Significance
More than 70 years ago, Monod described the phenomenon of diauxic growth of bacteria, the observation that in the presence of two alternative sugars, cells first use one of them and then, after a short lag phase, switch to the other. Until now it had been assumed that all cells in a population engage in the outgrowth on the second sugar after major metabolic adaptation of enzymatic composition has occurred, which takes time (hence the lag phase in growth). Here, we show that actually only a subpopulation is fit enough to partake in the second growth phase and present an evolutionary model, suggesting that this phenomenon might entail a bet-hedging strategy that helps bacteria adapt to the unexpectedly changing environment.
Keywords: phenotypic heterogeneity, Gram-positive bacteria, metabolic fitness
Abstract
When bacteria grow in a medium with two sugars, they first use the preferred sugar and only then start metabolizing the second one. After the first exponential growth phase, a short lag phase of nongrowth is observed, a period called the diauxie lag phase. It is commonly seen as a phase in which the bacteria prepare themselves to use the second sugar. Here we reveal that, in contrast to the established concept of metabolic adaptation in the lag phase, two stable cell types with alternative metabolic strategies emerge and coexist in a culture of the bacterium Lactococcus lactis. Only one of them continues to grow. The fraction of each metabolic phenotype depends on the level of catabolite repression and the metabolic state-dependent induction of stringent response, as well as on epigenetic cues. Furthermore, we show that the production of alternative metabolic phenotypes potentially entails a bet-hedging strategy. This study sheds new light on phenotypic heterogeneity during various lag phases occurring in microbiology and biotechnology and adjusts the generally accepted explanation of enzymatic adaptation proposed by Monod and shared by scientists for more than half a century.
In nature, bacteria are confronted with a wide range of environmental conditions that change over time. These conditions often elicit specific metabolic responses that increase the division rate of a cell. For example, when bacterial cells are exposed to multiple sugars, they do not metabolize all sugars simultaneously, but rather use the sugar that allows the highest cell-division rate. Cells switch to the less-preferred sugar when the most-preferred one (in many cases glucose) is depleted. Jacques Monod coined this phenomenon “diauxie” (1). Diauxie is characterized by two growth cycles—the first one on the preferred sugar, followed by a second one on the less-preferred sugar. Both are separated by a short period during which the population apparently does not grow. This period is known as the diauxie lag phase. It is typically assumed that cells need time to make the necessary enzymatic adaptations to switch from one substrate to another (2). However, the behavior of individual cells during the lag phase has not been studied in detail. Here, we examine the diauxic shift at the single-cell level in Lactococcus lactis using time-lapse microscopy, in addition to the traditional approach of studying population growth characteristics. Surprisingly, the lag phase at the switch from glucose to cellobiose consumption by L. lactis largely results from the heterogeneous response of cells to the environmental change, rather than the time it takes for cells to make the necessary metabolic adaptations. This result challenges Monod’s view of enzymatic adaptation and asks for a revision on the interpretation of population lag phases.
Results
Phenotypic Heterogeneity During Diauxie.
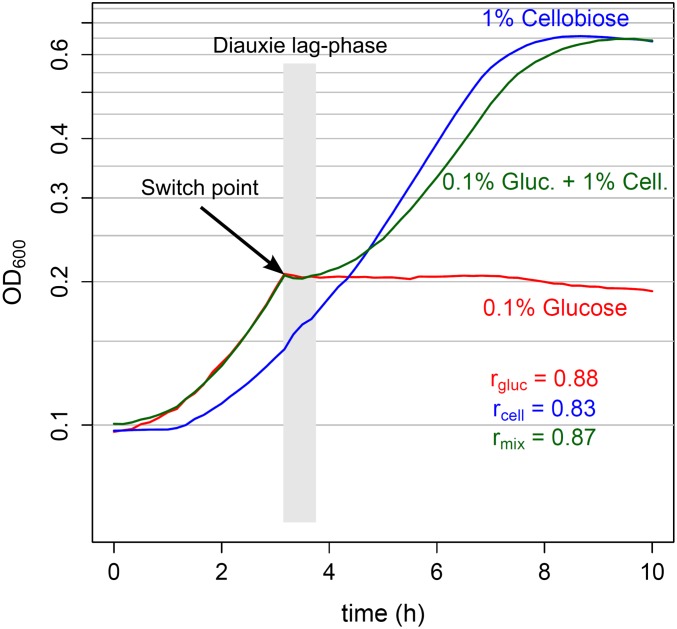
L. lactis M1 is a derivative of L. lactis MG1363, which grows well on cellobiose because of the activation of the Pcel promoter of the cellobiose uptake system IIC component CelB. An active cellobiose/lactose phosphotransferase system (PTSCel/Lac) consists of proteins PtcAB and CelB (3). A diauxic shift is seen when L. lactis M1 is propagated in a medium supplemented with both glucose (0.1%) and cellobiose (1%) (G-C medium; Fig. 1, green line). After a period of exponential growth, the bacterial culture stops growing at the same optical density at 600 nm (OD600), which is reached when the medium only contains glucose (Fig. 1, red line). After a lag phase of no or only little population growth following the switch point, apparent population growth is resumed, eventually leading to a density that is reached when the medium only contains cellobiose (Fig. 1, blue line). In this study we mainly focus on the switch from glucose to cellobiose consumption, although diauxie is also observed in a medium with glucose and lactose (SI Appendix, Fig. S1).
Fig. 1.
L. lactis diauxic shift. Growth (OD600) of L. lactis M1 in chemically defined medium with 0.1% glucose (red line), 1% cellobiose (blue line), or a mixture of 0.1% glucose and 1% cellobiose (green line) is shown; maximal growth rates (r) measured along the growth curves of the cultures are shown in the right lower corner. During biphasic growth in a medium with a mixture of glucose and cellobiose, cells first consume glucose. The diauxie lag phase, which follows the switch point after glucose depletion, is generally thought to result from adaptation of the metabolism of cells to using the second sugar (in this case, cellobiose). During the second exponential growth phase, cellobiose is used.
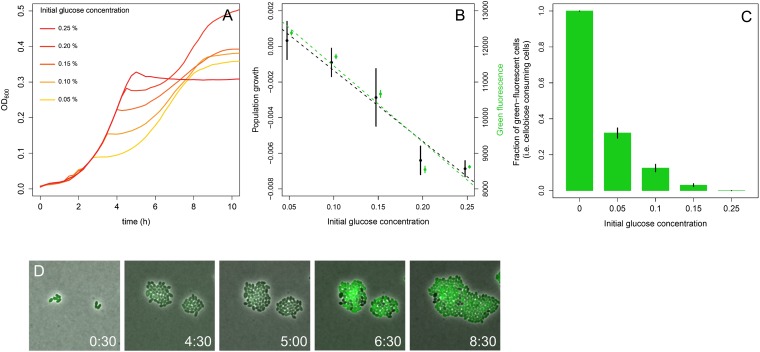
Fig. 2A shows the growth of L. lactis M1 in G-C medium containing 1% cellobiose and a varying concentration of glucose. As expected, the switch from glucose to cellobiose utilization occurs earlier when the initial glucose concentration is lower. We visualized population growth by plotting the change in OD600 of a culture as the difference between two subsequent OD600 measurements (SI Appendix, Fig. S2 B and D). Interestingly, just at the switch point (arrow in Fig. 1), the OD drops; thus, the population growth value (the change in OD between two points in a growth curve) becomes negative. When we plotted this lowest population growth value for each glucose concentration (Fig. 2B, black dots), it appeared to be negatively correlated to the initial glucose concentration—i.e., at the switch point, the lag phase is more pronounced when cells grow initially on higher glucose concentrations. If the whole population would switch from glucose to cellobiose consumption, the opposite were to be expected—the higher the initial glucose concentration, the higher the population density would be at the switch point. This process would result in a higher population growth rate on cellobiose because the population growth rate is the product of the population density and growth rate of the individual cell, given that the cellobiose concentration is very high and similar in all cases. We therefore speculated that only a fraction of the population commits to cellobiose consumption and that the size of this subpopulation depends on the glucose concentration during the first period of growth.
Fig. 2.
Effects of initial glucose concentration on the L. lactis shift to growth on cellobiose. (A) Growth (OD600) of L. lactis M1 in CDM, with various concentrations of glucose (0.05–0.25%; orange to red) and 1% cellobiose. (B) L. lactis M1gfp population growth rate expressed by the change in OD of a culture—i.e., the difference between two subsequent OD600 measurements (shown in SI Appendix, Fig. S2) (black; df = 13; R2 = 0.88; P = 2.267 × 10−7) after the switch point is negatively related to the initial glucose concentration in the medium. The same holds for the intensity of green fluorescence of the whole population (green; df = 13; R2 = 0.93; P = 1.03 × 10−8). Curiously, just after glucose depletion, the culture density slightly drops for all glucose–cellobiose combinations, resulting in negative values for population growth (see also SI Appendix, Fig. S2). Based on our microscopy data, it cannot be explained by cell lysis. This drop in culture OD is always observed at the transition point before L. lactis enters the stationary phase and is not a specific characteristic of diauxie. Rather, it is an intrinsic L. lactis property, probably attributable to cell-structure changes. However, the level of the OD drop (lowest population growth value) and the population growth rate during the second exponential growth phase correlates well with initial glucose concentration. (C) The fraction of cellobiose-using cells, determined on the basis of fluorescent microscopy from liquid culture samples (of identical experiments to those in A and B), also decreases with the increase of the initial glucose concentration. See also SI Appendix, Fig. S2. (D) Snapshots of the time-lapse experiment, performed in glucose and cellobiose containing CDM, illustrating appearance (at 6 h 30 min) and coexistence of two stable phenotypes: cellobiose-consuming (green cells) and nongrowing (black cells) (Movie S1). Error bars are means ± SD of three independent measurements.
We investigated how many cells activate cellobiose utilization as a function of the initial glucose concentration. Pcel is a promoter of the gene cluster llmg_0186celB, coding for the IIC component of the PTSCel/Lac. L. lactis M1gfp cells, in which Pcel was fused to the green fluorescent protein (GFP) gene, express GFP when they take up and metabolize cellobiose. The fluorescence intensity of the culture negatively correlates with the initial glucose concentration in the medium (Fig. 2B, green dots): The higher the glucose concentration, the lower the intensity. Using time-lapse fluorescence microscopy, we studied the diauxic shift at the single-cell level (Fig. 2D and Movies S1 and S2). Cells of an isogenic population of L. lactis M1gfp growing on a mixture of glucose and cellobiose do indeed show a heterogeneous response to the change in glucose availability. At the switch point, the population differentiates into two stable phenotypes, dividing and nondividing cells. These cells are furthermore characterized by the activity of Pcel (and the green fluorescence of GFP) being either ON or OFF, respectively. Cells in which Pcel is ON (Cel+ cells) express the cellobiose transporter CelB and are able to import cellobiose, use it, and grow; conversely, cells in which Pcel is OFF (Cel− cells) are not able to metabolize cellobiose and, consequently, do not divide. Fluorescence microscopy revealed that, in line with our earlier conclusion, the fraction of Cel+ cells is negatively related to the initial glucose concentration in the medium (Fig. 2C). Although this fraction strongly reflects the initial glucose concentration (SI Appendix, Fig. S3), it is not affected by the initial cellobiose concentration: Even very little cellobiose (0.01%) in combination with 0.05% glucose results in the same fraction of Cel+ cells as 1% of cellobiose with 0.05% glucose (SI Appendix, Fig. S4A). In conclusion, the microscopy results, which are confirmed by flow-cytometry data (SI Appendix, Fig. S4B), support our hypothesis that the diauxie lag phase results from a glucose concentration-dependent heterogeneous response of the population at the switch point.
The Regulation of Phenotypic Heterogeneity.
How can the emergence of phenotypic heterogeneity during the diauxic shift be explained? It is well known that the order in which bacterial cells use multiple sugars depends on the global regulatory system of carbon catabolite repression (CCR) (4–7). This mechanism allows the bacteria to first consume the sugar that supports the highest growth rate (most often glucose) by shutting down expression of alternative sugar utilization pathways. The fact that the heterogeneity in L. lactis M1 diauxie strongly correlates with the initial glucose concentration indicates that CCR might be involved. Indeed, the Pcel promoter region contains two binding sites (cre sites) for the CCR transcriptional regulator CcpA (SI Appendix, Fig. S5) (3). We deleted the ccpA gene from the chromosome of L. lactis M1gfp to examine whether population heterogeneity also occurs in the absence of CCR. The resulting strain neither shows diauxic growth in G-C medium nor exhibits population heterogeneity in the consumption of cellobiose. Instead of first consuming glucose, all cells immediately also start using cellobiose (Fig. 3; SI Appendix, Fig. S6; and Movie S3).
Fig. 3.
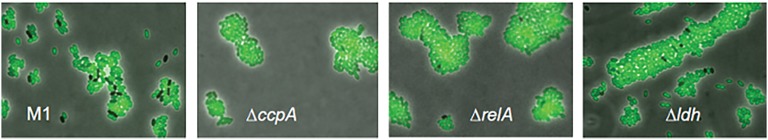
Deletion of ccpA, relA, or ldh from the chromosome of L. lactis M1gfp increases the fraction of Cel+ cells (green). Snapshots of time-lapse and -course experiments performed in G-C (0.1–1%) medium with different M1gfp deletion mutants. Overlays of phase-contrast and green fluorescence images are shown. The clumps of cells in the microscopy pictures resulted from their growth on agarose pads during time-lapse experiments and were chosen intentionally to show more cells in one picture. Neither L. lactis M1 nor its parent strain MG1363 forms aggregates under the conditions used in our experiments.
These data confirm that CCR in L. lactis is relieved at the switch point, after glucose is exhausted. This step then allows expression of the cellobiose gene cluster and growth of the cells on cellobiose. However, CCR is not the only factor in determining a cell’s capacity to switch, and it is not the only determinant of the heterogeneity observed because, after glucose depletion and consequential relief of CCR, eventually all cells would start consuming cellobiose. In contrast, we observe two stable phenotypes: cells that have switched and use cellobiose and cells that never make the switch (Fig. 2 and Movies S1 and S2).
Expression of the cellobiose gene cluster, production and assembly of the transporter, and import of the sugar are costly and depend on the energetic state of the cell (8, 9) (SI Appendix, Fig. S5). The nongrowing phenotype of Cel− cells suggests that their energetic state around the moment of glucose depletion may be too low to allow making the necessary investments in cellobiose utilization. It is important to note that the Cel− cells are not dead—they readily divide when supplemented with glucose (Movie S4). The Cel− cells probably remain viable because of induction of the stringent response, a protective mechanism that inhibits major energy-consuming processes in a coordinated manner as soon as bacterial cells encounter adverse conditions such as nutrient limitation or several other stresses (10). Stringent response has been observed during a lag phase in glucose–lactose diauxie of Escherichia coli (11) and carbon starvation in Staphylococcus aureus (12). The stringent response factor RelA produces the phosphorylated purine-derived alarmones (p)ppGpp in response to the presence of uncharged tRNA molecules (13–15). Stringent response depending on RelA has also been observed in L. lactis (16). An L. lactis relA knockout mutant would be expected to be unable to mount a RelA-dependent response. Postponing the stringent response might allow cells to allocate their last energy sources to the switch to cellobiose consumption. In this scenario, only those cells that are already completely energy deprived would stay Cel−. Indeed, when relA was deleted from the chromosome of L. lactis M1gfp, the fraction of Cel+ cells increased significantly for all combinations of glucose and cellobiose tested (Fig. 3, Movie S5, and SI Appendix, Fig. S7). The Cel− cells remaining in a culture of L. lactis M1gfpΔrelA upon glucose addition resume growth, only very slowly compared with the Cel− cells of L. lactis M1gfp. Interestingly, the fact that the relA mutant generates a larger fraction of Cel+ cells than the wild-type indicates that the metabolic switch is governed by a cellular decision—namely, to induce stringent response, rather than mere energy exhaustion. To further investigate the effect of the stringent response, we tested whether addition of a small amount of an energy source at the switch point could delay the response and prolong the time of cells to decide to become Cel+. Addition of 0.01% glucose at the switch point of an L. lactis M1gfp culture growing in G-C medium indeed increases the fraction of Cel+ cells (SI Appendix, Fig. S8).
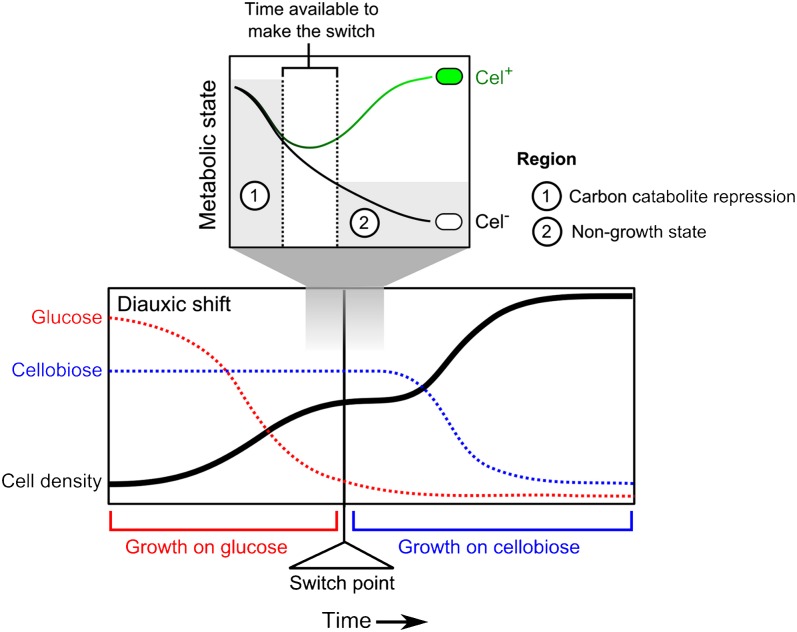
In conclusion, we propose that, upon glucose depletion, L. lactis cells only have limited time to switch from glucose to cellobiose consumption (Fig. 4). This window in time is defined by the point at which CCR is relieved in a cell until the moment that the (p)ppGpp level gets high, and the stringent response results in a cell entering into a nongrowth state. Cel+ cells express the cellobiose utilization system and switch to cellobiose consumption in a timely manner, replenishing their energy levels and avoiding the nongrowth state caused by the stringent response. Cel− cells do not make this switch on time, run out of energy after glucose is completely depleted, and enter the protective nongrowing state induced by the stringent response until another carbon source that is easy to metabolize (e.g., glucose or galactose) becomes available. This hypothesis could explain why the fraction of Cel− cells is larger for higher initial glucose concentrations. When there is more glucose in the medium, the population density at the switch point is higher and glucose depletes faster. Thus, the stringent response is triggered sooner, and, consequently, the window during which cells can switch is smaller (Figs. 2 and 4). On top of this hypothesis, accumulation or depletion of other factors in the medium could play a role in earlier induction of the stringent response when the cell density is higher.
Fig. 4.
Putative mechanism underlying the phenotypic heterogeneity in L. lactis sugar utilization. At the moment of glucose exhaustion from the medium (the switch point), the CCR level in a cell decreases. Once the repression is relieved, a cell can start expressing to the cel cluster and switch to cellobiose consumption, but it must have sufficient energy (“metabolic state”) to do so. This switch, however, is only possible if the cell switches early enough. If the cell runs out of energy before it makes the switch, the stringent response locks the cell in a nongrowing state (Cel−). If a cell is able to make the switch, it continues to grow using cellobiose (Cel+).
Given that the energetic state of a cell is very important in that cell’s ability to switch to another sugar, our next aim was to determine how the differences in metabolic capacity of cells arise. It is well known that L. lactis can activate two metabolic routes: homolactic and heterolactic fermentation. The homolactic pathway is faster but less efficient than the heterolactic pathway (less ATP molecules are produced per glucose molecule; SI Appendix, Fig. S5). When L. lactis grows on glucose, it mainly produces lactate (homolactic fermentation), whereas upon slower growth on cellobiose, it shifts to the energetically more-efficient heterolactic fermentation (17, 18). An essential enzyme in the latter pathway is acetate kinase AckA. The reaction catalyzed by this enzyme yields ATP; therefore, the expression strength of AckA can be considered as indicative of the ATP level, or metabolic fitness.
The promoter of ackA was shown to contain a cre site in L. lactis. Deletion of ccpA increased expression of the gene (7). We monitored ackA expression in time using the M1PackA-gfp strain. At the diauxie switch the green fluorescence of individual L. lactis M1PackA–gfp cells varies. To correlate PackA expression (and ATP level) of a single cell with a Cel+ or Cel− phenotype, we constructed a double-labeled strain: Besides PackA–gfp, we integrated a Pcel–mCherry into the chromosome of L. lactis M1. Only cells with a high enough PackA–gfp expression at the time of glucose exhaustion are able to switch to cellobiose consumption and become red fluorescent (activity of Pcel–mCherry) (SI Appendix, Fig. S9).
Early induction of acetate kinase and the heterolactic fermentation pathway might be important in obtaining a metabolic state high enough to allow switching to cellobiose consumption, through the additional ATP gained. To test whether heterolactic fermentation plays a role in determining the fraction of Cel+ cells, the ldh gene encoding the main enzyme of the homolactic fermentation pathway, lactate dehydrogenase, was deleted from the chromosome. L. lactis M1gfpΔldh only performs heterolactic fermentation and grows slower than its parent (Fig. 3 and SI Appendix, Fig. S10). During glucose–cellobiose diauxie, the fraction of Cel+ cells in the ldh deletion strain significantly increases for all sugar combinations tested. These observations confirm that the metabolic strategy that a cell performs at the moment of glucose depletion plays an important role in the cell’s ability to switch.
Epigenetics.
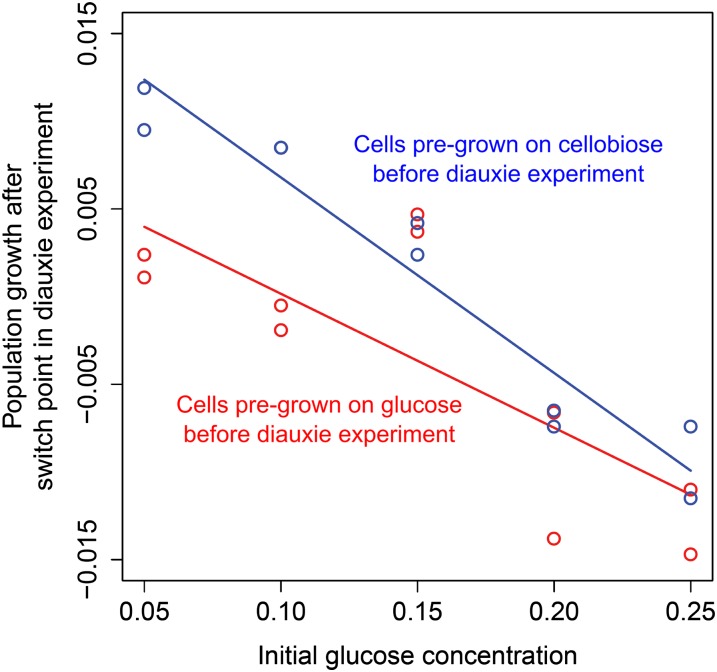
To investigate possible preculture effects on the fraction of switching cells, we compared the diauxic growth characteristics of cells originating from glucose- or cellobiose-containing medium. L. lactis M1 cells from a cellobiose preculture will most likely possess several copies of the cellobiose transporter, even after a number of divisions on glucose. The presence of cellobiose transporters in a cell before the switch could facilitate sensing of and responding to cellobiose at the switch point. Our experimental data appear to confirm this hypothesis: Cells precultured in a medium with cellobiose exhibited a shorter lag phase between the two sugar utilization phases in G-C medium than those pregrown with glucose (Fig. 5 and SI Appendix, Fig. S2). As shown above, a shorter lag phase could be explained by a higher fraction of cells that switch to cellobiose utilization. This finding suggests the involvement of an epigenetic mechanism in metabolic switching. An epigenetic memory has been described for expression of the lactose operon lac of E. coli, which lasted for two cell generations, presumably because of expression bursts of the regulator and transporter genes involved (19).
Fig. 5.
Epigenetic effects influence the decision making of L. lactis cells. Comparison of population growth rate after the switch point for populations precultured in CDM with cellobiose (blue dots and line) and those precultured in CDM containing glucose (red dots and line) is shown. A higher fraction of cellobiose precultured cells switch to cellobiose consumption after the switch point for all initial glucose concentrations tested compared with glucose precultured cells (df = 17; R2 = 0.7877; Pinitial glucose conc. = 9.407 × 10−7; Ppreculture effect = 0.014). See also SI Appendix, Fig. S2.
Fig. 5 shows that preculture conditions have the strongest effect on the fraction of Cel+ cells when the initial glucose concentration in the G-C medium is low—in other words, when only a few cell divisions are made before glucose is depleted. This finding can be explained by the fact that, during cell division, together with the membrane, a substantial fraction of the membrane proteome is being passed on to the progeny. The fewer cell divisions that occur before the change of carbon source, the more inherited cellobiose transporters that are present on the surface of a cell. To confirm this hypothesis, we placed celB on a plasmid under control of the nisin-inducible promoter PnisA and overexpressed it in L. lactis M1 carrying chromosomally integrated nisRK to allow expression of PnisA. When cells were grown in G-C medium with inducing amounts of nisin, no diauxie lag phase was observed (SI Appendix, Fig. S2F). When celB was overexpressed before transfer of cells to G-C medium, the diauxie lag phase became significantly shorter. This result suggests that the number of transporters produced in a cell during preculture with nisin was sufficient to support an immediate switch to cellobiose after glucose was depleted. We conclude that the destiny of a cell does not only depend on the environment of the cell but is also affected epigenetically by the environment to which the mother cell was exposed.
Heterogeneity as a Bet-Hedging Strategy.
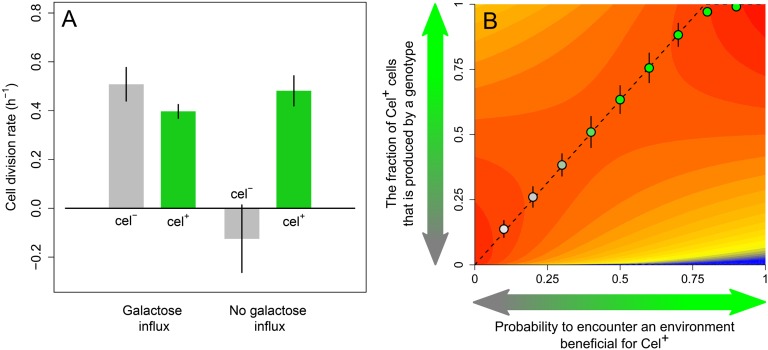
From an evolutionary perspective, the occurrence of Cel− cells seems puzzling: Why do Cel− cells not relieve CCR sooner to gain sufficient time to switch to cellobiose consumption? Might Cel− cells have a fitness benefit when supplemented with alternative sugars? To examine this possibility, we transferred a mixture of Cel+ and Cel− cells to a microscopy slide containing solidified medium with galactose as the sole carbon source. Indeed, we observed that the Cel− cells divided faster than the Cel+ cells on this alternate sugar (Movie S6). Apparently, there is a tradeoff: Cells that grow well on cellobiose (Cel+) perform worse on galactose than Cel− cells (Fig. 6A). Slower growth of Cel+ cells on galactose might be explained by the burden of expressing transporters and enzymes needed for cellobiose utilization, which are useless in galactose consumption.
Fig. 6.
Heterogeneity as a bet-hedging strategy. (A) Cell-division rates calculated from the time-lapse movies (Movies S1, S2, and S6) for L. lactis M1 Cel+ and Cel− cells (n = 3). (B) Geometric mean fitness (blue, low; red, high) as a function of the fraction of Cel+ cells produced by a genotype. The adaptive landscape shows the performance of genotypes (vertical axis) for various environmental conditions (horizontal axis) characterized by the probability P that conditions favor Cel+ cells. Dotted line indicates the best-performing genotypes. All genotypes that do not exclusively produce Cel− or Cel+ cells represent bet-hedging strategies. The evolved genotypes, from agent-based simulation, are superimposed on the adaptive landscape (circles, average evolved genotype; error bars, SD; n = 100; see SI Appendix for details).
The difference in performance of Cel+ and Cel− cells on various substrates suggests that the observed population heterogeneity could be the result of adaptive evolution. A genotype that produces a mixture of Cel+ and Cel− cells might have an advantage over one that produces only Cel+ or only Cel− cells when future environmental conditions are unpredictable. This advantage is because the former genotype reduces the variation in fitness over time, thereby maximizing its geometric fitness (SI Appendix).
To examine whether such a bet-hedging strategy (20) could indeed evolve, we constructed a model based on the growth rates of Cel− and Cel+ cells as derived from the time-lapse microscopy data (Movies S1, S2, and S6) and plotted the corresponding fitness landscape (Fig. 6B). For a variety of environments characterized by the probability P that conditions are favorable for Cel+ cells (i.e., galactose influx occurs late after the switch point), it indicates the fitness of a spectrum of genotypes. Each genotype is characterized by the fraction α of cells that become Cel+ following diauxie. A genotype producing a homogeneous population of Cel+ cells corresponds to α = 1; that generating only Cel− cells corresponds to α = 0. Fig. 6B shows that, for most environmental conditions (i.e., for most values of P), an intermediate value of α results in the highest fitness. In other words, a heterogeneous population, corresponding to a bet-hedging strategy, has a selective advantage. As indicated by the dashed line in Fig. 6B, the optimal value of α (i.e., the optimal fraction of Cel+ cells) increases linearly with the probability P that the environment is profitable for Cel+ cells. The predictions based on the fitness landscape are in good agreement with individual-based evolutionary simulations (Fig. 6B; see SI Appendix for modeling details). It is therefore likely that the population heterogeneity described in this study—and the underlying molecular mechanisms—are the result of natural selection.
Discussion
In this study, we examined the mechanisms that underlie diauxie in L. lactis. During the diauxic shift from glucose to a less-preferable carbon source like cellobiose, L. lactis differentiates into two distinct phenotypic subpopulations. One subpopulation stops dividing, whereas the other continues to divide and grow on the second carbon source. Our findings adjust the conventional concept of diauxie lag phase. The lag phase in the population growth curve of L. lactis is not a result of a temporal growth arrest of the whole population, but is caused by the presence of a nongrowing subpopulation of cells in the culture.
We propose that this phenotypic heterogeneity results from the differential capacity of cells to deal with the time constraint between CCR relief needed to grow on the second sugar and the activation of the stringent response. The metabolic state of individual cells determines whether the stringent response is induced and whether they can make the switch to cellobiose consumption. The fraction of switching cells furthermore depends on epigenetic cues, such as preculture conditions.
One of the best-studied examples of heterogeneity is the lac operon of E. coli. It has been shown in the lac operon that artificial, nonmetabolizable inducers can trigger a heterogeneous response in the E. coli population (19, 21–23). In contrast to these studies, two stable alternative metabolic strategies emerge and coexist in L. lactis population under natural conditions of diauxie. A variation in growth rates has recently been shown to occur in an E. coli population during diauxic shift as a result of stochastic transcription bursts of the permease gene lacY, a finding that is quite different from our system (24). Stringent response and CCR have been implicated in glucose–lactose diauxie of E. coli, but only at the level of the whole population (11). Our study in L. lactis demonstrates that the outcomes of the two global regulatory processes can vary at the single-cell level, creating populations of cells that behave differently. In fact, a stochastic increase in (p)ppGpp levels in single cells has been associated with the persister phenotype in E. coli (25). Cells with elevated (p)ppGpp levels slowed down their metabolism via activation of toxin–antitoxin loci and became insensitive to antibiotics affecting growing cells. A microstarvation model was proposed to explain these bursts of (p)ppGpp and subsequent activation of toxin–antitoxin modules in individual cells (25). Nutrient availability varies in a culture, exposing some cells to starvation conditions that induce the stringent response. Notably, an increase in persister formation was observed when E. coli was subjected to a shift in carbon sources (26). In this study, the E. coli RelA/SpoT couple was proposed to act as a toxin/antitoxin system locking cells in a condition of stasis. L. lactis does not possess a SpoT analog—RelA exhibits both (p)ppGpp synthesis and degradation activities (16, 27). Nevertheless, the stringent response-induced nongrowth state of E. coli and that of Cel− cells of L. lactis appear to have similarities.
The stringent responses in E. coli and L. lactis differ significantly with respect to their effect on central carbon metabolism (28). Instead of activating many catabolic processes—as is the case in E. coli—the stringent response in L. lactis results in the negative control of catabolism, minimizing energy use. Although ppGpp is needed for full induction of lac operon expression in E. coli (29) and stimulates the regulon of the CCR regulator Crp (11), a similar control mechanism has not been observed in Gram-positive bacteria. Differences in regulation are illustrated by the phenotype of the relA-deletion mutants. Although E. coli ΔrelA, despite the presence of the second ppGpp synthetase SpoT, shows a prolonged lag phase during glucose–lactose diauxie (11), the lag phase in glucose–cellobiose diauxie is significantly reduced in L. lactis M1ΔrelA. A comparison of the latter strain with an E. coli relA spoT double knockout is not possible because that strain exhibits auxotrophy for a number of amino acids and requires a specific medium in which diauxie cannot be studied (11).
Heterogeneous population responses are ubiquitous among differentiating bacteria (30–40). L. lactis is not known to initiate any form of differentiation—such as competence development, sporulation, or motility—that occur in many other bacteria. Here, to our knowledge, we show for the first time that heterogeneity plays a major role in the metabolism of this nondifferentiating bacterium. Furthermore, we demonstrate that the observed heterogeneity might represent a bet-hedging strategy. Application of such a strategy to nutrient transitions is previously unidentified. Even though our evolutionary model is based on the growth rates measured during the particular glucose-to-cellobiose switch studied here, it is likely that phenotypic differentiation is also beneficial for other carbon source combinations. Importantly, our work shows that many lag phases observed in microbiology and biotechnology might potentially result from heterogeneity, which contrasts the paradigm originally proposed by Monod and embraced by scientists for >70 y.
Materials and Methods
L. lactis strains were grown overnight in a chemically defined medium (CDM) supplemented with glucose or cellobiose (41). Next, they were washed with CDM and diluted 20x in fresh CDM containing cellobiose, glucose, or a mixture of both; growth and fluorescence development were monitored by using a microtiter-plate reader (Tecan Group Ltd.) or FACS (BD Biosciences). Cells for microscopy experiments were cultured in 0.1% glucose-containing CDM until they reached the midexponential growth phase; they were then washed and transferred to a microscopy slide carrying a thin layer of 1.5% (wt/vol) high-resolution agarose (Sigma-Aldrich) with G-C medium (CDM with various concentrations of glucose and 1% cellobiose). Cells were grown in a 30 °C environmental chamber and monitored with an IX71 Microscope (Olympus). Pictures were taken every 10–20 min. Experiments are described in detail in SI Appendix.
Supplementary Material
Acknowledgments
We thank Prof. Dr. Matthias Heinemann and Dr. Jan-Willem Veening for helpful discussions and Dr. Jeroen Siebring for his advice on how to use his developed chromosomal integration system for PackA-gfp. A.S. and H.B. were supported by a Stichting Technische Wetenschappen grant in the scope of Project 10619, “Understanding Preculture-Dependent Growth and Acidification Rates of Lactococcus lactis as the Result of Population Heterogeneity.” J.v.G. is supported by the Gratama Fund and a Netherlands Organization for Scientific Research grant.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1320063111/-/DCSupplemental.
References
- 1.Monod J. The growth of bacterial cultures. Annu Rev Microbiol. 1949;3:371–394. [Google Scholar]
- 2.Stanier RY. Enzymatic adaptation in bacteria. Annu Rev Microbiol. 1951;5:35–56. doi: 10.1146/annurev.mi.05.100151.000343. [DOI] [PubMed] [Google Scholar]
- 3.Solopova A, et al. A specific mutation in the promoter region of the silent cel cluster accounts for the appearance of lactose-utilizing Lactococcus lactis MG1363. Appl Environ Microbiol. 2012;78(16):5612–5621. doi: 10.1128/AEM.00455-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Loomis WF, Jr, Magasanik B. Glucose-lactose diauxie in Escherichia coli. J Bacteriol. 1967;93(4):1397–1401. doi: 10.1128/jb.93.4.1397-1401.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saier MH, Jr, et al. Catabolite repression and inducer control in Gram-positive bacteria. Microbiology. 1996;142(Pt 2):217–230. doi: 10.1099/13500872-142-2-217. [DOI] [PubMed] [Google Scholar]
- 6.Stülke J, Hillen W. Carbon catabolite repression in bacteria. Curr Opin Microbiol. 1999;2(2):195–201. doi: 10.1016/S1369-5274(99)80034-4. [DOI] [PubMed] [Google Scholar]
- 7.Zomer AL, Buist G, Larsen R, Kok J, Kuipers OP. Time-resolved determination of the CcpA regulon of Lactococcus lactis subsp. cremoris MG1363. J Bacteriol. 2007;189(4):1366–1381. doi: 10.1128/JB.01013-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Postma PW, Lengeler JW, Jacobson GR. Phosphoenolpyruvate:carbohydrate phosphotransferase systems of bacteria. Microbiol Rev. 1993;57(3):543–594. doi: 10.1128/mr.57.3.543-594.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saier MH, Jr, Reizer J. The bacterial phosphotransferase system: new frontiers 30 years later. Mol Microbiol. 1994;13(5):755–764. doi: 10.1111/j.1365-2958.1994.tb00468.x. [DOI] [PubMed] [Google Scholar]
- 10.Chang DE, Smalley DJ, Conway T. Gene expression profiling of Escherichia coli growth transitions: An expanded stringent response model. Mol Microbiol. 2002;45(2):289–306. doi: 10.1046/j.1365-2958.2002.03001.x. [DOI] [PubMed] [Google Scholar]
- 11.Traxler MF, Chang DE, Conway T. Guanosine 3′,5′-bispyrophosphate coordinates global gene expression during glucose-lactose diauxie in Escherichia coli. Proc Natl Acad Sci USA. 2006;103(7):2374–2379. doi: 10.1073/pnas.0510995103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Crosse AM, Greenway DL, England RR. Accumulation of ppGpp and ppGp in Staphylococcus aureus 8325-4 following nutrient starvation. Lett Appl Microbiol. 2000;31(4):332–337. doi: 10.1046/j.1472-765x.2000.00822.x. [DOI] [PubMed] [Google Scholar]
- 13.Cashel M. Regulation of bacterial ppGpp and pppGpp. Annu Rev Microbiol. 1975;29:301–318. doi: 10.1146/annurev.mi.29.100175.001505. [DOI] [PubMed] [Google Scholar]
- 14.Potrykus K, Cashel M. (p)ppGpp: Still magical? Annu Rev Microbiol. 2008;62:35–51. doi: 10.1146/annurev.micro.62.081307.162903. [DOI] [PubMed] [Google Scholar]
- 15.English BP, et al. Single-molecule investigations of the stringent response machinery in living bacterial cells. Proc Natl Acad Sci USA. 2011;108(31):E365–E373. doi: 10.1073/pnas.1102255108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rallu F, Gruss A, Ehrlich SD, Maguin E. Acid- and multistress-resistant mutants of Lactococcus lactis: Identification of intracellular stress signals. Mol Microbiol. 2000;35(3):517–528. doi: 10.1046/j.1365-2958.2000.01711.x. [DOI] [PubMed] [Google Scholar]
- 17.Jensen NB, Melchiorsen CR, Jokumsen KV, Villadsen J. Metabolic behavior of Lactococcus lactis MG1363 in microaerobic continuous cultivation at a low dilution rate. Appl Environ Microbiol. 2001;67(6):2677–2682. doi: 10.1128/AEM.67.6.2677-2682.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cocaign-Bousquet M, Even S, Lindley ND, Loubière P. Anaerobic sugar catabolism in Lactococcus lactis: Genetic regulation and enzyme control over pathway flux. Appl Microbiol Biotechnol. 2002;60(1-2):24–32. doi: 10.1007/s00253-002-1065-x. [DOI] [PubMed] [Google Scholar]
- 19.Robert L, et al. Pre-dispositions and epigenetic inheritance in the Escherichia coli lactose operon bistable switch. Mol Syst Biol. 2010;6:357. doi: 10.1038/msb.2010.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.de Jong IG, Veening JW, Kuipers OP. Single cell analysis of gene expression patterns during carbon starvation in Bacillus subtilis reveals large phenotypic variation. Environ Microbiol. 2012;14(12):3110–3121. doi: 10.1111/j.1462-2920.2012.02892.x. [DOI] [PubMed] [Google Scholar]
- 21.Novick A, Weiner M. Enzyme induction as an all-or-none phenomenon. Proc Natl Acad Sci USA. 1957;43(7):553–566. doi: 10.1073/pnas.43.7.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ozbudak EM, Thattai M, Lim HN, Shraiman BI, Van Oudenaarden A. Multistability in the lactose utilization network of Escherichia coli. Nature. 2004;427(6976):737–740. doi: 10.1038/nature02298. [DOI] [PubMed] [Google Scholar]
- 23.Narang A, Pilyugin SS. Bistability of the lac operon during growth of Escherichia coli on lactose and lactose+glucose. Bull Math Biol. 2008;70(4):1032–1064. doi: 10.1007/s11538-007-9289-7. [DOI] [PubMed] [Google Scholar]
- 24.Boulineau S, et al. Single-cell dynamics reveals sustained growth during diauxic shifts. PLoS ONE. 2013;8(4):e61686. doi: 10.1371/journal.pone.0061686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maisonneuve E, Castro-Camargo M, Gerdes K. (p)ppGpp controls bacterial persistence by stochastic induction of toxin-antitoxin activity. Cell. 2013;154(5):1140–1150. doi: 10.1016/j.cell.2013.07.048. [DOI] [PubMed] [Google Scholar]
- 26.Amato SM, Orman MA, Brynildsen MP. Metabolic control of persister formation in Escherichia coli. Mol Cell. 2013;50(4):475–487. doi: 10.1016/j.molcel.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 27.Mechold U, Cashel M, Steiner K, Gentry D, Malke H. Functional analysis of a relA/spoT gene homolog from Streptococcus equisimilis. J Bacteriol. 1996;178(5):1401–1411. doi: 10.1128/jb.178.5.1401-1411.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dressaire C, et al. Investigation of the adaptation of Lactococcus lactis to isoleucine starvation integrating dynamic transcriptome and proteome information. Microb Cell Fact. 2011;10(Suppl 1):S18. doi: 10.1186/1475-2859-10-S1-S18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Primakoff P, Artz SW. Positive control of lac operon expression in vitro by guanosine 5′-diphosphate 3′-diphosphate. Proc Natl Acad Sci USA. 1979;76(4):1726–1730. doi: 10.1073/pnas.76.4.1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rao CV, Wolf DM, Arkin AP. Control, exploitation and tolerance of intracellular noise. Nature. 2002;420(6912):231–237. doi: 10.1038/nature01258. [DOI] [PubMed] [Google Scholar]
- 31.Elowitz MB, Levine AJ, Siggia ED, Swain PS. Stochastic gene expression in a single cell. Science. 2002;297(5584):1183–1186. doi: 10.1126/science.1070919. [DOI] [PubMed] [Google Scholar]
- 32.Balaban NQ, Merrin J, Chait R, Kowalik L, Leibler S. Bacterial persistence as a phenotypic switch. Science. 2004;305(5690):1622–1625. doi: 10.1126/science.1099390. [DOI] [PubMed] [Google Scholar]
- 33.Thattai M, van Oudenaarden A. Stochastic gene expression in fluctuating environments. Genetics. 2004;167(1):523–530. doi: 10.1534/genetics.167.1.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kearns DB, Losick R. Cell population heterogeneity during growth of Bacillus subtilis. Genes Dev. 2005;19(24):3083–3094. doi: 10.1101/gad.1373905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dubnau D, Losick R. Bistability in bacteria. Mol Microbiol. 2006;61(3):564–572. doi: 10.1111/j.1365-2958.2006.05249.x. [DOI] [PubMed] [Google Scholar]
- 36.Smits WK, Kuipers OP, Veening JW. Phenotypic variation in bacteria: The role of feedback regulation. Nat Rev Microbiol. 2006;4(4):259–271. doi: 10.1038/nrmicro1381. [DOI] [PubMed] [Google Scholar]
- 37.Raj A, van Oudenaarden A. Nature, nurture, or chance: Stochastic gene expression and its consequences. Cell. 2008;135(2):216–226. doi: 10.1016/j.cell.2008.09.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Veening JW, Smits WK, Kuipers OP. Bistability, epigenetics, and bet-hedging in bacteria. Annu Rev Microbiol. 2008;62:193–210. doi: 10.1146/annurev.micro.62.081307.163002. [DOI] [PubMed] [Google Scholar]
- 39.Veening JW, et al. Bet-hedging and epigenetic inheritance in bacterial cell development. Proc Natl Acad Sci USA. 2008;105(11):4393–4398. doi: 10.1073/pnas.0700463105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rainey PB, et al. The evolutionary emergence of stochastic phenotype switching in bacteria. Microb Cell Fact. 2011;10(Suppl 1):S14. doi: 10.1186/1475-2859-10-S1-S14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Goel A, Santos F, Vos WM, Teusink B, Molenaar D. Standardized assay medium to measure Lactococcus lactis enzyme activities while mimicking intracellular conditions. Appl Environ Microbiol. 2012;78(1):134–143. doi: 10.1128/AEM.05276-11. [DOI] [PMC free article] [PubMed] [Google Scholar]