Abstract
The plasma membrane (PM) and endocytic protein quality control (QC) in conjunction with the endosomal sorting machinery either repairs or targets conformationally damaged membrane proteins for lysosomal/vacuolar degradation. Here, we provide an overview of emerging aspects of the underlying mechanisms of PM QC that fulfill a critical role in preserving cellular protein homeostasis in health and diseases.
Protein quality control (QC) is essential to preserve the functional and structural integrity of polypeptides during their lifespan and offset potentially deleterious consequences of misfolding. Multiple QC systems have evolved at the cellular and transcellular level to compensate for the limited fidelity of protein translation, maturation, transport, and conformational maintenance at various cellular and extracellular destinations (109). The QC surveillance of DNA replication (107), transcription (126, 150), and maturation/transport of mRNAs (134) and tRNAs (16, 145) also contribute indirectly to cellular protein homeostasis or proteostasis (109). Conformational surveillance mechanisms of mature polypeptides involve the repair (or refolding) of unfolded molecules by chaperone networks and/or the proteolytic degradation of irreversibly damaged molecules via the ubiquitin-proteasome system (UPS), autophagosomes and/or lysosomal/vacuolar proteolysis. Partially overlapping proteostasis networks have evolved to meet the distinct requirements of polypeptides' intrinsic designs at distinct locations, such as the nucleus, mitochondria, ER, cytosol, plasma membrane (PM), as well as the extracellular space (15, 40, 42, 60, 94, 98, 143) (FIGURE 1). Here, we survey the cellular and molecular basis of PM QC mechanisms that contribute to the refolding and/or elimination of nonnative polypeptides that either escaped the biosynthetic QC (3, 16) or were generated during aging and proteotoxic stress in situ.
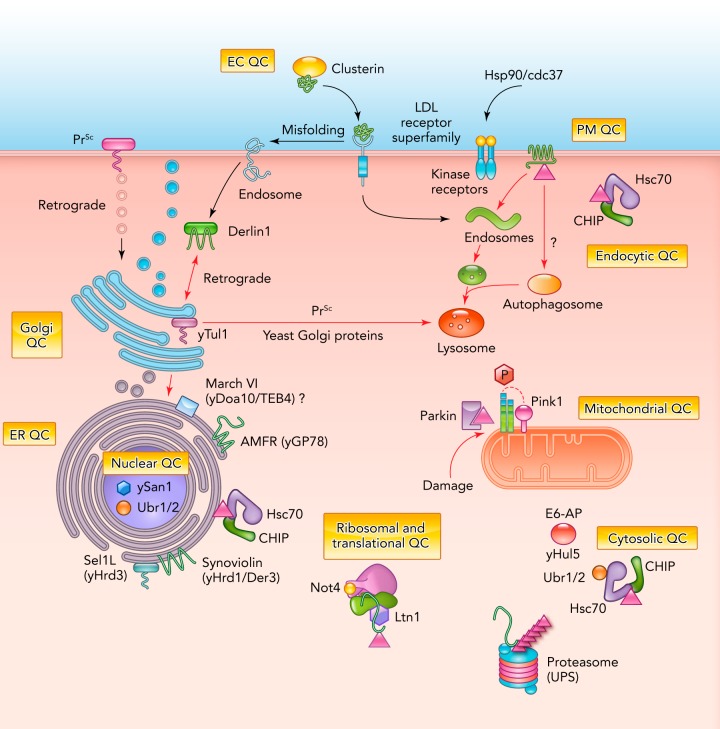
FIGURE 1.
The landscape of cellular protein quality controls
Multiple cellular organelles have folding sensor machineries to detect protein conformers; however, it is not clear how these systems discriminate terminally misfolded molecules from on-pathway folding intermediates. Aberrant or partially translated products are ubiquitinated by the ribosomal E3 ligases, Ltn1, and/or Not4 (98). Nonnative proteins in the cytosol are recognized by Hsp/Hsc70/Hsp40 and ubiquitinated by E3 ligases CHIP, Ubr1 and 2, and Hul5 (closest mammalian homologs UBE3B and UBE3C) (24, 38, 92) and directed to proteasomal degradation. In the yeast nucleus, the E3 San1 (42) can directly recognize misfolded proteins; however, it needs chaperone recruitment for transporting misfolded substrate into the nucleus (53). Ubr1–2 functions both in the cytosol and nucleus (53). In the ER-associated degradation (ERAD), the role of Hsp70/40/90 is conserved, but UDP-glucose:glycoprotein-glucosyltransferase (UGGT) can also directly bind nonnative proteins. Gp78 [mammalian autocrine motility factor receptor (AMFR)] functions in the ERAD with association of p97/VCP. The E3 Hrd1 and Hrd3 (mammalian Synoviolin and Sel1L, respectively) bind to misfolded transmembrane and luminal ER proteins (ERAD-M and ERAD-L pathways). Cytosolically exposed domains of membrane proteins (ERAD-C) as well as cytosolic polypeptides are ubiquitinated by CHIP and in yeast by Doa10 (mammalian MARCH VI) (15). In yeast, the Golgi QC prominently contributes to the vacuolar degradation pathways. Whether this mechanism exists in mammalian cells remains to be investigated, although both misfolded scrapie prions (Prsc) (45) and LDL-receptors use this route for lysosomal degradation or for ERAD (misfolded LDL-receptor) (25). At the PM, the Hsc70/Hsp90/CHIP complex ubiquitinates nonnative MPs, a prerequisite for MVB/lysosomal targeting via the ESCRT multiprotein complex, which contains multiple Ub-receptors (2, 95). Hsp90/Cdc37 may play a role in recognition of misfolded extracellular domains (35). Clusterin and other soluble chaperones mediate the clearance of misfolded extracellular proteins by delivering them via receptor-mediated endocytosis for lysosomal proteolysis (143). Following mitofusion and PINK1-dependent recruitment to damaged mitochondria, Parkin ubiquitinates damaged mitochondrial outer membrane proteins, a prerequisite for mitophagy (40). Degradation of ubiquitinated protein aggregates can occur in multiple cellular locations via autophagy relying on fusion with endosomes (130).
Conformational Surveillance of PM Proteins is Necessary for Cellular and Organismal Homeostasis
In addition to serving to maintain intracellular homeostasis and communication with the extracellular environment of unicellular organisms, integral PM proteins also constitute signaling networks with pivotal roles in the proliferation, development, polarity, migration, and maintenance of the extracellular milieu in multicellular organisms. To regulate the myriad of activities and protein-protein interactions, it is imperative to maintain the native conformation of PM proteins, despite their modest folding free energy at physiological temperature (27). The limited thermal stability in concert with unavoidable cellular and environmental stresses (e.g., thermal, mechanical, oxidative, osmotic, and metabolic) and/or mutations that escaped the recognition by the ER or Golgi QC can contribute to the appearance of unfolded, misfolded, and/or aggregation-prone cytotoxic polypeptides at the PM (FIGURE 2). Failure to repair or eliminate these damaged PM proteins could lead to the loss- or gain-of-function phenotype, with severe consequences for cellular and organismal proteostasis (102). Conversely, the PM QC may allow the disposal of modestly unfolded and partially functional proteins, which could exacerbate the phenotypic manifestation of conformational diseases and pose additional hurdles on pharmacological therapies of folding defects (2, 96, 118).
FIGURE 2.
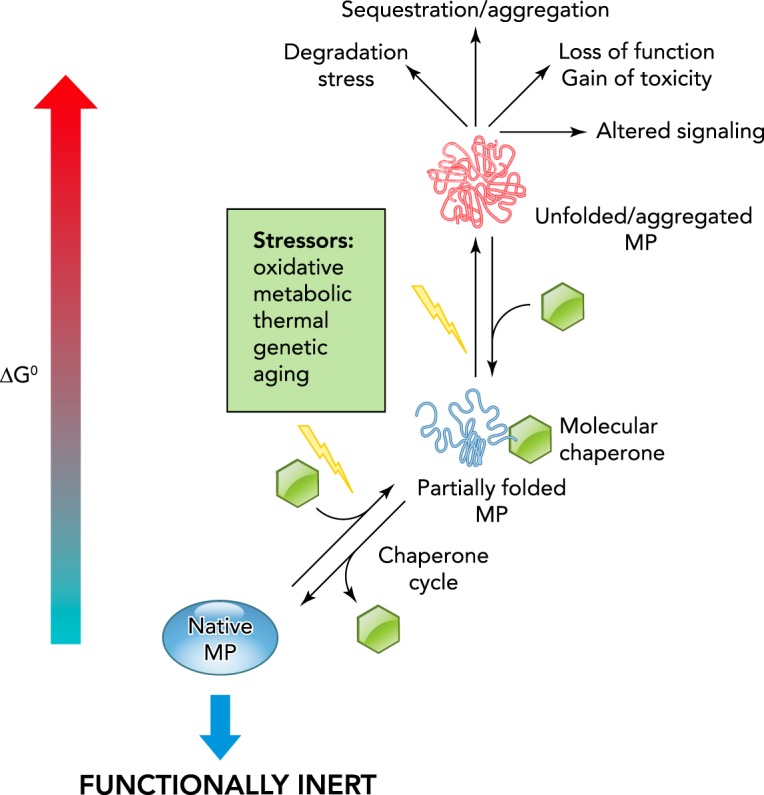
Protein folding and misfolding dynamics in the cellular environment
Multiple external and internal factors (e.g., molecular and chemical chaperones abundance and activity, ion composition, metabolic state, thermal and oxidative stress) can influence the conformational dynamics of PM proteins by shifting their folding-unfolding equilibrium. The energetically favorable conformers are represented by lower folding free energy (ΔG0) of the native polypeptides. Proteotoxic stress- and disease-causing mutations can either thermodynamically and/or kinetically interfere with the folding pathways, as also indicated by the increased ΔG0 of partially folded and unfolded conformers.
The first evidence for PM QC operation came from studies demonstrating that the stressed uracil transporter (Fur4p), as well as the mutant multidrug resistance (Pdr5) and pheromone (Ste6p) transporter are rapidly degraded from the yeast PM (33, 54, 76). The downregulation of these proteins via endocytosis and vacuolar proteolysis required ubiquitination, a posttranslational modification by the cellular ubiquitination machinery, consisting of an Ub-activating E1 enzyme, E2 Ub-conjugating enzyme (e.g., Ubc4/Ubc5) (116), and E3 Ub-ligase (e.g., Rsp5, the yeast homologue of the Nedd-4) (32). Subsequent work has confirmed this paradigm and extended its counterpart to mammalian cells (118).
The degradative PM QC can be divided into four steps. 1) Recognition of nonnative clients and the recruitment of ubiquitination machinery that leads to 2) the internalization and 3) the endosomal sorting of ubiquitinated cargo into budding intralumenal vesicles (ILV) by the endosomal sorting complexes required for transport (ESCRT) and the formation of multivesicular endosome (MVE). 4) The fusion of MVE with the lysosomal/vacuolar compartment ensures reutilization of amino acids from cleaved polypeptides (55, 81). Although the first stage of the PM QC displays unique features, subsequent steps appear to overlap mechanistically with the signal-dependent downregulation of ubiquitinated, native PM transporters, receptors, and structural proteins, topics covered in more depth by a number of excellent review articles (21, 100, 113).
Recognition and Ubiquitination of Misfolded PM Protein
Cytoplasmic Domain Recognition
Several yeast and mammalian integral PM proteins [e.g., mutant variants of the cystic fibrosis transmembrane conductance regulator (CFTR), α2-receptor, transferrin receptor, bile salt export pump (BSEP), megalencephalic leukoencephalopathy with subcortical cyst 1 (MLC1), vasopressin V2 receptor (V2R), dopamine D4.4 receptor (DRD4), Na+-H+ exchanger 6 (NHE6), unliganded MHC I and human ether-à-go-go K+ (hERG) channel] are prematurely eliminated from the cell surface as a result of documented or perceived structural defect (1–2, 10, 17, 39, 79–80, 95, 115, 117, 141, 151). The nature of the structural defect that signals substrate recognition by the PM QC remains poorly defined. Destabilizing mutations confined to cytoplasmic regions may disrupt the secondary, tertiary, or quaternary structure directly, whereas mutations that reside in the transmembrane (TM) or extracellular regions, similarly to various stressors, may be relayed to cytosolic domains and recognized by molecular chaperones as exemplified by a subset of misfolded ER membrane proteins (114) or recognized by specialized mechanisms (see following sections).
The highly conserved cytosolic chaperone system, Hsp70/Hsc70, transiently interacts with short extended peptide segments enriched in hydrophobic and basic amino acids (112, 154) in the cytoplamic region of unfolded PM proteins. Similar to Hsp70/Hsc70, the chaperone activity of the Hsp90 family members is coupled to ATP binding and hydrolysis, and is regulated by co-chaperones (57, 127). Based on the interaction of Hsp90 with a range of metastable mature polypeptides (e.g., kinases, E3 ligases, receptors, and steroid receptors) and biogenic folding intermediates, the Hsp90 machinery can recognize both partially folded as well as extended polypeptide conformation, with preference toward thermally unstable domains (127). As a result, chaperone interactions can maintain partially unfolded PM proteins in a folding competent conformation by protecting them from aggregation or, perhaps, by remodeling the folding landscape of client protein as observed in case of the GoES/GroEL prokaryotic chaperone (18).
The significance of conformational maintenance of the Hsp90 complex is exemplified by the pleiotropic consequences of the Hsp90 ATPase cycle inhibition upon preventing ATP/ADP binding with geldanamycin. Geldanamycin elicits the Ub-dependent degradation of several metastable PM tyrosine receptor kinases (e.g., ErbB2, Ron, EGF, Met, and EphA2) as a consequence of the unfolding of their catalytic domains (72, 129, 153). Ubiquitination of these kinases is usually facilitated by the recruitment of the COOH terminus of Hsc70 interacting protein (CHIP) via Hsc70/Hsp70, which is upregulated upon Hsp90 inhibition as a consequence of cellular stress response (22).
If structural perturbation by the PM protein unfolding cannot be counteracted by the profolding activity of chaperones, ubiquitination and lysosomal/vacuolar degradation prevail. The biochemical basis of this “triage” decision is only partly understood and probably relies on the extended residence time of Hsp70/Hsc70-CHIP complex on the misfolded polypeptide (22). The NH2-terminal tetratricopeptide repeat (TPR) of CHIP binds to the COOH terminus of Hsc70/Hsp90 chaperones with low affinity. The COOH-terminal U-box, a RING-like domain, serves as scaffold for E2 enzymes UbcH5 or Ubc13/UEV1 to assemble either K48- or K63-linked Ub chains (4). In case of native-like proteins, it is postulated that transient association-dissociation cycles of Hsc70-Hop-Hsp90 and co-chaperones ensure that the client protein rapidly (re)folds and undergoes only limited ubiquitination that is reversed by the activity of deubiquitinating (DUB) enzyme(s). In contrast, extended association of Hsc70-CHIP with slowly folding clients, promoted by the CHIP-induced inhibition of the Hsc70/Hsp70 ATPase activity, leads to more efficient ubiquitination (22). This model is supported by in vitro biochemical studies (95), demonstrating complex formation between Hsc70/Hsp90/Hsp40, CHIP, and the temperature rescued ΔF508-CFTR or the CD4t-λC chimeric model protein upon thermal unfolding of the nucleotide binding domains (NBDs) or the λC domain, respectively, at the PM (2, 95) (FIGURE 3).
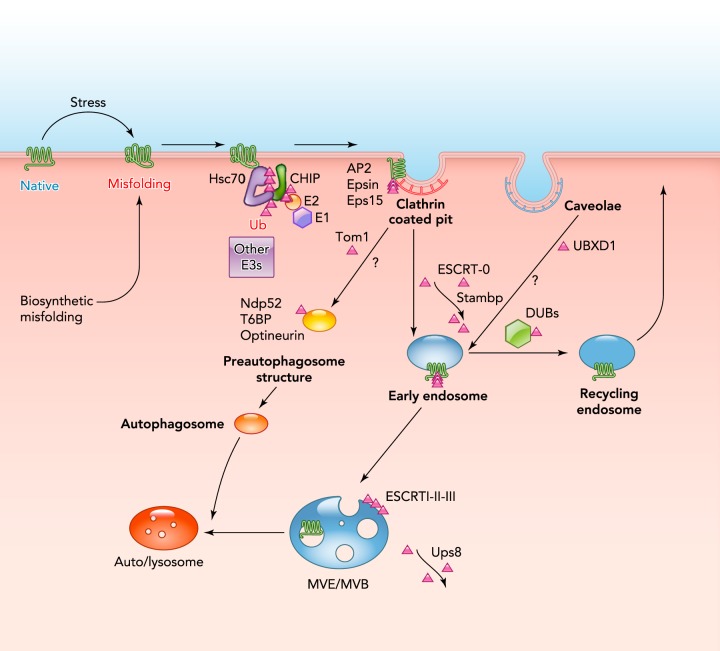
FIGURE 3.
Protein quality control at the plasma membrane
Conformational defects, caused by genetic mutations or various stress, may lead to the recognition of PM proteins by the peripheral QC machinery. Adaptors or molecular chaperones recognize the exposed degron in nonnative MP, which can instigate the recruitment of E3/E2 Ub-ligase(s). Some of the components of a chaperone network and the CHIP E3 Ub-ligase of the peripheral QC machinery in higher eukaryotes are indicated. Increased ubiquitination of nonnative PM proteins can serve both as an internalization and lysosomal/vaccuolar targeting signal that facilitates the irreversible disposal of damaged molecules. Contribution of the autophagocytic and caveolin-dependent degradation route of the nonnative PM proteins requires further experimental assessment.
Soluble client binding to and refolding activity of Hsc70/Hsp70 and Hsp90 are regulated by co-chaperones both in vitro and in vivo (49, 119, 140). A subset of co-chaperones, DNAJA1 (Hdj2), DNAJB2 (Hsj1), Aha1, and HOP have been invoked to influence the peripheral triage decision by facilitating the temperature-rescued ΔF508-CFTR ubiquitination in post-Golgi compartment (2, 95). Ablation of DNAJA1 by siRNA decreased the ubiquitination and concomitantly stabilized the ΔF508-CFTR at the PM, probably via impaired client recognition and transfer to Hsc70 similar to that observed at the ER QC (148–149). Although HOP and Aha1 can facilitate Hsp90 refolding activity in vitro (83, 138), depletion of these co-chaperones enhanced the biosynthetic maturation of ΔF508-CFTR and induced the rescued ΔF508-CFTR stabilization by a poorly understood mechanism (95). Interestingly, DNAJB2 harboring an Ub-interacting motif (UIM) and J-domain regulates rescued ΔF508-CFTR ubiquitination only at the post-endocytic stage (95) (Table 1).
Table 1.
Endocytic components capable of binding Ub
| Component | Adaptor | Binding Domains | Binding Partners | Function |
|---|---|---|---|---|
| ESCRT-0 | Hrs (Vps27) | DUIM | STAM, Ub | Ub-cargo binding and concentration |
| VHS | ESCRT-I, Ub | |||
| FYVE | Phosphodityl-inositol-3 phosphate | |||
| GAT | Ub | |||
| PTAP | Clathrin | |||
| Stam1-2 (Hse1) | VHS | Hrs, Ub | Ub-cargo binding and concentration | |
| UIM | Ub | |||
| SH3 | STAMBP and USP8 | |||
| GAT | Ub | |||
| ESCRT-I | Tsg101 (Vps23) | UEV | Ub, ESCRT-0, HDPTP/PTPN23 | Ub-cargo binding and concentration |
| Mvb12A (Mvb12) | Ub-binding in COOH terminus | Ub | Ub-cargo binding and concentration and complex stabilization | |
| ESCRT-I components | ||||
| UBAP1 | SOUBA | Ub | Ub-cargo binding and concentration | |
| Tsg101, HD-PTP/PTPN23 | ||||
| USP8, Vps37A (ESCRT-I) | ||||
| ESCRT-II | EAP45 (Vps36) | GLUE | Ub | Ub-cargo binding and concentration |
| WH2 | Phosphoinositide lipids | |||
| ESCRT-I | ||||
| ESCRT associated | Alix (Bro1) | Bro1 | K63 polyUb | Viral budding and |
| V-domain | CHMP4 (ESCRT-III) | cytokinesis | ||
| In yeast, Bro1 binds Doa4 (USP8) and ESCRT-0 | Ubiquitinated cargo, sorting to MVB | |||
| ARF associated | GGA1-3 | GAT | Ub, ARF1 and 3 | Trans Golgi-to-endosome transport |
| VHS | Tsg101, Ub | |||
| GAE | Accessory proteins with DFGXØ | |||
| Adaptor | Tom1 and Tom1L1 and L2 | VHS, GAT | Ub, Myosin VI, Endofin, Clathrin, ZFYVE16, Tollip | Endosomal trafficking |
| Adaptor | Tollip | CUE, C2, TBD | Ub or phosphodityl-inositol-3 phosphate, Tom1 Tom1L2 | Component of the signaling pathway for Toll-like receptor complex and interleukin receptor-1 |
| GTP exchange factor | Rabex-5 (Vps9) | A20 Zn-finger | Ub | Endosomal fusion |
| (CUE domain in yeast) | RGS14, Rabaptin-5 Rab4/5/21/22, GGAs | |||
| Molecular chaperone | DNAJB2 | J-domain | Hsp70/Hsc70 binding | Regulates activity of Hsp70 |
| (Hsp40 homolog) | UIM | Polyubiquitin | ||
| Molecular cochaperone | BAG1 | BAG-domain | Hsp70 binding | Negative regulator of Hsp70 chaperone activity |
| UBL | UBD interaction | Receptor interactions |
ARF, ADP ribosylation factor; BAG, apoptosis regulator Bcl-2; CUE, coupling of ubiquitin conjugation to endoplasmic reticulum degradation; DUIM, double-sided ubiquitin-interacting motif (UIM); FYVE, Fab1, YotB, Vac1p, and EEA1; GAE, γ-adaptin ear domain; GAT, GGA and Tom1; GGA, Golgi-localizing/γ-adaptin ear homology domain/ADP-ribosylation factor-binding; GLUE, GRAM-like ubiquitin binding in EAP45; PTAP, Pro-Thr-Ala-Pro; Rab, Ras superfamily of monomeric G proteins; RGS, regulator of G protein signaling; SH3, Src homology 3; SOUBA, solenoid of overlapping ubiquitin-associated domain; UEV, Ubiquitin E2 variant; VHS, Vps27p, Hrs, and STAM domain; WH2, WASP-homology 2 or Wiskott-Aldrich homology 2; UBL, Ubiquitin-like domains.
Contrary to the chaperone-dependent PM QC in higher eukaryotes, conformationally damaged yeast nutrient transporters are subjected to chaperone-independent ubiquitination upon exposure to cellular stress or excess substrate (44, 73, 76). Both heat and oxidative stress, similar to high concentration of substrates, lead to conformational perturbations that may destabilize the hydrogen bonded NH2-terminal “loop interaction domain” (or LID) association with multiple cytosolic loops in Fur4 and Mup1, the yeast uracil and methionine transporter, respectively (69). This structural alteration serves as a degron by exposing two Lys ubiquitin acceptor sites for Rsp5-mediated ubiquitination (69).
Both arrestin-related protein (ART)-dependent and -independent mechanisms have been implicated in degron recognition by Rsp5 (69, 77, 91, 152); however, the precise biochemical basis remains to be determined. Intriguingly, the Rsp5-dependent ubiquitination and elimination of unfolded Fur4p could not be substituted by Ubr1 or San1 E3 ligases that can recognize unfolded cytosolic and nuclear client protein (152). The possible redundancy of PM QC ligases is supported by the observation that CHIP ablation only partially inhibits the proteolytic downregulation of unfolded ΔF508-CFTR, DRD4, V2R, and hERG, whereas depletion of other E3 Ub-ligases (e.g., Hrd1 and Gp78) also impeded CFTR clearance from the PM (1, 2, 95). Similarly, at least two E3 ligases (Cullin5 and CHIP) are responsible for the geldanamycin-induced ubiquitination and lysosomal downregulation of ErbB2 (HER2/neu) (34, 153). These observations with the modest phenotypic manifestation of the CHIP knockout mouse (87) suggest that a complex network of QC E3 ligases has evolved to maintain PM proteostasis with overlapping substrate specificity.
Transmembrane Segment Recognition
Conformational defects caused by mutations in the TM segments may be recognized directly in the membrane plane and/or via allosteric perturbations of the cytosolic region due to rearrangement of exposed cytoslic loops and domains, as suggested by the phenotype of missense TM mutations in the V2R (W164S), DRD4 (M345T) (1, 2). These mutations introduce polar residues and induce the CHIP recruitment and polyubiquitination probably in a chaperone-dependent manner at the cytosolic surface of the G-protein-coupled receptors (GPCRs) (1, 2). It is plausible that intracellular and/or extracellular K+ depletion results in the global unfolding of the hERG channel, including its major cytosolic domains, leading to the CHIP recruitment (1). Unfolding is likely initiated by depletion of one or more K+ ions from the ion-conducting pore of the channel (1). In addition to mutations, reduction of the membrane sphingolipid content can also provoke conformational destabilization, followed by the ubiquitination-dependent vacuolar degradation of the yeast PM Pma-1 (H-ATPase) and Gap1p (general amino acid permease) transporters, conceivably by altering the TM domain packing (39, 73, 136, 151).
A distinct substrate-binding mode is envisioned for the membrane-associated RING-CH (MARCH) ligases, representing a small family of 11 RING-CH E3 Ub ligases with incompletely understood physiological functions in PM remodeling (37, 90). MARCH ligases are orthologs of the Kaposi sarcoma-associated herpes virus (KSVH) immunoevasion ligase K3 and K5 (137) and contain two transmembrane segments and a COOH-terminal cytosolic RING-CH domain. Endogenous or overexpressed MARCHs play a key role in the ubiquitination-dependent lysosomal downregulation of native cell surface receptors (e.g., MHC-I: K3, K5, MARCH-9 and -4; MHC-II: MARCH-2 and -8, CD4, CD44 and CD98, MARCH-4 and -8) (6, 7, 23, 26, 41, 46, 62, 65, 66, 90). MARCH-8 and -1 can recognize a transmembrane hydrophobic segment in concert with charged residues and results in the Ub-conjugation to a Lys residue at the membrane-cytosol interface in the case of the HLA-DR and transferrin receptor (41, 46). Although the consensus signal for TM recognition by most MARCHes remains to be identified, it is reasonable to assume that a similar recognition mechanism may come into play upon unfolding and/or disassembly of polytopic PM polypeptides. It is also possible that otherwise buried hydrophilic residues become exposed and are recognized by QC E3 ligases. Although the latter scenario has not been documented at the PM yet, a cluster of intramembrane hydrophilic residues was found in both ER and Golgi E3 ligases (Hrd1, gp78, Doa10, and Tul1) that can facilitate binding to charged TM residues of nonnative client proteins (8, 106).
QC at the Exofacial Surface of the PM
The QC machinery that directly recognizes the extracellular surface of nonnative PM proteins and renders them susceptible for rapid elimination has not been described. Conformational defects emanating from the exofacial surface of PM proteins, however, may be relayed allosterically to cytoplasmic regions. Impaired N-glycosylation of H+-K+-ATPase, κ- and δ-opiod receptor, Kv1.4 potassium channel, glucose transporter 1 (GLUT1), and CFTR has been associated with metabolic destabilization at the PM (43, 75, 82, 133, 139) and was sufficient to compromise the conformational stability of CFTR cytosolic domains, with consequential polyubiquitination in post-Golgi compartments (43). Unfolding of the extracellular domain of the LDL receptor provokes Derlin-1-/syntaxin-dependent retrograde transport of the internalized receptor from early endosomes to the Golgi complex and ultimately to the ER, where their degradation occurs (25). The signal that preferentially targets unfolded PM proteins toward ER-associated degradation remains to be elucidated.
Molecular chaperones secreted into the extracellular space may impact the PM homeostasis (89, 101, 144, 147). Hsp90 and its co-chaperone, the cell division cycle Cdc37, are involved in cell migration and invasion in normal and cancer cells (36, 123). The extracellular Hsp90-Cdc37 complex interacts with kinase receptors (HER2, EGF, and Erbb2) and metalloproteinases (35). In analogy to the stabilization of cytosolic kinase domains (127), Hsp90-Cdc37 in concert with extracellular Hsp70, Hop, and p23 maintains the active conformation of the extracellular domains of selected receptors (35).
Clusterin (apolipoprotein J), haptoglobin, and α2-macroglobulin accumulate extracellularly after stress induction and participate in conformational protection of PM proteins by their ATP-independent holdase activity (101). The scavenger capacity of these chaperones can prevent further aggregation by sequestering misfolded conformers of extracellular proteins (89, 101, 144, 147). Extended association of clusterin with client proteins culminates in receptor-mediated endocytosis and lysosomal degradation to circumvent the PM permeabilization, triggered by extracellular oligomers (89) (see FIGURE 1). It was demonstrated 20 years ago that expression of clusterin is increased in Alzheimer's disease (85), which since has been attributed to the neuroprotective response of the brain (135). By binding to β-amyloid peptides, clusterin prevents fibril formation (146) and may have a similar function in several subclasses of high-density lipoprotein (HDL) particles. Accordingly, loss-of-function mutations and haploinsufficiency of clusterin, as well as elevated cholesterol levels, are implicated as a risk factor for Alzheimer's disease (78, 122, 142).
Chaperone-Independent Ubiquitination of Nonnative PM Proteins
As an alternative route of ubiquitination, several adaptors have been invoked in the recruitment of E3 ligases to nonnative PM proteins. Ten arrestin-related trafficking adaptors or ARTs are implicated in client selection for Rsp5-mediated ubiquitination that invariably causes substrate and/or stress-induced downregulation of yeast PM proteins by accelerated internalization and ESCRT-dependent vacuolar degradation. Based on computer modeling and phylogenetic analysis, two subclasses of arrestins are distinguished (9). The more ancient alpha-arrestins (or ARTs) contain an NH2-terminal arrestin domain similar to beta-arrestins and a COOH-terminal PPxY motif(s) (9), which is responsible for the Rsp5 recruitment via the WW domains. Although multiple ARTs have overlapping substrate specificity (e.g., ART1, 2, 4, and 8 are involved in Hxt6, Fur4, and Tat2 downregulation), only ART1 is required to render Can1 and Mup1 metabolically unstable (77). Besides ARTs, arrestin-like adaptors also participate in the downregulation of PM transporters. Both Bul1/Bul2 and ART1–9 ablation are required to impede the downregulation of destabilized Fur4 from the PM (69). Mammalian cells have five members of the arrestin domain-containing proteins (ARRDC) in the alpha-arrestin subfamily, but their contribution to sensing unfolded PM proteins is not known (48). In contrast to alpha-arrestins, beta-arrestins have clathrin and AP-2 interacting scaffolding function to facilitate internalization and signaling of activated GPCRs upon phosphorylation in higher eukaryotes (120). Bsd2 is a transmembrane adaptor protein that harbors PY motifs, recruits Rsp5, and can recognize polar TM domains of unfolded or activated PM proteins (e.g., Smf1 metal transporter and mutant PM ATPase) at the endosomal and Golgi compartment (58). This mechanism complements the ART-dependent ubiquitination at the PM and facilitates the transporter vacuolar delivery along the endocytic pathway where deubiquitination may occur (19). Likewise, ubiquitination and lysosomal delivery of Smf1 was blocked if nine ARTs in concert with Bsd2 were deleted (91). ARTs can also recruit constituents of the ESCRT-I machinery (Vps28 and Vps23) to modulate cargo sorting efficiency via ESCRT-I ubiquitination (56, 104). Intriguingly, the human ortholog (NDFIP1) of Bsd2 is essential for the Nedd4–2-dependent polyubiquitination and downregulation of the divalent metal transporter (DMT1) to protect mammalian cells from toxic iron accumulation, suggesting a similar paradigm for the regulation of DMT1 PM density (63). In addition to being an adaptor, NDFIP1 also functions as an activator of multiple HECT domain E3 ligases of the Nedd4 family (88).
Configuration of Ubiquitin as Sorting Signal at the PM and Endosome
Ubiquitination of unfolded PM protein is one of the key sorting signals that ensures cargo recognition by the internalization and endocytic sorting apparatus to facilitate lysosomal/vacuolar targeting and degradation (47). The cellular aspects of this paradigm could be exemplified by several PM proteins, including a direct correlation between ubiquitination, internalization, and lysosomal delivery of the cell surface resident CD4T-λC on thermal unfolding (2, 44, 51, 95, 110, 118). Although a single Ub appears to be sufficient to direct internalization in yeast, MVE sorting requires poly- or multiple-mono-Ub for recognition by ESCRT0-II (2, 95, 105, 124). Notably, polyUb chain or multiple monoUb of numerous native and misfolded PM proteins, as well as model proteins in higher eukaryotes, significantly augments the internalization rate of cargo (5, 31, 50). In addition of K63-linked Ub chains, lysosomal sorting signals may contain K48-, K11-, and K29-linked chains to enhance the avidity for Ub-binding protein recognition (13). In misfolded CD4T-λC, the K63-linked Ub chain was more abundant than the K48-linked chain at the PM (2), and the Rsp5 synthesized K63-linked Ub-chain is susceptible to deubiquitination by the Ubp2 DUB (67, 68).
The Ub-chain configuration is dynamically determined by both the E2/E3 and the deubiquitinating enzyme (DUB) activities. Although some DUBs associate with specific E3 ligases (e.g., Ubp2-Rsp5), others [e.g., STAMBP (AMSH), STAMBP-LP (AMSH-LP), and USP8 (UBPY)] are confined to endosomes (19, 132), allowing regulated remodeling of cargo Ub configuration that can either facilitate lysosomal/vacuolar degradation or recycling, depending on the extent and configuration of ubiquitination. Interaction of USP20 and USP33 DUB with beta-arrestins ensures the agonist-stimulated beta2-adrenergic receptor deubiquitination (11, 121), whereas the USP2 variant 45 deubiquitinates ENaC (14). USP10 regulates CFTR recycling in airway epithelia (12), and USP46 prohibits lysosomal degradation of glutamate receptor in C. elegans (71). No specific DUB activity has been identified in association with the PM QC machinery.
Decoding Ubiquitin Modification as Sorting Signal Along the Endocytic Pathway
A large number of Ub-receptors containing a variety of Ub binding domains (UBD) are involved in the translation of Ub signals as internalization and lysosomal sorting motifs of PM cargo molecules (28). The UBDs may also protect against premature deubiquitination and proteasome-mediated cleavage of PM proteins, a prerequisite for MVE/lysosomal delivery. Although only a subset of the endocytic Ub receptors has been functionally identified in PM QC, it is reasonable to assume that the sorting machinery of native and nonnative ubiquitinated cargos partially overlaps.
Depending on their primary confinement, ubiquitinated PM cargo can be internalized via clathrin- or caveolin-dependent internalization route. Ubiquitinated cargo concentration into clathrin-coated pits are facilitated by the endocytic adaptors (Epsin 1–2 and Eps15/15R/15B), harnessing multiple UBDs with preferentially recognition of polyUb (5, 50, 111, 128). In the caveolin-dependent pathway, UBXD1 and the p97-UBXD1 ATPase complex are required for targeting of ubiquitinated caveolin-1 to early endosomes (70).
Following the delivery of ubiquitinated cargoes from clathrin- and caveolin-coated vesicles into tubular early endosomes (52), cargo segregation from recycling toward lysosomal delivery is mediated by components of the ESCRT0-III. The four multimeric ESCRT (0–III) complexes consecutively assemble and disassemble with each other and numerous accessory proteins to ultimately ensure the sorting and budding of most ubiquitinated PM proteins into intraluminal vesicles (ILVs). ESCRT-0 consists of Hrs and Stam1/2, encompassing five UBDs, and has modestly higher affinity to K63- over K48-linked polyUb chain with ∼50-fold increased binding preference to K63-linked tetra-Ub over mono-Ub (59, 108) (Table 1). Ubiquitination of constituents of the PM QC machinery (e.g., Hsc70, CHIP, and BAG1) may serve as a signal amplification mechanism to enhance the fidelity of the lysosomal targeting of nonnative membrane proteins. In concert, the Ub-like domain of BAG1, an Hsc70 cochaperone, could further facilitate nonnative cargo recognition by the Ub-receptor network both at the PM and endosomes, as observed for the misfolded CFTR mutant (95).
Subsequent interaction of ubiquitinated PM proteins with ESCRT I–III machinery is required for their delivery into MVE and the formation of ILV (55, 64). Depletion of Hrs, Stam1, or Tsg101 (ESCRT-I) attenuated the endocytosis and lysosomal delivery of misfolded ΔF508-CFTR, CD4T-λC, V2R-W164S, and DRD4-M345T from the cell surface. The polyubiquitinated nonnative rΔF508 and Δ70 CFTR (70 residues COOH-terminal truncation), but not the native channel, physically interact with ESCRT components (e.g., Hrs, Stam1/2, TSG101) (118). This functional and biochemical evidence supports the indispensible role of ESCRT complexes in the lysosomal degradation of nonnative PM proteins (2, 43, 95).
Several other Ub-receptors have been identified along the endocytic pathways that contribute to ubiquitinated PM protein recognition and clearance. TOM1 or TOM1 like-1–2 [TOML1–2] clathrin interacting ESCRT-0-like proteins (103) can form a complex with Tollip and Endofin and are capable of interfacing with Hrs and Tsg101 (131) (Table 1). The ESCRT-I proteins, Tsg101 and UBAP1, and the ESCRT-II EAP45 also incorporate UBDs. In addition, the Golgi protein gamma adaptin-ear-containing ARF-binding protein 3 (GGA3) has Hrs-like UBDs and binds both ubiquitinated cargo and Tsg101 (64, 103). The early endosomal Rab5 GTP/GDP exchange factor (Rabex-5) also contains two UBDs (74, 99); one of them (A20 Zn-finger) displays E3 Ub ligase activity (84) and can associate with ubiquitinated cargo both at PM and endosomes, as shown for the EGF receptor (99).
The ILV budding process is controlled by the assembly of ESCRT-III, lacking known UBDs, but has the capacity to recruit two DUBs, USP8 and STAMBP (20). Bro1 protein His domain protein tyrosine phosphatise (HD-PTP/PTPN23), which is an adaptor protein for ESCRTs, has been shown to compete with STAM2 for USP8 interaction, driving the ubiquitinated EGF receptor to ESCRT-III (125). In yeast, Bro1 interacts with ESCRT-0, recruits Doa4 (USP8), and selectively sorts ubiquitinated cargo to MVE (97). Bro1 is a distant relative to mammalian Alix, which captures K63-linked polyUb (30). HD-PTP may have inherited the yeast Bro1 role, representing the degradative Ub-adaptor link from sorting endosomes to MVE.
Following the removal of Ubs and targeting client proteins into the budding ILV from the limiting membrane of MVE, the cargo is considered to be at the point of no return, whereas MVE matures into lysosome. Endolysosomal trafficking pathway, however, may not be the exclusive degradation route of misfolded PM protein elimination. A possible link between clathrin-mediated endocytosis and autophagy has been proposed via the actin motor, myosin VI, and its adaptor proteins (Dab2, GIPC, Tom1, and LMTK2), harboring Ub, clathrin/AP2, and phospholipid binding domains (Table 2). Myosin VI links Tom1 to the autophagy adaptor proteins (T6BP, NDP52, and optineurin), which can recruit ubiquitinated cargo and via LC3 association may facilitate autophagosome formation (130). It is also possible that unfolded proteins are targeted for degradation in the absence of covalent Ub modification, either via complex formation with an E3 ligase, via component(s) of the ESCRT machinery, or by exposing a sorting signal that can be recognized, e.g., by ALIX, as exemplified by various transmembrane cargoes (e.g., Sna3, delta opioid receptor, and PAR1) (29, 61, 86, 93).
Table 2.
Possible adaptor proteins for directing cargo to autophagosomes at the endocytic pathway
| Component | Adaptor | Binding Domains | Binding Partners | Function |
|---|---|---|---|---|
| Endocytic vesicles | Vps34 | Phosphoinositide-3-kinase FYVE, PX | Phosphoinositide-3-kinase regulatory subunit 4, Beclin, either UVRAG or rubicon, or ATG14, Rab7A | Phoshoinositol to phosphatidylinositol (3)-phosphate formation, transport from early to late endosomes, lysosomal enzyme transport, autophagosome formation |
| Clathrin-dependent endocytosis complex | Dab2 | Clathrin binding AP2 binding Phosphotyrosine binding motif | Clathrin EH domain-containing proteins (EPS15, EPS15L1 and ITSN1) Myosin VI | Clathrin-associated sorting protein, through myosin VI dimerisation to autophagy |
| Endocytic vesicles | Tom1 and/or Tom1L2 | VHS, GAT | Ub | Ub-cargo binding, autophagy |
| Myosin VI, Endofin | Endosomal trafficking | |||
| Clathrin, ZFYVE16 | ||||
| Myosin VI | Coiled coil | Multiple, primary Dab2, actin | Membrane trafficking, autophagy | |
| Endocytic vesicles | GIPC | PDZ | PDZ-binding proteins Myosin VI, APPL1 | Endocytosis |
| Endocytic vesicles | LMTK2 | Transmembrane serine/threonine kinase | Myosin VI | Early endocytic trafficking and recycling |
| NDP52 | UBD/Zn-finger | Ub | Autophagy adaptor, | |
| (CALCOCO2) | LIR | LC3 | signaling, | |
| SKICH | Myosin VI | Actin cytoskeleton reorganization | ||
| NAP1, LC3 | Receptor for Ub-coated bacteria in macroautophagy | |||
| T6BP (TIFA) | UBD/Zn-finger | Ub | Autophagy adaptor | |
| LIR | LC3 | Signaling | ||
| SKICH | Myosin VI | |||
| FHA | ITCH (AIP4) | |||
| PtdIns(4,5)P2 binding | ||||
| Optineurin | UBD | Ub | Autophagy adaptor | |
| LIR | LC3 | Signaling, secretion | ||
| Zn-finger | Myosin VI | |||
| CYLD, TBK1, TBC1D17 | Myosin VI dimerisation | |||
| Huntingtin, Rab8 | ||||
| ATG16L1 | 7 WD40 repeats | Clathrin/AP2 | Early autophagosome formation, | |
| probably in the dynamin-dependent scission process |
AP, adaptin subunit of the adaptor protein; APPL1, adaptor protein, phosphotyrosine interaction; ATG, autophagy related gene; CYLD, cylindromatosis; Dab2, disabled-2; EH, Eps15 homology; FHA, fork head associated domain; FYVE, Fab1, YotB, Vac1p, and EEA1; GAT, GGA and Tom1; ITSN1, intersectin 1; LC3, microtubule-associated protein 1 light chain 3; LIR, LC3-interaction motif; LMTK2, lemur tyrosine kinase-2; NDP52/CALCOCO2, calcium binding and coiled-coil domain 2, also known as nuclear domain 10 protein 52; PDZ, postsynaptic density protein (PSD95), Drosophila disc large tumor suppressor (Dlg1), and zonula occludens-1 protein (zo-1) or Discs-large homologous regions; PX, phosphoinositide-binding domain; Rab, Ras-associated protein; SKICH, SKIP-carboxyl homology domain; TBC, Tre-2, Bub2p, and Cdc16p domain family (Rab GTPase-activating protein); TBK1, TANK-binding kinase 1; UVRAG, UV radiation resistance-associated gene protein; VHS, Vps27p, Hrs, and STAM domain, PH domain, and leucine zipper containing 1; Vps, class III phosphoinositide 3-kinase vacuolar protein sorting; WD, Trp-Asp solenoid repeat containing domain; UBD, ubiquitin binding domain; ZF, zinc finger domain.
Conclusions
Although significant progress has been made to uncover the peripheral or PM QC machinery, many questions remain unanswered regarding the inner working of this homeostatic mechanism in cellular and organismal physiology. Little is known about the conformational sensitivity of the peripheral QC toward metastable or partially unfolded membrane proteins and the redundancy of the recognition, ubiquitination, and sorting machinery involved. We still do not have a comprehensive inventory of adaptors and chaperones that account for the recognition of conformationally damaged PM proteins, and we lack knowledge of possible contribution of alternative disposal pathways, such as autophagosome, proteasome, and retrograde ER delivery. Finally, the regulation of molecular chaperones that may suppress conformational defects of PM proteins by their foldase activity and/or delay ubiquitination will have to be elucidated. Better understanding of the cellular proteostasis networks at the molecular level may lead to novel opportunities for influencing inherited or acquired diseases afflicting the conformational integrity of PM proteins.
Footnotes
Experimental work in G.L.L.'s laboratory was supported by funding from CIHR, National Institute of Diabetes and Digestive and Kidney Diseases, Cystic Fibrosis Canada, and Cystic Fibrosis Foundation, USA. G.L.L. is a Canada Research Chair.
No conflicts of interest, financial or otherwise, are declared by the author(s).
Author contributions: P.M.A. and G.L.L. edited and revised manuscript; P.M.A. and G.L.L. approved final version of manuscript; P.M.A. and G.L.L. prepared figures; P.M.A. and G.L.L. drafted manuscript.
References
- 1.Apaja PM, Foo B, Okiyoneda T, Valinsky WC, Barriere H, Atanasiu R, Ficker E, Lukacs GL, Shrier A. Ubiquitination-dependent quality control of hERG K+-channel with acquired and inherited conformational defect at the plasma membrane. Mol Biol Cell 24: 3787–3804, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Apaja PM, Xu H, Lukacs GL. Quality control for unfolded proteins at the plasma membrane. J Cell Biol 191: 553–570, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arvan P, Zhao X, Ramos-Castaneda J, Chang A. Secretory pathway quality control operating in Golgi, plasmalemmal, and endosomal systems. Traffic 3: 771–780, 2002 [DOI] [PubMed] [Google Scholar]
- 4.Ballinger CA, Connell P, Wu Y, Hu Z, Thompson LJ, Yin LY, Patterson C. Identification of CHIP, a novel tetratricopeptide repeat-containing protein that interacts with heat shock proteins and negatively regulates chaperone functions. Mol Cell Biol 19: 4535–4545, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barriere H, Nemes C, Lechardeur D, Khan-Mohammad M, Fruh K, Lukacs GL. Molecular basis of oligoubiquitin-dependent internalization of membrane proteins in Mammalian cells. Traffic 7: 282–297, 2006 [DOI] [PubMed] [Google Scholar]
- 6.Bartee E, Eyster CA, Viswanathan K, Mansouri M, Donaldson JG, Fruh K. Membrane-associated RING-CH proteins associate with Bap31 and target CD81 and CD44 to lysosomes. PLos One 5: e15132, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bartee E, Mansouri M, Hovey Nerenberg BT, Gouveia K, Fruh K. Downregulation of major histocompatibility complex class I by human ubiquitin ligases related to viral immune evasion proteins. J Virol 78: 1109–1120, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bays NW, Gardner RG, Seelig LP, Joazeiro CA, Hampton RY. Hrd1p/Der3p is a membrane-anchored ubiquitin ligase required for ER-associated degradation. Nat Cell Biol 3: 24–29, 2001 [DOI] [PubMed] [Google Scholar]
- 9.Becuwe M, Herrador A, Haguenauer-Tsapis R, Vincent O, Leon S. Ubiquitin-mediated regulation of endocytosis by proteins of the arrestin family. Biochem Res Int 2012: 242764, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Benharouga M, Haardt M, Kartner N, Lukacs GL. COOH-terminal truncations promote proteasome-dependent degradation of mature cystic fibrosis transmembrane conductance regulator from post-Golgi compartments. J Cell Biol 153: 957–970, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Berthouze M, Venkataramanan V, Li Y, Shenoy SK. The deubiquitinases USP33 and USP20 coordinate beta2 adrenergic receptor recycling and resensitization. EMBO J 28: 1684–1696, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bomberger JM, Barnaby RL, Stanton BA. The deubiquitinating enzyme USP10 regulates the endocytic recycling of CFTR in airway epithelial cells. Channels (Austin) 4: 150–154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boname JM, Thomas M, Stagg HR, Xu P, Peng J, Lehner PJ. Efficient internalization of MHC I requires lysine-11 and lysine-63 mixed linkage polyubiquitin chains. Traffic 11: 210–220, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boulkroun S, Ruffieux-Daidie D, Vitagliano JJ, Poirot O, Charles RP, Lagnaz D, Firsov D, Kellenberger S, Staub O. Vasopressin-inducible ubiquitin-specific protease 10 increases ENaC cell surface expression by deubiquitylating and stabilizing sorting nexin 3. Am J Physiol Renal Physiol 295: F889–F900, 2008 [DOI] [PubMed] [Google Scholar]
- 15.Brodsky JL. Cleaning up: ER-associated degradation to the rescue. Cell 151: 1163–1167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Buchberger A, Bukau B, Sommer T. Protein quality control in the cytosol and the endoplasmic reticulum: brothers in arms. Mol Cell 40: 238–252, 2010 [DOI] [PubMed] [Google Scholar]
- 17.Capdevila-Nortes X, Lopez-Hernandez T, Apaja PM, Lopez de Heredia M, Sirisi S, Callejo G, Arnedo T, Nunes V, Lukacs GL, Gasull X, Estevez R. Insights into MLC pathogenesis: GlialCAM is an MLC1 chaperone required for proper activation of volume-regulated anion currents. Hum Mol Genet 22: 4405–4416, 2013 [DOI] [PubMed] [Google Scholar]
- 18.Chakraborty K, Chatila M, Sinha J, Shi Q, Poschner BC, Sikor M, Jiang G, Lamb DC, Hartl FU, Hayer-Hartl M. Chaperonin-catalyzed rescue of kinetically trapped states in protein folding. Cell 142: 112–122, 2010 [DOI] [PubMed] [Google Scholar]
- 19.Clague MJ, Barsukov I, Coulson JM, Liu H, Rigden DJ, Urbe S. Deubiquitylases from genes to organism. Physiol Rev 93: 1289–1315, 2013 [DOI] [PubMed] [Google Scholar]
- 20.Clague MJ, Hammond DE. Membrane traffic: catching the lysosome express. Curr Biol 16: 416–418, 2006 [DOI] [PubMed] [Google Scholar]
- 21.Clague MJ, Liu H, Urbe S. Governance of endocytic trafficking and signaling by reversible ubiquitylation. Dev Cell 23: 457–467, 2012 [DOI] [PubMed] [Google Scholar]
- 22.Connell P, Ballinger CA, Jiang J, Wu Y, Thompson LJ, Hohfeld J, Patterson C. The co-chaperone CHIP regulates protein triage decisions mediated by heat-shock proteins. Nat Cell Biol 3: 93–96, 2001 [DOI] [PubMed] [Google Scholar]
- 23.Coscoy L, Ganem D. PHD domains and E3 ubiquitin ligases: viruses make the connection. Trends Cell Biol 13: 7–12, 2003 [DOI] [PubMed] [Google Scholar]
- 24.Dai Q, Qian SB, Li HH, McDonough H, Borchers C, Huang D, Takayama S, Younger JM, Ren HY, Cyr DM, Patterson C. Regulation of the cytoplasmic quality control protein degradation pathway by BAG2. J Biol Chem 280: 38673–38681, 2005 [DOI] [PubMed] [Google Scholar]
- 25.Dang H, Klokk TI, Schaheen B, McLaughlin BM, Thomas AJ, Durns TA, Bitler BG, Sandvig K, Fares H. Derlin-dependent retrograde transport from endosomes to the Golgi apparatus. Traffic 12: 1417–1431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.De Gassart A, Camosseto V, Thibodeau J, Ceppi M, Catalan N, Pierre P, Gatti E. MHC class II stabilization at the surface of human dendritic cells is the result of maturation-dependent MARCH I down-regulation. Proc Natl Acad Sci USA 105: 3491–3496, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.DePristo MA, Weinreich DM, Hartl DL. Missense meanderings in sequence space: a biophysical view of protein evolution. Nat Rev Genet 6: 678–687, 2005 [DOI] [PubMed] [Google Scholar]
- 28.Dikic I, Wakatsuki S, Walters KJ. Ubiquitin-binding domains: from structures to functions. Nat Rev Mol Cell Biol 10: 659–671, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dores MR, Chen B, Lin H, Soh UJ, Paing MM, Montagne WA, Meerloo T, Trejo J. ALIX binds a YPX(3)L motif of the GPCR PAR1 and mediates ubiquitin-independent ESCRT-III/MVB sorting. J Cell Biol 197: 407–419, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dowlatshahi DP, Sandrin V, Vivona S, Shaler TA, Kaiser SE, Melandri F, Sundquist WI, Kopito RR. ALIX is a Lys63-specific polyubiquitin binding protein that functions in retrovirus budding. Dev Cell 23: 1247–1254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Duncan LM, Piper S, Dodd RB, Saville MK, Sanderson CM, Luzio JP, Lehner PJ. Lysine-63-linked ubiquitination is required for endolysosomal degradation of class I molecules. EMBO J 25: 1635–1645, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dupre S, Urban-Grimal D, Haguenauer-Tsapis R. Ubiquitin and endocytic internalization in yeast and animal cells. Biochim Biophys Acta 1695: 89–111, 2004 [DOI] [PubMed] [Google Scholar]
- 33.Egner R, Mahe Y, Pandjaitan R, Kuchler K. Endocytosis and vacuolar degradation of the plasma membrane-localized Pdr5 ATP-binding cassette multidrug transporter in Saccharomyces cerevisiae. Mol Cell Biol 15: 5879–5887, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ehrlich ES, Wang T, Luo K, Xiao Z, Niewiadomska AM, Martinez T, Xu W, Neckers L, Yu XF. Regulation of Hsp90 client proteins by a Cullin5-RING E3 ubiquitin ligase. Proc Natl Acad Sci USA 106: 20330–20335, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.El Hamidieh A, Grammatikakis N, Patsavoudi E. Cell surface Cdc37 participates in extracellular HSP90 mediated cancer cell invasion. PLos One 7: e42722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Eustace BK, Jay DG. Extracellular roles for the molecular chaperone, hsp90. Cell Cycle 3: 1098–1100, 2004 [PubMed] [Google Scholar]
- 37.Eyster CA, Cole NB, Petersen S, Viswanathan K, Fruh K, Donaldson JG. MARCH ubiquitin ligases alter the itinerary of clathrin-independent cargo from recycling to degradation. Mol Biol Cell 22: 3218–3230, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fang NN, Ng AH, Measday V, Mayor T. Hul5 HECT ubiquitin ligase plays a major role in the ubiquitylation and turnover of cytosolic misfolded proteins. Nat Cell Biol 13: 1344–1352, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fayadat L, Kopito RR. Recognition of a single transmembrane degron by sequential quality control checkpoints. Mol Biol Cell 14: 1268–1278, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fischer F, Hamann A, Osiewacz HD. Mitochondrial quality control: an integrated network of pathways. Trends Biochem Sci 37: 284–292, 2012 [DOI] [PubMed] [Google Scholar]
- 41.Fujita H, Iwabu Y, Tokunaga K, Tanaka Y. Membrane-associated RING-CH (MARCH) 8 mediates the ubiquitination and lysosomal degradation of the transferrin receptor. J Cell Sci 126: 2798–2809, 2013 [DOI] [PubMed] [Google Scholar]
- 42.Gardner RG, Nelson ZW, Gottschling DE. Degradation-mediated protein quality control in the nucleus. Cell 120: 803–815, 2005 [DOI] [PubMed] [Google Scholar]
- 43.Glozman R, Okiyoneda T, Mulvihill CM, Rini JM, Barriere H, Lukacs GL. N-glycans are direct determinants of CFTR folding and stability in secretory and endocytic membrane traffic. J Cell Biol 184: 847–862, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gong X, Chang A. A mutant plasma membrane ATPase, Pma1–10, is defective in stability at the yeast cell surface. Proc Natl Acad Sci USA 98: 9104–9109, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Goold R, McKinnon C, Rabbanian S, Collinge J, Schiavo G, Tabrizi SJ. Alternative fates of newly formed PrPSc upon prion conversion on the plasma membrane. J Cell Sci 126: 3552–3562, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Goto E, Ishido S, Sato Y, Ohgimoto S, Ohgimoto K, Nagano-Fujii M, Hotta H. c-MIR, a human E3 ubiquitin ligase, is a functional homolog of herpesvirus proteins MIR1 and MIR2 and has similar activity. J Biol Chem 278: 14657–14668, 2003 [DOI] [PubMed] [Google Scholar]
- 47.Haglund K, Dikic I. The role of ubiquitylation in receptor endocytosis and endosomal sorting. J Cell Sci 125: 265–275, 2012 [DOI] [PubMed] [Google Scholar]
- 48.Han SO, Kommaddi RP, Shenoy SK. Distinct roles for beta-arrestin2 and arrestin-domain-containing proteins in beta2 adrenergic receptor trafficking. EMBO Rep 14: 164–171, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hartl FU. Molecular chaperones in cellular protein folding. Nature 381: 571–579, 1996 [DOI] [PubMed] [Google Scholar]
- 50.Hawryluk MJ, Keyel PA, Mishra SK, Watkins SC, Heuser JE, Traub LM. Epsin 1 is a polyubiquitin-selective clathrin-associated sorting protein. Traffic 7: 262–281, 2006 [DOI] [PubMed] [Google Scholar]
- 51.Hayashi H, Sugiyama Y. Short-chain ubiquitination is associated with the degradation rate of a cell-surface-resident bile salt export pump (BSEP/ABCB11). Mol Pharmacol 75: 143–150, 2009 [DOI] [PubMed] [Google Scholar]
- 52.Hayer A, Stoeber M, Ritz D, Engel S, Meyer HH, Helenius A. Caveolin-1 is ubiquitinated and targeted to intralumenal vesicles in endolysosomes for degradation. J Cell Biol 191: 615–629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Heck JW, Cheung SK, Hampton RY. Cytoplasmic protein quality control degradation mediated by parallel actions of the E3 ubiquitin ligases Ubr1 and San1. Proc Natl Acad Sci USA 107: 1106–1111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hein C, Springael JY, Volland C, Haguenauer-Tsapis R, Andre B. NPl1, an essential yeast gene involved in induced degradation of Gap1 and Fur4 permeases, encodes the Rsp5 ubiquitin-protein ligase. Mol Microbiol 18: 77–87, 1995 [DOI] [PubMed] [Google Scholar]
- 55.Henne WM, Buchkovich NJ, Emr SD. The ESCRT pathway. Dev Cell 21: 77–91, 2011 [DOI] [PubMed] [Google Scholar]
- 56.Herrador A, Herranz S, Lara D, Vincent O. Recruitment of the ESCRT machinery to a putative seven-transmembrane-domain receptor is mediated by an arrestin-related protein. Mol Cell Biol 30: 897–907, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hessling M, Richter K, Buchner J. Dissection of the ATP-induced conformational cycle of the molecular chaperone Hsp90. Nat Struct Mol Biol 16: 287–293, 2009 [DOI] [PubMed] [Google Scholar]
- 58.Hettema EH, Valdez-Taubas J, Pelham HR. Bsd2 binds the ubiquitin ligase Rsp5 and mediates the ubiquitination of transmembrane proteins. EMBO J 23: 1279–1288, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hirano S, Kawasaki M, Ura H, Kato R, Raiborg C, Stenmark H, Wakatsuki S. Double-sided ubiquitin binding of Hrs-UIM in endosomal protein sorting. Nat Struct Mol Biol 13: 272–277, 2006 [DOI] [PubMed] [Google Scholar]
- 60.Hirsch C, Gauss R, Horn SC, Neuber O, Sommer T. The ubiquitylation machinery of the endoplasmic reticulum. Nature 458: 453–460, 2009 [DOI] [PubMed] [Google Scholar]
- 61.Hislop JN, Henry AG, Marchese A, von Zastrow M. Ubiquitination regulates proteolytic processing of G protein-coupled receptors after their sorting to lysosomes. J Biol Chem 284: 19361–19370, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hor S, Ziv T, Admon A, Lehner PJ. Stable isotope labeling by amino acids in cell culture and differential plasma membrane proteome quantitation identify new substrates for the MARCH9 transmembrane E3 ligase. Mol Cell Proteomics 8: 1959–1971, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Howitt J, Putz U, Lackovic J, Doan A, Dorstyn L, Cheng H, Yang B, Chan-Ling T, Silke J, Kumar S, Tan SS. Divalent metal transporter 1 (DMT1) regulation by Ndfip1 prevents metal toxicity in human neurons. Proc Natl Acad Sci USA 106: 15489–15494, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hurley JH, Stenmark H. Molecular mechanisms of ubiquitin-dependent membrane traffic. Annu Rev Biophys 40: 119–142, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jahnke M, Trowsdale J, Kelly AP. Ubiquitination of HLA-DO by MARCH family E3 ligases. Eur J Immunol 43: 1153–1161, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jahnke M, Trowsdale J, Kelly AP. Ubiquitination of human leukocyte antigen (HLA)-DM by different membrane-associated RING-CH (MARCH) protein family E3 ligases targets different endocytic pathways. J Biol Chem 287: 7256–7264, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kee Y, Lyon N, Huibregtse JM. The Rsp5 ubiquitin ligase is coupled to and antagonized by the Ubp2 deubiquitinating enzyme. EMBO J 24: 2414–2424, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kee Y, Munoz W, Lyon N, Huibregtse JM. The deubiquitinating enzyme Ubp2 modulates Rsp5-dependent Lys63-linked polyubiquitin conjugates in Saccharomyces cerevisiae. J Biol Chem 281: 36724–36731, 2006 [DOI] [PubMed] [Google Scholar]
- 69.Keener JM, Babst M. Quality control and substrate-dependent downregulation of the nutrient transporter Fur4. Traffic 14: 412–427, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kirchner P, Bug M, Meyer H. Ubiquitination of the N-terminal region of caveolin-1 regulates endosomal sorting by the VCP/p97 AAA-ATPase. J Biol Chem 288: 7363–7372, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kowalski JR, Dahlberg CL, Juo P. The deubiquitinating enzyme USP-46 negatively regulates the degradation of glutamate receptors to control their abundance in the ventral nerve cord of Caenorhabditis elegans. J Neurosci 31: 1341–1354, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Krishnamoorthy GP, Guida T, Alfano L, Avilla E, Santoro M, Carlomagno F, Melillo RM. Molecular mechanism of 17-allylamino-17-demethoxygeldanamycin (17-AAG)-induced AXL receptor tyrosine kinase degradation. J Biol Chem 288: 17481–17494, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lauwers E, Grossmann G, Andre B. Evidence for coupled biogenesis of yeast Gap1 permease and sphingolipids: essential role in transport activity and normal control by ubiquitination. Mol Biol Cell 18: 3068–3080, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lee S, Tsai YC, Mattera R, Smith WJ, Kostelansky MS, Weissman AM, Bonifacino JS, Hurley JH. Structural basis for ubiquitin recognition and autoubiquitination by Rabex-5. Nat Struct Mol Biol 13: 264–271, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Li JG, Chen C, Liu-Chen L.-Y. N-glycosylation of the human κ opioid receptor enhances its stability but slows its trafficking along the biosynthesis pathway. Biochemistry 46: 10960–10970, 2007 [DOI] [PubMed] [Google Scholar]
- 76.Li Y, Kane T, Tipper C, Spatrick P, Jenness DD. Yeast mutants affecting possible quality control of plasma membrane proteins. Mol Cell Biol 19: 3588–3599, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lin CH, MacGurn JA, Chu T, Stefan CJ, Emr SD. Arrestin-related ubiquitin-ligase adaptors regulate endocytosis and protein turnover at the cell surface. Cell 135: 714–725, 2008 [DOI] [PubMed] [Google Scholar]
- 78.Ling IF, Bhongsatiern J, Simpson JF, Fardo DW, Estus S. Genetics of clusterin isoform expression and Alzheimer's disease risk. PLos One 7: e33923, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ljunggren HG, Stam NJ, Ohlen C, Neefjes JJ, Hoglund P, Heemels MT, Bastin J, Schumacher TN, Townsend A, Karre K, et al. Empty MHC class I molecules come out in the cold. Nature 346: 476–480, 1990 [DOI] [PubMed] [Google Scholar]
- 80.Lukacs GL, Chang XB, Bear C, Kartner N, Mohamed A, Riordan JR, Grinstein S. The delta F508 mutation decreases the stability of cystic fibrosis transmembrane conductance regulator in the plasma membrane. Determination of functional half-lives on transfected cells. J Biol Chem 268: 21592–21598, 1993 [PubMed] [Google Scholar]
- 81.MacGurn JA, Hsu PC, Emr SD. Ubiquitin and membrane protein turnover: from cradle to grave. Annu Rev Biochem 81: 231–259, 2012 [DOI] [PubMed] [Google Scholar]
- 82.Markkanen PMH, Petäjä-Repo UE. N-glycan-mediated quality control in the endoplasmic reticulum is required for the expression of correctly folded δ-opioid receptors at the cell surface. J Biol Chem 283: 29086–29098, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Marozkina NV, Yemen S, Borowitz M, Liu L, Plapp M, Sun F, Islam R, Erdmann-Gilmore P, Townsend RR, Lichti CF, Mantri S, Clapp PW, Randell SH, Gaston B, Zaman K. Hsp 70/Hsp 90 organizing protein as a nitrosylation target in cystic fibrosis therapy. Proc Natl Acad Sci USA 107: 11393–11398, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mattera R, Tsai YC, Weissman AM, Bonifacino JS. The Rab5 guanine nucleotide exchange factor Rabex-5 binds ubiquitin (Ub) and functions as a Ub ligase through an atypical Ub-interacting motif and a zinc finger domain. J Biol Chem 281: 6874–6883, 2006 [DOI] [PubMed] [Google Scholar]
- 85.May PC, Lampert-Etchells M, Johnson SA, Poirier J, Masters JN, Finch CE. Dynamics of gene expression for a hippocampal glycoprotein elevated in Alzheimer's disease and in response to experimental lesions in rat. Neuron 5: 831–839, 1990 [DOI] [PubMed] [Google Scholar]
- 86.McNatt MW, McKittrick I, West M, Odorizzi G. Direct binding to Rsp5 mediates ubiquitin-independent sorting of Sna3 via the multivesicular body pathway. Mol Biol Cell 18: 697–706, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Min JN, Whaley RA, Sharpless NE, Lockyer P, Portbury AL, Patterson C. CHIP deficiency decreases longevity, with accelerated aging phenotypes accompanied by altered protein quality control. Mol Cell Biol 28: 4018–4025, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mund T, Pelham HR. Regulation of PTEN/Akt and MAP kinase signaling pathways by the ubiquitin ligase activators Ndfip1 and Ndfip2. Proc Natl Acad Sci USA 107: 11429–11434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Narayan P, Meehan S, Carver JA, Wilson MR, Dobson CM, Klenerman D. Amyloid-beta oligomers are sequestered by both intracellular and extracellular chaperones. Biochemistry 51: 9270–9276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Nathan JA, Lehner PJ. The trafficking and regulation of membrane receptors by the RING-CH ubiquitin E3 ligases. Exp Cell Res 315: 1593–1600, 2009 [DOI] [PubMed] [Google Scholar]
- 91.Nikko E, Sullivan JA, Pelham HR. Arrestin-like proteins mediate ubiquitination and endocytosis of the yeast metal transporter Smf1. EMBO Rep 9: 1216–1221, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Nillegoda NB, Theodoraki MA, Mandal AK, Mayo KJ, Ren HY, Sultana R, Wu K, Johnson J, Cyr DM, Caplan AJ. Ubr1 and Ubr2 function in a quality control pathway for degradation of unfolded cytosolic proteins. Mol Biol Cell 21: 2102–2116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Oestreich AJ, Aboian M, Lee J, Azmi I, Payne J, Issaka R, Davies BA, Katzmann DJ. Characterization of multiple multivesicular body sorting determinants within Sna3: a role for the ubiquitin ligase Rsp5. Mol Biol Cell 18: 707–720, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Okiyoneda T, Apaja PM, Lukacs GL. Protein quality control at the plasma membrane. Curr Opin Cell Biol 23: 483–491, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Okiyoneda T, Barriere H, Bagdany M, Rabeh WM, Du K, Hohfeld J, Young JC, Lukacs GL. Peripheral protein quality control removes unfolded CFTR from the plasma membrane. Science 329: 805–810, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Okiyoneda T, Veit G, Dekkers JF, Bagdany M, Soya N, Xu H, Roldan A, Verkman AS, Kurth M, Simon A, Hegedus T, Beekman JM, Lukacs GL. Mechanism-based corrector combination restores DeltaF508-CFTR folding and function. Nat Chem Biol 9: 444–454, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Pashkova N, Gakhar L, Winistorfer SC, Sunshine AB, Rich M, Dunham MJ, Yu L, Piper RC. The yeast alix homolog bro1 functions as a ubiquitin receptor for protein sorting into multivesicular endosomes. Dev Cell 25: 520–533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Pechmann S, Willmund F, Frydman J. The ribosome as a hub for protein quality control. Mol Cell 49: 411–421, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Penengo L, Mapelli M, Murachelli AG, Confalonieri S, Magri L, Musacchio A, Di Fiore PP, Polo S, Schneider TR. Crystal structure of the ubiquitin binding domains of rabex-5 reveals two modes of interaction with ubiquitin. Cell 124: 1183–1195, 2006 [DOI] [PubMed] [Google Scholar]
- 100.Piper RC, Lehner PJ. Endosomal transport via ubiquitination. Trends Cell Biol 21: 647–655, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Poon S, Easterbrook-Smith SB, Rybchyn MS, Carver JA, Wilson MR. Clusterin is an ATP-independent chaperone with very broad substrate specificity that stabilizes stressed proteins in a folding-competent state. Biochemistry 39: 15953–15960, 2000 [DOI] [PubMed] [Google Scholar]
- 102.Powers ET, Balch WE. Diversity in the origins of proteostasis networks: a driver for protein function in evolution. Nat Rev Mol Cell Biol 14: 237–248, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Puertollano R. Interactions of TOM1L1 with the multivesicular body sorting machinery. J Biol Chem 280: 9258–9264, 2005 [DOI] [PubMed] [Google Scholar]
- 104.Rauch S, Martin-Serrano J. Multiple interactions between the ESCRT machinery and arrestin-related proteins: implications for PPXY-dependent budding. J Virol 85: 3546–3556, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Reggiori F, Pelham HR. Sorting of proteins into multivesicular bodies: ubiquitin-dependent and -independent targeting. EMBO J 20: 5176–5186, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Reggiori F, Pelham HRB. A transmembrane ubiquitin ligase required to sort membrane proteins into multivesicular bodies. Nat Cell Biol 4: 117–123, 2002 [DOI] [PubMed] [Google Scholar]
- 107.Reha-Krantz LJ. DNA polymerase proofreading: multiple roles maintain genome stability. Biochim Biophys Acta 1804: 1049–1063, 2010 [DOI] [PubMed] [Google Scholar]
- 108.Ren X, Hurley JH. VHS domains of ESCRT-0 cooperate in high-avidity binding to polyubiquitinated cargo. EMBO J 29: 1045–1054, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Roth DM, Balch WE. Modeling general proteostasis: proteome balance in health and disease. Curr Opin Cell Biol 23: 126–134, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Roxrud I, Raiborg C, Gilfillan GD, Stromme P, Stenmark H. Dual degradation mechanisms ensure disposal of NHE6 mutant protein associated with neurological disease. Exp Cell Res 315: 3014–3027, 2009 [DOI] [PubMed] [Google Scholar]
- 111.Roxrud I, Raiborg C, Pedersen NM, Stang E, Stenmark H. An endosomally localized isoform of Eps15 interacts with Hrs to mediate degradation of epidermal growth factor receptor. J Cell Biol 180: 1205–1218, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Rudiger S, Germeroth L, Schneider-Mergener J, Bukau B. Substrate specificity of the DnaK chaperone determined by screening cellulose-bound peptide libraries. EMBO J 16: 1501–1507, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Rusten TE, Vaccari T, Stenmark H. Shaping development with ESCRTs. Nat Cell Biol 14: 38–45, 2012 [DOI] [PubMed] [Google Scholar]
- 114.Sato BK, Schulz D, Do PH, Hampton RY. Misfolded membrane proteins are specifically recognized by the transmembrane domain of the Hrd1p ubiquitin ligase. Mol Cell 34: 212–222, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Schaheen B, Dang H, Fares H. Derlin-dependent accumulation of integral membrane proteins at cell surfaces. J Cell Sci 122: 2228–2239, 2009 [DOI] [PubMed] [Google Scholar]
- 116.Scheffner M, Nuber U, Huibregtse JM. Protein ubiquitination involving an E1–E2-E3 enzyme ubiquitin thioester cascade. Nature 373: 81–83, 1995 [DOI] [PubMed] [Google Scholar]
- 117.Sharma M, Benharouga M, Hu W, Lukacs GL. Conformational and temperature-sensitive stability defects of the delta F508 cystic fibrosis transmembrane conductance regulator in post-endoplasmic reticulum compartments. J Biol Chem 276: 8942–8950, 2001 [DOI] [PubMed] [Google Scholar]
- 118.Sharma M, Pampinella F, Nemes C, Benharouga M, So J, Du K, Bache KG, Papsin B, Zerangue N, Stenmark H, Lukacs GL. Misfolding diverts CFTR from recycling to degradation: quality control at early endosomes. J Cell Biol 164: 923–933, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Sharma S, Chakraborty K, Muller BK, Astola N, Tang YC, Lamb DC, Hayer-Hartl M, Hartl FU. Monitoring protein conformation along the pathway of chaperonin-assisted folding. Cell 133: 142–153, 2008 [DOI] [PubMed] [Google Scholar]
- 120.Shenoy SK, Lefkowitz RJ. Beta-arrestin-mediated receptor trafficking and signal transduction. Trends Pharmacol Sci 32: 521–533, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Shenoy SK, Modi AS, Shukla AK, Xiao K, Berthouze M, Ahn S, Wilkinson KD, Miller WE, Lefkowitz RJ. Beta-arrestin-dependent signaling and trafficking of 7-transmembrane receptors is reciprocally regulated by the deubiquitinase USP33 and the E3 ligase Mdm2. Proc Natl Acad Sci USA 106: 6650–6655, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Shobab LA, Hsiung GY, Feldman HH. Cholesterol in Alzheimer's disease. Lancet Neurol 4: 841–852, 2005 [DOI] [PubMed] [Google Scholar]
- 123.Sidera K, Samiotaki M, Yfanti E, Panayotou G, Patsavoudi E. Involvement of cell surface HSP90 in cell migration reveals a novel role in the developing nervous system. J Biol Chem 279: 45379–45388, 2004 [DOI] [PubMed] [Google Scholar]
- 124.Sigismund S, Woelk T, Puri C, Maspero E, Tacchetti C, Transidico P, Di Fiore PP, Polo S. Clathrin-independent endocytosis of ubiquitinated cargos. Proc Natl Acad Sci USA 102: 2760–2765, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Stefani F, Zhang L, Taylor S, Donovan J, Rollinson S, Doyotte A, Brownhill K, Bennion J, Pickering-Brown S, Woodman P. UBAP1 is a component of an endosome-specific ESCRT-I complex that is essential for MVB sorting. Curr Biol 21: 1245–1250, 2011 [DOI] [PubMed] [Google Scholar]
- 126.Sydow JF, Cramer P. RNA polymerase fidelity and transcriptional proofreading. Curr Opin Struct Biol 19: 732–739, 2009 [DOI] [PubMed] [Google Scholar]
- 127.Taipale M, Krykbaeva I, Koeva M, Kayatekin C, Westover KD, Karras GI, Lindquist S. Quantitative analysis of HSP90-client interactions reveals principles of substrate recognition. Cell 150: 987–1001, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Traub LM, Lukacs GL. Decoding ubiquitin sorting signals for clathrin-dependent endocytosis by CLASPs. J Cell Sci 120: 543–553, 2007 [DOI] [PubMed] [Google Scholar]
- 129.Trepel J, Mollapour M, Giaccone G, Neckers L. Targeting the dynamic HSP90 complex in cancer. Nat Rev Cancer 10: 537–549, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Tumbarello DA, Waxse BJ, Arden SD, Bright NA, Kendrick-Jones J, Buss F. Autophagy receptors link myosin VI to autophagosomes to mediate Tom1-dependent autophagosome maturation and fusion with the lysosome. Nat Cell Biol 14: 1024–1035, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Urbe S. Ubiquitin and endocytic protein sorting. Essays Biochem 41: 81–98, 2005 [DOI] [PubMed] [Google Scholar]
- 132.Urbe S, Liu H, Hayes SD, Heride C, Rigden DJ, Clague MJ. Systematic survey of deubiquitinase localization identifies USP21 as a regulator of centrosome- and microtubule-associated functions. Mol Biol Cell 23: 1095–1103, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Vagin O, Turdikulova S, Sachs G. The H,K-ATPase beta subunit as a model to study the role of N-glycosylation in membrane trafficking and apical sorting. J Biol Chem 279: 39026–39034, 2004 [DOI] [PubMed] [Google Scholar]
- 134.van Hoof A, Wagner EJ. A brief survey of mRNA surveillance. Trends Biochem Sci 36: 585–592, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Verbeek MM, Otte-Holler I, Veerhuis R, Ruiter DJ, De Waal RM. Distribution of A beta-associated proteins in cerebrovascular amyloid of Alzheimer's disease. Acta Neuropathol (Berl) 96: 628–636, 1998 [DOI] [PubMed] [Google Scholar]
- 136.Wang Q, Chang A. Sphingoid base synthesis is required for oligomerization and cell surface stability of the yeast plasma membrane ATPase, Pma1. Proc Natl Acad Sci USA 99: 12853–12858, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Wang X, Herr RA, Hansen T. Viral and cellular MARCH ubiquitin ligases and cancer. Semin Cancer Biol 18: 441–450, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Wang X, Venable J, LaPointe P, Hutt DM, Koulov AV, Coppinger J, Gurkan C, Kellner W, Matteson J, Plutner H, Riordan JR, Kelly JW, Yates JR, 3rd, Balch WE. Hsp90 cochaperone Aha1 downregulation rescues misfolding of CFTR in cystic fibrosis. Cell 127: 803–815, 2006 [DOI] [PubMed] [Google Scholar]
- 139.Watanabe I, Zhu J, Recio-Pinto E, Thornhill WB. Glycosylation affects the protein stability and cell surface expression of Kv1.4 but not Kv11 potassium channels. J Biol Chem 279: 8879–8885, 2004 [DOI] [PubMed] [Google Scholar]
- 140.Wegele H, Muller L, Buchner J. Hsp70 and Hsp90: a relay team for protein folding. Rev Physiol Biochem Pharmacol 151: 1–44, 2004 [DOI] [PubMed] [Google Scholar]
- 141.Wilson MH, Highfield HA, Limbird LE. The role of a conserved inter-transmembrane domain interface in regulating alpha(2a)-adrenergic receptor conformational stability and cell-surface turnover. Mol Pharmacol 59: 929–938, 2001 [DOI] [PubMed] [Google Scholar]
- 142.Wu ZC, Yu JT, Li Y, Tan L. Clusterin in Alzheimer's disease. Adv Clin Chem 56: 155–173, 2012 [DOI] [PubMed] [Google Scholar]
- 143.Wyatt A, Yerbury J, Poon S, Dabbs R, Wilson M. Chapter 6: The chaperone action of Clusterin and its putative role in quality control of extracellular protein folding. Adv Cancer Res 104: 89–114, 2009 [DOI] [PubMed] [Google Scholar]
- 144.Wyatt AR, Yerbury JJ, Ecroyd H, Wilson MR. Extracellular chaperones and proteostasis. Annu Rev Biochem 82: 295–322 [DOI] [PubMed] [Google Scholar]
- 145.Yadavalli SS, Ibba M. Quality control in aminoacyl-tRNA synthesis its role in translational fidelity. Adv Protein Chem Struct Biol 86: 1–43, 2012 [DOI] [PubMed] [Google Scholar]
- 146.Yerbury JJ, Poon S, Meehan S, Thompson B, Kumita JR, Dobson CM, Wilson MR. The extracellular chaperone clusterin influences amyloid formation and toxicity by interacting with prefibrillar structures. FASEB J 21: 2312–2322, 2007 [DOI] [PubMed] [Google Scholar]
- 147.Yerbury JJ, Stewart EM, Wyatt AR, Wilson MR. Quality control of protein folding in extracellular space. EMBO Rep 6: 1131–1136, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Young JC, Agashe VR, Siegers K, Hartl FU. Pathways of chaperone-mediated protein folding in the cytosol. Nat Rev Mol Cell Biol 5: 781–791, 2004 [DOI] [PubMed] [Google Scholar]
- 149.Younger JM, Ren HY, Chen L, Fan CY, Fields A, Patterson C, Cyr DM. A foldable CFTRDeltaF508 biogenic intermediate accumulates upon inhibition of the Hsc70-CHIP E3 ubiquitin ligase. J Cell Biol 167: 1075–1085, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Zaher HS, Green R. Fidelity at the molecular level: lessons from protein synthesis. Cell 136: 746–762, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Zaliauskiene L, Kang S, Brouillette CG, Lebowitz J, Arani RB, Collawn JF. Down-regulation of cell surface receptors is modulated by polar residues within the transmembrane domain. Mol Biol Cell 11: 2643–2655, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Zhao Y, Macgurn JA, Liu M, Emr S. The ART-Rsp5 ubiquitin ligase network comprises a plasma membrane quality control system that protects yeast cells from proteotoxic stress. Elife 2: e00459, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Zhou P, Fernandes N, Dodge IL, Reddi AL, Rao N, Safran H, DiPetrillo TA, Wazer DE, Band V, Band H. ErbB2 degradation mediated by the co-chaperone protein CHIP. J Biol Chem 278: 13829–13837, 2003 [DOI] [PubMed] [Google Scholar]
- 154.Zhu X, Zhao X, Burkholder WF, Gragerov A, Ogata CM, Gottesman ME, Hendrickson WA. Structural analysis of substrate binding by the molecular chaperone DnaK. Science 272: 1606–1614, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]