Abstract
Buddhist meditation practices have become a topic of widespread interest in both science and medicine. Traditional Buddhist formulations describe meditation as a state of relaxed alertness that must guard against both excessive hyperarousal (restlessness) and excessive hypoarousal (drowsiness, sleep). Modern applications of meditation have emphasized the hypoarousing and relaxing effects without as much emphasis on the arousing or alertness-promoting effects. In an attempt to counterbalance the plethora of data demonstrating the relaxing and hypoarousing effects of Buddhist meditation, this interdisciplinary review aims to provide evidence of meditation’s arousing or wake-promoting effects by drawing both from Buddhist textual sources and from scientific studies, including subjective, behavioral, and neuroimaging studies during wakefulness, meditation, and sleep. Factors that may influence whether meditation increases or decreases arousal are discussed, with particular emphasis on dose, expertise, and contemplative trajectory. The course of meditative progress suggests a nonlinear multiphasic trajectory such that early phases that are more effortful may produce more fatigue and sleep propensity, while later stages produce greater wakefulness as a result of neuroplastic changes and more efficient processing.
Keywords: Buddhist meditation, relaxation, sleep, arousal, alertness, wakefulness
Introduction
Meditation practices that are derived from Buddhist traditions have been gradually adopted in the West and have become a topic of widespread interest in both science and medicine. In order to fit with modern Western values and worldviews, the practices have been largely decontextualized from their original Buddhist goal of awakening and recontextualized as clinical interventions or forms of psychological and physiological self-maintenance.1 This recontextualization has resulted in a modification of the traditional goals of practice and also in the way scientific studies report on the effects of meditation, which may emphasize or de-emphasize certain aspects of Buddhist formulations to serve modern needs and values.2,3
For example, Buddhist texts describe meditation practice as aiming to cultivate a state of relaxed alertness, and therefore must continually balance between the extremes of hyperarousal, agitation, and restlessness on the one hand, and hypoarousal, excessive relaxation, mental dullness, and sleep on the other (Fig. 1).4–6 Within the modern context, however, much more emphasis has been placed on the relaxing effects of meditation without as much attention to the arousing or wake-promoting effects. In some modern formulations, the goal of meditation has expanded beyond relaxation and decreased arousal7–10 to include a state of consciousness that is deliberately half-asleep, a “physiological twilight condition between waking and sleep,”11–13 equivalent to sleep,14, 15 or a form of sleep-like hibernation, a “shallow torpor”16 that is reversed by (i.e., the opposite of) wakefulness.17 As a result, meditation practices are often equated with non-specific relaxation techniques7 where hypoarousal and sleep are seen as desirable rather than obstacles to concentration (samâdhi).18
Figure 1.

Meditation maintains a state of relaxed alertness by guarding against the extremes of hyperarousal (excitation, restlessness, anxiety) and hypoarousal (laxity, drowsiness, sleep).
In contrast to modern formulations that emphasize the hypoarousal end of the spectrum, traditional Buddhist texts tend to emphasize vigilant wakefulness as a means to a profound shift in perception called awakening (bodhi). Because meditation must balance mental laxity and agitation, relaxation is infused with courageous energy (viriyâ), zeal (atapi), and an alert vigilant awareness such that a practitioner is devoted to wakefulness (jâgariya), and guarded against the hindrances of drowsiness and sleep.19,20 Scholars familiar with the traditional Buddhist goals of meditation have criticized Western scientists for their overemphasis on relaxation.1,21 In his Science and Buddhism: A Guide for the Perplexed, Buddhist studies scholar Donald Lopez laments “Where is the insistence that meditation is not intended to induce relaxation but rather a vital transformation of one’s vision of reality?”22 Others warn how “a practice that only relaxes the mind might eventually prove harmful.”23
As an attempt to promote a more balanced view of the goal of mindfulness meditation, Jon Kabat-Zinn changed the name of his program from Stress Reduction and Relaxation Program24 to Mindfulness-Based Stress Reduction (MBSR), characterized the term mindfulness as being awake,24 and removed the word relaxation from audiotapes and handouts. Similar attempts to point out the arousing effects of meditation have been widespread11,16,23 but also lost in the well-established enthusiasm around relaxation and its closely-related cousin, sleep.
In an attempt to counterbalance the plethora of data demonstrating the relaxing and hypoarousing effects of Buddhist meditation, this interdisciplinary review aims to provide evidence of meditation’s arousing or wake-promoting effects by drawing from both scientific studies and Buddhist textual sources. The construct of arousal is complex and multidimensional, with multiple distinct but overlapping inputs, including from cortical, autonomic, endocrine, cognitive, and affective systems.25 While other reviews have focused more on somatic forms of arousal,26 this review will focus on the cognitive dimensions of arousal that pertain to wakefulness and alertness.
The link between attention and wakefulness
While there are many different forms of meditation, most agree that the deliberate training of attention, either in a focused and directed or open and receptive way, is a foundational part of any meditation. This review emphasizes two common forms of attention training, focused attention (FA) and open monitoring (OM), which are foundational in Buddhist and many non-Buddhist meditation practices.27
Attention has been typically divided into three basic functionally and anatomically distinct types: orienting, executive attention, and alerting.28,29 Orienting directs and limits attention to a selected stimulus. Executive attention, also called conflict monitoring or selective or focused attention, involves prioritizing among competing tasks or responses. Alerting consists of achieving and maintaining a state of mental preparedness and a high sensitivity to incoming stimuli, and is subdivided into phasic alertness and tonic alertness. Phasic alertness refers to the ability to increase response readiness briefly following a cue or warning signal. Tonic alertness, also called intrinsic alertness or vigilant attention,30 is a general level of arousal, alertness, vigilance, wakefulness, or state of mental preparedness to detect or respond to infrequent or unexpected stimuli. Tonic alertness can also be measured by its deficit: a general level of sleepiness, fatigue, or lack of sustained attention characterized by an attentional lapse or mind wandering.31 Because tonic alertness provides the cognitive tone for performing more complicated functions, such as working memory and executive control,32,33 it is thought that all other forms of attention are dependent on tonic alertness.34 Studies of alertness training35–38 or pharmacological enhancement of alertness39 leading to improvements on a broad range of attentional tasks also support the idea that tonic alertness is a foundational prerequisite for other forms of attention. While all three types of attention have been researched in meditation studies,40–53 less emphasis has been placed on tonic alertness. Furthermore, tonic alertness has been described in terms of increased sustained attention or vigilance40,49,54,55 but not in terms of increased wakefulness or arousal, an approach influenced by the modern Western view that meditation promotes relaxation and sleep rather than wakefulness.
The positive relationship between attention and arousal or wakefulness is evident in common everyday experiences. We have all noticed, for example that paying attention, as well as learning, remembering, and regulating emotion, are more difficult when we are tired, or that intensely engaging attention before bed can inhibit sleep. The idea that attention is positively correlated with greater arousal and wakefulness is well-established within the fields of sleep and chronobiology,56 but this correlation has been relatively unexplored within the field of meditation research. Thus, this review will adopt a sleep researcher’s approach in order to examine the effects of contemplative practices on tonic alertness as defined by the complementary domains of sleep propensity and wakefulness. Specifically, this review will investigate the effects of Buddhist meditation on the following indices of sleep propensity/wakefulness: (1) the activation of sleep/wake–related brain areas, (2) subjective reports of sleepiness/fatigue, (3) electroencephalogram (EEG) activity during wakefulness, (4) behavioral measures of vigilance/sustained attention, (5) EEG during sleep, and 6) sleep duration.
Meditation effects on tonic alertness
Neuroimaging studies
Variations in wakefulness and sleep propensity correspond to specific brain areas that are also affected by meditation practices, both in a state-related way (during meditation) and a trait-related way (at rest or during tasks). Generally speaking, decreased alertness and the transition of wake to sleep onset is associated with a global decrease in most brain areas, particularly frontal areas, but an increase in areas in the default mode network (DMN).57–59 Tonic alertness is associated with activity in right hemisphere cortical areas and subcortical networks, particularly the dorsal anterior cingulate cortex (dACC), the dorsolateral prefrontal cortex (DLPFC), the anterior insula, the inferior parietal lobule, the thalamus, and the brainstem (Table 1).30–32,35,60–64 A number of studies have found that meditation-related activation or volume increases are lateralized to the right hemisphere and correspond to tonic alertness–related brain areas (lateral PFC, inferior parietal, and anterior insula).65–70
Table 1.
Effects of meditation practice on brain areas underlying tonic alertness/sleep
| DLPFC | dACC | AIC | IPL | SPL | BG | THAL | DMN | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sleep | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↑ | ||||
| Tonic alertness | ↑R | ↑R | ↑R | ↑R | ↑R | ↑ | ↑ | |||||
| Meditation Studies | Type of meditation | Sample | Design | Task | ||||||||
| Allen et al.80 | 6-week mindfulness | MOD | LRCT, CS | Affective Stroop at R | ↑L | ↑ | ↑R | ↑B | ||||
| Baerensten et al.109 (onset vs. rest) | Zen, Mantra | EX | WS | M vs. R | ↑B | ↓R | ↑B | ↓ | ||||
| Baerensten et al.109 (sustained vs. rest) | Zen, Mantra | EX | WS | M vs. R | ↓R | ↑ | ||||||
| Baerentsen et al.109 (meditation vs. rest) | Mixed | EX | WS | M vs. R | ↓B | ↑B | ↑R | ↑ | ↓ | |||
| Baron Short et al.78 (less experienced meditators) | Tibetan, Zen, OM | EX, NM | CS, WS | M vs. C (picture viewing) | ↓ | |||||||
| Baron Short et al.78 (meditators vs. controls) | Tibetan, Zen, OM | EX, NM | CS, WS | M vs. C (picture viewing) | ↑ | |||||||
| Baron Short et al.78 (more experienced meditators) | Tibetan, Zen, OM | EX, NM | CS, WS | M vs. C (picture viewing) | ↑ | |||||||
| Brefczynski- Lewis et al.45 | Tibetan FA | EX, NM | CS | M vs. R | ↑L | ↓R | ↑ | ↑B | ↑L | ND | ↑↓L | |
| Brefczynski- Lewis et al.45 (less hours EX) | Tibetan FA | EX, NM | CS | M vs. R | ↑ | |||||||
| Brefczynski- Lewis et al.45 (more hours EX) | Tibetan FA | EX, NM | CS | M vs. R | ↓ | |||||||
| Brewer et al.99 (F) | FA, OM, LKM | EX, NM | WS, CS | M vs. R | ↓ | ↓ | ||||||
| Farb et al.67 | 8-week MBSR | MOD, NM | CS | M while reading self-related vs.control words | ↑L | ↑R | ↑ | ↓ | ||||
| Farb et al.87 | 8-week MBSR | MOD, NM | CS | Watching sad videos vs. R | ↑↓L | ↑R | ↑R | ↓R | ↑ | |||
| Gard et al.89 | Vipassana | EX, NM | CS, WS | Anticipation/pain during M and C | ↑↓ | ↑R | ↑R | ↓ | ↓ | |||
| Goldin and Gross189 | 8-week MBSR | MOD, NM | LRCT, WS | M vs. C (count backwards) | ↑ | ↑ | ↑ | |||||
| Goldin and Gross189 | 8-week MBSR | MOD, NM | LRCT, CS | Self-reference vs. control words at rest | ↑ | |||||||
| Goldin and Gross189 | 8-week MBSR | NOV | LRCT, WS | Self-reference vs. Control words at Rest | ↓ | ↑ | ↑ | ↑ ↓ | ||||
| Grant et al.93 (S) | Zen | EX, NM | CS, WS | R | ↑R | ↑R | ↑ | |||||
| Grant et al.88 | Zen | EX, NM | CS | During pain | ↓B | ↑B | ↑B | ↑B | ↑B | |||
| Hasenkamp et al.65 | FA—AWARE Phase | EX | WS | M | ↑L | ↑B | ↑B | ↑L | ||||
| Hasenkamp et al.65 | FA—FOCUS Phase | EX | WS | M | ↑R | |||||||
| Hasenkamp et al.65 | FA—SHIFT phase | EX | WS | M | ↓B | ↑B | ↓L | ↑B | ↑ B | ↓B | ||
| Hölzel et al.92 (S) | Vipassana | EX, NM | CS | R | ↑ | ↑ | ||||||
| Hölzel et al.86 | Vipassana | EX, NM | CS, WS | M vs. C (arithmetic) | ↑ | ↑B | ||||||
| Hölzel et al.92 (S) | 8-week MBSR | MOD | LRCT, WS | R | ↑ | |||||||
| Ives-Deliperi et al.190 | MBSR-trained experts | EX | WS | M vs. C (generation of numbers) | ↓R | ↓L | ↓B | ↓B | ↑R | |||
| Klimecki et al.191 | 6 h LKM | NOV | WS | Neutral vs. distressing videos at R | ↑R | ↑R | ||||||
| Lazar et al.66 | Insight | EX, NM | CS | R | ↑ | ↑ | ||||||
| Lee et al.81 | FA, LKM | EX, NM | CS, WS | Emotion photos vs. cognitive task at R and while M | ↑ | |||||||
| Lee et al.81 (FA only) | FA | EX, NM | CS, WS | Emotion photos vs. cognitive task at R and while M | ↑L | ↑R | ↓R | |||||
| Lee et al.81 (LK only) | LKM | EX, NM | CS, WS | Emotion processing (photos) vs. cognitive task at R and while M | ↑L | ↑L | ↑R | ↑L | ||||
| Luders et al.83 (S) | Mixed | EX, NM | CS | R | ↑ | ↑R | ||||||
| Luders et al.95 (Cortical gyrification) (S) | Mixed | EX, NM | CS | R | ↑B | ↑L | ↑R | |||||
| Manna et al.76 (monks FA> rest) | Theravada FA | EX, NOV | WS | M vs. R | ↑R ↓L | ↑B | ↓L | |||||
| Manna et al.76 (monks OM> Rest) | Theravada OM | EX, NOV | WS | M vs. R | ↑L | ↑L | ||||||
| Manna et al.76 (monks OM>FA) | Theravada FA, OM | EX, NOV | WS | M vs. R | ↑B | R | ↑L | ↑L | ||||
| Manna et al.76 (NOV FA> rest) | 10-day FA | EX, NOV | WS | M vs. R | ↑L | |||||||
| Manna et al.76 (NOV OM> rest) | 10-day FA | EX, NOV | WS | M vs. R | ↑R | ↑B | ||||||
| Pagnoni and Cekic48 (S) | Zen | EX, NM | CS | Attention task | ↑L | |||||||
| Ritskes R et al.79 | Zen | EX | WS | M vs. R | ↑R | ↓ | ↑ | |||||
| Taylor et al.94 | Zen and mindfulness | EX, NOV | CS | M vs. R, while viewing emotional images | ↓R | |||||||
| Yu et al.77 | Zen FA | NOV | WS | M | ↑ | |||||||
| Zeidan et al.70 | FA 4 days | NOV | L, WS | During pain | ↑ | ↑B | ↑R | ↓B |
NOTE: Type of study: S= structural (gyrification, volume), all other S = functional (fMRI, PET); type of meditation: FA= focused attention, OM= open monitoring, LKM= loving kindness; design: CS= cross-sectional, WS= within-subjects, RCT= randomized controlled trial, L = longitudinal; sample: EX=expert, MOD = moderate, NOV= novice, NM= non-meditator; task: M= meditation, R= rest, C= control task; brain areas: follow Brodmann area (BA), when provided, or authors designation; L = left; R = right; B= bilateral; ND=no difference; DLPFC= dorsolateral prefrontal cortex (BA 9–12, 45, 46, 47) dACC= dorsal anterior cingulate cortex (BA 11, 24, 25, 32); AIC = anterior insular cortex (BA 13, 14, 47, 48); IPL= inferior parietal lobule (BA 39, 40, 43) (also called the temporal-parietal junction or TPJ); SPL= superior parietal lobule (BA 7, precuneus); BG=basal ganglia (caudate, putamen, globuspallidus); THAL = thalamus; DMN= default mode network (mPFC, PCC).
Dorsolateral prefrontal cortex
High levels of activity in the DLPFC correspond to alert wakefulness,71–73 while a hypoactive DLPFC is associated with fatigue and increased sleep propensity.71,74,75 Buddhist meditation practices have been found to be associated with increased activity in the DLPFC, both during meditation45,65,76–79 and during tasks,67,70,80,81 as well as larger frontal gray matter volumes.66,82,83
Dorsal anterior cingulate cortex
As part of its many functions, the dACC is thought to control arousal via brainstem noradrenergic activation and multiple thalamic nuclei.31 The ACC has been implicated in the intentional modulation of arousal, with the right-sided ACC proposed to be more associated with increased arousal and left-sided activation with decreased arousal.84,85 Buddhist meditation practices have been found to be associated with increases in activation in the dACC both during meditation45,65,76,78,86 and during tasks.70,80,81,87–89
Insula
Often thought to underlie interoception,90 the anterior insula has also been proposed as the substrate of basic awareness or consciousness,91 is a central node in the intrinsic alertness network,30,62 and corresponds with states of wakefulness and autonomic arousal during meditation.45,68 The (right) insula has been found to have greater gray matter concentration82,92 and thickness66,93 in meditators and greater activation both during meditation65,76 and on tasks following mindfulness training.67,70,80,87–89,94 Larger volume and increased gyrification in the right anterior insula has been found to correlate with duration of meditation training.82,95
Default mode network
The default mode is a network of midline brain structures, including the medial PFC and posterior cingulate, that is active during rest or when the brain is not otherwise engaged, and is thought to be involved in stimulus-independent, self-referential thought and mind wandering.96 Converging evidence suggests that meditation training may be associated with decreased DMN activity,67, 70, 87, 94, 97–99 Because increased DMN activity is associated with negative mental health outcomes,100, 101 it has been posited that “one mechanism through which meditation may be efficacious is by repeated disengagement or reduction of DMN activity.”65
Instead of being a sign of mental excitation, mind wandering and DMN activation is associated with decreased sympathetic arousal,102 lower levels of alertness and vigilance,103 and increases in delta power that indicate sleepiness.104–106 The DMN includes some of the only brain regions that actually increase their activity during the transition from wakefulness to stage-1 sleep,57–59 and vigilance can be pharmacologically induced by suppressing the DMN.107 Thus, DMN activation and mind wandering can be viewed as a sign of mental laxity and drowsiness on a continuum with stage-1 sleep and dreaming.108 In this view, when meditation decreases DMN activation/mind wandering, the result is not a calming in the direction of relaxation/sleep, but rather a move in the opposite direction: towards an increased alertness and vigilance that counteracts mental laxity and sleepiness.
Subcortical and brainstem structures
Studies of subcortical structures have found increased activation or gray matter density in tonic alertness–related areas in meditators, such as the thalamus,83,87,88 basal ganglia,48,88,109,110 and arousal-related areas of the brainstem, such as the reticular formation.111
Neurotransmitters
Increases in wake-related/sleep-inhibiting neurotransmitters, such as norepinephrine,16,26,47,112, 113 dopamine,114 and serotonin,77,115–118 have been found in studies of non-Buddhist forms of meditation, although the relationship between peripheral and brain levels is unclear.
Functional connectivity
In addition to activation of alertness-related areas and deactivation sleep-related areas, functional connectivity (FC) or correlated activity between brain areas can also serve as an index of sleepiness or wakefulness. The fading of consciousness during non-rapid eye movement (NREM) sleep is thought to be associated with the breakdown of effective connectivity between multiple cortical areas,119 specifically within nodes of the DMN, and between the DMN and attention network.
Intra-DMN connectivity
Connectivity between anterior and posterior nodes of the DMN remains intact during wake and into light and REM sleep120–122 but decouples during slow-wave sleep and vegetative states, when consciousness is at a minimum. Thus, the functional integrity of the DMN has been thought to be indicative of the level of consciousness.122 Functional connectivity studies of meditation have indicated increased connectivity between anterior and posterior nodes of the DMN at rest123,124 and during meditation125,126 in experienced meditators with 10,000 h of practice but not following 8 weeks of training,127 which suggests that increases in default mode connectivity (and potentially an associated increase in wakefulness) correspond with later but not earlier stages of practice.
DMN and attention network connectivity
Typically, vigilance is associated with maximal anticorrrelation between the DMN and attention network.128 Impaired vigilance and lack of anticorrelation is caused by insufficient DMN deactivation so that self-referential processes and mind wandering intrude into (and impair) task performance.129 Participants with more meditation experience exhibited increased connectivity between attentional regions and medial frontal regions (i.e., less anticorrelation), both during meditation99,125,130 and during rest.99,123 In experienced meditators, the decreased anticorrelation is associated with attention systems remaining active at rest when the DMN is active, and faster suppression of the DMN during attentional engagement and task performance.65,99
Although there is much variability in the methodologies, practice, and sample characteristics, more than 25 neuroimaging studies have found that Buddhist meditation practices are associated with activation/enlargement of the areas that underlie tonic alertness and/or prevent sleep both during meditation (state) and at rest or during tasks (trait). In order to promote sleep, the brain areas activated in meditation would need to be deactivated.131 Furthermore, changes in functional connectivity both at rest and during meditation also correspond with greater wakefulness, especially in more experienced meditators. Based on the brain imaging findings, one might predict that meditators would be generally more awake and less sleepy, especially as practice progresses.
Less sleepy during wake
Subjective measures
While many meditation studies have described subjective reports of decreased arousal, especially in the hyper-aroused,132 reports of increased energy and arousal following meditation are also prevalent. Early uncontrolled studies of mindfulness training in clinical samples found increased energy and decreased fatigue as secondary outcomes.133–135 Later randomized control trials specifically targeted fatigue136–140 and called mindfulness meditation “vitality training.”141 Improvements in fatigue were maintained up to one year136,138,139,141 and correlated with improvements in neuropsychological functioning.140 Studies in nonclinical populations with active controls have also found improvements in fatigue, alertness, and attention following Buddhist meditation training.46,142
Behavioral measures
Several studies have found improvements on behavioral measures of tonic alertness following both brief and long-term practice of both FA and OM meditations. Valentine and Sweet40 compared experienced Buddhist meditators to controls on a sustained attention task that included both phasic and tonic alerting (i.e., expected and unexpected stimuli). Alerting capabilities were associated with length of practice and OM practitioners outperformed FA meditators on indices of tonic (but not phasic) alertness. Jha et al.49 investigated multiple forms of attention in novices undergoing MBSR training and long-term meditators on a 1-month intensive Vipassana retreat. Orienting and directed attention improved in the MBSR group, but tonic alertness improved only in long-term meditators and was directly associated with prior meditation practice.
While the above studies suggest that only OM practice in advanced meditators supports increased tonic alertness, other studies have found increases in tonic alertness following both long- and short-focused attention training. Following 3 months of intensive Tibetan-style shamatha training, MacLean et al.54 found increases in vigilance that were related to improvements in visual sensitivity which persisted at least 3 months after the retreat ended. Lee81 found that expert (2 h/day for 5 years) FA and loving-kindness (LKM) meditators made significantly fewer omission errors on a continuous performance task than novices, indicating greater level of tonic alertness. Kaul et al.55 found that meditation naive university students who performed a 40-min FA mediation improved their performance on the psychomotor vigilance task (PVT) in comparison to a nap or a control activity. This study also found that meditation improved decrements in PVT vigilance following a night of sleep deprivation, so that meditation practice increased wakefulness. This study suggests that some brief increases in tonic alertness may be apparent after only a single practice session of focused attention training.
Waking EEG
In some of the early meditation studies using objective polysomnographic measurements of sleep propensity, both Elson et al.143 and Banquet and Sailhan144 found that meditators were more likely to remain awake and less likely to fall asleep (defined as stage-2 sleep or delta EEG, respectively) than non-meditator controls during a comparable period of relaxed wakefulness.143, 144 This finding is consistent with the notion that meditative training involves learning to keep a relaxed and yet aroused mental state for successively longer periods of time.
While many early studies of waking EEG during meditation reported enhanced theta and alpha power consistent with a possibly near–sleep-like state, recent investigations with more rigorous methodology have failed to replicate these findings.11 Instead, localized frontal midline theta increases have been more consistently obtained in meditators,145–147 especially during FA practices, indicative of the concentrated cognitive engagement indexed by frontal midline theta.148,149 Other studies have reported higher baseline (trait) and state-related increases in gamma power in long-term Tibetan Buddhist practitioners,124 Vipassana meditators,146 as well as meditators in other traditions,150 which are indicative of a more highly alert and aware brain state.
Reports of increases in midline frontal delta power151 or decreases in gamma power97 that are thought to indicate decreased DMN activity also support the idea of the wake-promoting and sleep-inhibiting effects of meditation. Additionally, Cahn146 reported decreased bilateral frontal delta power as a state effect of Vipassana meditation in long-term practitioners, consistent with a more highly alert brain state. A comprehensive analysis of waking EEG during breath-focused awareness before, during, and after an intensive 3-month meditation retreat indicated decreases in central and parietal beta power and a concomitant significant decrease in peak alpha frequency.152 The decreased beta activity might be conceived of in terms of decreased active processing consistent with the traditional understanding that beta activity is associated with an active and aroused brain processing state.153 However, the authors note that decreased beta activity has been correlated with increased blood oxygen level–dependent (BOLD) signal in precentral motor areas154 and with facilitating sensory processing of attended somatosensory stimuli,155 and thus this decrease in beta power was interpreted as indicative of enhanced cortical activation. The decrease in peak alpha frequency was thought to correspond to a decrease in cognitive effort because increased cognitive load leads to increases in peak alpha frequency.156 This has also been previously reported as a trait effect of meditation.11
In addition to spontaneous EEG, investigations of event-related EEG dynamics in relation to sensory stimuli further bolster the notion that meditation is associated with a concurrently more alert and efficient brain state. Decreased P300 amplitude to initial visual stimuli on an attentional blink task,157 and increased frontal theta synchronization158 to normally unattended subsequent stimuli following an intensive 3-month retreat, indicates a broadening of attentional capacities across time that facilitates increased processing of difficult-to-detect stimulus trains. This same group of subjects also showed an enhanced perception and concomitant theta synchronization in response to challenging subtle auditory targets in an auditory oddball paradigm, further delineating brain mechanisms underlying meditative practice effects in improving attentional engagement to demanding cognitive tasks.51 Using a simpler and passive auditory oddball paradigm in assessing state effects of Vipassana meditation, it has also been shown that while later signatures (P300, delta synchronization) of automated frontal cognitive engagement to unexpected distracter stimuli are decreased, markers of enhanced early stimulus representation (N100, gamma synchronization) are concomitantly observed, consistent with the notion of a highly aware brain state that is simultaneously less reactive.159,160
Overall, findings in more recent awake EEG studies of meditators, whether assessed by increased frontal theta power associated with highly focused cognitive states, increased gamma power associated with more active cortical processing, decreases in measures of DMN activation or measures of enhanced sensory and attentional processing with concomitantly decreased automated reactivity, have been consistently indicative of increased brain alertness.
More awake during sleep
In many Tibetan Buddhist161 and also non-Buddhist traditions,162 the goal of cultivating of vigilant awareness in every waking moment is extended beyond the waking state to sleep states as well. One teacher instructs “When you fall asleep, you are asleep with virtually full awareness.”161 Several studies have described meditators’ self-reports of ongoing conscious awareness during sleep.162,163 In support of such increased wakefulness during sleep, several polysomnographic sleep studies have found greater cortical arousal during sleep in meditators, including increased stage-1 sleep, alpha-theta (wake-like) EEG, arousals, reduced slow-wave sleep,144, 162,164 and increased gamma.165
Ferrarelli et al.165 found that long-term meditators had increased parietal-occipital EEG gamma power (25–40 Hz) during whole-night non-rapid eye movement (NREM) sleep compared to non-meditating controls. Increased gamma was positively correlated with the length of lifetime daily meditation practice (Spearman’s rho= 0.475, P< 0.02). In an EEG-based longitudinal randomized controlled trial (RCT), Britton et al.164 found increased wakefulness and decreased sleep propensity following Mindfulness-Based Cognitive Therapy (MBCT) that was correlated with improved mood and meditation practice amount. Over the course of the trial, the MBCT group increased in stage-1 minutes, awakenings, and arousals, and decreased in percent of slow-wave (stage 3 and 4) sleep compared to waitlist controls. Each of these objective indices of increased cortical arousal was correlated with amount of home meditation practice in a linear dose-dependent fashion, which further supports that the increased arousal was due to meditation. Although there was no overall treatment effect on sleep duration, average (diary) total sleep at post-treatment was negatively correlated with minutes of daily practice, so that more meditation was associated with less sleep (Fig. 2A–D).
Figure 2.

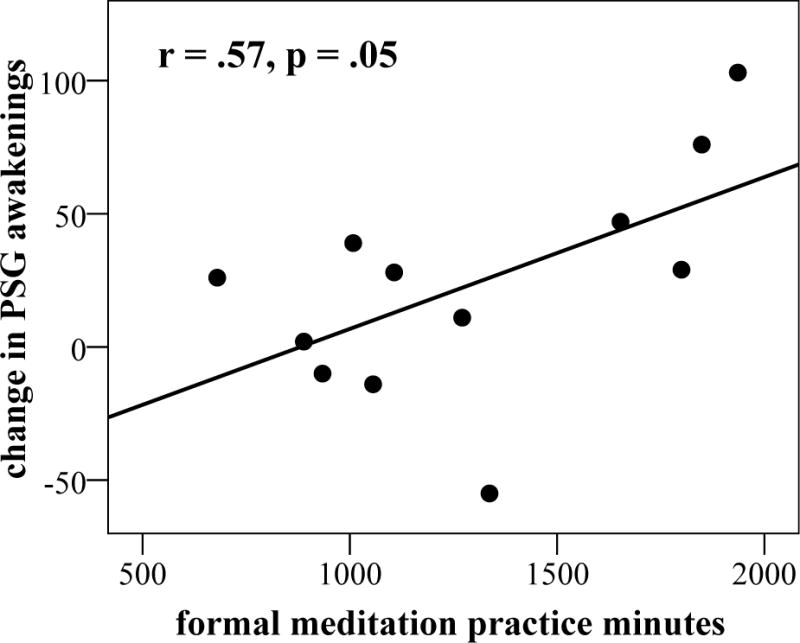

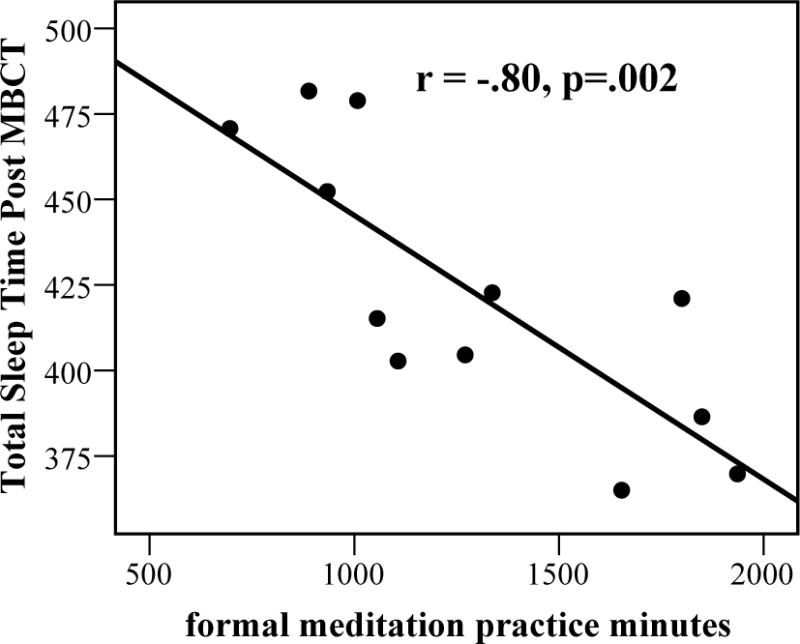
Correlation between meditation practice and changes in sleep or arousal variables following MBCT, including (A) stage-1 minutes, (B) arousal and awakenings, (C) slow-wave sleep (SWS) percent, and (D) total (diary) sleep time. Figures A–C reprinted, with permission, from Britton et al.164
There are several lines of evidence to suggest that these increases in cortical arousal are beneficial and desirable, rather than indicators of poor sleep or insomnia. First, these indices of increases in cortical arousal were also associated with an improvement in depression (Beck depression inventory (BDI) scores)), including increased awakenings, arousals, stage-1 minutes, as well as reduced overall sleep time (r= –0.60), so that the larger increase in cortical arousal, the larger the decrease in depression scores.
Meditation and sleep amount
Decreased sleep duration in meditators during periods of intensive practice, such as multiday silent retreats is a well-known phenomenon27 and is considered a sign of meditative proficiency and progress.19 Kornfield163 documented decreased sleep duration in Theravada Vipassana meditators on 3-month retreats (12–15 h of meditation/day) compared to a control group who meditated 1–2 h/day. Seventy percent of the retreat participants reported an average 2 h decrease in sleep duration in comparison to 10% of controls, who also reported increases in sleep duration (10%). The periods of sleep decrease were associated with periods of greatest self-reported and teacher-observed mindfulness.
In a qualitative study in our laboratory, a meditator who had completed a 3-month Tibetan-style shamatha retreat (12–15 h/day), reported that for the first 2 weeks of the retreat, her sleep duration initially increased to 8 h/night before gradually diminishing to 1.5–3 h/night by the eighth week. Buddhist texts suggest a nocturnal sleep time among proficient meditators of approximately 4 hours.19
Other studies have begun to assess the effects of daily meditation practice on sleep duration outside of a retreat context. Kaul et al.,55 measured 15 days of diary-reported sleep in a small sample of experienced Indian meditators (n=7) who practice focused (breath) awareness techniques 1.5–3.5 h/day (for at least 3 years) compared to matched non-meditating controls (n=23). Sleep diaries indicated a significantly shorter average nocturnal sleep duration in meditators than controls (5.2 h vs. 7.8 h, P< 0.00001).
A recent study examined sleep duration of meditators in daily life, using objective, EEG-based measures of sleep in a much larger sample. This cross-sectional study165 recorded single-night polysomnographic sleep studies (high-density EEG) in long-term (8700 average lifetime meditation hours) Tibetan and Theravada Buddhist meditators and age- and gender-matched non-meditating controls. The meditators had a significantly reduced total sleep time (6.2 vs. 6.7 h, P= 0.001) as well as increased minutes of wake after sleep onset (74 vs. 44 min, P =0.003) compared to controls.
In contrast to advanced Buddhist mediators who view decreased sleep as a sign of progress, modern clinical applications seek to increase sleep duration and depth with meditation practice. High-quality empirical support for clinically-oriented meditation programs, such as MBSR, promoting sleep have not been particularly strong (see Ref. 166 for a review) and often lack objective measures of sleep, particularly EEG. While some RCTs have found positive effects of meditation training on self-reported sleep quality167–169 other RCTs have found no difference from controls.170–172 Subjective ratings of sleep quality appear to be more affected by meditation than specific sleep parameters (such as sleep duration)173,174 with both positive and negative changes in arousal when measured by objective measures of quality.167–169 After many mixed or equivocal studies of MBSR, some sleep researchers have suggested that “mindfulness meditation as a single intervention might not yield strong effects on sleep”132 and instead created programs that include mindfulness as part of a multicomponent cognitive-behavioral sleep treatment.132,175 As an indication of the current state of the research, the most recent (2012) U.S. Government Report judged the level of evidence of clinically-oriented meditation programs for improving sleep to be insufficient.176
What factors determine whether meditation training will increase or decrease arousal, and promote wakefulness or sleep? This is an important research question that is largely unknown, but probably encompasses a number of factors, including dose and type of practice, sample characteristics (condition/disorder), degree/type of sleep disturbance, comparison group, concurrent medication,167 and age and method of measuring sleep or wakefulness (subjective/objective).
Non-linear progress
In order to make sense of the mixed and contradictory findings, it may help to approach the trajectory of meditative expertise as non-linear in regard to arousal and tonic alertness.177 Specifically, relaxing and sleep-promoting effects might be expected at low doses or early stages of practice (such as an introductory 8-week program), but more alertness, wakefulness, and less sleep as dose or practice progresses. Britton et al.175 found that as little as 5–10 min/day for 2–3 days/week (<1 h/week) increased self-reported sleep duration by more than an hour, but that as practice times approached 30 min/day (>3 h/week), then sleep duration began to decrease (Fig. 2D) and cortical arousal began to increase (Fig. 2A–C).164 Similarly, our 3-month retreat participants slept more during the first 2 weeks, then progressively less, in line with findings of decreased sleep following intensive163 or long-term daily practice.55,165
Both Buddhist sources and recent research support the idea that early stages of practice require more effort and produce more fatigue than later stages of practice.27,178,179 Most beginning meditators start with a form of focused attention training, which relies on orienting, executive, and phasic alerting forms of attention. Like training a muscle, attention is redirected from self-referential thought back to the meditative object, over and over until attentional stability can be established. Proficient meditators can disengage faster and more easily from self-referential thought (DMN activity).65 Attention enhances efficiency of sensory information processing and enhances sensory acuity by increasing the ratio of signal to noise in neural communications.180 Meditation-related increases in perceptual sensitivity and sensory acuity43, 181–183 require less cognitive resources and effort for stimulus detection and therefore free up resources to be available for other functions.43,54 Like having stronger muscles, more efficient processing is less taxing or fatiguing and supports more enduring alertness. Reports that increases in directed attention/phasic alerting leads to improved tonic alertness49,184 support Kabat-Zinn’s statement that “By paying attention you literally become more awake.”185 Indeed, many studies have found positive correlations between meditative expertise (practice amount) and subjective, neurological, and behavioral measures of wakefulness (see Table 2).
Table 2.
Correlation between meditation experience/expertise and alertness
| Study | Expertise | Alertness/sleepiness | r value (Pearson) |
|---|---|---|---|
| Self-report measures | |||
| Ong et al.132 | KIMS score | Diary-rated daytime sleepiness | –0.65*a (post-treatment) –0.71**a (6 months) –0.54*a (12 months) |
| Diary-rated daytime tiredness | –0.66**a (post-treatment) –0.62**a (6 months) |
||
| Cahn and Polich160 | Years of daily practice | Drowsiness during control condition | –0.60* |
| Brittonet al.175 | Frequency of practice in mindfulness-based sleep treatment (5–10 min/day, 2–3 days/week) |
Diary sleep duration | +0.56* |
| Minutes of home practice during MBCT | Total (diary) sleep time | –0.80** | |
| Neuroimaging measures (EEG, fMRI) | |||
| Brittonet al.164 | Minutes of home practice during MBCT | Increase in stage-1 minutes during sleep | 0.80** |
| Minutes of home practice during MBCT | Increase PSG awakenings and arousals during sleep | 0.57* | |
| Frequency of practice (times per week) | Decrease in percent of slow-wave sleep (stages 3/4) | –0.62* | |
| Ferrarelli et al.165 | Lifetime daily practice hours (non-retreat) | Increased occipital-parietal gamma during NREM sleep | 0.48**a |
| Luderset al.95 | Lifetime hours of meditation practice | Increased gyrification in R anterior insula | NR |
| Holzel et al.82 | Lifetime hours of Vipassana meditation | Increased volume of R anterior insula | 0.36# |
| Hasenkampet al.65 | Lifetime hours of meditation practice | Decreased activity in DMN | –0.74*** |
| Baron et al.78 | Lifetime hours of meditation practice (<10 years vs.>10 years) | Activation of DLPFC and ACC during mindfulness meditation | NR |
| Behavioral Measures | |||
| Jhaet al.49 | Lifetime meditation experience | Magnitude of the alerting score post-retreat (negative value indicated better performance) | –0.52* |
| Valentine and Sweet40 | Meditation experience | Performance on tonic alerting task | NR |
Spearman’s rho
KIMS= Kentucky Inventory of Mindfulness Scale; NR= not reported.
P< 0.05
P< 0.01
P< 0.005
However, the relationship between phasic and tonic alertness is more likely bi-directional and mutually reinforcing,35–38 as the resources that are freed up by more efficient processing (sensory acuity) can promote a form of tonic alertness that makes the meditator more aware of internal and external stimuli,43, 54, 186 thus leading to more efficient processing, until the system runs so effortlessly that it may require much less recovery time (i.e., sleep).
Neuroimaging studies are beginning to support a non-linear trajectory of meditative practice and proficiency. Brain areas underlying tonic alertness are activated in early stages of training, compared to non-meditators, but these areas begin to deactivate as meditative expertise becomes more proficient and effortless in later stages.45,86,178,187 This eventual effortlessness that accompanies increased efficiency in later stages is thought to arise from increased connectivity between brain areas that were previously not co-activated.99,125,178,188
Thus, the point when meditation practice no longer produces drowsiness and sleep, but instead engenders increased and sustained wakefulness, may be an indicator of the neuroplastic changes that signify meditative proficiency. In this sense, “awakening” is not a metaphor, but rather an iterative process of neuroplastic modifications and increased efficiency that supports a new level of perceptual sensitivity and insight.
Conclusion
The purpose of this review was to provide evidence, by drawing from both scientific studies and Buddhist textual sources, of meditation’s arousing or wake-promoting effects in an attempt to counterbalance the common modern characterization of Buddhist meditation as a relaxation technique that promotes hypoarousal and sleep. Traditional Buddhist formulations and a host of recent subjective, behavioral, and neuroimaging studies of meditation suggest that Buddhist meditation practices may promote greater wakefulness and lower sleep propensity, especially as practice progresses. With a more interdisciplinary approach that includes traditional Buddhist formulations, the scientific study of meditation may gain a more comprehensive view of the full range of possible effects and applications of contemplative practices.
Acknowledgments
Funding for this research was provided by Grants T32-AT001287 and MH067553-05 from the National Institutes of Health (NIH) and the Mind and Life Institute. W.B.B. is supported by an NIH Career Development Award K23-AT006328-01A1 and by Lenz and Hershey Foundation Grants to the Brown University Contemplative Studies Initiative.
Footnotes
Conflicts of interest
The authors declare no conflicts of interest.
References
- 1.McMahan DL. The Making of Buddhist Modernism. Oxford: Oxford University Press; 2008. [Google Scholar]
- 2.Williams M, Kabat-Zinn J. Mindfulness: diverse perspectives on its meaning, origins, and multiple applications at the intersection of science and dharma. Contemporary Buddhism. 2011;12:1–18. [Google Scholar]
- 3.Davidson RJ. Empirical explorations of mindfulness: conceptual and methodological conundrums. Emotion. 2010;10:8–11. doi: 10.1037/a0018480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pandita SU. In this very life: the liberation teachings of the Buddha. Boston: Wisdom; 1992. [Google Scholar]
- 5.Gunaratana H. Mindfulness in Plain English. Boston: Wisdom; 2002. [Google Scholar]
- 6.Bodhi B. The Numerical Discourses of the Buddha: A New Translation of the Anguttara Nikaya. Boston: Wisdom; 2012. [Google Scholar]
- 7.Beary JF, Benson H. A simple psychophysiologic technique which elicits the hypometabolic changes of the relaxation response. Psychosomatic Medicine. 1974;36:115–120. doi: 10.1097/00006842-197403000-00003. [DOI] [PubMed] [Google Scholar]
- 8.Morse DR, Martin JS, Furst ML, et al. A physiological and subjective evaluation of meditation, hypnosis, and relaxation. Psychosomatic medicine. 1977;39:304–324. doi: 10.1097/00006842-197709000-00004. [DOI] [PubMed] [Google Scholar]
- 9.Cooper MJ, Aygen MM. A relaxation technique in the management of hypercholesterolemia. Journal of Human Stress. 1979;5:24–27. doi: 10.1080/0097840x.1979.10545991. [DOI] [PubMed] [Google Scholar]
- 10.Cardoso R, de Souza E, Camano L, et al. Meditation in health: an operational definition. Brain Research Protocols. 2004;14:58–60. doi: 10.1016/j.brainresprot.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 11.Cahn BR, Polich J. Meditation states and traits: EEG, ERP, and neuroimaging studies. Psychological Bulletin. 2006;132:180–211. doi: 10.1037/0033-2909.132.2.180. [DOI] [PubMed] [Google Scholar]
- 12.Fenwick PB, Donaldson S, Gillis L, et al. Metabolic and EEG changes during transcendental meditation: an explanation. Biol Psychol. 1977;5:101–18. doi: 10.1016/0301-0511(77)90007-2. [DOI] [PubMed] [Google Scholar]
- 13.Williams P, West M. EEG responses to photic stimulation in persons experienced at meditation. Electroencephalography and Clinical Neurophysiology. 1975;39:519–522. doi: 10.1016/0013-4694(75)90054-1. [DOI] [PubMed] [Google Scholar]
- 14.Pagano RR, Rose RM, Stivers RM, et al. Sleep during transcendental meditation. Science. 1976;191:308–10. doi: 10.1126/science.1108200. [DOI] [PubMed] [Google Scholar]
- 15.Younger J, Adriance W, Berger RJ. Sleep during transcendental meditation. Perceptual and Motor Skills. 1975;40:953–954. doi: 10.2466/pms.1975.40.3.953. [DOI] [PubMed] [Google Scholar]
- 16.Young JD, Taylor E. Meditation as a Voluntary Hypometabolic State of Biological Estivation. News Physiol Sci. 1998;13:149–153. doi: 10.1152/physiologyonline.1998.13.3.149. [DOI] [PubMed] [Google Scholar]
- 17.Walker JM, Berger RJ. Sleep as an adaptation for energy conservation functionally related to hibernation and shallow torpor. Adaptive Capabilities of the Nervous System, Progress in Brain Research. 1980;53:255–278. doi: 10.1016/S0079-6123(08)60068-0. [DOI] [PubMed] [Google Scholar]
- 18.Kyabgon T. Mind at Ease: Self-Liberation through Mahamudra Meditation. Boston: Shambhala; 2003. [Google Scholar]
- 19.Ñanamoli B, Bodhi B. The Middle Length Discourses of the Buddha: A New Translation of the Majjhima Nikaya. Wisdom Publications; Boston: 1995. Sekha-patipada Sutta (The Practice for One in Training) [Google Scholar]
- 20.Gyatso T. Stages of Meditation. Ithaca: Snow Lion; 2001. [Google Scholar]
- 21.Wallace BA. The Attention Revolution: Unlocking the Power of the Focused Mind. Boston: Wisdom; 2006. [Google Scholar]
- 22.Lopez DS., Jr . Buddhism and science: A guide for the perplexed. University of Chicago Press; 2009. [Google Scholar]
- 23.Lutz A, Dunne JP, Davidson RJ. Meditation and the Neuroscience of Consciousness: An Introduction. In: Zelazo MMP, Thompson E, editors. Cambridge Handbook of Consciousness. Cambridge University Press; New York: 2007. [Google Scholar]
- 24.Kabat-Zinn J. An out-patient program in Behavioral Medicine for chronic pain patients based on the practice of mindfulness meditation: Theoretical considerations and preliminary results. General Hospital Psychiatry. 1982;4:33–47. doi: 10.1016/0163-8343(82)90026-3. [DOI] [PubMed] [Google Scholar]
- 25.Derryberry D, Rothbart MK. Arousal, affect, and attention as components of temperament. Journal of personality and social psychology. 1988;55:958–66. doi: 10.1037//0022-3514.55.6.958. [DOI] [PubMed] [Google Scholar]
- 26.Holmes DS. Meditation and somatic arousal reduction. American Psychologist. 1984;39:1–10. doi: 10.1037//0003-066x.39.1.1. [DOI] [PubMed] [Google Scholar]
- 27.Lutz A, Slagter HA, Dunne JD, et al. Attention regulation and monitoring in meditation. Trends in cognitive sciences. 2008;12:163–9. doi: 10.1016/j.tics.2008.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fan J, Raz A, Posner M. Attentional Mechanisms. In: Aminoff M, Daroff R, editors. Encyclopedia of Neurological Sciences. Elsevier; New York: 2003. [Google Scholar]
- 29.Fan J, McCandliss BD, Sommer T, et al. Testing the efficiency and independence of attentional networks. Journal of Cognitive Neuroscience. 2002;14:340–7. doi: 10.1162/089892902317361886. [DOI] [PubMed] [Google Scholar]
- 30.Langner R, Eickhoff SB. Sustaining attention to simple tasks: A meta-analytic review of the neural mechanisms of vigilant attention. Psychol Bull. 2013;139:870–900. doi: 10.1037/a0030694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sturm W, Willmes K. On the functional neuroanatomy of intrinsic and phasic alertness. NeuroImage. 2001;14:S76–84. doi: 10.1006/nimg.2001.0839. [DOI] [PubMed] [Google Scholar]
- 32.Sturm W, de Simone A, Krause BJ, et al. Functional anatomy of intrinsic alertness: evidence for a fronto-parietal-thalamic-brainstem network in the right hemisphere. Neuropsychologia. 1999;37:797–805. doi: 10.1016/s0028-3932(98)00141-9. [DOI] [PubMed] [Google Scholar]
- 33.Posner MI. Measuring alertness. Annals of the New York Academy of Sciences. 2008;1129:193–199. doi: 10.1196/annals.1417.011. [DOI] [PubMed] [Google Scholar]
- 34.Raz A, Buhle J. Typologies of attentional networks. Nature Reviews Neuroscience. 2006;7:367–379. doi: 10.1038/nrn1903. [DOI] [PubMed] [Google Scholar]
- 35.O’Connell RG, Bellgrove MA, Dockree PM, et al. Cognitive remediation in ADHD: effects of periodic non-contingent alerts on sustained attention to response. Neuropsychol Rehabil. 2006;16:653–65. doi: 10.1080/09602010500200250. [DOI] [PubMed] [Google Scholar]
- 36.Robertson I, Tegnér R, Tham K, et al. Sustained attention training for unilateral neglect: theoretical and rehabilitation implications. Journal of Clinical and Experimental Neuropsychology. 1995;17:416–430. doi: 10.1080/01688639508405133. [DOI] [PubMed] [Google Scholar]
- 37.Manly T. Cognitive rehabilitation for unilateral neglect: Review. Neuropsychological Rehabilitation. 2002;12:289–310. [Google Scholar]
- 38.Sturm W, Thimm M, Kust J, et al. Alertness-training in neglect: behavioral and imaging results. Restorative neurology and neuroscience. 2006;24:371–84. [PubMed] [Google Scholar]
- 39.Arnsten AF, Pliszka SR. Catecholamine influences on prefrontal cortical function: relevance to treatment of attention deficit/hyperactivity disorder and related disorders. Pharmacology, biochemistry, and behavior. 2011;99:211–6. doi: 10.1016/j.pbb.2011.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Valentine E, Sweet P. Meditation and attention: A comparison of the effects of concentrative and mindfulness meditation on sustained attention. Mental Health, Religion, and culture. 1999;2:59–70. [Google Scholar]
- 41.Chan D, Woollacott M. Effects of level of meditation experience on attentional focus: is the efficiency of executive or orientation networks improved? Journal of Alternative and Complementary Medicine. 2007;13:651–7. doi: 10.1089/acm.2007.7022. [DOI] [PubMed] [Google Scholar]
- 42.Davidson RJ, Goleman DJ, Schwartz GE. Attentional and affective concomitants of meditation: a cross-sectional study. Journal of Abnormal Psychology. 1976;85:235–8. doi: 10.1037//0021-843x.85.2.235. [DOI] [PubMed] [Google Scholar]
- 43.Slagter HA, Lutz A, Greischar LL, et al. Mental training affects distribution of limited brain resources. PLoS Biology. 2007;5:e138. doi: 10.1371/journal.pbio.0050138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lazar SW, Bush G, Gollub RL, et al. Functional brain mapping of the relaxation response and meditation. Neuroreport. 2000;11:1581–5. [PubMed] [Google Scholar]
- 45.Brefczynski-Lewis JA, Lutz A, Schaefer HS, et al. Neural correlates of attentional expertise in long-term meditation practitioners. Proc Natl Acad Sci U S A. 2007;104:11483–8. doi: 10.1073/pnas.0606552104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tang YY, Ma Y, Wang J, et al. Short-term meditation training improves attention and self-regulation. Proceedings of the National Academy of Science USA. 2007;104:17152–6. doi: 10.1073/pnas.0707678104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Srinivasan N, Baijal S. Concentrative meditation enhances preattentive processing: a mismatch negativity study. Neuroreport. 2007;18:1709–12. doi: 10.1097/WNR.0b013e3282f0d2d8. [DOI] [PubMed] [Google Scholar]
- 48.Pagnoni G, Cekic M. Age effects on gray matter volume and attentional performance in Zen meditation. Neurobiology of Aging. 2007;28:1623–7. doi: 10.1016/j.neurobiolaging.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 49.Jha AP, Krompinger J, Baime MJ. Mindfulness training modifies subsystems of attention. Cognitive, Affective, & Behavioral Neuroscience. 2007;7:109–19. doi: 10.3758/cabn.7.2.109. [DOI] [PubMed] [Google Scholar]
- 50.Bushell WC. New beginnings: evidence that the meditational regimen can lead to optimization of perception, attention, cognition, and other functions. Ann N Y Acad Sci. 2009;1172:348–61. doi: 10.1111/j.1749-6632.2009.04960.x. [DOI] [PubMed] [Google Scholar]
- 51.Lutz A, Slagter HA, Rawlings NB, et al. Mental training enhances attentional stability: neural and behavioral evidence. J Neurosci. 2009;29:13418–27. doi: 10.1523/JNEUROSCI.1614-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wenk-Sormaz H. Meditation can reduce habitual responding. Advances in Mind-Body Medicine. 2005 [PubMed] [Google Scholar]
- 53.Chambers R, Lo BCY, Allen NB. The impact of intensive mindfulness training on attentional control, cognitive style, and affect. Cognitive Therapy and Research. 2008;32:303–322. [Google Scholar]
- 54.MacLean KA, Ferrer E, Aichele SR, et al. Intensive meditation training improves perceptual discrimination and sustained attention. Psychol Sci. 2010;21:829–39. doi: 10.1177/0956797610371339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kaul P, Passafiume J, Sargent C, et al. Meditation acutely improves psychomotor vigilance, and may decrease sleep need. Behavioral and brain Functions. 2010;6:47–56. doi: 10.1186/1744-9081-6-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Killgore WD. Effects of sleep deprivation on cognition. Progress in brain research. 2010;185:105–29. doi: 10.1016/B978-0-444-53702-7.00007-5. [DOI] [PubMed] [Google Scholar]
- 57.Olbrich S, Mulert C, Karch S, et al. EEG-vigilance and BOLD effect during simultaneous EEG/fMRI measurement. NeuroImage. 2009;45:319. doi: 10.1016/j.neuroimage.2008.11.014. [DOI] [PubMed] [Google Scholar]
- 58.Rolls ET, Inoue K, Browning A. Activity of primate subgenual cingulate cortex neurons is related to sleep. Journal of Neurophysiology. 2003;90:134–142. doi: 10.1152/jn.00770.2002. [DOI] [PubMed] [Google Scholar]
- 59.Picchioni D, Fukunaga M, Carr WS, et al. fMRI differences between early and late stage-1 sleep. Neurosci Lett. 2008;441:81–5. doi: 10.1016/j.neulet.2008.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Posner MI, Petersen SE. The attention system of the human brain. 1989. DTIC Document. [DOI] [PubMed] [Google Scholar]
- 61.Sadaghiani S, Hesselmann G, Kleinschmidt A. Distributed and antagonistic contributions of ongoing activity fluctuations to auditory stimulus detection. The Journal of Neuroscience. 2009;29:13410–13417. doi: 10.1523/JNEUROSCI.2592-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sadaghiani S, Scheeringa R, Lehongre K, et al. Intrinsic connectivity networks, alpha oscillations, and tonic alertness: a simultaneous electroencephalography/functional magnetic resonance imaging study. The Journal of Neuroscience. 2010;30:10243–10250. doi: 10.1523/JNEUROSCI.1004-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.O’Connell RG, Bellgrove MA, Dockree PM, et al. Self-Alert Training: volitional modulation of autonomic arousal improves sustained attention. Neuropsychologia. 2008;46:1379–90. doi: 10.1016/j.neuropsychologia.2007.12.018. [DOI] [PubMed] [Google Scholar]
- 64.Mottaghy FM, Willmes K, Horwitz B, et al. Systems level modeling of a neuronal network subserving intrinsic alertness. NeuroImage. 2006;29:225–233. doi: 10.1016/j.neuroimage.2005.07.034. [DOI] [PubMed] [Google Scholar]
- 65.Hasenkamp W, Wilson-Mendenhall CD, Duncan E, et al. Mind wandering and attention during focused meditation: a fine-grained temporal analysis of fluctuating cognitive states. Neuroimage. 2012;59:750–60. doi: 10.1016/j.neuroimage.2011.07.008. [DOI] [PubMed] [Google Scholar]
- 66.Lazar SW, Kerr CE, Wasserman RH, et al. Meditation experience is associated with increased cortical thickness. Neuroreport. 2005;16:1893–7. doi: 10.1097/01.wnr.0000186598.66243.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Farb NA, Segal ZV, Mayberg H, et al. Attending to the present: mindfulness meditation reveals distinct neural modes of self-reference. Social Cognitive and Affective Neuroscience. 2007;2:313–322. doi: 10.1093/scan/nsm030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lutz A, Brefczynski-Lewis J, Johnstone T, et al. Regulation of the neural circuitry of emotion by compassion meditation: effects of meditative expertise. PloS one. 2008;3:e1897. doi: 10.1371/journal.pone.0001897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sato JR, Kozasa EH, Russell TA, et al. Brain imaging analysis can identify participants under regular mental training. PloS one. 2012;7:e39832. doi: 10.1371/journal.pone.0039832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zeidan F, Martucci KT, Kraft RA, et al. Brain mechanisms supporting the modulation of pain by mindfulness meditation. J Neurosci. 2011;31:5540–8. doi: 10.1523/JNEUROSCI.5791-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Buchsbaum MS, Gillin JC, Wu J, et al. Regional cerebral glucose metabolic rate in human sleep assessed by positron emission tomography. Life Sciences. 1989;45:1349–56. doi: 10.1016/0024-3205(89)90021-0. [DOI] [PubMed] [Google Scholar]
- 72.Sawaya R, Ingvar DH. Cerebral blood flow and metabolism in sleep. Acta Neurol Scand. 1989;80:481–91. doi: 10.1111/j.1600-0404.1989.tb03915.x. [DOI] [PubMed] [Google Scholar]
- 73.Maquet P, Dive D, Salmon E, et al. Cerebral glucose utilization during sleep-wake cycle in man determined by positron emission tomography and [18F]2-fluoro-2-deoxy-D-glucose method. Brain Res. 1990;513:136–43. doi: 10.1016/0006-8993(90)91099-3. [DOI] [PubMed] [Google Scholar]
- 74.Braun AR, Balkin TJ, Wesenten NJ, et al. Regional cerebral blood flow throughout the sleep-wake cycle. An H2(15)O PET study. Brain. 1997;120(Pt 7):1173–97. doi: 10.1093/brain/120.7.1173. [DOI] [PubMed] [Google Scholar]
- 75.Maquet P, Peters J, Aerts J, et al. Functional neuroanatomy of human rapid-eye-movement sleep and dreaming. Nature. 1996;383:163–6. doi: 10.1038/383163a0. [DOI] [PubMed] [Google Scholar]
- 76.Manna A, Raffone A, Perrucci MG, et al. Neural correlates of focused attention and cognitive monitoring in meditation. Brain Res Bull. 2010;82:46–56. doi: 10.1016/j.brainresbull.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 77.Yu X, Fumoto M, Nakatani Y, et al. Activation of the anterior prefrontal cortex and serotonergic system is associated with improvements in mood and EEG changes induced by Zen meditation practice in novices. International Journal of Psychophysiology. 2011;80:103–111. doi: 10.1016/j.ijpsycho.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 78.Baron Short E, Kose S, Mu Q, et al. Regional brain activation during meditation shows time and practice effects: an exploratory FMRI study. Evidence-based complementary and alternative medicine: eCAM. 2010;7:121–7. doi: 10.1093/ecam/nem163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ritskes R, Ritskes-Hoitinga M, Stodkilde-Jorgensen H, et al. MRI scanning during Zen meditation: the picture of enlightenment? Constructivism in Human Sciences. 2003;8:85–90. [Google Scholar]
- 80.Allen M, Dietz M, Blair KS, et al. Cognitive-affective neural plasticity following active-controlled mindfulness intervention. J Neurosci. 2012;32:15601–10. doi: 10.1523/JNEUROSCI.2957-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lee TM, Leung MK, Hou WK, et al. Distinct neural activity associated with focused-attention meditation and loving-kindness meditation. PLoS ONE. 2012;7:e40054. doi: 10.1371/journal.pone.0040054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Holzel BK, Ott U, Gard T, et al. Investigation of mindfulness meditation practitioners with voxel-based morphometry. Soc Cogn Affect Neurosci. 2008;3:55–61. doi: 10.1093/scan/nsm038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Luders E, Toga A, Lepore N, et al. The underlying anatomical correlates of long-term meditation: Larger frontal and hippocampal volumes of gray matter. Neuroimage. 2009;45:672–678. doi: 10.1016/j.neuroimage.2008.12.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Critchley HD, Melmed R, Featherstone E, et al. Brain activity during biofeedback relaxation A functional neuroimaging investigation. Brain. 2001;124:1003–1012. doi: 10.1093/brain/124.5.1003. [DOI] [PubMed] [Google Scholar]
- 85.Critchley HD, Corfield DR, Chandler MP, et al. Cerebral correlates of autonomic cardiovascular arousal: a functional neuroimaging investigation in humans. The Journal of physiology. 2000;523(Pt 1):259–70. doi: 10.1111/j.1469-7793.2000.t01-1-00259.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Holzel BK, Ott U, Hempel H, et al. Differential engagement of anterior cingulate and adjacent medial frontal cortex in adept meditators and non-meditators. Neurosci Lett. 2007;421:16–21. doi: 10.1016/j.neulet.2007.04.074. [DOI] [PubMed] [Google Scholar]
- 87.Farb NA, Anderson AK, Mayberg H, et al. Minding one’s emotions: mindfulness training alters the neural expression of sadness. Emotion. 2010;10:25–33. doi: 10.1037/a0017151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Grant JA, Courtemanche J, Rainville P. A non-elaborative mental stance and decoupling of executive and pain-related cortices predicts low pain sensitivity in Zen meditators. Pain. 2011;152:150–6. doi: 10.1016/j.pain.2010.10.006. [DOI] [PubMed] [Google Scholar]
- 89.Gard T, Holzel BK, Sack AT, et al. Pain attenuation through mindfulness is associated with decreased cognitive control and increased sensory processing in the brain. Cereb Cortex. 2012;22:2692–702. doi: 10.1093/cercor/bhr352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Critchley HD, Wiens S, Rotshtein P, et al. Neural systems supporting interoceptive awareness. Nature neuroscience. 2004;7:189–95. doi: 10.1038/nn1176. [DOI] [PubMed] [Google Scholar]
- 91.Craig AD. How do you feel–now? The anterior insula and human awareness. Nature reviews Neuroscience. 2009;10:59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- 92.Hölzel BK, Carmody J, Vangel M, et al. Mindfulness practice leads to increases in regional brain gray matter density. Psychiatry Research: Neuroimaging. 2011;191:36–43. doi: 10.1016/j.pscychresns.2010.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Grant JA, Courtemanche J, Duerden EG, et al. Cortical thickness and pain sensitivity in zen meditators. Emotion. 2010;10:43–53. doi: 10.1037/a0018334. [DOI] [PubMed] [Google Scholar]
- 94.Taylor VA, Grant J, Daneault V, et al. Impact of mindfulness on the neural responses to emotional pictures in experienced and beginner meditators. Neuroimage. 2011;57:1524–33. doi: 10.1016/j.neuroimage.2011.06.001. [DOI] [PubMed] [Google Scholar]
- 95.Luders E, Kurth F, Mayer EA, et al. The unique brain anatomy of meditation practitioners: alterations in cortical gyrification. Frontiers in human neuroscience. 2012;6 doi: 10.3389/fnhum.2012.00034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Qin P, Northoff G. How is our self related to midline regions and the default-mode network? NeuroImage. 2011;57:1221–33. doi: 10.1016/j.neuroimage.2011.05.028. [DOI] [PubMed] [Google Scholar]
- 97.Berkovich-Ohana A, Glicksohn J, Goldstein A. Mindfulness-induced changes in gamma band activity - Implications for the default mode network, self-reference and attention. Clinical Neurophysiology. 2011 doi: 10.1016/j.clinph.2011.07.048. [DOI] [PubMed] [Google Scholar]
- 98.Travis F, Haaga DA, Hagelin J, et al. A self-referential default brain state: patterns of coherence, power, and eLORETA sources during eyes-closed rest and Transcendental Meditation practice. Cognitive processing. 2010;11:21–30. doi: 10.1007/s10339-009-0343-2. [DOI] [PubMed] [Google Scholar]
- 99.Brewer JA, Worhunsky PD, Gray JR, et al. Meditation experience is associated with differences in default mode network activity and connectivity. Proc Natl Acad Sci U S A. 2011;108:20254–9. doi: 10.1073/pnas.1112029108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Broyd SJ, Demanuele C, Debener S, et al. Default-mode brain dysfunction in mental disorders: a systematic review. Neurosci Biobehav Rev. 2009;33:279–96. doi: 10.1016/j.neubiorev.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 101.Buckner RL, Andrews-Hanna JR, Schacter DL. The Brain’s Default Network. Annals of the New York Academy of Sciences. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- 102.Nagai Y, Critchley H, Featherstone E, et al. Activity in ventromedial prefrontal cortex covaries with sympathetic skin conductance level: a physiological account of a “default mode” of brain function. NeuroImage. 2004;22:243–251. doi: 10.1016/j.neuroimage.2004.01.019. [DOI] [PubMed] [Google Scholar]
- 103.Carciofo R, Du F, Song N, et al. Chronotype and time-of-day correlates of mind wandering and related phenomena. Biological Rhythm Research. 2013 null-null. [Google Scholar]
- 104.Braboszcz C, Delorme A. Lost in thoughts: neural markers of low alertness during mind wandering. NeuroImage. 2011;54:3040–3047. doi: 10.1016/j.neuroimage.2010.10.008. [DOI] [PubMed] [Google Scholar]
- 105.Sabri M, Labelle S, Gosselin A, et al. Effects of sleep onset on the mismatch negativity (MMN) to frequency deviants using a rapid rate of presentation. Cognitive brain research. 2003;17:164–176. doi: 10.1016/s0926-6410(03)00090-9. [DOI] [PubMed] [Google Scholar]
- 106.Oken B, Salinsky M, Elsas S. Vigilance, alertness, or sustained attention: physiological basis and measurement. Clinical Neurophysiology. 2006;117:1885–1901. doi: 10.1016/j.clinph.2006.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hahn B, Ross TJ, Yang Y, et al. Nicotine enhances visuospatial attention by deactivating areas of the resting brain default network. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2007;27:3477–89. doi: 10.1523/JNEUROSCI.5129-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Fox K, Nijeboer S, Solomonova E, et al. Dreaming as mindwandering: evidence from functional neuroimaging and first-person content reports. Frontiers in Human Neuroscience. 7:1–18. doi: 10.3389/fnhum.2013.00412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Baerentsen KB. Onset of meditation explored with fMRI. Neuroimage. 2001;13:S297. [Google Scholar]
- 110.Baerentsen KB, Stodkilde-Jorgensen H, Sommerlund B, et al. An investigation of brain processes supporting meditation. Cogn Process. 2009 doi: 10.1007/s10339-009-0342-3. [DOI] [PubMed] [Google Scholar]
- 111.Vestergaard-Poulsen P, van Beek M, Skewes J, et al. Long-term meditation is associated with increased gray matter density in the brain stem. Neuroreport. 2009;20:170–4. doi: 10.1097/WNR.0b013e328320012a. [DOI] [PubMed] [Google Scholar]
- 112.Lang R, Dehof K, Meurer KA, et al. Sympathetic activity and transcendental meditation. Journal of Neural Transmission. 1979;44:117–35. doi: 10.1007/BF01252706. [DOI] [PubMed] [Google Scholar]
- 113.Morrell EM, Hollandsworth JG., Jr Norepinephrine alterations under stress conditions following the regular practice of meditation. Psychosomatic Medicine. 1986;48:270–7. doi: 10.1097/00006842-198603000-00012. [DOI] [PubMed] [Google Scholar]
- 114.Kjaer TW, Bertelsen C, Piccini P, et al. Increased dopamine tone during meditation-induced change of consciousness. Cognitive Brain Research. 2002;13:255–9. doi: 10.1016/s0926-6410(01)00106-9. [DOI] [PubMed] [Google Scholar]
- 115.Walton KG, Pugh ND, Gelderloos P, et al. Stress reduction and preventing hypertension: Preliminary support for a psychoneuroendocrine mechanism. Journal of Alternative and Complementary Medicine. 1995;1:263–83. doi: 10.1089/acm.1995.1.263. [DOI] [PubMed] [Google Scholar]
- 116.Bujatti M, Rierderer P. Serotonin, norepinephrine, dopamine metabolites in transcendental meditation-technique. Journal of Neural Transmission. 1976;39:257–267. doi: 10.1007/BF01256514. [DOI] [PubMed] [Google Scholar]
- 117.Loliger S. Relationship between subjective bliss, 5-hydroxy-3-indoleacetic acid and the collective practice of Maharishi’s Transcendental Meditation and TM-Sidhi Program. Dissertation Abstracts International: Section B: The Sciences and Engineering. 1991;52:551. [Google Scholar]
- 118.Solberg E, Holen A, Ekeberg Ø, et al. The effects of long meditation on plasma melatonin and blood serotonin. Medical Science Monitor. 2004;10:96–101. [PubMed] [Google Scholar]
- 119.Massimini M, Ferrarelli F, Huber R, et al. Breakdown of cortical effective connectivity during sleep. Science. 2005;309:2228–32. doi: 10.1126/science.1117256. [DOI] [PubMed] [Google Scholar]
- 120.Horovitz SG, Braun AR, Carr WS, et al. Decoupling of the brain’s default mode network during deep sleep. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:11376–81. doi: 10.1073/pnas.0901435106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Samann PG, Wehrle R, Hoehn D, et al. Development of the brain’s default mode network from wakefulness to slow wave sleep. Cereb Cortex. 2011;21:2082–93. doi: 10.1093/cercor/bhq295. [DOI] [PubMed] [Google Scholar]
- 122.Chow HM, Horovitz SG, Carr WS, et al. Rhythmic alternating patterns of brain activity distinguish rapid eye movement sleep from other states of consciousness. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:10300–5. doi: 10.1073/pnas.1217691110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Taylor VA, Daneault V, Grant J, et al. Impact of meditation training on the default mode network during a restful state. Soc Cogn Affect Neurosci. 2013;8:4–14. doi: 10.1093/scan/nsr087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Lutz A, Greischar LL, Rawlings NB, et al. Long-term meditators self-induce high-amplitude gamma synchrony during mental practice. Proc Natl Acad Sci U S A. 2004;101:16369–73. doi: 10.1073/pnas.0407401101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Hasenkamp W, Barsalou LW. Effects of meditation experience on functional connectivity of distributed brain networks. Frontiers in human neuroscience. 2012;6:38. doi: 10.3389/fnhum.2012.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Jang JH, Jung WH, Kang DH, et al. Increased default mode network connectivity associated with meditation. Neurosci Lett. 2011;487:358–62. doi: 10.1016/j.neulet.2010.10.056. [DOI] [PubMed] [Google Scholar]
- 127.Kilpatrick LA, Suyenobu BY, Smith SR, et al. Impact of Mindfulness-Based Stress Reduction training on intrinsic brain connectivity. Neuroimage. 2011;56:290–8. doi: 10.1016/j.neuroimage.2011.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Thompson GJ, Magnuson ME, Merritt MD, et al. Short-time windows of correlation between large-scale functional brain networks predict vigilance intraindividually and interindividually. Human brain mapping. 2012 doi: 10.1002/hbm.22140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.De Havas JA, Parimal S, Soon CS, et al. Sleep deprivation reduces default mode network connectivity and anti-correlation during rest and task performance. NeuroImage. 2012;59:1745–51. doi: 10.1016/j.neuroimage.2011.08.026. [DOI] [PubMed] [Google Scholar]
- 130.Josipovic Z, Dinstein I, Weber J, et al. Influence of meditation on anti-correlated networks in the brain. Frontiers in human neuroscience. 2012;5:183. doi: 10.3389/fnhum.2011.00183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Nofzinger E. Neuroimaging of primary insomnia. In: Nofzinger E, Maquet P, Thorpy M, editors. Neuroimaging of sleep and sleep disorders. Cambridge University Press; Cambridge: 2013. pp. 197–208. [Google Scholar]
- 132.Ong JC, Shapiro SL, Manber R. Combining mindfulness meditation with cognitive-behavior therapy for insomnia: a treatment-development study. Behav Ther. 2008;39:171–82. doi: 10.1016/j.beth.2007.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Carlson LE, Speca M, Faris P, et al. One year pre-post intervention follow-up of psychological, immune, endocrine and blood pressure outcomes of mindfulness-based stress reduction (MBSR) in breast and prostate cancer outpatients. Brain Behav Immun. 2007;21:1038–49. doi: 10.1016/j.bbi.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 134.Surawy C, Roberts J, Silver A. The effect of mindfulness training on mood and measures of fatigue, activity, and quality of life in patients with chronic fatigue syndrome on a hospital waiting list: A series of exploratory studies. Behavioural and Cognitive Psychotherapy. 2005;33:103–109. [Google Scholar]
- 135.Singh BB, Berman BM, Hadhazy VA, et al. A pilot study of cognitive behavioral therapy in fibromyalgia. Altern Ther Health Med. 1998;4:67–70. [PubMed] [Google Scholar]
- 136.van der Lee ML, Garssen B. Mindfulness-based cognitive therapy reduces chronic cancer-related fatigue: a treatment study. Psycho-Oncology. 2012;21:264–272. doi: 10.1002/pon.1890. [DOI] [PubMed] [Google Scholar]
- 137.Sampalli T, Berlasso E, Fox R, et al. A controlled study of the effect of a mindfulness-based stress reduction technique in women with multiple chemical sensitivity, chronic fatigue syndrome, and fibromyalgia. Journal of multidisciplinary healthcare. 2009;2:53. doi: 10.2147/jmdh.s5220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Rimes KA, Wingrove J. Mindfulness-Based Cognitive Therapy for People with Chronic Fatigue Syndrome Still Experiencing Excessive Fatigue after Cognitive Behaviour Therapy: A Pilot Randomized Study. Clinical Psychology & Psychotherapy. 2011 doi: 10.1002/cpp.793. [DOI] [PubMed] [Google Scholar]
- 139.Grossman P, Kappos L, Gensicke H, et al. MS quality of life, depression, and fatigue improve after mindfulness training A randomized trial. Neurology. 2010;75:1141–1149. doi: 10.1212/WNL.0b013e3181f4d80d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Johansson B, Bjuhr H, Rönnbäck L. Mindfulness-based stress reduction (MBSR) improves long-term mental fatigue after stroke or traumatic brain injury. Brain Injury. 2012;26:1621–1628. doi: 10.3109/02699052.2012.700082. [DOI] [PubMed] [Google Scholar]
- 141.Zangi H, Finset A, Steen E, et al. The effects of a vitality training programme on psychological distress in patients with inflammatory rheumatic diseases and fibromyalgia: a 1-year follow-up. Scandinavian journal of rheumatology. 2009;38:231–232. doi: 10.1080/03009740802474680. [DOI] [PubMed] [Google Scholar]
- 142.Zeidan F, Johnson SK, Diamond BJ, et al. Mindfulness meditation improves cognition: Evidence of brief mental training. Consciousness and cognition. 2010;19:597–605. doi: 10.1016/j.concog.2010.03.014. [DOI] [PubMed] [Google Scholar]
- 143.Elson B, Hauri P, Cunis D. Physiological changes in Yoga meditation. Psychophysiology. 1977;14:52–57. doi: 10.1111/j.1469-8986.1977.tb01155.x. [DOI] [PubMed] [Google Scholar]
- 144.Banquet JP, Sailhan M. EEG analysis of spontaneous and induced states of consciousness. Revue d’Electroencephalographie et de Neurophysiologie Clinique. 1974;4:445–453. doi: 10.1016/s0370-4475(74)80056-0. [DOI] [PubMed] [Google Scholar]
- 145.Aftanas LI, Golocheikine SA. Human anterior and frontal midline theta and lower alpha reflect emotionally positive state and internalized attention: high-resolution EEG investigation of meditation. Neurosci Lett. 2001;310:57–60. doi: 10.1016/s0304-3940(01)02094-8. [DOI] [PubMed] [Google Scholar]
- 146.Cahn BR, Delorme A, Polich J. Occipital gamma activation during Vipassana meditation. Cogn Process. 2010;11:39–56. doi: 10.1007/s10339-009-0352-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Baijal S, Srinivasan N. Theta activity and meditative states: spectral changes during concentrative meditation. Cogn Process. 2010;11:31–8. doi: 10.1007/s10339-009-0272-0. [DOI] [PubMed] [Google Scholar]
- 148.Asada H, Fukuda Y, Tsunoda S, et al. Frontal midline theta rhythms reflect alternative activation of prefrontal cortex and anterior cingulate cortex in humans. Neurosci Lett. 1999;274:29–32. doi: 10.1016/s0304-3940(99)00679-5. [DOI] [PubMed] [Google Scholar]
- 149.Ishii R, Shinosaki K, Ukai S, et al. Medial prefrontal cortex generates frontal midline theta rhythm. Neuroreport. 1999;10:675–9. doi: 10.1097/00001756-199903170-00003. [DOI] [PubMed] [Google Scholar]
- 150.Braboszcz C, Cahn B, Levy J, et al. Increased gamma brainwave amplitude in di fferent meditation traditions. in preparation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Tei S, Faber PL, Lehmann D, et al. Meditators and non-meditators: EEG source imaging during resting. Brain Topogr. 2009;22:158–65. doi: 10.1007/s10548-009-0107-4. [DOI] [PubMed] [Google Scholar]
- 152.Saggar M, King BG, Zanesco AP, et al. Intensive training induces longitudinal changes in meditation state-related EEG oscillatory activity. Frontiers in human neuroscience. 2012;6:256. doi: 10.3389/fnhum.2012.00256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Engel AK, Fries P. Beta-band oscillations–signalling the status quo? Current opinion in neurobiology. 2010;20:156–65. doi: 10.1016/j.conb.2010.02.015. [DOI] [PubMed] [Google Scholar]
- 154.Ritter P, Moosmann M, Villringer A. Rolandic alpha and beta EEG rhythms’ strengths are inversely related to fMRI-BOLD signal in primary somatosensory and motor cortex. Human brain mapping. 2009;30:1168–87. doi: 10.1002/hbm.20585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Schubert R, Haufe S, Blankenburg F, et al. Now you’ll feel it, now you won’t: EEG rhythms predict the effectiveness of perceptual masking. J Cogn Neurosci. 2009;21:2407–19. doi: 10.1162/jocn.2008.21174. [DOI] [PubMed] [Google Scholar]
- 156.Moran RJ, Campo P, Maestu F, et al. Peak frequency in the theta and alpha bands correlates with human working memory capacity. Front Hum Neurosci. 2010;4:200. doi: 10.3389/fnhum.2010.00200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Slagter HA, Lutz A, Greischar LL, et al. Mental training affects distribution of limited brain resources. PLoS Biol. 2007;5:e138. doi: 10.1371/journal.pbio.0050138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Slagter HA, Lutz A, Greischar LL, et al. Theta phase synchrony and conscious target perception: impact of intensive mental training. J Cogn Neurosci. 2009;21:1536–49. doi: 10.1162/jocn.2009.21125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Cahn BR, Delorme A, Polich J. Event-related delta, theta, alpha and gamma correlates to auditory oddball processing during Vipassana meditation. Soc Cogn Affect Neurosci. 2013;8:100–11. doi: 10.1093/scan/nss060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Cahn BR, Polich J. Meditation (Vipassana) and the P3a event-related brain potential. Int J Psychophysiol. 2009;72:51–60. doi: 10.1016/j.ijpsycho.2008.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Norbu N. Dream Yoga and the Practice of Natural Light. Ithaca: Snow Lion Publications; 2002. [Google Scholar]
- 162.Mason L, Alexander C, Travis F, et al. Electrophysiological correlates of higher states of consciousness during sleep in long term practitioners of the TM program. Sleep. 1997;20:102–110. doi: 10.1093/sleep/20.2.102. [DOI] [PubMed] [Google Scholar]
- 163.Kornfield J. Intensive insight meditation: A phenomenological study. Journal of Transpersonal Psychology 1979 [Google Scholar]
- 164.Britton WB, Haynes PL, Fridel KW, et al. Polysomnographic and subjective profiles of sleep continuity before and after mindfulness-based cognitive therapy in partially remitted depression. Psychosom Med. 2010;72:539–48. doi: 10.1097/PSY.0b013e3181dc1bad. [DOI] [PubMed] [Google Scholar]
- 165.Ferrarelli F, Smith R, Dentico D, et al. Experienced mindfulness meditators exhibit higher parietal-occipital EEG gamma activity during NREM sleep. PloS one. doi: 10.1371/journal.pone.0073417. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Winbush NY, Gross CR, Kreitzer MJ. The effects of mindfulness-based stress reduction on sleep disturbance: a systematic review. Explore (NY) 2007;3:585–91. doi: 10.1016/j.explore.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 167.Britton WB, Haynes PL, Fridel KW, et al. Mindfulness-based cognitive therapy improves polysomnographic and subjective sleep profiles in antidepressant users with sleep complaints. Psychother Psychosom. 2012;81:296–304. doi: 10.1159/000332755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Gross CR, Kreitzer MJ, Reilly-Spong M, et al. Mindfulness-based stress reduction versus pharmacotherapy for chronic primary insomnia: a randomized controlled clinical trial. Explore (NY) 2011;7:76–87. doi: 10.1016/j.explore.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Klatt MD, Buckworth J, Malarkey WB. Effects of low-dose mindfulness-based stress reduction (MBSR-ld) on working adults. Health Educ Behav. 2009;36:601–14. doi: 10.1177/1090198108317627. [DOI] [PubMed] [Google Scholar]
- 170.Shapiro SL, Bootzin RR, Figueredo AJ, et al. The efficacy of mindfulness-based stress reduction in the treatment of sleep disturbance in women with breast cancer: an exploratory study. J Psychosom Res. 2003;54:85–91. doi: 10.1016/s0022-3999(02)00546-9. [DOI] [PubMed] [Google Scholar]
- 171.Roth B, Robbins D. Mindfulness-based stress reduction and health-related quality of life: findings from a bilingual inner-city patient population. Psychosom Med. 2004;66:113–23. doi: 10.1097/01.psy.0000097337.00754.09. [DOI] [PubMed] [Google Scholar]
- 172.Goldenberg D, Kaplan K, Nadeau M, et al. A controlled trial of a stress-reduction, cognitive behavioral treatment program in fibromyalgia. J Muskuloskel Pain. 1994;2:53–56. [Google Scholar]
- 173.Andersen SR, Wurtzen H, Steding-Jessen M, et al. Effect of mindfulness-based stress reduction on sleep quality: results of a randomized trial among Danish breast cancer patients. Acta Oncol. 2013;52:336–44. doi: 10.3109/0284186X.2012.745948. [DOI] [PubMed] [Google Scholar]
- 174.Shapiro SL, Bootzin RR, Figueredo AJ, et al. The efficacy of mindfulness-based stress reduction in the treatment of sleep disturbance in women with breast cancer: An exploratory study. J Psychosom Res. 2003;54:85–91. doi: 10.1016/s0022-3999(02)00546-9. [DOI] [PubMed] [Google Scholar]
- 175.Britton WB, Bootzin RR, Cousins JC, et al. The contribution of mindfulness practice to a multicomponent behavioral sleep intervention following substance abuse treatment in adolescents: a treatment-development study. Subst Abus. 2010;31:86–97. doi: 10.1080/08897071003641297. [DOI] [PubMed] [Google Scholar]
- 176.Meditation Programs for Stress and Well-being: Evidence Report. Agency for Healthcare Research and Quality. U.S. Department of Health and Human Services; 2012. [Google Scholar]
- 177.Tang YY, Posner MI. Tools of the trade: theory and method in mindfulness neuroscience. Soc Cogn Affect Neurosci. 2013;8:118–20. doi: 10.1093/scan/nss112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Tang YY, Posner MI. Attention training and attention state training. Trends in cognitive sciences. 2009;13:222–7. doi: 10.1016/j.tics.2009.01.009. [DOI] [PubMed] [Google Scholar]
- 179.Posner M, Rothbart MK, Rueda M, et al. Training effortless attention. In: Bruya B, editor. Effortless attention: A new perspective in the cognitive science of attention and action. MIT press; Boston: 2010. [Google Scholar]
- 180.Briggs F, Mangun GR, Usrey WM. Attention enhances synaptic efficacy and the signal-to-noise ratio in neural circuits. Nature. 2013;499:476–80. doi: 10.1038/nature12276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Silverstein RG, Brown AC, Roth HD, et al. Effects of mindfulness training on body awareness to sexual stimuli: implications for female sexual dysfunction. Psychosom Med. 2011;73:817–25. doi: 10.1097/PSY.0b013e318234e628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Fox KC, Zakarauskas P, Dixon M, et al. Meditation experience predicts introspective accuracy. PLoS ONE. 2012;7:e45370. doi: 10.1371/journal.pone.0045370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Kerr CE, Sacchet MD, Lazar SW, et al. Mindfulness starts with the body: somatosensory attention and top-down modulation of cortical alpha rhythms in mindfulness meditation. Frontiers in Human Neuroscience. 2013;7 doi: 10.3389/fnhum.2013.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Robertson IH, Mattingley JB, Rorden C, et al. Phasic alerting of neglect patients overcomes their spatial deficit in visual awareness. Nature. 1998;395:169–72. doi: 10.1038/25993. [DOI] [PubMed] [Google Scholar]
- 185.Kabat-Zinn J. Full catastrophe living: Using the wisdom of your body and mind to face stress, pain, and illness. 1990. Delta. [Google Scholar]
- 186.Thompson E, Davis JH. From the Five Aggregates to Phenomenal Consciousness: Towards a Cross-Cultural Cognitive Science. In: Emmanuel SM, editor. A Companion to Buddhist Philosophy. Wiley-Blackwell; 2013. [Google Scholar]
- 187.Tang YY, Ma Y, Fan Y, et al. Central and autonomic nervous system interaction is altered by short-term meditation. Proc Natl Acad Sci U S A. 2009;106:8865–70. doi: 10.1073/pnas.0904031106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Tang YY, Lu Q, Fan M, et al. Mechanisms of white matter changes induced by meditation. Proc Natl Acad Sci U S A. 2012;109:10570–4. doi: 10.1073/pnas.1207817109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Goldin PR, Gross JJ. Effects of mindfulness-based stress reduction (MBSR) on emotion regulation in social anxiety disorder. Emotion. 2010;10:83–91. doi: 10.1037/a0018441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Ives-Deliperi VL, Solms M, Meintjes EM. The neural substrates of mindfulness: an fMRI investigation. Soc Neurosci. 2011;6:231–242. doi: 10.1080/17470919.2010.513495. [DOI] [PubMed] [Google Scholar]
- 191.Klimecki OM, Leiberg S, Lamm C, Singer T. Functional neural plasticity and associated changes in positive affect after compassion training. Cereb Cortex. 2013;23:1552–1561. doi: 10.1093/cercor/bhs142. [DOI] [PubMed] [Google Scholar]


