Abstract
The beta-amyloid peptide, the hallmark of Alzheimer disease, forms fibrillar toxic aggregates in brain tissue that can be dissolved only by strong denaturing agents. To study beta-amyloid formation and its inhibition, we prepared immune complexes with two monoclonal antibodies (mAbs), AMY-33 and 6F/3D, raised against beta-amyloid fragments spanning amino acid residues 1-28 and 8-17 of the beta-amyloid peptide chain, respectively. In vitro aggregation of beta-amyloid peptide was induced by incubation for 3 h at 37 degrees C and monitored by ELISA, negative staining electron microscopy, and fluorimetric studies. We found that the mAs prevent the aggregation of beta-amyloid peptide and that the inhibitory effect appears to be related to the localization of the antibody-binding sites and the nature of the aggregating agents. Preparation of mAbs against "aggregating epitopes," defined as sequences related to the sites where protein aggregation is initiated, may lead to the understanding and prevention of protein aggregation. The results of this study may provide a foundation for using mAbs in vivo to prevent the beta-amyloid peptide aggregation that is associated with Alzheimer disease.
Full text
PDF
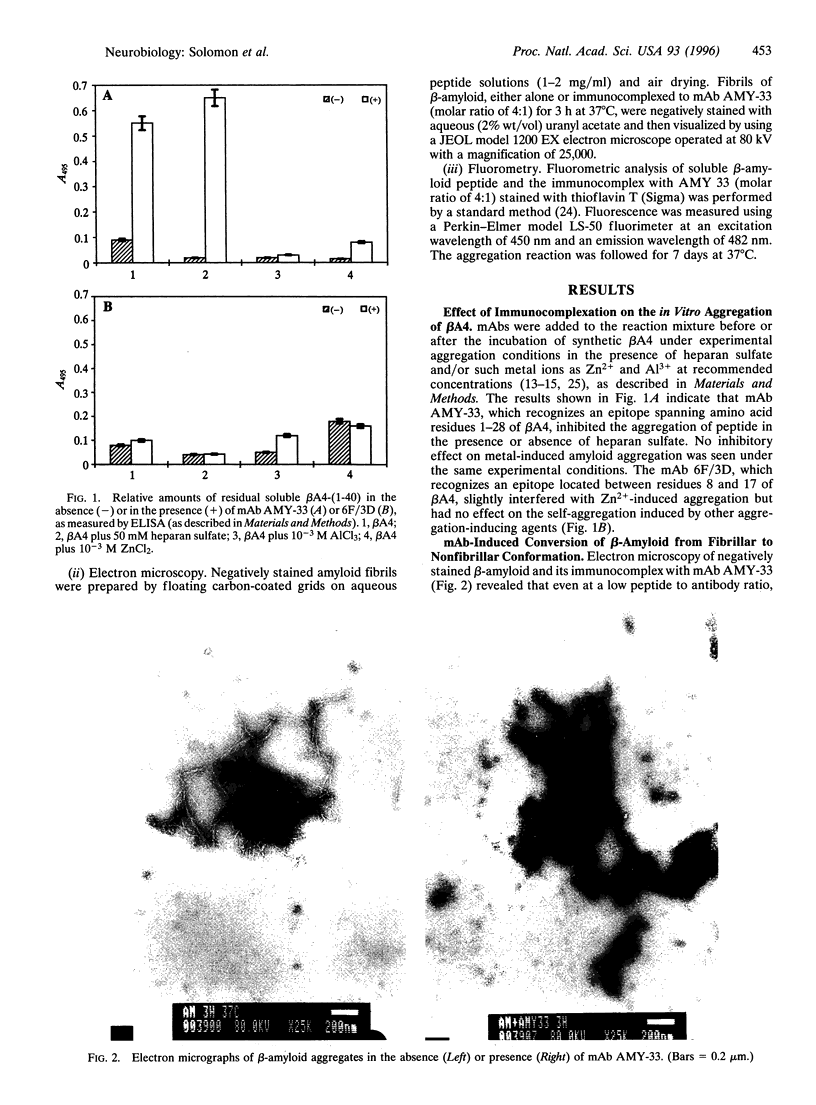
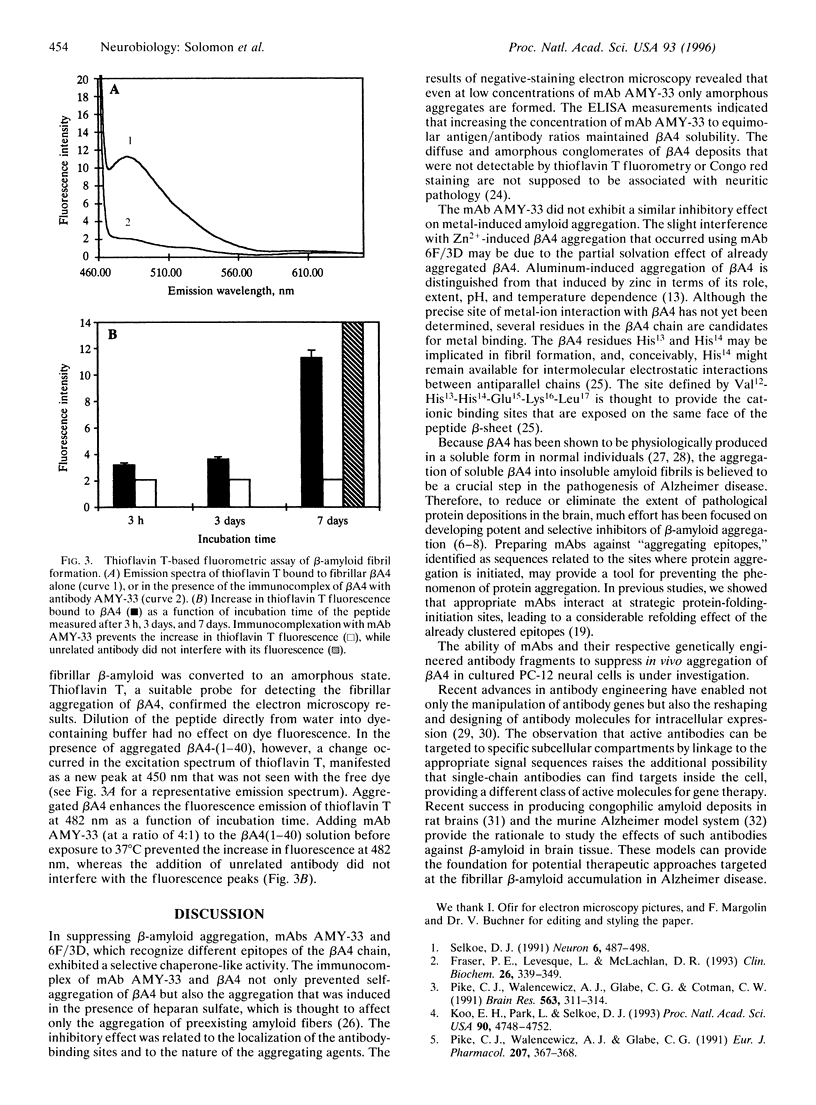

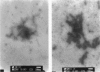
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blond S., Goldberg M. Partly native epitopes are already present on early intermediates in the folding of tryptophan synthase. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1147–1151. doi: 10.1073/pnas.84.5.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdick D., Soreghan B., Kwon M., Kosmoski J., Knauer M., Henschen A., Yates J., Cotman C., Glabe C. Assembly and aggregation properties of synthetic Alzheimer's A4/beta amyloid peptide analogs. J Biol Chem. 1992 Jan 5;267(1):546–554. [PubMed] [Google Scholar]
- Carlson J. D., Yarmush M. L. Antibody assisted protein refolding. Biotechnology (N Y) 1992 Jan;10(1):86–91. doi: 10.1038/nbt0192-86. [DOI] [PubMed] [Google Scholar]
- Crapper McLachlan D. R., Dalton A. J., Kruck T. P., Bell M. Y., Smith W. L., Kalow W., Andrews D. F. Intramuscular desferrioxamine in patients with Alzheimer's disease. Lancet. 1991 Jun 1;337(8753):1304–1308. doi: 10.1016/0140-6736(91)92978-b. [DOI] [PubMed] [Google Scholar]
- Ellis R. J., van der Vies S. M. Molecular chaperones. Annu Rev Biochem. 1991;60:321–347. doi: 10.1146/annurev.bi.60.070191.001541. [DOI] [PubMed] [Google Scholar]
- Fraser P. E., Lévesque L., McLachlan D. R. Biochemistry of Alzheimer's disease amyloid plaques. Clin Biochem. 1993 Oct;26(5):339–349. doi: 10.1016/0009-9120(93)90110-r. [DOI] [PubMed] [Google Scholar]
- Fraser P. E., Nguyen J. T., Chin D. T., Kirschner D. A. Effects of sulfate ions on Alzheimer beta/A4 peptide assemblies: implications for amyloid fibril-proteoglycan interactions. J Neurochem. 1992 Oct;59(4):1531–1540. doi: 10.1111/j.1471-4159.1992.tb08470.x. [DOI] [PubMed] [Google Scholar]
- Frederickson C. J. Neurobiology of zinc and zinc-containing neurons. Int Rev Neurobiol. 1989;31:145–238. doi: 10.1016/s0074-7742(08)60279-2. [DOI] [PubMed] [Google Scholar]
- Games D., Adams D., Alessandrini R., Barbour R., Berthelette P., Blackwell C., Carr T., Clemens J., Donaldson T., Gillespie F. Alzheimer-type neuropathology in transgenic mice overexpressing V717F beta-amyloid precursor protein. Nature. 1995 Feb 9;373(6514):523–527. doi: 10.1038/373523a0. [DOI] [PubMed] [Google Scholar]
- Koo E. H., Park L., Selkoe D. J. Amyloid beta-protein as a substrate interacts with extracellular matrix to promote neurite outgrowth. Proc Natl Acad Sci U S A. 1993 May 15;90(10):4748–4752. doi: 10.1073/pnas.90.10.4748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeVine H., 3rd Thioflavine T interaction with synthetic Alzheimer's disease beta-amyloid peptides: detection of amyloid aggregation in solution. Protein Sci. 1993 Mar;2(3):404–410. doi: 10.1002/pro.5560020312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantyh P. W., Ghilardi J. R., Rogers S., DeMaster E., Allen C. J., Stimson E. R., Maggio J. E. Aluminum, iron, and zinc ions promote aggregation of physiological concentrations of beta-amyloid peptide. J Neurochem. 1993 Sep;61(3):1171–1174. doi: 10.1111/j.1471-4159.1993.tb03639.x. [DOI] [PubMed] [Google Scholar]
- Marasco W. A., Haseltine W. A., Chen S. Y. Design, intracellular expression, and activity of a human anti-human immunodeficiency virus type 1 gp120 single-chain antibody. Proc Natl Acad Sci U S A. 1993 Aug 15;90(16):7889–7893. doi: 10.1073/pnas.90.16.7889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masters C. L., Simms G., Weinman N. A., Multhaup G., McDonald B. L., Beyreuther K. Amyloid plaque core protein in Alzheimer disease and Down syndrome. Proc Natl Acad Sci U S A. 1985 Jun;82(12):4245–4249. doi: 10.1073/pnas.82.12.4245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pike C. J., Burdick D., Walencewicz A. J., Glabe C. G., Cotman C. W. Neurodegeneration induced by beta-amyloid peptides in vitro: the role of peptide assembly state. J Neurosci. 1993 Apr;13(4):1676–1687. doi: 10.1523/JNEUROSCI.13-04-01676.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pike C. J., Walencewicz A. J., Glabe C. G., Cotman C. W. Aggregation-related toxicity of synthetic beta-amyloid protein in hippocampal cultures. Eur J Pharmacol. 1991 Aug 14;207(4):367–368. doi: 10.1016/0922-4106(91)90014-9. [DOI] [PubMed] [Google Scholar]
- Pike C. J., Walencewicz A. J., Glabe C. G., Cotman C. W. In vitro aging of beta-amyloid protein causes peptide aggregation and neurotoxicity. Brain Res. 1991 Nov 1;563(1-2):311–314. doi: 10.1016/0006-8993(91)91553-d. [DOI] [PubMed] [Google Scholar]
- Schwarzman A. L., Gregori L., Vitek M. P., Lyubski S., Strittmatter W. J., Enghilde J. J., Bhasin R., Silverman J., Weisgraber K. H., Coyle P. K. Transthyretin sequesters amyloid beta protein and prevents amyloid formation. Proc Natl Acad Sci U S A. 1994 Aug 30;91(18):8368–8372. doi: 10.1073/pnas.91.18.8368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selkoe D. J. The molecular pathology of Alzheimer's disease. Neuron. 1991 Apr;6(4):487–498. doi: 10.1016/0896-6273(91)90052-2. [DOI] [PubMed] [Google Scholar]
- Seubert P., Vigo-Pelfrey C., Esch F., Lee M., Dovey H., Davis D., Sinha S., Schlossmacher M., Whaley J., Swindlehurst C. Isolation and quantification of soluble Alzheimer's beta-peptide from biological fluids. Nature. 1992 Sep 24;359(6393):325–327. doi: 10.1038/359325a0. [DOI] [PubMed] [Google Scholar]
- Shoji M., Golde T. E., Ghiso J., Cheung T. T., Estus S., Shaffer L. M., Cai X. D., McKay D. M., Tintner R., Frangione B. Production of the Alzheimer amyloid beta protein by normal proteolytic processing. Science. 1992 Oct 2;258(5079):126–129. doi: 10.1126/science.1439760. [DOI] [PubMed] [Google Scholar]
- Snow A. D., Sekiguchi R., Nochlin D., Fraser P., Kimata K., Mizutani A., Arai M., Schreier W. A., Morgan D. G. An important role of heparan sulfate proteoglycan (Perlecan) in a model system for the deposition and persistence of fibrillar A beta-amyloid in rat brain. Neuron. 1994 Jan;12(1):219–234. doi: 10.1016/0896-6273(94)90165-1. [DOI] [PubMed] [Google Scholar]
- Snyder S. W., Ladror U. S., Wade W. S., Wang G. T., Barrett L. W., Matayoshi E. D., Huffaker H. J., Krafft G. A., Holzman T. F. Amyloid-beta aggregation: selective inhibition of aggregation in mixtures of amyloid with different chain lengths. Biophys J. 1994 Sep;67(3):1216–1228. doi: 10.1016/S0006-3495(94)80591-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon B., Fleminger G., Schwartz F., Doolman R., Sela B. A. Microalbuminuria immunoassay based on antibodies covalently conjugated to Eupergit C-coated beads. Diabetes Care. 1992 Nov;15(11):1451–1454. doi: 10.2337/diacare.15.11.1451. [DOI] [PubMed] [Google Scholar]
- Solomon B., Koppel R., Kenett D., Fleminger G. Localization of a highly immunogenic region of carboxypeptidase A recognized by three different monoclonal antibodies and their use in the detection of subtle conformational alterations in this enzyme region. Biochemistry. 1989 Feb 7;28(3):1235–1241. doi: 10.1021/bi00429a042. [DOI] [PubMed] [Google Scholar]
- Solomon B., Moav N., Pines G., Katchalski-Katzir E. Interaction of carboxypeptidase A with monoclonal antibodies. Mol Immunol. 1984 Jan;21(1):1–11. doi: 10.1016/0161-5890(84)90083-x. [DOI] [PubMed] [Google Scholar]
- Solomon B., Schmitt S., Schwartz F., Levi A., Fleminger G. Eupergit C-coated membranes as solid support for a sensitive immunoassay of human albumin. J Immunol Methods. 1993 Jan 4;157(1-2):209–215. doi: 10.1016/0022-1759(93)90089-p. [DOI] [PubMed] [Google Scholar]
- Solomon B., Schwartz F. Chaperone-like effect of monoclonal antibodies on refolding of heat-denatured carboxypeptidase A. J Mol Recognit. 1995 Jan-Apr;8(1-2):72–76. doi: 10.1002/jmr.300080113. [DOI] [PubMed] [Google Scholar]
- Talafous J., Marcinowski K. J., Klopman G., Zagorski M. G. Solution structure of residues 1-28 of the amyloid beta-peptide. Biochemistry. 1994 Jun 28;33(25):7788–7796. doi: 10.1021/bi00191a006. [DOI] [PubMed] [Google Scholar]
- Tomiyama T., Asano S., Suwa Y., Morita T., Kataoka K., Mori H., Endo N. Rifampicin prevents the aggregation and neurotoxicity of amyloid beta protein in vitro. Biochem Biophys Res Commun. 1994 Oct 14;204(1):76–83. doi: 10.1006/bbrc.1994.2428. [DOI] [PubMed] [Google Scholar]
- Travis J. Putting antibodies to work inside cells. Science. 1993 Aug 27;261(5125):1114–1114. doi: 10.1126/science.8356445. [DOI] [PubMed] [Google Scholar]
- Wisniewski T., Golabek A., Matsubara E., Ghiso J., Frangione B. Apolipoprotein E: binding to soluble Alzheimer's beta-amyloid. Biochem Biophys Res Commun. 1993 Apr 30;192(2):359–365. doi: 10.1006/bbrc.1993.1423. [DOI] [PubMed] [Google Scholar]