Abstract
In female mammals, the postpartum period involves dramatic shifts in many socioemotional behaviors. This includes a suppression of anxiety-related behaviors that requires recent physical contact with offspring. Factors contributing to differences among females in their susceptibility to the anxiety-modulating effect of offspring contact are unknown, but could include their innate anxiety and brain monoaminergic activity. Anxiety behavior was assessed in a large group of nulliparous female rats and the least-anxious and most-anxious tertiles were mated. Anxiety was assessed again postpartum after females were permitted or prevented from contacting their offspring 4 h before testing. Levels of dopamine β-hydroxylase (DBH, norepinephrine synthesizing enzyme) and tryptophan hydroxylase- 2 (TPH2, serotonin synthesizing enzyme) were measured in the brainstem and dorsal raphe, respectively. It was found that anxiety-related behavior in the two groups did not differ when dams were permitted contact with offspring before testing. Removal of the offspring before testing, however, differentially affected anxiety based on dams’ innate anxiety. Specifically, dams reverted back to their pre-mating levels of anxiety such that offspring removal slightly increased anxiety in the most-anxious females but greatly lowered anxiety in the least-anxious females. This reduction in anxiety in the least-anxious females after litter removal was associated with lower brainstem DBH. There was no relationship between females’ anxiety and dorsal raphe TPH2. Thus, a primary effect of recent contact with offspring on anxiety-related behavior in postpartum rats is to shift females away from their innate anxiety to a more moderate level of responding. This effect is particularly true for females with the lowest anxiety, may be mediated by central noradrenergic systems, and has implications for their ability to attend to their offspring.
Keywords: anxiety, female, peripartum, norepinephrine, serotonin, touch
INTRODUCTION
The onset and maintenance of motherhood is a time of tremendous neurobehavioral flux for female mammals (Numan et al., 2006; Lonstein et al., 2013; Sisk et al., 2013). This flux involves salient changes in how females process social stimuli, most obviously resulting in heightened positive responses to neonates, as well as changes in the new mother’s emotional state that help or hinder these positive responses. While the early postpartum period has been characterized for some women as a time of particular susceptibility to anxiety and other types of emotional dysregulation, the majority of women and other female animals studied show stable or even improved emotional regulation during the postpartum period (Neumann, 2003; Heron et al., 2004; Ross and McLean, 2006; Lonstein, 2007). Indeed, most studies find that anxiety-related behavior in early postpartum laboratory rodents is lower than what is found in females that have not given birth (see Lonstein, 2007 for review). In rats, this reduction depends on recent suckling or non-suckling physical contact with offspring, and dams’ anxiety-related behavior rises to levels found in nulliparous females if the litter is removed even for a few hours before testing (Lonstein, 2005; Figueira et al., 2008; Smith and Lonstein, 2008; Miller and Lonstein, 2011). A similar anxiolytic effect of recent suckling or non-suckling contact with infants has been found in human mothers (Heinrichs et al., 2001).
Studies on this topic in laboratory rats have provided valuable information about what can be expected for the anxiety-related behaviors of most postpartum females in response to infant contact. However, postpartum female rats may be heterogeneous in how their anxiety is affected by physical contact with neonates. This is suggested by the fact noted above that women are differentially susceptible to anxiety dysregulation during the postpartum period, with one of the best predictors of their postpartum anxiety being their history of anxiety before giving birth (Engle et al., 1990; Hundley et al., 1998; O’Connor et al., 2002; Heron et al., 2004; Britton, 2008; Grant et al., 2008). Such innate or “trait” anxiety could also contribute to heterogeneity in the anxiety-related behavior of postpartum laboratory rats, and there is a burgeoning body of research demonstrating the stability of emotional traits (including anxiety) in individual non-human animals across the lifespan (Burtt, 1967; Lister, 1987; Clarke and Boinski, 1995; Leibsch et al., 1998; Henniger et al., 2000; Gosling, 2001; Landgraf and Wigger, 2002; Cavigelli et al., 2007; Uher et al., 2008; Quinn et al., 2011; Curley et al., 2012; Cavigelli et al., 2013). Furthermore, in both rodents and humans, differences among individuals in anxiety or the experimental instillation of anxious states has been observed to affect somatosensory functioning (Jorum, 1988; van Meeteren et al., 1997; Kain et al., 2000; Rhudy and Meagher, 2000; Geerse et al., 2006; Devall et al., 2009; Aron et al., 2012; Corral-Frias et al., 2013). If the same is true for postpartum rats, mothers with the highest anxiety could be the most sensitive to, and benefit the most from, tactile inputs provided by the litter. One could alternatively conjecture that if maternal tactile sensitivity is too high, interacting with pups could be aversive and not reduce anxiety.
The neurochemicals underlying postpartum anxiety in general or its modulation by offspring contact are not very well understood. Research on this topic has traditionally focused on ovarian hormones (e.g., estradiol, progesterone) and peptides (e.g., oxytocin, prolactin) (Neumann, 2003; Lonstein, 2007), but classic neurotransmitter systems that modulate anxiety in nulliparous animals, such as norepinephrine and serotonin, are also involved. Noradrenergic neurons located in the locus coeruleus, ventrolateral medulla, and elsewhere in the brainstem have reciprocal connections with many areas of the limbic system and hypothalamus that are involved in emotion regulation (McKeller and Loewy, 1982; Woulfe et al., 1990). Elevated activity of these noradrenergic pathways is associated with anxiety in both laboratory rats (Tanaka et al., 2000; Neophytou et al., 2001; Dazzi et al., 2002; Fendt et al., 2005; Debiec and LeDoux, 2006) and humans (Sullivan et al., 1999; Tanaka et al., 2000; Ravindran and Stein, 2010; Kalk et al., 2011). Compared to nulliparous rats, postpartum rats have lower noradrenergic activity in some areas of the forebrain involved in the behavioral and physiological responses to anxiogenic stimuli (Toufexis and Walker, 1996; Windle et al., 1997; Toufexis et al., 1998; Douglas, 2005) and this may partly be mediated by brainstem noradrenergic neurons that are sensitive to tactile cues from pups (Li et al., 1999). The serotonin-synthesizing neurons in the brain are mostly located in the midbrain dorsal raphe nucleus and are also interconnected to many neural structures underlying anxiety and other emotional behaviors (Feldman et al., 1987; Chen et al., 1992; Hensler et al., 1994; Dinan, 1996; Ziegler and Herman, 2002; Lechin et al., 2006). The relationship between serotonin and anxiety in rodents is equivocal, though, as experimental manipulations of central serotonin systems have been seen to either increase or decrease anxiety-related behavior (Briley et al., 1990; Critchley et al., 1992; Kalueff et al., 2007; Olivier et al., 2008; Mosienko et al., 2012). Even so, peripartum plasticity of serotonergic cells in the dorsal raphe may render this system particularly influential for how postpartum state and physical interaction with pups affect maternal anxiety (Klink et al., 2002; Robichaud and Debonnel, 2005; Holschbach and Lonstein, 2013).
In the present experiment, we examined if mother laboratory rats differ in how contact with pups influences their anxiety-related behavior, based on whether the mothers were characterized as having a low-anxiety or a high-anxiety profile before giving birth. We then assessed the relationships between their anxiety-related behavior and brainstem expression of dopamine β-hydroxylase (DBH, the rate-limiting enzyme for norepinephrine synthesis), which is very highly correlated with levels of brain norepinephrine (Coyle and Axelrod, 1972; Hartman et al., 1972), and midbrain dorsal raphe expression of tryptophan hydroxylase-2 (TPH2, the rate-limiting enzyme for serotonin synthesis), which is highly correlated with brain serotonin content (Walther et al., 2003; Donner and Handa, 2009). We hypothesized that, unlike randomly selected postpartum laboratory rats that mostly show reduced anxiety in response to recent contact with the litter (Lonstein, 2005; Figueira et al., 2008; Smith and Lonstein, 2008; Miller and Lonstein, 2011), mother rats with the highest anxiety would be the most sensitive to the anxiolytic effect of physical contact with pups whereas mothers with the lowest anxiety would not be affected at all due to a floor effect. Considering the relationship between noradrenergic activity and anxiety in non-postpartum mammals, we predicted an inverse relationship between brainstem levels of DBH and dams’ anxiety-related behavior, while determining the relationship between dams’ anxiety and dorsal raphe levels of TPH2 was more exploratory.
EXPERIMENTAL PROCEDURES
Subjects
Subjects were adult female Long–Evans rats, descended from rats purchased from Harlan Laboratories (Indianapolis, IN) that were born and raised in our colony and housed as described previously (Smith and Lonstein, 2008). Beginning at 65 days of age, subjects’ estrous cycles were monitored daily by vaginal smear and pre-mating anxiety testing occurred on a day of diestrus (details below). Diestrus was chosen because it is characterized by low circulating ovarian hormone titers that are similar to lactational diestrus (Tsukamura and Maeda, 2001). Between 90 and 100 days of age, estrous cycles were again monitored daily with a vaginal impedance meter (Fine Science Tools, Foster City, CA, USA) and on a day of proestrus the females were housed with sexually experienced males from our colony for 2 days. After mating, females were housed with another pregnant female until being singly housed 5–7 days before expected parturition. Litters were culled to contain four males and four females within 24 h after birth. All work was conducted in accordance with the National Institutes of Health Guide for Care and Use of Laboratory Animals and the Institutional Animal Care and Use Committee at Michigan State University.
Anxiety-related behavior testing
Behavior testing occurred between 1400 and 1600 h. To avoid complications such as habituation to the testing apparatus or changes in the neurobiological underpinning of behavior sometimes associated with repeated testing in the same behavioral paradigm (File, 1990; Bourin and Hascoët, 2003), pre-mating anxiety was assessed for 10 min with a light–dark box and postpartum anxiety was assessed for 10 min with an elevated plus maze using methods previously described in detail (Lonstein, 2005; Miller et al., 2011; Smith et al., 2012). Both paradigms are based on rats’ innate aversion to bright light and open spaces, such that lower anxiety is associated with more time spent in the light chamber of the light–dark box and a greater percentage of time spent in the open arms of the elevated plus maze. The behaviors displayed by cycling female laboratory rats tested in both paradigms are highly correlated (Henniger et al., 2000) and the behaviors displayed in these paradigms are more highly correlated with each other than between either paradigm and the similarly popular open field (Ramos et al., 2008). A mirror was suspended near the ceiling above each apparatus and the images in the mirror were recorded for later scoring with a computerized data-acquisition system. Apparatuses were cleaned with 70% ethanol and allowed to dry between subjects. The duration of time spent in the light chamber of the light–dark box, and the percentage of time spent in and frequency of entries into the open arms of the elevated plus maze, were used as the primary measures of anxiety-related behavior with high durations or percentages of time indicative of low anxiety (Pellow et al., 1985; Costall et al., 1989). The frequency of entries made into closed arms of the elevated plus maze was used as an indicator of general locomotor activity (e.g., Cruz et al., 1994; Ramos et al., 2008). Similar to our previous reports (Lonstein, 2005; Figueira et al., 2008; Miller et al., 2011), other behavioral variables were measured in these paradigms, but were found here to not have significant relationships with each other or with the neurochemical measures and are not reported.
Sixty cycling nulliparous female rats were screened for anxiety behavior and the 20 least-anxious and 20 most-anxious (based on a tertile split of the duration of time spent in the light chamber of the light–dark box) were mated at least 3 days after testing. This tertile split produced very divergent groups, with the least-anxious females spending 79.2±9.7 s in the light chamber (range 35–163 s) and the most-anxious females spending 2.6±0.4 s in the light chamber (range 1–7 s) (t(34) = 17.97, P < 0.0001). On postpartum day 7, these least- and most-anxious females were randomly assigned to one of two groups to assess their sensitivity to offspring contact. Half of the 20 least-anxious and half of the 20 most-anxious mothers had their offspring removed 4 h before testing, which increases anxiety-related behavior in groups of randomly selected postpartum rats from our colony (Lonstein, 2005; Smith and Lonstein, 2008; Miller et al., 2011). The remaining half of the subjects in each group remained with their pups, but had their cage lids briefly lifted 4 h before testing to control for the mild cage disturbance during offspring removal in the separated group.
Analysis of brainstem DBH and midbrain TPH2
Sacrifice and brain processing
Immediately after elevated plus-maze testing, dams were narcotized by being placed for ~2 min in a cage prefilled with CO2. After decapitation, brains were removed from the skull, flash frozen in isopentane, and stored at −80 °C until further processing. Brainstems were isolated to obtain the noradrenergic cell groups, corresponding to plates 57–72 from Swanson’s atlas of the rat brain (1998). For homogenization, the brainstem was placed in a solution of 1 mL of RIPA, 10 μL Na3 VO4, 10μL PMSF, and 10 μL protease inhibitor (SC-24948, all Santa Cruz Biotechnology, Santa Cruz, CA, USA) and homogenized on ice with pulses of a sonic dismembrator (Fisher Scientific, Pittsburgh, PA, USA) for 20 s at 100% amplitude. Midbrains were cut coronally into 500-μm thick sections using a cryostat (Leica CM1950, Nussloch, Germany) and three sections including the dorsal raphe (plates 44–50 from Swanson, 1998) was obtained from each brain. The dorsal raphe was collected using a 1-mm-diameter brain punch (Stoelting CO, Wood Dale, IL, USA) and the samples were then placed in a microcentrifuge tube containing a solution of 50 μL RIPA buffer, 0.5 μL NAOtn, 0.5 μL PMSF, and 1 μL protease inhibitor then homogenized by pulsed sonication for 10 s at 20% amplitude. The sonication wand was cleaned with 100% ethanol and dried between samples. Homogenates were centrifuged at 4 °C at 15,000 rpm for 20 min. The supernatants (cell lysates) were collected, placed in clean microcentrifuge tubes, and stored at −80 °C. Protein concentrations in lysates were determined using a Pierce BCA Protein Assay kit (#23227, Thermo Scientific, Rockford, IL, USA) and Bio-Rad iMark microplate reader (Hercules, CA, USA).
Western blotting
All incubations occurred at room temperature with agitation unless otherwise noted. Samples (10 μg of total protein, DBH; 1 μg of total protein, TPH2) were denatured at 95 °C for 5 min and run on 10% Tris–Glycine NB NuSep precast gels (NuSep, Bogart, GA, USA). The gels were then transferred to polyvinylidene difluoride membranes (iBlot gel transfer PVDF, Invitrogen #iB4010, Grand Island, NY, USA), washed three times for 10 min each in Tris-buffered saline (TBS) with 0.05% Tween-20 (TBS-T) and blocked in TBS-T with 5% nonfat dry milk for 1 h. Membranes were then incubated in the appropriate primary antiserum (DBH: 1:1000, #AB1585, Lot: 2159603, Millipore, Billerica, MA, USA; TPH2: 1:500, #ABN60, Lot: 2069370, Millipore, Billerica, MA, USA) in TBS-T with 5% non-fat dry milk overnight at 4 °C. Membranes were washed three times for 10 min each in TBS-T, then incubated in a peroxidase-conjugated antirabbit IgG secondary antiserum with 5% milk (1:2000, #7074, Lot: 24, Cell Signaling Technology, St. Louis, MO, USA) for 1 h, and rinsed in TBS three times for 10 min each. Immunoreactive bands were detected with an enhanced chemiluminescence kit (Western Blotting Luminol Reagent, #SC-2048; Santa Cruz Biotechnology, Santa Cruz, CA, USA) and membranes were immediately exposed to film (Blue Sensitive X-ray film, Laboratory Products Sales, Rochester, NY, USA), developed, and fixed using a Kodak X-OMAT 1000A Processor (Kodak, Rochester, NY, USA).
Membranes were stripped and probed for glyceraldehyde 3-phosphate dehydrogenase (GAPDH) as the “housekeeping” protein to control for any differences in total protein among lanes. Membranes were rinsed twice for 10 min in TBS-T and incubated in stripping buffer (Restore Plus Western blot, #46430, Thermo Scientific, Rockford, IL, USA) for 15 min at 37 °C. Membranes were then washed four times for 10 min each in TBS-T, blocked again for 1 h in TBS-T with 5% nonfat dry milk and incubated overnight at 4 °C with a mouse polyclonal antiserum raised against GAPDH (1:500; MAB374; Lot: 2145925, Millipore, Billerica, MA, USA), rinsed with TBS-T three times, then incubated for 1 h with peroxidase-conjugated rabbit antimouse secondary antiserum (1:80,000; #A9044, Lot: 010M4797, Sigma–Aldrich, St. Louis, MO, USA). The remaining procedures were as described above for DBH and TPH2 blotting. Control blots included using bovine adrenal medulla (#ab140410, lot: APN113621-1, Abcam, Cambridge, MA, USA) run alongside subjects’ brainstem samples, which revealed the expected single band at ~67 kDa for DBH, and preabsorption of the TPH2 primary antiserum with 1 mg/ml TPH2 peptide (#EBP11011, Lot: 21 Abcore, Ramona, CA, USA) run in parallel with non-preabsorbed dorsal raphe samples, which eliminated TPH2-immunoreactive bands.
Following developing, films were placed on a light box (Model BL1824 # 24762, Hall-Productions Co., Grover Beach, CA, USA) and images of the immunoreactive bands were captured using a digital camera (Roper Scientific Photometrics, Tucson, AZ, USA). ImageJ (National Institutes of Health, Bethesda, MD, USA) was used to determine the integrated density of the immunoreactive bands. The values for the DBH and TPH2 integrated densities were standardized using the subjects’ corresponding GAPDH integrated density measurements.
Statistical analyses
Non-normal data were log-transformed before parametric analyses. Two-way analysis of variance (ANOVA) was used to compare the anxiety-related behavior and brain amine content of the least- and most-anxious females that were either permitted or denied contact with offspring before testing. Significant interactions were further analyzed with simple main effects analyses. Two subjects from the low-anxious group did not get pregnant and were not tested on the elevated plus maze. Two subjects (one from the least-anxious group and one from the most-anxious group) were revealed on a quantile–quantile plot as significant outliers for their behavior in the elevated plus maze and were removed from the analyses of those data. Spearman correlations were used to examine the relationships among females’ pre-mating anxiety, postpartum anxiety, and brain amine expression. Statistical significance was indicated by p ≤ 0.05.
RESULTS
Effects of offspring contact on postpartum anxiety
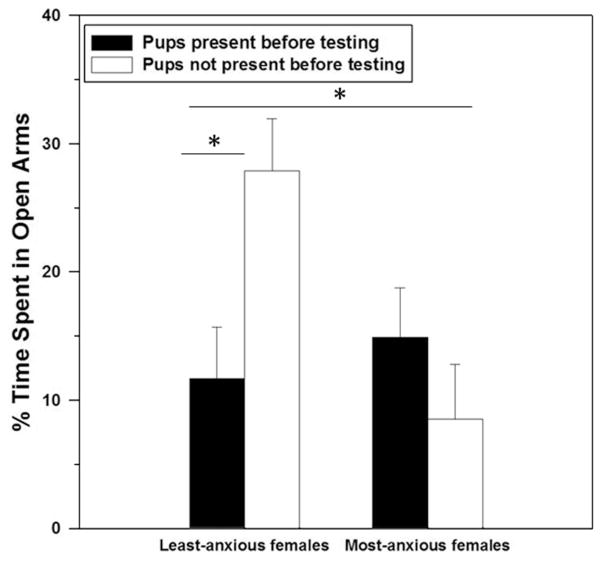
As expected, when collapsed across separation group the females assigned to the most-anxious group (based on their pre-mating behavior in the light–dark box) later spent a lower percentage of time in the open arms of the elevated plus maze when tested postpartum compared to the least-anxious group of females (F(1, 34) = 4.16, P < 0.05). Although our laboratory previously found that removing offspring four hours before testing increases anxiety in unselected postpartum rats from our colony (Lonstein, 2005; Figueira et al., 2008; Smith and Lonstein, 2008; Miller et al., 2011), there was no main effect of offspring contact on the percentage of time spent in the open arms of the elevated plus maze by the selected dams used in this study (F(1, 34) = 0.34, P = 0.56). However, there was a significant interaction between anxiety group and offspring contact on the percentage of time spent in the open arms (F(1, 34) = 6.35, P = 0.02), which was mostly driven by an anxiogenic effect of offspring contact in the least-anxious mothers (F(1, 15) = 6.50, P = 0.02, Fig. 1). Importantly, offspring presence did not significantly affect the percentage of time that the most-anxious mothers spent in the open arms of elevated plus maze (F(1, 17) = 0.86, P = 0.37).
Fig. 1.
Percentage of time (Mean ± SEM) spent in the open arms of an elevated plus maze by postpartum female laboratory rats that displayed low or high anxiety-related behavior before mating and had contact with offspring or not before postpartum anxiety testing. Data were log-transformed for analysis but the untransformed data are shown. There was a significant main effect of the anxiety group, and an interaction between the anxiety group and offspring contact that was driven by the least-anxious females (both indicated by asterisks, P < 0.05).
There was a small but significant main effect of offspring contact on the percentage of entries made into the open arms of the elevated plus maze (F(1, 34) = 5.67, P = 0.02), with females denied offspring contact making more entries into open arms compared to females permitted contact before testing (11±2 vs. 7±1 entries; F(34) = 6.28, P = 0.02). There was no significant main effect of anxiety group (F(1, 34) = 0.56, P = 0.46), and no significant interaction between anxiety group and offspring contact (F(1, 34) = 0.14, P = 0.71), on the percentage of entries that were made in the open arms of the elevated plus maze.
There was no significant main effect of offspring contact on the frequency of entries made into the closed arms of the elevated plus maze (10±1 vs. 10±1; F(1, 31) = 0.03, P = 0.87), but there was a marginally significant main effect of anxiety group on this measure (least-anxious vs. most-anxious females, 11±1 and 9±1 entries, respectively; F(1, 31) = 3.31, P = 0.08) as well as a marginally significant interaction between anxiety group and offspring contact (F(1, 31) = 3.25, P = 0.08).
Correlations between pre-mating anxiety and postpartum anxiety
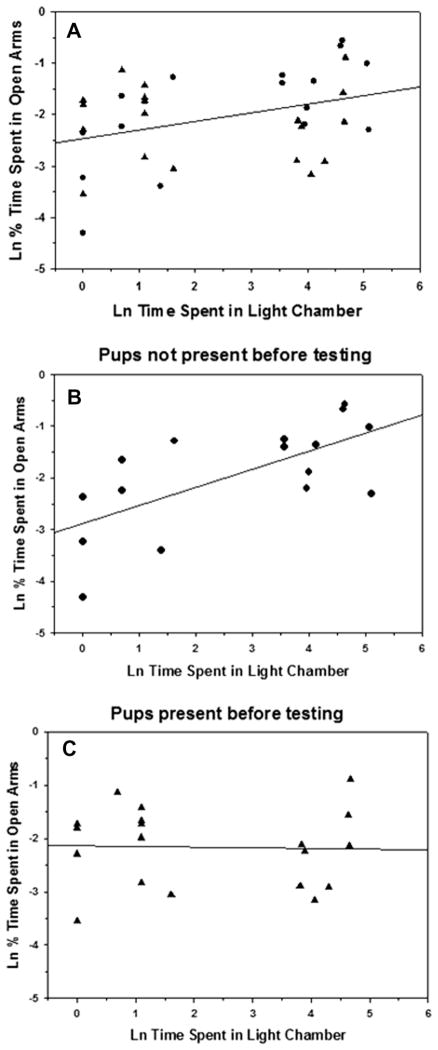
Females’ pre-mating anxiety behavior (i.e., the duration of time spent in the light chamber of the light–dark box) was significantly positively correlated with their postpartum anxiety in the elevated plus maze (i.e., the percentage of time spent in the open arms) (r(34) = 0.35, P < 0.05; Fig. 2A). Consistent with the results described above, the strength of this relationship was influenced by offspring contact because while pre-mating anxiety was a particularly strong predictor of postpartum anxiety in the females denied offspring contact 4 h before testing (r(15) = 0.64, P < 0.01; Fig. 2B), pre-mating anxiety was not significantly associated with postpartum anxiety behavior in females that were permitted contact with offspring until testing (r(19) = −0.01, P = 0.97; Fig. 2C).
Fig. 2.
Correlations between the percentage of time postpartum female laboratory rats spent in the open arms of an elevated plus maze and the time they had spent in the light chamber of the light–dark box before mating. (A) All subjects, (B) subjects with no offspring contact for 4 h before elevated plus-maze testing, (C) subjects with offspring contact until elevated plus-maze testing. Data were log-transformed before analysis. Circles – offspring were present before testing; triangles – offspring were not present before testing.
Pre-mating anxiety was not significantly associated with the percentage of entries made into the open arms of the elevated plus maze (r(34) = 0.26, P = 0.13). This was true for females that had offspring removed before testing (r(15) = 0.32, P < 0.23), and also for females that remained with offspring until testing (r(19) = 0.21, P < 0.39).
Relationships among anxiety, offspring contact and brain amine content
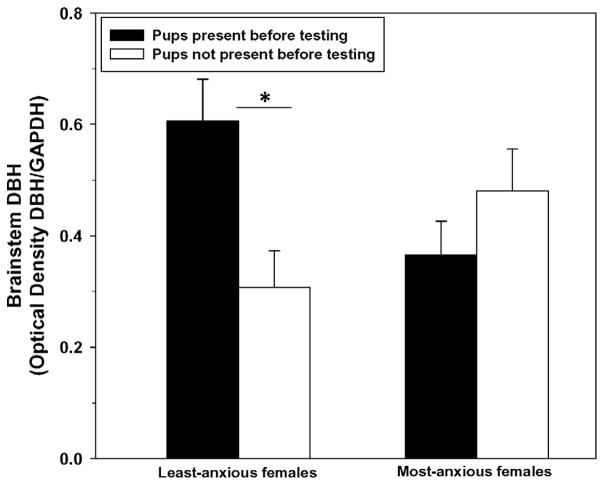
There were no significant main effects of anxiety group (F(1, 33) = 0.28, P = 0.60) or offspring presence (F(1, 33) = 1.74, P = 0.20) on brainstem DBH expression. Similar to the elevated plus-maze results, there was a significant interaction between these factors (F(1, 33 = 4.66, P = 0.04; Fig. 3), such that offspring presence before testing was associated with higher DBH expression in the brainstem of least-anxious females (F(1,15) = 9.59, P < 0.01) but there was no difference between the two groups of most-anxious females (F(1,17) = 0.27, P = 0.61). There were no significant correlations between the duration of time females spent in the light chamber of the light–dark box before mating (r(34) = 0.15, P = 0.39), or the percentage of time they spent in the open arms of the elevated plus maze postpartum (r(33) = 0.09, P = 0.60), and brainstem DBH expression. This was also true when examined within each anxiety group and within each offspring-contact group.
Fig. 3.
Optical density of dopamine beta-hydroxylase (DBH) in the brainstem of the least-anxious and most-anxious postpartum female rats (Means ± SEM). Data were log-transformed for analysis but the untransformed data are shown. There was a significant interaction between the anxiety group and offspring contact, driven by the least-anxious females and indicated by the asterisk, P < 0.05.
There were no significant main effects of anxiety group (F(1,35) = 1.53, P = 0.22) or offspring presence (F(1,35) = 0.16, P = 0.69) on TPH2 expression in the dorsal raphe. There was also no significant interaction between these factors on TPH2 expression in the dorsal raphe (F(1,35) = 0.43, P = 0.51). We found no significant correlations between the duration of time females spent in the light chamber of the light–dark box before mating (r(36) = 0.15, P = 0.38) or the percentage of time mothers spent in the open arms of the elevated plus maze and their TPH2 expression in the dorsal raphe (r(35) = 0.05, P = 0.76).
DISCUSSION
The endocrine consequences of pregnancy and parturition very strongly motivate female mammals to interact with neonates, but these hormonal influences quickly wane, and physical contact with offspring (either suckling or non-suckling) is instead necessary to maintain the suite of behavioral changes that have occurred in the mother (Numan et al., 2006; Lonstein and Morrell, 2007; Lonstein et al., 2013). For most animals studied, these changes include a reduction in anxiety and other indicators of emotional reactivity (Fleming and Luebke, 1981; Hard and Hansen, 1985; Neumann, 2003; Lonstein, 2007). This blunted emotional reactivity in postpartum mothers requires recent physical interaction with the litter (Lonstein, 2005; Figueira et al., 2008; Smith and Lonstein, 2008; Miller et al., 2011), and can even be observed in nulliparous laboratory rats that are induced to express maternal behavior and interact with pups (Ferreira et al., 2002; Pereira et al., 2005; Agrati and Zuluaga, 2008).
We herein found that this is not the case for all postpartum rats because, depending on their innate anxiety, recent litter contact produced different effects on dams’ elevated plus-maze behavior. Contrary to what we had hypothesized based on the relationship between anxiety and tactile sensitivity (Jorum, 1988; van Meeteren et al., 1997; Kain et al., 2000; Rhudy and Meagher, 2000; Geerse et al., 2006; Devall et al., 2009; Aron et al., 2012; Corral-Frias et al., 2013), anxiety behavior in the most-anxious females was almost unaffected by the presence of offspring before testing. Instead, the least-anxious females were the most affected by offspring presence. In addition, the relationship between pre-mating and postpartum anxiety was the strongest in the females that were denied contact with offspring before testing, suggesting that one consequence of infant contact is to shift some mothers’ anxiety away from their innate or trait levels of anxiety. While aspects of these findings may appear inconsistent with some of our previous work demonstrating that the absence of offspring increases anxiety in postpartum rats (Lonstein, 2005; Figueira et al., 2008; Smith and Lonstein, 2008; Miller et al., 2011), the difference is very likely due to the fact that our previous studies did not involve prescreening females for anxiety and subsequently testing only the females lying at the extremes.
The striking decrease in anxiety-related behavior after the least-anxious females were separated from their litters might suggest that heightened emotional reactivity when pups are present is optimal for successful mothering in this population. This was also suggested by the small but significantly higher number of open entries made by all dams that were interacting with pups before testing compared to those that had pups removed. An increase in anxiety in low-anxious dams while they interact with pups could help focus their maternal attention to the needs of the young, possibly refining mother–offspring interactions and enhancing the mother’s ability to protect the nest (Lonstein and Gammie, 2002; Bosch, 2011). Some support for this suggestion in laboratory rats comes from the findings that mothers genetically selected for low anxiety or other emotional reactivity are less maternally responsive than high-anxiety dams, particularly when tested under novel or stressful conditions (Holland, 1965; Driscoll et al., 1979; Fuemm and Driscoll, 1981; Neumann et al., 2005; Clinton et al., 2007; Kessler et al., 2011; Curley et al., 2012). Additionally, low anxiety-related behavior in postpartum rhesus monkey mothers is associated with less interest in and concern about the infant (Maestripieri, 1993a,b, 1998). A similar suggestion has been made for women, such that individual differences in maternal psychological factors are a critical influence on maternal behavior (Barrett and Fleming, 2011) and that unusually low anxiety and inadequate arousal could contribute to inattentiveness to infant cues and a lack of preoccupation about the needs of the infant (Pryce, 1992; Fleming et al., 1997; Leckman et al., 1999, 2004; Stallings et al., 2001; Bosch, 2011).
Our finding that offspring presence only slightly and non-significantly affected anxiety in the most-anxious mothers was unexpected, because we predicted that these rat mothers could benefit the most from an anxiolytic effect of interacting with young. It may be that these most-anxious dams are less sensitive to tactile inputs from offspring compared to other mothers, but this is inconsistent with the positive relationship between anxiety and tactile sensitivity that exists at least in humans (Jorum, 1988; van Meeteren et al., 1997; Kain et al., 2000; Rhudy and Meagher, 2000; Geerse et al., 2006; Devall et al., 2009; Aron et al., 2012; Corral-Frias et al., 2013). Furthermore, female rats that are the most effective retrievers and more prone to display kyphosis (i.e., crouched nursing), two behaviors that depend on sensitivity of the dam’s perioral region and ventrum to pup-related tactile inputs (Stern, 1996), are relatively more anxious rather than less anxious (Caldji et al., 1998; Bosch, 2011). A ceiling effect could instead have contributed to these results, such that high-anxiety mothers are immune to factors such as litter removal that could potentially increase anxiety. For instance, intracerebroventricular infusion of corticotropin-releasing factor cannot increase anxiety in female rats that are already highly anxious (Klampfl et al., 2013). On the other hand, anxiolytic drugs cannot further decrease anxiety in already low-anxious animals (Wegener et al., 2012).
Our study revealed a significant positive correlation between females’ pre-mating and postpartum anxiety-related behaviors. This relationship is reminiscent of the stability in anxiety before and after giving birth in women (Engle et al., 1990; Heron et al., 2004; Breitkopf et al., 2006; Bussel et al., 2006; Britton, 2008; Grant et al., 2008). Anxiety-related behavior in nulliparous female laboratory rodents is stable across many months (Leibsch et al., 1998; Henniger et al., 2000; Curley et al., 2012), suggesting the existence of an ongoing “trait” anxiety (Gosling, 2001). It is unknown whether or not anxiety-related behavior in our least-anxious and most-anxious rats continues to be predictable after the first week postpartum, but this may be likely because there is a significant correlation between the anxiety of randomly selected female mice tested during the first postpartum week and again several days post-weaning (Rödel et al., 2012). Furthermore, mother rhesus monkeys show stable anxiety across multiple births spanning years (Maestripieri, 2000). In the only other study including an assessment of trait-dependent anxiety across reproductive states in laboratory rats, Neumann et al. (2005) reported that female rats selectively bred for high- or low-anxiety profiles maintained their trait anxiety after giving birth for the first time. Our results extend these findings to rats that are not genetically selected for their emotional behavior and these genetically heterogeneous rats may more closely represent the least-anxious and most-anxious female rats found in most laboratory colonies. Studying such rats can complement studies of genetically predisposed animals by addressing the substantial non-genetic influences on anxiety (Hettema et al., 2001a,b; Clément et al., 2002; Francis et al., 2003; Caldji et al., 2004; Priebe et al., 2005) and humans (Kendler et al., 1992; Legrand et al., 1999; Hettema et al., 2001a,b; Lau et al., 2006).
While anxiety seems to be an inherent trait in female rats, it cannot be forgotten that mother–infant interactions are necessarily dyadic, so the emotional reactivity of the mother could be influenced by that of her offspring and vice versa (Britton, 2011). Emotional reactivity in rat pups is reflected behaviorally by the rate they emit ultrasonic vocalizations (Shair et al., 1997; Barron et al., 2000; Branchi et al., 2001; Brunelli, 2005; D’Amato et al., 2005; Wöhr and Schwarting, 2008) and the presence of frequently vocalizing and emotionally reactive pups may not be soothing for their mothers. In fact, pups of females bred for low anxiety and high novelty-seeking are especially demanding of maternal attention (Clinton et al., 2010) and a respite from their frequent solicitations may reduce anxiety in their dams. Because the increased demand for maternal care by low-anxious pups is found even when they are cross-fostered to high-anxious dams (Clinton et al., 2010), a future study could determine if the elevated plus-maze behavior of low- or high-anxiety rats is differently affected by separation from the litter depending on the phenotype of the pups they interact with before testing.
Based on the positive association most often found between central noradrenergic activity and anxiety in nulliparous laboratory rodents (Charney, 1996; Tanaka et al., 2000; Cecchi et al., 2002; Fendt et al., 2005; Schweimer et al., 2005), we hypothesized that high brainstem DBH expression (which is highly correlated with elevated norepinephrine synthesis; Coyle and Axelrod, 1972; Hartman et al., 1972) would be related to greater anxiety-related behavior in dams. This hypothesis was supported, such that group differences in the DBH expression generally reflected the group differences in dams’ anxiety-related behavior, with DBH significantly reduced after litter removal in the least-anxious females but non-significantly affected by offspring contact in the most-anxious females. It could have been possible that brainstem DBH was affected by suckling for the dams permitted physical contact with the litter before testing and sacrifice (Moyer et al., 1979; Crowley et al., 1987; Kendrick et al., 1992), but it is important to note there was no significant main effect of offspring presence on maternal brainstem DBH expression. Forebrain sites that receive noradrenergic input possibly mediating these offspring-associated changes in anxiety in the least-anxious females are unknown, but a good candidate is the ventral bed nucleus of the stria terminalis (BSTv). The BSTv contains the densest noradrenergic plexus in the forebrain (Kilts and Anderson, 1986; Fendt et al., 2005) and is intimately associated with anxiety in mammals (Davis et al., 2010). Although we have found that acute suppression or stimulation of norepinephrine release in the BSTv has little effect on anxiety-related behavior in randomly selected postpartum female rats (Smith et al., 2013), longer term manipulation of noradrenergic activity in the BSTv (hours or days rather than minutes) could be found to have consequences for their anxiety (Choi et al., 2008). In addition to affecting the BSTv, high brainstem DBH synthesis in low-anxiety mothers while they interact with pups could be associated with activation of forebrain-projecting noradrenergic cells of the locus coeruleus that increase maternal arousal and attention to external sensory information (Aston-Jones et al., 1999; Berridge and Waterhouse, 2003) or even brainstem adrenergic cells that influence autonomic function (Madden and Sved, 2003).
As noted above, the relationship between central serotonergic activity and anxiety is sometimes unclear (Briley et al., 1990; Critchley et al., 1992; Kalueff et al., 2007; Hovatta and Barlow, 2008; Olivier et al., 2008; Huh et al., 2011; Mosienko et al., 2012). Nonetheless, TPH2 expression in the dorsal raphe has repeatedly been reported to be negatively correlated with anxiety-related behavior in nulliparous female and male laboratory rodents (Gutknecht et al., 2007; Reuter et al., 2007; Beaulieu et al., 2008; Berger et al., 2012). We did not find that this relationship extended to postpartum female rats. Reproductive state has been seen to determine the relationship between brain serotonergic activity and anxiety, including that serotonin-based anxiolytics that are effective in nulliparous laboratory rats have no effect in early postpartum rats (Fernández-Guasti et al., 1998; Picazo et al., 2000; Heikkinen et al., 2002). This may be due to alterations in numerous aspects of central serotonergic functioning across reproductive states (Handley et al., 1977, 1980; Okatani et al., 1990; Schrocksnadel et al., 1996; Klink et al., 2002; Suda et al., 2008).
CONCLUSION
Mother rats differing in their innate or “trait” levels of anxiety-related behavior are differentially susceptible to the anxiolytic effects of physical contact with offspring. These differences were associated with the capacity for norepinephrine synthesis, but not serotonin synthesis, in the maternal brain. If these findings translate to humans, the present and previous data collectively suggest that women with modest levels of anxiety would benefit the most from enhanced physical contact with infants. Indeed, there is evidence that “kangaroo care” promoting physical contact between mothers and infants reduces anxiety in postpartum women (Shiau, 1998; Lee and Shin, 2007), which complements the larger literature on the anxiety-reducing effects of touch outside the context of the mother–infant dyad (Field et al., 1992; Field, 1998). For women with relatively low anxiety, however, infant touch may have the opposite effect (i.e., raise anxiety), possibly by activating noradrenergic pathways that beneficially increase vigilance and focus maternal attention. Lastly, anxiety in the most anxious mothers may be less easily ameliorated or susceptible to the potential anxiety-modulating effects of infant touch.
Acknowledgments
This research was supported by NICHD grant #R01HD057962 to JSL. The authors would like to thank Eman Ahmed, Sarah Armstrong, Michael Donlin, Katie Harding, M. Allie Holschbach, and Katrina Linning for their assistance with various components of this project.
Abbreviations
- BSTv
ventral bed nucleus of the stria terminalis
- DBH
dopamine β-hydroxylase
- EPM
elevated plus maze
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- TBS
Tris-buffered saline
- TBS-T
Tris-buffered saline with 0.05% Tween-20
- TPH2
tryptophan hydroxylase-2
Footnotes
CONTRIBUTORS
C.M. Ragan is the primary author who conceptualized and performed experiments and substantially contributed to the writing of this manuscript. J.S. Lonstein is the principal investigator of the laboratory, conceptualized the experiments, and substantially contributed to the writing of this manuscript. All authors have approved the final article.
FINANCIAL DISCLOSURES
The authors have nothing to declare.
References
- Agrati D, Zuluaga MJ, Fernández-Guasti A, Meikle A, Ferreira A. Maternal condition reduces fear behaviors but not the endocrine response to an emotional threat in virgin female rats. Horm Behav. 2008;53(1):232–240. doi: 10.1016/j.yhbeh.2007.10.002. [DOI] [PubMed] [Google Scholar]
- Aron EN, Aron A, Jagiellowicz J. Sensory processing sensitivity: a review in the light of the evolution of biological responsivity. Pers Soc Psychol Rev. 2012;16:262–282. doi: 10.1177/1088868311434213. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Rajkowski J, Cohen J. Role of locus coeruleus in attention and behavioral flexibility. Biol Psychiatry. 1999;46:1309–1320. doi: 10.1016/s0006-3223(99)00140-7. [DOI] [PubMed] [Google Scholar]
- Barrett J, Fleming AS. Annual research review: all mothers are not created equal: neural and psychobiological perspectives on mothering and the importance of individual differences. J Child Psychol Psychiatry. 2011;52:368–397. doi: 10.1111/j.1469-7610.2010.02306.x. [DOI] [PubMed] [Google Scholar]
- Barron S, Segar T, Yahr J, Baseheart B, Willford J. The effects of neonatal ethanol and/or cocaine exposure on isolation-induced ultrasonic vocalizations. Pharmacol Biochem Behav. 2000;67:1–9. doi: 10.1016/s0091-3057(00)00304-x. [DOI] [PubMed] [Google Scholar]
- Beaulieu JM, Zhang X, Rodriguiz RM, Sotnikova TD, Cools MJ, Wetsel WC, Gainetdinov RR, Caron MG. Role of GSK3 beta in behavioral abnormalities induced by serotonin deficiency. Proc Natl Acad Sci U S A. 2008;105:1333–1338. doi: 10.1073/pnas.0711496105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger SM, Weber T, Perreau-Lenz S, Vogt MA, Gartside SE, Maser-Gluth C. A functional Tph2 C1473G polymorphism causes an anxiety phenotype via compensatory changes in the serotonergic system. Neuropsychopharmacology. 2012;37:1986–1998. doi: 10.1038/npp.2012.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge CW, Waterhouse BD. The locus coeruleus–noradrenergic system: modulation of behavioral state and state-dependent cognitive processes. Brain Res Brain Res Rev. 2003;42:33–84. doi: 10.1016/s0165-0173(03)00143-7. [DOI] [PubMed] [Google Scholar]
- Bosch OJ. Maternal nurturing is dependent on her innate anxiety: the behavioral roles of brain oxytocin and vasopressin. Horm Behav. 2011;59:202–212. doi: 10.1016/j.yhbeh.2010.11.012. [DOI] [PubMed] [Google Scholar]
- Bourin M, Hascoët M. The mouse light/dark box test. Eur J Pharmacol. 2003;463:55–65. doi: 10.1016/s0014-2999(03)01274-3. [DOI] [PubMed] [Google Scholar]
- Branchi I, Santucci D, Alleva E. Ultrasonic vocalisation emitted by infant rodents: a tool for assessment of neurobehavioural development. Behav Brain Res. 2001;125:49–56. doi: 10.1016/s0166-4328(01)00277-7. [DOI] [PubMed] [Google Scholar]
- Breitkopf CR, Primeau LA, Levine RE, Olson GL, Wu ZH, Berenson AB. Anxiety symptoms during pregnancy and postpartum. J Psychosom Obstet Gynaecol. 2006;27:157–162. doi: 10.1080/01674820500523521. [DOI] [PubMed] [Google Scholar]
- Briley M, Chopin P, Moret C. Effect of serotonergic lesion on “anxious” behaviour measured in the elevated plus-maze test in the rat. Psychopharmacology. 1990;101:187–189. doi: 10.1007/BF02244124. [DOI] [PubMed] [Google Scholar]
- Britton JR. Maternal anxiety: course and antecedents during the early postpartum period. Depress Anxiety. 2008;25:793–800. doi: 10.1002/da.20325. [DOI] [PubMed] [Google Scholar]
- Britton JR. Infant temperament and maternal anxiety and depressed mood in the early postpartum period. Women Health. 2011;51:55–71. doi: 10.1080/03630242.2011.540741. [DOI] [PubMed] [Google Scholar]
- Brunelli SA. Selective breeding for an infant phenotype: rat pup ultrasonic vocalization (USV) Behav Genet. 2005;35:53–65. doi: 10.1007/s10519-004-0855-6. [DOI] [PubMed] [Google Scholar]
- Burtt HE. The psychology of birds. New York: Macmillan; 1967. [Google Scholar]
- Van Bussel JCH, Spitz B, Demyttenaere K. Women’s mental health before, during, and after pregnancy: a population-based controlled cohort study. Birth. 2006;33:297–302. doi: 10.1111/j.1523-536X.2006.00122.x. [DOI] [PubMed] [Google Scholar]
- Caldji C, Tannenbaum B, Sharma S, Francis D, Plotsky PM, Meaney MJ. Maternal care during infancy regulates the development of neural systems mediating the expression of fearfulness in the rat. Proc Natl Acad Sci U S A. 1998;95:5335–5340. doi: 10.1073/pnas.95.9.5335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavigelli SA, Stine MM, Kovacsics C, Jefferson A, Diep MN, Barrett CE. Behavioral inhibition and glucocorticoid dynamics in a rodent model. Physiol Behav. 2007;92:897–905. doi: 10.1016/j.physbeh.2007.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavigelli SA, Michael KC, Ragan CM. Behavioral, physiological, and health biases in laboratory rodents: a basis for understanding mechanistic links between human personality and health. In: Carere C, Maestripieri D, editors. Animal personalities: behavior, physiology and evolution. Chicago: University of Chicago Press; 2013. [Google Scholar]
- Cecchi M, Khoshbouei H, Morilak DA. Modulatory effects of norepinephrine, acting on alpha 1 receptors in the central nucleus of the amygdala, on behavioral and neuroendocrine responses to acute immobilization stress. Neuropharmacology. 2002;43:1139–1147. doi: 10.1016/s0028-3908(02)00292-7. [DOI] [PubMed] [Google Scholar]
- Charney DS. Noradrenergic mechanisms in stress and anxiety: I. Preclinical studies. Synapse. 1996;23:28–38. doi: 10.1002/(SICI)1098-2396(199605)23:1<28::AID-SYN4>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Choi DC, Furay AR, Evanson NK, Ulrich-Lai YM, Nguyen MM, Ostrander MM, Herman JP. The role of the posterior medial bed nucleus of the stria terminalis in modulating hypothalamic–pituitary–adrenocortical axis responsiveness to acute and chronic stress. Psychoneuroendocrinology. 2008;33:659–669. doi: 10.1016/j.psyneuen.2008.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke AS, Boinski S. Temperament in nonhuman primates. Am J Primatol. 1995;37:103–125. doi: 10.1002/ajp.1350370205. [DOI] [PubMed] [Google Scholar]
- Clément Y, Calatayud F, Belzung C. Genetic basis of anxiety-like behaviour: a critical review. Brain Res Bull. 2002;57:57–71. doi: 10.1016/s0361-9230(01)00637-2. [DOI] [PubMed] [Google Scholar]
- Clinton SM, Vázquez DM, Kabbaj M, Kabbaj M-H, Watson SJ, Akil H. Individual differences in novelty-seeking and emotional reactivity correlate with variation in maternal behavior. Horm Behav. 2007;51:655–664. doi: 10.1016/j.yhbeh.2007.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clinton SM, Bedrosian TA, Abraham AD, Watson SJ, Akil H. Neural and environmental factors impacting maternal behavior differences in high- versus low-novelty-seeking rats. Horm Behav. 2010;57:463–473. doi: 10.1016/j.yhbeh.2010.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corral-Frias NS, Lahood RP, Edelman-Vogelsang KE, French ED, Fellous JM. Involvement of the ventral tegmental area in a rodent model of post-traumatic stress disorder. Neuropsychopharmacology. 2013;38:350–363. doi: 10.1038/npp.2012.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costall B, Jones BJ, Kelly ME, Naylor RJ, Tomkins DM. Exploration of mice in a black and white test box: validation as a model of anxiety. Pharm Biochem Behav. 1989;32:777–785. doi: 10.1016/0091-3057(89)90033-6. [DOI] [PubMed] [Google Scholar]
- Coyle JT, Axelrod J. Dopamine-β-hydroxylase in the rat brain: developmental characteristics. J Neurochem. 1972;19:449–459. doi: 10.1111/j.1471-4159.1972.tb01354.x. [DOI] [PubMed] [Google Scholar]
- Critchley MA, Njung’e K, Handley SL. Actions and some interactions of 5-HT1A ligands in the elevated X-maze and effects of dorsal raphe lesions. Psychopharmacology. 1992;106:484–490. doi: 10.1007/BF02244819. [DOI] [PubMed] [Google Scholar]
- Crowley WR, Shyr SW, Kacsoh B, Grosvenor CE. Evidence for stimulatory noradrenergic and inhibitory dopaminergic regulation of oxytocin release in the lactating rat. Endocrinology. 1987;121:4–20. doi: 10.1210/endo-121-1-14. [DOI] [PubMed] [Google Scholar]
- Cruz APM, Frei F, Graeff FG. Ethopharmacological analysis of rat behavior on the elevated plus-maze. Pharmacol Biochem Behav. 1994;49:171–176. doi: 10.1016/0091-3057(94)90472-3. [DOI] [PubMed] [Google Scholar]
- Curley JP, Jensen CL, Franks B, Champagne FA. Variation in maternal and anxiety-like behavior associated with discrete patterns of oxytocin and vasopressin 1a receptor density in the lateral septum. Horm Behav. 2012;61:454–461. doi: 10.1016/j.yhbeh.2012.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Amato FR, Scalera E, Sarli C, Moles A. Pup calls, mothers rush: does maternal responsiveness affect the amount of ultrasonic vocalization in mouse pups? Behav Genet. 2005;35:103–112. doi: 10.1007/s10519-004-0860-9. [DOI] [PubMed] [Google Scholar]
- Davis M, Walker DL, Miles L, Grillon C. Phasic vs. sustained fear in rats and humans: role of the extended amygdala in fear vs. anxiety. J Neuropsychopharmacol. 2010;35:105–135. doi: 10.1038/npp.2009.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dazzi L, Vignone V, Seu E, Ladu S, Vacca G, Biggio G. Inhibition by venlafaxine of the increase in norepinephrine output in rat prefrontal cortex elicited by acute stress or by the anxiogenic drug FG 7142. J Psychopharmacol. 2002;16:125–131. doi: 10.1177/026988110201600202. [DOI] [PubMed] [Google Scholar]
- Debiec J, LeDoux LE. Noradrenergic signaling in the amygdala contributes to the reconsolidation of fear memory: treatment implications for PTSD. Ann N Y Acad Sci. 2006;1071:521–524. doi: 10.1196/annals.1364.056. [DOI] [PubMed] [Google Scholar]
- Devall AJ, Liu ZW, Lovick TA. Hyperalgesia in the setting of anxiety: sex differences and effects of the oestrous cycle in Wistar rats. Psychoneuroendocrinology. 2009;34:587–596. doi: 10.1016/j.psyneuen.2008.10.021. [DOI] [PubMed] [Google Scholar]
- Dinan T. Serotonin and the regulation of hypothalamic–pituitary–adrenal axis function. Life Sci. 1996;58:1683–1694. doi: 10.1016/0024-3205(96)00066-5. [DOI] [PubMed] [Google Scholar]
- Donner N, Handa RJ. Estrogen receptor beta regulates the expression of tryptophan-hydroxylase 2 mRNA within serotonergic neurons of the rat dorsal raphe nuclei. Neuroscience. 2009;163:705–718. doi: 10.1016/j.neuroscience.2009.06.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas AJ. Central noradrenergic mechanisms underlying acute stress responses of the hypothalamo–pituitary–adrenal axis: adaptations through pregnancy and lactation. Stress. 2005;8:5–18. doi: 10.1080/10253890500044380. [DOI] [PubMed] [Google Scholar]
- Driscoll P, Fuemm H, Battig K. Maternal behavior in two rat lines selected for differences in the acquisition of two-way avoidance. Experientia. 1979;35:786–788. doi: 10.1007/BF01968248. [DOI] [PubMed] [Google Scholar]
- Engle PL, Scrimshaw SC, Zambrana RE, Dunkel-Schetter C. Prenatal and postnatal anxiety in Mexican women giving birth in Los Angeles. Health Psychol. 1990;9:285–299. doi: 10.1037//0278-6133.9.3.285. [DOI] [PubMed] [Google Scholar]
- Feldman S, Conforti N, Melamed E. Paraventricular nucleus serotonin mediates neurally stimulated adrenocortical secretion. Brain Res Bull. 1987;18:165–168. doi: 10.1016/0361-9230(87)90186-9. [DOI] [PubMed] [Google Scholar]
- Fendt M, Siegl S, Steiniger-Brach B. Noradrenaline transmission within the ventral bed nucleus of the stria terminalis is critical for fear behavior induced by trimethylthiazoline, a component of fox odor. J Neurosci. 2005;25:5998–6004. doi: 10.1523/JNEUROSCI.1028-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández-Guasti A, Picazo O, Ferreira A. Blockade of the anxiolytic action of 8-OH-DPAT in lactating rats. Pharmacol Biochem Behav. 1998;59:45–50. doi: 10.1016/s0091-3057(97)00392-4. [DOI] [PubMed] [Google Scholar]
- Ferreira A, Pereira M, Agrati D, Uriarte N, Fernández-Guasti A. Role of maternal behavior on aggression, fear and anxiety. Physiol Behav. 2002;77:197–204. doi: 10.1016/s0031-9384(02)00845-4. [DOI] [PubMed] [Google Scholar]
- Field TM. Touch therapies. In: Hoffman RR, Sherrick MF, Warm JS, editors. Viewing psychology as a whole: the integrative science of William N. Dember. Washington, DC: APA; 1998. pp. 603–624. [Google Scholar]
- Field T, Morrow C, Valdeon C, Larson S, Kuhn C, Schanberg S. Massage reduces anxiety in child and adolescent psychiatric patients. J Am Acad Child Adolesc Psychiatry. 1992;31:125–131. doi: 10.1097/00004583-199201000-00019. [DOI] [PubMed] [Google Scholar]
- Figueira RJ, Peabody MF, Lonstein JS. Oxytocin receptor activity in the ventrocaudal periaqueductal gray modulates anxiety-related behavior in postpartum rats. Behav Neurosci. 2008;122:618–628. doi: 10.1037/0735-7044.122.3.618. [DOI] [PubMed] [Google Scholar]
- File SE. New strategies in the search for anxiolytics. Drug Des Deliv. 1990;5:195–201. [PubMed] [Google Scholar]
- Fleming AS, Luebke A. Timidity prevents the virgin female rat from being a good mother: emotionality differences between nulliparous and parturient females. Physiol Behav. 1981;27:863–868. doi: 10.1016/0031-9384(81)90054-8. [DOI] [PubMed] [Google Scholar]
- Francis DD, Szegda K, Campbell G, Martin WD, Insel TR. Epigenetic sources of behavioral differences in mice. Nat Neurosci. 2003;6:445–446. doi: 10.1038/nn1038. [DOI] [PubMed] [Google Scholar]
- Fuemm H, Driscoll P. Litter size manipulations do not alter maternal behaviour traits in selected lines of rats. Anim Behav. 1981;29:1267–1269. [Google Scholar]
- Geerse GJ, van Grup LC, Wiegant VM, Stam R. Individual reactivity to the open-field predicts the expression of stress-induced behavioral and somatic pain sensitization. Behav Brain Res. 2006;174:112–118. doi: 10.1016/j.bbr.2006.07.010. [DOI] [PubMed] [Google Scholar]
- Gosling SD. From mice to men: what can we learn about personality from animal research? Psychol Bull. 2001;127:45–86. doi: 10.1037/0033-2909.127.1.45. [DOI] [PubMed] [Google Scholar]
- Grant K-A, McMahon C, Austin M-P. Maternal anxiety during the transition to parenthood: a prospective study. J Affect Disord. 2008;108:101–111. doi: 10.1016/j.jad.2007.10.002. [DOI] [PubMed] [Google Scholar]
- Gutknecht L, Jacob C, Strobel A, Kriegebaum C, Müller J, Zeng Y, et al. Tryptophan hydroxylase-2 gene variation influences personality traits and disorders related to emotional dysregulation. Int J Neuropsychopharmacol. 2007;10:309–320. doi: 10.1017/S1461145706007437. [DOI] [PubMed] [Google Scholar]
- Handley SL, Dunn TL, Baker JM, Cockshott C, Gould S. Mood changes in puerperium, and plasma tryptophan and cortisol concentrations. Br Med J. 1977;2:18–20. doi: 10.1136/bmj.2.6078.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handley SL, Dunn TL, Waldron G, Baker JM. Tryptophan, cortisol and puerperal mood. Br J Psychiatry. 1980;136:498–508. doi: 10.1192/bjp.136.5.498. [DOI] [PubMed] [Google Scholar]
- Hard E, Hansen S. Reduced fearfulness in the lactating rat. Physiol Behav. 1985;35:641–643. doi: 10.1016/0031-9384(85)90155-6. [DOI] [PubMed] [Google Scholar]
- Hartman BK, Zide D, Udenfriend S. The use of dopamine β-hydroxylase as a marker for the noradrenergic pathways of the central nervous system in the rat. Proc Natn Acad Sci U S A. 1972;69:2722–2726. doi: 10.1073/pnas.69.9.2722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heikkinen T, Ekblad U, Kero P, Ekblad S, Laine K. Citalopram in pregnancy and lactation. Clin Pharmacol Ther. 2002;72:184–191. doi: 10.1067/mcp.2002.126181. [DOI] [PubMed] [Google Scholar]
- Heinrichs M, Meinlschmidt G, Neumann I, Wagner S, Kirschbaum C, Ehlert U, Hellhammer DH. Effects of suckling on hypothalamic–pituitary–adrenal axis response to psychosocial stress in postpartum women. J Endocrinol Metab. 2001;86:4798–4804. doi: 10.1210/jcem.86.10.7919. [DOI] [PubMed] [Google Scholar]
- Henniger MS, Ohl F, Hölter SM, Weissenbacher P, Toschi N, Lörscher P, Wigger A, Spanagel R, Landgraf R. Unconditioned anxiety and social behaviour in two rat lines selectively bred for high and low anxiety-related behaviour. Behav Brain Res. 2000;111:153–163. doi: 10.1016/s0166-4328(00)00151-0. [DOI] [PubMed] [Google Scholar]
- Hensler JG, Ferry RC, Labow DM, Kovachich GB, Frazer A. Quantitative autoradiography of the serotonin transporter to assess the distribution of serotonergic projections from the dorsal raphe nucleus. Synapse. 1994;17:1–15. doi: 10.1002/syn.890170102. [DOI] [PubMed] [Google Scholar]
- Heron J, O’Connor TG, Evans J, Golding J, Glover V. The course of anxiety and depression through pregnancy and the postpartum in a community sample. J Affect Disord. 2004;80:65–73. doi: 10.1016/j.jad.2003.08.004. [DOI] [PubMed] [Google Scholar]
- Hettema JM, Neale MC, Kendler KS. A review and meta-analysis of the genetic epidemiology of anxiety disorders. Am J Psychiatry. 2001a;158:1568–1578. doi: 10.1176/appi.ajp.158.10.1568. [DOI] [PubMed] [Google Scholar]
- Hettema JM, Prescott CA, Kendler KS. A population-based twin study of generalized anxiety disorder in men and women. J Nerv Ment Dis. 2001b;189:413–420. doi: 10.1097/00005053-200107000-00001. [DOI] [PubMed] [Google Scholar]
- Holland HC. An apparatus note on A.M.B.A. (automatic maternal behaviour apparatus) Anim Behav. 1965;13:201–202. doi: 10.1016/0003-3472(65)90034-5. [DOI] [PubMed] [Google Scholar]
- Holschbach MA, Lonstein JS. Peripartum plasticity in the serotonergic dorsal raphe. Poster presented at Society for Behavioral Neuroendocrinology; Atlanta, Georgia. 2013. [Google Scholar]
- Hovatta I, Barlow C. Molecular genetics of anxiety in mice and men. Ann Med. 2008;40:92–109. doi: 10.1080/07853890701747096. [DOI] [PubMed] [Google Scholar]
- Huh J, Goebert D, Takeshita J, Lu BY, Kang M. Treatment of generalized anxiety disorder: a comprehensive review of the literature for psychopharmacologic alternatives to newer antidepressants and benzodiazepines. Prim Care Companion CNS Disord. 2011;13 doi: 10.4088/PCC.08r00709blu. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hundley V, Gurney E, Graham W, Rennie A. Can anxiety in pregnant women be measured using the state-trait anxiety inventory? Midwifery. 1998;14:118–121. doi: 10.1016/s0266-6138(98)90009-2. [DOI] [PubMed] [Google Scholar]
- Jorum E. Analgesia or hyperalgesia following stress correlates with emotional behavior in rats. Pain. 1988;32:341–348. doi: 10.1016/0304-3959(88)90046-2. [DOI] [PubMed] [Google Scholar]
- Kain ZN, Sevarino F, Alexander GM, Pincus S, Mayes LC. Preoperative anxiety and postoperative pain in women undergoing hysterectomy. A repeated-measures design. J Psychosom Res. 2000;49:417–422. doi: 10.1016/s0022-3999(00)00189-6. [DOI] [PubMed] [Google Scholar]
- Kalk NJ, Nutt DJ, Lingford-Hughes AR. The role of central noradrenergic dysregulation in anxiety disorders: evidence from clinical studies. J Psychopharmacol. 2011;25:3–16. doi: 10.1177/0269881110367448. [DOI] [PubMed] [Google Scholar]
- Kalueff AV, Fox MA, Gallagher PS, Murphy DL. Hypolocomotion, anxiety and serotonin syndrome-like behavior contribute to the complex phenotype of serotonin transporter knockout mice. Genes Brain Behav. 2007;6:389–400. doi: 10.1111/j.1601-183X.2006.00270.x. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Neale MC, Kessler RC, Heath AC, Eaves LJ. The genetic epidemiology of phobias in women: the interrelationship of agoraphobia, social phobia, situational phobia, and simple phobia. Arch Gen Psychiatry. 1992;49:273–281. doi: 10.1001/archpsyc.1992.01820040025003. [DOI] [PubMed] [Google Scholar]
- Kendrick KM, Keverne EB, Hinton MR, Goode JA. Oxytocin, amino acid and monoamine release in the region of the medial preoptic area and bed nucleus of the stria terminalis of the sheep during parturition and suckling. Brain Res. 1992;569:199–209. doi: 10.1016/0006-8993(92)90631-i. [DOI] [PubMed] [Google Scholar]
- Kessler MS, Bosch OJ, Bunck M, Landgraf R, Neumann ID. Maternal care differs in mice bred for high vs. low trait anxiety: impact of brain vasopressin and cross-fostering. Soc Neurosci. 2011;6:156–168. doi: 10.1080/17470919.2010.495567. [DOI] [PubMed] [Google Scholar]
- Kilts CD, Anderson CM. The simultaneous quantification of dopamine, norepinephrine, and epinephrine in micropunched rat brain nuclei by online trace enrichment HPLC with electrochemical detection: distribution of catecholamines in the limbic system. Neurochem Int. 1986;9:437–445. doi: 10.1016/0197-0186(86)90086-0. [DOI] [PubMed] [Google Scholar]
- Klampfl SM, Neumann ID, Bosch OJ. Reduced brain corticotropin-releasing factor receptor activation is required for adequate maternal care and maternal aggression in lactating rats. Eur J Neurosci. 2013:1–9. doi: 10.1111/ejn.12274. [DOI] [PubMed] [Google Scholar]
- Klink R, Robichaud M, Debonnel G. Gender and gonadal status modulation of dorsal raphe nucleus serotonergic neurons. Part I: effects of gender and pregnancy. Neuropharmacology. 2002;43:1119–1128. doi: 10.1016/s0028-3908(02)00219-8. [DOI] [PubMed] [Google Scholar]
- Landgraf R, Wigger A. High vs low anxiety-related behavior rats: an animal model of extremes in trait anxiety. Behav Genet. 2002;32:301–314. doi: 10.1023/a:1020258104318. [DOI] [PubMed] [Google Scholar]
- Lau JYF, Eley TC, Stevenson J. Examining the state–trait anxiety relationship: a behavioural genetic approach. J Abnorm Child Psychol. 2006;34:19–27. doi: 10.1007/s10802-005-9006-7. [DOI] [PubMed] [Google Scholar]
- Lechin F, van der Dijs B, Hernández G, Orozco B, Rodríguez S, Baez S. Acute effects of tianeptine on circulating neurotransmitters and cardiovascular parameters. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30:214–222. doi: 10.1016/j.pnpbp.2005.10.013. [DOI] [PubMed] [Google Scholar]
- Leckman JF, Mayes LC, Feldman R, Evans DW, King RA, Cohen DJ. Early parental preoccupations and behaviors and their possible relationship to the symptoms of obsessive–compulsive disorder. Acta Psychiatr Scand Suppl. 1999;396:1–26. doi: 10.1111/j.1600-0447.1999.tb10951.x. [DOI] [PubMed] [Google Scholar]
- Leckman JF, Feldman R, Swain JE, Eicher V, Thompson N, Mayes LC. Primary parental preoccupation: circuits, genes, and the crucial role of the environment. J Neural Transm. 2004;111:753–771. doi: 10.1007/s00702-003-0067-x. [DOI] [PubMed] [Google Scholar]
- Lee SB, Shin HS. Effects of Kangaroo Care on anxiety, maternal role confidence, and maternal infant attachment of mothers who delivered preterm infants. Taehan Kanho Hakhoe Chi. 2007;37:949–956. doi: 10.4040/jkan.2007.37.6.949. [DOI] [PubMed] [Google Scholar]
- Legrand LN, McGue M, Iacono WG. A twin study of state and trait anxiety in childhood and adolescence. J Child Psychol Psychiatry. 1999;40:953–958. [PubMed] [Google Scholar]
- Li C, Chen P, Smith MS. Neural populations in the rat forebrain and brainstem activated by the suckling stimulus as demonstrated by cFos expression. Neuroscience. 1999;94:117–129. doi: 10.1016/s0306-4522(99)00236-5. [DOI] [PubMed] [Google Scholar]
- Lister RG. The use of a plus-maze to measure anxiety in the mouse. Psychopharmacology (Berl) 1987;92:180–185. doi: 10.1007/BF00177912. [DOI] [PubMed] [Google Scholar]
- Lonstein JS. Reduced anxiety in postpartum rats requires recent physical interactions with pups, but is independent of suckling and peripheral sources of hormones. Horm Behav. 2005;47:241–255. doi: 10.1016/j.yhbeh.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Lonstein JS. Regulation of anxiety during the postpartum period. Front Neuroendocrinol. 2007;28:115–141. doi: 10.1016/j.yfrne.2007.05.002. [DOI] [PubMed] [Google Scholar]
- Lonstein JS, Gammie SC. Sensory, hormonal, and neural control of maternal aggression in laboratory rodents. Neurosci Biobehav Rev. 2002;26:869–888. doi: 10.1016/s0149-7634(02)00087-8. [DOI] [PubMed] [Google Scholar]
- Lonstein JS, Pereira M, Marler CA, Morrell JI. Parental behavior. In: Plant TM, Zeleznik AJ, editors. Knobil and Neill’s physiology of reproduction. 4. Amsterdam: Elsevier; 2013. [Google Scholar]
- Madden CJ, Sved AF. Rostral ventrolateral medulla C1 neurons and cardiovascular regulation. Cell Mol Neurobiol. 2003;23:739–749. doi: 10.1023/A:1025000919468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maestripieri D. Maternal anxiety in rhesus macaques (Macaca mulatta). I. Measurement of anxiety and identification of anxiety-eliciting situations. Ethology. 1993a;95:19–31. [Google Scholar]
- Maestripieri D. Maternal anxiety in rhesus macaques (Macaca mulatta). II. Emotional bases of individual differences in mothering style. Ethology. 1993b;95:32–42. [Google Scholar]
- Maestripieri D. Parenting styles of abusive mothers in group-living rhesus macaques. Anim Behavi. 1998;55:1–11. doi: 10.1006/anbe.1997.0578. [DOI] [PubMed] [Google Scholar]
- Maestripieri D. Measuring temperament in rhesus macaques: consistency and change in emotionality over time. Behav Processes. 2000;49:167–171. doi: 10.1016/s0376-6357(00)00083-8. [DOI] [PubMed] [Google Scholar]
- Miller SM, Lonstein JS. Autoradiographic analysis of GABA(A) and benzodiazepine binding in the neural anxiety network of virgin and postpartum female rats. Brain Res Bull. 2011;86:60–64. doi: 10.1016/j.brainresbull.2011.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller SM, Piasecki CC, Lonstein JS. Use of the light–dark box to compare the anxiety-related behavior of virgin and postpartum female rats. Pharmacol Biochem Behav. 2011;100:130–137. doi: 10.1016/j.pbb.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosienko V, Bert B, Beis D, Matthes S, Fink H, Bader M, Alenina N. Exaggerated aggression and decreased anxiety in mice deficient in brain serotonin. Transl Psychiatry. 2012;2:e122. doi: 10.1038/tp.2012.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyer JA, O’Donohue TL, Herrenkohl LR, Gala R, Jacobowitz DM. Effects of suckling on serum prolactin levels and catecholamine concentrations and turnover in discrete brain regions. Brain Res. 1979;176:125–133. doi: 10.1016/0006-8993(79)90874-6. [DOI] [PubMed] [Google Scholar]
- Neophytou SI, Aspley S, Butler S, Beckett S, Marsden CA. Effects of lesioning noradrenergic neurones in the locus coeruleus on conditioned and unconditioned aversive behaviour in the rat. Prog Neuropsychopharmacol Biol Psychiatry. 2001;25:1307–1321. doi: 10.1016/s0278-5846(01)00181-6. [DOI] [PubMed] [Google Scholar]
- Neumann ID. Brain mechanisms underlying emotional alterations in the peripartum period in rats. Depress Anxiety. 2003;17:111–121. doi: 10.1002/da.10070. [DOI] [PubMed] [Google Scholar]
- Neumann ID, Kromer SA, Bosch OJ. Effects of psycho-social stress during pregnancy on neuroendocrine and behavioural parameters in lactation depend on the genetically determined stress vulnerability. Psychoneuroendocrinology. 2005;30:791–806. doi: 10.1016/j.psyneuen.2005.03.008. [DOI] [PubMed] [Google Scholar]
- Okatani Y, Tamura S, Sagara Y. Serotonin metabolism in normal pregnant women and fetus. Nippon Sanka Fujinka Gakkai Zasshi. 1990;42:1503–1509. [PubMed] [Google Scholar]
- Olivier JDA, Van Der Hart MGC, Van Swelm RPL, Dederen PJ, Homberg JR, Cremers T, Ellenbroek BA. A study in male and female 5-HT transporter knockout rats: an animal model for anxiety and depression disorders. Neuroscience. 2008;152:573–584. doi: 10.1016/j.neuroscience.2007.12.032. [DOI] [PubMed] [Google Scholar]
- Pellow S, Chopin P, File SE, Briley M. Validation of open: closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J Neurosci Methods. 1985;14:149–167. doi: 10.1016/0165-0270(85)90031-7. [DOI] [PubMed] [Google Scholar]
- Pereira M, Uriarte N, Agrati D, Zuluaga MJ, Ferreira A. Motivational aspects of maternal anxiolysis in lactating rats. Psychopharmacology. 2005;180:241–248. doi: 10.1007/s00213-005-2157-y. [DOI] [PubMed] [Google Scholar]
- Picazo O, Rosenblatt JS, Fernández-Guasti A. The differential effect of the anxiolytic agent 8-OH-DPAT during lactation is independent of pup withdrawal and maternal behavior. Psychoneuroendocrinology. 2000;25:693–706. doi: 10.1016/s0306-4530(00)00019-6. [DOI] [PubMed] [Google Scholar]
- Priebe K, Romeo RD, Francis DD, Sisti HM, Mueller A, McEwen BS, Brake WG. Maternal influences on adult stress and anxiety-like behavior in C57BL/6J and BALB/cJ mice: a cross-fostering study. Dev Psychobiol. 2005;47:398–407. doi: 10.1002/dev.20098. [DOI] [PubMed] [Google Scholar]
- Pryce CR. A comparative systems model of the regulation of maternal motivation in mammals. Anim Behav. 1992;43:417–441. [Google Scholar]
- Quinn JL, Cole EF, Patrick SC, Sheldon BC. Scale and state dependence of the relationship between personality and dispersal in a great tit population. J Anim Ecol. 2011;80:918–928. doi: 10.1111/j.1365-2656.2011.01835.x. [DOI] [PubMed] [Google Scholar]
- Ramos A, Pereira E, Martins GC, Wehrmeister TD, Izídio GS. Integrating the open field, elevated plus maze and light/dark box to assess different types of emotional behaviors in one single trial. Behav Brain Res. 2008;193:277–288. doi: 10.1016/j.bbr.2008.06.007. [DOI] [PubMed] [Google Scholar]
- Reuter M, Kuepper Y, Hennig J. Association between a polymorphism in the promoter region of the TPH2 gene and the personality trait of harm avoidance. Int J Neuropsychopharmacol. 2007;10:401–404. doi: 10.1017/S1461145706007073. [DOI] [PubMed] [Google Scholar]
- Rhudy JL, Meagher MW. Fear and anxiety: divergent effects on human pain thresholds. Pain. 2000;84:65–75. doi: 10.1016/S0304-3959(99)00183-9. [DOI] [PubMed] [Google Scholar]
- Robichaud M, Debonnel G. Oestrogen and testosterone modulate the firing activity of dorsal raphe nucleus serotonergic neurones in both male and female rats. J Neuroendocrinol. 2005;17:179–185. doi: 10.1111/j.1365-2826.2005.01292.x. [DOI] [PubMed] [Google Scholar]
- Rödel HG, Hudson R, Rammler L, Sänger N, Schwarz L, Machnik P. Lactation does not alter the long-term stability of individual differences in behavior of laboratory mice on the elevated plus maze. J Ethol. 2012;30:263–270. [Google Scholar]
- Ross LE, McLean LM. Anxiety disorders during pregnancy and the postpartum period: a systematic review. J Clin Psychiatry. 2006;67:1285–1298. doi: 10.4088/jcp.v67n0818. [DOI] [PubMed] [Google Scholar]
- Schrocksnadel H, Baier-Bitterlich G, Dapunt O, Wachter H, Fuchs D. Decreased plasma tryptophan in pregnancy. Obstet Gynecol. 1996;88:47–50. doi: 10.1016/0029-7844(96)00084-1. [DOI] [PubMed] [Google Scholar]
- Schweimer J, Fendt M, Schnitzler HU. Effects of clonidine injections into the bed nucleus of the stria terminalis on fear and anxiety behavior in rats. Eur J Pharmacol. 2005;507(1–3):117–124. doi: 10.1016/j.ejphar.2004.11.044. [DOI] [PubMed] [Google Scholar]
- Shair HN, Masmela JR, Brunelli SA, Hofer MA. Potentiation and inhibition of ultrasonic vocalization of rat pups: regulation by social cues. Dev Psychobiol. 1997;30:195–200. doi: 10.1002/(sici)1098-2302(199704)30:3<195::aid-dev2>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- Shiau S-HH. Randomized controlled trial of kangaroo care with fullterm infants: effects on maternal anxiety, breastmilk maturation, breast engorgement, and breastfeeding status. Dissertation Abstr Int. 1998:5332. [Google Scholar]
- Sisk CL, Lonstein JS, Gore AC. Critical periods during development: hormonal influences on neurobehavioral transitions across the lifespan. In: Pfaff DW, editor. Neuroscience in the 21st century. Springer; 2013. pp. 1715–1752. [Google Scholar]
- Smith CD, Lonstein JS. Contact with infants modulates anxiety-generated c-fos activity in the brains of postpartum rats. Behav Brain Res. 2008;190(2):193–200. doi: 10.1016/j.bbr.2008.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CD, Holschbach MA, Olsewicz J, Lonstein JS. Effects of noradrenergic alpha-2 receptor antagonism or noradrenergic lesions in the ventral bed nucleus of the stria terminalis and medial preoptic area on maternal care in female rats. Psychopharmacology (Berl) 2012;224:263–276. doi: 10.1007/s00213-012-2749-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CD, Piasecki CC, Weera M, Olczewicz J, Lonstein JS. Noradrenergic alpha-2 receptor modulators in the ventral bed nucleus of the stria terminalis: effects on anxiety behavior in postpartum and virgin female rats. Behavioral Neuroscience. 2013;127:582–597. doi: 10.1037/a0032776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stallings J, Fleming AS, Corter C, Worthman C, Steiner M. The effects of infant cries and odors on sympathy, cortisol, and autonomic responses in new mothers and nonpostpartum women. Parenting. 2001;1:71–100. [Google Scholar]
- Suda S, Segi-Nishida E, Newton SS, Duman RS. A post-partum model in rat: behavioral and gene expression changes induced by ovarian steroid deprivation. Biol Psychiatry. 2008;64:311–319. doi: 10.1016/j.biopsych.2008.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan GM, Coplan JD, Kent JM, Gorman JM. The noradrenergic system in pathological anxiety: a focus on panic with relevance to generalized anxiety and phobias. Biol Psychiatry. 1999;46:1205–1218. doi: 10.1016/s0006-3223(99)00246-2. [DOI] [PubMed] [Google Scholar]
- Swanson LW. Brain maps: structure of the rat brain. Amsterdam: Elsevier; 1998. [Google Scholar]
- Tanaka M, Yoshida M, Emoto H, Ishii H. Noradrenaline systems in the hypothalamus, amygdala and locus coeruleus are involved in the provocation of anxiety: basic studies. Eur J Pharmacol. 2000;405:397–406. doi: 10.1016/s0014-2999(00)00569-0. [DOI] [PubMed] [Google Scholar]
- Toufexis DJ, Walker CD. Noradrenergic facilitation of the adrenocorticotropin response to stress is absent during lactation in the rat. Brain Res. 1996;737:71–77. doi: 10.1016/0006-8993(96)00627-0. [DOI] [PubMed] [Google Scholar]
- Toufexis DJ, Thrivikraman KV, Plotsky PM, Morilak DA, Huang N, Walker CD. Reduced noradrenergic tone to the hypothalamic paraventricular nucleus contributes to the stress hyporesponsiveness of lactation. J Neuroendocrinol. 1998;10:417–427. doi: 10.1046/j.1365-2826.1998.00223.x. [DOI] [PubMed] [Google Scholar]
- Tsukamura H, Maeda KI. Non-metabolic and metabolic factors causing lactational anestrus: rat models uncovering the neuroendocrine mechanism underlying the suckling-induced changes in the mother. Prog Brain Res. 2001;133:187–205. doi: 10.1016/s0079-6123(01)33014-5. [DOI] [PubMed] [Google Scholar]
- Uher J, Asendorpf JB, Call J. Personality in the behaviour of great apes: temporal stability, cross-situational consistency and coherence in response. Anim Behav. 2008;75:99–112. [Google Scholar]
- van Meeteren NL, Brakkee JH, Helders PJ, Croiset G, Gispen WH, Wiegant VM. Recovery of function after sciatic nerve crush lesion in rats selected for diverging locomotor activity in the open field. Neurosci Lett. 1997;238:131–134. doi: 10.1016/s0304-3940(97)00870-7. [DOI] [PubMed] [Google Scholar]
- Walther DJ, Peter JU, Bashammakh S, Hörtnagl H, Voits M, Fink H, Bader M. Synthesis of serotonin by a second tryptophan hydroxylase isoform. Science. 2003;299:76. doi: 10.1126/science.1078197. [DOI] [PubMed] [Google Scholar]
- Wegener G, Mathe AA, Neumann ID. Selectively bred rodents as models of depression and anxiety. Curr Topics Behav Neurosci. 2012;12:139–187. doi: 10.1007/7854_2011_192. [DOI] [PubMed] [Google Scholar]
- Windle RJ, Brady MM, Kunanandam T, Da Costa AP, Wilson BC, Harbuz M, Lightman SL, Ingram CD. Reduced response of the hypothalamo–pituitary–adrenal axis to alpha1-agonist stimulation during lactation. Endocrinology. 1997;138:3741–3748. doi: 10.1210/endo.138.9.5405. [DOI] [PubMed] [Google Scholar]
- Wöhr M, Schwarting RKW. Ultrasonic calling during fear conditioning in the rat: No evidence for an audience effect. Anim Behav. 2008;76:749–760. [Google Scholar]
- Woulfe JM, Flumerfelt BA, Hrycyshyn AW. Efferent connections of the A1 noradrenergic cell group: a DBH immunohistochemical and PHA-L anterograde tracing study. Exp Neurol. 1990;109:308–322. doi: 10.1016/s0014-4886(05)80022-6. [DOI] [PubMed] [Google Scholar]
- Ziegler DR, Herman JP. Neurocircuitry of stress integration: anatomical pathways regulating the hypothalamo–pituitary–adrenocortical axis of the rat. Integr Comp Biol. 2002;42:541–551. doi: 10.1093/icb/42.3.541. [DOI] [PubMed] [Google Scholar]