Abstract
The light response in Neurospora is mediated by the photoreceptor and circadian transcription factor White Collar Complex (WCC). The expression rate of the WCC target genes adapts in daylight and remains refractory to moonlight, despite the extraordinary light sensitivity of the WCC. To explain this photoadaptation, feedback inhibition by the WCC interaction partner VIVID (VVD) has been invoked. Here we show through data-driven mathematical modeling that VVD allows Neurospora to detect relative changes in light intensity. To achieve this behavior, VVD acts as an inhibitor of WCC-driven gene expression and, at the same time, as a positive regulator that maintains the responsiveness of the photosystem. Our data indicate that this paradoxical function is realized by a futile cycle that involves the light-induced sequestration of active WCC by VVD and the replenishment of the activatable WCC pool through the decay of the photoactivated state. Our quantitative study uncovers a novel network motif for achieving sensory adaptation and defines a core input module of the circadian clock in Neurospora.
Keywords: adaptation, mathematical model, Neurospora , protein–protein interaction, VVD
Introduction
Circadian clocks are a fundamental adaptation to the day–night rhythm in all kingdoms of life. They are entrained by light cues from the environment, whose intensity varies due to the chosen habitat and fluctuates randomly because of weather conditions. Experimental work on the model organism Neurospora crassa indicates that its clock does not perceive directly the fluctuating environmental light intensity but, rather, that the light is processed by an input module of the clock that exhibits sensory adaptation (Schwerdtfeger and Linden, 2001; Heintzen et al, 2001; Malzahn et al, 2010). The idea of a light-processing unit has previously been suggested on theoretical grounds (Kronauer et al, 1999), but its molecular basis is unknown.
The circadian clock of the fungus Neurospora crassa exemplifies the transcriptional negative feedback design found in a multitude of organisms (Brunner and Káldi, 2008; Baker et al, 2012). Here the clock proteins White Collar 1 and 2 (WC-1 and WC-2) act as global activators of photoresponses. WC-1, a blue-light photoreceptor, and WC-2 form the transcription factor White Collar Complex (WCC) that drives the expression of target genes with ∼24 h periodicity. Among many other genes, WCC activates the expression of the clock gene frequency (frq); FRQ protein in turn inhibits the WCC. This negative feedback loop is thought to generate the circadian cycles of WCC activity in constant darkness—the hallmark of an autonomous clock (Gonze et al, 2000; Tseng et al, 2012).
The Neurospora light response exhibits sensory adaptation, rendering WCC-driven gene expression transient in constant light (Schwerdtfeger and Linden, 2001; Heintzen et al, 2001). Recently, Malzahn et al (2010) demonstrated directly that photoadaptation prevents the resetting of the circadian clock in Neurospora by moonlight. Strikingly, a mutant strain with defective photoadaptation has such high light sensitivity that it perceives moonlight and fails to entrain to natural day–night cycles. This finding shows that besides short-term adaptation, the Neurospora light response displays longer-term memory of experienced daylight that makes the organism insensitive to light input during the night and at dawn (Elvin et al, 2005; Malzahn et al, 2010).
Two kinds of mechanisms have been invoked to explain Neurospora photoadaptation: constitutive removal of light-activated WCC (Talora et al, 1999; He and Liu, 2005; Tsumoto et al, 2011) and feedback inhibition through the blue-light photoreceptor VIVID (VVD) (Heintzen et al, 2001; Schwerdtfeger and Linden, 2001, 2003; Shrode et al, 2001). By now, a large body of work has shown that photoadaptation in Neurospora requires the blue-light photoreceptor VVD (reviewed in Baker et al, 2012). VVD is induced by light-activated WCC and inhibits the function of the latter as a transcriptional activator. The combination of rapid induction and slow degradation of VVD might realize the adaptation of the photoresponse during the day and prolonged refractoriness during the night.
VVD physically interacts with WC-1 and represses the transcription of WCC target genes (Chen et al, 2010; Hunt et al, 2010; Malzahn et al, 2010). Specifically, the light-activated WCC complex dimerizes on the light-response elements (LREs), such as those found in the frq promoter, via an interaction of the photosensory light–oxygen–voltage (LOV) domains of WC-1. Light-activated VVD, which also harbors a LOV domain, disrupts WCC homodimerization by competitive binding to WC-1 (Malzahn et al, 2010). Thus, VVD acts as a feedback inhibitor of light-induced transcription by sequestering the active WCC. However, this downregulation of the light response is only one aspect of photoadaptation. At the same time, Neurospora can respond to further increases in light intensity, perceiving intensity changes rather than the absolute light level (Schwerdtfeger and Linden, 2003; Chen et al, 2010; Malzahn et al, 2010). As fungi may inhabit sites with very different ambient light intensities (e.g., underneath tree bark versus sun-exposed surfaces), the sensing of relative light changes will be crucial for the proper functioning of the circadian clock.
From a mechanistic point of view, however, it is perplexing that feedback inhibition of the WCC by VVD should bring about this finely tuned adaption behavior. If VVD efficiently sequesters the central activator of the photoresponse, one must ask how Neurospora stays responsive to increases in light intensity. This is a general problem, because adaptation involves tonic inhibition that tends to downregulate the sensitivity of a sensory system. Moreover, mathematical models have shown that direct deactivation of a positive regulator generally fails to produce good adaptation (Behar et al, 2007; Ma et al, 2009), further questioning a simple inhibition model.
To dissect the molecular mechanisms of photoadaptation in Neurospora on a quantitative basis, we have combined mathematical modeling with systematic, time-resolved measurements of the expression of the core genes, wc-1, frq and vvd. We identified a novel ‘adaptation motif’ in the WCC–FRQ–VVD reaction network and suggest that this motif allows Neurospora to sense relative changes in light intensity. This motif employs the futile cycling of photoactivated states to turn simple feedback inhibition into adaptation. Thus, our study defines an essential, non-oscillatory module of the circadian clock in Neurospora. In addition, it identifies a hitherto unrecognized network motif for achieving sensory adaptation.
Results
Rapid photoadaptation and continued light responsiveness of Neurospora depend on VVD
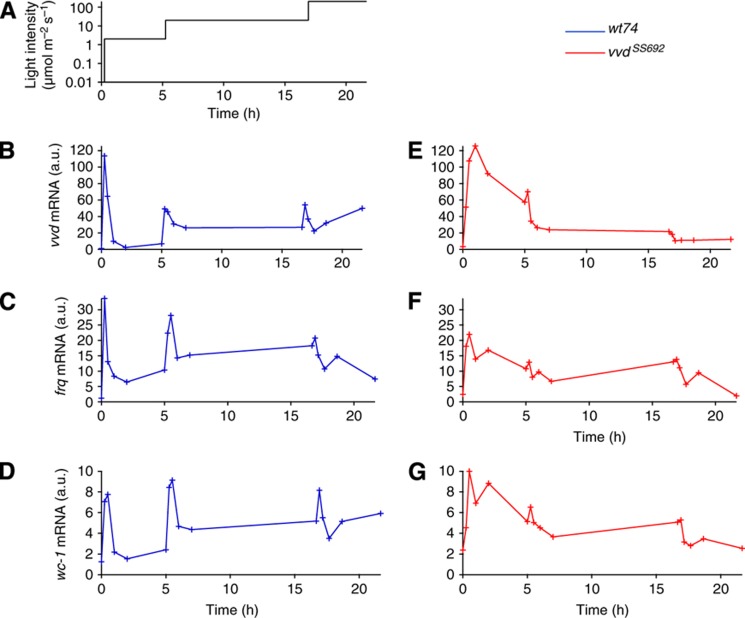
To quantify the light-sensing ability of Neurospora and systematically probe the role of VVD, we subjected the wild-type strain (wt74) and the corresponding VVD loss-of-function mutant vvd– (vvdSS692, Heintzen et al, 2001) to three increasing light steps on a logarithmic scale, from very dim light to the light intensity of a cloudy day (2, 20 and 200 μmol photons m–2 s–1, Figure 1A). The prolonged exposure to constant light intensity was chosen to quantify the transient light response and subsequent return to a steady state. The vvdSS692 mutant allele is transcribed but no functional protein is produced, allowing us to compare the transcription of the vvd gene in the presence (wild type) and absence (mutant) of VVD protein. Normalizing to vvd (t=0) in the wild type and correcting for the efficiency of the various RT–PCR probes (Supplementary Figure 1), we found that vvd was generally the most abundant mRNA species after light induction followed by frq and wc-1 (Figure 1A–G).
Figure 1.
Rapid adaptation and repeated induction depend on VVD. Light induction experiments for vvd, wc-1 and frq. Liquid cultures of the vvd loss-of-function mutant (vvdSS69) and its corresponding wild-type strain (wt74) were raised in light for 24 h and then transferred to constant darkness for 24 h before light induction. Initial light intensity was 2 μmol m−2 s−1 and was raised to 20 μmol m−2 s−1 after 300 min and again to 200 μmol m−2 s−1 after 1000 min (A). Samples were collected at the indicated time points and mRNA levels for vvd, frq and wc-1 were measured via qRT–PCR. The wild-type vvd mRNA levels at t=0 were set to 1. To compare the relative levels of the mRNAs, we corrected for the efficiency of the various RT–PCR probes used, by performing qPCR with a dilution series of genomic DNA. The values were corrected with reference to vvd (further details in the Supplementary Information). Wild-type data are shown in panels (B–D) and vvdSS692 mutant data are shown in panels (E–G). Throughout the paper, wild-type data are shown in blue and vvdSS692 mutant data are shown in red. Source data for this figure is available on the online supplementary information page.
After the transfer from dark to the lowest light intensity (t=0), the levels of all three mRNAs peaked within 30 min and then decreased rapidly in the wild-type strain (Figure 1B–D), and much more slowly in the mutant (Figure 1E–G). Subsequent light steps evoked further responses in the wild type, showing that the photosystem remains responsive to higher light intensities (Figure 1B–D; cf. Schwerdtfeger and Linden, 2003; Malzahn et al, 2010). By contrast, responsiveness to subsequent light steps was strongly diminished in the vvd– mutant (Figure 1E–G). Taken together, these data show that VVD has both an inhibitory function in light sensing, causing rapid inactivation of the photoresponse in constant light, and a positive role in mediating continued responsiveness to higher light intensities.
Mathematical model for Neurospora light sensing and adaptation
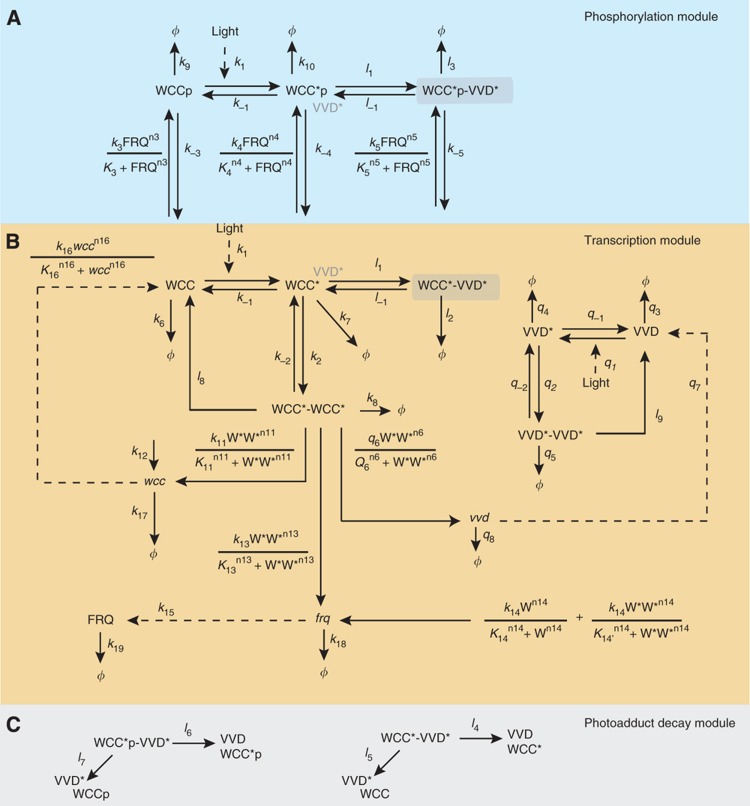
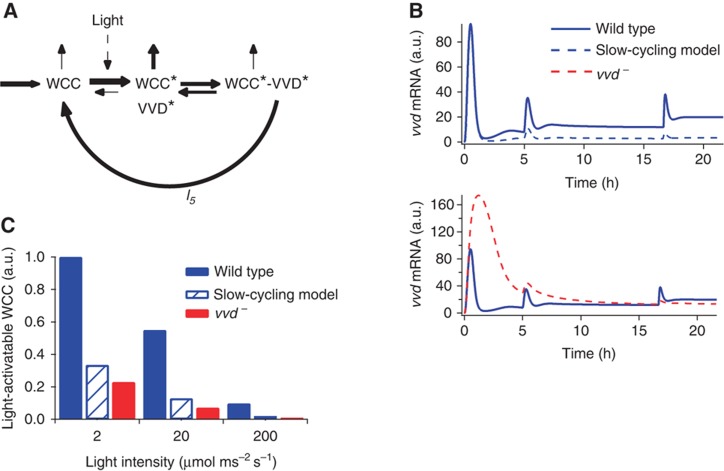
To understand how VVD can act as both an inhibitor and a positive regulator, we integrated the key molecular interactions described experimentally into a mathematical model (Figure 2). The core part of this model is the transcription module (Figure 2B) that describes the expression of the WCC target genes, vvd, frq and wc-1, and the regulation of the transcriptional activity of WCC by light and by VVD. Light converts the dark forms of the WCC and VVD into the light-activated forms WCC* and VVD* through the formation of a photoadduct in their respective LOV domains. The resulting conformational change of the proteins allows the homodimerization and heterodimerization of WCC* and VVD* (Zoltowski and Crane, 2008; Zoltowski et al, 2009; Malzahn et al, 2010; Vaidya et al, 2011; Schafmeier and Diernfellner, 2011). Light-activated WCC* homodimers activate the transcription of wc-1, vvd and frq by binding to LREs. In the dark, transcription of wc-1 is driven by a basal rate independent of the WCC, while the dark-form WCC drives the transcription of frq by binding to the Clock box (C-box) of its promoter (Froehlich et al, 2002, 2003), but not transcription of vvd (Káldi et al, 2006; Hunt et al, 2007). The WCC*–VVD* heterodimer is transcriptionally inactive (Malzahn et al, 2010). Therefore, VVD functions as a feedback inhibitor of the light-activated WCC. The expression of WCC, VVD and FRQ is balanced by protein and mRNA degradation. The light-activated, transcriptionally active WCC* homodimer is degraded rapidly, whereas the various VVD-bound and dark-form WCC species are more stable (He et al, 2005; Schafmeier et al, 2006; Schafmeier et al, 2008; Malzahn et al, 2010).
Figure 2.
Model scheme. Protein variables are indicated in normal font and mRNA variables are italicized. Light-activated forms of protein are denoted with an asterix. Phosphorylated states are of the form WCCp. The model is described in three modules: a phosphorylation module (A, shaded blue) involving FRQ-dependent phosphorylation of the WCC; a transcription module (B) that includes the light activation of WCC, subsequent homodimerization and transcription; and the photoadduct decay module (C, shaded gray). The photoadduct decay module shows the additional pathways for breakdown of the heterodimer complex.
The phosphorylation module (Figure 2A) describes an additional negative feedback loop on WCC activity through FRQ-mediated phosphorylation that contributes to controlling WCC-driven gene expression. In constant dark, it can generate self-sustained circadian oscillations, involving complex regulation of WCC and FRQ by phosphorylation, nucleocytoplasmic shuttling and degradation (Merrow et al, 2001; He et al, 2005; Schafmeier et al, 2005; Cha et al, 2008; Schafmeier et al, 2008; Diernfellner et al, 2009; Querfurth et al, 2011; Diernfellner and Schafmeier, 2011, Tseng et al, 2012).
The light-induced photoadducts in the LOV domains of WCC* and VVD* are metastable and revert to the dark form spontaneously (Guo et al, 2005). Therefore, the WCC*VVD* heterodimer can decompose through distinct pathways: (i) dissociation of the light-activated heterodimers and (ii) photoadduct decay in one binding partner, upon which the partners lose affinity for one another. This latter mechanism, depicted in the photoadduct decay module (Figure 2C), allows for the ‘futile cycling’ of WCC*–VVD* heterodimer formation through photoactivation followed by photoadduct decay. For consistency, we also include the dissociation of the WCC* and VVD* homodimers triggered by photoadduct decay into the model.
We translated the reaction scheme into coupled ordinary differential equations for the concentrations of the 14 mRNA and protein species (Supplementary Text S1).
The model accounts for photoresponse dynamics in wild type and vvd– mutant
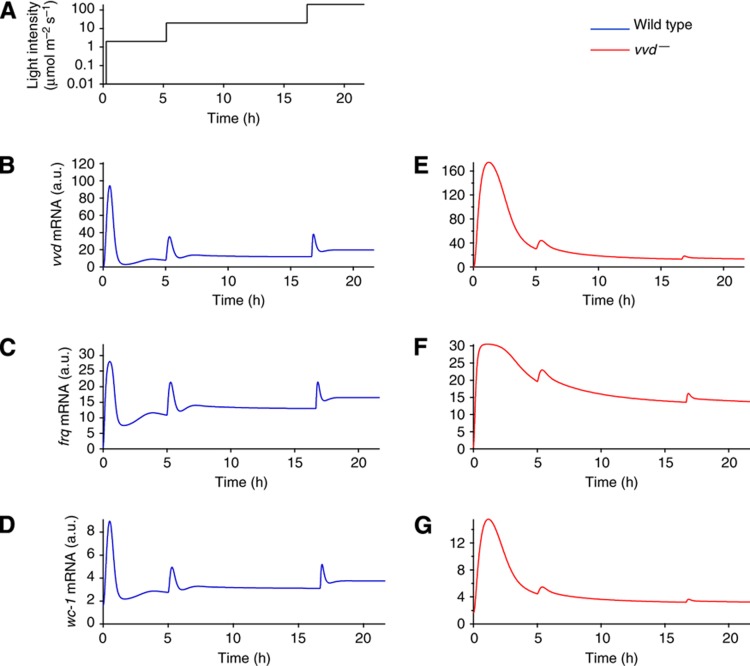
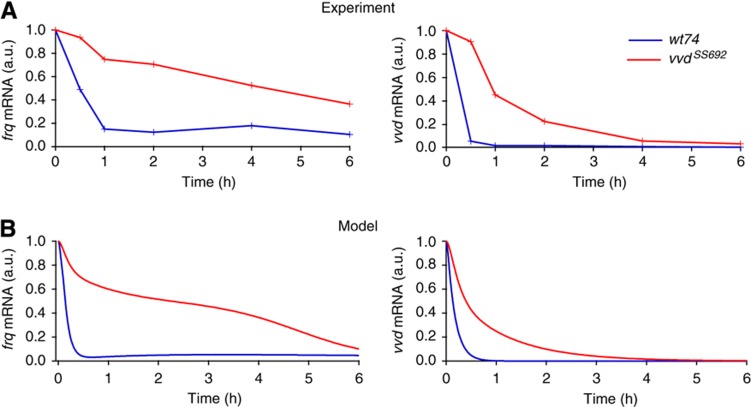
To fit the model to the light-step experiments in Figure 1, we first determined the kinetic parameters for the model without VVD protein, mimicking the vvd– mutant (using both a Bayesian Markov chain Monte Carlo algorithm and a maximum likelihood estimate; Supplementary Text S1). The available data constrain parameters of the model that govern VVD function (including VVD production, heterodimerization with WCC and photoadduct decay; Supplementary Figure S5). For a reduced, biologically less-detailed version of the model, all parameters can be identified from the data (Supplementary Text S2). The parameterized model reproduces the very slow decline of the response after the first light step, caused by degradation of light-activated WCC and inhibition of WCC by FRQ-mediated phosphorylation. The following two light steps do not elicit substantial responses (Figure 3E–G; cf. Figure 1E–G). Interestingly, the vvd–mutant data show an immediate drop of mRNA levels after the light pulse that cannot be explained by the present model and hints at additional mechanisms regulating mRNA synthesis or degradation.
Figure 3.
The model accounts for the photoresponse dynamics of the wild type and mutant. The model scheme was fitted to the light induction experiments with the light regime shown in (A), and, consistent with the experimental data, the wild type shows repeated activation in response to increasing light (B–D), while responsiveness is diminished in the mutant (E–G).
Next we introduced functional VVD protein to simulate the wild type. To fit the full model, we kept the kinetic parameters of all processes not involving VVD unchanged from the vvd– mutant model and only determined the VVD-related parameters. The introduction of VVD fully restored rapid photoadaptation and allowed repeated responses to increasing light steps (Figure 3A–D; cf. Figure 1A–D). Consistent with this positive role of VVD, the WCC protein levels were higher in the wild type than in the mutant (Supplementary Figure S2). Moreover, the model correctly accounts for vvd being the most abundant mRNA, followed by frq and wc-1.
In summary, the mathematical model reproduces salient features of Neurospora photoadaptation. Consistent with the experimental data, VVD mediates both rapid downregulation of the photoresponse in constant light and continued responsiveness to increases in light intensity.
VVD sets refractory phase for light sensing
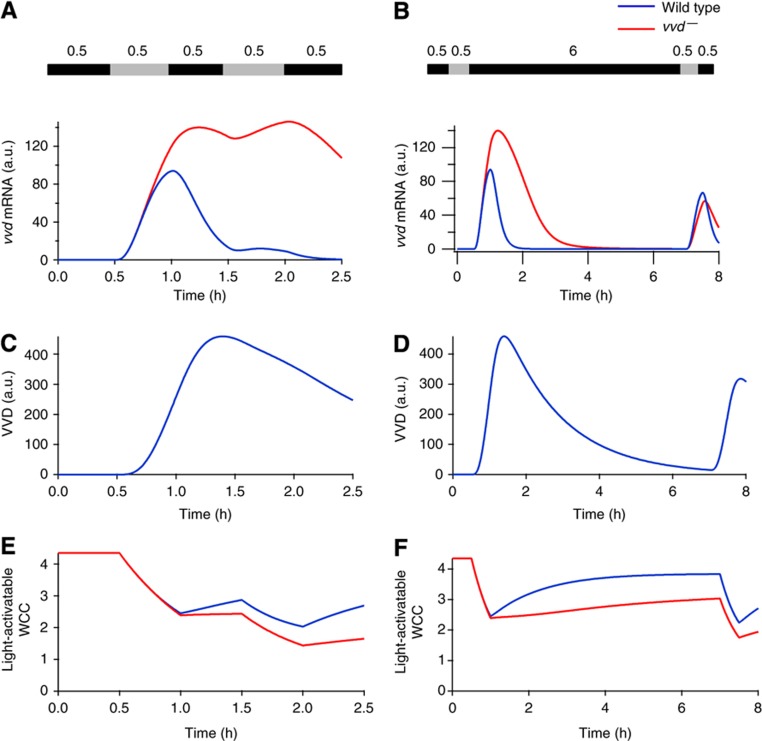
Next we asked whether the model parameterized with the light-step protocol exhibits a prolonged refractory phase that accounts for the unresponsiveness of the wild type to moonlight (Malzahn et al, 2010). We simulated successive transient light stimuli separated by a variable dark phase, displaying vvd mRNA as a measure of the transcriptional response. In the wild type, VVD protein efficiently suppresses the response to a rapidly following second stimulus (Figure 4A and C). Responsiveness to the second stimulus is restored only after a dark incubation of ∼6 h (Figure 4B), when the VVD level has dropped (Figure 4D). The key role of VVD is highlighted by comparing the wild type with the vvd– mutant: the latter reacts to the second light stimulus both after short (30 min) and long (6 h) dark incubation (Figure 4A and B). These simulations match previous experiments (Arpaia et al, 1999, Schwerdtfeger and Linden, 2001), showing that the model correctly explains the prolonged suppression of WCC-mediated transcription by VVD.
Figure 4.
VVD sets the refractory period after light response. Model-simulated responses to different dark/light regimes with light levels of 20 μmol m−2 s−1. The intervening dark incubation period between the two light pulses are 30 min (left panel) or 6 h (right panel). (A and B) After only 30 min of intervening darkness, the vvd– mutant responds to the second light pulse, whereas the wild type requires a period of 6 h in the dark before it can respond again. (C and D) A substantial pool of VVD still remains after 30 min in the dark, thus preventing a response to the second light pulse in the wild type. After 6 h, the VVD pool is depleted, restoring full responsiveness. (E and F) Light-activatable WCC (dark form) levels recover faster in the wild type than the vvd– mutant during both durations of dark period, illustrating the role of VVD in restoring the WCC. On exposure to the second light pulse, the WCC is activated in both the wild type and vvd− mutant. However, the VVD remaining after 30 min of dark suppresses the light response, and only after 6 h of darkness are VVD levels low such that the wild type is able to respond to the light pulse.
Remarkably, the model predicts that the pool of WCC that can be activated by light (cf. Figure 2) recovers during the dark periods more quickly in the wild type than in the vvd– mutant (Figure 4E and F), further illustrating that VVD also has a positive effect on the WCC. Taken together, the model simulations show that the kinetics of VVD degradation in the dark set the refractory period during which the photosystem remains blind to low-light input. At the same time, VVD supports the recovery of the light-activatable WCC.
VVD efficiently represses the transcriptional activity of light-activated WCC
Although VVD supports higher levels of WCC in the light, its inhibitory effect on the WCC should be dominant so as to suppress spurious responses to low-light input. Indeed, Hunt et al (2010) have reported that frq transcription in the wild-type strain was rapidly reduced to basal levels upon light–dark transfer, whereas frq mRNA remained elevated for several hours in a vvd-knockout strain. We corroborated this effect for frq mRNA. Furthermore, we observed it for vvd mRNA, albeit with somewhat different kinetics (Figure 5A). These data are consistent with the rapid inactivation of light-activated WCC by VVD. The model reproduces the data without parameter adjustment (Figure 5B). In particular, the lack of inhibition of light-activated WCC by VVD explains prolonged transcription in the dark in the vvd– mutant.
Figure 5.
VVD represses transcriptional activity after transfer to dark. (A) Repression of frq and vvd RNA synthesis upon light–dark transfer is slow in vvdSS692. Cultures of wt and vvdSS692 were grown in constant light (20 μmol m−2 s−1) for 2 days, transferred to darkness and collected at the indicated time points. Levels of vvd and frq mRNA are shown as determined by real-time PCR. mRNA levels are normalized to the value at the light-to-dark transfer point (t=0). (B) Simulations reproduce the experimental result showing frq and vvd mRNA levels elevated for up to 4 h in the vvd– mutant and wild-type levels reaching baseline within 1 h. Source data for this figure is available on the online supplementary information page.
The transcript level of frq declines more slowly than that of vvd in the mutant. Moreover, frq transcription in the wild type is maintained at a basal level in the long run, while vvd transcription vanishes (cf. Figure 5A). To explain this difference, we have implemented the different architectures of the vvd and frq promoters in the model. The vvd transcription is driven by light-activated WCC; frq transcription is activated by two promoter elements: the proximal LRE, mediating light induction, and the C-box, which can be activated by the dark-form WCC (Froehlich et al, 2002). This difference in gene regulation naturally accounts for the different kinetics of vvd and frq mRNA (cf. Figure 5B).
In prolonged darkness, self-sustained oscillations of the circadian clock are independent of VVD. The model reproduces this phenomenon (Supplementary Figure S3). In agreement with experiment (Elvin et al, 2005), the vvd– mutant shows a phase delay of ∼4 h.
To summarize, these results show that the competitive binding of VVD to WCC can fully account for the efficient quenching of the transcriptional activity of light-activated WCC. In this way, VVD produced during light exposure also times the onset of the free-running clock in constant darkness without being involved in generating the oscillations.
Futile cycling turns feedback inhibition into adaptation
The quantitative agreement of the model with diverse experimental scenarios provides strong evidence for VVD’s function as a competitive feedback inhibitor of the light-activated WCC. However, VVD is essential also for continuous light responsiveness of the photosystem (cf. Figure 1). Analyzing the dynamics of the WCC pool in the model, we observed that a futile cycle is the key to repeated light sensing (Figure 6A). This cycle is generated by the heterodimerization of light-activated WCC and VVD, followed by the spontaneous decay of the photoadduct in either protein. Heterodimerization inhibits photoactivated WCC and protects it from degradation, whereas photoadduct decay releases it directly back into the light-activatable pool WCC (Figure 6A).
Figure 6.
Photoadduct decay constitutes a futile cycle that ensures repeated sensitivity. (A) Schematic representation of the futile cycling arising from photoadduct decay of the WCC–VVD hetereodimer. Thickness of the arrows indicate the relative rate of reactions. (B) Model simulations show responsiveness to increasing light stimuli is maintained via the futile cycle. Upper panel: the rate of photoadduct decay, l5, is decreased by a factor of 10. The first response is identical to the wild type (solid blue) but the system loses responsiveness to increasing light stimuli (dashed blue), while still functioning as an inhibitor of light-mediated WCC activity. Lower panel: the vvd– mutant loses both the ability to respond to increasing light (similar to the slow-cycling model) and downregulation of the response in constant stimulus. (C) Repeated responsiveness is maintained by a sizeable pool of light-activatable WCC (WCC). Simulations show wild-type levels are higher than the vvd– mutant and the slow-cycling model.
To demonstrate the key role of this futile cycle, we introduced a variant of the model (the ‘slow-cycling model’) in which the WCC–VVD complex is artificially stabilized (by slowing down the rate of photoadduct decay, l5 in Figure 2, by a factor of 10). Otherwise, the model is unchanged; in particular, the binding of VVD to WCC is not altered. Indeed, the slow-cycling model fully retains the function of VVD as a competitive inhibitor of WCC. The response to the initial light step is unchanged (Figure 6B) the first spike in the original model (solid blue curve) and the slow-cycling model (dashed blue curve) are identical. However, the responses to subsequent light steps are abolished when photoadduct decay is slowed down; with respect to this feature, the slow-cycling model is very similar to the vvd– model. Thus, sufficiently rapid photoadduct decay is essential for maintaining the responsiveness of the photosystem.
In order to elicit a significant response, a sizeable pool of light-activatable WCC is required. Our simulations show that this pool diminishes with increasing light (Figure 6C; see also Supplementary Figure S2). However, the wild type always retains a larger pool than the vvd– mutant, explaining why it remains responsive to subsequent light steps but the mutant does not. Slowing photoadduct decay reduces the light-activatable WCC pool practically to the level of the vvd– mutant (Figure 6C). This shows that sequestration of WCC by VVD per se, which is intact in the slow-cycling model, does not replenish the light-activatable WCC but futile cycling is needed. In particular, note that the light-activatable WCC pool cannot be fueled by dissociation of the WCC–VVD heterodimer (reaction with rate constants l1 and l–1 Figure 2), because this reaction carries a forward net flux during the adaptation phase (newly synthesized VVD captures active WCC) and thereafter equilibrates (net flux ∼0).
To further examine this mechanism, we constructed a simplified model representing only the WCC and VVD components and their key interactions. This model recapitulates the adaptation behavior seen in the full model and the experiments, and, more generally, shows adaptation and maintained responsiveness, provided that VVD is produced at a sufficiently large rate and the WCC–VVD complex decays at a sufficiently large rate to fuel the pool of activatable WCC (Supplementary Text S2). This further demonstrates that indeed downregulation and repeated responsiveness are characteristics that arise from the futile cycle.
Thus, we have identified a futile cycling mechanism in the light-sensing network that is capable of turning feedback inhibition by VVD into sensory adaptation. Binding of VVD to WCC inhibits the WCC, whereas the dissociation of the WCC–VVD complex caused by photoadduct decay continuously replenishes the light-activatable WCC pool and retains the responsiveness of the photosystem in constant light. This regulatory ‘motif’ allows VVD to act as both an inhibitor and a positive regulator of the light response.
Futile cycling maintains a light-activatable WCC pool at steady state
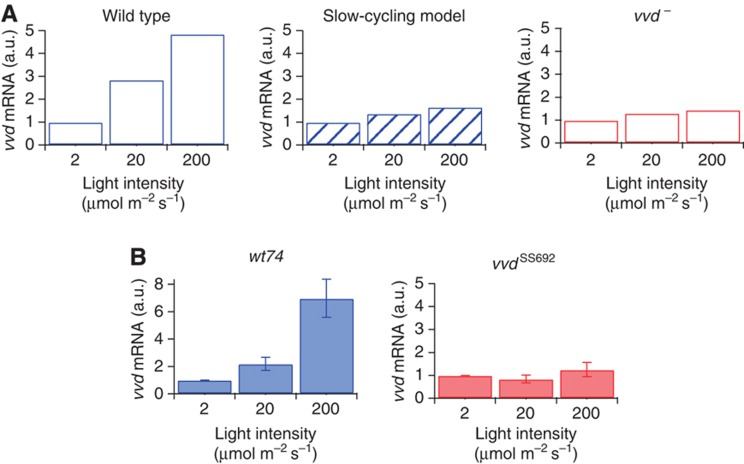
The futile cycling model implies that VVD maintains a pool of activatable WCC at steady state in constant light. Therefore, we asked whether we could measure a functional correlate of this pool. The rate of WCC-dependent gene transcription does not return to zero in constant light but settles to a basal value (cf. Figure 1 B–D). This indicates the existence of a light-activatable WCC pool, which allows steady-state gene expression. Focusing on the vvd gene as a WCC target, we simulated the steady-state level of vvd mRNA in the model, finding that it actually increases with light intensity (Figure 7A; fivefold increase from 2 to 200 μmol m–2 s–1). This increasing steady-state response in the model is due to a slowly decreasing size of the light-activatable WCC pool (cf. Figure 6) and the strong rise in light intensity, as the product of both controls the concentration of the transcriptionally active WCC. Indeed, vvd mRNA levels are directly related to the maintenance of the activatable WCC pool by VVD, because in the slow-cycling model the increase of vvd transcription with ambient light is much weaker (twofold increase from 2 to 200 μmol m–2 s–1) and barely noticeable in the absence of VVD.
Figure 7.
VVD tracks ambient light levels. (A) Model simulations show wild-type vvd mRNA levels rise up to fivefold with increasing light intensity. The simulated levels from both the slow-cycling model and the vvd− mutant remain constant over the three light intensities. All levels are normalized to the respective mRNA values at 2 μmol m−2 s−1. (B) Experimental results confirm the model simulations. Neurospora cultures of the indicated strains were grown for 48 h in constant light at 2, 20 and 200 μmol m−2 s−1, respectively. Samples were collected and mRNA was measured via qRT–PCR. Results shown are from at least two independent experiments measured in triplicates. Source data for this figure is available on the online supplementary information page.
To test this prediction, Neurospora cultures were grown for 48 h at constant light to ensure that a steady state of the photosystem is reached. In the wild type, we observed the expected increase of vvd mRNA with light intensity (Figure 7D; see also Malzahn et al, 2010). By contrast, no significant change of vvd transcription occurred in the vvd– mutant (Figure 7E), confirming the model prediction.
Discussion
Sensory adaptation comprises two components: the downregulation of the response in the presence of a constant stimulus and continued sensitivity of the sensory system to an increase in the stimulus. We have shown here that Neurospora light sensing exhibits bona fide adaptation and provides an underlying mechanism. Surprisingly, the negative and positive regulatory components of adaptation are mediated by a single molecule, the blue-light photoreceptor VVD. Our model suggests that the downregulation of the light response could efficiently be achieved by competitive heterodimerization of light-activated VVD with light-activated WCC (feedback inhibition) (Malzahn et al, 2010). At the same time, VVD can maintain the responsiveness to further increases in light intensity by channeling active WCC* away from degradation (of the WC-1 component) back into the light-activatable WCC pool (replenishment). This novel mechanism, requiring futile cycling through photoadduct formation and decay, could explain why VVD itself is a blue-light photoreceptor, rather than a light-independent inhibitor of the WCC.
A well-understood example of sensory adaption is vertebrate photoreception, which allows us to perceive objects at nearly constant contrast despite changes over many orders of magnitude in the level of ambient illumination (Fain et al, 2001). Most other sensory processes exhibit this type of behavior at least in some range of ambient stimulus intensity. In particular, photoadaptation has been studied for the entrainment of the circadian clock in mammals, with sophisticated light-stimulus protocols (Nelson and Takahashi, 1999; Kronauer et al, 1999; Rimmer et al, 2000). Much theoretical work on adaptation has focused on the precise downregulation of the response to prestimulus level (‘exact’ or ‘perfect’ adaptation; Knox et al, 1986; Yi et al, 2000; Behar et al, 2007; Ma et al, 2009); and this concept has informed recent experimental work (Muzzey et al, 2009; Houser et al, 2012). By comparison, the mechanisms that maintain responsiveness of a sensory system after downregulation of the acute response are much less well understood (e.g., Knox et al, 1986; Tang and Othmer, 1994; Behar et al, 2007).
Here we have found for Neurospora that light adaptation is not perfect. There is a remaining basal response, suggesting that perfect light adaptation is not of overriding physiological importance for this organism. Rather, the photosystem maintains its responsiveness within the natural span of light intensities, so that Neurospora perceives relative changes in intensity (Schwerdtfeger and Linden, 2003; Chen et al, 2010; Malzahn et al, 2010). This property is likely to be physiologically important for sessile organisms populating habitats with a wide range of ambient light intensities, as it ensures robust responses to the day–night (i.e., sunlight–moonlight) cycles irrespective of the actual habitat and support accurate entrainment of the clock (Malzahn et al, 2010; Thommen et al 2010; Tsumoto et al, 2011).
The data-driven model demonstrates that VVD mediates both downregulation and the maintained responsiveness of the photosystem. In principle, downregulation could be achieved through selective degradation of activated WCC. Indeed, light-activated WC-1 is degraded rapidly (Malzahn et al, 2010). However, the level of WC-1 in light is actually higher in the wild type than in adaptation-deficient mutants, arguing against a role for WC-1 degradation in adaptation. Of note, Kronauer et al (1999) have previously suggested a simple model of light adaptation where the rate of activation of a single light sensor is postulated as the output driving the (mammalian) circadian clock. Unlike this conceptual model, the model presented here is fully based on experimentally implicated molecular mechanisms, and its functional output is the concentration of the active WCC transcription factor.
Maintained responsiveness of the system requires the replenishment of the WCC pool. Enhanced WC-1 synthesis would be the most direct method. However, this would not be dependent on VVD, contradicting our finding that the vvd– mutant cannot respond to multiple increases in light intensity. Moreover, WC-1 synthesis is insufficient to compensate its light-induced degradation (Malzahn et al, 2010 and Supplementary Figure S2). Instead, we propose that heterodimerization by VVD not only downregulates light responses, but also has a prominent role through the subsequent rechanneling of the WCC back into the light-activatable pool. It is important to note that dissociation of the heterodimer complex cannot fulfill this function, because the net flux is in the direction of the WCC–VVD association to mediate inhibition of the WCC. Rather, the decay of the photoadduct, which resets the individual components to their inactivated forms, is the driving mechanism. The model accounts for the fact that the photocycle of the VVD LOV domain lies within the range of hours (Zoltowski et al, 2009). Decay rates for the WCC photoadduct are of the same order of magnitude as the VVD photoadduct (Malzahn et al, 2010). The model demonstrates that such a VVD-dependent ‘passive’ method of replenishment explains the differences in light adaptation between the wild type and the mutant, whereas the ‘active’ method (de novo synthesis) does not.
Important insights into circadian oscillators and adaptation have been gained from simplistic models that allow quite general analysis (e.g., Leloup et al, 1999; Leloup Goldbeter, 2000; Akman et al, 2008, 2010; Behar et al, 2007; Ma et al, 2009). Our analysis indicates that certain mechanistic principles might be missed by such conceptual analyses. For example, previous computational searches for network motifs for adaptation did not allow for inhibition by sequestration and, thus, could not come up with the futile-cycling motif described here (Behar et al, 2007; Ma et al, 2009). Iteration between theory and experiment clearly provides a complementary strategy to establish which mechanistic details matter for biological function (Boothby, 2009). Importantly, the model has been parameterized using the light-step protocol (Figure 1) and subsequently been tested successfully against diverse experimental data (Figures 5 and 7). Thus, we find that the parameters of VVD expression, degradation and heterodimerization with WCC, as well as the rate of replenishment of the active WCC pool by decay of the WCC–VVD complex are constrained by the experimental data, allowing us to identify the dual role of VVD for photoadaptation. Other parameters, in particular the ones pertaining to the action of FRQ, cannot be reliably estimated from our data. This is very likely to be of minor importance in the present context, as data by us and others indicate that VVD has the key role in photoadaptation (Chen et al, 2010; Hunt et al, 2010; Malzahn et al, 2010) However, given FRQs function in the mechanism of the clock our findings imply that further targeted measurements under well-defined conditions will be needed to arrive at quantitatively predictive models of the Neurospora circadian clock that link molecular mechanism to physiology (Tseng et al, 2012).
Finally, we note that the futile-cycling motif for adaptation could be realized through other mechanisms than photoadduct formation and decay. For example, the regulation of inhibitor affinity by phosphorylation and dephosphorylation (or any other reversible covalent modification) could realize the same regulatory motif, indicating that it might be employed more widely.
Materials and methods
Neurospora strains and culture conditions
Neurospora strains used in this study were wt74 and the vvd loss-of-function mutant vvdSS692 (Heintzen et al 2001). Standard growth medium contained 2% glucose, 0.5% L-arginine, 1 × Vogel’s and 10 ng ml−1 biotin. Cultures were incubated at 25 °C. The light-step protocol (Figure 1A) accommodates one long light step (20 μmol m−2 s−1) to accurately monitor the return to steady state while keeping the overall duration below 24 h to prevent nutrient depletion.
RNA analysis
Total RNA was extracted with the peqGOLD TriFast reagent (Peqlab). cDNA was synthesized from 1 μg total RNA with the QuantiTect Reverse Transcription Kit (QIAGEN). Transcript levels were quantified by RT−PCR with TaqMan Probes in a StepOne system (Applied Biosystems). Triplicate reactions (20 μl) containing cDNA equivalent to 0.05 μg RNA were analyzed. Primers and probes for measuring actin, frq and wc-1 RNA are described elsewhere (Malzahn et al, 2010). For the analysis of vvd RNA, the following primers and probes were used: VVDII fwd: 5′-TACCCTACCAACACACTGTCTCATAAC-3′; VVDII rev: 5′-TCAACCTCCCATGGGTTCAT-3′; VVDII Probe: 5′-CATGAGCCATACCGTGAACTCGAGCAC-3′.
Mathematical modeling and parameter estimation
We used a Markov chain Monte Carlo and Bayesian inference technique, implemented in Fortran 95, to fit the model parameters. Further details of the fitting technique are explained in the Supplementary Information. The model was fitted to the light induction experiments (Figure 1) and the system of ODEs was solved using the Matlab solver ode15s.
Supplementary Material
Supplementary Figures S1–3, Supplementary Tables S1–2
Acknowledgments
MB and TH are members of CellNetworks. This work was supported in part by the Initiative and Networking Fund of the Helmholtz Association within the Helmholtz Alliance on Systems Biology/SBCancer (TH). We thank Sabine Schultz for technical assistance and Andreas Raue for help with the profile likelihood analysis.
Author contributions: EG, MB and TH conceived the study. EG performed the modeling. ACRD carried out the experiments. EG, ACRD, MB and TH analyzed the data. EG, MB and TH wrote the paper.
Footnotes
The authors declare that they have no conflict of interest.
References
- Akman OE, Locke JC, Tang S, Carré I, Millar AJ, Rand DA (2008) Isoform switching facilitates period control in the Neurospora crassa circadian clock. Mol Syst Biol 4: 164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akman OE, Rand DA, Brown PE, Millar AJ (2010) Robustness from flexibility in the fungal circadian clock. BMC Syst Biol 4: 1–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arpaia G, Cerri F, Baima S, Macino G (1999) Involvement of protein kinase C in the response of Neurospora crassa to blue light. Mol Gen Genet 262: 314–322 [DOI] [PubMed] [Google Scholar]
- Baker CL, Loros JJ, Dunlap JC (2012) The circadian clock of Neurospora crassa. FEMS Microbiol Rev 36: 95–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behar M, Hao N, Dohlman HG, Elston TC (2007) Mathematical and computational analysis of adaptation via feedback inhibition in signal transduction pathways. Biophys J 93: 806–821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boothby M (2009) The calculus of integrating differentiation: timing control of T-bet. Immunity 30: 666–668 [DOI] [PubMed] [Google Scholar]
- Brunner M, Káldi K (2008) Interlocked feedback loops of the circadian clock of Neurospora crassa. Mol Microbiol 68: 255–262 [DOI] [PubMed] [Google Scholar]
- Cha J, Chang S, Huang G, Cheng P, Liu Y (2008) Control of WHITE COLLAR localization by phosphorylation is a critical step in the circadian negative feedback process. EMBO J 27: 3246–3255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, DeMay BS, Gladfelter AS, Dunlap JC, Loros JJ (2010) Physical interaction between VIVID and White Collar complex regulates photoadaptation in Neurospora. Proc Natl Acad Sci USA 107: 16715–16720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diernfellner A, Schafmeier T (2011) Phosphorylations: making the Neurospora crassa circadian clock tick. FEBS Lett 585: 1461–1466 [DOI] [PubMed] [Google Scholar]
- Diernfellner AC, Querfurth C, Salazar C, Höfer T, Brunner M (2009) Phosphorylation modulates rapid nucleocytoplasmic shuttling and cytoplasmic accumulation of Neurospora clock protein FRQ on a circadian time scale. Genes Dev 23: 2192–2200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elvin M, Loros JJ, Dunlap JC, Heintzen C (2005) The PAS/LOV protein VIVID supports a rapidly dampened daytime oscillator that facilitates entrainment of the Neurospora circadian clock. Genes Dev 19: 2593–2605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fain GL, Matthews HR, Cornwall MC, Koutalos Y (2001) Adaptation in vertebrate photoreceptors. Physiol Rev 81: 117–151 [DOI] [PubMed] [Google Scholar]
- Froehlich AC, Liu Y, Loros JJ, Dunlap JC (2002) White Collar-1, a circadian blue light photoreceptor, binding to the frequency promoter. Science 297: 815–819 [DOI] [PubMed] [Google Scholar]
- Froehlich AC, Loros JJ, Dunlap JC (2003) Rhythmic binding of a WHITE COLLAR-containing complex to the frequency promoter is inhibited by FREQUENCY. Proc Natl Acad Sci USA 100: 5914–5919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonze D, Leloup J-C, Goldbeter A (2000) Theoretical models for circadian rhythms in Neurospora and Drosophila. Life Sci 323: 57–67 [DOI] [PubMed] [Google Scholar]
- Guo H, Kottke T, Hegemann P, Dick B (2005) The phot LOV2 domain and its interaction with LOV1. Biophys J 89: 402–412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Q, Liu Y (2005) Molecular mechanism of light responses in Neurospora: from light-induced transcription to photoadaptation. Genes Dev 19: 2888–2899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Q, Shu H, Cheng P, Chen S, Wang L, Liu Y (2005) Light-independent phosphorylation of WHITE COLLAR-1 regulates its function in the Neurospora circadian negative feedback loop. J Biol Chem 280: 17526–17532 [DOI] [PubMed] [Google Scholar]
- Heintzen C, Loros JJ, Dunlap JC (2001) The PAS protein VIVID defines a clock-associated feedback loop that represses light input, modulates gating, and regulates clock resetting. Cell 104: 453–464 [DOI] [PubMed] [Google Scholar]
- Houser JR, Ford E, Nagiec MJ, Errede B, Elston TC (2012) Positive roles for negative regulators in the mating response of yeast. Mol Syst Biol 8: 586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt SM, Elvin M, Crosthwaite SK, Heintzen C (2007) The PAS/LOV protein VIVID controls temperature compensation of circadian clock phase and development in Neurospora crassa. Genes Dev 21: 1964–1974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt SM, Thompson S, Elvin M, Heintzen C (2010) VIVD interacts with the WHITE COLLAR complex and FREQUENCY-interacting RNA helicase to alter light and clock responses in Neurospora. Proc Natl Acad Sci USA 107: 16709–16714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Káldi K, González BH, Brunner M (2006) Transcriptional regulation of the Neurospora circadian clock gene wc-1 affects the phase of circadian output. EMBO Rep 7: 199–204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knox BE, Devreotes PN, Goldbeter A, Segel LA (1986) A molecular mechanism for sensory adaptation based on ligand-induced receptor modification. Proc Natl Acad Sci USA 83: 2345–2349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronauer RE, Forger DB, Jewett ME (1999) Quantifying human circadian pacemaker response to brief,extended, and repeated light stimuli over the phototopic range. J Biol Rhythms 14: 501–515 [DOI] [PubMed] [Google Scholar]
- Leloup J, Goldbeter A (2000) Modeling the molecular regulatory mechanism of circadian rhythms in Drosophila. BioEssays 22: 84–93 [DOI] [PubMed] [Google Scholar]
- Leloup J, Gonze D, Goldbeter A (1999) Limit cycle models for circadian rhythms based on transcriptional regulation in Drosophila and Neurospora. J Biol Rhythms 14: 433–448 [DOI] [PubMed] [Google Scholar]
- Ma W, Trusina A, El-Samad H, Lim WA, Tang C (2009) Defining network topologies that can achieve biochemical adaptation. Cell 138: 760–773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malzahn E, Ciprianidis S, Káldi K, Schafmeier T, Brunner M (2010) Photoadaptation in Neursopora by competitive interaction of activating and inhibitory LOV domains. Cell 142: 762–772 [DOI] [PubMed] [Google Scholar]
- Merrow M, Franchi L, Dragovic Z, Görl M, Johnson J, Brunner M, Macino G, Roenneberg T (2001) Circadian regulation of the light input pathway in Neurospora crassa. EMBO J 20: 307–315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muzzey D, Gómez-Uribe CA, Mettetal JT, van Oudenaarden A (2009) A systems-level analysis of perfect adaptation in yeast osmoregulation. Cell 138: 160–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson DE, Takahashi JS (1999) Integration and saturation within the circadian photic entrainment pathway of hamsters. Am J Physiol 277: R1351–R1361 [DOI] [PubMed] [Google Scholar]
- Querfurth C, Diernfellner A, Gin E, Malzahn E, Hoefer T, Brunner M (2011) Circadian conformational change of the Neurospora clock protein FREQUENCY triggered by clustered hyperphosphorylation of a basic domain. Mol Cell 43: 713–722 [DOI] [PubMed] [Google Scholar]
- Rimmer DW, Boivin DB, Shanahan TL, Kronauer RE, Duffy JF, Czeisler CA (2000) Dynamic resetting of the human circadian pacemaker by intermittent bright light. Am J Physiol Regul Integr Comp Physiol 279: R1574–R1579 [DOI] [PubMed] [Google Scholar]
- Schafmeier T, Diernfellner A (2011) Light input and processing in the circadian clock of Neurospora. FEBS Lett 585: 1467–1473 [DOI] [PubMed] [Google Scholar]
- Schafmeier T, Diernfellner A, Schäfer A, Dintsis O, Neiss A, Brunner M (2008) Circadian activity and abundance rhythms of the Neurospora clock transcription factor WCC associated with rapid nucleo-cytoplasmic shuttling. Genes Dev 22: 3397–3402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafmeier T, Haase A, Káldi K, Scholz J, Fuchs M, Brunner M (2005) Transcriptional Feedback of Neurospora Circadian Clock Gene by phosphorylation-dependent Inactivation of Its Transcription Factor. Cell 122: 235–246 [DOI] [PubMed] [Google Scholar]
- Schafmeier T, Káldi K, Diernfellner A, Mohr C, Brunner M (2006) Phosphorylation-dependent maturation of Neurospora circadian clock protein from a nuclear repressor towards a cytoplasmic activator. Genes Dev 20: 297–306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrode LB, Lewis ZA, White LD, Bell-Pedersen D, Ebbole DJ (2001) vvd is required for light adaptation of conidiation-specific genes of Neurospora crassa, but not circadian conidiation. Fungal Genet Biol 32: 169–181 [DOI] [PubMed] [Google Scholar]
- Schwerdtfeger C, Linden H (2003) VIVID is a flavoprotein and serves as a fungal blue light receptor for photoadaptation. EMBO J 22: 4846–4855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwerdtfeger C, Linden H (2001) Blue light adaptation and desensitization of light signal transduction in Neurospora crassa. Mol Microbiol 39: 1080–1087 [DOI] [PubMed] [Google Scholar]
- Talora C, Franchi L, Linden H, Ballario P, Macino G (1999) Role of a white collar-1–white collar-2 complex in blue-light signal transduction. EMBO J 18: 4961–4968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Y, Othmer HG (1994) A G protein-based model of adaptation in Dictyostelium discoideum. Math Biosci 120: 25–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thommen Q, Pfeuty B, Morant PE, Corellou F, Bouget FY, Lefranc M (2010) Robustness of circadian clocks to daylight fluctuations: hints from the picoeucaryote Ostreococcus tauri. PLoS Comput Biol 6: e1000990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng Y-Y, Hunt SM, Heintzen C, Crosthwaite SK, Schwartz J-M (2012) Comprehensive modelling of the Neurospora circadian clock and its temperature compensation. PLoS Comput Biol 8: 1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsumoto K, Kurosawa G, Yoshinaga T, Aihara K (2011) Modeling light adaptation in circadian clock: prediction of the response that stabilizes entrainment. PLoS One 6: e20880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaidya AT, Chen C, Dunlap JC, Loros JJ, Crane BR (2011) Structure of a light-activated LOV protein dimer that regulates transcription. Sci Signal 4: ra50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi TM, Huang Y, Simon MI, Doyle J (2000) Robust perfect adaptation in bacterial chemotaxis through integral feedback control. Proc Natl Acad Sci USA 97: 4649–4653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoltowski B, Crane B (2008) Light activation of the LOV protein Vivid generates a rapidly exchanging dimer. Biochemistry 47: 7012–7019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoltowski B, Vaccaro B, Crane B (2009) Mechanism-based tuning of a LOV domain photoreceptor. Nat Chem Biol 5: 827–834 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figures S1–3, Supplementary Tables S1–2