Abstract
PAQR-2 is a C. elegans homolog of the mammalian adiponectin receptors. We have recently shown that PAQR-2 is essential for the ability of C. elegans to grow at its lower temperature range, i.e., 15 °C, and that the likely role of PAQR-2 during cold adaptation is to regulate membrane fluidity by promoting fatty acid desaturation. Here we present a summary of this work, with an emphasis on placing our C. elegans findings in the context of mammalian biology.
Keywords: adiponectin, PAQR, membrane fluidity, AdipoR1, AdipoR2, cold adaptation, CREST proteins, desaturases, NHR-49, SBP-1
Why Study Adiponectin Receptor Homologs in C. elegans?
Adiponectin
Adiponectin, discovered in the mid-’90s, is a hormone expressed specifically by adipocytes.1-3 It is composed of 244 amino acids, with a collagenous domain that mediates multimerization, and a globular domain that interacts with the receptors. In mice, administration of adiponectin enhances insulin sensitivity, fatty acid oxidation4-7 and energy expenditure,8,9 protects from atherosclerosis,10 and causes decreased body weight.5,11 In human, serum concentration of adiponectin correlates negatively with BMI and insulin resistance,12 and low serum adiponectin level is a well-established risk factor for type 2 diabetes13,14 and myocardial infarction.15 Importantly, polymorphisms in adiponectin or its receptors are associated with an increased risk of type 2 diabetes and other metabolic syndrome complications.16-20 What emerges from the literature is that adiponectin is a key regulator of metabolism, and that pharmacomodulation of the adiponectin pathway has great potential in the context of the metabolic syndrome. How adiponectin signals to cause its important effects remains however far from clear.
Adiponectin receptors
Human adiponectin likely acts via two homologous receptors: AdipoR1 and AdipoR2.21 These are members of the poorly understood PAQR (progestin and adipoQ receptors) protein family characterized by seven transmembrane domains with a topology inverse that of G protein-coupled receptors (GPCRs): in PAQR proteins the N terminus is intracellular.22 Both receptors are expressed in the hypothalamus and many peripheral tissues.9,21 Knockout mice have confirmed the insulin-sensitizing roles of these receptors: they exhibit increased adiposity, insulin resistance, excess gluconeogenesis, and reduced fatty acid oxidation.23,24 Adiponectin receptors clearly regulate the balance between energy utilization and storage, but how?
Events immediately downstream of AdipoR1/2 are ill-defined, and several discrepancies exist in the literature. Recent work inspired by studying a distant yeast homolog indicates that the AdipoR1/2 receptors may have an associated ceramidase activity, but this remains to be experimentally confirmed in other organisms.25-27 Some groups also reported that adiponectin induces AMPK in liver,28 that globular and recombinant adiponectin are biologically active,10,23 that double knockout mice lacking AdipoR1 and AdipoR2 are viable,23 and that adiponectin regulates apetite.9 Other groups have contrary findings.24,25,29-32 It is not known whether the receptors act as dimers or monomers, and some investigators even suggest that ligand binding causes monomerization of inactive receptor dimers.33 This confusing situation about an important metabolic hormone calls for an unbiased approach to elucidate the adiponectin signaling pathway, which is one of our goals.
Adiponectin Receptor Homologs in C. elegans
The road to isolating paqr-2 suppressors in C. elegans
There is no obvious homolog of adiponectin in C. elegans. However, in 2011 we published a description of the two closest C. elegans adiponectin receptor homologs, which we named PAQR-1 and PAQR-2.34 These are expressed in metabolically important tissues, and paqr-2 is the most important of the two genes: the paqr-2 mutant exhibits a cold adaptation defect (inability to grow at 15 °C), a distinctive tail tip morphology defect, and an excess fat storage phenotype when combined with a paqr-1 mutation. Furthermore, paqr-2 is sterile or synthetic lethal with mutations in regulators of fatty acid metabolism, including the Δ9 desaturase fat-6, the SREBP homolog sbp-1, and the HNF4/PPARα homolog NHR-49.34
The greatest merit of using C. elegans is that it can be used as a tool to discover new molecular components that regulate or mediate the effects of proteins, such as AdipoR1 and AdipoR2. A powerful and unbiased approach is to use random mutagenesis screens to identify modifier mutations. In work recently published, we screened 15 000 mutagenized haploid genomes and identified nine novel mutations (alleles et6 to et14) that can suppress the growth arrest phenotype of paqr-2 mutants at 15 °C.35 Importantly, all nine suppressors alleviated the paqr-2 tail phenotype, at least to some degree. This indicates that they compensate specifically for loss of paqr-2 function, rather than generally improving cold tolerance. The suppressor mutations therefore likely affect genes downstream of PAQR-2, or genes acting in parallel pathways. This mutant collection is potentially an important resource to elucidate the adiponectin pathway, of which many components will certainly be conserved between mammals and worms.
Identity of the paqr-2 suppressors
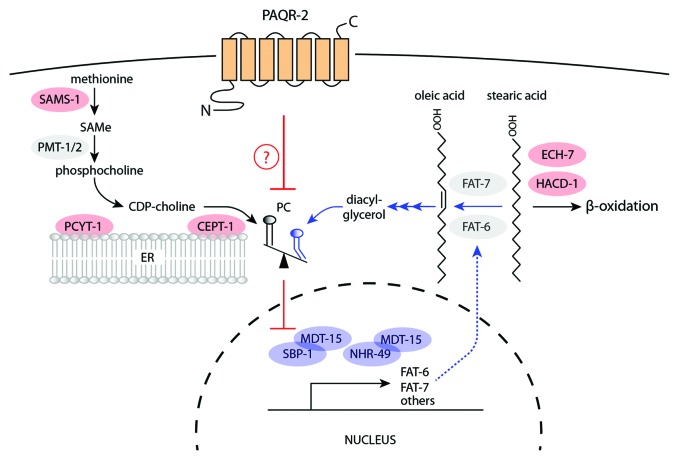
Whole genome sequencing and experimental confirmation allowed us to identify all nine suppressor alleles. The novel alleles affect genes belonging to two metabolic pathways: some mutations decrease phosphatidylcholine biosynthesis (loss of function [lof] mutations in pcyt-1 and cept-1) while other mutations likely result in increased fatty acid elongation and desaturation (gain-of-function [gof] mutations in mdt-15 and nhr-49 or loss of function mutations in ech-7 or hacd-1). Subsequent testing of a lof allele of sams-1 and of an overexpression sbp-1 transgene also revealed these to be paqr-2 suppressors. The following summarizes some of the literature that led us to construct the model shown in Figure 1, and discusses some implications of our findings.
Figure 1. Simplified pathway for paqr-2 suppressors. lof mutations affecting the proteins depicted in red, or gof mutations in proteins depicted in blue, can suppress the cold adaptation and withered tail tip phenotypes of the paqr-2(tm3410)-null mutant. Two main pathways are at work: on the left is the pathway for PC biosynthesis, and on the right are reactions important for FA metabolism. Lowering PC biosynthesis is expected to promote SBP-1 activation, which in turn activates Δ9 desaturases such as FAT-6 and FAT-7, ultimately resulting in more unsaturated fatty acids and increasing their relative abundance within membrane phospholipids. This increases membrane fluidity, which is adaptive at low temperatures. The role of PAQR-2 itself is unknown but one speculation is that it may regulate PC levels. lof mutations in aak-2 and nhr-80 can also partially suppress paqr-2 phenotypes (not shown).
paqr-2 suppressors and PC biosynthesis
SAMS-1 is the homolog of the mammalian methionine adenosyltransferase, while SAMe is the methyl donor for hundreds of methylation reactions, including RNA, DNA, proteins, and lipids,36 and methylation represents one of the earliest steps in de novo PC biosynthesis. PCs are produced by two alternative pathways: they can be synthesized either from choline (Kennedy pathway) or by methylation of phosphatidylethanolamine (PE) by PEMT in mammals, or of phosphoethanolamine by the PMT-1 and PMT-2 orthologs in C. elegans and plants.37-39 The Kennedy pathway includes the reactions performed by the C. elegans proteins PCYT-1 and CEPT-1.40 In mammals, these enzymes are named phosphocholine cytidylyltransferase (CCT) and cholinephosphotransferase (CPT), respectively, with CCT carrying out the rate-limiting step.41 The PC biosynthesis enzymes just mentioned have the following intracellular localizations: SAMS-1 is cytosolic;42,43 PMT-1 and PMT-2 work together and are also likely cytosolic;38,44 PCYT-1 is present in the nucleus where it may be connected to lipid signaling or simply sequestered in its inactive form, and associates with ER membranes where it may be regulated by bilayer elastic stress;45-48 CEPT-1 is associated with the ER and nuclear membranes.45
paqr-2 suppressors and FA metabolism
SBP-1 activation has two primary outcomes: the production of elongated and unsaturated fatty acids, and the accumulation of stored fat.49,50 The positive regulation of elo-2, fat-5, fat-6, and fat-7 by SBP-1 is supported by quantitative PCR and in vivo GFP reporters,49 and a role for SBP-1 as promoter of fat storage is evidenced by visualization of fat depots in sbp-1 mutants.49,50 The mediator subunit MDT-15 is required for SBP-1’s ability to control lipid homeostasis in C. elegans,51 and also interacts with NHR-49 to mediate its effects on fatty acid metabolism and response to dietary stress that, like SBP-1, include activation of the Δ9 desaturases.52,53 ECH-7 and HACD-1 are the worm homologs of the mammalian enoyl CoA hydratase and L-3-hydroxyacyl CoA dehydrogenase, mitrochondrial enzymes responsible for two of the four recurring steps of β-oxidation of fatty acids.54,55 lof mutations in these genes would be expected to decrease the rate of FA oxidation, hence increasing the time during which they are available to be modified by Δ9 desaturases. AAK-2 (the C. elegans AMP-activated kinase α2 catalytic subunit) acts to inhibit ATGL, hence slowing down energy utilization during periods of starvation;56,57 lof mutations in this gene also partially suppress paqr-2 phenotypes,34 and could act by releasing stored FAs for further exposure to Δ9 desaturases.
Connecting PC biosynthesis and FA metabolism
The profound regulatory effects of PC levels on SREBP activity have recently been discovered and are conserved between C. elegans and mammalian cells,58 although the precise mechanism is not yet clear. Our own work on PAQR-2 and its role in cold adaptation suggest that decreasing PC synthesis may be essential for cold adaptation by allowing adaptive remodeling of the structural lipids via elongases and desaturases regulated by SBP-1 and NHR-49.
The identity of the paqr-2 suppressors suggest that this adiponectin receptor homolog is a regulator of fatty acid metabolism in C. elegans. Furthermore, our results suggest a specific role for PAQR-2 in the regulation of membrane fluidity during cold adaptation in C. elegans: in the suppressor mutants there is a dramatic increase in the expression of a fat-7 transcriptional reporter that is accompanied by an increase in the abundance of unsaturated FAs.35 A greater proportion of unsaturated FAs is expected to increase membrane fluidity, which is an important adaptation to cold in poikilotherms. The role of paqr-2 as a regulator of membrane fluidity via influencing unsaturated FA levels could also explain the tail phenotype: the tail tip, being such a fine and delicate structure, may be particularly sensitive to defects in membrane fluidity. Remarkably, growth of the paqr-2 mutant was rescued by cultivating the worms in the presence of mild detergents used at concentrations expected to increase membrane fluidity.35 This observation provides elegant support for the hypothesis that one essential function of paqr-2 is to increase membrane fluidity during cold adaptation. Modulation of membrane fluidity is of great importance for many cellular processes, including regulating the function and clustering of membrane proteins, such as receptors and ion channels.59,60
PAQR-2, Adiponectin, and Cold Adaption
Human subjects wearing a 10 °C liquid-conditioned suit for 2 h have shown a near-doubling of circulating adiponectin levels.61 Although based on a relatively small study, cold exposure is the only known condition that can cause an acute increase in adiponectin levels. Furthermore, the increase was highest among individuals with the lowest basal levels of adiponectin, i.e., subjects with the highest BMI. Cold-induction of serum adiponectin levels has also been observed in rats kept at 4 °C for 24 h, with adiponectin mRNA levels also becoming elevated in brown adipose tissue (BAT).62 The induction of adiponectin by exposure to cold and the specific and significant increase of mRNA in BAT suggests that adiponectin might play a role in maintaining body temperature and basal metabolic rate in response to changing environmental conditions. Interestingly, as noted by Scherer and co-workers,3,63,64 adiponectin shows sequence homology with hibernation-associated plasma proteins (HP-27, HP-25, and HP20) and p88 HRP in the blood of Asian chipmunks (T. asiaticus) and hibernating woodchucks, respectively, which suggests that adiponectin may be part of a protein family that functions during cold adaptation, possibly by acting via the AdipoRs. Very few studies have been done that monitor the levels of adiponectin or its several CTRP homologs during hibernation. Based on scant evidence however, it does seem that the levels of adiponectin are lowest during hibernation, just as is the case for the hibernation-associated proteins;65,66 one effect of this may be to facilitate a drop in body temperature and cause a slow utilization of the fat stores during the many months of hibernation. Interestingly, some authors have suggested that the common ancestor to all mammals may have been a hibernator.67,68
In any case, adiponectin-type proteins may have been regulating fat storage vs. utilization in response to body temperature changes since the dawn of mammals, and probably even before that. It is therefore very interesting that an adiponectin receptor homolog is essential for cold adaptation in C. elegans, a poikilotherm. In C. elegans, adaptation to cold requires changes to the lipid composition so as to maintain membrane fluidity, as well as other changes that are currently not well defined.69-71 The paqr-2 suppressor mutants that we have identified suggest that homeostasis of lipid fluidity during cold adaptation is a primary function of this adiponectin receptor homolog. Given that the last common ancestor between worms and mammals was also a poikilotherm, the ancient function of the adiponectin receptors may perhaps, quite speculatively, be inferred from that of paqr-2 in C. elegans: to maintain membrane fluidity by promoting fatty acid desaturation, with these effects being essential for growth at lower temperature. In homeotherms such as mammals, the AdipoR1 and AdipoR2 have evidently retained their roles as metabolic regulators, and may even have retained a role in cold adaptation if the acute rise in adiponectin serum levels in response to cold exposure have a functional relevance.
Outstanding Question: What Does PAQR-2 Actually Do?
In spite of having identified many paqr-2 suppressors, we still have no idea of the actual biochemical function of the PAQR-2 protein, nor of its immediate downstream target. A PAQR-type protein exists in yeast, and was found to be associated with a ceramidase activity,27 a finding that prompted some investigators to examine this possible function for the mammalian AdipoR1/2 proteins.25 However, our own studies in C. elegans give no hint of a connection to ceramides, although it is certainly interesting to point out that ceramides are well known to have profound effects on membrane fluidity. A bioinformatics analysis of PAQR-type proteins led to the proposal that they are part of a much larger protein family, termed CREST (alkaline ceramidase, PAQR receptor, Per1, SID-1, and TMEM8).72 CREST proteins all have seven transmembrane domains inserted in an inverse orientation compared with GPCRs, i.e., having their N terminus facing the intracellular space, and all seem to act as hydrolases, including proteins with ceramidase and phospholipase A2 activities. One speculates, therefore, that PAQR-2 may also be a hydrolase. Specifically, and with an eye to the pathway that emerges from the study of the paqr-2 suppressors, one interesting possibility is that PAQR-2 may be a phospholipase that uses PCs as its substrates. In this scenario, the end effect of PAQR-2 would be similar to those mutations that inhibit PC synthesis, i.e., reducing PC levels. This hypothesis is particularly seducing in view of the fact that PCYT-1, which is responsible for the rate limiting step in PC synthesis, is regulated by membrane properties, such as curvature and/or fluidity that reflect a need for increased PCs.48 Thus, PAQR-2 and PCYT-1 could have opposite and complementary roles to fine-tune membrane properties. One way to test this is to examine the potential phospholipase activity of PAQR-2 using in vitro assays. Another is to identify new mutants that phenocopy the paqr-2 mutant, hoping that they will be more directly informative about the point of contact between paqr-2 and its downstream pathway. We are pursuing both avenues.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
This work was funded by the Swedish Research Council, Magnus Bergvalls Stiftelse, Diabetesfonden, and Carl Tryggers Stiftelse.
Glossary
Abbreviations:
- AAK-2
AMP-Activated Kinase-2
- AMPK
AMP kinase
- CCT
phosphocholine cytidylyltransferase
- CEPT-1
Choline/Ethanolamine PhosphoTransferase-1
- CPT
cholinephosphotransferase
- CREST
alkaline ceramidase, PAQR receptor, Per1, SID-1, and TMEM8
- ECH-7
Enoyl-CoA Hydratase
- FA
fatty acid
- gof
gain-of-function
- HACD-1
Hydroxy-Acyl-CoA Dehydrogenase
- lof
loss-of function
- MDT-15
MeDiaTor-15
- NHR-49
Nuclear Hormone Receptor family-15
- PC
phosphatidylcholine
- PE
phosphatidylethanolamine
- PCYT-1
CTP-phosphocholine cytidyltransferase
- PMT-1
Phosphoethanolamine MethylTransferase-1
- SAMS-1
S-Adenosyl Methionine Synthetase-1
- SBP-1
Sterol regulatory element Binding Protein-1
Footnotes
Previously published online: www.landesbioscience.com/journals/worm/article/27123
References
- 1.Hu E, Liang P, Spiegelman BM. AdipoQ is a novel adipose-specific gene dysregulated in obesity. J Biol Chem. 1996;271:10697–703. doi: 10.1074/jbc.271.18.10697. [DOI] [PubMed] [Google Scholar]
- 2.Maeda K, Okubo K, Shimomura I, Funahashi T, Matsuzawa Y, Matsubara K. cDNA cloning and expression of a novel adipose specific collagen-like factor, apM1 (AdiPose Most abundant Gene transcript 1) Biochem Biophys Res Commun. 1996;221:286–9. doi: 10.1006/bbrc.1996.0587. [DOI] [PubMed] [Google Scholar]
- 3.Scherer PE, Williams S, Fogliano M, Baldini G, Lodish HF. A novel serum protein similar to C1q, produced exclusively in adipocytes. J Biol Chem. 1995;270:26746–9. doi: 10.1074/jbc.270.45.26746. [DOI] [PubMed] [Google Scholar]
- 4.Berg AH, Combs TP, Du X, Brownlee M, Scherer PE. The adipocyte-secreted protein Acrp30 enhances hepatic insulin action. Nat Med. 2001;7:947–53. doi: 10.1038/90992. [DOI] [PubMed] [Google Scholar]
- 5.Fruebis J, Tsao TS, Javorschi S, Ebbets-Reed D, Erickson MR, Yen FT, Bihain BE, Lodish HF. Proteolytic cleavage product of 30-kDa adipocyte complement-related protein increases fatty acid oxidation in muscle and causes weight loss in mice. Proc Natl Acad Sci U S A. 2001;98:2005–10. doi: 10.1073/pnas.98.4.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yamauchi T, Kamon J, Minokoshi Y, Ito Y, Waki H, Uchida S, Yamashita S, Noda M, Kita S, Ueki K, et al. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat Med. 2002;8:1288–95. doi: 10.1038/nm788. [DOI] [PubMed] [Google Scholar]
- 7.Yamauchi T, Kamon J, Waki H, Terauchi Y, Kubota N, Hara K, Mori Y, Ide T, Murakami K, Tsuboyama-Kasaoka N, et al. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat Med. 2001;7:941–6. doi: 10.1038/90984. [DOI] [PubMed] [Google Scholar]
- 8.Klein I, Sanchez-Alavez M, Tabarean I, Schaefer J, Holmberg KH, Klaus J, Xia F, Marcondes MCG, Dubins JS, Morrison B, et al. AdipoR1 and 2 are expressed on warm sensitive neurons of the hypothalamic preoptic area and contribute to central hyperthermic effects of adiponectin. Brain Res. 2011;1423:1–9. doi: 10.1016/j.brainres.2011.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kubota N, Yano W, Kubota T, Yamauchi T, Itoh S, Kumagai H, Kozono H, Takamoto I, Okamoto S, Shiuchi T, et al. Adiponectin stimulates AMP-activated protein kinase in the hypothalamus and increases food intake. Cell Metab. 2007;6:55–68. doi: 10.1016/j.cmet.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 10.Yamauchi T, Kamon J, Waki H, Imai Y, Shimozawa N, Hioki K, Uchida S, Ito Y, Takakuwa K, Matsui J, et al. Globular adiponectin protected ob/ob mice from diabetes and ApoE-deficient mice from atherosclerosis. J Biol Chem. 2003;278:2461–8. doi: 10.1074/jbc.M209033200. [DOI] [PubMed] [Google Scholar]
- 11.Qi Y, Takahashi N, Hileman SM, Patel HR, Berg AH, Pajvani UB, Scherer PE, Ahima RS. Adiponectin acts in the brain to decrease body weight. Nat Med. 2004;10:524–9. doi: 10.1038/nm1029. [DOI] [PubMed] [Google Scholar]
- 12.Hotta K, Funahashi T, Arita Y, Takahashi M, Matsuda M, Okamoto Y, Iwahashi H, Kuriyama H, Ouchi N, Maeda K, et al. Plasma concentrations of a novel, adipose-specific protein, adiponectin, in type 2 diabetic patients. Arterioscler Thromb Vasc Biol. 2000;20:1595–9. doi: 10.1161/01.ATV.20.6.1595. [DOI] [PubMed] [Google Scholar]
- 13.Lindsay RS, Funahashi T, Hanson RL, Matsuzawa Y, Tanaka S, Tataranni PA, Knowler WC, Krakoff J. Adiponectin and development of type 2 diabetes in the Pima Indian population. Lancet. 2002;360:57–8. doi: 10.1016/S0140-6736(02)09335-2. [DOI] [PubMed] [Google Scholar]
- 14.Spranger J, Kroke A, Möhlig M, Bergmann MM, Ristow M, Boeing H, Pfeiffer AFH. Adiponectin and protection against type 2 diabetes mellitus. Lancet. 2003;361:226–8. doi: 10.1016/S0140-6736(03)12255-6. [DOI] [PubMed] [Google Scholar]
- 15.Pischon T, Girman CJ, Hotamisligil GS, Rifai N, Hu FB, Rimm EB. Plasma adiponectin levels and risk of myocardial infarction in men. JAMA. 2004;291:1730–7. doi: 10.1001/jama.291.14.1730. [DOI] [PubMed] [Google Scholar]
- 16.Hara K, Boutin P, Mori Y, Tobe K, Dina C, Yasuda K, Yamauchi T, Otabe S, Okada T, Eto K, et al. Genetic variation in the gene encoding adiponectin is associated with an increased risk of type 2 diabetes in the Japanese population. Diabetes. 2002;51:536–40. doi: 10.2337/diabetes.51.2.536. [DOI] [PubMed] [Google Scholar]
- 17.Kondo H, Shimomura I, Matsukawa Y, Kumada M, Takahashi M, Matsuda M, Ouchi N, Kihara S, Kawamoto T, Sumitsuji S, et al. Association of adiponectin mutation with type 2 diabetes: a candidate gene for the insulin resistance syndrome. Diabetes. 2002;51:2325–8. doi: 10.2337/diabetes.51.7.2325. [DOI] [PubMed] [Google Scholar]
- 18.Vasseur F, Helbecque N, Dina C, Lobbens S, Delannoy V, Gaget S, Boutin P, Vaxillaire M, Leprêtre F, Dupont S, et al. Single-nucleotide polymorphism haplotypes in the both proximal promoter and exon 3 of the APM1 gene modulate adipocyte-secreted adiponectin hormone levels and contribute to the genetic risk for type 2 diabetes in French Caucasians. Hum Mol Genet. 2002;11:2607–14. doi: 10.1093/hmg/11.21.2607. [DOI] [PubMed] [Google Scholar]
- 19.Vendramini MF, Pereira AC, Ferreira SR, Kasamatsu TS, Moisés RS, Japanese Brazilian Diabetes Study Group Association of genetic variants in the adiponectin encoding gene (ADIPOQ) with type 2 diabetes in Japanese Brazilians. J Diabetes Complications. 2010;24:115–20. doi: 10.1016/j.jdiacomp.2009.01.007. [DOI] [PubMed] [Google Scholar]
- 20.Waki H, Yamauchi T, Kamon J, Ito Y, Uchida S, Kita S, Hara K, Hada Y, Vasseur F, Froguel P, et al. Impaired multimerization of human adiponectin mutants associated with diabetes. Molecular structure and multimer formation of adiponectin. J Biol Chem. 2003;278:40352–63. doi: 10.1074/jbc.M300365200. [DOI] [PubMed] [Google Scholar]
- 21.Yamauchi T, Kamon J, Ito Y, Tsuchida A, Yokomizo T, Kita S, Sugiyama T, Miyagishi M, Hara K, Tsunoda M, et al. Cloning of adiponectin receptors that mediate antidiabetic metabolic effects. Nature. 2003;423:762–9. doi: 10.1038/nature01705. [DOI] [PubMed] [Google Scholar]
- 22.Tang YT, Hu T, Arterburn M, Boyle B, Bright JM, Emtage PC, Funk WD. PAQR proteins: a novel membrane receptor family defined by an ancient 7-transmembrane pass motif. J Mol Evol. 2005;61:372–80. doi: 10.1007/s00239-004-0375-2. [DOI] [PubMed] [Google Scholar]
- 23.Yamauchi T, Nio Y, Maki T, Kobayashi M, Takazawa T, Iwabu M, Okada-Iwabu M, Kawamoto S, Kubota N, Kubota T, et al. Targeted disruption of AdipoR1 and AdipoR2 causes abrogation of adiponectin binding and metabolic actions. Nat Med. 2007;13:332–9. doi: 10.1038/nm1557. [DOI] [PubMed] [Google Scholar]
- 24.Bjursell M, Ahnmark A, Bohlooly-Y M, William-Olsson L, Rhedin M, Peng X-R, Ploj K, Gerdin A-K, Arnerup G, Elmgren A, et al. Opposing effects of adiponectin receptors 1 and 2 on energy metabolism. Diabetes. 2007;56:583–93. doi: 10.2337/db06-1432. [DOI] [PubMed] [Google Scholar]
- 25.Holland WL, Miller RA, Wang ZV, Sun K, Barth BM, Bui HH, Davis KE, Bikman BT, Halberg N, Rutkowski JM, et al. Receptor-mediated activation of ceramidase activity initiates the pleiotropic actions of adiponectin. Nat Med. 2011;17:55–63. doi: 10.1038/nm.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kupchak BR, Garitaonandia I, Villa NY, Smith JL, Lyons TJ. Antagonism of human adiponectin receptors and their membrane progesterone receptor paralogs by TNFalpha and a ceramidase inhibitor. Biochemistry. 2009;48:5504–6. doi: 10.1021/bi9006258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Villa NY, Kupchak BR, Garitaonandia I, Smith JL, Alonso E, Alford C, Cowart LA, Hannun YA, Lyons TJ. Sphingolipids function as downstream effectors of a fungal PAQR. Mol Pharmacol. 2009;75:866–75. doi: 10.1124/mol.108.049809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Awazawa M, Ueki K, Inabe K, Yamauchi T, Kaneko K, Okazaki Y, Bardeesy N, Ohnishi S, Nagai R, Kadowaki T. Adiponectin suppresses hepatic SREBP1c expression in an AdipoR1/LKB1/AMPK dependent pathway. Biochem Biophys Res Commun. 2009;382:51–6. doi: 10.1016/j.bbrc.2009.02.131. [DOI] [PubMed] [Google Scholar]
- 29.Kusminski CM, McTernan PG, Schraw T, Kos K, O’Hare JP, Ahima R, Kumar S, Scherer PE. Adiponectin complexes in human cerebrospinal fluid: distinct complex distribution from serum. Diabetologia. 2007;50:634–42. doi: 10.1007/s00125-006-0577-9. [DOI] [PubMed] [Google Scholar]
- 30.Pajvani UB, Du X, Combs TP, Berg AH, Rajala MW, Schulthess T, Engel J, Brownlee M, Scherer PE. Structure-function studies of the adipocyte-secreted hormone Acrp30/adiponectin. Implications fpr metabolic regulation and bioactivity. J Biol Chem. 2003;278:9073–85. doi: 10.1074/jbc.M207198200. [DOI] [PubMed] [Google Scholar]
- 31.Schraw T, Wang ZV, Halberg N, Hawkins M, Scherer PE. Plasma adiponectin complexes have distinct biochemical characteristics. Endocrinology. 2008;149:2270–82. doi: 10.1210/en.2007-1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tullin S, Sams A, Brandt J, Dahl K, Gong W, Jeppesen CB, Krogh TN, Olsen GS, Liu Y, Pedersen AA, et al. Recombinant adiponectin does not lower plasma glucose in animal models of type 2 diabetes. PLoS One. 2012;7:e44270. doi: 10.1371/journal.pone.0044270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kosel D, Heiker JT, Juhl C, Wottawah CM, Blüher M, Mörl K, Beck-Sickinger AG. Dimerization of adiponectin receptor 1 is inhibited by adiponectin. J Cell Sci. 2010;123:1320–8. doi: 10.1242/jcs.057919. [DOI] [PubMed] [Google Scholar]
- 34.Svensson E, Olsen L, Mörck C, Brackmann C, Enejder A, Faergeman NJ, Pilon M. The adiponectin receptor homologs in C. elegans promote energy utilization and homeostasis. PLoS One. 2011;6:e21343. doi: 10.1371/journal.pone.0021343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Svensk E, Ståhlman M, Andersson CH, Johansson M, Borén J, Pilon M. PAQR-2 regulates fatty acid desaturation during cold adaptation in C. elegans. PLoS Genet. 2013;9:e1003801. doi: 10.1371/journal.pgen.1003801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mato JM, Lu SC. Homocysteine, the bad thiol. Hepatology. 2005;41:976–9. doi: 10.1002/hep.20708. [DOI] [PubMed] [Google Scholar]
- 37.Brendza KM, Haakenson W, Cahoon RE, Hicks LM, Palavalli LH, Chiapelli BJ, McLaird M, McCarter JP, Williams DJ, Hresko MC, et al. Phosphoethanolamine N-methyltransferase (PMT-1) catalyses the first reaction of a new pathway for phosphocholine biosynthesis in Caenorhabditis elegans. Biochem J. 2007;404:439–48. doi: 10.1042/BJ20061815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Palavalli LH, Brendza KM, Haakenson W, Cahoon RE, McLaird M, Hicks LM, McCarter JP, Williams DJ, Hresko MC, Jez JM. Defining the role of phosphomethylethanolamine N-methyltransferase from Caenorhabditis elegans in phosphocholine biosynthesis by biochemical and kinetic analysis. Biochemistry. 2006;45:6056–65. doi: 10.1021/bi060199d. [DOI] [PubMed] [Google Scholar]
- 39.Vance JE, Vance DE. Phospholipid biosynthesis in mammalian cells. Biochem Cell Biol. 2004;82:113–28. doi: 10.1139/o03-073. [DOI] [PubMed] [Google Scholar]
- 40.Lochnit G, Geyer R. Evidence for the presence of the Kennedy and Bremer- Greenberg pathways in Caenorhabditis elegans. Acta Biochim Pol. 2003;50:1239–43. [PubMed] [Google Scholar]
- 41.Gibellini F, Smith TK. The Kennedy pathway--De novo synthesis of phosphatidylethanolamine and phosphatidylcholine. IUBMB Life. 2010;62:414–28. doi: 10.1002/iub.354. [DOI] [PubMed] [Google Scholar]
- 42.Reytor E, Pérez-Miguelsanz J, Alvarez L, Pérez-Sala D, Pajares MA. Conformational signals in the C-terminal domain of methionine adenosyltransferase I/III determine its nucleocytoplasmic distribution. FASEB J. 2009;23:3347–60. doi: 10.1096/fj.09-130187. [DOI] [PubMed] [Google Scholar]
- 43.Mato JM, Alvarez L, Ortiz P, Pajares MA. S-adenosylmethionine synthesis: molecular mechanisms and clinical implications. Pharmacol Ther. 1997;73:265–80. doi: 10.1016/S0163-7258(96)00197-0. [DOI] [PubMed] [Google Scholar]
- 44.Bobenchik AM, Augagneur Y, Hao B, Hoch JC, Ben Mamoun C. Phosphoethanolamine methyltransferases in phosphocholine biosynthesis: functions and potential for antiparasite therapy. FEMS Microbiol Rev. 2011;35:609–19. doi: 10.1111/j.1574-6976.2011.00267.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Henneberry AL, Wright MM, McMaster CR. The major sites of cellular phospholipid synthesis and molecular determinants of Fatty Acid and lipid head group specificity. Mol Biol Cell. 2002;13:3148–61. doi: 10.1091/mbc.01-11-0540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jackowski S, Fagone P. CTP: Phosphocholine cytidylyltransferase: paving the way from gene to membrane. J Biol Chem. 2005;280:853–6. doi: 10.1074/jbc.R400031200. [DOI] [PubMed] [Google Scholar]
- 47.Cornell RB, Northwood IC. Regulation of CTP:phosphocholine cytidylyltransferase by amphitropism and relocalization. Trends Biochem Sci. 2000;25:441–7. doi: 10.1016/S0968-0004(00)01625-X. [DOI] [PubMed] [Google Scholar]
- 48.Attard GS, Templer RH, Smith WS, Hunt AN, Jackowski S. Modulation of CTP:phosphocholine cytidylyltransferase by membrane curvature elastic stress. Proc Natl Acad Sci U S A. 2000;97:9032–6. doi: 10.1073/pnas.160260697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nomura T, Horikawa M, Shimamura S, Hashimoto T, Sakamoto K. Fat accumulation in Caenorhabditis elegans is mediated by SREBP homolog SBP-1. Genes Nutr. 2010;5:17–27. doi: 10.1007/s12263-009-0157-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McKay RM, McKay JP, Avery L, Graff JM. C elegans: a model for exploring the genetics of fat storage. Dev Cell. 2003;4:131–42. doi: 10.1016/S1534-5807(02)00411-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yang F, Vought BW, Satterlee JS, Walker AK, Jim Sun Z-Y, Watts JL, DeBeaumont R, Saito RM, Hyberts SG, Yang S, et al. An ARC/Mediator subunit required for SREBP control of cholesterol and lipid homeostasis. Nature. 2006;442:700–4. doi: 10.1038/nature04942. [DOI] [PubMed] [Google Scholar]
- 52.Taubert S, Van Gilst MR, Hansen M, Yamamoto KR. A Mediator subunit, MDT-15, integrates regulation of fatty acid metabolism by NHR-49-dependent and -independent pathways in C. elegans. Genes Dev. 2006;20:1137–49. doi: 10.1101/gad.1395406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Taubert S, Hansen M, Van Gilst MR, Cooper SB, Yamamoto KR. The Mediator subunit MDT-15 confers metabolic adaptation to ingested material. PLoS Genet. 2008;4:e1000021. doi: 10.1371/journal.pgen.1000021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Houten SM, Wanders RJA. A general introduction to the biochemistry of mitochondrial fatty acid β-oxidation. J Inherit Metab Dis. 2010;33:469–77. doi: 10.1007/s10545-010-9061-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kompare M, Rizzo WB. Mitochondrial fatty-acid oxidation disorders. Semin Pediatr Neurol. 2008;15:140–9. doi: 10.1016/j.spen.2008.05.008. [DOI] [PubMed] [Google Scholar]
- 56.Narbonne P, Roy R. Caenorhabditis elegans dauers need LKB1/AMPK to ration lipid reserves and ensure long-term survival. Nature. 2009;457:210–4. doi: 10.1038/nature07536. [DOI] [PubMed] [Google Scholar]
- 57.Cunningham KA, Ashrafi K. Fat rationing in dauer times. Cell Metab. 2009;9:113–4. doi: 10.1016/j.cmet.2009.01.008. [DOI] [PubMed] [Google Scholar]
- 58.Walker AK, Jacobs RL, Watts JL, Rottiers V, Jiang K, Finnegan DM, Shioda T, Hansen M, Yang F, Niebergall LJ, et al. A conserved SREBP-1/phosphatidylcholine feedback circuit regulates lipogenesis in metazoans. Cell. 2011;147:840–52. doi: 10.1016/j.cell.2011.09.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.McIntosh TJ, Simon SA. Roles of bilayer material properties in function and distribution of membrane proteins. Annu Rev Biophys Biomol Struct. 2006;35:177–98. doi: 10.1146/annurev.biophys.35.040405.102022. [DOI] [PubMed] [Google Scholar]
- 60.van Meer G, Voelker DR, Feigenson GW. Membrane lipids: where they are and how they behave. Nat Rev Mol Cell Biol. 2008;9:112–24. doi: 10.1038/nrm2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Imbeault P, Dépault I, Haman F. Cold exposure increases adiponectin levels in men. Metabolism. 2009;58:552–9. doi: 10.1016/j.metabol.2008.11.017. [DOI] [PubMed] [Google Scholar]
- 62.Yoda M, Nakano Y, Tobe T, Shioda S, Choi-Miura NH, Tomita M. Characterization of mouse GBP28 and its induction by exposure to cold. Int J Obes Relat Metab Disord. 2001;25:75–83. doi: 10.1038/sj.ijo.0801482. [DOI] [PubMed] [Google Scholar]
- 63.Wong GW, Wang J, Hug C, Tsao T-S, Lodish HF. A family of Acrp30/adiponectin structural and functional paralogs. Proc Natl Acad Sci U S A. 2004;101:10302–7. doi: 10.1073/pnas.0403760101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Davis KE, Scherer PE. Adiponectin: no longer the lone soul in the fight against insulin resistance? Biochem J. 2008;416:e7–9. doi: 10.1042/BJ20082033. [DOI] [PubMed] [Google Scholar]
- 65.Takamatsu N, Ohba K, Kondo J, Kondo N, Shiba T. Hibernation-associated gene regulation of plasma proteins with a collagen-like domain in mammalian hibernators. Mol Cell Biol. 1993;13:1516–21. doi: 10.1128/mcb.13.3.1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Florant GL, Porst H, Peiffer A, Hudachek SF, Pittman C, Summers SA, Rajala MW, Scherer PE. Fat-cell mass, serum leptin and adiponectin changes during weight gain and loss in yellow-bellied marmots (Marmota flaviventris) J Comp Physiol B. 2004;174:633–9. doi: 10.1007/s00360-004-0454-0. [DOI] [PubMed] [Google Scholar]
- 67.Martin SL. Mammalian hibernation: a naturally reversible model for insulin resistance in man? Diab Vasc Dis Res. 2008;5:76–81. doi: 10.3132/dvdr.2008.013. [DOI] [PubMed] [Google Scholar]
- 68.Srere HK, Wang LC, Martin SL. Central role for differential gene expression in mammalian hibernation. Proc Natl Acad Sci U S A. 1992;89:7119–23. doi: 10.1073/pnas.89.15.7119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hayward SAL, Murray PA, Gracey AY, Cossins AR. Beyond the lipid hypothesis: mechanisms underlying phenotypic plasticity in inducible cold tolerance. Adv Exp Med Biol. 2007;594:132–42. doi: 10.1007/978-0-387-39975-1_12. [DOI] [PubMed] [Google Scholar]
- 70.Murray P, Hayward SAL, Govan GG, Gracey AY, Cossins AR. An explicit test of the phospholipid saturation hypothesis of acquired cold tolerance in Caenorhabditis elegans. Proc Natl Acad Sci U S A. 2007;104:5489–94. doi: 10.1073/pnas.0609590104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tanaka T, Ikita K, Ashida T, Motoyama Y, Yamaguchi Y, Satouchi K. Effects of growth temperature on the fatty acid composition of the free-living nematode Caenorhabditis elegans. Lipids. 1996;31:1173–8. doi: 10.1007/BF02524292. [DOI] [PubMed] [Google Scholar]
- 72.Pei J, Millay DP, Olson EN, Grishin NV. CREST--a large and diverse superfamily of putative transmembrane hydrolases. Biol Direct. 2011;6:37. doi: 10.1186/1745-6150-6-37. [DOI] [PMC free article] [PubMed] [Google Scholar]