of native TRPC6 channel activity by phosphatidylinositol 4,5-bisphosphate in mesenteric artery myocytes. J Physiol 586: 3087–3095, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Alcazar-Roman AR, Bolger TA, Wente SR. Control of mRNA export and translation termination by inositol hexakisphosphate requires specific interaction with Gle1. J Biol Chem 285: 16683–16692, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Alcazar-Roman AR, Wente SR. Inositol polyphosphates: a new frontier for regulating gene expression. Chromosoma 117: 1–13, 2008 [DOI] [PubMed] [Google Scholar]
- 30. Ali K, Bilancio A, Thomas M, Pearce W, Gilfillan AM, Tkaczyk C, Kuehn N, Gray A, Giddings J, Peskett E, Fox R, Bruce I, Walker C, Sawyer C, Okkenhaug K, Finan P, Vanhaesebroeck B. Essential role for the p110delta phosphoinositide 3-kinase in the allergic response. Nature 431: 1007–1011, 2004 [DOI] [PubMed] [Google Scholar]
- 31. Alimonti A, Carracedo A, Clohessy JG, Trotman LC, Nardella C, Egia A, Salmena L, Sampieri K, Haveman WJ, Brogi E, Richardson AL, Zhang J, Pandolfi PP. Subtle variations in Pten dose determine cancer susceptibility. Nat Genet 42: 454–458, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Allan D, Michell RH. Phosphatidylinositol cleavage in lymphocytes: requirement for Ca2+ at a low concentration and effects of other cations. Biochem J 142: 599–604, 1974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Allen V, Swigart P, Cheung R, Cockcroft S, Katan M. Regulation of inositol lipid-specific phospholipase Cdelta by changes in Ca2+ ion concentrations. Biochem J 327: 545–552, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Allison JH, Blisner ME, Holland WH, Hipps PP, Sherman WR. Increased brain myo-inositol 1-phosphate in lithium-treted rats. Biochem Biophys Res Commun 71: 664–670, 1976 [DOI] [PubMed] [Google Scholar]
- 35. Allison JH, Stewart MA. Reduced brain inositol in lithium-treated rats. Nature 233: 267–268, 1971 [DOI] [PubMed] [Google Scholar]
- 36. Alvarez-Venegas R, Avramova Z. SET-domain proteins of the Su(var)3–9, E(z) and trithorax families. Gene 285: 25–37, 2002 [DOI] [PubMed] [Google Scholar]
- 37. Alves E, Bartlett PJ, Garcia CR, Thomas AP. Melatonin and IP3-induced Ca2+ release from intracellular stores in the malaria parasite Plasmodium falciparum within infected red blood cells. J Biol Chem 286: 5905–5912, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Alvisi G, Madan V, Bartenschlager R. Hepatitis C virus and host cell lipids: an intimate connection. RNA Biol 8: 258–269, 2011 [DOI] [PubMed] [Google Scholar]
- 39. Amarilio R, Ramachandran S, Sabanay H, Lev S. Differential regulation of endoplasmic reticulum structure through VAP-Nir protein interaction. J Biol Chem 280: 5934–5944, 2005 [DOI] [PubMed] [Google Scholar]
- 40. Amatruda JF, Gattermeir DJ, Karpova TS, Cooper JA. Effects of null mutations and overexpression of capping protein on morphogenesis, actin distribution and polarized secretion in yeast. J Cell Biol 119: 1151–1162, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ameriks MK, Venable JD. Small molecule inhibitors of phosphoinositide 3-kinase (PI3K) delta and gamma. Curr Top Med Chem 9: 738–753, 2009 [DOI] [PubMed] [Google Scholar]
- 42. Amoras AL, Kanegane H, Miyawaki T, Vilela MM. Defective Fc-, CR1- and CR3-mediated monocyte phagocytosis and chemotaxis in common variable immunodeficiency and X-linked agammaglobulinemia patients. J Invest Allergol Clin Immunol 13: 181–188, 2003 [PubMed] [Google Scholar]
- 43. Ananthanarayanan B, Das S, Rhee SG, Murray D, Cho W. Membrane targeting of C2 domains of phospholipase C-delta isoforms. J Biol Chem 277: 3568–3575, 2002 [DOI] [PubMed] [Google Scholar]
- 44. Andreotti AH, Schwartzberg PL, Joseph RE, Berg LJ. T-cell signaling regulated by the Tec family kinase, Itk. Cold Spring Harb Perspect Biol 2: a002287, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Andrew N, Insall RH. Chemotaxis in shallow gradients is mediated independently of PtdIns 3-kinase by biased choices between random protrusions. Nat Cell Biol 9: 193–200, 2007 [DOI] [PubMed] [Google Scholar]
- 46. Ann K, Kowalchyk JA, Loyet KM, Martin TF. Novel Ca2+-binding protein (CAPS) related to UNC-31 required for Ca2+-activated exocytosis. J Biol Chem 272: 19637–19640, 1997 [DOI] [PubMed] [Google Scholar]
- 47. Antignano F, Ibaraki M, Kim C, Ruschmann J, Zhang A, Helgason CD, Krystal G. SHIP is required for dendritic cell maturation. J Immunol 184: 2805–2813, 2010 [DOI] [PubMed] [Google Scholar]
- 48. Antonescu CN, Aguet F, Danuser G, Schmid SL. Phosphatidylinositol-(4,5)-bisphosphate regulates clathrin-coated pit initiation, stabilization, and size. Mol Biol Cell 22: 2588–2600, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Aoyagi K, Sugaya T, Umeda M, Yamamoto S, Terakawa S, Takahashi M. The activation of exocytotic sites by the formation of phosphatidylinositol 4,5-bisphosphate microdomains at syntaxin clusters. J Biol Chem 280: 17346–17352, 2005 [DOI] [PubMed] [Google Scholar]
- 50. Araki N, Johnson MT, Swanson JA. A role for phosphoinositide 3-kinase in the completion of macropinocytosis and phagocytosis by macrophages. J Cell Biol 135: 1249–1260, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Arcaro A, Khanzada UK, Vanhaesebroeck B, Tetley TD, Waterfield MD, Seckl MJ. Two distinct phosphoinositide 3-kinases mediate polypeptide growth factor-stimulated PKB activation. EMBO J 21: 5097–5108, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Arcaro A, Wymann MP. Wortmannin is a potent phosphatidylinositol 3-kinase inhibitor: the role of phosphatidylinositol 3,4,5-trisphosphate in neutrophil responses. Biochem J 296: 297–301, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Arcaro A, Zvelebil MJ, Wallasch C, Ullrich A, Waterfield MD, Domin J. Class II phosphoinositide 3-kinases are downstream targets of activated polypeptide growth factor receptors. Mol Cell Biol 20: 3817–3830, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Arendt KL, Royo M, Fernandez-Monreal M, Knafo S, Petrok CN, Martens JR, Esteban JA. PIP3 controls synaptic function by maintaining AMPA receptor clustering at the postsynaptic membrane. Nat Neurosci 13: 36–44, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Arinami T. Analyses of the associations between the genes of 22q11 deletion syndrome and schizophrenia. J Hum Genet 51: 1037–1045, 2006 [DOI] [PubMed] [Google Scholar]
- 56. Arteaga CL, Johnson MD, Todderud G, Coffey RJ, Carpenter G, Page DL. Elevated content of the tyrosine kinase substrate phospholipase C-gamma 1 in primary human breast carcinomas. Proc Natl Acad Sci USA 88: 10435–10439, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Ashrafi K, Chang FY, Watts JL, Fraser AG, Kamath RS, Ahringer J, Ruvkun G. Genome-wide RNAi analysis of Caenorhabditis elegans fat regulatory genes. Nature 421: 268–272, 2003 [DOI] [PubMed] [Google Scholar]
- 58. Asteggiano C, Berberian G, Beauge L. Phosphatidyl inositol-4,5-bisphosphate bound to bovine cardiac Na+/Ca2+ exchanger displays a MgATP regulation similar to that of the exchange fluxes. Eur J Biochem 268: 437–442, 2001 [DOI] [PubMed] [Google Scholar]
- 59. Astle MV, Ooms LM, Cole AR, Binge LC, Dyson JM, Layton MJ, Petratos S, Sutherland C, Mitchell CA. Identification of a proline-rich inositol polyphosphate 5-phosphatase (PIPP): collapsin response mediator protein 2 (CRMP2) complex that regulates neurite elongation. J Biol Chem 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Atack JR. Inositol monophosphatase inhibitors–lithium mimetics? Med Res Rev 17: 215–224, 1997 [DOI] [PubMed] [Google Scholar]
- 61. Attree O, Olivos IM, Okabe I, Bauiley LC, Nelson DL, Lewis RA, McInnes RR, Nussbaum RL. The Lowe's oculocerebrorenal syndrome gene encodes a protein highly homologous to inositol polyphosphate-5-phosphatase. Nature 358: 239–242, 1992 [DOI] [PubMed] [Google Scholar]
- 62. Audhya A, Emr SD. Regulation of PI4,5P2 synthesis by nuclear-cytoplasmic shuttling of the Mss4 lipid kinase. Embo J 22: 4223–4236, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Audhya A, Emr SD. Stt4 PI 4-kinase localizes to the plasma membrane and functions in the Pkc1-mediated MAP kinase cascade. Dev Cell 2: 593–605, 2002 [DOI] [PubMed] [Google Scholar]
- 64. Audhya A, Foti M, Emr SD. Distinct roles for the yeast phosphatidylinositol 4-kinases, stt4p and pik1p, in secretion, cell growth, and organelle membrane dynamics. Mol Biol Cell 11: 2673–2689, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Audhya A, Loewith R, Parsons AB, Gao L, Tabuchi M, Zhou H, Boone C, Hall MN, Emr SD. Genome-wide lethality screen identifies new PI4,5P2 effectors that regulate the actin cytoskeleton. Embo J 23: 3747–3757, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Auger KR, Serunian LA, Soltoff SP, Libby P, Cantley LC. PDGF-dependent tyrosine phosphorylation stimulates production of novel polyphosphoinositides in intact cells. Cell 57: 167–175, 1989 [DOI] [PubMed] [Google Scholar]
- 67. Augustine JA, Secrist JP, Daniels JK, Leibson PJ, Abraham RT. Signal transduction through the T cell antigen receptor. Activation of phospholipase C through a G protein-independent coupling mechanism. J Immunol 146: 2889–2897, 1991 [PubMed] [Google Scholar]
- 68. Auvinen E, Kivi N, Vaheri A. Regulation of ezrin localization by Rac1 and PIPK in human epithelial cells. Exp Cell Res 313: 824–833, 2007 [DOI] [PubMed] [Google Scholar]
- 69. Azzedine H, Bolino A, Taieb T, Birouk N, Di Duca M, Bouhouche A, Benamou S, Mrabet A, Hammadouche T, Chkili T, Gouider R, Ravazzolo R, Brice A, Laporte J, LeGuern E. Mutations in MTMR13, a new pseudophosphatase homologue of MTMR2 and Sbf1, in two families with an autosomal recessive demyelinating form of Charcot-Marie-Tooth disease associated with early-onset glaucoma. Am J Hum Genet 72: 1141–1153, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Babst M, Katzmann DJ, Estepa-Sabal EJ, Meerloo T, Emr SD. Escrt-III: an endosome-associated heterooligomeric protein complex required for mvb sorting. Dev Cell 3: 271–282, 2002 [DOI] [PubMed] [Google Scholar]
- 71. Babst M, Katzmann DJ, Snyder WB, Wendland B, Emr SD. Endosome-associated complex, ESCRT-II, recruits transport machinery for protein sorting at the multivesicular body. Dev Cell 3: 283–289, 2002 [DOI] [PubMed] [Google Scholar]
- 72. Bach TL, Chen QM, Kerr WT, Wang Y, Lian L, Choi JK, Wu D, Kazanietz MG, Koretzky GA, Zigmond S, Abrams CS. Phospholipase cbeta is critical for T cell chemotaxis. J Immunol 179: 2223–2227, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Bache KG, Raiborg C, Mehlum A, Stenmark H. STAM and Hrs are subunits of a multivalent ubiquitin-binding complex on early endosomes. J Biol Chem 278: 12513–12521, 2003 [DOI] [PubMed] [Google Scholar]
- 74. Backer JM. The regulation and function of Class III PI3Ks: novel roles for Vps34. Biochem J 410: 1–17, 2008 [DOI] [PubMed] [Google Scholar]
- 75. Backer JM. The regulation of class IA PI 3-kinases by inter-subunit interactions. Curr Top Microbiol Immunol 346: 87–114, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Backer JM, Myers MG, Jr, Shoelson SE, Chin DJ, Sun XJ, Miralpeix M, Hu P, Margolis B, Skolnik EY, Schlessinger J. Phosphatidylinositol 3'-kinase is activated by association with IRS-1 during insulin stimulation. EMBO J 11: 3469–3479, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Bae YS, Cantley LG, Chen CS, Kim SR, Kwon KS, Rhee SG. Activation of phosphpolipase C-gamma by phosphatidylinositol 3,4,5-trisphosphate. J Biol Chem 273: 4465–4469, 1998 [DOI] [PubMed] [Google Scholar]
- 78. Bai J, Tucker WC, Chapman ER. PIP2 increases the speed of response of synaptotagmin and steers its membrane-penetration activity toward the plasma membrane. Nat Struct Mol Biol 11: 36–44, 2004 [DOI] [PubMed] [Google Scholar]
- 79. Baird D, Stefan C, Audhya A, Weys S, Emr SD. Assembly of the PtdIns 4-kinase Stt4 complex at the plasma membrane requires Ypp1 and Efr3. J Cell Biol 183: 1061–1074, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Bairstow SF, Ling K, Su X, Firestone AJ, Carbonara C, Anderson RA. Type Igamma661 phosphatidylinositol phosphate kinase directly interacts with AP2 and regulates endocytosis. J Biol Chem 281: 20632–20642, 2006 [DOI] [PubMed] [Google Scholar]
- 81. Balla A, Balla T. Phosphatidylinositol 4-kinases; old enzymes with emerging functions. Trends Cell Biol 16: 351–361, 2006 [DOI] [PubMed] [Google Scholar]
- 82. Balla A, Kim YJ, Varnai P, Szentpetery Z, Knight Z, Shokat KM, Balla T. Maintenance of hormone-sensitive phosphoinositide pools in the plasma membrane requires phosphatidylinositol 4-kinase IIIα. Mol Biol Cell 19: 711–721, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Balla A, Tuymetova G, Barshishat M, Geiszt M, Balla T. Characterization of type II phosphatidylinositol 4-kinase isoforms reveals association of the enzymes with endosomal vesicular compartments. J Biol Chem 277: 20041–22050, 2002 [DOI] [PubMed] [Google Scholar]
- 84. Balla A, Tuymetova G, Tsiomenko A, Varnai P, Balla T. A plasma membrane pool of phosphatidylinositol 4-phosphate is generated by phosphatidylinositol 4-kinase type-III alpha: studies with the PH domains of the oxysterol binding protein and FAPP1. Mol Biol Cell 16: 1282–1295, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Balla T. Inositol-lipid binding motifs: signal integrators through protein-lipid and protein-protein interactions. J Cell Sci 118: 2093–2104, 2005 [DOI] [PubMed] [Google Scholar]
- 86. Balla T, Baukal A, Hunyady L, Catt KJ. Agonist-induced regulation of inositol tetrakisphosphate isomers and inositol pentakisphosphate in adrenal glomerulosa cells. J Biol Chem 264: 13605–13611, 1989 [PubMed] [Google Scholar]
- 87. Balla T, Baukal AJ, Guillemette G, Catt KJ. Multiple pathways of inositol polyphosphate metabolism in angiotensin-stimulated adrenal glomerulosa cells. JBiolChem 263: 4083–4091, 1988 [PubMed] [Google Scholar]
- 88. Balla T, Baukal AJ, Guillemette G, Morgan RO, Catt KJ. Angiotensin-stimulated production of inositol trisphosphate isomers and rapid metabolism through inositol 4-monophosphate in adrenal glomerulosa cells. Proc Natl Acad Sci USA 83: 9323–9327, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Balla T, Downing GJ, Jaffe H, Kim S, Zolyomi A, Catt KJ. Isolation and molecular cloning of wortmannin-sensitive bovine type-III phosphatidylinositol 4-kinases. J Biol Chem 272: 18358–18366, 1997 [DOI] [PubMed] [Google Scholar]
- 90. Balla T, Enyedi P, Hunyady L, Spät A. Effects of lithium on angiotensin-stimulated phosphatidylinositol turnover and aldosterone production in adrenal glomerulosa cells: a possible causal relationship. FEBS Lett 171: 179–182, 1984 [DOI] [PubMed] [Google Scholar]
- 91. Balla T, Guillemette G, Baukal AJ, Catt KJ. Formation of inositol 1,3,4,6-tetrakisphosphate during angiotensin II action in bovine adrenal glomerulosa cells. Biochem Biophys Res Commun 148: 199–205, 1987 [DOI] [PubMed] [Google Scholar]
- 92. Balla T, Guillemette G, Baukal AJ, Catt KJ. Metabolism of inositol 1,3,4-trisphosphate to a new tetrakisphosphate isomer in angiotensin-stimulated adrenal glomerulosa cells. J Biol Chem 262: 9952–9955, 1987 [PubMed] [Google Scholar]
- 93. Balla T, Sim SS, Baukal AJ, Rhee SG, Catt KJ. Inositol polyphosphates are not increased by overexpression of Ins(1,4,5)P3 3-kinase but show cell-cycle dependent changes in growth factor-stimulated fibroblasts. Mol Biol Cell 5: 17–27, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Balla T, Varnai P. Visualization of cellular phosphoinositide pools with GFP-fused protein-domains. Curr Protoc Cell Biol Chapter 24: Unit 24 24, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Ballas LM, Bell RM. Topography of glycerolipid synthetic enzymes. Synthesis of phosphatidylserine, phosphatidylinositol and glycerolipid intermediates occurs on the cytoplasmic surface of rat liver microsomal vesicles. Biochim Biophys Acta 665: 586–595, 1981 [DOI] [PubMed] [Google Scholar]
- 96. Ballou CE, Lee YC. The structure of a myoinositol mannoside from Myobacterium tuberculosis glycolipid. Biochemistry 3: 682–685, 1964 [DOI] [PubMed] [Google Scholar]
- 97. Banerji S, Ngo M, Lane CF, Robinson CA, Minogue S, Ridgway ND. Oxysterol binding protein-dependent activation of sphingomyelin synthesis in the golgi apparatus requires phosphatidylinositol 4-kinase IIα. Mol Biol Cell 21: 4141–4150, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Bankaitis VA, Aitken JR, Cleves AE, Dowhan W. An essential role for a phospholipid transfer protein in yeast Golgi function. Nature 347: 561–562, 1990 [DOI] [PubMed] [Google Scholar]
- 99. Bankaitis VA, Cortese J, Phillips SE, Alb JG., Jr Phosphatidylinositol transfer protein function in the mouse. Adv Enzyme Regul 44: 201–218, 2004 [DOI] [PubMed] [Google Scholar]
- 100. Bankaitis VA, Garcia-Mata R, Mousley CJ. Golgi membrane dynamics and lipid metabolism. Curr Biol 22: R414–424, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Bankaitis VA, Johnson LM, Emr SD. Isolation of yeast mutants defective in protein targeting to the vacuole. Proc Natl Acad Sci USA 83: 9075–9079, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Bankaitis VA, Malehorn DE, Emr SD, Greene R. The Saccharomyces cerevisiae SEC 14 gene encodes a cytosolic factor that is required for transport of secretory proteins from the yeast Golgi complex. J Cell Biol 108: 1271–1281, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Bankaitis VA, Mousley CJ, Schaaf G. The Sec14 superfamily and mechanisms for crosstalk between lipid metabolism and lipid signaling. Trends Biochem Sci 35: 150–160, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Barber DF, Bartolome A, Hernandez C, Flores JM, Redondo C, Fernandez-Arias C, Camps M, Ruckle T, Schwarz MK, Rodriguez S, Martinez AC, Balomenos D, Rommel C, Carrera AC. PI3Kgamma inhibition blocks glomerulonephritis and extends lifespan in a mouse model of systemic lupus. Nat Med 11: 933–935, 2005 [DOI] [PubMed] [Google Scholar]
- 105. Barbier M, Attoub S, Calvez R, Laffargue M, Jarry A, Mareel M, Altruda F, Gespach C, Wu D, Lu B, Hirsch E, Wymann MP. Tumour biology: weakening link to colorectal cancer? Nature 413: 796, 2001 [DOI] [PubMed] [Google Scholar]
- 106. Barbieri MA, Heath CM, Peters EM, Wells A, Davis JN, Stahl PD. Phosphatidylinositol-4-phosphate 5-kinase-1beta is essential for epidermal growth factor receptor-mediated endocytosis. J Biol Chem 276: 47212–47216, 2001 [DOI] [PubMed] [Google Scholar]
- 107. Barnache S, Le Scolan E, Kosmider O, Denis N, Moreau-Gachelin F. Phosphatidylinositol 4-phosphatase type II is an erythropoietin-responsive gene. Oncogene 25: 1420–1423, 2006 [DOI] [PubMed] [Google Scholar]
- 108. Barylko B, Gerber SH, Binns DD, Grichine N, Khvotchev M, Sudhof TC, Albanesi JP. A novel family of phosphatidylinositol 4-kinases conserved from yeast to humans. J Biol Chem 276: 7705–7708, 2001 [DOI] [PubMed] [Google Scholar]
- 109. Barylko B, Mao YS, Wlodarski P, Jung G, Binns DD, Sun HQ, Yin HL, Albanesi JP. Palmitoylation controls the catalytic activity and subcellular distribution of phosphatidylinositol 4-kinase IIα. J Biol Chem 284: 9994–10003, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Barylko B, Wlodarski P, Binns DD, Gerber SH, Earnest S, Sudhof TC, Grichine N, Albanesi JP. Analysis of the catalytic domain of phosphatidylinositol 4-kinase type II. J Biol Chem 277: 44366–44375, 2002 [DOI] [PubMed] [Google Scholar]
- 111. Bassett AS, Chow EW, AbdelMalik P, Gheorghiu M, Husted J, Weksberg R. The schizophrenia phenotype in 22q11 deletion syndrome. Am J Psychiatry 160: 1580–1586, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Batty IH, van der Kaay J, Gray A, Telfer JF, Dixon MJ, Downes CP. The control of phosphatidylinositol 3,4-bisphosphate concentrations by activation of the Src homology 2 domain containing inositol polyphosphate 5-phosphatase 2, SHIP2. Biochem J 407: 255–266, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Baukrowitz T, Schulte U, Oliver D, Herlitze S, Krauter T, Tucker SJ, Ruppersberg JP, Fakler B. PIP2 and PIP as determinants for ATP inhibition of KATP channels. Science 282: 1141–1144, 1998 [DOI] [PubMed] [Google Scholar]
- 114. Bazenet CE, Ruano AR, Brockman JL, Anderson RA. The human erythrocyte contains two forms of phosphatidylinositol-4-phosphate 5-kinase which are differentially active toward membranes. J Biol Chem 265: 18012–18022, 1990 [PubMed] [Google Scholar]
- 115. Beemiller P, Zhang Y, Mohan S, Levinsohn E, Gaeta I, Hoppe AD, Swanson JA. A Cdc42 activation cycle coordinated by PI 3-kinase during Fc receptor-mediated phagocytosis. Mol Biol Cell 21: 470–480, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Beggs AH, Bohm J, Snead E, Kozlowski M, Maurer M, Minor K, Childers MK, Taylor SM, Hitte C, Mickelson JR, Guo LT, Mizisin AP, Buj-Bello A, Tiret L, Laporte J, Shelton GD. MTM1 mutation associated with X-linked myotubular myopathy in Labrador Retrievers. Proc Natl Acad Sci USA 107: 14697–14702, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Begley MJ, Taylor GS, Brock MA, Ghosh P, Woods VL, Dixon JE. Molecular basis for substrate recognition by MTMR2, a myotubularin family phosphoinositide phosphatase. Proc Natl Acad Sci USA 103: 927–932, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Begley MJ, Taylor GS, Kim SA, Veine DM, Dixon JE, Stuckey JA. Crystal structure of a phosphoinositide phosphatase, MTMR2: insights into myotubular myopathy and Charcot-Marie-Tooth syndrome. Mol Cell 12: 1391–1402, 2003 [DOI] [PubMed] [Google Scholar]
- 119. Benjamins JA, Agranoff BW. Distribution and properties of CDP-diacylglycerol:inositol transferase from brain. J Neurochem 16: 513–527, 1969 [DOI] [PubMed] [Google Scholar]
- 120. Beresford N, Patel S, Armstrong J, Szoor B, Fordham-Skelton AP, Tabernero L. MptpB, a virulence factor from Mycobacterium tuberculosis, exhibits triple-specificity phosphatase activity. Biochem J 406: 13–18, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Beresford NJ, Saville C, Bennett HJ, Roberts IS, Tabernero L. A new family of phosphoinositide phosphatases in microorganisms: identification and biochemical analysis. BMC Genomics 11: 457, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Berger KL, Cooper JD, Heaton NS, Yoon R, Oakland TE, Jordan TX, Mateu G, Grakoui A, Randall G. Roles for endocytic trafficking and phosphatidylinositol 4-kinase III alpha in hepatitis C virus replication. Proc Natl Acad Sci USA 106: 7577–7582, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Berger P, Berger I, Schaffitzel C, Tersar K, Volkmer B, Suter U. Multi-level regulation of myotubularin-related protein-2 phosphatase activity by myotubularin-related protein-13/set-binding factor-2. Hum Mol Genet 15: 569–579, 2006 [DOI] [PubMed] [Google Scholar]
- 124. Berger P, Bonneick S, Willi S, Wymann M, Suter U. Loss of phosphatase activity in myotubularin-related protein 2 is associated with Charcot-Marie-Tooth disease type 4B1. Hum Mol Genet 11: 1569–1579, 2002 [DOI] [PubMed] [Google Scholar]
- 125. Berndt A, Miller S, Williams O, Le DD, Houseman BT, Pacold JI, Gorrec F, Hon WC, Liu Y, Rommel C, Gaillard P, Ruckle T, Schwarz MK, Shokat KM, Shaw JP, Williams RL. The p110 delta structure: mechanisms for selectivity and potency of new PI(3)K inhibitors. Nat Chem Biol 6: 117–124, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Bernier LP, Ase AR, Chevallier S, Blais D, Zhao Q, Boue-Grabot E, Logothetis D, Seguela P. Phosphoinositides regulate P2X4 ATP-gated channels through direct interactions. J Neurosci 28: 12938–12945, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Bernier LP, Blais D, Boue-Grabot E, Seguella P. A dual polybasic motif determines phosphoinositide binding and regulation in the P2X channel family. Plos One 7: e40595, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Bernstein HG, Keilhoff G, Reiser M, Freese S, Wetzker R. Tissue distribution and subcellular localization of a G-protein activated phosphoinositide 3-kinase. An immunohistochemical study. Cell Mol Biol 44: 973–983, 1998 [PubMed] [Google Scholar]
- 129. Berridge MJ. Calcium signalling remodelling and disease. Biochem Soc Trans 40: 297–309, 2012 [DOI] [PubMed] [Google Scholar]
- 130. Berridge MJ. Inositol trisphosphate and diacylglycerol as intracellular messengers. Biochem J 220: 345–360, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Berridge MJ. Rapid accumulation of inositol trisphosphate reveals that agonists hydrolyze polyphosphoinositides instead of phosphatidylinositol. Biochem J 212: 849–858, 1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Berridge MJ, Dawson RMC, Downes CP, Heslop JP, Irvine RF. Changes in the levels of inositol phosphates after agonist-dependent hydrolysis of membrane phosphoinositides. Biochem J 212: 473–482, 1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Berridge MJ, Downes CP, Hanley MR. Lithium amplifies agonist-dependent phosphatidylinositol responses in brain and salivary glands. Biochem J 206: 587–595, 1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Berridge MJ, Downes CP, Hanley MR. Neural and developmental actions of lithium: a unifying hypothesis. Cell 59: 411–419, 1989 [DOI] [PubMed] [Google Scholar]
- 135. Berridge MJ, Irvine RF. Inositol trisphosphate, a novel second messenger in cellular signal transduction. Nature 312: 315–321, 1984 [DOI] [PubMed] [Google Scholar]
- 136. Berridge MJ, Lipp P, Bootman MD. The versatility and universality of calcium signalling. Nat Rev Mol Cell Biol 1: 11–21, 2002 [DOI] [PubMed] [Google Scholar]
- 137. Berry GT, Buccafusca R, Greer JJ, Eccleston E. Phosphoinositide deficiency due to inositol depletion is not a mechanism of lithium action in brain. Mol Genet Metab 82: 87–92, 2004 [DOI] [PubMed] [Google Scholar]
- 138. Berry GT, Wu S, Buccafusca R, Ren J, Gonzales LW, Ballard PL, Golden JA, Stevens MJ, Greer JJ. Loss of murine Na+/myo-inositol cotransporter leads to brain myo-inositol depletion and central apnea. J Biol Chem 278: 18297–18302, 2003 [DOI] [PubMed] [Google Scholar]
- 139. Berstein G, Blank JL, Jhon DY, Exton JH, Rhee SG, Ross EM. Phospholipase C-beta 1 is a GTPase-activating protein for Gq/11, its physiologic regulator. Cell 70: 411–418, 1992 [DOI] [PubMed] [Google Scholar]
- 140. Bertin A, McMurray MA, Thai L, Garcia G, 3rd, Votin V, Grob P, Allyn T, Thorner J, Nogales E. Phosphatidylinositol-4,5-bisphosphate promotes budding yeast septin filament assembly and organization. J Mol Biol 404: 711–731, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Betzig E, Patterson GH, Sougrat R, Lindwasser OW, Olenych S, Bonifacino JS, Davidson MW, Lippincott-Schwartz J, Hess HF. Imaging intracellular fluorescent proteins at nanometer resolution. Science 313: 1642–1645, 2006 [DOI] [PubMed] [Google Scholar]
- 142. Bi L, Okabe I, Bernard DJ, Nussbaum RL. Early embryonic lethality in mice deficient in the p110beta catalytic subunit of PI 3-kinase. Mamm Genome 13: 169–172, 2002 [DOI] [PubMed] [Google Scholar]
- 143. Bi L, Okabe I, Bernard DJ, Wynshaw-Boris A, Nussbaum RL. Proliferative defect and embryonic lethality in mice homozygous for a deletion in the p110alpha subunit of phosphoinositide 3-kinase. J Biol Chem 274: 10963–10968, 1999 [DOI] [PubMed] [Google Scholar]
- 144. Bianco A, Reghellin V, Donnici L, Fenu S, Alvarez R, Baruffa C, Peri F, Pagani M, Abrignani S, Neddermann P, De Francesco R. Metabolism of phosphatidylinositol 4-kinase iiialpha-dependent PI4P is subverted by HCV and is targeted by a 4-anilino quinazoline with antiviral activity. PLoS Pathog 8: e1002576, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Bielas SL, Silhavy JL, Brancati F, Kisseleva MV, Al-Gazali L, Sztriha L, Bayoumi RA, Zaki MS, Abdel-Aleem A, Rosti RO, Kayserili H, Swistun D, Scott LC, Bertini E, Boltshauser E, Fazzi E, Travaglini L, Field SJ, Gayral S, Jacoby M, Schurmans S, Dallapiccola B, Majerus PW, Valente EM, Gleeson JG. Mutations in INPP5E, encoding inositol polyphosphate-5-phosphatase E, link phosphatidyl inositol signaling to the ciliopathies. Nat Genet 41: 1032–1036, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Bilodeau PS, Urbanowski JL, Winistorfer SC, Piper RC. The Vps27p Hse1p complex binds ubiquitin and mediates endosomal protein sorting. Nat Cell Biol 4: 534–539, 2002 [DOI] [PubMed] [Google Scholar]
- 147. Blagoveshchenskaya A, Cheong FY, Rohde HM, Glover G, Knodler A, Nicolson T, Boehmelt G, Mayinger P. Integration of Golgi trafficking and growth factor signaling by the lipid phosphatase SAC1. J Cell Biol 180: 803–812, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Blaustein M, Pelisch F, Tanos T, Munoz MJ, Wengier D, Quadrana L, Sanford JR, Muschietti JP, Kornblihtt AR, Caceres JF, Coso OA, Srebrow A. Concerted regulation of nuclear and cytoplasmic activities of SR proteins by AKT. Nat Struct Mol Biol 12: 1037–1044, 2005 [DOI] [PubMed] [Google Scholar]
- 149. Blayney L, Gapper P, Rix C. Identification of phospholipase C beta isoforms and their location in cultured vascular smooth muscle cells of pig, human and rat. Cardiovasc Res 40: 564–572, 1998 [DOI] [PubMed] [Google Scholar]
- 150. Blesdale JE, Bundy GL, Bunting S, Fitzpatrick FA, Huff RM, Sun FF, Pike JE. Inhibition of phospholipase C-dependent processes by U-73,122. Adv Prostaglandin Thromboxane Leukotriene Res 19: 590–593, 1989 [PubMed] [Google Scholar]
- 151. Blind RD, Suzawa M, Ingraham HA. Direct modification and activation of a nuclear receptor-PIP(2) complex by the inositol lipid kinase IPMK. Sci Signal 5: ra44, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Blondeau F, Laporte J, Bodin S, Superti-Furga G, Payrastre B, Mandel JL. Myotubularin, a phosphatase deficient in myotubular myopathy, acts on phosphatidylinositol 3-kinase and phosphatidylinositol 3-phosphate pathway. Hum Mol Genet 9: 2223–2229, 2000 [DOI] [PubMed] [Google Scholar]
- 153. Bloomquist BT, Shortridge RD, Schneuwly S, Perdew M, Montell C, Steller H, Rubin G, Pak WL. Isolation of a putative phospholipase C gene of Drosophila, norpA, and its role in phototransduction. Cell 54: 723–733, 1988 [DOI] [PubMed] [Google Scholar]
- 154. Blumental-Perry A, Haney CJ, Weixel KM, Watkins SC, Weisz OA, Aridor M. Phosphatidylinositol 4-phosphate formation at ER exit sites regulates ER export. Dev Cell 11: 671–682, 2006 [DOI] [PubMed] [Google Scholar]
- 155. Bohdanowicz M, Balkin DM, De Camilli P, Grinstein S. Recruitment of OCRL and InpP5B to phagosomes by Rab5 and APPL1 depletes phosphoinositides and attenuates Akt signaling. Mol Biol Cell 23: 176–187, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Bohdanowicz M, Cosio G, Backer JM, Grinstein S. Class I and class III phosphoinositide 3-kinases are required for actin polymerization that propels phagosomes. J Cell Biol 191: 999–1012, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Bohnacker T, Marone R, Collmann E, Calvez R, Hirsch E, Wymann M. PI3Kgamma adapter subunits define coupling to degranulation and cell motility by distinct PtdIns(3,4,5)P3 pools. Sci Signal 2: ra27, 2009 [DOI] [PubMed] [Google Scholar]
- 158. Bolino A, Bolis A, Previtali SC, Dina G, Bussini S, Dati G, Amadio S, Del Carro U, Mruk DD, Feltri ML, Cheng CY, Quattrini A, Wrabetz L. Disruption of Mtmr2 produces CMT4B1-like neuropathy with myelin outfolding and impaired spermatogenesis. J Cell Biol 167: 711–721, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Bolino A, Muglia M, Conforti FL, LeGuern E, Salih MA, Georgiou DM, Christodoulou K, Hausmanowa-Petrusewicz I, Mandich P, Schenone A, Gambardella A, Bono F, Quattrone A, Devoto M, Monaco AP. Charcot-Marie-Tooth type 4B is caused by mutations in the gene encoding myotubularin-related protein-2. Nat Genet 25: 17–19, 2000 [DOI] [PubMed] [Google Scholar]
- 160. Bolis A, Coviello S, Bussini S, Dina G, Pardini C, Previtali SC, Malaguti M, Morana P, Del Carro U, Feltri ML, Quattrini A, Wrabetz L, Bolino A. Loss of Mtmr2 phosphatase in Schwann cells but not in motor neurons causes Charcot-Marie-Tooth type 4B1 neuropathy with myelin outfoldings. J Neurosci 25: 8567–8577, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Bolis A, Coviello S, Visigalli I, Taveggia C, Bachi A, Chishti AH, Hanada T, Quattrini A, Previtali SC, Biffi A, Bolino A. Dlg1, Sec8, and Mtmr2 regulate membrane homeostasis in Schwann cell myelination. J Neurosci 29: 8858–8870, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Bonangelino CJ, Nau JJ, Duex JE, Brinkman M, Wurmser AE, Gary JD, Emr SD, Weisman LS. Osmotic stress-induced increase of phosphatidylinositol 3,5-bisphosphate requires Vac14p, an activator of the lipid kinase Fab1p. J Cell Biol 156: 1015–1028, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Bondeva T, Pirola L, Bulgarelli-Leva G, Rubio I, Wetzker R, Wymann MP. Bifurcation of lipid and protein kinase signals of PI3Kgamma to the protein kinases PKB and MAPK. Science 282: 293–296, 1998 [DOI] [PubMed] [Google Scholar]
- 164. Bonneick S, Boentert M, Berger P, Atanasoski S, Mantei N, Wessig C, Toyka KV, Young P, Suter U. An animal model for Charcot-Marie-Tooth disease type 4B1. Hum Mol Genet 14: 3685–3695, 2005 [DOI] [PubMed] [Google Scholar]
- 165. Bonvini E, DeBell KE, Veri MC, Graham L, Stoica B, Laborda J, Aman MJ, DiBaldassarre A, Miscia S, Rellahan BL. On the mechanism coupling phospholipase Cgamma1 to the B- and T-cell antigen receptors. Adv Enzyme Regul 43: 245–269, 2003 [DOI] [PubMed] [Google Scholar]
- 166. Borawski J, Troke P, Puyang X, Gibaja V, Zhao S, Mickanin C, Leighton-Davies J, Wilson CJ, Myer V, Cornellataracido I, Baryza J, Tallarico J, Joberty G, Bantscheff M, Schirle M, Bouwmeester T, Mathy JE, Lin K, Compton T, Labow M, Wiedmann B, Gaither LA. Class III phosphatidylinositol 4-kinase alpha and beta are novel host factor regulators of hepatitis C virus replication. J Virol 83: 10058–10074, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Boronenkov IV, Anderson RA. The sequence of phosphatidylinositol-4-phosphate 5-kinase defines a novel family of lipid kinases. J Biol Chem 270: 2881–2884, 1995 [DOI] [PubMed] [Google Scholar]
- 168. Boronenkov IV, Loijens JC, Umeda M, Anderson RA. Phosphoinositide signaling pathways in nuclei are associated with nuclear speckles containing pre-mRNA processing factors. Mol Biol Cell 9: 3547–3560, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Bosch RR, Patel AM, Van Emst-de Vries SE, Smeets RL, De Pont JJ, Willems PH. U73122 and U73343 inhibit receptor-mediated phospholipase D activation downstream of phospholipase C in CHO cells. Eur J Pharmacol 346: 345–351, 1998 [DOI] [PubMed] [Google Scholar]
- 170. Botelho RJ, Efe JA, Teis D, Emr SD. Assembly of a Fab1 phosphoinositide kinase signaling complex requires the Fig4 phosphoinositide phosphatase. Mol Biol Cell 19: 4273–4286, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Botelho RJ, Harrison RE, Stone JC, Hancock JF, Philips MR, Jongstra-Bilen J, Mason D, Plumb J, Gold MR, Grinstein S. Localized diacylglycerol-dependent stimulation of Ras and Rap1 during phagocytosis. J Biol Chem 284: 28522–28532, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Botelho RJ, Teruel M, Dierckman R, Anderson R, Wells A, York JD, Meyer T, Grinstein S. Localized biphasic changes in phosphatidylinositol-4,5-bisphosphate at sites of phagocytosis. J Cell Biol 151: 1353–1368, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Bothwell SP, Chan E, Bernardini IM, Kuo YM, Gahl WA, Nussbaum RL. Mouse model for Lowe syndrome/Dent disease 2 renal tubulopathy. J Am Soc Nephrol 22: 443–448, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Boyer JL, Graber SG, Waldo GL, Harden TK, Garrison JC. Selective activation of phospholipase C by recombinant G-protein alpha- and betagamma-subunits. J Biol Chem 269: 2814–2819, 1994 [PubMed] [Google Scholar]
- 175. Boyle KB, Gyori D, Sindrilaru A, Scharffetter-Kochanek K, Taylor PR, Mocsai A, Stephens LR, Hawkins PT. Class IA phosphoinositide 3-kinase β and δ regulate neutrophil oxidase activation in response to Aspergillus fumigatus hyphae. J Immunol 186: 2978–2989, 2011 [DOI] [PubMed] [Google Scholar]
- 176. Brachmann SM, Yballe CM, Innocenti M, Deane JA, Fruman DA, Thomas SM, Cantley LC. Role of phosphoinositide 3-kinase regulatory isoforms in development and actin rearrangement. Mol Cell Biol 25: 2593–2606, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Brady JD, Rich ED, Martens JR, Karpen JW, Varnum MD, Brown RL. Interplay between PIP3 and calmodulin regulation of olfactory cyclic nucleotide-gated channels. Proc Natl Acad Sci USA 103: 15635–15640, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Breslow DK, Collins SR, Bodenmiller B, Aebersold R, Simons K, Shevchenko A, Ejsing CS, Weissman JS. Orm family proteins mediate sphingolipid homeostasis. Nature 463: 1048–1053, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Brice SE, Alford CW, Cowart LA. Modulation of sphingolipid metabolism by the phosphatidylinositol-4-phosphate phosphatase Sac1p through regulation of phosphatidylinositol in Saccharomyces cerevisiae. J Biol Chem 284: 7588–7596, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Bridges D, Ma JT, Park S, Inoki K, Weisman LS, Saltiel AR. PI(3,5)P2 plays a role in the activation and subcellular localization of mTORC1. Mol Biol Cell 23: 2955–2962, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Bright SR, Rich ED, Varnum MD. Regulation of human cone cyclic nucleotide-gated channels by endogenous phospholipids and exogenously applied phosphatidylinositol 3,4,5-trisphosphate. Mol Pharmacol 71: 176–183, 2007 [DOI] [PubMed] [Google Scholar]
- 182. Brill JA, Hime GR, Scharer-Schuksz M, Fuller MT. A phospholipid kinase regulates actin organization and intercellular bridge formation during germline cytokinesis. Development 127: 3855–3864, 2000 [DOI] [PubMed] [Google Scholar]
- 183. Bristol JA, Rhee SG. Regulation of phospholipase C-beta isozymes by G-proteins. Trends Endocrinol Metab 5: 402–406, 1994 [DOI] [PubMed] [Google Scholar]
- 184. Broad LM, Braun FJ, Lievremont JP, Bird GS, Kurosaki T, Putney JW., Jr Role of the phospholipase C-inositol 1,4,5-trisphosphate pathway in calcium release-activated calcium current and capacitative calcium entry. J Biol Chem 276: 15945–15952, 2001 [DOI] [PubMed] [Google Scholar]
- 185. Broberg CA, Zhang L, Gonzalez H, Laskowski-Arce MA, Orth K. A Vibrio effector protein is an inositol phosphatase and disrupts host cell membrane integrity. Science 329: 1660–1662, 2010 [DOI] [PubMed] [Google Scholar]
- 186. Brockerhoff H, Ballou CE. Phosphate incorporation in brain phosphoinositides. J Biol Chem 237: 49–52, 1962 [PubMed] [Google Scholar]
- 187. Bromann PA, Boetticher EE, Lomasney JW. A single amino acid substitution in the pleckstrin homology domain of phospholipase C delta1 enhances the rate of substrate hydrolysis. J Biol Chem 272: 16240–16246, 1997 [DOI] [PubMed] [Google Scholar]
- 188. Brombacher E, Urwyler S, Ragaz C, Weber SS, Kami K, Overduin M, Hilbi H. Rab1 guanine nucleotide exchange factor SidM is a major phosphatidylinositol 4-phosphate-binding effector protein of Legionella pneumophila. J Biol Chem 284: 4846–4856, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Brown FD, Rozelle AL, Yin HL, Balla T, Donaldson JG. Phosphatidylinositol 4,5-bisphosphate and Arf6-regulated membrane traffic. J Cell Biol 154: 1007–1017, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Brown JR, Auger KR. Phylogenomics of phosphoinositide lipid kinases: perspectives on the evolution of second messenger signaling and drug discovery. BMC Evol Biol 11: 4, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Brown RA, Domin J, Arcaro A, Waterfield MD, Shepherd PR. Insulin activates the alpha isoform of class II phosphoinositide 3-kinase. J Biol Chem 274: 14529–14532, 1999 [DOI] [PubMed] [Google Scholar]
- 192. Brown RA, Ho LK, Weber-Hall SJ, Shipley JM, Fry MJ. Identification and cDNA cloning of a novel mammalian C2 domain-containing phosphoinositide 3-kinase, HsC2-PI3K. Biochem Biophys Res Commun 233: 537–544, 1997 [DOI] [PubMed] [Google Scholar]
- 193. Bruns JR, Ellis MA, Jeromin A, Weisz OA. Multiple roles for phosphatidylinositol 4-kinase in biosynthetic transport in polarized Madin-Darby canine kidney cells. J Biol Chem 277: 2012–2018, 2002 [DOI] [PubMed] [Google Scholar]
- 194. Bryant DM, Mostov KE. From cells to organs: building polarized tissue. Nat Rev Mol Cell Biol 9: 887–901, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Buccafusca R, Venditti CP, Kenyon LC, Johanson RA, Van Bockstaele E, Ren J, Pagliardini S, Minarcik J, Golden JA, Coady MJ, Greer JJ, Berry GT. Characterization of the null murine sodium/myo-inositol cotransporter 1 (Smit1 or Slc5a3) phenotype: myo-inositol rescue is independent of expression of its cognate mitochondrial ribosomal protein subunit 6 (Mrps6) gene and of phosphatidylinositol levels in neonatal brain. Mol Genet Metab 95: 81–95, 2008 [DOI] [PubMed] [Google Scholar]
- 196. Buj-Bello A, Laugel V, Messaddeq N, Zahreddine H, Laporte J, Pellissier JF, Mandel JL. The lipid phosphatase myotubularin is essential for skeletal muscle maintenance but not for myogenesis in mice. Proc Natl Acad Sci USA 99: 15060–15065, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Bultsma Y, Keune WJ, Divecha N. PIP4Kbeta interacts with and modulates nuclear localization of the high-activity PtdIns5P-4-kinase isoform PIP4Kalpha. Biochem J 430: 223–235, 2010 [DOI] [PubMed] [Google Scholar]
- 198. Bunce MW, Boronenkov IV, Anderson RA. Coordinated activation of the nuclear ubiquitin ligase Cul3-SPOP by the generation of phosphatidylinositol 5-phosphate. J Biol Chem 283: 8678–8686, 2008 [DOI] [PubMed] [Google Scholar]
- 199. Bunney TD, Opaleye O, Roe SM, Vatter P, Baxendale RW, Walliser C, Everett KL, Josephs MB, Christow C, Rodrigues-Lima F, Gierschik P, Pearl LH, Katan M. Structural insights into formation of an active signaling complex between Rac and phospholipase C gamma 2. Mol Cell 34: 223–233, 2009 [DOI] [PubMed] [Google Scholar]
- 200. Burd CG, Emr SD. Phosphatidylinositol(3)-phosphate signaling mediated by specific binding to RING FYVE domains. MolCell 2: 157–162, 1998 [DOI] [PubMed] [Google Scholar]
- 201. Burda P, Padilla SM, Sarkar S, Emr SD. Retromer function in endosome-to-Golgi retrograde transport is regulated by the yeast Vps34 PtdIns 3-kinase. J Cell Sci 115: 3889–3900, 2002 [DOI] [PubMed] [Google Scholar]
- 202. Burgering BM, Coffer PJ. Protein kinase B (c-Akt) in phosphatidylinositol-3-OH kinase signal transduction. Nature 376: 599–602, 1995 [DOI] [PubMed] [Google Scholar]
- 203. Byfield MP, Murray JT, Backer JM. hVps34 is a nutrient-regulated lipid kinase required for activation of p70 S6 kinase. J Biol Chem 280: 33076–33082, 2005 [DOI] [PubMed] [Google Scholar]
- 204. Byrne RD, Barona TM, Garnier M, Koster G, Katan M, Poccia DL, Larijani B. Nuclear envelope assembly is promoted by phosphoinositide-specific phospholipase C with selective recruitment of phosphatidylinositol-enriched membranes. Biochem J 387: 393–400, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Cai H, Das S, Kamimura Y, Long Y, Parent CA, Devreotes PN. Ras-mediated activation of the TORC2-PKB pathway is critical for chemotaxis. J Cell Biol 190: 233–245, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. Cai H, Devreotes PN. Moving in the right direction: how eukaryotic cells migrate along chemical gradients. Semin Cell Dev Biol 22: 834–841, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207. Camps M, Carozzi A, Schnabel P, Scheer A, Parker PJ, Gierschik P. Isozyme-selective stimulation of phospholipase C-beta2 by G protein beta-gamma subunits. Nature 360: 684–686, 1992 [DOI] [PubMed] [Google Scholar]
- 208. Camps M, Ruckle T, Ji H, Ardissone V, Rintelen F, Shaw J, Ferrandi C, Chabert C, Gillieron C, Francon B, Martin T, Gretener D, Perrin D, Leroy D, Vitte PA, Hirsch E, Wymann MP, Cirillo R, Schwarz MK, Rommel C. Blockade of PI3Kgamma suppresses joint inflammation and damage in mouse models of rheumatoid arthritis. Nat Med 11: 936–943, 2005 [DOI] [PubMed] [Google Scholar]
- 209. Canobbio I, Stefanini L, Cipolla L, Ciraolo E, Gruppi C, Balduini C, Hirsch E, Torti M. Genetic evidence for a predominant role of PI3Kbeta catalytic activity in ITAM- and integrin-mediated signaling in platelets. Blood 114: 2193–2196, 2009 [DOI] [PubMed] [Google Scholar]
- 210. Cantley LC, Neel BG. New insights into tumor suppression: PTEN suppresses tumor formation by restraining the phosphoinositide 3-kinase/AKT pathway. Proc Natl Acad Sci USA 96: 4240–4245, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211. Cao C, Backer JM, Laporte J, Bedrick EJ, Wandinger-Ness A. Sequential actions of myotubularin lipid phosphatases regulate endosomal PI(3)P and growth factor receptor trafficking. Mol Biol Cell 19: 3334–3346, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212. Cao C, Laporte J, Backer JM, Wandinger-Ness A, Stein MP. Myotubularin lipid phosphatase binds the hVPS15/hVPS34 lipid kinase complex on endosomes. Traffic 8: 1052–1067, 2007 [DOI] [PubMed] [Google Scholar]
- 213. Carlton J, Bujny M, Peter BJ, Oorschot VM, Rutherford A, Mellor H, Klumperman J, McMahon HT, Cullen PJ. Sorting nexin-1 mediates tubular endosome-to-TGN transport through coincidence sensing of high-curvature membranes and 3-phosphoinositides. Curr Biol 14: 1791–1800, 2004 [DOI] [PubMed] [Google Scholar]
- 214. Carlton JG, Cullen PJ. Coincidence detection in phosphoinositide signaling. Trends Cell Biol 15: 540–547, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Caron E, Hall A. Identification of two distinct mechanisms of phagocytosis controlled by different Rho GTPases. Science 282: 1717–1721, 1998 [DOI] [PubMed] [Google Scholar]
- 216. Carrasco S, Merida I. Diacylglycerol, when simplicity becomes complex. Trends Biochem Sci 32: 27–36, 2007 [DOI] [PubMed] [Google Scholar]
- 217. Carrasco S, Meyer T. STIM proteins and the endoplasmic reticulum-plasma membrane junctions. Annu Rev Biochem 80: 973–1000, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218. Carricaburu V, Lamia KA, Lo E, Favereaux L, Payrastre B, Cantley LC, Rameh LE. The phosphatidylinositol (PI)-5-phosphate 4-kinase type II enzyme controls insulin signaling by regulating PI-3,4,5-trisphosphate degradation. Proc Natl Acad Sci USA 100: 9867–9872, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219. Carvou N, Holic R, Li M, Futter C, Skippen A, Cockcroft S. Phosphatidylinositol- and phosphatidylcholine-transfer activity of PITPbeta is essential for COPI-mediated retrograde transport from the Golgi to the endoplasmic reticulum. J Cell Sci 123: 1262–1273, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220. Casci T, Vinos J, Freeman M. Sprouty, an intracellular inhibitor of Ras signaling. Cell 96: 655–665, 1999 [DOI] [PubMed] [Google Scholar]
- 221. Castellano E, Downward J. Role of RAS in the regulation of PI 3-kinase. Curr Top Microbiol Immunol 346: 143–169, 2010 [DOI] [PubMed] [Google Scholar]
- 222. Castellino AM, Chao MV. Differential association of phosphatidylinositol-5-phosphate 4-kinase with the EGF/ErbB family of receptors. Cell Signal 11: 171–177, 1999 [DOI] [PubMed] [Google Scholar]
- 223. Castellino AM, Parker GJ, Boronenkov IV, Anderson RA, Chao MV. A novel interaction between the juxtamembrane region of the p55 tumor necrosis factor receptor and phosphatidylinositol-4-phosphate 5-kinase. J Biol Chem 272: 5861–5870, 1997 [DOI] [PubMed] [Google Scholar]
- 224. Caterina MJ, Julius D. The vanilloid receptor: a molecular gateway to the pain pathway. Annu Rev Neurosci 24: 487–517, 2001 [DOI] [PubMed] [Google Scholar]
- 225. Catimel B, Schieber C, Condron M, Patsiouras H, Connolly L, Catimel J, Nice EC, Burgess AW, Holmes AB. The PI(3,5)P2 and PI(4,5)P2 interactomes. J Proteome Res 7: 5295–5313, 2008 [DOI] [PubMed] [Google Scholar]
- 226. Chagpar RB, Links PH, Pastor MC, Furber LA, Hawrysh AD, Chamberlain MD, Anderson DH. Direct positive regulation of PTEN by the p85 subunit of phosphatidylinositol 3-kinase. Proc Natl Acad Sci USA 107: 5471–5476, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227. Chakravarti A. A compelling genetic hypothesis for a complex disease: PRODH2/DGCR6 variation leads to schizophrenia susceptibility. Proc Natl Acad Sci USA 99: 4755–4756, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228. Chan TO, Tanaka A, Bjorge JD, Fujita DJ. Association of type I phosphatidylinositol kinase activity with mutationally activated forms of human pp60c-src. Mol Cell Biol 10: 3280–3283, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229. Chang CJ, Mulholland DJ, Valamehr B, Mosessian S, Sellers WR, Wu H. PTEN nuclear localization is regulated by oxidative stress and mediates p53-dependent tumor suppression. Mol Cell Biol 28: 3281–3289, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230. Chang FS, Han GS, Carman GM, Blumer KJ. A WASp-binding type II phosphatidylinositol 4-kinase required for actin polymerization-driven endosome motility. J Cell Biol 171: 133–142, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231. Chang JD, Sukhova GK, Libby P, Schvartz E, Lichtenstein AH, Field SJ, Kennedy C, Madhavarapu S, Luo J, Wu D, Cantley LC. Deletion of the phosphoinositide 3-kinase p110gamma gene attenuates murine atherosclerosis. Proc Natl Acad Sci USA 104: 8077–8082, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232. Chang JT, Milligan S, Li Y, Chew CE, Wiggs J, Copeland NG, Jenkins NA, Campochiaro PA, Hyde DR, Zack DJ. Mammalian homolog of Drosophila retinal degeneration B rescues the mutant fly phenotype. J Neurosci 17: 5881–5890, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233. Chang Y, Schlenstedt G, Flockerzi V, Beck A. Properties of the intracellular transient receptor potential (TRP) channel in yeast, Yvc1. FEBS Lett 584: 2028–2032, 2010 [DOI] [PubMed] [Google Scholar]
- 234. Chang YY, Juhasz G, Goraksha-Hicks P, Arsham AM, Mallin DR, Muller LK, Neufeld TP. Nutrient-dependent regulation of autophagy through the target of rapamycin pathway. Biochem Soc Trans 37: 232–236, 2009 [DOI] [PubMed] [Google Scholar]
- 235. Changya L, Gallacher DV, Irvine RF, Potter BVL, Petersen OH. Inositol 1,3,4,5-tetrakisphosphate is essential for sustained activation of the Ca2+-dependent K+ current in single internally perfused mouse lacrimal acinar cells. J Membr Biol 109: 85–93, 1989 [DOI] [PubMed] [Google Scholar]
- 236. Charnas LR, Gahl WA. The oculocerebrorenal syndrome of Lowe. Adv Pediatr 38: 75–107, 1991 [PubMed] [Google Scholar]
- 237. Chaudhary A, Chen J, Gu QM, Witke W, Kwiatkowski DJ, Prestwich GD. Probing the phosphoinositide 4,5-bisphosphate binding site of human profilin. Chem Biol 5: 273–281, 1998 [DOI] [PubMed] [Google Scholar]
- 238. Cheeseman KL, Ueyama T, Michaud TM, Kashiwagi K, Wang D, Flax LA, Shirai Y, Loegering DJ, Saito N, Lennartz MR. Targeting of protein kinase C-epsilon during Fcgamma receptor-dependent phagocytosis requires the epsilonC1B domain and phospholipase C-gamma1. Mol Biol Cell 17: 799–813, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239. Cheever ML, Sato TK, de Beer T, Kutateladze TG, Emr SD, Overduin M. Phox domain interaction with PtdIns(3)P targets the Vam7 t-SNARE to vacuole membranes. Nat Cell Biol 3: 613–618, 2001 [DOI] [PubMed] [Google Scholar]
- 240. Chen CY, Ingram MF, Rosal PH, Graham TR. Role for Drs2p, a P-type ATPase and potential aminophospholipid translocase, in yeast late Golgi function. J Cell Biol 147: 1223–1236, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241. Chen D, Mauvais-Jarvis F, Bluher M, Fisher SJ, Jozsi A, Goodyear LJ, Ueki K, Kahn CR. p50alpha/p55alpha phosphoinositide 3-kinase knockout mice exhibit enhanced insulin sensitivity. Mol Cell Biol 24: 320–329, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242. Chen H, Rossier C, Morris MA, Scott HS, Gos A, Bairoch A, Antonarakis SE. A testis-specific gene, TPTE, encodes a putative transmembrane tyrosine phosphatase and maps to the pericentromeric region of human chromosomes 21 and 13, and to chromosomes 15, 22, and Y. Hum Genet 105: 399–409, 1999 [DOI] [PubMed] [Google Scholar]
- 243. Chen L, Zhou B, Zhang S, Wu L, Wang Y, Franzblau SG, Zhang ZY. Identification and characterization of novel inhibitors of mPTPB, an essential virulent phosphatase from Mycobacterium tuberculosis. ACS Med Chem Lett 1: 355–359, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244. Chen MZ, Zhu X, Sun HQ, Mao YS, Wei Y, Yamamoto M, Yin HL. Oxidative stress decreases phosphatidylinositol 4,5-bisphosphate levels by deactivating phosphatidylinositol- 4-phosphate 5-kinase beta in a Syk-dependent manner. J Biol Chem 284: 23743–23753, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245. Chen X, Talley EM, Patel N, Gomis A, McIntire WE, Dong B, Viana F, Garrison JC, Bayliss DA. Inhibition of a background potassium channel by Gq protein alpha-subunits. Proc Natl Acad Sci USA 103: 3422–3427, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246. Cheng X, Shen D, Samie M, Xu H. Mucolipins: intracellular TRPML1–3 channels. FEBS Lett 584: 2013–2021, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247. Cheung LW, Hennessy BT, Li J, Yu S, Myers AP, Djordjevic B, Lu Y, Stemke-Hale K, Zhang F, Ju Z, Cantley LC, Scherer SE, Liang H, Lu KH, Broaddus RR, Mills GB. High frequency of PIK3R1 and PIK3R2 mutations in endometrial cancer elucidates a novel mechanism for regulation of PTEN protein stability. Cancer Discov 1: 170–185, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248. Chi Y, Zhou B, Wang WQ, Chung SK, Kwon YU, Ahn YH, Chang YT, Tsujishita Y, Hurley JH, Zhang ZY. Comparative mechanistic and substrate specificity study of inositol polyphosphate 5-phosphatase Schizosaccharomyces pombe synaptojanin and SHIP2. J Biol Chem 279: 44987–44995, 2004 [DOI] [PubMed] [Google Scholar]
- 249. Choquette D, Hakim G, Filoteo AG, Plishker GA, Bostwick JR, Penniston JT. Regulation of plasma membrane Ca2+ ATPases by lipids of the phosphatidylinositol cycle. Biochem Biophys Res Commun 125: 908–915, 1984 [DOI] [PubMed] [Google Scholar]
- 250. Choudhury R, Diao A, Zhang F, Eisenberg E, Saint-Pol A, Williams C, Konstantakopoulos A, Lucocq J, Johannes L, Rabouille C, Greene LE, Lowe M. Lowe syndrome protein OCRL1 interacts with clathrin and regulates protein trafficking between endosomes and the trans-Golgi network. Mol Biol Cell 16: 3467–3479, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251. Choudhury R, Noakes CJ, McKenzie E, Kox C, Lowe M. Differential clathrin binding and subcellular localization of OCRL1 splice isoforms. J Biol Chem 284: 9965–9973, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252. Chow CY, Landers JE, Bergren SK, Sapp PC, Grant AE, Jones JM, Everett L, Lenk GM, McKenna-Yasek DM, Weisman LS, Figlewicz D, Brown RH, Meisler MH. Deleterious variants of FIG4, a phosphoinositide phosphatase, in patients with ALS. Am J Hum Genet 84: 85–88, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253. Chow CY, Zhang Y, Dowling JJ, Jin N, Adamska M, Shiga K, Szigeti K, Shy ME, Li J, Zhang X, Lupski JR, Weisman LS, Meisler MH. Mutation of FIG4 causes neurodegeneration in the pale tremor mouse and patients with CMT4J. Nature 448: 68–72, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254. Christoforidis S, Miaczynska M, Ashman K, Wilm M, Zhao L, Yip SC, Waterfield MD, Backer JM, Zerial M. Phosphatidylinositol-3-OH kinases are Rab5 effectors. Nat Cell Biol 1: 249–252, 1999 [DOI] [PubMed] [Google Scholar]
- 255. Christy AH, Kim AC, Marfatia SM, Lutchman M, Hanspal M, Jindal H, Liu SC, Low PS, Rouleau GA, Mohandas N, Chasis JA, Conboy Jg, Gascard P, Takakuwa Y, Huang SC, Benz EJ, Jr, Bretscher A, Fehon RG, Gusella JF, Ramesh V, Solomon F, Marchesi VT, Tsukita S, Arpin M, Louvard D, Tonks NK, Anderson JM, Fanning AS, Bryant PJ, Woods DF, Hoover KB. The FERM domain: a unique module involved in the linkage of cytoplasmic proteins to the membrane. Trends Biochem Sci 23: 281–282, 1998 [DOI] [PubMed] [Google Scholar]
- 256. Chua J, Deretic V. Mycobacterium tuberculosis reprograms waves of phosphatidylinositol 3-phosphate on phagosomal organelles. J Biol Chem 279: 36982–36992, 2004 [DOI] [PubMed] [Google Scholar]
- 257. Chuang HH, Prescott ED, Kong H, Shields S, Jordt SE, Basbaum AI, Chao MV, Julius D. Bradykinin and nerve growth factor release the capsaicin receptor from PtdIns(4,5)P2-mediated inhibition. Nature 411: 957–962, 2001 [DOI] [PubMed] [Google Scholar]
- 258. Chun JT, Puppo A, Vasilev F, Gragnaniello G, Garante E, Santella L. The biphasic increase of PIP2 in the fertilized eggs of starfish: new roles in actin polymerization and Ca2+ signaling. PLoS One 5: e14100, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259. Chung JK, Sekiya F, Kang HS, Lee C, Han JS, Kim SR, Bae YS, Morris AJ, Rhee SG. Synaptojanin inhibition of phospholipase D activity by hydrolysis of phosphatidylinositol 4,5-bisphosphate. J Biol Chem 272: 15980–15985, 1997 [DOI] [PubMed] [Google Scholar]
- 260. Chung SH, Song WJ, Kim K, Bednarski JJ, Chen J, Prestwich GD, Holz RW. The C2 domains of Rabphilin3A specifically bind phosphatidylinositol 4,5-bisphosphate containing vesicles in a Ca2+-dependent manner. In vitro characteristics and possible significance. J Biol Chem 273: 10240–10248, 1998 [DOI] [PubMed] [Google Scholar]
- 261. Ciraolo E, Iezzi M, Marone R, Marengo S, Curcio C, Costa C, Azzolino O, Gonella C, Rubinetto C, Wu H, Dastru W, Martin EL, Silengo L, Altruda F, Turco E, Lanzetti L, Musiani P, Ruckle T, Rommel C, Backer JM, Forni G, Wymann MP, Hirsch E. Phosphoinositide 3-kinase p110beta activity: key role in metabolism and mammary gland cancer but not development. Sci Signal 1: ra3, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262. Ciraolo E, Morello F, Hobbs RM, Wolf F, Marone R, Iezzi M, Lu X, Mengozzi G, Altruda F, Sorba G, Guan K, Pandolfi PP, Wymann MP, Hirsch E. Essential role of the p110beta subunit of phosphoinositide 3-OH kinase in male fertility. Mol Biol Cell 21: 704–711, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263. Ciruela A, Hinchliffe KA, Divecha N, Irvine RF. Nuclear targeting of the beta isoform of type II phosphatidylinositol phosphate kinase (phosphatidylinositol 5-phosphate 4-kinase) by its alpha-helix 7. Biochem J 346: 587–591, 2000 [PMC free article] [PubMed] [Google Scholar]
- 264. Citro S, Malik S, Oestreich EA, Radeff-Huang J, Kelley GG, Smrcka AV, Brown JH. Phospholipase C-epsilon is a nexus for Rho and Rap-mediated G protein-coupled receptor-induced astrocyte proliferation. Proc Natl Acad Sci USA 104: 15543–15548, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265. Clarke JH, Emson PC, Irvine RF. Distribution and neuronal expression of phosphatidylinositol phosphate kinase IIgamma in the mouse brain. J Comp Neurol 517: 296–312, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266. Clarke JH, Emson PC, Irvine RF. Localization of phosphatidylinositol phosphate kinase IIgamma in kidney to a membrane trafficking compartment within specialized cells of the nephron. Am J Physiol Renal Physiol 295: F1422–F1430, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267. Clarke JH, Wang M, Irvine RF. Localization, regulation and function of type II phosphatidylinositol 5-phosphate 4-kinases. Adv Enzyme Regul 50: 12–18, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268. Clayton E, Bardi G, Bell SE, Chantry D, Downes CP, Gray A, Humphries LA, Rawlings D, Reynolds H, Vigorito E, Turner M. A crucial role for the p110delta subunit of phosphatidylinositol 3-kinase in B cell development and activation. J Exp Med 196: 753–763, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269. Clayton EL, Minogue S, Waugh MG. Phosphatidylinositol 4-kinases and PI4P metabolism in the nervous system: roles in psychiatric and neurological diseases. Mol Neurobiol 47: 361–372, 2013 [DOI] [PubMed] [Google Scholar]
- 270. Cleeter M, Houlden H, Simons P, Al-Shawi R, Stevanin G, Durr A, Hsuan J, Warner TT. Screening for mutations in the phosphatidylinositol 4-kinase 2-alpha gene in autosomal recessive hereditary spastic paraplegia. Amyotroph Lateral Scler 2010 [DOI] [PubMed] [Google Scholar]
- 271. Clement S, Krause U, Desmedt F, Tanti JF, Behrends J, Pesesse X, Sasaki T, Penninger J, Doherty M, Malaisse W, Dumont JE, Le Marchand-Brustel Y, Erneux C, Hue L, Schurmans S. The lipid phosphatase SHIP2 controls insulin sensitivity. Nature 409: 92–97, 2001 [DOI] [PubMed] [Google Scholar]
- 272. Cleves AE, McGee TP, Whitters EA, Champion KM, Aitken JR, Dowhan W, Goebl M, Bankaitis VA. Mutations in the CDP-choline pathway for phospholipid biosynthesis bypass the requirement for an essential phospholipid transfer protein. Cell 64: 789–800, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273. Cleves AE, Novick PJ, Bankaitis VA. Mutations in the SAC1 gene suppress defects in yeast Golgi and yeast actin function. J Cell Biol 109: 2939–2950, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274. Coady MJ, Wallendorff B, Gagnon DG, Lapointe JY. Identification of a novel Na+/myo-inositol cotransporter. J Biol Chem 277: 35219–35224, 2002 [DOI] [PubMed] [Google Scholar]
- 275. Cocco L, Gilmour RS, Ognibene A, Letcher AJ, Manzoli FA, Irvine RF. Synthesis of polyphosphoinositides in nuclei of Friend cells. Evidence for polyphosphoinositide metabolism inside the nucleus which changes with cell differentiation. Biochem J 248: 765–770, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276. Cockcroft S, Bennett JP, Gomperts BD. fMet-Leu-Phe-stimulated phosphatidylinositol turnover in rabbit neutrophils is dependent on extracellular calcium. FEBS Lett 110: 115–118, 1980 [DOI] [PubMed] [Google Scholar]
- 277. Cockcroft S, Carvou N. Biochemical and biological functions of class I phosphatidylinositol transfer proteins. Biochim Biophys Acta 1771: 677–691, 2007 [DOI] [PubMed] [Google Scholar]
- 278. Cockcroft S, Garner K. 14-3-3 protein and ATRAP bind to the soluble class IIB phosphatidylinositol transfer protein RdgBbeta at distinct sites. Biochem Soc Trans 40: 451–456, 2012 [DOI] [PubMed] [Google Scholar]
- 279. Cockcroft S, Garner K. Function of the phosphatidylinositol transfer protein gene family: is phosphatidylinositol transfer the mechanism of action? Crit Rev Biochem Mol Biol 46: 89–117, 2011 [DOI] [PubMed] [Google Scholar]
- 280. Cockcroft S, Taylor JA, Judah JD. Subcellular localisation of inositol lipid kinases in rat liver. Biochim Biophys Acta 845: 163–170, 1985 [DOI] [PubMed] [Google Scholar]
- 281. Codogno P, Mehrpour M, Proikas-Cezanne T. Canonical and non-canonical autophagy: variations on a common theme of self-eating? Nat Rev Mol Cell Biol 13: 7–12, 2012 [DOI] [PubMed] [Google Scholar]
- 282. Collier JJ, White SM, Dick GM, Scott DK. Phosphatidylinositol 3-kinase inhibitors reveal a unique mechanism of enhancing insulin secretion in 832/13 rat insulinoma cells. Biochem Biophys Res Commun 324: 1018–1023, 2004 [DOI] [PubMed] [Google Scholar]
- 283. Colodzin M, Kennedy EP. Biosynthesis of diphosphoinositide in brain. J Biol Chem 240: 3771–3780, 1965 [PubMed] [Google Scholar]
- 284. Coluccio A, Malzone M, Neiman AM. Genetic evidence of a role for membrane lipid composition in the regulation of soluble NEM-sensitive factor receptor function in Saccharomyces cerevisiae. Genetics 166: 89–97, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285. Condliffe AM, Davidson K, Anderson KE, Ellson CD, Crabbe T, Okkenhaug K, Vanhaesebroeck B, Turner M, Webb L, Wymann MP, Hirsch E, Ruckle T, Camps M, Rommel C, Jackson SP, Chilvers ER, Stephens LR, Hawkins PT. Sequential activation of class IB and class IA PI3K is important for the primed respiratory burst of human but not murine neutrophils. Blood 106: 1432–1440, 2005 [DOI] [PubMed] [Google Scholar]
- 286. Connolly TM, Bross TE, Majerus PW. Isolation of a phosphomonoesterase from human platelets that specifically hydrolyses the 5-phosphate of inositol 1,4,5-trisphosphate. J Biol Chem 260: 7868–7874, 1985 [PubMed] [Google Scholar]
- 287. Cooke FT, Dove SK, McEwen RK, Painter G, Holmes AB, Hall MN, Michell RH, Parker PJ. The stress-activated phosphatidylinositol 3-phosphate 5-kinase Fab1p is essential for vacuole function in S. cerevisiae. Curr Biol 8: 1219–1222, 1998 [DOI] [PubMed] [Google Scholar]
- 288. Coon BG, Hernandez V, Madhivanan K, Mukherjee D, Hanna CB, Barinaga-Rementeria Ramirez I, Lowe M, Beales PL, Aguilar RC. The Lowe syndrome protein OCRL1 is involved in primary cilia assembly. Hum Mol Genet 21: 1835–1847, 2012 [DOI] [PubMed] [Google Scholar]
- 289. Coppolino MG, Dierckman R, Loijens J, Collins RF, Pouladi M, Jongstra-Bilen J, Schreiber AD, Trimble WS, Anderson R, Grinstein S. Inhibition of phosphatidylinositol-4-phosphate 5-kinase Ialpha impairs localized actin remodeling and suppresses phagocytosis. J Biol Chem 277: 43849–43857, 2002 [DOI] [PubMed] [Google Scholar]
- 290. Cossart P, Roy CR. Manipulation of host membrane machinery by bacterial pathogens. Curr Opin Cell Biol 22: 547–554, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291. Costa-Junior HM, Marques-da-Silva C, Vieira FS, Moncao-Ribeiro LC, Coutinho-Silva R. Lipid metabolism modulation by the P2X7 receptor in the immune system and during the course of infection: new insights into the old view. Purinergic Signal 7: 381–392, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292. Courtneidge SA, Heber A. An 81 kd protein complexed with middle T antigen and pp60c-src: a possible phosphatidylinositol kinase. Cell 50: 1031–1037, 1987 [DOI] [PubMed] [Google Scholar]
- 293. Cox D, Berg JS, Cammer M, Chinegwundoh JO, Dale BM, Cheney RE, Greenberg S. Myosin X is a downstream effector of PI(3)K during phagocytosis. Nat Cell Biol 4: 469–477, 2002 [DOI] [PubMed] [Google Scholar]
- 294. Cox D, Dale BM, Kashiwada M, Helgason CD, Greenberg S. A regulatory role for Src homology 2 domain-containing inositol 5'-phosphatase (SHIP) in phagocytosis mediated by Fc gamma receptors and complement receptor 3 [alpha(M)beta(2); CD11b/CD18]. J Exp Med 193: 61–71, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295. Cox D, Tseng CC, Bjekic G, Greenberg S. A requirement for phosphatidylinositol 3-kinase in pseudopod extension. J Biol Chem 274: 1240–1247, 1999 [DOI] [PubMed] [Google Scholar]
- 296. Cozier GE, Carlton J, Bouyoucef D, Cullen PJ. Membrane targeting by pleckstrin homology domains. Curr Top Microbiol Immunol 282: 49–88, 2004 [DOI] [PubMed] [Google Scholar]
- 297. Cozier GE, Carlton J, McGregor AH, Gleeson PA, Teasdale RD, Mellor H, Cullen PJ. The phox homology (PX) domain-dependent, 3-phosphoinositide-mediated association of sorting nexin-1 with an early sorting endosomal compartment is required for its ability to regulate epidermal growth factor receptor degradation. J Biol Chem 277: 48730–48736, 2002 [DOI] [PubMed] [Google Scholar]
- 298. Craige B, Salazar G, Faundez V. Phosphatidylinositol-4-kinase type II Alpha contains an AP-3 sorting motif and a kinase domain that are both required for endosome traffic. Mol Biol Cell 19: 1415–1426, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299. Creba JA, Downes CP, Hawkins PT, Brewster G, Michell RH, Kirk CJ. Rapid breakdown of phosphatidylinositol 4-phosphate and phosphatidylinositol 4,5-bisphosphate in rat hepatocytes stimulated by vasopressin and other Ca2+-mobilizing hormones. BiochemJ 212: 733–747, 1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300. Cremasco V, Benasciutti E, Cella M, Kisseleva M, Croke M, Faccio R. Phospholipase C gamma 2 is critical for development of a murine model of inflammatory arthritis by affecting actin dynamics in dendritic cells. PLoS One 5: e8909, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301. Cremona O, Di Paolo G, Wenk MR, Luthi A, Kim WT, Takei K, Daniell L, Nemoto Y, Shears SB, Flavell RA, McCormick DA, De Camilli P. Essential role of phosphoinositide metabolism in synaptic vesicle recycling. Cell 99: 179–188, 1999 [DOI] [PubMed] [Google Scholar]
- 302. Crljen V, Visnjic D, Banfic H. Presence of different phospholipase C isoforms in the nucleus and their activation during compensatory liver growth. FEBS Lett 571: 35–42, 2004 [DOI] [PubMed] [Google Scholar]
- 303. Cui S, Guerriero CJ, Szalinski CM, Kinlough CL, Hughey RP, Weisz OA. OCRL1 function in renal epithelial membrane traffic. Am J Physiol Renal Physiol 298: F335–F345, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304. Cullen PJ. Endosomal sorting and signalling: an emerging role for sorting nexins. Nat Rev Mol Cell Biol 9: 574–582, 2008 [DOI] [PubMed] [Google Scholar]
- 305. Cullen PJ, Carlton JG. Phosphoinositides in the mammalian endo-lysosomal network. Subcell Biochem 59: 65–110, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306. Cullen PJ, Hsuan JJ, Truong O, Letcher AJ, Jackson TR, Dawson AP, Irvine RF. Identification of a specific Ins(1,3,4,5)P4-binding protein as a member of the GAP1 family. Nature 376: 527–530, 1995 [DOI] [PubMed] [Google Scholar]
- 307. Cullen PJ, Korswagen HC. Sorting nexins provide diversity for retromer-dependent trafficking events. Nat Cell Biol 14: 29–37, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308. Cummings HE, Barbi J, Reville P, Oghumu S, Zorko N, Sarkar A, Keiser TL, Lu B, Ruckle T, Varikuti S, Lezama-Davila C, Wewers MD, Whitacre C, Radzioch D, Rommel C, Seveau S, Satoskar AR. Critical role for phosphoinositide 3-kinase gamma in parasite invasion and disease progression of cutaneous leishmaniasis. Proc Natl Acad Sci USA 109: 1251–1256, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309. Cunningham E, Tan SK, Swigart P, Hsuan J, Bankaitis V, Cockcroft S. The yeast and mammalian isoforms of phosphatidylinositol transfer protein can all restore phospholipase C-mediated inositol lipid signaling in cytosol-depleted RBL-2H3 and HL-60 cells. Proc Natl Acad Sci USA 93: 6589–6593, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310. Currie RA, MacLeod BMG, Downes CP. The lipid transfer activity of phosphatidylinositol transfer protein is sufficient to account for enhanced phospholipase C activity in turkey erythrocyte ghosts. Curr Biol 7: 184–190, 1997 [DOI] [PubMed] [Google Scholar]
- 311. Cutler NS, Heitman J, Cardenas ME. STT4 is an essential phosphatidylinositol 4-kinase that is a target of wortmannin in Saccharomyces cerevisiae. J Biol Chem 272: 27671–27677, 1997 [DOI] [PubMed] [Google Scholar]
- 312. Czech MP. Dynamics of phosphoinositides in membrane retrieval and insertion. Annu Rev Physiol 65: 791–815, 2003 [DOI] [PubMed] [Google Scholar]
- 313. D'Angelo G, Polishchuk E, Di Tullio G, Santoro M, Di Campli A, Godi A, West G, Bielawski J, Chuang CC, van der Spoel AC, Platt FM, Hannun YA, Polishchuk R, Mattjus P, De Matteis MA. Glycosphingolipid synthesis requires FAPP2 transfer of glucosylceramide. Nature 449: 62–67, 2007 [DOI] [PubMed] [Google Scholar]
- 314. D'Angelo G, Vicinanza M, Di Campli A, De Matteis MA. The multiple roles of PtdIns(4)P: not just the precursor of PtdIns(4,5)P2. J Cell Sci 121: 1955–1963, 2008 [DOI] [PubMed] [Google Scholar]
- 315. D'Angelo G, Vicinanza M, Wilson C, De Matteis MA. Phosphoinositides in Golgi complex function. Subcell Biochem 59: 255–270, 2012 [DOI] [PubMed] [Google Scholar]
- 316. Daboussi L, Costaguta G, Payne GS. Phosphoinositide-mediated clathrin adaptor progression at the trans-Golgi network. Nat Cell Biol 14: 239–248, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317. Dall'armi C, Devereaux KA, Di Paolo G. The role of lipids in the control of autophagy. Curr Biol 23: R33–45, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318. Dambournet D, Machicoane M, Chesneau L, Sachse M, Rocancourt M, El Marjou A, Formstecher E, Salomon R, Goud B, Echard A. Rab35 GTPase and OCRL phosphatase remodel lipids and F-actin for successful cytokinesis. Nat Cell Biol 13: 981–988, 2011 [DOI] [PubMed] [Google Scholar]
- 319. Daniels RL, Takashima Y, McKemy DD. Activity of the neuronal cold sensor TRPM8 is regulated by phospholipase C via the phospholipid phosphoinositol 4,5-bisphosphate. J Biol Chem 284: 1570–1582, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320. Danielsen SA, Cekaite L, Agesen TH, Sveen A, Nesbakken A, Thiis-Evensen E, Skotheim RI, Lind GE, Lothe RA. Phospholipase C isozymes are deregulated in colorectal cancer: insights gained from gene set enrichment analysis of the transcriptome. PLoS One 6: e24419, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321. Daquinag A, Fadri M, Jung SY, Qin J, Kunz J. The yeast PH domain proteins Slm1 and Slm2 are targets of sphingolipid signaling during the response to heat stress. Mol Cell Biol 27: 633–650, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322. Das B, Shu X, Day GJ, Han J, Krishna UM, Falck JR, Broek D. Control of intramolecular interactions between the pleckstrin homology and Dbl homology domains of Vav and Sos1 regulates Rac binding. J Biol Chem 275: 15074–15081, 2000 [DOI] [PubMed] [Google Scholar]
- 323. Das S, Dixon JE, Cho W. Membrane-binding and activation mechanism of PTEN. Proc Natl Acad Sci USA 100: 7491–7496, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324. Davidson JB, Stanacev NZ. Biosynthesis of cardiolipin in mitochondria. Can J Biochem 49: 1117–1124, 1971 [DOI] [PubMed] [Google Scholar]
- 325. Davidson L, Maccario H, Perera NM, Yang X, Spinelli L, Tibarewal P, Glancy B, Gray A, Weijer CJ, Downes CP, Leslie NR. Suppression of cellular proliferation and invasion by the concerted lipid and protein phosphatase activities of PTEN. Oncogene 29: 687–697, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326. Davis JN, Rock CO, Cheng M, Watson JB, Ashmun RA, Kirk H, Kay RJ, Roussel MF. Complementation of growth factor receptor-dependent mitogenic signaling by a truncated type I phosphatidylinositol 4-phosphate 5-kinase. Mol Cell Biol 17: 7398–7406, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327. Davis NY, McPhail LC, Horita DA. The NOXO1beta PX domain preferentially targets PtdIns(4,5)P(2) and PtdIns(3,4,5)P(3). J Mol Biol 417: 440–453, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328. Dawson RM. The measurement of 32P labelling of individual kephalins and lecithin in a small sample of tissue. Biochim Biophys Acta 14: 374–379, 1954 [DOI] [PubMed] [Google Scholar]
- 329. Dawson RM. Studies on the labelling of brain phospholipids with radioactive phosphorus. Biochem J 57: 237–245, 1954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330. Dbouk HA, Pang H, Fiser A, Backer JM. A biochemical mechanism for the oncogenic potential of the p110beta catalytic subunit of phosphoinositide 3-kinase. Proc Natl Acad Sci USA 107: 19897–19902, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331. Dbouk HA, Vadas O, Shymanets A, Burke JE, Salamon RS, Khalil BD, Barrett MO, Waldo GL, Surve C, Hsueh C, Perisic O, Harteneck C, Shepherd PR, Harden TK, Smrcka AV, Taussig R, Bresnick AR, Nurnberg B, Williams RL, Backer JM. G protein-coupled receptor-mediated activation of p110beta by Gbetagamma is required for cellular transformation and invasiveness. Sci Signal 5: ra89, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332. De Graaf P, Klapisz EE, Schulz TK, Cremers AF, Verkleij AJ, van Bergen en Henegouwen PM. Nuclear localization of phosphatidylinositol 4-kinase beta. J Cell Sci 115: 1769–1775, 2002 [DOI] [PubMed] [Google Scholar]
- 333. De Graaf P, Zwart WT, van Dijken RA, Deneka M, Schulz TK, Geijsen N, Coffer PJ, Gadella BM, Verkleij AJ, van der Sluijs P, van Bergen en Henegouwen PM. Phosphatidylinositol 4-kinasebeta is critical for functional association of rab11 with the Golgi complex. Mol Biol Cell 15: 2038–2047, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334. De Haro L, Quetglas S, Iborra C, Leveque C, Seagar M. Calmodulin-dependent regulation of a lipid binding domain in the v-SNARE synaptobrevin and its role in vesicular fusion. Biol Cell 95: 459–464, 2003 [DOI] [PubMed] [Google Scholar]
- 335. De Heuvel E, Bell AW, Ramjaun AR, Wong K, Sossin WS, McPherson PS. Identification of the major synaptojanin-binding proteins in brain. J Biol Chem 272: 8710–8716, 1997 [DOI] [PubMed] [Google Scholar]
- 336. De Lartigue J, Polson H, Feldman M, Shokat K, Tooze SA, Urbe S, Clague MJ. PIKfyve regulation of endosome-linked pathways. Traffic 10: 883–893, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337. De Matteis MA, Di Campli A, Godi A. The role of the phosphoinositides at the Golgi complex. Biochim Biophys Acta 1744: 396–405, 2005 [DOI] [PubMed] [Google Scholar]
- 338. De Robertis E. Molecular biology of synaptic receptors. Science 171: 963–971, 1971 [DOI] [PubMed] [Google Scholar]
- 339. De Saint-Jean M, Delfosse V, Douguet D, Chicanne G, Payrastre B, Bourguet W, Antonny B, Drin G. Osh4p exchanges sterols for phosphatidylinositol 4-phosphate between lipid bilayers. J Cell Biol 195: 965–978, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340. De Smedt F, Verjans B, Mailleux P, Erneux C. Cloning and expression of human brain type I inositol 1,4,5-trisphosphate 5-phosphatase. High levels of mRNA in cerebellar Purkinje cells. FEBS Lett 347: 69–72, 1994 [DOI] [PubMed] [Google Scholar]
- 341. De Vries KJ, Heinrichs AA, Cunningham E, Brunink F, Westerman J, Somerharju PJ, Cockcroft S, Wirtz KW, Snoek GT. An isoform of the phosphatidylinositol-transfer protein transfers sphingomyelin and is associated with the Golgi system. Biochem J 310: 643–649, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342. Deane JA, Trifilo MJ, Yballe CM, Choi S, Lane TE, Fruman DA. Enhanced T cell proliferation in mice lacking the p85beta subunit of phosphoinositide 3-kinase. J Immunol 172: 6615–6625, 2004 [DOI] [PubMed] [Google Scholar]
- 343. Demmel L, Beck M, Klose C, Schlaitz AL, Gloor Y, Hsu PP, Havlis J, Shevchenko A, Krause E, Kalaidzidis Y, Walch-Solimena C. Nucleocytoplasmic shuttling of the Golgi phosphatidylinositol 4-kinase Pik1 is regulated by 14-3-3 proteins and coordinates Golgi function with cell growth. Mol Biol Cell 19: 1046–1061, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344. Demmel L, Gravert M, Ercan E, Habermann B, Muller-Reichert T, Kukhtina V, Haucke V, Baust T, Sohrmann M, Kalaidzidis Y, Klose C, Beck M, Peter M, Walch-Solimena C. The clathrin adaptor Gga2p is a phosphatidylinositol 4-phosphate effector at the Golgi exit. Mol Biol Cell 19: 1991–2002, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345. Deng X, Wang Y, Zhou Y, Soboloff J, Gill DL. STIM and Orai: dynamic intermembrane coupling to control cellular calcium signals. J Biol Chem 284: 22501–22505, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346. Denley A, Kang S, Karst U, Vogt PK. Oncogenic signaling of class I PI3K isoforms. Oncogene 27: 2561–2574, 2008 [DOI] [PubMed] [Google Scholar]
- 347. Desrivieres S, Cooke FT, Morales-Johansson H, Parker PJ, Hall MN. Calmodulin controls organization of the actin cytoskeleton via regulation of phosphatidylinositol (4,5)-bisphosphate synthesis in Saccharomyces cerevisiae. Biochem J 366: 945–951, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348. Desrivieres S, Cooke FT, Parker PJ, Hall MN. MSS4, a phosphatidylinositol-4-phosphate 5-kinase required for organization of the actin cytoskeleton in Saccharomyces cerevisiae. J Biol Chem 273: 15787–15793, 1998 [DOI] [PubMed] [Google Scholar]
- 349. Di Cristofano A, Pandolfi PP. The multiple roles of PTEN in tumor suppression. Cell 100: 387–390, 2000 [DOI] [PubMed] [Google Scholar]
- 350. Di Daniel E, Cheng L, Maycox PR, Mudge AW. The common inositol-reversible effect of mood stabilizers on neurons does not involve GSK3 inhibition, myo-inositol-1-phosphate synthase or the sodium-dependent myo-inositol transporters. Mol Cell Neurosci 32: 27–36, 2006 [DOI] [PubMed] [Google Scholar]
- 351. Di Daniel E, Kew JN, Maycox PR. Investigation of the H+-myo-inositol transporter (HMIT) as a neuronal regulator of phosphoinositide signalling. Biochem Soc Trans 37: 1139–1143, 2009 [DOI] [PubMed] [Google Scholar]
- 352. Di Paolo G, Moskowitz HS, Gipson K, Wenk MR, Voronov S, Obayashi M, Flavell R, Fitzsimonds RM, Ryan Ta, De Camilli P. Impaired PtdIns(4,5)P2 synthesis in nerve terminals produces defects in synaptic vesicle trafficking. Nature 431: 415–422, 2004 [DOI] [PubMed] [Google Scholar]
- 353. Di Paolo G, Pellegrini L, Letinic K, Cestra G, Zoncu R, Voronov S, Chang S, Guo J, Wenk MR, De Camilli P. Recruitment and regulation of phosphatidylinositol phosphate kinase type 1gamma by the FERM domain of talin. Nature 420: 85–89, 2002 [DOI] [PubMed] [Google Scholar]
- 354. Diaz Anel AM. Phospholipase C beta3 is a key component in the Gbetagamma/PKCeta/PKD-mediated regulation of trans-Golgi network to plasma membrane transport. Biochem J 406: 157–165, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355. Dickeson SK, Lim CN, Schuyler GT, Dalton TP, Helmkamp GM, Jr, Yarbrough LR. Isolation and sequence of cDNA clones encoding rat phosphatidylinositol transfer protein. J Biol Chem 264: 16557–16564, 1989 [PubMed] [Google Scholar]
- 356. Didichenko SA, Thelen M. Phosphatidylinositol 3-kinase c2alpha contains a nuclear localization sequence and associates with nuclear speckles. J Biol Chem 276: 48135–48142, 2001 [DOI] [PubMed] [Google Scholar]
- 357. Dippold HC, Ng MM, Farber-Katz SE, Lee SK, Kerr ML, Peterman MC, Sim R, Wiharto PA, Galbraith KA, Madhavarapu S, Fuchs GJ, Meerloo T, Farquhar MG, Zhou H, Field SJ. GOLPH3 bridges phosphatidylinositol-4-phosphate and actomyosin to stretch and shape the Golgi to promote budding. Cell 139: 337–351, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358. Diringer H, Friis RR. Changes in phosphatidylinositol metabolism correlated with growth state of normal and Rous sarcoma virus-transformed Japanese quail cells. Cancer Res 37: 2978–2984, 1977 [PubMed] [Google Scholar]
- 359. Dittmer JC, Dawson RM. The isolation of a new lipid, triphosphoinositide, and monophosphoinositide from ox brain. Biochem J 81: 535–540, 1961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360. Divecha N, Banfic H, Irvine RF. The polyphosphoinositide cycle exists in the nuclei of Swiss 3T3 cells under the control of a receptor (IGF-I) in the plasma membrane, and stimulation of the cycle increases nuclear diacylglycerol and apparently induces translocation of protein kinase C to the nucleus. EMBO J 10: 3207–3214, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361. Divecha N, Brooksbank CE, Irvine RF. Purification and characterization of phosphatidylinositol 4-phosphate 5-kinases. Biochem J 288: 637–642, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362. Divecha N, Truong O, Hsuan JJ, Hinchliffe KA, Irvine RF. The cloning and sequence of the C isoform of PtdIns4P 5-kinase. Biochem J 309: 715–719, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363. Dixon MJ, Gray A, Schenning M, Agacan M, Tempel W, Tong Y, Nedyalkova L, Park HW, Leslie NR, van Aalten DM, Downes CP, Batty IH. IQGAP proteins reveal an atypical phosphoinositide (aPI) binding domain with a pseudo C2 domain fold. J Biol Chem 287: 22483–22496, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364. Doerks T, Strauss M, Brendel M, Bork P. GRAM, a novel domain in glucosyltransferases, myotubularins and other putative membrane-associated proteins. Trends Biochem Sci 25: 483–485, 2000 [DOI] [PubMed] [Google Scholar]
- 365. Doerner JF, Hatt H, Ramsey IS. Voltage- and temperature-dependent activation of TRPV3 channels is potentiated by receptor-mediated PI(4,5)P2 hydrolysis. J Gen Physiol 137: 271–288, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366. Doherty GJ, McMahon HT. Mechanisms of endocytosis. Annu Rev Biochem 78: 857–902, 2009 [DOI] [PubMed] [Google Scholar]
- 367. Domin J, Gaidarov I, Smith ME, Keen JH, Waterfield MD. The class II phosphoinositide 3-kinase PI3K-C2alpha is concentrated in the trans-Golgi network and present in clathrin-coated vesicles. J Biol Chem 275: 11943–11950, 2000 [DOI] [PubMed] [Google Scholar]
- 368. Domin J, Harper L, Aubyn D, Wheeler M, Florey O, Haskard D, Yuan M, Zicha D. The class II phosphoinositide 3-kinase PI3K-C2beta regulates cell migration by a PtdIns3P dependent mechanism. J Cell Physiol 205: 452–462, 2005 [DOI] [PubMed] [Google Scholar]
- 369. Domin J, Pages F, Volinia S, Rittenhouse SE, Zvelebil MJ, Stein RC, Waterfield MD. Cloning of a human phosphoinositide 3-kinase with a C2 domain that displays reduced sensitivity to the inhibitor wortmannin. Biochem J 326: 139–147, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370. Dominguez V, Raimondi C, Somanath S, Bugliani M, Loder MK, Edling CE, Divecha N, da Silva-Xavier G, Marselli L, Persaud SJ, Turner MD, Rutter GA, Marchetti P, Falasca M, Maffucci T. Class II phosphoinositide 3-kinase regulates exocytosis of insulin granules in pancreatic beta cells. J Biol Chem 286: 4216–4225, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371. Dong XP, Shen D, Wang X, Dawson T, Li X, Zhang Q, Cheng X, Zhang Y, Weisman LS, Delling M, Xu H. PI(3,5)P(2) controls membrane trafficking by direct activation of mucolipin Ca(2+) release channels in the endolysosome. Nat Commun 1: 38, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372. Donoso M, Cancino J, Lee J, van Kerkhof P, Retamal C, Bu G, Gonzalez A, Caceres A, Marzolo MP. Polarized traffic of LRP1 involves AP1B and SNX17 operating on Y-dependent sorting motifs in different pathways. Mol Biol Cell 20: 481–497, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373. Dormann D, Weijer G, Parent CA, Devreotes PN, Weijer CJ. Visualizing PI3 kinase-mediated cell-cell signaling during Dictyostelium development. Curr Biol 12: 1178–1188, 2002 [DOI] [PubMed] [Google Scholar]
- 374. Dou Z, Chattopadhyay M, Pan JA, Guerriero JL, Jiang YP, Ballou LM, Yue Z, Lin RZ, Zong WX. The class IA phosphatidylinositol 3-kinase p110-beta subunit is a positive regulator of autophagy. J Cell Biol 191: 827–843, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375. Doughman RL, Firestone AJ, Anderson RA. Phosphatidylinositol phosphate kinases put PI4,5P(2) in its place. J Membr Biol 194: 77–89, 2003 [DOI] [PubMed] [Google Scholar]
- 376. Dove SK, Cooke FT, Douglas MR, Sayers LG, Parker PJ, Michell RH. Osmotic stress activates phosphatidylinositol-3,5-bisphosphate synthesis. Nature 390: 187–192, 1997 [DOI] [PubMed] [Google Scholar]
- 377. Dove SK, Dong K, Kobayashi T, Williams FK, Michell RH. Phosphatidylinositol 3,5-bisphosphate and Fab1p/PIKfyve underPPIn endo-lysosome function. Biochem J 419: 1–13, 2009 [DOI] [PubMed] [Google Scholar]
- 378. Dove SK, McEwen RK, Mayes A, Hughes DC, Beggs JD, Michell RH. Vac14 controls PtdIns(3,5)P(2) synthesis and Fab1-dependent protein trafficking to the multivesicular body. Curr Biol 12: 885–893, 2002 [DOI] [PubMed] [Google Scholar]
- 379. Dove SK, Piper RC, McEwen RK, Yu JW, King MC, Hughes DC, Thuring J, Holmes AB, Cooke FT, Michell RH, Parker PJ, Lemmon MA. Svp1p defines a family of phosphatidylinositol 3,5-bisphosphate effectors. EMBO J 23: 1922–1933, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 380. Dowal L, Provitera P, Scarlata S. Stable association between G alpha(q) and phospholipase C beta 1 in living cells. J Biol Chem 281: 23999–24014, 2006 [DOI] [PubMed] [Google Scholar]
- 381. Dowler S, Currie RA, Campbell DG, Deak M, Kular G, Downes CP, Alessi DR. Identification of pleckstrin-homology-domain-containing proteins with novel phosphoinositide-binding specificities. Biochem J 351: 19–31, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 382. Dowling JJ, Low SE, Busta AS, Feldman EL. Zebrafish MTMR14 is required for excitation-contraction coupling, developmental motor function and the regulation of autophagy. Hum Mol Genet 19: 2668–2681, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 383. Downes CP, Michell RH. The polyphosphoinositide phosphodiesterase of erythrocyte membranes. Biochem J 198: 133–140, 1981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 384. Downes CP, Walker S, McConnachie G, Lindsay Y, Batty IH, Leslie NR. Acute regulation of the tumour suppressor phosphatase, PTEN, by anionic lipids and reactive oxygen species. Biochem Soc Trans 32: 338–342, 2004 [DOI] [PubMed] [Google Scholar]
- 385. Downes P, Michell RH. Phosphatidylinositol 4-phosphate and phosphatidylinositol 4,5-bisphosphate: lipids in search of a function. Cell Calcium 2: 467–502, 1982 [DOI] [PubMed] [Google Scholar]
- 386. Downing GJ, Kim S, Nakanishi S, Catt KJ, Balla T. Characterization of a soluble adrenal phosphatidylinositol 4-kinase reveals wortmannin-sensitivity of Type III phosphatidylinositol 4-kinases. Biochemistry 35: 3587–3594, 1996 [DOI] [PubMed] [Google Scholar]
- 387. Drayer AL, Van der Kaay J, Mayr GW, Van Haastert PJ. Role of phospholipase C in Dictyostelium: formation of inositol 1,4,5-trisphosphate and normal development in cells lacking phospholipase C activity. EMBO J 13: 1601–1609, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 388. Dressman MA, Olivos-Glander IM, Nussbaum RL, Suchy SF. Ocrl1, a PtdIns(4,5)P2 5-phosphatase, is localized to the trans-Golgi network of fibroblasts and epithelial cells. J Histochem Cytochem 48: 179–190, 2000 [DOI] [PubMed] [Google Scholar]
- 389. Duex JE, Nau JJ, Kauffman EJ, Weisman LS. Phosphoinositide 5-phosphatase Fig4p is required for both acute rise and subsequent fall in stress-induced phosphatidylinositol 3,5-bisphosphate levels. Eukaryot Cell 5: 723–731, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 390. Duex JE, Tang F, Weisman LS. The Vac14p-Fig4p complex acts independently of Vac7p and couples PI3,5P2 synthesis and turnover. J Cell Biol 172: 693–704, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 391. Dupraz S, Grassi D, Bernis ME, Sosa L, Bisbal M, Gastaldi L, Jausoro I, Caceres A, Pfenninger KH, Quiroga S. The TC10-Exo70 complex is essential for membrane expansion and axonal specification in developing neurons. J Neurosci 29: 13292–13301, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 392. Durell J, Garland JT, Friedal RO. Acetylcholine action: biochemical aspects. Science 165: 862–866, 1969 [DOI] [PubMed] [Google Scholar]
- 393. Durrell J. Acetylcholine-stimulated phosphodiesteratic cleavage of phosphoinositides: hypothetical role in membrane depolarization. Ann NY Acad Sci 165: 743–754, 1969 [PubMed] [Google Scholar]
- 394. Eagle H, Oyama VI, Levy M, Freeman AE. Myoinositol as an essential growth factor for normal and malignant human cells in culture. J Biol Chem 226: 191–206, 1957 [PubMed] [Google Scholar]
- 395. Eberhard DA, Cooper CL, Low MG, Holz RW. Evidence that the inositol phospholipids are necessary for exocytosis. Biochem J 268: 15–25, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 396. Echevarria W, Leite MF, Guerra MT, Zipfel WR, Nathanson MH. Regulation of calcium signals in the nucleus by a nucleoplasmic reticulum. Nat Cell Biol 5: 440–446, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 397. Efe JA, Botelho RJ, Emr SD. Atg18 regulates organelle morphology and Fab1 kinase activity independent of its membrane recruitment by phosphatidylinositol 3,5-bisphosphate. Mol Biol Cell 18: 4232–4244, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 398. El Sayegh TY, Arora PD, Ling K, Laschinger C, Janmey PA, Anderson RA, McCulloch CA. Phosphatidylinositol-4,5 bisphosphate produced by PIP5KIgamma regulates gelsolin, actin assembly, and adhesion strength of N-cadherin junctions. Mol Biol Cell 18: 3026–3038, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 399. Elabbadi N, Ancelin ML, Vial HJ. Characterization of phosphatidylinositol synthase and evidence of a polyphosphoinositide cycle in Plasmodium-infected erythrocytes. Mol Biochem Parasitol 63: 179–192, 1994 [DOI] [PubMed] [Google Scholar]
- 400. Elong Edimo W, Derua R, Janssens V, Nakamura T, Vanderwinden JM, Waelkens E, Erneux C. Evidence of SHIP2 Ser132 phosphorylation, its nuclear localization and stability. Biochem J 439: 391–401, 2011 [DOI] [PubMed] [Google Scholar]
- 401. Elong Edimo W, Vanderwinden JM, Erneux C. SHIP2 signalling at the plasma membrane, in the nucleus and at focal contacts. Adv Biol Regul 53: 28–37, 2013 [DOI] [PubMed] [Google Scholar]
- 402. Emori Y, Homma Y, Sorimachi H, Kawasaki H, Nakanishi O, Suzuki K, Takenawa T. A second type of rat phosphoinositide-specific phospholipase C containing a src-related sequence not essential for phosphoinositide-hydrolyzing activity. J Biol Chem 264: 21885–21890, 1989 [PubMed] [Google Scholar]
- 403. Endemann G, Dunn SN, Cantley LC. Bovine brain contains two types of phosphatidylinositol kinase. Biochemistry 26: 6845–6852, 1987 [DOI] [PubMed] [Google Scholar]
- 404. Engelman JA, Chen L, Tan X, Crosby K, Guimaraes AR, Upadhyay R, Maira M, McNamara K, Perera SA, Song Y, Chirieac LR, Kaur R, Lightbown A, Simendinger J, Li T, Padera RF, Garcia-Echeverria C, Weissleder R, Mahmood U, Cantley LC, Wong KK. Effective use of PI3K and MEK inhibitors to treat mutant Kras G12D and PIK3CA H1047R murine lung cancers. Nat Med 14: 1351–1356, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 405. Epand RM. Recognition of polyunsaturated acyl chains by enzymes acting on membrane lipids. Biochim Biophys Acta 1818: 957–962, 2012 [DOI] [PubMed] [Google Scholar]
- 406. Erdman S, Lin L, Malczynski M, Snyder M. Pheromone-regulated genes required for yeast mating differentiation. J Cell Biol 140: 461–483, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 407. Erdmann KS, Mao Y, McCrea HJ, Zoncu R, Lee S, Paradise S, Modregger J, Biemesderfer D, Toomre D, De Camilli P. A role of the Lowe syndrome protein OCRL in early steps of the endocytic pathway. Dev Cell 13: 377–390, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 408. Essen LO, Perisic O, Cheung R, Katan M, Williams RL. Crystal structure of a mammalian phosphoinositide-specific phospholipase C delta. Nature 380: 595–602, 1996 [DOI] [PubMed] [Google Scholar]
- 409. Essen LO, Perisic O, Lynch DE, Katan M, Williams RL. A ternary metal binding site in the C2 domain of phosphoinositide-specific phospholipase C-delta1. Biochemistry 36: 2753–2762, 1997 [DOI] [PubMed] [Google Scholar]
- 410. Estacion M, Sinkins WG, Schilling WP. Regulation of Drosophila transient receptor potential-like (TrpL) channels by phospholipase C-dependent mechanisms. J Physiol 530: 1–19, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 411. Eto K, Yamashita T, Tsubamoto Y, Terauchi Y, Hirose K, Kubota N, Yamashita S, Taka J, Satoh S, Sekihara H, Tobe K, Iino M, Noda M, Kimura S, Kadowaki T. Phosphatidylinositol 3-kinase suppresses glucose-stimulated insulin secretion by affecting post-cytosolic [Ca(2+)] elevation signals. Diabetes 51: 87–97, 2002 [DOI] [PubMed] [Google Scholar]
- 412. Evellin S, Nolte J, Tysack K, vom Dorp F, Thiel M, Weernink PA, Jakobs KH, Webb EJ, Lomasney JW, Schmidt M. Stimulation of phospholipase C-epsilon by the M3 muscarinic acetylcholine receptor mediated by cyclic AMP and the GTPase Rap2B. J Biol Chem 277: 16805–16813, 2002 [DOI] [PubMed] [Google Scholar]
- 413. Everett KL, Buehler A, Bunney TD, Margineanu A, Baxendale RW, Vatter P, Retlich M, Walliser C, Manning HB, Neil MA, Dunsby C, French PM, Gierschik P, Katan M. Membrane environment exerts an important influence on rac-mediated activation of phospholipase Cgamma2. Mol Cell Biol 31: 1240–1251, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 414. Everett KL, Bunney TD, Yoon Y, Rodrigues-Lima F, Harris R, Driscoll PC, Abe K, Fuchs H, de Angelis MH, Yu P, Cho W, Katan M. Characterization of phospholipase C gamma enzymes with gain-of-function mutations. J Biol Chem 284: 23083–23093, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 415. Fadri M, Daquinag A, Wang S, Xue T, Kunz J. The pleckstrin homology domain proteins Slm1 and Slm2 are required for actin cytoskeleton organization in yeast and bind phosphatidylinositol-4,5-bisphosphate and TORC2. Mol Biol Cell 16: 1883–1900, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 416. Faenza I, Matteucci A, Manzoli L, Billi AM, Aluigi M, Peruzzi D, Vitale M, Castorina S, Suh PG, Cocco L. A role for nuclear phospholipase Cbeta 1 in cell cycle control. J Biol Chem 275: 30520–30524, 2000 [DOI] [PubMed] [Google Scholar]
- 417. Fain JN, Berridge MJ. Relationship between phosphatidylinositol synthesis and recovery of 5-hydroxytryptamine-responsive Ca+2 flux in blowfly salivary glands. Biochem J 180: 655–661, 1979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 418. Falasca M, Hughes WE, Dominguez V, Sala G, Fostira F, Fang MQ, Cazzolli R, Shepherd PR, James DE, Maffucci T. The role of phosphoinositide 3-kinase C2alpha in insulin signaling. J Biol Chem 282: 28226–28236, 2007 [DOI] [PubMed] [Google Scholar]
- 419. Falasca M, Logan SK, Lehto VP, Baccante G, Lemmon MA, Schlessinger J. Activation of phospholipase Cgamma by PI 3-kinase-induced PH domain-mediated membrane targeting. EMBO J 17: 414–422, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 420. Falasca M, Maffucci T. Regulation and cellular functions of class II phosphoinositide 3-kinases. Biochem J 443: 587–601, 2012 [DOI] [PubMed] [Google Scholar]
- 421. Falkenburger BH, Jensen JB, Hille B. Kinetics of M1 muscarinic receptor and G protein signaling to phospholipase C in living cells. J Gen Physiol 135: 81–97, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 422. Falkenburger BH, Jensen JB, Hille B. Kinetics of PIP2 metabolism and KCNQ2/3 channel regulation studied with a voltage-sensitive phosphatase in living cells. J Gen Physiol 135: 99–114, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 423. Fan Z, Makielski JC. Anionic phospholipids activate ATP-sensitive potassium channels. J Biol Chem 272: 5388–5395, 1997 [DOI] [PubMed] [Google Scholar]
- 424. Fang M, Kearns BG, Gedvilaite A, Kagiwada S, Kearns M, Fung MK, Bankaitis VA. Kes1p shares homology with human oxysterol binding protein and participates in a novel regulatory pathway for yeast Golgi-derived transport vesicle biogenesis. EMBO J 15: 6447–6459, 1996 [PMC free article] [PubMed] [Google Scholar]
- 425. Farhan H, Weiss M, Tani K, Kaufman RJ, Hauri HP. Adaptation of endoplasmic reticulum exit sites to acute and chronic increases in cargo load. EMBO J 27: 2043–2054, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 426. Faulhammer F, Kanjilal-Kolar S, Knodler A, Lo J, Lee Y, Konrad G, Mayinger P. Growth control of Golgi phosphoinositides by reciprocal localization of sac1 lipid phosphatase and pik1 4-kinase. Traffic 8: 1554–1567, 2007 [DOI] [PubMed] [Google Scholar]
- 427. Faulhammer F, Konrad G, Brankatschk B, Tahirovic S, Knodler A, Mayinger P. Cell growth-dependent coordination of lipid signaling and glycosylation is mediated by interactions between Sac1p and Dpm1p. J Cell Biol 168: 185–191, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 428. Fedele CG, Ooms LM, Ho M, Vieusseux J, O'Toole SA, Millar EK, Lopez-Knowles E, Sriratana A, Gurung R, Baglietto L, Giles GG, Bailey CG, Rasko JE, Shields BJ, Price JT, Majerus PW, Sutherland RL, Tiganis T, McLean CA, Mitchell CA. Inositol polyphosphate 4-phosphatase II regulates PI3K/Akt signaling and is lost in human basal-like breast cancers. Proc Natl Acad Sci USA 107: 22231–22236, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 429. Feng JF, Rhee SG, Im MJ. Evidence that phospholipase delta1 is the effector in the Gh (transglutaminase II)-mediated signaling. J Biol Chem 271: 16451–16454, 1996 [DOI] [PubMed] [Google Scholar]
- 430. Ferguson GJ, Milne L, Kulkarni S, Sasaki T, Walker S, Andrews S, Crabbe T, Finan P, Jones G, Jackson S, Camps M, Rommel C, Wymann M, Hirsch E, Hawkins P, Stephens L. PI(3)Kgamma has an important context-dependent role in neutrophil chemokinesis. Nat Cell Biol 9: 86–91, 2007 [DOI] [PubMed] [Google Scholar]
- 431. Ferguson KM, Lemmon MA, Schlessinger J, Sigler PB. Structure of the high affinity complex of inositol trisphosphate with a phospholipase C plekstrin homology domain. Cell 83: 1037–1046, 1995 [DOI] [PubMed] [Google Scholar]
- 432. Ferguson SM, De Camilli P. Dynamin, a membrane-remodelling GTPase. Nat Rev Mol Cell Biol 13: 75–88, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 433. Ferguson SM, Raimondi A, Paradise S, Shen H, Mesaki K, Ferguson A, Destaing O, Ko G, Takasaki J, Cremona OEOT, De Camilli P. Coordinated actions of actin and BAR proteins upstream of dynamin at endocytic clathrin-coated pits. Dev Cell 17: 811–822, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 434. Ferron M, Vacher J. Characterization of the murine Inpp4b gene and identification of a novel isoform. Gene 376: 152–161, 2006 [DOI] [PubMed] [Google Scholar]
- 435. Feske S, Gwack Y, Prakriya M, Srikanth S, Puppel SH, Tanasa B, Hogan PG, Lewis RS, Daly M, Rao A. A mutation in Orai1 causes immune deficiency by abrogating CRAC channel function. Nature 441: 179–185, 2006 [DOI] [PubMed] [Google Scholar]
- 436. Field SJ, Madson N, Kerr ML, Galbraith KA, Kennedy CE, Tahiliani M, Wilkins A, Cantley LC. PtdIns(4,5)P2 functions at the cleavage furrow during cytokinesis. Curr Biol 15: 1407–1412, 2005 [DOI] [PubMed] [Google Scholar]
- 437. Fili N, Calleja V, Woscholski R, Parker PJ, Larijani B. Compartmental signal modulation: endosomal phosphatidylinositol 3-phosphate controls endosome morphology and selective cargo sorting. Proc Natl Acad Sci USA 103: 15473–15478, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 438. Filomatori CV, Rega AF. On the mechanism of activation of the plasma membrane Ca2+-ATPase by ATP and acidic phospholipids. J Biol Chem 278: 22265–22271, 2003 [DOI] [PubMed] [Google Scholar]
- 439. Finco TS, Kadlecek T, Zhang W, Samelson LE, Weiss A. LAT is required for TCR-mediated activation of PLCgamma1 and the Ras pathway. Immunity 9: 617–626, 1998 [DOI] [PubMed] [Google Scholar]
- 440. Firestein R, Nagy PL, Daly M, Huie P, Conti M, Cleary ML. Male infertility, impaired spermatogenesis, and azoospermia in mice deficient for the pseudophosphatase Sbf1. J Clin Invest 109: 1165–1172, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 441. Fisher DB, Mueller GC. An early alteration in the phospholipid metabolism of human lymphocytes by PHA. Proc Natl Acad Sci USA 60: 1396–1402, 1968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 442. Fisher DB, Mueller GC. Studies of the mechanism by which PHA stimulates phospholipid metabolism of human lymphocytes. Biochim Biophys Acta 248: 434–448, 1971 [Google Scholar]
- 443. Fiume R, Ramazzotti G, Teti G, Chiarini F, Faenza I, Mazzotti G, Billi AM, Cocco L. Involvement of nuclear PLCbeta1 in lamin B1 phosphorylation and G2/M cell cycle progression. FASEB J 23: 957–966, 2009 [DOI] [PubMed] [Google Scholar]
- 444. Flanagan CA, Schnieders EA, Emerick AW, Kunisawa R, Admon A, Thorner J. Phosphatidylinositol 4-kinase: gene structure and requirement for yeast cell viability. Science 262: 1444–1448, 1993 [DOI] [PubMed] [Google Scholar]
- 445. Flanagan CA, Thorner J. Purification and characterization of a soluble phosphatidylinositol 4-kinase from the yeast Saccharomyces cerevisiae. J Biol Chem 267: 24117–24125, 1992 [PubMed] [Google Scholar]
- 446. Flannagan RS, Jaumouille V, Grinstein S. The cell biology of phagocytosis. Annu Rev Pathol 7: 61–98, 2012 [DOI] [PubMed] [Google Scholar]
- 447. Flick JS, Thorner J. Genetic and biochemical characterization of a phosphatidylinositol-specific phospholiase C in Saccharomyces cerevisiae. Mol Cell Biol 13: 5861–5876, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 448. Fluckiger AC, Li Z, Kato RM, Wahl MI, Ochs RM, Longnecker R, Kinet JP, Witte ON, Scharenberg AM, Rawlings DJ. Btk/Tec kinases regulate sustained increases in intracellular Ca2+ following B-cell receptor activation. EMBO J 17: 1973–1985, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 449. Folch J. Complete fractionation of brain cephalin: isolation from it of phosphatidyl serine, phosphatidyl ethanolamine, and diphosphoinositide. J Biol Chem 177: 497–504, 1949 [PubMed] [Google Scholar]
- 450. Folch J, Wooley DW. Inositol, a constiuent of a brain phosphatide. J Biol Chem 142: 963–964, 1942 [Google Scholar]
- 451. Follo MY, Bosi C, Finelli C, Fiume R, Faenza I, Ramazzotti G, Gaboardi GC, Manzoli L, Cocco L. Real-time PCR as a tool for quantitative analysis of PI-PLCbeta1 gene expression in myelodysplastic syndrome. Int J Mol Med 18: 267–271, 2006 [PubMed] [Google Scholar]
- 452. Follo MY, Faenza I, Fiume R, Ramazzotti G, McCubrey JA, Martelli AM, Manzoli FA, Cocco L. Revisiting nuclear phospholipase C signalling in MDS. Adv Enzyme Regul 52: 2–6, 2012 [DOI] [PubMed] [Google Scholar]
- 453. Ford MGJ, Pearse BMF, Higgins MK, Vallis Y, Owen DJ, Gibson A, Hopkins CR, Evans PR, McMahon HT. Simultaneous binding of PI(4,5)P2 and clathrin by AP180 in the nucleation of clathrin lattices on membranes. Science 291: 1051–1055, 2001 [DOI] [PubMed] [Google Scholar]
- 454. Foster KG, Fingar DC. Mammalian target of rapamycin (mTOR): conducting the cellular signaling symphony. J Biol Chem 285: 14071–14077, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 455. Foti M, Audhya A, Emr SD. Sac1 lipid phosphatase and stt4 phosphatidylinositol 4-kinase regulate a pool of phosphatidylinositol 4-phosphate that functions in the control of the actin cytoskeleton and vacuole morphology. Mol Biol Cell 128: 2396–2411, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 456. Fougerat A, Gayral S, Gourdy P, Schambourg A, Ruckle T, Schwarz MK, Rommel C, Hirsch E, Arnal JF, Salles JP, Perret B, Breton-Douillon M, Wymann MP, Laffargue M. Genetic and pharmacological targeting of phosphoinositide 3-kinase-gamma reduces atherosclerosis and favors plaque stability by modulating inflammatory processes. Circulation 117: 1310–1317, 2008 [DOI] [PubMed] [Google Scholar]
- 457. Foukas LC, Claret M, Pearce W, Okkenhaug K, Meek S, Peskett E, Sancho S, Smith AJ, Withers DJ, Vanhaesebroeck B. Critical role for the p110alpha phosphoinositide-3-OH kinase in growth and metabolic regulation. Nature 441: 366–370, 2006 [DOI] [PubMed] [Google Scholar]
- 458. Fouladkou F, Landry T, Kawabe H, Neeb A, Lu C, Brose N, Stambolic V, Rotin D. The ubiquitin ligase Nedd4–1 is dispensable for the regulation of PTEN stability and localization. Proc Natl Acad Sci USA 105: 8585–8590, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 459. Franca-Koh J, Devreotes PN. Moving forward: mechanisms of chemoattractant gradient sensing. Physiology 19: 300–308, 2004 [DOI] [PubMed] [Google Scholar]
- 460. Franke TF, Kaplan DR, Cantley LC, Toker A. Direct regulation of the Akt protooncogene product by PI3,4P2. Science 275: 665–668, 1997 [DOI] [PubMed] [Google Scholar]
- 461. Franke TF, Yang SI, Chan TO, Datta K, Kazlauskas A, Morrison DK, Kaplan DR, Tsichlis PN. The protein kinase encoded by the Akt proto-oncogene is a target of the PDGF-activated phosphatidylinositol 3-kinase. Cell 81: 727–736, 1995 [DOI] [PubMed] [Google Scholar]
- 462. Franklin NE, Taylor GS, Vacratsis PO. Endosomal targeting of the phosphoinositide 3-phosphatase MTMR2 is regulated by an N-terminal phosphorylation site. J Biol Chem 286: 15841–15853, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 463. Frederick JP, Mattiske D, Wofford JA, Megosh LC, Drake LY, Chiou ST, Hogan BL, York JD. An essential role for an inositol polyphosphate multikinase, Ipk2, in mouse embryogenesis and second messenger production. Proc Natl Acad Sci USA 102: 8454–8459, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 464. Friedel RO, Brown JD, Durell J. The enzymic hydrolysis of phosphatidyl inositol by guinea pig brain: sub-cellular distribution and hydrolysis products. J Neurochem 16: 371–378, 1969 [DOI] [PubMed] [Google Scholar]
- 465. Frost A, Perera R, Roux A, Spasov K, Destaing O, Egelman EH, De Camilli P, Unger VM. Structural basis of membrane invagination by F-BAR domains. Cell 132: 807–817, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 466. Frost A, Unger VM, De Camilli P. The BAR domain superfamily: membrane-molding macromolecules. Cell 137: 191–196, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 467. Fruman DA, Bismuth G. Fine tuning the immune response with PI3K. Immunol Rev 228: 253–272, 2009 [DOI] [PubMed] [Google Scholar]
- 468. Fruman DA, Cantley LC, Carpenter CL. Structural organization and alternative splicing of the murine phosphoinositide 3-kinase p85 alpha gene. Genomics 37: 113–121, 1996 [DOI] [PubMed] [Google Scholar]
- 469. Fruman DA, Mauvais-Jarvis F, Pollard DA, Yballe CM, Brazil D, Bronson RT, Kahn CR, Cantley LC. Hypoglycaemia, liver necrosis and perinatal death in mice lacking all isoforms of phosphoinositide 3-kinase p85 alpha. Nat Genet 26: 379–382, 2000 [DOI] [PubMed] [Google Scholar]
- 470. Fruman DA, Snapper SB, Yballe CM, Davidson L, Yu JY, Alt FW, Cantley LC. Impaired B cell development and proliferation in absence of phosphoinositide 3-kinase p85alpha. Science 283: 393–397, 1999 [DOI] [PubMed] [Google Scholar]
- 471. Fu L, Qin YR, Xie D, Hu L, Kwong DL, Srivastava G, Tsao SW, Guan XY. Characterization of a novel tumor-suppressor gene PLC delta 1 at 3p22 in esophageal squamous cell carcinoma. Cancer Res 67: 10720–10726, 2007 [DOI] [PubMed] [Google Scholar]
- 472. Fujioka Y, Tsuda M, Hattori T, Sasaki J, Sasaki T, Miyazaki T, Ohba Y. The Ras-PI3K signaling pathway is involved in clathrin-independent endocytosis and the internalization of influenza viruses. PLoS One 6: e16324, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 473. Fujita M, Kinoshita T. GPI-anchor remodeling: potential functions of GPI-anchors in intracellular trafficking and membrane dynamics. Biochim Biophys Acta 1821: 1050–1058, 2012 [DOI] [PubMed] [Google Scholar]
- 474. Fujiwara Y, Kubo Y. Regulation of the desensitization and ion selectivity of ATP-gated P2X2 channels by phosphoinositides. J Physiol 576: 135–149, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 475. Fukami K, Inoue T, Kurokawa M, Fissore RA, Nakao K, Nagano K, Nakamura Y, Takenaka K, Yoshida N, Mikoshiba K, Takenawa T. Phospholipase Cdelta4: from genome structure to physiological function. Adv Enzyme Regul 43: 87–106, 2003 [DOI] [PubMed] [Google Scholar]
- 476. Fukami K, Nakao K, Inoue T, Kataoka Y, Kurokawa M, Fissore RA, Nakamura K, Katsuki M, Mikoshiba K, Yoshida N, Takenawa T. Requirement of phospholipase Cdelta4 for the zona pellucida-induced acrosome reaction. Science 292: 920–923, 2001 [DOI] [PubMed] [Google Scholar]
- 477. Fukami K, Yoshida M, Inoue T, Kurokawa M, Fissore RA, Yoshida N, Mikoshiba K, Takenawa T. Phospholipase Cdelta4 is required for Ca2+ mobilization essential for acrosome reaction in sperm. J Cell Biol 161: 79–88, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 478. Fukuda M, Kojima T, Kabayama H, Mikoshiba K. Mutation of the pleckstrin homology domain of Bruton's tyrosine kinase in immunodeficiency impaired inositol 1,3,4,5-tertakisphosphate binding capacity. J Biol Chem 271: 30303–30306, 1996 [DOI] [PubMed] [Google Scholar]
- 479. Fukui Y, Kornbluth S, Jong SM, Wang LH, Hanafusa H. Phosphatidylinositol kinase type I activity associates with various oncogene products. Oncogene Res 4: 283–292, 1989 [PubMed] [Google Scholar]
- 480. Fullwood Y, dos Santos M, Hsuan JJ. Cloning and characterization of a novel human phosphatidylinositol transfer protein, rdgBbeta. J Biol Chem 274: 31553–31558, 1999 [DOI] [PubMed] [Google Scholar]
- 481. Furnari FB, Huang HJ, Cavenee WK. The phosphoinositol phosphatase activity of PTEN mediates a serum-sensitive G1 growth arrest in glioma cells. Cancer Res 58: 5002–5008, 1998 [PubMed] [Google Scholar]
- 482. Gaidarov I, Keen JH. Phosphoinositide-AP-2 interactions required for targeting to plasma membrane clathrin-coated pits. J Cell Biol 146: 755–764, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 483. Gaidarov I, Krupnick JG, Falck JR, Benovic JL, Keen JH. Arrestin function in G protein-coupled receptor endocytosis requires phosphoinositide binding. EMBO J 18: 871–881, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 484. Gaidarov I, Smith ME, Domin J, Keen JH. The class II phosphoinositide 3-kinase C2alpha is activated by clathrin and regulates clathrin-mediated membrane trafficking. Mol Cell 7: 443–449, 2001 [DOI] [PubMed] [Google Scholar]
- 485. Gaidarov I, Zhao Y, Keen JH. Individual phosphoinositide 3-kinase C2alpha domain activities independently regulate clathrin function. J Biol Chem 280: 40766–40772, 2005 [DOI] [PubMed] [Google Scholar]
- 486. Gallego O, Betts MJ, Gvozdenovic-Jeremic J, Maeda K, Matetzki C, Aguilar-Gurrieri C, Beltran-Alvarez P, Bonn S, Fernandez-Tornero C, Jensen LJ, Kuhn M, Trott J, Rybin V, Muller CW, Bork P, Kaksonen M, Russell RB, Gavin AC. A systematic screen for protein-lipid interactions in Saccharomyces cerevisiae. Mol Syst Biol 6: 430, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 487. Gamper N, Reznikov V, Yamada Y, Yang J, Shapiro MS. Phosphatidylinositol 4,5-bisphosphate signals underlie receptor-specific Gq/11-mediated modulation of N-type Ca2+ channels. J Neurosci 24: 10980–10992, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 488. Gamper N, Shapiro MS. Regulation of ion transport proteins by membrane phosphoinositides. Nat Rev Neurosci 8: 921–934, 2007 [DOI] [PubMed] [Google Scholar]
- 489. Gan X, Wang J, Su B, Wu D. Evidence for direct activation of mTORC2 kinase activity by phosphatidylinositol 3,4,5-trisphosphate. J Biol Chem 286: 10998–11002, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 490. Gao J, Takeuchi H, Zhang Z, Fujii M, Kanematsu T, Hirata M. Binding of phospholipase C-related but catalytically inactive protein to phosphatidylinositol 4,5-bisphosphate via the PH domain. Cell Signal 21: 1180–1186, 2009 [DOI] [PubMed] [Google Scholar]
- 491. Gao X, Zhang Y, Arrazola P, Hino O, Kobayashi T, Yeung RS, Ru B, Pan D. Tsc tumour suppressor proteins antagonize amino-acid-TOR signalling. Nat Cell Biol 4: 699–704, 2002 [DOI] [PubMed] [Google Scholar]
- 492. Gao Z, Konrad RJ, Collins H, Matschinsky FM, Rothenberg PL, Wolf BA. Wortmannin inhibits insulin secretion in pancreatic islets and beta-TC3 cells independent of its inhibition of phosphatidylinositol 3-kinase. Diabetes 45: 854–862, 1996 [DOI] [PubMed] [Google Scholar]
- 493. Garcia-Bustos JF, Marini F, Stevenson I, Frei C, Hall MN. PIK1, an essential phosphatidylinositol 4-kinase associated with the yeast nucleus. EMBO J 13: 2352–2361, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 494. Garner K, Hunt AN, Koster G, Somerharju P, Groves E, Li M, Raghu P, Holic R, Cockcroft S. Phosphatidylinositol transfer protein, cytoplasmic 1 (PITPNC1) binds and transfers phosphatidic acid. J Biol Chem 287: 32263–32276, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 495. Garner K, Li M, Ugwuanya N, Cockcroft S. The phosphatidylinositol transfer protein RdgBbeta binds 14-3-3 via its unstructured C-terminus, whereas its lipid-binding domain interacts with the integral membrane protein ATRAP (angiotensin II type I receptor-associated protein). Biochem J 439: 97–111, 2011 [DOI] [PubMed] [Google Scholar]
- 496. Garnier-Lhomme M, Byrne RD, Hobday TM, Gschmeissner S, Woscholski R, Poccia DL, Dufourc EJ, Larijani B. Nuclear envelope remnants: fluid membranes enriched in sterols and polyphosphoinositides. PLoS One 4: e4255, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 497. Gary JD, Sato TK, Stefan CJ, Bonangelino CJ, Weisman LS, Emr SD. Regulation of Fab1 phosphatidylinositol 3-phosphate 5-kinase pathway by Vac7 protein and Fig4, a polyphosphoinositide phosphatase family member. Mol Biol Cell 13: 1238–1251, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 498. Gary JD, Wurmser AE, Bonangelino CJ, Weisman LS, Emr SD. Fab1p is essential for PtdIns(3)P 5-kinase activity and the maintenance of vacuolar size and membrane homeostasis. J Cell Biol 143: 65–79, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 499. Gassama-Diagne A, Yu W, ter Beest M, Martin-Belmonte F, Kierbel A, Engel J, Mostov K. Phosphatidylinositol-3,4,5-trisphosphate regulates the formation of the basolateral plasma membrane in epithelial cells. Nat Cell Biol 8: 963–970, 2006 [DOI] [PubMed] [Google Scholar]
- 500. Gatt MK, Glover DM. The Drosophila phosphatidylinositol transfer protein encoded by vibrator is essential to maintain cleavage-furrow ingression in cytokinesis. J Cell Sci 119: 2225–2235, 2006 [DOI] [PubMed] [Google Scholar]
- 501. Gaullier JM, Simonsen A, D'Arrigo A, Bremnes B, Stenmark H, Aasland R. FYVE fingers bind PtdIns(3)P. Nature 394: 432–433, 1998 [DOI] [PubMed] [Google Scholar]
- 502. Gavin AC, Bosche M, Krause R, Grandi P, Marzioch M, Bauer A, Schultz J, Rick JM, Michon AM, Cruciat CM, Remor M, Hofert C, Schelder M, Brajenovic M, Ruffner H, Merino A, Klein K, Hudak M, Dickson D, Rudi T, Gnau V, Bauch A, Bastuck S, Huhse B, Leutwein C, Heurtier MA, Copley RR, Edelmann A, Querfurth E, Rybin V, Drewes G, Raida M, Bouwmeester T, Bork P, Seraphin B, Kuster B, Neubauer G, Superti-Furga G. Functional organization of the yeast proteome by systematic analysis of protein complexes. Nature 415: 141–147, 2002 [DOI] [PubMed] [Google Scholar]
- 503. Geering B, Cutillas PR, Nock G, Gharbi SI, Vanhaesebroeck B. Class IA phosphoinositide 3-kinases are obligate p85-p110 heterodimers. Proc Natl Acad Sci USA 104: 7809–7814, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 504. Gehrmann T, Gulkan H, Suer S, Herberg FW, Balla A, Vereb G, Mayr GW, Heilmeyer LM., Jr Functional expression and characterisation of a new human phosphatidylinositol 4-kinase PI4K230. Biochim Biophys Acta 1437: 341–356, 1999 [DOI] [PubMed] [Google Scholar]
- 505. Gehrmann T, Heilmayer LG. Phosphatidylinositol 4-kinases. Eur J Biochem 253: 357–370, 1998 [DOI] [PubMed] [Google Scholar]
- 506. Georgescu MM, Kirsch KH, Akagi T, Shishido T, Hanafusa H. The tumor-suppressor activity of PTEN is regulated by its carboxyl-terminal region. Proc Natl Acad Sci USA 96: 10182–10187, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 507. Georgescu MM, Kirsch KH, Kaloudis P, Yang H, Pavletich NP, Hanafusa H. Stabilization and productive positioning roles of the C2 domain of PTEN tumor suppressor. Cancer Res 60: 7033–7038, 2000 [PubMed] [Google Scholar]
- 508. Giansanti MG, Bonaccorsi S, Kurek R, Farkas RM, Dimitri P, Fuller MT, Gatti M. The class I PITP giotto is required for Drosophila cytokinesis. Curr Biol 16: 195–201, 2006 [DOI] [PubMed] [Google Scholar]
- 509. Gillooly DJ, Morrow IC, Lindsay M, Gould R, Bryant NJ, Gaullier LM, Parton GP, Stenmark H. Localization of phosphatidylinositol 3-phosphate in yeast and mammalian cells. EMBO J 19: 4577–4588, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 510. Giudici ML, Emson PC, Irvine RF. A novel neuronal-specific splice variant of Type I phosphatidylinositol 4-phosphate 5-kinase isoform gamma. Biochem J 379: 489–496, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 511. Giudici ML, Lee K, Lim R, Irvine RF. The intracellular localisation and mobility of Type Igamma phosphatidylinositol 4P 5-kinase splice variants. FEBS Lett 580: 6933–6937, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 512. Gloor Y, Schone M, Habermann B, Ercan E, Beck M, Weselek G, Muller-Reichert T, Walch-Solimena C. Interaction between Sec7p and Pik1p: the first clue for the regulation of a coincidence detection signal. Eur J Cell Biol 89: 575–583, 2010 [DOI] [PubMed] [Google Scholar]
- 513. Godi A, Di Campi A, Konstantakopoulos A, Di Tullio G, Alessi DR, Kular GS, Daniele T, Marra P, Lucocq JM, De Matteis MA. FAPPs control Golgi-to-cell-surface membrane traffic by binding to ARF and PtdIns(4)P. Nat Cell Biol 6: 393–404, 2004 [DOI] [PubMed] [Google Scholar]
- 514. Godi A, Pertile P, Meyers R, Marra P, Di Tullio G, Iurisci C, Luini A, Corda D, De Matteis MA. ARF mediates recruitment of PtdIns-4-OH kinase-beta and stimulates synthesis of PtdIns(4,5)P2 on the Golgi complex. Nat Cell Biol 1: 280–287, 1999 [DOI] [PubMed] [Google Scholar]
- 515. Godin CM, Ferreira LT, Dale LB, Gros R, Cregan SP, Ferguson SS. The small GTPase Ral couples the angiotensin II type 1 receptor to the activation of phospholipase C-delta 1. Mol Pharmacol 77: 388–395, 2010 [DOI] [PubMed] [Google Scholar]
- 516. Goldschmidt-Clermont PJ, Machesky LM, Baldassare JJ, Pollard TD. The actin binding protein profilin binds to PIP2 and inhibits its hydrolysis by phospholipase C. Science 247: 1575–1578, 1990 [DOI] [PubMed] [Google Scholar]
- 517. Golebiewska U, Kay JG, Masters T, Grinstein S, Im W, Pastor RW, Scarlata S, McLaughlin S. Evidence for a fence that impedes the diffusion of phosphatidylinositol 4,5-bisphosphate out of the forming phagosomes of macrophages. Mol Biol Cell 22: 3498–3507, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 518. Golebiewska U, Nyako M, Woturski W, Zaitseva I, McLaughlin S. Diffusion coefficient of fluorescent phosphatidylinositol 4,5-bisphosphate in the plasma membrane of cells. Mol Biol Cell 19: 1663–1669, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 519. Gomes DA, Rodrigues MA, Leite MF, Gomez MV, Varnai P, Balla T, Bennett AM, Nathanson MH. C-met must translocate to the nucleus to initiate calcium signals. J Biol Chem 283: 4344–4351, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 520. Gong LW, Di Paolo G, Diaz E, Cestra G, Diaz ME, Lindau M, De Camilli P, Toomre D. Phosphatidylinositol phosphate kinase type I gamma regulates dynamics of large dense-core vesicle fusion. Proc Natl Acad Sci USA 102: 5204–5209, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 521. Gonzalez-Santamaria J, Campagna M, Ortega-Molina A, Marcos-Villar L, de la Cruz-Herrera CF, Gonzalez D, Gallego P, Lopitz-Otsoa F, Esteban M, Rodriguez MS, Serrano M, Rivas C. Regulation of the tumor suppressor PTEN by SUMO. Cell Death Dis 3: e393, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 522. Gozani O, Karuman P, Jones DR, Ivanov D, Cha J, Logovskoy AA, Baird CL, Zhu H, Field SJ, Lessnick SL, Villasenov J, Mehrotra B, Chen J, Rao VR, Brugge JS, Ferguson CG, Payrastre B, Myszka DG, Cantley LC, Wagner G, Divecha N, Prestwich GD, Yuan J. The PHD finger of the chromatin-associated protein ING2 functions as a nuclear phosphoinositide receptor. Cell 114: 99–111, 2003 [DOI] [PubMed] [Google Scholar]
- 523. Graham TR, Burd CG. Coordination of Golgi functions by phosphatidylinositol 4-kinases. Trends Cell Biol 21: 113–121, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 524. Graupera M, Guillermet-Guibert J, Foukas LC, Phng LK, Cain RJ, Salpekar A, Pearce W, Meek S, Millan J, Cutillas PR, Smith AJ, Ridley AJ, Ruhrberg C, Gerhardt H, Vanhaesebroeck B. Angiogenesis selectively requires the p110alpha isoform of PI3K to control endothelial cell migration. Nature 453: 662–666, 2008 [DOI] [PubMed] [Google Scholar]
- 525. Graziani A, Ling LE, Endemann G, Carpenter CL, Cantley LC. Purification and characterization of human erythrocyte phosphatidylinositol 4-kinase. Biochem J 284: 39–45, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 526. Gresset A, Hicks SN, Harden TK, Sondek J. Mechanism of phosphorylation-induced activation of phospholipase C-gamma isozymes. J Biol Chem 285: 35836–35847, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 527. Grieve AG, Daniels RD, Sanchez-Heras E, Hayes MJ, Moss SE, Matter K, Lowe M, Levine TP. Lowe Syndrome protein OCRL1 supports maturation of polarized epithelial cells. PLoS One 6: e24044, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 528. Griffith OH, Ryan M. Bacterial phosphatidylinositol-specific phospholipase C: structure, function, and interaction with lipids. Biochim Biophys Acta 1441: 237–254, 1999 [DOI] [PubMed] [Google Scholar]
- 529. Grishanin RN, Klenchin VA, Loyet KM, Kowalchyk JA, Ann K, Martin TF. Membrane association domains in Ca2+-dependent activator protein for secretion mediate plasma membrane and dense-core vesicle binding required for Ca2+-dependent exocytosis. J Biol Chem 277: 22025–22034, 2002 [DOI] [PubMed] [Google Scholar]
- 530. Grishanin RN, Kowalchyk JA, Klenchin VA, Ann K, Earles CA, Chapman ER, Gerona RR, Martin TF. CAPS acts at a prefusion step in dense-core vesicle exocytosis as a PIP2 binding protein. Neuron 43: 551–562, 2004 [DOI] [PubMed] [Google Scholar]
- 531. Gromada J, Bark C, Smidt K, Efanov AM, Janson J, Mandic SA, Webb DL, Zhang W, Meister B, Jeromin A, Berggren PO. Neuronal calcium sensor-1 potentiates glucose-dependent exocytosis in pancreatic beta cells through activation of phosphatidylinositol 4-kinase beta. Proc Natl Acad Sci USA 102: 10303–10308, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 532. Grubb DR, Vasilevski O, Huynh H, Woodcock EA. The extreme C-terminal region of phospholipase Cbeta1 determines subcellular localization and function; the “b” splice variant mediates alpha1-adrenergic receptor responses in cardiomyocytes. FASEB J 22: 2768–2774, 2008 [DOI] [PubMed] [Google Scholar]
- 533. Guan J, Stromhaug PE, George MD, Habibzadegah-Tari P, Bevan A, Dunn WA, Jr, Klionsky DJ. Cvt18/Gsa12 is required for cytoplasm-to-vacuole transport, pexophagy, and autophagy in Saccharomyces cerevisiae and Pichia pastoris. Mol Biol Cell 12: 3821–3838, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 534. Guillermet-Guibert J, Bjorklof K, Salpekar A, Gonella C, Ramadani F, Bilancio A, Meek S, Smith AJ, Okkenhaug K, Vanhaesebroeck B. The p110beta isoform of phosphoinositide 3-kinase signals downstream of G protein-coupled receptors and is functionally redundant with p110gamma. Proc Natl Acad Sci USA 105: 8292–8297, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 535. Guipponi M, Tapparel C, Jousson O, Scamuffa N, Mas C, Rossier C, Hutter P, Meda P, Lyle R, Reymond A, Antonarakis SE. The murine orthologue of the Golgi-localized TPTE protein provides clues to the evolutionary history of the human TPTE gene family. Hum Genet 109: 569–575, 2001 [DOI] [PubMed] [Google Scholar]
- 536. Gulati P, Gaspers LD, Dann SG, Joaquin M, Nobukuni T, Natt F, Kozma SC, Thomas AP, Thomas G. Amino acids activate mTOR complex 1 via Ca2+/CaM signaling to hVps34. Cell Metab 7: 456–465, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 537. Guo J, Yu FX. Cloning and characterization of human homologue of Drosophila retinal degeneration B: a candidate gene for degenerative retinal diseases. Dev Genet 20: 235–245, 1997 [DOI] [PubMed] [Google Scholar]
- 538. Guo S, Stolz LE, Lemrow SM, York JD. SAC1-like domains of yeast SAC1, INP52, and INP53 and of human synaptojanin encode polyphosphoinositide phosphatases. J Biol Chem 274: 12990–12995, 1999 [DOI] [PubMed] [Google Scholar]
- 539. Gupta S, Ramjaun AR, Haiko P, Wang Y, Warne PH, Nicke B, Nye E, Stamp G, Alitalo K, Downward J. Binding of ras to phosphoinositide 3-kinase p110alpha is required for ras-driven tumorigenesis in mice. Cell 129: 957–968, 2007 [DOI] [PubMed] [Google Scholar]
- 540. Gurung R, Tan A, Ooms LM, McGrath MJ, Huysmans RD, Munday AD, Prescott M, Whisstock JC, Mitchell CA. Identification of a novel domain in two mammalian inositol-polyphosphate 5-phosphatases that mediates membrane ruffle localization. The inositol 5-phosphatase skip localizes to the endoplasmic reticulum and translocates to membrane ruffles following epidermal growth factor stimulation. J Biol Chem 278: 11376–11385, 2003 [DOI] [PubMed] [Google Scholar]
- 541. Gustin JP, Karakas B, Weiss MB, Abukhdeir AM, Lauring J, Garay JP, Cosgrove D, Tamaki A, Konishi H, Konishi Y, Mohseni M, Wang G, Rosen DM, Denmeade SR, Higgins MJ, Vitolo MI, Bachman KE, Park BH. Knockin of mutant PIK3CA activates multiple oncogenic pathways. Proc Natl Acad Sci USA 106: 2835–2840, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 542. Gutman O, Walliser C, Piechulek T, Gierschik P, Henis YI. Differential regulation of phospholipase C-beta2 activity and membrane interaction by Galphaq, Gbeta1gamma2, and Rac2. J Biol Chem 285: 3905–3915, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 543. Gwack Y, Srikanth S, Oh-Hora M, Hogan PG, Lamperti ED, Yamashita M, Gelinas C, Neems DS, Sasaki Y, Feske S, Prakriya M, Rajewsky K, Rao A. Hair loss and defective T and B cell function in mice lacking ORAI1. Mol Cell Biol 28: 5209–5222, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 544. Ha SA, Bunch JT, Hama H, DeWald DB, Nothwehr SF. A novel mechanism for localizing membrane proteins to yeast trans-Golgi network requires function of synaptojanin-like protein. Mol Biol Cell 12: 3175–3190, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 545. Ha SA, Torabinejad J, DeWald DB, Wenk MR, Lucast L, De Camilli P, Newitt RA, Aebersold R, Nothwehr SF. The synaptojanin-like protein InP53/Sjl3 functions with clathrin in a yeast TGN-to-endosome pathway distinct from the GGA protein-dependent pathway. Mol Biol Cell 14: 1319–1333, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 546. Haarer BK, Lillie SH, Adams AE, Magdolen V, Bandlow W, Brown SS. Purification of profilin from Saccharomyces cerevisiae and analysis of profilin-deficient cells. J Cell Biol 110: 105–114, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 547. Haglund K, Di Fiore PP, Dikic I. Distinct monoubiquitin signals in receptor endocytosis. Trends Biochem Sci 28: 598–603, 2003 [DOI] [PubMed] [Google Scholar]
- 548. Hailey DW, Rambold AS, Satpute-Krishnan P, Mitra K, Sougrat R, Kim PK, Lippincott-Schwartz J. Mitochondria supply membranes for autophagosome biogenesis during starvation. Cell 141: 656–667, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 549. Hains MD, Wing MR, Maddileti S, Siderovski DP, Harden TK. Galpha12/13- and rho-dependent activation of phospholipase C-epsilon by lysophosphatidic acid and thrombin receptors. Mol Pharmacol 69: 2068–2075, 2006 [DOI] [PubMed] [Google Scholar]
- 550. Halaszovich CR, Schreiber DN, Oliver D. Ci-VSP is a depolarization-activated phosphatidylinositol-4,5-bisphosphate and phosphatidylinositol-3,4,5-trisphosphate 5'-phosphatase. J Biol Chem 284: 2106–2113, 2009 [DOI] [PubMed] [Google Scholar]
- 551. Halet G, Tunwell R, Balla T, Swann K, Carroll J. The dynamics of plasma membrane PtdIns(4,5)P(2) at fertilization of mouse eggs. J Cell Sci 115: 2139–2149, 2002 [DOI] [PubMed] [Google Scholar]
- 552. Halford S, Dulai KS, Daw SC, Fitzgibbon J, Hunt DM. Isolation and chromosomal localization of two human CDP-diacylgecrol Synthase (CDS) genes. Genomics 54: 140–144, 1998 [DOI] [PubMed] [Google Scholar]
- 553. Halstead JR, Savaskan NE, van den Bout I, Van Horck F, Hajdo-Milasinovic A, Snell M, Keune WJ, Ten Klooster JP, Hordijk PL, Divecha N. Rac controls PIP5K localisation and PtdIns(4,5)P synthesis, which modulates vinculin localisation and neurite dynamics. J Cell Sci 123: 3535–3546, 2010 [DOI] [PubMed] [Google Scholar]
- 554. Halstead JR, van Rheenen J, Snel MH, Meeuws S, Mohammed S, D'Santos CS, Heck AJ, Jalink K, Divecha N. A role for PtdIns(4,5)P2 and PIP5Kalpha in regulating stress-induced apoptosis. Curr Biol 16: 1850–1856, 2006 [DOI] [PubMed] [Google Scholar]
- 555. Hama H, Schnieders EA, Thorner J, Takemoto JY, DeWald DB. Direct involvement of phosphatidylinositol 4-phosphate in secretion in the yeast Saccharomyces cerevisiae. J Biol Chem 274: 34294–34300, 1999 [DOI] [PubMed] [Google Scholar]
- 556. Hamada K, Shimizu T, Matsui T, Tsukita S, Hakoshima T. Structural basis of the membrane targeting and unmasking mechanisms of the radixin FERM domain. EMBO J 19: 4449–4462, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 557. Hamilton BA, Smith DJ, Mueller KL, Kerrebrock aw Bronson RT, van Berkel V, Daly MJ, Kruglyak L, Reeve MP, Nemhauser JL, Hawkins TL, Rubin EM, Lander ES. The vibrator mutation causes neurodegeneration via reduced expression of PITP alpha: positional complementation cloning and extragenic suppression. Neuron 18: 711–722, 1997 [DOI] [PubMed] [Google Scholar]
- 558. Hammond GR, Dove SK, Nicol A, Pinxteren JA, Zicha D, Schiavo G. Elimination of plasma membrane phosphatidylinositol (4,5)-bisphosphate is required for exocytosis from mast cells. J Cell Sci 119: 2084–2094, 2006 [DOI] [PubMed] [Google Scholar]
- 559. Hammond GR, Fischer MJ, Anderson KE, Holdich J, Koteci A, Balla T, Irvine RF. PI4P and PI(4,5)P2 are essential but independent lipid determinants of membrane identity. Science 337: 727–730, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 560. Hammond GR, Schiavo G, Irvine RF. Immunocytochemical techniques reveal multiple, distinct cellular pools of PtdIns4P and PtdIns(4,5)P(2). Biochem J 422: 23–35, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 561. Hammond GR, Sim Y, Lagnado L, Irvine RF. Reversible binding and rapid diffusion of proteins in complex with inositol lipids serves to coordinate free movement with spatial information. J Cell Biol 184: 297–308, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 562. Han GS, Audhya A, Markley DJ, Emr SD, Carman GM. The Saccharomyces cerevisiae LSB6 gene encodes phosphatidylinositol 4-kinase activity. J Biol Chem 277: 47709–47718, 2002 [DOI] [PubMed] [Google Scholar]
- 563. Han SK, Mancino V, Simon MI. Phospholipase Cbeta 3 mediates the scratching response activated by the histamine H1 receptor on C-fiber nociceptive neurons. Neuron 52: 691–703, 2006 [DOI] [PubMed] [Google Scholar]
- 564. Hanada K, Kumagai K, Yasuda S, Miura Y, Kawano M, Fukasawa M, Nishijima M. Molecular machinery for non-vesicular trafficking of ceramide. Nature 426: 803–809, 2003 [DOI] [PubMed] [Google Scholar]
- 565. Hannan AJ, Blakemore C, Katsnelson A, Vitalis T, Huber KM, Bear M, Roder J, Kim D, Shin HS, Kind PC. PLC-beta1, activated via mGluRs, mediates activity-dependent differentiation in cerebral cortex. Nat Neurosci 4: 282–288, 2001 [DOI] [PubMed] [Google Scholar]
- 566. Hansen SB, Tao X, MacKinnon R. Structural basis of PIP2 activation of the classical inward rectifier K+ channel Kir2.2. Nature 477: 495–498, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 567. Hanson PI, Roth R, Lin Y, Heuser JE. Plasma membrane deformation by circular arrays of ESCRT-III protein filaments. J Cell Biol 180: 389–402, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 568. Hara S, Swigart P, Jones D, Cockcroft S. The first 5 amino acids of the carboxyl terminus of phosphatidylinositol transfer protein (PITP) alpha play a critical role in inositol lipid signaling. Transfer activity of PITP is essential but not sufficient for restoration of phospholipase C signaling. J Biol Chem 272: 14908–14913, 1997 [DOI] [PubMed] [Google Scholar]
- 569. Harada K, Truong AB, Cai T, Khavari PA. The class II phosphoinositide 3-kinase C2beta is not essential for epidermal differentiation. Mol Cell Biol 25: 11122–11130, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 570. Harden TK, Sondek J. Regulation of phospholipase C isozymes by ras superfamily GTPases. Annu Rev Pharmacol Toxicol 46: 355–379, 2006 [DOI] [PubMed] [Google Scholar]
- 571. Harden TK, Stephens L, Hawkins PT, Downes CP. Turkey erythrocyte membranes as a model for regulation of phospholipase C by guanine nucleotides. J Biol Chem 262: 9057–9061, 1987 [PubMed] [Google Scholar]
- 572. Harden TK, Waldo GL, Hicks SN, Sondek J. Mechanism of activation and inactivation of Gq/phospholipase C-beta signaling nodes. Chem Rev 111: 6120–6129, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 573. Hardie RC. Regulation of TRP channels via lipid second messengers. Annu Rev Physiol 65: 735–759, 2003 [DOI] [PubMed] [Google Scholar]
- 574. Harlan JE, Hajduk PJ, Yoon HS, Fesik SW. Plekstrin homology domains bind to phosphatidylinositol-4,5-bisphosphate. Nature 371: 168–170, 1994 [DOI] [PubMed] [Google Scholar]
- 575. Harrison RA, Roldan ER. Phosphoinositids and their products in the mammalian sperm acrosome reaction. J Reprod Fertil Suppl 42: 51–67, 1990 [PubMed] [Google Scholar]
- 576. Harterink M, Port F, Lorenowicz MJ, McGough IJ, Silhankova M, Betist MC, van Weering JR, van Heesbeen RG, Middelkoop TC, Basler K, Cullen PJ, Korswagen HC. A SNX3-dependent retromer pathway mediates retrograde transport of the Wnt sorting receptor Wntless and is required for Wnt secretion. Nat Cell Biol 13: 914–923, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 577. Harwood JL, Hawthorne JN. The properties and subcellular distribution of phosphatidylinositol kinase in mammalian tissues. Biochim Biophys Acta 171: 75–88, 1969 [DOI] [PubMed] [Google Scholar]
- 578. Hausser A, Link G, Hoene M, Russo C, Selchow O, Pfizenmaier K. Phospho-specific binding of 14-3-3 proteins to phosphatidylinositol 4-kinase III beta protects from dephosphorylation and stabilizes lipid kinase activity. J Cell Sci 119: 3613–3621, 2006 [DOI] [PubMed] [Google Scholar]
- 579. Hausser A, Storz P, Martens S, Link G, Toker A, Pfizenmaier K. Protein kinase D regulates vesicular transport by phosphorylating and activating phosphatidylinositol-4 kinase IIIbeta at the Golgi complex. Nat Cell Biol 7: 880–886, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 580. Hawkins PT, Michell RH, Kirk CJ. Analysis of the metabolic turnover of the individual phosphate groups of phosphatidylinositol 4-phosphate and phosphatidylinositol 4,5-bisphosphate. Validation of novel analytical techniques using 32P-labelled lipids from erythrocytes. Biochem J 218: 785–793, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 581. Hawthorne JN. The ethanol-insoluble phosphatides of mammalian liver. Biochem J 59: 2–2, 1955 [PubMed] [Google Scholar]
- 582. Hay JC, Fisette PL, Jenkins GH, Fukami K, Takenawa T, Anderson RA, Martin TFJ. ATP-dependent inositide phosphorylation required for Ca2+-activated secretion. Nature 374: 173–177, 1995 [DOI] [PubMed] [Google Scholar]
- 583. Hay JC, Martin TFJ. Phosphatidylinositol transfer protein required for ATP-dependent priming of Ca2+-activated secretion. Nature 366: 572–575, 1993 [DOI] [PubMed] [Google Scholar]
- 584. Hayakawa A, Hayes SJ, Lawe DC, Sudharshan E, Tuft R, Fogarty K, Lambright D, Corvera S. Structural basis for endosomal targeting by FYVE domains. J Biol Chem 279: 5958–5966, 2004 [DOI] [PubMed] [Google Scholar]
- 585. Hayakawa A, Leonard D, Murphy S, Hayes S, Soto M, Fogarty K, Standley C, Bellve K, Lambright D, Mello C, Corvera S. The WD40 and FYVE domain containing protein 2 defines a class of early endosomes necessary for endocytosis. Proc Natl Acad Sci USA 103: 11928–11933, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 586. Haynes LP, Thomas GM, Burgoyne RD. Interaction of neuronal calcium sensor-1 and ADP-ribosylation factor 1 allows bidirectional control of phosphatidylinositol 4-kinase beta and trans-Golgi network-plasma membrane traffic. J Biol Chem 280: 6047–6054, 2005 [DOI] [PubMed] [Google Scholar]
- 587. He J, Scott JL, Heroux A, Roy S, Lenoir M, Overduin M, Stahelin RV, Kutateladze TG. Molecular basis of phosphatidylinositol 4-phosphate and ARF1 GTPase recognition by the FAPP1 pleckstrin homology (PH) domain. J Biol Chem 286: 18650–18657, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 588. He Z, Feng S, Tong Q, Hilgemann DW, Philipson KD. Interaction of PIP(2) with the XIP region of the cardiac Na/Ca exchanger. Am J Physiol Cell Physiol 278: C661–C666, 2000 [DOI] [PubMed] [Google Scholar]
- 589. Heacock AM, Uhler MD, Agranoff BW. Cloning of CDP-diacylglycerol synthase from a human neuronal cell line. J Neurochem 67: 2200–2203, 1996 [DOI] [PubMed] [Google Scholar]
- 590. Heilmeyer LM, Jr, Vereb G, Jr, Vereb G, Kakuk A, Szivak I. Mammalian phosphatidylinositol 4-kinases. IUBMB Life 55: 59–65, 2003 [DOI] [PubMed] [Google Scholar]
- 591. Heit B, Robbins SM, Downey CM, Guan Z, Colarusso P, Miller BJ, Jirik FR, Kubes P. PTEN functions to “prioritize” chemotactic cues and prevent “distraction” in migrating neutrophils. Nat Immunol 9: 743–752, 2008 [DOI] [PubMed] [Google Scholar]
- 592. Heitman J, Movva NR, Hall MN. Targets for cell cycle arrest by the immunosuppressant rapamycin in yeast. Science 253: 905–909, 1991 [DOI] [PubMed] [Google Scholar]
- 593. Helgason CD, Damen JE, Rosten P, Grewal R, Sorensen P, Chappel SM, Borowski A, Jirik F, Krystal G, Humphries RK. Targeted disruption of SHIP leads to hemopoietic perturbations, lung pathology, and a shortened life span. Genes Dev 12: 1610–1620, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 594. Helliwell SB, Howald I, Barbet N, Hall MN. TOR2 is part of two related signaling pathways coordinating cell growth in Saccharomyces cerevisiae. Genetics 148: 99–112, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 595. Hellsten E, Bernard DJ, Owens JW, Eckhaus M, Suchy SF, Nussbaum RL. Sertoli cell vacuolization and abnormal germ cell adhesion in mice deficient in an inositol polyphosphate 5-phosphatase. Biol Reprod 66: 1522–1530, 2002 [DOI] [PubMed] [Google Scholar]
- 596. Hellsten E, Evans JP, Bernard DJ, Janne PA, Nussbaum RL. Disrupted sperm function and fertilin beta processing in mice deficient in the inositol polyphosphate 5-phosphatase Inpp5b. Dev Biol 240: 641–653, 2001 [DOI] [PubMed] [Google Scholar]
- 597. Helmkamp GM, Jr, Harvey MS, Wirtz KW, Van Deenen LL. Phospholipid exchange between membranes. Purification of bovine brain proteins that preferentially catalyze the transfer of phosphatidylinositol. J Biol Chem 249: 6382–6389, 1974 [PubMed] [Google Scholar]
- 598. Henderson P, van Limbergen JE, Wilson DC, Satsangi J, Russell RK. Genetics of childhood-onset inflammatory bowel disease. Inflamm Bowel Dis 17: 346–361, 2011 [DOI] [PubMed] [Google Scholar]
- 599. Hendricks KB, Wang BQ, Schnieders EA, Thorner J. Yeast homologue of neuronal frequenin is a regulator of phosphatidylinositol 4-OH-kinase. Nat Cell Biol 1: 234–241, 1999 [DOI] [PubMed] [Google Scholar]
- 600. Hennessy BT, Smith DL, Ram PT, Lu Y, Mills GB. Exploiting the PI3K/AKT pathway for cancer drug discovery. Nat Rev Drug Discov 4: 988–1004, 2005 [DOI] [PubMed] [Google Scholar]
- 601. Heo WD, Inoue T, Park WS, Kim ML, Park BO, Wandless TJ, Meyer T. PI(3,4,5)P3 and PI(4,5)P2 lipids target proteins with polybasic clusters to the plasma membrane. Science 314: 1458–1461, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 602. Hepler JR, Harden TK. Guanine nucleotide-dependent pertussis toxin-insensitive stimulation of inositol-phosphate formation in a membrane preparation from human astrocytoma cells. Biochem J 239: 141–146, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 603. Hepler JR, Jeffs RA, Huckle WR, Outlaw HE, Rhee SG, Earp HS, Harden TK. Evidence that the epidermal growth factor receptor and non-tyrosine kinase hormone receptors stimulate phosphoinositide hydrolysis by independent pathways. Biochem J 270: 337–344, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 604. Herman PK, Emr SD. Characterization of VPS34, a gene required for vacuolar protein sorting and vacuole segregation in Saccharomyces cerevisiae. Mol Cell Biol 10: 6742–6754, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 605. Herman PK, Stack JH, DeModena JA, Emr SD. A novel protein kinase homolog essential for protein sorting to the yeast lysosome-like vacuole. Cell 64: 425–437, 1991 [DOI] [PubMed] [Google Scholar]
- 606. Herman SE, Gordon AL, Wagner AJ, Heerema NA, Zhao W, Flynn JM, Jones J, Andritsos L, Puri KD, Lannutti BJ, Giese NA, Zhang X, Wei L, Byrd JC, Johnson AJ. Phosphatidylinositol 3-kinase-delta inhibitor CAL-101 shows promising preclinical activity in chronic lymphocytic leukemia by antagonizing intrinsic and extrinsic cellular survival signals. Blood 116: 2078–2088, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 607. Hernandez LD, Hueffer K, Wenk MR, Galan JE. Salmonella modulates vesicular traffic by altering phosphoinositide metabolism. Science 304: 1805–1807, 2004 [DOI] [PubMed] [Google Scholar]
- 608. Hernandez-Negrete I, Carretero-Ortega J, Rosenfeldt H, Hernandez-Garcia R, Calderon-Salinas JV, Reyes-Cruz G, Gutkind JS, Vazquez-Prado J. P-Rex1 links mammalian target of rapamycin signaling to Rac activation and cell migration. J Biol Chem 282: 23708–23715, 2007 [DOI] [PubMed] [Google Scholar]
- 609. Hertel F, Switalski A, Mintert-Jancke E, Karavassilidou K, Bender K, Pott L, Kienitz MC. A genetically encoded tool kit for manipulating and monitoring membrane phosphatidylinositol 4,5-bisphosphate in intact cells. PLoS One 6: e20855, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 610. Heslop JP, Irvine RF, Tashijan AH, Jr, Berridge MJ. Inositol tetrakis- and pentakisphosphates in GH4 cells. J Exp Biol 119: 395–401, 1985 [DOI] [PubMed] [Google Scholar]
- 611. Heymont J, Berenfeld L, Collins J, Kaganovich A, Maynes B, Moulin A, Ratskovskaya I, Poon PP, Johnston GC, Kamenetsky M, DeSilva J, Sun H, Petsko GA, Engebrecht J. TEP1, the yeast homolog of the human tumor suppressor gene PTEN/MMAC1/TEP1, is linked to the phosphatidylinositol pathway and plays a role in the developmental process of sporulation. Proc Natl Acad Sci USA 97: 12672–12677, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 612. Hicks SN, Jezyk MR, Gershburg S, Seifert JP, Harden TK, Sondek J. General and versatile autoinhibition of PLC isozymes. Mol Cell 31: 383–394, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 613. Hilbi H, Weber S, Finsel I. Anchors for effectors: subversion of phosphoinositide lipids by legionella. Front Microbiol 2: 91, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 614. Hiles ID, Otsu M, Volinia S, Fry MJ, Gout I, Dhand R, Panayotou G, Ruiz-Larrea F, Thompson A, Totty NF, Hsuan JJ, Courtneidge SA, Parker PJ, Waterfield MD. Phosphatidylinositol 3-kinase: structure and expression of the 110 kDa catalytic subunit. Cell 70: 419–429, 1992 [DOI] [PubMed] [Google Scholar]
- 615. Hilfiker S. Neuronal calcium sensor-1: a multifunctional regulator of secretion. Biochem Soc Trans 31: 828–832, 2003 [DOI] [PubMed] [Google Scholar]
- 616. Hilgemann DW, Ball R. Regulation of cardiac Na+,Ca2+ exchange and KATP potassium channels by PIP2. Science 273: 956–959, 1996 [DOI] [PubMed] [Google Scholar]
- 617. Hilgemann DW, Feng S, Nasuhoglu C. The complex and intriguing lives of PIP2 with ion channels and transporters. Sci STKE 2001: RE19, 2001 [DOI] [PubMed] [Google Scholar]
- 618. Hill EV, Hudson CA, Vertommen D, Rider MH, Tavare JM. Regulation of PIKfyve phosphorylation by insulin and osmotic stress. Biochem Biophys Res Commun 397: 650–655, 2010 [DOI] [PubMed] [Google Scholar]
- 619. Himmel B, Nagel G. Protein kinase-independent activation of CFTR by phosphatidylinositol phosphates. EMBO Rep 5: 85–90, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 620. Hinchliffe KA, Giudici ML, Letcher AJ, Irvine RF. Type IIalpha phosphatidylinositol phosphate kinase associates with the plasma membrane via interaction with type I isoforms. Biochem J 363: 563–570, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 621. Hinkes B, Wiggins RC, Gbadegesin R, Vlangos CN, Seelow D, Nurnberg G, Garg P, Verma R, Chaib H, Hoskins BE, Ashraf S, Becker C, Hennies HC, Goyal M, Wharram BL, Schachter AD, Mudumana S, Drummond I, Kerjaschki D, Waldherr R, Dietrich A, Ozaltin F, Bakkaloglu A, Cleper R, Basel-Vanagaite L, Pohl M, Griebel M, Tsygin AN, Soylu A, Muller D, Sorli CS, Bunney TD, Katan M, Liu J, Attanasio M, O'Toole JF, Hasselbacher K, Mucha B, Otto EA, Airik R, Kispert A, Kelley GG, Smrcka AV, Gudermann T, Holzman LB, Nurnberg P, Hildebrandt F. Positional cloning uncovers mutations in PLCE1 responsible for a nephrotic syndrome variant that may be reversible. Nat Genet 38: 1397–1405, 2006 [DOI] [PubMed] [Google Scholar]
- 622. Hirata M, Suzuki M, Ishii R, Satow R, Uchida T, Kitazumi T, Sasaki T, Kitamura T, Yamaguchi H, Nakamura Y, Fukami K. Genetic defect in phospholipase Cdelta1 protects mice from obesity by regulating thermogenesis and adipogenesis. Diabetes 60: 1926–1937, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 623. Hirsch E, Bosco O, Tropel P, Laffargue M, Calvez R, Altruda F, Wymann M, Montrucchio G. Resistance to thromboembolism in PI3Kgamma-deficient mice. FASEB J 15: 2019–2021, 2001 [DOI] [PubMed] [Google Scholar]
- 624. Hirsch E, Katanaev VL, Garlanda C, Azzolino O, Pirola L, Silengo L, Sozzani S, Mantovani A, Altruda F, Wymann MP. Central role for G protein-coupled phosphoinositide 3-kinase gamma in inflammation. Science 287: 1049–1053, 2000 [DOI] [PubMed] [Google Scholar]
- 625. Hnia K, Kretz C, Amoasii L, Bohm J, Liu X, Messaddeq N, Qu CK, Laporte J. Primary T-tubule and autophagy defects in the phosphoinositide phosphatase Jumpy/MTMR14 knockout mice muscle. Adv Enzyme Regul 52: 98–107, 2012 [DOI] [PubMed] [Google Scholar]
- 626. Hnia K, Tronchere H, Tomczak KK, Amoasii L, Schultz P, Beggs AH, Payrastre B, Mandel JL, Laporte J. Myotubularin controls desmin intermediate filament architecture and mitochondrial dynamics in human and mouse skeletal muscle. J Clin Invest 121: 70–85, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 627. Ho LK, Liu D, Rozycka M, Brown RA, Fry MJ. Identification of four novel human phosphoinositide 3-kinases defines a multi-isoform subfamily. Biochem Biophys Res Commun 235: 130–137, 1997 [DOI] [PubMed] [Google Scholar]
- 628. Hoeller O, Kay RR. Chemotaxis in the absence of PIP3 gradients. Curr Biol 17: 813–817, 2007 [DOI] [PubMed] [Google Scholar]
- 629. Hoeppner LH, Phoenix KN, Clark KJ, Bhattacharya R, Gong X, Sciuto TE, Vohra P, Suresh S, Bhattacharya S, Dvorak AM, Ekker SC, Dvorak HF, Claffey KP, Mukhopadhyay D. Revealing the role of phospholipase Cbeta3 in the regulation of VEGF-induced vascular permeability. Blood 120: 2167–2173, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 630. Hofmann SL, Majerus PW. Identification and properties of two distinct phosphatidylinositol-specific phospholipase C enzymes from sheep seminal vesicular glands. J Biol Chem 257: 6461–6469, 1982 [PubMed] [Google Scholar]
- 631. Hofmann SL, Majerus PW. Modulation of phosphatidylinositol-specific phospholipase C activity by phospholipid interactions, diglycerides, and calcium ions. J Biol Chem 257: 14359–14364, 1982 [PubMed] [Google Scholar]
- 632. Hokin LE. Autoradiographic localization of the acetylcholine-stimulated synthesis of phosphatidylinositol in the superior cervical ganglion. Proc Natl Acad Sci USA 53: 1369–1376, 1965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 633. Hokin LE. Effects of calcium omission on acteylcholine-stimulated amylase secretion and phospholipid secretion in pigeon pancreas slices. Biochim Biophys Acta 115: 219–221, 1966 [DOI] [PubMed] [Google Scholar]
- 634. Hokin LE. The road to the phosphoinositide-generated second messengers. Trends Pharmacol Sci 8: 53–56, 1987 [Google Scholar]
- 635. Hokin LE, Hokin MR. Effects of acetylcholine on the turnover of phosphoryl units in individual phospholipids of pancreas slices and brain cortex slices. Biochim Biophys Acta 18: 102–110, 1955 [DOI] [PubMed] [Google Scholar]
- 636. Hokin LE, Hokin MR. The incorporation of 32P from[32P]ATp into polyphosphoinositides and phosphatidic acid in erythrocyte membranes. Biochim Biophys Acta 84: 563–575, 1964 [DOI] [PubMed] [Google Scholar]
- 637. Hokin MR. Studies on chemical mechanisms of the action of neurotransmitters and hormones: relationship between hormone-stimulated 32P incorporation into phosphatidic acid and into phosphatidylinositol in pigeon pancreas slices. Arch Biochem Biophys 124: 271–279, 1968 [DOI] [PubMed] [Google Scholar]
- 638. Hokin MR, Hokin LE. Enzyme secretion and incorporation of P32 into phospholipides of pancreas slices. J Biol Chem 203: 967–977, 1953 [PubMed] [Google Scholar]
- 639. Hokin MR, Hokin LE. Interconversions of phosphatidylinositol and phosphatidic acid involved in the response to acetylcholine in the salt gland. In: Metabolism and Physiological Significance of Lipids. New York: Wiley, 1964, p. 423–434 [Google Scholar]
- 640. Hokin MR, Hokin LE. The synthesis of phosphatidic acid from diglyceride and adenosine triphosphate in extracts of brain microsomes. J Biol Chem 234: 1381–1386, 1959 [PubMed] [Google Scholar]
- 641. Hollander MC, Blumenthal GM, Dennis PA. PTEN loss in the continuum of common cancers, rare syndromes and mouse models. Nat Rev Cancer 11: 289–301, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 642. Holub BJ. The nutritional significance, metabolism, and function of myo-inositol and phosphatidylinositol in health and disease. Adv Nutr Res 4: 107–141, 1982 [DOI] [PubMed] [Google Scholar]
- 643. Holz RW, Hlubek MD, Sorensen SD, Fisher SK, Balla T, Ozaki S, Prestwich GD, Stuenkel EL, Bittner MA. A pleckstrin homology domain specific for Ptdins-4,5-P2 and fused to green fluorescent protein identifies plasma membrane Ptdins-4,5-P2 as being important in exocytosis. J Biol Chem 275: 17878–17885, 2000 [DOI] [PubMed] [Google Scholar]
- 644. Homma K, Terui S, Minemura M, Qadota H, Anraku Y, Kanaho Y, Ohya Y. Phosphatidylinositol-4-phosphate 5-kinase localized on the plasma membrane is essential for yeast cell morphogenesis. J Biol Chem 273: 15779–15786, 1998 [DOI] [PubMed] [Google Scholar]
- 645. Homma Y, Takenawa T, Emori Y, Sorimachi H, Suzuki K. Tissue- and cell type-specific expression of mRNAs for four types of inositol phospholipid-specific phospholipase C. Biochem Biophys Res Commun 164: 406–412, 1989 [DOI] [PubMed] [Google Scholar]
- 646. Honda A, Nogami M, Yokozeki T, Yamazaki M, Nakamura H, Watanabe H, Kawamoto K, Nakayama K, Morris AJ, Frohman MA, Kanaho Y. Phosphatidylinositol 4-phosphate 5-kinase alpha is a downstream effector of the small G protein ARF6 in membrane ruffle formation. Cell 99: 521–532, 1999 [DOI] [PubMed] [Google Scholar]
- 647. Hoopes RR, Jr, Shrimpton AE, Knohl SJ, Hueber P, Hoppe B, Matyus J, Simckes A, Tasic V, Toenshoff B, Suchy SF, Nussbaum RL, Scheinman SJ. Dent Disease with mutations in OCRL1. Am J Hum Genet 76: 260–267, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 648. Hoppe AD, Swanson JA. Cdc42, Rac1, and Rac2 display distinct patterns of activation during phagocytosis. Mol Biol Cell 15: 3509–3519, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 649. Horan KA, Watanabe K, Kong AM, Bailey CG, Rasko JE, Sasaki T, Mitchell CA. Regulation of FcgammaR-stimulated phagocytosis by the 72-kDa inositol polyphosphate 5-phosphatase: SHIP1, but not the 72-kDa 5-phosphatase, regulates complement receptor 3 mediated phagocytosis by differential recruitment of these 5-phosphatases to the phagocytic cup. Blood 110: 4480–4491, 2007 [DOI] [PubMed] [Google Scholar]
- 650. Hossain MI, Iwasaki H, Okochi Y, Chahine M, Higashijima S, Nagayama K, Okamura Y. Enzyme domain affects the movement of the voltage sensor in ascidian and zebrafish voltage-sensing phosphatases. J Biol Chem 283: 18248–18259, 2008 [DOI] [PubMed] [Google Scholar]
- 651. Hou WM, Zhang ZL, Tai HH. Purification and characterization of a phosphatidylinositol kinase from porcine liver microsomes. Biochim Biophys Acta 959: 67–75, 1988 [PubMed] [Google Scholar]
- 652. Hou X, Hagemann N, Schoebel S, Blankenfeldt W, Goody RS, Erdmann KS, Itzen A. A structural basis for Lowe syndrome caused by mutations in the Rab-binding domain of OCRL1. EMBO J 30: 1659–1670, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 653. Houlden H, King RH, Wood NW, Thomas PK, Reilly MM. Mutations in the 5' region of the myotubularin-related protein 2 (MTMR2) gene in autosomal recessive hereditary neuropathy with focally folded myelin. Brain 124: 907–915, 2001 [DOI] [PubMed] [Google Scholar]
- 654. Howell BW, Lanier LM, Frank R, Gertler FB, Cooper JA. The disabled 1 phosphotyrosine-binding domain binds to the internalization signals of transmembrane glycoproteins and to phospholipids. Mol Cell Biol 19: 5179–5188, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 655. Howes MT, Mayor S, Parton RG. Molecules, mechanisms, and cellular roles of clathrin-independent endocytosis. Curr Opin Cell Biol 22: 519–527, 2010 [DOI] [PubMed] [Google Scholar]
- 656. Hsu NY, Ilnytska O, Belov G, Santiana M, Chen YH, Takvorian PM, Pau C, van der Schaar H, Kaushik-Basu N, Balla T, Cameron CE, Ehrenfeld E, van Kuppeveld FJ, Altan-Bonnet N. Viral reorganization of the secretory pathway generates distinct organelles for RNA replication. Cell 141: 799–811, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 657. Hu Q, Klippel A, Muslin AJ, Fantl WJ, Williams LT. Ras-dependent induction of cellular responses by constitutively active phosphatidylinositol-3 kinase. Science 268: 100–102, 1995 [DOI] [PubMed] [Google Scholar]
- 658. Hu XT, Zhang FB, Fan YC, Shu XS, Wong AH, Zhou W, Shi QL, Tang HM, Fu L, Guan XY, Rha SY, Tao Q, He C. Phospholipase C delta 1 is a novel 3p22.3 tumor suppressor involved in cytoskeleton organization, with its epigenetic silencing correlated with high-stage gastric cancer. Oncogene 28: 2466–2475, 2009 [DOI] [PubMed] [Google Scholar]
- 659. Hu Z, Wu C, Shi Y, Guo H, Zhao X, Yin Z, Yang L, Dai J, Hu L, Tan W, Li Z, Deng Q, Wang J, Wu W, Jin G, Jiang Y, Yu D, Zhou G, Chen H, Guan P, Chen Y, Shu Y, Xu L, Liu X, Liu L, Xu P, Han B, Bai C, Zhao Y, Zhang H, Yan Y, Ma H, Chen J, Chu M, Lu F, Zhang Z, Chen F, Wang X, Jin L, Lu J, Zhou B, Lu D, Wu T, Lin D, Shen H. A genome-wide association study identifies two new lung cancer susceptibility loci at 13q12.12 and 22q122 in Han Chinese. Nat Genet 43: 792–796, 2011 [DOI] [PubMed] [Google Scholar]
- 660. Huang CH, Mandelker D, Schmidt-Kittler O, Samuels Y, Velculescu VE, Kinzler KW, Vogelstein B, Gabelli SB, Amzel LM. The structure of a human p110alpha/p85alpha complex elucidates the effects of oncogenic PI3Kalpha mutations. Science 318: 1744–1748, 2007 [DOI] [PubMed] [Google Scholar]
- 661. Huang CL, Feng S, Hilgemann DW. Direct activation of inward rectifier potassium channels by PIP2 and its stabilization by Gbetagamma. Nature 391: 803–806, 1998 [DOI] [PubMed] [Google Scholar]
- 662. Huang J, Liu CH, Hughes SA, Postma M, Schwiening CJ, Hardie RC. Activation of TRP channels by protons and phosphoinositide depletion in Drosophila photoreceptors. Curr Biol 20: 189–197, 2010 [DOI] [PubMed] [Google Scholar]
- 663. Huang J, Yan J, Zhang J, Zhu S, Wang Y, Shi T, Zhu C, Chen C, Liu X, Cheng J, Mustelin T, Feng GS, Chen G, Yu J. SUMO1 modification of PTEN regulates tumorigenesis by controlling its association with the plasma membrane. Nat Commun 3: 911, 2012 [DOI] [PubMed] [Google Scholar]
- 664. Huang W, Barrett M, Hajicek N, Hicks S, Harden TK, Sondek J, Zhang Q. Small molecule inhibitors of phospholipase C from a novel high-throughput screen. J Biol Chem 288: 5840–5844, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 665. Huang Y, Shah V, Liu T, Keshvara L. Signaling through Disabled 1 requires phosphoinositide binding. Biochem Biophys Res Commun 331: 1460–1468, 2005 [DOI] [PubMed] [Google Scholar]
- 666. Huang YE, Iijima M, Parent CA, Funamoto S, Firtel RA, Devreotes P. Receptor-mediated regulation of PI3Ks confines PI(3,4,5)P3 to the leading edge of chemotaxing cells. Mol Biol Cell 14: 1913–1922, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 667. Hughes WE, Woscholski R, Cooke FT, Patrick RS, Dove SK, McDonald NQ, Parker PJ. SAC1 encodes a regulated lipid phosphoinositide phosphatase, defects in which can be suppressed by the homologous Inp52p and Inp53p phosphatases. J Biol Chem 275: 801–808, 2000 [DOI] [PubMed] [Google Scholar]
- 668. Humbert MC, Weihbrecht K, Searby CC, Li Y, Pope RM, Sheffield VC, Seo S. ARL13B, PDE6D, and CEP164 form a functional network for INPP5E ciliary targeting. Proc Natl Acad Sci USA 109: 19691–19696, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 669. Hunter T. When is a lipid kinase not a lipid kinase? When it is a protein kinase. Cell 83: 1–4, 1995 [DOI] [PubMed] [Google Scholar]
- 670. Hunyady L, Baukal AJ, Gaborik Z, Olivares-Reyes JA, Bor M, Szaszak M, Lodge R, Catt KJ, Balla T. Differential PI 3-kinase dependence of early and late phases of recycling of the internalized AT1 angiotensin receptor. J Cell Biol 157: 1211–1222, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 671. Hurley JH. ESCRT complexes and the biogenesis of multivesicular bodies. Curr Opin Cell Biol 20: 4–11, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 672. Huttner IG, Strahl T, Osawa M, King DS, Ames JB, Thorner J. Molecular interactions of yeast frequenin (Frq1) with the phosphatidylinositol 4-kinase isoform, Pik1. J Biol Chem 278: 4862–4874, 2003 [DOI] [PubMed] [Google Scholar]
- 673. Hwang JI, Oh YS, Shin KJ, Kim H, Ryu SH, Suh PG. Molecular cloning and characterization of a novel phospholipase C, PLC-eta. Biochem J 389: 181–186, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 674. Hyvola N, Diao A, McKenzie E, Skippen A, Cockcroft S, Lowe M. Membrane targeting and activation of the Lowe syndrome protein OCRL1 by rab GTPases. EMBO J 25: 3750–3761, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 675. Ichimura Y, Kirisako T, Takao T, Satomi Y, Shimonishi Y, Ishihara N, Mizushima N, Tanida I, Kominami E, Ohsumi M, Noda T, Ohsumi Y. A ubiquitin-like system mediates protein lipidation. Nature 408: 488–492, 2000 [DOI] [PubMed] [Google Scholar]
- 676. Ichinohe M, Nakamura Y, Sai K, Nakahara M, Yamaguchi H, Fukami K. Lack of phospholipase C-delta1 induces skin inflammation. Biochem Biophys Res Commun 356: 912–918, 2007 [DOI] [PubMed] [Google Scholar]
- 677. Iijima M, Devreotes P. Tumor suppressor PTEN mediates sensing of chemoattractant gradients. Cell 109: 599–610, 2002 [DOI] [PubMed] [Google Scholar]
- 678. Iijima M, Huang YE, Luo HR, Vazquez F, Devreotes PN. Novel mechanism of PTEN regulation by its phosphatidylinositol 4,5-bisphosphate binding motif is critical for chemotaxis. J Biol Chem 279: 16606–16613, 2004 [DOI] [PubMed] [Google Scholar]
- 679. Ijuin T, Takenawa T. SKIP negatively regulates insulin-induced GLUT4 translocation and membrane ruffle formation. Mol Cell Biol 23: 1209–1220, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 680. Ijuin T, Yu YE, Mizutani K, Pao A, Tateya S, Tamori Y, Bradley A, Takenawa T. Increased insulin action in SKIP heterozygous knockout mice. Mol Cell Biol 28: 5184–5195, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 681. Ikeda H, Hideshima T, Fulciniti M, Perrone G, Miura N, Yasui H, Okawa Y, Kiziltepe T, Santo L, Vallet S, Cristea D, Calabrese E, Gorgun G, Raje NS, Richardson P, Munshi NC, Lannutti BJ, Puri KD, Giese NA, Anderson KC. PI3K/p110{delta} is a novel therapeutic target in multiple myeloma. Blood 116: 1460–1468, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 682. Ikonomov OC, Sbrissa D, Delvecchio K, Xie Y, Jin JP, Rappolee D, Shisheva A. The phosphoinositide kinase PIKfyve is vital in early embryonic development: preimplantation lethality of PIKfyve−/− embryos but normality of PIKfyve+/− mice. J Biol Chem 286: 13404–13413, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 683. Ikonomov OC, Sbrissa D, Fligger J, Delvecchio K, Shisheva A. ArPIKfyve regulates Sac3 protein abundance and turnover: disruption of the mechanism by Sac3I41T mutation causing Charcot-Marie-Tooth 4J disorder. J Biol Chem 285: 26760–26764, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 684. Ikonomov OC, Sbrissa D, Foti M, Carpentier JL, Shisheva A. PIKfyve controls fluid phase endocytosis but not recycling/degradation of endocytosed receptors or sorting of procathepsin D by regulating multivesicular body morphogenesis. Mol Biol Cell 14: 4581–4591, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 685. Ikonomov OC, Sbrissa D, Ijuin T, Takenawa T, Shisheva A. Sac3 is an insulin-regulated phosphatidylinositol 3,5-bisphosphate phosphatase: gain in insulin responsiveness through Sac3 down-regulation in adipocytes. J Biol Chem 284: 23961–23971, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 686. Ikonomov OC, Sbrissa D, Shisheva A. YM201636, an inhibitor of retroviral budding and PIKfyve-catalyzed PtdIns(3,5)P2 synthesis, halts glucose entry by insulin in adipocytes. Biochem Biophys Res Commun 382: 566–570, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 687. Ikuta S, Edamatsu H, Li M, Hu L, Kataoka T. Crucial role of phospholipase C epsilon in skin inflammation induced by tumor-promoting phorbol ester. Cancer Res 68: 64–72, 2008 [DOI] [PubMed] [Google Scholar]
- 688. Ile KE, Kassen S, Cao C, Vihtehlic T, Shah SD, Mousley CJ, Alb JG, Jr, Huijbregts RP, Stearns GW, Brockerhoff SE, Hyde DR, Bankaitis VA. Zebrafish class 1 phosphatidylinositol transfer proteins: PITPbeta and double cone cell outer segment integrity in retina. Traffic 11: 1151–1167, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 689. Ile KE, Schaaf G, Bankaitis VA. Phosphatidylinositol transfer proteins and cellular nanoreactors for lipid signaling. Nat Chem Biol 2: 576–583, 2006 [DOI] [PubMed] [Google Scholar]
- 690. Ilkaeva O, Kinch LN, Paulssen RH, Ross EM. Mutations in the carboxyl-terminal domain of phospholipase C-beta 1 delineate the dimer interface and a potential Galphaq interaction site. J Biol Chem 277: 4294–4300, 2002 [DOI] [PubMed] [Google Scholar]
- 691. Illenberger D, Schwald F, Pimmer D, Binder W, Maier G, Dietrich A, Gierschik P. Stimulation of phospholipase C-beta2 by the Rho GTPases Cdc42Hs and Rac1. EMBO J 17: 6241–6249, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 692. Illenberger D, Stephan I, Gierschik P, Schwald F. Stimulation of phospholipase C-beta 2 by Rho GTPases. Methods Enzymol 325: 167–177, 2000 [DOI] [PubMed] [Google Scholar]
- 693. Illenberger D, Walliser C, Strobel J, Gutman O, Niv H, Gaidzik V, Kloog Y, Gierschik P, Henis YI. Rac2 regulation of phospholipase C-beta 2 activity and mode of membrane interactions in intact cells. J Biol Chem 278: 8645–8652, 2003 [DOI] [PubMed] [Google Scholar]
- 694. Imai A, Gershengorn MC. Independent phosphatidylinositol synthesis in pituitary plasma membrane and endoplasmic reticulum. Nature 325: 726–728, 1987 [DOI] [PubMed] [Google Scholar]
- 695. Imai A, Gershengorn MC. Regulation by phosphatidylinositol of rat pituitary plasma membrane and endoplasmic reticulum phosphatidylinositol synthase activities. A mechanism for activation of phosphoinositide resynthesis during cell stimulation. J Biol Chem 262: 6457–6459, 1987 [PubMed] [Google Scholar]
- 696. Innocenti M, Frittoli E, Ponzanelli I, Falck JR, Brachmann SM, Di Fiore PP, Scita G. Phosphoinositide 3-kinase activates Rac by entering in a complex with Eps8, Abi1, and Sos-1. J Cell Biol 160: 17–23, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 697. Inoki K, Li Y, Zhu T, Wu J, Guan KL. TSC2 is phosphorylated and inhibited by Akt and suppresses mTOR signalling. Nat Cell Biol 4: 648–657, 2002 [DOI] [PubMed] [Google Scholar]
- 698. Inoue O, Suzuki-Inoue K, Dean WL, Frampton J, Watson SP. Integrin alpha2beta1 mediates outside-in regulation of platelet spreading on collagen through activation of Src kinases and PLCgamma2. J Cell Biol 160: 769–780, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 699. Inukai K, Anai M, Van Breda E, Hosaka T, Katagiri H, Funaki M, Fukushima Y, Ogihara T, Yazaki Y, Kikuchi Oka Y, Asano T. A novel 55-kDa regulatory subunit for phosphatidylinositol 3-kinase structurally similar to P55PIK is generated by alternative splicing of the p85alpha gene. J Biol Chem 271: 5317–5320, 1996 [DOI] [PubMed] [Google Scholar]
- 700. Irino Y, Cho H, Nakamura Y, Nakahara M, Furutani M, Suh PG, Takenawa T, Fukami K. Phospholipase C delta-type consists of three isozymes: bovine PLCdelta2 is a homologue of human/mouse PLCdelta4. Biochem Biophys Res Commun 320: 537–543, 2004 [DOI] [PubMed] [Google Scholar]
- 701. Irvine RF. 20 years of Ins(1,4,5)P3, and 40 years before. Nat Rev Mol Cell Biol 4: 586–590, 2003 [DOI] [PubMed] [Google Scholar]
- 702. Irvine RF, Dawson RM. The distribution of calcium-dependent phosphatidylinositol-specific phosphodiesterase in rat brain. J Neurochem 31: 1427–1434, 1978 [DOI] [PubMed] [Google Scholar]
- 703. Irvine RF, Hemington N, Dawson RM. The hydrolysis of phosphatidylinositol by lysosomal enzymes of rat liver and brain. Biochem J 176: 475–484, 1978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 704. Irvine RF, Letcher AJ, Dawson RMC. Phosphatidylinositol 4,5-bisphosphate phosphodiesterase activities of rat brain: some properties and possible control mechanisms. Biochem J 218: 177–185, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 705. Irvine RF, Letcher AJ, Heslop JP, Berridge MJ. The inositol tris/tetrakis phosphate pathway: demonstration of inositol (1,4,5)trisphosphate-3-kinase activity in mammalian tissues. Nature 320: 631–634, 1986 [DOI] [PubMed] [Google Scholar]
- 706. Irvine RF, Letcher AJ, Lander DJ, Berridge MJ. Specificity of inositol phosphate-stimulated Ca2+ mobilization from Swiss-mouse 3T3 cells. Biochem J 240: 301–304, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 707. Irvine RF, Letcher AJ, Lander DJ, Downes CP. Inositol trisphosphates in carbachol-stimulated rat parotid glands. Biochem J 223: 237–243, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 708. Ishihara H, Shibasaki Y, Kizuki N, Wada T, Yazaki Y, Asano T, Oka Y. Type I phosphatidylinositol-4-phosphate 5-kinases. Cloning of the third isoform and deletion/substitution analysis of members of this novel lipid kinase family. J Biol Chem 273: 8741–8748, 1998 [DOI] [PubMed] [Google Scholar]
- 709. Itakura E, Kishi C, Inoue K, Mizushima N. Beclin 1 forms two distinct phosphatidylinositol 3-kinase complexes with mammalian Atg14 and UVRAG. Mol Biol Cell 19: 5360–5372, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 710. Itoh T, Ijuin T, Takenawa T. A novel phosphatidylinositol-5-phosphate 4-kinase (phosphatidylinositol-phosphate kinase IIgamma) is phosphorylated in the endoplasmic reticulum in response to mitogenic signals. J Biol Chem 273: 20292–20299, 1998 [DOI] [PubMed] [Google Scholar]
- 711. Itoh T, Koshiba S, Kigawa T, Kikuchi A, Yokoyama S, Takenawa T. Role of the ENTH domain in phosphatidylinositol-4,5-bisphosphate binding and endocytosis. Science 291: 1047–1051, 2001 [DOI] [PubMed] [Google Scholar]
- 712. Ivetac I, Gurung R, Hakim S, Horan KA, Sheffield DA, Binge LC, Majerus PW, Tiganis T, Mitchell CA. Regulation of PI(3)K/Akt signalling and cellular transformation by inositol polyphosphate 4-phosphatase-1. EMBO Rep 10: 487–493, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 713. Iwasaki H, Murata Y, Kim Y, Hossain MI, Worby CA, Dixon JE, McCormack T, Sasaki T, Okamura Y. A voltage-sensing phosphatase, Ci-VSP, which shares sequence identity with PTEN, dephosphorylates phosphatidylinositol 4,5-bisphosphate. Proc Natl Acad Sci USA 105: 7970–7975, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 714. Jacoby M, Cox JJ, Gayral S, Hampshire DJ, Ayub M, Blockmans M, Pernot E, Kisseleva MV, Compere P, Schiffmann SN, Gergely F, Riley JH, Perez-Morga D, Woods CG, Schurmans S. INPP5E mutations cause primary cilium signaling defects, ciliary instability and ciliopathies in human and mouse. Nat Genet 41: 1027–1031, 2009 [DOI] [PubMed] [Google Scholar]
- 715. Jahn R, Fasshauer D. Molecular machines governing exocytosis of synaptic vesicles. Nature 490: 201–207, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 716. Jaiswal BS, Janakiraman V, Kljavin NM, Chaudhuri S, Stern HM, Wang W, Kan Z, Dbouk HA, Peters BA, Waring P, Dela Vega T, Kenski DM, Bowman KK, Lorenzo M, Li H, Wu J, Modrusan Z, Stinson J, Eby M, Yue P, Kaminker JS, de Sauvage FJ, Backer JM, Seshagiri S. Somatic mutations in p85alpha promote tumorigenesis through class IA PI3K activation. Cancer Cell 16: 463–474, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 717. Jakus Z, Simon E, Frommhold D, Sperandio M, Mocsai A. Critical role of phospholipase Cgamma2 in integrin and Fc receptor-mediated neutrophil functions and the effector phase of autoimmune arthritis. J Exp Med 206: 577–593, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 718. James DJ, Khodthong C, Kowalchyk JA, Martin TF. Phosphatidylinositol 4,5-bisphosphate regulates SNARE-dependent membrane fusion. J Cell Biol 182: 355–366, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 719. James DJ, Kowalchyk J, Daily N, Petrie M, Martin TF. CAPS drives trans-SNARE complex formation and membrane fusion through syntaxin interactions. Proc Natl Acad Sci USA 106: 17308–17313, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 720. Janmey PA. Phosphoinositides and calcium as regulators of cellular actin assembly and disassembly. Annu Rev Physiol 56: 169–191, 1994 [DOI] [PubMed] [Google Scholar]
- 721. Janmey PA, Stossel TP. Modulation of gelsolin function by phosphatidylinositol 4,5-bisphosphate. Nature 325: 362–364, 1987 [DOI] [PubMed] [Google Scholar]
- 722. Janne PA, Suchy SF, Bernard D, MacDonald M, Crawley J, Grinberg A, Wynshaw-Boris A, Westphal H, Nussbaum RL. Functional overlap between murine Inpp5b and Ocrl1 may explain why deficiency of the murine ortholog for OCRL1 does not cause Lowe syndrome in mice. J Clin Invest 101: 2042–2053, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 723. Jefferies HB, Cooke FT, Jat P, Boucheron C, Koizumi T, Hayakawa M, Kaizawa H, Ohishi T, Workman P, Waterfield MD, Parker PJ. A selective PIKfyve inhibitor blocks PtdIns(3,5)P(2) production and disrupts endomembrane transport and retroviral budding. EMBO Rep 9: 164–170, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 724. Jefferson AB, Majerus PW. Properties of type II inositol polyphosphate 5-phosphatase. J Biol Chem 270: 9370–9377, 1995 [DOI] [PubMed] [Google Scholar]
- 725. Jeffries TR, Dove SK, Michell RH, Parker PJ. PtdIns-specific MPR pathway association of a novel WD40 repeat protein, WIPI49. Mol Biol Cell 15: 2652–2663, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 726. Jenkins GH, Fisette PL, Anderson RA. Type I phosphatidylinositol 4-phosphate 5-kinase isoforms are specifically stimulated by phosphatidic acid. J Biol Chem 269: 11547–11554, 1994 [PubMed] [Google Scholar]
- 727. Jenkinson S, Nahorski SR, Challiss RA. Disruption by lithium of phosphatidylinositol-4,5-bisphosphate supply and inositol-1,4,5-trisphosphate generation in Chinese hamster ovary cells expressing human recombinant m1 muscarinic receptors. Mol Pharmacol 46: 1138–1148, 1994 [PubMed] [Google Scholar]
- 728. Jeske NA, Por ED, Belugin S, Chaudhury S, Berg KA, Akopian AN, Henry MA, Gomez R. A-kinase anchoring protein 150 mediates transient receptor potential family V type 1 sensitivity to phosphatidylinositol-4,5-bisphosphate. J Neurosci 31: 8681–8688, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 729. Jezyk MR, Snyder JT, Gershberg S, Worthylake DK, Harden TK, Sondek J. Crystal structure of Rac1 bound to its effector phospholipase C-beta2. Nat Struct Mol Biol 13: 1135–1140, 2006 [DOI] [PubMed] [Google Scholar]
- 730. Ji QS, Winnier GE, Niswender KD, Hortsman D, Wisdom R, Magnuson MA, Carpenter G. Essential role of the tyrosine kinase substrate phospholipase C-gamma1 in mammalian growth and development. Proc Natl Acad Sci USA 94: 2999–3003, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 731. Jia S, Liu Z, Zhang S, Liu P, Zhang L, Lee SH, Zhang J, Signoretti S, Loda M, Roberts TM, Zhao JJ. Essential roles of PI(3)K-p110beta in cell growth, metabolism and tumorigenesis. Nature 454: 776–779, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 732. Jiang H, Kuang Y, Wu Y, Xie W, Simon MI, Wu D. Roles of phospholipase C beta2 in chemoattractant-elicited responses. Proc Natl Acad Sci USA 94: 7971–7975, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 733. Jiang H, Lyubarsky A, Dodd R, Vardi N, Pugh E, Baylor D, Simon MI, Wu D. Phospholipase C beta 4 is involved in modulating the visual response in mice. Proc Natl Acad Sci USA 93: 14598–14601, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 734. Jin N, Chow CY, Liu L, Zolov SN, Bronson R, Davisson M, Petersen JL, Zhang Y, Park S, Duex JE, Goldowitz D, Meisler MH, Weisman LS. VAC14 nucleates a protein complex essential for the acute interconversion of PI3P and PI(3,5)P(2) in yeast and mouse. EMBO J 27: 3221–3234, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 735. Jin T, Zhang N, Long Y, Parent CA, Devreotes PN. Localization of the G protein betagamma complex in living cells during chemotaxis. Science 287: 1034–1036, 2000 [DOI] [PubMed] [Google Scholar]
- 736. Jin TG, Satoh T, Liao Y, Song C, Gao X, Kariya K, Hu CD, Kataoka T. Role of the CDC25 homology domain of phospholipase Cepsilon in amplification of Rap1-dependent signaling. J Biol Chem 276: 30301–30307, 2001 [DOI] [PubMed] [Google Scholar]
- 737. Jones DR, Bultsma Y, Keune WJ, Halstead JR, Elouarrat D, Mohammed S, Heck AJ, D'Santos CS, Divecha N. Nuclear PtdIns5P as a transducer of stress signaling: an in vivo role for PIP4Kbeta. Mol Cell 23: 685–695, 2006 [DOI] [PubMed] [Google Scholar]
- 738. Jongstra-Bilen J, Puig Cano A, Hasija M, Xiao H, Smith CI, Cybulsky MI. Dual functions of Bruton's tyrosine kinase and Tec kinase during Fcgamma receptor-induced signaling and phagocytosis. J Immunol 181: 288–298, 2008 [DOI] [PubMed] [Google Scholar]
- 739. Jope RS. Lithium and GSK-3: one inhibitor, two inhibitory actions, multiple outcomes. Trends Pharmacol Sci 24: 441–443, 2003 [DOI] [PubMed] [Google Scholar]
- 740. Jou ST, Carpino N, Takahashi Y, Piekorz R, Chao JR, Wang D, Ihle JN. Essential, nonredundant role for the phosphoinositide 3-kinase p110delta in signaling by the B-cell receptor complex. Mol Cell Biol 22: 8580–8591, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 741. Jovic M, Kean MJ, Szentpetery Z, Polevoy G, Gingras AC, Brill JA, Balla T. Two PI 4-kinases control lysosomal delivery of the Gaucher disease enzyme, beta-glucocerebrosidaae. Mol Biol Cell 23: 1533–1545, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 742. Ju M, Shi J, Saleh SN, Albert AP, Large WA. Ins(1,4,5)P3 interacts with PIP2 to regulate activation of TRPC6/C7 channels by diacylglycerol in native vascular myocytes. J Physiol 588: 1419–1433, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 743. Jung G, Wang J, Wlodarski P, Barylko B, Binns DD, Shu H, Yin HL, Albanesi JP. Molecular determinants of activation and membrane targeting of phosphoinositol 4-kinase IIbeta. Biochem J 409: 501–509, 2008 [DOI] [PubMed] [Google Scholar]
- 744. Kagawa S, Soeda Y, Ishihara H, Oya T, Sasahara M, Yaguchi S, Oshita R, Wada T, Tsuneki H, Sasaoka T. Impact of transgenic overexpression of SH2-containing inositol 5'-phosphatase 2 on glucose metabolism and insulin signaling in mice. Endocrinology 149: 642–650, 2008 [DOI] [PubMed] [Google Scholar]
- 745. Kagiwada S, Hashimoto M. The yeast VAP homolog Scs2p has a phosphoinositide-binding ability that is correlated with its activity. Biochem Biophys Res Commun 364: 870–876, 2007 [DOI] [PubMed] [Google Scholar]
- 746. Kaisaki PJ, Delepine M, Woon PY, Sebag-Montefiore L, Wilder SP, Menzel S, Vionnet N, Marion E, Riveline JP, Charpentier G, Schurmans S, Levy JC, Lathrop M, Farrall M, Gauguier D. Polymorphisms in type II SH2 domain-containing inositol 5-phosphatase (INPPL1, SHIP2) are associated with physiological abnormalities of the metabolic syndrome. Diabetes 53: 1900–1904, 2004 [DOI] [PubMed] [Google Scholar]
- 747. Kaiser SE, Brickner JH, Reilein AR, Fenn TD, Walter P, Brunger AT. Structural basis of FFAT motif-mediated ER targeting. Structure 13: 1035–1045, 2005 [DOI] [PubMed] [Google Scholar]
- 748. Kakuk A, Friedlander E, Vereb G, Jr, Kasa A, Balla A, Balla T, Heilmeyer LM, Jr, Gergely P, Vereb G. Nucleolar localization of phosphatidylinositol 4-kinase PI4K230 in various mammalian cells. Cytometry A 69: 1174–1183, 2006 [DOI] [PubMed] [Google Scholar]
- 749. Kamat A, Carpenter G. Phospholipase C-gamma1: regulation of enzyme function and role in growth factor-dependent signal transduction. Cytokine Growth Factor Rev 8: 109–117, 1997 [DOI] [PubMed] [Google Scholar]
- 750. Kamen LA, Levinsohn J, Swanson JA. Differential association of phosphatidylinositol 3-kinase, SHIP-1, and PTEN with forming phagosomes. Mol Biol Cell 18: 2463–2472, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 751. Kametaka S, Okano T, Ohsumi M, Ohsumi Y. Apg14p and Apg6/Vps30p form a protein complex essential for autophagy in the yeast, Saccharomyces cerevisiae. J Biol Chem 273: 22284–22291, 1998 [DOI] [PubMed] [Google Scholar]
- 752. Kanai F, Liu H, Field SJ, Akbary H, Matsuo T, Brown GE, Cantley LC, Yaffe MB. The PX domains of p47phox and p40phox bind to lipid products of PI(3)K. NatCell Biol 3: 675–678, 2001 [DOI] [PubMed] [Google Scholar]
- 753. Kanemaru K, Nakahara M, Nakamura Y, Hashiguchi Y, Kouchi Z, Yamaguchi H, Oshima N, Kiyonari H, Fukami K. Phospholipase C-eta2 is highly expressed in the habenula and retina. Gene Expr Patterns 10: 119–126, 2010 [DOI] [PubMed] [Google Scholar]
- 754. Kanematsu T, Jang IS, Yamaguchi T, Nagahama H, Yoshimura K, Hidaka K, Matsuda M, Takeuchi H, Misumi Y, Nakayama K, Yamamoto T, Akaike N, Hirata M. Role of the PLC-related, catalytically inactive protein p130 in GABA(A) receptor function. EMBO J 21: 1004–1011, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 755. Kanematsu T, Misumi Y, Watanabe Y, Ozaki S, Koga T, Iwanaga S, Ikehara Y, Hirata M. A new inositol 1,4,5-trisphosphate binding protein similar to phospholipase C-delta 1. Biochem J 313: 319–325, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 756. Kanematsu T, Takeya H, Watanabe Y, Ozaki S, Yoshida M, Koga T, Iwanaga S, Hirata M. Putative inositol 1,4,5-trisphosphate binding proteins in rat brain cytosol. J Biol Chem 267: 6518–6525, 1992 [PubMed] [Google Scholar]
- 757. Kanematsu T, Yasunaga A, Mizoguchi Y, Kuratani A, Kittler JT, Jovanovic JN, Takenaka K, Nakayama KI, Fukami K, Takenawa T, Moss SJ, Nabekura J, Hirata M. Modulation of GABA(A) receptor phosphorylation and membrane trafficking by phospholipase C-related inactive protein/protein phosphatase 1 and 2A signaling complex underlying brain-derived neurotrophic factor-dependent regulation of GABAergic inhibition. J Biol Chem 281: 22180–22189, 2006 [DOI] [PubMed] [Google Scholar]
- 758. Kang S, Denley A, Vanhaesebroeck B, Vogt PK. Oncogenic transformation induced by the p110beta, -gamma, and -delta isoforms of class I phosphoinositide 3-kinase. Proc Natl Acad Sci USA 103: 1289–1294, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 759. Kano M, Hashimoto K, Watanabe M, Kurihara H, Offermans S, Jiang H, Wu Y, Jun K, Shin HS, Inoue Y, Simon MI, Wu D. Phospholipase C beta4 is specifically involved in climbing fiber synapse elimination in the developing cerebellum. Proc Natl Acad Sci USA 95: 15724–15729, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 760. Kaplan DR, Whitman M, Schaffhausen B, Raptis L, Garcea RL, Pallas D, Roberts TM, Cantley L. Phosphatidylinositol metabolism and polyoma-mediated transformation. Proc Natl Acad Sci USA 83: 3624–3628, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 761. Karashima Y, Prenen J, Meseguer V, Owsianik G, Voets T, Nilius B. Modulation of the transient receptor potential channel TRPA1 by phosphatidylinositol 4,5-biphosphate manipulators. Pflügers Arch 457: 77–89, 2008 [DOI] [PubMed] [Google Scholar]
- 762. Katan M, Kriz RW, Totty N, Philp R, Meldrum E, Aldape RA, Knopf JL, Parker PJ. Determination of the primary structure of PLC-154 demonstrates diversity of phosphoinositide-specific phospholipase C activities. Cell 54: 171–177, 1988 [DOI] [PubMed] [Google Scholar]
- 763. Katan M, Parker PJ. Purification of phosphoinositide-specific phospholipase C from a particulate fraction of bovine brain. Eur J Biochem 168: 413–418, 1987 [DOI] [PubMed] [Google Scholar]
- 764. Katso RM, Pardo OE, Palamidessi A, Franz CM, Marinov M, De Laurentiis A, Downward J, Scita G, Ridley AJ, Waterfield MD, Arcaro A. Phosphoinositide 3-Kinase C2beta regulates cytoskeletal organization and cell migration via Rac-dependent mechanisms. Mol Biol Cell 17: 3729–3744, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 765. Katzmann DJ, Babst M, Emr SD. Ubiquitin-dependent sorting into the multivesicular body pathway requires the function of a conserved endosomal protein sorting complex, ESCRT-I. Cell 106: 145–155, 2001 [DOI] [PubMed] [Google Scholar]
- 766. Katzmann DJ, Stefan CJ, Babst M, Emr SD. Vps27 recruits ESCRT machinery to endosomes during MVB sorting. J Cell Biol 162: 413–423, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 767. Kauffmann-Zeh A, Thomas GM, Ball A, Prosser S, Cunningham E, Cockcroft S, Hsuan JJ. Requirement for phosphatidylinositol transfer protein in epidermal growth factor signaling. Science 268: 1188–1190, 1995 [DOI] [PubMed] [Google Scholar]
- 768. Kavran JM, Klein DE, Lee A, Falasca M, Isakoff SJ, Skolnik EY, Lemmon MA. Specificity and promiscuity in phosphoinositide binding by pleckstrin homology domains. J Biol Chem 273: 30497–30508, 1998 [DOI] [PubMed] [Google Scholar]
- 769. Kawano M, Kumagai K, Nishijima M, Hanada K. Efficient trafficking of ceramide from the endoplasmic reticulum to the Golgi apparatus requires a VAMP-associated protein-interacting FFAT motif of CERT. J Biol Chem 281: 30279–30288, 2006 [DOI] [PubMed] [Google Scholar]
- 770. Kazlauskas A, Cooper JA. Phosphorylation of the PDGF receptor beta subunit creates a tight binding site for phosphatidylinositol 3 kinase. EMBO J 9: 3279–3286, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 771. Keizer-Gunnink I, Kortholt A, Van Haastert PJ. Chemoattractants and chemorepellents act by inducing opposite polarity in phospholipase C and PI3-kinase signaling. J Cell Biol 177: 579–585, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 772. Kelley GG, Kaproth-Joslin KA, Reks SE, Smrcka AV, Wojcikiewicz RJ. G-protein-coupled receptor agonists activate endogenous phospholipase Cepsilon and phospholipase Cbeta3 in a temporally distinct manner. J Biol Chem 281: 2639–2648, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 773. Kelley GG, Reks SE, Ondrako JM, Smrcka AV. Phospholipase C(epsilon): a novel Ras effector. EMBO J 20: 743–754, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 774. Kelley GG, Reks SE, Smrcka AV. Hormonal regulation of phospholipase Cepsilon through distinct and overlapping pathways involving G12 and Ras family G-proteins. Biochem J 378: 129–139, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 775. Kemp P, Hubscher G, Hawthorne JN. A liver phospholipase hydrolysing phosphoinositides. Biochim Biophys Acta 31: 585–586, 1959 [DOI] [PubMed] [Google Scholar]
- 776. Kerk D, Moorhead GB. A phylogenetic survey of myotubularin genes of eukaryotes: distribution, protein structure, evolution, and gene expression. BMC Evol Biol 10: 196, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 777. Khattab MA. Targeting host factors: a novel rationale for the management of hepatitis C virus. World J Gastroenterol 15: 3472–3479, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 778. Khodthong C, Kabachinski G, James DJ, Martin TF. Munc13 homology domain-1 in CAPS/UNC31 mediates SNARE binding required for priming vesicle exocytosis. Cell Metab 14: 254–263, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 779. Kihara A, Noda T, Ishihara N, Ohsumi Y. Two distinct Vps34 phosphatidylinositol 3-kinase complexes function in autophagy and carboxypeptidase Y sorting in Saccharomyces cerevisiae. J Cell Biol 152: 519–530, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 780. Kim AY, Tang Z, Liu Q, Patel KN, Maag D, Geng Y, Dong X. Pirt, a phosphoinositide-binding protein, functions as a regulatory subunit of TRPV1. Cell 133: 475–485, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 781. Kim BC, Lee MN, Kim JY, Lee SS, Chang JD, Kim SS, Lee SY, Kim JH. Roles of phosphatidylinositol 3-kinase and Rac in the nuclear signaling by tumor necrosis factor-alpha in rat-2 fibroblasts. J Biol Chem 274: 24372–24377, 1999 [DOI] [PubMed] [Google Scholar]
- 782. Kim BJ, Kim MT, Jeon JH, Kim SJ, So I. Involvement of phosphatidylinositol 4,5-bisphosphate in the desensitization of canonical transient receptor potential 5. Biol Pharm Bull 31: 1733–1738, 2008 [DOI] [PubMed] [Google Scholar]
- 783. Kim D, Cavanaugh EJ, Simkin D. Inhibition of transient receptor potential A1 channel by phosphatidylinositol-4,5-bisphosphate. Am J Physiol Cell Physiol 295: C92–C99, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 784. Kim D, Jun KS, Lee SB, Kang NG, Min DS, Kim YH, Ryu SH, Suh PG, Shin HS. Phospholipase C isozymes selectively couple to specific neurotransmitter receptors. Nature 389: 290–293, 1997 [DOI] [PubMed] [Google Scholar]
- 785. Kim E, Goraksha-Hicks P, Li L, Neufeld TP, Guan KL. Regulation of TORC1 by Rag GTPases in nutrient response. Nat Cell Biol 10: 935–945, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 786. Kim HK, Kim JW, Zilberstein A, Margolis B, Kim JG, Schlessinger J, Rhee SG. PDGF stimulation of inositol phospholipid hydrolysis requires PLC-gamma1 phosphorylation on tyrosine residues 783 and 1254. Cell 65: 435–441, 1991 [DOI] [PubMed] [Google Scholar]
- 787. Kim JW, Roberts C, Maruyama Y, Berg S, Roper S, Chaudhari N. Faithful expression of GFP from the PLCbeta2 promoter in a functional class of taste receptor cells. Chem Senses 31: 213–219, 2006 [DOI] [PubMed] [Google Scholar]
- 788. Kim JW, Sim SS, Kim UH, Nishibe S, Wahl MI, Carpenter G, Rhee SG. Tyrosine residues in bovine phospholipase C-gamma phosphorylated by the epidermal growth factor receptor in vitro. J Biol Chem 265: 3940–3943, 1990 [PubMed] [Google Scholar]
- 789. Kim MJ, Bahk YY, Min DS, Lee SJ, Ryu SH, Suh PG. Cloning of cDNA encoding rat phospholipase C-beta 4, a new member of the phospholipase C. Biochem Biophys Res Commun 194: 706–712, 1993 [DOI] [PubMed] [Google Scholar]
- 790. Kim MJ, Min DS, Ryu SH, Suh PG. A cytosolic, galphaq- and betagamma-insensitive splice variant of phospholipase C-beta4. J Biol Chem 273: 3618–3624, 1998 [DOI] [PubMed] [Google Scholar]
- 791. Kim S, Kim SF, Maag D, Maxwell MJ, Resnick AC, Juluri KR, Chakraborty A, Koldobskiy MA, Cha SH, Barrow R, Snowman AM, Snyder SH. Amino acid signaling to mTOR mediated by inositol polyphosphate multikinase. Cell Metab 13: 215–221, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 792. Kim SA, Taylor GS, Torgersen KM, Dixon JE. Myotubularin and MTMR2, phosphatidylinositol 3-phosphatases mutated in myotubular myopathy and type 4B Charcot-Marie-Tooth disease. J Biol Chem 277: 4526–4531, 2002 [DOI] [PubMed] [Google Scholar]
- 793. Kim SA, Vacratsis PO, Firestein R, Cleary ML, Dixon JE. Regulation of myotubularin-related (MTMR)2 phosphatidylinositol phosphatase by MTMR5, a catalytically inactive phosphatase. Proc Natl Acad Sci USA 100: 4492–4497, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 794. Kim WT, Chang S, Daniell L, Cremona O, Di Paolo G, De Camilli P. Delayed reentry of recycling vesicles into the fusion-competent synaptic vesicle pool in synaptojanin 1 knockout mice. Proc Natl Acad Sci USA 99: 17143–17148, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 795. Kim YH, Park TJ, Lee YH, Baek KJ, Suh PG, Ryu SH, Kim KT. Phospholipase C-delta1 is activated by capacitative calcium entry that follows phospholipase C-beta activation upon bradykinin stimulation. J Biol Chem 274: 26127–26134, 1999 [DOI] [PubMed] [Google Scholar]
- 796. Kim YJ, Guzman-Hernandez ML, Balla T. A highly dynamic ER-derived phosphatidylinositol-synthesizing organelle supplies phosphoinositides to cellular membranes. Dev Cell 21: 813–824, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 797. Kim YJ, Sekiya F, Poulin B, Bae YS, Rhee SG. Mechanism of B-cell receptor-induced phosphorylation and activation of phospholipase C-gamma2. Mol Cell Biol 24: 9986–9999, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 798. Kimura T, Sakamoto H, Appella E, Siraganian RP. The negative signaling molecule SH2 domain-containing inositol-polyphosphate 5-phosphatase (SHIP) binds to the tyrosine-phosphorylated beta subunit of the high affinity IgE receptor. J Biol Chem 272: 13991–13996, 1997 [DOI] [PubMed] [Google Scholar]
- 799. Kioschis P, Wiemann S, Heiss NS, Francis F, Gotz C, Poustka A, Taudien S, Platzer M, Wiehe T, Beckmann G, Weber J, Nordsiek G, Rosenthal A. Genomic organization of a 225-kb region in Xq28 containing the gene for X-linked myotubular myopathy (MTM1) and a related gene (MTMR1). Genomics 54: 256–266, 1998 [DOI] [PubMed] [Google Scholar]
- 800. Kirk CJ, Creba JA, Downes CP, Michell RH. Hormone-stimulated metabolism of inositol lipids and its relationship to hepatic receptor function. Biochem Soc Trans 9: 377–379, 1981 [DOI] [PubMed] [Google Scholar]
- 801. Kisseleva MV, Wilson MP, Majerus PW. The isolation and characterization of a cDNA encoding phospholipid-specific inositol polyphosphate 5-phosphatase. J Biol Chem 275: 20110–20116, 2000 [DOI] [PubMed] [Google Scholar]
- 802. Klein RR, Bourdon DM, Costales CL, Wagner CD, White WL, Williams JD, Hicks SN, Sondek J, Thakker DR. Direct activation of human phospholipase C by its well known inhibitor u73122. J Biol Chem 286: 12407–12416, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 803. Klenk E, Sakai R. Inositomonophosphorsaure, ein spaltproducte der sojabohnenphosphatide. Hoppe-Zeylers Z Physiol Chem 258: 33–38, 1939 [Google Scholar]
- 804. Knight ZA, Gonzalez B, Feldman ME, Zunder ER, Goldenberg DD, Williams O, Loewith R, Stokoe D, Balla A, Toth B, Balla T, Weiss WA, Williams RL, Shokat KM. A pharmacological map of the PI3-K family defines a role for p110alpha in insulin signaling. Cell 125: 733–747, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 805. Knobbe CB, Lapin V, Suzuki A, Mak TW. The roles of PTEN in development, physiology and tumorigenesis in mouse models: a tissue-by-tissue survey. Oncogene 27: 5398–5415, 2008 [DOI] [PubMed] [Google Scholar]
- 806. Kobayashi T, Takematsu H, Yamaji T, Hiramoto S, Kozutsumi Y. Disturbance of sphingolipid biosynthesis abrogates the signaling of Mss4, phosphatidylinositol-4-phosphate 5-kinase, in yeast. J Biol Chem 280: 18087–18094, 2005 [DOI] [PubMed] [Google Scholar]
- 807. Kobrinsky E, Mirshahi T, Zhang H, Jin T, Logothetis DE. Receptor-mediated hydrolysis of plasma membrane messenger PIP2 leads to K+-current desensitization. Nat Cell Biol 2: 507–514, 2000 [DOI] [PubMed] [Google Scholar]
- 808. Kobrynski LJ, Sullivan KE. Velocardiofacial syndrome, DiGeorge syndrome: the chromosome 22q11.2 deletion syndromes. Lancet 370: 1443–1452, 2007 [DOI] [PubMed] [Google Scholar]
- 809. Kodaki T, Woscholski R, Hallberg B, Rodriguez-Viciana P, Downward J, Parker PJ. The activation of phosphatidylinositol 3-kinase by Ras. Curr Biol 4: 798–806, 1994 [DOI] [PubMed] [Google Scholar]
- 810. Koharudin LM, Furey W, Liu H, Liu YJ, Gronenborn AM. The phox domain of sorting nexin 5 lacks phosphatidylinositol 3-phosphate (PtdIns(3)P) specificity and preferentially binds to phosphatidylinositol 4,5-bisphosphate (PtdIns(4,5)P2). J Biol Chem 284: 23697–23707, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 811. Kohno T, Otsuka T, Takano H, Yamamoto T, Hamaguchi M, Terada M, Yokota J. Identification of a novel phospholipase C family gene at chromosome 2q33 that is homozygously deleted in human small cell lung carcinoma. Hum Mol Genet 4: 667–674, 1995 [DOI] [PubMed] [Google Scholar]
- 812. Kohout SC, Bell SC, Liu L, Xu Q, Minor DL, Jr, Isacoff EY. Electrochemical coupling in the voltage-dependent phosphatase Ci-VSP. Nat Chem Biol 6: 369–375, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 813. Kohout SC, Ulbrich MH, Bell SC, Isacoff EY. Subunit organization and functional transitions in Ci-VSP. Nat Struct Mol Biol 15: 106–108, 2008 [DOI] [PubMed] [Google Scholar]
- 814. Kok K, Geering B, Vanhaesebroeck B. Regulation of phosphoinositide 3-kinase expression in health and disease. Trends Biochem Sci 34: 115–127, 2009 [DOI] [PubMed] [Google Scholar]
- 815. Kolsch V, Charest PG, Firtel RA. The regulation of cell motility and chemotaxis by phospholipid signaling. J Cell Sci 121: 551–559, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 816. Komander D, Fairservice A, Deak M, Kular GS, Prescott AR, Peter Downes C, Safrany ST, Alessi DR, van Aalten DM. Structural insights into the regulation of PDK1 by phosphoinositides and inositol phosphates. EMBO J 23: 3918–3928, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 817. Kong AM, Horan KA, Sriratana A, Bailey CG, Collyer LJ, Nandurkar HH, Shisheva A, Layton MJ, Rasko JE, Rowe T, Mitchell CA. Phosphatidylinositol 3-phosphate [PtdIns3P] is generated at the plasma membrane by an inositol polyphosphate 5-phosphatase: endogenous PtdIns3P can promote GLUT4 translocation to the plasma membrane. Mol Cell Biol 26: 6065–6081, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 818. Kong AM, Speed CJ, O'Malley CJ, Layton MJ, Meehan T, Loveland KL, Cheema S, Ooms LM, Mitchell CA. Cloning and characterization of a 72-kDa inositol-polyphosphate 5-phosphatase localized to the Golgi network. J Biol Chem 275: 24052–24064, 2000 [DOI] [PubMed] [Google Scholar]
- 819. Koo SJ, Markovic S, Puchkov D, Mahrenholz CC, Beceren-Braun F, Maritzen T, Dernedde J, Volkmer R, Oschkinat H, Haucke V. SNARE motif-mediated sorting of synaptobrevin by the endocytic adaptors clathrin assembly lymphoid myeloid leukemia (CALM) and AP180 at synapses. Proc Natl Acad Sci USA 108: 13540–13545, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 820. Korzeniowski MK, Popovic MA, Szentpetery Z, Varnai P, Stojilkovic SS, Balla T. Dependence of STIM1/Orai1-mediated calcium entry on plasma membrane phosphoinositides. J Biol Chem 284: 21027–21035, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 821. Kostelansky MS, Schluter C, Tam YY, Lee S, Ghirlando R, Beach B, Conibear E, Hurley JH. Molecular architecture and functional model of the complete yeast ESCRT-I heterotetramer. Cell 129: 485–498, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 822. Kouchi Z, Fukami K, Shikano T, Oda S, Nakamura Y, Takenawa T, Miyazaki S. Recombinant phospholipase Czeta has high Ca2+ sensitivity and induces Ca2+ oscillations in mouse eggs. J Biol Chem 279: 10408–10412, 2004 [DOI] [PubMed] [Google Scholar]
- 823. Kouchi Z, Igarashi T, Shibayama N, Inanobe S, Sakurai K, Yamaguchi H, Fukuda T, Yanagi S, Nakamura Y, Fukami K. Phospholipase Cdelta3 regulates RhoA/Rho kinase signaling and neurite outgrowth. J Biol Chem 286: 8459–8471, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 824. Krauss M, Kinuta M, Wenk MR, De Camilli P, Takei K, Haucke V. ARF6 stimulates clathrin/AP-2 recruitment to synaptic membranes by activating phosphatidylinositol phosphate kinase type Igamma. J Cell Biol 162: 113–124, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 825. Krick R, Henke S, Tolstrup J, Thumm M. Dissecting the localization and function of Atg18, Atg21 and Ygr223c. Autophagy 4: 896–910, 2008 [DOI] [PubMed] [Google Scholar]
- 826. Kruger T, Sanchez CP, Lanzer M. Complementation of Saccharomyces cerevisiae pik1ts by a phosphatidylinositol 4-kinase from Plasmodium falciparum. Mol Biochem Parasitol 172: 149–151, 2010 [DOI] [PubMed] [Google Scholar]
- 827. Krugmann S, Anderson KE, Ridley SH, Risso N, McGregor A, Coadwell J, Davidson K, Eguinoa A, Ellson CD, Lipp P, Manifava M, Ktistakis N, Painter G, Thuring JW, Cooper MA, Lim ZY, Holmes AB, Dove SK, Michell RH, Grewal A, Nazarian A, Erdjument-Bromage H, Tempst P, Stephens LR, Hawkins PT. Identification of ARAP3, a novel PI3K effector regulating both Arf and Rho GTPases, by selective capture on phosphoinositide affinity matrices. Mol Cell 9: 95–108, 2002 [DOI] [PubMed] [Google Scholar]
- 828. Krylova IN, Sablin EP, Moore J, Xu RX, Waitt GM, MacKay JA, Juzumiene D, Bynum JM, Madauss K, Montana V, Lebedeva L, Suzawa M, Williams JD, Williams SP, Guy RK, Thornton JW, Fletterick RJ, Wilson TW, Ingraham HA. Structural analyses reveal phosphatidylinositols as ligands for the NR5 orphan receptors SF-1 and LRH-1. Cell 120: 343–355, 2005 [DOI] [PubMed] [Google Scholar]
- 829. Ktori C, Shepherd PR, O'Rourke L. TNF-alpha and leptin activate the alpha-isoform of class II phosphoinositide 3-kinase. Biochem Biophys Res Commun 306: 139–143, 2003 [DOI] [PubMed] [Google Scholar]
- 830. Kular GS, Chaudhary A, Prestwich G, Swigart P, Wetzker R, Cockcroft S. Co-operation of phosphatidylinositol transfer protein with phosphoinositide 3-kinase gamma in vitro. Adv Enzyme Regul 42: 53–61, 2002 [DOI] [PubMed] [Google Scholar]
- 831. Kumar A, Fernandez-Capetillo O, Carrera AC. Nuclear phosphoinositide 3-kinase beta controls double-strand break DNA repair. Proc Natl Acad Sci USA 107: 7491–7496, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 832. Kunz J, Wilson MP, Kisseleva M, Hurley JH, Majerus PW, Anderson RA. The activation loop of phosphatidylinositol phosphate kinases determines signaling specificity. Mol Cell 5: 1–11, 2000 [DOI] [PubMed] [Google Scholar]
- 833. Kurig B, Shymanets A, Bohnacker T, Prajwal Brock C, Ahmadian MR, Schaefer M, Gohla A, Harteneck C, Wymann MP, Jeanclos E, Nurnberg B. Ras is an indispensable coregulator of the class IB phosphoinositide 3-kinase p87/p110gamma. Proc Natl Acad Sci USA 106: 20312–20317, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 834. Kuroda K, Ito M, Shikano T, Awaji T, Yoda A, Takeuchi H, Kinoshita K, Miyazaki S. The role of X/Y linker region and N-terminal EF-hand domain in nuclear translocation and Ca2+ oscillation-inducing activities of phospholipase Czeta, a mammalian egg-activating factor. J Biol Chem 281: 27794–27805, 2006 [DOI] [PubMed] [Google Scholar]
- 835. Kurosu H, Maehama T, Okada T, Yamamoto T, Hoshino S, Fukui Y, Ui M, Hazeki O, Katada T. Heterodimeric phosphoinositide 3-kinase consisting of p85 and p110beta is synergistically activated by the betagamma subunits of G proteins and phosphotyrosyl peptide. J Biol Chem 272: 24252–24256, 1997 [DOI] [PubMed] [Google Scholar]
- 836. Kutateladze TG, Ogburn KD, Watson WT, deBeer T, Emr SD, Burd CG, Overduin M. Phosphatidylinositol 3-Phosphate recognition by the FYVE domain. Mol Cell 3: 805–811, 1999 [DOI] [PubMed] [Google Scholar]
- 837. Kuzmichev A, Zhang Y, Erdjument-Bromage H, Tempst P, Reinberg D. Role of the Sin3-histone deacetylase complex in growth regulation by the candidate tumor suppressor p33(ING1). Mol Cell Biol 22: 835–848, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 838. Kwabi-Addo B, Giri D, Schmidt K, Podsypanina K, Parsons R, Greenberg N, Ittmann M. Haploinsufficiency of the Pten tumor suppressor gene promotes prostate cancer progression. Proc Natl Acad Sci USA 98: 11563–11568, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 839. Kweon DH, Kim CS, Shin YK. Regulation of neuronal SNARE assembly by the membrane. Nat Struct Biol 10: 440–447, 2003 [DOI] [PubMed] [Google Scholar]
- 840. Kwik J, Boyle S, Fooksman D, Margolis L, Sheetz MP, Edidin M. Membrane cholesterol, lateral mobility, and the phosphatidylinositol 4,5-bisphosphate-dependent organization of cell actin. Proc Natl Acad Sci USA 100: 13964–13969, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 841. Kwon HM, Yamauchi A, Uchida S, Preston AS, Garcia-Perez A, Burg MB, Handler JS. Cloning of the cDNa for a Na+/myo-inositol cotransporter, a hypertonicity stress protein. J Biol Chem 267: 6297–6301, 1992 [PubMed] [Google Scholar]
- 842. Kwon J, Lee SR, Yang KS, Ahn Y, Kim YJ, Stadtman ER, Rhee SG. Reversible oxidation and inactivation of the tumor suppressor PTEN in cells stimulated with peptide growth factors. Proc Natl Acad Sci USA 101: 16419–16424, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 843. Kwon Y, Hofmann T, Montell C. Integration of phosphoinositide- and calmodulin-mediated regulation of TRPC6. Mol Cell 25: 491–503, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 844. LaBelle EF, Wilson K, Polyak E. Subcellular localization of phospholipase C isoforms in vascular smooth muscle. Biochim Biophys Acta 1583: 273–278, 2002 [DOI] [PubMed] [Google Scholar]
- 845. Lacalle RA, Peregil RM, Albar JP, Merino E, Martinez AC, Merida I, Manes S. Type I phosphatidylinositol 4-phosphate 5-kinase controls neutrophil polarity and directional movement. J Cell Biol 179: 1539–1553, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 846. Lacroix J, Halaszovich CR, Schreiber DN, Leitner MG, Bezanilla F, Oliver D, Villalba-Galea CA. Controlling the activity of a phosphatase and tensin homolog (PTEN) by membrane potential. J Biol Chem 286: 17945–17953, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 847. Laffargue M, Calvez R, Finan P, Trifilieff A, Barbier M, Altruda F, Hirsch E, Wymann MP. Phosphoinositide 3-kinase gamma is an essential amplifier of mast cell function. Immunity 16: 441–451, 2002 [DOI] [PubMed] [Google Scholar]
- 848. Lamia KA, Peroni OD, Kim YB, Rameh LE, Kahn BB, Cantley LC. Increased insulin sensitivity and reduced adiposity in phosphatidylinositol 5-phosphate 4-kinase beta−/− mice. Mol Cell Biol 24: 5080–5087, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 849. Lamond AI, Spector DL. Nuclear speckles: a model for nuclear organelles. Nat Rev Mol Cell Biol 4: 605–612, 2003 [DOI] [PubMed] [Google Scholar]
- 850. Langeslag M, Clark K, Moolenaar WH, van Leeuwen FN, Jalink K. Activation of TRPM7 channels by phospholipase C-coupled receptor agonists. J Biol Chem 282: 232–239, 2007 [DOI] [PubMed] [Google Scholar]
- 851. Langlois MJ, Bergeron S, Bernatchez G, Boudreau F, Saucier C, Perreault N, Carrier JC, Rivard N. The PTEN phosphatase controls intestinal epithelial cell polarity and barrier function: role in colorectal cancer progression. PLoS One 5: e15742, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 852. Lapetina EG, Michell RH. A membrane-bound activity catalysing phosphatidylinositol breakdown to 1,2,diacylglycerol, d-myoinositol 1:2-cyclic phosphate and d-myoinositol 1-phosphate: properties and subcellular distribution in rat cerebral cortex. Biochem J 131: 433–442, 1973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 853. Lapetina EG, Michell RH. Phosphatidylinositol metabolism in cells receiving extracellular stimulation. FEBS Lett 31: 1–10, 1973 [DOI] [PubMed] [Google Scholar]
- 854. Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell 149: 274–293, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 855. Laporte J, Bedez F, Bolino A, Mandel JL. Myotubularins, a large disease-associated family of cooperating catalytically active and inactive phosphoinositides phosphatases. Hum Mol Genet 12: R285–292, 2003 [DOI] [PubMed] [Google Scholar]
- 856. Laporte J, Blondeau F, Buj-Bello A, Tentler D, Kretz C, Dahl N, Mandel JL. Characterization of the myotubularin dual specificity phosphatase gene family from yeast to human. Hum Mol Genet 7: 1703–1712, 1998 [DOI] [PubMed] [Google Scholar]
- 857. Laporte J, Blondeau F, Gansmuller A, Lutz Y, Vonesch JL, Mandel JL. The PtdIns3P phosphatase myotubularin is a cytoplasmic protein that also localizes to Rac1-inducible plasma membrane ruffles. J Cell Sci 115: 3105–3117, 2002 [DOI] [PubMed] [Google Scholar]
- 858. Laporte J, Hu LJ, Kretz C, Mandel JL, Kioschis P, Coy JF, Klauck SM, Poustka A, Dahl N. A gene mutated in X-linked myotubular myopathy defines a new putative tyrosine phosphatase family conserved in yeast. Nat Genet 13: 175–182, 1996 [DOI] [PubMed] [Google Scholar]
- 859. Larsen EC, Ueyama T, Brannock PM, Shirai Y, Saito N, Larsson C, Loegering D, Weber PB, Lennartz MR. A role for PKC-epsilon in Fc gammaR-mediated phagocytosis by RAW 264.7 cells. J Cell Biol 159: 939–944, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 860. Lawlor MA, Alessi DR. PKB/Akt: a key mediator of cell proliferation, survival and insulin responses? J Cell Sci 114: 2903–2910, 2001 [DOI] [PubMed] [Google Scholar]
- 861. Lawson ND, Mugford JW, Diamond BA, Weinstein BM. Phospholipase C gamma-1 is required downstream of vascular endothelial growth factor during arterial development. Genes Dev 17: 1346–1351, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 862. Laxminarayan KM, Chan BK, Tetaz T, Bird PI, Mitchell CA. Characterization of a cDNA encoding the 43-kDa membrane-associated inositol-polyphosphate 5-phosphatase. J Biol Chem 269: 17305–17310, 1994 [PubMed] [Google Scholar]
- 863. Laxminarayan KM, Matzaris M, Speed CJ, Mitchell CA. Purification and characterization of a 43-kDa membrane-associated inositol polyphosphate 5-phosphatase from human placenta. J Biol Chem 268: 4968–4974, 1993 [PubMed] [Google Scholar]
- 864. Leber W, Skippen A, Fivelman QL, Bowyer PW, Cockcroft S, Baker DA. A unique phosphatidylinositol 4-phosphate 5-kinase is activated by ADP-ribosylation factor in Plasmodium falciparum. Int J Parasitol 39: 645–653, 2009 [DOI] [PubMed] [Google Scholar]
- 865. Lechner SG, Hussl S, Schicker KW, Drobny H, Boehm S. Presynaptic inhibition via a phospholipase C- and phosphatidylinositol bisphosphate-dependent regulation of neuronal Ca2+ channels. Mol Pharmacol 68: 1387–1396, 2005 [DOI] [PubMed] [Google Scholar]
- 866. Lee CH, Park D, Wu D, Rhee SG, Simon MI. Members of the Gq alpha subunit gene family activate phospholipase C beta isozymes. J Biol Chem 267: 16044–16047, 1992 [PubMed] [Google Scholar]
- 867. Lee CW, Lee KH, Lee SB, Park D, Rhee SG. Regulation of phospholipase C-beta 4 by ribonucleotides and the alpha subunit of Gq. J Biol Chem 269: 25335–25338, 1994 [PubMed] [Google Scholar]
- 868. Lee CW, Park DJ, Lee KH, Kim CG, Rhee SG. Purification, molecular cloning, and sequencing of phospholipase C-beta4. J Biol Chem 268: 21318–21327, 1993 [PubMed] [Google Scholar]
- 869. Lee J, Cha SK, Sun TJ, Huang CL. PIP2 activates TRPV5 and releases its inhibition by intracellular Mg2+. J Gen Physiol 126: 439–451, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 870. Lee JO, Yang H, Georgescu MM, Di Cristofano A, Maehama T, Shi Y, Dixon JE, Pandolfi P, Pavletich NP. Crystal structure of the PTEN tumor suppressor: implications for its phosphoinositide phosphatase activity and membrane association. Cell 99: 323–334, 1999 [DOI] [PubMed] [Google Scholar]
- 871. Lee SB, Rhee SG. Molecular cloning, splice variants, expression and purification of phospholipase C-delta4. J Biol Chem 271: 25–31, 1996 [DOI] [PubMed] [Google Scholar]
- 872. Lee SB, Varnai P, Balla A, Jalink K, Rhee SG, Balla T. The pleckstrin homology domain of phosphoinositide-specific phospholipase Cdelta4 is not a critical determinant of the membrane localization of the enzyme. J Biol Chem 279: 24362–24371, 2004 [DOI] [PubMed] [Google Scholar]
- 873. Lee SH, Poulogiannis G, Pyne S, Jia S, Zou L, Signoretti S, Loda M, Cantley LC, Roberts TM. A constitutively activated form of the p110beta isoform of PI3-kinase induces prostatic intraepithelial neoplasia in mice. Proc Natl Acad Sci USA 107: 11002–11007, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 874. Lee SR, Yang KS, Kwon J, Lee C, Jeong W, Rhee SG. Reversible inactivation of the tumor suppressor PTEN by H2O2. J Biol Chem 277: 20336–20342, 2002 [DOI] [PubMed] [Google Scholar]
- 875. Lee SY, Voronov S, Letinic K, Nairn AC, Di Paolo G, De Camilli P. Regulation of the interaction between PIPKI gamma and talin by proline-directed protein kinases. J Cell Biol 168: 789–799, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 876. Lee SY, Wenk MR, Kim Y, Nairn AC, De Camilli P. Regulation of synaptojanin 1 by cyclin-dependent kinase 5 at synapses. Proc Natl Acad Sci USA 101: 546–551, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 877. Legendre-Guillemin V, Wasiak S, Hussain NK, Angers A, McPherson PS. ENTH/ANTH proteins and clathrin-mediated membrane budding. J Cell Sci 117: 9–18, 2004 [DOI] [PubMed] [Google Scholar]
- 878. Lei EP, Stern CA, Fahrenkrog B, Krebber H, Moy TI, Aebi U, Silver PA. Sac3 is an mRNA export factor that localizes to cytoplasmic fibrils of nuclear pore complex. Mol Biol Cell 14: 836–847, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 879. Leibiger B, Moede T, Uhles S, Barker CJ, Creveaux M, Domin J, Berggren PO, Leibiger IB. Insulin-feedback via PI3K-C2alpha activated PKBalpha/Akt1 is required for glucose-stimulated insulin secretion. FASEB J 24: 1824–1837, 2010 [DOI] [PubMed] [Google Scholar]
- 880. Lemmon MA. Pleckstrin homology domains: not just for phosphoinositides. Biochem Soc Trans 32: 707–711, 2004 [DOI] [PubMed] [Google Scholar]
- 881. Lemmon MA, Falasca M, Ferguson KM, Schlessinger J. Regulatory recruitment of signalling molecules to the cell membrane by plekstrin-homology domains. Trends Cell Biol 7: 237–242, 1997 [DOI] [PubMed] [Google Scholar]
- 882. Lemmon MA, Ferguson KM, O'Brian R, Sigler PB, Schlessinger J. Specific and high-affinity binding of inositol phosphates to an isolated plekstrin homology domain. Proc Natl Acad Sci USA 92: 10472–10476, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 883. Lemonnier L, Trebak M, Putney JW., Jr Complex regulation of the TRPC3, 6 and 7 channel subfamily by diacylglycerol and phosphatidylinositol-4,5-bisphosphate. Cell Calcium 43: 506–514, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 884. Lenk GM, Ferguson CJ, Chow CY, Jin N, Jones JM, Grant AE, Zolov SN, Winters JJ, Giger RJ, Dowling JJ, Weisman LS, Meisler MH. Pathogenic mechanism of the FIG4 mutation responsible for Charcot-Marie-Tooth disease CMT4J. PLoS Genet 7: e1002104, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 885. Lenoir M, Coskun U, Grzybek M, Cao X, Buschhorn SB, James J, Simons K, Overduin M. Structural basis of wedging the Golgi membrane by FAPP pleckstrin homology domains. EMBO Rep 11: 279–284, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 886. Leonard D, Hayakawa A, Lawe D, Lambright D, Bellve KD, Standley C, Lifshitz LM, Fogarty KE, Corvera S. Sorting of EGF and transferrin at the plasma membrane and by cargo-specific signaling to EEA1-enriched endosomes. J Cell Sci 121: 3445–3458, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 887. Leopoldt D, Hanck T, Exner T, Maier U, Wetzker R, Nurnberg B. Gbetagamma stimulates phosphoinositide 3-kinase-gamma by direct interaction with two domains of the catalytic p110 subunit. J Biol Chem 273: 7024–7029, 1998 [DOI] [PubMed] [Google Scholar]
- 888. Leslie NR, Bennett D, Lindsay YE, Stewart H, Gray A, Downes CP. Redox regulation of PI 3-kinase signalling via inactivation of PTEN. EMBO J 22: 5501–5510, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 889. Leung DW, Tompkins C, Brewer J, Ball A, Coon M, Morris V, Waggoner D, Singer JW. Phospholipase C delta-4 overexpression upregulates ErbB1/2 expression, Erk signaling pathway, and proliferation in MCF-7 cells. Mol Cancer 3: 15, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 890. Lev S. Non-vesicular lipid transport by lipid-transfer proteins and beyond. Nat Rev Mol Cell Biol 11: 739–750, 2010 [DOI] [PubMed] [Google Scholar]
- 891. Lev S, Hernandez J, Martinez R, Chen A, Plowman G, Schlessinger J. Identification of a novel family of targets of PYK2 related to Drosophila retinal degeneration B (rdgB) protein. Mol Cell Biol 19: 2278–2288, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 892. Levin DE. Cell wall integrity signaling in Saccharomyces cerevisiae. Microbiol Mol Biol Rev 69: 262–291, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 893. Levine TP, Munro S. Dual targeting of Osh1p, a yeast homologue of oxysterol-binding protein, to both the Golgi and the nucleus-vacuole junction. Mol Biol Cell 6: 1633–1644, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 894. Levine TP, Munro S. The pleckstrin-homology domain of oxysterol-binding protein recognizes a determinant specific to Golgi membranes. Curr Biol 8: 729–739, 1998 [DOI] [PubMed] [Google Scholar]
- 895. Levine TP, Munro S. Targeting of Golgi-specific pleckstrin homology domains involves both PtdIns 4-kinase-dependent and -independent components. Curr Biol 12: 695–704, 2002 [DOI] [PubMed] [Google Scholar]
- 896. Lewis RS. The molecular choreography of a store-operated calcium channel. Nature 446: 284–287, 2007 [DOI] [PubMed] [Google Scholar]
- 897. Li DM, Sun H. TEP1, encoded by a candidate tumor suppressor locus, is a novel protein tyrosine phosphatase regulated by transforming growth factor beta. Cancer Res 57: 2124–2129, 1997 [PubMed] [Google Scholar]
- 898. Li J, Yen C, Liaw D, Podsypanina K, Bose S, Wang SI, Puc J, Miliaresis C, Rodgers L, McCombie R, Bigner SH, Giovanella BC, Ittmann M, Tycko B, Hibshoosh H, Wigler MH, Parsons R. PTEN, a putative protein tyrosine phosphatase gene mutated in human brain, breast, and prostate cancer. Science 275: 1943–1947, 1997 [DOI] [PubMed] [Google Scholar]
- 899. Li L, Gronning LM, Anderson PO, Li S, Edvardsen K, Johnston J, Kioussis D, Shepherd PR, Wang P. Insulin induces SOCS-6 expression and its binding to the p85 monomer of phosphoinositide 3-kinase, resulting in improvement in glucose metabolism. J Biol Chem 279: 34107–34114, 2004 [DOI] [PubMed] [Google Scholar]
- 900. Li M, Edamatsu H, Kitazawa R, Kitazawa S, Kataoka T. Phospholipase Cepsilon promotes intestinal tumorigenesis of Apc(Min/+) mice through augmentation of inflammation and angiogenesis. Carcinogenesis 30: 1424–1432, 2009 [DOI] [PubMed] [Google Scholar]
- 901. Li S, Tiab L, Jiao X, Munier FL, Zografos L, Frueh BE, Sergeev Y, Smith J, Rubin B, Meallet MA, Forster RK, Hejtmancik JF, Schorderet DF. Mutations in PIP5K3 are associated with Francois-Neetens mouchetee fleck corneal dystrophy. Am J Hum Genet 77: 54–63, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 902. Li X, Rivas MP, Fang M, Marchena J, Mehrotra B, Chaudhary A, Feng L, Prestwich GD, Bankaitis VA. Analysis of oxysterol binding protein homologue Kes1p function in regulation of Sec14p-dependent protein transport from the yeast Golgi complex. J Cell Biol 157: 63–77, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 903. Li Y, Choi M, Cavey G, Daugherty J, Suino K, Kovach A, Bingham NC, Kliewer SA, Xu EH. Crystallographic identification and functional characterization of phospholipids as ligands fro the orphan nuclear receptor steroidogenic factor-1. Mol Cell 17: 491–502, 2005 [DOI] [PubMed] [Google Scholar]
- 904. Li Y, Jia Y, Pichavant M, Loison F, Sarraj B, Kasorn A, You J, Robson BE, Umetsu DT, Mizgerd JP, Ye K, Luo HR. Targeted deletion of tumor suppressor PTEN augments neutrophil function and enhances host defense in neutropenia-associated pneumonia. Blood 113: 4930–4941, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 905. Li Z, Dong X, Wang Z, Liu W, Deng N, Ding Y, Tang L, Hla T, Zeng R, Li L, Wu D. Regulation of PTEN by Rho small GTPases. Nat Cell Biol 7: 399–404, 2005 [DOI] [PubMed] [Google Scholar]
- 906. Li Z, Jiang H, Xie W, Zhang Z, Smrcka AV, Wu D. Roles of PLC-beta2 and -beta3 and PI3Kgamma in chemoattractant-mediated signal transduction. Science 287: 1046–1049, 2000 [DOI] [PubMed] [Google Scholar]
- 907. Lian L, Wang Y, Draznin J, Eslin D, Bennett JS, Poncz M, Wu D, Abrams CS. The relative role of PLCbeta and PI3Kgamma in platelet activation. Blood 106: 110–117, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 908. Liang C, Lee JS, Inn KS, Gack MU, Li Q, Roberts EA, Vergne I, Deretic V, Feng P, Akazawa C, Jung JU. Beclin1-binding UVRAG targets the class C Vps complex to coordinate autophagosome maturation and endocytic trafficking. Nat Cell Biol 10: 776–787, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 909. Liao HJ, Kume T, McKay C, Xu MJ, Ihle JN, Carpenter G. Absence of erythrogenesis and vasculogenesis in Plcg1-deficient mice. J Biol Chem 277: 9335–9341, 2002 [DOI] [PubMed] [Google Scholar]
- 910. Lim YS, Hwang SB. Hepatitis C virus NS5A protein interacts with phosphatidylinositol 4-kinase type IIIalpha and regulates viral propagation. J Biol Chem 286: 11290–11298, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 911. Lindner M, Leitner MG, Halaszovich CR, Hammond GR, Oliver D. Probing the regulation of TASK potassium channels by PI(4,5)P2 with switchable phosphoinositide phosphatases. J Physiol 589: 3149–3162, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 912. Ling K, Bairstow SF, Carbonara C, Turbin DA, Huntsman DG, Anderson RA. Type I gamma phosphatidylinositol phosphate kinase modulates adherens junction and E-cadherin trafficking via a direct interaction with mu 1B adaptin. J Cell Biol 176: 343–353, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 913. Ling K, Doughman RL, Firestone AJ, Bunce MW, Anderson RA. Type I gamma phosphatidylinositol phosphate kinase targets and regulates focal adhesions. Nature 420: 89–93, 2002 [DOI] [PubMed] [Google Scholar]
- 914. Ling K, Doughman RL, Iyer VV, Firestone AJ, Bairstow SF, Mosher DF, Schaller MD, Anderson RA. Tyrosine phosphorylation of type Igamma phosphatidylinositol phosphate kinase by Src regulates an integrin-talin switch. J Cell Biol 163: 1339–1349, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 915. Liou HH, Zhou SS, Huang CL. Regulation of ROMK1 channel by protein kinase A via a phosphatidylinositol 4,5-bisphosphate-dependent mechanism. Proc Natl Acad Sci USA 96: 5820–5825, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 916. Liou J, Kim ML, Heo WD, Jones JT, Myers JW, Ferrell JE, Jr, Meyer T. STIM is a Ca2+ sensor essential for Ca2+-store-depletion-triggered Ca2+ influx. Curr Biol 15: 1235–1241, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 917. Lippincott-Schwartz J, Manley S. Putting super-resolution fluorescence microscopy to work. Nat Methods 6: 21–23, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 918. Liscovitch M, Chalifa V, Pertile P, Chen CS, Cantley LC. Novel function of phosphatidylinositol 4,5-biphosphate as a cofactor for brain membrane phospholipase D. J Biol Chem 269: 21403–21406, 1994 [PubMed] [Google Scholar]
- 919. Litvak V, Dahan N, Ramachandran S, Sabanay H, Lev S. Maintenance of the diacylglycerol level in the Golgi apparatus by the Nir2 protein is critical for Golgi secretory function. Nat Cell Biol 7: 225–234, 2005 [DOI] [PubMed] [Google Scholar]
- 920. Litvak V, Shaul YD, Shulewitz M, Amarilio R, Carmon S, Lev S. Targeting of Nir2 to lipid droplets is regulated by a specific threonine residue within its PI-transfer domain. Curr Biol 12: 1513–1518, 2002 [DOI] [PubMed] [Google Scholar]
- 921. Litvak V, Tian D, Carmon S, Lev S. Nir2, a human homolog of Drosophila melanogaster retinal degeneration B protein, is essential for cytokinesis. Mol Cell Biol 22: 5064–5075, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 922. Liu B, Qin F. Functional control of cold- and menthol-sensitive TRPM8 ion channels by phosphatidylinositol 4,5-bisphosphate. J Neurosci 25: 1674–1681, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 923. Liu D, Liman ER. Intracellular Ca2+ and the phospholipid PIP2 regulate the taste transduction ion channel TRPM5. Proc Natl Acad Sci USA 100: 15160–15165, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 924. Liu L, Damen JE, Cutler RL, Krystal G. Multiple cytokines stimulate the binding of a common 145-kilodalton protein to Shc at the Grb2 recognition site of Shc. Mol Cell Biol 14: 6926–6935, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 925. Liu N, Fukami K, Yu H, Takenawa T. A new phospholipase C delta 4 is induced at S-phase of the cell cycle and appears in the nucleus. J Biol Chem 271: 355–360, 1996 [DOI] [PubMed] [Google Scholar]
- 926. Liu P, Cheng H, Roberts TM, Zhao JJ. Targeting the phosphoinositide 3-kinase pathway in cancer. Nat Rev Drug Discov 8: 627–644, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 927. Liu Q, Sasaki T, Kozieradzki I, Wakeham A, Itie A, Dumont DJ, Penninger JM. SHIP is a negative regulator of growth factor receptor-mediated PKB/Akt activation and myeloid cell survival. Genes Dev 13: 786–791, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 928. Liu Q, Shalaby F, Jones J, Bouchard D, Dumont DJ. The SH2-containing inositol polyphosphate 5-phosphatase, ship, is expressed during hematopoiesis and spermatogenesis. Blood 91: 2753–2759, 1998 [PubMed] [Google Scholar]
- 929. Liu Y, Boukhelifa M, Tribble E, Morin-Kensicki E, Uetrecht A, Bear JE, Bankaitis VA. The Sac1 phosphoinositide phosphatase regulates Golgi membrane morphology and mitotic spindle organization in mammals. Mol Biol Cell 19: 3080–3096, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 930. Locasale JW, Cantley LC. Metabolic flux and the regulation of mammalian cell growth. Cell Metab 14: 443–451, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 931. Loewen CJ, Levine TP. A highly conserved binding site in vesicle-associated membrane protein-associated protein (VAP) for the FFAT motif of lipid-binding proteins. J Biol Chem 280: 14097–14104, 2005 [DOI] [PubMed] [Google Scholar]
- 932. Logothetis DE, Petrou VI, Adney SK, Mahajan R. Channelopathies linked to plasma membrane phosphoinositides. Pflügers Arch 460: 321–341, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 933. Loijens JC, Anderson RA. Type I phosphatidylinositol-4-phosphate 5-kinases are distinct members of this novel lipid kinase family. J Biol Chem 271: 32937–32943, 1996 [DOI] [PubMed] [Google Scholar]
- 934. Loijens JC, Boronenkov IV, Parker GJ, Anderson RA. The phosphatidylinositol 4-phosphate 5-kinase family. Adv Enzyme Regul 36: 115–140, 1996 [DOI] [PubMed] [Google Scholar]
- 935. Lomasney JW, Cheng HF, Roffler SR, King K. Activation of phospholipase C delta1 through C2 domain by a Ca2+-enzyme-phosphatidylserine ternary complex. J Biol Chem 274: 21995–22001, 1999 [DOI] [PubMed] [Google Scholar]
- 936. Lomasney JW, Cheng HF, Wang LP, Kuan Y, Liu S, Fesik SW, King K. Phosphatidylinositol 4,5-bisphosphate binding to the pleckstrin homology domain of phospholipase C-delta1 enhances enzyme activity. J Biol Chem 271: 25316–25326, 1996 [DOI] [PubMed] [Google Scholar]
- 937. Lopes CM, Rohacs T, Czirjak G, Balla T, Enyedi P, Logothetis DE. PIP2 hydrolysis underlies agonist-induced inhibition and regulates voltage gating of two-pore domain K+ channels. J Physiol 564: 117–129, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 938. Lopez I, Mak EC, Ding J, Hamm HE, Lomasney JW. A novel bifunctional phospholipase c that is regulated by Galpha 12 and stimulates the Ras/mitogen-activated protein kinase pathway. J Biol Chem 276: 2758–2765, 2001 [DOI] [PubMed] [Google Scholar]
- 939. Lopez-Rivas A, Mendoza SA, Nanberg E, Sinnett-Smith J, Rozengurt E. Ca2+-mobilizing actions of platelet-derived growth factor differ from those of bombesin and vasopressin in Swiss 3T3 mouse cells. Proc Natl Acad Sci USA 84: 5768–5772, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 940. Lorente-Rodriguez A, Barlowe C. Requirement for Golgi-localized PI(4)P in fusion of COPII vesicles with Golgi compartments. Mol Biol Cell 22: 216–229, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 941. Lorenzo O, Urbe S, Clague MJ. Analysis of phosphoinositide binding domain properties within the myotubularin-related protein MTMR3. J Cell Sci 118: 2005–2012, 2005 [DOI] [PubMed] [Google Scholar]
- 942. Lorenzo O, Urbe S, Clague MJ. Systematic analysis of myotubularins: heteromeric interactions, subcellular localisation and endosome related functions. J Cell Sci 119: 2953–2959, 2006 [DOI] [PubMed] [Google Scholar]
- 943. Love PE, Hayes SM. ITAM-mediated signaling by the T-cell antigen receptor. Cold Spring Harb Perspect Biol 2: a002485, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 944. Lowe CU, Terrey M, Mac LE. Organic-aciduria, decreased renal ammonia production, hydrophthalmos, and mental retardation; a clinical entity. AMA Am J Dis Child 83: 164–184, 1952 [DOI] [PubMed] [Google Scholar]
- 945. Lowe M. Structure and function of the Lowe syndrome protein OCRL1. Traffic 6: 711–719, 2005 [DOI] [PubMed] [Google Scholar]
- 946. Loyet KM, Kowalchyk JA, Chaudhary A, Chen J, Prestwitch GD, Martin TF. Specific binding of phosphatidylinositol 4,5-bisphosphate to calcium-dependent activator protein for secretion (CAPS), a potential phosphoinositide effector protein for regulated exocytosis. J Biol Chem 273: 8337–8343, 1998 [DOI] [PubMed] [Google Scholar]
- 947. Lu C, Peng YW, Shang J, Pawlyk BS, Yu F, Li T. The mammalian retinal degeneration B2 gene is not required for photoreceptor function and survival. Neuroscience 107: 35–41, 2001 [DOI] [PubMed] [Google Scholar]
- 948. Lu C, Vihtelic TS, Hyde DR, Li T. A neuronal-specific mammalian homolog of the Drosophila retinal degeneration B gene with expression restricted to the retina and dentate gyrus. J Neurosci 19: 7317–7325, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 949. Lu D, Sun HQ, Wang H, Barylko B, Fukata Y, Fukata M, Albanesi J, Yin HL. Phosphatidylinositol 4-kinase IIalpha is palmitoylated by Golgi-localized palmitoyl transferases in a cholesterol-dependent manner. J Biol Chem 287: 21856–21865, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 950. Lu Q, Yu J, Yan J, Wei Z, Zhang M. Structural basis of the myosin X PH1(N)-PH2-PH1(C) tandem as a specific and acute cellular PI(3,4,5)P(3) sensor. Mol Biol Cell 22: 4268–4278, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 951. Lukacs V, Thyagarajan B, Varnai P, Balla A, Balla T, Rohacs T. Dual regulation of TRPV1 by phosphoinositides. J Neurosci 27: 7070–7080, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 952. Lukinovic-Skudar V, Donlagic L, Banfic H, Visnjic D. Nuclear phospholipase C-beta1b activation during G2/M and late G1 phase in nocodazole-synchronized HL-60 cells. Biochim Biophys Acta 1733: 148–156, 2005 [DOI] [PubMed] [Google Scholar]
- 953. Lundby A, Akemann W, Knopfel T. Biophysical characterization of the fluorescent protein voltage probe VSFP2.3 based on the voltage-sensing domain of Ci-VSP. Eur Biophys J 39: 1625–1635, 2010 [DOI] [PubMed] [Google Scholar]
- 954. Luo HR, Huang YE, Chen JC, Saiardi A, Iijima M, Ye K, Huang Y, Nagata E, Devreotes P, Snyder SH. Inositol pyrophosphates mediate chemotaxis in Dictyostelium via pleckstrin homology domain-PtdIns(3,4,5)P3 interactions. Cell 114: 559–572, 2003 [DOI] [PubMed] [Google Scholar]
- 955. Luo J, Field SJ, Lee JY, Engelman JA, Cantley LC. The p85 regulatory subunit of phosphoinositide 3-kinase down-regulates IRS-1 signaling via the formation of a sequestration complex. J Cell Biol 170: 455–464, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 956. Luo N, West CC, Murga-Zamalloa CA, Sun L, Anderson RM, Wells CD, Weinreb RN, Travers JB, Khanna H, Sun Y. OCRL localizes to the primary cilium: a new role for cilia in Lowe syndrome. Hum Mol Genet 21: 3333–3344, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 957. Lykidis A, Jackson PD, Rock CO, Jackowski S. The role of CDP-diacylglycerol synthetase and phosphatidylinositol synthase activity levels in the regulation of cellular phosphatidylinositol content. J Biol Chem 272: 33402–33409, 1997 [DOI] [PubMed] [Google Scholar]
- 958. Lyon AM, Tesmer VM, Dhamsania VD, Thal DM, Gutierrez J, Chowdhury S, Suddala KC, Northup JK, Tesmer JJ. An autoinhibitory helix in the C-terminal region of phospholipase C-beta mediates Galphaq activation. Nat Struct Mol Biol 18: 999–1005, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 959. Ma H, Blake T, Chitnis A, Liu P, Balla T. Crucial role of phosphatidylinositol 4-kinase IIIalpha in development of zebrafish pectoral fin is linked to phosphoinositide 3-kinase and FGF signaling. J Cell Sci 122: 4303–4310, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 960. Ma HP, Saxena S, Warnock DG. Anionic phospholipids regulate native and expressed epithelial sodium channel (ENaC). J Biol Chem 277: 7641–7644, 2002 [DOI] [PubMed] [Google Scholar]
- 961. Ma R, Li WP, Rundle D, Kong J, Akbarali HI, Tsiokas L. PKD2 functions as an epidermal growth factor-activated plasma membrane channel. Mol Cell Biol 25: 8285–8298, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 962. Maag D, Maxwell MJ, Hardesty DA, Boucher KL, Choudhari N, Hanno AG, Ma JF, Snowman AS, Pietropaoli JW, Xu R, Storm PB, Saiardi A, Snyder SH, Resnick AC. Inositol polyphosphate multikinase is a physiologic PI3-kinase that activates Akt/PKB. Proc Natl Acad Sci USA 108: 1391–1396, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 963. Macara IG, Marinetti GV, Balduzzi PC. Transforming protein of avian sarcoma virus UR2 is associated with phosphatidylinositol kinase activity. Proc Natl Acad Sci USA 81: 2728–2732, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 964. Maccario H, Perera NM, Gray A, Downes CP, Leslie NR. Ubiquitination of PTEN (phosphatase and tensin homolog) inhibits phosphatase activity and is enhanced by membrane targeting and hyperosmotic stress. J Biol Chem 285: 12620–12628, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 965. MacDougall LK, Domin J, Waterfield MD. A family of phosphoinositide 3-kinases in Drosophila identifies a new mediator of signal transduction. Curr Biol 5: 1404–1415, 1995 [DOI] [PubMed] [Google Scholar]
- 966. MacDougall LK, Gagou ME, Leevers SJ, Hafen E, Waterfield MD. Targeted expression of the class II phosphoinositide 3-kinase in Drosophila melanogaster reveals lipid kinase-dependent effects on patterning and interactions with receptor signaling pathways. Mol Cell Biol 24: 796–808, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 967. Maddika S, Kavela S, Rani N, Palicharla VR, Pokorny JL, Sarkaria JN, Chen J. WWP2 is an E3 ubiquitin ligase for PTEN. Nat Cell Biol 13: 730–735, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 968. Maehama T, Dixon JE. PTEN: a tumour suppressor that functions as a phospholipid phosphatase. Trends Cell Biol 9: 125–128, 1999 [DOI] [PubMed] [Google Scholar]
- 969. Maehama T, Dixon JE. The tumor suppressor, PTEN/MMAC1, dephosphorylates the lipid second messenger, phosphatidylinositol 3,4,5-trisphosphate. J Biol Chem 273: 13375–13378, 1998 [DOI] [PubMed] [Google Scholar]
- 970. Maffucci T, Brancaccio A, Piccolo E, Stein RC, Falasca M. Insulin induces phosphatidylinositol-3-phosphate formation through TC10 activation. EMBO J 22: 4178–4189, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 971. Maffucci T, Cooke FT, Foster FM, Traer CJ, Fry MJ, Falasca M. Class II phosphoinositide 3-kinase defines a novel signaling pathway in cell migration. J Cell Biol 169: 789–799, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 972. Majerus PW, Connolly TM, Deckmyn H, Ross TS, Bross TE, Ishii H, Bansal VS, Wilson DB. The metabolism of phosphoinositide-derived messenger molecules. Science 234: 1519–1526, 1986 [DOI] [PubMed] [Google Scholar]
- 973. Majerus PW, Kisseleva MV, Norris FA. The role of phosphatases in inositol signaling reactions. J Biol Chem 274: 10669–10672, 1999 [DOI] [PubMed] [Google Scholar]
- 974. Malecz N, McCabe PC, Spaargaren C, Qiu R, Chuang Y, Symons M. Synaptojanin 2, a novel Rac1 effector that regulates clathrin-mediated endocytosis. Curr Biol 10: 1383–1386, 2000 [DOI] [PubMed] [Google Scholar]
- 975. Mallo GV, Espina M, Smith AC, Terebiznik MR, Aleman A, Finlay BB, Rameh LE, Grinstein S, Brumell JH. SopB promotes phosphatidylinositol 3-phosphate formation on Salmonella vacuoles by recruiting Rab5 and Vps34. J Cell Biol 182: 741–752, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 976. Manahan CL, Iglesias PA, Long Y, Devreotes PN. Chemoattractant signaling in dictyostelium discoideum. Annu Rev Cell Dev Biol 20: 223–253, 2004 [DOI] [PubMed] [Google Scholar]
- 977. Mandal M, Yan Z. Phosphatidylinositol (4,5)-bisphosphate regulation of N-methyl-d-aspartate receptor channels in cortical neurons. Mol Pharmacol 76: 1349–1359, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 978. Mandelker D, Gabelli SB, Schmidt-Kittler O, Zhu J, Cheong I, Huang CH, Kinzler KW, Vogelstein B, Amzel LM. A frequent kinase domain mutation that changes the interaction between PI3Kalpha and the membrane. Proc Natl Acad Sci USA 106: 16996–17001, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 979. Manford A, Xia T, Saxena AK, Stefan C, Hu F, Emr SD, Mao Y. Crystal structure of the yeast Sac1: implications for its phosphoinositide phosphatase function. EMBO J 29: 1489–1498, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 980. Manford AG, Stefan CJ, Yuan HL, Macgurn JA, Emr SD. ER-to-plasma membrane tethering proteins regulate cell signaling and ER morphology. Dev Cell 23: 1129–1140, 2012 [DOI] [PubMed] [Google Scholar]
- 981. Mani M, Lee SY, Lucast L, Cremona O, Di Paolo G, De Camilli P, Ryan TA. The dual phosphatase activity of synaptojanin1 is required for both efficient synaptic vesicle endocytosis and reavailability at nerve terminals. Neuron 56: 1004–1018, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 982. Mani T, Hennigan RF, Foster LA, Conrady DG, Herr AB, Ip W. FERM domain phosphoinositide binding targets merlin to the membrane and is essential for its growth-suppressive function. Mol Cell Biol 31: 1983–1996, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 983. Manji SS, Williams LH, Miller KA, Ooms LM, Bahlo M, Mitchell CA, Dahl HH. A mutation in synaptojanin 2 causes progressive hearing loss in the ENU-mutagenised mouse strain Mozart. PLoS One 6: e17607, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 984. Mankidy R, Hastings J, Thackeray JR. Distinct phospholipase C-gamma-dependent signaling pathways in the Drosophila eye and wing are revealed by a new small wing allele. Genetics 164: 553–563, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 985. Manning BD, Cantley LC. Rheb fills a GAP between TSC and TOR. Trends Biochem Sci 28: 573–576, 2003 [DOI] [PubMed] [Google Scholar]
- 986. Manning G, Plowman GD, Hunter T, Sudarsanam S. Evolution of protein kinase signaling from yeast to man. Trends Biochem Sci 27: 514–520, 2002 [DOI] [PubMed] [Google Scholar]
- 987. Manzoli L, Martelli AM, Billi AM, Faenza I, Fiume R, Cocco L. Nuclear phospholipase C: involvement in signal transduction. Prog Lipid Res 44: 185–206, 2005 [DOI] [PubMed] [Google Scholar]
- 988. Mao Y, Balkin DM, Zoncu R, Erdmann KS, Tomasini L, Hu F, Jin MM, Hodsdon ME, De Camilli P. A PH domain within OCRL bridges clathrin-mediated membrane trafficking to phosphoinositide metabolism. EMBO J 28: 1831–1842, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 989. Mao YS, Yamaga M, Zhu X, Wei Y, Sun HQ, Wang J, Yun M, Wang Y, Di Paolo G, Bennett M, Mellman I, Abrams CS, De Camilli P, Lu CY, Yin HL. Essential and unique roles of PIP5K-gamma and -alpha in Fcgamma receptor-mediated phagocytosis. J Cell Biol 184: 281–296, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 990. Maquenne L. Sur quelques derives de l'inosite. Cr hebd Seanc Acad Sci 104: 1719–1722, 1887 [Google Scholar]
- 991. Marcano AC, Burke B, Gungadoo J, Wallace C, Kaisaki PJ, Woon PY, Farrall M, Clayton D, Brown M, Dominiczak A, Connell JM, Webster J, Lathrop M, Caulfield M, Samani N, Gauguier D, Munroe PB. Genetic association analysis of inositol polyphosphate phosphatase-like 1 (INPPL1, SHIP2) variants with essential hypertension. J Med Genet 44: 603–605, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 992. Marcello MR, Evans JP. Multivariate analysis of male reproductive function in Inpp5b−/− mice reveals heterogeneity in defects in fertility, sperm-egg membrane interaction and proteolytic cleavage of sperm ADAMs. Mol Hum Reprod 16: 492–505, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 993. Marcus SL, Wenk MR, Steele-Mortimer O, Finlay BB. A synaptojanin-homologous region of Salmonella typhimurium SigD is essential for inositol phosphatase activity and Akt activation. FEBS Lett 494: 201–207, 2001 [DOI] [PubMed] [Google Scholar]
- 994. Margolis B, Li N, Koch A, Mohammadi M, Hurwitz DR, Zilberstein A, Ullrich A, Pawson T, Schlessinger J. The tyrosine phosphorylated carboxyterminus of the EGF receptor is a binding site for GAP and PLC-gamma. EMBO J 9: 4375–4380, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 995. Margolis B, Rhee SG, Felder S, Mervic M, Lyall R, Levitzki A, Ullrich A, Zilberstein A, Schlessinger J. EGF induces tyrosine phosphorylation of phospholipase C-II: a potential mechanism for EGF receptor signaling. Cell 57: 1101–1107, 1989 [DOI] [PubMed] [Google Scholar]
- 996. Marone R, Cmiljanovic V, Giese B, Wymann MP. Targeting phosphoinositide 3-kinase: moving towards therapy. Biochim Biophys Acta 1784: 159–185, 2008 [DOI] [PubMed] [Google Scholar]
- 997. Marquardt DL, Alongi JL, Walker LL. The phosphatidylinositol 3-kinase inhibitor wortmannin blocks mast cell exocytosis but not IL-6 production. J Immunol 156: 1942–1945, 1996 [PubMed] [Google Scholar]
- 998. Marques M, Kumar A, Poveda AM, Zuluaga S, Hernandez C, Jackson S, Pasero P, Carrera AC. Specific function of phosphoinositide 3-kinase beta in the control of DNA replication. Proc Natl Acad Sci USA 106: 7525–7530, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 999. Marsh DJ, Coulon V, Lunetta KL, Rocca-Serra P, Dahia PL, Zheng Z, Liaw D, Caron S, Duboue B, Lin AY, Richardson AL, Bonnetblanc JM, Bressieux JM, Cabarrot-Moreau A, Chompret A, Demange L, Eeles RA, Yahanda AM, Fearon ER, Fricker JP, Gorlin RJ, Hodgson SV, Huson S, Lacombe D, Eng C. Mutation spectrum and genotype-phenotype analyses in Cowden disease and Bannayan-Zonana syndrome, two hamartoma syndromes with germline PTEN mutation. Hum Mol Genet 7: 507–515, 1998 [DOI] [PubMed] [Google Scholar]
- 1000. Marshall JG, Booth JW, Stambolic V, Mak T, Balla T, Schreiber AD, Meyer T, Grinstein S. Restricted accumulation of phosphatidylinositol 3-kinase products in a plasmalemmal subdomain during Fc gamma receptor-mediated phagocytosis. J Cell Biol 153: 1369–1380, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1001. Martelli AM, Gilmour RS, Bertagnolo V, Neri LM, Manzoli L, Cocco L. Nuclear localization and signalling activity of phosphoinositidase C beta in Swiss 3T3 cells. Nature 358: 242–245, 1992 [DOI] [PubMed] [Google Scholar]
- 1002. Martin EL, Souza DG, Fagundes CT, Amaral FA, Assenzio B, Puntorieri V, Del Sorbo L, Fanelli V, Bosco M, Delsedime L, Pinho JF, Lemos VS, Souto FO, Alves-Filho JC, Cunha FQ, Slutsky AS, Ruckle T, Hirsch E, Teixeira MM, Ranieri VM. Phosphoinositide-3 kinase gamma activity contributes to sepsis and organ damage by altering neutrophil recruitment. Am J Respir Crit Care Med 182: 762–773, 2010 [DOI] [PubMed] [Google Scholar]
- 1003. Martin V, Guillermet-Guibert J, Chicanne G, Cabou C, Jandrot-Perrus M, Plantavid M, Vanhaesebroeck B, Payrastre B, Gratacap MP. Deletion of the p110beta isoform of phosphoinositide 3-kinase in platelets reveals its central role in Akt activation and thrombus formation in vitro and in vivo. Blood 115: 2008–2013, 2010 [DOI] [PubMed] [Google Scholar]
- 1004. Martin-Belmonte F, Gassama A, Datta A, Yu W, Rescher U, Gerke V, Mostov K. PTEN-mediated apical segregation of phosphoinositides controls epithelial morphogenesis through Cdc42. Cell 128: 383–397, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1005. Marwick JA, Caramori G, Casolari P, Mazzoni F, Kirkham PA, Adcock IM, Chung KF, Papi A. A role for phosphoinositol 3-kinase delta in the impairment of glucocorticoid responsiveness in patients with chronic obstructive pulmonary disease. J Allergy Clin Immunol 125: 1146–1153, 2010 [DOI] [PubMed] [Google Scholar]
- 1006. Matile P. Inositol deficiency resulting in death: an explanation of its occurrence in Neurospora crassa. Science 151: 86–88, 1966 [DOI] [PubMed] [Google Scholar]
- 1007. Matsuda M, Kanematsu T, Takeuchi H, Kukita T, Hirata M. Localization of a novel inositol 1,4,5-trisphosphate binding protein, p130 in rat brain. Neurosci Lett 257: 97–100, 1998 [DOI] [PubMed] [Google Scholar]
- 1008. Matsuda M, Paterson HF, Rodriguez R, Fensome AC, Ellis MV, Swann K, Katan M. Real time fluorescence imaging of PLC gamma translocation and its interaction with the epidermal growth factor receptor. J Cell Biol 153: 599–612, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1009. Matsuda M, Takeshita K, Kurokawa T, Sakata S, Suzuki M, Yamashita E, Okamura Y, Nakagawa A. Crystal structure of the cytoplasmic phosphatase and tensin homolog (PTEN)-like region of Ciona intestinalis voltage-sensing phosphatase provides insight into substrate specificity and redox regulation of the phosphoinositide phosphatase activity. J Biol Chem 286: 23368–23377, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1010. Matsui T, Yonemura S, Tsukita S. Activation of ERM proteins in vivo by Rho involves phosphatidyl-inositol 4-phosphate 5-kinase and not ROCK kinases. Curr Biol 9: 1259–1262, 1999 [DOI] [PubMed] [Google Scholar]
- 1011. Matsunaga K, Saitoh T, Tabata K, Omori H, Satoh T, Kurotori N, Maejima I, Shirahama-Noda K, Ichimura T, Isobe T, Akira S, Noda T, Yoshimori T. Two Beclin 1-binding proteins, Atg14L and Rubicon, reciprocally regulate autophagy at different stages. Nat Cell Biol 11: 385–396, 2009 [DOI] [PubMed] [Google Scholar]
- 1012. Matsushita Y, Ohya S, Suzuki Y, Itoda H, Kimura T, Yamamura H, Imaizumi Y. Inhibition of Kv1.3 potassium current by phosphoinositides and stromal-derived factor-1alpha in Jurkat T cells. Am J Physiol Cell Physiol 296: C1079–C1085, 2009 [DOI] [PubMed] [Google Scholar]
- 1013. Matzaris M, O'Malley CJ, Badger A, Speed CJ, Bird PI, Mitchell CA. Distinct membrane and cytosolic forms of inositol polyphosphate 5-phosphatase. II. Efficient membrane localization requires two discrete domains. J Biol Chem 273: 8256–8267, 1998 [DOI] [PubMed] [Google Scholar]
- 1014. Mayer BJ, Ren R, Clark KL, Baltimore D. A putative modular domain present in diverse signaling proteins. Cell 73: 629–630, 1993 [DOI] [PubMed] [Google Scholar]
- 1015. McConnachie G, Pass I, Walker SM, Downes CP. Interfacial kinetic analysis of the tumour suppressor phosphatase, PTEN: evidence for activation by anionic phospholipids. Biochem J 371: 947–955, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1016. McDonald B, Martin-Serrano J. No strings attached: the ESCRT machinery in viral budding and cytokinesis. J Cell Sci 122: 2167–2177, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1017. McEwen RK, Dove SK, Cooke FT, Painter GF, Holmes AB, Shisheva A, Ohya Y, Parker PJ, Michell RH. Complementation analysis in PtdInsP kinase-deficient yeast mutants demonstrates that Schizosaccharomyces pombe and murine Fab1p homologues are phosphatidylinositol 3-phosphate 5-kinases. J Biol Chem 274: 33905–33912, 1999 [DOI] [PubMed] [Google Scholar]
- 1018. McGee TP, Skinner HB, Whitters EA, Henry SA, Bankaitis VA. A phosphatidylinositol transfer protein controls the phosphatidylcholine content of yeast Golgi membranes. J Cell Biol 124: 273–287, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1019. McMahon HT, Boucrot E. Molecular mechanism and physiological functions of clathrin-mediated endocytosis. Nat Rev Mol Cell Biol 12: 517–533, 2011 [DOI] [PubMed] [Google Scholar]
- 1020. McOmish CE, Burrows E, Howard M, Scarr E, Kim D, Shin HS, Dean B, van den Buuse M, Hannan AJ. Phospholipase C-beta1 knockout mice exhibit endophenotypes modeling schizophrenia which are rescued by environmental enrichment and clozapine administration. Mol Psychiatry 13: 661–672, 2008 [DOI] [PubMed] [Google Scholar]
- 1021. McPhee F, Lowe G, Vaziri C, Downes CP. Phosphatidylinositol synthase and phosphatidylinositol/inositol exchange reactions in turkey erythrocyte membranes. Biochem J 275: 187–192, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1022. McPherson PS, Garcia EP, Slepnev VI, David C, Zhang X, Grabs D, Sossin WS, Bauerfeind R, Nemoto Y, De Camilli P. A presynaptic inositol-5-phosphatase. Nature 379: 353–357, 1996 [DOI] [PubMed] [Google Scholar]
- 1023. McPherson PS, Takei K, Schmid SL, De Camilli P. p145, a major Grb2-binding protein in brain, is co-localized with dynamin in nerve terminals where it undergoes activity-dependent dephosphorylation. J Biol Chem 269: 30132–30139, 1994 [PubMed] [Google Scholar]
- 1024. Mei J, Li Z, Gui JF. Cooperation of Mtmr8 with PI3K regulates actin filament modeling and muscle development in zebrafish. PLoS One 4: e4979, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1025. Mei J, Liu S, Li Z, Gui JF. Mtmr8 is essential for vasculature development in zebrafish embryos. BMC Dev Biol 10: 96, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1026. Meili R, Ellsworth C, Lee S, Reddy TB, Ma H, Firtel RA. Chemoattractant-mediated transient activation and membrane localization of Akt/PKB is required for efficient chemotaxis to cAMP in Dictyostelium. EMBO J 18: 2092–2105, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1027. Meisenhelder J, Suh PG, Rhee SG, Hunter T. Phospholipase C-gamma is a substrate for the PDGF and EGF receptor protein-tyrosine kinases in vivo and in vitro. Cell 57: 1109–1122, 1989 [DOI] [PubMed] [Google Scholar]
- 1028. Mellman DL, Anderson RA. A novel gene expression pathway regulated by nuclear phosphoinositides. Adv Enzyme Regul 49: 11–28, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1029. Mellman DL, Gonzales ML, Song C, Barlow CA, Wang P, Kendziorski C, Anderson RA. A PtdIns4,5P2-regulated nuclear poly(A) polymerase controls expression of select mRNAs. Nature 451: 1013–1017, 2008 [DOI] [PubMed] [Google Scholar]
- 1030. Mellman I, Nelson WJ. Coordinated protein sorting, targeting and distribution in polarized cells. Nat Rev Mol Cell Biol 9: 833–845, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1031. Menniti FS, Miller RN, Putney JW, Jr, Shears SB. Turnover of inositol polyphosphate pyrophosphates in pancreatoma cells. J Biol Chem 268: 3850–3856, 1993 [PubMed] [Google Scholar]
- 1032. Menniti FS, Miller RN, Putney JW, Jr, Shears SB. Turnover of inositol polyphosphates in pancreatoma cells. J Biol Chem 268: 3850–3856, 1993 [PubMed] [Google Scholar]
- 1033. Mercado J, Gordon-Shaag A, Zagotta WN, Gordon SE. Ca2+-dependent desensitization of TRPV2 channels is mediated by hydrolysis of phosphatidylinositol 4,5-bisphosphate. J Neurosci 30: 13338–13347, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1034. Meunier FA, Osborne SL, Hammond GR, Cooke FT, Parker PJ, Domin J, Schiavo G. Phosphatidylinositol 3-Kinase C2α is essential for ATP-dependent priming of neurosecretory granule exocytosis. Mol Biol Cell 16: 4841–4851, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1035. Meyer RD, Latz C, Rahimi N. Recruitment and activation of phospholipase Cgamma1 by vascular endothelial growth factor receptor-2 are required for tubulogenesis and differentiation of endothelial cells. J Biol Chem 278: 16347–16355, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1036. Meyers R, Cantley LC. Cloning and characterization of a wortmannin-sensitive human phosphatidylinositol 4-kinase. J Biol Chem 272: 4384–4390, 1997 [DOI] [PubMed] [Google Scholar]
- 1037. Michailidis IE, Helton TD, Petrou VI, Mirshahi T, Ehlers MD, Logothetis DE. Phosphatidylinositol-4,5-bisphosphate regulates NMDA receptor activity through alpha-actinin. J Neurosci 27: 5523–5532, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1038. Michailidis IE, Zhang Y, Yang J. The lipid connection-regulation of voltage-gated Ca2+ channels by phosphoinositides. Pflügers Arch 455: 147–155, 2007 [DOI] [PubMed] [Google Scholar]
- 1039. Michell B. Early steps along the road to inositol-lipid-based signalling. Trends Biochem Sci 20: 326–329, 1995 [DOI] [PubMed] [Google Scholar]
- 1040. Michell RH. Inositol phospholipids and cell surface receptor function. Biochim Biophys Acta 415: 81–147, 1975 [DOI] [PubMed] [Google Scholar]
- 1041. Michell RH. Is phosphotidylinositol really out of the calcium gate? Nature 296: 492–493, 1982 [DOI] [PubMed] [Google Scholar]
- 1042. Michell RH, Dove SK. A protein complex that regulates PtdIns(3,5)P2 levels. EMBO J 28: 86–87, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1043. Michell RH, Harwood JL, Coleman R, Hawthorne JN. Characteristics of rat liver phosphatidylinositol kinase and its presence in the plasma membrane. Biochim Biophys Acta 144: 649–658, 1967 [DOI] [PubMed] [Google Scholar]
- 1044. Michell RH, Hawthorne JN. The site of diphosphoinositide synthesis in rat liver. Biochem Biophys Res Commun 21: 333–338, 1965 [DOI] [PubMed] [Google Scholar]
- 1045. Michell RH, Heath VL, Lemmon MA, Dove SK. Phosphatidylinositol 3,5-bisphosphate: metabolism and cellular functions. Trends Biochem Sci 31: 52–63, 2005 [DOI] [PubMed] [Google Scholar]
- 1046. Michell RH, Kirk CJ, Jones LM, Downes CP, Creba JA. The stimulation of inositol lipid metabolism that accompanies calcium mobilization in stimulated cells: defined characteristics and unanswered questions. Philos Trans R Soc Lond B Biol Sci 296: 123–138, 1981 [DOI] [PubMed] [Google Scholar]
- 1047. Michell RH, Lapetina EG. Production of cyclic inositol phosphate in stimulated tissues. Nat New Biol 240: 258–260, 1972 [DOI] [PubMed] [Google Scholar]
- 1048. Miki H, Miura K, Takenawa T. N-WASP, a novel actin-depolymerizing protein, regulates the cortical cytoskeletal rearrangement in a PIP2-dependent manner downstream of tyrosine kinases. EMBO J 15: 5326–5335, 1996 [PMC free article] [PubMed] [Google Scholar]
- 1049. Mikitova V, Levine TP. Analysis of the key elements of FFAT-like motifs identifies new proteins that potentially bind VAP on the ER, including two AKAPs and FAPP2. PLoS One 7: e30455, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1050. Mikoshiba K. IP3 receptor/Ca2+ channel: from discovery to new signaling concepts. J Neurochem 102: 1426–1446, 2007 [DOI] [PubMed] [Google Scholar]
- 1051. Mikoshiba K. Role of IP3 receptor in development. Cell Calcium 49: 331–340, 2011 [DOI] [PubMed] [Google Scholar]
- 1052. Mikoshiba K, Fukuda M, Ibata K, Kabayama H, Mizutani A. Role of synaptotagmin, a Ca2+ and inositol polyphosphate binding protein, in neurotransmitter release and neurite outgrowth. Chem Phys Lipids 98: 59–67, 1999 [DOI] [PubMed] [Google Scholar]
- 1053. Miled N, Yan Y, Hon WC, Perisic O, Zvelebil M, Inbar Y, Schneidman-Duhovny D, Wolfson HJ, Backer JM, Williams RL. Mechanism of two classes of cancer mutations in the phosphoinositide 3-kinase catalytic subunit. Science 317: 239–242, 2007 [DOI] [PubMed] [Google Scholar]
- 1054. Miller S, Tavshanjian B, Oleksy A, Perisic O, Houseman BT, Shokat KM, Williams RL. Shaping development of autophagy inhibitors with the structure of the lipid kinase Vps34. Science 327: 1638–1642, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1055. Milligan SC, Alb JG, Jr, Elagina RB, Bankaitis VA, Hyde DR. The phosphatidylinositol transfer protein domain of Drosophila retinal degeneration B protein is essential for photoreceptor cell survival and recovery from light stimulation. J Cell Biol 139: 351–363, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1056. Milosevic I, Sorensen JB, Lang T, Krauss M, Nagy G, Haucke V, Jahn R, Neher E. Plasmalemmal phosphatidylinositol-4,5-bisphosphate level regulates the releasable vesicle pool size in chromaffin cells. J Neurosci 25: 2557–2565, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1057. Minagawa T, Ijuin T, Mochizuki Y, Takenawa T. Identification and characterization of a sac domain-containing phosphoinositide 5-phosphatase. J Biol Chem 276: 22011–22015, 2001 [DOI] [PubMed] [Google Scholar]
- 1058. Minogue S, Anderson JS, Waugh MG, dosSantos M, Corless S, Cramer R, Hsuan JJ. Cloning of a human type II phosphatidylinositol 4-kinase reveals a novel lipid kinase family. J Biol Chem 276: 16635–16640, 2001 [DOI] [PubMed] [Google Scholar]
- 1059. Minogue S, Chu KM, Westover EJ, Covey DF, Hsuan JJ, Waugh MG. Relationship between phosphatidylinositol 4-phosphate synthesis, membrane organization, and lateral diffusion of PI4KIIalpha at the trans-Golgi network. J Lipid Res 51: 2314–2324, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1060. Minogue S, Waugh MG, De Matteis MA, Stephens DJ, Berditchevski F, Hsuan JJ. Phosphatidylinositol 4-kinase is required for endosomal trafficking and degradation of the EGF receptor. J Cell Sci 119: 571–581, 2006 [DOI] [PubMed] [Google Scholar]
- 1061. Misawa H, Ohtsubo M, Copeland NG, Gilbert DJ, Jenkins NA, Yoshimura A. Cloning and characterization of a novel class II phosphoinositide 3-kinase containing C2 domain. Biochem Biophys Res Commun 244: 531–539, 1998 [DOI] [PubMed] [Google Scholar]
- 1062. Mishra SK, Keyel PA, Hawryluk MJ, Agostinelli NR, Watkins SC, Traub LM. Disabled-2 exhibits the properties of a cargo-selective endocytic clathrin adaptor. EMBO J 21: 4915–4926, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1063. Mishra SK, Watkins SC, Traub LM. The autosomal recessive hypercholesterolemia (ARH) protein interfaces directly with the clathrin-coat machinery. Proc Natl Acad Sci USA 99: 16099–16104, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1064. Misra S, Hurley JH. Crystal structure of a phosphatidylinositol 3-phosphate-specific membrane-targeting motif, the FYVE domain of Vps27p. Cell 97: 657–666, 1999 [DOI] [PubMed] [Google Scholar]
- 1065. Mitchell CA, Connoly TM, Majerus PW. Identification and isolation of a 75-kDa inositol polyphosphate-5-phosphatase from human platelets. J Biol Chem 264: 8873–8877, 1989 [PubMed] [Google Scholar]
- 1066. Mitchell CJ, Kelly MM, Blewitt M, Wilson JR, Biden TJ. Phospholipase C-gamma mediates the hydrolysis of phosphatidylinositol, but not of phosphatidylinositol 4,5-bisphoshate, in carbamylcholine-stimulated islets of langerhans. J Biol Chem 276: 19072–19077, 2001 [DOI] [PubMed] [Google Scholar]
- 1067. Mitchell J, Gutierrez J, Northup JK. Purification, characterization, and partial amino acid sequence of a G protein-activated phospholipase C from squid photoreceptors. J Biol Chem 270: 854–859, 1995 [DOI] [PubMed] [Google Scholar]
- 1068. Mitchell J, Wang X, Zhang G, Gentzsch M, Nelson DJ, Shears SB. An expanded biological repertoire for Ins(3,4,5,6)P4 through its modulation of ClC-3 function. Curr Biol 18: 1600–1605, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1069. Miyauchi K, Kim Y, Latinovic O, Morozov V, Melikyan GB. HIV enters cells via endocytosis and dynamin-dependent fusion with endosomes. Cell 137: 433–444, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1070. Miyoshi MA, Abe K, Emori Y. IP(3) receptor type 3 and PLCbeta2 are co-expressed with taste receptors T1R and T2R in rat taste bud cells. Chem Senses 26: 259–265, 2001 [DOI] [PubMed] [Google Scholar]
- 1071. Mizokami A, Kanematsu T, Ishibashi H, Yamaguchi T, Tanida I, Takenaka K, Nakayama KI, Fukami K, Takenawa T, Kominami E, Moss SJ, Yamamoto T, Nabekura J, Hirata M. Phospholipase C-related inactive protein is involved in trafficking of gamma2 subunit-containing GABA(A) receptors to the cell surface. J Neurosci 27: 1692–1701, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1072. Mizuno-Yamasaki E, Medkova M, Coleman J, Novick P. Phosphatidylinositol 4-phosphate controls both membrane recruitment and a regulatory switch of the Rab GEF Sec2p. Dev Cell 18: 828–840, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1073. Mizushima N, Komatsu M. Autophagy: renovation of cells and tissues. Cell 147: 728–741, 2011 [DOI] [PubMed] [Google Scholar]
- 1074. Mo G, Bernier LP, Zhao Q, Chabot-Dore AJ, Ase AR, Logothetis D, Cao CQ, Seguela P. Subtype-specific regulation of P2X3 and P2X2/3 receptors by phosphoinositides in peripheral nociceptors. Mol Pain 5: 47, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1075. Mochizuki Y, Majerus PW. Characterization of myotubularin-related protein 7 and its binding partner, myotubularin-related protein 9. Proc Natl Acad Sci USA 100: 9768–9773, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1076. Mochizuki Y, Takenawa T. Novel inositol polyphosphate 5-phosphatase localizes at membrane ruffles. J Biol Chem 274: 36790–36795, 1999 [DOI] [PubMed] [Google Scholar]
- 1077. Mohammadi M, Honegger AM, Rotin D, Fischer R, Bellot F, Li W, Dionne CA, Jaye M, Rubinstein M, Schlessinger J. A tyrosine-phosphorylated carboxy-terminal peptide of the fibroblast growth factor receptor (Flg) is a binding site for the SH2 domain of phospholipase C-gamma 1. Mol Cell Biol 11: 5068–5078, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1078. Monroe JG. ITAM-mediated tonic signalling through pre-BCR and BCR complexes. Nat Rev Immunol 6: 283–294, 2006 [DOI] [PubMed] [Google Scholar]
- 1079. Monserrate JP, York JD. Inositol phosphate synthesis and the nuclear processes they affect. Curr Opin Cell Biol 22: 365–373, 2010 [DOI] [PubMed] [Google Scholar]
- 1080. Moorhead AM, Jung JY, Smirnov A, Kaufer S, Scidmore MA. Multiple host proteins that function in phosphatidylinositol-4-phosphate metabolism are recruited to the chlamydial inclusion. Infect Immun 78: 1990–2007, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1081. Mora A, Komander D, Van Aalten DM, Alessi DR. PDK1, the master regulator of AGC kinase signal transduction. Semin Cell Dev Biol 15: 161–170, 2004 [DOI] [PubMed] [Google Scholar]
- 1082. Moradpour D, Penin F, Rice CM. Replication of hepatitis C virus. Nat Rev Microbiol 5: 453–463, 2007 [DOI] [PubMed] [Google Scholar]
- 1083. Moravcevic K, Mendrola JM, Schmitz KR, Wang YH, Slochower D, Janmey PA, Lemmon MA. Kinase associated-1 domains drive MARK/PAR1 kinases to membrane targets by binding acidic phospholipids. Cell 143: 966–977, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1084. Morita YS, Fukuda T, Sena CB, Yamaryo-Botte Y, McConville MJ, Kinoshita T. Inositol lipid metabolism in mycobacteria: biosynthesis and regulatory mechanisms. Biochim Biophys Acta 1810: 630–641, 2011 [DOI] [PubMed] [Google Scholar]
- 1085. Morita YS, Yamaryo-Botte Y, Miyanagi K, Callaghan JM, Patterson JH, Crellin PK, Coppel RL, Billman-Jacobe H, Kinoshita T, McConville MJ. Stress-induced synthesis of phosphatidylinositol 3-phosphate in mycobacteria. J Biol Chem 285: 16643–16650, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1086. Morrison DK, Kaplan DR, Rhee SG, Williams LT. Platelet-derived growth factor (PDGF)-dependent association of phospholipase C-gamma with the PDGF receptor signaling complex. Mol Cell Biol 10: 2359–2366, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1087. Mortier E, Wuytens G, Leenaerts I, Hannes F, Heung MY, Degeest G, David G, Zimmermann P. Nuclear speckles and nucleoli targeting by PIP2-PDZ domain interactions. EMBO J 24: 2556–2565, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1088. Mousley CJ, Davison JM, Bankaitis VA. Sec14 Like PITPs couple lipid metabolism with phosphoinositide synthesis to regulate Golgi functionality. Subcell Biochem 59: 271–287, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1089. Mousley CJ, Yuan P, Gaur NA, Trettin KD, Nile AH, Deminoff SJ, Dewar BJ, Wolpert M, Macdonald JM, Herman PK, Hinnebusch AG, Bankaitis VA. A sterol-binding protein integrates endosomal lipid metabolism with TOR signaling and nitrogen sensing. Cell 148: 702–715, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1090. Mu FT, Callaghan JM, Steele-Mortimer O, Stenmark H, Parton RG, Campbell PL, McCluskey J, Yeo JP, Tock EP, Toh BH. EEA1, an early endosome-associated protein. EEA1 is a conserved alpha-helical peripheral membrane protein flanked by cysteine “fingers” and contains a calmodulin-binding IQ motif. J Biol Chem 270: 13503–13511, 1995 [DOI] [PubMed] [Google Scholar]
- 1091. Mueller KL, Hoon MA, Erlenbach I, Chandrashekar J, Zuker CS, Ryba NJ. The receptors and coding logic for bitter taste. Nature 434: 225–229, 2005 [DOI] [PubMed] [Google Scholar]
- 1092. Mueller-Roeber B, Pical C. Inositol phospholipid metabolism in Arabidopsis. Characterized and putative isoforms of inositol phospholipid kinase and phosphoinositide-specific phospholipase C. Plant Physiol 130: 22–46, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1093. Munnik T, Testerink C. Plant phospholipid signaling: “in a nutshell”. J Lipid Res 50 Suppl: S260–265, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1094. Munugalavadla V, Borneo J, Ingram DA, Kapur R. p85alpha subunit of class IA PI-3 kinase is crucial for macrophage growth and migration. Blood 106: 103–109, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1095. Muraille E, Pesesse X, Kuntz C, Erneux C. Distribution of the src-homology-2-domain-containing inositol 5-phosphatase SHIP-2 in both non-haemopoietic and haemopoietic cells and possible involvement of SHIP-2 in negative signalling of B-cells. Biochem J 342: 697–705, 1999 [PMC free article] [PubMed] [Google Scholar]
- 1096. Murata Y, Iwasaki H, Sasaki M, Inaba K, Okamura Y. Phosphoinositide phosphatase activity coupled to an intrinsic voltage sensor. Nature 435: 1239–1243, 2005 [DOI] [PubMed] [Google Scholar]
- 1097. Murata Y, Okamura Y. Depolarization activates the phosphoinositide phosphatase Ci-VSP, as detected in Xenopus oocytes coexpressing sensors of PIP2. J Physiol 583: 875–889, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1098. Murphy TR, Vihtelic TS, Ile KE, Watson CT, Willer GB, Gregg RG, Bankaitis VA, Hyde DR. Phosphatidylinositol synthase is required for lens structural integrity and photoreceptor cell survival in the zebrafish eye. Exp Eye Res 93: 460–474, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1099. Murray DH, Tamm LK. Molecular mechanism of cholesterol- and polyphosphoinositide-mediated syntaxin clustering. Biochemistry 50: 9014–9022, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1100. Murray JT, Panaretou C, Stenmark H, Miaczynska M, Backer JM. Role of Rab5 in the recruitment of hVps34/p150 to the early endosome. Traffic 3: 416–427, 2002 [DOI] [PubMed] [Google Scholar]
- 1101. Mustelin T, Coggeshall KM, Isakov N, Altman A. T cell antigen receptor-mediated activation of phospholipase C requires tyrosine phosphorylation. Science 247: 1584–1587, 1990 [DOI] [PubMed] [Google Scholar]
- 1102. Myers MG, Jr, Backer JM, Sun XJ, Shoelson S, Hu P, Schlessinger J, Yoakim M, Schaffhausen B, White MF. IRS-1 activates phosphatidylinositol 3'-kinase by associating with src homology 2 domains of p85. Proc Natl Acad Sci USA 89: 10350–10354, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1103. Myers MP, Pass I, Batty IH, Van der Kaay J, Stolarov JP, Hemmings BA, Wigler MH, Downes CP, Tonks NK. The lipid phosphatase activity of PTEN is critical for its tumor supressor function. Proc Natl Acad Sci USA 95: 13513–13518, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1104. Myers MP, Stolarov JP, Eng C, Li J, Wang SI, Wigler MH, Parsons R, Tonks NK. P-TEN, the tumor suppressor from human chromosome 10q23, is a dual-specificity phosphatase. Proc Natl Acad Sci USA 94: 9052–9057, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1105. Nagano K, Fukami K, Minagawa T, Watanabe Y, Ozaki C, Takenawa T. A novel phospholipase C delta4 (PLCdelta4) splice variant as a negative regulator of PLC. J Biol Chem 274: 2872–2879, 1999 [DOI] [PubMed] [Google Scholar]
- 1106. Nakagawa T, Goto K, Kondo H. Cloning and characterization of a 92 kDa soluble phosphatidylinositol 4-kinase. Biochem J 320: 643–649, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1107. Nakagawa T, Goto K, Kondo H. Cloning, expression and localization of 230 kDa phosphatidylinositol 4-kinase. J Biol Chem 271: 12088–12094, 1996 [DOI] [PubMed] [Google Scholar]
- 1108. Nakahara M, Shimozawa M, Nakamura Y, Irino Y, Morita M, Kudo Y, Fukami K. A novel phospholipase C, PLC(eta)2, is a neuron-specific isozyme. J Biol Chem 280: 29128–29134, 2005 [DOI] [PubMed] [Google Scholar]
- 1109. Nakamura Y, Fukami K, Yu H, Takenaka K, Kataoka Y, Shirakata Y, Nishikawa S, Hashimoto K, Yoshida N, Takenawa T. Phospholipase Cdelta1 is required for skin stem cell lineage commitment. EMBO J 22: 2981–2991, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1110. Nakamura Y, Hamada Y, Fujiwara T, Enomoto H, Hiroe T, Tanaka S, Nose M, Nakahara M, Yoshida N, Takenawa T, Fukami K. Phospholipase C-delta1 and -delta3 are essential in the trophoblast for placental development. Mol Cell Biol 25: 10979–10988, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1111. Nakamura Y, Ichinohe M, Hirata M, Matsuura H, Fujiwara T, Igarashi T, Nakahara M, Yamaguchi H, Yasugi S, Takenawa T, Fukami K. Phospholipase C-delta1 is an essential molecule downstream of Foxn1, the gene responsible for the nude mutation, in normal hair development. FASEB J 22: 841–849, 2008 [DOI] [PubMed] [Google Scholar]
- 1112. Nakanishi S, Catt KJ, Balla T. A wortmannin-sensitive phosphatidylinositol 4-kinase that regulates hormone-sensitive pools of inositolphospholipids. Proc Natl Acad Sci USA 92: 5317–5321, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1113. Nakano-Kobayashi A, Yamazaki M, Unoki T, Hongu T, Murata C, Taguchi R, Katada T, Frohman MA, Yokozeki T, Kanaho Y. Role of activation of PIP5Kgamma661 by AP-2 complex in synaptic vesicle endocytosis. EMBO J 26: 1105–1116, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1114. Nakatsu F, Baskin JM, Chung J, Tanner LB, Shui G, Lee SY, Pirruccello M, Haio M, Ingolia NT, Wenk MR, De Camilli P. PtdIns4P synthesis by PI4KIIIa at the plasma membrane and its impact on plasma membrane identity. J Cell Biol. In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1115. Nakatsu F, Perera RM, Lucast L, Zoncu R, Domin J, Gertler FB, Toomre D, De Camilli P. The inositol 5-phosphatase SHIP2 regulates endocytic clathrin-coated pit dynamics. J Cell Biol 190: 307–315, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1116. Nanberg E, Rozengurt E. Temporal relationship between inositol polyphosphate formation and increases in cytosolic Ca2+ in quiescent 3T3 cells stimulated by platelet-dervived growth factor, bombesin and vasopressin. EMBO J 7: 2741–2747, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1117. Nandurkar HH, Caldwell KK, Whisstock JC, Layton MJ, Gaudet EA, Norris FA, Majerus PW, Mitchell CA. Characterization of an adapter subunit to a phosphatidylinositol (3)P 3-phosphatase: identification of a myotubularin-related protein lacking catalytic activity. Proc Natl Acad Sci USA 98: 9499–9504, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1118. Nandurkar HH, Layton M, Laporte J, Selan C, Corcoran L, Caldwell KK, Mochizuki Y, Majerus PW, Mitchell CA. Identification of myotubularin as the lipid phosphatase catalytic subunit associated with the 3-phosphatase adapter protein, 3-PAP. Proc Natl Acad Sci USA 100: 8660–8665, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1119. Narkis G, Ofir R, Landau D, Manor E, Volokita M, Hershkowitz R, Elbedour K, Birk OS. Lethal contractural syndrome type 3 (LCCS3) is caused by a mutation in PIP5K1C, which encodes PIPKI gamma of the phophatidylinsitol pathway. Am J Hum Genet 81: 530–539, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1120. Naslavsky N, Caplan S. EHD proteins: key conductors of endocytic transport. Trends Cell Biol 21: 122–131, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1121. Nasuhoglu C, Feng S, Mao J, Yamamoto M, Yin HL, Earnest S, Barylko B, Albanesi JP, Hilgemann DW. Nonradioactive analysis of phosphatidylinositides and other anionic phospholipids by anion-exchange high-performance liquid chromatography with suppressed conductivity detection. Anal Biochem 301: 243–254, 2002 [DOI] [PubMed] [Google Scholar]
- 1122. Naughtin MJ, Sheffield DA, Rahman P, Hughes WE, Gurung R, Stow JL, Nandurkar HH, Dyson JM, Mitchell CA. The myotubularin phosphatase MTMR4 regulates sorting from early endosomes. J Cell Sci 123: 3071–3083, 2010 [DOI] [PubMed] [Google Scholar]
- 1123. Nemoto Y, Arribas M, Haffner C, DeCamilli P. Synaptojanin 2, a novel synaptojanin isoform with a distinct targeting domain and expression pattern. J Biol Chem 272: 30817–30821, 1997 [DOI] [PubMed] [Google Scholar]
- 1124. Nemoto Y, De Camilli P. Recruitment of an alternatively spliced form of synaptojanin 2 to mitochondria by the interaction with the PDZ domain of a mitochondrial outer membrane protein. EMBO J 18: 2991–3006, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1125. Nemoto Y, Kearns BG, Wenk MR, Chen H, Mori K, Alb JG, Jr, De Camilli P, Bankaitis VA. Functional characterization of a mammalian Sac1 and mutants exhibiting substrate-specific defects in phosphoinositide phosphatase activity. J Biol Chem 275: 34293–34305, 2000 [DOI] [PubMed] [Google Scholar]
- 1126. Nemoto Y, Wenk MR, Watanabe M, Daniell L, Murakami T, Ringstad N, Yamada H, Takei K, De Camilli P. Identification and characterization of a synaptojanin 2 splice isoform predominantly expressed in nerve terminals. J Biol Chem 276: 41133–41142, 2001 [DOI] [PubMed] [Google Scholar]
- 1127. Neukomm LJ, Nicot AS, Kinchen JM, Almendinger J, Pinto SM, Zeng S, Doukoumetzidis K, Tronchere H, Payrastre B, Laporte JF, Hengartner MO. The phosphoinositide phosphatase MTM-1 regulates apoptotic cell corpse clearance through CED-5-CED-12 in C. elegans. Development 138: 2003–2014, 2011 [DOI] [PubMed] [Google Scholar]
- 1128. Newell-Litwa K, Salazar G, Smith Y, Faundez V. Roles of BLOC-1 and adaptor protein-3 complexes in cargo sorting to synaptic vesicles. Mol Biol Cell 20: 1441–1453, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1129. Nicot AS, Fares H, Payrastre B, Chisholm AD, Labouesse M, Laporte J. The phosphoinositide kinase PIKfyve/Fab1p regulates terminal lysosome maturation in Caenorhabditis elegans. Mol Biol Cell 17: 3062–3074, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1130. Niebuhr K, Giuriato S, Pedron T, Philpott DJ, Gaits F, Sable J, Sheetz MP, Parsot C, Sansonetti PJ, Payrastre B. Conversion of PtdIns(4,5)P(2) into PtdIns(5)P by the S. flexneri effector IpgD reorganizes host cell morphology. EMBO J 21: 5069–5078, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1131. Niggli V. A membrane-permeant ester of phosphatidylinositol 3,4, 5-trisphosphate [PIP(3)] is an activator of human neutrophil migration. FEBS Lett 473: 217–221, 2000 [DOI] [PubMed] [Google Scholar]
- 1132. Niggli V, Adunyah ES, Carafoli E. Acidic phospholipids, unsaturated fatty acids, and limited proteolysis mimic the effect of calmodulin on the purified erythrocyte Ca2+-ATPase. J Biol Chem 256: 8588–8592, 1981 [PubMed] [Google Scholar]
- 1133. Nikawa J, Kodaki T, Yamashita S. Primary structure and disruption of the phosphatidylinositol synthase gene of Saccharomyces cerevisiae. J Biol Chem 262: 4876–4881, 1987 [PubMed] [Google Scholar]
- 1134. Nikawa J, Yamashita S. Molecular cloning of the gene encoding CDPdiacylglycerol-inositol 3-phosphatidyl transferase in Saccharomyces cerevisiae. Eur J Biochem 143: 251–256, 1984 [DOI] [PubMed] [Google Scholar]
- 1135. Nikawa J, Yamashita S. Yeast mutant defective in synthesis of phosphatidylinositol. Isolation and characterization of a CDP diacylglycerol–inositol 3-phosphatidyltransferase Km mutant. Eur J Biochem 125: 445–451, 1982 [DOI] [PubMed] [Google Scholar]
- 1136. Nile AH, Bankaitis VA, Grabon A. Mammalian diseases of phosphatidylinositol transfer proteins and their homologs. Clin Lipidol 5: 867–897, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1137. Nilius B, Mahieu F, Prenen J, Janssens A, Owsianik G, Vennekens R, Voets T. The Ca2+-activated cation channel TRPM4 is regulated by phosphatidylinositol 4,5-biphosphate. EMBO J 25: 467–478, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1138. Nilius B, Owsianik G, Voets T. Transient receptor potential channels meet phosphoinositides. EMBO J 27: 2809–2816, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1139. Nishibe S, Wahl MI, Hernandez-Sotomayor SM, Tonks NK, Rhee SG, Carpenter G. Increase of the catalytic activity of phospholipase C-gamma 1 by tyrosine phosphorylation. Science 250: 1253–1256, 1990 [DOI] [PubMed] [Google Scholar]
- 1140. Nishio M, Watanabe KI, Sasaki J, Taya C, Takasuga S, Iizuka R, Balla T, Yamazaki M, Watanabe H, Itoh R, Kuroda S, Horie Y, Forster I, Mak TW, Yonekawa H, Penninger JM, Kanaho Y, Suzuki A, Sasaki T. Control of cell polarity and motility by the PtdIns(3,4,5)P(3) phosphatase SHIP1. Nat Cell Biol 9: 36–44, 2007 [DOI] [PubMed] [Google Scholar]
- 1141. Nishizuka Y. The molecular heterogeneity of protein kinase C and its implications for cellular regulation. Nature 34: 661–665, 1988 [DOI] [PubMed] [Google Scholar]
- 1142. Nishizuka Y. The role of protein kinase C in cell surface signal transduction and tumour promotion. Nature 308: 693–698, 1984 [DOI] [PubMed] [Google Scholar]
- 1143. Nobukuni T, Joaquin M, Roccio M, Dann SG, Kim SY, Gulati P, Byfield MP, Backer JM, Natt F, Bos JL, Zwartkruis FJ, Thomas G. Amino acids mediate mTOR/raptor signaling through activation of class 3 phosphatidylinositol 3OH-kinase. Proc Natl Acad Sci USA 102: 14238–14243, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1144. Nomikos M, Elgmati K, Theodoridou M, Calver BL, Nounesis G, Swann K, Lai FA. Phospholipase Czeta binding to PtdIns(4,5)P2 requires the XY-linker region. J Cell Sci 124: 2582–2590, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1145. Nomikos M, Elgmati K, Theodoridou M, Georgilis A, Gonzalez-Garcia JR, Nounesis G, Swann K, Lai FA. Novel regulation of PLCzeta activity via its XY-linker. Biochem J 438: 427–432, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1146. Norden AG, Lapsley M, Igarashi T, Kelleher CL, Lee PJ, Matsuyama T, Scheinman SJ, Shiraga H, Sundin DP, Thakker RV, Unwin RJ, Verroust P, Moestrup SK. Urinary megalin deficiency implicates abnormal tubular endocytic function in Fanconi syndrome. J Am Soc Nephrol 13: 125–133, 2002 [DOI] [PubMed] [Google Scholar]
- 1147. Norris FA, Atkins RC, Majerus PW. The cDNA cloning and characterization of inositol polyphosphate 4-phosphatase type II. Evidence for conserved alternative splicing in the 4-phosphatase family. J Biol Chem 272: 23859–23864, 1997 [DOI] [PubMed] [Google Scholar]
- 1148. Norris FA, Atkins RC, Majerus PW. Inositol polyphosphate 4-phosphatase is inactivated by calpain-mediated proteolysis in stimulated human platelets. J Biol Chem 272: 10987–10989, 1997 [DOI] [PubMed] [Google Scholar]
- 1149. Norris FA, Auethavekiat V, Majerus PW. The isolation and characterization of cDNA encoding human and rat brain inositol polyphosphate 4-phosphatase. J Biol Chem 270: 16128–16133, 1995 [DOI] [PubMed] [Google Scholar]
- 1150. Norris FA, Majerus PW. Hydrolysis of phosphatidylinositol 3,4-biphosphate by inositol polyphosphate 4-phosphatase isolated by affinity elution chromatography. J Biol Chem 269: 8716–8720, 1994 [PubMed] [Google Scholar]
- 1151. Norris FA, Wilson MP, Wallis TS, Galyov EE, Majerus PW. SopB, a protein required for virulence of Salmonella dublin, is an inositol phosphate phosphatase. Proc Natl Acad Sci USA 95: 14057–14059, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1152. Novick P, Osmond BC, Botstein D. Suppressors of yeast actin mutations. Genetics 121: 659–674, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1153. Nystuen A, Legare ME, Shultz LD, Frankel WN. A null mutation in inositol polyphosphate 4-phosphatase type I causes selective neuronal loss in weeble mutant mice. Neuron 32: 203–212, 2001 [DOI] [PubMed] [Google Scholar]
- 1154. O'Connell RM, Chaudhuri AA, Rao DS, Baltimore D. Inositol phosphatase SHIP1 is a primary target of miR-155. Proc Natl Acad Sci USA 106: 7113–7118, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1155. O'Malley CJ, McColl BK, Kong AM, Ellis SL, Wijayaratnam AP, Sambrook J, Mitchell CA. Mammalian inositol polyphosphate 5-phosphatase II can compensate for the absence of all three yeast Sac1-like-domain-containing 5-phosphatases. Biochem J 355: 805–817, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1156. Oak JS, Chen J, Peralta RQ, Deane JA, Fruman DA. The p85beta regulatory subunit of phosphoinositide 3-kinase has unique and redundant functions in B cells. Autoimmunity 42: 447–458, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1157. Obara K, Ohsumi Y. Atg14: a key player in orchestrating autophagy. Int J Cell Biol 2011: 713435, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1158. Odom AR, Stahlberg A, Wente SR, York JD. A role for nuclear inositol 1,4,5-trisphosphate kinase in transcriptional control. Science 287: 2026–2029, 2000 [DOI] [PubMed] [Google Scholar]
- 1159. Odorizzi G, Babst M, Emr SD. Fab1p PtdIns(3)P 5-kinase function essential for protein sorting in the multivesicular body. Cell 95: 847–858, 1998 [DOI] [PubMed] [Google Scholar]
- 1160. Odriozola L, Singh G, Hoang T, Chan AM. Regulation of PTEN activity by its carboxyl-terminal autoinhibitory domain. J Biol Chem 282: 23306–23315, 2007 [DOI] [PubMed] [Google Scholar]
- 1161. Oestreich EA, Wang H, Malik S, Kaproth-Joslin KA, Blaxall BC, Kelley GG, Dirksen RT, Smrcka AV. Epac-mediated activation of phospholipase C(epsilon) plays a critical role in beta-adrenergic receptor-dependent enhancement of Ca2+ mobilization in cardiac myocytes. J Biol Chem 282: 5488–5495, 2007 [DOI] [PubMed] [Google Scholar]
- 1162. Ojala PJ, Paavilainen V, Lappalainen P. Identification of yeast cofilin residues specific for actin monomer and PIP2 binding. Biochemistry 40: 15562–15569, 2001 [DOI] [PubMed] [Google Scholar]
- 1163. Okamura Y, Dixon JE. Voltage-sensing phosphatase: its molecular relationship with PTEN. Physiology 26: 6–13, 2011 [DOI] [PubMed] [Google Scholar]
- 1164. Okayama Y, Tkaczyk C, Metcalfe DD, Gilfillan AM. Comparison of Fc epsilon RI- and Fc gamma RI-mediated degranulation and TNF-alpha synthesis in human mast cells: selective utilization of phosphatidylinositol-3-kinase for Fc gamma RI-induced degranulation. Eur J Immunol 33: 1450–1459, 2003 [DOI] [PubMed] [Google Scholar]
- 1165. Okkenhaug K, Bilancio A, Farjot G, Priddle H, Sancho S, Peskett E, Pearce W, Meek SE, Salpekar A, Waterfield MD, Smith AJ, Vanhaesebroeck B. Impaired B and T cell antigen receptor signaling in p110delta PI 3-kinase mutant mice. Science 297: 1031–1034, 2002 [DOI] [PubMed] [Google Scholar]
- 1166. Olivos-Glander IM, Janne PA, Nussbaum RL. The oculocerebrorenal syndrome gene product is a 105-kD protein localized to the Golgi complex. Am J Hum Genet 57: 817–823, 1995 [PMC free article] [PubMed] [Google Scholar]
- 1167. Olkkonen VM, Lehto M. Oxysterols and oxysterol binding proteins: role in lipid metabolism and atherosclerosis. Ann Med 36: 562–572, 2004 [DOI] [PubMed] [Google Scholar]
- 1168. Olsen HL, Hoy M, Zhang W, Bertorello AM, Bokvist K, Capito K, Efanov AM, Meister B, Thams P, Yang sN, Rorsrman P, Berggren PO, Gromada J. Phosphatidylinositol 4-kinase serves as a metabolic sensor and regulates priming of secretory granules in pancreatic beta cells. Proc Natl Acad Sci USA 100: 5187–5192, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1169. Ombrello MJ, Remmers EF, Sun G, Freeman AF, Datta S, Torabi-Parizi P, Subramanian N, Bunney TD, Baxendale RW, Martins MS, Romberg N, Komarow H, Aksentijevich I, Kim HS, Ho J, Cruse G, Jung MY, Gilfillan AM, Metcalfe DD, Nelson C, O'Brien M, Wisch L, Stone K, Douek DC, Gandhi C, Wanderer AA, Lee H, Nelson SF, Shianna KV, Cirulli ET, Goldstein DB, Long EO, Moir S, Meffre E, Holland SM, Kastner DL, Katan M, Hoffman HM, Milner JD. Cold Urticaria, immunodeficiency, and autoimmunity related to PLCG2 deletions. N Engl J Med 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1170. Ong CJ, Ming-Lum A, Nodwell M, Ghanipour A, Yang L, Williams DE, Kim J, Demirjian L, Qasimi P, Ruschmann J, Cao LP, Ma K, Chung SW, Duronio V, Andersen RJ, Krystal G, Mui AL. Small-molecule agonists of SHIP1 inhibit the phosphoinositide 3-kinase pathway in hematopoietic cells. Blood 110: 1942–1949, 2007 [DOI] [PubMed] [Google Scholar]
- 1171. Ono F, Nakagawa T, Saito S, Owada Y, Sakagami H, Goto K, Suzuki M, Matsuno S, Kondo H. A novel class II phosphoinositide 3-kinase predominantly expressed in the liver and its enhanced expression during liver regeneration. J Biol Chem 273: 7731–7736, 1998 [DOI] [PubMed] [Google Scholar]
- 1172. Ooms LM, Fedele CG, Astle MV, Ivetac I, Cheung V, Pearson RB, Layton MJ, Forrai A, Nandurkar HH, Mitchell CA. The inositol polyphosphate 5-phosphatase, PIPP, is a novel regulator of phosphoinositide 3-kinase-dependent neurite elongation. Mol Biol Cell 17: 607–622, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1173. Oppelt A, Lobert VH, Haglund K, Mackey AM, Rameh LE, Liestol K, Oliver Schink K, Marie Pedersen N, Wenzel EM, Haugsten EM, Brech A, Erik Rusten T, Stenmark H, Wesche J. Production of phosphatidylinositol 5-phosphate via PIKfyve and MTMR3 regulates cell migration. EMBO Rep 14: 57–64, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1174. Oron Y, Lowe M, Selinger Z. Incorporation of inorganic [32P] phosphate into rat parotid phosphatidylinositol. Induction through activation of alpha adrenergic and cholinergic receptors and relation to K+ release. Mol Pharmacol 11: 79–86, 1975 [PubMed] [Google Scholar]
- 1175. Osborne MA, Zenner G, Lubinus M, Zhang X, Songyang Z, Cantley LC, Majerus P, Burn P, Kochan JP. The inositol 5'-phosphatase SHIP binds to immunoreceptor signaling motifs and responds to high affinity IgE receptor aggregation. J Biol Chem 271: 29271–29278, 1996 [DOI] [PubMed] [Google Scholar]
- 1176. Osborne SL, Thomas CL, Gschmeissner S, Schiavo G. Nuclear PtdIns(4,5)P2 assembles in a mitotically regulated particle involved in pre-mRNA splicing. J Cell Sci 114: 2501–2511, 2001 [DOI] [PubMed] [Google Scholar]
- 1177. Osborne SL, Wallis TP, Jimenez JL, Gorman JJ, Meunier FA. Identification of secretory granule phosphatidylinositol 4,5-bisphosphate-interacting proteins using an affinity pulldown strategy. Mol Cell Proteomics 6: 1158–1169, 2007 [DOI] [PubMed] [Google Scholar]
- 1178. Otsu M, Hiles I, Gout I, Fry MJ, Ruiz-Larrea F, Panayotou G, Thompson A, Dhand R, Hsuan JJ, Totty NF, Smith AD, Morgan SJ, Courtneidge SA, Parker PJ, Waterfield MD. Characterization of two 85 kDa proteins that associate with receptor tyrosine kinases, middle-T/pp60c-src complexes, and PI3-kinase. Cell 65: 91–104, 1991 [DOI] [PubMed] [Google Scholar]
- 1179. Otsu M, Urade R, Kito M, Omura F, Kikuchi M. A possible role of ER-60 protease in the degradation of misfolded proteins in the endoplasmic reticulum. J Biol Chem 270: 14958–14961, 1995 [DOI] [PubMed] [Google Scholar]
- 1180. Otsuguro K, Tang J, Tang Y, Xiao R, Freichel M, Tsvilovskyy V, Ito S, Flockerzi V, Zhu MX, Zholos AV. Isoform-specific inhibition of TRPC4 channel by phosphatidylinositol 4,5-bisphosphate. J Biol Chem 283: 10026–10036, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1181. Otsuki M, Fukami K, Kohno T, Yokota J, Takenawa T. Identification and characterization of a new phospholipase C-like protein, PLC-L(2). Biochem Biophys Res Commun 266: 97–103, 1999 [DOI] [PubMed] [Google Scholar]
- 1182. Oudit GY, Crackower MA, Eriksson U, Sarao R, Kozieradzki I, Sasaki T, Irie-Sasaki J, Gidrewicz D, Rybin VO, Wada T, Steinberg SF, Backx PH, Penninger JM. Phosphoinositide 3-kinase gamma-deficient mice are protected from isoproterenol-induced heart failure. Circulation 108: 2147–2152, 2003 [DOI] [PubMed] [Google Scholar]
- 1183. Ozato-Sakurai N, Fujita A, Fujimoto T. The distribution of phosphatidylinositol 4,5-bisphosphate in acinar cells of rat pancreas revealed with the freeze-fracture replica labeling method. PLoS One 6: e23567, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1184. Pacold ME, Suire S, Perisic O, Lara-Gonzalez S, Davis CT, Walker EH, Hawkins PT, Stephens L, Eccleston JF, Williams RL. Crystal structure and functional analysis of Ras binding to its effector phosphoinositide 3-kinase gamma. Cell 103: 931–943, 2000 [DOI] [PubMed] [Google Scholar]
- 1185. Padron D, Wang YJ, Yamamoto M, Yin H, Roth MG. Phosphatidylinositol phosphate 5-kinase Ibeta recruits AP-2 to the plasma membrane and regulates rates of constitutive endocytosis. J Cell Biol 162: 693–701, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1186. Pagliarini DJ, Wiley SE, Kimple ME, Dixon JR, Kelly P, Worby CA, Casey PJ, Dixon JE. Involvement of a mitochondrial phosphatase in the regulation of ATP production and insulin secretion in pancreatic beta cells. Mol Cell 19: 197–207, 2005 [DOI] [PubMed] [Google Scholar]
- 1187. Pagliarini DJ, Worby CA, Dixon JE. A PTEN-like phosphatase with a novel substrate specificity. J Biol Chem 279: 38590–38596, 2004 [DOI] [PubMed] [Google Scholar]
- 1188. Pan W, Pham VN, Stratman AN, Castranova D, Kamei M, Kidd KR, Lo BD, Shaw KM, Torres-Vazquez J, Mikelis CM, Gutkind JS, Davis GE, Weinstein BM. CDP-diacylglycerol synthetase-controlled phosphoinositide availability limits VEGFA signaling and vascular morphogenesis. Blood 120: 489–498, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1189. Panaretou C, Domin J, Cockcroft S, Waterfield MD. Characterization of p150, an adaptor protein for the human phosphatidylinositol (PtdIns) 3-kinase. Substrate presentation by phosphatidylinositol transfer protein to the p150/PtdIns 3-kinase complex. J Biol Chem 272: 2477–2485, 1997 [DOI] [PubMed] [Google Scholar]
- 1190. Pang H, Flinn R, Patsialou A, Wyckoff J, Roussos ET, Wu H, Pozzuto M, Goswami S, Condeelis JS, Bresnick AR, Segall JE, Backer JM. Differential enhancement of breast cancer cell motility and metastasis by helical and kinase domain mutations of class IA phosphoinositide 3-kinase. Cancer Res 69: 8868–8876, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1191. Pang ZP, Sudhof TC. Cell biology of Ca2+-triggered exocytosis. Curr Opin Cell Biol 22: 496–505, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1192. Papp B, Sarkadi B, Enyedi A, Caride AJ, Penniston JT, Gardos G. Functional domains of the in situ red cell membrane calcium pump revealed by proteolysis and monoclonal antibodies. Possible sites for regulation by calpain and acidic lipids. J Biol Chem 264: 4577–4582, 1989 [PubMed] [Google Scholar]
- 1193. Parekh AB, Putney JW., Jr Store-operated calcium channels. Phys Rev 85: 757–810, 2005 [DOI] [PubMed] [Google Scholar]
- 1194. Park D, Jhon DY, Kriz R, Knopf J, Rhee SG. Cloning, sequencing, expression and Gq-independent activation of phospholipase C-beta2. J Biol Chem 267: 16048–16055, 1992 [PubMed] [Google Scholar]
- 1195. Park D, Jhon DY, Lee CW, Lee KH,. Activation of phospholipase C isozymes by G protein betagamma subunits. J Biol Chem 268: 4573–4576, 1993 [PubMed] [Google Scholar]
- 1196. Park D, Jhon DY, Lee CW, Ryu SH, Rhee SG. Removal of the carboxyl-terminal region of phospholipase C-beta 1 by calpain abolishes activation by G alpha q. J Biol Chem 268: 3710–3714, 1993 [PubMed] [Google Scholar]
- 1197. Park DJ, Rho HW, Rhee SG. CD3 stimulation causes phosphorylation of phospholipase C-gamma 1 on serine and tyrosine residues in a human T-cell line. Proc Natl Acad Sci USA 88: 5453–5456, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1198. Parsons DW, Jones S, Zhang X, Lin JC, Leary RJ, Angenendt P, Mankoo P, Carter H, Siu IM, Gallia GL, Olivi A, McLendon R, Rasheed BA, Keir S, Nikolskaya T, Nikolsky Y, Busam DA, Tekleab H, Diaz LA, Jr, Hartigan J, Smith DR, Strausberg RL, Marie SK, Shinjo SM, Yan H, Riggins GJ, Bigner DD, Karchin R, Papadopoulos N, Parmigiani G, Vogelstein B, Velculescu VE, Kinzler KW. An integrated genomic analysis of human glioblastoma multiforme. Science 321: 1807–1812, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1199. Patrucco E, Notte A, Barberis L, Selvetella G, Maffei A, Brancaccio M, Marengo S, Russo G, Azzolino O, Rybalkin SD, Silengo L, Altruda F, Wetzker R, Wymann MP, Lembo G, Hirsch E. PI3Kgamma modulates the cardiac response to chronic pressure overload by distinct kinase-dependent and -independent effects. Cell 118: 375–387, 2004 [DOI] [PubMed] [Google Scholar]
- 1200. Paulick MG, Bertozzi CR. The glycosylphosphatidylinositol anchor: a complex membrane-anchoring structure for proteins. Biochemistry 47: 6991–7000, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1201. Paulssen RH, Woodson J, Liu Z, Ross EM. Carboxyl-terminal fragments of phospholipase C-beta1 with intrinsic Gq GTPase-activating protein (GAP) activity. J Biol Chem 271: 26622–26629, 1996 [DOI] [PubMed] [Google Scholar]
- 1202. Pawelczyk T, Matecki A. Localization of phospholipase C delta3 in the cell and regulation of its activity by phospholipids and calcium. Eur J Biochem 257: 169–177, 1998 [DOI] [PubMed] [Google Scholar]
- 1203. Pawelczyk T, Matecki A. Phospholipase C-delta3 binds with high specificity to phosphatidylinositol 4,5-bisphosphate and phosphatidic acid in bilayer membranes. Eur J Biochem 262: 291–298, 1999 [DOI] [PubMed] [Google Scholar]
- 1204. Payrastre B, Gaits-Iacovoni F, Sansonetti P, Tronchere H. Phosphoinositides and cellular pathogens. Subcell Biochem 59: 363–388, 2012 [DOI] [PubMed] [Google Scholar]
- 1205. Payrastre B, Nievers M, Boonstra J, Breton M, Verkleij AJ, VanBergen en Henegouwen PMP. A differential location of phosphoinositide kinases, diacylglycerol kinase, and phospholipase C in the nuclear matrix. J Biol Chem 267: 5078–5084, 1992 [PubMed] [Google Scholar]
- 1206. Pendaries C, Tronchere H, Arbibe L, Mounier J, Gozani O, Cantley L, Fry MJ, Gaits-Iacovoni F, Sansonetti PJ, Payrastre B. PtdIns5P activates the host cell PI3-kinase/Akt pathway during Shigella flexneri infection. EMBO J 25: 1024–1034, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1207. Pendl GG, Prieschl EE, Thumb W, Harrer NE, Auer M, Baumruker T. Effects of phosphatidylinositol-3-kinase inhibitors on degranulation and gene induction in allergically triggered mouse mast cells. Int Arch Allergy Immunol 112: 392–399, 1997 [DOI] [PubMed] [Google Scholar]
- 1208. Perera NM, Michell RH, Dove SK. Hypo-osmotic stress activates Plc1p-dependent phosphatidylinositol 4,5-bisphosphate hydrolysis and inositol Hexakisphosphate accumulation in yeast. J Biol Chem 279: 5216–5226, 2004 [DOI] [PubMed] [Google Scholar]
- 1209. Perera RM, Zoncu R, Lucast L, De Camilli P, Toomre D. Two synaptojanin 1 isoforms are recruited to clathrin-coated pits at different stages. Proc Natl Acad Sci USA 103: 19332–19337, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1210. Perry RJ, Ridgway ND. Oxysterol-binding protein and VAMP-associated protein are required for sterol-dependent activation of the ceramide transport protein. Mol Biol Cell 17: 2604–2616, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1211. Peter BJ, Kent HM, Mills IG, Vallis Y, Butler PJ, Evans PR, McMahon HT. BAR domains as sensors of membrane curvature: the amphiphysin BAR structure. Science 303: 495–499, 2004 [DOI] [PubMed] [Google Scholar]
- 1212. Peters KG, Marie J, Wilson E, Ives HE, Escobedo J, Del Rosario M, Mirda D, Williams LT. Point mutation of an FGF receptor abolishes phosphatidylinositol turnover and Ca2+ flux but not mitogenesis. Nature 358: 678–681, 1992 [DOI] [PubMed] [Google Scholar]
- 1213. Phee H, Jacob A, Coggeshall KM. Enzymatic activity of the Src homology 2 domain-containing inositol phosphatase is regulated by a plasma membrane location. J Biol Chem 275: 19090–19097, 2000 [DOI] [PubMed] [Google Scholar]
- 1214. Philip F, Guo Y, Scarlata S. Multiple roles of pleckstrin homology domains in phospholipase Cbeta function. FEBS Lett 531: 28–32, 2002 [DOI] [PubMed] [Google Scholar]
- 1215. Philip F, Kadamur G, Silos RG, Woodson J, Ross EM. Synergistic activation of phospholipase C-beta3 by Galpha(q) and Gbetagamma describes a simple two-state coincidence detector. Curr Biol 20: 1327–1335, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1216. Phillips SE, Ile KE, Boukhelifa M, Huijbregts RP, Bankaitis VA. Specific and nonspecific membrane-binding determinants cooperate in targeting phosphatidylinositol transfer protein beta-isoform to the mammalian trans-Golgi network. Mol Biol Cell 17: 2498–2512, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1217. Phillips SE, Sha B, Topalof L, Xie Z, Alb JG, Klenchin VA, Swigart P, Cockcroft S, Martin TF, Luo M, Bankaitis VA. Yeast Sec14p deficient in phosphatidylinositol transfer activity is functional in vivo. Mol Cell 4: 187–197, 1999 [DOI] [PubMed] [Google Scholar]
- 1218. Phillips SV, Yu Y, Rossbach A, Nomikos M, Vassilakopoulou V, Livaniou E, Cumbes B, Lai FA, George CH, Swann K. Divergent effect of mammalian PLCzeta in generating Ca2+ oscillations in somatic cells compared with eggs. Biochem J 438: 545–553, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1219. Philp AJ, Campbell IG, Leet C, Vincan E, Rockman SP, Whitehead RH, Thomas RJ, Phillips WA. The phosphatidylinositol 3'-kinase p85alpha gene is an oncogene in human ovarian and colon tumors. Cancer Res 61: 7426–7429, 2001 [PubMed] [Google Scholar]
- 1220. Pian P, Bucchi A, Robinson RB, Siegelbaum SA. Regulation of gating and rundown of HCN hyperpolarization-activated channels by exogenous and endogenous PIP2. J Gen Physiol 128: 593–604, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1221. Piechulek T, Rehlen T, Walliser C, Vatter P, Moepps B, Gierschik P. Isozyme-specific stimulation of phospholipase C-gamma2 by Rac GTPases. J Biol Chem 280: 38923–38931, 2005 [DOI] [PubMed] [Google Scholar]
- 1222. Pigeau GM, Kolic J, Ball BJ, Hoppa MB, Wang YW, Ruckle T, Woo M, Manning Fox JE, MacDonald PE. Insulin granule recruitment and exocytosis is dependent on p110gamma in insulinoma and human beta-cells. Diabetes 58: 2084–2092, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1223. Pirruccello M, De Camilli P. Inositol 5-phosphatases: insights from the Lowe syndrome protein OCRL. Trends Biochem Sci 37: 234–143, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1224. Pirruccello M, Swan LE, Folta-Stogniew E, De Camilli P. Recognition of the F&H motif by the Lowe syndrome protein OCRL. Nat Struct Mol Biol 18: 789–795, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1225. Pizarro-Cerda J, Cossart P. Subversion of phosphoinositide metabolism by intracellular bacterial pathogens. Nat Cell Biol 6: 1026–1033, 2004 [DOI] [PubMed] [Google Scholar]
- 1226. Pizarro-Cerda J, Payrastre B, Wang YJ, Veiga E, Yin HL, Cossart P. Type II phosphatidylinositol 4-kinases promote Listeria monocytogenes entry into target cells. Cell Microbiol 9: 2381–2390, 2007 [DOI] [PubMed] [Google Scholar]
- 1227. Plant PJ, Correa J, Goldenberg N, Bain J, Batt J. The inositol phosphatase MTMR4 is a novel target of the ubiquitin ligase Nedd4. Biochem J 419: 57–63, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1228. Plantard L, Arjonen A, Lock JG, Nurani G, Ivaska J, Stromblad S. PtdIns(3,4,5)P is a regulator of myosin-X localization and filopodia formation. J Cell Sci 123: 3525–3534, 2010 [DOI] [PubMed] [Google Scholar]
- 1229. Pochynyuk O, Bugaj V, Stockand JD. Physiologic regulation of the epithelial sodium channel by phosphatidylinositides. Curr Opin Nephrol Hypertens 17: 533–540, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1230. Pochynyuk O, Tong Q, Medina J, Vandewalle A, Staruschenko A, Bugaj V, Stockand JD. Molecular determinants of PI(4,5)P2 and PI(3,4,5)P3 regulation of the epithelial Na+ channel. J Gen Physiol 130: 399–413, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1231. Polevoy G, Wei HC, Wong R, Szentpetery Z, Kim YJ, Goldbach P, Steinbach SK, Balla T, Brill JA. Dual roles for the Drosophila PI 4-kinase four wheel drive in localizing Rab11 during cytokinesis. J Cell Biol 187: 847–858, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1232. Ponting CP. A novel domain suggests a ciliary function for ASPM, a brain size determining gene. Bioinformatics 22: 1031–1035, 2006 [DOI] [PubMed] [Google Scholar]
- 1233. Porter FD, Li YS, Deuel TF. Purification and characterization of a phosphatidylinositol 4-kinase from bovine uteri. J Biol Chem 263: 8989–8995, 1988 [PubMed] [Google Scholar]
- 1234. Posternak T. The Cyclitols. San Francisco: Holden Day, 1965, p. 431 [Google Scholar]
- 1235. Poussin MA, Leitges M, Goldfine H. The ability of Listeria monocytogenes PI-PLC to facilitate escape from the macrophage phagosome is dependent on host PKCbeta. Microb Pathog 46: 1–5, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1236. Pouyssegur J, Chambard JC, L'Allemain G, Magnaldo I, Seuwen K. Transmembrane signalling pathways initiating cell growth in fibroblasts. Philos Trans R Soc Lond B Biol Sci 320: 427–436, 1988 [DOI] [PubMed] [Google Scholar]
- 1237. Preuss LP, Schmitz AJ, Thole JM, Bonner HKS, Otegui MS, Nielsen E. A role for the RabA4b effector protein, PI4Kb1 in polarized expansion of root hair cells in Arabidopsis. J Cell Biol 172: 991–998, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1238. Proikas-Cezanne T, Ruckerbauer S, Stierhof YD, Berg C, Nordheim A. Human WIPI-1 puncta-formation: a novel assay to assess mammalian autophagy. FEBS Lett 581: 3396–3404, 2007 [DOI] [PubMed] [Google Scholar]
- 1239. Prussia A, Thepchatri P, Snyder JP, Plemper RK. Systematic approaches towards the development of host-directed antiviral therapeutics. Int J Mol Sci 12: 4027–4052, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1240. Pulcinelli FM, Gresele P, Bonuglia M, Gazzaniga PP. Evidence for separate effects of U73122 on phospholipase C and calcium channels in human platelets. Biochem Pharmacol 56: 1481–1484, 1998 [DOI] [PubMed] [Google Scholar]
- 1241. Punnonen K, Denning M, Lee E, Li L, Rhee SG, Yuspa SH. Keratinocyte differentiation is associated with changes in the expression and regulation of phospholipase C isoenzymes. J Invest Dermatol 101: 719–726, 1993 [DOI] [PubMed] [Google Scholar]
- 1242. Putney JW., Jr A model for receptor-regulated calcium entry. Cell Calcium 7: 1–12, 1986 [DOI] [PubMed] [Google Scholar]
- 1243. Qualmann B, Koch D, Kessels MM. Let's go bananas: revisiting the endocytic BAR code. EMBO J 30: 3501–3515, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1244. Quignard JF, Mironneau J, Carricaburu V, Fournier B, Babich A, Nurnberg B, Mironneau C, Macrez N. Phosphoinositide 3-kinase gamma mediates angiotensin II-induced stimulation of L-type calcium channels in vascular myocytes. J Biol Chem 276: 32545–32551, 2001 [DOI] [PubMed] [Google Scholar]
- 1245. Raabe AC, Wengelnik K, Billker O, Vial HJ. Multiple roles for Plasmodium berghei phosphoinositide-specific phospholipase C in regulating gametocyte activation and differentiation. Cell Microbiol 13: 955–966, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1246. Raboy V. Progress in breeding low phytate crops. J Nutr 132: 503S–505S, 2002 [DOI] [PubMed] [Google Scholar]
- 1247. Raftopoulou M, Etienne-Manneville S, Self A, Nicholls S, Hall A. Regulation of cell migration by the C2 domain of the tumor suppressor PTEN. Science 303: 1179–1181, 2004 [DOI] [PubMed] [Google Scholar]
- 1248. Ragaz C, Pietsch H, Urwyler S, Tiaden A, Weber SS, Hilbi H. The Legionella pneumophila phosphatidylinositol-4 phosphate-binding type IV substrate SidC recruits endoplasmic reticulum vesicles to a replication-permissive vacuole. Cell Microbiol 10: 2416–2433, 2008 [DOI] [PubMed] [Google Scholar]
- 1249. Rahdar M, Inoue T, Meyer T, Zhang J, Vazquez F, Devreotes PN. A phosphorylation-dependent intramolecular interaction regulates the membrane association and activity of the tumor suppressor PTEN. Proc Natl Acad Sci USA 106: 480–485, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1250. Raiborg C, Bache KG, Gillooly DJ, Madshus IH, Stang E, Stenmark H. Hrs sorts ubiquitinated proteins into clathrin-coated microdomains of early endosomes. Nat Cell Biol 4: 394–398, 2002 [DOI] [PubMed] [Google Scholar]
- 1251. Raiborg C, Rusten TE, Stenmark H. Protein sorting into multivesicular endosomes. Curr Opin Cell Biol 15: 446–455, 2003 [DOI] [PubMed] [Google Scholar]
- 1252. Rameh LE, Arvidsson A, Carraway Iii KL, Couvillon AD, Rathbun G, Crompton A, VanRentherghem B, Czech MP, Ravichandran KS, Burakoff SJ, Wang DS, Chen CS, Cantley LC. A comparative analysis of the phosphoinositide binding specificity of plekstrin homology domains. J Biol Chem 272: 22059–22066, 1997 [DOI] [PubMed] [Google Scholar]
- 1253. Rameh LE, Rhee SG, Spokes K, Kazlauskas A, Cantley LC, Cantley LG. Phosphoinositide 3-kinase regulates phospholipase Cgamma-mediated calcium signaling. J Biol Chem 273: 23750–23757, 1998 [DOI] [PubMed] [Google Scholar]
- 1254. Rameh LE, Tolias KF, Duckworth BC, Cantley LC. A new pathway for synthesis of phosphatidylinositol-4,5-bisphosphate. Nature 390: 192–196, 1997 [DOI] [PubMed] [Google Scholar]
- 1255. Ramel D, Lagarrigue F, Pons V, Mounier J, Dupuis-Coronas S, Chicanne G, Sansonetti PJ, Gaits-Iacovoni F, Tronchere H, Payrastre B. Shigella flexneri infection generates the lipid PI5P to alter endocytosis and prevent termination of EGFR signaling. Sci Signal 4: ra61, 2011 [DOI] [PubMed] [Google Scholar]
- 1256. Rao K, Paik WY, Zheng L, Jobin RM, Tomic M, Jiang H, Nakanishi S, Stojilkovic SS. Wortmannin-sensitive and -insensitive steps in calcium-controlled exocytosis in pituitary gonadotrophs: evidence that myosin light chain kinase mediates calcium-dependent and wortmannin-sensitive gonadotropin secretion. Endocrinology 138: 1440–1449, 1997 [DOI] [PubMed] [Google Scholar]
- 1257. Rao VD, Misra S, Boronenkov IV, Anderson RA, Hurley JH. Structure of type IIbeta phosphatidylinositol phosphate kinase: a protein kinase fold flattened for interfacial phosphorylation. Cell 94: 829–839, 1998 [DOI] [PubMed] [Google Scholar]
- 1258. Rapoport SI, Basselin M, Kim HW, Rao JS. Bipolar disorder and mechanisms of action of mood stabilizers. Brain Res Rev 61: 185–209, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1259. Ratzan WJ, Evsikov AV, Okamura Y, Jaffe LA. Voltage sensitive phosphoinositide phosphatases of Xenopus: their tissue distribution and voltage dependence. J Cell Physiol 226: 2740–2746, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1260. Rawlings DJ, Saffran DC, Tsukada S, Largaespada DA, Grimaldi JC, Cohen L, Mohr RN, Bazan JF, Howard M, Copeland NG, Jenkins NA, Witte ON. Mutation of the unique region of Bruton's tyrosine kinase in immunodeficient XID mice. Science 261: 358–361, 1993 [DOI] [PubMed] [Google Scholar]
- 1261. Rawlings DJ, Witte ON. The Btk subfamily of cytoplasmic tyrosine kinases: structure, regulation and function. Semin Immunol 7: 237–246, 1995 [DOI] [PubMed] [Google Scholar]
- 1262. Raychaudhuri S, Im YJ, Hurley JH, Prinz WA. Nonvesicular sterol movement from plasma membrane to ER requires oxysterol-binding protein-related proteins and phosphoinositides. J Cell Biol 173: 107–119, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1263. Rbaibi Y, Cui S, Mo D, Carattino M, Rohatgi R, Satlin LM, Szalinski CM, Swanhart LM, Folsch H, Hukriede NA, Weisz OA. OCRL1 modulates cilia length in renal epithelial cells. Traffic 9999: 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1264. Rebecchi MJ, Raghubir A, Scarlata S, Hartenstine MJ, Brown T, Stallings JD. Expression and function of phospholipase C in breast carcinoma. Adv Enzyme Regul 49: 59–73, 2009 [DOI] [PubMed] [Google Scholar]
- 1265. Rebecchi MJ, Rosen OM. Purification of a phosphoinositide-specific phospholipase C from bovine brain. J Biol Chem 262: 12526–12532, 1987 [PubMed] [Google Scholar]
- 1266. Redfern RE, Redfern D, Furgason ML, Munson M, Ross AH, Gericke A. PTEN phosphatase selectively binds phosphoinositides and undergoes structural changes. Biochemistry 47: 2162–2171, 2008 [DOI] [PubMed] [Google Scholar]
- 1267. Reiss S, Rebhan I, Backes P, Romero-Brey I, Erfle H, Matula P, Kaderali L, Poenisch M, Blankenburg H, Hiet MS, Longerich T, Diehl S, Ramirez F, Balla T, Rohr K, Kaul A, Buhler S, Pepperkok R, Lengauer T, Albrecht M, Eils R, Schirmacher P, Lohmann V, Bartenschlager R. Recruitment and activation of a lipid kinase by hepatitis C virus NS5A is essential for integrity of the membranous replication compartment. Cell Host Microbe 9: 32–45, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1268. Rescher U, Ruhe D, Ludwig C, Zobiack N, Gerke V. Annexin 2 is a phosphatidylinositol (4,5)-bisphosphate binding protein recruited to actin assembly sites at cellular membranes. J Cell Sci 117: 3473–3480, 2004 [DOI] [PubMed] [Google Scholar]
- 1269. Resnick AC, Snowman AM, Kang BN, Hurt KJ, Snyder SH, Saiardi A. Inositol polyphosphate multikinase is a nuclear PI3-kinase with transcriptional regulatory activity. Proc Natl Acad Sci USA 102: 12783–12788, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1270. Reyland ME. Protein kinase C isoforms: multi-functional regulators of cell life and death. Front Biosci 14: 2386–2399, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1271. Rhee SG, Suh PG, Ryu SH, Lee SY. Studies of inositol phospholipid-specific phospholipase C. Science 244: 546–550, 1989 [DOI] [PubMed] [Google Scholar]
- 1272. Richards DE, Irvine RF, Dawson RM. Hydrolysis of membrane phospholipids by phospholipases of rat liver lysosomes. Biochem J 182: 599–606, 1979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1273. Robinson FL, Dixon JE. Myotubularin phosphatases: policing 3-phosphoinositides. Trends Cell Biol 16: 403–412, 2006 [DOI] [PubMed] [Google Scholar]
- 1274. Robinson FL, Dixon JE. The phosphoinositide-3-phosphatase MTMR2 associates with MTMR13, a membrane-associated pseudophosphatase also mutated in type 4B Charcot-Marie-Tooth disease. J Biol Chem 280: 31699–31707, 2005 [DOI] [PubMed] [Google Scholar]
- 1275. Robinson FL, Niesman IR, Beiswenger KK, Dixon JE. Loss of the inactive myotubularin-related phosphatase Mtmr13 leads to a Charcot-Marie-Tooth 4B2-like peripheral neuropathy in mice. Proc Natl Acad Sci USA 105: 4916–4921, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1276. Rodnight R. Cerebral diphosphoinositide breakdown: activation, complexity and distribution in animal (mainly nervous) tissues. Biochem J 63: 223–231, 1956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1277. Rodrigues GA, Falasca M, Zhang Z, Ong SH, Schlessinger J. A novel positive feedback loop mediated by the docking protein Gab1 and phosphatidylinositol 3-kinase in epidermal growth factor receptor signaling. Mol Cell Biol 20: 1448–1459, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1278. Rodriguez R, Matsuda M, Perisic O, Bravo J, Paul A, Jones NP, Light Y, Swann K, Williams RL, Katan M. Tyrosine residues in phospholipase Cgamma 2 essential for the enzyme function in B-cell signaling. J Biol Chem 276: 47982–47992, 2001 [DOI] [PubMed] [Google Scholar]
- 1279. Rodriguez-Borlado L, Barber DF, Hernandez C, Rodriguez-Marcos MA, Sanchez A, Hirsch E, Wymann M, Martinez AC, Carrera AC. Phosphatidylinositol 3-kinase regulates the CD4/CD8 T cell differentiation ratio. J Immunol 170: 4475–4482, 2003 [DOI] [PubMed] [Google Scholar]
- 1280. Rodriguez-Viciana P, Warne PH, Dhand R, Vanhaesebroeck B, Gout I, Fry MJ, Waterfield MD, Downward J. Phosphatidylinositol-3-OH kinase as a direct target of Ras. Nature 370: 527–532, 1994 [DOI] [PubMed] [Google Scholar]
- 1281. Rohacs T, Chen J, Prestwich GD, Logothetis DE. Distinct specificities of inwardly rectifying K+ channels for phosphoinositides. J Biol Chem 274: 36065–36072, 1999 [DOI] [PubMed] [Google Scholar]
- 1282. Rohacs T, Lopes C, Mirshahi T, Jin T, Zhang H, Logothetis DE. Assaying phosphatidylinositol bisphosphate regulation of potassium channels. Methods Enzymol 345: 71–92, 2002 [DOI] [PubMed] [Google Scholar]
- 1283. Rohacs T, Lopes CM, Jin T, Ramdya PP, Molnar Z, Logothetis DE. Specificity of activation by phosphoinositides determines lipid regulation of Kir channels. Proc Natl Acad Sci USA 100: 745–750, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1284. Rohacs T, Lopes CM, Michailidis I, Logothetis DE. PI(4,5)P2 regulates the activation and desensitization of TRPM8 channels through the TRP domain. Nat Neurosci 8: 626–634, 2005 [DOI] [PubMed] [Google Scholar]
- 1285. Rohacs T, Nilius B. Regulation of transient receptor potential (TRP) channels by phosphoinositides. Pflügers Arch 455: 157–168, 2007 [DOI] [PubMed] [Google Scholar]
- 1286. Rohacs T, Thyagarajan B, Lukacs V. Phospholipase C mediated modulation of TRPV1 channels. Mol Neurobiol 37: 153–163, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1287. Rohatgi R, Ho HY, Kirschner MW. Mechanism of N-WASP activation by CDC42 and phosphatidylinositol 4, 5-bisphosphate. J Cell Biol 150: 1299–1310, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1288. Rohde G, Wenzel D, Haucke V. A phosphatidylinositol (4,5)-bisphosphate binding site within mu2-adaptin regulates clathrin-mediated endocytosis. J Cell Biol 158: 209–214, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1289. Rohde HM, Cheong FY, Konrad G, Paiha K, Mayinger P, Boehmelt G. The human phosphatidylinositol phosphatase SAC1 interacts with the coatomer I complex. J Biol Chem 278: 52689–52699, 2003 [DOI] [PubMed] [Google Scholar]
- 1290. Rohrschneider LR, Fuller JF, Wolf I, Liu Y, Lucas DM. Structure, function, and biology of SHIP proteins. Genes Dev 14: 505–520, 2000 [PubMed] [Google Scholar]
- 1291. Roifman CM, Wang G. Phospholipase C-gamma 1 and phospholipase C-gamma 2 are substrates of the B cell antigen receptor associated protein tyrosine kinase. Biochem Biophys Res Commun 183: 411–416, 1992 [DOI] [PubMed] [Google Scholar]
- 1292. Roll P, Massacrier A, Pereira S, Robaglia-Schlupp A, Cau P, Szepetowski P. New human sodium/glucose cotransporter gene (KST1): identification, characterization, and mutation analysis in ICCA (infantile convulsions and choreoathetosis) and BFIC (benign familial infantile convulsions) families. Gene 285: 141–148, 2002 [DOI] [PubMed] [Google Scholar]
- 1293. Romero-Suarez S, Shen J, Brotto L, Hall T, Mo C, Valdivia HH, Andresen J, Wacker M, Nosek TM, Qu CK, Brotto M. Muscle-specific inositide phosphatase (MIP/MTMR14) is reduced with age and its loss accelerates skeletal muscle aging process by altering calcium homeostasis. Aging 2: 504–513, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1294. Rommel C. Taking PI3Kdelta and PI3Kgamma one step ahead: dual active PI3Kdelta/gamma inhibitors for the treatment of immune-mediated inflammatory diseases. Curr Top Microbiol Immunol 346: 279–299, 2010 [DOI] [PubMed] [Google Scholar]
- 1295. Rommel C, Camps M, Ji H. PI3K delta and PI3K gamma: partners in crime in inflammation in rheumatoid arthritis and beyond? Nat Rev Immunol 7: 191–201, 2007 [DOI] [PubMed] [Google Scholar]
- 1296. Roos J, DiGregorio PJ, Yeromin AV, Ohlsen K, Lioudyno M, Zhang S, Safrina O, Kozak JA, Wagner SL, Cahalan MD, Velicelebi G, Stauderman KA. STIM1, an essential and conserved component of store-operated Ca2+ channel function. J Cell Biol 169: 435–445, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1297. Ross SH, Lindsay Y, Safrany ST, Lorenzo O, Villa F, Toth R, Clague MJ, Downes CP, Leslie NR. Differential redox regulation within the PTP superfamily. Cell Signal 19: 1521–1530, 2007 [DOI] [PubMed] [Google Scholar]
- 1298. Rosse C, Linch M, Kermorgant S, Cameron AJ, Boeckeler K, Parker PJ. PKC and the control of localized signal dynamics. Nat Rev Mol Cell Biol 11: 103–112, 2010 [DOI] [PubMed] [Google Scholar]
- 1299. Rossler P, Kroner C, Freitag J, Noe J, Breer H. Identification of a phospholipase C beta subtype in rat taste cells. Eur J Cell Biol 77: 253–261, 1998 [DOI] [PubMed] [Google Scholar]
- 1300. Rothman JH, Howald I, Stevens TH. Characterization of genes required for protein sorting and vacuolar function in the yeast Saccharomyces cerevisiae. EMBO J 8: 2057–2065, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1301. Rottbauer W, Just S, Wessels G, Trano N, Most P, Katus HA, Fishman MC. VEGF-PLCgamma1 pathway controls cardiac contractility in the embryonic heart. Genes Dev 19: 1624–1634, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1302. Rousset M, Cens T, Gouin-Charnet A, Scamps F, Charnet P. Ca2+ and phosphatidylinositol 4,5-bisphosphate stabilize a Gbeta gamma-sensitive state of Ca V2 Ca2+ channels. J Biol Chem 279: 14619–14630, 2004 [DOI] [PubMed] [Google Scholar]
- 1303. Routt SM, Ryan MM, Tyeryar K, Rizzieri KE, Mousley C, Roumanie O, Brennwald PJ, Bankaitis VA. Nonclassical PITPs activate PLD via the Stt4p PtdIns-4-kinase and modulate function of late stages of exocytosis in vegetative yeast. Traffic 6: 1157–1172, 2005 [DOI] [PubMed] [Google Scholar]
- 1304. Roy A, Levine TP. Multiple pools of phosphatidylinositol 4-phosphate detected using the pleckstrin homology domain of Osh2p. J Biol Chem 279: 44683–44689, 2004 [DOI] [PubMed] [Google Scholar]
- 1305. Rozelle AL, Machesky LM, Yamamoto M, Driessens MH, Insall RH, Roth MG, Luby-Phelps K, Marriott G, Hall A, Yin HL. Phosphatidylinositol 4,5-bisphosphate induces actin-based movement of raft-enriched vesicles through WASP-ArP2/3. Curr Biol 10: 311–320, 2000 [DOI] [PubMed] [Google Scholar]
- 1306. Rozengurt E. Protein kinase D signaling: multiple biological functions in health and disease. Physiology 26: 23–33, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1307. Rozenvayn N, Flaumenhaft R. Phosphatidylinositol 4,5-bisphosphate mediates Ca2+-induced platelet alpha-granule secretion: evidence for type II phosphatidylinositol 5-phosphate 4-kinase function. J Biol Chem 276: 22410–22419, 2001 [DOI] [PubMed] [Google Scholar]
- 1308. Rozenvayn N, Flaumenhaft R. Protein kinase C mediates translocation of type II phosphatidylinositol 5-phosphate 4-kinase required for platelet alpha-granule secretion. J Biol Chem 278: 8126–8134, 2003 [DOI] [PubMed] [Google Scholar]
- 1309. Rubio I, Rodriguez-Viciana P, Downward J, Wetzker R. Interaction of Ras with phosphoinositide 3-kinase gamma. Biochem J 326: 891–895, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1310. Ruckle T, Schwarz MK, Rommel C. PI3Kgamma inhibition: towards an “aspirin of the 21st century”? Nat Rev Drug Discov 5: 903–918, 2006 [DOI] [PubMed] [Google Scholar]
- 1311. Rudge SA, Anderson DM, Emr SD. Vacuole size control: regulation of PtdIns(3,5)P2 levels by the vacuole-associated Vac14-Fig4 complex, a PtdIns(3,5)P2-specific phosphatase. Mol Biol Cell 15: 24–36, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1312. Runnels LW, Yue LX, Clapham DE. The TRPM7 channel is inactivated by PIP2 hydrolysis. Nat Cell Biol 15: 370–378, 2002 [DOI] [PubMed] [Google Scholar]
- 1313. Rupnik M, Kreft M, Sikdar SK, Grilc S, Romih R, Zupancic G, Martin TF, Zorec R. Rapid regulated dense-core vesicle exocytosis requires the CAPS protein. Proc Natl Acad Sci USA 97: 5627–5632, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1314. Rusk N, Le PU, Mariggio S, Guay G, Lurisci C, Nabi IR, Corda D, Symons M. Synaptojanin 2 functions at an early step of clathrin-mediated endocytosis. Curr Biol 13: 659–663, 2003 [DOI] [PubMed] [Google Scholar]
- 1315. Rusten TE, Rodahl LM, Pattni K, Englund C, Samakovlis C, Dove S, Brech A, Stenmark H. Fab1 phosphatidylinositol 3-phosphate 5-kinase controls trafficking but not silencing of endocytosed receptors. Mol Biol Cell 17: 3989–4001, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1316. Rutherford AC, Traer C, Wassmer T, Pattni K, Bujny MV, Carlton JG, Stenmark H, Cullen PJ. The mammalian phosphatidylinositol 3-phosphate 5-kinase (PIKfyve) regulates endosome-to-TGN retrograde transport. J Cell Sci 119: 3944–3957, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1317. Rynkiewicz NK, Liu HJ, Balamatsias D, Mitchell CA. INPP4A/INPP4B and P-Rex proteins: related but different? Adv Enzyme Regul 52: 265–279, 2012 [DOI] [PubMed] [Google Scholar]
- 1318. Ryu SH, Cho KS, Lee KY, Suh PG, Rhee SG. Purification and characterization of two immunologically distinct phosphoinositide-specific phospholipases C from bovine brain. J Biol Chem 262: 12511–12518, 1987 [PubMed] [Google Scholar]
- 1319. Ryu SH, Suh PG, Cho KS, Lee KY, Rhee SG. Bovine brain cytosol contains three immunologically distinct forms of inositol phospholipid-specific phospholipase-C. Proc Natl Acad Sci USA 84: 6649–6653, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1320. Saarikangas J, Zhao H, Lappalainen P. Regulation of the actin cytoskeleton-plasma membrane interplay by phosphoinositides. Physiol Rev 90: 259–289, 2010 [DOI] [PubMed] [Google Scholar]
- 1321. Sachs AJ, David SA, Haider NB, Nystuen AM. Patterned neuroprotection in the Inpp4a(wbl) mutant mouse cerebellum correlates with the expression of Eaat4. PLoS One 4: e8270, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1322. Saha AK, Dowling JN, LaMarco KL, Das S, Remaley AT, Olomu N, Pope MT, Glew RH. Properties of an acid phosphatase from Legionella micdadei which blocks superoxide anion production by human neutrophils. Arch Biochem Biophys 243: 150–160, 1985 [DOI] [PubMed] [Google Scholar]
- 1323. Saha AK, Dowling JN, Pasculle AW, Glew RH. Legionella micdadei phosphatase catalyzes the hydrolysis of phosphatidylinositol 4,5-bisphosphate in human neutrophils. Arch Biochem Biophys 265: 94–104, 1988 [DOI] [PubMed] [Google Scholar]
- 1324. Saiardi A. Cell signalling by inositol pyrophosphates. Subcell Biochem 59: 413–443, 2012 [DOI] [PubMed] [Google Scholar]
- 1325. Saito S, Goto K, Tonosaki A, Kondo H. Gene cloning and characterization of CDP-diacylglycerol synthase from rat brain. J Biol Chem 272: 9503–9509, 1997 [DOI] [PubMed] [Google Scholar]
- 1326. Saito T, Stopkova P, Diaz L, Papolos DF, Boussemart L, Lachman HM. Polymorphism screening of PIK4CA: possible candidate gene for chromosome 22q11-linked psychiatric disorders. Am J Med Genet B Neuropsychiatr Genet 116: 77–83, 2003 [DOI] [PubMed] [Google Scholar]
- 1327. Saksena S, Wahlman J, Teis D, Johnson AE, Emr SD. Functional reconstitution of ESCRT-III assembly and disassembly. Cell 136: 97–109, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1328. Sakurai K, Hirata M, Yamaguchi H, Nakamura Y, Fukami K. Phospholipase Cdelta3 is a novel binding partner of myosin VI and functions as anchoring of myosin VI on plasma membrane. Adv Enzyme Regul 51: 171–181, 2011 [DOI] [PubMed] [Google Scholar]
- 1329. Sala G, Dituri F, Raimondi C, Previdi S, Maffucci T, Mazzoletti M, Rossi C, Iezzi M, Lattanzio R, Piantelli M, Iacobelli S, Broggini M, Falasca M. Phospholipase Cgamma1 is required for metastasis development and progression. Cancer Res 68: 10187–10196, 2008 [DOI] [PubMed] [Google Scholar]
- 1330. Salazar G, Craige B, Wainer BH, Guo J, De Camilli P, Faundez V. Phosphatidylinositol-4-kinase type II alpha is a component of adaptor protein-3-derived vesicles. Mol Biol Cell 16: 3692–3704, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1331. Saleh SN, Albert AP, Large WA. Obligatory role for phosphatidylinositol 4,5-bisphosphate in activation of native TRPC1 store-operated channels in vascular myocytes. J Physiol 587: 531–540, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1332. Salim K, Bottomley MJ, Querfurth E, Zvelebil MJ, Gout I, Scaife R, Margolis RL, Gigg R, Smith CIE, Driscoll PC, Waterfield MD, Panayotou G. Distinct specificity in the recognition of phosphoinositides by the plekstrin homology domains of dynamin and Bruton's tyrosine kinase. EMBO J 15: 6241–6250, 1996 [PMC free article] [PubMed] [Google Scholar]
- 1333. Samuels Y, Diaz LA, Jr, Schmidt-Kittler O, Cummins JM, Delong L, Cheong I, Rago C, Huso DL, Lengauer C, Kinzler KW, Vogelstein B, Velculescu VE. Mutant PIK3CA promotes cell growth and invasion of human cancer cells. Cancer Cell 7: 561–573, 2005 [DOI] [PubMed] [Google Scholar]
- 1334. Samuels Y, Wang Z, Bardelli A, Silliman N, Ptak J, Szabo S, Yan H, Gazdar A, Powell SM, Riggins GJ, Willson JK, Markowitz S, Kinzler KW, Vogelstein B, Velculescu VE. High frequency of mutations of the PIK3CA gene in human cancers. Science 304: 554, 2004 [DOI] [PubMed] [Google Scholar]
- 1335. Sancak Y, Bar-Peled L, Zoncu R, Markhard AL, Nada S, Sabatini DM. Ragulator-Rag complex targets mTORC1 to the lysosomal surface and is necessary for its activation by amino acids. Cell 141: 290–303, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1336. Sancak Y, Peterson TR, Shaul YD, Lindquist RA, Thoreen CC, Bar-Peled L, Sabatini DM. The Rag GTPases bind raptor and mediate amino acid signaling to mTORC1. Science 320: 1496–1501, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1337. Sandhu RS, Hokin MR. Amylase secretion and phosphatide synthesis in response to epinephrine in rat parotid gland slices and the role of cyclic 3',5'-AMP. Federation Proc 26: 391–391, 1967 [Google Scholar]
- 1338. Santi P, Solimando L, Zini N, Santi S, Riccio M, Guidotti L. Inositol-specific phospholipase C in low and fast proliferating hepatoma cell lines. Int J Oncol 22: 1147–1153, 2003 [DOI] [PubMed] [Google Scholar]
- 1339. Santiago-Calvo E, Mule S, Redman CR, Hokin MR. The chromatographic seperation of polyphosphoinositides and studies on their turnover in various tissues. Biochim Biophys Acta 84: 550–562, 1964 [DOI] [PubMed] [Google Scholar]
- 1340. Sarantis H, Balkin DM, De Camilli P, Isberg RR, Brumell JH, Grinstein S. Yersinia entry into host cells requires Rab5-dependent dephosphorylation of PI(4,5)P(2) and membrane scission. Cell Host Microbe 11: 117–128, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1341. Sarraj B, Massberg S, Li Y, Kasorn A, Subramanian K, Loison F, Silberstein LE, von Andrian U, Luo HR. Myeloid-specific deletion of tumor suppressor PTEN augments neutrophil transendothelial migration during inflammation. J Immunol 182: 7190–7200, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1342. Sasaki AT, Chun C, Takeda K, Firtel RA. Localized Ras signaling at the leading edge regulates PI3K, cell polarity, and directional cell movement. J Cell Biol 167: 505–518, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1343. Sasaki J, Kofuji S, Itoh R, Momiyama T, Takayama K, Murakami H, Chida S, Tsuya Y, Takasuga S, Eguchi S, Asanuma K, Horie Y, Miura K, Davies EM, Mitchell C, Yamazaki M, Hirai H, Takenawa T, Suzuki A, Sasaki T. The PtdIns(3,4)P(2) phosphatase INPP4A is a suppressor of excitotoxic neuronal death. Nature 465: 497–501, 2010 [DOI] [PubMed] [Google Scholar]
- 1344. Sasaki J, Sasaki T, Yamazaki M, Matsuoka K, Taya C, Shitara H, Takasuga S, Nishio M, Mizuno K, Wada T, Miyazaki H, Watanabe H, Iizuka R, Kubo S, Murata S, Chiba T, Maehama T, Hamada K, Kishimoto H, Frohman MA, Tanaka K, Penninger JM, Yonekawa H, Suzuki A, Kanaho Y. Regulation of anaphylactic responses by phosphatidylinositol phosphate kinase type I α. J Exp Med 201: 859–870, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1345. Sasaki T, Irie-Sasaki J, Horie Y, Bachmaier K, Fata JE, Li M, Suzuki A, Bouchard D, Ho A, Redston M, Gallinger S, Khokha R, Mak TW, Hawkins PT, Stephens L, Scherer SW, Tsao M, Penninger JM. Colorectal carcinomas in mice lacking the catalytic subunit of PI(3)Kgamma. Nature 406: 897–902, 2000 [DOI] [PubMed] [Google Scholar]
- 1346. Sasaki T, Irie-Sasaki J, Jones RG, Oliviera-dos-Santos AJ, Stanford WL, Bolon B, Wakeham A, Itie A, Bouchard D, Kozieradzki I, Joza N, Mak TW, Ohashi PS, Suzuki A, Penninger JM. Function of PI3Kgamma in thymocyte development, T cell activation, and neutrophil migration. Science 287: 1040–1046, 2000 [DOI] [PubMed] [Google Scholar]
- 1347. Sasaki T, Takasuga S, Sasaki J, Kofuji S, Eguchi S, Yamazaki M, Suzuki A. Mammalian phosphoinositide kinases and phosphatases. Prog Lipid Res 48: 307–343, 2009 [DOI] [PubMed] [Google Scholar]
- 1348. Sason H, Milgrom M, Weiss AM, Melamed-Book N, Balla T, Grinstein S, Backert S, Rosenshine I, Aroeti B. Enteropathogenic Escherichia coli subverts phosphatidylinositol 4,5-bisphosphate and phosphatidylinositol 3,4,5-trisphosphate upon epithelial cell infection. Mol Biol Cell 20: 544–555, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1349. Saunders CM, Larman MG, Parrington J, Cox LJ, Royse J, Blayney LM, Swann K, Lai FA. PLC zeta: a sperm-specific trigger of Ca2+ oscillations in eggs and embryo development. Development 129: 3533–3544, 2002 [DOI] [PubMed] [Google Scholar]
- 1350. Savitsky K, Bar-Shira A, Gilad S, Rotman G, Ziv Y, Vanagaite L, Tagle DA, Smith S, Uziel T, Sfez S, Ashkenazi M, Pecker I, Frydman M, Harnik R, Patanjali SR, Simmons A, Clines GA, Sartiel A, Gatti RA, Chessa L, Sanal O, Lavin MF, Jaspers NGJ, Taylor AMR, Arlett CF, Miki T, Weissman SM, Lovett M, Collins FS, Shiloh Y. A single ataxia telangiectasia gene with a product similar to PI-3 kinase. Science 268: 1749–1753, 1995 [DOI] [PubMed] [Google Scholar]
- 1351. Sbrissa D, Ikonomov OC, Fenner H, Shisheva A. ArPIKfyve homomeric and heteromeric interactions scaffold PIKfyve and Sac3 in a complex to promote PIKfyve activity and functionality. J Mol Biol 384: 766–779, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1352. Sbrissa D, Ikonomov OC, Filios C, Delvecchio K, Shisheva A. Functional dissociation between PIKfyve-synthesized PtdIns5P and PtdIns(3,5)P2 by means of the PIKfyve inhibitor YM201636. Am J Physiol Cell Physiol 303: C436–C446, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1353. Sbrissa D, Ikonomov OC, Fu Z, Ijuin T, Gruenberg J, Takenawa T, Shisheva A. Core protein machinery for mammalian phosphatidylinositol 3,5-bisphosphate synthesis and turnover that regulates the progression of endosomal transport. Novel Sac phosphatase joins the ArPIKfyve-PIKfyve complex. J Biol Chem 282: 23878–23891, 2007 [DOI] [PubMed] [Google Scholar]
- 1354. Schaaf G, Ortlund EA, Tyeryar KR, Mousley CJ, Ile KE, Garrett TA, Ren J, Woolls MJ, Raetz CR, Redinbo MR, Bankaitis VA. Functional anatomy of phospholipid binding and regulation of phosphoinositide homeostasis by proteins of the sec14 superfamily. Mol Cell 29: 191–206, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1355. Schaletzky J, Dove SK, Short B, Lorenzo O, Clague MJ, Barr FA. Phosphatidylinositol-5-phosphate activation and conserved substrate specificity of the myotubularin phosphatidylinositol 3-phosphatases. Curr Biol 13: 504–509, 2003 [DOI] [PubMed] [Google Scholar]
- 1356. Scharenberg AM, El-Hillal O, Fruman DA, Beitz LO, Li Z, Lin S, Gout I, Cantley LC, Rawlings DJ, Kinet JP. Phosphatidylinositol-3,4,5-trisphosphate (PtdIns-3,4,5-P3)/Tec kinase-dependent calcium signaling pathway: a target for SHIP-mediated inhibitory signals. EMBO J 17: 1961–1972, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1357. Scharenberg AM, Kinet JP. PtdIns-3,4,5-P3: a regulatory nexus between tyrosine kinases and sustained calcium signals. Cell 94: 5–8, 1998 [DOI] [PubMed] [Google Scholar]
- 1358. Schiavo G, Gu QM, Prestwich GD, Sollner TH, Rothmann JE. Calcium-dependent switching of the specificity of phosphoinositide binding to synaptotagmin. Proc Natl Acad Sci USA 93: 13327–13332, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1359. Schill NJ, Anderson RA. Two novel phosphatidylinositol-4-phosphate 5-kinase type Igamma splice variants expressed in human cells display distinctive cellular targeting. Biochem J 422: 473–482, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1360. Schlame M, Haldar D. Cardiolipin is synthesized on the matrix side of the inner membrane in rat liver mitochondria. J Biol Chem 268: 74–79, 1993 [PubMed] [Google Scholar]
- 1361. Schmid AC, Wise HM, Mitchell CA, Nussbaum R, Woscholski R. Type II phosphoinositide 5-phosphatases have unique sensitivities towards fatty acid composition and head group phosphorylation. FEBS Lett 576: 9–13, 2004 [DOI] [PubMed] [Google Scholar]
- 1362. Schmid SL, Frolov VA. Dynamin: functional design of a membrane fission catalyst. Annu Rev Cell Dev Biol 27: 79–105, 2011 [DOI] [PubMed] [Google Scholar]
- 1363. Schmidt A, Kunz J, Hall MN. TOR2 is required for organization of the actin cytoskeleton in yeast. Proc Natl Acad Sci USA 93: 13780–13785, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1364. Schmidt M, Evellin S, Weernink PA, von Dorp F, Rehmann H, Lomasney JW, Jakobs KH. A new phospholipase-C-calcium signalling pathway mediated by cyclic AMP and a Rap GTPase. Nat Cell Biol 3: 1020–1024, 2001 [DOI] [PubMed] [Google Scholar]
- 1365. Schmitz KR, Liu J, Li S, Setty TG, Wood CS, Burd CG, Ferguson KM. Golgi localization of glycosyltransferases requires a Vps74p oligomer. Dev Cell 14: 523–534, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1366. Schouten A, Agianian B, Westerman J, Kroon J, Wirtz KW, Gros P. Structure of apo-phosphatidylinositol transfer protein alpha provides insight into membrane association. EMBO J 21: 2117–2121, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1367. Schu PV, Takegawa K, Fry MJ, Stack JH, Waterfield MD, Emr SD. Phosphatidylinositol 3-kinase encoded by yeast VPS34 gene essential for protein sorting. Science 260: 88–91, 1993 [DOI] [PubMed] [Google Scholar]
- 1368. Schulze H, Korpal M, Hurov J, Kim SW, Zhang J, Cantley LC, Graf T, Shivdasani RA. Characterization of the megakaryocyte demarcation membrane system and its role in thrombopoiesis. Blood 107: 3868–3875, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1369. Sciorra VA, Audhya A, Parsons AB, Segev N, Boone C, Emr SD. Synthetic genetic array analysis of the PtdIns 4-kinase Pik1p identifies components in a Golgi-specific Ypt31/rab-GTPase signaling pathway. Mol Biol Cell 16: 776–793, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1370. Scott CC, Dobson W, Botelho RJ, Coady-Osberg N, Chavrier P, Knecht DA, Heath C, Stahl P, Grinstein S. Phosphatidylinositol-4,5-bisphosphate hydrolysis directs actin remodeling during phagocytosis. J Cell Biol 169: 139–149, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1371. Scott KL, Chin L. Signaling from the Golgi: mechanisms and models for Golgi phosphoprotein 3-mediated oncogenesis. Clin Cancer Res 16: 2229–2234, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1372. Seaman MN. Cargo-selective endosomal sorting for retrieval to the Golgi requires retromer. J Cell Biol 165: 111–122, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1373. Seaman MN, Marcusson EG, Cereghino JL, Emr SD. Endosome to Golgi retrieval of the vacuolar protein sorting receptor, Vps10p, requires the function of the VPS29, VPS30, and VPS35 gene products. J Cell Biol 137: 79–92, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1374. Secrist JP, Karnitz L, Abraham RT. T-cell antigen receptor ligation induces tyrosine phosphorylation of phospholipase C-gamma 1. J Biol Chem 266: 12135–12139, 1991 [PubMed] [Google Scholar]
- 1375. Seeds AM, Frederick JP, Tsui MM, York JD. Roles for inositol polyphosphate kinases in the regulation of nuclear processes and developmental biology. Adv Enzyme Regul 47: 10–25, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1376. Seelan RS, Lakshmanan J, Casanova MF, Parthasarathy RN. Identification of myo-inositol-3-phosphate synthase isoforms: characterization, expression, and putative role of a 16-kDa gamma(c) isoform. J Biol Chem 284: 9443–9457, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1377. Segui B, Allen-Baume V, Cockcroft S. Phosphatidylinositol transfer protein beta displays minimal sphingomyelin transfer activity and is not required for biosynthesis and trafficking of sphingomyelin. Biochem J 366: 23–34, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1378. Seifert JP, Wing MR, Snyder JT, Gershburg S, Sondek J, Harden TK. RhoA activates purified phospholipase C-epsilon by a guanine nucleotide-dependent mechanism. J Biol Chem 279: 47992–47997, 2004 [DOI] [PubMed] [Google Scholar]
- 1379. Seifert JP, Zhou Y, Hicks SN, Sondek J, Harden TK. Dual activation of phospholipase C-epsilon by Rho and Ras GTPases. J Biol Chem 283: 29690–29698, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1380. Sekiya F, Poulin B, Kim YJ, Rhee SG. Mechanism of tyrosine phosphorylation and activation of phospholipase C-gamma 1. Tyrosine 783 phosphorylation is not sufficient for lipase activation. J Biol Chem 279: 32181–32190, 2004 [DOI] [PubMed] [Google Scholar]
- 1381. Senderek J, Bergmann C, Weber S, Ketelsen UP, Schorle H, Rudnik-Schoneborn S, Buttner R, Buchheim E, Zerres K. Mutation of the SBF2 gene, encoding a novel member of the myotubularin family, in Charcot-Marie-Tooth neuropathy type 4B2/11p15. Hum Mol Genet 12: 349–356, 2003 [DOI] [PubMed] [Google Scholar]
- 1382. Seroude L, Brummel T, Kapahi P, Benzer S. Spatio-temporal analysis of gene expression during aging in Drosophila melanogaster. Aging Cell 1: 47–56, 2002 [DOI] [PubMed] [Google Scholar]
- 1383. Serrano CJ, Graham L, DeBell K, Rawat R, Veri MC, Bonvini E, Rellahan BL, Reischl IG. A new tyrosine phosphorylation site in PLC gamma 1: the role of tyrosine 775 in immune receptor signaling. J Immunol 174: 6233–6237, 2005 [DOI] [PubMed] [Google Scholar]
- 1384. Serunian LA, Haber MT, Fukui T, Kim JW, Rhee SG, Lowenstein JM, Cantley LC. Polyphosphoinositides produced by phosphatidylinositol 3-kinase are poor substrates for phospholipases C from rat liver and bovine brain. J Biol Chem 264: 17809–17815, 1989 [PubMed] [Google Scholar]
- 1385. Servant G, Weiner OD, Herzmark P, Balla T, Sedat JW, Bourne HR. Polarization of chemoattractant receptor signaling during neutrophil chemotaxis. Science 287: 1037–1040, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1386. Severin S, Gratacap MP, Lenain N, Alvarez L, Hollande E, Penninger JM, Gachet C, Plantavid M, Payrastre B. Deficiency of Src homology 2 domain-containing inositol 5-phosphatase 1 affects platelet responses and thrombus growth. J Clin Invest 117: 944–952, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1387. Sha B, Phillips SE, Bankaitis VA, Luo M. Crystal structure of the Saccharomyces cerevisiae phosphatidylinositol-transfer protein. Nature 391: 506–510, 1998 [DOI] [PubMed] [Google Scholar]
- 1388. Sharma M, Batra J, Mabalirajan U, Sharma S, Nagarkatti R, Aich J, Sharma SK, Niphadkar PV, Ghosh B. A genetic variation in inositol polyphosphate 4 phosphatase a enhances susceptibility to asthma. Am J Respir Crit Care Med 177: 712–719, 2008 [DOI] [PubMed] [Google Scholar]
- 1389. Shears SB. Understanding the biological significance of diphosphoinositol polyphosphates (“inositol pyrophosphates”). Biochem Soc Symp 211–221, 2007 [DOI] [PubMed] [Google Scholar]
- 1390. Shears SB, Ganapathi SB, Gokhale NA, Schenk TM, Wang H, Weaver JD, Zaremba A, Zhou Y. Defining signal transduction by inositol phosphates. Subcell Biochem 59: 389–412, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1391. Shears SB, Parry JB, Tang EKY, Irvine RF, Michell RH, Kirk CJ. Metabolism of d-myo-inositol 1,3,4,5-tetrakisphosphate by rat liver, including the synthesis of a novel isomer of myo-inositol tetrakisphosphate. Biochem J 246: 139–147, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1392. Shelton SN, Barylko B, Binns DD, Horazdovsky BF, Albanesi JP, Goodman JM. Saccharomyces cerevisiae contains a Type II phosphoinositide 4-kinase. Biochem J 371: 533–540, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1393. Shen J, Yu WM, Brotto M, Scherman JA, Guo C, Stoddard C, Nosek TM, Valdivia HH, Qu CK. Deficiency of MIP/MTMR14 phosphatase induces a muscle disorder by disrupting Ca2+ homeostasis. Nat Cell Biol 11: 769–776, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1394. Shen WH, Balajee AS, Wang J, Wu H, Eng C, Pandolfi PP, Yin Y. Essential role for nuclear PTEN in maintaining chromosomal integrity. Cell 128: 157–170, 2007 [DOI] [PubMed] [Google Scholar]
- 1395. Shen WL, Kwon Y, Adegbola AA, Luo J, Chess A, Montell C. Function of rhodopsin in temperature discrimination in Drosophila. Science 331: 1333–1336, 2011 [DOI] [PubMed] [Google Scholar]
- 1396. Sherman WR, Leavitt AL, Honchar MP, Hallcher LM, Phillips BE. Evidence that lithium alters phosphoinositide metabolism: chronic administration elevates primarily d-myo-inositol 1-phosphate in cerebral cortex of the rat. J Neurochem 36: 1947–1951, 1981 [DOI] [PubMed] [Google Scholar]
- 1397. Shewan A, Eastburn DJ, Mostov K. Phosphoinositides in cell architecture. Cold Spring Harb Perspect Biol 3: a004796, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1398. Shibatohge M, Kariya K, Liao Y, Hu CD, Watari Y, Goshima M, Shima F, Kataoka T. Identification of PLC210, a Caenorhabditis elegans phospholipase C, as a putative effector of Ras. J Biol Chem 273: 6218–6222, 1998 [DOI] [PubMed] [Google Scholar]
- 1399. Shimohama S, Homma Y, Suenaga T, Fujimoto S, Taniguchi T, Araki W, Yamaoka Y, Takenawa T, Kimura J. Aberrant accumulation of phospholipase C-delta in Alzheimer brains. Am J Pathol 139: 737–742, 1991 [PMC free article] [PubMed] [Google Scholar]
- 1400. Shimohama S, Perry G, Richey P, Takenawa T, Whitehouse PJ, Miyoshi K, Suenaga T, Matsumoto S, Nishimura M, Kimura J. Abnormal accumulation of phospholipase C-delta in filamentous inclusions of human neurodegenerative diseases. Neurosci Lett 162: 183–186, 1993 [DOI] [PubMed] [Google Scholar]
- 1401. Shin HW, Hayashi M, Christoforidis S, Lacas-Gervais S, Hoepfner S, Wenk MR, Modregger J, Uttenweiler-Joseph S, Wilm M, Nystuen A, Frankel WN, Solimena M, De Camilli P, Zeria LM. An enzymatic cascade of Rab5 effectors regulates phosphoinositide turnover in the endocytic pathway. J Cell Biol 170: 607–618, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1402. Shin N, Lee S, Ahn N, Kim SA, Ahn SG, YongPark Z, Chang S. Sorting nexin 9 interacts with dynamin 1 and N-WASP and coordinates synaptic vesicle endocytosis. J Biol Chem 282: 28939–28950, 2007 [DOI] [PubMed] [Google Scholar]
- 1403. Shin OH, Lu J, Rhee JS, Tomchick DR, Pang ZP, Wojcik SM, Camacho-Perez M, Brose N, Machius M, Rizo J, Rosenmund C, Sudhof TC. Munc13 C2B domain is an activity-dependent Ca2+ regulator of synaptic exocytosis. Nat Struct Mol Biol 17: 280–288, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1404. Shisheva A, Sbrissa D, Ikonomov O. Cloning, characterization, and expression of a novel Zn2+-binding FYVE finger-containing phosphoinositide kinase in insulin-sensitive cells. Mol Cell Biol 19: 623–634, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1405. Sidhu G, Li W, Laryngakis N, Bishai E, Balla T, Southwick F. Phosphoinositide 3-kinase is required for intracellular Listeria monocytogenes actin-based motility and filopod formation. J Biol Chem 280: 11379–11386, 2005 [DOI] [PubMed] [Google Scholar]
- 1406. Sidhu RS, Clough RR, Bhullar RP. Regulation of phospholipase C-delta1 through direct interactions with the small GTPase Ral and calmodulin. J Biol Chem 280: 21933–21941, 2005 [DOI] [PubMed] [Google Scholar]
- 1407. Sillence DJ, Downes CP. Subcellular distribution of agonist-stimulated phosphatidylinositol synthesis in 1321 N1 astrocytoma cells. Biochem J 290: 381–387, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1408. Simons JP, Al-Shawi R, Minogue S, Waugh MG, Wiedemann C, Evangelou S, Loesch A, Sihra TS, King R, Warner TT, Hsuan JJ. Loss of phosphatidylinositol 4-kinase 2alpha activity causes late onset degeneration of spinal cord axons. Proc Natl Acad Sci USA 106: 11535–11539, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1409. Simonsen A, Lippe R, Christoforidis S, Gaullier JM, Brech A, Callaghan J, Toh BH, Murphy C, Zerial M, Stenmark H. EEA1 links PI(3)K function to Rab5 regulation of endosome fusion. Nature 394: 494–498, 1998 [DOI] [PubMed] [Google Scholar]
- 1410. Singer AU, Waldo GL, Harden TK, Sondek J. A unique fold of phospholipase C-beta mediates dimerization and interaction with G alpha q. Nat Struct Biol 9: 32–36, 2002 [DOI] [PubMed] [Google Scholar]
- 1411. Singh R, Rao V, Shakila H, Gupta R, Khera A, Dhar N, Singh A, Koul A, Singh Y, Naseema M, Narayanan PR, Paramasivan CN, Ramanathan VD, Tyagi AK. Disruption of mptpB impairs the ability of Mycobacterium tuberculosis to survive in guinea pigs. Mol Microbiol 50: 751–762, 2003 [DOI] [PubMed] [Google Scholar]
- 1412. Skare P, Karlsson R. Evidence for two interaction regions for phosphatidylinositol(4,5)-bisphosphate on mammalian profilin I. FEBS Lett 522: 119–124, 2002 [DOI] [PubMed] [Google Scholar]
- 1413. Skinner HB, Alb JG, Jr, Whitters EA, Helmkamp GM, Jr, Bankaitis VA. Phospholipid transfer activity is relevant to but not sufficient for the essential function of the yeast SEC14 gene product. EMBO J 12: 4775–4784, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1414. Skinner HB, McGee TP, McMaster CR, Fry MR, Bell RM, Bankaitis VA. The Saccharomyces cerevisiae phosphatidylinositol-transfer protein effects a ligand-dependent inhibition of choline-phosphate cytidylyltransferase activity. Proc Natl Acad Sci USA 92: 112–116, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1415. Skippen A, Jones DH, Morgan CP, Li M, Cockcroft S. Mechanism of ADP ribosylation factor-stimulated phosphatidylinositol 4,5-bisphosphate synthesis in HL60 cells. J Biol Chem 277: 5823–5831, 2002 [DOI] [PubMed] [Google Scholar]
- 1416. Slagsvold T, Aasland R, Hirano S, Bache KG, Raiborg C, Trambaiolo D, Wakatsuki S, Stenmark H. EaP45 in mammalian ESCRT-II binds ubiquitin via a phosphoinositide-interacting GLUE domain. J Biol Chem 280: 19600–19606, 2005 [DOI] [PubMed] [Google Scholar]
- 1417. Sleeman MW, Wortley KE, Lai KM, Gowen LC, Kintner J, Kline WO, Garcia K, Stitt TN, Yancopoulos GD, Wiegand SJ, Glass DJ. Absence of the lipid phosphatase SHIP2 confers resistance to dietary obesity. Nat Med 11: 199–205, 2005 [DOI] [PubMed] [Google Scholar]
- 1418. Slepnev VI, De Camilli P. Accessory factors in clathrin-dependent synaptic vesicle endocytosis. Nat Rev Neurosci 1: 161–172, 2000 [DOI] [PubMed] [Google Scholar]
- 1419. Slepnev VI, Ochoa GC, Butler MH, Grabs D, De Camilli P. Role of phosphorylation in regulation of the assembly of endocytic coat complexes. Science 281: 821–824, 1998 [DOI] [PubMed] [Google Scholar]
- 1420. Sloan-Stanley GH. Anaerobic reactions of phospholipins in brain suspensions. Biochem J 53: 613–619, 1953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1421. Smith CD, Wells WW. Phosphorylation of rat liver nuclear envelopes. II. Characterization of in vitro lipid phosphorylation. J Biol Chem 258: 9368–9373, 1983 [PubMed] [Google Scholar]
- 1422. Smith GC, Jackson SP. The DNA-dependent protein kinase. Genes Dev 13: 916–934, 1999 [DOI] [PubMed] [Google Scholar]
- 1423. Smith RJ, Sam LM, Justen JM, Bundy GL, Bala GA, Bleasdale JE. Receptor-coupled signal transduction in human polymorphonuclear neutrophils: effects of a novel inhibitor of phospholipase C-dependent processes on cell responsiveness. J Pharmacol Exp Ther 253: 688–697, 1990 [PubMed] [Google Scholar]
- 1424. Smrcka AV, Brown JH, Holz GG. Role of phospholipase Cepsilon in physiological phosphoinositide signaling networks. Cell Signal 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1425. Smrcka AV, Hepler JR, Borwn KO, Sternweis PC. Regulation of polyphosphoinositide-specific phospholipase C activity by purified Gq. Science 251: 804–807, 1991 [DOI] [PubMed] [Google Scholar]
- 1426. Smrcka AV, Sternweis PC. Regulation of purified subtypes of phosphatidylinositol-specific phospholipase C beta by G protein alpha and betagamma subunits. J Biol Chem 268: 9667–9674, 1993 [PubMed] [Google Scholar]
- 1427. Smyth JT, Hwang SY, Tomita T, DeHaven WI, Mercer JC, Putney JW. Activation and regulation of store-operated calcium entry. J Cell Mol Med 14: 2337–2349, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1428. Snyder JT, Singer AU, Wing MR, Harden TK, Sondek J. The pleckstrin homology domain of phospholipase C-beta2 as an effector site for Rac. J Biol Chem 278: 21099–21104, 2003 [DOI] [PubMed] [Google Scholar]
- 1429. Soeda Y, Tsuneki H, Muranaka H, Mori N, Hosoh S, Ichihara Y, Kagawa S, Wang X, Toyooka N, Takamura Y, Uwano T, Nishijo H, Wada T, Sasaoka T. The inositol phosphatase SHIP2 negatively regulates insulin/IGF-I actions implicated in neuroprotection and memory function in mouse brain. Mol Endocrinol 24: 1965–1977, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1430. Soliman GA, Acosta-Jaquez HA, Dunlop EA, Ekim B, Maj NE, Tee AR, Fingar DC. mTOR Ser-2481 autophosphorylation monitors mTORC-specific catalytic activity and clarifies rapamycin mechanism of action. J Biol Chem 285: 7866–7879, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1431. Song C, Hu CD, Masago M, Kariyai K, Yamawaki-Kataoka Y, Shibatohge M, Wu D, Satoh T, Kataoka T. Regulation of a novel human phospholipase C, PLCepsilon, through membrane targeting by Ras. J Biol Chem 276: 2752–2757, 2001 [DOI] [PubMed] [Google Scholar]
- 1432. Song C, Satoh T, Edamatsu H, Wu D, Tadano M, Gao X, Kataoka T. Differential roles of Ras and Rap1 in growth factor-dependent activation of phospholipase C epsilon. Oncogene 21: 8105–8113, 2002 [DOI] [PubMed] [Google Scholar]
- 1433. Song MS, Carracedo A, Salmena L, Song SJ, Egia A, Malumbres M, Pandolfi PP. Nuclear PTEN regulates the APC-CDH1 tumor-suppressive complex in a phosphatase-independent manner. Cell 144: 187–199, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1434. Song MS, Salmena L, Pandolfi PP. The functions and regulation of the PTEN tumour suppressor. Nat Rev Mol Cell Biol 13: 283–296, 2012 [DOI] [PubMed] [Google Scholar]
- 1435. Songyang Z, Shoelson SE, Chaudhuri M, Gish G, Pawson T, Haser WG, King F, Roberts T, Ratnofsky S, Lechleider RJ. SH2 domains recognize specific phosphopeptide sequences. Cell 72: 767–778, 1993 [DOI] [PubMed] [Google Scholar]
- 1436. Sorli SC, Bunney TD, Sugden PH, Paterson HF, Katan M. Signaling properties and expression in normal and tumor tissues of two phospholipase C epsilon splice variants. Oncogene 24: 90–100, 2005 [DOI] [PubMed] [Google Scholar]
- 1437. Soulet F, Yarar D, Leonard M, Schmid SL. SNX9 regulates dynamin assembly and is required for efficient clathrin-mediated endocytosis. Mol Biol Cell 16: 2058–2067, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1438. Speed CJ, Matzaris M, Bird PI, Mitchell CA. Tissue distribution and intracellular localisation of the 75-kDa inositol polyphosphate 5-phosphatase. Eur J Biochem 234: 216–224, 1995 [DOI] [PubMed] [Google Scholar]
- 1439. Spudich G, Chibalina MV, Au JS, Arden SD, Buss F, Kendrick-Jones J. Myosin VI targeting to clathrin-coated structures and dimerization is mediated by binding to Disabled-2 and PtdIns(4,5)P2. Nat Cell Biol 9: 176–183, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1440. Srinivasan S, Seaman M, Nemoto Y, Daniell L, Suchy SF, Emr S, De Camilli P, Nussbaum R. Disruption of three phosphatidylinositol-polyphosphate 5-phosphatase genes from Saccharomyces cerevisiae results in pleiotropic abnormalities of vacuole morphology, cell shape, and osmohomeostasis. Eur J Cell Biol 74: 350–360, 1997 [PubMed] [Google Scholar]
- 1441. Srinivasan S, Wang F, Glavas S, Ott A, Hofmann F, Aktories K, Kalman D, Bourne HR. Rac and Cdc42 play distinct roles in regulating PI(3,4,5)P3 and polarity during neutrophil chemotaxis. J Cell Biol 160: 375–385, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1442. Srivastava S, Choudhury P, Li Z, Liu G, Nadkarni V, Ko K, Coetzee WA, Skolnik EY. Phosphatidylinositol 3-phosphate indirectly activates KCa3.1 via 14 amino acids in the carboxy terminus of KCa31. Mol Biol Cell 17: 146–154, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1443. Srivastava S, Ko K, Choudhury P, Li Z, Johnson AK, Nadkarni V, Unutmaz D, Coetzee WA, Skolnik EY. Phosphatidylinositol-3 phosphatase myotubularin-related protein 6 negatively regulates CD4 T cells. Mol Cell Biol 26: 5595–5602, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1444. Srivastava S, Li Z, Lin L, Liu G, Ko K, Coetzee WA, Skolnik EY. The phosphatidylinositol 3-phosphate phosphatase myotubularin- related protein 6 (MTMR6) is a negative regulator of the Ca2+-activated K+ channel KCa3.1 Mol Cell Biol 25: 3630–3638, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1445. Stack JH, DeWald DB, Takegawa K, Emr SD. Vesicle-mediated protein transport: regulatory interactions between the Vps15 protein kinase and the Vps34 PtdIns 3-kinase essential for protein sorting to the vacuole in yeast. J Cell Biol 129: 321–334, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1446. Stack JH, Emr SD. Vps34p required for yeast vacuolar protein sorting is a multiple specificity kinase that exhibits both protein kinase and phosphatidylinositol-specific PI 3-kinase activities. J Biol Chem 269: 31552–31562, 1994 [PubMed] [Google Scholar]
- 1447. Stahelin RV, Long F, Diraviyam K, Bruzik KS, Murray D, Cho W. Phosphatidylinositol 3-phosphate induces the membrane penetration of the FYVE domains of Vps27p and Hrs. J Biol Chem 277: 26379–26388, 2002 [DOI] [PubMed] [Google Scholar]
- 1448. Stallings JD, Tall EG, Pentyala S, Rebecchi MJ. Nuclear translocation of phospholipase C-delta1 is linked to the cell cycle and nuclear phosphatidylinositol 4,5-bisphosphate. J Biol Chem 280: 22060–22069, 2005 [DOI] [PubMed] [Google Scholar]
- 1449. Stallings JD, Zeng YX, Narvaez F, Rebecchi MJ. Phospholipase C-delta1 expression is linked to proliferation, DNA synthesis, and cyclin E levels. J Biol Chem 283: 13992–14001, 2008 [DOI] [PubMed] [Google Scholar]
- 1450. Stambolic V, Tsao MS, Macpherson D, Suzuki A, Chapman WB, Mak TW. High incidence of breast and endometrial neoplasia resembling human Cowden syndrome in pten+/− mice. Cancer Res 60: 3605–3611, 2000 [PubMed] [Google Scholar]
- 1451. Stauffer TP, Ahn S, Meyer T. Receptor-induced transient reduction in plasma membrane PtdIns(4,5)P2 concentration monitored in living cells. Curr Biol 8: 343–346, 1998 [DOI] [PubMed] [Google Scholar]
- 1452. Steck PA, Pershouse MA, Jasser SA, Yung WK, Lin H, Ligon AH, Langford LA, Baumgard ML, Hattier T, Davis T, Frye C, Hu R, Swedlund B, Teng DH, Tavtigian SV. Identification of a candidate tumour suppressor gene, MMAC1, at chromosome 10q23.3 that is mutated in multiple advanced cancers. Nat Genet 15: 356–362, 1997 [DOI] [PubMed] [Google Scholar]
- 1453. Stefan CJ, Audhya A, Emr SD. The yeast synaptojanin-like proteins control the cellular distribution of phosphatidylinositol (4,5)-bisphosphate. Mol Biol Cell 13: 542–557, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1454. Stefan CJ, Manford AG, Baird D, Yamada-Hanff J, Mao Y, Emr SD. Osh proteins regulate phosphoinositide metabolism at ER-plasma membrane contact sites. Cell 144: 389–401, 2011 [DOI] [PubMed] [Google Scholar]
- 1455. Steger DJ, Haswell ES, Miller AL, Wente SR, O'Shea EK. Regulation of chromatin remodeling by inositol polyphosphates. Science 299: 114–116, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1456. Steinberg F, Heesom KJ, Bass MD, Cullen PJ. SNX17 protects integrins from degradation by sorting between lysosomal and recycling pathways. J Cell Biol 197: 219–230, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1457. Stenmark H, Aasland R. FYVE-finger proteins–effectors of an inositol lipid. J Cell Sci 112: 4175–4183, 1999 [DOI] [PubMed] [Google Scholar]
- 1458. Stenmark H, Aasland R, Toh BH, D'Arrigo A. Endosomal localization of the autoantigen EEA1 is mediated by a Zinc-binding FYVE finger. J Biol Chem 271: 24048–24054, 1996 [DOI] [PubMed] [Google Scholar]
- 1459. Stenton GR, Mackenzie LF, Tam P, Cross JL, Harwig C, Raymond J, Toews J, Chernoff D, Macrury T, Szabo C. Characterization of AQX-1125, a small molecule SHIP1 activator: part 2. Efficacy studies in allergic and pulmonary inflammation models in vivo. Br J Pharmacol 168: 1519–1529, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1460. Stenton GR, Mackenzie LF, Tam P, Cross JL, Harwig C, Raymond J, Toews J, Wu J, Ogden N, Macrury T, Szabo C. Characterization of AQX-1125, a small molecule SHIP1 activator: part 1. Effects on inflammatory cell activation and chemotaxis in vitro and pharmacokinetic characterization in vivo. Br J Pharmacol 168: 1506–1518, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1461. Stephens L, Eguinoa A, Corey S, Jackson T, Hawkins PT. Receptor stimulated accumulation of phosphatidylinositol (3,4,5)-trisphosphate by G-protein mediated pathways in human myeloid derived cells. EMBO J 12: 2265–2273, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1462. Stephens L, Hawkins PT, Downes CP. Metabolic and structural evidence for the existence of a third species of polyphosphoinositide in cells: d-phosphatidyl-myo-inositol 3-phosphate. Biochem J 259: 267–276, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1463. Stephens L, Milne L, Hawkins P. Moving towards a better understanding of chemotaxis. Curr Biol 18: R485–494, 2008 [DOI] [PubMed] [Google Scholar]
- 1464. Stephens LR, Cooke FT, Walters R, Jackson TR, Volinia S, Gout I, Waterfield MD, Hawkins PT. Characterization of a phosphatidylinositol-specific phosphoinositide 3-kinase from mammalian cells. Curr Biol 4: 203–214, 1994 [DOI] [PubMed] [Google Scholar]
- 1465. Stephens LR, Eguinoa A, Erdjument-Bromage H, Lui M, Cooke F, Coadwell J, Smrcka AS, Thelen M, Cadwallader K, Tempst P, Hawkins PT. The G beta gamma sensitivity of a PI3K is dependent upon a tightly associated adaptor, p101. Cell 89: 105–114, 1997 [DOI] [PubMed] [Google Scholar]
- 1466. Stephens LR, Hawkins PT, Barker CJ, Downes CP. Synthesis of myo-inositol 1,3,4,5,6-pentakisphosphate from inositol phosphates generated by receptor activation. Biochem J 253: 721–733, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1467. Stephens LR, Jackson TR, Hawkins PT. Agonist-stimulated synthesis of phosphatidylinositol(3,4,5)-trisphosphate: a new intracellular signalling system? Biochim Biophys Acta 1179: 27–75, 1993 [DOI] [PubMed] [Google Scholar]
- 1468. Stephens LR, Smrcka AV, Cooke FT, Jackson TR, Sternweis PC, Hawkins PT. A novel phosphoinositide 3 kinase activity in myeloid-derived cells is activated by G protein beta-gamma subunits. Cell 77: 83–93, 1994 [DOI] [PubMed] [Google Scholar]
- 1469. Stevenson JM, Perera IY, Heilmann II, Persson S, Boss WF. Inositol signaling and plant growth. Trends Plant Sci 5: 357, 2000 [DOI] [PubMed] [Google Scholar]
- 1470. Stewart AJ, Morgan K, Farquharson C, Millar RP. Phospholipase C-eta enzymes as putative protein kinase C and Ca2+ signalling components in neuronal and neuroendocrine tissues. Neuroendocrinology 86: 243–248, 2007 [DOI] [PubMed] [Google Scholar]
- 1471. Stewart AJ, Mukherjee J, Roberts SJ, Lester D, Farquharson C. Identification of a novel class of mammalian phosphoinositol-specific phospholipase C enzymes. Int J Mol Med 15: 117–121, 2005 [PubMed] [Google Scholar]
- 1472. Stockinger W, Sailler B, Strasser V, Recheis B, Fasching D, Kahr L, Schneider WJ, Nimpf J. The PX-domain protein SNX17 interacts with members of the LDL receptor family and modulates endocytosis of the LDL receptor. EMBO J 21: 4259–4267, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1473. Stolz LE, Huynh CV, Thorner J, York JD. Identification and characterization of an essential family of inositol polyphosphate 5-phosphatases (INP51, INP52 and INP53 gene products) in the yeast Saccharomyces cerevisiae. Genetics 148: 1715–1729, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1474. Storey DJ, Shears SB, Kirk CJ, Michell RH. Stepwise enzymatic dephosphorylation of inositol 1,4,5-trisphosphate to inositol in liver. Nature 312: 374–376, 1984 [DOI] [PubMed] [Google Scholar]
- 1475. Stowell MH, Marks B, Wigge P, McMahon HT. Nucleotide-dependent conformational changes in dynamin: evidence for a mechanochemical molecular spring. Nat Cell Biol 1: 27–32, 1999 [DOI] [PubMed] [Google Scholar]
- 1476. Stoyanov B, Volinia S, Rubio I, Loubtchenkov M, Malek D, Stoyanova S, Vanhaesebroeck B, Dhand R, Nurnberg B, Gierschik P, Seedorf K, Hsuan JJ, Waterfield MD, Wetzker R. Cloning and characterization of a G protein-activated human phosphatidylinositol 3-kinase. Science 269: 690–693, 1995 [DOI] [PubMed] [Google Scholar]
- 1477. Stoyanova S, Bulgarelli-Leva G, Kirsch C, Hanck T, Klinger R, Wetzker R, Wymann MP. Lipid kinase and protein kinase activities of G-protein-coupled phosphoinositide kinase gamma: structure-activity analysis and interactions with wortmannin. Biochem J 324: 489–495, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1478. Strahl T, Hama H, Dewald DB, Thorner J. Yeast phosphatidylinositol 4-kinase, Pik1, has essential roles at the Golgi and in the nucleus. J Cell Biol 171: 967–979, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1479. Strahl T, Thorner J. Synthesis and function of membrane phosphoinositides in budding yeast, Saccharomyces cerevisiae. Biochim Biophys Acta 1771: 353–404, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1480. Straub SG, Sharp GW. A wortmannin-sensitive signal transduction pathway is involved in the stimulation of insulin release by vasoactive intestinal polypeptide and pituitary adenylate cyclase-activating polypeptide. J Biol Chem 271: 1660–1668, 1996 [DOI] [PubMed] [Google Scholar]
- 1481. Streb H, Irvine RF, Berridge MJ, Schulz I. Release of Ca2+ from a nonmitochondrial intracellular store in pancreatic acinar cells by inositol-1,4,5-trisphosphate. Nature 306: 67–68, 1983 [DOI] [PubMed] [Google Scholar]
- 1482. Suchy SF, Nussbaum RL. The deficiency of PIP2 5-phosphatase in Lowe syndrome affects actin polymerization. Am J Hum Genet 71: 1420–1427, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1483. Suchy SF, Olivos-Glander IM, Nussbaum RL. Lowe syndrome, a deficiency of phosphatidylinositol 4,5-bisphosphate 5-phosphatase in the Golgi apparatus. Hum Mol Genet 4: 2245–2250, 1995 [DOI] [PubMed] [Google Scholar]
- 1484. Sue AQAK, Fialkow L, Vlahos CJ, Schelm JA, Grinstein S, Butler J, Downey GP. Inhibition of neutrophil oxidative burst and granule secretion by wortmannin: potential role of MAP kinase and renaturable kinases. J Cell Physiol 172: 94–108, 1997 [DOI] [PubMed] [Google Scholar]
- 1485. Suer S, Sickmann A, Meyer HE, Herberg FW, Heilmeyer LM., Jr Human phosphatidylinositol 4-kinase isoform PI4K92. Expression of the recombinant enzyme and determination of multiple phosphorylation sites. Eur J Biochem 268: 2099–2106, 2001 [DOI] [PubMed] [Google Scholar]
- 1486. Sugimoto Y, Whitman M, Cantley LC, Erikson RL. Evidence that the Rous sarcoma virus transforming gene product phosphorylates phosphatidylinositol and diacylglycerol. Proc Natl Acad Sci USA 81: 2117–2121, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1487. Suh BC, Hille B. PIP2 is a necessary cofactor for ion channel function: how and why? Annu Rev Biophys 37: 175–195, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1488. Suh BC, Hille B. Recovery from muscarinic modulation of M current channels requires phosphatidylinositol 4,5-bisphosphate synthesis. Neuron 35: 507–520, 2002 [DOI] [PubMed] [Google Scholar]
- 1489. Suh BC, Inoue T, Meyer T, Hille B. Rapid chemically induced changes of PtdIns(4,5)P2 gate KCNQ ion channels. Science 314: 1454–1457, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1490. Suh BC, Kim DI, Falkenburger BH, Hille B. Membrane-localized beta-subunits alter the PIP2 regulation of high-voltage activated Ca2+ channels. Proc Natl Acad Sci USA 109: 3161–3166, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1491. Suh BC, Leal K, Hille B. Modulation of high-voltage activated Ca2+ channels by membrane phosphatidylinositol 4,5-bisphosphate. Neuron 67: 224–238, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1492. Suh HY, Lee DW, Lee KH, Ku B, Choi SJ, Woo JS, Kim YG, Oh BH. Structural insights into the dual nucleotide exchange and GDI displacement activity of SidM/DrrA. EMBO J 29: 496–504, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1493. Suh PG, Ryu SH, Moon KH, Suh HW, Rhee SG. Cloning and sequence of multiple forms of phospholipase-C. Cell 54: 161–169, 1988 [DOI] [PubMed] [Google Scholar]
- 1494. Suh PG, Ryu SH, Moon KH, Suh HW, Rhee SG. Inositol phospholipid-specific phospholipase C: complete cDNA and protein sequences and sequence homology to tyrosine kinase-related oncogene products. Proc Natl Acad Sci USA 85: 5419–5423, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1495. Sui JL, Petit-Jacques J, Logothetis DE. Activation of the atrial KACh channel by the betagamma subunits of G proteins or intracellular Na+ ions depends on the presence of phosphatidylinositol phosphates. Proc Natl Acad Sci USA 95: 1307–1312, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1496. Suire S, Coadwell J, Ferguson GJ, Davidson K, Hawkins P, Stephens L. p84, a new Gbetagamma-activated regulatory subunit of the type IB phosphoinositide 3-kinase p110gamma. Curr Biol 15: 566–570, 2005 [DOI] [PubMed] [Google Scholar]
- 1497. Suire S, Condliffe AM, Ferguson GJ, Ellson CD, Guillou H, Davidson K, Welch H, Coadwell J, Turner M, Chilvers ER, Hawkins PT, Stephens L. Gbetagammas and the Ras binding domain of p110gamma are both important regulators of PI(3)Kgamma signalling in neutrophils. Nat Cell Biol 8: 1303–1309, 2006 [DOI] [PubMed] [Google Scholar]
- 1498. Sun Y, Ling K, Wagoner MP, Anderson RA. Type I gamma phosphatidylinositol phosphate kinase is required for EGF-stimulated directional cell migration. J Cell Biol 178: 297–308, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1499. Sun Y, Turbin DA, Ling K, Thapa N, Leung S, Huntsman DG, Anderson RA. Type I gamma phosphatidylinositol phosphate kinase modulates invasion and proliferation and its expression correlates with poor prognosis in breast cancer. Breast Cancer Res 12: R6, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1500. Suzuki A, de la Pompa JL, Stambolic V, Elia AJ, Sasaki T, del Barco Barrantes I, Ho A, Wakeham A, Itie A, Khoo W., Fukumoto M, Mak TW. High cancer susceptibility and embryonic lethality associated with mutation of the PTEN tumor suppressor gene in mice. Curr Biol 8: 1169–1178, 1998 [DOI] [PubMed] [Google Scholar]
- 1501. Suzuki A, Nakano T, Mak TW, Sasaki T. Portrait of PTEN: messages from mutant mice. Cancer Sci 99: 209–213, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1502. Suzuki E, Hirosawa K. Immunolocalization of a Drosophila phosphatidylinositol transfer protein (rdgB) in normal and rdgA mutant photoreceptor cells with special reference to the subrhabdomeric cisternae. J Electron Microsc 43: 183–189, 1994 [PubMed] [Google Scholar]
- 1503. Suzuki H, Terauchi Y, Fujiwara M, Aizawa S, Yazaki Y, Kadowaki T, Koyasu S. Xid-like immunodeficiency in mice with disruption of the p85alpha subunit of phosphoinositide 3-kinase. Science 283: 390–392, 1999 [DOI] [PubMed] [Google Scholar]
- 1504. Suzuki T, Banno Y, Nozawa Y. Partial purification and characterization of two forms of phosphatidylinositol 4-phosphate 5-kinase from human platelet membrane. Thromb Res 64: 45–56, 1991 [DOI] [PubMed] [Google Scholar]
- 1505. Swaney KF, Huang CH, Devreotes PN. Eukaryotic chemotaxis: a network of signaling pathways controls motility, directional sensing, and polarity. Annu Rev Biophys 39: 265–289, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1506. Sweitzer SM, Hinshaw JE. Dynamin undergoes a GTP-dependent conformational change causing vesiculation. Cell 93: 1021–1029, 1998 [DOI] [PubMed] [Google Scholar]
- 1507. Sylvia V, Curtin G, Norman J, Stec J, Busbee D. Activation of a low specific activity form of DNA polymerase alpha by inositol-1,4-bisphosphate. Cell 54: 651–658, 1988 [DOI] [PubMed] [Google Scholar]
- 1508. Sylvia VL, Joe CO, Norman JO, Curtin GM, Tilley RD, Busbee DL. Interaction of phosphatidylinositol-4-monophosphate with a low activity form of DNA polymerase alpha: a potential mechanism for enzyme activation. Int J Biochem 21: 347–353, 1989 [DOI] [PubMed] [Google Scholar]
- 1509. Szentpetery Z, Varnai P, Balla T. Acute manipulation of Golgi phosphoinositides to assess their importance in cellular trafficking and signaling. Proc Natl Acad Sci USA 107: 8225–8230, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1510. Tabuchi M, Audhya A, Parsons AB, Boone C, Emr SD. The PI(4,5)P2 and TORC2 binding proteins, Slm1 and Slm2, function in sphingolipid regulation. Mol Biol Cell 26: 5861–5875, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1511. Tadano M, Edamatsu H, Minamisawa S, Yokoyama U, Ishikawa Y, Suzuki N, Saito H, Wu D, Masago-Toda M, Yamawaki-Kataoka Y, Setsu T, Terashima T, Maeda S, Satoh T, Kataoka T. Congenital semilunar valvulogenesis defect in mice deficient in phospholipase C epsilon. Mol Cell Biol 25: 2191–2199, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1512. Taddei A. Active genes at the nuclear pore complex. Curr Opin Cell Biol 19: 305–310, 2007 [DOI] [PubMed] [Google Scholar]
- 1513. Tai AW, Benita Y, Peng LF, Kim SS, Sakamoto N, Xavier RJ, Chung RT. A functional genomic screen identifies cellular cofactors of hepatitis C virus replication. Cell Host Microbe 5: 298–307, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1514. Tai AW, Salloum S. The role of the phosphatidylinositol 4-kinase PI4KA in hepatitis C virus-induced host membrane rearrangement. PLoS One 6: e26300, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1515. Takahashi H, Masuda K, Ando T, Kobayashi T, Honda H. Prognostic predictor with multiple fuzzy neural models using expression profiles from DNA microarray for metastases of breast cancer. J Biosci Bioeng 98: 193–199, 2004 [DOI] [PubMed] [Google Scholar]
- 1516. Takahashi T, Yamaguchi S, Chida K, Shibuya M. A single autophosphorylation site on KDR/Flk-1 is essential for VEGF-A-dependent activation of PLC-gamma and DNA synthesis in vascular endothelial cells. EMBO J 20: 2768–2778, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1517. Takahashi Y, Coppola D, Matsushita N, Cualing HD, Sun M, Sato Y, Liang C, Jung JU, Cheng JQ, Mule JJ, Pledger WJ, Wang HG. Bif-1 interacts with Beclin 1 through UVRAG and regulates autophagy and tumorigenesis. Nat Cell Biol 9: 1142–1151, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1518. Takeda K, Sasaki AT, Ha H, Seung HA, Firtel RA. Role of phosphatidylinositol 3-kinases in chemotaxis in Dictyostelium. J Biol Chem 282: 11874–11884, 2007 [DOI] [PubMed] [Google Scholar]
- 1519. Takenaka K, Fukami K, Otsuki M, Nakamura Y, Kataoka Y, Wada M, Tsuji K, Nishikawa S, Yoshida N, Takenawa T. Role of phospholipase C-L2, a novel phospholipase C-like protein that lacks lipase activity, in B-cell receptor signaling. Mol Cell Biol 23: 7329–7338, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1520. Takenawa T. Phosphoinositide-binding interface proteins involved in shaping cell membranes. Proc Jpn Acad Ser B Phys Biol Sci 86: 509–523, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1521. Takenawa T, Nagai Y. Purification of phosphatidylinositol-specific phospholipase C from rat liver. J Biol Chem 256: 6769–6775, 1981 [PubMed] [Google Scholar]
- 1522. Takenawa T, Saito M, Nagai Y, Egawa K. Solubilization of the enzyme catalyzing CDP-diglyceride-independent incorporation of myo-inositol into phosphatidyl inositol and its comparison to CDP-diglyceride:inositol transferase. Arch Biochem Biophys 182: 244–250, 1977 [DOI] [PubMed] [Google Scholar]
- 1523. Takeshita S, Namba N, Zhao JJ, Jiang Y, Genant HK, Silva MJ, Brodt MD, Helgason CD, Kalesnikoff J, Rauh MJ, Humphries RK, Krystal G, Teitelbaum SL, Ross FP. SHIP-deficient mice are severely osteoporotic due to increased numbers of hyper-resorptive osteoclasts. Nat Med 8: 943–949, 2002 [DOI] [PubMed] [Google Scholar]
- 1524. Takeuchi H, Oike M, Paterson HF, Allen VV, Kanematsu T, Ito Y, Erneux C, Katan M, Hirata M. Inhibition of Ca2+ signalling by p130, a phospholipase-C-related catalytically inactive protein: critical role of the p130 pleckstrin homology domain. Biochem J 349: 357–368, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1525. Takezawa R, Schmitz C, Demeuse P, Scharenberg AM, Penner R, Fleig A. Receptor-mediated regulation of the TRPM7 channel through its endogenous protein kinase domain. Proc Natl Acad Sci USA 101: 6009–6014, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1526. Tanaka O, Kondo H. Localization of mRNAs for three novel members (beta 3, beta 4 and gamma 2) of phospholipase C family in mature rat brain. Neurosci Lett 182: 17–20, 1994 [DOI] [PubMed] [Google Scholar]
- 1527. Tanaka S, Hosaka K. Cloning of a cDNA encoding a second phosphatidylinositol transfer protein of rat brain by complementation of the yeast sec14 mutation. J Biochem 115: 981–984, 1994 [DOI] [PubMed] [Google Scholar]
- 1528. Tanaka S, Nikawa J, Imai H, Yamashita S, Hosaka K. Molecular cloning of rat phosphatidylinositol synthase cDNA by functional complementation of the yeast Saccharomyces cerevisiae pis mutation. FEBS Lett 393: 89–92, 1996 [DOI] [PubMed] [Google Scholar]
- 1529. Tani M, Kuge O. Requirement of a specific group of sphingolipid-metabolizing enzyme for growth of yeast Saccharomyces cerevisiae under impaired metabolism of glycerophospholipids. Mol Microbiol 78: 395–413, 2010 [DOI] [PubMed] [Google Scholar]
- 1530. Taniguchi CM, Winnay J, Kondo T, Bronson RT, Guimaraes AR, Aleman JO, Luo J, Stephanopoulos G, Weissleder R, Cantley LC, Kahn CR. The phosphoinositide 3-kinase regulatory subunit p85alpha can exert tumor suppressor properties through negative regulation of growth factor signaling. Cancer Res 70: 5305–5315, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1531. Tapparel C, Reymond A, Girardet C, Guillou L, Lyle R, Lamon C, Hutter P, Antonarakis SE. The TPTE gene family: cellular expression, subcellular localization and alternative splicing. Gene 323: 189–199, 2003 [DOI] [PubMed] [Google Scholar]
- 1532. Tassa A, Roux MP, Attaix D, Bechet DM. Class III phosphoinositide 3-kinase–Beclin1 complex mediates the amino acid-dependent regulation of autophagy in C2C12 myotubes. Biochem J 376: 577–586, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1533. Tawk L, Chicanne G, Dubremetz JF, Richard V, Payrastre B, Vial HJ, Roy C, Wengelnik K. Phosphatidylinositol 3-phosphate, an essential lipid in Plasmodium, localizes to the food vacuole membrane and the apicoplast. Eukaryot Cell 9: 1519–1530, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1534. Taylor CW, Prole DL. Ca2+ signalling by IP3 receptors. Subcell Biochem 59: 1–34, 2012 [DOI] [PubMed] [Google Scholar]
- 1535. Taylor CW, Tovey SC. IP3 receptors: toward understanding their activation. Cold Spring Harb Perspect Biol 2: a004010, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1536. Taylor GS, Maehama T, Dixon JE. Myotubularin, a protein tyrosine phosphatase mutated in myotubular myopathy, dephosphorylates the lipid second messenger, phosphatidylinositol 3-phosphate. Proc Natl Acad Sci USA 97: 8910–8915, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1537. Taylor MJ, Perrais D, Merrifield CJ. A high precision survey of the molecular dynamics of mammalian clathrin-mediated endocytosis. PLoS Biol 9: e1000604, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1538. Taylor SJ, Chae HZ, Rhee SG, Exton JH. Activation of the beta 1 isozyme of phospholipase C by alpha subunits of the Gq class of G proteins. Nature 350: 516–518, 1991 [DOI] [PubMed] [Google Scholar]
- 1539. Taylor SJ, Exton JH. Guanine-nucleotide and hormone regulation of polyphosphoinositide phospholipase C activity of rat liver plasma membranes. Bivalent-cation and phospholipid requirements. Biochem J 248: 791–799, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1540. Teasdale RD, Collins BM. Insights into the PX (phox-homology) domain and SNX (sorting nexin) protein families: structures, functions and roles in disease. Biochem J 441: 39–59, 2012 [DOI] [PubMed] [Google Scholar]
- 1541. Tee AR, Fingar DC, Manning BD, Kwiatkowski DJ, Cantley LC, Blenis J. Tuberous sclerosis complex-1 and -2 gene products function together to inhibit mammalian target of rapamycin (mTOR)-mediated downstream signaling. Proc Natl Acad Sci USA 99: 13571–13576, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1542. Teis D, Saksena S, Emr SD. Ordered assembly of the ESCRT-III complex on endosomes is required to sequester cargo during MVB formation. Dev Cell 15: 578–589, 2008 [DOI] [PubMed] [Google Scholar]
- 1543. Terauchi Y, Tsuji Y, Satoh S, Minoura H, Murakami K, Okuno A, Inukai K, Asano T, Kaburagi Y, Ueki K, Nakajima H, Hanafusa T, Matsuzawa Y, Sekihara H, Yin Y, Barrett JC, Oda H, Ishikawa T, Akanuma Y, Komuro I, Suzuki M, Yamamura K, Kodama T, Suzuki H, Kadowaki T. Increased insulin sensitivity and hypoglycaemia in mice lacking the p85 alpha subunit of phosphoinositide 3-kinase. Nat Genet 21: 230–235, 1999 [DOI] [PubMed] [Google Scholar]
- 1544. Terebiznik MR, Vieira OV, Marcus SL, Slade A, Yip CM, Trimble WS, Meyer T, Finlay BB, Grinstein S. Elimination of host cell PtdIns(4,5)P2 by bacterial SigD promotes membrane fission during invasion by Salmonella. Nat Cell Biol 4: 766–773, 2002 [DOI] [PubMed] [Google Scholar]
- 1545. Tersar K, Boentert M, Berger P, Bonneick S, Wessig C, Toyka KV, Young P, Suter U. Mtmr13/Sbf2-deficient mice: an animal model for CMT4B2. Hum Mol Genet 16: 2991–3001, 2007 [DOI] [PubMed] [Google Scholar]
- 1546. Terunuma M, Jang IS, Ha SH, Kittler JT, Kanematsu T, Jovanovic JN, Nakayama KI, Akaike N, Ryu SH, Moss SJ, Hirata M. GABAA receptor phospho-dependent modulation is regulated by phospholipase C-related inactive protein type 1, a novel protein phosphatase 1 anchoring protein. J Neurosci 24: 7074–7084, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1547. Thakur PC, Stuckenholz C, Rivera MR, Davison JM, Yao JK, Amsterdam A, Sadler KC, Bahary N. Lack of de novo phosphatidylinositol synthesis leads to endoplasmic reticulum stress and hepatic steatosis in cdipt-deficient zebrafish. Hepatology 54: 452–462, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1548. Tham TN, Gouin E, Rubinstein E, Boucheix C, Cossart P, Pizarro-Cerda J. Tetraspanin CD81 is required for Listeria monocytogenes invasion. Infect Immun 78: 204–209, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1549. Theoharides TC, Cochrane DE. Critical role of mast cells in inflammatory diseases and the effect of acute stress. J Neuroimmunol 146: 1–12, 2004 [DOI] [PubMed] [Google Scholar]
- 1550. Thieman JR, Mishra SK, Ling K, Doray B, Anderson RA, Traub LM. Clathrin regulates the association of PIPKIgamma661 with the AP-2 adaptor beta2 appendage. J Biol Chem 284: 13924–13939, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1551. Thomas GMH, Cunningham E, Fensome A, Ball A, Totty NF, Truong O, Hsuan JJ, Cockcroft S. An essential role of phosphatidylinositol transfer protein in phospholipase C-mediated inositol lipid signaling. Cell 74: 919–928, 1993 [DOI] [PubMed] [Google Scholar]
- 1552. Thompson AK, Mostafapour SP, Denlinger LC, Bleasdale JE, Fisher SK. The aminosteroid U-73122 inhibits muscarinic receptor sequestration and phosphoinositide hydrolysis in SK-N-SH neuroblastoma cells: a role for Gp in receptor compartmentation. J Biol Chem 266: 23856–23862, 1991 [PubMed] [Google Scholar]
- 1553. Thompson JL, Shuttleworth TJ. Orai channel-dependent activation of phospholipase C-delta: a novel mechanism for the effects of calcium entry on calcium oscillations. J Physiol 589: 5057–5069, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1554. Thompson W, Dawson RM. The hydrolysis of triphosphoinositide by extracts of ox brain. Biochem J 91: 233–236, 1964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1555. Thompson W, Dawson RMC. The triphosphoinositide phosphodiesterase of brain tissue. Biochem J 91: 237–243, 1964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1556. Thyagarajan B, Lukacs V, Rohacs T. Hydrolysis of phosphatidylinositol 4,5-bisphosphate mediates calcium-induced inactivation of TRPV6 channels. J Biol Chem 283: 14980–14987, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1557. Tilley SJ, Skippen A, Murray-Rust J, Swigart PM, Stewart A, Morgan CP, Cockcroft S, McDonald NQ. Structure-function analysis of human phosphatidylinositol transfer protein alpha bound to phosphatidylinositol. Structure 12: 317–326, 2004 [DOI] [PubMed] [Google Scholar]
- 1558. To Y, Ito K, Kizawa Y, Failla M, Ito M, Kusama T, Elliott WM, Hogg JC, Adcock IM, Barnes PJ. Targeting phosphoinositide-3-kinase-delta with theophylline reverses corticosteroid insensitivity in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 182: 897–904, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1559. Toker A. Phosphoinositide 3-kinases-a historical perspective. Subcell Biochem 58: 95–110, 2012 [DOI] [PubMed] [Google Scholar]
- 1560. Tolias KF, Couvillon AD, Cantley LC, Carpenter CL. Characterization of a Rac1- and RhoGDI-associated lipid kinase signaling complex. Mol Cell Biol 18: 762–770, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1561. Tolkacheva T, Boddapati M, Sanfiz A, Tsuchida K, Kimmelman AC, Chan AM. Regulation of PTEN binding to MAGI-2 by two putative phosphorylation sites at threonine 382 and 383. Cancer Res 61: 4985–4989, 2001 [PubMed] [Google Scholar]
- 1562. Tolkacheva T, Chan AM. Inhibition of H-Ras transformation by the PTEN/MMAC1/TEP1 tumor suppressor gene. Oncogene 19: 680–689, 2000 [DOI] [PubMed] [Google Scholar]
- 1563. Tomlinson RV, Ballou CE. Complete characterization of the myo-inositol polyphosphates from beef brain phosphoinositide. J Biol Chem 236: 1902–1906, 1961 [PubMed] [Google Scholar]
- 1564. Tong Q, Gamper N, Medina JL, Shapiro MS, Stockand JD. Direct activation of the epithelial Na+ channel by phosphatidylinositol 3,4,5-trisphosphate and phosphatidylinositol 3,4-bisphosphate produced by phosphoinositide 3-OH kinase. J Biol Chem 279: 22654–22663, 2004 [DOI] [PubMed] [Google Scholar]
- 1565. Tooze SA, Yoshimori T. The origin of the autophagosomal membrane. Nat Cell Biol 12: 831–835, 2010 [DOI] [PubMed] [Google Scholar]
- 1566. Tosch V, Rohde HM, Tronchere H, Zanoteli E, Monroy N, Kretz C, Dondaine N, Payrastre B, Mandel JL, Laporte J. A novel PtdIns3P and PtdIns(3,5)P2 phosphatase with an inactivating variant in centronuclear myopathy. Hum Mol Genet 15: 3098–3106, 2006 [DOI] [PubMed] [Google Scholar]
- 1567. Toth B, Balla A, Ma H, Knight ZA, Shokat KM, Balla T. Phosphatidylinositol 4-kinase IIIbeta regulates the transport of ceramide between the endoplasmic reticulum and Golgi. J Biol Chem 281: 36369–36377, 2006 [DOI] [PubMed] [Google Scholar]
- 1568. Toth B, Csanady L. Pore collapse underlies irreversible inactivation of TRPM2 cation channel currents. Proc Natl Acad Sci USA 109: 13440–13445, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1569. Toth DJ, Toth J, Gulyas G, Balla A, Balla T, Hunyady L, Varnai P. Acute depletion of plasma membrane phosphatidylinositol 4,5-bisphosphate impairs specific steps in G protein-coupled receptor endocytosis. J Cell Sci 125: 2185–2197, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1570. Touchberry CD, Bales IK, Stone JK, Rohrberg TJ, Parelkar NK, Nguyen T, Fuentes O, Liu X, Qu CK, Andresen JJ, Valdivia HH, Brotto M, Wacker MJ. Phosphatidylinositol 3,5-bisphosphate [PI(3,5)P2] potentiates cardiac contractility via activation of the ryanodine receptor. J Biol Chem 285: 40312–40321, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1571. Traer CJ, Rutherford AC, Palmer KJ, Wassmer T, Oakley J, Attar N, Carlton JG, Kremerskothen J, Stephens DJ, Cullen PJ. SNX4 coordinates endosomal sorting of TfnR with dynein-mediated transport into the endocytic recycling compartment. Nat Cell Biol 9: 1370–1380, 2007 [DOI] [PubMed] [Google Scholar]
- 1572. Traynor-Kaplan AE, Thompson BL, Harris AL, Taylor P, Omann GM, Sklar LA. Transient increase in phosphatidylinositol 3,4-bisphosphate and phosphatidylinositol trisphosphate during activation of human neutrophils. J Biol Chem 264: 15668–15673, 1989 [PubMed] [Google Scholar]
- 1573. Trebak M, Lemonnier L, DeHaven WI, Wedel BJ, Bird GS, Putney JW., Jr Complex functions of phosphatidylinositol 4,5-bisphosphate in regulation of TRPC5 cation channels. Pflügers Arch 457: 757–769, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1574. Trifaro JM, Gasman S, Gutierrez LM. Cytoskeletal control of vesicle transport and exocytosis in chromaffin cells. Acta Physiol 192: 165–172, 2008 [DOI] [PubMed] [Google Scholar]
- 1575. Trivedi D, Padinjat R. RdgB proteins: functions in lipid homeostasis and signal transduction. Biochim Biophys Acta 1771: 692–699, 2007 [DOI] [PubMed] [Google Scholar]
- 1576. Tronchere H, Laporte J, Pendaries C, Chaussade C, Liaubet L, Pirola L, Mandel JL, Payrastre B. Production of phosphatidylinositol 5-phosphate by the phosphoinositide 3-phosphatase myotubularin in mammalian cells. J Biol Chem 279: 7304–7312, 2004 [DOI] [PubMed] [Google Scholar]
- 1577. Trotard M, Lepere-Douard C, Regeard M, Piquet-Pellorce C, Lavillette D, Cosset FL, Gripon P, Le Seyec J. Kinases required in hepatitis C virus entry and replication highlighted by small interference RNA screening. FASEB J 23: 3780–3789, 2009 [DOI] [PubMed] [Google Scholar]
- 1578. Trotman LC, Niki M, Dotan ZA, Koutcher JA, Di Cristofano A, Xiao A, Khoo AS, Roy-Burman P, Greenberg NM, Van Dyke T, Cordon-Cardo C, Pandolfi PP. Pten dose dictates cancer progression in the prostate. PLoS Biol 1: E59, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1579. Trotman LC, Wang X, Alimonti A, Chen Z, Teruya-Feldstein J, Yang H, Pavletich NP, Carver BS, Cordon-Cardo C, Erdjument-Bromage H, Tempst P, Chi SG, Kim HJ, Misteli T, Jiang X, Pandolfi PP. Ubiquitination regulates PTEN nuclear import and tumor suppression. Cell 128: 141–156, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1580. Trotter PJ, Wu WI, Pedretti J, Yates R, Voelker DR. A genetic screen for aminophospholipid transport mutants identifies the phosphatidylinositol 4-kinase, Stt4p, as an essential component in phosphatidylserine metabolism. J Biol Chem 273: 13189–13196, 1998 [DOI] [PubMed] [Google Scholar]
- 1581. Tsujishita Y, Guo S, Stolz LE, York JD, Hurley JH. Specificity determinants in phosphoinositide dephosphorylation: crystal structure of an archetypal inositol polyphosphate 5-phosphatase. Cell 105: 379–389, 2001 [DOI] [PubMed] [Google Scholar]
- 1582. Tucker WC, Chapman ER. Role of synaptotagmin in Ca2+-triggered exocytosis. Biochem J 366: 1–13, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1583. Turner SJ, Domin J, Waterfield MD, Ward SG, Westwick J. The CC chemokine monocyte chemotactic peptide-1 activates both the class I p85/p110 phosphatidylinositol 3-kinase and the class II PI3K-C2alpha. J Biol Chem 273: 25987–25995, 1998 [DOI] [PubMed] [Google Scholar]
- 1584. Tyers M, Rachubinski RA, Stewart MI, Varrichio AM, Shorr RG, Haslam RJ, Harley CB. Molecular cloning and expression of the major protein kinase C substrate of platelets. Nature 333: 470–473, 1988 [DOI] [PubMed] [Google Scholar]
- 1585. Ueki K, Fruman DA, Brachmann SM, Tseng YH, Cantley LC, Kahn CR. Molecular balance between the regulatory and catalytic subunits of phosphoinositide 3-kinase regulates cell signaling and survival. Mol Cell Biol 22: 965–977, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1586. Ueno T, Falkenburger BH, Pohlmeyer C, Inoue T. Triggering actin comets versus membrane ruffles: distinctive effects of phosphoinositides on actin reorganization. Sci Signal 4: ra87, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1587. Ufret-Vincenty CA, Klein RM, Hua L, Angueyra J, Gordon SE. Localization of the PIP2 sensor of TRPV1 ion channels. J Biol Chem 286: 9688–9698, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1588. Ugolev Y, Berdichevsky Y, Weinbaum C, Pick E. Dissociation of Rac1(GDP)RhoGDI complexes by the cooperative action of anionic liposomes containing phosphatidylinositol 3,4,5-trisphosphate, Rac guanine nucleotide exchange factor, and GTP. J Biol Chem 283: 22257–22271, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1589. Uldry M, Ibberson M, Horisberger JD, Chatton JY, Riederer BM, Thorens B. Identification of a mammalian H+-myo-inositol symporter expressed predominantly in the brain. EMBO J 20: 4467–4477, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1590. Uldry M, Steiner P, Zurich MG, Beguin P, Hirling H, Dolci W, Thorens B. Regulated exocytosis of an H+/myo-inositol symporter at synapses and growth cones. EMBO J 23: 531–540, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1591. Umeki N, Jung HS, Sakai T, Sato O, Ikebe R, Ikebe M. Phospholipid-dependent regulation of the motor activity of myosin X. Nat Struct Mol Biol 18: 783–788, 2011 [DOI] [PubMed] [Google Scholar]
- 1592. Underhill DM, Ozinsky A. Phagocytosis of microbes: complexity in action. Annu Rev Immunol 20: 825–852, 2002 [DOI] [PubMed] [Google Scholar]
- 1593. Ungewickell A, Hugge C, Kisseleva M, Chang SC, Zou J, Feng Y, Galyov EE, Wilson M, Majerus PW. The identification and characterization of two phosphatidylinositol-4,5-bisphosphate 4-phosphatases. Proc Natl Acad Sci USA 102: 18854–18859, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1594. Ungewickell A, Ward ME, Ungewickell E, Majerus PW. The inositol polyphosphate 5-phosphatase Ocrl associates with endosomes that are partially coated with clathrin. Proc Natl Acad Sci USA 101: 13501–13506, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1595. Urade R, Nasu M, Moriyama T, Wada K, Kito M. Protein degradation by the phosphoinositide-specific phospholipase C-alpha family from rat liver endoplasmic reticulum. J Biol Chem 267: 15152–15159, 1992 [PubMed] [Google Scholar]
- 1596. Vaid A, Ranjan R, Smythe WA, Hoppe HC, Sharma P. PfPI3K, a phosphatidylinositol-3 kinase from Plasmodium falciparum, is exported to the host erythrocyte and is involved in hemoglobin trafficking. Blood 115: 2500–2507, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1597. Vaillancourt FH, Pilote L, Cartier M, Lippens J, Liuzzi M, Bethell RC, Cordingley MG, Kukolj G. Identification of a lipid kinase as a host factor involved in hepatitis C virus RNA replication. Virology 387: 5–10, 2009 [DOI] [PubMed] [Google Scholar]
- 1598. Vaithianathan T, Bukiya A, Liu J, Liu P, Asuncion-Chin M, Fan Z, Dopico A. Direct regulation of BK channels by phosphatidylinositol 4,5-bisphosphate as a novel signaling pathway. J Gen Physiol 132: 13–28, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1599. Valente EM, Brancati F, Dallapiccola B. Genotypes and phenotypes of Joubert syndrome and related disorders. Eur J Med Genet 51: 1–23, 2008 [DOI] [PubMed] [Google Scholar]
- 1600. Van 't Veer LJ, Dai H, van de Vijver MJ, He YD, Hart AA, Mao M, Peterse HL, van der Kooy K, Marton MJ, Witteveen AT, Schreiber GJ, Kerkhoven RM, Roberts C, Linsley PS, Bernards R, Friend SH. Gene expression profiling predicts clinical outcome of breast cancer. Nature 415: 530–536, 2002 [DOI] [PubMed] [Google Scholar]
- 1601. Van Dam EM, Ten Broeke T, Jansen K, Spijkers P, Stoorvogel W. Endocytosed transferrin receptors recycle via distinct dynamin and phosphatidylinositol 3-kinase-dependent pathways. J Biol Chem 277: 48876–48883, 2002 [DOI] [PubMed] [Google Scholar]
- 1602. Van den Bogaart G, Meyenberg K, Diederichsen U, Jahn R. Phosphatidylinositol 4,5-bisphosphate increases the Ca2+ affinity of synaptotagmin-1 40-fold. J Biol Chem 287: 16447–16453, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1603. Van den Bogaart G, Meyenberg K, Risselada HJ, Amin H, Willig KI, Hubrich BE, Dier M, Hell SW, Grubmuller H, Diederichsen U, Jahn R. Membrane protein sequestering by ionic protein-lipid interactions. Nature 479: 552–555, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1604. Van Keymeulen A, Wong K, Knight ZA, Govaerts C, Hahn KM, Shokat KM, Bourne HR. To stabilize neutrophil polarity, PIP3 and Cdc42 augment RhoA activity at the back as well as signals at the front. J Cell Biol 174: 437–445, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1605. Van Tiel CM, Luberto C, Snoek GT, Hannun YA, Wirtz KW. Rapid replenishment of sphingomyelin in the plasma membrane upon degradation by sphingomyelinase in NIH3T3 cells overexpressing the phosphatidylinositol transfer protein beta. Biochem J 346: 537–543, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1606. Van Weering JR, Verkade P, Cullen PJ. SNX-BAR proteins in phosphoinositide-mediated, tubular-based endosomal sorting. Semin Cell Dev Biol 21: 371–380, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1607. Van Weering JR, Verkade P, Cullen PJ. SNX-BAR-mediated endosome tubulation is co-ordinated with endosome maturation. Traffic 13: 94–107, 2012 [DOI] [PubMed] [Google Scholar]
- 1608. Vander Heiden MG, Lunt SY, Dayton TL, Fiske BP, Israelsen WJ, Mattaini KR, Vokes NI, Stephanopoulos G, Cantley LC, Metallo CM, Locasale JW. Metabolic pathway alterations that support cell proliferation. Cold Spring Harb Symp Quant Biol 2012 [DOI] [PubMed] [Google Scholar]
- 1609. Vanhaesebroeck B, Ali K, Bilancio A, Geering B, Foukas LC. Signalling by PI3K isoforms: insights from gene-targeted mice. Trends Biochem Sci 30: 194–204, 2005 [DOI] [PubMed] [Google Scholar]
- 1610. Vanhaesebroeck B, Guillermet-Guibert J, Graupera M, Bilanges B. The emerging mechanisms of isoform-specific PI3K signalling. Nat Rev Mol Cell Biol 11: 329–341, 2010 [DOI] [PubMed] [Google Scholar]
- 1611. Vanhaesebroeck B, Higashi K, Raven C, Welham M, Anderson S, Brennan P, Ward SG, Waterfield MD. Autophosphorylation of p110delta phosphoinositide 3-kinase: a new paradigm for the regulation of lipid kinase in vitro and in vivo. EMBO J 18: 1292–1302, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1612. Vanhaesebroeck B, Stephens L, Hawkins P. PI3K signalling: the path to discovery and understanding. Nat Rev Mol Cell Biol 13: 195–203, 2012 [DOI] [PubMed] [Google Scholar]
- 1613. Vanhaesebroeck B, Vogt PK, Rommel C. PI3K: from the bench to the clinic and back. Curr Top Microbiol Immunol 347: 1–19, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1614. Vanhaesebroeck B, Welham MJ, Kotani K, Stein R, Warne PH, Zvelebil MJ, Higashi K, Volinia S, Downward J, Waterfield MD. P110delta, a novel phosphoinositide 3-kinase in leukocytes. Proc Natl Acad Sci USA 94: 4330–4335, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1615. Várnai P, Balla T. Visualization of phosphoinositides that bind pleckstrin homology domains: calcium-and agonist-induced dynamic changes and relationship to myo-[3H]inositol-labeled phosphoinositide pools. J Cell Biol 143: 501–510, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1616. Varnai P, Lin X, Lee SB, Tuymetova G, Bondeva T, Spat A, Rhee SG, Hajnoczky G, Balla T. Inositol lipid binding and membrane localization of isolated pleckstrin homology (PH) domains. Studies on the PH domains of phospholipase C delta 1 and p130. J Biol Chem 277: 27412–27422, 2002 [DOI] [PubMed] [Google Scholar]
- 1617. Varnai P, Thyagarajan B, Rohacs T, Balla T. Rapidly inducible changes in phosphatidylinositol 4,5-bisphosphate levels influence multiple regulatory functions of the lipid in intact living cells. J Cell Biol 175: 377–382, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1618. Varnai P, Toth B, Toth DJ, Hunyady L, Balla T. Visualization and manipulation of plasma membrane-endoplasmic reticulum contact sites indicates the presence of additional molecular components within the STIM1-Orai1 Complex. J Biol Chem 282: 29678–29690, 2007 [DOI] [PubMed] [Google Scholar]
- 1619. Varsanyi M, Tolle HG, Heilmeyer MG, Jr, Dawson RM, Irvine RF. Activation of sarcoplasmic reticular Ca2+ transport ATPase by phosphorylation of an associated phosphatidylinositol. EMBO J 2: 1543–1548, 1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1620. Vasudevan KM, Garraway LA. AKT signaling in physiology and disease. Curr Top Microbiol Immunol 347: 105–133, 2010 [DOI] [PubMed] [Google Scholar]
- 1621. Vasudevan L, Jeromin A, Volpicelli-Daley L, De Camilli P, Holowka D, Baird B. The beta- and gamma-isoforms of type I PIP5K regulate distinct stages of Ca2+ signaling in mast cells. J Cell Sci 122: 2567–2574, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1622. Vaziri C, Downes CP, Macfarlane SC. Direct labelling of hormone-sensitive phosphoinositides by a plasma-membrane-associated PtdIns synthase in turkey erythrocytes. Biochem J 294: 793–799, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1623. Vecchione C, Patrucco E, Marino G, Barberis L, Poulet R, Aretini A, Maffei A, Gentile MT, Storto M, Azzolino O, Brancaccio M, Colussi GL, Bettarini U, Altruda F, Silengo L, Tarone G, Wymann MP, Hirsch E, Lembo G. Protection from angiotensin II-mediated vasculotoxic and hypertensive response in mice lacking PI3Kgamma. J Exp Med 201: 1217–1228, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1624. Vedham V, Phee H, Coggeshall KM. Vav activation and function as a rac guanine nucleotide exchange factor in macrophage colony-stimulating factor-induced macrophage chemotaxis. Mol Cell Biol 25: 4211–4220, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1625. Velichkova M, Juan J, Kadandale P, Jean S, Ribeiro I, Raman V, Stefan C, Kiger AA. Drosophila Mtm and class II PI3K coregulate a PI(3)P pool with cortical and endolysosomal functions. J Cell Biol 190: 407–425, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1626. Veltman DM, Keizer-Gunnik I, Van Haastert PJ. Four key signaling pathways mediating chemotaxis in Dictyostelium discoideum. J Cell Biol 180: 747–753, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1627. Venkateswarlu K, Oatey PB, Tavare JM, Cullen PJ. Insulin-dependent translocation of ARNO to the plasma membrane of adipocytes requires phosphatidylinositol 3-kinase. Curr Biol 8: 463–466, 1998 [DOI] [PubMed] [Google Scholar]
- 1628. Verbsky J, Lavine K, Majerus PW. Disruption of the mouse inositol 1,3,4,5,6-pentakisphosphate 2-kinase gene, associated lethality, and tissue distribution of 2-kinase expression. Proc Natl Acad Sci USA 102: 8448–8453, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1629. Verbsky JW, Chang SC, Wilson MP, Mochizuki Y, Majerus PW. The pathway for the production of inositol hexakisphosphate in human cells. J Biol Chem 280: 1911–1920, 2005 [DOI] [PubMed] [Google Scholar]
- 1630. Vergne I, Chua J, Lee HH, Lucas M, Belisle J, Deretic V. Mechanism of phagolysosome biogenesis block by viable Mycobacterium tuberculosis. Proc Natl Acad Sci USA 102: 4033–4038, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1631. Vergne I, Deretic V. The role of PI3P phosphatases in the regulation of autophagy. FEBS Lett 584: 1313–1318, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1632. Vergne I, Roberts E, Elmaoued RA, Tosch V, Delgado MA, Proikas-Cezanne T, Laporte J, Deretic V. Control of autophagy initiation by phosphoinositide 3-phosphatase Jumpy. EMBO J 28: 2244–2258, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1633. Viard P, Butcher AJ, Halet G, Davies A, Nurnberg B, Heblich F, Dolphin AC. PI3K promotes voltage-dependent calcium channel trafficking to the plasma membrane. NatNeurosci 7: 939–946, 2004 [DOI] [PubMed] [Google Scholar]
- 1634. Vicinanza M, Di Campli A, Polishchuk E, Santoro M, Di Tullio G, Godi A, Levtchenko E, De Leo MG, Polishchuk R, Sandoval L, Marzolo MP, De Matteis MA. OCRL controls trafficking through early endosomes via PtdIns4,5P(2)-dependent regulation of endosomal actin. EMBO J 30: 4970–4985, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1635. Vig M, Peinelt C, Beck A, Koomoa DL, Rabah D, Koblan-Huberson M, Kraft S, Turner H, Fleig A, Penner R, Kinet JP. CRACM1 is a plasma membrane protein essential for store-operated Ca2+ entry. Science 312: 1220–1223, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1636. Vihtelic TS, Hyde DR, O'Tousa JE. Isolation and characterization of the Drosophila retinal degeneration B (rdgB) gene. Genetics 127: 761–768, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1637. Villalba-Galea CA, Miceli F, Taglialatela M, Bezanilla F. Coupling between the voltage-sensing and phosphatase domains of Ci-VSP. J Gen Physiol 134: 5–14, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1638. Virbasius JV, Guilherme A, Czech MP. Mouse p170 is a novel phosphatidylinositol 3-kinase containing a C2 domain. J Biol Chem 271: 13304–13307, 1996 [DOI] [PubMed] [Google Scholar]
- 1639. Voglmaier SM, Keen JH, Murphy JE, Ferris CD, Prestwich GD, Snyder SH, Theibert AB. Inositol hexakisphosphate receptor identified as the clathrin assembly protein AP-2. Biochem Biophys Res Commun 187: 158–163, 1992 [DOI] [PubMed] [Google Scholar]
- 1640. Vogt PK, Hart JR, Gymnopoulos M, Jiang H, Kang S, Bader AG, Zhao L, Denley A. Phosphatidylinositol 3-kinase: the oncoprotein. Curr Top Microbiol Immunol 347: 79–104, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1641. Voigt P, Dorner MB, Schaefer M. Characterization of p87PIKAP, a novel regulatory subunit of phosphoinositide 3-kinase gamma that is highly expressed in heart and interacts with PDE3B. J Biol Chem 281: 9977–9986, 2006 [DOI] [PubMed] [Google Scholar]
- 1642. Volinia S, Dhand R, Vanhaesebroeck B, MacDougall LK, Stein R, Zvelebil MJ, Domin J, Panaretou C, Waterfield MD. A human phosphatidylinositol 3-kinase complex related to the yeast Vps34p-Vps15p protein sorting system. EMBO J 14: 3339–3348, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1643. Vollert CS, Uetz P. The PX domain protein interaction network in yeast. Mol Cell Proteomics 3: 1053–1064, 2004 [DOI] [PubMed] [Google Scholar]
- 1644. Volpicelli-Daley LA, Lucast L, Gong LW, Liu L, Sasaki J, Sasaki T, Abrams CS, Kanaho Y, De Camilli P. Phosphatidylinositol-4-phosphate 5-kinases and phosphatidylinositol 4,5-bisphosphate synthesis in the brain. J Biol Chem 285: 28708–28714, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1645. Volta M, Bulfone A, Gattuso C, Rossi E, Mariani M, Consalez GG, Zuffardi O, Ballabio A, Banfi S, Franco B. Identification and characterization of CDS2, a mammalian homolog of the Drosophila CDP-diacylglycerol synthase gene. Genomics 55: 68–77, 1999 [DOI] [PubMed] [Google Scholar]
- 1646. Volwerk JJ, Koke JA, Wetherwax PB, Griffith OH. Functional characteristics of phosphatidylinositol-specific phospholipases C from Bacillus cereus and Bacillus thuringiensis. FEMS Microbiol Lett 52: 237–241, 1989 [DOI] [PubMed] [Google Scholar]
- 1647. Voronov SV, Frere SG, Giovedi S, Pollina EA, Borel C, Zhang H, Schmidt C, Akeson EC, Wenk MR, Cimasoni L, Arancio O, Davisson MT, Antonarakis SE, Gardiner K, De Camilli P, Di Paolo G. Synaptojanin 1-linked phosphoinositide dyshomeostasis and cognitive deficits in mouse models of Down's syndrome. Proc Natl Acad Sci USA 105: 9415–9420, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1648. Vorstman JA, Chow EW, Ophoff RA, van Engeland H, Beemer FA, Kahn RS, Sinke RJ, Bassett AS. Association of the PIK4CA schizophrenia-susceptibility gene in adults with the 22q11.2 deletion syndrome. Am J Med Genet B Neuropsychiatr Genet 150B: 430–433, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1649. Vyas P, Norris FA, Joseph R, Majerus PW, Orkin SH. Inositol polyphosphate 4-phosphatase type I regulates cell growth downstream of transcription factor GATA-1. Proc Natl Acad Sci USA 97: 13696–13701, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1650. Wada T, Sasaoka T, Funaki M, Hori H, Murakami S, Ishiki M, Haruta T, Asano T, Ogawa W, Ishihara H, Kobayashi M. Overexpression of SH2-containing inositol phosphatase 2 results in negative regulation of insulin-induced metabolic actions in 3T3-L1 adipocytes via its 5'-phosphatase catalytic activity. Mol Cell Biol 21: 1633–1646, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1651. Wagner H, Holzl J, Lissau A, Horhammer L. Papierchromatographische von phosphatiden,III: quantitative papierchromatographische bestimmung von phosphatiden und phosphatidsuaren in rattenorganen. Biochem J 339: 34–45, 1961 [PubMed] [Google Scholar]
- 1652. Wahl MI, Nishibe S, Kim JW, Kim H, Rhee SG, Carpenter G. Identification of two epidermal growth factor-sensitive tyrosine phosphorylation sites of phospholipase C-gamma in intact HSC-1 cells. J Biol Chem 265: 3944–3948, 1990 [PubMed] [Google Scholar]
- 1653. Wahl MI, Nishibe S, Suh PG, Rhee SG, Carpenter G. Epidermal growth factor stimulates tyrosine phosphorylation of phospholipase C-II independently of receptor internalization and extracellular calcium. Proc Natl Acad Sci USA 86: 1568–1572, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1654. Wahl MI, Olashaw NE, Nishibe S, Rhee SG, Pledger WJ, Carpenter G. Platelet-derived growth factor induces rapid and sustained tyrosine phosphorylation of phospholipase C-gamma in quiescent BALB/c 3T3 cells. Mol Cell Biol 9: 2934–2943, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1655. Walch-Solimena C, Novick P. The yeast phosphatidylinositol-4-OH kinase Pik1 regulates secretion at the Golgi. Nat Cell Biol 1: 523–525, 1999 [DOI] [PubMed] [Google Scholar]
- 1656. Waldo GL, Boyer JL, Morris AJ, Harden TK. Purification of an AlF4- and G-protein beta gamma-subunit-regulated phospholipase C-activating protein. J Biol Chem 266: 14217–14225, 1991 [PubMed] [Google Scholar]
- 1657. Waldo GL, Ricks TK, Hicks SN, Cheever ML, Kawano T, Tsuboi K, Wang X, Montell C, Kozasa T, Sondek J, Harden TK. Kinetic scaffolding mediated by a phospholipase C-beta and Gq signaling complex. Science 330: 974–980, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1658. Walker DH, Dougherty N, Pike LJ. Purification and characterization of a phosphatidylinositol kinase from A431 cells. Biochemistry 27: 6504–6511, 1988 [DOI] [PubMed] [Google Scholar]
- 1659. Walker DM, Urbe S, Dove SK, Tenza D, Raposo G, Clague MJ. Characterization of MTMR3, an inositol lipid 3-phosphatase with novel substrate specificity. Curr Biol 11: 1600–1605, 2001 [DOI] [PubMed] [Google Scholar]
- 1660. Walker EM, Bispham JR, Hill SJ. Nonselective effects of the putative phospholipase C inhibitor, U73122, on adenosine A1 receptor-mediated signal transduction events in Chinese hamster ovary cells. Biochem Pharmacol 1455: 1462–1460, 1998 [DOI] [PubMed] [Google Scholar]
- 1661. Walker MB, Miller CT, Swartz ME, Eberhart JK, Kimmel CB. Phospholipase C, beta 3 is required for Endothelin1 regulation of pharyngeal arch patterning in zebrafish. Dev Biol 304: 194–207, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1662. Walker SM, Downes CP, Leslie NR. TPIP: a novel phosphoinositide 3-phosphatase. Biochem J 360: 277–283, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1663. Walliser C, Retlich M, Harris R, Everett KL, Josephs MB, Vatter P, Esposito D, Driscoll PC, Katan M, Gierschik P, Bunney TD. rac regulates its effector phospholipase Cgamma2 through interaction with a split pleckstrin homology domain. J Biol Chem 283: 30351–30362, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1664. Walsh CM, Chvanov M, Haynes LP, Petersen OH, Tepikin AV, Burgoyne RD. Role of phosphoinositides in STIM1 dynamics and store-operated calcium entry. Biochem J 425: 159–168, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1665. Walz HA, Shi X, Chouinard M, Bue CA, Navaroli DM, Hayakawa A, Zhou QL, Nadler J, Leonard DM, Corvera S. Isoform-specific regulation of Akt signaling by the endosomal protein WDFY2. J Biol Chem 285: 14101–14108, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1666. Wang D, Feng J, Wen R, Marine JC, Sangster MY, Parganas E, Hoffmeyer A, Jackson CW, Cleveland JL, Murray PJ, Ihle JN. Phospholipase Cgamma2 is essential in the functions of B cell and several Fc receptors. Immunity 13: 25–35, 2000 [DOI] [PubMed] [Google Scholar]
- 1667. Wang F, Herzmark P, Weiner OD, Srinivasan S, Servant G, Bourne HR. Lipid products of PI(3)Ks maintain persistent cell polarity and directed motility in neutrophils. Nat Cell Biol 4: 513–518, 2002 [DOI] [PubMed] [Google Scholar]
- 1668. Wang H, Oestreich EA, Maekawa N, Bullard TA, Vikstrom KL, Dirksen RT, Kelley GG, Blaxall BC, Smrcka AV. Phospholipase C epsilon modulates beta-adrenergic receptor-dependent cardiac contraction and inhibits cardiac hypertrophy. Circ Res 97: 1305–1313, 2005 [DOI] [PubMed] [Google Scholar]
- 1669. Wang J, Auger KR, Jarvis L, Shi Y, Roberts TM. Direct association of Grb2 with the p85 subunit of phosphatidylinositol 3-kinase. J Biol Chem 270: 12774–12780, 1995 [DOI] [PubMed] [Google Scholar]
- 1670. Wang J, Gambhir A, Hangyas-Mihalyne G, Murray D, Golebiewska U, McLaughlin S. Lateral sequestration of phosphatidylinositol 4,5-bisphosphate by the basic effector domain of myristoylated alanine-rich C kinase substrate is due to nonspecific electrostatic interactions. J Biol Chem 277: 34401–34412, 2002 [DOI] [PubMed] [Google Scholar]
- 1671. Wang J, Sun HQ, Macia E, Kirchhausen T, Watson H, Bonifacino JS, Yin HL. PI4P promotes the recruitment of the GGA adaptor proteins to the trans-Golgi network and regulates their recognition of the ubiquitin sorting signal. Mol Biol Cell 18: 2646–2655, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1672. Wang M, Bond NJ, Letcher AJ, Richardson JP, Lilley KS, Irvine RF, Clarke JH. Genomic tagging reveals a random association of endogenous PtdIns5P 4-kinases IIalpha and IIbeta and a partial nuclear localization of the IIalpha isoform. Biochem J 430: 215–221, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1673. Wang T, Pentyala S, Rebecchi MJ, Scarlata S. Differential association of the pleckstrin homology domains of phospholipases C-beta 1, C-beta 2, and C-delta 1 with lipid bilayers and the beta gamma subunits of heterotrimeric G proteins. Biochemistry 38: 1517–1524, 1999 [DOI] [PubMed] [Google Scholar]
- 1674. Wang X, Trotman LC, Koppie T, Alimonti A, Chen Z, Gao Z, Wang J, Erdjument-Bromage H, Tempst P, Cordon-Cardo C, Pandolfi PP, Jiang X. NEDD4–1 is a proto-oncogenic ubiquitin ligase for PTEN. Cell 128: 129–139, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1675. Wang X, Zhou C, Qiu G, Yang Y, Yan D, Xing T, Fan J, Tang H, Peng Z. Phospholipase C epsilon plays a suppressive role in incidence of colorectal cancer. Med Oncol 2011 [DOI] [PubMed] [Google Scholar]
- 1676. Wang Y, Chen X, Lian L, Tang T, Stalker TJ, Sasaki T, Kanaho Y, Brass LF, Choi JK, Hartwig JH, Abrams CS. Loss of PIP5KIbeta demonstrates that PIP5KI isoform-specific PIP2 synthesis is required for IP3 formation. Proc Natl Acad Sci USA 105: 14064–14069, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1677. Wang Y, Lian L, Golden JA, Morrisey EE, Abrams CS. PIP5KI gamma is required for cardiovascular and neuronal development. Proc Natl Acad Sci USA 104: 11748–11753, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1678. Wang Y, Litvinov RI, Chen X, Bach TL, Lian L, Petrich BG, Monkley SJ, Kanaho Y, Critchley DR, Sasaki T, Birnbaum MJ, Weisel JW, Hartwig J, Abrams CS. Loss of PIP5KIgamma, unlike other PIP5KI isoforms, impairs the integrity of the membrane cytoskeleton in murine megakaryocytes. J Clin Invest 118: 812–819, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1679. Wang YJ, Li WH, Wang J, Xu K, Dong P, Luo X, Yin HL. Critical role of PIP5KIgamma 87 in InsP3-mediated Ca2+ signaling. J Cell Biol 167: 1005–1010, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1680. Wang YJ, Wang J, Sun HQ, Martinez M, Sun YX, Macia E, Kirschhausen T, Albanesi JP, Roth MG, Yin HL. Phosphatidylinositol 4 phosphate regulates targeting of clathrin adaptor AP-1 complexes to the Golgi. Cell 114: 299–310, 2003 [DOI] [PubMed] [Google Scholar]
- 1681. Wang Z, Thurmond DC. Mechanisms of biphasic insulin-granule exocytosis: roles of the cytoskeleton, small GTPases and SNARE proteins. J Cell Sci 122: 893–903, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1682. Warashina A. Light-evoked recovery from wortmannin-induced inhibition of catecholamine secretion and synaptic transmission. Arch Biochem Biophys 367: 303–310, 1999 [DOI] [PubMed] [Google Scholar]
- 1683. Waselle L, Gerona RR, Vitale N, Martih TF, Bader MF, Regazzi R. Role of phosphoinositide signaling in the control of insulin exocytosis. Mol Endocrinol 19: 3097–3106, 2005 [DOI] [PubMed] [Google Scholar]
- 1684. Watt SA, Kular G, Fleming IN, Downes CP, Lucocq JM. Subcellular localization of phosphatidylinositol 4,5-bisphosphate using the pleckstrin homology domain of phospholipase C delta1. Biochem J 363: 657–666, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1685. Waugh MG, Minogue S, Anderson JS, Balinger A, Blumenkrantz D, Calnan DP, Cramer R, Hsuan JJ. Localization of a highly active pool of type II phosphatidylinositol 4-kinase in a p97/valosin-containing-protein-rich fraction of the endoplasmic reticulum. Biochem J 373: 57–63, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1686. Waugh MG, Minogue S, Chotai D, Berditchevski F, Hsuan JJ. Lipid and peptide control of phosphatidylinositol 4-kinase IIalpha activity on Golgi-endosomal rafts. J Biol Chem 281: 3757–3763, 2006 [DOI] [PubMed] [Google Scholar]
- 1687. Wee S, Wiederschain D, Maira SM, Loo A, Miller C, deBeaumont R, Stegmeier F, Yao YM, Lengauer C. PTEN-deficient cancers depend on PIK3CB. Proc Natl Acad Sci USA 105: 13057–13062, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1688. Weeks R, Dowhan W, Shen H, Balantac N, Meengs B, Nudelman E, Leung DW. Isolation and expression of an isoform of human CDP-diacylglycerol synthase cDNA. DNA Cell Biol 16: 281–289, 1997 [DOI] [PubMed] [Google Scholar]
- 1689. Wei HC, Sanny J, Shu H, Baillie DL, Brill JA, Price JV, Harden N. The Sac1 lipid phosphatase regulates cell shape change and the JNK cascade during dorsal closure in Drosophila. Curr Biol 13: 1882–1887, 2003 [DOI] [PubMed] [Google Scholar]
- 1690. Wei YJ, Sun HQ, Yamamoto M, Wlodarski P, Kunii K, Martinez M, Barylko B, Albanesi JP, Yin HL. Type II phosphatidylinositol 4-kinase beta is a cytosolic and peripheral membrane protein that is recruited to the plasma membrane and activated by Rac-GTP. J Biol Chem 277: 46586–46593, 2002 [DOI] [PubMed] [Google Scholar]
- 1691. Weiger MC, Parent CA. Phosphoinositides in chemotaxis. Subcell Biochem 59: 217–254, 2012 [DOI] [PubMed] [Google Scholar]
- 1692. Weimar WR, Lane PW, Sidman RL. Vibrator (vb): a spinocerebellar system degeneration with autosomal recessive inheritance in mice. Brain Res 251: 357–364, 1982 [DOI] [PubMed] [Google Scholar]
- 1693. Weiner OD, Neilsen PO, Prestwich GD, Kirschner MW, Cantley LC, Bourne HR. A PtdInsP(3)- and Rho GTPase-mediated positive feedback loop regulates neutrophil polarity. Nat Cell Biol 4: 509–513, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1694. Weiss A, Koretzky G, Schatzman RC, Kadlecek T. Functional activation of the T-cell antigen receptor induces tyrosine phosphorylation of phospholipase C-gamma 1. Proc Natl Acad Sci USA 88: 5484–5488, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1695. Weisz OA, Gibson GA, Leung SM, Roder J, Jeromin A. Overexpression of frequenin, a modulator of phosphatidylinositol 4-kinase, inhibits biosynthetic delivery of an apical protein in polarized madin-darby canine kidney cells. J Biol Chem 275: 24341–24347, 2000 [DOI] [PubMed] [Google Scholar]
- 1696. Weisz OA, Rodriguez-Boulan E. Apical trafficking in epithelial cells: signals, clusters and motors. J Cell Sci 122: 4253–4266, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1697. Weixel KM, Blumental-Perry A, Watkins SC, Aridor M, Weisz OA. Distinct Golgi populations of phosphatidylinositol 4-phosphate regulated by phosphatidylinositol 4-kinases. J Biol Chem 280: 10501–10508, 2005 [DOI] [PubMed] [Google Scholar]
- 1698. Welch HC, Coadwell WJ, Ellson CD, Ferguson GJ, Andrews SR, Erdjument-Bromage H, Tempst P, Hawkins PT, Stephens LR. P-Rex1, a PtdIns(3,4,5)P3- and Gbetagamma-regulated guanine-nucleotide exchange factor for Rac. Cell 108: 809–821, 2002 [DOI] [PubMed] [Google Scholar]
- 1699. Welch HC, Coadwell WJ, Stephens LR, Hawkins PT. Phosphoinositide 3-kinase-dependent activation of Rac. FEBS Lett 546: 93–97, 2003 [DOI] [PubMed] [Google Scholar]
- 1700. Wen PJ, Osborne SL, Morrow IC, Parton RG, Domin J, Meunier FA. Ca2+-regulated pool of phosphatidylinositol-3-phosphate produced by phosphatidylinositol 3-kinase C2alpha on neurosecretory vesicles. Mol Biol Cell 19: 5593–5603, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1701. Wen PJ, Osborne SL, Zanin M, Low PC, Wang HT, Schoenwaelder SM, Jackson SP, Wedlich-Soldner R, Vanhaesebroeck B, Keating DJ, Meunier FA. Phosphatidylinositol(4,5)bisphosphate coordinates actin-mediated mobilization and translocation of secretory vesicles to the plasma membrane of chromaffin cells. Nat Commun 2: 491, 2011 [DOI] [PubMed] [Google Scholar]
- 1702. Wenk MR, De Camilli P. Protein-lipid interactions and phosphoinositide metabolism in membrane traffic: insights from vesicle recycling in nerve terminals. Proc Natl Acad Sci USA 101: 8262–8269, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1703. Wenk MR, Lucast L, Di Paolo G, Romanelli AJ, Suchy SF, Nussbaum RL, Cline GW, Shulman GI, McMurray W, De Camilli P. Phosphoinositide profiling in complex lipid mixtures using electrospray ionization mass spectrometry. Nat Biotechnol 21: 813–817, 2003 [DOI] [PubMed] [Google Scholar]
- 1704. Wenk MR, Pellegrini L, Klenchin VA, Di Paolo G, Chang S, Daniell L, Arioka M, Martin TF, De Camilli P. PIP kinase Igamma is the major PI(4,5)P(2) synthesizing enzyme at the synapse. Neuron 31: 79–88, 2001 [DOI] [PubMed] [Google Scholar]
- 1705. Wetzker R, Klinger R, Hsuan J, Fry MJ, Kauffmann-Zeh A, Muller E, Frunder H, Waterfield MD. Purification and characterization of phosphatidylinositol 4-kinase from human erythrocyte membranes. Eur J Biochem 200: 179–185, 1991 [DOI] [PubMed] [Google Scholar]
- 1706. Wheeler M, Domin J. The N-terminus of phosphoinositide 3-kinase-C2beta regulates lipid kinase activity and binding to clathrin. J Cell Physiol 206: 586–593, 2006 [DOI] [PubMed] [Google Scholar]
- 1707. Whitaker M. Polyphosphoinositide hydrolysis is associated with exocytosis in adrenal medullary cells. FEBS Lett 189: 137–140, 1985 [DOI] [PubMed] [Google Scholar]
- 1708. Whitaker M, Aitchison M. Calcium-dependent polyphosphoinositide hydrolysis is associated with exocytosis in vitro. FEBS Lett 182: 119–124, 1985 [DOI] [PubMed] [Google Scholar]
- 1709. White ES, Sagana RL, Booth AJ, Yan M, Cornett AM, Bloomheart CA, Tsui JL, Wilke CA, Moore BB, Ritzenthaler JD, Roman J, Muro AF. Control of fibroblast fibronectin expression and alternative splicing via the PI3K/Akt/mTOR pathway. Exp Cell Res 316: 2644–2653, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1710. Whitley P, Reaves BJ, Hashimoto M, Riley AM, Potter BV, Holman GD. Identification of mammalian Vps24p as an effector of phosphatidylinositol 3,5-bisphosphate-dependent endosome compartmentalization. J Biol Chem 278: 38786–38795, 2003 [DOI] [PubMed] [Google Scholar]
- 1711. Whitman M, Downes CP, Keeler M, Keller T, Cantley L. Type-I phosphatidylinositol kinase makes a novel inositol phospholipid, phosphatidylinositol-3-phosphate. Nature 332: 644–646, 1988 [DOI] [PubMed] [Google Scholar]
- 1712. Whitman M, Kaplan D, Roberts T, Cantley L. Evidence for two distinct phosphatidylinositol kinases in fibroblasts. Implications for cellular regulation. Biochem J 247: 165–174, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1713. Whitman M, Kaplan DR, Schaffhausen B, Cantley LC, Roberts TM. Association of phosphatidylinositol kinase activity with polyoma middle-T competent for transformation. Nature 315: 239–242, 1985 [DOI] [PubMed] [Google Scholar]
- 1714. Whitters EA, Cleves AE, McGee TP, Skinner HB, Bankaitis VA. SAC1p is an integral membrane protein that influences the cellular requirement for phospholipid transfer protein function and inositol in yeast. J Cell Biol 122: 79–94, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1715. Whorton MR, MacKinnon R. Crystal structure of the mammalian GIRK2 K+ channel and gating regulation by G proteins, PIP2, and sodium. Cell 147: 199–208, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1716. Wiedemann C, Schäfer T, Burger MM. Chromaffin granule-associated phosphatidylinositol 4-kinase activity is required for stimulated secretion. EMBO J 15: 2094–2101, 1996 [PMC free article] [PubMed] [Google Scholar]
- 1717. Wild AC, Yu JW, Lemmon MA, Blumer KJ. The p21-activated protein kinase-related kinase Cla4 is a coincidence detector of signaling by Cdc42 and phosphatidylinositol 4-phosphate. J Biol Chem 279: 17101–17110, 2004 [DOI] [PubMed] [Google Scholar]
- 1718. Williams C, Choudhury R, McKenzie E, Lowe M. Targeting of the type II inositol polyphosphate 5-phosphatase INPP5B to the early secretory pathway. J Cell Sci 120: 3941–3951, 2007 [DOI] [PubMed] [Google Scholar]
- 1719. Wilson MP, Majerus PW. Isolation of inositol 1,3,4-trisphosphate 5/6-kinase, cDNA cloning and expression of the recombinant enzyme. J Biol Chem 271: 11904–11910, 1996 [DOI] [PubMed] [Google Scholar]
- 1720. Winters JJ, Ferguson CJ, Lenk GM, Giger-Mateeva VI, Shrager P, Meisler MH, Giger RJ. Congenital CNS hypomyelination in the Fig4 null mouse is rescued by neuronal expression of the PI(3,5)P(2) phosphatase Fig4. J Neurosci 31: 17736–17751, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1721. Wishart MJ, Taylor GS, Dixon JE. Phoxy lipids: revealing PX domains as phosphoinositide binding modules. Cell 105: 817–820, 2001 [DOI] [PubMed] [Google Scholar]
- 1722. Wisniewski D, Strife A, Swendeman S, Erdjument-Bromage H, Geromanos S, Kavanaugh WM, Tempst P, Clarkson B. A novel SH2-containing phosphatidylinositol 3,4,5-trisphosphate 5-phosphatase (SHIP2) is constitutively tyrosine phosphorylated and associated with src homologous and collagen gene (SHC) in chronic myelogenous leukemia progenitor cells. Blood 93: 2707–2720, 1999 [PubMed] [Google Scholar]
- 1723. Wollert T, Wunder C, Lippincott-Schwartz J, Hurley JH. Membrane scission by the ESCRT-III complex. Nature 458: 172–177, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1724. Womack KB, Gordon SE, He F, Wensel TG, Lu CC, Hilgemann DW. Do phosphatidylinositides modulate vertebrate phototransduction? J Neurosci 20: 2792–2799, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1725. Wong K, Cantley LC. Cloning and characterization of a human phosphatidylinositol 4-kinase. J Biol Chem 269: 28878–28884, 1994 [PubMed] [Google Scholar]
- 1726. Wong K, Meyers R, Cantley LC. Subcellular localization of phosphatidylinositol 4-kinase isoforms. J Biol Chem 272: 13236–13241, 1997 [DOI] [PubMed] [Google Scholar]
- 1727. Wong KK, Engelman JA, Cantley LC. Targeting the PI3K signaling pathway in cancer. Curr Opin Genet Dev 20: 87–90, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1728. Wong R, Hadjiyanni I, Wei HC, Polevoy G, McBride R, Sem KP, Brill JA. PIP2 hydrolysis and calcium release are required for cytokinesis in Drosophila spermatocytes. Curr Biol 15: 1401–1406, 2005 [DOI] [PubMed] [Google Scholar]
- 1729. Wonodi I, Hong LE, Avila MT, Buchanan RW, Carpenter WT, Jr, Stine OC, Mitchell BD, Thaker GK. Association between polymorphism of the SNAP29 gene promoter region and schizophrenia. Schizophr Res 78: 339–341, 2005 [DOI] [PubMed] [Google Scholar]
- 1730. Wonodi I, Stine OC, Mitchell BD, Buchanan RW, Thaker GK. Association between Val108/158 Met polymorphism of the COMT gene and schizophrenia. Am J Med Genet B Neuropsychiatr Genet 120B: 47–50, 2003 [DOI] [PubMed] [Google Scholar]
- 1731. Wood CS, Schmitz KR, Bessman NJ, Setty TG, Ferguson KM, Burd CG. PtdIns4P recognition by Vps74/GOLPH3 links PtdIns 4-kinase signaling to retrograde Golgi trafficking. J Cell Biol 187: 967–975, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1732. Woodcock EA, Lambert KA. The inositol phosphate response to thrombin in rat right atria differs from the response to noradrenaline. Eur J Pharmacol 291: 213–216, 1995 [DOI] [PubMed] [Google Scholar]
- 1733. Wu H, Shekar SC, Flinn RJ, El-Sibai M, Jaiswal BS, Sen KI, Janakiraman V, Seshagiri S, Gerfen GJ, Girvin ME, Backer JM. Regulation of Class IA PI 3-kinases: C2 domain-iSH2 domain contacts inhibit p85/p110alpha and are disrupted in oncogenic p85 mutants. Proc Natl Acad Sci USA 106: 20258–20263, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1734. Wu J, McNicholas CM, Bevensee MO. Phosphatidylinositol 4,5-bisphosphate (PIP2) stimulates the electrogenic Na/HCO3 cotransporter NBCe1-A expressed in Xenopus oocytes. Proc Natl Acad Sci USA 106: 14150–14155, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1735. Wu L, Bauer CS, Zhen XG, Xie C, Yang J. Dual regulation of voltage-gated calcium channels by PtdIns(4,5)P2. Nature 419: 947–952, 2002 [DOI] [PubMed] [Google Scholar]
- 1736. Wu L, Niemeyer B, Colley N, Socolich M, Zuker CS. Regulation of PLC-mediated signalling in vivo by CDP-diacylglycerol synthase. Nature 373: 216–222, 1995 [DOI] [PubMed] [Google Scholar]
- 1737. Wu WI, Routt S, Bankaitis VA, Voelker DR. A new gene involved in the transport-dependent metabolism of phosphatidylserine, PSTB2/PDR17, shares sequence similarity with the gene encoding the phosphatidylinositol/phosphatidylcholine transfer protein, SEC14. J Biol Chem 275: 14446–14456, 2000 [DOI] [PubMed] [Google Scholar]
- 1738. Wu Y, Dowbenko D, Pisabarro MT, Dillard-Telm L, Koeppen H, Lasky LA. PTEN 2, a Golgi-associated testis-specific homologue of the PTEN tumor suppressor lipid phosphatase. J Biol Chem 276: 21745–21753, 2001 [DOI] [PubMed] [Google Scholar]
- 1739. Wurmser AE, Emr SD. Novel PtdIns(3)P-binding protein Etf1 functions as an effector of the Vps34 PtdIns 3-kinase in autophagy. J Cell Biol 158: 761–772, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1740. Wymann MP, Bjorklof K, Calvez R, Finan P, Thomast M, Trifilieff A, Barbier M, Altruda F, Hirsch E, Laffargue M. Phosphoinositide 3-kinase gamma: a key modulator in inflammation and allergy. Biochem Soc Trans 31: 275–280, 2003 [DOI] [PubMed] [Google Scholar]
- 1741. Xia Y, Irvine RF, Giudici ML. Phosphatidylinositol 4-phosphate 5-kinase Igamma_v6, a new splice variant found in rodents and humans. Biochem Biophys Res Commun 411: 416–420, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1742. Xiao W, Ando T, Wang HY, Kawakami Y, Kawakami T. Lyn- and PLC-beta3-dependent regulation of SHP-1 phosphorylation controls Stat5 activity and myelomonocytic leukemia-like disease. Blood 116: 6003–6013, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1743. Xiao W, Hong H, Kawakami Y, Kato Y, Wu D, Yasudo H, Kimura A, Kubagawa H, Bertoli LF, Davis RS, Chau LA, Madrenas J, Hsia CC, Xenocostas A, Kipps TJ, Hennighausen L, Iwama A, Nakauchi H, Kawakami T. Tumor suppression by phospholipase C-beta3 via SHP-1-mediated dephosphorylation of Stat5. Cancer Cell 16: 161–171, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1744. Xiao Z, Zhang N, Murphy DB, Devreotes PN. Dynamic distribution of chemoattractant receptors in living cells during chemotaxis and persistent stimulation. J Cell Biol 139: 365–374, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1745. Xie J, Sun B, Du J, Yang W, Chen HC, Overton JD, Runnels LW, Yue L. Phosphatidylinositol 4,5-bisphosphate [PIP(2)] controls magnesium gatekeeper TRPM6 activity. Sci Rep 1: 146, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1746. Xie W, Samoriski GM, McLaughlin JP, Romoser VA, Smrcka A, Hinkle PM, Bidlack JM, Gross RA, Jiang H, Wu D. Genetic alteration of phospholipase C beta3 expression modulates behavioral and cellular responses to mu opioids. Proc Natl Acad Sci USA 96: 10385–10390, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1747. Xie Y, Ding YQ, Hong Y, Feng Z, Navarre S, Xi CX, Zhu XJ, Wang CL, Ackerman SL, Kozlowski D, Mei L, Xiong WC. Phosphatidylinositol transfer protein-alpha in netrin-1-induced PLC signalling and neurite outgrowth. Nat Cell Biol 7: 1124–1132, 2005 [DOI] [PubMed] [Google Scholar]
- 1748. Xie Z, Fang M, Rivas MP, Faulkner AJ, Sternweis PC, Engebrecht JA, Bankaitis VA. Phospholipase D activity is required for suppression of yeast phosphatidylinositol transfer protein defects. Proc Natl Acad Sci USA 95: 12346–12351, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1749. Xiong X, Xu Q, Huang Y, Singh RD, Anderson R, Leof E, Hu J, Ling K. An association between type Igamma PI4P 5-kinase and Exo70 directs E-cadherin clustering and epithelial polarization. Mol Biol Cell 23: 87–98, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1750. Xu Y, Hortsman H, Seet L, Wong SH, Hong W. SNX3 regulates endosomal function through its PX-domain-mediated interaction with PtdIns(3)P. Nat Cell Biol 3: 658–666, 2001 [DOI] [PubMed] [Google Scholar]
- 1751. Yablonski D, Kadlecek T, Weiss A. Identification of a phospholipase C-gamma1 (PLC-gamma1) SH3 domain-binding site in SLP-76 required for T-cell receptor-mediated activation of PLC-gamma1 and NFAT. Mol Cell Biol 21: 4208–4218, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1752. Yablonski D, Kuhne MR, Kadlecek T, Weiss A. Uncoupling of nonreceptor tyrosine kinases from PLC-gamma1 in an SLP-76-deficient T cell. Science 281: 413–416, 1998 [DOI] [PubMed] [Google Scholar]
- 1753. Yagisawa H, Okada M, Naito Y, Sasaki K, Yamaga M, Fujii M. Coordinated intracellular translocation of phosphoinositide-specific phospholipase C-delta with the cell cycle. Biochim Biophys Acta 1761: 522–534, 2006 [DOI] [PubMed] [Google Scholar]
- 1754. Yagisawa H, Sakuma K, Paterson HF, Cheung R, Allen V, Hirata H, Watanabe Y, Hirata M, Williams RL, Katan M. Replacement of single basic amino acids in the plekstrin homology domain of phospholipase C-delta1 alter the ligand binding, phospholipase activity and interaction with the plasma membrane. J Biol Chem 273: 417–424, 1998 [DOI] [PubMed] [Google Scholar]
- 1755. Yamaga M, Fujii M, Kamata H, Hirata H, Yagisawa H. Phospholipase C-delta1 contains a functional nuclear export signal sequence. J Biol Chem 274: 28537–28541, 1999 [DOI] [PubMed] [Google Scholar]
- 1756. Yamaguchi S, Homma K, Natori S. A novel egg-derived tyrosine phosphatase, EDTP, that participates in the embryogenesis of Sarcophaga peregrina (flesh fly). Eur J Biochem 259: 946–953, 1999 [DOI] [PubMed] [Google Scholar]
- 1757. Yamamoto A, DeWald DB, Boronenkov IV, Anderson RA, Emr SD, Koshland D. Novel PI(4)P 5-kinase homologue, Fab1p, essential for normal vacuole function and morphology in yeast. Mol Biol Cell 6: 525–539, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1758. Yamamoto M, Chen MZ, Wang YJ, Sun HQ, Wei Y, Martinez M, Yin HL. Hypertonic stress increases phosphatidylinositol 4,5-bisphosphate levels by activating PIP5KIbeta. J Biol Chem 281: 32630–32638, 2006 [DOI] [PubMed] [Google Scholar]
- 1759. Yamamoto M, Hilgemann DH, Feng S, Bito H, Ishihara H, Shibasaki Y, Yin HL. Phosphatidylinositol 4,5-bisphosphate induces actin stress-fiber formation and inhibits membrane ruffling in CV1 cells. J Cell Biol 152: 867–876, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1760. Yamamoto T, Takeuchi H, Kanematsu T, Allen V, Yagisawa H, Kikkawa U, Watanabe Y, Nakasima A, Katan M, Hirata M. Involvement of EF hand motifs in the Ca2+-dependent binding of the pleckstrin homology domain to phosphoinositides. Eur J Biochem 265: 481–490, 1999 [DOI] [PubMed] [Google Scholar]
- 1761. Yano H, Nakanishi S, Kimura K, Hanai N, Saitoh Y, Fukui Y, Nonomura Y, Matsuda Y. Inhibition of histamine secretion by wortmannin through the blockade of phosphatidylinositol 3-kinase in RBL-2H3 cells. J Biol Chem 268: 25846–25856, 1993 [PubMed] [Google Scholar]
- 1762. Yao J, Gaffaney JD, Kwon SE, Chapman ER. Doc2 is a Ca2+ sensor required for asynchronous neurotransmitter release. Cell 147: 666–677, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1763. Yaradanakul A, Feng S, Shen C, Lariccia V, Lin MJ, Yang J, Kang TM, Dong P, Yin HL, Albanesi JP, Hilgemann DW. Dual control of cardiac Na+ Ca2+ exchange by PIP2: electrophysiological analysis of direct and indirect mechanisms. J Physiol 582: 991–1010, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1764. Yarar D, Surka MC, Leonard MC, Schmid SL. SNX9 activities are regulated by multiple phosphoinositides through both PX and BAR domains. Traffic 9: 133–146, 2008 [DOI] [PubMed] [Google Scholar]
- 1765. Yarar D, Waterman-Storer CM, Schmid SL. SNX9 couples actin assembly to phosphoinositide signals and is required for membrane remodeling during endocytosis. Dev Cell 13: 43–56, 2007 [DOI] [PubMed] [Google Scholar]
- 1766. Yeung T, Ozdamar B, Paroutis P, Grinstein S. Lipid metabolism and dynamics during phagocytosis. Curr Opin Cell Biol 18: 429–437, 2006 [DOI] [PubMed] [Google Scholar]
- 1767. Yeung T, Terebiznik M, Yu L, Silvius J, Abidi WM, Philips M, Levine T, Kapus A, Grinstein S. Receptor activation alters inner surface potential during phagocytosis. Science 313: 347–351, 2006 [DOI] [PubMed] [Google Scholar]
- 1768. Yin HL, Janmey PA. Phosphoinositide regulation of the actin cytoskeleton. Annu Rev Physiol 65: 761–789, 2003 [DOI] [PubMed] [Google Scholar]
- 1769. Ying H, Kimmelman AC, Lyssiotis CA, Hua S, Chu GC, Fletcher-Sananikone E, Locasale JW, Son J, Zhang H, Coloff JL, Yan H, Wang W, Chen S, Viale A, Zheng H, Paik JH, Lim C, Guimaraes AR, Martin ES, Chang J, Hezel AF, Perry SR, Hu J, Gan B, Xiao Y, Asara JM, Weissleder R, Wang YA, Chin L, Cantley LC, Depinho RA. Oncogenic Kras maintains pancreatic tumors through regulation of anabolic glucose metabolism. Cell 149: 656–670, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1770. Yoda A, Oda S, Shikano T, Kouchi Z, Awaji T, Shirakawa H, Kinoshita K, Miyazaki S. Ca2+ oscillation-inducing phospholipase C zeta expressed in mouse eggs is accumulated to the pronucleus during egg activation. Dev Biol 268: 245–257, 2004 [DOI] [PubMed] [Google Scholar]
- 1771. Yoko-o T, Matsui Y, Yagisawa H, Nojima H, Uno I, Toh-e A. The putative phosphoinositide-specific phospholipase C gene, PLC1, of the yeast Saccharomyces cerevisiae is important for cell growth. Proc Natl Acad Sci USA 90: 1804–1808, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1772. Yonezawa N, Nishida E, Iida K, Yahara I, Sakai H. Inhibition of the interactions of cofilin, destrin, and deoxyribonuclease I with actin by phosphoinositides. J Biol Chem 265: 8382–8386, 1990 [PubMed] [Google Scholar]
- 1773. Yoon MS, Du G, Backer JM, Frohman MA, Chen J. Class III PI-3-kinase activates phospholipase D in an amino acid-sensing mTORC1 pathway. J Cell Biol 195: 435–447, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1774. Yoon SY, Jellerette T, Salicioni AM, Lee HC, Yoo MS, Coward K, Parrington J, Grow D, Cibelli JB, Visconti PE, Mager J, Fissore RA. Human sperm devoid of PLC, zeta 1 fail to induce Ca2+ release and are unable to initiate the first step of embryo development. J Clin Invest 118: 3671–3681, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1775. York JD, Majerus PW. Isolation and heterologous expression of a cDNA encoding bovine inositol polyphosphate 1-phosphatase. Proc Natl Acad Sci USA 87: 9548–9552, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1776. York JD, Majerus PW. Nuclear phosphatidylinositols decrease during S-phase of the cell cycle in HeLa cells. J Biol Chem 269: 7847–7850, 1994 [PubMed] [Google Scholar]
- 1777. York JD, Odom AR, Murphy R, Ives EB, Wente SR. A phospholipase C-dependent inositol polyphosphate kinase pathway required for efficient messenger RNA export. Science 285: 96–100, 1999 [DOI] [PubMed] [Google Scholar]
- 1778. Yoshida S, Ohya Y, Goebl M, Nakano A, Anrakur Y. A novel gene, STT4, encodes a phosphatidylionositol 4-kinase in the PKC1 protein kinase pathway of Saccharomyces cerevisiae. J Biol Chem 269: 1166–1171, 1994 [PubMed] [Google Scholar]
- 1779. Yoshida S, Ohya Y, Nakano A, Anraku Y. Genetic interactions among genes involved in the STT4-PKC1 pathway of Saccharomyces cerevisiae. Mol Gen Genet 242: 631–640, 1994 [DOI] [PubMed] [Google Scholar]
- 1780. Yu J, Pan L, Qin X, Chen H, Xu Y, Chen Y, Tang H. MTMR4 attenuates transforming growth factor beta (TGFbeta) signaling by dephosphorylating R-Smads in endosomes. J Biol Chem 285: 8454–8462, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1781. Yu J, Zhang Y, McIlroy J, Rordorf-Nikolic T, Orr GA, Backer JM. Regulation of the p85/p110 phosphatidylinositol 3'-kinase: stabilization and inhibition of the p110alpha catalytic subunit by the p85 regulatory subunit. Mol Cell Biol 18: 1379–1387, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1782. Yu JW, Lemmon MA. All phox homology (PX) domains from Saccharomyces cerevisiae specifically recognize phosphatidylinositol 3-phosphate. J Biol Chem 276: 44179–44184, 2001 [DOI] [PubMed] [Google Scholar]
- 1783. Yu JW, Mendrola JM, Audhya A, Singh S, Keleti D, DeWald DB, Murray D, Emr SD, Lemmon MA. Genome-wide analysis of membrane targeting by S. cerevisiae pleckstrin homology domains. Mol Cell 13: 677–688, 2004 [DOI] [PubMed] [Google Scholar]
- 1784. Yu Y, Nomikos M, Theodoridou M, Nounesis G, Lai FA, Swann K. PLCzeta causes Ca2+ oscillations in mouse eggs by targeting intracellular and not plasma membrane PI(4,5)P2. Mol Biol Cell 23: 371–380, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1785. Yuan TL, Cantley LC. PI3K pathway alterations in cancer: variations on a theme. Oncogene 27: 5497–5510, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1786. Yuan Y, Gao X, Guo N, Zhang H, Xie Z, Jin M, Li B, Yu L, Jing N. rSac3, a novel Sac domain phosphoinositide phosphatase, promotes neurite outgrowth in PC12 cells. Cell Res 17: 919–932, 2007 [DOI] [PubMed] [Google Scholar]
- 1787. Yue G, Malik B, Eaton DC. Phosphatidylinositol 4,5-bisphosphate (PIP2) stimulates epithelial sodium channel activity in A6 cells. J Biol Chem 277: 11965–11969, 2002 [DOI] [PubMed] [Google Scholar]
- 1788. Yun M, Keshvara L, Park CG, Zhang YM, Dickerson JB, Zheng J, Rock CO, Curran T, Park HW. Crystal structures of the Dab homology domains of mouse disabled 1 and 2. J Biol Chem 278: 36572–36581, 2003 [DOI] [PubMed] [Google Scholar]
- 1789. Zakharian E, Cao C, Rohacs T. Intracellular ATP supports TRPV6 activity via lipid kinases and the generation of PtdIns(4,5) P. FASEB J 25: 3915–3928, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1790. Zawalich WS, Zawalich KC. A link between insulin resistance and hyperinsulinemia: inhibitors of phosphatidylinositol 3-kinase augment glucose-induced insulin secretion from islets of lean, but not obese, rats. Endocrinology 141: 3287–3295, 2000 [DOI] [PubMed] [Google Scholar]
- 1791. Zelenka PS. Changes in phosphatidylinositol metabolism during differentiation of lens fibres into lens epithelial cells in the embryonic chick. J Biol Chem 255: 1296–1300, 1980 [PubMed] [Google Scholar]
- 1792. Zhai C, Li K, Markaki V, Phelan JP, Bowers K, Cooke FT, Panaretou B. Ypp1/YGR198w plays an essential role in phosphoinositide signalling at the plasma membrane. Biochem J 415: 455–466, 2008 [DOI] [PubMed] [Google Scholar]
- 1793. Zhainazarov AB, Spehr M, Wetzel CH, Hatt H, Ache BW. Modulation of the olfactory CNG channel by Ptdlns(3,4,5)P3. J Membr Biol 201: 51–57, 2004 [DOI] [PubMed] [Google Scholar]
- 1794. Zhang H, Craciun LC, Mirshahi T, Rohacs T, Lopes CM, Jin T, Logothetis DE. PIP2 activates KCNQ channels, and its hydrolysis underlies receptor-mediated inhibition of M currents. Neuron 37: 963–975, 2003 [DOI] [PubMed] [Google Scholar]
- 1795. Zhang H, He C, Yan X, Mirshaki T, Logothetis DE. Activation of inwardly rectifying K+ channels by distinct PtdIns(4,5)P2 interactions. Nat Cell Biol 1: 183–188, 1999 [DOI] [PubMed] [Google Scholar]
- 1796. Zhang J, Banfic H, Straforini F, Tosi L, Volinia S, Rittenhouse SE. A type II phosphoinositide 3-kinase is stimulated via activated integrin in platelets. A source of phosphatidylinositol 3-phosphate. J Biol Chem 273: 14081–14084, 1998 [DOI] [PubMed] [Google Scholar]
- 1797. Zhang J, Guan Z, Murphy AN, Wiley SE, Perkins GA, Worby CA, Engel JL, Heacock P, Nguyen OK, Wang JH, Raetz CR, Dowhan W, Dixon JE. Mitochondrial phosphatase PTPMT1 is essential for cardiolipin biosynthesis. Cell Metab 13: 690–700, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1798. Zhang J, Ravichandran KS, Garrison JC. A key role for the phosphorylation of Ser440 by the cyclic AMP-dependent protein kinase in regulating the activity of the Src homology 2 domain-containing Inositol 5'-phosphatase (SHIP1). J Biol Chem 285: 34839–34849, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1799. Zhang L, Malik S, Kelley GG, Kapiloff MS, Smrcka AV. Phospholipase C epsilon scaffolds to muscle-specific A kinase anchoring protein (mAKAPbeta) and integrates multiple hypertrophic stimuli in cardiac myocytes. J Biol Chem 286: 23012–23021, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1800. Zhang L, Mao YS, Janmey PA, Yin HL. Phosphatidylinositol 4,5 bisphosphate and the actin cytoskeleton. Subcell Biochem 59: 177–215, 2012 [DOI] [PubMed] [Google Scholar]
- 1801. Zhang P, Wang Y, Sesaki H, Iijima M. Proteomic identification of phosphatidylinositol (3,4,5) triphosphate-binding proteins in Dictyostelium discoideum. Proc Natl Acad Sci USA 107: 11829–11834, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1802. Zhang SL, Yeromin AV, Zhang XH, Yu Y, Safrina O, Penna A, Roos J, Stauderman KA, Cahalan MD. Genome-wide RNAi screen of Ca(2+) influx identifies genes that regulate Ca(2+) release-activated Ca(2+) channel activity. Proc Natl Acad Sci USA 103: 9357–9362, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1803. Zhang W, Sloan-Lancaster J, Kitchen J, Trible RP, Samelson LE. LAT: the ZAP-70 tyrosine kinase substrate that links T cell receptor to cellular activation. Cell 92: 83–92, 1998 [DOI] [PubMed] [Google Scholar]
- 1804. Zhang X, Hartz PA, Philip E, Racusen LC, Majerus PW. Cell lines from kidney proximal tubules of a patient with Lowe syndrome lack OCRL inositol polyphosphate 5-phosphatase and accumulate phosphatidylinositol 4,5-bisphosphate. J Biol Chem 273: 1574–1582, 1998 [DOI] [PubMed] [Google Scholar]
- 1805. Zhang X, Jefferson AB, Auethavekiat V, Majerus PW. The protein deficient in Lowe syndrome is a phosphatidylinositol-4,5-bisphosphate 5-phosphatase. Proc Natl Acad Sci USA 92: 4853–4856, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1806. Zhang X, Tang N, Hadden TJ, Rishi AK. Akt, FoxO and regulation of apoptosis. Biochim Biophys Acta 1813: 1978–1986, 2011 [DOI] [PubMed] [Google Scholar]
- 1807. Zhang X, Vadas O, Perisic O, Anderson KE, Clark J, Hawkins PT, Stephens LR, Williams RL. Structure of lipid kinase p110beta/p85beta elucidates an unusual SH2-domain-mediated inhibitory mechanism. Mol Cell 41: 567–578, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1808. Zhang Y, Hoon MA, Chandrashekar J, Mueller KL, Cook B, Wu D, Zuker CS, Ryba NJ. Coding of sweet, bitter, and umami tastes: different receptor cells sharing similar signaling pathways. Cell 112: 293–301, 2003 [DOI] [PubMed] [Google Scholar]
- 1809. Zhang Y, Liao M, Dufau ML. Phosphatidylinositol 3-kinase/protein kinase Czeta-induced phosphorylation of Sp1 and p107 repressor release have a critical role in histone deacetylase inhibitor-mediated derepression [corrected] of transcription of the luteinizing hormone receptor gene. Mol Cell Biol 26: 6748–6761, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1810. Zhang Y, Zolov SN, Chow CY, Slutsky SG, Richardson SC, Piper RC, Yang B, Nau JJ, Westrick RJ, Morrison SJ, Meisler MH, Weisman LS. Loss of Vac14, a regulator of the signaling lipid phosphatidylinositol 3,5-bisphosphate, results in neurodegeneration in mice. Proc Natl Acad Sci USA 104: 17518–17523, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1811. Zhang Z, Okawa H, Wang Y, Liman ER. Phosphatidylinositol 4,5-bisphosphate rescues TRPM4 channels from desensitization. J Biol Chem 280: 39185–39192, 2005 [DOI] [PubMed] [Google Scholar]
- 1812. Zhao H, Hakala M, Lappalainen P. ADF/cofilin binds phosphoinositides in a multivalent manner to act as a PIP2-density sensor. Biophys J 98: 2327–2336, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1813. Zhao JJ, Liu Z, Wang L, Shin E, Loda MF, Roberts TM. The oncogenic properties of mutant p110alpha and p110beta phosphatidylinositol 3-kinases in human mammary epithelial cells. Proc Natl Acad Sci USA 102: 18443–18448, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1814. Zhao R, Qi Y, Chen J, Zhao ZJ. FYVE-DSP2, a FYVE domain-containing dual specificity protein phosphatase that dephosphorylates phosphotidylinositol 3-phosphate. Exp Cell Res 265: 329–338, 2001 [DOI] [PubMed] [Google Scholar]
- 1815. Zhen XG, Xie C, Yamada Y, Zhang Y, Doyle C, Yang J. A single amino acid mutation attenuates rundown of voltage-gated calcium channels. FEBS Lett 580: 5733–5738, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1816. Zheng J, Cahill SM, Lemmon MA, Fushman D, Schlessinger J, Cowburn D. Identification of the binding site for acidic phospholipids on the pH domain of dynamin: implications for stimulation of GTPase activity. J Mol Biol 255: 14–21, 1996 [DOI] [PubMed] [Google Scholar]
- 1817. Zheng L, Paik WY, Cesnjaj M, Balla T, Tomic M, Catt KJ, Stojilkovic SS. Effects of the phospholipase-C inhibitor, U73122, on signaling and secretion in pituitary gonadotrophs. Endocrinology 136: 1079–1088, 1995 [DOI] [PubMed] [Google Scholar]
- 1818. Zhong Y, Wang QJ, Li X, Yan Y, Backer JM, Chait BT, Heintz N, Yue Z. Distinct regulation of autophagic activity by Atg14L and Rubicon associated with Beclin 1-phosphatidylinositol-3-kinase complex. Nat Cell Biol 11: 468–476, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1819. Zhou Y, Sondek J, Harden TK. Activation of human phospholipase C-eta2 by Gbetagamma. Biochemistry 47: 4410–4417, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1820. Zhou Y, Wing MR, Sondek J, Harden TK. Molecular cloning and characterization of PLC-eta2. Biochem J 391: 667–676, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1821. Zhou Z, Yu X. Phagosome maturation during the removal of apoptotic cells: receptors lead the way. Trends Cell Biol 18: 474–485, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1822. Zhu G, Yoshida S, Migita K, Yamada J, Mori F, Tomiyama M, Wakabayashi K, Kanematsu T, Hirata M, Kaneko S, Ueno S, Okada M. Dysfunction of extrasynaptic GABAergic transmission in PRIP-1 KO mice is associated with an epilepsy phenotype. J Pharmacol Exp Ther 343: 520–528, 2012 [DOI] [PubMed] [Google Scholar]
- 1823. Zhu W, Trivedi CM, Zhou D, Yuan L, Lu MM, Epstein JA. Inpp5f is a polyphosphoinositide phosphatase that regulates cardiac hypertrophic responsiveness. Circ Res 105: 1240–1247, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1824. Zhu Y, Hu L, Zhou Y, Yao Q, Liu L, Shao F. Structural mechanism of host Rab1 activation by the bifunctional Legionella type IV effector SidM/DrrA. Proc Natl Acad Sci USA 107: 4699–4704, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1825. Zimmermann P, Meerschaert K, Reekmans G, Leenaerts I, Small JV, Vandekerckhove J, David G, Gettemans J. PIP2-PDZ domain binding controls association of syntenin with the plasma membrane. Mol Cell 9: 1215–1225, 2002 [DOI] [PubMed] [Google Scholar]
- 1826. Zolles G, Klocker N, Wenzel D, Weisser-Thomas J, Fleischmann BK, Roeper J, Fakler B. Pacemaking by HCN channels requires interaction with phosphoinositides. Neuron 52: 1027–1036, 2006 [DOI] [PubMed] [Google Scholar]
- 1827. Zolov SN, Bridges D, Zhang Y, Lee WW, Riehle E, Verma R, Lenk GM, Converso-Baran K, Weide T, Albin RL, Saltiel AR, Meisler MH, Russell MW, Weisman LS. In vivo, Pikfyve generates PI(3,5)P2, which serves as both a signaling lipid and the major precursor for PI5P. Proc Natl Acad Sci USA 109: 17472–17477, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1828. Zolyomi A, Zhao X, Downing GJ, Balla T. Localization of two distinct type III phosphatidylinositol 4-kinase enzyme mRNAs in the rat. Am J Physiol Cell Physiol 278: C914–C920, 2000 [DOI] [PubMed] [Google Scholar]
- 1829. Zoncu R, Bar-Peled L, Efeyan A, Wang S, Sancak Y, Sabatini DM. mTORC1 senses lysosomal amino acids through an inside-out mechanism that requires the vacuolar H+-ATPase. Science 334: 678–683, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1830. Zoncu R, Efeyan A, Sabatini DM. mTOR: from growth signal integration to cancer, diabetes and ageing. Nat Rev Mol Cell Biol 12: 21–35, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1831. Zoncu R, Perera RM, Sebastian R, Nakatsu F, Chen H, Balla T, Ayala G, Toomre D, De Camilli PV. Loss of endocytic clathrin-coated pits upon acute depletion of phosphatidylinositol 4,5-bisphosphate. Proc Natl Acad Sci USA 104: 3793–3798, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1832. Zou J, Chang SC, Marjanovic J, Majerus PW. MTMR9 increases MTMR6 enzyme activity, stability, and role in apoptosis. J Biol Chem 284: 2064–2071, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1833. Zou J, Marjanovic J, Kisseleva MV, Wilson M, Majerus PW. Type I phosphatidylinositol-4,5-bisphosphate 4-phosphatase regulates stress-induced apoptosis. Proc Natl Acad Sci USA 104: 16834–16839, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]