Abstract
Hepatocyte growth factor, produced by stromal and follicular dendritic cells, and present at high concentrations in the sera of patients with chronic lymphocytic leukemia, prolongs the survival of leukemic B cells by interacting with their receptor, c-MET. It is, however, unknown whether hepatocyte growth factor influences microenvironmental cells, such as nurse-like cells, which deliver survival signals to the leukemic clone. We evaluated the expression of c-MET on nurse-like cells and monocytes from patients with chronic lymphocytic leukemia and searched for phenotypic/functional features supposed to be influenced by the hepatocyte growth factor/c-MET interaction. c-MET is expressed at high levels on nurse-like cells and at significantly higher levels than normal on monocytes from patients. Moreover, the hepatocyte growth factor/c-MET interaction activates STAT3TYR705 phosphorylation in nurse-like cells. Indoleamine 2,3-dioxygenase, an enzyme modulating T-cell proliferation and induced on normal monocytes after hepatocyte growth factor treatment, was detected together with interleukin-10 on nurse-like cells, and on freshly-prepared patients’ monocytes. Immunohistochemical/immunostaining analyses demonstrated the presence of c-MET+ and indoleamine 2,3-dioxygenase+ cells in lymph node biopsies, co-expressed with CD68 and vimentin. Furthermore nurse-like cells and chronic lymphocytic monocytes significantly inhibited T-cell proliferation, prevented by anti-transforming growth factor beta and interleukin-10 antibodies and indoleamine 2,3-dioxygenase inhibitors, and supported CD4+CD25high+/FOXP3+ T regulatory cell expansion. We suggest that nurse-like cells display features of immunosuppressive type 2 macrophages: higher hepatocyte growth factor levels, produced by leukemic or other microenvironmental surrounding cells, may cooperate to induce M2 polarization. Hepatocyte growth factor may thus have a dual pathophysiological role: directly through enhancement of survival of the leukemic clone and indirectly by favoring T-cell immunosuppression.
Introduction
B-cell chronic lymphocytic leukemia (CLL) is characterized by the relentless accumulation of monoclonal B cells in the blood, secondary lymphoid tissue and bone marrow. Despite their apparent longevity in vivo, CLL cells undergo spontaneous apoptosis when cultured in vitro, suggesting that cell-cell contacts and factors produced in the microenvironment are essential to prolong their survival. Apoptosis of CLL B cells can be prevented by co-culture with mesenchymal bone marrow stromal cells,1–3 follicular dendritic cells4 and monocyte-derived nurse-like cells.5 Nurse-like cells (NLC) have been described as a peculiar subset of cells derived from cultures of blood mononuclear cells of CLL patients and differentiate into large, adherent cells capable of protecting leukemic B cells from apoptosis:6 indeed CLL cells experience a rapid decline in viability when removed from co-culture with NLC. Several studies identified factors and cellular contacts involved in increasing CLL viability. NLC attract CLL cells by secreting CXCL126 and CXCL137 and protect CLL from apoptosis through CXCL12, BAFF, APRIL,6,8 CD319 and plexin-B1.10 These peculiar cells have also been observed in spleens and lymphoid tissues of CLL patients, where they possibly facilitate the expansion of leukemic cells. Despite expressing myelomonocytic antigens, NLC are believed to be distinct from monocytes-macrophages or monocyte-derived dendritic cells. They express low levels of CD14 and CD33 but high levels of CD68, and are vimentin+, CD1a− and CD83−.5 In addition, unlike NLC found in synovia from patients with rheumatoid arthritis,11 they are negative for CD106.5 Many findings support the hypothesis that NLC differentiate in vitro under the influence of leukemic B cells and specific microenviromental factors: CLL cells (but not normal B cells) are capable of inducing specific changes in CD14+ monocytes.5 Moreover NLC derived from co-culture of monocytes and autologous leukemic cells are characterized by a gene expression profile typical of cells with dysregulated immunocompetence which could be relevant in the context of the acquired immunodeficiency commonly found in CLL patients.12 Whether NLC represent CLL-specific tumor-associated macrophages, as recently suggested,13 is still debated.
We have recently reported that hepatocyte growth factor (HGF), together with CXCL12, is produced at high levels by stromal cells and is capable of prolonging the survival of CLL cells which are positive for the HGF receptor, c-MET.14 HGF is a multifunctional cytokine that induces multiple biological responses in target cells, including adhesion, motility, growth, survival and morphogenesis by activating its tyrosine kinase receptor, c-MET.15,16 In normal individuals, HGF is constitutively produced by fibroblast-like stromal cells in lymphoid tissue and by follicular dendritic cells within the germinal center microenvironment.15,17,18 NLC are present in the lymph nodes of CLL patients, where they are interspersed with stromal, dendritic and T cells to form proliferation centers.19 The intriguing finding that HGF levels are higher in sera from CLL patients than from normal controls,20 together with a still undefined pattern of effects induced by HGF on myelomonocytic cells, prompted us to determine c-MET expression on NLC and on monocytes-macrophages as well as investigate potential downstream events caused by the interaction between HGF and c-MET.
Methods
This study was approved by the review board of the IRCCS AOU San Martino –IST, Genoa, Italy. Full details are provided in the Online Supplementary Methods file.
Cell preparation
Heparinized blood or bone marrow samples were taken from CLL patients untreated for at least 3 months. The patients’ characteristics are summarized in Online Supplementary Table S1. NLC were derived and characterized as described below and in the Online Supplementary Methods. CD14+ monocytes were purified from patients or healthy donors by magnetic beads. The human monocytic cell line THP-1 was utilized in selected experiments.
Fluorescence microscopy and flow cytometry
NLC or fresh monocytes from CLL or normal donors were challenged with antibodies against c-MET, indoleamine 2,3-dioxygenase (IDO), vimentin, CD68, interleukin (IL)-10, CD14, CXCR4, CD163 and pSTAT3TYR705 and analyzed by immunofluorescence or flow cytometry. The quantification of pSTAT3 immunopositive cells area is described in detail in the Online Supplementary Methods.
Analysis of STAT3 and pSTAT3 activation by western blotting
Western blotting was used to evaluate STAT3, pSTAT3 and β-actin in monocytes from normal or CLL donors and in NLC with or without HGF treatment.
Immunohistochemistry and immunofluorescence analysis of lymph node and bone marrow biopsies
Tissue sections were probed with anti-human c-MET, -IDO, -vimentin, -CD68, and -CD163. 3,3′-Diaminobenzidine (DAB) substrate chromogen and hematoxylin counterstaining, or Alexa 488- and Alexa 568-fluorescein isothiocyanate conjugated monoclonal antibodies were used in the histochemistry and immunofluorescence analysis, respectively.
RNA isolation and quantitative real-time polymerase chain reaction
The relative expression of GAPDH, HGF, c-MET, stromal derived factor-1 (SDF-1/CXCL12), CD68, CXCR4, transforming growth factor β-1 (TGF-β1) and IL-10 was assessed by SYBR green quantitative real-time reverse transcriptase polymerase chain reaction (PCR) analysis.
Evaluation of c-MET and indoleamine 2,3-dioxygenase expression on normal monocytes following their co-culture with chronic lymphocytic leukemia cells
Purified monocytes from healthy donors were cultured alone or with CD14-depleted B cells from CLL patients or normal donors. After 10 days detached monocytes were stained with antibodies against c-MET and IDO.
Quantification of hepatocyte growth factor in cell cultures
HGF production was detected by enzyme-linked immunoassays in supernatants from cultures of monocytes, CLL cells, or monocytes/NLC plus CLL or NLC alone, CLL sera and CLL-bone marrow stromal cell media, prepared as detailed in the Online Supplementary Methods. HGF expression was further quantified by multiple-color staining on CLL cells, CD19+/CD5+ gated, after co-culture with monocytes/NLC for different periods (0, 4, 8, 11, and 14 days); single-color staining was used for monocytes/NLC.
Blocking chronic lymphocytic leukemia cell survival by monoclonal antibodies against hepatocyte growth factor
CLL cells co-cultured with NLC for 12 days were recovered and re-plated with or without NLC. When needed anti-HGF (L2G7) and anti-CXCR4 monoclonal antibodies were added. Apoptosis was evaluated at different time-points by DiOC6 staining.21
Determination of T-cell proliferation inhibition
To assess the suppression of T-cell proliferation, CFSE-labeled-preactivated T cells were co-cultured with monocytes from normal or CLL donors or with NLC. The reduction in CFSE intensity was evaluated on gated TCRαβ+ lymphocytes. Anti-IL-10, -TGFβ or IDO inhibitors were added when needed.
Determination of T regulatory cell expansion in long-term co-cultures
The presence of T regulatory cells was evaluated in peripheral blood monocytes from CLL patients either fresh or after autologous monocyte co-culture (for 8, 11, and 14 days). T regulatory cells were identified by FOXP3 expression on CD4+/CD25high+ cells.
Statistical analysis
A two-sided Student t-test or Mann-Whitney test was applied. P values are indicated as *, ** or ***, for 0.05≥P≥ 0.01, 0.01>P≥ 0.001, or P<0.001, respectively.
Results
Nurse-like cells express the hepatocyte growth factor receptor, c-MET
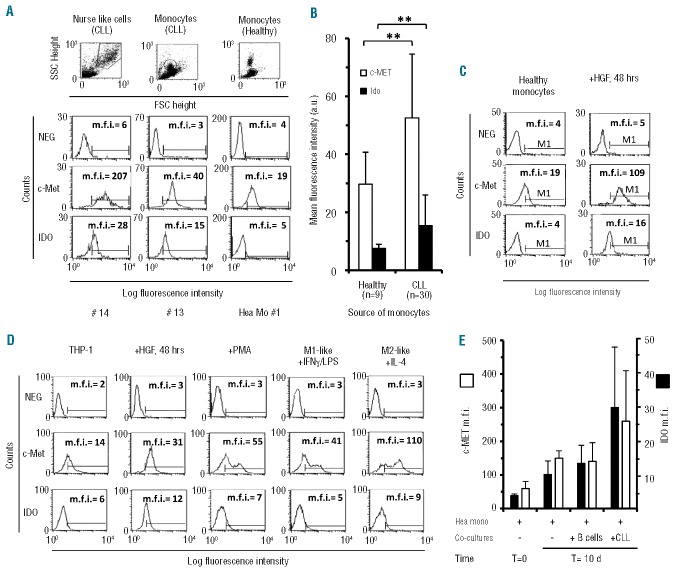
We first analyzed expression of the HGF receptor c-MET on NLC by immunofluorescence. Typical large round cells with morphological features of NLC were obtained after cultures of fresh CLL in chamber slides for 14 days. As shown in Figure 1A and B, we observed high levels of intra-cytoplasmic expression of c-MET in large adherent cells. Moreover a thin cytoplasmic fluorescent rim was detectable on small leukemic cells, in agreement with recent data.14 Two-color immunofluorescence for cMET and CD68 or vimentin, showed that c-MET+ cells were also positive for CD68 and vimentin, two markers strongly expressed by NLC.5 Furthermore, in agreement with the notion of a nurturing function of NLC, the large round cells were surrounded by smaller CD19+ leukemic cells (Figure 1C).
Figure 1.
c-MET, IDO and IL-10 expression on NLC and pSTAT3 activation upon HGF treatment. (A) c-MET immunopositivity detection by specific fluorescence isothiocyanate (FITC)-conjugated monoclonal antibodies, on in vitro-derived NLC from different CLL patients (#). Nuclear counter-staining was performed by DAPI and the images are presented at 40× magnification. (B) Immunofluorescence analysis of the coexpression of c-MET and CD68 (FITC-conjugated) with phycoerythrin (PE)-conjugated vimentin, the latter typically highly expressed on NLC.5 Images are representative of NLC derived from one of four CLL cases studied for each coexpression. Residual DAPI+ small B cells are dispersed throughout the visual field. Images are shown at 20× magnification. Inserts depict isotypic controls (20X) (C) Images evidence nurturing features of a large NLC depicted as c-MET-FITC positive, CD19-PE negative. Red arrows identify CD19+ B cells attached to a large CD19− NLC (green arrow). (D) STAT3 phosphorylation was induced by treating NLC with HGF for 40 min and detected by specific PE-conjugated anti-pSTAT3 monoclonal antibodies. NLC from three different CLL cases (case id# 3, 26 and 27) are represented before (untreated:Untr) and after HGF exposure (HGF). Images are depicted at 20× magnification, inserts at 40X. (E) Image analysis of the immunopositive integrated area in untreated (CN) versus treated (HGF) cells. The average cell area in treated cells was normalized to the area of untreated cells in the same patient. Bars represent the mean integrated area ± SD (Student t test) (F) Immunofluorescence analysis of the coexpression of IDO-FITC and CD68-PE on NLC from a representative CLL case of three studied, as demonstrated by the merged images. Photographs and inserts as in (D). (G) Immunofluorescence analysis of the coexpression of IDO-FITC and IL-10-PE on NLC as demonstrated by the merged images. Photographs and inserts as in (D).
Hepatocyte growth factor induces STAT3 phosphorylation in nurse-like cells
STAT3, a downstream effector activated by HGF,22 is also considered one of the master regulators of M2 polarization in monocytes-macrophages.23 We, therefore, investigated whether HGF could induce STAT3 phosporylation in NLC: indeed we recently described rapid STAT3 activation in leukemic CLL cells following interaction between HGF and c-MET.14 Although a weak phosphorylation signal is sometimes observed already on NLC in basal conditions, treatment with HGF for 40 min induced de novo, or strongly increased, STAT3TYR705 phosphorylation, as shown in three representative cases (Figure 1D). When we quantified the pSTAT3 immunopositive integrated area of HGF-treated versus untreated cells, the phosphorylation was statistically significant in the three cases analyzed (Figure 1E). The finding of rapid STAT3 activation demonstrates that the interaction of HGF with its receptor exerts a direct effect on NLC through STAT3 signaling. Western blot analysis confirmed STAT3 activation in CLL-monocytes and in NLC after HGF treatment (Online Supplementary Figure S1).
Nurse-like cells express indoleamine 2,3-dioxygenase and interleukin-10
Here we investigated whether NLC from CLL patients constitutively expressed IDO, a key enzyme in the tryptophan catabolism that modulates T-cell proliferation and activation. The rationale for these tests is based upon the observation that normal monocytes-macrophages express IDO usually upon exposure to HGF24 and that CLL patients have higher than normal HGF levels.21 We derived NLC as previously described (see Online Supplementary File) and after 14 days of culture we analyzed large CD68+ cells for IDO expression. As shown in Figure 1F we observed IDO positivity on CD68+ cells and also found that, on the 14-day derived NLC, IDO appeared to be co-expressed with IL-10 (Figure 1G), a cytokine typically involved in immunosuppression. In agreement with the immunofluorescence analysis, quantitative reverse transcriptase PCR confirmed that NLC, and not CLL cells, expressed IL-10. c-MET expression was also confirmed on NLC by real-time reverse transcriptase PCR, at higher levels than on leukemic B cells (Online Supplementary Figure S2). We further observed that substantial amounts of HGF mRNA were expressed by NLC co-cultured with CLL cells, correlating with a moderate release of HGF by NLC (300–400 pg/mL), along with other characteristic mRNA (CD68, CXCL12) (Online Supplementary Figure S2).
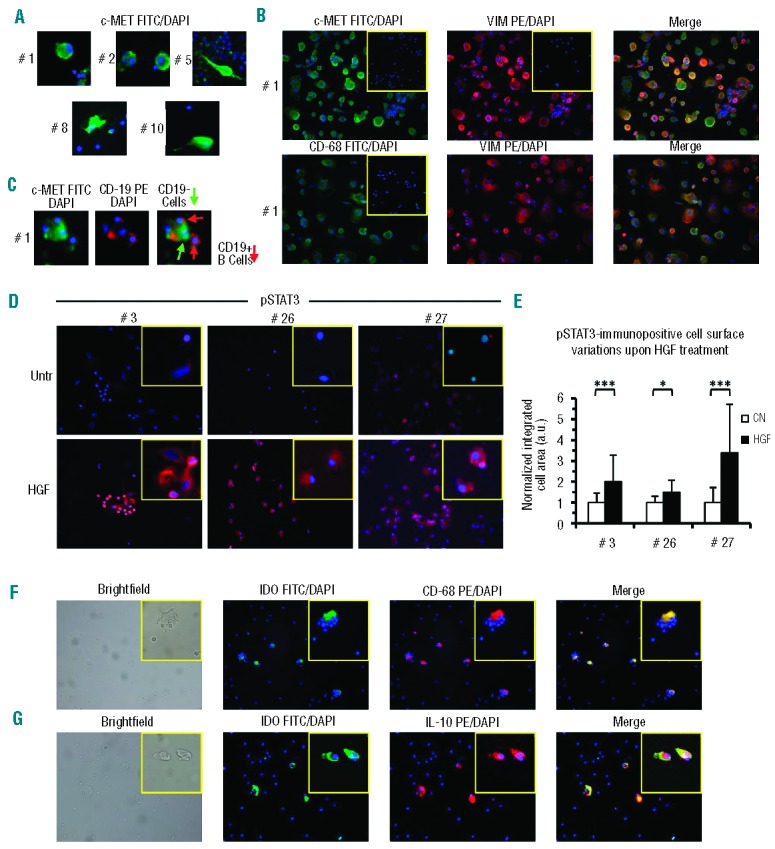
Cells from CLL-bone marrow aspirates were cultured at high cell concentration in chamber slides for 14 days. As for peripheral blood from CLL patients we again obtained a significant number of adherent cells with features of NLC which were also c-MET+ and IDO+, as well as CD68+ and CD14+ (Online Supplementary Figure S3: one representative cases of two analyzed). This finding indicates that NLC precursors may also be present in the bone marrow and suggests a possible recirculation between peripheral blood, bone marrow and lymphoid tissues.
Interestingly, immunohistochemical staining demonstrated c-MET and IDO expression by lymph node and bone marrow cells with a morphology resembling that of NLC (Figure 2). In addition, c-MET and IDO were co-expressed by CD68+ and vimentin+ cells (Figure 2) in lymph nodes. The expression of these markers is consistent with a NLC phenotype.5 Furthermore IDO was co-expressed in vivo with CD163, an antigen typically displayed by tumor associated macrophages (Figure 2), as well as with CXCR4 on NLC derived in vitro from peripheral blood (Online Supplementary Figure S4). Altogether the present data demonstrate that c-MET, IDO and CD163 are already expressed in vivo by NLC and not induced by in vitro culture.
Figure 2.
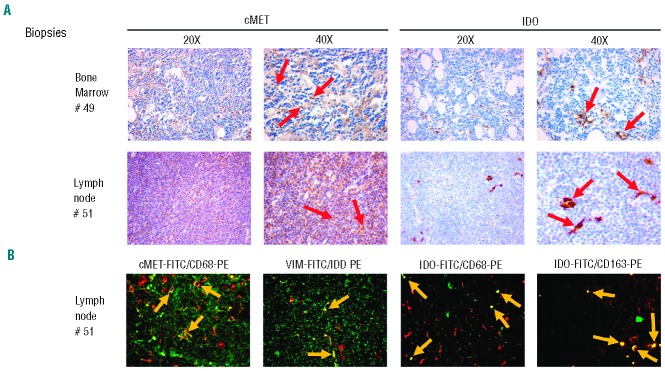
Immunofluorescence and immunohistochemical analysis of c-MET and IDO expression on CD68+ or vimentin+ cells in bone marrow and lymph node biopsies. (A) Immunohistochemical analysis of c-MET and IDO expression in bone marrow and lymph nodes. Red arrows indicate c-MET and IDO positivity on large cells resembling NLC: one representative case (#49 or #51) of eight analyzed. Images are shown at 20× and 40× magnification. (B) Immunofluorescence analysis of the coexpression of c-MET-fluorescein isothiocyanate (FITC) and CD68-phycoerythrin (PE), of VIM-FITC and IDO-PE, or of IDO-FITC and CD68- or CD163-PE in lymph nodes. Yellow arrows identify double-positive cells. Single-positive cells are also present in the images. Images are at 40× magnification.
Indoleamine 2,3-dioxygenase is present on monocytes freshly isolated from patients with chronic lymphocytic leukemia
Using flow cytometry, we further analyzed the expression of IDO by monocytes from CLL patients and healthy donors as well as by NLC. Substantial IDO expression was detected in CLL-monocytes and in NLC but not in monocytes from healthy donors (Figure 3A,B; P=0.005; n=9 healthy-monocytes versus n=30 CLL-monocytes). In addition, c-MET levels were significantly higher than normal in CLL-monocytes (Figure 3B; P=0.008). We then confirmed that exposure to HGF for 48 h caused IDO and c-MET upregulation in normal monocytes (Figure 3C). The same HGF treatment induced upregulation in the THP-1 cell line, a valuable in vitro model for studying monocyte-macrophage differentiation25 (Figure 3D). Interestingly when THP-1 cells were treated with phorbol myristate (PMA) to induce macrophage differentiation, and then cultured with interferon-γ/lipopolysaccharide or interleukin-4 to induce M1 or M2 polarization respectively, c-MET upregulation was seen only on the M2 polarized cells (Figure 3D). IDO also resulted increased, albeit weakly, on M2 cells (Figure 3D). High c-MET and to a lesser extent IDO levels appear to be features of type 2 monocytes-macrophages, thus suggesting an M2-oriented phenotype of CLL-monocytes.
Figure 3.
Flow cytometric analysis of c-MET and IDO expression on NLC or monocytes from CLL patients or healthy donors and study of their induction following HGF treatment. (A) Upper row: c-MET and IDO were analyzed on NLC or monocytes by setting a gate based on the side- and forward-angle light scatter and including at least 95% of CD14+ cells. The second row depicts fluorochrome-conjugated isotype control monoclonal antibodies while the third and fourth rows depict c-MET and IDO expression, respectively, indicated as mean fluorescence intensity (m.f.i.). One representative case for each cell type is shown, Id numbers refer to patients as reported in Online Supplementary Table S1, HeaMo#1: healthy monocytes (B) Comparison between c-MET and IDO expression on monocytes from healthy donors (n=9) or from CLL patients (n=30), as detected by flow cytometry. c-MET and IDO expression was significantly higher on CLL-monocytes (P=0.005 and P=0.008 respectively, Mann-Whitney test). (C) c-MET and IDO surface expression was enhanced after 48 h HGF treatment on healthy monocytes (one representative cases of three analyzed). (D) The THP-1 monocytic cell line was evaluated for c-MET and IDO expression after HGF treatment or M1-, M2-induced polarization. A significat increase in c-MET and IDO expression was observed in HGF-treated cells; c-MET resulted even more expressed upon M2-like driven polarization. (E) Representation of consistent c-MET and IDO upregulation on normal monocytes following long-term co-cultures (10 days) with CLL cells. Data are representative of four different samples of healthy monocytes matched with CLL cells from seven different patients or normal B cells from two healthy donors. c-MET and IDO were analyzed by flow cytometry and values are expressed as mean fluorescence intensity (m.f.i.) ± SD.
Chronic lymphocytic leukemia cells induce indoleamine 2,3-dioxygenase and c-MET expression by normal monocytes
CLL cells from seven patients were co-cultured with purified monocytes from healthy donors for 10 days. IDO and c-MET were consistently upregulated in monocytes co-cultured with CLL cells, compared to their levels in monocytes freshly-prepared, cultured with medium only or co-cultured with normal B cells (Figure 3E). These experiments demonstrate the capacity of CLL cells to induce IDO and c-MET expression by normal monocytes.
Co-cultures of chronic lymphocytic leukemia B and nurse-like cells stimulate hepatocyte growth factor production in vitro
We analyzed HGF levels in media of purified CLL-monocytes, or differentiated NLC or CLL cells cultured for different periods in vitro. HGF, absent from the media of CLL cells or CLL-monocytes cultured alone for 48 h, was instead found at significant levels in the 14-day supernatants of CLL/NLC co-cultures. However, no HGF was detected in the media of NLC re-cultured for an additional 48 h (Figure 4A). Whether CLL cells and/or NLC are induced to produce HGF in co-cultures was analyzed by flow cytometry on cells cultured for different periods. As shown in Figure 4B, HGF, absent from fresh cells and expressed at a low level until day 8, was significantly upregulated between days 11 and 14 by both NLC and CLL cells (P<0.001 for both types at day 11; P=0.04 for monocytes/NLC and P=0.004 for CLL cells at day 14, n=4, Mann-Whitney test). CLL cells, previously co-cultured with NLC, were re-cultured with medium alone or with NLC in the presence or absence of a neutralizing anti-HGF monoclonal antibody. The anti-HGF monoclonal antibody caused a substantial inhibition of viability when B cells were re-cultured alone (Figure 4C; P=0.027). This inhibition was not observed when B cells were re-cultured with NLC. The association of an anti-CXCR4 with the anti-HGF monoclonal antibody could, however, induce a greater reduction of survival (Figure 4C), in agreement with putative amounts of CXCL12 expressed by NLC. These data demonstrate that, upon mutual stimulation, both CLL cells and NLC are capable of producing HGF which, in turn, inhibits CLL apoptosis.
Figure 4.
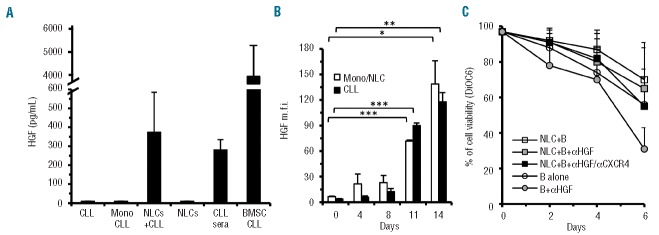
Determination of HGF production through different experimental approaches. (A) Evaluation of HGF released in 48 h cultures by different cell types alone or in the indicated combinations: CLL cells (CLL) (mean ±SD of four CLL patients: n=4), CLL-monocytes (Mono CLL, n=7), NLC (n=7) in medium alone, or co-cultured, NLC+CLL (n=7) for 14 days. Sera (n=2) and spent medium of bone marrow stromal cells from CLL patients (n=2) were also tested. (B) Flow cytometry determination of HGF expression during a 14-day co-culture of monocytes and CLL cells. HGF, absent in freshly prepared monocytes and in CLL cells, remained low in both cell types until day 8, but was significantly upregulated between days 11 and 14. Data represent means of four cases studied at day 0 and day 14, two of which were also studied at intermediate time points. (C) Viability of CLL cells cultured alone or in the presence of NLC with/without anti-HGF monoclonal antibodies alone or in association with anti-CXCR4 monoclonal antibodies. The letter B in the graph indicates CLL cells. Data are mean values of four different cases ±SD. Percentage of apoptosis was determined by DiOC6 staining. Significant differences were detected between CLL B cells cultured alone versus B cells plus anti-HGF (P=0.027, Mann-Whitney).
Inhibitory effect of chronic lymphocytic leukemia monocytes and nurse-like cells on T-cell proliferation
To explore whether monocytes from CLL patients or NLC can exert immuno-regulatory functions, allogeneic pre-activated T cells were cultured with graded concentrations of monocytes from CLL or normal donors. CLL-monocytes, but not monocytes from healthy subjects, caused a consistent (almost significant, P=0.057) decrease of T-cell proliferation, which was significantly reduced to less than 50% when NLC were added in the place of normal monocytes (P=0.016 Figure 5A). The cultures were also carried out in the presence of anti-TGFβ or -IL-10 neutralizing antibodies or IDO inhibitors. Anti-TGFβ antibodies almost completely restored T-cell proliferation inhibited by NLC, indicating a prominent role of this factor. All three agents, taken separately, had a partial effect in restoring the proliferation inhibited by the presence of CLL-monocytes, but exhibited a synergistic effect when added together to the cultures, suggesting that IDO, IL-10 and TGFβ may all contribute to suppress T-cell proliferation (Figure 5B). Histograms of T-cell proliferation inhibition by CLL monocytes/NLC from representative cases, as determined by CFSE staining, are shown in Online Supplementary Figure S5. Reverse transcriptase PCR analysis showed increasing levels of TGFβ and IL-10 mRNA in NLC and CLL-monocytes compared to normal monocytes, in accordance with the above functional data (Figure 5C). A minimal although significant increase of TGFβ mRNA expression was detected in HGF-treated THP-1 cells (P<0.05). It is of further note that in PMA-treated THP-1 cells there was an increase in TGFβ mRNA expression, which was even more remarkable after exposure to HGF (0.01>P≥0.001). M2 phenotype-oriented THP-1 cells also demonstrated this increase in TGFβ mRNA (Figure 5D).
Figure 5.
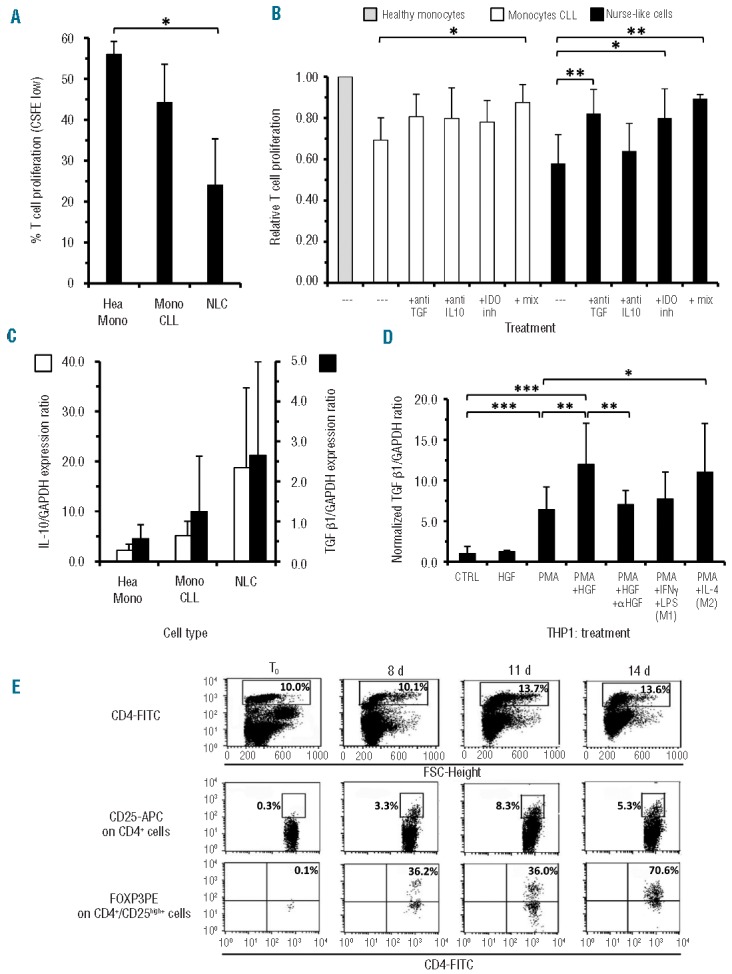
Suppression of T-cell proliferation by monocytes from CLL and NLC, determination of the factors involved and of T regulatory cell expansion in autologous monocyte/peripheral blood mononuclear cell co-cultures. (A) Proliferation of activated T cells, as determined by CFSE staining, was inhibited by addition of either monocytes or NLC (P=0.016, Mann-Whitney) derived from CLL patients but not monocytes from healthy donors. Results are the mean of two independent experiments each with four cases/cell type (n=4). (B) T-cell proliferation in the presence of CLL monocytes (white bars) or NLC (black bars) was restored by the addition of anti-TGFβ, or anti-IL-10 neutralizing antibodies, or IDO inhibitors (inh), as detected by CFSE staining. The combination of these three blocking agents (mix) induced a stronger effect for CLL-monocytes (**) while for NLC even the use of the anti-TGFβ alone (**) was sufficient to restore T-cell proliferation to almost normal values (n=6 for CLL-monocytes; n= 5 for NLC). Data were normalized to proliferation values obtained with T cells and monocytes from healthy subjects (gray bar), P values were calculated by a two sided Mann-Whitney test. (C) According to functional data shown in panel B, higher trends of IL-10 (left y axis, white bars) and TGFβ (right y axis, black bars) mRNA levels were observed in NLC and CLL-monocytes with respect to healthy monocytes as detected by real-time reverse transcriptase-PCR. (D) HGF increased, weakly but significantly, TGFβ mRNA expression in THP-1 cells at basal conditions and in a more marked manner in PMA pre-treated cells: this latter effect was significantly inhibited by the neutralizing anti-HGF humanized monoclonal antibody L2G7. Increased expression of TGFβ mRNA was further induced in THP-1 only on cells polarized towards the M2 phenotype but not towards M1. Data are relative fold increase compared to untreated THP-1 cells of the TGFβ/GAPDH mRNA expression ratio, P values for the Mann-Whitney test. (E) Co-cultures of enriched CLL monocytes with autologous peripheral blood mononuclear cells favor T regulatory cell expansion as determined by three-color staining (CD4/CD25/FOXP3). The lymphocyte subpopulation was gated on forward side scatter (FSC) versus CD4+ cells. Only the CD4+/CD25high+population was further assessed for FOXP3 expression. CD4+/CD25high+/FOXP3+ T regulatory cells progressively expanded in autologous co-culture by day 8 to 14. The maximal FOXP3 expression was reached at day 14 (70.6%).
CD4+CD25high+FOXP3+ T regulatory cells are expanded in long-term co-cultures of enriched monocytes with autologous peripheral blood monocytes from patients with chronic lymphocytic leukemia
Next we examined whether co-culturing purified and enriched CLL monocytes with total peripheral blood monocytes from the same patient could favor the expansion of T regulatory cells (identified as CD4+ cells, with high CD25 expression, co-expressing the transcription factor FOXP3). As shown in one representative case depicted in Figure 5E, a number of CD4+ T cells appeared to be activated by day 8 of culture because of CD25 upregulation. Moreover, a consistent percentage of these CD4+/CD25high+ T cells exhibited a progressive increase of FOXP3 expression (average =72% at day14; n=2). In our in vitro culture system, T-cell activation is due to endogenously, and not exogenously, produced factors: this system putatively models what may occur physiologically when monocytes/macrophages infiltrate lymph nodes and drive the T-cell repertoire towards an immunosuppressive T regulatory phenotype.
Discussion
Increasing attention has recently been focused on the interactions between CLL cells and their microenvironment. Soluble factors as well as contact between malignant cells and bystander NLC, T cells, stromal cells or follicular dendritic cells prolong the survival of CLL cells and facilitate clonal expansion. HGF, produced by mesenchymal and follicular dendritic cells in bone marrow or lymph nodes,18,26 increases CLL cell viability.14 The levels of HGF are higher than normal in sera from CLL patients,20 a finding suggesting a possible role for this factor in CLL clonal expansion. Here we investigated whether HGF could interfere in the control of this mechanism by regulating the function of NCL and monocytes.
We demonstrated that c-MET is highly expressed by NLC and that the interaction between HGF and c-MET leads to rapid STAT3 phosphorylation. We further observed that the expression of c-MET is significantly higher than normal on circulating CLL-monocytes. Since the expression of c-MET is constitutively low in monocytes from healthy donors but is upregulated upon activation by endotoxin or IL-1β,27 high c-MET expression by CLL-monocytes suggests a continuous in vivo activation.
We also observed that IDO, which is relevant in immunosuppressive mechanisms, is constitutively expressed on NLC and on CLL-monocytes. Monocytes from normal individuals, on the other hand, are IDO-negative but become IDO-positive following exposure to HGF.24 IDO is an intracellular enzyme that catalyzes the conversion of the essential amino-acid tryptophan into kynurinine.28 The role of IDO, initially thought to be antimicrobial, has been recently extended to the regulation of prevention of T-cell-driven rejection of the fetus during pregnancy and to tumor immune escape phenomena.29–31 In diffuse large B-cell lymphomas, IDO expression correlates with prognosis.32 While in some cases of diffuse large B-cell lymphoma, the lymphoma cells are themselves IDO-producers, in others cases only the cells of the microenvironment are IDO-positive.32 Furthermore, kynurinine-tryptophan ratios, reflecting increased IDO activity, have been found to be higher than normal in sera from CLL patients.33 Since the genes encoding IDO are downregulated in CLL cells, higher kynurinine-tryptophan ratios appear putatively related to the IDO activity of microenvironmental cells. This is in agreement with IDO expression by CD68+, vimentin+, CD163+ cells in lymph nodes (see Figure 2); a finding which may suggest ongoing immunosuppression of tumor-specific T cells in vivo.34 Moreover, the simultaneous expression of IL-10 and IDO by NLC may also indicate the acquisition of an immunosuppressive phenotype, similar to that of type 2 macrophages.35 Since in the context of immune responses, the cytokine milieu compels mononuclear phagocytes to express specialized and polarized functional properties, we also hypothesized that HGF, possibly present at high amounts in the CLL-microenvironment, could contribute to drive polarization of CLL-monocytes and NLC. This suggestion appears strengthened by the observation that STAT3 is rapidly activated by HGF on NLC. STAT3 activation promotes tumor evasion by inhibiting the production of Th1-type immunostimulating molecules and, at the same time, enhances expression of immunosuppressive factors with subsequent inhibition of dendritic cell maturation. While M1 macrophages have immunostimulatory, Th1-orienting properties, M2 cells exhibit high IL-10 and low IL-12 production, poor antigen-presenting capacities and suppress Th1 adaptive immunity.23 In relation to this, NLC exhibit a gene expression profile consistent with impaired immunocompetence, with reduced levels of lysozyme activity, CD74 and DR-antigens and increased FCGR2B expression.12 The findings that CLL-monocytes, and to a greater extent NLC, significantly inhibit T-cell proliferation, as demonstrated here, provide important support to the notion of ongoing immunosuppression. Soluble factors produced by NLC should also favor the expansion of T regulatory cells within the CLL microenvironment. Indeed higher than normal numbers of T regulatory cells have been found in CLL patients.36–39 We show here that T regulatory cells (CD4+/CD25high+/FOXP3+) are significantly expanded by the presence of CLL-monocytes or NLC: this observation strengthens a link between impairment of immune response and CLL progression.
We further demonstrated that TGFβ was the major cytokine involved in suppression of T-cell proliferation (Figure 5B) and, in agreement with functional studies, NLC showed higher levels of TGFβ mRNA expression than did normal monocytes. However, since simultaneous addition of antibodies against TGFβ and IL-10, and IDO inhibitors resulted in greater and more significant effects when CLL-monocytes were co-cultured with T cells, it is likely that multiple factors cooperate in immunosuppression. Recently CLL-monocytes have been shown to have diminished HLA-DR or CD86 expression and reduced immune stimulatory activity.40 In addition, an increased proportion of CD14+HLA-DRlow/neg monocytes correlate with a shorter time to progression in CLL: interestingly monocytes characterized by low/negative HLA-DR expression were observed in prostate and ovarian cancers and induced immunosuppression through IL-10 and TGFβ secretion.40–42 IL-10 affects many important functions of monocytes-macrophages and dendritic cells by inhibiting the secretion of various cytokines (IL-2, IL-12, tumor necrosis factor-α, interferon-γ), downregulating MHC class I expression and recruiting T regulatory cells.43 On the other hand TGFβ suppresses anti-tumor activity of T/NK cells and monocytes-macrophages44 and has a key role in mesenchymal stem cell-induced immunosuppression.45 HGF, released by mesenchymal bone marrow cells, also displays immunosuppressive activities, and may inhibit T-cell proliferation in synergy with TGFβ.46 We further demonstrated here that TGFβ expression is significantly increased following HGF treatment and that M2-like cells appear characterized by higher levels of c-MET than M1-like cells in the PMA-treated THP-1 model. The expression of HGF by leukemic B cells themselves intriguingly suggests that, in the CLL microenvironment, HGF may be derived in both autocrine and paracrine manners. In naïve splenic B cells from mice, HGF is produced after an interaction between CD74 and macrophage migration inhibitory factor and regulates the survival of these cells.47 Interestingly, high levels of macrophage migration inhibitory factor have been detected in sera from CLL patients and lack or inhibition of this factor delays CLL development in the Eμ-TCL1 mouse model of the disease.48
In keeping with our data, a phenotype resembling tumor-associated M2 macrophages has been recently proposed for NLC49 as well as deregulation of genes involved in phagocytosis and inflammation for CLL-monocytes.50 Intriguingly, acquisition of an M2 phenotype appears to be induced under the influence of malignant CLL cells as we demonstrated in normal monocytes, through IDO and c-MET upregulation.
Collectively our findings support a model for ongoing immunosuppression in CLL. Cross-talk between monocytes/NLC and CLL cells results in autocrine HGF production: HGF in turn induces activation of the former cells, causes elevated secretion of TGFβ, IL-10 and IDO, and depresses anti-tumor immune responses, through M2 differentiation and T regulatory cell expansion (Online Supplementary Figure S6). Collectively our observations provide new bases for investigating novel therapies to revert polarization of monocytes-macrophages towards a classical immune-responsive M1 phenotype. These therapies would facilitate the potential induction of a tumor-specific response while preventing the onset of immunosuppressive mechanisms.
Footnotes
The online version of this article has a Supplementary Appendix.
Funding
Supported by the Italian Ministry of Health project 2007 (DdT), Italian Association for Cancer Research (AIRC) IG10492(MF), 5 X mille 9980(MF), MFAG6384(GP) and MIUR-PRIN2009T4TC33_004(MCM)
Authorship and Disclosures
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Kay NE, Shanafelt TD, Strege AK, Lee YK, Bone ND, Raza A. Bone biopsy derived marrow stromal elements rescue chronic lymphocytic leukemia B-cells from spontaneous and drug induced cell death and facilitates an “angiogenic switch”. Leuk Res 2007; 31(7):899–906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kurtova AV, Balakrishnan K, Chen R, Ding W, Schnabl S, Quiroga MP, et al. Diverse marrow stromal cells protect CLL cells from spontaneous and drug-induced apoptosis: development of a reliable and reproducible system to assess stromal cell adhesion-mediated drug resistance. Blood 2009;114(20): 4441–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lagneaux L, Delforge A, Bron D, De Bruyn C, Stryckmans P. Chronic lymphocytic leukemic B cells but not normal B cells are rescued from apoptosis by contact with normal bone marrow stromal cells. Blood 1998;91(7):2387–96 [PubMed] [Google Scholar]
- 4.Pedersen IM, Kitada S, Leoni LM, Zapata JM, Karras JG, Tsukada N, et al. Protection of CLL B cells by a follicular dendritic cell line is dependent on induction of Mcl-1. Blood 2002;100(5):1795–801 [PubMed] [Google Scholar]
- 5.Tsukada N, Burger JA, Zvaifler NJ, Kipps TJ. Distinctive features of “nurselike” cells that differentiate in the context of chronic lymphocytic leukemia. Blood 2002;99(3):1030–7 [DOI] [PubMed] [Google Scholar]
- 6.Burger JA, Tsukada N, Burger M, Zvaifler NJ, Dell’Aquila M, Kipps TJ. Blood-derived nurse-like cells protect chronic lymphocytic leukemia B cells from spontaneous apoptosis through stromal cell-derived factor-1. Blood 2000;96(8):2655–63 [PubMed] [Google Scholar]
- 7.Burkle A, Niedermeier M, Schmitt-Graff A, Wierda WG, Keating MJ, Burger JA. Overexpression of the CXCR5 chemokine receptor, and its ligand, CXCL13 in B-cell chronic lymphocytic leukemia. Blood 2007;110(9):3316–25 [DOI] [PubMed] [Google Scholar]
- 8.Nishio M, Endo T, Tsukada N, Ohata J, Kitada S, Reed JC, et al. Nurselike cells express BAFF and APRIL, which can promote survival of chronic lymphocytic leukemia cells via a paracrine pathway distinct from that of SDF-1alpha. Blood 2005; 106(3):1012–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Poggi A, Prevosto C, Catellani S, Rocco I, Garuti A, Zocchi MR. Engagement of CD31 delivers an activating signal that contributes to the survival of chronic lymphocytic leukaemia cells. Br J Haematol 2010;151(3): 252–64 [DOI] [PubMed] [Google Scholar]
- 10.Deaglio S, Vaisitti T, Bergui L, Bonello L, Horenstein AL, Tamagnone L, et al. CD38 and CD100 lead a network of surface receptors relaying positive signals for B-CLL growth and survival. Blood 2005;105(8): 3042–50 [DOI] [PubMed] [Google Scholar]
- 11.Shimaoka Y, Attrep JF, Hirano T, Ishihara K, Suzuki R, Toyosaki T, et al. Nurse-like cells from bone marrow and synovium of patients with rheumatoid arthritis promote survival and enhance function of human B cells. J Clin Invest 1998;102(3):606–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bhattacharya N, Diener S, Idler IS, Rauen J, Habe S, Busch H, et al. Nurse-like cells show deregulated expression of genes involved in immunocompetence. Br J Haematol 2011; 154(3):349–56 [DOI] [PubMed] [Google Scholar]
- 13.Ysebaert L, Fournie JJ. Genomic and phenotypic characterization of nurse-like cells that promote drug resistance in chronic lymphocytic leukemia. Leuk Lymphoma 2011;52(7):1404–6 [DOI] [PubMed] [Google Scholar]
- 14.Giannoni P, Scaglione S, Quarto R, Narcisi R, Parodi M, Balleari E, et al. An interaction between hepatocyte growth factor and its receptor (c-MET) prolongs the survival of chronic lymphocytic leukemic cells through STAT3 phosphorylation: a potential role of mesenchymal cells in the disease. Haematologica 2011;96(7):1015–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boros P, Miller CM. Hepatocyte growth factor: a multifunctional cytokine. Lancet 1995;345(8945):293–5 [DOI] [PubMed] [Google Scholar]
- 16.Naldini L, Vigna E, Narsimhan RP, Gaudino G, Zarnegar R, Michalopoulos GK, et al. Hepatocyte growth factor (HGF) stimulates the tyrosine kinase activity of the receptor encoded by the proto-oncogene c-MET. Oncogene 1991;6(4):501–4 [PubMed] [Google Scholar]
- 17.Skibinski G, Skibinska A, James K. The role of hepatocyte growth factor and its receptor c-met in interactions between lymphocytes and stromal cells in secondary human lymphoid organs. Immunology 2001;102(4): 506–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van der Voort R, Taher TE, Keehnen RM, Smit L, Groenink M, Pals ST. Paracrine regulation of germinal center B cell adhesion through the c-met-hepatocyte growth factor/scatter factor pathway. J Exp Med 1997;185(12):2121–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Burger JA, Ghia P, Rosenwald A, Caligaris-Cappio F. The microenvironment in mature B-cell malignancies: a target for new treatment strategies. Blood 2009;114(16):3367–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aguayo A, Kantarjian H, Manshouri T, Gidel C, Estey E, Thomas D, et al. Angiogenesis in acute and chronic leukemias and myelodysplastic syndromes. Blood 2000;96(6):2240–5 [PubMed] [Google Scholar]
- 21.De Totero D, Meazza R, Zupo S, Cutrona G, Matis S, Colombo M, et al. Interleukin-21 receptor (IL-21R) is up-regulated by CD40 triggering and mediates proapoptotic signals in chronic lymphocytic leukemia B cells. Blood 2006;107(9):3708–15 [DOI] [PubMed] [Google Scholar]
- 22.Gentile A, Trusolino L, Comoglio PM. The Met tyrosine kinase receptor in development and cancer. Cancer Metastasis Rev 2008;27(1):85–94 [DOI] [PubMed] [Google Scholar]
- 23.Sica A, Allavena P, Mantovani A. Cancer related inflammation: the macrophage connection. Cancer Lett 2008;267(2):204–15 [DOI] [PubMed] [Google Scholar]
- 24.Rutella S, Bonanno G, Procoli A, Mariotti A, de Ritis DG, Curti A, et al. Hepatocyte growth factor favors monocyte differentiation into regulatory interleukin (IL)-10++IL-12low/neg accessory cells with dendritic-cell features. Blood 2006;108(1):218–27 [DOI] [PubMed] [Google Scholar]
- 25.Auwerx J. The human leukemia cell line, THP-1: a multifacetted model for the study of monocyte-macrophage differentiation. Experientia 1991;47(1):22–31 [DOI] [PubMed] [Google Scholar]
- 26.van der Voort R, Taher TE, Derksen PW, Spaargaren M, van der Neut R, Pals ST. The hepatocyte growth factor/Met pathway in development, tumorigenesis, and B-cell differentiation. Adv Cancer Res 2000;79:39–90 [DOI] [PubMed] [Google Scholar]
- 27.Galimi F, Cottone E, Vigna E, Arena N, Boccaccio C, Giordano S, et al. Hepatocyte growth factor is a regulator of monocyte-macrophage function. J Immunol 2001;166(2):1241–7 [DOI] [PubMed] [Google Scholar]
- 28.Mellor AL, Munn DH. Tryptophan catabolism and T-cell tolerance: immunosuppression by starvation? Immunol Today. 1999;20(10):469–73 [DOI] [PubMed] [Google Scholar]
- 29.Munn DH, Shafizadeh E, Attwood JT, Bondarev I, Pashine A, Mellor AL. Inhibition of T cell proliferation by macrophage tryptophan catabolism. J Exp Med 1999;189(9): 1363–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Munn DH, Zhou M, Attwood JT, Bondarev I, Conway SJ, Marshall B, et al. Prevention of allogeneic fetal rejection by tryptophan catabolism. Science 1998;281(5380):1191–3 [DOI] [PubMed] [Google Scholar]
- 31.Pfefferkorn ER. Interferon gamma blocks the growth of Toxoplasma gondii in human fibroblasts by inducing the host cells to degrade tryptophan. Proc Natl Acad Sci USA 1984;81(3):908–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ninomiya S, Hara T, Tsurumi H, Hoshi M, Kanemura N, Goto N, et al. Indoleamine 2,3-dioxygenase in tumor tissue indicates prognosis in patients with diffuse large B-cell lymphoma treated with R-CHOP. Ann Hematol 2011;90(4):409–16 [DOI] [PubMed] [Google Scholar]
- 33.Lindstrom V, Aittoniemi J, Jylhava J, Eklund C, Hurme M, Paavonen T, et al. Indoleamine 2,3-dioxygenase activity and expression in patients with chronic lymphocytic leukemia. Clin Lymphoma Myeloma Leuk 2012;12(5):363–5 [DOI] [PubMed] [Google Scholar]
- 34.Munn DH, Mellor AL. Indoleamine 2,3-dioxygenase and tumor-induced tolerance. J Clin Invest 2007;117(5):1147–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mantovani A, Sozzani S, Locati M, Allavena P, Sica A. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol 2002;23(11):549–55 [DOI] [PubMed] [Google Scholar]
- 36.D’Arena G, Laurenti L, Minervini MM, Deaglio S, Bonello L, De Martino L, et al. Regulatory T-cell number is increased in chronic lymphocytic leukemia patients and correlates with progressive disease. Leuk Res 2011;35(3):363–8 [DOI] [PubMed] [Google Scholar]
- 37.Deutsch V, Perry C, Polliack A. Expansion of regulatory T cells in B chronic lymphocytic leukemia: enhanced ‘brakes’ on host immunity. Leuk Lymphoma 2009;50(5):687–8 [DOI] [PubMed] [Google Scholar]
- 38.Giannopoulos K, Schmitt M, Wlasiuk P, Chen J, Bojarska-Junak A, Kowal M, et al. The high frequency of T regulatory cells in patients with B-cell chronic lymphocytic leukemia is diminished through treatment with thalidomide. Leukemia 2008;22(1): 222–4 [DOI] [PubMed] [Google Scholar]
- 39.Jak M, Mous R, Remmerswaal EB, Spijker R, Jaspers A, Yague A, et al. Enhanced formation and survival of CD4+ CD25hi Foxp3+ T-cells in chronic lymphocytic leukemia. Leuk Lymphoma 2009;50(5):788–801 [DOI] [PubMed] [Google Scholar]
- 40.Gustafson MP, Abraham RS, Lin Y, Wu W, Gastineau DA, Zent CS, et al. Association of an increased frequency of CD14+ HLA-DR lo/neg monocytes with decreased time to progression in chronic lymphocytic leukaemia (CLL). Br J Haematol 2011;156(5):674–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Loercher AE, Nash MA, Kavanagh JJ, Platsoucas CD, Freedman RS. Identification of an IL-10-producing HLA-DR-negative monocyte subset in the malignant ascites of patients with ovarian carcinoma that inhibits cytokine protein expression and proliferation of autologous T cells. J Immunol 1999;163(11):6251–60 [PubMed] [Google Scholar]
- 42.Vuk-Pavlovic S, Bulur PA, Lin Y, Qin R, Szumlanski CL, Zhao X, et al. Immunosuppressive CD14+HLA-DRlow/-monocytes in prostate cancer. Prostate 2010;70(4):443–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Banchereau J, Pascual V, O’Garra A. From IL-2 to IL-37: the expanding spectrum of anti-inflammatory cytokines. Nat Immunol 2013;13(10):925–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li MO, Wan YY, Sanjabi S, Robertson AK, Flavell RA. Transforming growth factor-beta regulation of immune responses. Annu Rev Immunol 2006;24:99–146 [DOI] [PubMed] [Google Scholar]
- 45.Han Z, Jing Y, Zhang S, Liu Y, Shi Y, Wei L. The role of immunosuppression of mesenchymal stem cells in tissue repair and tumor growth. Cell Biosci 2012;2(1):8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Di Nicola M, Carlo-Stella C, Magni M, Milanesi M, Longoni PD, Matteucci P, et al. Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood 2002;99(10):3838–43 [DOI] [PubMed] [Google Scholar]
- 47.Gordin M, Tesio M, Cohen S, Gore Y, Lantner F, Leng L, et al. c-Met and its ligand hepatocyte growth factor/scatter factor regulate mature B cell survival in a pathway induced by CD74. J Immunol 2010;185(4):2020–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Reinart N, Nguyen PH, Boucas J, Rosen N, Kvasnicka HM, Heukamp L, et al. Delayed development of chronic lymphocytic leukemia in the absence of macrophage migration inhibitory factor. Blood 2013;121(5):812–21 [DOI] [PubMed] [Google Scholar]
- 49.Filip AA, Cisel B, Koczkodaj D, Wasik-Szczepanek E, Piersiak T, Dmoszynska A. Circulating microenvironment of CLL: are nurse-like cells related to tumor-associated macrophages? Blood Cells Mol Dis. 2013;50(4):263–70 [DOI] [PubMed] [Google Scholar]
- 50.Maffei R, Bulgarelli J, Fiorcari S, Bertoncelli L, Martinelli S, Guarnotta C, et al. The monocytic population in chronic lymphocytic leukemia shows altered composition and deregulation of genes involved in phagocytosis and inflammation. Haematologica 2013;98(7):1115–23 [DOI] [PMC free article] [PubMed] [Google Scholar]