Abstract
The immunoregulatory protein T cell immunoglobulin- and mucin-domain-containing molecule-3 (Tim-3) mediates T cell exhaustion and contributes to the suppression of immune responses in both viral infections and tumors. Tim-3 blockade reverses the exhausted phenotype of CD4+ and CD8+ T cells in several chronic diseases including melanoma. Interestingly, natural killer (NK) cells constitutively express Tim-3; however, its role in modulating the function of these innate effector cells remains unclear, particularly in human disease. In this study, we compared the function of Tim-3 in NK cells from healthy donors and patients with metastatic melanoma. NK cells from the latter were functionally impaired/exhausted and Tim-3 blockade reversed this exhausted phenotype. Moreover, Tim-3 expression levels correlated with the stage of the disease and poor prognostic factors. These data indicate that Tim-3 can function as an NK cell exhaustion marker in advanced melanoma and supports the development of Tim-3-targeted therapies to restore antitumor immunity.
Keywords: Melanoma, NK cells, Tim-3, Exhaustion, Immune checkpoint
INTRODUCTION
Malignant melanoma (MM) is the fifth most common cancer in men with incidence increasing more rapidly than that of any other malignancy (1). It is the most aggressive type of skin cancer, accounting for more than 70% of skin cancer-related deaths (2). The median survival of advanced melanoma patients (stage IV) was until recently less than 1 year (3), but new therapeutic options are improving response rates and overall survival (OS) (4, 5).
One such intervention is Ipilimumab (5), a monoclonal antibody against cytotoxic T-lymphocyte antigen-4 (CTLA-4), a negative regulator that is up-regulated on exhausted T cells in cancers and chronic infections (6). The exhaustion phenotype has been described for T cells as a state of cellular dysfunction that arises as a consequence of continuous and chronic stimulation by viral or tumor antigens, as well as by immunosuppressive cytokines. It is characterized by an early loss of proliferative capacity, cytotoxic potential, and the ability to produce cytokines, as well as sustained expression of inhibitory receptors (7). Besides CTLA-4 (8, 9), Program Death-1 (PD-1) (10) and T cell immunoglobulin- and mucin-domain-containing molecule-3 (Tim-3) (11) are additional inhibitory receptors that can be up-regulated in the setting of chronic infections or progressive cancers.
Tim-3 is an inhibitory receptor that plays a crucial role in mediating T cell exhaustion in both viral infections and tumors (12-14). Indeed, Tim-3 blockade reverses the exhausted phenotype of CD4+ and CD8+ T cells in several chronic diseases including melanoma (11, 15, 16). Unlike resting T cells, where it is minimally expressed and up-regulated only after chronic stimulation, Tim-3 is constitutively expressed at considerably higher levels on resting natural killer (NK) cells (17). Recent publications, however, have reported conflicting data regarding Tim-3 function on NK cells (17, 18). Ndhlovu and colleagues showed that Tim-3 inhibits NK cell-mediated cytotoxicity (17) while another study suggested that Tim-3 may enhance IFNγ production instead (18). Both studies only evaluated healthy donors and not patients with chronic diseases such as cancer, where Tim-3 expression on NK cells may have a more significant immunomodulatory role.
NK cells, through IFNγ production and direct cytotoxicity, are able to eliminate tumor cells. Accordingly, NK cell infiltration in tumor tissue is associated with better prognosis (19-21), while low activity of peripheral blood NK cells is associated with increased risk of cancer (22). These findings have prompted interest in the development of cancer therapies based on NK cells, such as NK cell adoptive transfer or targeting NK cell inhibitory receptors (23). In this regard, a better understanding of Tim-3 function in the context of advanced tumors could potentially impact treatment.
In this study, we characterized Tim-3 expression and function in NK cells from advanced melanoma patients. We found that NK cells from these subjects expressed high levels of Tim-3 and were functionally impaired/exhausted. Importantly, we showed that Tim-3 blockade reversed this exhausted phenotype. Furthermore, we found that Tim-3 expression in NK cells increases as the stage of melanoma advances, and is higher in melanoma patients with poor prognostic factors. These data open exciting avenues for new therapies targeting Tim-3 in tumor immunotherapy.
MATERIALS AND METHODS
Reagents
For staining, Tim-3 antibody used for staining experiments was purchased from R&D. Anti-CD279 (PD-1) was purchased from BD Bioscience and anti-CTLA-4 was purchased from LifeSpan Biosciences. To check the purity of selected NK cells they were stained for CD56, CD16, CD3, CD14 and CD19 purchased from Biolegend. Anti-CD25 was purchased from Miltenyi, anti-CD122, anti-CD132 and anti-IL-5R were from Biolegend. Anti-CD107-FITC and anti-IFNγ-FITC antibodies were purchased from Biolegend. Anti-NKG2D, anti-NKp46, anti-DNAM-1, anti-KIR3DL1, anti-KIR2DL3 antibodies (BD Bioscience) were used as indicated by the manufacturer. Anti-Eomes and anti-T-bet were purchased from eBioscience. Anti-galectin-9 was purchased from BioLegend. CellTrace™ CFSE Cell Proliferation Kit was purchased from Life Technologies. FITC Annexin V Apoptosis Detection Kit with 7-AAD was purchased from BioLegend.
For blocking experiments, 10 or 20μg/ml of Tim-3 blocking antibody (Biolegend, clone 2E2; R&D #AF2365), galectin-9 blocking antibody (BioLegend), IL-2R blocking antibody (α, β and γ chains – R&D Biosystems) or CD16 blocking antibody (BioLegend) was added to the culture 1 hour before starting the functional assays.
For crosslinking experiments, the same anti-Tim-3 antibody (Biolegend) was used. Anti-CD16 and anti-CD94 from Biolegend were also used to coat beads and in the reverse-ADCC assay and in our crosslinking experiments. For both experiments, crosslinking and blocking, we used IgG1 isotype control from BD. 7AAD was purchased from Biolegend.
For stimulation, the cytokines rhIL-2, rhIL-12, rhIL-15 and rhIL18 were purchased from R&D System. Recombinant Human Galectin-9, purchased from R&D Systems, was used to stimulate NK cells 1h before the cytotoxicity assay.
Cell lines
K562 cells, Gmel and FM29 cells were cultured in complete media (RPMI-1640; Life Technologies) supplemented with 10% FBS, 2mM L-glutamine, 100U/ml penicillin, and 100μg/ml streptomycin. K562 is a chronic myelogenous leukemia cell line, which was used as target cells in experiments from figures 2B, 4A, 5A, 5B, 6D, 6F, S3A, S3D, S4B, S5A, S5C, S6A, S6B, S7A, S7D, S7E, S7F, S7G and S8B. Gmel is a melanoma cell line that expresses galectin-9 intracellularly and 5% of these cells express galectin-9 in the membrane. Gmel cells were used as target cells in the experiments shown in figure S6C and S6D. FM29 cells were used as target cells in the experiment shown in figure S7B. Melanoma cell lines WM1552, WM3248, WM793b were purchased from the Wistar Institute. They were used as target cells in the experiment shown in figure S7B. P815 cell line was purchased from ATCC. This cell line is FcR+ and was used for the reverse ADCC-assay (Figures 4C, S5B S5D). All cell lines were mycoplasma free; no other authentication assays were performed.
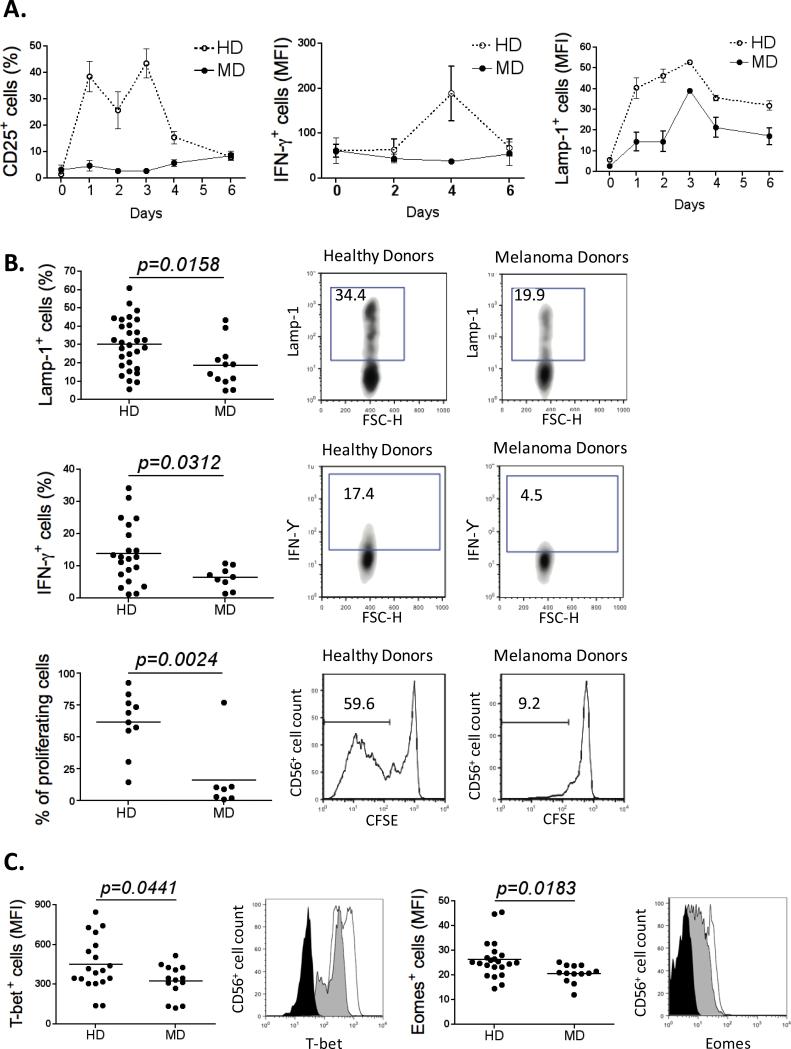
Figure 2. MD NK cells are functionally impaired/exhausted.
(A) Freshly purified NK cells (HD n=12; MD n=5) were stimulated with 200U/ml of IL-2. Expression of IL-2R (α chain), IFNγ production, and cytotoxicty were monitored every two days over 6 days (day 0, 2, 4 and 6) by flow cytometry. (B) The percentage of Lamp-1+ NK cells from healthy (n=30) and melanoma donors (n=12) after a cytotoxic assay is shown using K562 cells as target cells (upper left panel). The percentage of IFNγ+ NK cells from healthy (n=22) and melanoma donors (n=9) is shown after 4h stimulation with IL-12 (middle left panel). The percentage of proliferating NK cells from healthy (n=10) and melanoma donors (n=7) is shown after 6 days of culture in presence of 200U/ml of IL-2 (lower left panel). On the right panel of each graph, plots depicting the expression of Lamp-1, IFNγ, and CFSE in NK cells purified from a representative healthy donor and a representative melanoma patient are shown. (C) Graphs representing the MFI of T-bet and Eomes on NK cells purified from healthy (n=19) and melanoma donors (n=14). Representative plots are shown (Isotype control: black; HD: unfilled; MD: gray). All experiments were performed in duplicate.
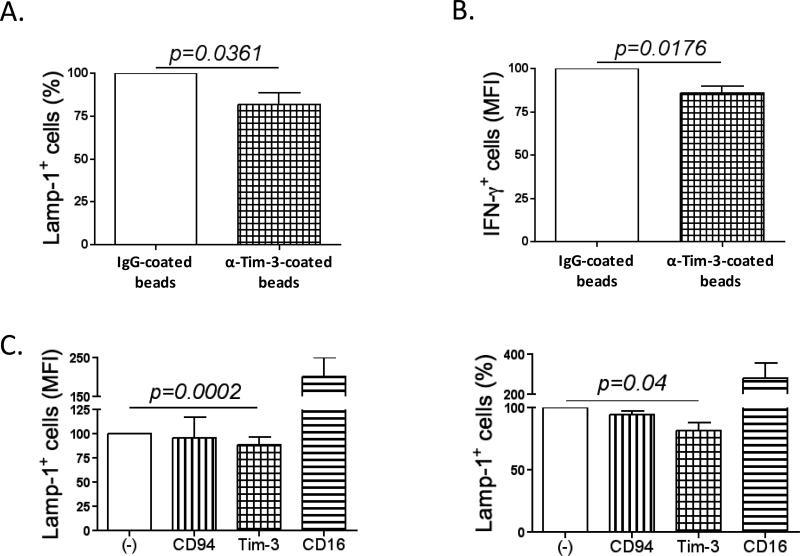
Figure 4. Tim-3 engagement inhibits NK cell functions.
(A) The percentage of LAMP-1+ (n=8) and (B) the MFI of IFNγ+ cells (n=6) of NK cells from melanoma donors pre-incubated with IgG-coated beads or anti-Tim-3-coated beads for 2h prior to evaluating the cytotoxic function or IFNγ production. (C) Reverse-ADCC assay using FcR+ P815 cells. NK cells from melanoma or healthy donors were co-cultured with P815 cells and different antibodies were added to the reaction: anti-Tim-3, anti-CD94 (negative control) or anti-CD16 (positive control). Data were normalized to the values obtained for the condition: (A and B) with IgG-coated beads (100%); (C) with no antibody. All experiments were performed in duplicate.
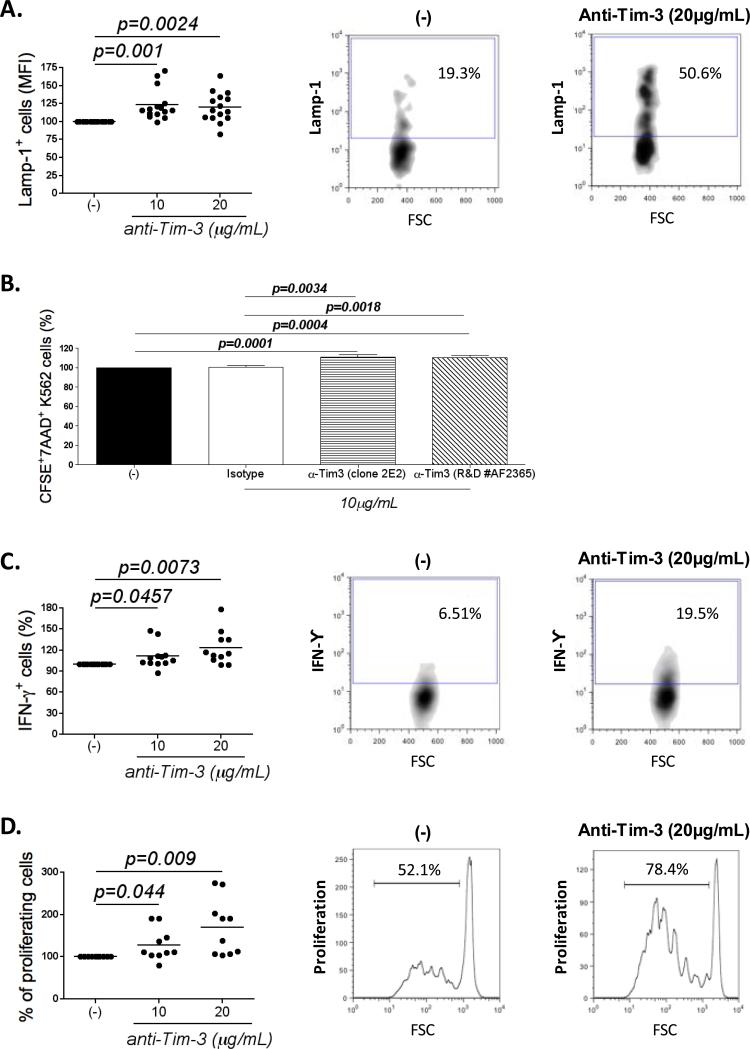
Figure 5. NK cell exhaustion can be reversed by Tim-3 blockade.
(A) Graph represents the expression of LAMP-1 (n=15; MFI) in NK cells from melanoma donors incubated with 10 or 20μg/ml of soluble Tim-3 blocking antibody 1h before the cytotoxic assay. (B) Plot shows the percentage of CFSE+7AAD+ K562 cells (% of killed K562 cells) in the presence of 10μg/ml of two different Tim-3 blocking antibodies (clone 2E2 or R&D #AF2365) 1h before the killing assay (n=9 MD). Graphs represent the expression of (C) IFNγ (n=12; MFI) and (D) the percentage of proliferating cells (n=10; %) in NK cells from melanoma donors incubated with 10 or 20μg/ml of soluble Tim-3 blocking antibody 1h before the functional assays. (A, C and D) Plots depicting the expression of LAMP-1, IFNγ production and proliferation with (right panel) and without (middle panel) Tim-3 blockade from a representative melanoma patient. Data were normalized to the values obtained for the condition without blocking antibody. All experiments were performed in duplicate.
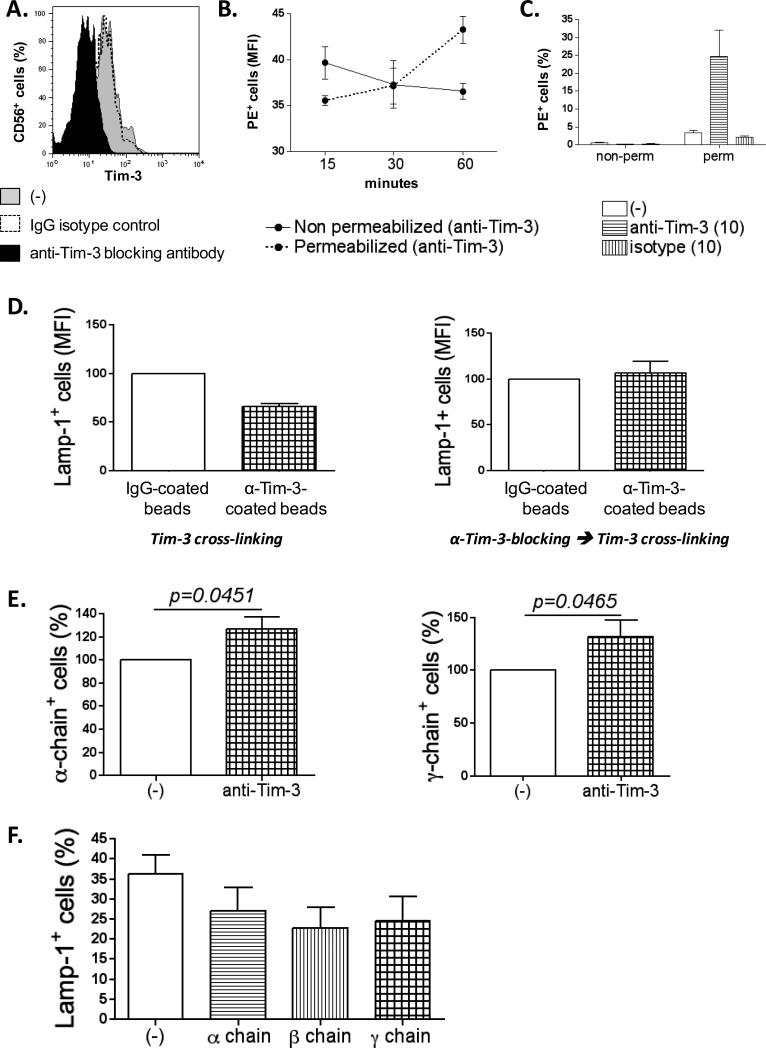
Figure 6. Soluble Tim-3 blocking antibody induces internalization of Tim-3 and up-regulation of IL-2R (α and γ chains).
(A) Plot depicting the decrease of Tim-3 expression in the membrane of NK cells after 1h treatment with soluble Tim-3 blocking antibody. (B) Graph represents the MFI of NK cells positive for the PE-conjugated, anti-mouse IgG antibody, with or without permeabilization (n=6). (C) The previous experiment was repeated also including an isotype control. Graph shows the percentage of cells positive for the PE-conjugated, anti-mouse IgG antibody after 1h of incubation with antibodies (n=6). (D) The represents the MFI of Lamp-1+ NK cells from healthy donors (n=6) after a cytotoxic assay with K562 cells. (Left panel) NK cells were pre-incubated (2h) with anti-Tim-3-coated beads or IgG-coated beads. (Right panel) Soluble Tim-3 blocking antibody was added 1h before crosslinking with anti-Tim-3-coated beads as described previously. Data were normalized to the values obtained for the condition with beads alone. (E) The plots show the expression of IL-2R α and γ chains (% of positive cells) in NK cells untreated or after 1h of treatment with 10μg/ml of soluble Tim-3 blocking antibody (n=7). Data were normalized to the values obtained for the condition without blocking antibody. (F) The graph depicts the percentage of Lamp-1+ NK cells after two days of culture with 200U/mL of IL-2, untreated or treated with blocking antibody for α, β or β chain (n=5). All experiments were performed in duplicate.
Human samples
Blood samples from healthy donors (HD) were purchased from the New York Blood Center. Blood samples were obtained under the Interdisciplinary Melanoma Cooperative Group IRB-approved protocols (#H10362) from 113 untreated melanoma patients (melanoma donors – MD; stages I, II and III/IV).
NK cell purification
Peripheral blood mononuclear cells (PBMC) were purified from HD- or MD-derived buffy coats by Ficoll-Paque Plus centrifugation. NK cell enrichment was performed by negative selection using Easy-Sep Human NK cell Enrichment Kit (StemCell Technologies) according to the manufacturer's recommendations, obtaining more than 95% CD3−CD56+ populations.
NK cell stimulation
For short-term functional assays (cytotoxicity and IFNγ production), purified NK cells were activated overnight in the presence of 1000U/ml of IL-2 complete media (RPMI-1640; Life Technologies, Carlsbad, CA) supplemented with 10% FBS, 2mM L-glutamine, 100U/ml penicillin, and 100μg/ml streptomycin. For the long-term experiments and proliferation assays, NK cells were cultured in the same media and stimulated with 200U/ml of IL-2.
Cell Staining and Flow Cytometry Analysis
Before staining, cells were washed twice with PBS supplemented with 0.5% bovine serum albumin and 2 mM EDTA (MACS Buffer). For surface staining, cells were incubated with specific antibodies for 30min at 4°C. For intracellular staining, cells were first fixed with 4% paraformaldehyde (PFA) for 10min at room temperature, permeabilized with MACS buffer supplemented with 0.1% saponin and stained with specific antibodies for 30min at room temperature. Cells were then washed twice with MACS buffer and analyzed by FACS. Data analysis was performed using FlowJo software.
Cell sorting
Gmel cells were stained for galectin-9 (Gal-9), following the protocol described above. After Gal-9 staining, these cells were sorted into populations of either Gal-9−-Gmel and Gal-9+-Gmel by flow cytometry. We followed the same procedure to sort NK cells into Tim-3+ and Tim-3− NK cells.
Crosslinking experiment: (A) Coated–Beads
Dynabeads® Pan Mouse IgG (Invitrogen) were labeled with purified mouse IgG1, κ isotype control (BDbiosciences) or purified human anti-TIM-3 (Biolegend) according to the manufacturer's instructions. Antibody labeled beads were added to NK cells at a 1:1 ratio for 30min at 4°C, and then incubated at 37°C for 90min. Functional assays were performed as described below. (B) Reverse-ADCC: NK cells were co-cultured with FcR+ P815 cells and different antibodies were added to the reaction: either anti-Tim-3, anti-CD94 (negative control) or anti-CD16 (positive control) antibody.
Endocytosis
To assess whether Tim-3 was endocytosed after Tim-3 blockade, IL-2-stimulated NK cells from healthy donors were incubated with the Tim-3 blocking Ab or IgG1 isotype control, as previously described, for 15min, 30min and 1h. Cells were then fixed, permeabilized or not and stained with a PE anti-mouse Ab in order to detect Tim-3 blocking antibody on the surface and/or in the cytoplasm of the cells.
Cytotoxicity Assay
Purified NK cells were co-cultured with target cells at a 5:1 effector/target ratio in combination of anti-CD107 antibody (0.5μg/ml) and monensin 1000X. After 4h incubation, CD107 expression on CD56+ cells was quantified by flow cytometry.
IFNγ production assay
Purified NK cells were cultured for 4h in the presence of 1μg/ml of IL-12 and brefeldin A (10μg/ml). After 4h cells were fixed (4% paraformaldehyde) and permeabilized (0.1% saponin). Permeabilized cells were stained for intracellular IFNγ and analyzed by flow cytometry.
Proliferation assay
Purified NK cells were loaded with 2μM CFSE and cultured in complete media supplemented with 200U/ml of IL-2. After 6 days of culture, CFSE dilution was analyzed by flow cytometry as a measure of cell proliferation.
Blocking experiment
10 or 20μg/ml of Tim-3 blocking antibodies (Biolegend, clone 2E2 or R&D #AF2365) was added to the culture 1 hour before starting the functional assays (before adding K562 cells in the cytotoxicity assay, IL-12 in the cytokine production assay and IL-2 in the proliferation assay). We used IgG1 isotype control from BD Biosciences.
Statistical analyses
Separate analyses were performed for each experiment individually. Two tailed t tests were used. Analyses take into account paired observations within donors when appropriate or unpaired observations when we compare parameters between healthy and melanoma NK cells. To analyze Tim-3 expression according to demographic and clinical parameters we used unpaired t test to compare two groups, and ANOVA to compare more than two groups.
RESULTS
MD NK cells display an exhausted phenotype
Advanced tumors are characterized by an environment that promotes T cell exhaustion (7). Exhausted T cells are characterized by: 1) over-expression of inhibitory receptors such as CTLA-4 and PD-1; 2) down-regulation of cytokine receptors, rendering them refractory to cytokine stimulation; 3) loss of function (cytotoxicity, cytokine production and proliferation); and 4) down-regulation of transcription factors T-bet and Eomesodermin (Eomes) (24). In metastatic melanoma, peripheral blood CD8+ T cells are functionally exhausted (11), however, NK cell phenotype and function in the same context has not been evaluated.
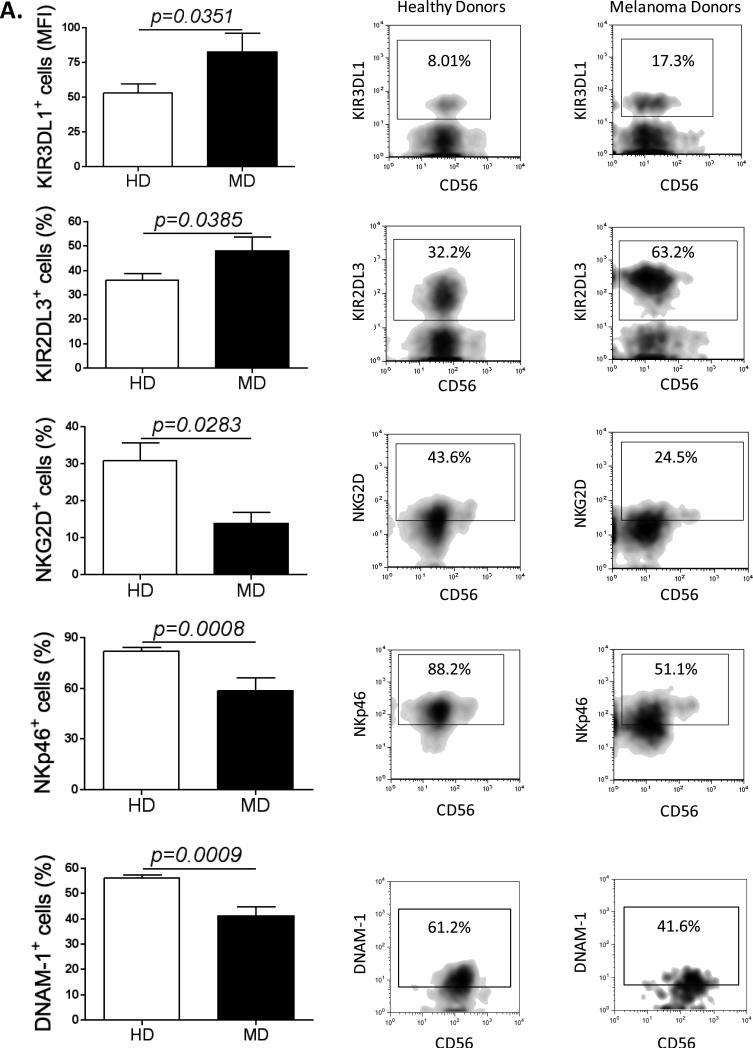
To determine whether peripheral NK cells from advanced melanoma patients display an exhausted phenotype, we examined whether they expressed features associated with T cell exhaustion. NK cells were purified from peripheral blood of advanced melanoma patients and healthy donors (HD) (Fig. S1A). Interestingly, melanoma donors (MD) NK cells down-regulate the NK cell markers CD16 and CD56 (Fig. S1B), and consequently display a decrease in the percentage of both CD56bright and CD56dim NK cell subsets (Fig. S1C). We then measured the expression of activating and inhibitory receptors in NK cells from MD and HD. Clearly, NK cells from MD expressed higher levels of inhibitory receptors (KIR3DL1 and KIR2DL3) and lower levels of activating receptors (NKG2D, NKp46 and DNAM-1) when compared with NK cells from HD (Fig. 1 and Fig. S1D). We also evaluated PD-1 and CTLA-4 expressions; however there were no significant differences between MD and HD (Fig. S1E).
Figure 1. MD NK cells up-regulate inhibitory receptors and down-regulate activating receptors.
(A) Graphs compare the expression of the inhibitory receptors KIR3DL1 (HD n=18; MD n=8) and KIR2DL3 (HD n=26, MD n=12) and the activating receptors NKG2D (HD n=25; MD n=12), NKp46 (HD n=20, MD n=11) and DNAM-1 (HD n=10; MD n=10), in peripheral blood NK cells purified from healthy donors and from melanoma patients. On the right panel, plots depicting the expression of KIR3DL1, KIR2DL3, NKG2D, NKp46 and DNAM-1 according to CD56 expression in NK cells from a representative healthy donor and a representative melanoma patient. All experiments were performed in duplicate.
Second, we measured the levels of the three IL-2 receptor (IL-2R) chains (α chain (CD25), β chain (CD122) and γ chain (CD132)). The expression of each subunit was measured in steady state conditions (day 0) and on days 2, 4 and 6 after IL-2 stimulation. HD or MD NK cells did not express significant levels of CD25 at rest. Interestingly, after IL-2 stimulation, HD NK cells up-regulated CD25 to very high levels whereas MD NK cells failed to do so (Fig. 2A and Fig. S2A). CD122 expression was similar in NK cells from healthy and melanoma subjects and decreased after IL-2 addition (Fig. S2A and Fig. S2B). CD132 expression was slightly higher in resting HD NK cells compared to MD NK cells. After IL-2 stimulation, both sources of NK cells showed an increase of this receptor, followed by a plateau and then a decrease. However, the overall expression of CD132 on HD NK cells was substantially higher than that of MD NK cells (Fig. S2A and Fig. S2B). We next tested whether altered IL-2R expression translated into a differential response to IL-2 stimulation. While cytotoxicity was induced in both sources of NK cells, the levels increased more significantly over time in HD NK cells. Whereas IFNγ production was induced by and increased in HD NK cells in response to IL-2, it did not change from baseline in the case of MD NK cells (Fig. 2A). Moreover, HD NK cells up-regulated both NKG2D and KIR3DL1, while MD NK cells did not (Fig. S2C). We also measured the levels of the IL-15 receptor (IL-15R), another significant NK cell stimulatory molecule. As observed for IL-2R, IL-15R levels are significantly lower in MD NK cells when compared with HD NK cells (Fig. S2D).
Next, we determined whether NK cells from melanoma patients were functionally impaired, by assessing cytotoxicity, IFNγ production, and cell proliferation. We found that, in comparison with HD NK cells, MD NK cells failed to efficiently kill target cells as assessed by LAMP-1 expression, after stimulation by either IL-2 (p=0.0158) or IL-15 (p=0.04) (Fig. 2B, Fig. S3A and Fig. S3D). In addition, they produced less IFNγ in response to different stimuli: IL-12 (p=0.0312) (Fig. 2B and Fig. S3D), the combination of IL-12 and IL-18 (p=0.001), IL-15 (p=0.05), or after coculture with K562 cells (p=0.0173) (Fig. S3B). Finally, NK cells from melanoma patients lost their ability to proliferate when cultured with IL-2 (p=0.0024), IL-12 (p=0.04), IL-15 (p=0.05) or IL-18 (p=0.01) (Fig. 2B, Fig. S3C and Fig. S3D). The transcription factors T-bet and Eomes regulate the function of NK cells and a recent study showed that down-modulation of these transcription factors is a characteristic of NK cell exhaustion (25). As expected, Fig. 2C shows that the levels of Eomes and T-bet are lower in NK cells from melanoma donors when compared with NK cells from healthy donors.
These results clearly demonstrate that NK cells from advanced melanoma patients are functionally exhausted as shown by over-expression of inhibitory receptors and down-regulation of activating receptors, an impaired response to IL-2 stimulation possibly due to IL-2R down-modulation, defects in cytokine production, proliferation and cytotoxicity, and a down-modulation of the transcription factors T-bet and Eomes.
Tim-3 is up-regulated in MD NK cells and is associated with clinical parameters that predict a poorer prognosis
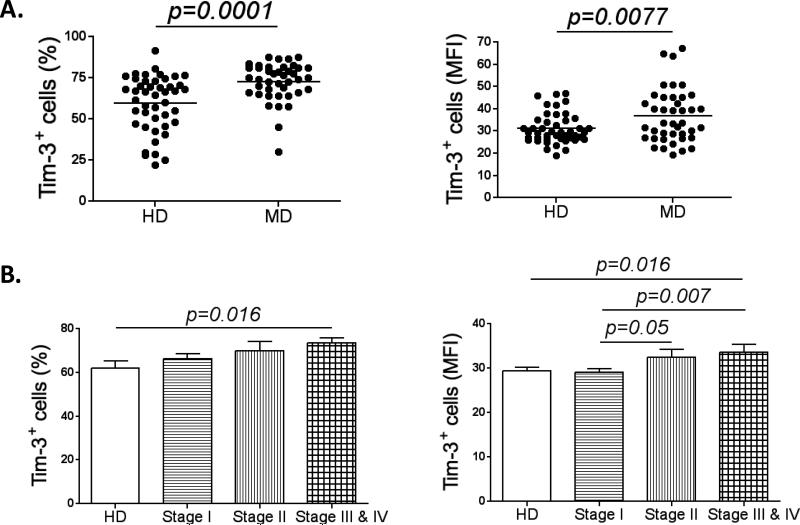
Tim-3 is another immune checkpoint expressed by CD8+ T cells in the context of advanced tumors, such as melanoma. However, the role of Tim-3 in NK cells under the same circumstances remains unknown. Therefore, we first measured Tim-3 expression in MD NK cells. Compared to HD NK cells, they expressed significantly higher levels of surface Tim-3 (p=0.0001) (Fig. 3A). Moreover, we checked the expression of Tim-3 in steady state conditions and after IL-2 stimulation in both CD56bright and CD56dim NK cell subsets. In unstimulated conditions, both subsets of NK cells from melanoma patients express higher levels of Tim-3 when compared with their counterparts from healthy donors (both % and MFI). However, after stimulation, while Tim-3 expression increases significantly in both subsets in HD (% and MFI) we did not observe any substantial increase in either subset from MD (Fig S4A).
Figure 3. Tim-3 is up-regulated in MD NK cells.
(A) Graph comparing Tim-3 expression in NK cells from healthy donors (HD; n=45) and melanoma patients (MD; n=41). Represented as the percentage of Tim-3+ cells (left panel) and the MFI of the Tim-3+ population (right panel). (B) The graphs show the percentage (left panel) and MFI (right panel) of Tim-3+ NK cells from healthy donors (n=30) and patients with melanoma stage I (n=47), II (n=18) and III/IV (n=18). All experiments were performed in duplicate.
We next measured the percentage of Lamp-1+ cells (Fig S4B) and IFNγ+ cells (Fig S4C) in the fraction of Tim-3+ vs Tim-3− NK cells, in unstimulated and stimulated conditions. Interestingly, in unstimulated conditions, in both HD and MD, the percentage of Lamp-1+ cells and IFNγ+ cells was higher in the Tim-3−fraction; however after IL-2 stimulation, the most active cells (Lamp-1+ cells or IFNγ+) were Tim-3+ (Fig. S4B and Fig. S4C). Indeed, as previously described (17, 18), Tim-3 is a marker of differentiation. However, when we compare the percentage of active cells in the Tim-3+ NK cells from healthy donors vs melanoma patients, clearly MD NK cells are less active, suggesting that these cells are exhausted.
We then evaluated whether Tim-3 expression was also increased at early stages of melanoma (stage I and II) and how its expression is related to clinical parameters. A cohort of 83 melanoma patients, distributed as stage I (n=47), stage II (n=18) and stage III/IV (n=18) was studied according to Tim-3 expression. As the stage of melanoma advanced, we observed a pattern of gradually increasing Tim-3+ NK cell numbers (%) and intensity (MFI) (Fig. 3B). In the same cohort of patients we were able to analyze Tim-3 expression (percentage and MFI) according to demographic and clinical parameters. Interestingly, the expression of Tim-3 is higher in melanoma patients with poor prognostic factors, such as thickness >1mm, mitotic rate >=1/mm2 and ulceration. Moreover, the intensity of Tim-3 expression (MFI) was higher in patients with metastases. Significantly, Tim-3 expression did not differ according to age, gender or lymph node status (Table 1).
Table 1.
Tim-3 expression (%) and (MFI) according to demographic and clinical parameters with well-known prognostic value
| Demographic and clinical parameters | Number of patients (N=83(%)) | Tim-3+ cells (%) Mean(SD) | P value | Tim-3+ cells (MFI) Mean(SD) | P value | |
|---|---|---|---|---|---|---|
| Age Groupsa | <= 45y | 22 (27) | 71.77(10.78) | 0.4122 | 31.30(5.953) | 0.9363 |
| 46-70y | 30 (36) | 69.51(13.52) | 30.94(6.650) | |||
| >=71y | 31 (37) | 66.34(18.15) | 30.62(7.319) | |||
| Genderb | Female | 27 (33) | 69.19(12.67) | 0.7544 | 30.09(6.630) | 0.4382 |
| Male | 56 (67) | 69.28(15.85) | 31.31(6.701) | |||
| Stagea | I | 47 (56) | 66.01(15.78) | 0.2116 | 29.07(5.303) | 0.0114 |
| II | 18 (22) | 69.93(17.45) | 32.39(7.895) | |||
| III/IV | 18 (22) | 73.28(9.973) | 34.30(8.479) | |||
| Thicknessb | <=1mm | 47 (57) | 66.03(15.47) | 0.041 | 29.18(5.642) | 0.0059 |
| >1mm | 36 (43) | 72.70(13.20) | 33.18(7.166) | |||
| Mitotic Indexb | <1/mm2 | 35 (42) | 65.01(15.83) | 0.0056 | 29.28(5.771) | 0.0278 |
| >=1/mm2 | 42 (51) | 73.54(10.21) | 32.46(6.540) | |||
| Unclassified | 6 (7) | |||||
| Ulcerationb | Absent | 58 (70) | 67.51(15.34) | 0.0351 | 30.19(6.263) | 0.0466 |
| Present | 19 (23) | 75.58(9.979) | 33.61(6.811) | |||
| Unclassified | 6 (7) | |||||
| LN statusb | Negative | 70 (84) | 68.24(15.58) | 0.3280 | 30.85(6.875) | 0.8242 |
| Positive | 13 (16) | 72.64(9.389) | 31.30(5.647) | |||
| Metastasisb | Absent | 74 (89) | 68.10(15.22) | 0.1476 | 30.26(6.105) | 0.0092 |
| Present | 9 (11) | 75.70(8.996) | 36.31(8.840) | |||
One-way analysis of variance (ANOVA);
Unpaired T test
In conclusion, our data suggest that higher Tim-3 expression is associated with clinical parameters such as thickness, mitotic rate and ulceration, well-known prognostic factors.
Tim-3 negatively regulates MD NK cell function
In order to determine the role of Tim-3 in NK cell exhaustion, we developed a system to engage Tim-3 through crosslinking, by using anti-Tim-3-coated beads. As shown in Fig. 4A and 4B, Tim-3 activation in MD NK cells reduced cytotoxicity by approximately 20% (p=0.0361) and IFNγ production by 25% (p=0.0176) as compared to controls. We performed a reverse-ADCC assay using FcR+ P815 cells that bind the Fc portion of antibodies, allowing them to crosslink their relevant ligands on other cells. We co-cultured NK cells from melanoma patients with P815 cells and added the following antibodies to the reaction: anti-Tim-3, anti-CD94 (negative control) or anti-CD16 (positive control). As expected, CD16 crosslinking activated NK cells rendering them more cytotoxic, while CD94 crosslinking reduced NK cell cytotoxicity. When Tim-3 was crosslinked, we observed a decrease in NK cell cytotoxicity (p=0.0002) (Fig. 4C). This finding confirms that Tim-3 acts as an inhibitory receptor on NK cells and that its ligation down-modulates NK cell function in the context of advanced melanoma. In HD, Tim-3 ligation also resulted in a significant decrease of cytotoxicity as previously described (17). We used the same two systems to crosslink Tim-3 on HD NK cells, anti-Tim-3-coated beads (Fig. S5A) and reverse-ADCC (Fig. S5B), as well as, anti-Tim-3-coated K562 cells (Fig. S5C), and showed an inhibition of Lamp-1 expression by NK cells (first two systems), and a decrease of K562 cell apoptosis (third system). It is important to point out that NK cells from melanoma patients may have already undergone significant Tim-3-mediated inhibition in vivo; therefore, it is not surprising that Tim-3 ligation did not achieve greater inhibition of MD NK cell function when compared with NK cells from healthy donors. Tim-3 also acts as an inhibitory receptor on T cells that when chronically activated, induces T cell apoptosis. However, in our system chronic Tim-3 stimulation did not induce NK cell apoptosis as we show in Fig. S5D.
Galectin-9 is the most studied Tim-3 ligand identified thus far. Therefore, we used recombinant and surface-bound galectin-9 (Gal9) as Tim-3 agonists. NK cells from HD were incubated with 25 and 50μg of rh-Gal9 1h before assessing cytotoxicity, and displayed a significant decrease in Lamp-1 expression, but without significant toxicity (Fig. S6A). We were able to abrogate this effect by blocking Gal-9 with a specific blocking antibody or β-lactose (Fig. S6B). Next we employed a more physiological approach by using the melanoma cell line Gmel as a source of Gal-9. Gmel cells were sorted according to their surface expression of Gal-9 into Gal9+-Gmel and Gal9−-Gmel (Fig. S6C) and used as target cells. We found that NK cell-mediated cytotoxicity from both HD or MD was lower in the presence of Gal9+-Gmel cells compared with Gal9−-Gmel (Fig. S6C). We then sorted NK cells according to Tim-3 expression into Tim-3+ vs Tim-3− NK cells, and repeated the same experiment. The suppressive effect of galectin-9 was only evident in the presence of Tim-3+ NK cells (Fig. S6D).
Therefore, our data indicate that following activation, Tim-3 functions as an inhibitory receptor in NK cells from melanoma patients by reducing their cytotoxicity and cytokine secretion potential.
Tim-3 blockade reverses exhaustion in MD NK cells
In T cells, Tim-3 has been described as a marker of T cell exhaustion, which when blocked can reverse the function of these cells (11, 14, 15). In order to study whether Tim-3 blockade could also reverse the function of MD NK cells, we blocked the Tim-3 receptor by adding a soluble Tim-3 blocking antibody (10 and 20μg/mL; clone 2E2) 1h before assessing NK cell functions in various assays. Fig. 5A shows that blocking Tim-3 significantly improved cytotoxicity by 20-25% (p=0.002). We obtained the same results with a different Tim-3 blocking antibody (R&D #AF2365; Fig. S7A). Both blocking antibodies were validated in a killing assay (Fig. 5B). IFNγ production also increased in the presence of Tim-3 blocking antibody by 15-20% (Fig. 5C; p=0.007). Finally the percentage of proliferating cells dramatically increased by 30% and 60% with the addition of 10 and 20μg/mL anti-Tim-3 antibody, respectively (Fig. 5D; p=0.009). Our results were confirmed with another Tim-3 blocking antibody (R&D #AF2365; Fig. S7A). Importantly, Tim-3 blockade improved NK cell cytotoxicity against four different melanoma cell lines (Fig. S7B). All four melanoma cell lines express galectin-9 (Fig. S7C), however the expression levels do not correlate with the increase of cytotoxicity that is observed with the Tim-3 blocking antibody. Isotype control antibody did not affect NK cell cytotoxicity, IFNγ production, or proliferation capacity (Fig. S7D).
As expected, Tim-3 blockade of NK cells from healthy donors also improved their cytotoxicity (Fig. S7E and S7F). However, addition of Galectin-9 blocking antibody did not affect cytotoxicity, suggesting that other Tim-3 natural ligands may participate since K562 cells do not express galectin-9 (Fig S7G). Altogether, these results demonstrate that Tim-3 blockade enhances NK cell function, including that of exhausted NK cells derived from advanced melanoma patients.
Tim-3 blockade induces internalization of the receptor and up-regulation of IL-2R
We investigated the mechanism underlying the reversal of NK cell exhaustion by the blockade of Tim-3. NK cells treated with Tim-3 blocking antibody showed a clear decrease in surface membrane Tim-3 levels (Fig. 6A). We hypothesized that Tim-3 expression decreased due to internalization. To address this possibility, NK cells from HD were first incubated with Tim-3 blocking antibody or isotype control, then fixed, permeabilized, and stained with a PE-conjugated anti-mouse IgG antibody in order to detect Tim-3 antibody both on the surface and in the cytoplasm of cells. As control, we also assessed antibody levels on cells that were fixed but not permeabilized. These experiments demonstrated that soluble Tim-3 blocking antibody induced internalization of Tim-3 (Fig. 6B and 6C). To demonstrate that reduced Tim-3 levels in the membrane account for reversal of NK cell exhaustion, NK cells were pre-treated with soluble Tim-3 blocking antibody and residual Tim-3 was crosslinked using anti-Tim-3-coated beads. As expected, in this context, the beads had no effect on cytotoxicity, due to prior internalization of Tim-3 by the soluble blocking antibody (Fig. 6D).
The effect of Tim-3 blockade was not due to altered viability, as assessed after treatment with 5, 10 and 20μg/mL of Tim-3 blocking antibody (Fig. S8A). Moreover, we confirmed that this effect is really due to Tim-3 blockade and not consequent to the engagement of the Fc portion of our Tim-3 blocking antibody with CD16 receptor (FcγRIII) in NK cells. Indeed, before adding the Tim-3 blocking antibody, we incubated MD NK cells with a CD16 (FcγRIII) blocking antibody for one hour. This anti-CD16 antibody blocks the possibility that the Fc portion of other antibodies added to the reaction (in our case Tim-3 blocking antibody) binds to CD16 receptor (FcγRIII). Fig. S8B shows an increase of cytotoxicity with Tim-3 blocking antibody, even after CD16 blockade.
Levels of activating and inhibitory receptors in the membrane of NK cells treated with Tim-3 blocking antibody were also measured. CD16 expression was increased by 10% after Tim-3 blockade (Fig. S8C). Likewise, there was a significant increase in the expression of α and γ chains of the IL-2R (Fig. 6E). As shown in Fig. 2A and Fig. S2C, HD NK cells respond to IL-2 stimulation with increased expression of activating and inhibitory receptors, cytotoxicity, and IFNγ production. In order to define the role of each chain of IL-2R in this NK cell response, we cultured purified NK cells from HD with 200U/mL of IL-2 and separately blocked with each chain of the IL-2R (α, β, and γ). After two days the expression of the activating and inhibitory receptors, and cytotoxicity was assessed. Our data demonstrate that all three chains affect the expression of the activating and inhibitory receptors, although the γ chain appears to elicit the more pronounced effect (Fig. S8D); all three IL-2R chains also enhance NK cell-mediated cytotoxicity after IL-2 stimulation (Fig. 6F). Taken together, Tim-3 blockade increases the expression of IL-2R, making NK cells more responsive to IL-2 stimulation, and consequently improving NK cell-mediated cytotoxicity.
Collectively, our findings show that NK cells in advanced melanoma patients display an exhausted phenotype. Furthermore, Tim-3 is overexpressed in MD NK cells and its levels correlate with the stage of the disease and the poor prognostic factors. More importantly, through Tim-3 blockade it is possible to reverse exhaustion in NK cells from patients with advanced melanoma.
DISCUSSION
T cell exhaustion has been extensively studied in the context of chronic infectious diseases and different types of cancer, however, little is known about the exhaustion of NK cells. Recently Mamessier and colleagues have shown that NK cells from breast cancer patients depict some dysfunctional phenotype (26), however our results provide the first demonstration that NK cell from advanced melanoma patients display the four main characteristics that define T cell exhaustion, and more important, that Tim-3 is an exhaustion marker in NK cells. Therefore, similarly to T cells, exhausted NK cells up-regulate inhibitory receptors while down-regulating IL-2 receptors and consequently are unable to respond appropriately to IL-2 stimulation. In addition, they are functionally impaired (reduced cytotoxicity, cytokine production, and proliferation) and express reduced levels of activating receptors and the transcription factors Eomes and T-bet. This exhaustion phenotype is associated with a higher expression of the inhibitory receptor Tim-3. Even though NK cells express Tim-3 in steady state, the NK cell exhausted phenotype is characterized by an up-regulation of this receptor. Therefore, the levels of Tim-3 in association with functional defects seem to be of key importance in defining the role of Tim-3. The exact mechanism of NK cell exhaustion is still unclear; it is possible that NK cells become exhausted due to systemic production of cytokines, or within the tumor microenvironment in response to specific ligands. Indeed, chronic stimulation with IL-2 and IL-15 (27, 28), shed MICA (tumor-derived ligand for NKG2D) (29, 30), or CD155 (a tumor-derived ligand for DNAM-1) (31) has been described to induce NK cell exhaustion. Exhausted T cells also up-regulate CTLA-4 and PD-1; however we found no significant expression of these receptors in the membrane of MD NK cells. Altogether, our results show that Tim-3 is constitutively expressed on NK cells from HD. More importantly, we demonstrate for the first time that exhausted NK cells up-regulate Tim-3, which functions as an inhibitory receptor/exhaustion marker, similarly to its described role in T cells. We found that the expression of Tim-3 in NK cells is higher in melanoma patients with bad prognostic factors, and increases as the disease stages progress.
Contrasting roles have been described for Tim-3 in NK cells from healthy donors. While one study showed that Tim-3 inhibits normal donor NK cell-mediated cytotoxicity (17), another suggested that Tim-3 may instead enhance IFNγ production (18). While the two studies are difficult to align, the different model systems and contexts could explain these divergent results, as has been reported for other NK cell receptors such as 2B4 and KIR2DL4 (32, 33). Our data are consistent with Tim-3 acting as an inhibitory receptor on NK cells from healthy, and most importantly, from melanoma donors. We also show that when triggered Tim-3 does not promote NK cell death. In our system Tim-3 negatively regulates NK cell function in a galectin-9-independent manner, suggesting a role for other Tim-3-ligands. Phosphatidylserine (PTS), exposed on the surface of apoptotic cells, has been reported as a Tim-3 ligand and may be a candidate for conferring NK cell exhaustion in vivo after tumor cell death.
When we block Tim-3 receptor with a soluble antibody we are able to recover, in part, NK cells’ function. This reversal is comparable to that in T cells after in vitro blockade of other immune checkpoints such as PD-1 blockade (11, 34) that has been used in clinical trials with impressive clinical responses (35). The Tim-3 blocking antibody binds and internalizes the receptor, decreasing its expression in the membrane of NK cells and the possibility of binding to the natural ligands. Another possibility is that we are blocking the intrinsic inhibitory pathway of Tim-3, independently of any ligand. We also showed that Tim-3 blockade induces a 10% increase of CD16 expression (MFI) that could provide another explanation for the increase of NK cell function. Thus CD16, an activating receptor that is directly involved in the lysis of tumor cells, may function not only through ADCC but also independent of antibody binding. Finally, we demonstrated that Tim-3 blockade increases the expression of the IL-2R in the membrane of MD NK cells, augmenting their ability to respond to IL-2 stimulation. The enhanced responsiveness may contribute towards the partial reversal of MD NK cell function after Tim-3 blockade.
Similar to CTLA-4 and PD-1, Tim-3 belongs to the group of immune checkpoint molecules and is a potential therapeutic target. Although there is no clinical data yet, Tim-3 has been reported to be co-expressed with PD-1 on human tumor-specific CD8+ T cells, and dual blockade of both molecules significantly enhances the in vitro proliferation and cytokine production of human T cells (11). Furthermore, in vivo studies have shown that Tim-3 blockade alone, or in combination with PD-1 blockade, is able to control tumor growth in four different tumor models, including melanoma (14, 36). A recent study showed that Tim-3 blockade stimulates potent antitumor responses against established melanoma via NK cell-dependent mechanisms when associated with a vaccine (37). However, in those studies it was not clear if Tim-3 had a direct effect on NK cells. Our findings provide the first evidence that Tim-3 blockade can directly reverse NK cell exhaustion and improve the function of NK cells from melanoma patients. Even though the recovery of melanoma NK cell function is significant, it is not complete. It is possible that Tim-3 works with other receptors to regulate NK cell exhaustion, although we could not detect a role for either CTLA-4 or PD-1. Nevertheless, combinatorial strategies that also target other inhibitory NK cell receptors may enable the recovery of NK cell phenotype more completely. Our study has direct clinical relevance since it shows for the first time that blocking Tim-3 improves, ex vivo, the function of NK cells, which could be used for NK cell adoptive transfer therapy. Moreover, our studies support the concept that systemic Tim-3 blockade could improve antitumor response in the context of melanoma, as is the case with systemic CTLA-4 and PD-1 blockade. Less adverse events should be expected with Tim-3 blockade since Tim-3-deficient mice are viable and do not develop autoimmune or lymphoproliferative diseases (12), as opposed to CTLA-4-deficient mice (38).
In conclusion, this study suggests that higher Tim-3 expression on NK cells is associated with advanced stages of melanoma and with poor prognostic clinical parameters. We show for the first time that Tim-3 is an exhaustion marker expressed in NK cells from advanced melanoma patients and that its blockade reverses their exhausted phenotype. Tim-3, therefore, represents a promising therapeutic target that could enhance antitumor immunity with the potential to produce durable clinical responses that are dependent not only upon T cells but also the innate immune system.
Supplementary Material
ACKNOWLEDGEMENTS
We thank E. Godefroy, O. Manches and D. Frleta for critical reading of the manuscript.
Funding:
This study was supported by grants from the US National Institutes of Health (R01AI081848, R01AI071078, R43CA153669), the Melanoma Research Alliance and the Cancer Research Institute. I.P.S was previously supported by a Fellowship from the Programme for Advanced Medical Education.
Footnotes
Disclosure of Potential Conflicts of Interest
Vijay K. Kuchroo is a founder of, and has a financial interest in, CoStim Pharmaceuticals, a company that is developing novel biologics targeting immune checkpoint inhibitors. Ana C. Anderson is a paid member of the Scientific Advisory Board of CoStim Pharmaceuticals. The other authors have no conflicting financial interests.
REFERENCES
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Villanueva J, Herlyn M. Melanoma and the tumor microenvironment. Current Oncol Reports. 2008;10:439–46. doi: 10.1007/s11912-008-0067-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nikolaou VA, Stratigos AJ, Flaherty KT, Tsao H. Melanoma: new insights and new therapies. J Invest Dermatol. 2012;132:854–63. doi: 10.1038/jid.2011.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chapman PB, Hauschild A, Robert C, Haanen JB, Ascierto P, Larkin J, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011;364:2507–16. doi: 10.1056/NEJMoa1103782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–23. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baitsch L, Baumgaertner P, Devevre E, Raghav SK, Legat A, Barba L, et al. Exhaustion of tumor-specific CD8(+) T cells in metastases from melanoma patients. J Clin Invest. 2011;121:2350–60. doi: 10.1172/JCI46102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wherry EJ. T cell exhaustion. Nat Immunol. 2011;12:492–9. doi: 10.1038/ni.2035. [DOI] [PubMed] [Google Scholar]
- 8.Kaufmann DE, Kavanagh DG, Pereyra F, Zaunders JJ, Mackey EW, Miura T, et al. Upregulation of CTLA-4 by HIV-specific CD4+ T cells correlates with disease progression and defines a reversible immune dysfunction. Nat Immunol. 2007;8:1246–54. doi: 10.1038/ni1515. [DOI] [PubMed] [Google Scholar]
- 9.Baitsch L, Legat A, Barba L, Fuertes Marraco SA, Rivals JP, Baumgaertner P, et al. Extended co-expression of inhibitory receptors by human CD8 T-cells depending on differentiation, antigen-specificity and anatomical localization. PloS one. 2012;7:e30852. doi: 10.1371/journal.pone.0030852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barber DL, Wherry EJ, Masopust D, Zhu B, Allison JP, Sharpe AH, et al. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439:682–7. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- 11.Fourcade J, Sun Z, Benallaoua M, Guillaume P, Luescher IF, Sander C, et al. Upregulation of Tim-3 and PD-1 expression is associated with tumor antigen638 specific CD8+ T cell dysfunction in melanoma patients. J Exp Med. 2010;207:2175–86. doi: 10.1084/jem.20100637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sanchez-Fueyo A, Tian J, Picarella D, Domenig C, Zheng XX, Sabatos CA, et al. Tim-3 inhibits T helper type 1-mediated auto- and alloimmune responses and promotes immunological tolerance. Nat Immunol. 2003;4:1093–101. doi: 10.1038/ni987. [DOI] [PubMed] [Google Scholar]
- 13.Sabatos CA, Chakravarti S, Cha E, Schubart A, Sanchez-Fueyo A, Zheng XX, et al. Interaction of Tim-3 and Tim-3 ligand regulates T helper type 1 responses and induction of peripheral tolerance. Nat Immunol. 2003;4:1102–10. doi: 10.1038/ni988. [DOI] [PubMed] [Google Scholar]
- 14.Sakuishi K, Apetoh L, Sullivan JM, Blazar BR, Kuchroo VK, Anderson AC. Targeting Tim-3 and PD-1 pathways to reverse T cell exhaustion and restore anti648 tumor immunity. J Exp Med. 2010;207:2187–94. doi: 10.1084/jem.20100643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McMahan RH, Golden-Mason L, Nishimura MI, McMahon BJ, Kemper M, Allen TM, et al. Tim-3 expression on PD-1+ HCV-specific human CTLs is associated with viral persistence, and its blockade restores hepatocyte-directed in vitro cytotoxicity. J Clin Invest. 2010;120:4546–57. doi: 10.1172/JCI43127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jin HT, Anderson AC, Tan WG, West EE, Ha SJ, Araki K, et al. Cooperation of Tim-3 and PD-1 in CD8 T-cell exhaustion during chronic viral infection. Proc Natl Acad Sci USA. 2010;107:14733–8. doi: 10.1073/pnas.1009731107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ndhlovu LC, Lopez-Verges S, Barbour JD, Jones RB, Jha AR, Long BR, et al. Tim-3 marks human natural killer cell maturation and suppresses cell-mediated cytotoxicity. Blood. 2012;119:3734–43. doi: 10.1182/blood-2011-11-392951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gleason MK, Lenvik TR, McCullar V, Felices M, O'Brien MS, Cooley SA, et al. Tim-3 is an inducible human natural killer cell receptor that enhances interferon gamma production in response to galectin-9. Blood. 2012;119:3064–72. doi: 10.1182/blood-2011-06-360321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eckl J, Buchner A, Prinz PU, Riesenberg R, Siegert SI, Kammerer R, et al. Transcript signature predicts tissue NK cell content and defines renal cell carcinoma subgroups independent of TNM staging. J Mol Med. 2012;90:55–66. doi: 10.1007/s00109-011-0806-7. [DOI] [PubMed] [Google Scholar]
- 20.Ishigami S, Natsugoe S, Tokuda K, Nakajo A, Che X, Iwashige H, et al. Prognostic value of intratumoral natural killer cells in gastric carcinoma. Cancer. 2000;88:577–83. [PubMed] [Google Scholar]
- 21.Platonova S, Cherfils-Vicini J, Damotte D, Crozet L, Vieillard V, Validire P, et al. Profound coordinated alterations of intratumoral NK cell phenotype and function in lung carcinoma. Cancer Res. 2011;71:5412–22. doi: 10.1158/0008-5472.CAN-10-4179. [DOI] [PubMed] [Google Scholar]
- 22.Imai K, Matsuyama S, Miyake S, Suga K, Nakachi K. Natural cytotoxic activity of peripheral-blood lymphocytes and cancer incidence: an 11-year follow-up study of a general population. Lancet. 2000;356:1795–9. doi: 10.1016/S0140-6736(00)03231-1. [DOI] [PubMed] [Google Scholar]
- 23.Vivier E, Ugolini S, Blaise D, Chabannon C, Brossay L. Targeting natural killer cells and natural killer T cells in cancer. Nat Rev Immunol. 2012;12:239–52. doi: 10.1038/nri3174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jin HT, Jeong YH, Park HJ, Ha SJ. Mechanism of T cell exhaustion in a chronic environment. BMB reports. 2011;44:217–31. doi: 10.5483/BMBRep.2011.44.4.217. [DOI] [PubMed] [Google Scholar]
- 25.Gill S, Vasey AE, De Souza A, Baker J, Smith AT, Kohrt HE, et al. Rapid development of exhaustion and down-regulation of eomesodermin limit the antitumor activity of adoptively transferred murine natural killer cells. Blood. 2012;119:5758–68. doi: 10.1182/blood-2012-03-415364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mamessier E, Sylvain A, Thibult ML, Houvenaeghel G, Jacquemier J, Castellano R, et al. Human breast cancer cells enhance self tolerance by promoting evasion from NK cell antitumor immunity. J Clin Invest. 2011;121:3609–22. doi: 10.1172/JCI45816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Elpek KG, Rubinstein MP, Bellemare-Pelletier A, Goldrath AW, Turley SJ. Mature natural killer cells with phenotypic and functional alterations accumulate upon sustained stimulation with IL-15/IL-15Ralpha complexes. Proc Natl Acad Sci USA. 2010;107:21647–52. doi: 10.1073/pnas.1012128107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huenecke S, Zimmermann SY, Kloess S, Esser R, Brinkmann A, Tramsen L, et al. IL-2-driven regulation of NK cell receptors with regard to the distribution of CD16+ and CD16- subpopulations and in vivo influence after haploidentical NK cell infusion. J Immunother. 2010;33:200–10. doi: 10.1097/CJI.0b013e3181bb46f7. [DOI] [PubMed] [Google Scholar]
- 29.Ashiru O, Boutet P, Fernandez-Messina L, Aguera-Gonzalez S, Skepper JN, Vales-Gomez M, et al. Natural killer cell cytotoxicity is suppressed by exposure to the human NKG2D ligand MICA*008 that is shed by tumor cells in exosomes. Cancer Res. 2010;70:481–9. doi: 10.1158/0008-5472.CAN-09-1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Groh V, Wu J, Yee C, Spies T. Tumour-derived soluble MIC ligands impair expression of NKG2D and T-cell activation. Nature. 2002;419:734–8. doi: 10.1038/nature01112. [DOI] [PubMed] [Google Scholar]
- 31.Carlsten M, Norell H, Bryceson YT, Poschke I, Schedvins K, Ljunggren HG, et al. Primary human tumor cells expressing CD155 impair tumor targeting by down701 regulating DNAM-1 on NK cells. J Immunol. 2009;183:4921–30. doi: 10.4049/jimmunol.0901226. [DOI] [PubMed] [Google Scholar]
- 32.Faure M, Long EO. KIR2DL4 (CD158d), an NK cell-activating receptor with inhibitory potential. J Immunol. 2002;168:6208–14. doi: 10.4049/jimmunol.168.12.6208. [DOI] [PubMed] [Google Scholar]
- 33.Schlaphoff V, Lunemann S, Suneetha PV, Jaroszewicz J, Grabowski J, Dietz J, et al. Dual function of the NK cell receptor 2B4 (CD244) in the regulation of HCV706 specific CD8+ T cells. PLoS Pathogens. 2011;7:e1002045. doi: 10.1371/journal.ppat.1002045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Matsuzaki J, Gnjatic S, Mhawech-Fauceglia P, Beck A, Miller A, Tsuji T, et al. Tumor-infiltrating NY-ESO-1-specific CD8+ T cells are negatively regulated by LAG-3 and PD-1 in human ovarian cancer. Proc Natl Acad Sci USA. 2010;107:7875–80. doi: 10.1073/pnas.1003345107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–54. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ngiow SF, von Scheidt B, Akiba H, Yagita H, Teng MW, Smyth MJ. Anti-TIM3 antibody promotes T cell IFN-gamma-mediated antitumor immunity and suppresses established tumors. Cancer Res. 2011;71:3540–51. doi: 10.1158/0008-5472.CAN-11-0096. [DOI] [PubMed] [Google Scholar]
- 37.Baghdadi M, Nagao H, Yoshiyama H, Akiba H, Yagita H, Dosaka-Akita H, et al. Combined blockade of TIM-3 and TIM-4 augments cancer vaccine efficacy against established melanomas. Cancer Immunol Immunother. 2013;62:629–37. doi: 10.1007/s00262-012-1371-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Khattri R, Auger JA, Griffin MD, Sharpe AH, Bluestone JA. Lymphoproliferative disorder in CTLA-4 knockout mice is characterized by CD28-regulated activation of Th2 responses. J Immunol. 1999;162:5784–91. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.