Abstract
Proteasome inhibition is associated with substantial antitumor effects in preclinical models of multiple myeloma (MM) as well as in patients. However, results of recent clinical trials to evaluate the effect of the proteasome inhibitor bortezomib (Velcade®, also called PS-341) in MM patients have shown limited activity when used as a single agent. This underscores the need to find new efficacious and less toxic proteasome inhibitors. Recently, carfilzomib was approved for treatment of refractory/relapsed MM and several new agents have been introduced into the clinic, including marizomib, and MLN9708, and trials investigating these second generation proteasome inhibitors have demonstrated promising results. We have recently synthesized a novel proteasome inhibitor, BU-32, and tested its growth inhibitory effects in different human MM cells including RPMI8226, MM.1S, MM.1R, and U266. In this study, we evaluate the efficacy of the novel proteasome inhibitor BU-32 (NSC D750499) using an in vitro MM model. BU-32 exhibits strong cytotoxicity in a panel of multiple myeloma cell lines – RPMI8226, MM1S, MM1R, and U266. In addition, we demonstrate by proteasome inhibition assay that BU-32 potently inhibits the chymotryptic- and caspase-like activities of the 26S proteasome. We further show from Annexin V FITC binding studies that BU-32, like Bortezomib, induces apoptosis in a panel of MM cell lines but the effect is more pronounced with BU-32-treated cells. Invasion assay with the MM.1S cell line indicates that BU-32 inhibits the invasiveness of myeloma cells. Results from our studies using real-time PCR array analyses show that BU-32 effectively down-regulates an array of angiogenesis and inflammatory markers. Our results suggest that BU-32 might be a potential chemotherapeutic agent with promising antitumor activity for the treatment of MM.
Introduction
The Ubiquitin-Proteasome System (UPS) regulates the degradation of the majority of intracellular proteins and is the major pathway by which cells regulate protein homeostasis in eukaryotes [1]. During normal protein homeostasis, specific proteins are targeted for destruction via the attachment of polyubiquitin [2]. Once tagged, these polyubiquitin proteins are then degraded by the 26S proteasome into recyclable amino acids [3, 4]. Proteasome substrates include misfolded proteins and highly regulated members of critical signaling cascades, including proteins involved in growth and cell cycle control, and apoptosis. Proteasome inhibition results in the stabilization and accumulation of these proteins, leading to the activation of antiproliferative signals, cell cycle disruption, activation of apoptotic pathways, and, ultimately, cell death [2, 5, 6]. Since proteasomes play a central goal in the cytoplasmic turnover of the vast majority of proteins, the manipulation of proteasome activity is a key goal in controlling the stability of regulatory proteins [7, 8].
The 26S proteasome is a multienzyme complex that consists of two 19S regulatory subunits and the 20S multicatalytic core [9]. The 20S proteasome is a chambered, barrel-shaped structure containing two heptameric rings made from β subunits. The α rings perform capping and gating functions while the β subunits (β1, β2, and β5), which contain the NH2-terminal threonines responsible for the different proteasome proteolytic activities, and are referred to as caspase-like, trypsin-like, and chymotrypsin-like, respectively [7, 10, 11].
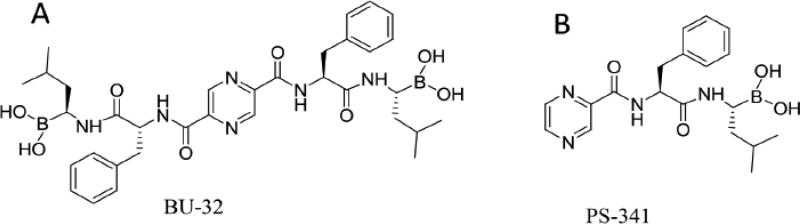
Bortezomib is the most studied and best characterized proteasome inhibitor and the first of the class to be approved for clinical use for treatment of refractory multiple myeloma and mantle cell lymphoma. It is a specific and reversible inhibitor of the chymotryptic activity of the 26S proteasome, and has a wide range of molecular effects, including inhibition of nuclear factor-κB (NF-κB), abrogation of tumor growth and survival, induction of apoptosis, upregulation of heat shock proteins, inhibition of DNA damage repair, and induction of ER stress [12]. The remarkable sensitivity of myeloma to this drug is not well understood, but there is some in vitro evidence that this may be related to the high level of immunoglobulin production and the activation of unfolded protein response (UPR) [13]. Despite the clinical success of Bortezomib, it has been associated with many shortcomings, including possible off-target toxicities and the development of drug resistance [14-16]. Recently, several new agents have been introduced into the clinic, including carfilzomib, marizomib, and MLN9708, and trials investigating these second generation proteasome inhibitors have demonstrated promising results[17]. Here, we describe in vitro data that support development of a new proteasome inhibitor BU-32 (Figure 1A), a second-generation bivalent analog of Bortezomib (also called PS-341, Figure 1B), possessing two boronic acid “war-heads” which is highly effective in killing myeloma cells. In breast cancer cell lines, BU-32 has been shown to selectively inhibit proteasome chymotrypsin-like activity and tumor cell proliferation, upregulate apoptosis and downregulate NF-κB expression and angiogenic marker genes, and is efficacious in blocking breast tumor growth and bone metastases [18]. Direct comparison with Bortezomib revealed that BU-32 has improved toxicity profile and is better tolerated at higher doses in mice and studies on the National Cancer Institute (NCI's) panel of 60 cell lines indicates it has broad spectrum of activity against various cancer cell lines. In the present study, we report the effects of BU-32 against sensitive MM cell lines and MM cells that are resistant to conventional therapies.
Figure 1.
Chemical structures of peptidyl boronic acids. A The structure of BU-32. B The structure of PS-341.
Methods
Cell lines
MM.1S (dexamethasone [Dex]-sensitive), MM.1R (Dex-resistant), RPMI-8226, U266 (IL6-dependent) human and 5TGM1 mouse MM cell lines were originally obtained from the American Type Tissue Culture Collection (ATCC). These cell lines were cultured in RPMI-8226 medium supplemented with 10% fetal bovine serum (FBS) and antibiotics as described previously [19].
Cellular proliferation assay
Cellular proliferation was assayed using the cell-titer glo luminescence-based kit (Promega, Madison, WI, USA) according to the manufacturer's directions [20]. Briefly, different cancer cells were planted in 96-well plates and incubated in RPMI media containing serum (5%) for 24h. Then the cells were treated with different concentrations of drug for 48h. The cell-titer glo substrate was added to the cells to allow for the production of luminescence signal. Luminescence of a minimum of 6 replicates per treatment condition was measured in a Fluoroscan Ascent FL luminometer (Thermo Labsystems Waltham, MA, USA).
20S proteasome assay
Proteasome chymotrypsin-like, caspase-like, and trypsin-like activities were determined using Proteasome-Glo™ Assay System from Promega (Madison, WI) according to the manufacturer's protocol as previously described [18].
Apoptosis assay
Annexin staining was conducted with the use of a kit, Annexin V-FITC Apoptosis detection kit, BD Pharmingen (San Jose, CA, USA). 106 cells per T25 flask was split and left to recover for 24 h before exposure to proteasome inhibitor. Then the cells were treated with drugs for 24 h. After incubation, cells were washed with DPBS and resuspended in 100μl of binding buffer (supplied by the vendor). Cells were stained with Annexin V-FITC and propydium iodide according to the manufacturer's protocol before analysis by flow cytometry [21].
In vitro cell invasion assay
The 3-D invasion assay modeling trans-mesothelial invasion was described previously [22, 23]. Briefly, after labeling with CellTracker Green® (Molecular Probes-Invitrogen, Carlsbad, CA), cells were placed on top of growth-factor-reduced Matrigel ™, coated on 8-μm pore membranes in 24-well invasion chambers (BD Bioscience, San Jose, CA, USA). The invasion chambers were incubated at 37 °C in 5% CO2 for 24 hr. Cells that do not invade through the matrigel, those on the upper surface of the membranes, were mechanically removed with cotton tip applicators. Invaded cells on the bottom of the coated membranes were visualized using a fluorescence microscope with a 20x objective. Images were obtained from four standardized, non-overlapping fields covering approximately 86% of the membrane. Invaded cells were counted using the Image J software (http://rsbweb.nih.gov/ij/). Invasion assays were performed in duplicates and repeated at least three times.
RNA expression studies
The expressions of a wide array of angiogenic marker genes for different cancer cell lines were examined by real-time quantitative reverse transcription-PCR (RT-PCR) after treatment with drug. Total RNA were extracted using RNeasy plus Mini Kit from Qiagen (Valencia, CA, USA) according to the manufacturer's protocol. Reverse transcription (RT) was carried out using the Applied Biosystems kit (Foster City, CA, USA). Real-time PCR and subsequent analysis were carried out using Smartmix PCR beeds (Cepheid, Sunnyvale, CA, USA) with 0.25 X SyberGreen in the Cepheid SmartCycler to detect different genes and the house keeping gene GAPDH. For quantification, the cycle threshold number (Ct) exhibiting the maximum curve growth rate was determined [24]. The relative gene expression of each sample, normalized to that of glyceraldehyde-3-phosphate dehydrogenase (GAPDH), was calculated by the formula:
PCR array analysis for angiogenesis was done using SA Biosciences kit (Frederick, MD) according to the manufacturer's protocol.
Statistical methods
Statistical analysis was conducted by the Student t test. Differences between the groups were considered significant when the P value was 0.05 or less.
Results
BU-32 inhibits tumor cell proliferation
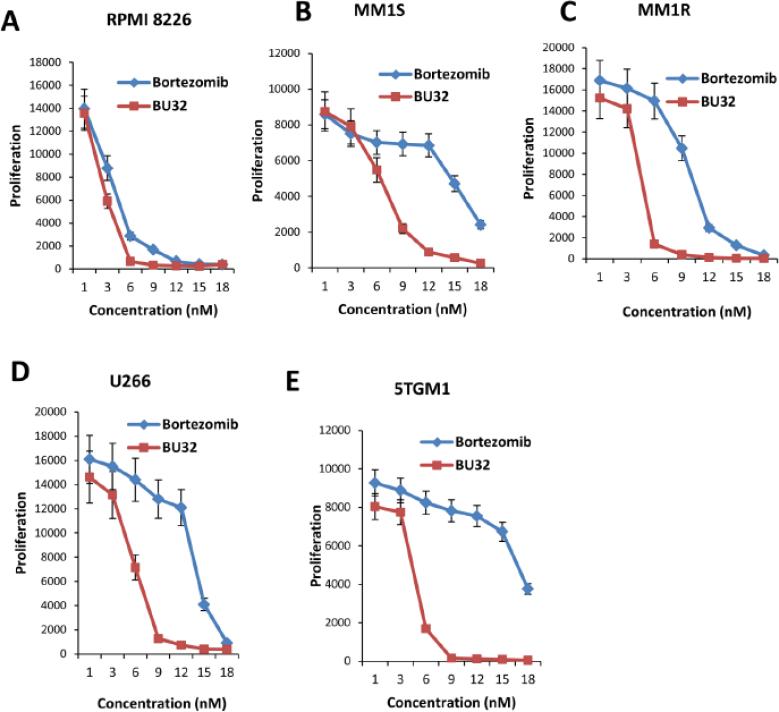
MM cell lines (RPMI-8226, MM.1S, MM.1R, and U266) were treated with low doses (1-18 nM) of BU-32 and PS-341 for 48 hours followed by assessment for cell viability using cell-glo assay. A significant decrease in viability of all cell lines was observed in response to treatment with BU-32 (Figs. 2A-E). BU-32, therefore, exhibits strong cytotoxicity on the cell lines with half-maximal inhibitory concentrations (IC50) of: RPMI-8226 cells (IC50 = 3.2 nM), MM.1S cells (IC50 = 7.5 nM), MM1R cells (IC50 = 4.5 nM), and U266 cells (IC50 = 6.1 nM),. These values are comparable with those obtained with PS-341 for the RPMI-8226 cells (IC50 = 4.5 nM), MM.1S cells (IC50 = 15 nM), MM1R cells (IC50 = 8.9 nM), and U266 cells (IC50 = 13.5 nM). The results indicate that the novel bivalent proteasome inhibitor, BU-32, has potent anti-MM cytotoxicity against a panel of both sensitive and resistant MM cell lines RPMI8226, MM1S, MM1R, and U266 (Supp. Fig. 1). To investigate the effect of exposure time of drug on cell viability, the cells were incubated with BU-32 and PS-341 and cell growth was measured at various time points. The results demonstrate that cell viability decreases with time, an indication that the BU-32 and PS-341 persistently inhibit the growth of MM cells (Supp. Figs. 2 A-E). Our results further demonstrate that BU-32 is more effective than PS-341 at reducing cell viability in MM cell lines.
Figure 2.
Effect of BU-32 on myeloma cell proliferation. Proliferation assay of RPMI8226 (A), MM1S (B), MM1R (C), U266 (D), and 5TGM1 (E). Myeloma cells were treated with different concentrations (1-18 nM) of Bortezomib and BU-32 for 48h. BU-32 persistently inhibits myeloma cell viability. Results are mean of three independent experiments.
BU-32 selectively inhibits proteasome chymotrypsin & caspase-like activity
Like the parent proteasome inhibitor PS-341, the primary target of BU-32 is the uniquitin proteasome system. The proteolytic activity of proteasomes is mediated by 3 active sites: chymotrypsin-like, caspase-like and trypsin-like [25]. To evaluate the potency and selectivity of BU-32 for the three proteasome active sites, we monitored the rates of fluorogenic peptide substrate hydrolysis by the proteasome in intact cells of a panel of cell lines. Our results showed that BU-32 primarily inhibits chymotrypsin-like activity and, to a lesser degree, the caspase-like activity of the proteasome. Incubation of a panel of MM cell lines (RPMI-8226, MM.1S, MM.1R, and U266,) with BU-32 for 24 hours resulted in a dose-dependent inhibition of all three proteasome catalytic activities, with the chymotrypsin-like and caspase-like activity exhibiting the greatest sensitivity for all MM cell lines (Table 1). Table 1 represents the IC50 value for chymotrypsin-like, caspase-like and trypsin-like activity of all MM cell lines. The potency of proteasome inhibition of BU-32 is cell line-dependent, with chymotrysin-like activity showing the greatest sensitivity for RPMI8226, MM1S, MM1R, and U266 cell lines (Supp. Fig. 3-7).
Table 1.
Selective inhibition of the proteasome chymotrypsin-like and caspase-like activity by BU-32
| Cell line | Chymotrypsin-like | Caspase-like | Trypsin-like | |||
|---|---|---|---|---|---|---|
| IC50 (nM) | IC50 (nM) | IC50 (nM) | ||||
| BU-32 | Bortezomib | BU-32 | Bortezomib | BU-32 | Bortezomib | |
| RPMI822G | 4.2 ± 0.38 | 6.7 ± 0.48 | 13.5 ± 0.87 | 15.1 ± 0.92 | >18 | >18 |
| MM1S | 2.9 ± 0.18 | 4.5 ± 0.32 | 9.2 ± 0.32 | 12.1 ± 0.87 | >18 | >18 |
| MM1R | 7.5 ± 0.56 | 8.6 ± 0.19 | 9.0 ± 0.42 | 10.1 ± 0.67 | >18 | >18 |
| U266 | 6.1 ± 0.42 | 8.4 ± 0.12 | 13.4 ± 0.56 | 18.0 ± 0.78 | >18 | >18 |
BU-32 induces apoptosis and inhibits the invasion of myeloma cells
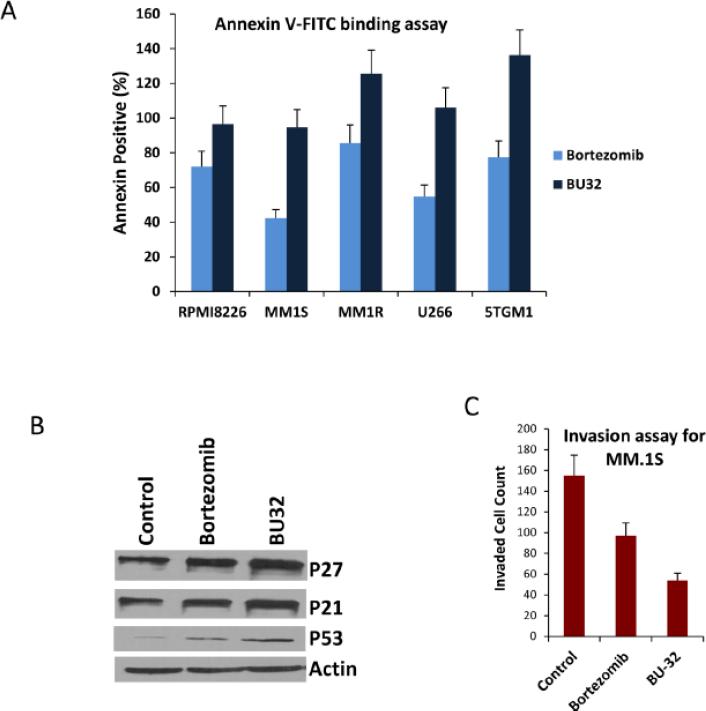
To gain further insight into the mechanism of the antiproliferative effect of BU-32 in myeloma, we investigated the effect of BU-32 on induction of apoptosis in a panel of MM cell lines. We have previously demonstrated that exposure to BU-32 induces the upregulation of the proapoptotic markers Bid and Bax, cell-cycle-dependent kinase inhibitors p21 and p27, and the tumor suppressor gene p53, and downregulates NF-κB expression in the breast cancer cell lines MDA-MB-231, MCF7, and SKBR3 [18]. After 24 h of incubation with BU-32, the percentage of apoptotic cells was determined by annexin-V/PI staining analyzed by flow cytometry. The induction of apoptosis exhibited by BU-32 is more than to that of Bortezomib (Fig. 3A). Western blot analysis (Fig 3B) indicates that p21, p27 and p53 marker gene expression are upregulated in presence of proteasome inhibitors. These results indicate that BU-32 inhibits MM cell proliferation by inducing apoptosis.
Figure 3.
Exposure to BU-32 induces apoptosis in myeloma cell lines. (A) Annexin staining was conducted with the use of Annexin V-FITC Apoptosis detection kit. The MM cells were treated with drug for 24 hours. Cells were stained with annexin V-FITC and propydium iodide according to the manufacturer's protocol before analysis by flow cytometry. Results are mean ± standard deviation of three independent experiments. (B) Western blot analysis of p21, p27 and p53 marker gene in MM.1S cells treated with Bortezomib and BU-32. (C) Invasion assay of MM.1S cells treated with Bortezomib and BU-32. A total of four randomly selected fields were examined for cells that had migrated from top to bottom chambers. Image is representative of 3 experiments with similar results.
Next, we investigated the impact of BU-32 on myeloma cell migration or invasion in MM.1S cells, since this plays a key role in the progression of MM. After labeling with CellTracker Green®, growth factor-deprived MM.1S cells were pretreated with BU-32 and bortezomib according to their IC50 value specific to particular cell line. Control and drug treated MM.1S cells were then plated on a top of growth-factor-reduced Matrigel ™, coated on 8-μm pore membranes in 24-well invasion chambers and exposed for 8 hours to serum containing medium in the lower chamber. The migrated cells on the bottom face of the membrane were visualized using a fluorescence microscope. Figure 3C represents the number of invaded cells in control and drug treatment. Our results show that BU-32 inhibits the invasiveness of MM1S cells by 65.16% compared to control while the corresponding level of inhibition for Bortezomib observed was 37.42%.
BU-32 downregulates angiogenic and inflammatory marker genes
As shown in breast cancer cells [18], exposure to BU-32 causes downregulation of an array of angiogenic marker genes in a panel myeloma cell lines. Bone marrow (BM) angiogenesis is emerging as a critical component of MM development and progression, and hence as an attractive therapeutic target for the disease. Complex interactions between myeloma, stroma (SCs) and endothelial cells (ECs) that are mediated by various cytokines with angiogenic properties promote myeloma cell survival and proliferation in the BM microenvironment [26, 27]. As such, agents that block angiogenesis are highly desired in cancer therapy. Suppression of angiogenesis is an important component of the anticancer activity of Bortezomib, and is thought to largely occur via inhibition of NF-κB – which induces the expression of many proangiogenic factors, including vascular endothelial growth factor (VEGF).
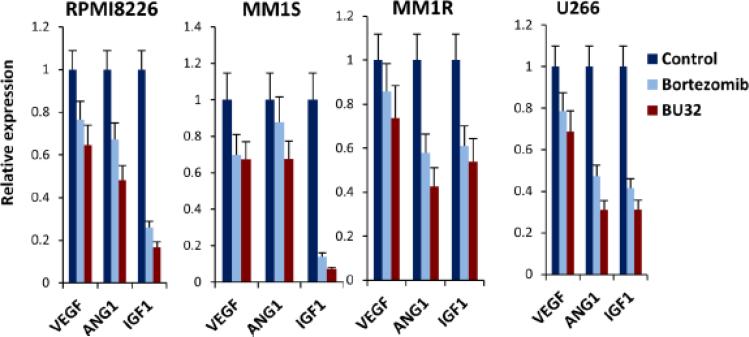
We investigated whether exposure to BU-32 affects the expression of VEGF, Ang1, and IGF-1 genes that are primarily involved in the angiogenic cascade using RPMI8226, MM1S, MM1R, and U266, (Fig 4) cell lines, and compared the effect with that of Bortezomib. We showed that exposure to BU-32 induced significant downregulation of angiogenic markers in all the cell lines, with BU-32 showing a more pronounced effect compared to Bortezomib. The results also indicate that the extent of downregulation of these genes is cell line dependent.
Figure 4.
Exposure of myeloma cells to BU-32 reduces the expression of angiogenesis marker genes. Expression levels of VEGF, ANG1, IGF1 angiogenic genes in RPMI8226, MM1S, MM1R, and U266 myeloma cell lines exposed to proteasome inhibitor, as evaluated by real-time RT-PCR. RT-PCR was done on RNA extracted from 24 h drug treated myeloma cell lines. Results are ± SD of three independent determination.
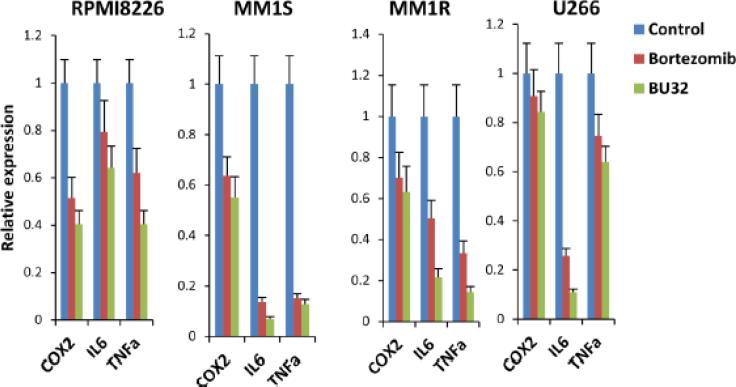
In addition, exposure to BU-32 induces the down-regulation of an array of inflammatory marker genes in MM cells. MM is known to involve a deregulated cytokine network with secretion of inflammatory mediators [28]. Several reports indicate that COX-2 inhibition might result in effective down-regulation of pathways that are relevant to MM pathogenesis. A study by Fujita et al [29] shows that thalidomide strongly inhibits COX-2 expression at the mRNA level, and suggests that some of the many clinical effects of this drug may be due to inhibition of this pathway. We investigated whether exposure to BU-32 affects the expression of genes involved in the inflammatory pathway, such as COX-2, IL-6, TNF-α in the RPMI8226, MM1S, MM1R, and U266, cell lines, and compared the effect with that of Bortezomib (Fig 5). We showed that exposure to BU-32 induces a significant downregulation of these inflammatory markers in all MM cell lines and that the effect was generally more pronounced for BU-32 than Bortezomib.
Figure 5.
Exposure to BU-32 reduces the expression of inflammatory marker genes. Expression COX2, IL6 and TNFa inflammatory genes in RPMI8226, MM1S, MM1R, and U266 myeloma cell lines exposed to proteasome inhibitor, as evaluated by real-time RT-PCR. RT-PCR was done on RNA extracted from 24 h drug treated myeloma cell lines. Results are ± SD of three independent determination.
Discussion
The ubiquitin proteasome pathway represents a promising target for cancer therapy and preclinical and clinical validation of its significance in cancer therapy have been provided through studies with the first in-class proteasome inhibitor Bortezomib (PS-341, Velcade®; Millenium Pharmaceuticals Inc., Cambridge, MA, USA), a dipeptide boronic acid, that works by reversibly inhibiting the effects of the proteasome and inducing apoptosis in several tumor cell lines and animal models [30-32]. Our recent study has shown that the novel diboronated analog of Bortezomib, BU-32 (NSC D750499), is a potent and selective inhibitor of the 20S proteasome and that it has potent in vitro anti-tumor activity against a panel of breast cancer cells as well as in vivo efficacy in mouse xenograft and metastasis models and has an excellent toxicity profile [18]. In the present article, we demonstrate that BU-32 has remarkable in vitro activity in multiple myeloma and provide a rationale for further clinical evaluation in patients.
In this study, we demonstrate for the first time that BU-32 is cytotoxic to various MM cell lines, including those that are resistant to conventional therapy (RPMI 8226, MM1S, MM1R, U266 and 5TGM1). Using Cell-Glo assay in the presence or absence of drug at various concentrations (1-18 nM), we demonstrate the striking dose- and time-dependent efficacy of BU-32 against these human MM cell lines and compare its activity to that of Bortezomib. Interestingly, consistent with our previous studies on breast cancer [18], the cytotoxicity of BU-32 against the panel of myeloma cell lines is comparable with that of Bortezomib in spite of the more bulky size of the former and the possibility of steric hindrance at the proteasome binding pockets. While a definitive evidence for the nature of the interactions of BU-32 with the binding pockets of the proteasome awaits further studies, it is obvious from the present data that the remarkable potency of BU-32 observed in these cell lines is the result of significant interactions with the proteasome active site.
We recently reported that BU-32 differentially blocks all three proteasome activities in the MDA-MB-231, MCF-7, and SKBR3 cell lines with the chymotrypsin-like and caspase-like activities being the more predominant than the trypsin-like activity. We therefore investigated the active site selectivity profile of BU-32 in a panel of myeloma cell lines. Interestingly, we find that both BU-32 and Bortezomib, inhibit all three proteasome activities in RPMI 8226, MM1S, MM1R, and U266, myeloma cell lines, with chymotrypsin- and caspase-like activities being the most predominant, and the trypsin-like activity being the lowest. Our observations are consistent with the active site selectivity profile reported for Bortezomib [7, 19, 33-38] and substantiate data from our recent study of BU-32 in breast cancer [18].
We next examined the effect of BU-32 on apoptosis in a panel of MM cell lines by Annexin VFITC staining and demonstrate that there is an increase in the percentage of apoptotic cells in response to BU-32 compared to Bortezomib. The apoptosis assay results are consistent with our cell proliferation experiments.
It is already reported that BU-32 downregulates the expression of NF-kB [18]. NF-kB regulates the expression of a wide variety of genes implicated in proliferation, angiogenesis, invasion, and metastasis and interruption of this signaling would be expected to inhibit all of these processes [39]. The decreased activity of NF-kB through proteasome inhibition is presumed to play an important role in the invasion of MM.1S cells. The 3-D invasion assay modeling in Boyden chamber with matrigel coated polycarbonate membrane demonstrate that BU-32 significantly reduce the invasiveness of MM.1S cells (65.16%) compared to Bortezomib (37.42%).
Angiogenesis is a hallmark process in the pathology of many diseases, including multiple myeloma. Using real-time PCR array, we analyzed the expression of few important genes (VEGF, ANG1, IGF-I) related to angiogenesis signal transduction pathways. This gene expression profiling analyzed the number of fold of expression change in BU-32 relative to Bortezomib (Fig. 4).
Our investigation revealed that both Bortezomib and BU-32 downregulate the expression of VEGF, ANG1, and IGF-I genes involved in the angiogenic cascade but the effect of BU-32 is more pronounced. VEGF production induces angiogenesis as well as myeloma cell proliferation and migration [40]. VEGF is present in the BM micro-environment of patients with MM and associated with the neovascularization at sites of MM cell infiltration [18]. In addition, VEGF is produced by MM cells and triggers IL-6 production from BMSCs, thereby augmenting MM cell growth and survival [41, 42]. The direct effect of VEGF on tumor cells, coupled with its effects in the BM micro-environment, suggest VEGF as a target for novel therapies to improve outcome in MM. ANG1 is mandatory for vessel sprouting and remodeling [42]. IGF-I is an angiogenic factor that acts both directly through activation of the relative receptor IGF receptor-I and indirectly through up-regulation of VEGF gene expression [43].
We found that treatment with BU-32 in MM cell lines resulted in the reduction of inflammatory enzymes including COX2 as well as the inhibition of proinflammatory cytokines (TNFa and IL6) which are the downstream target genes of NF-kB. The results also indicate that BU-32 is more effective compared to Bortezomib (Fig. 5). Down-regulation of COX2 by the treatment with BU-32 results in the attenuation of the neurotoxic effect of inflammation. In a variety of diseases, prostanoids produced by COX2 have been shown to be responsible for inflammatory processes by inducing vasodilation and perturbing the integrity of vascular barrier [44]. Bortezomib blocks IL-6 production, a key growth factor in myeloma cell proliferation [45].
Toxicity studies have shown that BU-32 has a broad safe dosing range compare to Bortezomib [18]. From the toxicity study, BU-32 showed a promising tolerance profile and therefore can offer a wide range of therapeutic index.[18] The clinical observations revealing that Bortezomib therapy can be associated with toxicity and resistance, coupled with our preclinical findings demonstrating that low doses of BU-32 trigger comparable potency in anti-MM effect in vitro without increased toxicity, suggests the promise of BU-32 treatment strategies.
In summary, we demonstrate from in vitro studies that BU-32 inhibits tumor cell proliferation and proteasome chymotrysin-like and caspase-like activities. In addition, BU-32 down-regulates angiogenic and inflammatory marker genes, induces apoptosis and inhibits invasion of myeloma cells. Our results suggest that BU-32 might be a potential chemotherapeutic agent with promising antitumor activity for the treatment of MM. Our earlier studies showed that BU-32 is less toxic than Bortezomib [18]. Further work is in progress to fully evaluate the therapeutic potential of BU-32 for myeloma therapy.
Supplementary Material
Acknowledgements
This work was supported by grants KO1 CA113468, R21 CA153155, and P30 CA054174 (to JKA) from the National Cancer Institute, and by grants from the Kerr, and William & Ella Owens Research Foundations (JKA).
Footnotes
DISCLOSURES: NONE
REFERENCES
- 1.Adams J. The proteasome: a suitable antineoplastic target. Nat Rev Cancer. 2004;4(5):349–360. doi: 10.1038/nrc1361. [DOI] [PubMed] [Google Scholar]
- 2.Kupperman E, Lee EC, Cao Y, Bannerman B, Fitzgerald M, Berger A, Yu J, Yang Y, Hales P, Bruzzese F, et al. Evaluation of the proteasome inhibitor MLN9708 in preclinical models of human cancer. Cancer Res. 70(5):1970–1980. doi: 10.1158/0008-5472.CAN-09-2766. [DOI] [PubMed] [Google Scholar]
- 3.Ciechanover A. Intracellular protein degradation: from a vague idea, through the lysosome and the ubiquitin-proteasome system, and onto human diseases and drug targeting (Nobel lecture). Angew Chem Int Ed Engl. 2005;44(37):5944–5967. doi: 10.1002/anie.200501428. [DOI] [PubMed] [Google Scholar]
- 4.Goldberg AL. Functions of the proteasome: from protein degradation and immune surveillance to cancer therapy. Biochem Soc Trans. 2007;35(Pt 1):12–17. doi: 10.1042/BST0350012. [DOI] [PubMed] [Google Scholar]
- 5.Orlowski RZ, Kuhn DJ. Proteasome inhibitors in cancer therapy: lessons from the first decade. Clin Cancer Res. 2008;14(6):1649–1657. doi: 10.1158/1078-0432.CCR-07-2218. [DOI] [PubMed] [Google Scholar]
- 6.Dalton WS. The proteasome. Semin Oncol. 2004;31(6 Suppl 16):3–9. doi: 10.1053/j.seminoncol.2004.10.012. discussion 33. [DOI] [PubMed] [Google Scholar]
- 7.Kisselev AF, Goldberg AL. Proteasome inhibitors: from research tools to drug candidates. Chem Biol. 2001;8(8):739–758. doi: 10.1016/s1074-5521(01)00056-4. [DOI] [PubMed] [Google Scholar]
- 8.Groll M, Huber R. Inhibitors of the eukaryotic 20S proteasome core particle: a structural approach. Biochim Biophys Acta. 2004;1695(1-3):33–44. doi: 10.1016/j.bbamcr.2004.09.025. [DOI] [PubMed] [Google Scholar]
- 9.Groll M, Berkers CR, Ploegh HL, Ovaa H. Crystal structure of the boronic acid-based proteasome inhibitor bortezomib in complex with the yeast 20S proteasome. Structure. 2006;14(3):451–456. doi: 10.1016/j.str.2005.11.019. [DOI] [PubMed] [Google Scholar]
- 10.Lowe J, Stock D, Jap B, Zwickl P, Baumeister W, Huber R. Crystal structure of the 20S proteasome from the archaeon T. acidophilum at 3.4 A resolution. Science. 1995;268(5210):533–539. doi: 10.1126/science.7725097. [DOI] [PubMed] [Google Scholar]
- 11.Brannigan JA, Dodson G, Duggleby HJ, Moody PC, Smith JL, Tomchick DR, Murzin AG. A protein catalytic framework with an N-terminal nucleophile is capable of self-activation. Nature. 1995;378(6555):416–419. doi: 10.1038/378416a0. [DOI] [PubMed] [Google Scholar]
- 12.Anderson KC. New insights into therapeutic targets in myeloma. Hematology Am Soc Hematol Educ Program. 2011:184–190. doi: 10.1182/asheducation-2011.1.184. [DOI] [PubMed] [Google Scholar]
- 13.Meister S, Schubert U, Neubert K, Herrmann K, Burger R, Gramatzki M, Hahn S, Schreiber S, Wilhelm S, Herrmann M, et al. Extensive immunoglobulin production sensitizes myeloma cells for proteasome inhibition. Cancer Res. 2007;67(4):1783–1792. doi: 10.1158/0008-5472.CAN-06-2258. [DOI] [PubMed] [Google Scholar]
- 14.Chauhan D, Singh AV, Aujay M, Kirk CJ, Bandi M, Ciccarelli B, Raje N, Richardson P, Anderson KC. A novel orally active proteasome inhibitor ONX 0912 triggers in vitro and in vivo cytotoxicity in multiple myeloma. Blood. 116(23):4906–4915. doi: 10.1182/blood-2010-04-276626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Richardson PG, Briemberg H, Jagannath S, Wen PY, Barlogie B, Berenson J, Singhal S, Siegel DS, Irwin D, Schuster M, et al. Frequency, characteristics, and reversibility of peripheral neuropathy during treatment of advanced multiple myeloma with bortezomib. J Clin Oncol. 2006;24(19):3113–3120. doi: 10.1200/JCO.2005.04.7779. [DOI] [PubMed] [Google Scholar]
- 16.Lonial S, Waller EK, Richardson PG, Jagannath S, Orlowski RZ, Giver CR, Jaye DL, Francis D, Giusti S, Torre C, et al. Risk factors and kinetics of thrombocytopenia associated with bortezomib for relapsed, refractory multiple myeloma. Blood. 2005;106(12):3777–3784. doi: 10.1182/blood-2005-03-1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moreau P, Richardson PG, Cavo M, Orlowski RZ, San Miguel JF, Palumbo A, Harousseau JL. Proteasome inhibitors in multiple myeloma: 10 years later. Blood. 2012;120(5):947–959. doi: 10.1182/blood-2012-04-403733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Agyin JK, Santhamma B, Nair HB, Roy SS, Tekmal RR. BU-32: a novel proteasome inhibitor for breast cancer. Breast cancer research : BCR. 2009;11(5):R74. doi: 10.1186/bcr2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chauhan D, Singh A, Brahmandam M, Podar K, Hideshima T, Richardson P, Munshi N, Palladino MA, Anderson KC. Combination of proteasome inhibitors bortezomib and NPI-0052 trigger in vivo synergistic cytotoxicity in multiple myeloma. Blood. 2008;111(3):1654–1664. doi: 10.1182/blood-2007-08-105601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu YG, Tekmal RR, Binkley PA, Nair HB, Schenken RS, Kirma NB. Induction of endometrial epithelial cell invasion and c-fms expression by transforming growth factor beta. Mol Hum Reprod. 2009;15(10):665–673. doi: 10.1093/molehr/gap043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Demo SD, Kirk CJ, Aujay MA, Buchholz TJ, Dajee M, Ho MN, Jiang J, Laidig GJ, Lewis ER, Parlati F, et al. Antitumor activity of PR-171, a novel irreversible inhibitor of the proteasome. Cancer Res. 2007;67(13):6383–6391. doi: 10.1158/0008-5472.CAN-06-4086. [DOI] [PubMed] [Google Scholar]
- 22.Ferreira MC, Witz CA, Hammes LS, Kirma N, Petraglia F, Schenken RS, Reis FM. Activin A increases invasiveness of endometrial cells in an in vitro model of human peritoneum. Mol Hum Reprod. 2008;14(5):301–307. doi: 10.1093/molehr/gan016. [DOI] [PubMed] [Google Scholar]
- 23.Nair AS, Nair HB, Lucidi RS, Kirchner AJ, Schenken RS, Tekmal RR, Witz CA. Modeling the early endometriotic lesion: mesothelium-endometrial cell co-culture increases endometrial invasion and alters mesothelial and endometrial gene transcription. Fertil Steril. 2008;90(4 Suppl):1487–1495. doi: 10.1016/j.fertnstert.2007.09.047. [DOI] [PubMed] [Google Scholar]
- 24.Kirma N, Hammes LS, Liu YG, Nair HB, Valente PT, Kumar S, Flowers LC, Tekmal RR. Elevated expression of the oncogene c-fms and its ligand, the macrophage colony-stimulating factor-1, in cervical cancer and the role of transforming growth factor-beta1 in inducing c-fms expression. Cancer Res. 2007;67(5):1918–1926. doi: 10.1158/0008-5472.CAN-06-1991. [DOI] [PubMed] [Google Scholar]
- 25.Visekruna A, Joeris T, Seidel D, Kroesen A, Loddenkemper C, Zeitz M, Kaufmann SH, Schmidt-Ullrich R, Steinhoff U. Proteasome-mediated degradation of IkappaBalpha and processing of p105 in Crohn disease and ulcerative colitis. J Clin Invest. 2006;116(12):3195–3203. doi: 10.1172/JCI28804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ferrarini M, Ferrero E. Proteasome inhibitors and modulators of angiogenesis in multiple myeloma. Curr Med Chem. 18(34):5185–5195. doi: 10.2174/092986711798184316. [DOI] [PubMed] [Google Scholar]
- 27.Anargyrou K, Dimopoulos MA, Sezer O, Terpos E. Novel anti-myeloma agents and angiogenesis. Leuk Lymphoma. 2008;49(4):677–689. doi: 10.1080/10428190701861686. [DOI] [PubMed] [Google Scholar]
- 28.Ladetto M, Vallet S, Trojan A, Dell'Aquila M, Monitillo L, Rosato R, Santo L, Drandi D, Bertola A, Falco P, et al. Cyclooxygenase-2 (COX-2) is frequently expressed in multiple myeloma and is an independent predictor of poor outcome. Blood. 2005;105(12):4784–4791. doi: 10.1182/blood-2004-11-4201. [DOI] [PubMed] [Google Scholar]
- 29.Fujita J, Mestre JR, Zeldis JB, Subbaramaiah K, Dannenberg AJ. Thalidomide and its analogues inhibit lipopolysaccharide-mediated Iinduction of cyclooxygenase-2. Clin Cancer Res. 2001;7(11):3349–3355. [PubMed] [Google Scholar]
- 30.Cardoso F, Durbecq V, Laes JF, Badran B, Lagneaux L, Bex F, Desmedt C, Willard-Gallo K, Ross JS, Burny A, et al. Bortezomib (PS-341, Velcade) increases the efficacy of trastuzumab (Herceptin) in HER-2-positive breast cancer cells in a synergistic manner. Mol Cancer Ther. 2006;5(12):3042–3051. doi: 10.1158/1535-7163.MCT-06-0104. [DOI] [PubMed] [Google Scholar]
- 31.Adams J, Kauffman M. Development of the proteasome inhibitor Velcade (Bortezomib). Cancer Invest. 2004;22(2):304–311. doi: 10.1081/cnv-120030218. [DOI] [PubMed] [Google Scholar]
- 32.Fribley A, Zeng Q, Wang CY. Proteasome inhibitor PS-341 induces apoptosis through induction of endoplasmic reticulum stress-reactive oxygen species in head and neck squamous cell carcinoma cells. Mol Cell Biol. 2004;24(22):9695–9704. doi: 10.1128/MCB.24.22.9695-9704.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Crawford LJ, Walker B, Ovaa H, Chauhan D, Anderson KC, Morris TC, Irvine AE. Comparative selectivity and specificity of the proteasome inhibitors BzLLLCOCHO, PS-341, and MG-132. Cancer Res. 2006;66(12):6379–6386. doi: 10.1158/0008-5472.CAN-06-0605. [DOI] [PubMed] [Google Scholar]
- 34.Arendt CS, Hochstrasser M. Identification of the yeast 20S proteasome catalytic centers and subunit interactions required for active-site formation. Proc Natl Acad Sci U S A. 1997;94(14):7156–7161. doi: 10.1073/pnas.94.14.7156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Heinemeyer W, Fischer M, Krimmer T, Stachon U, Wolf DH. The active sites of the eukaryotic 20 S proteasome and their involvement in subunit precursor processing. J Biol Chem. 1997;272(40):25200–25209. doi: 10.1074/jbc.272.40.25200. [DOI] [PubMed] [Google Scholar]
- 36.Kisselev AF, Callard A, Goldberg AL. Importance of the different proteolytic sites of the proteasome and the efficacy of inhibitors varies with the protein substrate. J Biol Chem. 2006;281(13):8582–8590. doi: 10.1074/jbc.M509043200. [DOI] [PubMed] [Google Scholar]
- 37.Chauhan D, Hideshima T, Anderson KC. Proteasome inhibition in multiple myeloma: therapeutic implication. Annu Rev Pharmacol Toxicol. 2005;45:465–476. doi: 10.1146/annurev.pharmtox.45.120403.100037. [DOI] [PubMed] [Google Scholar]
- 38.Berkers CR, Verdoes M, Lichtman E, Fiebiger E, Kessler BM, Anderson KC, Ploegh HL, Ovaa H, Galardy PJ. Activity probe for in vivo profiling of the specificity of proteasome inhibitor bortezomib. Nat Methods. 2005;2(5):357–362. doi: 10.1038/nmeth759. [DOI] [PubMed] [Google Scholar]
- 39.Nawrocki ST, Bruns CJ, Harbison MT, Bold RJ, Gotsch BS, Abbruzzese JL, Elliott P, Adams J, McConkey DJ. Effects of the proteasome inhibitor PS-341 on apoptosis and angiogenesis in orthotopic human pancreatic tumor xenografts. Mol Cancer Ther. 2002;1(14):1243–1253. [PubMed] [Google Scholar]
- 40.Podar K, Tai YT, Davies FE, Lentzsch S, Sattler M, Hideshima T, Lin BK, Gupta D, Shima Y, Chauhan D, et al. Vascular endothelial growth factor triggers signaling cascades mediating multiple myeloma cell growth and migration. Blood. 2001;98(2):428–435. doi: 10.1182/blood.v98.2.428. [DOI] [PubMed] [Google Scholar]
- 41.Dankbar B, Padro T, Leo R, Feldmann B, Kropff M, Mesters RM, Serve H, Berdel WE, Kienast J. Vascular endothelial growth factor and interleukin-6 in paracrine tumor- stromal cell interactions in multiple myeloma. Blood. 2000;95(8):2630–2636. [PubMed] [Google Scholar]
- 42.Roccaro AM, Hideshima T, Raje N, Kumar S, Ishitsuka K, Yasui H, Shiraishi N, Ribatti D, Nico B, Vacca A, et al. Bortezomib mediates antiangiogenesis in multiple myeloma via direct and indirect effects on endothelial cells. Cancer Res. 2006;66(1):184–191. doi: 10.1158/0008-5472.CAN-05-1195. [DOI] [PubMed] [Google Scholar]
- 43.Gerritsen ME, Tomlinson JE, Zlot C, Ziman M, Hwang S. Using gene expression profiling to identify the molecular basis of the synergistic actions of hepatocyte growth factor and vascular endothelial growth factor in human endothelial cells. Br J Pharmacol. 2003;140(4):595–610. doi: 10.1038/sj.bjp.0705494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gong C, Ennis SR, Hoff JT, Keep RF. Inducible cyclooxygenase-2 expression after experimental intracerebral hemorrhage. Brain Res. 2001;901(1-2):38–46. doi: 10.1016/s0006-8993(01)02186-2. [DOI] [PubMed] [Google Scholar]
- 45.Park J, Ayyappan V, Bae EK, Lee C, Kim BS, Kim BK, Lee YY, Ahn KS, Yoon SS. Curcumin in combination with bortezomib synergistically induced apoptosis in human multiple myeloma U266 cells. Mol Oncol. 2008;2(4):317–326. doi: 10.1016/j.molonc.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.