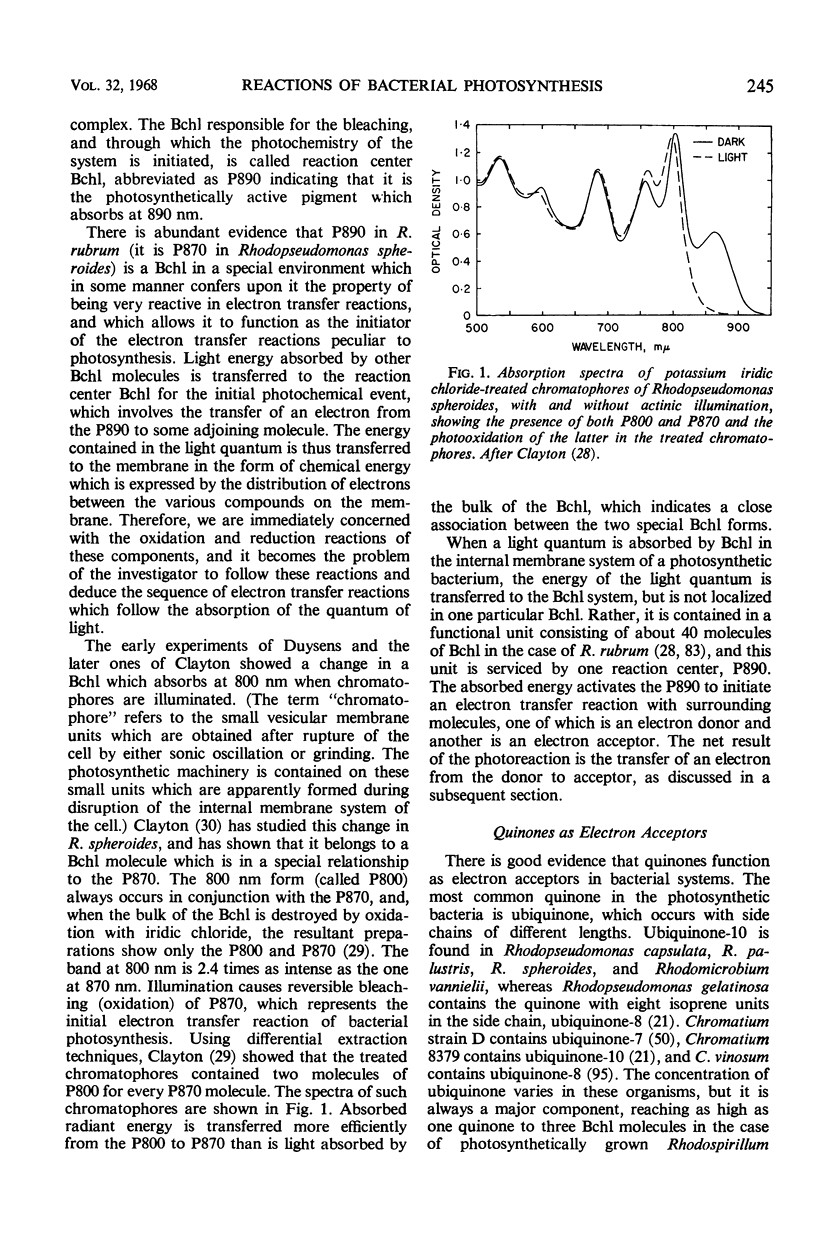
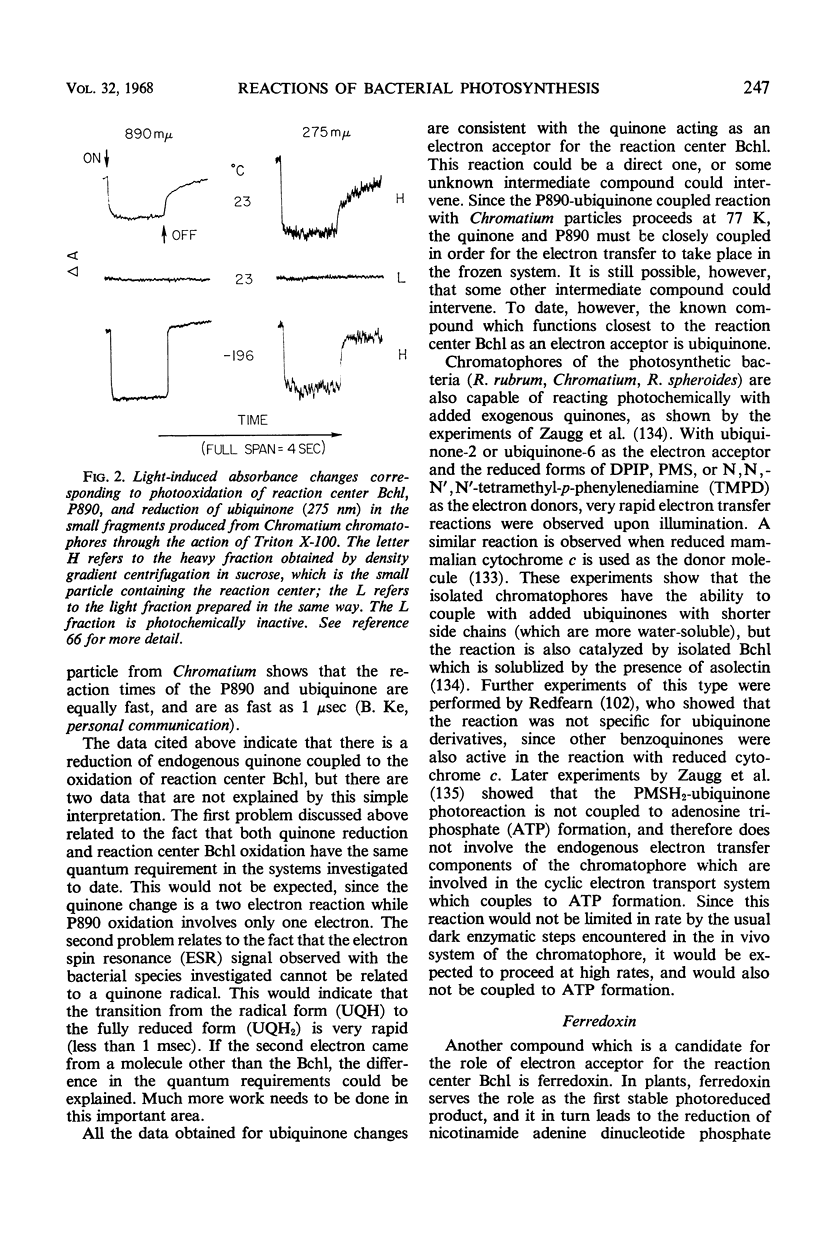
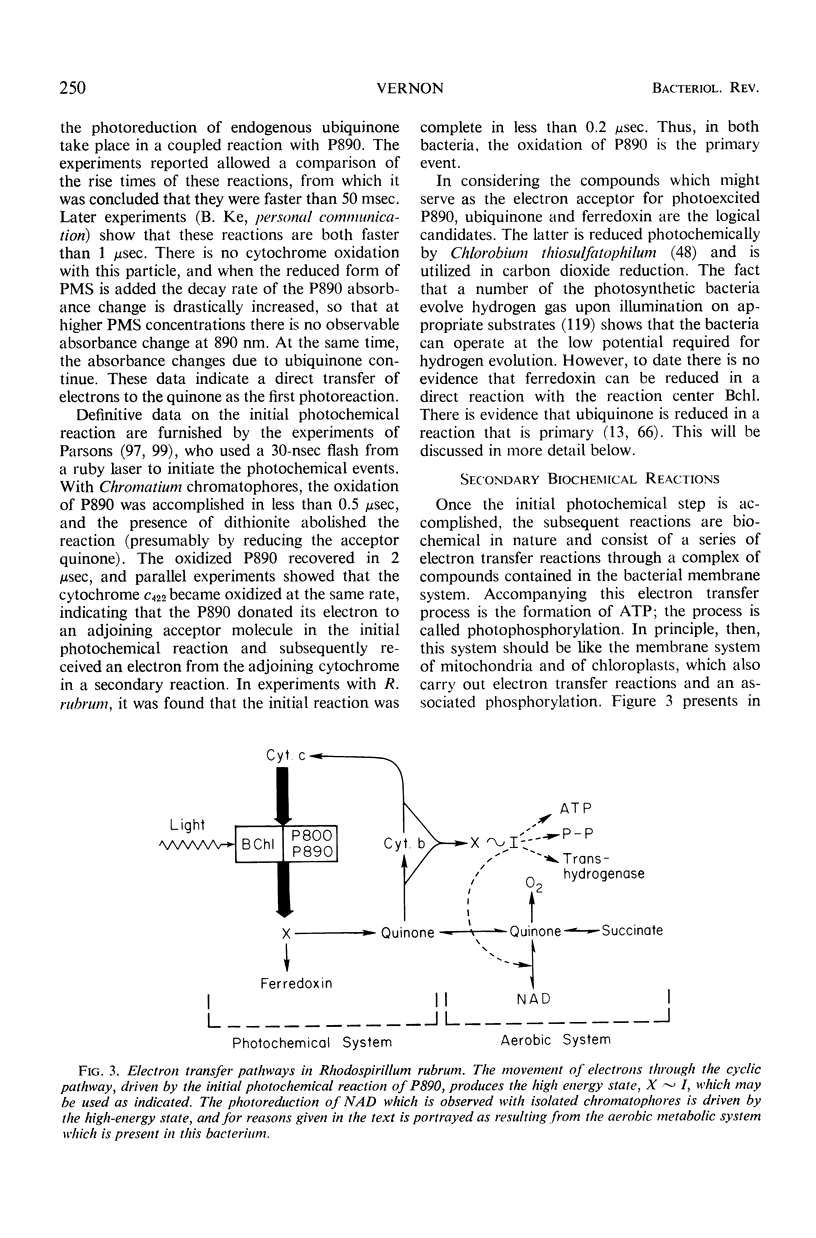
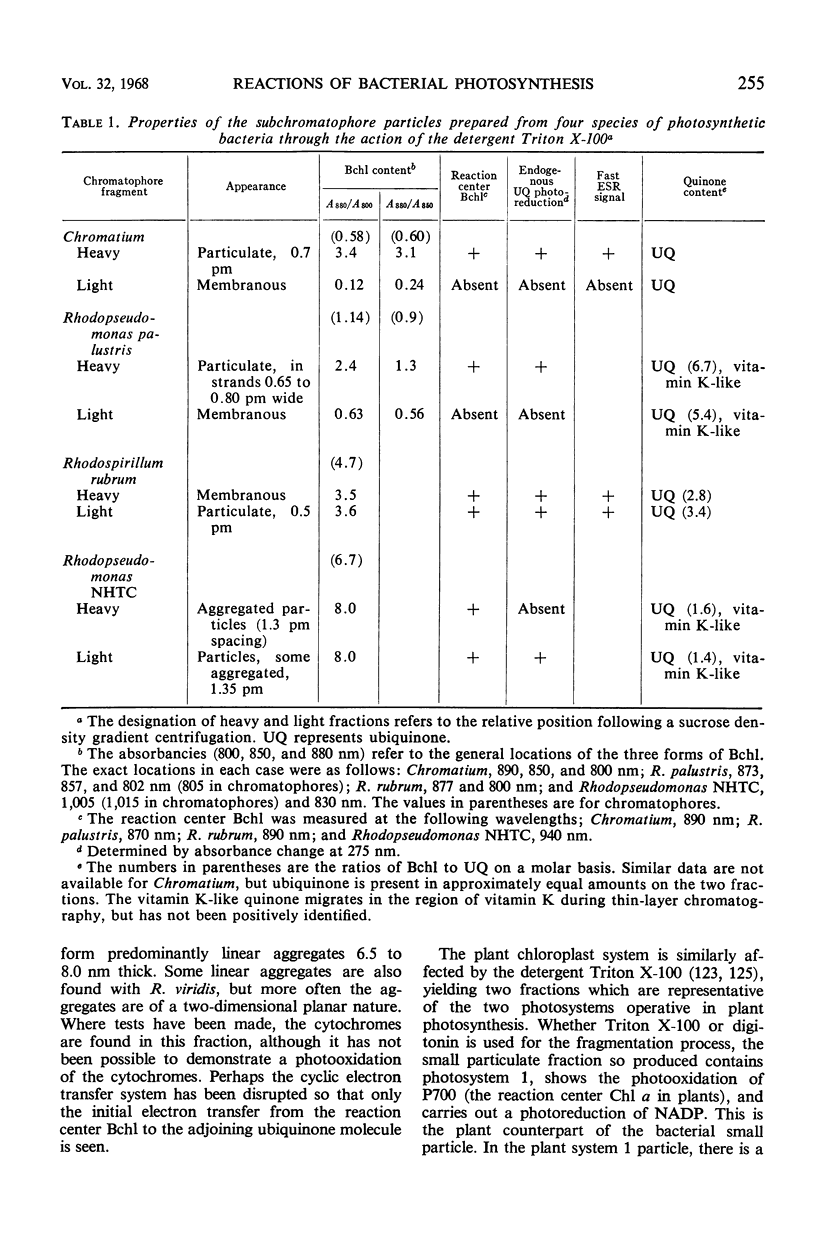
Full text
PDF














Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMESZ J. Kinetics, quantum requirement and action spectrum of light-induced phosphopyridine nucleotide reduction in Rhodospirillum rubrum and Rhodopseudomonas spheroides. Biochim Biophys Acta. 1963 Jan 15;66:22–36. doi: 10.1016/0006-3002(63)91164-8. [DOI] [PubMed] [Google Scholar]
- Arnold W., Clayton R. K. THE FIRST STEP IN PHOTOSYNTHESIS: EVIDENCE FOR ITS ELECTRONIC NATURE. Proc Natl Acad Sci U S A. 1960 Jun;46(6):769–776. doi: 10.1073/pnas.46.6.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BALTSCHEFFSKY H., ARWIDSSON B. Evidence for two phosphorylation sites in bacterial cyclic photophosphorylation. Biochim Biophys Acta. 1962 Dec 17;65:425–428. doi: 10.1016/0006-3002(62)90444-4. [DOI] [PubMed] [Google Scholar]
- BALTSCHEFFSKY H., BALTSCHEFFSKY M., OLSON J. M. The quantum efficiency of ATP production in bacterial light-in-duced phosphorylation. Biochim Biophys Acta. 1961 Jun 24;50:380–382. doi: 10.1016/0006-3002(61)90346-8. [DOI] [PubMed] [Google Scholar]
- BERGERON J. A., FULLER R. C. Influence of carotenoids on the infra-red spectrum of bacteriochlorophyll in Chromatium. Nature. 1959 Oct 24;184(Suppl 17):1340–1341. doi: 10.1038/1841340b0. [DOI] [PubMed] [Google Scholar]
- BOSE S. K., GEST H. Hydrogenase and light-stimulated electron transfer reactions in photosynthetic bacteria. Nature. 1962 Sep 22;195:1168–1171. doi: 10.1038/1951168a0. [DOI] [PubMed] [Google Scholar]
- BRIL C. Action of a non-ionic detergent on chromatophores of Rhodopseudomonas spheroides. Biochim Biophys Acta. 1958 Aug;29(2):458–458. doi: 10.1016/0006-3002(58)90223-3. [DOI] [PubMed] [Google Scholar]
- BRIL C. Studies on bacterial chromatophores. I. Reversible disturbance of transfer of electronic excitation energy between bacteriochlorophyll-types in Chromatium. Biochim Biophys Acta. 1960 Apr 8;39:296–303. doi: 10.1016/0006-3002(60)90166-9. [DOI] [PubMed] [Google Scholar]
- BRIL C. Studies on bacterial chromatophores. II. Energy transfer and photooxidative bleaching of bacteriochlorophyll in relation to structure in normal and carotenoid-depleted Chromatium. Biochim Biophys Acta. 1963 Jan 15;66:50–60. doi: 10.1016/0006-3002(63)91166-1. [DOI] [PubMed] [Google Scholar]
- Bachofen R., Arnon D. I. Crystalline ferredoxin from the photosynthetic bacterium Chromatium. Biochim Biophys Acta. 1966 Jun 8;120(2):259–265. doi: 10.1016/0926-6585(66)90345-1. [DOI] [PubMed] [Google Scholar]
- Baltscheffsky H., Von Stedingk L. V., Heldt H. W., Klingenberg M. Inorganic pyrophosphate: formation in bacterial photophosphorylation. Science. 1966 Sep 2;153(3740):1120–1122. doi: 10.1126/science.153.3740.1120. [DOI] [PubMed] [Google Scholar]
- Baltscheffsky M., Baltscheffsky H., von Stedingk L. V. Light-induced energy conversion and the inorganic pyrophosphatase reaction in chromatophores from Rhodospirillum rubrum . Brookhaven Symp Biol. 1966;19:246–257. [PubMed] [Google Scholar]
- Baltscheffsky M. Inorganic pyrophosphate and ATP as energy donors in chromatophores from Rhodospirillum rubrum. Nature. 1967 Oct 21;216(5112):241–243. doi: 10.1038/216241a0. [DOI] [PubMed] [Google Scholar]
- Beugeling T. Photochemical activities of K3Fe(CN)6-treated chromatophores from Rhodospirillum rubrum. Biochim Biophys Acta. 1968 Jan 15;153(1):143–153. doi: 10.1016/0005-2728(68)90155-2. [DOI] [PubMed] [Google Scholar]
- Black C. C., Jr Chloroplast reactions with dipyridyl salts. Biochim Biophys Acta. 1966 Jul 13;120(3):332–340. doi: 10.1016/0926-6585(66)90300-1. [DOI] [PubMed] [Google Scholar]
- CLAYTON R. K. Evidence for the photochemical reduction on coenzyme Q in chromatophores of photosynthetic bacteria. Biochem Biophys Res Commun. 1962 Sep 25;9:49–53. doi: 10.1016/0006-291x(62)90085-2. [DOI] [PubMed] [Google Scholar]
- Carr N. G., Exell G. Ubiquinone concentrations in athiorhodaceae grown under various environmental conditions. Biochem J. 1965 Sep;96(3):688–692. doi: 10.1042/bj0960688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chance B., Nishimura M., Avron M., Baltscheffsky M. Light-induced intravesicular pH changes in Rhodospirillum rubrum chromatophores. Arch Biochem Biophys. 1966 Oct;117(1):158–166. doi: 10.1016/0003-9861(66)90139-1. [DOI] [PubMed] [Google Scholar]
- Chance B., Nishimura M. ON THE MECHANISM OF CHLOROPHYLL-CYTOCHROME INTERACTION: THE TEMPERATURE INSENSITIVITY OF LIGHT-INDUCED CYTOCHROME OXIDATION IN CHROMATIUM. Proc Natl Acad Sci U S A. 1960 Jan;46(1):19–24. doi: 10.1073/pnas.46.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton R. K. The bacterial photosynthetic reaction center. Brookhaven Symp Biol. 1966;19:62–70. [PubMed] [Google Scholar]
- Clayton R. K. The biophysical problems of photosynthesis. Science. 1965 Sep 17;149(3690):1346–1354. doi: 10.1126/science.149.3690.1346. [DOI] [PubMed] [Google Scholar]
- Cost K., Frenkel A. W. Light-induced interactions of Rhodospirillum rubrum chromatophores with bromothymol blue. Biochemistry. 1967 Mar;6(3):663–667. doi: 10.1021/bi00855a004. [DOI] [PubMed] [Google Scholar]
- Cusanovich M. A., Bartsch R. G., Kamen M. D. Light-induced electron transport in Chromatium strain D. II. Light-induced absorbance changes in Chromatium chromatophores. Biochim Biophys Acta. 1968 Feb 12;153(2):397–417. doi: 10.1016/0005-2728(68)90083-2. [DOI] [PubMed] [Google Scholar]
- DUYSENS L. N., SWEEP G. Fluorescence spectrophotometry of pyridine nucleotide in photosynthesizing cells. Biochim Biophys Acta. 1957 Jul;25(1):13–16. doi: 10.1016/0006-3002(57)90409-2. [DOI] [PubMed] [Google Scholar]
- De Klerk H., Kamen M. D. A high-potential non-haem iron protein from the facultative photoheterotrophe Rhodopseudomonas gelatinosa. Biochim Biophys Acta. 1966 Jan 4;112(1):175–178. doi: 10.1016/s0926-6585(96)90022-9. [DOI] [PubMed] [Google Scholar]
- Dilley R. A., Vernon L. P. Quantum requirement of the light-induced proton uptake by spinach chloroplasts. Proc Natl Acad Sci U S A. 1967 Feb;57(2):395–400. doi: 10.1073/pnas.57.2.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dus K., De Klerk H., Sletten K., Bartsch R. G. Chemical characterization of high potential iron proteins from Chromatium and Rhodopseudomonas gelatinosa. Biochim Biophys Acta. 1967 Jun 27;140(2):291–311. doi: 10.1016/0005-2795(67)90470-9. [DOI] [PubMed] [Google Scholar]
- Duysens L. N., Amesz J. Quantum Requirement for Phosphopyridine Nucleotide Reduction in Photosynthesis. Plant Physiol. 1959 May;34(3):210–213. doi: 10.1104/pp.34.3.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eimhjellen K. E., Steensland H., Traetteberg J. A Thiococcus sp. nov. gen., its pigments and internal membrane system. Arch Mikrobiol. 1967;59(1):82–92. doi: 10.1007/BF00406319. [DOI] [PubMed] [Google Scholar]
- Evans M. C., Buchanan B. B., Arnon D. I. A new ferredoxin-dependent carbon reduction cycle in a photosynthetic bacterium. Proc Natl Acad Sci U S A. 1966 Apr;55(4):928–934. doi: 10.1073/pnas.55.4.928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans M. C., Buchanan B. B. Photoreduction of ferredoxin and its use in carbon dioxide fixation by a subcellular system from a photosynthetic bacterium. Proc Natl Acad Sci U S A. 1965 Jun;53(6):1420–1425. doi: 10.1073/pnas.53.6.1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FULLER R. C., SMILLIE R. M., RIGOPOULOS N., YOUNT V. Comparative studies of some quinones in photosynthetic systems. Arch Biochem Biophys. 1961 Nov;95:197–202. doi: 10.1016/0003-9861(61)90135-7. [DOI] [PubMed] [Google Scholar]
- GRIFFITHS M., SISTROM W. R., COHENBAZIRE G., STANIER R. Y., CALVIN M. Function of carotenoids in photosynthesis. Nature. 1955 Dec 24;176(4495):1211–1215. doi: 10.1038/1761211a0. [DOI] [PubMed] [Google Scholar]
- Garcia A., Vernon L. P., Ke B., Mollenhauer H. Some structural and photochemical properties of Rhodopseudomonas palustris subchromatophore particles obtained by treatment with Triton X-100. Biochemistry. 1968 Jan;7(1):319–325. doi: 10.1021/bi00841a040. [DOI] [PubMed] [Google Scholar]
- Garcia A., Vernon L. P., Ke B., Mollenhauer H. Some structural and photochemical properties of Rhodopseudomonas species NHTC 133 subchromatophore particles obtained by treatment with Triton X-100. Biochemistry. 1968 Jan;7(1):326–332. doi: 10.1021/bi00841a041. [DOI] [PubMed] [Google Scholar]
- Garcia A., Vernon L. P., Mollenhauer H. Properties of Chromatium subchromatophore particles obtained by treatment with Triton X-100. Biochemistry. 1966 Jul;5(7):2399–2407. doi: 10.1021/bi00871a033. [DOI] [PubMed] [Google Scholar]
- Garcia A., Vernon L. P., Mollenhauer H. Properties of Rhodospirillum rubrum subchromatophore particles obtained by treatment with Triton X-100. Biochemistry. 1966 Jul;5(7):2408–2416. doi: 10.1021/bi00871a034. [DOI] [PubMed] [Google Scholar]
- Gest H. Comparative biochemistry of photosynthetic processes. Nature. 1966 Feb 26;209(5026):879–882. doi: 10.1038/209879a0. [DOI] [PubMed] [Google Scholar]
- Gibson J. Aerobic metabolism of Chromatium sp. strain D. Arch Mikrobiol. 1967;59(1):104–112. doi: 10.1007/BF00406321. [DOI] [PubMed] [Google Scholar]
- HORIO T., YAMASHITA J. SITES OF PHOTOSYNTHETIC ELECTRON-TRANSPORT SYSTEMS COUPLING PHOSPHORYLATION WITH CHROMATOPHORES FROM RHODOSPIRILLUM RUBRUM. Biochim Biophys Acta. 1964 Sep 25;88:237–250. doi: 10.1016/0926-6577(64)90180-9. [DOI] [PubMed] [Google Scholar]
- Hinkson J. W. Nicotinamide adenine dinucleotide photoreduction with Chromatium and Rhodospirillum rubrum chromatophores. Arch Biochem Biophys. 1965 Dec;112(3):478–487. doi: 10.1016/0003-9861(65)90084-6. [DOI] [PubMed] [Google Scholar]
- JENSEN A., AASMUNDRUD O., EIMHJELLEN K. E. CHLOROPHYLLS OF PHOTOSYNTHETIC BACTERIA. Biochim Biophys Acta. 1964 Nov 29;88:466–479. doi: 10.1016/0926-6577(64)90089-0. [DOI] [PubMed] [Google Scholar]
- KOMEN J. G. Observations on the infrared absorption spectrum of bacteriochlorophyll. Biochim Biophys Acta. 1956 Oct;22(1):9–15. doi: 10.1016/0006-3002(56)90216-5. [DOI] [PubMed] [Google Scholar]
- Ke B., Vernon L. P., Garcia A., Ngo E. Coupled photooxidation of bacteriochlorophyll P890 and photoreduction of ubiquinone in a photochemically active subchromatophore particle derived from Chromatium. Biochemistry. 1968 Jan;7(1):311–318. doi: 10.1021/bi00841a039. [DOI] [PubMed] [Google Scholar]
- Keister D. L., Yike N. J. Energy-linked reactions in photosynthetic bacteria. I. Succinatelinked ATP-driven NAD reduction by Rhodospirillum rubrum chromatophores. Arch Biochem Biophys. 1967 Aug;121(2):415–422. doi: 10.1016/0003-9861(67)90095-1. [DOI] [PubMed] [Google Scholar]
- Keister D. L., Yike N. J. Studies on an energy-lined pyridine nucleotide transhydrogenase in photosynthetic bacteria. I. Demonstration of the reaction in Rhodospirillum rubrum. Biochem Biophys Res Commun. 1966 Aug 23;24(4):519–525. doi: 10.1016/0006-291x(66)90350-0. [DOI] [PubMed] [Google Scholar]
- Klemme J. H., Schlegel H. G. Photoreduktion von Pyridinnucleotid durch Chromatophoren aus Rhodopseudomonas capsulata mit molekularem Wasserstoff. Arch Mikrobiol. 1967;59(1):185–196. [PubMed] [Google Scholar]
- Kok B., Rurainski H. J., Owens O. V. The reducing power generated in photoact I of photosynthesis. Biochim Biophys Acta. 1965 Nov 29;109(2):347–356. doi: 10.1016/0926-6585(65)90162-7. [DOI] [PubMed] [Google Scholar]
- Lindstrom E. S. Photooxidase Activity of Heated Chromatophores of Rhodospirillum rubrum. Plant Physiol. 1962 Mar;37(2):127–129. doi: 10.1104/pp.37.2.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maclean F. I., Forrest H. S., Hoare D. S. Pteridine content of some photosynthetic bacteria. Arch Biochem Biophys. 1966 Oct;117(1):54–58. doi: 10.1016/0003-9861(66)90124-x. [DOI] [PubMed] [Google Scholar]
- NEWTON J. W. A disulfide photoreduction system in chromatophores of Rhodospirillum rubrum. J Biol Chem. 1962 Oct;237:3282–3286. [PubMed] [Google Scholar]
- NISHIMURA M. Studies on bacterial photophosphorylation. IV. On the maximum amount of delayed photophosphorylation induced by a single flash. Biochim Biophys Acta. 1962 May 7;59:183–188. doi: 10.1016/0006-3002(62)90709-6. [DOI] [PubMed] [Google Scholar]
- NOZAKI M., TAGAWA K., ARNON D. I. Noncyclic photophosphorylation in photosynthetic bacteria. Proc Natl Acad Sci U S A. 1961 Sep 15;47:1334–1340. doi: 10.1073/pnas.47.9.1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nugent N. A., Fuller R. C. Carotenoid biosynthesis in Rhodospirillum rubrum: effect of pteridine inhibitor. Science. 1967 Nov 17;158(3803):922–924. doi: 10.1126/science.158.3803.922. [DOI] [PubMed] [Google Scholar]
- OLSON J. M., AMESZ J. Action spectra for fluorescence excitation of pyridine nucleotide in photosynthetic bacteria and algae. Biochim Biophys Acta. 1960 Jan 1;37:14–24. doi: 10.1016/0006-3002(60)90072-x. [DOI] [PubMed] [Google Scholar]
- OLSON J. M., CHANCE B. Oxidation-reduction reactions in the photosynthetic bacterium Chromatium. I. Absorption spectrum changes in whole cells. Arch Biochem Biophys. 1960 May;88:26–39. doi: 10.1016/0003-9861(60)90193-4. [DOI] [PubMed] [Google Scholar]
- OLSON J. M., DUYSENS L. N., KRONENBERG G. H. Spectrofluorometry of pyridine nucleotide reactions in chromatium. Biochim Biophys Acta. 1959 Nov;36:125–131. doi: 10.1016/0006-3002(59)90076-9. [DOI] [PubMed] [Google Scholar]
- ORMEROD J. G., GEST H. Symposium on metabolism of inorganic compounds. IV. Hydrogen photosynthesis and alternative metabolic pathways in photosynthetic bacteria. Bacteriol Rev. 1962 Mar;26:51–66. doi: 10.1128/br.26.1.51-66.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OSNITSKAYA L. K., THRELFALL D. R., GOODWIN T. W. UBIQUINONE-40 AND VITAMIN K-2 (40) IN CHROMATIUM VINOSUM. Nature. 1964 Oct 3;204:80–81. doi: 10.1038/204080b0. [DOI] [PubMed] [Google Scholar]
- Olson J. M. Quantum Efficiency of Cytochrome Oxidation in a Photosynthetic Bacterium. Science. 1962 Jan 12;135(3498):101–102. doi: 10.1126/science.135.3498.101. [DOI] [PubMed] [Google Scholar]
- Orlando J. A., Sabo D., Curnyn C. Photoreduction of Pyridine Nucleotide by Subcellular Preparations from Rhodopseudomonas spheroides. Plant Physiol. 1966 Jun;41(6):937–945. doi: 10.1104/pp.41.6.937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PARSON W. W., RUDNEY H. THE BIOSYNTHESIS OF UBIQUINONE AND RHODOQUINONE FROM P-HYDROXYBENZOATE AND P-HYDROXYBENZALDEHYDE IN RHODOSPIRILLUM RUBRUM. J Biol Chem. 1965 Apr;240:1855–1863. [PubMed] [Google Scholar]
- Parson W. W. Observations on the changes in ultraviolet absorbance caused by succinate and light in Rhodospirillum rubrum. Biochim Biophys Acta. 1967 Jul 5;143(1):263–265. doi: 10.1016/0005-2728(67)90128-4. [DOI] [PubMed] [Google Scholar]
- Parson W. W. The role of P870 in bacterial photosynthesis. Biochim Biophys Acta. 1968 Jan 15;153(1):248–259. doi: 10.1016/0005-2728(68)90167-9. [DOI] [PubMed] [Google Scholar]
- Pfennig N. Photosynthetic bacteria. Annu Rev Microbiol. 1967;21:285–324. doi: 10.1146/annurev.mi.21.100167.001441. [DOI] [PubMed] [Google Scholar]
- Reed D. W., Clayton R. K. Isolation of a reaction center fraction from Rhodopseudomonas spheroides. Biochem Biophys Res Commun. 1968 Mar 12;30(5):471–475. doi: 10.1016/0006-291x(68)90075-2. [DOI] [PubMed] [Google Scholar]
- Schleyer H. Electron paramagnetic resonance studies on photosynthetic bacteria. I. Properties of photo-induced EPR-signals of Chromatium D. Biochim Biophys Acta. 1968 Feb 12;153(2):427–447. doi: 10.1016/0005-2728(68)90085-6. [DOI] [PubMed] [Google Scholar]
- Shavit N., Thore A., Keister D. L., San Pietro A. Inhibition by nigericin of the light-induced pH change in Rhodospirillum rubrum chromatophores. Proc Natl Acad Sci U S A. 1968 Mar;59(3):917–922. doi: 10.1073/pnas.59.3.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAGAWA K., ARNON D. I. Ferredoxins as electron carriers in photosynthesis and in the biological production and consumption of hydrogen gas. Nature. 1962 Aug 11;195:537–543. doi: 10.1038/195537a0. [DOI] [PubMed] [Google Scholar]
- Trebst A., Pistorius E., Baltscheffsky H. p-Phenylenediamines as electron donors for photosynthetic pyridine nucleotide reduction in chromatophores from Rhodospirillum rubrum. Biochim Biophys Acta. 1967 Jul 5;143(1):257–260. doi: 10.1016/0005-2728(67)90126-0. [DOI] [PubMed] [Google Scholar]
- VERNON L. P., ASH O. K. The photo-reduction of pyridine nucleotides by illuminated chromatophores of Rhodospirillum rubrum in the presence of succinate. J Biol Chem. 1959 Jul;234(7):1878–1882. [PubMed] [Google Scholar]
- VERNON L. P. Cytochrome c content of Rhodosperillum rubrum. Arch Biochem Biophys. 1953 Apr;43(2):492–493. doi: 10.1016/0003-9861(53)90144-1. [DOI] [PubMed] [Google Scholar]
- VERNON L. P., KAMEN M. D. Hematin compounds in photosynthetic bacteria. J Biol Chem. 1954 Dec;211(2):643–662. [PubMed] [Google Scholar]
- VERNON L. P., KAMEN M. D. Studies on the metabolism of photosynthetic bacteria. XV. Photoautoxidation of ferrocytochrome c in extracts of Rhodospirillum rubrum. Arch Biochem Biophys. 1953 Jun;44(2):298–311. doi: 10.1016/0003-9861(53)90047-2. [DOI] [PubMed] [Google Scholar]
- VERNON L. P. Photoreduction of pyridine nucleotides by cell-free extracts and chromatophores of Rhodospirillum rubrum. J Biol Chem. 1958 Jul;233(1):212–216. [PubMed] [Google Scholar]
- VREDENBERG W. J., AMESZ J., DUYSENS L. N. LIGHT-INDUCED SPECTRAL SHIFTS IN BACTERIOCHLOROPHYLL AND CAROTENOID ABSORPTION IN PURPLE BACTERIA. Biochem Biophys Res Commun. 1965 Feb 3;18:435–439. doi: 10.1016/0006-291x(65)90727-8. [DOI] [PubMed] [Google Scholar]
- VREDENBERG W. J., DUYSENS L. N. Transfer of energy from bacteriochlorophyll to a reaction centre during bacterial photosynthesis. Nature. 1963 Jan 26;197:355–357. doi: 10.1038/197355a0. [DOI] [PubMed] [Google Scholar]
- Vernon L. P., Garcia A. F. Pigment-protein complexes derived from Rhondospirillum rubrum chromatophores by enzymatic digestion. Biochim Biophys Acta. 1967 Jul 5;143(1):144–153. doi: 10.1016/0005-2728(67)90117-x. [DOI] [PubMed] [Google Scholar]
- Vernon L. P., Ke B., Shaw E. R. Relationship of P700, electron spin resonance signal, and photochemical activity of a small chloroplast particle obtained by the action of Triton X-100. Biochemistry. 1967 Jul;6(7):2210–2220. doi: 10.1021/bi00859a044. [DOI] [PubMed] [Google Scholar]
- Yamanaka T., Kamen M. D. An NADP reductase, an NADH-dye reductase and a non-haem iron protein isolated from a facultative photoheterotroph, Rhodopseudomonas palustris. Biochim Biophys Acta. 1967 Mar 8;131(2):317–329. doi: 10.1016/0005-2728(67)90145-4. [DOI] [PubMed] [Google Scholar]
- ZAUGG W. S. Coupled photoreduction of ubiquinone and photooxidation of ferrocytochrome C catalyzed by chromatophores of Rhodospirillum rubrum. Proc Natl Acad Sci U S A. 1963 Jul;50:100–106. doi: 10.1073/pnas.50.1.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZAUGG W. S., VERNON L. P., TIRPACK A. PHOTOREDUCTION OF UBIQUINONE AND PHOTOOXIDIATION OF PHENAZINE METHOSULFATE BY CHROMATOPHORES OF PHOTOSYNTHETIC BACTERIA AND BACTERIOCHLOROPHYLL. Proc Natl Acad Sci U S A. 1964 Feb;51:232–238. doi: 10.1073/pnas.51.2.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaugg W. S., Vernon L. P., Helmer G. Light-induced electron transfer reactions and adenosine triphosphate formation by Rhodospirillum rubrum chromatophores. Arch Biochem Biophys. 1967 Mar;119(1):560–571. doi: 10.1016/0003-9861(67)90491-2. [DOI] [PubMed] [Google Scholar]
- von Stedingk L. V., Baltscheffsky H. The light-induced, reversible pH change in chromatophores from Rhodospirillum rubrum. Arch Biochem Biophys. 1966 Nov;117(2):400–404. doi: 10.1016/0003-9861(66)90428-0. [DOI] [PubMed] [Google Scholar]


