Abstract
The prevention or delay of the onset of age-related diseases prolongs survival and improves quality of life while reducing the burden on the health care system. Activation of sirtuin 1 (SIRT1), an NAD+ deacetylase, improves metabolism and confers protection against physiological and cognitive disturbances in old age. SRT1720 is a specific SIRT1 activator that has health and lifespan benefits in adult mice fed a high-fat diet. We found extension in lifespan, delayed onset of age-related metabolic diseases, and improved general health in mice fed a standard diet after SRT1720 supplementation. Inhibition of pro-inflammatory gene expression both in the liver and muscle of SRT1720-treated animals was noted. SRT1720 lowered phosphorylation of NF-κB pathway regulators in vitro only when SIRT1 was functionally present. Combined with our previous work, the current study further supports the beneficial effects of SRT1720 on health across the lifespan in mice.
Keywords: SRT1720, healthspan, standard diet, mice, longevity, SIRT1
INTRODUCTION
Many of the chronic diseases that exist in older adults constitute a highly significant social and economic burden to the community. Long-term illness, diminished quality of life, and increased health care costs are all challenges that older adults are facing. Sirtuin 1 (SIRT1), an NAD+-dependent deacetylase, is one of the seven mammalian sirtuins and is known to play an important role in maintaining metabolic homeostasis in multiple tissues (Baur et al., 2012). SIRT1 has been proposed as an anti-aging protein, and its activation results in health benefits in multiple organisms (Baur et al., 2012). Pharmacological interventions aimed at increasing SIRT1 activity have been found to slow the onset of aging and delay age-associated diseases. There is mounting evidence that overexpression of SIRT1 in mice and use of small molecule activators of SIRT1, such as resveratrol and SRT1720, enhance insulin sensitivity and protect against diet-induced impairments in mitochondrial capacity and oxidative metabolism in laboratory animals (Banks et al., 2008; Feige et al., 2008; Milne et al., 2007; Pfluger et al., 2008). Even though SIRT1 activators improve health and extend lifespan of mice maintained on a high-fat diet (Baur et al., 2006; Minor et al., 2011), there has yet to be a demonstration of both improvements in lifespan and healthspan in mice fed a standard diet (SD). For example, resveratrol improves healthspan of some mice fed a SD; yet, no effect on lifespan was detected in C57BL6/J mice (Pearson et al., 2008) or in genetically heterogeneous mice (Strong et al., 2013). Other compounds that extend lifespan in mice include the immunomodulatory drug rapamycin and the insulin-sensitizing agent, metformin. However, chronic administration of rapamycin induces glucose intolerance, insulin resistance and incidence of diabetogenic effects (Deblon et al., 2012; Houde et al., 2010; Lamming et al., 2012) despite lifespan extension in mice (Harrison et al., 2009). Similarly, metformin is associated with adverse side effects, including lactic acidosis and kidney dysfunction in a dose-dependent manner (Martin-Montalvo et al., 2013). Here, we show that SRT1720 supplementation extends survival and improves health in mice fed a standard diet.
RESULTS AND DISCUSSION
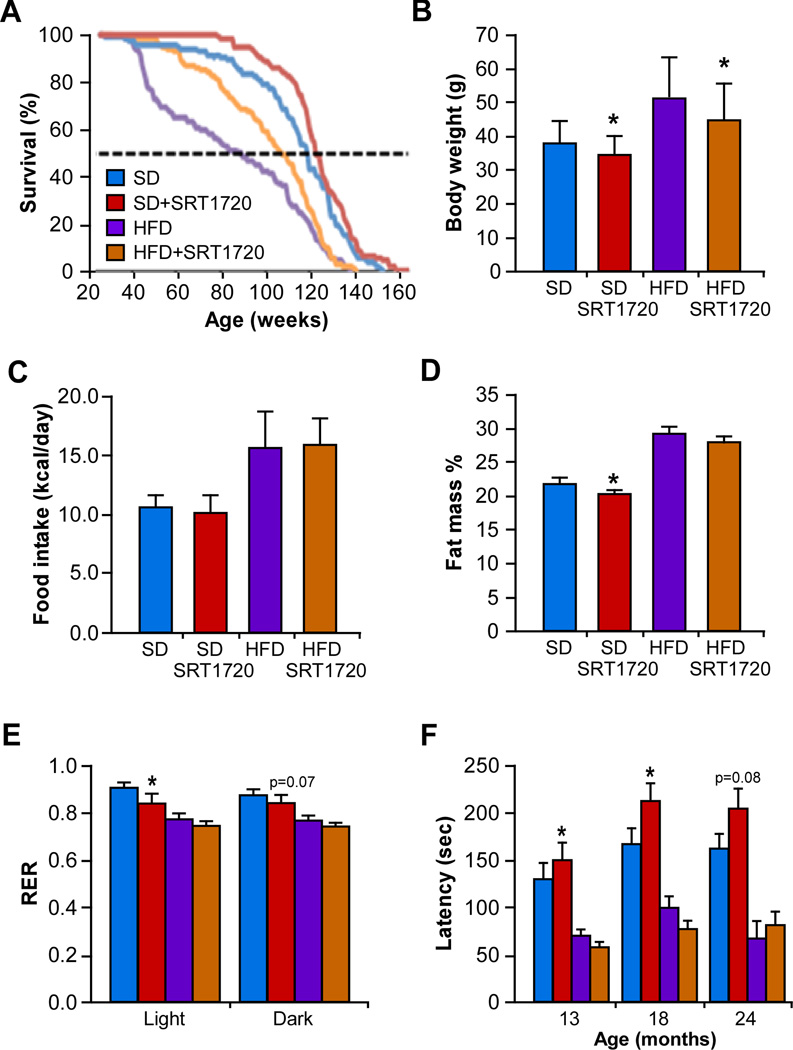
To test the effect of SRT1720 on the health and lifespan of C57BL6/J mice, these animals were fed either a high-fat diet (HFD) or a standard AIN-93G diet (SD) supplemented with 100mg/kg SRT1720 beginning at 6 months of age for the remainder of their life. SRT1720 treatment significantly extends mean lifespan of mice on both diets (Figure 1A). Survival among the SD-fed groups was significantly different as assessed via the non-parametric log-rank (Mantel-Haenszel) test (χ2=4.168, p=0.0412). Mean lifespan was increased 8.8% and we observed a trend towards an increase in median lifespan of 5 weeks (Su and Wei, 1993) with SRT1720 supplementation (χ2=2.77, p=0.096). However, there was no difference in 90th percentile survival (Wang et al., 2004) despite the overall significant effect on lifespan. This data are consistent with the notion that in the absence of a metabolic insult, interventions such as SRT1720 will have a more pronounced effect in delaying the onset of age-associated diseases. In the HFD groups, survival was significantly lower based on the non-parametric Log-rank (Mantel-Haenszel) test (χ2=5.470, p=0.0193), and SRT1720 supplementation increased mean lifespan by 21.7%, in agreement with our previous study (Minor et al., 2011), although maximum lifespan did not change (Figure 1A). Median survival was significantly increased by 22 weeks in HFD-fed mice treated with SRT1720 (χ2=5.23, p=0.022), indicating an improvement in general health in these mice (Figure 1A). The hazard ratio for death was significantly reduced with SRT1720 treatment (HR=0.73, 95% CI (0.59, 0.90)], p=0.0034), indicating a positive effect of SRT1720 on improving lifespan in mice. SRT1720 significantly reduced the average body weight of HFD-fed mice (Figure 1B) despite no difference in daily caloric intake (Figure 1C) or food consumption (Figure S1a). As expected, mice on HFD weighed significantly more than their SD-fed counterparts across the course of the study (Figure S1b), and SRT1720 supplementation also reduced both average body weight (Figure 1B) and body weight in SD-fed animals (Figure S1b) without impacting on daily caloric intake (Figure 1C). Treatment with SRT1720 significantly reduced body fat percentage in SD-fed animals (Figure 1D), despite no difference in lean-to-fat ratio (Figure S1c). However, the lack of difference for either parameter in HFD-treated mice is in contrast with our previous study (Minor et al., 2011), which may be due to the time-point at which the treatment was started (6 months vs. 12 months).
Figure 1. SRT1720 extends lifespan and improves health in mice fed a standard diet.
(A) Kaplan-Meier survival curves for mice fed either a standard diet (SD) or high-fat diet (HFD) supplemented without or with SRT1720 (SD-SRT1720, HFD-SRT1720); (B) Average body weight over the study; (C) Average daily caloric intake over the study; (D) Percentage fat mass measured by nuclear magnetic resonance spectroscopy at 13 months of age; (E) Respiratory Exchange Ratio (RER); (F) Rotarod performance. Data are shown as mean ± SEM. * p≤0.05 compared to diet without SRT1720.
Recent studies have illustrated the beneficial cardioprotective effects of SRT1720 against ischemia-reperfusion injury and the loss of cardiac function that occurs with aging (Tong et al., 2012). SRT1720 also confers protection from atherosclerosis in vitro (Zeng et al., 2013) via SIRT1 activation. The synchrony in circadian rhythms leads to increased longevity and improved health (Froy, 2011). Interestingly, SRT1720 is essential for the proper regulation of circadian rhythm in the liver of SD-fed mice through a SIRT1-dependent mechanism (Bellet et al., 2013). Although some controversy exists concerning the specificity of SRT1720 toward SIRT1 (Huber et al., 2010; Pacholec et al., 2010), the notion that SIRT1 is a target of SRT1720 is supported by recent data showing that it activates SIRT1 via a direct allosteric mechanism (Hubbard et al., 2013). Taken together, these results illustrate the involvement of multiple SIRT1-dependent mechanisms in the biological actions of SRT1720.
We assessed metabolism using the CLAMS system and found that SRT1720 supplementation significantly lowered respiratory exchange ratio (RER) in SD-fed animals during the light cycle, with a trend toward a reduction during the dark cycle (Figure 1E). No difference in locomotor activity between groups was observed (Figure S1d). The RER for HFD-fed mice was close to 0.7, indicating a preference for fatty acid oxidation (Figure 1E). Interestingly, increased fatty acid oxidation in SRT1720-treated SD-fed mice, as evidenced by a decrease of RER, could explain the lower percentage of fat mass in these animals (Figure 1D). In addition to the lifespan extension, it was also important to ascertain whether there was improvement in the quality of life of SRT1720-treated mice through assessment of balance and motor coordination. SRT1720 significantly improved rotarod performance at 13 and 18 months of age in SD-fed mice, with a nearly significant (p=0.08) improvement in performance at 24 months of age (Figure 1F). No beneficial effects of SRT1720 were observed in HFD-fed mice (Figure 1F). While it is recognized that there is both a learning and body weight component to the rotarod performance test, a recent study has shown that improvement in rotarod performance can be independent of body weight and that diet may have a larger effect on neurobehavior than either learning or body weight (Kovacs and Pearce, 2013). Overall these data indicate that SRT1720 improves muscle function and motor coordination over the life of SD-fed mice. For the remainder of the study, we focused on the effects of SRT1720 on various metabolic variables in SD-fed mice.
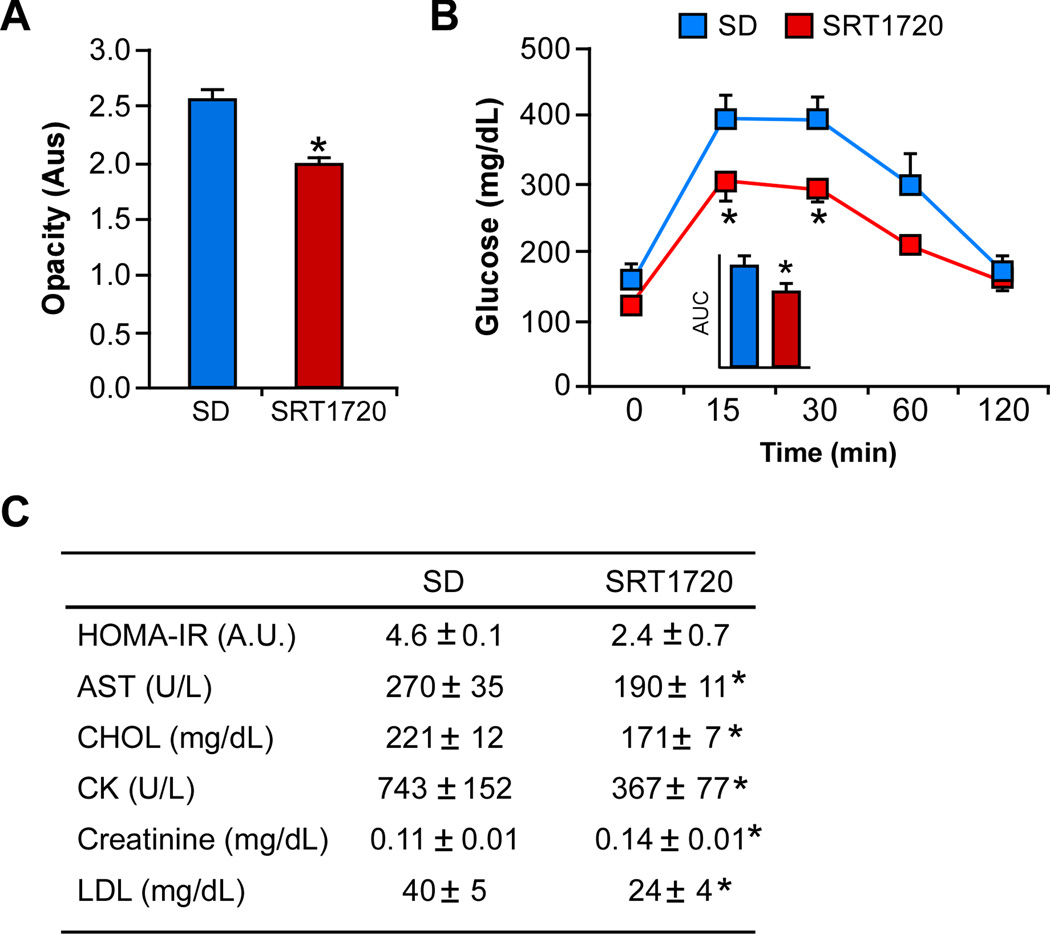
Because aging is associated with a functional and metabolic decline, we assessed the effects of SRT1720 on several variables of aging. The increase in cataract formation with age has been attributed, in part, to the accumulation of reactive oxygen species (Wolf et al., 2005). Here, a marked reduction in cataract opacity was observed in SRT1720-treated SD-fed mice (Figure 2A), which is consistent with our previous study on resveratrol (Pearson et al., 2008).
Figure 2. SRT1720 improves the quality of life of SD-fed mice.
(A) Cataract formation as assessed by lens opacity classification; (B) Oral glucose tolerance test with area under the curve (inset); (C) The homeostatic model assessment calculation of insulin resistance (HOMA-IR) and serum biochemical markers. Data are shown as mean ± SEM. * p≤0.05 compared to SD diet without SRT1720.
Another hallmark feature of aging is the increase in blood glucose and insulin levels, which leads to type 2 diabetes, cardiovascular disease and non-alcoholic fatty liver disease. In this study, an oral glucose tolerance test was performed to determine whether SRT1720 supplementation improved glucose disposal in SD-fed mice (Figure 2B). Our results indicated significant reduction in area under the curve values, which are consistent with greater glucose disposal in response to SRT1720 (Figure 2B, inset). Although not statistically significant, the reduction in the Homeostatic Model Assessment of Insulin Resistance (HOMA-IR) index was indicative of improved insulin sensitivity in SRT1720-treated animals (Figure 2C). Recent work has reported that SRT1720 lowers insulin resistance in mouse models of obesity (Minor et al., 2011; Yamazaki et al., 2009), which may be due to an enhancement of glucose-stimulated insulin secretion by SRT1720. This biological response was not measured in the current study. Our results are consistent with two previous studies showing that lifelong resveratrol supplementation and short-term SRT1720 treatment of HFD-fed mice both elicit beneficial effects on insulin sensitivity and protect against diet-induced impairments in mitochondrial capacity and oxidative metabolism in vivo (Feige et al., 2008; Pearson et al., 2008). Total cholesterol and low-density lipoprotein levels were lower in SRT1720-treated animals fed a SD (Figure 2C), which may be due to reduced expression of lipogenic enzymes that occur with SRT1720 treatment (Yamazaki et al., 2009). The ability of SRT1720 to influence serum lipid profile may contribute to an improvement in risk factors for metabolic syndrome that occurs as part of the normal progression of aging. Thus, mechanisms of lifespan and healthspan extension by SRT1720 could be linked to protection against age-associated metabolic and functional decline in laboratory mice.
SRT1720 treatment did not alter the number of pathologies in mice fed a SD on gross postmortem pathology screens (Table S1). Although the incidence of hepatocellular carcinoma and glomerulonephritis appeared higher in the SRT1720-treated group (Table S1), the mean age at which necropsy was performed was 10 weeks later, consistent with the notion that SRT1720 delays the onset of pathologies allowing mice to live a longer and healthier life. The incidence of lymphoma was not assessed in these mice. Upon further histological analysis of tissues by a blinded pathologist (Table S2 and Figure S2), we observed a significant reduction in steatosis in the SRT1720-treated mice relative to the SD-fed animals, despite a small but significant increase in lymphocyte infiltration in the kidneys (Table S2). SRT1720 has been shown to be effective in reducing HFD-induced pathologies (Minor et al., 2011), atherosclerosis (Zeng et al., 2013) and myocardial infarction (Tong et al., 2012). Although overexpression of SIRT1 confers a multidrug resistance phenotype in various cancer cell lines in vitro (de Jong et al., 2011; Oh et al., 2010; Zhu et al., 2012), pharmacological activation of SIRT1 by SRT1720 has been associated with reduction in multiple myeloma tumor growth (Chauhan et al., 2011), but with increased lung metastasis of breast cancer cells in a mouse xenograft model (Suzuki et al., 2012). Harmful effects of this kind warrant further studies on dosage, timing, and actions in various tissues of SRT1720 relevant to the development of therapeutically useful SIRT1 activators. In the study herein, low levels of serum markers of hepatic and renal function –AST, creatinine and CK– were found with SRT1720 treatment (Figure 2C), indicating that the compound was well tolerated. To ensure that the ingested SRT1720 reached adequate circulating concentrations, the serum levels of SRT1720 were measured in the morning and evening, and were found to be 193±30.0 and 339±23.0 ng/mL, respectively, consistent with the feeding cycle of the mice and with our previous study of high-fat diet-fed mice (Minor et al., 2011). To date, there has been no correlation between STAC concentrations (resveratrol or SRT1720) in vivo and activation of SIRT1 in vitro, the reason being that there is controversy surrounding the methods for measuring SIRT1 enzymatic activity (Dominy et al., 2013). Thus far, the measurement of the steady-state acetylation status of select sirtuin substrates in vivo (e.g., PGC-1α, p53, NF-κB and others) remains an important tool for evaluating changes in sirtuin activity (Dominy et al., 2013). Based on our NF-κB data (see below), we propose that the measured plasma concentration of SRT1720 in treated mice is sufficient to activate SIRT1 in vivo.
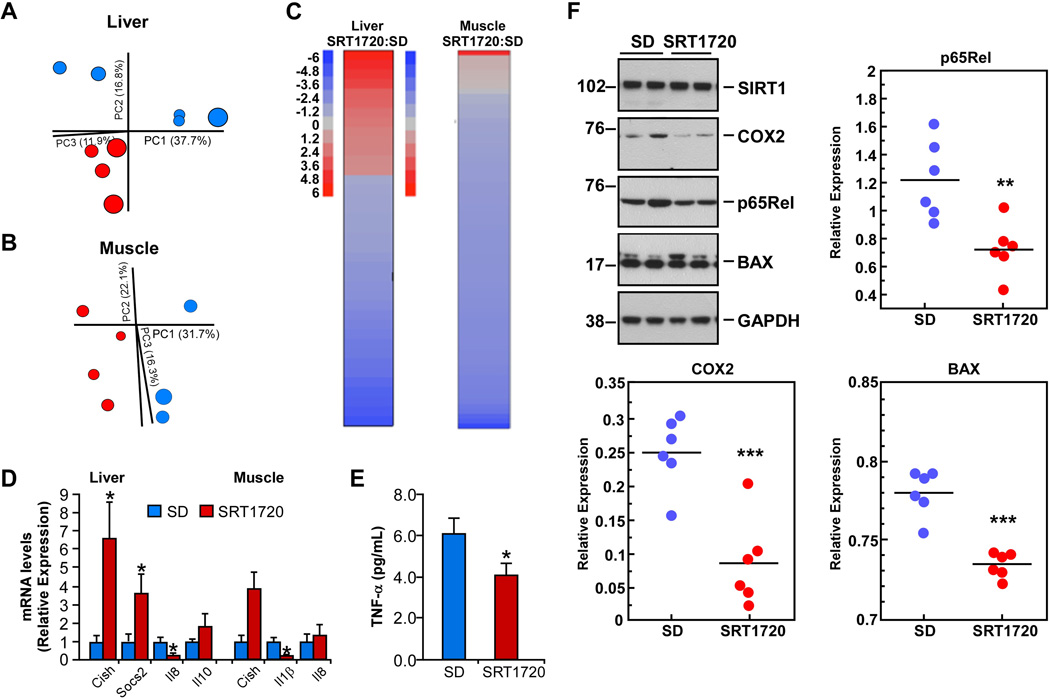
Whole-genome DNA microarrays and pathway analyses were performed on liver and muscle samples of SD-fed mice supplemented or not with SRT1720. Principal Component Analysis showed a distinct separation of both treatment groups, with the effect of SRT1720 supplementation being more pronounced in the muscle tissue (Figure 3A vs. 3B). The top 10 upand down-regulated genes in both tissues are shown in Table S3, with the complete dataset made available at http://www.ncbi.nlm.nih.gov/geo/. Among the top upregulated genes, members of the cytokine-induced STAT inhibitor (CIS) family (e.g., suppressor of cytokine signaling 2 (SOCS2) and cytokine-inducible SH2-containing protein (CISH)), which are associated with suppression of the inflammatory response (Kubo et al., 2003), were significantly higher in the liver and muscle of SRT1720-treated mice. Similarly, a 7-fold increase in hepatic expression of cytochrome P450 1A2, which has a role in xenobiotic, cholesterol and lipid metabolism (Hafner et al., 2011), was observed in SRT1720-treated mice and may explain the lower circulating serum cholesterol and LDL levels in these animals. These results are consistent with a recent report showing that SRT1720 ameliorates fatty liver through a reduction in lipogenic enzyme expression in monosodium glutamate-treated mice (Yamazaki et al., 2009). Lipocalin 2 (Lcn2) and serum amyloid A1 and A2 (Saa1, Saa2), which are involved in innate immunity and the acute phase response, respectively, were the top three most downregulated genes in the liver of SRT1720-treated animals. Downregulation of the cytokine-inducible transcription factor, Ankyrin repeat domain 1 (Ankrd1), was observed in the muscle following SRT1720 supplementation (Table S3).
Figure 3. SRT1720 elicits differential gene expression profiles in the liver and muscle of SD-fed mice.
Principal component analysis (PCA) was performed on (A) liver and (B) skeletal muscle of SD-fed mice supplemented without and with SRT1720. (C) Parametric analysis of gene-set enrichment (PAGE) analysis was performed on microarray data. Columns show significantly up- (red) and down-regulated (blue) pathways following SRT1720 supplementation. (D) mRNA expression analysis in liver and skeletal muscle by quantitative real-time PCR. Relative expression values were normalized to those of SD-fed control mice. (E) Serum tumor necrosis factor alpha (TNF-α) concentrations in SD-fed mice supplemented or not with SRT1720. Data are shown as mean ± SEM. (F) Western blotting of liver lysates from SD-fed mice supplemented or not with SRT1720. Upper left panel, representative blots; remaining panels, Signals associated with bands of interest were normalized to GAPDH and plotted. * p≤0.05; ** p≤0.01; *** p≤0.001 when compared to SD-fed control animals.
The effect of SRT1720 on gene expression was further investigated using parametric analysis of gene set enrichment (PAGE) (Figure 3C). Interestingly, the negative transcriptional effect of SRT1720 was stronger in the muscle than in the liver. The expanded list of top up- and down-regulated pathways in the liver and muscle of SRT1720-treated SD-fed mice are presented in Tables S4 and S5. Notably, ribosomal proteins were the most up-regulated pathways in the mouse liver (Table S4) and alterations in ribosomal biology and translation are tightly linked to longevity in other organisms (Heeren et al., 2009; Houtkooper et al., 2013; Rogers et al., 2011), which may provide another mechanism through which SRT1720 exerts its pro-longevity effects. One of the top pathways to be altered by SRT1720 supplementation in both the liver and the muscle was the inflammation pathway, and quantitative RT-PCR analysis showed significant reduction in select pro-inflammatory mediators in both tissues when compared to SD-fed control animals (Figure 3D). Consistent with this idea, circulating TNF-α levels were lower in the SRT1720 cohort (Figure 3E), and a number of NF-κB target genes were among the top downregulated genes following SRT1720 supplementation (Table S3). Similarly, Western blotting of liver lysates illustrated the significant reduction in protein levels encoding for p65Rel and two known targets of NF-κB, COX2 and BAX, in the SRT1720-fed cohort (Figure 3F). No alteration in SIRT1 protein expression was observed. Although the acetylation status of canonical SIRT1 targets was not directly assessed, these results led us to hypothesize that SRT1720 may inhibit NF-κB signaling via SIRT1 activation.
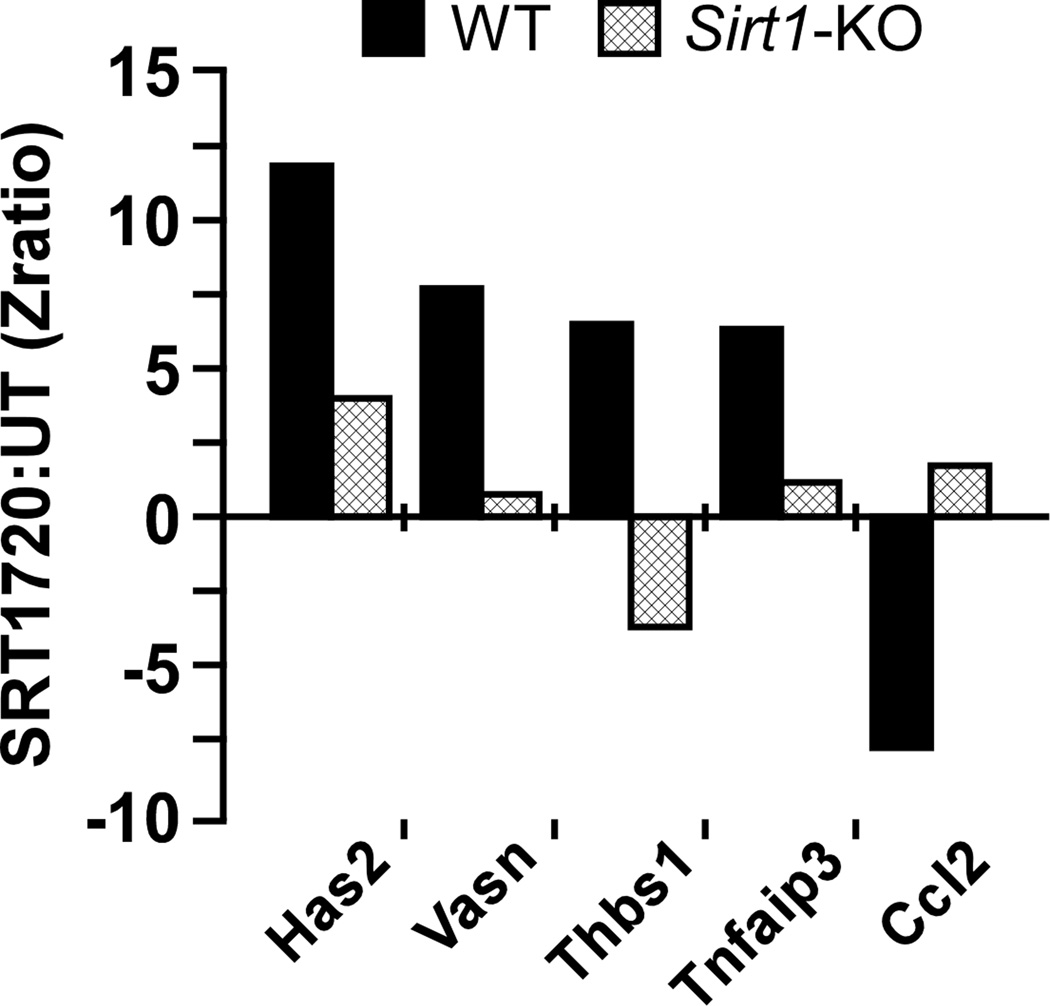
To explore the molecular mechanisms underlying SRT1720 control of inflammatory signaling, wild type and SIRT1 gene knockout murine embryonic fibroblast (MEF) cells were used for cDNA microarray analysis (Figure 4; Table S6) in conjunction with a commercial phospho-antibody microarray (Table S7). Using the latter method, it has previously been demonstrated that SIRT1 deletion is associated with constitutive activation of the pro-inflammatory NF-κB pathway (Bernier et al., 2011). Conversely, overexpression of SIRT1 is associated with reduced NF-κB activity in mice (Pfluger et al., 2008) while SIRT1 activation by SRT1720 reduces expression of NF-κB and its targets in foam cells (Zeng et al., 2013) and in multiple myeloma cells (Chauhan et al., 2011). Here, it was found that expression of few genes was influenced by SRT1720 in a SIRT1-independent manner (e.g., Id2), while expression of other genes, including Has2, Gadd45a, Ddit3, Thbs1, and Ifitm3, required SIRT1 following MEF stimulation with SRT1720 (Table S6). Importantly, SRT1720 induced significant changes in the phosphorylation status of several proteins involved in NF-κB activation in wild-type MEFs, but not Sirt1-KO MEFs (Table S7). Given that low-grade chronic inflammation is believed to contribute to aging and age-related diseases (Franceschi et al., 2000), our results indicate that SRT1720 may keep non-obese mice healthier and extend their mean lifespan by spurring robust inflammatory defense. In accordance with this notion is the fact that SRT1720 exerts antiinflammatory properties in mouse models of asthma (Ichikawa et al., 2013) and emphysema (Yao et al., 2012).
Figure 4. SIRT1 dependence in SRT1720-mediated gene expression in MEF cells.
Microarray data collected from untreated (UT) and SRT1720-treated wild-type (WT) and Sirt1-KO MEFs were used to illustrate enrichment of select genes. A larger list of genes can be found in Table S6.
In summary, we report data showing that SRT1720 elicits lifespan extension and improved healthspan in mice maintained on a standard diet. This work highlights the importance of examining the therapeutic value of small molecule activators of SIRT1 in general health and longevity.
EXPERIMENTAL PROCEDURES
Animals and Diets
Male C57BL/6J mice were obtained from the Jackson Laboratory (Bar Harbor, ME) at 15 weeks of age and housed at the Gerontology Research Center, in Baltimore, MD, in cages of four. Diets were started at 28 weeks of age after randomization into four groups of 100 mice per group. Mice were fed one of four diets: standard diet (SD) of AIN-93G (carbohydrate:protein:fat ratio of 64:19:17 percent of kcal), a SD supplemented with SRT1720 (SD-SRT1720), a high fat diet consisting of AIN-93G modified to provide 60% of calories from fat (HFD; carbohydrate:protein:fat ratio of 16:23:61), or a HFD supplemented with SRT1720 (HFD-SRT1720). SRT1720 was included in diets at a concentration of 1.33g/kg (SD) and 2g/kg (HFD; g drug/kg chow), respectively, which was formulated to provide mice with approximate daily doses of 100mg/kg (drug/kg body weight). Study diets were purchased from Dyets, Inc. (Bethlehem, PA). SRT1720 was provided by Sirtris Pharmaceuticals, Inc. (Cambridge, MA). All groups had ad libitum access to their prescribed diet and water throughout the study. Body weight and food intake were monitored bi-weekly. Animal rooms were maintained at 20–22°C with 30–70% relative humidity and a 12-hour light/dark cycle. All animal protocols were approved by the Animal Care and Use Committee (352-LEG-2012) of the National Institute on Aging.
Survival Study
Moribund animals were euthanized and every animal found dead or euthanized was necropsied. Additional information is available in the Supplemental section.
Histology
Organs were fixed in 4% paraformaldehyde then stained with hematoxylin and eosin for scoring of pathology by a histopathologist blinded to the treatment group.
Determination of serum marker concentrations and HOMA-IR calculation
Detailed information can be found in the Supplemental section.
Oral Glucose Tolerance Test (OGTT)
Following an overnight fast (18 hours), mice received an oral gavage of 30% glucose solution (Sigma Aldrich, St Louis, MO) at a dose of 2g/kg body weight. Blood glucose was measured using an Ascensia Elite glucose meter at 0, 15, 30, 60 and 120 minutes following gavage (n=6 SD, n=8 SD-SRT1720, 73 weeks age, 46 weeks on diet).
Body Composition
Measurements of lean body mass, fat and fluid mass in live mice were acquired by nuclear magnetic resonance (NMR) spectroscopy using the Minispec LF90 (Bruker Optics, Billerica, MA) (n=84 SD, n=93 SD-SRT1720, n=51 HFD, n=73 HFD-SRT1720, 76 weeks age, 49 weeks on diet).
Metabolic Assessment
Mouse metabolic rate was assessed by indirect calorimetry in opencircuit oxymax chambers using the Comprehensive Lab Animal Monitoring System (CLAMS; Columbus Instruments, Columbus, OH) as previously described (Minor et al., 2011) (n=8 SD, n=8 SD-SRT1720, n=7 HFD, n=8 HFD-SRT1720, 52 weeks age, 25 weeks on diet).
Rotarod
All mice were acclimated 15 min before testing. Mice were tested at the same time of day during their light cycle over a five-day period. Mice were given a habituation trial at a constant speed of 4 rpm for 1 minute before the first trial. On the same day there was a total of three trials given, separated by 30-minute rest periods, during which the rotarod accelerated from 4 to 40 rpm over a period of five minutes. Latency to fall was recorded and averaged over all three trials. This test was completed on the same mice at 13, 18 and 24 months of age.
Cataract Formation
Lens opacity reading was performed using a Kowa SL14 hand-held slit lamp (Kowa, Tokyo, Japan) by an experienced pathologist blinded to the experimental group, as described previously (Wolf et al., 2005) (n=138 eyes SD, n=142 eyes SD-SRT1720, 105 weeks age, 78 weeks diet).
Western blotting
Detailed information can be found in the Supplemental section.
Microarray
RNA from tissues was isolated using the RNeasy kit (Qiagen, Valencia, CA) and then hybridized to BD-202-0202 Illumina Beadchips. Raw data were subjected to Z-normalization, as described elsewhere (Cheadle et al., 2003; Lee et al., 2012). Additional information is available in the Supplemental section. All raw data are available in the Gene Expression Omnibus database (accession number GSE50987).
Quantitative Real-time RT-PCR
Total RNA was extracted from frozen tissue samples or cells using the RNeasy kit (Qiagen). Additional information can be found in the Supplemental section.
Phosphoprotein Profiling
The Phospho Explorer antibody microarray from Full Moon Biosystems, Inc. (Sunnyvale, CA) was used to survey the phosphorylation state of select proteins involved in the IKK/NF-κB activation pathway, according to the protocol outlined in Bernier et al. (Bernier et al., 2011). In brief, wild-type and Sirt1-KO murine embryonic fibroblasts (gift of Raul Mostoslavsky, Harvard Medical School, Boston, MA) were incubated with vehicle or 3 µM SRT1720 for 18 h, after which cell lysates were prepared and biotinylated. The antibody array experiment was performed by Full Moon Biosystems, Inc., and the data was analyzed with GenePix Pro 6.0 (Molecular Devices, Sunnyvale, CA). Each of the 1318 antibodies had two replicates printed on a coated microscope slide, along with multiple positive and negative controls.
Statistics
Data are expressed as means ± standard error of the mean (SEM). Student’s t-tests were used for all comparisons unless otherwise stated. Mortality during the survival study was assessed through the use of the non-parametric Log-rank (Mantel-Haenszel) test to compare the differences in Kaplan-Meier survival curves. Median survival was assessed using the non-parametric Chi-square test (Su and Wei, 1993). 90th percentile survival estimates were used as surrogate measures of maximum survival times according to the methods of Wang et al., (2004). The survival analyses were implemented in R (The R Development Core Team, Vienna) from scratch using the methodological references given. Comparisons for the histopathology data were performed using Fisher’s exact test. Analyses were performed using Excel 2010 (Microsoft Corp., Redmond, WA), IBM SPSS Statistics (Amonk, NY), or SigmaStat 3.0 (Aspire Software International, Ashburn, VA). A p value of ≤ 0.05 was considered statistically significant.
Supplementary Material
Highlights.
SRT1720 supplementation extends mean lifespan of mice fed a standard diet.
SRT1720 improves healthspan of mice fed a standard diet.
SRT1720 reduces the age-associated increase in risk factors for metabolic disease.
SRT1720 supplementation confers anti-inflammatory properties in target tissues.
ACKNOWLEDGMENTS
This research was conducted under a Cooperative Research and Development Agreement (CRADA) between Sirtris, a GSK Company, and the National Institute on Aging, National Institutes of Health (NIA/NIH). Funding was provided by the Intramural Research Program of the NIA/NIH. We are grateful to Dawn Nines, Justine Lucas and Dawn Phillips-Boyer for their excellent animal care. We thank Dr Elin Lehrmann for microarray assistance. We thank Drs. Hua-Jun He (National Institute of Standards and Technology, Gaithersburg, MD), Yaping Zong (Full Moon Biosystems, Inc., Sunnyvale, CA) and Xiong Li (Dept. of Mathematics and Computer Science, Emory University, Atlanta, GA) for their contribution in carrying out the phospho-antibody array experiment and data analysis. We thank Dr Norm Wolf for the assessment of cataracts in the mice and Olga Carlson for technical assistance with the multiplex assay. SJM was supported by a National Medical Health and Research Council of Australia CJ Martin Early Career Fellowship (RGMS ID 2010-01671). JD is supported by a University of Alabama at Birmingham Statistical Genetics Postdoctoral Training Program Grant (T32HL072757)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Banks AS, Kon N, Knight C, Matsumoto M, Gutiérrez-Juárez R, Rossetti L, Gu W, Accili D. SirT1 Gain of Function Increases Energy Efficiency and Prevents Diabetes in Mice. Cell Metab. 2008;8:333–341. doi: 10.1016/j.cmet.2008.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baur J, Pearson K, Price N, Jamieson H, Lerin C, Kalra A, Prabhu V, Allard J, Lopez-Lluch G, Lewis K, et al. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006;444:337–342. doi: 10.1038/nature05354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baur JA, Ungvari Z, Minor RK, Le Couteur DG, de Cabo R. Are sirtuins viable targets for improving healthspan and lifespan? Nat. Rev. Drug Discov. 2012;11:443–461. doi: 10.1038/nrd3738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellet MM, Nakahata Y, Boudjelal M, Watts E, Mossakowska DE, Edwards KA, Cervantes M, Astarita G, Loh C, Ellis JL, et al. Pharmacological modulation of circadian rhythms by synthetic activators of the deacetylase SIRT1. Proc. Natl. Acad. Sci. U.S.A. 2013;110:3333–3338. doi: 10.1073/pnas.1214266110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernier M, Paul RK, Martin-Montalvo A, Scheibye-Knudsen M, Song S, He HJ, Armour SM, Hubbard BP, Bohr VA, Wang L, et al. Negative regulation of STAT3 protein-mediated cellular respiration by SIRT1 protein. J. Biol. Chem. 2011;286:19270–19279. doi: 10.1074/jbc.M110.200311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang Y, Levy D, Horton JR, Peng J, Zhang X, Gozani O, Cheng X. Structural basis of SETD6-mediated regulation of the NF-κB network via methyl-lysine signaling. Nuc. Acids Res. 2011;39:6380–6389. doi: 10.1093/nar/gkr256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauhan D, Bandi M, Singh AV, Ray A, Raje N, Richardson P, Anderson KC. Preclinical evaluation of a novel SIRT1 modulator SRT1720 in multiple myeloma cells. Br. J. Haematol. 2011;155:588–598. doi: 10.1111/j.1365-2141.2011.08888.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheadle C, Vawter MP, Freed WJ, Becker KG. Analysis of Microarray Data Using Z Score Transformation. J. Mol. Diagnost. 2003;5:73–81. doi: 10.1016/S1525-1578(10)60455-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jong E, Winkel P, Poelstra K, Prakash J. Anticancer effects of 15d-prostaglandin- J2 in wild-type and doxorubicin-resistant ovarian cancer cells: novel actions on SIRT1 and HDAC. PLoS ONE. 2011;6:e25192. doi: 10.1371/journal.pone.0025192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deblon N, Bourgoin L, Veyrat-Durebex C, Peyrou M, Vinciguerra M, Caillon A, Maeder C, Fournier M, Montet X, Rohner-Jeanrenaud F, et al. Chronic mTOR inhibition by rapamycin induces muscle insulin resistance despite weight loss in rats. Br. J. Pharmacol. 2012;165:2325–2340. doi: 10.1111/j.1476-5381.2011.01716.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demarchi F, Bertoli C, Sandy P, Schneider C. Glycogen synthase kinase-3β regulates NF-κB1/p105 stability. J. Biol. Chem. 2003;278:39583–39590. doi: 10.1074/jbc.M305676200. [DOI] [PubMed] [Google Scholar]
- Dominy J, Puigserver P, Canto C. In vivo measurement of the acetylation state of sirtuin substrates as a proxy for sirtuin activity. Methods Mol Biol. 2013;1077:217–237. doi: 10.1007/978-1-62703-637-5_15. [DOI] [PubMed] [Google Scholar]
- Feige JrmN, Lagouge M, Canto C, Strehle A, Houten SM, Milne JC, Lambert PD, Mataki C, Elliott PJ, Auwerx J. Specific SIRT1 Activation Mimics Low Energy Levels and Protects against Diet-Induced Metabolic Disorders by Enhancing Fat Oxidation. Cell Metab. 2008;8:347–358. doi: 10.1016/j.cmet.2008.08.017. [DOI] [PubMed] [Google Scholar]
- Franceschi C, Bonafè M, Valensin S, Olivieri F, De Luca M, Ottaviani E, De Benedictis G. Inflamm-aging: An Evolutionary Perspective on Immunosenescence. Ann. N. Y. Acad. Sci. 2000;908:244–254. doi: 10.1111/j.1749-6632.2000.tb06651.x. [DOI] [PubMed] [Google Scholar]
- Froy O. Circadian rhythms, aging, and life span in mammals. Physiology (Bethesda) 2011;26:225–235. doi: 10.1152/physiol.00012.2011. [DOI] [PubMed] [Google Scholar]
- Hafner M, Rezen T, Rozman D. Regulation of hepatic cytochromes P450 by lipids and cholesterol. Curr. Drug Metab. 2011;12:173–185. doi: 10.2174/138920011795016890. [DOI] [PubMed] [Google Scholar]
- Harrison DE, Strong R, Sharp ZD, Nelson JF, Astle CM, Flurkey K, Nadon NL, Wilkinson JE, Frenkel K, Carter CS, et al. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature. 2009;460:392–395. doi: 10.1038/nature08221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heeren G, Rinnerthaler M, Laun P, von Seyerl P, Kossler S, Klinger H, Hager M, Bogengruber E, Jarolim S, Simon-Nobbe B, et al. The mitochondrial ribosomal protein of the large subunit, Afo1p, determines cellular longevity through mitochondrial back-signaling via TOR1. Aging. 2009;1:622–636. doi: 10.18632/aging.100065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houde VP, Brule S, Festuccia WT, Blanchard PG, Bellmann K, Deshaies Y, Marette A. Chronic rapamycin treatment causes glucose intolerance and hyperlipidemia by upregulating hepatic gluconeogenesis and impairing lipid deposition in adipose tissue. Diabetes. 2010;59:1338–1348. doi: 10.2337/db09-1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houtkooper RH, Mouchiroud L, Ryu D, Moullan N, Katsyuba E, Knott G, Williams RW, Auwerx J. Mitonuclear protein imbalance as a conserved longevity mechanism. Nature. 2013;497:451–457. doi: 10.1038/nature12188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard BP, Gomes AP, Dai H, Li J, Case AW, Considine T, Riera TV, Lee JE, E SY, Lamming DW, et al. Evidence for a Common Mechanism of SIRT1 Regulation by Allosteric Activators. Science. 2013;339:1216–1219. doi: 10.1126/science.1231097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber JL, McBurney MW, Distefano PS, McDonagh T. SIRT1-independent mechanisms of the putative sirtuin enzyme activators SRT1720 and SRT2183. Future Med. Chem. 2010;2:1751–1759. doi: 10.4155/fmc.10.257. [DOI] [PubMed] [Google Scholar]
- Ichikawa T, Hayashi R, Suzuki K, Imanishi S, Kambara K, Okazawa S, Inomata M, Yamada T, Yamazaki Y, Koshimizu Y, et al. The Sirt1 Activator SRT1720 Suppresses Inflammation in an OVA-induced Mouse Model of Asthma. Respirology. 2013;18:332–339. doi: 10.1111/j.1440-1843.2012.02284.x. [DOI] [PubMed] [Google Scholar]
- Karin M. Positive and negative regulation of IκB kinase activity through IKKβ subunit phosphorylation. Science. 1999;284:309–313. doi: 10.1126/science.284.5412.309. [DOI] [PubMed] [Google Scholar]
- Kim SY, Volsky DJ. PAGE: Parametric analysis of gene set enrichment. BMC Bioinformatics. 2005;6:1471–2105. doi: 10.1186/1471-2105-6-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs AD, Pearce DA. Location- and sex-specific differences in weight and motor coordination in two commonly used mouse strains. Sci Rep. 2013;3:2116. doi: 10.1038/srep02116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubo M, Hanada T, Yoshimura A. Suppressors of cytokine signaling and immunity. Nat. Immunol. 2003;4:1169–1176. doi: 10.1038/ni1012. [DOI] [PubMed] [Google Scholar]
- Lamming DW, Ye L, Katajisto P, Goncalves MD, Saitoh M, Stevens DM, Davis JG, Salmon AB, Richardson A, Ahima RS, et al. Rapamycin-induced insulin resistance is mediated by mTORC2 loss and uncoupled from longevity. Science. 2012;335:1638–1643. doi: 10.1126/science.1215135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C, Raffaghello L, Brandhorst S, Safdie FM, Bianchi G, Martin-Montalvo A, Pistoia V, Wei M, Hwang S, Merlino A, et al. Fasting Cycles Retard Growth of Tumors and Sensitize a Range of Cancer Cell Types to Chemotherapy. Sci. Translat. Med. 2012;4 doi: 10.1126/scitranslmed.3003293. 124ra127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Montalvo A, Mercken EM, Mitchell SJ, Palacios HH, Mote PL, Scheibye-Knudsen M, Gomes AP, Ward TM, Minor RK, Blouin MJ, et al. Metformin improves healthspan and lifespan in mice. Nat Commun. 2013;4:2192. doi: 10.1038/ncomms3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milne J, Lambert P, Schenk S, Carney D, Smith J, Gagne D, Jin L, Boss O, Perni R, Vu C, et al. Small molecule activators of SIRT1 as therapeutics for the treatment of type 2 diabetes. Nature. 2007;450:712–716. doi: 10.1038/nature06261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minor RK, Baur JA, Gomes AP, Ward TM, Csiszar A, Mercken EM, Abdelmohsen K, Shin YK, Canto C, Scheibye-Knudsen M, et al. SRT1720 improves survival and healthspan of obese mice. Sci. Rep. 2011;1:2045–2322. doi: 10.1038/srep00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh WK, Cho KB, Hien TT, Kim TH, Kim HS, Dao TT, Han HK, Kwon SM, Ahn SG, Yoon JH, et al. Amurensin G, a potent natural SIRT1 inhibitor, rescues doxorubicin responsiveness via down-regulation of multidrug resistance 1. Mol. Pharmacol. 2010;78:855–864. doi: 10.1124/mol.110.065961. [DOI] [PubMed] [Google Scholar]
- Ozes ON, Mayo LD, Gustin JA, Pfeffer SR, Pfeffer LM, Donner DB. NF-κB activation by tumour necrosis factor requires the Akt serine-threonine kinase. Nature. 1999;401:82–85. doi: 10.1038/43466. [DOI] [PubMed] [Google Scholar]
- Pacholec M, Bleasdale JE, Chrunyk B, Cunningham D, Flynn D, Garofalo RS, Griffith D, Griffor M, Loulakis P, Pabst B, et al. SRT1720, SRT2183, SRT1460, and Resveratrol Are Not Direct Activators of SIRT1. J. Biol. Chem. 2010;285:8340–8351. doi: 10.1074/jbc.M109.088682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson KJ, Baur JA, Lewis KN, Peshkin L, Price NL, Labinskyy N, Swindell WR, Kamara D, Minor RK, Perez E, et al. Resveratrol Delays Age-Related Deterioration and Mimics Transcriptional Aspects of Dietary Restriction without Extending Life Span. Cell Metab. 2008;8:157–168. doi: 10.1016/j.cmet.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfluger PT, Herranz D, Velasco-Miguel S, Serrano M, Tschöp MH. Sirt1 protects against high-fat diet-induced metabolic damage. Proc. Natl. Acad. Sci. U.S. A. 2008;105:9793–9798. doi: 10.1073/pnas.0802917105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers AN, Chen D, McColl G, Czerwieniec G, Felkey K, Gibson BW, Hubbard A, Melov S, Lithgow GJ, Kapahi P. Life span extension via eIF4G inhibition is mediated by posttranscriptional remodeling of stress response gene expression in Celegans. Cell Metab. 2011;14:55–66. doi: 10.1016/j.cmet.2011.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strong R, Miller RA, Astle CM, Baur JA, de Cabo R, Fernandez E, Guo W, Javors M, Kirkland JL, Nelson JF, et al. Evaluation of resveratrol, green tea extract, curcumin, oxaloacetic acid, and medium-chain triglyceride oil on life span of genetically heterogeneous mice. J. Gerontol. A Biol. Sci. Med. Sci. 2013;68:6–16. doi: 10.1093/gerona/gls070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki K, Hayashi R, Ichikawa T, Imanishi S, Yamada T, Inomata M, Miwa T, Matsui S, Usui I, Urakaze M, et al. SRT1720, a SIRT1 activator, promotes tumor cell migration, and lung metastasis of breast cancer in mice. Oncol. Rep. 2012;27:1726–1732. doi: 10.3892/or.2012.1750. [DOI] [PubMed] [Google Scholar]
- Tong C, Morrison A, Mattison S, Qian S, Bryniarski M, Rankin B, Wang J, Thomas DP, Li J. Impaired SIRT1 nucleocytoplasmic shuttling in the senescent heart during ischemic stress. FASEB J. 2013;27:1–11. doi: 10.1096/fj.12-216473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winston JT, Strack P, Beer-Romero P, Chu CY, Elledge SJ, Harper JW. The SCFβ-TRCP-ubiquitin ligase complex associates specifically with phosphorylated destruction motifs in IκBα and β-catenin and stimulates IκBα ubiquitination in vitro. Genes Dev. 1999;13:270–283. doi: 10.1101/gad.13.3.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf N, Penn P, Pendergrass W, Van Remmen H, Bartke A, Rabinovitch P, Martin GM. Age-related cataract progression in five mouse models for anti-oxidant protection or hormonal influence. Exp. Eye Res. 2005;81:276–285. doi: 10.1016/j.exer.2005.01.024. [DOI] [PubMed] [Google Scholar]
- Yamazaki Y, Usui I, Kanatani Y, Matsuya Y, Tsuneyama K, Fujisaka S, Bukhari A, Suzuki H, Senda S, Imanishi S, et al. Treatment with SRT1720, a SIRT1 activator, ameliorates fatty liver with reduced expression of lipogenic enzymes in MSG mice. Am. J. Physiol. Endocrinol-Metab. 2009;297:E1179–E1186. doi: 10.1152/ajpendo.90997.2008. [DOI] [PubMed] [Google Scholar]
- Yao H, Chung S, Hwang J-w, Rajendrasozhan S, Sundar IK, Dean DA, McBurney MW, Guarente L, Gu W, Rönty M, et al. SIRT1 protects against emphysema via FOXO3-mediated reduction of premature senescence in mice. J. Clin. Invest. 2012;122:2032–2045. doi: 10.1172/JCI60132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng HT, Fu YC, Yu W, Lin JM, Zhou L, Liu L, Wang W. SIRT1 prevents atherosclerosis via liverXreceptor and NFkappaB signaling in a U937 cell model. Mol. Med. Rep. 2013;8:23–28. doi: 10.3892/mmr.2013.1460. [DOI] [PubMed] [Google Scholar]
- Zhu H, Xia L, Zhang Y, Wang H, Xu W, Hu H, Wang J, Xin J, Gang Y, Sha S, et al. Activating transcription factor 4 confers a multidrug resistance phenotype to gastric cancer cells through transactivation of SIRT1 expression. PLoS ONE. 2012;7:e31431. doi: 10.1371/journal.pone.0031431. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.