Abstract
In the last two decades, it has become clear that γδ T cells recognize a diverse array of antigens including self and foreign, large and small, and peptidic and non-peptidic molecules. In this respect, γδ antigens as a whole resemble more the antigens recognized by antibodies than those recognized by αβ T cells. Because of this antigenic diversity, no single mechanism—such as the major histocompatibility complex (MHC) restriction of αβ T cells—is likely to provide a basis for all observed T-cell antigen receptor (TCR)-dependent γδ T-cell responses. Furthermore, available evidence suggests that many individual γδ T cells are poly-specific, probably using different modes of ligand recognition in their responses to unrelated antigens. While posing a unique challenge in the maintenance of self-tolerance, this broad reactivity pattern might enable multiple overlapping uses of γδ T-cell populations, and thus generate a more efficient immune response.
Keywords: antigen recognition, ligand, T-cell receptor, γδ T cells
Introduction
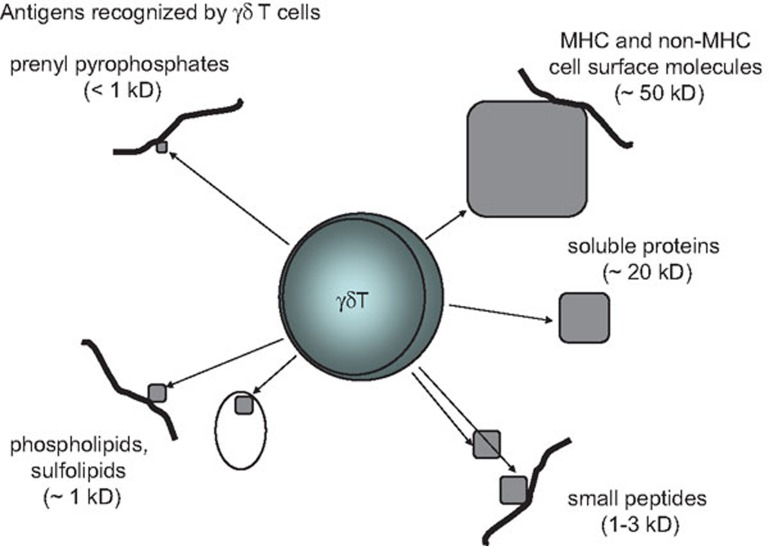
Determining which antigens (Ags) γδ T cells recognize with their T-cell antigen receptors (TCRs) arguably remains the biggest challenge in the field. Significant progress has been made during the last several years in collecting individual examples of Ag responses of γδ T cells and, in some cases, in delineating the mechanism of Ag recognition. The upshot of these studies is that γδ T cells recognize diverse Ags, quite different from the uniform, major histocompatibility complex (MHC)-packaged protein fragments recognized by αβ T cells, and more like the highly variable and diverse Ags recognized by B cells (Figure 1). This observation, along with a structural comparison of the γδ TCRs with immunoglobulins, encouraged the hypothesis that ligand recognition by γδ T cells is B cell-like.1 However, despite obvious similarities, the γδ TCRs are different from the B-cell receptors (BCRs) in structure and composition, and chances are that Ag recognition by γδ T cells is a unique feature of this lymphocyte type, even if it resembles in some aspects Ag recognition by other lymphocytes. In this review, we will examine the diversity of Ags recognized by γδ T cells, as well as cellular distributions of γδ specificities, and possible consequences for self-tolerance in comparison with αβ T cells and B cells.
Figure 1.
γδ T cells recognize a wide range of structurally different ligands. Unlike most αβ T cells, γδ T cells recognize ligands that vary much in size, composition and molecular structure, including MHC and non-MHC cell surface molecules, soluble proteins and smaller peptides, phospholipids, prenyl pyrophosphates and sulfatide. Some of these molecules appear to be recognized in complex with others (e.g. certain phospholipids in complex with CD1d or apo H), and most if not all are recognized while bound to or expressed on the cell surface. apo H, apolipoprotein H; MHC, major histocompatibility complex.
The γδ T-cell receptors
The γδ TCRs are encoded by two distinct sets of genes, γ and δ, which rearrange like the Ig genes to form templates for diverse TCR protein molecules.2 Although there are fewer Vγ and Vδ genes than IgV and TCRαβV genes, the recombinatorial possibilities for generating the γδ TCRs are almost infinite,3 largely due to the unique ability of the δ genes to rearrange D segments in tandem and to utilize all three reading frames. This creates multiple joints in the same single gene, each with the opportunity for N region additions and trimming. However, this unique mechanism focuses potential γδ TCR diversity on a relatively small region of the TCR surface, CDR3δ. The significance of this narrow focus is not yet clear as will be further discussed below. Despite the potential for diversity, some commonly occurring γδ TCRs are invariant,4 or nearly invariant, which has led to the concept of γδ TCRs as innate pattern recognition receptors. However, other γδ TCRs are diverse initially, and later selected during immune responses.5,6 At the level of lymphocyte populations, the γδ TCRs appear to be less evenly distributed than the other adaptive receptors. In mice, where this has been studied in detail, subsets of γδ T cells expressing different Vγs become segregated already during ontogeny due to their sequential developmental patterns in the thymus.7 This temporal segregation may also dictate their spacial partition, as cells expressing different Vγs colonize different peripheral tissues.2 γδ T cells expressing different Vγs have different effects on the immune responses.8 Recent studies revealed a correlation between TCR-Vγ expression and functional differentiation,9,10 thus emphasizing the biological significance of the γδ TCR segregation in the mouse. Nevertheless, specific evidence concerned with IL-17 producing γδ T cells suggests that, at least in this case, the correlation between γδ TCR expression and function is merely coincidental—waves of γδ T cells expressing certain TCRs coincide with a thymic environment that temporarily favors TH17 differentiation.11 Although there is still less experimental evidence, segregation of TCR-defined γδ T-cell subsets (both spacial and functional) has also been found in other species and might well be a universal feature of these cells. With regard to Ag specificity, one immediate consequence of this is a partial repertoire restriction, and the association of certain repertoires with certain functions. This might be necessary in maintaining self-tolerance, or to channel the responses of γδ T cells into patterns with predetermined outcomes. In any case, it suggests a degree of rigidity of the repertoire that seems to be absent in B cells. Along the same vein, there is no evidence of somatic mutation in the γδ TCRs. Therefore, any affinity maturation of γδ T cells has to be driven by CDR3 selection, although γδ TCR–ligand interactions described so far are low affinity by comparison with class-switched BCRs. On the other hand, ligand recognition by γδ T cells is not limited to MHC-presented peptides. Affinities of TCR–ligand interactions seem to vary considerably, and in some reported cases were of higher affinity than those of MHC-restricted αβ TCRs. In this regard and by comparison with αβ T cells at least, the γδ responses show more variation and plasticity.12
The density of cell surface-expressed γδ TCRs is generally similar to that of αβ TCRs, but there are differences between the two types of TCR in terms of their association with proteins of the CD3 complex. Of note, murine γδ TCR complexes lack CD3δ, and upon activation instead express FcεR1γ, in contrast to the αβ TCRs and to human γδ TCRs.13 At least some of the γδ TCRs appear to signal more efficiently than the αβ TCRs, which is described in detail elsewhere.13,14 This might lower the activation threshold for certain γδ T cells. Nevertheless, the γδ TCRs overall resemble the other cell surface-expressed adaptive lymphocyte receptors, the BCRs and the αβ TCRs, suggesting that they provide similar sensitivities to the lymphocytes that carry them.
Mechanism of ligand recognition
We already mentioned the hypothesis, proposed by Chien and collaborators,1 that ligand recognition by γδ T cells is B cell-like. This idea is based on structural and functional observations. Thus, CDR3 regions of the γδ TCRs resemble Ig CDRs in terms of length and variability.15 As is the case with Ig heavy and light chains, respectively, TCR-δ has extensive CDR3s whereas TCR-γ has short CDR3s. Moreover, TCR-δ CDR3s are far more diverse, similar to IgH CDR3s. In contrast, TCR-α and TCR-β CDR3s are intermediate in length, and similar to each other both in length and diversity, which may be a requirement for the docking on the surface of MHC molecules and the recognition of MHC-bound small peptides. However, with γδ TCRs, most of the potential for diversity is concentrated in CDR3δ, while BCRs are diverse in CDR1 and 2 as well, and have, in addition, the option of affinity maturation through somatic mutation, which implies large differences in ligand recognition. Not only will B cells recognize a wider array of ligands, but also will they interact with them through higher affinity.
Other observations support the idea of B cell-like ligand recognition by γδ T cells, but they also illustrate differences. Thus, there is a conspicuous absence of reports of MHC-restricted Ag recognition by γδ T cells,16 which is the main mode of ligand recognition by αβ T cells. Possible exceptions to this observation will be discussed below. However, in some cases it has been shown that Ags must be presented to be stimulatory for γδ T cells.17 These presented Ags tend to be small, and in soluble form might not be capable of TCR cross-linking, a prerequisite for activation via immunoreceptors. Whether any Ags in solution can be recognized and can trigger responses of γδ T cells is not yet known, although this seems likely when such Ags are multivalent.18 An example might be the response to an insulin peptide, which can be elicited from isolated single hybridoma cells (in the absence of antigen-presenting cells (APCs) or other hybridoma cells) expressing an insulin peptide-reactive γδ TCR.19 Whether responses to cell surface-expressed molecules such as CD1c, CD1d, MICA/B and T10/22 have a special significance in γδ TCR-mediated ligand recognition remains unclear. Unlike the αβ TCRs, which have an inherent bias for MHC recognition associated with certain dedicated amino acids,20,21 no such bias has been reported for the γδ TCRs. In fact, judging from the interaction of T10/22-reactive γδ TCRs with their ligand, where specificity is largely determined by a single D segment within TCR-δ,22 there is no reason to expect a similar bias for the γδ TCRs. Similarly, no inherent MHC bias seems to exist with the BCRs. However, it remains possible that γδ TCRs have inherent biases for the recognition of cell surface molecules other than MHC,23 and given the limitation of the repertoire outside of CDR3δ, this even seems likely.24 No such bias or restricting element has been firmly established, however. Perhaps the biggest difference to Ag recognition via BCRs is that so many conventional Ags seem to be incapable of eliciting responses by γδ T cells. To our knowledge, specific TCR-mediated responses of γδ T cells have not been elicited to Ags such as ovalbumin, hen egg lysozyme, cytochrome C and many others, all of which are recognized by antibodies. This is clearly not due to an inability of γδ T cells to recognize proteins—in fact, there may be more proteinaceous than non-proteinaceous ligands for the γδ TCRs. Nor is it due to an inability of γδ T cells to undergo clonal selection following immunization—there are well-documented examples of such selection among peripheral γδ T cells. It may have to do, however, with the fact that large portions of the γδ TCR are comparatively invariant, and the highly variable area is limited to CDR3δ, i.e. one segment of the γδ TCR combining site. It seems likely that this particular restriction of variability holds a clue that might eventually help to explain the Ag preferences of γδ T cells.24
Specific examples of ligands
The number of bona fide ligands for γδ TCRs is still relatively small. Nevertheless, our aim was not to provide a complete list but rather to highlight the differences and diversity of ligands recognized.
MHC-like ligands
Despite the fact that there may be no inherent MHC bias in the γδ TCRs—none has been reported as of this writing—MHC molecules were investigated as ligands for the γδ TCR even prior to the landmark studies by Matis and Bluestone.25,26 The pair of related T-locus Ags, T10/22, may be considered prototypic, because crystal structures of these Ags, as well as of a γδ TCR engaged with T22, have been available for some time now.27,28 These structures show that the T Ags do not present peptides, and that the γδ TCR (KN6) binds to T22 at an angle, mainly using CDR3δ amino-acid side chains for the interaction. This is much unlike the binding of αβ TCRs to MHC molecules, where CDR1 and 2 of both TCR-α and β, mainly interact with the MHC surface, and the CDR3s with the peptide in the groove. The repertoire of T10/22 recognizing γδ TCRs is diverse, including several Vγs and Vδs, with a shared motif in CDR3δ (W-(S)EGYEL).29 Although expressing the motif is sufficient for ligand recognition, these TCRs can have widely different affinities for T22, suggesting that non-motif amino-acid side chains are involved in the interaction as well. Approximately 0.4% of lymphoid γδ T cells in mice recognize T22. The biological significance of this specificity remains to be determined. However, because T10/22 appear to be induced by cell stress, it is possible that T10/22-specific γδ T cells play a role as monitors of tissue health.
Similarly to T10/22, the classical MHC molecules I-Ek,b,s have been identified early as potential ligands for γδ T cells. This has been confirmed later in binding studies, which again did not reveal any role for presented peptides.30 Post-translational modification of these classical MHC molecules appears to be critical for γδ T-cell recognition. However, binding affinities are low, the population of murine γδ T cells capable of recognizing these ligands remains to be investigated, and the biological role of I-E recognition by γδ T cells is still unclear.
Although a number of human γδ T-cell lines and clones were characterized early on as MHC-specific, it was not formally shown that their responses were TCR-mediated. This was rectified more recently in the case of a human γδ response to HLA B58 (Kaiser A, Fisch P, 5th International γδ T cell Conference, 31 May–12 June 2012, Freiburg, GER). Here, it was shown by TCR transfer into a mouse hybridoma cell line that the specific reactivity to the alloantigen HLA B58 is mediated by the γδ TCRs, and that this type of recognition supports cytolysis. Interestingly, because the human γδ TCR in question differentiates between HLA B*5802 and HLA B*5801, which differ only in three amino acids in the floor of the peptide binding groove, a bound peptide might play a role in this particular case.
Human γδ T cells (as well as αβ T cells) also recognize group 1 CD1 molecules (CD1a, b, c).31 These molecules are primarily expressed on professional Ag presenting cells where they present lipid Ags (glycolipids and certain microbial lipids) to the T cells. The Vδ1+ subset of human γδ T cells, which is mainly found in the tissues, shows prominent reactivity to CD1c, and produces IFN-γ and granulysin in the course of such responses. It has been suggested that the CD1c-reactive cells, dependent in part also on inflammatory cytokines and co-stimulation via NKG2D, provide protection against microbial infections prior to the more slowly developing responses of Ag-specific αβ T cells.31,32 Finally, it has been reported that group 1 CD1 molecules can present lipid A to human γδ T cells.33 The related group 2 CD1 molecule, CD1d, appears to be recognized by both human and murine γδ T cells.34,35,36,37 The same molecule has been studied in detail as a ligand for classical natural killer T cells,38 also both in mice and humans. With natural killer T cells, CD1d serves as presenter of certain lipids, and there is some evidence that γδ T cells in humans and mice also recognize a CD1d/lipid complex. Thus, cloned human γδ T cells responded to phosphatidyl ethanol amine (PE) in a manner dependent on CD1d, which suggested CD1d-restricted recognition of this phospholipid.34,35,39 The phospholipid cardiolipin (CL) binds to CD1d and murine γδ T cells responded both in vivo and in vitro to CL dependent on the presence of CD1d, suggesting that this phospholipid is also presented by CD1d, and is thus recognized by the γδ T cells.36 Finally, CD1d appears to present sulfatide, an abundant myelin glycosphingolipid, to human γδ T cells expressing Vδ1.40 Thus, the CD1d-restricted recognition by γδ T cells of small molecules might be the format closest to MHC-restricted Ag recognition by αβ T cells.
The stress-induced MHC class I-related molecules MICA and MICB were also found to be recognized by human γδ T cells derived from the intestinal epithelia. These cells expressed diverse Vδ1+ TCRs. Both α1 and α2 domains of MICA/B were involved in the recognition, but Ag processing was not required.41 Interestingly, the human γδ T cells also recognized MIC proteins derived from other primate species despite extensive amino-acid changes in the α1 and α2 domains, perhaps due to a single conserved site.42 Further examination revealed that MICA/B are broadly expressed on carcinomas of the lung, breast, kindney, ovary, prostate and colon where they are recognized by tumor-infiltrating Vδ1+ γδ T cells, which may affect tumor survival.43 However, MICA is also a ligand for NKG2D, which is expressed on natural killer cells, CD8+ αβ T cells and γδ T cells. All of these cells can be activated via NKG2D, which complicates analysis of the γδ responses.44 MICA engagement by NKG2D also enhances responses of Vγ9+Vδ2+ γδ T cells to non-peptide Ags.45 Nevertheless, a study with soluble MICA tetramers confirmed binding to Vδ1+ γδ TCRs and suggested that MIC delivers both TCR-dependent signal 1 and NKG2D-dependent signal 2 in the appropriate γδ T cells.46 A crystal structure of a MIC-reactive Vδ1+ γδ TCR is now available, revealing a surprisingly flat potential binding surface. Furthermore, it appears that MIC binding by the TCR and by NKG2D is mutually exclusive, perhaps forcing sequential recognition.47
Finally, a study presented by C. R. Willcox at the 5th International γδ T Cell Conference in Freiburg, Germany (31 May–2 June 2012) indicates that the endothelial protein C receptor has joined the ranks of MHC-like γδ TCR ligands too. Endothelial protein C receptor is a TCR ligand that is expressed on cytomegalovirus-infected cells and on tumor cells. It is recognized by a human Vγ4Vδ5+ clone sensitive to the conformation of the ligand. Recognition—via CDR3—also depends on endothelial protein C receptor expression levels and co-stimulation, both of which were found to be stress-regulated. This specificity was deemed Ig-like as well.
Other proteins expressed on the cell surface
A study of anti tumor responses by human γδ T cells revealed interactions of the Vγ9Vδ2 γδ TCR with an F1-ATPase-related structure expressed on the surface of the Burkitt's lymphoma cell line Daudi and certain other tumor lines but not another Burkitt's lymphoma, Raji.48 This observation showed that cell surface-expressed proteins other than MHC molecules can be recognized by γδ T cells. Moreover, response-inhibitory effects of antibodies directed against a serum protein, apolipoprotein A1, suggested involvement of this protein as well, and this was confirmed in molecular binding experiments using surface plasmon resonance, in which a soluble Vγ9Vδ2 TCR construct bound both purified F1-ATPase and a delipidated form of apolipoprotein A1. Finally, consistent with the idea that a trimolecular complex of these molecules provides the basis for γδ T-cell ligand recognition, apolipoprotein A1 was found to be required for optimal responses of Vγ9Vδ2 T cells by tumor target cell lines expressing F1-ATPase. Involvement of an apolipoprotein has been observed in another γδ response as well 49 and will be further discussed below.
Based on other studies, also with Daudi cells,50,51,52 it seems clear that F1-ATPase is not the only non-MHC-related protein on the cell surface that might be recognized by γδ T cells. In fact, screening experiments of murine cell lines and macrophages with soluble γδ TCR constructs have implied the presence of multiple additional ligands,53,54 although their precise molecular nature remains to be determined.
Soluble proteins
Probably the first defined Ag reported to stimulate specific responses of human γδ T cells was tetanus toxoid, a potent immunogen derived from the protein tetanospasmin of Clostridium tetani.55,56 Responses included IFN-γ production, induction of IL-2R expression and proliferation, and were limited to clones and T-cell somatic hybrids expressing certain γδ TCRs. Despite these auspicious beginnings, no further studies on the tetanus toxoid response of γδ T cells have been reported and the underlying mechanism remains uncertain.
Further bacterial proteins reported to elicit specific responses of human γδ T cells include the unrelated staphylococcal enterotoxin A (SEA) 57 and the toxin listeriolysin O (LLO).58 Several studies examined the responses of human γδ T cells to bacterial super-Ags, such as SEA, SEB, SEE and TSST.59 Interestingly, Vγ9+ clones killed SEA-pulsed targets but did not proliferate in response to such stimulation, whereas Vγ9-negative γδ clones proliferated. The cytotoxic reactivity of the γδ T cells was found to be more restricted such that a given clone might respond to SEA but not SEE and vice versa, in contrast to αβ T cells, which often respond to multiple SEs. Although limited to certain γδ TCRs, binding interactions between SEA or other super-Ags and the γδ TCR have not yet been demonstrated, and the analysis of the responses is complicated by observations indicating that some of the cytotoxic activity of the Vγ9+ clones is actually mediated by staphylococcal enterotoxin-specific antibodies that bind to Fc receptors of the γδ T cells.
Both rodent and primate γδ T cells respond to Listeria monocytogenes.58,60,61,62,63 In a study with human γδ T cells, live bacteria were required to elicit responses of γδ T cells in vitro. A γδ T cell line derived from such culture responded specifically to the large LLO-derived peptide LLO 470–508. Since this portion of LLO shows a high degree of homology with other Gram-positive bacterial toxins, it was deemed probable that such toxins might also be recognized.58 In this system, involvement of the γδ TCR remains to be demonstrated. The large size of the stimulatory peptide, which also contains a cysteine, raises the possibility that the LLO-reactive γδ T cells might recognize a conformational epitope, but this aspect has not been explored.
Early studies with mycobacterial extracts and purified protein derivative (PPD) revealed responses of human and murine γδ T cells to mycobacterial Ags.64,65,66 The murine response to PPD was found to be limited to cells expressing Vγ1,67 suggesting γδ TCR involvement in the response to a mycobacterial protein molecule. Nevertheless, subsequent studies with human γδ T cells focused on non-peptidic Ags contained within but not specific to mycobacteria (see below). More recently, defined mycobacterial proteins were also found to elicit γδ T-cell responses. One of these is ESAT-6, a small, highly immunogenic protein, which is part of a transmembrane secretion pathway in M. tuberculosis,68 and a critical virulence factor.69 A response to ESAT-6 was initially observed in γδ T cells of cattle experimentally infected with M. bovis.70 WC1+ bovine γδ T cells responded to this protein with proliferation and IFN-γ secretion and changes in CD45 isoforms.71,72 A γδ response to ESAT-6 was also observed in patients with active pulmonary tuberculosis.73 Others reported that ESAT-6 directly induces purified γδ T effector memory cells from tuberculin skin test-positive patients to produce IFN-γ, and that CD4+ αβ T cells regulate this response.74 However, this observation has been challenged and it requires further investigation.75,76 Since αβ T cells also respond to ESAT-6, and for possible application in a tuberculosis vaccine,77 it will be important to show that an αβ T cell-independent γδ response exists.
γδ T cells protect mice from herpes simplex virus (HSV) type 1-induced lethal encephalitis,78 and γδ T cells were isolated that recognize and respond to HSV glycoprotein 1.79 Further characterization of a clone expressing Vγ2Vδ8 revealed that this response does not require classical Ag-processing pathways and in fact can occur in the absence of any APCs,80 similar to the findings with small peptide Ags described below.19,81 The HSV glycoprotein 1-reactive γδ clone responded in a B cell-like manner to a conformational epitope, and it did not require any glycosylation of the Ag. The epitope was localized to the solvent-exposed amino terminus of the protein, and it was sensitive to sulfhydryl reduction.82 The same clone failed to respond to glycoprotein 1 derived from HSV-2, suggesting that amino acid differences near the N-terminus and specific to these orthologues are critical for recognition.79 These data provided convincing evidence that soluble non-MHC proteins can be recognized by γδ T cells and suggest that such reactivity might play an important role in host protection against viral infections.83
In a study of anti-tumor responses by human T cells, TCR-δ+ cytotoxic cells with specificity for an immunoglobulin idiotype of autologous B-cell tumors were detected.84 Further investigation showed that the γδ CTLs specifically recognized the λ light-chain protein.85 However, the anti-tumor response was not inhibited by λ-specific antibodies, and cells transfected with a λ-chain construct that could not be expressed on the cell surface were still lysed suggesting that the cytolytic γδ T cells recognized the λ-protein in a processed form. There was no indication of MHC-mediated Ag presentation, but because antibodies against a member of the HSP-70 family, which is expressed on the surface of the target cells, inhibited the anti tumor response, the authors suggested that λ might be recognized by the cytolytic γδ T cells in the form of an HSP-70-presented peptide Ag. Despite such intriguing findings, no further reports on this response have appeared. The postulated antigenic λ peptide has not been confirmed, and it remains unclear if the λ protein needs to retain some secondary structure to be recognized. Ig λ contains several cysteines, and in view of the studies with HSV gI,82 as well as our own recent observations with an insulin peptide (Kemal Aydintug M, unpublished, see below), it might be worthwhile to determine whether the redox state of the λ-chain influences its antigenicity.
Most recently, the target specificity of a human γδ TCR derived from a presumed autoreactive and pathogenic γδ T-cell clone has been determined. This amazing story goes back to a rare case of human autoimmune myositis where infiltration of skeletal muscle with γδ T cells instead of the more common autoreactive αβ T cells was observed.5 It was found that the muscle-infiltrating γδ T cells were clonally expanded and express an uncommon Vγ1.3Vδ2+ γδ TCR.86 Based on responses of transfectomas, as well as binding studies with soluble TCR constructs,87,88 it appears now that this γδ TCR (named M88) recognizes a common conformational motif on the surface of several proteins from different species, including bacterial and human aminoacyl-tRNA synthetases.89 For example, M88 recognizes histidyl-tRNA synthetase, which is also targeted by autoantibodies in patients with myositis. One of the soluble proteins recognized, the E. coli translation initition factor 1, has been mutagenized extensively to show that a short α-helical stretch including amino acids 39–42 of the E. coli translation initition factor 1 constitutes a critical part of the epitope for M88. Several aspects of this response seem noteworthy including the focus of a γδ TCR on a conformational epitope, which is reminiscent of the findings with HSV glycoprotein 1,82 the multispecificity of the γδ TCR, which recognizes an epitope shared between various unrelated proteins, and the convergence of γδ and antibody responses, which focus on the same Ags.
Smaller peptides
In addition to the γδ responses to intact native proteins, a number of responses to smaller peptide Ags have been reported as well. These have been listed elsewhere in greater detail.90 Briefly, using hybridomas with murine γδ T cells, responses to peptides as short as seven amino acids were observed.91 The first report of a response to a small, defined peptide Ag involved a peptide derived from a mycobacterial heat shock protein, HSP-65.81 The peptide (p180–196) was recognized by a large number of clones expressing murine Vγ1,92 and it was shown by TCR transfer that the hybridoma response was TCR-dependent.91 Unlike αβ T cells recognizing small peptides in an MHC-restricted fashion, γδ hybridomas responding to these peptides did not require APCs,92 in this regard resembling B cells.
Because the shortest HSP-derived stimulatory peptide (FGLQLEL) resembled a motif shared by unfolded proteins which bind to the molecular chaperone HSP-70 BiP,93 we also examined unrelated peptides having this motif for their ability to elicit γδ responses. Several were stimulatory, albeit not as strongly as the HSP peptide (data unpublished). Although we have previously suggested that this might reflect an ability of γδ T cells to recognize unfolded proteins, it might instead indicate that γδ T cells recognize, rather than singular amino-acid sequences, secondary structure of peptides such as, for example, α helices.
More recently, we have studied a different peptide response of murine γδ T cells involving a major insulin epitope in the non-obese diabetic mouse model of type 1 autoimmune diabetes. The Ins2 B:9-23 peptide is a naturally occurring Ag contained in certain β cells of the pancreatic islets,94 which is recognized by diabetogenic I-Ag7-restricted CD4+ αβ T cells, and by B lymphocytes.95 In a hybridoma collection generated to screen the TCR-Vβ repertoire of B:9-23 peptide-reactive αβ T cells, a peptide-reactive clone was found expressing a γδ TCR.19 This hybridoma (SP9D11) expressed Vγ4 paired with Vδ10. In addition to the peptide, the hybridoma responded to a preparation of pancreatic islet cells, but not to intact insulin. The peptide response was TCR-dependent and, like the γδ hybridomas recognizing HSP-65-derived peptides, SP9D11 did not require APCs, unlike all B:9-23-reactive αβ T cells. In fact, the γδ response did not require any accessory cells, because isolated individual SP9D11 hybridoma cells still responded to the peptide when tested in a single-cell assay.19 Unlike γδ cells recognizing HSP-derived peptides, the repertoire of B:9-23- reactive γδ T cells includes different Vγs in addition to several Vδs. Thus, different portions of the γδ TCR might be critical in recognizing the insulin peptide Ag. Whereas two C-terminal amino acids of the insulin peptide appear to be dispensable for the γδ response, the N-terminal one is required. Surprisingly, the γδ response also required the tyrosine in position 16, in this regard resembling I-Ag7-restricted αβ T cells. However, in contrast to the αβ T cells, γδ cells also require the cysteine in position 19,19 suggesting that the peptide might be stimulatory as a dimer. This was recently confirmed (Kemal Aydintug M, manuscript in preparation). It is also noteworthy that B:9-23 represents an α-helical segment of the insulin B chain. Some of this secondary structure might be retained by the peptide and could be important for γδ recognition.
Several other defined peptides have been either implied as Ag for γδ T cells or actually found to be stimulatory, including the already mentioned processed Ig λ light-chain protein,85 a peptide derived from tetanus toxin (C. tetani, 1235–1246)96 and a LLO-derived peptide (470–508).58 However, the molecular details of these responses remain to be determined.
Finally, some studies involving random heterocopolymers of two or more amino acids have been reported as well. Most prominently, a response of a γδ hybridoma to poly GT, a polymer of approximately 100 amino acids containing glutamic acid and tyrosine at a 1∶1 ratio, was published as early as 1989.97 We later showed that essentially all Vγ1+ γδ T cells respond to poly GT,18 as well as cells expressing other γδ TCRs, but we were unable to confirm the original claim that poly GT is recognized in the context of Qa-1b. With its random sequence, poly GT is not a defined Ag and does not have a defined structure. However, it seems probable that segments of individual pGT molecules assume secondary structures, some of which might meet stimulatory requirements. pGT is also anionic, and we have pointed out before that several polyanionic structures are capable of stimulating Vγ1 responses, perhaps due to particular properties of Vγ1 itself.16
Non-peptidic Ags
Original observations that large numbers of γδ T cells were responsive to mycobacteria and mycobacterial Ags included both human and murine cells.64,65,66,98 However, while many murine cells were found to react with mycobacterial PPD and HSP-65,66 human mycobacteria-reactive γδ T cells failed to react with PPD and HSP-65.99 Searching for alternative Ags, Pfeffer and colleagues100 found that most human mycobacteria-reactive γδ T cells responded to Ags contained in fractions of mycobacterial lysates with a molecular mass of <3 kDa. Moreover, these Ags were protease-resistant. In contrast, only few human γδ T cells responded to PPD and HSP-65. These surprising observations were rapidly followed by studies identifying several mycobacterial and synthetic non-peptidic Ags for human γδ T cells, first the mycobacteria-derived TUBag4, a 5′ triphosphorylated thymidine-containing compound with additional structurally related stimulatory molecules101 and then synthetic alkyl phosphates. In particular monoethyl phosphate,102 and subsequently mycobacteria-derived isopentenyl pyrophosphate and related prenyl pyrophosphate derivatives were found to be stimulatory.103 Since these early reports, many additional Ags of essentially the same type, now often somewhat imprecisely referred to as ‘phosphoantigens', have been described. Although some are only weak stimulators, others were found that are extremely potent, most prominently perhaps HMBPP,104 a metabolite in the 2-C-methyl-𝒹-erythritol-4 pathway for isoprenoid synthesis.
Despite their small size, recognition of the prenyl pyrophosphates by the Vγ9Vδ2 γδ TCRs depends on all CDRs.105 This may be more understandable considering that these small molecules appear to be presented on the surface of target cells17,106,107 and thus might be ‘seen' in a certain context. However, the mechanism of presentation remains mostly unclear, and it might vary between individual Ags.
Finally, there are small molecular compounds that stimulate prenyl pyrophosphate-reactive γδ T cells indirectly, by blocking farnesyl pyrophosphate synthase in the mevalonate pathway, which increases cellular IPP levels.108,109 These include bisphosphonates110 and alkylamines.111
It should be noted that the response to prenyl pyrophosphates is limited to a single subset of γδ T cells present in primates, both human and non-human. This response already received and clearly deserves much attention, because of its potential therapeutic significance and because so many human peripheral blood γδ T cells show this specificity, but there is no indication that it is representative of γδ specificities in general.
Phospholipids
In the 1990s, our lab used a collection of hybridomas generated with γδ T cells from normal untreated mice to screen for ligands recognized by the γδ TCR. Because we had already found responses to other anionic molecules (oligonucleotides and peptides), we also tested anionic phospholipids. We promptly found TCR-dependent responses to CL and the related phospholipids phosphatidylglycerol and phosphatidic acid, but not to other phospholipids (phosphatidylinositol, phosphatidylserine, phosphatidylcholine and phosphorylcholine). Only cells expressing Vγ1 responded, and the response was dependent on the presence of a serum factor, which we tentatively identified as apolipoprotein H (apo H, β2-glycoprotein 1). These findings suggested a connection with numerous observations made with so-called antiphospholipid antibodies, which develop in response to some infections but also in patients suffering from autoimmune diseases. Some such antibodies react with apo H, or complexes of apo H and CL.112 Because the γδ cells required CL and serum factors in stoichiometric ratios in order to respond optimally, we suggested that they might recognize CL and apo H in a complex as do antiphospholipid antibodies.49 This remains to be confirmed, however. Although we observed anti CL responses only with hybridomas, recently another group reported that normal splenic and hepatic γδ T cells from healthy mice proliferated in vitro in response to CL. Such cells were also activated in vivo following transfer of CL-pulsed dendritic cells and here, they noted a requirement for CD1d expression by the DC. They finally demonstrated that CL binds to CD1d and provided a crystal structure of this complex.36 They suggested that γδ T cells can recognize this complex but further analysis will be required to confirm this proposed mechanism.
Human γδ T cells were reported to recognize PE, apparently also in the context of (human) CD1d.35 The investigators found that cloned peripheral blood or nasal mucosa-derived γδ T cells from cypress pollen-sensitive subjects responded to pollen-derived PE after incubation in vitro. The response was specific for PE and limited to molecules with partially saturated fatty acid side chains. In a subsequent study, the same group found that human γδ T cells derived from the duodenal mucosa also contained a high percentage of CD1d-restricted PE-reactive γδ T cells.39 Taken together, the studies of three independent groups involving mice and humans make a good case that phospholipids also belong into the category of ligands recognized by γδ T cells.
Finally, a just published study already mentioned above indicates that the myelin-derived glycosphingolipid sulfatide is also recognized, by human Vδ1+ γδ T cells.40 This new observation hints at the possibility of other not yet identified categories of non-peptidic Ags for γδ T cells.
Distribution of γδ T-cell specificities
Despite an enormous potential for receptor diversity in CDR3δ,3 clear evidence of peripheral selection of γδ T cells 6 and the occasional emergence of γδ T-cell clones,5 most specificities identified so far are not clone-specific. It is not clear whether this is mainly a bias of the investigators—polyclonal responses are more easily spotted—or an intrinsic property of γδ T-cell recognition. The response to poly GT in mice, for example, is shared by Vγ1+ cells, Vγ2+ cells and others. Similarly, the response to PPD is common to most Vγ1+ cells. It could be argued that these examples are inadequate because both stimuli are composed of a heterogeneous mix of molecules, but the response to a fully defined peptide (HSP-65 p180–196) was also shared by most Vγ1+ cells. Similarly, many Vγ1+ cells respond to CL, and all of these responses are demonstrably TCR-dependent. In contrast to these responses, the insulin peptide B:9-23 is recognized by some γδ T cells expressing Vγ4, but also by some expressing Vγ1 and by others expressing at least one additional Vγ. B:9-23 reactive γδ T cells seem to be frequent in the non-obese diabetic mouse strain but not in C57BL/6 mice, but this needs to be confirmed. The much studied response to T10/22 is shared by many different cells expressing the TCR-δ CDR3 motif W(S)EGYEL, which is largely based on one reading frame of Dδ2, in murine γδ T cells expressing several different Vγs and Vδs. In humans, the responses to prenyl pyrophosphates are shared by many Vγ9Vδ2+ cells, with only some bias for particular γ-chain CDR3 amino acids,105 and many Vδ1+ cells respond to MICA/B. However, the recognition by a polymyositis associated γδ TCR of a peptide motif on amino-acyl tRNA synthetases is remarkable because the TCR is derived from clonally expanded γδ T cells, and both CDR3s seem to play a role. Here, the γδ TCR seems to provide specificity for a conformational motif shared by a number of related and some unrelated proteins. Albeit still sketchy, these data taken together suggest that ligand recognition by γδ T cells can occur in different modes, e.g. involving none, one or two CDR3s, and specificities might be distributed over larger and smaller subsets of γδ T cells, depending on the Ag. A logical consequence of this is that many individual γδ T cells are multispecific. Indeed, this is what we find. For example, a single murine γδ TCR expressed by a hybridoma clone might support responses to poly GT, PPD, HSP-60 p180–196 and CL. Over the years, we observed several cells with such broad reactivity pattern. More interestingly, perhaps, we recently found that a cell that recognizes T22, because it expresses the appropriate CDR3δ motif also responded to pGT and even to the insulin peptide B:9-23 (Kemal Aydintug M, unpubl. observ.). However, it seems that some γδ T cells are more likely to be multispecific than others. In mice, Vγ1+ γδ T cells seem to have a greater propensity for multispecificity than Vγ4+ or Vγ6+ cells. In this regard, Vγ1+ cells in mice are reminiscent of the polyspecific B1 B cells. In humans, multispecific γδ T cell clones have been observed as well, mostly with Vγ9Vδ2+ cells. Here, mycobacteria-reactive cells also might respond to tetanus toxoid, to non-mycobacteria-derived phosphoantigens, or to phospholipids in the context of CD1d.
Consequences of multi-specificity
With all multispecific lymphocytes, the question of self-tolerance looms heavily. Selection becomes more complex if there are several specificities of auto-Ags within one responder. Negative selection of high affinity clones also removes their low affinity specificities as well as possible specificities for non-self Ags. A requirement for intermediate to low affinities for several Ags might diminish a large cell population to a small one that fits all the selection criteria. On the other hand, surviving multispecific cells might be more useful than monospecific ones, simply because they can be employed in several different settings. Moreover, as has been argued before, specificities shared by many γδ T cells would make for faster responses by eliminating the need for prior clonal expansion.
Conclusions
A review of the Ags recognized by γδ T cells reveals a remarkable diversity (Figure 1). This likely precludes a single mechanism of ligand recognition akin to that of MHC-restricted αβ T cells, but does not preclude Ig-like Ag recognition. Some subsets of γδ T cells resemble B1 B cells in their broad ligand specificity, while others more resemble innate αβ T cells. How γδ T cells are kept in check to prevent auto-aggressive reactivity remains unclear.
References
- Chien YH, Jores R, Crowley MP. Recognition by γ/δ T cells. Annu Rev Immunol. 1996;14:511–532. doi: 10.1146/annurev.immunol.14.1.511. [DOI] [PubMed] [Google Scholar]
- Haas W, Pereira P, Tonegawa S. γ/δ T cells. Annu Rev Immunol. 1993;11:637–685. doi: 10.1146/annurev.iy.11.040193.003225. [DOI] [PubMed] [Google Scholar]
- Davis MM, Bjorkman PJ. T cell antigen receptor genes and T cell recognition. Nature. 1988;334:395–402. doi: 10.1038/334395a0. [DOI] [PubMed] [Google Scholar]
- Asarnow DM, Kuziel WA, Bonyhadi M, Tigelaar RE, Tucker PW, Allison JP. Limited diversity of γδ antigen receptor genes of Thy-1+ dendritic epidermal cells. Cell. 1988;55:837–847. doi: 10.1016/0092-8674(88)90139-0. [DOI] [PubMed] [Google Scholar]
- Hohlfeld R, Engel AG, Ii K, Harper MC. Polymyositis mediated by T lymphocytes that express that γ/δ receptor. N Engl J Med. 1991;324:877–881. doi: 10.1056/NEJM199103283241303. [DOI] [PubMed] [Google Scholar]
- Roark CL, French JD, Taylor MA, Bendele AM, Born WK, O'Brien RL. Exacerbation of collagen-induced arthritis by oligoclonal, iL-17-producing γδ T cells. J Immunol. 2007;179:5576–5583. doi: 10.4049/jimmunol.179.8.5576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havran W, Allison JP. Developmentally ordered appearance of thymocytes expressing different T cell antigen receptors. Nature. 1988;335:443–445. doi: 10.1038/335443a0. [DOI] [PubMed] [Google Scholar]
- O'Brien RL, Roark CL, Jin N, Aydintug MK, French JD, Chain JL, et al. γδ T cell receptors: functional correlations. Immunol Rev. 2007;215:77–88. doi: 10.1111/j.1600-065X.2006.00477.x. [DOI] [PubMed] [Google Scholar]
- Jin N, Roark CL, Miyahara N, Taube C, Aydintug MK, Wands JM, et al. Allergic airway hyperresponsiveness-enhancing γδ T cells develop in normal untreated mice and fail to produce IL-4/13, unlike Th2 and NKT cells. J Immunol. 2009;182:2002–2010. doi: 10.4049/jimmunol.0803280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayan K, Sylvia KE, Malhotra N, Yin CC, Martens G, Vallerskog T, et al. Intrathymic programming of effector fates in three molecularly distinct γδ T cell subtypes. Nature Immunol. 2012;13:511–518. doi: 10.1038/ni.2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas JD, Ravens S, Duber S, Sandrock I, Oberdorfer L, Kashani E, et al. Development of interleukin-17-producing γδ T cells is restricted to a functional embryonic wave. Immunity. 2012;37:48–59. doi: 10.1016/j.immuni.2012.06.003. [DOI] [PubMed] [Google Scholar]
- Bonneville M, O'Brien RL, Born WK. γδ T cell effector functions: a blend of innate programming and acquired plasticity. Nat Rev Immunol. 2010;10:467–478. doi: 10.1038/nri2781. [DOI] [PubMed] [Google Scholar]
- Hayes SM, Love PE. Distinct structure and signalling potential of the γδ TCR complex. Immunity. 2002;16:1–20. doi: 10.1016/s1074-7613(02)00320-5. [DOI] [PubMed] [Google Scholar]
- Hayes SM, Li L, Love PE. TCR signal strength influences αβ/γδ lineage fate. Immunity. 2005;22:583–593. doi: 10.1016/j.immuni.2005.03.014. [DOI] [PubMed] [Google Scholar]
- Rock EP, Sibbald PR, Davis MM, Chien YH. CDR3 length in antigen-specific immune receptors. J Exp Med. 1994;179:323–328. doi: 10.1084/jem.179.1.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Born W, Cady C, Jones-Carson J, Mukasa A, Lahn M, O'Brien R. Immunoregulatory functions of γδ T cells. Adv Immunol. 1999;71:77–144. [PubMed] [Google Scholar]
- Morita CT, Beckman EM, Bukowski JF, Tanaka Y, Band H, Bloom BR, et al. Direct presentation of nonpeptide prenyl pyrophosphate antigens to human γδ T cells. Immunity. 1995;3:495–507. doi: 10.1016/1074-7613(95)90178-7. [DOI] [PubMed] [Google Scholar]
- Cady CT, Lahn M, Vollmer M, Tsuji M, Seo SJ, Reardon CL, et al. Response of murine γδ T cells to the synthetic polypeptide poly-Glu50Tyr50. . J Immunol. 2000;165:1790–1798. doi: 10.4049/jimmunol.165.4.1790. [DOI] [PubMed] [Google Scholar]
- Zhang L, Jin N, Nakayama M, O'Brien RL, Eisenbarth GS, Born WK. γδ T cell receptors confer autonomous responsiveness to the insulin-peptide B:9-23. J Autoimmun. 2010;34:478–484. doi: 10.1016/j.jaut.2009.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackman M, Yague J, Kubo R, Gay D, Coleclough C, Palmer E, et al. The T cell recpertoire may be biased in favor of MHC recognition. Cell. 1986;47:349–357. doi: 10.1016/0092-8674(86)90591-x. [DOI] [PubMed] [Google Scholar]
- Garcia KC, Gapin L, Adams JJ, Birnbaum ME, Scott-Browne JP, Kappler JW, et al. A closer look at TCR germline recongition. Immunity. 2012;36:887–888. doi: 10.1016/j.immuni.2012.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams EJ, Strop P, Shin S, Chien YH, Garcia KC. An autonomous CDR3δ is sufficient for recognition of the nonclassical MHC class I molecules T10 and T22 by γδ T cells. Nat Immunol. 2008;9:777–784. doi: 10.1038/ni.1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyden LM, Lewis JM, Barbee SD, Bas A, Girardi M, Hayday AC, et al. Skint1, the prototype of a newly identified immunoglobulin superfamily gene cluster, positively selects epidermal γδ T cells. Nat Genet. 2008;40:656–662. doi: 10.1038/ng.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Born WK, O'Brien RL. Antigen-restricted γδ T-cell receptors. Arch Immunol Ther Exp (Warsz) 2009;57:129–135. doi: 10.1007/s00005-009-0017-x. [DOI] [PubMed] [Google Scholar]
- Matis LA, Cron R, Bluestone JA. Major histocompatibility complex-linked specificity of γδ receptor-bearing T lymphocytes. Nature. 1987;330:262–264. doi: 10.1038/330262a0. [DOI] [PubMed] [Google Scholar]
- Matis LA, Fry AM, Cron RQ, Cotterman MM, Dick RF, Bluestone JA. Structure and specificity of a class II alloreactive γδ T cell receptor heterodimer. Science. 1989;245:746–749. doi: 10.1126/science.2528206. [DOI] [PubMed] [Google Scholar]
- Wingren C, Crowley MP, Degano M, Chien YH, Wilson IA. Crystal structure of a γδ T cell receptor ligand T22: a truncated MHC-like fold. Science. 2000;287:310–314. doi: 10.1126/science.287.5451.310. [DOI] [PubMed] [Google Scholar]
- Adams EJ, Chien YH, Garcia KC. Structure of a γδ T cell receptor in complex with the nonclassical MHC T22. Science. 2005;308:227–231. doi: 10.1126/science.1106885. [DOI] [PubMed] [Google Scholar]
- Shin S, El-Diwany R, Schaffert S, Adams EJ, Garcia KC, Pereira P, et al. Antigen recognition determinants of γδ T cell receptors. Science. 2005;308:252–255. doi: 10.1126/science.1106480. [DOI] [PubMed] [Google Scholar]
- Hampl J, Schild H, Litzenberger C, Baron M, Crowley MP, Chien YH. The specificity of a weak γδ TCR interaction can be modulated by the glycosylation of the ligand. J Immunol. 1999;163:288–294. [PubMed] [Google Scholar]
- Sugita M, Brenner MB. T lymphocyte recognition of human group 1 CD1 molecules: implications for innate and acquired immunity. Semin Immunol. 2000;12:511–516. doi: 10.1006/smim.2000.0277. [DOI] [PubMed] [Google Scholar]
- Das H, Sugita M, Brenner MB. Mechanisms of Vδ1 γδ T cell activation by microbial components. J Immunol. 2004;172:6578–6586. doi: 10.4049/jimmunol.172.11.6578. [DOI] [PubMed] [Google Scholar]
- Cui Y, Kang L, Cui L, He W. Human γδ T cell recognition of lipid A is predominately presented by CD1b or CD1c on dendritic cells. Biol Direct. 2009;4:47–58. doi: 10.1186/1745-6150-4-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agea E, Russano A, Bistoni O, Mannucci R, Nicoletti I, Corazzi L, et al. Human CD1-restricted T cell recognition of lipids from pollens. J Exp Med. 2005;202:295–308. doi: 10.1084/jem.20050773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russano AM, Agea E, Corazzi L, Postle AD, de Libero G, Porcelli SA, et al. Recognition of pollen-derived phosphatidyl-ethanolamine by human CD1d-restricted gamma delta T cells. J Allergy Clin Immunol. 2006;117:1178–1184. doi: 10.1016/j.jaci.2006.01.001. [DOI] [PubMed] [Google Scholar]
- Dieude M, Striegl H, Tyznik AJ, Wang J, Behar SM, Piccirillo CA, et al. Cardiolipin binds to CD1d and stimulates CD1d-restricted γδ T cells in the normal murine repertoire. J Immunol. 2011;186:4771–4781. doi: 10.4049/jimmunol.1000921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber S, Sartini D, Exley M. Role of CD1d in Coxsackievirus B3-induced myocarditis. J Immunol. 2003;170:3147–3153. doi: 10.4049/jimmunol.170.6.3147. [DOI] [PubMed] [Google Scholar]
- Gapin L. iNKT cell autoreactivity: what is ‘self' and how is it recognized. Nat Rev Immunol. 2010;10:272–277. doi: 10.1038/nri2743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russano AM, Bassotti G, Agea E, Bistoni O, Mazzocchi A, Morelli A, et al. CD1-restricted recognition of exogenous and self-lipid antigens by duodenal γδ+ T lymphocytes. J Immunol. 2007;178:3620–3626. doi: 10.4049/jimmunol.178.6.3620. [DOI] [PubMed] [Google Scholar]
- Bai L, Picard D, Anderson B, Chaudhary V, Luoma A, Jabri B, et al. The majority of CD1d-sulfatide-specific T cells in human blood use a semiinvariant Vδ1 TCR. Eur J Immunol. 2012;42:2505–2510. doi: 10.1002/eji.201242531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groh V, Steinle A, Bauer S, Spies T. Recognition of stress-induced MHC molecules by intestinal epithelial γδ T cells. Science. 1998;279:1737–1740. doi: 10.1126/science.279.5357.1737. [DOI] [PubMed] [Google Scholar]
- Steinle A, Groh V, Spiess T. Diversification, expression, and γδ T cell recognition of evolutionarily distant members of the MIC family of major histocompatibility complex class I-related molecules. Proc Natl Acad Sci U S A. 1998;95:12510–12515. doi: 10.1073/pnas.95.21.12510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groh V, Rhinehart R, Secrist H, Bauer S, Grabstein KH, Spies T. Broad tumor-associated expression and recognition by tumor-derived gamma delta T cells of MICA and MICB. Proc Natl Acad Sci U S A. 1999;96:6879–6884. doi: 10.1073/pnas.96.12.6879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer S, Groh V, Wu J, Steinle A, Phillips JH, Lanier LL, et al. Activation of NK cells and T cells by NKG2D, a receptor for stress-inducible MICA. Science. 1999;285:727–729. doi: 10.1126/science.285.5428.727. [DOI] [PubMed] [Google Scholar]
- Das H, Groh V, Kuiji C, Sugita M, Morita CT, Spies T, et al. MICA engagement by human Vγ2Vδ2 T cells enhances their antigen-dependent effector function. Immunity. 2001;15:83–93. doi: 10.1016/s1074-7613(01)00168-6. [DOI] [PubMed] [Google Scholar]
- Wu J, Groh V, Spies T. T cell antigen receptor engagement and specificty in the recognition of stress-inducible MHC class I-related chains by human epithelial γδ T cells. J Immunol. 2002;169:1236–1240. doi: 10.4049/jimmunol.169.3.1236. [DOI] [PubMed] [Google Scholar]
- Xu B, Pizarro JC, Holmes MA, McBeth C, Groh V, Spies T, et al. Crystal structure of a γδ T-cell receptor specific for the human MHC class I homolog MICA. Proc Natl Acad Sci U S A. 2011;108:2414–2419. doi: 10.1073/pnas.1015433108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scotet E, Martinez LO, Grant E, Barbaras R, Jeno P, Saulquin X, et al. Tumor recognition following Vγ9Vδ2 T cell receptor interactions with a surface F1-ATPase-related structure and apolipoprotein A-1. Immunity. 2005;22:71–80. doi: 10.1016/j.immuni.2004.11.012. [DOI] [PubMed] [Google Scholar]
- Born WK, Vollmer M, Reardon C, Matsuura E, Voelker DR, Giclas PC, et al. Hybridomas expressing γδ T-cell receptors respond to cardiolipin and β2-glycoprotein 1 (apolipoprotein H) Scand J Immunol. 2003;58:374–381. doi: 10.1046/j.1365-3083.2003.01315.x. [DOI] [PubMed] [Google Scholar]
- Fisch P, Malkovsky M, Braakman E, Sturm E, Bolhuis RLH, Prieve A, et al. γ/δ T-cell clones and natural killer-cell clones mediate distinct patterns of non-major histocompatibility complex-restricted cytolysis. J Exp Med. 1990;171:1567–1579. doi: 10.1084/jem.171.5.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisch P, Malkovsky M, Kovats S, Sturm E, Braakman E, Klein BS, et al. Recognition by human Vγ9/Vδ2 T cells of a GroEL homolog on Daudi Burkitt's lymphoma cells. Science. 1990;250:1269–1273. doi: 10.1126/science.1978758. [DOI] [PubMed] [Google Scholar]
- Fisch P, Oettel K, Fudim N, Surfus JE, Malkovsky M, Sondel PM. MHC-unrestricted cytotoxic and proliferative responses of two distinct human γ/δ T cell subsets to Daudi cells. J Immunol. 1992;148:2315–2323. [PubMed] [Google Scholar]
- Aydintug MK, Roark CL, Yin X, Wands JM, Born WK, O'Brien RL. Detection of cell surface ligands for the γδ TCR using soluble TCRs. J Immunol. 2004;172:4167–4175. doi: 10.4049/jimmunol.172.7.4167. [DOI] [PubMed] [Google Scholar]
- Aydintug MK, Roark CL, Chain JL, Born WK, O'Brien RL. Macrophages express multiple ligands for γδ TCRs. Mol Immunol. 2008;45:3253–3263. doi: 10.1016/j.molimm.2008.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozbor D, Trinchieri G, Monos DS, Isobe M, Russo G, Haney JA, et al. Human TCR-γ+/δ+, CD8+ T lymphocytes recognize tetanus toxoid in an MHC-restricted fashion. J Exp Med. 1989;169:1847–1851. doi: 10.1084/jem.169.5.1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozbor D, Cassatella MA, Lessin S, Kagan J, Finver S, Faust J, et al. Expression and function of γδ- and αβ-T cell receptor heterodimers on human somatic T cell hybrids. J Immunol. 1990;144:3677–3683. [PubMed] [Google Scholar]
- Rust CJJ, Verreck F, Vietor H, Koning F. Specific recognition of staphylococcal enterotoxin A by human T cells bearing receptors with the Vγ9 region. Nature. 1990;346:572–574. doi: 10.1038/346572a0. [DOI] [PubMed] [Google Scholar]
- Guo Y, Ziegler HK, Safley SA, Niesel DW, Vaidya S, Klimpel GR. Human T-cell recognition of Listeria monocytogenes: recognition of listeriolysin O by TcRαβ+ and TcRγδ+ T cells. Infect Immunol. 1995;63:2288–2294. doi: 10.1128/iai.63.6.2288-2294.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rust CJJ, Koning F. γδ T cell reactivity towards bacterial superantigens. Semin Immunol. 1993;5:41–46. doi: 10.1006/smim.1993.1006. [DOI] [PubMed] [Google Scholar]
- Skeen MJ, Ziegler HK. Intercellular interactions and cytokine responsiveness of peritoneal α/β and γ/δ T cells from Listeria-infected mice: synergistic effects of interleukin 1 and 7 on γ/δ T cells. J Exp Med. 1993;178:985–996. doi: 10.1084/jem.178.3.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu YX, Roark CE, Kelly K, Drevets D, Campbell P, O'Brien R, et al. Immune protection and control of inflammatory tissue necrosis by γδ T cells. J Immunol. 1994;153:3101–3115. [PubMed] [Google Scholar]
- O'Brien RL, Xiang Y, Huber S, Ikuta K, Born WK. Depletion of a γδ T cell subset can increase host resistance to a bacterial infection. J Immunol. 2000;165:6472–6479. doi: 10.4049/jimmunol.165.11.6472. [DOI] [PubMed] [Google Scholar]
- Ryan-Payseur B, Frencher J, Shen L, Chen CY, Huang D, Chen ZW. Multieffector-functional immune responses of HMBPP-specific Vγ2Vδ2 T cells in non-human primates inoculated with Listeria monocytogenes DeltaactA prfA. J Immunol. 2012;189:1285–1293. doi: 10.4049/jimmunol.1200641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holoshitz J, Koning F, Coligan JE, de Bruyn J, Strober S. Isolation of CD4−CD8− mycobacteria-reactive T lymphocyte clones from rheumatoid arthritis synovial fluid. Nature. 1989;339:226–229. doi: 10.1038/339226a0. [DOI] [PubMed] [Google Scholar]
- Janis EM, Kaufmann SHE, Schwartz RH, Pardoll DM. Activation of γδ T cells in the primary immune response to Mycobacterium tuberculosis. . Science. 1989;244:713–716. doi: 10.1126/science.2524098. [DOI] [PubMed] [Google Scholar]
- O'Brien RL, Happ MP, Dallas A, Palmer E, Kubo R, Born WK. Stimulation of a major subset of lymphocytes expressing T cell receptor γδ by an antigen derived from Mycobacterium tuberculosis. . Cell. 1989;57:667–674. doi: 10.1016/0092-8674(89)90135-9. [DOI] [PubMed] [Google Scholar]
- Happ MP, Kubo RT, Palmer E, Born WK, O'Brien RL. Limited receptor repertoire in a mycobacteria-reactive subset of γδ T lymphocytes. Nature. 1989;342:696–698. doi: 10.1038/342696a0. [DOI] [PubMed] [Google Scholar]
- Bitter W, Houben ENG, Bottai D, Brodin P, Brown EJ, Cox JS, et al. Systematic genetic nomenclature for type VII secretion systems. PLOS Pathog. 2009;5:1–6, e1000507. doi: 10.1371/journal.ppat.1000507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottai D, Mailessi L, Simeone R, Friqui W, Laurent C, Lenormand P, et al. ESAT-6 secretion-independent impact of ESX-1 genes espF and espG1 on virulence of Mycobacterium tuberculosis. . J Infect Dis. 2011;203:1155–1164. doi: 10.1093/infdis/jiq089. [DOI] [PubMed] [Google Scholar]
- Rhodes SG, Buddle BM, Hewinson RG, Vordermaier HM. Bovine tuberculosis: immune responses in the peripheral blood and at the site of active disease. Immunology. 2000;99:195–202. doi: 10.1046/j.1365-2567.2000.00944.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh MD, Kennedy HE, Smyth AJ, Girvin RM, Andersen P, Pollock JM. Responses of bovine WC1+γδ T cells to protein and nonprotein antigens of Mycobacterium bovis. . Infect Immun. 2002;70:6114–6120. doi: 10.1128/IAI.70.11.6114-6120.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maue AC, Waters WR, Davis WC, Palmer MV, Minion FC, Estes DM. Analysis of immune responses directed toward a recombinant early secretory antigenic target six-kilodalton protein-culture filtrate protein 10 fusion protein in Mycobacterium bovis infected cattle. Infect Immun. 2005;73:6659–6667. doi: 10.1128/IAI.73.10.6659-6667.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gioia C, Agrati C, Goletti D, Vincenti D, Carrara S, Amicosante M, et al. Different cytokine production and effector/memory dynamics of αβ+ or γδ+ T-cell subsets in the peripheral blood of patients with active pulmonary tuberculosis. Int J Immunopathol Pharmacol. 2003;16:247–252. doi: 10.1177/039463200301600310. [DOI] [PubMed] [Google Scholar]
- Li L, Wu CY. CD4+CD25+ Treg cells inhibit human memory γδ T cells to produce IFN-γ in response to M. tuberculosis antigen ESAT-6. Blood. 2008;111:5629–5636. doi: 10.1182/blood-2008-02-139899. [DOI] [PubMed] [Google Scholar]
- Casetti R, Martino A, Sacchi A, Agrati C, Goletti D, Martini F. Do human γδ T cells respond to M.tuberculosis protein antigens. Blood. 2008;112:4776. doi: 10.1182/blood-2008-07-167619. [DOI] [PubMed] [Google Scholar]
- Li L, Wu CY.Human memory but not naive γδ T cells from TST-positive individuals respond to M. tuberculosis antigen. Blood 2008112477719029458 [Google Scholar]
- Cendron D, Ingoure S, Martino A, Casetti R, Horand F, Romagne F, et al. A tuberculosis vaccine based on phosphoantigens and fusion proteins induces distinct γδ and αβ T cell responses in primates. Eur J Immunol. 2007;37:549–565. doi: 10.1002/eji.200636343. [DOI] [PubMed] [Google Scholar]
- Sciammas R, Kodukula P, Tang Q, Hendricks RL, Bluestone JA. T cell receptor-γ/δ cells protect mice from herpes simplex virus type 1-induced lethal encephalitis. J Exp Med. 1997;185:1969–1975. doi: 10.1084/jem.185.11.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson RM, Lancki DW, Sperling AI, Dick RF, Spear PG, Fitch FW, et al. A murine CD4−, CD8− T cell receptor-γδ T lymphocyte clone specific for Herpes simplex virus glycoprotein I. J Immunol. 1992;148:983–988. [PubMed] [Google Scholar]
- Sciammas R, Johnson RM, Sperling AI, Brady W, Linsley PS, Spear PG, et al. Unique antigen recognition by a herpsevirus-specific TCR-γδ cell. J Immunol. 1994;152:5392–5397. [PubMed] [Google Scholar]
- Born W, Hall L, Dallas A, Boymel J, Shinnick T, Young D, et al. Recognition of a peptide antigen by heat shock reactive γδ T lymphocytes. Science. 1990;249:67–69. doi: 10.1126/science.1695022. [DOI] [PubMed] [Google Scholar]
- Sciammas R, Bluestone JA. HSV-1 glycoprotein I-reactive TCRγδ cells directly recognize the peptide backbone in a conformationally dependent manner. J Immunol. 1998;161:5187–5192. [PubMed] [Google Scholar]
- Sciammas R, Bluestone JA. TCRγδ cells and viruses. Microbes Infect. 1999;1:203–212. doi: 10.1016/s1286-4579(99)80035-5. [DOI] [PubMed] [Google Scholar]
- Wright A, Lee JE, Link MP, Smith SD, Carroll W, Levy R, et al. Cytotoxic T lymphocytes specific for self tumor immunoglobulin express T cell receptor δ chain. J Exp Med. 1989;169:1557–1564. doi: 10.1084/jem.169.5.1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HT, Nelson EL, Clayberger C, Sanjanwala M, Sklar J, Krensky AM. γδ T cell recognition of tumor Ig peptide. J Immunol. 1995;154:1614–1623. [PubMed] [Google Scholar]
- Pluschke G, Rüegg D, Hohlfeld R, Engel AG. Autoaggressive myocytotoxic T lymphocytes expressing an unusual γ/δ T cell receptor. J Exp Med. 1992;176:1785–1789. doi: 10.1084/jem.176.6.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiendl H, Malotka J, Holzwarth B, Weltzien HU, Wekerle H, Hohlfeld R, et al. An autoreactive γδ TCR derived from a polymyositis lesion. J Immunol. 2002;169:515–521. doi: 10.4049/jimmunol.169.1.515. [DOI] [PubMed] [Google Scholar]
- Dornmair K, Schneider CK, Malotka J, Dechant G, Wiendl H, Hohlfeld R. Antigen recongition properties of a Vγ1.3Vδ2-T-cell receptor from a rare variant of polymyositis. J Neuroimmunol. 2004;152:168–175. doi: 10.1016/j.jneuroim.2004.03.016. [DOI] [PubMed] [Google Scholar]
- Bruder J, Siewert K, Obermeier B, Malotka J, Scheinert P, Kellermann J, et al. Target specificity of an autoreactive pathogenic human γδ-T cell receptor in myositis. J Biol Chem. 2012;287:20986–20995. doi: 10.1074/jbc.M112.356709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Born WK, Zhang L, Nakayama M, Jin N, Chain JL, Huan Y, et al. Peptide antigens for γδ T cells. Cell Mol Life Sci. 2011;68:2335–23343. doi: 10.1007/s00018-011-0697-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu YX, Kersh G, Vollmer M, Kalataradi H, Heyborne K, Reardon C, et al. Structural requirements for peptides that stimulate a subset of γδ T cells. J Immunol. 1994;152:1578–1588. [PubMed] [Google Scholar]
- O'Brien RL, Fu YX, Cranfill R, Dallas A, Reardon C, Lang J, et al. Heat shock protein Hsp-60 reactive γδ cells: a large, diversified T lymphocyte subset with highly focused specificity. Proc Natl Acad Sci U S A. 1992;89:4348–4352. doi: 10.1073/pnas.89.10.4348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blond-Elguindi S, Cwirla SE, Dower WJ, Lipshutz RJ, Sprang SR, Sambrook JF, et al. Affinity panning of a library of peptides displayed on bacteriophages reveals the binding specificity of BiP. Cell. 1993;75:717–728. doi: 10.1016/0092-8674(93)90492-9. [DOI] [PubMed] [Google Scholar]
- Mohan JF, Levisetti MG, Calderon B, Herzog JW, Petzold SJ, Unanue ER. Unique autoreactive T cells recognize insulin peptides generated within the islets of Langerhans in autoimmune diabetes. Nat Immunol. 2010;4:350–354. doi: 10.1038/ni.1850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Nakayama M, Eisenbarth GS. Insulin as an autoantigen in NOD/human diabetes. Curr Opin Immunol. 2008;20:111–118. doi: 10.1016/j.coi.2007.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holoshitz J, Vila LM, Keroack BJ, McKinley DR, Bayne NK. Dual antigenic recognition by cloned human γδ T cells. J Clin Invest. 1992;89:308–314. doi: 10.1172/JCI115577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidovic D, Roglic M, McKune K, Guerder S, MacKay C, Dembic Z. Qa-1 restricted recognition of foreign antigen by a γδ T-cell hybridoma. Nature. 1989;340:646–650. doi: 10.1038/340646a0. [DOI] [PubMed] [Google Scholar]
- Modlin RL, Pirmez C, Hofman FM, Torigian V, Uyemura K, Rea TH, et al. Lymphocytes bearing antigen-specific γδ T-cell receptors in human infectious disease lesions. Nature. 1989;339:544–548. doi: 10.1038/339544a0. [DOI] [PubMed] [Google Scholar]
- Kabelitz D, Bender A, Schondelmaier S, Schoel B, Kaufmann SHE. A large fraction of human peripheral blood γδ+ T cells is activated by Mycobacterium tuberculosis but not by its 65 kD heat shock protein. J Exp Med. 1990;171:667–679. doi: 10.1084/jem.171.3.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeffer K, Schoel B, Gulle H, Kaufmann SHE, Wagner H. Primary responses to human T cells to mycobacteria: a frequent set of γ/δ T cells are stimulated by protease-resistant ligands. Eur J Immunol. 1990;20:1175–1179. doi: 10.1002/eji.1830200534. [DOI] [PubMed] [Google Scholar]
- Constant P, Davodeau F, Peyrat MA, Poquet Y, Puzo G, Bonneville M, et al. Stimulation of human γδ T cells by nonpeptidic mycobacterial ligands. Science. 1994;264:267–270. doi: 10.1126/science.8146660. [DOI] [PubMed] [Google Scholar]
- Tanaka Y, Sano S, Nieves E, de Libero G, Rosa D, Modlin RL, et al. Nonpeptide ligands for human γδ T cells. Proc Natl Acad Sci U S A. 1994;91:8175–8179. doi: 10.1073/pnas.91.17.8175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka Y, Morita CT, Tanaka Y, Nieves E, Brenner MB, Bloom BR. Natural and synthetic non-peptide antigens recognized by human γδ T cells. Nature. 1995;375:155–158. doi: 10.1038/375155a0. [DOI] [PubMed] [Google Scholar]
- Hintz M, Reichenberg A, Altincicek B, Bahr U, Gschwind RM, Kollas AK, et al. Identification of (E)-4-hydroxy-3-methyl-but-2-enyl pyrophosphate as a major activator for human γδ T cells in Escherichia coli. . FEBS Lett. 2001;509:317–322. doi: 10.1016/s0014-5793(01)03191-x. [DOI] [PubMed] [Google Scholar]
- Wang H, Fang Z, Morita C. Vγ2Vδ2 T cell receptor recognition of prenyl pyrophosphates is dependent on all CDRs. J Immunol. 2010;184:6209–6222. doi: 10.4049/jimmunol.1000231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarikonda G, Wang H, Puan KJ, Liu XH, Lee HK, Song Y, et al. Photoaffinity antigens for human γδ T cells. J Immunol. 2008;181:7738–7750. doi: 10.4049/jimmunol.181.11.7738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei H, Huang D, Lai X, Chen M, Zhong W, Wang R, et al. Definition of APC presentation of phosphoantigen (E)-4-hydroxy-3-methyl-but-2-enyl pyrophosphate to Vγ2Vδ2 TCR. J Immunol. 2008;181:4798–4806. doi: 10.4049/jimmunol.181.7.4798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gober HJ, Kistowska M, Angman L, Jeno P, Mori L, de Libero G. Human T cell receptor γδ cells recognize endogenous mevalonate metabolites in tumor cells. J Exp Med. 2003;197:163–168. doi: 10.1084/jem.20021500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson K, Rojas-Navea J, Rogers MJ. Alkylamines cause Vγ9Vδ2 T cell activation and proliferation by inhibiting the mevalonate pathway. Blood. 2006;107:651–654. doi: 10.1182/blood-2005-03-1025. [DOI] [PubMed] [Google Scholar]
- Kunzmann V, Bauer E, Wilhelm M. γ/δ T-cell stimulation by pamidronate. New Engl J Med. 1999;340:737–738. doi: 10.1056/NEJM199903043400914. [DOI] [PubMed] [Google Scholar]
- Bukowski JF, Morita CT, Brenner MB. Human γδ T cells recognize alkylamines derived from microbes, edible plants, tea: implications for innate immunity. Immunity. 1999;11:57–65. doi: 10.1016/s1074-7613(00)80081-3. [DOI] [PubMed] [Google Scholar]
- Matsuura E, Lopez LR, Shoenfeld Y, Ames PRJ.β2 glycoprotein I and oxidative inflammation in early atherogenesis: a progression from innate to adaptive immunity Autoimmun Reve-pub ahead of print 2012 Apr 27; doi: 10.1016/j.autrev.2012.04.003. [DOI] [PubMed]