Abstract
Antiarrhythmic drugs are a group of pharmaceuticals that suppress or prevent abnormal heart rhythms, which are often associated with substantial morbidity and mortality. Current antiarrhythmic drugs that typically target plasma membrane ion channels have limited clinical success and in some cases have been described as being pro-arrhythmic. However, recent studies suggest that pathological release of calcium (Ca2+) from the sarcoplasmic reticulum via cardiac ryanodine receptors (RyR2) could represent a promising target for antiarrhythmic therapy. Diastolic SR Ca2+ release has been linked to arrhythmogenesis in both the inherited arrhythmia syndrome 'catecholaminergic polymorphic ventricular tachycardia' and acquired forms of heart disease (eg, atrial fibrillation, heart failure). Several classes of pharmaceuticals have been shown to reduce abnormal RyR2 activity and may confer protection against triggered arrhythmias through reduction of SR Ca2+ leak. In this review, we will evaluate the current pharmacological methods for stabilizing RyR2 and suggest treatment modalities based on current evidence of molecular mechanisms.
Keywords: arrhythmias, atrial fibrillation, calcium, heart failure, ryanodine receptor, sarcoplasmic reticulum
Introduction
The cardiac ryanodine receptor (RyR2) is a homotetrameric Ca2+ release channel located in the sarcoplasmic reticulum (SR) membrane1, 2. During the normal cardiac cycle, plasma membrane depolarization initiates opening of L-type Ca2+ channels (LTCC), by which extracellular Ca2+ enters the cytoplasm. Ca2+ influx acts as a trigger that subsequently activates RyR2 channels, leading to a ten-fold greater release of SR Ca2+ into the cytoplasm. During systolic contraction of the heart, elevated cytoplasmic Ca2+ binds to troponin-C, allowing actin and myosin to interdigitate and cause sarcomere shortening, thus causing myocardial contraction. Diastolic relaxation occurs when cytoplasmic [Ca2+] decreases as Ca2+ is extruded through the Na+/Ca2+-exchanger (NCX) or is actively pumped back into the SR through the sarco/endoplasmic reticulum Ca2+-ATPase (SERCA2a)3. Concomitantly, myocardial relaxation is directly associated with diastolic reduction in Ca2+ levels. Thus, physiologic control of Ca2+ release from the SR is necessary for timely contraction and relaxation during the cardiac cycle. Pathological “leak” of Ca2+ during diastole may be detrimental and lead to cardiac arrhythmias4, 5.
There is now considerable evidence that abnormal RyR2-mediated Ca2+ release from the SR can lead to both atrial6, 7 and ventricular arrhythmias associated with sudden cardiac death8, 9, 10. Increased SR Ca2+ release during diastole can lead to activation of the Na+/Ca2+-exchanger11, which in turn generates a transient inward current that can cause afterdepolarizations and triggered action potentials. These afterdepolarizations have been observed in humans and have been directly linked to arrhythmogenesis in animal models of arrhythmias12.
Genetic susceptibility to cardiac arrhythmias may arise directly from genetic mutations in RyR2, such as in patients with catecholaminergic polymorphic ventricular tachycardia (CPVT)9, 13. Mutations in other proteins that bind to the pore-forming subunits within the RyR2 macromolecular complex (eg, calsequestrin, junctophilin) also have been reported to confer genetic susceptibility to cardiac arrhythmias and/or cardiomyopathy14, 15. These observations provide direct evidence that a perturbation in RyR2 function can facilitate the development of cardiac arrhythmias.
Additionally, acquired structural heart disease, for example heart failure or myocardial ischemia, has been shown to modify the post-translational regulation of RyR2 through nitrosylation, oxidation, and phosphorylation, which might also increase susceptibility to diastolic Ca2+ release and arrhythmias16, 17, 18, 19, 20. Given that there are many excellent reviews on strategies to modify intracellular signaling to reduce RyR2 activation21, 22, we will restrict the scope of this review mainly to pharmacological strategies to stabilize RyR2 directly to reduce arrhythmic potential.
Because RyR2 also plays an important role during excitation-contraction coupling, it is important that antiarrhythmic compounds targeting the RyR2 channel complex will not interfere with systolic SR Ca2+ release. At the same time, inhibition of diastolic SR Ca2+ release is a desirable feature of compounds that could prevent arrhythmias22. RyR2 activity can be modulated by numerous natural and pharmacological compounds, as reviewed elsewhere in more detail22, 23, 24. These compounds may modulate RyR2 in various ways, including by modulating channel gating, ion channel translocation, RyR2 subunit composition, or posttranslational modifications. Some of these compounds have emerged as strong candidates for antiarrhythmic drugs, and will be discussed in more detail below.
Dantrolene
Dantrolene sodium is a hydrantoin derivative that was initially described as a muscle relaxant25, but later found to be a potent therapeutic agent for patients suffering from the rare life-threatening condition known as malignant hyperthermia (MH)26. Patients susceptible to MH typically have inherited mutations in the type 1 ryanodine receptor (RyR1) primarily found in skeletal muscle27. Exposure to inhaled halogenated anesthetics during surgery can trigger massive RyR1-mediated Ca2+ release associated with muscle breakdown, elevation of serum creatinine kinase (CK), hypotension, hyperthermia, and tachycardia, which often results in intraoperative death 28. Dantrolene has been shown to directly bind to the N-terminus of RyR1 and to prevent SR Ca2+ leak in skeletal muscle, thereby improving clinical outcomes29. Dantrolene is believed to stabilize interdomain interactions between the N-terminal and central domains of RyR130 and RyR231, although the effects of dantrole on single RyR channels remains controversial32.
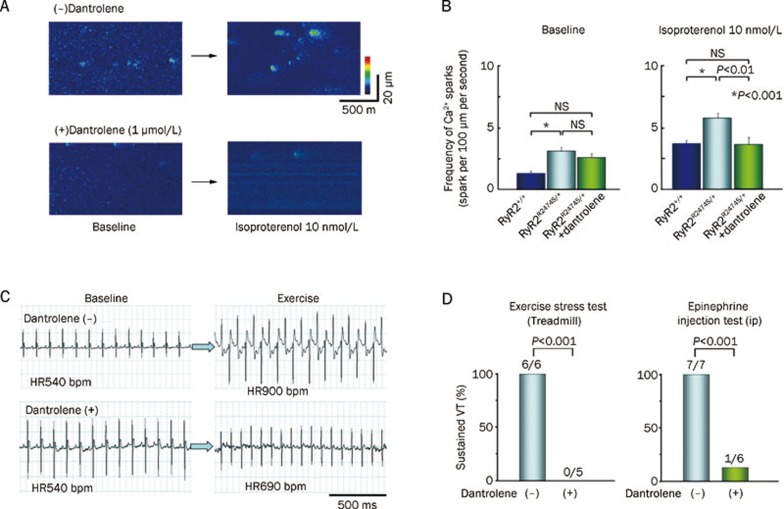
Given that dantrolene improves the stability of both RyR1 and RyR2, dantrolene has become a molecule of interest for preventing cardiac arrhythmias. Dantrolene has previously been described as an inhibitor of arrhythmias in animal models of ischemia-reperfusion33, 34, 35. More recently, dantrolene was demonstrated to inhibit catecholaminergic polymorphic ventricular tachycardia in a knock-in mouse model heterozygous for mutation R2474S in RyR236 (Figure 1). Dantrolene was shown to suppress isoproterenol-induced spontaneous SR Ca2+ releases (ie, sparks) in intact myocytes isolated from RyR2-R2474A/+ mice. The mechanisms by which dantrolene prevented CPVT was attributed to stabilization of mutant RyR2 channels, and possibly also by preventing the PKA-induced reduction in calmodulin binding to RyR237. In this study, dantrolene did not exert any appreciable effects on cardiac function in hearts of wild-type mice. However, dantrolene did correct defective interdomain interactions within RyR2 isolated from dogs with heart failure, associated with suppression of delayed afterdepolarizations31.
Figure 1.
Dantrolene inhibits catecholaminergic polymorphic ventricular tachycardia in mice. (A) Representative images of Ca2+ sparks in cardiomyocytes isolated from heterozygous RyR2-R2474S/+ knock-in mice, showing that dantrolene reduces Ca2+ spark frequency following isoproterenol exposure. (B) Bar graph showing that dantrolene suppresses abnormal Ca2+ spark frequency in RyR2-R2474S/+ mutant mice after isoproterenol exposure. (C) Telemetric ECG recordings reveal exercise-induced ventricular tachycardia in a RyR2-R2474S/+ mouse, which was suppressed by dantrolene. (D) Bar graphs revealing that dantrolene suppresses the incidence of exercise or epinephrine induced ventricular tachycardia (VT) in RyR2-R2474S/+ mice. Adapted from Kobayashi et al36.
In other studies, dantrolene has been described to improve cardiac contractility in failing hearts, which may contribute to its role in reducing arrhythmias in structural heart disease. Congestive heart failure (CHF) has been associated with a negative force-frequency relationship (Bowditch effect) in failing myocardium. Dantrolene has been shown to ameliorate the negative force-frequency relationship in explanted failing myocardial muscle strips by improving inotropic response to isoproterenol38. This improved contractility was not associated with overall changes in cytoplasmic [Ca2+], and was postulated to be associated with improvement of diastolic Ca2+ release. Further evidence for reduction of Ca2+ release events in improved contractility was shown by Kobayashi et al31, who examined the effects of dantrolene on cardiac function on failing hearts. Dantrolene was shown to directly bind near the N-terminal domain in RyR2 at amino acids 601–620, a site critical for N-terminal and central domain interactions. Whereas unzipping of N-terminal and central domains was associated with spontaneous SR Ca2+ leak, dantrolene suppressed both unzipping and SR Ca2+ leak (sparks) and ultimately delayed afterdepolarizations, which are common in heart failure. Additionally, dantrolene restores calmodulin (CaM) binding to RyR2, which is usually attenuated in heart failure39. Thus, dantrolene appears to be a promising molecule to treat arrhythmias in patients with CPVT and may attenuate cardiac Ca2+ handling dysfunction associated with heart failure.
1,4-Benzothiazepines
The benzothiazepine derivative JTV519 (4-[3(1-(4-benzyl)piperidinyl)propionyl]-7-methoxy-2,2,4,5-tetrahydro-1,4-benzothiazepine; also known as K201) was first identified as a compound able to suppress intracellular Ca2+ overload associated with cardiac cell death40. The drug has been reported to have antiarrhythmic effects in a canine model of atrial fibrillation due to sterile pericarditis41 and Langendorff-perfused rat hearts subjected to ischemia-reperfusion42, 43. JTV519 interacts with annexin-V and at higher doses inhibits various voltage-gated ion channels in the heart44, 45. Subsequently, it has become clear that RyR2 represents an important target of JTV51946, 47.
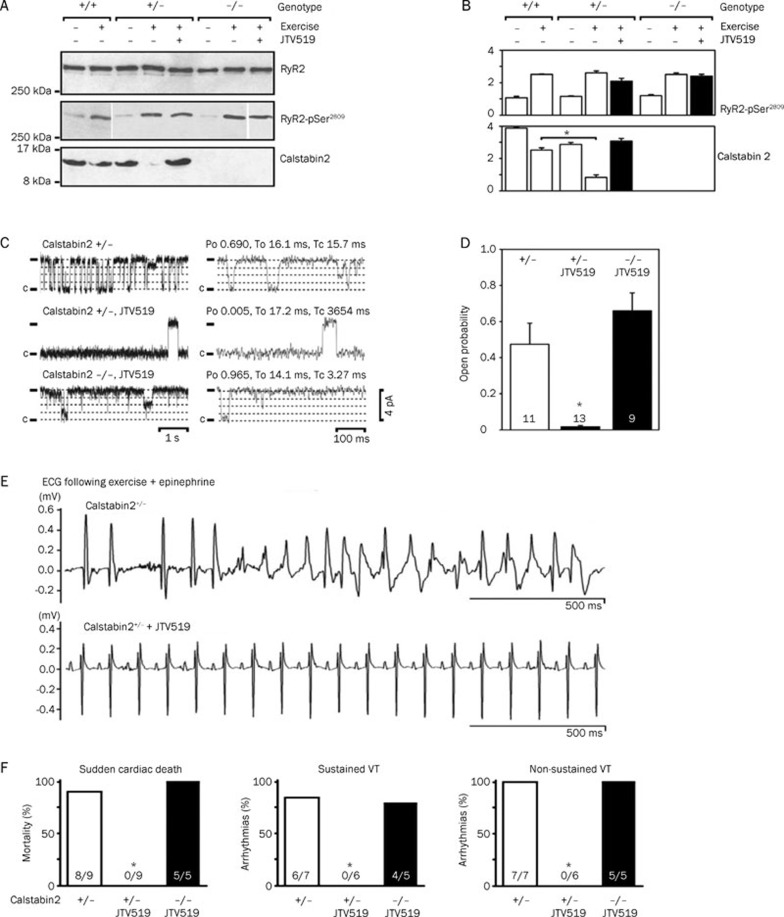
JTV519 was described to normalize RyR2 gating in dogs with tachycardia-induced heart failure48. In this study, Kohno et al48 demonstrated that JTV519 reversed the SR Ca2+ release defects indicative of RyR2 dysfunction. The concept of RyR2 stabilization by FKBP12.6 was further supported by the findings that JTV519 treatment of dogs with pacing-induced heart failure increased the amount of FKBP12.6 immunoprecipitated with RyR246. Moreover, JTV519 was shown to prevent lethal ventricular arrhythmias in mice haploinsufficient for FKBP12.6 by increasing FKBP12.6 binding to RyR247 (Figure 2). The lack of efficacy of JTV519 in FKBP12.6 deficient mice suggests that FKBP12.6 binding to RyR2 is associated with the therapeutic effects of this compound47. On the other hand, normalizing FKBP12.6 levels within the RyR2 macromolecular complex stabilizes the closed state of the channel, thereby preventing aberrant openings during diastole5. Follow-up in vitro studies of human RyR2 mutations found in patients with CPVT (P2328S, Q4201R, and V4653F) showed that JTV519 can also normalize mutant channel gating as evidenced by single channel recordings49.
Figure 2.
Anti-arrhythmic effects of 1,4-benzothiazepine JTV-519 in FKBP12.6+/− mice. (A–B) Representative immunoblots and quantifications for RyR2, RyR2-pSer2809 (PKA phosphorylation site on RyR2), and calstabin2 (FKBP12.6) from wildtype (WT), calstabin2+/− heterozygous, and calstabin2−/− (FKBP12.6−/−) knockout mice. Whereas exercise increased PKA phosphorylation of RyR2 and decreased calstabin2 (FKBP12.6) binding to RyR2, JTV-519 prevented calstabin2 dissociation. (C–D) Representative single channel recordings in planar lipid bilayers showing that JVT519 reduced the open probability of RyR2 isolated from calstabin2+/− but not calstabin2−/− mice, consistent with calstabin2 (FKBP12.6) being required for the therapeutic effects of JTV519. (E–F) ECG tracings showing that JTV-519 reduces ventricular arrhythmias in calstabin2+/− but not calstabin2−/− mice. *P<0.05. Adapted from Wehrens et al47.
There has been some controversy whether the antiarrhythmic effects of JTV519 require modification of RyR2-FKBP12.6 interactions50. In a mouse model of CPVT caused by the R4496C mutation in RyR2, it was shown that this mutation did not alter FKBP12.6 binding affinity for RyR2. Moreover, JTV519 did not prevent delayed afterdepolarizations in myocytes isolated from heterozygous RyR2-R4496C/+ mice51. However, subsequent studies by other groups have shown that JTV519 did reduce the occurrence of spontaneous action potentials in ouabain-treated WT and RyR2-R4496/+ mouse myocytes, presumably independent of FKBP12.652. Additionally, in vitro studies in HEK293 cells suggest that JTV519 suppresses store-overload induced Ca2+ release independently of FKBP12.6 binding, though the relevance of these observations have yet to be determined in vivo50. Also, Yamamoto et al53 reported that JTV519 directly bound to RyR2 between amino acids 2114 and 2149, and that JTV519 can normalize defective interdomain interactions associated with RyR2 dysfunction.
Recently S107, a new 1,4-benzothiazepine similar to JTV519, has been found to prevent ventricular arrhythmias in a CPVT mouse model heterozygous for mutation R2474S in RyR2 54. In contrast to JTV519, S107 has been reported to lack off-target activity for ion channels other than RyR2 at concentrations up to 10 mmol/L54, 55. S107 provided protection against epinephrine-induced ventricular tachycardias caused by abnormal SR Ca2+ leak in RyR2-R2474S/+ mice54. Further, S107 was recently shown to be effective at preventing ventricular arrhythmias in the mdx mouse model of muscular dystrophy56. Thus, 1,4-benzothiazepine derivatives JTV519 and S107 hold promise as RyR2-stabilizing molecules that could reduce the risk of arrhythmias57.
Flecainide
Flecainide is a trifluoroethoxybenzamide that was discovered to be a potent antiarrhythmic agent in 197758. Flecainide initially showed promise as an antiarrhythmic agent against both ventricular59 and atrial arrhythmias60. Because a predominant mechanism of action on inactivation of voltage-gated Na+ channels, it was classified as a type IC anti-arrhythmic drug. However, clinical trial results indicated that in patients with structural heart disease susceptible to ventricular arrhythmias, flecainide might in fact be pro-arrhythmogenic61, 62.
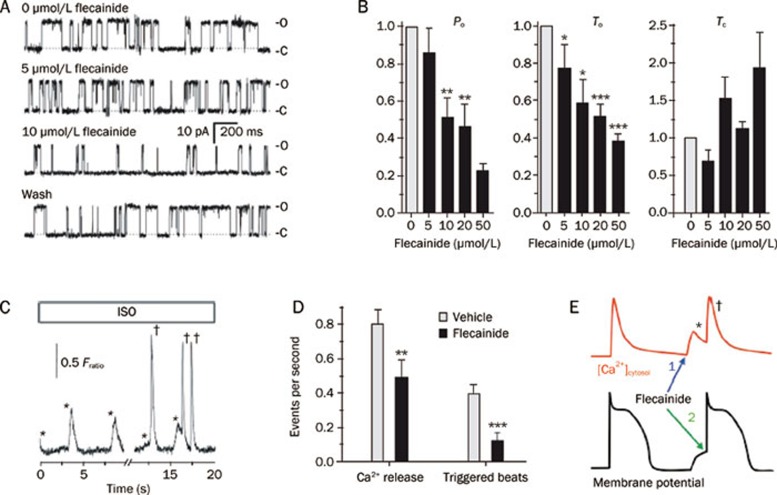
Recently, there has been a resurgence of enthusiasm for the use of flecainide in a select group of CPVT patients with genetic predisposition to ventricular arrhythmias and SCD. Watanabe et al63 found that flecainide inhibited the RyR2 channel by reducing the duration of RyR2 channel openings without affecting closed channel duration. Flecainide reduced SR Ca2+ release events and triggered arrhythmic beats in a calsequestrin deficient (Casq2−/−) mouse model of CPVT 63 (Figure 3). Moreover, it was shown that flecainide significantly reduced the incidence of exercise-induced arrhythmias in patients with mutations in CASQ263. Follow-up studies from the same group showed that flecainide reduced Ca2+ spark mass but increased spark frequency, resulting in a net neutral effect on SR Ca2+ leak and SR Ca2+ content64. This finding is distinct from the reported mechanism of tetracaine, another RyR2 channel blocking agent, which reduces Ca2+ sparks and SR leak, thereby increasing SR Ca2+ content. Therefore, it was concluded that flecainide promoted block of the RyR2 open state, reducing the “probability of saltatory wave propagation between adjacent Ca2+ release units”64.
Figure 3.
Prevention of triggered arrhythmias by flecainide. (A–B) Concentration-dependent effects of flecainide on single sheep RyR2 channels in lipid bilayers. Flecainide decreases open probability (Po) and mean open time (To), and does not significantly alter the mean closed time (Tc) of RyR2. *P<0.02, **P<0.01 and ***P<0.001. (C–D) Effects of flecainide on isoproterenol (ISO) stimulated calsequestrin-deficient cardiomyocytes. Whereas ISO evoked spontaneous SR Ca2+ release events (*), flecainide reduced the number of Ca2+ releases and triggered beats (†). **P=0.0078 and ***P <0.001. (E) Cartoon illustrating dual effects of flecainide action on SR Ca2+ release (red tracing) and inhibition of premature beats triggered by delayed after depolarization (black tracing). Adapted from Watanabe et al63.
Other groups have applied these findings to further delineate the mechanism of arrhythmogenesis in another mouse model of CPVT. Knock-in mice heterozygous for mutation R4496/+ in RyR2 were crossed with Cntn2-EGFP transgenic mice expressing a fluorescent marker for the cardiac conduction system65. Whereas tetracaine reduced spontaneous SR Ca2+ release events in ventricular myocytes and Purkinje cells equally, flecainide more specifically targeted mutant RyR2 in Purkinje cells, implicating the Purkinje conduction system as a potent mediator of ventricular arrhythmias in CPVT. Thus, flecainide may have a unique role in the prevention and suppression of ventricular arrhythmias in patients with genetically inherited CPVT.
Modulation of RyR2 posttranslational modification
In addition to inherited mutations, RyR2 channel function may also be perturbed due to acquired changes in, for example, channel posttranslational modulation2. Xu et al20 demonstrated that increased S-nitrosylation leads to enhanced RyR2 activity and promotes SR Ca2+ release. Increased S-nitrosylation of RyR2 has been associated with cardiac arrhythmias in a mouse model of Duchenne's muscular dystrophy, and inhibition with S107 (see above) was shown to normalize both RyR1 and RyR2 function and prevent arrhythmias56, 66. In contrast, Gonzalez et al17 demonstrated that decreased rather than increased S-nitrosylation of RyR2 might promote SR Ca2+ leak and arrhythmogenesis. One explanation of this apparent paradox relates to nitroso-redox imbance, a condition in which excess formation of reactive oxygen species (ROS) can modify the same thiols that are also target of S-nitrosylation67. Indeed, Gonzalez et al67 reported evidence for increased oxidation of RyR2 associated with an increased tendency towards SR Ca2+ leak in rats with heart failure. In this particular study, increased oxidative stress was primarily the result of enhanced xanthine oxidase (XO) activity. Pharmacological inhibition of XO restored both the nitroso-redox imbalance and intracellular Ca2+ release defects in these rats with heart failure67. Moreover, Niggli's group showed that anti-oxidants (ie, MPG and Mn-cpx3) normalized abnormal RyR sensitivity and hypersensitive E-C coupling in dystrophic cardiomyocytes68.
Increased oxidative stress might also promote activation of Ca2+/calmodulin-dependent protein kinase, which can phosphorylate RyR2 along with other Ca2+ handling proteins, and increase the propensity towards cardiac arrhythmias69. We have previously shown that CaMKII phosphorylation of RyR2 leads to increased channel open probability19. Recently, we demonstrated that constitutive phosphorylation of RyR2 by CaMKII in RyR2-S2814D knock-in mice promoted abnormal SR Ca2+ release events associated with ectopic activity and ventricular arrhythmias10. On the other hand, genetic inhibition of RyR2 phosphorylation at S2814 in RyR2-S2814A knock-in mice conferred protection against ventricular arrhythmias in mice with heart failure induced by transverse aortic banding10. These studies suggest that inhibition of RyR2 phosphorylation by CaMKII might provide a very specific way of preventing ventricular and also atrial arrhythmias6. Moreover, pharmacological inhibition of the enzyme CaMKII itself might also provide protection against arrhythmias6, 70, 71.
RyR2 is also regulated by protein kinase A (PKA) phosphorylation, and increased PKA phosphorylation of RyR2 has been observed in patients with atrial fibrillation7. Shan et al57 demonstrated that mice in which RyR2 was constitutively by PKA (RyR2-S2808D knock-in mice) exhibited an increased open probability, more calcium sparks, and increased SR Ca2+ leak. Inhibition of PKA phosphorylation of RyR2 in RyR2-S2808A mice was shown to protect against catecholamine-induced ventricular arrhythmias72. Although there are currently no drugs that specifically reduce RyR2 phosphorylation, beta blockers such as carvedilol have been shown to reduce RyR2 phosphorylation and thereby RyR2 open probability in patients with atrial fibrillation73. In addition, some beta blockers such as carvedilol also have antioxidant properties in addition to beta-adrenergic blockade, and may be useful in prolongation of arrhythmia-free survival in patients with congestive heart failure versus beta blockers lacking anti-oxidant properties74. Clearly, further pharmacological studies would be needed to determine whether modulating post-translational modifications of RyR2 represents a suitable anti-arrhythmic strategy.
Conclusions
Cardiac arrhythmias is a potentially life-threatening complication of genetic and structural heart disease. Recent insights into excitation-contraction coupling have implicated release of SR Ca2+ through RyR2 as a key mechanism for the initiation and maintenance of both atrial and ventricular arrhythmias. RyR2-mediated release of SR Ca2+ is a tightly regulated process that involves discrete release of Ca2+ during systole, and cessation of Ca2+ release during diastole. For timely rhythmic release of Ca2+ from RyR2, the channel must succinctly open in response to cytoplasmic Ca2+ flux, but remain closed during diastolic SR Ca2+ filling. Destabilization of RyR2 may occur as a result of genetic mutations (ie, CPVT) or acquired (eg, oxidation, nitrosylation, phosphorylation) modifications, resulting in pathologic diastolic Ca2+ release, and subsequent arrhythmias.
Given the prominent role of RyR2 in SR Ca2+ release, pharmacological strategies to modulate RyR2 stability and gating have shown great promise as a therapy for cardiac arrhythmias. Several drugs targeting RyR2, such as benzothiazepine derivatives and flecainide, bind RyR2 directly and reduce the open probability of RyR2, thereby reducing pathological SR Ca2+ “leak”. As benzothiazepines and flecainide have an additional role in blockade of voltage-gated sodium channels and delayed rectifier potassium channels, there may be an additive effect on anti-arrhythmic action, however further studies are necessary to evaluate whether this may occur independently of RyR2 blockade or enhancement of RyR2-FKBP12.6 binding. Additionally, dantrolene has been shown to bind directly to RyR2 and stabilize inter-domain regions, although the effects on RyR2 open probability are still controversial. Each of these drugs has a unique mechanism of action, as dantrolene stabilizes N-terminal and central domain interactions, benzothiazepines increase FKBP12.6 (calstabin2) binding to RyR2 (among other mechanisms), and flecainide blocks the open state of the channel. These drugs have proven particularly helpful in CPVT, in which stabilization of RyR2 reduces diastolic SR Ca2+ leak, and therefore reduces delayed afterdepolarizations.
In patients with structural heart disease, such as in congestive heart failure, acquired alterations in RyR2 function occur primarily due to post-translational modification of the channel. There is extensive evidence that hyperphosphorylation of RyR2 in heart failure also promotes the occurrence of SR Ca2+ leak10, 18, 72, 75. By genetic or pharmacological blockade of RyR2 phosphorylation at CaMKII or PKA site, animal models have shown that effective arrhythmia prophylaxis is possible, especially under catecholaminergic conditions or after stress exercise. Recent insights also indicate that adverse redox remodeling of RyR2 may predispose to cardiac arrhythmias. Emerging data suggest that certain beta-adrenergic blocking agents, such as carvedilol, may also exert a redox-stabilizing effect on RyR2, which may potentially increase survival in patients with acquired heart disease. Ultimately, these insights will guide the design of future studies in human patients, whereby stabilization of the RyR2 channel might lead to improved outcomes in morbidity and mortality.
Acknowledgments
Dr McCauley is supported by NHLBI training grant 5T32HL066991-07. Dr WEHRENS is a WM Keck Foundation Distinguished Young Scholar in Medical Research and is supported by NHLBI grants R01-HL089598 and R01-HL091947, and Muscular Dystrophy Association grant 186531. This work was also supported in part by the Fondation Leducq Alliance for CaMKII Signaling in Heart.
References
- Bers DM. Cardiac excitation-contraction coupling. Nature. 2002;415:198–205. doi: 10.1038/415198a. [DOI] [PubMed] [Google Scholar]
- Wehrens XH, Lehnart SE, Marks AR. Intracellular calcium release and cardiac disease. Annu Rev Physiol. 2005;67:69–98. doi: 10.1146/annurev.physiol.67.040403.114521. [DOI] [PubMed] [Google Scholar]
- Dobrev D, Wehrens XH. Calmodulin kinase II, sarcoplasmic reticulum Ca2+ leak, and atrial fibrillation. Trends Cardiovasc Med. 2010;20:30–4. doi: 10.1016/j.tcm.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laitinen PJ, Brown KM, Piippo K, Swan H, Devaney JM, Brahmbhatt B, et al. Mutations of the cardiac ryanodine receptor (RyR2) gene in familial polymorphic ventricular tachycardia. Circulation. 2001;103:485–90. doi: 10.1161/01.cir.103.4.485. [DOI] [PubMed] [Google Scholar]
- Wehrens XH, Lehnart SE, Huang F, Vest JA, Reiken SR, Mohler PJ, et al. FKBP12.6 deficiency and defective calcium release channel (ryanodine receptor) function linked to exercise-induced sudden cardiac death. Cell. 2003;113:829–40. doi: 10.1016/s0092-8674(03)00434-3. [DOI] [PubMed] [Google Scholar]
- Chelu MG, Sarma S, Sood S, Wang S, van Oort RJ, Skapura DG, et al. Calmodulin kinase II-mediated sarcoplasmic reticulum Ca2+ leak promotes atrial fibrillation in mice. J Clin Invest. 2009;119:1940–51. doi: 10.1172/JCI37059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vest JA, Wehrens XH, Reiken SR, Lehnart SE, Dobrev D, Chandra P, et al. Defective cardiac ryanodine receptor regulation during atrial fibrillation. Circulation. 2005;111:2025–32. doi: 10.1161/01.CIR.0000162461.67140.4C. [DOI] [PubMed] [Google Scholar]
- Mathur N, Sood S, Wang S, van Oort RJ, Sarma S, Li N, et al. Sudden infant death syndrome in mice with an inherited mutation in RyR2. Circ Arrhythm Electrophysiol. 2009;2:677–85. doi: 10.1161/CIRCEP.109.894683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priori SG, Napolitano C, Tiso N, Memmi M, Vignati G, Bloise R, et al. Mutations in the cardiac ryanodine receptor gene (hRyR2) underlie catecholaminergic polymorphic ventricular tachycardia. Circulation. 2001;103:196–200. doi: 10.1161/01.cir.103.2.196. [DOI] [PubMed] [Google Scholar]
- van Oort RJ, McCauley MD, Dixit SS, Pereira L, Yang Y, Respress JL, et al. Ryanodine receptor phosphorylation by calcium/calmodulin-dependent protein kinase II promotes life-threatening ventricular arrhythmias in mice with heart failure. Circulation. 2010;122:2669–79. doi: 10.1161/CIRCULATIONAHA.110.982298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehnart SE, Terrenoire C, Reiken S, Wehrens XH, Song LS, Tillman EJ, et al. Stabilization of cardiac ryanodine receptor prevents intracellular calcium leak and arrhythmias. Proc Natl Acad Sci U S A. 2006;103:7906–10. doi: 10.1073/pnas.0602133103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paavola J, Viitasalo M, Laitinen-Forsblom PJ, Pasternack M, Swan H, Tikkanen I, et al. Mutant ryanodine receptors in catecholaminergic polymorphic ventricular tachycardia generate delayed afterdepolarizations due to increased propensity to Ca2+ waves. Eur Heart J. 2007;28:1135–42. doi: 10.1093/eurheartj/ehl543. [DOI] [PubMed] [Google Scholar]
- McCauley MD, Wehrens XH. Animal models of arrhythmogenic cardiomyopathy. Dis Model Mech. 2009;2:563–70. doi: 10.1242/dmm.002840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landstrom AP, Weisleder N, Batalden KB, Bos JM, Tester DJ, Ommen SR, et al. Mutations in JPH2-encoded junctophilin-2 associated with hypertrophic cardiomyopathy in humans. J Mol Cell Cardiol. 2007;42:1026–35. doi: 10.1016/j.yjmcc.2007.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song L, Alcalai R, Arad M, Wolf CM, Toka O, Conner DA, et al. Calsequestrin 2 (CASQ2) mutations increase expression of calreticulin and ryanodine receptors, causing catecholaminergic polymorphic ventricular tachycardia. J Clin Invest. 2007;117:1814–23. doi: 10.1172/JCI31080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belevych AE, Terentyev D, Viatchenko-Karpinski S, Terentyeva R, Sridhar A, Nishijima Y, et al. Redox modification of ryanodine receptors underlies calcium alternans in a canine model of sudden cardiac death. Cardiovasc Res. 2009;84:387–95. doi: 10.1093/cvr/cvp246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez DR, Beigi F, Treuer AV, Hare JM. Deficient ryanodine receptor S-nitrosylation increases sarcoplasmic reticulum calcium leak and arrhythmogenesis in cardiomyocytes. Proc Natl Acad Sci U S A. 2007;104:20612–7. doi: 10.1073/pnas.0706796104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marx SO, Reiken S, Hisamatsu Y, Jayaraman T, Burkhoff D, Rosemblit N, et al. PKA phosphorylation dissociates FKBP12.6 from the calcium release channel (ryanodine receptor): defective regulation in failing hearts. Cell. 2000;101:365–76. doi: 10.1016/s0092-8674(00)80847-8. [DOI] [PubMed] [Google Scholar]
- Wehrens XH, Lehnart SE, Reiken SR, Marks AR. Ca2+/calmodulin-dependent protein kinase II phosphorylation regulates the cardiac ryanodine receptor. Circ Res. 2004;94:e61–70. doi: 10.1161/01.RES.0000125626.33738.E2. [DOI] [PubMed] [Google Scholar]
- Xu L, Eu JP, Meissner G, Stamler JS. Activation of the cardiac calcium release channel (ryanodine receptor) by poly-S-nitrosylation. Science. 1998;279:234–7. doi: 10.1126/science.279.5348.234. [DOI] [PubMed] [Google Scholar]
- Anderson ME, Higgins LS, Schulman H. Disease mechanisms and emerging therapies: protein kinases and their inhibitors in myocardial disease. Nat Clin Pract Cardiovasc Med. 2006;3:437–45. doi: 10.1038/ncpcardio0585. [DOI] [PubMed] [Google Scholar]
- Santonastasi M, Wehrens XH. Ryanodine receptors as pharmacological targets for heart disease. Acta Pharmacol Sin. 2007;28:937–44. doi: 10.1111/j.1745-7254.2007.00582.x. [DOI] [PubMed] [Google Scholar]
- Dulhunty AF, Beard NA, Pouliquin P, Casarotto MG. Agonists and antagonists of the cardiac ryanodine receptor: potential therapeutic agents. Pharmacol Ther. 2007;113:247–63. doi: 10.1016/j.pharmthera.2006.08.007. [DOI] [PubMed] [Google Scholar]
- Wehrens XH, Lehnart SE, Marks AR. Ryanodine receptor-targeted anti-arrhythmic therapy. Ann N Y Acad Sci. 2005;1047:366–75. doi: 10.1196/annals.1341.032. [DOI] [PubMed] [Google Scholar]
- Snyder HR Jr, Davis CS, Bickerton RK, Halliday RP. 1-[(5-arylfurfurylidene)amino]hydantoins. A new class of muscle relaxants. J Med Chem. 1967;10:807–10. doi: 10.1021/jm00317a011. [DOI] [PubMed] [Google Scholar]
- Harrison GG. Control of the malignant hyperpyrexic syndrome in MHS swine by dantrolene sodium. Br J Anaesth. 1975;47:62–5. doi: 10.1093/bja/47.1.62. [DOI] [PubMed] [Google Scholar]
- Durham WJ, Aracena-Parks P, Long C, Rossi AE, Goonasekera SA, Boncompagni S, et al. RyR1 S-nitrosylation underlies environmental heat stroke and sudden death in Y522S RyR1 knockin mice. Cell. 2008;133:53–65. doi: 10.1016/j.cell.2008.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denborough M. Malignant hyperthermia. Lancet. 1998;352:1131–6. doi: 10.1016/S0140-6736(98)03078-5. [DOI] [PubMed] [Google Scholar]
- Paul-Pletzer K, Yamamoto T, Bhat MB, Ma J, Ikemoto N, Jimenez LS, et al. Identification of a dantrolene-binding sequence on the skeletal muscle ryanodine receptor. J Biol Chem. 2002;277:34918–23. doi: 10.1074/jbc.M205487200. [DOI] [PubMed] [Google Scholar]
- Kobayashi S, Bannister ML, Gangopadhyay JP, Hamada T, Parness J, Ikemoto N. Dantrolene stabilizes domain interactions within the ryanodine receptor. J Biol Chem. 2005;280:6580–7. doi: 10.1074/jbc.M408375200. [DOI] [PubMed] [Google Scholar]
- Kobayashi S, Yano M, Suetomi T, Ono M, Tateishi H, Mochizuki M, et al. Dantrolene, a therapeutic agent for malignant hyperthermia, markedly improves the function of failing cardiomyocytes by stabilizing interdomain interactions within the ryanodine receptor. J Am Coll Cardiol. 2009;53:1993–2005. doi: 10.1016/j.jacc.2009.01.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szentesi P, Collet C, Sarkozi S, Szegedi C, Jona I, Jacquemond V, et al. Effects of dantrolene on steps of excitation-contraction coupling in mammalian skeletal muscle fibers. J Gen Physiol. 2001;118:355–75. doi: 10.1085/jgp.118.4.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balam Ortiz EO, Carvajal K, Cruz D. Protective effect of dantrolene in post-ischemic reperfusion myocardial damage. Arch Inst Cardiol Mex. 1999;69:311–9. [PubMed] [Google Scholar]
- Boys JA, Toledo AH, Anaya-Prado R, Lopez-Neblina F, Toledo-Pereyra LH. Effects of dantrolene on ischemia-reperfusion injury in animal models: a review of outcomes in heart, brain, liver, and kidney. J Investig Med. 2010;58:875–82. doi: 10.231/JIM.0b013e3181e5d719. [DOI] [PubMed] [Google Scholar]
- Pelleg A, Roth A, Shargordsky B, Belhassen B, Chagnac A, Laniado S. Effects of dantrolene sodium on occlusion and reperfusion arrhythmias in the canine heart. Methods Find Exp Clin Pharmacol. 1985;7:239–43. [PubMed] [Google Scholar]
- Kobayashi S, Yano M, Uchinoumi H, Suetomi T, Susa T, Ono M, et al. Dantrolene, a therapeutic agent for malignant hyperthermia, inhibits catecholaminergic polymorphic ventricular tachycardia in a RyR2(R2474S/+) knock-in mouse model. Circ J. 2010;74:2579–84. doi: 10.1253/circj.cj-10-0680. [DOI] [PubMed] [Google Scholar]
- Xu X, Yano M, Uchinoumi H, Hino A, Suetomi T, Ono M, et al. Defective calmodulin binding to the cardiac ryanodine receptor plays a key role in CPVT-associated channel dysfunction. Biochem Biophys Res Commun. 2010;394:660–6. doi: 10.1016/j.bbrc.2010.03.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meissner A, Min JY, Haake N, Hirt S, Simon R. Dantrolene sodium improves the force-frequency relationship and beta-adregenic res-ponsiveness in failing human myocardium. Eur J Heart Fail. 1999;1:177–86. doi: 10.1016/s1388-9842(99)00017-3. [DOI] [PubMed] [Google Scholar]
- Ono M, Yano M, Hino A, Suetomi T, Xu X, Susa T, et al. Dissociation of calmodulin from cardiac ryanodine receptor causes aberrant Ca2+ release in heart failure. Cardiovasc Res. 2010;87:609–17. doi: 10.1093/cvr/cvq108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko N. New 1,4-benzothiazepine derivative, K201, demonstrates cardioprotective effects against sudden cardiac cell death and intracellular calcium blocking action. Drug Dev Res. 1994;33:429–38. [Google Scholar]
- Kumagai K, Nakashima H, Gondo N, Saku K. Antiarrhythmic effects of JTV-519, a novel cardioprotective drug, on atrial fibrillation/flutter in a canine sterile pericarditis model. J Cardiovasc Electrophysiol. 2003;14:880–4. doi: 10.1046/j.1540-8167.2003.03050.x. [DOI] [PubMed] [Google Scholar]
- Hachida M, Lu H, Kaneko N, Nonoyama M, Koyanagi H. Protective effect of JTV519 on prolonged myocardial preservation. Transplant Proc. 1999;31:1094. doi: 10.1016/s0041-1345(98)02104-6. [DOI] [PubMed] [Google Scholar]
- Inagaki K, Kihara Y, Hayashida W, Izumi T, Iwanaga Y, Yoneda T, et al. Anti-ischemic effect of a novel cardioprotective agent, JTV519, is mediated through specific activation of delta-isoform of protein kinase C in rat ventricular myocardium. Circulation. 2000;101:797–804. doi: 10.1161/01.cir.101.7.797. [DOI] [PubMed] [Google Scholar]
- Kimura J, Kawahara M, Sakai E, Yatabe J, Nakanishi H. Effects of a novel cardioprotective drug, JTV-519, on membrane currents of guinea pig ventricular myocytes. Jpn J Pharmacol. 1999;79:275–81. doi: 10.1254/jjp.79.275. [DOI] [PubMed] [Google Scholar]
- Nakaya H, Furusawa Y, Ogura T, Tamagawa M, Uemura H. Inhibitory effects of JTV-519, a novel cardioprotective drug, on potassium currents and experimental atrial fibrillation in guinea-pig hearts. Br J Pharmacol. 2000;131:1363–72. doi: 10.1038/sj.bjp.0703713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yano M, Kobayashi S, Kohno M, Doi M, Tokuhisa T, Okuda S, et al. FKBP12.6-mediated stabilization of calcium-release channel (ryanodine receptor) as a novel therapeutic strategy against heart failure. Circulation. 2003;107:477–84. doi: 10.1161/01.cir.0000044917.74408.be. [DOI] [PubMed] [Google Scholar]
- Wehrens XH, Lehnart SE, Reiken SR, Deng SX, Vest JA, Cervantes D, et al. Protection from cardiac arrhythmia through ryanodine receptor-stabilizing protein calstabin2. Science. 2004;304:292–6. doi: 10.1126/science.1094301. [DOI] [PubMed] [Google Scholar]
- Kohno M, Yano M, Kobayashi S, Doi M, Oda T, Tokuhisa T, et al. A new cardioprotective agent, JTV519, improves defective channel gating of ryanodine receptor in heart failure. Am J Physiol Heart Circ Physiol. 2003;284:H1035–42. doi: 10.1152/ajpheart.00722.2002. [DOI] [PubMed] [Google Scholar]
- Lehnart SE, Wehrens XH, Laitinen PJ, Reiken SR, Deng SX, Cheng Z, et al. Sudden death in familial polymorphic ventricular tachycardia associated with calcium release channel (ryanodine receptor) leak. Circulation. 2004;109:3208–14. doi: 10.1161/01.CIR.0000132472.98675.EC. [DOI] [PubMed] [Google Scholar]
- Hunt DJ, Jones PP, Wang R, Chen W, Bolstad J, Chen K, et al. K201 (JTV519) suppresses spontaneous Ca2+ release and [3H]ryanodine binding to RyR2 irrespective of FKBP12.6 association. Biochem J. 2007;404:431–8. doi: 10.1042/BJ20070135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu N, Colombi B, Memmi M, Zissimopoulos S, Rizzi N, Negri S, et al. Arrhythmogenesis in catecholaminergic polymorphic ventricular tachycardia: insights from a RyR2 R4496C knock-in mouse model. Circ Res. 2006;99:292–8. doi: 10.1161/01.RES.0000235869.50747.e1. [DOI] [PubMed] [Google Scholar]
- Sedej S, Heinzel FR, Walther S, Dybkova N, Wakula P, Groborz J, et al. Na+-dependent SR Ca2+ overload induces arrhythmogenic events in mouse cardiomyocytes with a human CPVT mutation. Cardiovasc Res. 2010;87:50–9. doi: 10.1093/cvr/cvq007. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Yano M, Xu X, Uchinoumi H, Tateishi H, Mochizuki M, et al. Identification of target domains of the cardiac ryanodine receptor to correct channel disorder in failing hearts. Circulation. 2008;117:762–72. doi: 10.1161/CIRCULATIONAHA.107.718957. [DOI] [PubMed] [Google Scholar]
- Lehnart SE, Mongillo M, Bellinger A, Lindegger N, Chen BX, Hsueh W, et al. Leaky Ca2+ release channel/ryanodine receptor 2 causes seizures and sudden cardiac death in mice. J Clin Invest. 2008;118:2230–45. doi: 10.1172/JCI35346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellinger AM, Reiken S, Dura M, Murphy PW, Deng SX, Landry DW, et al. Remodeling of ryanodine receptor complex causes “leaky” channels: a molecular mechanism for decreased exercise capacity. Proc Natl Acad Sci U S A. 2008;105:2198–202. doi: 10.1073/pnas.0711074105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fauconnier J, Thireau J, Reiken S, Cassan C, Richard S, Matecki S, et al. Leaky RyR2 trigger ventricular arrhythmias in Duchenne muscular dystrophy. Proc Natl Acad Sci U S A. 2010;107:1559–64. doi: 10.1073/pnas.0908540107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shan J, Betzenhauser MJ, Kushnir A, Reiken S, Meli AC, Wronska A, et al. Role of chronic ryanodine receptor phosphorylation in heart failure and beta-adrenergic receptor blockade in mice. J Clin Invest. 2010;120:4375–87. doi: 10.1172/JCI37649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banitt EH, Bronn WR, Coyne WE, Schmid JR. Antiarrhythmics. 2. Synthesis and antiarrhythmic activity of N-(piperidylalkyl)trifluoroethoxybenzamides. J Med Chem. 1977;20:821–6. doi: 10.1021/jm00216a016. [DOI] [PubMed] [Google Scholar]
- Anderson JL, Stewart JR, Perry BA, Van Hamersveld DD, Johnson TA, Conard GJ, et al. Oral flecainide acetate for the treatment of ventricular arrhythmias. N Engl J Med. 1981;305:473–7. doi: 10.1056/NEJM198108273050901. [DOI] [PubMed] [Google Scholar]
- Anderson JL, Gilbert EM, Alpert BL, Henthorn RW, Waldo AL, Bhandari AK, et al. Prevention of symptomatic recurrences of paroxysmal atrial fibrillation in patients initially tolerating antiarrhythmic therapy. A multicenter, double-blind, crossover study of flecainide and placebo with transtelephonic monitoring. Flecainide Supraventricular Tachycardia Study Group. Circulation. 1989;80:1557–70. doi: 10.1161/01.cir.80.6.1557. [DOI] [PubMed] [Google Scholar]
- Muhiddin K, Nathan AW, Hellestrand KJ, Banim SO, Camm AJ. Ventricular tachycardia associated with flecainide. Lancet. 1982;2:1220–1. doi: 10.1016/s0140-6736(82)91238-7. [DOI] [PubMed] [Google Scholar]
- Preliminary report: effect of encainide and flecainide on mortality in a randomized trial of arrhythmia suppression after myocardial infarction. The Cardiac Arrhythmia Suppression Trial (CAST) Investigators. N Engl J Med. 1989;321:406–12. doi: 10.1056/NEJM198908103210629. [DOI] [PubMed] [Google Scholar]
- Watanabe H, Chopra N, Laver D, Hwang HS, Davies SS, Roach DE, et al. Flecainide prevents catecholaminergic polymorphic ventricular tachycardia in mice and humans. Nat Med. 2009;15:380–3. doi: 10.1038/nm.1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilliard FA, Steele DS, Laver D, Yang Z, Le Marchand SJ, Chopra N, et al. Flecainide inhibits arrhythmogenic Ca2+ waves by open state block of ryanodine receptor Ca2+ release channels and reduction of Ca2+ spark mass. J Mol Cell Cardiol. 2010;48:293–301. doi: 10.1016/j.yjmcc.2009.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang G, Giovannone SF, Liu N, Liu FY, Zhang J, Priori SG, et al. Purkinje cells from RyR2 mutant mice are highly arrhythmogenic but responsive to targeted therapy. Circ Res. 2010;107:512–9. doi: 10.1161/CIRCRESAHA.110.221481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellinger AM, Reiken S, Carlson C, Mongillo M, Liu X, Rothman L, et al. Hypernitrosylated ryanodine receptor calcium release channels are leaky in dystrophic muscle. Nat Med. 2009;15:325–30. doi: 10.1038/nm.1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez DR, Treuer AV, Castellanos J, Dulce RA, Hare JM. Impaired S-nitrosylation of the ryanodine receptor caused by xanthine oxidase activity contributes to calcium leak in heart failure. J Biol Chem. 2010;285:28938–45. doi: 10.1074/jbc.M110.154948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullrich ND, Fanchaouy M, Gusev K, Shirokova N, Niggli E. Hypersensitivity of excitation-contraction coupling in dystrophic cardiomyocytes. Am J Physiol Heart Circ Physiol. 2009;297:H1992–2003. doi: 10.1152/ajpheart.00602.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson JR, Joiner ML, Guan X, Kutschke W, Yang J, Oddis CV, et al. A dynamic pathway for calcium-independent activation of CaMKII by methionine oxidation. Cell. 2008;133:462–74. doi: 10.1016/j.cell.2008.02.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sag CM, Wadsack DP, Khabbazzadeh S, Abesser M, Grefe C, Neumann K, et al. Calcium/calmodulin-dependent protein kinase II contributes to cardiac arrhythmogenesis in heart failure. Circ Heart Fail. 2009;2:664–75. doi: 10.1161/CIRCHEARTFAILURE.109.865279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson JR, Anderson ME. CaMKII and its role in cardiac arrhythmia. J Cardiovasc Electrophysiol. 2008;19:1332–6. doi: 10.1111/j.1540-8167.2008.01295.x. [DOI] [PubMed] [Google Scholar]
- Lehnart SE, Wehrens XH, Reiken S, Warrier S, Belevych AE, Harvey RD, et al. Phosphodiesterase 4D deficiency in the ryanodine-receptor complex promotes heart failure and arrhythmias. Cell. 2005;123:25–35. doi: 10.1016/j.cell.2005.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiken S, Wehrens XH, Vest JA, Barbone A, Klotz S, Mancini D, et al. Beta-blockers restore calcium release channel function and improve cardiac muscle performance in human heart failure. Circulation. 2003;107:2459–66. doi: 10.1161/01.CIR.0000068316.53218.49. [DOI] [PubMed] [Google Scholar]
- Poole-Wilson PA, Swedberg K, Cleland JG, Di Lenarda A, Hanrath P, Komajda M, et al. Comparison of carvedilol and metoprolol on clinical outcomes in patients with chronic heart failure in the Carvedilol Or Metoprolol European Trial (COMET): randomised controlled trial. Lancet. 2003;362:7–13. doi: 10.1016/S0140-6736(03)13800-7. [DOI] [PubMed] [Google Scholar]
- Wehrens XH, Lehnart SE, Reiken S, Vest JA, Wronska A, Marks AR. Ryanodine receptor/calcium release channel PKA phosphorylation: a critical mediator of heart failure progression. Proc Natl Acad Sci U S A. 2006;103:511–8. doi: 10.1073/pnas.0510113103. [DOI] [PMC free article] [PubMed] [Google Scholar]