Abstract
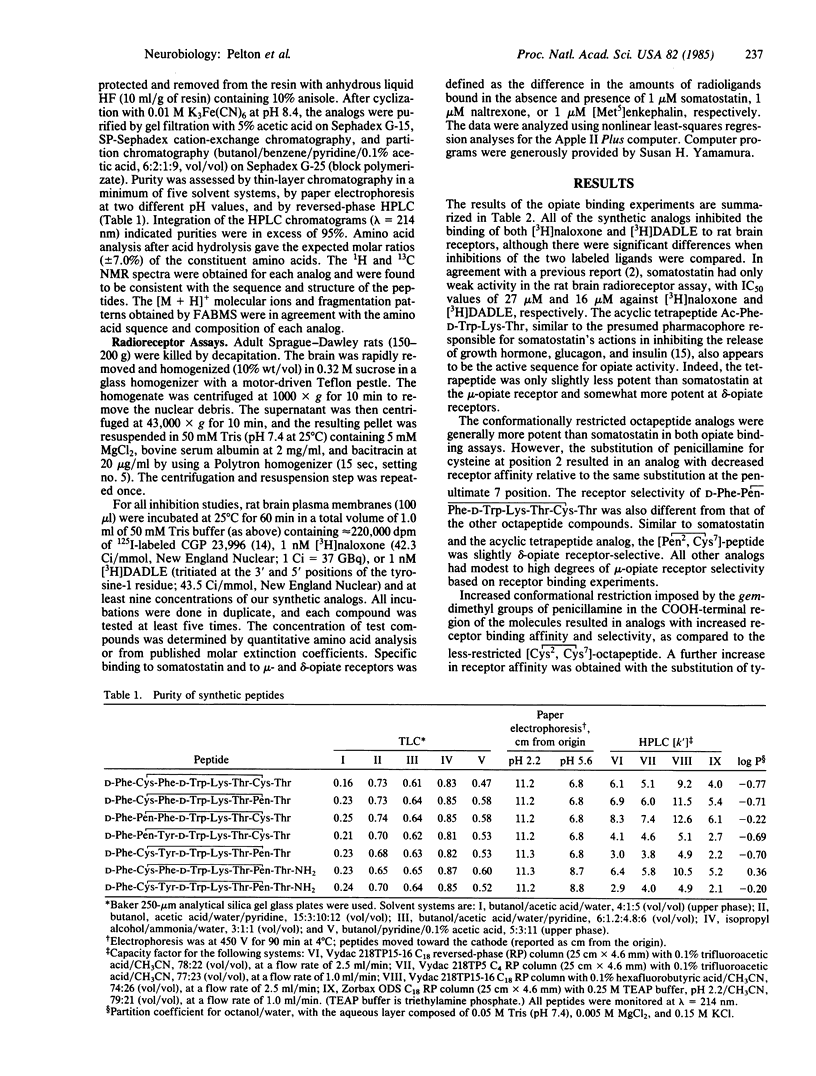
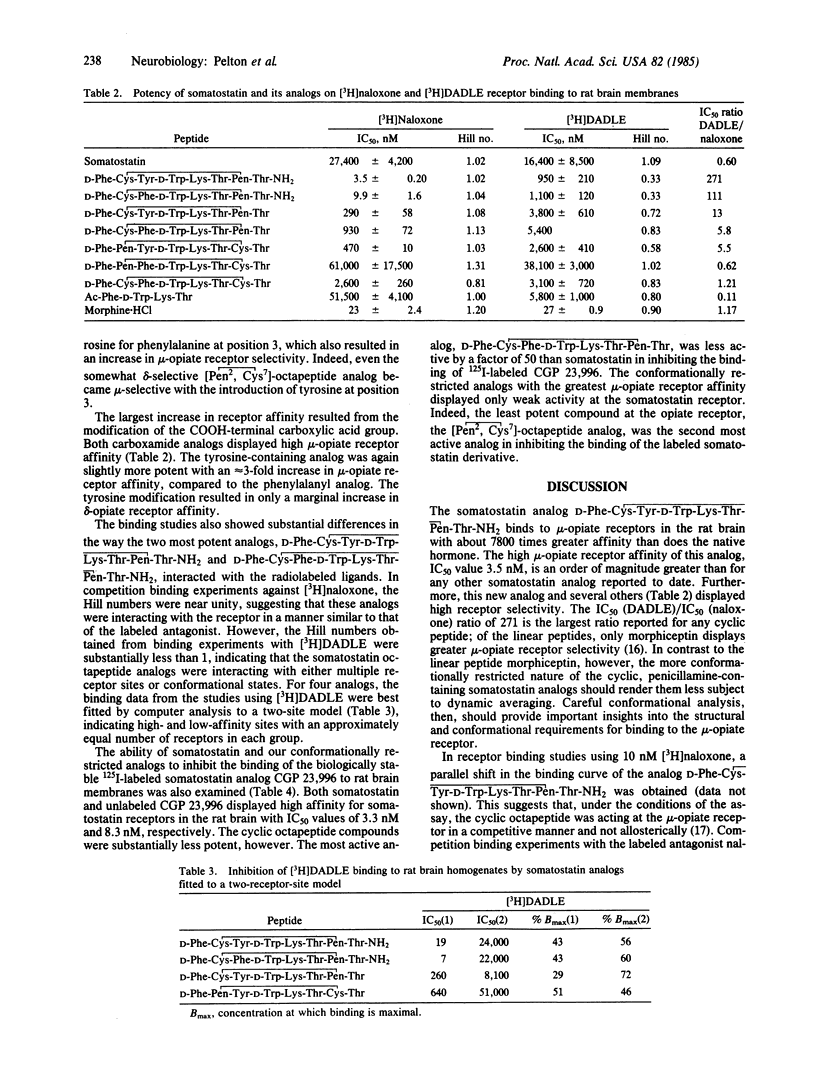
A series of cyclic, conformationally restricted analogs of somatostatin have been prepared and tested for their ability to inhibit the binding of [3H]naloxone and [D-Ala2, D-Leu5] [3H]enkephalin to rat brain membranes. The most potent analog, D-Phe-Cys-Tyr-D-Trp-Lys-Thr-Pen-Thr-NH2 where Pen is penicillamine in [D-Phe5, Cys6, Tyr7, D-Trp8, Pen11]somatostatin-(5-12)-octapeptide amide, exhibited high affinity for mu-opiate receptors (IC50 value of [3H]naloxone = 3.5 nM), being 7800 times more potent than somatostatin. The cyclic octapeptide also displayed high mu-opiate receptor selectivity with an IC50 [( D-Ala2,D-Leu5]enkephalin)/IC50 (naloxone) ratio of 271. The high affinity and selectivity of the somatostatin analog for mu-opiate receptors may be of use in examining the physiological role(s) of the mu-opiate receptor.
Full text
PDF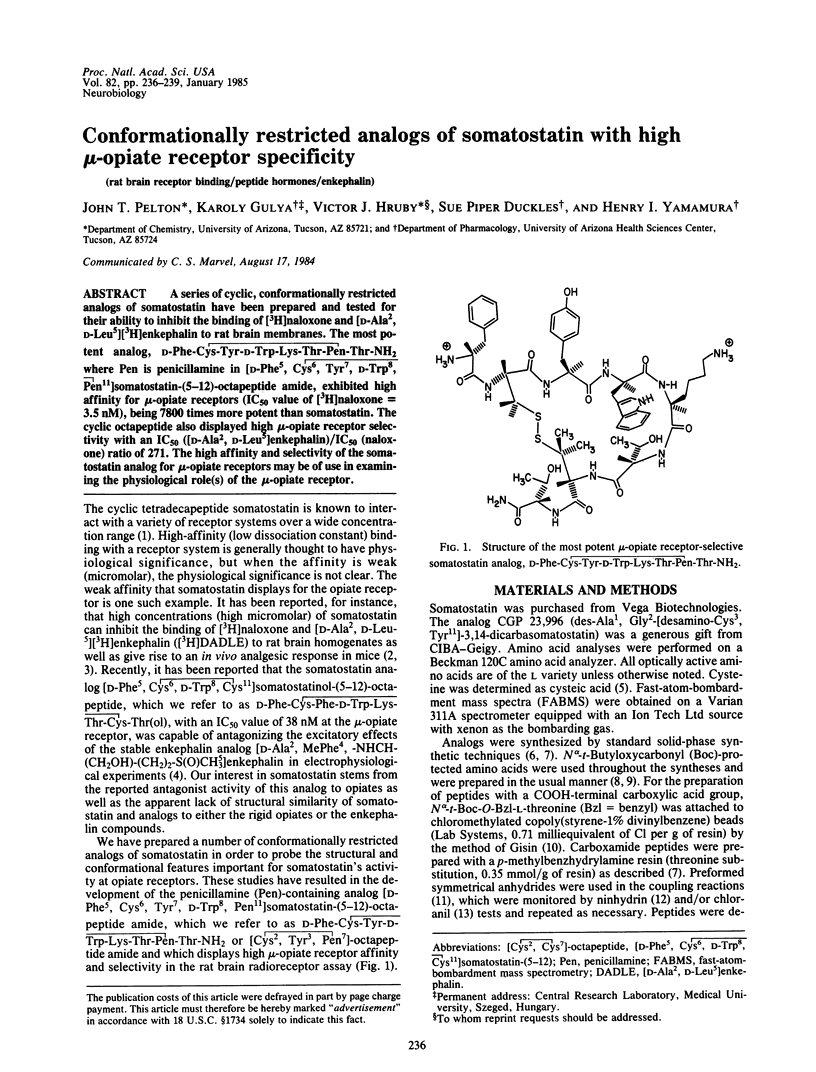

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chang K. J., Lillian A., Hazum E., Cuatrecasas P., Chang J. K. Morphiceptin (NH4-tyr-pro-phe-pro-COHN2): a potent and specific agonist for morphine (mu) receptors. Science. 1981 Apr 3;212(4490):75–77. doi: 10.1126/science.6259732. [DOI] [PubMed] [Google Scholar]
- Czernik A. J., Petrack B. Somatostatin receptor binding in rat cerebral cortex. Characterization using a nonreducible somatostatin analog. J Biol Chem. 1983 May 10;258(9):5525–5530. [PubMed] [Google Scholar]
- Ehlert F. J., Roeske W. R., Itoga E., Yamamura H. I. The binding of [3H]nitrendipine to receptors for calcium channel antagonists in the heart, cerebral cortex, and ileum of rats. Life Sci. 1982 Jun 21;30(25):2191–2202. doi: 10.1016/0024-3205(82)90293-4. [DOI] [PubMed] [Google Scholar]
- Hagenmaier H., Frank H. Increased coupling yields in solid phase peptide synthesis with a modified carbodiimide coupling procedure. Hoppe Seylers Z Physiol Chem. 1972 Dec;353(12):1973–1976. [PubMed] [Google Scholar]
- Kaiser E., Colescott R. L., Bossinger C. D., Cook P. I. Color test for detection of free terminal amino groups in the solid-phase synthesis of peptides. Anal Biochem. 1970 Apr;34(2):595–598. doi: 10.1016/0003-2697(70)90146-6. [DOI] [PubMed] [Google Scholar]
- Lundbaek K. Somatostatin: clinical importance and outlook. Metabolism. 1978 Sep;27(9 Suppl 1):1463–1469. doi: 10.1016/0026-0495(78)90093-8. [DOI] [PubMed] [Google Scholar]
- Maurer R., Gaehwiler B. H., Buescher H. H., Hill R. C., Roemer D. Opiate antagonistic properties of an octapeptide somatostatin analog. Proc Natl Acad Sci U S A. 1982 Aug;79(15):4815–4817. doi: 10.1073/pnas.79.15.4815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moroder L., Hallett A., Wünsch E., Keller O., Wersin G. Di-tert.-butyldicarbonat--ein vorteilhaftes Reagenz zur Eingührung der tert.-Butyloxycarbonyl-Schutzgruppe. Hoppe Seylers Z Physiol Chem. 1976 Nov;357(11):1651–1653. [PubMed] [Google Scholar]
- Rezek M., Havlicek V., Leybin L., LaBella F. S., Friesen H. Opiate-like naloxone-reversible actions of somatostatin given intracerebrally. Can J Physiol Pharmacol. 1978 Apr;56(2):227–231. doi: 10.1139/y78-033. [DOI] [PubMed] [Google Scholar]
- Terenius L. Somatostatin and ACTH are peptides with partial antagonist-like selectivity for opiate receptors. Eur J Pharmacol. 1976 Jul;38(1):211–213. doi: 10.1016/0014-2999(76)90221-1. [DOI] [PubMed] [Google Scholar]
- Upson D. A., Hruby V. J. Synthesis of specifically deuterated S-benzylcysteines and of oxytocin and related diastereomers deuterated in the half-cystine positions. J Org Chem. 1976 Apr 16;41(8):1353–1358. doi: 10.1021/jo00870a014. [DOI] [PubMed] [Google Scholar]
- Veber D. F., Holly F. W., Nutt R. F., Bergstrand S. J., Brady S. F., Hirschmann R., Glitzer M. S., Saperstein R. Highly active cyclic and bicyclic somatostatin analogues of reduced ring size. Nature. 1979 Aug 9;280(5722):512–514. doi: 10.1038/280512a0. [DOI] [PubMed] [Google Scholar]


