Abstract
Nearly half of the world’s population harbors helminth infections or suffers from allergic disorders. A common feature of this population is the so-called “type 2 immune response,” which confers protection against helminths, but also promotes pathologic responses associated with allergic inflammation. However, the mechanisms that initiate and control type 2 responses remain enigmatic. Recent advances have revealed a role for the innate immune system in orchestrating type 2 responses against a bewildering array of stimuli, from nanometer-sized allergens to 20-meter-long helminth parasites. Here, we review these advances and suggest that the human immune system has evolved multiple mechanisms of sensing such stimuli, from recognition of molecular patterns via innate immune receptors to detecting metabolic changes and tissue damage caused by these stimuli.
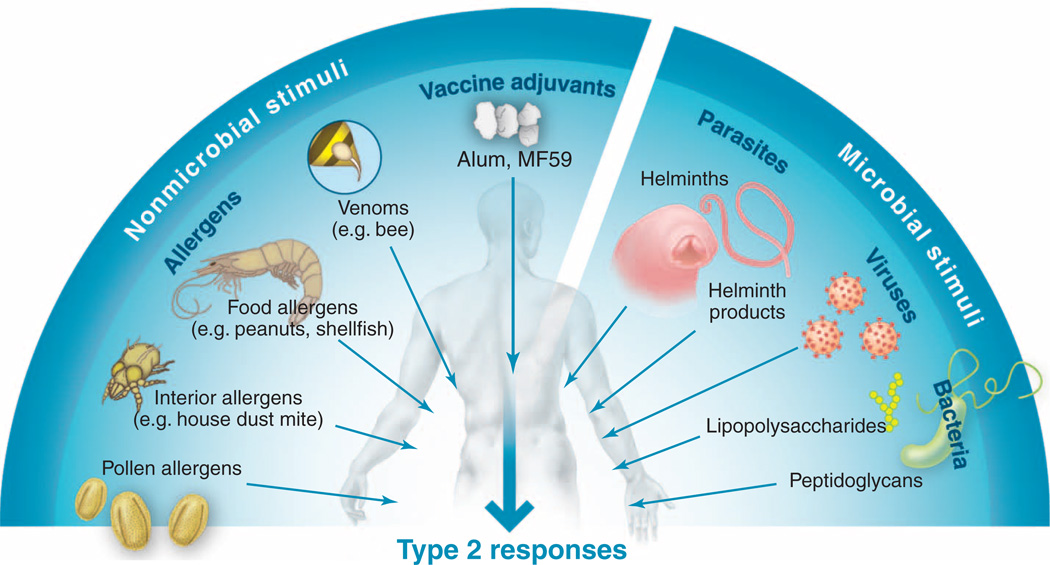
More than 3 billion people worldwide are infected with parasitic worms called helminths or suffer allergic disorders such as asthma, allergic rhinitis, food allergies, and eczema (1, 2). A common feature of these infectious or inflammatory conditions is the so-called allergic or “type 2” immune response (3–6). Type 2 immune responses are induced by and confer protection against helminths, but can also play pathologic roles, promoting acute and chronic inflammatory responses against a myriad of allergens. Although type 2 immune responses have been explored largely in the context of helminth infections and allergies, they are also induced by venoms, vaccine adjuvants such as alum (7, 8), several endogenous ligands in the host, and some bacterial and viral infections (although allergic reactions to the latter are the exception rather than the rule) (Fig. 1). Despite the medical and economic impact of type 2 inflammatory responses, how such diverse stimuli trigger prototypic type 2 responses, the nature of the cellular and molecular networks that orchestrate these responses, and whether there are distinct kinds of type 2 responses that play protective versus pathologic roles in various infectious or inflammatory settings are still unclear.
Fig. 1.
Diversity of stimuli that induce type 2 immune responses. Such stimuli range from nanometersized allergens to 20-m-long helminthic parasites. Despite marked differences in size, shape, structure, and physical and chemical properties, all of these stimuli induce type 2 immune responses.
Type 2 Immunity: An Overview
Type 2 responses are characterized by the induction of CD4+ T helper (TH) 2 cells, which secrete cytokines such as interleukin-4 (IL-4), IL-5, IL-9, and IL-13. TH2 cells promote B cell responses and immunoglobulin E (IgE) secretion through their production of IL-4 (3–6). IgE immune complexes bind to high-affinity IgE receptors (FcεR1) on basophils and mast cells, leading to their activation and secretion of several cytokines and inflammatory mediators such as histamine, heparin, and serotonin (3–6). These factors mediate a range of effector functions characteristic of type 2 inflammation, including recruitment of alternatively activated macrophages and granulocytes, smooth muscle contractility, and mucus hypersecretion (3–6). Because many different cell types are involved in the orchestration of TH2 cell responses, the term “type 2 response” will be used to describe the overall response.
TH2 cell responses belong to a larger spectrum of distinct TH responses that have evolved to protect the host against a spectrum of pathogens. Different types of TH cells are characterized by distinct cytokines and transcription factor profiles and also the types of pathogens they control. For example, infection with intracellular bacteria such as Mycobacterium tuberculosis or viruses typically induces strong CD4+ TH1 cell responses that result in the secretion of interferon-γ and the elicitation of CD8+ cytotoxic T cells that can kill infected cells (3, 4). In contrast, TH2 cell responses are typically induced by helminths but play a central role in mediating allergic disorders and asthma. Other TH subsets include TH17 cells, which contribute to immunity against extracellular bacteria and fungi and the pathogenesis of multiple chronic inflammatory diseases (3, 4); T follicular (TFH) cells, which promote differentiation of memory B cells (4); and regulatory T cells (Tregs), which suppress TH1, TH2, and TH17 responses (4).
Despite the notable developments in understanding the cellular and molecular mechanisms that control TH1 and TH17 cell responses, much less is understood about how TH2 cell responses are initiated and orchestrated. Furthermore, the question of why type 2 responses are generated to allergens and helminths remains a mystery. The diversity of stimuli that induce type 2 responses (Fig. 1), the assembly of different cell types that seem to play key roles, and the fact that there appear to be variants of type 2 responses are all challenges in studying type 2 inflammation. However, several conceptual advances in recent years have begun to shed light on the pathways that initiate and regulate type 2 responses. In the present Review, we examine this recent progress. First, we discuss the apparent heterogeneity of cytokine profiles within the TH2 cells (the TH2 medley) and consider the physiological relevance of this heterogeneity in vivo. Second, we reflect on how the immune system senses a staggering diversity of allergens, helminth products, and other microbes to initiate type 2 responses. Third, we consider how type 2 responses are initiated and orchestrated. We discuss the current knowledge of the cell types, innate receptors, and signaling pathways that orchestrate TH2 responses. Finally, we conclude by highlighting some key questions that must be answered to facilitate the rational design of therapeutics against type 2 inflammatory disorders.
The TH2 Medley
Traditionally TH2 cells have been defined as those T cells that produce IL-4, IL-5, IL-13, IL-9, and IL-10 and express the transcription factors GATA-binding protein 3 (GATA-3), signal transducer and activator of transcription–5 (STAT-5), and STAT-6 (3–6). However, single-cell analysis has revealed a marked degree of heterogeneity in the cytokine profiles of TH2 cells (9). Furthermore, ex vivo restimulation of human T cells isolated from blood revealed a population of TH2 cells that produce IL-4, IL-5, and IL-13 but not IL-10. In contrast, this cell population expressed high levels of the proinflammatory cytokine tumor necrosis factor–α (TNF-α) (10). Because IL-10 is a cytokine that is known to suppress allergic inflammation, TH2 cells that coexpress proinflammatory cytokines have been termed “inflammatory TH2 cells” to distinguish them from the canonical “noninflammatory TH2 cells” that do produce IL-10. Consistent with this, IL-13+/TNF-α+ inflammatory TH2 cells have also been isolated from human breast cancer biopsies and are believed to contribute to an inflammatory milieu that promotes tumor progression (11). Whether the TH2 cells that cause allergic disease display a more proinflammatory phenotype is poorly understood. Moreover, certain microbial stimuli can stimulate TH2-like cells that cannot be pigeonholed into the canonical TH2 phenotype. For example, Porphyromonas gingivalis–derived lipopolysaccharide (LPS) promotes TH2 cell–like responses in which TH cells produce IL-13, IL-5, and IL-10 but not IL-4 (12). Other stimuli, such as certain ligands for Toll-like receptor 2 (TLR2), promote a mixed TH2 and TH17 response (13), and subsets of Tcells that secrete both IL-4 and IL-17 and coexpress the transcription factors GATA3 and RORγt have been described and linked to bronchial hypereactivity in humans (14). In addition, TH2 cells can acquire IL-9 production when stimulated with transforming growth factor–β (15).
It is important to note that many studies that indicate heterogeneity within a population of TH2 cells have employed restimulation of T cells ex vivo. Although these approaches reveal the potential of a given TH2 cell to express different effector cytokines, they do not illuminate what the cell actually secretes within the tissue microenvironment. Therefore, an important challenge for the field is to evaluate the heterogeneity of TH2 cell responses in vivo. Employing genetically engineered reporter mice in which the temporal and spatial expression of a given cytokine by antigen-specific T cells can be visualized in vivo has proven to be a fruitful approach to addressing this question. For example, the use of this approach to assess the temporal and spatial production of these cytokines in vivo revealed that in lymph nodes, IL-4 but not IL-13 was produced by TFH cells (16). In contrast, TH2 cells in tissues produced both cytokines.
Such heterogeneity in cytokine expression may exist within populations of TH2 cells because subsets of TH2 cells may be functionally distinct. Thus, IL-4–producing TFH cells in the germinal center may induce isotype switching of B cells to IgE, whereas IL-4+IL-13+ TH2 cells in tissues may contribute to tissue remodeling. An alternative, though not mutually exclusive, possibility is that the heterogeneity represents the dynamic nature of TH2 cell responses, in which the cytokine profiles of individual T cells within the TH2 compartment are dependent on the number of cell divisions and the tissue microenvironment in which the T cells reside. If the TH2 cell variants indeed have distinct functions, then learning whether one variant (for instance, pathologic) can be reprogrammed to another (e.g., protective) has therapeutic relevance in the context of allergic disorders and asthma.
How Are Type 2–Inducing Stimuli Sensed?
In the immune system, pathogen recognition receptors (PRRs) have evolved to sense a diverse array of stimuli (17–19). PRRs include TLRs, C-type lectin–like receptors (CLRs), and NOD-like receptors (NLRs), which are expressed on the surface and in intracellular compartments of a variety of cell types, including dendritic cells (DCs) and epithelial cells. Triggering of such PRRs results in the activation of DCs, which then stimulate antigen-specific T cells and tune the type of TH cell response that is generated. Indirect activation of DCs can also occur, for example, via epithelial cell–derived factors such as thymic stromal lymphopoietin (TSLP), which conditions DCs to induce TH2 cell responses (3–6).
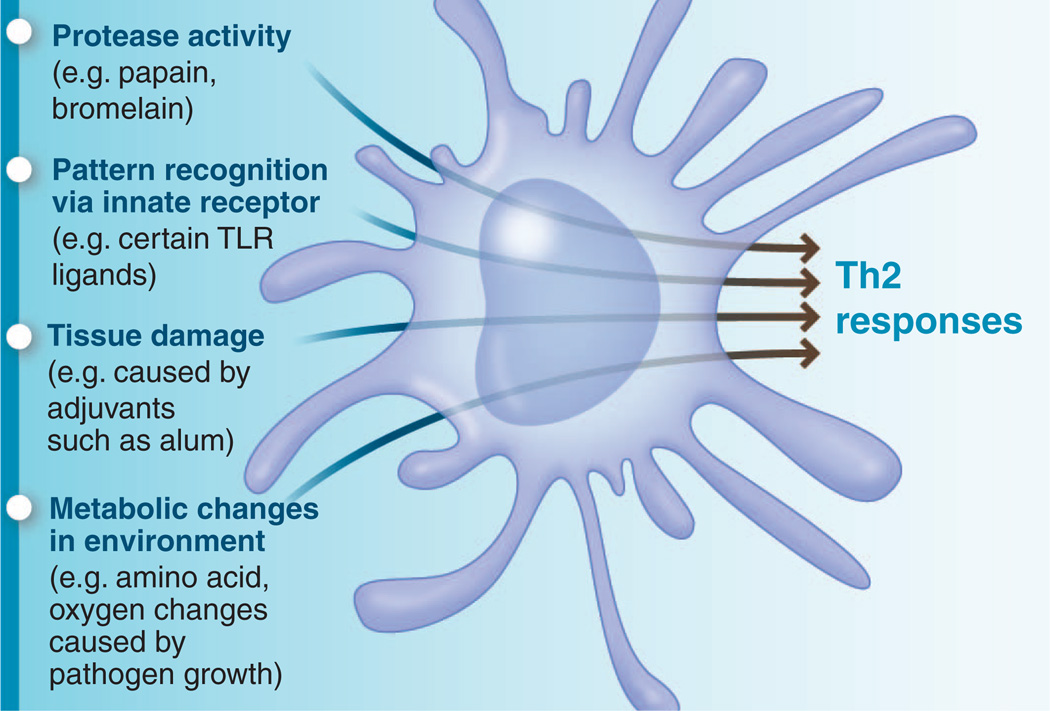
Although recent studies have highlighted the role of PRR triggering of DCs in orchestrating type 2 responses, emerging insights suggest that this paradigm may not be the only way to induce type 2 immunity. Other pathways such as the enzymatic activity of allergens, or recognition of tissue damage and/or metabolic changes caused by allergens or helminths, may also trigger type 2 immunity (Fig. 2).
Fig. 2.
Diverse mechanisms by which the innate immune system senses type 2–inducing stimuli. The host appears to have evolved multiple mechanisms to sense a bewildering array of stimuli that induce type 2 immune responses. Many pathogens and allergens can be sensed by pattern recognition receptors. In addition, proteolytic cleavage of host proteins by the protease activity of allergens, as well as tissue damage and metabolic changes caused by stimuli, may also be sensed by cells such as dendritic cells. Such diverse signals are decoded by dendritic cells to program type 2 immunity.
Given the notable array of microbial (e.g., helminth products, bacterial LPS, viruses) and nonmicrobial (allergens from pollen, mites, animal dander, insects, molds, foods, venoms, and adjuvants) stimuli that provoke type 2 responses, it may be necessary to look beyond the classical PRR-DC paradigm when thinking about how type 2 responses are generated. Are the known sets of PRRs sufficient to account for type 2–inducing stimuli, or do we have to posit an undiscovered family of PRRs or other innate sensors that promote these responses? Although the role of TLRs in inducing TH1 cell responses is appreciated, there are also several examples in which stimuli that activate TLRs (e.g., P. gingivalis LPS, synthetic TLR2 ligands, helminth extracts, low doses of lipopolysaccharides) signal DCs to induce TH2 cell responses (6). Furthermore, recognition of bacterial peptidoglycans by NOD1 and NOD2 or by the C-type lectin DC-SIGN induces type 2 responses in some scenarios (6).
In the case of allergens, there is evidence that they can be sensed by PRRs. For example, extracts of the ubiquitous house dust mite (HDM) allergens signal through TLR4 on airway structural cells to produce cytokines such as TSLP, granulocyte-monocyte colony-stimulating factor (GM-CSF), IL-25, and IL-33, which condition DCs and other accessory cells to promote a TH2 response and airway inflammation (20). HDM extract contains endotoxin, which signals through TLR4 and modulates allergic sensitization in children (21). Furthermore, it has been proposed that low-dose LPS promotes TH2 cell responses, whereas high doses promote TH1 cell responses (22). The induction of TSLP, GM-CSF, IL-25, and IL-33 in a TLR4-dependent manner may reflect contaminating LPS (20). In addition, the main kind of HDM called Der p 2 is structurally and functionally homologous to MD2 (the LPS binding component of the TLR4 complex) and directly interacts with TLR4 to facilitate TLR4 signaling-dependent, TH2-mediated allergic inflammation (23). Now, there is increasing evidence that other allergens also trigger TLR4 by a similar mechanism. For example, a nickel allergy, one of the most frequent causes of contact hypersensitivity in industrialized countries, is triggered by an inflammatory response via TLR4 signaling (24). Interestingly, the cysteine protease papain also induces type 2 in vivo, through a mechanism dependent on TLR4 (25). Finally, HDMs can also activate DCs through the C-type lectin dectin-2 to induce TH2 responses (26).
Sensing a universe of allergens
Currently more than 1000 protein allergens from mites, animal dander, pollens, insects, and foods have been cloned and sequenced, and through structural biology and bioinformatics approaches, the secondary and tertiary structures of more than 200 allergens have been determined (27–29). Although most allergens can be classified into structural families on the basis of their protein folds (e.g., antiparallel β strands and antiparallel β strands associated with one or more α helices), these structural features do not allow discrimination between allergens and nonallergens (27–29). Moreover, functional analyses of allergens reveal that they consist of proteins with diverse biological functions, such as proteases, lipid-transfer proteins, and calcium-binding proteins (27–30).
One feature that contributes to the induction of type 2 responses by some allergens is the serine or cysteine protease activity of allergens such as the HDM group I allergens Der p1 and Der f1, papain, and allergens from Aspergillus and ragweed (27–29). The proteolytic activity of Der p1 and papain seem essential, as inactive forms of these proteins do not induce type 2 responses, although whether this is due to loss of enzymatic activity or to the shorter half life of inactive allergens is unknown (Fig. 2). It has also been reported that a cysteine protease from the parasite Leishmania mexicana induces type 2 responses in mice and that this effect could be abrogated by protease inhibitors (27–29). It is not clear what targets of such proteases are relevant for the induction of type 2 responses. Although protease-activated receptors (PARs) are activated by serine proteases, the ability of PARs to recognize cysteine protease activity and mediate TH2 responses is poorly understood (30). Recently, PAR-2 was shown to mediate, in part, the induction of the TH2 cell–inducing cytokine TSLP from airway epithelial cells in vitro in response to the protease activity of the common environmental fungus Alternaria alternata (31). Moreover, the serine protease kallikrein-5 induces atopic dermatitis–like lesions through PAR-2–mediated induction of TSLP in Netherton syndrome, a persistent atopic skin disease caused by mutations in the gene encoding serine protease inhibitor Kazal-type-5 (SPINK5, also known as LEKTI) (32). This protease inhibitor mutation results in unregulated kallikrein-5 activation of PAR-2, with the induction of the pro-TH2 mediators TSLP, thymus and activation-regulated chemokine, and macrophage-derived chemokine. However, the targets of other proteases such as papain are not known. Moreover, other allergens such as those isolated from cats and cockroaches do not appear to have any protease activity, indicating that protease activity is not the only pathway to promote type 2 inflammation. Consistent with this observation, many proteases such as bacterial proteases,metalloproteases, and aspartic proteases do not appear to induce type 2 responses, suggesting that protease activity alone may not be sufficient.
Tissue damage and metabolic changes
Besides pattern recognition and protease activity, it is conceivable that certain allergens and helminth-derived factors induce type 2 immune responses as a result of tissue damage caused by cell death (33). Many endogenous molecules released by tissue damage are potent inducers of type 2 responses. For example, induction of TH2 responses by the vaccine adjuvant alum does not seem to depend on any of the known PRRs (6, 7), but rather on tissue damage that results in the release of uric acid crystals, which programs DCs to induce type 2 responses (34). Furthermore, a recent report suggests that in mice, alum causes cell death and the subsequent release of host DNA, which acts as a potent endogenous adjuvant that triggers TH2 responses, partly through an interferon response factor 3–dependent mechanism (35). In addition, our recent work suggests that immunization with papain results in the induction of reactive oxygen species (ROS) in epithelial cells at the site. ROS orchestrated TH2 cell responses, in part, by inducing oxidized lipids that triggered the induction of TSLP by epithelial cells mediated by TLR4 and the adaptor protein TRIF (25). Consistent with this observation, two other signals of tissue damage—high mobility group nucleosome binding protein 1 (a nonhistone chromatin binding protein that is released upon cell death) and matrix metalloproteinase-2 (a proteolytic enzyme that degrades the extracellular matrix)—activate DCs via a TLR4-dependent pathway to induce TH2 cell responses (36, 37).
Given these examples, a critical question is why such tissue damage–associated molecules induce type 2 responses? It has been proposed that type 2 responses represent a rapid repair response to tissue damage (33). Consistent with this idea, synthesis of collagen I and III during wound repair after helminth infections is dependent on IL-4 and IL-13. Furthermore, in an experimental model of helminth infection in mice, although there was initial production of IL-17 and concomitant inflammation and tissue damage, subsequent signaling via the IL-4 receptor resulted in a suppression of IL-17 production, enhanced expression of IL-10, and generation of anti-inflammatory macrophages, all of which lead to rapid tissue repair (38).
Finally, we can speculate that metabolic changes in the local milieu [for example, amino acid starvation or oxygen deprivation leading to the so-called integrated stress response (39)] may program DCs and other innate cells at the site of inflammation to induce type 2 responses. How might TH2-inducing stimuli cause such metabolic changes? In the case of helminths, it is possible that growth and development consumes nutrients such as amino acids and oxygen from the local milieu, thus triggering the stress response. Furthermore, tissue damage caused by the migration of helminths may also trigger stress responses (i.e., oxidative stress) that are associated with type 2 inflammation. In the case of allergens such as papain, their protease activity may trigger tissue damage and elicit the stress response. Future experimental evaluation of such scenarios will be necessary to elucidate their influence on type 2 inflammation in vivo. Taken together, there is growing evidence that TH2-inducing stimuli are sensed by a broad range of mechanisms, including PRR-mediated activation of DCs, as well as by the enzymatic activity of allergens or recognition of tissue damage and/or metabolic changes caused by allergens or helminthes.
How Are Type 2 Responses Orchestrated?
As stated above, the current paradigm in innate immunity ascribes a central role for DCs in sensing microbial stimuli via PRRs and influencing the differentiation of lymphocytes into distinct effector cell populations. However, exposure to an allergen or pathogen can result in the activation of a spectrum of cell types of the innate immune system, including basophils, mast cells, natural killer T cells, innate lymphoid cells (ILCs), tissue epithelial cells, and stromal cells (Fig. 3).
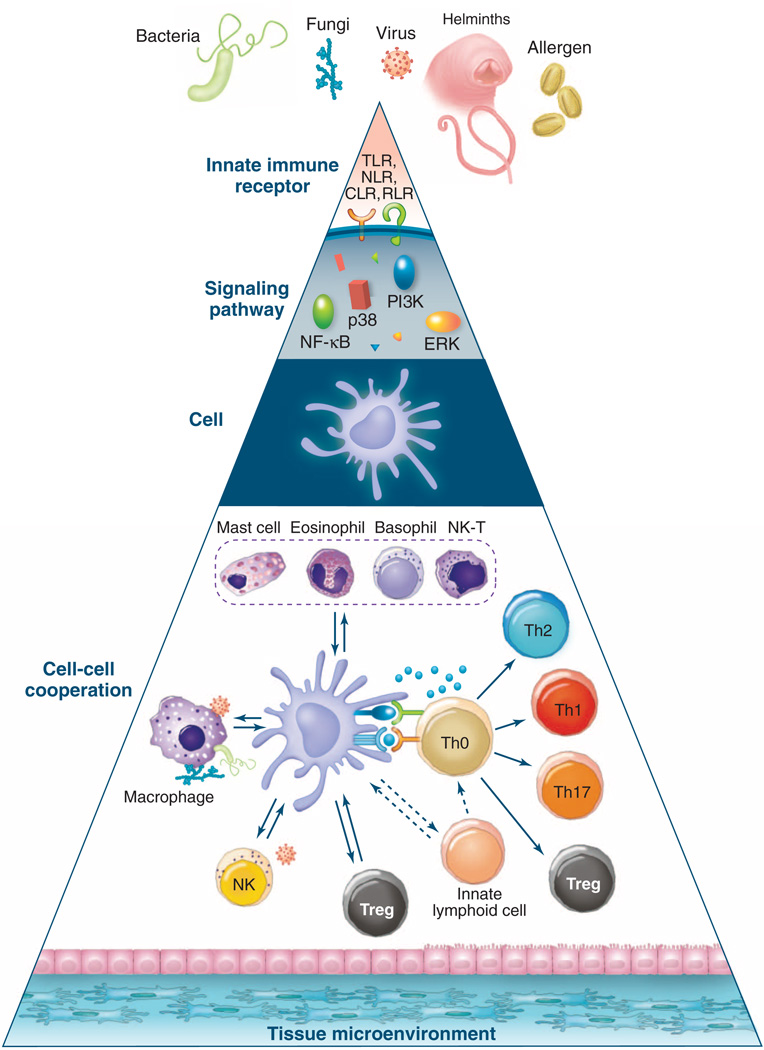
Fig. 3.
Hierarchies of organization in the innate immune system. The complexity of the innate immune system in sensing stimuli and orchestrating type 2 immune responses can be conceptualized as occurring in different hierarchies of organization. The cell (that is, the dendritic cell) can be considered as the ground level; the innate receptors expressed by the cell and the signaling networks within the cell represent higher-resolution levels of the hierarchy. Conversely, cell-cell cooperation and the impact of the tissue microenvironment represent more global views of the hierarchy. This model offers a conceptual framework for therapeutic interventions to allergic inflammation. Thus, one might envision targeting the signaling level (for instance, by inhibiting ROS in DCs), the cellular level (for example, by inhibiting migration of cells such as DCs or basophils), or the cell-cell cooperation level (by inhibiting molecules such as TSLP, IL-25, and IL-33 that mediate cell interactions). RLR, RIG-I–like receptors; PI3K, phosphatidylinositol 3-kinase; NK-T, natural killer T cells.
DCs have been shown to play a central role in coordinating the actions of such cell types. Therefore, a unified picture of how allergens and helminths initiate type 2 responses must account for the interaction of a network of many different cell types in the innate immune system. In this context, it is instructive to consider the different hierarchies or organization in the innate immune system (Fig. 3) (6). Given their key role in orchestrating immune responses, one could envision DCs as the “ground level” and the innate receptors and signaling networks representing higher-resolution views of the hierarchy. A more global view of the hierarchy includes multicellular cooperation (for example, between DCs, basophils, ILCs, and stromal cells) and the influence of tissue microenvironments (for instance, intestine versus lung). Such a hierarchical model can be applied to most biological systems, but given that recent studies have highlighted the diverse cell types that orchestrate type 2 responses, the model may be especially apt here. Importantly, such a view may provide insights into how allergic inflammation can be prevented at one or more levels of the hierarchy (Fig. 3).
The cell level
A key question in the field is what cell types are responsible for initiating TH2 responses. DCs play a central role in this process. For example, particular subsets of DCs seem to have an intrinsic capacity to induce type 2 responses. Thus, in mouse spleens the CD11c+CD8α+ DCs versus CD11c+CD8α− DCs produce different amounts of the TH1-inducing cytokine subunit IL-12p70 and differentially induce TH1 versus TH2 cell responses, respectively (6, 19). In mouse lungs, it has been demonstrated that type 2 responses after exposure to house dust mite allergens are induced by a subset of inflammatory DCs that express Fcε receptor 1 (40). In humans, plasmacytoid DCs (6, 19, 41) in the blood and Langerhans cells (42) in the skin can preferentially induce type 2 responses. Besides their functional specializations, DCs also show a great deal of functional plasticity. Thus, pathogen products such as LPS from P. gingivalis (12), omega-1 [a T2 ribonuclease glycoprotein derived from Schistosoma mansoni egg antigen (SEA)] (43, 44), helminth-derived stimuli (45), cholera toxin (46), or allergens (47, 48) can all condition DCs to promote type 2 responses. Furthermore, the mediators of the allergic response, such as TSLP (which is produced by stromal cells and histamine), can stimulate DCs to induce TH2 responses (49). Thus, certain DC subsets appear to have an intrinsic capacity to induce type 2 responses, and a multitude of microbial stimuli are reported to “condition” DCs to stimulate TH2 responses.
But are DCs necessary and sufficient to induce type 2 responses against helminths or allergens? Several recent studies demonstrate that DCs are necessary for type 2 responses in vivo. For example, conditional ablation of DCs in vivo using CD11c–diphtera toxin (DTR) mice abrogates TH2 cell responses and allergen-induced asthma in an ovalbumin-driven asthma model (50). Furthermore, ablation of DCs in CD11c- DTR mice before immunization with antigen plus papain resulted in diminished TH2 cell responses (25) and diminished TH2 responses to Nippostrongylus brasiliensis and S. mansoni (51, 52). Finally, a subset of FcεR1+ CD11c+ inflammatory DCs were shown to be necessary for the induction of TH2 responses to inhaled HDM antigens (40).
Although DCs are important in the development of type 2 responses in many circumstances, several studies suggest that DCs were not sufficient for optimal type 2 responses after exposure to some allergens or helminth parasites (53–55). Subsequent studies demonstrated a role for basophils in cooperation with DCs in promoting TH2 responses (25). Basophils constitute a minor population (<1%) of leukocytes in the blood and spleen and, upon activation, produce several effector molecules such as histamines, platelet-activating factors, leukotrienes, and the TH2 cytokines IL-4 and IL-13 (56). These cells were believed to represent an innate source of TH2 cytokines required for optimal type 2 responses. Consistent with this idea, basophils are rapidly recruited to the draining lymph nodes of mice injected with helminths or allergens within 24 hours and before the appearance of TH2 cells (25, 53–55). Besides producing TH2-associated cytokines, in some circumstances basophils, and potentially other granulocytes, can contribute to antigen presentation to T cells during the initiation of TH2 cell responses to allergens and helminths (53–55). Consistent with this notion, although DCs were required for TH2 cell responses, depletion of basophils using an antibody against basophils resulted in impaired TH2 responses to Trichuris muris (55). New genetic tools that have allowed the deletion of the majority of basophils in vivo suggest that type 2 responses can develop in the absence of basophils in some circumstances (57, 58). However, in these mice, basophils were constitutively deleted from birth, but not all basophils were deleted, so it is not clear whether compensatory mechanisms such as enhanced TH2-inducing capacities of other cells such as mast cells and DCs may contribute to developing type 2 responses in these settings. The extent to which DCs or basophils contribute to developing type 2 responses appears to be context-dependent. Differences in the relative roles of these two cell types observed in different studies may reflect differences in experimental conditions and models or, alternatively, may illuminate heterogeneity in the pathways that promote type 2 inflammation that can be influenced by stimulus- or tissue-dependent factors. This is clearly an area requiring further investigation.
What signals do DCs and basophils impart to promote TH2 cell differentiation? In the case of TH1 cells, IL-12 produced by DCs is essential, and it has been proposed that stimuli that fail to induce IL-12 in DCs program them to induce TH2 responses (3, 4, 6). Evidence for this “default mechanism” of TH2 cell response is observed with stimuli such as certain TLR2 ligands or allergens or helminth products that do not induce IL-12 (6). However, despite their failure to produce IL-12, DCs exposed to papain were incapable of inducing TH2 responses (25). Rather, such DCs induced greatly diminished TH1 cell responses, but TH2 cell responses were not induced. An exogenous source of IL-4 (from basophils) was necessary for optimal TH2 cell development (25). This is consistent with a number of previous studies demonstrating a key role for IL-4 in TH2 cell responses after helminth infection. Basophils and mast cells are key producers of IL-4 during TH2 cell development and rapidly produce IL-4 upon cross-linking of their FcεRI receptors through preexisting antigen-IgE complexes (3, 4, 56, 59). This may be relevant in memory responses, in which preexisting antigen-specific TH2 cells would have helped the production of antigen-specific IgE (59). However, in primary immune responses, activation of basophils and mast cells by allergens and helminths may lead to IL-4 production. Indeed, a recent report of heterogeneity within the basophil population has revealed that TSLP-dependent basophils are activated independently of IgE and are more potent sources of IL-4 compared with classical IL-3–dependent basophils (60). Besides IL-4, other molecules on DCs, such as OX40-l (10) and the Notch ligands Jagged-1 and Jagged-2, can promote TH2 responses (61).
Innate immune receptors
As discussed above, many TLR2 ligands induce type 2 responses (6). Furthermore, low doses of LPS have also been shown to induce TH2 cells in response to intranasal immunization with protein antigens, by means of a TLR4- andMyD88-dependent mechanism (22). NLRs, which include Nod1 and Nod2, are a family of cytosolic proteins that sense peptidoglycans or peptides derived from their degradation. Immunization of mice with ligands specific for Nod1 or Nod2 induces TH2-biased responses (62). CLRs belong to a large superfamily of transmembrane and soluble proteins that sense carbohydrate components of several pathogens, as well as self-glycoproteins. Certain microbial stimuli that signal via DC-SIGN, a CLR with broad pathogen recognition specificity, induce TH2 responses. For example, the LPS Lewis antigen (Le) of Helicobacter pylori induces TH2-biased responses through DC-SIGN (63).
Signaling networks
Very little is known about the signaling networks that program innate cells such as DCs to induce type 2 responses. Enhanced duration and magnitude of the extracellular signal–regulated mitogen-activated protein kinase (ERK MAPK), induced in DCs by certain TLR2 ligands or SEA, inhibits the production of IL-12p70, enhances IL-10 production, and programs DCs to stimulate TH2 cell–biased responses (64). Enhanced and sustained ERK signaling results in phosphorylation and stabilization of the early growth transcription factor c-Fos, which suppresses IL-12p70 production (64). Additionally, more recent studies have revealed a role for the MAPK3 (Tpl2) in ERK activation in DCs. Macrophages from Tpl2−/− mice display reduced c-Fos expression (65). Taken together, these results demonstrate an important role for ERK-fos signaling in inhibiting IL-12 in DCs and promoting TH2-biased responses. There are now several examples of diverse stimuli (e.g., Lactobacilli strains, complement proteins C5a and iC3b, ligands that activate DC-SIGN) that suppress IL-12 production via this pathway (6). Further work is required to assess the degree to which allergens and helminth products activate this pathway to promote type 2 responses.
Cell-cell cooperation and tissue microenvironment
DCs and basophils have been demonstrated to cooperate in the induction of type 2 immune responses, and this has been reviewed in detail elsewhere (6, 25). Furthermore, recent studies identified heterogeneous populations of ILCs (including natural helper cells, multipotent progenitor type 2 cells, nuocytes, and innate type 2 helper cells) as potent sources of TH2-associated cytokines that can promote type 2 responses. ILCs are activated by IL-25 and IL-33 secreted by epithelial cells and are a critical and early source of IL-5 and IL-13 (66–69). Influenza virus–induced airway hyperreactivity is mediated by ILCs and activated by IL-33 produced from virus-triggered macrophages and epithelial cells. In allergen-mediated asthma, IL-25, IL-33, and TSLP produced by epithelial cells activate ILCs and can promote type 2 inflammation (66–69). Finally, the influence of Tregs on type 2 inflammation has been well characterized and discussed extensively elsewhere. The recent report that extrathymically generated Tregs control mucosal type 2 inflammation (70) and that deletion of the transcription factor interferon regulatory factor 4 (which is essential for TH2 cell differentiation) from Tregs led to allergic inflammation (71) highlights the intricate cellular network that promotes and regulates type 2 responses.
Summary and Future Challenges
A marked feature of type 2 responses is their diversity. There is diversity in terms of the array of stimuli that trigger type 2 responses, the mechanisms by which the innate immune system senses such stimuli, and the cellular and molecular pathways that orchestrate the response. Indeed, there even appears to be diversity in the cytokine profiles of responding cells in a TH2 cell response, although whether such variants are functionally distinct remains to be established. We propose a conceptual framework to obtain an integrated understanding of how type 2 responses are initiated and controlled: The cell (the dendritic cell in this case) can be considered as the ground level, with innate receptors and signaling networks representing the subcellular levels and cell-cell cooperation and tissue microenvironments representing the supercellular levels (Fig. 3) (6). This hierarchy illuminates major challenges for the future, and we believe that such integrated understanding will come about from studying every level of the hierarchy with the use of systems biology approaches. Such approaches are likely to be particularly fruitful when used to study type 2 immune responses in humans in the context of helminth infections, allergic disorders, or vaccination (72). Greater access to human samples coupled with standardization of high-throughput technologies and reagents will accelerate progress in identifying putative molecular networks that orchestrate type 2 responses in human disease. The functional validation of such networks, involving their perturbation, will be facilitated by the establishment of more sophisticated animal models of type 2 inflammation.
Acknowledgments
Research in the Pulendran lab is supported by the NIH (grants U19AI090023, HHSN266200700006C, U54AI057157, R37AI48638, R37DK057665, U19AI057266, and N01 AI50025 to B.P.) and the Bill and Melinda Gates Foundation. Research in the Artis lab is supported by the NIH (grants AI061570, AI087990, AI074878, AI083480, AI095466, and AI095608 to D.A.) and the Burroughs Wellcome Fund Investigator in Pathogenesis of Infectious Disease Award (D.A.). We sincerely apologize to our colleagues that, owing to limitations of space, we were unable to cite many relevant references.
References and Notes
- 1.Pawankar R, Canonica GW, Holgate ST, Lockey RF. Curr. Opin. Allergy Clin. Immunol. 2012;12:39. doi: 10.1097/ACI.0b013e32834ec13b. [DOI] [PubMed] [Google Scholar]
- 2.Hotez PJ, et al. N. Engl. J. Med. 2007;357:1018. doi: 10.1056/NEJMra064142. [DOI] [PubMed] [Google Scholar]
- 3.Paul WE, Zhu J. Nat. Rev. Immunol. 2010;10:225. doi: 10.1038/nri2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhu J, Yamane H, Paul WE. Annu. Rev. Immunol. 2010;28:445. doi: 10.1146/annurev-immunol-030409-101212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lambrecht BN, Hammad H. Annu. Rev. Immunol. 2012;30:243. doi: 10.1146/annurev-immunol-020711-075021. [DOI] [PubMed] [Google Scholar]
- 6.Pulendran B, Tang H, Manicassamy S. Nat. Immunol. 2010;11:647. doi: 10.1038/ni.1894. [DOI] [PubMed] [Google Scholar]
- 7.Coffman RL, Sher A, Seder RA. Immunity. 2010;33:492. doi: 10.1016/j.immuni.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Palm NW, Rosenstein RK, Medzhitov R. Nature. 2012;484:465. doi: 10.1038/nature11047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kelso A, Groves P, Ramm L, Doyle AG. Int. Immunol. 1999;11:617. doi: 10.1093/intimm/11.4.617. [DOI] [PubMed] [Google Scholar]
- 10.Ito T, et al. J. Exp. Med. 2005;202:1213. doi: 10.1084/jem.20051135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pedroza-Gonzalez A, et al. J. Exp. Med. 2011;208:479. doi: 10.1084/jem.20102131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pulendran B, et al. J. Immunol. 2001;167:5067. doi: 10.4049/jimmunol.167.9.5067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wenink MH, et al. J. Immunol. 2009;183:6960. doi: 10.4049/jimmunol.0900713. [DOI] [PubMed] [Google Scholar]
- 14.Wang YH, et al. J. Exp. Med. 2010;207:2479. doi: 10.1084/jem.20101376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Veldhoen M, et al. Nat. Immunol. 2008;9:1341. doi: 10.1038/ni.1659. [DOI] [PubMed] [Google Scholar]
- 16.Liang H-E, et al. Nat. Immunol. 2012;13:58. doi: 10.1038/ni.2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ronald PC, Beutler B. Science. 2010;330:1061. doi: 10.1126/science.1189468. [DOI] [PubMed] [Google Scholar]
- 18.Kawai T, Akira S. Nat. Immunol. 2010;11:373. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- 19.Steinman RM, Banchereau J. Nature. 2007;449:419. doi: 10.1038/nature06175. [DOI] [PubMed] [Google Scholar]
- 20.Hammad H, et al. Nat. Med. 2009;15:410. doi: 10.1038/nm.1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Braun-Fahrländer C, et al. N. Engl. J. Med. 2002;347:869. doi: 10.1056/NEJMoa020057. [DOI] [PubMed] [Google Scholar]
- 22.Eisenbarth SC, et al. J. Exp. Med. 2002;196:1645. doi: 10.1084/jem.20021340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Trompette A, et al. Nature. 2009;457:585. doi: 10.1038/nature07548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schmidt M, et al. Nat. Immunol. 2010;11:814. doi: 10.1038/ni.1919. [DOI] [PubMed] [Google Scholar]
- 25.Tang H, et al. Nat. Immunol. 2010;11:608. doi: 10.1038/ni.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barrett NA, et al. J. Exp. Med. 2011;208:593. doi: 10.1084/jem.20100793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Poulsen LK. Clin. Exp. Allergy. 2009;39:623. doi: 10.1111/j.1365-2222.2009.03245.x. [DOI] [PubMed] [Google Scholar]
- 28.Traidl-Hoffmann C, Jakob T, Behrendt H. J. Allergy Clin. Immunol. 2009;123:558. doi: 10.1016/j.jaci.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 29.Chapman MD, Pomés A, Breiteneder H, Ferreira F. J. Allergy Clin. Immunol. 2007;119:414. doi: 10.1016/j.jaci.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 30.Ramachandran R, Noorbakhsh F, Defea K, Hollenberg MD. Nat. Rev. Drug Discov. 2012;11:69. doi: 10.1038/nrd3615. [DOI] [PubMed] [Google Scholar]
- 31.Kouzaki H, O’Grady SM, Lawrence CB, Kita H. J. Immunol. 2009;183:1427. doi: 10.4049/jimmunol.0900904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Briot A, et al. J. Exp. Med. 2009;206:1135. doi: 10.1084/jem.20082242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Allen JE, Wynn TA. PLoS Pathog. 2011;7:e1002003. doi: 10.1371/journal.ppat.1002003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kool M, et al. Immunity. 2011;34:527. doi: 10.1016/j.immuni.2011.03.015. [DOI] [PubMed] [Google Scholar]
- 35.Marichal T, et al. Nat. Med. 2011;17:996. doi: 10.1038/nm.2403. [DOI] [PubMed] [Google Scholar]
- 36.Yang D, et al. J. Exp. Med. 2012;209:157. doi: 10.1084/jem.20101354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Godefroy E, et al. Cancer Cell. 2011;19:333. doi: 10.1016/j.ccr.2011.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen F, et al. Nat. Med. 2012;18:260. doi: 10.1038/nm.2628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Harding HP, Calfon M, Urano F, Novoa I, Ron D. Annu. Rev. Cell Dev. Biol. 2002;18:575. doi: 10.1146/annurev.cellbio.18.011402.160624. [DOI] [PubMed] [Google Scholar]
- 40.Hammad H, et al. J. Exp. Med. 2010;207:2097. doi: 10.1084/jem.20101563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rissoan M-C, et al. Science. 1999;283:1183. doi: 10.1126/science.283.5405.1183. [DOI] [PubMed] [Google Scholar]
- 42.Klechevsky E, et al. Immunity. 2008;29:497. doi: 10.1016/j.immuni.2008.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Everts B, et al. J. Exp. Med. 2009;206:1673. doi: 10.1084/jem.20082460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Steinfelder S, et al. J. Exp. Med. 2009;206:1681. doi: 10.1084/jem.20082462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Whelan M, et al. J. Immunol. 2000;164:6453. doi: 10.4049/jimmunol.164.12.6453. [DOI] [PubMed] [Google Scholar]
- 46.Braun MC, He J, Wu CY, Kelsall BL. J. Exp. Med. 1999;189:541. doi: 10.1084/jem.189.3.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Traidl-Hoffmann C, et al. J. Exp. Med. 2005;201:627. doi: 10.1084/jem.20041065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shreffler WG, et al. J. Immunol. 2006;177:3677. doi: 10.4049/jimmunol.177.6.3677. [DOI] [PubMed] [Google Scholar]
- 49.Soumelis V, et al. Nat. Immunol. 2002;3:673. doi: 10.1038/ni805. [DOI] [PubMed] [Google Scholar]
- 50.van Rijt LS, et al. J. Exp. Med. 2005;201:981. doi: 10.1084/jem.20042311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ohnmacht C, et al. Immunity. 2010;33:364. doi: 10.1016/j.immuni.2010.08.011. [DOI] [PubMed] [Google Scholar]
- 52.Phythian-Adams AT, et al. J. Exp. Med. 2010;207:2089. doi: 10.1084/jem.20100734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yoshimoto T, et al. Nat. Immunol. 2009;10:706. doi: 10.1038/ni.1737. [DOI] [PubMed] [Google Scholar]
- 54.Sokol CL, et al. Nat. Immunol. 2009;10:713. doi: 10.1038/ni.1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Perrigoue JG, et al. Nat. Immunol. 2009;10:697. doi: 10.1038/ni.1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Karasuyama H, Mukai K, Obata K, Tsujimura Y, Wada T. Annu. Rev. Immunol. 2011;29:45. doi: 10.1146/annurev-immunol-031210-101257. [DOI] [PubMed] [Google Scholar]
- 57.Voehringer D. Curr. Opin. Immunol. 2011;23:789. doi: 10.1016/j.coi.2011.09.005. [DOI] [PubMed] [Google Scholar]
- 58.Sullivan BM, et al. Nat. Immunol. 2011;12:527. doi: 10.1038/ni.2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Khodoun MV, Orekhova T, Potter C, Morris S, Finkelman FD. J. Exp. Med. 2004;200:857. doi: 10.1084/jem.20040598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Siracusa MC, et al. Nature. 2011;477:229. doi: 10.1038/nature10329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Amsen D, et al. Cell. 2004;117:515. doi: 10.1016/s0092-8674(04)00451-9. [DOI] [PubMed] [Google Scholar]
- 62.Fritz JH, et al. Immunity. 2007;26:445. doi: 10.1016/j.immuni.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 63.Bergman MP, et al. J. Exp. Med. 2004;200:979. doi: 10.1084/jem.20041061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Agrawal S, et al. J. Immunol. 2003;171:4984. doi: 10.4049/jimmunol.171.10.4984. [DOI] [PubMed] [Google Scholar]
- 65.Kaiser F, et al. J. Exp. Med. 2009;206:1863. doi: 10.1084/jem.20091059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Moro K, et al. Nature. 2010;463:540. doi: 10.1038/nature08636. [DOI] [PubMed] [Google Scholar]
- 67.Saenz SA, et al. Nature. 2010;464:1362. doi: 10.1038/nature08901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Neill DR, et al. Nature. 2010;464:1367. doi: 10.1038/nature08900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Spits H, Cupedo T. Annu. Rev. Immunol. 2012;30:647. doi: 10.1146/annurev-immunol-020711-075053. [DOI] [PubMed] [Google Scholar]
- 70.Josefowicz SZ, et al. Nature. 2012;482:395. doi: 10.1038/nature10772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zheng Y, et al. Nature. 2009;458:351. doi: 10.1038/nature07674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Querec TD, et al. Nat. Immunol. 2008;10:116. doi: 10.1038/ni.1688. [DOI] [PMC free article] [PubMed] [Google Scholar]