Summary
Frailty is the most problematic expression of population ageing. It is a state of vulnerability to poor resolution of homeostasis following a stress and is a consequence of cumulative decline in multiple physiological systems over a lifespan. This cumulative decline erodes homeostatic reserve until relatively minor stressor events trigger disproportionate changes in health status, typically a fall or delirium. Landmark studies have developed valid models for frailty and these have allowed epidemiological studies that demonstrate the association of frailty with adverse health outcomes. New research is needed to develop more efficient methods to detect and severity grade frailty as part of routine clinical practice, particularly methods with utility for primary care. This would greatly inform the appropriate selection of older people for invasive procedures or medications and would be the basis for a paradigm shift in the care of frail older people towards a more appropriate goal-directed care.
Background
Population ageing worldwide is rapidly accelerating from 461 million people aged over 65 years in 2004 to an estimated 2 billion people by 2050 (1, 2), which has profound implications for the planning and delivery of health and social care. The most problematic expression of population ageing is the clinical condition of frailty. Frailty develops as a consequence of age-related decline in multiple physiological systems, which collectively results in a vulnerability to sudden health status changes triggered by relatively minor stressor events. It is estimated that a quarter to a half of people over 85 years are frail and these people have significantly increased risk of falls, disability, long-term care and death (3, 4). Importantly, up to three quarters of people over 85 years might not be frail, raising the questions of how frailty develops, how it might be prevented and how it can be detected reliably. This review addresses these issues.
Frailty definition and presentations
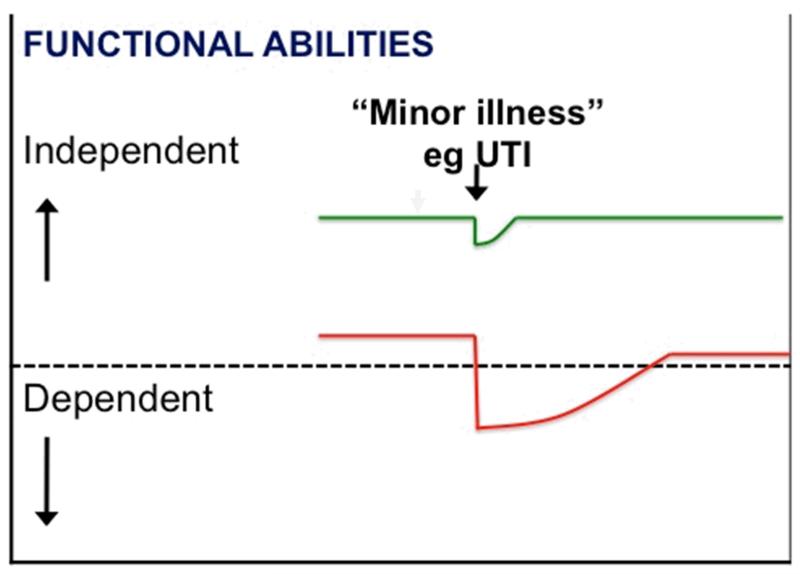
Frailty is a state of increased vulnerability to poor resolution of homeostasis following a stress, which increases the risk of adverse outcomes including falls, delirium and disability (3, 5, 6). It is a long established clinical expression that implies concern over an older person’s vulnerability and prognosis. This is shown diagrammatically in figure 1, in which an apparently small insult (e.g. a new drug; “minor” infection; or “minor” surgery) results in a dramatic and disproportionate change in health state: from independent to dependent; mobile to immobile; postural stability to falling; lucid to delirious. The dependency oscillations observed in frail older people has been referred to as “unstable disability” to reflect the often marked changes in functional ability that are familiar to practitioners working with older people (7). The common clinical presentations of frailty are given in table 1.
Figure 1.
Vulnerability of frail older people to a sudden change in health status following a minor illness. The green line represents a fit older person who, following a minor stress such as an infection, experiences a relatively small deterioration in function and then returns to homeostasis. The red line represents a frail older person who, following a similar stress, experiences a larger deterioration which may manifest as functional dependency and who does not return to baseline homeostasis.
Key: UTI: Urinary tract infection
Table 1.
Common clinical presentations of frailty.
| Non-specific: | Extreme fatigue, unexplained weight loss, frequent infections |
| Falls: | Balance and gait impairment are core features of frailty, and are important risk factors for falls. A “hot” fall is related to a minor illness that reduces postural balance below a critical threshold necessary to maintain gait integrity. “Spontaneous“ falls occur in more severe frailty when vital postural systems (vision, balance, strength) are no longer consistent with safe navigation through undemanding environments. Spontaneous falls are typically repeated and are closely associated with the psychological reaction of “fear of further falls“ so that the person develops severely impaired mobility. |
| Delirium: | Delirium (sometimes called acute confusion) is characterised by the rapid onset of fluctuating confusion and impaired awareness. It is related to a reduction in the integrity of brain function and is independently associated with adverse outcomes. Approximately 30% of older people admitted to hospital will develop delirium and the point prevalence estimate for delirium in long-term care is 15%. |
| Fluctuating disability: | Day-to-day instability resulting in patients with ‘good’, independent days, and ‘bad’ days on which (professional) care is often needed. |
Pathophysiology of frailty
Frailty is a disorder of multiple inter-related physiological systems. There is a gradual decline in physiological reserve with ageing but, in frailty, this decline is accelerated and homeostatic mechanisms start failing (8, 9). An important perspective for frailty, therefore, is to consider how the complex mechanisms of ageing promote cumulative decline in multiple physiological systems, consequent erosion of homeostatic reserve and vulnerability to disproportionate changes in health status following relatively minor stressor events. These complex ageing mechanisms are influenced by underlying genetic and environmental factors (10) in combination with epigenetic mechanisms, which regulate the differential expression of genes in cells and may be especially important in ageing (11, 12).
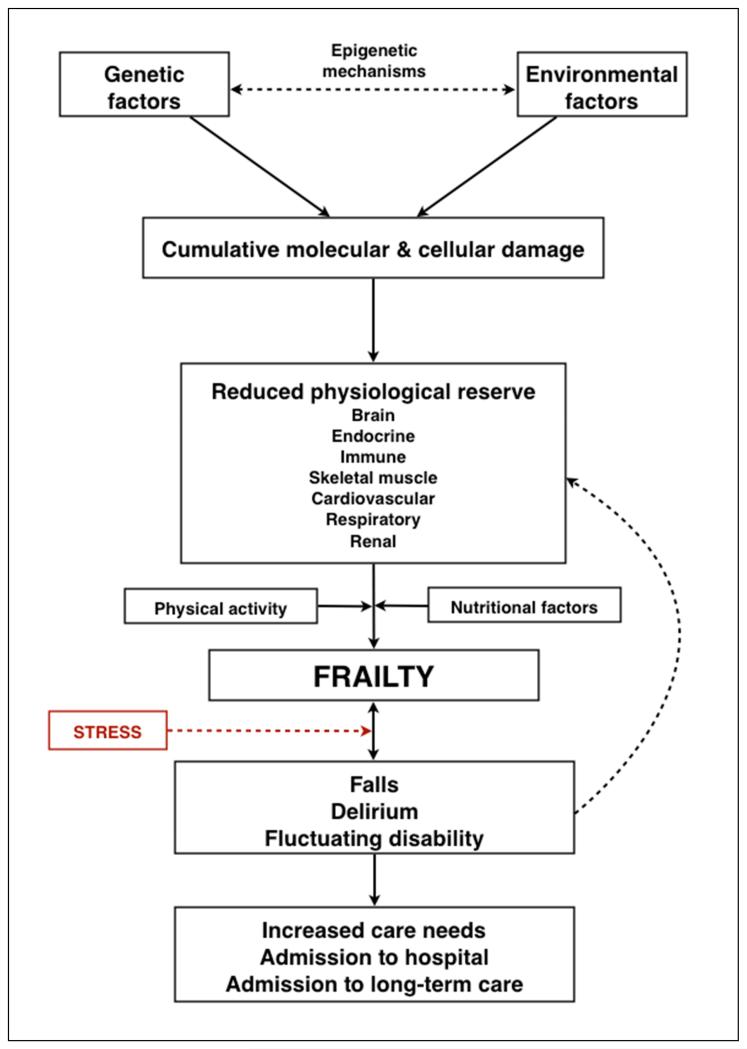
A schematic representation of frailty is provided in figure 2.
Figure 2.
A schematic representation of the pathophysiology of frailty.
Ageing is considered to result from the lifelong accumulation of molecular and cellular damage caused by multiple mechanisms regulated by a complex maintenance and repair network under the influence of genetic, environmental and epigenetic mechanisms. There is uncertainty regarding the precise level of cellular damage required to cause impaired organ physiology but, importantly, many organ systems exhibit considerable redundancy, which provides the physiological reserve required to compensate for age and disease-related changes. The brain, endocrine system, immune system and skeletal muscle are intrinsically inter-related and are currently the organ systems best studied in the development of frailty. Loss of physiological reserve in other systems including the cardiovascular, respiratory and renal systems also contributes. Influenced by level of physical activity and nutritional factors, cumulative loss of physiological reserve in these organ systems can lead to frailty, which is characterised by increased vulnerability to poor resolution of homeostasis following a stress, increasing the risk of adverse outcomes including falls, delirium and disability. These are common clinical presentations of frailty, are common reasons for admission to hospital and can accelerate further decline. Both frailty itself and these common clinical presentations identify those at increased risk of requiring care at home and long-term care admission.
The pathway to frailty
Ageing is considered to result from the lifelong accumulation of molecular and cellular damage caused by multiple mechanisms under the regulation of a complex maintenance and repair network (10). There is uncertainty regarding the precise level of cellular damage required to cause impaired organ physiology but, importantly, many organ systems exhibit considerable redundancy, which provides the physiological reserve required to compensate for age and disease-related changes (13). For example, the brain contains more neurons and skeletal muscle more myocytes than are required for survival (13). Therefore, a key question is whether there is a critical threshold of age-related, cumulative decline in multiple physiological systems beyond which frailty becomes evident. A 2009 cross-sectional study involving 1,002 female participants investigated cumulative physiological dysfunction in six different systems (haematological, inflammatory, hormonal, adiposity, neuromuscular and micronutrients) using 12 measures and reported a non-linear relationship between the number of abnormal systems and frailty, independent of age and comorbidity (14). The presence of abnormal results in three or more systems was a significant predictor of frailty. Importantly, the number of abnormal systems was more predictive than abnormalities in any particular system. This provides evidence to suggest that when physiological decline reaches an aggregate critical mass, frailty becomes evident (14).
The brain, endocrine system, immune system and skeletal muscle are intrinsically inter-related and are currently the organ systems best studied in the development of frailty (5). These systems will be considered in greater detail, but it is important to recognise that frailty has also been associated with loss of physiological reserve in the respiratory (15), cardiovascular (16), renal (17) and haemopoietic and clotting systems (18, 19) and that nutritional status can also be a mediating factor (3, 20-22).
The frail brain
Ageing is associated with characteristic structural and physiological changes in the brain. The loss of individual neurons in the majority of cortical regions is minimal (23), but neurons with high metabolic demands, for example the hippocampal pyramidal neurons, may be disproportionally affected by altered synaptic function, protein transport and mitochondrial function (23). The hippocampus has been identified as an important mediator in the pathophysiology of cognitive decline and Alzheimer’s dementia (24) and is a key component of the stress response, sensing increased glucocorticoid levels and relaying information to the hypothalamus in a negative feedback loop (25).
The ageing brain is also characterised by structural and functional changes to microglial cells, which are the resident immune cell population of the central nervous system (CNS) and are the CNS equivalent of macrophages. They are activated by brain injury and local and systemic inflammation and become primed (hyper-responsive) to small stimuli with ageing, which can potentially cause damage and neuronal death (26-28). Primed microglia are postulated to play an important role in the pathophysiology of delirium (28, 29). A prospective cohort study involving 273 hospitalised older people identified that frailty is associated with both increased risk of developing delirium (odds ratio, OR, 8.5, 95% confidence interval, CI, 4.8-14.8) and subsequent reduced survival (median survival in frail older patients with delirium 88 days (95% CI 5-171 days); median survival in non-frail older patients with delirium 359 days (95% CI 118-600 days)) (6). This indicates that the combination of delirium and frailty identifies older people at particularly high risk of adverse outcomes.
There is accumulating evidence from observational studies to support a temporal association between frailty, cognitive impairment and dementia. A prospective cohort study (n=750) of older people without cognitive impairment at baseline reported that frailty was associated with an increased risk of developing mild cognitive impairment over 12 years of follow-up (hazard ratio, HR, 1.63, CI, 1.27-2.08) (30). Increasing degree of frailty was also associated with a faster rate of cognitive decline. An independent association between frailty and dementia has been reported in two large prospective cohort studies (31, 32).
The frail endocrine system
The brain and endocrine system are intrinsically linked through the hypothalamo-pituitary axis, which controls metabolism and energy use via the signaling action of a series of homeostatic hormones (23). During ageing, there is a decline in production of three major circulating hormones. Firstly, a decline in growth hormone synthesis by the pituitary causes a reduction in insulin-like growth factor-1 (IGF-1) production by the liver and other organs. IGFs are a family of small peptides that increase anabolic activity in many cells. Promotion of neuronal plasticity and increased skeletal muscle strength are considered particularly important effects (33). Secondly, decreased oestradiol and testosterone cause increased release of luteinising hormone (LH) and follicle stimulating hormone (FSH). Thirdly, the adrenocortical cells that produce the major sex steroid precursor dehydroepiandrosterone (DHEA) and DHEA sulphate (DHEAS) decrease in activity, often mirrored by a gradual increase in cortisol release (34, 35).
Changes to IGF signaling, sex hormone, DHEA/DHEAS production and cortisol secretion are considered important in frailty, although the exact relationships remain uncertain and require further investigation. One cross-sectional study reported significantly lower levels of IGF-1 in people identified as frail when compared to age-matched controls (36). However, if IGF-1 has a key aetiological role in frailty an association between IGF-1 and mortality might be anticipated but a series of observational studies have reported inconsistent associations (37-41). Furthermore, although the muscles of frail older people appear to retain the capability to respond to IGF-1 (42) trials of IGF-1 supplementation in older people have failed to demonstrate benefit (43).
Although an association between testosterone levels and frailty has been identified (44) this may be a sensitive marker rather than a pathological mechanism (45). A cross-sectional study reported an association between DHEAS and frailty but the influence of comorbid conditions could not be confidently excluded (46). A U-shaped association between DHEAS and mortality has been reported in disabled older women (47).
One cross sectional study (n=214) reported that frailty was independently associated with chronically elevated diurnal cortisol levels (48). A link between chronically elevated cortisol and frailty is plausible, as persistently high levels of cortisol are associated with increased catabolism, leading to loss of muscle mass, anorexia, weight loss and reduced energy expenditure - cardinal clinical features of frailty (49).
The frail immune system
The ageing immune system is characterised by a decline in stem cells, alterations in T-lymphocyte production, blunting of the B-cell led antibody response and reduced phagocytic activity of neutrophils, macrophages and natural killer cells (50, 51). This senescent immune system may function adequately in the quiescent state but fail to respond appropriately to the stress of acute inflammation (50). There is evidence that inflammation has a key role in the pathophysiology of frailty through an abnormal, low-grade inflammatory response that is hyper-responsive to stimuli and that persists for a prolonged period following removal of the initial inflammatory stimulus (19, 52-57). A range of inflammatory cytokines have been independently associated with frailty including interleukin-6 (IL-6), C-reactive protein (CRP), tumour necrosis factor-α (TNFα) and CXC chemokine ligand-10 (CXCL-10), a potent pro-inflammatory mediator (52, 54-58). However, higher CRP levels in very old people have also been associated with better memory function (59). Advanced glycation end products (AGEs) are a group of molecules produced by the glycation of proteins, lipids and nucleic acid that have potential to cause widespread cellular damage by upregulation of inflammation (60). They have been associated with ageing, chronic disease and mortality and may have an important role in frailty (60).
Inflammation is associated with anorexia and catabolism of skeletal muscle and adipose tissue, which may contribute to the nutritional compromise, muscle weakness and weight loss that characterise frailty (41, 61, 62). Furthermore, frailty is associated with an impaired antibody response to influenza (63) and pneumococcal vaccine (64), which helps explain the observation that vaccination in older people is associated with only relatively modest clinical effectiveness (65).
Frail skeletal muscle - sarcopenia
Sarcopenia has been defined as progressive loss of skeletal muscle mass, strength and power and is considered a key component of frailty (66, 67). Loss of muscle strength and power may be more important than changes to muscle mass (68). Under normal circumstances, muscle homeostasis is maintained in a delicate balance between new muscle cell formation, hypertrophy and protein loss. This delicate balance is coordinated by the brain, endocrine system and immune system and is influenced by nutritional factors and level of physical activity. The adverse neurological, endocrine and immune components of frailty have the potential to upset this delicate homeostatic balance and accelerate the development of sarcopenia. Inflammatory cytokines including IL-6 and TNFα activate muscle breakdown to generate amino acids for energy and cleave antigenic peptides (69). This fundamentally protective response may become pathological in the presence of an overactive, insufficiently regulated inflammatory response that characterises frailty, leading to loss of muscle mass and strength, with attendant decline in functional ability.
Frailty models
Reliable frailty models should be assessed against their success in predicting both natural history and response to therapeutic interventions and be underpinned by biological principles of causality (70). The two principal emerging models of frailty are the phenotype model (3) and the cumulative deficit model underpinning the Canadian Study of Health and Aging (CSHA) Frailty Index (71).
The phenotype model
In a landmark study, Fried and colleagues (3) undertook a secondary analysis of data obtained from a prospective cohort study (the Cardiovascular Health Study (CHS)) involving 5210 men and women aged 65 years and older. A frailty phenotype was operationalised using a cluster of variables: unintentional weight loss; self-reported exhaustion; low energy expenditure; slow gait speed; weak grip strength (table 2). The lowest quintile values were used to define absence/presence of these variables. People with Parkinson’s disease, previous stroke, cognitive impairment or depression were excluded. People with three of the five factors were considered frail, one or two factors as pre-frail, and no factors as robust older-people. The population so defined was categorised as 7% frail, 47% pre-frail and 46% not frail. Follow-up assessments were undertaken at 3 and 5 years with the outcomes of falls; mobility and function; hospitalisation; and death.
Table 2.
The five phenotype model indicators of frailty and their associated measures.
| Frailty indictor | Measure |
|---|---|
| Weight loss | Self-reported weight loss of more than 10 pounds or recorded weight loss of ≥ 5% per annum |
| Self-reported exhaustion | Self-reported exhaustion on CES-D depression score (3-4 days per week or most of the time) |
| Low energy expenditure | Energy expenditure <383 KCal/week (males) or <270 KCal/week (females) |
| Slow gait speed | Standardised cut-off times to walk 15 feet, stratified for sex and height |
| Weak grip strength | Grip strength, stratified by sex and BMI |
Key. CES-D, Center for Epidemiological Studies Depression; BMI, body mass index.
People categorised as frail were observed to have more adverse outcomes compared to people categorised as not frail, with the pre-frail group having outcomes intermediate between the two. Observed mortality at 7 years was 12%, 23% and 43% for not frail, pre-frail and frail groups respectively. The 7-year adjusted hazard rate for mortality was 1.63 (95% CI 1.27-2.08) for the frail group (3).
This work is important as it suggests a frailty phenotype can be defined and might be a basis to detect frailty in routine care. However, it is not presently clear how the variables might reliably be translated into clinical practice. Additionally, the five factors were fortuitously available and selected from a prospective cohort study that was not designed to investigate frailty. Other potentially important factors such as cognitive impairment, a highly prevalent condition associated with functional decline and disability, were not included as a component of the phenotype (72). Nonetheless, despite these criticisms, the general approach of clusters of variables to define a frailty phenotype has been independently validated (73, 74).
The cumulative deficit model
The Frailty Index (FI) was developed as part of the CSHA (71); a five year prospective cohort study (n=10,263) designed to investigate the epidemiology and burdens of dementia in older people in Canada (mean age: 82 years). Ninety-two baseline parameters of symptoms (e.g. low mood), signs (e.g. tremor) and abnormal laboratory values, disease states and disabilities, collectively referred to as deficits, were used to define frailty (75). The FI was a simple calculation of the presence or absence of each variable as a proportion of the total (e.g. 20 deficits present out of a possible 92 gives a FI of 20/92 = 0.22). Thus frailty is defined as the cumulative effect of individual deficits - ‘the more individuals have wrong with them, the more likely they are to be frail’ (76).
The statistical distribution of the FI (a gamma distribution) was consistent with a probability model that typically describes systems with inbuilt redundancy. This is an attractive mathematical model for frailty as it implies that the FI has properties that fully support the concept of reduced reserve. Thus, although each individual deficit carries no obvious or imminent threat for mortality (e.g. hearing impairment), the deficits cumulatively contribute to an increased risk of death. This is consistent with the increased vulnerability and threat of impending homeostatic failure that is essential to the frailty concept. Importantly, the cumulative deficit model expresses the notion of a gradation of frailty with progressive accumulation of deficits each of which has an equal weight in mathematical modeling of the FI. This is attractive clinically because it allows frailty to be considered as gradable rather than present/absent. Moreover, it is the number of equally weighted deficits, as a measure of accumulated vulnerability, that is related to adverse outcomes, rather than particular clusters of deficits (77). Importantly, a value of 0.67 appears to identify a level of frailty beyond which further deficit accumulation is not sustainable and death is likely to supervene (78). This value may represent the warning sign of being close to the ‘tipping point’ that characterises a complex system on the brink of collapse (79).
Subsequent work has demonstrated that the rather daunting initial list of 92 variables can be reduced to a more manageable 30 or so without loss of predictive validity (4). The criteria for variable inclusion into the FI are: biologically sensible; accumulates with age; does not saturate too early (80). This makes the FI very adaptable as a conceptual approach.
Several studies using data from the CSHA demonstrated that the FI was strongly related to the risk of death and institutionalisation (4, 77). The 10-year adjusted hazard rate for mortality was 1.57 (95% CI 1.41-1.74) for the frail group (4), a statistically similar estimate to the phenotype model (3). Mortality has previously been demonstrated to be exponentially related to the value of the FI (81).
Comparing the phenotype model with the cumulative deficit model
The phenotype and cumulative deficit models demonstrate overlap in identification of frailty (82) and considerable statistical convergence (83). This is especially important because the demonstration of convergent predictive validity for adverse health outcomes between two conceptually different models of frailty may help advance the debate regarding whether frailty is best considered as a syndrome or a state by lending support for recognition of the condition as a unified construct.
The continuous FI demonstrated greater discriminatory ability for people with moderate and severe frailty when compared to the categorical phenotype model, a finding that has been independently validated (84). Use of continuous models may facilitate more precise identification of frail older people for interventions to improve outcomes and a 2011 study re-scaled the phenotype model to make it more continuous, providing better discriminatory capacity (85).
Epidemiology
The importance of frailty as a leading cause of death in older people comes from a ten-year prospective cohort study involving community dwelling older people (n=754) (86). Cause of death was based on clinical home-based assessments conducted at 18-month intervals and death certificates. The most common condition leading to death was frailty (27.9%) compared to organ failure (21.4%), cancer (19.3%), dementia (13.8%) and other causes (14.9%).
Prevalence of frailty
A recent systematic review investigated the prevalence of frailty (87). Twenty-one community based cohort studies involving 61,500 older people were identified. The operational definitions for frailty and the inclusion/exclusion criteria varied between the studies, which largely explained the considerable variation in reported frailty prevalence rates of 4.0 to 59.1%. When the reported rates were restricted to the studies that used the phenotype model, the weighted average frailty prevalence rate was 9.9% (95% CI 9.6-10.2), and 44.2% for pre-frailty prevalence (95% CI 44.2-44.7). Frailty was statistically more prevalent in women (11 studies, 9.6%, 95% CI 9.2-10) than men (5.2%; 95% CI 4.9-5.5). Frailty increased steadily with age: 65-69 years: 4%; 70-74 years: 7%; 75-79 years: 9% 80-84 years: 16%; >85 years: 26%. Rates appear to be higher in studies that employed the graded frailty index, which would count as frail some people whose increased risk is captured in the pre-frail category of the phenotype model (4).
Most frailty models were developed in Caucasian populations and the prevalence of frailty may be higher in inhabitants of Southern Europe (88) and older Hispanic and African Americans (3, 89) so different cut-offs for frailty may be required in different populations.
Adverse outcomes of frailty
Associations between frailty and adverse outcomes reported in four large prospective cohort studies (3, 90-92) are presented in table 3.
Table 3.
Covariate adjusted associations between frailty and adverse outcomes (falls, disability, hospitalisation, care home admission and mortality) from four large prospective cohort studies.
| Study | Year | Country | Number of participants | Length of follow-up | Falls HR/OR 95% CI | Worsening disability HR/OR 95% CI | Hospitalisation HR/OR 95% CI | Care home admission HR/OR 95% CI | Mortality HR/OR 95% CI | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Intermed frailty | Severe frailty | Intermed frailty | Severe frailty | Intermed frailty | Severe frailty | Intermed frailty | Severe frailty | Intermed frailty | Severe frailty | |||||
| Cardiovascular Health Study (CHS) (3) | 2001 | US | 5317 | 7 years | HR 1.12 1.00-1.26 | HR 1.23 1.50-2.21 | HR 1.55 1.38-1.75 | HR 1.79 1.47-2.17 | HR 1.11 1.03-1.19 | HR 1.27 1.11-1.46 | NA | NA | HR 1.32 1.13-1.55 | HR 1.63 1.27-2.08 |
| Canadian Study of Health & Aging (CSHA) (90) | 2004 | Canada | 9008 | 5 years | NA | NA | NA | NA | NA | NA | OR 2.54 1.67-3.86 | OR 2.60 1.36-4.96 | OR 2.54 1.92-3.37 | OR 3.69 2.26-6.02 |
| Women’s Health & Aging Study (WHAS) (91) | 2006 | US | 1438 | 3 years | HR 0.92 0.63-1.64 | HR 1.18 0.63-2.19 | NA | NA | HR 0.99 0.67-1.47 | HR 0.67 0.33-1.35 | HR 5.16 0.81-32.8 | HR 23.98 4.45-129 | HR 3.50 1.91-6.39 | HR 6.03 3.00-12.0 |
| Study of Osteoporotic Fractures (SOF) (92) | 2008 | US | 6701 | 4.5 years | OR 1.23 1.02-1.48 | OR 2.44 1.95-3.04 | OR 1.89 1.66-2.14 | OR 2.79 2.31-3.37 | NA | NA | NA | NA | OR 1.54 1.40-1.69 | HR 2.75 2.46-3.07 |
Key: HR, hazard ratio; OR, odds ratio; CI, confidence interval; intermed, intermediate; US, United States; NA, not available
Frailty transitions
Frailty is a dynamic process (93) but a transition to a level of greater frailty is more common than improvement and the development of frailty frequently results in a spiral of decline that leads to increasing frailty and risk of worsening disability, falls, admission to hospital and death (94). Risk of admission to long-term care is also increased in those with mild frailty (adjusted risk ratio (RR) 2.54, 95% CI 1.67-3.86) and moderate/severe frailty (RR 2.60, 95% CI 1.36-4.96) (95).
Frailty, comorbidity and disability
The CHS population was used to investigate the overlap between frailty, comorbidity and disability (3, 96). Frailty and comorbidity (defined as two or more of the following nine diseases: myocardial infarction; angina; congestive heart failure; claudication; arthritis; cancer; diabetes; hypertension; chronic obstructive pulmonary disease) was present in 46.2% of the population, frailty and disability (defined as the presence of restriction in at least one activity of daily living) was present in 5.7%, and the combination of frailty, disability and comorbidity was present in 21.5% of the study group. Importantly, frailty was present without comorbidity or disability in 26.6% of the study group. This finding provides support for frailty as an independent concept, distinct from comorbidity and disability. However, more recent work suggests that the overlap is more frequent and increases with greater frailty (97). The contribution of subclinical disease may be particularly important and physiological measurements to identify older people at risk of frailty may help guide the development of earlier preventative interventions (85).
The instrumentation of frailty
The demonstration of large between-group differences for people who are frail compared to not frail (4) is important because it entreats clinicians away from judgments based on chronological age towards the notion of frailty. Researchers and clinicians, therefore, require simple, valid, accurate and reliable tools to detect frailty. Monitoring outcomes of interventions in frail people additionally requires tools that are sensitive to change (98).
Standardised questionnaires to identify frailty
A systematic review with a broadly based study selection criteria identified 20 candidate tools for frailty (99). However, most of the included studies described either primary research to investigate models of frailty or the focus was on functional restriction, which, although an important manifestation of frailty, is insufficient for reliable identification of the condition. The Frail Elderly Functional Questionnaire (19 items) was identified as a potential outcome measure for frailty intervention studies as it is suitable for use by telephone or proxy, valid and reliable (100), and is sensitive to change (101).
The Groningen Frailty Indicator (102) and the Tilburg Frailty Indicator (103) are simple and similar (104) questionnaire based approaches to detecting people with frailty. Aspects of validity have been investigated but, importantly, studies of diagnostic accuracy against well-defined community populations of older people are not yet available. Moreover, both these questionnaires require new information to be gathered. The option of using existing patient data held in primary care records to construct a frailty index consistent with the cumulative deficit model requires investigation.
Assessments to identify frailty
The timed-up-and-go test (TUGT), a simple standardised measure of gait speed that requires a stop watch (105), and hand grip strength that requires a hand-held dynamometer (106), have been investigated as potential single assessments to detect frailty. Pulmonary function is associated with frailty (15) and may have utility as a straightforward detection test. However, diagnostic accuracy of these assessments has not been confirmed. A systematic review identified nine prospective studies (n=34,485) investigating slow gait speed (107). Slow gait speed successfully characterised the sub-group of older people who had adverse outcomes and had similar accuracy to complex multivariate models that included itemising chronic conditions.
The Edmonton Frail Scale is a multi-dimensional assessment instrument that includes the TUGT, and a test for cognitive impairment (108). It is quick to administer (less than 5 minutes) and is valid, reliable and feasible for routine use by non-geriatricians but the diagnostic accuracy has not been investigated.
The various interRAI instruments are widely used internationally to standardise the assessment of older people. Nine items that are embedded in many of the instruments can be extracted and comprise the Changes in Health, End-Stage Disease and Signs and Symptoms (CHESS) scale. Although not explicitly a frailty measure (109), CHESS has been demonstrated to be a strong predictor of mortality and further validation studies are ongoing.
Comprehensive geriatric assessment (CGA) has become the internationally established method to assess older people in clinical practice. It is a multidisciplinary diagnostic process to determine an older person’s medical, psychological and functional capability to develop a plan for treatment and follow up (110). The process, provided it is closely linked to interventions, is associated with superior outcomes (111) and has been applied successfully beyond elderly care medicine (112, 113). Two studies embedded in the CSHA programme were used to investigate the predictive validity of CGA as conducted by over 70 clinicians (71, 114). In both studies, the clinically obtained CGA results were highly correlated with the research standard CSHA FI and were predictive of death and need for institutional care. These studies are the first objective confirmation that CGA is sensitive to the reliable detection of degrees of frailty. CGA is currently the gold standard to detect frailty and it should be more widely deployed. The practical limitation of CGA is the time and expertise required for the process.
Interventions for frailty
Reducing the prevalence or severity of frailty is likely to have large benefits for the individual, their families and for society. Several approaches have been investigated in clinical trials.
Interventions based on comprehensive geriatric assessment
Frail older people receiving inpatient CGA are more likely to return home, less likely to experience cognitive or functional decline and have lower in-hospital mortality (111). Complex interventions based on CGA delivered to older people in the community can increase the likelihood of continuing to live at home, principally through a reduced need for care home admission and reduced falls (115, 116), but those who are most frail appear to receive least benefit (115).
Exercise interventions
Exercise has physiological effects on the brain, endocrine system, immune system and skeletal muscle (42, 117-120). Three systematic reviews of home-based and group-based exercise interventions for frail older people concluded that exercise can improve outcomes of mobility and functional ability (121-123). Meta-analysis identified that the effect sizes are likely to be small to moderate (pooled standardised mean difference (SMD) for mobility 0.18, 95% CI 0.05-0.30; pooled SMD for functional ability 0.27, 95% CI 0.08-0.46) (121). The most effective intensity (duration and frequency) of exercise intervention remains uncertain, but adherence was characteristically high across a range of interventions.
Most trials did not use a validated measure or operationalised model to record frailty at baseline or follow-up but, where results were stratified by frailty, those who were most frail appeared to gain least benefit (124). However, this is contradictory to the results from a Cochrane review that incorporated 49 RCTs of exercise interventions for long-term care (LTC) residents (a group of older people who are likely to be very frail) and concluded that interventions, particularly those involving strength and balance training, can successfully increase muscle strength and functional abilities (125). It is therefore possible that even small gains in strength of LTC residents translate into important functional gains.
Nutritional interventions
Nutritional interventions may have potential to address the impaired nutrition and weight loss of frailty. However, there is a paucity of evidence. One RCT that investigated the effects of exercise and nutritional supplementation in 100 frail older people living in long-term care reported that nutritional supplementation had no effect on muscle strength, gait speed, stair climbing or physical activity (126). A Cochrane review of nutritional interventions for preventing and treating pressure ulcers in older hospital patients, a group who are likely to be frail, reported that it was not possible to draw any firm conclusions due to the absence of trials of high methodological quality (127).
Pharmacological agents
Few pharmacological agents have been investigated in frailty. Angiotensin converting enzyme (ACE) inhibitors have been demonstrated to improve the structure and biochemical function of skeletal muscle (128) and there is evidence that ACE inhibitors may halt or slow the decline in muscle strength in older age (129) and improve exercise capacity and quality of life (130). Testosterone improves muscle strength but also increases adverse cardiovascular and respiratory outcomes (131). IGFs have direct effects on skeletal muscle (5) but IGF-1 does not appear to improve muscle strength or bone density in healthy older women (132). Low vitamin D levels have been associated with frailty (133) and vitamin D has been demonstrated to improve neuromuscular function (134). Although vitamin D prescription for older people who are deficient may reduce falls (135) and use of calcium/vitamin D supplements for older people in long-term care can reduce fractures (136), the general use of vitamin D as treatment for frailty remains controversial (43). The use of pharmacological agents for the prevention and treatment of frailty is an important area for future research.
Conclusions
Modern healthcare systems are largely organised around a single system illness (137). Many older people, however, have multi-organ problems. Frailty is a practical, unifying concept in the care of these older people that directs attention away from organ specific diagnoses towards a more holistic viewpoint of the patient and their predicament. It is a state of vulnerability to poor resolution of homeostasis following a stress and is strongly associated with adverse outcomes. Distinguishing older people who are frail from people who are not frail should therefore form an essential aspect of assessment in any health care encounter that might result in an invasive procedure or potentially harmful medication. It allows practitioners to weigh up benefits and risks, and for patients to make properly informed choices. Failure to detect frailty potentially exposes patients to interventions from which they may not benefit and indeed may be harmed. Conversely, to exclude physiologically well (non-frail) older people simply on the basis of age is unacceptable.
The most evidence-based process to detect and severity grade frailty is the process of CGA. This is a resource intensive process and new research is urgently required to find equally reliable but more efficient and responsive methods for routine care. The necessary requirements of future frailty instruments, including the important issue of clinical sensibility, have been defined (138). This might be achieved by development and further validation of the currently available frailty-specific multi-dimensional questionnaires, but the utility of existing clinical data sets, particularly in primary care, is attractive. This approach would be underpinned by the cumulative deficits frailty model and implies that frailty could be both positively identified and severity graded. Such a simple tool would facilitate essential research to gain deeper insight into the complex mechanisms of frailty and aid the development and evaluation of interventions to improve outcomes. It would also have considerable clinical merit as it would be the basis for a paradigm shift in the care of frail older people towards a more appropriate goal-directed care in which individually framed clinical outcomes that span organ systems are negotiated with patients (139).
Search strategy and selection criteria.
We developed a structured search strategy with the assistance of an expert librarian at the University of Leeds. We searched the Cochrane Library (2000-2012), CINAHL (2000-2012), MEDLINE (2000-2012), EMBASE (2000-2012), PSYCINFO (2000-2012) and PEDRO (2000- 2012). We used the search terms “frailty”, “frail elderly ” or “sarcopenia” with terms “aged” or “aged, 80 and over” or “aging/genetics” or “longevity” or “centenarian” or “oldest old” or “very old” or “very elderly”. We performed a further search limiting the results to systematic reviews of interventions for the prevention and treatment of frailty. Additional papers were identified from personal libraries and reference lists of retrieved articles.
“It is more important to know what sort of person has a disease than to know what sort of disease a person has” Hippocrates c400BC
Acknowledgement
We would like to express our gratitude to Deirdre Andre, research support librarian at the University of Leeds, for her assistance in devising and running the search strategy for this review.
Footnotes
Conflict of interest
KR has applied to various Canadian government schemes to commercialise a new version of the frailty index based on a comprehensive geriatric assessment and a new screening tool, adapted from the Clinical Frailty Scale of the Canadian Study of Health and Aging. To do this, with colleagues he has formed a company called Videx Canada. He has not asserted copyright on any frailty measure discussed here.
AC, JY, SI and MOR declare that they have no conflicts of interest.
References
- 1.Kinsella K, Phillips D. Global Aging: The Challenge of Success. Population Reference Bureau; Washington, DC: 2005. ( Population Bulletin 60, no.1 ). [Google Scholar]
- 2.The World at Six Billion . Population Division. Department of Economic and Social Affairs. United Nations Secretariat; Oct 12, 1999. Available at www.un.org/esa/population/publications/sixbillion/sixbilpart1.pdf. [Google Scholar]
- 3.Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, Seeman T, Tracy R, Kop WJ, Burke G, McBurnie MA. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3):M146–56. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 4.Song X, Mitnitski A, Rockwood K. Prevalence and 10-year outcomes of frailty in older adults in relation to deficit accumulation. J Am Geriatr Soc. 2010;58(4):681–7. doi: 10.1111/j.1532-5415.2010.02764.x. [DOI] [PubMed] [Google Scholar]
- 5.Walston J, Hadley EC, Ferrucci L, Guralnik JM, Newman AB, Studenski SA, Ershler WB, Harris T, Fried LP. Research agenda for frailty in older adults: toward a better understanding of physiology and etiology: summary from the American Geriatrics Society/National Institute on Aging Research Conference on Frailty in Older Adults. J Am Geriatr Soc. 2006;54(6):991–1001. doi: 10.1111/j.1532-5415.2006.00745.x. [DOI] [PubMed] [Google Scholar]
- 6.Eeles EM, White SV, O’Mahony SM, Bayer AJ, Hubbard RE. The impact of frailty and delirium on mortality in older inpatients. Age Ageing. 2012;41(3):412–6. doi: 10.1093/ageing/afs021. [DOI] [PubMed] [Google Scholar]
- 7.Campbell AJ, Buchner DM. Unstable disability and the fluctuations of frailty. Age Ageing. 1997;26(4):315–8. doi: 10.1093/ageing/26.4.315. [DOI] [PubMed] [Google Scholar]
- 8.Ferrucci L, Cavazzini C, Corsi A, Bartali B, Russo CR, Lauretani F, Corsi AM, Bandinelli S, Guralnik JM. Biomarkers of frailty in older persons. J Endocrinol Invest. 2002;25(10 Suppl):10–5. [PubMed] [Google Scholar]
- 9.Taffett G. Physiology of Aging. In: Cassell C, editor. Geriatric Medicine An Evidence Based Approach. Springer-Verlag; New York: 2003. [Google Scholar]
- 10.Kirkwood TB. Understanding the odd science of aging. Cell. 2005;120(4):437–47. doi: 10.1016/j.cell.2005.01.027. [DOI] [PubMed] [Google Scholar]
- 11.McGowan PO, Szyf M. Environmental epigenomics: understanding the effects of parental care on the epigenome. Essays Biochem. 2010;48(1):275–87. doi: 10.1042/bse0480275. [DOI] [PubMed] [Google Scholar]
- 12.Kahn A, Fraga MF. Epigenetics and aging: status, challenges, and needs for the future. J Gerontol A Biol Sci Med Sci. 2009;64(2):195–8. doi: 10.1093/gerona/gln064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lipsitz LA. Dynamics of stability: the physiologic basis of functional health and frailty. J Gerontol A Biol Sci Med Sci. 2002;57(3):B115–25. doi: 10.1093/gerona/57.3.b115. [DOI] [PubMed] [Google Scholar]
- 14.Fried LP, Xue QL, Cappola AR, Ferrucci L, Chaves P, Varadhan R, Guralnik JM, Leng SX, Semba RD, Walston JD, Blaum CS, Bandeen-Roche K. Nonlinear multisystem physiological dysregulation associated with frailty in older women: implications for etiology and treatment. J Gerontol A Biol Sci Med Sci. 2009;64(10):1049–57. doi: 10.1093/gerona/glp076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vaz Fragoso CA, Enright PL, McAvay G, Van Ness PH, Gill TM. Frailty and respiratory impairment in older persons. Am J Med. 2012;125(1):79–86. doi: 10.1016/j.amjmed.2011.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Afilalo J, Karunananthan S, Eisenberg MJ, Alexander KP, Bergman H. Role of frailty in patients with cardiovascular disease. Am J Cardiol. 2009;103(11):1616–21. doi: 10.1016/j.amjcard.2009.01.375. [DOI] [PubMed] [Google Scholar]
- 17.Abadir PM. The frail renin-angiotensin system. Clin Geriatr Med. 2011;27(1):53–65. doi: 10.1016/j.cger.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chaves PH, Semba RD, Leng SX, Woodman RC, Ferrucci L, Guralnik JM, Fried LP. Impact of anemia and cardiovascular disease on frailty status of community-dwelling older women: the Women’s Health and Aging Studies I and II. J Gerontol A Biol Sci Med Sci. 2005;60(6):729–35. doi: 10.1093/gerona/60.6.729. [DOI] [PubMed] [Google Scholar]
- 19.Walston J, McBurnie MA, Newman A, Tracy RP, Kop WJ, Hirsch CH, Gottdiener J, Fried LP. Frailty and activation of the inflammation and coagulation systems with and without clinical comorbidities: results from the Cardiovascular Health Study. Arch Intern Med. 2002;162(20):2333–41. doi: 10.1001/archinte.162.20.2333. [DOI] [PubMed] [Google Scholar]
- 20.Sullivan DH, Patch GA, Walls RC, Lipschitz DA. Impact of nutrition status on morbidity and mortality in a select population of geriatric rehabilitation patients. Am J Clin Nutr. 1990;51(5):749–58. doi: 10.1093/ajcn/51.5.749. [DOI] [PubMed] [Google Scholar]
- 21.Payette H, Coulombe C, Boutier V, Gray-Donald K. Nutrition risk factors for institutionalization in a free-living functionally dependent elderly population. J Clin Epidemiol. 2000;53(6):579–87. doi: 10.1016/s0895-4356(99)00186-9. [DOI] [PubMed] [Google Scholar]
- 22.Newman AB, Yanez D, Harris T, Duxbury A, Enright PL, Fried LP. Weight change in old age and its association with mortality. J Am Geriatr Soc. 2001;49(10):1309–18. doi: 10.1046/j.1532-5415.2001.49258.x. [DOI] [PubMed] [Google Scholar]
- 23.Bishop NA, Lu T, Yankner BA. Neural mechanisms of ageing and cognitive decline. Nature. 2010;464(7288):529–35. doi: 10.1038/nature08983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Panegyres PK. The contribution of the study of neurodegenerative disorders to the understanding of human memory. QJM. 2004;97(9):555–67. doi: 10.1093/qjmed/hch096. [DOI] [PubMed] [Google Scholar]
- 25.Miller DB, O’Callaghan JP. Aging, stress and the hippocampus. Ageing Res Rev. 2005;4(2):123–40. doi: 10.1016/j.arr.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 26.Luo XG, Ding JQ, Chen SD. Microglia in the aging brain: relevance to neurodegeneration. Mol Neurodegener. 2010;5:12. doi: 10.1186/1750-1326-5-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Streit WJ. Microglial senescence: does the brain’s immune system have an expiration date? Trends Neurosci. 2006;29(9):506–10. doi: 10.1016/j.tins.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 28.Cunningham C, Wilcockson DC, Campion S, Lunnon K, Perry VH. Central and systemic endotoxin challenges exacerbate the local inflammatory response and increase neuronal death during chronic neurodegeneration. J Neurosci. 2005;25(40):9275–84. doi: 10.1523/JNEUROSCI.2614-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van Gool WA, van de Beek D, Eikelenboom P. Systemic infection and delirium: when cytokines and acetylcholine collide. Lancet. 2010;375(9716):773–5. doi: 10.1016/S0140-6736(09)61158-2. [DOI] [PubMed] [Google Scholar]
- 30.Boyle PA, Buchman AS, Wilson RS, Leurgans SE, Bennett DA. Physical frailty is associated with incident mild cognitive impairment in community-based older persons. J Am Geriatr Soc. 2010;58(2):248–55. doi: 10.1111/j.1532-5415.2009.02671.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Buchman AS, Boyle PA, Wilson RS, Tang Y, Bennett DA. Frailty is associated with incident Alzheimer’s disease and cognitive decline in the elderly. Psychosom Med. 2007;69(5):483–9. doi: 10.1097/psy.0b013e318068de1d. [DOI] [PubMed] [Google Scholar]
- 32.Song X, Mitnitski A, Rockwood K. Nontraditional risk factors combine to predict Alzheimer disease and dementia. Neurology. 2011;77(3):227–34. doi: 10.1212/WNL.0b013e318225c6bc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Florini JR, Ewton DZ, Magri KA. Hormones, growth factors, and myogenic differentiation. Annu Rev Physiol. 1991;53:201–16. doi: 10.1146/annurev.ph.53.030191.001221. [DOI] [PubMed] [Google Scholar]
- 34.Lamberts SW. The endocrinology of aging and the brain. Arch Neurol. 2002;59(11):1709–11. doi: 10.1001/archneur.59.11.1709. [DOI] [PubMed] [Google Scholar]
- 35.Lamberts SW, van den Beld AW, van der Lely AJ. The endocrinology of aging. Science. 1997;278(5337):419–24. doi: 10.1126/science.278.5337.419. [DOI] [PubMed] [Google Scholar]
- 36.Leng SX, Cappola AR, Andersen RE, Blackman MR, Koenig K, Blair M, Walston JD. Serum levels of insulin-like growth factor-I (IGF-I) and dehydroepiandrosterone sulfate (DHEA-S), and their relationships with serum interleukin-6, in the geriatric syndrome of frailty. Aging Clin Exp Res. 2004;16(2):153–7. doi: 10.1007/BF03324545. [DOI] [PubMed] [Google Scholar]
- 37.Kaplan RC, Buzkova P, Cappola AR, Strickler HD, McGinn AP, Mercer LD, Arnold AM, Pollak MN, Newman AB. Decline in circulating insulin-like growth factors and mortality in older adults: cardiovascular health study all-stars study. J Clin Endocrinol Metab. 2012;97(6):1970–6. doi: 10.1210/jc.2011-2967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cappola AR, Xue QL, Ferrucci L, Guralnik JM, Volpato S, Fried LP. Insulin-like growth factor I and interleukin-6 contribute synergistically to disability and mortality in older women. J Clin Endocrinol Metab. 2003;88(5):2019–25. doi: 10.1210/jc.2002-021694. [DOI] [PubMed] [Google Scholar]
- 39.Laughlin GA, Barrett-Connor E, Criqui MH, Kritz-Silverstein D. The prospective association of serum insulin-like growth factor I (IGF-I) and IGF-binding protein-1 levels with all cause and cardiovascular disease mortality in older adults: the Rancho Bernardo Study. J Clin Endocrinol Metab. 2004;89(1):114–20. doi: 10.1210/jc.2003-030967. [DOI] [PubMed] [Google Scholar]
- 40.Saydah S, Graubard B, Ballard-Barbash R, Berrigan D. Insulin-like growth factors and subsequent risk of mortality in the United States. Am J Epidemiol. 2007;166(5):518–26. doi: 10.1093/aje/kwm124. [DOI] [PubMed] [Google Scholar]
- 41.Payette H, Roubenoff R, Jacques PF, Dinarello CA, Wilson PW, Abad LW, Harris T. Insulin-like growth factor-1 and interleukin 6 predict sarcopenia in very old community-living men and women: the Framingham Heart Study. J Am Geriatr Soc. 2003;51(9):1237–43. doi: 10.1046/j.1532-5415.2003.51407.x. [DOI] [PubMed] [Google Scholar]
- 42.Singh MA, Ding W, Manfredi TJ, Solares GS, O’Neill EF, Clements KM, Ryan ND, Kehayias JJ, Fielding RA, Evans WJ. Insulin-like growth factor I in skeletal muscle after weight-lifting exercise in frail elders. Am J Physiol. 1999;277(1 Pt 1):E135–43. doi: 10.1152/ajpendo.1999.277.1.E135. [DOI] [PubMed] [Google Scholar]
- 43.Campbell S, Szoeke C. Pharmacological treatment of frailty in the elderly. Journal of pharmacy practice and research. 2009;39(2):147–51. [Google Scholar]
- 44.Cawthon PM, Ensrud KE, Laughlin GA, Cauley JA, Dam TT, Barrett-Connor E, Fink HA, Hoffman AR, Lau E, Lane NE, Stefanick ML, Cummings SR, Orwoll ES. Sex hormones and frailty in older men: the osteoporotic fractures in men (MrOS) study. J Clin Endocrinol Metab. 2009;94(10):3806–15. doi: 10.1210/jc.2009-0417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tajar A, O’Connell MD, Mitnitski AB, O’Neill TW, Searle SD, Huhtaniemi IT, Finn JD, Bartfai G, Boonen S, Casanueva FF, Forti G, Giwercman A, Han TS, Kula K, Labrie F, Lean ME, Pendleton N, Punab M, Silman AJ, Vanderschueren D, Rockwood K, Wu FC. Frailty in relation to variations in hormone levels of the hypothalamic-pituitary-testicular axis in older men: results from the European male aging study. J Am Geriatr Soc. 2011;59(5):814–21. doi: 10.1111/j.1532-5415.2011.03398.x. [DOI] [PubMed] [Google Scholar]
- 46.Voznesensky M, Walsh S, Dauser D, Brindisi J, Kenny AM. The association between dehydroepiandosterone and frailty in older men and women. Age Ageing. 2009;38(4):401–6. doi: 10.1093/ageing/afp015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cappola AR, Xue QL, Walston JD, Leng SX, Ferrucci L, Guralnik J, Fried LP. DHEAS levels and mortality in disabled older women: the Women’s Health and Aging Study I. J Gerontol A Biol Sci Med Sci. 2006;61(9):957–62. doi: 10.1093/gerona/61.9.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Varadhan R, Walston J, Cappola AR, Carlson MC, Wand GS, Fried LP. Higher levels and blunted diurnal variation of cortisol in frail older women. J Gerontol A Biol Sci Med Sci. 2008;63(2):190–5. doi: 10.1093/gerona/63.2.190. [DOI] [PubMed] [Google Scholar]
- 49.Attaix D, Mosoni L, Dardevet D, Combaret L, Mirand PP, Grizard J. Altered responses in skeletal muscle protein turnover during aging in anabolic and catabolic periods. Int J Biochem Cell Biol. 2005;37(10):1962–73. doi: 10.1016/j.biocel.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 50.Sahin E, Depinho RA. Linking functional decline of telomeres, mitochondria and stem cells during ageing. Nature. 2010;464(7288):520–8. doi: 10.1038/nature08982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Miller RA. The aging immune system: primer and prospectus. Science. 1996;273(5271):70–4. doi: 10.1126/science.273.5271.70. [DOI] [PubMed] [Google Scholar]
- 52.Barzilay JI, Blaum C, Moore T, Xue QL, Hirsch CH, Walston JD, Fried LP. Insulin resistance and inflammation as precursors of frailty: the Cardiovascular Health Study. Arch Intern Med. 2007;167(7):635–41. doi: 10.1001/archinte.167.7.635. [DOI] [PubMed] [Google Scholar]
- 53.Harris TB, Ferrucci L, Tracy RP, Corti MC, Wacholder S, Ettinger WH, Jr., Heimovitz H, Cohen HJ, Wallace R. Associations of elevated interleukin-6 and C-reactive protein levels with mortality in the elderly. Am J Med. 1999;106(5):506–12. doi: 10.1016/s0002-9343(99)00066-2. [DOI] [PubMed] [Google Scholar]
- 54.Hubbard RE, O’Mahony MS, Savva GM, Calver BL, Woodhouse KW. Inflammation and frailty measures in older people. J Cell Mol Med. 2009;13(9B):3103–9. doi: 10.1111/j.1582-4934.2009.00733.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Leng SX, Xue QL, Tian J, Walston JD, Fried LP. Inflammation and frailty in older women. J Am Geriatr Soc. 2007;55(6):864–71. doi: 10.1111/j.1532-5415.2007.01186.x. [DOI] [PubMed] [Google Scholar]
- 56.Qu T, Walston JD, Yang H, Fedarko NS, Xue QL, Beamer BA, Ferrucci L, Rose NR, Leng SX. Upregulated ex vivo expression of stress-responsive inflammatory pathway genes by LPS-challenged CD14(+) monocytes in frail older adults. Mech Ageing Dev. 2009;130(3):161–6. doi: 10.1016/j.mad.2008.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Qu T, Yang H, Walston JD, Fedarko NS, Leng SX. Upregulated monocytic expression of CXC chemokine ligand 10 (CXCL-10) and its relationship with serum interleukin-6 levels in the syndrome of frailty. Cytokine. 2009;46(3):319–24. doi: 10.1016/j.cyto.2009.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Collerton J, Martin-Ruiz C, Davies K, Hilkens CM, Isaacs J, Kolenda C, Parker C, Dunn M, Catt M, Jagger C, von Zglinicki T, Kirkwood TB. Frailty and the role of inflammation, immunosenescence and cellular ageing in the very old: Cross-sectional findings from the Newcastle 85+ Study. Mech Ageing Dev. 2012;133(6):456–66. doi: 10.1016/j.mad.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 59.Silverman JM, Beeri MS, Schmeidler J, Rosendorff C, Angelo G, Mavris RS, Grossman HT, Elder GA, Carrion-Baralt J, West R. C-reactive protein and memory function suggest antagonistic pleiotropy in very old nondemented subjects. Age Ageing. 2009;38(2):237–41. doi: 10.1093/ageing/afn278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Semba RD, Nicklett EJ, Ferrucci L. Does accumulation of advanced glycation end products contribute to the aging phenotype? J Gerontol A Biol Sci Med Sci. 2010;65(9):963–75. doi: 10.1093/gerona/glq074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Oldenburg HS, Rogy MA, Lazarus DD, Van Zee KJ, Keeler BP, Chizzonite RA, Lowry SF, Moldawer LL. Cachexia and the acute-phase protein response in inflammation are regulated by interleukin-6. Eur J Immunol. 1993;23(8):1889–94. doi: 10.1002/eji.1830230824. [DOI] [PubMed] [Google Scholar]
- 62.Schaap LA, Pluijm SM, Deeg DJ, Visser M. Inflammatory markers and loss of muscle mass (sarcopenia) and strength. Am J Med. 2006;119(6):526 e9–17. doi: 10.1016/j.amjmed.2005.10.049. [DOI] [PubMed] [Google Scholar]
- 63.Yao X, Hamilton RG, Weng NP, Xue QL, Bream JH, Li H, Tian J, Yeh SH, Resnick B, Xu X, Walston J, Fried LP, Leng SX. Frailty is associated with impairment of vaccine-induced antibody response and increase in post-vaccination influenza infection in community-dwelling older adults. Vaccine. 2011;29(31):5015–21. doi: 10.1016/j.vaccine.2011.04.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ridda I, Macintyre CR, Lindley R, Gao Z, Sullivan JS, Yuan FF, McIntyre PB. Immunological responses to pneumococcal vaccine in frail older people. Vaccine. 2009;27(10):1628–36. doi: 10.1016/j.vaccine.2008.11.098. [DOI] [PubMed] [Google Scholar]
- 65.Jefferson T, Rivetti D, Rivetti A, Rudin M, Di Pietrantonj C, Demicheli V. Efficacy and effectiveness of influenza vaccines in elderly people: a systematic review. Lancet. 2005;366(9492):1165–74. doi: 10.1016/S0140-6736(05)67339-4. [DOI] [PubMed] [Google Scholar]
- 66.Howard C, Ferrucci L, Sun K, Fried LP, Walston J, Varadhan R, Guralnik JM, Semba RD. Oxidative protein damage is associated with poor grip strength among older women living in the community. J Appl Physiol. 2007;103(1):17–20. doi: 10.1152/japplphysiol.00133.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, Martin FC, Michel JP, Rolland Y, Schneider SM, Topinkova E, Vandewoude M, Zamboni M. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010;39(4):412–23. doi: 10.1093/ageing/afq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Manini T, Clark B. Dynapenia and aging: an update. J Gerontol A Biol Sci Med Sci. 2012;67A(1):28–40. doi: 10.1093/gerona/glr010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gaczynska M, Rock KL, Spies T, Goldberg AL. Peptidase activities of proteasomes are differentially regulated by the major histocompatibility complex-encoded genes for LMP2 and LMP7. Proc Natl Acad Sci U S A. 1994;91(20):9213–7. doi: 10.1073/pnas.91.20.9213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bell J. Redefining Disease. The Harveian Oration. Royal College of Physicians; 2010. pp. 1–37. 2010. [Google Scholar]
- 71.Rockwood K, Song X, MacKnight C, Bergman H, Hogan DB, McDowell I, Mitnitski A. A global clinical measure of fitness and frailty in elderly people. CMAJ. 2005;173(5):489–95. doi: 10.1503/cmaj.050051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rothman MD, Leo-Summers L, Gill TM. Prognostic significance of potential frailty criteria. J Am Geriatr Soc. 2008;56(12):2211–116. doi: 10.1111/j.1532-5415.2008.02008.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Guilley E, Ghisletta P, Armi F, Berchtold A, d’Epinay C, Michel J, de Ribaupierre A. Dynamics of Frailty and ADL Dependence in a Five-Year Longitudinal Study of Octogenarians. Research on Aging. 2008;30:299–317. [Google Scholar]
- 74.Sourial N, Wolfson C, Bergman H, Zhu B, Karunananthan S, Quail J, Fletcher J, Weiss D, Bandeen-Roche K, Beland F. A correspondence analysis revealed frailty deficits aggregate and are multidimensional. J Clin Epidemiol. 2010;63(6):647–54. doi: 10.1016/j.jclinepi.2009.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mitnitski AB, Mogilner AJ, Rockwood K. Accumulation of deficits as a proxy measure of aging. ScientificWorldJournal. 2001;1:323–36. doi: 10.1100/tsw.2001.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rockwood K, Mitnitski A. Frailty in relation to the accumulation of deficits. J Gerontol A Biol Sci Med Sci. 2007;62(7):722–7. doi: 10.1093/gerona/62.7.722. [DOI] [PubMed] [Google Scholar]
- 77.Rockwood K, Mitnitski A, Song X, Steen B, Skoog I. Long-term risks of death and institutionalization of elderly people in relation to deficit accumulation at age 70. J Am Geriatr Soc. 2006;54(6):975–9. doi: 10.1111/j.1532-5415.2006.00738.x. [DOI] [PubMed] [Google Scholar]
- 78.Rockwood K, Mitnitski A. Limits to deficit accumulation in elderly people. Mech Ageing Dev. 2006;127(5):494–6. doi: 10.1016/j.mad.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 79.Scheffer M. Complex systems: Foreseeing tipping points. Nature. 2010;467(7314):411–2. doi: 10.1038/467411a. [DOI] [PubMed] [Google Scholar]
- 80.Searle SD, Mitnitski A, Gahbauer EA, Gill TM, Rockwood K. A standard procedure for creating a frailty index. BMC Geriatr. 2008;8:24. doi: 10.1186/1471-2318-8-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mitnitski A, Song X, Skoog I, Broe GA, Cox JL, Grunfeld E, Rockwood K. Relative fitness and frailty of elderly men and women in developed countries and their relationship with mortality. J Am Geriatr Soc. 2005;53(12):2184–9. doi: 10.1111/j.1532-5415.2005.00506.x. [DOI] [PubMed] [Google Scholar]
- 82.Cigolle CT, Ofstedal MB, Tian Z, Blaum CS. Comparing models of frailty: the Health and Retirement Study. J Am Geriatr Soc. 2009;57(5):830–9. doi: 10.1111/j.1532-5415.2009.02225.x. [DOI] [PubMed] [Google Scholar]
- 83.Rockwood K, Andrew M, Mitnitski A. A comparison of two approaches to measuring frailty in elderly people. J Gerontol A Biol Sci Med Sci. 2007;62(7):738–43. doi: 10.1093/gerona/62.7.738. [DOI] [PubMed] [Google Scholar]
- 84.Kulminski AM, Ukraintseva SV, Kulminskaya IV, Arbeev KG, Land K, Yashin AI. Cumulative deficits better characterize susceptibility to death in elderly people than phenotypic frailty: lessons from the Cardiovascular Health Study. J Am Geriatr Soc. 2008;56(5):898–903. doi: 10.1111/j.1532-5415.2008.01656.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sanders JL, Boudreau RM, Fried LP, Walston JD, Harris TB, Newman AB. Measurement of organ structure and function enhances understanding of the physiological basis of frailty: the Cardiovascular Health Study. J Am Geriatr Soc. 2011;59(9):1581–8. doi: 10.1111/j.1532-5415.2011.03557.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gill TM, Gahbauer EA, Han L, Allore HG. Trajectories of disability in the last year of life. N Engl J Med. 2010;362(13):1173–80. doi: 10.1056/NEJMoa0909087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Collard RM, Boter H, Schoevers RA, Oude Voshaar RC. Prevalence of frailty in community-dwelling older persons: a systematic review. J Am Geriatr Soc. 2012;60(8):1487–92. doi: 10.1111/j.1532-5415.2012.04054.x. [DOI] [PubMed] [Google Scholar]
- 88.Santos-Eggimann B, Cuenoud P, Spagnoli J, Junod J. Prevalence of frailty in middle-aged and older community-dwelling Europeans living in 10 countries. J Gerontol A Biol Sci Med Sci. 2009;64(6):675–81. doi: 10.1093/gerona/glp012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Espinoza SE, Hazuda HP. Frailty in older Mexican-American and European-American adults: is there an ethnic disparity? J Am Geriatr Soc. 2008;56(9):1744–9. doi: 10.1111/j.1532-5415.2008.01845.x. [DOI] [PubMed] [Google Scholar]
- 90.Rockwood K, Howlett SE, MacKnight C, Beattie BL, Bergman H, Hebert R, Hogan DB, Wolfson C, McDowell I. Prevalence, attributes, and outcomes of fitness and frailty in community-dwelling older adults: report from the Canadian study of health and aging. J Gerontol A Biol Sci Med Sci. 2004;59(12):1310–7. doi: 10.1093/gerona/59.12.1310. [DOI] [PubMed] [Google Scholar]
- 91.Bandeen-Roche K, Xue QL, Ferrucci L, Walston J, Guralnik JM, Chaves P, Zeger SL, Fried LP. Phenotype of frailty: characterization in the women’s health and aging studies. J Gerontol A Biol Sci Med Sci. 2006;61(3):262–6. doi: 10.1093/gerona/61.3.262. [DOI] [PubMed] [Google Scholar]
- 92.Ensrud KE, Ewing SK, Taylor BC, Fink HA, Cawthon PM, Stone KL, Hillier TA, Cauley JA, Hochberg MC, Rodondi N, Tracy JK, Cummings SR. Comparison of 2 frailty indexes for prediction of falls, disability, fractures, and death in older women. Arch Intern Med. 2008;168(4):382–9. doi: 10.1001/archinternmed.2007.113. [DOI] [PubMed] [Google Scholar]
- 93.Gill TM, Gahbauer EA, Allore HG, Han L. Transitions between frailty states among community-living older persons. Archives of Internal Medicine. 2006;166(4):418–23. doi: 10.1001/archinte.166.4.418. [DOI] [PubMed] [Google Scholar]
- 94.Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, Seeman T, Tracy R, Kop WJ, Burke G, McBurnie MA. Cardiovascular Health Study Collaborative Research G. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3):M146–56. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 95.Rockwood K, Howlett SE, MacKnight C, Beattie BL, Bergman H, Hebert R, Hogan DB, Wolfson C, McDowell I. Prevalence, attributes, and outcomes of fitness and frailty in community-dwelling older adults: report from the Canadian study of health and aging. J Gerontol A Biol Sci Med Sci. 2004;59(12):1310–7. doi: 10.1093/gerona/59.12.1310. [DOI] [PubMed] [Google Scholar]
- 96.Fried LP, Ferrucci L, Darer J, Williamson JD, Anderson G. Untangling the concepts of disability, frailty, and comorbidity: implications for improved targeting and care. J Gerontol A Biol Sci Med Sci. 2004;59(3):255–63. doi: 10.1093/gerona/59.3.m255. [DOI] [PubMed] [Google Scholar]
- 97.Theou O, Rockwood MR, Mitnitski A, Rockwood K. Disability and co-morbidity in relation to frailty: How much do they overlap? Arch Gerontol Geriatr. 2012;55(2):e1–8. doi: 10.1016/j.archger.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 98.de Vries NM, Staal JB, van Ravensberg CD, Hobbelen JS, Olde Rikkert MG, Nijhuis-van der Sanden MW. Outcome instruments to measure frailty: a systematic review. Ageing Res Rev. 2011;10(1):104–14. doi: 10.1016/j.arr.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 99.Sternberg SA, Wershof Schwartz A, Karunananthan S, Bergman H, Mark Clarfield A. The identification of frailty: a systematic literature review. J Am Geriatr Soc. 2011;59(11):2129–38. doi: 10.1111/j.1532-5415.2011.03597.x. [DOI] [PubMed] [Google Scholar]
- 100.Gloth FM, 3rd, Walston J, Meyer J, Pearson J. Reliability and validity of the Frail Elderly Functional Assessment questionnaire. Am J Phys Med Rehabil. 1995;74(1):45–53. doi: 10.1097/00002060-199501000-00008. [DOI] [PubMed] [Google Scholar]
- 101.Gloth FM, 3rd, Scheve AA, Shah S, Ashton R, McKinney R. The Frail Elderly Functional Assessment questionnaire: its responsiveness and validity in alternative settings. Arch Phys Med Rehabil. 1999;80(12):1572–6. doi: 10.1016/s0003-9993(99)90332-5. [DOI] [PubMed] [Google Scholar]
- 102.Schuurmans H, Steverink N, Lindenberg S, Frieswijk N, Slaets JP. Old or frail: what tells us more? J Gerontol A Biol Sci Med Sci. 2004;59(9):M962–5. doi: 10.1093/gerona/59.9.m962. [DOI] [PubMed] [Google Scholar]
- 103.Gobbens RJ, van Assen MA, Luijkx KG, Schols JM. The Predictive Validity of the Tilburg Frailty Indicator: Disability, Health Care Utilization, and Quality of Life in a Population at Risk. Gerontologist. 2012 doi: 10.1093/geront/gnr135. [DOI] [PubMed] [Google Scholar]
- 104.Metzelthin SF, Daniels R, van Rossum E, de Witte L, van den Heuvel WJ, Kempen GI. The psychometric properties of three self-report screening instruments for identifying frail older people in the community. BMC Public Health. 2010;10:176. doi: 10.1186/1471-2458-10-176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Podsiadlo D, Richardson S. The timed “Up & Go”: a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc. 1991;39(2):142–8. doi: 10.1111/j.1532-5415.1991.tb01616.x. [DOI] [PubMed] [Google Scholar]
- 106.Syddall H, Cooper C, Martin F, Briggs R, Aihie Sayer A. Is grip strength a useful single marker of frailty? Age Ageing. 2003;32(6):650–6. doi: 10.1093/ageing/afg111. [DOI] [PubMed] [Google Scholar]
- 107.Studenski S, Perera S, Patel K, Rosano C, Faulkner K, Inzitari M, Brach J, Chandler J, Cawthon P, Connor EB, Nevitt M, Visser M, Kritchevsky S, Badinelli S, Harris T, Newman AB, Cauley J, Ferrucci L, Guralnik J. Gait speed and survival in older adults. JAMA. 2011;305(1):50–8. doi: 10.1001/jama.2010.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Rolfson DB, Majumdar SR, Tsuyuki RT, Tahir A, Rockwood K. Validity and reliability of the Edmonton Frail Scale. Age Ageing. 2006;35(5):526–9. doi: 10.1093/ageing/afl041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Armstrong JJ, Stolee P, Hirdes JP, Poss JW. Examining three frailty conceptualizations in their ability to predict negative outcomes for home-care clients. Age Ageing. 2010;39(6):755–8. doi: 10.1093/ageing/afq121. [DOI] [PubMed] [Google Scholar]
- 110.Rubenstein LZ, Stuck AE, Siu AL, Wieland D. Impacts of geriatric evaluation and management programs on defined outcomes: overview of the evidence. J Am Geriatr Soc. 1991;39(9 Pt 2):8S–16S. doi: 10.1111/j.1532-5415.1991.tb05927.x. discussion 7S-8S. [DOI] [PubMed] [Google Scholar]
- 111.Ellis G, Whitehead MA, Robinson D, O’Neill D, Langhorne P. Comprehensive geriatric assessment for older adults admitted to hospital: meta-analysis of randomised controlled trials. BMJ. 2011;343:d6553. doi: 10.1136/bmj.d6553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Harari D, Hopper A, Dhesi J, Babic-Illman G, Lockwood L, Martin F. Proactive care of older people undergoing surgery (’POPS’): designing, embedding, evaluating and funding a comprehensive geriatric assessment service for older elective surgical patients. Age Ageing. 2007;36(2):190–6. doi: 10.1093/ageing/afl163. [DOI] [PubMed] [Google Scholar]
- 113.Extermann M, Aapro M, Bernabei R, Cohen HJ, Droz JP, Lichtman S, Mor V, Monfardini S, Repetto L, Sorbye L, Topinkova E. Use of comprehensive geriatric assessment in older cancer patients: recommendations from the task force on CGA of the International Society of Geriatric Oncology (SIOG) Crit Rev Oncol Hematol. 2005;55(3):241–52. doi: 10.1016/j.critrevonc.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 114.Jones DM, Song X, Rockwood K. Operationalizing a frailty index from a standardized comprehensive geriatric assessment. J Am Geriatr Soc. 2004;52(11):1929–33. doi: 10.1111/j.1532-5415.2004.52521.x. [DOI] [PubMed] [Google Scholar]
- 115.Beswick AD, Rees K, Dieppe P, Ayis S, Gooberman-Hill R, Horwood J, Ebrahim S. Complex interventions to improve physical function and maintain independent living in elderly people: a systematic review and meta-analysis. Lancet. 2008;371(9614):725–35. doi: 10.1016/S0140-6736(08)60342-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Stuck AE, Egger M, Hammer A, Minder CE, Beck JC. Home visits to prevent nursing home admission and functional decline in elderly people: systematic review and meta-regression analysis. JAMA. 2002;287(8):1022–8. doi: 10.1001/jama.287.8.1022. [DOI] [PubMed] [Google Scholar]
- 117.van Praag H. Exercise and the brain: something to chew on. Trends Neurosci. 2009;32(5):283–90. doi: 10.1016/j.tins.2008.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Barber SE, Clegg AP, Young JB. Is there a role for physical activity in preventing cognitive decline in people with mild cognitive impairment? Age Ageing. 2012;41(1):5–8. doi: 10.1093/ageing/afr138. [DOI] [PubMed] [Google Scholar]
- 119.Gleeson M, McFarlin B, Flynn M. Exercise and Toll-like receptors. Exerc Immunol Rev. 2006;12:34–53. [PubMed] [Google Scholar]
- 120.Handschin C, Spiegelman BM. The role of exercise and PGC1alpha in inflammation and chronic disease. Nature. 2008;454(7203):463–9. doi: 10.1038/nature07206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.de Vries NM, van Ravensberg CD, Hobbelen JS, Olde Rikkert MG, Staal JB, Nijhuis-van der Sanden MW. Effects of physical exercise therapy on mobility, physical functioning, physical activity and quality of life in community-dwelling older adults with impaired mobility, physical disability and/or multi-morbidity: a meta-analysis. Ageing Res Rev. 2012;11(1):136–49. doi: 10.1016/j.arr.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 122.Theou O, Stathokostas L, Roland KP, Jakobi JM, Patterson C, Vandervoort AA, Jones GR. The effectiveness of exercise interventions for the management of frailty: a systematic review. J Aging Res. 2011;2011:569194. doi: 10.4061/2011/569194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Clegg A, Barber S, Young J, Forster A, Iliffe S. Do home-based exercise interventions improve outcomes for frail older people? Findings from a systematic review. Reviews in clinical gerontology. 2012;22(1):68–78. doi: 10.1017/S0959259811000165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Gill TM, Baker DI, Gottschalk M, Peduzzi PN, Allore H, Byers A. A program to prevent functional decline in physically frail, elderly persons who live at home. N Engl J Med. 2002;347(14):1068–74. doi: 10.1056/NEJMoa020423. [DOI] [PubMed] [Google Scholar]
- 125.Forster A, Lambley R, Hardy J, Young J, Smith J, Green J, Burns E. Rehabilitation for older people in long-term care. Cochrane database of systematic reviews. 2009;1:CD004294. doi: 10.1002/14651858.CD004294.pub2. [DOI] [PubMed] [Google Scholar]
- 126.Fiatarone MA, O’Neill EF, Ryan ND, Clements KM, Solares GR, Nelson ME, Roberts SB, Kehayias JJ, Lipsitz LA, Evans WJ. Exercise training and nutritional supplementation for physical frailty in very elderly people. N Engl J Med. 1994;330(25):1769–75. doi: 10.1056/NEJM199406233302501. [DOI] [PubMed] [Google Scholar]
- 127.Langer G, Schloemer G, Knerr A, Kuss O, Behrens J. Nutritional interventions for preventing and treating pressure ulcers. Cochrane Database Syst Rev. 2003;(4):CD003216. doi: 10.1002/14651858.CD003216. [DOI] [PubMed] [Google Scholar]
- 128.Schaufelberger M, Andersson G, Eriksson BO, Grimby G, Held P, Swedberg K. Skeletal muscle changes in patients with chronic heart failure before and after treatment with enalapril. Eur Heart J. 1996;17(11):1678–85. doi: 10.1093/oxfordjournals.eurheartj.a014751. [DOI] [PubMed] [Google Scholar]
- 129.Onder G, Penninx BW, Balkrishnan R, Fried LP, Chaves PH, Williamson J, Carter C, Di Bari M, Guralnik JM, Pahor M. Relation between use of angiotensin-converting enzyme inhibitors and muscle strength and physical function in older women: an observational study. Lancet. 2002;359(9310):926–30. doi: 10.1016/s0140-6736(02)08024-8. [DOI] [PubMed] [Google Scholar]
- 130.Sumukadas D, Witham MD, Struthers AD, McMurdo ME. Effect of perindopril on physical function in elderly people with functional impairment: a randomized controlled trial. CMAJ. 2007;177(8):867–74. doi: 10.1503/cmaj.061339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Basaria S, Coviello AD, Travison TG, Storer TW, Farwell WR, Jette AM, Eder R, Tennstedt S, Ulloor J, Zhang A, Choong K, Lakshman KM, Mazer NA, Miciek R, Krasnoff J, Elmi A, Knapp PE, Brooks B, Appleman E, Aggarwal S, Bhasin G, Hede-Brierley L, Bhatia A, Collins L, LeBrasseur N, Fiore LD, Bhasin S. Adverse events associated with testosterone administration. N Engl J Med. 2010;363(2):109–22. doi: 10.1056/NEJMoa1000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Friedlander AL, Butterfield GE, Moynihan S, Grillo J, Pollack M, Holloway L, Friedman L, Yesavage J, Matthias D, Lee S, Marcus R, Hoffman AR. One year of insulin-like growth factor I treatment does not affect bone density, body composition, or psychological measures in postmenopausal women. J Clin Endocrinol Metab. 2001;86(4):1496–503. doi: 10.1210/jcem.86.4.7377. [DOI] [PubMed] [Google Scholar]
- 133.Puts MT, Visser M, Twisk JW, Deeg DJ, Lips P. Endocrine and inflammatory markers as predictors of frailty. Clin Endocrinol (Oxf) 2005;63(4):403–11. doi: 10.1111/j.1365-2265.2005.02355.x. [DOI] [PubMed] [Google Scholar]
- 134.Wicherts IS, van Schoor NM, Boeke AJ, Visser M, Deeg DJ, Smit J, Knol DL, Lips P. Vitamin D status predicts physical performance and its decline in older persons. J Clin Endocrinol Metab. 2007;92(6):2058–65. doi: 10.1210/jc.2006-1525. [DOI] [PubMed] [Google Scholar]
- 135.Gillespie LD, Robertson MC, Gillespie WJ, Lamb SE, Gates S, Cumming RG, Rowe BH. Interventions for preventing falls in older people living in the community. Cochrane Database Syst Rev. 2009;(2):CD007146. doi: 10.1002/14651858.CD007146.pub2. [DOI] [PubMed] [Google Scholar]
- 136.Avenell A, Gillespie WJ, Gillespie LD, O’Connel D. Vitamin D and vitamin D analogues for preventing fractures associated with involutional and post-menopausal osteoporosis. Cochrane Database Syst Rev. 2009;(2):CD000227. doi: 10.1002/14651858.CD000227.pub3. [DOI] [PubMed] [Google Scholar]
- 137.Rockwood K, Hubbard R. Frailty and the geriatrician. Age Ageing. 2004;33(5):429–30. doi: 10.1093/ageing/afh153. [DOI] [PubMed] [Google Scholar]
- 138.Rockwood K. What would make a definition of frailty successful? Age Ageing. 2005;34(5):432–4. doi: 10.1093/ageing/afi146. [DOI] [PubMed] [Google Scholar]
- 139.Reuben DB, Tinetti ME. Goal-oriented patient care--an alternative health outcomes paradigm. N Engl J Med. 2012;366(9):777–9. doi: 10.1056/NEJMp1113631. [DOI] [PubMed] [Google Scholar]