Abstract
In this study, we determined if vitamin D could inhibit oxidative stress-induced thromboxane production by placental trophoblasts. Trophoblast isolated from normal placentas were stimulated with CoCl2, a hypoxic mimicking agent, with or without pretreatment of 1,25(OH)2D3. Soluble phospholipase-A2, metabolites of thromboxane-A2 and prostacyclin, and 8-isoprostane were measured. Expression of cyclooxygenase-1 (COX-1), COX-2, and heme oxygenase-1 (HO-1) were determined. We found that pretreatment of trophoblasts with 1,25(OH)2D3 significantly reduced 8-isoprostane and the ratio of thromboxane-A2 to prostacyclin production, and blocked COX-2 expression induced by CoCl2. These results provide evidence of the beneficial effects of vitamin D on placental trophoblasts.
Keywords: vitamin D, COX-2, thromboxane, trophoblast
Introduction
Recent studies have shown that vitamin D is beneficial to both maternal and fetal health. Sufficient vitamin D intake during pregnancy reduces risk of pregnancy complications such as gestational diabetes and preterm birth [1,2]. In contrast, vitamin D deficiency during pregnancy has been linked to several adverse pregnancy outcomes including those associated with placental insufficiency such as preeclampsia and low birth weight [1,3–5]. Vitamin D exerts many biological effects on the placenta. It regulates lactogen expression [6], human chorionic gonadotropin secretion [7], and calcium transport [8]. Vitamin D also down-regulates inflammatory cytokine TNFα and IL-6 expression in placental trophoblasts [9].
Increased thromboxane and decreased prostacyclin production is a characteristic of placental trophoblast dysfunction in preeclampsia [10–12]. Thromboxane is a potent vasoconstrictor and prostacyclin is a potent vasodilator. However, effects of vitamin D on thromboxane and prostacyclin production have never been studied. Since hypoxia/oxidative stress promotes trophoblast thromboxane production [13], in this study we specifically investigated the role of vitamin D in hypoxia/oxidative stress-induced thromboxane production in placental trophoblasts.
Materials and Methods
Placental trophoblasts were isolated by trypsin digestion as previously described [14]. All placentas were delivered by uncomplicated pregnancies. Placental collection was approved by the IRB at Louisiana State University Health Sciences Center-Shreveport. Freshly isolated trophoblasts were seeded into 6 well/plates (5×106 cells/well) and cultured with Dulbecco’s Modified Eagle Medium containing 5% fetal bovine serum and antibiotics. On day-2, cells were treated with 1,25(OH)2D3 in the presence or absence of cobalt (II) chloride (CoCl2). CoCl2 is a chelate interfering Fe2+ on hemoglobin [15]. It can initiate oxygen-sensing signal transduction pathway and upregulates erythropoietin and HIFα expression [16,17]. Thus, CoCl2 has been widely used as a hypoxic mimicking agent to induce tissue or cell hypoxia/oxidative stress in in vivo and in vitro studies [18–22]. After 48hrs of culture, medium and total cellular protein were collected and stored at −80C until assay. All chemicals and reagents were from Sigma (St. Louis, MO) unless otherwise noted.
Medium levels of TXB2 and 6-keto PGF1α (stable metabolites of thromboxane-A2 and prostacyclin), soluble phospholipase-A2 (sPLA2), and 8-isoprostane were measured by enzyme immunoassay. Assay kits were purchased from Cayman (Ann Arbor, MI). An aliquot of 100μl of sample was assayed in duplicate. Within- and between-assay variations were < 8% for all assays.
Protein expression for cyclooxygenase-1 (COX-1), COX-2, and HO-1 were determined by Western blot. Antibody for COX-1 (sc-1752) and COX-2 (sc-19999) were from Santa Cruz (San Diego, CA) and for HO-1 (610713) was from BD Biosciences (San Jose, CA). β-actin expression was determined and used as the loading control for each sample. Densities were analyzed by NIH Image 1.16.
Data is presented as mean ± SE and analyzed by analysis of variance (ANOVA). Student-Newman-Keuls test was used as post hoc tests. A probability level less than 0.05 was set as statistically significant.
Results and Discussion
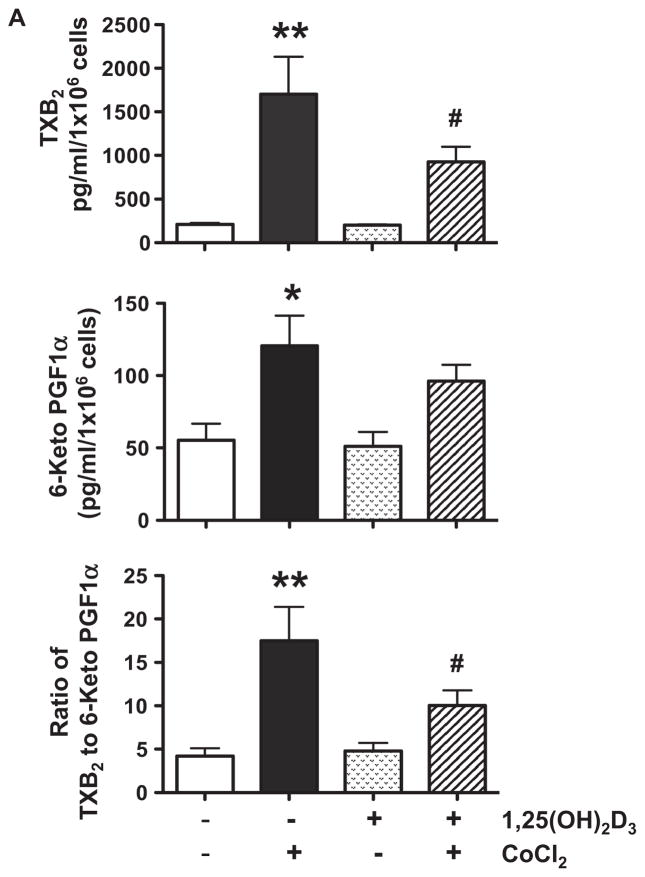
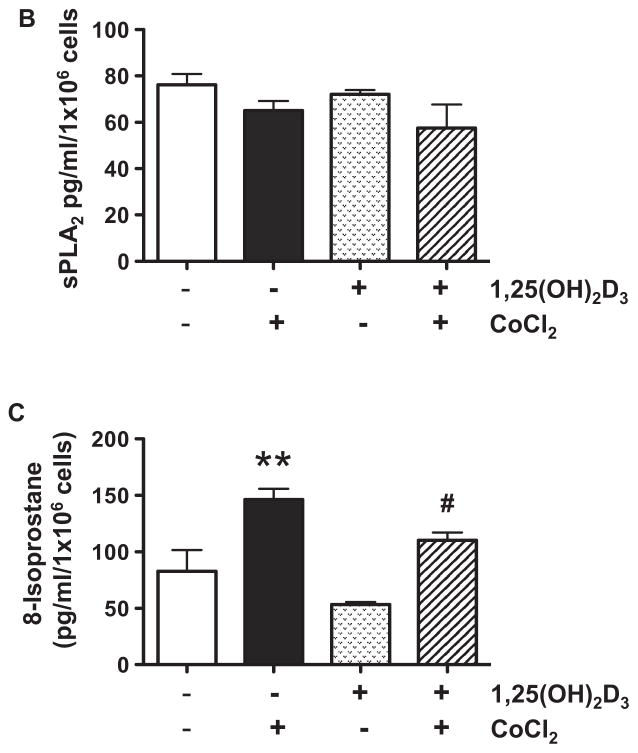
Results of trophoblast production of TXB2, 6-Keto PGF1α, and sPLA2 are shown in Figure 1. We found that cells treated with CoCl2 produced significantly more TXB2, p<0.01, over 6-Keto PGF1α, p<0.05, but had no effect on sPLA2 release. 1,25(OH)2D3 alone had no affect on TXB2, 6-Keto PGF1α, and sPLA2 production (Figure 1A and B), but significantly reduced TXB2 production and the ratio of TXB2 to 6-Keto PGF1α induced by CoCl2, p<0.05. To determine CoCl2-induce oxidative stress, we examined 8-isoprostane production. Isoprostanes are a family of eicosanoids of non-enzymatic origin produced by the random oxidation of tissue phospholipids by oxygen radicals. Increased 8-isoprostane production is a marker of increased oxidative stress and lipid peroxide production [23]. Our results showed that the pattern of 8-isoprostane production (Figure 1C) was similar to TXB2. Suppression of CoCl2-induced 8-isoprostane production by 1,25(OH)2D3 provided evidence of the anti-oxidative effect of vitamin D on placental trophoblasts. These observations are very important because oxidative stress-induced thromboxane production by trophoblasts is believed to contribute to increased placental vasoconstriction in preeclampsia [11]. Thromboxane facilitates platelet aggregation. Thus, reduce lipid peroxide and thromboxane production or reduced ratio of thromboxane to prostacyclin would result in prostacyclin dominance that promotes circulation and retards thrombosis in the placenta.
Figure 1.
Trophoblast production of TXB 2, 6-Keto PGF1α, sPLA2, and 8-isoprostane. Data are expressed as mean ± SE from 6 independent experiments. A: TXB2 and 6-Keto PGF1α production and the ratio of TXB2 to 6-Keto PGF1α. B. sPLA2 release. C. 8-isoprostane production. * p<0.05 and ** p<0.01: cells treated with CoCl2 vs. control untreated cells; # p<0.05: cells pretreated with 1,25(OH)2D3+ CoCl2 vs. cells treated with CoCl2. Cells treated with CoCl2 produced significantly more TXB2, 6-Keto PGF1α, and 8-isoprostane, but had no effect on sPLA2 release. 1,25(OH)2D3 significantly reduced TXB2 and 8-isoprostane production and the ratio of TXB2 to 6-Keto PGF1α induced by CoCl2.
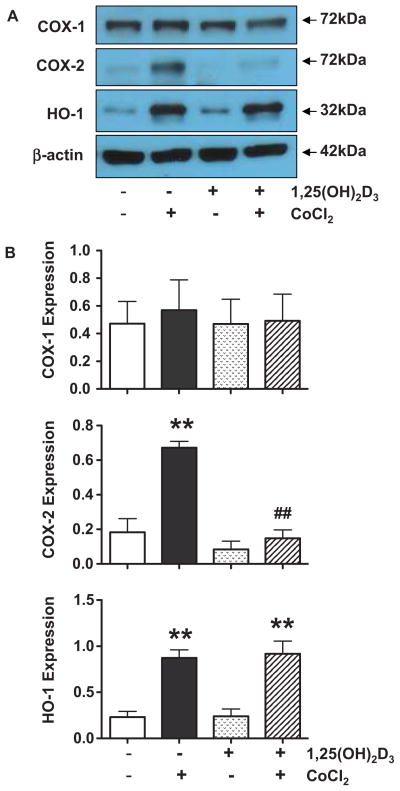
Inhibition of CoCl2-induced COX-2 up-regulation by 1,25(OH)2D3 is another important finding in this study (Figure 2). PLA2 liberates AA from membrane phospholipids and then COX and peroxidase convert AA to thromboxane, prostacyclin, and prostaglandins. Our results showed that CoCl2 had no effect on sPLA2 release and COX-1 expression, but significantly up-regulated COX-2 expression, which could be inhibited by 1,25(OH)2D3. We did not examine thromboxane synthase and prostacyclin synthase expression, because CoCl2-induced thromboxane and prostacyclin production could be blocked by specific COX-2 inhibitor NS-398 (Figure 1 supplement). Therefore, inhibition of CoCl2-induced COX-2 up-regulation could be a mechanism of 1,25(OH)2D3 suppression of hypoxia/oxidative stress-induced thromboxane and 8-isoprostane production.
Figure 2.
Trophoblast expression of COX-1, COX-2, and A:HO-1. Representative blots for COX-1, COX-2, and HO-1 expression in control cells, cells treated with CoCl2 with or without pretreated with 1,25(OH)2D3. B: Relative COX-1, COX-2, and HO-1 expression after normalized by β-actin expression in each sample. ** p<0.01: cells treated with CoCl2 vs. control untreated cells; ## p<0.01: cells pretreated with 1,25(OH)2D3+ CoCl2 vs. cells treated with CoCl2. CoCl2 had no effect on COX-1 expression, but significantly increased COX-2 and HO-1 expression. Pretreatment of the cells with 1,25(OH)2D3 blocked CoCl2 induced COX-2, but not HO-1, expression.
We also examined HO-1 expression. HO-1 is a fundamental ‘sensor’ of cellular stress and directly contributes to limit or prevent tissue damage [24]. HO-1 is induced not only by the substrate heme but also by a variety of agents causing inflammation and oxidative stress [24]. HO-1 induction participates in cellular adaptation to stress and are involved in the mechanisms of defense [25]. Our results showed that HO-1 expression was increased when cells were exposed to CoCl2 even in the presence of 1,25(OH)2D3 (Figure 2), which suggest that up-regulation of COX-2 and HO-1 induced by CoCl2 are regulated through different mechanisms and also imply the specificity of COX-2 suppression by 1,25(OH)2D3.
Maternal vitamin D levels are lower in preeclampsia than in normotensive pregnant women [5,26]. Vitamin D insufficiency/deficiency has emerged as an independent risk factor not only for preeclampsia [5] but also for cardiovascular diseases [27,28]. Although studies have suggested that vitamin D metabolic/generating system, including vitamin D binding protein, 25-hydroxylase, 1α-hydroxylase, 24-hydroxylase, and vitamin D receptor, is present in placental trophoblasts [3,21,29,30], the role of vitamin D and its down-stream effects on trophoblast function are largely unknown. Nonetheless, results from the present study, i.e. 1,25(OH)2D3 suppresses hypoxia/oxidative stress-induced COX-2 up-regulation and thromboxane production by trophoblasts, provides further evidence of the beneficial effects of vitamin D on the placenta.
Supplementary Material
Acknowledgments
This study was supported in part by grants from NIH, NHLBI HL65997 to YW.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Brannon PM. Vitamin D and adverse pregnancy outcomes: beyond bone health and growth. Proc Nutr Soc. 2012;71(2):205–12. doi: 10.1017/S0029665111003399. [DOI] [PubMed] [Google Scholar]
- 2.Urrutia RP, Thorp JM. Vitamin D in pregnancy: current concepts. Curr Opin Obstet Gynecol. 2012;24:57–64. doi: 10.1097/GCO.0b013e3283505ab3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shin JS, Choi MY, Longtine MS, Nelson DM. Vitamin D effects on pregnancy and the placenta. Placenta. 2010;31:1027–1034. doi: 10.1016/j.placenta.2010.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baker AM, Haeri S, Camargo CA, Jr, Espinola JA, Stuebe AM. A nested case-control study of midgestation vitamin D deficiency and risk of severe preeclampsia. J Clin Endocrinol Metab. 2010;95:5105–5019. doi: 10.1210/jc.2010-0996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bodnar LM, Catov JM, Simhan HN, Holick MF, Powers RW, Roberts JM. Maternal vitamin D deficiency increases the risk of preeclampsia. J Clin Endocrinol Metab. 2007;92:3517–3522. doi: 10.1210/jc.2007-0718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stephanou A, Ross R, Handwerger S. Regulation of human placental lactogen expression by 1,25-dihydroxyvitamin D3. Endocrinology. 1994;135:2651–2656. doi: 10.1210/endo.135.6.7988455. [DOI] [PubMed] [Google Scholar]
- 7.Barrera D, Avila E, Hernández G, Méndez I, González L, Halhali A, Larrea F, Morales A, Díaz L. Calcitriol affects hCG gene transcription in cultured human syncytiotrophoblasts. Reprod Biol Endocrinol. 2008;6:3. doi: 10.1186/1477-7827-6-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lester GE. Cholecalciferol and placental calcium transport. Fed Proc. 1986;45:2524–2527. [PubMed] [Google Scholar]
- 9.Noyola-Martínez N, Díaz L, Avila E, Halhali A, Larrea F, Barrera D. Calcitriol downregulates TNF-α and IL-6 expression in cultured placental cells from preeclamptic women. Cytokine. 2013;61:245–250. doi: 10.1016/j.cyto.2012.10.001. [DOI] [PubMed] [Google Scholar]
- 10.Walsh SW. Preeclampsia: An imbalance in placental prostacyclin and thromboxane production. Am J Obstet Gynecol. 1985;152:335–340. doi: 10.1016/s0002-9378(85)80223-4. [DOI] [PubMed] [Google Scholar]
- 11.Walsh SW, Parisi VM. The role of prostanoids and thromboxane in the regulation of placental blood flow. In: Rosenfeld CR, editor. The Uterine Circulation. Ithaca: Perinatology Press; 1989. pp. 273–298. [Google Scholar]
- 12.Walsh SW, Wang Y. Trophoblast and placental villous core production of lipid peroxides, thromboxane, and prostacyclin in preeclampsia. Journal of Clinical Endocrinology and Metabolism. 1995;80:1888–1893. doi: 10.1210/jcem.80.6.7775637. [DOI] [PubMed] [Google Scholar]
- 13.Bowen RS, Zhang Y, Gu Y, Lewis DF, Wang Y. Increased phospholipase A2 and thromboxane but not prostacyclin production by placental trophoblast cells from normal and preeclamptic pregnancies cultured under hypoxia condition. Placenta. 2005;26:402–409. doi: 10.1016/j.placenta.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 14.Ma R, Gu Y, Groome LJ, Wang Y. ADAM17 regulates TNFα production by placental trophoblasts. Placenta. 2011;32:975–980. doi: 10.1016/j.placenta.2011.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gluhcheva Y, Madzharova M, Zhorova R, Atanasov V, Ivanova Ju, Mitewa M. Cobalt(II)-induced changes in hemoglobin content and iron concentration in mice from different age groups. Medical Biotechnology. 2011:0023. [Google Scholar]
- 16.Goldberg MA, Schneider TJ. Similarities between the oxygen-sensing mechanisms regulating the expression of vascular endothelial growth factor and erythropoietin. J Biol Chem. 1994;269:4355–4359. [PubMed] [Google Scholar]
- 17.Dai ZJ, Gao J, Ma XB, Yan K, Liu XX, Kang HF, Ji ZZ, Guan HT, Wang XJ. Up-regulation of hypoxia inducible factor-1α by cobalt chloride correlates with proliferation and apoptosis in PC-2 cells. J Exp Clin Cancer Res. 2012;31:28. doi: 10.1186/1756-9966-31-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kamiya T, Hara H, Inagaki N, Adachi T. The effect of hypoxia mimetic cobalt chloride on the expression of EC-SOD in 3T3-L1 adipocytes. Redox Rep. 2010;15:131–134. doi: 10.1179/174329210X12650506623483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hayashi M, Sakata M, Takeda T, Yamamoto T, Okamoto Y, Sawada K, Kimura A, Minekawa R, Tahara M, Tasaka K, Murata Y. Induction of glucose transporter 1 expression through hypoxia-inducible factor 1alpha under hypoxic conditions in trophoblast-derived cells. J Endocrinol. 2004;183:145–154. doi: 10.1677/joe.1.05599. [DOI] [PubMed] [Google Scholar]
- 20.Chen JK, Zhan YJ, Yang CS, Tzeng SF. Oxidative stress-induced attenuation of thrombospondin-1 expression in primary rat astrocytes. J Cell Biochem. 2011;112:59–70. doi: 10.1002/jcb.22732. [DOI] [PubMed] [Google Scholar]
- 21.Ma R, Gu Y, Zhao S, Sun J, Groome LJ, Wang Y. Expressions of vitamin D metabolic components VDBP, CYP2R1, CYP27B1, CYP24A1, and VDR in placentas from normal and preeclamptic pregnancies. Am J Physiol Endocrinol Metab. 2012;303:E928–935. doi: 10.1152/ajpendo.00279.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ramkhelawon B, Yang Y, van Gils JM, Hewing B, Rayner KJ, Parathath S, Guo L, Oldebeken S, Feig JL, Fisher EA, Moore KJ. Hypoxia induces netrin-1 and Unc5b in atherosclerotic plaques: mechanism for macrophage retention and survival. Arterioscler Thromb Vasc Biol. 2013;33:1180–1188. doi: 10.1161/ATVBAHA.112.301008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fam SS, Morrow JD. The isoprostanes: unique products of arachidonic acid oxidation-a review. Curr Med Chem. 2003;10:1723–1740. doi: 10.2174/0929867033457115. [DOI] [PubMed] [Google Scholar]
- 24.Choi AM, Alam J. Heme oxygenase-1: function, regulation, and implication of a novel stress-inducible protein in oxidant-induced lung injury. Am J Respir Cell Mol Biol. 1996;15:9–19. doi: 10.1165/ajrcmb.15.1.8679227. [DOI] [PubMed] [Google Scholar]
- 25.Motterlini R, Foresti R. Heme oxygenase-1 as a target for drug discovery. Antioxid Redox Signal. 2003 Nov 1; doi: 10.1089/ars.2013.5658. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 26.Ainy E, Ghazi AA, Azizi F. Changes in calcium, 25(OH) vitamin D3 and other biochemical factors during pregnancy. J Endocrinol Invest. 2006;29:303–307. doi: 10.1007/BF03344100. [DOI] [PubMed] [Google Scholar]
- 27.AI Mheid I, Patel RS, Tangpricha V, Quyyumi AA. Vitamin D and cardiovascular disease: is the evidence solid? Eur Heart J. 2013 Jun 9; doi: 10.1093/eurheartj/eht166. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Motiwala SR, Wang TJ. Vitamin D and cardiovascular disease. Curr Opin Nephrol Hypertens. 2011;20:345–353. doi: 10.1097/MNH.0b013e3283474985. [DOI] [PubMed] [Google Scholar]
- 29.Díaz L, Sánchez I, Avila E, Halhali A, Vilchis F, Larrea F. Identification of a 25-hydroxyvitamin D3 1alpha-hydroxylase gene transcription product in cultures of human syncytiotrophoblast cells. J Clin Endocrinol Metab. 2000;85:2543–2549. doi: 10.1210/jcem.85.7.6693. [DOI] [PubMed] [Google Scholar]
- 30.Liu NQ, Hewison M. Vitamin D, the placenta and pregnancy. Arch Biochem Biophys. 2012;523:37–47. doi: 10.1016/j.abb.2011.11.018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.