Abstract
The gastrointestinal (GI) tract of poultry is densely populated with microorganisms which closely and intensively interact with the host and ingested feed. The gut microbiome benefits the host by providing nutrients from otherwise poorly utilized dietary substrates and modulating the development and function of the digestive and immune system. In return, the host provides a permissive habitat and nutrients for bacterial colonization and growth. Gut microbiome can be affected by diet, and different dietary interventions are used by poultry producers to enhance bird growth and reduce risk of enteric infection by pathogens. There also exist extensive interactions among members of the gut microbiome. A comprehensive understanding of these interactions will help develop new dietary or managerial interventions that can enhance bird growth, maximize host feed utilization, and protect birds from enteric diseases caused by pathogenic bacteria.
Keywords: AGPs, gut microbiome, interaction, nutrition, poultry, prebiotics, probiotics
Introduction
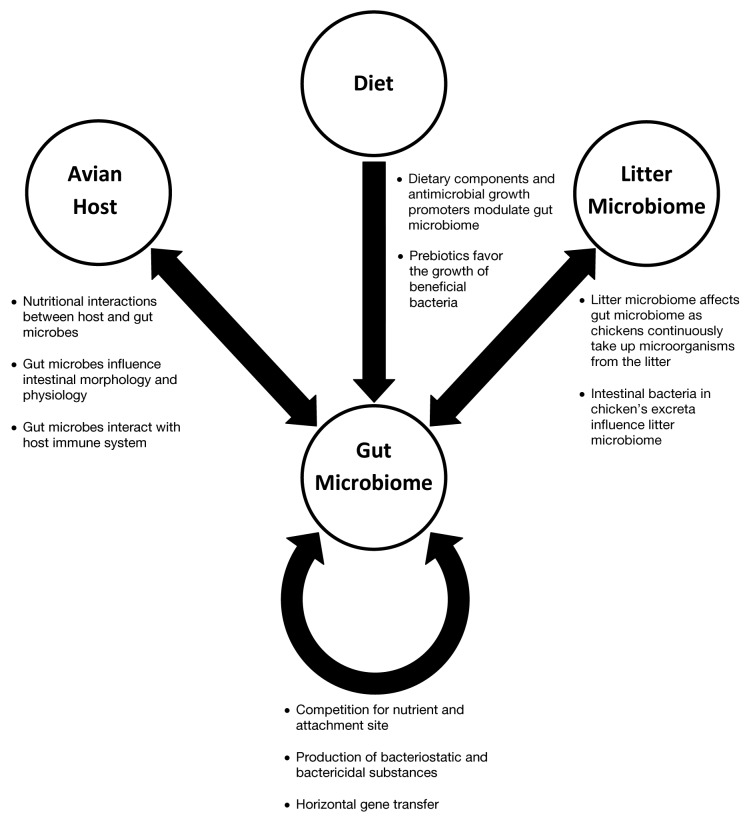
The gastrointestinal (GI) tract of poultry comes into contact with exogenous microorganisms immediately after hatch and thereafter it becomes a warm shelter for a complex microbiome consisting primarily anaerobic bacteria. As host grows, this microbiome becomes very diverse until it reaches a relatively stable yet dynamic state. Compared with other food animals that are mammalians, poultry (chicken, turkey, and duck) has a shorter GI tract and faster digesta transit. This anatomic feature selects a very different intestinal microbiome in poultry than in other food animals. There exist extensive interactions of this intestinal microbiome with poultry host and diet, and also interactions among individual gut microbes (Fig. 1), which have profound effects on poultry nutrition and health, and are therefore of great importance to poultry production. The objective of this review is to give an overview of the current state of knowledge of the microbe-host, microbe-diet, and microbe-microbe interactions in poultry (primarily chicken) gut.
Figure 1. Conceptual model of the interactions among gut microbiome, avian host, diet, and litter microbiome
Intestinal Microbiome of Poultry
The GI tract of poultry (e.g., chicken, turkey, and duck) consists of esophagus, crop, proventriculus, gizzard, small intestines (duodenum, jejunum, and ileum), cecum, colon, and cloaca. Relative to body length, the poultry GI tract is much shorter than that of mammalian animals. As such, the digesta passes through the entire GI tract faster in poultry than in mammalians. Although diet and feeding can have an effect on passage rate, the average whole tract transit time is less than 3.5 h.1 Such short retention time selects bacteria that can adhere to the mucosal layer and/or grow fast. However, the ceca, which are two blind pouches that have rather slow passage rate, are ideal habitats for a diverse microbiome that has considerable effect on host nutrition and health. The cecal microbiome is indeed the most studied intestinal microbiome of poultry.
The cecum of both chickens and turkeys harbors a complex microbiome, which is almost exclusively composed of bacteria.2 Early cultivation-based studies revealed low abundances of lactobacilli (>104/g colony forming units, CFUs) and clostridia (102–104/g CFUs) in the small intestines and high abundance (1010‒1011/g microscope counts) of anaerobic bacteria in the cecum of chickens.3,4 Identified bacteria included anaerobic Gram-negative cocci, facultative anaerobic cocci, and streptococci. Peptostreptococcus, Propionibacterium, Eubacterium, Bacteroides, and Clostridium were the major genera that were recovered from cecum by cultivation. Between 20–60% of the total cecal bacteria could be cultivated depending on the media used.3,4 Temporal changes were also observed as chicken aged.3 The first cultivation-based study on the intestinal microbiome of domesticated turkeys was reported in 1983.5 Most (77%) of the microbes were Gram-positive rods, followed by Gram-negative rods (14%), and Gram-positive cocci (9%). Bacteria of Eubacterium, Lactobacillus, Peptostreptococcus, Escherichia coli, Propionibacterium, and Bacteroides were isolated as predominant microorganisms. Although only revealing a limited number and diversity of bacteria, these early studies laid the foundation for microbiological studies of the intestinal microbiome in poultry.
Sequencing of 16S rRNA genes by first the Sanger sequencing technology and recently next-generation sequencing (NGS) technologies make it possible to comprehensively characterize the intestinal microbiome of poultry, and the sequence information has greatly expanded our knowledge on the bacterial diversity present in the intestinal tract, particularly the cecum, of chickens and turkeys.2 Through phylogenetic and statistical analysis of 16S rRNA gene sequences recovered from intestinal microbiome of both chickens and turkeys, a global bacterial census was created for poultry intestinal microbiome.2 Although this census is not complete, it serves as a phylogenetic framework for the bacterial diversity in the intestinal microbiome of both chickens and turkeys. In total, 13 phyla of bacteria were found, but Firmicutes, Bacteroidetes, and Proteobacteria accounted for most (> 90%) of the intestinal bacteria of chickens and turkeys. More than 900 species-equivalent operational taxonomic units (OTUs, defined at 0.03 phylogenetic distance) were found in chicken, and these OTUs represent 117 established genera of bacteria. For turkey, the census contained nearly 500 OTUs of bacteria within 69 existing genera. The most predominant genera found in both chicken and turkey were Clostridium, Ruminococcus, Lactobacillus, and Bacteroides, but with different distribution between the two bird species. Chickens and turkeys have distinct intestinal microbiomes, sharing only 16% similarity at species-equivalent level. Genetic and other factors (e.g., diet, digesta passage rate, and rearing environment) may be attributable to the difference in intestinal microbiome composition between chicken and turkey.
Interactions between Gut Microbiome and Host
Extensive interactions occur between poultry host and its gut microbiome (Fig. 1). These interactions are manifested particularly through exchange of nutrients, modulation of host gut morphology, physiology, and immunity.
Nutritional interactions
Most readily digestible dietary carbohydrates are digested and absorbed by the host in the proximal gut, leaving indigestible carbohydrates and residual digestible carbohydrates to bacteria residing the distal gut.6 Many intestinal bacteria can hydrolyze indigestible dietary polysaccharides, oligosaccharides, and disaccharides to their compositional sugars, which can then be fermented by intestinal bacteria, yielding short chain fatty acids (SCFAs), primarily acetate, propionate, and butyrate. The SCFAs can be utilized by the host as energy and carbon source.6-9 Such fermentation can be observed in most part of the avian gut (from crop to cecum) but primarily takes place in the cecum, which is densely populated with bacteria.10 The above fermentation increases as young birds grow. Cecal acetate, propionate, and butyrate are undetectable in 1-d-old broilers. As the cecal microbiome becomes established, these SCFAs reach high concentrations in 15-d-old broilers and remain stable afterwards.7 In the cecum, SCFAs are absorbed across the epithelium by passive diffusion and enter a variety of metabolic pathways.6 A previous study has provided evidence that SCFAs, especially butyrate, can serve as an important energy source for intestinal epithelial cells.11 In addition, it is reported that SCFAs can regulate intestinal blood flow, stimulate enterocyte growth and proliferation, regulate mucin production, and affect intestinal immune responses.6,9,12
Gut bacteria also contribute to host nitrogen metabolism. In birds, the intestinal and urogenital tracts meet at the cloaca where urine mixes with feces. Some urine may travel to the ceca due to the retrograde peristalsis in the rectum.13 Cecal bacteria can then catabolize uric acid to ammonia, which can be absorbed by the host and used to synthesize a few amino acids such as glutamine.14 Some of the dietary nitrogen is incorporated into bacterial cellular proteins. Therefore, gut bacteria themselves can be a source of amino acids.15 However, the majority of these bacterial proteins are lost to the host with the excretion of feces because most of the intestinal bacteria in birds reside in the cecum which does not have the ability to digest and absorb protein. Utilization of bacterial proteins is possible when chickens are housed on hard floors, where coprophagy (ingestion of feces) can occur and bacterial proteins can be digested and absorbed in proximal gut.8,14
A recent study demonstrated in vitro that the chicken intestinal microbiome required simple sugars and peptides for balanced growth whereas human intestinal microbiome preferred polysaccharides and proteins.16 Chicken microbiome also produced greater concentrations of SCFAs than human microbiome. Given the shorter digestive tract and faster digesta transit in poultry than in mammalian animals, more sugars and peptides may be available in the intestines of poultry than in the colon of human, which in turn selected an intestinal microbiome adapted to simple sugars and peptides.
Gut microbiome of poultry may also serve as a vitamin (especially B vitamins) supplier to its host.6,17 Similar as bacterial protein, most of the vitamins synthesized by gut bacteria are excreted with feces because they cannot be absorbed in the cecum.6 However, coprophagic birds may benefit from bacterial vitamin synthesis. This is evidenced by a greater vitamin requirement by chickens housed in wire cages, where coprophagy is prevented, than by chickens raised on hard floors.14
In a reciprocal manner, birds can also provide some nutrients to intestinal bacteria. For instance, mucins produced by goblet cells of the gut are important sources of carbon, nitrogen, and energy for some commensal and pathogenic bacteria.6,18 Few reports are available on mucin-utilizing bacteria of poultry origin, but studies on other animal species showed that a variety of bacteria can degrade mucins, including some species of Bifidobacterium,19,20 Bacteroides,6 and Akkermansia muciniphila.21 These bacteria are able to attach to the mucus layer and secrete specific enzymes for mucin degradation.18 Although mucin degradation by these bacteria has not been demonstrated in poultry yet, members of these species have been found in the gut of poultry, and it is reasonable to assume that some of the intestinal bacteria can and do degrade mucins in birds. The mucus layer of GI tract serves as a protective barrier for attached bacteria, and the constantly replenished mucin is an excellent source of nutrient for some gut bacteria. The ability to attach to and utilize mucin enables mucin-utilizing bacteria to outcompete other species on the surface of the mucus layer. As a result, these bacteria play an important role in enteric disease and health.
Despite the fact that birds and its intestinal inhabitants both benefit from the host-microbe nutrient exchange, some of the intestinal bacteria are sometimes found to compete with the host for nutrients. Gut microbiome has evolved with the host toward a symbiotic relationship, and in healthy birds direct competition for nutrients is limited, as most digestible nutrients are absorbed by the host in the small intestine, where bacterial density is low and bacterial utilization of nutrient is suppressed due to the low pH and short retention time.10 However, when bacteria overgrow in the small intestine under certain circumstances, nutrients are captured and utilized by bacteria before normal absorption by host can take place.22 In humans and mice, some intestinal bacteria can deconjugate bile acids thereby suppressing lipid digestion by the host.23,24 Clostridium perfringens, streptococci, and some of the bifidobacteria and lactobacilli isolated from chickens are able to deconjugate bile acids, but it remains to be determined to what extent bacterial deconjugation of bile acids decreases lipid digestion in chicken.
In modern broiler production industries, feed represents the major portion of production cost. Efficiency in converting feed into body mass is thus of critical concern for broiler producers. Because gut microbiome plays such an important role in feed digestion and absorption, attentions have been drawn to the associations between gut microbiome and host feed utilization efficiency. By using microbial profiling on broiler chickens across various feeding trails, Torok et al.25 were able to identify groups of bacteria that are potentially associated with broiler growth performance. Recently, more comprehensive analyses using a NGS technology also revealed certain bacteria that might be associated with growth performance of broiler chickens.26,27 Future studies are needed to determine if these bacteria are the cause or consequence of variations in feed utilization efficiency.
Microbiome affects intestinal morphology and physiology
The early post-hatch period is a critical stage for poultry growth and health as the new hatchling switches its nutrient source from the yolk to carbohydrate- and protein-based diet.28,29 In order to accommodate the rapid transition of nutrient source, the digestive organs of newly hatched poults undergo both anatomical and physiological changes and are the most rapidly developing organs during the early post-hatch period.30 The rapidly developed intestinal tract provides an ideal niche for microbial colonization. In the meantime, gut microbiome also plays an important role in intestinal development. Previous studies using germ-free (GF) chickens indicated that, comparing with conventional birds, the small intestine and cecum of GF birds had a reduced weight and a thinner wall.31,32 It has been suggested that SCFAs increases enterocyte growth and proliferation, which may partially explain the stimulating effect on intestinal growth by gut microbiome.33-35 This premise was supported by the study of Muramatsu et al.36 who reported that feeding fermentable carbohydrates, which can stimulate microbial fermentation and consequently SCFAs production, increased the gut weight in chicken.
Gut microbiome can also affect intestinal morphology of poultry. Intestinal villi are shorter and the crypts are shallower in GF birds or birds colonized with a low load of bacteria than in conventionally-raised birds.32,37 Dietary supplementation of three different probiotic species (Lactobacillus acidophilus, Bacillus subtilis, and Saccharomyces cerevisiae) also increased villus height in duodenum and villus height:crypt depth ratio in ileum of broilers.38 Similarly, it has been reported that dietary inclusion of prebiotics (e.g., fructooligosaccharide and mannanoligosaccharide) or fermented feed (e.g., fermented cottonseed, soybean, and rapeseed meal) also result in increased villus height and villus height:crypt depth ratio in the small intestine of chicken.39-43 Such morphology alterations are not likely a direct effect of these dietary supplements, but an indirect effect through the manipulation of gut microbiome structure.40 Intestinal morphology change can also be an outcome of infections caused by enteric pathogens. For instance, chickens with Eimeria spp/C. perfringens-induced necrotic enteritis had significantly reduced villus heights and villus height:crypt depth ratio in comparison to unchallenged controls or challenged chickens fed zinc bacitracin/monensin.44 Fasina et al.45 also demonstrated that mock-challenged chicks had significantly greater villus height, villus area, crypt depth, and villus height:crypt depth ratio than chicks challenged with Salmonella Typhimurium.
The activity of intestinal digestive enzymes can be affected by gut microbiome as well. Compared with GF chickens, conventional birds had greater activity of intestinal alkaline phosphatase.46 Diets that can induce changes in gut microbiome structure may also influence intestinal digestive enzyme activity. For instance, the activities of amylase and protease are elevated in broilers fed diets containing fermented cottonseed meal or fructooligosaccharides.40,43 Feeding broilers with fermented soybean meal instead of unfermented soybean meal increased the activities of protease, trypsin and lipase.41 It was concluded that these diets stimulate certain bacteria (e.g., Bifidobacterium and Lactobacillus) that can increase digestive enzyme activity, while suppressing some bacteria (e.g., Escherichia coli) that can either impair digestive enzyme secretion by damaging the villus and microvillus of mucosa or secrete proteolytic enzyme to degrade digestive enzymes.40
Microbiome and immunity
Colonization with microorganisms in the poultry gut occurs immediately after hatch and microbial succession follows until eventual establishment of a complex and dynamic microbiome.47 Digestive tract is the most important reservoir of microorganisms and extensive interaction between these non-self cells and host immune system takes place in the GI tract.
The inner surface of avian gut is coated with a gel-like mucus layer which is formed from mucin glycoprotein secreted by the goblet cells.48 This layer of mucin consists of an outer loose layer in which microorganisms can colonize and an inner compact layer which repels most bacteria.49 As a component of the intestinal mucosal innate immune system, the mucus layer prevents gut microorganisms from penetrating into the intestinal epithelium and serves as the first line of defense against infection.47 A good example is the different pathogenicity of Campylobacter jejuni in chicken and human. In vitro studies have shown that C. jejuni is able to adhere and invade both chicken and human intestinal epithelial cells.50,51 However, C. jejuni does not cause disease in chicken even though the chicken gut is heavily populated with this bacterium, whereas ingestion of C. jejuni-contaminated food may lead to severe gastroenteritis in human.52 It has been shown that the chicken intestinal mucus is able to attenuate C. jejuni virulence by inhibiting its ability to adhere and invade intestinal epithelial cells,51,53 whereas the human mucus-adherent E12 cell line was found to enhance C. jejuni adhesion and invasion.54 Thus, it has been suggested that the difference in intestinal mucus layer between chicken and human may contribute to the distinct pathogenesis of C. jejuni seen in these two hosts.55 Another study reported that sialylated mucin is more abundant in conventional chicks while sulfated mucin is more predominant in birds with a low bacterial load.37 Such change in mucin composition can be observed as early as 4 d post-hatch and indicates a potential role gut microbiome plays in regulating the establishment of mucus layer.37 By using a chicken necrotic enteritis model (coccidial infection followed by C. perfringens inoculation), Collier et al.56 also showed that infection with Eimeria acervulina and E. maxima enhanced host mucogenesis, which benefits the growth of mucolytic bacteria C. perfringens. Interestingly, as the severity of necrotic enteritis becomes greater, the expression of mucin gene (e.g., MUC2) decreases.48 Such decline in mucogenesis is probably due to the severe necrosis of the intestinal mucosa which results in extensive shedding of goblet cells.
Another important component of the innate immune system that functions in the avian gut is the antimicrobial peptides present on the intestinal epithelial surface.47 In poultry, the most important and well-studied antimicrobial peptides are β-defensins. They are small cationic peptides produced by avian macrophages, heterophils, and epithelial cells, and they can kill various intestinal pathogens by disrupting cell membrane permeability, which leads to cell lysis.57,58 Brisbin et al.47 indicated that Salmonella infection increased the expression of β-defensin genes in chicken, whereas administration of probiotics prior to Salmonella inoculation resulted in a decline in the gene expression of β-defensins. However, in a study conducted by Derache et al.,58 in vitro infection with live Salmonella Enteritidis did not increase the expression of β-defensin genes in avian epithelial cell cultures. A possible explanation for this discrepancy is that the increase in β-defensin gene expression after in vivo Salmonella challenge was due to the recruitment of heterophils to the gut in response to Salmonella infection. Interestingly, in their study, Derache et al.58 found that avian epithelial cells responded differently to live and heat-inactivated Salmonella Enteritidis: the expression of β-defensin gene AvBD2 in epithelial cells was increased after incubation with heat-inactivated Salmonella Enteritidis. Such finding indicates that live Salmonella Enteritidis may be able to block the induction of β-defensin gene expression in epithelial cells by a yet unknown mechanism and use this mechanism as a strategy to prevent itself from being eliminated by host immunity. Such a strategy may subsequently facilitate Salmonella Enteritidis to adhere to and invade the intestinal epithelium.
The cellular component of the avian innate system, such as macrophages and heterophils, also protects host from enteric infection. These cells can be found in peripheral circulation and the lamina propria. When intestinal microorganisms breach the intestinal epithelial barrier these immune cells are recruited to the site of infection, where they kill the invaders using a variety of strategies, such as phagocytosis and oxidative burst.47 The post-hatch colonization of avian gut by commensal microorganisms typically leads to a mild inflammation, which in turn results in macrophage and heterophil infiltration into the lamina propria.59 In addition, increased influx of macrophages and heterophils to the lamina propria and villus epithelium can be observed in chickens infected with enteric pathogens such as Salmonella Typhimurium and Salmonella Enteritidis.45,60 Although leukocyte infiltration is a defense mechanism against microbial infection during acute inflammatory response, it is worth noting that some pathogens are able to take advantage of this defense mechanism and use it to facilitate its pathogenicity. For instance, Salmonella is known as an intracellular pathogen which is able to survive and replicate in some host cells such as macrophages.61,62 The influx of macrophages to lamina propria and villus epithelium may therefore help spreading the pathogen to other organs and causing systemic infection.
The interaction between gut microbiome and host innate immune system can leads to subsequent adaptive immune response. B cells and T cells, which elicit antibody-mediated and cell-mediated immune responses, respectively, are the two primary types of lymphocytes that are of fundamental importance in the adaptive immune system. In avian gut, B cells and T cells can be found in organized lymphoid tissues (e.g., cecal tonsils, Peyer’s patches, and the bursa of Fabricius) and in more dispersed areas such as lamina propria and epithelium.47,63 It has been shown that manipulation of gut microbiome through administration of probiotics can influence antibody-mediated immune response. Birds receiving probiotics containing L. acidophilus, Bifidobacterium bifidum, and Streptococcus faecalis showed enhanced systemic antibody response to sheep red blood cells.64 In addition, intestinal IgG reactive to tetanus toxoid, and serum IgG and IgM reactive to tetanus toxoid and C. perfringens α-toxin were also increased in chickens fed the same probiotic product.65 Other studies suggest that various strains of lactobacilli have a stimulating effect on antibody-mediated response in chicken and such effect is dependent on the strain of Lactobacillus used and the type (layer- or meat-type) and age of the chicken.66,67 However, it remains to be elucidated how probiotics enhance antibody-mediated immune response. It is speculated that probiotics can stimulate the production of Th2 cytokines (e.g., IL-4 and IL-10), which may subsequently enhance the immune response mediated by antibody.64
Besides antibody-mediated response, cell-mediated immune response is also found to be affected by gut microbiome. By using germ-free, conventional, and gnotobiotic chickens, Mwangi et al.68 demonstrated that enteric microbiome complexity had a dramatic influence on the gut T cell repertoire. Brisbin et al.69 reported that various Lactobacillus species had the capacity to induce differential cytokine expression in T cells of chicken cecal tonsils which could contribute to intestinal homeostasis. In addition, it has been shown that after being challenged with Salmonella Typhimurium, broiler chickens treated with probiotics containing L. acidophilus, Bifidobacterium bifidum, and Streptococcus faecalis had a significant decrease in gene expression of IL-12 and IFN-γ, which are important cytokines in cell-mediated response against intracellular pathogens, in cecal tonsil.70 It should be noted that besides pathogens and probiotic strains, commensal bacteria, at least some of them, may also affect the immune response. Future studies are needed to determine the types of such commensal bacteria and their importance to immune response in poultry.
Interactions between Gut Microbiome and Diet
Dietary components affect gut microbiome
Diet has the greatest potential impact on the intestinal microbiome in poultry as dietary components that escape host digestion and absorption serve as the substrates for the growth of intestinal bacteria (Fig. 1). One of the most important impacts stems from the use of wheat-, barley-, or rye-based diets. These diets contain high levels of indigestible, water-soluble, non-starch polysaccharides, favor the proliferation of C. perfringens and predispose young chicks to necrotic enteritis, whereas diets poor in non-starch polysaccharides, such as corn-based diets, do not.71,72 It has been suggested that high level of non-starch polysaccharides leads to increased digesta viscosity, decreased digesta passage rate, and a decline in nutrient digestibility, which in turn favors the growth of C. perfringens.73,74 When compared with corn-based diet, wheat-based diets also affect a number of other bacteria.75,76 Even a small variation in dietary cereal grain composition can potentially affect the intestinal bacteria at strain level as demonstrated by Hammons et al.77 who showed that a standard corn-soybean ration favored Lactobacillus agilis type R5, whereas a ration high in wheat middlings favored L. agilis type R1. The source and level of dietary protein may also affect gut microbiome. It has been demonstrated that unlike soybean meal, which is widely used as a source of protein in poultry industry, fermented cottonseed meal as a protein source increases the population of lactobacilli and decreases the number of coliforms in cecum of broiler chickens.43 Diets with high percentages of animal protein (e.g., fishmeal) favors the growth of C. perfringens in the hind-gut of chicken and is considered as one of the predisposing factors of necrotic enteritis.78 In addition, it has been reported that C. perfringens was more abundant in the ileum of broiler chickens fed diet with animal fat (a mixture of lard and tallow) than chickens fed diet with soy oil, indicating that gut microbiome can also be influenced by dietary fat source.79
Various feed additives in poultry diet can influence gut microbiome and some of them are used to modulate intestinal microbiome to reduce enteric pathogens. Dietary enzymes, such as xylanase and β-glucanase, increase intestinal lactic acid bacteria and decrease the population of adverse and pathogenic bacteria such as E. coli.80 Dietary supplementation with xylanase and β-glucanase can also offer chickens some protection against necrotic enteritis as the enzymes break down the non-starch polysaccharides in the diet and reduce the digesta viscosity.81-83 Dietary inclusion of some plant-derived essential oils has also been used to protect chickens from enteric disease. For instance, plant-derived trans-cinnamaldehyde and eugenol were shown to be effective at reducing Salmonella Enteritidis colonization in 20-d-old broiler chickens.84 In addition, it has been demonstrated that a blend of essential oils containing thymol, carvacrol, eugenol, curcumin, and piperin reduced the colonization and proliferation of C. perfringens in the gut of broiler chickens.85
Antibiotic growth promoters
Another class of feed additives that has drastic effect on intestinal microbiome is antibiotic growth promoters (AGPs) (Fig. 1). AGPs are a group of dietary antibiotics used at sub-therapeutic levels to improve feed efficiency, increase animal growth, and maintain animal health.86 Dietary inclusion of AGPs has been practiced in food animal industry for more than 50 y.86,87 Although the precise mode of action of AGPs still remains to be elucidated, it is widely accepted that the growth-promoting effect of AGPs is primarily brought about through modulation of intestinal microbiome.88 Adverse and pathogenic bacteria in the GI tract of chicken, such as E. coli, Salmonella ssp., and C. perfringens, compete with the host for nutrient and may also damage the intestinal epithelium, which adversely affect the digestion and absorption function of the host.89 Inclusion of AGPs in poultry diet can inhibit the growth of enteric pathogens, reduce the incidence of disease and promote growth of the birds. However, due to the growing concern over widespread antibiotic resistance, there is a trend toward abolishing the use of AGPs. Most AGPs are banned in the European Union, and the United States has started to reduce the use of AGPs, with a possible ban on AGPs in the not-so-distant future.90 A negative outcome of banning AGPs is potential increase in incidence of disease in chicken. For instance, after the AGPs ban, C. perfringens-induced necrotic enteritis has become one of the most noticeable, emerging diseases of broiler chickens in Europe.91 Therefore, non-antibiotic alternatives which can control disease and promote growth of chicken are of great interest.
Prebiotics
Prebiotics are indigestible food ingredients which benefits the host animal by serving as a substrate for one or several beneficial bacteria present in the intestine (Fig. 1), granting these beneficial bacteria proliferative advantages over other bacteria.9,90,92 Most prebiotics are polysaccharides such as galactooligosaccharides (GOS) and fructooligosaccharides (FOS). It has been reported that dietary inclusion of GOS favored the growth of bifidobacteria in the GI tract of broiler chickens.93 Inclusion of FOS in an alfalfa molting diet significantly decreased cecal Salmonella Enteritidis counts in laying hens.94 Dietary supplementation with FOS also decreased C. perfringens and E. coli, and increased Lactobacillus diversity in chicken gut.95 Mannanoligosaccharides (MOS) is another prebiotic used in poultry industry. In addition to stimulating beneficial bacteria, MOS can also block pathogen binding to mannan receptors on the mucosal surface, thus hampering the attachment to and colonization of intestinal epithelia by certain pathogenic bacteria, particularly Salmonella Typhimurium.96
Interactions among Avian Gut Microbes
As in other microbiome, different members of the GI microbiome can have different interactions, such as competition, cooperation, and antagonism (Fig. 1). The interactions among different bacteria that are important to poultry production are overviewed.
Competition for nutrient and attachment site
Although the avian GI tract is an ideal habitat for microorganisms, it does not support unrestricted microbial growth or proliferation due to the limited availability of nutrient and space therein. Therefore, competition for these resources (i.e., nutrient and attachment site) among microorganisms is a common phenomenon in intestinal ecosystem.97 A good example is the competition for zinc among GI microbes. Zinc is an essential trace element required by both eukaryotic and prokaryotic cells and is involved in various cellular functions, such as enzymatic reactions and gene expression.98,99 Under low-zinc conditions, C. jejuni uses the high-affinity ZnuABC transporter to bring zinc into cell.100 In a recent study, Gielda and DiRita showed that both a wild-type C. jejuni strain and a znuABC- mutant strain of C. jejuni were able to colonize limited-mirobiota chicks at similar efficiencies, but only the wild-type C. jejuni strain was able to colonize conventional chicks.99 In the same study, it was also shown that the zinc level in cecal content was significantly lower in the conventional chicks than in the limited-microbiota chicks, suggesting that under low zinc conditions, C. jejuni lacking the high-affinity zinc uptake system was outcompeted by other bacteria present in the GI tract. The ZnuABC transporter system is found not only in C. jejuni but also in some pathogenic bacteria (e.g., Salmonella Typhimurium and E. coli), making it a potential target for development of broad-spectrum antimicrobials.99,101,102
In order to cause infections in birds, enteric pathogens need to first attach to and breach the intestinal epithelial barrier.103 In healthy birds, the commensal bacterial communities in the GI tract colonize intestinal mucosa and form a layer of bacteria covering the mucosal surface. By occupying a diverse array of adhering niches along the GI tract, this layer of dense and complex microbial communities can effectively block the attachment and subsequent colonization by most invading enteric pathogens.103,104 This phenomenon is called “competitive exclusion.”32 The GI tract of newly hatched chick is sterile, but is immediately colonized by microorganisms present in the surrounding environment.47 In the wild, the GI tract of the new hatchling is rapidly colonized by members of the gut microbiome from its mother’s feces and is therefore protected from pathogen invasion.105 However, in commercial poultry production the chicks are hatched in incubators and have no contact with hens. The surrounding environment is therefore relatively clean and usually has a microbial community distinct from the microbiome in a healthy adult chicken’s gut, which may lead to a delay in normal colonization and succession of intestinal microbiome.92,105 Enteric pathogens in the environment may thus have a greater opportunity to attach to and breach intestinal mucosal layer and cause infection in new hatchlings as a result of the absence of a normal gut microbiome. This may partially explain why newly hatched chicks are particularly vulnerable to enteric infections such as necrotic enteritis.92,103 In order to protect newly hatched chicks from enteric disease, competitive exclusion cultures have been used by poultry producers to help newly hatched chicks to rapidly establish a healthy gut microbiome. Competitive exclusion cultures are suspensions of intestinal contents obtained from healthy adult birds.106 By oral administration to newly hatched poults, competitive exclusion cultures have been shown to be effective in protecting new hatchings from being infected by some pathogens such as Salmonella and C. perfringens.107-109
Production of bacteriostatic and bactericidal substances
Another widely used strategy for some bacteria to gain competitive advantages is to produce bacteriostatic or bactericidal substances hostile to competitors. Previous studies have shown that lactic acid and other SCFAs produced by various commensal bacteria are inhibitory to certain pathogens. For instance, in vitro studies have shown that lactic acid bacteria ferment carbohydrates present in chickens’ feed and produce lactic acid, which lowers the pH in the surrounding environment and inhibits the growth of certain pathogens such as E. coli, Salmonella Typhimurium, and C. perfringens.110,111 An in vivo study also demonstrated a negative correlation between concentrations of SCFAs (in particular acetate, propionate, and butyrate) and abundance of the family Enterobacteriaceae in broilers’ ceca.7 Such a negative correlation was further substantiated by an in vitro study conducted by the same researchers. It was proposed that, in addition to lowering extracellular pH, SCFAs in undissociated form can diffuse freely across the bacterial cell membrane into the cell, where they dissociate, lowering the intracellular pH that inhibits some essential enzymes or metabolism.7,112,113
Certain bacteria can also produce bacteriocins to selectively inhibit the growth of other bacteria. Bacteriocins are a group of antimicrobial peptides produced by bacteria and archaea.114 Various strains of Lactobacillus salivarius isolated from chicken GI tract can produce bacteriocins which are inhibitory to some Gram-negative and Gram-positive bacteria such as Salmonella Enteritidis and C. jejuni.115-117 Bacteriocins produced by strains of Enterococcus faecium, Pediococcus pentosaceus, and Bacillus subtilis isolated from broiler chicken are able to inhibit C. perfringens and Listeria monocytogenes.118,119 In addition, it has been shown that several strains of E. faecium produce bacteriocins against the oocysts of poultry Eimeria spp.120 The inhibitory effect on various adverse bacteria and pathogens makes bacteriocin production a frequently considered trait in selection of probiotics. Nevertheless, it is worth noting that a variety of pathogenic bacteria (e.g., Staphylococcus aureus) also produce bacteriocin effective against competing bacteria.121
Horizontal gene transfer
Horizontal gene transfer is “the non-genealogical transmission of genetic material from one organism to another.”122 It is mediated by processes such as conjugation, transformation, and transduction and is an effective mechanism which contributes to bacterial diversification and facilitates bacterial adaptation to new environments.123,124 In modern poultry industry, litter, which contains bacteria excreted from chickens or turkey, is often used for multiple growth cycles. Once developed in the GI tract, antibiotic resistant bacteria can accumulate in the litter and recycle between the litter and GI tract over multiple growth cycles (Fig. 1). Such a practice can greatly increase the incidence of horizontal transfer of resistance genes and may contribute to the wide spread of antimicrobial resistance among adverse and pathogenic bacteria and is thus of particular interest.125-127 In addition, virulence genes can also be exchanged among poultry enteric pathogens, increasing the recipient’s pathogenicity.128 The predominant commensal intestinal microorganisms usually possess certain traits which enable them to outcompete other bacteria (especially adverse and pathogenic bacteria) and survive in the GI tract. These traits, however, may be acquired by pathogens via horizontal gene transfer, making those pathogens more competitive. On the other hand, commensal bacteria may also become pathogenic to the poultry host by obtaining virulence factors from pathogens.129 Therefore, caution should be taken when using direct-fed microbials such as probiotics.
Probiotics
Probiotics are live microbial feed supplement used by livestock and poultry producers to protect animals from enteric pathogen infection and improve animal health.92,130 The mode of action of probiotics can vary depending on the traits of the specific probiotic strains/species used, but most probiotics benefit the host through the following mechanisms: (1) inhibition of colonization by and proliferation of pathogenic bacteria through competition for nutrient and attachment site,103,104 (2) production of bacteriostatic and bactericidal substances against pathogens,7,111 (3) neutralizing enterotoxins,131 (4) enhancing gut barrier function,132 and (5) enhancing host immunity.132,133 The effect of different probiotics on chicken gut microbiome has been extensively investigated. Several lactobacilli strains have been shown to decrease the population of Salmonella, Campylobacter and some other non-beneficial bacterial groups in chicken gut.134,135 Molnár et al.136 reported that dietary supplementation of Bacillus subtilis significantly decreased E. coli population in the ileum of chicken. Another study demonstrated that spores of Bacillus licheniformis could prevent C. perfringens-induced necrotic enteritis in broiler chickens.131 Some strains of Clostridium butyricum are also potential probiotics that can be used in poultry production. This was demonstrated by Yang et al.132 who showed that C. butyricum HJCB998 significantly decreased cecal Salmonella and C. perfringens population while increasing Lactobacillus and Bifidobacterium populations in the cecum. The protective effect of multispecies probiotics has also been investigated. A multispecies probiotics containing Enterococcus faecium, Bifidobacterium animalis, Pediococcus acidilactici, L. salivarius, and Lactobacillus reuteri isolated from chicken gut decreased cecal coliform population.137 Ghareeb et al. also demonstrated that multispecies probiotics containing E. faecium, P. acidilactici, L. salivarius, and L. reuteri significantly reduced cecal colonization by C. jejuni, indicating that probiotic products can also be used to improve food safety by reducing the population of human pathogens, such as C. jejuni, in chicken.138
Poultry litter microorganisms influence gut microbiome
During their growth cycle, chickens continuously take up microorganisms from the surrounding environment. Poultry litter, the bedding material used in chicken houses, is usually mixed with chicken excreta and thus harbors a complex microbial community (mostly intestinal bacteria), and is thus of a potential impact on chicken gut microbiome (Fig. 1). Reusing litter for several growth cycles before a thorough clean-out is a management practice commonly used by poultry producers to reduce production cost and to help alleviate the challenges faced in litter disposal.139 Reuse of poultry litter influences the microbial community resident in the litter, which may in turn affect chicken gut microbiome. In a recent study, Cressman et al.140 demonstrated that more environmental bacteria were found in fresh litter, while more bacteria of intestinal origin resided in reused litter. It was also found in the same study that the ileal mucosal microbiome of chickens reared on fresh litter was dominated by Lactobacillus spp, whereas a group of unclassified Clostridiales were the dominating bacteria in chickens reared on reused litter. It was also reported that microorganisms in reused poultry litter can function as competitive exclusion culture and delay ileal mucosal colonization by C. perfringens during early post-hatch period.141 On the other hand, reused litter may also harbor disease-causing microorganisms from the previous flock and thus serves as a source of pathogens to the subsequent flock.142
Conclusions and Future Perspectives
Gut microbiome is now recognized as an essential component of the intestinal ecosystem and is referred to as a forgotten organ, which contributes to the wellbeing of animal host in a range of aspects, especially nutrition and disease resistance.143 Thanks to the new technologies such as NGS, it is now possible to gain a comprehensive knowledge of not only the compositional but also the metabolic characteristics of the gut microbiome, which allows researchers to better understand the interactions among gut microbiome, diet, and host. Manipulations of gut microbiome through dietary and managerial interventions have been used by poultry producers to enhance bird growth and reduce the incidence of disease. Undeniably, however, AGPs are still the most effective and cost-efficient strategy to do that job. Further studies on poultry gut microbiome and its interaction with host and diet can potentially provide the knowledge base needed to develop alternative strategies that can completely replace AGPs in modern poultry production.
Glossary
Abbreviations:
- GI
gastrointestinal
- CFUs
colony forming units
- NGS
next-generation sequencing
- SCFAs
short chain fatty acids
- GF
germ-free
- AGPs
antibiotic growth promoters
- GOS
galactooligosaccharides
- FOS
fructooligosaccharides
- MOS
mannanoligosaccharides
Disclosure of Potential Conflicts of Interest
No potential conflict of interest was disclosed.
References
- 1.Hughes RJ. Relationship between digesta transit time and apparent metabolisable energy value of wheat in chickens. Br Poult Sci. 2008;49:716–20. doi: 10.1080/00071660802449145. [DOI] [PubMed] [Google Scholar]
- 2.Wei S, Morrison M, Yu Z. Bacterial census of poultry intestinal microbiome. Poult Sci. 2013;92:671–83. doi: 10.3382/ps.2012-02822. [DOI] [PubMed] [Google Scholar]
- 3.Barnes EM, Mead GC, Barnum DA, Harry EG. The intestinal flora of the chicken in the period 2 to 6 weeks of age, with particular reference to the anaerobic bacteria. Br Poult Sci. 1972;13:311–26. doi: 10.1080/00071667208415953. [DOI] [PubMed] [Google Scholar]
- 4.Salanitro JP, Fairchilds IG, Zgornicki YD. Isolation, culture characteristics, and identification of anaerobic bacteria from the chicken cecum. Appl Microbiol. 1974;27:678–87. doi: 10.1128/am.27.4.678-687.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bedbury HP, Duke GE. Cecal microflora of turkeys fed low or high fiber diets: enumeration, identification, and determination of cellulolytic activity. Poult Sci. 1983;62:675–82. doi: 10.3382/ps.0620675. [DOI] [PubMed] [Google Scholar]
- 6.Hooper LV, Midtvedt T, Gordon JI. How host-microbial interactions shape the nutrient environment of the mammalian intestine. Annu Rev Nutr. 2002;22:283–307. doi: 10.1146/annurev.nutr.22.011602.092259. [DOI] [PubMed] [Google Scholar]
- 7.van Der Wielen PW, Biesterveld S, Notermans S, Hofstra H, Urlings BA, van Knapen F. Role of volatile fatty acids in development of the cecal microflora in broiler chickens during growth. Appl Environ Microbiol. 2000;66:2536–40. doi: 10.1128/AEM.66.6.2536-2540.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Koutsos E, Arias V. Intestinal ecology: Interactions among the gastrointestinal tract, nutrition, and the microflora. J Appl Poult Res. 2006;15:161–73. [Google Scholar]
- 9.Tellez G, Higgins S, Donoghue A, Hargis B. Digestive physiology and the role of microorganisms. J Appl Poult Res. 2006;15:136–44. [Google Scholar]
- 10.Rehman HU, Vahjen W, Awad WA, Zentek J. Indigenous bacteria and bacterial metabolic products in the gastrointestinal tract of broiler chickens. Arch Anim Nutr. 2007;61:319–35. doi: 10.1080/17450390701556817. [DOI] [PubMed] [Google Scholar]
- 11.Pryde SE, Duncan SH, Hold GL, Stewart CS, Flint HJ. The microbiology of butyrate formation in the human colon. FEMS Microbiol Lett. 2002;217:133–9. doi: 10.1111/j.1574-6968.2002.tb11467.x. [DOI] [PubMed] [Google Scholar]
- 12.Sanderson IR. Short chain fatty acid regulation of signaling genes expressed by the intestinal epithelium. J Nutr. 2004;134:2450S–4S. doi: 10.1093/jn/134.9.2450S. [DOI] [PubMed] [Google Scholar]
- 13.Denbow D. Gastrointestinal anatomy and physiology. Sturkie's avian physiology 2000; 5:299-325. [Google Scholar]
- 14.Vispo C, Karasov WH. The interaction of avian gut microbes and their host: An elusive symbiosis. In: Gastrointestinal Microbiology: Springer, 1997:116-155. [Google Scholar]
- 15.Metges CC. Contribution of microbial amino acids to amino acid homeostasis of the host. J Nutr. 2000;130:1857S–64S. doi: 10.1093/jn/130.7.1857S. [DOI] [PubMed] [Google Scholar]
- 16.Lei F, Yin Y, Wang Y, Deng B, Yu HD, Li L, Xiang C, Wang S, Zhu B, Wang X. Higher-level production of volatile fatty acids in vitro by chicken gut microbiotas than by human gut microbiotas as determined by functional analyses. Appl Environ Microbiol. 2012;78:5763–72. doi: 10.1128/AEM.00327-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.LeBlanc JG, Milani C, de Giori GS, Sesma F, van Sinderen D, Ventura M. Bacteria as vitamin suppliers to their host: a gut microbiota perspective. Curr Opin Biotechnol. 2013;24:160–8. doi: 10.1016/j.copbio.2012.08.005. [DOI] [PubMed] [Google Scholar]
- 18.Derrien M, van Passel MW, van de Bovenkamp JH, Schipper RG, de Vos WM, Dekker J. Mucin-bacterial interactions in the human oral cavity and digestive tract. Gut Microbes. 2010;1:254–68. doi: 10.4161/gmic.1.4.12778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ruas-Madiedo P, Gueimonde M, Fernández-García M, de los Reyes-Gavilán CG, Margolles A. Mucin degradation by Bifidobacterium strains isolated from the human intestinal microbiota. Appl Environ Microbiol. 2008;74:1936–40. doi: 10.1128/AEM.02509-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Killer J, Marounek M. Fermentation of mucin by bifidobacteria from rectal samples of humans and rectal and intestinal samples of animals. Folia Microbiol (Praha) 2011;56:85–9. doi: 10.1007/s12223-011-0022-4. [DOI] [PubMed] [Google Scholar]
- 21.Derrien M, Collado MC, Ben-Amor K, Salminen S, de Vos WM. The Mucin degrader Akkermansia muciniphila is an abundant resident of the human intestinal tract. Appl Environ Microbiol. 2008;74:1646–8. doi: 10.1128/AEM.01226-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fan X, Sellin JH. Review article: Small intestinal bacterial overgrowth, bile acid malabsorption and gluten intolerance as possible causes of chronic watery diarrhoea. Aliment Pharmacol Ther. 2009;29:1069–77. doi: 10.1111/j.1365-2036.2009.03970.x. [DOI] [PubMed] [Google Scholar]
- 23.Miyata M, Yamakawa H, Hamatsu M, Kuribayashi H, Takamatsu Y, Yamazoe Y. Enterobacteria modulate intestinal bile acid transport and homeostasis through apical sodium-dependent bile acid transporter (SLC10A2) expression. J Pharmacol Exp Ther. 2011;336:188–96. doi: 10.1124/jpet.110.171736. [DOI] [PubMed] [Google Scholar]
- 24.Kuribayashi H, Miyata M, Yamakawa H, Yoshinari K, Yamazoe Y. Enterobacteria-mediated deconjugation of taurocholic acid enhances ileal farnesoid X receptor signaling. Eur J Pharmacol. 2012;697:132–8. doi: 10.1016/j.ejphar.2012.09.048. [DOI] [PubMed] [Google Scholar]
- 25.Torok VA, Hughes RJ, Mikkelsen LL, Perez-Maldonado R, Balding K, MacAlpine R, Percy NJ, Ophel-Keller K. Identification and characterization of potential performance-related gut microbiotas in broiler chickens across various feeding trials. Appl Environ Microbiol. 2011;77:5868–78. doi: 10.1128/AEM.00165-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stanley D, Denman SE, Hughes RJ, Geier MS, Crowley TM, Chen H, Haring VR, Moore RJ. Intestinal microbiota associated with differential feed conversion efficiency in chickens. Appl Microbiol Biotechnol. 2012;96:1361–9. doi: 10.1007/s00253-011-3847-5. [DOI] [PubMed] [Google Scholar]
- 27.Stanley D, Geier MS, Denman SE, Haring VR, Crowley TM, Hughes RJ, Moore RJ. Identification of chicken intestinal microbiota correlated with the efficiency of energy extraction from feed. Vet Microbiol. 2013;164:85–92. doi: 10.1016/j.vetmic.2013.01.030. [DOI] [PubMed] [Google Scholar]
- 28.Gilbert ER, Williams PM, Ray WK, Li H, Emmerson DA, Wong EA, Webb KE., Jr. Proteomic evaluation of chicken brush-border membrane during the early posthatch period. J Proteome Res. 2010;9:4628–39. doi: 10.1021/pr1003533. [DOI] [PubMed] [Google Scholar]
- 29.Cheled-Shoval SL, Amit-Romach E, Barbakov M, Uni Z. The effect of in ovo administration of mannan oligosaccharide on small intestine development during the pre- and posthatch periods in chickens. Poult Sci. 2011;90:2301–10. doi: 10.3382/ps.2011-01488. [DOI] [PubMed] [Google Scholar]
- 30.Uni Z, Noy Y, Sklan D. Posthatch development of small intestinal function in the poult. Poult Sci. 1999;78:215–22. doi: 10.1093/ps/78.2.215. [DOI] [PubMed] [Google Scholar]
- 31.Furuse M, Okumura J. Nutritional and physiological characteristics in germ-free chickens. Comp Biochem Physiol A Physiol. 1994;109:547–56. doi: 10.1016/0300-9629(94)90193-7. [DOI] [PubMed] [Google Scholar]
- 32.Gabriel IL. M, Mallet SG, JF. Microflora of the digestive tract: Critical factors and consequences for poultry. Worlds Poult Sci J. 2006;62:499–512. [Google Scholar]
- 33.Le Blay G, Blottière HM, Ferrier L, Le Foll E, Bonnet C, Galmiche JP, Cherbut C. Short-chain fatty acids induce cytoskeletal and extracellular protein modifications associated with modulation of proliferation on primary culture of rat intestinal smooth muscle cells. Dig Dis Sci. 2000;45:1623–30. doi: 10.1023/A:1005529414765. [DOI] [PubMed] [Google Scholar]
- 34.Blottière HM, Buecher B, Galmiche JP, Cherbut C. Molecular analysis of the effect of short-chain fatty acids on intestinal cell proliferation. Proc Nutr Soc. 2003;62:101–6. doi: 10.1079/PNS2002215. [DOI] [PubMed] [Google Scholar]
- 35.Fukunaga T, Sasaki M, Araki Y, Okamoto T, Yasuoka T, Tsujikawa T, Fujiyama Y, Bamba T. Effects of the soluble fibre pectin on intestinal cell proliferation, fecal short chain fatty acid production and microbial population. Digestion. 2003;67:42–9. doi: 10.1159/000069705. [DOI] [PubMed] [Google Scholar]
- 36.Muramatsu T, Takasu O, Okumura J. Research note: fructose feeding increases lower gut weights in germ-free and conventional chicks. Poult Sci. 1993;72:1597–600. doi: 10.3382/ps.0721597. [DOI] [PubMed] [Google Scholar]
- 37.Forder RE, Howarth GS, Tivey DR, Hughes RJ. Bacterial modulation of small intestinal goblet cells and mucin composition during early posthatch development of poultry. Poult Sci. 2007;86:2396–403. doi: 10.3382/ps.2007-00222. [DOI] [PubMed] [Google Scholar]
- 38.Chae B, Ingale S, Kim J, Kim K, Sen S, Lee S, Khong C, Kim EK, Kwon IK. Effect of dietary supplementation of probiotics on performance, caecal microbiology and small intestinal morphology of broiler chickens. Animal Nutrition and Feed Technology. 2012;12:1–12. [Google Scholar]
- 39.Sonmez G, Eren M. Effects of supplementation of zinc bacitracin, mannan oligosaccharide, and probiotic into the broiler feeds on morphology of the small intestine. Vet.Fac.Dergisi Uludag Univ. 1999;18:125–38. [Google Scholar]
- 40.Xu ZR, Hu CH, Xia MS, Zhan XA, Wang MQ. Effects of dietary fructooligosaccharide on digestive enzyme activities, intestinal microflora and morphology of male broilers. Poult Sci. 2003;82:1030–6. doi: 10.1093/ps/82.6.1030. [DOI] [PubMed] [Google Scholar]
- 41.Feng J, Liu X, Xu ZR, Wang YZ, Liu JX. Effects of fermented soybean meal on digestive enzyme activities and intestinal morphology in broilers. Poult Sci. 2007;86:1149–54. doi: 10.1093/ps/86.6.1149. [DOI] [PubMed] [Google Scholar]
- 42.Chiang G, Lu W, Piao X, Hu J, Gong L, Thacker P. Effects of feeding solid-state fermented rapeseed meal on performance, nutrient digestibility, intestinal ecology and intestinal morphology of broiler chickens. Asian-Aust. J Anim Sci. 2010;23:263–71. [Google Scholar]
- 43.Sun H, Tang JW, Yao XH, Wu YF, Wang X, Feng J. Effects of dietary inclusion of fermented cottonseed meal on growth, cecal microbial population, small intestinal morphology, and digestive enzyme activity of broilers. Trop Anim Health Prod. 2013;45:987–93. doi: 10.1007/s11250-012-0322-y. [DOI] [PubMed] [Google Scholar]
- 44.Golder HM, Geier MS, Forder RE, Hynd PI, Hughes RJ. Effects of necrotic enteritis challenge on intestinal micro-architecture and mucin profile. Br Poult Sci. 2011;52:500–6. doi: 10.1080/00071668.2011.587183. [DOI] [PubMed] [Google Scholar]
- 45.Fasina YO, Hoerr FJ, McKee SR, Conner DE. Influence of Salmonella enterica serovar Typhimurium infection on intestinal goblet cells and villous morphology in broiler chicks. Avian Dis. 2010;54:841–7. doi: 10.1637/9055-090809-Reg.1. [DOI] [PubMed] [Google Scholar]
- 46.Palmer MF, Rolls BA. The activities of some metabolic enzymes in the intestines of germ-free and conventional chicks. Br J Nutr. 1983;50:783–90. doi: 10.1079/BJN19830149. [DOI] [PubMed] [Google Scholar]
- 47.Brisbin JT, Gong J, Sharif S. Interactions between commensal bacteria and the gut-associated immune system of the chicken. Anim Health Res Rev. 2008;9:101–10. doi: 10.1017/S146625230800145X. [DOI] [PubMed] [Google Scholar]
- 48.Forder RE, Nattrass GS, Geier MS, Hughes RJ, Hynd PI. Quantitative analyses of genes associated with mucin synthesis of broiler chickens with induced necrotic enteritis. Poult Sci. 2012;91:1335–41. doi: 10.3382/ps.2011-02062. [DOI] [PubMed] [Google Scholar]
- 49.Hansson GC, Johansson ME. The inner of the two Muc2 mucin-dependent mucus layers in colon is devoid of bacteria. Gut Microbes. 2010;1:51–4. doi: 10.4161/gmic.1.1.10470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Grant CC, Konkel ME, Cieplak W, Jr., Tompkins LS. Role of flagella in adherence, internalization, and translocation of Campylobacter jejuni in nonpolarized and polarized epithelial cell cultures. Infect Immun. 1993;61:1764–71. doi: 10.1128/iai.61.5.1764-1771.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Byrne CM, Clyne M, Bourke B. Campylobacter jejuni adhere to and invade chicken intestinal epithelial cells in vitro. Microbiology. 2007;153:561–9. doi: 10.1099/mic.0.2006/000711-0. [DOI] [PubMed] [Google Scholar]
- 52.Hermans D, Pasmans F, Messens W, Martel A, Van Immerseel F, Rasschaert G, Heyndrickx M, Van Deun K, Haesebrouck F. Poultry as a host for the zoonotic pathogen Campylobacter jejuni. Vector Borne Zoonotic Dis. 2012;12:89–98. doi: 10.1089/vbz.2011.0676. [DOI] [PubMed] [Google Scholar]
- 53.Alemka A, Whelan S, Gough R, Clyne M, Gallagher ME, Carrington SD, Bourke B. Purified chicken intestinal mucin attenuates Campylobacter jejuni pathogenicity in vitro. J Med Microbiol. 2010;59:898–903. doi: 10.1099/jmm.0.019315-0. [DOI] [PubMed] [Google Scholar]
- 54.Alemka A, Clyne M, Shanahan F, Tompkins T, Corcionivoschi N, Bourke B. Probiotic colonization of the adherent mucus layer of HT29MTXE12 cells attenuates Campylobacter jejuni virulence properties. Infect Immun. 2010;78:2812–22. doi: 10.1128/IAI.01249-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Alemka A, Corcionivoschi N, Bourke B. Defense and adaptation: the complex inter-relationship between Campylobacter jejuni and mucus. Front Cell Infect Microbiol. 2012;2:15. doi: 10.3389/fcimb.2012.00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Collier CT, Hofacre CL, Payne AM, Anderson DB, Kaiser P, Mackie RI, Gaskins HR. Coccidia-induced mucogenesis promotes the onset of necrotic enteritis by supporting Clostridium perfringens growth. Vet Immunol Immunopathol. 2008;122:104–15. doi: 10.1016/j.vetimm.2007.10.014. [DOI] [PubMed] [Google Scholar]
- 57.Jenssen H, Hamill P, Hancock RE. Peptide antimicrobial agents. Clin Microbiol Rev. 2006;19:491–511. doi: 10.1128/CMR.00056-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Derache C, Esnault E, Bonsergent C, Le Vern Y, Quéré P, Lalmanach AC. Differential modulation of beta-defensin gene expression by Salmonella Enteritidis in intestinal epithelial cells from resistant and susceptible chicken inbred lines. Dev Comp Immunol. 2009;33:959–66. doi: 10.1016/j.dci.2009.03.005. [DOI] [PubMed] [Google Scholar]
- 59.Crhanova M, Hradecka H, Faldynova M, Matulova M, Havlickova H, Sisak F, Rychlik I. Immune response of chicken gut to natural colonization by gut microflora and to Salmonella enterica serovar enteritidis infection. Infect Immun. 2011;79:2755–63. doi: 10.1128/IAI.01375-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Van Immerseel F, De Buck J, De Smet I, Mast J, Haesebrouck F, Ducatelle R. Dynamics of immune cell infiltration in the caecal lamina propria of chickens after neonatal infection with a Salmonella enteritidis strain. Dev Comp Immunol. 2002;26:355–64. doi: 10.1016/S0145-305X(01)00084-2. [DOI] [PubMed] [Google Scholar]
- 61.Buchmeier NA, Heffron F. Inhibition of macrophage phagosome-lysosome fusion by Salmonella typhimurium. Infect Immun. 1991;59:2232–8. doi: 10.1128/iai.59.7.2232-2238.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cirillo DM, Valdivia RH, Monack DM, Falkow S. Macrophage-dependent induction of the Salmonella pathogenicity island 2 type III secretion system and its role in intracellular survival. Mol Microbiol. 1998;30:175–88. doi: 10.1046/j.1365-2958.1998.01048.x. [DOI] [PubMed] [Google Scholar]
- 63.Bar-Shira E, Sklan D, Friedman A. Establishment of immune competence in the avian GALT during the immediate post-hatch period. Dev Comp Immunol. 2003;27:147–57. doi: 10.1016/S0145-305X(02)00076-9. [DOI] [PubMed] [Google Scholar]
- 64.Haghighi HR, Gong J, Gyles CL, Hayes MA, Sanei B, Parvizi P, Gisavi H, Chambers JR, Sharif S. Modulation of antibody-mediated immune response by probiotics in chickens. Clin Diagn Lab Immunol. 2005;12:1387–92. doi: 10.1128/CDLI.12.12.1387-1392.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Haghighi HR, Gong J, Gyles CL, Hayes MA, Zhou H, Sanei B, Chambers JR, Sharif S. Probiotics stimulate production of natural antibodies in chickens. Clin Vaccine Immunol. 2006;13:975–80. doi: 10.1128/CVI.00161-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Koenen ME, Kramer J, van der Hulst R, Heres L, Jeurissen SH, Boersma WJ. Immunomodulation by probiotic lactobacilli in layer- and meat-type chickens. Br Poult Sci. 2004;45:355–66. doi: 10.1080/00071660410001730851. [DOI] [PubMed] [Google Scholar]
- 67.Brisbin JT, Gong J, Orouji S, Esufali J, Mallick AI, Parvizi P, Shewen PE, Sharif S. Oral treatment of chickens with lactobacilli influences elicitation of immune responses. Clin Vaccine Immunol. 2011;18:1447–55. doi: 10.1128/CVI.05100-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mwangi WN, Beal RK, Powers C, Wu X, Humphrey T, Watson M, Bailey M, Friedman A, Smith AL. Regional and global changes in TCRalphabeta T cell repertoires in the gut are dependent upon the complexity of the enteric microflora. Dev Comp Immunol. 2010;34:406–17. doi: 10.1016/j.dci.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 69.Brisbin JT, Parvizi P, Sharif S. Differential cytokine expression in T-cell subsets of chicken caecal tonsils co-cultured with three species of Lactobacillus. Benef Microbes. 2012;3:205–10. doi: 10.3920/BM2012.0014. [DOI] [PubMed] [Google Scholar]
- 70.Haghighi HR, Abdul-Careem MF, Dara RA, Chambers JR, Sharif S. Cytokine gene expression in chicken cecal tonsils following treatment with probiotics and Salmonella infection. Vet Microbiol. 2008;126:225–33. doi: 10.1016/j.vetmic.2007.06.026. [DOI] [PubMed] [Google Scholar]
- 71.Annett CB, Viste JR, Chirino-Trejo M, Classen HL, Middleton DM, Simko E. Necrotic enteritis: effect of barley, wheat and corn diets on proliferation of Clostridium perfringens type A. Avian Pathol. 2002;31:598–601. doi: 10.1080/0307945021000024544. [DOI] [PubMed] [Google Scholar]
- 72.Jia W, Slominski BA, Bruce HL, Blank G, Crow G, Jones O. Effects of diet type and enzyme addition on growth performance and gut health of broiler chickens during subclinical Clostridium perfringens challenge. Poult Sci. 2009;88:132–40. doi: 10.3382/ps.2008-00204. [DOI] [PubMed] [Google Scholar]
- 73.Choct M, Hughes RJ, Wang J, Bedford MR, Morgan AJ, Annison G. Increased small intestinal fermentation is partly responsible for the anti-nutritive activity of non-starch polysaccharides in chickens. Br Poult Sci. 1996;37:609–21. doi: 10.1080/00071669608417891. [DOI] [PubMed] [Google Scholar]
- 74.Timbermont L, Haesebrouck F, Ducatelle R, Van Immerseel F. Necrotic enteritis in broilers: an updated review on the pathogenesis. Avian Pathol. 2011;40:341–7. doi: 10.1080/03079457.2011.590967. [DOI] [PubMed] [Google Scholar]
- 75.Torok VA, Ophel-Keller K, Loo M, Hughes RJ. Application of methods for identifying broiler chicken gut bacterial species linked with increased energy metabolism. Appl Environ Microbiol. 2008;74:783–91. doi: 10.1128/AEM.01384-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shakouri MD, Iji PA, Mikkelsen LL, Cowieson AJ. Intestinal function and gut microflora of broiler chickens as influenced by cereal grains and microbial enzyme supplementation. J Anim Physiol Anim Nutr (Berl) 2009;93:647–58. doi: 10.1111/j.1439-0396.2008.00852.x. [DOI] [PubMed] [Google Scholar]
- 77.Hammons S, Oh PL, Martínez I, Clark K, Schlegel VL, Sitorius E, Scheideler SE, Walter J. A small variation in diet influences the Lactobacillus strain composition in the crop of broiler chickens. Syst Appl Microbiol. 2010;33:275–81. doi: 10.1016/j.syapm.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 78.Drew MD, Syed NA, Goldade BG, Laarveld B, Van Kessel AG. Effects of dietary protein source and level on intestinal populations of Clostridium perfringens in broiler chickens. Poult Sci. 2004;83:414–20. doi: 10.1093/ps/83.3.414. [DOI] [PubMed] [Google Scholar]
- 79.Knarreborg A, Simon MA, Engberg RM, Jensen BB, Tannock GW. Effects of dietary fat source and subtherapeutic levels of antibiotic on the bacterial community in the ileum of broiler chickens at various ages. Appl Environ Microbiol. 2002;68:5918–24. doi: 10.1128/AEM.68.12.5918-5924.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rodríguez ML, Rebolé A, Velasco S, Ortiz LT, Treviño J, Alzueta C. Wheat- and barley-based diets with or without additives influence broiler chicken performance, nutrient digestibility and intestinal microflora. J Sci Food Agric. 2012;92:184–90. doi: 10.1002/jsfa.4561. [DOI] [PubMed] [Google Scholar]
- 81.Owens B, Tucker L, Collins MA, McCracken KJ. Effects of different feed additives alone or in combination on broiler performance, gut microflora and ileal histology. Br Poult Sci. 2008;49:202–12. doi: 10.1080/00071660802004890. [DOI] [PubMed] [Google Scholar]
- 82.Engberg RM, Hedemann MS, Steenfeldt S, Jensen BB. Influence of whole wheat and xylanase on broiler performance and microbial composition and activity in the digestive tract. Poult Sci. 2004;83:925–38. doi: 10.1093/ps/83.6.925. [DOI] [PubMed] [Google Scholar]
- 83.McDevitt R, Brooker J, Acamovic T, Sparks N. Necrotic enteritis; a continuing challenge for the poultry industry. Worlds Poult Sci J. 2006;62:221–48. doi: 10.1079/WPS200593. [DOI] [Google Scholar]
- 84.Kollanoor-Johny A, Mattson T, Baskaran SA, Amalaradjou MA, Babapoor S, March B, Valipe S, Darre M, Hoagland T, Schreiber D, et al. Reduction of Salmonella enterica serovar enteritidis colonization in 20-day-old broiler chickens by the plant-derived compounds trans-cinnamaldehyde and eugenol. Appl Environ Microbiol. 2012;78:2981–7. doi: 10.1128/AEM.07643-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mitsch P, Zitterl-Eglseer K, Köhler B, Gabler C, Losa R, Zimpernik I. The effect of two different blends of essential oil components on the proliferation of Clostridium perfringens in the intestines of broiler chickens. Poult Sci. 2004;83:669–75. doi: 10.1093/ps/83.4.669. [DOI] [PubMed] [Google Scholar]
- 86.Danzeisen JL, Kim HB, Isaacson RE, Tu ZJ, Johnson TJ. Modulations of the chicken cecal microbiome and metagenome in response to anticoccidial and growth promoter treatment. PLoS One. 2011;6:e27949. doi: 10.1371/journal.pone.0027949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lin J, Hunkapiller AA, Layton AC, Chang YJ, Robbins KR. Response of intestinal microbiota to antibiotic growth promoters in chickens. Foodborne Pathog Dis. 2013;10:331–7. doi: 10.1089/fpd.2012.1348. [DOI] [PubMed] [Google Scholar]
- 88.Dibner JJ, Richards JD. Antibiotic growth promoters in agriculture: history and mode of action. Poult Sci. 2005;84:634–43. doi: 10.1093/ps/84.4.634. [DOI] [PubMed] [Google Scholar]
- 89.Gunal M, Yayli G, Kaya O, Karahan N, Sulak O. The effects of antibiotic growth promoter, probiotic or organic acid supplementation on performance, intestinal microflora and tissue of broilers. Int J Poult Sci. 2006;5:149–55. doi: 10.3923/ijps.2006.149.155. [DOI] [Google Scholar]
- 90.Van Immerseel F, De Buck J, Pasmans F, Huyghebaert G, Haesebrouck F, Ducatelle R. Clostridium perfringens in poultry: an emerging threat for animal and public health. Avian Pathol. 2004;33:537–49. doi: 10.1080/03079450400013162. [DOI] [PubMed] [Google Scholar]
- 91.Van Immerseel F, Rood JI, Moore RJ, Titball RW. Rethinking our understanding of the pathogenesis of necrotic enteritis in chickens. Trends Microbiol. 2009;17:32–6. doi: 10.1016/j.tim.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 92.Dahiya J, Wilkie D, Van Kessel A, Drew M. Potential strategies for controlling necrotic enteritis in broiler chickens in post-antibiotic era. Anim Feed Sci Technol. 2006;129:60–88. doi: 10.1016/j.anifeedsci.2005.12.003. [DOI] [Google Scholar]
- 93.Jung SJ, Houde R, Baurhoo B, Zhao X, Lee BH. Effects of galacto-oligosaccharides and a Bifidobacteria lactis-based probiotic strain on the growth performance and fecal microflora of broiler chickens. Poult Sci. 2008;87:1694–9. doi: 10.3382/ps.2007-00489. [DOI] [PubMed] [Google Scholar]
- 94.Donalson LM, McReynolds JL, Kim WK, Chalova VI, Woodward CL, Kubena LF, Nisbet DJ, Ricke SC. The influence of a fructooligosaccharide prebiotic combined with alfalfa molt diets on the gastrointestinal tract fermentation, Salmonella enteritidis infection, and intestinal shedding in laying hens. Poult Sci. 2008;87:1253–62. doi: 10.3382/ps.2007-00166. [DOI] [PubMed] [Google Scholar]
- 95.Kim GB, Seo YM, Kim CH, Paik IK. Effect of dietary prebiotic supplementation on the performance, intestinal microflora, and immune response of broilers. Poult Sci. 2011;90:75–82. doi: 10.3382/ps.2010-00732. [DOI] [PubMed] [Google Scholar]
- 96.Spring P, Wenk C, Dawson KA, Newman KE. The effects of dietary mannaoligosaccharides on cecal parameters and the concentrations of enteric bacteria in the ceca of salmonella-challenged broiler chicks. Poult Sci. 2000;79:205–11. doi: 10.1093/ps/79.2.205. [DOI] [PubMed] [Google Scholar]
- 97.Soler JJ, Martín-Vivaldi M, Peralta-Sánchez JM, Ruiz-Rodríguez M. Antibiotic-producing bacteria as a possible defence of birds against pathogenic microorganisms. Open Ornithology Journal. 2010;3:93–100. [Google Scholar]
- 98.Berg JM, Shi Y. The galvanization of biology: a growing appreciation for the roles of zinc. Science. 1996;271:1081–5. doi: 10.1126/science.271.5252.1081. [DOI] [PubMed] [Google Scholar]
- 99.Gielda LM, DiRita VJ. Zinc competition among the intestinal microbiota. MBio. 2012;3:e00171–12. doi: 10.1128/mBio.00171-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Davis LM, Kakuda T, DiRita VJ. A Campylobacter jejuni znuA orthologue is essential for growth in low-zinc environments and chick colonization. J Bacteriol. 2009;191:1631–40. doi: 10.1128/JB.01394-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Patzer SI, Hantke K. The zinc-responsive regulator Zur and its control of the znu gene cluster encoding the ZnuABC zinc uptake system in Escherichia coli. J Biol Chem. 2000;275:24321–32. doi: 10.1074/jbc.M001775200. [DOI] [PubMed] [Google Scholar]
- 102.Campoy S, Jara M, Busquets N, Pérez De Rozas AM, Badiola I, Barbé J. Role of the high-affinity zinc uptake znuABC system in Salmonella enterica serovar typhimurium virulence. Infect Immun. 2002;70:4721–5. doi: 10.1128/IAI.70.8.4721-4725.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lan Y, Verstegen M, Tamminga S, Williams B. The role of the commensal gut microbial community in broiler chickens. Worlds Poult Sci J. 2005;61:95–104. doi: 10.1079/WPS200445. [DOI] [Google Scholar]
- 104.Lawley TD, Walker AW. Intestinal colonization resistance. Immunology. 2013;138:1–11. doi: 10.1111/j.1365-2567.2012.03616.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lutful Kabir SM. The role of probiotics in the poultry industry. Int J Mol Sci. 2009;10:3531–46. doi: 10.3390/ijms10083531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Nisbet D. Defined competitive exclusion cultures in the prevention of enteropathogen colonisation in poultry and swine. Antonie Van Leeuwenhoek. 2002;81:481–6. doi: 10.1023/A:1020541603877. [DOI] [PubMed] [Google Scholar]
- 107.Hollister AG, Corrier DE, Nisbet DJ, DeLoach JR. Effects of chicken-derived cecal microorganisms maintained in continuous culture on cecal colonization by Salmonella typhimurium in turkey poults. Poult Sci. 1999;78:546–9. doi: 10.1093/ps/78.4.546. [DOI] [PubMed] [Google Scholar]
- 108.McReynolds J, Moore R, McElroy A, Hargis B, Caldwell D. Evaluation of a competitive exclusion culture and megan vac 1 on Salmonella Typhimurium colonization in neonatal broiler chickens. J Appl Poult Res. 2007;16:456–63. [Google Scholar]
- 109.Craven SE, Stern NJ, Cox NA, Bailey JS, Berrang M. Cecal carriage of Clostridium perfringens in broiler chickens given Mucosal Starter Culture. Avian Dis. 1999;43:484–90. doi: 10.2307/1592646. [DOI] [PubMed] [Google Scholar]
- 110.Hinton A, Jr., Corrier D, DeLoach J. In vitro inhibition of Salmonella Typhimurium and Escherichia coli 0157: H7 by an anaerobic gram-positive coccus isolated from the cecal contents of adult chickens. J Food Prot. 1992;55 doi: 10.4315/0362-028X-55.3.162. [DOI] [PubMed] [Google Scholar]
- 111.Murry A, Jr., Hinton A, Jr., Morrison H. Inhibition of growth of Escherichia coli, Salmonella Typhimurium, and Clostridia perfringens on chicken feed media by Lactobacillus salivarius and Lactobacillus plantarum. Int J Poult Sci. 2004;3:603–7. doi: 10.3923/ijps.2004.603.607. [DOI] [Google Scholar]
- 112.Van Immerseel F, Fievez V, de Buck J, Pasmans F, Martel A, Haesebrouck F, Ducatelle R. Microencapsulated short-chain fatty acids in feed modify colonization and invasion early after infection with Salmonella enteritidis in young chickens. Poult Sci. 2004;83:69–74. doi: 10.1093/ps/83.1.69. [DOI] [PubMed] [Google Scholar]
- 113.Van Immerseel F, Russell JB, Flythe MD, Gantois I, Timbermont L, Pasmans F, Haesebrouck F, Ducatelle R. The use of organic acids to combat Salmonella in poultry: a mechanistic explanation of the efficacy. Avian Pathol. 2006;35:182–8. doi: 10.1080/03079450600711045. [DOI] [PubMed] [Google Scholar]
- 114.Dobson A, Cotter PD, Ross RP, Hill C. Bacteriocin production: a probiotic trait? Appl Environ Microbiol. 2012;78:1–6. doi: 10.1128/AEM.05576-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Stern NJ, Svetoch EA, Eruslanov BV, Perelygin VV, Mitsevich EV, Mitsevich IP, Pokhilenko VD, Levchuk VP, Svetoch OE, Seal BS. Isolation of a Lactobacillus salivarius strain and purification of its bacteriocin, which is inhibitory to Campylobacter jejuni in the chicken gastrointestinal system. Antimicrob Agents Chemother. 2006;50:3111–6. doi: 10.1128/AAC.00259-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Svetoch EA, Eruslanov BV, Levchuk VP, Perelygin VV, Mitsevich EV, Mitsevich IP, Stepanshin J, Dyatlov I, Seal BS, Stern NJ. Isolation of Lactobacillus salivarius 1077 (NRRL B-50053) and characterization of its bacteriocin, including the antimicrobial activity spectrum. Appl Environ Microbiol. 2011;77:2749–54. doi: 10.1128/AEM.02481-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Messaoudi S, Kergourlay G, Dalgalarrondo M, Choiset Y, Ferchichi M, Prévost H, Pilet MF, Chobert JM, Manai M, Dousset X. Purification and characterization of a new bacteriocin active against Campylobacter produced by Lactobacillus salivarius SMXD51. Food Microbiol. 2012;32:129–34. doi: 10.1016/j.fm.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 118.Teo AY, Tan HM. Inhibition of Clostridium perfringens by a novel strain of Bacillus subtilis isolated from the gastrointestinal tracts of healthy chickens. Appl Environ Microbiol. 2005;71:4185–90. doi: 10.1128/AEM.71.8.4185-4190.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Shin MS, Han SK, Ji AR, Kim KS, Lee WK. Isolation and characterization of bacteriocin-producing bacteria from the gastrointestinal tract of broiler chickens for probiotic use. J Appl Microbiol. 2008;105:2203–12. doi: 10.1111/j.1365-2672.2008.03935.x. [DOI] [PubMed] [Google Scholar]
- 120.Strompfová V, Lauková A, Marcinákova M, Vasilková Z. Testing of probiotic and bacteriocin-producing lactic acid bacteria towards Eimeria sp. Pol J Vet Sci. 2010;13:389–91. [PubMed] [Google Scholar]
- 121.Wladyka B, Wielebska K, Wloka M, Bochenska O, Dubin G, Dubin A, Mak P. Isolation, biochemical characterization, and cloning of a bacteriocin from the poultry-associated Staphylococcus aureus strain CH-91. Appl Microbiol Biotechnol. 2013;97:7229–39. doi: 10.1007/s00253-012-4578-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Goldenfeld N, Woese C. Biology’s next revolution. Nature. 2007;445:369. doi: 10.1038/445369a. [DOI] [PubMed] [Google Scholar]
- 123.Boto L. Horizontal gene transfer in evolution: facts and challenges. Proc Biol Sci. 2010;277:819–27. doi: 10.1098/rspb.2009.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Holmgren L. Horizontal gene transfer: you are what you eat. Biochem Biophys Res Commun. 2010;396:147–51. doi: 10.1016/j.bbrc.2010.04.026. [DOI] [PubMed] [Google Scholar]
- 125.Dhanarani TS, Shankar C, Park J, Dexilin M, Kumar RR, Thamaraiselvi K. Study on acquisition of bacterial antibiotic resistance determinants in poultry litter. Poult Sci. 2009;88:1381–7. doi: 10.3382/ps.2008-00327. [DOI] [PubMed] [Google Scholar]
- 126.Wimalarathna HM, Richardson JF, Lawson AJ, Elson R, Meldrum R, Little CL, Maiden MC, McCarthy ND, Sheppard SK. Widespread acquisition of antimicrobial resistance among Campylobacter isolates from UK retail poultry and evidence for clonal expansion of resistant lineages. BMC Microbiol. 2013;13:160. doi: 10.1186/1471-2180-13-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.You Y, Hilpert M, Ward MJ. Identification of Tet45, a tetracycline efflux pump, from a poultry-litter-exposed soil isolate and persistence of tet(45) in the soil. J Antimicrob Chemother. 2013;68:1962–9. doi: 10.1093/jac/dkt127. [DOI] [PubMed] [Google Scholar]
- 128.Johnson TJ, Thorsness JL, Anderson CP, Lynne AM, Foley SL, Han J, Fricke WF, McDermott PF, White DG, Khatri M, et al. Horizontal gene transfer of a ColV plasmid has resulted in a dominant avian clonal type of Salmonella enterica serovar Kentucky. PLoS One. 2010;5:e15524. doi: 10.1371/journal.pone.0015524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.van Reenen CA, Dicks LM. Horizontal gene transfer amongst probiotic lactic acid bacteria and other intestinal microbiota: what are the possibilities? A review. Arch Microbiol. 2011;193:157–68. doi: 10.1007/s00203-010-0668-3. [DOI] [PubMed] [Google Scholar]
- 130.Lutful Kabir SM. The role of probiotics in the poultry industry. Int J Mol Sci. 2009;10:3531–46. doi: 10.3390/ijms10083531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Knap I, Lund B, Kehlet AB, Hofacre C, Mathis G. Bacillus licheniformis prevents necrotic enteritis in broiler chickens. Avian Dis. 2010;54:931–5. doi: 10.1637/9106-101509-ResNote.1. [DOI] [PubMed] [Google Scholar]
- 132.Yang CM, Cao GT, Ferket PR, Liu TT, Zhou L, Zhang L, Xiao YP, Chen AG. Effects of probiotic, Clostridium butyricum, on growth performance, immune function, and cecal microflora in broiler chickens. Poult Sci. 2012;91:2121–9. doi: 10.3382/ps.2011-02131. [DOI] [PubMed] [Google Scholar]
- 133.Ng SC, Hart AL, Kamm MA, Stagg AJ, Knight SC. Mechanisms of action of probiotics: recent advances. Inflamm Bowel Dis. 2009;15:300–10. doi: 10.1002/ibd.20602. [DOI] [PubMed] [Google Scholar]
- 134.Nakphaichit M, Thanomwongwattana S, Phraephaisarn C, Sakamoto N, Keawsompong S, Nakayama J, Nitisinprasert S. The effect of including Lactobacillus reuteri KUB-AC5 during post-hatch feeding on the growth and ileum microbiota of broiler chickens. Poult Sci. 2011;90:2753–65. doi: 10.3382/ps.2011-01637. [DOI] [PubMed] [Google Scholar]
- 135.Pascual M, Hugas M, Badiola JI, Monfort JM, Garriga M. Lactobacillus salivarius CTC2197 prevents Salmonella enteritidis colonization in chickens. Appl Environ Microbiol. 1999;65:4981–6. doi: 10.1128/aem.65.11.4981-4986.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Molnár AK, Podmaniczky B, Kürti P, Tenk I, Glávits R, Virág G, Szabó Z. Effect of different concentrations of Bacillus subtilis on growth performance, carcase quality, gut microflora and immune response of broiler chickens. Br Poult Sci. 2011;52:658–65. doi: 10.1080/00071668.2011.636029. [DOI] [PubMed] [Google Scholar]
- 137.Mountzouris KC, Tsitrsikos P, Palamidi I, Arvaniti A, Mohnl M, Schatzmayr G, Fegeros K. Effects of probiotic inclusion levels in broiler nutrition on growth performance, nutrient digestibility, plasma immunoglobulins, and cecal microflora composition. Poult Sci. 2010;89:58–67. doi: 10.3382/ps.2009-00308. [DOI] [PubMed] [Google Scholar]
- 138.Ghareeb K, Awad WA, Mohnl M, Porta R, Biarnés M, Böhm J, Schatzmayr G. Evaluating the efficacy of an avian-specific probiotic to reduce the colonization of Campylobacter jejuni in broiler chickens. Poult Sci. 2012;91:1825–32. doi: 10.3382/ps.2012-02168. [DOI] [PubMed] [Google Scholar]
- 139.Coufal CD, Chavez C, Niemeyer PR, Carey JB. Measurement of broiler litter production rates and nutrient content using recycled litter. Poult Sci. 2006;85:398–403. doi: 10.1093/ps/85.3.398. [DOI] [PubMed] [Google Scholar]
- 140.Cressman MD, Yu Z, Nelson MC, Moeller SJ, Lilburn MS, Zerby HN. Interrelations between the microbiotas in the litter and in the intestines of commercial broiler chickens. Appl Environ Microbiol. 2010;76:6572–82. doi: 10.1128/AEM.00180-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Wei S, Gutek A, Lilburn M, Yu Z. Abundance of pathogens in the gut and litter of broiler chickens as affected by bacitracin and litter management. Vet Microbiol. 2013;166:595–601. doi: 10.1016/j.vetmic.2013.06.006. [DOI] [PubMed] [Google Scholar]
- 142.Stanley VG, Gray C, Daley M, Krueger WF, Sefton AE. An alternative to antibiotic-based drugs in feed for enhancing performance of broilers grown on Eimeria spp.-infected litter. Poult Sci. 2004;83:39–44. doi: 10.1093/ps/83.1.39. [DOI] [PubMed] [Google Scholar]
- 143.O’Hara AM, Shanahan F. The gut flora as a forgotten organ. EMBO Rep. 2006;7:688–93. doi: 10.1038/sj.embor.7400731. [DOI] [PMC free article] [PubMed] [Google Scholar]