Abstract
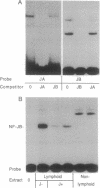
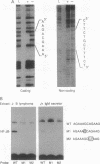
In a primary immune response a signal from interleukin 2 (IL-2) induces B lymphocytes to express the gene for the IgM joining component, the J chain. The signaling mechanism was pursued in this study by examining the J-chain gene 5' flanking region for regulatory sequences and interacting nuclear factors. The analyses identified a major control region located between -75 and -45 that encodes two adjacent elements: a T-rich sequence (JA) containing a single positive regulatory motif and an A+G-rich sequence (JB) containing overlapping positive and negative regulatory motifs. Dissection of the two elements indicated that the bifunctional JB sequence is the likely target of the IL-2 signal. The evidence was based on findings that (i) JB activity correlated with J-chain gene transcription--i.e., JB acts as a repressor in J-chain-silent B cells and as an activator in J-chain-expressing cells, and (ii) JB activator function is mediated by a B-cell-specific nuclear protein, NF-JB, that exhibits an IL-2-responsive binding pattern.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asao H., Takeshita T., Nakamura M., Nagata K., Sugamura K. Interleukin 2 (IL-2)-induced tyrosine phosphorylation of IL-2 receptor p75. J Exp Med. 1990 Mar 1;171(3):637–644. doi: 10.1084/jem.171.3.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackman M. A., Tigges M. A., Minie M. E., Koshland M. E. A model system for peptide hormone action in differentiation: interleukin 2 induces a B lymphoma to transcribe the J chain gene. Cell. 1986 Nov 21;47(4):609–617. doi: 10.1016/0092-8674(86)90625-2. [DOI] [PubMed] [Google Scholar]
- Cantrell D. A., Smith K. A. The interleukin-2 T-cell system: a new cell growth model. Science. 1984 Jun 22;224(4655):1312–1316. doi: 10.1126/science.6427923. [DOI] [PubMed] [Google Scholar]
- Durand D. B., Shaw J. P., Bush M. R., Replogle R. E., Belagaje R., Crabtree G. R. Characterization of antigen receptor response elements within the interleukin-2 enhancer. Mol Cell Biol. 1988 Apr;8(4):1715–1724. doi: 10.1128/mcb.8.4.1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eardley D. D., Koshland M. E. Glycosylphosphatidylinositol: a candidate system for interleukin-2 signal transduction. Science. 1991 Jan 4;251(4989):78–81. doi: 10.1126/science.1824727. [DOI] [PubMed] [Google Scholar]
- Gillies S. D., Morrison S. L., Oi V. T., Tonegawa S. A tissue-specific transcription enhancer element is located in the major intron of a rearranged immunoglobulin heavy chain gene. Cell. 1983 Jul;33(3):717–728. doi: 10.1016/0092-8674(83)90014-4. [DOI] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosschedl R., Baltimore D. Cell-type specificity of immunoglobulin gene expression is regulated by at least three DNA sequence elements. Cell. 1985 Jul;41(3):885–897. doi: 10.1016/s0092-8674(85)80069-6. [DOI] [PubMed] [Google Scholar]
- Karim F. D., Urness L. D., Thummel C. S., Klemsz M. J., McKercher S. R., Celada A., Van Beveren C., Maki R. A., Gunther C. V., Nye J. A. The ETS-domain: a new DNA-binding motif that recognizes a purine-rich core DNA sequence. Genes Dev. 1990 Sep;4(9):1451–1453. doi: 10.1101/gad.4.9.1451. [DOI] [PubMed] [Google Scholar]
- Kunkel T. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Proc Natl Acad Sci U S A. 1985 Jan;82(2):488–492. doi: 10.1073/pnas.82.2.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwabara M. D., Sigman D. S. Footprinting DNA-protein complexes in situ following gel retardation assays using 1,10-phenanthroline-copper ion: Escherichia coli RNA polymerase-lac promoter complexes. Biochemistry. 1987 Nov 17;26(23):7234–7238. doi: 10.1021/bi00397a006. [DOI] [PubMed] [Google Scholar]
- Mather E. L., Alt F. W., Bothwell A. L., Baltimore D., Koshland M. E. Expression of J chain RNA in cell lines representing different stages of B lymphocyte differentiation. Cell. 1981 Feb;23(2):369–378. doi: 10.1016/0092-8674(81)90132-x. [DOI] [PubMed] [Google Scholar]
- Matsui K., Nakanishi K., Cohen D. I., Hada T., Furuyama J., Hamaoka T., Higashino K. B cell response pathways regulated by IL-5 and IL-2. Secretory microH chain-mRNA and J chain mRNA expression are separately controlled events. J Immunol. 1989 Apr 15;142(8):2918–2923. [PubMed] [Google Scholar]
- McFadden H. J., Koshland M. E. Interleukin 2- and interleukin 5-induced changes in the binding of regulatory factors to the J-chain gene promoter. Proc Natl Acad Sci U S A. 1991 Dec 15;88(24):11027–11031. doi: 10.1073/pnas.88.24.11027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merida I., Gaulton G. N. Protein tyrosine phosphorylation associated with activation of the interleukin 2 receptor. J Biol Chem. 1990 Apr 5;265(10):5690–5694. [PubMed] [Google Scholar]
- Merida I., Pratt J. C., Gaulton G. N. Regulation of interleukin 2-dependent growth responses by glycosylphosphatidylinositol molecules. Proc Natl Acad Sci U S A. 1990 Dec;87(23):9421–9425. doi: 10.1073/pnas.87.23.9421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minie M. E., Koshland M. E. Accessibility of the promoter sequence in the J-chain gene is regulated by chromatin changes during B-cell differentiation. Mol Cell Biol. 1986 Nov;6(11):4031–4038. doi: 10.1128/mcb.6.11.4031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakanishi K., Cohen D. I., Blackman M., Nielsen E., Ohara J., Hamaoka T., Koshland M. E., Paul W. E. Ig RNA expression in normal B cells stimulated with anti-IgM antibody and T cell-derived growth and differentiation factors. J Exp Med. 1984 Dec 1;160(6):1736–1751. doi: 10.1084/jem.160.6.1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakanishi K., Malek T. R., Smith K. A., Hamaoka T., Shevach E. M., Paul W. E. Both interleukin 2 and a second T cell-derived factor in EL-4 supernatant have activity as differentiation factors in IgM synthesis. J Exp Med. 1984 Dec 1;160(6):1605–1621. doi: 10.1084/jem.160.6.1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul W. E. Pleiotropy and redundancy: T cell-derived lymphokines in the immune response. Cell. 1989 May 19;57(4):521–524. doi: 10.1016/0092-8674(89)90121-9. [DOI] [PubMed] [Google Scholar]
- Peterson C. L., Orth K., Calame K. L. Binding in vitro of multiple cellular proteins to immunoglobulin heavy-chain enhancer DNA. Mol Cell Biol. 1986 Dec;6(12):4168–4178. doi: 10.1128/mcb.6.12.4168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robb R. J., Munck A., Smith K. A. T cell growth factor receptors. Quantitation, specificity, and biological relevance. J Exp Med. 1981 Nov 1;154(5):1455–1474. doi: 10.1084/jem.154.5.1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saltzman E. M., Thom R. R., Casnellie J. E. Activation of a tyrosine protein kinase is an early event in the stimulation of T lymphocytes by interleukin-2. J Biol Chem. 1988 May 25;263(15):6956–6959. [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith K. A. The interleukin 2 receptor. Annu Rev Cell Biol. 1989;5:397–425. doi: 10.1146/annurev.cb.05.110189.002145. [DOI] [PubMed] [Google Scholar]
- Tigges M. A., Casey L. S., Koshland M. E. Mechanism of interleukin-2 signaling: mediation of different outcomes by a single receptor and transduction pathway. Science. 1989 Feb 10;243(4892):781–786. doi: 10.1126/science.2492678. [DOI] [PubMed] [Google Scholar]
- Waldmann T. A., Goldman C. K., Robb R. J., Depper J. M., Leonard W. J., Sharrow S. O., Bongiovanni K. F., Korsmeyer S. J., Greene W. C. Expression of interleukin 2 receptors on activated human B cells. J Exp Med. 1984 Nov 1;160(5):1450–1466. doi: 10.1084/jem.160.5.1450. [DOI] [PMC free article] [PubMed] [Google Scholar]