Abstract
Advances in stem cell biology have raised great expectations that diseases and injuries of the central nervous system (CNS) may be ameliorated by the development of non-hematopoietic stem cell medicines. Yet, the application of adult stem cells as CNS therapeutics is challenging and the interpretation of some of the outcomes ambiguous. In fact, the initial idea that stem cell transplants work only via structural cell replacement has been challenged by the observation of consistent cellular signaling between the graft and the host. Cellular signaling is the foundation of coordinated actions and flexible responses, and arises via networks of exchanging and interacting molecules that transmit patterns of information between cells. Sustained stem cell graft-to-host communication leads to remarkable trophic effects on endogenous brain cells and beneficial modulatory actions on innate and adaptive immune responses in vivo, ultimately promoting the healing of the injured CNS. Among a number of adult stem cell types, mesenchymal stem cells (MSCs) and neural stem/precursor cells (NPCs) are being extensively investigated for their ability to signal to the immune system upon transplantation in experimental CNS diseases. Here, we focus on the main cellular signaling pathways that grafted MSCs and NPCs use to establish a therapeutically relevant cross talk with host immune cells, while examining the role of inflammation in regulating some of the bidirectionality of these communications. We propose that the identification of the players involved in stem cell signaling might contribute to the development of innovative, high clinical impact therapeutics for inflammatory CNS diseases.
Keywords: stem cell–host interactions, neural stem cells, mesenchymal stem cells, immune modulation, inflammation
Introduction
Cell replacement therapies with adult stem cells have received much attention in recent years as a potential means of driving recovery after CNS damage (Martino and Pluchino, 2006), such as that accumulating during the course of neurological diseases characterized by inflammation, which include multiple sclerosis (MS), brain stroke, spinal cord injuries (SCI), Alzheimer’s disease (AD), Parkinson’s disease (PD), and amyotrophic lateral sclerosis (ALS) (Glass et al., 2010).
Bone marrow-derived mesenchymal stem cells (MSCs) and subventricular zone (SVZ)-derived neural stem/precursor cells (NPCs) exert remarkable trophic effects on endogenous neural cells and beneficial modulatory actions on inflammatory responses. The systemic injection of these stem cells into rodents and non-human primates with immune-mediated experimental CNS demyelination, stroke and injuries of the spinal cord has led to neuroprotection and recovery of function (Martino et al., 2011; Uccelli et al., 2011b). However, the concomitant observation that a surprisingly low number of transplanted stem cells survive, differentiate and integrate in vivo (Lees et al., 2012) has inspired the important new concept that stem cell grafts are capable of a multitude of bystander tissue healing effects where the initially expected differentiation potential loses the lead (Rossi and Cattaneo 2002). Thus, the emerging concept of stem cell therapeutic plasticity, or functional multipotency, recapitulates the multiple ways in which stem cell grafts can mediate systemic homeostasis. This concept also encompasses the interactions of stem cell grafts with CNS-resident versus CNS-infiltrating immune cells at the level of the inflammatory tissue area, in which they are either transplanted or to which they migrate after transplantation (Martino and Pluchino 2006; Teng et al., 2011).
While a comprehensive understanding of the mechanisms by which stem cell grafts work is still lacking, it may be likely that they exert some of their therapeutic effects by secreting a complex array of homeostatic molecules with immune regulatory and tissue trophic functions that ultimately reduce tissue damage and/or enhance endogenous repair (Li and Xie, 2005). Most of these properties are shared between different stem cell types and define key developmental conserved regulatory pathways (Ivanova et al., 2002), and anticipate the presence of a common stem cell extracellular (secreted) signature capable of modulating some key intrinsic reactions of cells and tissues that are ultimately responsible for the repair of injured tissues, including the CNS (Martino and Pluchino, 2006; Uccelli et al., 2008).
The idea that stem cell transplants work typically via structural cell replacement (Rossi and Cattaneo, 2002) is now being significantly challenged by the evidence of consistent cellular signaling between the stem cell graft and the host (Martino et al., 2011). Stem cell graft-to-host communication is delivered with secreted cytokines and/or growth factors, or through communicating cellular (Gap) junctional transfer of electrical, metabolic and immunological information (Ratajczak et al., 2012). Some very early work also suggests that extracellular membrane vesicles (EVs) might play a key role, and are transferred from donor grafted stem cells to target endogenous cells (Cossetti et al., 2012b).
The newest picture is therefore that stem cell therapies, contrary to single-molecule-based pharmaceutical interventions, hold the potential to deliver a complex series of information to a multitude of targets in the diseased microenvironment (Cossetti et al., 2012a). A number of studies are now focusing on the cellular signaling that exists between grafted stem cells and endogenous target cells, with the aim of clarifying its physiological or circumstantial nature, and elucidating its molecular signature and therapeutic potential.
Here, we will specifically focus on MSC- and NPC-based transplantation approaches in the context of brain diseases. We will examine the main cellular signaling pathways that grafted stem cells use to establish a therapeutically relevant cross talk with the host immune system, and discuss the potential role of local inflammation in regulating some of the bidirectionality of this cellular communication. Concurrently, we will examine how engrafted stem cells influence the initiation and maintenance of both innate and adaptive immune responses, while providing insights into how the understanding of the mechanisms regulating this reciprocal relationship might contribute to the development of innovative, high clinical impact therapeutic strategies for regenerative neurosciences.
Environmental Sensors and Stem Cell Graft-to-Host Immune System Interactions
The in vivo interactions between the stem cell graft and the host immune system are mediated by functional environmental sensors, which play significant roles in both the immunogenicity and the functional plasticity of the graft.
The Immunogenicity of the Stem Cell Graft
The immunogenicity is the ability of allogeneic stem cells to provoke an immune response when facing the host immune system after transplantation (e.g. at the level of the CNS tissue after focal transplantation, or into the blood stream immediately after systemic injection) (Schu et al., 2012). The mechanism of rejection by the host immune system implies that donor major histocompatibility complex (MHC)-expressing cells stimulate recipient CD8+ or CD4+ T cells, either directly in the presence of appropriate co-stimulatory molecules (such as CD80/B7.1 or CD86/B7.2), or indirectly through cross presentation of MHC alloantigens by professional APCs (Brevig et al., 2000; Kamoun, 2006).
Whether or not allogeneic (or xenogeneic) stem cell grafts are intrinsically immunogenic is still a matter of debate. Part of the immunogenicity of stem cells is determined by evaluating the expression of MHC-I and –II and co-stimulatory molecules, or by assessing their behavior in mixed lymphocyte reactions (MLR) in vitro, and in allogeneic transplantation settings in vivo (De Miguel et al., 2012). While this property of stem cells has virtually no impact on the outcome of MSC autografts in phase I/II clinical trials (Connick et al., 2012; Lee et al., 2012; Mazzini et al., 2012), or within experimental syngeneic settings (Morando et al., 2012), it becomes more relevant when (single donor) grafted allogeneic human NPCs are evaluated in phase I clinical trials for rare leukodystrophies (Gupta et al., 2012; Steiner et al., 2010), ALS (Glass et al., 2012; Riley et al., 2012) or stroke, with or without concomitant immune suppression (Aboody et al., 2011).
Recent evidence supports the possibility that undifferentiated adult stem cells are endowed with an immunologically privileged status and are capable of escaping the normal processes of allogeneic rejection (Bifari et al., 2010). However, there is some controversy regarding the expression of MHC and co-stimulatory molecules by MSCs and NPCs, as well as their upregulation upon exposure to cytokines mimicking an inflammatory environment, or when comparing different protocols for isolation and culture (Table 1). Induced pluripotent stem (iPS) cells are now being proposed as a potential source of autologous stem cells for therapy, and early evidence already exists that differentiated cells generated from autologous iPS cells can be applied for cell replacement therapy without eliciting immune rejection (Guha et al., 2013).
TABLE 1. Immunophenotype of MSCs and NPCs.
| MSCa | NPC | ||||
|---|---|---|---|---|---|
| Ligand(s) | Rodent | Human | Rodent | Human | |
| Chemokine receptors | |||||
| CCR1 (CD191) | Macrophage inflammatory protein (MIP)-1α/CCL3, Regulated on Activation Normal T Expressed and Secreted protein (RANTES)/CCL5, Monocyte chemoattractant protein (MCP)-3/CCL7, and Myeloid progenitor inhibitory factor (MPIF)-1/CCL23 | ± | + | ||
| CCR2 (CD192) | Monocyte chemoattractant protein (MCP)-1/CCL2 | + | +b | + | |
| CCR3 (CD193) | Eotaxin/CCL11, Eotaxin-3/CCL26, CCL7, MCP-4/CCL13, and CCL5 | ± b | |||
| CCR4 (CD194) | CCL2, MIP-1β/CCL4, CCL5, Thymus and Activation Regulated Chemokine (TARC)/CCL17, Macrophage-derived chemokine (MDC)/CCL22 | ± b | |||
| CCR5 (CD195) | CCL5, CCL3, CCL4, CCL31 | + | + | ± | |
| CCR5 (CD196) | MIP3 α/CCL6 | + | |||
| CCR7 (CD197) | EBI1 Ligand Chemokine (ELC)/CCL19 and secondary lymphoid-tissue chemokine (SLC)/CCL21 | + | |||
| CCR8 (CDw198) | CCL1 | + | |||
| CCR9 (CDw199) | Thymus-Expressed Chemokine (TECK)/CCL25 | + | + | ||
| CXCR1 (CD181) | Neutrophil chemotactic factor (IL-8) | + | |||
| CXCR2 (CD182) | IL-8, Neutrophil-activating protein 3 (NAP-3)/CXCL1, MIP-2α/CXCL2, MIP-2β/CXCL3 | + | |||
| CXCR3 (CD183) | Platelet Factor 4 (PF4)/CXCL4, Monokine induced by gamma interferon (MIG)/CXCL9), Interferon gamma-induced protein 10 (IP-10)/CXCL10, Interferon-inducible T-cell alpha chemoattractant (I-TAC)/CXCL11 | + | + | ||
| CXCR4 (CD184) | Stromal cell-derived factor (SDF)-1 α/CXCL12 | + | ± | + | + |
| CXCR5 (CD185) | B lymphocyte chemoattractant (BLC)/CXCL13 | + | |||
| CXCR6 (CD186) | CXCL16 | + | + | ||
| CX3CR1 (GPR 13) | Fractalkine/CXC3CL1 | + | + | ||
| Integrins | |||||
| α1 (CD49a) | Collagen and Laminin | + | − | ||
| α2 (CD49b) | Multiple on cellular membrane (role in cell adhesion) | +low | + | ||
| α4 (CD49d) | Galectins, Paxillin, multiple on ECM | ± | + | ||
| α5 (CD49e) | Fibronectin, role in cell-surface mediated signaling | + | ± | ||
| α6 (CD49f ) | Tetraspannins, role in cell-surface mediated signaling | low | + | ||
| αv (CD51) | Vitronectin receptor and multiple on ECM | +low | |||
| β1 (CD29) | Netrin-1 and Reelin, multiple on ECM | + | |||
| β2 (CD18) | Multiple, role in cell adhesion and cell-surface mediated signaling | − | + | ||
| β3(CD61) | Multiple, role in cell adhesion and cell-surface mediated signaling | + | |||
| β4(CD104) | Laminins, multiple on ECM | + | |||
| Clusters designation (CDs) | |||||
| CD11b | Inactivated complement component 3b (iC3b) | − | |||
| CD13 | Not known | ± | + | ||
| CD14 | Co-receptor for bacterial lipopolysaccharide (LPS) | − | |||
| CD19 | CD81, CD82, Complement receptor 2, VAV2 | − | |||
| CD24 | Multiple, role in cell adhesion | − | low | ||
| CD34 | Multiple on ECM, role in cell adhesion | ± | − | − | |
| CD40 | CD40L (CD154) | − | − | ||
| CD44 | Hyaluronic acid (HA), Osteopontin, Collagens, Matrix metalloproteinases (MMPs) | + | + | + | + |
| CD45 | Multiple, role in cell growth, differentiation, mitotic cycle, and oncogenic transformation | ± | − | − | − |
| CD54 | Multiple integrins, including CD11a/CD18 or CD11b/CD18 | + | |||
| CD56 | Multiple, role in cell–cell adhesion, neurite outgrowth, synaptic plasticity, and learning and memory | − | − | + | + |
| CD71 | Transferrin | + | |||
| CD73 | Not known | + | + | ||
| CD79 | Not known | − | |||
| CD80 | CD28 and Cytotoxic T-Lymphocyte Antigen 4 (CTLA)-4/CD152 | − | ± | ||
| CD86 | CD28 and CD152 | − | ± | ||
| CD90 | Not known | + | + | ||
| CD105 | Transforming growth factor (TGF)-β1 and β3, Activin A, bone morphogenetic protein 2 and 7 | + | + | ||
| CD106 | α and β integrins, role in cell adhesion | + | |||
| CD107 | Stem Cell Factor (SCF) | ± | − | ||
| CD133 | Not known | − | + | + | |
| CD166 | CD6 | + | |||
| CD271 | Nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF), neurotrophin (NT)-3 and –4 | ± | + | + | |
| Toll-like receptors (TLRs) | |||||
| TLR 1 (CD281) | Triacyl lipopeptides | + | + | ||
| TLR 2 (CD282) | Peptidoglycan, lipoteichoic acid, porins, lipopeptides, lipoglycans, zymosan | + | + | +b | +b |
| TLR 3 (CD283) | Double-stranded RNA | + | + | + | + |
| TLR 4 (CD284) | Lipopolysaccharide, glycans, envelope proteins, glycoinositolphospholipids | + | + | +b | +b |
| TLR 5 (CD285) | Flagellin | + | + | ||
| TLR 6 (CD286) | Lipoteichoic acid, lipopeptides, zymosan | + | + | ||
| TLR 7 (CD287) | Single-stranded RNA | ± | ± | ||
| TLR 8 (CD288) | Single-stranded RNA | ± | ± | ||
| TLR 9 (CD289) | CpG DNA, hemozoin | ± | ± | ||
| TLR 10 (CD2909) | Not known | ± | ± | ||
| Other | |||||
| Lin | Not known | + | |||
| MHC-I (HLA- ABC) | Not known | +lowb | + | ||
| MHC-II (HLA-DR) | Not known | − | − | − b | − b |
| Sca-1 | Not known | + | + | + | |
| Stro-1 | Not known | + | + | − | |
Evidence from bone marrow-derived MSCs only are quoted.
Upregulated under inflammatory conditions. Data in Table 1 are in part summarized in (Chamberlain et al., 2008; Hall et al., 2006; Martino and Pluchino 2006; Pluchino et al., 2009b; Rojewski et al., 2008; Uccelli et al., 2008; Yuan et al., 2011).
With regenerative medicine clinical trials on the horizon, and limited knowledge of potential stem cell graft-versus-host interactions to consider, it is imperative that the immunogenicity of stem cells is understood (Pearl et al., 2012).
In Vitro Stem Cell Immunogenicity
In vitro, both MSCs and NPCs are MHC-I low/medium and MHC-II negative (Klyushnenkova et al., 2005; Le Blanc et al., 2003; Pluchino et al., 2005, 2009a,b), but upregulation of MHC-I and -II expression is observed after long-term neurosphere expansion (Laguna Goya et al., 2011; Odeberg et al., 2005) or NPC exposure to proinflammatory cytokines such as interferon (IFN)-γ or tumor necrosis factor (TNF)-α (Johansson et al., 2008). Under basal growth conditions the co-stimulatory molecules CD80/B7.1, CD86/B7.2 and CD40 are also absent in both MSCs and NPCs (Odeberg et al., 2005; Tse et al., 2003). MSCs and NPCs fail to elicit a proliferative response when co-cultured with allogeneic mismatched peripheral blood mononuclear cells (PBMCs) (Laguna Goya et al., 2011; Odeberg et al., 2005; Tse et al., 2003). A single study investigating the immunogenicity of human NPCs in vitro, by one-way MLR with peripheral blood lymphocytes from human leukocyte antigen (HLA)-mismatched donors, shows than NPCs hold a low—but not negligible—immunogenic potential that is sufficient to activate peripheral lymphocytes. In this context, the transcription and release of transforming growth factor (TGF)-β1 by NPCs balance their immunogenicity (Ubiali et al., 2007). Alloreactive cytotoxic T lymphocytes and NK cells fail to lyse untreated NPCs, but readily kill IFN-γ-treated NPCs that have upregulated MHC-I and -II (Mammolenti et al., 2004). This implies that adult stem cells have the potential to induce T-cell anergy or T-cell unresponsiveness due to a lack of costimulation (Imitola et al., 2004a). Such an immune-privileged status of stem cells may be epiphenomenal to a critical balance of suppressing and activating effects in which the low immunogenicity of the stem cell prevails only when stem cell-mediated downregulation of immune cell activation overrides its own allostimulatory potential (Fang et al., 2006). From this perspective, the suppression exerted only by a large number of MSCs on MLRs in vitro more likely reflects cell dose rather than histocompatibility effects that would result from an overload of stimulatory mechanisms (Le Blanc et al., 2003) (Table 1).
In Vivo Graft Stem Cell Immunogenicity
In vivo, stem cells may not retain infinite immune privilege and the inflammatory context to which they are exposed upon transplantation may influence their immune phenotype (De Miguel et al., 2012; Laguna Goya et al., 2011). Allogeneic rodent MSCs are recognized by the host immune system in vivo, elicit a cellular and humoural immune response, and fail to induce tolerance in graft-versus-host disease (GVHD) (Badillo et al., 2008; Ringden and Le Blanc, 2011). When studied within a non-myeloablative allogeneic bone marrow (BM) transplantation setting in naive immune competent mice, infused allogeneic MSCs prime host T cells and induce a memory T-cell response, resulting in rejection of the BM graft (Eliopoulos et al., 2005; Nauta et al., 2006). This partial lack of immune competence has been attributed to defects in the expression of different components of antigen processing machinery by MSCs (e.g. chaperone ERp57, MB1, and zeta components of 20S proteasome and immunoproteasomal components LMP7 and LMP10), irrespective of IFN-γ stimulation. This lack has also been attributed to the presence of the immunosuppressive, non-polymorphic HLA class Ib molecule HLA-G (Morandi et al., 2008).
The low immunogenicity of grafted NPCs is suggested by the lack of acute immune response directed toward intracerebrally transplanted mouse NPCs in rats with middle cerebral artery occlusion (MCAo) (Modo et al., 2002). Similarly, low immunogenicity is observed when allo/xenogeneic NPCs are either directly injected into the blood stream (Lee et al., 2008), or co-transplanted under the kidney capsule with pancreatic islets in a fully mismatched allograft model (Melzi et al., 2010). This property appears restricted to NPCs, rather than to CNS cells in a broader sense, given that the neonatal or fetal CNS tissue is vulnerable to rejection (Brundin et al., 2010; Modo et al., 2002). The source of stem cells and delivery into an inflammatory environment can influence the degree of immunogenicity, while stem cell differentiation toward a mature (and immunogenic) phenotype can increase the likelihood of graft rejection (Imitola et al., 2004a). In agreement with this, IFN-γ-treated NPCs transplanted into the CNS of immune competent animals are actively rejected, even though the concomitant ischemic microenvironment should favor stem cell survival (Kim et al., 2006). Furthermore, the transplantation of MHC-mismatched (C57BL/6) NPCs into (BALB/c) mice with mouse hepatitis virus (JHMV)-induced CNS demyelination results in an increase in transcripts encoding the T-cell chemoattractants monokine induced by gamma interferon (MIG)/CXCL9 and interferon gamma-induced protein (IP)10/CXCL10, which correlate with increased T cell infiltration and NPC rejection (Weinger et al., 2012).
The Functional Plasticity of the Graft
The functional plasticity of the graft describes the dynamic sequence of actions that transplanted stem cells initiate upon exposure to the host inflammatory microenvironment thanks to the array of functional environmental sensors expressed on their surface (Freedman et al., 2010; Martino and Pluchino 2006; Muller et al., 2006). This includes not only integration and differentiation (Rossi and Cattaneo, 2002), but particularly for systemic stem cell therapies homing and the extravasation into the CNS, and modulation of immune responses in situ.
Homing and Extravasation
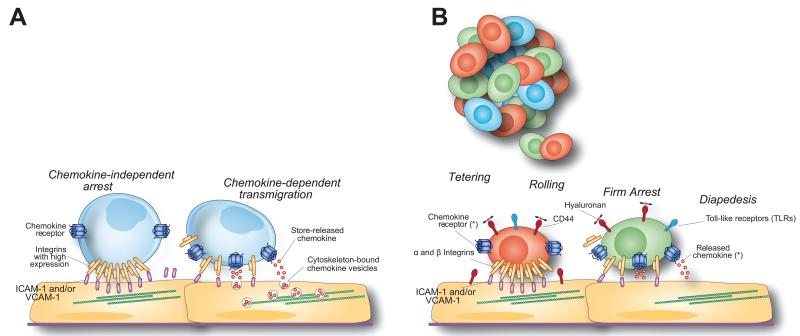
Early studies on models of CNS inflammation showed that the transmigration of naive and effector T cells into the CNS is differentially regulated with increased recruitment of the latter (Kivisakk et al., 2009; Sixt et al., 2001). Activated effector T cells have a high expression of integrins on the plasma membrane. These are able to support rapid tethering, rolling, and firm arrest under flow conditions in the absence of further chemokine-induced upregulation of ligand affinity (Ransohoff and Engelhardt, 2012). T cells cross the border by either paracellular diapedesis—literally, squeezing amongst endothelial cells—or transcellular diapedesis, that is, by creating pores through the cells. While the former requires the disassembly of the intercellular junction structure, the latter involves the formation of cell–cell interactions through the arrangement of docking structures, or transmigratory cups, enriched in intercellular adhesion molecule (ICAM)-1 and vascular cell adhesion molecule (VCAM)-1, which partially embrace migrating leukocytes (Peer et al., 2007). The precise contribution of integrins and G protein-coupled receptors (GPCRs) to the enhanced extravasation of effector T cells in response to inflammation has remained unclear. While T cell antigen-specificity is mandatory for crossing the blood–brain barrier (BBB) and reaching perivascular spaces, it becomes dispensable when further crossing the glia limitans and invading the CNS parenchyma (Archambault et al., 2005). A recent expansion of the view of T cell recruitment demonstrates that activated effector T cells rely on intracellular chemokines that are stored in the recycling vesicles of the inflamed endothelium and released at the cell surface to activate integrins through G protein-coupled receptors (GPCRs) for arrest and diapedesis (Shulman et al., 2012) (Fig. 1A).
FIGURE 1.
Rules for the migration of effector T cells and systemically injected stem cells to the CNS. (A) Activated effector T lymphocytes have a high expression of membrane-bound integrins that are able to support chemokine-independent arrest under flow. The transmigration of effector T cells requires chemokines that are stored in intracellular vesicles, ready to be released in close contact with crawling cells. (B) Tethering, rolling, and firm arrest of injected stem cells to activated endothelial cells and diapedesis into inflamed CNS areas are sequentially mediated by the constitutive expression of functional α and β integrins, cell adhesion molecules such as CD44, TLRs and chemokine receptors on the MSC/NPC surface. *The main chemokine signaling pathways responsible for stem cell migration are shown in Table 2.
Following the observation that intravenously injected NPCs target an intracranial tumor in rodents (Aboody et al., 2000), the value and the mechanisms behind this alternative stem cell injection route were extensively investigated, using different types of stem cells and experimental CNS disease models. The first studies in mice with experimental autoimmune encephalomyelitis (EAE), a model of multiple sclerosis (MS), showed that brain inflammation and endothelial cell activation at the level of the BBB is indispensable in promoting CNS homing and extravasation of systemically injected (e.g. intravenously or intracerebroventricularly) NPCs (Ben-Hur et al., 2003; Chu et al., 2003; Pluchino et al., 2003). NPCs injected systemically into healthy animals were in fact never found in the CNS, while exclusively accumulating (and persisting only for a short period) in peripheral organs (Pluchino et al., 2003). Specific homing of transplanted NPCs has been shown, so far, in experimental brain stroke (Chu et al., 2003; Lindvall and Kokaia, 2011), SCI (Takeuchi et al., 2007), epilepsy (Chu et al., 2004; Hattiangady et al., 2008), HD (Lee et al., 2006), and glioblastoma (Aboody et al., 2000; Ahmed et al., 2011).
Tethering, rolling and firm adhesion of injected stem cells to activated endothelial cells and subsequent diapedesis into inflamed CNS areas are sequentially mediated by the constitutive expression of functional α and β integrins (Campos et al., 2004, 2006; Leone et al., 2005; Pluchino et al., 2003, 2005), cell adhesion molecules such as CD44 (Pluchino et al., 2003, 2005) (Fig. 2), and chemokine receptors (e.g. CCR1, CCR2, CCR5, CXCR3, CXCR4) on the NPC surface (Imitola et al., 2004b; Ji et al., 2004; Pluchino et al., 2005) (Fig. 1B).
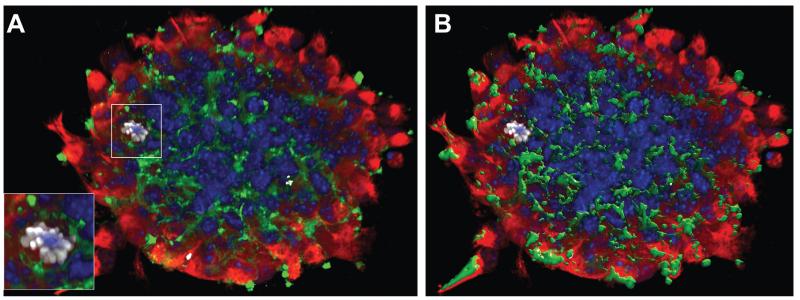
FIGURE 2.
NPCs express environmental sensors. (A) Z-stack confocal image (from a total of n5 40 Z-stacks of optical slices in 0.5 μm intervals) of a mouse neurosphere in vitro. Red is for vimentin, green is for CD44, white is for phosphor-histone H3 (pHH3) and blue is for cell nuclei (Dapi). (B) Volocity V®-based 3D reconstruction of the CD44 expression in A. The magnified frame in A shows a pHH3+/CD44+ NPC.
Human MSCs require coordinated P-selectin, VCAM-1 and α4β1 integrin, matrix metalloproteinase (MMP)-2 and cytokine involvement for rolling, firm adhesion, and transendothelial migration under shear stress conditions in vitro (Chamberlain et al., 2011; Ruster et al., 2006). Mouse NPCs migrate across endothelial cells using clusters of a4 integrin dimers (that bind to VCAM-1) (Pluchino et al., 2005) and CXCR4, a receptor for stromal cell-derived factor (SDF)-1α/CXCL12, both in vitro and in vivo (Corti et al., 2005; Peng et al., 2004). Significantly, the fluorescence-activated cell sorting (FACS)-based selection of either CXCR4- or CD49d-expressing mouse NPCs leads to more efficient CNS homing following intracerebroventricular cell injection into healthy mice (Corti et al., 2005), and intracarotid NPC transplantation into mice with brain stroke (Guzman et al., 2008), respectively.
The activation of the CXCR4/SDF-1α signaling pathway on NPCs and MSCs increases their migratory capacity, survival, and remyelinating capacity, both in vitro on slice cultures (Corti et al., 2005; Imitola et al., 2004b), as well as in vivo upon focal transplantation into rodents with experimental cerebral ischemia (Robin et al., 2006; Wang et al., 2008), and JHMV-induced demyelination (Carbajal et al., 2010, 2011). In human NPCs, integrins α2, α6, and β preferentially mediate the homing toward the vasculature, whereas the CXCR4/SDF-1α signaling pathway regulates homing through the brain parenchyma (Carbajal et al., 2010; Mueller et al., 2006; van der Meulen et al., 2009).
MSCs lack the expression of CD44, a cell-surface glycoprotein that binds to hyaluronic acid (HA) and is expressed in activated T cells (DeGrendele et al., 1997); yet, they acquire CD44 expression after extensive in vitro expansion (Qian et al., 2012). CD44-expressing NPCs (Fig. 2) induce the formation of transmigratory apical cups, enriched in ICAM-1 and VCAM-1, at the surface of CD44+ brain endothelial cells in vitro (Rampon et al., 2008), thus hijacking the endothelial signaling system previously shown to be involved in leukocyte extravasation (Butcher and Picker, 1996; Ley et al., 2007).
MSCs and NPCs constitutively express chemokine receptors and release chemokines, which are upregulated after cell exposure to TNF-α or IFN-γ (Croitoru-Lamoury et al., 2007; Turbic et al., 2011) and directly required for proliferation and differentiation of both stem cell types (Li et al., 2011; Rice and Scolding, 2010).
A number of chemokine/cytokine signaling pathways have been elucidated in the therapeutic application of stem cells, several of which influence the migratory properties of transplanted cells:
The activation of monocyte chemoattractant protein (MCP)-1/CCL2 signaling plays a central role in the transendothelial recruitment of intra-arterially delivered (CCR2-expressing) mouse NPCs in vivo (Andres et al., 2011), although it is not functional on human MSCs (Ringe et al., 2007);
Human NPCs express CXCR1 and CXCR5, which mediate in vitro migration across a monolayer of human brain ECs in response to IL-8/CXCL8 and B lymphocyte chemoattractant (BLC)/CXCL13, respectively (Weiss et al., 2010);
Fractalkine/CX3CL1 downstream signaling is functional in the trafficking of transplanted (CX3CR1-expressing) rat and human MSCs in rats with hypoglossal nerve injury and MCAo, respectively (Ji et al., 2004; Zhu et al., 2009);
Hepatocyte growth factor (HGF), a multifunctional cytokine originally characterized as a mitogen for hepatocytes, and its cognate receptor HGFR/c-met, are constitutively expressed in MSCs, where HGF signaling stimulates the chemotactic migration and recruitment of MSCs in vitro (Neuss et al., 2004; Son et al., 2006) (Table 2).
TABLE 2. Functional Environmental Sensors on MSCs and NPCs.
| Receptor | Ligand | Stem Cellsa | Described Functionb | References |
|---|---|---|---|---|
| MHC-I/II | MSCs/NPCs | Not known | Multiple refs. | |
| CD80/CD86/CD40 | MSCs/NPCs | Not known | Multiple refs. | |
| CD49d (VLA-4) | VCAM-1 | NPCs | Homing/adhesion | (Guzman et al., 2008; Pluchino et al., 2005) |
| P-selectin | P-selectin glycoprotein ligand (PSGL)-1 | MSCs | Homing/adhesion and migration | (Ruster et al., 2006) |
| CXCR4 | SDF-1α/CXCL12 | MSCs/NPCs | Migration | (Carbajal et al., 2010; Corti et al., 2005; Imitola et al., 2004b; Mueller et al., 2006; Wang et al., 2008) |
| CD44 | HA | Role in interaction with endothelial cells and extravasation | (Qian et al., 2012; Rampon et al., 2008; Zhu et al., 2006) | |
| CCR2 | MCP-1/CCL2 | NPCs | Transendothelial recruitment | (Andres et al., 2011) |
| CXCR1 and CXCR5 | IL-8/CXCL8 and CXCL13 | NPCs | Role in interaction with endothelial cells and extravasation | (Weiss et al., 2010) |
| CX3CR1 | Fractalkine/CX3CL1 | MSCs | Trafficking | (Ji et al., 2004; Zhu et al., 2009) |
| HGF | HGFR/cMet | MSCs | Migration and recruitment | (Neuss et al., 2004) |
| TLR | TLR agonists | MSCs | Migration and recruitment, immune modulation; secretion of cytokines and chemokines | (Liotta et al., 2008; Romieu-Mourez et al., 2009; Tomchuck et al., 2008) |
Evidence from bone marrow-derived MSCs only are quoted.
Function described in stem cell immune interactions.
Therefore, NPCs—much more so than MSCs—display CNS pathotropism upon transplantation (Martino and Pluchino, 2006; Muller et al., 2006), owing to the expression of a large variety of environmental sensors that work as “Velcro-like” biomimicries in response to a variety of mediators of inflammation involved in tissue damage and repair (Belmadani et al., 2006; Martino et al., 2011; Tran and Miller, 2003). The discovery of the specific homing ability of adult stem cells across the BBB has opened new frontiers for the treatment of CNS diseases, in particular those characterized by widely disseminated damage. This characteristic, allowing stem cell interactions with endogenous endothelial and ependymal cells in the context of inflammatory conditions, represents an essential requirement in the therapeutic paradigm of systemic (cellular) delivery for tissue-specific diseases.
Modulation of Immune Responses
The systemic injection of MSCs and NPCs is remarkably immune regulatory in vivo (Martino and Pluchino, 2006; Spaggiari and Moretta, 2012; Uccelli et al., 2008).
Syngeneic and xenogeneic MSCs ameliorate the clinical course of both myelin oligodendrocyte glycoprotein (MOG) and proteolipid protein (PLP)-induced chronic and relapsing EAE, respectively. MSC-transplanted EAE mice show reduced demyelination and T and B cell infiltration of the CNS, and decreased production of myelin-specific antibodies (Gerdoni et al., 2007; Zappia et al., 2005; Zhang et al., 2005). Importantly, MSCs produce these effects only when injected at disease onset or peak, while the injection of MSCs during the chronic phase has no effect (Zappia et al., 2005). The observation that T cells from MSC-transplanted EAE mice do not proliferate in vitro following a second antigen challenge, suggests that a state of peripheral tolerance is produced by MSCs (Zappia et al., 2005). Injected MSCs also prevent the differentiation and maturation of monocytes into dendritic cells (DCs) in vivo (Ramasamy et al., 2007). Furthermore, MSCs block almost instantaneously the migration of CCR7- and α4β1-expressing DCs into draining lymph nodes to hinder local antigen presentation to CD4+ T cells and cross-presentation to CD8+ T cells (Chiesa et al., 2011). Recently, when transplanted into the lesion epicenter of rats with contusion SCI, MSCs migrated within the injured spinal cord without differentiating into glial or neuronal elements. Additionally, significant skewing of the acute inflammatory cell infiltrate at the injured site with increased numbers of Arginase-1+ or CD206+ alternatively activated (M2-like) anti-inflammatory macrophages, and decreased numbers of iNOS+ or CD16/32+ classically activated (M1-like) proinflammatory macrophages were observed (Nakajima et al., 2012). Data from stroke research suggest that MSCs might also release neurotrophic factors, such as brain derived neurotrophic factor (BDNF), provide trophic support for vulnerable neurons (particularly in the ischemic penumbra), support endogenous oligodendrogenesis and regulate anti-inflammatory responses with a reduction of neural edema in situ, thus leading to enhanced tissue sparing (Chen et al., 2001, 2002, 2003).
NPCs attenuate brain inflammation and the amount of microglial activation, when injected into the biological fluids (blood stream, cerebrospinal fluid and lymph) of rodents or non-human primates with either chronic and relapsing EAE or stroke (Bacigaluppi et al., 2009; Capone et al., 2007; Daadi et al., 2010; Lee et al., 2008), thus reducing the amount of demyelination and axonal/neuronal pathology, promoting host-driven brain repair (Einstein et al., 2009), and in turn decreasing the clinical severity of the disease (Martino et al., 2011). Once within the CNS, systemically injected NPCs accumulate and persist around perivascular spaces where reactive astrocytes, inflamed endothelial cells and blood-borne infiltrating T cells co-reside. In these areas, named “CNS atypical ectopic niches,” the great majority of transplanted NPCs survive long term, displaying undifferentiated features, and promote neuroprotection through the in situ release of immune modulatory molecules and neurotrophic factors (Pluchino et al., 2005). These effects correlate well with a reduction in perivascular inflammatory infiltrates and CD3+ T cells (Pluchino et al., 2005), an increase in CD25+ and CD25+/CD62L+ Treg cells, and a significant reduction in the brain expression of the inflammation marker ICAM-1 and its ligand LFA-1 (Einstein et al., 2003). Moreover, NPCs injected systemically into EAE mice induce apoptosis of blood-borne CNS-infiltrating encephalitogenic Th1 cells in situ (Pluchino et al., 2005). When injected intraparenchymally at the proximal and distal ends of the contused mouse spinal cord, NPCs survive transplantation and establish cellular contacts with endogenous professional phagocytes, while also reducing the proportion of classically activated (M1-like) macrophages and promoting the healing of the injured cord (Cusimano et al., 2012).
Interestingly, only small numbers (between 1 and 5%) of systemically injected MSCs and NPCs traffic over the inflamed CNS, whereas a rather significant accumulation of transplanted stem cells is observed at the level of the spleen and the draining lymph nodes (Bacigaluppi et al., 2008, 2009; Capone et al., 2007; Chiesa et al., 2011; Gerdoni et al., 2007; Lee et al., 2008; Pluchino et al., 2009a,b; Sun et al., 2010). Here, stem cells extensively interact with the host immune system to inhibit the activation and proliferation of T cells (Gerdoni et al., 2007), the maturation of DCs (Pluchino et al., 2009b), or the emigration of spleen neutrophils toward the damaged brain (Lee et al., 2008). Overall, these pioneering studies demonstrated the therapeutic efficacy of MSCs and NPCs in animal models of inflammatory damage, but left (partly) open the question of whether or not stem cell integration in the nervous system is indispensable for the therapeutic outcomes observed.
In vitro, the full range of MSC and NPC immune regulatory properties is constitutive, as well as enhanced after licensing or priming with proinflammatory cytokines or Toll-like receptor (TLR) ligands that recapitulate some aspects of inflammatory cellular signaling (Bifari et al., 2010; Kokaia et al., 2012; Ren et al., 2008). TLRs are being proposed as first-line environmental danger sensors for stem cells (Delarosa et al., 2012; Martino and Pluchino 2007).
Human MSCs express TLR 1–6 and TLR 9 only (Raicevic et al., 2010), while mouse MSCs also express TLR 7 and 8, and exposure to TLR ligands controls MSC functions including: TLR 2-dependent regulation of IL-6 secretion; nuclear factor kappa B (NF-kB) translocation; reduced MSC basal motility; and increased MSC proliferation (Pevsner-Fischer et al., 2007). Activation of human MSCs by TLR ligands induces IL-6, IL-8 and CXCL10 secretion and NF-kB nuclear translocation in vitro (Liotta et al., 2008). TLR 3 and 4 ligation via lipopolysaccharide (LPS) reduces the suppressive activity of MSCs on proliferating T cells (Tomchuck et al., 2008), while increasing their reprogramming capacity on macrophages by a prostaglandin (PG) E2-dependent mechanism (Nemeth et al., 2009). The timing and the relative levels of the TLR ligation determine the final functional outcome of MSCs. This is suggested by the description of two major subsets of TLR-stimulated human MSCs, which either mature towards a pro-inflammatory phenotype after treatment if exposed to the TLR 4 agonist LPS (MSC1), or acquire an immune suppressive function after treatment with the TLR 3 agonist Poly(I:C) (MSC2) (Bunnell et al., 2010; Pevsner-Fischer et al., 2007; Waterman et al., 2010).
The veto-like (forbidding) activity of MSCs (Potian et al., 2003; Tscherning and Claesson, 1993) inhibits T-cell proliferation (Bartholomew et al., 2002; Bocelli-Tyndall et al., 2007; Di Nicola et al., 2002; Klyushnenkova et al., 2005) via induction of T-cell quiescence upon blockade into G0/G1 (Glennie et al., 2005) and downregulation of the T-cell activation markers CD25, CD38, and CD69 (Groh et al., 2005; Le Blanc et al., 2004) and of IL-2 production (Park et al., 2011). MSCs also inhibit Th17 differentiation from both naïve and memory T cell precursors in vitro, as well as preventing the efflux of naturally occurring Th17 cells derived from inflammation sites in vivo (Duffy et al., 2011). Human MSCs shift CD8+ cytotoxic cells towards a suppressive phenotype (Hof-Nahor et al., 2012). Some of the immune modulatory activities of MSCs have been ascribed to their ability to induce the generation of CD4+/CD25+/FoxP3+ T cells with regulatory functions (Treg) (Maccario et al., 2005; Prevosto et al., 2007; Tasso et al., 2012). MSCs also functionally interact with B cells, which are again arrested in G0/G1 (Tabera et al., 2008), thus failing to progress toward differentiation and production of immunoglobulins in vitro (Corcione et al., 2006). Conversely, MSCs strongly enhance the proliferation and differentiation into plasma cells of memory B cells in vitro and in vivo (Traggiai et al., 2008). Finally, rodent and human MSCs exert profound effects on immature DCs, by constraining their maturation to professional APCs (Chiesa et al., 2011; English et al., 2008; Gur-Wahnon et al., 2007; Jiang et al., 2005; Li et al., 2008; Liu et al., 2012; Zhang et al., 2004).
NPCs also express TLRs 2, 3, and 4 and respond to TLR agonists, which regulate stem cell proliferation and differentiation, both in vitro and in vivo. While TLR 2 ligation stimulates neurogenesis (Rolls et al., 2007), TLR 3 and 4 downstream signaling has inhibitory effects on both stem cell proliferation and self-renewal (Rolls et al., 2007; Yaddanapudi et al., 2011), partly by inhibiting Sonic Hedgehog (Shh) signaling. Remarkably, the exposure to TLR 2 and 4 ligands induces the secretion of TNF-α by NPCs (Covacu et al., 2009) (Tables 1 and 2).
Mouse NPCs inhibit the activation and proliferation of both antigen-specific and antigen non-specific Th1 and Th17 cells in vitro, as well as inducing T cell apoptosis (Einstein et al., 2003; Fainstein et al., 2008; Knight et al., 2010; Pluchino et al., 2005). Human NPCs suppress the proliferation and alter the cytokine secretion profiles of xenogeneic (e.g. marmoset), antigen-specific and allogeneic mitogen-activated T cells (Kim et al., 2009b; Pluchino et al., 2009a). Compared with rodent NPCs, human NPCs have a lower cytotoxicity towards T cells, but a higher cytotoxicity toward monocyte/macrophages (Ricci-Vitiani et al., 2007) in vitro. Human NPCs also hinder the differentiation of myeloid precursor cells (MPCs) into immature DCs, and the maturation of immature DC to functional APCs (Pluchino et al., 2009a).
Therefore, while most of the mechanisms underlying the immunosuppressive effects of MSCs and NPCs are yet to be clarified, it is very likely that at least some of them involve consistent cellular signaling between the stem cell graft and the target host immune cell.
Stem Cell Signaling and Regulation of the Host Immune Responses
Multicellular organisms have developed a range of very efficient and controlled cell-to-cell communication mechanisms that are deeply integrated, thus generating global and concerted response behaviors of neighboring and distant cells in the environment. This variety of mechanisms, known as cell signaling, is the foundation for coordinated cellular actions and flexible responses. Stem cell signaling takes place through different pathways that involve networks of interacting molecules transmitting information between the graft and the host. This exchange of signals entails either cell-to-cell contacts (juxtacrine) or gradients formed by soluble factors (paracrine) (Friedl et al., 2005) which also circulate in blood and body fluids and act in a regional or systemic manner (endocrine). Stem cell signaling may also involve the newly recognized release of extracellular membrane vesicles (EVs) (Kalra et al., 2012; Mathivanan et al., 2010; Thery et al., 2002). A schematic representation of the alternative cell signaling pathways regulating the interactions between the stem cell graft and the host immune system in inflammatory brain diseases is shown in Figure 3.
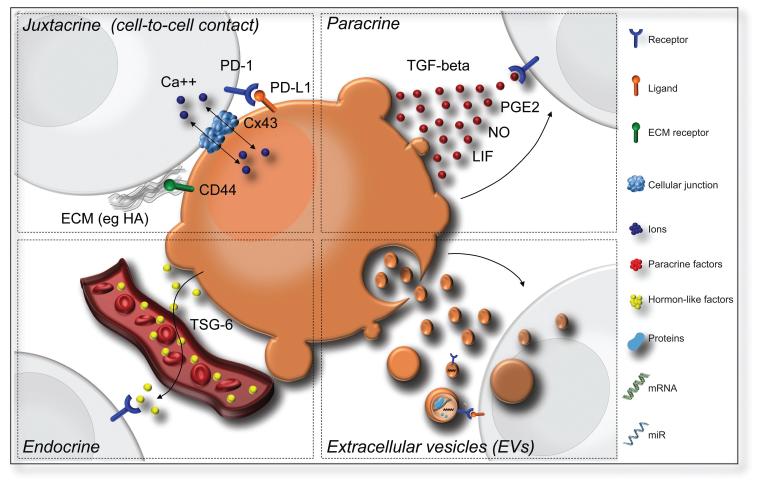
FIGURE 3.
Schematic representation of the alternative cell signaling pathways regulating the interactions between the stem cell graft and the host immune system in inflammatory brain diseases. (A) Juxtacrine signaling pathways (cell-to-cell contact) that include (i) receptor-ligand interaction such as PD-L1/PD-1; (ii) receptor binding to components of the extracellular matrix (ECM) released by neighboring cells (such as CD44 to HA); and iii) gap junction formation (via connexins); (B) Paracrine signaling with release of soluble factors that likely form a gradient (e.g. TGF-β, LIF, NO, PGE2); (C) Endocrine signaling (signaling at a distance) that implies the release of hormonelike factors such as TSG-6; and (D) EV release with the possibility to deliver a multitude of bioactive molecules such as mRNAs, micro-RNAs and proteins. Abbreviations: CD; cluster of differentiation; HA: hyaluronic acid; ECM: extracellular matrix; Cx43: Connexin-43; Ca++: calcium; TGF-β: transforming growth factor beta; PGE2: Prostaglandin E2; NO: nitric oxide; LIF: Leukaemia inhibitory factor; TSG-6: TNF-α-stimulated gene/protein 6; PD-1: programmed death-1; PD-L1: programmed cell death 1 ligand 1; EVs: extracellular vesicles.
Cell-to-Cell Contacts or Juxtacrine Signaling
Juxtacrine signaling (Anklesaria et al., 1990) refers to:
The direct membrane-to-membrane contact that is generally mediated by receptor–ligand binding between adjacent cells. A clear example of receptor–ligand interaction has been described for MSCs that acquire APC-like properties upon the expression of functional VCAM-1, which binds with high affinity to activated integrin α4 expressed by T lymphocytes in vitro (Majumdar et al., 2003).
The binding of a cell receptor to components of the extracellular matrix (ECM) released by a neighboring cell. An example of this is the adhesive and promigratory role of membrane CD44 bound to ECM hyaluronic acid (HA) in the transendothelial migration of grafted NPCs (Rampon et al., 2008) across brain endothelial cells.
The involvement of cellular junctional coupling where membrane regions of two neighboring cells (within a range of 2–4 nm) become closely apposed.
The interaction between transplanted stem cells and immune cells is well documented and it has also been shown that NPCs are able to establish immune synapse-like contacts with CD3+ T cells leading to CD3 redistribution at the level of the contact zone (Imitola et al., 2004a). Cell-to-cell contacts may also imply the formation of a network of nanotubes between neighboring cells, as described for cardiomyoblasts and MSCs in vitro (Cselenyak et al., 2010).
MSCs co-express a functional Fas/CD95 ligand (FasL) and non-functional Fas/CD90 that makes them in principle of low sensitivity to programmed cell death (Mazar et al., 2009). In experimental colitis and systemic sclerosis (SS), intravenously injected MSCs activate Fas-regulated MCP-1 secretion, which recruits T cells for FasL-mediated apoptosis, and ameliorate disease phenotypes in colitis and SS, while FASL−/− MSCs fail to do so (Akiyama et al., 2012). Similarly, in NPCs, membrane bound FasL is partly responsible for the NPC-regulated pro-apoptotic effect on encephalitogenic Th1 and Th17 (but not Th2) lymphocytes in EAE in vivo and in vitro (Knight et al., 2010; Pluchino et al., 2005). Interestingly, mouse NPCs are resistant to FasL-induced cell death and activation of Fas increases NPC survival through upregulation of the inhibitor of apoptosis protein (IAP), Birc3 (Knight et al., 2010).
IFN-γ primes some of the immunosuppressive properties of MSCs via upregulation of programmed cell death protein-ligand (PD-L) 1, which attenuates the activation and effector functions of PD-1-expressing Th1 and Th17 cells, via modulation of the expression of different cytokine receptors, such as IL-12R, and the signal transductors STAT5a and STAT5b (Luz-Crawford et al., 2012; Sheng et al., 2008).
Junctional coupling via connexins was first described between grafted NPCs and host Purkinje neurons in B05/+ spinocerebellar ataxia type 1 (SCA1) mice in vivo, and has been associated with neuronal rescue and behavior via the transcellular transfer of small molecules and Ca2+ waves (Jaderstad et al., 2010). More recently, connexin43+ cellular contacts have been described between endogenous macrophages and grafted NPCs in mice with experimental contusion SCI (Fig. 4). These NPC-transplanted SCI mice show a reduction of proinflammatory M1-like macrophages at the injured site and develop much less severe secondary cord damage, compared with sham-operated controls (Cusimano et al., 2012) (Table 3).
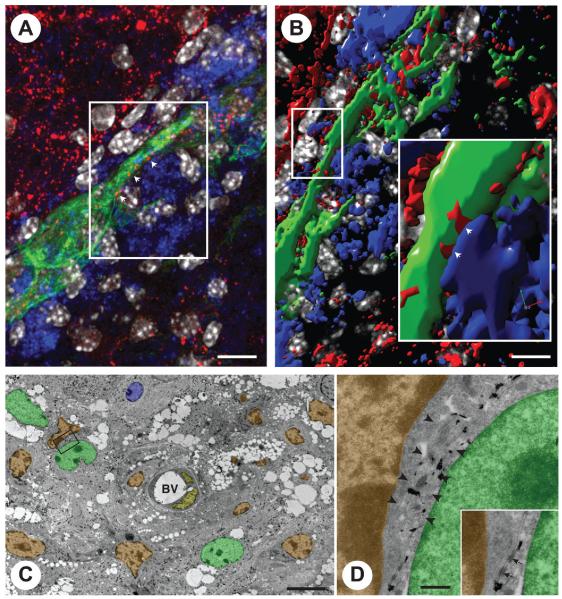
FIGURE 4.
NPC grafts establish juxtacrine signaling with endogenous professional phagocytes through junctional coupling. (A) Confocal microscopy image of GFP (green) NPCs contacting F4/80+ macrophages via connexin43+ cellular junctions (red; arrowheads). (B) Volocity V®-based 3D reconstruction of the confocal Z-stack in A. The magnified inset shows a structural junctional connexin43 pattern (red; arrowheads) between the process of one NPC (green) and one juxtaposed F4/80+ macrophage (blue). (C) Immunoelectron micrograph of GFP+ NPCs. The frame indicates one NPC whose processes are found to be in very close contact with a (GFP−) monocyte/macrophage. (D) High magnification of the frame in C showing the immunogold-labeled process of an NPC (arrowheads) running between a monocyte/macrophage and a second immunogold-labeled NPC. Cellular junctions between both NPC cytoplasms (inset, arrows) and between the NPC and the monocyte/macrophage can be observed in the inset. Pseudo colors in B and C: NPCs are in green; monocytes/macrophages are in orange; endothelial cells are in yellow and endogenous astrocytes are in blue. (Reproduced with permission from Cusimano et al., Brain, 2012, 135, 447-460, ©Oxford University Press.)
TABLE 3. Stem Cell Signaling and Modulation of Immune Functions.
| Cell Signaling | Pathway | Stem Cella | Target Cell | Described Functionb | References |
|---|---|---|---|---|---|
| Juxtacrine | FAS/FASL | MSCs/NPCs | T cell | T cell apoptosis | (Akiyama et al., 2012; Knight et al., 2010; Pluchino et al., 2005) |
| Juxtacrine | PD-1/PD-L1 PDL-2 | MSCs | T cells | Inhibition of T cell activation and effector function(s) | (Luz-Crawford et al., 2012; Sheng et al., 2008) |
| Juxtacrine | ICAM/VCAM | MSCs | T cells | Inhibition of T cell activation and effector function(s) | (Ren et al., 2010) |
| Juxtacrine | Cx43 | MSCs/NPCs | Neurons, macrophages | Transfer of Ca++, inhibition of M1-like functions | (Cusimano et al., 2012; Jaderstad et al., 2010) |
| aracrine | IDO-kynurenine | MSCs (h) | T cells, DCs | T cell apoptosis, inhibition of antigen presentation | (Lanz et al., 2010; Matysiak et al., 2008, 2011; Meisel et al., 2004; Plumas et al., 2005) |
| Paracrine | COX2-PGE2 | MSCs/NPCs | T cells | Inhibition T cell proliferation, inhibition Th17 cell functions | (Aggarwal and Pittenger, 2005; Bouffi et al., 2010; Duffy etal., 2011; Martinet et al., 2009; Wang et al., 2009) |
| Paracrine | iNOS-NO | MSCs (r) | T cells | Inhibition T cell proliferation | (Ren et al., 2008) |
| Paracrine | HO | MSCs/NPCs | T cells | Inhibition T cell proliferation, generation of Tr1 and Th3 Tregs | (Bonnamain et al., 2012; Chabannes et al., 2007; Moll et al., 2011) |
| Paracrine | VEGF | NPCs | Microglia/macrophages | Inhibition of microglial activation, proliferation and phagocytosis | (Horie et al., 2011; Kim et al., 2009a; Mosher et al., 2012) |
| Paracrine | LIF | NPCs | Th17 cells | Inhibition of Th17 cell differentiation | (Cao et al., 2011; Horie et al., 2011; Kim et al., 2009a; Mosher et al., 2012) |
| Paracrine | Galectins | MSCs/NPCs | T cells | Inhibition of T cell proliferation | (Gieseke et al., 2010; Sioud 2011; Yamane et al., 2010, 2011) |
| Endocrine/Paracrine | TSG-6 | MSCs | Macrophages | Inhibition of macrophage activation, proliferation and phagocytosis | (Fisher-Shoval et al., 2012; Lee et al., 2009; Roddy et al., 2011) |
| EVs | miR transfer | MSCs/NPCs | Multiple | Post-transcriptional regulation | (Bruno et al., 2009; Chen et al., 2010; Xin et al., 2012) |
Evidence from bone marrow-derived MSCs only are quoted.
Function described in stem cell immune interactions.
Paracrine Signaling
Paracrine (“para” = near) signaling refers to a local signaling system in which a factor secreted by a donor cell diffuses over small distances through the extracellular fluids and affects other nearby target cells. This results in the formation of a gradient of the signaling molecule with proximal target cells responding differently according to the concentration they are exposed to. In regenerative medicine grafted stem cells secrete a variety of paracrine factors, including interleukins, colony-stimulating factors, prostaglandins, and growth factors, which regulate interactions with the environment (Ratajczak et al., 2012).
Paracrine signaling mediated by factors released by the stem cells can play an essential role in the reparative process observed after stem cell transplantation, with MSCs and NPCs secreting growth factors, chemokines and cytokines, constitutively as well as in response to proinflammatory stimuli (Gnecchi et al., 2008; Ratajczak et al., 2012).
MSCs express indoleamine 2,3-dioxygenase (IDO)—an immune regulatory enzyme that catalyses the degradation of tryptophan via the kynurenine pathway—and exhibit functional IDO activity and IDO-dependent apoptotic effects on allogeneic human T cells upon stimulation with IFN-γ (Meisel et al., 2004; Plumas et al., 2005). In vivo in mice with EAE, transplanted syngeneic MSCs prevent relapses and promote myelin repair via an IFN-γ-dependent mechanism that induces IDO in CD11c+ DCs and leads to the inhibition of antigen reactivity and disease spread (Matysiak et al., 2008, 2011).
MSCs also modulate local allogeneic responses after transplantation through the secretion of nitric oxide (NO) and PGE2, which switch the host immune response from a Th1/Th17 towards an anti-inflammatory Th2-like secretory profile (Aggarwal and Pittenger, 2005; Bouffi et al., 2010). Recently, in vitro co-cultures have identified NO and PGE2 as being novel strong inhibitors of the NPC-induced impairment of T-cell proliferation, alternative to the interference with cell activation or induction of apoptosis (Wang et al., 2009).
Heme oxygenase-1 (HO-1) is another key contributor to MSC-mediated suppression of alloactivated T cells. Beyond the direct suppressive function of HO-1 (Chabannes et al., 2007), a recent study has identified a novel HO-1-driven mechanism of rat and human MSCs leading to the generation of IL-10+ Tr1 and TGF-β+ Th3 Tregs in allo- and T-cell receptor-activated lymphocytes. Again, observations from in vitro co-cultures with T cells have implicated HO-1 in the anti-proliferative effects of rat NPCs (Bonnamain et al., 2012).
Stem cells secrete neurotrophins and growth factors, some of which have been recently described to play a major role in stem cell–immune cell interactions (Ranganath et al., 2012; Thirant et al., 2012).
Conditioned medium from human MSCs (MSC-CM) reduces functional deficits in mouse MOG-induced chronic EAE and promotes the development of oligodendrocytes and neurons. MSC-CM contains HGF, and exogenously supplied HGF promotes recovery in EAE, whereas cMet and antibodies to HGF block the functional recovery mediated by HGF and MSC-CM (Bai et al., 2012).
Vascular endothelial growth factor (VEGF) is necessary for NPCs to modulate microglial activation, proliferation and phagocytosis (Mosher et al., 2012), while leukemia inhibitory factor (LIF) inhibits Th17 cell differentiation in vitro, and leads to amelioration of EAE in vivo (Horie et al., 2011) after NPC transplantation.
Finally, Galectins (Gal) are a family of carbohydrate-binding proteins with an affinity for β-galactosides, and Gal-1 and -3 exhibit profound and unique modulatory activities on immune effector functions by controlling cell activation, cytokine synthesis and viability through cross-linking of the cognate receptors on the surface of lymphatic cells (de la Fuente et al., 2012). The interaction of MSC-membrane-bound or secreted Gal-1 and Gal-3 with their receptors on T cells induces tolerogenic signals, resulting in T-cell suppression (Sioud, 2011). In line with this evidence, the transplantation of Gal-1-overexpressing human NPCs exhibits remarkable therapeutic efficacy in experimental SCI and brain stroke (Yamane et al., 2010, 2011) (Table 3).
Signaling at a Distance (Endocrine)
Endocrine signaling differs from paracrine signaling in that the signaling molecules (such as hormones) are released into the bloodstream and travel over much longer distances. Specialized cells localized in endocrine organs usually secrete the signaling molecules. Recent studies provide evidence of cellular signaling between grafted stem cells and host immune cells that do not necessarily fall under the current definition of endocrine signaling. These studies excitingly show that the systemic stem cell graft has the potential to exert some remote actions in vivo, regardless of the numbers of transplanted stem cells effectively reaching (and/or surviving at) the injury site.
Systemically injected human NPCs ameliorate the clinico-pathological signs of experimental intracerebral hemorrhage (ICH) in the mouse; yet, the majority of injected NPCs do not enter the brain, but rather accumulate and persist within the marginal zone area of the spleen, where they inhibit macrophage activation and induce significant attenuation of both cerebral and splenic activation of TNF-α, IL-6 secretion, and NF-kB. Splenectomy before ICH induction eliminates the beneficial effect of the stem cell graft and suggests that stem cell-driven protection from brain damage may then be attained, even without CNS entry of the transplanted cells (Lee et al., 2008).
Peripheral immune modulation has also been observed following systemic MSC and NPC transplantation in EAE, owing to a remarkable accumulation of transplanted cells at the level of secondary lymphoid organs, where T-cell proliferation and production of pro-inflammatory cytokines were inhibited (Einstein et al., 2007; Pluchino et al., 2009b; Zappia et al., 2005). Furthermore, exclusive targeting of the peripheral immune system has been reported in relapsing EAE in mice after subcutaneous NPC injection (Pluchino et al., 2009b). NPC-injected EAE mice showed significant clinical improvement, despite the absence of injected cells in the CNS. Instead, undifferentiated NPCs were consistently found at the level of the perivascular areas in draining lymph nodes, where they hindered the activation of myeloid DCs to APCs by a bone morphogenetic protein (BMP)-4-dependent mechanism that reduced the pathogenicity of antigen-specific T cells (Pluchino et al., 2009b).
Prockop et al. have demonstrated that MSCs injected systemically in mice with myocardial infarction reduce the infarct size, and improve cardiac function through the release of the anti-inflammatory factor tumor necrosis factor (TNF)-α-induced protein (TNAIP6/TSG-6) in response to the entrapment of the transplanted MSCs as small emboli in the lungs (Lee et al., 2009). Two recent reports have confirmed this TSG-6-dependent, but engraftment-independent, action at a distance of grafted MSCs, in mice with both EAE (Fisher-Shoval et al., 2012) and ethanol-induced corneal inflammation (Roddy et al., 2011) (Table 3).
Signaling Through Extracellular Membrane Vesicles
The evidence that secreted membrane vesicles (EVs) provide extracellular waves of information that are capable of inducing multiple functional responses in adjacent and distant target cells has only emerged over the last decade (Thery, 2011). EVs are actively secreted by most cell types and have been identified in body fluids such as urine, amniotic and cerebrospinal fluid, malignant ascites, bronchoalveolar lavage, synovial fluid, breast milk, saliva and blood, and they work as key players in the regulation of immune responses (Thery et al., 2009).
EVs, including shed vesicles and exosomes, have been demonstrated to be secreted by a number of different cell types, including stem cells, either constitutively or upon activation (Thery et al., 2009). These organelles can be considered as a miniature version of the parental cell with the very same complexity of signaling, and a tendency to participate in a wide spectrum of biochemical and cellular activities (Heijnen et al., 1999). EVs released by stem cells may interact through specific receptor ligands (e.g. CD44 and/or CD29) with target cells to transfer proteins, biological reactive lipids and receptors (Bruno et al., 2009), or mRNAs and microRNAs (miRNA) that may account for epigenetic changes in target cells (Ratajczak et al., 2006; Valadi et al., 2007). Moreover, EVs are considered to be paracrine or endocrine signaling vehicles, given that they can contain and transport signaling molecules, such as cytokines, to target cells at distant sites (Camussi et al., 2010; Ratajczak et al., 2012).
MSC-derived EVs shuttle lipids, mRNAs (Tomasoni et al., 2013), both double-strand precursor (pre-miR) and single-strand mature microRNAs (miR) (Chen et al., 2009; Collino et al., 2010; Xin et al., 2012) and are tolerogenic (Mokarizadeh et al., 2012) and neuritogenic (Xin et al., 2012) on target cells. Systemically injected EVs from human bone marrow-derived MSCs have been shown to accelerate kidney repair in a mouse model of acute kidney injury (AKI) by inhibiting apoptosis and stimulating tubular epithelial cell proliferation. EVs also significantly reduced the impairment of renal function. Pretreatment of EVs with RNase to inactivate their RNA cargo abrogated these protective effects. Moreover, EVs capable of reducing the acute injury also protected from later chronic kidney disease (Gatti et al., 2011). A recent study in an MCAo rat stroke model reported the possibility that MSCs might communicate with brain parenchymal cells via exosome-mediated miR-133b transfer, leading to specific gene expression regulation that in turn enhanced neurite outgrowth and contributed to functional recovery (Xin et al., 2012).
All these studies suggest the existence of a bidirectional exchange of genetic information between stem and target cells, or reciprocally from injured cells to bone marrow-derived or resident stem cells, that in turn leads to tissue repair (Camussi et al., 2010). In this context, embryonic and adult stem cell-derived EVs shuttle defined patterns of mRNAs and miRs that are internalized by a receptor-mediated mechanism in target cells, and may induce de-differentiation of cells surviving injury with cell cycle re-entry and tissue self-repair. Conversely, it might be envisaged that transcripts delivered by EVs from injured cells might reprogram the phenotype of stem cells to acquire specific features of the inflamed/damaged microenvironment.
Exosomes from human ES cell-derived MSCs have recently been shown to reduce infarct size in a mouse model of myocardial ischemia/reperfusion injury and in this setting exosomes have been identified as the cardioprotective component in the MSC paracrine secretion (Lai et al., 2010). These very same MSC-derived exosomes contained the miRs hsalet-7b and hsa-let-7g, predominantly in the precursor form (Chen et al., 2010).
The first tentative evidence of immune modulation by NPC-derived exosomes emerged from experiments in which the culture supernatant of human NPCs (HB1.F3) suppressed the activation and proliferation of human T cells by apoptosis and cell cycle arrest. Exosomes isolated from NPCs and added to the supernatant of cultured T cells resulted in similar suppression by G0/G1 cell cycle arrest. This reinforces the possibility that (at least part of) the immune modulatory effects of NPCs might be mediated by secreted EVs/exosomes (Kim et al., 2009c). The hypothesis of EV secretion by NPCs introduces a completely different dimension to the therapeutic applications of NPCs in regenerative medicine. By replacing transplantation of NPCs with administration of their secreted products (including EVs), many of the limitations and safety concerns associated with the transplantation of viable replicating cells, such as tumors arising from transplanted NPCs, could be mitigated (Amariglio et al., 2009).
EVs represent a vectorized novel signaling system operating from inside a donor cell towards the periphery, the cytosol, or possibly the nucleus of a target cell that is regarded as in-between paracrine (Ratajczak et al., 2012) and endocrine cellular signaling (Camussi et al., 2010). These signaling vesicles may therefore play a central, though previously hidden, regulatory action in a wide range of cellular processes, under both physiological and pathological circumstances.
Some recent evidence suggests that different cell types, including MSCs, traffic complex suites of proteins (Kim et al., 2012) and non-coding regulatory RNAs (Collino et al., 2010; Kalra et al., 2012; Koh et al., 2010)—which appear to be regulated by extracellular signals that mimic changes in the environment, including Wnts, cytokines, hypoxia and growth factor deprivation (Cossetti et al., 2012b). Current efforts are focusing on establishing the specificity versus reactivity of the vesicle content, as recent studies have suggested that certain patterns of extracellular miRs might not mirror the cellular miRome, but rather, being epiphenomenal to cellular machinery, regulating the mobility of small nucleic acids outside the cell via recycling vesicles (Kalra et al., 2012).
Based on this preliminary evidence, it would be more than reasonable to predict the existence of sophisticated cellular machinery controlling the trafficking of extracellular (signaling) RNAs via exosomes. These mechanisms are likely to occur at two main levels, acting either exclusively or cooperatively: (a) transcriptional, at the genetic loci of secreted non-coding RNAs; and/or (b) post-transcriptional, through ribonucleoproteins (Collino et al., 2010) that convey the correct non-coding RNAs from cells towards EVs and/or exosomes.
Thus, EVs represent a promising opportunity to develop novel cell-free therapy approaches that might overcome the obstacles and risks associated with the use of native or engineered stem cells. To put this in perspective, naturally occurring EV nano-factories may benefit from the expression of specific membrane molecules that would confer upon them a potential mechanism for homing to a specific tissue or microenvironment (El Andaloussi et al., 2013) (Table 3).
Conclusions and Perspectives
Consistent evidence challenges the old view that somatic, non-hematopoietic stem cell medicines, including those using MSCs and NPCs, protect the injured CNS exclusively throughout cell replacement (Rossi and Cattaneo, 2002). It is now accepted that the transplantation of stem cell transplantation via biological routes remarkably promotes the repair/healing of the CNS via several “bystander” or “chaperon” capacities that the graft exhibits within specific in vivo microenvironments after transplantation (Martino et al., 2011; Uccelli et al., 2011a). As such, compelling evidence is being provided that sustained stem cell graft-to-host exchange of signals leads to remarkable trophic effects on endogenous brain cells and beneficial modulatory actions on innate and adaptive immune responses that ultimately promote the healing of the injured CNS (Martino et al., 2011; Uccelli et al., 2007). Animal studies have taught much about the therapeutic potential of the physiological, and in some cases just circumstantial, stem cell graft-host immune cell interactions in the nervous system. However, convincing evidence of the value of the allo- or xenogeneic transplantation settings in the study of stem cell graft-to-host immune interactions is lacking; and some doubts as to the artifactual nature of certain observations persist.
After having established the significant role of diffusible secreted neuroprotective and immune regulatory molecules in this process (Martino et al., 2011; Uccelli et al., 2011a), recent evidence has highlighted the potential of cellular junctional coupling, as well as the (horizontal) transfer of different levels of information via EVs, between the stem cell graft and the host immune cells (Cossetti et al., 2012b; Huang et al., 2013). The physiological role of stem cell-derived EVs is currently not well understood. Nevertheless, encouraging results indicate that EVs have similar protective and reparative properties to their cellular counterparts in tissue repair and possibly anti-cancer therapy. Thus, EVs could represent a promising opportunity to develop novel cell-free therapy approaches that might overcome the obstacles and risks associated with the use of native or engineered stem cells.
Human studies have a great responsibility to help:
Establish the boundaries between the basal versus inflammation-reactive immunocompetence and immune regulatory potential of transplantable stem cell sources;
Determine the potential occurrence of undesired collateral immune suppression and/or deficiency; and
Define their overall safety profile in vivo, when sustained cell signaling with the host (human) immune system takes place within a pathogen-enriched environment (Aboody et al., 2011).
Therefore, parallel to the development of clinical trials looking at the safety of these novel stem cell-based therapeutics, some of which have just started (Bonab et al., 2012; Glass et al., 2012; Gupta et al., 2012; Mazzini et al., 2003, 2012; Steiner et al., 2010), we foresee that the exploitation of the mechanisms regulating their modalities of intercellular communication, including those addressing the mechanism of EV trafficking and secretion, has a realistic chance to revolutionize most of our current understanding of (stem) cell biology and its application in CNS repair.
Acknowledgment
Grant sponsor: National Multiple Sclerosis Society (NMSS); Grant number: RG-4001-A1; Grant sponsor: the Italian Multiple Sclerosis Association (AISM); Grant number: 2010/R/31; Grant sponsor: Italian Ministry of Health; Grant number: GR08-7; Grant sponsor: Wings for Life; Grant number: WFL-SE-013/09; Grant sponsor: Banca Agricola Popolare di Ragusa (BAPR) (to S.P.); Grant sponsor: European Research Council (ERC) under the ERC-2010-StG Grant Agreement; Grant number: 260511-SEM_SEM; Grant sponsor: European Community (EC) 7th Framework Program (FP7/2007-2013) under Grant Agreement; Grant number: 280772-iONE.
The authors thank Jayden A. Smith for critically reviewing the article and Paula Francis for proof edits. We acknowledge the technical assistance of Elena Giusto and Matteo Doneg a for providing the confocal images in Figure 2, and the contributions of past and present members of the Pluchino laboratory, who have contributed to (or inspired) this review.
Footnotes
Additional Supporting Information may be found in the online version of this article.
References
- Aboody K, Capela A, Niazi N, Stern JH, Temple S. Translating stem cell studies to the clinic for CNS repair: Current state of the art and the need for a Rosetta Stone. Neuron. 2011;70:597–613. doi: 10.1016/j.neuron.2011.05.007. [DOI] [PubMed] [Google Scholar]
- Aboody KS, Brown A, Rainov NG, Bower KA, Liu S, Yang W, Small JE, Herrlinger U, Ourednik V, Black PM, Breakefield XO, Snyder EY. Neural stem cells display extensive tropism for pathology in adult brain: evidence from intracranial gliomas. Proc Natl Acad Sci USA. 2000;97:12846–12851. doi: 10.1073/pnas.97.23.12846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005;105:1815–1822. doi: 10.1182/blood-2004-04-1559. [DOI] [PubMed] [Google Scholar]
- Ahmed AU, Thaci B, Alexiades NG, Han Y, Qian S, Liu F, Balyasnikova IV, Ulasov IY, Aboody KS, Lesniak MS. Neural stem cell-based cell carriers enhance therapeutic efficacy of an oncolytic adenovirus in an orthotopic mouse model of human glioblastoma. Mol Ther. 2011;19:1714–1726. doi: 10.1038/mt.2011.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiyama K, Chen C, Wang D, Xu X, Qu C, Yamaza T, Cai T, Chen W, Sun L, Shi S. Mesenchymal-stem-cell-induced immunoregulation involves FAS-ligand-/FAS-mediated T cell apoptosis. Cell Stem Cell. 2012;10:544–555. doi: 10.1016/j.stem.2012.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amariglio N, Hirshberg A, Scheithauer BW, Cohen Y, Loewenthal R, Trakhtenbrot L, Paz N, Koren-Michowitz M, Waldman D, Leider-Trejo L, Toren A, Constantini S, Rechavi G. Donor-derived brain tumor following neural stem cell transplantation in an ataxia telangiectasia patient. PLoS Med. 2009;6:e1000029. doi: 10.1371/journal.pmed.1000029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andres RH, Choi R, Pendharkar AV, Gaeta X, Wang N, Nathan JK, Chua JY, Lee SW, Palmer TD, Steinberg GK, Guzman R. The CCR2/CCL2 interaction mediates the transendothelial recruitment of intravascularly delivered neural stem cells to the ischemic brain. Stroke. 2011;42:2923–2931. doi: 10.1161/STROKEAHA.110.606368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anklesaria P, Teixido J, Laiho M, Pierce JH, Greenberger JS, Massague J. Cell-cell adhesion mediated by binding of membrane-anchored transforming growth factor alpha to epidermal growth factor receptors promotes cell proliferation. Proc Natl Acad Sci USA. 1990;87:3289–3293. doi: 10.1073/pnas.87.9.3289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archambault AS, Sim J, Gimenez MA, Russell JH. Defining antigen-dependent stages of T cell migration from the blood to the central nervous system parenchyma. Eur J Immunol. 2005;35:1076–1085. doi: 10.1002/eji.200425864. [DOI] [PubMed] [Google Scholar]
- Bacigaluppi M, Pluchino S, Martino G, Kilic E, Hermann DM. Neural stem/precursor cells for the treatment of ischemic stroke. J Neurol Sci. 2008;265:73–77. doi: 10.1016/j.jns.2007.06.012. [DOI] [PubMed] [Google Scholar]
- Bacigaluppi M, Pluchino S, Peruzzotti-Jametti L, Kilic E, Kilic U, Salani G, Brambilla E, West MJ, Comi G, Martino G, Hermann DM. Delayed post-ischaemic neuroprotection following systemic neural stem cell transplantation involves multiple mechanisms. Brain. 2009;132:2239–2251. doi: 10.1093/brain/awp174. [DOI] [PubMed] [Google Scholar]
- Badillo AT, Peranteau WH, Heaton TE, Quinn C, Flake AW. Murine bone marrow derived stromal progenitor cells fail to prevent or treat acute graft-versus-host disease. Br J Haematol. 2008;141:224–234. doi: 10.1111/j.1365-2141.2008.07040.x. [DOI] [PubMed] [Google Scholar]
- Bai L, Lennon DP, Caplan AI, DeChant A, Hecker J, Kranso J, Zaremba A, Miller RH. Hepatocyte growth factor mediates mesenchymal stem cell-induced recovery in multiple sclerosis models. Nat Neurosci. 2012;15:862–870. doi: 10.1038/nn.3109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartholomew A, Sturgeon C, Siatskas M, Ferrer K, McIntosh K, Patil S, Hardy W, Devine S, Ucker D, Deans R, Moseley A, Hoffman R. Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivo. Exp Hematol. 2002;30:42–48. doi: 10.1016/s0301-472x(01)00769-x. [DOI] [PubMed] [Google Scholar]
- Belmadani A, Tran PB, Ren D, Miller RJ. Chemokines regulate the migration of neural progenitors to sites of neuroinflammation. J Neurosci. 2006;26:3182–3191. doi: 10.1523/JNEUROSCI.0156-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Hur T, Einstein O, Mizrachi-Kol R, Ben-Menachem O, Reinhartz E, Karussis D, Abramsky O. Transplanted multipotential neural precursor cells migrate into the inflamed white matter in response to experimental autoimmune encephalomyelitis. Glia. 2003;41:73–80. doi: 10.1002/glia.10159. [DOI] [PubMed] [Google Scholar]
- Bifari F, Pacelli L, Krampera M. Immunological properties of embryonic and adult stem cells. World J Stem Cells. 2010;2:50–60. doi: 10.4252/wjsc.v2.i3.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bocelli-Tyndall C, Bracci L, Spagnoli G, Braccini A, Bouchenaki M, Ceredig R, Pistoia V, Martin I, Tyndall A. Bone marrow mesenchymal stromal cells (BM-MSCs) from healthy donors and auto-immune disease patients reduce the proliferation of autologous- and allogeneic-stimulated lymphocytes in vitro. Rheumatology (Oxford) 2007;46:403–408. doi: 10.1093/rheumatology/kel267. [DOI] [PubMed] [Google Scholar]
- Bonab MM, Sahraian MA, Aghsaie A, Karvigh SA, Hosseinian SM, Nikbin B, Lotfi J, Khorramnia S, Motamed MR, Togha M, Harirchian MH, Moghadam NB, Alikhani K, Yadegari S, Jafarian S, Gheini MR. Autologous mesenchymal stem cell therapy in progressive multiple sclerosis: an open label study. Curr Stem Cell Res Ther. 2012;7:407–414. doi: 10.2174/157488812804484648. [DOI] [PubMed] [Google Scholar]
- Bonnamain V, Mathieux E, Thinard R, Thebault P, Nerriere-Daguin V, Leveque X, Anegon I, Vanhove B, Neveu I, Naveilhan P. Expression of heme oxygenase-1 in neural stem/progenitor cells as a potential mechanism to evade host immune response. Stem Cells. 2012;30:2342–2353. doi: 10.1002/stem.1199. [DOI] [PubMed] [Google Scholar]
- Bouffi C, Bony C, Courties G, Jorgensen C, Noel D. IL-6-dependent PGE2 secretion by mesenchymal stem cells inhibits local inflammation in experimental arthritis. PLoS One. 2010;5:e14247. doi: 10.1371/journal.pone.0014247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brevig T, Pedersen EB, Finsen B. Molecular and cellular mechanisms in immune rejection of intracerebral neural transplants. Novartis Found Symp. 2000;231:166–177. doi: 10.1002/0470870834.ch11. discussion 177-183,302-306. [DOI] [PubMed] [Google Scholar]
- Brundin P, Barker RA, Parmar M. Neural grafting in Parkinson’s disease Problems and possibilities. Prog Brain Res. 2010;184:265–294. doi: 10.1016/S0079-6123(10)84014-2. [DOI] [PubMed] [Google Scholar]
- Bruno S, Grange C, Deregibus MC, Calogero RA, Saviozzi S, Collino F, Morando L, Busca A, Falda M, Bussolati B, Tetta C, Camussi G. Mesenchymal stem cell-derived microvesicles protect against acute tubular injury. J Am Soc Nephrol. 2009;20:1053–1067. doi: 10.1681/ASN.2008070798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunnell BA, Betancourt AM, Sullivan DE. New concepts on the immune modulation mediated by mesenchymal stem cells. Stem Cell Res Ther. 2010;1:34. doi: 10.1186/scrt34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butcher EC, Picker LJ. Lymphocyte homing and homeostasis. Science. 1996;272:60–66. doi: 10.1126/science.272.5258.60. [DOI] [PubMed] [Google Scholar]
- Campos LS, Decker L, Taylor V, Skarnes W. Notch, EGFR and beta 1 integrin pathways are coordinated in neural stem cells. J Biol Chem. 2006;281:5300–5309. doi: 10.1074/jbc.M511886200. [DOI] [PubMed] [Google Scholar]
- Campos LS, Leone DP, Relvas JB, Brakebusch C, Fassler R, Suter U, ffrench-Constant C. Beta1 integrins activate a MAPK signalling pathway in neural stem cells that contributes to their maintenance. Development. 2004;131:3433–3444. doi: 10.1242/dev.01199. [DOI] [PubMed] [Google Scholar]
- Camussi G, Deregibus MC, Tetta C. Paracrine/endocrine mechanism of stem cells on kidney repair: role of microvesicle-mediated transfer of genetic information. Curr Opin Nephrol Hypertens. 19:7–12. doi: 10.1097/MNH.0b013e328332fb6f. [DOI] [PubMed] [Google Scholar]
- Cao W, Yang Y, Wang Z, Liu A, Fang L, Wu F, Hong J, Shi Y, Leung S, Dong C, Zhang JZ. Leukemia inhibitory factor inhibits T helper 17 cell differentiation and confers treatment effects of neural progenitor cell therapy in autoimmune disease. Immunity. 2011;35:273–284. doi: 10.1016/j.immuni.2011.06.011. [DOI] [PubMed] [Google Scholar]
- Capone C, Frigerio S, Fumagalli S, Gelati M, Principato MC, Storini C, Montinaro M, Kraftsik R, De Curtis M, Parati E, De Simoni MG. Neurosphere-derived cells exert a neuroprotective action by changing the ischemic microenvironment. PLoS One. 2007;2:e373. doi: 10.1371/journal.pone.0000373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbajal KS, Miranda JL, Tsukamoto MR, Lane TE. CXCR4 signaling regulates remyelination by endogenous oligodendrocyte progenitor cells in a viral model of demyelination. Glia. 2011;59:1813–1821. doi: 10.1002/glia.21225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbajal KS, Schaumburg C, Strieter R, Kane J, Lane TE. Migration of engrafted neural stem cells is mediated by CXCL12 signaling through CXCR4 in a viral model of multiple sclerosis. Proc Natl Acad Sci USA. 2010;107:11068–11073. doi: 10.1073/pnas.1006375107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chabannes D, Hill M, Merieau E, Rossignol J, Brion R, Soulillou JP, Anegon I, Cuturi MC. A role for heme oxygenase-1 in the immunosuppressive effect of adult rat and human mesenchymal stem cells. Blood. 2007;110:3691–3694. doi: 10.1182/blood-2007-02-075481. [DOI] [PubMed] [Google Scholar]
- Chamberlain G, Smith H, Rainger GE, Middleton J. Mesenchymal stem cells exhibit firm adhesion, crawling, spreading and transmigration across aortic endothelial cells: Effects of chemokines and shear. PLoS One. 2011;6:e25663. doi: 10.1371/journal.pone.0025663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberlain G, Wright K, Rot A, Ashton B, Middleton J. Murine mesenchymal stem cells exhibit a restricted repertoire of functional chemokine receptors: Comparison with human. PLoS One. 2008;3:e2934. doi: 10.1371/journal.pone.0002934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Li Y, Katakowski M, Chen X, Wang L, Lu D, Lu M, Gautam SC, Chopp M. Intravenous bone marrow stromal cell therapy reduces apoptosis and promotes endogenous cell proliferation after stroke in female rat. J Neurosci Res. 2003;73:778–786. doi: 10.1002/jnr.10691. [DOI] [PubMed] [Google Scholar]
- Chen J, Li Y, Wang L, Zhang Z, Lu D, Lu M, Chopp M. Therapeutic benefit of intravenous administration of bone marrow stromal cells after cerebral ischemia in rats. Stroke. 2001;32:1005–1011. doi: 10.1161/01.str.32.4.1005. [DOI] [PubMed] [Google Scholar]
- Chen TS, Lai RC, Lee MM, Choo AB, Lee CN, Lim SK. Mesenchymal stem cell secretes microparticles enriched in pre-microRNAs. Nucleic Acids Res. 2009;38:215–224. doi: 10.1093/nar/gkp857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen TS, Lai RC, Lee MM, Choo AB, Lee CN, Lim SK. Mesenchymal stem cell secretes microparticles enriched in pre-microRNAs. Nucleic Acids Res. 2010;38:215–224. doi: 10.1093/nar/gkp857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Li Y, Wang L, Katakowski M, Zhang L, Chen J, Xu Y, Gautam SC, Chopp M. Ischemic rat brain extracts induce human marrow stromal cell growth factor production. Neuropathology. 2002;22:275–279. doi: 10.1046/j.1440-1789.2002.00450.x. [DOI] [PubMed] [Google Scholar]
- Chiesa S, Morbelli S, Morando S, Massollo M, Marini C, Bertoni A, Frassoni F, Bartolome ST, Sambuceti G, Traggiai E, Uccelli A. Mesenchymal stem cells impair in vivo T-cell priming by dendritic cells. Proc Natl Acad Sci USA. 2011;108:17384–17389. doi: 10.1073/pnas.1103650108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu K, Kim M, Jeong SW, Kim SU, Yoon BW. Human neural stem cells can migrate, differentiate, and integrate after intravenous transplantation in adult rats with transient forebrain ischemia. Neurosci Lett. 2003;343:129–133. doi: 10.1016/s0304-3940(03)00174-5. [DOI] [PubMed] [Google Scholar]
- Chu K, Kim M, Jung KH, Jeon D, Lee ST, Kim J, Jeong SW, Kim SU, Lee SK, Shin HS, Roh JK. Human neural stem cell transplantation reduces spontaneous recurrent seizures following pilocarpine-induced status epilepticus in adult rats. Brain Res. 2004;1023:213–221. doi: 10.1016/j.brainres.2004.07.045. [DOI] [PubMed] [Google Scholar]
- Collino F, Deregibus MC, Bruno S, Sterpone L, Aghemo G, Viltono L, Tetta C, Camussi G. Microvesicles derived from adult human bone marrow and tissue specific mesenchymal stem cells shuttle selected pattern of miRNAs. PLoS One. 2010;5:e11803. doi: 10.1371/journal.pone.0011803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connick P, Kolappan M, Crawley C, Webber DJ, Patani R, Michell AW, Du MQ, Luan SL, Altmann DR, Thompson AJ, Compston A, Scott MA, Miller DH, Chandran S. Autologous mesenchymal stem cells for the treatment of secondary progressive multiple sclerosis: An open-label phase 2a proof-of-concept study. Lancet Neurol. 2012;11:150–156. doi: 10.1016/S1474-4422(11)70305-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcione A, Benvenuto F, Ferretti E, Giunti D, Cappiello V, Cazzanti F, Risso M, Gualandi F, Mancardi GL, Pistoia V, Uccelli A. Human mesenchymal stem cells modulate B-cell functions. Blood. 2006;107:367–372. doi: 10.1182/blood-2005-07-2657. [DOI] [PubMed] [Google Scholar]
- Corti S, Locatelli F, Papadimitriou D, Donadoni C, Del Bo R, Fortunato F, Strazzer S, Salani S, Bresolin N, Comi GP. Multipotentiality, homing properties, and pyramidal neurogenesis of CNS-derived LeX(ssea-1)/CXCR4+ stem cells. FASEB J. 2005;19:1860–1862. doi: 10.1096/fj.05-4170fje. [DOI] [PubMed] [Google Scholar]
- Cossetti C, Alfaro-Cervello C, Donega M, Tyzack G, Pluchino S. New perspectives of tissue remodelling with neural stem and progenitor cell-based therapies. Cell Tissue Res. 2012a;349:321–329. doi: 10.1007/s00441-012-1341-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cossetti C, Smith JA, Iraci N, Leonardi T, Alfaro-Cervello C, Pluchino S. Extracellular membrane vesicles and immune regulation in the brain. Front Physiol. 2012b;3:117. doi: 10.3389/fphys.2012.00117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covacu R, Arvidsson L, Andersson A, Khademi M, Erlandsson-Harris H, Harris RA, Svensson MA, Olsson T, Brundin L. TLR activation induces TNF-alpha production from adult neural stem/progenitor cells. J Immunol. 2009;182:6889–6895. doi: 10.4049/jimmunol.0802907. [DOI] [PubMed] [Google Scholar]
- Croitoru-Lamoury J, Lamoury FM, Zaunders JJ, Veas LA, Brew BJ. Human mesenchymal stem cells constitutively express chemokines and chemokine receptors that can be upregulated by cytokines, IFN-beta, and Copaxone. J Interferon Cytokine Res. 2007;27:53–64. doi: 10.1089/jir.2006.0037. [DOI] [PubMed] [Google Scholar]
- Cselenyak A, Pankotai E, Horvath EM, Kiss L, Lacza Z. Mesenchymal stem cells rescue cardiomyoblasts from cell death in an in vitro ischemia model via direct cell-to-cell connections. BMC Cell Biol. 2010;11:29. doi: 10.1186/1471-2121-11-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cusimano M, Biziato D, Brambilla E, Donega M, Alfaro-Cervello C, Snider S, Salani G, Pucci F, Comi G, Garcia-Verdugo JM, De Palma M, Martino G, Pluchino S. Transplanted neural stem/precursor cells instruct phagocytes and reduce secondary tissue damage in the injured spinal cord. Brain. 2012;135:447–460. doi: 10.1093/brain/awr339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daadi MM, Davis AS, Arac A, Li Z, Maag AL, Bhatnagar R, Jiang K, Sun G, Wu JC, Steinberg GK. Human neural stem cell grafts modify microglial response and enhance axonal sprouting in neonatal hypoxic-ischemic brain injury. Stroke. 2010;41:516–523. doi: 10.1161/STROKEAHA.109.573691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Fuente H, Cibrian D, Sanchez-Madrid F. Immunoregulatory molecules are master regulators of inflammation during the immune response. FEBS Lett. 2012;586:2897–2905. doi: 10.1016/j.febslet.2012.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Miguel MP, Fuentes-Julian S, Blazquez-Martinez A, Pascual CY, Aller MA, Arias J, Arnalich-Montiel F. Immunosuppressive properties of mesenchymal stem cells: advances and applications. Curr Mol Med. 2012;12:574–591. doi: 10.2174/156652412800619950. [DOI] [PubMed] [Google Scholar]
- DeGrendele HC, Estess P, Siegelman MH. Requirement for CD44 in activated T cell extravasation into an inflammatory site. Science. 1997;278:672–675. doi: 10.1126/science.278.5338.672. [DOI] [PubMed] [Google Scholar]
- Delarosa O, Dalemans W, Lombardo E. Toll-like receptors as modulators of mesenchymal stem cells. Front Immunol. 2012;3:182. doi: 10.3389/fimmu.2012.00182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Nicola M, Carlo-Stella C, Magni M, Milanesi M, Longoni PD, Matteucci P, Grisanti S, Gianni AM. Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood. 2002;99:3838–3843. doi: 10.1182/blood.v99.10.3838. [DOI] [PubMed] [Google Scholar]
- Duffy MM, Pindjakova J, Hanley SA, McCarthy C, Weidhofer GA, Sweeney EM, English K, Shaw G, Murphy JM, Barry FP, Mahon BP, Belton O, Ceredig R, Griffin MD. Mesenchymal stem cell inhibition of T-helper 17 cell-differentiation is triggered by cell-cell contact and mediated by prostaglandin E2 via the EP4 receptor. Eur J Immunol. 2011;41:2840–2851. doi: 10.1002/eji.201141499. [DOI] [PubMed] [Google Scholar]
- Einstein O, Fainstein N, Vaknin I, Mizrachi-Kol R, Reihartz E, Grigoriadis N, Lavon I, Baniyash M, Lassmann H, Ben-Hur T. Neural precursors attenuate autoimmune encephalomyelitis by peripheral immunosuppression. Ann Neurol. 2007;61:209–218. doi: 10.1002/ana.21033. [DOI] [PubMed] [Google Scholar]
- Einstein O, Friedman-Levi Y, Grigoriadis N, Ben-Hur T. Transplanted neural precursors enhance host brain-derived myelin regeneration. J Neurosci. 2009;29:15694–15702. doi: 10.1523/JNEUROSCI.3364-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Einstein O, Karussis D, Grigoriadis N, Mizrachi-Kol R, Reinhartz E, Abramsky O, Ben-Hur T. Intraventricular transplantation of neural precursor cell spheres attenuates acute experimental allergic encephalomyelitis. Mol Cell Neurosci. 2003;24:1074–1082. doi: 10.1016/j.mcn.2003.08.009. [DOI] [PubMed] [Google Scholar]
- El Andaloussi S, Lakhal S, Mager I, Wood MJ. Exosomes for targeted siRNA delivery across biological barriers. Adv Drug Deliv Rev. 2013;65:391–397. doi: 10.1016/j.addr.2012.08.008. [DOI] [PubMed] [Google Scholar]
- Eliopoulos N, Stagg J, Lejeune L, Pommey S, Galipeau J. Allogeneic marrow stromal cells are immune rejected by MHC class I- and class II-mismatched recipient mice. Blood. 2005;106:4057–4065. doi: 10.1182/blood-2005-03-1004. [DOI] [PubMed] [Google Scholar]
- English K, Barry FP, Mahon BP. Murine mesenchymal stem cells suppress dendritic cell migration, maturation and antigen presentation. Immunol Lett. 2008;115:50–58. doi: 10.1016/j.imlet.2007.10.002. [DOI] [PubMed] [Google Scholar]
- Fainstein N, Vaknin I, Einstein O, Zisman P, Ben Sasson SZ, Baniyash M, Ben-Hur T. Neural precursor cells inhibit multiple inflammatory signals. Mol Cell Neurosci. 2008;39:335–341. doi: 10.1016/j.mcn.2008.07.007. [DOI] [PubMed] [Google Scholar]
- Fang L, Lange C, Engel M, Zander AR, Fehse B. Sensitive balance of suppressing and activating effects of mesenchymal stem cells on T-cell proliferation. Transplantation. 2006;82:1370–1373. doi: 10.1097/01.tp.0000232450.62408.f9. [DOI] [PubMed] [Google Scholar]
- Fisher-Shoval Y, Barhum Y, Sadan O, Yust-Katz S, Ben-Zur T, Lev N, Benkler C, Hod M, Melamed E, Offen D. Transplantation of placenta-derived mesenchymal stem cells in the EAE mouse model of MS. J Mol Neurosci. 2012;48:176–184. doi: 10.1007/s12031-012-9805-6. [DOI] [PubMed] [Google Scholar]
- Freedman MS, Bar-Or A, Atkins HL, Karussis D, Frassoni F, Lazarus H, Scolding N, Slavin S, Le Blanc K, Uccelli A. The therapeutic potential of mesenchymal stem cell transplantation as a treatment for multiple sclerosis: Consensus report of the International MSCT Study Group. Mult Scler. 2010;16:503–510. doi: 10.1177/1352458509359727. [DOI] [PubMed] [Google Scholar]
- Friedl P, den Boer AT, Gunzer M. Tuning immune responses: Diversity and adaptation of the immunological synapse. Nat Rev Immunol. 2005;5:532–545. doi: 10.1038/nri1647. [DOI] [PubMed] [Google Scholar]
- Gatti S, Bruno S, Deregibus MC, Sordi A, Cantaluppi V, Tetta C, Camussi G. Microvesicles derived from human adult mesenchymal stem cells protect against ischaemia-reperfusion-induced acute and chronic kidney injury. Nephrol Dial Transplant. 2011;26:1474–1483. doi: 10.1093/ndt/gfr015. [DOI] [PubMed] [Google Scholar]
- Gerdoni E, Gallo B, Casazza S, Musio S, Bonanni I, Pedemonte E, Mantegazza R, Frassoni F, Mancardi G, Pedotti R, Uccelli A. Mesenchymal stem cells effectively modulate pathogenic immune response in experimental autoimmune encephalomyelitis. Ann Neurol. 2007;61:219–227. doi: 10.1002/ana.21076. [DOI] [PubMed] [Google Scholar]
- Gieseke F, Bohringer J, Bussolari R, Dominici M, Handgretinger R, Muller I. Human multipotent mesenchymal stromal cells use galectin-1 to inhibit immune effector cells. Blood. 2010;116:3770–3779. doi: 10.1182/blood-2010-02-270777. [DOI] [PubMed] [Google Scholar]
- Glass CK, Saijo K, Winner B, Marchetto MC, Gage FH. Mechanisms underlying inflammation in neurodegeneration. Cell. 2010;140:918–934. doi: 10.1016/j.cell.2010.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass JD, Boulis NM, Johe K, Rutkove SB, Federici T, Polak M, Kelly C, Feldman EL. Lumbar intraspinal injection of neural stem cells in patients with amyotrophic lateral sclerosis: Results of a phase I trial in 12 patients. Stem cells. 2012;30:1144–1151. doi: 10.1002/stem.1079. [DOI] [PubMed] [Google Scholar]
- Glennie S, Soeiro I, Dyson PJ, Lam EW, Dazzi F. Bone marrow mesenchymal stem cells induce division arrest anergy of activated T cells. Blood. 2005;105:2821–2857. doi: 10.1182/blood-2004-09-3696. [DOI] [PubMed] [Google Scholar]
- Gnecchi M, Zhang Z, Ni A, Dzau VJ. Paracrine mechanisms in adult stem cell signaling and therapy. Circ Res. 2008;103:1204–1219. doi: 10.1161/CIRCRESAHA.108.176826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groh ME, Maitra B, Szekely E, Koc ON. Human mesenchymal stem cells require monocyte-mediated activation to suppress alloreactive T cells. Exp Hematol. 2005;33:928–934. doi: 10.1016/j.exphem.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Guha P, Morgan JW, Mostoslavsky G, Rodrigues NP, Boyd AS. Lack of immune response to differentiated cells derived from syngeneic induced pluripotent stem cells. Cell Stem Cell. 2013;12:1–6. doi: 10.1016/j.stem.2013.01.006. [DOI] [PubMed] [Google Scholar]
- Gupta N, Henry RG, Strober J, Kang SM, Lim DA, Bucci M, Caverzasi E, Gaetano L, Mandelli ML, Ryan T, Perry R, Farrell J, Jeremy RJ, Ulman M, Huhn SL, Barkovich AJ, Rowitch DH. Neural stem cell engraftment and myelination in the human brain. Sci Transl Med. 2012;4:155ra137. doi: 10.1126/scitranslmed.3004373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gur-Wahnon D, Borovsky Z, Beyth S, Liebergall M, Rachmilewitz J. Contact-dependent induction of regulatory antigen-presenting cells by human mesenchymal stem cells is mediated via STAT3 signaling. Exp Hematol. 2007;35:426–433. doi: 10.1016/j.exphem.2006.11.001. [DOI] [PubMed] [Google Scholar]
- Guzman R, De Los Angeles A, Cheshier S, Choi R, Hoang S, Liauw J, Schaar B, Steinberg G. Intracarotid injection of fluorescence activated cell-sorted CD49d-positive neural stem cells improves targeted cell delivery and behavior after stroke in a mouse stroke model. Stroke. 2008;39:1300–1306. doi: 10.1161/STROKEAHA.107.500470. [DOI] [PubMed] [Google Scholar]
- Hall PE, Lathia JD, Miller NG, Caldwell MA, ffrench-Constant C. Integrins are markers of human neural stem cells. Stem Cells. 2006;24:2078–2084. doi: 10.1634/stemcells.2005-0595. [DOI] [PubMed] [Google Scholar]
- Hattiangady B, Rao MS, Shetty AK. Grafting of striatal precursor cells into hippocampus shortly after status epilepticus restrains chronic temporal lobe epilepsy. Exp Neurol. 2008;212:468–481. doi: 10.1016/j.expneurol.2008.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heijnen HF, Schiel AE, Fijnheer R, Geuze HJ, Sixma JJ. Activated platelets release two types of membrane vesicles: microvesicles by surface shedding and exosomes derived from exocytosis of multivesicular bodies and alpha-granules. Blood. 1999;94:3791–3799. [PubMed] [Google Scholar]
- Hof-Nahor I, Leshansky L, Shivtiel S, Eldor L, Aberdam D, Itskovitz-Eldor J, Berrih-Aknin S. Human mesenchymal stem cells shift CD8+ T cells towards a suppressive phenotype by inducing tolerogenic monocytes. J Cell Sci. 2012;125:4640–4650. doi: 10.1242/jcs.108860. [DOI] [PubMed] [Google Scholar]
- Horie N, Pereira MP, Niizuma K, Sun G, Keren-Gill H, Encarnacion A, Shamloo M, Hamilton SA, Jiang K, Huhn S, Palmer TD, Bliss TM, Steinberg GK. Transplanted stem cell-secreted VEGF effects post-stroke recovery, inflammation, and vascular repair. Stem Cells. 2011;29:274–285. doi: 10.1002/stem.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YC, Parolini O, Deng L. The potential role of microvesicles in mesenchymal stem cell-based therapy. Stem Cells Dev. 2013;22:841–844. doi: 10.1089/scd.2012.0631. [DOI] [PubMed] [Google Scholar]
- Imitola J, Comabella M, Chandraker AK, Dangond F, Sayegh MH, Snyder EY, Khoury SJ. Neural stem/progenitor cells express costimulatory molecules that are differentially regulated by inflammatory and apoptotic stimuli. Am J Pathol. 2004a;164:1615–1625. doi: 10.1016/S0002-9440(10)63720-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imitola J, Raddassi K, Park KI, Mueller FJ, Nieto M, Teng YD, Frenkel D, Li J, Sidman RL, Walsh CA, Snyder EY, Khoury SJ. Directed migration of neural stem cells to sites of CNS injury by the stromal cell-derived factor 1alpha/CXC chemokine receptor 4 pathway. Proc Natl Acad Sci USA. 2004b;101:18117–18122. doi: 10.1073/pnas.0408258102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanova NB, Dimos JT, Schaniel C, Hackney JA, Moore KA, Lemischka IR. A stem cell molecular signature. Science. 2002;298:601–604. doi: 10.1126/science.1073823. [DOI] [PubMed] [Google Scholar]
- Jaderstad J, Jaderstad LM, Li J, Chintawar S, Salto C, Pandolfo M, Ourednik V, Teng YD, Sidman RL, Arenas E, Snyder EY, Herlenius E. Communication via gap junctions underlies early functional and beneficial interactions between grafted neural stem cells and the host. Proc Natl Acad Sci USA. 2010;107:5184–5189. doi: 10.1073/pnas.0915134107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji JF, He BP, Dheen ST, Tay SS. Expression of chemokine receptors CXCR4, CCR2, CCR5 and CX3CR1 in neural progenitor cells isolated from the subventricular zone of the adult rat brain. Neurosci Lett. 2004;355:236–240. doi: 10.1016/j.neulet.2003.11.024. [DOI] [PubMed] [Google Scholar]
- Jiang XX, Zhang Y, Liu B, Zhang SX, Wu Y, Yu XD, Mao N. Human mesenchymal stem cells inhibit differentiation and function of monocyte-derived dendritic cells. Blood. 2005;105:4120–4126. doi: 10.1182/blood-2004-02-0586. [DOI] [PubMed] [Google Scholar]
- Johansson S, Price J, Modo M. Effect of inflammatory cytokines on major histocompatibility complex expression and differentiation of human neural stem/progenitor cells. Stem Cells. 2008;26:2444–2454. doi: 10.1634/stemcells.2008-0116. [DOI] [PubMed] [Google Scholar]
- Kalra H, Simpson RJ, Ji H, Aikawa E, Altevogt P, Askenase P, Bond VC, Borras FE, Breakefield X, Budnik V, Buzas E, Camussi G, Clayton A, Cocucci E, Falcon-Perez JM, Gabrielsson S, Gho YS, Gupta D, Harsha HC, Hendrix A, Hill AF, Inal JM, Jenster G, Krämer-Albers EM, Lim SK, Llorente A, Lötvall J, Marcilla A, Mincheva-Nilsson L, Nazarenko I, Nieuwland R, Nolte-’t Hoen EN, Pandey A, Patel T, Piper MG, Pluchino S, Prasad TS, Rajendran L, Raposo G, Record M, Reid GE, Sánchez-Madrid F, Schiffelers RM, Siljander P, Stensballe A, Stoorvogel W, Taylor D, Thery C, Valadi H, van Balkom BW, Vázquez J, Vidal M, Wauben MH, Yáñez-Mó M, Zoeller M, Mathivanan S. Vesiclepedia: a compendium for extracellular vesicles with continuous community annotation. PLoS Biol. 2012;10:e1001450. doi: 10.1371/journal.pbio.1001450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamoun M. Mechanisms of chronic allograft dysfunction. Ther Drug Monit. 2006;28:14–18. doi: 10.1097/01.ftd.0000194499.32398.32. [DOI] [PubMed] [Google Scholar]
- Kim DE, Tsuji K, Kim YR, Mueller FJ, Eom HS, Snyder EY, Lo EH, Weissleder R, Schellingerhout D. Neural stem cell transplant survival in brains of mice: assessing the effect of immunity and ischemia by using real-time bioluminescent imaging. Radiology. 2006;241:822–830. doi: 10.1148/radiol.2413050466. [DOI] [PubMed] [Google Scholar]
- Kim HM, Hwang DH, Lee JE, Kim SU, Kim BG. Ex vivo VEGF delivery by neural stem cells enhances proliferation of glial progenitors, angiogenesis, and tissue sparing after spinal cord injury. PLoS One. 2009a;4:e4987. doi: 10.1371/journal.pone.0004987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HS, Choi DY, Yun SJ, Choi SM, Kang JW, Jung JW, Hwang D, Kim KP, Kim DW. Proteomic analysis of microvesicles derived from human mesenchymal stem cells. J Proteome Res. 2012;11:839–849. doi: 10.1021/pr200682z. [DOI] [PubMed] [Google Scholar]
- Kim SY, Cho HS, Yang SH, Shin JY, Kim JS, Lee ST, Chu K, Roh JK, Kim SU, Park CG. Soluble mediators from human neural stem cells play a critical role in suppression of T-cell activation and proliferation. J Neurosci Res. 2009b;87:2264–2272. doi: 10.1002/jnr.22050. [DOI] [PubMed] [Google Scholar]
- Kim SY, Cho HS, Yang SH, Shin JY, Kim JS, Park CG. Exosomes secreted from human neural stem cells suppress T cell activation. J Immunol. 2009c;182:90. [Google Scholar]
- Kivisakk P, Imitola J, Rasmussen S, Elyaman W, Zhu B, Ransohoff RM, Khoury SJ. Localizing central nervous system immune surveillance: Meningeal antigen-presenting cells activate T cells during experimental autoimmune encephalomyelitis. Ann Neurol. 2009;65:457–469. doi: 10.1002/ana.21379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klyushnenkova E, Mosca JD, Zernetkina V, Majumdar MK, Beggs KJ, Simonetti DW, Deans RJ, McIntosh KR. T cell responses to allogeneic human mesenchymal stem cells: immunogenicity, tolerance, and suppression. J Biomed Sci. 2005;12:47–57. doi: 10.1007/s11373-004-8183-7. [DOI] [PubMed] [Google Scholar]
- Knight JC, Scharf EL, Mao-Draayer Y. Fas activation increases neural progenitor cell survival. J Neurosci Res. 2010;88:746–757. doi: 10.1002/jnr.22253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh W, Sheng CT, Tan B, Lee QY, Kuznetsov V, Kiang LS, Tanavde V. Analysis of deep sequencing microRNA expression profile from human embryonic stem cells derived mesenchymal stem cells reveals possible role of let-7 microRNA family in downstream targeting of hepatic nuclear factor 4 alpha. BMC Genomics. 2010;11(Suppl 1):S6. doi: 10.1186/1471-2164-11-S1-S6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokaia Z, Martino G, Schwartz M, Lindvall O. Cross-talk between neural stem cells and immune cells: the key to better brain repair? Nat Neurosci. 2012;15:1078–1087. doi: 10.1038/nn.3163. [DOI] [PubMed] [Google Scholar]
- Laguna Goya R, Busch R, Mathur R, Coles AJ, Barker RA. Human fetal neural precursor cells can up-regulate MHC class I and class II expression and elicit CD4 and CD8 T cell proliferation. Neurobiol Dis. 2011;41:407–414. doi: 10.1016/j.nbd.2010.10.008. [DOI] [PubMed] [Google Scholar]
- Lai RC, Arslan F, Lee MM, Sze NS, Choo A, Chen TS, Salto-Tellez M, Timmers L, Lee CN, El Oakley RM, Pasterkamp G, de Kleijn DP, Lim SK. Exosome secreted by MSC reduces myocardial ischemia/reperfusion injury. Stem Cell Res. 2010;4:214–222. doi: 10.1016/j.scr.2009.12.003. [DOI] [PubMed] [Google Scholar]
- Lanz TV, Opitz CA, Ho PP, Agrawal A, Lutz C, Weller M, Mellor AL, Steinman L, Wick W, Platten M. Mouse mesenchymal stem cells suppress antigen-specific TH cell immunity independent of indoleamine 2,3-dioxygenase 1 (IDO1) Stem Cells Dev. 2010;19:657–668. doi: 10.1089/scd.2009.0385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Blanc K, Rasmusson I, Gotherstrom C, Seidel C, Sundberg B, Sundin M, Rosendahl K, Tammik C, Ringden O. Mesenchymal stem cells inhibit the expression of CD25 (interleukin-2 receptor) and CD38 on phytohaemagglutinin-activated lymphocytes. Scand J Immunol. 2004;60:307–315. doi: 10.1111/j.0300-9475.2004.01483.x. [DOI] [PubMed] [Google Scholar]
- Le Blanc K, Tammik L, Sundberg B, Haynesworth SE, Ringden O. Mesenchymal stem cells inhibit and stimulate mixed lymphocyte cultures and mitogenic responses independently of the major histocompatibility complex. Scand J Immunol. 2003;57:11–20. doi: 10.1046/j.1365-3083.2003.01176.x. [DOI] [PubMed] [Google Scholar]
- Lee PH, Lee JE, Kim HS, Song SK, Lee HS, Nam HS, Cheong JW, Jeong Y, Park HJ, Kim DJ, Nam CM, Lee JD, Kim HO, Sohn YH. A randomized trial of mesenchymal stem cells in multiple system atrophy. Ann Neurol. 2012;72:32–40. doi: 10.1002/ana.23612. [DOI] [PubMed] [Google Scholar]
- Lee RH, Pulin AA, Seo MJ, Kota DJ, Ylostalo J, Larson BL, Semprun-Prieto L, Delafontaine P, Prockop DJ. Intravenous hMSCs improve myocardial infarction in mice because cells embolized in lung are activated to secrete the anti-inflammatory protein TSG-6. Cell Stem Cell. 2009;5:54–63. doi: 10.1016/j.stem.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee ST, Chu K, Jung KH, Kim SJ, Kim DH, Kang KM, Hong NH, Kim JH, Ban JJ, Park HK, Kim SU, Park CG, Lee SK, Kim M, Roh JK. Anti-inflammatory mechanism of intravascular neural stem cell transplantation in haemorrhagic stroke. Brain. 2008;131:616–629. doi: 10.1093/brain/awm306. [DOI] [PubMed] [Google Scholar]
- Lee ST, Park JE, Lee K, Kang L, Chu K, Kim SU, Kim M, Roh JK. Noninvasive method of immortalized neural stem-like cell transplantation in an experimental model of Huntington’s disease. J Neurosci Methods. 2006;152:250–254. doi: 10.1016/j.jneumeth.2005.09.013. [DOI] [PubMed] [Google Scholar]
- Lees JS, Sena ES, Egan KJ, Antonic A, Koblar SA, Howells DW, Macleod MR. Stem cell-based therapy for experimental stroke: A systematic review and meta-analysis. Int J Stroke. 2012;7:582–588. doi: 10.1111/j.1747-4949.2012.00797.x. [DOI] [PubMed] [Google Scholar]
- Leone DP, Relvas JB, Campos LS, Hemmi S, Brakebusch C, Fassler R, Ffrench-Constant C, Suter U. Regulation of neural progenitor proliferation and survival by beta1 integrins. J Cell Sci. 2005;118:2589–2599. doi: 10.1242/jcs.02396. [DOI] [PubMed] [Google Scholar]
- Ley K, Laudanna C, Cybulsky MI, Nourshargh S. Getting to the site of inflammation: The leukocyte adhesion cascade updated. Nat Rev Immunol. 2007;7:678–689. doi: 10.1038/nri2156. [DOI] [PubMed] [Google Scholar]
- Li H, Guo Z, Jiang X, Zhu H, Li X, Mao N. Mesenchymal stem cells alter migratory property of T and dendritic cells to delay the development of murine lethal acute graft-versus-host disease. Stem Cells. 2008;26:2531–2541. doi: 10.1634/stemcells.2008-0146. [DOI] [PubMed] [Google Scholar]
- Li L, Xie T. Stem cell niche: Structure and function. Annu Rev Cell Dev Biol. 2005;21:605–631. doi: 10.1146/annurev.cellbio.21.012704.131525. [DOI] [PubMed] [Google Scholar]
- Li M, Chang CJ, Lathia JD, Wang L, Pacenta HL, Cotleur A, Ransohoff RM. Chemokine receptor CXCR4 signaling modulates the growth factor-induced cell cycle of self-renewing and multipotent neural progenitor cells. Glia. 2011;59:108–118. doi: 10.1002/glia.21080. [DOI] [PubMed] [Google Scholar]
- Lindvall O, Kokaia Z. Stem cell research in stroke: How far from the clinic? Stroke. 2011;42:2369–2375. doi: 10.1161/STROKEAHA.110.599654. [DOI] [PubMed] [Google Scholar]
- Liotta F, Angeli R, Cosmi L, Fili L, Manuelli C, Frosali F, Mazzinghi B, Maggi L, Pasini A, Lisi V, Santarlasci V, Consoloni L, Angelotti ML, Romagnani P, Parronchi P, Krampera M, Maggi E, Romagnani S, Annunziato F. Toll-like receptors 3 and 4 are expressed by human bone marrow-derived mesenchymal stem cells and can inhibit their T-cell modulatory activity by impairing Notch signaling. Stem Cells. 2008;26:279–289. doi: 10.1634/stemcells.2007-0454. [DOI] [PubMed] [Google Scholar]
- Liu X, Qu X, Chen Y, Liao L, Cheng K, Shao C, Zenke M, Keating A, Zhao RC. Mesenchymal stem/stromal cells induce the generation of novel IL-10-dependent regulatory dendritic cells by SOCS3 activation. J Immunol. 2012;189:1182–1192. doi: 10.4049/jimmunol.1102996. [DOI] [PubMed] [Google Scholar]
- Luz-Crawford P, Noel D, Fernandez X, Khoury M, Figueroa F, Carrion F, Jorgensen C, Djouad F. Mesenchymal stem cells repress Th17 molecular program through the PD-1 pathway. PLoS One. 2012;7:e45272. doi: 10.1371/journal.pone.0045272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maccario R, Podesta M, Moretta A, Cometa A, Comoli P, Montagna D, Daudt L, Ibatici A, Piaggio G, Pozzi S, Frassoni F, Locatelli F. Interaction of human mesenchymal stem cells with cells involved in alloantigen-specific immune response favors the differentiation of CD4+ T-cell subsets expressing a regulatory/suppressive phenotype. Haematologica. 2005;90:516–525. [PubMed] [Google Scholar]
- Majumdar MK, Keane-Moore M, Buyaner D, Hardy WB, Moorman MA, McIntosh KR, Mosca JD. Characterization and functionality of cell surface molecules on human mesenchymal stem cells. J Biomed Sci. 2003;10:228–241. doi: 10.1007/BF02256058. [DOI] [PubMed] [Google Scholar]
- Mammolenti M, Gajavelli S, Tsoulfas P, Levy R. Absence of major histocompatibility complex class I on neural stem cells does not permit natural killer cell killing and prevents recognition by alloreactive cytotoxic T lymphocytes in vitro. Stem Cells. 2004;22:1101–1110. doi: 10.1634/stemcells.22-6-1101. [DOI] [PubMed] [Google Scholar]
- Martinet L, Fleury-Cappellesso S, Gadelorge M, Dietrich G, Bourin P, Fournie JJ, Poupot R. A regulatory cross-talk between Vgamma9Vdelta2 T lymphocytes and mesenchymal stem cells. Eur J Immunol. 2009;39:752–762. doi: 10.1002/eji.200838812. [DOI] [PubMed] [Google Scholar]
- Martino G, Pluchino S. The therapeutic potential of neural stem cells. Nat Rev Neurosci. 2006;7:395–406. doi: 10.1038/nrn1908. [DOI] [PubMed] [Google Scholar]
- Martino G, Pluchino S. Neural stem cells: guardians of the brain. Nat Cell Biol. 2007;9:1031–1034. doi: 10.1038/ncb0907-1031. [DOI] [PubMed] [Google Scholar]
- Martino G, Pluchino S, Bonfanti L, Schwartz M. Brain regeneration in physiology and pathology: the immune signature driving therapeutic plasticity of neural stem cells. Physiol Rev. 2011;91:1281–1304. doi: 10.1152/physrev.00032.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathivanan S, Ji H, Simpson RJ. Exosomes: Extracellular organelles important in intercellular communication. J Proteomics. 2010;73:1907–1920. doi: 10.1016/j.jprot.2010.06.006. [DOI] [PubMed] [Google Scholar]
- Matysiak M, Orlowski W, Fortak-Michalska M, Jurewicz A, Selmaj K. Immunoregulatory function of bone marrow mesenchymal stem cells in EAE depends on their differentiation state and secretion of PGE2. J Neuroimmunol. 2011;233:106–111. doi: 10.1016/j.jneuroim.2010.12.004. [DOI] [PubMed] [Google Scholar]
- Matysiak M, Stasiolek M, Orlowski W, Jurewicz A, Janczar S, Raine CS, Selmaj K. Stem cells ameliorate EAE via an indoleamine 2,3-dioxygenase (IDO) mechanism. J Neuroimmunol. 2008;193:12–23. doi: 10.1016/j.jneuroim.2007.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazar J, Thomas M, Bezrukov L, Chanturia A, Pekkurnaz G, Yin S, Kuznetsov SA, Robey PG, Zimmerberg J. Cytotoxicity mediated by the Fas ligand (FasL)-activated apoptotic pathway in stem cells. J Biol Chem. 2009;284:22022–22028. doi: 10.1074/jbc.M109.032235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzini L, Fagioli F, Boccaletti R, Mareschi K, Oliveri G, Olivieri C, Pastore I, Marasso R, Madon E. Stem cell therapy in amyotrophic lateral sclerosis: A methodological approach in humans. Amyotroph Lateral Scler Other Motor Neuron Disord. 2003;4:158–161. doi: 10.1080/14660820310014653. [DOI] [PubMed] [Google Scholar]
- Mazzini L, Mareschi K, Ferrero I, Miglioretti M, Stecco A, Servo S, Carriero A, Monaco F, Fagioli F. Mesenchymal stromal cell transplantation in amyotrophic lateral sclerosis: a long-term safety study. Cytotherapy. 2012;14:56–60. doi: 10.3109/14653249.2011.613929. [DOI] [PubMed] [Google Scholar]
- Meisel R, Zibert A, Laryea M, Gobel U, Daubener W, Dilloo D. Human bone marrow stromal cells inhibit allogeneic T-cell responses by indoleamine 2,3-dioxygenase-mediated tryptophan degradation. Blood. 2004;103:4619–4621. doi: 10.1182/blood-2003-11-3909. [DOI] [PubMed] [Google Scholar]
- Melzi R, Antonioli B, Mercalli A, Battaglia M, Valle A, Pluchino S, Galli R, Sordi V, Bosi E, Martino G, Bonifacio E, Doglioni C, Piemonti L. Co-graft of allogeneic immune regulatory neural stem cells (NPC) and pancreatic islets mediates tolerance, while inducing NPC-derived tumors in mice. PLoS One. 2010;5:e10357. doi: 10.1371/journal.pone.0010357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modo M, Rezaie P, Heuschling P, Patel S, Male DK, Hodges H. Transplantation of neural stem cells in a rat model of stroke: assessment of short-term graft survival and acute host immunological response. Brain Res. 2002;958:70–82. doi: 10.1016/s0006-8993(02)03463-7. [DOI] [PubMed] [Google Scholar]
- Mokarizadeh A, Delirezh N, Morshedi A, Mosayebi G, Farshid AA, Mardani K. Microvesicles derived from mesenchymal stem cells: potent organelles for induction of tolerogenic signaling. Immunol Lett. 2012;147:47–54. doi: 10.1016/j.imlet.2012.06.001. [DOI] [PubMed] [Google Scholar]
- Moll G, Jitschin R, von Bahr L, Rasmusson-Duprez I, Sundberg B, Lonnies L, Elgue G, Nilsson-Ekdahl K, Mougiakakos D, Lambris JD, Ringden O, Le Blanc K, Nilsson B. Mesenchymal stromal cells engage complement and complement receptor bearing innate effector cells to modulate immune responses. PLoS One. 2011;6:e21703. doi: 10.1371/journal.pone.0021703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morandi F, Raffaghello L, Bianchi G, Meloni F, Salis A, Millo E, Ferrone S, Barnaba V, Pistoia V. Immunogenicity of human mesenchymal stem cells in HLA-class I-restricted T-cell responses against viral or tumor-associated antigens. Stem Cells. 2008;26:1275–1287. doi: 10.1634/stemcells.2007-0878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morando S, Vigo T, Esposito M, Casazza S, Novi G, Principato MC, Furlan R, Uccelli A. The therapeutic effect of mesenchymal stem cell transplantation in experimental autoimmune encephalomyelitis is mediated by peripheral and central mechanisms. Stem Cell Res Ther. 2012;3:3. doi: 10.1186/scrt94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosher KI, Andres RH, Fukuhara T, Bieri G, Hasegawa-Moriyama M, He Y, Guzman R, Wyss-Coray T. Neural progenitor cells regulate microglia functions and activity. Nat Neurosci. 2012;15:1485–1487. doi: 10.1038/nn.3233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller FJ, Serobyan N, Schraufstatter IU, DiScipio R, Wakeman D, Loring JF, Snyder EY, Khaldoyanidi SK. Adhesive interactions between human neural stem cells and inflamed human vascular endothelium are mediated by integrins. Stem Cells. 2006;24:2367–2372. doi: 10.1634/stemcells.2005-0568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller FJ, Snyder EY, Loring JF. Gene therapy: can neural stem cells deliver? Nat Rev Neurosci. 2006;7:75–84. doi: 10.1038/nrn1829. [DOI] [PubMed] [Google Scholar]
- Nakajima H, Uchida K, Guerrero AR, Watanabe S, Sugita D, Takeura N, Yoshida A, Long G, Wright KT, Johnson WE, Baba H. Transplantation of mesenchymal stem cells promotes an alternative pathway of macrophage activation and functional recovery after spinal cord injury. J Neurotrauma. 2012;29:1614–1625. doi: 10.1089/neu.2011.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nauta AJ, Westerhuis G, Kruisselbrink AB, Lurvink EG, Willemze R, Fibbe WE. Donor-derived mesenchymal stem cells are immunogenic in an allogeneic host and stimulate donor graft rejection in a nonmyeloablative setting. Blood. 2006;08:2114–2120. doi: 10.1182/blood-2005-11-011650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemeth K, Leelahavanichkul A, Yuen PS, Mayer B, Parmelee A, Doi K, Robey PG, Leelahavanichkul K, Koller BH, Brown JM, Hu X, Jelinek I, Star RA, Mezey E. Bone marrow stromal cells attenuate sepsis via prostaglandin E(2)-dependent reprogramming of host macrophages to increase their interleukin-10 production. Nat Med. 2009;15:42–49. doi: 10.1038/nm.1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuss S, Becher E, Woltje M, Tietze L, Jahnen-Dechent W. Functional expression of HGF and HGF receptor/c-met in adult human mesenchymal stem cells suggests a role in cell mobilization, tissue repair, and wound healing. Stem Cells. 2004;22:405–414. doi: 10.1634/stemcells.22-3-405. [DOI] [PubMed] [Google Scholar]
- Odeberg J, Piao JH, Samuelsson EB, Falci S, Akesson E. Low immunogenicity of in vitro-expanded human neural cells despite high MHC expression. J Neuroimmunol. 2005;161:1–11. doi: 10.1016/j.jneuroim.2004.11.016. [DOI] [PubMed] [Google Scholar]
- Park MJ, Shin JS, Kim YH, Hong SH, Yang SH, Shin JY, Kim SY, Kim B, Kim JS, Park CG. Murine mesenchymal stem cells suppress T lymphocyte activation through IL-2 receptor alpha (CD25) cleavage by producing matrix metalloproteinases. Stem Cell Rev. 2011;7:381–393. doi: 10.1007/s12015-010-9203-9. [DOI] [PubMed] [Google Scholar]
- Pearl JI, Kean LS, Davis MM, Wu JC. Pluripotent stem cells: Immune to the immune system? Sci Transl Med. 2012;4:164ps25. doi: 10.1126/scitranslmed.3005090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peer D, Zhu P, Carman CV, Lieberman J, Shimaoka M. Selective gene silencing in activated leukocytes by targeting siRNAs to the integrin lymphocyte function-associated antigen-1. Proc Natl Acad Sci USA. 2007;104:4095–4100. doi: 10.1073/pnas.0608491104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng X, Ueda H, Zhou H, Stokol T, Shen TL, Alcaraz A, Nagy T, Vassalli JD, Guan JL. Overexpression of focal adhesion kinase in vascular endothelial cells promotes angiogenesis in transgenic mice. Cardiovasc Res. 2004;64:421–430. doi: 10.1016/j.cardiores.2004.07.012. [DOI] [PubMed] [Google Scholar]
- Pevsner-Fischer M, Morad V, Cohen-Sfady M, Rousso-Noori L, Zanin-Zhorov A, Cohen S, Cohen IR, Zipori D. Toll-like receptors and their ligands control mesenchymal stem cell functions. Blood. 2007;109:1422–1432. doi: 10.1182/blood-2006-06-028704. [DOI] [PubMed] [Google Scholar]
- Pluchino S, Gritti A, Blezer E, Amadio S, Brambilla E, Borsellino G, Cossetti C, Del Carro U, Comi G, tHart B, Vescovi A, Martino G. Human neural stem cells ameliorate autoimmune encephalomyelitis in non-human primates. Ann Neurol. 2009a;66:343–354. doi: 10.1002/ana.21745. [DOI] [PubMed] [Google Scholar]
- Pluchino S, Quattrini A, Brambilla E, Gritti A, Salani G, Dina G, Galli R, Del Carro U, Amadio S, Bergami A, Furlan R, Comi G, Vescovi AL, Martino G. Injection of adult neurospheres induces recovery in a chronic model of multiple sclerosis. Nature. 2003;422:688–694. doi: 10.1038/nature01552. [DOI] [PubMed] [Google Scholar]
- Pluchino S, Zanotti L, Brambilla E, Querini P, Rovere-Capobianco A, Alfaro-Cervello C, Salani G, Cossetti C, Borsellino G, Battistini L, Ponzoni M, Doglioni C, Garcia-Verdugo JM, Comi G, Manfredi AA, Martino G. Immune regulatory neural stem/precursor cells protect from central nervous system autoimmunity by restraining dendritic cell function. PLoS One. 2009b;4:e5959. doi: 10.1371/journal.pone.0005959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pluchino S, Zanotti L, Rossi B, Brambilla E, Ottoboni L, Salani G, Martinello M, Cattalini A, Bergami A, Furlan R, Comi G, Constantin G, Martino G. Neurosphere-derived multipotent precursors promote neuroprotection by an immunomodulatory mechanism. Nature. 2005;436:266–271. doi: 10.1038/nature03889. [DOI] [PubMed] [Google Scholar]
- Plumas J, Chaperot L, Richard MJ, Molens JP, Bensa JC, Favrot MC. Mesenchymal stem cells induce apoptosis of activated T cells. Leukemia. 2005;19:1597–1604. doi: 10.1038/sj.leu.2403871. [DOI] [PubMed] [Google Scholar]
- Potian JA, Aviv H, Ponzio NM, Harrison JS, Rameshwar P. Veto-like activity of mesenchymal stem cells: functional discrimination between cellular responses to alloantigens and recall antigens. J Immunol. 2003;171:3426–3434. doi: 10.4049/jimmunol.171.7.3426. [DOI] [PubMed] [Google Scholar]
- Prevosto C, Zancolli M, Canevali P, Zocchi MR, Poggi A. Generation of CD4+ or CD8+ regulatory T cells upon mesenchymal stem cell-lymphocyte interaction. Haematologica. 2007;92:881–888. doi: 10.3324/haematol.11240. [DOI] [PubMed] [Google Scholar]
- Qian L, Huang Y, Spencer CI, Foley A, Vedantham V, Liu L, Conway SJ, Fu JD, Srivastava D. In vivo reprogramming of murine cardiac fibroblasts into induced cardiomyocytes. Nature. 2012;485:593–598. doi: 10.1038/nature11044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raicevic G, Rouas R, Najar M, Stordeur P, Boufker HI, Bron D, Martiat P, Goldman M, Nevessignsky MT, Lagneaux L. Inflammation modifies the pattern and the function of Toll-like receptors expressed by human mesenchymal stromal cells. Hum Immunol. 2010;71:235–244. doi: 10.1016/j.humimm.2009.12.005. [DOI] [PubMed] [Google Scholar]
- Ramasamy R, Fazekasova H, Lam EW, Soeiro I, Lombardi G, Dazzi F. Mesenchymal stem cells inhibit dendritic cell differentiation and function by preventing entry into the cell cycle. Transplantation. 2007;83:71–76. doi: 10.1097/01.tp.0000244572.24780.54. [DOI] [PubMed] [Google Scholar]
- Rampon C, Weiss N, Deboux C, Chaverot N, Miller F, Buchet D, Tricoire-Leignel H, Cazaubon S, Baron-Van Evercooren A, Couraud PO. Molecular mechanism of systemic delivery of neural precursor cells to the brain: Assembly of brain endothelial apical cups and control of transmigration by CD44. Stem Cells. 2008;26:1673–1682. doi: 10.1634/stemcells.2008-0122. [DOI] [PubMed] [Google Scholar]
- Ranganath SH, Levy O, Inamdar MS, Karp JM. Harnessing the mesenchymal stem cell secretome for the treatment of cardiovascular disease. Cell stem cell. 2012;10:244–258. doi: 10.1016/j.stem.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ransohoff RM, Engelhardt B. The anatomical and cellular basis of immune surveillance in the central nervous system. Nat Rev Immunol. 2012;12:623–635. doi: 10.1038/nri3265. [DOI] [PubMed] [Google Scholar]
- Ratajczak J, Miekus K, Kucia M, Zhang J, Reca R, Dvorak P, Ratajczak MZ. Embryonic stem cell-derived microvesicles reprogram hematopoietic progenitors: Evidence for horizontal transfer of mRNA and protein delivery. Leukemia. 2006;20:847–856. doi: 10.1038/sj.leu.2404132. [DOI] [PubMed] [Google Scholar]
- Ratajczak MZ, Kucia M, Jadczyk T, Greco NJ, Wojakowski W, Tendera M, Ratajczak J. Pivotal role of paracrine effects in stem cell therapies in regenerative medicine: Can we translate stem cell-secreted paracrine factors and microvesicles into better therapeutic strategies? Leukemia. 2012;26:1166–1173. doi: 10.1038/leu.2011.389. [DOI] [PubMed] [Google Scholar]
- Ren G, Zhang L, Zhao X, Xu G, Zhang Y, Roberts AI, Zhao RC, Shi Y. Mesenchymal stem cell-mediated immunosuppression occurs via concerted action of chemokines and nitric oxide. Cell Stem Cell. 2008;2:141–150. doi: 10.1016/j.stem.2007.11.014. [DOI] [PubMed] [Google Scholar]
- Ren G, Zhao X, Zhang L, Zhang J, L’Huillier A, Ling W, Roberts AI, Le AD, Shi S, Shao C, Shi Y. Inflammatory cytokine-induced intercellular adhesion molecule-1 and vascular cell adhesion molecule-1 in mesenchymal stem cells are critical for immunosuppression. J Immunol. 2010;184:2321–2328. doi: 10.4049/jimmunol.0902023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricci-Vitiani L, Lombardi DG, Signore M, Biffoni M, Pallini R, Parati E, Peschle C, De Maria R. Human neural progenitor cells display limited cytotoxicity and increased oligodendrogenesis during inflammation. Cell Death Differ. 2007;14:876–878. doi: 10.1038/sj.cdd.4402078. [DOI] [PubMed] [Google Scholar]
- Rice CM, Scolding NJ. Adult human mesenchymal cells proliferate and migrate in response to chemokines expressed in demyelination. Cell Adh Migr. 2010;4:235–240. doi: 10.4161/cam.4.2.11404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley J, Federici T, Polak M, Kelly C, Glass J, Raore B, Taub J, Kesner V, Feldman EL, Boulis NM. Intraspinal stem cell transplantation in amyotrophic lateral sclerosis: A phase I safety trial, technical note, and lumbar safety outcomes. Neurosurgery. 2012;71:405–416. doi: 10.1227/NEU.0b013e31825ca05f. [DOI] [PubMed] [Google Scholar]
- Ringden O, Le Blanc K. Mesenchymal stem cells for treatment of acute and chronic graft-versus-host disease, tissue toxicity and hemorrhages. Best Pract Res Clin Haematol. 2011;24:65–72. doi: 10.1016/j.beha.2011.01.003. [DOI] [PubMed] [Google Scholar]
- Ringe J, Strassburg S, Neumann K, Endres M, Notter M, Burmester GR, Kaps C, Sittinger M. Towards in situ tissue repair: human mesenchymal stem cells express chemokine receptors CXCR1, CXCR2 and CCR2, and migrate upon stimulation with CXCL8 but not CCL2. J Cell Biochem. 2007;101:135–146. doi: 10.1002/jcb.21172. [DOI] [PubMed] [Google Scholar]
- Robin AM, Zhang ZG, Wang L, Zhang RL, Katakowski M, Zhang L, Wang Y, Zhang C, Chopp M. Stromal cell-derived factor 1alpha mediates neural progenitor cell motility after focal cerebral ischemia. J Cereb Blood Flow Metab. 2006;26:125–134. doi: 10.1038/sj.jcbfm.9600172. [DOI] [PubMed] [Google Scholar]
- Roddy GW, Oh JY, Lee RH, Bartosh TJ, Ylostalo J, Coble K, Rosa RH, Jr., Prockop DJ. Action at a distance: systemically administered adult stem/progenitor cells (MSCs) reduce inflammatory damage to the cornea without engraftment and primarily by secretion of TNF-alpha stimulated gene/protein 6. Stem Cells. 2011;29:1572–1579. doi: 10.1002/stem.708. [DOI] [PubMed] [Google Scholar]
- Rojewski MT, Weber BM, Schrezenmeier H. Phenotypic characterization of mesenchymal stem cells from various tissues. Transfus Med Hemother. 2008;35:168–184. doi: 10.1159/000129013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolls A, Shechter R, London A, Ziv Y, Ronen A, Levy R, Schwartz M. Toll-like receptors modulate adult hippocampal neurogenesis. Nat Cell Biol. 2007;9:1081–1088. doi: 10.1038/ncb1629. [DOI] [PubMed] [Google Scholar]
- Rossi F, Cattaneo E. Opinion: Neural stem cell therapy for neurological diseases: dreams and reality. Nat Rev Neurosci. 2002;3:401–409. doi: 10.1038/nrn809. [DOI] [PubMed] [Google Scholar]
- Ruster B, Gottig S, Ludwig RJ, Bistrian R, Muller S, Seifried E, Gille J, Henschler R. Mesenchymal stem cells display coordinated rolling and adhesion behavior on endothelial cells. Blood. 2006;108:3938–3944. doi: 10.1182/blood-2006-05-025098. [DOI] [PubMed] [Google Scholar]
- Schu S, Nosov M, O’Flynn L, Shaw G, Treacy O, Barry F, Murphy M, O’Brien T, Ritter T. Immunogenicity of allogeneic mesenchymal stem cells. J Cell Mol Med. 2012;16:2094–2103. doi: 10.1111/j.1582-4934.2011.01509.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng H, Wang Y, Jin Y, Zhang Q, Zhang Y, Wang L, Shen B, Yin S, Liu W, Cui L, Li N. A critical role of IFNgamma in priming MSC-mediated suppression of T cell proliferation through up-regulation of B7-H1. Cell Res. 2008;18:846–857. doi: 10.1038/cr.2008.80. [DOI] [PubMed] [Google Scholar]
- Shulman Z, Cohen SJ, Roediger B, Kalchenko V, Jain R, Grabovsky V, Klein E, Shinder V, Stoler-Barak L, Feigelson SW, Meshel T, Nurmi SM, Goldstein I, Hartley O, Gahmberg CG, Etzioni A, Weninger W, Ben-Baruch A, Alon R. Transendothelial migration of lymphocytes mediated by intraendothelial vesicle stores rather than by extracellular chemokine depots. Nat Immunol. 2012;13:67–76. doi: 10.1038/ni.2173. [DOI] [PubMed] [Google Scholar]
- Sioud M. New insights into mesenchymal stromal cell-mediated T-cell suppression through galectins. Scand J Immunol. 2011;73:79–84. doi: 10.1111/j.1365-3083.2010.02491.x. [DOI] [PubMed] [Google Scholar]
- Sixt M, Engelhardt B, Pausch F, Hallmann R, Wendler O, Sorokin LM. Endothelial cell laminin isoforms, laminins 8 and 10, play decisive roles in T cell recruitment across the blood-brain barrier in experimental autoimmune encephalomyelitis. J Cell Biol. 2001;153:933–946. doi: 10.1083/jcb.153.5.933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Son BR, Marquez-Curtis LA, Kucia M, Wysoczynski M, Turner AR, Ratajczak J, Ratajczak MZ, Janowska-Wieczorek A. Migration of bone marrow and cord blood mesenchymal stem cells in vitro is regulated by stromal-derived factor-1-CXCR4 and hepatocyte growth factor-c-met axes and involves matrix metalloproteinases. Stem Cells. 2006;24:1254–1264. doi: 10.1634/stemcells.2005-0271. [DOI] [PubMed] [Google Scholar]
- Spaggiari GM, Moretta L. Interactions between mesenchymal stem cells and dendritic cells. Adv Biochem Eng Biotechnol. 2012:1–10. doi: 10.1007/10_2012_154. [DOI] [PubMed] [Google Scholar]
- Steiner R, Huhn S, Koch T, Al-Uzri A, Guillaime D, Sutcliffe T, Vogel H, Selden N. CNS transplantation of purified human neural stem cells in infantile and late-infantile neuronal ceroid lipofuscinoses: Summary of the Phase I trial. Mol Genet Metab. 2010;99:S35. [Google Scholar]
- Sun C, Zhang H, Li J, Huang H, Cheng H, Wang Y, Li P, An Y. Modulation of the major histocompatibility complex by neural stem cell-derived neurotrophic factors used for regenerative therapy in a rat model of stroke. J Transl Med. 2010;8:77. doi: 10.1186/1479-5876-8-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabera S, Perez-Simon JA, Diez-Campelo M, Sanchez-Abarca LI, Blanco B, Lopez A, Benito A, Ocio E, Sanchez-Guijo FM, Canizo C, San Miguel JF. The effect of mesenchymal stem cells on the viability, proliferation and differentiation of B-lymphocytes. Haematologica. 2008;93:1301–1309. doi: 10.3324/haematol.12857. [DOI] [PubMed] [Google Scholar]
- Takeuchi H, Natsume A, Wakabayashi T, Aoshima C, Shimato S, Ito M, Ishii J, Maeda Y, Hara M, Kim SU, Yoshida J. Intravenously transplanted human neural stem cells migrate to the injured spinal cord in adult mice in an SDF-1- and HGF-dependent manner. Neurosci Lett. 2007;426:69–74. doi: 10.1016/j.neulet.2007.08.048. [DOI] [PubMed] [Google Scholar]
- Tasso R, Ilengo C, Quarto R, Cancedda R, Caspi RR, Pennesi G. Mesenchymal stem cells induce functionally active T-regulatory lymphocytes in a paracrine fashion and ameliorate experimental autoimmune uveitis. Invest Ophthalmol Vis Sci. 2012;53:786–793. doi: 10.1167/iovs.11-8211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng YD, Yu D, Ropper AE, Li J, Kabatas S, Wakeman DR, Wang J, Sullivan MP, Redmond DE, Jr, Langer R, Snyder EY, Sidman RL. Functional multipotency of stem cells: A conceptual review of neurotrophic factor-based evidence and its role in translational research. Curr Neuropharmacol. 2011;9:574–585. doi: 10.2174/157015911798376299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thery C. Exosomes: Secreted vesicles and intercellular communications. F1000 Biol Rep. 2011;3:15. doi: 10.3410/B3-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thery C, Ostrowski M, Segura E. Membrane vesicles as conveyors of immune responses. Nat Rev Immunol. 2009;9:581–593. doi: 10.1038/nri2567. [DOI] [PubMed] [Google Scholar]
- Thery C, Zitvogel L, Amigorena S. Exosomes: Composition, biogenesis and function. Nat Rev Immunol. 2002;2:569–579. doi: 10.1038/nri855. [DOI] [PubMed] [Google Scholar]
- Thirant C, Galan-Moya EM, Dubois LG, Pinte S, Chafey P, Broussard C, Varlet P, Devaux B, Soncin F, Gavard J, Junier MP, Chneiweiss H. Differential proteomic analysis of human glioblastoma and neural stem cells reveals HDGF as a novel angiogenic secreted factor. Stem Cells. 2012;30:845–853. doi: 10.1002/stem.1062. [DOI] [PubMed] [Google Scholar]
- Tomasoni S, Longaretti L, Rota C, Morigi M, Conti S, Gotti E, Capelli C, Introna M, Remuzzi G, Benigni A. Transfer of growth factor receptor mRNA via exosomes unravels the regenerative effect of mesenchymal stem cells. Stem Cells Dev. 2013;22:772–780. doi: 10.1089/scd.2012.0266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomchuck SL, Zwezdaryk KJ, Coffelt SB, Waterman RS, Danka ES, Scandurro AB. Toll-like receptors on human mesenchymal stem cells drive their migration and immunomodulating responses. Stem Cells. 2008;26:99–107. doi: 10.1634/stemcells.2007-0563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traggiai E, Volpi S, Schena F, Gattorno M, Ferlito F, Moretta L, Martini A. Bone marrow-derived mesenchymal stem cells induce both polyclonal expansion and differentiation of B cells isolated from healthy donors and systemic lupus erythematosus patients. Stem Cells. 2008;26:562–569. doi: 10.1634/stemcells.2007-0528. [DOI] [PubMed] [Google Scholar]
- Tran PB, Miller RJ. Chemokine receptors: signposts to brain development and disease. Nat Rev Neurosci. 2003;4:444–455. doi: 10.1038/nrn1116. [DOI] [PubMed] [Google Scholar]
- Tscherning T, Claesson MH. Veto suppression: the peripheral way of T cell tolerization. Exp Clin Immunogenet. 1993;10:179–188. [PubMed] [Google Scholar]
- Tse WT, Pendleton JD, Beyer WM, Egalka MC, Guinan EC. Suppression of allogeneic T-cell proliferation by human marrow stromal cells: implications in transplantation. Transplantation. 2003;75:389–397. doi: 10.1097/01.TP.0000045055.63901.A9. [DOI] [PubMed] [Google Scholar]
- Turbic A, Leong SY, Turnley AM. Chemokines and inflammatory mediators interact to regulate adult murine neural precursor cell proliferation, survival and differentiation. PLoS One. 2011;6:e25406. doi: 10.1371/journal.pone.0025406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ubiali F, Nava S, Nessi V, Frigerio S, Parati E, Bernasconi P, Mantegazza R, Baggi F. Allorecognition of human neural stem cells by peripheral blood lymphocytes despite low expression of MHC molecules: Role of TGF-beta in modulating proliferation. Int Immunol. 2007;19:1063–1074. doi: 10.1093/intimm/dxm079. [DOI] [PubMed] [Google Scholar]
- Uccelli A, Benvenuto F, Laroni A, Giunti D. Neuroprotective features of mesenchymal stem cells. Best Pract Res Clin Haematol. 2011a;24:59–64. doi: 10.1016/j.beha.2011.01.004. [DOI] [PubMed] [Google Scholar]
- Uccelli A, Laroni A, Freedman MS. Mesenchymal stem cells for the treatment of multiple sclerosis and other neurological diseases. Lancet Neurol. 2011b;10:649–656. doi: 10.1016/S1474-4422(11)70121-1. [DOI] [PubMed] [Google Scholar]
- Uccelli A, Moretta L, Pistoia V. Mesenchymal stem cells in health and disease. Nat Rev Immunol. 2008;8:726–736. doi: 10.1038/nri2395. [DOI] [PubMed] [Google Scholar]
- Uccelli A, Pistoia V, Moretta L. Mesenchymal stem cells: A new strategy for immunosuppression? Trends Immunol. 2007;28:219–226. doi: 10.1016/j.it.2007.03.001. [DOI] [PubMed] [Google Scholar]
- Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9:654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- van der Meulen AA, Biber K, Lukovac S, Balasubramaniyan V, den Dunnen WF, Boddeke HW, Mooij JJ. The role of CXC chemokine ligand (CXCL)12-CXC chemokine receptor (CXCR)4 signalling in the migration of neural stem cells towards a brain tumour. Neuropathol Appl Neurobiol. 2009;35:579–591. doi: 10.1111/j.1365-2990.2009.01036.x. [DOI] [PubMed] [Google Scholar]
- Wang L, Shi J, van Ginkel FW, Lan L, Niemeyer G, Martin DR, Snyder EY, Cox NR. Neural stem/progenitor cells modulate immune responses by suppressing T lymphocytes with nitric oxide and prostaglandin E2. Exp Neurol. 2009;216:177–183. doi: 10.1016/j.expneurol.2008.11.017. [DOI] [PubMed] [Google Scholar]
- Wang Y, Deng Y, Zhou GQ. SDF-1alpha/CXCR4-mediated migration of systemically transplanted bone marrow stromal cells towards ischemic brain lesion in a rat model. Brain Res. 2008;1195:104–112. doi: 10.1016/j.brainres.2007.11.068. [DOI] [PubMed] [Google Scholar]
- Waterman RS, Tomchuck SL, Henkle SL, Betancourt AM. A new mesenchymal stem cell (MSC) paradigm: polarization into a pro-inflammatory MSC1 or an Immunosuppressive MSC2 phenotype. PLoS One. 2010;5:e10088. doi: 10.1371/journal.pone.0010088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinger JG, Weist BM, Plaisted WC, Klaus SM, Walsh CM, Lane TE. MHC mismatch results in neural progenitor cell rejection following spinal cord transplantation in a model of viral-induced demyelination. Stem Cells. 2012;30:2584–2595. doi: 10.1002/stem.1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss N, Deboux C, Chaverot N, Miller F, Baron-Van Evercooren A, Couraud PO, Cazaubon S. IL8 and CXCL13 are potent chemokines for the recruitment of human neural precursor cells across brain endothelial cells. J Neuroimmunol. 2010;223:131–134. doi: 10.1016/j.jneuroim.2010.03.009. [DOI] [PubMed] [Google Scholar]
- Xin H, Li Y, Buller B, Katakowski M, Zhang Y, Wang X, Shang X, Zhang ZG, Chopp M. Exosome-mediated transfer of miR-133b from multipotent mesenchymal stromal cells to neural cells contributes to neurite outgrowth. Stem Cells. 2012;30:1556–1564. doi: 10.1002/stem.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaddanapudi K, De Miranda J, Hornig M, Lipkin WI. Toll-like receptor 3 regulates neural stem cell proliferation by modulating the Sonic Hedgehog pathway. PloS One. 2011;6:e26766. doi: 10.1371/journal.pone.0026766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamane J, Ishibashi S, Sakaguchi M, Kuroiwa T, Kanemura Y, Nakamura M, Miyoshi H, Sawamoto K, Toyama Y, Mizusawa H, Okano H. Transplantation of human neural stem/progenitor cells overexpressing galectin-1 improves functional recovery from focal brain ischemia in the Mongolian gerbil. Mol Brain. 2011;4:35. doi: 10.1186/1756-6606-4-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamane J, Nakamura M, Iwanami A, Sakaguchi M, Katoh H, Yamada M, Momoshima S, Miyao S, Ishii K, Tamaoki N, Nomura T, Okano HJ, Kanemura Y, Toyama Y, Okano H. Transplantation of galectin-1-expressing human neural stem cells into the injured spinal cord of adult common marmosets. J Neurosci Res. 2010;88:1394–1405. doi: 10.1002/jnr.22322. [DOI] [PubMed] [Google Scholar]
- Yuan SH, Martin J, Elia J, Flippin J, Paramban RI, Hefferan MP, Vidal JG, Mu Y, Killian RL, Israel MA, Emre N, Marsala S, Marsala M, Gage FH, Goldstein LS, Carson CT. Cell-surface marker signatures for the isolation of neural stem cells, glia and neurons derived from human pluripotent stem cells. PLoS One. 2011;6:e17540. doi: 10.1371/journal.pone.0017540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zappia E, Casazza S, Pedemonte E, Benvenuto F, Bonanni I, Gerdoni E, Giunti D, Ceravolo A, Cazzanti F, Frassoni F, Mancardi G, Uccelli A. Mesenchymal stem cells ameliorate experimental autoimmune encephalomyelitis inducing T-cell anergy. Blood. 2005;106:1755–1761. doi: 10.1182/blood-2005-04-1496. [DOI] [PubMed] [Google Scholar]
- Zhang J, Li Y, Chen J, Cui Y, Lu M, Elias SB, Mitchell JB, Hammill L, Vanguri P, Chopp M. Human bone marrow stromal cell treatment improves neurological functional recovery in EAE mice. Exp Neurol. 2005;195:16–26. doi: 10.1016/j.expneurol.2005.03.018. [DOI] [PubMed] [Google Scholar]
- Zhang W, Ge W, Li C, You S, Liao L, Han Q, Deng W, Zhao RC. Effects of mesenchymal stem cells on differentiation, maturation, and function of human monocyte-derived dendritic cells. Stem Cells Dev. 2004;13:263–271. doi: 10.1089/154732804323099190. [DOI] [PubMed] [Google Scholar]
- Zhu H, Mitsuhashi N, Klein A, Barsky LW, Weinberg K, Barr ML, Demetriou A, Wu GD. The role of the hyaluronan receptor CD44 in mesenchymal stem cell migration in the extracellular matrix. Stem Cells. 2006;24:928–935. doi: 10.1634/stemcells.2005-0186. [DOI] [PubMed] [Google Scholar]
- Zhu J, Zhou Z, Liu Y, Zheng J. Fractalkine and CX3CR1 are involved in the migration of intravenously grafted human bone marrow stromal cells toward ischemic brain lesion in rats. Brain Res. 2009;1287:173–183. doi: 10.1016/j.brainres.2009.06.068. [DOI] [PubMed] [Google Scholar]