Abstract
Reversing the spread of antibiotic multiresistant bacteria is hampered by ignorance of the natural history of resistance genes, the mobile elements carrying them, and the bacterial hosts harboring them. Using traditional cultivation and cultivation-independent molecular techniques, we quantified antibiotic resistance genes and mobile elements called integrons in poultry house litter from commercial poultry farms. Unexpectedly, the major reservoir for Class 1 integrons in poultry litter is not their previously identified hosts, Gram-negative Enterobacteriaceae such as Escherichia coli. Rather, integrons and associated resistance genes abound in several genera of Gram-positive bacteria that constitute >85% of the litter community compared with Enterobacteriaceae that comprise <2% of this ecosystem. This finding warrants reexamination of our assumptions about the persistence and spread of antibiotic resistance genes.
Antibacterial use in livestock for disease treatment and to increase feed efficiency began in the 1940s. By the 1960s, concern with the increasing prevalence of antibiotic-resistant pathogenic bacteria in hospitals led to the interdiction for agricultural use of antibiotics also used in human medicine in the hope that resistance epidemics in hospital pathogens could thereby be quelled (1). Over the last 30 years, we have learned that this hope was unrealistic, because we share pathogenic and benign bacteria with other humans and animals and because bacteria readily transfer genes among themselves. Thus, once multiresistant bacteria proliferate in a clinical or agricultural ecosytem, they can spread to other ecosystems (2, 3).
In principle, the inter-ecosystem spread of resistance will depend on the concentrations of resistance genes and their host bacteria in an ecosystem and on the rate of their exchange between ecosystems. However, it is rare, apart from water quality testing, to determine the concentration of bacteria, much less of specific genes, in any environmental or medical specimen. Thus, we do not know the actual size of the resistance gene pool, their locations in the environment, or how rapidly they move among hosts and ecosystems. Lack of this information limits design of effective measures for control of resistant bacteria.
In commercial poultry meat (“broiler”) production, antibiotics can be administered to flocks of up to 20,000 birds occupying a single building (“house”) over the 6-wk growing period from day-old chick to adult bird (Table 1 and refs. 4–7). The floors of chicken houses are covered with a bedding material of softwood shavings that, during maturation of each flock, becomes mixed with chicken feces, urine, skin, feathers, insects, and small invertebrates. The resulting mixture, called poultry litter, is often replaced with fresh wood shavings between flocks and constitutes a significant component of waste from commercial poultry production (8). Litter has a unique largely aerobic microbiota, reflecting its various inocula and substrates (9). Rich in minerals, it can be recycled for fertilizer, among other uses (8).
Table 1. Antimicrobials used on broiler poultry farms.
| Purpose | Farm 1 | Farm 2 | Farms 3 and 4 | |
|---|---|---|---|---|
| Prophylactic | Gentamicin, 0.1 mg/egg | Gentamicin, 0.2 mg/chick, s.c. | Gentamicin, 0.2 mg/egg | |
| Starter feed (weeks 1 and 2) | Antibiotic | Bacitracin virginiamycin | Bacitracin | None |
| Coccidiostat | Diclazuril/semduramycin | Roxarsone/salinomycin | Salinomycin | |
| Grower feed (weeks 3 and 4) | Antibiotic | Bacitracin | None | None |
| Coccidiostat | Salinomycin | Roxarsone/salinomycin | Salinomycin | |
| Finisher feed (weeks 5 and 6) | Antibiotic | None | None | None |
| Coccidiostat | None | None | None | |
| Withdrawal feed (week 7) | Antibiotic | Virginiamycin | None | None |
Semduramycin and salinomycin are ionophores, and roxarsone is an arsenical.
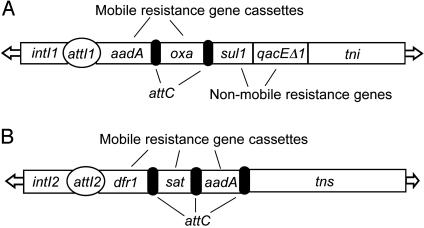
Because Gram-negative bacteria of the family Enterobacteriaceae are common agents of food-borne diseases, great attention is focused on antibiotic resistance genes in this ubiquitous but numerically minor group of intestinal bacteria. The major agents of gene transfer in the Enterobacteriaceae include the conjugative plasmids in which antibiotic resistance genes typically occur within distinct genetic elements called transposons, which themselves can randomly recombine to other plasmids or the chromosome of the same cell (10). Plasmids also have genes that make it difficult to eliminate them from their host bacteria once acquired (11). Among Enterobacteriaceae, the greatest variety of plasmid-borne resistance genes is associated with integrons (12), transposable genetic loci that assemble tandem arrays of distinct antibiotic resistance genes (Fig. 1). The signature gene of an integron is the integrase (intI), a site-specific recombinase (13, 14) that inserts and removes small DNA cassettes, each encoding an antibiotic resistance gene at a site called attI. Cassettes also have a loosely conserved region called attC that interacts with the integrase during insertion and excision. The cassettes lack a promoter but, when inserted into attI1, the encoded gene is expressed from a promoter located in the adjacent intI1 gene. Over 50 distinct resistance gene cassettes have been described, and up to seven have been observed in a single integron in natural isolates (15).
Fig. 1.
Integrons common in poultry litter. (A) Typical Tn21-like Class 1 Integron (28). Nonmobile resistance genes lacking attC are sul1, sulfonamide resistance; and qacEΔ1, quaternary ammonium compound resistance. Example mobile resistance genes include aadA, aminoglycoside adenyltransferase; and oxa, β-lactamase. Other genes are attI1, specific insertion site; intI1, integrase; tni, integron transposition operon; and arrows, inverted repeats involved in transposition (B) Class 2 Integron, Tn7 (30, 40). Mobile resistance genes are dfr1, trimethoprim; sat, streptothricin; aadA, aminoglycoside adenyltransferase. Other genes are attI2, specific insertion site; intI2, integrase; tns, Tn7 transposition operon; arrows, inverted repeats involved in transposition (not to scale).
Of the nine described integron classes, Classes 1 (Tn21-like; Fig. 1 A) and 2 (Tn7-like; Fig. 1B) are most frequently found in bacteria that colonize food animals (16–18), in domesticated and wild birds (17), and in human clinical specimens (19, 20). Although studied exclusively in the Enterobacteriaceae, Class 1 integrons have been seen fortuitously on three occasions in single strains of Gram-positive bacteria (21–23). Less is known of the epidemiology of Class 2 Tn7-like integrons, although they have been found in human (24) and veterinary (17) enterobacterial isolates. We used a conventional measure of microbial prevalence, viable cell counts, and cultivation-independent qPCR of integron-related target genes to characterize litter microbiota on two independently owned and managed geographically separated commercial broiler farms in northeast Georgia.
Materials and Methods
Specimen Collection. Litter specimens were taken every 2 weeks from two sequential flocks in a single house at each farm over a 13-week period during the summer of 2001. The house sampled on Farm 1 (34 specimens) held 17,000 birds, and that on Farm 2 (40 specimens) held 20,000 birds. To prevent Escherichia coli peritonitis, gentamicin was administered either in ovo (Farm 1) or by injection of the chicks (Farm 2) just before placement in the broiler house (Table 1). Other antibiotics, antibacterials, and coccidiostats were used to prevent necrotic enteritis (Table 1 and ref. 4). Therapeutic use of antibiotics was not required on either farm. Old litter was replaced between the first and second flocks on each farm. Poultry litter samples were collected from each house at chick placement (week 0), and at ≈2 weeks, 4 weeks, and 6 weeks after placement. Litter samples were grabbed by gloved hand from under the nipple drinker and water lines and along the length of the flock house, pooled in a sterile plastic bag, and thoroughly mixed. Five such grabbed mixed samples were collected from each house at each sampling time.
DNA Purification and Quantification. Five grams of each thoroughly mixed pooled litter sample was suspended in 45 ml of PBS in a sterile 50-ml Falcon tube and shaken with a Burrell Scientific Wrist Action Shaker (Model 75, Burrell, Pittsburgh) at maximum setting for 5 min at room temperature. Debris was removed by centrifugation at 50 × g for 15 min at 24°C. The bacterial cells were pelleted from the resulting supernatant by centrifugation at 3,650 × g for 15 min at 24°C and stored at -70°C in 50% glycerol. The weight of each pellet of litter-washate cells was determined before processing for DNA recovery.
Total DNA was isolated from the litter washate cells by a bead-beater-phenol method. Typically, ≈150 mg of litter-derived cells was suspended in 500 μl of STE buffer (10 mM Tris·HCl/1 mM EDTA/1% SDS) in a 2.0-ml screw-cap microcentrifuge tube to which was added 500 μl of Tris-buffered phenol (Roche Molecular Biochemicals) and 300 μl of acid-washed 0.1-mm glass beads (Biospec Products, Bartlesville, OK). Cell were lysed in a Bead Beater (Biospec Products) for 1 min at 6,000 rpm at 24°C and centrifuged for 10 min at 10,000 × g at 24°C. The aqueous phase was transferred to a clean tube. This procedure was repeated with 500 μl of STE buffer, and this second aqueous phase was mixed with the first. Samples >150 mg received a second phenol extraction with 400 μl of STE buffer, 200 μl of phenol, and 100 μl of glass beads followed by washing with 300 μl of STE buffer. All aqueous phases were combined, split into tubes with a maximum volume of 900 μl each, and extracted with equal volumes of phenol/chloroform (25:24) and then with an equal volume of chloroform. The final aqueous phase was made 0.3 M in sodium acetate, and DNA was precipitated by adding 2 volumes of cold 100% ethanol and centrifuging at 12,000 × g at 4°C. The pellet was washed with cold 70% ethanol, air dried, and dissolved in TE buffer (10 mM Tris·HCl/1 mM EDTA, pH 7.5). DNA was treated with heat-treated RNase for 1 h at 37°C, passed through a spin column (Sephadex G-25, Sigma), and dialyzed in a 10,000-Da cut-off Slide-A-Lyzer Mini Dialysis Unit (Pierce) against autoclaved TE buffer at 4°C for two 1-hr changes of 1 liter each. DNA concentration was measured at OD260 in a Cary 100 Biospectrophotometer (Varian) by using an ε260 = 62.90 calibrated with λ DNA (Promega). When these methods were applied to pure E. coli standard strains, DNA recovery was ≈99% of theoretical maximum based on microscopic counts of formalinized cells. DNA recovery from litter specimens was ≈13% of theoretical maximum, assuming all material washed from litter consisted of prokaryotic cells, each with an E. coli-size genome (10 fg) (25). This recovery is consistent with that reported for soil DNA isolation (26, 27).
Gene Targets and Primers. Gene targets quantified by PCR included the eubacterial 16S rDNA, three genes strongly associated with Class 1 integrons (Fig. 1 A; the integrase gene, intI1, and the nonmobile cassettes conferring resistance to sulfadiazine, sulI, and to quaternary ammonium compounds, qacEΔ1; refs. 28 and 29); two genes uniquely associated with Class 2 integrons (Fig. 1B; the integrase gene, intI2, and the Tn7 transposase subunit, tnsB; ref. 30); and a mobile gene cassette conferring streptomycin resistance, aadA1, often found in Class 1 and 2 integrons. With three exceptions, the oligonucleotide primers have been described (Table 3, which is published as supporting information on the PNAS web site) (16, 17, 31). We designed sul1, aadA, and tnsB primer sets with oligo primer analysis software, Ver. 6.7 (National Biosciences, Plymouth, MN), and all primers were synthesized by Sigma–Genosys. Cassette arrays inserted at the attI1 site can be amplified by using primers based on the so-called 5′ conserved sequence (5′CS, within intI1) and the 3′ conserved sequence (3′CS, within sul1) (32). Standard E. coli strains carrying each target gene demonstrated detection limits for 16S rDNA (100 copies) and the other targets (1–10 copies; Table 2), consistent with other recent work (31, 33).
Table 2. intl1-positive aerobic Gram-positive bacteria from poultry litter.
| Isolate no. | 16S rDNA identity | Insert identity |
|---|---|---|
| Farm 1 | ||
| 693—7* | Staphylococcus sp. | ND |
| 693—8* | Staphylococcus lentus | ND |
| 693—9* | Staphylococcus lentus | ND |
| 693—14 | Staphylococcus nepalensis | ND |
| 693—17 | Aerococcus sp. | rRNA methylase† |
| 304—1 | Corynebacterium ammoniagenes | ND |
| 693—2* | C. ammoniagenes | ND |
| 693—10* | Cornyebacterium casei | Lipid-A-synthase† |
| 878—3 | Cornyebacterium glutamicum | ND |
| 878—10 | Brevibacterium thiogenitalis | ND |
| Farm 2 | ||
| 555—4* | Staphylococcus xylosus | Transketolase† |
| 619—11 | Aerococcus sp. | ND |
| 776—5 | Staphylococcus sp. | ND |
| 384—1 | C. ammoniagenes | dfrA1, aadA1 |
| 384—11 | C. ammoniagenes | aadA9 |
| 555—9‡ | C. ammoniagenes | ND |
| 619—6 | C. ammoniagenes | aadA1 |
| 619—12 | C. ammoniagenes | dfrA1, aadA1 |
| Farms 3 and 4 | ||
| L1—76-17 | C. casei | dfrA1, aadA1 |
| L2—07-09 | C. ammoniagenes | aadA2 |
| L2—64-11 | C. casei | aadA1 |
| L2—79-05 | C. ammoniagenes | aadA9 |
| L2—79-15 | C. ammoniagenes | aadA1 |
ND, none detected. Produced no insert amplicon.
Checked for larger intl1 amplicon, 740 bp, 100% identical to Tn21 intl1
Gene fragment; most similar GenBank loci (blast-x): 555—4, gi:15675539; 693—10; gi:15793098; and 693—17, gi:27315075
Missense in intl1 sequence: G127R
qPCR. Reactions were prepared in a UV sterilized laminar flow PCR hood and performed with Syber Green PCR Core Kit (PE Applied Biosystems) by using 1 ng of litter DNA per 50-μl reaction in 96-well thin-wall PCR plates in an iCycler using icycle iq software, Ver. 2.3 (Bio-Rad). Cycling conditions were optimized by gradient PCR in the same instrument. Size and purity of qPCR products from field specimens were assessed by melting curve analysis and in many cases also by conventional agarose gel electrophoresis. We found excellent agreement between these methods.
Computing Target Concentration per Gram of Litter. Using the observed total yield of DNA from each litter washate cell pellet and the total mass of the litter washate pellet, we converted the number of targets per milligram of DNA recovered (determined by qPCR) to the total number of targets per gram of original litter. The concentration of eubacterial targets is based on E. coli as a standard, without compensating for the fact that E. coli has seven copies of rDNA per cell, because this number lies in the middle of the range observed for eubacteria (1–15 copies; ref. 34). Thus, in our work the term “eubacterial genomes” is actually “E. coli-equivalent eubacterial genomes.” Interspecimen variation on any given day averaged 3–6% for high-concentration targets and for low-concentration targets never exceeded 2-fold (Table 4, which is published as supporting information on the PNAS web site), indicating a robust sampling protocol and reproducible analytical methods.
Total Aerobic Colony-Forming Units (cfu). Serial dilutions of each of five daily litter sample washates (see above) were made in PBS and plated on Brain–Heart Infusion agar (BHI, Difco) and on MacConkey agar (Mac, Difco) to obtain total bacterial cfu and Gram-negative bacterial cfu, respectively. This time- and labor-intensive process was limited to the first flock on each farm.
Isolation and Characterization of Gram-Positive Bacterial Strains from Litter. Cryogenically archived cell pellets from litter were plated on BHI agar and incubated at room temperature for 36–40 h. Isolated colonies were restreaked both on Phenethyl–Alcohol–Blood (PEAB) agar that inhibits Gram-negative bacteria and on Mac agar that inhibits Gram-positive bacteria until they showed growth only on PEAB but not on Mac agar. They were then streaked on BHI and used for colony PCR for 16S rDNA, intI1, and attI1 inserts. Resulting amplicons were sequenced by Lark Sequencing Technologies (Houston) and Macrogen (Seoul, South Korea).
Results and Discussion
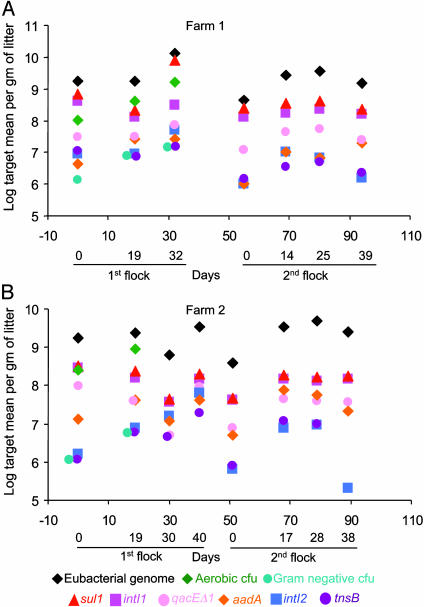
All targets increased ≈10-fold during each flock (Fig. 2) and decreased between flocks when the litter was replaced with fresh wood shavings. Total eubacterial genome targets ranged from 108 to 1010 per gram of litter (Fig. 2, black diamonds), ≈10-fold lower than the cecal digesta of chickens, which is largely anaerobic (35). The concentration of total aerobic bacteria (in cfu) in litter (Fig. 2, green diamonds) averaged ≈5-fold lower than that of the eubacterial target (Fig. 2, black diamonds). Because a eubacterial 16S rDNA library prepared from litter of these and other northeast Georgia broiler farms shows that only ≈4% of the population are anaerobes (9), this discrepancy may reflect impaired cultivation efficiency as seen in bacteria directly taken from other environments (36).
Fig. 2.
Concentrations of gene targets and cultivatable bacteria in poultry litter. Data points are means of five daily litter grab samples as described in Materials and Methods (percent SD, Table 3). Targets are: eubacterial genomes (black diamonds); total aerobic cfu (green diamonds); Gram-negative cfu (blue-green circles); integrase intI1 (magenta squares); sulfadiazine resistance, sul1 (red triangles); disinfectant resistance, qacEΔ1 (pink circles); streptomycin resistance, aadA (orange diamonds); integrase intI2 (blue squares); and transposase subunit, tnsB (purple circles). Some symbols are offset horizontally for visibility. There were only three sampling times for the first flock on Farm 1, because birds were sent to processor earlier than anticipated.
Unexpectedly, we found on both farms that the concentration of intI1 (Fig. 2, magenta squares) ranged from 50- to 500-fold greater than the concentration of cultivatable aerobic Gramnegative bacteria (Fig. 2, blue-green circles), the assumed major hosts for Class 1 integrons. The concentration of the other Class 1 hallmark gene, sul11 (Fig. 2, red triangles), was generally superimposable on the intI1 concentration on both farms (Fig. 2), consistent with these genes being physically linked (28). In contrast, another common Class 1 integron gene, qacEΔ1 (Fig. 2, pink circles), occurred at ≈7% of all eubacterial genomes on both farms, but well above the concentration of culturable Gram-negative bacteria (Fig. 2, blue-green circles). QacEΔ1 is routinely found in Gram-negative and -positive bacteria (37, 38), but its association with a Class 1 integron has been demonstrated only in two Gram-positive strains (21, 22).
The concentration of the Class 2 integrase, intI2 (Fig. 2, blue squares), ranges from 10- to 100-fold lower than that of the eubacterial target (Fig. 2, black diamonds) and of intI1 (magenta squares) on both farms. Because all of Tn7's resistance gene cassettes are mobile, the only markers, apart from intI2, uniquely associated with Tn7 are its own transposition genes, tnsABCDE. The concentration of tnsB (Fig. 2, purple circles) was largely superimposable on that of intI2 (Fig. 2, blue squares), consistent with these two targets being physically linked as expected. Moreover, both Tn7-associated targets had concentrations similar to that of culturable aerobic Gram-negative bacteria (blue-green circles), consistent with Tn7 residing in its expected host group in this ecosystem. Because Tn7 usually has a single insertion in a chromosome or plasmid (30), we can conclude that the lower limit of detection we observed for any single copy gene in the litter ecosystem was that for intI2 at approximately one per 10,000 eubacterial genomes (Fig. 2, Farm 2, second flock, 38 days).
The streptomycin-resistance gene aadA is on a mobile cassette (15, 29) and occurs in both Tn21 (Fig. 1 A) (28, 39) and Tn7 (Fig. 1B) (40). Here, the concentration of aadA1 (Fig. 2, orange diamond) was ≈4% or less of the eubacterial genome concentration on both farms, slightly above the concentrations of intI2 and tnsB and much lower than those of intI1 and sul1. That neither aadA1 nor qacEΔ1 is as abundant as the intI1 and sul1 targets(s) in this ecosystem suggests that the latter reside in linkage groups not entirely identical to Tn21.
We asked whether the unexpectedly high concentrations of intI1 and sul1 were due to there being multiple copies of these genes in aerobic Gram-negative bacteria. This explanation was ruled out by finding that 10 Gram-negative isolates from five distinct sampling times on both farms had only single copies of each of these genes (data not shown). Further, the possibility that cultivation had vastly underestimated the aerobic Gram-negative bacteria in litter was ruled out by a 16S rDNA library survey (9) that showed that γ-proteobacteria, which includes Enterobacteriaceae, comprise < 2% of the litter community; none of the 340 clones examined in that study were Enterobacteriaceae, suggesting that they are a very minor component of the γ-proteobacteria population in litter, just as they are in the bowel (41). In addition, litter concentration of the Q gene of the lysogenic coliphage, λ, in a random subset of samples from both farms differed by no more than 2-fold from that of tnsB, intI2, and MacConkey cfu (data not shown).
To test the remaining possibility, that Class 1 integrons reside in the aerobic Gram-positive bacteria that comprise ≈87% of litter eubacterial microbiota (9), we cultivated cryopreserved litter cells from several time points aerobically, purified single colonies from the highest dilutions, and subjected them to colony PCR. To test the generality of these findings, we did the same with litter cell samples collected in an unrelated project from two other, geographically distant, farms owned and operated by a third company (Farms 3 and 4; Table 1). Among 225 randomly selected aerobic Gram-positive clones from all four farms, 10–25% carried intI1, depending on the farm (data not shown). Twenty-three intI1-positive Gram-positive isolates were randomly chosen for further genotyping and proved to be aerococci, brevibacteria, staphylococcci, and corynebacteria (Table 4). With a single exception, all intI1 amplicons were identical to intI1 of Tn21 (≈260 bases considered). In a subset of six isolates, a larger overlapping 542-bp intI1 amplicon was also examined (for a total of 740 bp considered, including the entire catalytic motif), and all six were identical to Tn21 intI1. Fewer inserted cassettes were detected in Farm 1 isolates than in those from the other farms. Absence of an attI1 insert amplicon can arise because there is no cassette present [yields a ≈180-bp amplicon (42)], because a primer target has been deleted, or because so many cassettes are inserted at attI that the target fails to amplify under standard conditions. AadA was more often detected in Farm 2 isolates, although the concentration of this target did not differ much between the first two farms (Fig. 2, orange diamonds). AttI inserts from three genera on two farms were gene fragments, as also recently noted in a large survey of soil communities (ref. 43; Table 4). We do not have qPCR data for Farms 3 and 4, but aadA occurred in all isolates from these farms, indicating that Class 1 integron carriage by abundant aerobic Gram-positive bacteria in poultry litter is not unique to Farms 1 and 2.
Although antibiotics were used prophylactically on all farms and as a growth promotant only on Farms 1 and 2 (Table 1; Farms 3 and 4 used only coccidiostat growth promotants), resistance gene cassettes were similarly abundant in litter from Farms 1 and 2 and were present in numerically dominant isolates from all farms. Thus, a high prevalence of integron-related genes is not limited to farms using antibiotic growth promotants. Persistence of a specific resistance gene also occurs in human populations after decades without exposure to that antibiotic (44, 45). The likely basis for this persistence is physical linkage of genes for resistance to an older antibiotic with genes for resistance to a currently used antibiotic (46).
That our findings apply not only to these four farms in northeast Georgia is suggested by earlier reports of Class 1 integrase genes in two isolates of high GC Gram-positive bacteria from the Czech Republic (21) and France (23) and one low GC Gram-positive isolate from Minnesota (22). Thus, our observations explicitly expand the environmental “host space” for integron-based resistance genes by several orders of magnitude. We also document the previously undescribed occurrence in staphylococci, aerococci, and brevibacteria of intI1. In the latter two genera, production of 5′CS-3′CS amplicons shows that intI1 is linked to sul1I in the hallmark arrangement of Class 1 integrons. Staphylococci and corynebacteria are common skin commensals of humans (47), and the former readily become antibiotic resistant during treatment with oral or parenteral antibiotics (48). A similar phenomenon may occur in antibiotic-treated animals.
Our observations raise the question of whether large unrecognized reservoirs of integrons exist in the environmental or commensal microbiota of other intensively farmed animals and of humans. Qualitative sampling of soil communities for sequences linked to attC homologs revealed equal numbers of genes with possible origins in Gram-positive or -negative bacteria (43). Thus, as suggested earlier (49), attC-dependent integrases may engage widely in genome sculpting. As to mechanisms and frequencies of exchange of integron gene arrays between these Gram-positive bacteria and the Enterobacteriaceae, there is no information. However, the 100% identity of their intI1 genes to that of Tn21 suggests that intergenus exchanges take place currently, not just on historical or evolutionary time scales, and could contribute to real-time spread of integron-based resistances to medically significant bacteria. Although both are found on conjugative plasmids, Tn7 here manifests a more parochial distribution than the cosmopolitan Tn21-like integrons. Discovering what limits Tn7's wandering will be as important as discovering what fosters Tn21's promiscuity.
If antibiotic resistance were limited to spontaneous mutations in a few thousand bacteria among the hundreds of billions in one treated host, it would not be the epidemic problem it is today. The principle that has been slow to impact the practice of antibiotic use is that formerly assumed barriers to intergenus exchange are far from absolute. Our work underscores this point with respect to both the phylogenetic extent of dissemination and the actual physical prevalence of these agents in the environment. We thereby demonstrate the value of direct cultivation-independent quantification of target genes as a tool for the discovery of unexpected relationships in complex microbial ecosystems.
Supplementary Material
Acknowledgments
We thank Chris Oxendine for statistical analyses, Marie Maier and Julie Shearer for sequencing assistance, Valerie Hilliard for bacteriophage quantification, Laura Williams for critiquing the manuscript, and the three anonymous poultry-producing companies for the use of their facilities in this study. The work was supported by Grant 99-35212-8680 from the National Research Initiative of the U.S. Department of Agriculture (to J.M. and A.O.S.).
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: qPCR, quantitative PCR; cfu, colony-forming units.
References
- 1.Witte, W. (2000) Int. J. Antimicrob. Agents 16, S19-S24. [DOI] [PubMed] [Google Scholar]
- 2.O'Brien, T. F. (2002) Clin. Infect. Dis. 34, S78-S84.11988877 [Google Scholar]
- 3.Smith, D. L., Harris, A. D., Johnson, J. A., Silbergeld, E. K. & Morris, J. G., Jr. (2002) Proc. Natl. Acad. Sci. USA 99, 6434-6439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McEwen, S. A. & Fedorka-Cray, P. J. (2002) Clin. Infect. Dis. 34, S93-S106. [DOI] [PubMed] [Google Scholar]
- 5.Marshall, B., Petrowski, D. & Levy, S. B. (1990) Proc. Natl. Acad. Sci. USA 87, 6609-6613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hofacre, C. L., de Cotret, A. R., Maurer, J. J., Garritty, A. & Thayer, S. G. (2000) Avian Dis. 44, 963-967. [PubMed] [Google Scholar]
- 7.McDermott, P. F., Zhao, S., Wagner, D. D., Simjee, S., Walker, R. D. & White, D. G. (2002) Anim. Biotechnol. 13, 71-84. [DOI] [PubMed] [Google Scholar]
- 8.Williams, C. M., Barker, J. C. & Sims, J. T. (1999) Rev. Environ. Contam. Toxicol. 162, 105-157. [DOI] [PubMed] [Google Scholar]
- 9.Lu, J., Sanchez, S., Hofacre, C., Maurer, J. J., Harmon, B. G. & Lee, M. D. (2003) Appl. Environ. Microbiol. 69, 901-908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Toussaint, A. & Merlin, C. (2002) Plasmid 47, 26-35. [DOI] [PubMed] [Google Scholar]
- 11.Heinemann, J. A., Ankenbauer, R. G. & Amabile-Cuevas, C. F. (2000) Drug Discov. Today 5, 195-204. [DOI] [PubMed] [Google Scholar]
- 12.Leverstein-van Hall, M.A., Blok, H. E. M., Donders, A. R. T., Paauw, A., Fluit, A. C. & Verhoef, J. (2003) J. Infect. Dis. 187, 251-259. [DOI] [PubMed] [Google Scholar]
- 13.Sundstrom, L. (1998) APMIS Suppl. 84, 37-42. [DOI] [PubMed] [Google Scholar]
- 14.Rowe-Magnus, A. D., Davies, J. & Mazel, D. (2002) Curr. Top. Microbiol. Immunol. 264, 167-188. [PubMed] [Google Scholar]
- 15.Recchia, G. D. & Hall, R. M. (1995) Microbiology 141, 3015-3027. [DOI] [PubMed] [Google Scholar]
- 16.Bass, L., Liebert, C. A., Lee, M. D., Summers, A. O., White, D. G., Thayer, S. G. & Maurer, J. J. (1999) Antimicrob. Agents Chemother. 43, 2925-2929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goldstein, C., Lee, M. D., Sanchez, S., Hudson, C., Phillips, B., Register, B., Grady, M., Liebert, C., Summers, A. O., White, D. G. & Maurer, J. J. (2001) Antimicrob. Agents Chemother. 45, 723-726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sunde, M. & Sorum, H. (1999) Microb. Drug Resist. 5, 279-287. [DOI] [PubMed] [Google Scholar]
- 19.Normark, B. H. & Normark, S. (2002) J. Intern. Med. 252, 91-106. [DOI] [PubMed] [Google Scholar]
- 20.Wireman, J., Liebert, C. A., Smith, T. & Summers, A. O. (1997) Appl. Environ. Microbiol. 63, 4494-4503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nesvera, J., Hochmannova, J. & Patek, M. (1998) FEMS Microbiol. Lett. 169, 391-395. [DOI] [PubMed] [Google Scholar]
- 22.Clark, N. C., Olsvik, O., Swenson, J. M., Spiegel, C. A. & Tenover, F. C. (1999) Antimicrob. Agents Chemother. 43, 157-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martin, C., Timm, J., Rauzier, J., Gomez-Lus, R., Davies, J. & Gicquel, B. (1990) Nature 345, 739-743. [DOI] [PubMed] [Google Scholar]
- 24.McIver, C. J., White, P. A., Jones, L. A., Karagiannis, T., Harkness, J., Marriott, D. & Rawlinson, W. D. (2002) J. Clin. Microbiol. 40, 1538-1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Neidhardt, F. C. & Umbarger, H. E. (1996) in Escherichia coli and Salmonella Cellular and Molecular Biology, eds. Neidhardt, F. C., III, R. C., Ingraham, J. L., Lin, E. C. C., Low, K. B., Magasanik, B., Reznikoff, W. S., Riley, M., Schaechter, M. & Umbarger, H. E. (Am. Soc. Microbiol. Press, Washington, DC), 2nd Ed., Vol. 1, pp. 13-16. [Google Scholar]
- 26.Miller, D. N., Bryant, J. E., Madsen, E. L. & Ghiorse, W. C. (1999) Appl. Environ. Microbiol. 65, 4715-4724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Courtois, S., Frostegard, A., Goransson, P., Depret, G., Jeannin, P. & Simonet, P. (2001) Environ. Microbiol. 3, 431-439. [DOI] [PubMed] [Google Scholar]
- 28.Liebert, C. A., Hall, R. M. & Summers, A. O. (1999) Microbiol. Mol. Biol. Rev. 63, 507-522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fluit, A. C. & Schmitz, F. J. (1999) Eur. J. Clin. Microbiol. Infect. Dis. 18, 761-770. [DOI] [PubMed] [Google Scholar]
- 30.Peters, J. E. & Craig, N. L. (2001) Nat. Rev. Mol. Cell Biol. 2, 806-814. [DOI] [PubMed] [Google Scholar]
- 31.Suzuki, M. T., Taylor, L. T. & DeLong, E. F. (2000) Appl. Environ. Microbiol. 66, 4605-4614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Levesque, C., Piche, L., Larose, C. & Roy, P. H. (1995) Antimicrob. Agents Chemother. 39, 185-191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nadkarni, M. A., Martin, F. E., Jacques, N. A. & Hunter, N. (2002) Microbiology 148, 257-266. [DOI] [PubMed] [Google Scholar]
- 34.Klappenbach, J. A., Saxman, P. R., Cole, J. R. & Schmidt, T. M. (2001) Nucleic Acids Res. 29, 181-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gong, J., Forster, R. J., Yu, H., Chambers, J. R., Sabour, P. M., Wheatcroft, R. & Chen, S. (2002) FEMS Microbiol. Lett. 208, 1-7. [DOI] [PubMed] [Google Scholar]
- 36.Stackebrandt, E. & Embley, T. M. (2000) in Nonculturable Microorganisms in the Environment, eds. Rita R. Colwell & Grimes, D. J. (Am. Soc. Microbiol., Washington, DC), pp. 57-75.
- 37.Kazama, H., Hamashima, H., Sasatsu, M. & Arai, T. (1998) FEMS Microbiol. Lett. 165, 295-299. [DOI] [PubMed] [Google Scholar]
- 38.Kazama, H., Hamashima, H., Sasatsu, M. & Arai, T. (1998) FEMS Microbiol. Lett. 159, 173-178. [DOI] [PubMed] [Google Scholar]
- 39.Stokes, H. W., O'Gorman, D. B., Recchia, G. D., Parsekhian, M. & Hall, R. M. (1997) Mol. Microbiol. 26, 731-745. [DOI] [PubMed] [Google Scholar]
- 40.Hansson, K., Sundstrom, L., Pelletier, A. & Roy, P. H. (2002) J. Bacteriol. 184, 1712-1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Finegold, S. M., Sutter, V. L. & Mathisen, G. E. (1983) in Human Intestinal Microflora in Health and Disease, ed. Hentges, D. J. (Academic, New York), Vol. 3–32.
- 42.Bissonnette, L. & Roy, P. H. (1992) J. Bacteriol. 174, 1248-1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Holmes, A. J., Gillings, M. R., Nield, B. S., Mabbutt, B. C., Nevalainen, K. M. H. & Stokes, H. W. (2003) Environ. Microbiol. 5, 383-394. [DOI] [PubMed] [Google Scholar]
- 44.Chiew, Y.-F., Yeo, S.-F., Hall, L. M. C. & Livermore, D. M. (1998) J. Antimicrob. Chemother. 41, 247-251. [DOI] [PubMed] [Google Scholar]
- 45.Enne, V. I., Livermore, D. M., Stephens, P. & Hall, L. M. C. (2001) Lancet 357, 1325-1328. [DOI] [PubMed] [Google Scholar]
- 46.Summers, A. O. (2002) Clin. Infect. Dis. 34, S85-92. [DOI] [PubMed] [Google Scholar]
- 47.Chiller, K., Selkin, B. A. & Murakawa, G. J. (2001) J. Invest. Dermatol. Symp. Proc. 6, 170-174. [DOI] [PubMed] [Google Scholar]
- 48.Terpstra, S., Noordhoek, G. T., Voesten, H. G., Hendriks, B. & Degener, J. E. (1999) J. Hosp. Infect. 43, 195-202. [DOI] [PubMed] [Google Scholar]
- 49.Mazel, D., Dychinco, B., Webb, V. A. & Davies, J. (1998) Science 280, 605-608. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.