Abstract
The mechanisms involved in renal repair by mesenchymal stromal cells (MSCs) are not entirely elucidated. The paracrine secretion of bioactive molecules has been implicated in the protective effects. Besides soluble mediators, MSCs have been shown to release extracellular vesicles (EVs), involved in renal repair process for different injury models. EVs have been shown to mediate communication between cells through the transference of several molecules, like protein, bioactive lipids, mRNA, and microRNAs (miRNAs). The miRNAs are noncoding RNAs that posttranscriptionally modulate gene expression and are involved in the regulation of several cellular processes, including those related to repair. The aim of the present study was to investigate the role of MSC-EVs in the modulation of miRNAs inside renal proximal tubular epithelial cells (PTECs) in an in vitro model of ischemia-reperfusion injury induced by ATP depletion. In this model we evaluated whether changes in miRNA expression were dependent on direct miRNA transfer or on transcription induction by MSC-EVs. The obtained results showed an enhanced incorporation of MSC-EVs in injured PTECs with protection from cell death. This biological effect was associated with EV-mediated miRNA transfer and with transcriptional modulation of miRNAs expressed by injured PTECs. Prediction of miRNA targets showed that miRNAs modulated in PTECs are involved in process of renal recovery with downregulation of coding-mRNAs associated with apoptosis, cytoskeleton reorganization, and hypoxia, such as CASP3 and 7, SHC1 and SMAD4. In conclusion, these results indicate that MSC-EVs may transfer and modulate the expression of several miRNAs involved in the repair and recovery process in PTECs.
Introduction
Mesenchymal stromal cells (MSCs) are instrumental in renal regeneration and functional recovery. The mechanisms by which these cells act are not completely elucidated. However the main mechanism seems to be independent from the differentiation properties of these cells [1,2]. MSCs are transiently recruited in the injured kidney and act by a paracrine mechanism through a direct secretion of cytokines, growth factors, and several other bioactive molecules [3]. The interaction between MSCs and proximal tubular epithelial cells (PTECs) was also shown to be mediated through secretion of extracellular vesicles (EVs) [4]. Ratajczak et al. [5] demonstrated that hematopoietic progenitor cells can be reprogrammed by horizontal transfer of messenger RNAs and proteins by EVs derived from embryonic stem cells. Adult stem cells are also capable to secret EVs containing RNAs that can be transferred to target cells inducing phenotypic changes [6–8]. Based on this observation Quesenberry et al. proposed a new role of EVs in stem cell biology where EVs may shuttle information between stem and injured cells [8]. They also showed that the phenotypic changes in target cells exposed to vesicles were due to transfer of mRNA and transcriptional regulators [9]. In acute kidney injury (AKI) models, the administration of EVs derived from MSCs (MSC-EVs) led to tissue repair by stimulating proliferation and increasing apoptosis resistance of PTECs [4]. The beneficial effects of MSC-EVs were similar to those observed with the administration of MSCs, suggesting that the release of EVs represents an important mechanism in the regenerative action of these cells.
The internalization of EVs is followed by the transfer of several groups of molecules (lipids, proteins, and nuclei acids) [10] capable of inducing phenotypic changes in recipient cells [11]. Among these molecules, microRNAs (miRNAs) play an important role in the modulation of phenotype of recipient cells. In fact, miRNAs are noncoding RNAs that posttranscriptionally regulate genes by mRNA cleavage or translation repression through a sequence-dependent process that regulates the expression of many proteins involved in different cellular pathways, like proliferation, cell death, differentiation, and tumor development [12]. Collino et al. observed different patterns of miRNAs inside MSCs and their secreted MSC-EVs, suggesting a regulated mechanism of miRNA compartmentalization within MSC-EVs [7]. Subsequent analysis of possible miRNA targets inside MSC-EVs suggested that these molecules can regulate important cellular processes related to renal repair.
The aim of the present study was to investigate the role of MSC-EVs in the modulation of miRNAs inside renal PTECs in an in vitro model of ATP depletion injury. In this model we evaluated whether changes in miRNA expression were dependent on direct miRNA transfer or on transcription induction by MSC-EVs. Moreover, we evaluated whether variation in miRNA expression in the PTECs was followed by regulation of genes related to renal recovery and protection from cell death.
Materials and Methods
Renal epithelial cell culture
Human kidney 2 (HK-2) is a PTEC cell line derived from normal kidney (ATCC). The cells were cultured in low-glucose DMEM (Lonza) supplemented with 10% of fetal calf serum (FCS) under a humidified atmosphere of 5% CO2 at 37°C. During assays, the cells were cultured with DMEM in the absence of FCS.
Characterization of bone marrow MSCs
Human MSCs were purchased from Lonza and cultured and characterized as previously described [4,7]. The MSC characterization was performed by fluorescence activated cell sorting (FACS). The antibodies used, all phycoerythrin- or fluorescein-isothiocyanate- conjugated, were as follows: anti-CD146, anti-CD105, and anti-CD90 (Miltenyi Biotech); anti-CD73, anti-CD29, anti-CD34, anti-CD44, anti-CD45, anti-CD80, anti-CD86, anti-CD166, and anti-HLA-I (Becton Dickinson Biosciences Pharmingen). As control we used mouse isotypic IgG from Dakocytomation. MSCs expressed CD44, CD90, CD73, CD105, CD146, CD166, and HLA class I. MSCs did not express CD45, CD14, and CD34 hematopoietic markers and CD80, CD86, and CD40 costimulatory molecules.
Isolation and characterization of MSC-EVs
MSC-EVs were isolated from cell-free supernatants of MSCs and cultured overnight in Roswell Park Memorial Institute (RPMI) medium containing 0.5% of bovine serum albumin (Sigma). To remove debris supernatants were centrifuged first at 300 g and then at 6,000 g for 20 min. Subsequently, supernatants were ultracentrifuged at 150,000 g (Optima L-90K ultracentrifuge; Beckman Coulter) for 1 h at 4°C and the pellets containing MSC-EVs were resuspended in RPMI containing 1% DMSO and stored at −80°C. FACS analysis of MSC-EVs performed using Guava easyCyte™ (Millipore) showed the presence of several MSC markers, such as CD29, CD44, CD73, CD90, CD146, HLA-class I, and alpha-5, but not CD105. In addition MSC-EVs expressed the exosomal markers CD9, CD81, and CD107, but not CD63 (Supplementary Fig. S1). Nanoparticle tracking analysis using NanoSight LM10 was performed to determine size and number of MSC-EVs. The size of MSC-EVs ranged from 50 to 250 nm, with a mean value of 170 nm. The number of MSC-EVs ranged from 1,300 to 4,800 particles/cell, with a mean value of 2,200 particles/cell (corresponding to 2.7×108 particles/mL of medium). Contamination of endotoxin was excluded by Limulus test (Charles River Laboratories, Inc.).
MSC-EV incorporation by PTECs
To determine the MSC-EV incorporation dynamic by PTECs, we incubated the MSC-EVs (3×109 particles/mL) derived from MSCs double-labeled with SYTO® RNASelect and Vybrant® Dil (Fig. 1A) (both from Molecular Probes) with PTECs for periods of 6, 12, and 24 h in normal and injury conditions. The levels of MSC-EV incorporation were analyzed by FACS and confocal microscopy. To determine the specificity of SYTO RNASelect, MSC-EVs were incubated with RNAse as previously described [13]. The MSC-EVs that were RNAse treated were incubated with PTECs for 24 h. The intensity of RNA marker inside PTECs was significantly reduced in comparison to PTECs incubated with not treated MSC-EVs (Supplementary Fig. S2).
FIG. 1.
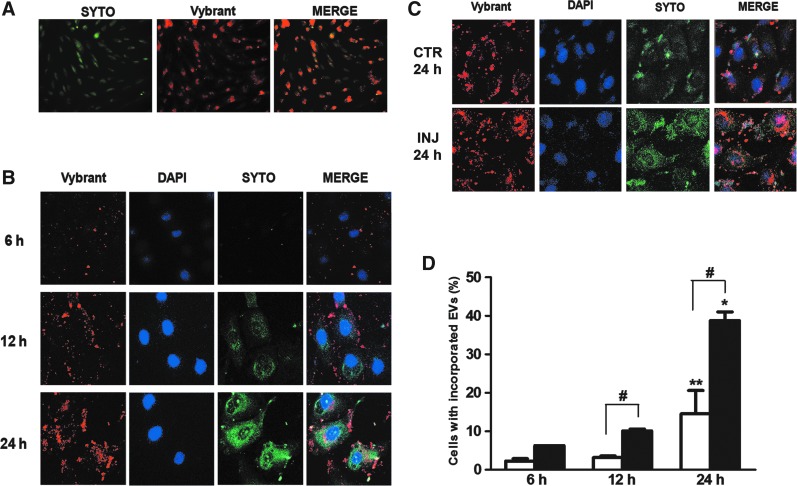
Incorporation of MSC-EVs and RNA transfer in proximal tubular epithelial cells (PTECs). (A) MSCs were double-stained in red (with Vybrant Dil, 15-min incubation) and green (with Syto-RNA, 30-min incubation). Original magnification: ×200. Labeled MSCs released double-labeled EVs (see “Materials and Methods” section). (B) Double-labeled MSC-EVs were incubated for 6, 12, and 24 h with PTECs in normal condition and after ATP depletion injury. The first column of panels from the left shows the internalization of MSC-EV membranes. The second column of panels is the nuclei of PTECs stained with DAPI (blue). The third column of panels shows the distribution of Syto-RNA carried by MSC-EVs inside PTECs. The fourth column of panels shows a merge between the two previous panels. These experiments were realized in normal culture condition. (C) MSC-EV incorporation in PTECs after 24 h of incubation in normal culture condition and after ATP depletion injury. The panel description is the same as indicated above. Three experiments were performed with similar results using MSC-EVs derived from different MSCs. Original magnification: ×630. (D) FACS analysis of Vybrant Dil-labeled MSC-EV incorporation rate by PTECs. White bars represent the experiments realized in normal control conditions and black bars represent incorporation rate by PTECs after ATP depletion injury. Statistical analysis was performed by ANOVA with Newman-Keuls multicomparison test: *,**statistical difference between the 6- and 24-h experimental conditions; #statistical difference between normal and injury conditions, in the same incubation time (P<0.05; n=4). EV, extracellular vesicle; MSCs, mesenchymal stromal cells; FACS, fluorescence activated cell sorting.
To determine the participation of CD29 and CD44 in the MSC-EV incorporation by PTECs, EVs were preincubated (15 min at 4°C) with blocking antibody (1 μg/mL) against adhesion molecule CD29 (β1-integrin; Becton Dickinson) and with hialuronic acid (sHA; 100 μg/mL from Rooster comb; Sigma) to block CD44 and then incubated with the cells. The incorporation was observed by confocal microscopy.
ATP depletion injury model
To promote an injury that mimics important aspects of renal tubule injury during acute kidney ischemia, 60%–70% confluent PTECs were incubated for 1 h in serum-free, low-glucose DMEM in the presence of 10 mM 2-deoxyglucose (Sigma) (to inhibit glycolysis) and 1 μM antimycin A (Sigma) (to block the mitochondrial respiratory chain at the level of complex III). These combinations of inhibitors avoid oxidation of any substrate and lead to almost complete exhaustion of ATP stores [14]. After this period, the cells were washed with PBS and incubated in low-glucose DMEM for 24 h at 37°C and 5% CO2, in the presence (1×109 particles/mL) or absence of MSC-EVs.
Cell death and proliferation analyses
The cell death analysis was performed using the Muse™ Annexin V & Dead Cell Assay (Millipore). The kit allows quantitative analysis of live, early, and late apoptosis. The assays were performed as indicated in the manufacturer's protocols. After submitted to the experimental conditions (normal, ATP depletion, and ATP depletion+MSC-EV conditions), the PTECs were harvested with trypsin, and resuspended in DMEM supplemented with 10% FCS so that the final concentration was 1×105 cells/mL. An aliquot of 100 μL of the cells was then mixed with 100 μL of Muse Annexin V & Dead Cell reagent, incubated for 20 min at room temperature, and subsequently analyzed by the Muse Cell Analyzer (Millipore). TUNEL assay was also performed with ApopTag® In Situ Apoptosis Detection Kit (S7111; Chemicon®) to determine the apoptosis via DNA fragmentation. The assays were performed according to the manufacturer's protocol. The TUNEL-positive cell rate was determined by the number of stained cells in relation to the total number of cells. Estimations were made by counting a total of 500 cells in random fields using fluorescent microscopy at a magnification of ×200. Proliferation was assessed by measuring BrdU incorporation.
Transespithelial resistance
To measure transespithelial resistance (TER), an epithelial Voltohmeter (World Precision Instruments) was used in confluent PTEC monolayers grown on permeable inserts (BD Falcon® 0.4-μm-pore-size PET membrane). Measurements of electrical resistance of cell-free membrane inserts were performed and subtracted from all subsequent measurements. The electrodes were equilibrated in sterile phosphate-buffered saline and placed to the same depth in the solutions bathing the cultured monolayer.
RNA extraction
The mirVana RNA isolation kit (Ambion) was used for RNA extraction from MSC-EVs and cell preparations and RNA was measured by spectrophotometry (Nanodrop ND-1000; Wilmington DE). RNA quality and the presence of small RNAs were evaluated by capillary electrophoresis on an Agilent 2100 Bioanalyzer (Agilent Technologies, Inc.)
miRNA and mRNA profiling by quantitative real-time polymerase chain reaction
To analyze the MSC-EV miRNA content and the changes in the miRNA levels inside renal cell after injury and treatment with MSC-EVs, the Applied Biosystems TaqManH MicroRNA Assay Human Panel Early Access kit (Applied Biosystems) was used to profile 365 mature miRNAs by sequential steps of reverse transcription (Megaplex™ RT Pools; Applied Biosystems) using an Applied Biosystems 7900HT real-time polymerase chain reaction (PCR) instrument. The SDS software version 2.3 was used to calculate Raw Ct values with automatic baseline and threshold [7].
miRNAs screened by microarray analysis were confirmed using miScript Reverse Transcription Kit and miScript SYBR Green PCR Kit (both from Qiagen). The following specific primers to hsa-miR-148b-3p, 375, 410, 495, 548c-3p, 548c-5p, 561, and 886-3p were used. The snoRNA RNU48 was used as normalize reference control. Fold change in miRNA expression was calculated as 2−ΔCt using the geometric mean in Ct values of all the card conditions as normalizer.
The mRNA expression in PTECs was assessed by quantitative real-time PCR using High Capacity cDNA Reverse Transcription Kit (Applied Biosystems) and the Power SYBR® Green PCR Master Mix (Applied Biosystems). Negative cDNA controls (no cDNA) were cycled in parallel with each run. Quantitative real-time polymerase chain reaction (qRT-PCR) was performed using a 96-well StepOne™ Real-Time System (Applied Biosystems). The sequence-specific oligonucleotide primers were all obtained from MWG-Biotech AG, Ebersberg, Germany (www.mwg-biotech.com).
Blockage of transcription in renal cells
PTECs were incubated with 10 μg/mL of actinomycin D (Sigma) in DMEM without FCS for 30 min. After this period the cells were washed with PBS and then submitted to ATP depletion injury and subsequently maintained in culture for 24 h in the presence (1×109 particles/mL) or absence of MSC-EVs. The control group, after incubation with actinomycin D, was maintained in DMEM without FCS.
miRNA target prediction
Predicted miRNA targets were obtained from Targetscan [15], release 6.1, using the “nonconserved targets” list downloaded from the TargetScan Web site. Gene ontology (GO) annotations were obtained from the NCBI Entrez Gene database. A list of genes expressed in HK-2 PTECs was obtained from the microarray data deposited in the Gene Expression Omnibus (www.ncbi.nlm.nih.gov/geo) under accession GSE23338. Gene analysis was performed in three different PTEC preparations and a gene was defined as expressed when present in at least two of them. We then generated a list of mRNAs that are expressed in HK-2 cells and a list of miRNAs that are upregulated when PTECs were treated with MSC-EVs. The GO of selected miRNAs was performed for “actin cytoskeleton reorganization,” “induction of apoptosis,” and “response to hypoxia.”
Statistical analyses
Statistical analyses were carried out using the one-way analysis of variance test and Newman-Keuls or Dunnett post-tests. Statistical significance was set at P<0.05. Data were analyzed using the GraphPad Prism 5.0 Demo program.
Results
MSC-EV incorporation by PTECs
The incorporation of labeled MSC-EVs by PTECs was observed at different culture times by confocal microscopy (Fig. 1B). Initial incorporation was observed after 6-h incubation, followed by a progressive increase until 24 h. Concomitant to EV incorporation, an increase of labeled RNA distribution in PTEC cytoplasm was observed, indicating the transfer of RNA from MSC-EVs to PTECs. To determine whether ATP injury could influence the MSC-EV incorporation, the vesicles were incubated with PTECs after injury and compared with cells cultured in normal condition. The results obtained showed that the MSC-EV incorporation was significantly increased in injured cells (Fig. 1C). FACS analysis showed that the uptake of MSC-EVs by PTECs presents a 2.7-fold increase when compared with normal condition group at 24 h (Fig. 1D).
CD29 and CD44 are involved in the MSC-EV incorporation by PTECs
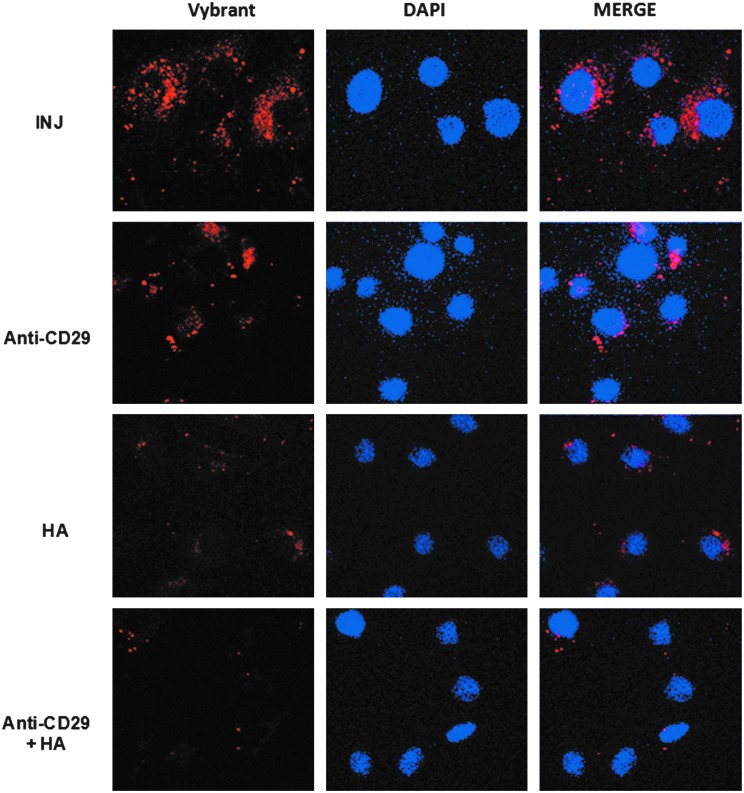
Since the adhesion molecules CD29 and CD44 are present on the surface of MSC-EVs, we evaluated their role in the internalization by PTECs. EVs derived from MSCs stained in red by Vybrant Dil were preincubated with anti-CD29 antibody and/or with sHA to block CD29 and CD44, respectively, and then incubated for 24 h with PTECs after injury (Fig. 2). CD29 and CD44 blockage significantly diminished the internalization of labeled MSC-EVs within PTECs. The simultaneous blockage of CD29 and CD44 almost completely inhibited the EV internalization.
FIG. 2.
Blockage of MSC-EV incorporation by PTECs. MSC-EVs stained with Vybrant Dil (red) were incubated for 24 h with PTECs submitted to ATP depletion injury. To block CD29 and CD44 integrins the MSC-EVs were previously incubated with anti-CD29 antibody, hialuronic acid (HA), or both simultaneously as indicated in the panels. Left panels indicate internalization of MSC-EV membrane. INJ indicated cells submitted to injury without any blockage. Middle panels show the nuclei of PTECs stained with DAPI (blue). Right panels show a merge of the two previous images. Three experiments were performed with similar results using MSC-EVs derived from different MSCs. Original magnification: ×630.
Biological effect of MSC-EVs on PTEC injury
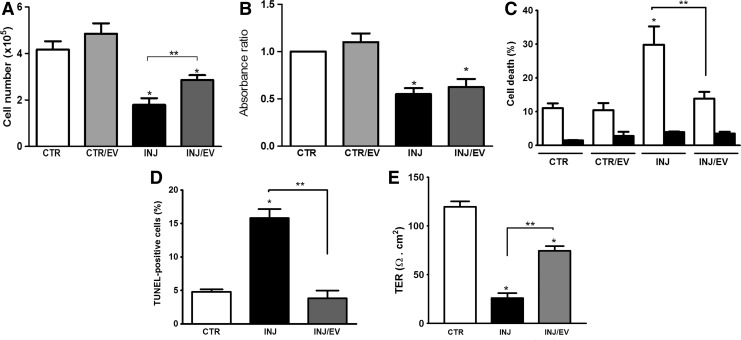
To observe the biological effects of MSC-EVs, cell death, proliferation, and TER in PTECs cultured were evaluated (Fig. 3). The initial analysis showed that MSC-EVs partially reversed the reduction of number of viable cells observed after injury (Fig. 3A). This phenomenon was not due to an increase in the PTEC proliferation (Fig. 3B), but to a protective effect of MSC-EVs, as confirmed by FACS and TUNEL analyses (Fig. 3C, D).
FIG. 3.
MSC-EVs promoted protection but not proliferation in PTECs after injury. After ATP depletion injury, MSC-EVs were incubated with PTECs for 24 h. (A) Number of viable cells by counting with Trypan blue staining. (B) Proliferation was performed by an ELISA for Brdu incorporation. (C) Cell death analysis by Muse Annexin V & Dead Cell Assay. Black bars indicate cell death rate by early apoptosis and white bars represent late apoptosis. (D) Apoptosis was also evaluated by TUNEL and expressed as percentage of positive cells (500 cells were counted in random fields using a fluorescent microscopy at a magnification of ×200). (E) Effect of MSC-EVs on TER of PTECs. TER was measured in all groups before submitted to the different conditions and no significant difference was observed (not shown). Final measures were performed 24 h after the cell incubation with antimycin A. Each group is indicated in the abscissa; in the control group (CTR) the cells were not submitted to injury. CTR/EV represents PTECs that were incubated with MSC-EVs; INJ indicates the PTECs submitted to injury, while INJ/EV is the group submitted to injury and then incubated with MSC-EVs. Statistical analysis was performed by ANOVA with Newman-Keuls multicomparison test: *statistical difference related to the control group; **statistical difference between injured group and injured group treated with MSC-EVs (P<0.05; n=5). ANOVA, analysis of variance; TER, transespithelial resistance.
The ATP depletion injury promotes disruption of renal epithelia integrity, affecting directly its function [16]. Evaluation of TER as a functional marker of epithelial integrity showed that ATP depletion injury induced a significant loss of TER (Fig. 3E). MSC-EVs significantly reduced the loss of TER, suggesting a protective effect on PTEC function.
Identification and modulation of mature miRNAs inside PTECs treated with MSC-EVs
miRNA content of MSC-EVs was evaluated. Table 1 shows the 20 miRNAs more expressed in the MSC-EVs that were confirmed by RT-PCR. miRNA content of PTECs was also evaluated in basal condition, after injury, and after incubation with MSC-EVs. Injury induced significant variations of several miRNAs within PTECs (Table 2). Some of the miRNAs were upregulated (h-miR-224, 296, 450, 548a, 548d, 570, 616, 618, 627, 642, 651, 655, and 873) and others were downregulated (h-miR-125a-3p, 148b-3p, 150, 219, 335, 451, 485-3p, 495, 518, 548c-3p, and 576-5p). MSC-EV treatment partially or completely reversed some of miRNA changes observed after injury as indicated in Table 2. In addition, some miRNAs that did not change during injury were modulated by MSC-EVs.
Table 1.
Identification of microRNAs Carried by Mesenchymal Stromal Cell–Extracellular Vesicles
| miRNAs more expressed inside MSC-EVs | |||
|---|---|---|---|
| MSC-EVs miRNAs | 2−ΔCt | MSC-EVs miRNAs | 2−ΔCt |
| miR-222 | 202 | miR-193b | 27 |
| miR-145 | 185 | h-let-7e | 24 |
| miR-125b | 93 | miR-191 | 23 |
| miR-199a-3p | 92 | miR-221 | 23 |
| miR-21 | 79 | miR-31 | 23 |
| miR-100 | 69 | h-let-7a | 14 |
| h-let-7b | 53 | miR-30b | 13 |
| miR-99a | 47 | miR-17 | 12 |
| miR-24 | 45 | miR-106a | 12 |
| miR-19b | 32 | miR-26a | 12 |
The table shows the fold change analysis of the 20 miRNAs more expressed in the MSC-EVs. The relative expression of miRNAs in MSC-EVs was defined as fold change evaluated as 2−ΔCt, as described in “Materials and Methods” section.
EV, extracellular vesicle; miRNAs, microRNAs; MSCs, mesenchymal stromal cells.
Table 2.
Changes in the Expression of microRNAs Inside Proximal Tubular Epithelial Cells
| Changes of miRNA expression inside PTECs | |||||
|---|---|---|---|---|---|
| miRNAs upregulated in injury | Treatment with MSC-EVs | miRNA downregulated in injury | Treatment with MSC-EVs | miRNAs upregulated only with MSC-EVs | miRNAs downregulated only with MSC-EVs |
| miR-224 | Reverted | miR-125a-3p | Not reverted | • let-7a | miR-217 |
| miR-296 | Reverted | • miR-148b-3p | Reverted | • miR-375 | miR-450b-5p |
| miR-450a | Reverted | miR-150 | Not reverted | • miR-410 | miR-548d-5p |
| miR-548a | Reverted | miR-219 | Not reverted | • miR-548c-5p | |
| miR-548d | Not reverted | miR-335 | Not reverted | • miR-561 | |
| miR-570 | Reverted | • miR-451 | Reverted | • miR-886-3p | |
| miR-616 | Reverted | • miR-485-3p | Reverted | ||
| miR-618 | Not reverted | • miR-495 | Reverted | ||
| miR-627 | Not reverted | miR-518 | Not reverted | ||
| miR-651 | Not reverted | miR-522 | Reverted | ||
| miR-642 | Reverted | • miR-548c-3p | Reverted | ||
| miR-655 | Reverted | ||||
List of miRNAs that significantly varied after injury and treatment with MSC-EVs in respect to the control (fold change ≥1.8). The PTECs cultivated in normal condition were established as the parameter to determine the variations in the miRNA expression (up- and downregulated). The relative expression of miRNAs of PTECs in the different conditions was defined as fold change evaluated as 2−ΔCt, as described in “Materials and Methods” section.
The symbol “•” indicates miRNAs that are possibly transferred or expression induced by MSC-EVs.
PTECs, proximal tubular epithelial cells.
In this condition, some miRNAs were downregulated (h-miRNA-217, 450b-5p, and 548d-5p) and others were upregulated (h-let7-a, h-miRNA 375, 410, 548c-5p, 561, and 886-3p).
miRNAs transferred or expression induced by MSC-EVs
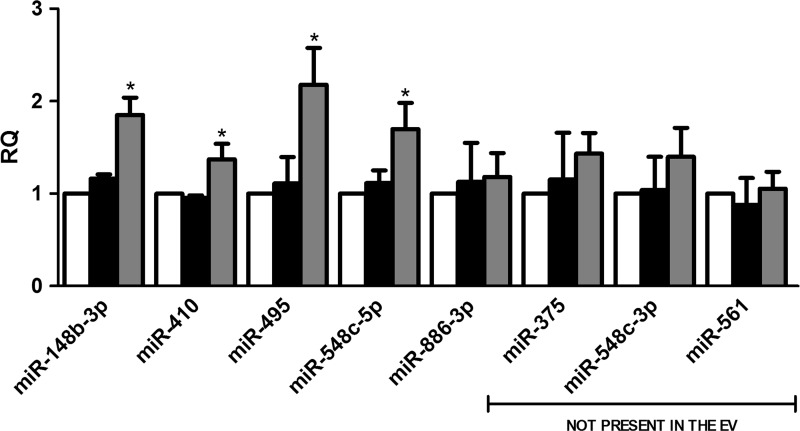
To assess whether miRNA variation in MSC-EV-treated PTECs was dependent on the delivery of miRNAs by MSC-EVs or on transcription induced within the recipient cells, we performed selected experiments in the presence of transcription inhibitor actinomycin D (Fig. 4). We observed an increase in the expression of miR-148b-3p, miR-410, miR-495, and miR-548c-5p within the recipient cells despite transcription inhibition, suggesting a direct transfer of these miRNAs. The effective transfer cannot be seen when basal levels of miRNA in the recipient cells were already high. This is the case of miR-886-3p that was present in the MSC-EVs but its increase inside of PTECs treated with the vesicles was not observed after transcription blockage. In fact the amount of miR-886-3p present in MSC-EVs was significantly lower than that present in PTECs in basal condition. Upregulation of miR-375, miR-548c-3p, miR-561, and miR-886-3p was inhibited by actinomycin D, which indicates that the increase was dependent on MSC-EV stimulation rather than by direct transfer. These results were supported by the screening of miRNAs present inside the MSC-EVs (Supplementary Table S1). Some miRNAs, not present in the vesicles, were upregulated inside PTECs after MSC-EV treatment, suggesting that vesicles stimulate their transcription. Moreover, several miRNAs were downregulated in PTECs after MSC-EV stimulation (Table 2), indicating that variation of miRNA content observed after incorporation of MSC-EVs was also dependent on transcription modulation.
FIG. 4.
Characterization of miRNAs transferred or upregulated by MSC-EVs. PTECs were first incubated with actinomycin D. After transcription blockage, the cells were submitted to injury and treated or not with MSC-EVs. White bars indicate control group maintained in normal condition. Black bars represent PTECs submitted to injury and gray bars represent the cells treated with MSC-EVs after injury. Three experiments were performed in triplicate. Analysis of upregulated miRNAs was performed by quantitative real-time polymerase chain reaction. The abscissa indicates the miRNAs evaluated. Data are expressed as relative quantification (RQ), normalized to RNU48. Statistical analysis was performed by ANOVA with Dunnett multicomparison test: *statistical difference to the injured group (P<0.05; n=6).
miRNA target prediction and modulation by MSC-EVs
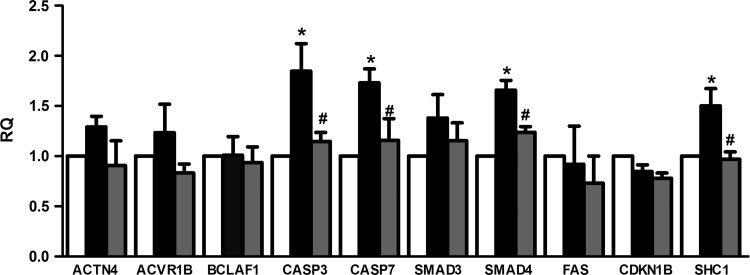
Among the modulated miRNAs, we were interested in those directly transferred (miRNAs detected inside MSC-EVs) or those whose expression was induced by EVs (miRNAs not present inside MSC-EVs) in the PTECs: let7-a, miR-148b-3p, 375, 410, 451, 485-3p, 495, 522, 548c-3p, 548c-5p, 561, and 886-3p (marked with black dot in Table 2). To determine the involvement of these miRNAs in the recovery process, we performed a GO analysis based on genes expressed on HK-2 PTECs and that were involved in important process of ATP depletion injury: cell death by apoptosis, cytoskeleton reorganization, and hypoxia (Supplementary Table S2). From the group of predicted targets, we choose those in which the downregulation was associated with an improvement in renal recovery (Table 3). A subsequent analysis on the modulation of these genes revealed that caspase-3 (CASP3), caspase 7 (CASP7), SMAD family member 4 (SMAD4), and Src homology 2 domain containing transforming protein 1 (SHC1) genes were upregulated after injury. The incubation of MSC-EVs after injury inhibited this upregulation (Fig. 5).
Table 3.
Gene Ontology Biological Functions of Upregulated microRNA Targets Inside Proximal Tubular Epithelial Cells
| Predicted targets of upregulated miRNAs in PTECs | |||
|---|---|---|---|
| Gene | Symbol | Process involved | Targeted by miRNA |
| Actinin, alpha 4 | ACTN4 | Hypoxia | miR-410, 485-3p, 548c-5p |
| Activin A receptor, type B | ACVR1B | Apoptosis | miR-148b-3p, 410, 495, h-let-7a |
| BCL2-associated transcription factor 1 | BCLAF1 | Apoptosis | miR-410, 495, 548c-5p, 561 |
| Caspase 3, apoptosis-related cysteine peptidase | CASP3 | Apoptosis | miR-410, 495, 548c-5p, h-let-7a |
| Caspase 7, apoptosis-related cysteine peptidase | CASP7 | Apoptosis | miR-375, 495, 548c-5p |
| Cyclin-dependent kinase inhibitor 1B (p27, Kip1) | CDKN1B | Apoptosis | miR-148b-3p, 485-3p, 495, 561 |
| Fas cell surface death receptor | FAS | Apoptosis | miR-410, 485-3p, 561, h-let-7a |
| SHC (Src homology 2 domain containing) transforming protein 1 | SHC1 | Cytoskeleton | miR-375, 548c-5p, 561 |
| SMAD family member 3 | SMAD3 | Apoptosis Hypoxia |
miR-410, 485-3p, 548c-5p, 561 |
| SMAD family member 4 | SMAD4 | Hypoxia | miR-410, 485-3p, 495, 548c-5p, 561 |
Gene ontology biological functions of predicted targets of miRNAs related to recovery process (apoptosis, cytoskeleton reorganization, and hypoxia) in PTECs. The list of predicted targets was established using as background all genes expressed in PTECs (HK-2 cells) and predicted to be a target of at least three miRNAs.
FIG. 5.
Changes in the expression of predicted miRNA targets modulated by MSC-EVs. Evaluation of the gene expression of miRNA targets predicted by GO analysis related to hypoxia, cytoskeleton reorganization, and apoptosis processes. The changes in gene expression were performed by quantitative real-time polymerase chain reaction. The analysis was performed in all three conditions: control (white bars), injury (black bars), and injury treated with MSC-EVs (gray bars). Data are expressed as RQ, normalized to GAPDH. The abscissa indicated the evaluated genes. Statistical analysis was performed by ANOVA with Dunnett multicomparison test: *statistical difference to the control group; #statistical difference to the injured group (P<0.05; n=4).
Discussion
In this study, we demonstrated that EVs secreted by MSCs protect renal epithelial cells after ATP depletion injury. The uptake of MSC-EVs by PTECs was increased after injury, resulting in cell death reduction and maintenance of TER. The incorporation of MSC-EVs modulated several miRNAs inside renal cells that were related to important processes in renal recovery.
Several studies demonstrated that MSCs favor renal recovery after AKI [17–20] and protect against chronic kidney disease [21–24]. The paracrine secretion has been shown to be the main mechanism related to the injured recovery, stimulating tubular surviving cells that reenter into cycle and proliferate, promoting a recovery in renal epithelia integrity [18, 25–33]. Recently, MSCs have been shown to be capable to secrete EVs that are small vesicles that compartmentalize several bioactive molecules and can interact through specific receptor–ligands with target cells, consequent transfer of proteins, lipids, and RNAs [5,10]. Moreover, our group demonstrated the EV-mediated transfer of functional mRNAs both in vitro and in vivo [4,34,35] and of miRNAs in vitro [7]. In the present study we observed a progressive increase in the MSC-EV incorporation with time, resulting also in the increase of RNA delivery inside the cells in normal and in injury conditions. Interestingly, MSC-EV incorporation rates were higher after PTECs were submitted to ATP depletion, indicating that under injury condition the renal cells were more responsive to the effects mediated by MSC-EVs. In addition, CD29 and CD44 integrins, present in the MSC-EV membrane, were directly involved in the renal uptake after injury. The participation of these integrins in the EV uptake was supported by experiments based on inhibition of EV incorporation after CD29/CD44 blockage [4]. The incorporation of MSC-EVs led to the protection of PTECs from apoptosis. Bruno et al. also pointed to the protective effect of MSC-EVs in ischemia-reperfusion model in vivo with effects similar to that observed with MSC administration [4].
The comparative screening of miRNA content in EVs and in normal-, injured-, and EV-treated PTECs revealed that miRNAs were modulated during injury and recovery. miRNAs are known to play an important role in the regulation of process involved in renal pathology, like proliferation, cell cycle, phenotype, and death [12]. miRNA involvement in the ischemia/reperfusion-induced AKI was suggested by experiments that show attenuated renal ischemic damage after miRNA depletion using knock-out mouse for Dicer, an enzyme involved in the maturation of miRNAs [36]. Subsequent studies pointed to the participation of miRNAs in the prevention of tubular cell death after ischemic injury [37–39]. In the present study, miRNA prediction targets indicated a possible regulation of apoptosis by miR-410, miR-495, miR-548c-5p, and let-7a that target CASP3 and miR-375, miR-495, and miR-548c-5p that target CASP7. Activation of caspases occurred before DNA fragmentation or cell death, and administration of pan-caspase blocking antibodies protected against hypoxia-induced damage [40,41]. The administration of MSC-EVs promoted reduction of CASP3 and CASP7, suggesting that these upregulated miRNAs may be, at least in part, responsible for the reduction of renal cell death promoted by ATP depletion (Fig. 5).
Another group of upregulated miRNAs—miR-375, miR-548c-5p, and miR-561—may be responsible for the downregulation of SHC1 that could be involved in the protective effect of renal cells. SHC1, also known as p66shc, is a signaling protein implicated in receptor tyrosine kinase signal transduction that is involved in the polymerization of actin [42]. Moreover, SHC1 is a recognized mediator of mitochondrial dysfunction, whose activation is associated with excessive generation of reactive oxygen species that depolarize the mitochondria [43]. SHC1 also contributes to cell death by inhibiting the prosurvival EGFR-ras-ERK pathway [44]. We also found that SMAD4 was reduced by MSC-EV incubation. Smad4 plays an important role in the epithelial-mesenchymal transition induced by TGF-β1 [45,46]. TGF-β1 promotes fibrosis by phosphorylation of Smad2 and Smad3 that form a complex with Smad4 and is translocated to the nuclei to regulated target genes [47]. The role of Smad4 in the fibrotic process inhibition seems to be associated with its capacity to influence the association of Smad3 with collagen promoter regions [48,49]. The modulation of SMAD4 by MSC-EVs suggests a participation of these vesicles in the prevention of fibrosis process that is associated with renal mass reduction and impairment of tubule regeneration.
In conclusion, our results show that renal cells increased MSC-EV uptake after injury and that the protective effects promoted by these vesicles was, at least in part, mediated by the transfer or induction of miRNAs that regulate important targets related to cell recovery.
Supplementary Material
Acknowledgments
Research reported in this publication was supported by a grant from Fresenius Medical Care; by grant from Associazione Italiana per la Ricerca sul Cancro (AIRC); by the Ministry of Health/DECIT, Ministry of Science and Technology/CNPq, Ministry of Education/CAPES, and Carlos Chagas Filho Rio de Janeiro State Foundation for Research Support/FAPERJ (Brazil); and by the National Center For Advancing Translational Sciences of the National Institutes of Health under award number UH2TR000880. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author Disclosure Statement
C.T. (Fresenius Medical Care) is employed by commercial companies and contributed to the study as researchers. C.T. and G.C. are named inventors in related patents.
References
- 1.Bussolati B, Hauser PV, Carvalhosa R. and Camussi G. (2009). Contribution of stem cells to kidney repair. Curr Stem Cell Res Ther 4:2–8 [DOI] [PubMed] [Google Scholar]
- 2.Asanuma H, Meldrum DR. and Meldrum KK. (2010). Therapeutic applications of mesenchymal stem cells to repair kidney injury. J Urol 184:26–33 [DOI] [PubMed] [Google Scholar]
- 3.Camussi G, Deregibus MC. and Tetta C. (2010). Paracrine/endocrine mechanism of stem cells on kidney repair: role of microvesicle-mediated transfer of genetic information. Curr Opin Nephrol Hypertens 19:7–12 [DOI] [PubMed] [Google Scholar]
- 4.Bruno S, Grange C, Deregibus MC, Calogero RA, Saviozzi S, Collino F, Morando L, Busca A, Falda M, et al. (2009). Mesenchymal stem cell-derived microvesicles protect against acute tubular injury. J Am Soc Nephrol 20:1053–1067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ratajczak J, Miekus K, Kucia M, Zhang J, Reca R, Dvorak P. and Ratajczak MZ. (2006). Embryonic stem cells-derived microvesicles reprogram hematopoietic progenitors: evidence for horizontal transfer of mRNA and protein delivery. Leukemia 20:847–856 [DOI] [PubMed] [Google Scholar]
- 6.Deregibus MC, Cantaluppi V, Calogero R, Lo Iacono M, Tetta C, Biancone L, Bruno S, Bussolati B. and Camussi G. (2007). Endothelial progenitor cell-derived microvescicles activate an angiogenic program in endothelial cells by a horizontal transfer of mRNA. Blood 110:2440–2448 [DOI] [PubMed] [Google Scholar]
- 7.Collino F, Deregibus MC, Bruno S, Sterpone L, Aghemo G, Viltono L, Tetta C. and Camussi G. (2010). Microvesicles derived from adult human bone marrow and tissue specific mesenchymal stem cells shuttle selected pattern of miRNAs. PLoS One 5:e11803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Quesenberry PJ, Dooner MS. and Aliotta JM. (2010). Stem cell plasticity revisited: the continuum marrow model and phenotypic changes mediated by microvesicles. Exp Hematol 38:581–592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aliotta JM, Pereira M, Li M, Amaral A, Sorokina A, Dooner MS, Sears EH, Brilliant K, Ramratnam B, Hixson DC. and Quesenberry PJ. (2012). Stable cell fate changes in marrow cells induced by lung-derived microvesicles. J Extracell Vesicles 16:1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Raposo G. and Stoorvogel W. (2013). Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol 200:373–383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Quesenberry PJ. and Aliotta JM. (2010). Cellular phenotype switching and microvesicles. Adv Drug Deliv Rev 62:1141–1148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bartel DP. (2004). MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 116:281–297 [DOI] [PubMed] [Google Scholar]
- 13.Fonsato V, Collino F, Herrera MB, Cavallari C, Deregibus MC, Cisterna B, Bruno S, Romagnoli R, Salizzoni M, Tetta C. and Camussi G. (2012). Human liver stem cell-derived microvesicles inhibit hepatoma growth in SCID mice by delivering antitumor microRNAs. Stem Cells 30:1985–1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dagher PC. (2000). Modeling ischemia in vitro: selective depletion of adenine and guanine nucleotide pools. Am J Physiol Cell Physiol 279:C1270–C1277 [DOI] [PubMed] [Google Scholar]
- 15.Friedman RC, Farh KK, Burge CB. and Bartel DP. (2009). Most mammalian mRNAs are conserved targets of microRNAs. Genome Res 19:92–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schwartz JH, Shih T, Menza SA. and Lieberthal W. (1999). ATP depletion increases tyrosine phosphorylation of beta-catenin and plakoglobin in renal tubular cells. J Am Soc Nephrol 10:2297–2305 [DOI] [PubMed] [Google Scholar]
- 17.Herrera MB, Bussolati B, Bruno S, Fonsato V, Romanazzi GM. and Camussi G. (2004). Mesenchymal stem cells contribute to the renal repair of acute tubular epithelial injury. Int J Mol Med 14:1035–1041 [PubMed] [Google Scholar]
- 18.Tögel F, Hu Z, Weiss K, Isaac J, Lange C. and Westenfelder C. (2005). Administered mesenchymal stem cells protect against ischemic acute renal failure through differentiation-independent mechanisms. Am J Physiol Renal Physiol 289:F31–F42 [DOI] [PubMed] [Google Scholar]
- 19.Morigi M, Introna M, Imberti B, Corna D, Abbate M, Rota C, Rottoli D, Benigni A, Perico N, et al. (2008). Human bone marrow mesenchymal stem cells accelerate recovery of acute renal injury and prolong survival in mice. Stem Cells 26:2075–2082 [DOI] [PubMed] [Google Scholar]
- 20.Beiral HJ, Rodrigues-Ferreira C, Fernandes AM, Gonsalez SR, Mortari NC, Takiya CM, Sorenson MM, Figueiredo-Freitas C, Galina A. and Vieyra A. (2014). The impact of stem cells on electron fluxes, proton translocation and ATP synthesis in kidney mitochondria after ischemia/reperfusion. Cell Transplant 23:207–220 [DOI] [PubMed] [Google Scholar]
- 21.Semedo P, Correa-Costa M, Antonio Cenedeze M, Maria Avancini Costa Malheiros D, Antonia dos Reis M, Shimizu MH, Seguro AC. and Pacheco-Silva A. (2009). Mesenchymal stem cells attenuate renal fibrosis through immune modulation and remodelling properties in a rat remnant kidney model. Stem Cells 27:3063–3073 [DOI] [PubMed] [Google Scholar]
- 22.Choi S, Park M, Kim J, Hwang S, Park S. and Lee Y. (2009). The role of mesenchymal stem cells in the functional improvement of chronic renal failure. Stem Cells Dev 18:521–529 [DOI] [PubMed] [Google Scholar]
- 23.Choi SJ, Kim JK. and Hwang SD. Mesenchymal stem cell therapy for chronic renal failure. Expert Opin Biol Ther 10:1217–1226 [DOI] [PubMed] [Google Scholar]
- 24.Verdoorn KS, Lindoso RS, Lowe J, Lara LS, Vieyra A. and Einicker-Lamas M. (2010). Bone marrow mononuclear cells shift bioactive lipid pattern in injured kidney towards tissue repair in rats with unilateral ureteral obstruction. Nephrol Dial Transplant 25:3867–3874 [DOI] [PubMed] [Google Scholar]
- 25.Lindoso RS, Araujo DS, Adão-Novaes J, Mariante RM, Verdoorn KS, Fragel-Madeira L, Caruso-Neves C, Linden R, Vieyra A. and Einicker-Lamas M. (2011). Paracrine interaction between bone marrow-derived stem cells and renal epithelial cells. Cell Physiol Biochem 28:267–278 [DOI] [PubMed] [Google Scholar]
- 26.Togel F, Weiss K, Yang Y, Hu Z, Zhang P. and Westenfelder C. (2007). Vasculotropic, paracrine actions of infused mesenchymal stem cells are important to the recovery from acute kidney injury. Am J Physiol Renal Physiol 292:F1626–F1635 [DOI] [PubMed] [Google Scholar]
- 27.Bi B, Schmitt R, Israilova M, Nishio H. and Cantley LG. (2007). Stromal cells protect against acute tubular injury via an endocrine effect. J Am Soc Nephrol 18:2486–2496 [DOI] [PubMed] [Google Scholar]
- 28.Tögel F, Zhang P, Hu Z. and Westenfelder C. (2009). VEGF is a mediator of the renoprotective effects of multipotent marrow stromal cells in acute kidney injury. J Cell Mol Med 13:2109–2114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bi B, Guo J, Marlier A, Lin SR. and Cantley LG. (2008). Erythropoietin expands a stromal cell population that can mediate renoprotection. Am J Physiol Renal Physiol 295:F1017–F1022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cantaluppi V, Biancone L, Romanazzi GM, Figliolini F, Beltramo S, Galimi F, Camboni MG, Deriu E, Conaldi P, et al. (2008). Macrophage stimulating protein may promote tubular regeneration after acute injury. J Am Soc Nephrol 19:1904–1918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Caplan AI. and Dennis JE. (2006). Mesenchymal stem cells as trophic mediators. J Cell Biochem 98:1076–1084 [DOI] [PubMed] [Google Scholar]
- 32.Vogetseder A, Picard N, Gaspert A, Walch M, Kaissling B. and Le Hir M. (2008). Proliferation capacity of the renal proximal tubule involves the bulk of differentiated epithelial cells. Am J Physiol Cell Physiol 294:C22–C28 [DOI] [PubMed] [Google Scholar]
- 33.Gnecchi M, Zhang Z, Ni A. and Dzau VJ. (2008). Paracrine mechanisms in adult stem cell signaling and therapy. Circ Res 103:1204–1219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Herrera MB, Fonsato V, Gatti S, Deregibus MC, Sordi A, Cantarella D, Calogero R, Bussolati B, Tetta C. and Camussi G. (2009). Human liver stem cell-derived microvesicles accelerate hepatic regeneration in hepatectomized rats. J Cell Mol Med 14:1605–1618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bruno S, Grange C, Collino F, Deregibus MC, Cantaluppi V, Biancone L, Tetta C. and Camussi G. (2012). Microvesicles derived from mesenchymal stem cells enhance survival in a lethal model of acute kidney injury. PLoS One 7:e33115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wei Q, Bhatt K, He HZ, Mi QS, Haase VH. and Dong Z. (2010). Targeted deletion of Dicer from proximal tubules protects against renal ischemia-reperfusion injury. J Am Soc Nephrol 21:756–761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Godwin JG, Ge X, Stephan K, Jurisch A, Tullius SG. and Iacomini J. (2010). Identification of a microRNA signature of renal ischemia reperfusion injury. Proc Natl Acad Sci U S A 107:14339–14344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kaucsár T, Révész C, Godó M, Krenács T, Albert M, Szalay CI, Rosivall L, Benyó Z, Bátkai S, et al. (2013). Activation of the miR-17 family and miR-21 during murine kidney ischemia-reperfusion injury. Nucleic Acid Ther 23:344–354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Saikumar J, Hoffmann D, Kim TM, Gonzalez VR, Zhang Q, Goering PL, Brown RP, Bijol V, Park PJ, Waikar SS. and Vaidya VS. (2012). Expression, circulation, and excretion profile of microRNA-21, -155, and -18a following acute kidney injury. Toxicol Sci 129:256–267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kaushal GP, Ueda N. and Shah SV. (1997). Role of caspases (ICE/CED 3 proteases) in DNA damage and cell death in response to a mitochondrial inhibitor, antimycin A. Kidney Int 52:438–445 [DOI] [PubMed] [Google Scholar]
- 41.Edelstein CL, Shi Y. and Schrier RW. (1999). Role of caspases in hypoxiainduced necrosis of rat renal proximal tubules. J Am Soc Nephrol 10:1940–1949 [DOI] [PubMed] [Google Scholar]
- 42.Natalicchio A, Tortosa F, Perrini S, Laviola L. and Giorgino F. (2011). p66Shc, a multifaceted protein linking Erk signalling, glucose metabolism, and oxidative stress. Arch Physiol Biochem 117:116–124 [DOI] [PubMed] [Google Scholar]
- 43.Orsini F, Migliaccio E, Moroni M, Contursi C, Raker VA, Piccini D, Martin-Padura I, Pelliccia G, Trinei M, et al. (2004). The life span determinant p66Shc localizes to mitochondria where it associates with mitochondrial heat shock protein 70 and regulates trans-membrane potential. J Biol Chem 279:25689–25695 [DOI] [PubMed] [Google Scholar]
- 44.Natalicchio A, Laviola L, De Tullio C, Renna LA, Montrone C, Perrini S, Valenti G, Procino G, Svelto M. and Giorgino F. (2004). Role of p66SHC isoform in IGF-I receptor signal through MEK/ERK and regulation of actin cytoskeleton in rat myoblasts. J Biol Chem 279:43900–43909 [DOI] [PubMed] [Google Scholar]
- 45.Liu SF, Chang SY, Lee TC, Chuang LY, Guh JY, Hung CY, Hung TJ, Hung YJ, Chen PY, Hsieh PF. and Yang YL. (2012). Dioscorea alata attenuates renal interstitial cellular fibrosis by regulating Smad- and epithelial-mesenchymal transition signaling pathways. PLoS One 7:e47482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Meng XM, Huang XR, Xiao J, Chung AC, Qin W, Chen HY. and Lan HY. (2012). Disruption of Smad4 impairs TGF-β/Smad3 and Smad7 transcriptional regulation during renal inflammation and fibrosis in vivo and in vitro. Kidney Int 81:266–279 [DOI] [PubMed] [Google Scholar]
- 47.Lan HY. (2011). Diverse roles of TGF-β/Smads in renal fibrosis and inflammation. Int J Biol Sci 7:1056–1067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Verrecchia F, Vindevoghel L, Lechleider RJ, Uitto J, Roberts AB. and Mauviel A. (2001). Smad3/AP-1 interactions control transcriptional responses to TGF-beta in a promoter-specific manner. Oncogene 20:3332–3340 [DOI] [PubMed] [Google Scholar]
- 49.Vindevoghel L, Lechleider RJ, Kon A, de Caestecker MP, Uitto J, Roberts AB. and Mauviel A. (1998). SMAD3/4-dependent transcriptional activation of the human type VII collagen gene (COL7A1) promoter by transforming growth factor beta. Proc Natl Acad Sci U S A 95:14769–14774 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.