Abstract
The mammalian olfactory neuroepithelium provides a unique system for understanding the regulation of neurogenesis by adult neural stem cells. Recently, mouse horizontal basal cells (HBCs) were identified as stem cells that regenerate olfactory receptor neurons (ORNs) and non-neuronal cell types only after extensive injury of the olfactory epithelium (OE). Here we report a broader spectrum of action for these cells. We show that even during normal neuronal turnover, HBCs actively generate neuronal and non-neuronal cells throughout adulthood. This occurs in a temporally controlled manner: an initial wave of HBC-derived neurogenesis was observed soon after birth, and a second wave of neurogenesis was observed at 4 months of age. Moreover, upon selective depletion of mature ORNs by olfactory bulbectomy, HBCs give rise to more neurons. Our findings demonstrate a crucial role for HBCs as multipotent progenitors in the adult OE, acting during normal neuronal turnover as well as in acute regeneration upon injury.
Keywords: Stem cell, Multipotent progenitor, Olfactory, Neurogenesis, Cell fate mapping
INTRODUCTION
The mammalian olfactory neuroepithelium has long been known for its unique characteristic of actively generating neurons throughout adulthood, at a rate that by far exceeds neurogenesis in the subventricular zone and dentate gyrus [1–7]. The adult olfactory epithelium (OE) is capable of rapid neuronal regeneration and functional recovery after extensive damage to the tissue, and even under normal physiological conditions, steady-state neurogenesis takes place to continuously replace apoptotic olfactory receptor neurons (ORNs) [4]. This robust regenerative capacity, together with the fact that the OE is a simple neuroepithelium containing only one type of neuron, namely the ORN, offers an ideal system for the study of neural stem cells in the adult.
The OE is a pseudostratified neuroepithelium, structurally and functionally highly conserved among mammals [8, 9]. It consists mainly of ORNs, which migrate to the apical region of the OE as they mature. The dendrites of mature ORNs are exposed to the apical surface of the OE, whereas their axons extend basally and exit the OE, eventually projecting to the olfactory bulb [10]. On the other hand, two types of non-neuronal cells are present in the OE: sustentacular cells and cells of the Bowman’s glands and ducts. Sustentacular cells are neuron-supporting cells and occupy the most apical layer of the OE, whereas Bowman’s glands secrete mucus to the outer surface of the epithelium via ducts that extend through the OE.
The progenitors of the ORN lineage reside in the basal compartment of the OE, which consists of two distinct cell types: globose basal cells (GBCs) and horizontal basal cells (HBCs). HBCs are situated most basally in the OE and are directly attached to the basal lamina, whereas GBCs lie immediately above the HBC layer. GBCs are associated with active proliferation and contain direct ORN precursor cells positive for early neuronal differentiation markers, such as Mash1 and Neurogenin1 [2, 11]. On the other hand, HBCs divide infrequently and express keratin 5 (K5) and keratin 14 (K14) but are negative for neuronal markers [12]. Retroviral lineage studies proposed that ORNs derive from GBCs but not from HBCs, which led to a long-prevailing model holding that the stem cells giving rise to ORNs reside in the GBC population and that HBCs are outside the ORN lineage [13, 14].
However, a recent fate-mapping study in the mouse, by Leung et al., showed that HBCs can regenerate both neuronal and non-neuronal cells after extensive lesioning of the OE by olfactotoxic reagents [15]. However, in intact mice, HBCs remained largely inactive, and selective depletion of mature ORNs by olfactory bulbectomy did not affect the behavior of HBCs. Therefore, it was proposed that under normal neuronal turnover conditions, as well as after selective neuronal loss, GBC progenitors were sufficient for OE regeneration and that only in the case of more extensive damage did HBCs become active and regenerate the OE [15]. These findings, however, raised a question as to whether this regenerative capacity of HBCs after severe lesioning represents a normal physiological process or, rather, abnormal dedifferentiation [16]. It is noteworthy that Leung et al. used an inducible Cre activation system, which marked only a small fraction of HBCs at a single time point, leaving the possibility that HBC behavior and potential had not been fully revealed.
We used a constitutively active Cre system that allowed us to mark a large number of HBCs in a temporally continuous manner. Our results demonstrate that contrary to the findings of Leung et al., in intact mice, HBCs give rise to a significant number of ORNs, as well as to non-neuronal sustentacular and Bowman’s gland/duct cells. HBC-derived ORNs were observed at as early as postnatal day 10 (P10), up to at least 1 year of age. These results together suggest that HBCs contain multipotent progenitor cells, which are active during normal neuronal turnover in the OE and capable of prolonged self-renewal for the lifetime of an organism. In addition, we performed a time-course study on HBC-derived neurogenesis through adulthood and observed two temporally distinct waves of neurogenesis: an initial wave occurring soon after birth, and a second wave peaking at 4 months of age. Furthermore, in contrast to the findings by Leung et al., selective loss of mature ORNs induced by olfactory bulbectomy led to a substantial increase in the number of HBC-derived neuronal and non-neuronal cells. Our findings collectively demonstrate a crucial role for HBCs as multipotent progenitors in the adult OE, acting in both normal neuronal turnover and acute regeneration upon injury.
MATERIALS AND METHODS
Mouse Strains and Olfactory Bulbectomy
K5.CrePR1 transgenic mice were generated in the laboratory of Dennis Roop (Baylor College of Medicine, Houston, TX) [17]. Gt(ROSA)26Sortm1Sor/J (R26R) mice were purchased from the Jackson Laboratory (Bar Harbor, ME, http://www.jax.org). Unilateral olfactory bulbectomy was performed on deeply anesthetized mice as described [11]. All procedures were approved by the Institutional Animal Care and Use Committee at University of Texas M.D. Anderson Cancer Center.
Tissue Preparation, X-Gal Staining, and Immunofluorescence
Olfactory tissue preparation was performed as described [12], with the exception that 2% periodate-lysine-paraformaldehyde in 0.1 M phosphate buffer, pH 7.4, was used for fixation. Cryostat sections were cut at 8–20 µm and processed for X-gal staining and immunofluorescence as described [18]. For cell counting experiments, cryostat sections were cut at 5–10 µm. Sections stained with X-gal were counterstained with eosin. The following primary antibodies were used for immunofluorescence: polyclonal rabbit anti-Cre recombinase (1:500; Covance, Princeton, NJ, http://www.covance. com), monoclonal mouse anti-K14 (1:100; Abcam, Cambridge, MA, http://www.abcam.com), polyclonal rabbit anti-β-galactosidase (1:500; MP Biomedicals, Irvine CA, http://www.mpbio.com), monoclonal mouse anti-neuronal class III β-tubulin (TuJ1; 1:200; Covance), polyclonal goat anti-olfactory marker protein (1:1,000; Wako Chemical, Osaka, Japan, http://www.wako-chem.co.jp/english), polyclonal rabbit anti-Mash1 (1:2,000; gift from Jane E. Johnson), polyclonal chicken anti-β-galactosidase (1:200; Abcam), and monoclonal rat anti-Notch 2 (1:50; Developmental Studies Hybridoma Bank, Iowa City, IA, http://www.uiowa.edu/~dshbwww). The following secondary antibodies were used: goat anti-rabbit Alexa 488, donkey anti-mouse Alexa 594, donkey anti-rabbit Alexa 594, goat anti-mouse Alexa 488, donkey anti-goat Alexa 488, goat anti-chicken Alexa 488, and goat anti-rat Alexa 488 (Molecular Probes, Eugene, OR, http://probes.invitrogen.com).
RESULTS
HBC-Specific Lineage Analysis in the Mouse
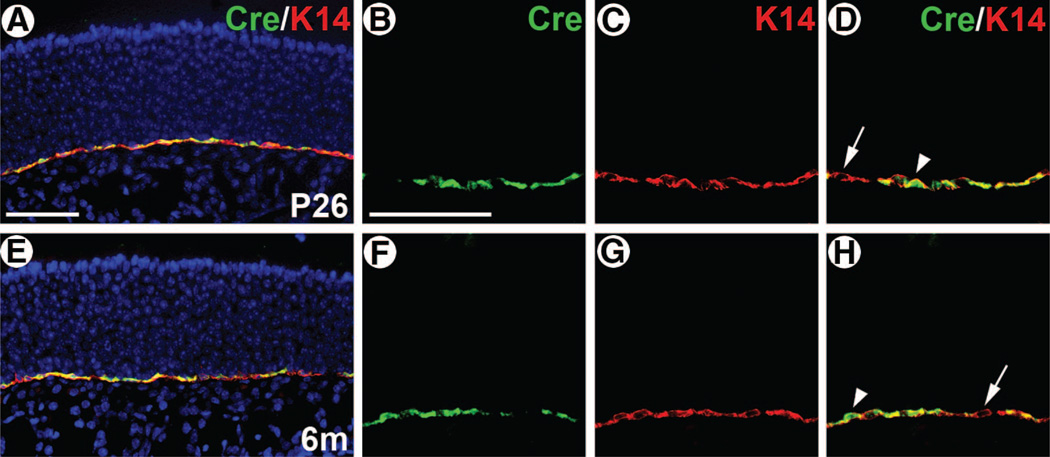
To investigate the neurogenic potential of HBCs in vivo, we performed a cell lineage analysis of HBCs using the Cre-loxP system in the mouse. We used K5.CrePR1 transgenic mice [17], in which the K5 promoter drives expression of a Cre-progesterone receptor fusion protein [19]. To test whether CrePR1 expression is HBC-specific in this strain, we performed double immunofluorescence using antibodies for Cre and K14. In K5.CrePR1 mice at P26, 3 months, and 6 months of age, a significant overlap of Cre and K14 expression was observed in the OE (Fig. 1; data not shown). Cre expression was seen exclusively in the HBC layer of the OE and absent in all other regions of the olfactory tissue. Although a subset of K14+ HBCs was negative for Cre expression (Fig. 1D, 1H, arrows), the majority were Cre+. Importantly, Cre+ cells always accompanied K14 expression, at all ages examined (Fig. 1; data not shown), indicating that K5.CrePR1 can be used as a specific Cre strain for HBCs.
Figure 1.
Horizontal basal cell (HBC)-specific Cre expression in K5.CrePR1. K5.CrePR1 tg/+ mice at P26 (A–D) and 6m (E–H) were examined for Cre and K14 expression by double immunofluorescence. The majority of HBCs were Cre+whereas a subset was Cre+ (arrows). No Cre+/K14+ cells were seen. The intracellular localization of Cre was primarily cytoplasmic, with a subset of cells showing Cre in the nucleus as well (arrowheads). Scale bars = 55 µm (A, E) and 55 µm (B–D, F–H). Abbreviations: m, months; P, postnatal day.
CrePR1 is a conditional Cre whose recombinase activity is induced by RU486 [17]. However, we noted that in the HBCs, CrePR1 does not require RU486 for its recombinase activity (supplemental online Fig. 1). In fact, similar background recombinase activity in the absence of RU486 has been reported in oral epithelium of K5.CrePR1 mice and may be caused by cryptic splice sites in CrePR1 mRNA, resulting in aberrantly spliced mRNA lacking part of the PR1 domain [20, 21]. Therefore, in the following experiments, K5.CrePR1 was used as a constitutively active Cre strain, and no RU486 administration was performed. To genetically mark HBCs and track their cell fate, K5.CrePR1 mice were crossed to the Cre reporter strain R26Rwhich expresses LacZ upon Cre-mediated recombination [22]. Embryonic ectoderm-specific recombination of R26R confirmed that this reporter locus was capable of driving LacZ expression in all cell types of the adult OE (supplemental online Fig. 2).
HBCs Give Rise to Neurons and Non-Neuronal Cells in the OE
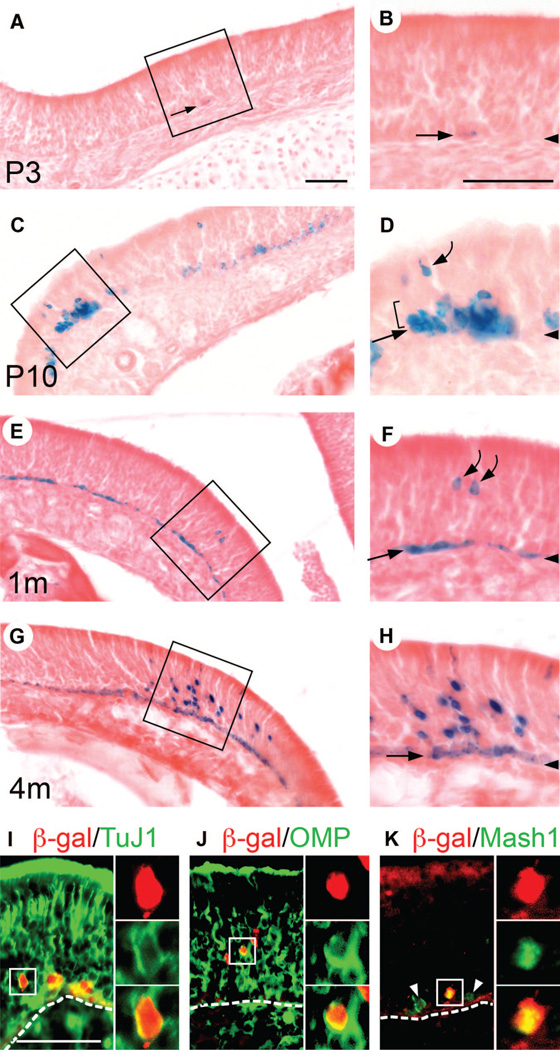
We followed the cell fate of HBCs by analyzing LacZ expression in K5.CrePR1: R26R double-heterozygous mice at various ages. At P3, rare cells that were weakly positive for β-galactosidase (β-gal) activity were seen scattered in the OE (Fig. 2A, 2B, arrows). These cells were seen as 1–2 cells tightly associated with the basal lamina, confirming that they were HBCs. P3 represented the onset of Cre reporter activity as β-gal activity could not be detected at earlier stages (data not shown). By P10, the number of β-gal+ HBCs, as well as the intensity of β-gal activity in HBCs, had increased (Fig. 2C, 2D, straight arrow). Notably, a small number of β-gal+ cell clusters containing non-HBCs were observed (Fig. 2D). The number of β-gal+ HBCs rapidly increased and by 2 weeks, approximately half of all HBCs were β-gal+ (data not shown). By 1 month, the majority of HBCs were β-gal+ (Fig. 2E, 2F, straight arrow), and a small number of β-gal+ non-HBCs were observed (Fig. 2E, 2F, curved arrows). These non-HBCs appeared to be a mixture of GBCs (Figs. 2D, 3B, 3C, brackets) and ORNs (Fig. 2D, 2F, curved arrows), as judged by their position in the OE and cell morphology. At 4 months, large β-gal+ cell clusters containing many tentative ORNs were observed (Fig. 2G, 2H). To test whether they indeed represented cells of the ORN lineage, we examined the expression of neuronal class III β-tubulin (detected by the TuJ1 antibody), olfactory marker protein (OMP) (which is a marker for mature ORNs [23]), and Mash1 (which is an early neuronal differentiation marker expressed in a subset of GBCs). Double immunofluorescence revealed β-gal+/TuJ1+ cells and β-gal+/OMP+ cells (Fig. 2I, 2J), demonstrating the presence of ORNs derived from HBCs, as well as β-gal+/Mash1+ cells, demonstrating the presence of GBCs derived from HBCs (Fig. 2K). HBC-derived GBCs and ORNs were in most cases grouped in a small number of clusters scattered throughout the tissue.
Figure 2.
Horizontal basal cells (HBCs) give rise to neuronal cells in the olfactory epithelium (OE). (A–H): K5.CrePR1 tg/+: R26R+/− mice at P3 (A, B), P10 (C, D)1m (E, F), and 4m (G, H) were examined for β-gal activity by X-gal staining. Insets in (A, C, E, G) are enlarged in (B, D, F, H), respectively. Arrowheads in (B, D, F, H) indicate the position of the basal lamina. (A, B): At P3, infrequent β-gal+ HBCs were observed (arrows). (C, D): By P10, the number of β-gal+ HBCs, as well as the intensity of β-gal activity in HBCs, had increased (straight arrow). Notably, β-gal+ cell clusters containing tentative globose basal cells (GBCs) (bracket) and olfactory receptor neurons (ORNs) (curved arrow) were observed. (E, F): By 1m, the majority of HBCs were β-gal+ (straight arrow). β-Gal+ tentative ORNs were observed (curved arrows). (G, H) At 4m, β-gal+ cell clusters containing many tentative ORNs were observed. (I, J, K): K5.CrePR1 tg/+: R26R+/− mice at 2–3m were examined for β-gal/TuJ1 (I)β-gal/OMP (J), and β-gal/Mash1 (K) expression by double immunofluorescence. Dotted lines indicate the position of the basal lamina. The presence of β-gal+/TuJ1+ (I) and β-gal+/OMP+ cells (J) demonstrated that HBCs had generated ORNs. The presence of β-gal+/Mash1+ cells (K) demonstrated that HBCs had generated Mash1+ GBCs. Arrowheads point to Mash1+ GBCs that are negative for β-gal (K). The red signal in the apical region of the OE corresponds to nonspecific binding of the secondary antibody (K). Scale bars = 60 µm (A, C, E, G)60 µm (B, D, F, H), and 55 µm (I, J, K). Abbreviations: β-gal, β-galactosidase; m, months; OMP, olfactory marker protein; P, postnatal day; TuJ1, neuronal class III β-tubulin.
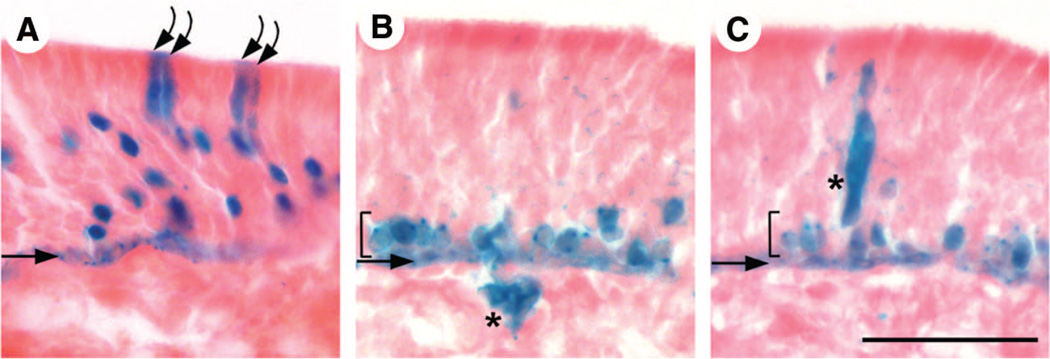
Figure 3.
Horizontal basal cells (HBCs) give rise to non-neuronal cell types in the olfactory epithelium (OE). Four-month-old K5.CrePR1 tg/+: R26R+/− mice were examined for β-galactosidase (β-gal) activity by X-gal staining. Straight arrows and brackets indicate the position of HBCs and globose basal cells, respectively. (B) and (C) are adjacent sections. In addition to multiple olfactory receptor neurons, sustentacular cells positive for β-gal were observed ([A], curved arrows). These cells have large, columnar cell bodies located in the most apical layer of the OE. β-Gal+ Bowman’s gland cells ([B], asterisk) and duct cells ([C], asterisk) were also observed. Gland cells are located in the lamina propria beneath the OE and extend a duct-like structure that traverses the OE. Scale bar = 50 µm (A–C).
In addition to cells of the ORN lineage, β-gal+ cells with the morphology and epithelial position characteristic of sustentacular cells (Fig. 3A, curved arrows) and Bowman’s gland and duct cells (Fig. 3B, 3C, asterisks) were observed. These β-gal+ non-neuronal cells were always in close proximity with β-gal+ ORN lineage cells. Non-neuronal cells derived from HBCs were rare; of 164 β-gal+ cell clusters from 18 animals, only 5 clusters contained Bowman’s gland/duct cells, whereas 20 contained sustentacular cells, in which sustentacular cells on average made up less than 5% of the total cell number in a cluster (data not shown). The scarcity of HBC-derived non-neuronal cells hampered marker analysis to further characterize these cells; however, we confirmed the presence of HBC-derived cells expressing a sustentacular cell-specific marker in bulbectomized mice (Fig. 5H). The vast majority of HBC-derived cells were of the ORN lineage, that is, GBCs (Fig. 3B, 3C, brackets) and/or ORNs (Fig. 2G, 2H). Moreover, all clusters observed contained at least one cell of the ORN lineage. In other words, clusters consisting of only non-neuronal cells were never observed. These data collectively suggest the presence of multipotent progenitors within the HBC population, which during normal neuronal turnover generate all cell types in the OE (i.e., the ORN lineage cells and the non-neuronal sustentacular cells and Bowman’s gland/duct cells).
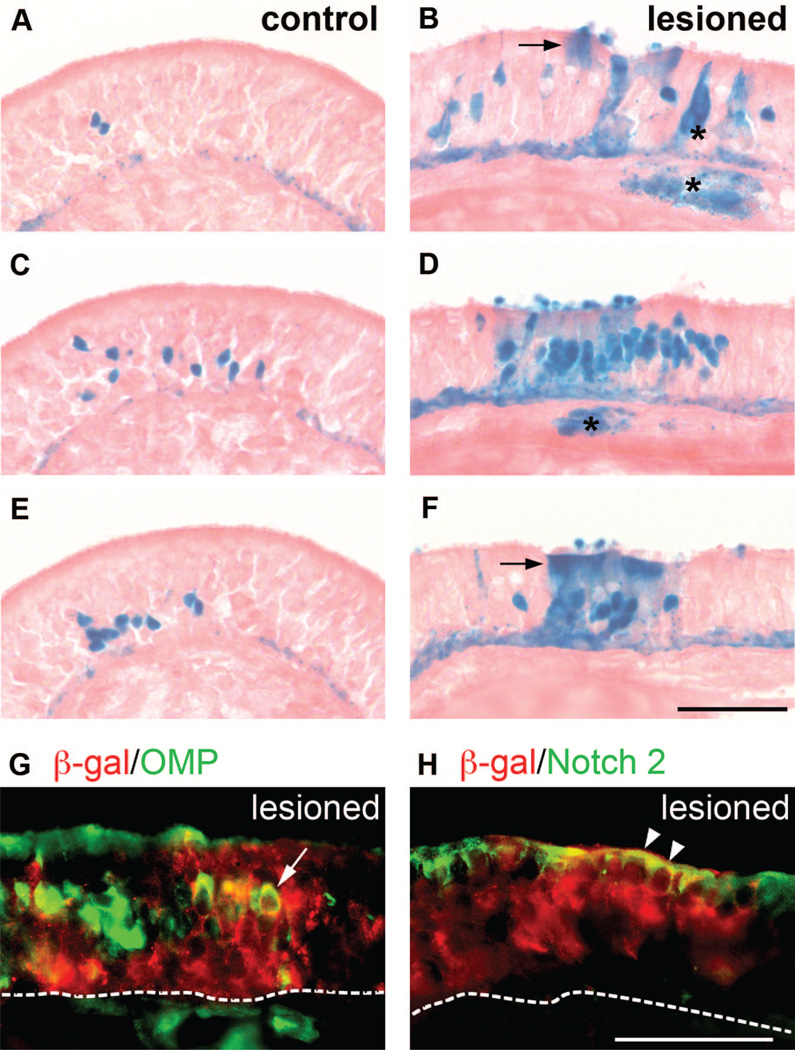
Figure 5.
Olfactory bulbectomy enhances horizontal basal cell-derived neurogenesis. Unilateral olfactory bulbectomy was performed on 2-month-old K5.CrePR1 tg/+: R26R+/− mice. Thirty days later, olfactory epithelium (OE) on the contralateral side of the lesioned bulb (control) and on the ipsilateral side of the lesioned bulb (lesioned) was examined for β-gal activity by X-gal staining (A–F). Both control (A, C, E) and lesioned (B, D, F) tissues shown are from the same animal. (A, C, E) represent every third section through the largest cluster found in control OE, whereas (B, D, F) represent every third section through the largest cluster found in lesioned OE. Arrows point to sustentacular cells, and asterisks indicate cells of Bowman’s glands and ducts (B, D, F). Lesioned OE was examined for β-gal/OMP (G) and β-gal/Notch 2 (H) expression by double immunofluorescence. Dotted lines indicate the position of the basal lamina. The arrow points to a β-gal+/OMP+ olfactory receptor neuron (G). Arrowheads point to β-gal+/Notch 2+ sustentacular cells (H). Scale bars = 60 µm (A–F) and 60 µm (G, H). Abbreviations: β-gal, β-galactosidase; OMP, olfactory marker protein.
Two Waves of HBC-Derived Neurogenesis
To comprehensively understand the function of HBCs during development, we made serial sections through the entire olfactory tissue of K5.CrePR1: R26R double-heterozygous mice at different ages and examined the number of HBC-derived neuronal clusters, as well as the cellular composition of individual clusters. Counting of clusters was not difficult, as they were relatively rare and widely dispersed in the tissue, which enabled the distinction of one cluster from another by visual inspection. However, to use an objective criterion for how to define a cluster, we chose 150 µm as the maximum distance allowed between two adjacent cells in the same cluster. The cellular composition of clusters was determined by classifying β-gal+ cells into GBCs, ORNs, and sustentacular cells on the basis of the morphology and/or position of a given cell in the OE. Thus, GBCs were defined as cells located immediately above HBCs (Fig. 3B, 3C, brackets). Sustentacular cells were defined as column-shaped cells in the most apical layer of the OE (Fig. 3A, curved arrows). Cells positioned between GBCs and sustentacular cells were counted as ORNs (Fig. 2F, curved arrows).
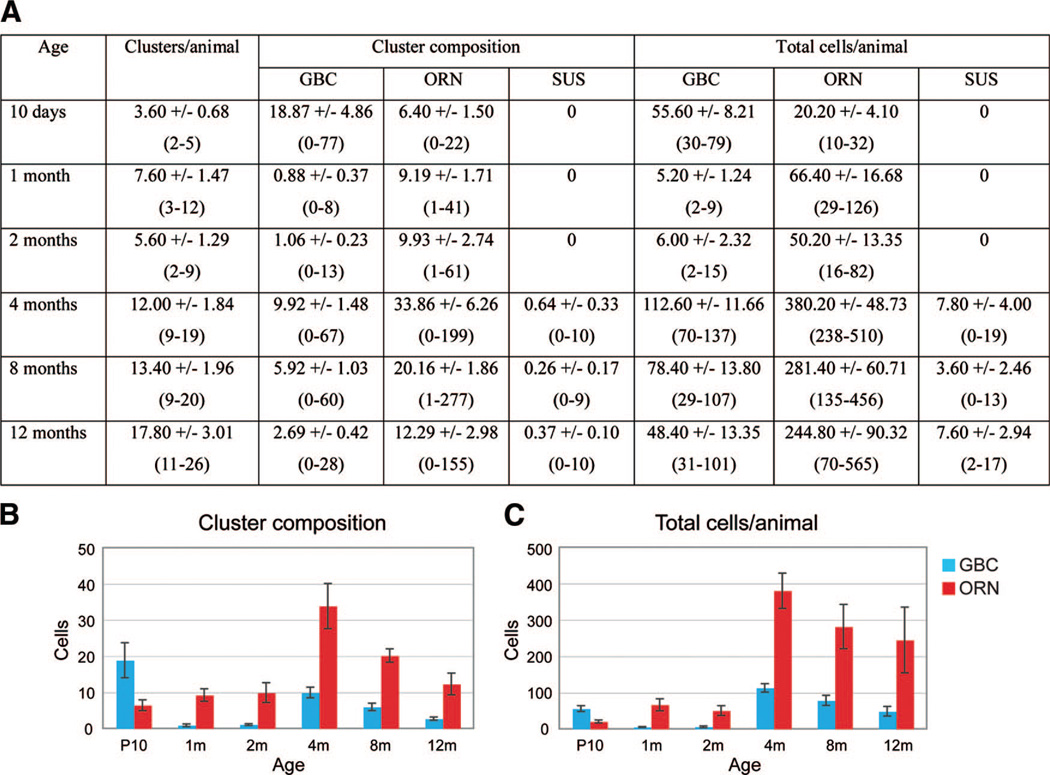
We started our quantification at P10, which is when the number of β-gal+ HBCs and the intensity of β-gal activity in HBCs increases and when we first started to see β-gal+ neuronal clusters. As shown in Figure 4A, the number of clusters per animal at P10 was small (3.60 ± 0.68 clusters; mean ± SEM; n = 5 animals). These clusters mostly consisted of GBCs with a smaller number of ORNs (18.87 ± 4.86 GBCs; 6.40 ± 1.50 ORNs; Fig. 4A, 4B), which is in accordance with a HBC-GBCORN sequence, with GBCs as intermediates in the neuronal differentiation pathway [15]. At 1 month, however, the cellular composition of clusters showed a reversal of the ratio between GBCs and ORNs, with only a small number of GBCs and more ORNs present (0.88 ± 0.37 GBCs, 9.19 ± 1.71 ORNs; Fig. 4A, 4B). These data suggest that during the period between P10 and 1 month, the initially produced GBCs had differentiated into ORNs, whereas no new GBCs were produced. The numbers of GBCs and ORNs per cluster remained alike at 2 months (1.06 ± 0.23 GBCs, 9.93 ± 2.74 ORNs; Fig. 4A, 4B). However, at 4 months, a significant rise in the numbers of GBCs and ORNs per cluster was observed (9.92 ± 1.48 GBCs, 33.86 ± 6.26 ORNs; Fig. 4A, 4B), which, together with an overall rise in the number of clusters (12.00 ± 1.84 clusters; Fig. 4A), resulted in an increase of more than sixfold in the total cell number per animal compared with earlier time points (Fig. 4C). This surge in neurogenesis implies a rising demand for neuronal replacement between 2 and 4 months, urging HBCs to initiate a new round of neurogenesis. These data demonstrate two waves of neurogenesis from HBCs, the first wave occurring soon after birth and the second wave at 4 months of age, suggesting a temporal control mechanism of neurogenic activity. The first wave of neurogenesis may correlate to epithelial expansion, which occurs during the early postnatal period [24]. Some studies have estimated the life span of ORNs to be 1–3 months long, which suggests that the two distinct waves reflect the intrinsic turnover rate of ORNs [3, 25]. On the other hand, sustentacular cells derived from HBCs were not observed until 4 months, and their number remained extremely low throughout adulthood (Fig. 4A).
Figure 4.
Two waves of horizontal basal cell (HBC)-derived neurogenesis. K5.CrePR1 tg/+: R26R+/− mice at various ages ranging from P10 to 12m were examined for the number of β-galactosidase (β-gal+) neuronal clusters in the olfactory epithelium per animal (clusters/animal), the cellular composition of individual clusters (cluster composition), and the total number of β-gal+ cells per animal (total cells/animal) (A). All numbers represent the average ± SEM of data obtained from five animals per age. In parentheses is shown the range of each data set. (B, C): Graphical presentations of (A), regarding cluster composition and total cells per animal, respectively. For simplicity, the data on SUSs are excluded. The first wave of HBC-derived neurogenesis started at P10, and the second wave peaked at 4m of age. Abbreviations: GBC, globose basal cell; m, months; ORN, olfactory receptor neuron; SUS, sustentacular cell.
As age increased, a modest rise in the number of neuronal clusters was observed between 4 and 12 months, whereas the overall cell number per cluster, as well as per animal, declined during this period (Fig. 4A–4C). However, compared with mice younger than 4 months, the total number of HBC-derived cells was significantly higher in aged mice (Fig. 4C). Previous studies reported an age-related increase in ORN cell death, which might contribute to the overall high number of HBC-derived neurons in old mice so as to compensate for more severe ORN loss [26].
Olfactory Bulbectomy Enhances HBC-Derived Neurogenesis
Next, we sought to understand the behavior of HBCs upon the need for rapid, large-scale neuronal regeneration in the OE. To do so, we performed olfactory bulbectomy, by which the olfactory axons connected to the olfactory bulb are destroyed. This leads to selective cell death of all mature ORNs in the OE and triggers a substantial increase in basal cell proliferation so as to reconstitute the OE [12, 27]. Unilateral olfactory bulbectomy was performed on 2-month-old K5.CrePR1: R26R double-heterozygous mice, and 30 days later the mice were sacrificed and analyzed for the number of HBC-derived neuronal clusters and their cellular composition. Remarkably, the OE on the ipsilateral side of the lesioned olfactory bulb (lesioned OE) showed very large clusters, some of which contained more than 1,000 ORNs (Fig. 5; Table 1). Interestingly, these clusters also contained a substantial number of sustentacular cells, which were never observed in the OE on the contralateral side of the lesioned olfactory bulb (control OE) (Fig. 5B, 5F, arrows; Table 1). Likewise, whereas clusters in control OE did not contain any Bowman’s gland/duct cells, 2 clusters of 45 examined in lesioned OE contained Bowman’s gland/duct cells (Fig. 5B, 5D; asterisks). The expanded clusters were found on various sites within lesioned OE, including the anterior region of the tissue, indicating that the increase of neurons and non-neurons did not result from direct damage due to a tear in the cribiform plate. We examined the expression of OMP and Notch 2, a marker for sustentacular cells [28], in clusters in lesioned OE. The number of ORNs expressing OMP was greatly reduced in lesioned OE (compare Fig. 5G with Fig. 2J), as was reported previously [29], yet several β-gal+/OMP+ cells were observed (Fig. 5G, arrow). In addition, clusters contained β-gal+/Notch 2+ cells (Fig. 5H, arrowheads), demonstrating the presence of HBC-derived sustentacular cells after bulbectomy. In each animal that we examined (n = 3 animals), there was an increase of more than sevenfold in the total number of GBCs and an increase of more than ninefold in the total number of ORNs in lesioned OE compared with control OE; these increases were attributed to the increase of GBCs and ORNs per cluster in lesioned versus control OE (Table 1). A slight overall increase in the number of clusters was observed as well, although this increase was statistically insignificant (t test: p = .38). Together, these data demonstrate that acute, selective depletion of mature ORNs leads to an increase in the number of neuronal cells and sustentacular cells derived from HBCs.
Table 1.
Analysis of horizontal basal cell-derived clusters after olfactory bulbectomy
| Cluster composition |
Total cells |
|||||||
|---|---|---|---|---|---|---|---|---|
| Animal | OE | Clusters | GBCs | ORNs | SUSs | GBCs | ORNs | SUSs |
| 1 | Control | 16 | 0.94 (0–10) | 8.13 (1–77) | 0 | 15 | 130 | 0 |
| Lesioned | 14 | 17.57 (0–213) | 126.79 (1–1,093) | 6.79 (0–94) | 246 | 1,775 | 95 | |
| 2 | Control | 7 | 1.86 (0–8) | 10.71 (1–38) | 0 | 13 | 75 | 0 |
| Lesioned | 11 | 9.27 (0–90) | 64.45 (1–611) | 4.73 (0–48) | 102 | 709 | 52 | |
| 3 | Control | 11 | 2.55 (0–20) | 24.18 (1–71) | 0 | 28 | 266 | 0 |
| Lesioned | 20 | 25.40 (0–302) | 130.75 (1–1,137) | 1.60 (0–15) | 508 | 2,615 | 32 | |
Unilateral olfactory bulbectomy was performed on three K5.CrePR1 tg/+: R26R+/− mice at 2 months of age, and mice were sacrificed 30 days later. Control OE and lesioned OE were examined for the number of β-galactosidase+ (β-gal+) neuronal clusters (clusters), the cellular composition of individual clusters (cluster composition), and the total number of β-gal+ cells (total cells). In parentheses is shown the range of each data set.
Abbreviations: GBC, globose basal cell; OE, olfactory epithelium; ORN, olfactory receptor neuron; SUS, sustentacular cell.
DISCUSSION
Our study demonstrates the ability of HBCs to give rise to all cell types, neuronal and non-neuronal, in the adult OE. Furthermore, the presence of HBC-derived cells throughout adulthood and the generation of large clusters from HBCs upon mature ORN depletion together strongly suggest the presence of long-lived, multipotent progenitors in the HBC population that maintain the OE during normal neuronal turnover and replenish the OE upon injury. We present a model in which HBCs undergo either self-renewal or differentiation into neuronal and non-neuronal lineages (Fig. 6). As the vast majority of HBC-derived cells were neuronal, both in normal and in injured OE, we propose that the primary course of action for HBCs is to give rise to GBCs and eventually to ORNs. At a lower frequency, HBCs also proceed toward non-neuronal differentiation into sustentacular cells or Bowman’s gland/duct cells.
Figure 6.
Model of HBC differentiation pathways. HBCs undergo self-renewal or differentiation mainly into GBCs, which eventually produce ORNs. HBCs can also proceed toward differentiation into SUSs or BG cells, albeit at a lower frequency. Abbreviations: BG, Bowman’s gland and duct; GBC, globose basal cell; HBC, horizontal basal cell; ORN, olfactory receptor neuron; SUS, sustentacular cell.
We performed a detailed time-course study of HBC-derived neurogenesis during normal development and uncovered temporal regulation of HBC activity. The first wave of HBC-derived neurogenesis was observed to occur at P10, which was shortly after β-gal+ HBCs had started to become vaguely visible at P3. We do not know whether HBCs give rise to neurons during embryogenesis, as β-gal activity in HBCs could not be detected until P3. The second wave of HBC-derived neurogenesis took place at 4 months of age, which suggested that after the first wave, HBCs remained quiescent for 2–3 months. The surge in neurogenesis at 4 months implies a rising demand for neuronal replacement, which could be at least in part due to the limited life span of ORNs generated at P10, urging HBCs to initiate a new round of neurogenesis.
We examined the effect of mature ORN depletion on HBC behavior by performing olfactory bulbectomy on our transgenic mice. As a result, we observed a dramatic increase in the total number of HBC-derived neurons and sustentacular cells in lesioned OE compared with control OE. However, the number of clusters in lesioned OE was not significantly increased, suggesting that olfactory bulbectomy does not increase the number of actively neurogenic HBCs. The overall increase in HBC-derived cells was instead due to the larger number of cells per cluster in lesioned versus control OE. Whereas the maximum number of ORNs per cluster in control OE was 77, some clusters in lesioned OE contained more than 1,000 ORNs. As it was reported previously that olfactory bulbectomy increases the number of bromodeoxyuridine (BrdU)-positive GBCs but not the number of BrdU-positive HBCs, we speculate that the increase of ORNs per cluster was caused mainly by GBCs undergoing more cell divisions [15]. Yet in addition to increased proliferation at the GBC level, it is possible that active HBCs underwent more cell divisions, which, given the limited number of these cells, would not necessarily contribute to a detectable change in overall HBC proliferation.
Our findings differ from those by Leung et al. who observed clusters of HBC-derived neurons and non-neuronal cells only after an extensive injury of the OE by exposure to methyl bromide gas, which destroys sustentacular cells, ORNs, and most GBCs. They reported that HBCs remain largely quiescent during normal neuronal turnover or even after mature ORN depletion and proposed that HBCs serve as a reservoir of stem cells that become active exclusively when GBC progenitors are depleted. In contrast, we observed a significant number of HBC-derived neurons and non-neuronal cells in normal mice and an increase of those cells after mature ORN depletion. We attribute this discrepancy to a difference in the experimental system used. Leung et al. used a tamoxifen-inducible Cre activation system in which a single dose of tamoxifen was given to 2–3-week-old mice, resulting in the marking of only approximately 10% of all HBCs, at a single time point [15]. We, on the other hand, used a constitutively active Cre system, which allowed us to mark approximately 70% of HBCs in a temporally continuous manner. As a result, our fate mapping was robust enough to detect HBC-derived clusters in normal mice, from as early as P10. Our findings present HBCs as a source of multipotent progenitors, which participate in normal OE maintenance even in the presence of GBC progenitors.
It is interesting to note that despite our broad cell marking system, the number of HBC-derived cells was limited even after olfactory bulbectomy, which causes massive ORN cell death [26, 31]. There are several possible explanations for this. With our cell marking system, Cre reporter activity in HBCs was first detected at P3, and it was not until 1 month of age that the majority of HBCs were β-gal+. Therefore, insufficient marking of early postnatal HBCs may have led to an underrepresentation of the number of HBC-derived neurons, especially in young mice. Considering the general ORN life span of 1–3 months [3, 25], with one study reporting that some ORNs survive for 12 months [32], it is possible that unmarked, early HBCs generated neurons that were not β-gal+ but long-lived, leading to an underestimation of HBC-derived neurons. However, it should also be noted that the number of HBC-derived neurons remained limited at 12 months of age (Fig. 4) and even in 2-year-old mice (data not shown). Therefore, it is highly unlikely that insufficient marking of early postnatal HBCs was the only factor accounting for the limited number of HBC-derived neurons. A more likely explanation would be that only a few multipotent progenitors exist within the HBC population or that all HBCs possess multipotency but only a few are mobilized, at least in normal and bulbectomized mice. In terms of gene expression, HBC-specific genes known to date are expressed homogeneously in all HBCs [12], and it remains to be answered whether a subpopulation of HBCs can be identified as active progenitors by means of gene expression or other characteristics.
Importantly, the limited number of active HBCs contributing to only a portion of the OE suggests that HBCs are not the sole source of multipotent progenitors in the OE. Several studies have suggested that GBCs contain not only committed neuronal progenitors but also multipotent progenitors, which, following methyl bromide gas-induced tissue damage, can generate both neuronal and non-neuronal cells [14, 33, 34]. Moreover, during early development, distinct HBCs cannot be observed until late embryogenesis [24, 35, 36], whereas GBCs are present from as early as embryonic day 10 [37], suggesting that HBCs may derive from GBCs in embryonic OE. In adult OE, a picture emerges where HBCs and GBCs each contain a group of multipotent progenitors that have similar abilities but function in different settings. In normal OE, with a steady demand for new neurons, multipotent GBCs may be the main workforce in maintaining the OE, whereas multipotent HBCs play a supplementary role. However, when the OE is damaged, multipotent HBCs become crucial for enhanced regeneration, and the robustness of HBC participation likely depends on the degree of damage, with methyl bromide gas exposure imposing more extensive injury compared with olfactory bulbectomy [15].
CONCLUSION
In this study we have shown the presence of HBCs that possess multipotency in vivo. However, it remains to be addressed whether they possess other stem cell characteristics, such as self-renewal and the ability to repopulate the entire epithelium. Of equal interest would be to decipher the relationship and balance between multipotent progenitors in HBCs and GBCs. In this context, the OE provides an excellent system for studying the interaction between multiple lineages in a dynamic environment. The OE is an ideal source of stem cells to be used for autologous transplantation, as the anatomy of the tissue facilitates biopsy, and further characterization of olfactory neural stem cells may therefore provide promising clinical implications [38–40].
ACKNOWLEDGMENTS
We thank Jane E. Johnson (University of Texas Southwestern, Dallas, TX) for providing us an anti-Mash1 antibody and Crestina L. Beites for commenting on the manuscript. This work was supported by the Ben F. Love Endowment (to R.R.B.), the Odyssey Program, and the Kimberly Clark Foundation Endowment for New and Innovative Research at the University of Texas M.D. Anderson Cancer Center (to N.I.), and National Institutes of Health Grants P01-AR47898 and R01-AR052263 (to D.R.R.). D.R.R. is currently affiliated with the Department of Dermatology, Charles C. Gates Regenerative Medicine and Stem Cell Biology Program, University of Colorado at Denver and Health Sciences Center, Aurora, CO.
Footnotes
DISCLOSURE OF POTENTIAL CONFLICTS OF INTEREST
The authors indicate no potential conflicts of interest.
See www.StemCells.com for supplemental material available online.
REFERENCES
- 1.Calof AL, Bonnin A, Crocker C, et al. Progenitor cells of the olfactory receptor neuron lineage. Microsc Res Tech. 2002;58:176–188. doi: 10.1002/jemt.10147. [DOI] [PubMed] [Google Scholar]
- 2.Beites CL, Kawauchi S, Crocker CE, et al. Identification and molecular regulation of neural stem cells in the olfactory epithelium. Exp Cell Res. 2005;306:309–316. doi: 10.1016/j.yexcr.2005.03.027. [DOI] [PubMed] [Google Scholar]
- 3.Graziadei PP, Graziadei GA. Neurogenesis and neuron regeneration in the olfactory system of mammals. I. Morphological aspects of differentiation and structural organization of the olfactory sensory neurons. J Neurocytol. 1979;8:1–18. doi: 10.1007/BF01206454. [DOI] [PubMed] [Google Scholar]
- 4.Schwob JE. Neural regeneration and the peripheral olfactory system. Anat Rec. 2002;269:33–49. doi: 10.1002/ar.10047. [DOI] [PubMed] [Google Scholar]
- 5.Alvarez-Buylla A, Garcia-Verdugo JM, Tramontin AD. A unified hypothesis on the lineage of neural stem cells. Nat Rev Neurosci. 2001;2:287–293. doi: 10.1038/35067582. [DOI] [PubMed] [Google Scholar]
- 6.Gage FH. Mammalian neural stem cells. Science. 2000;287:1433–1438. doi: 10.1126/science.287.5457.1433. [DOI] [PubMed] [Google Scholar]
- 7.Kempermann G, Kuhn HG, Gage FH. Genetic influence on neurogenesis in the dentate gyrus of adult mice. Proc Natl Acad Sci U S A. 1997;94:10409–10414. doi: 10.1073/pnas.94.19.10409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rawson NE, Gomez G. Cell and molecular biology of human olfaction. Microsc Res Tech. 2002;58:142–151. doi: 10.1002/jemt.10132. [DOI] [PubMed] [Google Scholar]
- 9.Nibu K, Li G, Zhang X, et al. Olfactory neuron-specific expression of NeuroD in mouse and human nasal mucosa. Cell Tissue Res. 1999;298:405–414. doi: 10.1007/s004419900098. [DOI] [PubMed] [Google Scholar]
- 10.Nagao H, Yamaguchi M, Takahash Y, et al. Grouping and representation of odorant receptors in domains of the olfactory bulb sensory map. Microsc Res Tech. 2002;58:168–175. doi: 10.1002/jemt.10146. [DOI] [PubMed] [Google Scholar]
- 11.Gordon MK, Mumm JS, Davis RA, et al. Dynamics of MASH1 expression in vitro and in vivo suggest a non-stem cell site of MASH1 action in the olfactory receptor neuron lineage. Mol Cell Neurosci. 1995;6:363–379. doi: 10.1006/mcne.1995.1028. [DOI] [PubMed] [Google Scholar]
- 12.Carter LA, MacDonald JL, Roskams AJ. Olfactory horizontal basal cells demonstrate a conserved multipotent progenitor phenotype. J Neurosci. 2004;24:5670–5683. doi: 10.1523/JNEUROSCI.0330-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Caggiano M, Kauer JS, Hunter DD. Globose basal cells are neuronal progenitors in the olfactory epithelium: A lineage analysis using a replication-incompetent retrovirus. Neuron. 1994;13:339–352. doi: 10.1016/0896-6273(94)90351-4. [DOI] [PubMed] [Google Scholar]
- 14.Huard JM, Youngentob SL, Goldstein BJ, et al. Adult olfactory epithelium contains multipotent progenitors that give rise to neurons and non-neural cells. J Comp Neurol. 1998;400:469–486. [PubMed] [Google Scholar]
- 15.Leung CT, Coulombe PA, Reed RR. Contribution of olfactory neural stem cells to tissue maintenance and regeneration. Nat Neurosci. 2007;10:720–726. doi: 10.1038/nn1882. [DOI] [PubMed] [Google Scholar]
- 16.Duggan CD, Ngai J. Scent of a stem cell. Nat Neurosci. 2007;10:673–674. doi: 10.1038/nn0607-673. [DOI] [PubMed] [Google Scholar]
- 17.Zhou Z, Wang D, Wang XJ, et al. In utero activation of K5.CrePR1 induces gene deletion. Genesis. 2002;32:191–192. doi: 10.1002/gene.10064. [DOI] [PubMed] [Google Scholar]
- 18.Wang SW, Kim BS, Ding K, et al. Requirement for math5 in the development of retinal ganglion cells. Genes Dev. 2001;15:24–29. doi: 10.1101/gad.855301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kellendonk C, Tronche F, Monaghan AP, et al. Regulation of Cre recombinase activity by the synthetic steroid RU 486. Nucleic Acids Res. 1996;24:1404–1411. doi: 10.1093/nar/24.8.1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Caulin C, Nguyen T, Longley MA, et al. Inducible activation of oncogenic K-ras results in tumor formation in the oral cavity. Cancer Res. 2004;64:5054–5058. doi: 10.1158/0008-5472.CAN-04-1488. [DOI] [PubMed] [Google Scholar]
- 21.Wunderlich FT, Wildner H, Rajewsky K, et al. New variants of inducible Cre recombinase: A novel mutant of Cre-PR fusion protein exhibits enhanced sensitivity and an expanded range of inducibility. Nucleic Acids Res. 2001;29:E47. doi: 10.1093/nar/29.10.e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Soriano P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nature Genet. 1999;21:70–71. doi: 10.1038/5007. [DOI] [PubMed] [Google Scholar]
- 23.Buiakova OI, Baker H, Scott JW, et al. Olfactory marker protein (OMP) gene deletion causes altered physiological activity of olfactory sensory neurons. Proc Natl Acad Sci U S A. 1996;93:9858–9863. doi: 10.1073/pnas.93.18.9858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Suzuki Y, Takeda M. Basal cells in the mouse olfactory epithelium during development: Immunohistochemical and electron-microscopic studies. Brain Res Dev Brain Res. 1993;73:107–113. doi: 10.1016/0165-3806(93)90052-c. [DOI] [PubMed] [Google Scholar]
- 25.Mackay-Sim A, Kittel PW. On the life span of olfactory receptor neurons. Eur J Neurosci. 1991;3:209–215. doi: 10.1111/j.1460-9568.1991.tb00081.x. [DOI] [PubMed] [Google Scholar]
- 26.Conley DB, Robinson AM, Shinners MJ, et al. Age-related olfactory dysfunction: Cellular and molecular characterization in the rat. Am J Rhinol. 2003;17:169–175. [PubMed] [Google Scholar]
- 27.Schwartz Levey M, Chikaraishi DM, Kauer JS. Characterization of potential precursor populations in the mouse olfactory epithelium using immunocytochemistry and autoradiography. J Neurosci. 1991;11:3556–3564. doi: 10.1523/JNEUROSCI.11-11-03556.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carson C, Murdoch B, Roskams AJ. Notch 2 and Notch 1/3 segregate to neuronal and glial lineages of the developing olfactory epithelium. Dev Dyn. 2006;235:1678–1688. doi: 10.1002/dvdy.20733. [DOI] [PubMed] [Google Scholar]
- 29.Carr VM, Walters E, Margolis FL, et al. An enhanced olfactory marker protein immunoreactivity in individual olfactory receptor neurons following olfactory bulbectomy may be related to increased neurogenesis. J Neurobiol. 1998;34:377–390. [PubMed] [Google Scholar]
- 30.Schwob JE, Youngentob SL, Mezza RC. Reconstitution of the rat olfactory epithelium after methyl bromide-induced lesion. J Comp Neurol. 1995;359:15–37. doi: 10.1002/cne.903590103. [DOI] [PubMed] [Google Scholar]
- 31.Holcomb JD, Mumm JS, Calof AL. Apoptosis in the neuronal lineage of the mouse olfactory epithelium: Regulation in vivo and in vitro. Dev Biol. 1995;172:307–323. doi: 10.1006/dbio.1995.0025. [DOI] [PubMed] [Google Scholar]
- 32.Hinds JW, Hinds PL, McNelly NA. An autoradiographic study of the mouse olfactory epithelium: Evidence for long-lived receptors. Anatomical Record. 1984;210:375–383. doi: 10.1002/ar.1092100213. [DOI] [PubMed] [Google Scholar]
- 33.Chen X, Fang H, Schwob JE. Multipotency of purified, transplanted globose basal cells in olfactory epithelium. J Comp Neurol. 2004;469:457–474. doi: 10.1002/cne.11031. [DOI] [PubMed] [Google Scholar]
- 34.Jang W, Youngentob SL, Schwob JE. Globose basal cells are required for reconstitution of olfactory epithelium after methyl bromide lesion. J Comp Neurol. 2003;460:123–140. doi: 10.1002/cne.10642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Holbrook EH, Szumowski KE, Schwob JE. An immunochemical, ultra-structural, and developmental characterization of the horizontal basal cells of rat olfactory epithelium. J Comp Neurol. 1995;363:129–146. doi: 10.1002/cne.903630111. [DOI] [PubMed] [Google Scholar]
- 36.Cuschieri A, Bannister LH. The development of the olfactory mucosa in the mouse: Electron microscopy. J Anat. 1975;119:471–498. [PMC free article] [PubMed] [Google Scholar]
- 37.Cau E, Gradwohl G, Fode C, et al. Mash1 activates a cascade of bHLH regulators in olfactory neuron progenitors. Development. 1997;124:1611–1621. doi: 10.1242/dev.124.8.1611. [DOI] [PubMed] [Google Scholar]
- 38.Winstead W, Marshall CT, Lu CL, et al. Endoscopic biopsy of human olfactory epithelium as a source of progenitor cells. Am J Rhinol. 2005;19:83–90. [PubMed] [Google Scholar]
- 39.Xiao M, Klueber KM, Lu C, et al. Human adult olfactory neural progenitors rescue axotomized rodent rubrospinal neurons and promote functional recovery. Exp Neurol. 2005;194:12–30. doi: 10.1016/j.expneurol.2005.01.021. [DOI] [PubMed] [Google Scholar]
- 40.Murrell W, Feron F, Wetzig A, et al. Multipotent stem cells from adult olfactory mucosa. Dev Dyn. 2005;233:496–515. doi: 10.1002/dvdy.20360. [DOI] [PubMed] [Google Scholar]