Abstract
Previously, we reported that (−)-xanthatin, a naturally occurring xanthanolide present in the Cocklebur plant, exhibits potent anti-proliferative effects on human breast cancer cells, accompanied by an induction of the growth arrest and DNA damage-inducible gene 45γ (GADD45γ), recognized recently as a novel tumor suppressor gene. However, the mechanisms mediating this activation were unknown. Topoisomerase IIα (Topo IIα) inhibition has been reported to produce a cell death response accompanied by an atypical DNA laddering fragmentation profile, similar to that noted previously for (–)-xanthatin. Therefore we hypothesized that (−)-xanthatin’s GADD45γ activation was mediated through the Topo IIα pathway. Here, we identify that (−)-xanthatin does function as a catalytic inhibitor of Topo IIα, promoting DNA damage. In addition, reactive oxygen species (ROS) were elevated in cells treated with this agent. Mechanistically, it was determined that the induced levels of GADD45γ mRNA resulting from (−)-xanthatin exposures were stabilized by coordinately produced ROS, and that the consequent induction of GADD45γ mRNA, GADD45γ protein and ROS generation were abrogated by co-treatment with N-acetyl-l-cysteine. Taken together, the data support the concept that Topo IIα inhibition by (−)-xanthatin is a trigger that stimulates expression of DNA damage-inducible GADD45γ mRNA and that concomitantly produced ROS act downstream to further enhance the GADD45γ mRNA/GADD45γ protein induction process, resulting in breast cancer cell death.
Keywords: Xanthatin, GADD45γ, Topoisomerase IIα, Breast cancer, ROS
1. Introduction
Accumulating evidence supports the view that the growth arrest and DNA damage-inducible gene 45γ (GADD45γ) functions as a tumor suppressor gene in cancers, leading to G2/M arrest and cell death (Ying et al., 2005; Zerbini and Liebermann, 2005). Further support for this concept lies in the observation that the GADD45γ gene is rarely mutated in human tumors (Ying et al., 2005; Zerbini and Liebermann, 2005). It is noteworthy that ectopic expression of GADD45γ is being explored as a novel strategy to inhibit tumor growth. In these respects, selective activators of GADD45γ appear to offer potential as chemotherapeutics. We recently reported that (−)-xanthatin (Fig. 1A), one of the major xanthanolides presented in Xanthium strumarium (the Cocklebur plant), possesses potent anti-proliferative activity for the highly invasive MDA-MB-231 line of breast cancer cells, apparently mediated through induction of GADD45γ (Takeda et al., 2011). Various sesquiterpene lactones have been recognized previously as toxic to both humans and animals, underscoring a view that these substances are non-selectively reactive with cellular macromolecules (Piovano et al., 2000). However, the sesquiterpene lactone, (−)-xanthatin, appears to exhibit minimal toxicity in animals, possessing an LD50 (50% Lethal Dose) value of ~800 mg/kg (Roussakis et al., 1994). Although the results of our prior investigation suggested that GADD45γ is a functional target of (−)-xanthatin’s anti-proliferative activities (Takeda et al., 2011), the pathways mediating these effects were not well established.
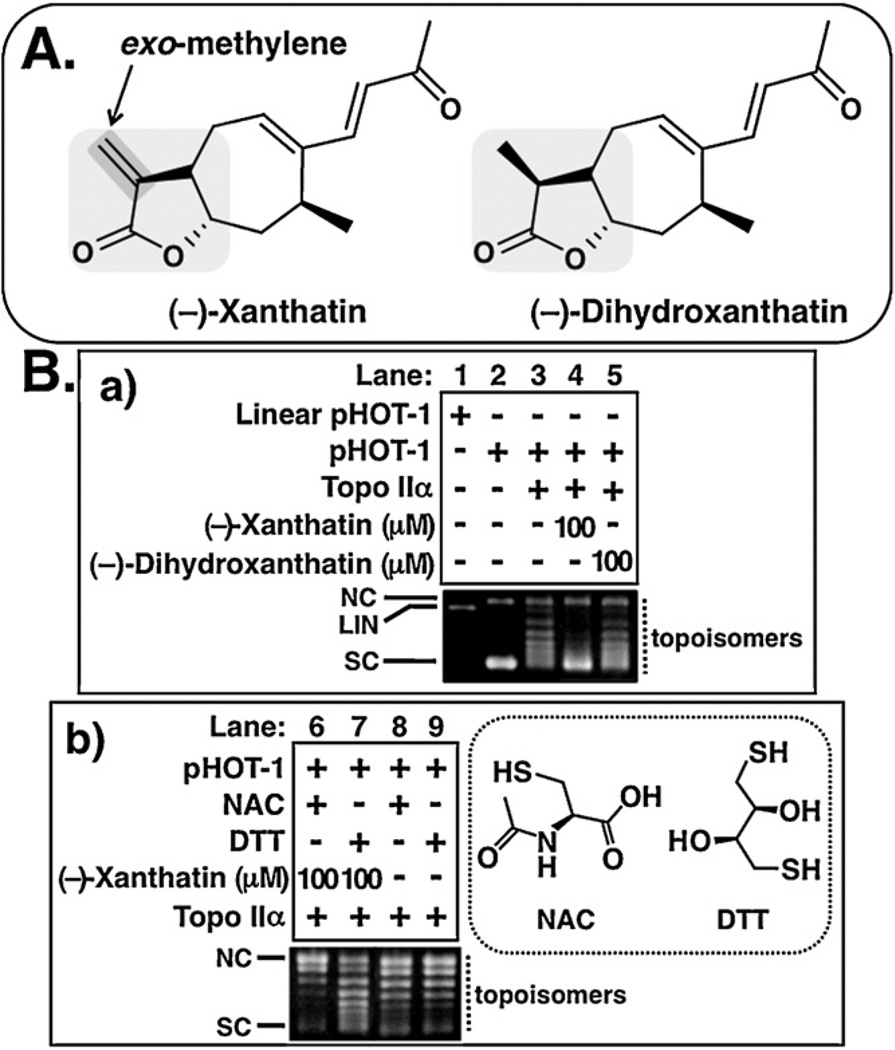
Fig. 1.
(−)-Xanthatin inhibits Topo IIα. (A) Chemical structures of (−)-xanthatin and (−)-dihydroxanthatin are shown. The exo-methylene lactone moiety, indicated with gray inclusion in the (−)-xanthatin, has been suggested as important for (−)-xanthatin-mediated biological effects, including suppression of MDA-MB-231 cell growth (Takeda et al., 2011). (−)-Dihydroxanthatin is an inactive congener of (−)-xanthatin, lacking the exo-methylene moiety (Takeda et al., 2011). (B-a) Effects of (−)-xanthatin and (−)-dihydroxanthatin (100 µM) on the DNA relaxation (pHOT-1) catalyzed by human Topo IIα (lanes 1–5). NC, nicked open circular DNA; LIN, linear DNA; SC, supercoiled DNA; topoisomers, relaxed forms of DNA. (B-b) Effects of –SH-containing agents, NAC and DTT (1 mM, respectively) on 100 µM (−)-xanthatin-mediated inhibition of Topo IIα activity (lanes 6–9). Structures of NAC and DTT are also shown (right portion of figure).
In the prior studies, GADD45γ activation resulted in a cell death response that was inhibited by the reactive oxygen species (ROS)-scavenging agent, N-acetyl-l-cysteine (NAC) (Takeda et al., 2011). These findings suggested that oxidative stress (i.e., ROS) may serve as a key mediator of (−)-xanthatin’s anti-proliferative effects. Further, the earlier investigations revealed that (−)-xanthatin treatment of MDA-MB-231 cells elicited a high molecular weight (HMW) DNA fragmentation pattern, not the DNA laddering profile typically associated with apoptosis (Takeda et al., 2011). HMW DNA fragmentation is a characteristic result of treatments with the important chemotherapeutic class of type II topoisomerase α (Topo IIα) inhibitors, such as etoposide (Hsiang and Liu, 1998; Solovyan et al., 2002). Since ROS generation and HMW fragmentation commonly characterize early stages of cell death (Ha et al., 1997; Quillet-Mary et al., 1997; Li et al., 1999), it was reasoned that inhibition of Topo IIα by (−)-xanthatin may underlie this agent’s ability to induce GADD45γ expression. We further hypothesized a functional interplay between Topo IIα inhibition and ROS in mediating (−)-xanthatin’s induction of GADD45γ.
The results presented in this report demonstrate that (i) (−)-xanthatin functions as a catalytic inhibitor of Topo IIα, and that (ii) ROS production is stimulated by (−)-xanthatin exposures. Mechanistically, these data suggest that transient induction of GADD45γ mRNA is triggered by (−)-xanthatin-mediated Topo IIα inhibition, and that concomitantly produced ROS stabilizes GADD45γ mRNA levels, in turn leading to enhanced GADD45γ protein expression. As (−)-xanthatin-stimulated expression of the GADD45γ induction response was abrogated by co-treatment with NAC, these data imply that ROS possibly act downstream to further enhance the GADD45γ induction process.
2. Materials and methods
2.1. Reagents
(−)-Xanthatin, (−)-dihydroxanthatin, and (+)-8-epi-xanthatin were chemically synthesized according to a published protocol (Matsuo et al., 2010). These synthesized compounds were purified by HPLC (High-performance liquid chromatography) or column chromatography, and their purity (>95%) was confirmed by 1H and 13C NMR (Nuclear Magnetic Resonance) spectroscopy. No ring-opened derivatives of the xanthanolides’ lactones were detected in these analyses (Takeda et al., 2011). 2′,7′-Dichlorofluorescein diacetate (DCFH-DA), etoposide, and reduced glutathione (GSH) were purchased from Sigma Co. (St. Louis, MO, USA). Actinomycin D (Act D) and NAC were purchased from Nacalai Tesque, Inc. (Kyoto, Japan). l-Buthionine-sulfoximine (BSO, a GSH-depleting compound) was purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Amsacrine (m-AMSA) was purchased from Sigma–Aldrich (St. Louis, MO, USA). Ellipticine was purchased from ChromaDex Inc. (Irvine, CA, USA). All other reagents were of the highest grade commercially available.
2.2. Topo IIα-mediated DNA relaxation and cleavage assays
The human Topo IIα enzyme was obtained from TopoGEN (Columbus, OH, USA) and negatively supercoiled pHOT1 DNA was used as a substrate for Topo IIα (TopoGEN, Columbus, OH, USA). The enzyme reaction and analysis of DNA relaxation/DNA cleavage were performed using the Topo II Drug Screening Kit according to the manufacturer’s protocol (TopoGEN, Columbus, OH, USA). To establish the effect of (−)-xanthatin co-incubation on etoposide-stabilized linear DNA formation, the relative intensity of the linear DNA band (normalized to the intensity of the nicked circular band) in the presence of (−)-xanthatin was compared to that in the presence of etoposide alone. In brief, for the detection of topoisomers that are relaxed DNA forms, reaction mixtures were subjected to 1% agarose gels cast without including 0.5 µg/mL ethidium bromide (EtBr) (non-EtBr gel) and electrophoresed in the absence of EtBr. When analyzing linear DNA (Cleavage assay), 1% agarose gels were cast and run in the absence of 0.5 µg/mL EtBr (EtBr gel). These gels were run at 50 V for 60 min and either stained with EtBr (non-EtBr gel) or destained with water (EtBr gel).
2.3. DNA damage analysis
(−)-Xanthatin-mediated DNA damage in MDA-MB-231 cells was assessed using EpiQuik™ in situ DNA Damage Assay Kit (Epigentek Group Inc., Farmingdale, NY, USA), which is a whole cell-based assay for the detection of DNA damage by measuring phosphorylation of H2AX at Ser139 (γH2AX).
2.4. Antibodies and Western blot analysis
Antibodies specific for GADD45γ (sc-33173; Santa Cruz Biotechnology, Santa. Cruz, CA, USA) and β-actin (A5060; Sigma Co., St. Louis, MO, USA) were used. Whole cell extracts were prepared as previously described (Takeda et al., 2009a). SDS–polyacrylamide gel electrophoresis/Western blot analysis was performed based on procedures described previously (Takeda et al., 2009a). Equal amounts of protein for each sample were confirmed by probing with β-actin.
2.5. (−)-Xanthatin-DNA interactions
An EtBr displacement fluorescence assay was employed to determine whether (−)-xanthatin binds in the minor groove of DNA (Fortune and Osheroff, 1998). Experiments using DNA-intercalating Topo II inhibitors (m-AMSA and ellipticine) and non-DNA-intercalating Topo II inhibitor (etoposide) were performed in the analyses.
2.6. Cell cultures and cytotoxicity assays
Cell culture conditions and methods were performed as described previously (Takeda et al., 2009b, 2011). Briefly, the human breast cancer cell line, MDA-MB-231 (obtained from the American Type Culture Collection, Rockville, MD, USA), was routinely grown in phenol red-containing minimum essential medium alpha (Invitrogen, Carlsbad, CA), supplemented with 10 mM HEPES [4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid], 5% fetal bovine serum, 100 U/mL of penicillin, and 100 µg/mL of streptomycin in a humidified incubator, within an atmosphere of 5% CO2, at 37 °C. Before chemical treatments, the medium was changed to phenol red-free minimum essential medium alpha (Invitrogen, Carlsbad, CA, USA) supplemented with 10 mM HEPES, 5% dextrin-coated charcoal-treated serum, 100 U/mL of penicillin, and 100 µg/mL of streptomycin. Cultures of approximately 60% confluence in a 100 mm Petri dish were used to seed the proliferation experiments. In the cytotoxicity assays, the cells were seeded into 96-well plates at a density of approximately 5000 cells/well, and test substances were introduced 4 h after cell seeding. Cells were treated with increasing concentrations of (−)-xanthatin for 48 h. After then, cell viability was analyzed using the CellTiter 96® Aqueous One Solution Cell Proliferation Assay (MTS reagent; Promega, Madison, WI, USA), according to the manufacturer’s instructions. Test chemicals were prepared in ethanol or dimethylsulfoxide (DMSO). Control incubations contained equivalent additions of ethanol or DMSO. No measurable influence of ethanol or DMSO was observed on cell viability at the final concentrations used.
2.7. ROS detection
Cellular ROS levels were quantified as according to reported methods using a DCFH-DA probe (Label et al., 1992).
2.8. Determination of GSH contents
Intracellular levels of GSH were determined using the GSH-Glo Glutathione Assay Kit. (Promega, Madison, WI, USA).
2.9. Analysis of GADD45γ, Topo IIα, and β-actin mRNAs by semiquantitative reverse transcription-polymerase chainreaction (RT-PCR)
Total RNA was prepared from MDA-MB-231 cells using the RNeasy kit (Qiagen, Inc. Hilden, Germany) and purified using RNeasy/QIAamp columns (Qiagen, Inc. Hilden, Germany). Complementary DNA (cDNA) synthesis, RT and PCR were performed using the SuperScript™ one-step RT-PCR System with Platinum® Taq polymerase (Invitrogen, Carlsbad, CA, USA). The primers used for Topo IIα were: Topo IIα (sense) 5′-CTA TTG AAG AAC TGG CTC CAA-3′ and Topo IIα (anti-sense) 5′-CTT TAA ACA GCC TAC GTG GTC-3′. PCR primers used for β-actin and GADD45γ were taken from previously published reports (Steuerwald et al., 2000; Takeda et al., 2011). PCR was performed under conditions producing template quantity-dependent amplification. PCR products were separated by 1.5% agarose gel electrophoresis in Tris–acetate EDTA (ethylenediamine-N,N,N′ ,N′-tetraacetic acid) buffer and stained with EtBr. When the RT reaction was omitted, no signal was detected in any of the samples. β-Actin was used as an internal control for RT-PCR. Quantification of band intensity was performed using NIH Image 1.61 software (http://rsb.info.nih.gov/nih-image/).
2.10. Determination of GADD45γ mRNA half-life
GADD45γ transcript half-life (t1/2) was determined after treatment by the transcription inhibitor, Act D. Samples were collected every 2 h for 8 h after transcription inhibition. Total RNA was extracted and mRNA relative abundance was determined by semiquantitative RT-PCR. The values were normalized relative to the value prior to Act D treatment, plotted as a function of time and subjected to a regression analysis according to Rishi et al. (1999). Quantification of band intensity was performed using NIH Image 1.61 software.
2.11. Data analysis
IC50 values were determined using SigmaPlot 11® software (Systat Software, Inc., San Jose, CA, USA), according to analyses described previously (Takeda et al., 2009b, 2011). Differences were considered significant when the p value was calculated as less than 0.05. Statistical differences between two groups were calculated by Student’s t test. Other statistical analyses were performed by Scheffe’s F test, a post hoc test for analyzing results of ANOVA testing. The calculations were performed using Statview 5.0 J software (SAS Institute Inc., Cary, NC, USA).
3. Results
3.1. (−)-Xanthatin inhibits Topo IIα activity and induces DNA damage
Cellular DNA cleavage accompanied by HMW DNA fragmentation during cell death characterizes cells treated with Topo IIα inhibitors (Lagarkova et al., 1995; Stanulla et al., 1997; Hsiang and Liu, 1998; Solovyan et al., 2002). Since (−)-xanthatin-mediated cell death is also accompanied by the appearance of HMW DNA fragments (Takeda et al., 2011), the potential involvement of Topo IIα in this process was examined. Topo IIα catalyzes DNA relaxation after transient introduction into DNA double-strand breaks (i.e., linear DNA) (Jensen et al., 2002). After incubation with (−)-xanthatin and the Topo IIα enzyme, but in the absence of EtBr, the reaction products were subjected to agarose gel electrophoresis under conditions allowing for the detection of topoisomers (i.e., relaxed DNA forms) that are feature for Topo IIα catalytic activity. As presented in Fig. 1B-a (lane 2 vs. 3), supercoiled DNA (indicated as SC) was converted to the relaxed form (indicated as RLX) by Topo IIα-mediated enzymatic conversion. When the experiments were conducted in the presence of (−)-xanthatin and (−)-dihydroxanthatin (see structures in Fig. 1A), the relaxation of DNA from its SC conformation was inhibited by (−)-xanthatin at 100 µM, while the same concentration of (−)-dihydroxanthatin exhibited no effect (Fig. 1B-a, lane 3 vs. 4 or 5).
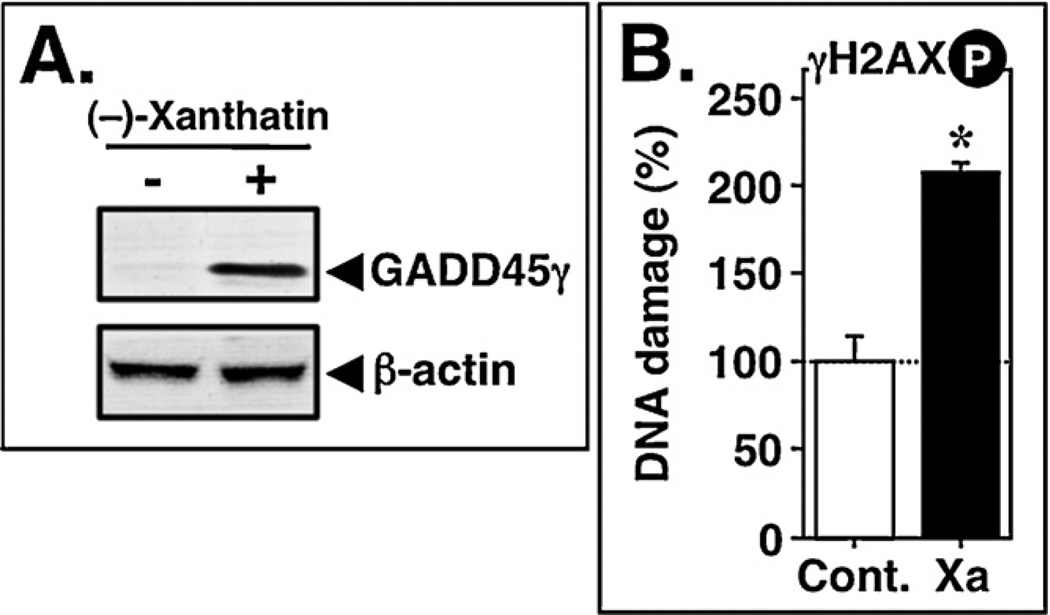
Since (−)-xanthatin contains an exo-methylene lactone group as a possible reactive electrophilic moiety (Zhang et al., 2005; Ghantous et al., 2010; Takeda et al., 2011), and since Topo IIα activity is sensitive to –SH alkylation (via Michael addition with alkylating agents) (Jensen et al., 2002; Wang et al., 2001; Chen et al., 2012), we reasoned that (−)-xanthatin may function in its activity through an –SH reactor mechanism. We tested whether additions of the –SH-containing agents, NAC or dithiothreitol (DTT), would block the Topo IIα inhibitory activity elicited by (−)-xanthatin. Although neither NAC nor DTT alone exhibited detectable inhibitory activity on Topo IIα-mediated DNA relaxation (Fig. 1B-b, lanes 8 and 9), the inhibitory activities of (−)-xanthatin were abrogated by these respective sulfhydryl (SH) reagents (Fig. 1B-b, lanes 6 and 7). In particular, DTT, which possesses two free –SH groups, was a more effective inhibitor than that of NAC (see structures in Fig. 1B-b). It has been reported that Topo IIα inhibitors, such as ICRF-193, can induce DNA damage response marked by phosphorylation of histone H2AX (γH2AX) (Park and Avraham, 2006). Therefore, we examined whether (−) xanthatin triggers DNA damage in MDA-MB-231 cells. As shown in Fig. 2B, after 12 h exposure, (−)-xanthatin at 10 µM evoked the formation γH2AX (205.4 ± 4.8% vs. control = 100%, p < 0.05), which is one of the earliest chromatin modification events in DNA damage response (Mah et al., 2010), a response that coincided with the up-regulation of the DNA damage-sensitive GADD45γ expression (Fig. 2A). It should be noted that the formation of γH2AX in MDA-MB-231 cells was already detected within 2 h of exposure to (−)-xanthatin (120.2 ± 5.1% vs. control = 100%, p < 0.05).
Fig. 2.
(−)-Xanthatin induces DNA damage. (A) MDA-MB-231 cells were treated with 10 µM (−)-xanthatin (indicated as +) or vehicle (indicated as −) for 48 h. Total cell lysates were prepared, and Western blot analyses were performed using antibodies specific for GADD45γ and β-actin, respectively. β-Actin was used an internal loading control. (B) MDA-MB-231 cells were treated with 10 µM (−)-xanthatin (indicated as Xa) or vehicle (indicated as Cont.) for 12 h. DNA damage was determined by ELISA using an anti-phospho-H2AX (Ser139) antibody. Data are expressed as the percent of the control, as mean ± S.D. (n = 6). *Significantly different (p < 0.05) from the control.
Taken together with the evidence that (−)-xanthatin did not affect the endogenous expression of Topo IIα in MDA-MB-231 cells (data not shown), these results suggest that (−)-xanthatin inhibits Topo IIα enzymatic activity via its interaction with –SH residue(s) present within the protein, leading to the production of DNA damage.
3.2. (−)-Xanthatin is a catalytic Topo IIα inhibitor
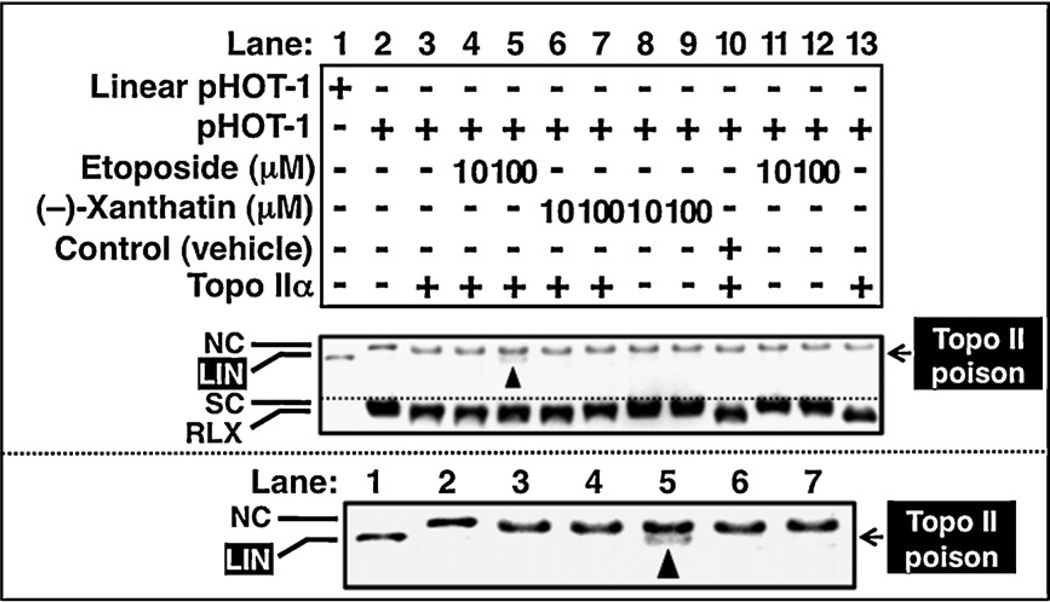
To clarify the inhibitory mode of (−)-xanthatin, we performed Topo IIα-mediated DNA relaxation assays utilizing a method that distinguishes the appearance of linear DNA from more complex forms (indicated as LIN, DNA cleavage assay). Given that (−)-xanthatin acts as a catalytic inhibitor of Topo IIα (Fig. 1B) and not a Topo II poison, such as etoposide, (−)-xanthatin treatments should not yield a “LIN” DNA species. After incubation with 10 µM or 100 µM (−)-xanthatin, or etoposide, in the presence of EtBr, the reaction products were analyzed with agarose gel electrophoresis. The appearance of LIN DNA was only detected when incubated with 100 µM etoposide + Topo IIα, but not with 100 µM (−)-xanthatin + Topo IIα (Fig. 3A upper panel, lane 5 vs. 7 and see also enlarged image placed below). In the absence of Topo IIα, LIN was not detected either in the presence of (−)-xanthatin or etoposide (Fig. 3A, lanes 8, 9 and 11, 12). LIN was also detected in the presence of 100 µM m-AMSA, another Topo II poison. Even concentrations up to 500 µM of (−)-xanthatin were ineffective in producing LIN DNA (data not shown).
Fig. 3.
Unlike etoposide, inhibition of Topo IIα by (−)-xanthatin does not linearize DNA. Effects of (−)-xanthatin (10, 100 µM) or etoposide (10, 100 µM) on the human Topo IIα-mediated cleavage of DNA (pHOT-1) (lanes 1–13). Lane 10 shows a vehicle-treated sample (indicated as Control). The lower panel is an enlarged view of a portion of the upper panel, better visualizing linearized DNA in lane 5. NC, nicked open circular DNA; LIN, linear DNA; SC, supercoiled DNA.
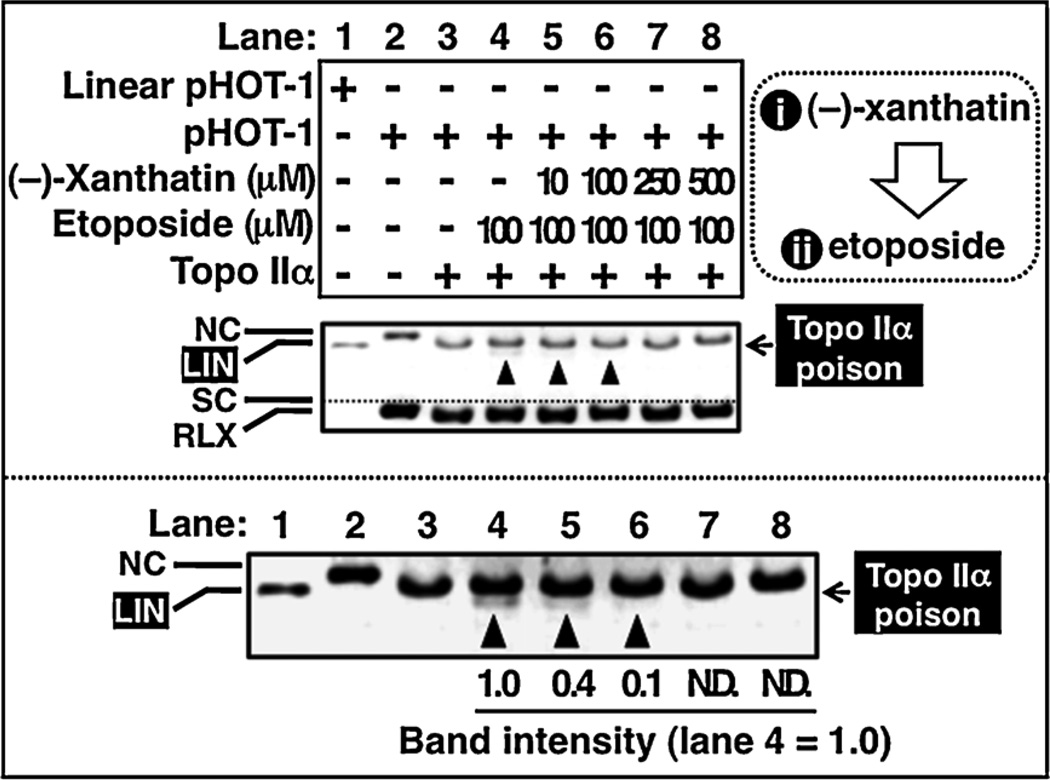
To further examine the mechanisms of (−)-xanthatin’s Topo IIα inhibition, we assessed whether (−)-xanthatin might antagonize the effect of etoposide in the DNA cleavage assay, as Topo II catalytic inhibitors are known to antagonize the effects of Topo II poisons (Jensen et al., 2002; Bau and Kurz, 2011). Pre-incubation with (−)-xanthatin was effective in antagonizing etoposide-induced LIN formation, in a concentration-dependent manner (Fig. 4B, lane 4 vs. 5–8). Neither co-incubation nor post-incubation with (−)-xanthatin was effective in this respect (data not shown). Together, the results imply that (−)-xanthatin functions mechanistically as a catalytic inhibitor of Topo IIα.
Fig. 4.
(−)-Xanthatin antagonizes etoposide-mediated generation of linear DNA. Topo IIα enzyme was pre-incubated in a buffer containing increasing concentrations of (−)-xanthatin (lanes 5–8; from 10 µM to 500 µM) in the presence or absence of etoposide (100 µM) for 30 min. Subsequently, linear DNA (pHOT-1) was detected as described in Section 2 (lanes 1–8). The lower panel shows an enlarged view of the upper panel to better allow visualization of linear DNA (indicated as LIN). The intensity of linear bands was quantified and expressed as fold-change (lane 4). NC, nicked open circular DNA; LIN, linear DNA; SC, supercoiled DNA.
3.3. (−)-Xanthatin does not intercalate into DNA
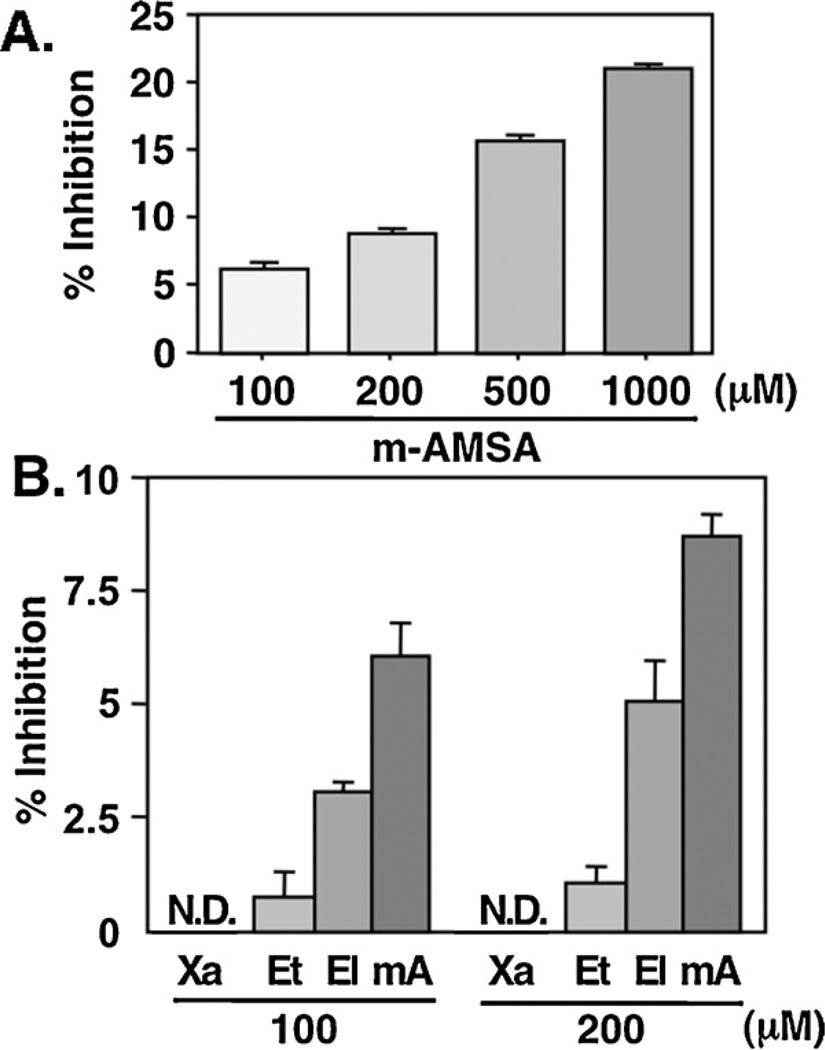
It is reported that certain chemicals that alter the gross structure of DNA can dramatically effect the catalytic activity of Topo IIα (Zechiedrich and Osheroff, 1990; Boos and Stopper, 2000), and that some –SH interacting agents can bind directly to DNA (Zhang et al., 2005; Ghantous et al., 2010). Thus, we examined whether (−)-xanthatin’s inhibition of Topo IIα might occur via direct interaction with DNA. The ability of (−)-xanthatin to displace EtBr from the minor groove of DNA was determined by an established fluorescence emission assay (Fortune and Osheroff, 1998), as DNA-bound EtBr exhibits much stronger fluorescence emission than free EtBr. Although the DNA intercalators, m-AMSA (mA) and ellipticine (El), were capable of displacing EtBr (Fig. 5A and B), neither (−)-xanthatin nor the non-DNA-intercalator, etoposide (Et), demonstrated any significant displacement of EtBr, with <1% displacement detected even at 500 µM (−)-xanthatin (data not shown). Therefore these results suggest that (−)-xanthatin’s ability to inhibit Topo IIα activity does not result from this agent’s direct interaction with DNA.
Fig. 5.
(−)-Xanthatin does not displace EtBr from the minor groove of DNA. (A) The ability of m-AMSA to interact with the minor groove of DNA was determined by a fluorescence-based ethidium displacement assay. Samples contained 1 µM EtBr and 5 nM double-stranded 40-mer DNA oligonucleotide. Increasing concentrations of m-AMSA were added, and ethidium fluorescence at 605 nm (λmax) was monitored (510 nm excitation wavelength). (B) The ability of 100 µM or 200 µM (−)-xanthatin (Xa), etoposide (Et), ellipticine (El), or m-AMSA (mA) to interact with the minor groove of DNA was determined. Data are expressed as the percent of vehicle-treated group (Control), as mean ± S.D. (n = 8). N.D., not detectable.
3.4. (−)-Xanthatin increases ROS levels and stabilizes GADD45γ mRNA in MDA-MB-231 cells
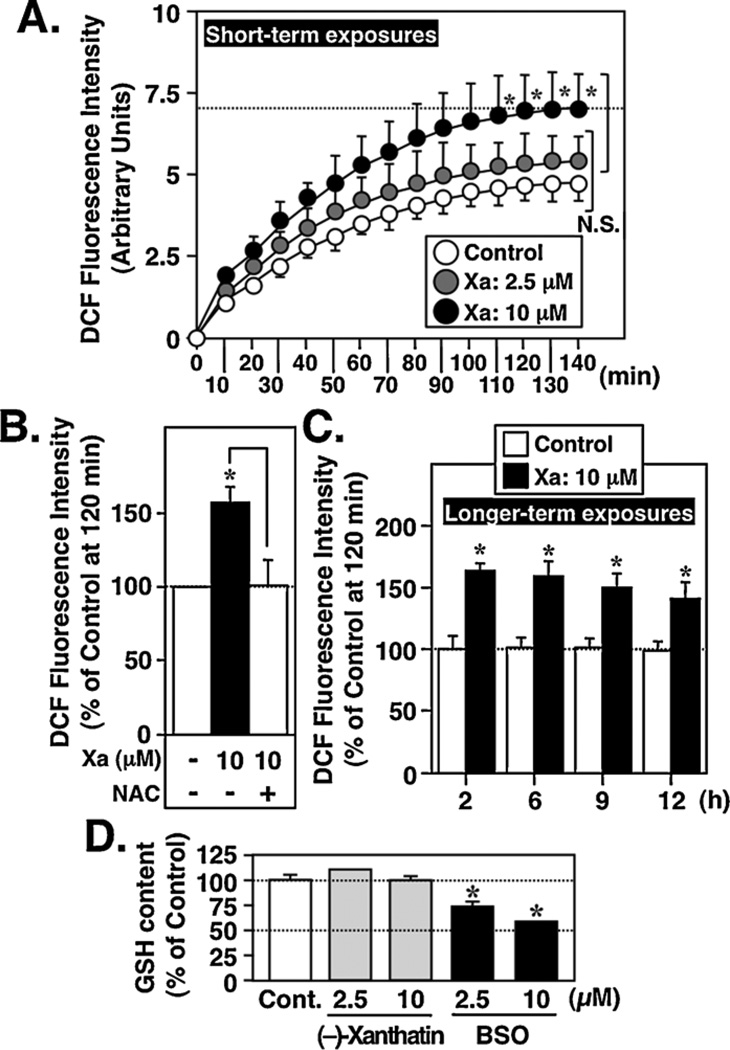
Since 10 µM (−)-xanthatin mediates anti-proliferative effects on MDA-MB-231 cells that are abrogated by ROS scavengers such as NAC (Takeda et al., 2011) (see also Fig. 8B), we assessed the respective levels of intracellular ROS in MDA-MB-231 cells following (−)-xanthatin treatment, using a DCFH-DA probe (Label et al., 1992). Compared to vehicle control, 10 µM (−)-xanthatin resulted in ROS generation in a time-dependent manner, reaching a plateau at 120 min (Fig. 6A), effects that were blocked by NAC (Fig. 6B), whereas lower concentrations of (−)-xanthatin (2.5 µM) did not produce significant levels of ROS (Fig. 6A). ROS levels were further assessed following 10 µM (–)-xanthatin exposures for 12 h. ROS production was sustained even over this time course (Fig. 6C, ‘longer-term exposures’). It should be noted that the concentrations of (−)-xanthatin (i.e., 10 µM vs. 2.5 µM) required to generate ROS concurred with those required to mediate its anti-proliferative effects on MDA-MB-231 cells (Takeda et al., 2011).
Fig. 8.
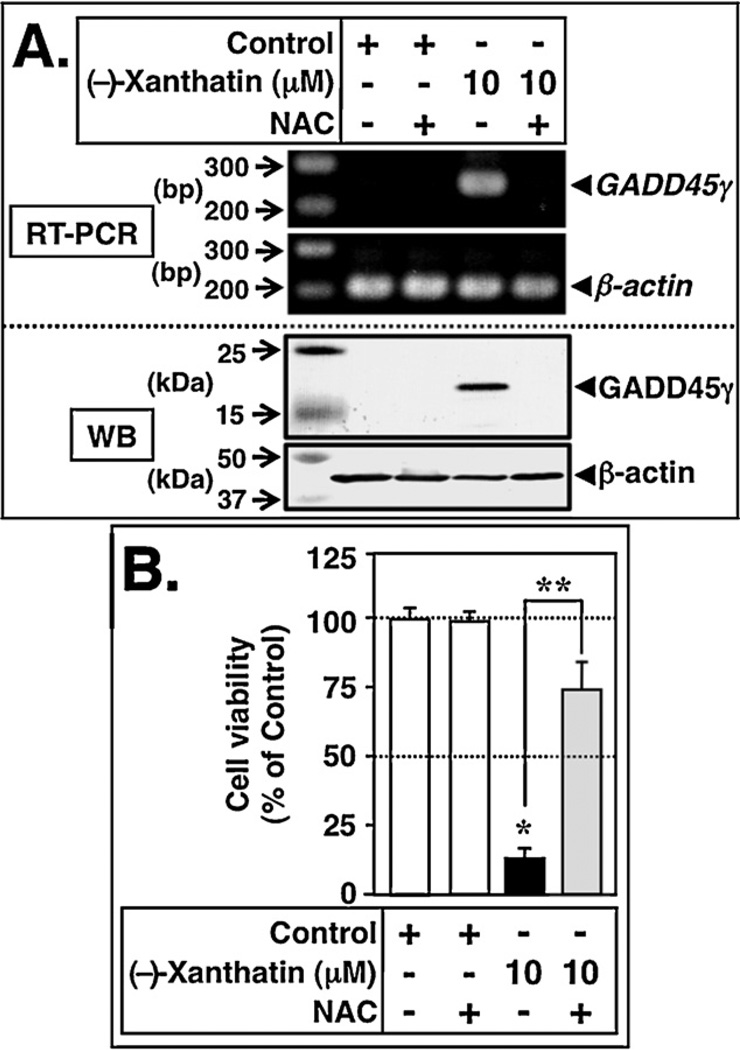
Concomitantly produced ROS are involved in the (−)-xanthatin-mediated up-regulation of GADD45γ. (A) RT-PCR (upper panel) and Western blot (WB, lower panel) analyses of GADD45γ levels in MDA-MB-231 cells 48 h after treatment with 10 µM (−)-xanthatin or vehicle in the presence or absence of 1 mM NAC. NAC was added as a pretreatment, 2 h prior to (−)-xanthatin additions. β-Actin was used an internal loading control. A 100-bp DNA ladder marker for RT-PCR and protein molecular weight marker (kDa) were also loaded. (B) MDA-MB-231 cells were treated for 48 h with 10 µM (–)-xanthatin or vehicle in the presence or absence of 1 mM NAC. NAC was added as a pretreatment 2 h prior to (−)-xanthatin additions. Data are expressed as the percent of the vehicle-treated group (Control; +−/−group), as mean ± S.D. (n = 6). *Significantly different (p < 0.05) from the control. **Significantly different (p < 0.05) from the 10 µM (−)-xanthatin alone-treated group.
Fig. 6.
(−)-Xanthatin produces intracellular ROS as a function of time. (A) (Short-term exposures) MDA-MB-231 cells were exposed for up to 140 min to (−)-xanthatin (Xa; 2.5, 10 µM). Intracellular ROS were measured as described in Section 2. Data are expressed as the percent of vehicle-treated group (Control), as mean ± S.D. (n = 6). *Significantly different (p < 0.05) from the control. N.S., not significant. (B) MDA-MB-231 cells were exposed for 2 h to (−)-xanthatin (Xa; 10 µM) in the presence or absence of 1 mM NAC. Intracellular ROS were measured as described in Section 2. *Significantly different (p < 0.05) from the control at 120 min. (C) (Longer-term exposures) MDA-MB-231 cells were exposed for up to 12 h to (−)-xanthatin (Xa; 10 µM). Intracellular ROS were measured as described in Materials and Methods. Data are expressed as the percent of vehicle-treated group (control), as mean ± S.D. (n = 6). *Significantly different (p < 0.05) from the control at 120 min. (D) Effect of (−)-xanthatin on the GSH levels in MDA-MB-231 cells. Cells were treated with 2.5 or 10 µM (−)-xanthatin, or 2.5 or 10 µM BSO, for 12 h and the levels of GSH were measured. Data are expressed as the percent of vehicle-treated group (indicated as Cont.), as mean ± S.D. (n = 6). *Significantly different (p < 0.05) from the control.
To examine whether (−)-xanthatin may be generating ROS indirectly, through depletion of cellular GSH levels, cells were exposed to the agent for 12 h then assessed for GSH content. As shown in Fig. 6D, these treatments resulted in no change in GSH levels. However, BSO, an established inhibitor of GSH synthesis, was effective in depleting GSH levels in a concentration-dependent manner. These results support the conclusion that (−)-xanthatin triggers sustained production of ROS in MDA-MB-231 cells via mechanisms independent of GSH depletion.
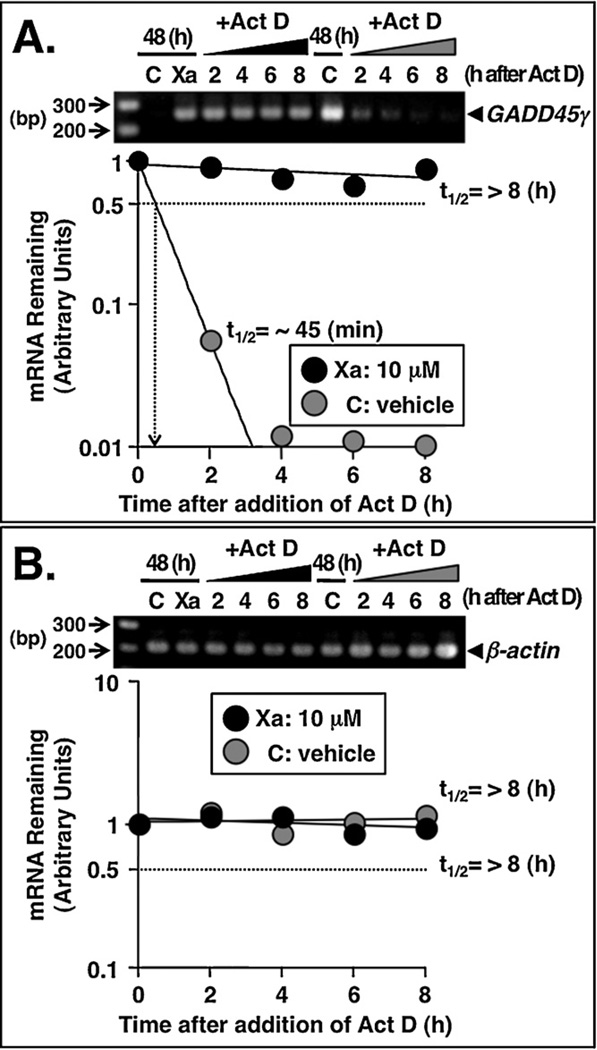
Based on the reports suggesting that changes in GADD45γ mRNA stability are modulated upon treatment with ROS-producing agents (Adler et al., 1999; Zheng et al., 2005), we next investigated whether (−)-xanthatin may affect the stability of GADD45γ mRNA. MDA-MB-231 cells were incubated for 48 h in the presence or absence of 10 µM (−)-xanthatin (indicated as Xa) or vehicle (indicated as C). Act D (4 µg/mL), an established transcriptional inhibitor, was then added to the cell cultures. GADD45γ and β-actin mRNA levels were determined by semiquantitative RT-PCR at various time intervals. β-Actin mRNA stability was not affected by either (−)-xanthatin or vehicle treatments (Fig. 7B). It was reported previously that the half-life of GADD45γ mRNA under normal conditions is approximately ~60 min (Bruemmer et al., 2003). In the current study, (−)-xanthatin remarkably enhanced the stability of the GADD45γ mRNA transcript, >11-fold when compared with vehicle-treated controls (~45 min half-life; Fig. 7A, see also inset of Fig. 7A). Further, in parallel experiments we tested independent RT-PCR primer sets and those experiments yielded the same quantitative conclusions (data not shown). Together, these results indicate that (−)-xanthatin stabilizes GADD45γ mRNA in MDA-MB-231 cells, possibly through a ROS generation mechanism.
Fig. 7.
(−)-Xanthatin stabilizes GADD45γ mRNA. (A and B) Effects of (−)-xanthatin or vehicle on the mRNA stability of GADD45γ and β-actin in MDA-MB-231 cells. MDA-MB-231 cells were treated for 48 h with 10 µM (−)-xanthatin (Xa) or vehicle (C), and subsequently, the cells were exposed to the transcriptional inhibitor, actinomycin D (Act D, 4 µg/mL) for 2, 4, 6, or 8 h. The Act D concentration used was determined based on both efficacy and lack of toxicity following dose-response experiments. After the respective Act D exposures, total cellular RNA was isolated and RT-PCR analyses were performed as described in Section 2. β-Actin was also used as an RNA internal control. (A) Semi-logarithmic plot of the decay of GADD45γ mRNA is shown. The number of optimized PCR cycles used for the amplification of GADD45γ was 33. Due to the very low expression levels of GADD45γ, for the determination of half-life of GADD45γ mRNA after ‘vehicle exposure (indicated as C)’, the number of optimized PCR cycles used for the amplification of GADD45γ was 42. A 100 bp DNA ladder marker was also loaded. (B) Semi-logarithmic plot of the decay of β-actin mRNA is shown. The number of optimized PCR cycles used for the amplification of β-actin was 29. The mRNA decay plots and calculation of the mRNA half-life were performed according to reported methods (Rishi et al., 1999). A 100 bp DNA ladder marker was also loaded.
3.5. ROS is involved in (−)-xanthatin’s up-regulation of GADD45γ
We reasoned that if ROS generation is involved as a mediator of (−)-xanthatin’s up-regulation of GADD45γ expression (i.e., Figs. 6 and 7), then NAC, an effective ROS scavenger (Zhang et al., 2011), should interfere with this pathway. As demonstrated in Fig. 8A, pre-treatment with 1 mM NAC largely blocked the induction of GADD45γ mRNA (indicated as RT-PCR) and GADD45γ protein (indicated as WB) resulting from 10 µM (−)-xanthatin exposures (upper and lower panels, respectively). Treatment with NAC alone had no effect on the GADD45γ mRNA/GADD45γ protein expression status (Fig. 8A). In accord with these results, 1 mM NAC inhibited the anti-proliferative activity exerted by 10 µM (−)-xanthatin treatment of MDA-MB-231 cells (Fig. 8B) (see also Takeda et al., 2011). These data support the concept that ROS-dependent pathways underlie (−)-xanthatin’s up-regulation of the GADD45γ mRNA/GADD45γ protein.
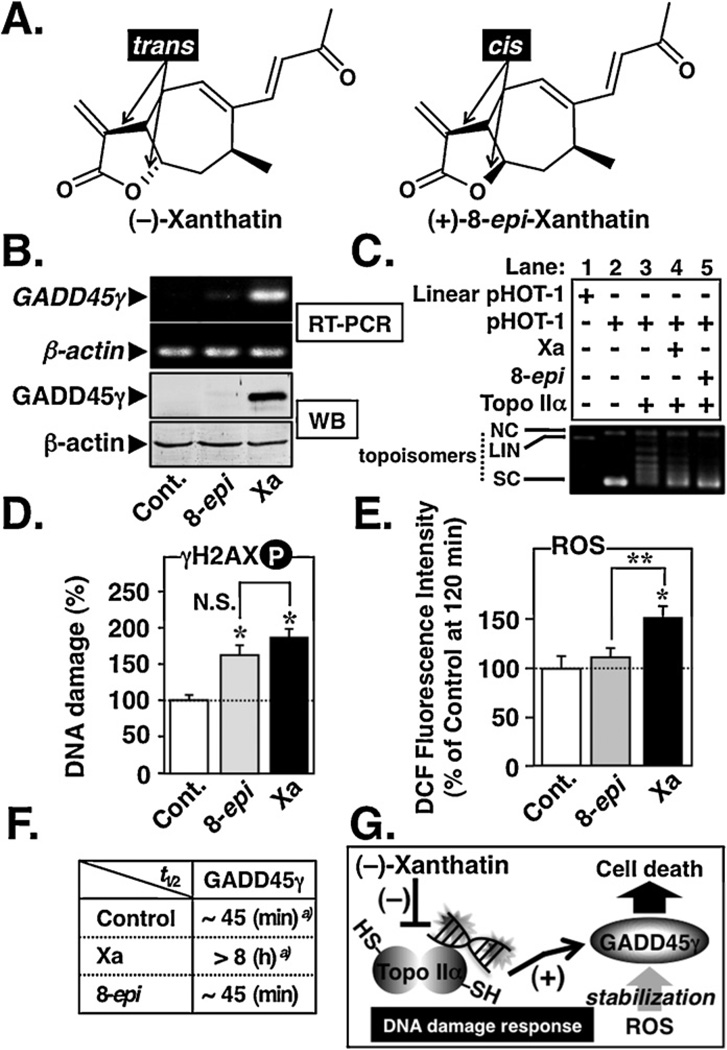
Based on these results, a working model for (−)-xanthatin-mediated up-regulation of GADD45γ is suggested. It is proposed that GADD45γ activation results from (−)-xanthatin’s ability to inhibit Topo IIα, thereby enhancing cellular DNA damage, and that these activities are stabilized by concomitantly generated ROS also induced by this agent (Fig. 9G). In support of this model, a cis-isomer of (−)-xanthatin, (+)-8-epi-xanthatin (8-epi) (Fig. 9A), was also tested, but exhibited only very weak GADD45γ induction potential (Fig. 9B). It was noteworthy that although (+)-8-epi-xanthatin exhibited Topo IIα inhibitory activity, accompanied by DNA damage at levels comparable to (−)-xanthatin (Fig. 9C, lanes 4, 5 and Fig. 9D), (+)-8-epi-xanthatin lacked ROS-producing potential (Fig. 9E). These results were further supported by the data presented in Fig. 9F, demonstrating that (+)-8-epi-xanthatin does not stabilize GADD45γ mRNA, as does (−)-xanthatin (see also Fig. 7A). Neither was (+)-8-epi-xanthatin effective in eliciting ROS generation at 12 h incubation times (data not shown).
Fig. 9.
Topo IIα inhibition followed by ROS generation is involved in the (−)-xanthatin’s up-regulation of GADD45γ. (A) Chemical structures of (−)-xanthatin (trans) and its cis-isomer (+)-8-epi-xanthatin. These xanthanolies contain the exo-methylene lactone moiety in their structures. (B) RT-PCR (upper panel) and Western blot (WB, lower panel) analyses of GADD45γ levels in MDA-MB-231 cells 48 h after treatment with 10 µM (−)-xanthatin (Xa), (+)-8-epi-xanthatin (8-epi), or vehicle (Cont.). β-Actin was used the internal loading control. The number of optimized PCR cycles used for the amplification of GADD45γ and β-actin were 33 and 29, respectively. (C) Effects of 100 µM Xa or 8-epi on the DNA relaxation (pHOT-1) catalyzed by human Topo IIα. NC, nicked open circular DNA; LIN, linear DNA; SC, supercoiled DNA; topoisomers, relaxed forms of DNA. (D) MDA-MB-231 cells were treated with 10 µM (−)-Xa or 8-epi for 12 h. DNA damage was determined by ELISA using an anti-phospho-H2AX (Ser139) antibody. Data are expressed as the percent of the control, as mean ± S.D. (n = 6). *Significantly different (p < 0.05) from the vehicle-treated group (Cont.). N.S., not significant. (E) MDA-MB-231 cells were exposed for 2 h to 10 µM Xa or 8-epi. Intracellular ROS were measured as described in Section 2. *Significantly different (p < 0.05) from the vehicle-treated control (Cont.). **Significantly different (p < 0.05) from the 8-epi-treated group. (F) Summary of the effects of 10 µM Xa or 8-epi on the GADD45γ mRNA stability in MDA-MB-231 cells. For the data of vehicle-treated group (Control)a) and Xaa) presented in the figure, half-life data (t1/2) were taken from Fig. 7. Determination of the GADD45γ mRNA stability after 8-epi treatment was performed as described in Fig. 7. (G) A working model for the (−)-xanthatin-mediated up-regulation of tumor suppressor GADD45γ. In this study, it was revealed that (−)-xanthatin requires “two pathways” to stimulate GADD45γ expression that is highly suppressed in human breast cancer MDA-MB-231 cells: (i) (−)-xanthatin inhibits catalytic activity of Topo IIα (accompanied by DNA damage) through interaction with –SH residues on the molecule, followed by GADD45γ induction, and (ii) the up-regulated GADD45γ mRNA/GADD45γ protein is stabilized by concomitantly generated ROS.
Together, these data demonstrate that (−)-xanthatin’s inhibition of Topo IIα is a prerequisite for the induction of GADD45γ and that mechanistically, this inhibition is sustained by concomitantly produced ROS that act downstream in the process to further enhance and prolong the GADD45γ tumor suppressor induction process, resulting in breast cancer cell death (see Fig. 9G).
4. Discussion
In the present study, it is demonstrated that (−)-xanthatin inhibits Topo IIα, and that DNA damage was induced by this agent. Topo IIα is one of the targets for some of the most active anti-cancer drugs utilized in the treatment of human malignancies since Topo IIα is known to be highly expressed in rapidly proliferating human cells, including breast cancer cells (Depowski et al., 2000; Larsen et al., 2003a,b; Chikamori et al., 2010).
Compounds that target Topo IIα are classified into two categories: (i) Topo II poisons and (ii) Topo II inhibitors (Larsen et al., 2003a; Chikamori et al., 2010). Topo II poisons, such as etoposide, stabilize the Topo II-DNA covalent cleavage complex leading to an accumulation of DNA double-strand breaks (i.e., linear DNA), whereas agents that inhibit the enzyme during other reaction steps are designated as catalytic inhibitors (Larsen et al., 2003a). Several studies have shown that Topo IIα catalytic activity is sensitive to thiol (–SH)-reactive agents such as maleimide, orthoquinone, cisplatin, and other quinones, leading to an inactivation of the enzyme via interactions with cysteine residues (Wang et al., 2001; Jensen et al., 2002; Lu et al., 2005; Chen et al., 2012). The Topo IIα monomer contains 13 cysteine residues and at least five of these cysteines are present principally as reduced free –SH groups (Hasinoff et al., 2005). Sesquiterpene lactones containing an exo-methylene-lactone group are highly reactive with cellular thiols, resulting in alkylation of sulfhydryl residues via Michael type addition (Zhang et al., 2005; Ghantous et al., 2010). As shown in Fig. 1B-b, (−)-xanthatin-mediated Topo IIα inhibition was interfered with by –SH-containing compounds, abrogating (−)-xanthatin’s inhibitory ability (Fig. 3). These results suggest that (−)-xanthatin inhibits the catalytic activity of Topo IIα via interaction with free –SH groups present within the molecule.
In the present analysis, it was not possible to unambiguously delineate the mechanism(s) underlying ROS generation subsequent to (−)-xanthatin exposures. However, given (−)-xanthatin utilizes two pathways for the induction of GADD45γ including (i) Topo IIα inhibition/DNA damage and (ii) ROS production, the demonstration that Topo IIα-inhibitory (+)-8-epi-xanthatin, a cis-isomer of (−)-xanthatin lacking ROS production potential, did not induce GADD45γ expression (Fig. 9E), may imply that ROS generation is a critical modality mediating (−)-xanthatin’s anti-proliferative activities via induction of GADD45γ. These findings provide support for the consideration of (−)-xanthatin as an intriguing chemical entity for inclusion with the other sesquiterpene lactones in future cancer clinical trials (Zhang et al., 2005; Ghantous et al., 2010). In support of this, several possibilities of (−)-xanthatin’s potential for cancer prevention and therapy are being reported (Nibret et al., 2011; Zhang et al., 2012a,b).
Acknowledgements
This work was performed under the Cooperative Research Program of Network Joint Research Center for Materials and Devices [Research No. 2011290, (H.A.)]. This study was supported in part by the Program for Promotion of Basic and Applied Researches for Innovations in Bio-oriented Industry [(BRAIN) (M.S.)], and supported by Grant-in-Aid for Young Scientists (B) [Research Nos. 20790149 and 22790176 (S.T.)] from the Ministry of Education, Culture, Sport, Science and Technology of Japan. This study was also supported by the donation from NEUES Corporation, Japan (H.A.). K.M. also acknowledges the support of the JSPS. C.J.O. was supported by a USPHS award, ES016358.
Abbreviations
- GADD45γ
growth arrest and DNA damage-inducible gene 45γ
- Topo IIα
topoisomerase IIα
- ROS
reactive oxygen species
- NAC
N-acetyl-l-cysteine
Footnotes
Conflicts of interest
No potential conflicts of interest were disclosed.
References
- Adler V, Yin Z, Tew KD, Ronai Z. Role of redox potential and reactive oxygen species in stress signaling. Oncogene. 1999;18:6104–6111. doi: 10.1038/sj.onc.1203128. [DOI] [PubMed] [Google Scholar]
- Bau JT, Kurz EU. Sodium salicylate is a novel catalytic inhibitor of human DNA topoisomerase II alpha. Biochem. Pharmacol. 2011;81:345–354. doi: 10.1016/j.bcp.2010.10.009. [DOI] [PubMed] [Google Scholar]
- Boos G, Stopper H. Genotoxicity of several clinically used topoisomerase II inhibitors. Toxicol. Lett. 2000;116:7–16. doi: 10.1016/s0378-4274(00)00192-2. [DOI] [PubMed] [Google Scholar]
- Bruemmer D, Yin F, Liu J, Berger JP, Sakai T, Blaschke F, Fleck E, Van Herle AJ, Forman BM, Law RE. Regulation of the growth arrest and DNA damage-inducible gene 45 (GADD45) by peroxisome proliferator-activated receptor gamma in vascular smooth muscle cells. Circ. Res. 2003;93:e38–e47. doi: 10.1161/01.RES.0000088344.15288.E6. [DOI] [PubMed] [Google Scholar]
- Chen YT, Collins TR, Guan Z, Chen VB, Hsieh TS. Probing conformational changes in human DNA topoisomerase IIα by pulsed alkylation mass spectrometry. J. Biol. Chem. 2012;287:25660–25668. doi: 10.1074/jbc.M112.377606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chikamori K, Grozav AG, Kozuki T, Grabowski D, Ganapathi R, Ganapathi MK. DNA topoisomerase II enzymes as molecular targets for cancer chemotherapy. Curr. Cancer Drug Targets. 2010;10:758–771. doi: 10.2174/156800910793605785. [DOI] [PubMed] [Google Scholar]
- Depowski PL, Rosenthal SI, Brien TP, Stylos S, Johnson RL, Ross JS. Topoisomerase IIα expression in breast cancer: correlation with outcome variables. Mod. Pathol. 2000;13:542–547. doi: 10.1038/modpathol.3880094. [DOI] [PubMed] [Google Scholar]
- Fortune JM, Osheroff N. Merbarone inhibits the catalytic activity of human topoisomerase IIα by blocking DNA cleavage. J. Biol. Chem. 1998;273:17643–17650. doi: 10.1074/jbc.273.28.17643. [DOI] [PubMed] [Google Scholar]
- Ghantous A, Gali-Muhtasib H, Vuorela H, Saliba NA, Darwiche N. What made sesquiterpene lactones reach cancer clinical trials? Drug Discov. Today. 2010;15:668–678. doi: 10.1016/j.drudis.2010.06.002. [DOI] [PubMed] [Google Scholar]
- Ha HC, Woster PM, Yager JD, Casero RA., Jr The role of polyamine catabolism in polyamine analogue-induced programmed cell death. Proc. Natl. Acad. Sci. U.S.A. 1997;94:11557–11562. doi: 10.1073/pnas.94.21.11557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasinoff BB, Wu X, Krokhin OV, Ens W, Standing KG, Nitiss JL, Sivaram T, Giorgianni A, Yang S, Jiang Y, Yalowich JC. Biochemical and proteomics approaches to characterize topoisomerase IIα cysteines and DNA as targets responsible for cisplatin-induced inhibition of topoisomerase IIα. Mol. Pharmacol. 2005;67:937–947. doi: 10.1124/mol.104.004416. [DOI] [PubMed] [Google Scholar]
- Hsiang YH, Liu LF. Evidence for the reversibility of cellular DNA lesion induced by mammalian topoisomerase II poisons. J. Biol. Chem. 1998;264:9713–9715. [PubMed] [Google Scholar]
- Jensen LH, Renodon-Corniere A, Wessel I, Langer SW, Søkilde B, Carstensen EV, Sehested M, Jensen PB. Maleimide is a potent inhibitor of topoisomerase II in vitro and in vivo: a new mode of catalytic inhibition. Mol. Pharmacol. 2002;61:1235–1243. doi: 10.1124/mol.61.5.1235. [DOI] [PubMed] [Google Scholar]
- Label CP, Ischiropoulos H, Bondy SC. Evaluation of the probe 2′,7′-dichlorofluorescin as an indicator of reactive oxygen species formation and oxidative stress. Chem. Res. Toxicol. 1992;5:227–231. doi: 10.1021/tx00026a012. [DOI] [PubMed] [Google Scholar]
- Lagarkova MA, Iarovaia OV, Razin SV. Large-scale fragmentation of mammalian DNA in the course of apoptosis proceeds via excision of chromosomal DNA loops and their oligomers. J. Biol. Chem. 1995;270:20239–20241. doi: 10.1074/jbc.270.35.20239. [DOI] [PubMed] [Google Scholar]
- Larsen AK, Escargueil AE, Skladanowski A. Catalytic topoisomerase II inhibitors in cancer therapy. Pharmacol. Ther. 2003a;99:167–181. doi: 10.1016/s0163-7258(03)00058-5. [DOI] [PubMed] [Google Scholar]
- Larsen AK, Escargueil AE, Skladanowski A. From DNA damage to G2 arrest: the many roles of topoisomerase II. Prog. Cell Cycle Res. 2003b;5:295–300. [PubMed] [Google Scholar]
- Li TK, Chen AY, Yu C, Mao Y, Wang H, Liu LF. Activation of topoisomerase II-mediated excision of chromosomal DNA loops during oxidative stress. Genes Dev. 1999;13:1553–1560. doi: 10.1101/gad.13.12.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mah LJ, El-Osta A, Karagiannis TC. γH2AX: a sensitive molecular marker of DNA damage and repair. Leukemia. 2010;24:679–686. doi: 10.1038/leu.2010.6. [DOI] [PubMed] [Google Scholar]
- Matsuo K, Ohtsuki K, Yoshikawa T, Shisho K, Yokotani-Tomita K, Shinto M. Total synthesis of xanthanolides. Tetrahedron. 2010;66:8407–8419. [Google Scholar]
- Nibret E, Youns M, Krauth-Siegel RL, Wink M. Biological activities of xanthatin from xanthium strumarium leaves. Phytother. Res. 2011;25:1883–1890. doi: 10.1002/ptr.3651. [DOI] [PubMed] [Google Scholar]
- Park I, Avraham HK. Cell cycle-dependent DNA damage signaling induced by ICRF-193 involves ATM, ATR, CHK2, and BRCA1. Exp. Cell Res. 2006;312:1996–2008. doi: 10.1016/j.yexcr.2006.02.029. [DOI] [PubMed] [Google Scholar]
- Piovano M, Chamy MC, Garbarino JA, Quilhot W. Secondary metabolites in the genus sticta (lichens) Biochem. Syst. Ecol. 2000;28:589–590. doi: 10.1016/s0305-1978(99)00092-7. [DOI] [PubMed] [Google Scholar]
- Quillet-Mary A, Jaffrézou JP, Mansat V, Bordier C, Naval J, Laurent G. Implication of mitochondrial hydrogen peroxide generation in creamed-induced apoptosis. J. Biol. Chem. 1997;272:21388–21395. doi: 10.1074/jbc.272.34.21388. [DOI] [PubMed] [Google Scholar]
- Rishi AK, Sun RJ, Gao Y, Hsu CK, Gerald TM, Sheikh MS, Dawson MI, Reichert U, Shroot B, Fornace AJ, Jr, Brewer G, Fontana JA. Post-transcriptional regulation of the DNA damage-inducible gadd45 gene in human breast carcinoma cells exposed to a novel retinoid CD437. Nucleic. Acids Res. 1999;27:3111–3119. doi: 10.1093/nar/27.15.3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roussakis C, Chinou I, Vayas C, Harvala C, Verbist JF. Cytotoxic activity of xanthatin and the crude extracts of Xanthium strumarium. Planta Med. 1994;60:473–474. doi: 10.1055/s-2006-959537. [DOI] [PubMed] [Google Scholar]
- Solovyan VT, Bezvenyuk ZA, Salminen A, Austin CA, Courtney MJ. The role of topoisomerase II in the excision of DNA loop domains during apoptosis. J. Biol. Chem. 2002;277:21458–21467. doi: 10.1074/jbc.M110621200. [DOI] [PubMed] [Google Scholar]
- Stanulla M, Wang J, Chervinsky DS, Thandla S, Aplan PD. DNA cleavage within the MLL breakpoint cluster region is a specific event which occurs as part of higher-order chromatin fragmentation during the initial stages of apoptosis. Mol. Cell. Biol. 1997;17:4070–4079. doi: 10.1128/mcb.17.7.4070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steuerwald N, Cohen J, Herrera RJ, Brenner CA. Quantification of mRNA in single oocytes and embryos by real-time rapid cycle fluorescence monitored RT-PCR. Mol. Hum. Reprod. 2000;6:448–453. doi: 10.1093/molehr/6.5.448. [DOI] [PubMed] [Google Scholar]
- Takeda S, Ishii Y, Iwanaga M, Nurrochmad A, Ito Y, Mackenzie PI, Nagata K, Yamazoe Y, Oguri K, Yamada H. Interaction of cytochrome P450 3A4 and UDP-glucuronosyltransferase 2B7: evidence for protein-protein association and possible involvement of CYP3A4 J-helix in the interaction. Mol. Pharmacol. 2009a;75:956–964. doi: 10.1124/mol.108.052001. [DOI] [PubMed] [Google Scholar]
- Takeda S, Yamamoto I, Watanabe K. Modulation of (9-Tetrahydrocannabinol-induced MCF-7 breast cancer cell growth by cyclooxygenase and aromatase. Toxicology. 2009b;259:25–32. doi: 10.1016/j.tox.2009.01.024. [DOI] [PubMed] [Google Scholar]
- Takeda S, Matsuo K, Yaji K, Okajima-Miyazaki S, Harada M, Miyoshi H, Okamoto Y, Amamoto T, Shindo M, Omiecinski CJ, Aramaki H. (−)-Xanthatin selectively induces and stimulates capsize-independent cell death in human breast cancer MDA-MB-231 cells. Chem. Res. Toxicol. 2011;24:855–865. doi: 10.1021/tx200046s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Mao Y, Chen AY, Zhou N, LaVoie EJ, Liu LF. Stimulation of topoisomerase II-mediated DNA damage via a mechanism involving protein thiolation. Biochemistry. 2001;40:3316–3323. doi: 10.1021/bi002786j. [DOI] [PubMed] [Google Scholar]
- Ying J, Srivastava G, Hsieh WS, Gao Z, Murray P, Liao SK, Ambinder R, Tao Q. The stress-responsive gene GADD45G is a functional tumor suppressor, with its response to environmental stresses frequently disrupted epigenetically in multiple tumors. Clin. Cancer Res. 2005;11:6442–66449. doi: 10.1158/1078-0432.CCR-05-0267. [DOI] [PubMed] [Google Scholar]
- Zechiedrich EL, Osheroff N. Eukaryotic topoisomerases recognize nucleic acid topology by preferentially interacting with DNA crossovers. EMBO J. 1990;9:4555–4562. doi: 10.1002/j.1460-2075.1990.tb07908.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerbini LF, Liebermann TA. GADD45 deregulation in cancer: frequently methylated tumor suppressors and potential therapeutic targets. Clin. Cancer Res. 2005;11:6409–6413. doi: 10.1158/1078-0432.CCR-05-1475. [DOI] [PubMed] [Google Scholar]
- Zhang F, Lau SS, Monks TJ. The cytoprotective effect of N-acetyl-l-cysteine against ROS-induced cytotoxicity is independent of its ability to enhance glutathione synthesis. Toxicol. Sci. 2011;120:87–97. doi: 10.1093/toxsci/kfq364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Ruan J, Yan L, Li W, Wu Y, Tao L, Zhang F, Zheng S, Wang A, Lu Y. Xanthatin induces cell cycle arrest at G2/M checkpoint and apoptosis via disrupting NF-κB pathway in A549 non-small-cell lung cancer cells. Molecules. 2012a;26:3736–3750. doi: 10.3390/molecules17043736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Tao L, Ruan J, Li W, Wu Y, Yan L, Zhang F, Fan F, Zheng S, Wang A, Lu Y. Xanthatin induces G2/M cell cycle arrest and apoptosis in human gastric carcinoma MKN-45 cells. Planta Med. 2012b;78:890–895. doi: 10.1055/s-0031-1298481. [DOI] [PubMed] [Google Scholar]
- Zhang S, Won YK, Ong CN, Shen HM. Anti-cancer potential of sesquiterpene lactones: bioactivity and molecular mechanisms. Curr. Med. Chem. Anticancer Agents. 2005;5:239–249. doi: 10.2174/1568011053765976. [DOI] [PubMed] [Google Scholar]
- Zheng X, Zhang Y, Chen YQ, Castranova V, Shi X, Chen F. Inhibition of NF-κB stabilizes gadd45α mRNA. Biochem. Biophys. Res. Commun. 2005;329:95–99. doi: 10.1016/j.bbrc.2005.01.105. [DOI] [PubMed] [Google Scholar]