Summary
Categorical choices are preceded by the accumulation of sensory evidence in favour of one action or another. Current models describe evidence accumulation as a continuous process occurring at a constant rate, but this view is inconsistent with accounts of a psychological refractory period during sequential information processing. During multi-sample perceptual categorisation, we found that the neural encoding of momentary evidence in human electrical brain signals and its subsequent impact on choice fluctuated rhythmically according to the phase of ongoing parietal delta oscillations (1-3 Hz). By contrast, lateralised beta-band power (10-30 Hz) overlying human motor cortex encoded the integrated evidence as a response preparation signal. These findings draw a clear distinction between central and motor stages of perceptual decision making, with successive samples of sensory evidence competing to pass through a serial processing bottleneck before being mapped onto action.
Keywords: perception, decision making, cortical oscillations, phase coding, human electrophysiology, EEG, psychophysics
Introduction
Integrating multiple samples of evidence helps optimise behaviour by allowing the true state of the environment to be estimated more precisely (Wald and Wolfowitz, 1949; Bogacz et al., 2006). Current decision-theoretic models assume that momentary evidence is accumulated at a constant rate in the form of a decision variable, a quantity that maps the integrated evidence onto an appropriate action (Link, 1975; Ratcliff and Smith, 2004). These linear integration models have drawn support from neurophysiological recordings in the non-human primate which have demonstrated a gradual build-up of neuronal firing rates in the lateral intraparietal cortex (LIP) during evidence accumulation (Shadlen and Newsome, 2001; Roitman and Shadlen, 2002; Gold and Shadlen, 2003, 2007). This work has led to the prevailing view that sensory information is converted fluidly and continuously into action, with the encoding of momentary evidence and its integration in sensorimotor cortex forming an indivisible precursor to choice.
However, the notion that sensory evidence is integrated linearly and continuously is at odds with a rich psychological literature describing how human perception is limited by a central processing bottleneck (Marois and Ivanoff, 2005), giving rise to a psychological refractory period of a few hundreds of milliseconds during which relevant sensory information is perceived as lagging (Pashler, 1984) or even missed (Raymond et al., 1992). One intuitive explanation for these refractory periods is that humans are constrained to sample the environment discretely in rhythmic frames lasting up to hundreds of milliseconds (VanRullen and Koch, 2003) – thereby allocating processing resources to incoming sensory information depending on its position within the sampling cycle (Busch and VanRullen, 2010). In accordance with this rhythmic sampling view, an emerging neurophysiological framework proposes that slow cortical oscillations in the delta band (1-3 Hz) can serve as instruments of attentional selection by modulating rhythmically the gain of information processing (Lakatos et al., 2008; Schroeder and Lakatos, 2009). However, these temporally structured slow fluctuations in neural excitability have only been observed in early sensory cortex, and at frequencies that match the presentation rate of relevant stimuli, making it unclear whether they reflect a temporal constraint on sequential information processing.
One central prediction arising from this rhythmic account of information processing is that humans should exhibit slow rhythmic fluctuations in their rate of evidence accumulation during decision making – in other words, that samples of evidence which strongly influence choice should be succeeded by a refractory period during which new samples have a weaker impact on the same choice. Critically, this push-pull pattern of decision ‘weighting’ should follow the phase of cortical delta oscillations. Here we tested these predictions by recording human electroencephalographic (EEG) signals during a perceptual categorisation task that required the integration of multiple samples of evidence over time. We found that the neural encoding of individual samples of evidence and their decision weight on a categorical choice occurring several hundreds of milliseconds later fluctuated rhythmically according to the phase of delta oscillations overlying human parietal cortex.
Results
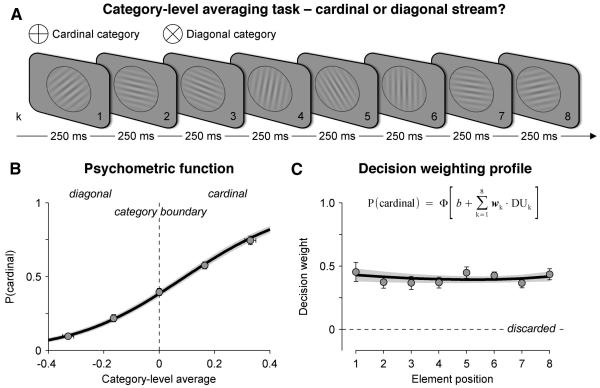
We recorded scalp EEG signals from 15 human participants who viewed a rapid stream of eight oriented Gabor patterns presented at a rate of 4 Hz (Figure 1A). Following each stream, participants reported whether, on average, the tilt of the eight elements fell closer to the cardinal or diagonal axes. We defined two parametric quantities indexing two types of information provided by each element on each trial: (i) the perceptual update (or PUk), corresponding to the absolute difference in tilt between a given element k and the previous element k−1, and (ii) the decision update (or DUk), corresponding to the amount of categorical evidence provided by element k.
Figure 1. Experimental design and behaviour.
(A) Category-level averaging task. Rapid visual streams of eight oriented Gabor patterns were presented at a stimulation rate of 4 Hz. Participants reported whether, on average, the tilt of the eight elements fell closer to the cardinal or diagonal axes.
(B) Psychometric function relating the category-level average (x-axis), divided into five equally spaced levels, to the probability of judging the stream as being cardinal (y-axis). Dots and attached error bars indicate participants’ data (mean ± s.e.m.), the black line (and its shaded error bar indicating s.e.m) indicates the best-fitting cumulative normal function estimated via a logistic regression of choice against the category-level average.
(C) Decision weighting profile across the eight elements. Individual decision weights are estimated using a multivariate logistic regression of choice against a linear combination of the eight decision updates (inset equation). Dots and attached error bars indicate participants’ data, the black line (and its shaded error bar) indicates the best-fitting exponential profile. Same conventions as in (B).
In other words, perceptual updates reflect how much each new element differs visually from the previous one – i.e., the successive visual transients occurring at the onset of each new element, whereas decision updates reflect how much each new element differs from the decision criterion – i.e., the incremental quantity that the internal decision variable should be updated with (Figure S1). Critically, the use of a cardinal/diagonal decision axis ensured that PUk and DUk were uncorrelated across trials – i.e., two elements could give rise to identical perceptual updates, but different decision updates (see Experimental Procedures and Figure S1). Perceptual updates are irrelevant to perform the task, whereas decision updates correspond to the quantity that subjects should integrate over time: the sum of the eight decision updates, each signed with its corresponding category. Finally, we ensured that successive decision updates were not correlated across trials by sampling them randomly from independent uniform distributions (Figure S1): this feature allowed us to regress individual decision weights with the highest statistical power.
Categorisation accuracy was titrated for each participant prior to the experiment by adjusting the average categorical evidence available at the end of the trial over five evenly spaced levels (Figure 1B). We then estimated the decision weight (or wk) associated with each element k, defined as its multiplicative contribution to the subsequent choice. We calculated these weights across trials via a multivariate parametric regression of choice on the basis of a linear combination of the eight decision updates:
where P(cardinal) corresponds to the probability of judging the stream as cardinal, ϕ to the cumulative normal function, and b to an additive response bias towards one of the two categories. We found that decision weights were all positive (t-test against zero, all p < 0.001) and statistically indistinguishable across successive elements (repeated-measures ANOVA, F7,98 < 1, p > 0.5), indicating that across trials, participants weighted the eight elements equally, irrespective of their position within the stream (Figure 1C).
We further tested whether successive elements contributed independently to the final choice – e.g., whether past decision updates did not influence the contribution of future elements to the final choice. We found that subjects indeed used the decision information provided by successive elements in an orthogonal fashion (Figure S1): for any given element, the magnitude of previous and next decision updates did not influence the contribution of the current element to choice (see Supplemental Information).
Neural encoding of perceptual and decision updates
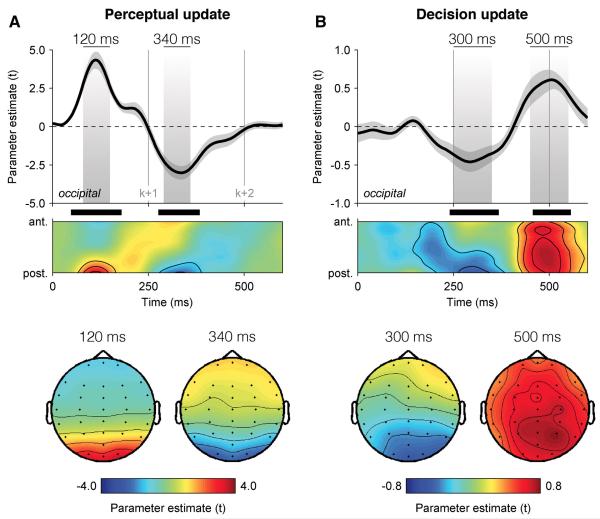
We began by identifying the neural correlates of perceptual and decision updates by regressing single-trial EEG signals, filtered at 1-16 Hz, against these two parametric quantities at successive time samples following the onset of each element. The resulting encoding time courses are not event-related potentials, but estimates of the extent to which single-trial EEG signals encode PUk and DUk in a parametric fashion (see Experimental Procedures).
This analysis revealed spatially and temporally distinct correlates of perceptual and decision updates (Figure 2). The encoding of PUk peaked at 120 ms following the onset of element k at occipital electrodes (t-test against zero, peak t14 = 8.2, cluster-level p < 0.001), whereas the encoding of DUk showed a negative component at 300 ms followed by a positive one at 500 ms, the latter being more distributed across the scalp but peaking at parietal electrodes (peak t14 = 5.6, cluster-level p < 0.001). In other words, elements were processed perceptually before 100 ms, and converted into decision-relevant signals by 250 ms.
Figure 2. Dissociable neural encoding of perceptual and decision updates.
(A) Neural encoding of perceptual update PUk in EEG signals, expressed as parameter estimate in t units. Upper panel: encoding time course at occipital electrodes (above), and following a spatial antero-posterior gradient from electrode FPz to electrode Oz (below). Shaded areas highlight positive and negative components peaking respectively at 120 ms (left) and 340 ms (right) following element k. Lower panel: encoding scalp topographies at 120 ms (left) and 340 ms (right) following element k. Shaded error bars indicate s.e.m. Thick black lines indicate cluster-level significance at p < 0.001.
(B) Neural encoding of decision update DUk in EEG signals. Upper panel: encoding time course at occipital electrodes (above), and following the antero-posterior gradient (below). Shaded areas highlight negative and positive components peaking respectively at 300 ms and 500 ms following element k. Lower panel: encoding scalp topographies at 300 ms (left) and 500 ms (right) following element k. Same conventions as in (A).
The fact that the encoding of each perceptual/decision update was not completed by 250 ms – i.e., at the onset of the next element, suggests that the encoding of successive updates was partially overlapping (Figure S2). To confirm this, we entered simultaneously previous, current and next perceptual/decision updates as multiple regressors of single-trial EEG signals (see Supplemental Information), and observed overlapping encoding time courses that were indistinguishable from those obtained via univariate regression. Importantly, this finding demonstrates that the neural encoding of element k following the onset of element k+1 is not contaminated by the neural encoding of element k+1.
Neural decoding of individual decision weights
Subsequently, we estimated the extent to which the neural encoding of decision updates for each element k predicted the decision weight wk assigned to that element in the eventual choice. This decoding analysis measures the subjective choice-predictive information available in neural encoding signals, over and above the objective categorical information provided by each element. Capitalising on trial-to-trial variability in the encoding of DUk, we tested whether encoding residuals – trial-to-trial fluctuations in EEG signals unexplained by DUk – co-varied with wk across trials (see Experimental Procedures). We calculated the strength wk,t of this psycho-physiological interaction at successive time points following each element k via a multivariate parametric regression for which the interaction between each decision update DUk and the corresponding encoding residuals rk,t at time t was modelled as an additional predictor of choice:
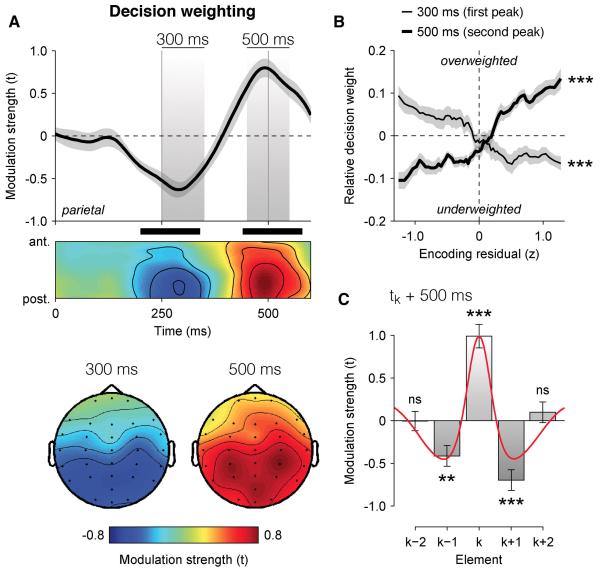
The time course of this psycho-physiological modulation wk,t (Figure 3A) matched that observed for the neural encoding of DUk, with a negative component at 300 ms followed by a positive one at 500 ms at parietal electrodes (t-test against zero, peak t14 = 7.2, cluster-level p < 0.001). In other words, elements for which the neural encoding of DUk was stronger were overweighted in the subsequent choice, whereas elements for which the neural encoding of DUk was weaker were underweighted (Figure 3B).
Figure 3. Neural decoding of individual decision weights.
(A) Neural decoding of multiplicative decision weighting in EEG signals, expressed as modulation strength in t units. Upper panel: decision weight decoding time course at parietal electrodes (above), and following the antero-posterior gradient (below). Shaded areas highlight negative and positive components peaking respectively at 300 ms and 500 ms following element k. Lower panel: decoding scalp topographies at 300 ms (left) and 500 ms (right) following element k. Shaded error bars indicate s.e.m. Thick black bars indicate cluster-level significance at p < 0.001.
(B) Relationship between encoding residuals at 300 ms (thin) and 500 ms (thick) following element k at parietal electrodes, binned in 64 overlapping quartiles, and the relative (meansubtracted) decision weight wk. Shaded error bars indicate s.e.m.
(C) Neural decoding of successive decision weights assigned to elements k−1 and k+1 at 500 ms following element k. Error bars indicate s.e.m. Two stars indicate a significant modulation at p < 0.01, three stars at p < 0.001, ns a non-significant modulation. The red line indicates the raw auto-correlation profile of parietal EEG signals.
Our main hypothesis was that the weighting of momentary evidence during its accumulation would fluctuate rhythmically. We thus assessed whether the strength of the neural encoding of DUk also influenced the decision weights associated with temporally adjacent elements in the stream (Figure 3C and Figure S3). Consistent with a fluctuating gain of evidence accumulation, the neural encoding of DUk was inversely related to the decision weight wk+1 associated with the subsequent element. In other words, a stronger neural encoding of DUk predicted not only the overweighting of the current element, but also the underweighting of the next element presented 250 ms later. This inverse relationship was maximal at parietal electrodes at 500 ms following element k (t-test against zero, t14 = −6.0, p < 0.001). This ‘push-pull’ pattern of decision weighting was also significant for the previous element k−1 (t14 = −3.8, p = 0.001), but not for further elements k−2 and k+2 (both p > 0.1), indicating that this competitive interaction between decision weights was focal in time – i.e., strongest for immediately adjacent elements in the stream.
Slow rhythmic fluctuations in neural encoding and decision weighting
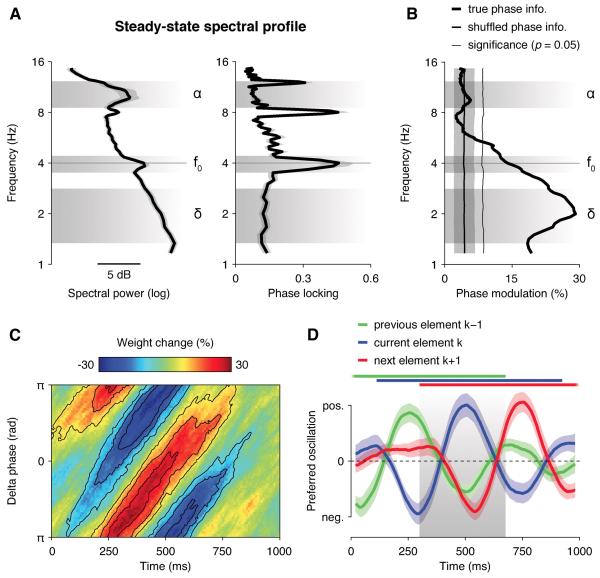
If momentary evidence is sampled in a rhythmic fashion, then the neural encoding of DUk and its decision weight wk should depend on the phase of slow cortical oscillations, possibly in the delta band (1-3 Hz) where the period matches the refractory pattern of decision weighting observed across successive elements (Figure 3C).
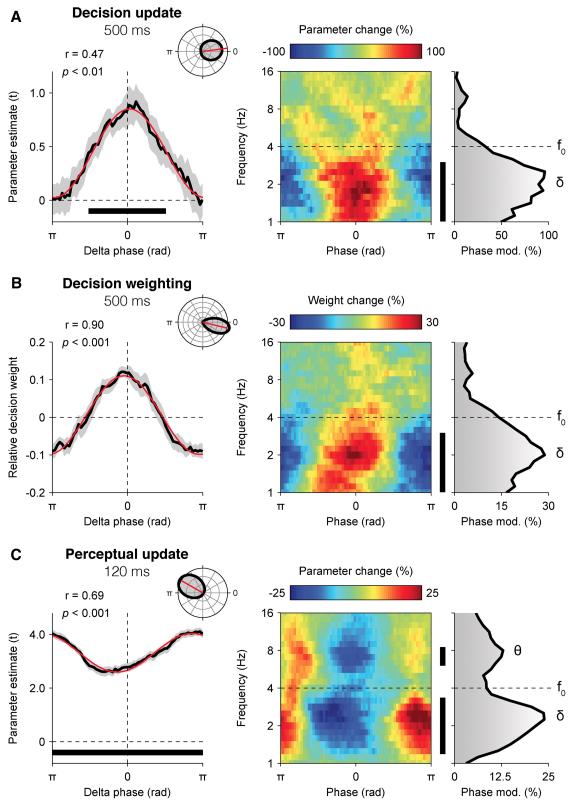
We thus assessed whether the phase of EEG oscillations between 1 and 16 Hz influenced the neural encoding of DUk (see Experimental Procedures). Sorting single trials according to their phase at 2 Hz measured at 500 ms following element k at parietal electrodes (Figure 4A and Figure S4), we observed that the neural encoding of DUk was stronger at the peak and weaker at the trough of the delta cycle at 2 Hz (Rayleigh test, r14 = 0.47, p = 0.01). Importantly, the decision weight wk assigned to element k also depended on delta phase (Figure 4B), following the same phase relationship (r14 = 0.90, p < 0.001) – in other words, the samples of evidence that fell at the preferred delta phase were encoded more strongly and overweighted in the subsequent choice. By contrast, those that fell in the opposite delta phase – typically the following element presented 250 ms later – were poorly encoded and underweighted in the same choice. This phasic modulation of decision weighting was significant for all elements (t-test against zero, all p < 0.05), and did not interact with the position of element k (repeated-measures ANOVA, F7,98 < 1, p > 0.5), or with the amount of categorical evidence available at the end of the stream (F2,28 < 1, p > 0.2).
Figure 4. Slow rhythmic fluctuations in neural encoding and decision weighting.
(A) Phase-dependent fluctuations in the neural encoding of decision updates at parietal electrodes. Left panel: relationship between delta phase and the encoding of decision update DUk at 500 ms following element k at parietal electrodes. Inset: distribution of preferred phase across participants (0: peak, π: trough). Shaded error bars indicate s.e.m. The red sinusoid indicates the best fit. The thick black line indicates significant neural encoding at p < 0.01 (two-tailed). Right panel: relationship between the phase of EEG oscillations form 1 to 16 Hz and the neural encoding of decision update DUk at 500 ms following element k at parietal electrodes. Phase modulation corresponds to the relative amplitude of the best sinusoidal fit. f0 indicates stimulation frequency (4 Hz).
(B) Phase-dependent fluctuations in decision weight wk at 500 ms following element k at parietal electrodes. Same conventions as in (A).
(C) Phase-dependent fluctuations in the neural encoding of perceptual update PUk at 120 ms following element k at occipital electrodes. Same conventions as in (A).
For completeness, we also assessed whether the phase of EEG oscillations between 1 and 16 Hz influenced the neural encoding of perceptual updates (Figure 4C and Figure S4). As observed for DUk, we found that the neural encoding of PUk at 120 ms following element k at occipital electrodes co-varied with delta phase at 2 Hz (Rayleigh test, r14 = 0.69, p < 0.001). However, in contrast to DUk, the neural encoding of PUk was strongest at the trough and weakest at the peak of the delta cycle, and also depended on theta phase at 8 Hz (r14 = 0.45, p < 0.05) following the same phase relationship, thereby matching previous observations (Busch and VanRullen, 2010; Stefanics et al., 2010).
To verify that this phasic effect at 2 Hz was not a consequence of our rhythmic presentation rate, we calculated steady-state spectral power and phase locking across trials between 1 and 16 Hz and found anticipated peaks at the stimulation frequency (4 Hz) and its higher harmonics, but no peak in the delta band (Figure 5A). Subtracting the average steady-state broadband response from the EEG data (Figure S5) before estimating delta phase did not change the observed pattern of results, either qualitatively or quantitatively. Slow fluctuations in decision weighting thus followed the phase of endogenous, non-phase-locked delta oscillations, not the phase of a fixed subharmonic of the stimulation frequency. Importantly, shuffling phase information across trials confirmed that this phasic modulation of decision weighting could not be due to the entrainment of EEG oscillations to the stimulation frequency: indeed, shuffling phase information kept phase locking constant, but fully abolished the phasic modulation of decision weighting (Figure 5B).
Figure 5. Oscillatory properties of slow fluctuations in decision weighting.
(A) Steady-state spectral power (left) and phase locking (right) across trials between 1 and 16 Hz, averaged across occipital and parietal electrodes, showing anticipated peaks at stimulation frequency f0 and its higher harmonics, but no peak in the delta band (1-4 Hz). Shaded error bars indicate s.e.m.
(B) Phase modulation of decision weight wk at 500 ms following element k. The thick black line indicates modulation strength using the true phase information, the thinner black line (mean ± s.d.m.) using trial-shuffled phase information. The thin black line indicates the boot-strapped p = 0.05 significance threshold (two-tailed).
(C) Relationship between delta phase, estimated as the analytic phase of band-pass-filtered EEG signals between 1 and 4 Hz, and decision weight wk between 0 and 1000 ms following element k. The preferred delta phase with respect to decision weight wk follows a damped delta oscillation at 2 Hz peaking around 500 ms following element k.
(D) Overlapping oscillations with opposite preferred phases for current (blue) vs. previous (green) and next (blue) elements. Shaded error bars indicate s.e.m. Thick horizontal lines indicate significant time windows (p < 0.05, two-tailed) for previous, current and next elements, overlapping at 300-650 ms following element k.
Transient changes in neural signals can resemble oscillations when analysed using Fourier-based decompositions. To further test whether the observed fluctuations in decision weighting reflected a truly cyclic process, not just a transient change in broadband EEG signals, we first varied the temporal spread σ of the Gaussian envelope used to estimate delta phase, and measured the temporal spread for which the effect of parietal delta phase on wk was strongest at 500 ms following element k (see Experimental Procedures). This analysis identified an optimal temporal spread of 4 cycles – i.e., a full width at half maximum of 750 ms, indicating that the slow fluctuations in decision weighting spanned more than one delta cycle. Besides, time-frequency decompositions of transient changes in EEG signals typically show low frequency specificity, spanning frequencies across multiple octaves. Here, by contrast, the phasic modulation of decision weighing was fully circumscribed to the delta range (Figure S5), consistent with a genuinely rhythmic process.
Finally, we re-estimated delta phase using a non-Fourier-based approach, namely, the Hilbert transform, and obtained the same phasic modulation of decision weighting. To do so, we band-pass-filtered single-trial EEG signals between 1 and 4 Hz and estimated the analytic phase of the EEG signals at each time point from 0 to 1000 ms following element k at parietal electrodes (see Experimental Procedures). The preferred phase with respect to the decision weight wk shifted linearly over time from 100 to 750 ms following element k – hence spanning more than one delta cycle and confirming that the phasic modulation of decision weighting is not due to a single transient change in EEG signals (Figure 5C). Besides, entering simultaneously previous (k−1), current (k), and next (k+1) elements as separate interaction terms showed overlapping influences of delta phase on the weighting of successive elements from 300 to 650 ms following element k (p < 0.05), with opposite preferred phases for current vs. previous/next elements (Figure 5D).
Endogenous, not exogenous dynamics of neural encoding and decision weighting
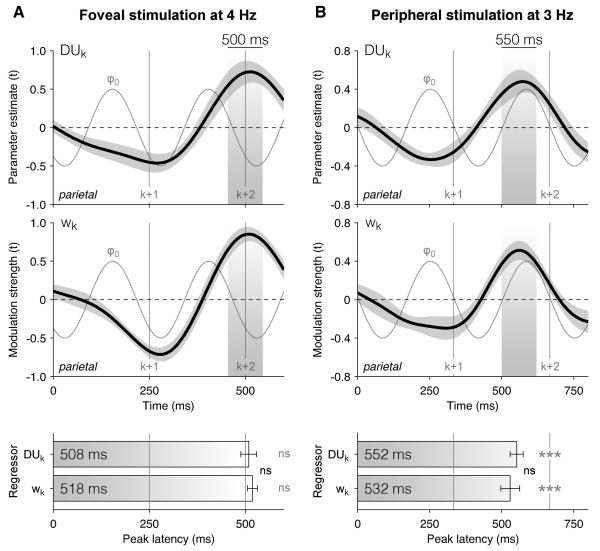
Several features of the data strongly suggest that the phasic modulation of neural encoding and decision weighting was not occurring at a fixed subharmonic of the 4 Hz stimulation rate. Nevertheless, we sought to confirm that the time courses of neural encoding (Figure 2) and decision weighting (Figure 3) also reflected endogenous cortical dynamics, rather than being mainly driven by the stimulation frequency f0.
To do so, we obtained additional EEG data from an independent group of 17 participants who performed the same categorisation task at a different stimulation rate of 3 Hz (see Supplemental Information). We compared the estimated neural encoding and decision weighting time courses between these two datasets (Figure 6 and Figure S6). At both stimulation rates, the peak latencies of neural encoding and decision weighting did not differ significantly (paired t-test, both p > 0.5). And critically, we found no difference in peak latencies for neural encoding and decision weighting between the two stimulation rates (two-sample t-test, neural encoding: 508 ± 20 ms at 4 Hz, 552 ± 22 ms at 3 Hz, t30 = 1.4, p > 0.1, decision weighting: 518 ± 12 ms at 4 Hz, 532 ± 34 ms at 3 Hz, t30 < 1, p > 0.5). Furthermore, while the neural encoding and decision weighting profiles for element k peaked around the onset of element k+2 at a stimulation rate of 4 Hz (t-test against 500 ms, neural encoding: t14 < 1, p > 0.5, decision weighting: t14 = 1.4, p > 0.1), they peaked significantly earlier than the onset of element k+2 at 3 Hz (t-test against 667 ms, neural encoding: t16 = −5.1, p < 0.001, decision weighting: t16 = −4.0, p = 0.001). The relative stability of peak latencies across stimulation frequencies confirms that the two profiles do not follow a fixed subharmonic of f0.
Figure 6. Preserved neural encoding and decoding profiles at different stimulation rates.
(A) Neural encoding and decoding profiles at a stimulation rate of 4 Hz. Upper panels: neural encoding (of decision update DUk) and decoding (of decision weight wk) profiles estimated from delta-band-filtered EEG signals (1-4 Hz) at parietal electrodes. Shaded areas highlight the positive encoding/decoding component peaking at 500 ms following element k. The thin grey line indicates the phase-locked oscillation ϕ0 at the stimulation frequency. Shaded error bars indicate s.e.m. Lower panel: peak latencies of the positive encoding/decoding component. Error bars indicate s.e.m. Three stars indicate a significant latency difference at p < 0.001, ns a non-significant latency difference.
(B) Preserved neural encoding and decoding profiles obtained from an independent dataset at a stimulation rate of 3 Hz. Same conventions as in (A). Note that the positive encoding/decoding components peak at different parts of the phase-locked oscillation ϕ0 between the two stimulation frequencies.
Neural encoding of the integrated evidence in motor beta-band activity
Previous non-invasive studies in humans have identified a different neural correlate of evidence accumulation, in the form of lateralised beta-band power (10-30 Hz) over the motor cortex preceding a left- or right-handed response (Donner et al., 2009). However, it remains unclear whether this neural signal contributes to the weighting of momentary evidence, or rather reflects its downstream integration as a response preparation signal.
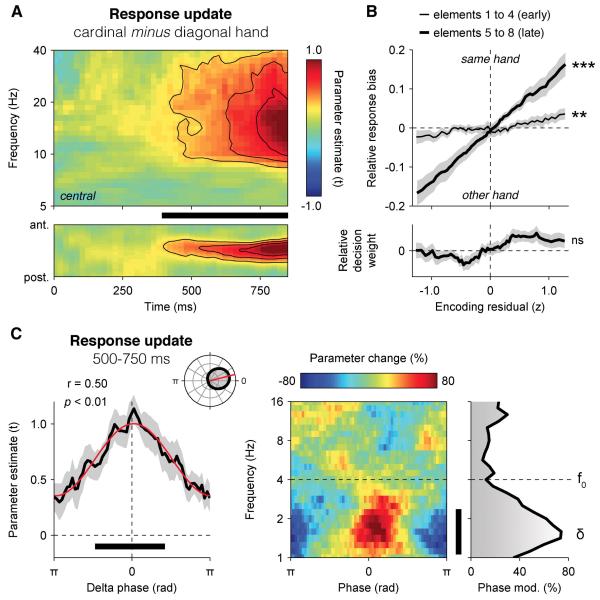
To arbitrate between these two possibilities, we carried out further analyses. First, we assessed the neural encoding of response updates – i.e., decision updates signed according to the stimulus-response mapping used by each participant, in lateralised beta-band power. In other words, we estimated the extent to which inter-hemispheric differences in beta-band activity (see Experimental Procedures) co-varied with response update RUk across trials at successive time samples following element k (Figure 7A). The neural encoding of RUk in motor beta-band activity (10-30 Hz) ramped up gradually from 500 ms onwards at central electrodes (500-750 ms: t-test against zero, t14 = 3.4, p < 0.01), notably later than its encoding in broadband signals at parietal electrodes (Figure 2B). This sustained encoding of successive response updates in motor beta-band activity contrasts sharply with the transient encoding of successive decision updates observed in parietal broadband signals.
Figure 7. Neural encoding of the running decision variable in motor beta-band activity.
(A) Neural encoding of response update RUk in motor beta-band activity (10-30 Hz), expressed as parameter estimate in t units. Upper panel: encoding time-frequency profile at motor electrodes (above), and following the antero-posterior gradient (below). The thick black bar indicates cluster-level significance at p < 0.001.
(B) Relationship between encoding residuals in motor beta-band activity and the relative response bias (upper panel) or decision weight (lower panel) at 500-750 ms following early elements 1-4 (thin line) and late elements 5-8 (thick line). Shaded error bars indicate s.e.m. Two stars indicate a significant correlation at p < 0.01, three stars at p < 0.001, ns a non-significant correlation.
(C) Phase-dependent fluctuations in the neural encoding of response update RUk in motor beta-band activity (10-30 Hz). Left panel: relationship between the phase of parietal delta oscillations at 500 ms following element k and the encoding of RUk in motor beta-band activity at 500-750 ms. Right panel: relationship between the phase of parietal EEG oscillations from 1 to 16 Hz at 500 ms following element k and the neural encoding of response update RUk in motor beta-band activity. Same conventions as in Figure 4.
We then asked whether the neural encoding of RUk in motor beta-band activity predicted the multiplicative decision weight wk assigned to element k in the subsequent choice, or instead co-varied with an additive change in response bias – i.e., the probability of a left- or right-handed response irrespective of element k (see Experimental Procedures). To this end, we again related trial-to-trial variability in neural encoding to variability in choice. But in this psycho-physiological analysis, choice was predicted via two separate modulatory terms: (i) the interaction between each decision update DUk and the corresponding encoding residuals rk,t at time t (parameterised by wk,t), and (ii) the main effect of encoding residuals rk,t at time t (parameterised by bk,t):
Consistent with a response preparation signal, we found that encoding residuals following element k predicted bk,t (500-750 ms, t-test against zero, t14 = 6.7, p < 0.001), but not wk,t (t14 = −1.6, p > 0.1) – indicating that motor beta-band activity had an additive, not a multiplicative, influence on decision making (Figure 7B). In accordance with this interpretation, the strength of this psycho-physiological modulation increased parametrically across successive elements (repeated-measures ANOVA, F7,98 = 18.6, p < 0.001) – i.e., with temporal proximity from the motor response. This is to be expected from a response preparation signal driven by large temporal fluctuations in sensory input (Yang and Shadlen, 2007).
We carried out additional analyses locked to the onset of the response period, which all confirmed that motor beta-band activity behaved as a response preparation signal (Figure S7): (i) the neural encoding of the sum of response updates distinguished correct choices from errors from more than 500 ms before the onset of the response period (paired t-test, t14 = 4.8, p < 0.001), (ii) the neural decoding of choice (i.e., left- vs. right-handed response) showed similar predictive profiles preceding correct choices and errors (see Supplemental Information), and (iii) the between-element variability in neural encoding of response updates correlated positively with the between-element weighting profile estimated behaviourally (r = +0.44 ± 0.10, t-test against zero, t14 = 4.4, p < 0.001).
Finally, we assessed whether the neural encoding of DUk in motor beta-band activity also fluctuated rhythmically according to the phase of parietal delta oscillations (Figure 7C), and found that it followed the same phase relationship as its earlier encoding in broadband parietal signals (Rayleigh test, r14 = 0.50, p < 0.01). This phase dependency suggests that motor beta-band activity reflects a computation which occurs downstream from the weighting of momentary evidence according the phase of parietal delta oscillations.
Discussion
Together, these findings chart the electrophysiological substrates of the sensorimotor cascade whereby successive samples of sensory evidence are processed from lower to higher levels, integrated, and converted into an appropriate response. By linking trial-to-trial fluctuations in neural signals to variability in choice, these findings draw a clear distinction between the computations performed by two neural mechanisms during categorical decision-making. Firstly, momentary evidence undergoes a multiplicative weighting according to the phase of ongoing delta oscillations (1-3 Hz) overlying human parietal cortex. Subsequently, lateralised beta-band activity (10-30 Hz) over the motor cortex integrates the weighted evidence in an additive fashion, consistent with the formation of a decision variable. Categorical choices are thus preceded by discrete central and motor stages, both of which follow an early perceptual stage confined to early visual cortex. These findings thus call into question the widely held view that evidence accumulation is indistinguishable from the gradual engagement of a response effector – in other words, that the neural encoding of decision-relevant evidence reduces to a preparatory signal that precedes motor output (Shadlen and Newsome, 2001; Roitman and Shadlen, 2002; Gold and Shadlen, 2003, 2007).
Moreover, while current decision-theoretic models describe evidence accumulation as a linear process whereby successive samples of evidence are integrated at a constant rate over hundreds of milliseconds (Ratcliff and Smith, 2004; Gold and Shadlen, 2007), we show that both the encoding of momentary evidence in the human EEG and its impact on decision-making fluctuate in a rhythmic fashion: samples of evidence processed in the preferred delta cycle are overweighted, and followed by underweighted samples processed in the opposite cycle 250 ms later. The timing of this push-pull pattern of decision weighting forges a link between a recent decision-making literature and classic psychological accounts of capacity limits in human perception (Pashler, 1984; Raymond et al., 1992; Marois and Ivanoff, 2005). Our findings suggest that the serial attentional bottleneck identified as responsible for refractory phenomena such as the attentional blink (Sergent et al., 2005; Sigman and Dehaene, 2008; Tombu et al., 2011) might impose a general sampling constraint on decision-making over several hundreds of milliseconds. This finding points to a previously unaccounted-for source of variability in human decisions, and imposes an important limitation on decision-theoretic models – such as diffusion or ‘race’ models – in which successive samples are totted up linearly towards a decision bound, suggesting that they are a suitable descriptor of sensorimotor decisions only when occurring over very short timescales.
Our findings invite obvious parallels with a literature describing how a second target stimulus (T2) is often missed if it occurs shortly after a first target stimulus (T1), not least because the attentional blink is maximal when T1 and T2 are separated by approximately 250 ms, or ‘lag-2’ (Raymond et al., 1992) – i.e., the peak-to-trough latency with respect to a 2 Hz cycle. Even more notably, the finding that decision weighting fluctuates rhythmically at approximately 2 Hz is consistent with a related finding, namely that when T2 follows T1 at a very short latency (e.g., 125 ms), then it is less likely to be missed, a phenomenon known as ‘lag-1 sparing’ (Chun and Potter, 1995). Assuming that the phase of ongoing delta oscillations is at least partially reset by the occurrence of T1, then an unexpected T2 occurring at 125 ms post-T1 (lag-1) will fall in the waning portion of the delta cycle, whereas a T2 occurring at 250 ms (lag-2) will fall close to its nadir, such that T2 is more likely to be processed (and hence detected) at lag-1 than at lag-2. Finally, subliminal effects of ‘blinked’ stimuli on subsequent decisions have been interpreted as indicating a preserved perceptual processing of blinked stimuli (Dehaene et al., 2006). Accordingly, we find that delta phase has a much stronger modulatory influence on the encoding of decision-relevant information than that of perceptual information.
However, our findings also offer an explanation for a phenomenon that has long puzzled researchers interested in modelling decision latencies – that serial models of the decision process (such as diffusion-to-bound models) can only account for the relative speeds of errors and correct choices if drift rate (i.e., the rate of evidence accumulation) is allowed to vary across trials. Varying drift rate randomly across successive trials allows different admixtures of trials with high and low drift rates to drive correct and incorrect choices, leading to the widely observed phenomenon that decision latencies for errors typically exceed those for correct choices (Ratcliff and Rouder, 1998). Even within each trial, the gradual build-up of neuronal firing rates in sensorimotor cortex is known to vary stochastically in a fashion that predicts the dynamics of the eventual movement (Hanes and Schall, 1996). Our findings suggest a neurophysiological explanation for these two phenomena – that the rate of evidence accumulation varies within the course of a single trial according to the phase of ongoing slow cortical oscillations.
Measuring the temporal spread of this cyclic modulation of information processing revealed that it lasted for several delta cycles, and was thus not simply a transient, discrete activity evoked by each element. Nevertheless, the influence of parietal delta phase on decision weighting tapered off quite rapidly, indicating that this rhythmic mechanism is not a rigid oscillatory mechanism, but rather can be flexibly aligned to account for the changing demands of information processing – e.g., become entrained to the onset of relevant stimuli when they are presented at predictable times at delta-band (< 3 Hz) stimulation frequencies (Lakatos et al., 2008; Schroeder and Lakatos, 2009; Stefanics et al., 2010). However, the strongest competition was observed between a given element and its immediate neighbours – within each delta cycle – as expected if successive samples of information were competing to pass through a serial processing bottleneck.
Finally, these findings shed light on the role of slow cortical oscillations in sensory selection (Lakatos et al., 2008; Schroeder and Lakatos, 2009; Stefanics et al., 2010). Previous research has demonstrated that the encoding of sensory information depends on the phase of delta oscillations in sensory cortex, but because stimulation occurred in the delta band, it is hard to tell whether this modulation was dependent on being driven exogenously (entrained) by the external stimulation rhythm. Here we show that the neural encoding of both perceptual and categorical information in the human EEG was modulated by an internal rhythm distinct from the stimulation frequency. This finding demonstrates unambiguously that the selection of perceptual and categorical information is dependent on endogenous delta oscillations, not on the entrainment of neural oscillations to any stimulation frequency. Moreover, although the source of observed neural activity is hard to pinpoint with scalp EEG recordings, we observed the encoding and decoding of decision information relatively diffusely across the scalp, with maxima over the parietal cortex, suggesting that slow cortical oscillations contribute to information processing beyond primary sensory cortices.
One possibility is that this rhythmic sampling mechanism may have evolved to ensure that the neural processing of currently available information is not corrupted by potentially distracting information arriving in its immediate wake. It might also be that slow reverberatory activity may inject stochasticity into a neural circuitry that, coupled with attractor dynamics, helps mediate the trade-off between exploratory and exploitative behaviour (Soltani and Wang, 2008, 2010). Interestingly, unlike the neural encoding of decision-relevant information which depended exclusively on the phase of delta oscillations, the gain of visual responses also followed the phase of faster cortical rhythms around 8 Hz. This finding is consistent with recent reports that evoked visual responses and signal detectability depend on the phase of EEG oscillations in this frequency range in humans (Busch et al., 2009; Wyart and Sergent, 2009; Scheeringa et al., 2011). The particular frequency of fluctuations in neural excitability may reflect the predominant time constants of synaptic activity in the corresponding cortical area (Wang, 2010; Bernacchia et al., 2011).
To conclude, we found that during extended categorical decisions, the rate of evidence accumulation fluctuates over time, in a fashion that can be predicted from the ongoing phase of slow EEG oscillations in the delta band (1-3 Hz) overlying human parietal cortex. Large-scale delta oscillations thus appear as an excellent candidate substrate for the serial attentional bottleneck known to give rise to a range of cognitive phenomena such as the attentional blink and the psychological refractory period. These findings suggest that slow rhythmic changes in cortical excitability form a tight temporal constraint on sequential information processing.
Experimental Procedures
Participants
Sixteen students were recruited from the University of Oxford (age range: 18-25 years). All had normal or corrected-to-normal vision, and reported no history of neurologic or psychiatric disorders. They provided written consent before the experiment and received £30 in compensation for their participation, in addition to bonuses depending on their categorisation performance (approximately £5). The experiment followed local ethics guidelines. The data from one participant was not included because of excessive eye blinks.
Stimuli
Visual stimuli were presented using the Psychophysics-3 toolbox (Brainard, 1997; Pelli, 1997) and additional custom scripts written for MATLAB (The Mathworks, Natick, MA). The display CRT monitor had a resolution of 1024 × 768 pixels, a refresh rate of 60 Hz, and was gamma-corrected using a decoding exponent of 2.2. Participants viewed the stimuli from a distance of approximately 80 cm in a darkened room.
Each trial comprised a sequence of eight centrally presented Gabor patterns presented every 250 ms (4 Hz), preceded by two visual masks and followed by one visual mask presented at the same frequency. All Gabor patterns had identical parameters (contrast: 50%, diameter: 4 degrees of visual angle, spatial frequency: 2 cycles per degree of visual angle, Gaussian envelope s.d.: 1 degree of visual angle), except for their tilt. Masks were created from the linear superposition of the four cardinal and diagonal Gabor patterns. Each stimulus was presented on the screen for 233.3 ms (14 frames), and followed by a blank period of 16.7 ms (1 frame) to avoid visual ‘tearing’ artefacts across successive elements, thus resulting in a stimulus onset asynchrony of 250 ms (i.e., 4 Hz).
Task design
On each trial, the tilt of each Gabor pattern (or element) was drawn randomly from a probability density function whose generating parameters were titrated for each participant prior to the experiment (see below). Across trials, the tilt of each Gabor pattern was distributed uniformly. Following each stream, participants reported whether, on average, the tilt of the eight elements fell closer to the cardinal or diagonal axes. Positive or negative feedback was provided on the basis of the average of eight decision values corresponding to the angular distance between the tilt of each element to the cardinal or diagonal axes, normalised between −1 (diagonal) and +1 (cardinal). The unsigned decision value, or decision update, associated with each element was also distributed uniformly. Trials corresponding to a negative average decision value were associated with the diagonal response, while those corresponding to a positive average decision value were associated with the cardinal response.
Participants responded by pressing either of the two Ctrl keys of a standard keyboard with their left or right index finger, using a cardinal/diagonal response mapping (e.g., cardinal: left, diagonal: right) fully counter-balanced across participants. Auditory feedback was given at the end of each trial – 250 ms following each response – depending on the agreement between the response and the sign of the average decision value (or category-level average) across the eight elements. Increasing pairs of tones (440/880 Hz) followed correct responses, whereas decreasing ones (880/440 Hz) followed errors.
Prior to the experiment, each participant undertook a short practice session, followed by a titration session during which his or her psychophysical threshold – i.e., the unsigned category-level average corresponding to a categorisation accuracy of 75 % – was estimated using an adaptive staircase procedure (Kaernbach, 1991). This threshold estimate was then used to determine five evenly spaced levels of category-level average, from a diagonal to a cardinal average, split into three difficulty levels. Easy cardinal/diagonal trials (1/3 of all trials) corresponded to a categorisation sensitivity d′ of 2.12 ± 0.18 (mean ± s.e.m.), whereas difficult cardinal/diagonal trials (1/3 of all trials) corresponded to a d′ of 1.00 ± 0.09. Neutral trials (1/3 of all trials) corresponded to a null category-level average, and were associated with a pseudo-random feedback, positive on 60 % of neutral trials. The experiment consisted of 672 trials, divided into 7 sessions of 96 trials.
After each session, participants were presented with a wheel of fortune which selected randomly one trial from the block: participants won an additional £1 bonus if their response on that trial was correct. The titration procedure ensured that participants typically won £5 in additional bonuses across the 7 experimental sessions.
EEG acquisition and pre-processing
A Neuroscan system with Synamps-2 digital amplifiers was used to record EEG signals from 32 Ag/AgCl electrodes, located at FP1, FPz, FP2, F7, F3, Fz, F4, F8, FT7, FC3, FCz, FC4, FT8, T7, C3, Cz, C4, T8, TP7, CP3, CPz, CP4, TP8, P7, P3, Pz, P4, P8, POz, O1, Oz, O2, plus four additional electrodes used in a bipolar montage as horizontal and vertical electro-oculograms (EOG), and two electrodes located at the mastoids used as reference. All electrode impedances were kept below 50 kΩ. EEG signals were recorded at a sampling rate of 1000 Hz and high-pass filtered online at 0.1 Hz.
Pre-processing was carried out using the EEGLAB toolbox for MATLAB (Delorme and Makeig, 2004). The data was down-sampled to 250 Hz, band-pass-filtered between 1 and 40 Hz, then epoched from 500 ms before the onset of the first pre-mask to 1 s following the offset of the post-mask (i.e., 1 s following the onset of the response period). We visually inspected these epochs: (i) to remove trials containing non-stereotypical artefacts (such as transient muscular activity), and (ii) to identify ‘bad’ electrode(s) showing frequent amplifier ‘jumps’ or other electrical artifacts (e.g., spikes), which were interpolated to the weighted average of neighbouring electrodes. A maximum of one electrode was identified as bad per participant, and only for three of the fifteen recorded participants.
Independent component analysis (ICA) was then performed on the epoched data as implemented in EEGLAB – excluding the EOG, reference, and interpolated electrodes from the analysis – and ICA components were visually inspected to reject the ones capturing stereotypical artefacts (in particular eye blinks and sustained high-frequency noise). Finally, single epochs were re-inspected visually to ensure that no artefact remained. Rejected trials were excluded from all further analyses, resulting in an average of 565 ± 15 trials per participant (mean ± s.e.m.).
Steady-state frequency spectra were estimated using a standard FFT from the onset of the first element (i.e., following the two pre-masks) until the offset of the last element (i.e., preceding the post-mask). Frequency power was defined as the average square amplitude of complex Fourier components, whereas phase locking was defined as the length of the vector average of single-trial phase estimates.
Time-frequency analyses were carried out using the Fieldtrip toolbox for MATLAB (Oostenveld et al., 2011). The phase of slow EEG oscillations was estimated using either a wavelet transform (Morlet wavelets, frequency range: 1-16 Hz, 4 cycles per window), or a Hilbert transform applied to band-pass-filtered EEG signals in the delta band (1-4 Hz). While both methods provided time-resolved estimates of EEG phase at the single-trial level, the Hilbert transform did not make any assumption regarding the sinusoidal nature of narrow-band EEG signals. The spectral power of beta-band EEG oscillations (>10 Hz) was estimated using a ‘multi-tapering’ time-frequency transform (Mitra and Pesaran, 1999; Pesaran et al., 2002), as implemented in Fieldtrip (Slepian tapers, frequency range: 5-40 Hz, 5 cycles and 3 tapers per window). The purpose of this multi-tapering approach is to obtain more precise power estimates by smoothing across frequencies. Note that both time-frequency transforms use a constant number of cycles across frequencies, hence a time window whose duration decreases inversely with increasing frequency.
For simplicity, we report statistical tests on EEG data averaged across electrode sites. Occipital electrodes correspond to electrodes O1, Oz, and O2. Parietal electrodes correspond to electrodes P3, Pz, P4, and POz. Central/motor electrodes correspond to electrodes C3 and C4 – analysed as their difference to calculate an inter-hemispheric asymmetry index.
EEG analysis – neural encoding of parametric information
We regressed single-trial EEG signals against several parametric quantities associated with individual elements at successive time samples following the onset of the corresponding element. These analyses were carried out separately for each of the eight elements in the stream, then averaged across elements, and finally averaged across participants to produce a group-level grand-average.
For each element k, a general linear regression model (GLM) was used, in which we included the perceptual update PUk and the decision update DUk as two parametric regressors to predict the trial-to-trial variability in EEG signals at a given time t following element k. This parametric regression was done separately at successive times from 0 to 600 ms following element k. The time course of the corresponding parameter estimates – i.e., the normalised best-fitting regression coefficients, expressed in between-trial t units – measured the sensitivity of single-trial EEG signals to perceptual and decision updates. Because these time courses are time series of the between-trial correlation between the EEG and element k, we refer to them as describing the neural encoding of perceptual/decision updates provided by element k.
Baselining for this regression-based analysis was performed by decorrelating the EEG signal at each electrode and each time following the onset of element k from trial-to-trial variability in the EEG signal at the last time sample before the onset of element k. The rationale behind this baselining strategy is that trial-to-trial variability in the EEG signal before the onset of element k could not be related to the encoding of element k. Applying or not this baseline did not change the pattern or overall significance of the observed effects.
A comparable approach was adopted to assess how response-mapped decision updates were encoded in inter-hemispheric beta-band activity (10-30 Hz) at central electrodes. For each participant, we calculated single-trial spectral power from 5 to 40 Hz at electrodes C3 (overlying the left motor cortex) and C4 (overlying the right motor cortex), and subtracted spectral power between these electrodes, C3 minus C4 or C4 minus C3, depending on the cardinal/diagonal response mapping used for each participant: the motor electrode associated with ‘cardinal’ responses (C4 if the participant responded ‘cardinal’ with his or her left index finger, or C3 otherwise) was counted positively, whereas the motor electrode associated with ‘diagonal’ responses was counted negatively.
EEG analysis – psycho-physiological interaction analysis
We used an approach analogous to a psycho-physiological interaction analysis (Friston et al., 1997) to assess the relationship between the encoding of DUk and the decision weight wk assigned to that element in the subsequent categorical choice. We refer to this analysis scheme as a neural decoding approach because it quantifies how trial-to-trial variability in the neural encoding of element k in the EEG – i.e., residuals from the encoding regression described above – co-varied with its decision weighting across trials.
To do so, we quantified whether and how much trial-to-trial fluctuations in EEG signals exerted a modulatory influence on the relationship between the eight decision updates and choice via multivariate parametric regression. In other words, we determined whether EEG-informed regressions of choice led to a significant increase in prediction accuracy. This type of approach is often called psycho-physiological because it assesses how trial-to-trial variability in the EEG (i.e., a physiological variable) modulates the relationship between decision updates and the subsequent categorical choice (i.e., a psychological variable).
Within the general linear model framework, a psycho-physiological modulation can take either of two forms: (i) a multiplicative modulation, or interaction, corresponding to a modulation of the decision weight wk assigned to one (or several) of the eight elements in the subsequent choice, or (ii) an additive modulation, corresponding to a modulation of response bias – i.e., the probability of a ‘cardinal’ or ‘diagonal’ response irrespective of element k. In all psycho-physiological analyses, choice was thus predicted via two separate modulatory terms on top of the weighted decision updates wk. DUk and the overall response bias b entered as offset terms in a multivariate parametric regression: (i) the interaction between each decision update DUk and the corresponding EEG encoding residuals rk,t, and (ii) the main effect of EEG encoding residuals rk,t. Importantly, psycho-physiological analyses assume that EEG encoding residuals co-vary linearly either with the multiplicative decision weight assigned to successive elements, or with the additive response bias in favour of one of the two categories.
This psycho-physiological regression provided parameter estimates for the two modulatory terms: (i) the strength of the multiplicative modulation of the decision weight assigned to element k, and (ii) the strength of the additive modulation of response bias. A non-zero parameter estimate for the multiplicative modulation term indicates that the physiological signal modulates the contribution of the corresponding decision update on choice. By contrast, a non-zero parameter estimate for the additive modulation term indicates that the physiological signal biases responses towards either the cardinal or diagonal category irrespectively of the corresponding decision update.
We first used this psycho-physiological modulatory approach to investigate whether trial-to-trial variability in the neural encoding of DUk at parietal electrodes modulated the decision weight wk assigned to element k in the eventual choice. To address this question, we applied the approach described above by taking as physiological variable the trial-to-trial encoding residuals from DUk at parietal electrodes, calculated at each electrode and each time from 0 to 600 ms following the onset of element k. We then extended this analysis to temporally adjacent elements in the stream, by including not only the interaction between encoding residuals from element k and DUk, but also the interaction between encoding residuals from element k and adjacent decision updates DUk−1 and DUk+1.
We also applied this psycho-physiological modulatory approach to the phase ϕ of EEG oscillations, a circular quantity defined between −π and π. We took into account the notion that ϕ is a complex-valued physiological variable by performing separate real-valued psychophysiological analyses for sin(ϕ) and for cos(ϕ), and by recovering the strength of the modulation and the corresponding preferred phase using the quadratic pair relationship.
When assessing how trial-to-trial fluctuations in lateralised beta-band activity at motor electrodes influenced the subsequent choice, we acknowledged that beta-band activity did not encode successive decision updates discretely and transiently as observed in broadband signals at parietal electrodes, but rather in a cumulative ramping-up fashion, and used the encoding residuals from the running decision variable – i.e., the cumulative sum of individual updates up to element k – rather than the encoding residuals from DUk in isolation.
EEG analysis – statistical procedures
The neural encoding and decoding analyses described above were performed separately for each participant and each element. At the group level, we used standard parametric tests (e.g., paired t-tests and repeated-measures ANOVAs) to assess the statistical significance of observed effects across the group. The type-1 error rate arising from multiple comparisons was controlled for using non-parametric cluster-level statistics (Maris and Oostenveld, 2007) computed across electrodes, time samples, and frequencies.
Supplementary Material
Highlights.
Sensory evidence is accumulated discontinuously during decision making.
Unique computations occur at central and motor stages of evidence processing.
The phase of slow cortical oscillations predicts the gain of evidence accumulation.
Fast oscillatory motor activity reflects the accumulated decision information.
Acknowledgements
The authors would like to thank Nick Yeung and Kia Nobre for providing access to their EEG equipment, Nick Myers for assistance with EEG acquisition, Tim Behrens, Etienne Koechlin, Benjamin Morillon and Mark Stokes for useful suggestions and comments. V.W. is supported by a postdoctoral research grant from the Fyssen Foundation.
References
- Bernacchia A, Seo H, Lee D, Wang XJ. A reservoir of time constants for memory traces in cortical neurons. Nat. Neurosci. 2011;14:366–372. doi: 10.1038/nn.2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogacz R, Brown E, Moehlis J, Holmes P, Cohen JD. The physics of optimal decision making: a formal analysis of models of performance in two-alternative forced-choice tasks. Psychol. Rev. 2006;113:700–765. doi: 10.1037/0033-295X.113.4.700. [DOI] [PubMed] [Google Scholar]
- Brainard DH. The Psychophysics Toolbox. Spat. Vis. 1997;10:433–436. [Google Scholar]
- Busch NA, Dubois J, VanRullen R. The phase of ongoing EEG oscillations predicts visual perception. J. Neurosci. 2009;29:7869–7876. doi: 10.1523/JNEUROSCI.0113-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busch NA, VanRullen R. Spontaneous EEG oscillations reveal periodic sampling of visual attention. Proc. Natl. Acad. Sci. U. S. A. 2010;107:16048–16053. doi: 10.1073/pnas.1004801107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun MM, Potter MC. A two-stage model for multiple target detection in rapid serial visual presentation. J. Exp. Psychol. Hum. Percept. Perform. 1995;21:109–127. doi: 10.1037//0096-1523.21.1.109. [DOI] [PubMed] [Google Scholar]
- Dehaene S, Changeux JP, Naccache L, Sackur J, Sergent C. Conscious, preconscious, and subliminal processing: a testable taxonomy. Trends in cognitive sciences. 2006;10:204–211. doi: 10.1016/j.tics.2006.03.007. [DOI] [PubMed] [Google Scholar]
- Delorme A, Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J. Neurosci. Methods. 2004;134:9–21. doi: 10.1016/j.jneumeth.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Donner TH, Siegel M, Fries P, Engel AK. Buildup of choice-predictive activity in human motor cortex during perceptual decision making. Curr. Biol. 2009;19:1581–1585. doi: 10.1016/j.cub.2009.07.066. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Buechel C, Fink GR, Morris J, Rolls E, Dolan RJ. Psychophysiological and modulatory interactions in neuroimaging. Neuroimage. 1997;6:218–229. doi: 10.1006/nimg.1997.0291. [DOI] [PubMed] [Google Scholar]
- Gold JI, Shadlen MN. The influence of behavioral context on the representation of a perceptual decision in developing oculomotor commands. J. Neurosci. 2003;23:632–651. doi: 10.1523/JNEUROSCI.23-02-00632.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold JI, Shadlen MN. The neural basis of decision making. Annu. Rev. Neurosci. 2007;30:535–574. doi: 10.1146/annurev.neuro.29.051605.113038. [DOI] [PubMed] [Google Scholar]
- Hanes DP, Schall JD. Neural control of voluntary movement initiation. Science. 1996;274:427–430. doi: 10.1126/science.274.5286.427. [DOI] [PubMed] [Google Scholar]
- Kaernbach C. Simple adaptive testing with the weighted up-down method. Percept. Psychophys. 1991;49:227–229. doi: 10.3758/bf03214307. [DOI] [PubMed] [Google Scholar]
- Lakatos P, Karmos G, Mehta AD, Ulbert I, Schroeder CE. Entrainment of neuronal oscillations as a mechanism of attentional selection. Science. 2008;320:110–113. doi: 10.1126/science.1154735. [DOI] [PubMed] [Google Scholar]
- Link SW. The relative judgement theory of two-choice response times. J. Math. Psychol. 1975;12:114–135. [Google Scholar]
- Maris E, Oostenveld R. Nonparametric statistical testing of EEG- and MEG-data. J. Neurosci. Methods. 2007;164:177–190. doi: 10.1016/j.jneumeth.2007.03.024. [DOI] [PubMed] [Google Scholar]
- Marois R, Ivanoff J. Capacity limits of information processing in the brain. Trends Cogn. Sci. 2005;9:296–305. doi: 10.1016/j.tics.2005.04.010. [DOI] [PubMed] [Google Scholar]
- Mitra PP, Pesaran B. Analysis of dynamic brain imaging data. Biophys. J. 1999;76:691–708. doi: 10.1016/S0006-3495(99)77236-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oostenveld R, Fries P, Maris E, Schoffelen JM. FieldTrip: open source software for advanced analysis of MEG, EEG, and invasive electrophysiological data. Comput. Intell. Neurosci. 2011;2011:156869. doi: 10.1155/2011/156869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pashler H. Processing stages in overlapping tasks: evidence for a central bottleneck. J. Exp. Psychol. Hum. Percept. Perform. 1984;10:358–377. doi: 10.1037//0096-1523.10.3.358. [DOI] [PubMed] [Google Scholar]
- Pelli DG. The VideoToolbox software for visual psychophysics: transforming numbers into movies. Spat. Vis. 1997;10:437–442. [Google Scholar]
- Pesaran B, Pezaris JS, Sahani M, Mitra PP, Andersen RA. Temporal structure in neuronal activity during working memory in macaque parietal cortex. Nat. Neurosci. 2002;5:805–811. doi: 10.1038/nn890. [DOI] [PubMed] [Google Scholar]
- Ratcliff R, Rouder JN. Modeling response times for two-choice decisions. Psychol. Sci. 1998;9:347–356. [Google Scholar]
- Ratcliff R, Smith PL. A comparison of sequential sampling models for two-choice reaction time. Psychol. Rev. 2004;111:333–367. doi: 10.1037/0033-295X.111.2.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond JE, Shapiro KL, Arnell KM. Temporary suppression of visual processing in an RSVP task: an attentional blink? J Exp Psychol Hum Percept Perform. 1992;18:849–860. doi: 10.1037//0096-1523.18.3.849. [DOI] [PubMed] [Google Scholar]
- Roitman JD, Shadlen MN. Response of neurons in the lateral intraparietal area during a combined visual discrimination reaction time task. J. Neurosci. 2002;22:9475–9489. doi: 10.1523/JNEUROSCI.22-21-09475.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheeringa R, Mazaheri A, Bojak I, Norris DG, Kleinschmidt A. Modulation of visually evoked cortical fMRI responses by phase of ongoing occipital alpha oscillations. J. Neurosci. 2011;31:3813–3820. doi: 10.1523/JNEUROSCI.4697-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder CE, Lakatos P. Low-frequency neuronal oscillations as instruments of sensory selection. Trends Neurosci. 2009;32:9–18. doi: 10.1016/j.tins.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sergent C, Baillet S, Dehaene S. Timing of the brain events underlying access to consciousness during the attentional blink. Nat. Neurosci. 2005;8:1391–1400. doi: 10.1038/nn1549. [DOI] [PubMed] [Google Scholar]
- Shadlen MN, Newsome WT. Neural basis of a perceptual decision in the parietal cortex (area LIP) of the rhesus monkey. J. Neurophysiol. 2001;86:1916–1936. doi: 10.1152/jn.2001.86.4.1916. [DOI] [PubMed] [Google Scholar]
- Sigman M, Dehaene S. Brain mechanisms of serial and parallel processing during dual-task performance. J. Neurosci. 2008;28:7585–7598. doi: 10.1523/JNEUROSCI.0948-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soltani A, Wang XJ. From biophysics to cognition: reward-dependent adaptive choice behavior. Curr. Opin. Neurobiol. 2008;18:209–216. doi: 10.1016/j.conb.2008.07.003. [DOI] [PubMed] [Google Scholar]
- Soltani A, Wang XJ. Synaptic computation underlying probabilistic inference. Nat. Neurosci. 2010;13:112–119. doi: 10.1038/nn.2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanics G, Hangya B, Hernadi I, Winkler I, Lakatos P, Ulbert I. Phase entrainment of human delta oscillations can mediate the effects of expectation on reaction speed. J. Neurosci. 2010;30:13578–13585. doi: 10.1523/JNEUROSCI.0703-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tombu MN, Asplund CL, Dux PE, Godwin D, Martin JW, Marois R. A unified attentional bottleneck in the human brain. Proc. Natl. Acad. Sci. U. S. A. 2011;108:13426–13431. doi: 10.1073/pnas.1103583108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanRullen R, Koch C. Is perception discrete or continuous? Trends Cogn. Sci. 2003;7:207–213. doi: 10.1016/s1364-6613(03)00095-0. [DOI] [PubMed] [Google Scholar]
- Wald A, Wolfowitz J. Bayes solutions of sequential decision problems. Proc. Natl. Acad. Sci. U. S. A. 1949;35:99–102. doi: 10.1073/pnas.35.2.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XJ. Neurophysiological and computational principles of cortical rhythms in cognition. Physiol. Rev. 2010;90:1195–1268. doi: 10.1152/physrev.00035.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyart V, Sergent C. The phase of ongoing EEG oscillations uncovers the fine temporal structure of conscious perception. J. Neurosci. 2009;29:12839–12841. doi: 10.1523/JNEUROSCI.3410-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang T, Shadlen MN. Probabilistic reasoning by neurons. Nature. 2007;447:1075–1080. doi: 10.1038/nature05852. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.