Abstract
A central function of epithelia is the control of the volume and electrolyte composition of bodily fluids through vectorial transport of electrolytes and the obligatory H2O. In exocrine glands fluid and electrolyte secretion is carried out by both acinar and duct cells, with the portion of fluid secreted by each cell type vary among glands. All acinar cells secrete isotonic, plasma-like fluid, while the duct determines the final electrolyte composition of the fluid by absorbing most of the Cl− and secreting HCO3−. The key transporters mediating acinar fluid and electrolyte secretion are the basolateral Na+/K+/2Cl− cotransporter, the luminal Ca2+-activated Cl− channel ANO1 and basolateral and luminal Ca2+-activated K+ channels. Ductal fluid and HCO3− secretion are mediated by the basolateral membrane Na+-HCO3− cotransporter NBCe1-B and the luminal membrane Cl−/HCO3− exchanger slc26a6 and the Cl− channel CFTR. The function of the transporters is regulated by multiple inputs, which in the duct include major regulation by the WNK/SPAK pathway that inhibit secretion and the IRBIT/PP1 pathway that antagonize the effects of the WNK/SPAK pathway to both stimulate and coordinate the secretion. The function of these regulatory pathways in secretory glands acinar cells is yet to be examined. An important concept in biology is synergism among signaling pathways to generate the final physiological response that ensures regulation with high fidelity and guards against cell toxicity. While synergism is observed in all epithelial functions, the molecular mechanism mediating the synergism is not known. Recent work reveals a central role for IRBIT as a third messenger that integrates and synergizes the function of the Ca2+ and cAMP signaling pathways in activation of epithelial fluid and electrolyte secretion. These concepts are discussed in this review using secretion by the pancreatic and salivary gland ducts as model systems.
Keywords: Secretory glands, WNK/SPAK pathway, IRBIT/PP1 pathway, coordination, synergism
Introduction
Fluid and electrolyte secretion is a fundamental physiological function that regulates the systemic fluid and electrolyte composition and bodily volume. Specialized tissues like secretory epithelia also generate biological fluids with defined volume and electrolyte composition that are tuned to their specific functions. Anomalous fluid and electrolyte transport is associated with many diseases, most commonly hypertension and diarrheal diseases and inflammatory diseases. Formation and secretion of fluid with defined composition is determined by vectorial ion transport to generate osmotic gradients that drive water flow through water channels down the osmotic gradient. The vectorial transport is mediated by placing selective transporters in defined cellular membranes, such as the basolateral and luminal membranes of epithelia. This by necessity requires coordination of the transport events in the two membranes, and in many cases in microdomains within membranes. Fluid and electrolyte transport in all cells and tissues is thus tightly controlled by multiple inputs than regulate both the availability and activity of the transporters in the cells and in the plasma membrane. Importantly, the multiple inputs are integrated into a synergized final response that allows high fidelity with better on/off control of the systems. The WNK/SPAK kinases and IRBIT/PP1 pathways emerged as pathways that regulate and coordinate epithelial transport. Moreover, regulation of these pathways serves to integrate and synergize multiple inputs. The present review uses secretory glands fluid and HCO3− secretion and its regulation by the WNK/SPAK and IRBIT/PP1 pathways as models to discuss the principal transporters, regulation and coordination of the transport and synergism among the Ca2+ and cAMP signaling pathways.
The major transporters mediating secretory glands fluid and electrolyte secretion
The major cell types engaged in secretory glands fluid and electrolyte secretion are the acinar clusters and the ductal system or surface epithelial cells. The volume secreted by each cell type differs among glands. For example, in the pancreas the acinar cells secrete small isotonic, NaCl-rich fluid and the duct secretes most of the fluid in the pancreatic juice. In the salivary glands, the acinar cells also secrete isotonic NaCl-rich fluid but secrete most of the fluid in saliva. However, in all glands the duct determines the final composition of the secreted fluid with its main function is absorption of the Cl− and secretion of HCO3− to generate highly alkaline fluid containing as much as 140 mM HCO3− and about 20 mM Cl− (72).
Key Acinar Cell Transporters
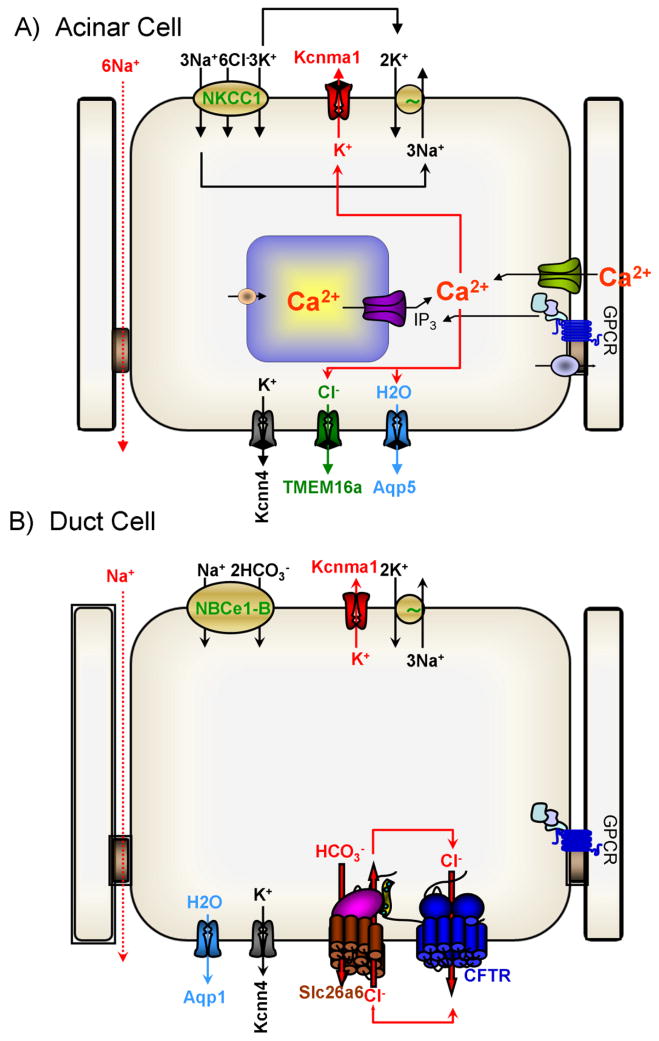
The basic transporters and the mechanism of fluid secretion are similar in most acinar cells and are illustrated in the model in Fig. 1A. As is the case in all cells, the Na+ and K+ gradients and membrane potential are set by the Na+/K+ ATPase pump that in polarized cells is located in the basolateral membrane (23, 68, 109). The Na+/K+ ATPase pump converts the chemical energy in ATP to osmotic energy in the form of the Na+ and K+ gradients. Salt uptake by acinar cells to fuel the fluid transport is mediated mostly by the ubiquitous basolateral Na+/K+/2Cl− cotransporter NKCC1 and partly by the Na+/H+ exchanger NHE1 and the Cl−/HCO3− exchanger AE2. NKCC1 and NHE1 also feed the Na+/K+ ATPase pump with Na+ (163, 164). NKCC1 is the main Cl− influx pathway in acinar cells that together with AE2 sets intracellular Cl− at 40–60 mM (165). The basolateral Ca2+-activated K+ channels set the membrane potential at −50 to −60 mV (107). NHE1 and AE2 also regulate cytoplasmic pH and maintain it at about 7.2 (88, 92). The main transporters at the luminal membrane are the Ca2+-activate Cl− channel TMEM16A/Ano1 (70, 119) and the water channel AQP1 (pancreas) and AQP5 (salivary glands) (26, 81). The acinar cells tight junction is permeable to Na+ and is the main route of transcellular Na+ flux (71, 84, 135).
Fig. 1. Mechanism of fluid and electrolyte secretion by secretory glands acinar and duct cells.
The panels show the key transporters and the relationships between them that mediate the bulk of fluid and electrolyte secretion by secretory glands acinar (A) and duct (B) cells.
Acinar cells fluid and electrolyte secretion is regulated by multiple inputs, although most inputs transmit their signals by changes in cytoplasmic Ca2+, but the secretion is augmented by the cAMP/PKA system. The physiological Ca2+ signal is in the form of Ca2+ oscillations that are initiated at the apical pole and often propagate to the basal pole (60, 63, 139). Acinar cell secretion is initiated by an increase in [Ca2+]i at the apical pole that activates the Ano1 Ca2+-activated Cl− channel (70, 119, 160). The Ca2+ signal then propagates to the basolateral membrane and activates the K+ channels (91, 107, 120). The identity of the basolateral K+ channels is not known with certainty, although acinar cells express both the large-conductance K(Ca)1.1 and the intermediate-conductance K(Ca)3.1 channels (121). However, K(Ca2+)1.1 is expressed largely in the apical membrane (89) and the localization of K(Ca)3.1 is not known with certainty. Activation of the Cl− and K+ channels by Ca2+ leads to Cl− efflux into the luminal space and K+ efflux to the interstitial space with Na+ flow through the tight junction from the basal side to the apical side, resulting in the secretion of NaCl and generation of an osmotic gradient. The Ca2+ increase also activates AQP5 in acinar cells (56) that mediates the water efflux to the luminal space and cell shrinkage (3, 7, 108). Cell shrinkage causes reduction in [Ca2+]i and activates the volume sensitive basolateral membrane ion transporters, NKCC1 (42, 48), NHE1 (2) and AE2 (3). Activation of NKCC1 by cell shrinkage is being studied extensively since NKCC1 activation involves phosphorylation by the volume sensitive SPAK kinase (35). The cycle of activation of luminal and basolateral membrane transporters is repeated with each Ca2+ spike during Ca2+ oscillations making acinar cells functioning as a Ca2+-driven ion and water pump.
Key Ductal Transporters
Fluid and electrolyte secretion by secretory gland ducts varies between tissues. A well-established example is Na+ and K+ handling by the salivary and pancreatic ducts. While the pancreatic duct does not absorb Na+ and secrete K+, the salivary duct expressed the epithelial Na+ channel ENaC and the K(Ca)1.1 K+ channel in the luminal membrane and absorb the Na+ and secret K+ to the saliva (72). However, common to all ducts is the absorption of Cl− and secretion of HCO3− into the fluid secreted by acinar cells, while secreting some or most of the fluid generated by the glans. Fluid and HCO3− secretion is the cardinal functions of the ducts and are the activities altered in disease states. The main transporters mediating ductal fluid and HCO3− secretion are shown in Fig. 1B.
Ductal transport uses the energy in the Na+ gradient and the membrane potential to secrete. The Na+/K+ ATPase pump is abundantly expressed in the basolateral membrane of the ducts (123, 133). The duct cells membrane potential is closed to the K+ diffusion potential although, remarkably, the molecular identity of the K+ channel(s) that set membrane potential is still not known with certainty. K(Ca2+)1.1 (MaxiK) channels is expressed in the luminal membrane of the pancreatic (141) and salivary gland ducts (90), indicating expression of yet unidentified K+ channel in the basolateral membrane of duct cells that sets the membrane potential.
Ductal HCO3− secretion requires HCO3− influx across the basolateral membrane and HCO3− exit across the luminal membrane. Na+-HCO3− co-transport activity was found in the basolateral membrane of the rat pancreatic duct (164) that was later identified in the guinea pig (54, 112) and salivary gland ducts (80). The transporter was cloned from the pancreas and named pNBC1 (1). After identification of all members of the superfamily of Na+-driven HCO3− transporters it was re-named NBCe1-B (13). NBCe1-B is an electrogenic transporter with 1Na+-2HCO3− stoichiometry (40), resulting in accumulation of cytoplasmic HCO3− and a net influx of osmolytes. The activity of NBCe1-B is regulated by multiple inputs, including IRBIT (131, 157), the WNK/SPAK pathway (159) and PI(4,5)P2 (51), all of which converge on the NBCe1-B N terminal autoinhibitory domain (51). Regulation of NBCe1-B is discussed further bellow because of its central role in ductal HCO3− secretion.
HCO3− exit across the luminal membrane is mediated by the interrelated activity of the Cl− channel Cystic Fibrosis Transmembrane conductance Regulator (CFTR) and the Cl−/HCO3− exchanger slc26a6 (72). CFTR (ABCC7) was discovered as the protein mutated in cystic fibrosis (61, 117, 122) and is a member of the ATP-binding cassette (ABC) superfamily. CFTR functions as a Cl− channel that is activated by the cAMP/PKA pathway (138). CFTR is expressed and functioning in the luminal membrane of the pancreatic and salivary gland ducts (16, 135). Although CFTR functions primarily as a Cl− channel, CFTR has finite HCO3− permeability (77, 111, 126) and CFTR-mediated HCO3− flux becomes important at the distal portion of the ducts when luminal and cytoplasmic Cl− are very low. CFTR is inhibited by extracellular and intracellular Cl− and at extracellular Cl− higher than 30 mM CFTR does not participate in HCO3− transport (126, 150). However, at the distal portion of the duct luminal and cytoplasmic Cl− are at about 30 and 5–7 mM, respectively, CFTR likely participates in HCO3− secretion (55, 100).
Importantly, in addition to functioning as the main Cl− channel CFTR integrates the entire transport function at the luminal membrane by interacting and regulating the function of many major luminal transporters. Thus, CFTR exists in a macromolecular complex assembled by scaffolding proteins with multiple PDZ binding domains (132, 145). In the complexes, functional interactions with CFTR were reported for ENaC, outwardly rectifying Cl− channels, ROMK2 and KvLQT1 K+ channels, the SLC26 transporters, NBCn1-A (NBC3) and perhaps aquaporins (69, 71). When activated by PKA, CFTR activates the SLC26 transporters to facilitate HCO3− secretion (66) and at the same time inhibits ENaC (137) and NBCn1-A (101) to inhibit HCO3− and Na+ absorption.
The bulk of ductal HCO3− secretion is mediated by members of the SLC26 transporters, most prominently by slc26a6 (29, 103). The SLC26A family consists of 10 transporters, several of which are associated with human diseases (31, 102). The transporters appear to function as dimmers (21) or tetramers (43). The basic structure includes a transmembrane sector that is homologous to the core structure of the ClC Cl− channels (96), and a STAS domain (21, 105), that functions as a protein-protein interacting domain (66). A low resolution structure of the dimer with imposed transmembrane sector and a STAS domain is shown in Fig. 2. Members of the family transport divers substrates, including Cl−, HCO3−, I−, SO42−, oxalate and formate and can function as electroneutral and as electrogenic exchangers and as ion channels (98). SLC26A1 and SLC26A2 functioning as SO42− transporters (82), with SLC26A2 mediating SO42−/Cl−/OH− exchanger (95). Slc26a3 and Slc26a6 function as 2Cl−/1HCO3− or 1Cl−/2HCO3−, respectively, electrogenic exchangers (65, 152) that can also mediate uncoupled ion fluxes (96). Slc26a6 is probably the most versatile transporter mediating 1Cl−/2HCO3− exchange (128), Cl−/oxalate and Cl−/formate exchange (59, 96) and uncoupled ion fluxes (24). Slc26a4 mediates electroneutral Cl−/HCO3−/I− exchange (129). SLC26A5 functions as an anion regulated, voltage sensing motor protein (124). SLC26A7 (62) and SLC26A9 (30) function as selective Cl− channels with minimal or no HCO3− permeability, but can conduct other anions, in particular NO3−. Recent work suggested that SLC26A11 functions as a Cl− channel (113). Secretory glands express several SLC26 transporters in the same cell, both at the basolateral and luminal membranes. Several Mendelian diseases are associated with the SLC26 transporters. Mutations in SLC26A2 causes chondrodysplasias (45), mutations in SLC26A3 causes congenital chloride diarrhea (49) and mutations in SLC26A4 are associated with Pendred syndrome (33).
Fig. 2. The major domains of the SLC26 transporters.
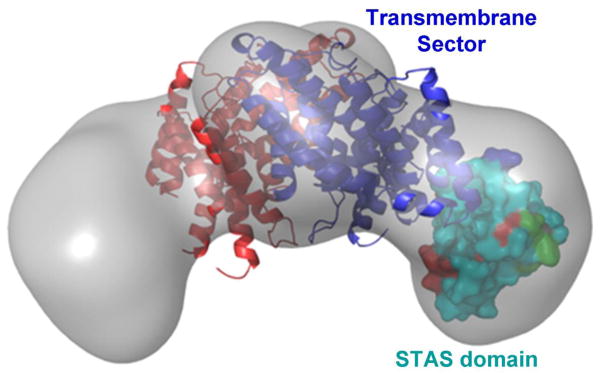
The gray image is the global structure of an SLC26 transporters obtained by small angle neutron scattering (22). The core transmembrane sector is modeled based on similarity to the ClC transmembrane sector (97), and the STAS domain is the solved crystal structure of Slc26a5 (106). The images were taken from the respective references.
Most HCO3− secretion in secretory glands is mediated by Slc26a6, where it functions mainly as1Cl−/2HCO3− exchanger in the duct luminal membrane (129, 136, 147). As electrogenic exchanger slc26a6 mediates net solute transport and thus is essential for fluid secretion by the duct. Slc26a6 was found as part of a search for novel SLC26 transporters (78), and as the oxalate transporter in the renal proximal tubule (64). Slc26a6 mediates oxalate homeostasis by secreting oxalate into the intestinal lumen. There is no known human mutation in SLC26A6, but deletion of Slc26a6 in mice results in urolithiasis due to increased renal oxalate load (57) and enhanced activity of the Na+-dicarboxylic acid transporter NaDC-1 (94). The enhanced NaDC-1 activity absorbs urinary citrate to reduced urine Ca2+ buffering and together with the high oxalate resulting in kidney stones. The Slc26a6 STAS domain and NaDC-1 first intracellular loop interacts to activate slc26a6 and inhibits NaDC-1 (94). Oxalate transport by Slc26a6 may also have a role in salivary gland stones (Sialolithiasis) (44).
A more relevant mode of regulation of slc26a6 is through the STAS domain that interacts with CFTR R domain to mediate the mutual activation of slc26a6 and CFTR. The STAS domains of Slc26a3 and slc26a6 were shown to interact with the phosphorylated CFTR R domain (66). The unphosphorylated R domain interacts with the first nucleotide binding domain (NBD1) of CFTR to hinder formation of the ATP binding sites between NBD1 and NBD2 and inhibit CFTR (9). The isolated STAS domain was sufficient to fully activate CFTR (66) and when the R domain is targeted to the plasma membrane it activated the SLC26 transporters (127). Similar interaction between CFTR was demonstrated for Slc26a4 (38, 129), Slc26a5 (50), Slc26a8 (118) and Slc26a9 (8, 11). These findings indicate that in the resting state the unphosphorylated CFTR R domain interacts with NBD1 to inhibit CFTR and at the same time to sequester the R domain away from the STAS domain, thus maintain the SLC26 transporters n the inactive state by their STAS domain. Cell stimulation that increases phosphorylation of the R domain causes its dissociation from NBD1 and facilitates its binding to and trapping by the STAS domain. These cause activation of both CFTR and the SLC26 transporters. The SLC26 transporters mediate Cl−/HCO3− exchange to absorb the Cl− and secrete HCO3−. The Cl− is returned to the luminal space by CFTR to maintain the transport by the SLC26 transporters (see Fig. 1B). In the pancreatic duct, net osmolyte secretion in the form of HCO3− by slc26a6 and Cl− efflux by CFTR together with basal to luminal paracellular Na+ flow result in osmotic water secretion to generate the pancreatic juice.
Regulation of fluid and HCO3− secretion by the WNK/SPAK pathway
The With-No lysine (K) Kinases (WNK) are members of the MAP kinases superfamily that lack the conserved lysine in subdomain II (53) that is contributed by a lysine in subdomain I (86). Mammals have four WNK kinases (83), with WNK1 (19, 93) and WNK4 (59, 142) being widely express. The domain structure of the WNKs is known only in part and includes the homologous kinase domain, an autoinhibitory domain (AID), multiple putative coiled-coil domains, and several proline-rich domains. The WNKs likely have additional domains in the large stretch between the kinase domain and the C terminus that may participate in the numerous functions of the WNK kinases (53, 83, 143).
A major role of the WNKs is the regulation of Na+, K+, Cl−, HCO3− and Ca2+ transporters in epithelia (53, 83, 143) that is associated with hypertension. The WNKs regulate the transporters either by determining their surface expression or by regulating their activity. Extensive discussion of this topic can be found in reference (83). The present discussion refers only to regulation of transporters in secretory glands. Regulation of the NaCl cotransporter NCC by the WNKs and SPAK is the most extensively studied transporter and serves as a model system to understand the various functions of the WNKs. For example, WNK1 and WNK4 reduce the expression of NCC in the plasma membrane (15, 39, 153). WNK1 can also indirectly regulates NCC activity by phosphorylating the SPAK and OSR1 kinases (143), which activate NCC without affecting its surface expression (83). Notably, the effect of the WNKs (125, 155) is independent of their kinase function, indicating that in this case the WNKs function as scaffolds. Similarly, in secretory glands the WNKs act as SPAK scaffold to inhibit the surface expression and the activity of NBCe1-B (156), Slc26a3, Slc26a6 (100), Slc26a9 (29) and CFTR (154, 156).
Many of the WNK functions were learned from studying mutations causing pseudohypoaldosteroism type II (PHAII) that result in hypertension. Such analysis identified mutations in WNK1 and WNK4 that reduce the function of the kinases, leading in increased membrane expression of NCC and hypertension (149). Numerous subsequent studies examined the role of the WNKs in many transport events in the kidney and other epithelia (52, 83, 103). However, a new mechanism by which the mutations affect the function of the WNKs was clarified recently with further analysis of mutations leading to PHAII. These analyses identified the kelck-like 3 (KLHL3) (14, 79) and cullin 3 (CUL3) (14) as regulators of WNK1 and WNK4 levels (99, 130, 144). CUL3 is a component of an E3 Cullin Ring ligase (110) that interact with the adaptor KLHL3, a member of the BTB domain-containing kelck proteins. The E3 ligase ubiquitinate proteins to mark them for degradation by the proteasome (67). KLHL3 binds both CUL3 and WNK1 and WNK4, resulting in their ubiquitination and degradation. Disease causing mutations in the WNKs, KLHL3 and CUL3 inhibits the WNKs ubiquitination to increase their cellular level, and thus regulation of the level of transporters in the plasma membrane (99, 130, 144). Understanding this mode of regulation is in its early stage and likely to be extensively examined in the coming years.
The WNKs and the sterile 20 family stress kinases SPAK/OSR1 function in the same pathway in regulating ion transporters with the WNKs phosphorylating and activating the SPAK/OSR1 kinases (24, 25). SPAK and OSR1 are related kinases with apparent redundant function in many cellular and biochemical assays (36, 116, 143). The WNKs function as scaffolds for the kinases but also activate them by phosphorylating the conserved T185 and S325 of OSR1 and T243 and S383 of SPAK. The SPAK and OSR1 interacts with the [R/K]Fx[V/I] motif that is present in the WNKs and transporters regulated by SPAK/OSR1 such as NKCC1 (25, 116), Slc26a3, Slc26a6 and CFTR. The effect of the SPAK/OSR1 is specific for a given transporter. The SPAK/OSR1 activates NKCC1 and the kinase function of both the WNKs and SPAK/OSR1 is required (25, 116). On the other hand, SPAK/OSR1 inhibits the activity of NBCe1-B and CFTR (156).
The WNKs and SPAK/OSR1 pathway (WNK/SPAK pathway) is a potent negative regulator of the Cl− and HCO3− transporters NBCe1-B (156), Slc26a3, Slc26a6 (100), Slc26a9 (29) and CFTR (154, 156). The WNK/SPAK pathway inhibits surface expression and the activity of NBCe1-B, Slc26a9 (29, 156) and CFTR (154, 156). The kinase-dead WNK mutants were as effective as wild-type WNKs in reducing surface expression and inhibiting the transporters and, moreover, the WNK11–119 fragment recapitulates all the effects of WNK1 (156). SPAK has the same effect as the WNKs. however the kinase-dead SPAK mutant reversed the effect of the WNKs, indicating that the WNKs act upstream of SPAK. In vivo, knockdown of the WNKs and of SPAK in the native pancreatic duct enhanced stimulated fluid secretion, indicating that the kinases exert tonic inhibition of the secretion (156) to set the basal non-secretory state of the epithelia (see below the model in Fig. 5).
Fig. 5. IRBIT-mediated synergistic activation of epithelial fluid and HCO3− secretion by the cAMP and Ca2+ signaling pathways.
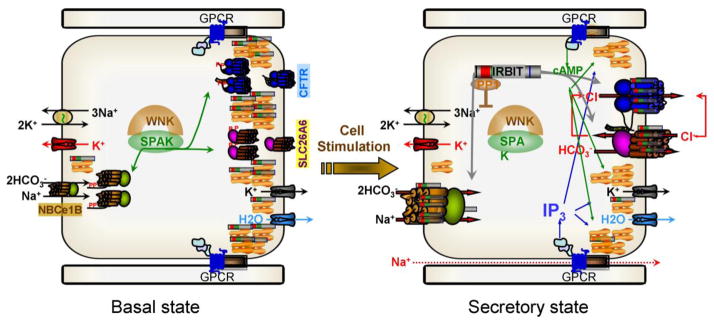
In the resting state the WNK/SPAK kinases associate with the transporters and SPAK phosphorylates NBCe1-B AID, Slc26a6 STAS domain and CFTR R domain to sequester most of them in intracellular organelles. Al low cytoplasmic IP3 IRBIT is bound to the IP3 receptors that in secretory glands are clustered at the apical pole. When the cells are stimulated with a combination of physiological concentrations of IP3 and cAMP generating agonists, PKA phosphorylates the IP3Rs to facilitate release of IRBIT from the IP3Rs by IP3 binding. IRBIT recruits PP1 to the transporters to dephosphorylate them and at the SPAK phosphorylation sites and target them to the plasma membrane. IRBIT remains bound to the transporters AIDs to relieve their constitutive inhibition resulting in activation of the transporters and of fluid and electrolyte secretion.
The IRBIT/PP1 pathway antagonizes the function of the WNK/SPAK pathway
IRBIT (IP3 binding protein released with IP3) was discovered in different contexts and most recently as a protein that binds to the IP3 binding site of the IP3Rs (6) and as an activator of the NBCe1-B (131). The various functions of IRBIT have been discussed recently (158) and here we limit the discussion to its role in ion transport. The IRBIT domains identified include (from the N terminus) a PP1 binding motif, a PEST domain, a coiled-coil domain and a PDZ ligand at the end of the C-terminus (28, 158). The PEST domain has multiple phosphorylation sites, several of which are required for all known IRBIT functions (28, 158). Deletion of the coiled-coil domain prevents activation of target proteins by IRBIT (159), by acting as a dominant negative (104). The PDZ ligand is required for interaction of IRBIT with the IP3Rs (27) and formation of complexes with the HCO3− transporters (156). Within the complexes IRBIT activates several Cl− and HCO3− transporters, including the Na+-HCO3− transporters NBCe1-B (51, 131, 159) and NBCn1-A (51), CFTR (159), NHE3 (46) and Slc26a6 (104). Activation by IRBIT has two components; IRBIT increases the surface expression of the transporters and then increases their transport activity (turnover rate).
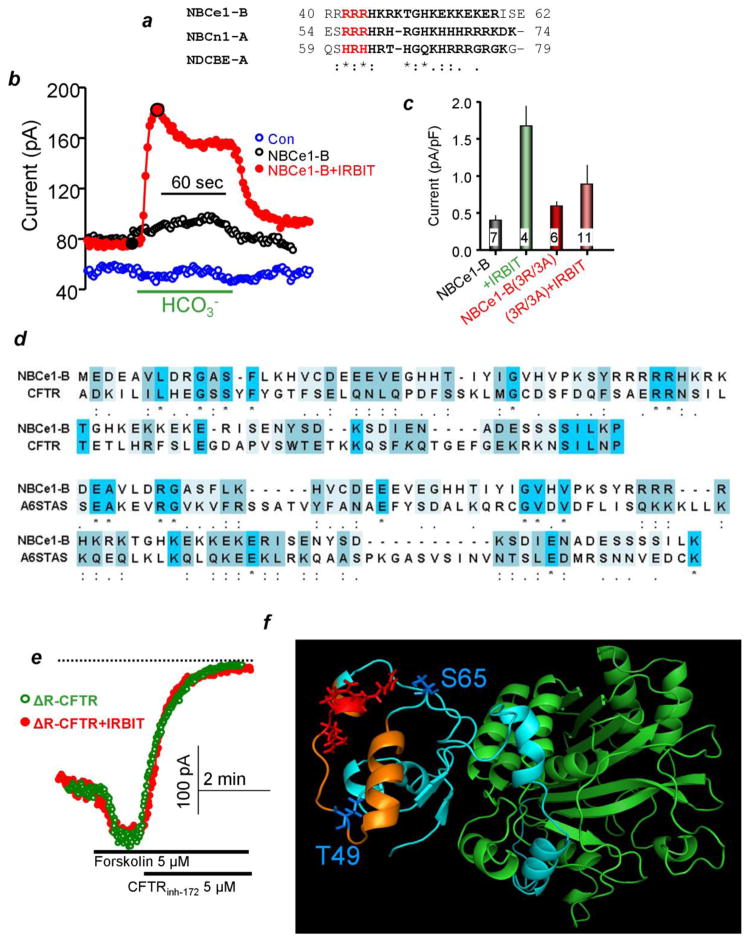
The mechanism by which IRBIT activate transporters activity is only partially understood. The best understood mechanism is activation of NBCe1-B. The first 85 residues of NBCe1-B form an autoinhibitory domain (AID) (75). Removal of AID activates NBCe1-B to the same extent as IRBIT, and IRBIT no longer activates NBCe1-B lacking the AID (131). Recently, we analyzed further the role of the AID in the regulation of NBCe1-B by IRBIT and was able to narrow the IRBIT interacting domain to NBCe1-B(40-62) that is conserved in all Na+-HCO3− cotransporters family but NBCe1-A (51). NBCe1-B(40-62) is shown in Fig. 3a, which also highlight three conserved arginines. Neutralizing the arginines eliminates activation of NBCe1-B by IRBIT (Figs. 3b and 3c). The tree arginines also mediate activation of NBCe1-B by PI(4,5)P2 and the activation by IRBIT and PI(4,5)P2 is not additive (51). Finally, negative regulation of NBCe1-B by SPAK is mediated by phosphorylation of Thr49 and Ser65 that are also located within the AID (51). The location of the arginines and the SPAK phosphorylation sites in a structural model of the N terminus domain of NBCe1-B is shown in Fig. 3f. Interestingly, Fig. 3d shows that a sequence similar to the NBCe1-B(1-85) is present in CFTR R domain and slc26a6 STAS domain. Moreover, the STAS and R domains interact with IRBIT (51), and deletion of the R domain prevents activation of CFTR by IRBIT (Fig. 3e). In addition, residues 591–696 in the C terminus of NHE3 was proposed as the IRBIT binding site (46). Further analysis showed that NHE3(606-651) has good homology with NBCe1-B(1-85) (unpublished observation). Together, these findings raise the possibility that the R and the STAS domains and NHE3(606-651) function as AID of CFTR, NBCe1-B and NHE3, respectively. IRBIT activates the transporters by relieving the autoinhibition.
Fig. 3. The autoinhibitory domain (AID) of NBCe1-B and a potential AIDs in slc26a6 and CFTR.
Panel (a) show alignment of a sequence within NBCe1-B AID to which IRBIT and PI(4,5)P2 bind that require the conserved arginines (highlighted in red) and the homologous sequence in NBCn1-A and NDCBE-A. Panel (b) shows traces of NBCe1-B current in HeLa cells transfected with vector (blue), NBCe1-B (black) and NBCe1-B+IRBIT (red). Panel (c) shows the mea±s.e.m of the NBCe1-B current measured with the indicated mutants in the NBCe1-B AID. Panel (d) is an alignment of the NBCe1-B AID with sequences within the CFTR R domain and slc26a6 STAS domain. Panel (e) shows that IRBIT does not activate ΔR-CFTR. Panel (f) shows a model of the first 400 residues of NBCe1-B. The green domain encompasses residues 100–400 of NBCe1-B and is similar to the structure of the N terminus of AE1. Residues 40–62 are in orange and the rest in turquoise. The mutated arginines are shown in red in sticks form and the residues phosphorylated by SPAK are in blue. The results were taken from (51).
A key action of IRBIT in activating the Cl− and HCO3− transporters is antagonizing the effect of the WNK/SPAK pathway to surface expression of the transporters. Although by itself it has no effect on transporters surface expression, when it is co-expressed with the WNKs or SPAK IRBIT reverses the reduction in transporters surface expression, indicating that IRBIT antagonizes the effects of the WNK/SPAK pathway (156). IRBIT achieves this by recruiting the protein phosphatase 1 (PP1) to the transporter complexes. IRBIT has a PP1 binding site and actively recruit PP1 to NBCe1-B, CFTR and slc26a6 (104, 156). Significantly, mutation of the IRBIT PP1 binding motif inhibits activation of NBCe1-B and CFTR by IRBIT and overexpression of PP1 restores surface expression and partially activates the transporters in a manner similar to IRBIT (156). Accordingly, knockdown of IRBIT markedly inhibited fluid secretion by the stimulated pancreatic duct. Moreover, the reduced secretion due to IRBIT knockdown was partially recovered by knockdown of SPAK (156), further emphasizing the interplay between the IRBIT/PP1 and the WNK/SPAK pathways. Although by recruiting PP1 IRBIT dephosphorylate NBCe1-B, CFTR and slc26a6 (104, 156), it remains to be determined if PP1 specifically dephosphorylates NBCe1-B Thr49 and Ser65 that are phosphorylated by SPAK (51) and identify the phosphorylation sites in CFTR, slc26a6, NBCn1-A, NHE3 and other transporters that may be regulated by the WNK/SPAK and IRBIT/PP1 pathways.
IRBIT acts as a third messenger to mediate synergistic activation of epithelial transport by the Ca2+ and cAMP signaling pathways
Synergism is a fundamental concept in biology. All physiological functions are determined and regulated by multiple signaling pathways that are integrated into a physiological response. Every one of these inputs have positive regulatory functions but, with no exception, over activation of any signaling pathway is highly toxic or lead to uncontrolled cellular and tissue functions. In fact, many diseases are caused by mutations that result in over activation of signaling by G protein coupled receptors (GPCRs) (134, 140) and tyrosine kinase receptors (10, 76) that are common in many forms of cancers. Signaling pathways interact and cooperate in several manners. A common interaction is through cross-talk (32, 151). In Ca2+ and cAMP signaling, the Ca2+ signal regulates cAMP production (148), and cAMP regulates the Ca2+ signal by affecting the function of IP3 receptors (12). Cross-talk coordinates the function of multiple inputs but does guard against signaling toxicity and is not very efficient in ensuring fidelity of the integrated response. To guard against cell damaged caused by over-activation of signaling pathway and ensure high fidelity of the integrated response while achieving maximal physiological response, signaling pathways function at 5–10% of capacity but synergize to generate the maximal response.
Synergism occurs between all signaling pathways and mediates all cellular responses including gene transcription (34, 41), energy metabolism (115, 146) and secretion (37). A common signaling synergism in epithelia is between the Ca2+ and cAMP signaling pathways. Just to name a few examples, synergism between Ca2+ and cAMP signaling regulates stomach pepsinogen (114) and acid (17) secretion, pancreatic enzyme secretion (85), catecholamine secretion (4), mucus secretion by the airway (18, 20), ciliary beat frequency (87), activation of K+ and Cl− channels and fluid secretion by salivary acinar cells (47), intestinal fluid secretion (161) and fluid secretion by the airway (58, 74). Yet, we know very little about the molecular mechanism of synergism. This begun to change with the discovery of the function of IRBIT as a third messengers and its central role in synergizing the Ca2+ and cAMP signaling (104). Competition between IRBIT and IP3 for binding to the IP3 binding domain of the IP3Rs (5), the release of IRBIT from the IP3Rs by IP3 (6), PKA-mediated increased responsiveness of the IP3Rs to IP3 (12, 162), clustering of IP3Rs at the apical pole of secretory epithelia (73), the expression of slc26a6 and CFTR al the luminal membrane, and activation of epithelial Cl− and HCO3− transporters by IRBIT (46, 104, 156, 159), raised the possibility that IRBIT may mediate the synergistic activation of transporters by the Ca2+ and cAMP pathways. We used multiple experimental systems to examine the role of IRBIT as a third messenger that mediates the synergism between the Ca2+ and cAMP signaling pathways (104).
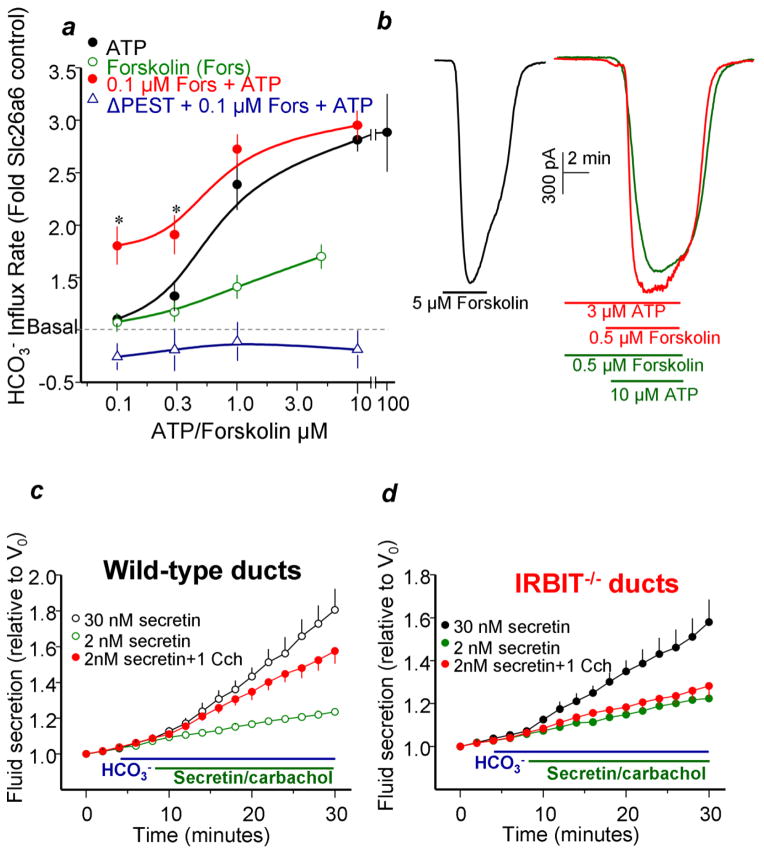
Expression studies in model system showed that CFTR is fully activated by maximal stimulation of the cAMP pathway and slc26a6 is fully activated by the Ca2+ signaling pathway (Figs. 4a, 4b). Co-expression of the transporters with IRBIT was sufficient to partially or maximally activate them, and knockdown of IRBIT reduced their activation by the signaling pathways, indicating regulation of the transporters by IRBIT. The PKA pathway poorly activated slc26a6, but markedly left shifted the concentration-dependence for activation of slc26a6 by the Ca2+ signaling pathway (Fig. 4a). Conversely, the Ca2+ signaling pathway poorly activated CFTR, but markedly enhanced activation of CFTR by partial stimulation of PKA (Fig. 4b). These effects required elevation of cellular IP3 but were independent on cytoplasmic Ca2+, indicating that elevation of cellular IP3 rather than Ca2+ synergizes with PKA. Hence, the PKA and Ca2+ signaling pathways synergize in activation of transporters that are primarily activated by the Ca2+ signaling (slc26a6) or PKA (CFTR) pathways. Notably, dominant negative IRBIT constructs (Fig. 4a) and knockdown of IRBIT eliminated the synergistic activation of the transporters (104). The molecular mechanism of the synergism was revealed by showing that co-activation of the cells with low levels of the PKA and Ca2+ signaling pathways resulted in release of IRBIT from the IP3 receptors and its interaction with slc26a6 and CFTR. Further direct evidence were obtained using IP3Rs mutants in which the PKA phosphorylable sites were mutated to the phosphormimetic glutamates (IP3Rs(SS/EE) or the non-phosphorylable alanines (IP3Rs(SS/AA) (12). IP3Rs(SS/EE) facilitated release of IRBIT from the IP3Rs and the synergistic activation of slc26a6 and CFTR, whereas IP3Rs(SS/AA) retarded release of IRBIT from the IP3Rs and prevented the synergistic activation of slc26a6 and CFTR by the PKA and Ca2+ signaling pathways (104).
Fig. 4. IRBIT mediates the synergism between PKA and Ca2+ signaling pathways.
Panel (a) depict the synergistic activation of slc26a6 by 0.1μM forskolin and 0.1 and 0.3μM ATP. HeLa cells transfected with slc26a6 or slc26a6 and IRBIT(ΔPEST) were stimulated with ATP alone (close black circles), forskolin alone (open green circles) or with 0.1μM forskolin and the various concentrations of ATP (close red circles and open purple triangles). The results are the mea±s.e.m of 4–6 experiments. Panel (b) shows the synergistic activation of CFTR by 0.5μM forskolin and 3μM ATP. Maximal CFTR current is evoked by stimulation with 5 μM forskolin. Panels (c, d) show that IRBIT is required for synergistic activation of ductal fluid secretion. Fluid secretion in pancreatic ducts from wild-type and IRBIT−/− mice was measured in sealed ducts in HCO3−-buffered media and stimulated with 5μM forskolin or 30nM secretin (black circles), low concentration of 0.1μM forskolin or 2nM secretin (green circles), 1μM carbachol (blue circles) and the combination of 2nM secretin and 1μM carbachol (red circles).
The findings in model systems were extended to in vivo by showing that stimulation of salivary and pancreatic ducts with low concentration of cAMP-generating and IP3-generating agonists synergize in activation of slc26a6 and of CFTR. Importantly, viral delivery of IP3Rs(SS/AA) to salivary gland ducts eliminated the synergistic activation of slc26a6 and CFTR. Accordingly, stimulating pancreatic and salivary gland cells with IP3 generating agonists resulted in interaction of IRBIT with slc26a6 and CFTR. Importantly, deletion of IRBIT in mice eliminated the synergistic activation of slc26a6 and CFTR by weak stimulation of the cAMP- and IP3-signaling pathways (104). Most notably, Figs. 4c and 4d show that knockout of IRBIT eliminated the synergistic stimulation by the Ca2+ and PKA pathway of fluid secretion by the pancreatic duct.
Together, regulation of epithelial fluid and electrolyte transporters by the WNK/SPAK and IRBIT/PP1 pathways and the role of IRBIT in mediating the synergism between the PKA and Ca2+ signaling pathways lead to the model in Fig. 5 for the mechanism and synergism in epithelial fluid and electrolyte secretion. In the resting state, the WNK/SPAK pathway is associated with the transporters where SPAK phosphorylates the autoinhibitory domains of NBCe1-B, Slc26a2 and CFTR to keep most transporters internalized and inactive. At the same time, cellular IP3 levels are low and most IRBIT is sequestered by the IP3Rs that are clustered at the apical pole of polarized cells (73). Hence, IP3Rs function to buffer the level of IRBIT available for binding to all IRBIT targets.
Upon cell stimulation with physiological concentrations of agonists that activate both the PKA and Ca2+ signaling pathway but only at 5% strength the IP3Rs are phosphorylated by PKA. Phosphorylation of the IP3Rs increases their affinity for IP3 and reduces their affinity for IRBIT (104), resulting in dissociation of IRBIT from the IP3Rs. The IP3Rs and IRBIT are present in the same microdomain or within a distance that allows the released IRBIT to interact with CFTR and slc26a6 present intracellularly and in the luminal membrane. IRBIT recruits PP1 that dephosphorylates the transporters at the sites phosphorylated by SPAK. This results in insertion of the transporters in the plasma membrane. In addition, binding of IRBIT to the transporters AIDs relieve their inhibitory effect, resulting in activation of the transporters. By translocating from its binding to IP3Rs in the ER to the plasma membrane where it activates the transporters, IRBIT functions as a third messenger that transmit the information of the second messengers cAMP and IP3 to the plasma membrane. At the same time IRBIT integrates and synergizes the activity of the PKA and Ca2+ signaling system, providing a molecular mechanism for the synergism in epithelial transport. So far, the synergism has been demonstrated for the function of the salivary gland and pancreatic ducts. It will be of particular interest to determine whether IRBIT mediates the synergism in other forms of epithelial fluid and electrolyte secretion, such as that by acinar cells, and in other epithelia such as the airway, intestine and the liver and non-epithelial cells, such as the neuronal and immune systems.
Acknowledgments
The work in the authors’ laboratory was funded by Intramural Research Program of the NIH, NIDCR grant DE000735.
References
- Abuladze N, Lee I, Newman D, Hwang J, Boorer K, Pushkin A, Kurtz I. Molecular cloning, chromosomal localization, tissue distribution, and functional expression of the human pancreatic sodium bicarbonate cotransporter. J Biol Chem. 1998;273:17689–95. doi: 10.1074/jbc.273.28.17689. [DOI] [PubMed] [Google Scholar]
- Alexander RT, Grinstein S. Na+/H+ exchangers and the regulation of volume. Acta Physiol (Oxf) 2006;187:159–67. doi: 10.1111/j.1748-1716.2006.01558.x. [DOI] [PubMed] [Google Scholar]
- Alper SL. Molecular physiology and genetics of Na+-independent SLC4 anion exchangers. J Exp Biol. 2009;212:1672–83. doi: 10.1242/jeb.029454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderova M, Duchene AD, Barbara JG, Takeda K. Vasoactive intestinal peptide potentiates and directly stimulates catecholamine secretion from rat adrenal chromaffin cells. Brain Res. 1998;809:97–106. doi: 10.1016/s0006-8993(98)00856-7. [DOI] [PubMed] [Google Scholar]
- Ando H, Mizutani A, Kiefer H, Tsuzurugi D, Michikawa T, Mikoshiba K. IRBIT suppresses IP3 receptor activity by competing with IP3 for the common binding site on the IP3 receptor. Mol Cell. 2006;22:795–806. doi: 10.1016/j.molcel.2006.05.017. [DOI] [PubMed] [Google Scholar]
- Ando H, Mizutani A, Matsu-ura T, Mikoshiba K. IRBIT, a novel inositol 1,4,5-trisphosphate (IP3) receptor-binding protein, is released from the IP3 receptor upon IP3 binding to the receptor. J Biol Chem. 2003;278:10602–12. doi: 10.1074/jbc.M210119200. [DOI] [PubMed] [Google Scholar]
- Arreola J, Melvin JE, Begenisich T. Activation of calcium-dependent chloride channels in rat parotid acinar cells. J Gen Physiol. 1996;108:35–47. doi: 10.1085/jgp.108.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avella M, Loriol C, Boulukos K, Borgese F, Ehrenfeld J. SLC26A9 stimulates CFTR expression and function in human bronchial cell lines. J Cell Physiol. 2011;226:212–23. doi: 10.1002/jcp.22328. [DOI] [PubMed] [Google Scholar]
- Baker JM, Hudson RP, Kanelis V, Choy WY, Thibodeau PH, Thomas PJ, Forman-Kay JD. CFTR regulatory region interacts with NBD1 predominantly via multiple transient helices. Nat Struct Mol Biol. 2007;14:738–45. doi: 10.1038/nsmb1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belden S, Flaherty KT. MEK and RAF inhibitors for BRAF-mutated cancers. Expert Rev Mol Med. 2012;14:e17. doi: 10.1017/erm.2012.11. [DOI] [PubMed] [Google Scholar]
- Bertrand CA, Zhang R, Pilewski JM, Frizzell RA. SLC26A9 is a constitutively active, CFTR-regulated anion conductance in human bronchial epithelia. J Gen Physiol. 2009;133:421–38. doi: 10.1085/jgp.200810097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betzenhauser MJ, Fike JL, Wagner LE, 2nd, Yule DI. Protein kinase A increases type-2 inositol 1,4,5-trisphosphate receptor activity by phosphorylation of serine 937. J Biol Chem. 2009;284:25116–25. doi: 10.1074/jbc.M109.010132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boron WF, Chen L, Parker MD. Modular structure of sodium-coupled bicarbonate transporters. J Exp Biol. 2009;212:1697–706. doi: 10.1242/jeb.028563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyden LM, Choi M, Choate KA, Nelson-Williams CJ, Farhi A, Toka HR, Tikhonova IR, Bjornson R, Mane SM, Colussi G, Lebel M, Gordon RD, Semmekrot BA, Poujol A, Valimaki MJ, De Ferrari ME, Sanjad SA, Gutkin M, Karet FE, Tucci JR, Stockigt JR, Keppler-Noreuil KM, Porter CC, Anand SK, Whiteford ML, Davis ID, Dewar SB, Bettinelli A, Fadrowski JJ, Belsha CW, Hunley TE, Nelson RD, Trachtman H, Cole TR, Pinsk M, Bockenhauer D, Shenoy M, Vaidyanathan P, Foreman JW, Rasoulpour M, Thameem F, Al-Shahrouri HZ, Radhakrishnan J, Gharavi AG, Goilav B, Lifton RP. Mutations in kelch-like 3 and cullin 3 cause hypertension and electrolyte abnormalities. Nature. 2012;482:98–102. doi: 10.1038/nature10814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai H, Cebotaru V, Wang YH, Zhang XM, Cebotaru L, Guggino SE, Guggino WB. WNK4 kinase regulates surface expression of the human sodium chloride cotransporter in mammalian cells. Kidney international. 2006;69:2162–70. doi: 10.1038/sj.ki.5000333. [DOI] [PubMed] [Google Scholar]
- Catalan MA, Nakamoto T, Gonzalez-Begne M, Camden JM, Wall SM, Clarke LL, Melvin JE. Cftr and ENaC ion channels mediate NaCl absorption in the mouse submandibular gland. J Physiol. 2010;588:713–24. doi: 10.1113/jphysiol.2009.183541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chew CS, Petropoulos AC. Thapsigargin potentiates histamine-stimulated HCl secretion in gastric parietal cells but does not mimic cholinergic responses. Cell Regul. 1991;2:27–39. doi: 10.1091/mbc.2.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho HJ, Joo NS, Wine JJ. Mucus secretion from individual submucosal glands of the ferret trachea. Am J Physiol Lung Cell Mol Physiol. 2010;299:L124–36. doi: 10.1152/ajplung.00049.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choate KA, Kahle KT, Wilson FH, Nelson-Williams C, Lifton RP. WNK1, a kinase mutated in inherited hypertension with hyperkalemia, localizes to diverse Cl−-transporting epithelia. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:663–8. doi: 10.1073/pnas.242728499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi JY, Khansaheb M, Joo NS, Krouse ME, Robbins RC, Weill D, Wine JJ. Substance P stimulates human airway submucosal gland secretion mainly via a CFTR-dependent process. J Clin Invest. 2009;119:1189–200. doi: 10.1172/JCI37284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compton EL, Karinou E, Naismith JH, Gabel F, Javelle A. Low resolution structure of a bacterial SLC26 transporter reveals dimeric stoichiometry and mobile intracellular domains. The Journal of biological chemistry. 2011;286:27058–67. doi: 10.1074/jbc.M111.244533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compton EL, Karinou E, Naismith JH, Gabel F, Javelle A. Low resolution structure of a bacterial SLC26 transporter reveals dimeric stoichiometry and mobile intracellular domains. J Biol Chem. 2011;286:27058–67. doi: 10.1074/jbc.M111.244533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compton J, Martinez JR, Martinez AM, Young JA. Fluid and electrolyte secretion from the isolated, perfused submandibular and sublingual glands of the rat. Arch Oral Biol. 1981;26:555–61. doi: 10.1016/0003-9969(81)90017-0. [DOI] [PubMed] [Google Scholar]
- Delpire E. The mammalian family of sterile 20p-like protein kinases. Pflugers Archiv : European journal of physiology. 2009;458:953–67. doi: 10.1007/s00424-009-0674-y. [DOI] [PubMed] [Google Scholar]
- Delpire E, Austin TM. Kinase regulation of Na+-K+-2Cl− cotransport in primary afferent neurons. The Journal of physiology. 2010;588:3365–73. doi: 10.1113/jphysiol.2010.190769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delporte C, Steinfeld S. Distribution and roles of aquaporins in salivary glands. Biochim Biophys Acta. 2006;1758:1061–70. doi: 10.1016/j.bbamem.2006.01.022. [DOI] [PubMed] [Google Scholar]
- Devogelaere B, Nadif Kasri N, Derua R, Waelkens E, Callewaert G, Missiaen L, Parys JB, De Smedt H. Binding of IRBIT to the IP3 receptor: determinants and functional effects. Biochem Biophys Res Commun. 2006;343:49–56. doi: 10.1016/j.bbrc.2006.02.119. [DOI] [PubMed] [Google Scholar]
- Devogelaere B, Sammels E, De Smedt H. The IRBIT domain adds new functions to the AHCY family. Bioessays. 2008;30:642–52. doi: 10.1002/bies.20772. [DOI] [PubMed] [Google Scholar]
- Dorwart MR, Shcheynikov N, Wang Y, Stippec S, Muallem S. SLC26A9 is a Cl(−) channel regulated by the WNK kinases. The Journal of physiology. 2007;584:333–45. doi: 10.1113/jphysiol.2007.135855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorwart MR, Shcheynikov N, Wang Y, Stippec S, Muallem S. SLC26A9 is a Cl(−) channel regulated by the WNK kinases. J Physiol. 2007;584:333–45. doi: 10.1113/jphysiol.2007.135855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorwart MR, Shcheynikov N, Yang D, Muallem S. The solute carrier 26 family of proteins in epithelial ion transport. Physiology (Bethesda) 2008;23:104–14. doi: 10.1152/physiol.00037.2007. [DOI] [PubMed] [Google Scholar]
- El-Showk S, Ruonala R, Helariutta Y. Crossing paths: cytokinin signalling and crosstalk. Development. 2013;140:1373–83. doi: 10.1242/dev.086371. [DOI] [PubMed] [Google Scholar]
- Everett LA, Glaser B, Beck JC, Idol JR, Buchs A, Heyman M, Adawi F, Hazani E, Nassir E, Baxevanis AD, Sheffield VC, Green ED. Pendred syndrome is caused by mutations in a putative sulphate transporter gene (PDS) Nat Genet. 1997;17:411–22. doi: 10.1038/ng1297-411. [DOI] [PubMed] [Google Scholar]
- Ferre S, Karcz-Kubicha M, Hope BT, Popoli P, Burgueno J, Gutierrez MA, Casado V, Fuxe K, Goldberg SR, Lluis C, Franco R, Ciruela F. Synergistic interaction between adenosine A2A and glutamate mGlu5 receptors: implications for striatal neuronal function. Proc Natl Acad Sci U S A. 2002;99:11940–5. doi: 10.1073/pnas.172393799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagnon KB, Delpire E. Molecular physiology of SPAK and OSR1: two Ste20-related protein kinases regulating ion transport. Physiological reviews. 2012;92:1577–617. doi: 10.1152/physrev.00009.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagnon KB, Delpire E. Molecular physiology of SPAK and OSR1: two Ste20-related protein kinases regulating ion transport. Physiol Rev. 2012;92:1577–617. doi: 10.1152/physrev.00009.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner JD. Regulation of pancreatic exocrine function in vitro: initial steps in the actions of secretagogues. Annu Rev Physiol. 1979;41:55–66. doi: 10.1146/annurev.ph.41.030179.000415. [DOI] [PubMed] [Google Scholar]
- Garnett JP, Hickman E, Tunkamnerdthai O, Cuthbert AW, Gray MA. Protein phosphatase 1 coordinates CFTR-dependent airway epithelial HCO3− secretion by reciprocal regulation of apical and basolateral membrane Cl(−)-HCO3− exchangers. Br J Pharmacol. 2013;168:1946–60. doi: 10.1111/bph.12085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golbang AP, Cope G, Hamad A, Murthy M, Liu CH, Cuthbert AW, O’Shaughnessy KM. Regulation of the expression of the Na/Cl cotransporter by WNK4 and WNK1: evidence that accelerated dynamin-dependent endocytosis is not involved. American journal of physiology. Renal physiology. 2006;291:F1369–76. doi: 10.1152/ajprenal.00468.2005. [DOI] [PubMed] [Google Scholar]
- Gross E, Hawkins K, Abuladze N, Pushkin A, Cotton CU, Hopfer U, Kurtz I. The stoichiometry of the electrogenic sodium bicarbonate cotransporter NBC1 is cell-type dependent. J Physiol. 2001;531:597–603. doi: 10.1111/j.1469-7793.2001.0597h.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu W, Shi XL, Roeder RG. Synergistic activation of transcription by CBP and p53. Nature. 1997;387:819–23. doi: 10.1038/42972. [DOI] [PubMed] [Google Scholar]
- Haas M, Forbush B., 3rd The Na-K-Cl cotransporter of secretory epithelia. Annu Rev Physiol. 2000;62:515–34. doi: 10.1146/annurev.physiol.62.1.515. [DOI] [PubMed] [Google Scholar]
- Hallworth R, Stark K, Zholudeva L, Currall BB, Nichols MG. The conserved tetrameric subunit stoichiometry of Slc26 proteins. Microscopy and microanalysis : the official journal of Microscopy Society of America, Microbeam Analysis Society, Microscopical Society of Canada. 2013;19:799–807. doi: 10.1017/S1431927613000457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison JD. Causes, natural history, and incidence of salivary stones and obstructions. Otolaryngol Clin North Am. 2009;42:927–47. doi: 10.1016/j.otc.2009.08.012. [DOI] [PubMed] [Google Scholar]
- Hastbacka J, de la Chapelle A, Mahtani MM, Clines G, Reeve-Daly MP, Daly M, Hamilton BA, Kusumi K, Trivedi B, Weaver A, et al. The diastrophic dysplasia gene encodes a novel sulfate transporter: positional cloning by fine-structure linkage disequilibrium mapping. Cell. 1994;78:1073–87. doi: 10.1016/0092-8674(94)90281-x. [DOI] [PubMed] [Google Scholar]
- He P, Zhang H, Yun CC. IRBIT, inositol 1,4,5-triphosphate (IP3) receptor-binding protein released with IP3, binds Na+/H+ exchanger NHE3 and activates NHE3 activity in response to calcium. J Biol Chem. 2008;283:33544–53. doi: 10.1074/jbc.M805534200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirono C, Sugita M, Furuya K, Yamagishi S, Shiba Y. Potentiation by isoproterenol on carbachol-induced K+ and Cl− currents and fluid secretion in rat parotid. J Membr Biol. 1998;164:197–203. doi: 10.1007/s002329900405. [DOI] [PubMed] [Google Scholar]
- Hoffmann EK, Lambert IH, Pedersen SF. Physiology of cell volume regulation in vertebrates. Physiol Rev. 2009;89:193–277. doi: 10.1152/physrev.00037.2007. [DOI] [PubMed] [Google Scholar]
- Hoglund P, Haila S, Socha J, Tomaszewski L, Saarialho-Kere U, Karjalainen-Lindsberg ML, Airola K, Holmberg C, de la Chapelle A, Kere J. Mutations of the Down-regulated in adenoma (DRA) gene cause congenital chloride diarrhoea. Nat Genet. 1996;14:316–9. doi: 10.1038/ng1196-316. [DOI] [PubMed] [Google Scholar]
- Homma K, Miller KK, Anderson CT, Sengupta S, Du GG, Aguinaga S, Cheatham M, Dallos P, Zheng J. Interaction between CFTR and prestin (SLC26A5) Biochim Biophys Acta. 2010;1798:1029–40. doi: 10.1016/j.bbamem.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong JH, Yang D, Shcheynikov N, Ohana E, Shin DM, Muallem S. Convergence of IRBIT, phosphatidylinositol (4,5) bisphosphate, and WNK/SPAK kinases in regulation of the Na+-HCO3− cotransporters family. Proc Natl Acad Sci U S A. 2013;110:4105–10. doi: 10.1073/pnas.1221410110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoorn EJ, Ellison DH. WNK kinases and the kidney. Exp Cell Res. 2012;318:1020–6. doi: 10.1016/j.yexcr.2012.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang CL, Cha SK, Wang HR, Xie J, Cobb MH. WNKs: protein kinases with a unique kinase domain. Experimental & molecular medicine. 2007;39:565–73. doi: 10.1038/emm.2007.62. [DOI] [PubMed] [Google Scholar]
- Ishiguro H, Naruse S, Kitagawa M, Suzuki A, Yamamoto A, Hayakawa T, Case RM, Steward MC. CO2 permeability and bicarbonate transport in microperfused interlobular ducts isolated from guinea-pig pancreas. J Physiol. 2000;528(Pt 2):305–15. doi: 10.1111/j.1469-7793.2000.00305.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishiguro H, Steward MC, Naruse S, Ko SB, Goto H, Case RM, Kondo T, Yamamoto A. CFTR functions as a bicarbonate channel in pancreatic duct cells. J Gen Physiol. 2009;133:315–26. doi: 10.1085/jgp.200810122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa Y, Yuan Z, Inoue N, Skowronski MT, Nakae Y, Shono M, Cho G, Yasui M, Agre P, Nielsen S. Identification of AQP5 in lipid rafts and its translocation to apical membranes by activation of M3 mAChRs in interlobular ducts of rat parotid gland. Am J Physiol Cell Physiol. 2005;289:C1303–11. doi: 10.1152/ajpcell.00211.2005. [DOI] [PubMed] [Google Scholar]
- Jiang Z, Asplin JR, Evan AP, Rajendran VM, Velazquez H, Nottoli TP, Binder HJ, Aronson PS. Calcium oxalate urolithiasis in mice lacking anion transporter Slc26a6. Nat Genet. 2006;38:474–8. doi: 10.1038/ng1762. [DOI] [PubMed] [Google Scholar]
- Joo NS, Cho HJ, Khansaheb M, Wine JJ. Hyposecretion of fluid from tracheal submucosal glands of CFTR-deficient pigs. J Clin Invest. 2010;120:3161–6. doi: 10.1172/JCI43466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahle KT, Gimenez I, Hassan H, Wilson FH, Wong RD, Forbush B, Aronson PS, Lifton RP. WNK4 regulates apical and basolateral Cl− flux in extrarenal epithelia. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:2064–9. doi: 10.1073/pnas.0308434100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasai H, Augustine GJ. Cytosolic Ca2+ gradients triggering unidirectional fluid secretion from exocrine pancreas. Nature. 1990;348:735–8. doi: 10.1038/348735a0. [DOI] [PubMed] [Google Scholar]
- Kerem B, Rommens JM, Buchanan JA, Markiewicz D, Cox TK, Chakravarti A, Buchwald M, Tsui LC. Identification of the cystic fibrosis gene: genetic analysis. Science. 1989;245:1073–80. doi: 10.1126/science.2570460. [DOI] [PubMed] [Google Scholar]
- Kim KH, Shcheynikov N, Wang Y, Muallem S. SLC26A7 is a Cl− channel regulated by intracellular pH. J Biol Chem. 2005;280:6463–70. doi: 10.1074/jbc.M409162200. [DOI] [PubMed] [Google Scholar]
- Kiselyov K, Wang X, Shin DM, Zang W, Muallem S. Calcium signaling complexes in microdomains of polarized secretory cells. Cell Calcium. 2006;40:451–9. doi: 10.1016/j.ceca.2006.08.009. [DOI] [PubMed] [Google Scholar]
- Knauf F, Yang CL, Thomson RB, Mentone SA, Giebisch G, Aronson PS. Identification of a chloride-formate exchanger expressed on the brush border membrane of renal proximal tubule cells. Proc Natl Acad Sci U S A. 2001;98:9425–30. doi: 10.1073/pnas.141241098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko SB, Shcheynikov N, Choi JY, Luo X, Ishibashi K, Thomas PJ, Kim JY, Kim KH, Lee MG, Naruse S, Muallem S. A molecular mechanism for aberrant CFTR-dependent HCO(3)(−) transport in cystic fibrosis. EMBO J. 2002;21:5662–72. doi: 10.1093/emboj/cdf580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko SB, Zeng W, Dorwart MR, Luo X, Kim KH, Millen L, Goto H, Naruse S, Soyombo A, Thomas PJ, Muallem S. Gating of CFTR by the STAS domain of SLC26 transporters. Nat Cell Biol. 2004;6:343–50. doi: 10.1038/ncb1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornitzer D, Ciechanover A. Modes of regulation of ubiquitin-mediated protein degradation. J Cell Physiol. 2000;182:1–11. doi: 10.1002/(SICI)1097-4652(200001)182:1<1::AID-JCP1>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- Kuijpers GA, Van Nooy IG, De Pont JJ, Bonting SL. The mechanism of fluid secretion in the rabbit pancreas studied by means of various inhibitors. Biochim Biophys Acta. 1984;778:324–31. doi: 10.1016/0005-2736(84)90376-6. [DOI] [PubMed] [Google Scholar]
- Kunzelmann K. CFTR: interacting with everything? News Physiol Sci. 2001;16:167–70. doi: 10.1152/physiologyonline.2001.16.4.167. [DOI] [PubMed] [Google Scholar]
- Kunzelmann K, Kongsuphol P, Aldehni F, Tian Y, Ousingsawat J, Warth R, Schreiber R. Bestrophin and TMEM16-Ca(2+) activated Cl(−) channels with different functions. Cell Calcium. 2009;46:233–41. doi: 10.1016/j.ceca.2009.09.003. [DOI] [PubMed] [Google Scholar]
- Lee MG, Muallem S. Physiology of duct cell secretion. In: Beger H, Buchler M, Kozarek R, Lerch M, Neoptolemos J, Warshaw A, Whitcomb D, Shiratori K, editors. Pancreas: An Integrated Textbook of Basic Science, Medicine, and Surgery. Blackwell Publishing; Oxford, U.K: 2008. pp. 78–90. [Google Scholar]
- Lee MG, Ohana E, Park HW, Yang D, Muallem S. Molecular mechanism of pancreatic and salivary gland fluid and HCO3 secretion. Physiological reviews. 2012;92:39–74. doi: 10.1152/physrev.00011.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MG, Xu X, Zeng W, Diaz J, Wojcikiewicz RJ, Kuo TH, Wuytack F, Racymaekers L, Muallem S. Polarized expression of Ca2+ channels in pancreatic and salivary gland cells. Correlation with initiation and propagation of [Ca2+]i waves. J Biol Chem. 1997;272:15765–70. doi: 10.1074/jbc.272.25.15765. [DOI] [PubMed] [Google Scholar]
- Lee RJ, Foskett JK. cAMP-activated Ca2+ signaling is required for CFTR-mediated serous cell fluid secretion in porcine and human airways. J Clin Invest. 2010;120:3137–48. doi: 10.1172/JCI42992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SK, Boron WF, Parker MD. Relief of autoinhibition of the electrogenic Na-HCO(3) [corrected] cotransporter NBCe1-B: role of IRBIT vs. amino-terminal truncation. American journal of physiology. Cell physiology. 2012;302:C518–26. doi: 10.1152/ajpcell.00352.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lennartsson J, Ronnstrand L. Stem cell factor receptor/c-Kit: from basic science to clinical implications. Physiol Rev. 2012;92:1619–49. doi: 10.1152/physrev.00046.2011. [DOI] [PubMed] [Google Scholar]
- Linsdell P, Tabcharani JA, Rommens JM, Hou YX, Chang XB, Tsui LC, Riordan JR, Hanrahan JW. Permeability of wild-type and mutant cystic fibrosis transmembrane conductance regulator chloride channels to polyatomic anions. J Gen Physiol. 1997;110:355–64. doi: 10.1085/jgp.110.4.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohi H, Kujala M, Kerkela E, Saarialho-Kere U, Kestila M, Kere J. Mapping of five new putative anion transporter genes in human and characterization of SLC26A6, a candidate gene for pancreatic anion exchanger. Genomics. 2000;70:102–12. doi: 10.1006/geno.2000.6355. [DOI] [PubMed] [Google Scholar]
- Louis-Dit-Picard H, Barc J, Trujillano D, Miserey-Lenkei S, Bouatia-Naji N, Pylypenko O, Beaurain G, Bonnefond A, Sand O, Simian C, Vidal-Petiot E, Soukaseum C, Mandet C, Broux F, Chabre O, Delahousse M, Esnault V, Fiquet B, Houillier P, Bagnis CI, Koenig J, Konrad M, Landais P, Mourani C, Niaudet P, Probst V, Thauvin C, Unwin RJ, Soroka SD, Ehret G, Ossowski S, Caulfield M, Bruneval P, Estivill X, Froguel P, Hadchouel J, Schott JJ, Jeunemaitre X. KLHL3 mutations cause familial hyperkalemic hypertension by impairing ion transport in the distal nephron. Nat Genet. 2012;44:456–60. S1–3. doi: 10.1038/ng.2218. [DOI] [PubMed] [Google Scholar]
- Luo X, Choi JY, Ko SB, Pushkin A, Kurtz I, Ahn W, Lee MG, Muallem S. HCO3− salvage mechanisms in the submandibular gland acinar and duct cells. J Biol Chem. 2001;276:9808–16. doi: 10.1074/jbc.M008548200. [DOI] [PubMed] [Google Scholar]
- Ma T, Song Y, Gillespie A, Carlson EJ, Epstein CJ, Verkman AS. Defective secretion of saliva in transgenic mice lacking aquaporin-5 water channels. J Biol Chem. 1999;274:20071–4. doi: 10.1074/jbc.274.29.20071. [DOI] [PubMed] [Google Scholar]
- Markovich D, Aronson PS. Specificity and regulation of renal sulfate transporters. Annu Rev Physiol. 2007;69:361–75. doi: 10.1146/annurev.physiol.69.040705.141319. [DOI] [PubMed] [Google Scholar]
- McCormick JA, Ellison DH. The WNKs: atypical protein kinases with pleiotropic actions. Physiological reviews. 2011;91:177–219. doi: 10.1152/physrev.00017.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melvin JE, Yule D, Shuttleworth T, Begenisich T. Regulation of fluid and electrolyte secretion in salivary gland acinar cells. Annu Rev Physiol. 2005;67:445–69. doi: 10.1146/annurev.physiol.67.041703.084745. [DOI] [PubMed] [Google Scholar]
- Metz DC, Patto RJ, Mrozinski JE, Jr, Jensen RT, Turner RJ, Gardner JD. Thapsigargin defines the roles of cellular calcium in secretagogue-stimulated enzyme secretion from pancreatic acini. J Biol Chem. 1992;267:20620–9. [PubMed] [Google Scholar]
- Min X, Lee BH, Cobb MH, Goldsmith EJ. Crystal structure of the kinase domain of WNK1, a kinase that causes a hereditary form of hypertension. Structure. 2004;12:1303–11. doi: 10.1016/j.str.2004.04.014. [DOI] [PubMed] [Google Scholar]
- Morales B, Barrera N, Uribe P, Mora C, Villalon M. Functional cross talk after activation of P2 and P1 receptors in oviductal ciliated cells. Am J Physiol Cell Physiol. 2000;279:C658–69. doi: 10.1152/ajpcell.2000.279.3.C658. [DOI] [PubMed] [Google Scholar]
- Muallem S, Loessberg PA. Intracellular pH-regulatory mechanisms in pancreatic acinar cells. I. Characterization of H+ and HCO3− transporters. J Biol Chem. 1990;265:12806–12. [PubMed] [Google Scholar]
- Nakamoto T, Romanenko VG, Takahashi A, Begenisich T, Melvin JE. Apical maxi-K (KCa1.1) channels mediate K+ secretion by the mouse submandibular exocrine gland. American journal of physiology. Cell physiology. 2008;294:C810–9. doi: 10.1152/ajpcell.00511.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamoto T, Romanenko VG, Takahashi A, Begenisich T, Melvin JE. Apical maxi-K (KCa1.1) channels mediate K+ secretion by the mouse submandibular exocrine gland. Am J Physiol Cell Physiol. 2008;294:C810–9. doi: 10.1152/ajpcell.00511.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nehrke K, Quinn CC, Begenisich T. Molecular identification of Ca2+-activated K+ channels in parotid acinar cells. Am J Physiol Cell Physiol. 2003;284:C535–46. doi: 10.1152/ajpcell.00044.2002. [DOI] [PubMed] [Google Scholar]
- Nguyen HV, Stuart-Tilley A, Alper SL, Melvin JE. Cl(−)/HCO(3)(−) exchange is acetazolamide sensitive and activated by a muscarinic receptor-induced [Ca(2+)](i) increase in salivary acinar cells. Am J Physiol Gastrointest Liver Physiol. 2004;286:G312–20. doi: 10.1152/ajpgi.00158.2003. [DOI] [PubMed] [Google Scholar]
- O’Reilly M, Marshall E, Speirs HJ, Brown RW. WNK1, a gene within a novel blood pressure control pathway, tissue-specifically generates radically different isoforms with and without a kinase domain. Journal of the American Society of Nephrology : JASN. 2003;14:2447–56. doi: 10.1097/01.asn.0000089830.97681.3b. [DOI] [PubMed] [Google Scholar]
- Ohana E, Shcheynikov N, Moe OW, Muallem S. SLC26A6 and NaDC-1 Transporters Interact to Regulate Oxalate and Citrate Homeostasis. Journal of the American Society of Nephrology : JASN. 2013 doi: 10.1681/ASN.2013010080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohana E, Shcheynikov N, Park M, Muallem S. Solute carrier family 26 member a2 (Slc26a2) protein functions as an electroneutral SOFormula/OH-/Cl− exchanger regulated by extracellular Cl. The Journal of biological chemistry. 2012;287:5122–32. doi: 10.1074/jbc.M111.297192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohana E, Shcheynikov N, Yang D, So I, Muallem S. Determinants of coupled transport and uncoupled current by the electrogenic SLC26 transporters. The Journal of general physiology. 2011;137:239–51. doi: 10.1085/jgp.201010531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohana E, Shcheynikov N, Yang D, So I, Muallem S. Determinants of coupled transport and uncoupled current by the electrogenic SLC26 transporters. J Gen Physiol. 2011;137:239–51. doi: 10.1085/jgp.201010531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohana E, Yang D, Shcheynikov N, Muallem S. Diverse transport modes by the solute carrier 26 family of anion transporters. J Physiol. 2009;587:2179–85. doi: 10.1113/jphysiol.2008.164863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta A, Schumacher FR, Mehellou Y, Johnson C, Knebel A, Macartney TJ, Wood NT, Alessi DR, Kurz T. The CUL3-KLHL3 E3 ligase complex mutated in Gordon’s hypertension syndrome interacts with and ubiquitylates WNK isoforms: disease-causing mutations in KLHL3 and WNK4 disrupt interaction. Biochem J. 2013;451:111–22. doi: 10.1042/BJ20121903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park HW, Nam JH, Kim JY, Namkung W, Yoon JS, Lee JS, Kim KS, Venglovecz V, Gray MA, Kim KH, Lee MG. Dynamic regulation of CFTR bicarbonate permeability by [Cl−]i and its role in pancreatic bicarbonate secretion. Gastroenterology. 2010;139:620–31. doi: 10.1053/j.gastro.2010.04.004. [DOI] [PubMed] [Google Scholar]
- Park M, Ko SB, Choi JY, Muallem G, Thomas PJ, Pushkin A, Lee MS, Kim JY, Lee MG, Muallem S, Kurtz I. The cystic fibrosis transmembrane conductance regulator interacts with and regulates the activity of the HCO3− salvage transporter human Na+-HCO3− cotransport isoform 3. J Biol Chem. 2002;277:50503–9. doi: 10.1074/jbc.M201862200. [DOI] [PubMed] [Google Scholar]
- Park S, Hong JH, Ohana E, Muallem S. The WNK/SPAK and IRBIT/PP1 pathways in epithelial fluid and electrolyte transport. Physiology. 2012;27:291–9. doi: 10.1152/physiol.00028.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S, Hong JH, Ohana E, Muallem S. The WNK/SPAK and IRBIT/PP1 pathways in epithelial fluid and electrolyte transport. Physiology (Bethesda) 2012;27:291–9. doi: 10.1152/physiol.00028.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S, Shcheynikov N, Hong JH, Zheng C, Suh SH, Kawaai K, Ando H, Mizutani A, Abe T, Kiyonari H, Seki G, Yule D, Mikoshiba K, Muallem S. Irbit mediates synergy between ca(2+) and cAMP signaling pathways during epithelial transport in mice. Gastroenterology. 2013;145:232–41. doi: 10.1053/j.gastro.2013.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasqualetto E, Aiello R, Gesiot L, Bonetto G, Bellanda M, Battistutta R. Structure of the cytosolic portion of the motor protein prestin and functional role of the STAS domain in SLC26/SulP anion transporters. Journal of molecular biology. 2010;400:448–62. doi: 10.1016/j.jmb.2010.05.013. [DOI] [PubMed] [Google Scholar]
- Pasqualetto E, Aiello R, Gesiot L, Bonetto G, Bellanda M, Battistutta R. Structure of the cytosolic portion of the motor protein prestin and functional role of the STAS domain in SLC26/SulP anion transporters. J Mol Biol. 2010;400:448–62. doi: 10.1016/j.jmb.2010.05.013. [DOI] [PubMed] [Google Scholar]
- Petersen OH. Calcium-activated potassium channels and fluid secretion by exocrine glands. Am J Physiol. 1986;251:G1–13. doi: 10.1152/ajpgi.1986.251.1.G1. [DOI] [PubMed] [Google Scholar]
- Petersen OH, Philpott HG. Mouse pancreatic acinar cells: the anion selectivity of the acetylcholine-opened chloride pathway. J Physiol. 1980;306:481–92. doi: 10.1113/jphysiol.1980.sp013409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen OH, Ueda N. Secretion of fluid and amylase in the perfused rat pancreas. J Physiol. 1977;264:819–35. doi: 10.1113/jphysiol.1977.sp011696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petroski MD, Deshaies RJ. Function and regulation of cullin-RING ubiquitin ligases. Nat Rev Mol Cell Biol. 2005;6:9–20. doi: 10.1038/nrm1547. [DOI] [PubMed] [Google Scholar]
- Poulsen JH, Fischer H, Illek B, Machen TE. Bicarbonate conductance and pH regulatory capability of cystic fibrosis transmembrane conductance regulator. Proc Natl Acad Sci U S A. 1994;91:5340–4. doi: 10.1073/pnas.91.12.5340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinton PM. The neglected ion: HCO3−. Nat Med. 2001;7:292–3. doi: 10.1038/85429. [DOI] [PubMed] [Google Scholar]
- Rahmati N, Kunzelmann K, Xu J, Barone S, Sirianant L, De Zeeuw CI, Soleimani M. Slc26a11 is prominently expressed in the brain and functions as a chloride channel: expression in Purkinje cells and stimulation of V H-ATPase. Pflugers Archiv : European journal of physiology. 2013 Jun 4; doi: 10.1007/s00424-013-1300-6. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raufman JP, Kasbekar DK, Jensen RT, Gardner JD. Potentiation of pepsinogen secretion from dispersed glands from rat stomach. Am J Physiol. 1983;245:G525–30. doi: 10.1152/ajpgi.1983.245.4.G525. [DOI] [PubMed] [Google Scholar]
- Ribeiro MO. Effects of thyroid hormone analogs on lipid metabolism and thermogenesis. Thyroid. 2008;18:197–203. doi: 10.1089/thy.2007.0288. [DOI] [PubMed] [Google Scholar]
- Richardson C, Alessi DR. The regulation of salt transport and blood pressure by the WNK-SPAK/OSR1 signalling pathway. Journal of cell science. 2008;121:3293–304. doi: 10.1242/jcs.029223. [DOI] [PubMed] [Google Scholar]
- Riordan JR, Rommens JM, Kerem B, Alon N, Rozmahel R, Grzelczak Z, Zielenski J, Lok S, Plavsic N, Chou JL, et al. Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA. Science. 1989;245:1066–73. doi: 10.1126/science.2475911. [DOI] [PubMed] [Google Scholar]
- Rode B, Dirami T, Bakouh N, Rizk-Rabin M, Norez C, Lhuillier P, Lores P, Jollivet M, Melin P, Zvetkova I, Bienvenu T, Becq F, Planelles G, Edelman A, Gacon G, Toure A. The testis anion transporter TAT1 (SLC26A8) physically and functionally interacts with the cystic fibrosis transmembrane conductance regulator channel: a potential role during sperm capacitation. Hum Mol Genet. 2012;21:1287–98. doi: 10.1093/hmg/ddr558. [DOI] [PubMed] [Google Scholar]
- Romanenko VG, Catalan MA, Brown DA, Putzier I, Hartzell HC, Marmorstein AD, Gonzalez-Begne M, Rock JR, Harfe BD, Melvin JE. Tmem16A encodes the Ca2+-activated Cl− channel in mouse submandibular salivary gland acinar cells. J Biol Chem. 2010;285:12990–3001. doi: 10.1074/jbc.M109.068544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanenko VG, Nakamoto T, Srivastava A, Begenisich T, Melvin JE. Regulation of membrane potential and fluid secretion by Ca2+-activated K+ channels in mouse submandibular glands. J Physiol. 2007;581:801–17. doi: 10.1113/jphysiol.2006.127498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanenko VG, Roser KS, Melvin JE, Begenisich T. The role of cell cholesterol and the cytoskeleton in the interaction between IK1 and maxi-K channels. American journal of physiology. Cell physiology. 2009;296:C878–88. doi: 10.1152/ajpcell.00438.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rommens JM, Iannuzzi MC, Kerem B, Drumm ML, Melmer G, Dean M, Rozmahel R, Cole JL, Kennedy D, Hidaka N, et al. Identification of the cystic fibrosis gene: chromosome walking and jumping. Science. 1989;245:1059–65. doi: 10.1126/science.2772657. [DOI] [PubMed] [Google Scholar]
- Roussa E. Channels and transporters in salivary glands. Cell Tissue Res. 2011;343:263–87. doi: 10.1007/s00441-010-1089-y. [DOI] [PubMed] [Google Scholar]
- Rybalchenko V, Santos-Sacchi J. Anion control of voltage sensing by the motor protein prestin in outer hair cells. Biophys J. 2008;95:4439–47. doi: 10.1529/biophysj.108.134197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- San-Cristobal P, Ponce-Coria J, Vazquez N, Bobadilla NA, Gamba G. WNK3 and WNK4 amino-terminal domain defines their effect on the renal Na+-Cl− cotransporter. American journal of physiology. Renal physiology. 2008;295:F1199–206. doi: 10.1152/ajprenal.90396.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shcheynikov N, Kim KH, Kim KM, Dorwart MR, Ko SB, Goto H, Naruse S, Thomas PJ, Muallem S. Dynamic control of cystic fibrosis transmembrane conductance regulator Cl−/HCO3− selectivity by external Cl−. J Biol Chem. 2004;279:21857–65. doi: 10.1074/jbc.M313323200. [DOI] [PubMed] [Google Scholar]
- Shcheynikov N, Ko SB, Zeng W, Choi JY, Dorwart MR, Thomas PJ, Muallem S. Regulatory interaction between CFTR and the SLC26 transporters. Novartis Found Symp. 2006;273:177–86. discussion 186–92, 261–4. [PubMed] [Google Scholar]
- Shcheynikov N, Wang Y, Park M, Ko SB, Dorwart M, Naruse S, Thomas PJ, Muallem S. Coupling modes and stoichiometry of Cl−/HCO3− exchange by slc26a3 and slc26a6. The Journal of general physiology. 2006;127:511–24. doi: 10.1085/jgp.200509392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shcheynikov N, Yang D, Wang Y, Zeng W, Karniski LP, So I, Wall SM, Muallem S. The Slc26a4 transporter functions as an electroneutral Cl−/I−/HCO3− exchanger: role of Slc26a4 and Slc26a6 in I− and HCO3− secretion and in regulation of CFTR in the parotid duct. J Physiol. 2008;586:3813–24. doi: 10.1113/jphysiol.2008.154468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata S, Zhang J, Puthumana J, Stone KL, Lifton RP. Kelch-like 3 and Cullin 3 regulate electrolyte homeostasis via ubiquitination and degradation of WNK4. Proc Natl Acad Sci U S A. 2013;110:7838–43. doi: 10.1073/pnas.1304592110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirakabe K, Priori G, Yamada H, Ando H, Horita S, Fujita T, Fujimoto I, Mizutani A, Seki G, Mikoshiba K. IRBIT, an inositol 1,4,5-trisphosphate receptor-binding protein, specifically binds to and activates pancreas-type Na+/HCO3− cotransporter 1 (pNBC1) Proc Natl Acad Sci U S A. 2006;103:9542–7. doi: 10.1073/pnas.0602250103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Short DB, Trotter KW, Reczek D, Kreda SM, Bretscher A, Boucher RC, Stutts MJ, Milgram SL. An apical PDZ protein anchors the cystic fibrosis transmembrane conductance regulator to the cytoskeleton. J Biol Chem. 1998;273:19797–801. doi: 10.1074/jbc.273.31.19797. [DOI] [PubMed] [Google Scholar]
- Smith ZD, Caplan MJ, Forbush B, 3rd, Jamieson JD. Monoclonal antibody localization of Na+-K+-ATPase in the exocrine pancreas and parotid of the dog. Am J Physiol. 1987;253:G99–109. doi: 10.1152/ajpgi.1987.253.2.G99. [DOI] [PubMed] [Google Scholar]
- Smrcka AV. Molecular targeting of Galpha and Gbetagamma subunits: a potential approach for cancer therapeutics. Trends Pharmacol Sci. 2013;34:290–8. doi: 10.1016/j.tips.2013.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steward MC, Ishiguro H, Case RM. Mechanisms of bicarbonate secretion in the pancreatic duct. Annu Rev Physiol. 2005;67:377–409. doi: 10.1146/annurev.physiol.67.031103.153247. [DOI] [PubMed] [Google Scholar]
- Stewart AK, Yamamoto A, Nakakuki M, Kondo T, Alper SL, Ishiguro H. Functional coupling of apical Cl−/HCO3− exchange with CFTR in stimulated HCO3− secretion by guinea pig interlobular pancreatic duct. Am J Physiol Gastrointest Liver Physiol. 2009;296:G1307–17. doi: 10.1152/ajpgi.90697.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stutts MJ, Canessa CM, Olsen JC, Hamrick M, Cohn JA, Rossier BC, Boucher RC. CFTR as a cAMP-dependent regulator of sodium channels. Science. 1995;269:847–50. doi: 10.1126/science.7543698. [DOI] [PubMed] [Google Scholar]
- Tabcharani JA, Chang XB, Riordan JR, Hanrahan JW. Phosphorylation-regulated Cl− channel in CHO cells stably expressing the cystic fibrosis gene. Nature. 1991;352:628–31. doi: 10.1038/352628a0. [DOI] [PubMed] [Google Scholar]
- Thorn P, Lawrie AM, Smith PM, Gallacher DV, Petersen OH. Local and global cytosolic Ca2+ oscillations in exocrine cells evoked by agonists and inositol trisphosphate. Cell. 1993;74:661–8. doi: 10.1016/0092-8674(93)90513-p. [DOI] [PubMed] [Google Scholar]
- Vassart G, Costagliola S. G protein-coupled receptors: mutations and endocrine diseases. Nat Rev Endocrinol. 2011;7:362–72. doi: 10.1038/nrendo.2011.20. [DOI] [PubMed] [Google Scholar]
- Venglovecz V, Hegyi P, Rakonczay Z, Jr, Tiszlavicz L, Nardi A, Grunnet M, Gray MA. Pathophysiological relevance of apical large-conductance Ca2+-activated potassium channels in pancreatic duct epithelial cells. Gut. 2011;60:361–9. doi: 10.1136/gut.2010.214213. [DOI] [PubMed] [Google Scholar]
- Verissimo F, Jordan P. WNK kinases, a novel protein kinase subfamily in multi-cellular organisms. Oncogene. 2001;20:5562–9. doi: 10.1038/sj.onc.1204726. [DOI] [PubMed] [Google Scholar]
- Vitari AC, Deak M, Morrice NA, Alessi DR. The WNK1 and WNK4 protein kinases that are mutated in Gordon’s hypertension syndrome phosphorylate and activate SPAK and OSR1 protein kinases. The Biochemical journal. 2005;391:17–24. doi: 10.1042/BJ20051180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakabayashi M, Mori T, Isobe K, Sohara E, Susa K, Araki Y, Chiga M, Kikuchi E, Nomura N, Mori Y, Matsuo H, Murata T, Nomura S, Asano T, Kawaguchi H, Nonoyama S, Rai T, Sasaki S, Uchida S. Impaired KLHL3-mediated ubiquitination of WNK4 causes human hypertension. Cell Rep. 2013;3:858–68. doi: 10.1016/j.celrep.2013.02.024. [DOI] [PubMed] [Google Scholar]
- Wang S, Raab RW, Schatz PJ, Guggino WB, Li M. Peptide binding consensus of the NHE-RF-PDZ1 domain matches the C-terminal sequence of cystic fibrosis transmembrane conductance regulator (CFTR) FEBS Lett. 1998;427:103–8. doi: 10.1016/s0014-5793(98)00402-5. [DOI] [PubMed] [Google Scholar]
- Wang Y, Li G, Goode J, Paz JC, Ouyang K, Screaton R, Fischer WH, Chen J, Tabas I, Montminy M. Inositol-1,4,5-trisphosphate receptor regulates hepatic gluconeogenesis in fasting and diabetes. Nature. 2012;485:128–32. doi: 10.1038/nature10988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Soyombo AA, Shcheynikov N, Zeng W, Dorwart M, Marino CR, Thomas PJ, Muallem S. Slc26a6 regulates CFTR activity in vivo to determine pancreatic duct HCO3− secretion: relevance to cystic fibrosis. EMBO J. 2006;25:5049–57. doi: 10.1038/sj.emboj.7601387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willoughby D. Organization of cAMP signalling microdomains for optimal regulation by Ca2+ entry. Biochem Soc Trans. 2012;40:246–50. doi: 10.1042/BST20110613. [DOI] [PubMed] [Google Scholar]
- Wilson FH, Disse-Nicodeme S, Choate KA, Ishikawa K, Nelson-Williams C, Desitter I, Gunel M, Milford DV, Lipkin GW, Achard JM, Feely MP, Dussol B, Berland Y, Unwin RJ, Mayan H, Simon DB, Farfel Z, Jeunemaitre X, Lifton RP. Human hypertension caused by mutations in WNK kinases. Science. 2001;293:1107–12. doi: 10.1126/science.1062844. [DOI] [PubMed] [Google Scholar]
- Wright AM, Gong X, Verdon B, Linsdell P, Mehta A, Riordan JR, Argent BE, Gray MA. Novel regulation of cystic fibrosis transmembrane conductance regulator (CFTR) channel gating by external chloride. J Biol Chem. 2004;279:41658–63. doi: 10.1074/jbc.M405517200. [DOI] [PubMed] [Google Scholar]
- Wurzinger B, Mair A, Pfister B, Teige M. Cross-talk of calcium-dependent protein kinase and MAP kinase signaling. Plant Signal Behav. 2011;6:8–12. doi: 10.4161/psb.6.1.14012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Q, Welch R, Mercado A, Romero MF, Mount DB. Molecular characterization of the murine Slc26a6 anion exchanger: functional comparison with Slc26a1. Am J Physiol Renal Physiol. 2002;283:F826–38. doi: 10.1152/ajprenal.00079.2002. [DOI] [PubMed] [Google Scholar]
- Yang CL, Angell J, Mitchell R, Ellison DH. WNK kinases regulate thiazide-sensitive Na-Cl cotransport. The Journal of clinical investigation. 2003;111:1039–45. doi: 10.1172/JCI17443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang CL, Liu X, Paliege A, Zhu X, Bachmann S, Dawson DC, Ellison DH. WNK1 and WNK4 modulate CFTR activity. Biochemical and biophysical research communications. 2007;353:535–40. doi: 10.1016/j.bbrc.2006.11.151. [DOI] [PubMed] [Google Scholar]
- Yang CL, Zhu X, Wang Z, Subramanya AR, Ellison DH. Mechanisms of WNK1 and WNK4 interaction in the regulation of thiazide-sensitive NaCl cotransport. The Journal of clinical investigation. 2005;115:1379–87. doi: 10.1172/JCI22452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang D, Li Q, So I, Huang CL, Ando H, Mizutani A, Seki G, Mikoshiba K, Thomas PJ, Muallem S. IRBIT governs epithelial secretion in mice by antagonizing the WNK/SPAK kinase pathway. The Journal of clinical investigation. 2011;121:956–65. doi: 10.1172/JCI43475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang D, Shcheynikov N, Muallem S. IRBIT: It Is Everywhere. Neurochem Res. 2011;36:1166–74. doi: 10.1007/s11064-010-0353-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang D, Shcheynikov N, Muallem S. IRBIT: it is everywhere. Neurochemical research. 2011;36:1166–74. doi: 10.1007/s11064-010-0353-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang D, Shcheynikov N, Zeng W, Ohana E, So I, Ando H, Mizutani A, Mikoshiba K, Muallem S. IRBIT coordinates epithelial fluid and HCO3− secretion by stimulating the transporters pNBC1 and CFTR in the murine pancreatic duct. J Clin Invest. 2009;119:193–202. doi: 10.1172/JCI36983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang YD, Cho H, Koo JY, Tak MH, Cho Y, Shim WS, Park SP, Lee J, Lee B, Kim BM, Raouf R, Shin YK, Oh U. TMEM16A confers receptor-activated calcium-dependent chloride conductance. Nature. 2008;455:1210–5. doi: 10.1038/nature07313. [DOI] [PubMed] [Google Scholar]
- Yue GG, Yip TW, Huang Y, Ko WH. Cellular mechanism for potentiation of Ca2+-mediated Cl− secretion by the flavonoid baicalein in intestinal epithelia. J Biol Chem. 2004;279:39310–6. doi: 10.1074/jbc.M406787200. [DOI] [PubMed] [Google Scholar]
- Yule DI, Betzenhauser MJ, Joseph SK. Linking structure to function: Recent lessons from inositol 1,4,5-trisphosphate receptor mutagenesis. Cell Calcium. 2010;47:469–79. doi: 10.1016/j.ceca.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H, Muallem S. Agonist-specific regulation of [Na+]i in pancreatic acinar cells. J Gen Physiol. 1995;106:1243–63. doi: 10.1085/jgp.106.6.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H, Muallem S. Na+, K+, and Cl− transport in resting pancreatic acinar cells. J Gen Physiol. 1995;106:1225–42. doi: 10.1085/jgp.106.6.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H, Xu X, Diaz J, Muallem S. Na+, K+, and H+/HCO3− transport in submandibular salivary ducts. Membrane localization of transporters. J Biol Chem. 1995;270:19599–605. [PubMed] [Google Scholar]