Abstract
The use of prostaglandins and NSAIDS in the clinic has proven that lipid mediators and their associated pathways make attractive therapeutic targets. When contemplating therapies involving lipid pathways, several basic agents come to mind. There are the enzymes and accessory proteins that lead to the metabolism of lipid substrates, provided through diet or through actions of lipases, the subsequent lipid products, and finally the lipid sensors or receptors. There is abundant evidence that molecules along this lipid continuum can serve as prognostic and diagnostic indicators and are in fact viable therapeutic targets. Furthermore, lipids themselves can be used as therapeutics. Despite this, the vernacular dialog pertaining to “biomarkers” does not routinely include mention of lipids, though this is rapidly changing. Collectively these agents are becoming more appreciated for their respective roles in diverse disease processes from cancer to preterm labor and are receiving their due appreciation after decades of ground work in the lipid field. By relating examples of disease processes that result from dysfunction along the lipid continuum, as well as examples of lipid therapies and emerging technologies, this review is meant to inspire further reading and discovery.
Keywords: Cancer, Bioactive lipids, Raman, Therapeutics, Biomarkers, Drug synergism
1. Introduction
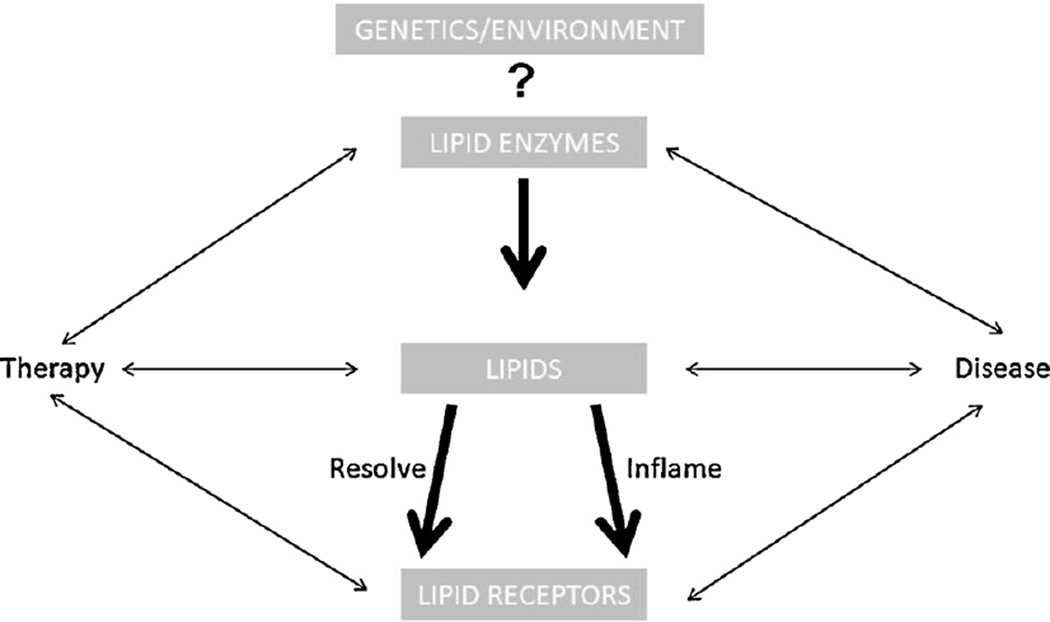
The imbalance between lipid mediators that regulate inflammation or resolution, leads to a number of diseases, many of which are only now being appreciated for their underlying lipid origins. “Physiological” inflammation has its place. In the gastrointestinal tract it is necessary for proper development of the mucosal immune system, and is a tempered response that does not manifest in disease [1]. Ovulation can be considered another example of regulated physiological inflammation [2–4]. However, dysregulated lipid signaling can lead to a host of ailments including fibrosis, cancer, and neurological disorders to name a few. The term rheostat has been used frequently in the lipids field to refer to the yin and yan, or balance of activities along the continuum depicted in Fig. 1. A case can be made for nearly every direction suggested by the figure and for nearly all lipid pathways. There are volumes written on lipid mediators or bioactive lipids and their associated pathways, these include the leukotrienes, lysophospholipids, prostaglandins, platelet activating factor, endocannabinoids, and prostanoids. Collectively the pathways with their receptors, enzymes, accessory proteins and associated lipid products are the subject of intense development for their usefulness as diagnostic and prognostic biomarkers as well as therapeutic targets, particularly in cancer. While this cast of players is better known for the roles they play in many diseases as a result of environment (e.g., diet) or genetics, it is the context of the activity along lipid pathways that determines outcome with regard to disease development, prevention or healing. New characters continue to emerge including novel receptors that are providing additional therapeutic targets, and lipids themselves are being used therapeutically.
Fig. 1. The continuum of bioactive lipid pathways.
Whether prostaglandins or resolvins bind lipid receptors in a therapeutic context, or HETE molecules interact with receptors to push cancer toward metastasis, there is evidence to support nearly every direction and sub circuit depicted on the schematic. Every major class of lipid enzymes has been determined to contribute in some form or other to disease progression and/or healing. Lipidomics, systems biology, and biophysics technologies are paving the paths between the mile markers established over decades of ground breaking work.
Recommended reading: Chemical Reviews, Special Issue: 2011 Lipid Biochemistry, Metabolism, and Signaling (October 2011) 111(10). ISSN 0009-2665 Useful sites:
http://www.lipidomicnet.org/index.php/Main_Page
http://en.wikipedia.org/wiki/Raman_spectroscopy)
http://en.wikipedia.org/wiki/Coherent_anti-Stokes_Raman_spectroscopy.
Instead of a class by class comparison, which would again require volumes, the purpose of this review is to relate some loosely associated new and revisited ideas from the overall field by using pointed examples as they relate to the theme of this issue, and to highlight some exciting technologies being adapted to lipidomics from our colleagues in bioengineering/physics. It is hoped that for the veterans of the field as well as the uninitiated and curious, this review will encourage exploration into new ways to use lipids and their pathways to our advantage.
2. Lipids used as therapeutic pharmacologic agents
The omega-6 polyunsaturated fatty acid arachidonic acid (AA) is an eicosanoid precursor that can be acquired in cells by metabolism of linoleic acid or can be taken up by cells directly through dietary intake, which is the most common source. Once in the cell, this precursor of many lipid mediators is mobilized to lipid pools that are actively remodeled, such as the nuclear membrane. From there it is subsequently reassigned back to other cellular membranes that are the presumed sites of synthesis [5]. Arachidonic acid becomes anchored to membrane phospholipids like phosphatidylethanolamine, but primarily phosphatidylcholine and phosphatidylinositol, by esterification catalyzed by a fatty-acylCoA synthetase. The release of AA from the phospholipids is the signaling cascade lynchpin. The most common release mechanism is through the action of a Ca2+-dependent cytosolic phospholipase A2 (cPLA2), particularly cPLA2α (group IVA), (also referred to as GIVA PLA2), in response to a cell stimulus [6–8]. The cPLA2 apparently evolved simultaneously with eicosanoid receptors, and more than 30 genes for phospholipase are encoded in mammalian genomes, including the secreted (sPLA2), the Ca2+-independent (iPLA2), and Ca2+-dependent (cPLA2) enzymes. These three classes have been the most studied to date [9,10]. Similarly the omega 3 fatty acids, eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) can be released from the membrane stores as well. The stimuli that lead down the signaling pathway are obviously diverse and include those that are “complete activators” such as calcium ionophores, microorganisms, and oxidants, as well as agonists that potentiate the cascade by “priming” the response in preparation for a more complete stimulus [11]. Furthermore, the regulatory mechanisms for biosynthesis and secretion of lipid mediators are highly varied and depend on factors such as the expression levels and functional state of the metabolic enzymes, compartmentalization of the enzymes and finally expression levels and functional state of accessory proteins such as the transport proteins [11]. The cyclooxygenase (COX), lipoxygenase (LOX) and P450 epoxygenase enzymatic pathways can metabolize AA after its release from the cell membrane to generate numerous lipid effector molecules.
One of the most exciting recent advances in lipid research involves the discovery of a subset of eicosanoid lipid mediators that are actively involved in mitigating inflammatory responses. The first hint that recovery from inflammation was not just a waning of proinflammatory signals, but in fact an active recovery process came from studies of “spontaneously resolving inflammatory exudates” [12]. Through cooperative interactions of cells within the inflammatory microenvironment, transcellular synthesis of the pro-resolving lipoxins occurs by two routes [13]. As part of this functional redundancy, leukocytes can provide LTA4, a leukotriene intermediate from 5-LOX metabolism of AA, to neighboring cells such as platelets that in turn use this substrate to produce lipoxins LXA4 and LXB4 through the action of their own 12-LOX. Alternatively, LXA4 and LXB4 can be synthesized by cells through the uptake of a 15-LOX intermediate, and subsequent 5-LOX activity. Aspirin-triggered lipoxins also exist, but production of these occurs either through the COX-2 or P450 pathway [14]. The yin and yan transition of pro-inflammatory lipid production to that which resolves inflammation is modulated by still other lipid mediators such as a subset of prostaglandins.
The resolvins include the E-series (RvE1–E2) and D-series (RvD1–D6) lipid metabolites that originate from the omega 3 fatty acids, EPA and DHA respectively. DHA is also the source of maresins (MaR1) and neuro/protectins (NPD1/PD1). These newly characterized lipid mediators are thought to drive the benefits attributed to omega 3 up take [14].
Lipoxins, resolvins, maresins, and protectins (not to be confused with the membrane complement attack complex inhibitor protein (CD59) with the same name [15]) all target specific cell populations through defined surface receptors such as GPR32, FPRL1 (ALX/FPR2), cysLT1, ChemR23 (also activated by the protein chemerin), and BLT1 and have various immunoregulatory functions so as to regulate acute inflammation (reviewed in [16–18]).
This “resolution pharmacology” has already had a major impact on treatment modalities of, for example, periodontal disease [19,20]. In a lapine periodontitis model, leukocyte-mediated bone loss was prevented by RvE1, and it was demonstrated further that osteoclast differentiation was also abolished with resolvin treatment ([21] and references therein). These exciting preliminary studies on the effects of the pro-resolving arm of lipid mediators portend a blow to the resistance mechanisms of many diseases. The therapeutic effects of lipids in general, as well as from targeting their pathways, are heralding major advances in diverse disciplines.
2.1. Types of therapeutic lipids
Highly or polyunsaturated fatty acids (HUFA, PUFA) are the predominant source of lipid mediators, with both the eicosanoid (C20) and docosanoid (C22) fatty acid metabolites driving many phenotypes targeted for therapy including apoptotic pathways [22,23]. The direct consumption of preformed DHA and EPA has been demonstrated to have many therapeutic effects, particularly when derived from marine sources, as these substrates tend to skew the lipid continuum toward a healthy balance [24,25]. However, defined lipid mediators are being singled out of numerous lipid reaction pathways to treat specific diseases.
2.2. Specific examples of disease/pathobiology studies that suggest beneficial impact of lipids
Gynecological applications
Numerous studies on prostaglandins and their receptors in pregnancy have been reported [26]. Cervical ripening and the onset of labor are induced through the E and F series prostaglandins with a concomitant reduction of progesterone [27,28]. In a recent study that garnered national attention, midtrimester administration of progesterone was shown to decrease preterm delivery [29], an effect that is likely due to antagonistic effects on the lipid mediators.
Oral use of the prostaglandin E1 analog, misoprostol, was indicated for treating peptic ulcers and then adopted in the 1990s for use as a uterotonic (contracts the womb after child birth) in management of postpartum hemorrhage [30]. Oxytocin and ergometrine are two uterotonic drugs that are conventionally used to manage postpartum hemorrhage. While considered the gold standard, there are some drawbacks when considering their application in areas of the world where standard medical care may not be readily available. They are heat labile, require injection, and can elevate blood pressure. A recent Cochrane review of 72 randomized trials (52,678 women) reported that misoprostol was effective in reducing severe hemorrhage and reduced the need for blood transfusion. Given the lower cost, ease of administration, and its temperature stability, in addition to the fact that it can be used in hypertensives, oral administration of misoprostol was determined to be a viable treatment option particularly in low or middle-income countries despite some of the side effects such as fever and nausea that could impact maternal-infant bonding [31].
Misoprostol is now being used prophylactically to maintain uterine tone during C-section under anesthesia and to reduce blood loss during myomectomy for fibroids [32,33]. In 1995, and again in 2012, misoprostol was evaluated for its ability to reduce oral mucositis in response to chemotherapy and radiation [34,35]. Unfortunately the radiation study was cut short due to funding cuts and the chemotherapy pilot was never expanded to greater patient numbers. Therefore, the efficacy of prostaglandin treatments on oral mucositis in response to cancer treatments awaits further evaluation.
Diabetes studies
Gurgul-Convey et al. have proposed a new mechanism that involves prostacyclin or PGI2 to improve the treatment of type II diabetes. Rat islet cells have low levels of the enzyme prostacyclin synthase (PGIS). However, they ramp-up production in response to glucose stimulation. While the parental version of the beta-cell line, INS1E, did not have the same response to glucose, they were made to artificially overproduce PGIS, which then significantly increased insulin production and secretion in response to nutritional cues. The prostacyclin analog iloprost, also used in other studies to mitigate pulmonary fibrosis [36], induced insulin secretion in parental INS1E cells, and the IP (prostacyclin) receptor antagonist CAY10441 attenuated the response in the PGIS over expressing transfectants. In addition to the IP receptor, it was determined that Epac2, a protein responsive to cAMP and present in exocytic machinery as a guanine nucleotide exchange factor (GEF), was also required for the observed effects [37]. Interestingly, in another study, palmitate was shown to induce autophagy and cell death in the same INS1E cell line as well as in isolated rat and human islet cells [38].
In a meta-analysis to determine the association of Omega-3 fatty acids and type II diabetes, Wu et al. concluded that regular intake levels were neither harmful nor helpful and that alphalinoleic acid was associated with “nonsignificant trends toward lower risk” [39]. A group looking at the cardioprotective effects of pioglitazone in diabetic patients, found that, like aspirin, it elevated the pro-resolving lipoxin metabolite of the omega 6 arachidonic acid, 15-epi-lipoxin A(4) (15-epi-LXA(4)) [40,41]. Clearly more research into specific effects of lipid mediators in diabetes phenotypes is warranted.
Cardiovascular studies
Similar questions have arisen with regard to efficacy of n-3 long chain polyunsaturated fatty acids (LCPUFA) in cardiovascular disease. However, the disparate study results on the beneficial effects of omega 3 on arrhythmia were recently reconciled by proposing that they serve as an “upstream therapy” by suppressing cell damage responses to inflammation, apoptosis, ischemia, etc., that is to say that they impart their benefits by preventing pathological structural remodeling of cardiac tissue particularly when applied during early stages of disease [42,43].
A recent study by Khairallah et al. has demonstrated that DHA supplementation increases overall mitochondrial n-3 PUFA content and dramatically alters mitochondrial cardiolipin, a tetraacyl phospholipid that is required to form the junctions between inner and outer mitochondrial membranes [44,45]. In general, depletion of total cardiac mitochondrial cardiolipin, and reduction of the tetralinoleoyl cardiolipin (L4CL) that makes up 60–80% of the cardiac mitochondrial cardiolipin pool, is associated with cardiac pathologies. Moreover, mitochondrial dysfunction overall has diverse repercussions and is known to impact diabetes and neurodegeneration in addition to heart failure (reviewed in [45]).
The findings by Stanley et al. that fish oil supplementation could prevent cardiomyocyte apoptosis in a rat model of chronic aortic hypertension led the investigators to uncover a link between DHA and the mitochondrial permeability transition pore (MPTP) [46]. Specifically it was determined that moderate DHA supplementation delayed Ca2+-induced opening of the pore, which is significant as opening of the MPTP leads to a permeability transition that results in depolarization and cell death [46]. Therefore, the contribution of mitochondrial dysfunction to cardiac pathologies may be mitigated by DHA-mediated membrane remodeling. In the clinic, administration of 0.9–3.6 g/d of DHA + EPA to heart failure patients lessened hospitalization and mortality as well as improved left ventricular function [46]. It is known that increased uptake of DHA and EPA decreases inflammatory mediators in the lipid pathways, reduces pro-inflammatory cytokine gene expression, and elevates beneficial resolvins and eicosanoids such as PGE3 and LTB5, for example. An additional potential benefit stems from elevation of the less cytotoxic peroxidation products from the long chain n-3 PUFAs, such as 4-hydroxy-2-hexanal (HHE), in comparison to those generated from n-6 PUFAs, such as 4-hydroxy-2-nonenal (HNE), which has deleterious effects on mitochondria and is also associated with left ventricular function [46]. The complexity and impact of dietary ratios of beneficial fats to carbohydrates in relation to healthy versus unhealthy weight, and the role of these ratios in cardiovascular disease onset or progression is an area actively being investigated [46].
Nevertheless, despite the numerous benefits of targeting the eicosanoids in cardiovascular diseases, there are significant consequences to global disruption of the physiological yin and yan, and specific targeting of individual components on the lipid continuum such as the lipid receptors or lipid enzymes would be more desirable. This has been covered in an excellent review by Capra et al. [47].
Critical care
In critically ill patients, such as those with sepsis, elevated circulating PLA2 can predict the risk of acute respiratory distress syndrome (ARDS), and it has long been recognized that modulation of systemic inflammatory response syndrome (SIRS) by targeting the lipid continuum could benefit critical care medicine [48,49]. In clinical trials, enteral administration of EPA and gamma-linolenic acid (GLA) could prevent progression to sepsis [50]. However, others reported that this regimen did more harm and could not be recommended for treating the critically ill patient [51]. In the pediatric setting, as reported by Shimizu, alprostadil (PGE1) and dinosprostone (PGE2) are used to treat primary pulmonary hypertension as well as ductus-dependent congenital heart disease. Similarly, misoprostol has improved juvenile rheumatoid arthritis and has been used to stave-off NSAID and corticosteroid damage to the gut in children [52]. For premature infants with hypoxemic respiratory failure due to respiratory syncytial virus (RSV) pneumonia, it is the inhalation of PGI2 together with high frequency oscillatory ventilation that improves clinical outcome [53]. Thus lipids continue to be utilized in novel critical care treatments.
Fibrosis and kidney disease
Epithelial to mesenchymal transition (EMT) occurs in both normal biology, as in development and response to injury, and pathobiology, as in cancer and organ fibrosis and is classified according to context [54]. Tissue and organ fibrosis are currently being debated as a form of EMT (reviewed in [55]). EMT onset and regulation is governed by numerous factors such as growth factors, reactive oxygen species, epigenetic factors, and relay of mechanosensory information [56,57]. As an aside, the mechanosensory stimulation of PGE2 production by low-intensity pulsed ultrasound (LIPU) has been demonstrated to induce cell differentiation albeit in osteoblasts through osteocytes, which leaves open the possibility of modulating EMT phenotypes in the future [58]. Currently though, diseases such as systemic sclerosis (SSc) and idiopathic pulmonary fibrosis (IPF) are still an enigma and remain difficult to treat [59]. Interstitial fibrosis in end-stage renal failure has also been correlated with EMT phenotypes [57]. Increasingly, lipid mediators are being substantively implicated in fibrosis and are currently the targets of emerging therapies (reviewed in [59]). In a study out of Italy, EPA and DHA were demonstrated to counter the kind of inflammation and extracellular matrix (ECM) accumulation seen in renal disease [60]. Meanwhile, several animal models have demonstrated that renal proximal tubule inflammation and dysfunction in diabetic nephropathy could be linked to altered levels of cannabinoid receptors, where for example AM251, a CB1 receptor agonist, ameliorated albuminuria and CB2 receptor agonists such as AM1241 could reduce monocyte chemoattractant protein MCP-1 as well as chemokine (C-C motif) receptor 2 (CCR2) (reviewed in [61]). In another report of the antifibrotic effects of lipid mediators, dihydrosphingosine-1-phosphate (dhS1P) was shown to affect scleroderma fibroblasts through its up regulation of phosphatase and tensin homolog (PTEN), and normalization of proteases and transcription factors [62].
Neurobiology
Lipid mediators are associated with traumatic brain injury, cerebral ischemia, subarachnoid hemorrhage, and exicitotoxic injury. Numerous diseases including epilepsy, Alzheimer’s, amyotrophic lateral sclerosis, Parkinson, and Creutzfeldt-Jakob disease have some form of underlying component along the lipid continuum [63,64]. Arachidonic acid metabolites are implicated in affective disorders, where studies have shown a clear benefit associated with omega 3 fatty acid consumption [65,66], and neuroprotectin D1 (NPD1; 10R,17S-dihydroxy-docosa-4Z,7Z,11E,13E,15E,19Z hexaenoic acid), the 15-LOX product of DHA, effectively improves lipid bilayer properties, reduces apoptosis, and down regulates inflammation to positively impact many neurological diseases [67]. Furthermore, omega 3 fatty acids also protect against long term injury from neonatal hypoxicischemic brain injury [68], perhaps also through the actions of resolvins. A competitive inhibitor of the COX enzymes and 5-LOX called licofelone is able to abate symptoms of Huntington’s disease in a mouse model, and may prove useful in the treatment of Alzheimer’s disease as well, as a recent study has demonstrated that transfer of the 5-LOX gene to mice worsened their Alzheimer’s phenotypes [69,70]. Simultaneous inhibition of the COX enzymes and 5-LOX with licofelone appears to improve drug delivery of riluzole in spinal cord injuries by lowering the efflux drug transporter P-glycoprotein (abcb1), which is upregulated in many cancers as well [71]. However, in Alzheimer’s β-amyloid itself can down regulate Abcb1 at the blood brain barrier to exacerbate the condition [72], which again speaks to the balance that pharmaceutical targeting of lipid mediators must achieve.
Arthritis
Proresolving lipid mediators have been steadily developed alongside synthetic anti-rheumatic drugs to target rheumatic diseases with success, though side effects have sidelined some prominent drugs [73–77]. A new lipidomics study has begun to identify and profile those natural products within synovial fluids of rheumatoid arthritis patients that could be targeted for further development in treatment including the maresins, the D-series resolvins, and lipoxins [78]. Conceptually the high density lipoproteins (HDL), like resolvins, are protective and anti-inflammatory, and part of their function is to prevent the oxidation of low density lipoprotein (LDL). However, in an acute phase response, they become pro-inflammatory (pi-HDL), and should the acute phase become chronic then diseases associated with chronic inflammation such as arthritis develop [79]. As oxidized LDL has also been found in synovial fluid, and LDL are seeded with metabolites of linoleic and arachidonic acids, it will be interesting to see what crosstalk occurs between the pro-resolving fatty acid derivatives and the pi-HDL or oxidized LDL [40].
Cancer
Studies to modulate the lipid continuum have been most concentrated in an effort to impact cancer in all its forms and permutations. The use of natural lipids has many benefits not the least of which is patient acceptability. The usefulness of nutritional agents and their derivatives has repeatedly been borne out in their impact on a diverse array of cancers including colorectal cancers as well as breast cancer [80,81]. In a newly published breast cancer study, it was determined that colony stimulating factor 1 (CSF1) could be suppressed by the effects of fish oil induced elevation of PTEN levels mediated by the reduced expression of the microRNA miR21 [82]. Another study has suggested that omega 3 consumption would counter the potential for breast cancer invasion by progestin hormone replacement therapy [83]. On the flip side where estrogen depletion is desired, there are a host of issues associated with antiestrogen therapy with agents such as Tamoxifen and Raloxifene. Aside from the fact that they do not prevent the expansion of estrogen receptor negative breast cancer, they can also cause thromboembolism [84]. Therefore studies are underway to determine if Raloxifene in combination with an omega 3 regimen, like Lovaza could be of some benefit. More promise for the benefit of omega 3 in skewing the lipid continuum comes from a recent study that suggested it may ameliorate the debilitating side-effects of peripheral neuropathy associated with paclitaxel therapy [85].
Just as has been reported in animal models of prostate cancer, the administration of EPA and DHA can also attenuate the growth of pancreatic cells in a xenograft model of MIA-PaCa-2 and is even more effective in combination with inhibitors of autophagy [86]. In the same study, growth of both Capan 2 and MIA-PaCa-2 cells were attenuated by EPA and DHA through the activation of caspase-8 and production of reactive oxygen species (ROS).
Unfortunately, cancer cells are always evolving mechanisms to outsmart our best efforts, and a fatty acid binding protein (FABP) has just been described in triple negative breast cancer that is associated with poor prognosis. When bound to DHA, FABP7 can stimulate the retinoid-activated nuclear receptor, RXRbeta, to promote transcription and cell survival [87]. Therefore, despite the growing arsenal of lipid mediators used to attack cancer, there is still a need to evaluate combinatorial therapies and to explore new sources of therapeutic molecules. Ultimately though, such strategies together with the anti-inflammatory, or pro-resolving, lipid mediators may soon finally force recalcitrant cancers, such as those of the pancreas and colon, to meet their match [88,89].
3. Targeting lipid pathways
The therapeutic use of lipids is coming into its own. However, there are plenty of examples along the lipid continuum where lipids and their signaling pathways are the culprits of disease, and often these lipid systems are inter-related by means of shared regulatory nodes [90,91]. The ceramide pathway is involved in many patho/biological functions including cancer and metabolic syndrome to name a few [92,93]. The eicosanoids are culprits in asthma and allergic inflammation [94]. Likewise, they are intimately associated with carcinogenesis and metastasis and have been shown to regulate nearly every phenotype associated with cancer and its progression, from angiogenesis to integrin modulation for adhesion, and endothelial cell retraction to tumor cell invasion [95]. Lipoxygenases have been the targets for development of many cancer chemopreventive inhibitors [96,97]. However, many additional enzymes along the lipid continuum can be targeted for pharmaceutical intervention, including those in the ceramide/sphingolipid as well as the endocannabinoid pathways [98,99].
Most lipid mediators initiate cell signals through either paracrine or autocrine mechanisms by binding to Rhodopsin-like seven-transmembrane G-protein coupled receptors (GPCR), and these too have been the focus of targeted therapeutic development [100]. As of 2010 there were five prostanoid receptors with an additional four subtypes, four leukotriene receptors, and eleven lysophospholipid receptors that include six lysophosphatidic acid and 5 sphingosine 1-phosphate receptors [101]. Additionally there are two cannabinoid receptors and the platelet activating receptor. The classic and currently accepted International Union of Pharmacology (IUPHAR) receptor names, according to class of lipid as well as the historical context of their discovery, as well as a phylogenetic study are summarized in succinct reviews by Howlett and Mizutani [101,102]. A number of receptors whose ligands were previously undefined, the so-called “orphan” receptors, have been paired with bioactive lipids [103,104]. Historically, thromboxane and the prostaglandins, which are both products of arachidonic acid metabolism by the COX enzyme, were the first bioactive lipids to have their receptors characterized, followed a decade later by the receptors for leukotrienes and lipoxins, which are arachidonic acid metabolites of the 5-LOX and 15-LOX enzymes respectively. Recently receptors for 12(S)-HETE and several autacoids, small molecules with short half lives similar to hormones that act near the site of synthesis, such as maresins, resolvins, protectins and lipoxins have been identified [16,103]. The receptor for 12(S)-HETE was identified as the orphan receptor GPR31 and christened 12-HETER1 [103]. Its expression and signaling activity was associated with prostate cancer progression and was demonstrated to be essential for cancer cell invasion in vitro.
In an unrelated study meant to determine the underlying mechanism of periventricular leukomalacia, linked to cerebral palsy in premature infants, 12-LOX enzymatic activity (leukocyte type) was determined to contribute to oligodendrocyte death in response to glutathione depletion [105]. This would suggest that attenuation of neuronal receptor isoforms of 12HETER1 identified in the prostate study would benefit oligodendrocytes by inhibiting cell death, whereas in prostate cancer, receptor inhibition would promote cell death. Furthermore, in a study of neuronal excitotoxicity from cerebral ischemia, several HETE isoforms, particularly 12(S)-HETE, could actually protect against AMPA receptor-mediated toxicity [106]. While the 15(S)-HETE and 5(S)-HETE isoforms also offered some protection, it is unlikely that they are acting through the same receptor. In diseases such as amyotrophic lateral sclerosis, epilepsy, and ischemia, direct targeting of the AMPA receptor function has not met with much success [107]. Therefore, in the future the novel 12(S)-HETE receptor may offer an attractive alternative target. These observations speak again to the lipid continuum where the yin and yan balance of lipid mediators and targeted therapy depends on context.
Endocannabinoid receptors
There are two known cannabinoid receptors, CB1 and CB2 [108]. However, suggestive evidence is accumulating in support of additional receptors such as in the endothelium as well as a novel isoform for analgesia [109]. GPR55 shares a mere 14–15% homology with either CB1 or CB2. However, it is responsive to cannabinoids as well as to lysophosphoinositol (LPI) and its crosstalk with CB1 and CB2 has been proposed based on pharmacological data. It is also upregulated in enteric epithelial cells and neurons in response to systemic inflammation and is being investigated for its role in the gastrointestinal tract, where it has also been associated with mechanoreceptors in the gut [110]. The CB1 antagonists SR141716A and AM251 have the opposite effect on, and apparently stimulate, GPR55, which may speak to the fact that the CB receptors are interacting with different downstream G-proteins than those interacting with GPR55, whose interaction with Gα13 predominates and activates RhoA and ROCK, leading ultimately to transcriptional activation. Additionally, it has been shown that GW405833, which is a CB2 ligand, can serve as an agonist of GPR55 and stimulate LPI signaling [111]. Because of the putative role of GPR55 in such diverse processes such as cancer, nociception, and insulin secretion, and its involvement in many organ systems, it is currently the focus of much attention in the lipid receptor field [110]. GPR55 clearly binds LPI, which has also been shown to be protective for cerebral ischemia and glutamate excitotoxicity in culture. Furthermore, GPR55 and its signaling pathway are involved in decidual tissue regression, angiogenesis, bone resorption (in addition to CB2 [112]), and cancer cell proliferation and migration, where elevated GPR55 has been correlated to poor prognosis in a number of cancers, and arachidonyl-LPI is elevated in the plasma and ascites of ovarian cancer patients compared to their healthy counterparts (reviewed in [113]). In light of the recent issues with the antagonists/inverse agonists of CB1, and their adverse effects such as nausea and psychiatric disturbances, such as were seen with rimonabant [114], GPR55 may offer an alternative or augmented route for therapies meant to target the endocannabinoid system.
Sphingosine 1-phosphate (S1P) receptors
S1P is the end product of a complex lipid pathway that originates at the plasma membrane with the release of sphingomyelin by sphingomyelinases followed by the action of ceramidases and ultimately the sphingosine kinase to produce the ligand for the S1P receptors [115]. The S1P receptors (S1P1-05, called previously Edg-1,3,5,6,8) represent another example of a lipid continuum that requires perfect balance. Their ligands are essential for endothelial health and vascular development. However, acute ligand excess can cause cardiac death (reviewed in [116]). S1P can also be found on HDL particles, and dependent on the receptor subtype present, can mediate numerous phenotypes including cancer-associated angiogenesis, proliferation and stem cell migration, as well as oligodendrocyte progenitor and immune cell migration [117–119]. The S1P3 receptor has been associated with a worsening of sepsis, and can potentiate epidermal growth factor receptor (EGFR)-mediated cancer phenotypes in lung cancer cells [116,120]. Reportedly, the first antagonizing antibody of a lipid-activated receptor was developed for S1P3 that shows therapeutic promise for treating sepsis and breast cancer progression [117]. S1P1 receptor antagonists have also been forthcoming,as the latest to be developed show promise in treating autoimmune inflammation such as in multiple sclerosis (reviewed in [119]). Other lysophospholipid receptors have been reviewed nicely by Ishii et al. [121].
The S1P1 receptor structure has just been resolved [122]. Moreover, the recognition of lipid receptors as therapeutic targets has given rise to intensive comparative modeling of these challenging membrane bound receptors, where careful selection of the template on which to model can give insight into the development of antagonists [123]. Likewise, the exciting development of new infrared (IR) probes that are genetically encoded is enabling us to track receptor activation [124].
Others
The prostanoid and thromboxane receptors as druggable targets are the subject of nice reviews by Anderson and Kontogiorgis [125,126]. Likewise the cognate nuclear lipid GPCRs can be found described by Marrache et al. [127].
3.1. Natural products
Natural products have been interrogated for use in anti-cancer therapies as well as in cancer prevention [128]. It has been appreciated for some time that flavonoids modulate lipid mediators [129–131]. They continue to inspire drug design as new inhibitors of the lipid pathways as well as antimicrobials are discovered [132–135].
3.1.1. Flavonoids
Baicalin, a glucuronide of Baicalein (12-LOX inhibitor), is a flavonoid out of Asian herbals that is known for its anti-inflammatory properties. It has been reported to prevent differentiation of Th17 cells, which are a CD4+ T helper (Th) subset that is currently the subject of intense study for their role in immunity and inflammation [136–138]. Differentiation of Treg and Th17 cells is reciprocally regulated by TGFβ and accessory cytokines through differentially utilized transcriptional regulators such as the Forkhead family transcription factor (Foxp3), the Retinoic Acid-related Orphan Receptor (RORgammaT), as well as the Aryl Hydrocarbon Receptor (AHR), which is best known for regulating gene expression in response to environmental contaminants but is also stimulated by non-toxic endogenous ligands [137,139–141]. The latter receptor appears to harbor a promiscuous ligand binding site insomuch as it binds numerous synthetic and natural ligands including the flavonoids and lipid products. Both lipoxin A4 and prostaglandin G2 are AHR ligands that lead to AHR-dependent gene expression including, CYP1A1 (Cytochrome P4501A1), prostaglandin endoperoxide H synthase 2 (COX2), murine epiregulin (EREG), and multidrug resistance genes such as MDR1 and BRCP (breast cancer resistance protein) [142,143].
Th17 cells are implicated in numerous pathological and physiological processes including rheumatoid arthritis, immune tolerance, mucosal infections and allergy, and tumor cell microenvironment (Reviewed in [140,144,145] and references therein). Th17 cells have also been invoked as a link between inflammatory bowel diseases and preterm birth [146], but their role in cancer is unclear [147]. A study of Th17 cells and their associated cytokines in pancreatic cancer by He et al., found that Th17 cells were in greater abundance in the circulation of cancer patients versus healthy individuals [148]. Likewise, Th17 cells were positively correlated with microvessel density and were more abundant in lymph node metastases and cancer tissues, particularly Stage III and IV, than in neighboring normal tissue. While there was no apparent association of Th17 cells with histological grade, age or gender, specific cytokines from this Th subset were reportedly higher in patients with poorer prognosis [148]. Interestingly, although Th17 cells were associated more with breast cancer than with healthy tissue, the study by Yang et al. reported a negative association between Th17 numbers and stage, lymph node metastases, and blood vessel invasion, and suggested that the increased presence of Th17 cells in breast cancer was indicative of a favorable prognosis [149]. In the future it may be determined that the balance between Treg, the regulatory T cells, and Th17 cells in relation to lipid mediators is the reason for such disparate results.
As with Baicalin, the catechin of green tea, epigallocatechin-3-gallate (EGCG) also impedes Th17 differentiation, regardless of whether they are induced under normally polarizing stimuli such as TGF-β +IL-6, or through combinations of IL-6 or IL-23 with IL-1β [150]. EGCG down regulated phosphorylated STAT3, which resulted in reduced levels of RORgammaT, a transcription factor necessary for Th-17 development. In their model of autoimmune encephalomyelitis (EAE), Wang et al. likewise showed that EGCG could lower circulating IL6 as well as the soluble form of the IL6 receptor [150].
With regard to other cancers and cancer-related phenotypes, in HepG2 hepatocellular carcinoma cells EGCG suppresses PGE2 secretion as well as EP1 prostanoid receptor levels. Moreover, HepG2 viability and motility in response to either PGE2 or ONO-DI-004, an EP1 receptor agonist, was also inhibited by addition of EGCG [151]. It should be noted that PGE2 can promote Th17 differentiation through EP2 receptor activation, which suggests that additional targets of EGCG may be forthcoming (reviewed in [152]).
EGCG has anti-thrombotic activity in that it can inhibit release of AA as well as AA-induced platelet aggregation [151]. Coadministration of EGCG with photodynamic therapy (PDT) in a mouse mammary tumor model was determined to increase responsiveness to therapy by reducing PDT-induced PGE2 and promoting apoptosis through attenuation of both GRP-78 and survivin [153].
The soy isoflavones have also featured in recent prostate cancer studies where it was determined that they could be used to sensitize cancer cells to radiotherapy, and in a porcine model they were shown to affect lipid metabolism in adipose, liver, and skeletal muscle through their activity on adipokines and myokines [154,155]. The effect of lipid mediators on adipocyte function and metabolic syndrome is another area that is rapidly expanding in lipidomics [156].
3.1.2. Others
Scientific literature is rich with compelling examples, as presented by Wallace in 2002, of why lipid mediators should be at the forefront when considering complementary cancer therapies [157]. Nutritional pharmaceuticals or nutraceuticals continue to be developed as we collectively gain a greater appreciation for the wisdom of ancient ways. Curcumin, a compound derived from the spice turmeric, has been the subject of many studies and the source of emerging drugs that target bioactive lipid pathways. It is an anti-inflammatory agent and can sensitize existing chemotherapies. This synergism is likely due to its concomitant effect on both COX and LOX enzymes as well as cPLA2 phosphorylation, which has been described [158–160]. While curcumin can be consumed in great quantity without adverse effect, its bioavailability is limited [161]. In a study meant to address the underlying mechanisms of colon cancer resistance to chemotherapy, a synthetic analogue of curcumin (known as 3, 4-difluorobenzo curcumin or CDF) in synergy with 5-fluorouracil and oxaliplatin (5-FU + Ox) dramatically inhibited the growth of chemo-resistant cells including CD44 positive stem cells [162]. In the latest effort to improve bioavailability, a curcumin-cyclodextrin conjugate was tested for its efficacy in reducing lung tumor burden in an orthotopic mouse model with some success [163]. Additionally, this conjugated form potentiated the effect of Gemcitabine, a nucleoside analog used for chemotherapy of a number of cancers. The IC50 of CDF in cultured cells was also improved with cyclodextrin. When tested in a mouse model this combination resulted in the pancreas having tenfold more CDF over serum concentrations [164]. While the relatedness of the following observations remains to be determined, it is noteworthy that CDF regulates the tumor suppressive microRNAs miR34a/miR34c, and that a regulatory binding site for these microRNAs, which are actually elevated in a model of hepatic fibrosis, was identified in the gene for acyl-CoA synthetase long-chain family member 1 (ACSL1) [165,166]. Furthermore, a study of palmitate-induced pancreatic β-cell dysfunction, found that miR34a modulated VAMP2, a protein on secretory vesicles involved in insulin secretion [167]. The apparent disparate functions of miR34 likely reflect tissue-based differences of gene expression perhaps related to bioactive lipid pathways. Curcumin and its derivatives appear to be viable therapeutics in the treatment of cancer, and have the additional benefit of serving as useful tools for studying several disease states that may fall under the regulation of curcumin-responsive microRNAs in relation to lipid mediators. Synthetic curcuminoids have been identified through protein modeling studies of known LOX enzyme structures in combination with biochemical assays [168]. Using this approach, two such inhibitors were identified (E22C, E26C) that successfully inhibited endothelial sprout formation, an early step in angiogenesis, in an in vitro assay with human umbilical vascular endothelial cells (HUVEC).
As a result of impaired AA mobilization, cPLA2α knockout mice are protected from metabolic syndrome, ischemic injury of the brain, and Alzheimer’s, but have impairments with regard to implantation and parturition [9]. In an effort to knockout cPLA2 activity from a pharmacological standpoint, several curcumin analogs, identified as rosmarinic acid, tetrahydrocurcumin, dihydrocurcumin and hexahydrocurcumin, have been discovered that can inhibit the PLA2 hydrophobic binding pocket from binding substrate thereby preventing the release of AA and downstream metabolic events [169]. Extracts from the white oatmeal Avena sativa can also attenuate the stimulated mobilization of AA in keratinocytes, though not completely, through the reduction of PLA2 transcription and protein production [170]. Phlorotannins from the brown algae Eisenia bicyclis are also potent inhibitors of PLA2, albeit of the secretory type, and are even more effective in some instances at inhibiting LOX enzymes than EGCG [171].
3.2. Pharmaceuticals
In a search for additional drugs that could target lipoxygenases and protect against, for example, neurodegenerative diseases, an initial computer screen of compounds that could bind the 15-LOX active site was paired with a screen for inhibition of recombinant enzymatic activity. Then, using a cellular assay to screen 20 hits that were originally found from the virtual screen of 50,000 compounds, two novel lipoxygenase inhibitors were discovered, called LOXBlock-1, and −3, that could protect neuronal and oligodendroglial cells from oxidative stress-induced cell death [172].
The Cytochrome P450 enzymes add epoxides to arachidonic acid to generate epoxyeicosatrienoic acids or EETs, which are additional lipid mediators that have a role in inflammation and platelet aggregation, as well as cancer [173]. The soluble epoxide hydrolase, or sEH, in turn degrades the EETs into dihydroxyeicosatrienoic acids or DHETs, thereby attenuating EETs. Small molecule inhibitors of the sEH are currently in development for the treatment of chronic inflammation [174].
3.2.1. New agents
In 2012, several new, modified NSAIDs have been reported that have differing effects on the lipid pathways. Elkady et al. have developed membrane prostaglandin E synthase (mPGES1) and 5-LOX inhibitors that show no COX-1 inhibition [175]. This was done by modifying the carboxylic acid functional group to sulfonamide derivatives, where “lonazolac derivative 22” and “indomethacin derivative 17” were found to be the best at inhibiting mPGES1 and 5-LOX respectively.
Additionally, another class of modified NSAIDS in a new form of aspirin penned NOSH-aspirin for “nitric oxide- and hydrogen sulfide-releasing hybrid (compound NBS-1120) has been in the news for its extremely potent inhibition of mouse colon cancer xenografts in vivo, in addition to its attenuation of numerous other malignant cell types, including pancreatic, breast, lung, prostate and leukemic cells [176,177]. Derivatization such that nitric oxide and hydrogen sulfide are released, has the dual benefit of protecting against the gastric side-effects as well as potently killing cancer cells by apoptosis and attenuating cell cycle progression. Whereas the indomethacin and lonazolac derivatives of the former aspirin compound did not impact COX1, the NOSH aspirin did. The impact of these drugs on the lipid continuum with regard to other mediators such as the lipoxins will be of great interest.
3.2.2. Manufacturing of therapeutic lipids
The creative use of natural systems such as single cell green algae to successfully manufacture fully functional antibodies was described nearly a decade ago and pharmaceutical production of proteins in algae continues to expand [178,179]. Similarly, the study of lipid production in algae from the field of aquaculture has led to novel insight and the potential for scale-up production of therapeutic lipids in addition to alternative energy lipids [180– 182]. Cross-discipline studies have led to the identification of novel lipid enzymes such as elongases and destaturases in eukaryotic algae that can be directly exploited for DHA production [183,184]. Organic extracts of microalgae have potential antioxidant and antiproliferative effects, and contain antineoplastic lipids, including glyco- and phospholipids, from brown and red algae, which have been determined to impact Meth-A fibrosarcoma [185–189]. Endogenous lipids from marine algae have proven to be useful antineoplastic agents as well. The EPA fraction of diethyl ether extracts of the diatom Cocconeis scutellum reportedly attenuated BT2 breast cancer cells, and marine derived EPA and DHA have been shown to sensitize animal tumor models to numerous cancer drugs, an observation that is being supported in various phase II and III clinical trials [190,191]. The commercial use of microalgae in the development of therapeutic lipids is a burgeoning area of development [192,193]. Additionally, and as an alternative to microalgae, the budding yeast Saccharomyces cerevisiae has the capacity to produce lipids of pharmaceutical interest whose production can be monitored at the single cell level by another technology coming into the lipidomics field; Coherent Anti-Stokes Raman Spectroscopy or CARS [194] (described in Section 4.2 of this review).
3.3. Applications (single and multi target)
The term rheostat has been used frequently in the lipidomics field to refer to the yin and yan of lipid activities along the continuum where the balance in the lipid signaling pathways may dictate health or disease progression. The diversity of lipid signaling pathways that can be targeted by lipids and novel drugs to impact diseases is offering a wealth of therapeutic possibilities that is increasingly recognized and being developed in both academia and by pharmaceutical companies alike, as both single and multi-targeted, synergistic therapies continue to be explored.
3.3.1. Single target (lipid centered)
Co-administration of the arachidonic acid metabolite 12-HETE with NSAIDs and opioids has been shown to reduce reperfusion injury. Recently the drug Fingolimod (FTY-720), which is an S1P1 antagonist that has been approved for treatment of multiple sclerosis, was shown to be effective as well [195,196]. While it did not afford protection against glutamate excitotoxicity, it did reduce infarct size. Incidentally, FTY-720 has also been used in animal models of fibrosis (reviewed in [59]).
Regardless of context be it sepsis or cancer, and regardless of where the lipid continuum is targeted either at the level of enzymes, lipid products, or receptors, clearly lipid mediators are leading us into new therapeutic avenues.
3.3.2. Multi-target (synergism of lipid therapy with existing chemotherapy)
A prime example of our knowledge gap with regard to the synergistic effects of targeting the lipid enzymes was revealed with the advent of COX inhibition [197]. As another recent study demonstrated, Celecoxib, a COX-2 inhibitor was found to cause off-target effects that led to increased amphiregulin (AREG) expression by myofibroblasts and subsequent stimulation of the EGFR. The study found that while Celecoxib was able to stave-off new adenomas, patients who were treated with the drug did not experience a reduced incidence of colon cancer, and in fact saw a greater incidence of adenomas which were more aggressive within 2 years [198]. While the desired benefit may be ultimately achieved by targeting the growth factor pathways in combination with COX-2, it nevertheless illustrates the need for alternate strategies. Using a different approach, Lim et al. demonstrated that administration of DHA in combination with Sulindac sulfide did suppress growth of human colon cancer xenografts, and this was through the death receptor (DR)5-dependent extrinsic apoptotic pathway [199]. DHA has also been shown to synergize with the effects of clioquinol (5-chloro-7-iodo-8-hydroxyquinoline) against tumor cells in a PPARalpha-dependent mechanism to affect a lower IC50 of clioquinol. The effect was attributed to the attenuation of NF-κB and prosurvival molecules such as Bcl-2, Akt and p65 [200]. Subsequently DHA was shown to target SOD1 in a PPARalpha dependent manner [201]. DHA also synergizes with and enhances curcumin activity against breast cancer cell proliferation [158].
Tumor cell-induced platelet aggregation (TCIPA) is a recognized process involving crosstalk between the two cell types in their microenvironment, whereby lipid metabolism of the platelets can induce integrin upregulation of tumor cells and promote tumor cell metastasis [202,203]. Rao et al. have demonstrated that DHA can inhibit platelet AA metabolism by COX into the bioactive thromboxane A2, resulting in less platelet aggregation [204]. After nearly three decades of research, thromboxane inhibitors are still actively being developed for a host of other diseases impacted by COX activity including lupus nephritis, asthma, and septic shock to name a few, and many are used in a combinatorial approach to restore the yin and yan along the lipid continuum [126,205].
As another example of the pleiotropic effects wrought by some of the lipid mediators, the enzyme 12-LOX from the lipoxygenase pathway produces a metabolic product from arachidonic acid called 12(S)-HETE that is clearly linked with much pathology (Table 1). It is known to stimulate angiogenesis in tumors as well as induce metastasis, and it can lead to TCIPA [202,206–209]. However, there is evidence that it could be therapeutic as well. In the context of wound healing, 12(S)-HETE is known to be a mitogen for endothelial cells and inhibition of the 12-LOX enzyme with BMD122 (formerly called BHPP; N-benzyl-N-hydroxy-5-phenylpentamide) prevents injured endothelial cell monolayers from responding to serum-induced repair [210]. Another potential benefit from 12(S)-HETE is implied by the results from a study meant to evaluate the cardioprotective effects of concomitant administration of NSAIDS with opioids [211]. Administration of 12(S)-HETE prior to reperfusion appeared to reduce infarct size. When 12(S)-HETE production was inhibited by the 12-LOX enzyme inhibitor baicalein, prior to the coadministration of ibuprofen and morphine, the protective effects of the NSAID-opioid combination were lost.
Table 1.
Disease etiologies regulated by 12(S)-HETE and potentially 12-HETER1.
| Cancer | **12(S)-HETE:pleiotropic effects: angiogenesis, cell proliferation,migration, invasion, endothelial cell retraction [95,278,279] |
| 13(S)-HODE: circadian regulation and cancer [280–282] | |
| Zellweger spectrum of peroxisome biogenesis disorders (PBD-ZSD) | |
| **impaired peroxisome fcn,cells unable to metabolize 12-HETE; blood, organ system metabolism affected, leads to damage of brain white matter [283,284] | |
| Hypertension | **mediation of angiotensin II–induced intracellular calcium transients [223,285,286] |
| Atherosclerosis | **plaque formation [287,288] |
| Diabetes | **ischemic/proliferative retinopathy, human islet cell death [223,289–292] |
| Parkinson’s | ** Glutathione (GSH) depletion–related cell death [293,294] |
| Alzheimer’s | ** c-jun-dependent apoptosis pathway, regulates BACE proteolytic pathway [172,295–297] |
| Neurological functions | **modulates neural peptide secretion, LHRH, the target of chemical castration in PCa treatment, intermediates activate TRPV1 in sensory neurons [298–300] |
Synergism between lipid pathways and chemotherapies has been studied in other models too. In a model of acute lymphoblastic leukemia, manipulation of the sphingolipid pathway could overcome cell resistance to the retinoid 4HPR N-(4-hydroxyphenyl)retinamide (4-HPR, fenretinide) [212], and ceramide has been proposed to drive resistance to doxorubicin [213]. Decursin, which is a putative anti-cancer coumarin out of the roots of the Korean Angelica gigas was determined to induce cell death in myeloid leukemia cells through its inhibition of COX2-dependent expression of survivin protein [214]. These data suggest there could be additional targets in lipid pathways that could provide a beneficial synergism with existing pharmaceutical applications. For years studies have been reporting on lipid pathways being modulated in combination as a means to therapy and a sample of recent studies are listed in Table 2. While there are numerous reports on the efficacy of modulating lipid mediators in tandem with standard chemotherapy, there does not appear to be a standard that correlates the class of pharmaceutical, e.g. microtubule inhibitors, with the lipid pathway most likely to impact it. Systems biology may eventually allow us to establish such correlations.
Table 2.
Studies on drug development and efficacy of co-modulation of lipid mediator pathways.
| LOX(s)/COX- |
|
| LOX/TXA2S- |
|
| COX/TPR- |
|
| Other combinations- |
|
4. Lipidomic methodologies
With the advent of “omics” technologies, proteomics and genomics have advanced at breakneck speeds and are performed routinely at many institutions. However, after decades of ground-breaking lipid research, the field of lipidomics is also having a major impact on numerous basic and clinical disciplines. Therefore, a brief overview of methodologies to study lipids is presented here in the context of their applications.
The tools and techniques commonly applied to the study of proteins such as chemical probes or antibody-based assays are currently available and are actively being developed for lipidomics [215,216]. Likewise, molecular sensors have been described recently for in situ quantitative imaging of cellular lipids, where lipid binding motifs from lipid receptors are repurposed as they are engineered together with inducible fluorophores to yield reporters of spatio-temporal signals on binding of the lipid ligand [217]. The translation of this technology to interrogate lipid mediators in general will become possible as more lipid receptor binding sites are described. Other technologies are described below.
4.1. LC–MS
Liquid chromatography–mass spectrometry (LC–MS), coupled with electrospray ionization techniques, is most commonly used in lipidomics [218–220]. The basic principle couples liquid phase separation with spectroscopy, which allows for quick and precise separation, identification and quantitative analyses of lipid species by providing size, sequence, and structural information.
Lipids present in biological fluids such as urine have been analyzed by separation and spectroscopy methods to evaluate disease states such as essential hypertension and prostatic diseases including prostate cancer [221–223]. Further application of the method has been extended recently to an LC–MS/MS screen for lipids and lipid mediators of synovial fluid from rheumatoid arthritis patients where the profiles of various inflammatory mediators and resolvins have been reported [78]. While LC–MS is routinely used for discovery and/or quantitative lipidomics of biological fluids, it has been proposed that use of these methods could discover regulatory lipids in breath aerosols that could be predictive of pulmonary pathobiology [224,225]. The use of breath aerosols to test for bioactive lipids is being applied to study conditions such as aspirin-exacerbated respiratory disease (AERD), where high baseline levels of 15(S)-HETE are associated with aspirin sensitivity and activate the TRPV1 cough receptor [226– 228]. These methods are also being applied to study exercise induced asthma brought on by inflammatory lipid mediators [229]. The use of LC–MS on breath condensates could also be reapplied to earlier studies such as those on the effects of ozone injury to airway epithelial cells at an early phase [230] as well as to detection of lung cancer, whose feasibility was reported using gas chromatography coupled with mass spectroscopy [231].
Mass spectroscopy is the most commonly used tool to study lipids. However, as biophysics and bioengineering blend with lipidomics, terms such as Raman spectroscopy as well as Coherent anti-Stokes Raman Spectroscopy (CARS) are entering the lipidomics parlance. These techniques are powerful for their ability to enable label-free study of molecules in three dimensions [232,233].
4.2. Raman spectroscopy
One of the perceived drawbacks of LC–MS is that by nature it cannot give spatial information about lipids in defined cellular compartments and relies on bulk or fractionated analyses of extracted lipids [234]. While matrix-assisted laser desorption/ ionization (MALDI) can give spatial information and identify precise molecular compositions of large biomolecules, the equipment is more expensive than for Raman and it requires some initial processing such as pre-coating of the samples with crystal matrix material. The analysis also tends to destroy the tissue. The following references offer an excellent accounting of the process either alone or in combination with imaging modalities [235–237].
Raman spectroscopy (RS) is an optical technique that exploits inherent differences between the vibration frequencies of molecules in response to laser excitation and the consequent effect on inelastic light scattering to generate unique spectra or “fingerprints” for individual molecules. One of the most exciting adaptations of RS has been the use of intrinsic RS spectra to profile biological samples in vitro and in vivo. This type of label-free, pattern profiling from spontaneous Raman scattering can reflect cell-specific protein, lipid and phosphate content, and can even reveal intracellular distribution of metabolites. There are numerous permutations of RS, including Tip-enhanced RS for optical spectroscopy on a nano-scale (TERS), Coherent anti-Stokes RS for overcoming fluorescence interference of basic RS and increasing resolution (CARS), and Resonance RS for polypeptide analysis. Raman scattering can be amplified (e.g., Stimulated Raman Scattering-SRS), which can facilitate in vivo imaging at speeds approaching real time [238]. Utility of the method has been demonstrated in a variety of applications, but particularly in cancer studies [239]. Raman spectroscopy overcomes the need for label that could interfere with lipid packing, and can provide real time information on lipid accumulation and metabolism throughout a living cell over brief or extended time courses [240]. To examine the compartmentalization of EPA after uptake into living cells, spontaneous Raman spectra were generated for DHA, EPA, AA and OA (oleic acid), which have 6, 5, 4 and 1 C=C bond(s) respectively. The Raman shift for the unsaturated fatty acids was mapped to a band at 3015 cm−1, where the peak intensities are roughly proportional to the amount of unsaturation in the lipids, so that the tallest peak at 3015 cm−1 is DHA and the lowest is OA. After incubating A549 human lung carcinoma cells in EPA, cells were profiled and subcellular localization of EPA was found in lipid drops based on the peak height at 3015 cm−1 [241]. Another advantage to the method is the absence of photo damage on repeated imaging. Additional studies have used the technique to examine cholesterol and DHA in the outer segment of retinal rods, and to look at lipid distribution in Caenorhabditis elegans [242–244]. Direct label-free observation of these molecules in relation to the relative microenvironment without the need for biochemical or histochemical analyses is proving to be an exciting new tool in lipidomics [245]. Single-cell laser-trapping Raman spectroscopy has been used for lipid profiling in real time of microalgae under varied growth conditions. Therefore, the technique will likely find additional applications in cell culture overall [238,246]. To that end, the Raman-generated spectra on autophagic cells was recently used to identify a reporter peak of phospholipids at 718 cm(−1) that appeared to reflect cellular autophagy on starvation [247].
Early neoplastic changes can be detected with RS, and malignancies can be discriminated from normal tissue based on the multiplexed RS spectral profiles [248–250]. The desired Raman scattering can be filtered from spectral background noise and fluorescence to generate cellular “fingerprints” that are unique and precise [234]. Algorithms that organize the comprehensive Raman spectrum, and that sort malignant and normal profiles through assignment by discriminant function analyses, already exist and have been applied, for example, to the analysis of human ductal carcinoma cells in a mouse model of human pancreatic cancer [251]. In addition to tissue analyses, biofluids such as serum appear to have potential spectral reporters of carcinoma as well with this methodology [252–254]. The technique was appreciated for its potential in medical applications more than a decade ago and has been steadily proving its value since then [255–257]. Raman spectra of neoplastic tissue are now actively being explored for novel cancer biomarkers [258]. Using Raman for optical biopsy, inflamed buccal mucosa has been profiled for oral cancers [259]. The applications have even found their way into dermatological research where Raman can report on the reaction of skin to cosmetics or therapeutic creams at a molecular level, including the packing of ceramides [260–262]. The source of tissue being imaged can be fresh or frozen and non-invasive measurements of tissue up to 50 mm have become possible with surface-enhanced RS (SERS) probes [263,264]. Theranostic SERS probes have also been developed that allow them to be used for both imaging and heat ablation [265]. With the arrival of near infrared receptor-targeted nanoprobes, the multimodal platform of Raman spectroscopy and microscopy is proving to be a powerful imaging combination whose application yields precise information in both time and space [266].
A multi-platform or “multi-modal” approach, where several technologies are bundled with Raman spectroscopy, can be used to examine tumor heterogeneity. For example, hypoxic regions can be visualized in breast solid tumor models using signatures of elevated lipid metabolism and other molecules that occur in response to hypoxia by using magnetic resonance (MR) imaging, MR spectroscopic imaging (MRSI), and optical imaging in combination [267]. Likewise, confocal Raman microscopy revealed that there are more lipid bodies (13.8%) in human colorectal adenocarcinoma Caco-2 cells than in the “normal” rat intestinal epithelial cell line IEC6 (1.8%). Furthermore, from the spectra of the two cell lines in comparison to reference standards, it was determined that lipid bodies in Caco-2 cells have a larger peak corresponding to arachidonic acid [268]. RS has been used in several breast cancer studies [248,269,270]. In the study by Brozek-Pluska et al., cancerous and non cancerous cells from the same patient were profiled for lipid content. The Raman spectra from the cancer cells were similar to the spectral profiles of gamma linolenic acid and arachidonic acid, a known precursor of procarcinogenic lipid metabolites, whereas the spectra from the normal tissue were comparable to those seen for oleic acid and omega 3 fatty acids. It should be noted though, that in the cancer cells the spectrum was dominated more by protein rather than lipid peaks as might be expected for cells that are actively growing.
The determination of lipid localization in addition to cellular lipid composition by Raman spectroscopy is proving to be valuable for studying such issues as cell polarity, which is an important factor in metastasis. The basal and apical sides of 3D-cultured breast acini were probed by Raman spectroscopy where it was found that lipids on the apical side were more ordered in comparison to the basal side. When polarity was disrupted by calcium chelation or by addition of arachidonic acid, which caused two tight junction proteins (ZO1 and ZO2) to move from the poles, the inverse order was observed [271]. Interestingly, in endothelial cells ZO1 is also mobilized by the activity of sphingosine-1-phosphate [272].
Defects in phosphatidylinositol processing are associated with dissolution of the primary cilium in response to serum, and inhibition of ceramide synthesis severely impairs cilium formation in Madin-Darby Canine Kidney (MDCK) cells but can be rescued by exogenous application of ceramide or ceramide analogs [273,274]. Centrosomes and primary cilia have been shown to be effective reporters of cell polarity, and the loss of the primary cilium appears to be associated with breast cancer [275–277]. Therefore, given the examples of Raman spectroscopy to study lipids in breast cancer, this would appear to be another novel application of the technology, i.e., linkage of lipids, cell organelles, polarity and cancer.
5. Conclusion
The paths less traveled along the lipid continuum are actively being paved by researchers in their quest for additional therapeutics targeted at diseases whose underlying culprit is lipid-mediated inflammation. New therapeutic targets have emerged as novel receptors are de-orphaned and paired with their lipid ligands or as the synergistic effects of lipids with existing chemotherapies are revealed through systems biology and proteo/ lipidomic approaches. Lipids are also being applied as therapeutic agents, as novel pathways that mitigate inflammation are mapped out and marine-enhanced sources are explored for new activities. The evolving convergence of basic and clinical science with biophysics and bioengineering has opened exploration into what lipids have to tell us regarding their place and function in such complex diseases such as cancer. On September 19, 2012 the United States House of Representatives passed the Recalcitrant Cancer Research Act (H.R. 733) in a unanimous vote. The goal of the National Cancer Institute under this bill is to identify major advances for prevention and treatment as well as detection and diagnosis of cancer. Lipidomics together with emerging spectroscopy methods will be leading the way.
Acknowledgements
Where possible the seminal treatises or recent reviews on the various topics have been included. It would require additional volumes to thank the leaders of the field and highlight their many contributions. We are indebted for their ground-breaking efforts that have brought lipidomics to the forefront. I gratefully thank my mentors and colleagues in the lipid field- Drs. K.V. Honn, K.R. Maddipati, and M-J. Lee, and in bioengineering- Drs. G.W. Auner and R.E. Kast. This work was supported in part by funding from the USAMRMC/Department of Defense grant W81XWH-1-0519, USAMRMC/Department of Defense grant W81XWH-08-1-0550, USAMRMC/Department of Defense grant W81XWH-06-1–0226, the National Institute of Health grant CA029997-21, the National Institute of Health grant CA114051 and the Fund for Cancer Research grant 440761.
Footnotes
Dysfunctional cell signaling dynamics in oncology-diagnostic, prognostic and treatment opportunities.
References
- 1.Fiocchi C. What is physiological “intestinal inflammation and how does it differ from pathological” inflammation? Inflamm Bowel Dis. 2008;14(Suppl. 2):S77–S78. doi: 10.1002/ibd.20618. [DOI] [PubMed] [Google Scholar]
- 2.Jabbour HN, Sales KJ, Catalano RD, Norman JE. Inflammatory pathways in female reproductive health and disease. Reproduction. 2009;138:903–919. doi: 10.1530/REP-09-0247. [DOI] [PubMed] [Google Scholar]
- 3.Richards JS, Russell DL, Ochsner S, Espey LL. Ovulation: new dimensions and new regulators of the inflammatory-like response. Annu Rev Physiol. 2002;64:69–92. doi: 10.1146/annurev.physiol.64.081501.131029. [DOI] [PubMed] [Google Scholar]
- 4.Spanel-Borowski K. Ovulation as danger signaling event of innate immunity. Mol Cell Endocrinol. 2011;333:1–7. doi: 10.1016/j.mce.2010.12.008. [DOI] [PubMed] [Google Scholar]
- 5.Neufeld EJ, Majerus PW, Krueger CM, Saffitz JE. Uptake and subcellular distribution of [3H]arachidonic acid in murine fibrosarcoma cells measured by electron microscope autoradiography. J Cell Biol. 1985;101:573–581. doi: 10.1083/jcb.101.2.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dennis EA. The growing phospholipase A2 superfamily of signal transduction enzymes. Trends Biochem Sci. 1997;22:1–2. doi: 10.1016/s0968-0004(96)20031-3. [DOI] [PubMed] [Google Scholar]
- 7.Burke JE, Dennis EA. Phospholipase A2 structure/function, mechanism, and signaling. J Lipid Res. 2009;50(Suppl.):S237–S242. doi: 10.1194/jlr.R800033-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Murakami M, Lambeau G. Emerging roles of secreted phospholipase A(2) enzymes: an update. Biochimie. 2013;95:43–50. doi: 10.1016/j.biochi.2012.09.007. [DOI] [PubMed] [Google Scholar]
- 9.Murakami M, Taketomi Y, Miki Y, Sato H, Hirabayashi T, Yamamoto K. Recent progress in phospholipase A2 research: from cells to animals to humans. Prog Lipid Res. 2011;50:152–192. doi: 10.1016/j.plipres.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 10.Schaloske RH, Dennis EA. The phospholipase A2 superfamily and its group numbering system. Biochim Biophys Acta. 2006;1761:1246–1259. doi: 10.1016/j.bbalip.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 11.Brock TG, Peters-Golden M. Activation and regulation of cellular eicosanoid biosynthesis. Sci World J. 2007;7:1273–1284. doi: 10.1100/tsw.2007.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Serhan CN. Systems approach with inflammatory exudates uncovers novel anti-inflammatory and pro-resolving mediators. Prostaglandins Leukot Essent Fatty Acids. 2008;79:157–163. doi: 10.1016/j.plefa.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brock TG. Lipoxins and resolvins. Inflammation 2011. Ann Arbor Cayman Chem. 2012 [Google Scholar]
- 14.Serhan CN, Petasis NA. Resolvins and protectins in inflammation resolution. Chem Rev. 2011;111:5922–5943. doi: 10.1021/cr100396c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hakulinen J, Meri S. Expression and function of the complement membrane attack complex inhibitor protectin (CD59) on human breast cancer cells. Lab Invest. 1994;71:820–827. [PubMed] [Google Scholar]
- 16.Serhan CN, Krishnamoorthy S, Recchiuti A, Chiang N. Novel anti-inflammatory-pro-resolving mediators and their receptors. Curr Top Med Chem. 2011;11:629–647. doi: 10.2174/1568026611109060629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee CH. Resolvins as new fascinating drug candidates for inflammatory diseases. Arch Pharm Res. 2012;35:3–7. doi: 10.1007/s12272-012-0121-z. [DOI] [PubMed] [Google Scholar]
- 18.Weylandt KH, Chiu CY, Gomolka B, Waechter SF, Wiedenmann B. Omega-3 fatty acids and their lipid mediators: towards an understanding of resolvin and protectin formation. Prostaglandins Other Lipid Mediat. 2012;97:73–82. doi: 10.1016/j.prostaglandins.2012.01.005. [DOI] [PubMed] [Google Scholar]
- 19.Hasturk H, Kantarci A, Van Dyke TE. Paradigm shift in the pharmacological management of periodontal diseases. Front Oral Biol. 2012;15:160–176. doi: 10.1159/000329678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Van Dyke TE. Proresolving lipid mediators: potential for prevention and treatment of periodontitis. J Clin Periodontol. 2011;38(Suppl. 11):119–125. doi: 10.1111/j.1600-051X.2010.01662.x. [DOI] [PubMed] [Google Scholar]
- 21.Herrera BS, Ohira T, Gao L, Omori K, Yang R, Zhu M, et al. An endogenous regulator of inflammation, resolvin E1, modulates osteoclast differentiation and bone resorption. Br J Pharmacol. 2008;155:1214–1223. doi: 10.1038/bjp.2008.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Calder PC. Omega-3 polyunsaturated fatty acids and inflammatory processes: nutrition or pharmacology? Br J Clin Pharmacol. 2012 doi: 10.1111/j.1365-2125.2012.04374.x. http://dx.doi.org/10.1111/j.1365-2125.2012.04374.x [Epub ahead of print] [DOI] [PMC free article] [PubMed]
- 23.Davidson J, Rotondo D, Rizzo MT, Leaver HA. Therapeutic implications of disorders of cell death signalling: membranes, micro-environment, and eicosanoid and docosanoid metabolism. Br J Pharmacol. 2012;166:1193–1210. doi: 10.1111/j.1476-5381.2012.01900.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.D’Orazio N, Gammone MA, Gemello E, De Girolamo M, Cusenza S, Riccioni G. Marine bioactives: pharmacological properties and potential applications against inflammatory diseases. Mar Drugs. 2012;10:812–833. doi: 10.3390/md10040812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Siriwardhana N, Kalupahana NS, Moustaid-Moussa N. Health benefits of n-3 polyunsaturated fatty acids: eicosapentaenoic acid and docosahexaenoic acid. Adv Food Nutr Res. 2012;65:211–222. doi: 10.1016/B978-0-12-416003-3.00013-5. [DOI] [PubMed] [Google Scholar]
- 26.Khan AH, Carson RJ, Nelson SM. Prostaglandins in labor-a translational approach. Front Biosci. 2008;13:5794–5809. doi: 10.2741/3117. [DOI] [PubMed] [Google Scholar]
- 27.Denison FC, Calder AA, Kelly RW. The action of prostaglandin E2 on the human cervix: stimulation of interleukin 8 and inhibition of secretory leukocyte protease inhibitor. Am J Obstet Gynecol. 1999;180:614–620. doi: 10.1016/s0002-9378(99)70263-2. [DOI] [PubMed] [Google Scholar]
- 28.Vladic-Stjernholm Y, Vladic T, Blesson CS, Ekman-Ordeberg G, Sahlin L. Prostaglandin treatment is associated with a withdrawal of progesterone and androgen at the receptor level in the uterine cervix. Reprod Biol Endocrinol. 2009;7:116. doi: 10.1186/1477-7827-7-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Romero R, Nicolaides K, Conde-Agudelo A, Tabor A, O’Brien JM, Cetingoz E, et al. Vaginal progesterone in women with an asymptomatic sonographic short cervix in the midtrimester decreases preterm delivery and neonatal morbidity: a systematic review and metaanalysis of individual patient data. Am J Obstet Gynecol. 2012;206:124. doi: 10.1016/j.ajog.2011.12.003. e1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.el-Refaey H, O’Brien P, Morafa W, Walder J, Rodeck C. Use of oral misoprostol in the prevention of postpartum haemorrhage. Br J Obstet Gynaecol. 1997;104:336–339. doi: 10.1111/j.1471-0528.1997.tb11464.x. [DOI] [PubMed] [Google Scholar]
- 31.Tuncalp O, Hofmeyr GJ, Gulmezoglu AM. Prostaglandins for preventing postpartum haemorrhage. Cochrane Database Syst Rev. 2012;8 doi: 10.1002/14651858.CD000494.pub4. CD000494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kongnyuy EJ, Wiysonge CS. Interventions to reduce haemorrhage during myomectomy for fibroids. Cochrane Database Syst Rev. 2011 doi: 10.1002/14651858.CD005355.pub2. CD005355. [DOI] [PubMed] [Google Scholar]
- 33.El Tahan MR, Warda OM, Rashad A, Yasseen AM, Ramzy EA, Ahmady MS, et al. Effects of preoperative sublingual misoprostol on uterine tone during isoflurane anesthesia for cesarean section. Rev Bras Anestesiol. 2012;62:625–635. doi: 10.1016/S0034-7094(12)70162-9. [DOI] [PubMed] [Google Scholar]
- 34.Hanson WR, Marks JE, Reddy SP, Simon S, Mihalo WE, Tova Y. Protection from radiation-induced oral mucositis by misoprostol, a prostaglandin E(1) analog: a placebo-controlled, double-blind clinical trial. Am J Ther. 1995;2:850–857. doi: 10.1097/00045391-199511000-00005. [DOI] [PubMed] [Google Scholar]
- 35.Lalla RV, Gordon GB, Schubert M, Silverman S, Jr, Hutten M, Sonis ST, et al. A randomized, double-blind, placebo-controlled trial of misoprostol for oral mucositis secondary to high-dose chemotherapy. Support Care Cancer. 2012;20:1797–1804. doi: 10.1007/s00520-011-1277-7. [DOI] [PubMed] [Google Scholar]
- 36.Zhu Y, Liu Y, Zhou W, Xiang R, Jiang L, Huang K, et al. A prostacyclin analogue, iloprost, protects from bleomycin-induced pulmonary fibrosis in mice. Respir Res. 2010;11:34. doi: 10.1186/1465-9921-11-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gurgul-Convey E, Hanzelka K, Lenzen S. Mechanism of prostacyclin-induced potentiation of glucose-induced insulin secretion. Endocrinology. 2012;153:2612–2622. doi: 10.1210/en.2011-2027. [DOI] [PubMed] [Google Scholar]
- 38.Martino L, Masini M, Novelli M, Beffy P, Bugliani M, Marselli L, et al. Palmitate activates autophagy in INS-1E beta-cells and in isolated rat and human pancreatic islets. PLoS One. 2012;7:e36188. doi: 10.1371/journal.pone.0036188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wu JH, Micha R, Imamura F, Pan A, Biggs ML, Ajaz O, et al. Omega-3 fatty acids and incident type 2 diabetes: a systematic review and meta-analysis. Br J Nutr. 2012;107(Suppl. 2):S214–S227. doi: 10.1017/S0007114512001602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Spite M, Serhan CN. Novel lipid mediators promote resolution of acute inflammation: impact of aspirin and statins. Circ Res. 2010;107:1170–1184. doi: 10.1161/CIRCRESAHA.110.223883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gutierrez AD, Sathyanarayana P, Konduru S, Ye Y, Birnbaum Y, Bajaj M. The effect of pioglitazone treatment on 15-epi-lipoxin A4 levels in patients with type 2 diabetes. Atherosclerosis. 2012;223:204–208. doi: 10.1016/j.atherosclerosis.2012.04.016. [DOI] [PubMed] [Google Scholar]
- 42.Calder PC. The role of marine omega-3 (n-3) fatty acids in inflammatory processes, atherosclerosis and plaque stability. Mol Nutr Food Res. 2012;56:1073–1080. doi: 10.1002/mnfr.201100710. [DOI] [PubMed] [Google Scholar]
- 43.Ramadeen A, Dorian P. How are n-3 LCPUFAs antiarrhythmic? A reassessment of n-3 LCPUFAs in cardiac disease. Cardiol Res Pract. 2012;2012:746709. doi: 10.1155/2012/746709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Khairallah RJ, Kim J, O’Shea KM, O’Connell KA, Brown BH, Galvao T, et al. Improved mitochondrial function with diet-induced increase in either docosahexaenoic acid or arachidonic acid in membrane phospholipids. PLoS One. 2012;7:e34402. doi: 10.1371/journal.pone.0034402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stanley WC, Khairallah RJ, Dabkowski ER. Update on lipids and mitochondrial function: impact of dietary n-3 polyunsaturated fatty acids. Curr Opin Clin Nutr Metab Care. 2012;15:122–126. doi: 10.1097/MCO.0b013e32834fdaf7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stanley WC, Dabkowski ER, Ribeiro RF, Jr, O’Connell KA. Dietary fat and heart failure: moving from lipotoxicity to lipoprotection. Circ Res. 2012;110:764–776. doi: 10.1161/CIRCRESAHA.111.253104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Capra V, Back M, Barbieri SS, Camera M, Tremoli E, Rovati GE. Eicosanoids and their drugs in cardiovascular diseases: focus on atherosclerosis and stroke. Med Res Rev. 2012 doi: 10.1002/med.21251. http://dx.doi.org/10.1002/med.21251[Epub ahead of print] [DOI] [PubMed]
- 48.Bulger EM, Maier RV. Lipid mediators in the pathophysiology of critical illness. Crit Care Med. 2000;28:N27–N36. doi: 10.1097/00003246-200004001-00004. [DOI] [PubMed] [Google Scholar]
- 49.Ott J, Hiesgen C, Mayer K. Lipids in critical care medicine. Prostaglandins Leukot Essent Fatty Acids. 2011;85:267–273. doi: 10.1016/j.plefa.2011.04.011. [DOI] [PubMed] [Google Scholar]
- 50.Pontes-Arruda A, Martins LF, de Lima SM, Isola AM, Toledo D, Rezende E, et al. Enteral nutrition with eicosapentaenoic acid, gamma-linolenic acid and antioxidants in the early treatment of sepsis: results from a multicenter, prospective, randomized, double-blinded, controlled study: the INTERSEPT study. Crit Care. 2011;15:R144. doi: 10.1186/cc10267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rice TW, Wheeler AP, Thompson BT, deBoisblanc BP, Steingrub J, Rock P. Enteral omega-3 fatty acid, gamma-linolenic acid, and antioxidant supplementation in acute lung injury. JAMA. 2011;306:1574–1581. doi: 10.1001/jama.2011.1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shimizu T. The future potential of eicosanoids and their inhibitors in paediatric practice. Drugs. 1998;56:169–176. doi: 10.2165/00003495-199856020-00001. [DOI] [PubMed] [Google Scholar]
- 53.Gupta M, Guertin S, Martin S, Omar S. Inhaled prostacyclin and high-frequency oscillatory ventilation in a premature infant with respiratory syncy-tial virus-associated respiratory failure. Pediatrics. 2012;130:e442–e445. doi: 10.1542/peds.2011-0239. [DOI] [PubMed] [Google Scholar]
- 54.Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139:871–890. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 55.Kriz W, Kaissling B, Le Hir M. Epithelial-mesenchymal transition (EMT) in kidney fibrosis: fact or fantasy? J Clin Invest. 2011;121:468–474. doi: 10.1172/JCI44595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gjorevski N, Boghaert E, Nelson CM. Regulation of epithelial-mesenchymal transition by transmission of mechanical stress through epithelial tissues. Cancer Microenviron. 2012;5:29–38. doi: 10.1007/s12307-011-0076-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lee K, Nelson CM. New insights into the regulation of epithelial-mesenchymal transition and tissue fibrosis. Int Rev Cell Mol Biol. 2012;294:171–221. doi: 10.1016/B978-0-12-394305-7.00004-5. [DOI] [PubMed] [Google Scholar]
- 58.Li L, Yang Z, Zhang H, Chen W, Chen M, Zhu Z. Low-intensity pulsed ultrasound regulates proliferation and differentiation of osteoblasts through osteocytes. Biochem Biophys Res Commun. 2012;418:296–300. doi: 10.1016/j.bbrc.2012.01.014. [DOI] [PubMed] [Google Scholar]
- 59.Castelino FV. Lipids and eicosanoids in fibrosis: emerging targets for therapy. Curr Opin Rheumatol. 2012;24:649–655. doi: 10.1097/BOR.0b013e328356d9f6. [DOI] [PubMed] [Google Scholar]
- 60.Priante G, Musacchio E, Valvason C, Clari G, Bordin L, Sartori L, et al. Further insights about the beneficial effects of n-3 fatty acids in the early molecular events of renal fibrosis in vitro. J Nephrol. 2012 doi: 10.5301/jn.5000193. http://dx.doi.org/10.5301/jn.5000193 [Epub ahead of print] [DOI] [PubMed]
- 61.Jenkin KA, Verty AN, McAinch AJ, Hryciw DH. Endocannabinoids and the renal proximal tubule: an emerging role in diabetic nephropathy. Int J Biochem Cell Biol. 2012;44:2028–2031. doi: 10.1016/j.biocel.2012.07.008. [DOI] [PubMed] [Google Scholar]
- 62.Bu S, Asano Y, Bujor A, Highland K, Hant F, Trojanowska M. Dihydrosphingosine 1-phosphate has a potent antifibrotic effect in scleroderma fibroblasts via normalization of phosphatase and tensin homolog levels. Arthritis Rheum. 2010;62:2117–2126. doi: 10.1002/art.27463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Phillis JW, Horrocks LA, Farooqui AA. Cyclooxygenases, lipoxygenases, and epoxygenases in CNS: their role and involvement in neurological disorders. Brain Res Rev. 2006;52:201–243. doi: 10.1016/j.brainresrev.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 64.Farooqui AA. Lipid mediators and their metabolism in the nucleous: implications for Alzheimer’s disease. J Alzheimers Dis. 2012;30(Suppl. 2):S163–S178. doi: 10.3233/JAD-2011-111085. [DOI] [PubMed] [Google Scholar]
- 65.Su KP. Mind–body interface: the role of n-3 fatty acids in psychoneuroim-munology, somatic presentation, and medical illness comorbidity of depression. Asia Pac J Clin Nutr. 2008;17(Suppl. 1):151–157. [PubMed] [Google Scholar]
- 66.Sublette ME, Russ MJ, Smith GS. Evidence for a role of the arachidonic acid cascade in affective disorders: a review. Bipolar Disord. 2004;6:95–105. doi: 10.1046/j.1399-5618.2003.00094.x. [DOI] [PubMed] [Google Scholar]
- 67.Palacios-Pelaez R, Lukiw WJ, Bazan NG. Omega-3 essential fatty acids modulate initiation and progression of neurodegenerative disease. Mol Neurobiol. 2010;41:367–374. doi: 10.1007/s12035-010-8139-z. [DOI] [PubMed] [Google Scholar]
- 68.Zhang W, Hu X, Yang W, Gao Y, Chen J. Omega-3 polyunsaturated fatty acid supplementation confers long-term neuroprotection against neonatal hypoxic-ischemic brain injury through anti-inflammatory actions. Stroke. 2010;41:2341–2347. doi: 10.1161/STROKEAHA.110.586081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kumar P, Kalonia H, Kumar A. Role of LOX/COX pathways in 3-nitropropionic acid-induced Huntington’s disease-like symptoms in rats: protective effect of licofelone. Br J Pharmacol. 2011;164:644–654. doi: 10.1111/j.1476-5381.2011.01418.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chu J, Giannopoulos PF, Ceballos-Diaz C, Golde TE, Pratico D. 5-Lipoxygenase gene transfer worsens memory, amyloid, and tau brain pathologies in a mouse model of alzheimer disease. Ann Neurol. 2012;72:442–454. doi: 10.1002/ana.23642. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 71.Dulin JN, Moore ML, Grill RJ., Jr The dual COX/5-LOX inhibitor licofelone attenuates P-glycoprotein-mediated drug resistance in the injured spinal cord. J Neurotrauma. 2012 doi: 10.1089/neu.2012.2587. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Brenn A, Grube M, Peters M, Fischer A, Jedlitschky G, Kroemer HK, et al. Betaamyloid downregulates MDR1-P-glycoprotein (Abcb1) expression at the blood-brain barrier in mice. Int J Alzheimers Dis. 2011;2011:690121. doi: 10.4061/2011/690121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chen YF, Jobanputra P, Barton P, Bryan S, Fry-Smith A, Harris G, et al. Cyclooxygenase-2 selective non-steroidal anti-inflammatory drugs (etodo-lac, meloxicam, celecoxib, rofecoxib, etoricoxib, valdecoxib and lumiracoxib) for osteoarthritis and rheumatoid arthritis: asystematic review and economic evaluation. Health Technol Assess. 2008;12:1–278. doi: 10.3310/hta12110. iii. [DOI] [PubMed] [Google Scholar]
- 74.Yacoubian S, Serhan CN. New endogenous anti-inflammatory and proresolving lipid mediators: implications for rheumatic diseases. Nat Clin Pract Rheumatol. 2007;3:570–509. doi: 10.1038/ncprheum0616. quiz 571 p following 589. [DOI] [PubMed] [Google Scholar]
- 75.Agarwal SK. Biologic agents in rheumatoid arthritis: an update for managed care professionals. J Manag Care Pharm. 2011;17:S14–S18. doi: 10.18553/jmcp.2011.17.s9-b.S14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Miles EA, Calder PC. Influence of marine n-3 polyunsaturated fatty acids on immune function and a systematic review of their effects on clinical outcomes in rheumatoid arthritis. Br J Nutr. 2012;107(Suppl. 2):S171–S184. doi: 10.1017/S0007114512001560. [DOI] [PubMed] [Google Scholar]
- 77.Rontoyanni VG, Sfikakis PP, Kitas GD, Protogerou AD. Marine n-3 fatty acids for cardiovascular risk reduction and disease control in rheumatoid arthritis: kill two birds with one stone? Curr Pharm Des. 2012;18:1531–1542. doi: 10.2174/138161212799504722. [DOI] [PubMed] [Google Scholar]
- 78.Giera M, Ioan-Facsinay A, Toes R, Gao F, Dalli J, Deelder AM, et al. Lipid and lipid mediator profiling of human synovial fluid in rheumatoid arthritis patients by means of LC-MS/MS. Biochim Biophys Acta. 2012;1821:1415–1424. doi: 10.1016/j.bbalip.2012.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.McMahon M, Brahn E. Inflammatory lipids as a target for therapy in the rheumatic diseases. Expert Opin Invest Drugs. 2008;17:1213–1224. doi: 10.1517/13543784.17.8.1213. [DOI] [PubMed] [Google Scholar]
- 80.Gerber M. Omega-3 fatty acids and cancers: a systematic update review of epidemiological studies. Br J Nutr. 2012;107(Suppl. 2):S228–S239. doi: 10.1017/S0007114512001614. [DOI] [PubMed] [Google Scholar]
- 81.Hull MA. Nutritional agents with anti-lnflammatory properties in chemoprevention of colorectal neoplasia. Recent Results Cancer Res. 2012;191:143–156. doi: 10.1007/978-3-642-30331-9_8. [DOI] [PubMed] [Google Scholar]
- 82.Mandal CC, Ghosh-Choudhury T, Dey N, Choudhury GG, Ghosh-Choudhury N. miR-21 is targeted by omega-3 polyunsaturated fatty acid to regulate breast tumor CSF-1 expression. Carcinogenesis. 2012;33:1897–1908. doi: 10.1093/carcin/bgs198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Moore MR, King RA. Effects ofomega-3 fatty acids on progestin stimulation of invasive properties in breast cancer. Horm Cancer. 2012;3:205–217. doi: 10.1007/s12672-012-0118-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Signori C, DuBrock C, Richie JP, Prokopczyk B, Demers LM, Hamilton C, et al. Administration of omega-3 fatty acids and Raloxifene to women at high risk of breast cancer: interim feasibility and biomarkers analysis from a clinical trial. Eur J Clin Nutr. 2012;66:878–884. doi: 10.1038/ejcn.2012.60. [DOI] [PubMed] [Google Scholar]
- 85.Ghoreishi Z, Esfahani A, Djazayeri A, Djalali M, Golestan B, Ayromlou H, et al. Omega-3 fatty acids are protective against paclitaxel-induced peripheral neuropathy:a randomized double-blind placebo controlled trial. BMC Cancer. 2012;355(12) doi: 10.1186/1471-2407-12-355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Fukui M, Kang KS, Okada K, Zhu BT. EPA, an omega-3 fatty acid, induces apoptosis in human pancreatic cancer cells: role of ROS accumulation, cas-pase- 8 activation, and autophagy induction. J Cell Biochem. 2013;114:192–203. doi: 10.1002/jcb.24354. [DOI] [PubMed] [Google Scholar]
- 87.Liu RZ, Graham K, Glubrecht DD, Lai R, Mackey JR, Godbout R. A fatty acidbinding protein 7/RXRbeta pathway enhances survival and proliferation in triple-negative breast cancer. J Pathol. 2012;228:310–21. doi: 10.1002/path.4001. [DOI] [PubMed] [Google Scholar]
- 88.Janakiram NB, Mohammed A, Rao CV. Role of lipoxins, resolvins, and other bioactive lipids in colon and pancreatic cancer. Cancer Metastasis Rev. 2011;30:507–523. doi: 10.1007/s10555-011-9311-2. [DOI] [PubMed] [Google Scholar]
- 89.Janakiram NB, Rao CV. Role of lipoxins and resolvins as anti-inflammatory and proresolving mediators in colon cancer. Curr Mol Med. 2009;9:565–579. doi: 10.2174/156652409788488748. [DOI] [PubMed] [Google Scholar]
- 90.Rouzer CA, Marnett LJ. Endocannabinoid oxygenation by cyclooxygenases, lipoxygenases, and cytochromes P450: cross-talk between the eicosanoid and endocannabinoid signaling pathways. Chem Rev. 2011;111:5899–5921. doi: 10.1021/cr2002799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wymann MP, Schneiter R. Lipid signalling in disease. Nat Rev Mol Cell Biol. 2008;9:162–176. doi: 10.1038/nrm2335. [DOI] [PubMed] [Google Scholar]
- 92.Mullen TD, Hannun YA, Obeid LM. Ceramide synthases at the centre of sphingolipid metabolism and biology. Biochem J. 2012;441:789–802. doi: 10.1042/BJ20111626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Summers SA. Ceramides in insulin resistance and lipotoxicity. Prog Lipid Res. 2006;45:42–72. doi: 10.1016/j.plipres.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 94.Boyce JA. Eicosanoids in asthma, allergic inflammation, and host defense. Curr Mol Med. 2008;8:335–349. doi: 10.2174/156652408785160989. [DOI] [PubMed] [Google Scholar]
- 95.Krishnamoorthy S, Honn KV. Eicosanoids in tumor progression and metasta sis. Subcell Biochem. 2008;49:145–168. doi: 10.1007/978-1-4020-8831-5_6. [DOI] [PubMed] [Google Scholar]
- 96.Steele VE, Holmes CA, Hawk ET, Kopelovich L, Lubet RA, Crowell JA, et al. Lipoxygenase inhibitors as potential cancer chemopreventives. Cancer Epidemiol Biomarkers Prev. 1999;8:467–483. [PubMed] [Google Scholar]
- 97.Steele VE, Holmes CA, Hawk ET, Kopelovich L, Lubet RA, Crowell JA, et al. Potential use of lipoxygenase inhibitors for cancer chemoprevention. Expert Opin Invest Drugs. 2000;9:2121–2138. doi: 10.1517/13543784.9.9.2121. [DOI] [PubMed] [Google Scholar]
- 98.Nixon GF. Sphingolipids in inflammation: pathological implications and potential therapeutic targets. Br J Pharmacol. 2009;158:982–993. doi: 10.1111/j.1476-5381.2009.00281.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Huwiler A, Pfeilschifter J. Lipids as targets for novel anti-inflammatory therapies. Pharmacol Ther. 2009;124:96–112. doi: 10.1016/j.pharmthera.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 100.Shimizu T. Lipid mediators in health and disease: enzymes and receptors as therapeutic targets for the regulation of immunity and inflammation. Annu Rev Pharmacol Toxicol. 2009;49:123–150. doi: 10.1146/annurev.pharmtox.011008.145616. [DOI] [PubMed] [Google Scholar]
- 101.Mizutani S, Tanaka M, Wheelock CE, Kanehisa M, Goto S. Phylogenetic analysis of lipid mediator GPCRs. Genome Inform. 2010;24:116–126. [PubMed] [Google Scholar]
- 102.Howlett AC. A short guide to the nomenclature of seven-transmembrane spanning receptors for lipid mediators. Life Sci. 2005;77:1522–1530. doi: 10.1016/j.lfs.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 103.Guo Y, Zhang W, Giroux C, Cai Y, Ekambaram P, Dilly AK, et al. Identification of the orphan G protein-coupled receptor GPR31 as a receptor for 12-(S)-hydroxyeicosatetraenoic acid. J Biol Chem. 2011;286:33832–33840. doi: 10.1074/jbc.M110.216564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kostenis E. A glance at G-protein-coupled receptors for lipid mediators: a growing receptor family with remarkably diverse ligands. Pharmacol Ther. 2004;102:243–257. doi: 10.1016/j.pharmthera.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 105.Wang H, Li J, Follett PL, Zhang Y, Cotanche DA, Jensen FE, et al. 12-Lipox-ygenase plays a key role in cell death caused by glutathione depletion and arachidonic acid in rat oligodendrocytes. Eur J Neurosci. 2004;20:2049–2058. doi: 10.1111/j.1460-9568.2004.03650.x. [DOI] [PubMed] [Google Scholar]
- 106.Hampson AJ, Grimaldi M. 12-hydroxyeicosatetrenoate (12-HETE) attenuates AMPA receptor-mediated neurotoxicity: evidence for a G-protein-coupled HETE receptor. J Neurosci. 2002;22:257–264. doi: 10.1523/JNEUROSCI.22-01-00257.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Chang PK, Verbich D, McKinney RA. AMPA receptors as drug targets in neurological disease-advantages, caveats, and future outlook. Eur J Neurosci. 2012;35:1908–1916. doi: 10.1111/j.1460-9568.2012.08165.x. [DOI] [PubMed] [Google Scholar]
- 108.Di Marzo V. The endocannabinoid system: its general strategy of action, tools for its pharmacological manipulation and potential therapeutic exploitation. Pharmacol Res. 2009;60:77–84. doi: 10.1016/j.phrs.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 109.Kreitzer FR, Stella N. The therapeutic potential of novel cannabinoid receptors. Pharmacol Ther. 2009;122:83–96. doi: 10.1016/j.pharmthera.2009.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Schicho R, Storr M. A potential role for GPR55 in gastrointestinal functions. Curr Opin Pharmacol. 2012;12:653–658. doi: 10.1016/j.coph.2012.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Anavi-Goffer S, Baillie G, Irving AJ, Gertsch J, Greig IR, Pertwee RG, et al. Modulation of L-alpha-lysophosphatidylinositol/GPR55 mitogen-activated protein kinase (MAPK) signaling by cannabinoids. J Biol Chem. 2012;287:91–104. doi: 10.1074/jbc.M111.296020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ofek O, Karsak M, Leclerc N, Fogel M, Frenkel B, Wright K, et al. Peripheral cannabinoid receptor, CB2, regulates bone mass. Proc Natl Acad Sci USA. 2006;103:696–701. doi: 10.1073/pnas.0504187103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Henstridge CM, Balenga NA, Kargl J, Andradas C, Brown AJ, Irving A, et al. Minireview: recent developments in the physiology and pathology of the lysophosphatidylinositol-sensitive receptor GPR55. Mol Endocrinol. 2011;25:1835–1848. doi: 10.1210/me.2011-1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Janero DR, Makriyannis A. Cannabinoid receptor antagonists: pharmacological opportunities, clinical experience, and translational prognosis. Expert Opin Emerg Drugs. 2009;14:43–65. doi: 10.1517/14728210902736568. [DOI] [PubMed] [Google Scholar]
- 115.Huwiler A, Pfeilschifter J. New players on the center stage: sphingosine 1-phosphate and its receptors as drug targets. Biochem Pharmacol. 2008;75:1893–1900. doi: 10.1016/j.bcp.2007.12.018. [DOI] [PubMed] [Google Scholar]
- 116.Rosen H, Goetzl EJ. Sphingosine 1-phosphate and its receptors: an autocrine and paracrine network. Nat Rev Immunol. 2005;5:560–570. doi: 10.1038/nri1650. [DOI] [PubMed] [Google Scholar]
- 117.Harris GL, Creason MB, Brulte GB, Herr DR. In vitro and in vivo antagonism of a G protein-coupled receptor (S1P3) with a novel blocking monoclonal antibody. PLoS One. 2012;7:e35129. doi: 10.1371/journal.pone.0035129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Novgorodov AS, El-Alwani M, Bielawski J, Obeid LM, Gudz TI. Activation of sphingosine-1-phosphate receptor S1P5 inhibits oligodendrocyte progenitor migration. FASEB J. 2007;21:1503–1514. doi: 10.1096/fj.06-7420com. [DOI] [PubMed] [Google Scholar]
- 119.Obinata H, Hla T. Fine-tuning S1P therapeutics. Chem Biol. 2012;19:1080–1082. doi: 10.1016/j.chembiol.2012.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Hsu A, Zhang W, Lee JF, An J, Ekambaram P, Liu J, et al. Sphingosine-1-phosphate receptor-3 signaling up-regulates epidermal growth factor receptor and enhances epidermal growth factor receptor-mediated carcinogenic activities in cultured lung adenocarcinoma cells. Int J Oncol. 2012;40:1619–1626. doi: 10.3892/ijo.2012.1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ishii I, Fukushima N, Ye X, Chun J. Lysophospholipid receptors: signaling and biology. Annu Rev Biochem. 2004;73:321–354. doi: 10.1146/annurev.biochem.73.011303.073731. [DOI] [PubMed] [Google Scholar]
- 122.Parrill AL, Lima S, Spiegel S. Structure of the first sphingosine 1-phosphate receptor. Sci Signal. 2012;5:pe23. doi: 10.1126/scisignal.2003160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Parrill AL. Comparative modeling of lipid receptors. Methods Mol Biol. 2012;914:207–218. doi: 10.1007/978-1-62703-023-6_12. [DOI] [PubMed] [Google Scholar]
- 124.Ye S, Zaitseva E, Caltabiano G, Schertler GF, Sakmar TP, Deupi X, et al. Tracking G-protein-coupled receptor activation using genetically encoded infrared probes. Nature. 2010;464:1386–1389. doi: 10.1038/nature08948. [DOI] [PubMed] [Google Scholar]
- 125.Andersson KE. Prostanoid receptor subtypes: new targets for OAB drugs? J Urol. 2009;182:2099–2100. doi: 10.1016/j.juro.2009.08.100. [DOI] [PubMed] [Google Scholar]
- 126.Kontogiorgis C, Hadjipavlou-Litina D. Thromboxane synthase inhibitors and thromboxane A2 receptor antagonists: a quantitative structure activity relationships (QSARs) analysis. Curr Med Chem. 2010;17:3162–3214. doi: 10.2174/092986710792231978. [DOI] [PubMed] [Google Scholar]
- 127.Marrache AM, Gobeil F, Zhu T, Chemtob S. Intracellular signaling of lipid mediators via cognate nuclear G protein-coupled receptors. Endothelium. 2005;12:63–72. doi: 10.1080/10623320590933815. [DOI] [PubMed] [Google Scholar]
- 128.Naithani R, Huma LC, Moriarty RM, McCormick DL, Mehta RG. Comprehensive review of cancer chemopreventive agents evaluated in experimental carcinogenesis models and clinical trials. Curr Med Chem. 2008;15:1044–1071. doi: 10.2174/092986708784221403. [DOI] [PubMed] [Google Scholar]
- 129.Chi YS, Jong HG, Son KH, Chang HW, Kang SS, Kim HP. Effects of naturally occurring prenylated flavonoids on enzymes metabolizing arachidonic acid: cyclooxygenases and lipoxygenases. Biochem Pharmacol. 2001;62:1185–1191. doi: 10.1016/s0006-2952(01)00773-0. [DOI] [PubMed] [Google Scholar]
- 130.Baumann J, von Bruchhausen F, Wurm G. Flavonoids and related compounds as inhibition of arachidonic acid peroxidation. Prostaglandins. 1980;20:627–639. doi: 10.1016/0090-6980(80)90103-3. [DOI] [PubMed] [Google Scholar]
- 131.Alcaraz MJ, Ferrandiz ML. Modification of arachidonic metabolism by flavonoids. J Ethnopharmacol. 1987;21:209–229. doi: 10.1016/0378-8741(87)90101-2. [DOI] [PubMed] [Google Scholar]
- 132.Sun D, Hurdle JG, Lee R, Cushman M, Pezzuto JM. Evaluation of flavonoid and resveratrol chemical libraries reveals abyssinone II as a promising antibacterial lead. ChemMedChem. 2012;7:1541–1545. doi: 10.1002/cmdc.201200253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Mascayano C, Nunez G, Acevedo W, Rezende MC. Binding of arachidonic acid and two flavonoid inhibitors to human 12- and 15-lipoxygenases: a steered molecular dynamics study. J Mol Model. 2010;16:1039–1045. doi: 10.1007/s00894-009-0616-9. [DOI] [PubMed] [Google Scholar]
- 134.Basabe P, de Roman M, Marcos IS, Diez D, Blanco A, Bodero O, et al. Prenylflavonoids and prenyl/alkyl-phloroacetophenones: synthesis and antitumour biological evaluation. Eur J Med Chem. 2010;45:4258–4269. doi: 10.1016/j.ejmech.2010.06.025. [DOI] [PubMed] [Google Scholar]
- 135.Burnett BP, Jia Q, Zhao Y, Levy RM. A medicinal extract of Scutellaria baicalensis and Acacia catechu acts as a dual inhibitor of cyclooxygenase and 5-lipoxygenase to reduce inflammation. J Med Food. 2007;10:442–451. doi: 10.1089/jmf.2006.255. [DOI] [PubMed] [Google Scholar]
- 136.Yang J, Yang X, Chu Y, Li M. Identification of Baicalin as an immunoregulatory compound by controlling T(H)17 cell differentiation. PLoS One. 2011;6:e17164. doi: 10.1371/journal.pone.0017164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Basso AS, Cheroutre H, Mucida D. More stories on Th17 cells. Cell Res. 2009;19:399–411. doi: 10.1038/cr.2009.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Park SJ, Lee YC. Interleukin-17 regulation: an attractive therapeutic approach for asthma. Respir Res. 2010;78(11) doi: 10.1186/1465-9921-11-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Zhou L, Lopes JE, Chong MM, Ivanov II, Min R, Victora GD, et al. TGF-beta-induced Foxp3 inhibits T(H)17 cell differentiation by antagonizing RORgammat function. Nature. 2008;453:236–240. doi: 10.1038/nature06878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Fan H, Liu Z, Jiang S. Th17 and regulatory T cell subsets in diseases and clinical application. Int Immunopharmacol. 2011;11:533–535. doi: 10.1016/j.intimp.2011.02.020. [DOI] [PubMed] [Google Scholar]
- 141.Labrecque MP, Takhar MK, Hollingshead BD, Prefontaine GG, Perdew GH, Beischlag TV. Distinct roles for aryl hydrocarbon receptor nuclear translocator and ah receptor in estrogen-mediated signaling in human cancer cell lines. PLoS One. 2012;7:e29545. doi: 10.1371/journal.pone.0029545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Denison MS, Nagy SR. Activation of the aryl hydrocarbon receptor by structurally diverse exogenous and endogenous chemicals. Annu Rev Pharmacol Toxicol. 2003;43:309–34. doi: 10.1146/annurev.pharmtox.43.100901.135828. [DOI] [PubMed] [Google Scholar]
- 143.Beischlag TV, Luis Morales J, Hollingshead BD, Perdew GH. The aryl hydrocarbon receptor complex and the control of gene expression. Crit Rev Eukaryot Gene Expr. 2008;18:207–250. doi: 10.1615/critreveukargeneexpr.v18.i3.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Crome SQ, Wang AY, Levings MK. Translational mini-review series on Th17 cells: function and regulation of human T helper 17 cells in health and disease. Clin Exp Immunol. 2010;159:109–119. doi: 10.1111/j.1365-2249.2009.04037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Louten J, Boniface K, de Waal Malefyt R. Development and function of TH17 cells in health and disease. J Allergy Clin Immunol. 2009;123:1004–1011. doi: 10.1016/j.jaci.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 146.Nasef NA, Ferguson LR. Inflammatory bowel disease and pregnancy: overlapping pathways. Transl Res. 2012;160:65–83. doi: 10.1016/j.trsl.2011.12.002. [DOI] [PubMed] [Google Scholar]
- 147.Wilke CM, Kryczek I, Wei S, Zhao E, Wu K, Wang G, et al. Th17 cells in cancer: help or hindrance? Carcinogenesis. 2011;32:643–649. doi: 10.1093/carcin/bgr019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.He S, Fei M, Wu Y, Zheng D, Wan D, Wang L, et al. Distribution and clinical significance of th17 cells in the tumor microenvironment and peripheral blood of pancreatic cancer patients. Int J Mol Sci. 2011;12:7424–7437. doi: 10.3390/ijms12117424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Yang L, Qi Y, Hu J, Tang L, Zhao S, Shan B. Expression of Th17 cells in breast cancer tissue and its association with clinical parameters. Cell Biochem Biophys. 2012;62:153–159. doi: 10.1007/s12013-011-9276-3. [DOI] [PubMed] [Google Scholar]
- 150.Wang J, Pae M, Meydani SN, Wu D. Green tea epigallocatechin-3-gallate modulates differentiation of naive CD4(+) T cells into specific lineage effector cells. J Mol Med (Berl) 2012 doi: 10.1007/s00109-012-0964-2. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 151.Jin J, Chang Y, Wei W, He YF, Hu SS, Wang D, et al. Prostanoid EP1 receptor as the target of (−)-epigallocatechin-3-gallate in suppressing hepatocellular carcinoma cells in vitro. Acta Pharmacol Sin. 2012;33:701–709. doi: 10.1038/aps.2012.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Sreeramkumar V, Fresno M, Cuesta N. Prostaglandin E2 and T cells: friends or foes? Immunol Cell Biol. 2012;90:579–586. doi: 10.1038/icb.2011.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Ferrario A, Luna M, Rucker N, Wong S, Gomer CJ. Pro-apoptotic and antiinflammatory properties of the green tea constituent epigallocatechin gallate increase photodynamic therapy responsiveness. Lasers Surg Med. 2011;43:644–650. doi: 10.1002/lsm.21081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Hillman GG, Singh-Gupta V. Soy isoflavones sensitize cancer cells to radiotherapy. Free Radic Biol Med. 2011;51:289–298. doi: 10.1016/j.freeradbiomed.2011.04.039. [DOI] [PubMed] [Google Scholar]
- 155.Yang H, Li F, Xiong X, Kong X, Zhang B, Yuan X, et al. Soy isoflavones modulate adipokines and myokines to regulate lipid metabolism in adipose tissue, skeletal muscle and liver of male Huanjiang mini-pigs. Mol Cell Endocrinol. 2013;365:44–51. doi: 10.1016/j.mce.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 156.Iyer A, Fairlie DP, Prins JB, Hammock BD, Brown L. Inflammatory lipid mediators in adipocyte function and obesity. Nat Rev Endocrinol. 2010;6:71–82. doi: 10.1038/nrendo.2009.264. [DOI] [PubMed] [Google Scholar]
- 157.Wallace JM. Nutritional and botanical modulation of the inflammatory cascade-eicosanoids, cyclooxygenases, and lipoxygenases-as an adjunct in cancer therapy. Integr Cancer Ther. 2002;1:7–37. doi: 10.1177/153473540200100102. discussion 37. [DOI] [PubMed] [Google Scholar]
- 158.Altenburg JD, Bieberich AA, Terry C, Harvey KA, Vanhorn JF, Xu Z, et al. A synergistic antiproliferation effect of curcumin and docosahexaenoic acid in SK-BR-3 breast cancer cells: unique signaling not explained by the effects of either compound alone. BMC Cancer. 2011;11:149. doi: 10.1186/1471-2407-11-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Hong J, Bose M, Ju J, Ryu JH, Chen X, Sang S, et al. Modulation of arachidonic acid metabolism by curcumin and related beta-diketone derivatives: effects on cytosolic phospholipase A(2), cyclooxygenases and 5-lipoxygenase. Carcinogenesis. 2004;25:1671–1679. doi: 10.1093/carcin/bgh165. [DOI] [PubMed] [Google Scholar]
- 160.Rao CV. Regulation of COX and LOX by curcumin. Adv Exp Med Biol. 2007;595:213–226. doi: 10.1007/978-0-387-46401-5_9. [DOI] [PubMed] [Google Scholar]
- 161.Anand P, Kunnumakkara AB, Newman RA, Aggarwal BB. Bioavailability of curcumin: problems and promises. Mol Pharm. 2007;4:807–818. doi: 10.1021/mp700113r. [DOI] [PubMed] [Google Scholar]
- 162.Kanwar SS, Yu Y, Nautiyal J, Patel BB, Padhye S, Sarkar FH, et al. Difluorinatedcurcumin (CDF): a novel curcumin analog is a potent inhibitor of colon cancer stem-like cells. Pharm Res. 2011;28:827–838. doi: 10.1007/s11095-010-0336-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Rocks N, Bekaert S, Coia I, Paulissen G, Gueders M, Evrard B, et al. Curcumincyclodextrin complexes potentiate gemcitabine effects in an orthotopic mouse model of lung cancer. Br J Cancer. 2012;107:1083–1092. doi: 10.1038/bjc.2012.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Dandawate PR, Vyas A, Ahmad A, Banerjee S, Deshpande J, Swamy KV, et al. Inclusion complex of novel curcumin analogue CDF and beta-cyclodextrin (1:2) and its enhanced in vivo anticancer activity against pancreatic cancer. Pharm Res. 2012;29:1775–1786. doi: 10.1007/s11095-012-0700-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Roy S, Levi E, Majumdar AP, Sarkar FH. Expression of miR-34 is lost in colon cancer which can be re-expressed by a novel agent CDF. J Hematol Oncol. 2012;5:58. doi: 10.1186/1756-8722-5-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Li WQ, Chen C, Xu MD, Guo J, Li YM, Xia QM, et al. The rno-miR-34 family is upregulated and targets ACSL1 in dimethylnitrosamine-induced hepatic fibrosis in rats. FEBS J. 2011;278:1522–1532. doi: 10.1111/j.1742-4658.2011.08075.x. [DOI] [PubMed] [Google Scholar]
- 167.Lovis P, Roggli E, Laybutt DR, Gattesco S, Yang JY, Widmann C, et al. Alterations in microRNA expression contribute to fatty acid-induced pancreatic beta-cell dysfunction. Diabetes. 2008;57:2728–2736. doi: 10.2337/db07-1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Jankun J, Aleem AM, Malgorzewicz S, Szkudlarek M, Zavodszky MI, Dewitt DL, et al. Synthetic curcuminoids modulate the arachidonic acid metabolism of human platelet 12-lipoxygenase and reduce sprout formation of human endothelial cells. Mol Cancer Ther. 2006;5:1371–1382. doi: 10.1158/1535-7163.MCT-06-0021. [DOI] [PubMed] [Google Scholar]
- 169.Dileep KV, Tintu I, Sadasivan C. Molecular docking studies of curcumin analogs with phospholipase A2. Interdiscip Sci. 2011;3:189–197. doi: 10.1007/s12539-011-0090-9. [DOI] [PubMed] [Google Scholar]
- 170.Aries MF, Vaissiere C, Pinelli E, Pipy B, Charveron M. Avena Rhealba inhibits A23187-stimulated arachidonic acid mobilization, eicosanoid release, and cPLA2 expression in human keratinocytes: potential in cutaneous inflammatory disorders. Biol Pharm Bull. 2005;28:601–606. doi: 10.1248/bpb.28.601. [DOI] [PubMed] [Google Scholar]
- 171.Shibata T, Nagayama K, Tanaka R, Yamaguchi K, Nakamura T. Inhibitory effects of brown algal phlorotannins on secretory phospholipase A<sub>2</sub>s, lipoxygenases and cyclooxygenases. J Appl Phycol. 2003;15:61–66. [Google Scholar]
- 172.van Leyen K, Arai K, Jin G, Kenyon V, Gerstner B, Rosenberg PA, et al. Novel lipoxygenase inhibitors as neuroprotective reagents. J Neurosci Res. 2008;86:904–909. doi: 10.1002/jnr.21543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Panigrahy D, Kaipainen A, Greene ER, Huang S. Cytochrome P450-derived eicosanoids: the neglected pathway in cancer. Cancer Metastasis Rev. 2010;29:723–7235. doi: 10.1007/s10555-010-9264-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Pillarisetti S, Khanna I. Targeting soluble epoxide hydrolase for inflammation and pain - an overview of pharmacology and the inhibitors. Inflamm Allergy Drug Targets. 2012;11:143–158. doi: 10.2174/187152812800392823. [DOI] [PubMed] [Google Scholar]
- 175.Elkady M, Niess R, Schaible AM, Bauer J, Luderer S, Ambrosi G, et al. Modified acidic nonsteroidal anti-inflammatory drugs as dual inhibitors of mPGES-1 and 5-LOX. J Med Chem. 2012;55:8958–8962. doi: 10.1021/jm3010543. [DOI] [PubMed] [Google Scholar]
- 176.Kodela R, Chattopadhyay M, Kashfi K. NOSH-aspirin: a novel nitric oxide-hydrogen sulfide-releasing hybrid: a new class of anti-inflammatory pharmaceuticals. ACS Med Chem Lett. 2012;3:257–262. doi: 10.1021/ml300002m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Chattopadhyay M, Kodela R, Olson KR, Kashfi K. NOSH-aspirin (NBS-1120), a novel nitric oxide- and hydrogen sulfide-releasing hybrid is a potent inhibitor of colon cancer cell growth in vitro and in a xenograft mouse model. Biochem Biophys Res Commun. 2012;419:523–528. doi: 10.1016/j.bbrc.2012.02.051. [DOI] [PubMed] [Google Scholar]
- 178.Mayfield SP, Franklin SE, Lerner RA. Expression and assembly of a fully active antibody in algae. Proc Natl Acad Sci USA. 2003;100:438–442. doi: 10.1073/pnas.0237108100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Rasala BA, Mayfield SP. The microalga Chlamydomonas reinhardtii as a platform for the production of human protein therapeutics. Bioeng Bugs. 2011;2:50–54. doi: 10.4161/bbug.2.1.13423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Guschina IA, Harwood JL. Lipids and lipid metabolism in eukaryotic algae. Prog Lipid Res. 2006;45:160–186. doi: 10.1016/j.plipres.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 181.Pruvost J, Van Vooren G, Cogne G, Legrand J. Investigation of biomass and lipids production with Neochloris oleoabundans in photobioreactor. Bioresour Technol. 2009;100:5988–5995. doi: 10.1016/j.biortech.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 182.Davis RW, Volponi JV, Jones HD, Carvalho BJ, Wu H, Singh S. Multiplex fluorometric assessment of nutrient limitation as a strategy for enhanced lipid enrichment and harvesting of Neochloris oleoabundans. Biotechnol Bioeng. 2012;109:2503–2512. doi: 10.1002/bit.24517. [DOI] [PubMed] [Google Scholar]
- 183.Pereira SL, Leonard AE, Huang YS, Chuang LT, Mukerji P. Identification of two novel microalgal enzymes involved in the conversion of the omega3-fatty acid, eicosapentaenoic acid, into docosahexaenoic acid. Biochem J. 2004;384:357–366. doi: 10.1042/BJ20040970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Meireles LA, Guedes AC, Malcata FX. Lipid class composition of the microalga Pavlova lutheri: eicosapentaenoic and docosahexaenoic acids. J Agric Food Chem. 2003;51:2237–2241. doi: 10.1021/jf025952y. [DOI] [PubMed] [Google Scholar]
- 185.Dembitsky VM. Anticancer activity of natural and synthetic acetylenic lipids. Lipids. 2006;41:883–924. doi: 10.1007/s11745-006-5044-3. [DOI] [PubMed] [Google Scholar]
- 186.Uma R, Sivasubramanian V, Niranjali Devaraj S. Evaluation of in vitro antioxidant activities and antiproliferative activity of green microalgae, Desmococcus olivaceous and Chlorococcum humicola. J Algal Biomass Utln. 2011;2:82–93. [Google Scholar]
- 187.Harada H, Noro T, Kamei Y. Selective antitumor activity in vitro from marine algae from Japan coasts. Biol Pharm Bull. 1997;20:541–546. doi: 10.1248/bpb.20.541. [DOI] [PubMed] [Google Scholar]
- 188.Talero E, Avila-Roman J, Motilva V. Chemoprevention with phytonutrients and microalgae products in chronic inflammation and colon cancer. Curr Pharm Des. 2012;18:3939–3965. doi: 10.2174/138161212802083725. [DOI] [PubMed] [Google Scholar]
- 189.Sithranga Boopathy N, Kathiresan K. Anticancer drugs from marine flora: an overview. J Oncol. 2010;2010:214186. doi: 10.1155/2010/214186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Nappo M, Berkov S, Massucco C, Di Maria V, Bastida J, Codina C, et al. Apoptotic activity of the marine diatom Cocconeis scutellum and eicosapentaenoic acid in BT20 cells. Pharm Biol. 2012;50:529–535. doi: 10.3109/13880209.2011.611811. [DOI] [PubMed] [Google Scholar]
- 191.Hajjaji N, Bougnoux P. Selective sensitization of tumors to chemotherapy by marine-derived lipids:a review. Cancer Treat Rev. 2012 doi: 10.1016/j.ctrv.2012.07.001. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 192.Raja R, Hemaiswarya S, Kumar NA, Sridhar S, Rengasamy R. A perspective on the biotechnological potential of microalgae. Crit Rev Microbiol. 2008;34:77–88. doi: 10.1080/10408410802086783. [DOI] [PubMed] [Google Scholar]
- 193.Pulz O, Gross W. Valuable products from biotechnology of microalgae. Appl Microbiol Biotechnol. 2004;65:635–648. doi: 10.1007/s00253-004-1647-x. [DOI] [PubMed] [Google Scholar]
- 194.Chumnanpuen P, Brackmann C, Nandy SK, Chatzipapadopoulos S, Nielsen J, Enejder A. Lipid biosynthesis monitored at the single-cell level in Saccharo-myces cerevisiae. Biotechnol J. 2012;7:594–601. doi: 10.1002/biot.201000386. [DOI] [PubMed] [Google Scholar]
- 195.Wei Y, Yemisci M, Kim HH, Yung LM, Shin HK, Hwang SK, et al. Fingolimod provides long-term protection in rodent models of cerebral ischemia. Ann Neurol. 2011;69:119–129. doi: 10.1002/ana.22186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Brinkmann V, Billich A, Baumruker T, Heining P, Schmouder R, Francis G, et al. Fingolimod (FTY720): discovery and development of an oral drug to treat multiple sclerosis. Nat Rev Drug Discov. 2010;9:883–897. doi: 10.1038/nrd3248. [DOI] [PubMed] [Google Scholar]
- 197.Kashfi K, Rigas B. Non-COX-2 targets and cancer: expanding the molecular target repertoire of chemoprevention. Biochem Pharmacol. 2005;70:969–986. doi: 10.1016/j.bcp.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 198.Benelli R, Vene R, Minghelli S, Carlone S, Gatteschi B, Ferrari N. Celecoxib induces proliferation and Amphiregulin production in colon subepithelial myofibroblasts, activating erk1-2 signaling in synergy with EGFR. Cancer Lett. 2013;328:73–82. doi: 10.1016/j.canlet.2012.09.008. [DOI] [PubMed] [Google Scholar]
- 199.Lim SJ, Lee E, Lee EH, Kim SY, Cha JH, Choi H, et al. Docosahexaenoic acid sensitizes colon cancer cells to sulindac sulfide-induced apoptosis. Oncol Rep. 2012;27:2023–2030. doi: 10.3892/or.2012.1706. [DOI] [PubMed] [Google Scholar]
- 200.Ding WQ, Liu B, Vaught JL, Palmiter RD, Lind SE. Clioquinol and docosahexaenoic acid act synergistically to kill tumor cells. Mol Cancer Ther. 2006;5:1864–1872. doi: 10.1158/1535-7163.MCT-06-0067. [DOI] [PubMed] [Google Scholar]
- 201.Tuller ER, Beavers CT, Lou JR, Ihnat MA, Benbrook DM, Ding WQ. Docosahexaenoic acid inhibits superoxide dismutase 1 gene transcription in human cancer cells: the involvement of peroxisome proliferator-activated receptor alpha and hypoxia-inducible factor-2alpha signaling. Mol Pharmacol. 2009;76:588–595. doi: 10.1124/mol.109.057430. [DOI] [PubMed] [Google Scholar]
- 202.Steinert BW, Tang DG, Grossi IM, Umbarger LA, Honn KV. Studies on the role of platelet eicosanoid metabolism and integrin alpha IIb beta 3 in tumor-cellinduced platelet aggregation. Int J Cancer. 1993;54:92–101. doi: 10.1002/ijc.2910540116. [DOI] [PubMed] [Google Scholar]
- 203.Honn KV, Tang DG, Crissman JD. Platelets and cancer metastasis: a causal relationship? Cancer Metastasis Rev. 1992;11:325–351. doi: 10.1007/BF01307186. [DOI] [PubMed] [Google Scholar]
- 204.Rao GH, Radha E, White JG. Effect of docosahexaenoic acid (DHA) on arachidonic acid metabolism and platelet function. Biochem Biophys Res Commun. 1983;117:549–555. doi: 10.1016/0006-291x(83)91235-4. [DOI] [PubMed] [Google Scholar]
- 205.Coccheri S. Antiplatelet drugs-do we need new options? With a reappraisal of direct thromboxane inhibitors. Drugs. 2010;70:887–908. doi: 10.2165/11536000-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 206.Menter DG, Onoda JM, Taylor JD, Honn KV. Effects of prostacyclin on tumor cell-induced platelet aggregation. Cancer Res. 1984;44:450–456. [PubMed] [Google Scholar]
- 207.Tang K, Honn KV. 12(S)-HETE in cancer metastasis. Adv Exp Med Biol. 1999;447:181–191. doi: 10.1007/978-1-4615-4861-4_17. [DOI] [PubMed] [Google Scholar]
- 208.Jurasz P, Alonso-Escolano D, Radomski MW. Platelet-cancer interactions: mechanisms and pharmacology of tumour cell-induced platelet aggregation. Br J Pharmacol. 2004;143:819–826. doi: 10.1038/sj.bjp.0706013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Moreno JJ. New aspects of the role of hydroxyeicosatetraenoic acids in cell growth and cancer development. Biochem Pharmacol. 2009;77:1–10. doi: 10.1016/j.bcp.2008.07.033. [DOI] [PubMed] [Google Scholar]
- 210.Tang DG, Renaud C, Stojakovic S, Diglio CA, Porter A, Honn KV. 12(S)-HETE is a mitogenic factor for microvascular endothelial cells: its potential role in angiogenesis. Biochem Biophys Res Commun. 1995;211:462–468. doi: 10.1006/bbrc.1995.1836. [DOI] [PubMed] [Google Scholar]
- 211.Gross ER, Hsu AK, Gross GJ. Acute aspirin treatment abolishes, whereas acute ibuprofen treatment enhances morphine-induced cardioprotection: role of 12-lipoxygenase. J Pharmacol Exp Ther. 2004;310:185–191. doi: 10.1124/jpet.103.064667. [DOI] [PubMed] [Google Scholar]
- 212.Apraiz A, Idkowiak-Baldys JK, Boyano MD, Perez-Yarza G, Hannun YA, Asumendi A. Evaluation of bioactive sphingolipids in 4-HPR-resistant leukemia cells. BMC Cancer. 2011;11:477. doi: 10.1186/1471-2407-11-477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Liu YY, Yu JY, Yin D, Patwardhan GA, Gupta V, Hirabayashi Y, et al. A role for ceramide in driving cancer cell resistance to doxorubicin. FASEB J. 2008;22:2541–2551. doi: 10.1096/fj.07-092981. [DOI] [PubMed] [Google Scholar]
- 214.Ahn Q, Jeong SJ, Lee HJ, Kwon HY, Han I, Kim HS, et al. Inhibition of cyclooxygenase-2-dependent survivin mediates decursin-induced apo-ptosis in human KBM-5 myeloid leukemia cells. Cancer Lett. 2010;298:212–221. doi: 10.1016/j.canlet.2010.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Wenk MR. Lipidomics: new tools and applications. Cell. 2010;143:888–895. doi: 10.1016/j.cell.2010.11.033. [DOI] [PubMed] [Google Scholar]
- 216.Islam MO, Lim YT, Chan CE, Cazenave-Gassiot A, Croxford JL, Wenk MR, et al. Generation and characterization of a novel recombinant antibody against 15-ketocholestane isolated by phage-display. Int J Mol Sci. 2012;13:4937–4948. doi: 10.3390/ijms13044937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Yoon Y, Lee PJ, Kurilova S, Cho W. In situ quantitative imaging of cellular lipids using molecular sensors. Nat Chem. 2011;3:868–8674. doi: 10.1038/nchem.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Yang K, Han X. Accurate quantification of lipid species by electrospray ionization mass spectrometry - meet a key challenge in lipidomics. Metabolites. 2011;1:21–40. doi: 10.3390/metabo1010021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Hartler J, Tharakan R, Kofeler HC, Graham DR, Thallinger GG. Bioinformatics tools and challenges in structural analysis of lipidomics MS/MS data. Brief Bioinform. 2012 doi: 10.1093/bib/bbs030. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 220.Brown HA, editor. Lipidomics and Bioactive Lipids: Mass Spectrometry– Based Lipid Analysis. San Diego, California: Academic Press; 2007. p. 387p. [Google Scholar]
- 221.Yang YJ, Lee SH, Hong SJ, Chung BC. Comparison of fatty acid profiles in the serum of patients with prostate cancer and benign prostatic hyperplasia. Clin Biochem. 1999;32:405–409. doi: 10.1016/s0009-9120(99)00036-3. [DOI] [PubMed] [Google Scholar]
- 222.Norwood DL, Kodo N, Millington DS. Application of continuous-flow liquid chromatography/fast-atom bombardment mass spectrometry to the analysis of diagnostic acylcarnitines in human urine. Rapid Commun Mass Spectrom. 1988;2:269–272. doi: 10.1002/rcm.1290021205. [DOI] [PubMed] [Google Scholar]
- 223.Gonzalez-Nunez D, Claria J, Rivera F, Poch E. Increased levels of 12(S)-HETE in patients with essential hypertension. Hypertension. 2001;37:334–338. doi: 10.1161/01.hyp.37.2.334. [DOI] [PubMed] [Google Scholar]
- 224.White DC, Geyer R, Cantu J, Jo SC, Peacock AD, Saxton AM, et al. Feasibility of assessment of regulatory lipids in breath condensate as potential presymp-tomatic harbingers of pulmonary pathobiology. J Microbiol Methods. 2005;62:293–302. doi: 10.1016/j.mimet.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 225.Lundstrom SL, Balgoma D, Wheelock AM, Haeggstrom JZ, Dahlen SE, Wheelock CE. Lipid mediator profiling in pulmonary disease. Curr Pharm Biotechnol. 2011;12:1026–1052. doi: 10.2174/138920111795909087. [DOI] [PubMed] [Google Scholar]
- 226.Adcock JJ. TRPV1 receptors in sensitisation of cough and pain reflexes. Pulm Pharmacol Ther. 2009;22:65–70. doi: 10.1016/j.pupt.2008.12.014. [DOI] [PubMed] [Google Scholar]
- 227.Schafer D, Maune S. Pathogenic mechanisms and in vitro diagnosis of AERD. J Allergy (Cairo) 2012;2012:789232. doi: 10.1155/2012/789232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Koskela H, Purokivi M, Nieminen R, Moilanen E. The cough receptor TRPV1 agonists 15(S)-HETE and LTB4 in the cough response to hypertonicity. Inflamm Allergy Drug Targets. 2012;11:102–108. doi: 10.2174/187152812800392814. [DOI] [PubMed] [Google Scholar]
- 229.Syslova K, Kacer P, Vilhanova B, Kuzma M, Lipovova P, Fenclova Z, et al. Determination of cysteinyl leukotrienes in exhaled breath condensate: method combining immunoseparation with LC-ESI-MS/MS. J Chromatogr B Analyt Technol Biomed Life Sci. 2011;879:2220–2208. doi: 10.1016/j.jchromb.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 230.Leikauf GD, Simpson LG, Santrock J, Zhao Q, Abbinante-Nissen J, Zhou S, et al. Airway epithelial cell responses to ozone injury. Environ Health Perspect. 1995;103(Suppl. 2):91–95. doi: 10.1289/ehp.95103s291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Peled N, Hakim M, Bunn PA, Jr, Miller YE, Kennedy TC, Mattei J, et al. Noninvasive breath analysis of pulmonary nodules. J Thorac Oncol. 2012;7:1528–1533. doi: 10.1097/JTO.0b013e3182637d5f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Krafft C, Dietzek B, Schmitt M, Popp J. Raman and coherent anti-Stokes Raman scattering microspectroscopy for biomedical applications. J Biomed Opt. 2012;17:040801. doi: 10.1117/1.JBO.17.4.040801. [DOI] [PubMed] [Google Scholar]
- 233.Folick A, Min W, Wang MC. Label-free imaging of lipid dynamics using Coherent Anti-stokes Raman Scattering (CARS) and Stimulated Raman Scattering (SRS) microscopy. Curr Opin Genet Dev. 2011;21:585–590. doi: 10.1016/j.gde.2011.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Le TT, Yue S, Cheng JX. Shedding new light on lipid biology with coherent anti-Stokes Raman scattering microscopy. J Lipid Res. 2010;51:3091–3102. doi: 10.1194/jlr.R008730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Bakry R, Rainer M, Huck CW, Bonn GK. Protein profiling for cancer biomarker discovery using matrix-assisted laser desorption/ionization time-of-flight mass spectrometry and infrared imaging: a review. Anal Chim Acta. 2011;690:26–34. doi: 10.1016/j.aca.2011.01.044. [DOI] [PubMed] [Google Scholar]
- 236.Walch A, Rauser S, Deininger SO, Hofler H. MALDI imaging mass spectrometry for direct tissue analysis: a new frontier for molecular histology. Histo-chem Cell Biol. 2008;130:421–434. doi: 10.1007/s00418-008-0469-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Berry KA, Hankin JA, Barkley RM, Spraggins JM, Caprioli RM, Murphy RC. MALDI imaging of lipid biochemistry in tissues by mass spectrometry. Chem Rev. 2011;111:6491–6512. doi: 10.1021/cr200280p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Fu D, Lu FK, Zhang X, Freudiger C, Pernik DR, Holtom G, et al. Quantitative chemical imaging with multiplex stimulated Raman scattering microscopy. J Am Chem Soc. 2012;134:3623–3626. doi: 10.1021/ja210081h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Zavaleta CL, Kircher MF, Gambhir SS. Raman’s effect’on molecular imaging. J Nucl Med. 2011;52:1839–1844. doi: 10.2967/jnumed.111.087775. [DOI] [PubMed] [Google Scholar]
- 240.Enejder A, Brackmann C, Svedberg F. Coherent anti-Stokes Raman scattering microscopy of cellular lipid storage. IEEE J Selected Top Quantum Electron. 2010;16:506–515. [Google Scholar]
- 241.Freudiger CW, Min W, Saar BG, Lu S, Holtom GR, He C, et al. Label-free biomedical imaging with high sensitivity by stimulated Raman scattering microscopy. Science. 2008;322:1857–1861. doi: 10.1126/science.1165758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Schultz ZD. Raman spectroscopic imaging of cholesterol and docosahexaenoic acid distribution in the retinal rod outer segment. Aust J Chem. 2011;64:611–616. doi: 10.1071/ch11019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Freudiger CW, Min W, Holtom GR, Xu B, Dantus M, Xie XS. Highly specific label-free molecular imaging with spectrally tailored excitation stimulated Raman scattering (STE-SRS) microscopy. Nat Photonics. 2011;5:103–109. doi: 10.1038/nphoton.2010.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Hellerer T, Axang C, Brackmann C, Hillertz P, Pilon M, Enejder A. Monitoring of lipid storage in Caenorhabditis elegans using coherent anti-Stokes Raman scattering (CARS) microscopy. Proc Natl Acad Sci USA. 2007;104:14658–14663. doi: 10.1073/pnas.0703594104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Nan X, Yang WY, Xie XS. CARS microscopy: lights up lipids in living cells. Bioph Int. 2004;11:44–47. [Google Scholar]
- 246.Huang YY, Beal CM, Cai WW, Ruoff RS, Terentjev EM. Micro-Raman spectroscopy of algae: composition analysis and fluorescence background behavior. Biotechnol Bioeng. 2010;105:889–898. doi: 10.1002/bit.22617. [DOI] [PubMed] [Google Scholar]
- 247.Konorov SO, Jardon MA, Piret JM, Blades MW, Turner RF. Raman microspec-troscopy of live cells under autophagy-inducing conditions. Analyst. 2012;137:4662–4668. doi: 10.1039/c2an35477b. [DOI] [PubMed] [Google Scholar]
- 248.Kast RE, Serhatkulu GK, Cao A, Pandya AK, Dai H, Thakur JS, et al. Raman spectroscopy can differentiate malignant tumors from normal breast tissue and detect early neoplastic changes in a mouse model. Biopolymers. 2008;89:235–241. doi: 10.1002/bip.20899. [DOI] [PubMed] [Google Scholar]
- 249.Kast R, Rabah R, Wills H, Poulik J, Auner GW, Klein MD. Differentiation of small round blue cell tumors using Raman spectroscopy. J Pediatr Surg. 2010;45:1110–1114. doi: 10.1016/j.jpedsurg.2010.02.072. [DOI] [PubMed] [Google Scholar]
- 250.Silveira L, Silveira FL, Bodanese B, Za Ngaro RA, Pacheco MT. Discriminating model for diagnosis of basal cell carcinoma and melanoma in vitro based on the Raman spectra of selected biochemicals. J Biomed Opt. 2012;17:077003. doi: 10.1117/1.JBO.17.7.077003. [DOI] [PubMed] [Google Scholar]
- 251.Pandya AK, Serhatkulu GK, Cao A, Kast RE, Dai H, Rabah R, et al. Evaluation of pancreatic cancer with Raman spectroscopy in a mouse model. Pancreas. 2008;36:e1–e8. doi: 10.1097/MPA.0b013e31815a3f1c. [DOI] [PubMed] [Google Scholar]
- 252.Li X, Yang T, Li S. Discrimination of serum Raman spectroscopy between normal and colorectal cancer using selected parameters and regression-discriminant analysis. Appl Opt. 2012;51:5038–5043. doi: 10.1364/AO.51.005038. [DOI] [PubMed] [Google Scholar]
- 253.Wang G, Lipert RJ, Jain M, Kaur S, Chakraboty S, Torres MP, et al. Detection of the potential pancreatic cancer marker MUC4 in serum using surface-enhanced Raman scattering. Anal Chem. 2011;83:2554–2561. doi: 10.1021/ac102829b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Pichardo-Molina JL, Frausto-Reyes C, Barbosa-Garcia O, Huerta-Franco R, Gonzalez-Trujillo JL, Ramirez-Alvarado CA, et al. Raman spectroscopy and multivariate analysis of serum samples from breast cancer patients. Lasers Med Sci. 2007;22:229–236. doi: 10.1007/s10103-006-0432-8. [DOI] [PubMed] [Google Scholar]
- 255.Manoharan R, Shafer K, Perelman L, Wu J, Chen K, Deinum G, et al. Raman spectroscopy and fluorescence photon migration for breast cancer diagnosis and imaging. Photochem Photobiol. 1998;67:15–22. [PubMed] [Google Scholar]
- 256.Choo-Smith LP, Edwards HG, Endtz HP, Kros JM, Heule F, Barr H, et al. Medical applications of Raman spectroscopy: from proof of principle to clinical implementation. Biopolymers. 2002;67:1–9. doi: 10.1002/bip.10064. [DOI] [PubMed] [Google Scholar]
- 257.Das RS, Agrawal YK. Raman spectroscopy: recent advancements, techniques and applications. Vib Spectrosc. 2011;57:163–176. [Google Scholar]
- 258.Fenn M, Pappu V. Data mining for cancer biomarkers with Raman spectroscopy. In: Pardalos PM, Xanthopoulos P, Zervakis M, editors. Data Mining for Biomarker Discovery. US: Springer; 2012. pp. 143–168. [Google Scholar]
- 259.de Carvalho LF, Bitar RA, Arisawa EA, Brandao AA, Honorio KM, Cabral LA, et al. Spectral region optimization for Raman-based optical biopsy of inflammatory lesions. Photomed Laser Surg. 2010;28(Suppl. 1):S111–S117. doi: 10.1089/pho.2009.2673. [DOI] [PubMed] [Google Scholar]
- 260.Tfayli A, Guillard E, Manfait M, Baillet-Guffroy A. Molecular interactions of penetration enhancers within ceramides organization: a Raman spectroscopy approach. Analyst. 2012;137:5002–5010. doi: 10.1039/c2an35220f. [DOI] [PubMed] [Google Scholar]
- 261.Simpson E, Bohling A, Bielfeldt S, Bosc C, Kerrouche N. Improvement of skin barrier function in atopic dermatitis patients with a new moisturizer containing a ceramide precursor. J Dermatolog Treat. 2012 doi: 10.3109/09546634.2012.713461. [DOI] [PubMed] [Google Scholar]
- 262.Tosato MG, Alves RS, Dos Santos EA, Raniero L, Menezes PF, Belletti KM, et al. Raman spectroscopic investigation of the effects of cosmetic formulations on the constituents and properties of human skin. Photomed Laser Surg. 2012;30:85–91. doi: 10.1089/pho.2011.3059. [DOI] [PubMed] [Google Scholar]
- 263.Wills H, Kast R, Stewart C, Rabah R, Pandya A, Poulik J, et al. Raman spectroscopy detects and distinguishes neuroblastoma and related tissues in fresh and (banked) frozen specimens. J Pediatr Surg. 2009;44:386–391. doi: 10.1016/j.jpedsurg.2008.10.095. [DOI] [PubMed] [Google Scholar]
- 264.Stone N, Kerssens M, Lloyd GR, Faulds K, Graham D, Matousek P. Surface enhanced spatially offset Raman spectroscopic (SESORS) imaging - the next dimension. Chem Sci. 2011;2:776–780. [Google Scholar]
- 265.von Maltzahn G, Centrone A, Park JH, Ramanathan R, Sailor MJ, Hatton TA, et al. SERS-coded gold nanorods as a multifunctional platform for densely multiplexed near-infrared imaging and photothermal heating. Adv Mater. 2009;21:3175–3180. doi: 10.1002/adma.200803464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Cheng K, Cheng Z. Near infrared receptor-targeted nanoprobes for early diagnosis of cancers. Curr Med Chem. 2012;19:4767–4785. doi: 10.2174/092986712803341458. [DOI] [PubMed] [Google Scholar]
- 267.Jiang L, Greenwood TR, Artemov D, Raman V, Winnard PT, Jr, Heeren RM, et al. Localized hypoxia results in spatially heterogeneous metabolic signatures in breast tumor models. Neoplasia. 2012;14:732–741. doi: 10.1593/neo.12858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Scalfi-Happ C, Udart M, Hauser C, Ru¨ck A. Investigation of lipid bodies in a colon carcinoma cell line by confocal Raman microscopy. Med Laser Appl. 2011;26:152–157. [Google Scholar]
- 269.Nieva C, Marro M, Santana-Codina N, Rao S, Petrov D, Sierra A. The lipid phenotype of breast cancer cells characterized by Raman microspectroscopy: towards a stratification of malignancy. PLoS One. 2012;7:e46456. doi: 10.1371/journal.pone.0046456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Brozek-Pluska B, Musial J, Kordek R, Bailo E, Dieing T, Abramczyk H. Raman spectroscopy and imaging: applications in human breast cancer diagnosis. Analyst. 2012;137:3773–3780. doi: 10.1039/c2an16179f. [DOI] [PubMed] [Google Scholar]
- 271.Yue S, Cárdenas-Mora Juan M, Chaboub Lesley S, Lelie`vre Sophie A, Cheng J-X. Label-free analysis of breast tissue polarity by Raman imaging of lipid phase. Biophys J. 2012;102:1215–1223. doi: 10.1016/j.bpj.2012.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Lee JF, Zeng Q, Ozaki H, Wang L, Hand AR, Hla T, et al. Dual roles of tight junction-associated protein, zonula occludens-1, in sphingosine 1-phos- phate-mediated endothelial chemotaxis and barrier integrity. J Biol Chem. 2006;281:29190–29200. doi: 10.1074/jbc.M604310200. [DOI] [PubMed] [Google Scholar]
- 273.Bielas SL, Silhavy JL, Brancati F, Kisseleva MV, Al-Gazali L, Sztriha L, et al. Mutations in INPP5E, encoding inositol polyphosphate-5-phosphatase E, link phosphatidyl inositol signaling to the ciliopathies. Nat Genet. 2009;41:1032–1036. doi: 10.1038/ng.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Wang G, Krishnamurthy K, Bieberich E. Regulation of primary cilia formation by ceramide. J Lipid Res. 2009;50:2103–2110. doi: 10.1194/jlr.M900097-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Mazzorana M, Montoya G, Mortuza GB. The centrosome: a target for cancer therapy. Curr Cancer Drug Targets. 2011;11:600–612. doi: 10.2174/156800911795655949. [DOI] [PubMed] [Google Scholar]
- 276.Yuan K, Frolova N, Xie Y, Wang D, Cook L, Kwon YJ, et al. Primary cilia are decreased in breast cancer: analysis of a collection of human breast cancer cell lines and tissues. J Histochem Cytochem. 2010;58:857–870. doi: 10.1369/jhc.2010.955856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Christensen ST, Clement CA, Satir P, Pedersen LB. Primary cilia and coordination of receptor tyrosine kinase (RTK) signalling. J Pathol. 2012;226:172–184. doi: 10.1002/path.3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Pidgeon GP, Lysaght J, Krishnamoorthy S, Reynolds JV, O’Byrne K, Nie D, et al. Lipoxygenase metabolism: roles in tumor progression and survival. Cancer Metastasis Rev. 2007;26:503–524. doi: 10.1007/s10555-007-9098-3. [DOI] [PubMed] [Google Scholar]
- 279.Szekeres CK, Trikha M, Honn KV. 12(S)-HETE, pleiotropic functions, multiple signaling pathways. Adv Exp Med Biol. 2002;507:509–515. doi: 10.1007/978-1-4615-0193-0_78. [DOI] [PubMed] [Google Scholar]
- 280.Blask DE, Hill SM, Dauchy RT, Xiang S, Yuan L, Duplessis T, et al. Circadian regulation of molecular, dietary, and metabolic signaling mechanisms of human breast cancer growth by the nocturnal melatonin signal and the consequences of its disruption by light at night. J Pineal Res. 2011;51:259–269. doi: 10.1111/j.1600-079X.2011.00888.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Sahar S, Sassone-Corsi P. Metabolism and cancer: the circadian clock connection. Nat Rev Cancer. 2009;9:886–896. doi: 10.1038/nrc2747. [DOI] [PubMed] [Google Scholar]
- 282.Wood PA, Yang X, Hrushesky WJ. Clock genes and cancer. Integr Cancer Ther. 2009;8:303–308. doi: 10.1177/1534735409355292. [DOI] [PubMed] [Google Scholar]
- 283.Gordon JA, Figard PH, Spector AA. Hydroxyeicosatetraenoic acid metabolism in cultured human skin fibroblasts. Evidence for peroxisomal beta-oxidation. J Clin Invest. 1990;85:1173–1181. doi: 10.1172/JCI114550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Mayatepek E, Lehmann WD. 12- and 15-hydroxyeicosatetraenoic acid are excreted in the urine of peroxisome-deficient patients: evidence for peroxi-somal metabolism in vivo. Pediatr Res. 1996;39:146–149. doi: 10.1203/00006450-199601000-00022. [DOI] [PubMed] [Google Scholar]
- 285.Dolegowska B, Blogowski W, Kedzierska K, Safranow K, Jakubowska K, Olszewska M, et al. Platelets arachidonic acid metabolism in patients with essential hypertension. Platelets. 2009;20:242–249. doi: 10.1080/09537100902849836. [DOI] [PubMed] [Google Scholar]
- 286.Xie C, Wang DH. Inhibition of renin release by arachidonic acid metabolites, 12(s)-HPETE and 12-HETE: role of TRPV1 channels. Endocrinology. 2011;152:3811–3819. doi: 10.1210/en.2011-0141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Hajjar DP, Pomerantz KB. Eicosanoids and their role in atherosclerosis. Arch Mal Coeur Vaiss. 1989;82(Spec No 4):21–26. [PubMed] [Google Scholar]
- 288.Poeckel D, Zemski Berry KA, Murphy RC, Funk CD. Dual 12/15- and 5-lipoxygenase deficiency in macrophages alters arachidonic acid metabolism and attenuates peritonitis and atherosclerosis in ApoE knock-out mice. J Biol Chem. 2009;284:21077–21089. doi: 10.1074/jbc.M109.000901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Abdel-Rahman EM, Abadir PM, Siragy HM. Regulation of renal 12(S)-hydroxyeicosatetraenoic acid in diabetes by angiotensin AT1 and AT2 receptors. Am J Physiol Regul Integr Comp Physiol. 2008;295:R1473–R1478. doi: 10.1152/ajpregu.90699.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Al-Shabrawey M, Mussell R, Kahook K, Tawfik A, Eladl M, Sarthy V, et al. Increased expression and activity of 12-lipoxygenase in oxygen-induced ischemic retinopathy and proliferative diabetic retinopathy: implications in retinal neovascularization. Diabetes. 2011;60:614–624. doi: 10.2337/db10-0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Ma K, Nunemaker CS, Wu R, Chakrabarti SK, Taylor-Fishwick DA, Nadler JL. 12-Lipoxygenase products reduce insulin secretion and {beta}-cell viability in human islets. J Clin Endocrinol Metab. 2010;95:887–893. doi: 10.1210/jc.2009-1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Obrosova IG, Stavniichuk R, Drel VR, Shevalye H, Vareniuk I, Nadler JL, et al. Different roles of 12/15-lipoxygenase in diabetic large and small fiber peripheral and autonomic neuropathies. Am J Pathol. 2010;177:1436–1447. doi: 10.2353/ajpath.2010.100178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Canals S, Casarejos MJ, de Bernardo S, Rodriguez-Martin E, Mena MA. Nitric oxide triggers the toxicity due to glutathione depletion in midbrain cultures through 12-lipoxygenase. J Biol Chem. 2003;278:21542–21549. doi: 10.1074/jbc.M213174200. [DOI] [PubMed] [Google Scholar]
- 294.Li Y, Maher P, Schubert D. A role for 12-lipoxygenase in nerve cell death caused by glutathione depletion. Neuron. 1997;19:453–463. doi: 10.1016/s0896-6273(00)80953-8. [DOI] [PubMed] [Google Scholar]
- 295.Lebeau A, Terro F, Rostene W, Pelaprat D. Blockade of 12-lipoxygenase expression protects cortical neurons from apoptosis induced by beta-amyloid peptide. Cell Death Differ. 2004;11:875–884. doi: 10.1038/sj.cdd.4401395. [DOI] [PubMed] [Google Scholar]
- 296.Pratico D, Zhukareva V, Yao Y, Uryu K, Funk CD, Lawson JA, et al. 12/15-lipoxygenase is increased in Alzheimer’s disease: possible involvement in brain oxidative stress. Am J Pathol. 2004;164:1655–1662. doi: 10.1016/S0002-9440(10)63724-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Succol F, Pratico D. A role for 12/15 lipoxygenase in the amyloid beta precursor protein metabolism. J Neurochem. 2007;103:380–387. doi: 10.1111/j.1471-4159.2007.04742.x. [DOI] [PubMed] [Google Scholar]
- 298.Negro-Vilar A, Conte D, Valenca M. Transmembrane signals mediating neural peptide secretion: role of protein kinase C activators and arachidonic acid metabolites in luteinizing hormone-releasing hormone secretion. Endocrinology. 1986;119:2796–2802. doi: 10.1210/endo-119-6-2796. [DOI] [PubMed] [Google Scholar]
- 299.Suh YG, Oh U. Activation and activators of TRPV1 and their pharmaceutical implication. Curr Pharm Des. 2005;11:2687–2698. doi: 10.2174/1381612054546789. [DOI] [PubMed] [Google Scholar]
- 300.Du YR, Xiao Y, Lu ZM, Ding J, Xie F, Fu H, et al. Melittin activates TRPV1 receptors in primary nociceptive sensory neurons via the phospholipase A2 cascade pathways. Biochem Biophys Res Commun. 2011;408:32–37. doi: 10.1016/j.bbrc.2011.03.110. [DOI] [PMC free article] [PubMed] [Google Scholar]