Significance
MicroRNAs normally function to regulate gene expression through RNA-interference–mediated gene silencing. Here, we demonstrate that microRNAs can inhibit HIV-1 virus production by a novel mechanism not involving RNAi-mediated interference. The new mechanism involves interactions between microRNA and HIV-1 Gag protein’s RNA-binding (nucleocapsid) domain. These interactions prevent Gag proteins from effectively multimerizing into viral complexes at the plasma membrane and lead to inhibition of viral particle production. The microRNA–Gag interactions further result in Gag proteins being redirected into the endocytic pathway where they are degraded in lysosomes. These findings have significant implications for understanding how cells modulate HIV-1 infection and raise the possibility of manipulating total expression levels of host miRNAs to combat HIV-1 replication.
Abstract
MicroRNAs (miRNAs) are small, 18–22 nt long, noncoding RNAs that act as potent negative gene regulators in a variety of physiological and pathological processes. To repress gene expression, miRNAs are packaged into RNA-induced silencing complexes (RISCs) that target mRNAs for degradation and/or translational repression in a sequence-specific manner. Recently, miRNAs have been shown to also interact with proteins outside RISCs, impacting cellular processes through mechanisms not involving gene silencing. Here, we define a previously unappreciated activity of miRNAs in inhibiting RNA–protein interactions that in the context of HIV-1 biology blocks HIV virus budding and reduces virus infectivity. This occurs by miRNA binding to the nucleocapsid domain of the Gag protein, the main structural component of HIV-1 virions. The resulting miRNA–Gag complexes interfere with viral–RNA-mediated Gag assembly and viral budding at the plasma membrane, with imperfectly assembled Gag complexes endocytosed and delivered to lysosomes. The blockade of virus production by miRNA is reversed by adding the miRNA’s target mRNA and stimulated by depleting Argonaute-2, suggesting that when miRNAs are not mediating gene silencing, they can block HIV-1 production through disruption of Gag assembly on membranes. Overall, our findings have significant implications for understanding how cells modulate HIV-1 infection by miRNA expression and raise the possibility that miRNAs can function to disrupt RNA-mediated protein assembly processes in other cellular contexts.
To trigger viral budding, Gag proteins must extensively multimerize at the plasma membrane (PM), forming a tightly packed lattice that remodels into a virus particle containing a few thousand Gag molecules (1). Whereas recruitment of the viral genome by Gag is driven by specific and highly efficient interactions between Gag and the genome’s Psi-element (2, 3), increasing evidence suggests Gag multimerization can also be mediated by nonspecific Gag–RNA interactions. For example, long-stranded RNAs not possessing a Psi-element can drive Gag assembly, serving as a scaffold for concentrating Gag molecules (4–7). In in vitro systems, Gag can multimerize by binding nonspecifically to RNA through its nucleocapsid (NC) domain, with the degree of multimerization proportional to the length of the input RNA (4). Finally, in living cells, Gag binding to either HIV-1 viral RNA or cellular mRNA promotes viral particle assembly (5, 6). The proposed role of nonspecific Gag–RNA interactions in facilitating Gag multimerization and viral budding prompted us to investigate whether miRNAs could prevent viral budding by competing with viral RNA for Gag binding via an unconventional mechanism not involving gene silencing like others have shown (8–11).
Results and Discussion
To test whether miRNAs could block virus production without silencing HIV gene expression, we created a HEK293 cell line stably overexpressing an exogenous human microRNA (miRNA) (i.e., hsa-miR-146a) that is neither present in the parent cell line nor has target sites on HIV transcripts (12–14) (SI Appendix, Fig. S1). This cell line is referred to as miR+. The parent cell line, HEK293, is referred to as wild type (WT). The viral RNA transcript and its encoded viral proteins were expressed in miR+ or WT cells by transiently transfecting either a full-length HIV-1 proviral clone (HIV-1pNL43) or pNL43 derivatives in which the polymerase (pol) and envelope (env) genes were deleted and Gag was tagged with GFP (i.e., pNL43ΔPolΔEnvGag-GFP). The pNL43ΔPolΔEnvGag-GFP construct was always expressed in a 1:3 ratio with pNL43ΔPolΔEnvGag (lacking GFP) to ensure proper viral particle formation (15, 16) (Materials and Methods for details regarding derivative expression).
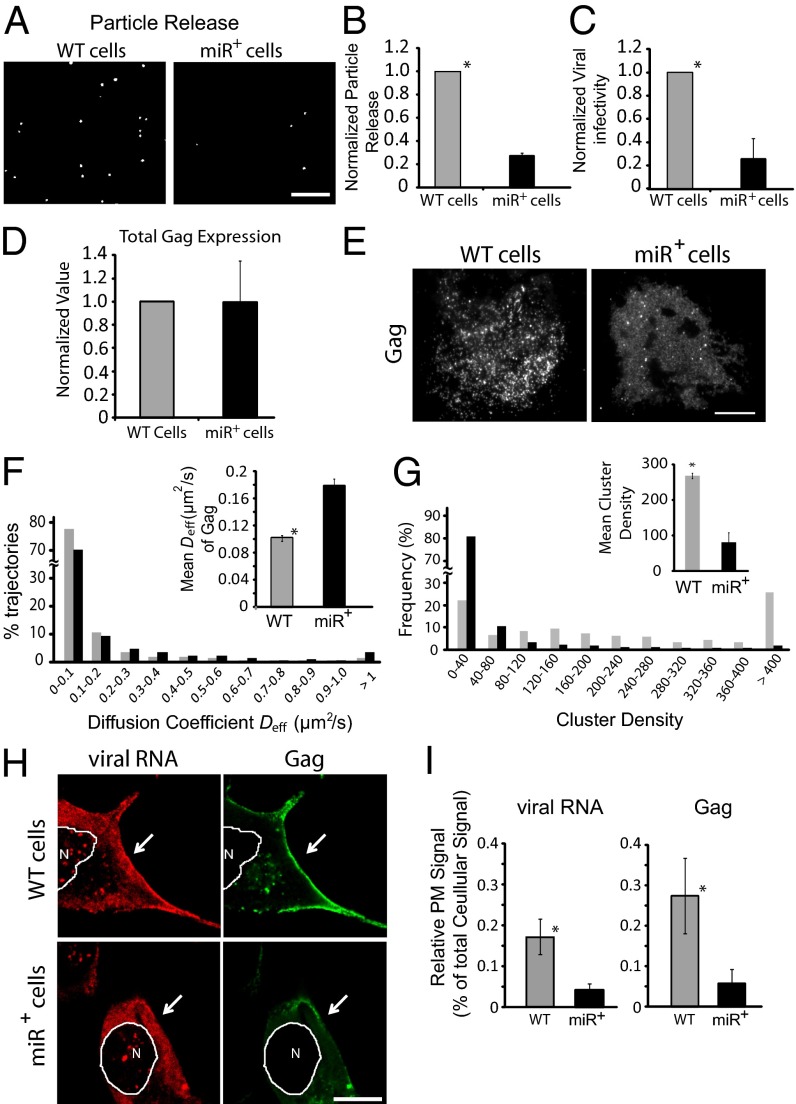
Compared with WT cells or empty-vector control cells, Gag in miR+ cells showed reduced capacity to assemble into particles, assessed by the number of released Gag-GFP particles in the supernatant (Fig. 1 A and B and SI Appendix, Fig. S2). In addition, the viral supernatant from miR+ cells contained fewer infectious virions (Fig. 1C). A reduction in released infectious viral particles was also observed in cells stably expressing a different exogenous miRNA (hsa-miR-888, referred to as miR+888) after transfection of the viral constructs (SI Appendix, Fig. S3). Given the exogenous miRNAs expressed in miR+ and miR+888 cells were derived from different precursor families, with little sequence homology in the seed sequences (SI Appendix, Fig. S4) and no known target sites on the viral transcript (12–14), we concluded that overexpression of exogenous miRNAs can inhibit HIV-1 particle production within cells by a mechanism independent of RNA-interference silencing of HIV-1 protein translation (12–14). Consistent with this, total Gag expression levels after transfection with the viral clones was similar in WT and miRNA-overexpressing cells (Fig. 1D and SI Appendix, Fig. S3).
Fig. 1.
Effect of miRNA overexpression on HIV-1 particle production and Gag dynamics at the PM. (A) Representative images of virus-like particles containing Gag-GFP released from WT or miR+ cells transiently transfected with pNL43ΔPolΔEnvGag-GFP. Released viral particles from equivalent numbers of transfected cells were concentrated and absorbed onto polylysine-coated coverslips for imaging. Fewer viral particles were released from miR+ cells. (B) Quantification of virus particle release from WT or miR+ cells transiently transfected with pNL43ΔPolΔEnvGag-GFP. Plotted is the average number of particles per field in 50–60 different fields acquired from three different experiments, with particle counts normalized to those from WT cell supernatants. (C) Viral infectivity of supernatants from WT or miR+ cells transfected with full-length HIV-1 proviral clone, normalized to WT cell supernatants. (D) Total Gag expression levels in WT or miR+ cells after transfection with the full-length HIV-1 proviral clone, normalized to Gag expression in infected WT cells. (E) Representative TIRF images of Gag at the PM in WT or miR+ cells transfected with pNL43ΔPolΔEnvGag-GFP. (F) spt-PALM measurements of single Gag molecules within Gag complexes diffusing across the PM in WT and miR+ cells transfected with pNL43ΔPolΔEnvGag-mEOS2. The histogram shows the distribution of diffusion coefficients of single Gag complexes. Total trajectories of the WT cells (2,316 tracks) versus miR+ cells (1,693 tracks) were acquired from >10 cells. Inset shows the mean ± SEM diffusion coefficients. (G) Cluster density distribution of Gag at the PM of WT and miR+ cells. WT and miR+ cells were transfected with pNL43ΔPolΔEnvGag-mEOS2 and imaged by PALM. For each cell, the cluster densities are normalized with respect to the mean density of Gag at the PM. Clusters are from more than four cells. Error bars, SEM (n = 1,252 for WT, n = 900 for miR+). (H) Representative images of unspliced HIV-1 viral RNA (detected by FISH) and Gag (detected by Gag-GFP) in WT and miR+ cells transfected with pNL43ΔPolΔEnvGag-GFP. Arrows point to the PM. Note the relative PM depletion of viral RNA and Gag from the PM in miR+ cells compared with WT cells. (I) Quantification of HIV-1 viral RNA and Gag levels at the PM of cells from H. The fraction of PM versus total labeling per cell is depicted (n > 5). All data are represented as mean ± SD of at least three replicate experiments unless otherwise noted. (Scale bar, 10 µm.)
To investigate how viral budding was inhibited in cells overexpressing exogenous miRNAs, we imaged Gag molecules at the PM in WT and miR+ cells by total internal reflection fluorescence (TIRF) microscopy, which restricts the field of view to regions at or close to the PM (Fig. 1E). In WT cells, the majority of Gag molecules appeared within small punctate structures characteristic of viral buds. In miR+ cells, by contrast, few Gag puncta were observed, with most of the PM pool of Gag widely distributed. This suggested that inhibition of viral particle budding in miRNA-overexpressing cells occurs by a mechanism involving disruption of Gag multimerization and coalescence into viral particles at the PM.
Hindrance of Gag multimerization and coalescence by exogenous miRNAs should alter the diffusional mobility of Gag at the PM. To test this, we used photoactivated localization microscopy (PALM), which can reveal protein dynamics and distribution patterns with tens of nanometer precision (17, 18). Gag was tagged with the photoactivable fluorescent protein mEOS2 (Gag-mEOS2) and expressed from the pNL4-3 derivative pNL43ΔPolΔEnvGag-mEOS2 in a 1:3 ratio with pNL43ΔPolΔEnvGag (Materials and Methods). Single-particle tracking PALM (spt-PALM) (18) was then used to measure the diffusional mobility of Gag. Gag’s statistical distribution of diffusion coefficients (Deff) shifted to more rapid diffusion in miRNA-overexpressing cells compared with WT cells (Fig. 1F and SI Appendix, Fig. S5). The mean Deff of Gag at the PM of WT cells was 0.10 ± 0.00 µm2/s (Fig. 1F, Inset and SI Appendix, Fig. S5, Inset), consistent with previous measurements (18). In miR+ and miR+888 cells, by contrast, Gag moved at the PM with an average diffusion coefficient of 0.18 ± 0.01 µm2/s and 0.22 ± 0.02 µm2/s, respectively. Hence, Gag molecules exhibit greater mobility at the PM of cells overexpressing miRNAs.
We next used PALM cluster analysis to characterize the relative distribution and density of Gag molecules at the PM of WT versus miRNA-overexpressing cells (Fig. 1G, SI Appendix, Fig. S6). The mean Gag cluster size in WT cells was 1.64 times greater than that in the miR+ cells, with 75% of Gag molecules associated in clusters in WT cells compared with ∼28% in miR+ cells. Within each cluster, the average density of Gag was 3.3 times greater in WT cells than in miR+ cells. Gag molecules at the PM in miR+888 cells showed a similar distribution pattern as seen in miR+ cells (SI Appendix, Fig. S6). Therefore, the extent that Gag clusters at the PM is reduced in cells overexpressing miRNAs.
The above results indicated that the organization and dynamics of Gag at the PM of miRNA-overexpressing cells is different from WT cells, with Gag less clustered and more highly mobile in miRNA-overexpressing cells. We speculated this was due to expressed miRNAs interfering with the binding of viral RNA to Gag at the PM. To test this possibility, we performed fluorescent in situ hybridization (FISH) to look for any changes in Gag–viral RNA interactions. Notably, less viral RNA and Gag were associated with the PM in miR+ cells compared with WT cells (Fig. 1 H and I). In addition, fewer particles containing viral RNA or Gag-GFP were released by miR+ cells compared with WT cells (SI Appendix, Fig. S7). These findings supported the view that exogenous miRNAs in miRNA-overexpressing cells disrupt viral RNA-mediated assembly of Gag into viral particles by competing with viral RNA for binding to Gag at the PM.
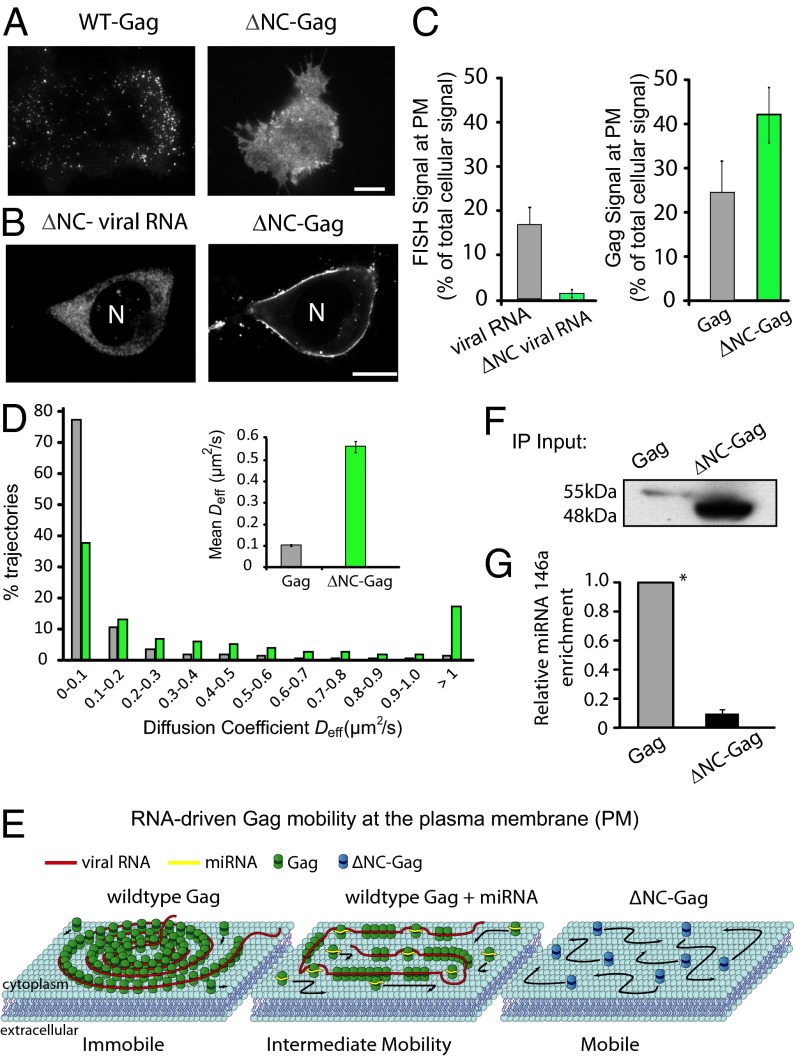
Previous studies have shown that deletion of the NC domain of Gag attenuates viral production (19). To investigate whether this arises from Gag’s failure to undergo viral RNA-mediated clustering into viral-like particles, analogous to that occurring in miRNA-overexpressing cells, we generated an HIV-1 proviral clone containing a mutant Gag-GFP, whose main RNA binding domain (NC) was deleted (ΔNC-Gag-GFP) (see SI Appendix, Gag-ZiL and ΔNC-Gag). We found ΔNC-Gag-GFP (coexpressed with ΔNC-Gag in a 1:3 ratio) in WT cells was more widely distributed and less punctate at the PM than when Gag-GFP was expressed (Fig. 2 A and C). Moreover, in ΔNC-Gag-GFP–expressing cells, very little viral RNA (assessed by FISH of ΔNC viral RNA) colocalized with ΔNC-Gag at the PM (Fig. 2 B and C). In addition, spt-PALM analysis showed ΔNC-Gag to be much more mobile than Gag in WT cells, with mobile ΔNC-Gag molecules having a mean diffusion coefficient of 0.56 ± 0.03 µm2/s compared with Gag molecules having a diffusion coefficient of ∼0.10 µm2/s (Fig. 2D). PALM cluster analysis further revealed that less than 1% of ΔNC-Gag molecules form clusters. Thus, when the NC domain of Gag is deleted, viral RNA recruitment to the PM is inhibited, Gag increases its mobility at the PM, and Gag coalescence into tight clusters is impeded.
Fig. 2.
miRNAs interact with the nucleocapsid (NC) domain of Gag to disrupt Gag assembly. (A) Representative TIRF images of Gag or ΔNC-Gag at the PM in WT cells. (B) WT cells were transfected with pNL43ΔPolΔNC-Gag-GFP, which has the NC domain of Gag deleted. Confocal images of unspliced HIV-1 viral RNA detected by FISH (Left) and ΔNC-Gag-GFP detected by fluorescence (Right). Note that viral RNA is distributed cytoplasmically, whereas ΔNC-Gag is enriched at the PM in these cells. (C) Quantification of HIV-1 viral RNA (assessed by FISH) and Gag (assessed by GFP signal) at the PM in WT cells. Depicted is the fraction of PM versus total labeling per cell. (D) Single-particle tracking PALM analysis of ΔNC-Gag-mEOS2 in WT cells expressing pNL43ΔPolΔNC-Gag-mEOS2. The histogram shows the distribution of lateral diffusion coefficients of single ΔNC-Gag molecules (1,526 tracks) within Gag complexes acquired from 10 cells (green bar), plotted together with the distribution of diffusion coefficients of the WT Gag at the PM of the WT cells (gray bar) as in Fig. 1G. (Inset) Mean ± SEM diffusion coefficients for the three conditions. Note that ΔNC-Gag exhibited the fastest diffusion rate. (E) Schematic model of how miRNA could interfere with Gag assembly at the PM, based on spt-PALM analysis. (F and G) Immunoprecipitation (IP) of miRNA-Gag complexes. miR+ cells were transfected with either pNL43ΔPolΔEnvGag or pNL43ΔPolΔNC-Gag. At 48 h posttransfection, the cells were lysed and immunoprecipitation of Gag or ΔNC-Gag was performed. (F) Representative Western blot image of Gag and ΔNC-Gag proteins immunoprecipated from the miR+ cells. (G) The presence of miR-146a in the IP material was confirmed by qRT-PCR, normalized to the mean ΔNC-Gag protein (E) input (1:7.5). All data are represented as mean ± SD of at least three replicate experiments unless otherwise noted. (Scale bar, 10 µm.)
The above results suggested that Gag dynamics at the PM change depending on whether or not Gag interacts with viral RNA (Fig. 2E). Under conditions where Gag efficiently interacts with viral RNA (i.e., Fig. 2E, Left, WT Gag), Gag–RNA complexes wind up into tight clusters that are immobile. When Gag cannot interact with RNA (i.e., Fig. 2E, Right, ΔNC-Gag), the protein freely diffuses at the PM without clustering. When Gag–RNA interactions are partially disrupted by overexpression of miRNA (i.e., Fig. 2E, Center, WT Gag + miRNA), Gag–RNA complexes do not wind up into tight clusters and exhibit an intermediate mobility between ΔNC-Gag and Gag in WT cells. Evidence that exogenous miRNAs in miRNA-overexpressing cells in fact bind to Gag came from RNA-immunoprecipitation experiments demonstrating a binding interaction between Gag and miRNA-146a (Fig. 2 F and G). The quantity of miRNA-146a retrieved by immunoprecipitating Gag from miR+ cells expressing WT Gag was much greater than that observed during immunoprecipitation of ΔNC-Gag from miR+ cells expressing ΔNC-Gag, suggesting miRNA-146a binds to Gag via the protein’s NC domain.
The observed effect of miRNA overexpression on the dynamics of Gag at the PM (i.e., reduced clustering of Gag and decreased budding of Gag particles from the PM), prompted us to examine the fate of Gag in miR+ cells. Fluorescence imaging showed that large vacuoles enriched in Gag were formed over time (Fig. 3A, arrow). These vacuoles ranged in size from 1 to 10 µm in diameter. Gag proteins increasingly appeared in such vacuoles in miR+ cells with time, reaching a plateau in which 50.3 ± 4.6% of the cells contained one or more Gag-positive vacuoles at the end of 48 h (Fig. 3B). Similar results were observed for miR+888 cells (SI Appendix, Fig. S8). By contrast, only 14.3 ± 6.9% of WT cells and 14.1 ± 4.3% of empty vector control cells expressing Gag contained such vacuoles at the end of 48 h (SI Appendix, Fig. S9). Large vacuoles were not present in miR+ or miR+888 cells that were not expressing Gag, suggesting that the vacuoles were induced by Gag expression. Processing of Gag in cells containing exogenous miRNA was decreased relative to WT cells, as expected if Gag assembly at the PM is impaired (SI Appendix, Fig. S10). Thus, under conditions of improperly assembled Gag complexes at the PM in miRNA-overexpressing cells, Gag redistributes into large intracellular vacuoles.
Fig. 3.
miRNA–Gag interactions drive the internalization of Gag complexes at the PM, leading to formation of large, Lamp1-enriched intracellular vacuoles. (A) Representative images of Gag expressed in miR+ cells through pNL43ΔPolΔEnvGag-GFP transfection. Note the appearance of large intracellular vacuoles enriched in Gag. (B) Percentage of cells having large intracellular vacuoles enriched in Gag as a function of time after transfection. (C) Percentage of Gag (expressed via pNL43ΔPolΔEnv) or Gag-ZiL–positive vacuoles (detected by immunofluorescence with anti-p24 antibodies) seen in cells expressing Gag or Gag-ZiL in WT or miR+ cells. (D) Reduction in vacuole formation in response to dynamin-K44A, a dominant-negative mutant that inhibits dynamin-dependent endocytosis. (E) miR+ cells expressing pNL43ΔPolΔEnvGag-GFP were fixed and immunofluorescently labeled with anti–Lamp-1 antibodies to stain lysosomes. Lamp-1 distribution colocalized with Gag-GFP in large intracellular vacuoles, identifying these structures as lysosomes. Nucleus, blue (stained with DAPI). (F) Representative images of miR-146a or unspliced HIV-1 RNA (detected by FISH) and Gag (detected by Gag-GFP) in WT and miR+ cells transfected with pNL43ΔPolΔEnvGag-GFP. Arrows point to the vacuoles. All data are represented as mean ± SD of at least three replicate experiments. Each replicate experiment is performed by visually examining at least 200 transfected cells. (Scale bar, 10 µm.)
Few, if any, Gag-containing vacuoles formed in miR+ cells expressing ΔNC-Gag (Fig. 2B). This suggested that vacuole formation was dependent on miRNA–Gag interactions. To further examine this dependence, we expressed a Gag mutant whose NC domain was replaced with an isoleucine-zipper domain (i.e., Gag-ZiL), expressed using the pNL4-3-ZiLΔPolΔEnv construct. Expressed Gag-ZiL molecules do not bind RNA because they lack the NC domain of Gag. Because of their leucine-zipper domain, however, they can still multimerize and form virus-like particles (19). The Gag-ZiL particles are noninfectious, lacking detectable viral RNA (SI Appendix, Gag-ZiL and ΔNC-Gag). We found that miR+ cells expressing Gag-ZiL had significantly reduced numbers of induced vacuoles (∼13% of cells observed 48 h posttransfection) relative to that seen in miR+ cells expressing Gag (Fig. 3C). In addition, the numbers of Gag-ZiL particles released by WT or miR+ cells expressing Gag-ZiL were similar (SI Appendix, Fig. S11). This indicated that miRNAs do not affect multimerization and budding of Gag-ZiL, which requires only Gag-ZiL’s isoleucine-zipper domain. Therefore, the increased production of large, Gag-enriched vacuoles in miR+ cells requires interactions between miRNA and Gag’s NC domain.
To clarify the nature of the Gag-enriched vacuoles seen in miRNA-overexpressing cells, we tested if they arose by endocytosis of Gag from the PM. Coexpression of HIV-1 constructs and mutant dynamin (i.e., Dyn-K44A, which inhibits severing of clathrin-coated vesicles) in miR+ cells led to a reduction in vacuole formation (Fig. 3D). This indicated that dynamin-dependent endocytosis facilitates Gag accumulation within vacuoles. Immunofluorescent labeling with lysosomal-associated membrane protein (LAMP)-specific antibodies identified the vacuoles as being late endosomal or lysosomal compartments (SI Appendix, Fig. S12 and Fig. 3E). Treatment with the lysosomal protease inhibitor leupeptin led to Gag accumulation inside the vacuoles rather than just at their membrane periphery (SI Appendix, Fig. S13). This suggested that Gag is normally destroyed after being transferred inside the vacuole. FISH experiments further showed that HIV-1 RNA and the miRNA-146a were both enriched within Gag vacuoles, colocalizing with Gag (Fig. 3F and SI Appendix, Fig. S14). Overall, these results revealed that Gag-enriched, lysosome-related vacuoles are formed through endocytosis of Gag complexes containing not only Gag but also HIV-1 RNA and miRNA. This explains the overall reduced levels of Gag at the PM of miRNA-overexpressing cells.
To assess what levels of miRNAs are required to disrupt Gag assembly and cause Gag delivery to vacuoles, we quantified endogenous miRNA levels relative to exogenous miRNA (i.e., miRNA-146a) levels using quantitative RT-PCR (qRT-PCR) analysis (Fig. 4A). miRNA-146a levels were similar to those of several highly abundant endogenous miRNAs (Fig. 4A). This prompted us to examine whether Gag could bind to endogenous miRNAs, not just exogenous miRNAs. Supporting this hypothesis, Gag-miRNA immunoprecipitation experiments revealed that in addition to miRNA-146a, other host miRNA species coimmunoprecipitated with Gag, including miR-17, miR-19, and miR-16 (Fig. 4B), consistent with previous work showing that Gag can nonspecifically bind to nucleic acids (20). Nonetheless, there was a nearly threefold enrichment of miRNA-146a relative to that of the retrieved endogenous miRNAs (i.e., miR-16) in the immunoprecipitate. The similar expression levels of endogenous and exogenous miRNAs within cells, yet significantly different levels in the immunoprecipitates, raised the question of why Gag would preferentially bind to exogenous over endogenous miRNAs.
Fig. 4.
miRNAs disrupt Gag assembly when they do not participate in RNAi activity. (A) Expression levels of the exogenous (exo) miR-146a and endogenous miR-16, -17, and -19 in the miR+ cells. Levels determined by qRT-PCR were normalized to the miR-146a level per cell. Note that miR-146a and miR-16 were expressed at similar levels. (B) Levels of the miR-146a and the endogenous miR-16, -17, and -19 retrieved by immunoprecipitating Gag from the miR+ cells. Note that significantly more miR-146a than miR-16 is retrieved by IP of Gag. (C) Addition of mRNA targets for the miR-146a reduced vacuole formation. miR+ cells were cotransfected with pNL43ΔPolΔEnv and the mRNA target or empty plasmids (Materials and Methods for construct details). At 24 h posttransfection, percentage of Gag expressing miR+ cells containing at least one vacuole were assayed by fluorescence microscopy. (D–F) The effect of knockdown of Ago2, Dicer, and Drosha on vacuole formation and Gag particle release. shRNA-Ago2, shRNA-Dicer, shRNA-Drosha (or shRNA-ctrl, a control shRNA) and pNL43ΔPolΔEnvGag-GFP were cotransfected in WT HEK293 cells. At 24 h posttransfection, Western blot was performed to assess the knockdown efficiency. Ago2 shRNA treatment reduced the levels of Ago2 by ∼50%, whereas Dicer shRNA and Drosha shRNA treatments reduced the Dicer and Drosha levels by 23% and 20%, respectively. Vacuoles and particle release were assayed by fluorescence microscopy as described in Materials and Methods. All data are represented as mean ± SD of at least three replicate experiments. Results were normalized to the number of vacuoles observed in the cells cotransfected with shRNA-ctrl. For the vacuole analysis in C–F, each replicate experiment is performed by visually examining at least 150 transfected cells. (G) Schematic model of Gag–miRNA complexes interfering with Gag assembly at the plasma membrane. In the absence of Gag–miRNA complexes, Gag and the HIV-1 viral RNA form stable complexes at the PM, resulting in viral budding. When Gag–miRNA complexes are present within the viral RNA-mediated assembling complexes, they interfere with proper Gag assembly, resulting in internal vacuole formation.
It is thought that most endogenous miRNAs within cells are bound to RNA-induced silencing complexes (RISCs), forming miRNA-RISCs that regulate the expression of a variety of host cellular genes (21). Therefore, most endogenous miRNAs within cells should be unable to interact with Gag at the PM to interfere with Gag assembly. By contrast, exogenous miRNAs that we introduced in HEK293 cells with no known mRNA targets would be available to bind Gag and interfere with Gag assembly. To test this idea, plasmid constructs encoding target mRNAs for miRNA-146a were coexpressed with Gag-GFP–expressing HIV-1 proviral clones in miR+ cells. Our assumption was that if our hypothesis was correct, then in these cells fewer Gag proteins should be internalized and delivered into vacuoles because miRNA-146a would interact with the expressed target mRNA within silencing machinery rather than with Gag at the PM. Consistent with this possibility, we found that fewer Gag vacuoles formed in miR+ cells expressing Gag plus the target mRNA for miRNA-146a compared with miR+ cells expressing Gag plus the empty vector control (Fig. 4C). Exogenous miRNAs, therefore, may interact with Gag more efficiently than endogenous miRNAs because they lack mRNA targets.
The above findings led us to investigate whether depletion of Argonaute 2 (Ago2), which binds miRNAs to form RISCs, makes the endogenous miRNAs available for interacting with Gag to disrupt viral assembly. Supporting this possibility, knockdown of Ago2 led to increased numbers of Gag-enriched intracellular vacuoles and decreased viral particle formation in WT HEK293 cells expressing Gag (Fig. 4D and SI Appendix, Fig. S15). The effects were analogous to those seen in Gag-expressing cells overexpressing foreign miRNAs. Gag-enriched intracellular vacuoles were also seen in Gag-expressing WT HEK293T and HeLa cells after knockdown of Ago2 (SI Appendix, Fig. S16). Endogenous miRNAs, therefore, may also interact with Gag to disrupt Gag assembly when not packaged with RISCs.
To test this idea further, we depleted proteins necessary for the biogenesis of miRNAs, including Dicer or Drosha (22). We reasoned that in cells depleted of either Dicer or Drosha, there would be few miRNAs available to bind to Gag and disrupt viral assembly. Supporting this hypothesis, partial knockdown of Dicer or Drosha led to reduced numbers of Gag-enriched intracellular vacuoles but enhanced viral particle formation in WT HEK293 cells expressing Gag (Fig. 4 E and F). This supported the idea that any miRNA when not mediating RNAi activities can obstruct viral assembly via miRNA–Gag interactions.
In summary, our results suggest that miRNA, when unbound to silencing machinery, can bind to Gag proteins and disrupt Gag clustering into viral particles at the PM (Fig. 4G). Improperly assembled Gag complexes so formed undergo enhanced endocytic delivery to lysosomes. This effect of miRNA on Gag assembly is more pronounced in cells that have miRNAs not engaged in RNAi activities. These findings linking miRNA with Gag physiology have significant implications for understanding how cells modulate HIV-1 infection. Gene expression is increasingly known to occur stochastically, with cell-to-cell variations in mRNA and protein levels (23–27). Moreover, transcription rates often exhibit pulsatile variations (23–27). Production of miRNAs may therefore sometimes be out of sync with mRNA transcription, leading to buildup of miRNAs not bound to mRNA or RISCs. The RISC-free miRNAs could then become potent inhibitors of viral production and regulators of Gag physiology. In this context, expression of total miRNAs may play an equal if not a more important role than specific miRNAs in regulating HIV-1 biogenesis. Given the ever-growing development of antiviral agents designed to target HIV-1 assembly, our results suggest the possibility of manipulating total expression levels of host miRNAs to combat HIV-1 transmission and infectivity.
Materials and Methods
Cell Culture.
Human embryonic kidney 293 (293), 293T, HeLa cell lines (American Type Culture Collection), the HIV reporter cell line TZM-bl [National Institutes of Health (NIH) AIDS Research and Reference Reagent Program, Germantown, MD], and miRNA-overexpressing cells (miR+ and miR+888) were cultured in Dulbecco’s modified eagle medium (DMEM; Mediatech), supplemented with 10% (vol/vol) FBS (Invitrogen) at 37 °C, 5% (vol/vol) CO2, and 90% relative humidity. All experiments were performed with cells at passage numbers between 10 and 25.
miRNA Expression Plasmids.
Precursor miR-146a expression plasmid (backbone pBApo-CMV Neo) was described previously (28). Precursor miR888 expression vector was constructed by PCR amplification of the human genomic DNA with forward primer ACCTGCGGGATCCCTTCTGGTCCTGGCAATCAT and reverse primer ACTGCTAAGCTTACCAGTCTGAGGACCACCAC. The PCR product was then inserted into pBApo-CMV Neo digested with BamH1 and HindIII. The neo-resistant miRNA constructs also served as the PCR templates for constructing hygromycin-resistant precursor miRNA expression vectors. For the hygro precursor miR-146a vector, forward and reverse primers were ACCTGCGCTAGCTTTACAGGGCTGGGACAGG and ACTGCTGCGGCCGCAAGCTCTTCAGCAGACTGAAAA. For the hygro precursor-miR-888 vector, the forward and reverse primers were ACCTGCGCTAGCCTTCTGGTCCTGGCAATCAT and ACTGCTGCGGCCGCACCAGTCTGAGGACCACCAC, respectively. The resulting PCR products were then inserted into the NheI and NotI sites of Hyg-N1, constructed by excising out the sequences encoding DsRed-Monomer from the pDsRed-Monomer-Hyg-N1 vector with NheI and NotI. The functionality of all precursor miR constructs were confirmed by miR-reporter assays (see below).
miR-Reporter Constructs.
The construction of miRNA reporter construct for miR-146a was described previously (28). miRNA reporter construct for miR-888 was constructed similarly by introducing multiple tandem repeats of binding sites perfectly complementary to miR888 into the NotI site of psiCHECK2 (Promega). For the miR888 reporter construct, the following sequences were inserted: GCGGCCGCTGACTGACAGCTTTTTGAGTAAAGAATTCTTTGACTGACAGCTTTTTGAGTAAAGAATTCTTTGACTGACAGCTTTTTGAGTAGCGGCCGC. (The underlined sequence is complementary to miR888.)
Viral Constructs.
The pNL43 proviral construct (29) was used to construct other pNL43 derivatives used in this study. These constructs including pNL43ΔPolΔEnv, pNL43ΔPolΔEnvGag-GFP, pNL43ΔPolΔEnvGag-mEOS2, pNL4-3ΔNCΔPolΔEnv, pNL43ΔNCΔPolΔEnvGag-GFP, pNL43ΔNCΔPolΔEnvGag-GFP, ΔNCΔPolGag-mEOS2, and pNL43ZiLΔPolΔEnv are described below.
The pNL43ΔPolΔEnv was constructed by subcloning the SpeI–SalI and SalI–BamHI regions from pNL43 into pBluescript (SK+), deleting the BclI–NsiI and NsiI–BglII regions from the pol and env genes, respectively, and inserting the resulting SpeI–SalI and SalI–BamHI regions back to pNL43. The pNL43ΔPolΔEnvGag-GFP was constructed by inserting GFP from pNL4-3/iGFP [a kind gift from Benjamin Chen (Mount Sinai School of Medicine, New York)] into the BssHII–SpeI region of pNL43ΔPolΔEnv. The pNL43ΔPolΔEnvGag-mEOS2 was constructed by amplification of pCMV-mEosFP2-C1 with BsiE-AclI flanking primers. The amplified PCR products were subcloned into pBluescript (SK+) containing the HindIII–SpeI region of pNL43, where the synthetic cleavage site at the C terminus of the matrix domain and the cloning sites (BstBI and PacI) were introduced. The inserted sequences are indicated in uppercase in the following: gtcTCGCAGAACTATCCAATTGTACAATTCGAAGGTTAATTAagccaa (the sequences for the synthetic cleavage site are in italics and the restriction sites are underlined). The resulting partial fluorescent proteins (FPs) were cloned into pNL43ΔPolΔEnv by insertion into the BssHII–SpeI site. The pNL43ΔPolΔEnvΔNC was made by subcloning the SpeI–EcoRI region from pNL43 ΔNC [a kind gift from David Ott (National Cancer Institute, Frederick, MD)], deleting the BcII–NsiI fragment of the Pol region, and cloning into pNL43ΔPolΔEnv by insertion into the SpeI–EcoRI site. The pNL43ΔPolΔEnvΔNC-Gag-GFP was constructed by insertion of the SpeI–EcoRI site from pNL43 ΔPolΔEnvΔNC into pNL43ΔPolΔEnvGag-GFP. The pNL43ΔPolΔNC-Gag-mEOS2 was constructed by insertion of the SpeI–EcoRI fragment from pNL43ΔPolΔEnvΔNC into pNL43/imEosFP2. The pNL4-3 Zil ΔPolΔEnv construct was made by amplifying the SpeI–ApaI region of Zil-p1p6 (30) and cloning into pNL43ΔPolΔEnv.
shRNA Constructs.
pTER-shAgo2 and pTER-shDicer plasmids were kind gifts from P. Svoboda (Friedrich Miescher Institute for Biomedical Research, Basel, Switzerland). The shRNA Drosha plasmid was a kind gift from Tyler Jacks (Massachusetts Institute of Technology, Cambridge, MA). To construct the pTER-shCtrl plasmid, the sequences encoding shAgo2 in the pTER-shAgo2 were replaced with sequences AAGAGATAGTTGCGGACAATCTGACTTTTTT using BglII and HindIII restriction sites.
Transfection.
Transfection was performed with Fugene 6 (Promega) unless otherwise noted as per manufacturer’s protocol when cells reached 50–70% confluency. For fluorescent microscopic studies where FPs were used, cells seeded on clean coverslips or labtek chambers (Nunc) were transfected with a mixture containing both FP-tagged and untagged PNL43 derivative constructs at a 1:3 ratio. This is to avoid potential morphological defects in Gag assembly caused by expressing Gag tagged with FP alone. For shRNA knockdown studies, equal amounts of shRNA constructs and pNL43 derivative constructs were transfected.
Establishment of Cell Lines Stably Expressing Exogenous miRNAs.
HEK293 cell lines stably expressing hsa-miR-146a or hsa-miR888 were generated by two successive selections using miRNA expressing plasmids with neomycin- and hygromycin-resistant genes. This allowed the establishment of stable cell lines with high miRNA activity. First, cells transfected with miRNA-expressing vectors (derived from neomycin-resistant plasmids) were cultured in media containing 1–2 mg/mL geneticin. After 2 wk, single colonies were isolated, maintained in 0.5–1 mg/mL geneticin for 3 wk, and thereafter cultured without geneticin. The clone that exhibits the highest miRNA activity (as determined by miR-reporter assay) was further used to generate double stable cell lines by further transfecting it with hygromycin-resistant plasmids containing hsa-miR-146a or hsa-miR888. After selection in culture media containing 20–60 ng/mL hygromycin, single colonies were isolated and maintained in 10–30 ng/mL hygromycin for 3 wk and thereafter cultured without any antibiotics. The colony with the highest microRNA activity was selected for further studies.
Assessment of Total Gag Expression Levels.
Radioimmunoprecipitation assays were carried out as previously described (31). Briefly, WT HEK293 and miRNA-expressing cells were transfected with the HIV-1 molecular clone pNL4-3 by using Lipofectamine 2000 reagent (Invitrogen). At 24 h posttransfection, HIV-1–expressing cells were starved in [35S]Met/Cys-free medium (MPBio) for 30 min and metabolically labeled for 2 h with [35S]Met/Cys-Promix (Perkin-Elmer). Virions released into the medium were collected by ultracentrifugation at 100,000 × g for 45–60 min. Cell and virus lysates were immunoprecipitated with pooled Ig from HIV-1–infected patients (HIV-Ig) obtained from the NIH AIDS Research and Reference Reagent Program. Immunoprecipitated proteins were separated on 12% (wt/vol) acrylamide gels by SDS/PAGE, exposed to a phosphorimager plate (Fuji), and quantified by Quantity One software (Bio-Rad). In general, Gag detected in the supernatant represents only a minor fraction of total Gag labeled during a 2-h labeling period; therefore, virion-associated Gag is neglected for the measurements of total Gag expression levels. In some measurements, Gag expression was divided by the Envelope (Env) expression to correct for variations in transfection efficiency (SI Appendix, Fig. S17).
Infectivity Assay.
For luciferase-based, single-cycle infectivity assays, viral supernatant from WT cells and miRNA-expressing derivatives transfected with pNL4-3 were used to infect the CD4+/CXCR4+/CCR5+ HeLa cell derivative TZM-bl (obtained from J. Kappes and X. Wu through the AIDS Research and Reference Reagent Program) as previously described (32). Infection was carried out over a range of inputs from 1 to 10 μL of viral supernatant collected from the WT or miRNA-overexpressng cells for 2 h. At 48 h postinfection, cells were lysed and infectivity was measured using the Britelite Plus (Perkin-Elmer) Luciferase Reporter Gene Assay system. Percent infectivity was normalized based on reverse transcriptase (RT) activity. Datasets in the linear range of sensitivity were used as a means to assess the effect of miRNAs on virus infectivity.
miRNA Functional Analysis.
Cells were plated at 50,000 cells per well in a 24-well tissue culture plate overnight. The next day, cells were transfected with 0.5 μg miR-reporter plasmids using Fugene 6 as per manufacturer’s instructions. Twenty-four hours posttransfection, the samples were harvested and luciferase signals were measured using the Dual Luciferase Reporter Assay kit (Promega) following the manufacturer’s instructions. Signals were acquired on a luminometer (LMaxII, Molecular Devices), and the ratio between the Renilla and the firefly luciferase internal control was used to quantify the repression efficiency indicative of the activity of the microRNAs.
Fluorescent Microscopy.
Confocal imaging was performed with a Marianas spinning disk (Intelligent Imaging Innovations) attached to a Zeiss Observer.Z1 microscope (Carl Zeiss) equipped with a 63× and a 100× Plan Apochromat 1.4 NA (Carl Zeiss) objective lens or a Zeiss 710 with a 63× oil-immersion objective. Maximum-intensity projection images were constructed using ImageJ. TIRF imaging was performed on an Elyra PS.1 system (Carl Zeiss) using a 100× 1.46 NA objective and a 488-nm excitation laser (100 mW).
PALM Acquisition.
Eighteen- or 25-mm 1.5 thickness coverslips (Warner Instruments) were cleaned as previously described (33). Cells were grown on cleaned coverslips coated with fibronectin (2 μg × mL−1 in PBS, pH 7.4; Sigma) and transfected with pNL4-3ΔPΔE and pNL4-3-imEOS2-ΔPΔE as described above. For spt-PALM experiments, cells were placed in phenol red free DMEM containing 25 mM Hepes and 1% FBS and imaged at 37 °C. Imaging was performed on either a custom Olympus IX81 microscope with modified TIRF illuminator port and 60× 1.45 NA PlanApoN objective (Olympus) or a commercial TIRF Elyra PS.1 system using a 100× 1.46 NA objective (Carl Zeiss). Fluorescence emission was detected using an EM-CCD camera (Andor Technology, DU-897). Images were obtained by spontaneous photoconversion of mEOS2 probes using 500–900 µW of 561 nm light measured from the rear aperture of the objective. Upon significant depletion of mEOS2, 405 nm light was used at 5–10 µW in 500-ms pulses on intervals of 1–3 min to recover additional tracks. Images were acquired at 20 frames per second.
Spt-PALM Analysis.
Single molecules were localized using either a previously described algorithm written in IDL (Research Systems) (17) or commercially available software (Elyra PS.1 system, Carl Zeiss). Peaks identified in each frame were fit with a cylindrically symmetric Gaussian point spread function. The average localization precision of the peaks ranged between 20 and 35 nm. PALM images were collected under conditions where average distance between peaks in a single frame was substantially larger than the maximum distance that a single molecule is expected to move between two consecutive frames. The rest of the analysis was performed using code custom written in MATLAB (Mathworks). Single molecule peaks localized to better than 25 nm were only used for the final analysis. For single molecules diffusing with an average diffusion coefficient of 0.1 μm2/s (previously reported diffusion coefficient of Gag), 99% of molecules are expected to move less than 300 nm between two consecutive frames. Peaks present in consecutive frames within a distance of 300 nm were assigned to the same trajectory, representing the successive positions of the same molecule. Trajectories containing at least 15 steps were selected for calculating the average short-term diffusion coefficient (Deff). The mean square displacement (MSD) corresponding to different time lags (Δτ) was calculated by averaging the MSD over overlapping time windows. Finally, the Deff of each trajectory was obtained from a linear fitting of MSD vs. Δτ plot, using the first five time lags (0 < Δτ < 250 ms).
Cluster Analysis.
The single molecule localizations (with localization precision 30 nm or less) from all of the acquisition frames of a PALM time series of live cell (10,000 frames acquired over 8.3 min at 20 frames per second) were combined to form a composite, superresolution image. Hoshen–Kopelman algorithm (34)-based cluster analysis was performed on this composite image to identify individual clusters of Gag molecules. Briefly, Gag molecules with neighboring molecules within a distance of 300 nm were identified and grouped together with all of the shared neighbors into the same cluster. From the set of clusters obtained from this operation, only clusters with a radius less than or equal to 150 nm and density greater than three times the average density of Gag over the plasma membrane were considered as Gag platforms (nascent viral buds) arising from oligomerization of Gag at the plasma membrane. Next, the size of each cluster was determined by calculating the convex hull (the smallest convex set) for the set of molecules belonging to the cluster. The area of the convex hull and the radius of a circle of equivalent area (as the convex hull) were used as estimates of cluster area and cluster radius, respectively. The density of Gag-mEos2 within the convex hull was normalized with respect to the average density of Gag-mEos2 over the entire plasma membrane of the cell to obtain the cluster density.
Quantification of Particle Release.
Supernatant containing particles encapsidating Gag and the Gag-GFP were harvested 48 h posttransfecton. Cell debris and large aggregates were removed by centrifugation at 200 × g for 10 min followed by filtration through a 0.45-μm filter. Subsequently, an aliquot of the purified supernatant was incubated on a polylysine-coated coverslip at room temperature for 1 h before being imaged and/or subjected to fluorescent in situ hybridization according to procedures below. All images were processed by setting an appropriate intensity threshold in the GFP channel to obtain a good contrast. The same threshold setting was used for an entire dataset, which included at least 50 unique fields of view. Images were analyzed using the “analyze particles” command on NIH ImageJ.
Fluorescent in Situ Hybridization of HIV-1 Viral RNA.
Cells were fixed in PBS solution containing 4% (wt/vol) paraformaldehyde for 30 min at room temperature, washed with 1× PBS, and permeabilized at 4 °C in 70% (vol/vol) ethanol overnight. A pool of 48 oligonucleotides (listed in SI Appendix, Table S1), each labeled with a single Quasar 670 dye on the 3′ end and complementary to a different region of the target HIV-1 mRNA, were designed using custom FISH design software and manufactured by Biosearch Technologies. FISHs were performed according to the manufacturer’s protocol with slight modifications. In brief, after overnight permeabilization in 70% ethanol, the cells were washed with wash buffer [2× SSC, 10% (vol/vol) formamide] and then incubated in hybridization buffer [10% (wt/vol) dextran sulfate, 2× SSC, 10% (vol/vol) formamide] containing 500-nM probes for 4 h at 37 °C in a humidified chamber. Slides were washed with wash buffer to remove the unbound probes.
MicroRNA-Fluorescent in Situ Hybridization.
After the fixation and permeabilization step as described above, the cells were wash once with 0.2% (wt/vol) glycine/TBS, twice with 1× TBS, and then crosslinked with l-ethyl-3-(3–dimethylaminopropyl) carbodiimide (EDC) similar to the procedure previously described (35). In brief, cells were incubated twice for 10 min in a freshly prepared solution containing 0.13 M 1-methylimidazole, 300 mM NaCl, pH 8.0 adjusted with HCl. Then 0.16 M EDC (Pierce) was added to the cells and incubated for 1–2 h at room temperature. Before probe hybridization, the slides were washed in 0.2% (wt/vol) glycine/TBS, 1× TBS, and dehydrated in 70% ethanol followed by 99% (vol/vol) ethanol. Hybridization was carried out in hybridization buffer (Exiqon) containing 100 nM of the antisense miR-146a or scrambled locked nucleic acid probes (Exiqon) in a humidified chamber at 55 °C overnight. After stringent washes in 5× SSC, 1× SSC, and 0.2× SSC, the slides were blocked in 1× blocking solution (1% blocking reagent, 100 mM maleic acid, 150 mM NaCl, PH 7.5) (Roche) for 15 min and then incubated with anti-Dig-POD (1:500) in the blocking solution overnight at 4 °C. After washing the slides with wash buffer (0.1 M Tris⋅HCl, 0.15 M NaCl, 0.05% Tween20 pH 7.5), the samples were subject to Cy5-plus tyramide (1:100 dilution of stock solution in 1X Plus Amplification Diluent) using the TSA plus amplification system (Perkin-Elmer) according to the manufacturer’s protocol.
Quantitative RT-PCR of miRNA.
Total RNA was isolated from 106 cells using TRIzol according to the manufacturer's instructions. RNA concentrations were determined using a Cary100 UV-Vis spectrophotometer (Varian). cDNA synthesis was performed in parallel with RNA standards using the Taqman microRNA Reverse Transcription kit according to the manufacturer’s instructions. RNA oligonucleotides with the same sequence as matured miRNA (miRNA-16, miRNA-17, miR-19, miR146a, and miR888) (Integrated DNA Technologies) were used as the standards for miRNA quantification. All PCR experiments were performed using Taqman gene expression assays on an ABI StepOnePlus Real-Time PCR system (Applied Biosystems) according to the manufacturer’s instructions.
Immunoprecipitation of MicroRNA–Gag Complexes.
Immunoprecipitation of microRNA–Gag complexes was performed according to a procedure described previously with modifications (36). In brief, 1 × 107 cells were transiently transfected with 4 μg of pNL43ΔPolΔEnvGag or pNL43ΔPolΔEnvΔNC-Gag constructs. At 2 d posttransfection, cells were harvested in 500 μL of lysis buffer (100 mM KCl/5 mM MgCl2/10 mM Hepes, pH 7.05/0.5% Nonidet P-40/1 mM DTT/100). Following gentle mixing for 30 min at 4 °C, lysate was centrifuged at 16,000 × g for 30 min before being subjected to precleaning with proteinA-agarose beads for 1 h at 4 °C. A total of 500 μL of the precleaned lysates was recovered. HIV-Ig was added at 25 μg/mL of the recovered lysates. After overnight incubation with gentle mixing at 4 °C, 20 μL of 50% (vol/vol) protein-A agarose bead slurry was added to each sample and mixed for 4 h at 4 °C. The beads were then washed with lysis buffer with and without 1 M urea. A total of 50% of the sample was spun down at 16,000 × g for 20 min and the beads were resuspended in elution buffer and an aliquot was taken to determine the input of Gag or ΔNC-Gag by Western blot analysis. The other 50% of the sample was spun down at 16,000 × g for 20 min, resuspended, and incubated in polysome lysis buffer containing 0.1% SDS and 30 μg proteinase K at 50 °C for 30 min. Following phenol–chloroform extraction and ethanol precipitation, RNA was further purified from residual contaminants by lithium chloride precipitation. The purified RNA was subjected to cDNA synthesis and qRT-PCR analysis according to procedures described above.
Data Analysis.
All experiments were repeated at least three times unless otherwise stated. Statistics were performed using one-way ANOVA with post hoc testing of pairwise comparisons using Fisher’s protected least significant difference. Significant difference was taken at the P < 0.05 level.
Supplementary Material
Acknowledgments
The authors thank Dr. Nobuyoshi Kosaka for providing the miR-146a and its reporter plasmids and the members of J.L.-S.’s laboratory for helpful discussions. This project was supported in part by the National Natural Science Foundation of China (Grant 81371613 to A.K.C.) and China’s 1000 Young Talent Award program (A.K.C.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1408037111/-/DCSupplemental.
References
- 1.Briggs JA, et al. The stoichiometry of Gag protein in HIV-1. Nat Struct Mol Biol. 2004;11(7):672–675. doi: 10.1038/nsmb785. [DOI] [PubMed] [Google Scholar]
- 2.De Guzman RN, et al. Structure of the HIV-1 nucleocapsid protein bound to the SL3 psi-RNA recognition element. Science. 1998;279(5349):384–388. doi: 10.1126/science.279.5349.384. [DOI] [PubMed] [Google Scholar]
- 3.Amarasinghe GK, et al. NMR structure of the HIV-1 nucleocapsid protein bound to stem-loop SL2 of the psi-RNA packaging signal. Implications for genome recognition. J Mol Biol. 2000;301(2):491–511. doi: 10.1006/jmbi.2000.3979. [DOI] [PubMed] [Google Scholar]
- 4.Campbell S, Vogt VM. Self-assembly in vitro of purified CA-NC proteins from Rous sarcoma virus and human immunodeficiency virus type 1. J Virol. 1995;69(10):6487–6497. doi: 10.1128/jvi.69.10.6487-6497.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Muriaux D, Mirro J, Harvin D, Rein A. RNA is a structural element in retrovirus particles. Proc Natl Acad Sci USA. 2001;98(9):5246–5251. doi: 10.1073/pnas.091000398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jouvenet N, Simon SM, Bieniasz PD. Imaging the interaction of HIV-1 genomes and Gag during assembly of individual viral particles. Proc Natl Acad Sci USA. 2009;106(45):19114–19119. doi: 10.1073/pnas.0907364106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kutluay SB, Bieniasz PD. Analysis of the initiating events in HIV-1 particle assembly and genome packaging. PLoS Pathog. 2010;6(11):e1001200. doi: 10.1371/journal.ppat.1001200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eiring AM, et al. miR-328 functions as an RNA decoy to modulate hnRNP E2 regulation of mRNA translation in leukemic blasts. Cell. 2010;140(5):652–665. doi: 10.1016/j.cell.2010.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fabbri M, et al. MicroRNAs bind to Toll-like receptors to induce prometastatic inflammatory response. Proc Natl Acad Sci USA. 2012;109(31):E2110–E2116. doi: 10.1073/pnas.1209414109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lehmann SM, et al. An unconventional role for miRNA: let-7 activates Toll-like receptor 7 and causes neurodegeneration. Nat Neurosci. 2012;15(6):827–835. doi: 10.1038/nn.3113. [DOI] [PubMed] [Google Scholar]
- 11.Vickers KC, Palmisano BT, Shoucri BM, Shamburek RD, Remaley AT. MicroRNAs are transported in plasma and delivered to recipient cells by high-density lipoproteins. Nat Cell Biol. 2011;13(4):423–433. doi: 10.1038/ncb2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang J, et al. Cellular microRNAs contribute to HIV-1 latency in resting primary CD4+ T lymphocytes. Nat Med. 2007;13(10):1241–1247. doi: 10.1038/nm1639. [DOI] [PubMed] [Google Scholar]
- 13.Nathans R, et al. Cellular microRNA and P bodies modulate host-HIV-1 interactions. Mol Cell. 2009;34(6):696–709. doi: 10.1016/j.molcel.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sun G, et al. Interplay between HIV-1 infection and host microRNAs. Nucleic Acids Res. 2012;40(5):2181–2196. doi: 10.1093/nar/gkr961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jouvenet N, Bieniasz PD, Simon SM. Imaging the biogenesis of individual HIV-1 virions in live cells. Nature. 2008;454(7201):236–240. doi: 10.1038/nature06998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Larson DR, Johnson MC, Webb WW, Vogt VM. Visualization of retrovirus budding with correlated light and electron microscopy. Proc Natl Acad Sci USA. 2005;102(43):15453–15458. doi: 10.1073/pnas.0504812102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Betzig E, et al. Imaging intracellular fluorescent proteins at nanometer resolution. Science. 2006;313(5793):1642–1645. doi: 10.1126/science.1127344. [DOI] [PubMed] [Google Scholar]
- 18.Manley S, et al. High-density mapping of single-molecule trajectories with photoactivated localization microscopy. Nat Methods. 2008;5(2):155–157. doi: 10.1038/nmeth.1176. [DOI] [PubMed] [Google Scholar]
- 19.Accola MA, Strack B, Göttlinger HG. Efficient particle production by minimal Gag constructs which retain the carboxy-terminal domain of human immunodeficiency virus type 1 capsid-p2 and a late assembly domain. J Virol. 2000;74(12):5395–5402. doi: 10.1128/jvi.74.12.5395-5402.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rein A, Datta SA, Jones CP, Musier-Forsyth K. Diverse interactions of retroviral Gag proteins with RNAs. Trends Biochem Sci. 2011;36(7):373–380. doi: 10.1016/j.tibs.2011.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bartel DP. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell. 2004;116(2):281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 22.Kim VN. MicroRNA biogenesis: Coordinated cropping and dicing. Nat Rev Mol Cell Biol. 2005;6(5):376–385. doi: 10.1038/nrm1644. [DOI] [PubMed] [Google Scholar]
- 23.Muramoto T, et al. Live imaging of nascent RNA dynamics reveals distinct types of transcriptional pulse regulation. Proc Natl Acad Sci USA. 2012;109(19):7350–7355. doi: 10.1073/pnas.1117603109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Raj A, van Oudenaarden A. Nature, nurture, or chance: Stochastic gene expression and its consequences. Cell. 2008;135(2):216–226. doi: 10.1016/j.cell.2008.09.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chubb JR, Trcek T, Shenoy SM, Singer RH. Transcriptional pulsing of a developmental gene. Curr Biol. 2006;16(10):1018–1025. doi: 10.1016/j.cub.2006.03.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Larson DR, Singer RH, Zenklusen D. A single molecule view of gene expression. Trends Cell Biol. 2009;19(11):630–637. doi: 10.1016/j.tcb.2009.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Raj A, Peskin CS, Tranchina D, Vargas DY, Tyagi S. Stochastic mRNA synthesis in mammalian cells. PLoS Biol. 2006;4(10):e309. doi: 10.1371/journal.pbio.0040309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kosaka N, et al. Secretory mechanisms and intercellular transfer of microRNAs in living cells. J Biol Chem. 2010;285(23):17442–17452. doi: 10.1074/jbc.M110.107821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Adachi A, et al. Production of acquired immunodeficiency syndrome-associated retrovirus in human and nonhuman cells transfected with an infectious molecular clone. J Virol. 1986;59(2):284–291. doi: 10.1128/jvi.59.2.284-291.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Popova E, Popov S, Göttlinger HG. Human immunodeficiency virus type 1 nucleocapsid p1 confers ESCRT pathway dependence. J Virol. 2010;84(13):6590–6597. doi: 10.1128/JVI.00035-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Waki K, et al. Structural and functional insights into the HIV-1 maturation inhibitor binding pocket. PLoS Pathog. 2012;8(11):e1002997. doi: 10.1371/journal.ppat.1002997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Waheed AA, et al. Inhibition of HIV-1 replication by amphotericin B methyl ester: Selection for resistant variants. J Biol Chem. 2006;281(39):28699–28711. doi: 10.1074/jbc.M603609200. [DOI] [PubMed] [Google Scholar]
- 33.Sengupta P, et al. Probing protein heterogeneity in the plasma membrane using PALM and pair correlation analysis. Nat Methods. 2011;8(11):969–975. doi: 10.1038/nmeth.1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hoshen J, Kopelman R. Percolation and cluster distribution. 1. Cluster multiple labeling technique and critical concentration algorithm. Phys Rev B. 1976;14(8):3438–3445. [Google Scholar]
- 35.Pena JT, et al. miRNA in situ hybridization in formaldehyde and EDC-fixed tissues. Nat Methods. 2009;6(2):139–141. doi: 10.1038/nmeth.1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Peritz T, et al. Immunoprecipitation of mRNA-protein complexes. Nat Protoc. 2006;1(2):577–580. doi: 10.1038/nprot.2006.82. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.