Abstract
OBJECTIVE
Abdominal aortic aneurysms (AAA) are age-associated, life-threatening inflammatory dilations of the abdominal aorta. Human population studies have shown an association between obesity and AAA formation, but the molecular mechanisms underlying this connection remain largely unexplored. Adiponectin is an anti-inflammatory adipokine that that is downregulated in obesity. In this study we evaluated the role of adiponectin in a model of AAA using apolipoprotein E/adiponectin double-knockout (Apoe−/− Apn−/−) mice.
APPROACH AND RESULTS
Angiotensin II (Ang II)-infusion in male Apoe−/− Apn−/− mice led to a higher incidence of AAA and a significant increase of maximal aortic diameter compared with that of Apoe−/− mice (2.12 ± 0.07 mm vs. 1.67 ± 0.09 mm, respectively at 28 days). Adiponectin-deficiency augmented the early infiltration of macrophages and increased the expression of pro-inflammatory factors in the dilated aortic wall. MMP-2 and MMP-9 activation was also augmented in the aorta of Apoe−/− Apn−/− mice compared to Apoe−/− mice. These data suggest that the downregulation of adiponectin could directly contribute to the elevated incidence of AAA observed in obese individuals.
CONCLUSIONS
Adiponectin attenuates Ang II-induced vascular inflammation and AAA formation in mice.
Keywords: adiponectin, abdominal aortic aneurysm, inflammation
Introduction
Abdominal aortic aneurysm (AAA) is a life-threatening, age-associated vascular pathology that represents one of the leading causes of death in developed countries where a growing percentage of the population is over 65 years of age1. AAAs are localized dilations of the abdominal aorta exceeding the normal aortic diameter by more than 50%, which frequently lead to aortic rupture with a mortality rate as high as 90%. It is widely accepted that the primary events in AAA development involve inflammation and proteolytic degradation of extracellular matrix in the vessel wall. However, more precise knowledge of the molecular mechanisms underlying AAA is required because this disease is underdiagnosed, and the only available therapies are open surgery or endovascular aortic repair, that are costly and associated with high mortality rates1, 2.
Classical risk factors for AAA formation include smoking, male sex, age (>60 years), hypertension and family history1. Although not considered a traditional risk factor for AAA, an increasing number of human population studies have found an independent association of obesity or visceral adiposity with AAA presence or increasing abdominal aortic diameter3–8. In addition, studies in mouse models have shown that obesity promotes AAA formation9. Despite this compelling body of evidence, the molecular mechanisms that link obesity to AAA development remain largely unknown.
It is widely accepted that the unbalanced production of adipokines by dysfunctional adipose tissue in obese individuals greatly contributes to the increased cardiovascular risk associated with obesity10. However, the direct role of adipokines in AAA pathobiology remains relatively unexplored. Adiponectin is an adipokine, produced almost exclusively by the adipose tissue, that exerts anti-inflammatory effects on vascular and immune cells10 and is downregulated in obese individuals11, 12. Here we show that adiponectin protects against AAA formation and growth in a validated model of experimental AAA13.
Methods
Animal and Animal Protocol
We used a widely-accepted model of experimental AAA formation based on the infusion of Ang II to hyperlipidemic mice13. Adiponectin-deficient (Apn−/−) mice were crossed with Apoe−/− mice in the C57BL/6J background (The Jackson Laboratory) to generate apoE and adiponectin double knockout (Apoe−/− Apn−/−) mice. Water and regular mouse diet were available ad libitum. Alzet mini-osmotic pumps (Model 2004, Durect Corp.) were used to deliver 1000ng/kg/min of Ang II (Sigma-Aldrich) to 10-week-old mice for a period of 28 days. Pumps were implanted subcutaneously in the murine mild-scapular region through a small incision in the back of the neck that was closed with clips. Aged-matched mice were used as non-AAA controls (baseline). Both aneurysmal and non-aneurysmal animals were analyzed together. All experimental procedures were performed in accordance with the guidelines of the Institutional Animal Care and Use Committee at Boston University.
Ultrasonography
Abdominal aorta expansion was measured by high resolution ultrasonography with a small animal ultrasonography system (Vevo 770, Fujifilm VisualSonics Inc.) at baseline and 7, 14, 21 and 28 days after aneurysm induction by Ang II infusion, as previously described14. In brief, mice were anesthetized using 2% isoflurane and laid supine on a heated 37°C plate. B-mode ultrasound (US) imaging was used to assess suprarenal abdominal aortic diameter using a real-time microvisualization scan head (RMV 704) with a central frequency of 40 MHz (frame rate of 30 Hz). AAA incidence was determined based on an increase in the diameter of the suprarenal aorta between the diaphragm and the renal arteries of at least 50% or greater compared with baseline.
Serum Analyses
Blood samples were collected from mice in the fasting (14 hours) state. Serum glucose, total cholesterol and triglyceride concentrations were measured with enzymatic kits (Wako Pure Chemical Industries, Ltd.).
Histological Analyses and Immunohistochemistry
Twenty-eight days after Ang II infusion, mice were euthanized and perfused briefly with PBS, followed by prolonged whole-body perfusion with 4% paraformaldehyde solution. After dissection from the surrounding tissue, the suprarenal aorta was further fixed in 4% paraformaldehyde overnight, and then embedded in paraffin. Suprarenal abdominal aortic tissue was sectioned into 5 µm-thick serial sections. To visualize elastic lamina, histological sections were stained with the Elastic Stain kit (Sigma-Aldrich) following manufacturer’s instructions. To evaluate macrophage infiltration, sections were immunostained with a mouse F4/80 antibody. In brief, sections were subjected to heat-mediated antigen retrieval, blocked with 5% horse serum for 1.5 hour at room temperature, and then incubated with rat polyclonal anti-mouse F4/80 antibody (AbD Serotec) overnight at 4 °C. Detection of F4/80+ cells was achieved using the biotin/streptavidin-HRP system (Vector Laboratories). Histological sections were examined under a light microscope (Biorevo, Keyence). Four random microscopic fields in each mouse (n=4 in each group) were counted, and macrophage number was expressed as number of F4/80-positive cells per square millimeter.
RNA and protein extraction from the aortic samples
Suprarenal aortic samples were from abdominal aorta between the diaphragm and the renal arteries. These samples were cut approximately in half (one piece for RNA and the other for protein). Wet weights of tissues typically ranged from 1–2 mg at baseline and at day 3, and 4–10 mg at day 28. For RNA isolation, samples were homogenized in 1 ml of TRIzol Reagent with stainless steel beads using TissueLyser II (Qiagen). 200 microliters of chloroform was added, and the tubes were shaken for 15 seconds and incubated for 5 minutes at room temperature. The samples were centrifuged at 12,000×g for 15 minutes at 4°C, and the aqueous phase was transferred to a fresh tube. 500 microliters of isopropyl alcohol was added and incubated for 10 minutes at room temperature. After the samples were centrifuged at 12,000×g for 15 minutes at 4°C, the small RNA pellet could be visualized. The supernatant was carefully removed, and the RNA pellets were washed once with 1 ml of 75 % ethanol. The samples were centrifuged at 10,000×g for 10 minutes at 4°C. The supernatant was decanted carefully, and the RNA pellets were dried briefly and dissolved in 6–10 µl RNase-free water (DEPC water) accordingly to adjust for differences in the RNA pellet size. The concentration of RNA was measured using NanoDrop 1000 (Thermo Scientific), typically obtaining between 1 and 8 µg RNA. For protein extraction, samples were homogenized in 50 or 100 µl of RIPA Buffer (Thermo Scientific) according to aortic sample size (50 µl for baseline samples, 100 µl for day 28 samples) with stainless steel beads using TissueLyser II (Qiagen). The material was then centrifuged at 15,000×g for 15 minutes at 4°C to pellet the debris and the supernatant was transferred new tubes. Protein concentration was measured using the BCA Protein Assay Kit (Thermo Scientific). These preparations typically yielded between 34–83 µg protein from the baseline samples and 117–466 µg protein from the day 28 samples.
Quantitative Real-Time (RT) PCR
RNA (1 µg) was used for cDNA synthesis with a QuantiTect Reverse Transcription Kit (Qiagen) according to manufacturer’s instructions. SYBR Green reagent (SYBR Select Master Mix, Life Technologies) was used to evaluate PCR product amplification using a ViiA 7 Real-Time PCR System (Applied Biosystems). Specific primers are described in the Supplemental Table. Results were analyzed with the ViiA 7 software, and transcript levels were adjusted relative to the average of expression of Gapdh, Actb and Rplp0 as an internal control.
Gelatin Zymography
Suprarenal abdominal aortic protein extracts (30 µg) were obtained from the same mice used for qPCR analysis, loaded onto gelatin gels (Zymogram Ready Gels, Bio-Rad) and subjected to electrophoresis. After renaturation, gels were incubated for 12 hours at 37 °C in development solution (Bio-Rad) to promote gelatin digestion by MMPs. Gels were stained with Coomassie Brilliant Blue and total MMP levels were determined according to the manufacturer’s instructions. MMP activities were quantified using Image J software.
Data and Statistical Analysis
Data were expressed as means ±SEM. All statistical analyses were performed using GraphPad Prism version 6 (GraphPad Software, Inc.). The statistical significance of differences was assessed with Mann-Whitney U test, Wilcoxon signed-rank test, two-way ANOVA and Log-rank test, where appropriate. P-values of <0.05 were considered to be statistically significant.
Results
Adiponectin protects against Ang II-Induced AAA formation
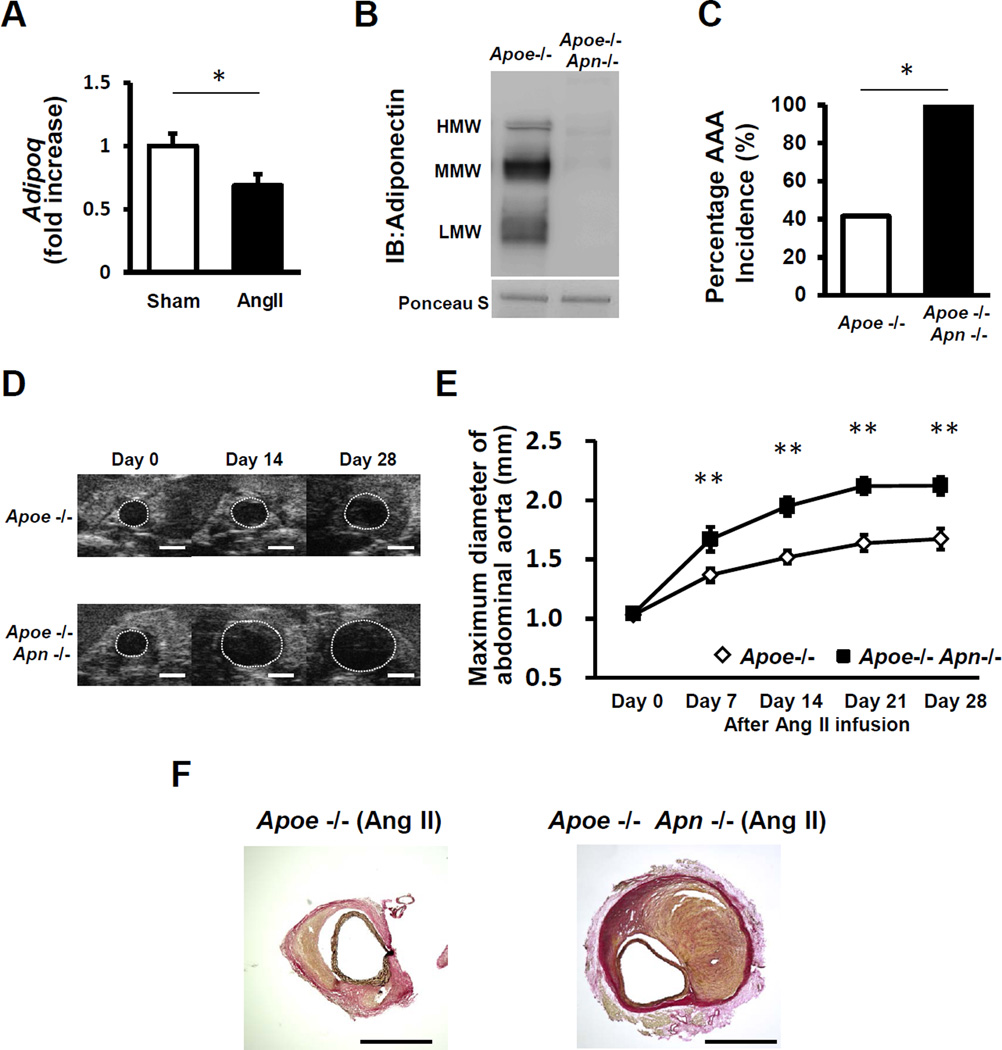
To evaluate the role of adiponectin in AAA formation and progression, we used an experimental model where angiotensin II (AngII) is infused to hyperlipidemic apoE-KO mice for 4 weeks via a subcutaneous osmotic mini-pump to induce the formation of suprarenal AAA13. We found that AngII infusion for 7 days leads to a significant decrease in the expression of adiponectin in white adipose tissue in this model (Figure 1A), consistent with previous reports in other systems15–17, but levels returned to baseline by 28 days (data not shown). Next, we evaluated AngII-induced AAA formation in Apoe/Apn (Apoe−/− Apn−/−) double knockout mice and Apoe−/− controls. Apn inactivation in Apoe−/− Apn−/− mice was confirmed by Western Blot (Figure 1B). Body weight and epididymal fat weight did not differ between Apoe−/− Apn−/− and Apoe−/− either at baseline or after 4 weeks of Ang II infusion. Similarly, adiponectin deficiency had no effect on total serum cholesterol, triglyceride or fasting glucose levels either at baseline or after 4 weeks of Ang II infusion (Table 1), demonstrating that adiponectin inactivation does not affect systemic metabolism in this mouse model. During the time course of the experiment, 33% (6 out of 18) of the Apoe−/− mice and 44% (8 out of 18) of Apoe−/− Apn−/− mice infused with Ang II died due to acute aortic rupture although this trend toward greater mortality in adiponectin-deficient mice was not statistically significant (Supplemental Figure 1). Among the surviving mice, Ang II infusion led to a 100% (10 out of 10) incidence of AAA in the Apoe−/− Apn−/− mice, in contrast to a 42% (5 out of 12) incidence of AAA in Apoe−/− mice as determined by ultrasound imaging (Figure 1C,D). Consistently, there was also a significant increase in maximal suprarenal aortic diameter in Apoe−/− Apn−/− relative to Apoe−/− mice at 7, 14, 21 and 28 days after Ang II infusion (Figure 1E). After 28 days of Ang II infusion average suprarenal aortic diameter was 2.12±0.07 mm in Apoe−/− Apn−/− mice compared with 1.67±0.09 mm in Apoe−/− controls.
Figure 1. Adiponectin deficiency exacerbates Ang II-induced AAA formation.
A. Adipoq mRNA expression levels as determined by quantitative real time-PCR in white adipose tissues of Apoe−/− mice after 7 days of Ang II infusion. The value of sham is set as 1. Results are mean±SEM. n=8 in each group. *P<0.05 using Mann-Whitney U test. B, Native western blot of mouse serum using anti-adiponectin primary antibody. C, Incidence of Ang II-induced AAA in Apoe−/− mice (n=12) and Apoe−/− Apn−/− mice (n=10) after Ang II infusion for 4 weeks. *P<0.05 using Wilcoxon signed-rank test. D, Representative B-mode ultrasonographic images of maximum diameter of aorta at various time points Scale bars indicate 1mm. E, Maximum suprarenal abdominal aortic diameter quantified by ultrasonography at baseline, and at 7, 14, 21 and 28 days after Ang II infusion. Results are mean±SEM. n=12 in Apoe−/− mice and n=10 in Apoe−/− Apn−/− mice. **P<0.01, Apoe−/− mice vs. Apoe−/− Apn−/− mice using two-way ANOVA with repeated measures. F, Representative photographs of elastin-stained histologic section showing macroscopic features of aneurysms induced by Ang II at 28 days. These sections highlight the intramural involvement, but not differences in lumen size because a stabilizing substance was not infused prior to harvest to preserve the lumen’s architecture. Scale bars indicate 1mm.
Table 1.
Characteristics of Apoe−/− and Apoe−/− Apn−/− mice.
| Apoe−/− | Apoe−/− Apn−/− | Apoe−/− | Apoe−/− Apn−/− | |
|---|---|---|---|---|
| Baseline | Ang II Day 28 | |||
| Body weight (g) | 30.2 ± 0.5 | 30.1 ± 0.7 | 29.8 ± 0.4 | 29.7 ± 0.5 |
| Epididymal fat weight (mg) | 217.1 ± 6.8 | 212.9 ± 9.2 | 165.8 ± 8.0** | 177.0 ±12.3* |
| Fasting Glucose (mg/dL) | 116.5 ± 6.5 | 115.7 ± 5.4 | 133.2 ± 5.4 | 134.2 ± 9.0 |
| Total Cholesterol (mg/dL) | 462.5 ± 33.6 | 440.2 ± 49.2 | 511.1 ± 23.4 | 557.8 ± 36.1 |
| Triglyceride (mg/dL) | 125.7 ± 8.5 | 126.3 ± 12.3 | 132.3 ± 9.3 | 159.7 ± 19.8 |
Ang II; angiotensin II. Results are mean±SEM.
n=10–18 in body weight, n=7–12 in epididymal fat weight, n=6 in fasting glucose, total cholesterol and triglyceride in each group.
P<0.01,
P<0.05, baseline vs. day 28 using two-way ANOVA.
Morphologically, the aortae of Apoe−/− Apn−/− mice did not differ from those of Apoe−/− mice at baseline (data was not shown), but AngII infusion led to a larger expansion of the vascular wall in adiponectin-deficient mice due to the development of a tissue mass comprised of mural thrombus, extracellular matrix and cellular materials (Figure 1F). As expected, AngII infusion increased systolic blood pressure, although there was no difference between Apoe−/− mice and Apoe−/− Apn−/− mice at any time point (Supplemental Figure 2A), demonstrating that the increased AAA size observed in adiponectin-deficient mice is not due to changes in blood pressure. Similarly, there were no differences in heart rate between the two strains (Supplemental Figure 2B).
Adiponectin-deficiency increases macrophage content in AngII-induced AAA
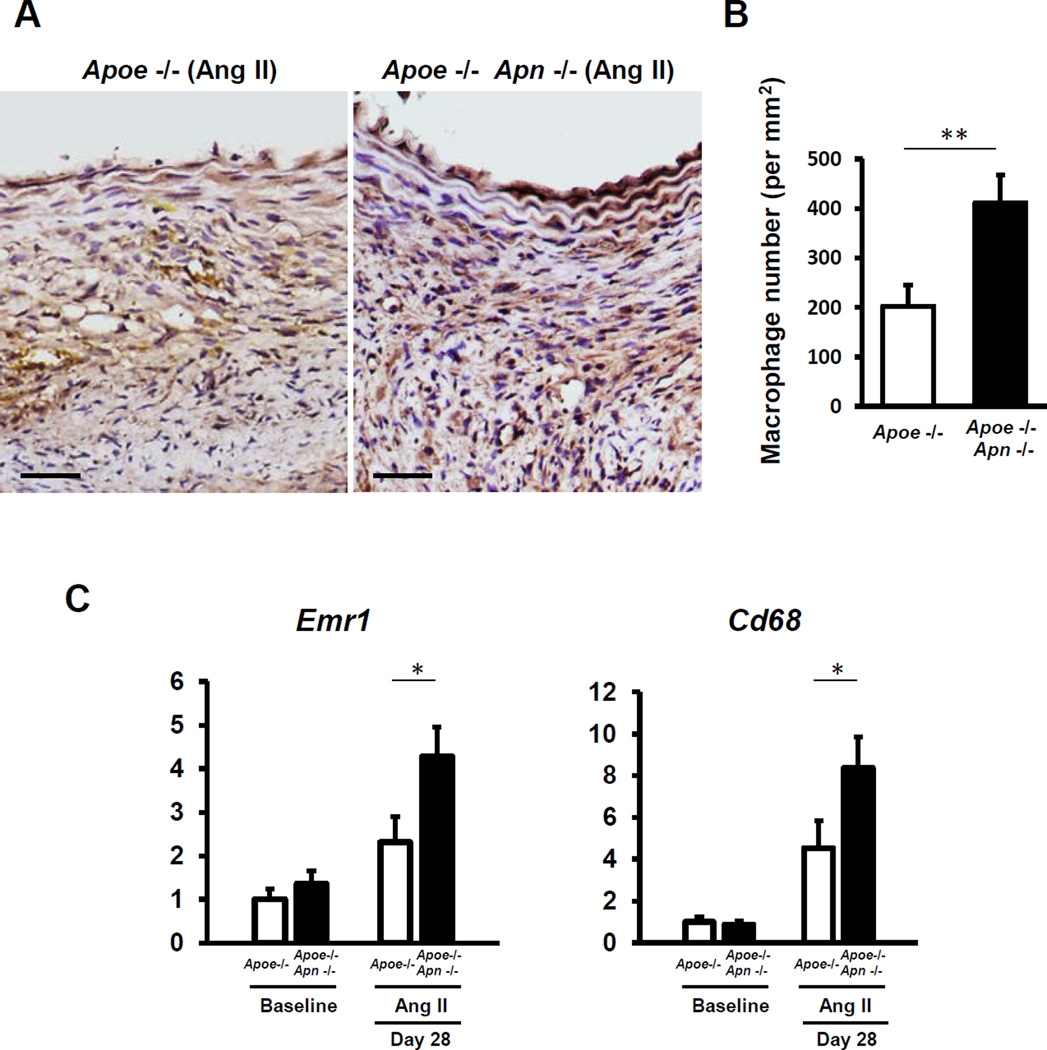
Macrophage-mediated inflammation plays an important role in AngII-induced AAA18, 19. To examine the effects of adiponectin deficiency on macrophage recruitment to AAA, we performed immunohistochemical studies to detect F4/80+ macrophages in sections of the suprarenal aorta of Apoe−/− Apn−/− mice and Apoe−/− controls at the 28 day time point. As shown in Figure 2A,B, macrophage content in AAAs was significantly increased in adiponectin-deficient mice. Consistently, increased expression of the macrophage-specific transcripts Emr1 (F4/80) and Cd68 was found in whole suprarenal AAA samples of Apoe−/− Apn−/− (Figure 2C). These data suggest that adiponectin prevents macrophage infiltration into the vascular wall in AngII-infused mice.
Figure 2. Adiponectin deficiency increases macrophage content in AAA.
A, Micrographs of representative F4/80 immunohistochemical staining (brown) of AAA in Apoe−/− mice and Apoe−/− Apn−/− mice at day 28 after Ang II infusion. Scale bars indicate 50µm. B, F4/80-positive macrophage content in the AAA vessel wall. Results are mean±SEM. n=4 in each group. **P<0.01 using Mann–Whitney U test. C, Quantitative real time-PCR analysis of the macrophage-specific transcripts Emr1 (F4/80) and Cd68 in suprarenal abdominal aortae at day 28 after Ang II infusion. Transcript levels of Gapdh, Actb and Rplp0 serves as an internal control. The value of gene expression in Apoe−/− mice at baseline is set as 1. Results are mean±SEM. n=8 in each group. *P<0.05, Apoe−/− mice vs. Apoe−/− Apn−/− mice using two-way ANOVA.
Adiponectin-deficiency promotes vascular inflammation in AAA
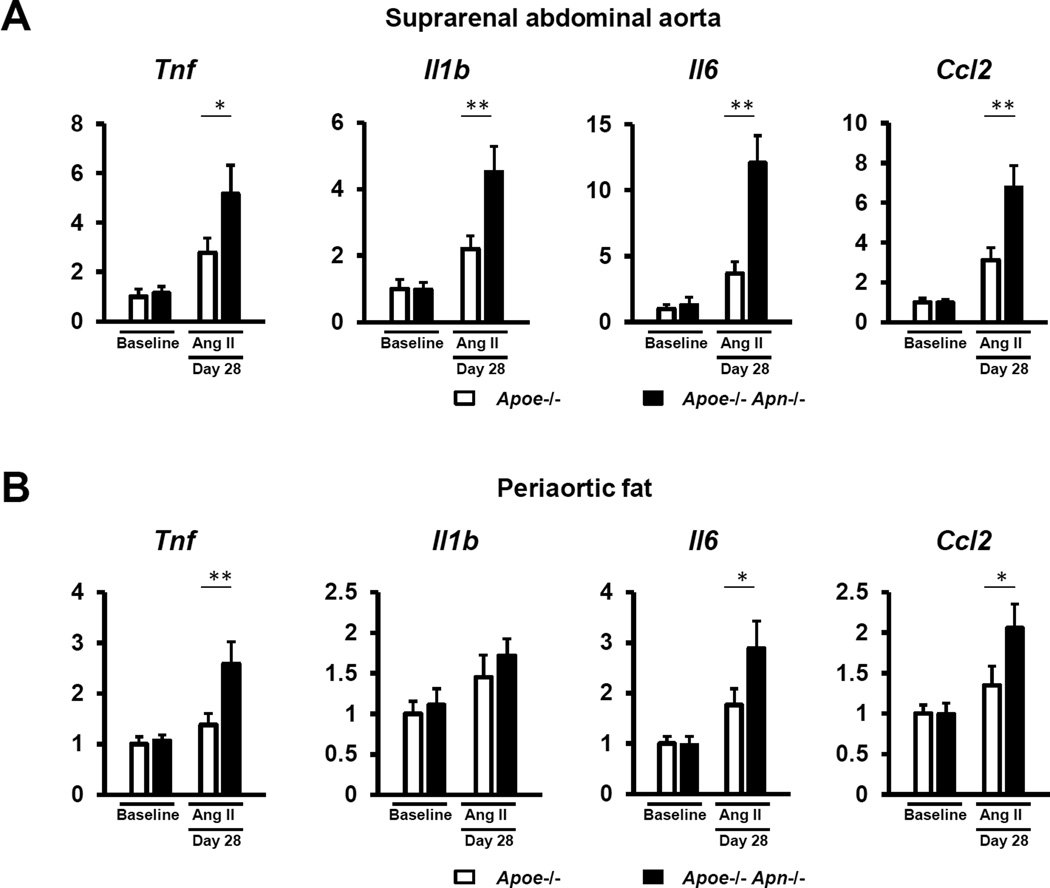
Experimental AAA have been shown to be mediated by various pro-inflammatory cytokines20–22. Therefore, we evaluated whether adiponectin deficiency affected the expression of these cytokines in AngII-induced AAAs. Gene expression of the pro-inflammatory cytokines Tnf, Il1b, and Il6 and the chemotactic factor Ccl2/Mcp1 were significantly increased in the suprarenal aorta of Apoe−/− Apn−/− relative to Apoe−/− mice after Ang II infusion for 28 days (Figure 3A). Perivascular fat inflammation has also been suggested to contribute to AAA formation in particular in obese mice9. In this regard, similar to the vascular wall, adiponectin deficiency resulted in higher gene expression of Tnf, Il6, and Ccl2/Mcp1 in periaortic fat of Ang II-treated mice (Figure 3B). Overall, these results suggest that adiponectin exerts anti-inflammatory actions in the vascular wall that may protect against AAA formation and growth.
Figure 3. Adiponectin deficiency increases the expression of pro-inflammatory cytokines in the aneurysmal vascular wall.
Quantitative real time-PCR analysis of the expression of pro-inflammatory cytokines in the aneurysmal suprarenal aorta (A) and the adjacent periaortic adipose tissue (B) at baseline and day 28 after Ang II stimulation. Transcript levels of Gapdh, Actb and Rplp0 serves as an internal control. The value of gene expression in Apoe−/− mice at baseline is set as 1. Results are mean±SEM. n=8 in each group. *P<0.05, **P<0.01, Apoe−/− mice vs. Apoe−/− Apn−/− mice using two-way ANOVA.
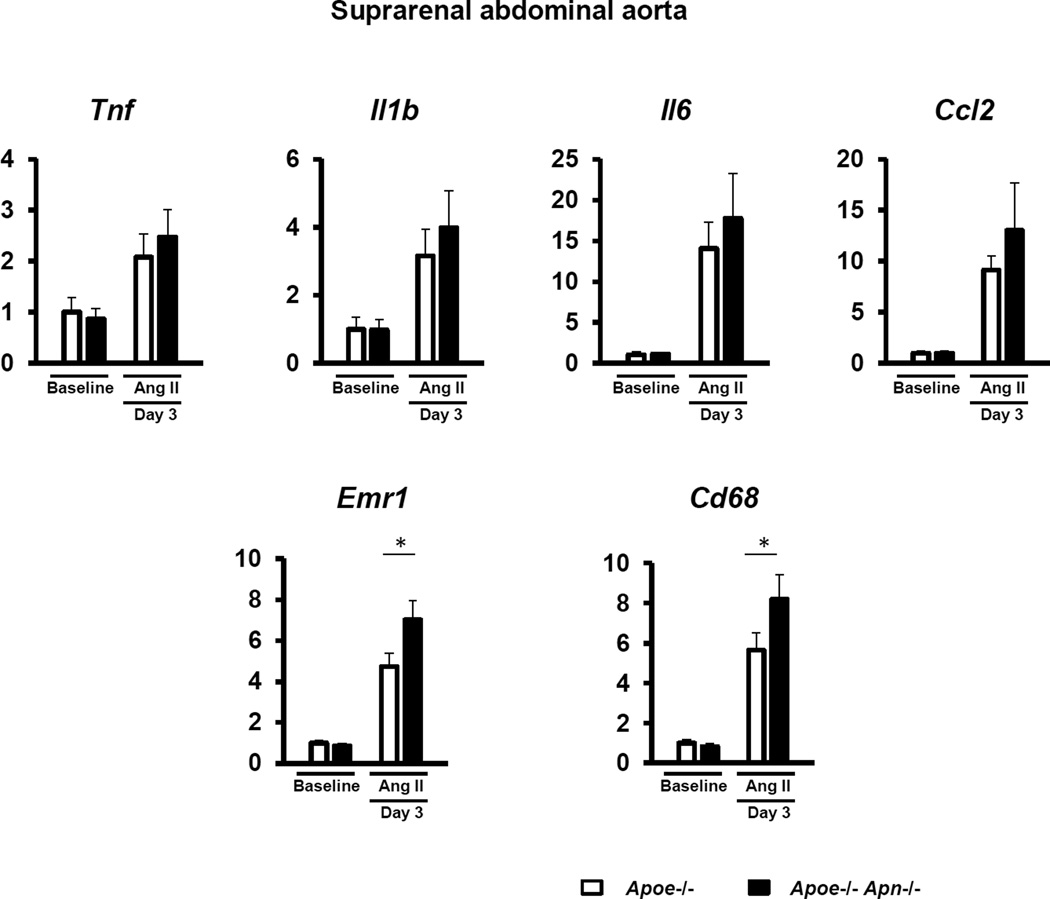
Separate sets of mice were also analyzed at earlier time points to determine the effects of adiponectin deficiency on the temporal sequence of events that give rise to aneurysm formation. At the 3 day time point, there was no difference between the diameters of the abdominal aortae of the Apoe−/− and Apoe−/− Apn−/− mice (1.22 ± 0.03 mm and 1.21 ± 0.03 mm, respectively; n=8 per group), and there were statistically significant increases in the macrophage markers Emr1 (F4/80) and Cd68 (Figure 4). In contrast, transcript levels of Tnf, Il1b, Il6 and Mcp1 were not significantly elevated at this time point (Figure 4) and no differences in MMP levels could be detected (not shown). However, Tnf, Il6, Mcp1, and MMP levels were elevated in the vessel wall at the 7 day time point (data not shown). Thus, these data suggest that adiponectin inhibits medial accumulation of macrophages, one of the earliest steps in aneurysm formation19.
Figure 4. Adiponectin deficiency increases the accumulation of macrophages in the aortic wall in the very early phase.
Quantitative real time-PCR analysis of the expression of pro-inflammatory cytokines and macrophage-specific markers in the suprarenal aorta at baseline and day 3 after Ang II stimulation. Transcript levels of Gapdh, Actb and Rplp0 serves as an internal control. The value of gene expression in Apoe−/− mice at baseline is set as 1. Results are mean±SEM. n=8 in each group. *P<0.05, Apoe−/− mice vs. Apoe−/− Apn−/− mice using two-way ANOVA.
Adiponectin-deficiency increases proteolytic activity in AAA
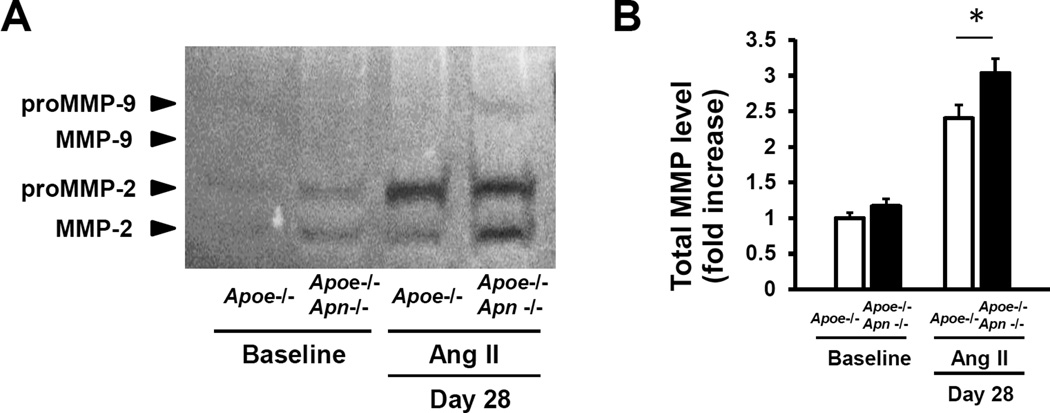
MMP2 and MMP9 have been shown to play essential and complementary roles in the formation of AAA and both are upregulated in human AAA23–25. To test whether adiponectin deficiency affects MMP-mediated proteolysis in the setting of AAA, the activities of MMP-2 and MMP-9 were assessed by gelatin zymography in protein extracts obtained from the suprarenal aorta of mice infused with AngII for 28 days. As shown in Figure 5, Apoe−/− Apn−/− mice exhibited a significant increase in combined MMP2/9 levels compared to Apoe−/− controls.
Figure 5. Adiponectin deficiency increases MMP level in AAA.
A, Representative gelatin zymography of aortic protein at baseline and day 28 after Ang II infusion. B, Quantification of total MMP level in aorta. The value of Apoe−/− mice at baseline is set as 1. Results are mean±SEM. n=4 in each group. *P<0.05, Apoe−/− mice vs. Apoe−/− Apn−/− mice using two-way ANOVA.
Discussion
AAA is a life-threatening, age-associated vascular pathology that affects between 5 and 9% of the population over the age of 65 years and is the tenth leading cause of death in western countries1. AAA development is characterized by the degradation of elastic and collagen fibers in the vascular wall due to the activity of proteolytic enzymes secreted by resident vascular cells and recruited inflammatory cells. However, the specific molecular insults that trigger AAA formation remain largely unknown. In this regard, the increasing body of evidence suggesting a connection between obesity and AAA development has opened new avenues of study in this field. Although obesity has not been traditionally considered a risk factor for AAA, population-based studies examining the etiology of this disorder have demonstrated that it is significantly associated with increased body weight or visceral adiposity3–8. However, the molecular mechanisms underlying this connection remain largely unknown. In the present study we show that adiponectin, an adipocyte-derived hormone typically downregulated in obese individuals, attenuates the formation of AAA in an experimental mouse model.
Adiponectin circulates at high levels (3 to 30 µg/ml) in the blood of lean individuals, but is markedly downregulated in obese human subjects11, 12. While adiponectin partially protects against metabolic dysfunction in obese mice26, 27, adiponectin-deficient mice display normal body and fat mass and normal metabolic parameters when fed a normal chow diet. Because of this, lean adiponectin-deficient mice have been used extensively to evaluate the effects of obesity-associated hypoadiponectinemia in the absence of the confounding factors associated with systemic metabolic dysfunction in obese mice. In this regard, our laboratory has previously shown using this model that Adiponectin has direct protective effects at different levels in the cardiovascular system, promoting revascularization of ischemic limbs28, and protecting against pressure overload- and angiotensin II-induced cardiac hypertrophy29, cardiac ischemia/reperfusion injury30, and systolic and diastolic heart failure31, 32. Here, we show that adiponectin attenuates AngII-induced AAA development in hyperlipidemic ApoE-deficient mice. In this experimental model, adiponectin-deficiency led to a higher incidence of AAA and an increase in the maximal suprarenal aortic diameter compared with that of mice that were deficient in ApoE alone. Mechanistically, our results suggest that increased AAA formation and growth in adiponectin-deficient mice is due, at least in part, to augmented vascular inflammation. Macrophage recruitment to the vascular wall is one of the early events leading to AAA formation in AngII-infused mice, and also contributes to the expansion of the aneurysmal artery in advanced stages of the disease18, 19. In this regard, we found increased macrophage content in the aneurysmal suprarenal abdominal aorta of Apoe−/− Apn−/− mice compared to Apoe−/− controls, suggesting that adiponectin prevents vascular infiltration of macrophages in this model. Supporting this notion, previous studies have shown that physiological concentrations of adiponectin inhibit TNF-α-induced monocyte adhesion to cultured endothelial cells33. In addition, we found that adiponectin-deficiency increased the expression of the proinflammatory cytokines IL-6, TNFα, IL-1β and CCL2/MCP-1 in AngII-induced AAAs. These data are consistent with previous studies showing that macrophages isolated from different tissues of adiponectin-deficient mice exhibit reduced expression of pro-inflammatory factors34, 35.
Here, we employed the AngII-induced model of AAA in mice13. Ang-II type I receptor and angiotensin-converting enzyme polymorphisms are associated with AAA in humans36, 37. The AngII model exhibits several similarities with human AAA, including a strong male gender preference38. However, the location of the thrombus is different from the human disease, being intramural in mice and intraluminal in human. Another difference is that the AngII-induced lesion in mice commonly involves the suprarenal abdominal aorta, while in human the most common site of AAA is the infrarenal aorta.
Once thought to be a consequence of advanced atherosclerosis, AAAs is widely accepted now to represent a distinct vascular disorder. Interestingly, while our results herein strongly suggest a significant protective action of adiponectin against AAA development, the role of adiponectin in atherosclerosis remains controversial. Some studies in animal models have suggested an atheroprotective role of adiponectin39–41, while others have found no effect of adiponectin gain or loss of function in atherosclerosis development42. Future studies are warranted to conclusively elucidate the macrovascular protective actions of adiponectin, and to evaluate the potential mechanisms by which adiponectin differentially affects aneurysmal versus atherosclerotic disorders.
It will be of interest to delineate the receptor-mediated pathways that contribute to adiponectin’s actions in maintaining vessel wall integrity. Because adiponectin is unusually abundant in serum and present as a high molecular weight multimer, its receptor interaction is likely to be atypical. In this regard, we have reported that adiponectin can interact with LRP1 (also referred to as CD91), an abundant, multifunctional cell surface receptor44. The interaction of LRP1/CD91 with adiponectin is similar to its interactions with the complement factor C1q45, a protein that is structurally similar to adiponectin and capable of binding adiponectin in blood46, 47. Of interest, LRP1/CD91 has been shown to be required for vascular integrity in animal models48, 49 and genetic studies in humans have implicated this gene locus in abdominal aortic aneurysm formation50, 51. Thus, in addition to affecting macrophage-mediated vascular inflammation as we report here, adiponectin could affect aneurysm formation through interactions with LRP1/CD91 and the complement cascade proteins.
In addition to adiponectin, the adipose tissue secretes a plethora of other adipokines. However, in contrast to adiponectin, most of these adipokines are pro-inflammatory and are upregulated in the obese state. Two of these pro-inflammatory adipokines, leptin and resistin, have been previously linked with AAA development. Leptin, which is typically secreted by adipocytes, has been shown to be produced locally by macrophages and smooth muscle cells in human AAAs, and the periaortic application of recombinant leptin to Apoe−/− mice promotes extracellular matrix digestion and aneurismal dilations of the aortic wall43. On the other hand, serum concentration of resistin, which in humans is expressed by monocytes/macrophages, has been shown to be independently associated with AAA and aortic diameter in humans3. However, the potential role of resistin in AAA pathogenesis has yet to be evaluated in experimental models.
In summary, our study shows that endogenous adiponectin protects against the development of Ang II-induced AAA in mice and identifies Apn as the first adipokine with a direct protective action against AAA development. Since adiponectin expression is significantly decreased in obese individuals11, 12, these results provide a potential mechanistic connection between obesity and AAA development.
Conclusion
Adiponectin attenuates AngII-induced AAA formation and growth in mice. Since clinical AAAs arise through multiple, interdependent pathogenic mechanisms, further research is warranted to clarify the role of adiponectin in human AAA.
Supplementary Material
Highlights.
Adiponectin attenuates Ang II-induced AAA formation and growth in hyperlipidemic mice.
Adiponectin decreases AAA inflammation.
Adiponectin is the first identified adipokine with protective actions against AAA.
Acknowledgments
Sources of funding
This work was supported by National Institutes of Health grants AG034972, HL081587 and HL068758 (to KW) and an American Heart Association Postdoctoral Fellowship grant 13POST17060028 (to JJF).
Abbreviations
- AAA
abdominal aortic aneurysm
- Apoe−/− Apn−/−
apolipoprotein E/adiponectin double-knockout
- angiotensin II
(Ang II)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
The authors have nothing to disclose.
References
- 1.Nordon IM, Hinchliffe RJ, Loftus IM, Thompson MM. Pathophysiology and epidemiology of abdominal aortic aneurysms. Nat Rev Cardiol. 2011;8:92–102. doi: 10.1038/nrcardio.2010.180. [DOI] [PubMed] [Google Scholar]
- 2.Sakalihasan N, Limet R, Defawe OD. Abdominal aortic aneurysm. Lancet. 2005;365:1577–1589. doi: 10.1016/S0140-6736(05)66459-8. [DOI] [PubMed] [Google Scholar]
- 3.Golledge J, Clancy P, Jamrozik K, Norman PE. Obesity, adipokines, and abdominal aortic aneurysm: Health in men study. Circulation. 2007;116:2275–2279. doi: 10.1161/CIRCULATIONAHA.107.717926. [DOI] [PubMed] [Google Scholar]
- 4.Stackelberg O, Bjorck M, Sadr-Azodi O, Larsson SC, Orsini N, Wolk A. Obesity and abdominal aortic aneurysm. Br J Surg. 2013;100:360–366. doi: 10.1002/bjs.8983. [DOI] [PubMed] [Google Scholar]
- 5.Allison MA, Kwan K, DiTomasso D, Wright CM, Criqui MH. The epidemiology of abdominal aortic diameter. J Vasc Surg. 2008;48:121–127. doi: 10.1016/j.jvs.2008.02.031. [DOI] [PubMed] [Google Scholar]
- 6.Kent KC, Zwolak RM, Egorova NN, Riles TS, Manganaro A, Moskowitz AJ, Gelijns AC, Greco G. Analysis of risk factors for abdominal aortic aneurysm in a cohort of more than 3 million individuals. J Vasc Surg. 2010;52:539–548. doi: 10.1016/j.jvs.2010.05.090. [DOI] [PubMed] [Google Scholar]
- 7.Lederle FA, Johnson GR, Wilson SE, Chute EP, Hye RJ, Makaroun MS, Barone GW, Bandyk D, Moneta GL, Makhoul RG. The aneurysm detection and management study screening program: Validation cohort and final results. Aneurysm detection and management veterans affairs cooperative study investigators. Arch Intern Med. 2000;160:1425–1430. doi: 10.1001/archinte.160.10.1425. [DOI] [PubMed] [Google Scholar]
- 8.Long A, Bui HT, Barbe C, Henni AH, Journet J, Metz D, Nazeyrollas P. Prevalence of abdominal aortic aneurysm and large infrarenal aorta in patients with acute coronary syndrome and proven coronary stenosis: A prospective monocenter study. Ann Vasc Surg. 2010;24:602–608. doi: 10.1016/j.avsg.2009.12.010. [DOI] [PubMed] [Google Scholar]
- 9.Police SB, Thatcher SE, Charnigo R, Daugherty A, Cassis LA. Obesity promotes inflammation in periaortic adipose tissue and angiotensin ii-induced abdominal aortic aneurysm formation. Arterioscler Thromb Vasc Biol. 2009;29:1458–1464. doi: 10.1161/ATVBAHA.109.192658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ouchi N, Parker JL, Lugus JJ, Walsh K. Adipokines in inflammation and metabolic disease. Nat Rev Immunol. 2011;11:85–97. doi: 10.1038/nri2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arita Y, Kihara S, Ouchi N, Takahashi M, Maeda K, Miyagawa J, Hotta K, Shimomura I, Nakamura T, Miyaoka K, Kuriyama H, Nishida M, Yamashita S, Okubo K, Matsubara K, Muraguchi M, Ohmoto Y, Funahashi T, Matsuzawa Y. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem Biophys Res Commun. 1999;257:79–83. doi: 10.1006/bbrc.1999.0255. [DOI] [PubMed] [Google Scholar]
- 12.Weyer C, Funahashi T, Tanaka S, Hotta K, Matsuzawa Y, Pratley RE, Tataranni PA. Hypoadiponectinemia in obesity and type 2 diabetes: Close association with insulin resistance and hyperinsulinemia. J Clin Endocrinol Metab. 2001;86:1930–1935. doi: 10.1210/jcem.86.5.7463. [DOI] [PubMed] [Google Scholar]
- 13.Daugherty A, Manning MW, Cassis LA. Angiotensin ii promotes atherosclerotic lesions and aneurysms in apolipoprotein e-deficient mice. J Clin Invest. 2000;105:1605–1612. doi: 10.1172/JCI7818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barisione C, Charnigo R, Howatt DA, Moorleghen JJ, Rateri DL, Daugherty A. Rapid dilation of the abdominal aorta during infusion of angiotensin ii detected by noninvasive high-frequency ultrasonography. J Vasc Surg. 2006;44:372–376. doi: 10.1016/j.jvs.2006.04.047. [DOI] [PubMed] [Google Scholar]
- 15.Clasen R, Schupp M, Foryst-Ludwig A, Sprang C, Clemenz M, Krikov M, Thone-Reineke C, Unger T, Kintscher U. Ppargamma-activating angiotensin type-1 receptor blockers induce adiponectin. Hypertension. 2005;46:137–143. doi: 10.1161/01.HYP.0000168046.19884.6a. [DOI] [PubMed] [Google Scholar]
- 16.Furuhashi M, Ura N, Higashiura K, Murakami H, Tanaka M, Moniwa N, Yoshida D, Shimamoto K. Blockade of the renin-angiotensin system increases adiponectin concentrations in patients with essential hypertension. Hypertension. 2003;42:76–81. doi: 10.1161/01.HYP.0000078490.59735.6E. [DOI] [PubMed] [Google Scholar]
- 17.Ran J, Hirano T, Fukui T, Saito K, Kageyama H, Okada K, Adachi M. Angiotensin ii infusion decreases plasma adiponectin level via its type 1 receptor in rats: An implication for hypertension-related insulin resistance. Metabolism. 2006;55:478–488. doi: 10.1016/j.metabol.2005.10.009. [DOI] [PubMed] [Google Scholar]
- 18.Rateri DL, Howatt DA, Moorleghen JJ, Charnigo R, Cassis LA, Daugherty A. Prolonged infusion of angiotensin ii in apoe(−/−) mice promotes macrophage recruitment with continued expansion of abdominal aortic aneurysm. Am J Pathol. 2011;179:1542–1548. doi: 10.1016/j.ajpath.2011.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saraff K, Babamusta F, Cassis LA, Daugherty A. Aortic dissection precedes formation of aneurysms and atherosclerosis in angiotensin ii-infused, apolipoprotein e-deficient mice. Arterioscler Thromb Vasc Biol. 2003;23:1621–1626. doi: 10.1161/01.ATV.0000085631.76095.64. [DOI] [PubMed] [Google Scholar]
- 20.Ishibashi M, Egashira K, Zhao Q, Hiasa K, Ohtani K, Ihara Y, Charo IF, Kura S, Tsuzuki T, Takeshita A, Sunagawa K. Bone marrow-derived monocyte chemoattractant protein-1 receptor ccr2 is critical in angiotensin ii-induced acceleration of atherosclerosis and aneurysm formation in hypercholesterolemic mice. Arterioscler Thromb Vasc Biol. 2004;24:e174–e178. doi: 10.1161/01.ATV.0000143384.69170.2d. [DOI] [PubMed] [Google Scholar]
- 21.Tieu BC, Lee C, Sun H, Lejeune W, Recinos A, 3rd, Ju X, Spratt H, Guo DC, Milewicz D, Tilton RG, Brasier AR. An adventitial il-6/mcp1 amplification loop accelerates macrophage-mediated vascular inflammation leading to aortic dissection in mice. J Clin Invest. 2009;119:3637–3651. doi: 10.1172/JCI38308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xiong W, MacTaggart J, Knispel R, Worth J, Persidsky Y, Baxter BT. Blocking tnf-alpha attenuates aneurysm formation in a murine model. J Immunol. 2009;183:2741–2746. doi: 10.4049/jimmunol.0803164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Davis V, Persidskaia R, Baca-Regen L, Itoh Y, Nagase H, Persidsky Y, Ghorpade A, Baxter BT. Matrix metalloproteinase-2 production and its binding to the matrix are increased in abdominal aortic aneurysms. Arterioscler Thromb Vasc Biol. 1998;18:1625–1633. doi: 10.1161/01.atv.18.10.1625. [DOI] [PubMed] [Google Scholar]
- 24.Longo GM, Xiong W, Greiner TC, Zhao Y, Fiotti N, Baxter BT. Matrix metalloproteinases 2 and 9 work in concert to produce aortic aneurysms. J Clin Invest. 2002;110:625–632. doi: 10.1172/JCI15334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thompson RW, Holmes DR, Mertens RA, Liao S, Botney MD, Mecham RP, Welgus HG, Parks WC. Production and localization of 92-kilodalton gelatinase in abdominal aortic aneurysms. An elastolytic metalloproteinase expressed by aneurysm-infiltrating macrophages. J Clin Invest. 1995;96:318–326. doi: 10.1172/JCI118037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maeda N, Shimomura I, Kishida K, Nishizawa H, Matsuda M, Nagaretani H, Furuyama N, Kondo H, Takahashi M, Arita Y, Komuro R, Ouchi N, Kihara S, Tochino Y, Okutomi K, Horie M, Takeda S, Aoyama T, Funahashi T, Matsuzawa Y. Diet-induced insulin resistance in mice lacking adiponectin/acrp30. Nat Med. 2002;8:731–737. doi: 10.1038/nm724. [DOI] [PubMed] [Google Scholar]
- 27.Nawrocki AR, Rajala MW, Tomas E, Pajvani UB, Saha AK, Trumbauer ME, Pang Z, Chen AS, Ruderman NB, Chen H, Rossetti L, Scherer PE. Mice lacking adiponectin show decreased hepatic insulin sensitivity and reduced responsiveness to peroxisome proliferator-activated receptor gamma agonists. J Biol Chem. 2006;281:2654–2660. doi: 10.1074/jbc.M505311200. [DOI] [PubMed] [Google Scholar]
- 28.Shibata R, Ouchi N, Kihara S, Sato K, Funahashi T, Walsh K. Adiponectin stimulates angiogenesis in response to tissue ischemia through stimulation of amp-activated protein kinase signaling. J Biol Chem. 2004;279:28670–28674. doi: 10.1074/jbc.M402558200. [DOI] [PubMed] [Google Scholar]
- 29.Shibata R, Ouchi N, Ito M, Kihara S, Shiojima I, Pimentel DR, Kumada M, Sato K, Schiekofer S, Ohashi K, Funahashi T, Colucci WS, Walsh K. Adiponectin-mediated modulation of hypertrophic signals in the heart. Nat Med. 2004;10:1384–1389. doi: 10.1038/nm1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shibata R, Sato K, Pimentel DR, Takemura Y, Kihara S, Ohashi K, Funahashi T, Ouchi N, Walsh K. Adiponectin protects against myocardial ischemia-reperfusion injury through ampk- and cox-2-dependent mechanisms. Nat Med. 2005;11:1096–1103. doi: 10.1038/nm1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sam F, Duhaney TA, Sato K, Wilson RM, Ohashi K, Sono-Romanelli S, Higuchi A, De Silva DS, Qin F, Walsh K, Ouchi N. Adiponectin deficiency, diastolic dysfunction, and diastolic heart failure. Endocrinology. 2010;151:322–331. doi: 10.1210/en.2009-0806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shimano M, Ouchi N, Shibata R, Ohashi K, Pimentel DR, Murohara T, Walsh K. Adiponectin deficiency exacerbates cardiac dysfunction following pressure overload through disruption of an ampk-dependent angiogenic response. J Mol Cell Cardiol. 2010;49:210–220. doi: 10.1016/j.yjmcc.2010.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ouchi N, Kihara S, Arita Y, Maeda K, Kuriyama H, Okamoto Y, Hotta K, Nishida M, Takahashi M, Nakamura T, Yamashita S, Funahashi T, Matsuzawa Y. Novel modulator for endothelial adhesion molecules: Adipocyte-derived plasma protein adiponectin. Circulation. 1999;100:2473–2476. doi: 10.1161/01.cir.100.25.2473. [DOI] [PubMed] [Google Scholar]
- 34.Ohashi K, Parker JL, Ouchi N, Higuchi A, Vita JA, Gokce N, Pedersen AA, Kalthoff C, Tullin S, Sams A, Summer R, Walsh K. Adiponectin promotes macrophage polarization toward an anti-inflammatory phenotype. J Biol Chem. 2010;285:6153–6160. doi: 10.1074/jbc.M109.088708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lovren F, Pan Y, Quan A, Szmitko PE, Singh KK, Shukla PC, Gupta M, Chan L, Al-Omran M, Teoh H, Verma S. Adiponectin primes human monocytes into alternative anti-inflammatory m2 macrophages. Am J Physiol Heart Circ Physiol. 2010;299:H656–H663. doi: 10.1152/ajpheart.00115.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jones GT, Thompson AR, van Bockxmeer FM, Hafez H, Cooper JA, Golledge J, Humphries SE, Norman PE, van Rij AM. Angiotensin ii type 1 receptor 1166c polymorphism is associated with abdominal aortic aneurysm in three independent cohorts. Arterioscler Thromb Vasc Biol. 2008;28:764–770. doi: 10.1161/ATVBAHA.107.155564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fatini C, Pratesi G, Sofi F, Gensini F, Sticchi E, Lari B, Pulli R, Dorigo W, Azas L, Pratesi C, Gensini GF, Abbate R. Ace dd genotype: A predisposing factor for abdominal aortic aneurysm. Eur J Vasc Endovasc Surg. 2005;29:227–232. doi: 10.1016/j.ejvs.2004.12.018. [DOI] [PubMed] [Google Scholar]
- 38.Bruemmer D, Daugherty A, Lu H, Rateri DL. Relevance of angiotensin ii-induced aortic pathologies in mice to human aortic aneurysms. Ann N Y Acad Sci. 2011;1245:7–10. doi: 10.1111/j.1749-6632.2011.06332.x. [DOI] [PubMed] [Google Scholar]
- 39.Okamoto Y, Folco EJ, Minami M, Wara AK, Feinberg MW, Sukhova GK, Colvin RA, Kihara S, Funahashi T, Luster AD, Libby P. Adiponectin inhibits the production of cxc receptor 3 chemokine ligands in macrophages and reduces t-lymphocyte recruitment in atherogenesis. Circ Res. 2008;102:218–225. doi: 10.1161/CIRCRESAHA.107.164988. [DOI] [PubMed] [Google Scholar]
- 40.Okamoto Y, Kihara S, Ouchi N, Nishida M, Arita Y, Kumada M, Ohashi K, Sakai N, Shimomura I, Kobayashi H, Terasaka N, Inaba T, Funahashi T, Matsuzawa Y. Adiponectin reduces atherosclerosis in apolipoprotein e-deficient mice. Circulation. 2002;106:2767–2770. doi: 10.1161/01.cir.0000042707.50032.19. [DOI] [PubMed] [Google Scholar]
- 41.Yamauchi T, Kamon J, Waki H, Imai Y, Shimozawa N, Hioki K, Uchida S, Ito Y, Takakuwa K, Matsui J, Takata M, Eto K, Terauchi Y, Komeda K, Tsunoda M, Murakami K, Ohnishi Y, Naitoh T, Yamamura K, Ueyama Y, Froguel P, Kimura S, Nagai R, Kadowaki T. Globular adiponectin protected ob/ob mice from diabetes and apoedeficient mice from atherosclerosis. J Biol Chem. 2003;278:2461–2468. doi: 10.1074/jbc.M209033200. [DOI] [PubMed] [Google Scholar]
- 42.Nawrocki AR, Hofmann SM, Teupser D, Basford JE, Durand JL, Jelicks LA, Woo CW, Kuriakose G, Factor SM, Tanowitz HB, Hui DY, Tabas I, Scherer PE. Lack of association between adiponectin levels and atherosclerosis in mice. Arterioscler Thromb Vasc Biol. 2010;30:1159–1165. doi: 10.1161/ATVBAHA.109.195826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tao M, Yu P, Nguyen BT, Mizrahi B, Savion N, Kolodgie FD, Virmani R, Hao S, Ozaki CK, Schneiderman J. Locally applied leptin induces regional aortic wall degeneration preceding aneurysm formation in apolipoprotein e-deficient mice. Arterioscler Thromb Vasc Biol. 2013;33:311–320. doi: 10.1161/ATVBAHA.112.300543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Takemura Y, Ouchi N, Shibata R, Aprahamian T, Kirber MT, Summer RS, Kihara S, Walsh K. Adiponectin modulates inflammatory reactions via calreticulin receptor-dependent clearance of early apoptotic bodies. J Clin Invest. 2007;117:375–386. doi: 10.1172/JCI29709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gardai SJ, McPhillips KA, Frasch SC, Janssen WJ, Starefeldt A, Murphy-Ullrich JE, Bratton DL, Oldenborg PA, Michalak M, Henson PM. Cell-surface calreticulin initiates clearance of viable or apoptotic cells through trans-activation of lrp on the phagocyte. Cell. 2005;123:321–334. doi: 10.1016/j.cell.2005.08.032. [DOI] [PubMed] [Google Scholar]
- 46.Kogan AE, Filatov VL, Kolosova OV, Katrukha IA, Mironova EV, Zhuravleva NS, Nagibin OA, Kara AN, Bereznikova AV, Katrukha AG. Oligomeric adiponectin forms and their complexes in the blood of healthy donors and patients with type 2 diabetes mellitus. J Immunoassay Immunochem. 2013;34:180–196. doi: 10.1080/15321819.2012.699494. [DOI] [PubMed] [Google Scholar]
- 47.Nakatsuji H, Kobayashi H, Kishida K, Nakagawa T, Takahashi S, Tanaka H, Akamatsu S, Funahashi T, Shimomura I. Binding of adiponectin and c1q in human serum, and clinical significance of the measurement of c1q-adiponectin/total adiponectin ratio. Metabolism. 2013;62:109–120. doi: 10.1016/j.metabol.2012.06.006. [DOI] [PubMed] [Google Scholar]
- 48.Boucher P, Gotthardt M, Li WP, Anderson RG, Herz J. Lrp: Role in vascular wall integrity and protection from atherosclerosis. Science. 2003;300:329–332. doi: 10.1126/science.1082095. [DOI] [PubMed] [Google Scholar]
- 49.Muratoglu SC, Belgrave S, Hampton B, Migliorini M, Coksaygan T, Chen L, Mikhailenko I, Strickland DK. Lrp1 protects the vasculature by regulating levels of connective tissue growth factor and htra1. Arterioscler Thromb Vasc Biol. 2013;33:2137–2146. doi: 10.1161/ATVBAHA.113.301893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chan CY, Chan YC, Cheuk BL, Cheng SW. A pilot study on low-density lipoprotein receptor-related protein-1 in chinese patients with abdominal aortic aneurysm. Eur J Vasc Endovasc Surg. 2013;46:549–556. doi: 10.1016/j.ejvs.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 51.Galora S, Saracini C, Pratesi G, Sticchi E, Pulli R, Pratesi C, Abbate R, Giusti B. Association of rs1466535 lrp1 but not rs3019885 slc30a8 and rs6674171 tdrd10 gene polymorphisms with abdominal aortic aneurysm in italian patients. J Vasc Surg. 2014 doi: 10.1016/j.jvs.2013.10.090. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.