Abstract
Heterologous prime-boost vaccination using plasmid DNA followed by replication-defective adenovirus vector generates a large number of specific CD8+ T effector memory (TEM) cells that provide long-term immunity against a variety of pathogens. In the present study, we initially characterized the frequency, phenotype, and function of these T cells in vaccinated mice that were subjected to infectious challenge with the human protozoan parasite Trypanosoma cruzi. We observed that the frequency of the specific CD8+ T cells in the spleens of the vaccinated mice increased after challenge. Specific TEM cells differentiated into cells with a KLRG1High CD27Low CD43Low CD183LowT-betHigh EomesLow phenotype and capable to produce simultaneously the antiparasitic mediators IFNγ and TNF. Using the gzmBCreERT2/ROSA26EYFP transgenic mouse line, in which the cells that express Granzyme B after immunization, are indelibly labeled with enhanced yellow fluorescent protein, we confirmed that CD8+ T cells present after challenge were indeed TEM cells that had been induced by vaccination. Subsequently, we observed that the in vivo increase in the frequency of the specific CD8+ T cells was not because of an anamnestic immune response. Most importantly, after challenge, the increase in the frequency of specific cells and the protective immunity they mediate were insensitive to treatment with the cytostatic toxic agent hydroxyurea. We have previously described that the administration of the drug FTY720, which reduces lymphocyte recirculation, severely impairs protective immunity, and our evidence supports the model that when large amounts of antigen-experienced CD8+ TEM cells are present after heterologous prime-boost vaccination, differentiation, and recirculation, rather than proliferation, are key for the resultant protective immunity.
Introduction
Genetic vaccination using the heterologous prime-boost regimen is being actively pursued to elicit specific immune responses mediated by cytotoxic CD8+ T cells. This strategy employs two different vaccine vectors, both of which carry the same foreign gene encoding the target antigen for priming and boosting immunizations. A number of different vector combinations have been tested, and the application of this strategy has been shown to successfully provide immunity against various infections, such as simian immunodeficiency virus malaria, Ebola virus, leishmaniasis, tuberculosis, Chagas disease, and toxoplasmosis (reviewed by Lasaro and Ertl, 2009; Ranasinghe and Ramshaw, 2009; Hill et al., 2010; Rollier et al., 2011; Vasconcelos et al., 2012b). The knowledge accumulated using preclinical experimental models is now being translated into a number of ongoing phase I/II trials in humans (Freel et al., 2010; Jaoko et al., 2010; Koup et al., 2010; De Rosa et al., 2011; O'Connell et al., 2012; Casazza et al., 2013; Chuang et al., 2013; Hammer et al., 2013).
Although the heterologous prime-boost regimen is being increasingly used for vaccine development, the precise reason for its success is still not fully understood. Distinct, but not mutually exclusively, explanations may account for its enhanced vaccination efficacy. This regimen elicits a high number of T effector (TE) cells (CD11aHigh, CD44High, CD127Low, and CD62LLow), which subsequently become long-lived T effector memory (TEM) cells (CD11aHigh, CD44High, CD127High, and CD62LLow) (reviewed by Vasconcelos et al., 2012b). It is conceivable that the large number of TE(M) cells might be the most important factor contributing to the efficacy of this immunization strategy. Their high frequency becomes even more important because in many cases, they are multifunctional T cells that are capable of lysing target cells and secreting multiple effector cytokines (reviewed by Vasconcelos et al., 2012b). In addition, other reasons that might explain the high efficacy of this regimen include the capacity of these cells to proliferate and/or to recirculate after infectious challenge.
Within the past few years, we reported that the heterologous prime-boost strategy successfully vaccinates highly susceptible A/Sn mice against systemic infection with the human intracellular protozoan parasite Trypanosoma cruzi, the causative agent of Chagas disease (American trypanosomiasis) (De Alencar et al., 2009; Haolla et al., 2009; Dominguez et al., 2011, 2012; Rigato et al., 2011). In these experiments, we used a recombinant plasmid DNA for priming and the human replication-defective recombinant adenovirus type 5 for boosting, both expressing the amastigote surface protein-2 (ASP-2) of T. cruzi. Our vaccination protocol elicited a strong immune response mediated by CD8+ TE cells (CD11aHigh, CD44High, CD127Low, and CD62LLow). These cells developed to form a stable pool of functional long-lived CD8+ TEM cells (CD11aHigh, CD44High, CD127High, and CD62LLow). Classical TCM (CD11aHigh CD44High CD62LHigh CD127High) are not seen in our system until 180 days after immunization. Their long-term survival required a functional IL-12/IL23 signaling pathway, but not the presence of CD4+ T cells. These cells were resistant to immunological erosion and, most important for vaccine development, they mediated strong protective immunity against experimental systemic infection with T. cruzi (Rigato et al., 2011).
In a subsequent study, we hypothesized that the T cells elicited by heterologous prime-boost vaccination must recirculate to mediate their antiparasitic effects. We approached this subject by administering the immunosuppressive drug FTY720 to vaccinated mice. This drug reduces lymphocyte recirculation by interfering with T cell signaling via sphingosine-1-phosphate receptor-1 (S1Pr1). This interference results in sustained inhibition of S1Pr1 signaling, effectively trapping T cells within the secondary lymphoid without inhibiting T cell activation. FTY720 administration significantly impaired protective immunity, supporting the hypothesis that T cell recirculation is critical for the protective immunity they mediate (Dominguez et al., 2012).
Because it is not well established whether in addition to recirculation, TE(M) cells must differentiate and proliferate to eliminate the parasite, the purpose of the present study was determine the frequencies, phenotypes, and functions of the TE(M) cells that are present after infectious challenge and whether protective immunity can be inhibited by treatment with the cytostatic toxic agent hydroxyurea (HU).
Materials and Methods
Ethics statement
This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the Brazilian National Council of Animal Experimentation (www.cobea.org.br/). The protocol was approved by the Committee on the Ethics of Animal Experiments of the Institutional Animal Care and Use Committee at the Federal University of Sao Paulo (Id No. CEP 0426/09).
Mice and parasites
Female 5–8-week-old C57BL/6 and A/Sn mice were purchased from CEDEME (Federal University of São Paulo). gzmBCreERT2/ROSA26EYFP or green fluorescent protein (GFP) transgenic mouse line C57BL/6-Tg(CAG-EGFP)131Osb/LeySopJ was generated as described by Bannard et al. (2009) or Okabe et al. (1997) and bred in our own animal facility. Bloodstream trypomastigotes of the Y strain of T. cruzi were obtained from mice infected 7 days earlier (De Alencar et al., 2009). The concentration of parasites was estimated and adjusted to 105 or 750 parasites/ml. Each mouse was inoculated with 104 (C57BL/6 or gzmBCreERT2/ROSA26EYFP) or 150 (A/Sn) trypomastigotes diluted in 0.1 ml phosphate buffered saline (PBS) and administrated subcutaneously in the base of the tail. HU (Sigma) was administered as cycle of 2 intraperitoneal injections of 1 gr/kg/day, 7 hr apart. Mice received four cycles every 2 days.
Peptides
Synthetic peptides were purchased from Genscript. Peptide purity was higher than 90%. Peptide identities were confirmed by a Q-TOF Micro equipped with an electrospray ionization source (Micromass). The immunodominant epitopes of ASP-2 were represented by AA VNHRFTLV. The pentamer H2Kb-VNHRFTLV was purchased from ProImmune Inc.
Genetic vaccination
Plasmid pIgSPclone9 and human replication-deficient adenovirus type 5 expressing the ASP-2 gene (AdASP-2) were generated, grown, and purified as described earlier (Boscardin et al., 2003; Machado et al., 2006). Control mice were immunized with pcDNA3 and human replication-deficient adenovirus type 5 expressing β-galactosidase (Adβ-gal). Mice were inoculated intramuscularly in each tibialis anterioris muscle with 50 μg of plasmid DNA. Twenty-one days later, these mice received 50 μl of a viral suspension containing 2×108 plaque forming units of adenovirus in the same locations. Immunological assays were performed at the days indicated in each figure.
Immunological assays
For flow cytometry analyses, we used mouse splenocytes treated with ACK buffer (NH4Cl, 0.15 M; KHCO3, 10 mM; Na2-EDTA 0.1 mM; pH 7.4) for lysing the erythrocytes. Single-cell suspensions were washed in PBS, stained for 10 min at room temperature with biotinylated MHC I multimer H-2Kb-VNHRFTLV, and stained for 20 min at 4°C with avidin-APC- or avidin-APC-Cy7-labeled and PerCP- or Pacific Blue-labeled anti-CD8 antibodies (BD Pharmingen). For the analyses of other cell-surface markers, single-cell suspensions from spleens of mice were stained with multimers and anti-CD8 antibody as described above. Then, cells were incubated with PE-labeled anti-CD27 (clone LG.3A10) and FITC-labeled anti-KLRG1 (clone MAFA) purchased from eBioscience. Alternatively, cells were incubated with PercP-labeled anti-CD43 (1B11) and PE-Cy7-labeled anti-CD183 (CXCR3-173) purchased from Biolegend. Isotype control antibodies were PE-labeled hamster IgG1k and rat IgG1k, and FITC- labeled IgG2ak (BD Pharmingen). At least 200,000 cells were acquired on a BD FacsCanto flow cytometer and then analyzed with FlowJo (Tree Star).
For the surface intracellular expression of cytokines (IFNγ and TNF; ICS), splenocytes collected from C57BL/6 mice were treated with ACK buffer. ICS were evaluated after in vitro culture of splenocytes in the presence or absence of the antigenic stimulus. Cells were washed 3 times in plain RPMI and resuspended in cell culture medium consisting of RPMI 1640 medium, pH 7.4, supplemented with 10 mM HEPES, 0.2% sodium bicarbonate, 59 mg/liter of penicillin, 133 mg/liter of streptomycin, and 10% Hyclone fetal bovine sera (Hyclone). The viability of the cells was evaluated using 0.2% trypan blue exclusion dye to discriminate between live and dead cells. Cell concentration was adjusted to 5×106 cells/ml in cell culture medium containing anti-CD28 (2 μg/ml), BdGolgiPlug, and monensin (5 μg/ml). In half of the cultures, a final concentration of 10 μM of the VNHRFTLV peptide was added. The cells were cultivated in flat-bottom 96-well plates (Corning) in a final volume of 200 μl in duplicate, at 37°C in a humid environment containing 5% CO2. After 12 hr incubation, cells were stained for surface markers with PerCP- and PE-labeled anti-CD8, on ice for 20 min. To detect IFNγ and TNF by intracellular staining, cells were then washed twice in buffer containing PBS, 0.5% BSA, and 2 mM EDTA, fixed, and permeabilized with BD perm/wash buffer. After being washed twice with BD perm/wash buffer, cells were stained for intracellular markers using APC-labeled anti-IFNγ (Clone XMG1.2) and PE-labeled anti-TNF (clone MP6-XT22), and surface markers FITC-labeled anti-KLRG1, FITC-labeled CD27 (LG.7F9), Alexa-fluor-labeled CD43, and PercP-labeled CD183 for 20 min on ice. Finally, cells were washed twice with BD perm/wash buffer and fixed in 1% PBS–paraformaldehyde. At least 300,000 cells were acquired on a BD FacsCanto flow cytometer and then analyzed with FlowJo.
For detection of bromodeoxiuridine (BrdU), mice were injected intraperitoneally with 2 mg of BrdU (Sigma) 7 times for 14 days. The cells were treated according to the manufacturer's instructions and stained with anti-BrdU-FITC (BD Pharmingen). At least 200,000 cells were analyzed by FACS as described above.
Statistical analysis
The values of parasitemia were log transformed before being compared using one-way ANOVA followed by Tukey's HSD tests. The log-rank test was used to compare mouse survival rates after challenge with T. cruzi. The differences were considered significant when the p-value was <0.05.
Results
Frequencies, phenotypes, and functions of specific CD8+ T cells in vaccinated mice after infectious challenge with T. cruzi
Protective immunity against T. cruzi infection can be achieved by genetic vaccination with the ASP-2 gene following a heterologous plasmid DNA priming-recombinant adenovirus boosting regimen (De Alencar et al., 2009; Haolla et al., 2009; Dominguez et al., 2011, 2012; Rigato et al., 2011). In C57BL/6 mice, protective immunity is mediated by CD8+ T cells that are specific for the immunodominant epitope VNHRFTLV (De Alencar et al., 2009; Rigato et al., 2011). Fourteen days after boosting with AdASP-2, specific CD8+ cells expressed the TE cell phenotype (CD11aHigh, CD44High, CD127Low, and CD62LLow). Later, 98 days after boosting, their phenotype developed into that of TEM cells (CD11aHigh, CD44High, CD127High, and CD62LLow) (Rigato et al., 2011).
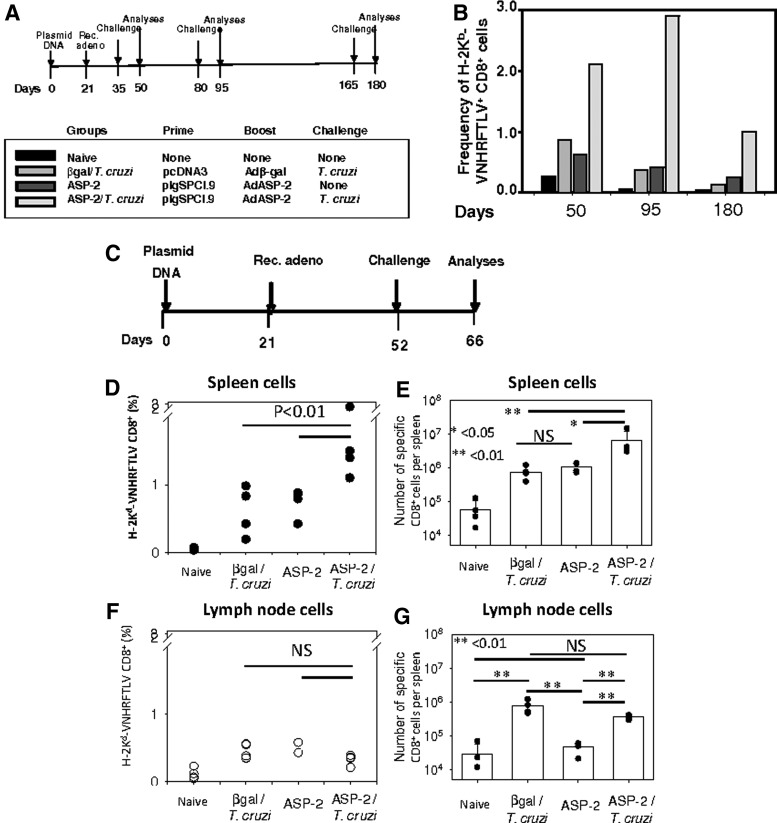
To study the frequencies of the specific CD8+ T cells, the vaccinated mice were challenged at different times after the last immunizing dose, as depicted in Fig. 1A. The frequencies of the CD8+ H-2Kb-VNHRFTLV+ cells in the spleen were compared among mice:
1. Naïve: nonmanipulated
2. β-Gal/T. cruzi: immunized with the control plasmid pcDNA3/Adβ-Gal and challenged with T. cruzi
3. ASP-2: immunized with pIgSP Cl.9/AdASP-2
4. ASP-2/T. cruzi: immunized pIgSP Cl.9/AdASP-2 and challenged with T. cruzi
FIG. 1.
Frequencies of specific splenic CD8+ T cells increase after infectious challenge with T. cruzi in mice vaccinated with the heterologous prime-boost vaccination regimen. (A) C57BL/6 mice were immunized and challenged as depicted. Priming and boosting immunizations were performed as described in the Materials and Methods section. Mouse groups: naïve, nonmanipulated; β-Gal/T. cruzi, immunized with the control vector pcDNA3/Adb-Gal and challenged with T. cruzi; ASP-2, immunized with pIgSP Cl.9/AdASP-2; ASP-2/T. cruzi, immunized pIgSP Cl.9/AdASP-2 and challenged with T. cruzi. (B) H-2Kb-VNHRFTLV+CD8+ splenic cell frequencies were estimated in pools of splenic cells from 4 mice. (C) GzmBCreERT2/ROSA26EYFP transgenic mice were immunized and challenged as depicted. (D) H-2Kb-VNHRFTLV+CD8+ cell frequencies were estimated in the spleen of individual mouse (n=4). (E) The total number of H-2Kb-VNHRFTLV+CD8+ cells was estimated in the spleen of each individual mouse (n=4). (F) H-2Kb-VNHRFTLV+CD8+ cell frequencies were estimated in the inguinal and popliteal lymph nodes of each individual mouse. (G) The total numbers of H-2Kb-VNHRFTLV+CD8+ cells were estimated in the inguinal and popliteal lymph nodes of each individual mouse (n=4). The dots represent each mouse, and the bars represent the means±SD.
After infectious challenge with T. cruzi, the frequency of the CD8+ H-2Kb-VNHRFTLV+ cells consistently increased among the splenic CD8+ T cells of the ASP-2/T. cruzi mice. This increase was always observed, irrespective of the day on which the challenge was performed after the last immunizing dose. The frequencies of the specific CD8+ T cells in the spleen of the ASP-2/T. cruzi mice were higher (from 2.46- to 7.83-fold) than those of the β-Gal/T. cruzi or ASP-2 mice (Fig. 1B).
Because we used pools of splenic T cells in the experiments described above, we reproduced the experiment in which we measured the individual frequencies of the specific CD8+ T cells using mice that were immunized according to the protocol described in Fig. 1C. The frequency of the CD8+ H-2Kb-VNHRFTLV+ cells was analyzed in the spleen and draining lymph nodes (popliteal and inguinal) of each individual mouse. After challenge, we detected a significant increase in the frequency of the splenic H-2Kb-VNHRFTLV+ CD8+ T cells (Fig. 1D). This event was not observed in the lymph nodes of the same mice (Fig. 1F).
Because of the splenomegaly in the mice challenged with T. cruzi, we also calculated the total number of CD8+ H-2Kb-VNHRFTLV+ cells per spleen and draining lymph nodes. We found that the total number of specific T cells was even higher in the ASP-2/T. cruzi mice than in the other mouse groups (Fig. 1E). In the case of the lymph nodes, we did not detect a difference between the ASP-2/T. cruzi mice and the βGal/T. cruzi mice (p=NS). However, there was a difference between the βGal/T. cruzi or ASP-2/T. cruzi mice and the ASP-2 mice. It reflected that the total number of recovered cells was significantly higher in the T. cruzi–challenged mice (Fig. 1G).
Analyses of the frequencies of specific CD8+ T cells in the blood of the different mouse groups revealed that immune and/or infected mice had a higher frequency than control βGal mice (p<0.001). ASP-2/T. cruzi mice had a higher frequency-specific CD8+ T cells; however, they were not statistically different from βGal/T. cruzi or ASP-2 mice (p=NS, data not shown).
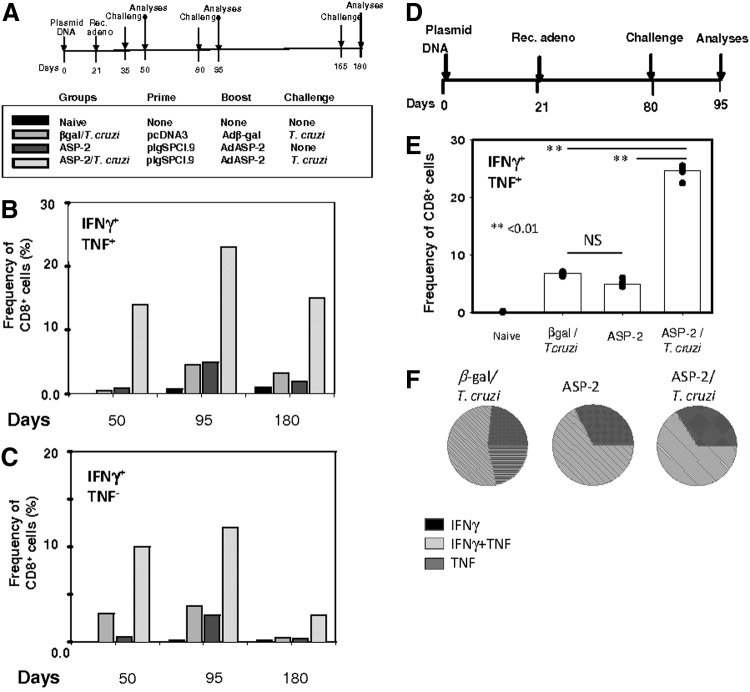
To determine whether these specific CD8+ T cell subpopulations accounted for any relevant antiparasitic effector functions, we evaluated the pattern of IFNγ/TNF expression upon in vitro stimulation with the antigen. Splenic mouse cells were collected from mice challenged 14 days earlier, at different days after the booster injection, as described in the timeline shown in Fig. 2A. These cells were cultured in vitro in the presence or absence of the cognate peptide VNHRFTLV. In the presence of the antigen, the CD8+ T cells from the β-Gal/T. cruzi, ASP-2, and ASP-2/T. cruzi mice expressed IFNγ and/or TNF (Fig. 2B). In contrast, the CD8+ T cells from the naïve control mice did not. Splenic T cells cultured in vitro in the absence of peptide presented very low frequencies of CD8+ cells that expressed either IFNγ or TNF (data not shown). The frequencies of the IFNγ+/TNF+ cells were higher in the population of splenic CD8+ cells from the ASP-2/T. cruzi mice (Fig. 1B). Similarly, these mice also presented higher frequencies of the IFNγ+ (Fig. 1C).
FIG. 2.
Frequencies of specific cytokine-secreting splenic CD8+ T cells increase after infectious challenge with T. cruzi in mice vaccinated with the heterologous prime-boost vaccination regimen. (A) C57BL/6 mice were immunized and challenged as depicted. Priming and boosting immunizations were performed as described in the Materials and Methods section. The mouse groups were the same as those described in the legend of Fig. 1. (B) Pools of splenic cells from 4 mice were restimulated in vitro in the absence (Med) or presence of the peptide VNHRFTLV (Pep), anti-CD28, BdGolgiPlug, and monensin. After 12 hr, the cells were stained with anti-CD8, anti-IFNγ, and anti-TNF. (C) The same as above expect that they represent only the cells stained with anti-CD8 and anti-IFNγ but not with anti-TNF. (D) C57BL/6 mice were immunized and challenged as depicted. (E) Splenic cells were restimulated in vitro in the presence of the peptide VNHRFTLV, anti-CD28, BdGolgiPlug, and monensin. After 12 hr, the cells were stained with anti-CD8, anti-IFNγ, and anti-TNF. The results are represented by medians (bars) from four individual mouse per group (dots). Two asterisks denote that the values of these groups were higher than those of the naïve control mice or all other groups (p<0.01), respectively. (F) Pie charts show the fraction of peptide-specific cells expressing the indicated molecules. The results are expressed as the mean values for four mice per group.
Because we used pools of splenic T cells in the experiments described above, we reproduced these experiments using individual mouse that were immunized and challenged according to the protocol described in Fig. 2D. The frequencies of specific CD8+ T cells expressing IFNγ and TNF in the spleen of each individual mouse after challenge were significantly higher than in the other groups (Fig. 2E). These results corroborated with our multimer staining results (Fig. 1B and D), confirming that the specific CD8+ T cells are indeed functional. The quality of the immune response was also compared by estimating the frequencies of cells expressing different cytokines. We did not find significant differences between the ASP-2 and ASP-2/T. cruzi mice in this analysis (Fig. 2F).
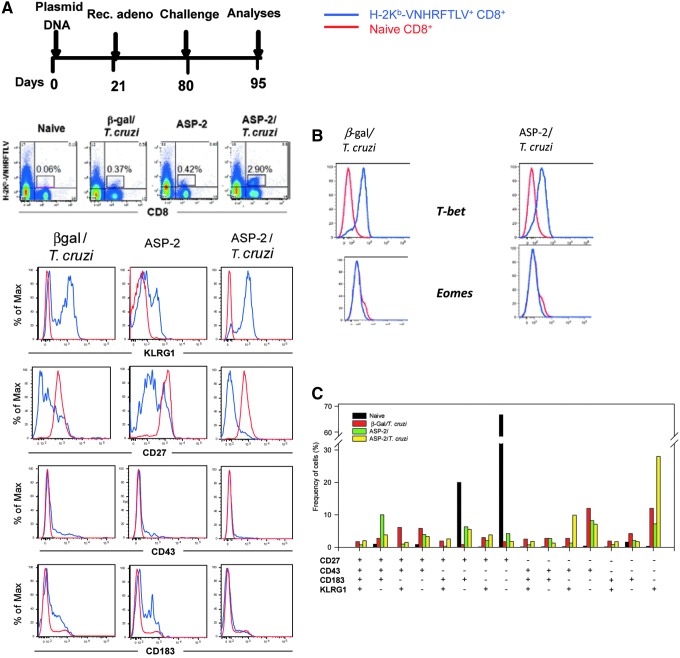
From our evaluation of the expression of the markers CD44, CD62L, and CD127, the specific CD8+ T cells always presented a TE(M) phenotype (Rigato et al., 2011). Thus, we then searched for other surface markers that could subdivide this population. On the basis of a previous study by Hikono et al. (2007), we used antibodies against KLRG1, CD27, CD43 (glycosylated form), and CD183 (chemokine receptor CXCR3) to compare the phenotype of resting naïve splenic CD8+ T cells to that of H-2Kb-VNHRFTLV+ CD8+ T cells from the β-Gal/T. cruzi, ASP-2, and ASP-2/T. cruzi mice. When comparing the expression of KLRG-1 or CD27, the phenotype of the H-2Kb-VNHRFTLV+ CD8+ cells varied dramatically in the different groups of mice (Fig. 3A). The ASP-2/T. cruzi mice had significantly higher frequencies of KLRG1+ and CD27− cells in their H-2Kb-VNHRFTLV+ CD8+ T cell population. The expression of CD43 or CD183 on the H-2Kb-VNHRFTLV+ CD8+ T cells was low in all mouse groups and was comparable to the staining observed for naïve CD8+ T cells. We also found that these cells were all T-bet+ and Eomes− (Fig. 3B).
FIG. 3.
Phenotype of specific splenic CD8+ T cells after infectious challenge with T. cruzi in mice vaccinated with the heterologous prime-boost vaccination regimen. (A) C57BL/6 mice were immunized and challenged as depicted. Priming and boosting immunizations were performed as described in the Materials and Methods section. The mouse groups were the same as those described in the legend of Fig. 1. The red and blue lines depict the naïve CD8+ cells and H-2Kb-VNHRFTLV CD8+ cells, respectively. Pools of splenic cells from 4 mice were stained with anti-CD8, H-2Kb-VNHRFTLV, and anti-KLRG, anti-CD27, anti-CD43, or anti-CD183. The red and blue lines denote the naïve CD8+ cells and H-2Kb-VNHRFLTV CD8+ cells, respectively. Analysis of individual mouse in each group provided similar results. (B) Pools of splenic cells from four mice were stained with anti-CD8, H-2Kb-VNHRFTLV, and anti-T-bet or anti-Eomes. (C) Boolean analyses of pools of splenic cells from 4 mice that were simultaneously stained with anti-CD8, H-2Kb-VNHRFLTV, anti-KLRG, anti-CD27, anti-CD43, and anti-CD183. The red and blue lines denote the naïve CD8+ cells and H-2Kb-VNHRFLTV CD8+ cells, respectively. Analysis of individual mouse in each group provided similar results.
To compare the different populations of H-2Kb-VNHRFTLV+ CD8+ T cells stained for the four different markers, we performed Boolean analyses. For these analyses, splenic cells were simultaneously labeled for CD8, H-2Kb-VNHRFTLV, KLRG1, CD27, CD43, and CD183. As depicted in Fig. 3C, the majority of the cells in the ASP-2/T. cruzi mice were KLRG1+CD27−CD43−CD183− cells. A number of isotype control antibodies were used described in the Materials and Methods section. None of them stained the CD8+H-2Kb-VNHRFTLV+ cells (data not shown).
In addition to these markers, the H-2Kb-VNHRFTLV+ CD8+ from the ASP-2/T. cruzi mice were CD11aHigh, CD44High, CD62LLow, CD127Low, CD11CHigh, CD49d High, PD1Low, CD95Low, CD38High, PDL-1Low, CD134High, LAG-3High, TRAILLow, TRAIL-R2Low, TIM3Low, and TRANCELow (Supplementary Fig. S1; Supplementary Data are available online at www.liebertpub.com/hum).
To determine the phenotype of the IFNγ- and TNF-producing cells, we combined the ICS analysis with staining for the surface markers KLRG1, CD27, CD43, and CD183. The CD8+ T cells expressing IFNγ or TNF were not proportionally equal in the different mouse groups. Higher frequencies of IFNγ+KLRG1+ and TNF+ KLRG1+ were observed in the splenic CD8+ cell population from the ASP-2/T. cruzi mice compared with the β-Gal/T. cruzi and ASP-2 mice. Similar analyses were also performed using CD27, CD43, and CD183. In these cases, we found that the predominant phenotype for the CD8+ IFNγ+ and CD8+ TNF+ cells was always CD27−, CD43−, and CD183− (Supplementary Fig. S2).
The specific CD8+ T cells observed after infectious challenge with T. cruzi are immune TE(M) cells previously expanded by vaccination
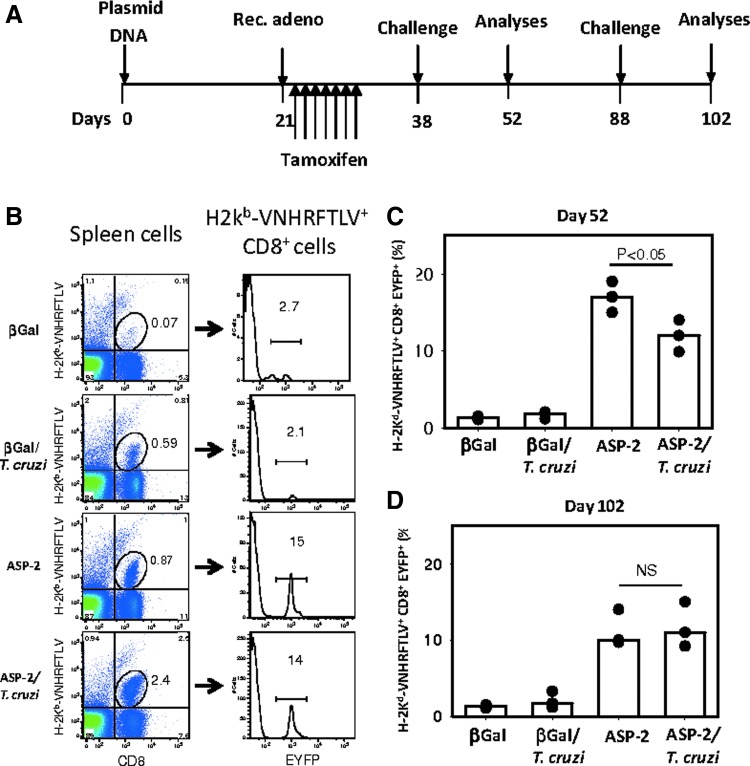
The results described above that before and after an infectious challenge, specific CD8+ T cells differ with respect to their frequency and phenotype raised the possibility that cells other than the vaccination-induced CD8+ TE(M) cells accounted for a proportion of the cells observed after challenge. To examine this possibility, we made use of the gzmBCreERT2/ROSA26EYFP transgenic mouse line. After antigenic stimulation in vivo and treatment with tamoxifen, cells expressing Granzyme B are indelibly labeled with EYFP (Bannard et al., 2009). Therefore, in these mice, antigen-specific CD8+ T cells with an effector function (TE cells) will become fluorescent (EYFP+). In Fig. 4A, the detailed protocol used for these experiments is shown. The tamoxifen treatment was performed over 7 days after the booster injection with the recombinant adenovirus (days 22–28).
FIG. 4.
The specific CD8+ T cells detected after challenge with T. cruzi are TE(M) cells. (A) GzmBCreERT2/ROSA26EYFP transgenic mice were immunized, treated with tamoxifen, and challenged as depicted. The mouse groups were the same as those described in the legend of Fig. 1. (B) Splenocytes were stained with anti-CD8 and H-2Kb-VNHRFTLV. The EYFP+ TE cell frequency in each group was then estimated. (C) Splenocytes isolated at day 52 were stained with anti-CD8 and H-2Kb-VNHRFTLV. The frequency of EYFP+ cells (Granzyme B+ TE) in each group is shown for each individual mouse (dots). Bars represent the median of each mouse group. (D) The same as above except that the cells were obtained from mice at day 102. The asterisk denotes a higher frequency of EYFP+ H-2Kb-VNHRFTLV + CD8+ cells (p<0.05).
The strategy used for quantifying the H-2Kb-VNHRFTLV+ CD8+ cells labeled with EYFP is depicted in Fig. 4B. We initially gated splenic H-2Kb-VNHRFTLV+ CD8+ cells, and the frequency of EYFP fluorescent cells was then estimated. The results are expressed as the proportion of EYFP+ cells within the total VNHRFTLV+ CD8+ cell population. As depicted in Fig. 4C, at day 52, 15–19% of the H-2Kb-VNHRFTLV+ CD8+ cells in the ASP-2 mice were labeled with EYFP. In vaccinated and challenged mice (ASP-2/T. cruzi), the frequency of H-2Kb-VNHRFTLV+ CD8+ cells labeled with EYFP ranged from 9.9% to 14% (Fig. 4C). The EYFP-labeled activated specific cells never reached 20%. This partial labeling was described earlier as a result of inefficient Cre-mediated recombination (Bannard et al., 2009).
Similar measurements made at day 102 detected 9.7% to 14% of the H-2Kb-VNHRFTLV+ CD8+ cells in the ASP-2 mice labeled with EYFP (Fig. 4D). Similarly, in vaccinated and challenged mice (ASP-2/T. cruzi), the frequency of H-2Kb-VNHRFTLV+ CD8+ cells labeled with EYFP ranged from 9.2% to 15% (Fig. 4D). In the β-Gal and β-Gal/T. cruzi mice, the frequencies of H-2Kb-VNHRFTLV+ CD8+ cells labeled with EYFP ranged between 1.2% and 3.3% (Fig. 4C and D).
Analysis of these results showed that although there was a statistically significant difference (p<0.05) between the frequencies of 2Kb-VNHRFTLV+ CD8+ EYFP+ cells observed in the ASP-2 and ASP-2/T. cruzi groups at day 52, we found no difference at day 102 (p=NS). Subsequent experiments confirmed that there was no significant difference between these groups. Thus, we concluded that the H-2Kb-VNHRFTLV+ CD8+ cells observed after the infectious challenge are the same antigen-specific effector CD8+ T cells that expressed Granzyme B after immunization with AdASP-2.
To further confirm that naïve T cells do not participate in the immune response after the infectious challenge of vaccinated mice, we performed experiments in which we transferred naïve GFP-transgenic splenic cells to vaccinate mice at the same day of the challenge (Supplementary Fig. S3A). After 20 days, we estimated the frequencies of specific T cells using in vitro restimulation and ICS. As shown earlier, the frequencies of specific CD8+ T cells were higher in the ASP-2/T. cruzi mice than those in the β-gal/T. cruzi mice (Supplementary Fig. S3B and E). Because the naïve cells were GFP+, we were able to localize these naïve CD8+ cells (Supplementary Fig. S3C). We then estimated the frequencies of the CD8+ IFNγ+ GFP+ cells (Supplementary Fig. S3D and F). A statistical comparison showed that the frequencies of the CD8+ IFNγ+ GFP+ cells in the β-gal/T. cruzi mice were significantly higher than those in the ASP-2/T. cruzi mice (Supplementary Fig. S3F). In fact, very few CD8+ IFNγ+ GFP+ cells were detected in these mice, showing that the presence of specific CD8+ TE cells completely inhibited naïve T cell activation.
Proliferation plays a minor role in the increase in the frequency of specific CD8+ T cells in the spleen and the protective immunity resulting from vaccination
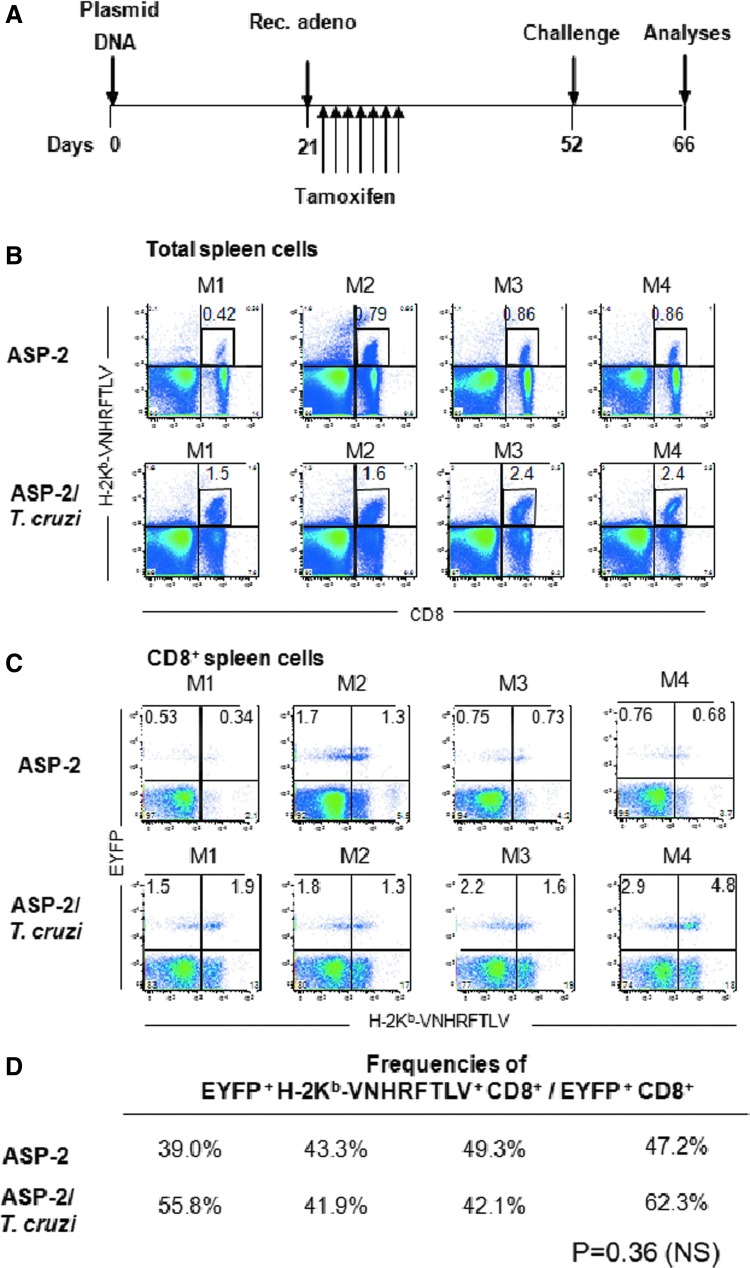
A plausible explanation for the increase in the frequencies of specific splenic CD8+ T cells after challenge is proliferation upon antigen restimulation (anamnestic response). Alternatively, this increase could be because of recirculation, for example. To evaluate these hypotheses, we immunized and challenged GzmBCreERT2/ROSA26EYFP mice line as depicted in Fig. 5A. We then analyzed the frequencies of specific cells (stained for H-2Kb-VNHRFTLV) within the total EYFP+ CD8+ T cells in the ASP-2 or ASP-2/T. cruzi mice. Our rationale was that if antigen-specific proliferation occurs after challenge, the frequency of the EYFP+ H-2Kb-VNHRFTLV+ CD8+ cells (within the EYFP+ CD8+ cells) should be much higher in the ASP-2/T. cruzi mice than in the ASP-2 mice because of the anamnestic proliferation.
FIG. 5.
EYFP+ H-2Kb-VNHRFTLV+ and EYFP+ H-2Kb-VNHRFTLV− CD8+ T cell frequencies increase after infectious challenge. (A) GzmBCreERT2/ROSA26EYFP transgenic mice were immunized, treated with tamoxifen, and challenged as depicted. The mouse groups were the same those as described in the legend of Fig. 1. M1–M4 denote each individual mouse analyzed. (B) The frequency of the splenic H-2Kb-VNHRFTLV+ CD8+ cells was higher in the ASP-2/T. cruzi mice compared with the ASP-2 mice (p<0.01). (C) Frequencies of the EYFP+ H-2Kb-VNHRFTLV+ and EYFP+ H-2Kb-VNHRFTLV− CD8+ cells in the ASP-2 and ASP-2/T. cruzi mice. (D) Frequencies of EYFP+ H-2Kb-VNHRFTLV+ CD8+ T cells/total EYFP+ CD8+ T cells. No significant difference was found between the frequency of the EYFP+ H-2Kb-VNHRFTLV+ CD8+ cells in the ASP-2/T. cruzi mice compared with that of the ASP-2 animals (p=NS). Results are from one of three independent experiments performed.
As described above, when analyzed among total spleen cells, we found that the frequency of splenic H-2Kb-VNHRFTLV+ CD8+ cells was higher in the ASP-2/T. cruzi mice compared with the ASP-2 mice (Fig. 5B). In contrast, when analyzed among the EYFP+ CD8+ cells, we found that there was little increase in the frequency of EYFP+ H-2Kb-VNHRFTLV+ CD8+ cells in the spleen of ASP-2/T. cruzi mice compared with the ASP-2 animals (Fig. 5C and D). Among the EYFP+ CD8+ cells, in the ASP-2 mice, the frequency of EYFP+ H-2Kb-VNHRFTLV+ CD8+ cells ranged from 39% to 49.3%. In the ASP-2/T. cruzi mice, this frequency ranged from 41.9% to 62.3% (p=NS). Thus, we concluded that the increase in these frequencies was not T. cruzi antigen specific.
The limited expansion of the H-2Kb-VNHRFTLV+ CD8+ cells was further corroborated by experiments of in vivo BrdU incorporation. Vaccinated or control mice were challenged or not with T. cruzi. In the subsequent days, these animals were treated with BrdU. Fourteen days later, the frequencies of the H-2Kb-VNHRFTLV+ CD8+ cells that incorporated BrdU or stained for KLRG1 were estimated. As shown in Supplementary Fig. S4B, there is a significant increase in the frequency of specific CD8+ T cells from ASP-2/T. cruzi mice when compared with the other mouse groups as described earlier.
We also observed that 22% of CD8+ T cells from control mice (β-gal) proliferated during the period of 2 weeks as estimated by BrdU incorporation. In contrast, as expected, a much higher frequency (66%) of H-2Kb-VNHRFTLV+ CD8+ cells from T. cruzi–infected mice incorporated this marker. In mice that were vaccinated but not challenged (ASP-2), proliferation of specific CD8+ T cells was minor (14%). Finally, we observed that only 36% of the H-2Kb-VNHRFTLV+ CD8+ cells from ASP-2/T. cruzi incorporated BrdU. We concluded that although a fraction of the specific T cells proliferated, the vast majority did not. Similar experiment is also shown in Supplementary Fig. S5C. In this case, the incorporation of BrdU was detected only in 12.1% of the specific CD8+ T cells. In this figure, we also show that the BrdU incorporation in splenic cells in this mouse group (ASP-2/T. cruzi) is not altered and occurred in parallel (Supplementary Fig. 5SD).
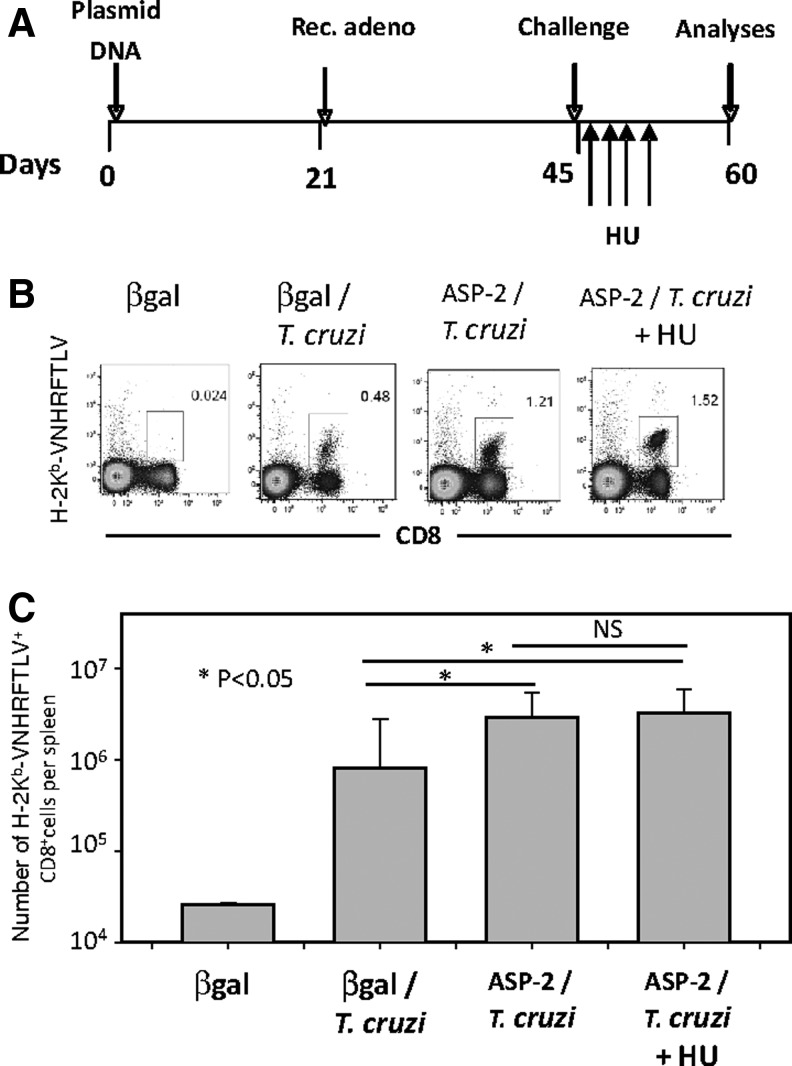
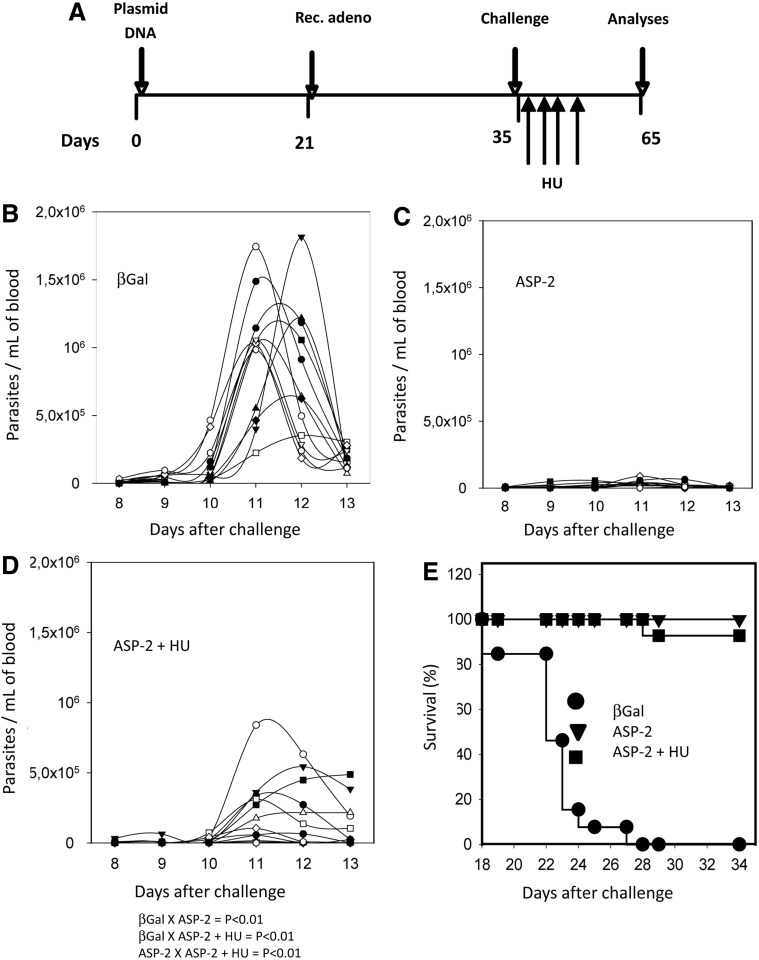
To determine whether the increase in frequency of specific TE(M) cells and the protective immunity they mediate is dependent on proliferation, we treated the immunized mice with the cytostatic toxic agent HU. We followed the immunization and treatment schedule shown in Fig. 6A. Fifteen days after challenge, we estimated the frequency of H-2Kb-VNHRFTLV+ CD8+ cells in the spleens of ASP-2/T. cruzi mice that were treated or not with HU. We did not detect a significant difference when compared the frequency or total number of H-2Kb-VNHRFTLV+ CD8+ cells per spleen between these two groups (p=NS, Fig. 6B and C, respectively). The HU treatment in this experiment led to a significant decrease in the total number of spleen cells. On average, the spleen cell number of the untreated mice was 1.56-fold higher than that of the HU-treated mice (p<0.05). Also, we performed BrdU incorporation experiments to confirm whether the treatment indeed inhibited proliferation as we expected. As shown in Supplementary Fig. S5D, BrdU was incorporated by 69.4% or 41.1% of splenic cells from T. cruzi–infected mice (βGal/T. cruzi or ASP-2/T. cruzi, respectively; Supplementary Fig. 5SD). In contrast, only 12.2% of the cells from ASP-2/T. cruzi mice treated with HU incorporated BrdU. From this experiment we could confirm that the treatment with HU severely inhibits the proliferation of all splenic cells. Because our ultimate goal was to determine the importance of T cell proliferation in protective immunity, we used the highly susceptible mouse strain A/Sn. In these mice, protective immunity is mediated by CD8+ cells that are specific for the epitopes TEWETGQI, PETLGHEI, and YIEVAGII (De Alencar et al., 2009; Haolla et al., 2009; Dominguez et al., 2011, 2012). These mice were vaccinated and treated according to the protocol described in Fig. 7A. After challenge, the vaccinated mice were treated or not with HU. The mice treated with HU (Fig. 7D) displayed significantly higher parasitemia than the vaccinated mice that were not treated with HU (Fig. 7C; p<0.01). However, their parasitemia was significantly lower than that of the infected βGal control mice (Fig. 7B; p<0.01).
FIG. 6.
The increase in the specific splenic CD8+ T cell frequency after infectious challenge is not inhibited by treatment with hydroxyurea (HU). (A) C57BL/6 mice were immunized, infected, and treated with HU as depicted. The mouse groups were the same as those described in the legend of Fig. 1 except that βGal denotes mice that were injected with pcDNA3 and Adβ-Gal. (B) Fourteen days after challenge, the H-2Kb-VNHRFTLV+ CD8+ cell frequency was estimated. Representative plot of each group. (C) Number of splenic H-2Kb-VNHRFTLV+ CD8+ cell expressed as mean±SD (n=4). Results are from one of two independent experiments performed.
FIG. 7.
The protective immunity elicited by heterologous prime-boost vaccination is not inhibited by treatment with HU. (A) A/Sn mice were immunized, infected, and treated with HU as depicted. (B) Parasitemia of each individual control mouse primed with pcDNA3 and boosted with Adβ-Gal (n=12). (C) Parasitemia of each individual ASP-2-immunized mouse (n=12). (D) Parasitemia of each individual ASP-2-immunized mouse treated with HU (n=10). Although the day of peak parasitemia was different for each mouse, we used the maximal values for statistical comparison. The results of the statistical comparisons are below panel D. (E) Kaplan–Meier survival curves of the different groups were compared, and the results showed that ASP-2-immunized mice treated or not with HU survived significantly longer (p<0.01) than control animals injected with βGal. Results are from two experiments pooled.
Despite their higher parasitemia, the vaccinated mice treated with HU recovered, and 90% of them survived the lethal challenge (Fig. 7E). The increase in survival was not statistically significant between the vaccinated mice treated or not with HU. These results led us to conclude that neither the increase in frequency of splenic T cells nor the observed protective immunity is sensitive to treatment with the cytostatic toxic drug HU.
Discussion
In the present study, we described the changes in frequency, phenotype, and function of CD8+ T cells after infectious challenge with T. cruzi in mice vaccinated by heterologous prime-boost with plasmid DNA and an adenoviral vector. The most important observations made in this study were that after the infectious challenge, the frequency of splenic CD8+ specific T cells increased and that these cells differentiated into KLGR1HighCD27Low cells. Most of the cells were multifunctional and produced IFNγ and TNF upon antigen restimulation. Using transgenic gzmBCreERT2/ROSA26EYFP mice, we confirmed that the specific T cells in the spleen were in fact CD8+ T cells that had been expanded after vaccination. Not only did these vaccine-induced T cells dominate the immune response after infectious challenge, but they also completely inhibited the expansion of specific naïve T cells (Supplementary Fig. S4). Although the mechanism that leads to inhibition of those specific naïve T cells was beyond the scope of our study, it can be hypothesized that the inhibition of parasite multiplication reduced the amount of antigen available for efficient priming. In addition, it has been described that antigen-specific activated CD8+ T cells are potent inhibitors of the priming of naive cells, as they rapidly eliminate antigen-presenting cells (Hafalla et al., 2003; Wong et al., 2003).
Despite the increase in the frequency of specific T cells, we could not find evidence that this rise was because of antigen-specific proliferation. Indeed our results showed that the ratio of activated CD8+ EYFP-expressing cells that were specific for the T. cruzi epitope or the adenoviral carrier did not change in mice challenged with the parasite (Fig. 5). In addition, treatment with the drug HU did not significantly reduce the frequencies of specific T cells after challenge infection (Fig. 6 and Supplementary Fig. S5). These observations are in agreement with BrdU incorporation experiments. We did not observe significant labeling of specific CD8+ cells after the infectious challenge of vaccinated mice. In contrast, in nonvaccinated and challenged mice, the majority of T cells were labeled with BrdU, denoting an intense proliferation (Supplementary Figs. S4 and S5). The anamnestic potential of CD8+ T cells primed by adenoviral vectors has been long shown to be excellent when boosted with recombinant vaccinia viruses (Bruña-Romero et al., 2001). However, more recently, using other antigenic stimuli, different groups have described a poor anamnestic response of CD8+ T cells primed with AdHu5 vector when compared with other adenovirus vector serotypes (Penaloza-MacMaster et al., 2013; Tan et al., 2013). Perhaps, in our case, T. cruzi belongs to this class of poor AdHu5-induced TE(M) cell activators.
Secondary responses depend on the timing of the booster injection (Bruna-Romero et al., 2001). This is probably because memory T cells need to rest before they can be restimulated with the antigen. In our case, we tested at different periods of time until 165 days and could not find remarkable differences in the increase in frequencies of specific T cells after challenge (Fig. 1B). These observations confirm that T. cruzi challenge at any timing after the immunization led to similar changes in frequencies of splenic specific CD8+ TE(M) cells. BrdU incorporation further corroborates with these observations. Also, these observations are important because they may indicate a path for vaccine improvement. Other adenovirus vectors of distinct serotypes expressing T. cruzi antigens may provide better priming for parasite boosting.
The increase in frequencies of specific CD8+ T cells in the spleen after challenge infection alone does not seem to account for the observed protective immunity. In our previous study, we showed that vaccinated mice that were administered FTY720 were significantly more susceptible to infection than untreated, vaccinated control animals (Dominguez et al., 2012). In FTY720-treated and vaccinated mice, we still observed a similar increase in the frequencies of specific CD8+ T cells after challenge (J.R.V., M.R.D., R.L., and M.M.R., unpublished results). Therefore, we concluded that the increase in these frequencies does not always correlate with or predict efficient protective immunity.
The increase in the frequency of the H-2Kb-VNHRFTLV+ CD8+ cells in the spleens of the ASP-2/T. cruzi mice possibly represents a major recirculation event of the activated CD8+ T cells. As mentioned above, the frequency of the splenic EYFP+ cells increases in a manner that is apparently independent of their specificity. This ability to rapidly recirculate might be associated with their phenotype, as discussed below. Alternatively, it might be caused by the infection. These mice display visible signs of splenomegaly. The factors that cause this splenomegaly and the influx of activated T cells after an infectious challenge are still poorly understood and remain an interesting aspect to be investigated.
The predominant phenotype of the specific CD8+ T cells detected after challenge was KLRG1High CD27Low CD43Low CD183Low− T-betHigh EomesLow (Fig. 3C and D). These cells were also CD62LLow and CD127Low (Supplementary Fig. S2). This phenotype resembles the recently described phenotype of effector-like memory CD8+ T cells, which persist to the memory phase, providing efficient control of infection with Listeria monocytogenes or vaccinia virus (Olson et al., 2013). These authors described that the number of these effector-like memory CD8+ T cells is greatly increased in the blood and splenic red pulp and is greatly reduced in the lymph nodes (Olson et al., 2013). Although we showed that these cells are reduced in the draining lymph nodes, we have yet to determine if our T cells also share other characteristics that could explain the observed rapid recirculation event.
In addition to their phenotype, these two protective CD8+ T cell types share functional similarities. Both of them express Granzyme B (Figs. 4 and 5) (Olson et al., 2013). They also require the expression of perforin to mediate efficient protective immunity (De Alencar et al., 2009; Olson et al., 2013).
The CD8+ TEM cells described herein also resemble CD8+ TEM cells that have been previously detected after heterologous prime-boost vaccination using different viral vectors (Fraser et al., 2013). However, it is important to note that in our case, the expression of PD1 is much lower. In fact, the expression of molecules that control cell survival/apoptosis, such as PD1, PDL-1, CD95, TRAIL, TRAIL2, and TIM3, is always low (Supplementary Fig. S1). In contrast, high levels of the activation markers CD44, CD11a, CD11c, CD38, and CD49d are a hallmark (Supplementary Fig. S1). A similar phenotype (PD1Low) was described for the specific CD8+ T cells elicited by heterologous prime-boost vaccination with plasmid DNA and recombinant, replication-defective adenovirus (AdHu5 and AdHu35) (Steffensen et al., 2013). Therefore, PD1Low phenotype may represent a general characteristic of the CD8+ T cells elicited by adenovirus vector immunization, as we have previously described (Vasconcelos et al., 2012a).
Treatment with HU after challenge did not significantly modify the frequency of splenic specific CD8+ T cells or impair the observed protective immunity. Earlier studies using HU treatment have shown exactly the opposite effects. Treatment with HU was found to completely abolish memory responses to lymphocytic choriomeningitis virus antigens (LCMV) (Bellier et al., 2003). These authors postulate that memory T cells must continue to proliferate to maintain immunological memory. The discrepancies in these results may reflect the fact that the memory T cells elicited by LCMV infection are mostly TCM cells (CD62LHighCD127High) (Wherry et al., 2003). TCM cells display high homeostatic proliferation. In contrast, TEM cells have limited homeostatic and antigen-driven proliferation (Wherry et al., 2003). Therefore, TCM cells might be more susceptible to HU treatment than TEM cells. The possibility that the HU treatment acts upon parasite elimination is unlikely. C57BL/6 mice infected with T. cruzi and treated HU from day 7 to day 13 have been found to uniformly succumb to infection (M.R.D. and M.M.R., unpublished results). The result that the T cells from vaccinated mice do not need to proliferate supports the concept that the high frequency of TE(M) cells present after the prime-boost immunization regimen in our model is sufficient to mediate protective immunity. Because our experiments were performed only 2 weeks after boost immunization (Figs. 5–7), it is possible that at longer times, when the frequency of specific T CD8+ T cells declines (more than 120 days), they may need to proliferate to mediate protective immunity. Alternatively, protective immunity may be lost as the number of cells decline. Although we consider these experiments important, because of their long duration, we plan to study this issue in the future.
These results complement our previous observation that CD8+ T cells must recirculate to mediate protective immunity against T. cruzi infection (Dominguez et al., 2012). To corroborate this observation, the molecular mechanisms used by these T cells to recirculate are currently being explored. We observed that in addition to FTY720, antibodies against integrins and chemokines drastically impact the protective immune response without impairing the specific immune response (F.V., M.M.R., and J.R.V., unpublished results). In addition to integrins and chemokines, a molecular mechanism that controls the ability of the CD8+ T cells to recirculate has been very recently described. Recirculating CD8+ T cells express the transcription factor KLF2. This factor increases the expression of the S1Pr1 receptor on CD8+ TE cells, allowing them to continue to recirculate (Skon et al., 2013). Our protective T cells certainly express the S1Pr1 receptor because they are targets of the drug FTY720.
In summary, our evidence supports the model that when large amounts of antigen-experienced CD8+ TEM cells are present after heterologous prime-boost vaccination, differentiation and recirculation, rather than proliferation, are key for the resultant protective immunity.
Supplementary Material
Acknowledgments
This work was supported by grants from Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP; 2009/06820-4, 2013/13668/0, and 2012/22514-3) and Instituto Nacional de Ciência e Tecnologia em Vacina (INCTV-CNPq). J.R.V., M.R.D., J.E., and A.A. were recipients of fellowships from FAPESP. O.B.-R., R.T.G., and M.M.R. are recipients of fellowships from CNPq.
Author Disclosure Statement
R.T.G., M.M.R., A.V.M., and O.B.-R. are named inventors on patent applications covering Trypanosoma cruzi–vectored vaccines and immunization regimens. No competing financial interests exist for the remaining authors.
References
- Bannard O., Kraman M., and Fearon D.T. (2009). Secondary replicative function of CD8+ T cells that had developed an effector phenotype. Science 323, 505–509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellier B., Thomas-Vaslin V., Saron M.F., and Klatzmann D. (2003). Turning immunological memory into amnesia by depletion of dividing T cells. Proc. Natl. Acad. Sci. USA 100, 15017–15022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boscardin S.B., Kinoshita S.S., Fujimura A.E., and Rodrigues M.M. (2003). Immunization with cDNA expressed by amastigotes of Trypanosoma cruzi elicits protective immune response against experimental infection. Infect. Immun. 71, 2744–2757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruña-Romero O., et al. (2001). Complete, long-lasting protection against malaria of mice primed and boosted with two distinct viral vectors expressing the same plasmodial antigen. Proc. Natl. Acad. Sci. USA 98, 11491–11496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casazza J.P., et al. (2013). Therapeutic vaccination expands and improves the function of the HIV-specific memory T-cell repertoire. J. Infect. Dis. 207, 1829–1840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang I., et al. (2013). DNA prime/Adenovirus boost malaria vaccine encoding P. falciparum CSP and AMA1 induces sterile protection associated with cell-mediated immunity PLoS One 8, e55571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Alencar B.C., et al. (2009). Perforin and gamma interferon expression are required for CD4+ and CD8+ T-cell-dependent protective immunity against a human parasite, Trypanosoma cruzi, elicited by heterologous plasmid DNA prime-recombinant adenovirus 5 boost vaccination. Infect. Immun. 77, 4383–4395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Rosa S.C., et al. (2011). HIV-DNA priming alters T cell responses to HIV-adenovirus vaccine even when responses to DNA are undetectable. J. Immunol. 187, 3391–3401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominguez M.R., et al. (2011). Subdominant/cryptic CD8 T cell epitopes contribute to resistance against experimental infection with a human protozoan parasite. PLoS One 6, e22011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominguez M.R., et al. (2012). Re-circulation of lymphocytes mediated by sphingosine-1-phosphate receptor-1 contributes to resistance against experimental infection with the protozoan parasite Trypanosoma cruzi. Vaccine 30, 2882–2891 [DOI] [PubMed] [Google Scholar]
- Fraser K.A., et al. (2013). Preexisting high frequencies of memory CD8+ T cells favor rapid memory differentiation and preservation of proliferative potential upon boosting. Immunity 39, 171–183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freel S.A., et al. (2010). Phenotypic and functional profile of HIV-inhibitory CD8 T cells elicited by natural infection and heterologous prime/boost vaccination. J. Virol. 84, 4998–5006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafalla J.C., et al. (2003). Early self-regulatory mechanisms control the magnitude of CD8+ T cell responses against liver stages of murine malaria. J. Immunol. 171, 964–970 [DOI] [PubMed] [Google Scholar]
- Hammer S.M., et al. (2013). Efficacy trial of a DNA/rAd5 HIV-1 preventive vaccine. N. Engl. J. Med. 369, 2083–2092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haolla F.A., et al. (2009). Strain-specific protective immunity following vaccination against experimental Trypanosoma cruzi infection. Vaccine 27, 5644–5653 [DOI] [PubMed] [Google Scholar]
- Hikono H., et al. (2007). Activation phenotype, rather than central- or effector-memory phenotype, predicts the recall efficacy of memory CD8+ T cells. J. Exp. Med. 204, 1625–1636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill A.V., et al. (2010). Prime-boost vectored malaria vaccines: progress and prospects. Hum. Vaccin. 6, 78–83 [DOI] [PubMed] [Google Scholar]
- Jaoko W., et al. (2010). Safety and immunogenicity study of Multiclade HIV-1 adenoviral vector vaccine alone or as boost following a multiclade HIV-1 DNA vaccine in Africa. PLoS One 5, e12873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koup R.A., et al. (2010). Priming immunization with DNA augments immunogenicity of recombinant adenoviral vectors for both HIV-1 specific antibody and T-cell responses. PLoS One 5, e9015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasaro M.O., and Ertl H.C. (2009). New insights on adenovirus as vaccine vectors. Mol. Ther. 17, 1333–1339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado A.V., et al. (2006). Long-term protective immunity induced against Trypanosoma cruzi infection after vaccination with recombinant adenoviruses encoding amastigote surface protein-2 and trans-sialidase. Hum. Gene Ther. 17, 898–908 [DOI] [PubMed] [Google Scholar]
- O'Connell R.J., Kim J.H., Corey L., and Michael N.L. (2012). Human immunodeficiency virus vaccine trials. (2012). Cold Spring Harb. Perspect. Med. 2, a007351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okabe M., et al. (1997). “Green mice” as a source of ubiquitous green cells. FEBS Lett. 407, 313–319 [DOI] [PubMed] [Google Scholar]
- Olson J.A., McDonald-Hyman C., Jameson S.C., and Hamilton S.E. (2013). Effector-like CD8+ T cells in the memory population mediate potent protective immunity. Immunity 38, 1250–1260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penaloza-MacMaster P., et al. (2013). Alternative serotype adenovirus vaccine vectors elicit memory T cells with enhanced anamnestic capacity compared to Ad5 vectors. J. Virol. 87, 1373–1384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranasinghe C., and Ramshaw I.A. (2009). Genetic heterologous prime-boost vaccination strategies for improved systemic and mucosal immunity. Exp. Rev. Vaccines 8, 1171–1181 [DOI] [PubMed] [Google Scholar]
- Rigato P.O., et al. (2011). Heterologous plasmid DNA prime-recombinant human adenovirus 5 boost vaccination generates a stable pool of protective long-lived CD8(+) T effector memory cells specific for a human parasite, Trypanosoma cruzi. Infect. Immun. 79, 2120–2130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rollier C.S., et al. (2011). Viral vectors as vaccine platforms: deployment in sight. Curr. Opin. Immunol. 23, 377–382 [DOI] [PubMed] [Google Scholar]
- Skon C.N., et al. (2013). Transcriptional downregulation of S1pr1 is required for the establishment of resident memory CD8+ T cells. Nat. Immunol. 14, 1285–1293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffensen M.A., et al. (2013). Qualitative and quantitative analysis of adenovirus type 5 vector-induced memory CD8 T cells: not as bad as their reputation J. Virol. 87, 6283–6295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan W.G., et al. (2013). Comparative analysis of simian immunodeficiency virus gag-specific effector and memory CD8+ T cells induced by different adenovirus vectors. J. Virol. 87, 1359–1372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasconcelos J.R., et al. (2012a). Pathogen-induced proapoptotic phenotype and high CD95 (Fas) expression accompany a suboptimal CD8+ T-cell response: reversal by adenoviral vaccine. PLoS Pathog. 8, e1002699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasconcelos J.R., et al. (2012b). Relevance of long-lived CD8(+) T effector memory cells for protective immunity elicited by heterologous prime-boost vaccination. Front. Immunol. 3, 358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong P., and Pamer E.G. (2003). Feedback regulation of pathogen-specific T cell priming. Immunity 18, 499–511 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.