Abstract
Sensory cilia are often encapsulated by an extracellular matrix (ECM). In Caenorhabditis elegans, Drosophila melanogaster, and vertebrates, this ECM is thought to be directly involved in ciliary mechanosensing by coupling external forces to the ciliary membrane. Drosophila mechano- and chemosensory cilia are both associated with an ECM, indicating that the ECM may have additional roles that go beyond mechanosensory cilium function. Here, we identify Artichoke (ATK), an evolutionarily conserved leucine-rich repeat ECM protein that is required for normal morphogenesis and function of ciliated sensilla in Drosophila. atk is transiently expressed in accessory cells in all ciliated sensory organs during their late embryonic development. Antibody stainings show ATK protein in the ECM that surrounds sensory cilia. Loss of ATK protein in atk null mutants leads to cilium deformation and disorientation in chordotonal organs, apparently without uncoupling the cilia from the ECM, and consequently to locomotion defects. Moreover, impaired chemotaxis in atk mutant larvae suggests that, based on ATK protein localization, the ECM is also crucial for the correct assembly of chemosensory receptors. In addition to defining a novel ECM component, our findings show the importance of ECM integrity for the proper morphogenesis of ciliated organs in different sensory modalities.
Keywords: chordotonal organ, extracellular matrix, LRR proteins, sensory cilium, dendritic cap
CILIA are microtubule-based membrane projections with a microtubule-based cytoskeleton, or axoneme, which consists of nine circularly arranged microtubule doublets. Cilia have been traditionally classified into motile or primary ones on the basis of an additional central microtubule pair that can be present (motile cilia) or not (primary cilia) (Gerdes et al. 2009). Recent data suggest nonetheless that it is the presence of outer and inner dynein arms that determines cilia motility. In invertebrates, cilia are restricted to certain sensory neurons that innervate specialized chemo- and mechanosensory organs and to sperm cells (Dubruille et al. 2002; Keil 2012).
Cilia can protrude freely into the extracellular space or be associated with an extracellular matrix (ECM) (McGlashan et al. 2006). This ECM seems especially important in the context of mechanotransduction. Given the exquisite speed of mechanotransduction, it is generally assumed that mechano-electrical transduction channels are directly gated by mechanical stimuli and detect the deflection of an external structure relative to an internal cellular structure (Gillespie and Walker 2001). The ECM acts as a linker between the external structure and the channels and is therefore an essential component of the system. Consistent with this hypothesis, different mec genes that were isolated in genetic screens in Caenorhabditis elegans for mechanosensitive mutants encode ECM proteins (Chalfie and Sulston 1981; Chalfie and Au 1989). Although the nature of the interaction between the ECM and the transduction channel is not fully understood, further studies in C. elegans have shown that the ECM proteins MEC-1 and MEC-5 are necessary for the localization of the mechanosensory degenerin channel complex and for touch sensitivity (Emtage et al. 2004).
In Drosophila, this sensory ECM has been described for all ciliated mechanosensory organs, and it is specifically known as the dendritic cap. Ciliated mechanosensory organs are type I sensory organs, which also include chemosensory organs. Type I sensory organs are multicellular and characteristically innervated by ciliated sensory neurons. Unlike type I organs, type II organs are unicellular, consisting of single multidendritic neurons whose dendrites lack cilia. In Drosophila embryos, mechanosensory type I organs can be further subdivided into two types: chordotonal (ch) organs that are attached internally to the cuticle and lack external structures and external sensory (es) organs whose stimulus-detecting structures protrude from the cuticle (reviewed in Kernan 2007). Both ch and es organs are innervated by a ciliated sensory neuron that is enveloped by accessory cells. They possess a dendritic cap at the cilium tip that is secreted by the accessory cells. The molecular composition of the dendritic cap and how it is assembled is largely unknown: the only protein that has been reported to localize to the dendritic cap is the zona pellucida (ZP)-domain protein NOMPA, which presumably serves as a scaffold to which other proteins bind (Chung et al. 2001). nompA mutants display disorganized dendritic caps, abolishing ch and es neuron function (Chung et al. 2001; Göpfert and Robert 2003). NOMPA also localizes to the dendritic tips of the ciliated neurons innervating Drosophila chemosensory organs (Chung et al. 2001), but the role of the supporting ECM has not yet been reported for these organs.
Since ECMs are common to all ciliated sensory organs, and are known to be essential at least in mechanosensation, it seems crucial to understand how the connections within the ECM scaffold occur. Adhesion molecules like leucine-rich repeat (LRR) proteins are likely to play an important role in this process. LRR are protein–protein interaction motifs of 20–30 amino acids enriched in leucines (Dolan et al. 2007). In C. elegans, the LRR proteins LET-4 and EGG-6 and the secreted LRR protein SYM-1 are required for the organization of the apical ECM in the excretory duct and pore (Mancuso et al. 2012). In Drosophila, the LRR-secreted protein Convoluted (Conv) is necessary for apical matrix organization during tracheal tube morphogenesis (Swanson et al. 2009). However, whether LRR proteins also play a role in the organization of the ECM supporting sensory organs is unknown.
Here we report the identification of Artichoke (ATK, CG5195), a novel Drosophila-secreted LRR protein that localizes to the supporting ECM of all ciliated sensory organs during the late embryonic stages of their morphogenesis. atk mutants generated by mobilization of the P{EPgy2} element Uhg8EY07139 show defects in the stretching of mechanosensory cilia in the lateral pentaloscolopidial (lch5) organ, the most prominent embryonic ch organ, and are locomotion impaired. These anatomical and behavioral phenotypes are rescued if the P{EPgy2} element Uhg8EY07139 is removed. Colocalization of ATK with NOMPA in embryonic chemosensory organs and mutant defects in chemotaxis document that ECM components are also required for chemosensation.
Materials and Methods
Fly strains
w1118 and the P{EPgy2} element line Uhg8EY07139 were obtained from the Bloomington Stock Center. ATK subcellular localization was analyzed using the line GFP-nompA (Chung et al. 2001) (kindly provided by D. F. Eberl, University of Iowa, Iowa City).
Mutagenesis
The hypomorhic alleles atk8 and atk23 were generated by imprecise excision of the P{EPgy2} insertion Uhg8EY07139. The null allele atk33 was generated by P-element-induced male recombination (Preston and Engels 1996; Preston et al. 1996). PCR and sequencing to detect genomic deletions were performed using the primers 5′-CCTGCCTTCTTGATGACAACTT-3′ (forward) and 5′-GATTCCTCCGGCTGAAGAG-3′ (reverse). atk33 was sequenced from the inverse PCR product of the ligation of whole genomic DNA after digestion with HpaII, using the primers 5′-CCTTTCACTCGCACTTATTG-3′(forward) and 5′-GTGAGACAGCGATATGATTGT-3′ (reverse).
In situ hybridization
Whole-mount in situ hybridization was performed using digoxigenin RNA-labeled probes (Tautz and Pfeifle 1989). A 2.5-kb EcoRI atk antisense RNA probe was synthesized using the cDNA clone RE27764. Genotypes examined were w1118, Uhg8EY07139, atk8, atk23, atk33, and Uhg8EY07139rv. Staining was detected using a nitro-blue tetrazolium and 5-bromo-4-chloro-3’-indolyphosphate reaction (Roche, Basel, Switzerland).
Antibody generation
A fusion protein containing amino acids 1403–1523 of ATK was generated using genomic DNA as a template and the primer pair 5′-GCGAATTCAGAGAATCTCCTTGTGCAATCG-3′ (forward) and 5′-ATGGATCCCCACCAGCTTCTCCTCCACT-3′ (reverse) containing EcoRI and BamHI restriction sites, respectively (underlined sequence). The digested fragment was cloned in the glutathione-S-transferase (GST) gene fusion vector pGEX-2T (Promega, Madison, WI) and transformed in Escherichia coli BL21 DE3. Selected clones were verified by sequencing.
After induction, the GST-ATK1403-1523 protein was purified using the Profinia Protein Purification System (BioRad, Hercules, CA) and used for antibody generation in guinea pig following conventional procedures according to directives of the European Commission (2003/65/CE) and Spain (RD 1201/2005; BOE 252/34367-91, 2005) regarding the protection of animals used for experimental and other scientific purposes. Purified anti-ATK serum was used at 1:250.
Immunohistochemistry
Eggs were grown at 25° and collected at the stage required based on midgut morphology (Hartenstein and Campos-Ortega 1985). Staining of whole-mount embryos was performed using standard techniques. Combined detection of atk messenger RNA and marker proteins in Drosophila embryos was performed as described in Patel (1994). The following primary antibodies were used: neuronal marker mAb22C10 (anti-Futsch), mAb2B10 (anti-Cut), anti-Prospero (Vaessin et al. 1991), and mAb8D12 (anti-Repo) (1:50, all from Developmental Studies Hybridoma Bank, Iowa City), anti-α-Tub85E (Matthews et al. 1990) (1:10, kindly provided by Adi Salzberg, Israel Institute of Technology, Haifa, Israel), anti-Suppressor of Hairless (1:100, Santa Cruz Primary Antibodies, Dallas), and mouse anti-GFP (1:100, Roche, Basel, Switzerland). Secondary antibodies used were Alexa 488 donkey anti-rabbit, Alexa 555 donkey anti-mouse, and Alexa 555 goat anti-guinea pig. Additionally, biotinylated anti-rabbit and goat anti-mouse were used for nonfluorescent stainings (all antibodies at 1:500 from Invitrogen, Carlsbad, CA). Imaging was performed on a Zeiss LSM710 confocal microscope (Carl Zeiss, Jena, Germany). Z-series were projected to obtain cross-sectional views using ImageJ v1.42 National Institutes of Health (NIH) software.
Larval crawling analysis
Single wandering third instar larvae were placed on the center of a 135-mm petri dish filled with 1% agarose. Larval movement was recorded over a period of 100 sec in a 25° room. Wild-type, Uhg8EY07139, atk8, atk23, atk33, and Uhg8EY07139rv larvae were tested. Digital video movies were captured with Moticam 350 camera (Edmund Scientific, Tonawanda, NY) and digitalized with Motic Images 2000 (MicroscopeWorld, Carlsbad, CA) 1.2 at 15 frames per second.
Dynamic image analysis
ImageJ v1.42 (NIH) software was used for processing of the digitalized images. High-contrast digital movie frames were analyzed using the ImageJ plugin Mtrack2, which generates a coordinate matrix with the centroid positions of larvae over time. Variables were calculated using Excel (Microsoft, Redmond, WA). Direction changes were counted when the difference between the two angles formed by three vectors binding four consecutive time points was >30°. Path length was calculated by adding all the cartesian distances between two centroid coordinates for consecutive frames.
Gustatory tests
A rectangular surface was divided into two halves filled with either 0.5 M sucrose in 1% agarose or 1% agarose (Heimbeck et al. 1999). To avoid diffusion, plates were poured immediately before testing. Thirty third instar feeding larvae were placed on the edge between surfaces and were allowed to freely move. The distribution of larvae was counted after 15 min. A response index (RI) was calculated for each genotype (RI = Ns − Nc/Ns + Nc, where Ns and Nc are the numbers of larvae present on sucrose and control areas, respectively).
Statistical analysis
The variable “artichoke-like” phenotype or its usual transformations did not follow a normal distribution. Hence, we used the Kruskal–Wallis test, followed by the proper post-hoc procedure to assess statistical differences (Conover 1999). Larval crawling data were normally distributed, while gustatory RIs were transformed into their arccosine that followed a normal distribution. Both sets of data were analyzed by a one-way ANOVA followed by planned multiple pairwise comparisons (Bonferroni correction). All statistical analyses were performed with SPSS13.
Results
atk is specifically expressed in accessory cells of embryonic ciliated sensory organs
To determine which cells express atk, we performed RNA in situ hybridization to whole embryos. Positive hybridization signal was observed in the peripheral nervous system (PNS) in a pattern consistent with atk expression in type I embryonic sensory organs, including ch and es mechanosensory organs (Figure 1A) and chemosensory organs of the head and tail (Figure 1B). Judging from the hybridizations, atk transcription takes place at embryonic stages 15–17. Transcription commenced in the head and the terminal sensilla at stage 15 (image not shown). At early stage 16, transcription started in mechanosensory organs along the body segments. Stage 16 embryos showed the strongest expression. At stage 17, expression vanished in mechanosensory organs along the body segments and weakened in the head and terminal sensilla (not shown). This temporal expression pattern suggests a role for atk in the late development of embryonic ciliated sensory organs that are undergoing the final developmental steps to become functional during larval stages.
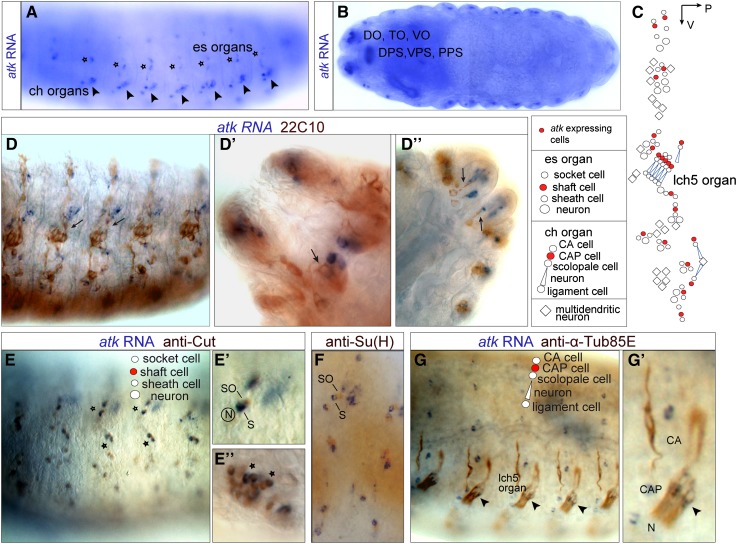
Figure 1.
atk is expressed by an accessory cell in every ciliated sensory organ. (A and B) atk in situ hybridization in wild-type stage 16 embryos. (A) Lateral view showing atk in situ signal in a pattern consistent with expression in ch organs (arrowheads) and es organs (stars). (B) Horizontal view showing atk expression in chemosensory organs: dorsal organ (DO), terminal organ (TO), ventral organ (VO), dorsal, ventral and posterior pharyngeal organs (DPS, VPS, and PPS, respectively). (C) Schematic diagram of mechanosensory organs per hemisegment showing atk-expressing cells (red). (D–G) Embryos double-stained for atk in situ (blue signal) and different primary antibodies (brown signal). (D) atk is not expressed by ciliated neurons labeled by the neuronal marker mAb 22C10 (arrows, D–D′′). (E and F) In es and chemosensory organs, atk is expressed by the shaft cell: it colocalizes with anti-Cut signal (stars, E–E′′), which labels socket (SO) and shaft (S) cells, but does not colocalize with anti-Su(H), which labels the socket (F). (G) In ch organs, atk is expressed by the cap cell (CAP) based on atk-expressing cell position and colocalization with anti-α-Tub85E (arrowheads, G and G′). CA: cap attachment cell; N: neuron.
Type I sensory organs are multicellular, consisting of ciliated sensory neurons and several accessory cells. To assess which of these cells express atk, we counterstained atk hybridization signals with different antibodies. Lack of colocalization of atk RNA with the neuronal marker mAb22C10 signaled that atk is not expressed in the sensory neurons but in associated accessory cells (Figure 1, D–D′′). It also showed that atk is not expressed in multidendritic type II sensory organs.
Chordotonal organs contain five accessory cells: the scolopale cell, which enwraps the cilium and creates a receptor lymph cavity; the ligament cell that proximally suspends the organ (Bodmer et al. 1989); the cap cell, which connects the tip of the cilium to the cuticle via an extracellular cap (Hartenstein 1988; Carlson et al. 1997a; Yack 2004); and two epidermal attachment cells that, suspending the cap and the ligament cells, respectively, seem to keep the organ under stretch (Brewster and Bodmer 1995; Inbal et al. 2004). Most accessory cells, including the ligament, the cap, and the two epidermal attachment cells, express α-Tubulin85E (α-Tub85E) (Matthews et al. 1990; Inbal et al. 2004). Double staining with atk RNA and anti-α-Tub85E yielded colocalization in one of these accessory cells that, judging from its position, is deemed to be the cap cell (Figure 1, G and G′). This notion was corroborated when we counterstained with anti-Repo and anti-Prospero, which stain the ligament cell (Halter et al. 1995) and scolopale cell (Vaessin et al. 1991), respectively, but not the cap cell. No colocalization was seen between atk RNA and these antibodies (data not shown), as expected for a cap cell expression of atk.
Similarly to ch organs, atk is not expressed by the neuron in es organs (Figure 1, D–D′′). Cell lineage studies suggest that the es organ equivalent of the cap cell of ch organs is the socket or tormogen cell (Lai and Orgogozo 2004). This cell secretes the receptor lymph (Keil 1997), with high potassium content and low calcium, and expresses the transcription factor Suppressor of Hairless [Su(H)] (Barolo et al. 2000). atk RNA, however, did not colocalize with anti-Su(H) (Figure 1F), suggesting that the atk-positive cell of es organs is not the socket cell. The other es accessory cells compose the sheath or thecogen cell that encloses the cilium and the trichogen or shaft cell that secretes the cuticular bristle shaft that is seated in the socket; in the es lineage, the cell corresponding to the ligament cell of ch organs enters apoptosis (Fichelson and Gho 2003). All cells of es organs express the transcription factor cut, yet after the in situ hybridization, anti-Cut antibody labeled only the shaft and socket cells. One of these cells expressed atk RNA (Figure 1, E–E′′), and since the socket cell was already ruled out as a candidate, we unambiguously identify the shaft cell as the atk-positive cell. Hence, atk is specifically expressed in certain accessory cells in type I sensory organs, i.e., the cap cells of ch organs and the shaft cells of es organs (Figure 1C).
Generation and expression pattern of atk mutant alleles
atk is located on the third chromosome at 77C3-C4 and is 10,172 bp long. The gene mag is encoded in the opposite DNA strand within the first intron of atk. To generate mutations in the atk locus, we mobilized the P{EPgy2} element Uhg8EY07139 that is inserted 170 bp downstream of the 3′ end of atk (Figure 2A). Two hypomorphic alleles, atk8 and atk23, were generated by imprecise excision of this P-element. Both alleles delete approximately half a kilobase from the 3′ region of the gene; atk8 deletes 514 bp including 142 C-terminal amino acids and atk23 deletes 436 bp including 122 C-terminal amino acids (Figure 2A). Analysis of the atk expression profile in atk8 and atk23 mutant embryos by in situ hybridization revealed that atk expression persists in the chemosensory organs of the embryonic head, but it is no longer detectable in the mechanosensory organs of the body segments (Figure 2C, data for atk8 not shown). Because both mutations equivalently caused this partial loss of atk expression, we used only atk23 in subsequent experiments.
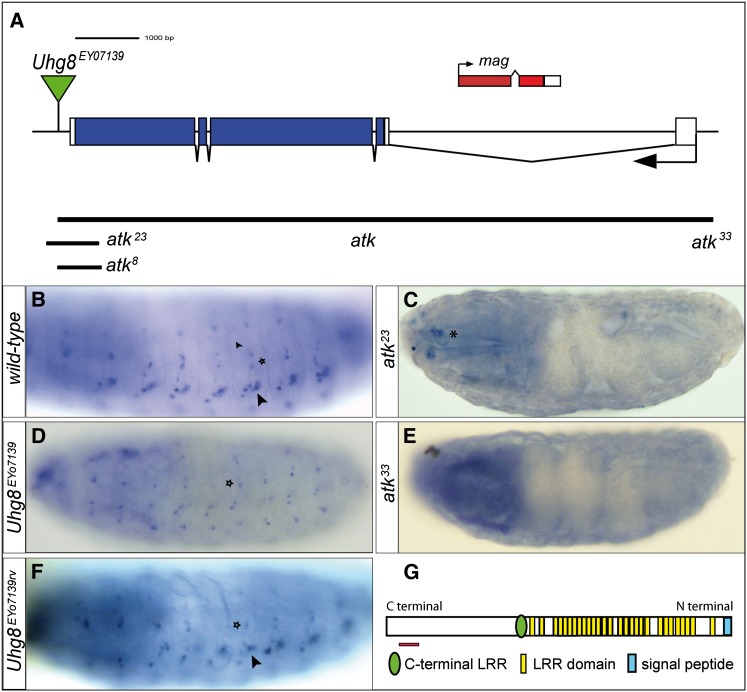
Figure 2.
Generation of atk mutant alleles and anti-ATK antibody. (A) Schematic of atk locus. Shown are the location of the P{EPgy2} element Uhg8EY07139, extent of deletion in the alleles generated—hypomorphs atk8 and atk23 and null allele atk33— and location of the gene mag. (B–F) Stage 16 embryos hybridized with an atk antisense RNA probe. (B) Wild-type embryo showing hybridization in es organs (star) and ch organs (arrowhead). (C) atk23 mutant embryos lack atk expression in mechanosensory organs, but expression persists in chemosensory organs (asterisk). (D) Embryos homozygous for Uhg8EY07139 lose atk expression in ch organs (arrowhead in B and F) but keep it in es organs (star). (E) atk33 homozygous embryos show no atk expression. (F) Uhg8EY07139rv show normal atk expression. (G) Predicted ATK protein with 29 LRRs. Red line indicates the region against which anti-ATK antibody was raised.
Even though a large number of P{EPgy2} element Uhg8EY07139 excisions were analyzed, we did not obtain an atk null mutant. We therefore used P-element-induced male recombination (see Materials and Methods), which produced the null allele atk33 that contained a 18-kb deletion proximal to the P-element insertion site, deleting also the genes mag, CG42764, and CG5955. In situ hybridization of atk33 mutant embryos revealed no detectable expression of atk (Figure 2E), indicating that atk33 is a bonafide null allele. All homozygous atk mutants are viable and fertile.
Partial loss of atk expression, as observed in hypomorphic atk mutants, also characterized the P{EPgy2} line Uhg8EY07139 that was used to generate the mutant atk alleles: judging from in situ hybridizations, atk expression persisted in head sensilla and es organs, but not in ch organs (Figure 2D). The P-element Uhg8EY07139 is not located within the atk-coding region, pointing to an atk expression regulatory unit located at the Uhg8EY07139 insertion site. Removing the P-element Uhg8EY07139 in the line Uhg8EY07139rv restored the complete pattern of atk expression observed in wild-type embryos (Figure 2F).
ATK localizes to the supporting ECM of type I sensory organs
The predicted ATK protein contains 1535 amino acids (Figure 2G), including 29 predicted LRRs. Sequence analysis identifies a signal peptide in amino acids 1–19 (Signal 4.0) and a highly hydrophobic short C-terminal sequence (ProtScale, Swiss Institute of Bioinformatics, Fribourg, Switzerland). Thus, ATK could be either secreted or attached to the external membrane surface of the atk-expressing cell through its carboxy tail. We generated an antibody against the C-terminal region of ATK (amino acids 1403–1523), which is free of LRR domains, to avoid a cross-reaction of antibody recognition sites with other LRR-containing proteins. Antibody specificity was confirmed using Western Blot (Figure 3D). The antiserum labeled all mechanosensory ch and es organs in immunostained embryos, producing a punctate staining adjacent to—and distally from—the tip of the sensory cilium (Figure 3) This staining persists in late stage 17 embryos when atk is no longer being transcribed but disappears during larval stages (Figure 3, E and F). Therefore, ATK protein is present only in a narrow time window, which coincides with the late development of the larval sensilla.
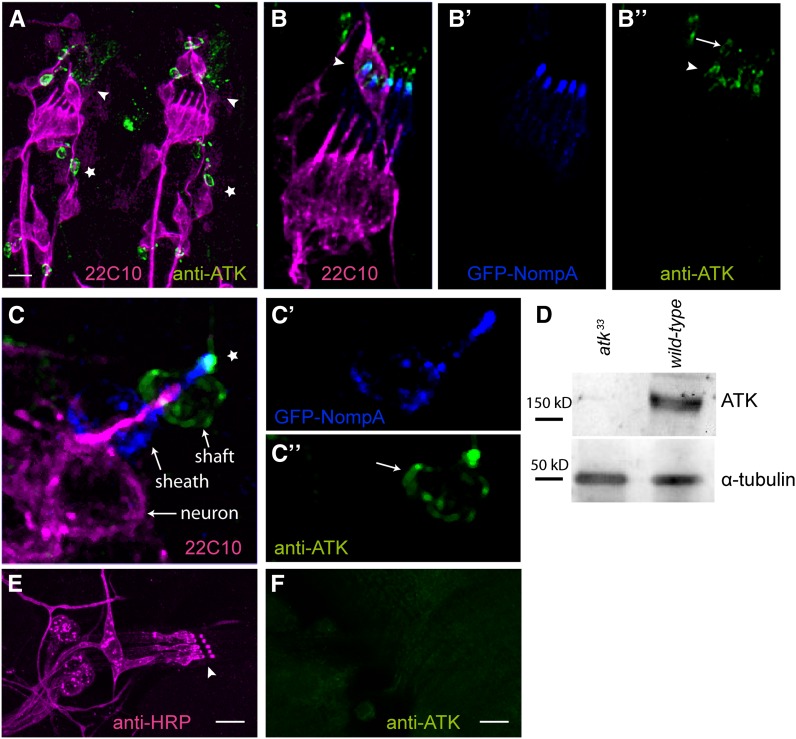
Figure 3.
ATK localizes to the distal region of the dendritic cap in late embryonic mechanosensory organs. (A) Stage 16 embryonic hemisegments immunostained with the neuronal marker mAb22C10 (magenta) and with anti-ATK (green). ATK localizes apically to the sensory cilia in ch (arrowhead) and es (star) organs. (B and C) Detailed view of embryonic mechanosensory organs additionally showing GFP-NOMPA (blue) in the dendritic cap. Single channel images are shown for GFP-NOMPA (B′ and C′) and anti-ATK (B″ and C″). In lch5 (B) and es (C) organs, ATK localizes to the distal region of the dendritic cap (arrowhead in B, star in C), where it partially overlaps with GFP-NOMPA. The cytoplasm of cap and shaft cells are also stained (arrows). (D) Western blot with anti-ATK antibody. (E and F) ATK protein is not present in larval mechanosensory organs. Second instar larval lch5 organ stained with anti-HRP (E) shows no detectable anti-ATK staining. (F) Bars, 10 µm.
We double-immunostained embryos with GFP-NOMPA, a functional fusion protein reporting the dendritic cap localization of NOMPA, which is produced by the scolopale cell of ch organs and the thecogen (sheath) cell of es organs (Chung et al. 2001). Colocalization of ATK and GFP-NOMPA in both ch (Figure 3B) and es (Figure 3C) organs placed ATK in the dendritic cap. ATK antibody also stained the cytoplasm of those accessory cells (cap or shaft) that express atk. Consistent with the hybridization signals, no antibody staining was observed in multidendritic type II sensory organs or outside the PNS (not shown).
Strong anti-ATK staining was seen in embryonic chemosensory organs (Figure 4). The larval head chemosensory system includes three pharyngeal organs [dorsal pharyngeal organ (DPS), ventral pharyngeal organ (VPS), and posterior pharyngeal organs (PPS)] that may serve gustation and mechanosensation (Singh 1997), as well as three external sensory organs, the dorsal organ (DO), the terminal organ (TO), and the ventral organ (VO) organs. Each of these organs consists of several sensilla, with each sensillum comprising one to nine sensory neurons and three accessory cells. The DO ends in a multiporous “dome,” suggesting an olfactory function, and is surrounded by six peripheral sensilla with gustatory function. The TO and VO respond to tastants only and are characterized by a terminal pore (Stocker 2008). We found that ATK is present in all these organ types in stage 16 embryos.
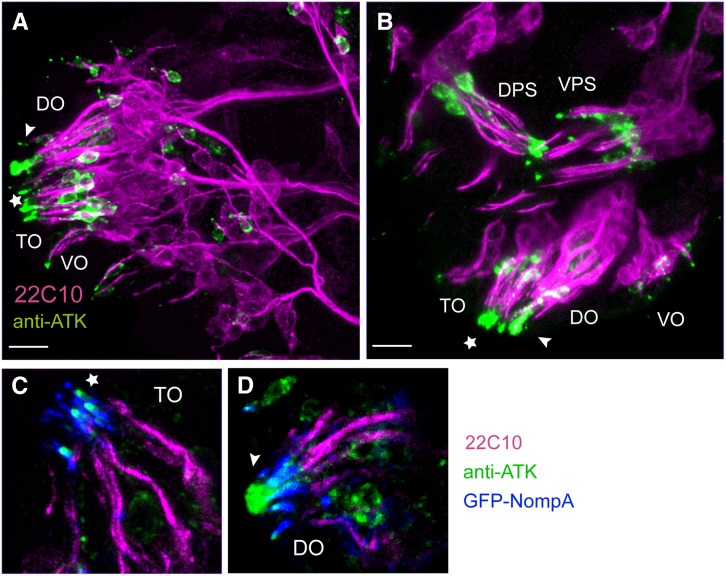
Figure 4.
ATK localizes to a supporting ECM in late embryonic chemosensory organs. (A and B) Stage 16 embryonic chemosensory organs immunostained with the neuronal marker mAb22C10 (magenta) and anti-ATK (green). DO: dorsal organ; TO: terminal organ; VO: ventral organ; DPS: dorsal pharyngeal organ; VPS: ventral pharyngeal organ. In DO (arrowhead) and TO (star), ATK localizes to the tip of the chemosensory cilia. Cell bodies of atk-expressing cells are also stained. Bars, 10 µm. (C and D) Detailed view of TO (C) and DO (D) additionally showing GFP-NOMPA (blue). (C) In TO, ATK localizes at the tip of the GFP-NOMPA region (star), where gustatory neurons are exposed to the environment. (D) In DO, ATK forms a vacuole-like structure in the center of the cilia cluster (arrowhead).
Although the neuronal composition of the larval chemosensory system is well established in Drosophila (Grillenzoni et al. 2007; Stocker 2008), much less is known about the accessory structures. Studies in Musca domestica show that the DO is filled by a large fluid-containing vacuole from the proximal region where the cellular bodies lie to the distal lumen of the dome. Microvilli from the trichogen and tormogen cells protrude into the vacuole and are thought to secret compounds into it. The sensory cilia surround the vacuole and branch after reaching the dome, but are not exposed to the exterior, so that, to reach their olfactory receptors, the chemicals should cross pores that go through the dome (Chu-Wang and Axtell 1971). Our characterization of ATK localization in the Drosophila chemosensory sensilla extends previous descriptions and shows that their structure is remarkably similar to that reported in M. domestica (Chu-Wang and Axtell 1971, 1972a,b). The distribution of the anti-ATK antibody in the late embryonic DO, where it labels a vacuolar-like elongated structure in the middle of the cluster of sensory cilia (Figure 4D), suggests that ATK is probably secreted by the trichogen cell into the central vacuole during the late stages of cilium growth. The protruding appearance of ATK-localizing region suggests that it might fill the dome cavity. On the other hand, in the late embryonic TO and VO, ATK localizes to the supporting ECM that enwraps each cilium up to the point where the cilium enters the cuticular pore and exposes itself to the environment (Figure 4C) (Chu-Wang and Axtell 1972a,b). This is also the case in the gustatory organs from the pharyngeal region. Strong expression was also seen in the anal sensory cones in stage 16 embryos (not shown).
Lack of atk causes defects in the stretching of the chordotonal organs
Since ATK transiently localizes to the supporting ECM of ciliated mechanosensory organs, we analyzed atk mutant embryos for morphological defects of the lch5 organ, the most prominent embryonic ch organ.
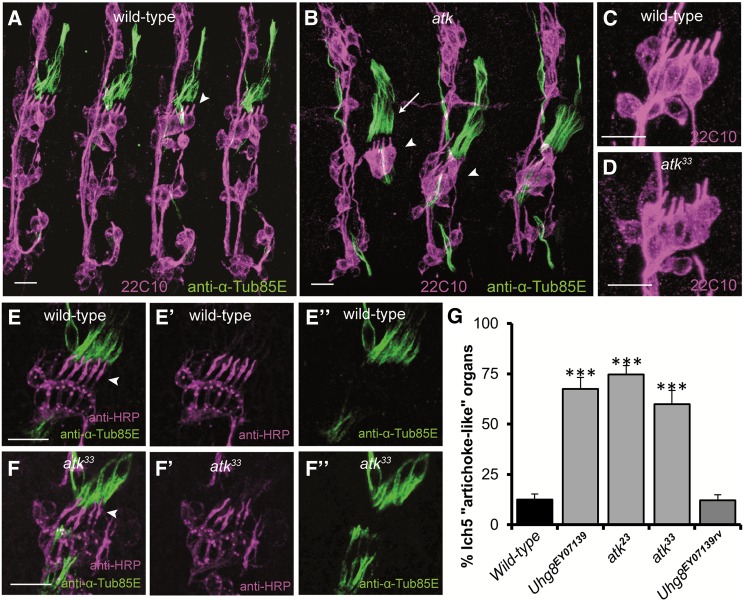
To visualize the sensory neurons and cap cells, we double-stained late-stage embryos with either neuronal marker mAb22C10 or anti-HRP and with anti-α-Tub85E (Matthews et al. 1990). The lch5 organ comprises five scolopidia that are arranged in a row with their cilia pointing to the dorsal posterior region of the embryo (Figure 5, A and C). Their tips penetrate the cap cells and contact them through the dendritic cap (Figure 5E). Cilia are parallel and equidistant to each other. All atk mutant alleles showed defects in the morphology of embryonic lch5 organs (Figure 5, B and D). Both cilia and neuronal cell bodies were affected. Cilia were not parallel to each other, but pointed out in different directions (Figure 5, B and D). However, staining mutant embryos with anti-HRP, a neuronal marker that stains the cilia up to their tips (mAb22C10 stains only the inner dendritic segment that ends at the basal body; compare Figure 5, C and E), revealed that atk mutant cilia still contact the cap cell (Figure 5F). Neuronal cell bodies also appeared frequently disorganized in atk mutant lh5 organs (Figure 5, B and D). We considered a lch5 organ to be “artichoke-like” when both the orientation of its cilia and the arrangement of its cell bodies were defective. A morphological blind scoring showed that defects were equally penetrant in hypomorphic atk23 and null atk33 mutants, as well as in homozygous Uhg8EY07139 mutants (Figure 5G), consistent with the complete loss of atk expression in ch organs observed in these strains (Figure 2D). Removing the P element in Uhg8EY07139rv flies fully rescued the morphological defects (Figure 5G).
Figure 5.
atk mutants show abnormal embryonic lch5 organ morphology. (A–D) Stage 16 embryonic hemisegments stained with neuronal marker mAb22C10 (magenta, labeling cell body and inner dendritic segment) and anti-α-Tub85E (green), which labels all ch organ accessory cells except the scolopale cell. (A and C) In wild-type lch5 organs, sensory cilia are parallel and point in the same direction (arrowhead). Neuronal cell bodies are aligned in a row. (B and D) In atk33 embryos, many lch5 organs show an artichoke-like phenotype with nonparallel dendrites and misaligned cell bodies (arrowheads in B). Cap cells also show defects (arrow in B). (E and F) Lch5 organs of stage 16 embryos, stained with anti-HRP (magenta, E′ and F′) that labels the entire cilium, and anti-α-Tub85E (green, E″ and F″). The cilium tip contacts the dendritic cap in both wild-type (E, arrowhead) and atk33 mutants (F, arrowhead). Bars, 10 µm. (G) Percentage of artichoke-like lch5 organs per hemisegment and genotype. Embryos were stained with mAb22C10 to label the sensory neurons and scored blind to genotype. Significant difference was established by the Kruskal–Wallis test followed by a nonparametric post-hoc test to compare each genotype with wild type. ***P < 0.001.
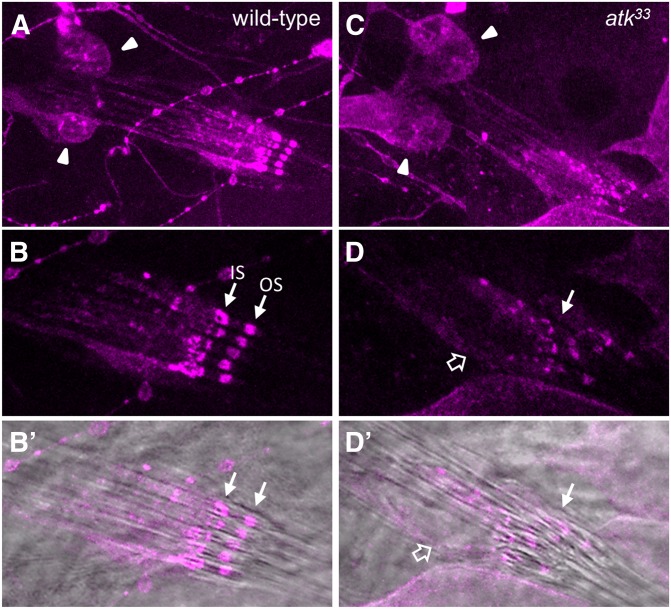
Despite its transient expression, ATK is required for the correct embryonic development of chordotonal organs which, although grossly normal, often show misplacement of cilia and/or neuronal soma. We analyzed the consequences of these defects at later stages, during the second and third larval instars (Figure 6). Cap and ligament cells of larval lch5 were normally stretched in atk mutant larvae (not shown), but, as in embryos, neuronal cilia were frequently disorganized (compare Figure 6, B and D). Additionally, anti-HRP signal was reduced in all lch5 mutant cilia and severely mislocalized, even though neuronal soma had similar levels of immunoreactivity (Figure 6). These alterations suggest functional defects in mutant larval mechanosensory receptors.
Figure 6.
atk mutant larvae show abnormal lch5 organ morphology and altered anti-HRP signal in cilia. Third instar larval lch5 organs stained with anti-HRP (magenta) in control (A and B) and atk33 mutants (C and D). (A and C) General view of neuronal cell bodies (arrowheads) and cilia. (B and D) High magnification of cilia showing anti-HRP signal, alone (B and D) or merged with a bright-field image (B′ and D′). In control lh5 organs (B), cilia are parallel and aligned with anti-HRP signal concentrated in two bands corresponding to inner (IS) and outer (OS) segments (Ma and Jarman 2011). In atk mutants (D), alignment of cilia is lost (open arrow points to a cilium that is bent and separated from the others), and distribution of anti-HRP immunoreactivity is severely disrupted.
atk mutant larvae exhibit locomotion defects
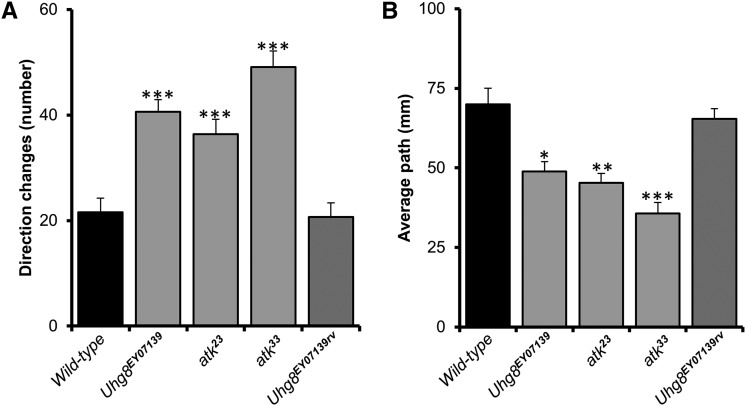
Many mutations affecting larval ch organs cause locomotion defects. Although muscle contractions that direct larval locomotion are controlled by the central nervous system, feedback from ch organs is required to coordinate the larval movement (Caldwell et al. 2003; Cheng et al. 2010). To test whether atk mutants display impaired locomotion, we assayed the crawling behavior of third instar larvae. Consistent with the anatomical defects in lch5 organs, all atk mutants exhibited severe defects in larval locomotion: the number of direction changes was significantly increased (Figure 7A) and the total path length was significantly reduced (Figure 7B). In the mutants, phases of translational movement were scarce and short, and larvae often stalled at the same point, turning their bodies several times in different directions. The severity of the defect was similar in all atk alleles, including Uhg8EY07139. The fact that Uhg8EY07139 homozygous larvae—which no longer show detectable atk expression in ch organs but keep a detectable expression in es organs—also show locomotive defects confirms previous works that propose that ch organs, in contrast to es organs, are major contributors to locomotive sensory feedback (Caldwell et al. 2003; Cheng et al. 2010).
Figure 7.
atk mutants show larval locomotion defects. Locomotion was scored for 100 sec by measuring the total number of direction changes (A) and the average path length (mm) (B). Means are represented. Error bars indicate SEM. Significant difference with respect to wild type was established by Bonferroni-corrected t-test. *P < 0.05; ** P < 0.01; ***P < 0.005.
Locomotion defects were fully rescued in Uhg8EY07139rv revertants, highlighting the importance of ATK and ch organs for coordinated larval movements.
ATK is required for an adequate larval response to sucrose
ATK is transitorily present in all gustatory organs of Drosophila embryos. In the main embryonic gustatory organs, VO and TO, ATK localizes just underneath the base of the pore through which each gustatory cilium projects (Figure 4C). Our attempt to detect anatomical defects in atk mutants was unsuccessful because of the lack of a simple stereotyped cilium pattern in chemosensory organs that renders the identification of anatomical defects a difficult task.
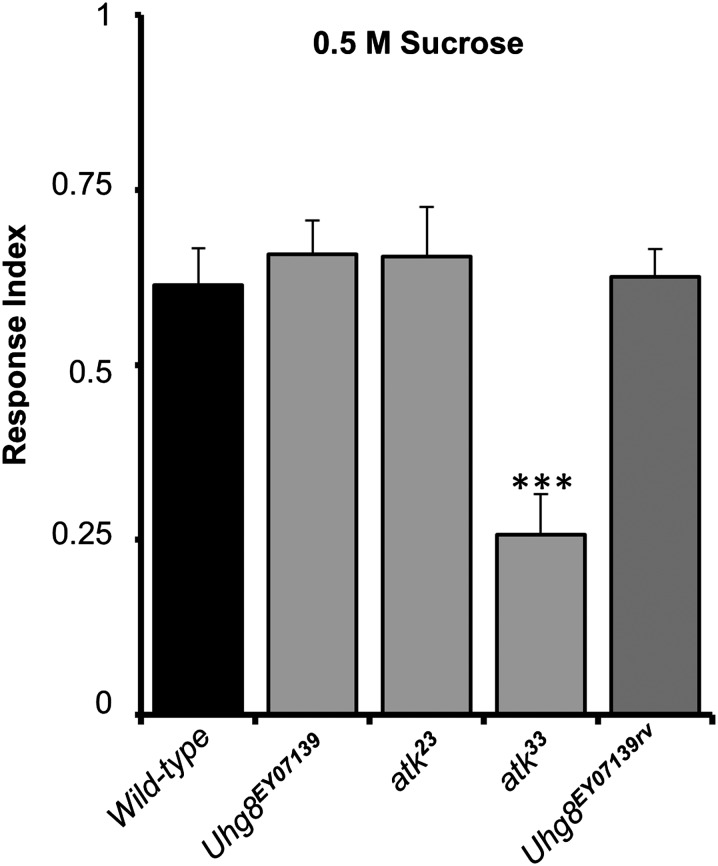
Therefore, to assess the requirement of ATK in chemosensation, we tested the response of larvae to sucrose, which induces positive chemotaxis (Miyakawa 1982). RI was calculated after allowing larvae to choose for 15 min between 1% agarose and 1% agarose supplemented 0.5 M sucrose. Among the atk mutant alleles, only atk33 null mutants showed a reduced response to sucrose; neither the hypomorphic atk23 and Uhg8EY07139 larvae nor the Uhg8EY07139rv revertants displayed chemosensory defects (Figure 8). These results seem consistent with the finding that atk expression in gustatory organs is abolished in the null mutants but persists in hypomorphic animals. However, we should be cautious interpreting these results because we cannot exclude the effect of additional genes removed by the atk33 deletion. Our attempts to rescue the anatomical and behavioral phenotypes with a Gal4-driven UAS-atk construct failed (not shown), probably as a consequence of the inability to reproduce the transient expression of the gene. Still, the gustatory impairment is consistent with ATK expression in gustatory sensilla and suggests that the ECM also plays essential roles in this type of ciliated sensory organs.
Figure 8.
atk mutant larvae show a significantly reduced response to sucrose. Response index for election between 1% agarose and 1% agarose + 0.5 M sucrose. atk33 null mutants show defects in chemosensation. atk23 hypomorphs and Uhg8EY07139 homozygous larvae, which keep atk expression in chemosensory organs, do not show defects. Therefore, ATK seems to be required for chemosensation, although an effect of additional genes deleted by the atk33 deficiency cannot be ruled out. Error bars indicate SEM. Significant difference with respect to wild type was established by Bonferroni-corrected t-test. ***P < 0.001.
Discussion
In this study we have tried to shed some light on the function of the supporting ECM in sensory organs by characterizing the novel secreted LRR protein Artichoke in Drosophila melanogaster. ATK transiently localizes to the most distal region of the ECM in ciliated sensory organs undergoing their late embryonic developmental steps. The presence of ATK in all ciliated sensory organs, its conservation among organisms with primary cilia (Avidor-Reiss et al. 2004), and the behavioral defects shown by atk mutants suggest a role for ATK in cilia assembly and function. Our work additionally suggests that a proper coupling between the sensory cilium and the supporting ECM is also essential for the correct assembly of chemosensory organs. To our knowledge, these results provide the first experimental evidence for an ECM role in Drosophila chemosensation.
ATK is a component of the sensilla ECM secreted by the Cap and Shaft cells
During the late stages of sensillum morphogenesis, atk is expressed in all embryonic ciliated sensory organs, including both chemo- and mechanoreceptors. ATK is the first molecule to be described that is secreted into the ECM by the shaft cell in es and chemosensory organs and by the cap cell in ch organs. In Musca, the matrix of the chemoreceptors is thought to be secreted by the trichogen and tormogen cells (Chu-Wang and Axtell 1971), as is the case for ATK. In Drosophila, this matrix was previously believed to be secreted only by the thecogen or scolopale cell (Hartenstein 1988; Carlson et al. 1997b). Our data provide the first direct evidence for assigning to cap and shaft cells a role in the secretion of essential components of the extracellular matrix, reinforcing the importance of these accessory cells for the development of the ciliary sensilla (Mrkusich et al. 2010).
The observation that the mutant alleles differentially affect atk expression in ch, es, and chemosensory sensilla suggests the presence of organ-specific regulatory units that control atk transcription in different types of sensory organs. A similar phenomenon has been described for the ciliogenesis regulator regulatory factor X (RFX): although RFX is expressed in both ch and es organs, it is activated by different enhancers in both organ types and seems to be a direct target of the proneural gene atonal in ch organs but an indirect target of the scute proneural gene in es organs (Cachero et al. 2011). The existence of different transcriptional regulatory elements could explain the temporal and quantitative variations in atk expression observed in mechanosensory ch, es, and chemosensory sensilla.
ATK is an ECM protein implicated in the late morphogenesis of ciliated sensory organs
Since ATK is present in all embryonic ciliated sensory organs in Drosophila, it seems likely that it was already present in the ancestral ciliated sensory organ and has been preserved in the diversified sensory organs to play a similar, essential function (Lai and Orgogozo 2004). Previous support for an evolutionarily conserved association between ATK and cilia came from the study by Avidor-Reiss et al. (2004), in which several ciliogenesis genes were identified. Through comparative genomic analysis of ciliated and nonciliated organisms, atk was shown to be specifically present in ciliated organisms, including Trypanosoma brucei, Drosophila, and humans. Because all the atk-containing organisms assemble at least some of their cilia through compartmentalized ciliogenesis—the process in which the cilium protrudes gradually from the basal bodies located under the plasma membrane—ATK was classified as a compartment gene (Avidor-Reiss et al. 2004). However, our results show that ATK is not strictly required for cilium growth because atk mutants apparently form cilia of normal length. Instead, we propose that ATK-like proteins are extracellular proteins involved in connecting cilia to accessory structures during late organ refinement.
Our data showing partial extracellular colocalization of ATK and NOMPA indicate that ATK transiently localizes to the site of contact between the cilium and distal accessory cells in embryonic mechanosensory sensilla and are consistent with a similar distribution in chemoreceptors (Hartenstein 1988; Yack 2004; Hartenstein 2005; Keil 2012). Although ch organs reach their basic final shape and position at stage 15, further refinement occurs during later stages, at the time when ATK expression peaks (Carlson et al. 1997b; Inbal et al. 2003; Kraut and Zinn 2004; Hartenstein 2005). Several studies suggest the importance of the accessory cells in this final process of organ refinement: in the v’ch1 chordotonal organ (Mrkusich et al. 2010), the positioning of the growing ciliary dendrite has shown to result from pulling forces that the dorsally extending cap cell exerts on the dendrite tip; and in the lch5 organ, the interaction between ligament and ligament attachment cells ventrally straightens the whole organ (Inbal et al. 2004; Klein et al. 2010). Thus, to ensure proper spatial and temporal transmission of the morphogenetic forces, the different components of the sensillum must interact adequately. The finding that lack of ATK during just these late stages has dramatic consequences on the overall morphology and functioning of the organ may reflect the requirement of a specialized ECM that maintains the apical components intimately communicated by conferring particular adhesive and signaling properties. Our results further suggest that the ECM in chemoreceptors not only plays a physiological function (Ebbs and Amrein 2007), but also is required for the proper maturation also of these organs.
The temporal and spatial localization of ATK suggests a developmental role for this protein in the very late morphogenetic events leading to the assembly of ciliated sensory sensilla. The role that ATK is playing in other ciliated organisms remains an interesting question. The fact that an extracellular molecule expressed in Drosophila by an accessory cell rather than by the ciliated cell is conserved among ciliated organisms is intriguing, especially for unicellular organisms like Trypanosoma brucei (Avidor-Reiss et al. 2004). However, this organism uses its flagellum to attach to the gut of the tsetse fly prior to infection (Dyer et al. 2013), and an adhesion molecular machinery is required for this process. A speculative, attractive hypothesis is that adhesion mechanisms that facilitate the connection of cilia are conserved in evolution independently of whether their purpose is infection or properly connecting sensory cilia to the stimulus-detecting apparatus.
In summary, our work shows that terminal differentiation of ciliated sensory organs in Drosophila is finely tuned by proteins like ATK that, although transiently expressed, have long-lasting functional effects. As far as we know, there is only one other protein that has a comparable influence: the β3-tubulin, which is expressed transitorily by the cap cell in ch organs at the same stages as atk, seems to confer permanent features of microtubule organization that persist in the fully differentiated cell, even after β3 is no longer present (Dettman et al. 2001). This highlights the importance of accessory cellular and extracellular structures not only for the process of sensory transduction, but also for the correct organ maturation.
Acknowledgments
We thank the J. F. de Celis Lab, especially C. Molnar and M. Organista, for helping us to carry out some of the experiments in this work; A. Salzberg, D. F. Eberl, and A. Baonza for providing us with flies and antibodies; J. M. Barcia for the design of the software to analyze the digital movies from the larval locomotion assay; and I. Monedero for assistance with confocal imaging. This study was supported by research grants from the Ministry of Science and Innovation of Spain (BFU2008-04683-C02-02/BMC to L.T.) and the German Science Foundation (SFB 889-C2, to M.C.G.). M.A. was supported by a fellowship from Universidad Autónoma de Madrid (Ayuda para la Formación de Personal Investigador) during 2008–2011.
Footnotes
Communicating editor: J. Sekelsky
Literature Cited
- Avidor-Reiss T., Maer A. M., Koundakjian E., Polyanovsky A., Keil T., et al. , 2004. Decoding cilia function: defining specialized genes required for compartmentalized cilia biogenesis. Cell 117: 527–539. [DOI] [PubMed] [Google Scholar]
- Barolo S., Walker R. G., Polyanovsky A. D., Freschi G., Keil T., et al. , 2000. A notch-independent activity of suppressor of hairless is required for normal mechanoreceptor physiology. Cell 103: 957–969. [DOI] [PubMed] [Google Scholar]
- Bodmer R., Carretto R., Jan Y. N., 1989. Neurogenesis of the peripheral nervous system in Drosophila embryos: DNA replication patterns and cell lineages. Neuron 3: 21–32. [DOI] [PubMed] [Google Scholar]
- Brewster R., Bodmer R., 1995. Origin and specification of type II sensory neurons in Drosophila. Development 121: 2923–2936. [DOI] [PubMed] [Google Scholar]
- Cachero S., Simpson T. I., Lage P. I. Z., Ma L., Newton F. G., et al. , 2011. The gene regulatory cascade linking proneural specification with differentiation in Drosophila sensory neurons. PLoS Biol. 9: e1000568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell J. C., Miller M. M., Wing S., Soll D. R., Eberl D. F., 2003. Dynamic analysis of larval locomotion in Drosophila chordotonal organ mutants. Proc. Natl. Acad. Sci. USA 100: 16053–16058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson S. D., Hilgers S. L., Juang J. L., 1997a First developmental signs of the scolopale (glial) cell and neuron comprising the chordotonal organ in the Drosophila embryo. Glia 19: 269–274. [PubMed] [Google Scholar]
- Carlson S. D., Hilgers S. L., Juang J. L., 1997b Ultrastructure and blood-nerve barrier of chordotonal organs in the Drosophila embryo. J. Neurocytol. 26: 377–388. [DOI] [PubMed] [Google Scholar]
- Chalfie M., Au M., 1989. Genetic control of differentiation of the Caenorhabditis elegans touch receptor neurons. Science 243: 1027–1033. [DOI] [PubMed] [Google Scholar]
- Chalfie M., Sulston J., 1981. Developmental genetics of the mechanosensory neurons of Caenorhabditis elegans. Dev. Biol. 82: 358–370. [DOI] [PubMed] [Google Scholar]
- Cheng L. E., Song W., Looger L. L., Jan L. Y., Jan Y. N., 2010. The role of the TRP channel NompC in Drosophila larval and adult locomotion. Neuron 67: 373–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung Y. D., Zhu J., Han Y., Kernan M. J., 2001. NompA encodes a PNS-specific, ZP domain protein required to connect mechanosensory dendrites to sensory structures. Neuron 29: 415–428. [DOI] [PubMed] [Google Scholar]
- Chu-Wang I., Axtell R. C., 1971. Fine structure of the dorsal organ of the house fly larva, Musca domestica L. Z. Zellforsch. Mikrosk. Anat. 117: 17–34. [DOI] [PubMed] [Google Scholar]
- Chu-Wang I., Axtell R. C., 1972a Fine structure of the terminal organ if the house fly, Musca domestica L. Z. Zellforsch. Mikrosk. Anat. 127: 287–305. [DOI] [PubMed] [Google Scholar]
- Chu-Wang I., Axtell R. C., 1972b Fine structure of the ventral organ of the house fly larva, Musca domestica L. Z. Zellforsch. Mikrosk. Anat. 130: 489–495. [DOI] [PubMed] [Google Scholar]
- Conover, W. J., 1999 Measures of rank correlation, pp. 312–332 in Practical Nonparametric Statistics, Ed. 3. John Wiley & Sons, New York. [Google Scholar]
- Dettman R. W., Turner F. R., Hoyle H. D., Raff E. C., 2001. Embryonic expression of the divergent Drosophila beta3-tubulin isoform is required for larval behavior. Genetics 158: 253–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolan J., Walshe K., Alsbury S., Hokamp K., O’Keeffe S., et al. , 2007. The extracellular leucine-rich repeat superfamily: a comparative survey and analysis of evolutionary relationships and expression patterns. BMC Genomics 8: 320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubruille R., Laurencon A., Vandaele C., Shishido E., Coulon-Bublex M., et al. , 2002. Drosophila regulatory factor X is necessary for ciliated sensory neuron differentiation. Development 129: 5487–5498. [DOI] [PubMed] [Google Scholar]
- Dyer N. A., Rose C., Ejeh N. O., Acosta-Serrano A., 2013. Flying tryps: survival and maturation of trypanosomes in tsetse flies. Trends Parasitol. 29: 188–196. [DOI] [PubMed] [Google Scholar]
- Ebbs M. L., Amrein H., 2007. Taste and pheromone perception in the fruit fly Drosophila melanogaster. Pflugers Arch. 454: 735–747. [DOI] [PubMed] [Google Scholar]
- Emtage L., Gu G. Q., Hartwieg E., Chalfie M., 2004. Extracellular proteins organize the mechanosensory channel complex in C. elegans touch receptor neurons. Neuron 44: 795–807. [DOI] [PubMed] [Google Scholar]
- Fichelson P., Gho M., 2003. The glial cell undergoes apoptosis in the microchaete lineage of Drosophila. Development 130: 123–133. [DOI] [PubMed] [Google Scholar]
- Gerdes J. M., Davis E. E., Katsanis N., 2009. The vertebrate primary cilium in development, homeostasis, and disease. Cell 137: 32–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie P. G., Walker R. G., 2001. Molecular basis of mechanosensory transduction. Nature 413: 194–202. [DOI] [PubMed] [Google Scholar]
- Göpfert M. C., Robert D., 2003. Motion generation by Drosophila mechanosensory neurons. Proc. Natl. Acad. Sci. USA 100: 5514–5519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillenzoni N., de Vaux V., Meuwly J., Vuichard S., Jarman A., et al. , 2007. Role of proneural genes in the formation of the larval olfactory organ of Drosophila. Dev. Genes Evol. 217: 209–219. [DOI] [PubMed] [Google Scholar]
- Halter D. A., Urban J., Rickert C., Ner S. S., Ito K., et al. , 1995. The homeobox gene repo is required for the differentiation and maintenance of glia function in the embryonic nervous system of Drosophila melanogaster. Development 121: 317–332. [DOI] [PubMed] [Google Scholar]
- Hartenstein V., 1988. Development of Drosophila larval sensory organs: spatiotemporal pattern of sensory neurons, peripheral axonal pathways and sensilla differentiation. Development 102: 869–886. [Google Scholar]
- Hartenstein, V., 2005 Development of insect sensilla, pp. 379–419 in Comprehensive Molecular Insect Science, edited by L. I. Gilbert, K. Iatrou, and S. S. Gill. Elsevier, Amsterdam; New York. [Google Scholar]
- Hartenstein V., Campos-Ortega J., 1985. Fate-mapping in wild type Drosophila melanogaster. Rouxs Arch. Dev. Biol. 194: 181–195. [Google Scholar]
- Heimbeck G., Bugnon V., Gendre N., Haberlin C., Stocker R. F., 1999. Smell and taste perception in Drosophila melanogaster larva: toxin expression studies in chemosensory neurons. J. Neurosci. 19: 6599–6609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inbal A., Levanon D., Salzberg A., 2003. Multiple roles for u-turn/ventral veinless in the development of Drosophila PNS. Development 130: 2467–2478. [DOI] [PubMed] [Google Scholar]
- Inbal A., Volk T., Salzberg A., 2004. Recruitment of ectodermal attachment cells via an EGFR-dependent mechanism during the organogenesis of Drosophila proprioceptors. Dev. Cell 7: 241–250. [DOI] [PubMed] [Google Scholar]
- Keil T. A., 1997. Functional morphology of insect mechanoreceptors. Microsc. Res. Tech. 39: 506–531. [DOI] [PubMed] [Google Scholar]
- Keil T. A., 2012. Sensory cilia in arthropods. Arthropod Struct. Dev. 41: 515–534. [DOI] [PubMed] [Google Scholar]
- Kernan M. J., 2007. Mechanotransduction and auditory transduction in Drosophila. Pflugers Arch. 454: 703–720. [DOI] [PubMed] [Google Scholar]
- Klein Y., Halachmi N., Egoz-Matia N., Toder M., Salzberg A., 2010. The proprioceptive and contractile systems in Drosophila are both patterned by the EGR family transcription factor Stripe. Dev. Biol. 337: 458–470. [DOI] [PubMed] [Google Scholar]
- Kraut R., Zinn K., 2004. Roundabout 2 regulates migration of sensory neurons by signaling in trans. Curr. Biol. 14: 1319–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai E. C., Orgogozo V., 2004. A hidden program in Drosophila peripheral neurogenesis revealed: fundamental principles underlying sensory organ diversity. Dev. Biol. 269: 1–17. [DOI] [PubMed] [Google Scholar]
- Ma L., Jarman A. P., 2011. Dilatory is a Drosophila protein related to AZI1 (CEP131) that is located at the ciliary base and required for cilium formation. J. Cell Sci. 124: 2622–2630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancuso V. P., Parry J. M., Storer L., Poggioli C., Nguyen K. C. Q., et al. , 2012. Extracellular leucine-rich repeat proteins are required to organize the apical extracellular matrix and maintain epithelial junction integrity in C. elegans. Development 139: 979–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews K. A., Miller D. F., Kaufman T. C., 1990. Functional implications of the unusual spatial distribution of a minor alpha-tubulin isotype in Drosophila: a common thread among chordotonal ligaments, developing muscle, and testis cyst cells. Dev. Biol. 137: 171–183. [DOI] [PubMed] [Google Scholar]
- McGlashan S. R., Jensen C. G., Poole C. A., 2006. Localization of extracellular matrix receptors on the chondrocyte primary cilium. J. Histochem. Cytochem. 54: 1005–1014. [DOI] [PubMed] [Google Scholar]
- Miyakawa Y., 1982. Behavioural evidence for the existence of sugar, salt and amino acid taste receptor cells and some of their properties in Drosophila larvae. J. Insect Physiol. 28: 405–410. [Google Scholar]
- Mrkusich E. M., Osman Z. B., Bates K. E., Marchingo J. M., Duman-Scheel M., et al. , 2010. Netrin-guided accessory cell morphogenesis dictates the dendrite orientation and migration of a Drosophila sensory neuron. Development 137: 2227–2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel N. H., 1994. Imaging neuronal subsets and other cell-types in whole-mount Drosophila embryos and larvae using antibody probes. Methods Cell Biol. 44: 445–487. [DOI] [PubMed] [Google Scholar]
- Preston C. R., Engels W. R., 1996. P-element-induced male recombination and gene conversion in Drosophila. Genetics 144: 1611–1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston C. R., Sved J. A., Engels W. R., 1996. Flanking duplications and deletions associated with P-induced male recombination in Drosophila. Genetics 144: 1623–1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh R. N., 1997. Neurobiology of the gustatory systems of Drosophila and some terrestrial insects. Microsc. Res. Tech. 39: 547–563. [DOI] [PubMed] [Google Scholar]
- Stocker, R. F., 2008 Design of the larval chemosensory system. Adv. Exp. Med. Biol. 628: 69–81. [DOI] [PubMed] [Google Scholar]
- Swanson L. E., Yu M., Nelson K. S., Laprise P., Tepass U., et al. , 2009. Drosophila convoluted/dALS is an essential gene required for tracheal tube morphogenesis and apical matrix organization. Genetics 181: 1281–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tautz D., Pfeifle C., 1989. A non-radioactive in situ hybridization method for the localization of specific RNAs in Drosophila embryos reveals translational control of the segmentation gene hunchback. Chromosoma 98: 81–85. [DOI] [PubMed] [Google Scholar]
- Vaessin H., Grell E., Wolff E., Bier E., Jan L. Y., et al. , 1991. Prospero is expressed in neuronal precursors and encodes a nuclear protein that is involved in the control of axonal outgrowth in Drosophila. Cell 29: 941–953. [DOI] [PubMed] [Google Scholar]
- Yack J. E., 2004. The structure and function of auditory chordotonal organs in insects. Microsc. Res. Tech. 63: 315–337. [DOI] [PubMed] [Google Scholar]