Abstract
Mechanical forces influence many aspects of cell behavior. Forces are detected and transduced into biochemical signals by force bearing molecular elements located at the cell surface, in adhesion complexes or in cytoskeletal structures1. The nucleus is physically connected to the cell surface through the cytoskeleton and the linker of nucleoskeleton and cytoskeleton (LINC) complex, allowing rapid mechanical stress transmission from adhesions to the nucleus2. Whereas it has been demonstrated that nuclei experience force3, the direct effect of force on the nucleus is not known. Here we show that isolated nuclei are able to respond to force by adjusting their stiffness to resist the applied tension. Using magnetic tweezers, we found that applying force on nesprin-1 triggers nuclear stiffening that does not involve chromatin or nuclear actin, but requires an intact nuclear lamina and emerin, a protein of the inner nuclear membrane. Emerin becomes tyrosine phosphorylated in response to force and mediates the nuclear mechanical response to tension. Our results demonstrate that mechanotransduction is not restricted to cell surface receptors and adhesions but can occur within the nucleus.
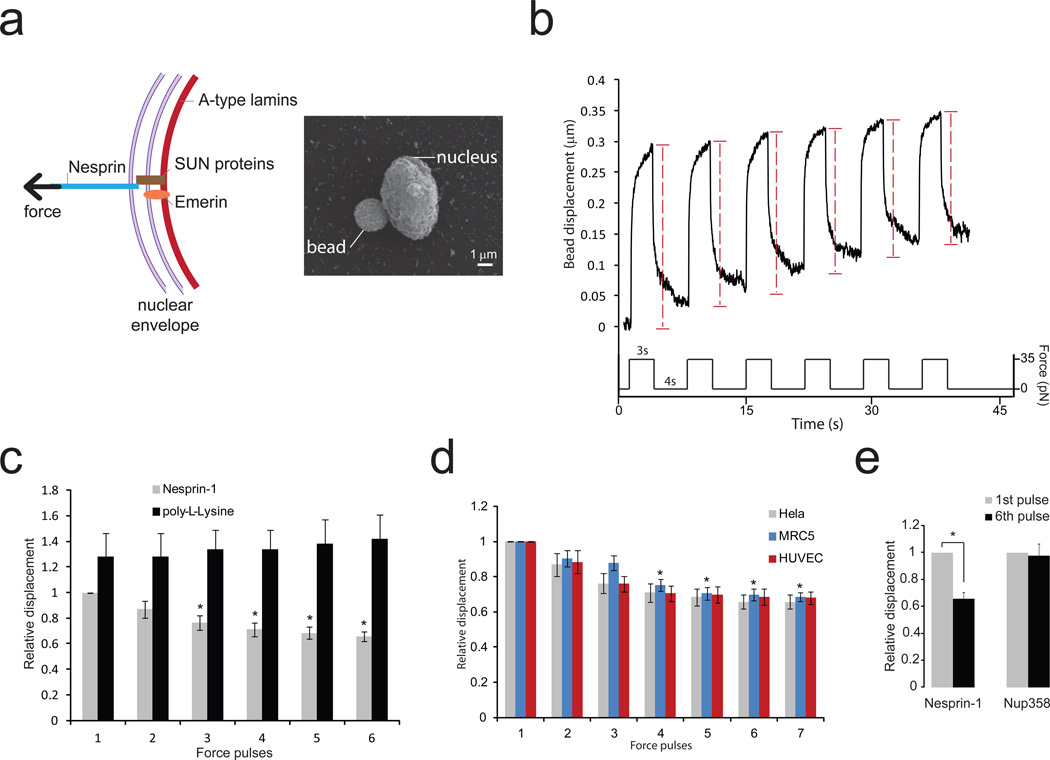
To mimic the transmission of mechanical stress from the cytoskeleton to the nucleus, we applied force directly on isolated nuclei via the LINC complex component nesprin-1 (Figure 1a). We used magnetic tweezers to stimulate magnetic beads coated with anti nesprin-1 antibody and we measured bead displacements due to a known force induced by a magnetic field. Application of successive pulses of constant force triggered an increase in nuclear stiffness, resulting in decreased bead displacement (Figure 1b, Supplementary Figure 1a and Supplementary figure 2). The "relative bead displacement" was calculated by normalizing the displacement for pulses 2, 3, 4, 5 and 6 to that observed during the first pulse. The decrease in bead displacement was significant after the third pulse (Figure 1c) and reached a maximum of 35% after the 6th pulse (Figure 1c). A similar decrease in bead displacement was observed when we stimulated nuclei isolated from endothelial cells or fibroblasts with pulses of force (Figure 1d), whereas no change in bead displacement was observed when beads were coated with poly-L-lysine (Figure 1c) or when pulses of force were applied to nuclear pores using beads coated with anti Nup358 antibody (Figure 1e). These results show that applying tension on the LINC complex triggers a mechanotransduction pathway that adjusts the mechanical properties of the nucleus. We next wanted to investigate the molecular events that mediate this nuclear response to force.
Figure 1. Isolated nuclei stiffen in response to force applied on nesprin-1.
a, Diagram of the LINC complex (left panel) showing where tensional forces were applied in order to mimic the transmission of mechanical stress from the cytoskeleton to the nucleus. Scanning electron microscope picture of a magnetic bead attached to a nucleus isolated from a Hela cell (right panel). Result is representative from 6 independent experiments.
b, Typical displacement of a 2.8 µm bead coated with anti nesprin-1 antibody bound to an isolated nucleus during force pulse application. Stiffening is indicated by decreased displacement during later pulses.
c, Change in bead displacement during 6 force pulses applied to beads coated with anti nesprin-1 antibody (n=18 beads) or poly-L-lysine (n=14 beads) and bound to nuclei isolated from Hela cells. Displacements were calculated relative to the first pulse of force applied to beads coated with anti nesprin-1 (Error bars represent s.e.m., *P<0.05, data were collected from 3 independent experiments and analyzed by one way ANOVA).
d, Change in bead displacement during 7 force pulses applied to beads coated with anti nesprin-1 bound to nuclei isolated from Hela cells (n=18 beads), MRC5 cells (n=21 beads) or HUVECs (n=15 beads). Displacements were calculated relative to the first pulse of force (Error bars represent s.e.m., *P<0.05, data were collected from 3 independent experiments and analyzed by one way ANOVA).
e, Change in bead displacement between the first and 6th pulse of force applied to beads coated with anti nesprin-1 antibody (n=18 beads) or anti NUP358 antibody (n=16 beads) and bound to nuclei isolated from Hela cells. Displacements were calculated relative to the first pulse of force (Error bars represent s.e.m., *P<0.05, data were collected from 3 independent experiments and analyzed by two-tailed unpaired t-test).
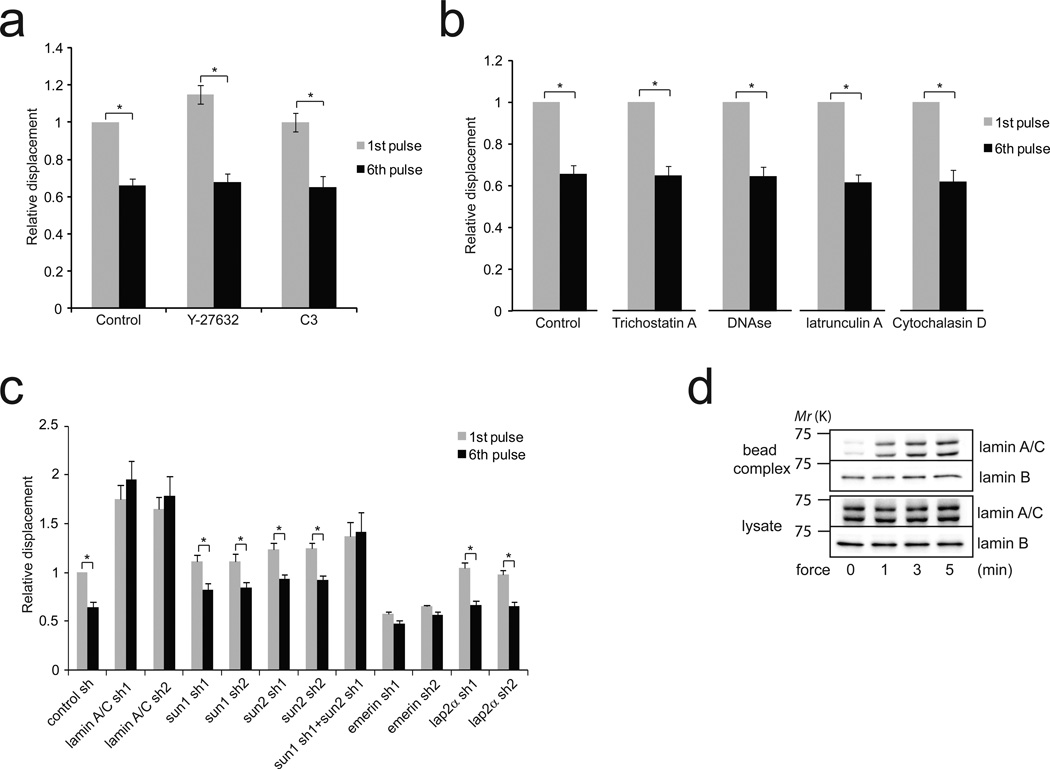
The application of force on integrins induces a local stiffening response 4,5, also called reinforcement6, that involves remodeling of the actin cytoskeleton and that requires the RhoA pathway5,7. Interestingly, both actin and RhoA have been reported to localize to the nucleus8,9. To determine the effect of force on nuclear RhoA activity, we used a permanent magnet to apply constant force on anti nesprin-1 antibody-coated beads. We observed that force on nesprin-1 activates RhoA in isolated nuclei (Supplementary figure 3b), however pharmacological inhibition of Rho or Rho-associated kinase (ROCK), respectively with C3 transferase or Y-27632, did not prevent nuclear stiffening in response to force (Figure 2a). Consistent with this, we found that treatment of nuclei with agents that disrupt actin filaments (latrunculin A or cytochalasin D) did not affect stiffening of isolated nuclei in response to force (Figure 2b). These results indicate that distinct molecular mechanisms regulate the mechanical adaptation to force that occurs at the cell surface and in the nucleus.
Figure 2. The nucleoskeleton mediates nuclear stiffening in response to force.
a, Change in bead displacement between the first and 6th pulse of force applied to beads coated with anti nesprin-1 antibody bound to nuclei treated with Y27632 (n=17 beads) or cell-permeable C3 transferase (n=25 beads) for 30 min. Displacements were calculated relative to the first pulse of force applied to untreated nuclei (Error bars represent s.e.m., *P<0.05, data were collected from 3 independent experiments and analyzed by two-tailed unpaired t-test).
b, Change in bead displacement between the first and 6th pulse of force applied to beads coated with anti nesprin-1 antibody bound to nuclei treated with trichostatin A (n=14 beads), DNAse1 (n=16 beads), latrunculin A (10 µM ; n=19 beads) or cytochalasin D (5 µM ; n=22 beads). Displacements were calculated relative to the first pulse of force (Error bars represent s.e.m., *P<0.05, data were collected from 3 independent experiments and analyzed by two-tailed unpaired t-test).
c, Change in bead displacement between the first and 6th pulse of force applied to beads coated with anti nesprin-1 antibody bound to nuclei isolated from stable cell lines depleted for lamin A/C (sh1 n=12 bead ; sh2 n=17 beads), SUN1 (sh1 n=19 beads; sh2 n=15 beads), SUN2 (sh1 n=18 beads; sh2 n=14 beads), SUN1 sh1+SUN2 sh1(n=14 beads), emerin (sh1 n=21 beads; sh2 n=15 beads) or LAP2α (sh1 n=14 beads; sh2 n=19 beads). Displacements were calculated relative to the first pulse of force applied to nuclei isolated from cells expressing control shRNA (Error bars represent s.e.m., *P<0.05, data were collected from 3 independent experiments and analyzed by two-tailed unpaired t-test).
d, Nuclei isolated from Hela cells were incubated with anti nesprin-1 coated magnetic beads. After stimulation with a permanent magnet for different amounts of time, the nuclei were lysed with detergent (1% NP-40 in Tris buffer). Then, the protein complexes associated with the beads (bead complex) were isolated from the lysate using a magnetic separation stand and both fractions were denatured, reduced in Laemmli buffer and analyzed by western blotting. All results are representative of at least three independent experiments.
Both the nucleoskeleton and chromatin contribute to the mechanical properties of the nucleus10,11. To test whether a change in the mechanical properties of DNA contributes to the nuclear stiffening in response to force, we used nuclei isolated from cells treated with Trichostatin A, a histone deacetylase inhibitor that causes DNA decondensation. Treatment with Trichostatin A did not prevent force-induced nuclear stiffening (Figure 2b), although trichostatin did induce a 2.3 fold increase in the average size of the nuclei. Similar results were obtained when nuclei were treated with DNAse I (Figure 2b), indicating that chromatin and DNA do not participate in the nuclear adaptation to force. Whereas our results show that DNA does not contribute to nuclear stiffening when mechanical stress is applied on the LINC complex, we cannot exclude that force may affect chromatin organization.
In order to determine if the nucleoskeleton mediates the mechanical response of the nucleus to force, we generated stable cell lines depleted for specific nucleoskeletal components using shRNA (Figure 2c – Supplementary Figure 3b) and monitored the change in stiffness of isolated nuclei during pulses of force application. As previously reported by others12, we observed that depletion of lamin A/C decreased nuclear rigidity (Figure 2c). Significantly, we found that nuclei isolated from lamin A/C knockdown cells not only displayed large bead displacements but also failed to stiffen after multiple pulses of force (Figure 2c). This result shows that lamin A/C is a major determinant of the nuclear strain when mechanical stress is applied on nesprin-1. Thus, strengthening the connection between the LINC complex and lamin A/C could potentially decrease nuclear deformation and contribute to stiffening in response to force on nesprin-1. To test this hypothesis, we isolated the LINC complex in nuclei submitted to force. We found that tension induced the recruitment of lamin A/C, but not lamin B to the LINC complex in response to force (Figure 2d), indicating that force on nesprin-1 triggers a reinforcement of the physical connection between lamin A/C and the LINC complex. SUN proteins interact with the KASH domain of nesprins to form the LINC complex and connect nesprins to lamin A/C2,10,13. To test if SUN proteins are required for the nuclear stiffening response, we analyzed the mechanical adaptation of nuclei isolated from SUN1 or SUN2 knockdown cells. We found that nuclei depleted of either SUN1 or SUN2 were still able to significantly increase their stiffness following force application, even though they displayed a decreased stiffening response compared to the control (Figure 2c). Simultaneous knockdown of both SUN1 and SUN2 completely prevented the nuclear response (Figure 2c), suggesting that SUN1 and SUN2 both participate in the force response and may have partially redundant roles, as reported by others 14. Emerin is a LEM-domain containing protein of the inner nuclear membrane that binds lamin A/C and whose depletion has been shown to affect nuclear mechanics15,16. Interestingly, we found that emerin depletion increased nuclear rigidity and prevented the nuclear adaptation to force (Figure 2c), whereas depletion of LAP2α (Figure 2c) or MAN1 (Supplementary Figure 3c–d), two other LEM-domain proteins, did not affect nuclear stiffening.
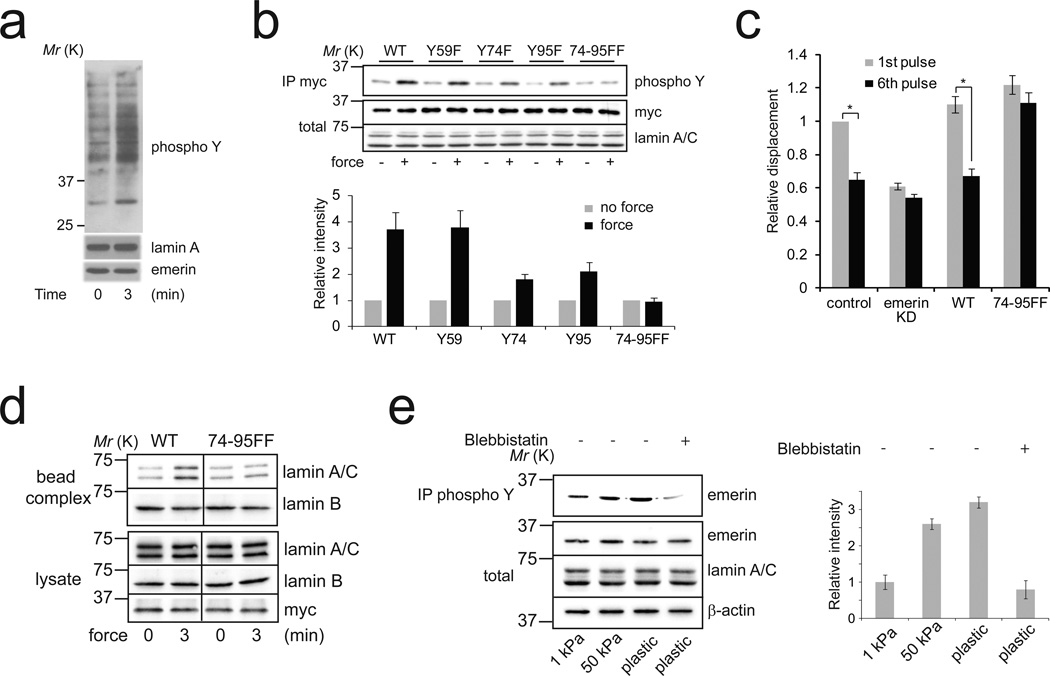
Induction of protein phosphorylation is one of the first events that occurs when mechanical force is applied to cells1,17. To understand the molecular process that regulates the nuclear adaptation to force, we compared tyrosine phosphorylation of nuclear proteins from isolated nuclei subjected to force or not. We found that force moderately stimulates tyrosine phosphorylation of multiple nuclear proteins (Figure 3a), but strongly induces tyrosine phosphorylation of a ~ 35 kD nuclear protein that we identified as emerin (Figure 3a, Supplementary figure 4a). Multiple tyrosine kinases have been described within the nucleus, including Src family kinases (SFKs)18,19, Abl20 and FAK21. To identify the tyrosine kinase which phosphorylates nuclear proteins in response to force, we used pharmacological inhibitors of SFKs, Abl and FAK and analyzed their effects on tyrosine phosphorylation of nuclear proteins induced by applying force on nesprin-1. We found that SFK inhibition (2.5 µM SU66056) prevented force-induced nuclear protein phosphorylation (Supplementary Figure 4b), including emerin phosphorylation, while FAK inhibition (5 µM FAK inhibitor 14) or Abl inhibition (10 µM gleevec) did not affect the increase in tyrosine phosphorylation of nuclear proteins. Additionally, we observed that force increased Src phosphorylation on the activation loop tyrosine (Y416) (Supplementary Figure 4c), indicating that force on nesprin-1 activates Src in isolated nuclei. Using proteomic analysis of emerin phosphorylation, a recent study reported that Src specifically phosphorylates emerin at Y59, Y74 and Y9522. We generated shRNA-resistant emerin mutants with tyrosine to phenylalanine substitution for each of these three residues (Y59F, Y74F and Y95F). We then expressed these mutants in emerin knockdown cells and analyzed their tyrosine phosphorylation in isolated nuclei subjected to force. We found that application of force on nesprin-1 induced phosphorylation of both wild-type (WT) emerin and the Y59F emerin mutant, whereas mutation of Y74 (Y74F) or to a lesser extent mutation of Y95 (Y95F) decreased emerin phosphorylation in response to force (Figure 3b). Consistent with these observations, we found no increase in tyrosine phosphorylation of the double mutant (74-95FF) after force application (Figure 3b). Together these results indicate that force on nesprin-1 activates Src, which, in turn, phosphorylates emerin on Y74 and Y95.
Figure 3. Emerin phosphorylation on Y74 and Y95 mediates the mechanical adaptation of isolated nuclei to force.
a, Nuclei isolated from Hela cells were incubated with anti nesprin-1-coated magnetic beads and stimulated with a permanent magnet for 3 min. Tyrosine phosphorylation of nuclear proteins was analyzed by western blot. All results are representative of at least three independent experiments.
b, Nuclei isolated from emerin knockdown Hela cells re-expressing WT, Y59F, Y74F, Y95F or 74-95FF emerin mutants were incubated with anti nesprin-1-coated magnetic beads and stimulated with a permanent magnet for 3 min. Tyrosine phosphorylation of emerin mutants was analyzed by western blot after immunoprecipitation ("total" refers to the emerin level in nuclear lysates). Corresponding densitometric analysis (lower panel) of emerin phosphorylation normalized to emerin levels and expressed as relative to the control in the absence of stimulation by force (Error bars represent s.e.m, densitometric data were analyzed from n=4 independent experiments).
c, Change in bead displacement between the first and 6th pulse of force applied to beads coated with anti nesprin-1 antibody bound to nuclei isolated from emerin knockdown cells re-expressing WT (n=15 beads) or 74-95FF emerin mutants (n=18 beads). Displacements were calculated relative to the first pulse of force applied to nuclei isolated from emerin knockdown cells (Error bars represent s.e.m., *P<0.05, data were collected from 3 independent experiments and analyzed by two-tailed unpaired t-test).
d, Nuclei isolated from emerin knockdown Hela cells re-expressing WT or 74-95FF emerin mutants were incubated with anti nesprin-1-coated magnetic beads and stimulated with a permanent magnet for 3 min. After stimulation the nuclei were lysed with detergent (1% NP-40 in Tris buffer). Then, the protein complexes associated with the beads (bead complex) were isolated from the lysate using a magnetic separation stand and both fractions were denatured and reduced in Laemmli buffer. All results are representative of at least three independent experiments.
e, Emerin tyrosine phosphorylation was analyzed after immunoprecipitation in MRC5 cells cultured on matrix with different rigidity (polyacrylamide gels of 1 kPa and 50 kPa and plastic) and treated with blebbistatin. ("total" refers to the emerin level in nuclear lysates). Corresponding densitometric analysis (left panel) of emerin phosphorylation normalized to emerin levels and expressed as relative to the 1 kPa condition (Error bars represent s.e.m., densitometric data were analyzed from n=4 independent experiments).
Next, we wanted to test whether emerin phosphorylation on Y74 and Y95 was necessary for the nuclear adaptation to force. As expected, we found that expression of WT emerin in emerin knockdown cells restored the stiffening of isolated nuclei in response to force (Figure 3c). In contrast, nuclei expressing 74-95FF emerin mutant failed to adapt to force (Figure 3c). In line with this, we did not observe lamin A/C recruitment to the LINC complex in response to force in nuclei expressing 74-95FF emerin mutant (Figure 3d) and SFK inhibition prevented the nuclear stiffening in response to force (Supplementary figure 4d). Our results show that Src-dependent emerin phosphorylation on Y74 and Y95 mediates the mechanical adaptation of isolated nuclei to force. However, how emerin phosphorylation affects lamin A/C interaction with the LINC complex remains to be elucidated. Interestingly, SUN2 and emerin interact with the same part of lamin A/C23, suggesting that they may compete for binding to lamin A/C and force-induced emerin phosphorylation may potentially affect SUN2 interaction with lamin A/C and reinforce the connection between nesprin and the nucleoskeleton. Nesprin-1 binds actin filaments and transmits both externally applied force and cell-generated force to the nucleoskeleton. To investigate if emerin phosphorylation is regulated by cell-generated contractility, we analyzed emerin phosphorylation during cell adhesion to fibronectin. Emerin phosphorylation increased during adhesion and this increase was blocked by inhibiting actomyosin contractility with blebbistatin (Supplementary Figure 5a). Substrate rigidity regulates cell contractility; cells on rigid substrates have been shown to exhibit greater contractility than cells plated on soft substrates24. We observed that fibroblasts grown on rigid substrates have increased emerin phosphorylation (Figure 3e). Additionally, we found that application of tensional force on integrin, using fibronectin-coated beads, increased emerin phosphorylation (Supplementary Figure 5b). These results demonstrate that emerin phosphorylation is regulated by cell-generated contractility and externally applied force, and indicate that emerin regulates nuclear rigidity in response to mechanical cues experienced by the whole cell.
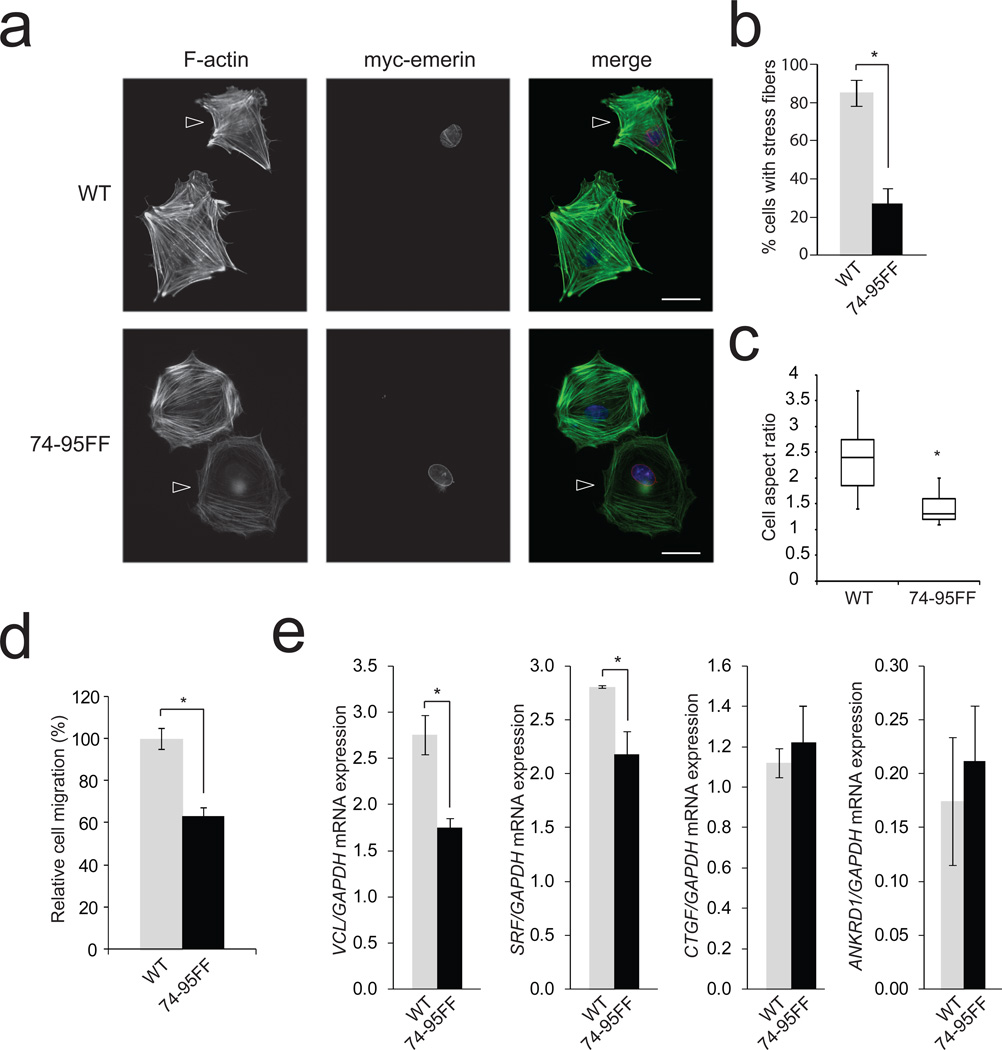
LINC complex components interact with perinuclear actin filaments25,26 and it has been reported that disruption of the LINC complex or depletion of lamin A/C affects the organization of the actin cytoskeleton,23,27,28 presumably because a subset of actin filaments require attachment at the nuclear surface. As emerin phosphorylation on Y74 and 95 regulates the magnitude of strain when tension is applied on the LINC complex, we hypothesized that emerin phosphorylation may be important for anchoring actin filaments to the LINC complex. We found that emerin deficient fibroblasts which expressed the phosphoresistant emerin mutant (74-95FF) displayed less bundled actin filaments (Figure 4a–b). This indicates that nuclear adaptation to force is critical for actin cytoskeletal organization, reinforcing the idea that structural elements are physically interdependent in cells, as proposed previously2,3. Impaired connection of the actin cytoskeleton with the nucleus has been shown to affect polarization and motility23. Remarkably, expression of phosphoresistant emerin 74-95FF resulted in defects in polarization and migration through pores (Figure 4c and 4d).
Figure 4. Emerin phosphorylation on Y74 and Y95 affects stress fiber formation and SRF-dependent gene expression.
a, Emerin knockdown MRC5 cells re-expressing WT or 74-95FF emerin mutant (arrowhead) were grown on fibronectin-coated coverslips, fixed, permeabilized and stained for F-actin (Alexa488-phalloidin) and myc-tagged emerin. Bar scale=25 µm. All results are representative of 4 independent experiments.
b, Cells were treated as above and analyzed for stress fibers. Graph represents the mean of n=64 myc positive cells expressing WT emerin and n=67 myc positive cells expressing 74-95FF. Data were analyzed by a blinded observer (Error bars represent s.e.m., *P<0.05, data were collected from 4 independent experiments and analyzed by two-tailed unpaired t-test).
c, Cells were treated as above and the cell aspect ratio analyzed. A number of n=64 myc positive cells expressing WT emerin and n=67 myc positive cells expressing 74-95FF were analyzed. Box plots indicate median values and capture 50% of data in boxes and 75% between the lines (*P<0.05, data were collected from 4 independent experiments and analyzed by two-tailed unpaired t-test).
d, Invasion of emerin knockdown Hela cells re-expressing WT or 74-95FF emerin mutant was evaluated by Transwell migration assays. Cells were plated in the upper chamber of the filters and after 8 hours cells that had migrated to the underside of the filters were fixed. Relative cell migration was determined by the number of cells that had migrated to the underside of the filter normalized to the total number of cells. A number of n=24 fields were observed per condition. The value from control shRNA Hela cells was arbitrarily set at 100% (Error bars represent s.e.m., *P<0.05 compared to WT, data were collected from 4 independent experiments and analyzed by one way ANOVA).
e, VCL, SRF, CTGF, ANKRD1 and GAPDH mRNA levels were analyzed by real-time qPCR in emerin knockdown MRC5 cells re-expressing WT or 74-95FF emerin mutant. Results are expressed as relative mRNA expression levels (error bars represent s.e.m., *P<0.05, data were collected from n=4 independent experiments and analyzed by two-tailed unpaired t-test).
We next wanted to analyze the effect of emerin phosphorylation on mechanosensitive gene expression. Using real-time qPCR, we first examined serum response factor (SRF)-dependent transcription. We found that expression of phosphoresistant emerin (74-95FF) decreased expression of VCL (vinculin) and SRF (Figure 4e). The transcription regulators YAP and TAZ have been recently described as sensors and mediators of mechanical cues. We observed that emerin deficient fibroblasts which expressed the 74-95FF emerin mutant displayed less nuclear localization of YAP and TAZ (Supplementary Figure 5d). However, we detected no effect on connective tissue growth factor (CTGF) and ankyrin repeat domain 1 (ANKRD1) mRNA levels (Figure 4e), two YAP/TAZ regulated genes. Emerin deficiency has been shown to impact IEX1 expression in response to strain15, interestingly we found that expression of 74-95FF emerin mutant decreased IEX1 basal level but it did not prevent IEX1 induction by tensional force application (Supplementary figure 5e). Our results are consistent with recent findings that emerin regulates megakaryoblastic leukaemia 1 (MKL1, also known as MAL or MRTF) nuclear localization and SRF-dependent transcription29. This previous work indicated that emerin regulates MKL1 signaling by controlling polymerization of nuclear actin29. Whereas we found that nuclear actin did not contribute to the nuclear stiffening observed in response to force (Figure 2b), this previous work raises the possibility that emerin phosphorylation regulates nuclear mechanics and transcription through potentially different pathways.
Nuclear mechanics affect many features of cell behavior including motility28,30, polarity and cell survival23. Previous work showed that nuclear rigidity can be modulated during differentiation11 or in response to long term application of shear stress on cells31. Here we show that isolated nuclei are able to adjust their rigidity within seconds in response to tension, suggesting that nuclei adapt their mechanical properties to the stress they experience, whether it is externally applied to the cell or generated within the cell itself. Our finding that isolated nuclei produce a mechanical response to force suggests that other organelles may similarly contribute to the integrated cellular mechanoresponse. Mechanical stress transmission to the nucleus depends on many factors, including cytoskeletal pre-stress, LINC complex structure and nucleoskeleton organization. All these elements are known to vary substantially between cell types2,11,23, possibly reflecting the need for the nuclei of these cells to respond differently to mechanical cues. Future work will help to determine in which physiological or pathological contexts nuclear mechanotransduction pathways are regulated.
Supplementary Material
ACKNOWLEDGEMENTS
This study was supported by National Institutes of Health Grants #GM029860 (to K.B.), P41-EB002025-23A1 (RS) and R01-HL077546-03A2 (RS), and a grant from the University Cancer Research Fund from the Lineberger Comprehensive Cancer Center. CG is supported by a Marie Curie Outgoing International Fellowship from the European Union Seventh Framework Programme (FP7/2007–2013) under grant agreement n° 254747.
Footnotes
AUTHOR CONTRIBUTIONS:
C.G designed and performed experiments. L. S., L. D. O., L.V.L., R.S. and R.G.M helped with experimental design and procedures. C.G and K.B wrote the manuscript. K.B. directed the project. All authors provided detailed comments.
References
- 1.Hoffman BD, Grashoff C, Schwartz MA. Dynamic molecular processes mediate cellular mechanotransduction. Nature. 2011;475:316–323. doi: 10.1038/nature10316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang N, Tytell JD, Ingber DE. Mechanotransduction at a distance: mechanically coupling the extracellular matrix with the nucleus. Nat Rev Mol Cell Biol. 2009;10:75–82. doi: 10.1038/nrm2594. [DOI] [PubMed] [Google Scholar]
- 3.Maniotis AJ, Chen CS, Ingber DE. Demonstration of mechanical connections between integrins, cytoskeletal filaments, and nucleoplasm that stabilize nuclear structure. Proc Natl Acad Sci U S A. 1997;94:849–854. doi: 10.1073/pnas.94.3.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang N, Butler JP, Ingber DE. Mechanotransduction across the cell surface and through the cytoskeleton. Science. 1993;260:1124–1127. doi: 10.1126/science.7684161. [DOI] [PubMed] [Google Scholar]
- 5.Matthews BD, Overby DR, Mannix R, Ingber DE. Cellular adaptation to mechanical stress: role of integrins Rho, cytoskeletal tension and mechanosensitive ion channels. J Cell Sci. 2006;119:508–518. doi: 10.1242/jcs.02760. [DOI] [PubMed] [Google Scholar]
- 6.Choquet D, Felsenfeld DP, Sheetz MP. Extracellular matrix rigidity causes strengthening of integrin-cytoskeleton linkages. Cell. 1997;88:39–48. doi: 10.1016/s0092-8674(00)81856-5. [DOI] [PubMed] [Google Scholar]
- 7.Guilluy C, et al. The Rho GEFs LARG and GEF-H1 regulate the mechanical response to force on integrins. Nat Cell Biol. 2011;13:724–729. doi: 10.1038/ncb2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hofmann WA, de Lanerolle P. Nuclear actin: to polymerize or not to polymerize. J Cell Biol. 2006;172:495–496. doi: 10.1083/jcb.200601095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dubash AD, et al. The small GTPase RhoA localizes to the nucleus and is activated by Net1 and DNA damage signals. PloS one. 2011;6:e17380. doi: 10.1371/journal.pone.0017380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dahl KN, Kalinowski A. Nucleoskeleton mechanics at a glance. J Cell Sci. 2011;124:675–678. doi: 10.1242/jcs.069096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pajerowski JD, Dahl KN, Zhong FL, Sammak PJ, Discher DE. Physical plasticity of the nucleus in stem cell differentiation. Proc Natl Acad Sci U S A. 2007;104:15619–15624. doi: 10.1073/pnas.0702576104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lammerding J, et al. Lamin A/C deficiency causes defective nuclear mechanics and mechanotransduction. J Clin Invest. 2004;113:370–378. doi: 10.1172/JCI19670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sosa BA, Rothballer A, Kutay U, Schwartz TU. LINC complexes form by binding of three KASH peptides to domain interfaces of trimeric SUN proteins. Cell. 2012;149:1035–1047. doi: 10.1016/j.cell.2012.03.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lei K, et al. SUN1 and SUN2 play critical but partially redundant roles in anchoring nuclei in skeletal muscle cells in mice. Proc Natl Acad Sci U S A. 2009;106:10207–10212. doi: 10.1073/pnas.0812037106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lammerding J, et al. Abnormal nuclear shape and impaired mechanotransduction in emerindeficient cells. J Cell Biol. 2005;170:781–791. doi: 10.1083/jcb.200502148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rowat AC, Lammerding J, Ipsen JH. Mechanical properties of the cell nucleus and the effect of emerin deficiency. Biophys J. 2006;91:4649–4664. doi: 10.1529/biophysj.106.086454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sawada Y, et al. Force sensing by mechanical extension of the Src family kinase substrate p130Cas. Cell. 2006;127:1015–1026. doi: 10.1016/j.cell.2006.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Takahashi A, et al. Nuclear localization of Src-family tyrosine kinases is required for growth factor-induced euchromatinization. Exp Cell Res. 2009;315:1117–1141. doi: 10.1016/j.yexcr.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 19.Chu I, et al. p27 phosphorylation by Src regulates inhibition of cyclin E-Cdk2. Cell. 2007;128:281–294. doi: 10.1016/j.cell.2006.11.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Taagepera S, et al. Nuclear-cytoplasmic shuttling of C-ABL tyrosine kinase. Proc Natl Acad Sci U S A. 1998;95:7457–7462. doi: 10.1073/pnas.95.13.7457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lim ST, et al. Nuclear FAK promotes cell proliferation and survival through FERM-enhanced p53 degradation. Mol Cell. 2008;29:9–22. doi: 10.1016/j.molcel.2007.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tifft KE, Bradbury KA, Wilson KL. Tyrosine phosphorylation of nuclear-membrane protein emerin by Src, Abl and other kinases. J Cell Sci. 2009;122:3780–3790. doi: 10.1242/jcs.048397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ho CY, Lammerding J. Lamins at a glance. J Cell Sci. 2012;125:2087–2093. doi: 10.1242/jcs.087288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Provenzano PP, Keely PJ. Mechanical signaling through the cytoskeleton regulates cell proliferation by coordinated focal adhesion and Rho GTPase signaling. J Cell Sci. 2011;124:1195–1205. doi: 10.1242/jcs.067009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Khatau SB, et al. A perinuclear actin cap regulates nuclear shape. Proc Natl Acad Sci U S A. 2009;106:19017–19022. doi: 10.1073/pnas.0908686106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Luxton GW, Gomes ER, Folker ES, Vintinner E, Gundersen GG. Linear arrays of nuclear envelope proteins harness retrograde actin flow for nuclear movement. Science. 2010;329:956–959. doi: 10.1126/science.1189072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Folker ES, Ostlund C, Luxton GW, Worman HJ, Gundersen GG. Lamin A variants that cause striated muscle disease are defective in anchoring transmembrane actin-associated nuclear lines for nuclear movement. Proc Natl Acad Sci U S A. 2011;108:131–136. doi: 10.1073/pnas.1000824108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khatau SB, et al. The distinct roles of the nucleus and nucleus-cytoskeleton connections in three-dimensional cell migration. Scientific reports. 2012;2:488. doi: 10.1038/srep00488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ho CY, Jaalouk DE, Vartiainen MK, Lammerding J. Lamin A/C and emerin regulate MKL1-SRF activity by modulating actin dynamics. Nature. 2013;497:507–511. doi: 10.1038/nature12105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Friedl P, Wolf K, Lammerding J. Nuclear mechanics during cell migration. Curr Opin Cell Biol. 2011;23:55–64. doi: 10.1016/j.ceb.2010.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Philip JT, Dahl KN. Nuclear mechanotransduction: response of the lamina to extracellular stress with implications in aging. Journal of biomechanics. 2008;41:3164–3170. doi: 10.1016/j.jbiomech.2008.08.024. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.