Abstract
Although metastasis is the most lethal attribute of cancer, critical gaps in our knowledge of how cancer cells effectively colonize distant sites remain. For example, little is known about the cellular and molecular events that occur during the timecourse of metastatic colonization. To address this we are using the mitogen-activated protein kinase kinase 4 (MKK4) metastasis suppressor as a tool to identify these events. Specifically, we report a microarray expression-based strategy to identify genes whose transcription is altered in SKOV3ip.1 human ovarian cancer cells that express ectopic MKK4 throughout the course of in vivo metastatic colonization. The majority of genes identified fell into the categories of cytokinesis, cytoskeleton remodeling, and cell adhesion, and their expression was repressed in MKK4-expressing cells relative to vector controls. The greatest transcriptional divergence was concomitant with impaired proliferation at 14 days post injection (dpi). Specifically, 763 genes were differentially expressed (FDR < 0.05) between lesions that expressed ectopic MKK4 and paired controls. In contrast, only seven genes were differentially expressed at the experimental endpoint, when MKK4-expressing and control cells had formed macroscopic metastases. Application of our cohort of differentially expressed genes to three independent clinical datasets demonstrated a strong correlation between our findings and metastatic phenotypes in patient samples. Our results highlight the dynamic nature of metastatic colonization and reinforce the importance of examining both molecular and cellular phenotypes over time when studying metastasis formation.
Keywords: Metastasis suppressor, MAP kinase kinase 4, Ovarian cancer, Transcriptional profiling, Omentum Microarray timecourse
Introduction
Significant progress has been made in specifically characterizing the cellular and molecular events responsible for the pathogenesis of metastasis since Fidler's systematic description of the process in 1978 [1, 2]. While the general steps of invasion, dissemination, lodging, and metastasis formation are well accepted, critical gaps in our knowledge remain. Perhaps the most important of these is our understanding of the final step in this process: metastatic colonization of target organs. A complete understanding of the molecular and cellular events required for cells lodged within target secondary sites to form metastases is needed to develop strategies to target and control the growth of disseminated cells. Such information could also provide insights into the molecular evolution of cells that ultimately grow within the microenvironment of the metastatic site. Unfortunately, most of the current concepts of cancer cell dormancy and subsequent outgrowth are based upon inferences from endpoint assays. To improve our understanding, we must dissect cellular and molecular events that occur during the timecourse of metastatic colonization. To address these critical gaps in knowledge, our laboratory is using the c-Jun NH2-terminal kinase (JNK) kinase 1/mitogen-activated protein kinase (MAPK) kinase 4 metastasis suppressor (hereafter referred to as MKK4) as a tool to interrogate ovarian cancer metastatic colonization of the omentum, a preferred metastatic site for clinical ovarian cancer [3, 4].
MKK4 is a dual-specificity kinase that can activate JNK or p38 MAPKs in response to diverse extracellular stimuli. Reduced MKK4 expression is correlated with disease progression in human patients with ovarian, prostate, and gastric cancers [5–9]. In the case of ovarian cancer, expression profiling studies identify elevated MKK4 expression as a significant predictor of improved response to surgical debulking [10]. In xenograft models using highly metastatic SKOV3ip.1 human ovarian cancer cells, ectopic MKK4 expression decreases the overall number of metastases by 88% (p < 0.0001) and correspondingly prolongs animal lifespan by 70% Wilcoxon (p = 0.0045) [6].
Studies designed to evaluate the effect(s) of MKK4 on omental metastasis formation over time yielded insights that could not be gleaned from endpoint studies. In brief, work from our laboratory showed that the metastasis suppressor function of MKK4 neither impairs omental localization of cancer cells nor induces apoptosis in malignant cells following attachment, but instead depends on signaling by the p38 MAPK to inhibit proliferation via the upregulation of the cell cycle inhibitory protein p21 [11, 12]. Despite the strong effect of MKK4 expression on metastatic development, the proliferative block is transient and MKK4-expressing cells lodged within the omentum ultimately resume proliferation, causing animals to succumb to metastatic disease [12]. Our cumulative findings raised the question of what mechanism(s) might regulate the dynamic antiproliferative behavior of MKK4-expressing cells over time.
The temporal and spatial events involved in MKK4-mediated suppression of colonization, coupled with the transient nature of its effect, prompted the hypothesis that MKK4 suppresses metastasis formation in part through a transcriptional mechanism. In this paper we report findings from a microarray study to identify genes whose transcription is altered at specific timepoints that correlate with the discrete phenotypic changes we have observed during MKK4-mediated metastasis suppression. In support of our hypothesis we found that widespread differential expression of transcripts coincides with impaired proliferation of MKK4-expressing cells. These changes primarily correspond to altered expression of a cohort of genes in MKK4-expressing cells at an early timepoint during metastatic colonization which primarily encode cell surface molecules and proteins involved in cytokinesis and cytoskeletal remodeling. Remarkably, these differences were dramatically reduced after MKK4-expressing cells bypassed suppression and formed overt lesions, with only seven of the ~22,000 genes evaluated on the array significantly differentially expressed at the experimental endpoints. A subset of these expression changes was subsequently confirmed at the protein level in a separate cohort of animals. As a measure of the broader clinical relevance of the expression signatures identified in our experiment, we demonstrate that the probe sets identified in our experiment differentiate between states of disease progression in published clinical expression data sets, in addition to the specific phenotype of MKK4-mediated metastasis suppression interrogated in our experiment. Taken together, our data provide a critical link between MKK4 pathway activation and cellular outcomes that control metastatic colonization and suggest that gene expression patterns concurrent with MKK4-mediated metastasis suppression are relevant to clinical disease.
Materials and methods
Cell culture and animal experimental metastasis assays
Cell lines generated from the human ovarian carcinoma cell line SKOV3ip.1 were cultured as previously described [11]. Intraperitoneal metastasis assays using 6–8 week old female Hsd: Athymic Nude-Fox1nu mice (Harlan Laboratories; Madison, WI) were conducted as previously described using 1 × 9 106 SKOV3ip.1-HA-MKK4 cells (expressing HA-tagged-MKK4 protein) or SKOV3ip.1-pLNCX2 cells (vector-only control) [11, 12]. Transgene expression was confirmed by immunoblotting as described in the Supplemental methods (Supplemental Fig. S1; [11]). Mice were weighed and observed 2–3 times per week and euthanized at the timepoints indicated in the study design in accordance with institutional animal research guidelines.
Rationale for study design
This study design is based upon our previous work showing that metastatic colonization is a dynamic process in which cellular behaviors are linked to specific molecular attributes over time [12]. The results of these experiments suggested that ectopic expression of MKK4 alters SKOV3ip.1's inherent colonization program to cause a delay in metastasis formation, and that SKOV3ip.1-pLNCX2 and SKOV3ip.1-HA-MKK4 endpoint metastases subsequently converge in both biological and molecular attributes. Quantitative real-time PCR experiments showed that MKK4 expression does not have a substantial effect on the number of cancer cells initially adhering to the omentum and parallel studies found no significant increase in apoptosis in these cells [12]. Instead, immunohistochemical quantitation of cell cycle proteins revealed that at 14 dpi SKOV3ip.1-HA-MKK4 cells have decreased BrdU incorporation and upregulate p21 expression, showing that proliferation is impaired [12]. Consistent with the in vivo timecourse data, in vitro kinase assays and in vivo passaging of cell lines derived from macroscopic metastases show that the eventual outgrowth of MKK4-expressing cells is not due to a discrete selection event [12]. Taken together, the findings of Lotan et al. show that the three timepoints chosen for the current study represent fundamentally different MKK4-dependent metastatic states. Thus they are an ideal starting point for studies that test the relationship between the metastatic phenotype and changes in gene expression.
Tissue collection and laser capture microdissection
The omental-pancreas complex was embedded in Tissue-Tek optimal cutting temperature (OCT) compound (Sakura, Torrance, CA), frozen in liquid N2 within 60 min after euthanasia and stored at -80°C until use. Frozen sections (8 lm) were cut onto PEN-membrane-coated slides (W. Nuhsbaum, Inc.) and kept at -80°C overnight. Slides were individually brought to room temperature and rapidly stained with H&E solution and UV-assisted Laser Capture Microdissection (LCM) on a Leica AS LMD 6000 was used specifically to isolate ovarian cancer cells from the omentum. An average of 40,000 cells was collected from each sample into RLT lysis buffer (Qiagen) containing bmercaptoethanol using a nuclease-free thin-walled 0.5 ml collection tube (Labsource).
RNA extraction and amplification
We optimized our approach for preparation, preservation, and handling of intact high quality RNA for array hybridization (Supplemental methods, Supplemental Fig. S1). In brief, RNA was extracted from microdissected cells using RNEasy Micro (Qiagen) and treated with DNase I. RNA from cells in culture was extracted from 5 × 9 105 cells by adding 350 ll of RLT with β-mercaptoethanol to three wells of a 12-well plate (Fisher Scientific). In the case of RNA prepared from cells grown in vitro, lysates from each cultured cell type were homogenized, and aliquots were distributed into three tubes. RNA was extracted in triplicate using RNEasy Micro columns as above. After all of the total RNA samples had been collected and extracted, one round of whole genome amplification was performed using Arcturus RiboAmp Plus RNA Amplification kit (Molecular Devices, MDS, Sunnyvale, CA) using 8 h for in vitro transcription. Total RNA and amplified RNA samples were checked for quality via Agilent Bioanalyzer and then hybridized onto Affymetrix HG U133 Plus 2.0 Arrays. We hybridized a total of 34 RNA samples to arrays, corresponding to three technical replicates of each cell line (e.g. SKOV3ip.1-HA-MKK4 and SKOV3ip.1-pLNCX2), and five biological replicates of cells collected at each timepoint with the exception of the SKOV3ip.1-HA-MKK4 endpoint, where only three high-quality samples could be obtained. (See Supplemental methods for further experimental detail and quality control data.)
Array processing, quality control, and statistical analyses
All array hybridization and scanning was carried out at the Functional Genomics Facility at the University of Chicago according to the manufacturer's protocols, and all analysis was conducted using R (http://www.r-project.org). Raw intensity values were background-corrected using the normexp method implemented in the package RMA [13], normalized and summarized using the Invariant Set method, and then the summarized intensities for each probe set were log 2 transformed [14]. All background correction, normalization, and summarization methods are implemented in the R package affy [15]. We assessed the quality of the array data by comparing correlations of the absolute intensities from microarray hybridizations of replicates to correlations of data from hybridizations of samples across different experimental conditions (Supplementary Table ST1; Supplementary Fig. S3), as well as by examining boxplots of overall intensity to ensure that signal distributions were similar throughout the entire experiment (Supplementary Fig. S2). Finally, MA plots were used to visually inspect concordance between biological replicates (Supplementary Fig. S4).
Once the data were confirmed to be of high quality, we analyzed the transformed background-corrected, normalized data by focusing on the arrays hybridized with RNA obtained exclusively from microdissected samples. In our model, each summarized probe set is treated independently. Let yijkl denote the normalized log 2 intensity for a probe set in biological replicate (l) with MKK4 state (i) (ectopic or endogenous expression of MKK4) in tumors microdissected at 14 dpi (j) or at the endpoint (k). We then consider the following probe set-specific mixed-effects linear model:
Here, β denotes the intercept, γi is a fixed effect that accounts for differences in baseline expression between cells of differing MKK4 status, and nj and kk are fixed effects that account for overall differences in expression at the second and final timepoints, respectively. (γν)ij and (γk)ik denote an interaction between MKK4 expression status and the respective timepoints (expression-by-time-point effects). Furthermore, ρijkl is a Normally distributed random effect with mean 0 and variance that takes into account the fact that within each expression-timepoint group there are multiple separately-isolated biological replicates. Finally, εijkl denotes the residual error, assumed to be Normally distributed with mean 0 and variance σ2. We fitted this model to each gene using the maximum likelihood approach implemented in the lme function in the R package nlme.
To identify probe sets mapping to genes whose expression levels were altered as a result of ectopic MKK4 expression, we used likelihood ratio tests within the framework of the linear model by comparing the fits of the full and reduced nested model for each parameter and then calculating p-values under the assumption that the likelihood ratio follows a χ2 distribution with the appropriate degrees of freedom (see Supplementary Methods for the exact models). We corrected for multiple testing by estimating the false discovery rate (FDR) using the approach of Storey and Tibshirani [16]. In addition, a similar probe-wise mixed effects linear model was used to compare expression levels before and after i.p. injection in a balanced subset of the in vitro and 3 dpi expression arrays to confirm that ectopic expression of MKK4 does not introduce widespread transcriptional variability to SKOV3ip.1-HA-MKK4 and SKOV3ip.1-pLNCX2 cell lines. All analyses were conducted at the level of probe sets, and gene-level statistics reported elsewhere in the manuscript correspond to the number of unique genes identified regardless of the underlying number of differentially expressed probe sets. Details of the above analyses are presented in full in the Supplemental methods.
We identified ontological categories that were enriched within each set of differentially expressed genes using the DAVID Bioinformatics Database as described in [17, 18] using the full set of 54,675 probe sets present on the arrays as the background set. See Supplemental Table ST3 for a complete list of categories significantly enriched within each gene set (p ≤ 0.001, Fisher's exact test).
Immunohistochemistry
To confirm the location of disseminated human epithelial cells to be isolated by LCM, we used immunohistochemistry (IHC) to detect expression of CD45, an antigen expressed on myeloid cells. IHC for these antigens was carried out using 5 lm-thick frozen sections. Sections were fixed in cold acetone for 2 min. Fixed sections were incubated with rat anti-mouse CD45 primary antibody (BD Pharmingen #550539) at 1:10 dilution followed by anti-rat IgG secondary antibody (BD Pharmingen, #551013) at 1:50 dilution. Slides were incubated with Streptavidin– HRP complex and DAB substrate at room temperature and counterstained with Hematoxylin.
To confirm the differential expression of candidate proteins identified in our microarray studies, IHC was conducted on tissues harvested from an independent cohort of 20 animals (performed by The Human Tissue Resource Center of The University of Chicago Cancer Research Center). Following our experimental design, tissues were harvested and embedded at 14 dpi. Sections were fixed in methanol-acetone and incubated with anti-ASPM antibody at 1:40 dilutions (Novus Biologicals, #NB100-2278). For PECAM-1/CD31, staining was performed on unfixed frozen sections with primary rabbit anti-PECAM-1 (Santa Cruz, #SC-1506-R) at 1:100 dilution. Following incubation with anti-rabbit secondary antibody, protein was determined using the EnVision? System-HRP non-biotin detection kit (DakoCytomation, #K4010) with DAB substrate. Slides were scanned using the ACIS Chromavision platform and scored for intensity of staining as described in the Supplemental methods. The average IOD/10 lm2 was calculated for each section (Supplemental methods), and the difference in protein levels was determined by comparing the distributions of average IOD/10 lm2 estimates of SKOV3ip.1-HA-MKK4 cells to SKOV3ip.1-pLNCX2 controls using a one-tailed t-test. Staining was measured in regions consisting exclusively of tumor cells.
Description of public datasets used for enrichment analysis
To compare our findings with published expression data from patient cohorts, we first turned our attention to four studies that used Affymetrix arrays to evaluate transcriptional changes associated with disease progression (e.g. changes in metastatic state). This approach allowed us to compare our data directly with the published results of Hendrix et al. [19], Riker et al. [20], and Pantaleo et al. [21]. Briefly, Hendrix et al. hybridized arrays with RNA from 99 ovarian tumors and 4 control samples of noncancerous ovarian tissue. Riker et al. compared gene expression in 16 subcutaneous melanoma samples to 24 metastases. In contrast, Pantaleo et al. examined gene expression in a group of 18 colon cancer liver metastases, comprised of 10 synchronous and 8 metachronous lesions.
We also compared our data to that of Scotlandi et al. who examined primary tumors and metastatic lesions in Ewing's sarcoma [22]. These analyses found that all of the 14 dpi gene sets were significantly enriched for differential expression in the Scotlandi data at the nominal level (the set of genes upregulated in SKOV3ip.1-HA-MKK4 cells was enriched at the level of p = 0.027; the set downregulated in SKOV3ip.1-HA-MKK4 at p = 0.042; and the set of all probe sets differentially expressed at 14 dpi p = 0.026), but were not significant following multiple test correction and we consequently chose to omit this analysis from our results.
Finally, two large datasets of ovarian cancers were also analyzed in order to determine whether the gene sets identified in our analysis could be used to partition late-stage tumors by patient survival or therapeutic response [23], or by tumor grade or malignancy [24]. In all cases we found no evidence that our gene sets differentiated between late-stage primary tumors, possibly because our experiment is designed to identify transcriptional changes specific to metastatic colonization and not ones that differentiate classes of late-stage primary tumors.
Public dataset enrichment analysis
We used a permutation-based approach to test whether the probe sets identified in our analysis were enriched for transcripts characteristic of human cancer development and malignant progression. For this analysis we chose to focus on probe sets rather than genes so as to avoid ambiguities in instances where probe sets with different expression patterns map to the same transcript. Within each public dataset, we assessed differential expression for each probe set identified in our analysis using a t-test (nominal p value ≤ 0.05) and then tested to see whether an unexpectedly high proportion of the probe sets identified in our experiment was nominally differentially expressed between conditions in the corresponding public dataset. Specifically, we compared the proportion of probe sets identified in our experiment that were differentially expressed between conditions in the public dataset with an empirical null distribution of identically-assessed proportions taken from 10,000 datasets in which the row and column indices were randomly permuted. We defined empirical enrichment p-values as the fraction of randomly-permuted data-sets that contained as many or more differentially expressed probe sets as were observed in the original data.
We applied the above analysis to each dataset using a total of four groups of probe sets. These corresponded to all of the probe sets mapping to transcripts differentially expressed at 14 dpi (1,020 probe sets, FDR = 0.05), two subsets of the 14 dpi list corresponding to the probe sets that indicated higher (326) and lower (694) expression of the associated transcript in the MKK4-expressing cells, respectively, and the full set of differentially expressed probe sets at the endpoint (9 probe sets, FDR = 0.05). In order to correct for multiple testing, we generated as many permuted datasets as there were tests, and compared the maximum proportion of differential expression across the four permuted sets. We then calculated corrected empirical p values as the fraction of these maxima that met or surpassed the number of probe sets differentially expressed in the corresponding list identified in our analysis.
Results
Broad transcriptional changes correlate with metastasis suppression during disease progression
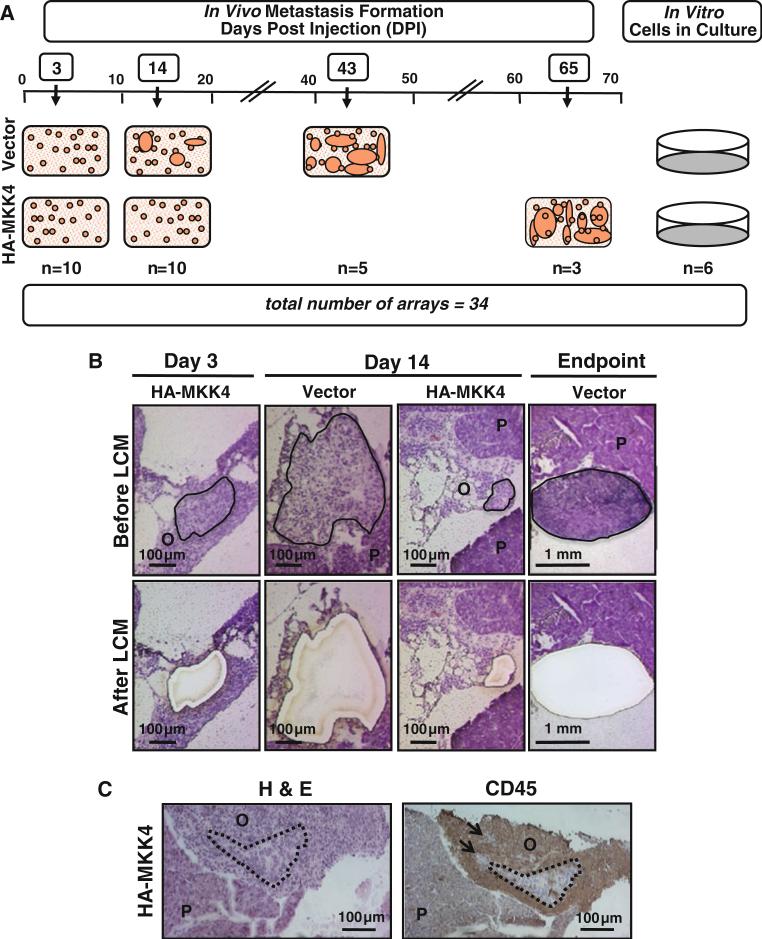
In order to identify the transcriptional basis of MKK4-mediated metastasis suppression in vivo, SKOV3ip.1-HAMKK4 cells were collected by LCM at 3 dpi, 14 dpi, and the experimental endpoints (43 and 65 dpi for SKOV3ip.1-HA-pLNCX2 and SKOV3ip.1-HA-MKK4, respectively) (Fig. 1). RNA was extracted from five biological replicates of each condition and in three replicates from each cell culture. Two of the five SKOV3ip.1-HA-MKK4 endpoint mice did not develop metastases suitable for excision by the endpoint and were not used, resulting in a total of 34 samples that were used in this study (28 from animals and 6 from cell cultures). RNA samples were hybridized to Affymetrix HG U133 Plus 2.0 expression arrays (Supplemental Fig. S2).
Fig. 1.
Summary of experimental approach to examine gene expression changes during omental metastatic colonization. Panel A, left schematic representation of SKOV3ip.1-pLNCX2 (Vector) and SKOV3ip.1-HA-MKK4 (HA-MKK4) lesions during the timecourse of metastatic colonization. Specifically, at 3 dpi, animals injected with either Vector cells or HA-MKK4 cells have histologic lesions, but not overt metastases. By 14 dpi, animals injected with Vector cells have approximately 10 overt metastases, while the majority of those injected with HA-MKK4 cells still only have histological metastases. Finally, at the experimental endpoints (i.e. 43 dpi for Vector cells and 65 dpi for HA-MKK4 cells) animals have 80–100 peritoneal metastases. For expression profiling, cells were collected by LCM from omental lesions at 3 and 14 dpi and at the experimental endpoints of each cell line in five biological replicates. Panel A, right: Three technical replicates of RNA prepared from cells grown in vitro were used as controls. Panel B sections stained with Hematoxylin and Eosin (H&E) show representative examples of SKOV3ip.1-pLNCX2 (Vector) and SKOV3ip.1-HA-MKK4 (HA-MKK4) lesions at 3 and 14 dpi. Panels in the upper and lower rows show the samples pre- and post-LCM, respectively. Outlined areas in the upper panels indicate the microdissectable cancer cells that have been excised with the laser in lower panels. The mouse pancreas, (P), and omentum, (O), are also indicated. Panel C representative images of histology and IHC of disseminated SKOV3ip.1-MKK4 cells in the mouse omentum. Mouse omenta and pancreas were harvested at 14 dpi, embedded in OCT, and frozen. Serial sections were cut and H&E staining or immuno-staining with the CD45 antibody was performed. Human ovarian cancer cells have large irregular nuclei and pale abundant cytoplasm that can be distinguished from surrounding mouse immune cells in H&E-stained sections. IHC for CD45 antigen stains the surrounding mouse immune cells, clearly showing the locations of clusters of disseminated cancer cells. Dotted lines outline clusters of cancer cells
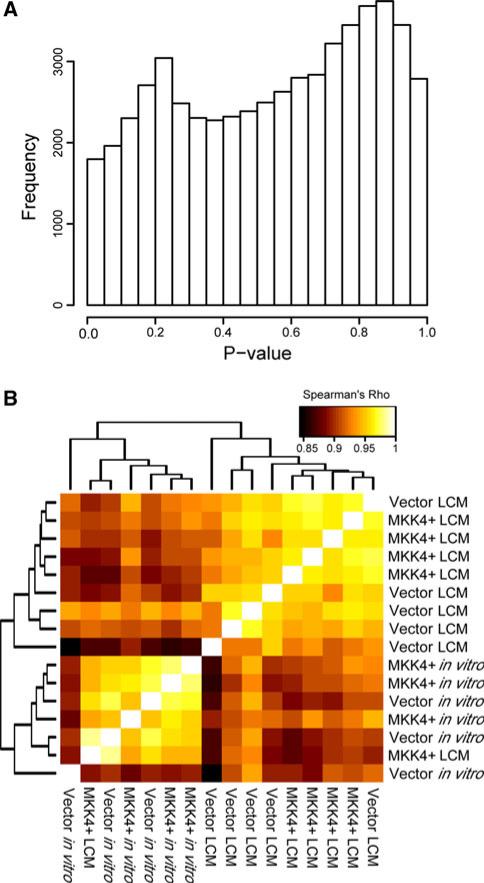
It has been well established that metastasis suppressors such as MKK4 generally do not confer measurable differences in phenotype to cancer cells in vitro [3, 4, 25]. Similarly, previous studies in our laboratory did not find any differences in the numbers of SKOV3ip.1-HA-MKK4 and SKOV3ip-pLNCX2 lodging on the omentum at 3 dpi. We used a mixed-effects linear model to identify genes differentially expressed between SKOV3ip.1-HA-MKK4 and SKOV3ip.1-pLNCX2 cell lines both in vitro and at 3 dpi (see Supplemental methods). Using this approach, no genes were significantly differentially expressed between the cell lines either in vitro or in vivo at 3 dpi (FDR = 0.05; Fig. 2a), although a subset of transcripts showed some limited evidence for divergent expression when all arrays were considered jointly (see Supplemental methods). In addition, we clustered the pairwise Spearman correlation coefficients of the 3 dpi and in vitro arrays by Euclidean distance and found that the arrays did not cluster by class and were highly correlated (mean ρ = 0.933), suggesting that SKOV3ip.1-HA-MKK4 and SKOV3ip.1-pLNCX2 cells are not significantly different at the transcriptional level when maintained in vitro and in the earliest timepoints of omental colonization (Fig. 2b). Thus, we consequently chose to focus on only the microdissected samples for subsequent analyses.
Fig. 2.
There are no discernable differences in gene expression among SKOV3ip.1-HA-MKK4 and SKOV3ip.1-pLNCX2 cells grown in vitro or harvested from omental lesions at 3 dpi. Panel A histogram of the uncorrected p values generated from comparison of the SKOV3ip.1-HA-MKK4 and SKOV3ip.1-pLNCX2 cells grown in vitro using a two-sided t-test. The y-axis corresponds to the number of genes differentially expressed at the nominal p value quantile depicted on the x-axis. Panel B correlation structure of expression estimates from arrays hybridized with samples either derived from SKOV3ip.1-HA-MKK4 or SKOV3ip.1-pLNCX2 cells isolated by LCM from histologic lesions at 3 dpi or harvested from cells growing in vitro. Note the high overall correlation between all arrays and the absence of clustering within class in this comparison
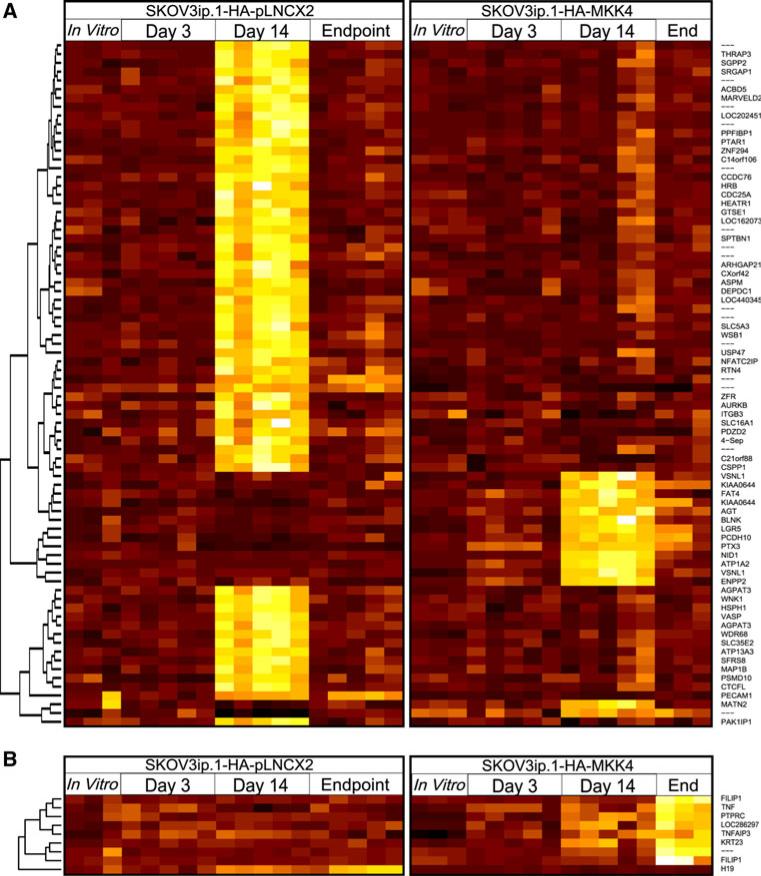
As described in the Introduction, work from Lotan et al. in our group showed that SKOV3ip.1-HA-MKK4 cells underwent a reversible growth arrest as compared to SKOV3ip.1-pLNCX2 controls [12]. Based upon these findings, we hypothesized that the greatest difference in gene expression overall could be detected at the pheno-typically divergent 14 dpi timepoint where MKK4-expressing cells are growth-arrested as compared to vector-only controls. To test this we fit a mixed-effects linear model to the expression estimates derived from each probe set to identify genes that were differentially expressed between SKOV3ip.1-pLNCX2 and SKOV3ip.1-HAMKK4 cells (see “Materials and methods” for additional details). At 14 dpi we identified 763 differentially expressed genes at an FDR of 0.05 (p < 0.001). Of these, 521 genes (68%) were more highly expressed in SKOV3ip.1-HA-pLNCX2 metastases relative to SKOV3ip.1-HAMKK4 cells (Fig. 3a). These genes were disproportionately likely to encode phosphoproteins (p = 1.90 × 10-31, Fisher's exact test) and belong to the broad categories of genes relating to cell division, cytoskeletal rearrangement, and alternative splicing (p < 4.0 × 10-7, FET). In contrast, the genes specifically upregulated in MKK4-expressing cells were disproportionately involved in cell adhesion (p = 6.65 × 10-4, FET) and proteins with EGF-like domains (p = 4.10 × 10-5, FET). A heatmap depicting the intensities of probe sets mapping to genes with highly significant differences in gene expression throughout MKK4-mediated metastasis suppression is shown in Fig. 3. The full results of the gene expression analysis are available in Supplementary Table ST2, and the full results of the Gene Ontology Analysis are provided in Supplementary Table ST3.
Fig. 3.
Distinct changes in gene expression in SKOV3ip.1-HAMKK4 and SKOV3ip.1-pLNCX2 cells during metastatic colonization. Panel A heatmap depicting summarized expression estimates from 78 probe sets differentially expressed between phenotypically divergent metastases at the day 14 timepoint at an estimated FDR of 0.001 (p < 1.5 × 10-6). The left and right sections depict expression estimates for each probe set on each array hybridized with RNA within each cell type from (left to right) in vitro cell cultures, cells microdissected after 3 dpi, 14 dpi, or at the endpoint. Black indicates low relative expression and yellow/white indicates high relative expression; note that expression estimates across probe sets are not directly comparable, and so the color scale is normalized within each probe set for clarity. Panel B summarized expression estimates for the nine probe sets differentially expressed between endpoint tumors at an FDR of 0.05 (p < 5.85 × 10-6)
Despite MKK4's initially strong suppressive effect on metastasis formation, SKOV3ip.1-HA-MKK4 cells lodged within the omentum ultimately resume proliferation. Indeed, by 65 dpi animals injected with SKOV3ip.1-HAMKK4 cells have a metastatic burden comparable to SKOV3ip.1-pLNCX2 controls at 43 dpi. Given the similarity of these phenotypes, we hypothesized that gene expression patterns of SKOV3ip.1-pLNCX2 and SKOV3ip.1-HA-MKK4 cells would converge at the experimental endpoints. In support of this hypothesis, our analyses found only nine probe sets are significantly differentially expressed between SKOV3ip.1-HA-MKK4 and SKOV3ip1-pLNCX2 endpoint metastases (FDR = 0.05, p < 6 × 10-6). These nine probe sets mapped to seven known genes: FILIP1, PTPRC, KRT23, TNF, TNFAIP3, LOC286297, and H19; of these, all but H19 had significantly elevated expression levels in SKOV3ip.1-HA-MKK4 metastases (Fig. 3b). To ensure that the reduced sample size at the experimental endpoints did not have a significant effect on our findings, a subsampling-based analysis was performed. This study indicated that the reduced sample size at the endpoint had only a minor effect on the overall scale of differential expression, and likely resulted in a (2.1 ± 0.95)-fold reduction in the number of differentially expressed probe sets identified (see Supplemental methods for details).
Transcriptional changes result in differential protein expression during MKK4-mediated metastasis suppression
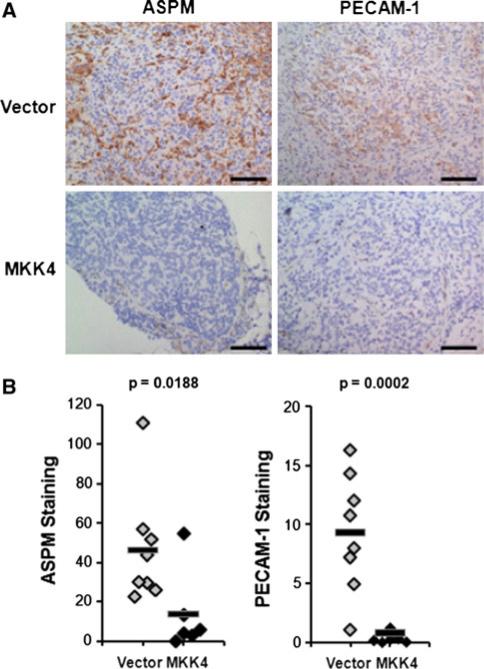
As stated in the preceding section, 763 genes were identified as being significantly differentially expressed between SKOV3ip.1-HA-MKK4 and SKOV3ip.1-pLNCX2 microscopic lesions at 14 dpi (FDR = 0.05; p < 0.001). In order to identify differentially expressed genes for validation at the protein level, we focused on a set of 78 genes with highly significant evidence for differential expression (FDR = 0.001; p < 1.5 × 10-6). While most of these transcripts corresponded to known genes, relatively few had corresponding antibodies which were validated for use in IHC. We identified IHC-tested antibodies to 15 different proteins which were generated in species other than mouse. This group consisted of seven transcripts upregulated in SKOV3ip.1-HA-MKK4 lesions (VSNL1, LGR5/GPR49, PCDH10, NID1, ENPP2, BLNK, MATN2) and eight transcripts upregulated in SKOV3ip.1-pLNCX2 lesions (SGPP2, CDC25A, SEPT4, AURKB, ASPM, VASP, SPTBN1, PECAM1). From these 15 proteins, we selected four candidates, BLNK, AURKB, ASPM, and PECAM1, for validation by IHC. These candidates were chosen because they belong to two categories (e.g. cell adhesion and cytokinesis/cytoskeletal remodeling) that our functional enrichment analysis indicated were highly enriched among the differentially expressed genes. Preliminary optimization studies using control tissues showed that the BLNK antibody did not detect BLNK expression in experimental samples. Similarly, we also met a technical challenge staining for AURKB expression in that the antibody detected both mouse and human AURKB and the background staining was too high to obtain a reasonable signal in the cells of interest. The ASPM and PECAM1 antibodies were optimized and were used for subsequent validation of differential expression a separate cohort of 20 animals injected with either with either SKOV3ip.1-HA-MKK4 or SKOV3ip.1-pLNCX2 cells. Tissues were collected at 14 dpi and ASPM and PECAM levels were assessed by IHC. Consistent with the patterns predicted by the array analysis, IHC evaluation confirmed that PECAM1 and ASPM protein levels were respectively increased more than 12-fold (p = 0.0002, one-tailed t-test) and threefold (p = 0.0188) in SKOV3ip.1-pLNCX2 cells relative to SKOV3ip.1-HA-MKK4 cells (Fig. 4). Preliminary staining of endpoint metastases for ASPM supported the array inference of convergent gene expression (data not shown).
Fig. 4.
Early metastases expressing MKK4 have decreased ASPM and PECAM-1 staining in the cancer cells. Panel A IHC was performed on tissues from an independent cohort of 20 animals. Staining was performed as described in “Materials and methods” on frozen sections from tissues harvested 14 dpi. Representative images of ASPM and PECAM-1 staining in metastases formed by the two cell types are shown. Scale bars represent 100 μm. Panel B quantitative estimate of stain intensity using the ACIS Chromavision platform. The γ-axis was calculated by determining the average intensity of the brown pixels per unit area (IOD/10 mm2) for one sample from each animal. Differential protein expression was determined by comparing the staining intensity of MKK4-expressing metastases to metastases formed from cells expressing empty vector using a one-tailed t-test. Average staining intensity is indicated with a solid bar
MKK4 transcriptional signatures stratify human samples in studies of clinical tumor progression
To evaluate the ability of our system to provide insight into clinical metastases, we tested the possibility that the probe sets differentially expressed during MKK4-mediated metastasis suppression are also differentially expressed in published microarray data from studies of clinical tumor progression and metastatic disease. A Monte Carlo strategy was employed to determine whether the probe sets identified in our analysis distinguish between tumor phenotypes in three public datasets comparing: (1) ovarian tumors with primary tissue [19], (2) primary tumors with secondary metastases in melanoma [20], and (3) synchronous and metachronous colorectal cancer metastases in liver, which compare metastases present at diagnosis to newly-formed relapse metastases [21] (see “Materials and methods” and Supplementary Methods). The results of these analyses are presented in Table 1.
Table 1.
MKK4-mediated transcriptional signatures in clinical dataa
| Set | Probe sets | On array | Differentially expressed | Corrected empirical p value | 95% CI +/− |
|---|---|---|---|---|---|
| Genes differentially expressed between ovarian cancer and normal tissue [19] (dataset comprised of 99 tumors and 4 normal samples) | |||||
| All genes differentially expressed at 14 dpi | 1,020 | 464 | 275 | <0.0001 | NAa |
| Upregulated in SKOV3ip.1-HA-MKK4+, 14 dpi | 326 | 146 | 87 | <0.0001 | NAa |
| Downregulated in SKOV3ip.1-HA-MKK4+, 14 dpi | 694 | 318 | 188 | <0.0001 | NAa |
| All genes differentially expressed at endpoint | 9 | 4 | 3 | 0.0020 | 0.0003 |
| Genes differentially expressed between primary and metastatic melanoma [20] (dataset comprised of 14 primary tumors and 40 metastatic samples) | |||||
| All genes differentially expressed at 14 dpi | 1,020 | 1020 | 165 | 0.0020 | 0.0002 |
| Upregulated in SKOV3ip.1-HA-MKK4+, 14 dpi | 326 | 326 | 54 | <0.0001 | NAa |
| Downregulated in SKOV3ip.1-HA-MKK4+, 14 dpi | 694 | 694 | 111 | 0.0046 | 0.0007 |
| All genes differentially expressed at endpoint | 9 | 9 | 2 | 0.2828 | 0.0036 |
| Genes differentially expressed between metachronous colorectal metastases in liver [21] (dataset comprised of 10 synchronous and 8 metachronous metastases) | |||||
| All genes differentially expressed at 14 dpi | 1,020 | 1,020 | 157 | 0.0109 | 0.0006 |
| Upregulated in SKOV3ip.1-HA-MKK4+, 14 dpi | 326 | 326 | 12 | 0.8466 | 0.0149 |
| Downregulated in SKOV3ip.1-HA-MKK4+, 14 dpi | 694 | 694 | 145 | 0.0004 | 0.001 |
| All genes differentially expressed at endpoint | 9 | 9 | 0 | – | – |
Simulation results demonstrating enrichment of differential expression of identified probe sets in clinical data
Overall, we found that transcriptional signatures identified in our model system closely resemble those observed in clinical datasets characterizing disease progression in humans. This consistency of transcriptional patterns across cancer types suggests that the gene expression changes that we observe during MKK4-mediated metastasis suppression reflect global changes relevant to aspects of metastatic disease progression in spontaneous human tumors. Specifically, the probe sets distinguishing between SKOV3ip.1-HA-MKK4 cells and SKOV3ip.1-pLNCX2 metastases at 14 dpi strongly differentiate between ovarian cancers and non-cancerous tissues (p < 0.0001). These same probe sets disproportionately mapped to transcripts whose expression levels distinguish between primary and metastatic tumors in the melanoma dataset (p = 0.0020) and differentiate synchronous and metachronous metastases (p = 0.0109), suggesting that the differences in transcriptional and phenotypic state observed in our model are consistent with changes present during cancer development in clinical disease and are specifically consistent with metastatic progression.
Interestingly, these differences in transcriptional state are more strongly correlated with metastatic phenotypes when we partition probe sets by their response to ectopic expression of MKK4. Specifically, the probe sets with elevated expression in SKOV3ip.1-HA-MKK4 cells at 14 dpi most strongly differentiated between primary tumors and their established metastases in the melanoma dataset (p < 0.0001), but did not distinguish between established and proliferating metastases in the data set contrasting synchronous and metachronous lesions (p = 0.85). In contrast, probe sets downregulated in MKK4-expressing lesions strongly differentiated between synchronous metastases and metachronous metastases (p = 0.0004), and more weakly segregated primary and metastatic lesions (p = 0.0046). These data suggest the possibility that MKK4 suppresses metastasis at the transcriptional level by inducing factors that prevent the initial formation of metastases from primary lesions while simultaneously suppressing factors involved in the subsequent proliferation of disseminated cells at distant sites.
Discussion
Previous genome-wide studies aimed at identifying the transcriptional mechanisms that underlie metastasis formation have primarily focused on comparisons between end-stage primary and metastatic tumors of mouse or human origin [20, 26], broad characterization of transcriptional changes [27], or between primary tumors of differing metastatic phenotypes [28–30]. In particular, experiments using in vivo selection of metastatic cell lines have yielded considerable insight into the transcriptional basis for metastatic localization [31–33]. However, studies to date have not specifically evaluated transcriptional events or patterns at discrete timepoints during metastatic colonization. We postulated that metastasis suppressors, which can specifically disrupt this process, could be used to identify important transcriptional events related to metastasis suppression.
In the current study we used a well-characterized xenograft model to rigorously characterize the in vivo biological, cellular, and transcriptional effects resulting from ectopic expression of a single protein throughout the course of omental metastatic colonization. The application of these results to three independent clinical datasets demonstrated a strong correlation between our findings and metastatic phenotypes in patient samples, underscoring the clinical relevance of our data. Specifically, the cohort of genes upregulated in SKOV3ip.1-HA-MKK4 cells at 14 dpi in our animal model distinguishes between the transcriptional states of primary tumors and distant metastases in melanoma, suggesting that MKK4 may transcriptionally regulate a set of factors central to the differentiation of metastases from primary tumors. At the same time, ectopic MKK4 suppressed a set of factors that strongly differentiated between established synchronous metastases and newly-formed metachronous metastases in colorectal cancer, suggesting a role in the subsequent proliferation of metastases at different sites.
The delay in metastasis formation mediated by MKK4 is accompanied by altered expression of a large set of genes broadly related to cytoskeletal remodeling, cell division, and cell adhesion. Among these we observed low expression of the cytokinesis-related protein ASPM within MKK4-expressing lesions, accompanied by reduced levels of the membrane protein PECAM1, which has established roles in metastasis formation via its interaction with the tumor microenvironment [34]. Interestingly, expression of PECAM1 and ASPM have both been associated with MAP kinase signaling via p38 activation in the context of inflammatory response [35] and viral infection [36], but have not previously been explicitly linked to MKK4-mediated metastasis suppression. Given our previous observations that MKK4 signals through p38 to suppress cellular proliferation and consequently impairs metastatic colonization [12, 37], these data suggest a mechanism whereby MKK4 inhibits transcription of genes involved in cell cycle progression and proliferation via activation of p38. Additional studies are necessary to fully elucidate the functional relationship between MKK4-mediated repression of genes involved in cytokinesis and the broader phenotype of MKK4's suppression of metastasis formation.
Although our work provides significant insight into the molecular basis of MKK4-mediated metastasis suppression, we are cognizant of the technical limitations associated with our approach. First, cross-hybridization of mouse RNA to human features on the arrays is a theoretical concern, although the high precision of the microdissection procedure and the lack of correlation between tumor size and the probe-wise coefficients of variance within each replicate group suggest that the potential effect of cross-hybridization is very limited. Second, due to the inherently small size of microscopic samples, it was necessary to amplify RNA prior to hybridization, which may have introduced biases in a subset of gene-specific expression estimates. Finally, the rigorously defined in vivo model that enables our experiment requires the use of immunocom-promised mice, and so aspects of the immune response, which may in turn have an effect on the transcriptional state of developing tumors, are absent from this study. Nevertheless, the observation that our findings are consistent with transcriptional patterns observed in human clinical datasets suggests that our model closely mimics the human microenvironment in key ways, and that our findings may have considerable translational value.
Data presented herein challenge the widely held view that all of the information one needs to control metastasis can be gleaned from interrogating primary tumors and/or endpoint metastases, as most MKK4-dependent gene expression changes are dynamic during the timecourse of metastatic colonization. These findings prompt important experimental questions. At what point in the timecourse of omental colonization do the gene expression profiles between SKOV3ip.1-pLNCX2 and SKOV3ip.1-HAMKK4 lesions diverge? Does divergence precede the 14 dpi timepoint, which was examined in the current study? Which genes are the key downstream effectors of activated MKK4? What are the microenvironmental cues that activate MKK4 and thus repression of gene expression? How do MKK4-expressing cells become “resistant” to this activation and bypass suppression? Ongoing studies are designed to address these pragmatic questions. We are also developing complementary in vivo and in vitro approaches to identify the cancer cell microenvironment interactions, which contribute to the gene expression patterns and metastasis suppression phenotypes presented herein. Evidence from our laboratory and others indicate that ovarian cancer cells specifically localize to omental structures known as milky spots. This is the specific microenvironment in which MKK4 activation, repression of gene transcription, and the reversible cell cycle arrest takes place. Subsequently, SKOV3ip.1 cells expressing MKK4 bypass suppression and resume proliferation. As shown by our cumulative studies, these metastases are virtually indistinguishable from controls at the molecular level. This may be due resistance to an external stimulus, secretion of a paracrine factor, or perhaps metastases modulate their microenvironment through mechanisms such as stromal reprogramming or modulation of the phenotype of cancer cells themselves via EMT [38]. In addition to increasing our understanding of metastatic colonization, this information may enable the development of approaches that extend the duration of suppression of omental metastatic colonization and provide insight into the microenvironmental pressures that ovarian cancer cells experience as they progress from disseminated cells to widespread, overt metastases. Such findings could have significant implications for the control of clinical ovarian cancers.
Supplementary Material
Acknowledgments
This study was supported by the funds received from Pardee Foundation to J.T.V. and C.W.R.-S.; from NCI/NIH 2RO1CA089569 to J.T.V., C.W.R-S., and M.W.L.; from DOD W81XWH-09-1-0127 to C.W.R.-S.; from Lederer Fund to J.T.V.; from Section of Urology Research Funds to C.W.R-S and J.T.V.; from NIH Grant T32 GM007197 to R.B.; and from Graduate Training in Growth and Development T32 HD07009 to J.T.V.
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s10585-011-9448-y) contains supplementary material, which is available to authorized users.
Contributor Information
Russell O. Bainer, Department of Human Genetics, The University of Chicago, Chicago, IL, USA
Jennifer Taylor Veneris, The Committee on Cancer Biology, The University of Chicago, Chicago, IL, USA; The Pritzker School of Medicine, The University of Chicago, Chicago, IL, USA.
S. Diane Yamada, Department of Obstetrics and Gynecology, The University of Chicago, Chicago, IL, USA.
Anthony Montag, Department of Pathology, The University of Chicago, Chicago, IL, USA.
Mark W. Lingen, Department of Pathology, The University of Chicago, Chicago, IL, USA
Yoav Gilad, Department of Human Genetics, The University of Chicago, Chicago, IL, USA.
Carrie W. Rinker-Schaeffer, The Committee on Cancer Biology, The University of Chicago, Chicago, IL, USA The Pritzker School of Medicine, The University of Chicago, Chicago, IL, USA; Department of Obstetrics and Gynecology, The University of Chicago, Chicago, IL, USA; Section of Urology, Department of Surgery, The University of Chicago, Room J653, MC 6038, 5841 S. Maryland Ave, Chicago, IL 60637, USA.
References
- 1.Fidler IJ. Tumor heterogeneity and the biology of cancer invasion and metastasis. Cancer Res. 1978;38(9):2651–2660. [PubMed] [Google Scholar]
- 2.Poste G, Fidler IJ. The pathogenesis of cancer metastasis. Nature. 1980;283(5743):139–146. doi: 10.1038/283139a0. [DOI] [PubMed] [Google Scholar]
- 3.Taylor JL, et al. New paradigms for the function of JNKK1/MKK4 in controlling growth of disseminated cancer cells. Cancer Lett. 2008;272(1):12–22. doi: 10.1016/j.canlet.2008.05.012. [DOI] [PubMed] [Google Scholar]
- 4.Thiolloy S, et al. Thinking outside the box: using metastasis suppressors as molecular tools. Semin Cancer Biol. 2011;21(2):89–98. doi: 10.1016/j.semcancer.2010.12.008. [DOI] [PubMed] [Google Scholar]
- 5.Cunningham SC, et al. MKK4 status predicts survival after resection of gastric adenocarcinoma. Arch Surg. 2006;141(11):1095–1099. doi: 10.1001/archsurg.141.11.1095. discussion 1100. [DOI] [PubMed] [Google Scholar]
- 6.Yamada SD, et al. Mitogen-activated protein kinase kinase 4 (MKK4) acts as a metastasis suppressor gene in human ovarian carcinoma. Cancer Res. 2002;62(22):6717–6723. [PubMed] [Google Scholar]
- 7.Yeasmin S, et al. MKK4 acts as a potential tumor suppressor in ovarian cancer. Tumour Biol. 2011;32(4):661–670. doi: 10.1007/s13277-011-0166-5. [DOI] [PubMed] [Google Scholar]
- 8.Kim HL, et al. Mitogen-activated protein kinase kinase 4 metastasis suppressor gene expression is inversely related to histological pattern in advancing human prostatic cancers. Cancer Res. 2001;61(7):2833–2837. [PubMed] [Google Scholar]
- 9.Szmulewitz RZ, et al. MKK4 suppresses metastatic colonization by multiple highly metastatic prostate cancer cell lines through a transient impairment in cell cycle progression. Int J Cancer. 2011 doi: 10.1002/ijc.26005. doi:10.1002/ijc.26005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Berchuck A, et al. Prediction of optimal versus suboptimal cytoreduction of advanced-stage serous ovarian cancer with the use of microarrays. Am J Obstet Gynecol. 2004;190(4):910–925. doi: 10.1016/j.ajog.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 11.Hickson JA, et al. The p38 kinases MKK4 and MKK6 suppress metastatic colonization in human ovarian carcinoma. Cancer Res. 2006;66(4):2264–2270. doi: 10.1158/0008-5472.CAN-05-3676. [DOI] [PubMed] [Google Scholar]
- 12.Lotan TL, et al. c-Jun NH2-terminal kinase activating kinase 1/mitogen-activated protein kinase kinase 4-mediated inhibition of SKOV3ip. 1 ovarian cancer metastasis involves growth arrest and p21 up-regulation. Cancer Res. 2008;68(7):2166–2175. doi: 10.1158/0008-5472.CAN-07-1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smyth GK, Speed T. Normalization of cDNA microarray data. Methods. 2003;31(4):265–273. doi: 10.1016/s1046-2023(03)00155-5. [DOI] [PubMed] [Google Scholar]
- 14.Li C, Wong WH. Model-based analysis of oligonucleotide arrays: expression index computation and outlier detection. Proc Natl Acad Sci USA. 2001;98(1):31–36. doi: 10.1073/pnas.011404098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gautier L, et al. affy—analysis of Affymetrix GeneChip data at the probe level. Bioinformatics. 2004;20(3):307–315. doi: 10.1093/bioinformatics/btg405. [DOI] [PubMed] [Google Scholar]
- 16.Storey JD, Tibshirani R. Statistical significance for genomewide studies. Proc Natl Acad Sci USA. 2003;100(16):9440–9445. doi: 10.1073/pnas.1530509100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dennis G, Jr, et al. DAVID: database for annotation, visualization, and integrated discovery. Genome Biol. 2003;4(5):P3. [PubMed] [Google Scholar]
- 18.Huang DW, Sherman B, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009;4(1):44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- 19.Hendrix ND, et al. Fibroblast growth factor 9 has oncogenic activity and is a downstream target of Wnt signaling in ovarian endometrioid adenocarcinomas. Cancer Res. 2006;66(3):1354–1362. doi: 10.1158/0008-5472.CAN-05-3694. [DOI] [PubMed] [Google Scholar]
- 20.Riker AI, et al. The gene expression profiles of primary and metastatic melanoma yields a transition point of tumor progression and metastasis. BMC Med Genomics. 2008;1:13. doi: 10.1186/1755-8794-1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pantaleo MA, et al. Gene expression profiling of liver metastases from colorectal cancer as potential basis for treatment choice. Br J Cancer. 2008;99(10):1729–1734. doi: 10.1038/sj.bjc.6604681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scotlandi K, et al. Overcoming resistance to conventional drugs in Ewing sarcoma and identification of molecular predictors of outcome. J Clin Oncol. 2009;27(13):2209–2216. doi: 10.1200/JCO.2008.19.2542. [DOI] [PubMed] [Google Scholar]
- 23.Dressman HK, et al. An integrated genomic-based approach to individualized treatment of patients with advanced-stage ovarian cancer. J Clin Oncol. 2007;25(5):517–525. doi: 10.1200/JCO.2006.06.3743. [DOI] [PubMed] [Google Scholar]
- 24.Tothill RW, et al. Novel molecular subtypes of serous and endometrioid ovarian cancer linked to clinical outcome. Clin Cancer Res. 2008;14(16):5198–5208. doi: 10.1158/1078-0432.CCR-08-0196. [DOI] [PubMed] [Google Scholar]
- 25.Shoushtari AN, et al. Metastasis-suppressor genes in clinical practice: lost in translation? Nat Rev Clin Oncol. 2011;8(6):333–342. doi: 10.1038/nrclinonc.2011.65. [DOI] [PubMed] [Google Scholar]
- 26.Lee JY, et al. Farnesoid X receptor, overexpressed in pancreatic cancer with lymph node metastasis promotes cell migration and invasion. Br J Cancer. 2011;104(6):1027–1037. doi: 10.1038/bjc.2011.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saito N, et al. Dynamics of global gene expression changes during brain metastasis formation. Neuropathology. 2009;29(4):389–397. doi: 10.1111/j.1440-1789.2008.00984.x. [DOI] [PubMed] [Google Scholar]
- 28.Dutertre M, et al. Exon-based clustering of murine breast tumor transcriptomes reveals alternative exons whose expression is associated with metastasis. Cancer Res. 2010;70(3):896–905. doi: 10.1158/0008-5472.CAN-09-2703. [DOI] [PubMed] [Google Scholar]
- 29.van de Vijver MJ, et al. A gene-expression signature as a predictor of survival in breast cancer. N Engl J Med. 2002;347(25):1999–2009. doi: 10.1056/NEJMoa021967. [DOI] [PubMed] [Google Scholar]
- 30.Ye QH, et al. Predicting hepatitis B virus-positive meta-static hepatocellular carcinomas using gene expression profiling and supervised machine learning. Nat Med. 2003;9(4):416–423. doi: 10.1038/nm843. [DOI] [PubMed] [Google Scholar]
- 31.Gupta PB, et al. The melanocyte differentiation program predisposes to metastasis after neoplastic transformation. Nat Genet. 2005;37(10):1047–1054. doi: 10.1038/ng1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Matsuda Y, et al. WNT signaling enhances breast cancer cell motility and blockade of the WNT pathway by sFRP1 suppresses MDA-MB-231 xenograft growth. Breast Cancer Res. 2009;11(3):R32. doi: 10.1186/bcr2317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Minn AJ, et al. Genes that mediate breast cancer metastasis to lung. Nature. 2005;436(7050):518–524. doi: 10.1038/nature03799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.DeLisser H, et al. Vascular endothelial platelet endothelial cell adhesion molecule 1 (PECAM-1) regulates advanced meta-static progression. Proc Natl Acad Sci USA. 2010;107(43):18616–18621. doi: 10.1073/pnas.1004654107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McGilvray ID, et al. Monocyte adhesion and transmigration induce tissue factor expression: role of the mitogen-activated protein kinases. Shock. 2002;18(1):51–57. doi: 10.1097/00024382-200207000-00010. [DOI] [PubMed] [Google Scholar]
- 36.Wu SC, et al. Hepatitis C virus NS5A protein down-regulates the expression of spindle gene Aspm through PKR-p38 signaling pathway. J Biol Chem. 2008;283(43):29396–29404. doi: 10.1074/jbc.M802821200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marasa BS, et al. Increased MKK4 abundance with replicative senescence is linked to the joint reduction of multiple microRNAs. Sci Signal. 2009;2(94):ra69. doi: 10.1126/scisignal.2000442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yeasmin S, et al. Loss of MKK4 expression in ovarian cancer: a potential role for the epithelial to mesenchymal transition. Int J Cancer. 2011;128(1):94–104. doi: 10.1002/ijc.25332. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.