Abstract
Background.
Vascular endothelial growth factor (VEGF) and VEGF receptor 2 (VEGFR-2) are believed to mediate angiogenesis in colorectal cancer (CRC). Ramucirumab (RAM; IMC-1121B) is a human IgG1 monoclonal antibody that inhibits VEGF ligand binding to VEGFR-2, inhibiting VEGFR-2 activation and signaling.
Methods.
Patients with metastatic CRC, Eastern Cooperative Oncology Group performance status 0–1, and adequate organ function who had not received chemotherapy for metastatic disease received RAM and the modified FOLFOX-6 regimen every 2 weeks. Endpoints included progression-free survival (PFS), objective response rate, overall survival, and safety. The sample size was based on a potentially improved median PFS from 8 months to 11 months.
Results.
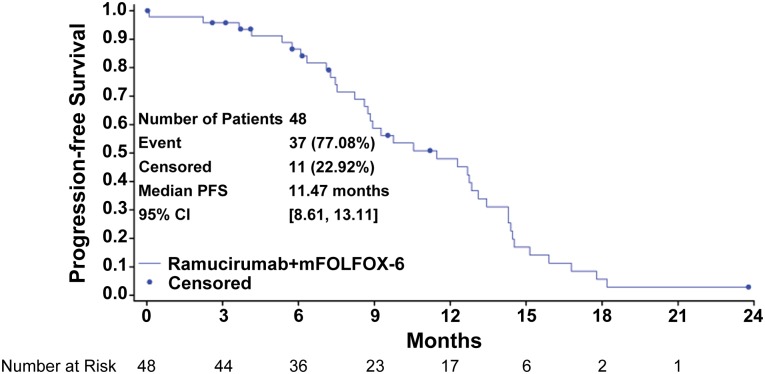
Forty-eight patients received therapy. Median PFS was 11.5 months (95% confidence interval [CI]: 8.6–13.1 months). The objective response rate was 58.3% (95% CI: 43.21–72.39). The disease control rate (complete or partial response plus stable disease) was 93.8% (95% CI: 82.8–98.7). Median overall survival was 20.4 months (95% CI: 18.5–25.1 months). The most frequent grade 3–4 adverse events included neutropenia (grade 3: 33.3%; grade 4: 8.3%), hypertension (grade 3: 16.7%), and neuropathy (grade 3: 12.5%). Two patients died during the study due to myocardial infarction and cardiopulmonary arrest.
Conclusion.
RAM may enhance the efficacy of modified FOLFOX-6 chemotherapy with an acceptable safety profile in metastatic CRC.
Author Summary
Discussion
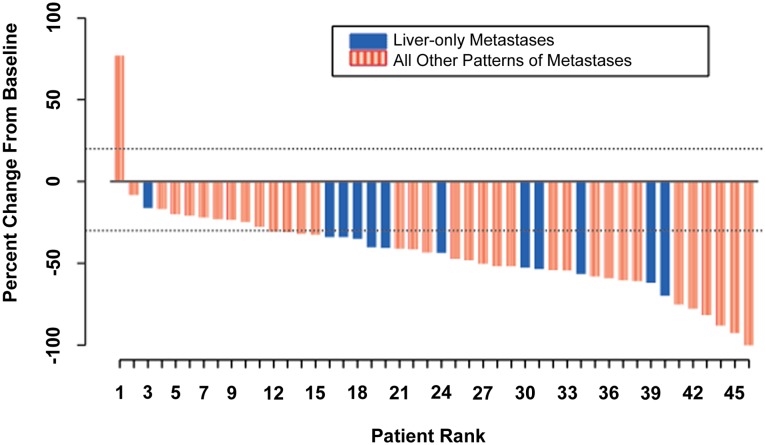
The combination of ramucirumab (RAM) and the modified FOLFOX-6 regimen (mFOLFOX-6) appears efficacious in the first-line treatment of patients with metastatic colorectal cancer (mCRC). The median progression-free survival (PFS) of 11.5 months (Fig. 1), an objective response rate of 58.3%, a disease control rate of 93.8% (stable disease defined as neither shrinkage sufficient to qualify for partial response nor increase sufficient to qualify for progressive disease, taking as a reference the smallest sum longest diameter since the start of treatment), and median overall survival (OS) of 20.4 months are encouraging and suggest that RAM may enhance the efficacy of mFOLFOX-6 in mCRC. Figure 2 shows that the majority of the study population experienced some tumor burden reduction, including patients with liver-only disease and those with more extensive patterns of metastases. Although many patients discontinued oxaliplatin after 5–8 months of therapy, 23% continued to receive RAM and 5-fluorouracil with ongoing disease control for more than 5 months after discontinuation of oxaliplatin. The median OS was 20.4 months.
Figure 1.
Progression-free survival curve: Kaplan-Meier plot for progression-free survival for all patients.
Abbreviations: CI, confidence interval; mFOLFOX-6, modified FOLFOX-6 regimen; PFS, progression-free survival.
Figure 2.
Waterfall plot of best percentage change from baseline in size of target tumor lesions. Best change in target-lesion size is maximum reduction from baseline or minimum increase in absence of reduction.
The incidence of most adverse events in patients receiving RAM and mFOLFOX-6 was consistent with the known adverse event profile of mFOLFOX-6 in mCRC [1–6]. Hypertension (including 16.7% at grade 3 and no grade 4) and proteinuria (12.5% at grade 2 and one grade 4 nephrotic syndrome) were observed. Two patients experienced grade 5 potential arterial thromboembolic events (myocardial infarction and cardiopulmonary arrest), and three patients had grade 3–4 venous thromboembolic events (pulmonary embolism, deep vein thrombosis, jugular vein thrombosis).
Exploratory pharmacokinetic, pharmacodynamic, and correlative analyses were conducted in samples collected from nine patients. Mean trough levels after repeated dosing of 8 mg/kg of RAM every 2 weeks exceeded concentrations associated with antitumor activity in preclinical models. Higher baseline levels of soluble Flt-1 (soluble VEGFR-1) and VEGF-A and lower baseline levels of VEGF-D appeared to be associated with longer PFS and OS. Because this was a single-arm trial, no conclusions can be drawn regarding whether these potential associations are prognostic or predictive. Conclusions are also limited by the sample size and should be considered hypothesis generating.
In conclusion, RAM may enhance the efficacy of mFOLFOX-6 in mCRC. The overall adverse event profile of the combination appears to be largely consistent with the toxicity profile of the constituent chemotherapeutic agents and the known safety profile of RAM to date. However, the modest sample size and the single-arm design of the study preclude definitive assessment regarding these conclusions.
Supplementary Material
Acknowledgments
We thank Ashwini Dhume and Anastasia Perkowski of ImClone Systems, a wholly-owned subsidiary of Eli Lilly and Company, for their medical writing and editorial assistance with the manuscript.
Footnotes
Access the full results at: Tabernero-14-28.theoncologist.com
ClinicalTrials.gov Identifier: NCT00862784
Sponsor(s): Eli Lilly and Company
Principal Investigator: Rocio Garcia-Carbonero
IRB Approved: Yes
Author disclosures and references available online.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.