Abstract
Chronic activation of plasmacytoid dendritic cells (pDCs) is an important contributor to the immunopathogenesis of HIV infection. The quinolone derivative chloroquine (CQ) prevents endosomal acidification, required for toll-like receptor sensing of HIV by pDCs, and is currently under clinical trial as an immunotherapeutic approach. We tested three different 6-desfluoroquinolones (6-DFQs), structurally related to CQ and endowed with antiretroviral activity, for their ability to inhibit HIV-induced pDC activation and interferon (IFN)-α production in peripheral blood mononuclear cells (PBMCs) in vitro. PBMCs from six healthy donors were cultured overnight with aldrithiol-2 (AT-2)-inactivated HIV-1MN in the presence or absence of 6-DFQs or CQ. IFN-α production was measured by ELISA; pDC and monocyte activation was analyzed by flow cytometry. Incubation with HIV labeled with the fluorescent dye DyLight-488 (DL488) was used to test virus uptake by flow cytometry. We found that the 6-DFQs effectively inhibited HIV-induced IFN-α similar to CQ, but only 6-DFQs also inhibited the upregulation of the pDC activation marker CD83. Interestingly, HIV-induced expression of the costimulatory molecule CD80 and, to a lesser extent CD86, was further enhanced on pDCs by 6-DFQs, but not CQ. Conversely, 6-DFQs and CQ had similar inhibitory effects on HIV-induced monocyte activation, consistent with the primary mechanism being associated with IFN-α signaling. Finally, 6-DFQs interfered with HIV interaction with pDCs and monocytes, but not myeloid DCs. Our data indicate that 6-DFQs may interfere with pDC-mediated and IFN-α-dependent immunopathogenesis while supporting pDC differentiation into mature antigen-presenting cells by favoring expression of costimulatory molecules.
Introduction
Dendritic cells (DCs) govern the transition from innate to adaptive immune responses and are critical in tweaking the balance between immune stimulation and suppression. Peripheral blood DCs include plasmacytoid dendritic cells (pDCs) and myeloid DCs (mDCs).1 Both mDCs and pDCs are found in the peripheral blood in an immature state, but can mature into fully competent antigen-presenting cells (APCs) after recognition of pathogen-associated molecular patterns (PAMPs) by Toll-like receptors (TLRs) or signaling via cytokines or ligand-receptor pathways.1 Human pDCs express TLR7 and TLR9, which are respectively triggered by single-stranded RNA and unmethylated CpG-rich DNA, allowing pDC activation in response to most viruses.2 Upon TLR7/9-mediated activation, pDCs produce large amounts of type I interferons (IFN-Is; including IFN-α and IFN-β).
IFN-I are produced early during viral infections and act both as immunostimulatory cytokines favoring APC maturation and as antiviral factors. IFN-α/β exert their antiviral function by activating intracellular restriction mechanisms and induce both antiproliferative and proapoptotic effects on multiple cell types, including T lymphocytes.3
The human immunodeficiency virus (HIV) type 1 is a potent activator of pDC.4 HIV-induced pDC activation and IFN-I production have been suggested to contribute to several aspects of HIV immunopathogenesis,5 including (1) apoptosis of uninfected CD4 T cells,6,7 (2) increased expression of immunosuppressive ligands such as PDL1,8 (3) upregulation of T cell activation markers,9,10 and (4) chemoattraction of CCR5+ CD4 T cells at the site of infection, thus favoring systemic diffusion of the virus.11 Chronic upregulation of IFN-stimulated genes (ISGs) beyond the acute phase is observed in pathogenic simian immunodeficiency virus (SIV) infection of disease-susceptible Rhesus macaques, but not in natural hosts of SIV (Sooty mangabeys and African green monkeys) that do not display signs of disease despite elevated levels of viral replication.12,13 We have recently shown that the degree of pDC activation depends on the dynamics of virus–cell interaction,14 and that HIV-induced pDC overactivation suppresses HIV-specific CD8 T cell responses. Thus, although IFN-α/β may act as potent inhibitors of HIV replication, particularly during the acute phase of infection, the prolonged activation of pDC during the chronic phase may eventually become harmful for the immune system. Hence, pharmacological interventions aimed at modulating HIV-induced pDC activation are currently being considered as immunotherapeutic approaches to be used in combination with classic antiretroviral therapy (ART).
Chloroquine (CQ) and hydroxychloroquine (HCQ) (Supplementary Fig. S1; Supplementary Data are available online at www.liebertpub.com/aid) are quinoline derivatives known for their application as antimalaria drugs. Quinolones are a class of compounds serendipitously discovered as byproducts of the synthesis of CQ, and were mainly developed as antibacterial agents. However, different biological activities have been reported during the years for the quinolone “privileged-structure.”15–17 Both CQ and certain quinolone derivatives have been described as exerting inhibitory activity on HIV replication in vitro.16,18,19 CQ can prevent HIV-dependent pDC activation by interfering with endosomal acidification and HIV trafficking into the TLR7/9-bearing endosomes.4,20,21 CQ and HCQ are being tested in clinical trials aimed at mitigating HIV-induced immune activation (clinicaltrials.gov ID: NCT00308620, NCT01650558, NCT00819390, NCT01067417, and NCT01232660, at doses ranging between 250 and 500 mg/day for 8–48 weeks) or enhancing the immune response against therapeutic vaccines (clinicaltrials.gov ID: NCT00972725, single dose 300 mg). Both CQ and HCQ gave promising results in two studies in selected groups of HIV-infected patients.22,23 Another trial showed no effect of HCQ on immune activation, and reported increased viral load and more rapid CD4 decline in HCQ-treated patients.24
Here we analyzed the immunomodulatory properties of three different 6-desfluoroquinolone compounds (6-DFQs: WM21, WM5, and HM13N, Supplementary Fig. S1) in the setting of HIV-stimulated peripheral blood mononuclear cells (PBMCs). These compounds were chosen based on their reported ability to inhibit HIV replication in vitro.25–27 We chose to use concentrations comparable to the ones described for the antiretroviral activity of both 6-DFQs and CQ (up to 1 μg/ml), which are lower than those described for the inhibition of pDC activation by CQ (51.6 μg/ml).18,19 The anti-HIV activity of 6-DFQs depends on the inhibition of Tat-mediated transcription.16,25–27 Thus, 6-DFQs do not appear to affect the initial stages of the HIV replicative cycle, from virus entry to integration, but rather interfere with the production of new virions from productively infected cells. Here we tested the ability of HM13N, WM5, and WM21 to interfere with HIV-induced pDC activation, which does not require reverse transcription and integration but depends on virus attachment and endocytosis.4 Furthermore, we investigated the effect of HM13N on the interaction of fluorescently labeled HIV with pDCs, mDCs, and monocytes among PBMCs.
We found that all three 6-DFQs, as well as CQ, inhibited HIV-induced interferon (IFN)-α production in PBMCs. The expressions of the costimulatory molecules CD80 and CD86, which were increased on pDCs after incubation with HIV, were not inhibited by the compounds tested and were, in some cases, enhanced. Finally, the naphthyridone derivative HM13N partially inhibited the uptake of fluorescently labeled HIV by monocytes and pDCs, but not mDCs.
Materials and Methods
Peripheral blood mononuclear cells (PBMCs) isolation and culture
Peripheral blood was provided by healthy donor volunteers recruited among the staff of the Immunology Section, Chelsea and Westminster Hospital. Verbal consent was given by informed donors in the presence of a witness not involved in the study and a record of donations is kept in the Immunology Section to ensure that the total donations for each volunteer (including donations elsewhere) do not exceed 500 ml in a 6-month period for men or 250 ml in 6 months for women, as per approval by the Riverside Ethical Committee and following the institution's occupational health regulations. PBMCs were isolated by density gradient centrifugation using Lymphocyte separation medium-1077 (PAA Laboratories GmbH, Pasching, Austria) and cultured at 2×106 cells/ml in RPMI 1640 (PAA Laboratories GmbH), 10% fetal bovine serum (FBS, PAA Laboratories GmbH), and 1% Pen-Strep-Glut (PAA Laboratories GmbH).
Stimulation of PBMCs with aldrithiol-2 (AT-2)-inactivated HIV-1MN, 6-DFQs, and CQ
PBMCs were cultured overnight with or without chemically inactivated HIV-1 in the presence or absence of the four compounds tested. HIV-1MN/CEMx174 was obtained from the AIDS and Cancer Vaccine Program (SAIC-NCI at Frederick). Inactivation of HIV-1MN with AT-2 renders the virus reverse transcription deficient while preserving the functional integrity of the envelope, and was performed as previously described.28 AT-2 HIV (referred to as HIV from now on) was added to PBMC cultures at 230 ng/ml p24 final concentration. The three 6-DFQs (HM13N, WM5, and WM21) were synthesized as previously described25,27,29; CQ was purchased from Sigma Aldrich. All compounds were solubilized in dimethyl sulfoxide (DMSO). Each compound was used in different experiments at 0.01, 0.1, and 1 μg/ml final concentrations (corresponding to 0.001%, 0.01%, and 0.1% DMSO, respectively) as described in the Results section. In each experiment, control conditions were run using 0.1% DMSO in media alone or in the presence of HIV. All compounds had no effect on cell viability, tested by Trypan blue exclusion.
Stimulation of PBMCs with synthetic TLR9 ligand, 6-DFQs, and CQ
PBMCs were stimulated overnight with 0.75 μM human-specific type A CpG ODN sequence 2216 (5′-ggG GGA CGA TCG TCg ggg gg-3′) (InvivoGen, San Diego, CA) resuspended in endotoxin-free water, in the presence or absence of 1 μg/ml of HM13N, WM5, WM21, or CQ.
IFN-α ELISA
Supernatants were collected from cell cultures and frozen at −80°C until the assay was performed. IFN-α was quantified in thawed culture supernatants using the human IFN-α multisubtype ELISA kit (PBL Interferon Source, Piscataway, NJ) following manufacturer's instruction.
DyLight-488 HIV preparation and HIV uptake assay
Labeling of HIV with DyLight-488 and the virus uptake assay were performed as previously described.14 Briefly, AT-2 HIV-1MN was pelleted at 100,000×g for 1 h at 4°C, resuspended in 0.05 M sodium borate buffer, pH 8.5, added to one vial of DyLight 488 (DL488), and incubated for 1 h at room temperature. PBMCs from uninfected donors were cultured with DL488 HIV in the presence or absence of each compound for 30 min, 2 h, or 18 h at either 37°C or 4°C before flow cytometry analysis. To avoid uptake saturation, DL488 HIV was used at a concentration 10-fold lower than that used for the biological assays. PBMCs incubated with unlabeled HIV were used to set the positivity threshold for DL488.
Dextran uptake assay
PBMCs from uninfected donors were cultured with FITC-labeled dextran (70 kDa) in the presence or absence of HM13N or CQ for 30 min or 2 h at either 37°C or 4°C (Table 1), then washed three times with ice-cold phosphate-buffered saline (PBS)+2% FBS before flow cytometry analysis.
Table 1.
Uptake of Dextran-Fluorescein Isothiocyanate by Plamacytoid Dendritic Cells, Monocytes, and Myeloid Dendritic Cells in Peripheral Blood Mononuclear Cells Cultured with Dimethyl Sulfoxide, Chloroquine, or HM13N
| pDC | Monocytes | mDC | |||
|---|---|---|---|---|---|
| 30 min | 4°C | DMSO | 37.8% (25.6–44.5%) | 31.8% (24.9–33.1%) | 23.0% (19.1–27.3%) |
| 371 (352–386) | 2,217 (1,771–2,380) | 1,009 (964–1,401) | |||
| CQ | 40.1% (27.7–58.3%) | 32.8% (23.7–46.5%) | 25.3 (22.5–27.6%) | ||
| 407 (362–604) | 1,832 (1,646–1,992) | 1,365 (1,065–2,001) | |||
| HM13N | 51.6% (39.5–64.3%) | 37.2% (30.0–45.9%) | 29.2% (26.3–34.8%) | ||
| 441 (423–443) | 2,007 (1,772–2,322) | 1,010 (1,007–1,274) | |||
| 37°C | DMSO | 79.5% (58.1–84.5%) | 55.8% (53.8–76.9%) | 89.2% (87.1–90.7%) | |
| 490 (459–510) | 1,912 (1,595–2,762) | 903 (810–1,133) | |||
| CQ | 79.4% (57.6–80.5%) | 71.3% (69.7–84.3%) | 84.4% (81.6–86.2%) | ||
| 743 (663–1,382) | 2,978 (2,283–3,264) | 1,474 (1,300–1,560) | |||
| HM13N | 78.3% (59.2–78.7%) | 64.1% (55.0–80.6%) | 89.6% (89.1–90.6%) | ||
| 517 (505–555) | 2,259 (2,092–3,454) | 1,570 (1,432–1,677) | |||
| 2 h | 4°C | DMSO | 68.9% (58.8–71.3%) | 58.4% (47.1–60.5%) | 63.9% (4.9–75.2%) |
| 552 (472–576) | 1,538 (1,361–1,924) | 2,842 (2,523–4,331) | |||
| CQ | 82.3% (69.5–82.4%) | 54.5% (47.0–59.8%) | 69.5% (46.1–80.3%) | ||
| 549 (475–592) | 1,643 (1,447–1,956) | 1,332 (1,198–3,254) | |||
| HM13N | 40.1% (37.9–60.3%) | 41.1% (40.1–45.3%) | 61.8% (39.1–70.7%) | ||
| 430 (409–448) | 1,775 (1,674–1,915) | 1,320 (1,177–3,344) | |||
| 37°C | DMSO | 100% (99.7–100%) | 99.7% (99.2–99.7%) | 97.3% (96.4–97.5%) | |
| 799 (790–1,026) | 3,408 (3,324–7,204) | 3,921 (2,918–5,333) | |||
| CQ | 99.6% (97.7–99.8%) | 99.7% (99.4–99.8%) | 97.9% (96.3–98.2%) | ||
| 813 (762–855) | 3,427 (3,284–5,278) | 2,907 (2,373–3,899) | |||
| HM13N | 98.9% (93.1–99.4%) | 99.7% (98.9–99.8%) | 95.0% (94.6–96.6%) | ||
| 875 (754–960) | 3,414 (3,262–6,671) | 3,197 (2,617–3,336) |
Median and IQR for N=3 independent experiments are shown for frequencies of dextran-FITC+ cells (numbers on top) and dextran-FITC MFI within the dextran-FITC+ gate (numbers on bottom).
pDC, plamacytoid dendritic cells; mDC, myeloid dendritic cells; DMSO, dimethyl sulfoxoid; CQ, chloroquine.
Flow cytometry
Cells were incubated for 20 min at room temperature with different combinations of the following antihuman antibodies: fluorescein isothiocyanate (FITC)-conjugated antihuman CD80 clone 2D10.4 (eBioscence, Hatfield, UK); phycoerythrin (PE)-conjugated antihuman CD83 clone HB15e (eBioscience); Peridinin Chlorophyll Protein Complex (PerCP)-CyChrome (Cy)5.5-conjugated antihuman CD86 clone IT2.2 (Biolegend, London, UK); PE-Cy7-conjugated antihuman CD123 clone 6H6 (Biolegend); allophycyanin (APC)-conjugated antihuman CD303 (BDCA2) clone AC144 (Miltenyi Biotec, Surrey, UK); APC-H7-conjugated antihuman CD14 clone MφP9 (BD biosciences, Oxford, UK); PerCP-Cy5.5-conjugated antihuman CD8 clone SK1 (Biolegend); PacificBlue-conjugated antihuman CD8 clone SK1 (Biolegend); PE-Cy7-conjugated antihuman CD19 clone SJ25C1 (BD biosciences); brilliant violet 421-conjugated antihuman CD1c (BDCA1) clone L161 (Biolegend); and PerCP-eFluor 710-conjugated antihuman CD1c (BDCA1) clone L161 (Biolegend). Appropriate isotype controls were used to establish positivity thresholds. FACS analysis was performed on an LSR-II flow cytometer using FACSDiva software (BD Bioscience). FlowJo software (Treestar, Ashland, OR) was used for data analysis. Data are presented both as frequencies of cells expressing specific markers and as relative changes induced by each compound compared to HIV-treated cells. Gating strategies for all experiments are shown in Supplementary Figs. S2 and S3.
Statistical analysis
Statistical analyses were performed using SPSS 19.0 software (SPSS Inc., Chicago, IL). Changes in IFN-α production in response to different concentrations of each compound were analyzed using Friedman's two-way analysis of variance (ANOVA) by ranks, and pairwise comparisons were subjected to Dunn's post-hoc correction for multiple analyses. Wilcoxon sign rank test was used to compare alterations in IFN-α secretion in response to CpG alone and in the presence of each compound. In all flow cytometry experiments, pairwise comparisons between HIV-treated cells with and without compounds were performed using the nonparametric Wilcoxon sign rank test. p values lower than 0.05 were considered statistically significant.
Results
6-DFQs inhibit HIV-induced IFN-α production with efficacy similar to CQ
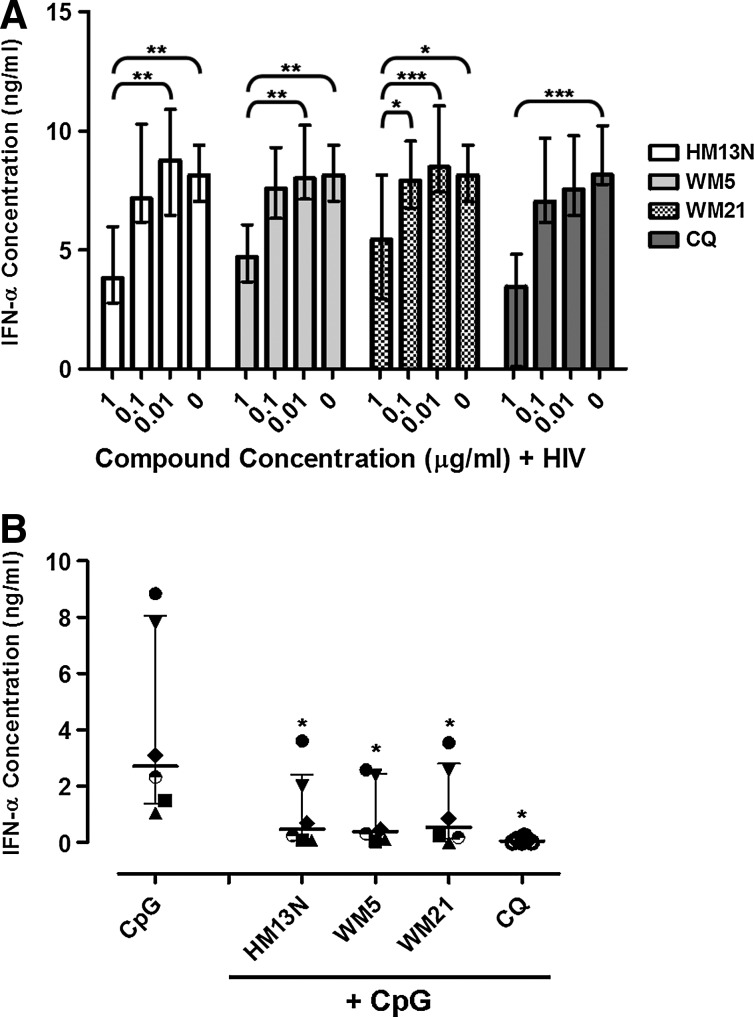
HIV is a potent activator of pDCs and induces high levels of IFN-α production in vitro.4 Inhibition of endosomal acidification using CQ efficiently prevents HIV-induced pDC activation and IFN-α production.20 PBMCs from HIV-uninfected donors (n=6) were cultured with reverse transcription-deficient HIV in the presence or absence of 6-DFQs or CQ at 0.01 μg/ml, 0.1 μg/ml, or 1 μg/ml. Supernatants were collected after overnight culture and IFN-α concentrations were measured by ELISA. Supernatants from non-HIV-treated cultures tested negative for IFN-α, and HIV-mediated IFN-α production was unaffected by 0.1% DMSO (data not shown). All four compounds significantly inhibited HIV-induced IFN-α production when used at 1 μg/ml, but not at 0.1 μg/ml or 0.01 μg/ml (Fig. 1A).
FIG. 1.
Effect of 6-desfluoroquinolone compounds (6-DFQs) and chloroquine (CQ) on HIV-induced interferon (IFN)-α production. Peripheral blood mononuclear cells (PBMCs) from healthy donors were cultured in the presence of (A) 0.1% dimethyl sulfoxide (DMSO)+HIV in the presence or absence of 0.01 μg/ml, 0.1 μg/ml, and 1 μg/ml of HM13N (N=8), WM5 (N=8), WM21 (N=8), or CQ (N=5) and (B) 0.75 μM human-specific type A CpG ODN in the presence or absence of 1 μg/ml HM13N, WM5, WM21, or CQ (N=6). IFN-α was measured in supernatants by ELISA after overnight culture. In (B), each symbol represents one individual donor and horizontal and vertical lines indicate medians and interquartile ranges (IQRs). *p<0.05, **p<0.01, and ***p<0.001 using (A) Friedman's two-way ANOVA by ranks with pairwise comparisons subjected to Dunn's post-hoc correction for multiple analyses and (B) Wilcoxon sign rank test.
Furthermore we demonstrated that all four compounds were able to dampen IFN-α secretion in PBMCs stimulated with the synthetic TLR 9 ligand CpG 2216. These data suggest that the effect of 6-DFQs is not restricted to HIV (Fig. 1B).
6-DFQs inhibit HIV-induced pDC activation while preserving the expression of costimulatory molecules
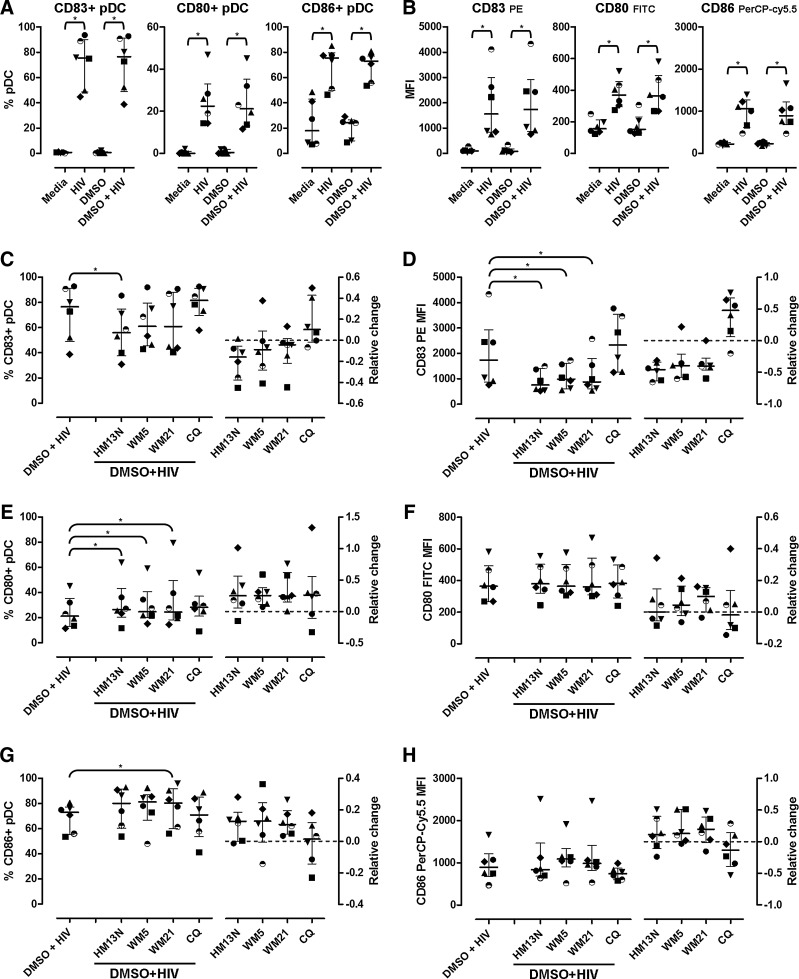
We tested whether 6-DFQs inhibited the upregulation of the activation marker CD83 and costimulatory molecules CD80 and CD86 induced by HIV in pDCs (Supplementary Fig. S2). As expected, overnight incubation with HIV resulted in increased frequency of pDCs expressing CD83, CD80, and CD86 as well as increased expression of each marker on a per cell basis (mean fluorescent intensity, MFI), which was not affected by 0.1% DMSO (Fig. 2A and B). All three 6-DFQs significantly reduced HIV-induced CD83 MFI on pDCs when used at 1 μg/ml, whereas a significant reduction of CD83+ pDCs was observed only with HM13N (Fig. 2C and D). Surprisingly, the frequency of CD80+ pDCs was further enhanced by the three 6-DFQs tested (Fig. 2E), but the minor changes were not statistically significant when CD80 MFI was analyzed (Fig. 2F). The frequency of CD86+ pDCs was significantly enhanced by WM21 (Fig. 2G), and similar trends approaching statistical significance were observed for HM13N and WM5 (p=0.075 for both HM13N and WM5; Fig. 2G), but no significant change was observed in CD86 MFI (Fig. 2H). CQ had no significant effect on CD83, CD80, and CD86 (Fig. 2C–H), despite the observed and documented effect on HIV-induced IFN-α production (Fig. 1A).20
FIG. 2.
Activation of plasmacytoid dendritic cells (pDCs) and expression of costimulatory molecules. Frequencies of pDCs (CD14− BDCA2+ CD123+; Supplementary Fig. S2A) expressing CD83, CD80, and CD86 (A) and mean fluorescent intensity (MFI) (B) in PBMCs from healthy donors (N=6) cultured overnight in media alone, HIV, 0.1% DMSO, and 0.1% DMSO+HIV. Frequencies of pDCs expressing CD83, CD80, and CD86 (C, E, G, respectively) and MFI (D, F, H, respectively) in PBMCs from the same donors (N=6) cultured overnight in 0.1% DMSO+HIV in the presence or absence of 1 μg/ml HM13N, WM5, WM21, or CQ. In all panels, left plots represent pDC frequencies (C, E, G) or MFI (D, F, H) and right plots represent the relative change caused by each compound compared to 0.1% DMSO+HIV, calculated as [(compound+HIV) – (DMSO+HIV)]/(DMSO+HIV). In all graphs, each symbol represents one individual donor and horizontal and vertical lines indicate medians and IQRs. *p<0.05 using Wilcoxon sign rank test on raw frequencies and MFIs.
These data indicate that 6-DFQs may differentially affect multiple functions of HIV-activated pDCs, inhibiting their ability to produce IFN-α, while enhancing their ability to develop an APC phenotype by enhancing the upregulation of costimulatory molecules.
Effect of 6-DFQs and CQ on HIV-induced activation of monocytes in PBMC cultures
Maturation of pDCs into APCs and IFN-producing cells is directly dependent on TLR7/9 signaling.1,2 During in vitro HIV stimulation of PBMCs, maturation of other myeloid DCs and monocytes into APCs occurs indirectly following IFN-α signaling, rather than by direct virus-mediated activation.8,14 Thus, monocytes are poorly responsive to HIV exposure in vitro, and although other cytokines produced by activated pDCs, such as tumor necrosis factor (TNF)-α, can promote monocyte activation, our previous data indicate that IFN-α-signaling blockade prevented monocyte activation in HIV-exposed PBMCs.8
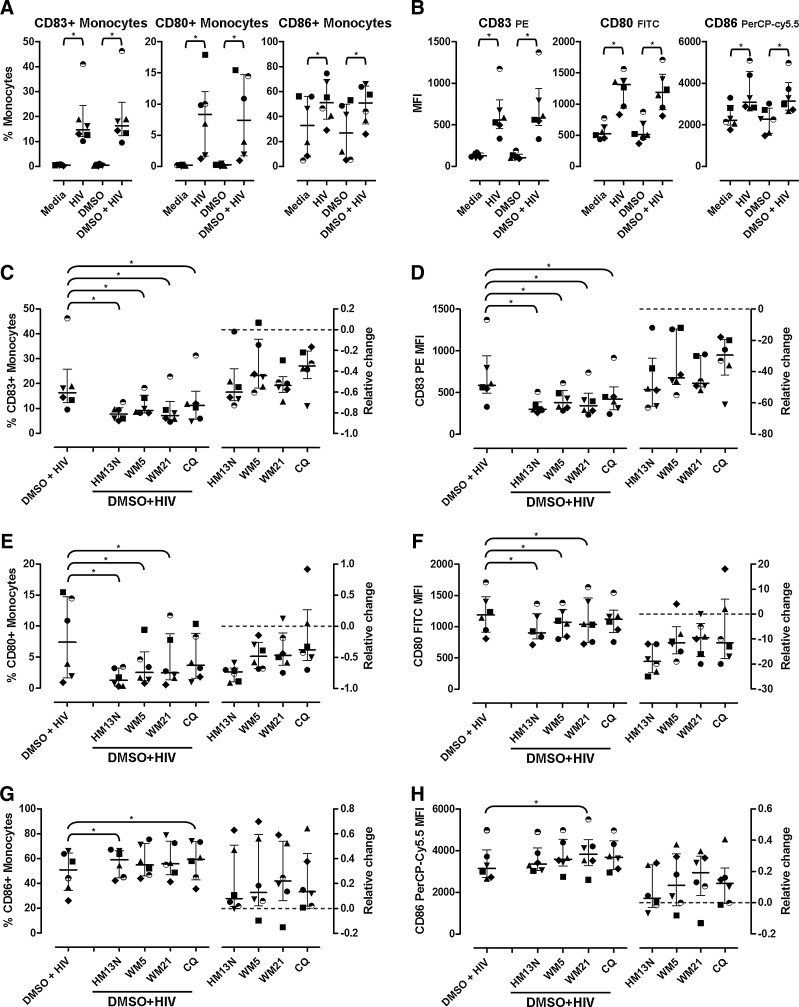
We tested the effect of 6-DFQs on activation markers and costimulatory molecules in monocytes (CD14+ cells). As previously described,8,14 stimulation of PBMCs with HIV resulted in upregulation of CD83, CD80, and CD86 in monocytes, which was unaltered by 0.1% DMSO (Fig. 3A and B). Treatment with 6-DFQs or CQ significantly inhibited HIV-induced upregulation of CD83 on monocytes, measured both as frequency of CD83+ cells and CD83 MFI (Fig. 3C and D). CD80 expression on monocytes (frequency and MFI) was significantly reduced by 6-DFQs but not CQ (Fig. 3E and F). Surprisingly, a partial enhancement of HIV-induced CD86 expression on monocytes was observed with HM13N (CD86+ monocytes), CQ (CD86+ monocytes), and WM21 (CD86 MFI) (Fig. 3G and H).
FIG. 3.
Activation of monocytes and expression of costimulatory molecules. Frequencies of monocytes (CD14+; Supplementary Fig. S2A) expressing CD83, CD80, and CD86 (A) and MFI (B) in PBMCs from healthy donors (N=6) cultured overnight in media alone, HIV, 0.1% DMSO, and 0.1% DMSO+HIV. Frequencies of monocytes expressing CD83, CD80, and CD86 (C, E, G, respectively) and MFI (D, F, H, respectively) in PBMCs from the same donors (N=6) cultured overnight in 0.1% DMSO+HIV in the presence or absence of 1 μg/ml HM13N, WM5, WM21, or CQ. In all panels, the left plots represent raw data on monocyte frequencies (C, E, G) or MFI (D, F, H) and the right plots represent the relative change caused by each compound compared to 0.1% DMSO+HIV, calculated as [(compound+HIV) – (DMSO+HIV)]/(DMSO+HIV). In all graphs, each symbol represents one individual donor and horizontal and vertical lines indicate medians and IQRs. *p<0.05 using Wilcoxon sign rank test on raw frequencies and MFIs.
These data confirm our previous observation that monocyte activation and upregulation of CD80, but not CD86, in HIV-treated PBMCs are prevented by inhibition of IFN-α signaling or production.8,14
HM13N interferes with the uptake of DL488-labeled HIV by pDCs and monocytes, but not mDCs and CD4 T cells
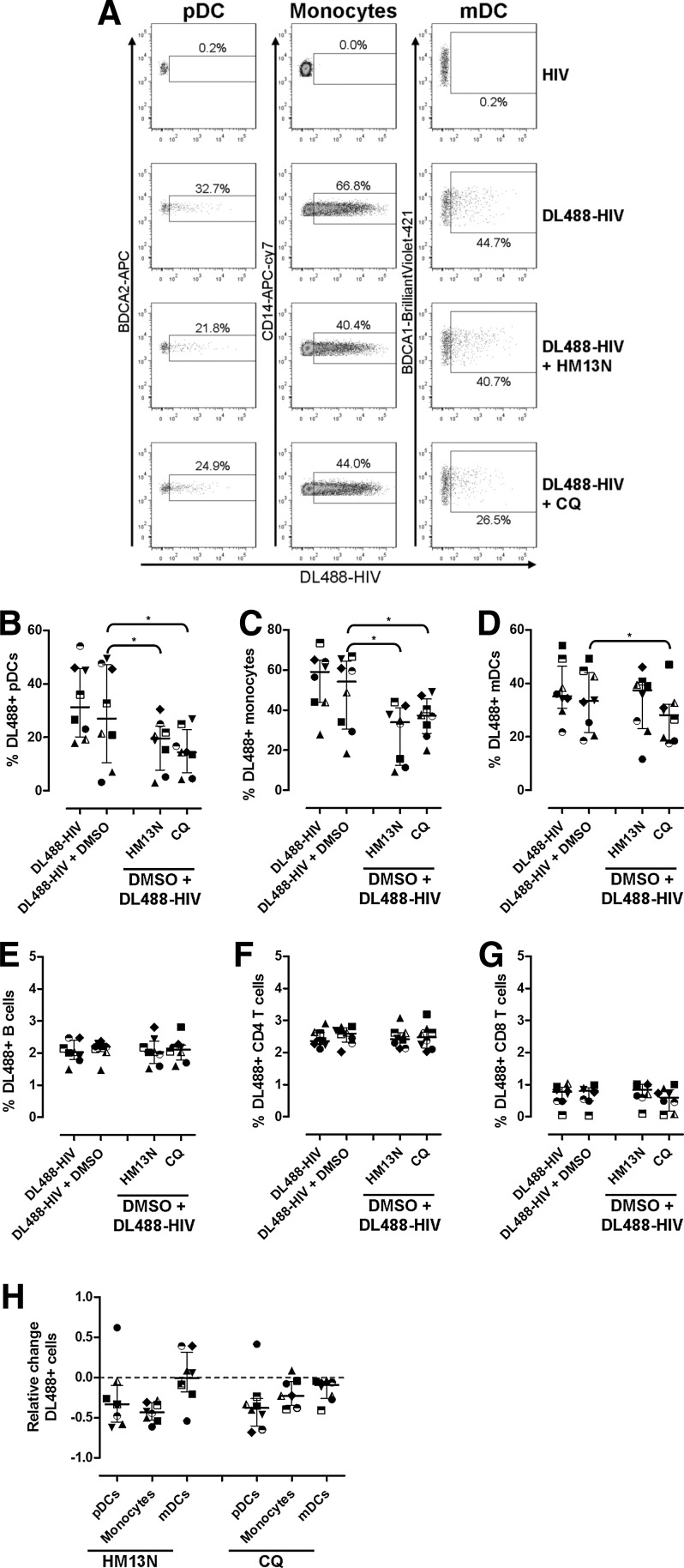
To determine the effect of 6-DFQs and CQ on HIV uptake by different cell types, we incubated PBMCs with DL488-labeled HIV as previously described.14 Cells that acquired HIV were visualized by flow cytometry as DL488+14 (Supplementary Figs. S3 and S4 and Fig. 4A). Minor increases of DL488 staining were observed in pDCs, monocytes, mDCs, B cells, and CD4 T cells, but not CD8 T cells, after 30 min and 2 h incubation at 37°C (Supplementary Fig. S4). Of note, DL488 staining was comparable in cells incubated at 37°C and 4°C, at both 30 min and 2 h (Supplementary Fig. S4), suggesting that the signal observed is due to virus–cell binding, not active endocytosis. DL488 staining at 4°C after 18 h was comparable to that observed after 30 min and 2 h (data not shown). Conversely, following 18 h incubation, approximately 30% of pDC, 60% of monocytes, and 30% of mDC stained positive for DL488 (Fig. 4A–D), suggesting efficient uptake of HIV by DCs and monocytes, likely via endocytosis or micropinocytosis.
FIG. 4.
Effect of HM13N and CQ on DL488-HIV interaction with pDCs, monocytes, myeloid dendritic cells (mDCs), B cells, and T cells. (A) Flow cytometry dot-plots for pDCs, monocytes, and mDCs (gated as shown in Supplementary Fig. S3) in PBMCs from one representative healthy donor (out of N=8) cultured overnight at 37°C with unlabeled HIV or DL488-HIV in the presence or absence of HM13N or CQ. (B–G) Frequencies of DL844+ pDCs (B), monocytes (C), mDCs (D), B cells (E), CD4 T cells (F), and CD8 T cells (G) in PBMCs from healthy donors (N=8) cultured overnight at 37°C with DL488-HIV in the presence or absence of 0.1% DMSO, 1 μg/ml HM13N, or 1 μg/ml CQ. (H) The relative changes caused by HM13N and CQ compared to 0.1% DMSO+HIV in pDCs, monocytes, and mDCs, calculated as [(compound+HIV) – (DMSO+HIV)]/(DMSO+HIV). In all graphs, each symbol represents one individual donor and horizontal and vertical lines indicate medians and IQRs. *p<0.05 using Wilcoxon sign rank test on raw frequencies of DL488+ cells.
Approximately 2% of B cells, 2.5% of CD4 T cells, and less than 1% of CD8 T cells stained DL488+ after 18 h, indicating limited reactivity with HIV (Fig. 4E, F, and G). CD4 T cells express high levels of the HIV receptor CD4, but have poor endocytotic activity in resting conditions. Thus, the low level of DL488 staining on CD4 T cells is consistent with internalization rather than surface binding as the main mechanism by which DCs and monocytes acquired DL488-HIV. Although DMSO appeared to interfere with HIV uptake in some samples, the overall effect was not statistically significant and median frequencies of DL488+ pDCs, mDCs, and monocytes in the presence or absence of DMSO were comparable.
HM13N significantly inhibited DL488-HIV uptake by pDCs (Fig. 4B and H) and monocytes (Fig. 4C and H), but showed no significant effect on mDCs (Fig. 4D and H). Conversely, CQ significantly inhibited DL488-HIV uptake by pDCs, monocytes (Fig. 4B and H), and to a lesser extent mDCs (Fig. 4D and 4H). Neither HM13N nor CQ exerted any effect on DL488-HIV association with any cell type analyzed after 30 min or 2 h (data not shown), and no effect was observed after 18 h on B cells, CD4 T cells, and CD8 T cells (Fig. 4E–G).
We tested whether HM13N or CQ interfered with the uptake of soluble antigens by DCs and monocytes (Supplementary Fig. S5). FITC-labeled dextran partially stained pDCs, monocytes, and mDCs after incubation at 4°C for 30 min or 2 h, indicating surface-bound dextran in the absence of active endocytosis. When cells were incubated at 37°C, efficient uptake of FITC-labeled dextran was observed in all three cell types, which was not reduced by CQ or HM13N.
Discussion
The immunologic hallmark of HIV infection is a status of chronic and progressive immune activation, which drives the immune system to exhaustion and ultimately leads to severe immunodeficiency.30 The mechanisms connecting HIV infection to chronic immune activation, while still subject to debate, are likely to involve alterations of multiple immunologic functions.30 A growing body of evidence indicates that overactivation of pDCs beyond the acute and throughout the chronic phase of HIV infection may play a critical role in driving HIV disease progression.5 CQ and HCQ are known inhibitors of HIV-induced pDC activation, and are currently under trial as immunotherapeutic agents to control immune exhaustion and disease progression.31 Here we provide evidence that 6-DFQs with antiretroviral properties inhibit pDC activation similar to CQ, while promoting the expression of costimulatory molecules, arguing in favor of further exploring their potential use as immunotherapeutic agents.
The antiretroviral activity of 6-DFQs relies on the inhibition of Tat-mediated transcription, and therefore affects the production of new virions from infected cells.16,25–27 However, in our study we used AT-2-treated HIV-1, which is reverse transcription deficient and does not establish productive infection in the target cells.28 Therefore, the immunomodulatory effect of 6-DFQs is independent of their antiviral activity, and seemingly is mediated by a different mechanism. Inhibition of endosomal acidification is considered the mechanism by which CQ inhibits HIV-induced pDC activation.4,20 The inhibition of CpG-induced IFN-α observed with both CQ and 6-DFQs argues in favor of a common mechanism targeting the endosomal pathway. However, we found that CQ, as well as HM13N, reduced the number of pDCs that acquired fluorescently labeled HIV after overnight incubation. DL488 staining in our experiment may indicate virions bound to the cell surface or internalized particles. The reported high rate of CD4 turnover via clathrin-dependent endocytosis in DCs32,33 and the minimal DL488 staining observed after incubation at 4°C, independent of the culture time, suggest that HIV uptake by pDCs occurs primarily by the endocytotic route. The inhibition of HIV uptake observed with CQ and HM13N may therefore indicate reduced internalization of viral particles, possibly due to the accumulation of endosomal vesicles and subsequent congestion of the endocytotic machinery.
Production of IFN-α and maturation into APCs are the main functional changes that pDCs undergo after TLR7 or TLR9 stimulation.1 We have recently shown that costimulatory molecule upregulation can be dissociated from IFN-α production in HIV-stimulated pDCs by modifying the native organization of the viral envelope, which contains a functional substructure pivotal for virus–cell interactions.14 Treatment with 6-DFQs partially reproduced this phenomenon, by suppressing IFN-α production and CD83 expression, but simultaneously enhancing HIV-induced upregulation of CD80 and CD86 on pDCs. Treatment with CQ differed from that observed with 6-DFQs, in that it showed poor or no inhibition of CD83 expression and no significant effect on costimulatory molecules. These results are in apparent contrast with previous reports in which CQ showed efficient inhibition of HIV-induced expression of CD83 and CD86.20,21 However, the concentration of CQ used by Ma et al. and Martinson et al. was 100 μM (51.6 μg/ml),20,21 compared to 1.94 μM (1 μg/ml) in our study.
Our intention was to compare CQ to the 6-DFQs within a range of concentrations consistent with the reported antiviral activity of the compounds.18,19,25–27,34 Therefore, our data suggest that 6-DFQs and CQ modulate HIV-induced IFN-α production at concentrations that are compatible with their antiretroviral activity. At this concentration 6-DFQs are more efficient inhibitors of pDC activation (as measured by CD83 expression), but do not decrease and even enhance costimulatory molecule expression.
The effect of 6-DFQs and CQ on monocyte activation reflects in part the inhibitory activity of these compounds on IFN-α production and on the presence of pDCs in the culture system, showing efficient inhibition of CD83 and CD80 upregulation by 6-DFQs. Expression of CD86 on monocytes was instead further upregulated by 6-DFQs and CQ, following the trend observed for pDCs. This result is not surprising, and corroborates our previous observations that HIV-induced CD86 upregulation on monocytes was insensitive to IFN-α inhibition.14 The mechanism by which these compounds tweak the balance from a primarily IFN-producing to an APC phenotype in pDCs remains obscure at this stage, but this phenomenon may have important implications for the development of quinolone-based immunotherapy. Thus, we have shown that IFN-I is a key suppressor of HIV-specific memory CD8 T cell responses,14 which are critical for controlling viral replication during chronic HIV infection.
By inhibiting IFN-I production while promoting costimulatory molecules expression, quinolones may relieve the immunosuppressive pressure and simultaneously promote APC activity, which is required to prime and maintain efficient antiviral T cell responses. In this perspective, it is noteworthy that HM13N showed no inhibitory effect on HIV uptake by mDCs, which are potent APCs and may continue to process and present HIV antigens in the absence of the IFN-I-mediated immunosuppressive activity of pDCs. The binding of HIV to receptors other than CD4 may influence viral uptake by different cell subsets. For example, interaction with integrins or scavenger receptors may efficiently allow HIV uptake independent of CD4.
Notably, neither HM13N nor CQ interfered with the uptake of dextran by DCs and monocytes, suggesting that the ability to uptake soluble antigens via micropinocytosis or clathrin-dependent endocytosis is unaffected by the compounds. The reasons for the different effects of HM13N and CQ on the uptake of HIV and dextran remain unclear. It is possible that soluble antigens of relatively small size, such as dextran (70 kDa, 5.3 nm in size), are endocytosed more efficiently than large particles such as whole HIV virions (100–120 nm), and therefore are not affected by the compounds tested.
It is important to note that the changes in costimulatory molecules expression that we observed in vitro need to be verified in an in vivo system, possibly using nonhuman primate models of HIV infection. In addition, Kader et al. have recently reported that mDCs and monocytes, rather than pDCs, become important IFN-α-producing cells during the early phases of postacute SIV infection in macaques.35 Thus, the biological relevance of our findings and the effectiveness of 6-DFQs as immunomodulatory agents in vivo remain to be determined, but our data indicate that compounds other than CQ and HCQ may be considered and tested for their immunomodulatory properties in the setting of HIV infection. Combining unique immunomodulatory properties with potent antiretroviral activity, 6-DFQs may represent the prototype of a new class of multifunctional drugs.
Supplementary Material
Acknowledgments
This study was supported by the Wellcome Trust (ref. 085164/Z/08/Z), by the Italian Ministry of Health (AIDS, UPR-2009-1301355), and by the NIH (RO1 MH085554 and R01 MH097233).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Steinman RM. and Hemmi H: Dendritic cells: Translating innate to adaptive immunity. Curr Top Microbiol Immunol 2006;311:17–58 [DOI] [PubMed] [Google Scholar]
- 2.Takeuchi O. and Akira S: Recognition of viruses by innate immunity. Immunol Rev 2007;220:214–224 [DOI] [PubMed] [Google Scholar]
- 3.Barber GN: Host defense, viruses, and apoptosis. Cell Death Differ 2001;8(2):113–126 [DOI] [PubMed] [Google Scholar]
- 4.Beignon AS, McKenna K, Skoberne M, et al.: Endocytosis of HIV-1 activates plasmacytoid dendritic cells via Toll-like receptor-viral RNA interactions. J Clin Invest 2005;115(11):3265–3275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boasso A. and Shearer GM: Chronic innate immune activation as a cause of HIV-1 immunopathogenesis. Clin Immunol 2008;126(3):235–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Herbeuval JP, Hardy AW, Boasso A, et al. : Regulation of TNF-related apoptosis-inducing ligand on primary CD4+ T cells by HIV-1: Role of type I IFN-producing plasmacytoid dendritic cells. Proc Natl Acad Sci USA 2005;102(39):13974–13979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stary G, Klein I, Kohlhofer S, et al. : Plasmacytoid dendritic cells express TRAIL and induce CD4+ T-cell apoptosis in HIV-1 viremic patients. Blood 2009;114(18):3854–3863 [DOI] [PubMed] [Google Scholar]
- 8.Boasso A, Hardy AW, Landay AL, et al. : PDL-1 upregulation on monocytes and T cells by HIV via type I interferon: Restricted expression of type I interferon receptor by CCR5-expressing leukocytes. Clin Immunol 2008;129(1):132–144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rodriguez B, Lederman MM, Jiang W, et al. : Interferon-alpha differentially rescues CD4 and CD8 T cells from apoptosis in HIV infection. AIDS 2006;20(10):1379–1389 [DOI] [PubMed] [Google Scholar]
- 10.Boasso A, Hardy AW, Anderson SA, Dolan MJ, and Shearer GM: HIV-induced type I interferon and tryptophan catabolism drive T cell dysfunction despite phenotypic activation. PLoS One 2008;3(8):e2961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li Q, Estes JD, Schlievert PM, et al. : Glycerol monolaurate prevents mucosal SIV transmission. Nature 2009;458(7241):1034–1038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bosinger SE, Li Q, Gordon SN, et al. : Global genomic analysis reveals rapid control of a robust innate response in SIV-infected sooty mangabeys. J Clin Invest 2009;119(12):3556–3572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jacquelin B, Mayau V, Targat B, et al. : Nonpathogenic SIV infection of African green monkeys induces a strong but rapidly controlled type I IFN response. J Clin Invest 2009;119(12):3544–3555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boasso A, Royle CM, Doumazos S, et al. : Overactivation of plasmacytoid dendritic cells inhibits antiviral T-cell responses: A model for HIV immunopathogenesis. Blood 2011;118(19):5152–5162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ahmed A. and Daneshtalab M: Nonclassical biological activities of quinolone derivatives. J Pharm Pharm Sci 2012;15(1):52–72 [DOI] [PubMed] [Google Scholar]
- 16.Tabarrini O, Massari S, and Cecchetti V: 6-Desfluoroquinolones as HIV-1 Tat-mediated transcription inhibitors. Future Med Chem 2010;2(7):1161–1180 [DOI] [PubMed] [Google Scholar]
- 17.Tabarrini O, Sabatini S, Massari S, Pieroni M, Franzblau SG, and Cecchetti V: 6-Hydrogen-8-methylquinolones active against replicating and non-replicating mycobacterium tuberculosis. Chem Biol Drug Des 2012;80(5):781–786 [DOI] [PubMed] [Google Scholar]
- 18.Tsai WP, Nara PL, Kung HF, and Oroszlan S: Inhibition of human immunodeficiency virus infectivity by chloroquine. AIDS Res Hum Retroviruses 1990;6(4):481–489 [DOI] [PubMed] [Google Scholar]
- 19.Savarino A, Gennero L, Chen HC, et al. : Anti-HIV effects of chloroquine: Mechanisms of inhibition and spectrum of activity. AIDS 2001;15(17):2221–2229 [DOI] [PubMed] [Google Scholar]
- 20.Martinson JA, Montoya CJ, Usuga X, Ronquillo R, Landay AL, and Desai SN: Chloroquine modulates HIV-1-induced plasmacytoid dendritic cell alpha interferon: Implication for T-cell activation. Antimicrob Agents Chemother 2010;54(2):871–881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ma JP, Xia HJ, Zhang GH, Han JB, Zhang LG, and Zheng YT: Inhibitory effects of chloroquine on the activation of plasmacytoid dendritic cells in SIVmac239-infected Chinese rhesus macaques. Cell Mol Immunol 2012;9(5):410–416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Piconi S, Parisotto S, Rizzardini G, et al. : Hydroxychloroquine drastically reduces immune activation in HIV-infected, ART-treated, immunological non-responders. Blood 2011; 118(12):3263–3272 [DOI] [PubMed] [Google Scholar]
- 23.Murray SM, Down CM, Boulware DR, et al. : Reduction of immune activation with chloroquine therapy during chronic HIV infection. J Virol 2010;84(22):12082–12086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Paton NI, Goodall RL, Dunn DT, et al. : Effects of hydroxychloroquine on immune activation and disease progression among HIV-infected patients not receiving antiretroviral therapy: A randomized controlled trial. JAMA 2012;308(4):353–361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tabarrini O, Stevens M, Cecchetti V, et al. : Structure modifications of 6-aminoquinolones with potent anti-HIV activity. J Med Chem 2004;47(22):5567–5578 [DOI] [PubMed] [Google Scholar]
- 26.Tabarrini O, Massari S, Daelemans D, et al. : Structure-activity relationship study on anti-HIV 6-desfluoroquinolones. J Med Chem 2008;51(17):5454–5458 [DOI] [PubMed] [Google Scholar]
- 27.Massari S, Daelemans D, Barreca ML, et al. : A 1,8-naphthyridone derivative targets the HIV-1 Tat-mediated transcription and potently inhibits the HIV-1 replication. J Med Chem 2010;53(2):641–648 [DOI] [PubMed] [Google Scholar]
- 28.Rossio JL, Esser MT, Suryanarayana K, et al. : Inactivation of human immunodeficiency virus type 1 infectivity with preservation of conformational and functional integrity of virion surface proteins. J Virol 1998;72(10):7992–8001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cecchetti V, Parolin C, Moro S, et al. : 6-Aminoquinolones as new potential anti-HIV agents. J Med Chem 2000;43(20):3799–3802 [DOI] [PubMed] [Google Scholar]
- 30.Moir S, Chun TW, and Fauci AS: Pathogenic mechanisms of HIV disease. Annu Rev Pathol 2011;6:223–248 [DOI] [PubMed] [Google Scholar]
- 31.Ellegard R, Shankar EM, and Larsson M: Targeting HIV-1 innate immune responses therapeutically. Curr Opin HIV AIDS 2011;6(5):435–443 [DOI] [PubMed] [Google Scholar]
- 32.Pelchen-Matthews A, da Silva RP, Bijlmakers MJ, Signoret N, Gordon S, and Marsh M: Lack of p56lck expression correlates with CD4 endocytosis in primary lymphoid and myeloid cells. Eur J Immunol 1998;28(11):3639–3647 [DOI] [PubMed] [Google Scholar]
- 33.Pitcher C, Honing S, Fingerhut A, Bowers K, and Marsh M: Cluster of differentiation antigen 4 (CD4) endocytosis and adaptor complex binding require activation of the CD4 endocytosis signal by serine phosphorylation. Mol Biol Cell 1999;10(3):677–691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stevens M, Balzarini J, Tabarrini O, et al. : Cell-dependent interference of a series of new 6-aminoquinolone derivatives with viral (HIV/CMV) transactivation. J Antimicrob Chemother 2005;56(5):847–855 [DOI] [PubMed] [Google Scholar]
- 35.Kader M, Smith AP, Guiducci C, et al. : Blocking TLR7- and TLR9-mediated IFN-alpha production by plasmacytoid dendritic cells does not diminish immune activation in early SIV infection. PLoS Pathog 2013;9(7):e1003530. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.