Significance
Hematophagy is key to mosquito reproductive success and an important link in pathogen transmission cycles. Vertebrate hemostasis, specifically platelet aggregation, vasoconstriction, and coagulation, presents a significant challenge to successful blood feeding. Mosquitoes have been selected to secrete salivary proteins with powerful antihemostatic activities. Aegyptin, a salivary protein from the dengue, chikungunya, and yellow fever vector, Aedes aegypti, binds collagen and inhibits platelet aggregation and adhesion. A transgene-based, gene-silencing approach elicited a specific and significant reduction in Aegyptin mRNA and protein levels in female salivary glands. The resulting prolonged probing times and reductions in feeding quality and quantity support the conclusion that platelet aggregation inhibition is a vital salivary function and that Aegyptin plays a significant and nonredundant role in successful feeding.
Keywords: hematophagy, evolution, saliva, transgenesis, RNAi
Abstract
Mosquito salivary glands have important roles in blood feeding and pathogen transmission. However, the biological relevance of many salivary components has yet to be determined. Aegyptin, a secreted salivary protein from Aedes aegypti, binds collagen and inhibits platelet aggregation and adhesion. We used a transgenic approach to study the relevance of Aegyptin in mosquito blood feeding. Aedes aegypti manipulated genetically to express gene-specific inverted-repeat RNA sequences exhibited significant reductions in Aegyptin mRNA accumulation (85–87%) and protein levels (>80-fold) in female mosquito salivary glands. Transgenic mosquitoes had longer probing times (78–300 s, P < 0.0001) when feeding on mice compared with controls (15–56 s), feeding success was reduced, and those feeding took smaller blood meals. However, no differences in feeding success or blood meal size were found in membrane feeding experiments using defibrinated human blood. Salivary gland extracts from transgenic mosquitoes failed to inhibit collagen-induced platelet aggregation in vitro. Reductions of Aegyptin did not affect salivary ADP-induced platelet aggregation inhibition or disturb anticlotting activities. Our results demonstrate the relevance of Aegyptin for A. aegypti blood feeding, providing further support for the hypothesis that platelet aggregation inhibition is a vital salivary function in blood feeding arthropods. It has been suggested that the multiple mosquito salivary components mediating platelet aggregation (i.e., Aegyptin, apyrase, D7) represent functional redundancy. Our findings do not support this hypothesis; instead, they indicate that multiple salivary components work synergistically and are necessary to achieve maximum blood feeding efficiency.
Aedes aegypti, the main vector of dengue, chikungunya, and yellow fever viruses, is an anautogenous mosquito that requires a blood meal for egg development. Female mosquitoes probe the skin of a suitable vertebrate host to acquire a blood meal. Hematophagy (blood feeding) to repletion usually takes from seconds to a few minutes (1). The stylets used to pierce the vascular beds of the vertebrate hosts cause local damage that triggers host hemostatic responses.
Primary hemostasis is characterized by vascular contraction, platelet adhesion, and formation of a soft aggregate plug that begins immediately after endothelial disruption. Injury causes temporary local contraction of vascular smooth muscle. Vasoconstriction slows blood flow, enhancing platelet adhesion and activation. The soft platelet plug is stabilized to form a clot during secondary hemostasis (2, 3). Hematophagic arthropods have evolved a complex mixture of salivary components that counteract platelet aggregation, blood coagulation, vasoconstriction, and inflammation to overcome this efficient and redundant vertebrate hemostatic system (4–6).
Aedes aegypti, Anopheles stephensi, and Simulim nigrimanum express salivary collagen-binding proteins that prevent collagen-induced platelet aggregation and adhesion by blocking its interaction with the platelet receptors glycoprotein VI and Integrin α2β1, and von Willebrand factor (vWF) (7–10). Aegyptin is an A. aegypti salivary protein that binds directly through its C-terminal domain to the vWF binding site in collagen (7, 8). Moreover, Aegyptin prevents carotid thrombus formation in vivo without causing excessive bleeding in mice.
We used a transgenic approach to reduce the products of the Aegyptin gene (also known as the 30K b gene; ref. 11) to study its relevance in blood feeding. Our results show that reduced levels of Aegyptin mRNAs and protein result in a phenotype in which the probing times of transgenic mosquitoes are increased significantly, and their salivary gland extracts fail to inhibit collagen-induced platelet aggregation in vitro. Reductions in Aegyptin did not affect either the salivary gland anticoagulant or ADP-induced platelet aggregation activities. We interpret these data to indicate that the altered feeding capabilities of transgenic insects results from their inability to inhibit collagen-induced platelet aggregation.
Although mosquito salivary antihemostatic activity has been demonstrated, it remains to be proven whether each particular salivary component is relevant or necessary to acquire a successful blood meal. It was proposed that redundancy of salivary function reinforces the efficiency of blood feeders (12, 13). However, our results do not support this general hypothesis. It appears that the diversity and complexity of antihemostatic proteins found in salivary secretions is not entirely redundant because reduction of Aegyptin alone impairs significantly the ability of the mosquito to acquire a blood meal.
Results
Generation of Transgenic Mosquitoes Expressing dsRNAi Against Aegyptin.
A transgene was engineered to produce a self-complementary inverted-repeat RNA derived from Exon-2 of the Aegyptin gene (Fig. 1A and Fig. S1). One transgenic line, designated dsAegyptin, was maintained by intercrossing transgenic siblings. Genomic integration of the dsAegyptin transgene was demonstrated by PCR. Gene-specific primers for Aegyptin and actin (Aaeact-1; ref. 14) were used as positive controls, whereas dsAegyptin and eGFP gene-specific primers were used as markers of transgene insertion (Fig. S2A and Table S1). Both eGFP and dsRNAi genes are under control of the bidirectional 30K gene promoter, therefore their transcripts are expected to be colocalized in the distal portion of the salivary glands lateral lobes (11). Fluorescence microscopy visualization reveals that eGFP is accumulated exclusively in the distal lateral lobes of salivary gland of dsAegyptin mosquitoes (Fig. S2B). No fluorescence was detected in control (Higgs) salivary glands (Fig. S2C). These results demonstrate the functionality of the 30K promoter to drive expression of the transgenes in the salivary gland compartment where the endogenous Aegyptin mRNA is transcribed (15). Temporal and spatial colocalization of Aegyptin mRNA and dsRNAi was designed to maximize the reduction of Aegyptin in salivary glands and reduce putative fitness costs associated with transgenesis.
Fig. 1.
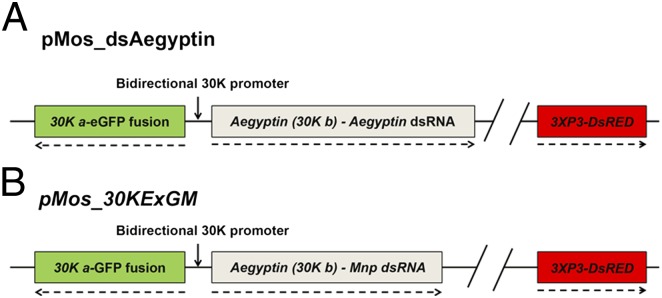
Schematic representations of the dsAegyptin and 30KExGM transgenes. (A) The plasmid containing the transgene dsAegyptin is engineered to express an anti-Aegyptin dsRNA under the control of the 30K bidirectional promoter. 30K a drives the expression of the reporter gene, eGPF, and 30K b, the Aegyptin promoter, drives the Aegyptin-specific dsRNA. The transformation marker gene DsRED is under control of the 3XP3 eye-specific promoter (11). (B) The transgene inserted in the control transgenic line 30KExGM has a similar organization and was engineered to express Mnp, an antidengue dsRNA. Detailed descriptions of pMos_dsAegyptin and pMos_30KExGM are in Fig. S1 and Mathur et al. (11), respectively. Dashed arrows indicate direction of transcription.
Aegyptin Expression in Transgenic Mosquitoes.
A previously described transgenic line, 30KExGM (11), was used as the biological control because of its transgene structural and expression similarities with dsAegyptin. The 30KExGM transgene consists of full-length 30K a fused to the eGFP ORF flanked by the 30K promoter and 30K a 5′-and 3′-end untranslated regions (UTRs) in one orientation and the dsRNAi Mnp antidengue effector gene (16) flanked by 30K promoter and Aegyptin (30K b) 5′ and 3′ UTRs in the cDNA (Fig. 1B). Both transgenic lines express eGFP and dsRNAi in their salivary glands. Real-time quantitative RT-PCR (qRT-PCR) was carried out with total RNA extracted from salivary glands of dsAegyptin or 30KExGM mosquitoes. Expression of dsRNAi in dsAegyptin mosquitoes reduced Aegyptin mRNAs to levels 85–87% (P < 0.0001) lower than those found in 30KExGM (Fig. 2A). We also found that silencing of Aegyptin gene transcripts resulted in a significant reduction of Aegyptin at the protein level (Fig. 2B, Table 1). Measured Aegyptin (Table 1) in the salivary glands of dsAegyptin mosquitoes (0.406 ± 0.03 ng) was ≥80-fold lower than in the parental strain Higgs (36.35 ± 0.98 ng) and the transgenic control 30KExGM (33.21 ± 1.21 ng).
Fig. 2.
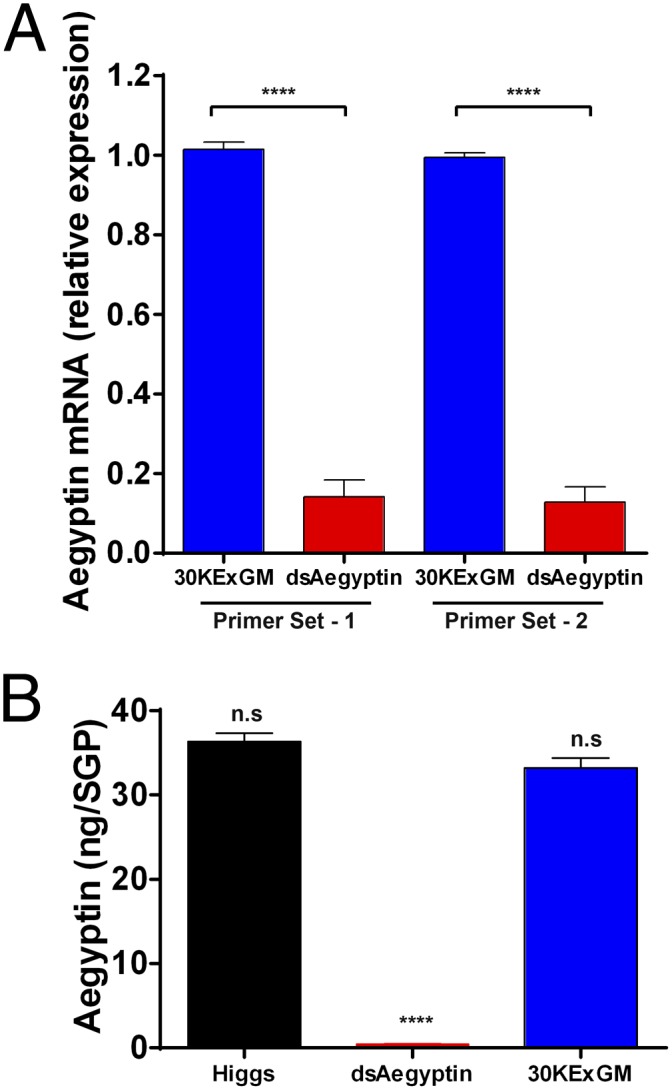
Quantification of Aegyptin gene expression in transgenic mosquitoes. (A) Accumulation of Aegyptin mRNA in salivary glands was quantified by using qRT-PCR. Expression of dsRNAi in dsAegyptin mosquitoes reduced significantly the Aegyptin mRNA level relative to the 30KExGM control (P < 0.0001). Biological replicates (n = 3) containing 20 salivary gland pairs each were assayed in duplicate. (B) dsRNA-mediated silencing of Aegyptin transcription products reduces significantly corresponding protein levels in transgenic dsAegyptin mosquitoes. ****P < 0.0001. n.s, not significant.
Table 1.
dsRNA-mediated silencing of Aegyptin significantly reduced its protein levels in transgenic mosquitoes
| Group | Higgs | dsAegyptin | 30KExGM |
| Aegyptin per salivary gland pair, ng | 36.35 ± 0.98 | 0.406 ± 0.03 | 33.21 ± 1.21 |
Effect of Reduced Aegyptin on Salivary Collagen-Binding Activity and Platelet Aggregation.
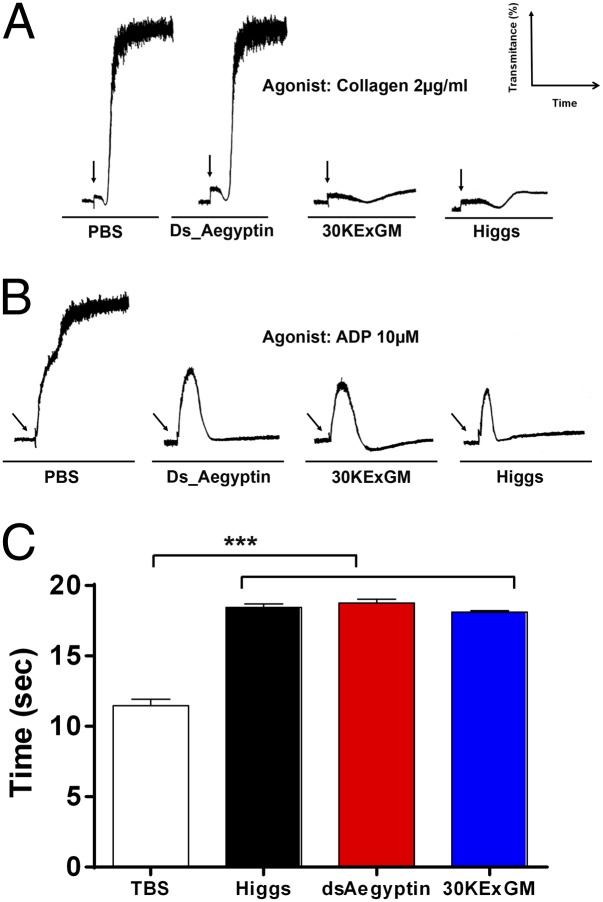
It was reported that Aegyptin binds to collagen and that it is the only salivary protein responsible for this activity (7, 8). A surface plasmon resonance (SPR) experiment was carried out to determine whether dsRNAi-mediated suppression of Aegyptin transcription products in salivary glands resulted in a phenotype lacking the salivary collagen-binding activity reported in wild-type mosquitoes. Collagen binding was reduced significantly in dsAegyptin mosquito saliva and salivary gland extracts (Fig. 3 A and B). Because collagen is recognized as the main thrombogenic component of the subendothelial matrix, collagen-induced platelet aggregation was assayed in vitro. dsAegyptin salivary gland extracts failed to inhibit collagen-induced platelet aggregation whereas those from control mosquitoes effectively abrogated platelet aggregation (Fig. 4A). ADP, another platelet aggregation agonist, was assayed as a control. A. aegypti salivary glands express a secreted, female-specific apyrase, hydrolyzing both ADP and ATP (17). Transcripts of the Apyrase gene are accumulated in the medial and distal lateral lobes of the adult female salivary glands (15, 18). Our results show that dsAegyptin mosquito salivary glands display the same ADP-induced platelet aggregation inhibition as control mosquitoes (Fig. 4B).
Fig. 3.
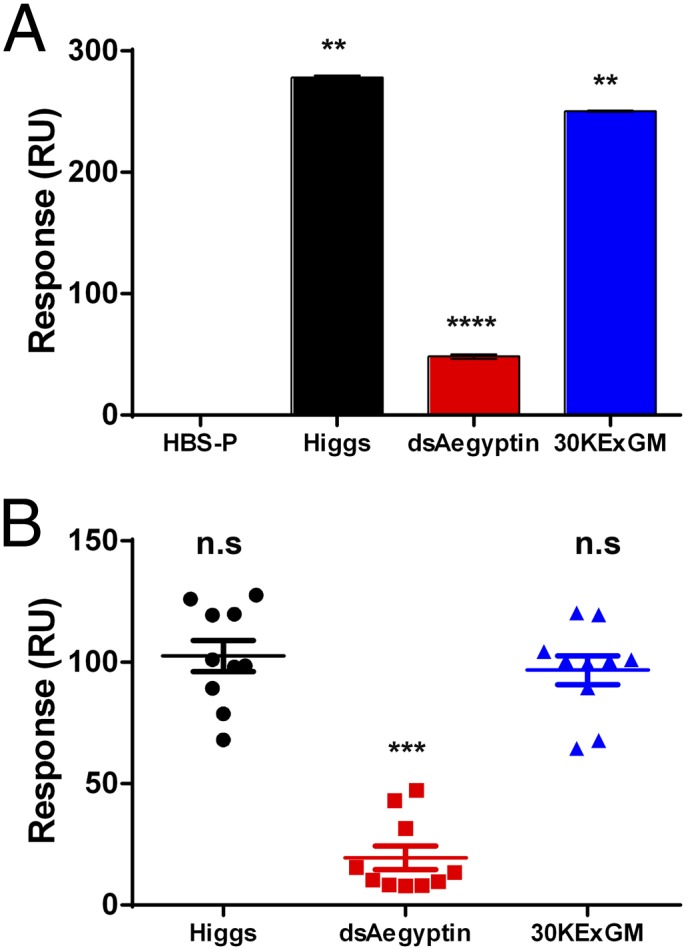
Suppressing Aegyptin transcription products results in the reduction of collagen binding activity of salivary gland extracts and saliva. SPR experiments were carried out to evaluate collagen binding activity of salivary glands and saliva of transgenic dsAegyptin mosquitoes. (A) Saliva of dsAegyptin mosquitoes showed lower collagen-binding capacity compared with control mosquitoes (Higgs and 30KExGM). (B) Single mosquito salivary gland pairs were homogenized in Hepes saline-buffered solution supplemented with 0.05% (vol/vol) surfactant P20 and flowed over immobilized collagen (HBS-V). Salivary gland homogenates from dsAegyptin have a significantly lower collagen-binding activity than Higgs and 30KExGM control mosquitoes. **P < 0.01, ***P < 0.001, ****P < 0.0001. n.s, not significant.
Fig. 4.
Effect of reduced Aegyptin on platelet aggregation and blood clotting. (A) Salivary gland extracts of dsAegyptin mosquitoes failed to inhibit collagen-induced platelet aggregation. Platelet-rich plasma was preincubated with two pairs of salivary glands for 60 s before adding 2 µg/mL of fibrillar collagen. Platelet aggregation was measured by light transmittance. (B) Suppressing Aegyptin did not impair the ability of salivary gland homogenates to inhibit ADP-induced platelet aggregation. (C) Prothrombin time was measured in a coagulometer by using normal reference plasma from healthy donors. Homogenates of two pairs of salivary gland were incubated with human plasma for 60 s before triggering clotting with thromboplastin reagent. No significant differences were found in the anticoagulant activity of salivary gland homogenates from dsAegyptin, Higgs and 30KExGM mosquitoes. All samples were assayed in triplicate. Arrows indicate addition of either collagen (A) or ADP (B). ***P < 0.001.
Reduced Aegyptin Has No Effect on the Anticoagulant Activity of Salivary Glands.
Platelet aggregation and blood coagulation are the two main components of the hemostatic response that lead to stoppage of blood loss. The main salivary anticoagulant in A. aegypti belongs to the superfamily of SERPINs targeting coagulation factor Xa (19, 20). Accumulation of transcripts for this anticoagulant shows a similar pattern as Aegyptin (15). Prothrombin time (PT) was measured to assess salivary gland anticoagulant activity in dsAegyptin mosquitoes (Fig. 4C). Reducing Aegyptin has no effect on the anticoagulant activity of salivary gland extracts. This result indicates that RNA interference against Aegyptin did not affect the function of other major proteins expressed in the same salivary gland compartments.
Reduced Aegyptin Significantly Increases Probing Time.
dsAegyptin mosquitoes had an exceptionally long probing time (median of 203 s), ranging from 78 to 300 s, whereas mosquitoes from the control groups started to ingest blood in 15–21 s (Fig. 5, Table 2). Because suppressing Aegyptin did not affect blood coagulation or ADP-induced platelet aggregation inhibition by salivary gland homogenates, we can conclude that the resulting phenotype observed in transgenic mosquitoes is due exclusively to Aegyptin reduction and the decrease of salivary collagen-binding and collagen-induced platelet aggregation activities found in dsAegyptin mosquitoes.
Fig. 5.
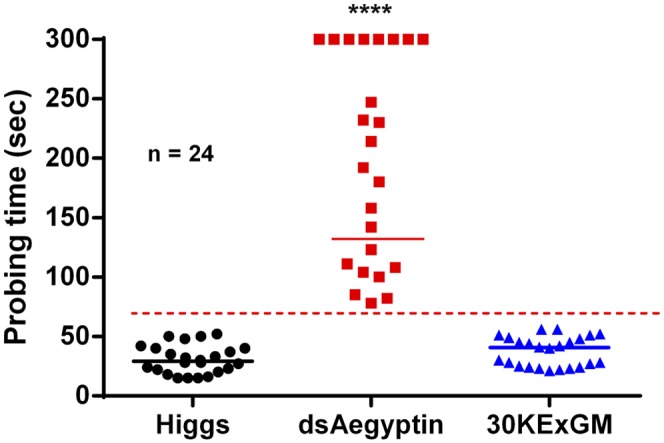
Probing time on mice is prolonged by reduced Aegyptin in transgenic mosquitoes. Probing time was significantly longer in dsAegyptin mosquitoes then in controls (n = 24, P < 0.0001). Transgenic mosquitoes expressing dsRNAi had the first sign of blood in their midgut at least 78 s from insertion of their mouth part in the host’s skin, whereas control mosquitoes were able to start ingesting blood at 15–21 s. dsAegyptin mosquitoes with a probing time of more than 300 s were not included in the statistical analysis. Horizontal bars represent the median value. ****P < 0.0001.
Table 2.
Probing time in dsAegyptin is significantly increased compared with control mosquitoes
| Group | Range, s | Median, s |
| Higgs | 15–52 | 29 |
| dsAegyptin | 78–300* | 203 |
| 30KExGM | 21–56 | 40.5 |
dsAegyptin mosquitoes with probing time of 300 s were not included in our statistical analysis.
Reduced Expression of Aegyptin Decreases the Feeding Success of Mosquitoes.
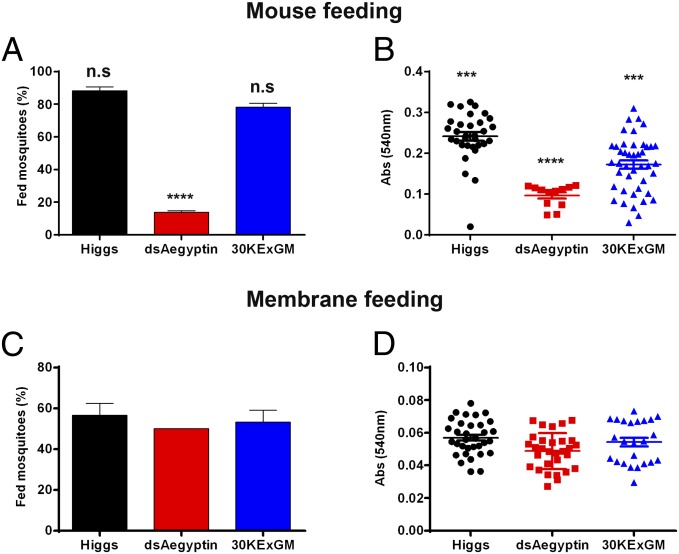
We used feeding success as an orthogonal method to assess blood meal intake. Starved female mosquitoes in three groups (20 individuals each) were offered an anesthetized mouse for 12 min and scored by visual inspection as fed or unfed. Mosquitoes fully engorged or with any visual sign of blood in their abdomen were recorded as fed. dsAegyptin mosquitoes were significantly less successful in obtaining a blood meal compared with the controls (P = 0.0001, χ2 test; Fig. 6A). An additional analysis was performed to evaluate the ingested blood meal size as an indication of meal quality acquired by the mosquitoes. Abdomens of fed mosquitoes were homogenized in 1 mL of Drabkin’s reagent, and their absorbance at 540 nm was measured. Suppressing Aegyptin also reduces significantly the amount of blood ingested by transgenic dsAegyptin mosquitoes compared with control mosquitoes (Fig. 6B). Although we cannot rule out the possibility of different feeding rates as a result of attraction/landing of mosquitoes, these results are in agreement with the longer probing times displayed by dsAegyptin mosquitoes. We used an artificial feeding system with parafilm membrane and defibrinated human blood to complement the previous experiments. We hypothesized that reduced Aegyptin would not have any effect on artificial feeding because this system lacks the collagen needed for normal platelet activating and aggregation. Starved mosquitoes were allowed to feed on membrane feeders for 30 min and scored by visual inspection as described above. No significant differences in feeding success or blood meal size were found in dsAegyptin mosquitoes compared with control mosquitoes (Fig. 6 C and D).
Fig. 6.
Feeding success of transgenic mosquitoes on mice and artificial membranes. (A) Reduced levels of Aegyptin significantly lowered the feeding success of mosquitoes on anesthetized mice. Percentages of successful feeding were analyzed by using a χ2 test. (B) Transgenic mosquitoes expressing dsRNAi against Aegyptin mRNA had significantly smaller blood content in their abdomens. Blood meal size (expressed as OD540nm units) ingested by mosquitoes that fed on mice was quantified by measuring the total hemoglobin content using Drabkin’s reagent as described in Materials and Methods. (C) dsAegyptin mosquitoes did not differ in their feeding success compared with controls when fed on an artificial membrane and a bloodmeal lacking collagen and collagen-induced platelet activation and aggregation. (D) Measurement of blood meal size revealed that dsAegyptin and 30KExGM had ingested similar amounts of blood when fed on artificial membrane system. Results were analyzed by using a χ2 test with 95% confidence interval. ***P < 0.001 and ****P < 0.0001. n.s, not significant.
Discussion
Saliva and salivary glands from blood feeding arthropods have been studied for their role in blood feeding and pathogen transmission. It is now clear that salivary secretions suppress the hemostatic system of vertebrate hosts helping the invertebrate to obtain a blood meal. Platelet interaction with the exposed extracellular matrix at the probe site is an early event in the hemostatic cascade. Exposed collagen, along with ADP released by broken cells, is one of the most thrombogenic substrates at the site. On vascular injury at sites of high shear rates, circulating vWF binds to exposed collagen fibers initiating the tethering of circulating platelets to the vessel wall via the GPIbα complex (21). Tethered platelets subsequently roll on the damaged vessel wall, a process that is amplified by the activation of the platelet integrin GPIIb/IIIa (21). Under high shear stress, platelet adhesion and activation begins immediately (4–20 s) after endothelial disruption (22). Activated platelets then provide a catalytic membrane surface for the generation of thrombin that, in turn, accelerates recruitment of circulating resting platelets and, particularly, formation of the fibrin necessary to stabilize thrombi and prevent their detachment by flowing blood (23).
An efficient and rapid mechanism for stopping the hemostatic response to blood loss at the biting site is essential for survival of hematophagic arthropods. Prolonged probing and feeding time can trigger awareness in the vertebrate hosts and result in aborted feeding or death of the arthropod. Hence, faster probing and feeding times would reduce the duration of vector-host contact and increase survival of the feeder.
We demonstrated here the relevance of Aegyptin in A. aegypti blood feeding success. Aegyptin, an abundantly expressed salivary protein of A. aegypti, is a collagen-binding protein that inhibits platelet aggregation and adhesion under static and flow conditions (7, 8). Reducing Aegyptin transcript abundance by using transgene-mediated dsRNA interference results in a phenotype with prolonged probing time and less successful ingestion of a blood meal. However, it is important to note that suppressing Aegyptin by the transgenic approach did not abrogate completely the collagen binding activity present in salivary glands and saliva. This residual binding might indicate that other salivary molecules also contribute to this activity, or alternatively, the residual activity could result from translation and accumulation of Aegyptin before the dsRNA silencing took effect.
Because transgenic mosquitoes did not have altered anticlotting or ADP-induced platelet aggregation inhibition activities, increased probing time in dsAegyptin mosquitoes can be attributed to reducing Aegyptin abundance and the resulting decrease in collagen-binding and collagen-induced platelet aggregation activities. In contrast, Das et al. (24) did not find any effect on probing time and feeding success in adult Anopheles gambiae following transient silencing of a member of the Aegyptin family (NCBI accession no. AGAP009974-RA). These authors attributed their results to the relatively low silencing efficiency of AGAP009974-RA transcripts in salivary glands or to the existence of redundant salivary functions in the mosquito. Although it has been proposed that redundancy of salivary function reinforces the efficiency of the antihemostatic response by blood feeders (12, 13), our results support alternative hypotheses. It appears that the diversity and complexity of antihemostatic proteins found in salivary secretions is not completely redundant because suppression of a single component, Aegyptin, significantly impairs the ability of the mosquito to acquire a blood meal.
The complexity of salivary components rather may be a response to the redundant and efficient hemostatic system of the vertebrate host. For example, multiple agonists can activate platelets, including collagen, ADP, thromboxane A2 (TXA2), serotonin, and thrombin (25). To overcome platelet activation and aggregation, mosquitoes have evolved several salivary compounds that can scavenge or neutralize these agonists. Apyrase, a ubiquitous enzyme found in A. aegypti and other mosquitoes (17), can efficiently hydrolyze ATP and ADP limiting their accumulation in the extracellular matrix (26). Removal of ATP and ADP from the biting site reduces the pain cause by these extracellular nucleotides and limits their effect on platelet activation. In contrast, the D7 protein, a multifunctional molecule found in mosquitoes, serves the important functions of inhibiting platelet aggregation, vasoconstriction, inflammation, and host behavioral responses that might disturb feeding (27–32).
In conclusion, we demonstrated that the salivary gland protein Aegyptin contributes significantly to blood feeding success in A. aegypti. Our combined results reinforce the role of platelet antiaggregation inhibitors in hematophagic arthropods as a primordial salivary function in blood feeding. Furthermore, we provide evidence supporting the conclusion that the multiple antiplatelet factors in mosquito saliva do not represent a redundant system. Instead they perform complementary functions to optimize successful feeding. Our data and conclusions are supported further by data on the partial silencing of salivary apyrase and gSG6 genes in A. gambiae, which resulted in reduced mosquito feeding efficiency (33, 34). Finally, our results support transgenic approaches for studies of gain and loss of salivary functions.
Materials and Methods
Ethic Statement.
Public Health Service Animal Welfare Assurance A4149-01 guidelines were followed according to the National Institute of Allergy and Infectious Diseases (NIAID), National Institutes of Health (NIH) Animal Office of Animal Care and Use. These studies were carried out according to the NIAID study protocol (Animal Study Proposal; ASP) approved by the NIH Office of Animal Care and User Committee, with approval ID ASP-LMVR3.
Animals.
Adult female mice weighing 10–15 g were housed under controlled temperature (24 ± 1 °C) and light (12 h light starting at 0700 hours) conditions, and all experiments were conducted in accordance with standards of animal care defined by the Institutional Committees (NIH).
Mosquito Rearing and Salivary Gland Dissections.
Mosquitoes were reared in the Laboratory of Malaria and Vector Biology, NIH. Adult females (2- to 3-d-old) were anesthetized with CO2, transferred to an ice-chilled plate, and salivary glands were dissected under a stereomicroscope in 25 mM Tris⋅HCl and 150 mM NaCl at pH 7.4. Individual or pools of 20 salivary glands pairs were sonicated and centrifuged at 12,000 × g for 5 min. Alternatively, saliva was collected by oil-induced salivation (35). Briefly, female mosquitoes were immobilized on glass slides, injected intrathoracically with 69 nL of 1 mM serotonin in water (Sigma-Aldrich), and their proboscides were inserted into capillary tubes filled with mineral oil. After 15 min of salivation, saliva from of 10–15 mosquito was pooled and partitioned at 14,000 × g in a bench top centrifuge, and the lower phase containing the saliva was transferred to a clean 1.5-mL tube. Salivary gland extracts and saliva were stored at −80 °C until used.
Transgene Construction and Embryo Microinjections.
The transgene was designed to carry inverted-repeat RNA sequences derived from Exon-2 of the Aegyptin gene (Fig. 1A). Transgene DNA synthesis and subcloning into pMOS-DsRED plasmid were performed by BioBasic. Microinjection of pMos_dsAegyptin and helper plasmids in preblastoderm embryos from A. aegypti Higgs white-eyed strain was carried out as described by Jasinskiene et al. (36). Microinjection of 1,039 mosquito embryos resulted in 494 surviving G0 mosquitoes (268 males and 226 females). Surviving G0s were outcrossed to mosquitoes of the opposite sex, and female mosquitoes were fed on mice to produce offspring. Progeny larvae of these crosses (G1) were screened for DsRED expression in their eyes by using a fluorescence microscope equipped with a DsRED filter set. Only one G1 transgenic line expressing the DsRED marker gene (red-eyed) was obtained. Transgenic G1 mosquitoes were outcrossed to the Higgs recipient strain, and their progeny (G2) were analyzed for the gene of interest. One transgenic line was established and maintained by intercrossing transgenic siblings.
Genomic DNA Extraction and PCR.
Genomic DNA was isolated from five adult mosquitoes by using the DNAzol reagent (Life Technologies). DNA samples were solubilized in 100 µL of TE buffer (10 mM Tris⋅HCl and 1 mM EDTA at pH 7.5) and used as templates for PCR amplification with Platinum PCR SuperMix (Life Technologies). The gene-specific primers TransE2rcF, TransE2rcR, eGFP-F, eGFP-R, Actin-F, Actin-R, Aegyptin-F, and Aegyptin-R were used for amplification (Table S1). The amplicons resolved in a 1.2% (wt/vol) agarose precast E-Gel (Life Technologies) containing ethidium bromide were visualized under UV light.
Fluorescence Microscopy.
Salivary glands of sugar-fed (3- to 5-d-old) transgenic and control Higgs mosquitoes were dissected as described above and immediately fixed in 4% paraformaldehyde in PBS to visualize the expression and accumulation of the reporter gene eGFP. After 24 h incubation at 4 °C, fixed salivary glands were rinsed three times for 10 min with PBS. Salivary glands then were incubated for 30 min at room temperature with PBS supplemented with 0.1% (vol/vol) BSA. Nuclear DNA was stained with Hoechst dye 1:30,000 (Molecular Probes) for 10 min and rinsed in PBS to remove excess dye. Salivary glands were mounted with Fluoroshield (Sigma-Aldrich), and images were obtained by using a DMI6000B fluorescence microscope (Leica).
qRT-PCR.
Total RNA from dissected salivary glands was isolated by using the TRIZOL reagent (Life Technologies). The yield of total RNA from 20 salivary gland pairs (three groups) was determined by using a NanoDrop-1000. Total RNA samples were treated with gDNA Wipeout (Qiagen) to remove any genomic DNA contamination. Gene-specific primers were designed to generate cDNA amplicons of 80–90 bp by using the following primers (5′-3′): dsAegyptin-1F: AAGAAACGACCGATGATGCT; dsAegyptin-1R: GCCAGCATCCTTATCTCCAG; dsAegyptin-2F: TGTCTGGTAGGCATTGTGCT; dsAegyptin-2R: AGACTCTCCGCTTGCATCAT; Ribosomal S7F: GGGACAAATCGGCCAGGCTATC; and Ribosomal S7R: TCGTGGACGCTTCTGCTTGTTG. First-strand cDNA synthesis was carried out by using QuantiTect reverse transcriptase kit (Qiagen). Aegyptin mRNA abundance was measured by SYBR green quantitative real-time PCR (DyNAmo HS) by using the CFX96 system (Bio-Rad) and was normalized by using the A. aegypti 40S ribosomal protein S7 transcript (AAEL009496-RA) as the reference. Each sample was assayed in duplicate from three biological replicates (20 pairs of salivary glands each). Fold-change values were derived by using the 2−ΔΔCt method.
Polyclonal Antibody Production.
Polyclonal antibodies against recombinant Aegyptin (7) were raised in rabbits by Spring Valley Laboratories as described in ref. 37.
Quantification of Aegyptin.
Quantification of Aegyptin in salivary glands was carried out by ELISA. Recombinant Aegyptin or salivary gland homogenates (pools of three pairs) were diluted in 100 µL of carbonate-bicarbonate buffer (pH 9.0) and immobilized on a 96-well, flat-bottom plate (Costar, Corning) overnight at 4 °C. Wells were washed with PBS and blocked with BSA (2% vol/vol, in PBS). After 2 h, wells were washed three times in PBS supplemented with 0.05% (vol/vol) Tween 20 (PBS-T) and incubated with anti-Aegyptin (1:2,500) in the PBS-T supplemented with 1% (vol/vol) BSA for 1 h. Wells were washed three times and incubated with alkaline phosphatase-coupled anti-rabbit IgG (1:10,000, in PBS-T). After 1-h incubation, the wells were washed four times with PBS-T and the stabilized p-nitrophenyl phosphate liquid substrate (Sigma) was added. Colorimetric analysis was performed by measuring absorbance values at 405 nm in a VERSA max microplate reader (Molecular Devices). Standards (0.01–1,000 ng of recombinant Aegyptin) were fitted by using a nonlinear four-parameter regression to generate a standard curve. Aegyptin concentration in salivary glands was calculated by using the standard curve.
Platelet Aggregation.
Platelet aggregation was measured by using an aggregometer (Chrono-Log Corporation) and platelet-rich plasma (PRP) from healthy, medication-free, human donors obtained by plateletpheresis [Department of Transfusion Medicine (DTM)/NIH Blood Bank]. Briefly, 300 µL of PRP, diluted 1:2 in Tyrode’s buffer, were prestirred in the aggregometer for 3 min to monitor preaggregation effects. Homogenates of two pairs of salivary glands in Tyrode’s buffer of each mosquito line or PBS were added to the PRP before adding the agonists. Aggregation then was induced by using 2 µg/mL (final concentration) of native collagen type I fibrils from equine tendons (Chrono-Log Corporation) or 10 µM ADP (Sigma-Aldrich). All experiments were carried out in triplicate by using PRP from three different donors.
Anticoagulant Assay.
Salivary gland extracts were assayed for the PT by using a Start4 Hemostasis Analyzer (Diagnostica Stago). Experimental samples (two pairs of salivary glands or PBS) and normal reference human plasma (50 μL) plasma were incubated for 1 min at 37 °C before adding 100 μL of Neoplastine CI Plus reagent (Diagnostica Stago), and the clotting time was recorded under stirring conditions. All samples were assayed in triplicate.
SPR Analysis.
All SPR experiments were carried out in a T100 instrument (GE Healthcare). The Biacore T100 evaluation software was used for binding measurements. SPR experiments were performed as described by Calvo et al (7). Briefly, collagen type I (20 µg/mL) in acetate buffer, pH 4.5, was immobilized over a CM5 sensor via amine coupling. Blank flow cells were used to subtract the buffer effect on sensograms. Binding experiments were carried out with a contact time of 120 s at a flow rate of 30 µL/min at 25 °C. Complex dissociation was monitored for 300 s, and the sensor surface was regenerated by a 45-s pulse of 10 mM HCl at 30 µL/min.
Probing Time.
Probing time in transgenic and control mosquitoes was measured according to the method described by Ribeiro (38). Probing time is defined as the time taken from initial insertion of the mouth parts into the mouse skin until the initial observable ingurgitation of blood. Briefly, single female mosquitoes (3- to 5-d-old, never blood fed) were caged in transparent polystyrene vials and starved for sugar and water the night before the tests were carried out. Probing time experiments were carried out in polystyrene vials attached directly to the mouse, thus minimizing the scattering of mosquitoes around the host. Mosquitoes were offered the shaved back of an anesthetized mouse kept on a slide warmer set to 37 °C. Probing time was measured on three different mice, alternating mosquitoes from each group (dsAegyptin, and the controls Higgs and 30KExGM). Mosquitoes that did not initiate probing or showed no interest for the host after 5-min exposure were eliminated from the analysis. All measurements were terminated at 300 s, and mosquitoes that did not start to imbibe blood at this cumulative time were recorded as 300 s.
Feeding Success on Mice and Artificial Feeder.
Feeding success was measured on anesthetized mice and artificial glass feeders. All mosquitoes used in this experiment were not blood fed previously. For mice feeding, three groups of 20 female mosquitoes (3- to 5-d-old) were caged and deprived of sugar and water the day before the experiments was carried out. Each cage containing starved mosquitoes was offered an anesthetized naïve mouse. Mosquitoes were allowed to feed for 12 min, the mice were removed, and the mosquitoes scored as either fed or unfed. Females with either their entire abdomens fully engorged or partially fed but with visible blood were considered as fed. For artificial membrane feeding, mosquitoes (20 mosquitoes, 3 groups) were allowed to feed on a mini glass feeder (Chemglass Life Sciences) covered with a stretched parafilm membrane. The blood meal consisted of human defibrinated blood diluted in RPMI incomplete medium [50% (vol/vol)] and supplemented with 10% (vol/vol) of human normal reference plasma (Diagnostica Stago). Glass feeders were kept at 37 °C during feeding. Mosquitoes were assessed after 30 min for blood in their abdomens as described above.
Blood Meal Size.
The blood meal size ingested by each mosquito fed on mice or artificial feeders was determined by quantification of total hemoglobin content in the abdomen by using Drabkin’s reagent (Sigma-Aldrich). Abdomens of fed mosquitoes were homogenized in 1 mL of freshly prepared Drabkin’s reagent supplemented with Brij 35 Solution (Sigma-Aldrich). Samples were cleared in an Eppendorf bench-top centrifuge for 15 min at 13,500 × g, and their absorbance at 540 nm was measured in a VERSA max microplate reader (Molecular Devices).
Statistical Analysis.
Data were analyzed by using GraphPad Prism v 6 software (GraphPad Software) and plotted as bar graphs or scatter plots. Comparisons were made with the two-tailed t test with a 95% confidence interval and two-way analysis of variance. P < 0.05 was considered significant. A χ2 test with a 95% confidence interval was used for feeding success measurements.
Supplementary Material
Acknowledgments
We thank V. M. Pham for technical support; Judy Coleman, Aniko Fazekas, Andre Laughinghouse, and Kevin Lee for expert mosquito care; DTM/National Institutes of Health (NIH) for providing platelet-rich plasma; and Brenda Marshall [Department of Public Social Services, Division of Intramural Research, National Institute of Allergy and Infectious Diseases (NIAID)] for editorial assistance. This work was supported by the Intramural Research Program of the Division of Intramural Research, NIAID, NIH. O.M., N.J., and A.A.J. were supported in part by NIH NIAID Grant AI29746.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1404179111/-/DCSupplemental.
References
- 1.Ribeiro JMC, Rossignol PA, Spielman A. Aedes aegypti: Model for blood finding strategy and prediction of parasite manipulation. Exp Parasitol. 1985;60(1):118–132. doi: 10.1016/s0014-4894(85)80029-1. [DOI] [PubMed] [Google Scholar]
- 2.Kauskot A, Hoylaerts MF. Platelet receptors. Handb Exp Pharmacol. 2012;(210):23–57. doi: 10.1007/978-3-642-29423-5_2. [DOI] [PubMed] [Google Scholar]
- 3.Hoffman R. Hematology: Basic Principles and Practice. 3rd Ed. New York: Churchill-Livingstone; 2000. p. 1783. [Google Scholar]
- 4.Ribeiro JM, Mans BJ, Arcà B. An insight into the sialome of blood-feeding Nematocera. Insect Biochem Mol Biol. 2010;40(11):767–784. doi: 10.1016/j.ibmb.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ribeiro JMC, Arca B. From sialomes to the sialoverse: An insight into the salivary potion of blood feeding insects. Adv Insect Physiol. 2009;37(2):59–118. [Google Scholar]
- 6.Mans BJ. Evolution of vertebrate hemostatic and inflammatory control mechanisms in blood-feeding arthropods. J Innate Immun. 2011;3(1):41–51. doi: 10.1159/000321599. [DOI] [PubMed] [Google Scholar]
- 7.Calvo E, et al. Aegyptin, a novel mosquito salivary gland protein, specifically binds to collagen and prevents its interaction with platelet glycoprotein VI, integrin alpha2beta1, and von Willebrand factor. J Biol Chem. 2007;282(37):26928–26938. doi: 10.1074/jbc.M705669200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Calvo E, et al. Aegyptin displays high-affinity for the von Willebrand factor binding site (RGQOGVMGF) in collagen and inhibits carotid thrombus formation in vivo. FEBS J. 2010;277(2):413–427. doi: 10.1111/j.1742-4658.2009.07494.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yoshida S, et al. Inhibition of collagen-induced platelet aggregation by anopheline antiplatelet protein, a saliva protein from a malaria vector mosquito. Blood. 2008;111(4):2007–2014. doi: 10.1182/blood-2007-06-097824. [DOI] [PubMed] [Google Scholar]
- 10.Chagas AC, Calvo E, Pimenta PF, Ribeiro JM. An insight into the sialome of Simulium guianense (DIPTERA:SIMulIIDAE), the main vector of River Blindness Disease in Brazil. BMC Genomics. 2011;12:612. doi: 10.1186/1471-2164-12-612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mathur G, et al. Transgene-mediated suppression of dengue viruses in the salivary glands of the yellow fever mosquito, Aedes aegypti. Insect Mol Biol. 2010;19(6):753–763. doi: 10.1111/j.1365-2583.2010.01032.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Champagne DE. Antihemostatic molecules from saliva of blood-feeding arthropods. Pathophysiol Haemost Thromb. 2005;34(4-5):221–227. doi: 10.1159/000092428. [DOI] [PubMed] [Google Scholar]
- 13.Fontaine A, et al. Mosquito salivary gland protein preservation in the field for immunological and biochemical analysis. Parasit Vectors. 2011;4:33. doi: 10.1186/1756-3305-4-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ibrahim MS, Eisinger SW, Scott AL. Muscle actin gene from Aedes aegypti (Diptera:Culicidae) J Med Entomol. 1996;33(6):955–962. doi: 10.1093/jmedent/33.6.955. [DOI] [PubMed] [Google Scholar]
- 15.Juhn J, et al. Spatial mapping of gene expression in the salivary glands of the dengue vector mosquito, Aedes aegypti. Parasit Vectors. 2011;4:1. doi: 10.1186/1756-3305-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Franz AW, et al. Engineering RNA interference-based resistance to dengue virus type 2 in genetically modified Aedes aegypti. Proc Natl Acad Sci USA. 2006;103(11):4198–4203. doi: 10.1073/pnas.0600479103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ribeiro JM, Sarkis JJ, Rossignol PA, Spielman A. Salivary apyrase of Aedes aegypti: Characterization and secretory fate. Comp Biochem Physiol B. 1984;79(1):81–86. doi: 10.1016/0305-0491(84)90081-6. [DOI] [PubMed] [Google Scholar]
- 18.Smartt CT, Kim AP, Grossman GL, James AA. The Apyrase gene of the vector mosquito, Aedes aegypti, is expressed specifically in the adult female salivary glands. Exp Parasitol. 1995;81(3):239–248. doi: 10.1006/expr.1995.1114. [DOI] [PubMed] [Google Scholar]
- 19.Stark KR, James AA. A factor Xa-directed anticoagulant from the salivary glands of the yellow fever mosquito Aedes aegypti. Exp Parasitol. 1995;81(3):321–331. doi: 10.1006/expr.1995.1123. [DOI] [PubMed] [Google Scholar]
- 20.Stark KR, James AA. Isolation and characterization of the gene encoding a novel factor Xa-directed anticoagulant from the yellow fever mosquito, Aedes aegypti. J Biol Chem. 1998;273(33):20802–20809. doi: 10.1074/jbc.273.33.20802. [DOI] [PubMed] [Google Scholar]
- 21.Szántó T, Joutsi-Korhonen L, Deckmyn H, Lassila R. New insights into von Willebrand disease and platelet function. Semin Thromb Hemost. 2012;38(1):55–63. doi: 10.1055/s-0031-1300952. [DOI] [PubMed] [Google Scholar]
- 22.Ruggeri ZM, Orje JN, Habermann R, Federici AB, Reininger AJ. Activation-independent platelet adhesion and aggregation under elevated shear stress. Blood. 2006;108(6):1903–1910. doi: 10.1182/blood-2006-04-011551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Scharf RE. Drugs that affect platelet function. Semin Thromb Hemost. 2012;38(8):865–883. doi: 10.1055/s-0032-1328881. [DOI] [PubMed] [Google Scholar]
- 24.Das S, et al. Transcriptomic and functional analysis of the Anopheles gambiae salivary gland in relation to blood feeding. BMC Genomics. 2010;11:566. doi: 10.1186/1471-2164-11-566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Davì G, Patrono C. Platelet activation and atherothrombosis. N Engl J Med. 2007;357(24):2482–2494. doi: 10.1056/NEJMra071014. [DOI] [PubMed] [Google Scholar]
- 26.Clark G, Roux SJ. Apyrases, extracellular ATP and the regulation of growth. Curr Opin Plant Biol. 2011;14(6):700–706. doi: 10.1016/j.pbi.2011.07.013. [DOI] [PubMed] [Google Scholar]
- 27.Alvarenga PH, et al. The function and three-dimensional structure of a thromboxane A2/cysteinyl leukotriene-binding protein from the saliva of a mosquito vector of the malaria parasite. PLoS Biol. 2010;8(11):e1000547. doi: 10.1371/journal.pbio.1000547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Calvo E, deBianchi AG, James AA, Marinotti O. The major acid soluble proteins of adult female Anopheles darlingi salivary glands include a member of the D7-related family of proteins. Insect Biochem Mol Biol. 2002;32(11):1419–1427. doi: 10.1016/s0965-1748(02)00062-0. [DOI] [PubMed] [Google Scholar]
- 29.Calvo E, Mans BJ, Andersen JF, Ribeiro JM. Function and evolution of a mosquito salivary protein family. J Biol Chem. 2006;281(4):1935–1942. doi: 10.1074/jbc.M510359200. [DOI] [PubMed] [Google Scholar]
- 30.Calvo E, Mans BJ, Ribeiro JM, Andersen JF. Multifunctionality and mechanism of ligand binding in a mosquito antiinflammatory protein. Proc Natl Acad Sci USA. 2009;106(10):3728–3733. doi: 10.1073/pnas.0813190106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Isawa H, et al. Identification and characterization of a new kallikrein-kinin system inhibitor from the salivary glands of the malaria vector mosquito Anopheles stephensi. Insect Biochem Mol Biol. 2007;37(5):466–477. doi: 10.1016/j.ibmb.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 32.Malafronte Rdos S, Calvo E, James AA, Marinotti O. The major salivary gland antigens of Culex quinquefasciatus are D7-related proteins. Insect Biochem Mol Biol. 2003;33(1):63–71. doi: 10.1016/s0965-1748(02)00168-6. [DOI] [PubMed] [Google Scholar]
- 33.Boisson B, et al. Gene silencing in mosquito salivary glands by RNAi. FEBS Lett. 2006;580(8):1988–1992. doi: 10.1016/j.febslet.2006.02.069. [DOI] [PubMed] [Google Scholar]
- 34.Lombardo F, et al. The Anopheles gambiae salivary protein gSG6: An anopheline-specific protein with a blood-feeding role. Insect Biochem Mol Biol. 2009;39(7):457–466. doi: 10.1016/j.ibmb.2009.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rossignol PA, Spielman A. Fluid transport across the ducts of the salivary glands of a mosquito. J Insect Physiol. 1982;28(7):579–583. [Google Scholar]
- 36.Jasinskiene N, Juhn J, James AA. Microinjection of A. aegypti embryos to obtain transgenic mosquitoes. J Vis Exp. 2007;(5):219. doi: 10.3791/219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chagas AC, et al. Lundep, a sand fly salivary endonuclease increases Leishmania parasite survival in neutrophils and inhibits XIIa contact activation in human plasma. PLoS Pathog. 2014;10(2):e1003923. doi: 10.1371/journal.ppat.1003923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ribeiro JM. Blood-feeding in mosquitoes: Probing time and salivary gland anti-haemostatic activities in representatives of three genera (Aedes, Anopheles, Culex) Med Vet Entomol. 2000;14(2):142–148. doi: 10.1046/j.1365-2915.2000.00227.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.