Abstract
Nonphotochemical quenching (NPQ) refers to a process that regulates photosynthetic light harvesting in plants as a response to changes in incident light intensity. By dissipating excess excitation energy of chlorophyll molecules as heat, NPQ balances the input and utilization of light energy in photosynthesis and protects the plant against photooxidative damage. To understand the physical mechanism of NPQ, we have performed femtosecond transient absorption experiments on intact thylakoid membranes isolated from spinach and transgenic Arabidopsis thaliana plants. These plants have well defined quenching capabilities and distinct contents of xanthophyll (Xan) cycle carotenoids. The kinetics probed in the spectral region of the S1 → Sn transition of Xans (530–580 nm) were found to be significantly different under the quenched and unquenched conditions, corresponding to maximum and no NPQ, respectively. The lifetime and the spectral characteristics indicate that the kinetic difference originated from the involvement of the S1 state of a specific Xan, zeaxanthin, in the quenched case.
Green plants live with a continual paradox: They have evolved to both use and dissipate solar energy with high efficiency. Highly reactive, photooxidative intermediates are inevitable byproducts of photosynthesis. An excess photon flux can exacerbate the damage caused by these intermediates, leading to problems ranging from reversible decreases in photosynthetic efficiency, to, in the worst case, death of the plant. Nonphotochemical quenching (NPQ) is a process that thermally dissipates the absorbed light energy in photosystem (PS) II that exceeds a plant's capacity for CO2 fixation, minimizing the deleterious effects of high light. Although NPQ has been phenomenologically documented for years, a fundamental understanding of its physical mechanism remains elusive (1–3).
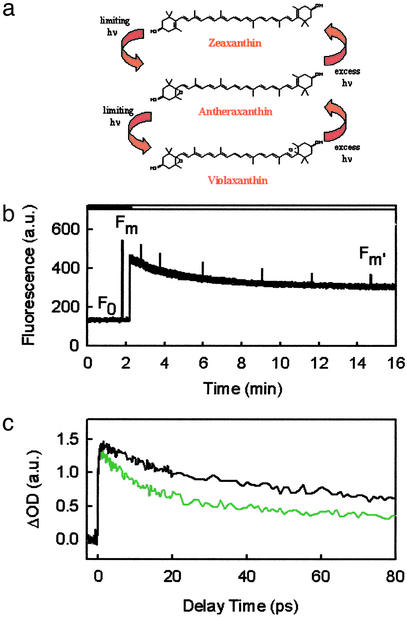
Feedback deexcitation or energy-dependent quenching (qE) (2, 3) is the major, rapidly reversible component of NPQ in a variety of plants, including spinach and Arabidopsis thaliana (4, 5), and is the focus of this study. qE is characterized by a light-induced absorbance change at 535 nm (ΔA535) (6) and the shortening of specific components of chlorophyll (Chl) fluorescence lifetimes, the exact numerical value for the shortened lifetime depending on the specific form of the photosynthetic system and the measurement conditions. For isolated thylakoid systems with closed reaction centers, a Chl lifetime component is reduced from ≈2.0 to ≈0.4 ns (4). It requires the buildup of a pH gradient (ΔpH) under high light conditions (2, 3), which triggers the enzymatic conversion of carotenoids, violaxanthin (Vio) to zeaxanthin (Zea), by means of the xanthophyll (Xan) cycle (Fig. 1a).
Fig 1.
(a) Xan cycle. (b) Chl fluorescence during induction of NPQ in isolated spinach thylakoid membranes. White and black bars above the graph indicate periods of illumination with high light and darkness, respectively. (c) TA profiles measured at 540 nm on excitation at 664 nm under unquenched (black line) and quenched (green line) conditions. The experimental data are the averages of five independent measurements.
Currently two hypotheses concerning the mechanism of qE exist, one in which the effect of Zea is solely structural (termed indirect quenching) and the other in which Zea acts as an energy acceptor for excitation transfer from the first Chl singlet excited state (termed direct quenching). The direct quenching mechanism was proposed based on both the previously determined, smaller So → S1 transition energy of Zea (7) and its short S1 lifetime (≈10 ps) (8) with respect to the first excited singlet state of Chl (Qy). The short S1 lifetime would allow for rapid thermal dissipation of excitation energy, preventing the generation of photooxidative intermediates. One major difficulty in assessing the feasibility of the direct quenching mechanism is that the carotenoid S1 state is optically dark. As a result, the potential involvement of this state in qE cannot be detected with time-resolved fluorescence experiments.
Although the formation of Zea is necessary for the majority of qE it is not sufficient (9). Along with ΔpH, a specific pigment-binding protein, PsbS (CP22), has been found to be essential for qE (5). In addition, proton binding to PSII proteins, including PsbS, is believed be significant in the activation of Zea's quenching role (2, 6). In this article, we present femtosecond transient absorption (TA) data on intact, active thylakoid membranes from spinach and A. thaliana. By combining a technique that allows us to detect nonfluorescent, short-lived excited states and a system with fully functional qE capability, we should be able to detect excitation of the S1 state of Zea after selective excitation of the Chl Qy band. The evidence shows direct involvement of Zea solely under conditions of maximum qE and supports a role for carotenoids as direct quenchers of excess excitation energy.
Materials and Methods
Sample Preparation.
A. thaliana plants in the Col-0 WT background were grown in potting soil in a greenhouse under short-day conditions (8 h light/16 h dark; maximum photon flux density of 500 μmol photons⋅m−2⋅s−1). Fresh spinach was purchased from a local market. Thylakoid membranes were isolated as described by Gilmore et al. (10) before each day's experiment.
Regulation and Detection of the Energized States of the Thylakoids.
A heat-filtered, intense incandescent light source producing a photon flux density of 800 μmol photons⋅m−2⋅s−1 at the sample position was used to induce and maintain the quenched state of the thylakoids. The samples were considered quenched when a maximum, steady-state amount of qE existed in reaction buffer (which occurred ≈5 min after high light illumination). Determinations of the energized states of thylakoids, unquenched and quenched, were made by recording the fluorescence emission with a FMS2 fluorometer (Hansatech Instruments, Pentney King's Lynn, U.K.).
Determination of the Xan Pigment Composition.
The concentrations of various pigments were determined by using reversed-phase HPLC (11).
Femtosecond TA Measurements.
The femtosecond TA measurements were performed by using a 250-kHz regenerative titanium/sapphire amplifier pumped optical parametric amplifier system (12), producing pump pulses centered at 664 and 683 nm. The probe pulses were selected from a white-light continuum with a band-pass filter ahead of the sample. The typical cross-correlation was 80–120 fs depending on the probe wavelength, and the maximal pump pulse energy was kept at 22 nJ, corresponding to a photon flux of 4.7 × 1013 photons⋅pulse−1⋅cm−2. The dimensions of the thylakoids led to extremely intense scattering of the pump beam. Effective suppression of the scattering was achieved by use of appropriate band-pass or short pass filters behind the sample, in conjunction with a monochromator (H20, ISA, Edison, NJ). The transmission changes with a spectral resolution of 2–4 nm were monitored by a silicon photodiode (DET 210, Thorlabs, Newton, NJ) connected to a lock-in amplifier. A sample cell with path length of 0.5 or 1 mm was used and translated during the data acquisition to avoid sample degradation. The typical sample OD was ≈0.3–0.8/mm at 683 nm. Sample stability was confirmed by measuring the absorption spectra before and after the time-resolved measurements.
Results
TA Kinetics Change in Response to Nonphotochemical Quenching.
On active spinach thylakoids, defined as isolated membranes with fully functional qE capability, TA measurements were carried out in two states, under conditions where no qE existed (unquenched) and where maximum, steady-state qE had been induced and maintained with constant illumination of high light (quenched) (Fig. 1b). The samples were excited at 683 or 664 nm, corresponding to the absorption maximum or the blue edge of the Chl Qy band, respectively. The kinetics were probed in two distinct spectral regions: the characteristic S1 → Sn transition of Xans (530–580 nm) and the excited-state absorption (ESA) and photobleaching and/or stimulated emission region of the Chl Qy band (650–720 nm). In the former region, the kinetics obtained depended on the state of the system. The measurements preformed under quenched conditions showed a faster initial decay and slightly smaller initial amplitude with respect to the unquenched case. In addition, no resolvable rise time was observed in the quenched traces within our time resolution of ≈120 fs (Fig. 1c). The distinctly different kinetics were found to be independent of the measurement order of an individual sample, i.e., whether the sample was unquenched first and then quenched, or vice versa. Performing a third measurement on a single sample, either quenched or unquenched depending on the order of the first two measurements, further verified that the kinetics obtained were completely reversible (data not shown).
In the experiments in which the unquenched measurement was carried out first, Vio is the dominant carotenoid in the Xan cycle pool. Illumination of the sample with high light induces an increase of ΔpH and, in turn, triggers the formation of Zea from Vio, by means of the Xan cycle (Fig. 1a). When the system is first measured in the quenched state and then unquenched, the increased ΔpH in the former case is fully dissipated in the latter case, whereas the Zea formed remains unchanged as the reaction buffer lacks the necessary cofactors for enzymatic conversion of Zea back to Vio. It thus indicates that the kinetic difference observed requires the coexistence of both Zea and the transmembrane ΔpH and is, therefore, fully consistent with previous studies on qE (1, 3). The induction of the Xan cycle in forming the quenched state was verified by HPLC analysis of pigments, showing that the relative content of Zea with respect to the total Xan cycle pool (Vio, antheraxanthin, and Zea) increases from 7.2 ± 1.7% to 64.2 ± 4.3% with a concomitant decrease in Vio content.
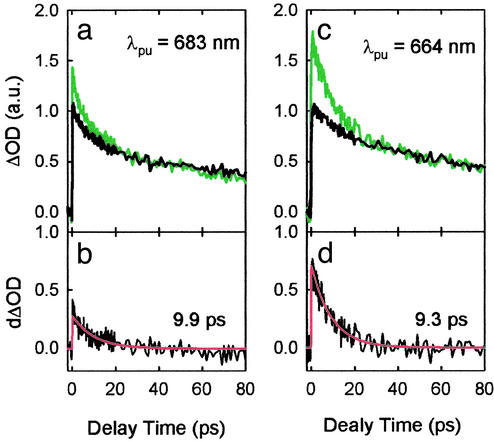
Upon closer inspection of the profiles in Fig. 1c, in which the probe was set to 540 nm, we find the quenched and unquenched measurements are kinetically indistinguishable at longer delay times (≥40 ps). The same observation holds true for the sets of traces probed at other wavelengths between 530 and 580 nm. For convenient visualization of these TA kinetics, we scaled the decay profile under quenched conditions to match the respective one measured under unquenched conditions at a longer delay time (60 ps) according to their deconvolution fits (Fig. 2c). We then calculated their difference, which was satisfactorily fit with a monoexponential decay (Fig. 2d) with a lifetime of 9.3 ps at 540 nm.
Fig 2.
(a and c) The scaled TA kinetics probed at 540 nm for spinach thylakoids under unquenched (black line) and quenched (green line) conditions on excitation at 683 and 664 nm. The profiles were normalized to 1.0 at the maximum amplitudes of the corresponding unquenched kinetics. (b and d) The difference between the quenched and unquenched curves in a and c and their monoexponential fits (red line) with the corresponding lifetimes shown in the plots.
Generally similar decay features were observed in the kinetics probed at other wavelengths in the spectral region between 530 and 580 nm; however, the relative difference between the kinetics obtained under quenched and unquenched conditions appears smaller at both the shorter and longer probe wavelengths. The smaller differences, together with the relatively low signal-to-noise (S/N) ratio of the raw data leads to a difference profile with even poorer S/N ratio and thus results in larger uncertainty in the time constant resolved by direct fitting of the differences. We therefore chose a different procedure for data analysis, global lifetime analysis (13), to extract the time constant associated with the additional decay component in the quenched kinetics. The global lifetime analysis was performed for each pair of kinetics (quenched and unquenched) recorded by using a single sample. The lifetimes and/or amplitudes associated with the three common components for the two kinetics were forced to be identical (global parameters), and the lifetime and amplitude of an additional component were taken as independent, free variables for the quenched decay. This analysis gives a lifetime of 10.5 ± 2.5 ps at 540 nm on excitation at 683 nm. The lifetime obtained by this procedure varies from 5 ps at 530 nm to 30 ps at 580 nm at the same excitation wavelength. The small kinetic differences for the probe wavelengths on either side of 540 nm and consequent poor S/N ratio make it difficult to be definitive on this point.
Calculation of the difference profiles (Fig. 2) and the global lifetime analysis are based on a common assumption: The TA signal at 530–580 nm observed after excitation of Chls in the Qy band originates from Chl ESA in the unquenched case, and in the quenched case, a superimposed signal from an additional, so far undefined but not Chl-related transition. The assumption is justified if the change in decay of the TA signal probed directly in the Chl Qy band is undetectable or at least significantly smaller with respect to the changes observed at 540 nm under quenched and unquenched conditions. Within our temporal window of detection (≈80 ps), on excitation at 683 nm, the decay profiles measured at both the magic angle and perpendicular polarizations (the latter used exclusively for wavelengths that were 20 nm or closer to the excitation wavelength due to sample scattering considerations) yielded indistinguishable kinetics, within the experimental uncertainty.
In addition, measurements on spinach thylakoids in the presence of nigericin were performed. Nigericin is an uncoupler that prevents the formation of ΔpH, and, subsequently, the conversion of Vio to Zea upon exposure to high light conditions (14). Identical decay traces were observed at 540 nm with and without high light illumination, confirming the necessity of ΔpH for the observed kinetic differences (data not shown).
The Kinetic Difference Is Enhanced When the Blue Edge of the Qy Band Is Preferentially Excited.
An increase in the magnitude of the kinetic differences was observed when the pump wavelength was tuned to 664 nm, the blue edge of the Qy band. A simple method to quantify this difference is the scaling factor used to match the quenched to the unquenched decay at 60 ps. For the kinetics probed at 540 nm, a factor of 1.3 was obtained for 683-nm excitation, which increased to 1.8 for 664 nm (Fig. 2).
Despite the overall amplitude enhancement observed for the kinetics at 664 nm, the lifetime associated with the difference is relatively constant. A decay with a lifetime of 8.6 ± 2.3 ps (standard deviation for four independent measurements obtained from global analysis) was obtained. In addition, measurements in the Qy region, probed at 680 nm after excitation at 664 nm, show differences between the quenched and unquenched kinetics that are significantly smaller in amplitude than those observed at 540 nm. The normalization factors were 1.8 and 1.1 for the probe at 540 and 680 nm, respectively (latter data not shown). Therefore, the same method of data analysis, including scaling the experimental decay profiles as well as calculating their difference, used in the case of 683-nm excitation is again justified.
TA Differences Are Proportional to the Amount of qE.
Although the faster TA kinetics obtained in the quenched vs. unquenched measurements with spinach thylakoids seem to correlate with qE, experiments on various A. thaliana mutants with well characterized qE capabilities were performed to confirm this result. Three mutants, two completely deficient and one enhanced in qE with respect to the WT, but all normal in their ability to carry out direct light-induced photochemistry, were studied.
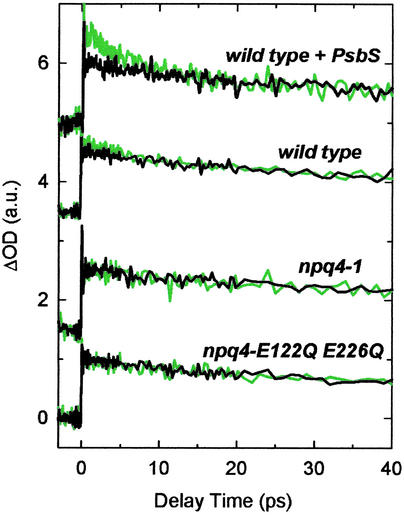
The amount of qE in the mutants is related to the protein PsbS (5). The two plant genotypes studied that are qE-deficient are npq4-1 and npq4-E122Q E226Q. The former completely lacks the PsbS gene (5), whereas the latter contains only overexpressed, inactive PsbS (15). The reason for the qE deficiency in npq4-E122Q E226Q is proposed to be related to the interruption of proton binding by the mutation of two glutamate residues on the luminal side of the PsbS protein (15). Previous studies found that npq4-1 and npq4-E122Q E226Q show no qE. In contrast, a strain that overexpresses PsbS, WT + PsbS, has ≈2.5-fold more PsbS than the WT and shows the largest qE (16).
The TA measurements of these mutants and the WT were performed at a probe wavelength of 540 nm with Chl excitation at 664 nm (Fig. 3). The largest kinetic difference was found for the WT + PsbS mutant, a significantly smaller difference for the WT, and no difference for the two qE-deficient mutants. These differences can be quantified by comparing the factors used to scale the kinetics, 1.8 for WT + PsbS, 1.3 for the WT, and 1.0 for both npq4-1 and npq4-E122Q E226Q. The results show a direct relationship between the amount of qE and the amplitude of the kinetic differences. They confirm that the TA kinetic differences are truly correlated to qE.
Fig 3.
The scaled TA kinetics measured at 540 nm on 664-nm excitation with the WT and three qE mutants of A. thaliana. The green and black lines indicate quenched and unquenched conditions for the samples with qE capability, which corresponds to the high light illumination on and off for the qE-deficient mutants. The decay profiles were normalized to 1.0 at the maximum amplitudes of the unquenched (light off) kinetics and vertically offset for clarity.
Global lifetime analysis of the TA measurements of the thylakoids from the two functional qE plants generated values of ≈7 ps for both the WT + PsbS and the WT. The values are similar to each other, but slightly shorter than the values obtained for the spinach thylakoids.
Mutants with Distinct Carotenoid Composition Show Zea Is Necessary for the Kinetic Difference.
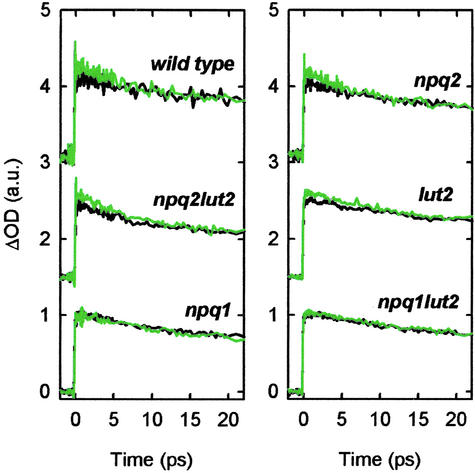
A series of TA measurements was also carried out on five A. thaliana mutant plant strains that have varying carotenoid composition. Previously, two carotenoids were experimentally shown to affect qE, Zea and lutein (Lut). Zea is found to be primarily responsible for the amount of quenching in Arabidopsis, whereas Lut seems to play a more minor role. Lut influences the rate of induction of qE and, to a smaller extent than Zea, the amount of qE (17, 18). Among the five mutants used, npq1 and npq1lut2 are severely qE-deficient. The npq1 mutant lacks Zea because of a defect in the structural gene for Vio deepoxidase and has a very small amount of qE, if any (19). The npq1lut2 mutant completely lacks both Zea and Lut and thus has no qE (20). The third mutant, lut2, lacks Lut but is capable of accumulating Zea in high light and shows somewhat less qE than the WT (17). In the other two mutants, npq2 and npq2lut2, the content of Zea remains constitutively high because of lack of Zea epoxidase, and both of them show qE levels comparable with the WT (17, 21). The distinct difference between these last two mutants is that only the npq2 mutant contains Lut (21).
The TA kinetics for the carotenoid mutants probed at 540 nm on excitation at 664 nm are shown in Fig. 4. Different kinetics were observed, in order of decreasing magnitude of qE, in npq2, npq2lut2, and lut2. For npq1 and npq1lut2, the kinetics detected were independent of the high light illumination. The result confirms that the kinetic differences exist exclusively when both qE and Zea are present. In addition, the experimental uncertainty does not allow us to detect kinetic differences of the amount expected for the npq1 mutant and corroborates information that supports a minor role for Lut in qE with respect to Zea (17, 21).
Fig 4.
The scaled TA kinetics measured at 540 nm on 664-nm excitation with the five mutants of A. thaliana with distinct carotenoid composition. For comparison, the data obtained from the WT are also shown. The coloring of lines and the data normalization are the same as in Fig. 4, and the decays recorded on each sample were vertically offset for clarity.
Discussion
The markedly different kinetics in response to the induction/relaxation of quenching are unique to the characteristic spectral region of S1 → Sn transition of Xans. Experiments on spinach thylakoids and a variety of well characterized A. thaliana mutants verified that the appearance of the kinetic difference is truly qE-related, because of the necessity of Zea, ΔpH, and PsbS for the existence of the faster kinetics observed exclusively in the quenched case. Furthermore, the Arabidopsis mutants showed a direct correlation between the magnitudes of the observed differences and the amount of qE induced (Fig. 3).
The correlation of our current observations with qE is further supported by the general consistency between the quenched and unquenched TA decay traces probed in the Chl bleaching region on excitation at 664 nm with previous fluorescence lifetime measurements performed under similar conditions. Because of differences in time resolution and detection window, the kinetics in this work cannot be directly compared with those reported (4, 22). For an approximate estimate, we chose to compare the relative amplitudes of the quenched and unquenched TA kinetics probed in the Chl at a time delay of 80 ps. The comparison shows that for the measurements on isolated pea thylakoids (22), the two normalized fluorescence decays at 682 nm differ by 4% with respect to the maximum amplitude. Similarly, for the isolated spinach thylakoids, the corresponding amplitude difference was 5% and 1% with high and low concentrations of Zea and antheraxanthin, respectively (4). The 5% difference in the amplitudes is consistent with the TA changes we see at 680 nm on excitation at 664 nm. In contrast, the indistinguishable kinetics probed in the same region after excitation at 683 nm suggest that Chls absorbing at different wavelengths have different responses to qE.
To identify the source of the faster initial decay measured under quenched conditions, we begin by examining the lifetime of the species giving rise to the new decay component, obtained either from fitting the calculated difference or by means of global lifetime analysis. The time constants obtained at 540 and 550 nm for both spinach and Arabidopsis, where the largest differences in amplitude were observed, yielded lifetimes ranging from ≈7 to 11 ps. Within the experimental uncertainty, this value is identical to the intrinsic S1 lifetime of carotenoids with 11 conjugated double bonds (23, 24). Measurements of the S1 lifetime of Zea in a variety of solvents produced a value of ≈9 ps (8, 25), whereas recently a value of 11 ps was obtained by using a reconstituted LHCII protein with Zea as the sole carotenoid species (26). Although several other carotenoids are present in thylakoid membranes, namely β-carotene, Lut, Vio, and neoxanthin with intrinsic S1 lifetimes of 9, 15, 23, and 35, respectively (8, 27–29), the data obtained from the mutants with different carotenoid composition show the generated kinetic difference is selective to Zea. Therefore, the most straightforward assignment for this unknown species is the S1 state of Zea. Accordingly, the decay profiles measured under quenched conditions originate from the ESA of Zea as a result of its S1 → Sn transition, together with the additional contribution from Chl ESA.
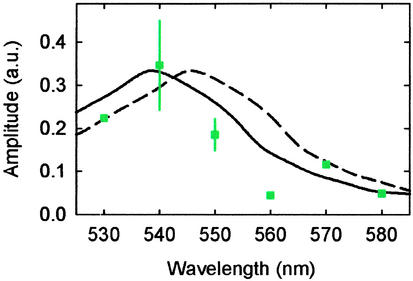
The spectrum of the kinetic differences further supports the assignment of the unknown species as the S1 state of Zea. Reconstruction of this spectrum involves first normalizing the unquenched kinetics probed at different wavelengths to a constant amplitude, based on the fact that the TA signal from Chl ESA in this region is rather flat (30). We then scaled the corresponding quenched kinetics with the value necessary to normalize the unquenched kinetics. The amplitudes of the quenched and unquenched kinetics at different wavelengths were obtained from the experimental profiles at a fixed delay time of 1 ps, and their difference was calculated and plotted in Fig. 5. Our reconstructed spectrum shows a maximum at ≈540 nm, whereas the experimental spectrum measured by using purified Zea in methanol at the corresponding delay time peaks at 547 nm (31). The calculated results, despite their large uncertainty owing to the small TA signals (on the order of 0.2 mOD or less) and their correspondingly much smaller differences, show reasonably good agreement with the experimental TA spectrum after the introduction of a 7-nm blue shift for ease of comparison (Fig. 5, solid line).
Fig 5.
The reconstructed spectrum (green squares) for the kinetic difference measured in spinach thylakoids at a delay time of 1 ps on 683-nm excitation. The error bars at 540 and 550 nm are given by the standard deviation of amplitudes determined in four and eight independent measurements, respectively. The solid line shows the TA spectrum obtained by using purified Zea in methanol at 1 ps (taken from ref. 31), and the dashed line is the same spectrum but blue-shifted by 7 nm. The amplitude of the spectrum was scaled to give the best match with the reconstructed spectrum.
The temporal and spectral characteristics of the kinetic differences, coupled with the necessity of Zea for the observation, strongly favor the S1 state of Zea as the most plausible and straightforward explanation for the faster kinetics observed under quenched conditions. Observation of the TA signal caused by the S1 → Sn transition of Zea could simply originate from direct optical excitation of the Zea. Because the electronic transition between the ground state and the S1 state is highly symmetry-forbidden for those carotenoids with more than nine conjugated double bonds (32), direct one-photon excitation of the S1 state of Zea should be extremely difficult and has never been reported. The possibility of this scenario was tested by using purified Zea dissolved in organic solvents (methanol and benzene). We failed to detect any TA signal at 540 or 550 nm on excitation at 664 and 683 nm, with the exception of a solvent response, even with a Zea concentration much higher than in thylakoid membranes (data not shown). The broad two-photon excitation spectrum of the S1 state determined for carotenoids with a similar number of double bonds (33) should ensure electronic resonance at both excitation wavelengths. Even though we cannot completely exclude an extreme change in the symmetry of Zea under qE conditions, we believe that the probability of direct excitation is exceedingly small.
Other experiments were performed to explore the possibility of direct optical excitation in isolated pigment–protein complexes or thylakoid membrane. Isolated, dark-adapted trimers of LHCII from WT (which lack Zea) and npq2 (which accumulate Zea in both the L2 and V1 binding sites) (34–36) plants of Arabidopsis were studied. The kinetics probed at 540 nm for both LHCII complexes are identical (data not shown) on excitation at 664 nm. Previous measurements of WT LHCII (in monomeric and trimeric form) showed that only Chl ESA exists in the region from 510 to 660 nm (30), therefore, simply introducing Zea into this pigment–protein complex cannot result in the kinetic difference we observed. Furthermore, the npq2 and npq2lut2 mutants, which accumulate Zea constitutively, display a faster decay only when qE is present. In addition, the measurements of LHCII and thylakoids under the unquenched condition all lack a lifetime component of ≈10 ps, which supports the notion that one-photon excitation of Zea is also unlikely when it is bound in a protein environment. All of the results are consistent with the idea that an electronic modification of some Zea molecules during the induction of qE, most likely in PsbS itself, is responsible for our observations (37).
A transient electrochromic shift of Zea (38), driven by the dipole moment change on excitation of neighboring Chls, could be the source of the kinetic differences observed; however, this seems unlikely for three reasons. The first is that the probe wavelengths from 530 to 580 nm are located on the red side of the spectrally red-shifted Zea absorption (37). For a given probe wavelength, λpr, and a symmetric S0 → S2 absorption band with an absorption maximum λm, a band shift <≈2|λpr − λm| will lead to increased absorption. Our observations contrast with this expected change because the initial amplitude of the quenched kinetics is smaller than the respective unquenched ones. Secondly, any electrochromic shift of the red-shifted Zea, either on the order previously observed (38) or significantly larger, is not consistent with our observation that the signal difference does not change sign over the probe range of 50 nm. Finally, the rapid phase of excitation equilibrium occurring in <5 ps (39) within the antenna of PSII makes it highly improbable that excitation remains sufficiently close to a Zea for the time scale of 10 ps that we observed.
The possible mechanisms for the formation of the excited state of Zea under conditions of qE that we observe are excitation energy transfer from Chls to the S1 state of Zea and the formation of a Zea–Chl heterodimer. In the case of energy transfer, the potential transfer process apparently needs to be fast enough to compete with the transfer processes to the other Chls in PSII. In addition, back transfer from Zea to Chl must be prevented if the 10-ps component represents the normal Zea S1 lifetime. Mutually canceling TA signals corresponding to the decay of the donor(s) and rise of the acceptor(s) may result in an undetectable rise corresponding to the transfer kinetics. In this case, identification of the exact donor(s), if possible, and its corresponding spectral properties would be crucial. For the heterodimer model, the influence of Xan–Chl interactions on the excited-state lifetimes was addressed by van Amerongen and van Grondelle (40), and a simple calculation showed that mixing the S1 state of Xan and the Qy state of Chl may hardly affect the S1 lifetime of Xan. In a qualitative sense, this mechanism could account for several features of our observations, namely the instantaneous onset of the kinetic difference with the pump pulse and the temporal and spectral features of the kinetic difference.
The nature of the kinetics differences in the Zea S1 → Sn spectral region strongly suggests that buildup of Zea population observed on optical excitation of Chl molecules in quenched thylakoid membranes occurs before excitation equilibrium within the entire PSII (39). Therefore, the observed excitation of Zea by means of Chl(s) does not represent the quenching of Chl singlet states during qE, because the quenching process occurs after excitation equilibrium in PSII. However, what we observe may be the kinetics of the quencher, which we identify as the S1 state of Zea or a Zea–Chl heterodimer. Because the lifetime of this species is ≈10 ps, and no further changes are seen in the Zea spectral region after ≈20 ps, it is reasonable to suggest that the rate of transfer from the Chls to the quencher is slow enough to preclude the observation of Zea excitation during qE. Modeling that incorporates structural and spectral information on the pigments involved, when available, may elucidate both the mechanism for the formation of the excited state of Zea and its role in qE.
Conclusions
We observed strikingly different TA kinetics in the spectral region between 530 and 580 nm that are correlated to the presence of both qE and Zea by using various thylakoid membranes with fully functional qE capability. Based on the lifetime and spectral characteristics, we attribute the observed differences to the excitation of the S1 state of Zea, which may result from energy transfer from excited Chl or formation of a Chl–Xan heterodimer. The response of Zea may represent the kinetics of the quenching species/complex involved in qE. The observations provide evidence for direct Zea involvement in the regulation of photosynthetic light harvesting and provide a key step in progress toward understanding the molecular mechanism of qE.
Acknowledgments
We thank Talila Golan for help with Arabidopsis growth, thylakoid preparations, and comments on the manuscript; Luca dall'Osto for kindly preparing and providing the WT and npq2 LHCII trimers; Prof. Roberto Bassi for comments on the manuscript; Hoffmann–La Roche for the generous gift of Zea; and Amer Deen for laser expertise. This work was supported by the director, Office of Science, Office of Basic Energy Sciences, Chemical Sciences Division, of the U.S. Department of Energy under Contract DE-AC03-76SF00098.
Abbreviations
NPQ, nonphotochemical quenching
PS, photosystem
qE, energy-dependent quenching
Chl, chlorophyll
Vio, violaxanthin
Zea, zeaxanthin
Xan, xanthophyll
TA, transient absorption
ESA, excited-state absorption
Lut, lutein
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Demmig B., Winter, K., Kruger, A. & Czygan, F.-C. (1987) Plant Physiol. 84, 218-224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Horton P., Ruban, A. V. & Walters, R. G. (1996) Annu. Rev. Plant Physiol. Plant Mol. Biol. 47, 655-684. [DOI] [PubMed] [Google Scholar]
- 3.Müller P., Li, X.-P. & Niyogi, K. K. (2001) Plant Physiol. 125, 1558-1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gilmore A. M., Hazlett, T. L. & Govindjee, (1995) Proc. Natl. Acad. Sci. USA 92, 2273-2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li X.-P., Björkman, O., Shih, C., Grossman, A. R., Rosenquist, M., Jansson, S. & Niyogi, K. K. (2000) Nature 403, 391-395. [DOI] [PubMed] [Google Scholar]
- 6.Gilmore A. M. (1997) Physiol. Plant. 99, 197-209. [Google Scholar]
- 7.Frank H. A., Farhoosh, R., Gebhard, R., Lugtenburg, J., Gosztola, D. & Wasielewski, M. R. (1993) Chem. Phys. Lett. 207, 88-92. [Google Scholar]
- 8.Frank H. A., Cua, A., Chynwat, V., Young, A., Gosztola, D. & Wasielewski, M. R. (1994) Photosynth. Res. 41, 389-395. [DOI] [PubMed] [Google Scholar]
- 9.Niyogi K. K. (1999) Annu. Rev. Plant Physiol. Plant Mol. Biol. 50, 333-359. [DOI] [PubMed] [Google Scholar]
- 10.Gilmore A. M., Shinkarev, V. P., Hazlett, T. L. & Govindjee, (1998) Biochemistry 37, 13582-13593. [DOI] [PubMed] [Google Scholar]
- 11.Müller-Moulé P., Conklin, P. L. & Niyogi, K. K. (2002) Plant Physiol. 128, 970-977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu Q. H., Ma, Y. Z. & Fleming, G. R. (2001) Chem. Phys. Lett. 338, 254-262. [Google Scholar]
- 13.Holzwarth A. R. (1996) in Biophysical Techniques in Photosynthesis, eds. Amesz, J. & Hoff, A. J. (Kluwer, Dordrecht, The Netherlands), pp. 75–92.
- 14.Gilmore A. M. & Yamamoto, H. Y. (1992) Proc. Natl. Acad. Sci. USA 89, 1899-1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li X.-P., Phippard, A., Pasari, J. & Niyogi, K. K. (2002) Funct. Plant Biol. 29, 1131-1139. [DOI] [PubMed] [Google Scholar]
- 16.Li X.-P., Müller-Moulé, P., Gilmore, A. M. & Niyogi, K. K. (2002) Proc. Natl. Acad. Sci. USA 99, 15222-15227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pogson B., Niyogi, K. K., Björkman, O. & DellaPenna, D. (1998) Proc. Natl. Acad. Sci. USA 94, 13324-13329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Niyogi K. K., Björkman, O. & Grossman, A. R. (1997) Proc. Natl. Acad. Sci. USA 94, 14162-14167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Havaux M. & Niyogi, K. K. (1999) Proc. Natl. Acad. Sci. USA 96, 8762-8767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Niyogi K. K., Shih, C., Chow, W. S., Pogson, B., DellaPenna, D. & Björkman, O. (2001) Photosynth. Res. 67, 139-145. [DOI] [PubMed] [Google Scholar]
- 21.Niyogi K. K., Grossman, A. R. & Björkman, O. (1998) Plant Cell 10, 1121-1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Richter M., Goss, R., Wagner, B. & Holzwarth, A. R. (1999) Biochemistry 38, 12718-12726. [DOI] [PubMed] [Google Scholar]
- 23.Koyama Y., Kuki, M., Andersson, P. O. & Gillbro, T. (1996) Photochem. Photobiol. B 63, 243-256. [Google Scholar]
- 24.Christensen R. L., Goyette, M., Gallagher, L., Duncan, J., DeCoster, B., Lugtenburg, J., Jansen, F. J. & van der Hoef, I. (1999) J. Phys. Chem. A 103, 2399-2407. [Google Scholar]
- 25.Debreczeny M. P., Wasielewski, M. R., Shinoda, S. & Osuka, A. (1997) J. Am. Chem. Soc. 119, 6407-6414. [Google Scholar]
- 26.Polívka T., Zigmantas, D., Sundström, V., Formaggio, E., Cinque, G. & Bassi, R. (2002) Biochemistry 41, 439-450. [DOI] [PubMed] [Google Scholar]
- 27.Frank H. A., Bautista, J. A., Josue, J., Pendon, Z., Hiller, R. G., Sharples, F. P., Gosztola, D. & Wasielewski, M. R. (2000) J. Phys. Chem. B 104, 4569-4577. [Google Scholar]
- 28.Polívka T., Herek, J. L., Zigmantas, D., Åkerlund, H.-E. & Sundström, V. (1999) Proc. Natl. Acad. Sci. USA 96, 4914-4917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wasielewski M. R. & Kispert, L. D. (1986) Chem. Phys. Lett. 128, 238-243. [Google Scholar]
- 30.Gradinaru C. C., van Stokkum, I. H. M., Pascal, A. A., van Grondelle, R. & van Amerongen, H. (2000) J. Phys. Chem. B 104, 9330-9342. [Google Scholar]
- 31.Billsten H. H., Zigmantas, D., Sundström, V. & Polívka, T. (2002) Chem. Phys. Lett. 355, 465-470. [Google Scholar]
- 32.Walla P. J., Linden, P. A., Ohta, K. & Fleming, G. R. (2002) J. Phys. Chem. B 106, 1909-1916. [Google Scholar]
- 33.Walla P. J., Yom, J., Krueger, B. P. & Fleming, G. R. (2000) J. Phys. Chem. B 104, 4799-4806. [Google Scholar]
- 34.Formaggio E., Cinque, G. & Bassi, R. (2001) J. Mol. Biol. 314, 1157-1166. [DOI] [PubMed] [Google Scholar]
- 35.Croce R., Weiss, S. & Bassi, R. (1999) J. Biol. Chem. 274, 29613-29623. [DOI] [PubMed] [Google Scholar]
- 36.Caffarri S., Croce, R., Breton, J. & Bassi, R. (2001) J. Biol. Chem. 276, 35924-35933. [DOI] [PubMed] [Google Scholar]
- 37.Ruban A. V., Pascal, A. A., Robert, B. & Horton, P. (2002) J. Biol. Chem. 277, 7785-7789. [DOI] [PubMed] [Google Scholar]
- 38.Herek J. L., Polívka, T., Pullerits, T., Fowler, G. J. S., Hunter, C. N. & Sundström, V. (1998) Biochemistry 37, 7057-7061. [DOI] [PubMed] [Google Scholar]
- 39.Vasil'ev S., Orth, P., Zouni, A., Owens, T. G. & Bruce, D. (2001) Proc. Natl. Acad. Sci. USA 98, 8602-8607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.van Amerongen H. & van Grondelle, R. (2001) J. Phys. Chem. B 105, 604-617. [Google Scholar]