Abstract
It is important for the anesthesiologist to understand the etiology of free radical damage and how free-radical scavengers attenuate this, so that this knowledge can be applied to diverse neuro-pathological conditions. This review will concentrate on the role of reactive species of oxygen in the pathophysiology of organ dysfunction, specifically sub arachnoid hemorrhage (SAH), traumatic brain injury (TBI) as well as global central nervous system (CNS) hypoxic, ischemic and reperfusion states. We enumerate potential therapeutic modalities that are been currently investigated and of interest for future trials. Antioxidants are perhaps the next frontier of translational research, especially in neuro-anesthesiology.
Keywords: Antioxidants, cerebroprotection, neuro-anesthesiology, neurocritical care
Introduction
Oxygen acts like a double-edged sword; it can be lifesaving or destructing. Oxidation reactions involve transfer of an electron from a substance to an oxidizing agent producing highly reactive and unstable free radicals.[1] Produced in response to a variety of stimuli, these reactive oxygen species (ROS), can cause oxidative damage to the cell structure and disrupt function through lipid peroxidation of cell membranes, degradation of nucleic acids and attraction/activation of neutrophils.[2] Thus they play a pivotal role in the mechanism of cell injury, cell death, and organ dysfunction. The role of ROS as a contributor to the overwhelming inflammatory response has been emphasized in adult respiratory distress syndrome (ARDS), traumatic and hypoxic central nervous system (CNS) states, and ischemic neurological deficits after sub arachnoid hemorrhage (SAH).
Free-radical scavengers, the protective systems operating in the mammalian cells, either prevent these ROS from being formed, or remove them before they can damage vital components of cells. Some antioxidants present in the body are catalases, glutathione peroxidase, superoxide dismutase (SOD); cofactors, like selenium, zinc, copper, iron and manganese; non-enzymatic antioxidants such as α-tocopherol, ascorbic acid, β-carotene; heme-binding proteins like ceruloplasmin, transferrin, albumin and haptoglobin.[3]
“Oxidative stress” (OS) defines an imbalance between production of oxidizing chemical species and their effective removal by protective antioxidants and scavenger enzymes.[4] Evidence of massive OS is established in adult critical illness characterized by tissue ischemia — reperfusion (I/R) injury and by an intense systemic inflammatory response such as during sepsis and ARDS. About 4000 antioxidants, including Vitamin E and C, carotenoids, phenolic/polyphenolic compounds (present as non-nutrient food substances) have been identified.
This review explores the role of ROS in various neurological conditions including global CNS hypoxic, ischemia — reperfusion (I/R) states, SAH, as well as traumatic brain injury (TBI). We provide a review of literature concerning endogenous and exogenous antioxidants being currently investigated at molecular level, animal studies, and human trials. This may be the time for elaborate translational studies unraveling the untapped potential of antioxidants in neurocritical care.
Materials and Methods
A thorough Pubmed and Medline literature search was conducted for studies investigating the use of antioxidants in neurocritical care. We utilized the following key words: Antioxidants, neurocritical care, ‘name of the antioxidant’ plus at least one of the following additional terms: SAH, ischemia — reperfusion injury, TBI, clinical trials, and clinical outcome. Complete manuscripts were studied and only those that reported on original studies with human subjects or preclinical studies and were published between 1990 and 2013 were included.
The Physiology of Oxidative Stress
Reactive oxygen species are constantly produced in small amounts as the result of cellular metabolism of oxygen. Tissue damage due to trauma, ischemia or infection leads to increased production of ROS and reactive nitrogen species (RNS). This complicated by an increased consumption, subsequent reduced stores and decreased activity of antioxidants, leads to OS and cell death. It is the major cause of increased morbidity and mortality among the critically ill.[5] At the cellular level, increased production of ROS and RNS during critical illness is related to Ca++ overload and excitotoxicity; activation of nitric oxide synthase (NOS) and production of NO; activation of phagocytes; release of iron, copper, and metalloproteins; conversion of xanthine dehydrogenase to xanthine oxidase; activation of eicosanoid pathway and inflammatory response ultimately culminating in mitochondrial failure.[6]
High levels of ROS and intracellular Ca++ overload leads to structural alteration of mitochondria and electron transport chain, resulting in further increase in ROS generation. ROS oxidize fatty acids in the cell membrane by lipid peroxidation causing direct cell damage or attack the DNA through self-perpetuating chain reactions and toxic byproducts.[7,8] They form cytotoxic aldehydes and alkoxy radicals which diffuse through the cell membrane destroying adjacent cells.[8]
ROS damages the DNA by attacking either the deoxyribose molecule, purine or pyrimidine bases, leading to sugar fragmentation, base loss, and strand breaks. They may alter DNA polymerases activity and decrease its fidelity.[9]
At the vascular endothelial level, NO· is normally produced by enzyme NOS from arginine and molecular oxygen. Nitric oxide (NO) produces vasodilation by relaxing smooth muscles in vessel.[10] During OS, an ‘inducible’ form of NOS is induced by cytokines and endotoxins. The higher level of NO· produced leads to hypotension, increase in intestinal epithelial permeability, and shuts down cellular respiration by inactivating mitochondrial cytochromes and effect cellular proliferation by inhibiting ribonucleotide reductase.[10] NO· rapidly reacts with ·O2 to form cytotoxic peroxynitrite.[11] This ends with depression of mitochondrial enzymes, change in the conformation of proteins, oxidative damage to lipids and DNA damage.[12]
The phagocytic cells of immune system require ROS to destroy bacteria and other ingested material.[13] ROS are formed as a result of “respiratory burst” following cell activation in the presence of a highly efficient enzyme ‘nicotinamide adenine dinucleotide oxidase’. Activation of large number of phagocytic cells results in OS.[7] The release of ·O2 activates chemotaxis, and cause tissue injury via oxidative and hydrolytic enzymes.[14] Peroxidases, in the phagocytic cells generate hypochlorous acid[13] causing cell lysis in vitro.[15]
Several metal ions including iron and copper are promoters of free radical reactions in vitro and are responsible for ROS production, but the presence of transferrin, lactoferrin, ceruloplasmin, and albumin, in healthy persons causes sequestration of these transition metal ions avoiding excessive ROS formation.[7,8] Toxins cause ruptured cells to release their contents into blood. The released free iron induces tissue injury and cell death by a sequence of reaction known as Haber-Weiss reaction [Fe++ + H2O2→ Fe+++ + OH· + OH·] in which superoxide and H2O2 are converted to highly reactive and toxic hydroxyl radicals.[8,16] I/R injury induces OS during circulatory shock, resulting in lysis of endothelial cells, which leads to microvascular thrombosis and loss of organ function.[7,17]
Oxidative Stress in Various Neurological Conditions
Brain is one of the most sensitive organs vulnerable to OS mediated injury following increased production of ROS because of its high oxidative metabolic activity, low antioxidant capacity, high content of polyunsaturated fatty acids and regional iron and high surface to membrane ratio.[6] Therefore, the discussion of the role of OS in conditions encountered in neurocritical care settings and a review of the clinical studies providing a rationale to support a potential therapeutic role of antioxidants in patients with ischemic and hemorrhagic strokes, SAH, TBI is important.
Ischemic stroke
Lower plasma concentrations of α- and β-carotene were found in patients with ischemic stroke as compared to healthy controls.[18] Significantly increased serum levels of NO, malondialdehyde (MDA) and glutathione along with worsening of neurological scale score has been observed in stroke patients[19] suggesting that changes in NO metabolism may be a marker of brain injury following ischemic stroke.
ROS are produced during ischemia and subsequent reperfusion leads to neuronal injury due to oxidative modification of DNA, protein and lipids. This suggests that OS enhances the deleterious effect on stroke severity and clinical outcome. Ischemia and vasospasm induced Delayed Ischemic Neurological Injury (DIND), if followed by reperfusion activates several deleterious pathways including cytochrome-C release, caspase induction and expression of matrix metalloproteinase (MMP) leading to blood brain barrier (BBB) injury and associated reduction of DNA-repair enzymes.[20]
Intracerebral hemorrhage
Iron induced neurotoxicity and delayed cerebral edema following intracerebral hemorrhage (ICH) has been implicated in release of hemoglobin containing red blood cells into brain parenchyma. This occurs through the release of iron containing heme yielding ferric ion by heme-oxygenase and subsequent OH· radical formation through Haber-Weiss reaction.[8,16] There is increased brain edema and up-regulation of heme-oxygenase after stereotactic infusion of ferric chloride, hemoglobin or hemin into basal ganglia.[21] Iron induced neurotoxicity and OS mediated injury was confirmed by Goldstein et al.,[22] who found hemin, the ferric ion oxidation product of heme toxic in human neuron like cells when compared to protoporphyrin IX exposure. The role of OS, investigated in a small study of 13 patients with spontaneous ICH and 15 patients with traumatic ICH compared to 40 healthy controls, observed that ICH patients had significantly lower plasma levels of vitamin C. The severity of neurological impairment inversely correlated with vitamin C levels as assessed by Glasgow Coma Scale, the National Institute of Health Stroke Scale (NIHSS) score and diameter of hematoma.[23]
Clinical studies in ICH have identified genome associations between Apo-lipoprotein ε (APO ε) variants ε2, ε4 and ICH. The APO ε4 allele amplifies inflammatory responses, increasing cerebral edema and hence worsening outcome after ICH.[24] COG1410, an APO ε analogue when studied in rat models showed decreased concentration of inflammatory proteins, reduced functional deficit and decreased cerebral edema.[25]
Subarachnoid hemorrhage
In one study, where rats were fed with a high salt diet for 3 months in addition to a lysyl oxidase inhibitor, ROS have been related to aneurysm formation. This led to up-regulation of ROS producing genes, suppression of ROS-eliminating genes and production of many highly oxidative species such as heme oxygenase, NOS, MMP and a 47 KDa portion of NADPH oxidase as examined using reverse transcriptase polymerase chain reaction (PCR) analysis, western blot, and immunohistochemistry.[26] They also observed decreased aneurysm size and production of oxidizing agents within the aneurysm in rats pretreated with Edavarone, a free-radical scavenger.[26] Further p47 NADPH oxidase knocked out mouse made Edavarone induced effects, indicating that it may be one of the key oxidizing agents required in aneurysm signaling cascade. Another rodent study, on the models of SAH demonstrated increase in superoxide anion and NADPH. However, pretreatment in these models with α lipoic acid alleviated OS and improved neurological outcome.[27]
Traumatic brain injury
Cat models with induced TBI revealed significantly more superoxide anion production after TBI compared to controls.[28] Persistent cerebral arteriolar dilation and reduced responsiveness to hypocapnic vasoconstriction after TBI have been attributed to OS (supported by reduced arteriolar dilation in animal models pretreated with SOD and catalase).[29] In rat models of TBI, administration of progesterone before the insult reduced isoprostane levels and improved neurological recovery.[30] The phase II, randomized double-blind placebo controlled study in 100 adult trauma patients showed moderate to good outcome in moderate TBI survivors with progesterone.[31] Progesterone for Traumatic Brain Injury: Experimental Clinical Treatment (ProTECT III), a phase III clinical study is currently enrolling patients to determine whether or not progesterone will be the first experimentally vetted treatment for TBI.
Apo E is another possible target for ameliorating neurological injury after TBI. Apo E reduces ROS and other inflammatory markers after different insults.[32] When Apo E or its analogues are given to rats before TBI; improved neurological outcome and reduced size of contusion was observed, compared to controls.[33] Conversely, as in ICH patients, the Apo E ε4 allele predisposes to worse outcomes after neurological injury, including TBI.[34] Thus, the use of Apo E or its analogue in the treatment of TBI is still controversial.
Use of Antioxidants in Neurocritical Care
Antioxidants are either exogenous or endogenous substances that inhibit oxidation. For antioxidants to achieve high efficacy, like other neuro protectants, they must penetrate the BBB and be given as early as possible preempting a massive OS. The natural antioxidants are classified in Table 1.
Table 1.
Naturally occurring antioxidants
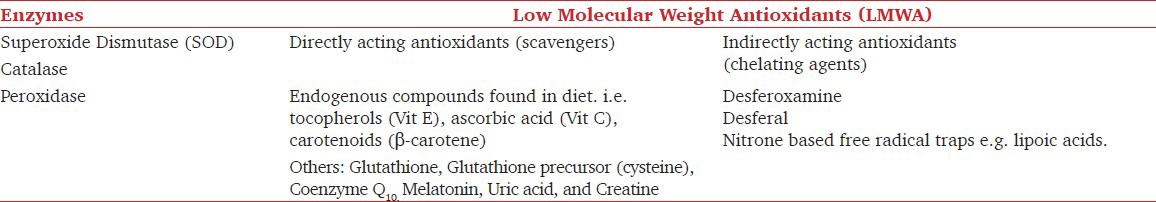
The potential antioxidant strategies would be restoring the naturally occurring endogenous substances, supplementation with exogenous antioxidants and administration of a few synthetic free radical scavengers. The utility of antioxidants in neuroanesthesiology for preventive and therapeutic purposes is reviewed below.
Vitamins and micronutrients
Preexisting low levels of antioxidants in brain, complicated by reduction during critical illness supports the rationale of supplementing antioxidant vitamins and nutrients during precarious illness.[23] Vitamin E and C, β-carotene and selenium act as free-radical scavengers contributing to prevention and helping in therapy of head injury and stroke. Supplementation of these antioxidant vitamins and nutrients either alone or in combination is referred to as “immune nutrition”.
Vitamin E, acts by breaking the propagation of free-radical chain reaction in the lipids of biological membranes. Deficiency of Vitamin E leads to peripheral neuropathy and ataxia[35] and influences the activity of antioxidant enzymes SOD, catalase and glutathione peroxidase.[36] β-carotene, a best known lipid soluble carotenoid due to its importance as a vitamin A (retinol) precursor possesses antioxidant properties analogous to that of vitamin E and C (ascorbic acid). It is a water soluble antioxidant with the brain concentration of 10 fold higher than its plasma levels.[37] High concentrations in the brain indicate its role as a cerebroprotective agent (inhibiting peroxidation of membrane phospholipids and free-radical scavenging action). It also possesses an important role in the regeneration of vitamin E.[38] Vitamin B12 use as a micronutrient in critical illness has been encouraged due to its glutathione sparing antioxidant properties along with NOS inhibition and decrease in nuclear factor-κB (NF-κB)activation.[39]
Selenium improves antioxidant capacity by increasing activity of glutathione peroxidase, and reducing plasma isoprostane and thiobarbituric acid reacting substances.[40] It also improves phagocytosis and immunoglobin synthesis, thus reducing infectious complications.
The results of the randomized, placebo-controlled trials[41,42] for the use of “immune nutrition” were encouraging but were not clearly recognized because of the inadequate sample size, uncertainties regarding the correct dose, appropriate timing, and route of administration (enteral vs parenteral). A meta-analysis of 11 studies of early immune nutrition in 886 well-defined intensive care patients found significant reduction in mortality and concluded that since none of the trials using immune nutrition reported any deleterious effect, supplementation with antioxidant trace elements and vitamins in intensive care patients is safe and possibly beneficial.[43] They asserted on very early start of immune nutrition at high dose parenterally to have a strong impact on outcomes.
The conflicting results on the efficacy of the use of vitamins to prevent stroke is explained by the fact that vitamin C and E may be oxidized to form ascorbyl radical and α-tocopherol radical which may act as toxic pro-oxidants in some ischemic circumstances.[44,45] Thus, vitamins may reduce stroke complications only if they are given at a specific dose and within a specific time window. Moreover, vitamins are beneficial only when the severity of stroke is mild with a low OS level in the ischemic zone.
Coenzyme Q10
Coenzyme Q10 (ubiquinone), is a lipid soluble, essential cofactor of the electron-transport chain, primarily present in mitochondria generating energy in form of ATP.[46] Recently, the use of coenzyme Q10 in treating OS in cardiovascular diseases, diabetes, and cancer has been reviewed.[47] The effect of coenzyme Q10 in mongolian gerbils with unilateral carotid ligation induced stroke exhibited a 45% survival improvement at four weeks.[48] Rabbit model of SAH induced symptomatic vasospasm showed that coenzyme Q10 prevented the development of ischemic brain lesions.[49] In TBI model of rats, coenzyme Q10 administration was protective because of significantly lesser increase of MDA levels. Neuronal degenerative findings and secondary brain damage caused by OS are decreased by its use in rats with TBI.[50]
α-Lipoic acid
α-Lipoic acid is found in every cell of our body and converts glucose into energy. α-Lipoic acid is both fat and water soluble, hence can easily cross the BBB.[51] Intracellularly, it is reduced to dihydrolipoate which is exported to extracellular medium. Both α-Lipoate and dihydrolipoate are potent antioxidants, and help in reducing lipid peroxidation, scavenging hydroxyl radicals, singlet oxygen and nitric oxide. In addition, α-lipoic acid chelates transition metals, recycles other antioxidants (such as vitamin C and E), raises intracellular glutathione levels and modulates transcription factor activity, especially that of NF-κB.[52]
In rat models, with MCA occlusion reduction in infarct size was seen with dihydrolipoate but not with α-lipoic acid[53] whereas a protective effect of α-lipoate against I/R injury in mongolian gerbil models were observed.[54] α-Lipoate was effective only when given subcutaneously but not intraperitoneally or into the cistern magna.[55] Similarly, few studies have found lipoic acid to be protective against cell death when administered 30 minutes prior to ischemia. However, reduction in infarct volume was not seen if lipoic acid was administered immediately prior to reperfusion.[56]
Melatonin
Melatonin is known as a biological modulator that contributes to the regulation of circadian rhythms, induces sleep and has a strong antioxidant action. It appears to influence aging and contribute to protection from age- related processes and disease state.[57]
A study on stroke model in rats with MCA occlusion found that animals treated with three daily doses of 5 mg/kg melatonin, intraperitoneally, started one hour after the onset of ischemia, had significantly inhibited induced NOS activity, resulting in decreased total NOS activity and tissue nitrite levels.[58] Oral administration of melatonin one hour before MCA occlusion in rats significantly enhanced glial cell survival.[59] Injection of melatonin (4 mg/kg) in pinealectomized rats before both ischemia and reperfusion reduced infarct volume by 40% and significantly improved neurological deficit scores.[60] Currently, melatonin appears to be a potent neuro-protectant due to its versatile antioxidative, anti-nitrosative, and immunomodulatory actions.[61]
Human Superoxide Dismutase (SOD)/Superoxide Dismutase like Molecules
SOD catalyzes dismutation of superoxide into oxygen and hydrogen peroxide, thus, representing the first line of defense against oxygen toxicity. Three forms of SOD are present in humans: SOD1 (located in cytoplasm), SOD2 (mitochondria) and SOD3 (extracellular). SOD1 and SOD3 contain copper, zinc, whereas SOD2 has manganese in its reactive centre. Traces of copper, zinc, and manganese metals are essential for maintaining the antioxidant activity.[62]
Ye N et al., in 100 male Sprague Dawley rats found protective effect of intraperitoneal injection of TAT-SOD (a protein transduced across the cell membrane to scavenge superoxide) against focal I/R injury. TAT-SOD effectively enhanced cerebral antioxidant ability, decreased MDA content, reduced lipid peroxidation and inhibited nerve cell apoptosis in an effective treatment window extending from two hours before and two hours after I/R. However, it had no influence if treatment was instituted four hours after I/R.[63] A multicentre randomized controlled clinical study in patients with severe head injury, given polyethylene glycol conjugated SOD therapy (pegorgotein) failed to improve outcome. The relatively long therapeutic time window of eight hours in the administration of the drug may explain the failure of SOD to exhibit protective effect in this clinical trial.[64]
Glutathione
Glutathione (GSH) is a ubiquitous tripeptide synthesized by two ATP-dependant enzymatic reactions.[65] It is generated by metabolism of N-acetyl cysteine. The sulfhydryl group of cysteine serves as a proton donor and is responsible for the major intracellular antioxidant activity. It plays a critical role in detoxification of peroxides and electrophilic toxins as a substrate for GSH peroxidase and GSH transferase.[66]
GSH depletion enhances the cerebral ischemic injury in rats.[67] Rapid restoration of thiol homeostasis in the brain during reperfusion may help the brain to recover from I/R injury.[68] GSH analogue YM737 given immediately after ischemia in cerebral ischemic rats reduced lethality, increased brain water levels and decreased MDA levels suggesting its anti-ischemic effects are due, in part, to inhibition of lipid peroxidative responses.[69] Glutathione monoethyl ester, in rat model of stroke increased cellular GSH and was particularly effective in increasing the mitochondrial pool. This increase in GSH levels following the glutathione monoethyl ester administration into the third ventricle during two hours of MCA occlusion and 48 hours of reperfusion decreased the infarct size by 46%.[70] More recently reduction of ischemic brain damage and increase of GSH by a liposomal preparation of quercetin in permanent focal ischemia was found in rats suggesting that endogenous brain GSH is critical in defense mechanisms after ischemia.[71] Though, GSH undoubtedly is a powerful antioxidant, the short therapeutic time windows for intervention with GSH system makes reproduction of these results in clinical trials more difficult.
Metal Ion Chelators
Free-metal ions are associated with the pathophysiology of various neurodegenerative diseases (e.g. copper in Wilson's disease and iron in Parkinson's disease). Their role in the mediation of OS following hemorrhage is well-known, therefore, proteins that are involved in the binding of metal ions were suggested as antioxidants.[8,16] These may include transferrin (binds iron), ceruloplasmin (binds copper) and hemopexin (binds heme, a catalyst in oxidative reactions).
Deferoxamine, decreases the availability of free iron by forming a stable complex with ferric ions. It attenuates the production of ROS, reduces brain MDA concentration and induces recovery of sodium-potassium pump activity.[72] However, the beneficial effects of deferoxamine may be partly related to its iron chelating abilities, because deferoxamine alters iron regulatory genes and protein binding activity; prevents apoptosis induced by glutathione depletion and OS by activating a signal transduction pathway; it exerts anti-inflammatory effects by stimulating cyclooxygenase; blocks glutamate mediated exicito-toxicity and exerts antiphagocytic effects.[73] Deferoxamine alleviates reperfusion induced injury following ischemia,[74] exert diverse protective effect after ICH,[75] and some evidence exists supporting the potential therapeutic role following intraventricular[76] and subarachnoid hemorrhage in experimental models.[77]
Owing to the potential therapeutic effects seen in the animal studies, clinical investigations of deferoxamine in stroke have gained attention in the recent years. A phase I, small open label safety and dose finding study of deferoxamine, enrolling 20 subjects divided into 5 dose tiers of deferoxamine started within 16 hours of ICH symptom onset was recently completed.[78] Analysis of clinical and radiological data from this study showed encouraging results; deferoxamine seemed to slow down the rate of peri-hematoma edema progression as compared to historic controls. Half (50%) of the deferoxamine treated patients had a modified Rankin Scale (mRS) score 0 to 2 at three months. Phase-II trial identified maximum tolerated dose of deferoxamine mesylate (62 mg/kg/day up to max daily dose of 6000 mg/day) given by a continuous I.V. infusion for 5 consecutive days beginning within 24 hours of ICH symptom onset is expected to complete in 2017. Similarly, phase II double blind, placebo, dose finding study to evaluate the safety and pharmacokinetics of deferoxamine in patients treated with I.V. recombinant tissue Plasminogen Activator (rt-PA) (The Thrombolysis and Deferoxamine in Middle Cerebral Artery Occlusion (TANDEM I) is complete and results awaited. Bipyridyl, a liposoluble iron chelating agent shows beneficial effects both in vitro by preventing death of cerebral endothelial cells and in vivo by diminishing BBB disruption after focal cerebral ischemia.[79]
Nitrones
Nitrones (X-CH = NO-Y) react with oxygen-free radicals and form nitroxyl-free radicals (more stable than oxygen-free radicals).[80] These spin trap scavenging agents have been studied in experimental animals and have shown to protect them from pathology associated with I/R injury, physical trauma and aging.[81] Phenyl-α-tert-butyl nitrone (PBN) is a synthetic antioxidant capable of scavenging oxygen and carbon based free radicals.[82] Various animal studies found that PBN administration before or within one hour after I/R, reduced infarct size, enhanced post ischemic reperfusion and significantly improved survival.[83,84] However, no benefit was observed if administered more than six hours after ischemic event.[85]
NXY-059 (2,4-disufophenyl-N-tert-butylnitrone) is a PBN, developed for treatment of ischemic stroke. Stroke-acute Ischemic NXY Treatment I (SAINT I) was a large clinical trial conducted on 1771 patients suffering from acute ischemic stroke (AIS). NXY-059 or placebo was administered intravenously to patients within 6 hours after the onset of stroke for 72 hours. Though NXY-059 significantly reduced disability after 90 days, it was not able to significantly change other neurological parameters and mortality.[86] SAINT II was a larger trial recruiting 3306 patients with AIS to further investigate the effect of NXY-059, but, this trial too did not find any efficacy for any of the end points.[87] Unfortunately, inappropriate treatment window of 6 hours, inclusion of disparate patients and issues related to the design of the SAINT II trial are a matter of concern.[88]
Ebselen
Ebselen (2-phenyl-1,2-benzisoselenazol-3(2H)-one), mimics glutathione peroxidase which reacts with peroxynitrite to inhibit enzymes such as lipooxygenases, NO synthetases, NADPH oxidase, Protein Kinase C and H+-K+ ATPase. Ebselen has low toxicity as its metabolism does not liberate the selenium moiety.[89] Safety and tolerability are virtuous with no apparent adverse effects.
Experimental studies in animals revealed that ebselen inhibits both vasospasm and tissue damage in stroke models, correlating with its inhibitory effect on oxidative processes.[90] Results from randomized, placebo controlled, double blind studies on the neurological consequences of AIS, SAH and acute MCA occlusion, revealed that ebselen significantly enhanced outcome in patients experiencing occlusive cerebral ischemia of limited duration.[91,92,93] The benefits with ebselen are closely related to the promptness with which the treatment is initiated, following the onset of stroke.
N-AcetylCysteine (NAC)
NAC is recommended for several OS related diseases.[94] NAC's multiple putative antioxidant properties stem from its ability to decrease NF-κB activation and cytokines production, regenerate NO as a sulfhydryl donor, replenish glutathione and scavenge ROS especially OH· and H2O2. NAC has been extensively studied in critically ill patients with ARDS, organ failure,[95] septic shock,[41] for prevention of exacerbation of COPD,[96] prevention of radiographic contrast induced nephropathy[97] and many others but the results have been inconsistent and inconclusive.
Rodent studies indicate the beneficial effect of NAC in treatment of I/R induced oxidant injury in stroke models and in experimental models with closed head trauma.[98,99] NAC is not synthesized endogenously and cannot cross the BBB after exogenous administration. This limits the efficacy of NAC in vivo. We found no studies in humans of use of NAC in neurocritical care but because NAC was helpful in various non neurological diseases, its brain-penetrated derivatives could be evaluated for use in patients with stroke.
Flavanoids
Flavanoids are a large group of naturally occurring phenolic compounds, present in high levels in human diet and have recently been reported to possess therapeutic potential in ischemic stroke. They have been studied for their vast antioxidant properties in-vitro[100] and many other biological activities including anti-tumor, cardio-protective and anti-inflammatory properties.[101] There role in cerebral protection from ischemia are promising.[102] Galangin, a naturally occurring flavanoid from the rhizome of Alpina offcinarum hances is widely used antioxidant. Administration of Galangin in rats (with MCA occlusion induced focal cerebral ischemia) exhibited a protective role through improvement in regional cerebral blood flow, attenuating mitochondrial dysfunction with decreased production of mitochondrial ROS and inhibiting caspase-dependent mitochondrial cell death pathway.[103] Similar neuroprotective effect of other potent flavanoids: Quercitin,[104] Luteolin,[105] Soy Isoflavone (Genistein),[106] Xanthohumol[107] and many others have been seen in ischemic stroke experimental models.
Minocycline
Minocycline is a second generation tetracycline derivative with established anti-inflammatory and anti-apoptotic use. It effectively crosses the BBB and demonstrates neuroprotective qualities in experimental models of CNS trauma, stroke, spinal cord injury and neurodegenerative diseases.[108] The main effects of minocycline include inhibition of microglial activation, attenuation of apoptosis and suppression of ROS generation. It is thus, an effective antioxidant and radical scavenger.[108] It inhibits polyadenosine diphosphate ribose polymerase I and MMP, chelates iron and prevents the neuronal death induced by ferrous sulphate.[109] In an open label, evaluator blinded study; oral administration of 200 mg/day of minocycline for 5 days, in patients after acute CNS infarction significantly decreased NIHSS and mRS score, signifying better clinical outcome.[110] However, this study had several limitations including open label design and small sample size. Fagan et al.,[111] in their open label, dose escalation, safety and dose finding study (Minocycline to Improve Neurological Outcome in Stroke (MINOS), concluded that minocycline may be an ideal agent to use with tissue plasminogen activator(tPA.) They found that minocycline was well tolerated up to doses of 10 mg/kg intravenously alone or in combination with tPA. They also found that half-life of minocycline was 24 hours, allowing once a day dosing.[111] Interestingly, in the MINOS trial authors found lower plasma MMP-9 levels (which predict post tPA hemorrhage) among tPA-treated subjects. Thus, combining minocycline with tPA may prevent adverse consequences of thrombolytic therapy.[112]
Edavarone
Edavarone (3-Methyl-1-phenyl-pyrazolin-5-one, MCI-186, Radicut®) is an antioxidant, approved for treatment of patients in AIS in Japan.[113] Edavarone scavenges ROS and inhibits pro-inflammatory responses after brain ischemia. In particular, post ischemic inflammation, leading to brain edema and infarction due to neuronal damage and endothelial cell death, is ameliorated by edavarone.[114] It also improves functional outcome in ischemic stroke patients.[115] Fifty percent of edavarone exists in an anionic form at physiological pH, which strongly reacts with ROS in brain. Byproducts formed after edavarone reacts with free radicals are stable and do not cause oxidation.[116] The mechanisms for reducing brain edema in acute ischemic stroke possibly are; inhibition of vascular endothelial growth factor,[117] or inhibition of aquaporin-4 (a membrane water channel) expression.[118] It has shown improvement in nerve growth factor expression in human astrocytes subjected to hypoxia-reoxygenation. This suggests edavarone's neurotrophic therapeutic effect in brain injury (ischemia-reperfusion).[119] It does reduce the oxidative damage in rodent brain after ischemic injury.[120]
Clinical studies on patients with acute lacunar infarction have shown efficacy of edavarone in improving functional outcome (especially motor palsy).[121] Though edavarone is a safe drug, some studies have reported nephrotoxicity which ultimately recovered in 45% of patients.[114]
Lazaroids
Lazaroids (21 amino steroids derived from glucocorticoids) specifically inhibits lipid peroxidation and act as membrane stabilizer without glucocorticoid and mineralocorticoid receptor dependant activity.[122] They are 100 times more potent antioxidants, than corticosteroids and therefore may be efficacious in management of acute CNS injury.
Tirilazad mesylate (U-74006F), one of the lazaroids has been clinically developed as a parental neuroprotective drug. It is a lipophilic compound with high affinity for vascular endothelium.[62,122] It has shown to protect the BBB against traumatic and SAH induced permeability. The penetration of tirilazad into brain parenchyma is enhanced after acute CNS injury and disruption of BBB.[62] Functional outcome studies in animal models of closed head injury,[123] I/R[124] and SAH[125] demonstrated beneficial effects of tirilazad.
However, there is a disparity between the beneficial effects of tirilazad in animal studies when compared with clinical trials, where tirilazad as a cerebroprotectant had inconclusive results. The randomized trial of Tirilazad For Acute Stroke (RANTTAS I and II) in stroke patients failed to show statistically significant reduction in mortality or improvement in functional outcome.[126,127] Another study however, showed better functional recovery at 3 months and overall reduction in mortality in patients receiving Tirilazad.[128] Similar study where anticonvulsant usage was much higher,[129] failed to reproduce the same. Evidence from a clinical trial in women given higher dosage of Tirilazad following aneurysmal SAH showed improvement in outcome.[130]
Several reasons like a single mechanism of action (i.e. lipid peroxidation), use of enzyme inducers (anticonvulsants), lack of bioavailability due to inability to cross BBB and suboptimal dosages might make it difficult to extrapolate the results of pre-clinical trials in humans. At present the clinical evidence is not strong enough to justify the routine use of tirilazad in the management of CNS trauma, ischemia, SAH.
Conclusions and Future Strategies
Antioxidants have a vital role in human physiology with several therapeutical implications in pathological states. Research in this field is evolving. Hence, better understanding of use of these antioxidants is likely to open a full spectrum of possibilities to make them an integral component of the treatment strategy. The role of OS in pathophysiological mechanism underlying acute CNS injury is established and is aggravated by reduction in endogenous antioxidants either due to genetic or environmental factors. Antioxidants have shown efficiency in animal models, but beneficial effects have not materialized in clinical trials. Several reasons have been advocated, which include wide variability of the nature and severity of illness in neurointensive care patients, low bioavailability of the antioxidant at desired site, lack of penetration through the BBB or suboptimal drug dosages and poor target specificity; and narrow therapeutic “time window”. Thus, to achieve the efficacy, antioxidants must be disease specific.
Future research demands improvement in the molecular design of antioxidants, increasing potency, site specificity and with better BBB penetration. Studies need to be designed to evaluate the efficacy and application of antioxidant cocktails with neuroprotectants; optimizing time and duration of therapy to achieve drug levels during the vascular event and irreversible neuronal loss along with the use of biomarkers to identify and assess the severity of OS along with monitoring the response to antioxidant therapy.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Hatwalne MS. Free radical scavengers in anaesthesiology and critical care. Indian J Anaesth. 2012;56:227–33. doi: 10.4103/0019-5049.98760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Coleman NA. Antioxidants in critical care medicine. Environ Toxicol Pharmacol. 2001;10:183–8. doi: 10.1016/s1382-6689(01)00082-5. [DOI] [PubMed] [Google Scholar]
- 3.Harris RA, Amor S. Sweet and sour-oxidative and carbonyl stress in neurological disorders. CNS Neurol Disord Drug Targets. 2011;10:82–107. doi: 10.2174/187152711794488656. [DOI] [PubMed] [Google Scholar]
- 4.Hanafy KA, Selim MH. Antioxidant strategies in neurocritical care. Neurotherapeutics. 2012;9:44–55. doi: 10.1007/s13311-011-0085-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gelain DP, de Bittencourt Pasquali MA, M Comim C, Grunwald MS, Ritter C, Tomasi CD, et al. Serum heat shock protein 70 levels, oxidant status, and mortality in sepsis. Shock. 2011;35:466–70. doi: 10.1097/SHK.0b013e31820fe704. [DOI] [PubMed] [Google Scholar]
- 6.Ghosh N, Ghosh R, Mandal SC. Antioxidant protection: A promising therapeutic intervention in neurodegenerative disease. Free Radic Res. 2011;45:888–905. doi: 10.3109/10715762.2011.574290. [DOI] [PubMed] [Google Scholar]
- 7.Schiller HJ, Reilly PM, Bulkley GB. Tissue perfusion in critical illnesses. Antioxidant therapy. Crit Care Med. 1993;21(2 Suppl):S92–102. [PubMed] [Google Scholar]
- 8.Cheeseman KH, Slater TF. An introduction to free radical biochemistry. Br Med Bull. 1993;49:481–93. doi: 10.1093/oxfordjournals.bmb.a072625. [DOI] [PubMed] [Google Scholar]
- 9.Dizdaroglu M. Chemical determination of free radical-induced damage to DNA. Free Radic Biol Med. 1991;10:225–42. doi: 10.1016/0891-5849(91)90080-m. [DOI] [PubMed] [Google Scholar]
- 10.Fink MP, Payen D. the role of nitric oxide in sepsis and ARDS: Synopsis of a roundtable conference held in Brussels on 18-20 March 1995. Intensive Care Med. 1996;22:158–65. doi: 10.1007/BF01720723. [DOI] [PubMed] [Google Scholar]
- 11.Halliwell B, Murcia MA, Chirico S, Aruoma OI. Free radicals and antioxidants in food and in vivo: What they do and how they work. Crit Rev Food Sci Nutr. 1995;35:7–20. doi: 10.1080/10408399509527682. [DOI] [PubMed] [Google Scholar]
- 12.Szabó C. Multiple pathways of peroxynitrite cytotoxicity. Toxicol Lett. 2003;140-141:105–12. doi: 10.1016/s0378-4274(02)00507-6. [DOI] [PubMed] [Google Scholar]
- 13.Baggiolini M, Thelen M. The phagocytes and the respiratory burst. In: Sies H, editor. Oxidative Stress: Oxidants and Antioxidants. San Diego: Academic Press; 1991. pp. 399–420. [Google Scholar]
- 14.Ferrari R, Ceconi C, Curello S, Cargnoni A, Pasini E, De Giuli F, et al. Role of oxygen free radicals in ischemic and reperfused myocardium. Am J Clin Nutr. 1991;53(1 Suppl):215S–22. doi: 10.1093/ajcn/53.1.215S. [DOI] [PubMed] [Google Scholar]
- 15.Cochrane CG. Cellular injury by oxidants. Am J Med. 1991;91:23–30S. doi: 10.1016/0002-9343(91)90280-b. [DOI] [PubMed] [Google Scholar]
- 16.Halliwell B, Gutteridge JM, Cross CE. Free radicals, antioxidants and human disease: Where are we now? J Lab Clin Med. 1992;119:598–620. [PubMed] [Google Scholar]
- 17.Rangan U, Bulkley GB. Prospects of treatment of free radical-mediated tissue injury. Br Med Bull. 1993;49:700–18. doi: 10.1093/oxfordjournals.bmb.a072641. [DOI] [PubMed] [Google Scholar]
- 18.Chang CY, Chen JY, Ke D, Hu ML. Plasma levels of lipophilic antioxidant vitamins in acute ischemic stroke patients: Correlation to inflammation markers and neurological deficits. Nutrition. 2005;21:987–93. doi: 10.1016/j.nut.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 19.Taffi R, Nanetti L, Mazzanti L, Bartolini M, Vignini A, Raffaelli F, et al. Plasma levels of nitric oxide and stroke outcome. J Neurol. 2008;255:94–8. doi: 10.1007/s00415-007-0700-y. [DOI] [PubMed] [Google Scholar]
- 20.Chan PH. Mitochondria and neuronal death/survival signalling pathways in cerebral ischemia. Neurochem Res. 2004;29:1943–9. doi: 10.1007/s11064-004-6869-x. [DOI] [PubMed] [Google Scholar]
- 21.Huang FP, Xi G, Keep RF, Hua Y, Nemoianu A, Hoff JT. Brain edema after experimental intracerebral hemorrhage: Role of haemoglobin degradation products. J Neurosurg. 2002;96:287–93. doi: 10.3171/jns.2002.96.2.0287. [DOI] [PubMed] [Google Scholar]
- 22.Goldstein L, Teng ZP, Zeserson E, Patel M, Regan RF. Hemin induces an iron-dependent, oxidative injury to human neuron like cells. J Neurosci Res. 2003;73:113–21. doi: 10.1002/jnr.10633. [DOI] [PubMed] [Google Scholar]
- 23.Polidori MC, Mecocci P, Frei B. Plasma vitamin C levels are decreased and correlated with brain damage in patients with intracranial hemorrhage or head trauma. Stroke. 2001;32:898–902. doi: 10.1161/01.str.32.4.898. [DOI] [PubMed] [Google Scholar]
- 24.Biffi A, Anderson CD, Jagiella JM, Schmidt H, Kissela B, Hansen BM, et al. APOE genotype and extent of bleeding and outcome in lobar intracerebral haemorrhage: A genetic association study. Lancet Neurol. 2011;10:702–9. doi: 10.1016/S1474-4422(11)70148-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Laskowitz DT, Lei B, Dawson HN, Wang H, Bellows ST, Christensen DJ, et al. The apoE-mimetic peptide, COG1410, improves functional recovery in a murine model of intracerebral hemorrhage. Neurocrit Care. 2011;16:316–26. doi: 10.1007/s12028-011-9641-5. [DOI] [PubMed] [Google Scholar]
- 26.Aoki T, Nishimura M, Kataoka H, Ishibashi R, Nozaki K, Hashimoto N. Reactive oxygen species modulate growth of cerebral aneurysms: A study using the free radical scavenger edaravone and p47phox(−/−) mice. Lab Invest. 2009;89:730–41. doi: 10.1038/labinvest.2009.36. [DOI] [PubMed] [Google Scholar]
- 27.Erşahin M, Toklu HZ, Cetinel S, Yüksel M, Erzik C, Berkman MZ, et al. Alpha lipoic acid alleviates oxidative stress and preserves blood brain permeability in rats with subarachnoid hemorrhage. Neurochem Res. 2010;35:418–28. doi: 10.1007/s11064-009-0072-z. [DOI] [PubMed] [Google Scholar]
- 28.Kontos HA, Wei EP. Superoxide production in experimental brain injury. J Neurosurg. 1986;64:803–7. doi: 10.3171/jns.1986.64.5.0803. [DOI] [PubMed] [Google Scholar]
- 29.Wali B, Sayeed I, Stein DG. Improved behavioral outcomes after progesterone administration in aged male rats with traumatic brain injury. Restor Neurol Neurosci. 2011;29:61–71. doi: 10.3233/RNN-2011-0579. [DOI] [PubMed] [Google Scholar]
- 30.Roof RL, Hoffman SW, Stein DG. Progesterone protects against lipid peroxidation following traumatic brain injury in rats. Mol Chem Neuropathol. 1997;31:1–11. doi: 10.1007/BF02815156. [DOI] [PubMed] [Google Scholar]
- 31.Wright DW, Kellermann AL, Hertzberg VS, Clark PL, Frankel M, Goldstein FC, et al. ProTECT: A randomized clinical trial of progesterone for acute traumatic brain injury. Ann Emerg Med. 2007;49:391–402. doi: 10.1016/j.annemergmed.2006.07.932. [DOI] [PubMed] [Google Scholar]
- 32.Poirier J. Apolipoprotein E in animal models of CNS injury and in Alzheimer's disease. Trends Neurosci. 1994;17:525–30. doi: 10.1016/0166-2236(94)90156-2. [DOI] [PubMed] [Google Scholar]
- 33.Hoane MR, Pierce JL, Holland MA, Birky ND, Dang T, Vitek MP, et al. The novel apolipoprotein E-based peptide COG1410 improves sensorimotor performance and reduces injury magnitude following cortical contusion injury. J Neurotrauma. 2007;24:1108–18. doi: 10.1089/neu.2006.0254. [DOI] [PubMed] [Google Scholar]
- 34.Smith C, Graham DI, Murray LS, Stewart J, Nicoll JA. Association of APOE e4 and cerebrovascular pathology in traumatic brain injury. J Neurol Neurosurg Psychiatry. 2006;77:363–6. doi: 10.1136/jnnp.2005.074617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Traber MG, Sies H. Vitamin E in humans: Demand and delivery. Annu Rev Nutr. 1996;16:321–47. doi: 10.1146/annurev.nu.16.070196.001541. [DOI] [PubMed] [Google Scholar]
- 36.De AK, Darad R. Physiological antioxidants and antioxidative enzymes in vitamin E-deficient rats. Toxicol Lett. 1988;44:47–54. doi: 10.1016/0378-4274(88)90128-2. [DOI] [PubMed] [Google Scholar]
- 37.Rose RC, Bote AM. Biology of free radical scavengers: An evaluation of Ascorbate. FASEB J. 1993;7:1135–42. [PubMed] [Google Scholar]
- 38.Chan AC. Partners in defense. Vitamin E and vitamin C. Can J Physiol Pharmacol. 1993;7:725–31. doi: 10.1139/y93-109. [DOI] [PubMed] [Google Scholar]
- 39.Manzanares W, Hardy G. Vitamin B12: The forgotten micronutrient for critical care. Curr Opin Clin Nutr Metab Care. 2010;13:662–8. doi: 10.1097/MCO.0b013e32833dfaec. [DOI] [PubMed] [Google Scholar]
- 40.Crimi E, Liguori A, Condorelli M, Cioffi M, Astuto M, Bontempo P, et al. The beneficial effects of antioxidant supplementation in enteral feeding in critically ill patients: A prospective, randomized, double-blind, placebo controlled trial. Anesth Analg. 2004;99:857–63. doi: 10.1213/01.ANE.0000133144.60584.F6. [DOI] [PubMed] [Google Scholar]
- 41.Crimi E, Sica V, Williams-Ignarro S, Zhang H, Slutsky AS, Ignarro LJ, et al. The role of oxidative stress in adult critical care. Free Radic Biol Med. 2006;40:398–406. doi: 10.1016/j.freeradbiomed.2005.10.054. [DOI] [PubMed] [Google Scholar]
- 42.Andrews PJ, Avenell A, Noble DW, Campbell MK, Croal BL, Simpson WG, et al. Scottish Intensive Care Glutamine or Selenium Evaluative Trial Trials Group. Randomised trial of glutamine, selenium, or both, to supplement parenteral nutrition for critically ill patients. BMJ. 2011;342:d1542. doi: 10.1136/bmj.d1542. [DOI] [PubMed] [Google Scholar]
- 43.Heyland DK, Dhaliwal R, Suchner U, Berger MM. Antioxidant nutrients: A systematic review of trace elements and vitamins in the critically ill patient. Intensive Care Med. 2005;31:327–37. doi: 10.1007/s00134-004-2522-z. [DOI] [PubMed] [Google Scholar]
- 44.Dyatlov VA, Makovetskaia VV, Leonhardt R, Lawrence DA, Carpenter DO. Vitamin E enhances Ca (2+)-mediated vulnerability of immature cerebellar granule cells to ischemia. Free Radic Biol Med. 1998;25:793–802. doi: 10.1016/s0891-5849(98)00157-9. [DOI] [PubMed] [Google Scholar]
- 45.Rice ME. Ascorbate regulation and its neuroprotective role in the brain. Trends Neurosci. 2000;23:209–16. doi: 10.1016/s0166-2236(99)01543-x. [DOI] [PubMed] [Google Scholar]
- 46.Ernster L, Dallner G. Biochemical, physiological and medical aspects of ubiquinon function. Biochem Biophys Acta. 1995;127:195–204. doi: 10.1016/0925-4439(95)00028-3. [DOI] [PubMed] [Google Scholar]
- 47.Lance J, McCabe S, Clancy RL, Pierce J. Coenzyme Q10 - a therapeutic agent. Medsurg Nurs. 2012;21:367–71. [PubMed] [Google Scholar]
- 48.Ogawa N, Tsukamoto S, Hirose Y, Kuroda H. Survival effect of coenzyme Q10, and naloxone on experimental stroke. Pharmacol Biochem Behav. 1986;24:315–7. doi: 10.1016/0091-3057(86)90357-6. [DOI] [PubMed] [Google Scholar]
- 49.Grieb P, Ryba MS, Sawicki J, Chrapusta SJ. Oral coenzyme Q10 administration prevents the development of ischemic brain lesions in rabbit model of symptomatic vasospasm. Acta Neuropathol. 1997;94:363–8. doi: 10.1007/s004010050720. [DOI] [PubMed] [Google Scholar]
- 50.Kalayci M, Unal MM, Gul S, Acikgoz S, Kandemir N, Hanci V, et al. Effect of coenzyme Q10 on ischemia and neuronal damage in an experimental traumatic brain-injury model in rats. BMC Neurosci. 2011;12:75. doi: 10.1186/1471-2202-12-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Packer L. New horizons in antioxidant research: Action of the thioctic acid/dihydrolipoic acid couple in biological systems, in Thioctsaure. In: Schmidt K, Ulrich H, editors. 2nd International Thictic Acid Workshop. Frankfurt: Universimed Verlag GmbH; 1992. pp. 35–44. [Google Scholar]
- 52.Packer L. Alpha-Lipoic acid: A metabolic antioxidant which regulates NF-kappa B signal transduction and protects against oxidative injury. Drug Metab Rev. 1998;30:245–75. doi: 10.3109/03602539808996311. [DOI] [PubMed] [Google Scholar]
- 53.Prehn JH, Karkoutly C, Nuglisch J, Peruche B, Krieglstein J. Dihydrolipoate reduces neuronal injury after cerebral ischemia. J Cereb Blood Flow Metab. 1992;12:78–87. doi: 10.1038/jcbfm.1992.10. [DOI] [PubMed] [Google Scholar]
- 54.Cao X, Phillis JW. The free radical scavenger alpha-lipoic acid protects against cerebral ischemia-reperfusion injury in gerbils. Free Radic Res. 1995;23:365–70. doi: 10.3109/10715769509065257. [DOI] [PubMed] [Google Scholar]
- 55.Woltz P, Krieglstein J. Neuroprotective effects of alpha-lipoic acid and its enantiomers demonstrated in rodent models of focal cerebral ischemia. Neuropharmacology. 1996;35:369–75. doi: 10.1016/0028-3908(95)00172-7. [DOI] [PubMed] [Google Scholar]
- 56.Connell BJ, Saleh M, Khan BV, Saleh TM. Lipoic acid protects against reperfusion injury in the early stages of cerebral ischemia. Brain Res. 2011;1375:128–36. doi: 10.1016/j.brainres.2010.12.045. [DOI] [PubMed] [Google Scholar]
- 57.Kostoglou-Athanassiou I. Therapeutic applications of melatonin. Ther Adv Endocrinol Metab. 2013;4:13–24. doi: 10.1177/2042018813476084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nair SM, Rahman RM, Clarkson AN, Sutherland BA, Taurin S, Sammut IA, et al. Melatonin treatment following stroke induction modulates L-arginine metabolism. J Pineal Res. 2011;51:313–23. doi: 10.1111/j.1600-079X.2011.00891.x. [DOI] [PubMed] [Google Scholar]
- 59.Borlongan CV, Yamamoto M, Takei N, Kumazaki M, Ungsuparkorn C, Hida H, et al. Glial survival is enhanced during melatonin induced neuroprotection against cerebral ischemia. FASEB J. 2000;14:1307–17. doi: 10.1096/fj.14.10.1307. [DOI] [PubMed] [Google Scholar]
- 60.Kilic E, Ozdemir YG, Bolay H, Kelestimur H, Dalkara T. Pinealectomy aggravates and melatonin administration attenuates brain damage in focal ischemia. J Cereb Blood Flow Metab. 1999;19:511–6. doi: 10.1097/00004647-199905000-00005. [DOI] [PubMed] [Google Scholar]
- 61.Samantaray S, Das A, Thakore NP, Matzelle DD, Reiter RJ, Ray SK, et al. Therapeutic potential of melatonin in traumatic central nervous system injury. J Pineal Res. 2009;47:134–42. doi: 10.1111/j.1600-079X.2009.00703.x. [DOI] [PubMed] [Google Scholar]
- 62.Halliwell B. Free radicals, antioxidants, and human disease: Curiosity, cause or consequence? Lancet. 1994;344:721–4. doi: 10.1016/s0140-6736(94)92211-x. [DOI] [PubMed] [Google Scholar]
- 63.Ye N, Liu S, Lin Y, Rao P. Protective effects of intraperitoneal injection of TAT-SOD against focal cerebral ischemia/reperfusion injury in rats. Life Sci. 2011;89:868–74. doi: 10.1016/j.lfs.2011.09.015. [DOI] [PubMed] [Google Scholar]
- 64.Young B, Runge JW, Waxman KS, Harrington T, Wilberger J, Muizelaar JP, et al. Effects of pegorgotein on neurologic outcome of patient with severe head injury: A multicenter, randomized controlled trial. JAMA. 1996;276:538–43. [PubMed] [Google Scholar]
- 65.Meister A, Anderson ME. Glutathione. Annu Rev Biochem. 1983;52:711–60. doi: 10.1146/annurev.bi.52.070183.003431. [DOI] [PubMed] [Google Scholar]
- 66.Larsson A, Orrenius S, Holngren A, Mannervik B. New York: Raven Press; 1983. Functions of glutathione. biochemical physiological toxicological and clinical aspects. [Google Scholar]
- 67.Mizui T, Kinouchi H, Chan PH. Depletion of brain glutathione by buthionine sulfoximine enhances cerebral ischemic injury in rats. Am J Physiol. 1992;262:H313–7. doi: 10.1152/ajpheart.1992.262.2.H313. [DOI] [PubMed] [Google Scholar]
- 68.Zaidan E, Sims NR. Alternations in the glutathione content of mitochondria following short term forebrain ischemia in rats. Neurosci Lett. 1996;218:75–8. doi: 10.1016/s0304-3940(96)13128-1. [DOI] [PubMed] [Google Scholar]
- 69.Yamamoto M, Sakamoto N, Iwai A, Yatsugi S, Hidaka K, Noguchi K, et al. Protective actions of YM737, a new glutathione analog, against cerebral ischemia in rats. Res Commun Chem Pathol Pharmacol. 1993;81:221–32. [PubMed] [Google Scholar]
- 70.Anderson MF, Nilsson M, Eriksson PS, Sims NR. Glutathione monoethyl ester provides neuroprotection in a rat model of stroke. Neurosci Lett. 2004;354:163–5. doi: 10.1016/j.neulet.2003.09.067. [DOI] [PubMed] [Google Scholar]
- 71.Rivera F, Costa G, Abin A, Urbanavicius J, Arruti C, Casanova G, et al. Reduction of ischemic brain damage and increase of glutathione by a liposomal preparation of quercetin in permanent focal ischemia in rats. Neurotox Res. 2008;13:105–14. doi: 10.1007/BF03033562. [DOI] [PubMed] [Google Scholar]
- 72.Bilgihan A, Turkozkan N, Aricioglu A, Aykol S, Cevik C, Göksel M. The effect of deferoxamine on brain lipid peroxide levels and Na-K ATPase activity following experimental subarachnoid hemorrhage. Gen Pharmacol. 1994;25:495–7. doi: 10.1016/0306-3623(94)90204-6. [DOI] [PubMed] [Google Scholar]
- 73.Selim M. Deferoxamine mesylate: A new hope for intracerebral hemorrhage: From bench to clinical trials. Stroke. 2009;40(3 Suppl):S90–1. doi: 10.1161/STROKEAHA.108.533125. [DOI] [PubMed] [Google Scholar]
- 74.Xing Y, Hua Y, Keep RF, Xi G. Effects of deferoxamine on brain injury after transient focal cerebral ischemia in rats with hyperglycemia. Brain Res. 2009;1291:113–21. doi: 10.1016/j.brainres.2009.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Okauchi M, Hua Y, Keep RF, Morgenstern LB, Xi G. Effects of deferoxamine on intracerebral hemorrhage-induced brain injury in aged rats. Stroke. 2009;40:1858–63. doi: 10.1161/STROKEAHA.108.535765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chen Z, Gao C, Hua Y, Keep RF, Muraszko K, Xi G. Role of iron in brain injury after intraventricular hemorrhage. Stroke. 2011;42:465–70. doi: 10.1161/STROKEAHA.110.602755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lee JY, Keep RF, He Y, Sagher O, Hua Y, Xi G. Hemoglobin and iron handling in brain after subarachnoid hemorrhage and the effect of deferoxamine on early brain injury. J Cereb Blood Flow Metab. 2010;30:1793–803. doi: 10.1038/jcbfm.2010.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Selim M, Yeatts S, Goldstein JN, Gomes J, Greenberg S, Morgenstern LB, et al. Deferoxamine Mesylate in Intracerebral Hemorrhage Investigators. Safety and tolerability of deferoxamine mesylate in patients with acute intracerebral hemorrhage. Stroke. 2011;42:3067–74. doi: 10.1161/STROKEAHA.111.617589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Méthy D, Bertrand N, Prigent-Tessier A, Mossiat C, Stanimirovic D, Beley A, et al. Beneficial effect of dipyridyl, a liposoluble iron chelator against focal cerebral ischemia: In vivo and in vitro evidence of protection of cerebral endothelial cells. Brain Res. 2008;1193:136–42. doi: 10.1016/j.brainres.2007.11.063. [DOI] [PubMed] [Google Scholar]
- 80.Janzen E. Spin trapping. Acc Chem Res. 1971;4:31–40. [Google Scholar]
- 81.Hensley K, Carney JM, Stewart CA, Tabatabaie T, Pye Q, Floyd RA. Nitrone-based free radical traps as neuroprotective agents in cerebral ischemia and other pathologies. Int Rev Neurobiol. 1997;40:299–317. doi: 10.1016/s0074-7742(08)60725-4. [DOI] [PubMed] [Google Scholar]
- 82.Kotake Y. Pharmacologic properties of phenyl N-tert-butylnitrone. Antioxid Redox Signal. 1999;1:481–99. doi: 10.1089/ars.1999.1.4-481. [DOI] [PubMed] [Google Scholar]
- 83.Carney JM, Floyd RA. Protection against oxidative damage to CNS by alpha-phenyl-tert-butyl nitrone (PBN) and other spin trapping agents: A novel series of nonlipid free radical scavengers. J Mol Neurosci. 1991;3:47–57. doi: 10.1007/BF02896848. [DOI] [PubMed] [Google Scholar]
- 84.Zhao Q, Pahlmark K, Smith ML, Siesjo BK. Delayed treatment with the spin trap alpha-phenyl-tert-butyl nitrone (PBN) reduces infarct size following transient middle cerebral artery occlusion in rats. Acta Physiol Scand. 1994;152:349–50. doi: 10.1111/j.1748-1716.1994.tb09816.x. [DOI] [PubMed] [Google Scholar]
- 85.Pahlmark K, Siesjo BK. Effect of the spin trap-alpha-phenyl-N-tert-butylnitrone (PBN) in transient forebrain ischemia in the rat. Acta Physiol Scand. 1996;157:41–51. doi: 10.1046/j.1365-201X.1996.440167000.x. [DOI] [PubMed] [Google Scholar]
- 86.Lees KR, Zivin JA, Ashwood T, Davalos A, Davis SM, Diener HC, et al. Stroke-Acute Ischemic NXY Treatment (SAINT I) Trial Investigators. NXY-059 for acute ischemic stroke. N Engl J Me. 2006;354:588–600. doi: 10.1056/NEJMoa052980. [DOI] [PubMed] [Google Scholar]
- 87.Shuaib A, Lees KR, Lyden P, Grotta J, Davalos A, Davis SM, et al. SAINT II Trial Investigators. NXY-059 for the treatment of acute ischemic stroke. N Engl J Med. 2007;357:562–71. doi: 10.1056/NEJMoa070240. [DOI] [PubMed] [Google Scholar]
- 88.Savitz SI. A critical appraisal of the NXY-059 neuroprotection studies for acute stroke: A need for more rigorous testing of neuroprotective agents in animal models of stroke. Exp Neurol. 2007;205:20–5. doi: 10.1016/j.expneurol.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 89.Parnham M, Sies H. Ebselen: Prospective therapy for cerebral ischaemia. Expert Opin Investig Drugs. 2000;9:607–19. doi: 10.1517/13543784.9.3.607. [DOI] [PubMed] [Google Scholar]
- 90.Takasago T, Peters EE, Graham DI, Masayasu H, Macrae IM. Neuroprotective efficacy of ebselen an antioxidant with anti-inflammatory actions, in rodent model of permanent middle cerebral artery occlusion. Br J Pharmacol. 1997;122:1251–6. doi: 10.1038/sj.bjp.0701426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Saito I, Asano T, Takakura K, Abe H, Yoshimoto T, Kikuchi H, et al. Neuroprotective efficacy of an antioxidant ebselen, in patients with delayed neurological deficits after aneurysmal subarachnoid hemorrhage. Neurosurgery. 1998;42:269–77. doi: 10.1097/00006123-199802000-00038. [DOI] [PubMed] [Google Scholar]
- 92.Yamaguchi T, Sano K, Takakura K, Saito I, Shinohara T, Asano T, et al. Ebselen in acute ischemic stroke: A placebo-controlled double-blind clinical trial. Ebselen study group. Stroke. 1998;29:12–7. doi: 10.1161/01.str.29.1.12. [DOI] [PubMed] [Google Scholar]
- 93.Ogawa A, Yoshimoto T, Kikuchi H, Sano K, Saito I, Yamaguchi T, et al. Ebselen in acute middle cerebral artery occlusion: A placebo-controlled double-blind clinical trial. Cerebrovas Dis. 1999;9:112–8. doi: 10.1159/000015908. [DOI] [PubMed] [Google Scholar]
- 94.Sheffner AL. The mucolytic action of acetylcysteine. Tuberculol Thorac Dis. 1966;23:31–3. [PubMed] [Google Scholar]
- 95.Molnár Z, Shearer E, Lowe D. N-Acetylcysteine treatment to prevent the progression of multisystem organ failure: A prospective, randomized, placebo-controlled study. Crit Care Med. 1999;27:1100–4. doi: 10.1097/00003246-199906000-00028. [DOI] [PubMed] [Google Scholar]
- 96.Sadowska AM, Verbraecken J, Darquennes K, De Backer WA. Role of N-acetylcysteine in the management of COPD. Int J Chron Obstruct Pulmon Dis. 2006;1:425–34. doi: 10.2147/copd.2006.1.4.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Fishbane S. N-acetylcysteine in the prevention of contrast-induced nephropathy. Clin J Am Soc Nephrol. 2008;3:281–7. doi: 10.2215/CJN.02590607. [DOI] [PubMed] [Google Scholar]
- 98.Khan M, Sekhon B, Jatana M, Giri S, Gilg AG, Sekhon C, et al. Administration of N-acetylcysteine after focal cerebral ischemia protects brain and reduces inflammation in a rat model of experimental stroke. J Neurosci Res. 2004;76:519–27. doi: 10.1002/jnr.20087. [DOI] [PubMed] [Google Scholar]
- 99.Hicdonmez T, Kanter M, Tiryaki M, Parsak T, Cobanoglu S. Neuroprotective effects of N-acetylcysteine on experimental closed head trauma in rats. Neurochem Res. 2006;31:473–81. doi: 10.1007/s11064-006-9040-z. [DOI] [PubMed] [Google Scholar]
- 100.Firuzi O, Lacanna A, Petrucci R, Marrosu G, Saso L. Evaluation of the antioxidant activity of flavonoids by “ferric reducing antioxidant power” assay and cyclic voltammetry. Biochim Biophys Acta. 2005;1721:174–84. doi: 10.1016/j.bbagen.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 101.Benavente-Garcia O, Castillo J. Update on uses and properties of citrus flavonoids: New findings in anticancer, cardiovascular, and anti-inflammatory activity. J Agric Food Chem. 2008;56:6185–205. doi: 10.1021/jf8006568. [DOI] [PubMed] [Google Scholar]
- 102.Vlasov TD. Mechanisms of cerebral protection from ischemia by tea constituents. Ross Fiziol Zh Im I M Sechenova. 2012;98:929–42. [PubMed] [Google Scholar]
- 103.Li S, Wu C, Zhu L, Gao J, Fang J, Li D, et al. By improving regional cortical blood flow, attenuating mitochondrial dysfunction and sequential apoptosis galangin acts as a potential neuroprotective agent after acute ischemic stroke. Molecules. 2012;17:13403–23. doi: 10.3390/molecules171113403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Pandey AK, Patnaik R, Muresanu DF, Sharma A, Sharma HS. Quercetin in hypoxia-induced oxidative stress: Novel target for neuroprotection. Int Rev Neurobiol. 2012;102:107–46. doi: 10.1016/B978-0-12-386986-9.00005-3. [DOI] [PubMed] [Google Scholar]
- 105.Qiao H, Dong L, Zhang X, Zhu C, Zhang X, Wang L, et al. Protective effect of luteolin in experimental ischemic stroke: Upregulated SOD1, CAT, Bcl-2 and claudin-5, down-regulated MDA and Bax expression. Neurochem Res. 2012;37:2014–24. doi: 10.1007/s11064-012-0822-1. [DOI] [PubMed] [Google Scholar]
- 106.Qian Y, Guan T, Huang M, Cao L, Li Y, Cheng H, et al. Neuroprotection by the soy isoflavone, genistein, via inhibition of mitochondria-dependent apoptosis pathways and reactive oxygen induced-NF-B activation in a cerebral ischemia mouse model. Neurochem Int. 2012;60:759–67. doi: 10.1016/j.neuint.2012.03.011. [DOI] [PubMed] [Google Scholar]
- 107.Yen TL, Hsu CK, Lu WJ, Hsieh CY, Hsiao G, Chou DS, et al. Neuroprotective effects of xanthohumol, a prenylated flavonoid from hops (Humulus lupulus), in ischemic stroke of rats. J Agric Food Chem. 2012;60:1937–44. doi: 10.1021/jf204909p. [DOI] [PubMed] [Google Scholar]
- 108.Plane JM, Shen Y, Pleasure DE, Deng W. Prospects for minocycline neuroprotection. Arch Neurol. 2010;67:1442–8. doi: 10.1001/archneurol.2010.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Chen-Roetling J, Chen L, Regan RF. Minocycline attenuates iron neurotoxicity in cortical cell cultures. Biochem Biophys Res Commun. 2009;386:322–6. doi: 10.1016/j.bbrc.2009.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lampl Y, Boaz M, Gilad R, Lorberboym M, Dabby R, Rapoport A, et al. Minocycline treatment in acute stroke: An open-label, evaluator-blinded study. Neurology. 2007;69:1404–10. doi: 10.1212/01.wnl.0000277487.04281.db. [DOI] [PubMed] [Google Scholar]
- 111.Fagan SC, Waller JL, Nichols FT, Edwards DJ, Pettigrew LC, Clark WM, et al. Minocycline to improve neurologic outcome in stroke (MINOS): A dose-finding study. Stroke. 2010;41:2283–7. doi: 10.1161/STROKEAHA.110.582601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Switzer JA, Hess DC, Ergul A, Waller JL, Machado LS, Portik-Dobos V, et al. Matrix Metalloproteinase-9 in an exploratory trial of intravenous minocycline for acute ischemic stroke. Stroke. 2011;42:2633–5. doi: 10.1161/STROKEAHA.111.618215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Watanabe T, Tahara M, Todo S. The novel antioxidant edaravone: From bench to bedside. Cardiovasc Ther. 2008;26:101–14. doi: 10.1111/j.1527-3466.2008.00041.x. [DOI] [PubMed] [Google Scholar]
- 114.Hishida A. Clinical analysis of 207 patients who developed renal disorders during or after treatment with edaravone reported during post-marketing surveillance. Clin Exp Nephrol. 2007;11:292–6. doi: 10.1007/s10157-007-0495-2. [DOI] [PubMed] [Google Scholar]
- 115.Unno Y, Katayama M, Shimizu H. Does functional outcome in acute ischaemic stroke patients correlate with the amount of free-radical scavenger treatment? A retrospective study of edaravone therapy. Clin Drug Investig. 2010;30:143–55. doi: 10.2165/11535500-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 116.Ono S, Okazaki K, Sakurai M, Inoue Y. Density Functional Study of the Radical Reactions of 3-Methyl-1-phenyl-2-pyrazolin-5-one (MCI-186): Implication for the Biological Function of MCI-186 as a Highly Potent Antioxidative Radical Scavenger. J Phys Chem. 1997;101:3769–75. [Google Scholar]
- 117.Ishikawa A, Yoshida H, Metoki N, Toki T, Imaizumi T, Matsumiya T, et al. Edaravone inhibits the expression of vascular endothelial growth factor in human astrocytes exposed to hypoxia. Neurosci Res. 2007;59:406–12. doi: 10.1016/j.neures.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 118.Kikuchi K, Tancharoen S, Matsuda F, Biswas KK, Ito T, Morimoto Y, et al. Edaravone attenuates cerebral ischemic injury by suppressing aquaporin-4. Biochem Biophys Res Commun. 2009;390:1121–5. doi: 10.1016/j.bbrc.2009.09.015. [DOI] [PubMed] [Google Scholar]
- 119.Yoshida H, Metoki N, Ishikawa A, Imaizumi T, Matsumiya T, Tanji K, et al. Edaravone improves the expression of nerve growth factor in human astrocytes subjected to hypoxia/reoxygenation. Neurosci Res. 2010;66:284–9. doi: 10.1016/j.neures.2009.11.011. [DOI] [PubMed] [Google Scholar]
- 120.Zhang P, Li W, Li L, Wang N, Li X, Gao M, et al. Treatment with edaravone attenuates ischemic brain injury and inhibits neurogenesis in the subventricular zone of adult rats after focal cerebral ischemia and reperfusion injury. Neuroscience. 2012;201:297–306. doi: 10.1016/j.neuroscience.2011.11.005. [DOI] [PubMed] [Google Scholar]
- 121.Ohta Y, Takamatsu K, Fukushima T, Ikegami S, Takeda I, Ota T, et al. Efficacy of the free radical scavenger, edaravone, for motor palsy of acute lacunar infarction. Intern Med. 2009;48:593–6. doi: 10.2169/internalmedicine.48.1871. [DOI] [PubMed] [Google Scholar]
- 122.Kavanagh RJ, Kam PC. Lazaroids: Efficacy and mechanism of action of the 21-aminosteroids in neuroprotection. Br J Anaesth. 2001;86:110–9. doi: 10.1093/bja/86.1.110. [DOI] [PubMed] [Google Scholar]
- 123.Sanada T, Nakamura T, Nishimura MC, Isayama K, Pitts LH. Effects of U-74006F on neurologic function and brain edema after fluid percussion injury in rats. Neurotrauma. 1993;10:65–71. doi: 10.1089/neu.1993.10.65. [DOI] [PubMed] [Google Scholar]
- 124.Xue D, Bruederlin B, Heinecke E, Li H, Slivka A, Buchan AM. U-74006F reduces neocortical infarction, but does not attenuate selective hippocampal CA1 necrosis. Stroke. 1990;21:178. [Google Scholar]
- 125.Matsui T, Kaizu H, Itoh S, Asano T. The role of active smooth muscle contraction in the occurrence of chronic vasospasm in the canine two-hemorrhage model. J Neurosurg. 1994;80:276–82. doi: 10.3171/jns.1994.80.2.0276. [DOI] [PubMed] [Google Scholar]
- 126.A randomized trial of tirilazad mesylate in patients with acute stroke (RANTTAS). The RANTTAS Investigators. Stroke. 1996;27:1453–8. doi: 10.1161/01.str.27.9.1453. [DOI] [PubMed] [Google Scholar]
- 127.Haley EC., Jr High-dose tirilazad for acute stroke (RANTTAS II).RANTTAS II Investigators. Stroke. 1998;29:1256–7. [PubMed] [Google Scholar]
- 128.Kassell NF, Haley EC, Apperson-Hansen C, Alves WM. Randomised double-blind vehicle controlled trial of tirilazad mesylate in patients with aneurysmal subarachnoid haemorrhage: A co-operative study in Europe, Australia and New Zealand. J Neurosurg. 1996;84:221. doi: 10.3171/jns.1996.84.2.0221. [DOI] [PubMed] [Google Scholar]
- 129.Haley EC, Kassell NF, Apperson-Hansen C, Maile MH, Alves WM. A randomised double blind vehicle controlled trial of tirilazad mesylate in patients with aneurysmal subarachnoid haemorrhage: A co-operative study in North America. J Neurosurg. 1997;86:467. doi: 10.3171/jns.1997.86.3.0467. [DOI] [PubMed] [Google Scholar]
- 130.Lanzino G, Kassell NF. Double blind randomised, vehicle controlled study of high-dose tirilazad mesylate in women with aneurysmal subarachnoid haemorrhage. Part 2. A co-operative study in North America. J Neurosurg. 1999;90:1018. doi: 10.3171/jns.1999.90.6.1018. [DOI] [PubMed] [Google Scholar]


