Abstract
SUMMARY
Treatment of tuberculosis (TB) remains challenging, with lengthy treatment durations and complex drug regimens that are toxic and difficult to administer. Similar to the vast majority of antibiotics, drugs for Mycobacterium tuberculosis are directed against microbial targets. Although more effective drugs that target the bacterium may lead to faster cure of patients, it is possible that a biological limit will be reached that can be overcome only by adopting a fundamentally new treatment approach. TB regimens might be improved by including agents that target host pathways. Recent work on host-pathogen interactions, host immunity, and host-directed interventions suggests that supplementing anti-TB therapy with host modulators may lead to shorter treatment times, a reduction in lung damage caused by the disease, and a lower risk of relapse or reinfection. We undertook this review to identify molecular pathways of the host that may be amenable to modulation by small molecules for the treatment of TB. Although several approaches to augmenting standard TB treatment have been proposed, only a few have been explored in detail or advanced to preclinical and clinical studies. Our review focuses on molecular targets and inhibitory small molecules that function within the macrophage or other myeloid cells, on host inflammatory pathways, or at the level of TB-induced lung pathology.
INTRODUCTION
The effective treatment of tuberculosis (TB) using current antibiotics faces obstacles that include a lengthy duration of treatment, potential drug toxicity, drug interactions with HIV medications, and rising rates of drug resistance. Efforts to develop new TB drugs have focused on mechanisms that target the bacillus. Recently, attention has turned to potential host-directed therapeutics (HDTs) in the hope that novel treatment strategies might overcome many of the obstacles faced by antibiotic therapies for TB. The goal of HDTs might be to shorten the course of treatment, reduce the number of agents required in combination drug therapy, simplify treatment of drug-resistant TB by improving the efficacy of second-line therapy, and/or preserve lung function of TB patients. The goal of treatment would determine the host target selected for intervention. HDTs that manipulate immune responses or the metabolic state of the bacteria to enhance host cell function, optimize inflammatory responses at the cell and organ level, or modify lung pathology might be employed during treatment. To identify pathways involved in the host response to TB and compounds that modulate these pathways, we searched PubMed for papers published from 2000 onwards and, with a few exceptions, focused primarily on small-molecule compounds that modulate host target pathways involved in control of TB, rather than larger-molecule “biologics.” Our review examines three broad target areas of HDTs. First, we discuss the biological pathways and compounds that act primarily within the macrophage or other host cells of TB. Second, we focus on HDTs that modulate the immune response and inflammatory pathways in the lung. Third, we consider pathways that modulate lung pathology and tissue homeostasis. These categories are not mutually exclusive, and some compounds and pathways belong to more than one category. We hope that this review will stimulate further studies of HDTs for TB that could shorten treatment duration, lower the number of drugs needed for treatment, and/or improve lung function and clinical outcomes.
OVERVIEW OF IMMUNE RESPONSE TO M. TUBERCULOSIS AND POTENTIAL HDT TARGETS
From recognition to killing, the macrophage plays a central role in Mycobacterium tuberculosis pathogenesis. First, M. tuberculosis binds to receptors on macrophages and other myeloid cells, where it is detected by the innate immune system (Fig. 1). Several receptors are critical for recognition, including Toll-like receptors (TLRs) (TLR1/2/6/8/9), Nod-like receptors (NLRs) (NOD2), C-type lectin receptors (CLRs) (CLEC4E or Mincle), mannose receptor (MR), dendritic cell-specific intracellular adhesion molecule 3 (ICAM-3)-grabbing nonintegrin (DC-SIGN) (CD209), complement receptors, Fc receptors, and DNA sensors (STING) (1–9). After binding and recognition by innate immune receptors, M. tuberculosis enters a phagocytic vacuole and prevents its maturation and fusion with lysosomes as an immune evasion strategy. Under some conditions, activation of the macrophage leads to phagolysosomal fusion, secretion of cytokines such as tumor necrosis factor (TNF), alpha interferon (IFN-α), IFN-β, interleukin-6 (IL-6), IL-12, and IL-1β, and production of antimicrobial reactive nitrogen intermediates (RNIs) and reactive oxygen intermediates (ROIs), all of which may lead to killing of M. tuberculosis. With a central role in the host response to M. tuberculosis, macrophage functions offer many potential targets for HDTs.
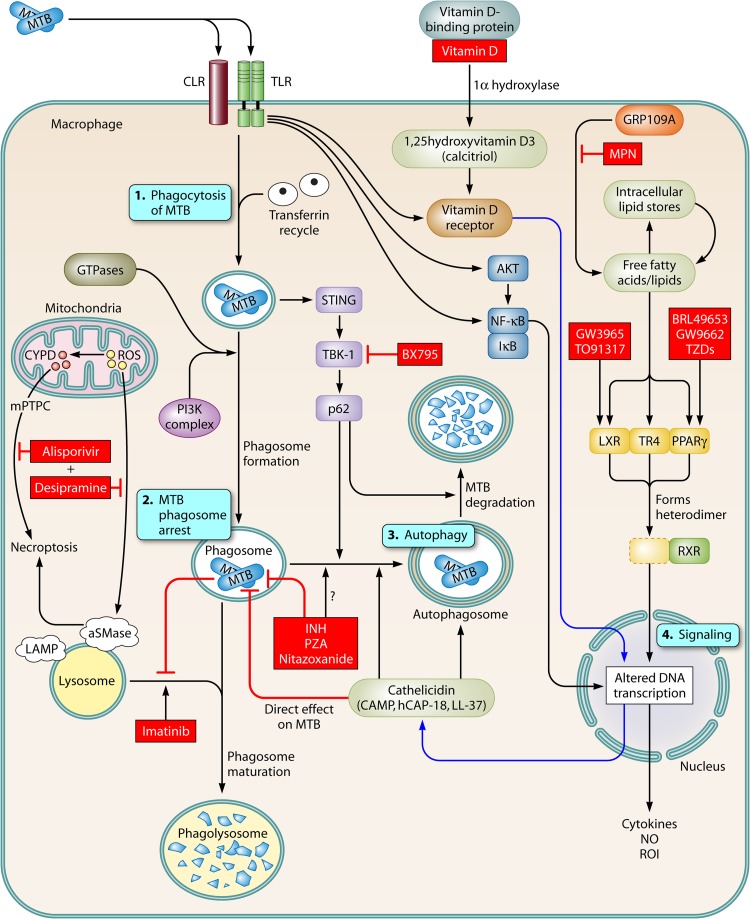
Fig 1.
HDTs within the macrophage. Upon infection of a macrophage by M. tuberculosis, several pathways that may serve as targets for host-directed therapeutics are activated. 1. After binding and uptake of M. tuberculosis (MTB) by macrophages through innate immune receptors (e.g., C-type lectin receptors [CLRs] and Toll-like receptors [TLRs]), the bacilli are taken into a macrophage and contained in phagosomes. Several signaling pathways and molecules, including Rab proteins, IRGM1, and phosphatidylinositol 3-kinase (PI3K), promote maturation of phagosomes and fusion with lysosomes. 2. M. tuberculosis arrests the development of phagolysosomes, preventing their acidification and enabling intracellular survival of M. tuberculosis. 3. Autophagy pathways can be stimulated by M. tuberculosis or other conditions, which leads to autophagosome formation and control of M. tuberculosis growth. 4. Several pathways mediate activation of signaling molecules. TLRs activate key elements of the signaling processes, including AKT, NF-κB, and components of the vitamin D pathway. The lipid-sensing nuclear receptors, LXR, TR4, and PPARγ, bind with RXR to modulate gene expression. HDT targets described in the text are marked in boxes with red shading.
The pulmonary and systemic immune response to M. tuberculosis also offers numerous HDT targets. After inhalation of M. tuberculosis into the lung, resident alveolar macrophages are one of the initial target cells of infection, in addition to dendritic cells and neutrophils, which traffic to the site of infection. M. tuberculosis-infected cells migrate to pulmonary lymph nodes, where an adaptive immune response is mounted with T cell production of IFN-γ and B cell production of antibodies (10, 11). The classical model of a successful immune response to M. tuberculosis includes secretion of IFN-γ by T cells, which activates macrophages to kill M. tuberculosis. When this is unsuccessful, M. tuberculosis continues to grow intracellularly until it lyses the cell and either reinfects new cells or replicates extracellularly. Extracellular TB can be associated with high numbers of bacteria (e.g., in lung cavities), which, due to their growth rate and metabolic state, likely have varying susceptibilities to TB drugs in comparison with intracellular bacilli. In addition, the extracellular niche can be a source of drug-resistant organisms due to the high bacterial burden and known ability of M. tuberculosis to develop drug resistance. When the immune response is partially successful, activated macrophages and other host cells (T cells, B cells, and fibroblasts) surround the M. tuberculosis-infected cells in an organized display, a granuloma, creating hypoxic, acidic, nutrient-poor conditions that are less permissive for M. tuberculosis replication. However, the lesions are not always sterilized, as M. tuberculosis employs a number of strategies to ensure its survival (12), including resisting toxic molecules produced by the host, modifying phagosome biogenesis to create an environment suitable for survival and growth, coopting the trafficking of cells within the granuloma to expand the number of infected cells, and inhibiting macrophage apoptosis to preserve its host niche (13, 14). At the adaptive level, an unusual and important feature of early immune responses to M. tuberculosis is the delayed appearance of T cell responses. M. tuberculosis adopts a nonreplicating state and can persist for many years until HIV or other factors restore conditions permissive for bacterial replication and development of active disease. Although the immune response in the lung is directed at eliminating the bacillus, activation of pathways that damage lung tissue result in fibrosis, scar formation, and impaired lung function.
The pulmonary immune response to M. tuberculosis contains many potential HDT targets. The stimulation of an antibacterial, M. tuberculosis-specific, T cell and B cell response is an HDT target that is currently hampered by lack of knowledge about the mechanisms underlying protective immunity. The majority of effort in this area is directed toward defining components of a vaccine that would stimulate protective M. tuberculosis-specific T cells. In addition, several lines of evidence suggest that M. tuberculosis-specific antibodies are important for a protective host response to M. tuberculosis (10). Antibody therapies are also potentially useful for TB treatment. Due to the large scope of this area, vaccine strategies involving stimulation of T cell- and B cell-mediated immune responses are not covered in this review article.
HDTs AND MACROPHAGE FUNCTION
Binding and M. tuberculosis Uptake
Upon recognition by the macrophage, uptake of M. tuberculosis is mediated by several surface receptors, including complement receptors, C-type lectin receptors, and TLRs. Inhibition of binding and uptake might be an attractive therapeutic target, since infection of the host cell would be prevented; however, the fate of the extracellular bacilli would determine progression of the infection and the course of disease. Imatinib is a tyrosine kinase inhibitor of BRC-ABL and was developed for treatment of chronic myelogenous leukemia (CML). ABL and related tyrosine kinases are involved in the uptake of M. tuberculosis and Mycobacterium marinum into macrophages, and treatment of macrophages with imatinib partially impaired uptake of M. tuberculosis into the cell (15) (Table 1). The mechanism of how imatinib inhibits entry and uptake is unknown.
Table 1.
Small molecules that target host pathways and regulate M. tuberculosis pathogenesis
| Compound (host target) | Host target pathway | FDA approval | Reference(s) |
|---|---|---|---|
| Desipramine | Acid sphingomyelinase | Yes | 155 |
| Nitazoxanide (quinone oxidoreductase NQO1) | Autophagy | Yes | 39 |
| Rapamycin (unclear target) | Autophagy | No | 30 |
| CC-3052 (PDE4) | cAMP | No | 148–150 |
| Cilostazol (PDE3) | cAMP | Yes | 144 |
| Pentoxifylline (nonselective PDE) | cAMP | Yes | 152 |
| Sildenafil (PDE5) | cAMP | Yes | 144 |
| Alisporivir | Cyclophilin D | No (phase III) | 155 |
| Acetylsalicylic acid/aspirin (COX) | Eicosanoids | Yes | 83, 93, 94 |
| PD146176 (15-LOX) | Eicosanoids | No | 82, 83 |
| U75302 and bestatin (LTB4 receptor) | Eicosanoids | No | 82, 83 |
| D4476 (CSNK1 and TGF-β receptor type 1) | Kinase | No | 198 |
| H-89 and ETB067 (PKB/ATK1) | Kinase | No | 34 |
| Imatinib (ABL tyrosine kinase) | Kinase | Yes | 15 |
| N-[2-(1H-Indol-3-yl)ethyl]-4-(2-methyl-1H-indol-3-yl)pyrimidin-2-amine, “compound 51” (PKR) | Kinase | No | 100, 199 |
| Mepenzolate bromide (GPR109A) | Lipid body formation | No | 58 |
| Berberine (PPARγ) | Lipid-sensing nuclear receptors | No | |
| BR49653 (PPARγ) | Lipid-sensing nuclear receptors | No | 59 |
| GW9662 (PPARγ) | Lipid-sensing nuclear receptors | No | 59, 61 |
| Perfluorononaoic acid (PPARγ) | Lipid-sensing nuclear receptors | No | |
| Thiazolidinediones (PPARγ) | Lipid-sensing nuclear receptors | No | |
| Oxyphenbutazone | Nonsteroidal anti-inflammatory | Yes | 40 |
| Ro32-3555 (MMP1, -8, -13) | MMP | No | 156 |
| Vitamin D (VDR) | Vitamin D | Yes | 53–56, 200, 201 |
Phagosome Maturation and Function
After binding and uptake by macrophages, M. tuberculosis resides in the phagosome. Normally, phagosomes mature by fusing with acidic lysosomes, which contain antimicrobial proteases and lipases. The proteases and lipases, together with ROIs and RNIs produced by the macrophage, degrade and destroy bacteria. M. tuberculosis arrests phagosome maturation, preserving features of an early endosome, including a higher pH and the absence of many molecules that degrade bacteria (5, 16, 17). Activation of macrophages with IFN-γ and other agonists increases macrophage restriction of M. tuberculosis replication (18). The complex interaction between M. tuberculosis and the phagosome suggests that multiple pathways could potentially be manipulated to control M. tuberculosis intracellular survival and replication.
Phagosome acidification.
Phagosomal maturation and acidification offer potential targets for intervention. ABL tyrosine kinase appears to control the phagosomal acidification required for M. tuberculosis growth restriction in human macrophages by regulating the vacuolar proton pump vATPase (19) (Fig. 1). CML patients receiving imatinib therapy had greater numbers of circulating monocytes with acidified lysosomes than controls. In addition, the intracellular survival of M. tuberculosis was reduced in macrophages treated with sera from patients receiving imatinib (19). In mice infected with M. marinum, imatinib treatment reduced the number of granulomatous lesions and the mycobacterial load in infected organs (15). In addition, the antimycobacterial effect of imatinib was synergistic with rifamycin antibiotics in mice infected with M. marinum and also in THP-1 cells infected with M. tuberculosis (15).
GTPases and phagosome maturation.
GTPases are enzymes that hydrolyze GTP to GDP, regulating the cell cycle, immunity, organelle trafficking, and phagosome formation (20) (Fig. 1). In mice, there are several families of GTPases, including the 47-kDa immunity-related GTPases (p47 IRGs), the 65- to 73-kDa guanylate-binding proteins (p65 Gpbs), the 285-kDa very large inducible GTPases (Vligs/Gvins) (18, 21), and the Rab GTPases (22). The p47, p65, and Rab families of GTPases are important mediators of the immune response to M. tuberculosis. GTPases regulate several important cellular processes that affect M. tuberculosis growth, including phagosome formation, phagosome maturation, and autophagy. IRGM1 (also known as LRG47) is the best-studied member of the p47 family, with a demonstrated role in M. tuberculosis pathogenesis (23). Lrg47 knockout mice have increased M. tuberculosis growth compared to that of wild-type (WT) mice, and Lrg47-deficient macrophage phagosomes show decreased fusion with lysosomes and incomplete acidification (24). Interestingly, Lrg47 also regulates survival of CD4 T cells through a mechanism that might be separate from macrophage functions regulated by this gene (25). Several members of the p65 GTPase family are also involved in autophagy induction (26). Although these findings in the mouse model are suggestive of an important role of IRGM1 in M. tuberculosis pathogenesis, the extrapolation to humans may not be straightforward due to gene family differences. There are 18 to 23 mouse p47 IRG genes, all of which are IFN-γ inducible, and only 3 in humans, none of which are IFN-γ inducible. In a loss-of-function screen of the 11 members of the p65 family, the absence of Gbp1 led to increased growth of Mycobacterium bovis bacillus Calmette-Guérin (BCG) in mice; Gbp1, -6, -7, and -10 regulated BCG growth in macrophages in vitro, and Gbp7 localized to phagosomes and regulated reactive oxygen production and subsequent mycobacterial killing (via p67phox translocation) (26). Although these studies have shown that GTPases regulate numerous steps in the cellular response to M. tuberculosis, no drug candidates targeting the GTPases have yet been identified.
Effector Function
Drugs may trigger effector functions of macrophages that lead directly to M. tuberculosis killing. For example, molecules that increase RNI and ROI or induce autophagy could potentially control M. tuberculosis replication.
Autophagy.
Autophagy is a process through which cells degrade and recycle their cytoplasmic contents using lysosomes. The targets of autophagy include cellular macromolecules and organelles, as well as intracellular pathogens such as M. tuberculosis, whose survival depends on avoiding degradation by the host (27, 28) (Fig. 1). Autophagy occurs under normal physiological conditions, but it is typically studied in human leukocytes as a response to starvation or IFN-γ stimulation. Steps in autophagy include formation of a phagophore and elongation and closure of the autophagosome, followed by fusion with lysosomes to form autolysosomes and degradation of contents. During an effective host response, the M. tuberculosis-containing phagosome matures into autolysosomes to degrade M. tuberculosis. Targeting of microbes to the autophagosome occurs through sequestasome-like receptors (SLRs) (p62/SQSTM1) that use ubiquitin tags and an LC3-interacting region. It may be possible to activate autophagy with small molecules that broadly modulate autophagic pathways involved in bulk processing of cytoplasmic contents (29) or by selectively targeting host proteins that pathogens interact with when manipulating autophagy, as has recently been shown for viruses and the intracellular pathogen Listeria monocytogenes (29).
Several lines of evidence suggest that autophagy is important in M. tuberculosis pathogenesis and might be an attractive target for an HDT. Some of these key papers are discussed in a recent review article of autophagy and TB pathogenesis (28). When autophagy is induced by IFN-γ, starvation, or rapamycin, macrophages effectively restrict M. tuberculosis growth (30, 31). Furthermore, Deretic and colleagues have demonstrated that knockdown of the molecules Rab8b, p62 and TBK1, which are involved in membrane trafficking and autophagy, reduce autophagosome initiation and maturation and limit the ability of macrophages to control M. tuberculosis or BCG replication (32, 33). AKT1 is a kinase involved in regulation of many signaling pathways, including activation of autophagy. Kuijl et al. performed a small interfering RNA (siRNA) screen of 658 human kinases using automated microscopy measuring Salmonella enterica serovar Typhimurium growth and identified 10 host kinases that reduced the growth of intracellular S. Typhimurium. The study also demonstrated that treatment with H-89 and ETB067, two structurally similar molecules that inhibit AKT-1, reduced the growth of multidrug-resistant (MDR) M. tuberculosis in primary human macrophages (34). Consistent with these findings, Kumar et al. found that knockdown of AKT1 and AKT2 caused reduced M. tuberculosis growth in THP1 cells (35).
On the bacterial side, a recent study found that the ESX-1 secretion system of M. tuberculosis triggers autophagy by release of bacterial DNA into the cytosol, where it is recognized by a STING-dependent DNA sensor pathway (36). This leads to ubiquitination of M. tuberculosis and delivery to autophagosomes in a p62-, NDP52-, and TBK1-dependent process. This study identified Atg5, STING, and NDP52 as regulators of M. tuberculosis growth in vitro in macrophages. Mice lacking Atg5 were also highly susceptible to M. tuberculosis in an in vivo infection model.
Recent studies have raised the intriguing idea that TB antibiotics may trigger autophagy. Kim et al. demonstrated that isoniazid (INH) and pyrazinamide (PZA) induce autophagy in M. tuberculosis-infected murine macrophages in a process that involves reactive oxygen species (ROS) (37). In experiments with in vitro infection of macrophages, inhibition of autophagy impaired the efficacy of INH. Interestingly, INH and PZA did not induce autophagy in uninfected cells, suggesting that autophagy is not induced directly by the antibiotics. It is possible that INH and PZA trigger autophagy indirectly through their antibacterial activities, with autophagy occurring only after the dead bacillus activates autophagy pathways. Nitazoxanide (NTZ) is an antiparasitic drug that also has activity against replicating and nonreplicating M. tuberculosis (38). Lam et al. demonstrated that NTZ can promote autophagy by inhibiting mTORC1 signaling (a negative regulator of autophagy) and stimulating processing of LC3 (39). NTZ inhibited the growth of M. tuberculosis in THP1 cells, and the human enzyme quinone oxidoreductase NQO1 was identified as the putative host target. These data suggest that NTZ might be a dual-acting antibiotic that exerts its antimicrobial effect directly on M. tuberculosis as well as by inducing autophagy in the host cell. Although not identified to play a role in autophagy, other small molecules also have dual host and antibacterial activities. The FDA-approved drugs oxyphenbutazone, a nonsteroidal anti-inflammatory agent, and zafirlukast, a leukotriene receptor antagonist that appears to dysregulate mycobacterial transcription, both kill M. tuberculosis in vitro (40, 41). The potential role of these drugs as dual host-directed and anti-M. tuberculosis therapies in models of infection remains to be defined.
Vitamin D.
Vitamin D has intrigued the TB field for many years, with early interest stemming from theories about whether cod liver oil and/or sunlight provided benefit through effects of vitamin D. Vitamin D has two forms in humans: ergocalciferol (vitamin D2) and cholecalciferol (vitamin D3). D2 and D3 can be obtained from diet, and D3 is also derived from sun exposure (via conversion of 7-dehydrocholesterol to D3). D3 is a prohormone that is converted into 1,25-hydroxy-D3 (25-OH-D3) (also called calcifediol and calcidiol), which then circulates in serum. 25-OH-D3 can be taken up by macrophages through the vitamin D-binding protein (DBP) and converted to 1,25-dihydroxy-D3 [1,25-(OH)2-D3, also called calcitriol], which is the active molecule. During M. tuberculosis infection of a macrophage, two convergent processes take place that result in antimycobacterial activity (42, 43). First, stimulation of the TLR1 and TLR2 receptors by M. tuberculosis promotes production of 1α-hydroxylase and vitamin D receptor (VDR). Second, 25-OH-D3 that has entered the macrophage is converted by 1α-hydroxylase into calcitriol by an IL-15-dependent mechanism (44). Calcitriol interacts with the VDR to stimulate a signaling pathway that produces the cathelicidin antimicrobial peptide (CAMP) (also called hCAP-18 or LL-37). CAMP is toxic to M. tuberculosis and also modulates phagosome maturation (45) and autophagosome formation (45–50) (Fig. 1).
Vitamin D is the most intensively studied TB HDT, with five randomized control trials (RCTs) in humans (Table 2). A case-control study found that vitamin D deficiency was associated with an increased risk of TB (51). A double-blind RCT with an immunologic endpoint demonstrated that a single dose of vitamin D improved M. tuberculosis growth restriction in supplemented patients versus controls (52). Several RCTs evaluated the effect of vitamin D on clinical outcomes. Some of the initial smaller trials reported a benefit from vitamin D. Nursyam et al. found an improved 6-week sputum conversion rate in the vitamin D arm (100% versus 76.7%; P = 0.002, n = 67) (53). Kota et al. found a trend toward more rapid sputum smear conversion time in a cohort from India (6 weeks versus 8 weeks; P = 0.067, n = 30) (54). In a larger study, Martineau et al. found no difference in sputum culture conversion time in an intention-to-treat analysis, though the trend favored vitamin D (36 days versus 43.5 days; P = 0.14, n = 126) (55). In a follow-up study with a per-protocol analysis of 95 subjects and adjustments for factors associated with sputum conversion, the median time to smear conversion was lower for the vitamin D group (23 versus 36 days; P = 0.04) (56). However, the time to culture conversion was still not significantly different (35 versus 46.5 days; P = 0.36). In additional exploratory analyses, vitamin D was associated with higher lymphocyte and monocyte counts as well as several cytokines (56). Finally, Wejse et al. found no difference in time to sputum smear conversion or mortality in a study of 365 TB patients in Guinea Bissau (57). In addition to treatment trials, several epidemiologic studies measured associations between genetic polymorphisms in VDR genes and susceptibility to TB. Although some studies found positive associations, results have been inconsistent, possibly due to heterogeneity of study design (1). Interestingly, Martineau et al. (55) found a significant difference in time to sputum conversion among those with the tt genotype of the TaqI gene but no difference among those with the TT and Tt genotypes. Overall, the smaller studies tended to report positive results, whereas the two larger, more comprehensive RCTs did not show a consistent benefit in terms of clinical outcomes (sputum culture conversion and mortality). The clinical trial results may differ due to substantial differences in the intervention employed in terms of vitamin D dose, schedule, location (different sunlight exposure), and vitamin D deficiency levels of the population.
Table 2.
Human clinical trial data for host-directed therapy for TBa
| Drug (reference[s]) | Sample size, n (population) | Intervention | Key results and comments |
|---|---|---|---|
| Aspirin (83, 94) | 119 (TBM) | RCT, aspirin 150 mg p.o. daily vs placebo; all received standard TB treatment; some received prednisolone | Stroke risk, aspirin vs placebo, OR 0.42 (95% CI 0.12–1.39, P = 0.18); death risk, placebo vs aspirin, OR 2.76 (95% CI 1.05–7.39, P = 0.03); prednisolone use was not standardized and was used more frequently in survivors and those who did not develop a new stroke |
| Aspirin (93) | 159 (TBM) | RCT, placebo vs aspirin 75 mg daily vs aspirin 100 mg/kg daily; all received standard TB treatment with prednisolone | No difference in mortality or morbidity among groups |
| Etanercept (141, 142) | 16 (PTB) | Single-arm trial of etanercept (n = 16) started on day 4 of TB treatment compared to historical controls (n = 42) | SCC slightly more rapid in etanercept group (median, 56 vs 63 days; P = 0.05) |
| IFN-γ (138) | 96 (PTB) | RCT, IFN-γ nebulized vs IFN-γ subcutaneous vs placebo; all received standard TB treatment | Higher SSC at 4 wk in IFN-γ group (P = 0.03); trend to higher SCC at 4 wk in IFN-γ group (P = 0.15); disease symptoms were less in both IFN-γ treatment groups |
| IFN-γ (137) | 32 (nontuberculous mycobacterial lung disease) | RCT, IFN-γ intramuscularly vs placebo; all received standard NTM treatment | IFN-γ group with improvement of symptoms compared to controls (6-mo complete responders, 72% vs 36%; P = 0.037); higher SCC at 18 mo in IFN-γ group (P = 0.04); radiographic improvement higher in IFN-γ group at 18 mo (P = 0.036) |
| IFN-γ (202) | 5 (PTB, MDR) | Open-label aerosol IFN-γ | Aerosolized IFN-γ was well tolerated by 5 MDR TB patients; treated patients showed steady wt gain; 4/5 treated patients switched from sputum smear positive to negative after 4 wk of treatment; chest CT scans showed improvement in all 5 treated patients |
| Pentoxifylline (152) | 120 (PTB) | RCT, pentoxifylline vs placebo; all received standard TB treatment | No difference in M. tuberculosis culture conversion, radiographic improvement, or death |
| Thalidomide (147) | 47 (TBM) | RCT, thalidomide vs placebo; all received standard TB treatment including prednisolone | Study stopped early because all adverse events (rash, hepatitis, death) occurred in thalidomide group |
| Vitamin D2 (200) | 192 (contacts of TB cases, United Kingdom) | RCT, placebo vs vitamin D2 (single dose of 2.5 mg) | Primary outcome, BCG growth in whole-blood assay; 24-h growth down in vitamin D group; no difference at 96 h |
| Vitamin D (53) | 67 (PTB, Indonesia) | RCT, placebo vs vitamin D (type not defined) (25 mg/day over 6 wk); all received standard TB treatment | Primary outcome, not specified; longer SSC in vitamin D group than in placebo group at 6 wk (77 vs 100%, P = 0.002) |
| Vitamin D3 (55, 56) | 146 (PTB, United Kingdom) | RCT, placebo vs vitamin D3 (2.5 mg × 4 doses over 42 days); all received standard TB treatment | Primary outcome, SCC; trend toward shorter SCC in vitamin D group but not significant; VDR TaqI tt genotype significantly lower time to conversion (but not Tt or TT genotype) |
| Vitamin D3 (57) | 365 (PTB, Guinea-Bissau) | RCT, placebo vs vitamin D3 (100,000 IU × 3 over 8 mo); all received standard TB treatment | Primary outcome, clinical improvement as assessed by clinical severity TB score; no difference in TB score, SSC, wt gain, or all-cause mortality |
| Vitamin D3 (54) | 30 (PTB, India, all with diabetes) | RCT, placebo vs vitamin D3 (60,000 IU p.o. per wk for 6 wk) + calcium carbonate (1,000 mg/day); all received standard TB treatment | Primary outcome, SSC; trend toward shorter SSC in vitamin D group (8 wk vs 6 wk; P = 0.067) |
Abbreviations: MDR, multidrug resistant; OR, odds ratio; CI, confidence interval; p.o., orally; PTB, pulmonary tuberculosis; RCT, randomized controlled trial; SCC, sputum culture conversion; SSC, sputum smear conversion; TBM, TB meningitis; NTM, nontuberculous mycobacteria; CT, computed tomography.
Lipid Metabolism
Lipid bodies and foamy macrophages.
One mechanism by which M. tuberculosis hijacks the cellular machinery of host macrophages is by promoting the formation of lipid bodies, giving rise to the foamy cell phenotype observed in TB. Fatty acids derived from lipid bodies might provide an important energy source for the bacterium, allowing it to survive and grow within host macrophages. Lipid body-derived fatty acids are also substrates for eicosanoid biosynthesis, which can either be pro- or anti-inflammatory. Recent data suggest that M. tuberculosis induces lipid bodies by induction of ketogenesis, which activates GPR109A and an antilipolytic pathway, leading to accumulation of lipid bodies that may have a protective effect for M. tuberculosis (58) (Fig. 1). Inhibition of GPR109A with mepenzolate bromide (MPN) led to increased killing of M. tuberculosis in THP1 cells and human peripheral blood monocyte-derived macrophages, as well as in murine in vivo studies (58).
Lipid-sensing nuclear receptors: PPARγ, LXRα,β, and TR4.
Macrophage lipid body formation and metabolism in M. tuberculosis pathogenesis is regulated by lipid-sensing nuclear receptors, including peroxisome proliferator-activated receptor gamma (PPARγ), liver X receptors alpha and beta (LXRα,β), and testicular receptor 4 (TR4) (59–61). PPARγ, LXRα,β, and TR4 share certain structural and functional characteristics. All three are expressed in macrophages, are localized in the cell nucleus, and bind to specific fatty acids or fatty acid metabolites. After ligand binding, these nuclear receptors form heterodimers with the retinoid X receptor (RXR) and bind to specific DNA elements in the promoter regions of target genes, regulating the expression of those genes. These nuclear receptors (especially PPARγ and LXRα) also play important roles in the systemic inflammation that contributes to cardiovascular disease, and for this reason small-molecule therapeutic interventions targeting these nuclear receptors have been developed and could potentially be repurposed to treat TB.
(i) PPARγ.
Humans have three PPAR nuclear receptors, PPARα, PPARβ/δ, and PPARγ, all of which are ligand-activated transcription factors that regulate fatty acid catabolism and lipid storage. The three PPARs differ in tissue distribution and function (62). Upon infection of macrophages by M. tuberculosis or BCG, PPARγ expression is upregulated (59, 60, 63). Several macrophage receptors are likely important for stimulating expression of PPARγ, including the mannose receptor (recognizing sugars such as ManLam on the M. tuberculosis cell wall) and the scavenger receptors (61). Potential ligands for PPARγ include fatty acids (host or M. tuberculosis) and products of the eicosanoid pathway. The signaling pathway leading to activation of PPARγ expression by M. tuberculosis is not well understood.
PPARγ is a potential HDT target in macrophages due to its regulation of cytokine production, lipid body biogenesis, and M. tuberculosis replication. Mahajan et al. reported that PPARγ appears to be involved in the M. tuberculosis-induced polarization of macrophages to a less microbicidal, alternative phenotype characterized by increased surface expression of CD36, lipid body formation, IL-10 secretion, arginase synthesis, and reduced ROI/RNI production (60). Inhibition of PPARγ (with siRNA or the GW9662 antagonist) was associated with decreased M. tuberculosis and/or BCG growth in human and mouse macrophages (59–61). Although there are data supporting a cytokine-modulatory effect of PPARγ, the results of three studies using siRNA knockdown or chemical modulation of PPARγ differ with regard to the direction of the effect (59–61). With respect to lipid bodies, Almeida et al. found that macrophage lipid body formation was induced by BCG, and this was enhanced by PPARγ agonist BRL49653 and inhibited by PPARγ antagonist GW9662 (59).
(ii) LXRα,β and TR4.
LXRα,β and TR4 are additional lipid-sensing nuclear receptors that mediate immune responses to M. tuberculosis. Similar to PPARγ, TR4 also appears to mediate mycobacterial programming of macrophages toward an alternative phenotype. RNA interference (RNAi)-mediated knockdown of TR4 decreased lipid body formation and enhanced the mycobactericidal activity of human THP-1 macrophages (60). In the same study, knockdown of LXRα had the opposite effect, leading to increased M. tuberculosis growth in THP-1 macrophages. In an in vivo murine intratracheal infection model, Lxrα/β−/− mice were more susceptible to H37Rv M. tuberculosis, with increased numbers of bacilli, more severe lung pathology, and a decreased TH1 and TH17 T cell response (64). In addition, prophylactic and therapeutic treatment of WT mice with an LXR agonist (TO91317 or GW3965) resulted in improved clearance of M. tuberculosis. Together, these results suggest that TR4 and PPARγ activation favors M. tuberculosis during infection, whereas LXRα,β activity protects the host.
(iii) HDTs for lipid-sensing nuclear receptors.
Several modulators of PPAR exist, including compounds approved for human use. PPARγ modulators include the thiazolidinediones (TZDs), which activate PPARγ and are used in diabetes treatment. TZDs include rosiglitazone (Avandia), pioglitazone (Actos), and troglitazone (Rezulin), all of which were formerly available in the United States or Europe but have been withdrawn or restricted due to adverse side effects (65). The adverse side effects include increased risks of liver failure and cardiovascular disease. Experimental agents include netoglitazone (an antidiabetic agent), rivoglitazone, ciglitazone, and rhodanine. A second class of PPAR activators includes the fenofibrate compounds, which activate PPARα and are FDA approved. Other activators of PPARγ include perfluorononanoic acid and berberine. Finally, there are experimental compounds used in the studies described here, including the PPARγ antagonist GW9662 and the PPARγ agonist BR49653. The use of FDA-approved PPARγ agents would be predicted to increase susceptibility to TB infection and/or disease. To our knowledge, an increased risk of TB has not been reported as an adverse side effect of PPARγ agonist use in humans. Small-molecule modulators for TR4 and LXRα,β were not identified in the literature. Together, these data suggest that lipid-sensing nuclear receptors are potential targets for interventions in TB pathogenesis. Inhibition of PPARγ and TR4 or activation of LXRα,β could lead to control of M. tuberculosis replication and favorable outcomes for the host.
Phospholipases
Phospholipases are enzymes that catalyze the breakdown of phospholipids into fatty acids and other lipophilic substances. Some of the resulting products are secondary messengers that are important for membrane trafficking, cell proliferation, and apoptosis. Phospholipases are classified according to the site of the phospholipid ester bond that is broken. The classes most relevant to TB are phospholipase D (PLD) and phospholipase A2 (PLA2) (Fig. 2).
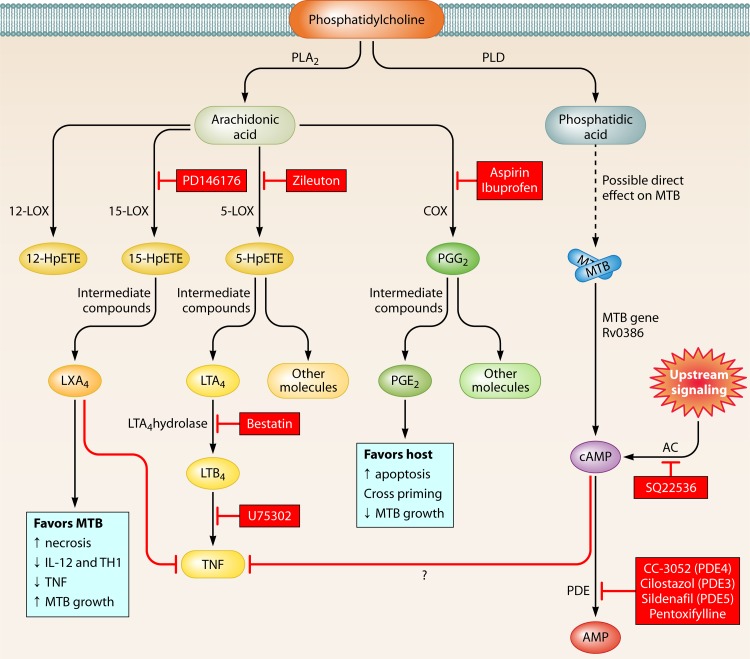
Fig 2.
Eicosanoid pathway and regulation of inflammation and HDTs. Phosphatidylcholine in the plasma membrane is broken down by phospholipase A2 into arachidonic acid (AA) and then converted into several eicosanoids by cyclooxygenase (COX), lipoxygenase (LOX), or cytochrome P450 enzymes (not shown). 15-LOX leads to LXA4 production, which promotes cell necrosis and facilitates M. tuberculosis replication. COX enzymes lead to PGE2, which is associated with increased apoptosis and restricted M. tuberculosis growth. However, inhibition of COX enzymes with NSAIDs can lead to improved outcomes for the host. Host- or M. tuberculosis-derived adenylate cyclases also lead to increased cAMP, which is broken down by phosphodiesterases and may modulate TNF levels. HDT targets described in the text are marked in boxes with red shading.
PLA2 has secreted (sPLA2), cytosolic (cPLA2), and calcium-independent (iPLA2) forms. iPLA2 cleaves cell membrane phospholipids to form arachidonic acid (AA) and other free fatty acids. AA acts as a signaling molecule and also has direct antimycobacterial properties against M. tuberculosis in culture and possibly within macrophages as well (66). There is mixed evidence for the role of PLA2 in control of M. tuberculosis. Arachidonyl trifluoromethyl ketone (ATFMK) (also known as AACOCF3) and methyl arachidonyl fluorophosphate (MAFP) are inhibitors of group IV cPLA2 and group VI iPLA2. ATMFK and MAFP reduced the activity of human macrophages against avirulent M. tuberculosis (strain H37Ra), and the addition of AA to macrophages treated with PLA2 inhibitors restored macrophage control of bacterial replication, possibly through increased cellular apoptosis (66). When quinacrine, another PLA2 inhibitor, and ATMFK were applied to M. tuberculosis in murine peritoneal macrophages, the antimycobacterial activity of macrophages was also reduced (67). However, bone marrow-derived macrophages lacking cPLA2 or treated with the PLA2 inhibitor ATMFK, MAFP, quinacrine, pyrrolidine-2, or indoxam (the last two being inhibitors of cPLA2 and sPLA2, respectively) were not deficient in their ability to restrict the growth of M. tuberculosis (68). It is not clear whether these contrary findings were a result of differences in the strain of M. tuberculosis used (avirulent H37Ra), source of macrophages, or other factors. Genetic and phenotypic differences between avirulent H37Ra and virulent H37Rv M. tuberculosis limit extrapolation of findings between the two organisms (10, 11). These data suggest a possible role for PLA2 in restricting M. tuberculosis growth, but further experimental studies are required to fully validate and define their function in host defense against M. tuberculosis.
PLD catalyzes the hydrolysis of phosphatidylcholine into phosphatidic acid (PA) and a choline head group. Although the pathway is unknown, in macrophages PA appears to facilitate phagosome maturation, phagolysosome formation, the production of ROIs, and M. tuberculosis killing. The activation of PLD has been achieved by several means, including ATP, sphingosine 1-phosphate (S1P), lysophosphatidic acid (LPA), and CpG DNA, all of which are nonspecific activators and thus limit conclusions from these studies about a specific role for PLD in control of M. tuberculosis. The activation of PLD by ATP, which stimulates PLD through the P2X7 ATP receptor and leads to multiple, diverse changes in macrophages, produces an antimycobacterial effect that is fully dependent on an increase in cytosolic Ca2+ levels (69). An increase in cytosolic Ca2+ is essential for the activation of several pathways that respond to mycobacteria, including phagosome-lysosome fusion and production of cyclic AMP (cAMP). Similarly, the induction of PLD with S1P, LPA, or CpG also results in an antimycobacterial effect in vitro in human macrophages (70–73). Strategies that activate PLD might be desirable as a host therapeutic, but the precise role of this molecule in host defense against M. tuberculosis needs to be better understood.
Eicosanoids, Inflammation, and Mechanisms of Cell Death
The eicosanoids are signaling molecules derived from fatty acids that mediate complex control over inflammatory reactions (74, 75), including the macrophage response to M. tuberculosis. The biochemical precursor for many eicosanoids is arachidonic acid (AA), which is generated by phospholipases as described above (Fig. 2). Metabolism of AA into the eicosanoids is carried out by three groups of enzymes (76): (i) cyclooxygenases (COX1 and COX2) metabolize AA to prostaglandins and thromboxane, (ii) lipoxygenases (e.g., 5-LOX, 12-LOX, and 15-LOX) catalyze the formation of AA into leukotrienes and lipoxins, and (iii) cytochrome P450 metabolizes AA into hydroxyeicosatetraenoic acids and epoxyeicosatrienoic acids, which function primarily as autocrine and paracrine effectors in the cardiovascular and renal systems (77). Several FDA-approved drugs that inhibit enzymes in the eicosanoid pathway are available: aspirin and other nonsteroidal anti-inflammatory drugs (NSAIDs), which inhibits COX1 and COX2, and zileuton, which inhibits 5-LOX and is used for the treatment of asthma.
The eicosanoids regulate several steps in M. tuberculosis pathogenesis, including cytokine production, mechanisms of macrophage death, T cell responses, and bacterial replication (78–80). The mechanism of macrophage cell death is fundamental to pathogenesis of M. tuberculosis, and studies suggest that necrotic cell death favors M. tuberculosis replication and survival, while apoptotic cell death favors the host with restriction of bacterial replication (78–80). Apoptosis of M. tuberculosis-infected dendritic cells promotes TH1 T cell responses, which are important for an effective host response against M. tuberculosis (80). The recognition of apoptotic cells by phagocytes appears to be beneficial during infection with M. tuberculosis, as has recently been shown with phosphatidylserine (PS)-coated liposomes that resemble apoptotic bodies (81). Chen et al. demonstrated that avirulent M. tuberculosis (strain H37Ra) promotes apoptosis by increasing cAMP levels and activating protein kinase A (PKA), which induces high prostaglandin E2 (PGE2) levels, whereas virulent M. tuberculosis (H37Rv) promotes necrosis via induction of LXA4, which downregulates PGE2 and blocks TNF (79). This suggests that interventions that alter the balance of these lipids and promote host cell apoptosis might hold promise in the treatment of M. tuberculosis.
One of the downstream effects of leukotriene B4 (LTB4) is to induce production of TNF, a neutrophil chemoattractant. Various methods for manipulating this branch of the AA cascade pathway in zebrafish (15-LOX inhibitor, LTB4 antagonist, and LTA4H mutant) suggest that regulation of TNF is potentially amenable to therapeutic intervention via the eicosanoid pathway. The desired effects depend on the overall balance of LXA4 and LTB4 levels (and effects on TNF). Tobin et al. found that mutations in LTA4H were associated with hypersusceptibility to M. marinum in a zebrafish model (82). The increase in mycobacterial proliferation in the LTA4H mutants was due to increased levels of anti-inflammatory lipoxins (such as LXA4) and decreased levels of TNF. At the same time, excess production of TNF via proinflammatory eicosanoids such as LTB4 was also detrimental, resulting in increased M. marinum growth. These findings in zebrafish were accompanied by human genetic studies conducted as part of a case-control study in Vietnam, which identified two LTA4H polymorphisms associated with protection from pulmonary and meningeal TB. Further studies discovered an lta4h promoter region polymorphism, which regulated transcriptional levels of the gene and was associated with increased inflammation in the cerebrospinal fluid of infected patients (83). However, the lta4h gene polymorphisms identified by Tobin et al. did not appear to play a role in protection against pulmonary TB in a Russian population (84). This difference may be attributable to multiple factors, including a different genetic background of the population. TB meningitis is currently treated with dexamethasone along with anti-TB drugs. Dexamethasone and other steroids (e.g., prednisone) are HDTs with pleiotropic effects that dampen the immune response through poorly understood mechanisms. Although dexamethasone is routinely used in the treatment of TB meningitis, many individuals still have poor outcomes (85). Interestingly, the proinflammatory LTA4H genotype was associated with increased survival in those treated with dexamethasone but not in those who were not treated (85). This observation indicates that the benefit of dexamethasone is likely dependent on the inflammatory state of the host at the time of treatment. Together, these studies suggest that LTA4H is an important checkpoint in the eicosanoid pathway that regulates TNF and other inflammatory phenotypes. The zebrafish and human data indicate that protection from TB depends on an optimal balance of inflammatory products of the eicosanoid pathway and that the benefits of immunomodulatory treatment will likely depend on genetically regulated levels of inflammation in human populations.
Recent findings have demonstrated that 15-LOX-dependent lipoxins, such as LXA4, are key mediators in resistance to M. tuberculosis. The lipoxins have broad counterinflammatory activities, including inhibiting neutrophil and monocyte migration, modulating matrix metalloproteinase (MMP) production, and suppressing Toxoplasma gondii-induced IL-12 secretion from dendritic cells and polarization of TH1 T cells (86, 87). Mice deficient in 5-LOX had lower levels of LXA4 than their WT counterparts and were hypersusceptible to infection with T. gondii due to an uncontrolled inflammatory response (88). Interestingly, the opposite phenotype was seen after infection with M. tuberculosis, with 5-LOX-deficient mice showing lower levels of LXA4, increased expression of IL-12, IFN-γ, and inducible nitric oxide synthase (iNOS) in the lungs, and a lower bacterial burden in lungs and spleens (89, 90). Inconsistent with this, however, M. tuberculosis-infected mice treated with a 5-LOX inhibitor, MK866, had higher bacterial loads and a shorter survival time than untreated mice (91). It was speculated that these differences might have been due to various levels of lipoxins observed in each study, the mouse model, infection severity, or genetic versus pharmacological approaches. In a Ghanian study group of 1,916 TB patients and 2,269 healthy, exposed controls, Herb et al. found variant 5-LOX alleles that were associated with susceptibility to pulmonary TB (92). Further studies in animal models and humans, possibly with zileuton, a 5-LOX inhibitor approved for the treatment of asthma, might help elucidate whether modulation of lipoxin production through this pathway might be a potential target for the therapy of TB.
Efforts to modulate the eicosanoid pathway for the treatment of TB have been attempted in human clinical trials with aspirin (an inhibitor of COX1 and COX2) (Table 2). Schoeman et al. examined 159 patients with TB meningitis through randomization to placebo and two different doses of aspirin; all patients also received standard TB drug therapy plus prednisolone (93). There were no differences in morbidity or mortality among the 3 groups. Misra et al. examined 119 subjects with TB meningitis and randomized them to aspirin versus placebo, along with standard TB drug therapy. The aspirin group had a lower risk of death (P = 0.03) and a trend toward lower stroke risk (P = 0.18) (94). Although these data suggest a possible benefit, data from this trial are difficult to interpret as prednisolone was used inconsistently and was not a standardized part of the protocol. Recent work in the C3HeB/FeJ mouse, which develops human-like necrotic lesions after infection with M. tuberculosis, suggests that use of NSAIDs might help in TB treatment. Administration of the COX inhibitor ibuprofen in M. tuberculosis-infected C3HeB/FeJ mice led to decreases in the size and number of lung lesions, decreases in the bacterial burden, and improvements in survival compared to those for untreated animals (95). Other studies of NSAIDs in TB therapy have demonstrated synergistic as well as antagonistic effects of certain NSAIDs in combination with TB drugs in the mouse model of TB (96–99).
Protein Kinase R
Protein kinase R (PKR) mediates cellular responses to stress through recognition of double-stranded RNA (dsRNA) and has pleiotropic effects on the cell, including regulation of cytokine responses. Mice lacking the gene for PKR infected with M. tuberculosis had lower mycobacterial loads and less pulmonary pathology than WT mice (100). In addition, M. tuberculosis-infected PKR-deficient macrophages exhibited increased apoptosis and increased expression of iNOS and TNF in response to IFN-γ, indicating that in the absence of PKR, macrophages were more fully activated (100). PKR has been suggested as a therapeutic target in neuronal degeneration and influenza (101–105). Although the mechanism underlying its role in restricting the host response to M. tuberculosis needs to be better understood, this study suggests that a PKR inhibitor might also be useful for the treatment of TB.
Siderophores and Iron Sequestration
The importance of host iron metabolism for M. tuberculosis pathogenesis has long been recognized. M. tuberculosis has evolved strategies to manipulate host iron homeostasis in order to acquire the iron that the bacterium needs to survive and grow. Several host molecules that regulate iron availability in solid tissues, blood, and macrophages have been identified, and their possible roles in TB pathogenesis are discussed in a recent review article (106). Of these molecules, we focus on lipocalin 2 (also called siderocalin, LCN2, or NGAL), Nramp1, and hepcidin, with brief mentions of lactoferrin and ferroportin. Small molecules that modulate these host factors involved in iron metabolism were not identified in the literature.
M. tuberculosis acquires iron by releasing siderophores, small compounds that chelate iron ions with extremely high affinity. The iron-bound siderophores are retrieved by M. tuberculosis using receptors that internalize them into the bacterium, where the iron is released for use in metabolic processes. Human hosts have proteins that bind bacterial siderophores, preventing bacteria from scavenging host iron. One such human protein is lipocalin 2, which has been shown to bind the M. tuberculosis siderophore carboxymycobactin, potentially protecting the host against mycobacterial infection through iron sequestration (107, 108). Three studies found that lipocalin 2 protein added to liquid cultures inhibited mycobacterial growth (52, 109, 110). In addition, lipocalin 2 knockout mice infected with M. tuberculosis had worse lung pathology and greater bacterial loads than WT mice (110). However, a recent study found that although an increase in lipocalin 2 production was part of the early response to M. avium infection, the bacterial load did not differ substantially between lipocalin 2-deficient and WT mice (109).
The protein NRAMP1, encoded by the Slc11a1 gene, is a membrane ion channel that functions in iron and manganese transport (111). Natural polymorphisms in Nramp1 in humans are associated with variable outcomes with respect to TB (112). Common laboratory mouse strains have coding allele variations in Nramp1 and have been intensively studied over the past 20 years. Early studies demonstrated that Nramp1-deficient mice (natural allelic variant) were more susceptible to BCG, Salmonella, and Leishmania (113). Nramp1 knockout mice were unable to control BCG growth early in infection but at later time points were similar to WT mice, with comparable liver and spleen histopathology (114). A similar study in which Nramp1-deficient mice were infected with M. tuberculosis found no differences compared to WT mice in bacterial growth, histopathology of the lung, liver, and spleen, or mouse survival (115).
Hepcidin is a liver hormone that regulates iron homeostasis by regulating iron transport across the gut mucosa. It binds to ferroportin, an iron channel on gut cells as well as in macrophages. Two studies found that hepcidin was produced by a variety of cell types, including human and mouse macrophages and dendritic cells, in response to mycobacterial infection (116, 117). Sow et al. showed that hepcidin localized to M. tuberculosis phagosomes in mouse macrophages and that treating M. tuberculosis in vitro with hepcidin resulted in reduced bacterial growth in a dose-dependent fashion (116). It has also been shown that mouse macrophages overexpressing ferroportin had reduced M. tuberculosis growth compared to control macrophages (118). Finally, lactoferrin is a protein contained in secretions, including milk, that binds to iron and has antimicrobial properties. Mice infected with M. tuberculosis and fed lactoferrin had lower bacterial loads and less lung pathology than M. tuberculosis-infected mice not fed lactoferrin (119).
Overall, these studies highlight that targeting M. tuberculosis iron acquisition and metabolism may be a strategy for HDT development for TB. However, targeting many of these pathways will be challenging, as it will require strategies to selectively upregulate the activity or expression of host iron-scavenging molecules such as lipocalin 2, hepcidin, or lactoferrin, perhaps throughout the full course of infection and without altering iron homeostasis of the host.
HDTs AND THE PULMONARY IMMUNE RESPONSE
After the early stages of the innate immune response to M. tuberculosis, a cascade of immune responses occurs. Macrophages and dendritic cells secrete cytokines and chemokines, which recruit cells to the site of infection and modulate the adaptive immune response. Antigen-presenting cells polarize T cell subsets through secretion of cytokines. For example, high IL-12/IL-10 ratios induce TH1 effector responses, IL-12/IFN-α ratios modulate differential induction of central and effector memory T cells, combinations of IL-1β, IL-6, transforming growth factor β (TGF-β), and/or IL-23 induce Th17 cells, and IL-10 and TGF-β may preferentially induce Tregs (120–125). Some cytokines, such as IFN-γ from TH1 T cells, activate macrophages to kill M. tuberculosis (11). TNF also activates macrophages to restrict M. tuberculosis growth. Despite the benefit of some proinflammatory cytokines such as IFN-γ and TNF, other cytokines such as IFN-α may be detrimental. For example, some studies suggest that IFN-α can exacerbate murine TB infection (126, 127). Anti-inflammatory cytokines such as IL-10 and TGF-β also mediate important aspects of M. tuberculosis infection. Elevated levels of IL-10 promote TH2 T cell polarization and an immune response that is not beneficial for the host (128, 129). IL-10 and TGF-β promote Treg development, which suppresses effector T cell responses and dampens the antimicrobial response during M. tuberculosis infection (130, 131). The pro- and anti-inflammatory responses culminate in different outcomes, including development of activated macrophages that form a granuloma, a collection of macrophages and T cells that surround the M. tuberculosis-infected cells. The classic view of the granuloma is that it has hypoxic, acidic, nutrient-poor conditions that are less permissive for M. tuberculosis replication. More recent data suggest that M. tuberculosis may utilize the granuloma as a mechanism for cell-to-cell spread of infection (132). Indeed, some of the bacilli adapt by entering into a nonreplicating state that can persist for many years until HIV or other factors restore conditions permissive for active disease. Within this environment of competing immune responses, which steps are plausible therapeutic intervention points for HDT with promising lead compounds? In this section, we highlight pathways where such lead compounds exist with data that suggest possible effects on M. tuberculosis infection and disease progression.
Cytokine Modulation
Cytokine regulation of the immune response offers numerous intervention points. Two major cytokines for consideration are IFN-γ and TNF, both of which activate macrophages and promote bacterial killing.
IFN-γ.
Interferons stimulate hundreds of genes in macrophages and induce antimicrobial effector responses, including inducible nitric oxide synthase (iNOS) and the nitric oxide pathway, NADPH oxidase (NOX) and the ROS pathway, indoleamine 2,3-dioxygenase (IDO), NRAMP1, GTPases, and autophagy (18, 133). IFN-γ-deficient mice are highly susceptible to in vivo M. tuberculosis infection (134). In addition, children with rare mutations in IFN-γ pathway genes are highly susceptible to BCG and nontuberculous mycobacterial infections (135). This central role of IFN-γ in M. tuberculosis pathogenesis led to human clinical trials to test its efficacy for treatment as an adjuvant to antibiotic therapy (Table 2). These trials included a safety study and two RCTs. In the safety study, aerosolized IFN-γ was well tolerated by five patients with MDR TB, and these patients showed suggestive evidence of clinical improvement (136). A small Cuban study of patients (n = 32) with nontuberculous mycobacterial lung disease (mostly M. avium complex) receiving antibiotic therapy were randomized to injections of IFN-γ (n = 18) versus placebo (n = 14) (137). Patients treated with IFN-γ showed greater improvement in pulmonary symptoms and a higher percentage of complete responders (72% versus 36% at 6 months; P = 0.037). Sputum culture conversion rates showed a trend toward faster resolution (P = 0.04), and radiographic improvement was greater (P = 0.036) in IFN-γ-treated individuals at 18 months (137). In a three-arm RCT, TB patients receiving antibiotic therapy with nebulized IFN-γ1b (n = 30) or subcutaneous injections of IFN-γ1b (n = 27) were compared with TB patients receiving antibiotic therapy alone (n = 30) (138). Patients in the aerosolized IFN-γ1b group were more likely to have negative sputum smears at 4 weeks (P = 0.03). In addition, culture conversion rates at 4 weeks showed a trend favoring aerosolized IFN-γ treatment that was not statistically significant (P = 0.15). Disease symptoms were less in both IFN-γ1b treatment groups. Together, these studies suggest that IFN-γ is beneficial for the treatment of nontuberculous mycobacteria as well as TB. Although some benefit was associated with IFN-γ treatment, several hurdles would prevent its development as an HDT. These obstacles include a small potential magnitude of benefit, high cost, and difficult supply chain logistics. In addition, the delivery of inhaled or subcutaneous IFN-γ to target tissues is challenging. With sequestration of M. tuberculosis within granulomas, cavities, and/or extrapulmonary sites, penetration issues would be formidable. In light of such challenges, these studies support efforts to identify the pathways activated by IFN-γ that could be modulated with small-molecule drugs.
TNF.
TNF mediates many important immune responses, including immune cell activation, differentiation, and cell death. TNF is produced by a number of cells, including macrophages, dendritic cells, neutrophils, and T cells. In vivo studies in mice (neutralization with anti-TNF antibodies and TNFp55R−/−) demonstrated that TNF is important for control of M. tuberculosis replication as well as granuloma formation (139). The mechanism of TNF regulation of M. tuberculosis growth is partially attributed to macrophage activation with increased phagocytosis and production of reactive nitrogen and oxygen intermediates. In humans, TNF antagonist therapy is used for treatment of rheumatologic disorders as well as inflammatory bowel disease (140). Patients with latent tuberculosis infection (LTBI) treated with TNF antagonists have a significantly increased risk of developing active TB disease (140). In addition to the protective role of TNF, excess levels may contribute to immunopathology (82). Ultimately, antibacterial effects need to be balanced with avoidance of immunopathology. Achieving this balance is a major goal of HDTs and is discussed above with manipulation of leukotriene pathways and below with phosphodiesterase (PDE) mechanisms.
The granuloma may benefit the host by containing the bacteria, restricting the spread of infection, and permitting the focusing of an immune response. However, the granuloma may also benefit the bacilli by facilitating their propagation to neighboring tissue via the trafficking of host cells that harbor M. tuberculosis (132). Bacilli in the granuloma are presumed to exist predominantly in a dormant state due to hypoxic conditions and other stresses that impose nonreplication of TB. This state of dormancy may be a barrier to successful drug treatment, since certain TB drugs have greater potency, or are only active, against replicating populations of M. tuberculosis. This is the basis for adjunctive therapy with immunosuppressive treatments that reactivate the bacilli and increase their susceptibility to standard TB drugs. One mechanism of granuloma disruption and bacillus reactivation is through TNF inhibition. In a single-arm trial with historical controls, Wallis et al. examined this concept by treating 16 HIV-1-infected patients with pulmonary TB with etanercept at the initiation of TB drug treatment. Interestingly, the sputum culture conversion rate showed a trend toward faster conversion in those receiving etanercept compared to historical controls (P = 0.05) (141, 142). Clinical and radiographic improvement did not differ between the two groups. Although the sample size was small and the design was not randomized, these data suggest that TNF inhibition could lead to more rapid clearance of M. tuberculosis from the lung and that manipulation of TNF levels offers the potential to alter the balance of inflammation to benefit the host. Another strategy for manipulating TNF via modulation of phosphodiesterase enzymes and cAMP levels is described below.
(i) cAMP and phosphodiesterase inhibitors.
cAMP is a second messenger in the cell that has pleiotropic effects, including the activation of signaling molecules such as PKA and immunomodulation of the cell. cAMP is formed by adenylate cyclases (ACs) and degraded by phosphodiesterases (PDEs). One of the effects of cAMP is inhibition of TNF production by monocytes and macrophages (143, 144). Phosphodiesterase inhibitors cause accumulation of cAMP, which then inhibits TNF production. Mammals have up to 11 classes of PDEs that differ in cell and tissue distribution. Two lines of investigation converged on its identification as an important regulator of M. tuberculosis survival in macrophages (Fig. 2). On the bacterial side, M. tuberculosis induces cAMP production in infected J774 macrophages following phagosome formation (145). Restricting production of cAMP through an AC inhibitor (SQ22536) or addition of a PKA inhibitor (H89) led to reduced mycobacterial growth (145). Agarwal et al. found that M. tuberculosis induced cAMP in macrophages and that an M. tuberculosis mutant lacking Rv0386, one of the 17 AC genes in M. tuberculosis, was associated with lower cAMP levels, CREB phosphorylation, and TNF production in macrophages, along with decreased growth of the bacterium in macrophages and in mice compared to WT M. tuberculosis (146). A similar phenotype was observed with an M. tuberculosis mutant that overexpressed the phosphodiesterase gene Rv0805, which also decreases cAMP levels in the macrophage. Together, these data suggest that cAMP induction by M. tuberculosis favors bacterial survival and that inhibiting cAMP levels results in reduced TNF levels and better control of M. tuberculosis by the host.
Manipulation of cAMP levels via host pathways also modulates M. tuberculosis growth during infection. Studies for HDTs were initiated with thalidomide, which is effective for treating erythema nodosum leprosum, an inflammatory reaction associated with high levels of TNF (147). Due to teratogenic side effects associated with thalidomide, a search for analogues that regulate cAMP and TNF led to the identification of a class of phosphodiesterase inhibitors (PDEi) which lead to increased levels of cAMP. Although thalidomide does not appear to directly inhibit PDEs, both thalidomide and PDEi share a property of inhibition of TNF, indirectly affecting M. tuberculosis growth in macrophages. cAMP modulates TNF, potentially through a pathway that includes PKA and the transcription factors cAMP response element-binding protein (CREB) and NF-κB.
The beneficial effect of PDEi on M. tuberculosis infection led to testing of these compounds with animal models in combination with standard anti-TB drugs. The effects of these treatments may be due to an alteration in the physiologic state of the bacterium (the hypothesis being that elevated cAMP levels prompt M. tuberculosis to maintain an active metabolic state, allowing the anti-TB drugs to be more effective) or through modulation of the host immune response (e.g., via TNF and immune activation). Experiments in animal models show that inhibition of certain PDEs restricts bacterial growth in vivo and decreases inflammation, with improved pathological outcomes compared with standard treatment. These studies included PDE3, PDE4, and PDE5 inhibitors. PDE4 hydrolyzes cAMP and is expressed in monocytes and macrophages but not in T cells. Mice and rabbits treated with the PDE4 inhibitor CC-3052, in combination with INH, had improved resolution of lung pathology and a lower burden of M. tuberculosis compared to mice treated with INH alone (148–150). However, a separate study found that administration of the PDE4 inhibitors rolipram and cilomilast decreased survival time of M. tuberculosis-infected mice and that addition of rolipram to the standard TB treatment resulted in a higher bacterial burden than with the standard treatment alone (151). The latter study suggests that certain PDE4 inhibitors might be detrimental in the mouse model, but further work is needed to better understand the impact of adjunctive use of PDE4 inhibitors in TB. PDE3 hydrolyzes both cAMP and cyclic GMP (cGMP) and is expressed in macrophages, endothelial cells, platelets, and airway smooth muscle cells. PDE5 hydrolyzes cGMP and is expressed in pulmonary vascular smooth muscle of pulmonary arteries and veins, bronchial blood vessels, and airway smooth muscle. PDE3 and PDE5 are inhibited by cilostazol and sildenafil, respectively. Use of these inhibitors in combination with standard TB drugs provides some benefit in the mouse model of infection, as measured by bacterial burden and time to sterilization (144). Pentoxifylline, a nonspecific and relatively weak PDE inhibitor, was tested as adjunctive TB treatment in an RCT in 107 HIV-infected patients in Uganda (152). Pentoxifyulline was not associated with any difference in M. tuberculosis culture conversion, radiographic improvement, or death. Although the data were negative, more potent and selective PDE inhibitors are now available to test this concept. Further work is required to better understand which PDEs are the most promising targets for the treatment of TB and whether select PDEi might be beneficial in combination with standard treatment.
The benefit of anti-inflammatory treatment with steroids has been demonstrated in RCTs for TB meningitis and pericarditis (85, 153). Despite clinical benefit, attempts to discover which immune pathways are inhibited by steroids during TB meningitis treatment were unsuccessful (154). Given the broad range of immunomodulatory effects and side effects of steroids, more selective immunomodulation is a major goal of HDT. Although no studies of newer, more specific PDEi have been performed for treatment of TB, an RCT of thalidomide with 47 TB meningitis patients was reported (147) (Table 2). The study was stopped early due to adverse side effects in the thalidomide arm.
(ii) ROS and mechanisms of cell death.
Although TNF is critical for the antibacterial response, TNF's proinflammatory effects can be detrimental. One therapeutic goal is to maximize the M. tuberculosis killing activity of TNF and avoid detrimental effects such as TNF-induced necrosis of macrophages. Using a zebrafish model with M. marinum, Roca and Ramakrishnan recently demonstrated that TNF induces ROS production by mitochondria, which initially leads to increased killing of M. tuberculosis but also culminates in macrophage necrosis, thus favoring growth of the bacillus (155). The necrosis pathway was mediated by mitochondrial cyclophilin D and acid sphingomyelinase-dependent activities. When these pathways were inhibited by alisporivir and desipramine, respectively, necrosis was blocked without impairing killing of M. tuberculosis. Desipramine is an FDA-approved drug used for treating depression; however, the tricyclic antidepressants are not used as first-line therapy due to their narrow therapeutic index, and alisporivir is in phase III trials for treating hepatitis C. These studies demonstrate that it may be feasible to selectively block a detrimental host pathway while preserving essential antimicrobial mechanisms of M. tuberculosis killing.
HDTs, PATHOLOGY, AND TISSUE HOMEOSTASIS
Matrix Metalloproteinases
Lung cavities are a hallmark of clinical TB and are formed through the breakdown of the extracellular matrix (156) (Fig. 3). The matrix metalloproteinases (MMPs) comprise a family of 24 zinc- and calcium-dependent proteases that break down proteins of the extracellular matrix and basement membrane and modulate lung remodeling, fibrosis, and inflammation (157–161) The MMPs regulate important aspects of the immune response and are involved in chronic diseases such as arthritis, psoriasis, chronic obstructive pulmonary diseases, and cancer (162). MMPs can be categorized by the type of tissue degraded, including collagenases (MMP1), gelatinases (MMP9), stromelysins, and elastases. M. tuberculosis induces the expression of several MMPs, including MMP1, MMP2, MMP7, MMP9, and MMP10 (158, 159, 163, 164).
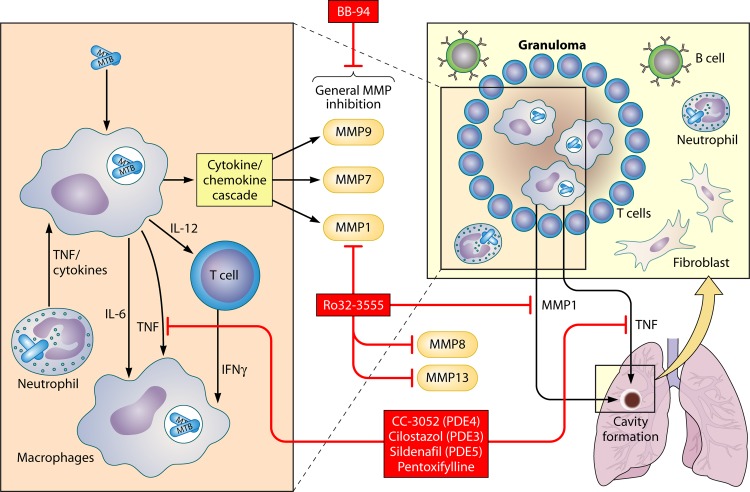
Fig 3.
Pulmonary immune response to M. tuberculosis infection and HDTs. M. tuberculosis-infected macrophages and dendritic cells secrete cytokines such as IL-6, IL-10, IL-12, and TNF. This cytokine response results in recruitment of macrophages, priming and differentiation of T cells, and formation of a granuloma. The nature of the host response determines M. tuberculosis replication, lung pathology, and cavity formation. HDT targets described in the text are marked in boxes with red shading.
MMP9 is upregulated in M. tuberculosis granulomas as well as in the cerebrospinal fluid (CSF) of patients with TB meningitis (165, 166). Mmp9-deficient mice infected with TB had decreased numbers of granulomas and lower lung bacterial counts compared to WT mice (167). In a zebrafish model of mycobacterial infection with M. marinum, MMP9 was shown to promote the recruitment of macrophages into granulomas (168). Inhibition of MMP9 with morpholinos resulted in decreased numbers of granulomas and decreased growth of M. marinum in zebrafish. These studies suggest that MMP9 regulates granuloma formation in TB and that inhibition of MMP9 may lead to improved clearance of bacteria in the host.
The role of MMP1 in tissue homeostasis and healing has been extensively studied. MMP1 was the most highly induced MMP in primary human monocytes infected with M. tuberculosis and was blocked by the MMP inhibitor Ro32-3555 (also called Trocade) (156). As mice do not express an orthologue of human MMP1, WT mice cannot be used to evaluate the role of MMP in TB. M. tuberculosis infection of mice transgenically expressing human MMP1 in activated macrophages under the control of the scavenger receptor A promoter/enhancer resulted in greater alveolar destruction and breakdown of collagen, suggesting a possible role for MMP1 in lung remodeling in human disease (156). However, no difference in bacterial burden was observed between wild-type and MMP1 transgenic mice.
Inhibition of several MMPs has been studied with BB-94, a nonselective MMP inhibitor. When mice were treated with BB94 and infected with M. tuberculosis, there was decreased mouse survival in the BB94-treated animals accompanied by increased numbers of granulomas at late time points (169). In contrast, a second study found that BB94-treated mice had decreased numbers of M. tuberculosis and granulomas in the blood and lungs at some of the early time points (170). In a third study, BB94-treated mice infected with M. tuberculosis had decreased CFU in blood and spleen but not the lung (167). Though the results of these studies are inconsistent, they suggest an effect of MMP modulation on TB phenotypes.
Neutrophils
Short-lived, phagocytic neutrophils are recruited early to foci of infection by chemokines and cytokines expressed by resident macrophages and other cells (Fig. 3). At the infectious site, neutrophils produce a number of antimicrobial products to eliminate pathogens, including reactive oxygen species, preformed oxidizing agents, and hydrolytic enzymes such as elastase, which are stored in intracellular granules (171). In the case of TB, the early and ongoing presence of neutrophils appears to play an important role in animal models of infection and in human disease. In zebrafish infected with M. marinum, neutrophils recruited to granulomas phagocytose dying bacterium-laden macrophages and kill internalized mycobacteria through oxidative mechanisms (172). Although the exact role of neutrophils in TB animal models and human patients remains to be better defined, it has become increasingly appreciated that neutrophils can harbor a large fraction of the M. tuberculosis bacillary burden. In humans with active pulmonary TB, neutrophils are the predominant cell type containing bacilli in the sputum, bronchoalveolar lavage fluid, and cavities of patients (173). Neutrophils also appear to be major hosts of M. tuberculosis in the mouse model of infection (174). Although the neutrophil response is designed to kill pathogens, the release of toxic antimicrobial factors can paradoxically contribute to the destruction of bystander immune and nonimmune cells, dissolution of tissue, and damage to the overall architecture of the lung. Rigorous downregulation of neutrophil activity is therefore important for preventing an excessive inflammatory response (175). In chronic infections such as TB, neutrophils likely continuously cycle into active or reactivating disease lesions, where they help control M. tuberculosis replication but also contribute to the progression of disease pathology (176). Reversing neutrophil damage is a major goal in inflammatory diseases such as asthma and chronic obstructive pulmonary disease (COPD), but a major challenge has been inhibiting excessive inflammation without impacting the beneficial innate response to pathogens. In TB, the timing of therapy targeting neutrophils will be critical, as early neutrophil responses are required to contain and limit infection, whereas later in disease sustained neutrophil responses can be damaging to the host and might help spread infection. A multitude of pathways in neutrophil recruitment, migration, and activation can be targeted. However, few currently available drugs modulate activity of neutrophils, and those that do act broadly on the immune response, are nonselective, and have high toxicity risks (177).
Among the many antimicrobial mechanisms of neutrophils is the release of neutrophil extracellular traps (NETs), which consist of DNA, histones, and antimicrobial proteins that help kill pathogens. Other innate cells, such as mast cells, eosinophils, and macrophages, may also release similar extracellular traps (ETs) (178). Much remains unknown about the role of ETs in defense against M. tuberculosis. NET and macrophage ET formation can be induced by M. tuberculosis, though a direct antituberculoidal role of these extracellular structures has not been shown (174, 179, 180). ETs produced by phagocytes may directly kill M. tuberculosis, but they may also sequester bacilli and promote their eventual destruction by other cells of the immune system. Conversely, ET components can be immunogenic and damaging to host tissue; limiting their production and accumulation might be beneficial in some diseases (181, 182). Although the role of ETs in host control of M. tuberculosis remains to be better defined, the regulation of ET formation by phagocytes might be modulated for the treatment of TB.
Antifibrotics
A central feature of TB is the formation of aggregates of immune and nonimmune cells called the granuloma, which contains the bacilli and concentrates a protective host response but conversely may also serve as a safe haven for M. tuberculosis and permit the dissemination of bacteria (183). Advanced granulomas consist of many cell types, including neutrophils, macrophages, natural killer cells, B and T cells, fibroblasts, and epithelial cells, encapsulated by a fibrous rim with extensive deposition of extracellular matrix (ECM) components and a central region of cellular necrosis and liquefied tissue. Progression of the granuloma and the process of lesion development and lesion healing leads to fibrosis and scarring of tissue. Fibrotic processes might be beneficial in that they contain infection; however, remodeling of the lung can lead to loss of alveolar spaces and long-lasting anatomical and structural changes that distort lung function and worsen patient outcomes (184). Pulmonary fibrosis may also lead to poor penetration of TB antibiotics into the lesion and thus to longer treatment times and drug resistance. In addition, lung fibrosis occurs dramatically during TB antibiotic therapy, presumably due to bacterial death, antigen release, and a boosting of the immune response. Therefore, the management of fibrosis might be particularly important during treatment. It may become even more critical if faster-acting TB regimens are developed that induce accelerated bacterial clearance, more robust immunity, and, as a result, greater fibrosis and lung remodeling.
Antifibrotic therapies, many of which are in various stages of development for pulmonary inflammatory diseases such as COPD, idiopathic pulmonary fibrosis (IPF), and asthma, have potential for use in TB as they may reduce TB-induced lung damage, improve lesional pharmacokinetics of TB drugs, and allow for better entry of protective immune cells into diseased tissues (184–187). However, many of the treatment modalities aimed at reducing lung fibrosis, including the recently approved agent pirfenidone for IPF (188), have pleiotropic effects and potential toxicities. In addition, many of these treatments prevent further fibrosis and progression of disease, and thus it is unlikely that they would restore complete lung function in pulmonary inflammatory diseases or in TB. A number of fibrosis targets have been implicated in COPD, IPF, and asthma, including cytokines and factors such as TGF-β (189), IL-13 (190), monocyte chemoattractant protein 1 (MCP-1) (191), MMPs (157), relaxin (192), and platelet-derived growth factor (PDGF) (193) and signaling molecules such as the tyrosine kinases inhibited by imatinib (194). Lung remodeling has been characterized to some extent in TB (184), but the mechanisms underlying the fibrotic process need to be better defined, and new tests and measures of airway remodeling need to be applied (195, 196). Insights are also required from preclinical and clinical studies of antifibrotic agents in the appropriate models and settings in combination with TB antibiotics to determine whether these have a role to play in treatment shortening or improving patient outcomes in TB.
CONCLUSIONS
HDTs offer great promise to expand therapeutic options for improved TB treatment. The pathways that might be targeted depend on the goal of treatment with an HDT and encompass broad, non-mutually exclusive categories of biologic processes, including modifying macrophage and host cell function, optimizing inflammatory responses at the cell and organ levels, and improving pulmonary pathology. Although the body of work in this area is certainly encouraging, significant challenges remain to advance mechanistic concepts and preclinical data into tangible results. Targets in these pathways have various degrees of validation, ranging from in vitro cell culture to animal models of TB, with limited evidence coming from human studies or clinical trials. Pathways and targets have been identified through classic “candidate gene” approaches of selecting biologically plausible targets as well as through agnostic genome-wide screening strategies. Efforts to identify candidate therapeutics are under way in the form of screening compound libraries for activity in M. tuberculosis-infected host cells. Additional targets might be identified by examining data from human clinical trials or observational studies of drugs carried out for non-TB indications in populations where TB is endemic. Insights into promising host targets may also come from human population genetic studies or studies from iatrogenic interventions such as TNF blockade therapy for rheumatologic disorders, as this may direct us to relevant host pathways in TB without bias regarding underlying mechanisms. Further hypothesis-based basic research and host-wide screens, as well as fundamental clinical research in human genetics and epidemiology of iatrogenic interventions in TB, may reveal new therapeutic opportunities and advance our understanding of host-pathogen interactions.
Although many opportunities exist in the development of HDTs for TB, their use comes with risks and challenges that need to be carefully considered. Given the complexity and pleiotropic functions of many target pathways, the potential toxicity of HDTs is a major risk. If HDTs are being repurposed from other therapeutic areas, the level of side effects may need to be reconsidered. For example, many oncologic conditions are associated with high mortality, and drug treatment may be more toxic than would otherwise be acceptable for other diseases. HDTs that stimulate immune responses carry risks of increased hyperinflammatory reactions such as the Koch phenomenon, immune reconstitution syndrome, and systemic inflammatory response syndrome. These inflammatory effects could lead to tissue damage, remodeling of airway space, and loss of lung function, as well as poorer penetration of TB drugs into TB lesions. Depending on their mechanism of action, HDTs may also lead to reactivation of quiescent bacterial subpopulations, including drug-resistant TB organisms. Conversely, HDTs could promote nonreplicating states of TB, which are refractory to standard TB antibiotics. Importantly, HDTs used in TB will also need to be compatible with therapeutic constraints imposed by comorbidities, in that they must not exacerbate the pathophysiology or affect the pharmacotherapy of the other diseases, such as diabetes, HIV/AIDS, and other infections, that might exist in people with TB. Finally, the cost and availability of HDTs may be a significant barrier for their deployment and use in developing countries and underserved populations.
The development of TB HDTs presents many challenges. In general, a major obstacle to TB drug discovery is the limited relevance and predictive power of animal models used to evaluate new TB chemotherapeutics. This might be particularly problematic for the evaluation of HDTs that interact with host factors and are likely to have complex, pleiotropic mechanisms of action that are highly dependent on the model in which they are tested. The host targets and pathways might be absent (e.g., MMP1 in mice) or highly divergent between animal models and humans (e.g., the p47 family of GTPases). A major challenge of HDTs, particularly those whose efficacy depends on immunomodulatory effects, is the diversity of patient populations. Patients present in diverse positions on the “damage response” framework, where they range on a spectrum from hypoinflammatory to hyperinflammatory (197). Moreover, the same patient may be at different points on the spectrum in different anatomic regions at the same time, which can be challenging to replicate in animal models of TB due to the homogeneity of disease in some animals. However, stratification of patients beyond HIV status is difficult in most areas where TB is endemic due to the lack of tools and weak health care infrastructure. Therefore, we will likely need HDTs that are applicable to a diverse range of TB patients, and developing a “one-size-fits-all” HDT treatment for TB patients will be challenging. Furthermore, as HDTs will be administered as adjuvants to anti-TB therapy, they will need to partner well with the current standard of care, as well as new regimens that are currently in development, and the optimal timing of their administration during treatment will have to be determined.
Future directions in HDT discovery should include head-to-head investigations of the most promising targets and compounds in relevant model systems in order to obtain comparable data for decision making and prioritization of opportunities. At the same time, several compounds that target distinct pathways are already FDA approved for non-TB indications and could be tested in humans with TB in the near future. Some of these drugs are generally safe, with years of clinical data, and have various levels of experimental data from TB patients to suggest that they could be beneficial for TB treatment. Given the limitations of current model systems for predicting human treatment efficacy, it might be prudent to proceed soon with human testing of some of these approved drugs or advanced clinical candidates.
ACKNOWLEDGMENTS
We thank Lisa E. Manhart and Kingsley Ndoh for helpful discussions and input and Ken Duncan for conceptual advice and helpful discussions. We thank Sabine Ehrt, Chetan Seshadri, Javeed Shah, and David Tobin for review of the manuscript.
We thank the Bill & Melinda Gates Foundation and University of Washington Strategic Analysis and Research Training (START) Program for their support of this project.
REFERENCES
- 1.Berrington WR, Hawn TR. 2007. Mycobacterium tuberculosis, macrophages, and the innate immune response: does common variation matter? Immunological Rev. 219:167–186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ishikawa E, Ishikawa T, Morita YS, Toyonaga K, Yamada H, Takeuchi O, Kinoshita T, Akira S, Yoshikai Y, Yamasaki S. 2009. Direct recognition of the mycobacterial glycolipid, trehalose dimycolate, by C-type lectin Mincle. J. Exp. Med. 206:2879–2888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu PT, Modlin RL. 2008. Human macrophage host defense against Mycobacterium tuberculosis. Curr. Opin. Immunol. 20:371–376 [DOI] [PubMed] [Google Scholar]
- 4.Manzanillo PS, Shiloh MU, Portnoy DA, Cox JS. 2012. Mycobacterium tuberculosis activates the DNA-dependent cytosolic surveillance pathway within macrophages. Cell Host Microbe 11:469–480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Russell DG. 2011. Mycobacterium tuberculosis and the intimate discourse of a chronic infection. Immunol. Rev. 240:252–268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Russell DG, Barry CE, III, Flynn JL. 2010. Tuberculosis: what we don't know can, and does, hurt us. Science 328:852–856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tailleux L, Pham-Thi N, Bergeron-Lafaurie A, Herrmann JL, Charles P, Schwartz O, Scheinmann P, Lagrange PH, de Blic J, Tazi A, Gicquel B, Neyrolles O. 2005. DC-SIGN induction in alveolar macrophages defines privileged target host cells for mycobacteria in patients with tuberculosis. PLoS Med. 2:e381. 10.1371/journal.pmed.0020381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tailleux L, Schwartz O, Herrmann JL, Pivert E, Jackson M, Amara A, Legres L, Dreher D, Nicod LP, Gluckman JC, Lagrange PH, Gicquel B, Neyrolles O. 2003. DC-SIGN is the major Mycobacterium tuberculosis receptor on human dendritic cells. J. Exp. Med. 197:121–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Torrelles JB, Azad AK, Henning LN, Carlson TK, Schlesinger LS. 2008. Role of C-type lectins in mycobacterial infections. Curr. Drug Targets 9:102–112 [DOI] [PubMed] [Google Scholar]
- 10.Achkar JM, Casadevall A. 2013. Antibody-mediated immunity against tuberculosis: implications for vaccine development. Cell Host Microbe 13:250–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Flynn JL. 2006. Lessons from experimental Mycobacterium tuberculosis infections. Microbes Infect. 8:1179–1188 [DOI] [PubMed] [Google Scholar]
- 12.Ernst JD. 2012. The immunological life cycle of tuberculosis. Nat. Rev. Immunol. 12:581–591 [DOI] [PubMed] [Google Scholar]
- 13.Cosma CL, Sherman DR, Ramakrishnan L. 2003. The secret lives of the pathogenic mycobacteria. Annu. Rev. Microbiol. 57:641–676 [DOI] [PubMed] [Google Scholar]
- 14.Ehrt S, Schnappinger D. 2009. Mycobacterial survival strategies in the phagosome: defence against host stresses. Cell. Microbiol. 11:1170–1178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Napier RJ, Rafi W, Cheruvu M, Powell KR, Zaunbrecher MA, Bornmann W, Salgame P, Shinnick TM, Kalman D. 2011. Imatinib-sensitive tyrosine kinases regulate mycobacterial pathogenesis and represent therapeutic targets against tuberculosis. Cell Host Microbe 10:475–485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rohde K, Yates RM, Purdy GE, Russell DG. 2007. Mycobacterium tuberculosis and the environment within the phagosome. Immunol. Rev. 219:37–54 [DOI] [PubMed] [Google Scholar]
- 17.Vergne I, Chua J, Lee HH, Lucas M, Belisle J, Deretic V. 2005. Mechanism of phagolysosome biogenesis block by viable Mycobacterium tuberculosis. Proc. Natl. Acad. Sci. U. S. A. 102:4033–4038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.MacMicking JD. 2012. Interferon-inducible effector mechanisms in cell-autonomous immunity. Nat. Rev. Immunol. 12:367–382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bruns H, Stegelmann F, Fabri M, Döhner K, van Zandbergen G, Wagner M, Skinner M, Modlin RL, Stenger S. 2012. Abelson tyrosine kinase controls phagosomal acidification required for killing of Mycobacterium tuberculosis in human macrophages. J. Immunol. 189:4069–4078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rajni Meena LS. 2010. Guanosine triphosphatases as novel therapeutic targets in tuberculosis. Int. J. Infect. Dis. 14:e682–e687 [DOI] [PubMed] [Google Scholar]
- 21.Martens S, Howard J. 2006. The interferon-inducible GTPases. Annu. Rev. Cell Dev. Biol. 22:559–589 [DOI] [PubMed] [Google Scholar]
- 22.Haas AK, Fuchs E, Kopajtich R, Barr FA. 2005. A GTPase-activating protein controls Rab5 function in endocytic trafficking. Nat. Cell Biol. 7:887–893 [DOI] [PubMed] [Google Scholar]
- 23.Singh SB, Davis AS, Taylor GA, Deretic V. 2006. Human IRGM induces autophagy to eliminate intracellular mycobacteria. Science 313:1438–1441 [DOI] [PubMed] [Google Scholar]
- 24.MacMicking JD, Taylor GA, McKinney JD. 2003. Immune control of tuberculosis by IFN-gamma-inducible LRG-47. Science 302:654–659 [DOI] [PubMed] [Google Scholar]
- 25.Feng CG, Collazo-Custodio CM, Eckhaus M, Hieny S, Belkaid Y, Elkins K, Jankovic D, Taylor GA, Sher A. 2004. Mice deficient in LRG-47 display increased susceptibility to mycobacterial infection associated with the induction of lymphopenia. J. Immunol. 172:1163–1168 [DOI] [PubMed] [Google Scholar]
- 26.Kim BH, Shenoy AR, Kumar P, Das R, Tiwari S, MacMicking JD. 2011. A family of IFN-γ-inducible 65-kD GTPases protects against bacterial infection. Science 332:717–721 [DOI] [PubMed] [Google Scholar]
- 27.Deretic V. 2011. Autophagy in immunity and cell-autonomous defense against intracellular microbes. Immunol. Rev. 240:92–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Songane M, Kleinnijenhuis J, Netea MG, van Crevel R. 2012. The role of autophagy in host defence against Mycobacterium tuberculosis infection. Tuberculosis (Edinb.) 92:388–396 [DOI] [PubMed] [Google Scholar]
- 29.Rubinsztein DC, Codogno P, Levine B. 2012. Autophagy modulation as a potential therapeutic target for diverse diseases. Nat. Rev. Drug Discov. 11:709–730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gutierrez MG, Master SS, Singh SB, Taylor GA, Colombo MI, Deretic V. 2004. Autophagy is a defense mechanism inhibiting BCG and Mycobacterium tuberculosis survival in infected macrophages. Cell 119:753–766 [DOI] [PubMed] [Google Scholar]
- 31.Harris J, De Haro SA, Master SS, Keane J, Roberts EA, Delgado M, Deretic V. 2007. T helper 2 cytokines inhibit autophagic control of intracellular Mycobacterium tuberculosis. Immunity 27:505–517 [DOI] [PubMed] [Google Scholar]
- 32.Pilli M, Arko-Mensah J, Ponpuak M, Roberts E, Master S, Mandell MA, Dupont N, Ornatowski W, Jiang S, Bradfute SB, Bruun JA, Hansen TE, Johansen T, Deretic V. 2012. TBK-1 promotes autophagy-mediated antimicrobial defense by controlling autophagosome maturation. Immunity 37:223–234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ponpuak M, Davis AS, Roberts EA, Delgado MA, Dinkins C, Zhao Z, Virgin HW, Kyei GB, Johansen T, Vergne I, Deretic V. 2010. Delivery of cytosolic components by autophagic adaptor protein p62 endows autophagosomes with unique antimicrobial properties. Immunity 32:329–341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kuijl C, Savage ND, Marsman M, Tuin AW, Janssen L, Egan DA, Ketema M, van den Nieuwendijk R, van den Eeden SJ, Geluk A, Poot A, van der Marel G, Beijersbergen RL, Overkleeft H, Ottenhoff TH, Neefjes J. 2007. Intracellular bacterial growth is controlled by a kinase network around PKB/AKT1. Nature 450:725–730 [DOI] [PubMed] [Google Scholar]
- 35.Kumar D, Nath L, Kamal MA, Varshney A, Jain A, Singh S, Rao KV. 2010. Genome-wide analysis of the host intracellular network that regulates survival of Mycobacterium tuberculosis. Cell 140:731–743 [DOI] [PubMed] [Google Scholar]
- 36.Watson RO, Manzanillo PS, Cox JS. 2012. Extracellular M. tuberculosis DNA targets bacteria for autophagy by activating the host DNA-sensing pathway. Cell 150:803–815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim JJ, Lee HM, Shin DM, Kim W, Yuk JM, Jin HS, Lee SH, Cha GH, Kim JM, Lee ZW, Shin SJ, Yoo H, Park YK, Park JB, Chung J, Yoshimori T, Jo EK. 2012. Host cell autophagy activated by antibiotics is required for their effective antimycobacterial drug action. Cell Host Microbe 11:457–468 [DOI] [PubMed] [Google Scholar]
- 38.de Carvalho LP, Lin G, Jiang X, Nathan C. 2009. Nitazoxanide kills replicating and nonreplicating Mycobacterium tuberculosis and evades resistance. J. Med. Chem. 52:5789–5792 [DOI] [PubMed] [Google Scholar]
- 39.Lam KK, Zheng X, Forestieri R, Balgi AD, Nodwell M, Vollett S, Anderson HJ, Andersen RJ, Av-Gay Y, Roberge M. 2012. Nitazoxanide stimulates autophagy and inhibits mTORC1 signaling and intracellular proliferation of Mycobacterium tuberculosis. PLoS Pathog. 8:e1002691. 10.1371/journal.ppat.1002691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gold B, Pingle M, Brickner SJ, Shah N, Roberts J, Rundell M, Bracken WC, Warrier T, Somersan S, Venugopal A, Darby C, Jiang X, Warren JD, Fernandez J, Ouerfelli O, Nuermberger EL, Cunningham-Bussel A, Rath P, Chidawanyika T, Deng H, Realubit R, Glickman JF, Nathan CF. 2012. Nonsteroidal anti-inflammatory drug sensitizes Mycobacterium tuberculosis to endogenous and exogenous antimicrobials. Proc. Natl. Acad. Sci. U. S. A. 109:16004–16011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pinault L, Han JS, Kang CM, Franco J, Ronning DR. 2013. Zafirlukast inhibits complexation of Lsr2 with DNA and growth of Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 57:2134–2140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Adams JS, Liu PT, Chun R, Modlin RL, Hewison M. 2007. Vitamin D in defense of the human immune response. Ann. N. Y. Acad. Sci. 1117:94–105 [DOI] [PubMed] [Google Scholar]
- 43.Hewison M. 2011. Antibacterial effects of vitamin D. Nat. Rev. Endocrinol. 7:337–345 [DOI] [PubMed] [Google Scholar]
- 44.Krutzik SR, Hewison M, Liu PT, Robles JA, Stenger S, Adams JS, Modlin RL. 2008. IL-15 links TLR2/1-induced macrophage differentiation to the vitamin D-dependent antimicrobial pathway. J. Immunol. 181:7115–7120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Anand SP, Selvaraj P. 2009. Effect of 1,25 dihydroxyvitamin D(3) on matrix metalloproteinases MMP-7, MMP-9 and the inhibitor TIMP-1 in pulmonary tuberculosis. Clin. Immunol. 133:126–131 [DOI] [PubMed] [Google Scholar]
- 46.Fabri M, Stenger S, Shin DM, Yuk JM, Liu PT, Realegeno S, Lee HM, Krutzik SR, Schenk M, Sieling PA, Teles R, Montoya D, Iyer SS, Bruns H, Lewinsohn DM, Hollis BW, Hewison M, Adams JS, Steinmeyer A, Zugel U, Cheng G, Jo EK, Bloom BR, Modlin RL. 2011. Vitamin D is required for IFN-gamma-mediated antimicrobial activity of human macrophages. Sci. Transl. Med. 3:104ra102. 10.1126/scitranslmed.3003045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu PT, Krutzik SR, Modlin RL. 2007. Therapeutic implications of the TLR and VDR partnership. Trends Mol. Med. 13:117–124 [DOI] [PubMed] [Google Scholar]
- 48.Liu PT, Stenger S, Li H, Wenzel L, Tan BH, Krutzik SR, Ochoa MT, Schauber J, Wu K, Meinken C, Kamen DL, Wagner M, Bals R, Steinmeyer A, Zugel U, Gallo RL, Eisenberg D, Hewison M, Hollis BW, Adams JS, Bloom BR, Modlin RL. 2006. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science 311:1770–1773 [DOI] [PubMed] [Google Scholar]
- 49.Liu PT, Stenger S, Tang DH, Modlin RL. 2007. Vitamin D-mediated human antimicrobial activity against Mycobacterium tuberculosis is dependent on the induction of cathelicidin. J. Immunol. 179:2060–2063 [DOI] [PubMed] [Google Scholar]
- 50.Yuk JM, Shin DM, Lee HM, Yang CS, Jin HS, Kim KK, Lee ZW, Lee SH, Kim JM, Jo EK. 2009. Vitamin D3 induces autophagy in human monocytes/macrophages via cathelicidin. Cell Host Microbe 6:231–243 [DOI] [PubMed] [Google Scholar]
- 51.Wilkinson R, Llewelyn M, Toossi Z, Patel P, Pasvol G, Lalvani A, Wright D, Latif M, Davidson R. 2000. Influence of vitamin D receptor and vitamin D receptor polymorphisms on tuberculosis among Gujarati Asians in west London: a case-control study. Lancet 355:618–621 [DOI] [PubMed] [Google Scholar]
- 52.Martineau AR, Newton SM, Wilkinson KA, Kampmann B, Hall BM, Nawroly N, Packe GE, Davidson RN, Griffiths CJ, Wilkinson RJ. 2007. Neutrophil-mediated innate immune resistance to mycobacteria. J. Clin. Invest. 117:1988–1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nursyam EW, Amin Z, Rumende CM. 2006. The effect of vitamin D as supplementary treatment in patients with moderately advanced pulmonary tuberculous lesion. Acta Med. Indones. 38:3–5 [PubMed] [Google Scholar]
- 54.Kota SK, Jammula S, Kota SK, Tripathy PR, Panda S, Modi KD. 2011. Effect of vitamin D supplementation in type 2 diabetes patients with pulmonary tuberculosis. Diabetes Metab. Syndr. 5:85–89 [DOI] [PubMed] [Google Scholar]
- 55.Martineau AR, Timms PM, Bothamley GH, Hanifa Y, Islam K, Claxton AP, Packe GE, Moore-Gillon JC, Darmalingam M, Davidson RN, Milburn HJ, Baker LV, Barker RD, Woodward NJ, Venton TR, Barnes KE, Mullett CJ, Coussens AK, Rutterford CM, Mein CA, Davies GR, Wilkinson RJ, Nikolayevskyy V, Drobniewski FA, Eldridge SM, Griffiths CJ. 2011. High-dose vitamin D(3) during intensive-phase antimicrobial treatment of pulmonary tuberculosis: a double-blind randomised controlled trial. Lancet 377:242–250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Coussens AK, Wilkinson RJ, Hanifa Y, Nikolayevskyy V, Elkington PT, Islam K, Timms PM, Venton TR, Bothamley GH, Packe GE, Darmalingam M, Davidson RN, Milburn HJ, Baker LV, Barker RD, Mein CA, Bhaw-Rosun L, Nuamah R, Young DB, Drobniewski FA, Griffiths CJ, Martineau AR. 2012. Vitamin D accelerates resolution of inflammatory responses during tuberculosis treatment. Proc. Natl. Acad. Sci. U. S. A. 10.1073/pnas.1200072109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wejse C, Gomes VF, Rabna P, Gustafson P, Aaby P, Lisse IM, Andersen PL, Glerup H, Sodemann M. 2009. Vitamin D as supplementary treatment for tuberculosis: a double-blind, randomized, placebo-controlled trial. Am. J. Respir. Crit. Care Med. 179:843–850 [DOI] [PubMed] [Google Scholar]
- 58.Singh V, Jamwal S, Jain R, Verma P, Gokhale R, Rao KV. 2012. Mycobacterium tuberculosis-driven targeted recalibration of macrophage lipid homeostasis promotes the foamy phenotype. Cell Host Microbe 12:669–681 [DOI] [PubMed] [Google Scholar]
- 59.Almeida PE, Silva AR, Maya-Monteiro CM, Töröcsik D, D'Avila H, Dezsö B, Magalhães KG, Castro-Faria-Neto HC, Nagy L, Bozza PT. 2009. Mycobacterium bovis bacillus Calmette-Guérin infection induces TLR2-dependent peroxisome proliferator-activated receptor gamma expression and activation: functions in inflammation, lipid metabolism, and pathogenesis. J. Immunol. 183:1337–1345 [DOI] [PubMed] [Google Scholar]
- 60.Mahajan S, Dkhar HK, Chandra V, Dave S, Nanduri R, Janmeja AK, Agrewala JN, Gupta P. 2012. Mycobacterium tuberculosis modulates macrophage lipid-sensing nuclear receptors PPARγ and TR4 for survival. J. Immunol. 188:5593–5603 [DOI] [PubMed] [Google Scholar]
- 61.Rajaram MV, Brooks MN, Morris JD, Torrelles JB, Azad AK, Schlesinger LS. 2010. Mycobacterium tuberculosis activates human macrophage peroxisome proliferator-activated receptor gamma linking mannose receptor recognition to regulation of immune responses. J. Immunol. 185:929–942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Michalik L, Auwerx J, Berger JP, Chatterjee VK, Glass CK, Gonzalez FJ, Grimaldi PA, Kadowaki T, Lazar MA, O'Rahilly S, Palmer CN, Plutzky J, Reddy JK, Spiegelman BM, Staels B, Wahli W. 2006. International Union of Pharmacology. LXI. Peroxisome proliferator-activated receptors. Pharmacol. Rev. 58:726–741 [DOI] [PubMed] [Google Scholar]
- 63.Almeida PE, Carneiro AB, Silva AR, Bozza PT. 2012. PPARγ expression and function in mycobacterial infection: roles in lipid metabolism, immunity, and bacterial killing. PPAR Res. 2012:383829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Korf H, Vander Beken S, Romano M, Steffensen KR, Stijlemans B, Gustafsson JA, Grooten J, Huygen K. 2009. Liver X receptors contribute to the protective immune response against Mycobacterium tuberculosis in mice. J. Clin. Invest. 119:1626–1637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Staels B, Fruchart JC. 2005. Therapeutic roles of peroxisome proliferator-activated receptor agonists. Diabetes 54:2460–2470 [DOI] [PubMed] [Google Scholar]
- 66.Duan L, Gan H, Arm J, Remold HG. 2001. Cytosolic phospholipase A2 participates with TNF-alpha in the induction of apoptosis of human macrophages infected with Mycobacterium tuberculosis H37Ra. J. Immunol. 166:7469–7476 [DOI] [PubMed] [Google Scholar]
- 67.Akaki T, Tomioka H, Shimizu T, Dekio S, Sato K. 2000. Comparative roles of free fatty acids with reactive nitrogen intermediates and reactive oxygen intermediates in expression of the anti-microbial activity of macrophages against Mycobacterium tuberculosis. Clin. Exp. Immunol. 121:302–310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Vandal OH, Gelb MH, Ehrt S, Nathan CF. 2006. Cytosolic phospholipase A2 enzymes are not required by mouse bone marrow-derived macrophages for the control of Mycobacterium tuberculosis in vitro. Infect. Immun. 74:1751–1756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kusner DJ, Barton JA. 2001. ATP stimulates human macrophages to kill intracellular virulent Mycobacterium tuberculosis via calcium-dependent phagosome-lysosome fusion. J. Immunol. 167:3308–3315 [DOI] [PubMed] [Google Scholar]
- 70.Auricchio G, Garg SK, Martino A, Volpe E, Ciaramella A, De Vito P, Baldini PM, Colizzi V, Fraziano M. 2003. Role of macrophage phospholipase D in natural and CpG-induced antimycobacterial activity. Cell. Microbiol. 5:913–920 [DOI] [PubMed] [Google Scholar]
- 71.Garg SK, Valente E, Greco E, Santucci MB, De Spirito M, Papi M, Bocchino M, Saltini C, Fraziano M. 2006. Lysophosphatidic acid enhances antimycobacterial activity both in vitro and ex vivo. Clin. Immunol. 121:23–28 [DOI] [PubMed] [Google Scholar]
- 72.Garg SK, Volpe E, Palmieri G, Mattei M, Galati D, Martino A, Piccioni MS, Valente E, Bonanno E, De Vito P, Baldini PM, Spagnoli LG, Colizzi V, Fraziano M. 2004. Sphingosine 1-phosphate induces antimicrobial activity both in vitro and in vivo. J. Infect. Dis. 189:2129–2138 [DOI] [PubMed] [Google Scholar]
- 73.Greco E, Santucci MB, Quintiliani G, Papi M, De Spirito M, Fraziano M. 2009. CpG oligodeoxynucleotides promote phospholipase D dependent phagolysosome maturation and intracellular mycobacterial killing in M. tuberculosis infected type II alveolar epithelial cells. Cell. Immunol. 259:1–4 [DOI] [PubMed] [Google Scholar]
- 74.Campbell EL, Serhan CN, Colgan SP. 2011. Antimicrobial aspects of inflammatory resolution in the mucosa: a role for proresolving mediators. J. Immunol. 187:3475–3481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Serhan CN. 2007. Resolution phase of inflammation: novel endogenous anti-inflammatory and proresolving lipid mediators and pathways. Annu. Rev. Immunol. 25:101–137 [DOI] [PubMed] [Google Scholar]
- 76.Needleman P, Turk J, Jakschik BA, Morrison AR, Lefkowith JB. 1986. Arachidonic acid metabolism. Annu. Rev. Biochem. 55:69–102 [DOI] [PubMed] [Google Scholar]
- 77.Spector AA, Fang X, Snyder GD, Weintraub NL. 2004. Epoxyeicosatrienoic acids (EETs): metabolism and biochemical function. Prog. Lipid Res. 43:55–90 [DOI] [PubMed] [Google Scholar]
- 78.Behar SM, Divangahi M, Remold HG. 2010. Evasion of innate immunity by Mycobacterium tuberculosis: is death an exit strategy? Nat. Rev. Microbiol. 8:668–674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chen M, Divangahi M, Gan H, Shin DS, Hong S, Lee DM, Serhan CN, Behar SM, Remold HG. 2008. Lipid mediators in innate immunity against tuberculosis: opposing roles of PGE2 and LXA4 in the induction of macrophage death. J. Exp. Med. 205:2791–2801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Divangahi M, Desjardins D, Nunes-Alves C, Remold HG, Behar SM. 2010. Eicosanoid pathways regulate adaptive immunity to Mycobacterium tuberculosis. Nat. Immunol. 11:751–758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Greco E, Quintiliani G, Santucci MB, Serafino A, Ciccaglione AR, Marcantonio C, Papi M, Maulucci G, Delogu G, Martino A, Goletti D, Sarmati L, Andreoni M, Altieri A, Alma M, Caccamo N, Di Liberto D, De Spirito M, Savage ND, Nisini R, Dieli F, Ottenhoff TH, Fraziano M. 2012. Janus-faced liposomes enhance antimicrobial innate immune response in Mycobacterium tuberculosis infection. Proc. Natl. Acad. Sci. U. S. A. 109:E1360–E1368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tobin DM, Vary JC, Jr, Ray JP, Walsh GS, Dunstan SJ, Bang ND, Hagge DA, Khadge S, King MC, Hawn TR, Moens CB, Ramakrishnan L. 2010. The lta4h locus modulates susceptibility to mycobacterial infection in zebrafish and humans. Cell 140:717–730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tobin DM, Roca FJ, Oh SF, McFarland R, Vickery TW, Ray JP, Ko DC, Zou Y, Bang ND, Chau TT, Vary JC, Hawn TR, Dunstan SJ, Farrar JJ, Thwaites GE, King MC, Serhan CN, Ramakrishnan L. 2012. Host genotype-specific therapies can optimize the inflammatory response to mycobacterial infections Cell 148:434–446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Curtis J, Kopanitsa L, Stebbings E, Speirs A, Ignatyeva O, Balabanova Y, Nikolayevskyy V, Hoffner S, Horstmann R, Drobniewski F, Nejentsev S. 2011. Association analysis of the LTA4H gene polymorphisms and pulmonary tuberculosis in 9115 subjects. Tuberculosis (Edinb.) 91:22–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Thwaites GE, Nguyen DB, Nguyen HD, Hoang TQ, Do TT, Nguyen TC, Nguyen QH, Nguyen TT, Nguyen NH, Nguyen TN, Nguyen NL, Nguyen HD, Vu NT, Cao HH, Tran TH, Pham PM, Nguyen TD, Stepniewska K, White NJ, Tran TH, Farrar JJ. 2004. Dexamethasone for the treatment of tuberculous meningitis in adolescents and adults. N. Engl. J. Med. 351:1741–1751 [DOI] [PubMed] [Google Scholar]
- 86.Clish CB, O'Brien JA, Gronert K, Stahl GL, Petasis NA, Serhan CN. 1999. Local and systemic delivery of a stable aspirin-triggered lipoxin prevents neutrophil recruitment in vivo. Proc. Natl. Acad. Sci. U. S. A. 96:8247–8252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sodin-Semrl S, Taddeo B, Tseng D, Varga J, Fiore S. 2000. Lipoxin A4 inhibits IL-1 beta-induced IL-6, IL-8, and matrix metalloproteinase-3 production in human synovial fibroblasts and enhances synthesis of tissue inhibitors of metalloproteinases. J. Immunol. 164:2660–2666 [DOI] [PubMed] [Google Scholar]
- 88.Aliberti J, Serhan C, Sher A. 2002. Parasite-induced lipoxin A4 is an endogenous regulator of IL-12 production and immunopathology in Toxoplasma gondii infection. J. Exp. Med. 196:1253–1262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Aliberti J, Hieny S, CReis e Sousa Serhan CN, Sher A. 2002. Lipoxin-mediated inhibition of IL-12 production by DCs: a mechanism for regulation of microbial immunity. Nat. Immunol. 3:76–82 [DOI] [PubMed] [Google Scholar]
- 90.Bafica A, Scanga CA, Serhan C, Machado F, White S, Sher A, Aliberti J. 2005. Host control of Mycobacterium tuberculosis is regulated by 5-lipoxygenase-dependent lipoxin production. J. Clin. Invest. 115:1601–1606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Peres CM, de Paula L, Medeiros AI, Sorgi CA, Soares EG, Carlos D, Peters-Golden M, Silva CL, Faccioli LH. 2007. Inhibition of leukotriene biosynthesis abrogates the host control of Mycobacterium tuberculosis. Microbes Infect. 9:483–489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Herb F, Thye T, Niemann S, Browne EN, Chinbuah MA, Gyapong J, Osei I, Owusu-Dabo E, Werz O, Rusch-Gerdes S, Horstmann RD, Meyer CG. 2008. ALOX5 variants associated with susceptibility to human pulmonary tuberculosis. Hum. Mol. Genet. 17:1052–1060 [DOI] [PubMed] [Google Scholar]
- 93.Schoeman JF, Janse van Rensburg A, Laubscher JA, Springer P. 2011. The role of aspirin in childhood tuberculous meningitis. J. Child Neurol. 26:956–962 [DOI] [PubMed] [Google Scholar]
- 94.Misra UK, Kalita J, Nair PP. 2010. Role of aspirin in tuberculous meningitis: a randomized open label placebo controlled trial. J. Neurol. Sci. 293:12–17 [DOI] [PubMed] [Google Scholar]
- 95.Vilaplana C, Marzo E, Tapia G, Diaz J, Garcia V, Cardona PJ. 2013. Ibuprofen therapy resulted in significantly decreased tissue bacillary loads and increased survival in a new murine experimental model of active tuberculosis. J. Infect. Dis. 208:199–202 [DOI] [PubMed] [Google Scholar]
- 96.Byrne ST, Denkin SM, Zhang Y. 2007. Aspirin and ibuprofen enhance pyrazinamide treatment of murine tuberculosis. J. Antimicrob. Chemother. 59:313–316 [DOI] [PubMed] [Google Scholar]
- 97.Byrne ST, Denkin SM, Zhang Y. 2007. Aspirin antagonism in isoniazid treatment of tuberculosis in mice. Antimicrob. Agents Chemother. 51:794–795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Dutta NK, Mazumdar K, Dastidar SG, Park JH. 2007. Activity of diclofenac used alone and in combination with streptomycin against Mycobacterium tuberculosis in mice. Int. J. Antimicrob. Agents 30:336–340 [DOI] [PubMed] [Google Scholar]
- 99.Ivanyi J, Zumla A. 2013. Nonsteroidal antiinflammatory drugs for adjunctive tuberculosis treatment. J. Infect. Dis. 208:185–188 [DOI] [PubMed] [Google Scholar]
- 100.Wu K, Koo J, Jiang X, Chen R, Cohen SN, Nathan C. 2012. Improved control of tuberculosis and activation of macrophages in mice lacking protein kinase R. PLoS One 7:e30512. 10.1371/journal.pone.0030512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bando Y, Onuki R, Katayama T, Manabe T, Kudo T, Taira K, Tohyama M. 2005. Double-strand RNA dependent protein kinase (PKR) is involved in the extrastriatal degeneration in Parkinson's disease and Huntington's disease. Neurochem. Int. 46:11–18 [DOI] [PubMed] [Google Scholar]
- 102.Chang RC, Suen KC, Ma CH, Elyaman W, Ng HK, Hugon J. 2002. Involvement of double-stranded RNA-dependent protein kinase and phosphorylation of eukaryotic initiation factor-2alpha in neuronal degeneration. J. Neurochem. 83:1215–1225 [DOI] [PubMed] [Google Scholar]
- 103.Goodman AG, Fornek JL, Medigeshi GR, Perrone LA, Peng X, Dyer MD, Proll SC, Knoblaugh SE, Carter VS, Korth MJ, Nelson JA, Tumpey TM, Katze MG. 2009. P58(IPK): a novel “CIHD” member of the host innate defense response against pathogenic virus infection. PLoS Pathog. 5:e1000438. 10.1371/journal.ppat.1000438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hu JH, Zhang H, Wagey R, Krieger C, Pelech SL. 2003. Protein kinase and protein phosphatase expression in amyotrophic lateral sclerosis spinal cord. J. Neurochem. 85:432–442 [DOI] [PubMed] [Google Scholar]
- 105.Morel M, Couturier J, Lafay-Chebassier C, Paccalin M, Page G. 2009. PKR, the double stranded RNA-dependent protein kinase as a critical target in Alzheimer's disease. J. Cell. Mol. Med. 13:1476–1488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Johnson EE, Wessling-Resnick M. 2012. Iron metabolism and the innate immune response to infection. Microbes Infect. 14:207–216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hoette TM, Clifton MC, Zawadzka AM, Holmes MA, Strong RK, Raymond KN. 2011. Immune interference in Mycobacterium tuberculosis intracellular iron acquisition through siderocalin recognition of carboxymycobactins. ACS Chem. Biol. 6:1327–1331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Holmes MA, Paulsene W, Jide X, Ratledge C, Strong RK. 2005. Siderocalin (Lcn 2) also binds carboxymycobactins, potentially defending against mycobacterial infections through iron sequestration. Structure 13:29–41 [DOI] [PubMed] [Google Scholar]
- 109.Halaas O, Steigedal M, Haug M, Awuh JA, Ryan L, Brech A, Sato S, Husebye H, Cangelosi GA, Akira S, Strong RK, Espevik T, Flo TH. 2010. Intracellular Mycobacterium avium intersect transferrin in the Rab11(+) recycling endocytic pathway and avoid lipocalin 2 trafficking to the lysosomal pathway. J. Infect. Dis. 201:783–792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Saiga H, Nishimura J, Kuwata H, Okuyama M, Matsumoto S, Sato S, Matsumoto M, Akira S, Yoshikai Y, Honda K, Yamamoto M, Takeda K. 2008. Lipocalin 2-dependent inhibition of mycobacterial growth in alveolar epithelium. J. Immunol. 181:8521–8527 [DOI] [PubMed] [Google Scholar]
- 111.Skamene E, Schurr E, Gros P. 1998. Infection genomics: Nramp1 as a major determinant of natural resistance to intracellular infections. Annu. Rev. Med. 49:275–287 [DOI] [PubMed] [Google Scholar]
- 112.Li X, Yang Y, Zhou F, Zhang Y, Lu H, Jin Q, Gao L. 2011. SLC11A1 (NRAMP1) polymorphisms and tuberculosis susceptibility: updated systematic review and meta-analysis. PLoS One 6:e15831. 10.1371/journal.pone.0015831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Govoni G, Gros P. 1998. Macrophage NRAMP1 and its role in resistance to microbial infections. Inflamm. Res. 47:277–284 [DOI] [PubMed] [Google Scholar]
- 114.Vidal S, Tremblay ML, Govoni G, Gauthier S, Sebastiani G, Malo D, Skamene E, Olivier M, Jothy S, Gros P. 1995. The Ity/Lsh/Bcg locus: natural resistance to infection with intracellular parasites is abrogated by disruption of the Nramp1 gene. J. Exp. Med. 182:655–666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.North RJ, LaCourse R, Ryan L, Gros P. 1999. Consequence of Nramp1 deletion to Mycobacterium tuberculosis infection in mice. Infect. Immun. 67:5811–5814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Sow FB, Alvarez GR, Gross RP, Satoskar AR, Schlesinger LS, Zwilling BS, Lafuse WP. 2009. Role of STAT1, NF-kappaB, and C/EBPbeta in the macrophage transcriptional regulation of hepcidin by mycobacterial infection and IFN-gamma. J. Leukoc. Biol. 86:1247–1258 [DOI] [PubMed] [Google Scholar]
- 117.Sow FB, Nandakumar S, Velu V, Kellar KL, Schlesinger LS, Amara RR, Lafuse WP, Shinnick TM, Sable SB. 2011. Mycobacterium tuberculosis components stimulate production of the antimicrobial peptide hepcidin. Tuberculosis (Edinb.) 91:314–321 [DOI] [PubMed] [Google Scholar]
- 118.Johnson EE, Sandgren A, Cherayil BJ, Murray M, Wessling-Resnick M. 2010. Role of ferroportin in macrophage-mediated immunity. Infect. Immun. 78:5099–5106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Welsh KJ, Hwang SA, Boyd S, Kruzel ML, Hunter RL, Actor JK. 2011. Influence of oral lactoferrin on Mycobacterium tuberculosis induced immunopathology. Tuberculosis (Edinb.) 91(Suppl 1):S105–S113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Gerosa F, Baldani-Guerra B, Lyakh LA, Batoni G, Esin S, Winkler-Pickett RT, Consolaro MR, De Marchi M, Giachino D, Robbiano A, Astegiano M, Sambataro A, Kastelein RA, Carra G, Trinchieri G. 2008. Differential regulation of interleukin 12 and interleukin 23 production in human dendritic cells. J. Exp. Med. 205:1447–1461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Lutz M, Knaus P. 2002. Integration of the TGF-beta pathway into the cellular signalling network. Cell Signal. 14:977–988 [DOI] [PubMed] [Google Scholar]
- 122.Manel N, Unutmaz D, Littman DR. 2008. The differentiation of human T(H)-17 cells requires transforming growth factor-beta and induction of the nuclear receptor RORgammat. Nat. Immunol. 9:641–649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ramos HJ, Davis AM, Cole AG, Schatzle JD, Forman J, Farrar JD. 2009. Reciprocal responsiveness to interleukin-12 and interferon-alpha specifies human CD8+ effector versus central memory T-cell fates. Blood 113:5516–5525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Trinchieri G. 2003. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat. Rev. Immunol. 3:133–146 [DOI] [PubMed] [Google Scholar]
- 125.Volpe E, Servant N, Zollinger R, Bogiatzi SI, Hupe P, Barillot E, Soumelis V. 2008. A critical function for transforming growth factor-beta, interleukin 23 and proinflammatory cytokines in driving and modulating human T(H)-17 responses. Nat. Immunol. 9:650–657 [DOI] [PubMed] [Google Scholar]
- 126.Antonelli LR, Gigliotti Rothfuchs A, Goncalves R, Roffe E, Cheever AW, Bafica A, Salazar AM, Feng CG, Sher A. 2010. Intranasal poly-IC treatment exacerbates tuberculosis in mice through the pulmonary recruitment of a pathogen-permissive monocyte/macrophage population. J. Clin. Invest. 120:1674–1682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Teles RM, Graeber TG, Krutzik SR, Montoya D, Schenk M, Lee DJ, Komisopoulou E, Kelly-Scumpia K, Chun R, Iyer SS, Sarno EN, Rea TH, Hewison M, Adams JS, Popper SJ, Relman DA, Stenger S, Bloom BR, Cheng G, Modlin RL. 2013. Type I interferon suppresses type II interferon-triggered human anti-mycobacterial responses. Science 339:1448–1453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Redford PS, Boonstra A, Read S, Pitt J, Graham C, Stavropoulos E, Bancroft GJ, O'Garra A. 2010. Enhanced protection to Mycobacterium tuberculosis infection in IL-10-deficient mice is accompanied by early and enhanced Th1 responses in the lung. Eur. J. Immunol. 40:2200–2210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Redford PS, Murray PJ, O'Garra A. 2011. The role of IL-10 in immune regulation during M. tuberculosis infection. Mucosal Immunol. 4:261–270 [DOI] [PubMed] [Google Scholar]
- 130.Kursar M, Koch M, Mittrucker HW, Nouailles G, Bonhagen K, Kamradt T, Kaufmann SH. 2007. Regulatory T cells prevent efficient clearance of Mycobacterium tuberculosis. J. Immunol. 178:2661–2665 [DOI] [PubMed] [Google Scholar]
- 131.Larson RP, Shafiani S, Urdahl KB. 2013. Foxp3(+) regulatory T cells in tuberculosis. Adv. Exp. Med. Biol. 783:165–180 [DOI] [PubMed] [Google Scholar]
- 132.Davis JM, Ramakrishnan L. 2009. The role of the granuloma in expansion and dissemination of early tuberculous infection. Cell 136:37–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Ehrt S, Schnappinger D, Bekiranov S, Drenkow J, Shi S, Gingeras TR, Gaasterland T, Schoolnik G, Nathan C. 2001. Reprogramming of the macrophage transcriptome in response to interferon-gamma and Mycobacterium tuberculosis: signaling roles of nitric oxide synthase-2 and phagocyte oxidase. J. Exp. Med. 194:1123–1140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Flynn JL, Chan J, Triebold KJ, Dalton DK, Stewart TA, Bloom BR. 1993. An essential role for interferon gamma in resistance to Mycobacterium tuberculosis infection. J. Exp. Med. 178:2249–2254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Casanova JL, Abel L. 2002. Genetic dissection of immunity to mycobacteria: the human model. Annu. Rev. Immunol. 20:581–620 [DOI] [PubMed] [Google Scholar]
- 136.Condos R, Raju B, Canova A, Zhao BY, Weiden M, Rom WN, Pine R. 2003. Recombinant gamma interferon stimulates signal transduction and gene expression in alveolar macrophages in vitro and in tuberculosis patients. Infect. Immun. 71:2058–2064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Milanés-Virelles MT, García-García I, Santos-Herrera Y, Valdés-Quintana M, Valenzuela-Silva CM, Jiménez-Madrigal G, Ramos-Gómez TI, Bello-Rivero I, Fernández-Olivera N, Sánchez-de la Osa RB, Rodríguez-Acosta C, González-Méndez L, Martínez-Sánchez G, López-Saura PA, MACGAM Study Group 2008. Adjuvant interferon gamma in patients with pulmonary atypical mycobacteriosis: a randomized, double-blind, placebo-controlled study. BMC Infect. Dis. 8:17. 10.1186/1471-2334-8-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Dawson R, Condos R, Tse D, Huie ML, Ress S, Tseng CH, Brauns C, Weiden M, Hoshino Y, Bateman E, Rom WN. 2009. Immunomodulation with recombinant interferon-gamma1b in pulmonary tuberculosis. PLoS One 4:e6984. 10.1371/journal.pone.0006984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Flynn JL, Goldstein MM, Chan J, Triebold KJ, Pfeffer K, Lowenstein CJ, Schreiber R, Mak TW, Bloom BR. 1995. Tumor necrosis factor-alpha is required in the protective immune response against Mycobacterium tuberculosis in mice. Immunity 2:561–572 [DOI] [PubMed] [Google Scholar]
- 140.Solovic I, Sester M, Gomez-Reino JJ, Rieder HL, Ehlers S, Milburn HJ, Kampmann B, Hellmich B, Groves R, Schreiber S, Wallis RS, Sotgiu G, Scholvinck EH, Goletti D, Zellweger JP, Diel R, Carmona L, Bartalesi F, Ravn P, Bossink A, Duarte R, Erkens C, Clark J, Migliori GB, Lange C. 2010. The risk of tuberculosis related to tumour necrosis factor antagonist therapies: a TBNET consensus statement. Eur. Respir. J. 36:1185–1206 [DOI] [PubMed] [Google Scholar]
- 141.Wallis RS. 2005. Reconsidering adjuvant immunotherapy for tuberculosis. Clin. Infect. Dis. 41:201–208 [DOI] [PubMed] [Google Scholar]
- 142.Wallis RS, Kyambadde P, Johnson JL, Horter L, Kittle R, Pohle M, Ducar C, Millard M, Mayanja-Kizza H, Whalen C, Okwera A. 2004. A study of the safety, immunology, virology, and microbiology of adjunctive etanercept in HIV-1-associated tuberculosis. AIDS 18:257–264 [DOI] [PubMed] [Google Scholar]
- 143.Corral LG, Kaplan G. 1999. Immunomodulation by thalidomide and thalidomide analogues. Ann. Rheum. Dis. 58(Suppl 1):I107–I113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Maiga M, Agarwal N, Ammerman NC, Gupta R, Guo H, Maiga MC, Lun S, Bishai WR. 2012. Successful shortening of tuberculosis treatment using adjuvant host-directed therapy with FDA-approved phosphodiesterase inhibitors in the mouse model. PLoS One 7:e30749. 10.1371/journal.pone.0030749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Kalamidas SA, Kuehnel MP, Peyron P, Rybin V, Rauch S, Kotoulas OB, Houslay M, Hemmings BA, Gutierrez MG, Anes E, Griffiths G. 2006. cAMP synthesis and degradation by phagosomes regulate actin assembly and fusion events: consequences for mycobacteria. J. Cell Sci. 119:3686–3694 [DOI] [PubMed] [Google Scholar]
- 146.Agarwal N, Lamichhane G, Gupta R, Nolan S, Bishai WR. 2009. Cyclic AMP intoxication of macrophages by a Mycobacterium tuberculosis adenylate cyclase. Nature 460:98–102 [DOI] [PubMed] [Google Scholar]
- 147.Schoeman JF, Springer P, van Rensburg AJ, Swanevelder S, Hanekom WA, Haslett PA, Kaplan G. 2004. Adjunctive thalidomide therapy for childhood tuberculous meningitis: results of a randomized study. J. Child Neurol. 19:250–257 [DOI] [PubMed] [Google Scholar]
- 148.Koo MS, Manca C, Yang G, O'Brien P, Sung N, Tsenova L, Subbian S, Fallows D, Muller G, Ehrt S, Kaplan G. 2011. Phosphodiesterase 4 inhibition reduces innate immunity and improves isoniazid clearance of Mycobacterium tuberculosis in the lungs of infected mice. PLoS One 6:e17091. 10.1371/journal.pone.0017091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Subbian S, Tsenova L, O'Brien P, Yang G, Koo MS, Peixoto B, Fallows D, Dartois V, Muller G, Kaplan G. 2011. Phosphodiesterase-4 inhibition alters gene expression and improves isoniazid-mediated clearance of Mycobacterium tuberculosis in rabbit lungs. PLoS Pathog. 7:e1002262. 10.1371/journal.ppat.1002262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Subbian S, Tsenova L, O'Brien P, Yang G, Koo MS, Peixoto B, Fallows D, Zeldis JB, Muller G, Kaplan G. 2011. Phosphodiesterase-4 inhibition combined with isoniazid treatment of rabbits with pulmonary tuberculosis reduces macrophage activation and lung pathology. Am. J. Pathol. 179:289–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Maiga M, Ammerman NC, Maiga MC, Tounkara A, Siddiqui S, Polis M, Murphy R, Bishai WR. 2013. Adjuvant host-directed therapy with types 3 and 5 but not type 4 phosphodiesterase inhibitors shortens the duration of tuberculosis treatment. J. Infect. Dis. 208:512–519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Wallis RS, Nsubuga P, Whalen C, Mugerwa RD, Okwera A, Oette D, Jackson JB, Johnson JL, Ellner JJ. 1996. Pentoxifylline therapy in human immunodeficiency virus-seropositive persons with tuberculosis: a randomized, controlled trial. J. Infect. Dis. 174:727–733 [DOI] [PubMed] [Google Scholar]
- 153.Strang JI, Kakaza HH, Gibson DG, Girling DJ, Nunn AJ, Fox W. 1987. Controlled trial of prednisolone as adjuvant in treatment of tuberculous constrictive pericarditis in Transkei. Lancet ii:1418–1422 [DOI] [PubMed] [Google Scholar]
- 154.Simmons CP, Thwaites GE, Quyen NT, Torok E, Hoang DM, Chau TT, Mai PP, Lan NT, Dung NH, Quy HT, Bang ND, Hien TT, Farrar J. 2006. Pretreatment intracerebral and peripheral blood immune responses in Vietnamese adults with tuberculous meningitis: diagnostic value and relationship to disease severity and outcome. J. Immunol. 176:2007–2014 [DOI] [PubMed] [Google Scholar]
- 155.Roca FJ, Ramakrishnan L. 2013. TNF dually mediates resistance and susceptibility to mycobacteria via mitochondrial reactive oxygen species. Cell 153:521–534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Elkington P, Shiomi T, Breen R, Nuttall RK, Ugarte-Gil CA, Walker NF, Saraiva L, Pedersen B, Mauri F, Lipman M, Edwards DR, Robertson BD, D'Armiento J, Friedland JS. 2011. MMP-1 drives immunopathology in human tuberculosis and transgenic mice. J. Clin. Invest. 121:1827–1833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Clutterbuck AL, Asplin KE, Harris P, Allaway D, Mobasheri A. 2009. Targeting matrix metalloproteinases in inflammatory conditions. Curr. Drug Targets 10:1245–1254 [DOI] [PubMed] [Google Scholar]
- 158.Coussens A, Timms PM, Boucher BJ, Venton TR, Ashcroft AT, Skolimowska KH, Newton SM, Wilkinson KA, Davidson RN, Griffiths CJ, Wilkinson RJ, Martineau AR. 2009. 1α,25-Dihydroxyvitamin D3 inhibits matrix metalloproteinases induced by Mycobacterium tuberculosis infection. Immunology 127:539–548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Elkington PT, Emerson JE, Lopez-Pascua LD, O'Kane CM, Horncastle DE, Boyle JJ, Friedland JS. 2005. Mycobacterium tuberculosis up-regulates matrix metalloproteinase-1 secretion from human airway epithelial cells via a p38 MAPK switch. J. Immunol. 175:5333–5340 [DOI] [PubMed] [Google Scholar]
- 160.Gurusamy N, Das DK. 2009. Autophagy, redox signaling, and ventricular remodeling. Antioxid. Redox Signal. 11:1975–1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Parks WC, Wilson CL, López-Boado YS. 2004. Matrix metalloproteinases as modulators of inflammation and innate immunity. Nat. Rev. Immunol. 4:617–629 [DOI] [PubMed] [Google Scholar]
- 162.Kumar D, Kumar M, Saravanan C, Singh SK. 2012. Curcumin: a potential candidate for matrix metalloproteinase inhibitors. Expert Opin. Ther. Targets 16:959–972 [DOI] [PubMed] [Google Scholar]
- 163.Friedland JS, Shaw TC, Price NM, Dayer JM. 2002. Differential regulation of MMP-1/9 and TIMP-1 secretion in human monocytic cells in response to Mycobacterium tuberculosis. Matrix Biol. 21:103–110 [DOI] [PubMed] [Google Scholar]
- 164.Quiding-Jarbrink M, Smith DA, Bancroft GJ. 2001. Production of matrix metalloproteinases in response to mycobacterial infection. Infect. Immun. 69:5661–5670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Green JA, Tran CT, Farrar JJ, Nguyen MT, Nguyen PH, Dinh SX, Ho ND, Ly CV, Tran HT, Friedland JS, Thwaites GE. 2009. Dexamethasone, cerebrospinal fluid matrix metalloproteinase concentrations and clinical outcomes in tuberculous meningitis. PLoS One 4:e7277. 10.1371/journal.pone.0007277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Price NM, Gilman RH, Uddin J, Recavarren S, Friedland JS. 2003. Unopposed matrix metalloproteinase-9 expression in human tuberculous granuloma and the role of TNF-alpha-dependent monocyte networks. J. Immunol. 171:5579–5586 [DOI] [PubMed] [Google Scholar]
- 167.Taylor JL, Hattle JM, Dreitz SA, Troudt JM, Izzo LS, Basaraba RJ, Orme IM, Matrisian LM, Izzo AA. 2006. Role for matrix metalloproteinase 9 in granuloma formation during pulmonary Mycobacterium tuberculosis infection. Infect. Immun. 74:6135–6144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Volkman HE, Pozos TC, Zheng J, Davis JM, Rawls JF, Ramakrishnan L. 2010. Tuberculous granuloma induction via interaction of a bacterial secreted protein with host epithelium. Science 327:466–469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Hernandez-Pando 2000. Treatment with BB-94, a broad spectrum inhibitor of zinc-dependent metalloproteinases, causes deviation of the cytokine profile towards type-2 in experimental pulmonary tuberculosis in Balb/c mice. J. Exp. Pathol. 81:199–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Izzo AA, Izzo LS, Kasimos J, Majka S. 2004. A matrix metalloproteinase inhibitor promotes granuloma formation during the early phase of Mycobacterium tuberculosis pulmonary infection. Tuberculosis (Edinb.) 84:387–396 [DOI] [PubMed] [Google Scholar]
- 171.Nathan C. 2006. Neutrophils and immunity: challenges and opportunities. Nat. Rev. Immunol. 6:173–182 [DOI] [PubMed] [Google Scholar]
- 172.Yang CT, Cambier CJ, Davis JM, Hall CJ, Crosier PS, Ramakrishnan L. 2012. Neutrophils exert protection in the early tuberculous granuloma by oxidative killing of mycobacteria phagocytosed from infected macrophages. Cell Host Microbe 12:301–312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Eum SY, Kong JH, Hong MS, Lee YJ, Kim JH, Hwang SH, Cho SN, Via LE, Barry CE., III 2010. Neutrophils are the predominant infected phagocytic cells in the airways of patients with active pulmonary TB. Chest 137:122–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Repasy T, Lee J, Marino S, Martinez N, Kirschner DE, Hendricks G, Baker S, Wilson AA, Kotton DN, Kornfeld H. 2013. Intracellular bacillary burden reflects a burst size for Mycobacterium tuberculosis in vivo. PLoS Pathog. 9:e1003190. 10.1371/journal.ppat.1003190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Nathan C. 2002. Points of control in inflammation. Nature 420:846–852 [DOI] [PubMed] [Google Scholar]
- 176.Lowe DM, Redford PS, Wilkinson RJ, O'Garra A, Martineau AR. 2012. Neutrophils in tuberculosis: friend or foe? Trends Immunol. 33:14–25 [DOI] [PubMed] [Google Scholar]
- 177.Burgos RA, Hidalgo MA, Figueroa CD, Conejeros I, Hancke JL. 2009. New potential targets to modulate neutrophil function in inflammation. Mini Rev. Med. Chem. 9:153–168 [DOI] [PubMed] [Google Scholar]
- 178.Goldmann O, Medina E. 2012. The expanding world of extracellular traps: not only neutrophils but much more. Front. Immunol. 3:420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Ramos-Kichik V, Mondragon-Flores R, Mondragon-Castelan M, Gonzalez-Pozos S, Muniz-Hernandez S, Rojas-Espinosa O, Chacon-Salinas R, Estrada-Parra S, Estrada-Garcia I. 2009. Neutrophil extracellular traps are induced by Mycobacterium tuberculosis. Tuberculosis (Edinb.) 89:29–37 [DOI] [PubMed] [Google Scholar]
- 180.Wong KW, Jacobs WR., Jr 2013. Mycobacterium tuberculosis exploits human interferon gamma to stimulate macrophage extracellular trap formation and necrosis. J. Infect. Dis. 10.1093/infdis/jit097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Cheng OZ, Palaniyar N. 2013. NET balancing: a problem in inflammatory lung diseases. Front. Immunol. 4:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Kaplan MJ, Radic M. 2012. Neutrophil extracellular traps: double-edged swords of innate immunity. J. Immunol. 189:2689–2695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Ramakrishnan L. 2012. Revisiting the role of the granuloma in tuberculosis. Nat. Rev. Immunol. 12:352–366 [DOI] [PubMed] [Google Scholar]
- 184.Dheda K, Booth H, Huggett JF, Johnson MA, Zumla A, Rook GA. 2005. Lung remodeling in pulmonary tuberculosis. J. Infect. Dis. 192:1201–1209 [DOI] [PubMed] [Google Scholar]
- 185.Adamali HI, Maher TM. 2012. Current and novel drug therapies for idiopathic pulmonary fibrosis. Drug Des. Dev. Ther. 6:261–271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Hakim A, Adcock IM, Usmani OS. 2012. Corticosteroid resistance and novel anti-inflammatory therapies in chronic obstructive pulmonary disease: current evidence and future direction. Drugs 72:1299–1312 [DOI] [PubMed] [Google Scholar]
- 187.Royce SG, Cheng V, Samuel CS, Tang ML. 2012. The regulation of fibrosis in airway remodeling in asthma. Mol. Cell. Endocrinol. 351:167–175 [DOI] [PubMed] [Google Scholar]
- 188.Maher TM. 2010. Pirfenidone in idiopathic pulmonary fibrosis. Drugs Today (Barc.) 46:473–482 [DOI] [PubMed] [Google Scholar]
- 189.Moore B, Murphy RF, Agrawal DK. 2008. Interaction of TGF-beta with immune cells in airway disease. Curr. Mol. Med. 8:427–436 [DOI] [PubMed] [Google Scholar]
- 190.Hancock A, Armstrong L, Gama R, Millar A. 1998. Production of interleukin 13 by alveolar macrophages from normal and fibrotic lung. Am. J. Respir. Cell Mol. Biol. 18:60–65 [DOI] [PubMed] [Google Scholar]
- 191.Rose CE, Jr, Sung SS, Fu SM. 2003. Significant involvement of CCL2 (MCP-1) in inflammatory disorders of the lung. Microcirculation 10:273–288 [DOI] [PubMed] [Google Scholar]
- 192.Bennett RG. 2009. Relaxin and its role in the development and treatment of fibrosis. Transl. Res. 154:1–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Allen JT, Spiteri MA. 2002. Growth factors in idiopathic pulmonary fibrosis: relative roles. Respir. Res. 3:13. 10.1186/rr162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Aono Y, Nishioka Y, Inayama M, Ugai M, Kishi J, Uehara H, Izumi K, Sone S. 2005. Imatinib as a novel antifibrotic agent in bleomycin-induced pulmonary fibrosis in mice. Am. J. Respir. Crit. Care Med. 171:1279–1285 [DOI] [PubMed] [Google Scholar]
- 195.Aysola RS, Hoffman EA, Gierada D, Wenzel S, Cook-Granroth J, Tarsi J, Zheng J, Schechtman KB, Ramkumar TP, Cochran R, Xueping E, Christie C, Newell J, Fain S, Altes TA, Castro M. 2008. Airway remodeling measured by multidetector CT is increased in severe asthma and correlates with pathology. Chest 134:1183–1191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Via LE, Schimel D, Weiner DM, Dartois V, Dayao E, Cai Y, Yoon YS, Dreher MR, Kastenmayer RJ, Laymon CM, Carny JE, Flynn JL, Herscovitch P, Barry CE., III 2012. Infection dynamics and response to chemotherapy in a rabbit model of tuberculosis using [(1)(8)F]2-fluoro-deoxy-d-glucose positron emission tomography and computed tomography. Antimicrob. Agents Chemother. 56:4391–4402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Pirofski LA, Casadevall A. 2008. The damage-response framework of microbial pathogenesis and infectious diseases. Adv. Exp. Med. Biol. 635:135–146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Jayaswal S, Kamal MA, Dua R, Gupta S, Majumdar T, Das G, Kumar D, Rao KV. 2010. Identification of host-dependent survival factors for intracellular Mycobacterium tuberculosis through an siRNA screen. PLoS Pathog. 6:e1000839. 10.1371/journal.ppat.1000839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Bryk R, Wu K, Raimundo BC, Boardman PE, Chao P, Conn GL, Anderson E, Cole JL, Duffy NP, Nathan C, Griffin JH. 2011. Identification of new inhibitors of protein kinase R guided by statistical modeling. Bioorg. Med. Chem. Lett. 21:4108–4114 [DOI] [PubMed] [Google Scholar]
- 200.Martineau AR, Wilkinson RJ, Wilkinson KA, Newton SM, Kampmann B, Hall BM, Packe GE, Davidson RN, Eldridge SM, Maunsell ZJ, Rainbow SJ, Berry JL, Griffiths CJ. 2007. A single dose of vitamin D enhances immunity to mycobacteria. Am. J. Respir. Crit. Care Med. 176:208–213 [DOI] [PubMed] [Google Scholar]
- 201.Yamshchikov AV, Desai NS, Blumberg HM, Ziegler TR, Tangpricha V. 2009. Vitamin D for treatment and prevention of infectious diseases: a systematic review of randomized controlled trials. Endocr. Pract. 15:438–449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Condos R, Rom WN, Schluger NW. 1997. Treatment of multidrug-resistant pulmonary tuberculosis with interferon-gamma via aerosol. Lancet 349:1513–1515 [DOI] [PubMed] [Google Scholar]