Abstract
Viruses use different strategies to overcome the host defense system. Recent studies have shown that viruses can induce DNA damage response (DDR). Many of these viruses use DDR signaling to benefit their replication, while other viruses block or inactivate DDR signaling. This review focuses on the effects of DDR and DNA repair on human cytomegalovirus (HCMV) replication. Here, we review the DDR induced by HCMV infection and its similarities and differences to DDR induced by other viruses. As DDR signaling pathways are critical for the replication of many viruses, blocking these pathways may represent novel therapeutic opportunities for the treatment of certain infectious diseases. Lastly, future perspectives in the field are discussed.
Keywords: HCMV, cell cycle, DNA damage response, DDR, ATM
1. Introduction
Human cytomegalovirus (HCMV) is a β-herpesvirus and is genetically the most complex viral pathogen of humans. Though HCMV infection rarely causes symptomatic disease in immunocompetent individuals, it can establish lifelong latency/persistence following primary infection and can be reactivated under some conditions. In general, it is the causative agent of a variety of disorders in immunocompromised and immunosuppressed individuals. HCMV-associated pneumonitis and retinitis are among the most prevalent complications following primary infection or reactivation of latent HCMV reservoirs. HCMV infections are serious threats to the health of HIV-positive individuals and transplant patients. Primary or reactivated HCMV infections can place pregnancies at risk as the virus can be transmitted to fetuses. In the United States, about 1% of newborns are congenitally infected with HCMV. Most congenitally infected infants and children do not present with health problems, but 22%–38% of infected infants are born with symptoms, including microcephaly and mental retardation [1,2,3]. HCMV is also the leading cause of nonfamilial hearing loss. A strong association between HCMV and glioblastomas has been established [4], but its direct role in tumorigenesis is, at this juncture, unclear.
Many environmental factors and physiological processes can damage DNA. The presence of abnormal DNA structures can induce many types of DNA signaling pathways. Mammalian viruses use different strategies to antagonize host defense systems, including altering host DNA damage response (DDR) to facilitate their replication. This review concentrates on the effects of DDR on HCMV replication and draws comparison to other viruses that induce DDR.
2. HCMV
HCMV virions are structurally complex. It is an enveloped DNA virus that contains a dsDNA genome of ~235 kilobase pairs, which is the largest genome of any human virus [5]. The genome encodes approximately 200 open reading frames [6,7] although a recent study suggests that many additional, small open reading frames are also transcribed and translated [8]. Recent study shows that HCMV genomes exists as complex mixtures of variants in patients, which may add another layer of genetic complexity to viral infections [9]. The viral genome is encased within a capsid and surrounded by a protein layer called the tegument [10,11]. This structure contains proteins that are delivered to cells upon infection and can act before the onset of viral immediate early (IE) gene expression to help initiate a productive infection. As examples of tegument proteins with such activity, pp71, promotes the degradation of hypophosphorylated forms of pRB, p107, and p130, thus stimulating activities associated with cell cycle progression, whereas tegument delivered pUL69 arrests the cell cycle in a late G1/S-like state [12].
Productive HCMV replication and gene expression has been subdivided into three kinetic classes: immediate early (IE), early (E), and late (L) [13,14]. IE genes are the first to be expressed and do not require de novo protein synthesis for their expression. The IE proteins have many functions which collectively prepare the host cell and viral genome for E gene expression and viral DNA replication. In general terms, E gene products are associated with promoting viral DNA replication. The replication of viral DNA is closely associated with expression of L genes. IE and E gene products also regulate late gene expression [15]. The 72-kDa IE1 protein and 86-kDa IE2 proteins are the first and, for IE1 at least, among the most abundantly expressed proteins during HCMV infection. Both proteins have long been recognized as transcriptional regulators, but they also interact with numerous cellular proteins including RB family members [16,17]. They are produced from differentially spliced transcripts under the control of strong promoter-enhancer element known as the major immediate early promoter (MIEP).
HCMV early genes require prior de novo synthesis of viral IE and cellular proteins for their transcription. The earliest of the early gene transcripts appear and accumulate to peak levels by 8 hours postinfection (e.g., UL112–113), while temporally later early transcripts can be detected just prior to the onset of viral DNA replication (e.g.,TRL4) and accumulate to peak levels when viral DNA replication is allowed to proceed [18]. Most of the viral early genes function in one of two ways. Some of the early genes are directly involve in viral DNA synthesis, cleavage and packaging of the viral genome, and contribute to assembly of the virus particles. Some other genes function to produce cellular and extracellular environments that are suitable for viral gene expression and replication, either by modulating factors involved in the regulation of cellular DNA synthesis or by altering the host’s immune response to the virus. Some examples of the early genes are the UL112-113 nuclear phosphoproteins and HCMV viral DNA replication proteins, including DNA polymerase processivity factor (UL44) and single-stranded DNA binding protein (SSB) (UL57), which are localized in nuclear replication compartments [19].
Following viral DNA replication, delayed early and late viral genes are expressed which, in general, encode the structural components of the virion. UL55 (gB), UL75 (gH) and UL99 (pp28) as well as components of the capsid are products of late genes. While much study has been carried on the regulation of HCMV IE and E gene expression, little is known about the specific mechanisms of regulating late gene expression.
A key biological property of HCMV is to maintain a lifelong relationship with its host by way of latent or persistent infections. During latency, only a subset of viral genes is expressed. The mechanisms governing the establishment and maintenance of latency and reactivation of HCMV from latency are complex and now coming into focus. HCMV resides latently in hematopoietic cells of the bone marrow. Several in vitro systems have been developed as models for HCMV latency. Nelson and colleagues [20,21] have used allogenic stimulation to study HCMV reactivation in monocytes that harbor viral genomes. CD4+ and CD8+ T lymphocytes, cytokines, IFN-γ, and tumor necrosis factor-α can facilitate viral reactivation [21,22]. Mocarski and colleagues [23,24] have studied HCMV latency in granulocyte–macrophage progenitors expressing CD33 and dendritic cell markers. They have identified several HCMV transcripts expressed during latency following in vivo or in vitro infection. Goodrum and colleagues have investigated a primary CD34 (+) hematopoietic progenitor cell system as an experimental model to study HCMV latency and reactivation [25]. Using an HCMV gene array, they examined HCMV gene expression in these cells. CD34+ cells exhibit distinct patterns of viral gene expression from that observed during productive or nonproductive infections. Furthermore, pUL138 was identified as an HCMV protein that promotes an infection with the hallmarks of latency [26,27]. Sinclair and colleagues analyzed the secretome of cells carrying latent HCMV and have identified changes in several secreted cellular proteins known to be involved in regulation of the immune response and chemoattraction [28]. Their results identified a strategy by which sites of latent HCMV can firstly recruit CD4+ T cells and then inhibit their antiviral effector functions. All told, much more needs to be learned in order to develop a clear understanding of HCMV latency.
In sum, HCMV infection strategies and viral replication are complex and reflective of the large genome, numbers of proteins (and miRNAs), and broad cell tropism.
3. Cell Cycle Checkpoints
Cell cycle checkpoints are regulatory steps in pathways that govern the order and timing of cell cycle transitions to ensure completion of one cellular event prior to commencement of another. Checkpoints also offer the opportunity to repair damaged DNA. Most eukaryotic cells proceed through an ordered cell cycle, G1→S→G2→M phase, during which the chromosomes and other cell material double in number with each of the daughter cells receiving one copy of the doubled genetic material. The cell cycle is completed when each daughter cell has its own intact outer membrane. Regulation of the cell cycle is the key for the normal development of multi cellular organisms.
In the cell cycle, the G1 phase represents an organizing state prior to DNA replication and where decisions regarding cell cycle progress are made. Factors that influence progression through G1 include cell size, metabolic state, cell signaling, and perhaps a need to repair damaged DNA. Inconsistency among these states or excessive DNA damage can lead cells to exit the cell cycle and undergo senescence or apoptosis. S phase is where DNA synthesis takes place resulting in the duplication of the cellular genome. In the G2 phase, the cell prepares for the process of mitosis, and the associated cell division to form two daughter cells. This stage provides another opportunity for recognition and repair of damaged DNA. Thus, under normal conditions, the progression of DNA replication and mitosis is signaled by the intracellular checkpoints primarily at the G1 and G2, respectively.
4. DNA Damage Response (DDR)
Many external and internal effectors, such as ionizing radiation and reactive oxygen species, can directly damage DNA. The presence of abnormal DNA structures, including single-stranded break, double-strand breaks, modification of incorporated nucleotides or aberrant replication fork structures, as well as alterations in higher-order chromatin structure, can induce one or more DDR. For a more complete review of DNA damage and responses, please refer to these publications [29,30,31,32,33].
Activation of the DDR plays a key role in avoiding or reducing errors. Although cells use different signaling pathways (Figure 1) to deal with different environment stresses, there are common elements. Signaling networks in response to DNA damage consists of sensors, transducers, and effectors. Sensors detect damaged DNA and signal to transducers. Transducers amplify and transfer the signal to effectors. Effectors then execute the cellular response to initiate cell cycle checkpoint activation, DNA repair or apoptosis. Cellular responses to DNA damage are crucial for maintaining genome integrity. Defects in the DDR system are also associated with several inherited human disorders [34,35,36] and cancers [37,38]. The DDR system tends to be more error prone than genomic replication and any remaining damage or incorrectly repaired damage may play a role in the development of pathologies such as birth defects, cancer or aging.
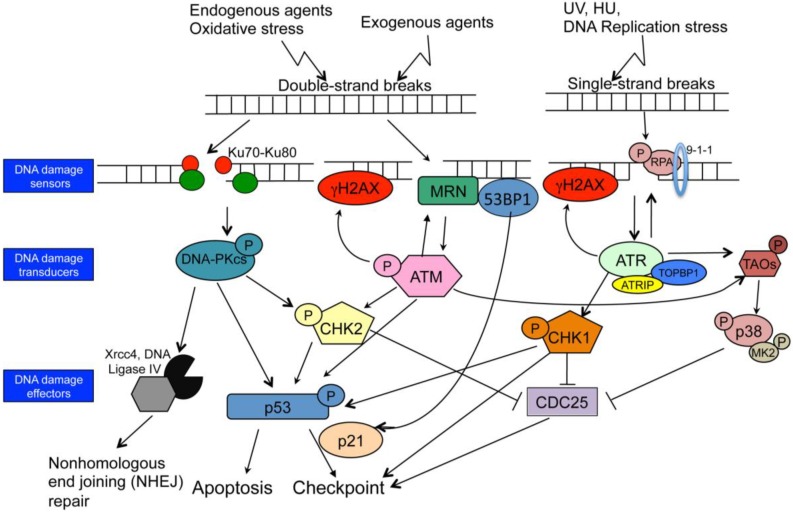
Figure 1.
DNA damage-induced cell cycle checkpoint network. Schematic representation of ATM, ATR and DNA-PK signaling pathways. DNA-PK responds to DNA double-strand breaks and regulates nonhomologous end joining (NHEJ). The DNA ends are captured by the KU heterodimer. Ku regulatory proteins recruit DNA-PK to double-strand breaks; two DNA-PKcs molecules in concert tether DNA ends together and recruit the DNA Ligase IV–XRCC4 complex to rejoin broken DNA ends. ATM responds to DNA double-strand breaks; phosphorylates H2AX and NBS1, which localize to sites of DNA damage, where upon the MRN complexes form. ATM activation regulates cell-cycle checkpoints through the phosphorylation of CHK2 and p53. ATR is activated in response to single-stranded DNA (ssDNA). Activation of ATR requires TopBP1. ATR is recruited to RPA-coated ssDNA by its binding partner ATRIP. ATR regulates the cell-cycle through activation of CHK1. Both ATM and ATR are required to activate the p38MAPK/MK2 effector kinase complex downstream of TAO kinases in response to DNA damage. The three effector kinases, CHK1, CHK2, and MK2 are directly responsible for inhibitory phosphorylation on members of the Cdc25 family. Arrows indicate the flow of the respective DDR pathways.
DNA lesions trigger the activation of various kinases, which play important roles in DDR (Figure 1). The phosphatidylinositol-3-kinase-like family, including ataxia-telangiectasia mutated (ATM), ataxia-telangiectasia Rad3-related (ATR) and DNA-dependent protein kinase catalytic subunit (DNA-PKcs) play central roles in DNA damage checkpoints. ATM is defective in ataxia-telangiectasia mutated disease (A–T), which is characterized by cancer susceptibility, radiosensitivity and neurological defects [39]. ATM is the primary mediator of the response to DNA double strand breaks (DSBs); ATM has been traditionally considered a nuclear protein that functions in response to genotoxic damage though ATM also participates in the oxidative stress response and cytoplasmic signaling [40,41,42,43,44]. ATR activation is generally associated with single-stranded DNA breaks and stalled DNA replication forks. DNA-Pkcs is an important enzyme involved in the non-homologous-end-joining pathway of double strand break repair [45]. The phosphorylation of these proteins plays a crucial role in the activation of various effector proteins. A large-scale proteomic study on ATM and ATR substrates identified more than 700 proteins that are phosphorylated in response to DNA damage [46].
5. The pRB-E2F Complex
The RB protein family consists of three members—pRB, p107, and p130—which maintain cells in a quiescent state as well as regulate the transition from G0/G1- to S-phase by modulating the activity of the E2F family of transcription factors. E2F transcription factors are the major downstream targets of the RB family of proteins, and are necessary for the expression of many genes that are required for cell cycle progression [47,48,49,50]. RB family members exert their growth regulatory functions partly by inhibiting the transcriptional activity of E2F [51,52,53,54,55]. Conversely, disruption of the RB gene by deletion or mutation, or inactivation of pRB by phosphorylation or interaction with viral oncoproteins, cause the release of free or now depressed and transcriptionally active E2F1-3 resulting in cell cycle progression [56,57,58]. Sustained inactivation of pRB often results in apoptosis or deregulated proliferation.
The E2F family, including E2F1-8, can be divided into two subgroups based on their primary function, activator E2Fs: E2F1, 2 and E2F3a, and suppressor E2Fs: E2F3b, E2F4-8. E2Fs 1–3a are required for the transactivation of target genes involved in the G1/S transition. E2F3 encodes two proteins, E2F3a and E2F3b that differ in expression pattern and function. E2F3a is a transcriptional activator mainly expressed during S phase, while E2F3b acts as a transcriptional repressor, which is constantly expressed during cell cycle. In contrast, E2F4 and E2F5 possess predominantly repressive activity. E2F4 and E2F5 bind to p107 and p130 with high affinity, and recent studies have demonstrated that they also interact with pRB [59,60,61,62,63]. E2F6 and E2F7 are also considered to be transcriptional repressors [64,65,66,67,68,69]. E2F6–8 are distinct from the other E2Fs in that they do not bind to pocket proteins. E2F6 is known to interact and form complexes with the Polycomb Group (PcG) proteins. It is not yet clear what the identity is of the interacting partners of these E2Fs. Deregulation of the pRB-E2F interaction results in hyperproliferation, lack of differentiation, genomic instability and can lead to cancer.
6. The Link between Cell Cycle and DDR
6.1. CHK1 and CHK2 Kinases Control the Cell Cycle in Response to DNA Damage
DNA damage poses a continuous threat to genomic integrity in mammalian cells. In order to prevent the propagation of damaged DNA through the cell cycle, cells have evolved DDR that coordinate cell cycle progression and checkpoints with the repair of DNA lesions. Mammalian cells initiate cell cycle arrest at different phases of the cell cycle in response to various forms of genotoxic stress to allow time for DNA repair. Cell cycle arrest and apoptosis are two of the downstream consequences of DDR [70]. CHK1 and CHK2 are checkpoint kinases that are activated by ATM or ATR and phosphorylate cell-cycle components to cause the arrest of the cell cycle [71,72] (Figure 1). Although CHK1 and CHK2 have overlapping roles, CHK1 kinase is restricted to S and G2 where its activity is amplified in the presence of different types of DNA damage [73]. CHK2 is expressed throughout the cell cycle and is also activated in the presence of DNA damage [74]. Usually, CHK1 or CHK2 phosphorylated p53 and CDC25 propagate signals to arrest cells or to undergo apoptosis depending on cell type and extent of DNA damage. However, DNA lesions sometimes do not induce cell cycle checkpoint responses, such as DNA damage during G2 phase of the green alga, Scenedesmus quadricauda [75]; or the level of DNA damage is low enough that the cell can deal with the lesions in the absence of a checkpoint response.
6.2. A Novel Cell Cycle Checkpoint Kinase Pathway, MK2, that also Induced Cell Cycle Arrest
Over the last decade, a number of publications point to a crucial role for the p38MAPK/MAPKAP-K2 (MK2) complex as an integral part of the DDR network [76,77]. There are four p38MAPK isoforms denoted α, β, γ and δ [78]. p38α and p38β have been shown to be activated by DNA damage-specific agents, such as cisplatin, doxorubicin, and temozolomiode [76,77,79,80,81]. p38α forms a nuclear complex with its downstream substrate MK2 and that upon activation of p38MAPK in this complex, p38MAPK phosphorylates and activates MK2. p38MAPK/MK2 complex is a third checkpoint effect or module that operates parallel to CHK1 and is activated downstream of ATM and ATR [76,77,82] (Figure 1). More detailed information on this DNA damage checkpoint signaling pathways can be found elsewhere [82,83,84].
7. Many Viruses can Induce DNA Damage Responses and Modulate Cell Cycle Progression
DDR can be activated not only by external sources of DNA damage, but also by intracellular conditions, such as oncogene overexpression, loss of tumor suppressors, and viral infections. Recent studies demonstrate that infections by DNA viruses or viruses with a DNA genome stage during infection induce host DDR (Table 1) [85,86,87,88,89]. Somewhat unexpectedly, some viruses with RNA genomes can also induce DDR. The mechanisms responsible for DDR induction by RNA viruses are less clear and possibly indirect.
Table 1.
A list of viruses that both induce and require host DNA damage responses (DDR) for productive infections.
| Virus that Induce DNA damage response (DDR) | Abbreviation | Virus type | DDR factors activated | DDR factors required for virus replication | References |
|---|---|---|---|---|---|
| Human cytomegalovirus | HCMV | dsDNA, β-herpesvirus | ATM, CHK2, p53, H2AX NBS1, CHK1 | ATM, p53, H2AX | [94,98] |
| Herpes simplex virus type 1 | HSV-1 | dsDNA, α-herpesvirus | ATM, CHK2, 53BP1, NBS1 | ATM, Mre11 | [95] |
| Epstein-Barr virus | EBV | dsDNA, γ-herpesvirus | ATM, CHK2, Nbs1, H2AX, p53, CHK1 | XPC | [96,99] |
| Murine gammaherpesvirus 68 | γHV68 | dsDNA, γ-herpesvirus | ATM, H2AX, p53, CHK1 | ATM, H2AX | [100,101] |
| Simian virus type 40 | SV40 | dsDNA, polyomavirus | ATM, CHK1, CHK2, p53 | ATM, Rad51, FancD2 | [97,102,103] |
| Human papillomavirus | HPV | dsDNA, papillomavirus | ATM, CHK2, H2AX, NBS1, CHK1, BRCA1 | ATM, CHK2 | [104,105,106,107,108] |
| Human parvovirus B19 | B19V | ssDNA, parvovirus | ATM, CHK2, ATR, DNA-PKcs, CHK1, Ku70/Ku80, H2AX, RPA-32 | ATR, CHK1, DNA-PKcs, Ku70/ku80 | [109,110,111] |
| Adeno-associated virus | AAV | ssDNA, parvovirus | ATM, CHK2, DNA-PKcs, SMC1, H2AX, CHK1, RPA32 | DNA-Pkcs | [112,113] |
| Human T-cell lymphotrophic virus type 1 | HTLV1 | ssRNA/dsDNA, retrovirus | ATM, CHK2, H2AX, NBS1, DNA-PKcs | N/A | [114,115,116,117,118,119] |
| Human immunodeficiency virus type 1 | HIV-1 | ssRNA/dsDNA, lentivirus | ATM, H2AX, p53, NBS1, ATR, CHK1, P38MAPK | ATM | [120,121,122] |
| Rift Valley fever virus | RVFV | ssRNA, arbovirus | ATM, CHK2, H2AX, p53 | ATM, CHK2, p53 | [123,124] |
| Hepatitis C virus | HCV | ssRNA, flavivirus | ATM, CHK2, H2AX, CHK1 | ATM, CHK2 | [125,126] |
The manipulation of the cell cycle by viruses is closely related to activation of the DDR and is usually associated with DNA double-strand break signaling pathways. As examples, oncoproteins of DNA tumor viruses, such as adenovirus E1A, simian virus 40 T antigen, and papillomavirus E7, each interact with the RB family of tumor suppressors, leading to E2F-mediated cell cycle stimulation, apoptosis induction, and cellular transformation. These proteins utilize a conserved LXCXE motif, which is also found in cellular proteins, to target the RB family. The subsequent induction of cell cycle checkpoints and activation of ATM/ATR/DNAPKcs pathways have been reported to accompany infection by a number of different viruses. Given that DDR usually results in cell cycle checkpoints and apoptosis, infection-associated DDR were initially considered to be antiviral, as in the case of adenovirus serotype 5 (Ad5) [90]. However, for many other viruses that induce a host DDR, the response appears to modulate the cell cycle at a precise point that favors virus replication [91,92,93]. Thus, manipulation of the cell cycle and the associated DDR may be a commonly employed strategy of viruses to create a favorable cellular environment for replication.
Several mammalian viruses evolved mechanisms to manipulate DDR pathways for their own benefit by exploiting or actively inhibiting different parts of the pathways [91]. For example, simian virus type 40 (SV40), herpes simplex virus 1(HSV-1), HCMV, and Epstein-Barr virus (EBV) all activate ATM and downstream signaling during infection, which is accompanied by recruitment of ATM and other repair proteins to sites of viral DNA replication [94,95,96,97] (Table 1).
HSV-1, an alphaherpesvirus, has a complex relationship with the DDR, in that it activates many components of the ATM-dependent signaling pathway, such as phosphorylation of CHK2, 53BP1, and NBS1, while inhibiting the DNA-PKcs and ATR kinases [95,127,128,129]. MRE11-RAD40-NBS1 (MRN) complex formation (Figure 1) and activated ATM promote HSV-1 replication [95]. HSV-1 codes for an immediate early transcription factor, ICP0 that promotes cell cycle arrest by inducing the tumor suppressor p53 and its downstream target proteins (p21, GADD45, and MDM2).
Epstein-Barr virus (EBV) is a gammaherpesvirus that induces the phosphorylation of ATM, NBS1, H2AX, CHK2, and p53 during lytic infection [99]. Phosphorylated ATM, NBS1 and Mre11 proteins are recruited to EBV replication compartments. XPC, a sensor of DDR that functions in nucleotide excision repair, is required for EBV replication [96].
The murine gammaherpesvirus 68 (γHV68) latency-associated, anti-interferon M2 protein induces ATM activation and histone acetylation [100] presumably to limit the induction of a virus-induced DNA damage signaling cascade. However, γHV68 protein kinase orf36 activates the DDR and facilitates lytic replication in primary macrophages. H2AX, an orf36 substrate, can enhance MHV68 replication [101].
The large T antigen encoded by the SV40 polyomavirus, deregulates multiple DNA damage signaling and repair pathways [97]. ATM mediated phosphorylation of SV40 large T antigen is detected at the onset of viral replication, and is required for optimal viral DNA synthesis [102]. Inhibition of ATM activity decreases SV40 DNA accumulation [102,103], and delays the assembly of viral replication compartments and recruitment of cellular DNA repair proteins to these sites.
Human papillomavirus (HPV) proteins induce a DDR characterized by the activation of the ATM kinase substrates CHK2, NBS1, and BRCA1 [104,105,106,107,108]. ATM kinase activity is required for HPV genome amplification in differentiating cells but not for episome maintenance in undifferentiated cells [106]. HPV does not induce degradation of MRN components but instead keeps them at high levels throughout differentiation [106].
As a single stranded DNA virus, human parvovirus B19 (B19V) induces a broad range of DDR by triggering activation of all PI-3-like kinases associated with DNA repair pathways during infection [109,110,111]. Phosphorylated ATM, ATR, and DNA-PKcs, and their downstream targets (CHK2, CHK1, and Ku70/Ku80 complex, respectively) are all localized within B19V replication compartments. However, B19 virus apparently only uses ATR-CHK1 signaling to promote its replication [111].
Relatedly, adeno-associated viruses (AAV), another member of Parvoviridae, do not have an absolute requirement for ATM kinase activity. DNA-PK is the primary mediator of damage signaling in response to AAV replication [113]. Immunofluorescence revealed that some activated damage proteins are found in a pan-nuclear pattern (phosphorylated ATM, SMC1, and H2AX), while others such as DNA-PK components (DNA-PKcs, Ku70, and Ku86) and RPA32 accumulate at AAV replication compartments. DNA-PK enhances recombinant AAV (rAAV) replication through the interaction of Ku proteins and AAV-ITRs [112].
ATM protein can also enhance the replication of retroviruses and lentiviruses [120,121], such as human immunodeficiency virus type 1 (HIV-1) where ATM can enhance HIV replication by stimulating Rev function [120,122]. Similar observations have been made during human T‑cell lymphotropic virus (HTLV1) infections [114,115,116,117,118,119].
In most cases, ATM signaling has been demonstrated to be beneficial for DNA virus replication. It has also been suggested that RNA viruses activate DDR functions that can be beneficial. A study shows the induction of DNA damage signaling upon infection with Rift Valley Fever Virus (RVFV), an RNA virus, that results in cell cycle arrest and increased viral replication [124]. ATM and a number of its substrates, CHK2, H2AX, and p53, were phosphorylated following RVFV infection. The use of ATM and CHK2 inhibitors or p53-null cells demonstrates that they are required for RVFV replication [123,124]. Another recently identified example of an RNA virus that activates DDR is HCV, which replicates better in the presence of ATM and CHK2, and expresses viral proteins that bind ATM and sensitize cells to DNA damage [125,126]. Other studies observe that HCV NS2 protein inhibits DNA damage signaling by sequestering p53 in the cytoplasm while the viral core protein interacts with NBS1 protein, leading to inhibition of MRN complex formation thereby blocking ATM activation and signaling in response to DSBs [130,131]. Clearly the association between RNA virus infection and DDR is complex, with additional study needed to better understand the molecular underpinnings and biology of this relationship.
In contrast, some viruses do not use DDR signaling for replication [91]. Specially, serotype-specific inactivation of the cellular DDR during adenovirus infection has been found [132]. For example, human adenovirus serotype 5 (Ad5) encoded E4 proteins inactivate the MRN complex early in infection, either via E1b55K/E4orf6-mediated degradation of MRN [90,133] or E4orf3-mediated mislocalization of MRN into nuclear tracks [134,135] and cytoplasmic aggresomes [136,137]. In addition to preventing ATM and ATR-mediated damage signaling, inactivation of MRN promotes Ad5 DNA replication [134,138,139]. Likewise, Kaposi’s sarcoma-associated herpesvirus (KSHV) viral interferon regulatory factor 1 (vIRF1) compromises an ATM/p53-mediated DNA damage checkpoint by targeting both upstream ATM kinase and downstream p53 tumor suppressor [140]. Whether this activity is essential for productive or latent infection awaits further study.
In summary, though there are some viruses that do not use DDR for their replication (i.e., Ad5), there are numerous viruses (Table 1) that induce DDR pathways and require ATM or other PI-3-like kinases for productive infection. For more detailed information of DDR and viruses in general, see the following reviews [85,86,87,88,89].
8. HCMV Modulates the Cell Cycle and Checkpoints
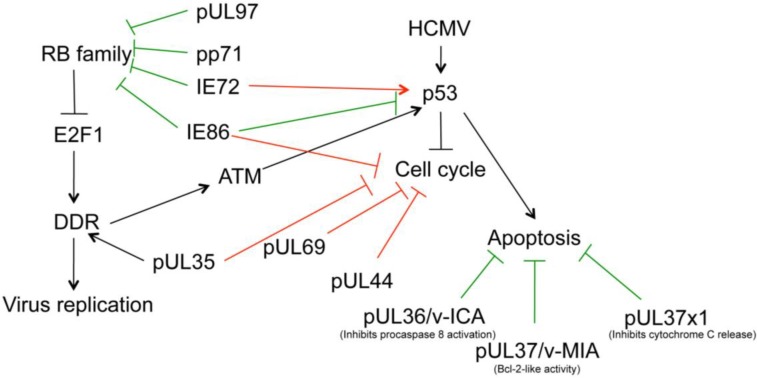
HCMV infection can alter the cell cycle status (Figure 2) and induce a DDR (Figure 3). Most cells infected with HCMV are driven into a unique G1/S-like state that provides enzymes and metabolites necessary for viral DNA replication [141,142,143]. At the same time, the virus directly suppresses competitive cellular DNA synthesis [144,145,146,147]. This unusual G1/S-like state is dependent upon several viral proteins including at least the tegument proteins pUL69 [148], pp71 [149], and pUL97 [150], pUL35 [151], the immediate early proteins, IE1 [152] and IE2 protein [153], and polymerase accessory protein pUL44 [154], while several other viral proteins prevent apoptosis signaling that would normally result from deregulating the cell cycle (Figure 2).
Figure 2.
The relationship between cell cycle and the DDR induced by HCMV. Lines depicted in green represent activities that promote cell cycle progression or prevent the cells from undergoing apoptosis. Lines depicted in red represent activities that can negatively affect cell cycle progress within the cell.
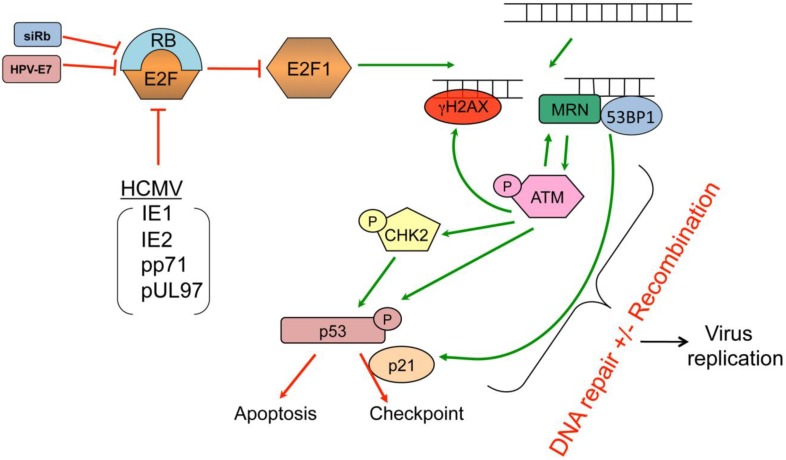
Figure 3.
Model of the host DNA damage response induced by HCMV infection.
The p53 checkpoint protein functions primarily as a transcriptional activator with target genes including the CDK inhibitor, p21, as well as Mdm2, a negative regulator of p53. The levels of p53 and its phosphorylation are increased during HCMV infection [94,98,141]. Phosphorylation-mediated activation of p53 induces an arrest of the cell cycle, which is mainly due to the induction of p21, sustained arrest leading to apoptosis. During HCMV infection, phosphorylation of p53 following DDR signaling prevents its interaction with MDM2, thereby stabilizing p53 by preventing its ubiquitin-mediated degradation [155], and contributing to the G1/S-like cell cycle arrest through the induction of p21 expression [152]. HCMV infection also activates several factors that normally induce cell cycle progression, including E2Fs [156]. In summary, the interaction between HCMV and cell cycle regulatory mechanisms is complex, with some viral factors eliciting cell cycle arrest and others promoting cell cycle progression. Thus, HCMV infection-induces a cell cycle arrest that leaves cells in a unique state that favors its replication, a theme that appears to be common to herpesviruses during productive infections [92].
9. HCMV, Deregulation of the Cell Cycle, and DNA Damage Signaling
HCMV infection induces a DDR that includes activation of ATM, H2AX, NBS1, CHK2, CHK1, and p53 [94,98,157]. How does HCMV induce DDR signaling? HCMV encode four proteins, IE1, IE2, pp71 and pUL97 that can either bind to or phosphorylate RB family members (Figure 2) [17,149,150,158,159,160,161,162,163]. Binding by these viral proteins to or phosphorylation of pRB causes the release of E2F proteins. Of these, the now de-repressed E2F1 can induce DSBs [164]. The resultant ATM activation and its downstream phosphorylation targets, including H2AX and p53, contribute to HCMV replication [94]. These DDR phenotypes are similar to productive infection with another herpesvirus, HSV-1 [85,89,95,128]. In addition, HCMV pUL35 can active DDR, causing γH2AX and 53BP1 foci formation and induce a cell cycle arrest [151] which likely supports viral replication [151].
HCMV, in particular, encodes several proteins that both modulate cell cycle controls and the host DDR to promote viral replication. The following subsections focus on how some of these viral proteins, in particular, IE1, IE2, pp71, pUL97, pUL69, mediate these activities.
9.1. IE1 Can Inactivate p107 and p130
UL123, encoding IE1 (sometimes referred to IE1-72 or IE72), is the first transcribed HCMV gene. As a predominantly nuclear protein, IE1 is a promiscuous transactivator that also interacts and modulates the function of p107, a member of the RB protein family [17,165]. IE1 has been suggested to exhibit kinase activity [156,163]. In vitro kinase assays suggest that both p107 and p130 can be phosphorylated by IE1 and that phosphorylation of these two RB proteins is sufficient to disrupt their interaction with E2F4 [163]. These findings imply that IE1 specifically targets at least two RB proteins for inactivation, which causes the derepression of E2F-responsive promoters. Even with RB family member inactivation and derepression of E2Fs, IE1 expression is unable to induce S-phase entry in primary fibroblasts due to a p53-dependent arrest [152]. This ability of p53 to block the IE1-mediated induction of S-phase is dependent on the p21 CDK inhibitor [157].
IE1 also disrupts PML bodies [166,167,168] (also known as promyelocytic oncogenic domains (PODs) or nuclear domains-10 (ND-10)). PML is involved in cellular growth regulation, transcription, DNA replication and repair, and posttranscriptional regulation of gene expression [169]. PML body integrity is also a component of the host intrinsic antiviral defense and the displacement of PML protein from PML bodies by IE1 contributes for sustained viral gene expression [170,171].
9.2. IE2 Binds pRB and p53
Studies have shown that the protein encoded by the largest transcript of UL122, IE2 (also known as IE2-86 or IE86) specifically interacts with pRB [16,160,172,173]. Expression of IE2 is sufficient to alleviate pRB repression of E2F-responsive promoters [16,47,160]. IE2 can also interact with p53 [174,175,176]. However, expression of IE2 in a human cell line blocked cell cycle progression in G1-phase similar to the phenotype observed when permissive cells are infected with HCMV [153]. Moreover, a study using DNA microarrays to analyze the effects of IE2 protein on cellular gene expression reveals that the 86kDa form of IE2 induces the expression of numerous factors associated with cell cycle regulation and bioenzymatic machinery necessary for DNA replication. For example, IE2 expression results in an increase in the mRNA levels of B-myb, cyclin E, cdk-2, E2F1, ribonucleotide reductase subunit-1 and -2, thymidine synthetase, MCM3, and MCM7, among other factors associated with S phase [177]. This study shows that most of the genes induced by IE2 are E2F targets. What is striking is that even though IE2 inactivates pRB, derepresses E2F activity, and can inhibit cell cycle arrest functions of p53, this viral protein does not induce S phase as measured by cellular DNA replication. This observation is in stark contrast to pRB and p53 targeting proteins encoded by other viruses such as SV40 lg T or the combination of adenovirus E1A and E1B-55K proteins, which are potent inducers of cell cycle progression and S phase. A clearer understanding is needed as to why IE2 does not induce S phase.
9.3. pp71 Binds to pRB, p107 and p130
The pp71 phosphoprotein, expressed from the UL82 ORF, is a tegument protein that localizes to the nucleus immediately after virion entry during productive infections [178,179]. Functionally, pp71 is a transcription factor that is packaged within the viral tegument and is essential for the adequate accumulation of IE1 and IE2 by transactivating the major IE promoter and accelerating the infection cycle of HCMV [180]. Studies examining the relationship between pp71 and the cell cycle reveal that pp71 contains a sequence (LACSD) that is similar to the RB-binding motif (LxCxE) present in viral oncoproteins encoded by the small DNA tumor viruses. This motif is required for the induction of DNA synthesis in quiescent cells and for degradation of the RB family by viral oncoproteins [149,162]. pp71 binds to all three RB family members and promotes the G1/S cell cycle state during infection [149].
9.4. pUL97 Phosphorylates RB Protein
pUL97, another tegument protein, is a multifunctional viral protein kinase which is required at multiple steps during viral replication. Deletion of the pUL97 region from the viral genome or pharmacological inhibition of pUL97 kinase activity drastically reduces viral replication. A number of cellular and viral interacting proteins and substrates of pUL97 have been described, including viral pUL69 [181], pUL44 [182], autophosphorylated pUL97 [183,184] , and, given its cdk-like activity, cellular RB family proteins are also phosphorylated by pUL97 [150]. In addition, pUL97 is able to phosphorylate nuclear lamins, which contributes to the HCMV-induced reorganization of the nuclear lamina [185].
9.5. pUL69 Modulates CDK Function
As mentioned earlier, IE1, IE2 and pUL97 can interact or phosphorylate RB family members to inactivate protein function, which can, under certain circumstances, result in stimulation of cell cycle progression. However, during HCMV infection, cell cycle progression is blocked at multiple points, including the G1-to-S-phase transition. pUL69, a phosphorylated virion tegument protein [186], is reported to be a factor responsible for this cell cycle block [148]. Host CDKs and pUL97 phosphorylate pUL69 and modulate its nuclear localization and activity [181]. Although the mechanism by which pUL69 induces an accumulation of cells in G1 is not clear, these findings indicate that HCMV tegument proteins (pUL69, pp71, and pUL97) can have an immediate effect on the cell cycle. It seems that HCMV has developed a strategy to inhibit cellular DNA replication and, at the same time, alter the host environment in a manner that ensures the replication of viral DNA.
9.6. E2F1-mediated DNA Damage Response
In the normal condition, RB family members bind to E2F family members to limit cell cycle progression. During HCMV infection, at least four viral proteins (IE1, IE2, pp71 and pUL97) inactivate RB family proteins resulting in the release of E2F proteins. These derepressed E2F proteins then alter the expression of S phase genes that contribute activities and substrates that support viral DNA replication. In addition, deregulated E2F1 induces DSBs and stimulates a robust host DDR [94]. The mechanism responsible for this phenomenon is unclear but it is specific to E2F1 as other RB-targeted E2Fs, namely E2F2 or E2F3, do not induce double strand DNA breaks (DSBs) or DDR [164]. This relationship between E2F1 and DSBs appears relevant to HCMV, as it is the only activator E2F that contributes significantly to viral replication [94]. Moreover, ATM, the signal transducing kinase of many DDR, functions downstream of E2F1 deregulation and is also required for HCMV replication [94].
10. Which DDR Factors Contribute to HCMV Replication?
ATM has been implicated as a target of several DNA viruses (Table 1), which activate or inhibit the ATM signaling pathway [85]. Depletion or inhibition of ATM by RNA interference or by pharmacological compounds, respectively, demonstrate that ATM is a key kinase in DDR and also the most common DDR factor contributing to virus replication [94]. ATM and at least some downstream targets, like H2AX [94], p53 [187] contribute to HCMV replication. In addition, at least one DNA repair factor, DDB2 [188], influences HCMV replication.
11. Difference and Similarities in the DDR Induced by HCMV and Other Viruses
11.1. HCMV Is Similar to Other Viruses that Use DDR (ATM Signaling) for Replication
As listed in Table 1, many viruses can activate ATM, and most of them can use ATM signaling to promote viral replication [94,95,97,99,100,101,102,103,104,106,108,109,110,113,114,116,120,122,124,125]. HCMV infection as well as IE1 or IE2 transduction can activate ATM [94,98,157]. Using caffeine, a PI3 kinase-like inhibitor, KU 55933, an ATM-specific inhibitor [189], AT cells derived from patients with ataxia telangiectasia, or siRNAs to deplete ATM, results in reduced or blocked HCMV replication [94]. Here, HCMV is similar to many other viruses with DNA stages in their replication strategy, such as HSV-1, HIV, MHV-68, SV-40, and HPV, in its requirement for ATM signaling for replication.
11.2. HCMV is Different from Other Viruses Not Using ATM or that Block DDR for Replication
HCMV is different from Ad5 and KSHV or B19V in its requirement for ATM signaling for efficient replication. Ad5 has evolved mechanisms to inhibit DNA damage signaling and repair during infection by degrading and mislocalizing components of the Mre11–Rad50–NBS1 (MRN) DNA damage recognition complex [135]. In addition, the Ad5 E3 ligase complex (comprised of E1B-55K and E4 adenoviral proteins) is able to target a number of cellular DNA repair proteins for proteasomal degradation including the RecQ helicase, bloom helicase (BLM) [190]. KSHV vIRF1 protein compromises an ATM/p53-mediated DDR by targeting both upstream ATM kinase activity and also downstream p53 tumor suppressor function by facilitating its proteasome-mediated degradation [140]. However, a recent study shows that during early de novo infection of primary endothelial cells, KSHV induces DDR signaling and ATM kinase activation as measured by H2AX phosphorylation, which are essential for KSHV’s latent gene expression and establishment of latency [191]. Taken together, it seems that KSHV is able to both inhibit, and induce the cellular DDR dependent upon its replication strategy. Infection with B19V induces a broad range of DNA damage responses by activating three upstream kinases: ATM, ATR, and DNA-PKcs. Disruption of either the ATR or DNA-PKcs, but not ATM, signaling pathways significantly reduced the efficiency of B19V replication without affecting the resultant cell cycle arrest [111]. Thus, it appears that B19V uses ATR and DNAPKcs, but not ATM to facilitate its replication. Likewise, DNA-PKcs contribute to the replication of adeno-associated virus (AAV), another parvovirus [112]. Interestingly, AAV and Ad5 coinfections activate a broad DDR that is different from that seen during Ad5 or AAV infection alone [113].
12. HCMV Infection Results in the Relocalization of DDR Proteins to Virus Replication Compartments (RCs)
Viral RCs are sites of viral DNA replication and maturation. RCs begin as multiple, discrete structures early in infection, then move and coalesce into a single, large structure that can be referred to as a “mature” RC [94,192]. HCMV DNA replication happens in the RCs, similar to HSV-1 [95,193,194]. During HCMV infection, many DDR proteins are relocalized to the RCs including γH2AX, p53, pATM, MRE11, CHK2, NBS1, Rad50, ATRIP, and CHK1 [94,98]. Although the mechanism(s) responsible for this relocation is unclear, these DDR proteins might interact with the viral DNA replication machinery in RCs to regulate viral DNA replication, gene expression, or recruit DNA repair proteins to repair damage to viral DNA.
13. Does DNA Damage Exist in Cellular or Viral DNA During Infection?
Many DNA viruses and retroviruses can tether or integrate their genome into the host DNA during infection [195,196]. It has been suggested that viral genetic material can be recognized by the host as damage DNA and stimulate cellular DNA repair mechanisms [197]. Given these observations, questions arise regarding whether host DNA is damaged during infections and whether DNA damage exists in viral DNA.
Host chromosome breaks have been observed in HCMV-infected cells. Infection of cells during S-phase results in two specific breaks on chromosome 1 at positions 1q42 and 1q21 [198]. HCMV infection-associated damage to chromosome 1 appears not to be cell-type-or-strain specific. Cells infected with HSV also show an increased incidence of chromosome breaks [199,200]. Chromatid gaps and breaks were found to accumulate in region 3 of the X chromosome and in region 7 of chromosome 1 [201]. Adenovirus type 12 E1B protein can induce damage specifically at 17q21–22, lp36, 1q21, and lq42-43 and at random sites in cellular chromosomes [202]. For HCMV, it is not clear if infection causes extensive host DNA damage or whether the two DNA break is sufficient to initiate the observed host DDR.
Some studies suggest that viral adsorption and penetration is required for inducing chromosomal breaks whereas viral protein expression is not required for induction of damage [198]. Alternately, a viral or cellular protein component of the incoming virion may be responsible for the induced damage. Our laboratory has observed DDR at very early times of infection, even before de novo viral protein expression. Input tegument proteins have been suggested to be partly responsible for DDR soon after viral entry, perhaps by inactivating pRB and derepressing E2F1 [199].
Another possible source of the DDR is that incoming, virion-delivered viral DNA or the nascent viral DNA generated during replication, is damaged or contains mutations. Evidence for these possibilities exists for HSV [194,200,201,202,203,204]. One can imagine that the oxidative state of virally infected cells, in particular HCMV infected cells [205] may result in oxidation of nucleotides such as guanines in viral DNA. It is also possible that, even though herpesviruses encode replicase complexes with proof reading activity, the high levels of (viral) DNA replication during infection may result in an accumulation of mutations that stimulate the relocalization of DDR proteins to viral RCs.
Activation of DDR does not necessarily require DNA lesions. Prolonged binding of DNA repair factors to chromatin can elicit DNA damage response in an ATM- and DNA-PK dependent manner in the absence of DNA lesions [206]. The herpesviral genomes are synthesized in a rolling circle manner to produce head-to-tail concatemers that are subsequently cleaved into unit-length genomes [207] and either the replication complexes or the cleaved DNA may be recognized as damaged DNA and trigger a DDR. In addition, viral infections confront cells with large amounts of exogenous genetic material that might be broadly recognized as abnormal [197] or the physical interaction of DNA repair factors with chromatin can be sufficient to activate the DDR signaling cascade [206]. Another possibility is that infected cells recognize viral replication as a genotoxic stress and elicit a DDR. In the case of HCMV infection, the inactivation of RB family members by IE1, IE2, pp71, and pUL97 and subsequent deregulation of E2F1 appears to result in DSBs in human fibroblasts [94]. Thus, it is possible that a trigger of the virus-induced DDR is not necessarily the recognition of linear viral DNA as double-strand breaks or actual damage to DNA, but rather, it is the recruitment of DNA damage repair factors to RCs or, very likely, a combination of these possibilities. Clearly, much work is needed to understand the interplay of viral infection, the presence of viral DNA and proteins, the remodeling of host cells function and the resultant DDR.
14. Future Perspectives
Although it is well accepted that activation of host DDR is a common theme of infections with DNA viruses, many of which require DDR signaling to replicate, more detailed study is still needed to better understand the “hows” and “whys” of this relationship. Most small DNA viruses capable of infecting nondividing cells induce S phase in order to activate the host DNA replication machinery to provide the nucleotide triphosphates and host replication machinery necessary for viral DNA replication [93]. However, many large DNA viruses, such as herpesviruses, code for not only their own viral replicase enzymes, but also factors involved in deoxynucleotide synthesis and do not require a canonical S phase to support viral replication [92]. It has been thought that the host DNA polymerases do not play a role in herpesviral DNA replication. However, our unpublished studies suggest that host DNA polymerases may contribute to HCMV replication with the implication that theses enzymes may contribute activities that are different from the viral polymerase [208]. It should be interesting to explore the roles of host DNA polymerases in the replication of HCMV and other viruses.
Components of the ATM-pathway are activated and recruited to sites of viral DNA synthesis including HCMV infected cells. Although ATR- and ATM-mediated pathways are related and both can be activated by similar genotoxic events, HSV-1 distinguishes between these two pathways, inactivating ATR pathway and potentially using ATM pathway. In contrast, it has been noted that the steady-state levels of ATR increase in HCMV infected cells at 48 hpi accompanied by a shift in mobility [98]. The ATR pathway also responds to DSBs, generally more slowly than ATM [209]. Determining the contribution of ATR signaling in relation to ATM signaling during HCMV infection should provide important insight into the specifics of DDR signaling as it pertains to HCMV infection and viral DNA replication,
HSV and HCMV infections, and the adenovirus type 12 E1B protein induce chromosome breaks in specific chromosome regions. The particular nonrandom distribution of chromosome aberrations in the HSV and HCMV infected cells raise a question as to whether virion genome deposition or early replication localized to particular chromosome regions are responsible for these effects.
The mechanism by which E2F1 stimulates host DDR is not well understood. Inactivation of RB and the subsequent deregulation of E2F1, but not the related family members, E2F2 or E2F3, leads to an accumulation of DSBs in human fibroblasts as observed by the γH2AX immunostaining and neutral comet assays [164]. The mechanism by which E2F1 stimulates host DDR needs to be further studied during infection by HCMV and other viruses.
Ubiquitination and sumolyation is one of the most common mechanisms by which viruses target cellular proteins. Viruses can encode their own ubiquitin ligases, such as the ICP0 protein of HSV-1. ICP0 interacts with PML isoform I and induces its SUMO-independent degradation [210]. Other viral proteins, such as Ad-E1B55K/E4orf6, KSHV-LANA, HPV-E6, and HIV-Vpr, can recruit and redirect cellular ubiquitin ligase complexes to target proteins. A recent study shows that a DNA repair factor, DDB2, can contribute to HCMV replication [188]. DDB2 is a component of a Cul4A-Ub ligase complex. The DDB1-CUL4ADDB2 complex is a cullin-RING (i.e., E3) Ub-ligase that targets histone H2A at UV-damaged DNA sites. Whether the ubiquitination ability of this complex is involved in HCMV replication needs further investigation.
One wonders if the intimate relationship between the host DDR and HCMV infection can be leveraged to treat HCMV-associated diseases. For example, there are no licensed treatments for pregnant women who undergo primary infection with HCMV during pregnancy, which places the fetus at risk for symptomatic congenital infection. This is at least partly due to the fact that drugs that are effective against HCMV infection have serious teratogenic side effects, which make them inappropriate for use during pregnancy. HCMV is also a common complication during tissue transplantation. Here, ganciclovir is commonly used to treat HCMV disease. However, ganciclovir induces leukocytopenia, an unwanted side effect in patients already receiving immunosuppressants. Perhaps, ATM inhibitors or more appropriately, drugs that target ATM responsive factors can be used alone or as adjuvant therapies to prevent congenital CMV infections or other diseases associated with infection. Continued study of infection-induced DDR pathways is essential for a better understanding of host-pathogen interactions and the development of host-targeted therapeutics.
Acknowledgments
We thank the members of the Kowalik laboratory for comments on the manuscript. We apologize to the many groups whose primary research papers were not cited due to unintentional omissions on our part. This publication was supported by grants from the National Institutes of Health (R01AI076189; R01HD061959). The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Authors Contributions
Both authors wrote the manuscript and generated the figures.
Conflicts of Interest
The authors declare no conflicts of interest.
References and Notes
- 1.Vancikova Z., Dvorak P. Cytomegalovirus infection in immunocompetent and immunocompromised individuals—A review. Curr. Drug Targets Immune Endocr. Metabol. Disord. 2001;1:179–187. doi: 10.2174/1568008013341334. [DOI] [PubMed] [Google Scholar]
- 2.Cheeran M.C., Lokensgard J.R., Schleiss M.R. Neuropathogenesis of congenital cytomegalovirus infection: disease mechanisms and prospects for intervention. Clin. Microbiol. Rev. 2009;22:99–126. doi: 10.1128/CMR.00023-08. Table of Contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Griffiths P.D., Walter S. Cytomegalovirus. Curr. Opin. Infect. Dis. 2005;18:241–245. doi: 10.1097/01.qco.0000168385.39390.1b. [DOI] [PubMed] [Google Scholar]
- 4.Dziurzynski K., Chang S.M., Heimberger A.B., Kalejta R.F., McGregor Dallas S.R., Smit M., Soroceanu L., Cobbs C.S. Consensus on the role of human cytomegalovirus in glioblastoma. Neuro Oncol. 2012;14:246–255. doi: 10.1093/neuonc/nor227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dolan A., Cunningham C., Hector R.D., Hassan-Walker A.F., Lee L., Addison C., Dargan D.J., McGeoch D.J., Gatherer D., Emery V.C., et al. Genetic content of wild-type human cytomegalovirus. J. Gen. Virol. 2004;85:1301–1312. doi: 10.1099/vir.0.79888-0. [DOI] [PubMed] [Google Scholar]
- 6.Chee M.S., Bankier A.T., Beck S., Bohni R., Brown C.M., Cerny R., Horsnell T., Hutchison C.A., 3rd, Kouzarides T., Martignetti J.A., et al. Analysis of the protein-coding content of the sequence of human cytomegalovirus strain AD169. Curr. Top. Microbiol. Immunol. 1990;154:125–169. doi: 10.1007/978-3-642-74980-3_6. [DOI] [PubMed] [Google Scholar]
- 7.Rigoutsos I., Novotny J., Huynh T., Chin-Bow S.T., Parida L., Platt D., Coleman D., Shenk T. In silico pattern-based analysis of the human cytomegalovirus genome. J. Virol. 2003;77:4326–4344. doi: 10.1128/JVI.77.7.4326-4344.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stern-Ginossar N., Weisburd B., Michalski A., Le V.T., Hein M.Y., Huang S.X., Ma M., Shen B., Qian S.B., Hengel H., et al. Decoding human cytomegalovirus. Science. 2012;338:1088–1093. doi: 10.1126/science.1227919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Renzette N., Bhattacharjee B., Jensen J.D., Gibson L., Kowalik T.F. Extensive genome-wide variability of human cytomegalovirus in congenitally infected infants. PLoS Pathog. 2011;7:e1001344. doi: 10.1371/journal.ppat.1001344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Varnum S.M., Streblow D.N., Monroe M.E., Smith P., Auberry K.J., Pasa-Tolic L., Wang D., Camp D.G., 2nd, Rodland K., Wiley S., et al. Identification of proteins in human cytomegalovirus (HCMV) particles: The HCMV proteome. J. Virol. 2004;78:10960–10966. doi: 10.1128/JVI.78.20.10960-10966.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baldick C.J., Jr., Shenk T. Proteins associated with purified human cytomegalovirus particles. J. Virol. 1996;70:6097–6105. doi: 10.1128/jvi.70.9.6097-6105.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hayashi M.L., Blankenship C., Shenk T. Human cytomegalovirus UL69 protein is required for efficient accumulation of infected cells in the G1 phase of the cell cycle. Proc. Natl. Acad. Sci. USA. 2000;97:2692–2696. doi: 10.1073/pnas.050587597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.DeMarchi J.M., Schmidt C.A., Kaplan A.S. Patterns of transcription of human cytomegalovirus in permissively infected cells. J. Virol. 1980;35:277–286. doi: 10.1128/jvi.35.2.277-286.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wathen M.W., Stinski M.F. Temporal patterns of human cytomegalovirus transcription: Mapping the viral RNAs synthesized at immediate early, early, and late times after infection. J. Virol. 1982;41:462–477. doi: 10.1128/jvi.41.2.462-477.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stamminger T., Fleckenstein B. Immediate-early transcription regulation of human cytomegalovirus. Curr. Top. Microbiol. Immunol. 1990;154:3–19. doi: 10.1007/978-3-642-74980-3_1. [DOI] [PubMed] [Google Scholar]
- 16.Fortunato E.A., Sommer M.H., Yoder K., Spector D.H. Identification of domains within the human cytomegalovirus major immediate-early 86-kilodalton protein and the retinoblastoma protein required for physical and functional interaction with each other. J. Virol. 1997;71:8176–8185. doi: 10.1128/jvi.71.11.8176-8185.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Poma E.E., Kowalik T.F., Zhu L., Sinclair J.H., Huang E.S. The human cytomegalovirus IE1–72 protein interacts with the cellular p107 protein and relieves p107-mediated transcriptional repression of an E2F-responsive promoter. J. Virol. 1996;70:7867–7877. doi: 10.1128/jvi.70.11.7867-7877.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fortunato E.A., Spector D.H. Regulation of human cytomegalovirus gene expression. Adv. Virus Res. 1999;54:61–128. doi: 10.1016/S0065-3527(08)60366-8. [DOI] [PubMed] [Google Scholar]
- 19.Penfold M.E., Mocarski E.S. Formation of cytomegalovirus DNA replication compartments defined by localization of viral proteins and DNA synthesis. Virology. 1997;239:46–61. doi: 10.1006/viro.1997.8848. [DOI] [PubMed] [Google Scholar]
- 20.Soderberg-Naucler C., Fish K.N., Nelson J.A. Reactivation of latent human cytomegalovirus by allogeneic stimulation of blood cells from healthy donors. Cell. 1997;91:119–126. doi: 10.1016/S0092-8674(01)80014-3. [DOI] [PubMed] [Google Scholar]
- 21.Soderberg-Naucler C., Streblow D.N., Fish K.N., Allan-Yorke J., Smith P.P., Nelson J.A. Reactivation of latent human cytomegalovirus in CD14(+) monocytes is differentiation dependent. J. Virol. 2001;75:7543–7554. doi: 10.1128/JVI.75.16.7543-7554.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Soderberg-Naucler C., Fish K.N., Nelson J.A. Interferon-gamma and tumor necrosis factor-alpha specifically induce formation of cytomegalovirus-permissive monocyte-derived macrophages that are refractory to the antiviral activity of these cytokines. J. Clin. Invest. 1997;100:3154–3163. doi: 10.1172/JCI119871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kondo K., Kaneshima H., Mocarski E.S. Human cytomegalovirus latent infection of granulocyte-macrophage progenitors. Proc. Natl. Acad. Sci. USA. 1994;91:11879–11883. doi: 10.1073/pnas.91.25.11879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hahn G., Jores R., Mocarski E.S. Cytomegalovirus remains latent in a common precursor of dendritic and myeloid cells. Proc. Natl. Acad. Sci. USA. 1998;95:3937–3942. doi: 10.1073/pnas.95.7.3937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goodrum F.D., Jordan C.T., High K., Shenk T. Human cytomegalovirus gene expression during infection of primary hematopoietic progenitor cells: A model for latency. Proc. Natl. Acad. Sci. USA. 2002;99:16255–16260. doi: 10.1073/pnas.252630899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Petrucelli A., Rak M., Grainger L., Goodrum F. Characterization of a novel Golgi apparatus-localized latency determinant encoded by human cytomegalovirus. J. Virol. 2009;83:5615–5629. doi: 10.1128/JVI.01989-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goodrum F., Reeves M., Sinclair J., High K., Shenk T. Human cytomegalovirus sequences expressed in latently infected individuals promote a latent infection in vitro. Blood. 2007;110:937–945. doi: 10.1182/blood-2007-01-070078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mason G.M., Poole E., Sissons J.G., Wills M.R., Sinclair J.H. Human cytomegalovirus latency alters the cellular secretome, inducing cluster of differentiation (CD)4+ T-cell migration and suppression of effector function. Proc. Natl. Acad. Sci. USA. 2012;109:14538–14543. doi: 10.1073/pnas.1204836109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jackson S.P., Bartek J. The DNA-damage response in human biology and disease. Nature. 2009;461:1071–1078. doi: 10.1038/nature08467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Halazonetis T.D., Gorgoulis V.G., Bartek J. An oncogene-induced DNA damage model for cancer development. Science. 2008;319:1352–1355. doi: 10.1126/science.1140735. [DOI] [PubMed] [Google Scholar]
- 31.Nyberg K.A., Michelson R.J., Putnam C.W., Weinert T.A. Toward maintaining the genome: DNA damage and replication checkpoints. Annu. Rev. Genet. 2002;36:617–656. doi: 10.1146/annurev.genet.36.060402.113540. [DOI] [PubMed] [Google Scholar]
- 32.Elledge S.J. Cell cycle checkpoints: Preventing an identity crisis. Science. 1996;274:1664–1672. doi: 10.1126/science.274.5293.1664. [DOI] [PubMed] [Google Scholar]
- 33.Sancar A., Lindsey-Boltz L.A., Unsal-Kacmaz K., Linn S. Molecular mechanisms of mammalian DNA repair and the DNA damage checkpoints. Annu. Rev. Biochem. 2004;73:39–85. doi: 10.1146/annurev.biochem.73.011303.073723. [DOI] [PubMed] [Google Scholar]
- 34.O’Driscoll M., Gennery A.R., Seidel J., Concannon P., Jeggo P.A. An overview of three new disorders associated with genetic instability: LIG4 syndrome, RS-SCID and ATR-Seckel syndrome. DNA Repair (Amst) 2004;3:1227–1235. doi: 10.1016/j.dnarep.2004.03.025. [DOI] [PubMed] [Google Scholar]
- 35.Shiloh Y. ATM and related protein kinases: Safeguarding genome integrity. Nat. Rev. Cancer. 2003;3:155–168. doi: 10.1038/nrc1011. [DOI] [PubMed] [Google Scholar]
- 36.Taylor A.M., Groom A., Byrd P.J. Ataxia-telangiectasia-like disorder (ATLD)-its clinical presentation and molecular basis. DNA Repair (Amst) 2004;3:1219–1225. doi: 10.1016/j.dnarep.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 37.Kastan M.B., Bartek J. Cell-cycle checkpoints and cancer. Nature. 2004;432:316–323. doi: 10.1038/nature03097. [DOI] [PubMed] [Google Scholar]
- 38.Khanna K.K., Jackson S.P. DNA double-strand breaks: Signaling, repair and the cancer connection. Nat. Genet. 2001;27:247–254. doi: 10.1038/85798. [DOI] [PubMed] [Google Scholar]
- 39.Shiloh Y., Kastan M.B. ATM: Genome stability, neuronal development, and cancer cross paths. Adv. Cancer Res. 2001;83:209–254. doi: 10.1016/S0065-230X(01)83007-4. [DOI] [PubMed] [Google Scholar]
- 40.Alexander A., Cai S.L., Kim J., Nanez A., Sahin M., MacLean K.H., Inoki K., Guan K.L., Shen J., Person M.D., et al. ATM signals to TSC2 in the cytoplasm to regulate mTORC1 in response to ROS. Proc. Natl. Acad. Sci. USA. 2010;107:4153–4158. doi: 10.1073/pnas.0913860107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Guo Z., Kozlov S., Lavin M.F., Person M.D., Paull T.T. ATM activation by oxidative stress. Science. 2010;330:517–521. doi: 10.1126/science.1192912. [DOI] [PubMed] [Google Scholar]
- 42.Hinz M., Stilmann M., Arslan S.C., Khanna K.K., Dittmar G., Scheidereit C. A cytoplasmic ATM-TRAF6-cIAP1 module links nuclear DNA damage signaling to ubiquitin-mediated NF-kappaB activation. Mol. Cell. 2010;40:63–74. doi: 10.1016/j.molcel.2010.09.008. [DOI] [PubMed] [Google Scholar]
- 43.Li B., Wang X., Rasheed N., Hu Y., Boast S., Ishii T., Nakayama K., Nakayama K.I., Goff S.P. Distinct roles of c-Abl and Atm in oxidative stress response are mediated by protein kinase C delta. Genes Dev. 2004;18:1824–1837. doi: 10.1101/gad.1223504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Abraham R.T. Cell cycle checkpoint signaling through the ATM and ATR kinases. Genes Dev. 2001;15:2177–2196. doi: 10.1101/gad.914401. [DOI] [PubMed] [Google Scholar]
- 45.Burma S., Chen B.P., Chen D.J. Role of non-homologous end joining (NHEJ) in maintaining genomic integrity. DNA Repair (Amst) 2006;5:1042–1048. doi: 10.1016/j.dnarep.2006.05.026. [DOI] [PubMed] [Google Scholar]
- 46.Matsuoka S., Ballif B.A., Smogorzewska A., McDonald E.R., 3rd, Hurov K.E., Luo J., Bakalarski C.E., Zhao Z., Solimini N., Lerenthal Y., et al. ATM and ATR substrate analysis reveals extensive protein networks responsive to DNA damage. Science. 2007;316:1160–1166. doi: 10.1126/science.1140321. [DOI] [PubMed] [Google Scholar]
- 47.Nevins J.R. E2F: A link between the Rb tumor suppressor protein and viral oncoproteins. Science. 1992;258:424–429. doi: 10.1126/science.1411535. [DOI] [PubMed] [Google Scholar]
- 48.Dyson N. pRB, p107 and the regulation of the E2F transcription factor. J. Cell. Sci. Suppl. 1994;18:81–87. doi: 10.1242/jcs.1994.Supplement_18.12. [DOI] [PubMed] [Google Scholar]
- 49.La Thangue N.B. DP and E2F proteins: Components of a heterodimeric transcription factor implicated in cell cycle control. Curr. Opin. Cell Biol. 1994;6:443–450. doi: 10.1016/0955-0674(94)90038-8. [DOI] [PubMed] [Google Scholar]
- 50.La Thangue N.B. E2F and the molecular mechanisms of early cell-cycle control. Biochem. Soc. Trans. 1996;24:54–59. doi: 10.1042/bst0240054. [DOI] [PubMed] [Google Scholar]
- 51.Chellappan S.P., Hiebert S., Mudryj M., Horowitz J.M., Nevins J.R. The E2F transcription factor is a cellular target for the RB protein. Cell. 1991;65:1053–1061. doi: 10.1016/0092-8674(91)90557-F. [DOI] [PubMed] [Google Scholar]
- 52.Helin K., Lees J.A., Vidal M., Dyson N., Harlow E., Fattaey A. A cDNA encoding a pRB-binding protein with properties of the transcription factor E2F. Cell. 1992;70:337–350. doi: 10.1016/0092-8674(92)90107-N. [DOI] [PubMed] [Google Scholar]
- 53.Kaelin W.G., Jr., Krek W., Sellers W.R., DeCaprio J.A., Ajchenbaum F., Fuchs C.S., Chittenden T., Li Y., Farnham P.J., Blanar M.A., et al. Expression cloning of a cDNA encoding a retinoblastoma-binding protein with E2F-like properties. Cell. 1992;70:351–364. doi: 10.1016/0092-8674(92)90108-O. [DOI] [PubMed] [Google Scholar]
- 54.Qin X.Q., Livingston D.M., Ewen M., Sellers W.R., Arany Z., Kaelin W.G., Jr. The transcription factor E2F-1 is a downstream target of RB action. Mol. Cell. Biol. 1995;15:742–755. doi: 10.1128/mcb.15.2.742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ikeda M.A., Jakoi L., Nevins J.R. A unique role for the Rb protein in controlling E2F accumulation during cell growth and differentiation. Proc. Natl. Acad. Sci. USA. 1996;93:3215–3220. doi: 10.1073/pnas.93.8.3215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chellappan S., Kraus V.B., Kroger B., Munger K., Howley P.M., Phelps W.C., Nevins J.R. Adenovirus E1A, simian virus 40 tumor antigen, and human papillomavirus E7 protein share the capacity to disrupt the interaction between transcription factor E2F and the retinoblastoma gene product. Proc. Natl. Acad. Sci. USA. 1992;89:4549–4553. doi: 10.1073/pnas.89.10.4549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nevins J.R. Disruption of cell-cycle control by viral oncoproteins. Biochem. Soc. Trans. 1993;21:935–938. doi: 10.1042/bst0210935. [DOI] [PubMed] [Google Scholar]
- 58.Nevins J.R. Cell cycle targets of the DNA tumor viruses. Curr. Opin. Genet. Dev. 1994;4:130–134. doi: 10.1016/0959-437x(94)90101-5. [DOI] [PubMed] [Google Scholar]
- 59.Moberg K., Starz M.A., Lees J.A. E2F-4 switches from p130 to p107 and pRB in response to cell cycle reentry. Mol. Cell. Biol. 1996;16:1436–1449. doi: 10.1128/mcb.16.4.1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ginsberg D., Vairo G., Chittenden T., Xiao Z.X., Xu G., Wydner K.L., DeCaprio J.A., Lawrence J.B., Livingston D.M. E2F-4, a new member of the E2F transcription factor family, interacts with p107. Genes Dev. 1994;8:2665–2679. doi: 10.1101/gad.8.22.2665. [DOI] [PubMed] [Google Scholar]
- 61.Hijmans E.M., Voorhoeve P.M., Beijersbergen R.L., van’t Veer L.J., Bernards R. E2F-5, a new E2F family member that interacts with p130 in vivo. Mol. Cell. Biol. 1995;15:3082–3089. doi: 10.1128/mcb.15.6.3082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vairo G., Livingston D.M., Ginsberg D. Functional interaction between E2F-4 and p130: Evidence for distinct mechanisms underlying growth suppression by different retinoblastoma protein family members. Genes Dev. 1995;9:869–881. doi: 10.1101/gad.9.7.869. [DOI] [PubMed] [Google Scholar]
- 63.Beijersbergen R.L., Kerkhoven R.M., Zhu L., Carlee L., Voorhoeve P.M., Bernards R. E2F-4, a new member of the E2F gene family, has oncogenic activity and associates with p107 in vivo. Genes Dev. 1994;8:2680–2690. doi: 10.1101/gad.8.22.2680. [DOI] [PubMed] [Google Scholar]
- 64.Morkel M., Wenkel J., Bannister A.J., Kouzarides T., Hagemeier C. An E2F-like repressor of transcription. Nature. 1997;390:567–568. doi: 10.1038/37507. [DOI] [PubMed] [Google Scholar]
- 65.Cartwright P., Muller H., Wagener C., Holm K., Helin K. E2F-6: A novel member of the E2F family is an inhibitor of E2F-dependent transcription. Oncogene. 1998;17:611–623. doi: 10.1038/sj.onc.1201975. [DOI] [PubMed] [Google Scholar]
- 66.Gaubatz S., Wood J.G., Livingston D.M. Unusual proliferation arrest and transcriptional control properties of a newly discovered E2F family member, E2F-6. Proc. Natl. Acad. Sci. USA. 1998;95:9190–9195. doi: 10.1073/pnas.95.16.9190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Trimarchi J.M., Fairchild B., Verona R., Moberg K., Andon N., Lees J.A. E2F-6, a member of the E2F family that can behave as a transcriptional repressor. Proc. Natl. Acad. Sci. USA. 1998;95:2850–2855. doi: 10.1073/pnas.95.6.2850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.de Bruin A., Maiti B., Jakoi L., Timmers C., Buerki R., Leone G. Identification and characterization of E2F7, a novel mammalian E2F family member capable of blocking cellular proliferation. J. Biol. Chem. 2003;278:42041–42049. doi: 10.1074/jbc.M308105200. [DOI] [PubMed] [Google Scholar]
- 69.Di Stefano L., Jensen M.R., Helin K. E2F7, a novel E2F featuring DP-independent repression of a subset of E2F-regulated genes. EMBO J. 2003;22:6289–6298. doi: 10.1093/emboj/cdg613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Roos W.P., Kaina B. DNA damage-induced cell death by apoptosis. Trends Mol. Med. 2006;12:440–450. doi: 10.1016/j.molmed.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 71.Zhou B.B., Bartek J. Targeting the checkpoint kinases: Chemosensitization versus chemoprotection. Nat. Rev. Cancer. 2004;4:216–225. doi: 10.1038/nrc1296. [DOI] [PubMed] [Google Scholar]
- 72.Bartek J., Lukas J. Chk1 and Chk2 kinases in checkpoint control and cancer. Cancer Cell. 2003;3:421–429. doi: 10.1016/S1535-6108(03)00110-7. [DOI] [PubMed] [Google Scholar]
- 73.Zhao H., Watkins J.L., Piwnica-Worms H. Disruption of the checkpoint kinase 1/cell division cycle 25A pathway abrogates ionizing radiation-induced S and G2 checkpoints. Proc. Natl. Acad. Sci. USA. 2002;99:14795–14800. doi: 10.1073/pnas.182557299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lukas C., Bartkova J., Latella L., Falck J., Mailand N., Schroeder T., Sehested M., Lukas J., Bartek J. DNA damage-activated kinase Chk2 is independent of proliferation or differentiation yet correlates with tissue biology. Cancer Res. 2001;61:4990–4993. [PubMed] [Google Scholar]
- 75.Hlavova M., Cizkova M., Vitova M., Bisova K., Zachleder V. DNA damage during G2 phase does not affect cell cycle progression of the green alga Scenedesmus quadricauda. PLoS One. 2011;6:e19626. doi: 10.1371/journal.pone.0019626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Manke I.A., Nguyen A., Lim D., Stewart M.Q., Elia A.E., Yaffe M.B. MAPKAP kinase-2 is a cell cycle checkpoint kinase that regulates the G2/M transition and S phase progression in response to UV irradiation. Mol. Cell. 2005;17:37–48. doi: 10.1016/j.molcel.2004.11.021. [DOI] [PubMed] [Google Scholar]
- 77.Reinhardt H.C., Aslanian A.S., Lees J.A., Yaffe M.B. p53-deficient cells rely on ATM- and ATR-mediated checkpoint signaling through the p38MAPK/MK2 pathway for survival after DNA damage. Cancer Cell. 2007;11:175–189. doi: 10.1016/j.ccr.2006.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kyriakis J.M., Avruch J. Mammalian mitogen-activated protein kinase signal transduction pathways activated by stress and inflammation. Physiol. Rev. 2001;81:807–869. doi: 10.1152/physrev.2001.81.2.807. [DOI] [PubMed] [Google Scholar]
- 79.Raman M., Earnest S., Zhang K., Zhao Y., Cobb M.H. TAO kinases mediate activation of p38 in response to DNA damage. EMBO J. 2007;26:2005–2014. doi: 10.1038/sj.emboj.7601668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hirose Y., Katayama M., Stokoe D., Haas-Kogan D.A., Berger M.S., Pieper R.O. The p38 mitogen-activated protein kinase pathway links the DNA mismatch repair system to the G2 checkpoint and to resistance to chemotherapeutic DNA-methylating agents. Mol. Cell. Biol. 2003;23:8306–8315. doi: 10.1128/MCB.23.22.8306-8315.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mikhailov A., Shinohara M., Rieder C.L. Topoisomerase II and histone deacetylase inhibitors delay the G2/M transition by triggering the p38 MAPK checkpoint pathway. J. Cell Biol. 2004;166:517–526. doi: 10.1083/jcb.200405167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Reinhardt H.C., Yaffe M.B. Kinases that control the cell cycle in response to DNA damage: Chk1, Chk2, and MK2. Curr. Opin. Cell Biol. 2009;21:245–255. doi: 10.1016/j.ceb.2009.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bouwman P., Jonkers J. The effects of deregulated DNA damage signalling on cancer chemotherapy response and resistance. Nat. Rev. Cancer. 2012;12:587–598. doi: 10.1038/nrc3342. [DOI] [PubMed] [Google Scholar]
- 84.Lazzaro F., Giannattasio M., Puddu F., Granata M., Pellicioli A., Plevani P., Muzi-Falconi M. Checkpoint mechanisms at the intersection between DNA damage and repair. DNA Repair (Amst) 2009;8:1055–1067. doi: 10.1016/j.dnarep.2009.04.022. [DOI] [PubMed] [Google Scholar]
- 85.Lilley C.E., Schwartz R.A., Weitzman M.D. Using or abusing: Viruses and the cellular DNA damage response. Trends Microbiol. 2007;15:119–126. doi: 10.1016/j.tim.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 86.McFadden K., Luftig M.A. Interplay between DNA tumor viruses and the host DNA damage response. Curr. Top. Microbiol. Immunol. 2013;371:229–257. doi: 10.1007/978-3-642-37765-5_9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Nikitin P.A., Luftig M.A. At a crossroads: Human DNA tumor viruses and the host DNA damage response. Future Virol. 2011;6:813–830. doi: 10.2217/fvl.11.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Turnell A.S., Grand R.J. DNA viruses and the cellular DNA-damage response. J. Gen. Virol. 2012;93:2076–2097. doi: 10.1099/vir.0.044412-0. [DOI] [PubMed] [Google Scholar]
- 89.Weitzman M.D., Lilley C.E., Chaurushiya M.S. Genomes in conflict: Maintaining genome integrity during virus infection. Annu. Rev. Microbiol. 2010;64:61–81. doi: 10.1146/annurev.micro.112408.134016. [DOI] [PubMed] [Google Scholar]
- 90.Stracker T.H., Carson C.T., Weitzman M.D. Adenovirus oncoproteins inactivate the Mre11-Rad50-NBS1 DNA repair complex. Nature. 2002;418:348–352. doi: 10.1038/nature00863. [DOI] [PubMed] [Google Scholar]
- 91.Chaurushiya M.S., Weitzman M.D. Viral manipulation of DNA repair and cell cycle checkpoints. DNA Repair (Amst) 2009;8:1166–1176. doi: 10.1016/j.dnarep.2009.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Flemington E.K. Herpesvirus lytic replication and the cell cycle: Arresting new developments. J. Virol. 2001;75:4475–4481. doi: 10.1128/JVI.75.10.4475-4481.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Nascimento R., Costa H., Parkhouse R.M. Virus manipulation of cell cycle. Protoplasma. 2012;249:519–528. doi: 10.1007/s00709-011-0327-9. [DOI] [PubMed] [Google Scholar]
- 94.E X., Pickering M.T., Debatis M., Castillo J., Lagadinos A., Wang S., Lu S., Kowalik T.F. An E2F1-mediated DNA damage response contributes to the replication of human cytomegalovirus. PLoS Pathog. 2011;7:e1001342. doi: 10.1371/journal.ppat.1001342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lilley C.E., Carson C.T., Muotri A.R., Gage F.H., Weitzman M.D. DNA repair proteins affect the lifecycle of herpes simplex virus 1. Proc. Natl. Acad. Sci. USA. 2005;102:5844–5849. doi: 10.1073/pnas.0501916102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lu C.C., Chen Y.C., Wang J.T., Yang P.W., Chen M.R. Xeroderma pigmentosum C is involved in Epstein Barr virus DNA replication. J. Gen. Virol. 2007;88:3234–3243. doi: 10.1099/vir.0.83212-0. [DOI] [PubMed] [Google Scholar]
- 97.Boichuk S., Hu L., Hein J., Gjoerup O.V. Multiple DNA damage signaling and repair pathways deregulated by simian virus 40 large T antigen. J. Virol. 2010;84:8007–8020. doi: 10.1128/JVI.00334-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Luo M.H., Rosenke K., Czornak K., Fortunato E.A. Human cytomegalovirus disrupts both ataxia telangiectasia mutated protein (ATM)- and ATM-Rad3-related kinase-mediated DNA damage responses during lytic infection. J. Virol. 2007;81:1934–1950. doi: 10.1128/JVI.01670-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kudoh A., Fujita M., Zhang L., Shirata N., Daikoku T., Sugaya Y., Isomura H., Nishiyama Y., Tsurumi T. Epstein-Barr virus lytic replication elicits ATM checkpoint signal transduction while providing an S-phase-like cellular environment. J. Biol. Chem. 2005;280:8156–8163. doi: 10.1074/jbc.M411405200. [DOI] [PubMed] [Google Scholar]
- 100.Liang X., Pickering M.T., Cho N.H., Chang H., Volkert M.R., Kowalik T.F., Jung J.U. Deregulation of DNA damage signal transduction by herpesvirus latency-associated M2. J. Virol. 2006;80:5862–5874. doi: 10.1128/JVI.02732-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Tarakanova V.L., Leung-Pineda V., Hwang S., Yang C.W., Matatall K., Basson M., Sun R., Piwnica-Worms H., Sleckman B.P., Virgin H.W. Gamma-herpesvirus kinase actively initiates a DNA damage response by inducing phosphorylation of H2AX to foster viral replication. Cell Host Microbe. 2007;1:275–286. doi: 10.1016/j.chom.2007.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Shi Y., Dodson G.E., Shaikh S., Rundell K., Tibbetts R.S. Ataxia-telangiectasia-mutated (ATM) is a T-antigen kinase that controls SV40 viral replication in vivo. J. Biol. Chem. 2005;280:40195–40200. doi: 10.1074/jbc.C500400200. [DOI] [PubMed] [Google Scholar]
- 103.Zhao X., Madden-Fuentes R.J., Lou B.X., Pipas J.M., Gerhardt J., Rigell C.J., Fanning E. Ataxia telangiectasia-mutated damage-signaling kinase- and proteasome-dependent destruction of Mre11-Rad50-Nbs1 subunits in Simian virus 40-infected primate cells. J. Virol. 2008;82:5316–5328. doi: 10.1128/JVI.02677-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Fradet-Turcotte A., Bergeron-Labrecque F., Moody C.A., Lehoux M., Laimins L.A., Archambault J. Nuclear accumulation of the papillomavirus E1 helicase blocks S-phase progression and triggers an ATM-dependent DNA damage response. J. Virol. 2011;85:8996–9012. doi: 10.1128/JVI.00542-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kadaja M., Isok-Paas H., Laos T., Ustav E., Ustav M. Mechanism of genomic instability in cells infected with the high-risk human papillomaviruses. PLoS Pathog. 2009;5:e1000397. doi: 10.1371/journal.ppat.1000397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Moody C.A., Laimins L.A. Human papillomaviruses activate the ATM DNA damage pathway for viral genome amplification upon differentiation. PLoS Pathog. 2009;5:e1000605. doi: 10.1371/journal.ppat.1000605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Reinson T., Toots M., Kadaja M., Pipitch R., Allik M., Ustav E., Ustav M. Engagement of the ATR-dependent DNA damage response at the human papillomavirus 18 replication centers during the initial amplification. J. Virol. 2013;87:951–964. doi: 10.1128/JVI.01943-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sakakibara N., Mitra R., McBride A.A. The papillomavirus E1 helicase activates a cellular DNA damage response in viral replication foci. J. Virol. 2011;85:8981–8995. doi: 10.1128/JVI.00541-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lou S., Luo Y., Cheng F., Huang Q., Shen W., Kleiboeker S., Tisdale J.F., Liu Z., Qiu J. Human parvovirus B19 DNA replication induces a DNA damage response that is dispensable for cell cycle arrest at phase G2/M. J. Virol. 2012;86:10748–10758. doi: 10.1128/JVI.01007-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Luo Y., Kleiboeker S., Deng X., Qiu J. Human parvovirus B19 infection causes cell cycle arrest of human erythroid progenitors at late S phase that favors viral DNA replication. J. Virol. 2013;87:12766–12775. doi: 10.1128/JVI.02333-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Luo Y., Lou S., Deng X., Liu Z., Li Y., Kleiboeker S., Qiu J. Parvovirus B19 infection of human primary erythroid progenitor cells triggers ATR-Chk1 signaling, which promotes B19 virus replication. J. Virol. 2011;85:8046–8055. doi: 10.1128/JVI.00831-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Choi Y.K., Nash K., Byrne B.J., Muzyczka N., Song S. The effect of DNA-dependent protein kinase on adeno-associated virus replication. PLoS One. 2010;5:e15073. doi: 10.1371/journal.pone.0015073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Schwartz R.A., Carson C.T., Schuberth C., Weitzman M.D. Adeno-associated virus replication induces a DNA damage response coordinated by DNA-dependent protein kinase. J. Virol. 2009;83:6269–6278. doi: 10.1128/JVI.00318-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Baydoun H.H., Bai X.T., Shelton S., Nicot C. HTLV-I tax increases genetic instability by inducing DNA double strand breaks during DNA replication and switching repair to NHEJ. PLoS One. 2012;7:e42226. doi: 10.1371/journal.pone.0042226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Belgnaoui S.M., Fryrear K.A., Nyalwidhe J.O., Guo X., Semmes O.J. The viral oncoprotein tax sequesters DNA damage response factors by tethering MDC1 to chromatin. J. Biol. Chem. 2010;285:32897–32905. doi: 10.1074/jbc.M110.146373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Chandhasin C., Ducu R.I., Berkovich E., Kastan M.B., Marriott S.J. Human T-cell leukemia virus type 1 tax attenuates the ATM-mediated cellular DNA damage response. J. Virol. 2008;82:6952–6961. doi: 10.1128/JVI.02331-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Chlichlia K., Khazaie K. HTLV-1 Tax: Linking transformation, DNA damage and apoptotic T-cell death. Chem. Biol. Interact. 2010;188:359–365. doi: 10.1016/j.cbi.2010.06.005. [DOI] [PubMed] [Google Scholar]
- 118.Durkin S.S., Guo X., Fryrear K.A., Mihaylova V.T., Gupta S.K., Belgnaoui S.M., Haoudi A., Kupfer G.M., Semmes O.J. HTLV-1 Tax oncoprotein subverts the cellular DNA damage response via binding to DNA-dependent protein kinase. J. Biol. Chem. 2008;283:36311–36320. doi: 10.1074/jbc.M804931200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kinjo T., Ham-Terhune J., Peloponese J.M., Jr., Jeang K.T. Induction of reactive oxygen species by human T-cell leukemia virus type 1 tax correlates with DNA damage and expression of cellular senescence marker. J. Virol. 2010;84:5431–5437. doi: 10.1128/JVI.02460-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ariumi Y., Trono D. Ataxia-telangiectasia-mutated (ATM) protein can enhance human immunodeficiency virus type 1 replication by stimulating Rev function. J. Virol. 2006;80:2445–2452. doi: 10.1128/JVI.80.5.2445-2452.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Lau A., Swinbank K.M., Ahmed P.S., Taylor D.L., Jackson S.P., Smith G.C., O’Connor M.J. Suppression of HIV-1 infection by a small molecule inhibitor of the ATM kinase. Nat. Cell Biol. 2005;7:493–500. doi: 10.1038/ncb1250. [DOI] [PubMed] [Google Scholar]
- 122.Perfettini J.L., Nardacci R., Bourouba M., Subra F., Gros L., Seror C., Manic G., Rosselli F., Amendola A., Masdehors P., et al. Critical involvement of the ATM-dependent DNA damage response in the apoptotic demise of HIV-1-elicited syncytia. PLoS One. 2008;3:e2458. doi: 10.1371/journal.pone.0002458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Austin D., Baer A., Lundberg L., Shafagati N., Schoonmaker A., Narayanan A., Popova T., Panthier J.J., Kashanchi F., Bailey C., et al. p53 Activation following Rift Valley fever virus infection contributes to cell death and viral production. PLoS One. 2012;7:e36327. doi: 10.1371/journal.pone.0036327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Baer A., Austin D., Narayanan A., Popova T., Kainulainen M., Bailey C., Kashanchi F., Weber F., Kehn-Hall K. Induction of DNA Damage Signaling upon Rift Valley Fever Virus Infection Results in Cell Cycle Arrest and Increased Viral Replication. J. Biol. Chem. 2012;287:7399–7410. doi: 10.1074/jbc.M111.296608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ariumi Y., Kuroki M., Dansako H., Abe K., Ikeda M., Wakita T., Kato N. The DNA damage sensors ataxia-telangiectasia mutated kinase and checkpoint kinase 2 are required for hepatitis C virus RNA replication. J. Virol. 2008;82:9639–9646. doi: 10.1128/JVI.00351-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Wen C., He X., Ma H., Hou N., Wei C., Song T., Zhang Y., Sun L., Ma Q., Zhong H. Hepatitis C virus infection downregulates the ligands of the activating receptor NKG2D. Cell. Mol. Immunol. 2008;5:475–478. doi: 10.1038/cmi.2008.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Parkinson J., Lees-Miller S.P., Everett R.D. Herpes simplex virus type 1 immediate-early protein vmw110 induces the proteasome-dependent degradation of the catalytic subunit of DNA-dependent protein kinase. J. Virol. 1999;73:650–657. doi: 10.1128/jvi.73.1.650-657.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Wilkinson D.E., Weller S.K. Recruitment of cellular recombination and repair proteins to sites of herpes simplex virus type 1 DNA replication is dependent on the composition of viral proteins within prereplicative sites and correlates with the induction of the DNA damage response. J. Virol. 2004;78:4783–4796. doi: 10.1128/JVI.78.9.4783-4796.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Wilkinson D.E., Weller S.K. Herpes simplex virus type I disrupts the ATR-dependent DNA-damage response during lytic infection. J. Cell Sci. 2006;119:2695–2703. doi: 10.1242/jcs.02981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Bittar C., Shrivastava S., Bhanja Chowdhury J., Rahal P., Ray R.B. Hepatitis C virus NS2 protein inhibits DNA damage pathway by sequestering p53 to the cytoplasm. PLoS One. 2013;8:e62581. doi: 10.1371/journal.pone.0062581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Machida K., McNamara G., Cheng K.T., Huang J., Wang C.H., Comai L., Ou J.H., Lai M.M. Hepatitis C virus inhibits DNA damage repair through reactive oxygen and nitrogen species and by interfering with the ATM-NBS1/Mre11/Rad50 DNA repair pathway in monocytes and hepatocytes. J. Immunol. 2010;185:6985–6998. doi: 10.4049/jimmunol.1000618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Forrester N.A., Sedgwick G.G., Thomas A., Blackford A.N., Speiseder T., Dobner T., Byrd P.J., Stewart G.S., Turnell A.S., Grand R.J. Serotype-specific inactivation of the cellular DNA damage response during adenovirus infection. J. Virol. 2011;85:2201–2211. doi: 10.1128/JVI.01748-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Karen K.A., Hoey P.J., Young C.S., Hearing P. Temporal regulation of the Mre11-Rad50-Nbs1 complex during adenovirus infection. J. Virol. 2009;83:4565–4573. doi: 10.1128/JVI.00042-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Evans J.D., Hearing P. Relocalization of the Mre11-Rad50-Nbs1 complex by the adenovirus E4 ORF3 protein is required for viral replication. J. Virol. 2005;79:6207–6215. doi: 10.1128/JVI.79.10.6207-6215.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Carson C.T., Orazio N.I., Lee D.V., Suh J., Bekker-Jensen S., Araujo F.D., Lakdawala S.S., Lilley C.E., Bartek J., Lukas J., et al. Mislocalization of the MRN complex prevents ATR signaling during adenovirus infection. EMBO J. 2009;28:652–662. doi: 10.1038/emboj.2009.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Liu Y., Shevchenko A., Berk A.J. Adenovirus exploits the cellular aggresome response to accelerate inactivation of the MRN complex. J. Virol. 2005;79:14004–14016. doi: 10.1128/JVI.79.22.14004-14016.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Araujo F.D., Stracker T.H., Carson C.T., Lee D.V., Weitzman M.D. Adenovirus type 5 E4orf3 protein targets the Mre11 complex to cytoplasmic aggresomes. J. Virol. 2005;79:11382–11391. doi: 10.1128/JVI.79.17.11382-11391.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Mathew S.S., Bridge E. The cellular Mre11 protein interferes with adenovirus E4 mutant DNA replication. Virology. 2007;365:346–355. doi: 10.1016/j.virol.2007.03.049. [DOI] [PubMed] [Google Scholar]
- 139.Lakdawala S.S., Schwartz R.A., Ferenchak K., Carson C.T., McSharry B.P., Wilkinson G.W., Weitzman M.D. Differential requirements of the C terminus of Nbs1 in suppressing adenovirus DNA replication and promoting concatemer formation. J. Virol. 2008;82:8362–8372. doi: 10.1128/JVI.00900-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Shin Y.C., Nakamura H., Liang X., Feng P., Chang H., Kowalik T.F., Jung J.U. Inhibition of the ATM/p53 signal transduction pathway by Kaposi’s sarcoma-associated herpesvirus interferon regulatory factor 1. J. Virol. 2006;80:2257–2266. doi: 10.1128/JVI.80.5.2257-2266.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Fortunato E.A., Spector D.H. p53 and RPA are sequestered in viral replication centers in the nuclei of cells infected with human cytomegalovirus. J. Virol. 1998;72:2033–2039. doi: 10.1128/jvi.72.3.2033-2039.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Fortunato E.A., McElroy A.K., Sanchez I., Spector D.H. Exploitation of cellular signaling and regulatory pathways by human cytomegalovirus. Trends Microbiol. 2000;8:111–119. doi: 10.1016/S0966-842X(00)01699-1. [DOI] [PubMed] [Google Scholar]
- 143.Kalejta R.F., Shenk T. Manipulation of the cell cycle by human cytomegalovirus. Front. Biosci. 2002;7:d295–d306. doi: 10.2741/kalejta. [DOI] [PubMed] [Google Scholar]
- 144.Biswas N., Sanchez V., Spector D.H. Human cytomegalovirus infection leads to accumulation of geminin and inhibition of the licensing of cellular DNA replication. J. Virol. 2003;77:2369–2376. doi: 10.1128/JVI.77.4.2369-2376.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Wiebusch L., Uecker R., Hagemeier C. Human cytomegalovirus prevents replication licensing by inhibiting MCM loading onto chromatin. EMBO Rep. 2003;4:42–46. doi: 10.1038/sj.embor.embor707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Bresnahan W.A., Boldogh I., Thompson E.A., Albrecht T. Human cytomegalovirus inhibits cellular DNA synthesis and arrests productively infected cells in late G1. Virology. 1996;224:150–160. doi: 10.1006/viro.1996.0516. [DOI] [PubMed] [Google Scholar]
- 147.Dittmer D., Mocarski E.S. Human cytomegalovirus infection inhibits G1/S transition. J. Virol. 1997;71:1629–1634. doi: 10.1128/jvi.71.2.1629-1634.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Lu M., Shenk T. Human cytomegalovirus UL69 protein induces cells to accumulate in G1 phase of the cell cycle. J. Virol. 1999;73:676–683. doi: 10.1128/jvi.73.1.676-683.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Kalejta R.F., Bechtel J.T., Shenk T. Human cytomegalovirus pp71 stimulates cell cycle progression by inducing the proteasome-dependent degradation of the retinoblastoma family of tumor suppressors. Mol. Cell. Biol. 2003;23:1885–1895. doi: 10.1128/MCB.23.6.1885-1895.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Hume A.J., Finkel J.S., Kamil J.P., Coen D.M., Culbertson M.R., Kalejta R.F. Phosphorylation of retinoblastoma protein by viral protein with cyclin-dependent kinase function. Science. 2008;320:797–799. doi: 10.1126/science.1152095. [DOI] [PubMed] [Google Scholar]
- 151.Salsman J., Jagannathan M., Paladino P., Chan P.K., Dellaire G., Raught B., Frappier L. Proteomic profiling of the human cytomegalovirus UL35 gene products reveals a role for UL35 in the DNA repair response. J. Virol. 2012;86:806–820. doi: 10.1128/JVI.05442-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Castillo J.P., Yurochko A.D., Kowalik T.F. Role of human cytomegalovirus immediate-early proteins in cell growth control. J. Virol. 2000;74:8028–8037. doi: 10.1128/JVI.74.17.8028-8037.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Wiebusch L., Hagemeier C. Human cytomegalovirus 86-kilodalton IE2 protein blocks cell cycle progression in G(1) J. Virol. 1999;73:9274–9283. doi: 10.1128/jvi.73.11.9274-9283.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Kwon Y., Kim M.N., Young Choi E., Heon Kim J., Hwang E.S., Cha C.Y. Inhibition of p53 transcriptional activity by human cytomegalovirus UL44. Microbiol. Immunol. 2012;56:324–331. doi: 10.1111/j.1348-0421.2012.00446.x. [DOI] [PubMed] [Google Scholar]
- 155.Pietenpol J.A., Stewart Z.A. Cell cycle checkpoint signaling: Cell cycle arrest versus apoptosis. Toxicology. 2002;181–182:475–481. doi: 10.1016/S0300-483X(02)00460-2. [DOI] [PubMed] [Google Scholar]
- 156.Margolis M.J., Pajovic S., Wong E.L., Wade M., Jupp R., Nelson J.A., Azizkhan J.C. Interaction of the 72-kilodalton human cytomegalovirus IE1 gene product with E2F1 coincides with E2F-dependent activation of dihydrofolate reductase transcription. J. Virol. 1995;69:7759–7767. doi: 10.1128/jvi.69.12.7759-7767.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Castillo J.P., Frame F.M., Rogoff H.A., Pickering M.T., Yurochko A.D., Kowalik T.F. Human cytomegalovirus IE1–72 activates ataxia telangiectasia mutated kinase and a p53/p21-mediated growth arrest response. J. Virol. 2005;79:11467–11475. doi: 10.1128/JVI.79.17.11467-11475.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Prichard M.N., Sztul E., Daily S.L., Perry A.L., Frederick S.L., Gill R.B., Hartline C.B., Streblow D.N., Varnum S.M., Smith R.D., et al. Human cytomegalovirus UL97 kinase activity is required for the hyperphosphorylation of retinoblastoma protein and inhibits the formation of nuclear aggresomes. J. Virol. 2008;82:5054–5067. doi: 10.1128/JVI.02174-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Zhang Z., Huong S.M., Wang X., Huang D.Y., Huang E.S. Interactions between human cytomegalovirus IE1–72 and cellular p107: Functional domains and mechanisms of up-regulation of cyclin E/cdk2 kinase activity. J. Virol. 2003;77:12660–12670. doi: 10.1128/JVI.77.23.12660-12670.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Hagemeier C., Caswell R., Hayhurst G., Sinclair J., Kouzarides T. Functional interaction between the HCMV IE2 transactivator and the retinoblastoma protein. EMBO J. 1994;13:2897–2903. doi: 10.1002/j.1460-2075.1994.tb06584.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Kalejta R.F. Human cytomegalovirus pp71: A new viral tool to probe the mechanisms of cell cycle progression and oncogenesis controlled by the retinoblastoma family of tumor suppressors. J. Cell. Biochem. 2004;93:37–45. doi: 10.1002/jcb.20177. [DOI] [PubMed] [Google Scholar]
- 162.Kalejta R.F., Shenk T. Proteasome-dependent, ubiquitin-independent degradation of the Rb family of tumor suppressors by the human cytomegalovirus pp71 protein. Proc. Natl. Acad. Sci. USA. 2003;100:3263–3268. doi: 10.1073/pnas.0538058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Pajovic S., Wong E.L., Black A.R., Azizkhan J.C. Identification of a viral kinase that phosphorylates specific E2Fs and pocket proteins. Mol. Cell. Biol. 1997;17:6459–6464. doi: 10.1128/mcb.17.11.6459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Pickering M.T., Kowalik T.F. Rb inactivation leads to E2F1-mediated DNA double-strand break accumulation. Oncogene. 2006;25:746–755. doi: 10.1038/sj.onc.1209103. [DOI] [PubMed] [Google Scholar]
- 165.Johnson R.A., Yurochko A.D., Poma E.E., Zhu L., Huang E.S. Domain mapping of the human cytomegalovirus IE1–72 and cellular p107 protein-protein interaction and the possible functional consequences. J. Gen. Virol. 1999;80:1293–1303. doi: 10.1099/0022-1317-80-5-1293. [DOI] [PubMed] [Google Scholar]
- 166.Ahn J.H., Hayward G.S. The major immediate-early proteins IE1 and IE2 of human cytomegalovirus colocalize with and disrupt PML-associated nuclear bodies at very early times in infected permissive cells. J. Virol. 1997;71:4599–4613. doi: 10.1128/jvi.71.6.4599-4613.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Wilkinson G.W., Kelly C., Sinclair J.H., Rickards C. Disruption of PML-associated nuclear bodies mediated by the human cytomegalovirus major immediate early gene product. J. Gen. Virol. 1998;79:1233–1245. doi: 10.1099/0022-1317-79-5-1233. [DOI] [PubMed] [Google Scholar]
- 168.Xiaofei E., Stadler B.M., Debatis M., Wang S., Lu S., Kowalik T.F. RNA interference-mediated targeting of human cytomegalovirus immediate-early or early gene products inhibits viral replication with differential effects on cellular functions. J. Virol. 2012;86:5660–5673. doi: 10.1128/JVI.06338-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Borden K.L. Pondering the promyelocytic leukemia protein (PML) puzzle: Possible functions for PML nuclear bodies. Mol. Cell. Biol. 2002;22:5259–5269. doi: 10.1128/MCB.22.15.5259-5269.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Saffert R.T., Kalejta R.F. Promyelocytic leukemia-nuclear body proteins: Herpesvirus enemies, accomplices, or both? Future Viro. 2008;3:265–277. doi: 10.2217/17460794.3.3.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Saffert R.T., Kalejta R.F. Inactivating a cellular intrinsic immune defense mediated by Daxx is the mechanism through which the human cytomegalovirus pp71 protein stimulates viral immediate-early gene expression. J. Virol. 2006;80:3863–3871. doi: 10.1128/JVI.80.8.3863-3871.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Sommer M.H., Scully A.L., Spector D.H. Transactivation by the human cytomegalovirus IE2 86-kilodalton protein requires a domain that binds to both the TATA box-binding protein and the retinoblastoma protein. J. Virol. 1994;68:6223–6231. doi: 10.1128/jvi.68.10.6223-6231.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Choi K.S., Kim S.J., Kim S. The retinoblastoma gene product negatively regulates transcriptional activation mediated by the human cytomegalovirus IE2 protein. Virology. 1995;208:450–456. doi: 10.1006/viro.1995.1175. [DOI] [PubMed] [Google Scholar]
- 174.Speir E., Modali R., Huang E.S., Leon M.B., Shawl F., Finkel T., Epstein S.E. Potential role of human cytomegalovirus and p53 interaction in coronary restenosis. Science. 1994;265:391–394. doi: 10.1126/science.8023160. [DOI] [PubMed] [Google Scholar]
- 175.Tsai H.L., Kou G.H., Chen S.C., Wu C.W., Lin Y.S. Human cytomegalovirus immediate-early protein IE2 tethers a transcriptional repression domain to p53. J. Biol. Chem. 1996;271:3534–3540. [PubMed] [Google Scholar]
- 176.Bonin L.R., McDougall J.K. Human cytomegalovirus IE2 86-kilodalton protein binds p53 but does not abrogate G1 checkpoint function. J. Virol. 1997;71:5861–5870. doi: 10.1128/jvi.71.8.5861-5870.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Song Y.J., Stinski M.F. Effect of the human cytomegalovirus IE86 protein on expression of E2F-responsive genes: A DNA microarray analysis. Proc. Natl. Acad. Sci. USA. 2002;99:2836–2841. doi: 10.1073/pnas.052010099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Ruger B., Klages S., Walla B., Albrecht J., Fleckenstein B., Tomlinson P., Barrell B. Primary structure and transcription of the genes coding for the two virion phosphoproteins pp65 and pp71 of human cytomegalovirus. J. Virol. 1987;61:446–453. doi: 10.1128/jvi.61.2.446-453.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Hensel G.M., Meyer H.H., Buchmann I., Pommerehne D., Schmolke S., Plachter B., Radsak K., Kern H.F. Intracellular localization and expression of the human cytomegalovirus matrix phosphoprotein pp71 (ppUL82): Evidence for its translocation into the nucleus. J. Gen. Virol. 1996;77:3087–3097. doi: 10.1099/0022-1317-77-12-3087. [DOI] [PubMed] [Google Scholar]
- 180.Baldick C.J., Jr., Marchini A., Patterson C.E., Shenk T. Human cytomegalovirus tegument protein pp71 (ppUL82) enhances the infectivity of viral DNA and accelerates the infectious cycle. J. Virol. 1997;71:4400–4408. doi: 10.1128/jvi.71.6.4400-4408.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Rechter S., Scott G.M., Eickhoff J., Zielke K., Auerochs S., Muller R., Stamminger T., Rawlinson W.D., Marschall M. Cyclin-dependent Kinases Phosphorylate the Cytomegalovirus RNA Export Protein pUL69 and Modulate Its Nuclear Localization and Activity. J. Biol. Chem. 2009;284:8605–8613. doi: 10.1074/jbc.M805693200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Marschall M., Freitag M., Suchy P., Romaker D., Kupfer R., Hanke M., Stamminger T. The protein kinase pUL97 of human cytomegalovirus interacts with and phosphorylates the DNA polymerase processivity factor pUL44. Virology. 2003;311:60–71. doi: 10.1016/S0042-6822(03)00147-8. [DOI] [PubMed] [Google Scholar]
- 183.Schregel V., Auerochs S., Jochmann R., Maurer K., Stamminger T., Marschall M. Mapping of a self-interaction domain of the cytomegalovirus protein kinase pUL97. J. Gen. Virol. 2007;88:395–404. doi: 10.1099/vir.0.82393-0. [DOI] [PubMed] [Google Scholar]
- 184.Michel D., Kramer S., Hohn S., Schaarschmidt P., Wunderlich K., Mertens T. Amino acids of conserved kinase motifs of cytomegalovirus protein UL97 are essential for autophosphorylation. J. Virol. 1999;73:8898–8901. doi: 10.1128/jvi.73.10.8898-8901.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Marschall M., Marzi A., aus dem Siepen P., Jochmann R., Kalmer M., Auerochs S., Lischka P., Leis M., Stamminger T. Cellular p32 recruits cytomegalovirus kinase pUL97 to redistribute the nuclear lamina. J. Biol. Chem. 2005;280:33357–33367. doi: 10.1074/jbc.M502672200. [DOI] [PubMed] [Google Scholar]
- 186.Winkler M., Stamminger T. A specific subform of the human cytomegalovirus transactivator protein pUL69 is contained within the tegument of virus particles. J. Virol. 1996;70:8984–8987. doi: 10.1128/jvi.70.12.8984-8987.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Casavant N.C., Luo M.H., Rosenke K., Winegardner T., Zurawska A., Fortunato E.A. Potential role for p53 in the permissive life cycle of human cytomegalovirus. J. Virol. 2006;80:8390–8401. doi: 10.1128/JVI.00505-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.E X., Savidis G., Chin C.R., Wang S., Lu S., Brass A.L., Kowalik T.F. A Novel DDB2-ATM Feedback Loop Regulates Human Cytomegalovirus Replication. J. Virol. 2014;88:2279–2290. doi: 10.1128/JVI.03423-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Hickson I., Zhao Y., Richardson C.J., Green S.J., Martin N.M., Orr A.I., Reaper P.M., Jackson S.P., Curtin N.J., Smith G.C. Identification and characterization of a novel and specific inhibitor of the ataxia-telangiectasia mutated kinase ATM. Cancer Res. 2004;64:9152–9159. doi: 10.1158/0008-5472.CAN-04-2727. [DOI] [PubMed] [Google Scholar]
- 190.Orazio N.I., Naeger C.M., Karlseder J., Weitzman M.D. The adenovirus E1b55K/E4orf6 complex induces degradation of the Bloom helicase during infection. J. Virol. 2011;85:1887–1892. doi: 10.1128/JVI.02134-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Singh V.V., Dutta D., Ansari M.A., Dutta S., Chandran B. Kaposi’s Sarcoma-Associated Herpesvirus Induces the ATM and H2AX DNA Damage Response Early during De Novo Infection of Primary Endothelial Cells, Which Play Roles in Latency Establishment. J. Virol. 2014;88:2821–2834. doi: 10.1128/JVI.03126-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Chang L., Godinez W.J., Kim I.H., Tektonidis M., de Lanerolle P., Eils R., Rohr K., Knipe D.M. Herpesviral replication compartments move and coalesce at nuclear speckles to enhance export of viral late mRNA. Proc. Natl. Acad. Sci. USA. 2011;108:E136–E144. doi: 10.1073/pnas.1103411108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Taylor T.J., Knipe D.M. Proteomics of herpes simplex virus replication compartments: Association of cellular DNA replication, repair, recombination, and chromatin remodeling proteins with ICP8. J. Virol. 2004;78:5856–5866. doi: 10.1128/JVI.78.11.5856-5866.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Wilkinson D.E., Weller S.K. The role of DNA recombination in herpes simplex virus DNA replication. IUBMB Life. 2003;55:451–458. doi: 10.1080/15216540310001612237. [DOI] [PubMed] [Google Scholar]
- 195.Kaufer B.B., Jarosinski K.W., Osterrieder N. Herpesvirus telomeric repeats facilitate genomic integration into host telomeres and mobilization of viral DNA during reactivation. J. Exp. Med. 2011;208:605–615. doi: 10.1084/jem.20101402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Li M., Mizuuchi M., Burke T.R., Jr., Craigie R. Retroviral DNA integration: Reaction pathway and critical intermediates. EMBO J. 2006;25:1295–1304. doi: 10.1038/sj.emboj.7601005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Weitzman M.D., Carson C.T., Schwartz R.A., Lilley C.E. Interactions of viruses with the cellular DNA repair machinery. DNA Repair (Amst) 2004;3:1165–1173. doi: 10.1016/j.dnarep.2004.03.018. [DOI] [PubMed] [Google Scholar]
- 198.Fortunato E.A., Dell’Aquila M.L., Spector D.H. Specific chromosome 1 breaks induced by human cytomegalovirus. Proc. Natl. Acad. Sci. USA. 2000;97:853–858. doi: 10.1073/pnas.97.2.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Kwak S., E X., Kowalik T.F. University of Massachusetts Medical School; Worcester, MA, USA: 2014. An HCMV virion-associated tegument protein initiates activation of the host DNA damage response to infection. to be submitted for publication. [Google Scholar]
- 200.Millhouse S., Su Y.H., Zhang X., Wang X., Song B.P., Zhu L., Oppenheim E., Fraser N.W., Block T.M. Evidence that herpes simplex virus DNA derived from quiescently infected cells in vitro, and latently infected cells in vivo, is physically damaged. J. Neurovirol. 2010;16:384–398. doi: 10.3109/13550284.2010.515651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Stich H.F., Hsu T.C., Rapp F. Viruses and Mammalian Chromosomes. I. Localization of Chromosome Aberrations after Infection with Herpes Simplex Virus. Virology. 1964;22:439–445. doi: 10.1016/0042-6822(64)90064-9. [DOI] [PubMed] [Google Scholar]
- 202.Peat D.S., Stanley M.A. Chromosome damage induced by herpes simplex virus type 1 in early infection. J. Gen. Virol. 1986;67:2273–2277. doi: 10.1099/0022-1317-67-10-2273. [DOI] [PubMed] [Google Scholar]
- 203.Schramayr S., Caporossi D., Mak I., Jelinek T., Bacchetti S. Chromosomal damage induced by human adenovirus type 12 requires expression of the E1B 55-kilodalton viral protein. J. Virol. 1990;64:2090–2095. doi: 10.1128/jvi.64.5.2090-2095.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.RW H.Y., Oakes J.E., Kudler L. In vitro repair of the preexisting nicks and gaps in herpes simplex virus DNA. Virology. 1977;76:286–294. doi: 10.1016/0042-6822(77)90303-8. [DOI] [PubMed] [Google Scholar]
- 205.Tilton C., Clippinger A.J., Maguire T., Alwine J.C. Human cytomegalovirus induces multiple means to combat reactive oxygen species. J. Virol. 2011;85:12585–12593. doi: 10.1128/JVI.05572-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Soutoglou E., Misteli T. Activation of the cellular DNA damage response in the absence of DNA lesions. Science. 2008;320:1507–1510. doi: 10.1126/science.1159051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.McVoy M.A., Adler S.P. Human cytomegalovirus DNA replicates after early circularization by concatemer formation, and inversion occurs within the concatemer. J. Virol. 1994;68:1040–1051. doi: 10.1128/jvi.68.2.1040-1051.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.E X., Kowalik T.F. University of Massachusetts Medical School; Worcester, MA, USA: 2014. A role for host DNA polymerases in the replication of herpesviral DNA. to be submitted for publication. [Google Scholar]
- 209.Zhou B.B., Elledge S.J. The DNA damage response: Putting checkpoints in perspective. Nature. 2000;408:433–439. doi: 10.1038/35044005. [DOI] [PubMed] [Google Scholar]
- 210.Cuchet-Lourenco D., Vanni E., Glass M., Orr A., Everett R.D. Herpes simplex virus 1 ubiquitin ligase ICP0 interacts with PML isoform I and induces its SUMO-independent degradation. J. Virol. 2012;86:11209–11222. doi: 10.1128/JVI.01145-12. [DOI] [PMC free article] [PubMed] [Google Scholar]