Abstract
Objective
Our objective was to examine the role of hypertriglyceridemia on the capacity of HDL to facilitate ABCA-1 mediated cholesterol efflux in type 2 diabetes (T2DM).
Methods
HDL mediated cholesterol efflux through the ABCA-1 transporter was measured using BHK cell lines in samples of 71 participants with T2DM in presence or absence of high triglyceride levels (TG). Additionally, HDL mediated efflux was measured in 13 diabetic and non-diabetic participants fasting and four hours after a high-fat test challenge.
Results
HDL mediated cholesterol efflux function was increased in participants with T2DM with hypertriglyceridemia when compared to participants with T2DM without hypertriglyceridemia (efflux ratio mean ± standard deviation (SD), T2DM + TG: 1.17 ± 0.25 vs. T2DM – TG: 1.03 ± 0.19, p = 0.0098). In the fat challenge study, we observed a significant increase in ABCA-1 mediated cholesterol efflux capacity following an ingestion of high-fat test meal by participants in both groups of T2DM (n = 6, efflux ratio, mean ± SD, pre: 0.86 ± 0.4 vs. post: 1.34 ± 0.6, p = 0.01) and non-diabetic participants (n = 7, efflux ratio mean ± SD pre : 1.24 ± 0.31 vs. post: 1.39 ± 0.42, p = 0.04) that was partly explained by the difference in CETP activity (r = 0.6, p = 0.03).
Conclusion
Our study suggests that high triglyceride levels facilitate ABCA-1 mediated efflux function of HDL in part by activating CETP.
Keywords: HDL, Efflux, ABCA-1, Triglycerides, CETP, diabetes
Introduction
High-density lipoprotein (HDL) particles participate in atheroprotection through several mechanisms, one of which is reverse cholesterol transport (RCT) – a mechanism by which HDL particles remove cholesterol from lipid-laden macrophages present in atherosclerotic plaques and transport it to the liver [1]. The first step in this macrophage RCT is efflux of cholesterol from macrophages and its transfer across the cell membranes to HDL particles, which involves adenosine triphosphate binding cassette transporter protein (ABCA-1) [1]. Two recent large studies [2, 3] presented conflicting reports on the relevance of HDL mediated cholesterol efflux on atherosclerosis and cardiovascular disease (CVD). In one study, Khera et al.[2] demonstrated that lower HDL mediated cholesterol efflux better correlated with measures of atherosclerosis by carotid ultrasound or angiography than HDL-C. In contrast, Li et al.[3] demonstrated that increased HDL mediated cholesterol efflux at baseline predicted incident CVD events at the 3 years follow-up in samples from a prospective CVD cohort. These conflicting reports underscore the need to better understand the factors that determine the capacity of HDL to facilitate ABCA-1 mediated cholesterol efflux.
Low levels of HDL cholesterol (HDL-C) combined with high levels of triglycerides are a common feature of diabetes and metabolic syndrome (MS) [4]. One mechanism for decreased levels of HDL cholesterol is through cholesteryl ester transfer protein (CEPT) mediated exchange of cholesterol for triglycerides. In hypertriglyceridemic states, increased flux of free fatty acids into liver results in increased levels of triglyceride-rich very-low density lipoproteins (VLDL) by increasing VLDL triglyceride secretion. Triglycerides in VLDL are exchanged for cholesteryl esters in HDL by an action of CETP, resulting in decrease of HDL cholesterol level [5], and subsequent formation of lipid-poor or pre-beta 1 HDL particles [6]. Pre-beta 1 HDL particles re-initiate ABCA-1 mediated efflux by accepting cholesterol from lipid-laden macrophages [7, 8]. However, whether increases in prebeta-1 HDL per se are sufficient to promote or maintain cholesterol excretion is not known.
The objective of this study was to investigate the relationship between the hypertriglyceridemic state and ABCA-1 mediated efflux in individuals with type 2 diabetes (T2DM). Initially, we compared ABCA-1 efflux function in individuals with T2DM in presence or absence of increased serum triglycerides levels. Cholesterol efflux measured in participants with diabetes and increased triglycerides levels was significantly increased compared to diabetic subjects without increased triglycerides levels. We subsequently measured the ABCA-1 efflux function in non-diabetic controls and participants with T2DM before and after an ingestion of standardized high-fat meal. The results of the fat challenge demonstrated that the efflux was significantly increased following an ingestion of high-fat meal. Combined, our results support that increased triglyceride levels facilitate the capacity of HDL to transport cholesterol by an ABCA-1 mediated interaction in T2DM.
Methods
Clinical Samples
We recruited study participants from the medicine clinics at University of Arizona. The study was approved by the Institutional Review Board, and all patients provided written informed consent prior to testing. Subjects reported to the Center for Clinical and Translational Sciences (CaTS Center) after an overnight fast. We recorded demographics (age, sex, ethnicity, medication profiles), clinical variables (blood pressure, waist circumference, weight, height, body mass index), and obtained biochemical measurements (fasting lipid profile, HbA1c) using a standard lab (Quest diagnostics). Participants were older than 18 years of age. Healthy volunteers were selected to be off any lipid, diabetes or blood pressure medications. The study excluded subjects if they met any of the following criteria: had type 1 diabetes, had prior cardiovascular events (CVD) event (clinical cardiovascular event was defined by prior coronary artery bypass surgery (CABG), percutaneous transluminal angioplasty (PTCA), or thrombotic stroke), were on an active weight loss program, had history of cancer, had HIV, or had history of steroid use. We attempted to limit secondary hypertriglyceridemia by excluding females on oral contraceptive pills or participants that used alcohol during the 24 hours before the study visit. All participants had glucose tolerance testing. Diagnosis of T2DM was based on medical history or an oral glucose tolerance test (OGTT) as per the American Diabetes Association guidelines with a 2hr OGTT > 200 mg/dL. High triglyceride levels were defined as fasting triglyceride levels ≥ than 150 mg/dL and ≤ than 500 mg/dL. For the fat challenge study, 13 participants (6 with diabetes and 7 non-diabetic controls) ingested a standardized test meal that consisted, per square meter of body surface area, of 175 mL of heavy whipped cream (Shamrock Farms, 99% fat). Of 700 calories per square meter, 99% (70 g) were derived from fat, <1% was derived from carbohydrates (<1 g), or from proteins (<1 g). The cholesterol content was 25 mg, and the ratio of total polyunsaturated to saturated fat was 0.06.
Apo A-I measurements
Total Apo A-I was measured in plasma using a commercial ELISA from Alerchek in 96 well plates.
Cholesterol Efflux
We assayed HDL mediated cholesterol efflux from samples of 6 subjects with low and high HDL-C using both J774 cells and BHK cells in multiple plates. We observed a strong correlation between efflux mediated in J774 and BHK (r=0.89, p<0.001). ABCA-1 mediated efflux in BHK cells had better (lower) replicate variability (both intra and interplate were <10%) than assessed in J774 cells. For that reason, we completed the study using the BHK cell system as previously described [9, 10]. Briefly, the BHK cell lines were transfected with ABCA-1 gene using a mifepristone switch. On day 1, cells were plated at concentration of 100k in high glucose/DMEM + 10% FBS. On day 2, cells were labeled using serum free high glucose/DMEM, 1mg/mL fatty-acid free albumin (FAFA: SIGMA), 2μg/mL Acetyl-Coenzyme A acetyltransferase (ACAT) inhibitor (Sandoz, SIGMA), 1 μCi cholesterol (Moravek) in 48 well plates. On day 3, ABCA-1 gene expression was induced by adding 10 nM of mifepristone. On day 4, 2.5% ApoB depleted plasma was added to each well and the % efflux was calculated by measuring the amount of cholesterol in the media after 4 hours of incubation with 2.5% ApoB depleted plasma using scintillation counting. ApoB depleted plasma was prepared as described previously [11]. ABCA-1 efflux is the difference between induced and control cells. The plate to plate variability was adjusted using the same standard sample (pooled for 30 random samples) in every plate. The result is a ratio of sample ABCA-1 efflux to standard pool ABCA-1 efflux and thus is a normalized efflux ratio. Samples were run in triplicates and the intraplate and interplate coefficient of variation (cv) were less than 10%. The linearity of the assay was verified, as shown in supplemental figure I. BHK cells were a gift from Dr. Chogren Tang, University of Washington.
LCAT, PLTP and CETP function assays
Fluorescence based assays (Roar Biomedical) described in prior clinical studies [12-15] were used to assess LCAT, PLTP and CETP functions. All of the assays utilized positive controls and standards. For LCAT, plasma assays were performed with 4 μl plasma / 100 μl assay buffer incubated in a 96 well microplate well at 37 oC for 6 hours. After 6 hours, 200 μl of read reagent was introduced into each well, mixed and then added to corresponding black plate wells. Samples were analyzed by dual wave at 390/470 using a fluorescent plate reader and ratio was calculated. Heat inactivated plasma served as a negative control. A lower ratio (1.2-1.8 range) translates into greater rate of cholesterol esterification reflecting increased LCAT activity, while heat inactivated plasma had ratios in the 2.8-3.0 range. For PLTP, 5 μl of plasma was added to 3 μl of PLTP standard donor with 42 μl of assay substrate and 50 μl of acceptor. After incubation for 20 min at 37 °C, fluorescence was read at excitation 465 nm and emission 535 nm. For CETP activity, 100 μl of freshly thawed plasma was added to 48 well black plates. 5 μl of Reagent C was added to the plasma an incubated in a water bath for 90 min at 37 °C. Following incubation assay fluorescence was read at excitation 465 nm and emission 535 nm. The results of these assays are reported in Median Fluorescence Intensities (MFI).
Statistical Analysis
The statistical program R version 3.1 was used (R development core team). Summary statistics for all outcomes were reported using means and standard deviations. The two groups were compared using an independent t-test. The associations between efflux and triglycerides or HDL-C were first evaluated using a general linear model approach (with log transformed triglyceride levels to normalize the distribution of the data). Using multivariate linear regression, we determined if HDL mediated efflux ratios were significantly different between the two groups after adjusting for HDL-C, Apo A-I levels, triglyceride levels, CETP and PLTP functions. Measurements after the fat challenge were compared using paired t-tests. CETP function was also not normally distributed, and the data was log transformed. Statistical significance for the comparisons in the major outcomes (HDL mediated cholesterol efflux, HDLC, triglyceride levels and CETP and PLTP functions) was judged at p < 0.05.
Results
45 participants with diabetes and hypertriglyceridemia and 26 participants with diabetes without hypertriglyceridemia were recruited to investigate the role of hypertriglyceridemia on HDL efflux function. Clinical and biochemical characteristics for both groups of participants are listed in table 1. The two groups were matched for sex (22 males and 23 females in first group vs. 13 males and 13 females in second group), age (59 ± 11 vs. 58 ± 12 years), and body mass index (35 ± 8 vs. 35 ± 10 kg/m2). The selection of these two groups was based on serum triglyceride levels between 150-500 mg/dL in the diabetes and hypertriglyceridemic (+TG) group vs ≤ 150 mg/dL in the group without hypertriglyceridemia (-TG). The mean ± SD of triglyceride levels between the two groups were (T2DM + TG: 226 ± 88 vs. T2DM-TG: 91 ± 22 mg/dL, p < 0.0001). As shown in table 1, values for systolic and diastolic blood pressure, as well as for hemoglobin A1c, were similar across both groups. However, participants with T2DM and hypertriglyceridemia had slightly greater concentrations of LDL cholesterol levels and measurements of waist circumference compared to the participants with diabetes without increased triglyceride levels.
TABLE 1.
Clinical and biochemical characteristics of study subjects.
| Characteristic | Diabetic Participants with Hypertriglyceridemia (n=45) Group 1 |
Diabetic Participants without Hypertriglyceridemia (n=26) Group 2 |
P |
|---|---|---|---|
| Demographic profile | |||
| Sex | 22 males, 23 females | 13 males, 13 females | 0.92 |
| Age (years) | 59 ± 11 | 58 ± 12 | 0.71 |
| Body mass index (kg/m2) | 35 ± 8 | 35 ± 10 | 0.74 |
| Waist Circumference (cm) | 118 ± 18 | 110 ± 16 | 0.09 |
| Vital signs | |||
| Systolic blood pressure (mm Hg) | 129 ± 15 | 131 ± 19 | 0.67 |
| Diastolic blood pressure (mm Hg) | 76 ± 8 | 77 ± 11 | 0.57 |
| Medications | |||
| Aspirin (%) | 31 | 53 | 0.06 |
| Statin (%) | 48 | 15 | 0.05 |
| Metformin (%) | 37 | 46 | 0.12 |
| Insulin (%) | 24 | 34 | 0.37 |
| Beta blocker (%) | 6 | 3 | 0.62 |
| ACE inhibitors or ARBs (%) | 35 | 38 | 0.82 |
| Ca channel blockers (%) | 1 | 0.07 | 0.64 |
| Niacin (%) | 0.08 | 0 | 0.12 |
| Fibrates (%) | 11 | 0 | 0.12 |
| Fish oil (%) | 0.04 | 0 | 0.1 |
Data are means ± SD
: Median Fluorescence Intensity
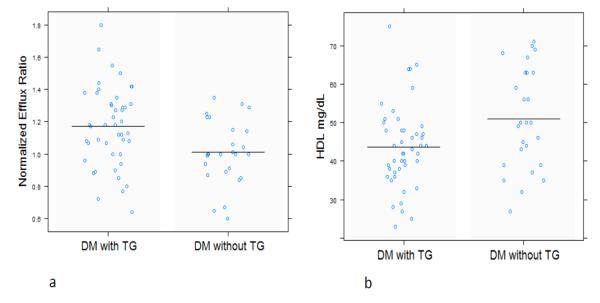
In the cross sectional study, we first investigated the relationship between levels of HDL cholesterol and ABCA-1 mediated efflux function. ABCA-1 mediated efflux ratio in diabetic subjects with hypertriglyceridemia was significantly increased when compared to diabetic subjects without hypertriglyceridemia (1.17 ± 0.25 vs. 1.03 ± 0.19, p = 0.0098, figure 1a). In contrast, HDL-C was lower in the diabetes and hypertriglyceridemia group compared to participants without triglyceridemia (44 ± 11 vs 50 ± 13 mg/dL, p=0.04, figure 1b), but total Apo A-I levels were not significantly different (135 ± 19 vs 138 ± 40, p=0.8). A positive correlation between HDL-C and efflux was noted in all the samples (r = 0.24, p = 0.04). The correlation, however, was observed in subjects with diabetes and hypertriglyceridemia (r = 0.34, p = 0.02), and not significant in subjects without hypertriglyceridemia (r = 0.16, p = 0.16). In both groups, triglyceride levels correlated with HDL mediated efflux ratio, even after adjusting for HDL-C levels (r=0.27, p=0.02). To understand the relationship between elevated triglyceride levels and ABCA-1 mediated cholesterol efflux function, CEPT, PLTP and LCAT activities were measured in plasma samples of these groups using validated fluorescent assays from Roar Biomedical. CETP and PLTP activities (assessed using median fluorescence intensities-MFI) were increased in hypertriglyceridemic subjects compared to subjects without elevated triglycerides levels: CETP activity (MFI: 417 ± 251 vs 335 ± 129, p=0.02) and PLTP activity (MFI: 45.3 ± 17.8 vs 39.8 ± 10.5, p=0.05). However, neither CETP activity (r=0.06, p=0.57), nor increased PLTP activity (r= -0.06, p=0.59) were associated with cholesterol efflux in this cross-sectional study. Triglyceride levels were significantly correlated with CETP activity (r=0.36, p=0.002) but the correlation with PLTP activity did not reach statistical significance (r=0.22, p=0.06). LCAT activity did not differ between the two groups (p=0.6).
Figure 1. ABCA-1 mediated cholesterol efflux in patients with type 2 diabetes and with or without hypertriglyceridemia.
Efflux ratio was greater in participants with both diabetes and increased triglyceride levels compared to participants with diabetes but without increased triglycerides (1a, p = 0.009, n=71). The efflux results were normalized to a standard pool. In contrast, HDL cholesterol levels were lower in subjects with both diabetes and increased triglyceride levels compared to participants with diabetes but without increased triglycerides (1b, p=0.04, n=72). These findings highlight the discrepancy between HDL cholesterol and ABCA-1 mediated efflux in hypertriglyceridemia. DM: type 2 diabetes, TG: Triglyceride levels between 150 mg/dL-500 mg/dL.
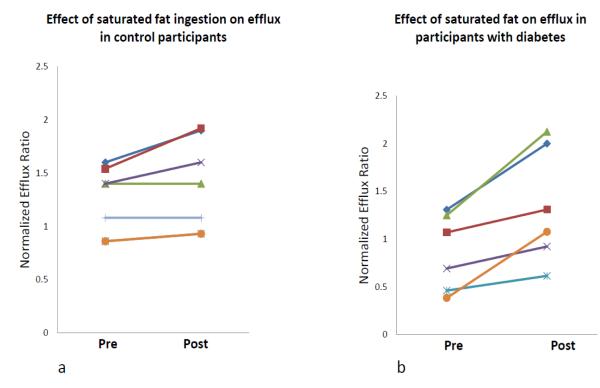
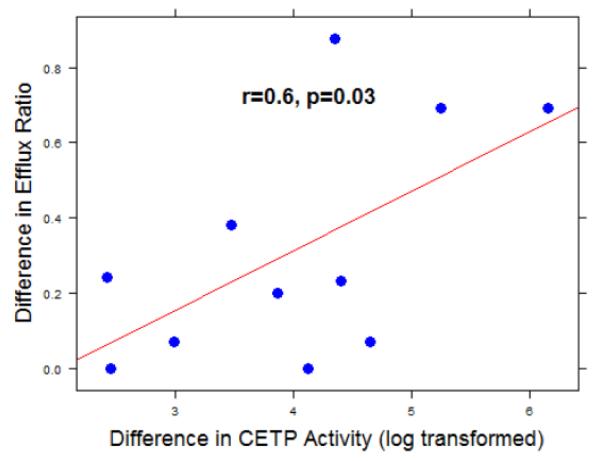
To test the effect of increased triglycerides on HDL mediated efflux, a fat challenge test was performed. For this part of our study, 6 participants with T2DM and 7 nominally healthy controls were recruited. HDL mediated ABCA-1 efflux function and lipid profiles were measured in all participants before and 4 hours following an ingestion of a standardized high-fat test as described above. CETP function was measured in plasma of 6 controls and 5 participants with diabetes before and after the fat ingestion. Control subjects were on average younger individuals compared to the subjects with diabetes (40 ± 14 vs. 59 ± 6 years, p<0.01). A summary of participants’ characteristics is provided in table 3. The baseline relative efflux ratio was lower in diabetic participants than in non-diabetic subjects, but the baseline difference did not reach statistical significance (0.861 vs. 1.24, p=0.08). Four hours after ingestion of a high-fat meal, significant increases in relative efflux ratio were observed in both groups (figures 2a and 2b). However, the increase in diabetic cohort was of a higher magnitude than in non-diabetic control participants as shown in table 3. The difference in efflux (efflux ratio post fat meal ingestion – efflux ratio pre fat meal ingestion) was significantly associated with difference in CETP activity following the fat challenge (r = 0.6, p = 0.03), figure 3. At baseline, both CETP activity and triglyceride levels did not correlate with efflux function in the control group (CETP: p=0.28, TG: p=0.7) nor the diabetic group (CETP: p=0.8, TG: p=0.2).
TABLE 3.
Fat challenge study.
| Control Participants (n=7) Group 1 |
Diabetic Participants (n=6) Group 2 |
P (Baseline) | |||||
|---|---|---|---|---|---|---|---|
| Age (years) | 40 ± 14 | 59± 6 | 0.001 | ||||
| Sex | 3M:4F | 4M: 2F | 0.4 | ||||
| BMI (kg/m2) | 25± 5 | 35± 5 | 0.009 | ||||
| Hemoglobin A1c (%) | 5.3± 0.36 | 8.5± 2.8 | 0.04 | ||||
| Fat challenge | Pre-Fat | Post-Fat | P | Pre-Fat | Post-Fat | P | |
| HDL (mg/dL) | 63 ±18 | 60 ±25 | 0.9 | 46 ±15 | 40 ±13 | 0.01 | 0.1 |
| Triglycerides (mg/dL) | 118 ± 108 | 251 ± 23 | 0.07 | 161 ± 73 | 351 ± 17 | 0.01 | 0.03 |
| CETP (MFI)* | 217 ± 75 | 246 ± 49 | 0.19 | 419 ± 221 | 527 ± 20 | 0.27 | 0.07 |
| Efflux Ratio ** | 1.24 ± 0.31 | 1.39 ± 0.42 | 0.04 | 0.86 ± 0.4 | 1.34 ± 0.6 | 0.01 | 0.08 |
Data are means ± SD, performed fasting and 4 hours following an ingestion of high-fat standardized test meal.
: Median Fluorescence Intensity. CETP activity was assessed in 6 controls and 5 participants with diabetes
Efflux measurements were normalized to a standard pool and reported as ratios.
Figure 2. Effect of a fat challenge on the ability of HDL to facilitate ABCA-1 mediated cholesterol efflux.
An increase in efflux ratio after ingestion of high-fat meal was observed in non-diabetic controls (figure 2a, p=0.04, n=6), and in participants with diabetes (figure 2b, p=0.01, n=7), although the magnitude of the increase was greater in diabetic participants. The efflux results were normalized to a standard pool.
Figure 3. The difference in CETP activity correlates with the difference in HDL mediated efflux ratio.
The difference in efflux ratio was significantly correlated to the difference in CETP activity. CETP activity was log transformed (non-normally distributed) pre and post the fat challenge, n=11 (6 controls and 5 participants with diabetes).
Discussion
Two previous studies suggested an increase in ABCA-1 mediated efflux function of HDL using the J774 macrophage cell system of type IV hypertriglyceridemic compared to normolipidemic subjects [8, 16]. We now extend this observation to patients with T2DM and hypertriglyceridemia using BHK cell system with mifepristone inducible ABCA-1 expression. Our results suggest that hypertriglyceridemia facilitates increased ABCA-1 mediated cholesterol efflux capacity that could partly be explained by changes in CETP function. The first part of our study compared ABCA-1 mediated cholesterol efflux function of HDL particles in diabetic subjects with presence and absence of hypertriglyceridemia. The results suggest that participants with hypertriglyceridemia had increased HDL mediated efflux despite low HDL-C. The second part of our study demonstrated an increase in HDL mediated efflux in diabetic and healthy subjects following a single ingestion of a saturated fatty drink that elevated triglyceride levels. Thus, our results suggest that HDL mediated cholesterol efflux through the ABCA-1 transporter may not be compromised in the setting of high triglycerides in T2DM. On the contrary, increased levels of triglycerides in diabetes may produce a compensatory effect of maintaining ABCA-1 mediated efflux, possibly through the actions of CETP to liberate prebeta-1 HDL. In one past study, hypertriglyceridemia did not alter cholesterol efflux out of J774 macrophages when these macrophages were not induced by c-AMP to express ABCA-1 [17]. Thus, the effect of hypertriglyceridemia is ABCA-1 mediated, likely involving an interaction with lipid-poor or prebeta-1 HDL. The discrepancy between HDL-C and HDL mediated ABCA-1 efflux capacity is illustrated in one prior study showing variable HDL mediated efflux capacities in samples from participants with the same Apo A-I or HDL-C concentrations [18]. CETP activity was increased in the group of subjects with hypertriglyceridemia and diabetes compared to diabetes without elevated triglyceride levels. However, we did not observe a significant relation between CETP activity and HDL mediated ABCA-1 efflux in this group. That could be related to the heterogeneity in the causes for the increased triglyceride levels in the fasting state of the cross sectional group. A more direct approach to examine this relationship was through the fat challenge. In this part of the study, differences in CETP activity after fat ingestion significantly explained differences in HDL mediated cholesterol efflux.
An earlier Study by Sasahara et al[19] suggested that the ability of plasma to facilitate cholesterol efflux out of fibroblasts differed between obese and lean individuals. Specifically, there was an increase in cholesterol efflux to prebeta-1 HDL particles together with a decrease in efflux to larger alpha HDL particles in obesity. More recently, the same group demonstrated that plasma from insulin resistant individuals demonstrated greater cholesterol efflux out of THP-1 macrophages compared to insulin sensitive individuals[20]. These changes were accompanied by an increase in prebeta-1 HDL in the setting of insulin resistance. Our findings here add to these studies suggesting a role for increased triglycerides and CETP activity for the increase in HDL mediated efflux in subjects with diabetes.
Two recent studies [2, 3] presented conflicting reports on the relevance of HDL mediated cholesterol efflux on atherosclerosis and CVD events. In a seminal study, Khera et al. demonstrated that lower HDL mediated cholesterol efflux better correlated with measures of atherosclerosis by carotid ultrasound or angiography than HDL-C. In agreement, Li et al. showed that lower cholesterol efflux was significantly associated with increased atherosclerosis by angiography in a cross-sectional cohort. In the same paper, Li et al. examined samples from a second cohort (prospective) where increased cholesterol efflux at baseline predicted incident CVD events at the 3 years follow-up. Our findings may provide an explanation for these apparently discrepant reports. Our study suggests that ABCA-1 efflux is increased in T2DM and hypertriglyceridemia, a condition that is associated with increased CVD risk. Thus, increases in ABCA-1 efflux might reflect states of HDL catabolism and alterations in several cardiac risk factors (such as oxidative stress, endothelial dysfunction), where the latter can offset benefits associated with increased ABCA-1 efflux. Alternatively, increases in ABCA-1 efflux might not translate into more efficient RCT. Recent findings suggest a “shuttle” role for lipid-poor Apo AI [21, 22] in RCT. Specifically, lipid-poor Apo A-I (and albumin) were shown to efficiently and synergistically efflux cholesterol, perhaps as a function of their plasma abundance and physicochemical properties. However, this cholesterol was then shuttled to accepters with a greater capacity for storage and transport: such as ApoB containing lipoproteins through CETP or RBCs through diffusional efflux pathways [23]. Thus, it is not clear if modest changes in lipid-poor Apo A-I activity alone (evidenced by change in ABCA-1 efflux) would be the rate limiting step for RCT. The relevance of diffusional efflux pathways (through RBCs, albumin, and large HDL) vs lipid-poor Apo A-I on net cholesterol excretion or CVD progression in clinical studies remains to be demonstrated.
We did not measure HDL TGs, but HDL TGs were previously shown to strongly associate with plasma TGs in hypertriglyceridemia of T2DM [24]. In addition, HDL TGs inversely correlated with ACAT activity [24]. Thus, HDL TG increase and HDL CE decrease in diabetes favor net cholesterol transport by decreasing the esterification of cholesterol donated from HDL. Since we used an ACAT inhibitor in all the efflux steps, this protocol masks the effect of endogenous ACAT activity by preventing cholesterol influx (HDL donated cholesterol) from getting esterified. Although this is a limitation, it is also an advantage as it standardizes the assay across all samples allowing examining the capacity of HDL to mediate efflux without having to adjust for influx (by preventing esterification). This approach was used in the two recent large studies associating HDL efflux with CVD [2, 3]. Indeed, the conflicting results of these two studies might reflect the limitation of focusing on ABCA-1 efflux as the sole indicator of HDL function or RCT. Our findings here would question the rational for therapies designed to improve ABCA-1 efflux in diabetes and hypertriglyceridemia where ABCA-1 activity is maintained, if not increased.
Limitations
In our study, we concentrated on the effect of hypertriglyceridemia on ABCA-1 mediated cholesterol efflux function by HDL, and did not investigate other RCT mechanisms, such as HDL mediated efflux of cholesterol and phospholipids by the interaction of ATP-binding cassette sub-family G member 1 protein (ABCG1 transporter) or scavenger receptor B1 (SR-B1). In a recent study, post prandial enrichment of HDL with triglycerides was shown to impair the capacity of hepatic cells expressing SR-BI receptors to uptake HDL despite facilitating ABCA-1, ABCG-1 and SR-BI efflux at the macrophage level [25]. Thus, the relative contributions of ABCA-1 efflux at the macrophage level together with the capacity of HDL for liver uptake or intestinal excretion is warranted to better assess the implications of hypertriglyceridemia on RCT. A second limitation of this study is that we did not assess whole plasma efflux. Recent findings suggest an important role for VLDL and LDL particles in accepting cholesterol from HDL [20, 21, 26]. However, whole plasma cholesterol efflux might involve several other cholesterol acceptors, making changes in efflux difficult to interpret without sample fractionation. A third limitation is that we did not measure prebeta-1 HDL particles in plasma. Such measurement would allow us to directly assess the relation between the concentrations of prebeta-1 HDL and its ability to facilitate ABCA-1 efflux function in hypertriglyceridemia and after the fat challenge. Finally, we observed a non-significant difference between HDL mediated efflux in the control and the diabetic groups, perhaps as a function of the small sample size. In addition, differences in the age and adiposity between the two groups could confound the response to the fat challenge.
Conclusions
Our results suggest that hypertriglyceridemia facilitates HDL mediated ABCA-1 mediated efflux in diabetes in part by activating CETP. Future studies are needed to address the relevance of HDL vs whole plasma mediated cholesterol efflux on CVD progression using not only ABCA-1 mediated expressing systems but through diffusional efflux pathways inorder to better define the critical steps of RCT.
Supplementary Material
Supplementary Figure I
Title: Dependence of efflux on apoB depleted plasma concentrations.
The data suggests that 2.5% apoB depleted plasma was in the mid-range of the standard curve and this concentration was used in the rest of the study.
TABLE 2.
Differences in the lipid measurements in this study between the diabetic subjects with and without high triglyceride levels
| Metabolic Measurements | Diabetic Participants with Hypertriglyceridemia (n=45) Group 1 |
Diabetic Participants without Hypertriglyceridemia (n=26) Group 2 |
|
|---|---|---|---|
| Hemoglobin A1c (%) | 8 ± 2 | 8 ± 2 | 0.28 |
| HDL cholesterol (mg/dL) | 44 ± 11 | 50 ± 13 | 0.04 |
| LDL cholesterol (mg/dL) | 112 ± 33 | 106 ± 30 | 0.41 |
| Triglycerides (mg/dL) | 226 ± 88 | 91 ± 22 | < 0.0001 |
| Apo A-I (mg/dL) | 135 ± 19 | 138 ± 40 | 0.8 |
| CETP activity (MFI)* | 417 ± 251 | 335 ± 129 | 0.02 |
| PLTP activity (MFI)* | 45.3 ± 17.8 | 39.8 ± 10.5 | 0.05 |
| LCAT activity (MFI)* | 1.41± 0.19 | 1.42± 0.12 | 0.6 |
| Efflux Ratio (Normalized)** | 1.17 ± 0.25 | 1.03 ± 0.19 | 0.0098 |
Data are means ± SD
: Median Fluorescence Intensity
Efflux measurements were normalized to a standard pool and reported as ratios.
Acknowledgements
We would like to thank Jianhua Fan with her assistance running the efflux assays.
Sources of funding: Dr. Yassine was supported by K23HL107389, AHA12CRP11750017 and USC CTSI pilot UL1TR000130. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Abbreviations
- HDL
High density lipoprotein
- HDL-C
High Density Lipoprotein cholesterol
- ABCA-1
adenosine triphosphate binding cassette transporter protein
- T2DM
Type 2 diabetes
- TG
Triglycerides
- CETP
Cholesteryl Ester Transfer Protein
- MS
Metabolic Syndrome
- RCT
Reverse Cholesterol Transport
- VLDL
Very Low Density Lipoprotein
- CaTS
Center for Clinical and Translational Sciences
- CABG
Coronary Artery Bypass Graft
- PTCA
Percutaneous transluminal angioplasty
- CVD
Cardiovascular Disease
- ABCG-1
ATP-binding cassette sub-family G member 1 protein
- SR-B1
Scavenger receptor B1
- OGTT
Oral Glucose Tolerance Test
- HNY
Design, Experiments, manuscript writing, statistical analysis
- AB
manuscript writing
- CS
running assays
- CSS
Design
- SSL
Design
- PDR
manuscript writing, study design
Footnotes
There are no competing interests by any of the authors.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cuchel M, Rader DJ. Macrophage reverse cholesterol transport: Key to the regression of atherosclerosis? Circulation. 2006;113(21):2548–55. doi: 10.1161/CIRCULATIONAHA.104.475715. [DOI] [PubMed] [Google Scholar]
- 2.Khera AV, Cuchel M, de la Llera-Moya M, et al. Cholesterol efflux capacity, high-density lipoprotein function, and atherosclerosis. N Engl J Med. 2011;364(2):127–35. doi: 10.1056/NEJMoa1001689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li XM, Tang WH, Mosior MK, et al. Paradoxical association of enhanced cholesterol efflux with increased incident cardiovascular risks. Arterioscler Thromb Vasc Biol. 2013;33(7):1696–705. doi: 10.1161/ATVBAHA.113.301373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grundy SM, Brewer HB, Jr., Cleeman JI, et al. Definition of metabolic syndrome: Report of the national heart, lung, and blood institute/american heart association conference on scientific issues related to definition. Circulation. 2004;109(3):433–8. doi: 10.1161/01.CIR.0000111245.75752.C6. [DOI] [PubMed] [Google Scholar]
- 5.Kwiterovich PO., Jr The metabolic pathways of high-density lipoprotein, low-density lipoprotein, and triglycerides: A current review. Am J Cardiol. 2000;86(12A):5L–10L. doi: 10.1016/s0002-9149(00)01461-2. [DOI] [PubMed] [Google Scholar]
- 6.Clay MA, Newnham HH, Forte TM, et al. Cholesteryl ester transfer protein and hepatic lipase activity promote shedding of apo a-i from hdl and subsequent formation of discoidal hdl. Biochim Biophys Acta. 1992;1124(1):52–8. doi: 10.1016/0005-2760(92)90125-f. [DOI] [PubMed] [Google Scholar]
- 7.Favari E, Lee M, Calabresi L, et al. Depletion of pre-beta-high density lipoprotein by human chymase impairs atp-binding cassette transporter a1- but not scavenger receptor class b type i-mediated lipid efflux to high density lipoprotein. J Biol Chem. 2004;279(11):9930–6. doi: 10.1074/jbc.M312476200. [DOI] [PubMed] [Google Scholar]
- 8.Attia N, Ramaharo A, Paul JL, et al. Enhanced removal of cholesterol from macrophage foam cells to serum from type iv hypertriglyceridemic subjects. Atherosclerosis. 2008;198(1):49–56. doi: 10.1016/j.atherosclerosis.2007.09.023. [DOI] [PubMed] [Google Scholar]
- 9.Oram JF, Wolfbauer G, Vaughan AM, et al. Phospholipid transfer protein interacts with and stabilizes atp-binding cassette transporter a1 and enhances cholesterol efflux from cells. J Biol Chem. 2003;278(52):52379–85. doi: 10.1074/jbc.M310695200. [DOI] [PubMed] [Google Scholar]
- 10.de la Llera-Moya M, Drazul-Schrader D, Asztalos BF, et al. The ability to promote efflux via abca1 determines the capacity of serum specimens with similar high-density lipoprotein cholesterol to remove cholesterol from macrophages. Arterioscler Thromb Vasc Biol. 2011;30(4):796–801. doi: 10.1161/ATVBAHA.109.199158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Asztalos BF, de la Llera-Moya M, Dallal GE, et al. Differential effects of hdl subpopulations on cellular abca1- and sr-bi-mediated cholesterol efflux. Journal of Lipid Research. 2005;46(10):2246–53. doi: 10.1194/jlr.M500187-JLR200. [DOI] [PubMed] [Google Scholar]
- 12.Bajnok L, Seres I, Varga Z, et al. Relationship of endogenous hyperleptinemia to serum paraoxonase 1, cholesteryl ester transfer protein, and lecithin cholesterol acyltransferase in obese individuals. Metabolism. 2007;56(11):1542–9. doi: 10.1016/j.metabol.2007.06.022. [DOI] [PubMed] [Google Scholar]
- 13.Kontush A, Therond P, Zerrad A, et al. Preferential sphingosine-1-phosphate enrichment and sphingomyelin depletion are key features of small dense hdl3 particles: Relevance to antiapoptotic and antioxidative activities. Arterioscler Thromb Vasc Biol. 2007;27(8):1843–9. doi: 10.1161/ATVBAHA.107.145672. [DOI] [PubMed] [Google Scholar]
- 14.Niesor EJ, Magg C, Ogawa N, et al. Modulating cholesteryl ester transfer protein activity maintains efficient pre-beta-hdl formation and increases reverse cholesterol transport. J Lipid Res. 51(12):3443–54. doi: 10.1194/jlr.M008706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Robins SJ, Lyass A, Brocia RW, et al. Plasma lipid transfer proteins and cardiovascular disease. The framingham heart study. Atherosclerosis. 2013 doi: 10.1016/j.atherosclerosis.2013.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fournier N, Francone O, Rothblat G, et al. Enhanced efflux of cholesterol from abca1-expressing macrophages to serum from type iv hypertriglyceridemic subjects. Atherosclerosis. 2003;171(2):287–93. doi: 10.1016/j.atherosclerosis.2003.08.011. [DOI] [PubMed] [Google Scholar]
- 17.Brites FD, Bonavita CD, De Geitere C, et al. Alterations in the main steps of reverse cholesterol transport in male patients with primary hypertriglyceridemia and low hdl-cholesterol levels. Atherosclerosis. 2000;152(1):181–92. doi: 10.1016/s0021-9150(99)00452-9. [DOI] [PubMed] [Google Scholar]
- 18.de la Llera-Moya M, Drazul-Schrader D, Asztalos BF, et al. The ability to promote efflux via abca1 determines the capacity of serum specimens with similar high-density lipoprotein cholesterol to remove cholesterol from macrophages. Arterioscler Thromb Vasc Biol. 2010;30(4):796–801. doi: 10.1161/ATVBAHA.109.199158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sasahara T, Nestel P, Fidge N, et al. Cholesterol transport between cells and high density lipoprotein subfractions from obese and lean subjects. J Lipid Res. 1998;39(3):544–54. [PubMed] [Google Scholar]
- 20.Nestel P, Hoang A, Sviridov D, et al. Cholesterol efflux from macrophages is influenced differentially by plasmas from overweight insulin-sensitive and -resistant subjects. Int J Obes (Lond) 2012;36(3):407–13. doi: 10.1038/ijo.2011.170. [DOI] [PubMed] [Google Scholar]
- 21.Hoang A, Drew BG, Low H, et al. Mechanism of cholesterol efflux in humans after infusion of reconstituted high-density lipoprotein. European heart journal. 2012;33(5):657–65. doi: 10.1093/eurheartj/ehr103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sankaranarayanan S, de la Llera-Moya M, Drazul-Schrader D, et al. Serum albumin acts as a shuttle to enhance cholesterol efflux from cells. Journal of lipid research. 2013;54(3):671–6. doi: 10.1194/jlr.M031336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hung KT, Berisha SZ, Ritchey BM, et al. Red blood cells play a role in reverse cholesterol transport. Arteriosclerosis, thrombosis, and vascular biology. 2012;32(6):1460–5. doi: 10.1161/ATVBAHA.112.248971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brinton EA, Oram JF, Bierman EL. The effect of variations in lipid composition of high-density lipoprotein on its interaction with receptors on human fibroblasts. Biochim Biophys Acta. 1987;920(1):68–75. doi: 10.1016/0005-2760(87)90312-2. [DOI] [PubMed] [Google Scholar]
- 25.Julia Z, Duchene E, Fournier N, et al. Postprandial lipemia enhances the capacity of large hdl2 particles to mediate free cholesterol efflux via sr-bi and abcg1 pathways in type iib hyperlipidemia. J Lipid Res. 2010;51(11):3350–8. doi: 10.1194/jlr.P009746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chan DC, Hoang A, Barrett PHR, et al. Apolipoprotein b-100 and apoa-ii kinetics as determinants of cellular cholesterol efflux. Journal of Clinical Endocrinology & Metabolism. 2012;97(9):E1658–E66. doi: 10.1210/jc.2012-1522. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure I
Title: Dependence of efflux on apoB depleted plasma concentrations.
The data suggests that 2.5% apoB depleted plasma was in the mid-range of the standard curve and this concentration was used in the rest of the study.